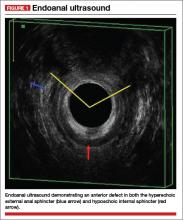
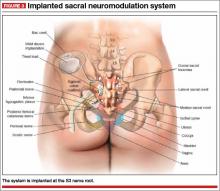
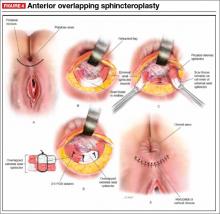
User login
Does vaginal prolapse repair using synthetic mesh confer long-term benefit over native-tissue colpopexy?
This is the third report from Gutman and colleagues on the outcomes of a double-blind, multicenter, randomized, controlled trial of vaginal prolapse repair using synthetic mesh versus native-tissue colpopexy in women with significant vaginal prolapse.
The trial involved 33 women who underwent mesh repair and 32 who underwent repair without mesh. (The mesh-free repair consisted primarily of uterosacral suspension and concurrent colporrhaphy.) It was halted when it reached a predetermined threshold for discontinuation, which was a mesh erosion rate of 15% or more.
Investigators found no difference in long-term cure rates between the mesh and no-mesh groups, regardless of the definition of cure (ie, anatomic, symptomatic, or combined). Nor was there a difference in the overall recurrence rate.
Summary of earlier reports
Three-month outcomes. The first report from this trial described 3-month objective treatment outcomes, with success described as prolapse no greater than stage 1.1 It found a high erosion rate (15.6%) for vaginal mesh, with no differences between groups in overall subjective or objective cure rates, with an overall recurrence rate of 59.4% (19 cases) in the mesh group versus 70.4% (24 cases) in the no-mesh group (P = .28), with recurrence defined as prolapse beyond stage 1 in any compartment. Investigators also observed potential benefit in the mesh group in the anterior vaginal wall at point Ba at a median of 9.7 months after surgery.
Related Article: Stop using synthetic mesh for routine repair of pelvic organ prolapse Cheryl B. Iglesia, MD (Stop/Start, April 2013)
One-year outcomes. The second report described 1-year objective and functional outcomes in all participants of the trial.2 It found comparable objective and subjective cure rates between groups but a higher reoperation rate for mesh repairs. Prolapse recurred in the anterior department in 46.9% of women in the mesh group versus 60.6% in the no-mesh group (P = .40).
Subjective quality-of-life assessments continued to reflect significant improvement in symptoms from baseline. Vaginal bulging was relieved in 96.2% of women in the mesh group, compared with 90.9% in the no-mesh group (P = .62).
More women in the mesh group required reoperation for recurrent prolapse or mesh exposure (5 in the mesh group vs 0 in the no-mesh group; P = .017).
Strengths and limitations of the trial
Gutman and colleagues are to be congratulated for continuing to monitor longer-term outcomes of vaginal prolapse repairs augmented with synthetic mesh, as data are sorely needed on both early complications and those more remote from surgery. However, it is regrettable that continued attrition in this trial led to minimal power to compare outcomes between groups.
Cure rates were assessed three ways: anatomically, by virtue of symptoms, and by a combination of the two measures. Participants had documentation of at least 2-year anatomic outcomes and 3-year subjective outcomes using validated measures.
Forty-one (63%) of the original 65 women in the trial had anatomic outcomes (20 in the mesh group vs 21 in the no-mesh group), and 51 (78%) of the original 65 women had evaluable subjective outcomes (25 in the mesh group vs 26 in the no-mesh group).
Women who underwent reoperation for recurrent prolapse were removed from any outcomes analysis and considered to have failed composite outcomes measures (anatomic and subjective assessment and whether reoperation or a pessary was required for recurrent prolapse).
The length of follow-up was similar between groups (median, 3 years; interquartile range, 2.97–3.15), and both groups demonstrated significant anatomic and subjective improvement from baseline.
No difference was observed between groups in the original primary anatomic outcome, which was a POP-Q stage no greater than 1 (45% in the mesh group vs 43% in the no-mesh group; P >.99). Nor was there a difference between groups in any other anatomic outcome, including POP-Q point Ba (median, –1.5 for mesh [range, –2.5, 1.0] vs –0.5 [range, –3.0, 4.0] for the no-mesh group; P = .21) and bulge symptoms (92% for the mesh group vs 81% for the no-mesh group; relative risk, 1.4; 95% confidence interval, 0.91–1.42).
Despite small numbers and markedly reduced comparative validity (readily acknowledged by the investigators), these longer-term outcomes were assessed by examiners blinded to treatment and using validated objective and subjective outcome measures.
The only other randomized trial of mesh versus native-tissue repair with 3-year outcomes had a much larger sample size and follow-up but addressed only anterior-compartment prolapse.3
What this evidence means for practice
The 3-year data presented by Gutman and colleagues should be viewed with caution, owing to the trial’s reduced sample size and power. However, they may be useful in designing future trials.
In the meantime, given the limited longer-term outcomes data available at present, I would recommend continued individualized use of mesh versus native-tissue repair in women presenting with prolapse, including educating patients about the risks and benefits of both approaches. It also is important that outcomes be followed in all of our patients in a robust, unbiased fashion. The new American Urogynecologic Society Pelvic Floor Disorders Registry provides the opportunity for this.
Holly E. Richter, PhD, MD
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
- Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: A randomized controlled trial. Obstet Gynecol. 2010;116(2 Pt 1):293–303.
- Sokol AI, Iglesia CB, Kudish BI, et al. One-year objective and functional outcomes of a randomized clinical trial of vaginal mesh for prolapse. Am J Obstet Gynecol. 2012;206(1):86.e1–e9.
- Nieminen K, Hiltunen R, Takala T, et al. Outcomes after anterior vaginal wall repair with mesh: A randomized, controlled trial with a 3-year follow-up. Am J Obstet Gynecol. 2010;203(3):235.e1–e8.
This is the third report from Gutman and colleagues on the outcomes of a double-blind, multicenter, randomized, controlled trial of vaginal prolapse repair using synthetic mesh versus native-tissue colpopexy in women with significant vaginal prolapse.
The trial involved 33 women who underwent mesh repair and 32 who underwent repair without mesh. (The mesh-free repair consisted primarily of uterosacral suspension and concurrent colporrhaphy.) It was halted when it reached a predetermined threshold for discontinuation, which was a mesh erosion rate of 15% or more.
Investigators found no difference in long-term cure rates between the mesh and no-mesh groups, regardless of the definition of cure (ie, anatomic, symptomatic, or combined). Nor was there a difference in the overall recurrence rate.
Summary of earlier reports
Three-month outcomes. The first report from this trial described 3-month objective treatment outcomes, with success described as prolapse no greater than stage 1.1 It found a high erosion rate (15.6%) for vaginal mesh, with no differences between groups in overall subjective or objective cure rates, with an overall recurrence rate of 59.4% (19 cases) in the mesh group versus 70.4% (24 cases) in the no-mesh group (P = .28), with recurrence defined as prolapse beyond stage 1 in any compartment. Investigators also observed potential benefit in the mesh group in the anterior vaginal wall at point Ba at a median of 9.7 months after surgery.
Related Article: Stop using synthetic mesh for routine repair of pelvic organ prolapse Cheryl B. Iglesia, MD (Stop/Start, April 2013)
One-year outcomes. The second report described 1-year objective and functional outcomes in all participants of the trial.2 It found comparable objective and subjective cure rates between groups but a higher reoperation rate for mesh repairs. Prolapse recurred in the anterior department in 46.9% of women in the mesh group versus 60.6% in the no-mesh group (P = .40).
Subjective quality-of-life assessments continued to reflect significant improvement in symptoms from baseline. Vaginal bulging was relieved in 96.2% of women in the mesh group, compared with 90.9% in the no-mesh group (P = .62).
More women in the mesh group required reoperation for recurrent prolapse or mesh exposure (5 in the mesh group vs 0 in the no-mesh group; P = .017).
Strengths and limitations of the trial
Gutman and colleagues are to be congratulated for continuing to monitor longer-term outcomes of vaginal prolapse repairs augmented with synthetic mesh, as data are sorely needed on both early complications and those more remote from surgery. However, it is regrettable that continued attrition in this trial led to minimal power to compare outcomes between groups.
Cure rates were assessed three ways: anatomically, by virtue of symptoms, and by a combination of the two measures. Participants had documentation of at least 2-year anatomic outcomes and 3-year subjective outcomes using validated measures.
Forty-one (63%) of the original 65 women in the trial had anatomic outcomes (20 in the mesh group vs 21 in the no-mesh group), and 51 (78%) of the original 65 women had evaluable subjective outcomes (25 in the mesh group vs 26 in the no-mesh group).
Women who underwent reoperation for recurrent prolapse were removed from any outcomes analysis and considered to have failed composite outcomes measures (anatomic and subjective assessment and whether reoperation or a pessary was required for recurrent prolapse).
The length of follow-up was similar between groups (median, 3 years; interquartile range, 2.97–3.15), and both groups demonstrated significant anatomic and subjective improvement from baseline.
No difference was observed between groups in the original primary anatomic outcome, which was a POP-Q stage no greater than 1 (45% in the mesh group vs 43% in the no-mesh group; P >.99). Nor was there a difference between groups in any other anatomic outcome, including POP-Q point Ba (median, –1.5 for mesh [range, –2.5, 1.0] vs –0.5 [range, –3.0, 4.0] for the no-mesh group; P = .21) and bulge symptoms (92% for the mesh group vs 81% for the no-mesh group; relative risk, 1.4; 95% confidence interval, 0.91–1.42).
Despite small numbers and markedly reduced comparative validity (readily acknowledged by the investigators), these longer-term outcomes were assessed by examiners blinded to treatment and using validated objective and subjective outcome measures.
The only other randomized trial of mesh versus native-tissue repair with 3-year outcomes had a much larger sample size and follow-up but addressed only anterior-compartment prolapse.3
What this evidence means for practice
The 3-year data presented by Gutman and colleagues should be viewed with caution, owing to the trial’s reduced sample size and power. However, they may be useful in designing future trials.
In the meantime, given the limited longer-term outcomes data available at present, I would recommend continued individualized use of mesh versus native-tissue repair in women presenting with prolapse, including educating patients about the risks and benefits of both approaches. It also is important that outcomes be followed in all of our patients in a robust, unbiased fashion. The new American Urogynecologic Society Pelvic Floor Disorders Registry provides the opportunity for this.
Holly E. Richter, PhD, MD
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
This is the third report from Gutman and colleagues on the outcomes of a double-blind, multicenter, randomized, controlled trial of vaginal prolapse repair using synthetic mesh versus native-tissue colpopexy in women with significant vaginal prolapse.
The trial involved 33 women who underwent mesh repair and 32 who underwent repair without mesh. (The mesh-free repair consisted primarily of uterosacral suspension and concurrent colporrhaphy.) It was halted when it reached a predetermined threshold for discontinuation, which was a mesh erosion rate of 15% or more.
Investigators found no difference in long-term cure rates between the mesh and no-mesh groups, regardless of the definition of cure (ie, anatomic, symptomatic, or combined). Nor was there a difference in the overall recurrence rate.
Summary of earlier reports
Three-month outcomes. The first report from this trial described 3-month objective treatment outcomes, with success described as prolapse no greater than stage 1.1 It found a high erosion rate (15.6%) for vaginal mesh, with no differences between groups in overall subjective or objective cure rates, with an overall recurrence rate of 59.4% (19 cases) in the mesh group versus 70.4% (24 cases) in the no-mesh group (P = .28), with recurrence defined as prolapse beyond stage 1 in any compartment. Investigators also observed potential benefit in the mesh group in the anterior vaginal wall at point Ba at a median of 9.7 months after surgery.
Related Article: Stop using synthetic mesh for routine repair of pelvic organ prolapse Cheryl B. Iglesia, MD (Stop/Start, April 2013)
One-year outcomes. The second report described 1-year objective and functional outcomes in all participants of the trial.2 It found comparable objective and subjective cure rates between groups but a higher reoperation rate for mesh repairs. Prolapse recurred in the anterior department in 46.9% of women in the mesh group versus 60.6% in the no-mesh group (P = .40).
Subjective quality-of-life assessments continued to reflect significant improvement in symptoms from baseline. Vaginal bulging was relieved in 96.2% of women in the mesh group, compared with 90.9% in the no-mesh group (P = .62).
More women in the mesh group required reoperation for recurrent prolapse or mesh exposure (5 in the mesh group vs 0 in the no-mesh group; P = .017).
Strengths and limitations of the trial
Gutman and colleagues are to be congratulated for continuing to monitor longer-term outcomes of vaginal prolapse repairs augmented with synthetic mesh, as data are sorely needed on both early complications and those more remote from surgery. However, it is regrettable that continued attrition in this trial led to minimal power to compare outcomes between groups.
Cure rates were assessed three ways: anatomically, by virtue of symptoms, and by a combination of the two measures. Participants had documentation of at least 2-year anatomic outcomes and 3-year subjective outcomes using validated measures.
Forty-one (63%) of the original 65 women in the trial had anatomic outcomes (20 in the mesh group vs 21 in the no-mesh group), and 51 (78%) of the original 65 women had evaluable subjective outcomes (25 in the mesh group vs 26 in the no-mesh group).
Women who underwent reoperation for recurrent prolapse were removed from any outcomes analysis and considered to have failed composite outcomes measures (anatomic and subjective assessment and whether reoperation or a pessary was required for recurrent prolapse).
The length of follow-up was similar between groups (median, 3 years; interquartile range, 2.97–3.15), and both groups demonstrated significant anatomic and subjective improvement from baseline.
No difference was observed between groups in the original primary anatomic outcome, which was a POP-Q stage no greater than 1 (45% in the mesh group vs 43% in the no-mesh group; P >.99). Nor was there a difference between groups in any other anatomic outcome, including POP-Q point Ba (median, –1.5 for mesh [range, –2.5, 1.0] vs –0.5 [range, –3.0, 4.0] for the no-mesh group; P = .21) and bulge symptoms (92% for the mesh group vs 81% for the no-mesh group; relative risk, 1.4; 95% confidence interval, 0.91–1.42).
Despite small numbers and markedly reduced comparative validity (readily acknowledged by the investigators), these longer-term outcomes were assessed by examiners blinded to treatment and using validated objective and subjective outcome measures.
The only other randomized trial of mesh versus native-tissue repair with 3-year outcomes had a much larger sample size and follow-up but addressed only anterior-compartment prolapse.3
What this evidence means for practice
The 3-year data presented by Gutman and colleagues should be viewed with caution, owing to the trial’s reduced sample size and power. However, they may be useful in designing future trials.
In the meantime, given the limited longer-term outcomes data available at present, I would recommend continued individualized use of mesh versus native-tissue repair in women presenting with prolapse, including educating patients about the risks and benefits of both approaches. It also is important that outcomes be followed in all of our patients in a robust, unbiased fashion. The new American Urogynecologic Society Pelvic Floor Disorders Registry provides the opportunity for this.
Holly E. Richter, PhD, MD
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected]
- Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: A randomized controlled trial. Obstet Gynecol. 2010;116(2 Pt 1):293–303.
- Sokol AI, Iglesia CB, Kudish BI, et al. One-year objective and functional outcomes of a randomized clinical trial of vaginal mesh for prolapse. Am J Obstet Gynecol. 2012;206(1):86.e1–e9.
- Nieminen K, Hiltunen R, Takala T, et al. Outcomes after anterior vaginal wall repair with mesh: A randomized, controlled trial with a 3-year follow-up. Am J Obstet Gynecol. 2010;203(3):235.e1–e8.
- Iglesia CB, Sokol AI, Sokol ER, et al. Vaginal mesh for prolapse: A randomized controlled trial. Obstet Gynecol. 2010;116(2 Pt 1):293–303.
- Sokol AI, Iglesia CB, Kudish BI, et al. One-year objective and functional outcomes of a randomized clinical trial of vaginal mesh for prolapse. Am J Obstet Gynecol. 2012;206(1):86.e1–e9.
- Nieminen K, Hiltunen R, Takala T, et al. Outcomes after anterior vaginal wall repair with mesh: A randomized, controlled trial with a 3-year follow-up. Am J Obstet Gynecol. 2010;203(3):235.e1–e8.
What's the appropriate lens to use in rigid cystoscopy to evaluate the bladder?
Dr. Gebhart says an angled lens is critical to viewing the bladder, but which angle is ideal?
When Dr. Gebhart surveyed attendees of the Pelvic Anatomy and Gynecology Symposium in Las Vegas, Nevada, in December 2013, as to which lens angle was the best option, the majority chose the 30-degree lens. Listen to why Dr. Gebhart recommends the 70-degree lens.
Dr. Gebhart says an angled lens is critical to viewing the bladder, but which angle is ideal?
When Dr. Gebhart surveyed attendees of the Pelvic Anatomy and Gynecology Symposium in Las Vegas, Nevada, in December 2013, as to which lens angle was the best option, the majority chose the 30-degree lens. Listen to why Dr. Gebhart recommends the 70-degree lens.
Dr. Gebhart says an angled lens is critical to viewing the bladder, but which angle is ideal?
When Dr. Gebhart surveyed attendees of the Pelvic Anatomy and Gynecology Symposium in Las Vegas, Nevada, in December 2013, as to which lens angle was the best option, the majority chose the 30-degree lens. Listen to why Dr. Gebhart recommends the 70-degree lens.
Vaginal pessaries VIDEO: Proper insertion and removal
Written and narrated by Teresa Tam, MD
For the related article, see: Pessaries for vaginal prolapse: Critical factors to successful fit and continued use Teresa Tam, MD, and Matthew Davies, MD (Surgical Techniques, December 2013)
Written and narrated by Teresa Tam, MD
For the related article, see: Pessaries for vaginal prolapse: Critical factors to successful fit and continued use Teresa Tam, MD, and Matthew Davies, MD (Surgical Techniques, December 2013)
Written and narrated by Teresa Tam, MD
For the related article, see: Pessaries for vaginal prolapse: Critical factors to successful fit and continued use Teresa Tam, MD, and Matthew Davies, MD (Surgical Techniques, December 2013)
Pessaries for vaginal prolapse: Critical factors to successful fit and continued use
CASE 1. EARLY-STAGE PELVIC ORGAN PROLAPSE
AC is a 64-year-old white woman with early stage III anterior and apical pelvic organ prolapse (POP). The prolapse is now affecting her ability to do some of the things that she enjoys, such as gardening and golfing.
She has hypertension controlled with medication and no other significant medical issues except mild arthritic changes in her hands and hips. She reports being sexually active with her husband on roughly a weekly basis.
On examination, the leading edge of her prolapse is the anterior vaginal wall, protruding 1 cm beyond the introitus, and the cervix is at the hymenal ring. There is no significant posterior wall prolapse.
After she is counseled about all possible treatment approaches for her early-stage POP, the patient elects to try the vaginal pessary. Now, it is your job to determine the optimal pessary based on the extent of her condition and to educate her about the potential side effects and best practices for its ongoing use.
The vaginal pessary is an important component of a gynecologist’s armamentarium. It is a low-risk, cost-effective, nonsurgical treatment option for the management of POP and genuine stress urinary incontinence (SUI).1,2 It is unfortunate that training in North America typically provides clinicians with only a cursory experience with pessary selection and care, minimizing the device’s importance as a viable tool in a practitioner’s ongoing practice. In fact, most clinicians tend to view the pessary with a mixture of reluctance and disregard.
This is regrettable, as a majority (89%) of patients can be successfully fitted with a pessary,3 regardless of their stage or site of prolapse.4 Although high-stage prolapse does not predict failure, ring pessaries are used most successfully with stage II (100%) and stage III (71%) prolapse, while Gellhorn pessaries are most successful with stage IV (64%) prolapse.5
In this article we review the several pessary options available to clinicians, as well as how to insert them and the best scenarios for their use. We also discuss the key requirements for patient assessment and in-office fitting (meant to optimize the fit and, thereby, the success of use), the possible side effects of pessary use that patients need to be aware of, and appropriate follow-up.
WHEN IS A PESSARY YOUR BEST MANAGEMENT APPROACH?
There are several indications for pessary use,6 namely when:
- the patient has significant comorbid risk factors for surgery
- the patient prefers a nonsurgical alternative
- a goal is to avoid reoperation
- POP or cervical insufficiency is present during pregnancy
- the patient desires future fertility
- surgery must be delayed due to treatment of vaginal ulcerations
- the pessary will be used as a postoperative adjunct to mesh-based repair.
Pessaries have very few contraindications (TABLE). However, factors that do negatively affect successful fitting include:
- prior pelvic surgery
- multiparity
- obesity
- SUI
- short vaginal length (<7 cm)
- wide vaginal introitus (>4 fingerbreadths)
- significant posterior vaginal wall defect.5,7-9
There are two main categories of vaginal pessaries: support and space-filling. All pessaries come in different sizes and shapes. Most are made of medical-grade silicone, rendering them durable and autoclavable as well as resistant to absorption of vaginal discharge and odors. The ring pessary with support is the most commonly used support pessary. The Gellhorn pessary is the most commonly used space-filling pessary. It is used as a second-line treatment for patients unable to retain the ring-with-support pessary.
Related Article: Pessary and pelvic floor exercises for incontinence—are two better than one? G. Willy Davila, MD (Examining the Evidence, May 2010)
SUPPORT PESSARY OPTIONS
The support pessaries are used to treat SUI and POP. These pessaries typically are the easiest types for patients to use because they are more comfortable and simpler to remove and insert than space-filling pessaries. For example, a ring pessary is two-dimensional and lies perpendicular to the long axis of the vagina, allowing patients to have intercourse with it in place. Support-type pessaries include the ring, Gehrung, Shaatz, and lever.
Ring
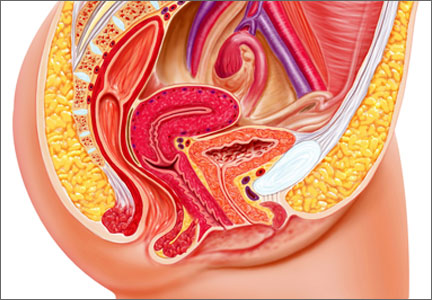
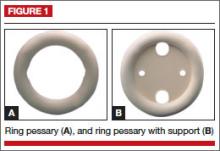
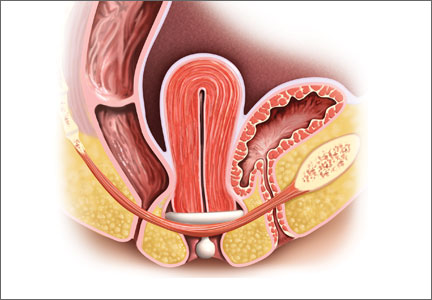
This is the most commonly used pessary because it fits most women. There are four types of ring pessaries: the ring (FIGURE 1A), ring with support (FIGURE 1B), incontinence ring, and incontinence ring with support. The ring pessary is appropriate for all stages of POP. The ring with support has a diaphragm that is useful in women who have uterine prolapse with or without cystocele. The incontinence ring has a knob that is placed beneath the urethra to increase urethral pressure and is useful in cases of SUI.
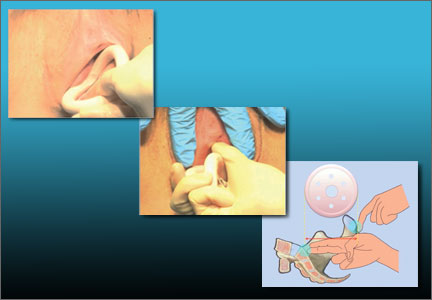
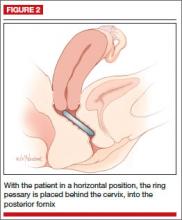
Insertion. Fold the pessary by bringing the two small holes together, and lubricate the leading edge. Insert it past the introitus with the folded edge facing down. Allow the pessary to reopen, and direct it behind the cervix into the posterior fornix (FIGURE 2). Give it a slight twist with your index finger to prevent expulsion.
To see insertion demonstrated, watch Vaginal pessaries: An instructional video
Gehrung
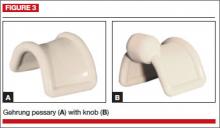
This pessary is designed with an arch-shaped malleable rim with wires incorporated into the arms (FIGURE 3). Use of the Gehrung pessary is rare; it is most often used in women with cystocele or rectocele.
Insertion. Fold the pessary to insert it into the vagina. Upon insertion, keep both heels of the pessary parallel to the posterior vagina with the back arch pushed over the cervix in the anterior fornix and the front arch resting behind the symphysis pubis. The concave surface and diaphragm support the anterior vagina. Place the convex portion of the curve beneath the bulge. The two bases rest on the posterior vagina against the lateral levator muscles.
Shaatz
This support pessary has a circular base similar to the Gellhorn pessary but without the rigid stem (FIGURE 4).
Insertion. Because it is stiff, insert this pessary vertically and then turn it to a horizontal position once it is inside the vagina.
Lever
The Hodge, Smith, and Risser pessaries are collectively called the lever pessaries. They are used to manage uterine retroversion and POP. They are rarely used.
The Hodge pessary is beneficial to patients with a narrow vaginal introitus, mild cystocele, and cervical insufficiency. The anterior portion of a Hodge pessary is rectangular (FIGURE 5A).
The Smith pessary is useful for patients with well-defined pubic notches because the anterior portion is rounded (FIGURE 5B).
For patients with a very shallow pubic notch, the Risser pessary is useful. The Risser’s anterior portion is rectangular with indentation but wider than the Hodge pessary (FIGURE 5C).
Insertion. Fold the pessary and insert it into the vagina with the index finger on the posterior curved bar until the pessary rests behind the cervix and the anterior horizontal bar rests behind the symphysis pubis.
SPACE-OCCUPYING PESSARIES
The second pessary category is the space-filling pessary. These pessaries are used primarily to support severe POP, especially posthysterectomy vaginal vault prolapse. They have larger bases to support the vaginal apex or cervix; therefore, they are more difficult to insert and remove. When this pessary type is in place, sexual intercourse is not possible. Examples include the Gellhorn, donut, cube, and inflatable pessaries.
Gellhorn
The Gellhorn pessary is the most commonly used space-filling pessary. It has a broad base with a stem (FIGURE 6). The broad base supports the vaginal apex while the stem keeps the circular base from rotating and prevents pessary expulsion. The stem comes in long or short lengths. The concave base provides vaginal suction and keeps the pessary in place. The holes in the stem and base provide vaginal drainage. The Gellhorn pessary is useful for women with more advanced prolapse and less perineal support.
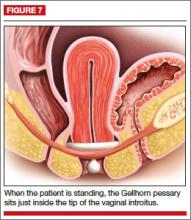
Insertion. Folding one side of the base to the stem, insert the Gellhorn pessary vertically inside the vagina. To facilitate insertion, separate the labia with the nondominant hand or depress the perineum with the index finger. Once the circular base is inside the vagina, push the pessary upward until the tip of the stem is just inside the vaginal introitus (FIGURE 7). Many medical illustrations inaccurately depict the Gellhorn pessary in a final placement that appears too high in the pelvis. This figure, which has the patient in a standing position, shows how low in the pelvis this space-filling pessary can sit in a patient with advanced prolapse.
Remove this pessary by gently pulling the stem while inserting the opposite hand beneath an edge of the pessary base to break the vaginal suction (Watch Vaginal pessaries: An instructional video).
Donut
The donut pessary is used for advanced prolapse because it fills a larger space. It is difficult to insert and remove because it is large, thick, and hollow (FIGURE 8).
Insertion. Insert it vertically and, once it is placed inside the vagina, rotate it to a horizontal position. A Kelly clamp can be used to grasp the pessary and facilitate removal.
Cube
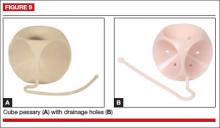
The cube pessary supports third-degree uterine prolapse by holding the vaginal wall with suction (FIGURE 9). Because of the risk of vaginal erosion and lack of drainage in some designs, the cube pessary requires nightly removal and cleaning.
Insertion. Squeezing the pessary with the thumb, index, and middle fingers, insert the cube pessary at the vaginal apex.
Removal requires breaking the suction by placing a fingertip between the vaginal mucosa and the pessary and compressing the cube between the thumb and forefinger to remove. Gently tugging on the string also helps with removal.
Inflatable
This space-filling pessary is an air-filled ball that is inflated via an attached stem that also enables insertion and removal. The older Inflatoball pessary is made of latex, so its use is contraindicated in patients with latex allergy. Newer inflatable pessaries are silicone-based and consist of an air-filled donut, a stem with a valve, and an air pump (FIGURE 10). Some models also include a deflation key. The inflatable pessary comes in small, medium, large, and extra-large sizes. This pessary type must be removed and cleaned daily.
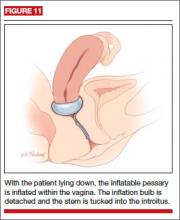
Insertion. Place the deflated pessary into the vagina. Move the ball-bearing valve within the stem (which controls the air flow) to a lateral projection on the side of the stem. To inflate, attach the inflation bulb. (Inflation typically requires 3 to 5 pumps of the bulb.) Move the ball bearing back into position to maintain the inflation, then detach the bulb. You can leave the stem outside the body or tuck it gently into the introitus (FIGURE 11).
INCONTINENCE PESSARIES
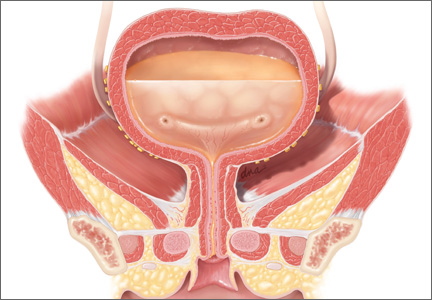
These devices are used specifically for SUI. The incontinence ring (FIGURE 12) and incontinence dish pessaries compress the urethra against the pubic symphysis. The knob is placed beneath the urethra, increasing the urethral closure pressure and thereby preventing urinary incontinence.
Related Article: Update on Urinary Incontinence Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (December 2011)
CASE 1 CONCLUDED
Given that AC has early-stage POP and is sexually active, a space-occupying pessary is not the optimal choice. Instead, a ring pessary with support is fitted for her trial.
What side effects might a patient anticipate with pessary use?
Vaginal discharge and slight odor are common. Pessary removal and cleaning are usually adequate to eliminate them. Temporary discontinuation of pessary use may be warranted until symptoms subside. If these maneuvers do not resolve the issue, then the patient should be examined to rule out other sources of infection.
Vaginal bleeding. Bleeding from vaginal abrasion and ulceration could be caused by trauma from pessary removal or vaginal impingement. Evaluation is warranted for any vaginal bleeding.
Changes in urinary function. Less commonly, women using a pessary may notice changes in their urinary function. Many women with anterior or apical prolapse will have altered urine streams with slow or trickling flow and possible hesitation upon initiation of voiding.
Alternatively, pessary placement may instigate stress-type incontinence akin to that seen after prolapse surgery. Changing pessary size may alleviate this condition. Otherwise, these side effects may reduce a patient’s willingness to continue pessary use.
How can a patient optimize her use of a pessary?
A patient can remove the pessary on a periodic basis or try to use it continuously. If she cannot or will not remove the pessary, then she will need to come back for scheduled visits, as described in the sidebar, “Essential components of a successfully fitted pessary.” If she is able to remove the pessary on her own, then she can use the device as needed or remove it for intercourse (though it is not necessary). She must remove it weekly, at a minimum, however, to both clean the pessary and give the vaginal walls a “rest,” which can minimize the potential for abrasions or erosions
ESSENTIAL COMPONENTS OF A SUCCESSFULLY FITTED PESSARY
Patient assessment
Accurate selection and placement of a pessary requires appropriate examination and fitting, beginning with determination of the patient’s stage of prolapse and introitus. Key steps include:
– Examine the patient with an empty bladder in the lithotomy position
– Perform bimanual pelvic and speculum examination using a Sims speculum (or bivalve speculum broken in half) with the patient in a supine position
– Administer the Pelvic Organ Prolapse Quantification (POP-Q) exam
– Perform digital examination
– Assess vaginal atrophy, vaginal introitus, and vaginal width and length
– Evaluate pelvic floor muscle strength (Kegel squeeze).
Next, gauge the correct pessary size by approximating the number of fingerbreadths accommodated across the vaginal width.
Another method of estimating pessary size is to insert two fingers inside the vagina and estimate the distance between the posterior fornix and the posterior pubic symphysis (Watch Vaginal pessaries: An instructional video). An easy reference is to start with a size 3 or 4 ring pessary if the vaginal introitus is 1 to 2 fingerbreadths in width and the prolapse is stage II to III. If the vagina accommodates 3 to 4 fingerbreadths, or there is stage IV prolapse, use a Gellhorn pessary.
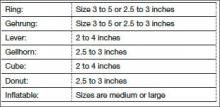
Here are the different types of pessaries and the most common sizes available. (Pessary sizes change in quarter-inch increments.)
In-office trial
Insert the pessary into the vagina using the dominant hand. Using the nondominant hand, separate the introitus and depress the perineal body. Apply a small amount of lubricant to the leading edge of the pessary.
After insertion, ask the patient to strain and cough, ambulate in the office, and void. Reexamine the patient to ensure that the pessary is still in the correct position and that placement has not shifted. Perform the cough leak test with the patient in a standing position and the pessary in place. Re-examine the patient while she is in a standing position. Use the largest pessary that is comfortable for her. Advise her to bring the pessary back to the office if it gets expelled.
This is a trial-and-error process; advise the patient of this. It may require a trial of several styles and sizes to find the right pessary fit. Once you find the correct size, document the final pessary size.
Follow-up
Schedule a follow-up appointment 1 to 2 weeks after insertion. Ask the patient whether she has experienced any discomfort, malodorous discharge, or vaginal bleeding. Also inquire about any changes in urinary habits or bowel movements and related complaints.
Remove the pessary and clean it with mild soap and water. Examine the vagina for pressure points, abrasions, ulcerations, and erosions.
Teach the patient how to remove, clean, and reinsert the pessary, and advise her to perform these tasks on a weekly basis.
Schedule a follow-up visit in 1 to 2 months, and another visit 6 to 12 months after that.
CASE 2. ADVANCED-STAGE POP
BD is an 82-year-old widow (G5P4014) with stage IV vaginal prolapse. She has noticed some scant blood staining on her clothing. She frequently voids small amounts of urine but never feels complete relief. She defecates normally.
Her medical history is significant for coronary artery disease with prior myocardial infarction, with multiple stent placements over the years. She has hypertension, reduced ejection fraction, and diabetes. She is morbidly obese and suffers from degenerative joint disease. She had a vaginal hysterectomy several years ago for benign indications.
Upon examination, BD’s prolapse is large, with excoriations and hyperkeratosis of the skin over the prolapse. It is easily reduced in the office.
What is the best pessary for this patient, and how should she be followed and counseled regarding ongoing care?
Since the failure rate for pessary usage increases with advancing prolapse stage, a space-occupying pessary is most appropriate to try initially. A trial with a support pessary could be useful to allow the excoriations to heal and provide a healthier vaginal environment. A Gellhorn pessary is commonly used. An inflatable pessary could be an alternative if the Gellhorn fails to stay in place. The cube pessary, known to cause more abrasions and erosions than other pessaries, is a poor choice given the state of the patient’s vaginal tissues at baseline.
Space-occupying pessaries are more difficult to insert and remove and have a higher risk of pain or trauma. Start with shorter time intervals between visits, eventually spacing them out for the patient’s convenience. The usual interval for follow-up is 3 to 4 months; longer intervals could be offered if the patient is reliable, adherent, and reports no complaints with pessary use.
Related Article: Update on pelvic floor dysfunction: Focus on urinary incontinence Alexis A. Dieter, MD, and Cindy L. Amundsen, MD (November 2013)
OUTCOMES
Only short- and medium-term outcomes for pessary use have been described in the literature. Short-term (2 months) satisfaction and continued use, along with resolution of prolapse, occurred in 92% of patients.7 Previous hysterectomy or prolapse surgery may influence the short-term success of pessary use.10
More than half of sexually active women achieved long-term use (up to 2 years), regardless of prolapse severity. Brincat and colleagues found that long-term pessary use (1 to 2 years) approached 60% in 132 women with both urinary incontinence and prolapse. Women being treated for POP were more likely to continue pessary use than women being treated for SUI.11 Age, parity, estrogen use, and sexual activity were characteristics also studied in pessary fitting. Neither sexual activity nor stage of prolapse was a contraindication to use of a pessary; long-term use was found to be acceptable in sexually active women.11
Successful fitting of a vaginal pessary has been associated with improvement in voiding, urinary and fecal urgency, and incontinence. A vaginal pessary is a viable nonsurgical option for the management of POP and urinary incontinence and remains an optimal minimally invasive approach to such disorders.
CASE 2 CONCLUDED
The patient returns to the clinic 1 month after the original insertion. The pessary is removed, and the vagina is inspected, with no abrasions or ulcerations found. The vaginal cavity and pessary are cleaned with a mild soap-and-water mixture. The pessary is lubricated and reinserted. This process is repeated 2 months later, with subsequent follow-up intervals doubled (up to 6 months between visits) when the patient has no complaints of discharge or odor.
- Colmer, WM Jr. Use of the pessary. Am J Obstet Gynecol. 1953;65(1):170–174.
- Culligan PJ. Nonsurgical management to pelvic organ prolapse. Obstet Gynecol. 2012;119(4):852–860.
- Nygaard IE, Heit M. Stress urinary incontinence. Obstet Gynecol. 2004;104(3):607–620.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 85: Pelvic organ prolapse. Obstet Gynecol. 2007;110(3):717–729.
- Clemons JL, Aguilar VC, Tillinghast TA, Jackson ND, Myers DL. Risk factors associated with an unsuccessful pessary fitting trial in women with pelvic organ prolapse. Am J Obstet Gynecol. 2004;190(2):345–350.
- Clemons JL, Brubaker L, Falk SJ. Vaginal pessary treatment of prolapse and incontinence. UpToDate. http://www.uptodate.com/contents/vaginal-pessary-treatment-of-prolapse-and-incontinence. Updated February 8, 2013. Accessed November 7, 2013.
- Mutone MF, Terry C, Hale DS, Benson JT. Factors which influence the short-term success of pessary management of pelvic organ prolapse. Am J Obstet Gynecol. 2005;193(1):89–94.
- Fernando RJ, Thakar R, Sultan AH, Shah SM, Jones PW. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108(1):93–99.
- Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol. 2005;106(3):615–634.
- Donnelly MJ, Powell-Morgan S, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(5):302–307.
- Brincat C, Kenton K, Fitzgerald MP, et al. Sexual activity predicts continued pessary use. Am J Obstet Gynecol. 2004;191(1):198–200.
CASE 1. EARLY-STAGE PELVIC ORGAN PROLAPSE
AC is a 64-year-old white woman with early stage III anterior and apical pelvic organ prolapse (POP). The prolapse is now affecting her ability to do some of the things that she enjoys, such as gardening and golfing.
She has hypertension controlled with medication and no other significant medical issues except mild arthritic changes in her hands and hips. She reports being sexually active with her husband on roughly a weekly basis.
On examination, the leading edge of her prolapse is the anterior vaginal wall, protruding 1 cm beyond the introitus, and the cervix is at the hymenal ring. There is no significant posterior wall prolapse.
After she is counseled about all possible treatment approaches for her early-stage POP, the patient elects to try the vaginal pessary. Now, it is your job to determine the optimal pessary based on the extent of her condition and to educate her about the potential side effects and best practices for its ongoing use.
The vaginal pessary is an important component of a gynecologist’s armamentarium. It is a low-risk, cost-effective, nonsurgical treatment option for the management of POP and genuine stress urinary incontinence (SUI).1,2 It is unfortunate that training in North America typically provides clinicians with only a cursory experience with pessary selection and care, minimizing the device’s importance as a viable tool in a practitioner’s ongoing practice. In fact, most clinicians tend to view the pessary with a mixture of reluctance and disregard.
This is regrettable, as a majority (89%) of patients can be successfully fitted with a pessary,3 regardless of their stage or site of prolapse.4 Although high-stage prolapse does not predict failure, ring pessaries are used most successfully with stage II (100%) and stage III (71%) prolapse, while Gellhorn pessaries are most successful with stage IV (64%) prolapse.5
In this article we review the several pessary options available to clinicians, as well as how to insert them and the best scenarios for their use. We also discuss the key requirements for patient assessment and in-office fitting (meant to optimize the fit and, thereby, the success of use), the possible side effects of pessary use that patients need to be aware of, and appropriate follow-up.
WHEN IS A PESSARY YOUR BEST MANAGEMENT APPROACH?
There are several indications for pessary use,6 namely when:
- the patient has significant comorbid risk factors for surgery
- the patient prefers a nonsurgical alternative
- a goal is to avoid reoperation
- POP or cervical insufficiency is present during pregnancy
- the patient desires future fertility
- surgery must be delayed due to treatment of vaginal ulcerations
- the pessary will be used as a postoperative adjunct to mesh-based repair.
Pessaries have very few contraindications (TABLE). However, factors that do negatively affect successful fitting include:
- prior pelvic surgery
- multiparity
- obesity
- SUI
- short vaginal length (<7 cm)
- wide vaginal introitus (>4 fingerbreadths)
- significant posterior vaginal wall defect.5,7-9
There are two main categories of vaginal pessaries: support and space-filling. All pessaries come in different sizes and shapes. Most are made of medical-grade silicone, rendering them durable and autoclavable as well as resistant to absorption of vaginal discharge and odors. The ring pessary with support is the most commonly used support pessary. The Gellhorn pessary is the most commonly used space-filling pessary. It is used as a second-line treatment for patients unable to retain the ring-with-support pessary.
Related Article: Pessary and pelvic floor exercises for incontinence—are two better than one? G. Willy Davila, MD (Examining the Evidence, May 2010)
SUPPORT PESSARY OPTIONS
The support pessaries are used to treat SUI and POP. These pessaries typically are the easiest types for patients to use because they are more comfortable and simpler to remove and insert than space-filling pessaries. For example, a ring pessary is two-dimensional and lies perpendicular to the long axis of the vagina, allowing patients to have intercourse with it in place. Support-type pessaries include the ring, Gehrung, Shaatz, and lever.
Ring
This is the most commonly used pessary because it fits most women. There are four types of ring pessaries: the ring (FIGURE 1A), ring with support (FIGURE 1B), incontinence ring, and incontinence ring with support. The ring pessary is appropriate for all stages of POP. The ring with support has a diaphragm that is useful in women who have uterine prolapse with or without cystocele. The incontinence ring has a knob that is placed beneath the urethra to increase urethral pressure and is useful in cases of SUI.
Insertion. Fold the pessary by bringing the two small holes together, and lubricate the leading edge. Insert it past the introitus with the folded edge facing down. Allow the pessary to reopen, and direct it behind the cervix into the posterior fornix (FIGURE 2). Give it a slight twist with your index finger to prevent expulsion.
To see insertion demonstrated, watch Vaginal pessaries: An instructional video
Gehrung
This pessary is designed with an arch-shaped malleable rim with wires incorporated into the arms (FIGURE 3). Use of the Gehrung pessary is rare; it is most often used in women with cystocele or rectocele.
Insertion. Fold the pessary to insert it into the vagina. Upon insertion, keep both heels of the pessary parallel to the posterior vagina with the back arch pushed over the cervix in the anterior fornix and the front arch resting behind the symphysis pubis. The concave surface and diaphragm support the anterior vagina. Place the convex portion of the curve beneath the bulge. The two bases rest on the posterior vagina against the lateral levator muscles.
Shaatz
This support pessary has a circular base similar to the Gellhorn pessary but without the rigid stem (FIGURE 4).
Insertion. Because it is stiff, insert this pessary vertically and then turn it to a horizontal position once it is inside the vagina.
Lever
The Hodge, Smith, and Risser pessaries are collectively called the lever pessaries. They are used to manage uterine retroversion and POP. They are rarely used.
The Hodge pessary is beneficial to patients with a narrow vaginal introitus, mild cystocele, and cervical insufficiency. The anterior portion of a Hodge pessary is rectangular (FIGURE 5A).
The Smith pessary is useful for patients with well-defined pubic notches because the anterior portion is rounded (FIGURE 5B).
For patients with a very shallow pubic notch, the Risser pessary is useful. The Risser’s anterior portion is rectangular with indentation but wider than the Hodge pessary (FIGURE 5C).
Insertion. Fold the pessary and insert it into the vagina with the index finger on the posterior curved bar until the pessary rests behind the cervix and the anterior horizontal bar rests behind the symphysis pubis.
SPACE-OCCUPYING PESSARIES
The second pessary category is the space-filling pessary. These pessaries are used primarily to support severe POP, especially posthysterectomy vaginal vault prolapse. They have larger bases to support the vaginal apex or cervix; therefore, they are more difficult to insert and remove. When this pessary type is in place, sexual intercourse is not possible. Examples include the Gellhorn, donut, cube, and inflatable pessaries.
Gellhorn
The Gellhorn pessary is the most commonly used space-filling pessary. It has a broad base with a stem (FIGURE 6). The broad base supports the vaginal apex while the stem keeps the circular base from rotating and prevents pessary expulsion. The stem comes in long or short lengths. The concave base provides vaginal suction and keeps the pessary in place. The holes in the stem and base provide vaginal drainage. The Gellhorn pessary is useful for women with more advanced prolapse and less perineal support.
Insertion. Folding one side of the base to the stem, insert the Gellhorn pessary vertically inside the vagina. To facilitate insertion, separate the labia with the nondominant hand or depress the perineum with the index finger. Once the circular base is inside the vagina, push the pessary upward until the tip of the stem is just inside the vaginal introitus (FIGURE 7). Many medical illustrations inaccurately depict the Gellhorn pessary in a final placement that appears too high in the pelvis. This figure, which has the patient in a standing position, shows how low in the pelvis this space-filling pessary can sit in a patient with advanced prolapse.
Remove this pessary by gently pulling the stem while inserting the opposite hand beneath an edge of the pessary base to break the vaginal suction (Watch Vaginal pessaries: An instructional video).
Donut
The donut pessary is used for advanced prolapse because it fills a larger space. It is difficult to insert and remove because it is large, thick, and hollow (FIGURE 8).
Insertion. Insert it vertically and, once it is placed inside the vagina, rotate it to a horizontal position. A Kelly clamp can be used to grasp the pessary and facilitate removal.
Cube
The cube pessary supports third-degree uterine prolapse by holding the vaginal wall with suction (FIGURE 9). Because of the risk of vaginal erosion and lack of drainage in some designs, the cube pessary requires nightly removal and cleaning.
Insertion. Squeezing the pessary with the thumb, index, and middle fingers, insert the cube pessary at the vaginal apex.
Removal requires breaking the suction by placing a fingertip between the vaginal mucosa and the pessary and compressing the cube between the thumb and forefinger to remove. Gently tugging on the string also helps with removal.
Inflatable
This space-filling pessary is an air-filled ball that is inflated via an attached stem that also enables insertion and removal. The older Inflatoball pessary is made of latex, so its use is contraindicated in patients with latex allergy. Newer inflatable pessaries are silicone-based and consist of an air-filled donut, a stem with a valve, and an air pump (FIGURE 10). Some models also include a deflation key. The inflatable pessary comes in small, medium, large, and extra-large sizes. This pessary type must be removed and cleaned daily.
Insertion. Place the deflated pessary into the vagina. Move the ball-bearing valve within the stem (which controls the air flow) to a lateral projection on the side of the stem. To inflate, attach the inflation bulb. (Inflation typically requires 3 to 5 pumps of the bulb.) Move the ball bearing back into position to maintain the inflation, then detach the bulb. You can leave the stem outside the body or tuck it gently into the introitus (FIGURE 11).
INCONTINENCE PESSARIES
These devices are used specifically for SUI. The incontinence ring (FIGURE 12) and incontinence dish pessaries compress the urethra against the pubic symphysis. The knob is placed beneath the urethra, increasing the urethral closure pressure and thereby preventing urinary incontinence.
Related Article: Update on Urinary Incontinence Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (December 2011)
CASE 1 CONCLUDED
Given that AC has early-stage POP and is sexually active, a space-occupying pessary is not the optimal choice. Instead, a ring pessary with support is fitted for her trial.
What side effects might a patient anticipate with pessary use?
Vaginal discharge and slight odor are common. Pessary removal and cleaning are usually adequate to eliminate them. Temporary discontinuation of pessary use may be warranted until symptoms subside. If these maneuvers do not resolve the issue, then the patient should be examined to rule out other sources of infection.
Vaginal bleeding. Bleeding from vaginal abrasion and ulceration could be caused by trauma from pessary removal or vaginal impingement. Evaluation is warranted for any vaginal bleeding.
Changes in urinary function. Less commonly, women using a pessary may notice changes in their urinary function. Many women with anterior or apical prolapse will have altered urine streams with slow or trickling flow and possible hesitation upon initiation of voiding.
Alternatively, pessary placement may instigate stress-type incontinence akin to that seen after prolapse surgery. Changing pessary size may alleviate this condition. Otherwise, these side effects may reduce a patient’s willingness to continue pessary use.
How can a patient optimize her use of a pessary?
A patient can remove the pessary on a periodic basis or try to use it continuously. If she cannot or will not remove the pessary, then she will need to come back for scheduled visits, as described in the sidebar, “Essential components of a successfully fitted pessary.” If she is able to remove the pessary on her own, then she can use the device as needed or remove it for intercourse (though it is not necessary). She must remove it weekly, at a minimum, however, to both clean the pessary and give the vaginal walls a “rest,” which can minimize the potential for abrasions or erosions
ESSENTIAL COMPONENTS OF A SUCCESSFULLY FITTED PESSARY
Patient assessment
Accurate selection and placement of a pessary requires appropriate examination and fitting, beginning with determination of the patient’s stage of prolapse and introitus. Key steps include:
– Examine the patient with an empty bladder in the lithotomy position
– Perform bimanual pelvic and speculum examination using a Sims speculum (or bivalve speculum broken in half) with the patient in a supine position
– Administer the Pelvic Organ Prolapse Quantification (POP-Q) exam
– Perform digital examination
– Assess vaginal atrophy, vaginal introitus, and vaginal width and length
– Evaluate pelvic floor muscle strength (Kegel squeeze).
Next, gauge the correct pessary size by approximating the number of fingerbreadths accommodated across the vaginal width.
Another method of estimating pessary size is to insert two fingers inside the vagina and estimate the distance between the posterior fornix and the posterior pubic symphysis (Watch Vaginal pessaries: An instructional video). An easy reference is to start with a size 3 or 4 ring pessary if the vaginal introitus is 1 to 2 fingerbreadths in width and the prolapse is stage II to III. If the vagina accommodates 3 to 4 fingerbreadths, or there is stage IV prolapse, use a Gellhorn pessary.
Here are the different types of pessaries and the most common sizes available. (Pessary sizes change in quarter-inch increments.)
In-office trial
Insert the pessary into the vagina using the dominant hand. Using the nondominant hand, separate the introitus and depress the perineal body. Apply a small amount of lubricant to the leading edge of the pessary.
After insertion, ask the patient to strain and cough, ambulate in the office, and void. Reexamine the patient to ensure that the pessary is still in the correct position and that placement has not shifted. Perform the cough leak test with the patient in a standing position and the pessary in place. Re-examine the patient while she is in a standing position. Use the largest pessary that is comfortable for her. Advise her to bring the pessary back to the office if it gets expelled.
This is a trial-and-error process; advise the patient of this. It may require a trial of several styles and sizes to find the right pessary fit. Once you find the correct size, document the final pessary size.
Follow-up
Schedule a follow-up appointment 1 to 2 weeks after insertion. Ask the patient whether she has experienced any discomfort, malodorous discharge, or vaginal bleeding. Also inquire about any changes in urinary habits or bowel movements and related complaints.
Remove the pessary and clean it with mild soap and water. Examine the vagina for pressure points, abrasions, ulcerations, and erosions.
Teach the patient how to remove, clean, and reinsert the pessary, and advise her to perform these tasks on a weekly basis.
Schedule a follow-up visit in 1 to 2 months, and another visit 6 to 12 months after that.
CASE 2. ADVANCED-STAGE POP
BD is an 82-year-old widow (G5P4014) with stage IV vaginal prolapse. She has noticed some scant blood staining on her clothing. She frequently voids small amounts of urine but never feels complete relief. She defecates normally.
Her medical history is significant for coronary artery disease with prior myocardial infarction, with multiple stent placements over the years. She has hypertension, reduced ejection fraction, and diabetes. She is morbidly obese and suffers from degenerative joint disease. She had a vaginal hysterectomy several years ago for benign indications.
Upon examination, BD’s prolapse is large, with excoriations and hyperkeratosis of the skin over the prolapse. It is easily reduced in the office.
What is the best pessary for this patient, and how should she be followed and counseled regarding ongoing care?
Since the failure rate for pessary usage increases with advancing prolapse stage, a space-occupying pessary is most appropriate to try initially. A trial with a support pessary could be useful to allow the excoriations to heal and provide a healthier vaginal environment. A Gellhorn pessary is commonly used. An inflatable pessary could be an alternative if the Gellhorn fails to stay in place. The cube pessary, known to cause more abrasions and erosions than other pessaries, is a poor choice given the state of the patient’s vaginal tissues at baseline.
Space-occupying pessaries are more difficult to insert and remove and have a higher risk of pain or trauma. Start with shorter time intervals between visits, eventually spacing them out for the patient’s convenience. The usual interval for follow-up is 3 to 4 months; longer intervals could be offered if the patient is reliable, adherent, and reports no complaints with pessary use.
Related Article: Update on pelvic floor dysfunction: Focus on urinary incontinence Alexis A. Dieter, MD, and Cindy L. Amundsen, MD (November 2013)
OUTCOMES
Only short- and medium-term outcomes for pessary use have been described in the literature. Short-term (2 months) satisfaction and continued use, along with resolution of prolapse, occurred in 92% of patients.7 Previous hysterectomy or prolapse surgery may influence the short-term success of pessary use.10
More than half of sexually active women achieved long-term use (up to 2 years), regardless of prolapse severity. Brincat and colleagues found that long-term pessary use (1 to 2 years) approached 60% in 132 women with both urinary incontinence and prolapse. Women being treated for POP were more likely to continue pessary use than women being treated for SUI.11 Age, parity, estrogen use, and sexual activity were characteristics also studied in pessary fitting. Neither sexual activity nor stage of prolapse was a contraindication to use of a pessary; long-term use was found to be acceptable in sexually active women.11
Successful fitting of a vaginal pessary has been associated with improvement in voiding, urinary and fecal urgency, and incontinence. A vaginal pessary is a viable nonsurgical option for the management of POP and urinary incontinence and remains an optimal minimally invasive approach to such disorders.
CASE 2 CONCLUDED
The patient returns to the clinic 1 month after the original insertion. The pessary is removed, and the vagina is inspected, with no abrasions or ulcerations found. The vaginal cavity and pessary are cleaned with a mild soap-and-water mixture. The pessary is lubricated and reinserted. This process is repeated 2 months later, with subsequent follow-up intervals doubled (up to 6 months between visits) when the patient has no complaints of discharge or odor.
CASE 1. EARLY-STAGE PELVIC ORGAN PROLAPSE
AC is a 64-year-old white woman with early stage III anterior and apical pelvic organ prolapse (POP). The prolapse is now affecting her ability to do some of the things that she enjoys, such as gardening and golfing.
She has hypertension controlled with medication and no other significant medical issues except mild arthritic changes in her hands and hips. She reports being sexually active with her husband on roughly a weekly basis.
On examination, the leading edge of her prolapse is the anterior vaginal wall, protruding 1 cm beyond the introitus, and the cervix is at the hymenal ring. There is no significant posterior wall prolapse.
After she is counseled about all possible treatment approaches for her early-stage POP, the patient elects to try the vaginal pessary. Now, it is your job to determine the optimal pessary based on the extent of her condition and to educate her about the potential side effects and best practices for its ongoing use.
The vaginal pessary is an important component of a gynecologist’s armamentarium. It is a low-risk, cost-effective, nonsurgical treatment option for the management of POP and genuine stress urinary incontinence (SUI).1,2 It is unfortunate that training in North America typically provides clinicians with only a cursory experience with pessary selection and care, minimizing the device’s importance as a viable tool in a practitioner’s ongoing practice. In fact, most clinicians tend to view the pessary with a mixture of reluctance and disregard.
This is regrettable, as a majority (89%) of patients can be successfully fitted with a pessary,3 regardless of their stage or site of prolapse.4 Although high-stage prolapse does not predict failure, ring pessaries are used most successfully with stage II (100%) and stage III (71%) prolapse, while Gellhorn pessaries are most successful with stage IV (64%) prolapse.5
In this article we review the several pessary options available to clinicians, as well as how to insert them and the best scenarios for their use. We also discuss the key requirements for patient assessment and in-office fitting (meant to optimize the fit and, thereby, the success of use), the possible side effects of pessary use that patients need to be aware of, and appropriate follow-up.
WHEN IS A PESSARY YOUR BEST MANAGEMENT APPROACH?
There are several indications for pessary use,6 namely when:
- the patient has significant comorbid risk factors for surgery
- the patient prefers a nonsurgical alternative
- a goal is to avoid reoperation
- POP or cervical insufficiency is present during pregnancy
- the patient desires future fertility
- surgery must be delayed due to treatment of vaginal ulcerations
- the pessary will be used as a postoperative adjunct to mesh-based repair.
Pessaries have very few contraindications (TABLE). However, factors that do negatively affect successful fitting include:
- prior pelvic surgery
- multiparity
- obesity
- SUI
- short vaginal length (<7 cm)
- wide vaginal introitus (>4 fingerbreadths)
- significant posterior vaginal wall defect.5,7-9
There are two main categories of vaginal pessaries: support and space-filling. All pessaries come in different sizes and shapes. Most are made of medical-grade silicone, rendering them durable and autoclavable as well as resistant to absorption of vaginal discharge and odors. The ring pessary with support is the most commonly used support pessary. The Gellhorn pessary is the most commonly used space-filling pessary. It is used as a second-line treatment for patients unable to retain the ring-with-support pessary.
Related Article: Pessary and pelvic floor exercises for incontinence—are two better than one? G. Willy Davila, MD (Examining the Evidence, May 2010)
SUPPORT PESSARY OPTIONS
The support pessaries are used to treat SUI and POP. These pessaries typically are the easiest types for patients to use because they are more comfortable and simpler to remove and insert than space-filling pessaries. For example, a ring pessary is two-dimensional and lies perpendicular to the long axis of the vagina, allowing patients to have intercourse with it in place. Support-type pessaries include the ring, Gehrung, Shaatz, and lever.
Ring
This is the most commonly used pessary because it fits most women. There are four types of ring pessaries: the ring (FIGURE 1A), ring with support (FIGURE 1B), incontinence ring, and incontinence ring with support. The ring pessary is appropriate for all stages of POP. The ring with support has a diaphragm that is useful in women who have uterine prolapse with or without cystocele. The incontinence ring has a knob that is placed beneath the urethra to increase urethral pressure and is useful in cases of SUI.
Insertion. Fold the pessary by bringing the two small holes together, and lubricate the leading edge. Insert it past the introitus with the folded edge facing down. Allow the pessary to reopen, and direct it behind the cervix into the posterior fornix (FIGURE 2). Give it a slight twist with your index finger to prevent expulsion.
To see insertion demonstrated, watch Vaginal pessaries: An instructional video
Gehrung
This pessary is designed with an arch-shaped malleable rim with wires incorporated into the arms (FIGURE 3). Use of the Gehrung pessary is rare; it is most often used in women with cystocele or rectocele.
Insertion. Fold the pessary to insert it into the vagina. Upon insertion, keep both heels of the pessary parallel to the posterior vagina with the back arch pushed over the cervix in the anterior fornix and the front arch resting behind the symphysis pubis. The concave surface and diaphragm support the anterior vagina. Place the convex portion of the curve beneath the bulge. The two bases rest on the posterior vagina against the lateral levator muscles.
Shaatz
This support pessary has a circular base similar to the Gellhorn pessary but without the rigid stem (FIGURE 4).
Insertion. Because it is stiff, insert this pessary vertically and then turn it to a horizontal position once it is inside the vagina.
Lever
The Hodge, Smith, and Risser pessaries are collectively called the lever pessaries. They are used to manage uterine retroversion and POP. They are rarely used.
The Hodge pessary is beneficial to patients with a narrow vaginal introitus, mild cystocele, and cervical insufficiency. The anterior portion of a Hodge pessary is rectangular (FIGURE 5A).
The Smith pessary is useful for patients with well-defined pubic notches because the anterior portion is rounded (FIGURE 5B).
For patients with a very shallow pubic notch, the Risser pessary is useful. The Risser’s anterior portion is rectangular with indentation but wider than the Hodge pessary (FIGURE 5C).
Insertion. Fold the pessary and insert it into the vagina with the index finger on the posterior curved bar until the pessary rests behind the cervix and the anterior horizontal bar rests behind the symphysis pubis.
SPACE-OCCUPYING PESSARIES
The second pessary category is the space-filling pessary. These pessaries are used primarily to support severe POP, especially posthysterectomy vaginal vault prolapse. They have larger bases to support the vaginal apex or cervix; therefore, they are more difficult to insert and remove. When this pessary type is in place, sexual intercourse is not possible. Examples include the Gellhorn, donut, cube, and inflatable pessaries.
Gellhorn
The Gellhorn pessary is the most commonly used space-filling pessary. It has a broad base with a stem (FIGURE 6). The broad base supports the vaginal apex while the stem keeps the circular base from rotating and prevents pessary expulsion. The stem comes in long or short lengths. The concave base provides vaginal suction and keeps the pessary in place. The holes in the stem and base provide vaginal drainage. The Gellhorn pessary is useful for women with more advanced prolapse and less perineal support.
Insertion. Folding one side of the base to the stem, insert the Gellhorn pessary vertically inside the vagina. To facilitate insertion, separate the labia with the nondominant hand or depress the perineum with the index finger. Once the circular base is inside the vagina, push the pessary upward until the tip of the stem is just inside the vaginal introitus (FIGURE 7). Many medical illustrations inaccurately depict the Gellhorn pessary in a final placement that appears too high in the pelvis. This figure, which has the patient in a standing position, shows how low in the pelvis this space-filling pessary can sit in a patient with advanced prolapse.
Remove this pessary by gently pulling the stem while inserting the opposite hand beneath an edge of the pessary base to break the vaginal suction (Watch Vaginal pessaries: An instructional video).
Donut
The donut pessary is used for advanced prolapse because it fills a larger space. It is difficult to insert and remove because it is large, thick, and hollow (FIGURE 8).
Insertion. Insert it vertically and, once it is placed inside the vagina, rotate it to a horizontal position. A Kelly clamp can be used to grasp the pessary and facilitate removal.
Cube
The cube pessary supports third-degree uterine prolapse by holding the vaginal wall with suction (FIGURE 9). Because of the risk of vaginal erosion and lack of drainage in some designs, the cube pessary requires nightly removal and cleaning.
Insertion. Squeezing the pessary with the thumb, index, and middle fingers, insert the cube pessary at the vaginal apex.
Removal requires breaking the suction by placing a fingertip between the vaginal mucosa and the pessary and compressing the cube between the thumb and forefinger to remove. Gently tugging on the string also helps with removal.
Inflatable
This space-filling pessary is an air-filled ball that is inflated via an attached stem that also enables insertion and removal. The older Inflatoball pessary is made of latex, so its use is contraindicated in patients with latex allergy. Newer inflatable pessaries are silicone-based and consist of an air-filled donut, a stem with a valve, and an air pump (FIGURE 10). Some models also include a deflation key. The inflatable pessary comes in small, medium, large, and extra-large sizes. This pessary type must be removed and cleaned daily.
Insertion. Place the deflated pessary into the vagina. Move the ball-bearing valve within the stem (which controls the air flow) to a lateral projection on the side of the stem. To inflate, attach the inflation bulb. (Inflation typically requires 3 to 5 pumps of the bulb.) Move the ball bearing back into position to maintain the inflation, then detach the bulb. You can leave the stem outside the body or tuck it gently into the introitus (FIGURE 11).
INCONTINENCE PESSARIES
These devices are used specifically for SUI. The incontinence ring (FIGURE 12) and incontinence dish pessaries compress the urethra against the pubic symphysis. The knob is placed beneath the urethra, increasing the urethral closure pressure and thereby preventing urinary incontinence.
Related Article: Update on Urinary Incontinence Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (December 2011)
CASE 1 CONCLUDED
Given that AC has early-stage POP and is sexually active, a space-occupying pessary is not the optimal choice. Instead, a ring pessary with support is fitted for her trial.
What side effects might a patient anticipate with pessary use?
Vaginal discharge and slight odor are common. Pessary removal and cleaning are usually adequate to eliminate them. Temporary discontinuation of pessary use may be warranted until symptoms subside. If these maneuvers do not resolve the issue, then the patient should be examined to rule out other sources of infection.
Vaginal bleeding. Bleeding from vaginal abrasion and ulceration could be caused by trauma from pessary removal or vaginal impingement. Evaluation is warranted for any vaginal bleeding.
Changes in urinary function. Less commonly, women using a pessary may notice changes in their urinary function. Many women with anterior or apical prolapse will have altered urine streams with slow or trickling flow and possible hesitation upon initiation of voiding.
Alternatively, pessary placement may instigate stress-type incontinence akin to that seen after prolapse surgery. Changing pessary size may alleviate this condition. Otherwise, these side effects may reduce a patient’s willingness to continue pessary use.
How can a patient optimize her use of a pessary?
A patient can remove the pessary on a periodic basis or try to use it continuously. If she cannot or will not remove the pessary, then she will need to come back for scheduled visits, as described in the sidebar, “Essential components of a successfully fitted pessary.” If she is able to remove the pessary on her own, then she can use the device as needed or remove it for intercourse (though it is not necessary). She must remove it weekly, at a minimum, however, to both clean the pessary and give the vaginal walls a “rest,” which can minimize the potential for abrasions or erosions
ESSENTIAL COMPONENTS OF A SUCCESSFULLY FITTED PESSARY
Patient assessment
Accurate selection and placement of a pessary requires appropriate examination and fitting, beginning with determination of the patient’s stage of prolapse and introitus. Key steps include:
– Examine the patient with an empty bladder in the lithotomy position
– Perform bimanual pelvic and speculum examination using a Sims speculum (or bivalve speculum broken in half) with the patient in a supine position
– Administer the Pelvic Organ Prolapse Quantification (POP-Q) exam
– Perform digital examination
– Assess vaginal atrophy, vaginal introitus, and vaginal width and length
– Evaluate pelvic floor muscle strength (Kegel squeeze).
Next, gauge the correct pessary size by approximating the number of fingerbreadths accommodated across the vaginal width.
Another method of estimating pessary size is to insert two fingers inside the vagina and estimate the distance between the posterior fornix and the posterior pubic symphysis (Watch Vaginal pessaries: An instructional video). An easy reference is to start with a size 3 or 4 ring pessary if the vaginal introitus is 1 to 2 fingerbreadths in width and the prolapse is stage II to III. If the vagina accommodates 3 to 4 fingerbreadths, or there is stage IV prolapse, use a Gellhorn pessary.
Here are the different types of pessaries and the most common sizes available. (Pessary sizes change in quarter-inch increments.)
In-office trial
Insert the pessary into the vagina using the dominant hand. Using the nondominant hand, separate the introitus and depress the perineal body. Apply a small amount of lubricant to the leading edge of the pessary.
After insertion, ask the patient to strain and cough, ambulate in the office, and void. Reexamine the patient to ensure that the pessary is still in the correct position and that placement has not shifted. Perform the cough leak test with the patient in a standing position and the pessary in place. Re-examine the patient while she is in a standing position. Use the largest pessary that is comfortable for her. Advise her to bring the pessary back to the office if it gets expelled.
This is a trial-and-error process; advise the patient of this. It may require a trial of several styles and sizes to find the right pessary fit. Once you find the correct size, document the final pessary size.
Follow-up
Schedule a follow-up appointment 1 to 2 weeks after insertion. Ask the patient whether she has experienced any discomfort, malodorous discharge, or vaginal bleeding. Also inquire about any changes in urinary habits or bowel movements and related complaints.
Remove the pessary and clean it with mild soap and water. Examine the vagina for pressure points, abrasions, ulcerations, and erosions.
Teach the patient how to remove, clean, and reinsert the pessary, and advise her to perform these tasks on a weekly basis.
Schedule a follow-up visit in 1 to 2 months, and another visit 6 to 12 months after that.
CASE 2. ADVANCED-STAGE POP
BD is an 82-year-old widow (G5P4014) with stage IV vaginal prolapse. She has noticed some scant blood staining on her clothing. She frequently voids small amounts of urine but never feels complete relief. She defecates normally.
Her medical history is significant for coronary artery disease with prior myocardial infarction, with multiple stent placements over the years. She has hypertension, reduced ejection fraction, and diabetes. She is morbidly obese and suffers from degenerative joint disease. She had a vaginal hysterectomy several years ago for benign indications.
Upon examination, BD’s prolapse is large, with excoriations and hyperkeratosis of the skin over the prolapse. It is easily reduced in the office.
What is the best pessary for this patient, and how should she be followed and counseled regarding ongoing care?
Since the failure rate for pessary usage increases with advancing prolapse stage, a space-occupying pessary is most appropriate to try initially. A trial with a support pessary could be useful to allow the excoriations to heal and provide a healthier vaginal environment. A Gellhorn pessary is commonly used. An inflatable pessary could be an alternative if the Gellhorn fails to stay in place. The cube pessary, known to cause more abrasions and erosions than other pessaries, is a poor choice given the state of the patient’s vaginal tissues at baseline.
Space-occupying pessaries are more difficult to insert and remove and have a higher risk of pain or trauma. Start with shorter time intervals between visits, eventually spacing them out for the patient’s convenience. The usual interval for follow-up is 3 to 4 months; longer intervals could be offered if the patient is reliable, adherent, and reports no complaints with pessary use.
Related Article: Update on pelvic floor dysfunction: Focus on urinary incontinence Alexis A. Dieter, MD, and Cindy L. Amundsen, MD (November 2013)
OUTCOMES
Only short- and medium-term outcomes for pessary use have been described in the literature. Short-term (2 months) satisfaction and continued use, along with resolution of prolapse, occurred in 92% of patients.7 Previous hysterectomy or prolapse surgery may influence the short-term success of pessary use.10
More than half of sexually active women achieved long-term use (up to 2 years), regardless of prolapse severity. Brincat and colleagues found that long-term pessary use (1 to 2 years) approached 60% in 132 women with both urinary incontinence and prolapse. Women being treated for POP were more likely to continue pessary use than women being treated for SUI.11 Age, parity, estrogen use, and sexual activity were characteristics also studied in pessary fitting. Neither sexual activity nor stage of prolapse was a contraindication to use of a pessary; long-term use was found to be acceptable in sexually active women.11
Successful fitting of a vaginal pessary has been associated with improvement in voiding, urinary and fecal urgency, and incontinence. A vaginal pessary is a viable nonsurgical option for the management of POP and urinary incontinence and remains an optimal minimally invasive approach to such disorders.
CASE 2 CONCLUDED
The patient returns to the clinic 1 month after the original insertion. The pessary is removed, and the vagina is inspected, with no abrasions or ulcerations found. The vaginal cavity and pessary are cleaned with a mild soap-and-water mixture. The pessary is lubricated and reinserted. This process is repeated 2 months later, with subsequent follow-up intervals doubled (up to 6 months between visits) when the patient has no complaints of discharge or odor.
- Colmer, WM Jr. Use of the pessary. Am J Obstet Gynecol. 1953;65(1):170–174.
- Culligan PJ. Nonsurgical management to pelvic organ prolapse. Obstet Gynecol. 2012;119(4):852–860.
- Nygaard IE, Heit M. Stress urinary incontinence. Obstet Gynecol. 2004;104(3):607–620.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 85: Pelvic organ prolapse. Obstet Gynecol. 2007;110(3):717–729.
- Clemons JL, Aguilar VC, Tillinghast TA, Jackson ND, Myers DL. Risk factors associated with an unsuccessful pessary fitting trial in women with pelvic organ prolapse. Am J Obstet Gynecol. 2004;190(2):345–350.
- Clemons JL, Brubaker L, Falk SJ. Vaginal pessary treatment of prolapse and incontinence. UpToDate. http://www.uptodate.com/contents/vaginal-pessary-treatment-of-prolapse-and-incontinence. Updated February 8, 2013. Accessed November 7, 2013.
- Mutone MF, Terry C, Hale DS, Benson JT. Factors which influence the short-term success of pessary management of pelvic organ prolapse. Am J Obstet Gynecol. 2005;193(1):89–94.
- Fernando RJ, Thakar R, Sultan AH, Shah SM, Jones PW. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108(1):93–99.
- Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol. 2005;106(3):615–634.
- Donnelly MJ, Powell-Morgan S, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(5):302–307.
- Brincat C, Kenton K, Fitzgerald MP, et al. Sexual activity predicts continued pessary use. Am J Obstet Gynecol. 2004;191(1):198–200.
- Colmer, WM Jr. Use of the pessary. Am J Obstet Gynecol. 1953;65(1):170–174.
- Culligan PJ. Nonsurgical management to pelvic organ prolapse. Obstet Gynecol. 2012;119(4):852–860.
- Nygaard IE, Heit M. Stress urinary incontinence. Obstet Gynecol. 2004;104(3):607–620.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 85: Pelvic organ prolapse. Obstet Gynecol. 2007;110(3):717–729.
- Clemons JL, Aguilar VC, Tillinghast TA, Jackson ND, Myers DL. Risk factors associated with an unsuccessful pessary fitting trial in women with pelvic organ prolapse. Am J Obstet Gynecol. 2004;190(2):345–350.
- Clemons JL, Brubaker L, Falk SJ. Vaginal pessary treatment of prolapse and incontinence. UpToDate. http://www.uptodate.com/contents/vaginal-pessary-treatment-of-prolapse-and-incontinence. Updated February 8, 2013. Accessed November 7, 2013.
- Mutone MF, Terry C, Hale DS, Benson JT. Factors which influence the short-term success of pessary management of pelvic organ prolapse. Am J Obstet Gynecol. 2005;193(1):89–94.
- Fernando RJ, Thakar R, Sultan AH, Shah SM, Jones PW. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108(1):93–99.
- Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol. 2005;106(3):615–634.
- Donnelly MJ, Powell-Morgan S, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(5):302–307.
- Brincat C, Kenton K, Fitzgerald MP, et al. Sexual activity predicts continued pessary use. Am J Obstet Gynecol. 2004;191(1):198–200.
![]()
Teresa Tam, MD
In this 15-minute video Dr. Tam demonstrates insertion and removal of the ring and Gellhorn pessaries and illustrates proper technique for estimating pessary size.
Update on pelvic floor dysfunction: Focus on urinary incontinence
Urinary incontinence (UI) affects almost half of all women in the United States.1,2 Estimates suggest that the prevalence of UI gradually rises during young adult life, comes to a broad plateau in middle age, and then steadily increases from that plateau after age 65. Therefore, over the next 40 years, as the elderly population expands in size, the number of women affected by UI will significantly grow.3
For patients with UI, a multitude of therapeutic options are available. Which option is the best for your patient? In this article, we aim to answer that question by interpreting the results of four randomized trials, each of which directly compare two available treatment options. The first study examines patients with stress urinary incontinence (SUI), comparing the patients’ subjective improvement in urinary leakage and bladder function at 12 months after randomization to treatment with physiotherapy or midurethral sling surgery.
The three other trials examine patients with overactive bladder (OAB) and urgency urinary incontinence (UUI). Each trial directly compares the use of anticholinergic medications to an alternate treatment modality. Currently, anticholinergic medications and behavioral therapy are the recommended first-line therapies for OAB. Unfortunately, anticholinergic medications have poor patient compliance and significant systemic side effects.4 Caution should be used when considering anticholinergic medications in patients with impaired gastric emptying or a history of urinary retention. They also should be used with caution in elderly patients who are extremely frail. Additionally, clearance from an ophthalmologist must be obtained prior to starting anticholinergic medication in patients with narrow-angle glaucoma.5 Due to poor adherence and potential side effects, there is a growing effort to discover alternative treatment modalities that are safe and effective. Therefore, we chose to examine trials comparing: mirabegron versus tolterodine, percutaneous tibial nerve stimulation versus tolterodine, and onabotulinumtoxinA versus anticholingeric medications.
UI defined
Before discussing treatment options, we want to clarify the main types of UI (FIGURE). UI is defined as the complaint of involuntary loss of urine. UI can be subdivided into SUI, OAB/UUI, or mixed urinary incontinence.6 While there are other less common genitourinary etiologies that can lead to UI, nongenitourinary etiologies are prevalent and can aggravate existing SUI or OAB (TABLE).
SUI is the complaint of involuntary loss of urine on effort or physical exertion (such as during sporting activities) or on sneezing or coughing. Often, SUI can be diagnosed by patient report alone and surgery can be considered in symptomatic patients who demonstrate cough leakage on physical examination and normal postvoid residual volumes.
UUI is the involuntary loss of urine associated with urgency; it often occurs in the setting of OAB, which is defined as the syndrome of urinary urgency, usually accompanied by frequency and nocturia, with or without UUI, in the absence of urinary tract infection or other obvious pathology (such as neurologic dysfunction, infection, or urologic neoplasm). OAB-dry is present when patients do not have leakage with urgency, but are bothered by urgency, frequency, and/or nocturia. OAB-wet occurs when a patient has urgencyincontinence.
The presence of both SUI and OAB/UUI is known as mixed urinary incontinence. Stress and urgency urinary symptoms often present together. In fact, 10% to 30% of women with stress symptoms are found to have bladder overactivity on subsequent evaluation.2,7 Therefore, it is important to take a good history and consider urodynamic evaluation to confirm the diagnosis of SUI prior to surgery in women with mixed stress and urge symptoms, a history of a previous surgery for incontinence, or when there is a poor correlation of physical examination findings to reported symptoms.
Is surgery a first-line option for patients with SUI?
Labrie J, Berghmans BL, Fischer K, et al. Surgery versus physiotherapy for stress urinary incontinence. NEJM. 2013;369(12):1124−1133.
Physiotherapy, including pelvic floor muscle training (“Kegel exercises”), is utilized as a first-line treatment option for women with SUI that carries minimal risk for the patient. Midurethral sling surgery is often recommended if an initial trial of conservative treatment fails.7 Up to 50% of women treated with pelvic floor physiotherapy will ultimately undergo surgery to treat their SUI.8
Related article: Does urodynamic testing before surgery for stress incontinence improve outcomes? G. Willy Davila, MD (Examining the Evidence, December 2012)
Details of the study
This was a randomized, multicenter trial of women aged 35 to 80 years with moderate to severe SUI. After excluding women with previous incontinence surgery and stage 2 or higher pelvic organ prolapse, 460 participants were randomly assigned to undergo either a midurethral sling surgery or physiotherapy (pelvic floor muscle training). The primary outcome was subjective improvement in urinary leakage and bladder function at 12 months, as measured by the Patient Global Impression of Improvement (PGI-I), a 7-point Likert scale ranging from “very much worse” to “very much better.”
In an intention-to-treat analysis, subjective improvement at 12 months was significantly higher in women randomized to midurethral sling surgery than in women randomized to physiotherapy (91% vs 64%, respectively).
Ten percent of patients had adverse events (AEs); all were related to surgery. The most common AEs were hematoma, vaginal epithelial perforation, and bladder perforation.
Notably, women had the option to cross over to the other treatment modality if they desired. In the physiotherapy group, 49% of women elected to cross over to surgery, while 11% of those who underwent midurethral sling surgery elected to cross over to physiotherapy during the 12-month follow-up period. When analyzing results by treatment received, the investigators found that the proportion of women who reported improvement was significantly lower among women who underwent physiotherapy only (32%), versus sling only (94%), or sling after physiotherapy (91%).
This randomized trial was well-designed and included a variety of treatment centers (university and general hospitals) with interventions performed by experienced surgeons (all of whom had performed at least 20 sling surgeries) and physiotherapists educated specifically in pelvic floor physiotherapy. The study population was limited to patients with moderate to severe SUI as defined by the Sandvik severity index.9 Therefore, these results may not be applicable to patients with milder symptoms, for whom physiotherapy has been recommended as first-line therapy with consideration of surgery if physiotherapy fails to sufficiently improve symptoms.7
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women with moderate to severe SUI without significant prolapse or a history of prior incontinence surgery have significantly better outcomes at 12 months after undergoing midurethral sling surgery versus physiotherapy. Physiotherapy carries little to no risk of adverse effects. Women with moderate to severe SUI should be counseled regarding the risks and benefits of both physiotherapy and midurethral sling surgery as initial treatment options.
Because stress and urgency urinary symptoms often present together, it is important to consider urodynamic evaluation to confirm SUI prior to surgery in women with:
• mixed stress and urge symptoms
• a history of a previous surgery for incontinence, or
• poor correlation of physical examination findings to reported symptoms.
Safety and tolerability of mirabegron versus tolterodine for OAB
Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a beta(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63(2):296−305.
In the bladder, beta3-receptors located within the detrusor smooth muscle facilitate urine storage by relaxing the detrusor, enabling the bladder to fill.10 The activation of beta3-receptors is thought to increase the bladder’s ability to store urine, with the goal of decreasing urgency, frequency, nocturia, and urgency incontinence. An alternative to anticholinergic medications, mirabegron is a beta3-agonist approved by the US Food and Drug Administration (FDA) in 2012 for clinical use in the treatment of OAB.
Details of the study
Chapple and colleagues aimed to assess the 12-month efficacy and safety of mirabegron in a randomized, double-blind active controlled trial. The primary outcome was incidence and severity of treatment-emergent adverse effects (TEAEs); the secondary outcome was the change in OAB symptoms from baseline to up to 12 months. Patients experiencing OAB symptoms for more than 3 months were eligible and were subsequently enrolled if they averaged 8 or more voids per day and 3 or more episodes of urgency with or without incontinence on a 3-day bladder diary. A total of 2,444 patients were randomly assigned in a 1:1:1 fashion to mirabegron 50 mg daily, mirabegron 100 mg daily, or tolterodine extended release (ER) 4 mg daily.
There was a similar incidence (60% to 63%) of TEAEs across all three groups. The most common TEAEs were hypertension (defined as average systolic blood pressure [BP] >140 mm Hg or average diastolic BP >90 mm Hg at two consecutive visits), UTI, headache, nasopharyngitis, and constipation. The adjusted mean changes in BP from baseline to final visit were less than 1 mm Hg for both systolic and diastolic BP for patients taking both doses of mirabegron, as well as for patients taking tolterodine. The incidence of dry mouth was higher in the tolterodine group than the mirabegron groups. Mirabegron 50 mg daily and 100 mg daily improved incontinence symptoms within 1 month of starting therapy; the degree of improvement was similar to that seen in the patients taking tolterodine ER 4 mg daily.
Related article: New overactive bladder treatment approved by the FDA (August 2012)
Some caveats
This study was well-designed to assess the safety and tolerability of mirabegron versus tolterodine. The doses utilized in the study were at or above the FDA-approved dosage of 25 mg to 50 mg daily for OAB treatment. Although investigators found mirabegron to be a safe alternative to anticholinergic medication, the study was not designed or powered to examine the efficacy of mirabegron versus tolterodine. No formal comparison of efficacy was made between mirabegron or tolterodine, or between the 50-mg and 100-mg doses of mirabegron. Moreover, 81% of participants had been treated with mirabegron in earlier Phase 3 studies, so most were not treatment naïve, limiting the applicability of results.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Mirabegron should be considered as a potential treatment option for patients who demonstrate poor tolerance of or response to anticholinergic medications; however, caution should be used in patients with severe uncontrolled high BP, end-stage kidney disease, or severe liver impairment.
Consider percutaneous tibial nerve stimulation over tolterodine for OAB in select patients
Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: Results from the overactive bladder innovative therapy trial. J Urol. 2009;182(3):1055−1061.
Neuromodulation utilizes electrical stimulation to improve bladder function and decrease OAB symptoms. First developed in the early 1980s by McGuire and colleagues, percutaneous tibial nerve stimulation (PTNS) was approved by the FDA in 2000 as Urgent PC and provides an outpatient, nonimplantable neuromodulation alternative to medication therapy for patients with OAB.11,12 By directly stimulating the posterior tibial nerve, PTNS works via the S3 sacral nerve plexus to alter the micturition reflex and improve bladder function.
Details of the study
Patients were eligible for the study if they demonstrated 8 or more voids per day on a 3-day bladder diary (whether or not they had a history of previous anticholinergic drug use). A total of 100 ambulatory adults with OAB symptoms were enrolled and randomly assigned to PTNS 30-minutes per week or tolterodine ER 4 mg daily.
At 12 weeks, both groups demonstrated a significant improvement in OAB measures as well as validated symptom severity and quality-of-life questionnaire scores. Subjective assessment of improvement in OAB symptoms was significantly greater in the PTNS group than in the tolterodine group (79.5% vs 54.8%, respectively; P = .01). However, mean reduction of voids for 24 hours was not significantly different between the two groups.
Both treatments were well tolerated, with only 15% to 16% of patients in both groups reporting mild to moderate side effects. The tolterodine group did have a significantly higher risk of dry mouth; however, the risk of constipation was not significantly different between the groups.
Study limitations
The authors performed an important multicenter, nonblinded, randomized, controlled trial, which was one of the first trials to directly compare two OAB therapies. The generalizability of the findings were limited, as the cohort included mostly patients with dry OAB who had no objective measures on UUI episodes. In addition, this trial had a limited observation period of only 12 weeks. Information regarding the effect of treatment after cessation of weekly PTNS therapy was not examined. Therefore, we are not able to determine whether repeat sessions provide adequate maintenance in the long term.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
PTNS 30 minutes daily is as effective as tolterodine ER 4 mg daily for 12 weeks in reducing OAB symptoms. PTNS is a safe alternative that should be considered in patients with OAB who poorly tolerate or have contraindications to medication therapy.
OnabotulinumtoxinA is an effective therapy for OAB
Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. NEJM. 2012;367(19):1803−1813.
The newest therapy for OAB is onabotulinumtoxinA, or Botox, which was FDA approved this year for the treatment of OAB in adults who cannot use or do not tolerate anticholinergic medications. Recommended doses are 100 U onabotulinumtoxinA in patients with idiopathic refractory OAB and 200 U onabotulinumtoxinA for patients with neurogenic OAB.
OnabotulinumtoxinA is a neurotoxin that blocks synaptic transmission at the neuromuscular junction to cause muscle paralysis and atrophy.13 Injecting onabotulinumtoxinA into the detrusor smooth muscle should relax the bladder and decrease sensations of urgency and frequency to achieve a longer duration of time for bladder filling and reduce the risk of urgency incontinence.
Effects of onabotulinumtoxinA appear to wear off over time, and patients may require repeat injections. Side effects of onabotulinumtoxinA therapy include an increased risk of UTI and the potential for urinary retention requiring intermittent self-catheterization.
Related article: Update on Pelvic Floor Dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
Details of the study
The Anticholinergic Versus Botulinum Toxin Comparison (ABC) study was a multicenter, randomized, double-blind, double-placebo–controlled trial conducted in women without known neurologic disease with moderate to severe UUI (defined as >5 UUI episodes on a 3-day bladder diary). Women were randomly assigned to a single intradetrusor injection of 100 U onabotulinumtoxinA plus oral placebo or to a single intradetrusor injection of saline plus solifenacin 5 mg daily (with the option of dose escalation and then switching to trospium XR if no improvement was seen).
Of the 241 women included in the final analysis, approximately 70% in each group reported adequate control of symptoms at 6 months. Adequate control was defined as a response of “agree strongly” or “agree” to the statement: “This treatment has given me adequate control of my urinary leakage.” Women in the onabotulinumtoxinA group were significantly more likely than women in the anticholinergic medication group to report complete resolution of UUI at 6 months (27% vs 13%, P = .003). However, the mean reduction in episodes of UUI per day and the improvements in quality-of-life questionnaire scores were found to be similar. Interestingly, worse baseline UUI was associated with greater reduction in episodes of UUI for both therapies.
This was a rigorous and well-executed double-blind, double-placebo−controlled randomized trial. By utilizing broad inclusion criteria and enrolling patients both with and without previous exposure to anticholinergic medications, the generalizability of study findings are greatly improved. Because this study did not examine the effect or efficacy of repeat injections, these findings have limited applicability to patients undergoing multiple onabotulinumtoxinA injections.
When considering use in your patient population, keep the possible side effects in mind.There were important differences in the side effects experienced with each therapy. Specifically, while the anticholinergic group had a higher frequency of dry mouth (46% anticholinergic vs 31% onabotulinumtoxinA, P = .02), the onabotulinumtoxinA group demonstrated higher rates of incomplete bladder emptying requiring catheterization (peak of 5% at 2 months) and greater risk of UTI (33% onabotulinumtoxinA vs 13% anticholinergic, P <.001).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This study showed that, among women with UUI, anticholinergic medication and onabotulinumtoxinA are equally effective in reducing UUI episodes and improving quality of life. It is important to consider the side effect profile, determine the patient’s preferences, and weigh the risks and benefits of each therapy when deciding what is the best treatment for your individual patient.
We want to hear from you! Tell us what you think.
- Anger JT, Saigal CS, Litwin MS. The prevalence of urinary incontinence among community dwelling adult women: Results from the National Health and Nutrition Examination Survey. J Urol. 2006;175(2):601–604.
- Dooley Y, Kenton K, Cao G, et al. Urinary incontinence prevalence: Results from the National Health and Nutrition Examination Survey. J Urol. 2008;179(2):656–661.
- Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstetr Gynecol. 2009;114(6):1278–1283.
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. Americal Urological Association. http://www.auanet.org/common/pdf/education/clinical-guidance/Overactive-Bladder.pdf. Published 2012. Revised June 11, 2013. Accessed October 21, 2013.
- Yu YF, Nichol MB, Yu AP, Ahn J. Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the California Medicaid program. Value Health. 2005;8(4):495–505.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- ACOG Practice Bulletin No. 63: Urinary incontinence in women. American College of Obstetricians and Gynecologists. Obstetr Gynecol. 2005;105(6):1533–1545.
- Bo K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstetr Gynecol. 2005;105(5 Pt 1):999–1005.
- Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47(6):497–499.
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–466.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129(1):78–79.
- Schiavo G, Santucci A, Dasgupta BR, et al. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335(1):99–103.
Urinary incontinence (UI) affects almost half of all women in the United States.1,2 Estimates suggest that the prevalence of UI gradually rises during young adult life, comes to a broad plateau in middle age, and then steadily increases from that plateau after age 65. Therefore, over the next 40 years, as the elderly population expands in size, the number of women affected by UI will significantly grow.3
For patients with UI, a multitude of therapeutic options are available. Which option is the best for your patient? In this article, we aim to answer that question by interpreting the results of four randomized trials, each of which directly compare two available treatment options. The first study examines patients with stress urinary incontinence (SUI), comparing the patients’ subjective improvement in urinary leakage and bladder function at 12 months after randomization to treatment with physiotherapy or midurethral sling surgery.
The three other trials examine patients with overactive bladder (OAB) and urgency urinary incontinence (UUI). Each trial directly compares the use of anticholinergic medications to an alternate treatment modality. Currently, anticholinergic medications and behavioral therapy are the recommended first-line therapies for OAB. Unfortunately, anticholinergic medications have poor patient compliance and significant systemic side effects.4 Caution should be used when considering anticholinergic medications in patients with impaired gastric emptying or a history of urinary retention. They also should be used with caution in elderly patients who are extremely frail. Additionally, clearance from an ophthalmologist must be obtained prior to starting anticholinergic medication in patients with narrow-angle glaucoma.5 Due to poor adherence and potential side effects, there is a growing effort to discover alternative treatment modalities that are safe and effective. Therefore, we chose to examine trials comparing: mirabegron versus tolterodine, percutaneous tibial nerve stimulation versus tolterodine, and onabotulinumtoxinA versus anticholingeric medications.
UI defined
Before discussing treatment options, we want to clarify the main types of UI (FIGURE). UI is defined as the complaint of involuntary loss of urine. UI can be subdivided into SUI, OAB/UUI, or mixed urinary incontinence.6 While there are other less common genitourinary etiologies that can lead to UI, nongenitourinary etiologies are prevalent and can aggravate existing SUI or OAB (TABLE).
SUI is the complaint of involuntary loss of urine on effort or physical exertion (such as during sporting activities) or on sneezing or coughing. Often, SUI can be diagnosed by patient report alone and surgery can be considered in symptomatic patients who demonstrate cough leakage on physical examination and normal postvoid residual volumes.
UUI is the involuntary loss of urine associated with urgency; it often occurs in the setting of OAB, which is defined as the syndrome of urinary urgency, usually accompanied by frequency and nocturia, with or without UUI, in the absence of urinary tract infection or other obvious pathology (such as neurologic dysfunction, infection, or urologic neoplasm). OAB-dry is present when patients do not have leakage with urgency, but are bothered by urgency, frequency, and/or nocturia. OAB-wet occurs when a patient has urgencyincontinence.
The presence of both SUI and OAB/UUI is known as mixed urinary incontinence. Stress and urgency urinary symptoms often present together. In fact, 10% to 30% of women with stress symptoms are found to have bladder overactivity on subsequent evaluation.2,7 Therefore, it is important to take a good history and consider urodynamic evaluation to confirm the diagnosis of SUI prior to surgery in women with mixed stress and urge symptoms, a history of a previous surgery for incontinence, or when there is a poor correlation of physical examination findings to reported symptoms.
Is surgery a first-line option for patients with SUI?
Labrie J, Berghmans BL, Fischer K, et al. Surgery versus physiotherapy for stress urinary incontinence. NEJM. 2013;369(12):1124−1133.
Physiotherapy, including pelvic floor muscle training (“Kegel exercises”), is utilized as a first-line treatment option for women with SUI that carries minimal risk for the patient. Midurethral sling surgery is often recommended if an initial trial of conservative treatment fails.7 Up to 50% of women treated with pelvic floor physiotherapy will ultimately undergo surgery to treat their SUI.8
Related article: Does urodynamic testing before surgery for stress incontinence improve outcomes? G. Willy Davila, MD (Examining the Evidence, December 2012)
Details of the study
This was a randomized, multicenter trial of women aged 35 to 80 years with moderate to severe SUI. After excluding women with previous incontinence surgery and stage 2 or higher pelvic organ prolapse, 460 participants were randomly assigned to undergo either a midurethral sling surgery or physiotherapy (pelvic floor muscle training). The primary outcome was subjective improvement in urinary leakage and bladder function at 12 months, as measured by the Patient Global Impression of Improvement (PGI-I), a 7-point Likert scale ranging from “very much worse” to “very much better.”
In an intention-to-treat analysis, subjective improvement at 12 months was significantly higher in women randomized to midurethral sling surgery than in women randomized to physiotherapy (91% vs 64%, respectively).
Ten percent of patients had adverse events (AEs); all were related to surgery. The most common AEs were hematoma, vaginal epithelial perforation, and bladder perforation.
Notably, women had the option to cross over to the other treatment modality if they desired. In the physiotherapy group, 49% of women elected to cross over to surgery, while 11% of those who underwent midurethral sling surgery elected to cross over to physiotherapy during the 12-month follow-up period. When analyzing results by treatment received, the investigators found that the proportion of women who reported improvement was significantly lower among women who underwent physiotherapy only (32%), versus sling only (94%), or sling after physiotherapy (91%).
This randomized trial was well-designed and included a variety of treatment centers (university and general hospitals) with interventions performed by experienced surgeons (all of whom had performed at least 20 sling surgeries) and physiotherapists educated specifically in pelvic floor physiotherapy. The study population was limited to patients with moderate to severe SUI as defined by the Sandvik severity index.9 Therefore, these results may not be applicable to patients with milder symptoms, for whom physiotherapy has been recommended as first-line therapy with consideration of surgery if physiotherapy fails to sufficiently improve symptoms.7
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women with moderate to severe SUI without significant prolapse or a history of prior incontinence surgery have significantly better outcomes at 12 months after undergoing midurethral sling surgery versus physiotherapy. Physiotherapy carries little to no risk of adverse effects. Women with moderate to severe SUI should be counseled regarding the risks and benefits of both physiotherapy and midurethral sling surgery as initial treatment options.
Because stress and urgency urinary symptoms often present together, it is important to consider urodynamic evaluation to confirm SUI prior to surgery in women with:
• mixed stress and urge symptoms
• a history of a previous surgery for incontinence, or
• poor correlation of physical examination findings to reported symptoms.
Safety and tolerability of mirabegron versus tolterodine for OAB
Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a beta(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63(2):296−305.
In the bladder, beta3-receptors located within the detrusor smooth muscle facilitate urine storage by relaxing the detrusor, enabling the bladder to fill.10 The activation of beta3-receptors is thought to increase the bladder’s ability to store urine, with the goal of decreasing urgency, frequency, nocturia, and urgency incontinence. An alternative to anticholinergic medications, mirabegron is a beta3-agonist approved by the US Food and Drug Administration (FDA) in 2012 for clinical use in the treatment of OAB.
Details of the study
Chapple and colleagues aimed to assess the 12-month efficacy and safety of mirabegron in a randomized, double-blind active controlled trial. The primary outcome was incidence and severity of treatment-emergent adverse effects (TEAEs); the secondary outcome was the change in OAB symptoms from baseline to up to 12 months. Patients experiencing OAB symptoms for more than 3 months were eligible and were subsequently enrolled if they averaged 8 or more voids per day and 3 or more episodes of urgency with or without incontinence on a 3-day bladder diary. A total of 2,444 patients were randomly assigned in a 1:1:1 fashion to mirabegron 50 mg daily, mirabegron 100 mg daily, or tolterodine extended release (ER) 4 mg daily.
There was a similar incidence (60% to 63%) of TEAEs across all three groups. The most common TEAEs were hypertension (defined as average systolic blood pressure [BP] >140 mm Hg or average diastolic BP >90 mm Hg at two consecutive visits), UTI, headache, nasopharyngitis, and constipation. The adjusted mean changes in BP from baseline to final visit were less than 1 mm Hg for both systolic and diastolic BP for patients taking both doses of mirabegron, as well as for patients taking tolterodine. The incidence of dry mouth was higher in the tolterodine group than the mirabegron groups. Mirabegron 50 mg daily and 100 mg daily improved incontinence symptoms within 1 month of starting therapy; the degree of improvement was similar to that seen in the patients taking tolterodine ER 4 mg daily.
Related article: New overactive bladder treatment approved by the FDA (August 2012)
Some caveats
This study was well-designed to assess the safety and tolerability of mirabegron versus tolterodine. The doses utilized in the study were at or above the FDA-approved dosage of 25 mg to 50 mg daily for OAB treatment. Although investigators found mirabegron to be a safe alternative to anticholinergic medication, the study was not designed or powered to examine the efficacy of mirabegron versus tolterodine. No formal comparison of efficacy was made between mirabegron or tolterodine, or between the 50-mg and 100-mg doses of mirabegron. Moreover, 81% of participants had been treated with mirabegron in earlier Phase 3 studies, so most were not treatment naïve, limiting the applicability of results.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Mirabegron should be considered as a potential treatment option for patients who demonstrate poor tolerance of or response to anticholinergic medications; however, caution should be used in patients with severe uncontrolled high BP, end-stage kidney disease, or severe liver impairment.
Consider percutaneous tibial nerve stimulation over tolterodine for OAB in select patients
Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: Results from the overactive bladder innovative therapy trial. J Urol. 2009;182(3):1055−1061.
Neuromodulation utilizes electrical stimulation to improve bladder function and decrease OAB symptoms. First developed in the early 1980s by McGuire and colleagues, percutaneous tibial nerve stimulation (PTNS) was approved by the FDA in 2000 as Urgent PC and provides an outpatient, nonimplantable neuromodulation alternative to medication therapy for patients with OAB.11,12 By directly stimulating the posterior tibial nerve, PTNS works via the S3 sacral nerve plexus to alter the micturition reflex and improve bladder function.
Details of the study
Patients were eligible for the study if they demonstrated 8 or more voids per day on a 3-day bladder diary (whether or not they had a history of previous anticholinergic drug use). A total of 100 ambulatory adults with OAB symptoms were enrolled and randomly assigned to PTNS 30-minutes per week or tolterodine ER 4 mg daily.
At 12 weeks, both groups demonstrated a significant improvement in OAB measures as well as validated symptom severity and quality-of-life questionnaire scores. Subjective assessment of improvement in OAB symptoms was significantly greater in the PTNS group than in the tolterodine group (79.5% vs 54.8%, respectively; P = .01). However, mean reduction of voids for 24 hours was not significantly different between the two groups.
Both treatments were well tolerated, with only 15% to 16% of patients in both groups reporting mild to moderate side effects. The tolterodine group did have a significantly higher risk of dry mouth; however, the risk of constipation was not significantly different between the groups.
Study limitations
The authors performed an important multicenter, nonblinded, randomized, controlled trial, which was one of the first trials to directly compare two OAB therapies. The generalizability of the findings were limited, as the cohort included mostly patients with dry OAB who had no objective measures on UUI episodes. In addition, this trial had a limited observation period of only 12 weeks. Information regarding the effect of treatment after cessation of weekly PTNS therapy was not examined. Therefore, we are not able to determine whether repeat sessions provide adequate maintenance in the long term.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
PTNS 30 minutes daily is as effective as tolterodine ER 4 mg daily for 12 weeks in reducing OAB symptoms. PTNS is a safe alternative that should be considered in patients with OAB who poorly tolerate or have contraindications to medication therapy.
OnabotulinumtoxinA is an effective therapy for OAB
Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. NEJM. 2012;367(19):1803−1813.
The newest therapy for OAB is onabotulinumtoxinA, or Botox, which was FDA approved this year for the treatment of OAB in adults who cannot use or do not tolerate anticholinergic medications. Recommended doses are 100 U onabotulinumtoxinA in patients with idiopathic refractory OAB and 200 U onabotulinumtoxinA for patients with neurogenic OAB.
OnabotulinumtoxinA is a neurotoxin that blocks synaptic transmission at the neuromuscular junction to cause muscle paralysis and atrophy.13 Injecting onabotulinumtoxinA into the detrusor smooth muscle should relax the bladder and decrease sensations of urgency and frequency to achieve a longer duration of time for bladder filling and reduce the risk of urgency incontinence.
Effects of onabotulinumtoxinA appear to wear off over time, and patients may require repeat injections. Side effects of onabotulinumtoxinA therapy include an increased risk of UTI and the potential for urinary retention requiring intermittent self-catheterization.
Related article: Update on Pelvic Floor Dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
Details of the study
The Anticholinergic Versus Botulinum Toxin Comparison (ABC) study was a multicenter, randomized, double-blind, double-placebo–controlled trial conducted in women without known neurologic disease with moderate to severe UUI (defined as >5 UUI episodes on a 3-day bladder diary). Women were randomly assigned to a single intradetrusor injection of 100 U onabotulinumtoxinA plus oral placebo or to a single intradetrusor injection of saline plus solifenacin 5 mg daily (with the option of dose escalation and then switching to trospium XR if no improvement was seen).
Of the 241 women included in the final analysis, approximately 70% in each group reported adequate control of symptoms at 6 months. Adequate control was defined as a response of “agree strongly” or “agree” to the statement: “This treatment has given me adequate control of my urinary leakage.” Women in the onabotulinumtoxinA group were significantly more likely than women in the anticholinergic medication group to report complete resolution of UUI at 6 months (27% vs 13%, P = .003). However, the mean reduction in episodes of UUI per day and the improvements in quality-of-life questionnaire scores were found to be similar. Interestingly, worse baseline UUI was associated with greater reduction in episodes of UUI for both therapies.
This was a rigorous and well-executed double-blind, double-placebo−controlled randomized trial. By utilizing broad inclusion criteria and enrolling patients both with and without previous exposure to anticholinergic medications, the generalizability of study findings are greatly improved. Because this study did not examine the effect or efficacy of repeat injections, these findings have limited applicability to patients undergoing multiple onabotulinumtoxinA injections.
When considering use in your patient population, keep the possible side effects in mind.There were important differences in the side effects experienced with each therapy. Specifically, while the anticholinergic group had a higher frequency of dry mouth (46% anticholinergic vs 31% onabotulinumtoxinA, P = .02), the onabotulinumtoxinA group demonstrated higher rates of incomplete bladder emptying requiring catheterization (peak of 5% at 2 months) and greater risk of UTI (33% onabotulinumtoxinA vs 13% anticholinergic, P <.001).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This study showed that, among women with UUI, anticholinergic medication and onabotulinumtoxinA are equally effective in reducing UUI episodes and improving quality of life. It is important to consider the side effect profile, determine the patient’s preferences, and weigh the risks and benefits of each therapy when deciding what is the best treatment for your individual patient.
We want to hear from you! Tell us what you think.
Urinary incontinence (UI) affects almost half of all women in the United States.1,2 Estimates suggest that the prevalence of UI gradually rises during young adult life, comes to a broad plateau in middle age, and then steadily increases from that plateau after age 65. Therefore, over the next 40 years, as the elderly population expands in size, the number of women affected by UI will significantly grow.3
For patients with UI, a multitude of therapeutic options are available. Which option is the best for your patient? In this article, we aim to answer that question by interpreting the results of four randomized trials, each of which directly compare two available treatment options. The first study examines patients with stress urinary incontinence (SUI), comparing the patients’ subjective improvement in urinary leakage and bladder function at 12 months after randomization to treatment with physiotherapy or midurethral sling surgery.
The three other trials examine patients with overactive bladder (OAB) and urgency urinary incontinence (UUI). Each trial directly compares the use of anticholinergic medications to an alternate treatment modality. Currently, anticholinergic medications and behavioral therapy are the recommended first-line therapies for OAB. Unfortunately, anticholinergic medications have poor patient compliance and significant systemic side effects.4 Caution should be used when considering anticholinergic medications in patients with impaired gastric emptying or a history of urinary retention. They also should be used with caution in elderly patients who are extremely frail. Additionally, clearance from an ophthalmologist must be obtained prior to starting anticholinergic medication in patients with narrow-angle glaucoma.5 Due to poor adherence and potential side effects, there is a growing effort to discover alternative treatment modalities that are safe and effective. Therefore, we chose to examine trials comparing: mirabegron versus tolterodine, percutaneous tibial nerve stimulation versus tolterodine, and onabotulinumtoxinA versus anticholingeric medications.
UI defined
Before discussing treatment options, we want to clarify the main types of UI (FIGURE). UI is defined as the complaint of involuntary loss of urine. UI can be subdivided into SUI, OAB/UUI, or mixed urinary incontinence.6 While there are other less common genitourinary etiologies that can lead to UI, nongenitourinary etiologies are prevalent and can aggravate existing SUI or OAB (TABLE).
SUI is the complaint of involuntary loss of urine on effort or physical exertion (such as during sporting activities) or on sneezing or coughing. Often, SUI can be diagnosed by patient report alone and surgery can be considered in symptomatic patients who demonstrate cough leakage on physical examination and normal postvoid residual volumes.
UUI is the involuntary loss of urine associated with urgency; it often occurs in the setting of OAB, which is defined as the syndrome of urinary urgency, usually accompanied by frequency and nocturia, with or without UUI, in the absence of urinary tract infection or other obvious pathology (such as neurologic dysfunction, infection, or urologic neoplasm). OAB-dry is present when patients do not have leakage with urgency, but are bothered by urgency, frequency, and/or nocturia. OAB-wet occurs when a patient has urgencyincontinence.
The presence of both SUI and OAB/UUI is known as mixed urinary incontinence. Stress and urgency urinary symptoms often present together. In fact, 10% to 30% of women with stress symptoms are found to have bladder overactivity on subsequent evaluation.2,7 Therefore, it is important to take a good history and consider urodynamic evaluation to confirm the diagnosis of SUI prior to surgery in women with mixed stress and urge symptoms, a history of a previous surgery for incontinence, or when there is a poor correlation of physical examination findings to reported symptoms.
Is surgery a first-line option for patients with SUI?
Labrie J, Berghmans BL, Fischer K, et al. Surgery versus physiotherapy for stress urinary incontinence. NEJM. 2013;369(12):1124−1133.
Physiotherapy, including pelvic floor muscle training (“Kegel exercises”), is utilized as a first-line treatment option for women with SUI that carries minimal risk for the patient. Midurethral sling surgery is often recommended if an initial trial of conservative treatment fails.7 Up to 50% of women treated with pelvic floor physiotherapy will ultimately undergo surgery to treat their SUI.8
Related article: Does urodynamic testing before surgery for stress incontinence improve outcomes? G. Willy Davila, MD (Examining the Evidence, December 2012)
Details of the study
This was a randomized, multicenter trial of women aged 35 to 80 years with moderate to severe SUI. After excluding women with previous incontinence surgery and stage 2 or higher pelvic organ prolapse, 460 participants were randomly assigned to undergo either a midurethral sling surgery or physiotherapy (pelvic floor muscle training). The primary outcome was subjective improvement in urinary leakage and bladder function at 12 months, as measured by the Patient Global Impression of Improvement (PGI-I), a 7-point Likert scale ranging from “very much worse” to “very much better.”
In an intention-to-treat analysis, subjective improvement at 12 months was significantly higher in women randomized to midurethral sling surgery than in women randomized to physiotherapy (91% vs 64%, respectively).
Ten percent of patients had adverse events (AEs); all were related to surgery. The most common AEs were hematoma, vaginal epithelial perforation, and bladder perforation.
Notably, women had the option to cross over to the other treatment modality if they desired. In the physiotherapy group, 49% of women elected to cross over to surgery, while 11% of those who underwent midurethral sling surgery elected to cross over to physiotherapy during the 12-month follow-up period. When analyzing results by treatment received, the investigators found that the proportion of women who reported improvement was significantly lower among women who underwent physiotherapy only (32%), versus sling only (94%), or sling after physiotherapy (91%).
This randomized trial was well-designed and included a variety of treatment centers (university and general hospitals) with interventions performed by experienced surgeons (all of whom had performed at least 20 sling surgeries) and physiotherapists educated specifically in pelvic floor physiotherapy. The study population was limited to patients with moderate to severe SUI as defined by the Sandvik severity index.9 Therefore, these results may not be applicable to patients with milder symptoms, for whom physiotherapy has been recommended as first-line therapy with consideration of surgery if physiotherapy fails to sufficiently improve symptoms.7
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women with moderate to severe SUI without significant prolapse or a history of prior incontinence surgery have significantly better outcomes at 12 months after undergoing midurethral sling surgery versus physiotherapy. Physiotherapy carries little to no risk of adverse effects. Women with moderate to severe SUI should be counseled regarding the risks and benefits of both physiotherapy and midurethral sling surgery as initial treatment options.
Because stress and urgency urinary symptoms often present together, it is important to consider urodynamic evaluation to confirm SUI prior to surgery in women with:
• mixed stress and urge symptoms
• a history of a previous surgery for incontinence, or
• poor correlation of physical examination findings to reported symptoms.
Safety and tolerability of mirabegron versus tolterodine for OAB
Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a beta(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63(2):296−305.
In the bladder, beta3-receptors located within the detrusor smooth muscle facilitate urine storage by relaxing the detrusor, enabling the bladder to fill.10 The activation of beta3-receptors is thought to increase the bladder’s ability to store urine, with the goal of decreasing urgency, frequency, nocturia, and urgency incontinence. An alternative to anticholinergic medications, mirabegron is a beta3-agonist approved by the US Food and Drug Administration (FDA) in 2012 for clinical use in the treatment of OAB.
Details of the study
Chapple and colleagues aimed to assess the 12-month efficacy and safety of mirabegron in a randomized, double-blind active controlled trial. The primary outcome was incidence and severity of treatment-emergent adverse effects (TEAEs); the secondary outcome was the change in OAB symptoms from baseline to up to 12 months. Patients experiencing OAB symptoms for more than 3 months were eligible and were subsequently enrolled if they averaged 8 or more voids per day and 3 or more episodes of urgency with or without incontinence on a 3-day bladder diary. A total of 2,444 patients were randomly assigned in a 1:1:1 fashion to mirabegron 50 mg daily, mirabegron 100 mg daily, or tolterodine extended release (ER) 4 mg daily.
There was a similar incidence (60% to 63%) of TEAEs across all three groups. The most common TEAEs were hypertension (defined as average systolic blood pressure [BP] >140 mm Hg or average diastolic BP >90 mm Hg at two consecutive visits), UTI, headache, nasopharyngitis, and constipation. The adjusted mean changes in BP from baseline to final visit were less than 1 mm Hg for both systolic and diastolic BP for patients taking both doses of mirabegron, as well as for patients taking tolterodine. The incidence of dry mouth was higher in the tolterodine group than the mirabegron groups. Mirabegron 50 mg daily and 100 mg daily improved incontinence symptoms within 1 month of starting therapy; the degree of improvement was similar to that seen in the patients taking tolterodine ER 4 mg daily.
Related article: New overactive bladder treatment approved by the FDA (August 2012)
Some caveats
This study was well-designed to assess the safety and tolerability of mirabegron versus tolterodine. The doses utilized in the study were at or above the FDA-approved dosage of 25 mg to 50 mg daily for OAB treatment. Although investigators found mirabegron to be a safe alternative to anticholinergic medication, the study was not designed or powered to examine the efficacy of mirabegron versus tolterodine. No formal comparison of efficacy was made between mirabegron or tolterodine, or between the 50-mg and 100-mg doses of mirabegron. Moreover, 81% of participants had been treated with mirabegron in earlier Phase 3 studies, so most were not treatment naïve, limiting the applicability of results.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Mirabegron should be considered as a potential treatment option for patients who demonstrate poor tolerance of or response to anticholinergic medications; however, caution should be used in patients with severe uncontrolled high BP, end-stage kidney disease, or severe liver impairment.
Consider percutaneous tibial nerve stimulation over tolterodine for OAB in select patients
Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: Results from the overactive bladder innovative therapy trial. J Urol. 2009;182(3):1055−1061.
Neuromodulation utilizes electrical stimulation to improve bladder function and decrease OAB symptoms. First developed in the early 1980s by McGuire and colleagues, percutaneous tibial nerve stimulation (PTNS) was approved by the FDA in 2000 as Urgent PC and provides an outpatient, nonimplantable neuromodulation alternative to medication therapy for patients with OAB.11,12 By directly stimulating the posterior tibial nerve, PTNS works via the S3 sacral nerve plexus to alter the micturition reflex and improve bladder function.
Details of the study
Patients were eligible for the study if they demonstrated 8 or more voids per day on a 3-day bladder diary (whether or not they had a history of previous anticholinergic drug use). A total of 100 ambulatory adults with OAB symptoms were enrolled and randomly assigned to PTNS 30-minutes per week or tolterodine ER 4 mg daily.
At 12 weeks, both groups demonstrated a significant improvement in OAB measures as well as validated symptom severity and quality-of-life questionnaire scores. Subjective assessment of improvement in OAB symptoms was significantly greater in the PTNS group than in the tolterodine group (79.5% vs 54.8%, respectively; P = .01). However, mean reduction of voids for 24 hours was not significantly different between the two groups.
Both treatments were well tolerated, with only 15% to 16% of patients in both groups reporting mild to moderate side effects. The tolterodine group did have a significantly higher risk of dry mouth; however, the risk of constipation was not significantly different between the groups.
Study limitations
The authors performed an important multicenter, nonblinded, randomized, controlled trial, which was one of the first trials to directly compare two OAB therapies. The generalizability of the findings were limited, as the cohort included mostly patients with dry OAB who had no objective measures on UUI episodes. In addition, this trial had a limited observation period of only 12 weeks. Information regarding the effect of treatment after cessation of weekly PTNS therapy was not examined. Therefore, we are not able to determine whether repeat sessions provide adequate maintenance in the long term.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
PTNS 30 minutes daily is as effective as tolterodine ER 4 mg daily for 12 weeks in reducing OAB symptoms. PTNS is a safe alternative that should be considered in patients with OAB who poorly tolerate or have contraindications to medication therapy.
OnabotulinumtoxinA is an effective therapy for OAB
Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. NEJM. 2012;367(19):1803−1813.
The newest therapy for OAB is onabotulinumtoxinA, or Botox, which was FDA approved this year for the treatment of OAB in adults who cannot use or do not tolerate anticholinergic medications. Recommended doses are 100 U onabotulinumtoxinA in patients with idiopathic refractory OAB and 200 U onabotulinumtoxinA for patients with neurogenic OAB.
OnabotulinumtoxinA is a neurotoxin that blocks synaptic transmission at the neuromuscular junction to cause muscle paralysis and atrophy.13 Injecting onabotulinumtoxinA into the detrusor smooth muscle should relax the bladder and decrease sensations of urgency and frequency to achieve a longer duration of time for bladder filling and reduce the risk of urgency incontinence.
Effects of onabotulinumtoxinA appear to wear off over time, and patients may require repeat injections. Side effects of onabotulinumtoxinA therapy include an increased risk of UTI and the potential for urinary retention requiring intermittent self-catheterization.
Related article: Update on Pelvic Floor Dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
Details of the study
The Anticholinergic Versus Botulinum Toxin Comparison (ABC) study was a multicenter, randomized, double-blind, double-placebo–controlled trial conducted in women without known neurologic disease with moderate to severe UUI (defined as >5 UUI episodes on a 3-day bladder diary). Women were randomly assigned to a single intradetrusor injection of 100 U onabotulinumtoxinA plus oral placebo or to a single intradetrusor injection of saline plus solifenacin 5 mg daily (with the option of dose escalation and then switching to trospium XR if no improvement was seen).
Of the 241 women included in the final analysis, approximately 70% in each group reported adequate control of symptoms at 6 months. Adequate control was defined as a response of “agree strongly” or “agree” to the statement: “This treatment has given me adequate control of my urinary leakage.” Women in the onabotulinumtoxinA group were significantly more likely than women in the anticholinergic medication group to report complete resolution of UUI at 6 months (27% vs 13%, P = .003). However, the mean reduction in episodes of UUI per day and the improvements in quality-of-life questionnaire scores were found to be similar. Interestingly, worse baseline UUI was associated with greater reduction in episodes of UUI for both therapies.
This was a rigorous and well-executed double-blind, double-placebo−controlled randomized trial. By utilizing broad inclusion criteria and enrolling patients both with and without previous exposure to anticholinergic medications, the generalizability of study findings are greatly improved. Because this study did not examine the effect or efficacy of repeat injections, these findings have limited applicability to patients undergoing multiple onabotulinumtoxinA injections.
When considering use in your patient population, keep the possible side effects in mind.There were important differences in the side effects experienced with each therapy. Specifically, while the anticholinergic group had a higher frequency of dry mouth (46% anticholinergic vs 31% onabotulinumtoxinA, P = .02), the onabotulinumtoxinA group demonstrated higher rates of incomplete bladder emptying requiring catheterization (peak of 5% at 2 months) and greater risk of UTI (33% onabotulinumtoxinA vs 13% anticholinergic, P <.001).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This study showed that, among women with UUI, anticholinergic medication and onabotulinumtoxinA are equally effective in reducing UUI episodes and improving quality of life. It is important to consider the side effect profile, determine the patient’s preferences, and weigh the risks and benefits of each therapy when deciding what is the best treatment for your individual patient.
We want to hear from you! Tell us what you think.
- Anger JT, Saigal CS, Litwin MS. The prevalence of urinary incontinence among community dwelling adult women: Results from the National Health and Nutrition Examination Survey. J Urol. 2006;175(2):601–604.
- Dooley Y, Kenton K, Cao G, et al. Urinary incontinence prevalence: Results from the National Health and Nutrition Examination Survey. J Urol. 2008;179(2):656–661.
- Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstetr Gynecol. 2009;114(6):1278–1283.
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. Americal Urological Association. http://www.auanet.org/common/pdf/education/clinical-guidance/Overactive-Bladder.pdf. Published 2012. Revised June 11, 2013. Accessed October 21, 2013.
- Yu YF, Nichol MB, Yu AP, Ahn J. Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the California Medicaid program. Value Health. 2005;8(4):495–505.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- ACOG Practice Bulletin No. 63: Urinary incontinence in women. American College of Obstetricians and Gynecologists. Obstetr Gynecol. 2005;105(6):1533–1545.
- Bo K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstetr Gynecol. 2005;105(5 Pt 1):999–1005.
- Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47(6):497–499.
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–466.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129(1):78–79.
- Schiavo G, Santucci A, Dasgupta BR, et al. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335(1):99–103.
- Anger JT, Saigal CS, Litwin MS. The prevalence of urinary incontinence among community dwelling adult women: Results from the National Health and Nutrition Examination Survey. J Urol. 2006;175(2):601–604.
- Dooley Y, Kenton K, Cao G, et al. Urinary incontinence prevalence: Results from the National Health and Nutrition Examination Survey. J Urol. 2008;179(2):656–661.
- Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstetr Gynecol. 2009;114(6):1278–1283.
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. Americal Urological Association. http://www.auanet.org/common/pdf/education/clinical-guidance/Overactive-Bladder.pdf. Published 2012. Revised June 11, 2013. Accessed October 21, 2013.
- Yu YF, Nichol MB, Yu AP, Ahn J. Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the California Medicaid program. Value Health. 2005;8(4):495–505.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- ACOG Practice Bulletin No. 63: Urinary incontinence in women. American College of Obstetricians and Gynecologists. Obstetr Gynecol. 2005;105(6):1533–1545.
- Bo K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstetr Gynecol. 2005;105(5 Pt 1):999–1005.
- Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47(6):497–499.
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–466.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129(1):78–79.
- Schiavo G, Santucci A, Dasgupta BR, et al. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335(1):99–103.
Fecal incontinence: New therapies, age-old problem
Fecal incontinence is a socially embarrassing condition that affects approximately 18 million adults in the United States.1 Its true incidence is likely much higher than reported, however, as many patients are reluctant to discuss it.
A recent study found that nearly 20% of women experience fecal incontinence at least once a year, and 9.5% experience it al least once a month.2 Only 28% of these women had ever discussed their symptoms with a physician, however.3 Women who did seek care were more likely to consult a family physician or internist (75%) than a gynecologist (7%).3
Until recently, few options were available for patients with fecal incontinence who had not benefited from conservative measures. Many patients simply had to live with their symptoms or undergo a diverting ostomy to control the chronic involuntary drainage.
Recent years have seen the development of new minimally invasive and highly successful techniques to treat fecal incontinence. Greater awareness of the prevalence of fecal incontinence and its devastating impact on quality of life is needed for this problem to be fully addressed, however. In this article, we review the recommended evaluation of a patient who reports fecal incontinence and describe the range of treatment options.
Fecal incontinence is a symptom, not a diagnosis
Although the most common historical factor contributing to fecal incontinence is obstetric trauma, there are several other causes of this condition. A detailed history and physical examination are vital to determine whether the patient is experiencing true fecal incontinence, or whether she is leaking for other reasons—so-called pseudo-incontinence.
Conditions that can mimic fecal incontinence include:
- prolapsing hemorrhoids
- anal fistula
- sexually transmitted infection
- benign or malignant anorectal neoplasms
- dermatologic conditions.
True fecal incontinence may be active (loss of stool despite the patient’s best effort to control it) or passive (loss of stool without the patient’s awareness). Among the causes of true fecal incontinence are:
- anal sphincter injury (obstetric tear, anorectal surgery such as fistulotomy, or trauma)
- denervation of the pelvic floor from pudendal nerve injury during childbirth
- chronic rectal prolapse
- neurologic conditions (spina bifida, myelomeningocele)
- noncompliant rectum from inflammatory bowel disease
- radiation proctitis.
The maintenance of continence requires a complex interaction between the sphincter muscle, the puborectalis muscle (which acts as a sling), rectal capacity and compliance, stool volume and frequency, and neurologic mechanisms.
Diagnosis and management require an experienced physician
We believe that patients reporting fecal incontinence are best worked up and managed by a physician who is well versed in the various diagnoses associated with fecal incontinence, as well as the most current treatments.
Diagnosis entails some detective work
When a patient reports fecal incontinence, she should be asked to elaborate on the circumstances surrounding the complaint and the frequency of its occurrence, duration of symptoms, and nature of the incontinence (gas, liquid, or solid).
Validated quality-of-life instruments, such as the Cleveland Clinic Florida Fecal Incontinence Score (CCF-FIS) may be helpful in documenting the severity of the symptoms and improvement after treatment (TABLE).4
One factor that current scoring systems fail to capture is urgency. In many instances, urgency is the symptom most distressing to the patient. Be sure to ask about it.
A detailed obstetric history also is important. It is not uncommon for a patient to develop symptoms 20 years or longer after the injury. Also review the patient’s medical history for inflammatory bowel disease, neurologic disorders, and any history of pelvic radiation for help in determining the cause of symptoms.
In addition, ask the patient about any other pelvic floor symptoms, such as voiding dysfunction and problems with pelvic organ prolapse. And question her about stool consistency and frequency. In some cases, diarrhea can lead to fecal incontinence and is usually managed conservatively.
Physical exam: Focus on the perineum and anus
A detailed physical examination is warranted to determine the state of the patient’s sphincter musculature and rule out other causes of pseudo-incontinence, such as hemorrhoids or anal fistula. Inspect the perineum for thinning of the perineal body and scars from prior surgery.
A patulous anus may be a sign of rectal prolapse. To check for it, ask the patient to strain on the commode. If rectal prolapse is present, it will become apparent upon straining. If prolapse is detected, surgical treatment of the prolapse would be the first step in managing the incontinence.
A simple test of neurologic function is to try to elicit an anal “wink” in response to a pinprick.
A digital rectal exam allows the assessment of resting and squeeze tone, as well as the use of accessory muscles, such as the gluteus maximus, during squeezing.
Rigid or flexible proctoscopy may be indicated to rule out inflammatory bowel disease, radiation proctitis, and rectal neoplasm, depending on the patient’s history.
A few diagnostic adjuncts can help
Several adjuncts to physical examination can provide more detailed information about the patient’s condition and facilitate the development of an individualized treatment plan. For example, if rectal prolapse, rectocele with delayed emptying, or enterocele is suspected, consider defecography. If voiding dysfunction coexists with the fecal incontinence, urodynamic testing and cystoscopy may be indicated.
We routinely perform physiology testing and endoanal ultrasound if surgery is planned to address the fecal incontinence, although routine use of these adjuncts is controversial. Because many patients can be managed with conservative medical measures, we do not find it necessary to perform these tests at the time of the first visit.
Anal physiology testing includes manometry (a measure of both resting and squeeze tone) and pudendal nerve terminal motor latency testing.
Manometry can help quantify the severity of muscle weakness and determine the presence or absence of normal anal reflexes. Pudendal nerve testing assesses the innervation of the anal sphincter. There is some evidence that patients who have a pudendal neuropathy have a poor outcome with sphincteroplasty,5 although that evidence is controversial. The findings from physiology studies have not been correlated with outcomes of newer treatments, such as sacral neuromodulation (InterStim, Medtronic, Minneapolis, Minnesota). Each physiology lab uses different equipment, so “normal” values vary between institutions.
Endoanal ultrasound is easily performed in an office setting. It is well tolerated and provides anatomic detail of the sphincter musculature. We use a 13-MHz rotating probe to provide 3D imaging of the anal canal. The internal sphincter is represented by a hypoechoic circle surrounded by the hyperechoic external sphincter (FIGURE 1).
In the hands of an experienced examiner, the sensitivity and specificity of endoanal ultrasound in detecting sphincter defects approaches 100%.6,7 Ultrasound also enables measurement of the perineal body. A normal perineal body measures 10 to 15 mm.
For treatment, try conservative measures first
Bulking agents (fiber), constipating agents (loperamide, etc.), or a laxative regimen with scheduled disimpactions (in patients who have pelvic outlet constipation and overflow incontinence) often can control the symptoms of fecal incontinence, making further interventions unnecessary.
Biofeedback is another option. It uses visual, auditory, and sensory information to train patients to better control anal sphincter muscle function.
A recent randomized study found manometric biofeedback to be superior to simple Kegel exercises in improving fecal continence.8 In this study, 76% of patients in the biofeedback group experienced symptom improvement, compared with 41% of patients in the pelvic floor exercise group (P <.001). The long-term benefits of biofeedback are less clear, and patients often need to be reminded to perform their exercises at home and to attend occasional refresher-training sessions. Nevertheless, biofeedback is an important noninvasive option for patients in whom medical management has failed.
Minimally invasive options are now available
Over the past 2 years, minimally invasive treatments for fecal incontinence have emerged, including an implantable sacral neuromodulation device (InterStim) and an injectable dextranomer (Solesta; Salix Pharmaceuticals, Raleigh, North Carolina). Previously, the only surgical option for fecal incontinence was a sphincter repair if a defect was present. The new options may help patients improve their quality of life without having to undergo major surgery.
No one has directly compared the outcomes of these procedures when they are performed by a colorectal surgeon versus a physician of another specialty. It is our belief that the treating physician should have a strong interest in caring for these complex patients and a good working knowledge of the various treatment options.
Related Article Obstetric anal sphincter injury: 7 critical questions about care Ranee Thakar, MD, MRCOG (February 2008)
Sacral neuromodulation
This technique initially was developed for the treatment of overactive bladder and nonobstructive urinary retention and has been used in the United States for the past 15 years for these indications. Improvement in fecal continence was observed in these patients, prompting further studies of its efficacy. In 2011, it was approved by the US Food and Drug Administration (FDA) for the treatment of fecal incontinence. It has since revolutionized the treatment of this disorder, offering a minimally invasive and highly successful alternative to sphincteroplasty.
The InterStim procedure is the only therapeutic modality to include a test phase. The outpatient procedure involves sterile placement of an electrode through the S3 foramen to stimulate the S3 nerve root using fluoroscopic guidance (FIGURES 2 and 3). Patients who experience at least 50% improvement in symptoms are then offered placement of a permanent stimulator.
In most series, approximately 90% of patients have a positive test and progress to implantation. A recent US multicenter clinical trial indicated that 86% of patients achieved an improvement in continence of at least 50%, and 40% of patients were completely continent at 3 years.9 The number of episodes of incontinence decreased from a mean of 9.4/week to 1.7/week.9 Quality of life also improved greatly. Few complications have been reported, the most notable of which is infection (10.8% in the US multicenter trial9).
Another advantage of sacral neuromodulation: It can be used successfully in patients with external sphincter defects as large as 120º. A study by Tjandra and colleagues found that 65% of patients experienced improvement in symptoms of at least 50%, and 47% of patients (more than 50% of whom had external sphincter defects as large as 120º) became completely continent.10
The only variable shown to predict success with sacral neuromodulation is a positive response to the test implant procedure.
In our experience, this procedure is easy to perform and well tolerated, even in elderly patients with multiple comorbidities. The procedure has the additional advantage of potentially improving concomitant urinary symptoms as well.
The major disadvantage of sacral neuromodulation is its cost, although most major insurance carriers cover it. There is no well-conducted cost-effectiveness analysis comparing this modality to other treatments.
Related Article Interstim: An implantable device for implacable urinary symptoms Deborah L. Myers, MD (October 2006)
Injectable agents
Several biocompatible bulking agents have been tested in the treatment of fecal incontinence. These compounds traditionally have been used to treat mild fecal incontinence, or to treat patients with isolated internal sphincter defects.
More recently, an injectable dextranomer in stabilized hyaluronic acid was approved by the FDA and marketed as Solesta. Graf and colleagues randomly allocated 136 patients to injection and 70 patients to sham injection. Patients with external sphincter defects were excluded. At 6 months, 52% of patients in the active treatment group experienced an improvement in continence of at least 50%, compared with 31% of patients injected with placebo.11
The advantage of this procedure is its minimally invasive nature (submucosal injection performed in the office). The disadvantage: a lack of long-term efficacy data, although unpublished data suggest that patients who improve after an injection see a durable response at 3 years.
This easy, office-based treatment is ideal for patients with minor incontinence or persistent symptoms after another procedure.
Sphincter repair
Anterior sphincteroplasty has been the mainstay of surgical treatment for patients with a sphincter defect. With the patient in a dorsal lithotomy or prone position on the operating-room table, a transverse perineal incision is made, and the ends of the severed sphincter muscle are located and mobilized. The repair then can be performed in an end-to-end manner or by overlapping the muscles in the anterior midline (FIGURE 4).
Some of the debatable technical issues of this procedure include:
- whether to overlap the muscles or scar tissue
- whether to repair internal and external defects together or separately
- how the age of the patient affects the outcome.
In regard to the first issue, there may be a superior outcome with overlapping repairs, but they carry a higher risk of dyspareunia and evacuation difficulties. Some surgeons will attempt a separate repair of the internal and external sphincter muscles if it appears feasible. Most often, both muscles are tethered together with scar tissue and separate repair is not possible. There are no conclusive data to demonstrate the superiority of either approach.
As for age, the traditional teaching was that older patients do not benefit from this procedure as much as younger patients do. However, a recent study found no differences in the CCF-FIS score in patients older than age 60, compared with younger patients.12 Investigators concluded that sphincteroplasty can be offered to both young and older patients.12
Although sphincteroplasty often leads to excellent short-term improvement, with 60% to 90% of patients experiencing a good or excellent outcome, nearly all series indicate a decline over the long term (>5 years). A recent systematic review found that as few as 12% of patients experience a good or excellent result, depending on the series.13
We offer sphincter repair to young women with a new sphincter defect after delivery. For older patients, we offer sacral neuromodulation as a first-line treatment.
Other surgical options
We believe that most patients with fecal incontinence can be managed using conservative measures, sacral neuromodulation, injectable dextranomer, or sphincter repair. However, several other options are available.
Artificial bowel sphincter
The artificial bowel sphincter was first described in 1987 and has been modified over the years. The system currently is marketed as the Acticon Neosphincter (American Medical Systems, Minnetonka, Minnesota). The procedure involves the creation of a subcutaneous tunnel around the anus so that an inflatable cuff can be positioned there. A pump then is tunneled through a Pfannenstiel incision to the labia or scrotum, and a reservoir is positioned in the space of Retzius. The device maintains continence by keeping the cuff inflated during the resting state and by pumping fluid from the cuff to the reservoir when the patient needs to evacuate.
The major barrier to utilization of the artificial bowel sphincter is infection. In a series of 112 patients who were implanted with the sphincter, 384 device-related adverse events occurred in 99 patients.14 A total of 73 revision operations were required in 51 patients (46%). Twenty-five percent of patients developed infection that required surgical revision, and 37% had the device explanted. Eighty-five percent of patients with a functional device had a successful outcome.14
Given the device-related challenges and infectious complications, patients should be considered for less invasive treatments before being offered an artificial bowel
sphincter.
Radiofrequency current
The Secca procedure (Curon Medical, Fremont, California) involves the application of radiofrequency current to the anal canal to generate thermal energy. This procedure causes contraction of collagen fibers, which are permanently shortened, and leads to tightening of the muscle. It is performed under intravenous sedation on an outpatient basis.
This approach is indicated for patients with mild to moderate fecal incontinence who have not responded to conservative management. An external sphincter defect is a contraindication.
Small, nonrandomized studies have found improvement in the CCF-FIS score in patients treated with this approach.15 The major limitation of this treatment is the lack of high-level clinical evidence demonstrating its efficacy and safety.
Antegrade continence enema
This approach, also known as the Malone procedure, is usually reserved for debilitating incontinence or constipation in the pediatric population. An appendicostomy is constructed at the navel, allowing daily introduction of a catheter and antegrade enema. The purpose is to perform rapid, controlled emptying of the colon at times chosen by the patient. It is also reserved as a last resort for patients considering an ostomy.
Adult patients with neurologic problems, such as spina bifida, may be candidates for this procedure, provided they are highly motivated.
Fecal diversion
Creation of a colostomy or ileostomy is usually the last resort for a patient with fecal incontinence. We are fortunate that there are an increasing number of options that may improve the patient’s condition before colostomy is required.
If fecal diversion is chosen by the patient, it is important to involve an enterostomal therapist for site marking and patient education. A well-constructed ostomy is essential, as this option often is permanent.
Up and coming options
A novel treatment approach for fecal incontinence is the magnetic anal sphincter. The device, marketed as the FENIX Continence Restoration System (Torax Medical, Shoreview, Minnesota) consists of a series of titanium beads with magnetic cores that are interlinked with titanium wires. The device is designed to encircle the external anal sphincter muscle, reinforce the sphincter, and expand to allow passage of stool at a socially appropriate time.
Preliminary data from 16 patients indicate a mean decrease in the number of episodes of incontinence from 7.2/week to 0.7/week, as well as a mean reduction in the CCF-FIS score from 17.2 to 8.7.16 Two devices were removed due to infection, and one device passed spontaneously after disconnection of the suture.16
This device is not approved by the FDA, but it may become a promising treatment if its safety and efficacy can be established in larger clinical trials.
The TOPAS sling (American Medical Systems) is currently being studied in a Phase 3, multicenter, nonrandomized, clinical trial (NCT01090739) for the treatment of fecal incontinence.17 The sling is implanted using a minimally invasive transobturator approach; two needle-passers deliver the sling assembly. Two small posterior incisions facilitate the postanal placement of the mesh.
This procedure replicates the anorectal angle created by the puborectalis muscle. Although it may become a minimally invasive treatment in the future, final results of the Phase 3 trial are not expected until 2016.
Tibial nerve stimulation is commonly used for urinary urge incontinence. Several small series have documented modest success with its application to fecal incontinence.18
The outpatient procedure involves the insertion of a needle electrode three fingerbreadths above the medial malleolus, followed by electrical stimulation. The current is slowly increased until a sensory or motor response (tingling under the sole of the foot or great toe plantar flexion) is elicited. Treatment necessitates several outpatient sessions.
In a recent series, the mean CCF-FIS decreased from 12.2/20 at baseline to 9.1/20 after treatment (P <.0001).18
The role of this procedure in the treatment algorithm for fecal incontinence remains to be determined.
What we offer patients
Fecal incontinence is a debilitating condition with an increasing number of potential therapeutic options. It clearly is under-recognized by patients and physicians alike.
After a thorough work-up, conservative treatment options should be offered first. When those fail, we generally recommend a trial of sacral neuromodulation for patients with no sphincter defect. When a sphincter defect is present, we counsel the patient about the merits of sphincter repair versus a trial of neuromodulation. These options have the most robust data supporting their clinical use, and have been used successfully in our own practices.
Given the continuous development of other therapeutic modalities, it is likely that future treatments will involve a stepwise progression of approaches. The need for colostomy should diminish in coming years as more minimally invasive techniques become available.
- Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512–517.
- Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66(11):1101–1108.
- Brown HW, Wexner SD, Segall MM, et al. Quality of life in women with accidental bowel leakage. Int Clin Pract. 2012;66(11):1109–1116.
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97.
- Sangwan YP, Collar JA, Barrett RC, et al. Unilateral pudendal neuropathy. Impact on outcomes of anal sphincter repair. Dis Colon Rectum. 1996;39(6):686–689.
- Deen KI, Kumar D, Williams JG, et al. Anal sphincter defects. Correlation between endoanal ultrasound and surgery. Ann Surg. 1993;218(2):201–205.
- Oberwalder M, Thaler K, Baig MK, et al. Anal ultrasound and endosonographic measurement of perineal body thickness: a new evaluation for fecal incontinence in females. Surg Endosc. 2004;18(4):650–654.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52(10):1730–1737.
- Mellgren AF, Wexner SD, Coller JA, et al. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2011:54(9):1065–1075.
- Tjandra JJ, Chan MK, Yeh CH, et al. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51(5):494–502.
- Graf W, Mellgren A, Matzel K, et al. Efficacy of a dextranomer in stabilized hyaluronic acid for treatment of faecal incontinence: a randomized, sham-controlled trial. Lancet. 2011;377(9770):997–1003.
- El-Gazzaz G, Zutshi M, Hannaway C, Gurland B, Hull T. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55(3):256–261.
- Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55(4):482–490.
- Wong WD, Congliosi SM, Spencer MP, et al. The safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45(9):1139–1153.
- Takahashi T, Morales M, Garcia-Osogobio S, et al. Secca procedure for the treatment of fecal incontinence: results of five-year follow-up. Dis Colon Rectum. 2008;51(3):355–359.
- Lehur PA, McNevin S, Buntzen S, et al. Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum. 2010;53(12):1604–1610.
- TOPAS sling. http://clinicaltrials.gov/ct2/show/NCT01090739. Accessed August 26, 2013.
- Hotouras A, Thaha MA, Allison ME, et al. Percutaneous tibial nerve stimulation (PTNS) in females with faecal incontinence: the impact of sphincter morphology and rectal sensation on the clinical outcome. Int J Colorectal Dis. 2012;27(7):927–930.
Fecal incontinence is a socially embarrassing condition that affects approximately 18 million adults in the United States.1 Its true incidence is likely much higher than reported, however, as many patients are reluctant to discuss it.
A recent study found that nearly 20% of women experience fecal incontinence at least once a year, and 9.5% experience it al least once a month.2 Only 28% of these women had ever discussed their symptoms with a physician, however.3 Women who did seek care were more likely to consult a family physician or internist (75%) than a gynecologist (7%).3
Until recently, few options were available for patients with fecal incontinence who had not benefited from conservative measures. Many patients simply had to live with their symptoms or undergo a diverting ostomy to control the chronic involuntary drainage.
Recent years have seen the development of new minimally invasive and highly successful techniques to treat fecal incontinence. Greater awareness of the prevalence of fecal incontinence and its devastating impact on quality of life is needed for this problem to be fully addressed, however. In this article, we review the recommended evaluation of a patient who reports fecal incontinence and describe the range of treatment options.
Fecal incontinence is a symptom, not a diagnosis
Although the most common historical factor contributing to fecal incontinence is obstetric trauma, there are several other causes of this condition. A detailed history and physical examination are vital to determine whether the patient is experiencing true fecal incontinence, or whether she is leaking for other reasons—so-called pseudo-incontinence.
Conditions that can mimic fecal incontinence include:
- prolapsing hemorrhoids
- anal fistula
- sexually transmitted infection
- benign or malignant anorectal neoplasms
- dermatologic conditions.
True fecal incontinence may be active (loss of stool despite the patient’s best effort to control it) or passive (loss of stool without the patient’s awareness). Among the causes of true fecal incontinence are:
- anal sphincter injury (obstetric tear, anorectal surgery such as fistulotomy, or trauma)
- denervation of the pelvic floor from pudendal nerve injury during childbirth
- chronic rectal prolapse
- neurologic conditions (spina bifida, myelomeningocele)
- noncompliant rectum from inflammatory bowel disease
- radiation proctitis.
The maintenance of continence requires a complex interaction between the sphincter muscle, the puborectalis muscle (which acts as a sling), rectal capacity and compliance, stool volume and frequency, and neurologic mechanisms.
Diagnosis and management require an experienced physician
We believe that patients reporting fecal incontinence are best worked up and managed by a physician who is well versed in the various diagnoses associated with fecal incontinence, as well as the most current treatments.
Diagnosis entails some detective work
When a patient reports fecal incontinence, she should be asked to elaborate on the circumstances surrounding the complaint and the frequency of its occurrence, duration of symptoms, and nature of the incontinence (gas, liquid, or solid).
Validated quality-of-life instruments, such as the Cleveland Clinic Florida Fecal Incontinence Score (CCF-FIS) may be helpful in documenting the severity of the symptoms and improvement after treatment (TABLE).4
One factor that current scoring systems fail to capture is urgency. In many instances, urgency is the symptom most distressing to the patient. Be sure to ask about it.
A detailed obstetric history also is important. It is not uncommon for a patient to develop symptoms 20 years or longer after the injury. Also review the patient’s medical history for inflammatory bowel disease, neurologic disorders, and any history of pelvic radiation for help in determining the cause of symptoms.
In addition, ask the patient about any other pelvic floor symptoms, such as voiding dysfunction and problems with pelvic organ prolapse. And question her about stool consistency and frequency. In some cases, diarrhea can lead to fecal incontinence and is usually managed conservatively.
Physical exam: Focus on the perineum and anus
A detailed physical examination is warranted to determine the state of the patient’s sphincter musculature and rule out other causes of pseudo-incontinence, such as hemorrhoids or anal fistula. Inspect the perineum for thinning of the perineal body and scars from prior surgery.
A patulous anus may be a sign of rectal prolapse. To check for it, ask the patient to strain on the commode. If rectal prolapse is present, it will become apparent upon straining. If prolapse is detected, surgical treatment of the prolapse would be the first step in managing the incontinence.
A simple test of neurologic function is to try to elicit an anal “wink” in response to a pinprick.
A digital rectal exam allows the assessment of resting and squeeze tone, as well as the use of accessory muscles, such as the gluteus maximus, during squeezing.
Rigid or flexible proctoscopy may be indicated to rule out inflammatory bowel disease, radiation proctitis, and rectal neoplasm, depending on the patient’s history.
A few diagnostic adjuncts can help
Several adjuncts to physical examination can provide more detailed information about the patient’s condition and facilitate the development of an individualized treatment plan. For example, if rectal prolapse, rectocele with delayed emptying, or enterocele is suspected, consider defecography. If voiding dysfunction coexists with the fecal incontinence, urodynamic testing and cystoscopy may be indicated.
We routinely perform physiology testing and endoanal ultrasound if surgery is planned to address the fecal incontinence, although routine use of these adjuncts is controversial. Because many patients can be managed with conservative medical measures, we do not find it necessary to perform these tests at the time of the first visit.
Anal physiology testing includes manometry (a measure of both resting and squeeze tone) and pudendal nerve terminal motor latency testing.
Manometry can help quantify the severity of muscle weakness and determine the presence or absence of normal anal reflexes. Pudendal nerve testing assesses the innervation of the anal sphincter. There is some evidence that patients who have a pudendal neuropathy have a poor outcome with sphincteroplasty,5 although that evidence is controversial. The findings from physiology studies have not been correlated with outcomes of newer treatments, such as sacral neuromodulation (InterStim, Medtronic, Minneapolis, Minnesota). Each physiology lab uses different equipment, so “normal” values vary between institutions.
Endoanal ultrasound is easily performed in an office setting. It is well tolerated and provides anatomic detail of the sphincter musculature. We use a 13-MHz rotating probe to provide 3D imaging of the anal canal. The internal sphincter is represented by a hypoechoic circle surrounded by the hyperechoic external sphincter (FIGURE 1).
In the hands of an experienced examiner, the sensitivity and specificity of endoanal ultrasound in detecting sphincter defects approaches 100%.6,7 Ultrasound also enables measurement of the perineal body. A normal perineal body measures 10 to 15 mm.
For treatment, try conservative measures first
Bulking agents (fiber), constipating agents (loperamide, etc.), or a laxative regimen with scheduled disimpactions (in patients who have pelvic outlet constipation and overflow incontinence) often can control the symptoms of fecal incontinence, making further interventions unnecessary.
Biofeedback is another option. It uses visual, auditory, and sensory information to train patients to better control anal sphincter muscle function.
A recent randomized study found manometric biofeedback to be superior to simple Kegel exercises in improving fecal continence.8 In this study, 76% of patients in the biofeedback group experienced symptom improvement, compared with 41% of patients in the pelvic floor exercise group (P <.001). The long-term benefits of biofeedback are less clear, and patients often need to be reminded to perform their exercises at home and to attend occasional refresher-training sessions. Nevertheless, biofeedback is an important noninvasive option for patients in whom medical management has failed.
Minimally invasive options are now available
Over the past 2 years, minimally invasive treatments for fecal incontinence have emerged, including an implantable sacral neuromodulation device (InterStim) and an injectable dextranomer (Solesta; Salix Pharmaceuticals, Raleigh, North Carolina). Previously, the only surgical option for fecal incontinence was a sphincter repair if a defect was present. The new options may help patients improve their quality of life without having to undergo major surgery.
No one has directly compared the outcomes of these procedures when they are performed by a colorectal surgeon versus a physician of another specialty. It is our belief that the treating physician should have a strong interest in caring for these complex patients and a good working knowledge of the various treatment options.
Related Article Obstetric anal sphincter injury: 7 critical questions about care Ranee Thakar, MD, MRCOG (February 2008)
Sacral neuromodulation
This technique initially was developed for the treatment of overactive bladder and nonobstructive urinary retention and has been used in the United States for the past 15 years for these indications. Improvement in fecal continence was observed in these patients, prompting further studies of its efficacy. In 2011, it was approved by the US Food and Drug Administration (FDA) for the treatment of fecal incontinence. It has since revolutionized the treatment of this disorder, offering a minimally invasive and highly successful alternative to sphincteroplasty.
The InterStim procedure is the only therapeutic modality to include a test phase. The outpatient procedure involves sterile placement of an electrode through the S3 foramen to stimulate the S3 nerve root using fluoroscopic guidance (FIGURES 2 and 3). Patients who experience at least 50% improvement in symptoms are then offered placement of a permanent stimulator.
In most series, approximately 90% of patients have a positive test and progress to implantation. A recent US multicenter clinical trial indicated that 86% of patients achieved an improvement in continence of at least 50%, and 40% of patients were completely continent at 3 years.9 The number of episodes of incontinence decreased from a mean of 9.4/week to 1.7/week.9 Quality of life also improved greatly. Few complications have been reported, the most notable of which is infection (10.8% in the US multicenter trial9).
Another advantage of sacral neuromodulation: It can be used successfully in patients with external sphincter defects as large as 120º. A study by Tjandra and colleagues found that 65% of patients experienced improvement in symptoms of at least 50%, and 47% of patients (more than 50% of whom had external sphincter defects as large as 120º) became completely continent.10
The only variable shown to predict success with sacral neuromodulation is a positive response to the test implant procedure.
In our experience, this procedure is easy to perform and well tolerated, even in elderly patients with multiple comorbidities. The procedure has the additional advantage of potentially improving concomitant urinary symptoms as well.
The major disadvantage of sacral neuromodulation is its cost, although most major insurance carriers cover it. There is no well-conducted cost-effectiveness analysis comparing this modality to other treatments.
Related Article Interstim: An implantable device for implacable urinary symptoms Deborah L. Myers, MD (October 2006)
Injectable agents
Several biocompatible bulking agents have been tested in the treatment of fecal incontinence. These compounds traditionally have been used to treat mild fecal incontinence, or to treat patients with isolated internal sphincter defects.
More recently, an injectable dextranomer in stabilized hyaluronic acid was approved by the FDA and marketed as Solesta. Graf and colleagues randomly allocated 136 patients to injection and 70 patients to sham injection. Patients with external sphincter defects were excluded. At 6 months, 52% of patients in the active treatment group experienced an improvement in continence of at least 50%, compared with 31% of patients injected with placebo.11
The advantage of this procedure is its minimally invasive nature (submucosal injection performed in the office). The disadvantage: a lack of long-term efficacy data, although unpublished data suggest that patients who improve after an injection see a durable response at 3 years.
This easy, office-based treatment is ideal for patients with minor incontinence or persistent symptoms after another procedure.
Sphincter repair
Anterior sphincteroplasty has been the mainstay of surgical treatment for patients with a sphincter defect. With the patient in a dorsal lithotomy or prone position on the operating-room table, a transverse perineal incision is made, and the ends of the severed sphincter muscle are located and mobilized. The repair then can be performed in an end-to-end manner or by overlapping the muscles in the anterior midline (FIGURE 4).
Some of the debatable technical issues of this procedure include:
- whether to overlap the muscles or scar tissue
- whether to repair internal and external defects together or separately
- how the age of the patient affects the outcome.
In regard to the first issue, there may be a superior outcome with overlapping repairs, but they carry a higher risk of dyspareunia and evacuation difficulties. Some surgeons will attempt a separate repair of the internal and external sphincter muscles if it appears feasible. Most often, both muscles are tethered together with scar tissue and separate repair is not possible. There are no conclusive data to demonstrate the superiority of either approach.
As for age, the traditional teaching was that older patients do not benefit from this procedure as much as younger patients do. However, a recent study found no differences in the CCF-FIS score in patients older than age 60, compared with younger patients.12 Investigators concluded that sphincteroplasty can be offered to both young and older patients.12
Although sphincteroplasty often leads to excellent short-term improvement, with 60% to 90% of patients experiencing a good or excellent outcome, nearly all series indicate a decline over the long term (>5 years). A recent systematic review found that as few as 12% of patients experience a good or excellent result, depending on the series.13
We offer sphincter repair to young women with a new sphincter defect after delivery. For older patients, we offer sacral neuromodulation as a first-line treatment.
Other surgical options
We believe that most patients with fecal incontinence can be managed using conservative measures, sacral neuromodulation, injectable dextranomer, or sphincter repair. However, several other options are available.
Artificial bowel sphincter
The artificial bowel sphincter was first described in 1987 and has been modified over the years. The system currently is marketed as the Acticon Neosphincter (American Medical Systems, Minnetonka, Minnesota). The procedure involves the creation of a subcutaneous tunnel around the anus so that an inflatable cuff can be positioned there. A pump then is tunneled through a Pfannenstiel incision to the labia or scrotum, and a reservoir is positioned in the space of Retzius. The device maintains continence by keeping the cuff inflated during the resting state and by pumping fluid from the cuff to the reservoir when the patient needs to evacuate.
The major barrier to utilization of the artificial bowel sphincter is infection. In a series of 112 patients who were implanted with the sphincter, 384 device-related adverse events occurred in 99 patients.14 A total of 73 revision operations were required in 51 patients (46%). Twenty-five percent of patients developed infection that required surgical revision, and 37% had the device explanted. Eighty-five percent of patients with a functional device had a successful outcome.14
Given the device-related challenges and infectious complications, patients should be considered for less invasive treatments before being offered an artificial bowel
sphincter.
Radiofrequency current
The Secca procedure (Curon Medical, Fremont, California) involves the application of radiofrequency current to the anal canal to generate thermal energy. This procedure causes contraction of collagen fibers, which are permanently shortened, and leads to tightening of the muscle. It is performed under intravenous sedation on an outpatient basis.
This approach is indicated for patients with mild to moderate fecal incontinence who have not responded to conservative management. An external sphincter defect is a contraindication.
Small, nonrandomized studies have found improvement in the CCF-FIS score in patients treated with this approach.15 The major limitation of this treatment is the lack of high-level clinical evidence demonstrating its efficacy and safety.
Antegrade continence enema
This approach, also known as the Malone procedure, is usually reserved for debilitating incontinence or constipation in the pediatric population. An appendicostomy is constructed at the navel, allowing daily introduction of a catheter and antegrade enema. The purpose is to perform rapid, controlled emptying of the colon at times chosen by the patient. It is also reserved as a last resort for patients considering an ostomy.
Adult patients with neurologic problems, such as spina bifida, may be candidates for this procedure, provided they are highly motivated.
Fecal diversion
Creation of a colostomy or ileostomy is usually the last resort for a patient with fecal incontinence. We are fortunate that there are an increasing number of options that may improve the patient’s condition before colostomy is required.
If fecal diversion is chosen by the patient, it is important to involve an enterostomal therapist for site marking and patient education. A well-constructed ostomy is essential, as this option often is permanent.
Up and coming options
A novel treatment approach for fecal incontinence is the magnetic anal sphincter. The device, marketed as the FENIX Continence Restoration System (Torax Medical, Shoreview, Minnesota) consists of a series of titanium beads with magnetic cores that are interlinked with titanium wires. The device is designed to encircle the external anal sphincter muscle, reinforce the sphincter, and expand to allow passage of stool at a socially appropriate time.
Preliminary data from 16 patients indicate a mean decrease in the number of episodes of incontinence from 7.2/week to 0.7/week, as well as a mean reduction in the CCF-FIS score from 17.2 to 8.7.16 Two devices were removed due to infection, and one device passed spontaneously after disconnection of the suture.16
This device is not approved by the FDA, but it may become a promising treatment if its safety and efficacy can be established in larger clinical trials.
The TOPAS sling (American Medical Systems) is currently being studied in a Phase 3, multicenter, nonrandomized, clinical trial (NCT01090739) for the treatment of fecal incontinence.17 The sling is implanted using a minimally invasive transobturator approach; two needle-passers deliver the sling assembly. Two small posterior incisions facilitate the postanal placement of the mesh.
This procedure replicates the anorectal angle created by the puborectalis muscle. Although it may become a minimally invasive treatment in the future, final results of the Phase 3 trial are not expected until 2016.
Tibial nerve stimulation is commonly used for urinary urge incontinence. Several small series have documented modest success with its application to fecal incontinence.18
The outpatient procedure involves the insertion of a needle electrode three fingerbreadths above the medial malleolus, followed by electrical stimulation. The current is slowly increased until a sensory or motor response (tingling under the sole of the foot or great toe plantar flexion) is elicited. Treatment necessitates several outpatient sessions.
In a recent series, the mean CCF-FIS decreased from 12.2/20 at baseline to 9.1/20 after treatment (P <.0001).18
The role of this procedure in the treatment algorithm for fecal incontinence remains to be determined.
What we offer patients
Fecal incontinence is a debilitating condition with an increasing number of potential therapeutic options. It clearly is under-recognized by patients and physicians alike.
After a thorough work-up, conservative treatment options should be offered first. When those fail, we generally recommend a trial of sacral neuromodulation for patients with no sphincter defect. When a sphincter defect is present, we counsel the patient about the merits of sphincter repair versus a trial of neuromodulation. These options have the most robust data supporting their clinical use, and have been used successfully in our own practices.
Given the continuous development of other therapeutic modalities, it is likely that future treatments will involve a stepwise progression of approaches. The need for colostomy should diminish in coming years as more minimally invasive techniques become available.
Fecal incontinence is a socially embarrassing condition that affects approximately 18 million adults in the United States.1 Its true incidence is likely much higher than reported, however, as many patients are reluctant to discuss it.
A recent study found that nearly 20% of women experience fecal incontinence at least once a year, and 9.5% experience it al least once a month.2 Only 28% of these women had ever discussed their symptoms with a physician, however.3 Women who did seek care were more likely to consult a family physician or internist (75%) than a gynecologist (7%).3
Until recently, few options were available for patients with fecal incontinence who had not benefited from conservative measures. Many patients simply had to live with their symptoms or undergo a diverting ostomy to control the chronic involuntary drainage.
Recent years have seen the development of new minimally invasive and highly successful techniques to treat fecal incontinence. Greater awareness of the prevalence of fecal incontinence and its devastating impact on quality of life is needed for this problem to be fully addressed, however. In this article, we review the recommended evaluation of a patient who reports fecal incontinence and describe the range of treatment options.
Fecal incontinence is a symptom, not a diagnosis
Although the most common historical factor contributing to fecal incontinence is obstetric trauma, there are several other causes of this condition. A detailed history and physical examination are vital to determine whether the patient is experiencing true fecal incontinence, or whether she is leaking for other reasons—so-called pseudo-incontinence.
Conditions that can mimic fecal incontinence include:
- prolapsing hemorrhoids
- anal fistula
- sexually transmitted infection
- benign or malignant anorectal neoplasms
- dermatologic conditions.
True fecal incontinence may be active (loss of stool despite the patient’s best effort to control it) or passive (loss of stool without the patient’s awareness). Among the causes of true fecal incontinence are:
- anal sphincter injury (obstetric tear, anorectal surgery such as fistulotomy, or trauma)
- denervation of the pelvic floor from pudendal nerve injury during childbirth
- chronic rectal prolapse
- neurologic conditions (spina bifida, myelomeningocele)
- noncompliant rectum from inflammatory bowel disease
- radiation proctitis.
The maintenance of continence requires a complex interaction between the sphincter muscle, the puborectalis muscle (which acts as a sling), rectal capacity and compliance, stool volume and frequency, and neurologic mechanisms.
Diagnosis and management require an experienced physician
We believe that patients reporting fecal incontinence are best worked up and managed by a physician who is well versed in the various diagnoses associated with fecal incontinence, as well as the most current treatments.
Diagnosis entails some detective work
When a patient reports fecal incontinence, she should be asked to elaborate on the circumstances surrounding the complaint and the frequency of its occurrence, duration of symptoms, and nature of the incontinence (gas, liquid, or solid).
Validated quality-of-life instruments, such as the Cleveland Clinic Florida Fecal Incontinence Score (CCF-FIS) may be helpful in documenting the severity of the symptoms and improvement after treatment (TABLE).4
One factor that current scoring systems fail to capture is urgency. In many instances, urgency is the symptom most distressing to the patient. Be sure to ask about it.
A detailed obstetric history also is important. It is not uncommon for a patient to develop symptoms 20 years or longer after the injury. Also review the patient’s medical history for inflammatory bowel disease, neurologic disorders, and any history of pelvic radiation for help in determining the cause of symptoms.
In addition, ask the patient about any other pelvic floor symptoms, such as voiding dysfunction and problems with pelvic organ prolapse. And question her about stool consistency and frequency. In some cases, diarrhea can lead to fecal incontinence and is usually managed conservatively.
Physical exam: Focus on the perineum and anus
A detailed physical examination is warranted to determine the state of the patient’s sphincter musculature and rule out other causes of pseudo-incontinence, such as hemorrhoids or anal fistula. Inspect the perineum for thinning of the perineal body and scars from prior surgery.
A patulous anus may be a sign of rectal prolapse. To check for it, ask the patient to strain on the commode. If rectal prolapse is present, it will become apparent upon straining. If prolapse is detected, surgical treatment of the prolapse would be the first step in managing the incontinence.
A simple test of neurologic function is to try to elicit an anal “wink” in response to a pinprick.
A digital rectal exam allows the assessment of resting and squeeze tone, as well as the use of accessory muscles, such as the gluteus maximus, during squeezing.
Rigid or flexible proctoscopy may be indicated to rule out inflammatory bowel disease, radiation proctitis, and rectal neoplasm, depending on the patient’s history.
A few diagnostic adjuncts can help
Several adjuncts to physical examination can provide more detailed information about the patient’s condition and facilitate the development of an individualized treatment plan. For example, if rectal prolapse, rectocele with delayed emptying, or enterocele is suspected, consider defecography. If voiding dysfunction coexists with the fecal incontinence, urodynamic testing and cystoscopy may be indicated.
We routinely perform physiology testing and endoanal ultrasound if surgery is planned to address the fecal incontinence, although routine use of these adjuncts is controversial. Because many patients can be managed with conservative medical measures, we do not find it necessary to perform these tests at the time of the first visit.
Anal physiology testing includes manometry (a measure of both resting and squeeze tone) and pudendal nerve terminal motor latency testing.
Manometry can help quantify the severity of muscle weakness and determine the presence or absence of normal anal reflexes. Pudendal nerve testing assesses the innervation of the anal sphincter. There is some evidence that patients who have a pudendal neuropathy have a poor outcome with sphincteroplasty,5 although that evidence is controversial. The findings from physiology studies have not been correlated with outcomes of newer treatments, such as sacral neuromodulation (InterStim, Medtronic, Minneapolis, Minnesota). Each physiology lab uses different equipment, so “normal” values vary between institutions.
Endoanal ultrasound is easily performed in an office setting. It is well tolerated and provides anatomic detail of the sphincter musculature. We use a 13-MHz rotating probe to provide 3D imaging of the anal canal. The internal sphincter is represented by a hypoechoic circle surrounded by the hyperechoic external sphincter (FIGURE 1).
In the hands of an experienced examiner, the sensitivity and specificity of endoanal ultrasound in detecting sphincter defects approaches 100%.6,7 Ultrasound also enables measurement of the perineal body. A normal perineal body measures 10 to 15 mm.
For treatment, try conservative measures first
Bulking agents (fiber), constipating agents (loperamide, etc.), or a laxative regimen with scheduled disimpactions (in patients who have pelvic outlet constipation and overflow incontinence) often can control the symptoms of fecal incontinence, making further interventions unnecessary.
Biofeedback is another option. It uses visual, auditory, and sensory information to train patients to better control anal sphincter muscle function.
A recent randomized study found manometric biofeedback to be superior to simple Kegel exercises in improving fecal continence.8 In this study, 76% of patients in the biofeedback group experienced symptom improvement, compared with 41% of patients in the pelvic floor exercise group (P <.001). The long-term benefits of biofeedback are less clear, and patients often need to be reminded to perform their exercises at home and to attend occasional refresher-training sessions. Nevertheless, biofeedback is an important noninvasive option for patients in whom medical management has failed.
Minimally invasive options are now available
Over the past 2 years, minimally invasive treatments for fecal incontinence have emerged, including an implantable sacral neuromodulation device (InterStim) and an injectable dextranomer (Solesta; Salix Pharmaceuticals, Raleigh, North Carolina). Previously, the only surgical option for fecal incontinence was a sphincter repair if a defect was present. The new options may help patients improve their quality of life without having to undergo major surgery.
No one has directly compared the outcomes of these procedures when they are performed by a colorectal surgeon versus a physician of another specialty. It is our belief that the treating physician should have a strong interest in caring for these complex patients and a good working knowledge of the various treatment options.
Related Article Obstetric anal sphincter injury: 7 critical questions about care Ranee Thakar, MD, MRCOG (February 2008)
Sacral neuromodulation
This technique initially was developed for the treatment of overactive bladder and nonobstructive urinary retention and has been used in the United States for the past 15 years for these indications. Improvement in fecal continence was observed in these patients, prompting further studies of its efficacy. In 2011, it was approved by the US Food and Drug Administration (FDA) for the treatment of fecal incontinence. It has since revolutionized the treatment of this disorder, offering a minimally invasive and highly successful alternative to sphincteroplasty.
The InterStim procedure is the only therapeutic modality to include a test phase. The outpatient procedure involves sterile placement of an electrode through the S3 foramen to stimulate the S3 nerve root using fluoroscopic guidance (FIGURES 2 and 3). Patients who experience at least 50% improvement in symptoms are then offered placement of a permanent stimulator.
In most series, approximately 90% of patients have a positive test and progress to implantation. A recent US multicenter clinical trial indicated that 86% of patients achieved an improvement in continence of at least 50%, and 40% of patients were completely continent at 3 years.9 The number of episodes of incontinence decreased from a mean of 9.4/week to 1.7/week.9 Quality of life also improved greatly. Few complications have been reported, the most notable of which is infection (10.8% in the US multicenter trial9).
Another advantage of sacral neuromodulation: It can be used successfully in patients with external sphincter defects as large as 120º. A study by Tjandra and colleagues found that 65% of patients experienced improvement in symptoms of at least 50%, and 47% of patients (more than 50% of whom had external sphincter defects as large as 120º) became completely continent.10
The only variable shown to predict success with sacral neuromodulation is a positive response to the test implant procedure.
In our experience, this procedure is easy to perform and well tolerated, even in elderly patients with multiple comorbidities. The procedure has the additional advantage of potentially improving concomitant urinary symptoms as well.
The major disadvantage of sacral neuromodulation is its cost, although most major insurance carriers cover it. There is no well-conducted cost-effectiveness analysis comparing this modality to other treatments.
Related Article Interstim: An implantable device for implacable urinary symptoms Deborah L. Myers, MD (October 2006)
Injectable agents
Several biocompatible bulking agents have been tested in the treatment of fecal incontinence. These compounds traditionally have been used to treat mild fecal incontinence, or to treat patients with isolated internal sphincter defects.
More recently, an injectable dextranomer in stabilized hyaluronic acid was approved by the FDA and marketed as Solesta. Graf and colleagues randomly allocated 136 patients to injection and 70 patients to sham injection. Patients with external sphincter defects were excluded. At 6 months, 52% of patients in the active treatment group experienced an improvement in continence of at least 50%, compared with 31% of patients injected with placebo.11
The advantage of this procedure is its minimally invasive nature (submucosal injection performed in the office). The disadvantage: a lack of long-term efficacy data, although unpublished data suggest that patients who improve after an injection see a durable response at 3 years.
This easy, office-based treatment is ideal for patients with minor incontinence or persistent symptoms after another procedure.
Sphincter repair
Anterior sphincteroplasty has been the mainstay of surgical treatment for patients with a sphincter defect. With the patient in a dorsal lithotomy or prone position on the operating-room table, a transverse perineal incision is made, and the ends of the severed sphincter muscle are located and mobilized. The repair then can be performed in an end-to-end manner or by overlapping the muscles in the anterior midline (FIGURE 4).
Some of the debatable technical issues of this procedure include:
- whether to overlap the muscles or scar tissue
- whether to repair internal and external defects together or separately
- how the age of the patient affects the outcome.
In regard to the first issue, there may be a superior outcome with overlapping repairs, but they carry a higher risk of dyspareunia and evacuation difficulties. Some surgeons will attempt a separate repair of the internal and external sphincter muscles if it appears feasible. Most often, both muscles are tethered together with scar tissue and separate repair is not possible. There are no conclusive data to demonstrate the superiority of either approach.
As for age, the traditional teaching was that older patients do not benefit from this procedure as much as younger patients do. However, a recent study found no differences in the CCF-FIS score in patients older than age 60, compared with younger patients.12 Investigators concluded that sphincteroplasty can be offered to both young and older patients.12
Although sphincteroplasty often leads to excellent short-term improvement, with 60% to 90% of patients experiencing a good or excellent outcome, nearly all series indicate a decline over the long term (>5 years). A recent systematic review found that as few as 12% of patients experience a good or excellent result, depending on the series.13
We offer sphincter repair to young women with a new sphincter defect after delivery. For older patients, we offer sacral neuromodulation as a first-line treatment.
Other surgical options
We believe that most patients with fecal incontinence can be managed using conservative measures, sacral neuromodulation, injectable dextranomer, or sphincter repair. However, several other options are available.
Artificial bowel sphincter
The artificial bowel sphincter was first described in 1987 and has been modified over the years. The system currently is marketed as the Acticon Neosphincter (American Medical Systems, Minnetonka, Minnesota). The procedure involves the creation of a subcutaneous tunnel around the anus so that an inflatable cuff can be positioned there. A pump then is tunneled through a Pfannenstiel incision to the labia or scrotum, and a reservoir is positioned in the space of Retzius. The device maintains continence by keeping the cuff inflated during the resting state and by pumping fluid from the cuff to the reservoir when the patient needs to evacuate.
The major barrier to utilization of the artificial bowel sphincter is infection. In a series of 112 patients who were implanted with the sphincter, 384 device-related adverse events occurred in 99 patients.14 A total of 73 revision operations were required in 51 patients (46%). Twenty-five percent of patients developed infection that required surgical revision, and 37% had the device explanted. Eighty-five percent of patients with a functional device had a successful outcome.14
Given the device-related challenges and infectious complications, patients should be considered for less invasive treatments before being offered an artificial bowel
sphincter.
Radiofrequency current
The Secca procedure (Curon Medical, Fremont, California) involves the application of radiofrequency current to the anal canal to generate thermal energy. This procedure causes contraction of collagen fibers, which are permanently shortened, and leads to tightening of the muscle. It is performed under intravenous sedation on an outpatient basis.
This approach is indicated for patients with mild to moderate fecal incontinence who have not responded to conservative management. An external sphincter defect is a contraindication.
Small, nonrandomized studies have found improvement in the CCF-FIS score in patients treated with this approach.15 The major limitation of this treatment is the lack of high-level clinical evidence demonstrating its efficacy and safety.
Antegrade continence enema
This approach, also known as the Malone procedure, is usually reserved for debilitating incontinence or constipation in the pediatric population. An appendicostomy is constructed at the navel, allowing daily introduction of a catheter and antegrade enema. The purpose is to perform rapid, controlled emptying of the colon at times chosen by the patient. It is also reserved as a last resort for patients considering an ostomy.
Adult patients with neurologic problems, such as spina bifida, may be candidates for this procedure, provided they are highly motivated.
Fecal diversion
Creation of a colostomy or ileostomy is usually the last resort for a patient with fecal incontinence. We are fortunate that there are an increasing number of options that may improve the patient’s condition before colostomy is required.
If fecal diversion is chosen by the patient, it is important to involve an enterostomal therapist for site marking and patient education. A well-constructed ostomy is essential, as this option often is permanent.
Up and coming options
A novel treatment approach for fecal incontinence is the magnetic anal sphincter. The device, marketed as the FENIX Continence Restoration System (Torax Medical, Shoreview, Minnesota) consists of a series of titanium beads with magnetic cores that are interlinked with titanium wires. The device is designed to encircle the external anal sphincter muscle, reinforce the sphincter, and expand to allow passage of stool at a socially appropriate time.
Preliminary data from 16 patients indicate a mean decrease in the number of episodes of incontinence from 7.2/week to 0.7/week, as well as a mean reduction in the CCF-FIS score from 17.2 to 8.7.16 Two devices were removed due to infection, and one device passed spontaneously after disconnection of the suture.16
This device is not approved by the FDA, but it may become a promising treatment if its safety and efficacy can be established in larger clinical trials.
The TOPAS sling (American Medical Systems) is currently being studied in a Phase 3, multicenter, nonrandomized, clinical trial (NCT01090739) for the treatment of fecal incontinence.17 The sling is implanted using a minimally invasive transobturator approach; two needle-passers deliver the sling assembly. Two small posterior incisions facilitate the postanal placement of the mesh.
This procedure replicates the anorectal angle created by the puborectalis muscle. Although it may become a minimally invasive treatment in the future, final results of the Phase 3 trial are not expected until 2016.
Tibial nerve stimulation is commonly used for urinary urge incontinence. Several small series have documented modest success with its application to fecal incontinence.18
The outpatient procedure involves the insertion of a needle electrode three fingerbreadths above the medial malleolus, followed by electrical stimulation. The current is slowly increased until a sensory or motor response (tingling under the sole of the foot or great toe plantar flexion) is elicited. Treatment necessitates several outpatient sessions.
In a recent series, the mean CCF-FIS decreased from 12.2/20 at baseline to 9.1/20 after treatment (P <.0001).18
The role of this procedure in the treatment algorithm for fecal incontinence remains to be determined.
What we offer patients
Fecal incontinence is a debilitating condition with an increasing number of potential therapeutic options. It clearly is under-recognized by patients and physicians alike.
After a thorough work-up, conservative treatment options should be offered first. When those fail, we generally recommend a trial of sacral neuromodulation for patients with no sphincter defect. When a sphincter defect is present, we counsel the patient about the merits of sphincter repair versus a trial of neuromodulation. These options have the most robust data supporting their clinical use, and have been used successfully in our own practices.
Given the continuous development of other therapeutic modalities, it is likely that future treatments will involve a stepwise progression of approaches. The need for colostomy should diminish in coming years as more minimally invasive techniques become available.
- Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512–517.
- Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66(11):1101–1108.
- Brown HW, Wexner SD, Segall MM, et al. Quality of life in women with accidental bowel leakage. Int Clin Pract. 2012;66(11):1109–1116.
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97.
- Sangwan YP, Collar JA, Barrett RC, et al. Unilateral pudendal neuropathy. Impact on outcomes of anal sphincter repair. Dis Colon Rectum. 1996;39(6):686–689.
- Deen KI, Kumar D, Williams JG, et al. Anal sphincter defects. Correlation between endoanal ultrasound and surgery. Ann Surg. 1993;218(2):201–205.
- Oberwalder M, Thaler K, Baig MK, et al. Anal ultrasound and endosonographic measurement of perineal body thickness: a new evaluation for fecal incontinence in females. Surg Endosc. 2004;18(4):650–654.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52(10):1730–1737.
- Mellgren AF, Wexner SD, Coller JA, et al. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2011:54(9):1065–1075.
- Tjandra JJ, Chan MK, Yeh CH, et al. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51(5):494–502.
- Graf W, Mellgren A, Matzel K, et al. Efficacy of a dextranomer in stabilized hyaluronic acid for treatment of faecal incontinence: a randomized, sham-controlled trial. Lancet. 2011;377(9770):997–1003.
- El-Gazzaz G, Zutshi M, Hannaway C, Gurland B, Hull T. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55(3):256–261.
- Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55(4):482–490.
- Wong WD, Congliosi SM, Spencer MP, et al. The safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45(9):1139–1153.
- Takahashi T, Morales M, Garcia-Osogobio S, et al. Secca procedure for the treatment of fecal incontinence: results of five-year follow-up. Dis Colon Rectum. 2008;51(3):355–359.
- Lehur PA, McNevin S, Buntzen S, et al. Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum. 2010;53(12):1604–1610.
- TOPAS sling. http://clinicaltrials.gov/ct2/show/NCT01090739. Accessed August 26, 2013.
- Hotouras A, Thaha MA, Allison ME, et al. Percutaneous tibial nerve stimulation (PTNS) in females with faecal incontinence: the impact of sphincter morphology and rectal sensation on the clinical outcome. Int J Colorectal Dis. 2012;27(7):927–930.
- Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137(2):512–517.
- Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66(11):1101–1108.
- Brown HW, Wexner SD, Segall MM, et al. Quality of life in women with accidental bowel leakage. Int Clin Pract. 2012;66(11):1109–1116.
- Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97.
- Sangwan YP, Collar JA, Barrett RC, et al. Unilateral pudendal neuropathy. Impact on outcomes of anal sphincter repair. Dis Colon Rectum. 1996;39(6):686–689.
- Deen KI, Kumar D, Williams JG, et al. Anal sphincter defects. Correlation between endoanal ultrasound and surgery. Ann Surg. 1993;218(2):201–205.
- Oberwalder M, Thaler K, Baig MK, et al. Anal ultrasound and endosonographic measurement of perineal body thickness: a new evaluation for fecal incontinence in females. Surg Endosc. 2004;18(4):650–654.
- Heymen S, Scarlett Y, Jones K, et al. Randomized controlled trial shows biofeedback to be superior to pelvic floor exercises for fecal incontinence. Dis Colon Rectum. 2009;52(10):1730–1737.
- Mellgren AF, Wexner SD, Coller JA, et al. Long-term efficacy and safety of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2011:54(9):1065–1075.
- Tjandra JJ, Chan MK, Yeh CH, et al. Sacral nerve stimulation is more effective than optimal medical therapy for severe fecal incontinence: a randomized, controlled study. Dis Colon Rectum. 2008;51(5):494–502.
- Graf W, Mellgren A, Matzel K, et al. Efficacy of a dextranomer in stabilized hyaluronic acid for treatment of faecal incontinence: a randomized, sham-controlled trial. Lancet. 2011;377(9770):997–1003.
- El-Gazzaz G, Zutshi M, Hannaway C, Gurland B, Hull T. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55(3):256–261.
- Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55(4):482–490.
- Wong WD, Congliosi SM, Spencer MP, et al. The safety and efficacy of the artificial bowel sphincter for fecal incontinence: results from a multicenter cohort study. Dis Colon Rectum. 2002;45(9):1139–1153.
- Takahashi T, Morales M, Garcia-Osogobio S, et al. Secca procedure for the treatment of fecal incontinence: results of five-year follow-up. Dis Colon Rectum. 2008;51(3):355–359.
- Lehur PA, McNevin S, Buntzen S, et al. Magnetic anal sphincter augmentation for the treatment of fecal incontinence: a preliminary report from a feasibility study. Dis Colon Rectum. 2010;53(12):1604–1610.
- TOPAS sling. http://clinicaltrials.gov/ct2/show/NCT01090739. Accessed August 26, 2013.
- Hotouras A, Thaha MA, Allison ME, et al. Percutaneous tibial nerve stimulation (PTNS) in females with faecal incontinence: the impact of sphincter morphology and rectal sensation on the clinical outcome. Int J Colorectal Dis. 2012;27(7):927–930.
What is causing her abdominal pain?
CASE: A healthy, nulliparous, 20-year-old woman visits her ObGyn to report a 2-day history of right lower quadrant pain. Her last menstrual period was appropriately timed and normal. She is not sexually active. Upon examination, she exhibits minimal left and right lower quadrant tenderness. Examination with the vaginal speculum reveals no vaginal or cervical abnormalities, and bimanual examination reveals a uterus of normal size, with cervical motion tenderness but no adnexal fullness or mass. For this reason, transvaginal ultrasound (TVUS) is performed. It reveals an enlarged, edematous appendix adjacent to a normal-appearing right ovary (FIGURE 1).
No uterine or ovarian pathology is noted. Because the appendix is enlarged on TVUS, Doppler interrogation is added, which shows abundant vascularity of the appendix (FIGURE 2).
What do these findings suggest?
Acute appendicitis is the most likely diagnosis, as both the physical findings and ultrasound imaging point to it. The patient is referred to a general surgeon, who examines her, noting that she is afebrile, with tenderness in the right lower quadrant. She exhibits localized guarding at McBurneys point, with mild rebound. There is no sign of organomegaly. Bowel sounds are normal, with no distention. The patient undergoes laparoscopy, which confirms the diagnosis. The appendix is resected successfully, and rupture is averted (FIGURE 3).
The patient is discharged home on the first postoperative day. At a follow-up visit 2 weeks later, she is fully recovered and has returned to full and normal activity.
Clinicians who are familiar with the appearance of an inflamed appendix on TVUS may be able to expedite the management of women with appendicitis, avoiding the potential delay, expense, and radiation exposure associated with computed tomography imaging of the abdomen and pelvis.
Acknowledgment
The authors are grateful to Grace J. Horton, RDMS, and Christine L. Bubier, AS, RT(R), RDMS, who generated the images in this case.
Do you have a DIAGNOSTIC IN-SIGHT?
Submit a query for your image-based case! [email protected]
CASE: A healthy, nulliparous, 20-year-old woman visits her ObGyn to report a 2-day history of right lower quadrant pain. Her last menstrual period was appropriately timed and normal. She is not sexually active. Upon examination, she exhibits minimal left and right lower quadrant tenderness. Examination with the vaginal speculum reveals no vaginal or cervical abnormalities, and bimanual examination reveals a uterus of normal size, with cervical motion tenderness but no adnexal fullness or mass. For this reason, transvaginal ultrasound (TVUS) is performed. It reveals an enlarged, edematous appendix adjacent to a normal-appearing right ovary (FIGURE 1).
No uterine or ovarian pathology is noted. Because the appendix is enlarged on TVUS, Doppler interrogation is added, which shows abundant vascularity of the appendix (FIGURE 2).
What do these findings suggest?
Acute appendicitis is the most likely diagnosis, as both the physical findings and ultrasound imaging point to it. The patient is referred to a general surgeon, who examines her, noting that she is afebrile, with tenderness in the right lower quadrant. She exhibits localized guarding at McBurneys point, with mild rebound. There is no sign of organomegaly. Bowel sounds are normal, with no distention. The patient undergoes laparoscopy, which confirms the diagnosis. The appendix is resected successfully, and rupture is averted (FIGURE 3).
The patient is discharged home on the first postoperative day. At a follow-up visit 2 weeks later, she is fully recovered and has returned to full and normal activity.
Clinicians who are familiar with the appearance of an inflamed appendix on TVUS may be able to expedite the management of women with appendicitis, avoiding the potential delay, expense, and radiation exposure associated with computed tomography imaging of the abdomen and pelvis.
Acknowledgment
The authors are grateful to Grace J. Horton, RDMS, and Christine L. Bubier, AS, RT(R), RDMS, who generated the images in this case.
Do you have a DIAGNOSTIC IN-SIGHT?
Submit a query for your image-based case! [email protected]
CASE: A healthy, nulliparous, 20-year-old woman visits her ObGyn to report a 2-day history of right lower quadrant pain. Her last menstrual period was appropriately timed and normal. She is not sexually active. Upon examination, she exhibits minimal left and right lower quadrant tenderness. Examination with the vaginal speculum reveals no vaginal or cervical abnormalities, and bimanual examination reveals a uterus of normal size, with cervical motion tenderness but no adnexal fullness or mass. For this reason, transvaginal ultrasound (TVUS) is performed. It reveals an enlarged, edematous appendix adjacent to a normal-appearing right ovary (FIGURE 1).
No uterine or ovarian pathology is noted. Because the appendix is enlarged on TVUS, Doppler interrogation is added, which shows abundant vascularity of the appendix (FIGURE 2).
What do these findings suggest?
Acute appendicitis is the most likely diagnosis, as both the physical findings and ultrasound imaging point to it. The patient is referred to a general surgeon, who examines her, noting that she is afebrile, with tenderness in the right lower quadrant. She exhibits localized guarding at McBurneys point, with mild rebound. There is no sign of organomegaly. Bowel sounds are normal, with no distention. The patient undergoes laparoscopy, which confirms the diagnosis. The appendix is resected successfully, and rupture is averted (FIGURE 3).
The patient is discharged home on the first postoperative day. At a follow-up visit 2 weeks later, she is fully recovered and has returned to full and normal activity.
Clinicians who are familiar with the appearance of an inflamed appendix on TVUS may be able to expedite the management of women with appendicitis, avoiding the potential delay, expense, and radiation exposure associated with computed tomography imaging of the abdomen and pelvis.
Acknowledgment
The authors are grateful to Grace J. Horton, RDMS, and Christine L. Bubier, AS, RT(R), RDMS, who generated the images in this case.
Do you have a DIAGNOSTIC IN-SIGHT?
Submit a query for your image-based case! [email protected]
Robotic vesicovaginal fistula repair: A systematic, endoscopic approach
In modern times in the United States, the vesicovaginal fistula (VVF) arises chiefly as a sequela of gynecologic surgery, usually hysterectomy. The injury most likely occurs at the time of dissection of the bladder flap off of the lower uterine segment and upper vagina.1 With increasing use of endoscopy and electrosurgery at the time of hysterectomy,2 the occurrence of VVF is likely to increase. Because of this, fistulas stemming from benign gynecologic surgical activity tend to occur above the trigone near the vaginal cuff.
Current technique of fistula repair involves either a vaginal approach, with the Latsko procedure,3 or an abdominal approach, involving a laparotomy; laparoscopy also is used with increasing frequency.4
Vaginal versus endoscopic approach. The vaginal approach can be straightforward, such as in cases of vault prolapse or a distally located fistula, or more difficult if the fistula is apical in location, especially if the apex is well suspended and the vagina is of normal length. I have found the abdominal approach to be optimal if the fistula is near the cuff and the vagina is of normal length and well suspended.
Classical teaching tells us that the first repair of the VVF is likely to be the most successful, with successively lower cure rates as the number of repair attempts increases. For this reason, I advocate the abdominal approach in most cases of apically placed VVFs.
Surgical approach
Why endoscopic, why robotic? Often, repair of the VVF is complicated by:
-
the challenge of locating the defect in the bladder
-
the technical difficulty in oversewing the bladder, which often must be done on the underside of the bladder, between the vaginal and bladder walls.
To tackle these challenges, an endoscopic approach promises improved visualization, and the robotic approach allows for surgical closure with improved visibility characteristic of endoscopy, while preserving the manual dexterity characteristic of open surgery.
Timing. It is believed that, in order to improve chances of successful surgical repair, the fistula should be approached either immediately (ie, within 1 to 2 weeks of the insult) or delayed by 8 to 12 or more weeks after the causative surgery.5
Preparation. Vesicovaginal fistulas can rarely involve the ureters, and this ureteric involvement needs to be ruled out. Accordingly, the workup of the VVF should begin with a thorough cystoscopic evaluation of the bladder, with retrograde pyelography to evaluate the integrity of the ureters bilaterally.
During this procedure, the location of the fistulous tract should be meticulously mapped. Care should be taken to document the location and extent of the fistula, as well as to identify the presence of multiple or separate tracts. If these tracts are present, they also need also to be catalogued. In my practice, the cystoscopy/retrograde pyelogram is performed as a separate surgical encounter.
Surgical technique
After the fistula is mapped and ureteric integrity is confirmed, the definitive surgical repair is performed. The steps to the surgical approach are straightforward.
1. Insert stents into the fistula and bilaterally into the ureters
Stenting the fistula permits rapid identification of the fistula tract without the need to enter the bladder separately. The ureteric stents you use should be one color, and the fistula stent should be a second color. I use 5 French yellow stents to cannulate the ureters and a 6 French blue stent for the fistula itself. Insert the fistula stent from the bladder side of the fistula. It should exit through the vagina (FIGURES 1A and 1B). In addition, place a 3-way Foley catheter for drainage and irrigate the bladder when indicated.
Figure 1. Stent insertion
Cystoscopically place a 6 French blue stent into the bladder side of the fistula. (A) The stent as seen from inside the bladder. (B) The stent as seen from the vaginal side.
2. Place the ports for optimal access
A 0° camera is adequate for visualization. Port placement is similar to that used for robotic sacral colpopexy; I use a supraumbilically placed camera port and three 8-mm robot ports (two on the left and one on the right of the umbilicus). Each port should be separated by about 10 cm. An assistant port is placed to the far right, for passing and retrieving sutures (FIGURE 2). An atraumatic grasper, monopolar scissors, and bipolar Maryland-type forceps are placed within the ports to begin the surgical procedure.
Figure 2. Ideal port placement
Place the camera port supraunbilically, with two 8-mm robot ports
3. Place a vaginal stent to aid dissection
The stent should be a sterile cylinder and have a diameter of 2 cm to 5 cm (to match the vaginal caliber). The tip should be rounded and flattened, with an extended handle available for manipulating the stent. The handle can be held by an assistant or attached to an external uterine positioning system (FIGURE 3); I use the Uterine Positioning SystemTM (Cooper Surgical, Trumbull, Connecticut).
4. Incise the vaginal cuff
Transversely incise the vaginal cuff with the monopolar scissors (VIDEO 1).
This allows entry into the vagina at the apex. The blue stent in the fistula should be visible at the anterior vaginal wall, as demonstrated in FIGURE 4.
5. Dissect the vaginal wall
Dissect the anterior vaginal wall down to the fistula, and dissect the bladder off of the vaginal wall for about 1.5 cm to 2 cm around the fistula tract (FIGURE 5 and VIDEO 2).
6. Cut the stent
Cut the stent passing thru the fistula, to move it out of the way.
7. Close the bladder
Stitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture (FIGURE 6 and Video 3 . I prefer polyglactin 910 (Vicryl; Ethicon, Somerville, New Jersey) because it is easier to handle.
FIGURE 6: Close the bladderStitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture. Keep the closure line free of tension.
8. Close the vaginal side of the fistula
Stitch the vaginal side of the fistula in a running fashion with 2-0 absorbable synthetic suture.
9. Verify closure
Check for watertight closure by retrofilling the bladder with 100 mL of sterile milk (obtained from the labor/delivery suite). Observe the suture line for any evidence of milk leakage. (Sterile milk does not stain the tissues, and this preserves tissue visibility. For this reason, milk is preferable to indigo carmine or methylene blue.)
10. Remove the stents from the bladder
Cystoscopically remove all stents.
11. Close the laparoscopic ports
12. Follow up to ensure surgical success
Leave the indwelling Foley catheter in place for 2 to 3 weeks. After such time, remove the catheter and perform voiding cystourethrogram to document bladder wall integrity.
Discussion
I have described a systematic approach to robotic VVF repair. The robotic portion of the procedure should require about 60 to 90 minutes in the absence of significant adhesions. The technique is amenable to a laparoscopic approach, when performed by an appropriately skilled operator.
Final takeaways. Important takeaways to this repair include:
- Stent the fistula to make it easy to find intraoperatively.
- Enter the vagina from above to rapidly locate the fistula tract.
- Use sterile milk to fill the bladder to look for leaks. This works without staining the tissues.
- Minimize tension on the bladder suture line.
In modern times in the United States, the vesicovaginal fistula (VVF) arises chiefly as a sequela of gynecologic surgery, usually hysterectomy. The injury most likely occurs at the time of dissection of the bladder flap off of the lower uterine segment and upper vagina.1 With increasing use of endoscopy and electrosurgery at the time of hysterectomy,2 the occurrence of VVF is likely to increase. Because of this, fistulas stemming from benign gynecologic surgical activity tend to occur above the trigone near the vaginal cuff.
Current technique of fistula repair involves either a vaginal approach, with the Latsko procedure,3 or an abdominal approach, involving a laparotomy; laparoscopy also is used with increasing frequency.4
Vaginal versus endoscopic approach. The vaginal approach can be straightforward, such as in cases of vault prolapse or a distally located fistula, or more difficult if the fistula is apical in location, especially if the apex is well suspended and the vagina is of normal length. I have found the abdominal approach to be optimal if the fistula is near the cuff and the vagina is of normal length and well suspended.
Classical teaching tells us that the first repair of the VVF is likely to be the most successful, with successively lower cure rates as the number of repair attempts increases. For this reason, I advocate the abdominal approach in most cases of apically placed VVFs.
Surgical approach
Why endoscopic, why robotic? Often, repair of the VVF is complicated by:
-
the challenge of locating the defect in the bladder
-
the technical difficulty in oversewing the bladder, which often must be done on the underside of the bladder, between the vaginal and bladder walls.
To tackle these challenges, an endoscopic approach promises improved visualization, and the robotic approach allows for surgical closure with improved visibility characteristic of endoscopy, while preserving the manual dexterity characteristic of open surgery.
Timing. It is believed that, in order to improve chances of successful surgical repair, the fistula should be approached either immediately (ie, within 1 to 2 weeks of the insult) or delayed by 8 to 12 or more weeks after the causative surgery.5
Preparation. Vesicovaginal fistulas can rarely involve the ureters, and this ureteric involvement needs to be ruled out. Accordingly, the workup of the VVF should begin with a thorough cystoscopic evaluation of the bladder, with retrograde pyelography to evaluate the integrity of the ureters bilaterally.
During this procedure, the location of the fistulous tract should be meticulously mapped. Care should be taken to document the location and extent of the fistula, as well as to identify the presence of multiple or separate tracts. If these tracts are present, they also need also to be catalogued. In my practice, the cystoscopy/retrograde pyelogram is performed as a separate surgical encounter.
Surgical technique
After the fistula is mapped and ureteric integrity is confirmed, the definitive surgical repair is performed. The steps to the surgical approach are straightforward.
1. Insert stents into the fistula and bilaterally into the ureters
Stenting the fistula permits rapid identification of the fistula tract without the need to enter the bladder separately. The ureteric stents you use should be one color, and the fistula stent should be a second color. I use 5 French yellow stents to cannulate the ureters and a 6 French blue stent for the fistula itself. Insert the fistula stent from the bladder side of the fistula. It should exit through the vagina (FIGURES 1A and 1B). In addition, place a 3-way Foley catheter for drainage and irrigate the bladder when indicated.
Figure 1. Stent insertion
Cystoscopically place a 6 French blue stent into the bladder side of the fistula. (A) The stent as seen from inside the bladder. (B) The stent as seen from the vaginal side.
2. Place the ports for optimal access
A 0° camera is adequate for visualization. Port placement is similar to that used for robotic sacral colpopexy; I use a supraumbilically placed camera port and three 8-mm robot ports (two on the left and one on the right of the umbilicus). Each port should be separated by about 10 cm. An assistant port is placed to the far right, for passing and retrieving sutures (FIGURE 2). An atraumatic grasper, monopolar scissors, and bipolar Maryland-type forceps are placed within the ports to begin the surgical procedure.
Figure 2. Ideal port placement
Place the camera port supraunbilically, with two 8-mm robot ports
3. Place a vaginal stent to aid dissection
The stent should be a sterile cylinder and have a diameter of 2 cm to 5 cm (to match the vaginal caliber). The tip should be rounded and flattened, with an extended handle available for manipulating the stent. The handle can be held by an assistant or attached to an external uterine positioning system (FIGURE 3); I use the Uterine Positioning SystemTM (Cooper Surgical, Trumbull, Connecticut).
4. Incise the vaginal cuff
Transversely incise the vaginal cuff with the monopolar scissors (VIDEO 1).
This allows entry into the vagina at the apex. The blue stent in the fistula should be visible at the anterior vaginal wall, as demonstrated in FIGURE 4.
5. Dissect the vaginal wall
Dissect the anterior vaginal wall down to the fistula, and dissect the bladder off of the vaginal wall for about 1.5 cm to 2 cm around the fistula tract (FIGURE 5 and VIDEO 2).
6. Cut the stent
Cut the stent passing thru the fistula, to move it out of the way.
7. Close the bladder
Stitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture (FIGURE 6 and Video 3 . I prefer polyglactin 910 (Vicryl; Ethicon, Somerville, New Jersey) because it is easier to handle.
FIGURE 6: Close the bladderStitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture. Keep the closure line free of tension.
8. Close the vaginal side of the fistula
Stitch the vaginal side of the fistula in a running fashion with 2-0 absorbable synthetic suture.
9. Verify closure
Check for watertight closure by retrofilling the bladder with 100 mL of sterile milk (obtained from the labor/delivery suite). Observe the suture line for any evidence of milk leakage. (Sterile milk does not stain the tissues, and this preserves tissue visibility. For this reason, milk is preferable to indigo carmine or methylene blue.)
10. Remove the stents from the bladder
Cystoscopically remove all stents.
11. Close the laparoscopic ports
12. Follow up to ensure surgical success
Leave the indwelling Foley catheter in place for 2 to 3 weeks. After such time, remove the catheter and perform voiding cystourethrogram to document bladder wall integrity.
Discussion
I have described a systematic approach to robotic VVF repair. The robotic portion of the procedure should require about 60 to 90 minutes in the absence of significant adhesions. The technique is amenable to a laparoscopic approach, when performed by an appropriately skilled operator.
Final takeaways. Important takeaways to this repair include:
- Stent the fistula to make it easy to find intraoperatively.
- Enter the vagina from above to rapidly locate the fistula tract.
- Use sterile milk to fill the bladder to look for leaks. This works without staining the tissues.
- Minimize tension on the bladder suture line.
In modern times in the United States, the vesicovaginal fistula (VVF) arises chiefly as a sequela of gynecologic surgery, usually hysterectomy. The injury most likely occurs at the time of dissection of the bladder flap off of the lower uterine segment and upper vagina.1 With increasing use of endoscopy and electrosurgery at the time of hysterectomy,2 the occurrence of VVF is likely to increase. Because of this, fistulas stemming from benign gynecologic surgical activity tend to occur above the trigone near the vaginal cuff.
Current technique of fistula repair involves either a vaginal approach, with the Latsko procedure,3 or an abdominal approach, involving a laparotomy; laparoscopy also is used with increasing frequency.4
Vaginal versus endoscopic approach. The vaginal approach can be straightforward, such as in cases of vault prolapse or a distally located fistula, or more difficult if the fistula is apical in location, especially if the apex is well suspended and the vagina is of normal length. I have found the abdominal approach to be optimal if the fistula is near the cuff and the vagina is of normal length and well suspended.
Classical teaching tells us that the first repair of the VVF is likely to be the most successful, with successively lower cure rates as the number of repair attempts increases. For this reason, I advocate the abdominal approach in most cases of apically placed VVFs.
Surgical approach
Why endoscopic, why robotic? Often, repair of the VVF is complicated by:
-
the challenge of locating the defect in the bladder
-
the technical difficulty in oversewing the bladder, which often must be done on the underside of the bladder, between the vaginal and bladder walls.
To tackle these challenges, an endoscopic approach promises improved visualization, and the robotic approach allows for surgical closure with improved visibility characteristic of endoscopy, while preserving the manual dexterity characteristic of open surgery.
Timing. It is believed that, in order to improve chances of successful surgical repair, the fistula should be approached either immediately (ie, within 1 to 2 weeks of the insult) or delayed by 8 to 12 or more weeks after the causative surgery.5
Preparation. Vesicovaginal fistulas can rarely involve the ureters, and this ureteric involvement needs to be ruled out. Accordingly, the workup of the VVF should begin with a thorough cystoscopic evaluation of the bladder, with retrograde pyelography to evaluate the integrity of the ureters bilaterally.
During this procedure, the location of the fistulous tract should be meticulously mapped. Care should be taken to document the location and extent of the fistula, as well as to identify the presence of multiple or separate tracts. If these tracts are present, they also need also to be catalogued. In my practice, the cystoscopy/retrograde pyelogram is performed as a separate surgical encounter.
Surgical technique
After the fistula is mapped and ureteric integrity is confirmed, the definitive surgical repair is performed. The steps to the surgical approach are straightforward.
1. Insert stents into the fistula and bilaterally into the ureters
Stenting the fistula permits rapid identification of the fistula tract without the need to enter the bladder separately. The ureteric stents you use should be one color, and the fistula stent should be a second color. I use 5 French yellow stents to cannulate the ureters and a 6 French blue stent for the fistula itself. Insert the fistula stent from the bladder side of the fistula. It should exit through the vagina (FIGURES 1A and 1B). In addition, place a 3-way Foley catheter for drainage and irrigate the bladder when indicated.
Figure 1. Stent insertion
Cystoscopically place a 6 French blue stent into the bladder side of the fistula. (A) The stent as seen from inside the bladder. (B) The stent as seen from the vaginal side.
2. Place the ports for optimal access
A 0° camera is adequate for visualization. Port placement is similar to that used for robotic sacral colpopexy; I use a supraumbilically placed camera port and three 8-mm robot ports (two on the left and one on the right of the umbilicus). Each port should be separated by about 10 cm. An assistant port is placed to the far right, for passing and retrieving sutures (FIGURE 2). An atraumatic grasper, monopolar scissors, and bipolar Maryland-type forceps are placed within the ports to begin the surgical procedure.
Figure 2. Ideal port placement
Place the camera port supraunbilically, with two 8-mm robot ports
3. Place a vaginal stent to aid dissection
The stent should be a sterile cylinder and have a diameter of 2 cm to 5 cm (to match the vaginal caliber). The tip should be rounded and flattened, with an extended handle available for manipulating the stent. The handle can be held by an assistant or attached to an external uterine positioning system (FIGURE 3); I use the Uterine Positioning SystemTM (Cooper Surgical, Trumbull, Connecticut).
4. Incise the vaginal cuff
Transversely incise the vaginal cuff with the monopolar scissors (VIDEO 1).
This allows entry into the vagina at the apex. The blue stent in the fistula should be visible at the anterior vaginal wall, as demonstrated in FIGURE 4.
5. Dissect the vaginal wall
Dissect the anterior vaginal wall down to the fistula, and dissect the bladder off of the vaginal wall for about 1.5 cm to 2 cm around the fistula tract (FIGURE 5 and VIDEO 2).
6. Cut the stent
Cut the stent passing thru the fistula, to move it out of the way.
7. Close the bladder
Stitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture (FIGURE 6 and Video 3 . I prefer polyglactin 910 (Vicryl; Ethicon, Somerville, New Jersey) because it is easier to handle.
FIGURE 6: Close the bladderStitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture. Keep the closure line free of tension.
8. Close the vaginal side of the fistula
Stitch the vaginal side of the fistula in a running fashion with 2-0 absorbable synthetic suture.
9. Verify closure
Check for watertight closure by retrofilling the bladder with 100 mL of sterile milk (obtained from the labor/delivery suite). Observe the suture line for any evidence of milk leakage. (Sterile milk does not stain the tissues, and this preserves tissue visibility. For this reason, milk is preferable to indigo carmine or methylene blue.)
10. Remove the stents from the bladder
Cystoscopically remove all stents.
11. Close the laparoscopic ports
12. Follow up to ensure surgical success
Leave the indwelling Foley catheter in place for 2 to 3 weeks. After such time, remove the catheter and perform voiding cystourethrogram to document bladder wall integrity.
Discussion
I have described a systematic approach to robotic VVF repair. The robotic portion of the procedure should require about 60 to 90 minutes in the absence of significant adhesions. The technique is amenable to a laparoscopic approach, when performed by an appropriately skilled operator.
Final takeaways. Important takeaways to this repair include:
- Stent the fistula to make it easy to find intraoperatively.
- Enter the vagina from above to rapidly locate the fistula tract.
- Use sterile milk to fill the bladder to look for leaks. This works without staining the tissues.
- Minimize tension on the bladder suture line.
Successful treatment of chronic vaginitis
Gadzooks! In preparing for the morning office practice session you notice that two patients with chronic vaginitis have been scheduled back to back in 15-minute slots.
Ms. A has chronic bacterial vaginosis. Ms. B has chronic yeast vaginitis. What are you going to do?
Chronic bacterial vaginosis
The normal vaginal microbiome is dominated by Lactobacillus crispatus and Lactobacillus jensenii. These organisms produce hydrogen peroxide and keep the vaginal pH ≤4.5. When Gardnerella vaginalis and associated anaerobic bacteria gain dominance in the vagina, bacterial vaginosis ensues. This infection is characterized by1:
- homogenous, thin, grayish-white discharge that smoothly coats the vaginal epithelium
- pH >4.5
- fishy odor when potassium hydroxide is added to a sample of the discharge
- clue cells on a saline wet mount.
Why is it prone to recur? If bacterial vaginosis was a simple infection, treatment with metronidazole or clindamycin should be very effective. But in many women the relief from symptoms provided by a single course of antibiotics is short-lived, and many patients experience recurrent bacterial vaginosis in the next few months.
The cause of this resistance to antibiotic treatment may be that G vaginalis and other anaerobes, such as Atopobium species, aggregate in vaginal biofilms that prevent the antibiotic from reaching the organism.2 The biofilm provides a safe haven for the bacteria to regrow following a single course of treatment.3 In addition, the nutrient-limited environment inside the encapsulated biofilm helps the bacteria to resist the toxic effects of the antibiotic.4
Another potential mechanism for bacterial vaginosis recurrence is that women destined to develop repeat infection often harbor G vaginalis encapsulated in biofilms in the mouth. These extravaginal bacteria often are found again in the vagina, suggesting that bacterial vaginosis can be acquired from extravaginal bacterial reservoirs.5 Investigators are developing approaches, such as intravaginal treatment with DNase, to destroy the vaginal biofilm in order to enhance the efficacy of antibiotic treatment.6
Treatment
Options for initial infection. There are three treatments for an initial occurrence of bacterial vaginosis7:
- oral metronidazole 500 mg twice daily for 7 days
- 0.75% metronidazole gel one applicator intravaginally once daily for 5 days, or
- 2% clindamycin cream one applicator intravaginally at bedtime for 7 days.
Long-term metronidazole for recurrence. Approximately half of women who respond to initial treatment will have bacterial vaginosis again within 1 year. If vaginitis caused by recurrent bacterial vaginosisis diagnosed, a prolonged course of antibiotic treatment is warranted. Treatment starts with an induction regimen of the standard treatments listed in the paragraph above. This is followed by a long-term maintenance regimen using 0.75% metronidazole vaginal gel one applicator twice weekly for 4 to 6 months.8
Recurrent Candida vulvovaginitis
Four or more occurrences of symptomatic Candida vulvovaginitis in 12 months indicates recurrent infection. Recurrence is usually caused by reinfection with the same organism from a vaginal reservoir. For women with such repeat infection, vaginal cultures should be obtained to confirm Candida and to search for treatment-resistant species, such as Candida glabrata. (Many C glabrata organisms are resistant to standard fluconazole treatment.)
Treatment options
Long courses of oral or vaginal antimycotic agents can be effective treatment for recurrent Candida vulvovaginitis.
Fluconazole. One regimen is fluconazole 150 mg orally every 72 hours for 3 doses, followed by fluconazole 150 mg once weekly for 6 months.9 If patients relapse from this regimen, then the vaginitis should be retreated with fluconazole 150 mg orally every 72 hours for 3 doses, followed by fluconazole 150 mg weekly for 12 months.
Boric acid. If C glabrata is thought to be the cause of the infection, it may be difficult to eradicate with fluconazole. A regimen to treat recurrent vaginitis caused by C glabrata is intravaginal boric acid, a 600 mg capsule once nightly for 14 days.10,11This medication is not FDA-approved for this purpose and must be made by a compounding pharmacy. Boric acid can be fatal if swallowed rather than used intravaginally. Care must be taken to avoid access to these capsules by children.
Boric acid vaginal capsules also can be used to treat chronic bacterial vaginosis in combination with antibiotic therapy.12
Flucytosine. An alternative regimen to treat C glabrata is flucytosine vaginal cream one applicator nightly for 14 days. This vaginal cream must be compounded because it is not available as a commercial medication.
You are armed and ready
In retrospect, you realize that the morning office session schedule is going to be fine. You will treat Ms. A with a long course of metronidazole and Ms. B with a long course of fluconazole. Hopefully, they will both find relief from their symptoms.
Tell us what you think, at [email protected]. Please include your name and city and state.
- Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988;158(4):819–828.
- Swidinski A, Mendling W, Loening-Baucke V, et al. Adherent biofilms in bacterial vaginosis.Obstet Gynecol. 2005;106(5 pt 1):1013–1023.
- Swidinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198(1):97e1–e6.
- Monds RD, O’Toole GA. The developmental model of microbial biofilm: ten years of a paradigm up for review. Trends Microbiol. 2009;17(2):73–87.
- Marrazzo JM, Friedler TL, Srinivasan S, et al. Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J Infect Dis. 2012;205(10):1580–1588.
- Hymes SR, Randis TM, Sun TY, Ratner AJ. DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J Infect Dis. 2013;207(10):1491–1497.
- Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010; 59(RR-12):1–110.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283–1289.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351(9):876–883.
- Savini V, Catavitello C, Bianco A, Balbinot A, D’Antonio F, D’Antonio D. Azole resistant Candida glabrata vulvovaginitis treated with boric acid. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):112.
- Iavazzo C, Gkegkes ID, Zarkada IM, Falagas ME. Boric acid for recurrent vulvovaginal candidiasis: the clinical evidence. J Womens Health(Larchmt). 2011;20(8):1245–1255.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732–734.
Gadzooks! In preparing for the morning office practice session you notice that two patients with chronic vaginitis have been scheduled back to back in 15-minute slots.
Ms. A has chronic bacterial vaginosis. Ms. B has chronic yeast vaginitis. What are you going to do?
Chronic bacterial vaginosis
The normal vaginal microbiome is dominated by Lactobacillus crispatus and Lactobacillus jensenii. These organisms produce hydrogen peroxide and keep the vaginal pH ≤4.5. When Gardnerella vaginalis and associated anaerobic bacteria gain dominance in the vagina, bacterial vaginosis ensues. This infection is characterized by1:
- homogenous, thin, grayish-white discharge that smoothly coats the vaginal epithelium
- pH >4.5
- fishy odor when potassium hydroxide is added to a sample of the discharge
- clue cells on a saline wet mount.
Why is it prone to recur? If bacterial vaginosis was a simple infection, treatment with metronidazole or clindamycin should be very effective. But in many women the relief from symptoms provided by a single course of antibiotics is short-lived, and many patients experience recurrent bacterial vaginosis in the next few months.
The cause of this resistance to antibiotic treatment may be that G vaginalis and other anaerobes, such as Atopobium species, aggregate in vaginal biofilms that prevent the antibiotic from reaching the organism.2 The biofilm provides a safe haven for the bacteria to regrow following a single course of treatment.3 In addition, the nutrient-limited environment inside the encapsulated biofilm helps the bacteria to resist the toxic effects of the antibiotic.4
Another potential mechanism for bacterial vaginosis recurrence is that women destined to develop repeat infection often harbor G vaginalis encapsulated in biofilms in the mouth. These extravaginal bacteria often are found again in the vagina, suggesting that bacterial vaginosis can be acquired from extravaginal bacterial reservoirs.5 Investigators are developing approaches, such as intravaginal treatment with DNase, to destroy the vaginal biofilm in order to enhance the efficacy of antibiotic treatment.6
Treatment
Options for initial infection. There are three treatments for an initial occurrence of bacterial vaginosis7:
- oral metronidazole 500 mg twice daily for 7 days
- 0.75% metronidazole gel one applicator intravaginally once daily for 5 days, or
- 2% clindamycin cream one applicator intravaginally at bedtime for 7 days.
Long-term metronidazole for recurrence. Approximately half of women who respond to initial treatment will have bacterial vaginosis again within 1 year. If vaginitis caused by recurrent bacterial vaginosisis diagnosed, a prolonged course of antibiotic treatment is warranted. Treatment starts with an induction regimen of the standard treatments listed in the paragraph above. This is followed by a long-term maintenance regimen using 0.75% metronidazole vaginal gel one applicator twice weekly for 4 to 6 months.8
Recurrent Candida vulvovaginitis
Four or more occurrences of symptomatic Candida vulvovaginitis in 12 months indicates recurrent infection. Recurrence is usually caused by reinfection with the same organism from a vaginal reservoir. For women with such repeat infection, vaginal cultures should be obtained to confirm Candida and to search for treatment-resistant species, such as Candida glabrata. (Many C glabrata organisms are resistant to standard fluconazole treatment.)
Treatment options
Long courses of oral or vaginal antimycotic agents can be effective treatment for recurrent Candida vulvovaginitis.
Fluconazole. One regimen is fluconazole 150 mg orally every 72 hours for 3 doses, followed by fluconazole 150 mg once weekly for 6 months.9 If patients relapse from this regimen, then the vaginitis should be retreated with fluconazole 150 mg orally every 72 hours for 3 doses, followed by fluconazole 150 mg weekly for 12 months.
Boric acid. If C glabrata is thought to be the cause of the infection, it may be difficult to eradicate with fluconazole. A regimen to treat recurrent vaginitis caused by C glabrata is intravaginal boric acid, a 600 mg capsule once nightly for 14 days.10,11This medication is not FDA-approved for this purpose and must be made by a compounding pharmacy. Boric acid can be fatal if swallowed rather than used intravaginally. Care must be taken to avoid access to these capsules by children.
Boric acid vaginal capsules also can be used to treat chronic bacterial vaginosis in combination with antibiotic therapy.12
Flucytosine. An alternative regimen to treat C glabrata is flucytosine vaginal cream one applicator nightly for 14 days. This vaginal cream must be compounded because it is not available as a commercial medication.
You are armed and ready
In retrospect, you realize that the morning office session schedule is going to be fine. You will treat Ms. A with a long course of metronidazole and Ms. B with a long course of fluconazole. Hopefully, they will both find relief from their symptoms.
Tell us what you think, at [email protected]. Please include your name and city and state.
Gadzooks! In preparing for the morning office practice session you notice that two patients with chronic vaginitis have been scheduled back to back in 15-minute slots.
Ms. A has chronic bacterial vaginosis. Ms. B has chronic yeast vaginitis. What are you going to do?
Chronic bacterial vaginosis
The normal vaginal microbiome is dominated by Lactobacillus crispatus and Lactobacillus jensenii. These organisms produce hydrogen peroxide and keep the vaginal pH ≤4.5. When Gardnerella vaginalis and associated anaerobic bacteria gain dominance in the vagina, bacterial vaginosis ensues. This infection is characterized by1:
- homogenous, thin, grayish-white discharge that smoothly coats the vaginal epithelium
- pH >4.5
- fishy odor when potassium hydroxide is added to a sample of the discharge
- clue cells on a saline wet mount.
Why is it prone to recur? If bacterial vaginosis was a simple infection, treatment with metronidazole or clindamycin should be very effective. But in many women the relief from symptoms provided by a single course of antibiotics is short-lived, and many patients experience recurrent bacterial vaginosis in the next few months.
The cause of this resistance to antibiotic treatment may be that G vaginalis and other anaerobes, such as Atopobium species, aggregate in vaginal biofilms that prevent the antibiotic from reaching the organism.2 The biofilm provides a safe haven for the bacteria to regrow following a single course of treatment.3 In addition, the nutrient-limited environment inside the encapsulated biofilm helps the bacteria to resist the toxic effects of the antibiotic.4
Another potential mechanism for bacterial vaginosis recurrence is that women destined to develop repeat infection often harbor G vaginalis encapsulated in biofilms in the mouth. These extravaginal bacteria often are found again in the vagina, suggesting that bacterial vaginosis can be acquired from extravaginal bacterial reservoirs.5 Investigators are developing approaches, such as intravaginal treatment with DNase, to destroy the vaginal biofilm in order to enhance the efficacy of antibiotic treatment.6
Treatment
Options for initial infection. There are three treatments for an initial occurrence of bacterial vaginosis7:
- oral metronidazole 500 mg twice daily for 7 days
- 0.75% metronidazole gel one applicator intravaginally once daily for 5 days, or
- 2% clindamycin cream one applicator intravaginally at bedtime for 7 days.
Long-term metronidazole for recurrence. Approximately half of women who respond to initial treatment will have bacterial vaginosis again within 1 year. If vaginitis caused by recurrent bacterial vaginosisis diagnosed, a prolonged course of antibiotic treatment is warranted. Treatment starts with an induction regimen of the standard treatments listed in the paragraph above. This is followed by a long-term maintenance regimen using 0.75% metronidazole vaginal gel one applicator twice weekly for 4 to 6 months.8
Recurrent Candida vulvovaginitis
Four or more occurrences of symptomatic Candida vulvovaginitis in 12 months indicates recurrent infection. Recurrence is usually caused by reinfection with the same organism from a vaginal reservoir. For women with such repeat infection, vaginal cultures should be obtained to confirm Candida and to search for treatment-resistant species, such as Candida glabrata. (Many C glabrata organisms are resistant to standard fluconazole treatment.)
Treatment options
Long courses of oral or vaginal antimycotic agents can be effective treatment for recurrent Candida vulvovaginitis.
Fluconazole. One regimen is fluconazole 150 mg orally every 72 hours for 3 doses, followed by fluconazole 150 mg once weekly for 6 months.9 If patients relapse from this regimen, then the vaginitis should be retreated with fluconazole 150 mg orally every 72 hours for 3 doses, followed by fluconazole 150 mg weekly for 12 months.
Boric acid. If C glabrata is thought to be the cause of the infection, it may be difficult to eradicate with fluconazole. A regimen to treat recurrent vaginitis caused by C glabrata is intravaginal boric acid, a 600 mg capsule once nightly for 14 days.10,11This medication is not FDA-approved for this purpose and must be made by a compounding pharmacy. Boric acid can be fatal if swallowed rather than used intravaginally. Care must be taken to avoid access to these capsules by children.
Boric acid vaginal capsules also can be used to treat chronic bacterial vaginosis in combination with antibiotic therapy.12
Flucytosine. An alternative regimen to treat C glabrata is flucytosine vaginal cream one applicator nightly for 14 days. This vaginal cream must be compounded because it is not available as a commercial medication.
You are armed and ready
In retrospect, you realize that the morning office session schedule is going to be fine. You will treat Ms. A with a long course of metronidazole and Ms. B with a long course of fluconazole. Hopefully, they will both find relief from their symptoms.
Tell us what you think, at [email protected]. Please include your name and city and state.
- Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988;158(4):819–828.
- Swidinski A, Mendling W, Loening-Baucke V, et al. Adherent biofilms in bacterial vaginosis.Obstet Gynecol. 2005;106(5 pt 1):1013–1023.
- Swidinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198(1):97e1–e6.
- Monds RD, O’Toole GA. The developmental model of microbial biofilm: ten years of a paradigm up for review. Trends Microbiol. 2009;17(2):73–87.
- Marrazzo JM, Friedler TL, Srinivasan S, et al. Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J Infect Dis. 2012;205(10):1580–1588.
- Hymes SR, Randis TM, Sun TY, Ratner AJ. DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J Infect Dis. 2013;207(10):1491–1497.
- Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010; 59(RR-12):1–110.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283–1289.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351(9):876–883.
- Savini V, Catavitello C, Bianco A, Balbinot A, D’Antonio F, D’Antonio D. Azole resistant Candida glabrata vulvovaginitis treated with boric acid. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):112.
- Iavazzo C, Gkegkes ID, Zarkada IM, Falagas ME. Boric acid for recurrent vulvovaginal candidiasis: the clinical evidence. J Womens Health(Larchmt). 2011;20(8):1245–1255.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732–734.
- Eschenbach DA, Hillier S, Critchlow C, Stevens C, DeRouen T, Holmes KK. Diagnosis and clinical manifestations of bacterial vaginosis. Am J Obstet Gynecol. 1988;158(4):819–828.
- Swidinski A, Mendling W, Loening-Baucke V, et al. Adherent biofilms in bacterial vaginosis.Obstet Gynecol. 2005;106(5 pt 1):1013–1023.
- Swidinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198(1):97e1–e6.
- Monds RD, O’Toole GA. The developmental model of microbial biofilm: ten years of a paradigm up for review. Trends Microbiol. 2009;17(2):73–87.
- Marrazzo JM, Friedler TL, Srinivasan S, et al. Extravaginal reservoirs of vaginal bacteria as risk factors for incident bacterial vaginosis. J Infect Dis. 2012;205(10):1580–1588.
- Hymes SR, Randis TM, Sun TY, Ratner AJ. DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J Infect Dis. 2013;207(10):1491–1497.
- Workowski KA, Berman S; Centers for Disease Control and Prevention (CDC). Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010; 59(RR-12):1–110.
- Sobel JD, Ferris D, Schwebke J, et al. Suppressive antibacterial therapy with 0.75% metronidazole vaginal gel to prevent recurrent bacterial vaginosis. Am J Obstet Gynecol. 2006;194(5):1283–1289.
- Sobel JD, Wiesenfeld HC, Martens M, et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N Engl J Med. 2004;351(9):876–883.
- Savini V, Catavitello C, Bianco A, Balbinot A, D’Antonio F, D’Antonio D. Azole resistant Candida glabrata vulvovaginitis treated with boric acid. Eur J Obstet Gynecol Reprod Biol. 2009;147(1):112.
- Iavazzo C, Gkegkes ID, Zarkada IM, Falagas ME. Boric acid for recurrent vulvovaginal candidiasis: the clinical evidence. J Womens Health(Larchmt). 2011;20(8):1245–1255.
- Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732–734.
Long-term increase seen in abdominal sacrocolpopexy failure rates
More than 225,000 operations are performed each year in the United States for pelvic organ prolapse (POP). Abdominal sacrocolpopexy is considered the most durable of these procedures, but long-term outcomes need to be studied, say researchers from University of Utah School of Medicine, Salt Lake City.1 Direct costs for these procedures exceed $1 billion per year, and, as the population ages, the need to treat POP and urinary incontinence will rise.1
The original Colpopexy and Urinary Reduction Efforts (CARE) trial included 322 women without stress urinary incontinence (SUI) who underwent abdominal sacrocolpopexy between 2002 and 2005 for symptomatic POP. Because SUI is a common adverse event following POP surgery, study patients were randomly assigned to receive concomitant Burch urethropexy or no urethropexy.
Details of the study
The extended CARE study enrolled 92% (215/233) of eligible 2-year CARE trial completers. A total of 181 (84%) of the 215 women went on to complete 5 years of follow-up, and 126 (56%) completed 7 years of follow-up. The primary goals of the extended CARE study, as reported in JAMA, were to compare long-term anatomic success rates, stress continence rates, overall pelvic floor symptoms, pelvic-floor–specific quality of life (QOL), and mesh-related adverse events.
RESULTS
Treatment failure probability. Treatment failure was considered symptomatic or anatomic POP, SUI, or overall urinary incontinence score of 3 or greater on the Incontinence Severity Index. The procedure’s failure rates showed a gradual increase over the follow-up, in both the urethropexy group and the no urethropexy group.
Urethropexy vs no urethropexy. By year 7, the estimated probabilities of treatment failure for the urethropexy group versus the no urethropexy group, respectively, were:
- for anatomic POP – 0.27 versus 0.22 (treatment difference of 0.05; 95% confidence interval [CI], 0.161 to 0.271)
- for symptomatic POP – 0.29 versus 0.24 (treatment difference of 0.049; 95% CI, 0.060 to 0.162)
- for composite POP – 0.48 versus 0.34 (treatment difference of 0.134; 95% CI, 0.096 to 0.322)
- for SUI – 0.62 versus 0.77 (treatment difference of 0.153; 95% CI, 0.268 to 0.030)
- for overall urinary incontinence – 0.75 versus 0.81 (treatment difference of 0.064; 95% CI, 0.161 to 0.032).
Mesh erosion probability. By year 2, 3 of the 322 women enrolled in CARE had suture erosion and 17 had mesh erosion. There were 2 additional cases of suture erosion and 6 additional cases of mesh erosion by year 7. All types of mesh eroded. The estimated probability of mesh erosion in the CARE and extended CARE trials at the time of the last known treatment failure (6.18 years) was 10.5% (95% CI, 6.8%-16.1%).
Repeat surgery probability. By year 7, at least 36 of 215 women (16.7%) in the extended CARE trial had additional surgery related to pelvic floor disorders, 11 for recurrent POP, 14 for SUI, and 11 for mesh complications.
ABDOMINAL SACROCOLPOPEXY FOR POP IS LESS EFFECTIVE THAN DESIRED
During 7 years of follow-up, abdominal sacrocolpopexy failure rates increased in both the urethropexy group and the no urethropexy group, although urethropexy prevented SUI longer than no urethropexy. “By 5 years, nearly one-third of women met our composite failure definition,” said the authors.1
“Based on our results,” they write, “women considering abdominal sacrocolpopexy should be counseled that this procedure effectively provides relief from POP symptoms; however, the anatomic support deteriorates over time. Adding an anti-incontinence procedure for women continent preoperatively decreases, but does not eliminate, the risk of de novo SUI. Surgical counseling about the ongoing risk of mesh-related events even for abdominal sacrocolpopexy is critical. Women should be aware that symptoms such as vaginal bleeding, discharge, and pain may be due to mesh erosion and should seek help accordingly.”1
We want to hear from you! Tell us what you think.
Reference
1. Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016–2024.
More than 225,000 operations are performed each year in the United States for pelvic organ prolapse (POP). Abdominal sacrocolpopexy is considered the most durable of these procedures, but long-term outcomes need to be studied, say researchers from University of Utah School of Medicine, Salt Lake City.1 Direct costs for these procedures exceed $1 billion per year, and, as the population ages, the need to treat POP and urinary incontinence will rise.1
The original Colpopexy and Urinary Reduction Efforts (CARE) trial included 322 women without stress urinary incontinence (SUI) who underwent abdominal sacrocolpopexy between 2002 and 2005 for symptomatic POP. Because SUI is a common adverse event following POP surgery, study patients were randomly assigned to receive concomitant Burch urethropexy or no urethropexy.
Details of the study
The extended CARE study enrolled 92% (215/233) of eligible 2-year CARE trial completers. A total of 181 (84%) of the 215 women went on to complete 5 years of follow-up, and 126 (56%) completed 7 years of follow-up. The primary goals of the extended CARE study, as reported in JAMA, were to compare long-term anatomic success rates, stress continence rates, overall pelvic floor symptoms, pelvic-floor–specific quality of life (QOL), and mesh-related adverse events.
RESULTS
Treatment failure probability. Treatment failure was considered symptomatic or anatomic POP, SUI, or overall urinary incontinence score of 3 or greater on the Incontinence Severity Index. The procedure’s failure rates showed a gradual increase over the follow-up, in both the urethropexy group and the no urethropexy group.
Urethropexy vs no urethropexy. By year 7, the estimated probabilities of treatment failure for the urethropexy group versus the no urethropexy group, respectively, were:
- for anatomic POP – 0.27 versus 0.22 (treatment difference of 0.05; 95% confidence interval [CI], 0.161 to 0.271)
- for symptomatic POP – 0.29 versus 0.24 (treatment difference of 0.049; 95% CI, 0.060 to 0.162)
- for composite POP – 0.48 versus 0.34 (treatment difference of 0.134; 95% CI, 0.096 to 0.322)
- for SUI – 0.62 versus 0.77 (treatment difference of 0.153; 95% CI, 0.268 to 0.030)
- for overall urinary incontinence – 0.75 versus 0.81 (treatment difference of 0.064; 95% CI, 0.161 to 0.032).
Mesh erosion probability. By year 2, 3 of the 322 women enrolled in CARE had suture erosion and 17 had mesh erosion. There were 2 additional cases of suture erosion and 6 additional cases of mesh erosion by year 7. All types of mesh eroded. The estimated probability of mesh erosion in the CARE and extended CARE trials at the time of the last known treatment failure (6.18 years) was 10.5% (95% CI, 6.8%-16.1%).
Repeat surgery probability. By year 7, at least 36 of 215 women (16.7%) in the extended CARE trial had additional surgery related to pelvic floor disorders, 11 for recurrent POP, 14 for SUI, and 11 for mesh complications.
ABDOMINAL SACROCOLPOPEXY FOR POP IS LESS EFFECTIVE THAN DESIRED
During 7 years of follow-up, abdominal sacrocolpopexy failure rates increased in both the urethropexy group and the no urethropexy group, although urethropexy prevented SUI longer than no urethropexy. “By 5 years, nearly one-third of women met our composite failure definition,” said the authors.1
“Based on our results,” they write, “women considering abdominal sacrocolpopexy should be counseled that this procedure effectively provides relief from POP symptoms; however, the anatomic support deteriorates over time. Adding an anti-incontinence procedure for women continent preoperatively decreases, but does not eliminate, the risk of de novo SUI. Surgical counseling about the ongoing risk of mesh-related events even for abdominal sacrocolpopexy is critical. Women should be aware that symptoms such as vaginal bleeding, discharge, and pain may be due to mesh erosion and should seek help accordingly.”1
We want to hear from you! Tell us what you think.
More than 225,000 operations are performed each year in the United States for pelvic organ prolapse (POP). Abdominal sacrocolpopexy is considered the most durable of these procedures, but long-term outcomes need to be studied, say researchers from University of Utah School of Medicine, Salt Lake City.1 Direct costs for these procedures exceed $1 billion per year, and, as the population ages, the need to treat POP and urinary incontinence will rise.1
The original Colpopexy and Urinary Reduction Efforts (CARE) trial included 322 women without stress urinary incontinence (SUI) who underwent abdominal sacrocolpopexy between 2002 and 2005 for symptomatic POP. Because SUI is a common adverse event following POP surgery, study patients were randomly assigned to receive concomitant Burch urethropexy or no urethropexy.
Details of the study
The extended CARE study enrolled 92% (215/233) of eligible 2-year CARE trial completers. A total of 181 (84%) of the 215 women went on to complete 5 years of follow-up, and 126 (56%) completed 7 years of follow-up. The primary goals of the extended CARE study, as reported in JAMA, were to compare long-term anatomic success rates, stress continence rates, overall pelvic floor symptoms, pelvic-floor–specific quality of life (QOL), and mesh-related adverse events.
RESULTS
Treatment failure probability. Treatment failure was considered symptomatic or anatomic POP, SUI, or overall urinary incontinence score of 3 or greater on the Incontinence Severity Index. The procedure’s failure rates showed a gradual increase over the follow-up, in both the urethropexy group and the no urethropexy group.
Urethropexy vs no urethropexy. By year 7, the estimated probabilities of treatment failure for the urethropexy group versus the no urethropexy group, respectively, were:
- for anatomic POP – 0.27 versus 0.22 (treatment difference of 0.05; 95% confidence interval [CI], 0.161 to 0.271)
- for symptomatic POP – 0.29 versus 0.24 (treatment difference of 0.049; 95% CI, 0.060 to 0.162)
- for composite POP – 0.48 versus 0.34 (treatment difference of 0.134; 95% CI, 0.096 to 0.322)
- for SUI – 0.62 versus 0.77 (treatment difference of 0.153; 95% CI, 0.268 to 0.030)
- for overall urinary incontinence – 0.75 versus 0.81 (treatment difference of 0.064; 95% CI, 0.161 to 0.032).
Mesh erosion probability. By year 2, 3 of the 322 women enrolled in CARE had suture erosion and 17 had mesh erosion. There were 2 additional cases of suture erosion and 6 additional cases of mesh erosion by year 7. All types of mesh eroded. The estimated probability of mesh erosion in the CARE and extended CARE trials at the time of the last known treatment failure (6.18 years) was 10.5% (95% CI, 6.8%-16.1%).
Repeat surgery probability. By year 7, at least 36 of 215 women (16.7%) in the extended CARE trial had additional surgery related to pelvic floor disorders, 11 for recurrent POP, 14 for SUI, and 11 for mesh complications.
ABDOMINAL SACROCOLPOPEXY FOR POP IS LESS EFFECTIVE THAN DESIRED
During 7 years of follow-up, abdominal sacrocolpopexy failure rates increased in both the urethropexy group and the no urethropexy group, although urethropexy prevented SUI longer than no urethropexy. “By 5 years, nearly one-third of women met our composite failure definition,” said the authors.1
“Based on our results,” they write, “women considering abdominal sacrocolpopexy should be counseled that this procedure effectively provides relief from POP symptoms; however, the anatomic support deteriorates over time. Adding an anti-incontinence procedure for women continent preoperatively decreases, but does not eliminate, the risk of de novo SUI. Surgical counseling about the ongoing risk of mesh-related events even for abdominal sacrocolpopexy is critical. Women should be aware that symptoms such as vaginal bleeding, discharge, and pain may be due to mesh erosion and should seek help accordingly.”1
We want to hear from you! Tell us what you think.
Reference
1. Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016–2024.
Reference
1. Nygaard I, Brubaker L, Zyczynski HM, et al. Long-term outcomes following abdominal sacrocolpopexy for pelvic organ prolapse. JAMA. 2013;309(19):2016–2024.