User login
For MD-IQ only
Clinical Edge Journal Scan Commentary: Prostate Cancer September 2021
In the study by Sternberg et al, radiographic progression-free survival (rPFS) and safety were compared between patients aged > 70 and younger than age 70 who were enrolled in the CARD study. In the CARD study, patients with metastatic castrate-resistant prostate cancer (mCRPC) were randomized to cabazitaxel versus abiraterone or enzlutamide after having failed previous treatment. Patients aged > 70 who received cabazitaxel had a higher rPFS than those who received abiraterone or enzalutamide. Grade > 3 adverse effects were identified in 58% of patients receiving cabazitaxel versus 49% in those receiving abiraterone or enzalutamide.
In the study by Smith et al., quality of life (QoL) as measured via time to deterioration of patient report outcomes (PRO) was evaluated in patients enrolled on the ARAMIS trial (darolutamide versus placebo in patients with non-metastatic castrate-resistant prostate cancer (nmCRPC). PRO was assessed via surveys as an exploratory endpoint in this study via FACT-P PCS (prostate cancer subscale) and EORTC QLQ-PR25. Overall, the findings were consistent with either an overall improvement in QoL or improvement in urinary and bowel symptoms over time. In a separate study, Fallah et al conducted a pooled analysis of survival and safety outcomes of 3 second generation androgen receptor blockers (apalutamide, darolutamide, and enzalutamide) in men with nmCRPC. They compared results for men age under 80 versus those 80 and above. Metastasis-free survival and overall survival were higher for the treatment groups compared with placebo groups for both age categories. Side effects were slightly higher in the group aged 80 and above.
In summary, quality of life is an endpoint of critical importance to patients. Inclusion of patient reported outcomes as measured via surveys that provide quantitative assessments aid providers in discussing treatment options with patients. In addition, such QoL instruments aid in assessments based on age. Age bias is common in oncology, and the included studies provide further evidence that age alone should not be a reason to adjust treatment recommendations. Inclusion of geriatric assessments into such studies may further aid in determining risks and benefits of particular prostate cancer treatments in future studies
In the study by Sternberg et al, radiographic progression-free survival (rPFS) and safety were compared between patients aged > 70 and younger than age 70 who were enrolled in the CARD study. In the CARD study, patients with metastatic castrate-resistant prostate cancer (mCRPC) were randomized to cabazitaxel versus abiraterone or enzlutamide after having failed previous treatment. Patients aged > 70 who received cabazitaxel had a higher rPFS than those who received abiraterone or enzalutamide. Grade > 3 adverse effects were identified in 58% of patients receiving cabazitaxel versus 49% in those receiving abiraterone or enzalutamide.
In the study by Smith et al., quality of life (QoL) as measured via time to deterioration of patient report outcomes (PRO) was evaluated in patients enrolled on the ARAMIS trial (darolutamide versus placebo in patients with non-metastatic castrate-resistant prostate cancer (nmCRPC). PRO was assessed via surveys as an exploratory endpoint in this study via FACT-P PCS (prostate cancer subscale) and EORTC QLQ-PR25. Overall, the findings were consistent with either an overall improvement in QoL or improvement in urinary and bowel symptoms over time. In a separate study, Fallah et al conducted a pooled analysis of survival and safety outcomes of 3 second generation androgen receptor blockers (apalutamide, darolutamide, and enzalutamide) in men with nmCRPC. They compared results for men age under 80 versus those 80 and above. Metastasis-free survival and overall survival were higher for the treatment groups compared with placebo groups for both age categories. Side effects were slightly higher in the group aged 80 and above.
In summary, quality of life is an endpoint of critical importance to patients. Inclusion of patient reported outcomes as measured via surveys that provide quantitative assessments aid providers in discussing treatment options with patients. In addition, such QoL instruments aid in assessments based on age. Age bias is common in oncology, and the included studies provide further evidence that age alone should not be a reason to adjust treatment recommendations. Inclusion of geriatric assessments into such studies may further aid in determining risks and benefits of particular prostate cancer treatments in future studies
In the study by Sternberg et al, radiographic progression-free survival (rPFS) and safety were compared between patients aged > 70 and younger than age 70 who were enrolled in the CARD study. In the CARD study, patients with metastatic castrate-resistant prostate cancer (mCRPC) were randomized to cabazitaxel versus abiraterone or enzlutamide after having failed previous treatment. Patients aged > 70 who received cabazitaxel had a higher rPFS than those who received abiraterone or enzalutamide. Grade > 3 adverse effects were identified in 58% of patients receiving cabazitaxel versus 49% in those receiving abiraterone or enzalutamide.
In the study by Smith et al., quality of life (QoL) as measured via time to deterioration of patient report outcomes (PRO) was evaluated in patients enrolled on the ARAMIS trial (darolutamide versus placebo in patients with non-metastatic castrate-resistant prostate cancer (nmCRPC). PRO was assessed via surveys as an exploratory endpoint in this study via FACT-P PCS (prostate cancer subscale) and EORTC QLQ-PR25. Overall, the findings were consistent with either an overall improvement in QoL or improvement in urinary and bowel symptoms over time. In a separate study, Fallah et al conducted a pooled analysis of survival and safety outcomes of 3 second generation androgen receptor blockers (apalutamide, darolutamide, and enzalutamide) in men with nmCRPC. They compared results for men age under 80 versus those 80 and above. Metastasis-free survival and overall survival were higher for the treatment groups compared with placebo groups for both age categories. Side effects were slightly higher in the group aged 80 and above.
In summary, quality of life is an endpoint of critical importance to patients. Inclusion of patient reported outcomes as measured via surveys that provide quantitative assessments aid providers in discussing treatment options with patients. In addition, such QoL instruments aid in assessments based on age. Age bias is common in oncology, and the included studies provide further evidence that age alone should not be a reason to adjust treatment recommendations. Inclusion of geriatric assessments into such studies may further aid in determining risks and benefits of particular prostate cancer treatments in future studies
Metastatic Hormone-Sensitive Prostate Cancer
Health-Related Quality of Life and Toxicity After Definitive High-Dose-Rate Brachytherapy Among Veterans With Prostate Cancer
Nearly 50,000 veterans are diagnosed with cancer within the Veterans Health Administration annually with prostate cancer (PC) being the most frequently diagnosed, accounting for 29% of all cancers diagnosed.1 The treatment of PC depends on the stage and risk group at presentation and patient preference. Men with early stage, localized PC can be managed with prostatectomy, radiation therapy, or active surveillance.2
Within the Veterans Health Administration, more patients are treated with radiation therapy than with radical prostatectomy.3 This is in contrast to the civil health system, where more patients are treated with radical prostatectomy than with radiation therapy.4,5 Radiation therapy for PC can be given externally with external beam radiation therapy or internally with brachytherapy (BT). BT is categorized by the rate at which the radiation dose is delivered and generally grouped as low-dose rate (LDR) or high-dose rate (HDR). LDRBT consists of permanently implanting radioactive seeds, which slowly deliver a radiation dose over an extended period. HDRBT consists of implanting catheters that allow delivery of a radioactive source to be placed temporarily in the prostate and removed after treatment. The utilization of HDRBT has become more common as treatment has evolved to consist of fewer, larger fractions in a shorter time, making it a convenient treatment option for men with PC.6 The veteran population has singular medical challenges. These patients differ from the general population and are often underrepresented in medical research and published studies.7 There are no studies exploring the treatment-associated toxicities from HDRBT treatment for PC specifically in the veteran population. The objective of this study is to report our findings regarding the veteran-reported and physician-graded toxicities associated with HDRBT as monotherapy in veterans treated through the US Department of Veterans Affairs (VA) for PC.
Methods
We performed a retrospective cohort study of a prospectively maintained, institutional review board-approved database of patients treated with HDRBT for PC. Veterans were seen in consultation at Edward Hines, Jr. VA Hospital (EHJVAH) in Hines, Illinois. This is the only VA hospital in Illinois that offers radiation therapy, so it acted as a tertiary center, receiving referrals from other, neighboring VA hospitals. If the veteran was deemed a good BT candidate and elected to proceed with HDRBT, HDR treatment was performed at a partnering academic institution equipped to provide HDRBT (Loyola University Medical Center).
We selected patients with National Cancer Center Network (NCCN) low- or intermediate-risk PC undergoing definitive HDRBT as monotherapy using 13.5 Gy x 2 fractions delivered over 2 implants that were 1 to 2 weeks apart. Patients who received androgen deprivation therapy (ADT) were excluded from this study. No patients received supplemental external beam radiation. Men with unfavorable intermediate risk PC were offered ADT and BT in accordance with NCCN guidelines. However, patients with unfavorable intermediate-risk PC who declined ADT or who were deemed poor ADT candidates due to comorbidities were treated with HDR as monotherapy and included in this study.8
HDR Treatment
Our HDRBT implant procedure and treatment planning details have been previously described.9 In brief, patients were implanted with between 17 and 22 catheters based on gland size under transrectal ultrasound guidance. After implantation, computed tomography and, when possible, magnetic resonance imaging of the prostate were obtained and registered for target delineation. The prostate was segmented, and an asymmetric planning target volume of 0 to 5 mm was created and extended to encompass the proximal seminal vesicles. The second fraction was given 1 to 2 weeks after initial treatment, based on patient, physician, and operating room availability.
Health-Related Quality of Life Assessment
Veteran-reported genitourinary (GU), gastrointestinal (GI), and sexual health-related quality of life (hrQOL) were assessed using the validated International Prostate Symptom Score (IPSS) and the Expanded Prostate Cancer Index Composite Short Form (EPIC-26) instruments.10,11 Baseline veteran-reported hrQOL scores in the GU, GI, and sexual domains were obtained prior to each veteran’s first HDR treatment. Veteran-reported hrQOL scores were assessed at each of the patient’s follow-up appointments. Physician-graded toxicity was assessed Common Terminology Criteria for Adverse Events (CTCAE) v 4.03 criteria.12 Physician-graded toxicity was assessed at each follow-up visit and reported as the highest grade reported during any follow-up examination.
Follow-up appointments typically occurred at 1 month, 3 months, 6 months, 12 months, and subsequently every 6 months after the second HDR treatment. Follow-up appointments were conducted in the radiation oncology department at EHJVAH.
Minimal Clinically Important Differences
To evaluate the veteran-reported hrQOL, we characterized statistically significant differences in IPSS or EPIC-26 scores over time as compared with baseline values as clinically important or not clinically important through the use of reported minimal clinically important difference (MCID) assessments.13-15 For the IPSS, we used reported data that showed a change of ≥ 3.0 points represented a clinically meaningful change in urinary function.14 For the EPIC-26 scores, we used reported data that showed a change of ≥ 6 points for urinary incontinence score, ≥ 5 points for urinary obstruction score, ≥ 4 points for bowel score, and ≥ 10 points for sexual score to represent an MCID.15
Statistical Analysis
Changes in veteran-reported hrQOL over time were compared using mixed linear effects models, with the time since the last BT implant serving as the fixed variable. Effects were deemed statistically significant if P < .05. If a statistically significant difference from baseline was found at any time point, additional evaluation was done to see if the numerical difference in the assessment led to an MCID as described above. IBM SPSS Statistics for Windows, version 25.0 was used for data analysis.
Results
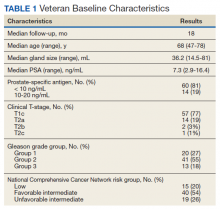
Seventy-four veterans were included in the study. The median follow-up was 18 months (range 1-43). The demographic and oncologic specifics of the treated veterans are outlined in Table 1.
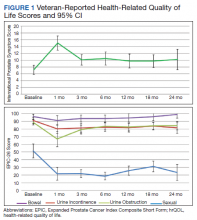
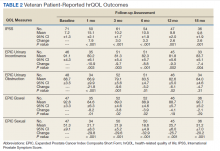
There was a significant increase in IPSS (P < .001) with reciprocal decline in EPIC-26 urinary incontinence (P = .008) and EPIC-26 urinary obstruction scores (P = .001) from baseline over time (Table 2 and Figure 1). At the 18-month follow-up assessment, there was no longer a significant difference in the EPIC-26 urinary obstruction score from baseline (88.7 vs 84.0, P = .31). The increases in IPSS at the 1-, 3-, and 6-month assessments met the criteria for MCID. The decrease in EPIC-26 urinary incontinence scores at the 1-, 3-, 6-, 12-, and 18-month assessments were found to be an MCID, as were the decrease in EPIC-26 urinary obstruction scores at the 1-, 3-, 6-, and 12-month assessments.
There was a significant decline in EPIC-26 bowel scores from baseline over time (P = .03). The decline in the EPIC-26 bowel hrQOL scores at the 1-, 3-, and 6-month follow-up assessment were significantly different from the baseline value. However, only the decrease seen at the 1-month assessment met criteria for MCID.
There was a significant decline in EPIC-26 sexual scores from baseline over time (P < .001). The decline in EPIC-26 sexual score noted at each follow-up compared with baseline was statistically significant. Each of these declines met criteria for an MCID.
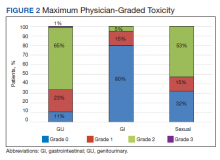
The rate of grade 2 GU, GI, and sexual physician-graded toxicity was 65%, 5%, and 53%, respectively (Figure 2). There was a single incident of grade 3 GU toxicity, which was a urethral stricture. There were no reported grade 3 GI or sexual toxicities, nor were there grade 4 or 5 toxicities. There were 5 total incidents of acute urinary retention for a 6.8% rate overall.
Discussion
We performed a retrospective study of veterans with low- or intermediate-risk PC undergoing definitive HDR prostate BT as monotherapy. We found that veterans experienced immediate declines in GU, GI, and sexual hrQOL after treatment. However, each trended toward a return to baseline over time, with the EPIC-26 urinary obstruction and the EPIC-26 bowel scores showing no difference from the baseline value within 18 months and 12 months, respectively. The physician-reported toxicities were low, with only 1 incidence of grade 3 GU toxicity, no grade 3 GI or sexual toxicities, and no grade 4 or 5 toxicity. This suggests that HDRBT is a well-tolerated and safe, definitive treatment for veterans with localized PC.
In a series similar to ours, Gaudet and colleagues reported on their single institutional results of treating 30 low- or intermediate-risk PC patients with HDRBT as monotherapy.16 Patients included in their study were civilians from the general population, treated in a similar fashion to the veterans treated in our study. Each patient received 27 Gy in 2 fractions given over 2 implants. The authors collected patient-reported hrQOL results using the IPSS and EPIC questionnaires and found that 57% of patients treated experienced moderate-to-severe urinary symptoms at the 1-month assessment after implantation, with a rapid recovery toward baseline over time. In contrast, GI symptoms did not change from baseline, while sexual symptoms worsened after implantation and failed to return to baseline.
Our results mirror this experience, with similar rates of patient-reported hrQOL scores and physician-graded toxicities. Patients reported similar rates of decline in GU, GI, and sexual hrQOL after treatment. The patient-reported GU and GI hrQOL scores worsened immediately after treatment, with a return toward baseline over time. However, the patient-reported sexual hrQOL dropped after treatment and had a subtle trend toward a return to baseline. Our data show higher rates of maximum physician-graded GU toxicity rates of 23%, 65%, and 1% grade 1, 2, and 3, respectively. This is likely due in part to our prophylactic use of tamsulosin. Patients who continued tamsulosin after the implant out of preference were technically grade 2 based on CTCAE v5.0 criteria. GI and sexual toxicity were substantially lower with rates of 15% and 5% grade 1 and grade 2 bowel toxicity with no grade 3 events, and 15% and 52% grade 1 and grade 2 sexual toxicity, respectively.
Contreras and colleagues also reported on treating civilian patients with HDRBT as monotherapy for PC.17 They, too, found similar results as in our veteran study, with a rapid decline in GU, GI, and sexual hrQOL scores immediately after treatment. They also found a gradual return to baseline in the GU hrQOL scores. Contrary to our results, they reported a return to baseline in sexual hrQOL scores, while their patients did not report a return to baseline in the GI hrQOL scores.
Limitations
To the authors’ knowledge, there are no other studies exploring HDR prostate BT toxicity in a veteran-specific population, and our study is novel in addressing this question. One limitation of the study is the relatively short median follow-up time of 18 months. With this limitation, our data were not yet sufficiently mature to perform biochemical control or overall survival analyses. The next step in our study is to calculate these clinical endpoints from our data after longer follow-up.
An additional limitation to our study is the single institutional nature of the design. While veterans from neighboring VA hospitals were included in the study by way of referral and treatment at our center, the only VA hospital in the state to provide radiation therapy, our patient population remains limited. Further multi-institutional and prospective data are needed to validate our findings.
Conclusions
HDR prostate BT as monotherapy is feasible with a favorable veteran-reported hrQOL and physician-graded toxicity profile. Veterans should be educated about this treatment modality when considering the optimal treatment for their localized prostate cancer.
1. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs health care system: 2010 update. Mil Med. 2017;182(7):e1883‐e1891. doi:10.7205/MILMED-D-16-00371
2. Skolarus TA, Hawley ST. Prostate cancer survivorship care in the Veterans Health Administration. Fed Pract. 2014;31(8):10‐17.
3. Nambudiri VE, Landrum MB, Lamont EB, et al. Understanding variation in primary prostate cancer treatment within the Veterans Health Administration. Urology. 2012;79(3):537‐545. doi:10.1016/j.urology.2011.11.013
4. Harlan LC, Potosky A, Gilliland FD, et al. Factors associated with initial therapy for clinically localized prostate cancer: prostate cancer outcomes study. J Natl Cancer Inst. 2001;93(24):1864-1871. doi:10.1093/jnci/93.24.1864
5. Burt LM, Shrieve DC, Tward JD. Factors influencing prostate cancer patterns of care: an analysis of treatment variation using the SEER database. Adv Radiat Oncol. 2018;3(2):170-180. doi:10.1016/j.adro.2017.12.008
6. Crook J, Marbán M, Batchelar D. HDR prostate brachytherapy. Semin Radiat Oncol. 2020;30(1):49‐60. doi:10.1016/j.semradonc.2019.08.003
7. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi: 10.1001/archinte.160.21.3252.
8. D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299(3):289-295. doi:10.1001/jama.299.3.289
9. Solanki AA, Mysz ML, Patel R, et al. Transitioning from a low-dose-rate to a high-dose-rate prostate brachytherapy program: comparing initial dosimetry and improving workflow efficiency through targeted interventions. Adv Radiat Oncol. 2019;4(1):103-111. doi:10.1016/j.adro.2018.10.004
10. Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549‐1564. doi:10.1016/s0022-5347(17)36966-5
11. Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899‐905. doi:10.1016/s0090-4295(00)00858-x
12. US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE). version 4.03. Updated June 14, 2010. Accessed June 15, 2021. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
13. McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312(13):1342-1343. doi:10.1001/jama.2014.13128
14. Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association Symptom Index and the Benign Prostatic Hyperplasia Impact Index is perceptible to patients? J Urol. 1995;154(5):1770-1774. doi:10.1016/S0022-5347(01)66780-6
15. Skolarus TA, Dunn RL, Sanda MG, et al. Minimally important difference for the Expanded Prostate Cancer Index Composite Short Form. Urology. 2015;85(1):101–105. doi:10.1016/j.urology.2014.08.044
16. Gaudet M, Pharand-Charbonneau M, Desrosiers MP, Wright D, Haddad A. Early toxicity and health-related quality of life results of high-dose-rate brachytherapy as monotherapy for low and intermediate-risk prostate cancer. Brachytherapy. 2018;17(3):524-529. doi:10.1016/j.brachy.2018.01.009
17. Contreras JA, Wilder RB, Mellon EA, Strom TJ, Fernandez DC, Biagioli MC. Quality of life after high-dose-rate brachytherapy monotherapy for prostate cancer. Int Braz J Urol. 2015;41(1):40-45. doi:10.1590/S1677-5538.IBJU.2015.01.07
Nearly 50,000 veterans are diagnosed with cancer within the Veterans Health Administration annually with prostate cancer (PC) being the most frequently diagnosed, accounting for 29% of all cancers diagnosed.1 The treatment of PC depends on the stage and risk group at presentation and patient preference. Men with early stage, localized PC can be managed with prostatectomy, radiation therapy, or active surveillance.2
Within the Veterans Health Administration, more patients are treated with radiation therapy than with radical prostatectomy.3 This is in contrast to the civil health system, where more patients are treated with radical prostatectomy than with radiation therapy.4,5 Radiation therapy for PC can be given externally with external beam radiation therapy or internally with brachytherapy (BT). BT is categorized by the rate at which the radiation dose is delivered and generally grouped as low-dose rate (LDR) or high-dose rate (HDR). LDRBT consists of permanently implanting radioactive seeds, which slowly deliver a radiation dose over an extended period. HDRBT consists of implanting catheters that allow delivery of a radioactive source to be placed temporarily in the prostate and removed after treatment. The utilization of HDRBT has become more common as treatment has evolved to consist of fewer, larger fractions in a shorter time, making it a convenient treatment option for men with PC.6 The veteran population has singular medical challenges. These patients differ from the general population and are often underrepresented in medical research and published studies.7 There are no studies exploring the treatment-associated toxicities from HDRBT treatment for PC specifically in the veteran population. The objective of this study is to report our findings regarding the veteran-reported and physician-graded toxicities associated with HDRBT as monotherapy in veterans treated through the US Department of Veterans Affairs (VA) for PC.
Methods
We performed a retrospective cohort study of a prospectively maintained, institutional review board-approved database of patients treated with HDRBT for PC. Veterans were seen in consultation at Edward Hines, Jr. VA Hospital (EHJVAH) in Hines, Illinois. This is the only VA hospital in Illinois that offers radiation therapy, so it acted as a tertiary center, receiving referrals from other, neighboring VA hospitals. If the veteran was deemed a good BT candidate and elected to proceed with HDRBT, HDR treatment was performed at a partnering academic institution equipped to provide HDRBT (Loyola University Medical Center).
We selected patients with National Cancer Center Network (NCCN) low- or intermediate-risk PC undergoing definitive HDRBT as monotherapy using 13.5 Gy x 2 fractions delivered over 2 implants that were 1 to 2 weeks apart. Patients who received androgen deprivation therapy (ADT) were excluded from this study. No patients received supplemental external beam radiation. Men with unfavorable intermediate risk PC were offered ADT and BT in accordance with NCCN guidelines. However, patients with unfavorable intermediate-risk PC who declined ADT or who were deemed poor ADT candidates due to comorbidities were treated with HDR as monotherapy and included in this study.8
HDR Treatment
Our HDRBT implant procedure and treatment planning details have been previously described.9 In brief, patients were implanted with between 17 and 22 catheters based on gland size under transrectal ultrasound guidance. After implantation, computed tomography and, when possible, magnetic resonance imaging of the prostate were obtained and registered for target delineation. The prostate was segmented, and an asymmetric planning target volume of 0 to 5 mm was created and extended to encompass the proximal seminal vesicles. The second fraction was given 1 to 2 weeks after initial treatment, based on patient, physician, and operating room availability.
Health-Related Quality of Life Assessment
Veteran-reported genitourinary (GU), gastrointestinal (GI), and sexual health-related quality of life (hrQOL) were assessed using the validated International Prostate Symptom Score (IPSS) and the Expanded Prostate Cancer Index Composite Short Form (EPIC-26) instruments.10,11 Baseline veteran-reported hrQOL scores in the GU, GI, and sexual domains were obtained prior to each veteran’s first HDR treatment. Veteran-reported hrQOL scores were assessed at each of the patient’s follow-up appointments. Physician-graded toxicity was assessed Common Terminology Criteria for Adverse Events (CTCAE) v 4.03 criteria.12 Physician-graded toxicity was assessed at each follow-up visit and reported as the highest grade reported during any follow-up examination.
Follow-up appointments typically occurred at 1 month, 3 months, 6 months, 12 months, and subsequently every 6 months after the second HDR treatment. Follow-up appointments were conducted in the radiation oncology department at EHJVAH.
Minimal Clinically Important Differences
To evaluate the veteran-reported hrQOL, we characterized statistically significant differences in IPSS or EPIC-26 scores over time as compared with baseline values as clinically important or not clinically important through the use of reported minimal clinically important difference (MCID) assessments.13-15 For the IPSS, we used reported data that showed a change of ≥ 3.0 points represented a clinically meaningful change in urinary function.14 For the EPIC-26 scores, we used reported data that showed a change of ≥ 6 points for urinary incontinence score, ≥ 5 points for urinary obstruction score, ≥ 4 points for bowel score, and ≥ 10 points for sexual score to represent an MCID.15
Statistical Analysis
Changes in veteran-reported hrQOL over time were compared using mixed linear effects models, with the time since the last BT implant serving as the fixed variable. Effects were deemed statistically significant if P < .05. If a statistically significant difference from baseline was found at any time point, additional evaluation was done to see if the numerical difference in the assessment led to an MCID as described above. IBM SPSS Statistics for Windows, version 25.0 was used for data analysis.
Results
Seventy-four veterans were included in the study. The median follow-up was 18 months (range 1-43). The demographic and oncologic specifics of the treated veterans are outlined in Table 1.
There was a significant increase in IPSS (P < .001) with reciprocal decline in EPIC-26 urinary incontinence (P = .008) and EPIC-26 urinary obstruction scores (P = .001) from baseline over time (Table 2 and Figure 1). At the 18-month follow-up assessment, there was no longer a significant difference in the EPIC-26 urinary obstruction score from baseline (88.7 vs 84.0, P = .31). The increases in IPSS at the 1-, 3-, and 6-month assessments met the criteria for MCID. The decrease in EPIC-26 urinary incontinence scores at the 1-, 3-, 6-, 12-, and 18-month assessments were found to be an MCID, as were the decrease in EPIC-26 urinary obstruction scores at the 1-, 3-, 6-, and 12-month assessments.
There was a significant decline in EPIC-26 bowel scores from baseline over time (P = .03). The decline in the EPIC-26 bowel hrQOL scores at the 1-, 3-, and 6-month follow-up assessment were significantly different from the baseline value. However, only the decrease seen at the 1-month assessment met criteria for MCID.
There was a significant decline in EPIC-26 sexual scores from baseline over time (P < .001). The decline in EPIC-26 sexual score noted at each follow-up compared with baseline was statistically significant. Each of these declines met criteria for an MCID.
The rate of grade 2 GU, GI, and sexual physician-graded toxicity was 65%, 5%, and 53%, respectively (Figure 2). There was a single incident of grade 3 GU toxicity, which was a urethral stricture. There were no reported grade 3 GI or sexual toxicities, nor were there grade 4 or 5 toxicities. There were 5 total incidents of acute urinary retention for a 6.8% rate overall.
Discussion
We performed a retrospective study of veterans with low- or intermediate-risk PC undergoing definitive HDR prostate BT as monotherapy. We found that veterans experienced immediate declines in GU, GI, and sexual hrQOL after treatment. However, each trended toward a return to baseline over time, with the EPIC-26 urinary obstruction and the EPIC-26 bowel scores showing no difference from the baseline value within 18 months and 12 months, respectively. The physician-reported toxicities were low, with only 1 incidence of grade 3 GU toxicity, no grade 3 GI or sexual toxicities, and no grade 4 or 5 toxicity. This suggests that HDRBT is a well-tolerated and safe, definitive treatment for veterans with localized PC.
In a series similar to ours, Gaudet and colleagues reported on their single institutional results of treating 30 low- or intermediate-risk PC patients with HDRBT as monotherapy.16 Patients included in their study were civilians from the general population, treated in a similar fashion to the veterans treated in our study. Each patient received 27 Gy in 2 fractions given over 2 implants. The authors collected patient-reported hrQOL results using the IPSS and EPIC questionnaires and found that 57% of patients treated experienced moderate-to-severe urinary symptoms at the 1-month assessment after implantation, with a rapid recovery toward baseline over time. In contrast, GI symptoms did not change from baseline, while sexual symptoms worsened after implantation and failed to return to baseline.
Our results mirror this experience, with similar rates of patient-reported hrQOL scores and physician-graded toxicities. Patients reported similar rates of decline in GU, GI, and sexual hrQOL after treatment. The patient-reported GU and GI hrQOL scores worsened immediately after treatment, with a return toward baseline over time. However, the patient-reported sexual hrQOL dropped after treatment and had a subtle trend toward a return to baseline. Our data show higher rates of maximum physician-graded GU toxicity rates of 23%, 65%, and 1% grade 1, 2, and 3, respectively. This is likely due in part to our prophylactic use of tamsulosin. Patients who continued tamsulosin after the implant out of preference were technically grade 2 based on CTCAE v5.0 criteria. GI and sexual toxicity were substantially lower with rates of 15% and 5% grade 1 and grade 2 bowel toxicity with no grade 3 events, and 15% and 52% grade 1 and grade 2 sexual toxicity, respectively.
Contreras and colleagues also reported on treating civilian patients with HDRBT as monotherapy for PC.17 They, too, found similar results as in our veteran study, with a rapid decline in GU, GI, and sexual hrQOL scores immediately after treatment. They also found a gradual return to baseline in the GU hrQOL scores. Contrary to our results, they reported a return to baseline in sexual hrQOL scores, while their patients did not report a return to baseline in the GI hrQOL scores.
Limitations
To the authors’ knowledge, there are no other studies exploring HDR prostate BT toxicity in a veteran-specific population, and our study is novel in addressing this question. One limitation of the study is the relatively short median follow-up time of 18 months. With this limitation, our data were not yet sufficiently mature to perform biochemical control or overall survival analyses. The next step in our study is to calculate these clinical endpoints from our data after longer follow-up.
An additional limitation to our study is the single institutional nature of the design. While veterans from neighboring VA hospitals were included in the study by way of referral and treatment at our center, the only VA hospital in the state to provide radiation therapy, our patient population remains limited. Further multi-institutional and prospective data are needed to validate our findings.
Conclusions
HDR prostate BT as monotherapy is feasible with a favorable veteran-reported hrQOL and physician-graded toxicity profile. Veterans should be educated about this treatment modality when considering the optimal treatment for their localized prostate cancer.
Nearly 50,000 veterans are diagnosed with cancer within the Veterans Health Administration annually with prostate cancer (PC) being the most frequently diagnosed, accounting for 29% of all cancers diagnosed.1 The treatment of PC depends on the stage and risk group at presentation and patient preference. Men with early stage, localized PC can be managed with prostatectomy, radiation therapy, or active surveillance.2
Within the Veterans Health Administration, more patients are treated with radiation therapy than with radical prostatectomy.3 This is in contrast to the civil health system, where more patients are treated with radical prostatectomy than with radiation therapy.4,5 Radiation therapy for PC can be given externally with external beam radiation therapy or internally with brachytherapy (BT). BT is categorized by the rate at which the radiation dose is delivered and generally grouped as low-dose rate (LDR) or high-dose rate (HDR). LDRBT consists of permanently implanting radioactive seeds, which slowly deliver a radiation dose over an extended period. HDRBT consists of implanting catheters that allow delivery of a radioactive source to be placed temporarily in the prostate and removed after treatment. The utilization of HDRBT has become more common as treatment has evolved to consist of fewer, larger fractions in a shorter time, making it a convenient treatment option for men with PC.6 The veteran population has singular medical challenges. These patients differ from the general population and are often underrepresented in medical research and published studies.7 There are no studies exploring the treatment-associated toxicities from HDRBT treatment for PC specifically in the veteran population. The objective of this study is to report our findings regarding the veteran-reported and physician-graded toxicities associated with HDRBT as monotherapy in veterans treated through the US Department of Veterans Affairs (VA) for PC.
Methods
We performed a retrospective cohort study of a prospectively maintained, institutional review board-approved database of patients treated with HDRBT for PC. Veterans were seen in consultation at Edward Hines, Jr. VA Hospital (EHJVAH) in Hines, Illinois. This is the only VA hospital in Illinois that offers radiation therapy, so it acted as a tertiary center, receiving referrals from other, neighboring VA hospitals. If the veteran was deemed a good BT candidate and elected to proceed with HDRBT, HDR treatment was performed at a partnering academic institution equipped to provide HDRBT (Loyola University Medical Center).
We selected patients with National Cancer Center Network (NCCN) low- or intermediate-risk PC undergoing definitive HDRBT as monotherapy using 13.5 Gy x 2 fractions delivered over 2 implants that were 1 to 2 weeks apart. Patients who received androgen deprivation therapy (ADT) were excluded from this study. No patients received supplemental external beam radiation. Men with unfavorable intermediate risk PC were offered ADT and BT in accordance with NCCN guidelines. However, patients with unfavorable intermediate-risk PC who declined ADT or who were deemed poor ADT candidates due to comorbidities were treated with HDR as monotherapy and included in this study.8
HDR Treatment
Our HDRBT implant procedure and treatment planning details have been previously described.9 In brief, patients were implanted with between 17 and 22 catheters based on gland size under transrectal ultrasound guidance. After implantation, computed tomography and, when possible, magnetic resonance imaging of the prostate were obtained and registered for target delineation. The prostate was segmented, and an asymmetric planning target volume of 0 to 5 mm was created and extended to encompass the proximal seminal vesicles. The second fraction was given 1 to 2 weeks after initial treatment, based on patient, physician, and operating room availability.
Health-Related Quality of Life Assessment
Veteran-reported genitourinary (GU), gastrointestinal (GI), and sexual health-related quality of life (hrQOL) were assessed using the validated International Prostate Symptom Score (IPSS) and the Expanded Prostate Cancer Index Composite Short Form (EPIC-26) instruments.10,11 Baseline veteran-reported hrQOL scores in the GU, GI, and sexual domains were obtained prior to each veteran’s first HDR treatment. Veteran-reported hrQOL scores were assessed at each of the patient’s follow-up appointments. Physician-graded toxicity was assessed Common Terminology Criteria for Adverse Events (CTCAE) v 4.03 criteria.12 Physician-graded toxicity was assessed at each follow-up visit and reported as the highest grade reported during any follow-up examination.
Follow-up appointments typically occurred at 1 month, 3 months, 6 months, 12 months, and subsequently every 6 months after the second HDR treatment. Follow-up appointments were conducted in the radiation oncology department at EHJVAH.
Minimal Clinically Important Differences
To evaluate the veteran-reported hrQOL, we characterized statistically significant differences in IPSS or EPIC-26 scores over time as compared with baseline values as clinically important or not clinically important through the use of reported minimal clinically important difference (MCID) assessments.13-15 For the IPSS, we used reported data that showed a change of ≥ 3.0 points represented a clinically meaningful change in urinary function.14 For the EPIC-26 scores, we used reported data that showed a change of ≥ 6 points for urinary incontinence score, ≥ 5 points for urinary obstruction score, ≥ 4 points for bowel score, and ≥ 10 points for sexual score to represent an MCID.15
Statistical Analysis
Changes in veteran-reported hrQOL over time were compared using mixed linear effects models, with the time since the last BT implant serving as the fixed variable. Effects were deemed statistically significant if P < .05. If a statistically significant difference from baseline was found at any time point, additional evaluation was done to see if the numerical difference in the assessment led to an MCID as described above. IBM SPSS Statistics for Windows, version 25.0 was used for data analysis.
Results
Seventy-four veterans were included in the study. The median follow-up was 18 months (range 1-43). The demographic and oncologic specifics of the treated veterans are outlined in Table 1.
There was a significant increase in IPSS (P < .001) with reciprocal decline in EPIC-26 urinary incontinence (P = .008) and EPIC-26 urinary obstruction scores (P = .001) from baseline over time (Table 2 and Figure 1). At the 18-month follow-up assessment, there was no longer a significant difference in the EPIC-26 urinary obstruction score from baseline (88.7 vs 84.0, P = .31). The increases in IPSS at the 1-, 3-, and 6-month assessments met the criteria for MCID. The decrease in EPIC-26 urinary incontinence scores at the 1-, 3-, 6-, 12-, and 18-month assessments were found to be an MCID, as were the decrease in EPIC-26 urinary obstruction scores at the 1-, 3-, 6-, and 12-month assessments.
There was a significant decline in EPIC-26 bowel scores from baseline over time (P = .03). The decline in the EPIC-26 bowel hrQOL scores at the 1-, 3-, and 6-month follow-up assessment were significantly different from the baseline value. However, only the decrease seen at the 1-month assessment met criteria for MCID.
There was a significant decline in EPIC-26 sexual scores from baseline over time (P < .001). The decline in EPIC-26 sexual score noted at each follow-up compared with baseline was statistically significant. Each of these declines met criteria for an MCID.
The rate of grade 2 GU, GI, and sexual physician-graded toxicity was 65%, 5%, and 53%, respectively (Figure 2). There was a single incident of grade 3 GU toxicity, which was a urethral stricture. There were no reported grade 3 GI or sexual toxicities, nor were there grade 4 or 5 toxicities. There were 5 total incidents of acute urinary retention for a 6.8% rate overall.
Discussion
We performed a retrospective study of veterans with low- or intermediate-risk PC undergoing definitive HDR prostate BT as monotherapy. We found that veterans experienced immediate declines in GU, GI, and sexual hrQOL after treatment. However, each trended toward a return to baseline over time, with the EPIC-26 urinary obstruction and the EPIC-26 bowel scores showing no difference from the baseline value within 18 months and 12 months, respectively. The physician-reported toxicities were low, with only 1 incidence of grade 3 GU toxicity, no grade 3 GI or sexual toxicities, and no grade 4 or 5 toxicity. This suggests that HDRBT is a well-tolerated and safe, definitive treatment for veterans with localized PC.
In a series similar to ours, Gaudet and colleagues reported on their single institutional results of treating 30 low- or intermediate-risk PC patients with HDRBT as monotherapy.16 Patients included in their study were civilians from the general population, treated in a similar fashion to the veterans treated in our study. Each patient received 27 Gy in 2 fractions given over 2 implants. The authors collected patient-reported hrQOL results using the IPSS and EPIC questionnaires and found that 57% of patients treated experienced moderate-to-severe urinary symptoms at the 1-month assessment after implantation, with a rapid recovery toward baseline over time. In contrast, GI symptoms did not change from baseline, while sexual symptoms worsened after implantation and failed to return to baseline.
Our results mirror this experience, with similar rates of patient-reported hrQOL scores and physician-graded toxicities. Patients reported similar rates of decline in GU, GI, and sexual hrQOL after treatment. The patient-reported GU and GI hrQOL scores worsened immediately after treatment, with a return toward baseline over time. However, the patient-reported sexual hrQOL dropped after treatment and had a subtle trend toward a return to baseline. Our data show higher rates of maximum physician-graded GU toxicity rates of 23%, 65%, and 1% grade 1, 2, and 3, respectively. This is likely due in part to our prophylactic use of tamsulosin. Patients who continued tamsulosin after the implant out of preference were technically grade 2 based on CTCAE v5.0 criteria. GI and sexual toxicity were substantially lower with rates of 15% and 5% grade 1 and grade 2 bowel toxicity with no grade 3 events, and 15% and 52% grade 1 and grade 2 sexual toxicity, respectively.
Contreras and colleagues also reported on treating civilian patients with HDRBT as monotherapy for PC.17 They, too, found similar results as in our veteran study, with a rapid decline in GU, GI, and sexual hrQOL scores immediately after treatment. They also found a gradual return to baseline in the GU hrQOL scores. Contrary to our results, they reported a return to baseline in sexual hrQOL scores, while their patients did not report a return to baseline in the GI hrQOL scores.
Limitations
To the authors’ knowledge, there are no other studies exploring HDR prostate BT toxicity in a veteran-specific population, and our study is novel in addressing this question. One limitation of the study is the relatively short median follow-up time of 18 months. With this limitation, our data were not yet sufficiently mature to perform biochemical control or overall survival analyses. The next step in our study is to calculate these clinical endpoints from our data after longer follow-up.
An additional limitation to our study is the single institutional nature of the design. While veterans from neighboring VA hospitals were included in the study by way of referral and treatment at our center, the only VA hospital in the state to provide radiation therapy, our patient population remains limited. Further multi-institutional and prospective data are needed to validate our findings.
Conclusions
HDR prostate BT as monotherapy is feasible with a favorable veteran-reported hrQOL and physician-graded toxicity profile. Veterans should be educated about this treatment modality when considering the optimal treatment for their localized prostate cancer.
1. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs health care system: 2010 update. Mil Med. 2017;182(7):e1883‐e1891. doi:10.7205/MILMED-D-16-00371
2. Skolarus TA, Hawley ST. Prostate cancer survivorship care in the Veterans Health Administration. Fed Pract. 2014;31(8):10‐17.
3. Nambudiri VE, Landrum MB, Lamont EB, et al. Understanding variation in primary prostate cancer treatment within the Veterans Health Administration. Urology. 2012;79(3):537‐545. doi:10.1016/j.urology.2011.11.013
4. Harlan LC, Potosky A, Gilliland FD, et al. Factors associated with initial therapy for clinically localized prostate cancer: prostate cancer outcomes study. J Natl Cancer Inst. 2001;93(24):1864-1871. doi:10.1093/jnci/93.24.1864
5. Burt LM, Shrieve DC, Tward JD. Factors influencing prostate cancer patterns of care: an analysis of treatment variation using the SEER database. Adv Radiat Oncol. 2018;3(2):170-180. doi:10.1016/j.adro.2017.12.008
6. Crook J, Marbán M, Batchelar D. HDR prostate brachytherapy. Semin Radiat Oncol. 2020;30(1):49‐60. doi:10.1016/j.semradonc.2019.08.003
7. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi: 10.1001/archinte.160.21.3252.
8. D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299(3):289-295. doi:10.1001/jama.299.3.289
9. Solanki AA, Mysz ML, Patel R, et al. Transitioning from a low-dose-rate to a high-dose-rate prostate brachytherapy program: comparing initial dosimetry and improving workflow efficiency through targeted interventions. Adv Radiat Oncol. 2019;4(1):103-111. doi:10.1016/j.adro.2018.10.004
10. Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549‐1564. doi:10.1016/s0022-5347(17)36966-5
11. Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899‐905. doi:10.1016/s0090-4295(00)00858-x
12. US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE). version 4.03. Updated June 14, 2010. Accessed June 15, 2021. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
13. McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312(13):1342-1343. doi:10.1001/jama.2014.13128
14. Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association Symptom Index and the Benign Prostatic Hyperplasia Impact Index is perceptible to patients? J Urol. 1995;154(5):1770-1774. doi:10.1016/S0022-5347(01)66780-6
15. Skolarus TA, Dunn RL, Sanda MG, et al. Minimally important difference for the Expanded Prostate Cancer Index Composite Short Form. Urology. 2015;85(1):101–105. doi:10.1016/j.urology.2014.08.044
16. Gaudet M, Pharand-Charbonneau M, Desrosiers MP, Wright D, Haddad A. Early toxicity and health-related quality of life results of high-dose-rate brachytherapy as monotherapy for low and intermediate-risk prostate cancer. Brachytherapy. 2018;17(3):524-529. doi:10.1016/j.brachy.2018.01.009
17. Contreras JA, Wilder RB, Mellon EA, Strom TJ, Fernandez DC, Biagioli MC. Quality of life after high-dose-rate brachytherapy monotherapy for prostate cancer. Int Braz J Urol. 2015;41(1):40-45. doi:10.1590/S1677-5538.IBJU.2015.01.07
1. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs health care system: 2010 update. Mil Med. 2017;182(7):e1883‐e1891. doi:10.7205/MILMED-D-16-00371
2. Skolarus TA, Hawley ST. Prostate cancer survivorship care in the Veterans Health Administration. Fed Pract. 2014;31(8):10‐17.
3. Nambudiri VE, Landrum MB, Lamont EB, et al. Understanding variation in primary prostate cancer treatment within the Veterans Health Administration. Urology. 2012;79(3):537‐545. doi:10.1016/j.urology.2011.11.013
4. Harlan LC, Potosky A, Gilliland FD, et al. Factors associated with initial therapy for clinically localized prostate cancer: prostate cancer outcomes study. J Natl Cancer Inst. 2001;93(24):1864-1871. doi:10.1093/jnci/93.24.1864
5. Burt LM, Shrieve DC, Tward JD. Factors influencing prostate cancer patterns of care: an analysis of treatment variation using the SEER database. Adv Radiat Oncol. 2018;3(2):170-180. doi:10.1016/j.adro.2017.12.008
6. Crook J, Marbán M, Batchelar D. HDR prostate brachytherapy. Semin Radiat Oncol. 2020;30(1):49‐60. doi:10.1016/j.semradonc.2019.08.003
7. Agha Z, Lofgren RP, VanRuiswyk JV, Layde PM. Are patients at Veterans Affairs medical centers sicker? A comparative analysis of health status and medical resource use. Arch Intern Med. 2000;160(21):3252-3257. doi: 10.1001/archinte.160.21.3252.
8. D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299(3):289-295. doi:10.1001/jama.299.3.289
9. Solanki AA, Mysz ML, Patel R, et al. Transitioning from a low-dose-rate to a high-dose-rate prostate brachytherapy program: comparing initial dosimetry and improving workflow efficiency through targeted interventions. Adv Radiat Oncol. 2019;4(1):103-111. doi:10.1016/j.adro.2018.10.004
10. Barry MJ, Fowler FJ Jr, O’Leary MP, et al. The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J Urol. 1992;148(5):1549‐1564. doi:10.1016/s0022-5347(17)36966-5
11. Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology. 2000;56(6):899‐905. doi:10.1016/s0090-4295(00)00858-x
12. US Department of Health and Human Services. Common terminology criteria for adverse events (CTCAE). version 4.03. Updated June 14, 2010. Accessed June 15, 2021. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
13. McGlothlin AE, Lewis RJ. Minimal clinically important difference: defining what really matters to patients. JAMA. 2014;312(13):1342-1343. doi:10.1001/jama.2014.13128
14. Barry MJ, Williford WO, Chang Y, et al. Benign prostatic hyperplasia specific health status measures in clinical research: how much change in the American Urological Association Symptom Index and the Benign Prostatic Hyperplasia Impact Index is perceptible to patients? J Urol. 1995;154(5):1770-1774. doi:10.1016/S0022-5347(01)66780-6
15. Skolarus TA, Dunn RL, Sanda MG, et al. Minimally important difference for the Expanded Prostate Cancer Index Composite Short Form. Urology. 2015;85(1):101–105. doi:10.1016/j.urology.2014.08.044
16. Gaudet M, Pharand-Charbonneau M, Desrosiers MP, Wright D, Haddad A. Early toxicity and health-related quality of life results of high-dose-rate brachytherapy as monotherapy for low and intermediate-risk prostate cancer. Brachytherapy. 2018;17(3):524-529. doi:10.1016/j.brachy.2018.01.009
17. Contreras JA, Wilder RB, Mellon EA, Strom TJ, Fernandez DC, Biagioli MC. Quality of life after high-dose-rate brachytherapy monotherapy for prostate cancer. Int Braz J Urol. 2015;41(1):40-45. doi:10.1590/S1677-5538.IBJU.2015.01.07
Bone Health in Patients With Prostate Cancer: An Evidence-Based Algorithm
Prostate cancer (PC) is the most commonly and newly diagnosed nonskin cancer and the second leading cause of cancer death in men in the United States. About 191,930 cases and about 33,330 deaths from PC were expected for the year 2020.1 About 1 in 41 men will die of PC. Most men diagnosed with PC are aged > 65 years and do not die of their disease. The 5-year survival rate of localized and regional disease is nearly 100%, and disease with distant metastases is 31%. As a result, more than 3.1 million men in the United States who have been diagnosed with PC are still alive today.1 Among veterans, there is a substantial population living with PC. Skolarus and Hawley reported in 2014 that an estimated 200,000 veterans with PC were survivors and 12,000 were newly diagnosed.2
In PC, skeletal strength can be affected by several factors, such as aging, malnutrition, androgen-deprivation therapy (ADT), and bone metastasis.3,4 In fact, most men can live the rest of their life with PC by using strategies to monitor and treat it, once it shows either radiographic or chemical signs of progression.5 ADT is the standard of care to treat hormone-sensitive PC, which is associated with significant skeletal-related adverse effects (AEs).6,7
Men undergoing ADT are 4 times more likely to develop substantial bone deficiency, Shahinian and colleagues found that in men surviving 5 years after PC diagnosis, 19.4% of those who received ADT had a fracture compared with 12% in men who did not (P < .001). The authors established a significant relation between the number of doses of gonadotropin-releasing hormone given in the first 12 months and the risk of fracture.8 Of those who progressed to metastatic disease, the first metastatic nonnodal site is most commonly to the bone.9 Advanced PC is characterized by increased bone turnover, which further raises concerns for bone health and patient performance.10
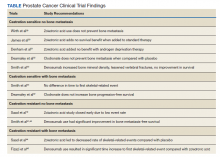
Skeletal-related events (SREs) include pathologic fracture, spinal cord compression, palliative radiation, or surgery to bone, and change in antineoplastic therapy secondary to bone pain. The concept of bone health refers to the prevention, diagnosis, and treatment of idiopathic, pathogenic, and treatment-related bone loss and delay or prevention of SREs.6,11 Guidelines and expert groups have recommended screening for osteoporosis at the start of ADT with bone mineral density testing, ensuring adequate calcium and vitamin D intake, modifying lifestyle behaviors (smoking cessation, alcohol moderation, and regular exercise), and prescribing bisphosphonates or receptor-activated nuclear factor κ-B ligand inhibitor, denosumab, for men with osteoporosis or who are at general high-fracture risk.12,13 The overuse of these medications results in undue cost to patients as well as AEs, such as osteonecrosis of the jaw (ONJ), hypocalcemia, and bone/joint pains.14-17 There are evidence-based guidelines for appropriate use of bisphosphonates and denosumab for delay and prevention of SREs in the setting of advanced PC.18 These doses also typically differ in frequency to those of osteoporosis.19 We summarize the evidence and guidance for health care providers who care for patients with PC at various stages and complications from both disease-related and treatment-related comorbidities.
Bone-Strengthening Agents
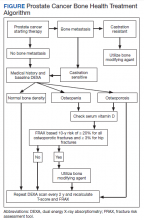
Overall, there is evidence to support the use of bone-strengthening agents in patients with osteopenia/osteoporosis in the prevention of SREs with significant risk factors for progressive bone demineralization, such as lifestyle factors and, in particular, treatments such as ADT. Bone-remodeling agents for treatment of bony metastasis have been shown to provide therapeutic advantage only in limited instances in the castration-resistant PC (CRPC) setting. Hence, in patients with hormone-sensitive PC due to medication-related AEs, treatment with bone-strengthening agents is indicated only if the patient has a significant preexisting risk for fracture from osteopenia/osteoporosis (Table). The Figure depicts an algorithm for the management of bone health in men with PC who are being treated with ADT.
Denosumab and bisphosphonates have an established role in preventing SREs in metastatic CRPC.20 The choice of denosumab or a bisphosphonate typically varies based on the indication, possible AEs, and cost of therapy. There are multiple studies involving initiation of these agents at various stages of disease to improve both time to progression as well as management of SREs. There is a lack of evidence that bisphosphonates prevent metastatic-bone lesions in castration-sensitive PC; therefore, prophylactic use of this agent is not recommended in patients unless they have significant bone demineralization.21,22
Medication-induced ONJ is a severe AE of both denosumab and bisphosphonate therapies. Data from recent trials showed that higher dosing and prolonged duration of denosumab and bisphosphonate therapies further increased risk of ONJ by 1.8% and 1.3%, respectively.15 Careful history taking and discussions with the patient and if possible their dentist on how to reduce risk are recommended. It is good practice for the patient to complete a dental evaluation prior to starting IV bisphosphonates or denosumab. Dental evaluations should be performed routinely at 3- to 12-month intervals throughout therapy based on individualized risk assessment.23 The benefits of using bisphosphonates to prevent fractures associated with osteoporosis outweigh the risk of ONJ in high-risk populations, but not in all patients with PC. A case-by-case basis and evaluation of risk factors should be performed prior to administering bone-modifying therapy. The long-term safety of IV bisphosphonates has not been adequately studied in controlled trials, and concerns regarding long-term complications, including renal toxicity, ONJ, and atypical femoral fractures, remain with prolonged therapy.24,25
The CALGB 70604 (Alliance) trial compared 3-month dosing to monthly treatment with zoledronic acid (ZA), showing no inferiority to lower frequency dosing.26 A Cochrane review of clinical trials found that in patients with advanced PC, bisphosphonates were found to provide roughly 58 fewer SREs per 1000 on average.27 A phase 3 study showed a modest benefit to denosumab vs ZA in the CRPC group regarding incidence of SREs. The rates of SREs were 289 of 951 patients in the bisphosphonate group, and 241 of 950 patients in the denosumab group (30.4% vs 25.3%; hazard ratio [HR], 0.78; 95% CI, 0.66-0.93; P = .005).28 In 2020, the American Society of Clinical Oncology endorsed the Cancer Care Ontario guidelines for prostate bone health care.18 Adequate supplementation is necessary in all patients treated with a bisphosphonate or denosumab to prevent treatment-related hypocalcemia. Typically, daily supplementation with a minimum of calcium 500 mg and vitamin D 400 IU is recommended.16
Bone Health in Patients
Nonmetastatic Hormone-Sensitive PC
ADT forms the backbone of treatment for patients with local and advanced metastatic castration-sensitive PC along with surgical and focal radiotherapy options. Cancer treatment-induced bone loss is known to occur with prolonged use of ADT. The ZEUS trial found no prevention of bone metastasis in patients with high-risk localized PC with the use of ZA in the absence of bone metastasis. A Kaplan-Meier estimated proportion of bone metastases after a median follow-up of 4.8 years was found to be not statistically significant: 14.7% in the ZA group vs 13.2% in the control/placebo group.29 The STAMPEDE trial showed no significant overall survival (OS) benefit with the addition of ZA to ADT vs ADT alone (HR, 0.94; 95% CI, 0.79-1.11; P = .45), 5-year survival with ADT alone was 55% compared to ADT plus ZA with 57% 5-year survival.30 The RADAR trial showed that at 5 years in high Gleason score patients, use of ZA in the absence of bone metastasis was beneficial, but not in low- or intermediate-risk patients. However, at 10-year analysis there was no significant difference in any of the high-stratified groups with or without ZA.31
The PR04 trial showed no effect on OS with clodronate compared with placebo in nonmetastatic castration-sensitive PC, with a HR of 1.12 (95% CI, 0.89-1.42; P = .94). The estimated 5-year survival was 80% with placebo and 78% with clodronate; 10-year survival rates were 51% with placebo and 48% with clodronate.32 Data from the HALT trial showed an increased bone mineral density and reduced risk of new vertebral fractures vs placebo (1.5% vs 3.9%, respectively) in the absence of metastatic bone lesions and a reduction in new vertebral fractures in patients with nonmetastatic PC.33 Most of these studies showed no benefit with the addition of ZA to nonmetastatic PC; although, the HALT trial provides evidence to support use of denosumab in patients with nonmetastatic PC for preventing vertebral fragility fractures in men receiving ADT.
Metastatic Hormone-Sensitive PC
ZA is often used to treat men with metastatic castration-sensitive PC despite limited efficacy and safety data. The CALGB 90202 (Alliance) trial authors found that the early use of ZA was not associated with increased time to first SRE. The median time to first SRE was 31.9 months in the ZA group (95% CI, 24.2-40.3) and 29.8 months in the placebo group (stratified HR, 0.97; 95% CI, 0-1.17; 1-sided stratified log-rank P = .39).34 OS was similar between the groups (HR, 0.88; 95% CI, 0.70-1.12; P = .29) as were reported AEs.34 Results from these studies suggest limited benefit in treating patients with metastatic hormone-sensitive PC with bisphosphonates without other medical indications for use. Additional studies suggest similar results for treatment with denosumab to that of bisphosphonate therapies.35
Nonmetastatic CRPC
Reasonable interest among treating clinicians exists to be able to delay or prevent the development of metastatic bone disease in patients who are showing biochemical signs of castration resistance but have not yet developed distant metastatic disease. Time to progression on ADT to castration resistance usually occurs 2 to 3 years following initiation of treatment. This typically occurs in patients with rising prostate-specific antigen (PSA). As per the Prostate Cancer Working Group 3, in the absence of radiologic progression, CRPC is defined by a 25% increase from the nadir (considering a starting value of ≥ 1 ng/mL), with a minimum rise of 2 ng/mL in the setting of castrate serum testosterone < 50 ng/dL despite good adherence to an ADT regimen, with proven serologic castration either by undetectable or a near undetectable nadir of serum testosterone concentration. Therapeutic implications include prevention of SREs as well as time to metastatic bone lesions. The Zometa 704 trial examined the use of ZA to reduce time to first metastatic bone lesion in the setting of patients with nonmetastatic CRPC.36 The trial was discontinued prematurely due to low patient accrual, but initial analysis provided information on the natural history of a rising PSA in this patient population. At 2 years, one-third of patients had developed bone metastases. Median bone metastasis-free survival was 30 months. Median time to first bone metastasis and OS were not reached. Baseline PSA and PSA velocity independently predicted a shorter time to first bone metastasis, metastasis-free survival, and OS.36
Denosumab was also studied in the setting of nonmetastatic CRPC in the Denosumab 147 trial. The study enrolled 1432 patients and found a significantly increased bone metastasis-free survival by a median of 4.2 months over placebo (HR, 0.85; 95% CI, 0.73-0.98; P = .03). Denosumab significantly delayed time to first bone metastasis (HR, 0.84; 95% CI, 0.71-0.98; P = .03). OS was similar between groups (HR, 1.01; 95% CI, 0.85-1.20; P = .91). Rates of AEs and serious AEs were similar between groups, except for ONJ and hypocalcemia. The rates of ONJ for denosumab were 1%, 3%, 4% in years 1,2, 3, respectively; overall, < 5% (n = 33). Hypocalcemia occurred in < 2% (n = 12) in denosumab-treated patients. The authors concluded that in men with CRPC, denosumab significantly prolonged bone metastasis–free survival and delayed time-to-bone metastasis.37 These 2 studies suggest a role of receptor-activated nuclear factor κ-B ligand inhibitor denosumab in patients with nonmetastatic CRPC in the appropriate setting. There were delays in bony metastatic disease, but no difference in OS. Rare denosumab treatment–related specific AEs were noted. Hence, denosumab is not recommended for use in this setting.
Metastatic CRPC
Castration resistance typically occurs 2 to 3 years following initiation of ADT and the most common extranodal site of disease is within the bone in metastatic PC. Disease progression within bones after ADT can be challenging given both the nature of progressive cancer with osteoblastic metastatic lesions and the prolonged effects of ADT on unaffected bone. The Zometa 039 study compared ZA with placebo and found a significant difference in SREs (38% and 49%, respectively; P .03). No survival benefit was observed with the addition of ZA. Use of other bisphosphonates pamidronate and clodronate did not have a similar degree of benefit.38,39
A phase 3 study of 1904 patients found that denosumab was superior to ZA in delaying the time to first on-study SRE (HR, 0.82; 95% CI, 0.71-0.95) and reducing rates of multiple SREs (HR, 0.82; 95% CI, 0.71-0.94).40 This was later confirmed with an additional study that demonstrated treatment with denosumab significantly reduced the risk of developing a first symptomatic SRE, defined as a pathologic fracture, spinal cord compression, necessity for radiation, or surgery (HR, 0.78; 95% CI, 0.66-0.93; P = .005) and first and subsequent symptomatic SREs (rate ratio, 0.78; 95% CI, 0.65-0.92; P = .004) compared with ZA.28 These findings suggest a continued role of denosumab in the treatment of advanced metastatic CRPC from both control of bone disease as well as quality of life and palliation of cancer-related symptoms.
Radium-223 dichloride (radium-223) is an α-emitting radionuclide for treatment of metastatic CRPC with bone metastasis, but otherwise no additional metastatic sites. Radium-223 is a calcium-mimetic that preferentially accumulates into areas of high-bone turnover, such as where bone metastases tend to occur. Radium-223 induces apoptosis of tumor cells through double-stranded DNA breaks. Studies have shown radium-223 to prolong OS and time-to-first symptomatic SRE.41 The ERA-223 trial showed that when radium-223 was combined with abiraterone acetate, there was an increase in fragility fracture risk compared with placebo combined with abiraterone. Data from the study revealed that the median symptomatic SRE-free survival was 22.3 months (95% CI, 20.4-24.8) in the radium-223 group and 26.0 months (21.8-28.3) in the placebo group. Concurrent treatment with abiraterone acetate plus prednisone or prednisolone and radium-223 was associated with increased fracture risk. Osteoporotic fractures were the most common type of fracture in the radium-223 group and of all fracture types, differed the most between the study groups.42
Conclusions
Convincing evidence supports the ongoing use of bisphosphonates and denosumab in patients with osteoporosis, significant osteopenia with risk factors, and in patients with CRPC with bone metastasis. Bone metastases can cause considerable morbidity and mortality among men with advanced PC. Pain, fracture, and neurologic injury can occur with metastatic bone lesions as well as with ADT-related bone loss. Prevention of SREs in patients with PC is a reasonable goal in PC survivors while being mindful of managing the risks of these therapies.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
2. Skolarus TA, Hawley ST. Prostate cancer survivorship care in the Veterans Health Administration. Fed Pract. 2014;31(8):10-17.
3. Gartrell BA, Coleman R, Efstathiou E, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. Eur Urol. 2015;68(5):850-858. doi:10.1016/j.eururo.2015.06.039
4. Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516-2527. doi:10.1056/NEJMoa0810095
5. Welch HG, Albertsen PC. Reconsidering Prostate cancer mortality—The future of PSA screening. N Engl J Med. 2020;382(16):1557-1563. doi:10.1056/NEJMms1914228
6. Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J; ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25 (suppl 3):iii124-137. doi:10.1093/annonc/mdu103
7. Saylor PJ, Smith MR. Adverse effects of androgen deprivation therapy: defining the problem and promoting health among men with prostate cancer. J Natl Compr Canc Netw. 2010;8(2):211-223. doi:10.6004/jnccn.2010.0014
8. Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154-164. doi:10.1056/NEJMoa041943
9. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657. doi:10.1056/NEJMra1701695
10. Saad F, Eastham JA, Smith MR. Biochemical markers of bone turnover and clinical outcomes in men with prostate cancer. Urol Oncol. 2012;30(4):369-378. doi:10.1016/j.urolonc.2010.08.007
11. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi:10.1007/s00198-014-2794-2
12. Alibhai SMH, Zukotynski K, Walker-Dilks C, et al; Cancer Care Ontario Genitourinary Cancer Disease Site Group. Bone health and bone-targeted therapies for prostate cancer: a programme in evidence-based care - Cancer Care Ontario Clinical Practice Guideline. Clin Oncol (R Coll Radiol). 2017;29(6):348-355. doi:10.1016/j.clon.2017.01.007
13. LEE CE. A comprehensive bone-health management approach with men with prostate cancer recieving androgen deprivation therapy. Curr Oncol. 2011;18(4):e163-172. doi:10.3747/co.v18i4.746
14. Kennel KA, Drake MT. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin Proc. 2009;84(7):632-638. doi:10.1016/S0025-6196(11)60752-0
15. Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23(5):1341-1347. doi:10.1093/annonc/mdr435
16. Body J-J, Bone HG, de Boer RH, et al. Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur J Cancer. 2015;51(13):1812-1821. doi:10.1016/j.ejca.2015.05.016
17. Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med. 2005;165(3):346-347. doi:10.1001/archinte.165.3.346-b
18. Saylor PJ, Rumble RB, Tagawa S, et al. Bone health and bone-targeted therapies for prostate cancer: ASCO endorsement of a cancer care Ontario guideline. J Clin Oncol. 2020;38(15):1736-1743. doi:10.1200/JCO.19.03148
19. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96(11):879-882. doi:10.1093/jnci/djh141
20. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic zcid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458-1468. doi:10.1093/jnci/94.19.1458
21. Aapro M, Saad F. Bone-modifying agents in the treatment of bone metastases in patients with advanced genitourinary malignancies: a focus on zoledronic acid. Ther Adv Urol. 2012;4(2):85-101. doi:10.1177/1756287212441234
22. Cianferotti L, Bertoldo F, Carini M, et al. The prevention of fragility fractures in patients with non-metastatic prostate cancer: a position statement by the international osteoporosis foundation. Oncotarget. 2017;8(43):75646-75663. doi:10.18632/oncotarget.17980
23. Ruggiero S, Gralow J, Marx RE, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract. 2006;2(1):7-14. doi:10.1200/JOP.2006.2.1.7
24. Corraini P, Heide-Jørgensen U, Schøodt M, et al. Osteonecrosis of the jaw and survival of patients with cancer: a nationwide cohort study in Denmark. Cancer Med. 2017;6(10):2271-2277. doi:10.1002/cam4.1173
25. Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565. doi:10.1210/jc.2009-1947
26. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58. doi:10.1001/jama.2016.19425
27. Macherey S, Monsef I, Jahn F, et al. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst Rev. 2017;12(12):CD006250. doi:10.1002/14651858.CD006250.pub2
28. Smith MR, Coleman RE, Klotz L, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol. 2015;26(2):368-374. doi:10.1093/annonc/mdu519
29. Wirth M, Tammela T, Cicalese V, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67(3):482-491. doi:10.1016/j.eururo.2014.02.014
30. James ND, Sydes MR, Clarke NW, et al; STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi:10.1016/S0140-6736(15)01037-5
31. Denham JW, Joseph D, Lamb DS, et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Lancet Oncol. 2019;20(2):267-281. doi:10.1016/S1470-2045(18)30757-5
32. Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10(9):872-876. doi:10.1016/S1470-2045(09)70201-3
33. Smith MR, Egerdie B, Toriz NH, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate Cancer. N Engl J Med. 2009;361(8):745-755. doi:10.1056/NEJMoa0809003
34. Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32(11):1143-1150. doi:10.1200/JCO.2013.51.6500
35. Kozyrakis D, Paridis D, Perikleous S, Malizos K, Zarkadas A, Tsagkalis A. The current role of osteoclast inhibitors in patients with prostate cancer. Adv Urol. 2018;2018:1525832. doi:10.1155/2018/1525832
36. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918-2925. doi:10.1200/JCO.2005.01.529
37. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39-46. doi:10.1016/S0140-6736(11)61226-9
38. Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21(23):4277-4284. doi:10.1200/JCO.2003.05.147
39. Ernst DS, Tannock IF, Winquist EW, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21(17):3335-3342. doi:10.1200/JCO.2003.03.042
40. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813-822. doi:10.1016/S0140-6736(10)62344-6
41. Parker C, Nilsson S, Heinrich D, et al; ALSYMPCA Investigators Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. doi:10.1056/NEJMoa1213755
42. Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):408-419. doi:10.1016/S1470-2045(18)30860-X
43. Smith MR, Saad F, Shore ND, et al. Effect of denosumab on prolonging bone-metastasis-free survival (BMFS) in men with nonmetastatic castrate-resistant prostate cancer (CRPC) presenting with aggressive PSA kinetics. J Clin Oncol. 2012;30(5_suppl):6-6.
44. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458-1468. doi:10.1093/jnci/94.19.1458
Prostate cancer (PC) is the most commonly and newly diagnosed nonskin cancer and the second leading cause of cancer death in men in the United States. About 191,930 cases and about 33,330 deaths from PC were expected for the year 2020.1 About 1 in 41 men will die of PC. Most men diagnosed with PC are aged > 65 years and do not die of their disease. The 5-year survival rate of localized and regional disease is nearly 100%, and disease with distant metastases is 31%. As a result, more than 3.1 million men in the United States who have been diagnosed with PC are still alive today.1 Among veterans, there is a substantial population living with PC. Skolarus and Hawley reported in 2014 that an estimated 200,000 veterans with PC were survivors and 12,000 were newly diagnosed.2
In PC, skeletal strength can be affected by several factors, such as aging, malnutrition, androgen-deprivation therapy (ADT), and bone metastasis.3,4 In fact, most men can live the rest of their life with PC by using strategies to monitor and treat it, once it shows either radiographic or chemical signs of progression.5 ADT is the standard of care to treat hormone-sensitive PC, which is associated with significant skeletal-related adverse effects (AEs).6,7
Men undergoing ADT are 4 times more likely to develop substantial bone deficiency, Shahinian and colleagues found that in men surviving 5 years after PC diagnosis, 19.4% of those who received ADT had a fracture compared with 12% in men who did not (P < .001). The authors established a significant relation between the number of doses of gonadotropin-releasing hormone given in the first 12 months and the risk of fracture.8 Of those who progressed to metastatic disease, the first metastatic nonnodal site is most commonly to the bone.9 Advanced PC is characterized by increased bone turnover, which further raises concerns for bone health and patient performance.10
Skeletal-related events (SREs) include pathologic fracture, spinal cord compression, palliative radiation, or surgery to bone, and change in antineoplastic therapy secondary to bone pain. The concept of bone health refers to the prevention, diagnosis, and treatment of idiopathic, pathogenic, and treatment-related bone loss and delay or prevention of SREs.6,11 Guidelines and expert groups have recommended screening for osteoporosis at the start of ADT with bone mineral density testing, ensuring adequate calcium and vitamin D intake, modifying lifestyle behaviors (smoking cessation, alcohol moderation, and regular exercise), and prescribing bisphosphonates or receptor-activated nuclear factor κ-B ligand inhibitor, denosumab, for men with osteoporosis or who are at general high-fracture risk.12,13 The overuse of these medications results in undue cost to patients as well as AEs, such as osteonecrosis of the jaw (ONJ), hypocalcemia, and bone/joint pains.14-17 There are evidence-based guidelines for appropriate use of bisphosphonates and denosumab for delay and prevention of SREs in the setting of advanced PC.18 These doses also typically differ in frequency to those of osteoporosis.19 We summarize the evidence and guidance for health care providers who care for patients with PC at various stages and complications from both disease-related and treatment-related comorbidities.
Bone-Strengthening Agents
Overall, there is evidence to support the use of bone-strengthening agents in patients with osteopenia/osteoporosis in the prevention of SREs with significant risk factors for progressive bone demineralization, such as lifestyle factors and, in particular, treatments such as ADT. Bone-remodeling agents for treatment of bony metastasis have been shown to provide therapeutic advantage only in limited instances in the castration-resistant PC (CRPC) setting. Hence, in patients with hormone-sensitive PC due to medication-related AEs, treatment with bone-strengthening agents is indicated only if the patient has a significant preexisting risk for fracture from osteopenia/osteoporosis (Table). The Figure depicts an algorithm for the management of bone health in men with PC who are being treated with ADT.
Denosumab and bisphosphonates have an established role in preventing SREs in metastatic CRPC.20 The choice of denosumab or a bisphosphonate typically varies based on the indication, possible AEs, and cost of therapy. There are multiple studies involving initiation of these agents at various stages of disease to improve both time to progression as well as management of SREs. There is a lack of evidence that bisphosphonates prevent metastatic-bone lesions in castration-sensitive PC; therefore, prophylactic use of this agent is not recommended in patients unless they have significant bone demineralization.21,22
Medication-induced ONJ is a severe AE of both denosumab and bisphosphonate therapies. Data from recent trials showed that higher dosing and prolonged duration of denosumab and bisphosphonate therapies further increased risk of ONJ by 1.8% and 1.3%, respectively.15 Careful history taking and discussions with the patient and if possible their dentist on how to reduce risk are recommended. It is good practice for the patient to complete a dental evaluation prior to starting IV bisphosphonates or denosumab. Dental evaluations should be performed routinely at 3- to 12-month intervals throughout therapy based on individualized risk assessment.23 The benefits of using bisphosphonates to prevent fractures associated with osteoporosis outweigh the risk of ONJ in high-risk populations, but not in all patients with PC. A case-by-case basis and evaluation of risk factors should be performed prior to administering bone-modifying therapy. The long-term safety of IV bisphosphonates has not been adequately studied in controlled trials, and concerns regarding long-term complications, including renal toxicity, ONJ, and atypical femoral fractures, remain with prolonged therapy.24,25
The CALGB 70604 (Alliance) trial compared 3-month dosing to monthly treatment with zoledronic acid (ZA), showing no inferiority to lower frequency dosing.26 A Cochrane review of clinical trials found that in patients with advanced PC, bisphosphonates were found to provide roughly 58 fewer SREs per 1000 on average.27 A phase 3 study showed a modest benefit to denosumab vs ZA in the CRPC group regarding incidence of SREs. The rates of SREs were 289 of 951 patients in the bisphosphonate group, and 241 of 950 patients in the denosumab group (30.4% vs 25.3%; hazard ratio [HR], 0.78; 95% CI, 0.66-0.93; P = .005).28 In 2020, the American Society of Clinical Oncology endorsed the Cancer Care Ontario guidelines for prostate bone health care.18 Adequate supplementation is necessary in all patients treated with a bisphosphonate or denosumab to prevent treatment-related hypocalcemia. Typically, daily supplementation with a minimum of calcium 500 mg and vitamin D 400 IU is recommended.16
Bone Health in Patients
Nonmetastatic Hormone-Sensitive PC
ADT forms the backbone of treatment for patients with local and advanced metastatic castration-sensitive PC along with surgical and focal radiotherapy options. Cancer treatment-induced bone loss is known to occur with prolonged use of ADT. The ZEUS trial found no prevention of bone metastasis in patients with high-risk localized PC with the use of ZA in the absence of bone metastasis. A Kaplan-Meier estimated proportion of bone metastases after a median follow-up of 4.8 years was found to be not statistically significant: 14.7% in the ZA group vs 13.2% in the control/placebo group.29 The STAMPEDE trial showed no significant overall survival (OS) benefit with the addition of ZA to ADT vs ADT alone (HR, 0.94; 95% CI, 0.79-1.11; P = .45), 5-year survival with ADT alone was 55% compared to ADT plus ZA with 57% 5-year survival.30 The RADAR trial showed that at 5 years in high Gleason score patients, use of ZA in the absence of bone metastasis was beneficial, but not in low- or intermediate-risk patients. However, at 10-year analysis there was no significant difference in any of the high-stratified groups with or without ZA.31
The PR04 trial showed no effect on OS with clodronate compared with placebo in nonmetastatic castration-sensitive PC, with a HR of 1.12 (95% CI, 0.89-1.42; P = .94). The estimated 5-year survival was 80% with placebo and 78% with clodronate; 10-year survival rates were 51% with placebo and 48% with clodronate.32 Data from the HALT trial showed an increased bone mineral density and reduced risk of new vertebral fractures vs placebo (1.5% vs 3.9%, respectively) in the absence of metastatic bone lesions and a reduction in new vertebral fractures in patients with nonmetastatic PC.33 Most of these studies showed no benefit with the addition of ZA to nonmetastatic PC; although, the HALT trial provides evidence to support use of denosumab in patients with nonmetastatic PC for preventing vertebral fragility fractures in men receiving ADT.
Metastatic Hormone-Sensitive PC
ZA is often used to treat men with metastatic castration-sensitive PC despite limited efficacy and safety data. The CALGB 90202 (Alliance) trial authors found that the early use of ZA was not associated with increased time to first SRE. The median time to first SRE was 31.9 months in the ZA group (95% CI, 24.2-40.3) and 29.8 months in the placebo group (stratified HR, 0.97; 95% CI, 0-1.17; 1-sided stratified log-rank P = .39).34 OS was similar between the groups (HR, 0.88; 95% CI, 0.70-1.12; P = .29) as were reported AEs.34 Results from these studies suggest limited benefit in treating patients with metastatic hormone-sensitive PC with bisphosphonates without other medical indications for use. Additional studies suggest similar results for treatment with denosumab to that of bisphosphonate therapies.35
Nonmetastatic CRPC
Reasonable interest among treating clinicians exists to be able to delay or prevent the development of metastatic bone disease in patients who are showing biochemical signs of castration resistance but have not yet developed distant metastatic disease. Time to progression on ADT to castration resistance usually occurs 2 to 3 years following initiation of treatment. This typically occurs in patients with rising prostate-specific antigen (PSA). As per the Prostate Cancer Working Group 3, in the absence of radiologic progression, CRPC is defined by a 25% increase from the nadir (considering a starting value of ≥ 1 ng/mL), with a minimum rise of 2 ng/mL in the setting of castrate serum testosterone < 50 ng/dL despite good adherence to an ADT regimen, with proven serologic castration either by undetectable or a near undetectable nadir of serum testosterone concentration. Therapeutic implications include prevention of SREs as well as time to metastatic bone lesions. The Zometa 704 trial examined the use of ZA to reduce time to first metastatic bone lesion in the setting of patients with nonmetastatic CRPC.36 The trial was discontinued prematurely due to low patient accrual, but initial analysis provided information on the natural history of a rising PSA in this patient population. At 2 years, one-third of patients had developed bone metastases. Median bone metastasis-free survival was 30 months. Median time to first bone metastasis and OS were not reached. Baseline PSA and PSA velocity independently predicted a shorter time to first bone metastasis, metastasis-free survival, and OS.36
Denosumab was also studied in the setting of nonmetastatic CRPC in the Denosumab 147 trial. The study enrolled 1432 patients and found a significantly increased bone metastasis-free survival by a median of 4.2 months over placebo (HR, 0.85; 95% CI, 0.73-0.98; P = .03). Denosumab significantly delayed time to first bone metastasis (HR, 0.84; 95% CI, 0.71-0.98; P = .03). OS was similar between groups (HR, 1.01; 95% CI, 0.85-1.20; P = .91). Rates of AEs and serious AEs were similar between groups, except for ONJ and hypocalcemia. The rates of ONJ for denosumab were 1%, 3%, 4% in years 1,2, 3, respectively; overall, < 5% (n = 33). Hypocalcemia occurred in < 2% (n = 12) in denosumab-treated patients. The authors concluded that in men with CRPC, denosumab significantly prolonged bone metastasis–free survival and delayed time-to-bone metastasis.37 These 2 studies suggest a role of receptor-activated nuclear factor κ-B ligand inhibitor denosumab in patients with nonmetastatic CRPC in the appropriate setting. There were delays in bony metastatic disease, but no difference in OS. Rare denosumab treatment–related specific AEs were noted. Hence, denosumab is not recommended for use in this setting.
Metastatic CRPC
Castration resistance typically occurs 2 to 3 years following initiation of ADT and the most common extranodal site of disease is within the bone in metastatic PC. Disease progression within bones after ADT can be challenging given both the nature of progressive cancer with osteoblastic metastatic lesions and the prolonged effects of ADT on unaffected bone. The Zometa 039 study compared ZA with placebo and found a significant difference in SREs (38% and 49%, respectively; P .03). No survival benefit was observed with the addition of ZA. Use of other bisphosphonates pamidronate and clodronate did not have a similar degree of benefit.38,39
A phase 3 study of 1904 patients found that denosumab was superior to ZA in delaying the time to first on-study SRE (HR, 0.82; 95% CI, 0.71-0.95) and reducing rates of multiple SREs (HR, 0.82; 95% CI, 0.71-0.94).40 This was later confirmed with an additional study that demonstrated treatment with denosumab significantly reduced the risk of developing a first symptomatic SRE, defined as a pathologic fracture, spinal cord compression, necessity for radiation, or surgery (HR, 0.78; 95% CI, 0.66-0.93; P = .005) and first and subsequent symptomatic SREs (rate ratio, 0.78; 95% CI, 0.65-0.92; P = .004) compared with ZA.28 These findings suggest a continued role of denosumab in the treatment of advanced metastatic CRPC from both control of bone disease as well as quality of life and palliation of cancer-related symptoms.
Radium-223 dichloride (radium-223) is an α-emitting radionuclide for treatment of metastatic CRPC with bone metastasis, but otherwise no additional metastatic sites. Radium-223 is a calcium-mimetic that preferentially accumulates into areas of high-bone turnover, such as where bone metastases tend to occur. Radium-223 induces apoptosis of tumor cells through double-stranded DNA breaks. Studies have shown radium-223 to prolong OS and time-to-first symptomatic SRE.41 The ERA-223 trial showed that when radium-223 was combined with abiraterone acetate, there was an increase in fragility fracture risk compared with placebo combined with abiraterone. Data from the study revealed that the median symptomatic SRE-free survival was 22.3 months (95% CI, 20.4-24.8) in the radium-223 group and 26.0 months (21.8-28.3) in the placebo group. Concurrent treatment with abiraterone acetate plus prednisone or prednisolone and radium-223 was associated with increased fracture risk. Osteoporotic fractures were the most common type of fracture in the radium-223 group and of all fracture types, differed the most between the study groups.42
Conclusions
Convincing evidence supports the ongoing use of bisphosphonates and denosumab in patients with osteoporosis, significant osteopenia with risk factors, and in patients with CRPC with bone metastasis. Bone metastases can cause considerable morbidity and mortality among men with advanced PC. Pain, fracture, and neurologic injury can occur with metastatic bone lesions as well as with ADT-related bone loss. Prevention of SREs in patients with PC is a reasonable goal in PC survivors while being mindful of managing the risks of these therapies.
Prostate cancer (PC) is the most commonly and newly diagnosed nonskin cancer and the second leading cause of cancer death in men in the United States. About 191,930 cases and about 33,330 deaths from PC were expected for the year 2020.1 About 1 in 41 men will die of PC. Most men diagnosed with PC are aged > 65 years and do not die of their disease. The 5-year survival rate of localized and regional disease is nearly 100%, and disease with distant metastases is 31%. As a result, more than 3.1 million men in the United States who have been diagnosed with PC are still alive today.1 Among veterans, there is a substantial population living with PC. Skolarus and Hawley reported in 2014 that an estimated 200,000 veterans with PC were survivors and 12,000 were newly diagnosed.2
In PC, skeletal strength can be affected by several factors, such as aging, malnutrition, androgen-deprivation therapy (ADT), and bone metastasis.3,4 In fact, most men can live the rest of their life with PC by using strategies to monitor and treat it, once it shows either radiographic or chemical signs of progression.5 ADT is the standard of care to treat hormone-sensitive PC, which is associated with significant skeletal-related adverse effects (AEs).6,7
Men undergoing ADT are 4 times more likely to develop substantial bone deficiency, Shahinian and colleagues found that in men surviving 5 years after PC diagnosis, 19.4% of those who received ADT had a fracture compared with 12% in men who did not (P < .001). The authors established a significant relation between the number of doses of gonadotropin-releasing hormone given in the first 12 months and the risk of fracture.8 Of those who progressed to metastatic disease, the first metastatic nonnodal site is most commonly to the bone.9 Advanced PC is characterized by increased bone turnover, which further raises concerns for bone health and patient performance.10
Skeletal-related events (SREs) include pathologic fracture, spinal cord compression, palliative radiation, or surgery to bone, and change in antineoplastic therapy secondary to bone pain. The concept of bone health refers to the prevention, diagnosis, and treatment of idiopathic, pathogenic, and treatment-related bone loss and delay or prevention of SREs.6,11 Guidelines and expert groups have recommended screening for osteoporosis at the start of ADT with bone mineral density testing, ensuring adequate calcium and vitamin D intake, modifying lifestyle behaviors (smoking cessation, alcohol moderation, and regular exercise), and prescribing bisphosphonates or receptor-activated nuclear factor κ-B ligand inhibitor, denosumab, for men with osteoporosis or who are at general high-fracture risk.12,13 The overuse of these medications results in undue cost to patients as well as AEs, such as osteonecrosis of the jaw (ONJ), hypocalcemia, and bone/joint pains.14-17 There are evidence-based guidelines for appropriate use of bisphosphonates and denosumab for delay and prevention of SREs in the setting of advanced PC.18 These doses also typically differ in frequency to those of osteoporosis.19 We summarize the evidence and guidance for health care providers who care for patients with PC at various stages and complications from both disease-related and treatment-related comorbidities.
Bone-Strengthening Agents
Overall, there is evidence to support the use of bone-strengthening agents in patients with osteopenia/osteoporosis in the prevention of SREs with significant risk factors for progressive bone demineralization, such as lifestyle factors and, in particular, treatments such as ADT. Bone-remodeling agents for treatment of bony metastasis have been shown to provide therapeutic advantage only in limited instances in the castration-resistant PC (CRPC) setting. Hence, in patients with hormone-sensitive PC due to medication-related AEs, treatment with bone-strengthening agents is indicated only if the patient has a significant preexisting risk for fracture from osteopenia/osteoporosis (Table). The Figure depicts an algorithm for the management of bone health in men with PC who are being treated with ADT.
Denosumab and bisphosphonates have an established role in preventing SREs in metastatic CRPC.20 The choice of denosumab or a bisphosphonate typically varies based on the indication, possible AEs, and cost of therapy. There are multiple studies involving initiation of these agents at various stages of disease to improve both time to progression as well as management of SREs. There is a lack of evidence that bisphosphonates prevent metastatic-bone lesions in castration-sensitive PC; therefore, prophylactic use of this agent is not recommended in patients unless they have significant bone demineralization.21,22
Medication-induced ONJ is a severe AE of both denosumab and bisphosphonate therapies. Data from recent trials showed that higher dosing and prolonged duration of denosumab and bisphosphonate therapies further increased risk of ONJ by 1.8% and 1.3%, respectively.15 Careful history taking and discussions with the patient and if possible their dentist on how to reduce risk are recommended. It is good practice for the patient to complete a dental evaluation prior to starting IV bisphosphonates or denosumab. Dental evaluations should be performed routinely at 3- to 12-month intervals throughout therapy based on individualized risk assessment.23 The benefits of using bisphosphonates to prevent fractures associated with osteoporosis outweigh the risk of ONJ in high-risk populations, but not in all patients with PC. A case-by-case basis and evaluation of risk factors should be performed prior to administering bone-modifying therapy. The long-term safety of IV bisphosphonates has not been adequately studied in controlled trials, and concerns regarding long-term complications, including renal toxicity, ONJ, and atypical femoral fractures, remain with prolonged therapy.24,25
The CALGB 70604 (Alliance) trial compared 3-month dosing to monthly treatment with zoledronic acid (ZA), showing no inferiority to lower frequency dosing.26 A Cochrane review of clinical trials found that in patients with advanced PC, bisphosphonates were found to provide roughly 58 fewer SREs per 1000 on average.27 A phase 3 study showed a modest benefit to denosumab vs ZA in the CRPC group regarding incidence of SREs. The rates of SREs were 289 of 951 patients in the bisphosphonate group, and 241 of 950 patients in the denosumab group (30.4% vs 25.3%; hazard ratio [HR], 0.78; 95% CI, 0.66-0.93; P = .005).28 In 2020, the American Society of Clinical Oncology endorsed the Cancer Care Ontario guidelines for prostate bone health care.18 Adequate supplementation is necessary in all patients treated with a bisphosphonate or denosumab to prevent treatment-related hypocalcemia. Typically, daily supplementation with a minimum of calcium 500 mg and vitamin D 400 IU is recommended.16
Bone Health in Patients
Nonmetastatic Hormone-Sensitive PC
ADT forms the backbone of treatment for patients with local and advanced metastatic castration-sensitive PC along with surgical and focal radiotherapy options. Cancer treatment-induced bone loss is known to occur with prolonged use of ADT. The ZEUS trial found no prevention of bone metastasis in patients with high-risk localized PC with the use of ZA in the absence of bone metastasis. A Kaplan-Meier estimated proportion of bone metastases after a median follow-up of 4.8 years was found to be not statistically significant: 14.7% in the ZA group vs 13.2% in the control/placebo group.29 The STAMPEDE trial showed no significant overall survival (OS) benefit with the addition of ZA to ADT vs ADT alone (HR, 0.94; 95% CI, 0.79-1.11; P = .45), 5-year survival with ADT alone was 55% compared to ADT plus ZA with 57% 5-year survival.30 The RADAR trial showed that at 5 years in high Gleason score patients, use of ZA in the absence of bone metastasis was beneficial, but not in low- or intermediate-risk patients. However, at 10-year analysis there was no significant difference in any of the high-stratified groups with or without ZA.31
The PR04 trial showed no effect on OS with clodronate compared with placebo in nonmetastatic castration-sensitive PC, with a HR of 1.12 (95% CI, 0.89-1.42; P = .94). The estimated 5-year survival was 80% with placebo and 78% with clodronate; 10-year survival rates were 51% with placebo and 48% with clodronate.32 Data from the HALT trial showed an increased bone mineral density and reduced risk of new vertebral fractures vs placebo (1.5% vs 3.9%, respectively) in the absence of metastatic bone lesions and a reduction in new vertebral fractures in patients with nonmetastatic PC.33 Most of these studies showed no benefit with the addition of ZA to nonmetastatic PC; although, the HALT trial provides evidence to support use of denosumab in patients with nonmetastatic PC for preventing vertebral fragility fractures in men receiving ADT.
Metastatic Hormone-Sensitive PC
ZA is often used to treat men with metastatic castration-sensitive PC despite limited efficacy and safety data. The CALGB 90202 (Alliance) trial authors found that the early use of ZA was not associated with increased time to first SRE. The median time to first SRE was 31.9 months in the ZA group (95% CI, 24.2-40.3) and 29.8 months in the placebo group (stratified HR, 0.97; 95% CI, 0-1.17; 1-sided stratified log-rank P = .39).34 OS was similar between the groups (HR, 0.88; 95% CI, 0.70-1.12; P = .29) as were reported AEs.34 Results from these studies suggest limited benefit in treating patients with metastatic hormone-sensitive PC with bisphosphonates without other medical indications for use. Additional studies suggest similar results for treatment with denosumab to that of bisphosphonate therapies.35
Nonmetastatic CRPC
Reasonable interest among treating clinicians exists to be able to delay or prevent the development of metastatic bone disease in patients who are showing biochemical signs of castration resistance but have not yet developed distant metastatic disease. Time to progression on ADT to castration resistance usually occurs 2 to 3 years following initiation of treatment. This typically occurs in patients with rising prostate-specific antigen (PSA). As per the Prostate Cancer Working Group 3, in the absence of radiologic progression, CRPC is defined by a 25% increase from the nadir (considering a starting value of ≥ 1 ng/mL), with a minimum rise of 2 ng/mL in the setting of castrate serum testosterone < 50 ng/dL despite good adherence to an ADT regimen, with proven serologic castration either by undetectable or a near undetectable nadir of serum testosterone concentration. Therapeutic implications include prevention of SREs as well as time to metastatic bone lesions. The Zometa 704 trial examined the use of ZA to reduce time to first metastatic bone lesion in the setting of patients with nonmetastatic CRPC.36 The trial was discontinued prematurely due to low patient accrual, but initial analysis provided information on the natural history of a rising PSA in this patient population. At 2 years, one-third of patients had developed bone metastases. Median bone metastasis-free survival was 30 months. Median time to first bone metastasis and OS were not reached. Baseline PSA and PSA velocity independently predicted a shorter time to first bone metastasis, metastasis-free survival, and OS.36
Denosumab was also studied in the setting of nonmetastatic CRPC in the Denosumab 147 trial. The study enrolled 1432 patients and found a significantly increased bone metastasis-free survival by a median of 4.2 months over placebo (HR, 0.85; 95% CI, 0.73-0.98; P = .03). Denosumab significantly delayed time to first bone metastasis (HR, 0.84; 95% CI, 0.71-0.98; P = .03). OS was similar between groups (HR, 1.01; 95% CI, 0.85-1.20; P = .91). Rates of AEs and serious AEs were similar between groups, except for ONJ and hypocalcemia. The rates of ONJ for denosumab were 1%, 3%, 4% in years 1,2, 3, respectively; overall, < 5% (n = 33). Hypocalcemia occurred in < 2% (n = 12) in denosumab-treated patients. The authors concluded that in men with CRPC, denosumab significantly prolonged bone metastasis–free survival and delayed time-to-bone metastasis.37 These 2 studies suggest a role of receptor-activated nuclear factor κ-B ligand inhibitor denosumab in patients with nonmetastatic CRPC in the appropriate setting. There were delays in bony metastatic disease, but no difference in OS. Rare denosumab treatment–related specific AEs were noted. Hence, denosumab is not recommended for use in this setting.
Metastatic CRPC
Castration resistance typically occurs 2 to 3 years following initiation of ADT and the most common extranodal site of disease is within the bone in metastatic PC. Disease progression within bones after ADT can be challenging given both the nature of progressive cancer with osteoblastic metastatic lesions and the prolonged effects of ADT on unaffected bone. The Zometa 039 study compared ZA with placebo and found a significant difference in SREs (38% and 49%, respectively; P .03). No survival benefit was observed with the addition of ZA. Use of other bisphosphonates pamidronate and clodronate did not have a similar degree of benefit.38,39
A phase 3 study of 1904 patients found that denosumab was superior to ZA in delaying the time to first on-study SRE (HR, 0.82; 95% CI, 0.71-0.95) and reducing rates of multiple SREs (HR, 0.82; 95% CI, 0.71-0.94).40 This was later confirmed with an additional study that demonstrated treatment with denosumab significantly reduced the risk of developing a first symptomatic SRE, defined as a pathologic fracture, spinal cord compression, necessity for radiation, or surgery (HR, 0.78; 95% CI, 0.66-0.93; P = .005) and first and subsequent symptomatic SREs (rate ratio, 0.78; 95% CI, 0.65-0.92; P = .004) compared with ZA.28 These findings suggest a continued role of denosumab in the treatment of advanced metastatic CRPC from both control of bone disease as well as quality of life and palliation of cancer-related symptoms.
Radium-223 dichloride (radium-223) is an α-emitting radionuclide for treatment of metastatic CRPC with bone metastasis, but otherwise no additional metastatic sites. Radium-223 is a calcium-mimetic that preferentially accumulates into areas of high-bone turnover, such as where bone metastases tend to occur. Radium-223 induces apoptosis of tumor cells through double-stranded DNA breaks. Studies have shown radium-223 to prolong OS and time-to-first symptomatic SRE.41 The ERA-223 trial showed that when radium-223 was combined with abiraterone acetate, there was an increase in fragility fracture risk compared with placebo combined with abiraterone. Data from the study revealed that the median symptomatic SRE-free survival was 22.3 months (95% CI, 20.4-24.8) in the radium-223 group and 26.0 months (21.8-28.3) in the placebo group. Concurrent treatment with abiraterone acetate plus prednisone or prednisolone and radium-223 was associated with increased fracture risk. Osteoporotic fractures were the most common type of fracture in the radium-223 group and of all fracture types, differed the most between the study groups.42
Conclusions
Convincing evidence supports the ongoing use of bisphosphonates and denosumab in patients with osteoporosis, significant osteopenia with risk factors, and in patients with CRPC with bone metastasis. Bone metastases can cause considerable morbidity and mortality among men with advanced PC. Pain, fracture, and neurologic injury can occur with metastatic bone lesions as well as with ADT-related bone loss. Prevention of SREs in patients with PC is a reasonable goal in PC survivors while being mindful of managing the risks of these therapies.
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
2. Skolarus TA, Hawley ST. Prostate cancer survivorship care in the Veterans Health Administration. Fed Pract. 2014;31(8):10-17.
3. Gartrell BA, Coleman R, Efstathiou E, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. Eur Urol. 2015;68(5):850-858. doi:10.1016/j.eururo.2015.06.039
4. Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516-2527. doi:10.1056/NEJMoa0810095
5. Welch HG, Albertsen PC. Reconsidering Prostate cancer mortality—The future of PSA screening. N Engl J Med. 2020;382(16):1557-1563. doi:10.1056/NEJMms1914228
6. Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J; ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25 (suppl 3):iii124-137. doi:10.1093/annonc/mdu103
7. Saylor PJ, Smith MR. Adverse effects of androgen deprivation therapy: defining the problem and promoting health among men with prostate cancer. J Natl Compr Canc Netw. 2010;8(2):211-223. doi:10.6004/jnccn.2010.0014
8. Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154-164. doi:10.1056/NEJMoa041943
9. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657. doi:10.1056/NEJMra1701695
10. Saad F, Eastham JA, Smith MR. Biochemical markers of bone turnover and clinical outcomes in men with prostate cancer. Urol Oncol. 2012;30(4):369-378. doi:10.1016/j.urolonc.2010.08.007
11. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi:10.1007/s00198-014-2794-2
12. Alibhai SMH, Zukotynski K, Walker-Dilks C, et al; Cancer Care Ontario Genitourinary Cancer Disease Site Group. Bone health and bone-targeted therapies for prostate cancer: a programme in evidence-based care - Cancer Care Ontario Clinical Practice Guideline. Clin Oncol (R Coll Radiol). 2017;29(6):348-355. doi:10.1016/j.clon.2017.01.007
13. LEE CE. A comprehensive bone-health management approach with men with prostate cancer recieving androgen deprivation therapy. Curr Oncol. 2011;18(4):e163-172. doi:10.3747/co.v18i4.746
14. Kennel KA, Drake MT. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin Proc. 2009;84(7):632-638. doi:10.1016/S0025-6196(11)60752-0
15. Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23(5):1341-1347. doi:10.1093/annonc/mdr435
16. Body J-J, Bone HG, de Boer RH, et al. Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur J Cancer. 2015;51(13):1812-1821. doi:10.1016/j.ejca.2015.05.016
17. Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med. 2005;165(3):346-347. doi:10.1001/archinte.165.3.346-b
18. Saylor PJ, Rumble RB, Tagawa S, et al. Bone health and bone-targeted therapies for prostate cancer: ASCO endorsement of a cancer care Ontario guideline. J Clin Oncol. 2020;38(15):1736-1743. doi:10.1200/JCO.19.03148
19. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96(11):879-882. doi:10.1093/jnci/djh141
20. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic zcid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458-1468. doi:10.1093/jnci/94.19.1458
21. Aapro M, Saad F. Bone-modifying agents in the treatment of bone metastases in patients with advanced genitourinary malignancies: a focus on zoledronic acid. Ther Adv Urol. 2012;4(2):85-101. doi:10.1177/1756287212441234
22. Cianferotti L, Bertoldo F, Carini M, et al. The prevention of fragility fractures in patients with non-metastatic prostate cancer: a position statement by the international osteoporosis foundation. Oncotarget. 2017;8(43):75646-75663. doi:10.18632/oncotarget.17980
23. Ruggiero S, Gralow J, Marx RE, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract. 2006;2(1):7-14. doi:10.1200/JOP.2006.2.1.7
24. Corraini P, Heide-Jørgensen U, Schøodt M, et al. Osteonecrosis of the jaw and survival of patients with cancer: a nationwide cohort study in Denmark. Cancer Med. 2017;6(10):2271-2277. doi:10.1002/cam4.1173
25. Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565. doi:10.1210/jc.2009-1947
26. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58. doi:10.1001/jama.2016.19425
27. Macherey S, Monsef I, Jahn F, et al. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst Rev. 2017;12(12):CD006250. doi:10.1002/14651858.CD006250.pub2
28. Smith MR, Coleman RE, Klotz L, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol. 2015;26(2):368-374. doi:10.1093/annonc/mdu519
29. Wirth M, Tammela T, Cicalese V, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67(3):482-491. doi:10.1016/j.eururo.2014.02.014
30. James ND, Sydes MR, Clarke NW, et al; STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi:10.1016/S0140-6736(15)01037-5
31. Denham JW, Joseph D, Lamb DS, et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Lancet Oncol. 2019;20(2):267-281. doi:10.1016/S1470-2045(18)30757-5
32. Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10(9):872-876. doi:10.1016/S1470-2045(09)70201-3
33. Smith MR, Egerdie B, Toriz NH, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate Cancer. N Engl J Med. 2009;361(8):745-755. doi:10.1056/NEJMoa0809003
34. Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32(11):1143-1150. doi:10.1200/JCO.2013.51.6500
35. Kozyrakis D, Paridis D, Perikleous S, Malizos K, Zarkadas A, Tsagkalis A. The current role of osteoclast inhibitors in patients with prostate cancer. Adv Urol. 2018;2018:1525832. doi:10.1155/2018/1525832
36. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918-2925. doi:10.1200/JCO.2005.01.529
37. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39-46. doi:10.1016/S0140-6736(11)61226-9
38. Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21(23):4277-4284. doi:10.1200/JCO.2003.05.147
39. Ernst DS, Tannock IF, Winquist EW, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21(17):3335-3342. doi:10.1200/JCO.2003.03.042
40. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813-822. doi:10.1016/S0140-6736(10)62344-6
41. Parker C, Nilsson S, Heinrich D, et al; ALSYMPCA Investigators Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. doi:10.1056/NEJMoa1213755
42. Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):408-419. doi:10.1016/S1470-2045(18)30860-X
43. Smith MR, Saad F, Shore ND, et al. Effect of denosumab on prolonging bone-metastasis-free survival (BMFS) in men with nonmetastatic castrate-resistant prostate cancer (CRPC) presenting with aggressive PSA kinetics. J Clin Oncol. 2012;30(5_suppl):6-6.
44. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458-1468. doi:10.1093/jnci/94.19.1458
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70(1):7-30. doi:10.3322/caac.21590
2. Skolarus TA, Hawley ST. Prostate cancer survivorship care in the Veterans Health Administration. Fed Pract. 2014;31(8):10-17.
3. Gartrell BA, Coleman R, Efstathiou E, et al. Metastatic prostate cancer and the bone: significance and therapeutic options. Eur Urol. 2015;68(5):850-858. doi:10.1016/j.eururo.2015.06.039
4. Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360(24):2516-2527. doi:10.1056/NEJMoa0810095
5. Welch HG, Albertsen PC. Reconsidering Prostate cancer mortality—The future of PSA screening. N Engl J Med. 2020;382(16):1557-1563. doi:10.1056/NEJMms1914228
6. Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J; ESMO Guidelines Working Group. Bone health in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol. 2014;25 (suppl 3):iii124-137. doi:10.1093/annonc/mdu103
7. Saylor PJ, Smith MR. Adverse effects of androgen deprivation therapy: defining the problem and promoting health among men with prostate cancer. J Natl Compr Canc Netw. 2010;8(2):211-223. doi:10.6004/jnccn.2010.0014
8. Shahinian VB, Kuo Y-F, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352(2):154-164. doi:10.1056/NEJMoa041943
9. Sartor O, de Bono JS. Metastatic prostate cancer. N Engl J Med. 2018;378(7):645-657. doi:10.1056/NEJMra1701695
10. Saad F, Eastham JA, Smith MR. Biochemical markers of bone turnover and clinical outcomes in men with prostate cancer. Urol Oncol. 2012;30(4):369-378. doi:10.1016/j.urolonc.2010.08.007
11. Cosman F, de Beur SJ, LeBoff MS, et al; National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2381. doi:10.1007/s00198-014-2794-2
12. Alibhai SMH, Zukotynski K, Walker-Dilks C, et al; Cancer Care Ontario Genitourinary Cancer Disease Site Group. Bone health and bone-targeted therapies for prostate cancer: a programme in evidence-based care - Cancer Care Ontario Clinical Practice Guideline. Clin Oncol (R Coll Radiol). 2017;29(6):348-355. doi:10.1016/j.clon.2017.01.007
13. LEE CE. A comprehensive bone-health management approach with men with prostate cancer recieving androgen deprivation therapy. Curr Oncol. 2011;18(4):e163-172. doi:10.3747/co.v18i4.746
14. Kennel KA, Drake MT. Adverse effects of bisphosphonates: Implications for osteoporosis management. Mayo Clin Proc. 2009;84(7):632-638. doi:10.1016/S0025-6196(11)60752-0
15. Saad F, Brown JE, Van Poznak C, et al. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23(5):1341-1347. doi:10.1093/annonc/mdr435
16. Body J-J, Bone HG, de Boer RH, et al. Hypocalcaemia in patients with metastatic bone disease treated with denosumab. Eur J Cancer. 2015;51(13):1812-1821. doi:10.1016/j.ejca.2015.05.016
17. Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med. 2005;165(3):346-347. doi:10.1001/archinte.165.3.346-b
18. Saylor PJ, Rumble RB, Tagawa S, et al. Bone health and bone-targeted therapies for prostate cancer: ASCO endorsement of a cancer care Ontario guideline. J Clin Oncol. 2020;38(15):1736-1743. doi:10.1200/JCO.19.03148
19. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96(11):879-882. doi:10.1093/jnci/djh141
20. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic zcid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458-1468. doi:10.1093/jnci/94.19.1458
21. Aapro M, Saad F. Bone-modifying agents in the treatment of bone metastases in patients with advanced genitourinary malignancies: a focus on zoledronic acid. Ther Adv Urol. 2012;4(2):85-101. doi:10.1177/1756287212441234
22. Cianferotti L, Bertoldo F, Carini M, et al. The prevention of fragility fractures in patients with non-metastatic prostate cancer: a position statement by the international osteoporosis foundation. Oncotarget. 2017;8(43):75646-75663. doi:10.18632/oncotarget.17980
23. Ruggiero S, Gralow J, Marx RE, et al. Practical guidelines for the prevention, diagnosis, and treatment of osteonecrosis of the jaw in patients with cancer. J Oncol Pract. 2006;2(1):7-14. doi:10.1200/JOP.2006.2.1.7
24. Corraini P, Heide-Jørgensen U, Schøodt M, et al. Osteonecrosis of the jaw and survival of patients with cancer: a nationwide cohort study in Denmark. Cancer Med. 2017;6(10):2271-2277. doi:10.1002/cam4.1173
25. Watts NB, Diab DL. Long-term use of bisphosphonates in osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1555-1565. doi:10.1210/jc.2009-1947
26. Himelstein AL, Foster JC, Khatcheressian JL, et al. Effect of longer interval vs standard dosing of zoledronic acid on skeletal events in patients with bone metastases: a randomized clinical trial. JAMA. 2017;317(1):48-58. doi:10.1001/jama.2016.19425
27. Macherey S, Monsef I, Jahn F, et al. Bisphosphonates for advanced prostate cancer. Cochrane Database Syst Rev. 2017;12(12):CD006250. doi:10.1002/14651858.CD006250.pub2
28. Smith MR, Coleman RE, Klotz L, et al. Denosumab for the prevention of skeletal complications in metastatic castration-resistant prostate cancer: comparison of skeletal-related events and symptomatic skeletal events. Ann Oncol. 2015;26(2):368-374. doi:10.1093/annonc/mdu519
29. Wirth M, Tammela T, Cicalese V, et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: efficacy and safety results of the Zometa European Study (ZEUS). Eur Urol. 2015;67(3):482-491. doi:10.1016/j.eururo.2014.02.014
30. James ND, Sydes MR, Clarke NW, et al; STAMPEDE Investigators. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi:10.1016/S0140-6736(15)01037-5
31. Denham JW, Joseph D, Lamb DS, et al. Short-term androgen suppression and radiotherapy versus intermediate-term androgen suppression and radiotherapy, with or without zoledronic acid, in men with locally advanced prostate cancer (TROG 03.04 RADAR): 10-year results from a randomised, phase 3, factorial trial. Lancet Oncol. 2019;20(2):267-281. doi:10.1016/S1470-2045(18)30757-5
32. Dearnaley DP, Mason MD, Parmar MK, Sanders K, Sydes MR. Adjuvant therapy with oral sodium clodronate in locally advanced and metastatic prostate cancer: long-term overall survival results from the MRC PR04 and PR05 randomised controlled trials. Lancet Oncol. 2009;10(9):872-876. doi:10.1016/S1470-2045(09)70201-3
33. Smith MR, Egerdie B, Toriz NH, et al; Denosumab HALT Prostate Cancer Study Group. Denosumab in men receiving androgen-deprivation therapy for prostate Cancer. N Engl J Med. 2009;361(8):745-755. doi:10.1056/NEJMoa0809003
34. Smith MR, Halabi S, Ryan CJ, et al. Randomized controlled trial of early zoledronic acid in men with castration-sensitive prostate cancer and bone metastases: results of CALGB 90202 (alliance). J Clin Oncol. 2014;32(11):1143-1150. doi:10.1200/JCO.2013.51.6500
35. Kozyrakis D, Paridis D, Perikleous S, Malizos K, Zarkadas A, Tsagkalis A. The current role of osteoclast inhibitors in patients with prostate cancer. Adv Urol. 2018;2018:1525832. doi:10.1155/2018/1525832
36. Smith MR, Kabbinavar F, Saad F, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23(13):2918-2925. doi:10.1200/JCO.2005.01.529
37. Smith MR, Saad F, Coleman R, et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39-46. doi:10.1016/S0140-6736(11)61226-9
38. Small EJ, Smith MR, Seaman JJ, Petrone S, Kowalski MO. Combined analysis of two multicenter, randomized, placebo-controlled studies of pamidronate disodium for the palliation of bone pain in men with metastatic prostate cancer. J Clin Oncol. 2003;21(23):4277-4284. doi:10.1200/JCO.2003.05.147
39. Ernst DS, Tannock IF, Winquist EW, et al. Randomized, double-blind, controlled trial of mitoxantrone/prednisone and clodronate versus mitoxantrone/prednisone and placebo in patients with hormone-refractory prostate cancer and pain. J Clin Oncol. 2003;21(17):3335-3342. doi:10.1200/JCO.2003.03.042
40. Fizazi K, Carducci M, Smith M, et al. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377(9768):813-822. doi:10.1016/S0140-6736(10)62344-6
41. Parker C, Nilsson S, Heinrich D, et al; ALSYMPCA Investigators Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223. doi:10.1056/NEJMoa1213755
42. Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(3):408-419. doi:10.1016/S1470-2045(18)30860-X
43. Smith MR, Saad F, Shore ND, et al. Effect of denosumab on prolonging bone-metastasis-free survival (BMFS) in men with nonmetastatic castrate-resistant prostate cancer (CRPC) presenting with aggressive PSA kinetics. J Clin Oncol. 2012;30(5_suppl):6-6.
44. Saad F, Gleason DM, Murray R, et al; Zoledronic Acid Prostate Cancer Study Group. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458-1468. doi:10.1093/jnci/94.19.1458
The Precision Oncology Program for Cancer of the Prostate (POPCaP) Network: A Veterans Affairs/Prostate Cancer Foundation Collaboration(FULL)
The US Department of Veterans Affairs (VA) is home to the Veterans Health Administration (VHA), which delivers care at 1,255 health care facilities, including 170 medical centers. The VA serves 6 million veterans each year and is the largest integrated provider of cancer care in the US. The system uses a single, enterprise-wide electronic health record. The detailed curation of clinical outcomes, laboratory results, and radiology is used in VA efforts to improve oncology outcomes for veterans. The VA also has a National Precision Oncology Program (NPOP), which offers system-wide DNA sequencing for veterans with cancer. Given its size, integration, and capabilities, the VA is an ideal setting for rapid learning cycles of testing and implementing best practices at scale.
Prostate cancer is the most common malignancy affecting men in the US. It is the most commonly-diagnosed solid tumor in the VA, and in 2014, there were 11,376 prostate cancer diagnoses in the VA.1 The clinical characteristics and treatment of veterans with prostate cancer largely parallel the broader population of men in the US.1 Although the majority of men diagnosed with prostate cancer have disease localized to the prostate, an important minority develop metastatic disease, which represents a risk for substantial morbidity and is the lethal form of the disease. Research has yielded transformative advances in the care of men with metastatic prostate cancer, including drugs targeting the testosterone/androgen signaling axis, taxane chemotherapy, the radionuclide radium-223, and a dendritic cell vaccine. Unfortunately, the magnitude and duration of response to these therapies varies widely, and determining the biology relevant to an individual patient that would better inform their treatment decisions is a critical next step. As the ability to interrogate the cancer genome has improved, relevant drivers of tumorigenesis and predictive biomarkers are being identified rapidly, and oncology care has evolved from a one-size-fits-all approach to a precision approach, which uses these biomarkers to assist in therapeutic decision making.
Precision Oncology for Prostate Cancer
A series of studies interrogating the genomics of metastatic prostate cancer have been critical to defining the relevance of precision oncology for prostate cancer. Most of what is known about the genomics of prostate cancer has been derived from analysis of samples from the prostate itself. These samples may not reflect the biology of metastasis and genetic evolution in response to treatment pressure, so the genomic alterations in metastatic disease remained incompletely characterized. Two large research teams supported by grants from the American Association for Cancer Research, Stand Up 2 Cancer, and Prostate Cancer Foundation (PCF) focused their efforts on sampling and analyzing metastatic tissue to define the most relevant genomic alterations in advanced prostate cancer.
These efforts defined a broad range of relatively common alterations in the androgen receptor, as well as the tumor suppressors TP53 and PTEN.2,3 Important subsets of less common alterations in pathways that were potentially targetable were also found, including new alterations in PIK3CA/B, BRAF/RAF1, and β-catenin. Most surprisingly, alterations of DNA repair pathways, including mismatch repair and homologous recombination were found in 20% of tumors, and half of these tumors contained germline alterations. The same groups performed a follow up analysis of germline DNA from men with metastatic prostate cancer, which confirmed that 12% of these patients carry a pathogenic germline alteration in a DNA repair pathway gene.4 These efforts immediately invigorated precision oncology clinical trials for prostate cancer and spurred an effort to find the molecular alterations that could be leveraged to improve care for men with advanced prostate cancer.
Targetable Alterations
Currently a number of genomic alterations are immediately actionable. There are several agents approved by the US Food and Drug Administration (FDA) that exploit these Achilles heels of prostate cancer. Mismatch repair deficiency occurs when any of a group of genes responsible for proofreading the fidelity of DNA replication is compromised by mutation or deletion. Imperfect reading and correction subsequently lead to many DNA mutations in a tissue (hypermutation), which then increases the risk of developing malignancy. If a defective gene in the mismatch repair pathway is inherited, a patient has a genetic predisposition to specific malignancies that are part of the Lynch syndrome.5 Prostate cancer is a relatively rare manifestation of Lynch syndrome, although it is considered one of the malignancies in the Lynch syndrome spectrum.6
Alteration of one of the mismatch repair genes also can occur spontaneously in a tumor, resulting in the same high frequency of spontaneous DNA mutations. Overall, between 3% and 5% of metastatic prostate cancers contain mismatch repair deficiency. The majority of these cases are a result of spontaneous loss or mutation of the relevant gene, but 1 in 5 of these tumors occurs as a component of Lynch syndrome.7 Identification of mismatch repair deficiency is critical because the resulting hypermutation makes these tumors particularly susceptible to intervention with immunotherapy. Up to half of patients with metastatic prostate cancer can have durable responses. This finding is consistent with the experience treating other malignancies with mismatch repair deficiency.8 Although screening for mismatch repair deficiency is standard of care for patients with malignancies such as colorectal cancer, few patients with prostate cancer may receive the mismatch repair deficiency screening (based on unpublished data). In contrast, screening is routine for patients with adenocarcinoma of the lung because their proportion of ROS1 and ALK alterations is similar to the frequency of mismatch repair deficiency when compared with patients with prostate cancer.9
Homologous recombination is another mechanism by which cells repair DNA damage and is responsible for repairing double strand breaks, the type of DNA damage most likely to lead to carcinogenesis. In advanced prostate cancer, BRCA2, ATM, BRCA1 and other members of the Fanconi Anemia/BRCA gene family are altered 20% of the time. These genes also are the most common germline alterations implicated in the development of prostate cancer.2,10 Prostate cancer is considered a BRCA-related cancer much like breast, ovarian, and pancreatic cancers. Defects in homologous recombination repair make BRCA-altered prostate cancers susceptible to DNA damaging chemotherapy, such as platinum and to the use of poly–(adenosine diphosphate–ribose) polymerase (PARP) inhibitors because cancer cells then accumulate cytotoxic and apoptotic levels of DNA.11
In May 2020, the FDA approved the use of PARP inhibitors for the treatment of prostate cancers that contain BRCA and other DNA repair alterations. Rucaparib received accelerated approval for the treatment of prostate cancers containing BRCA alterations and olaparib received full approval for treatment of prostate cancers containing an array of alterations in DNA repair genes.12,13 Both approvals were the direct result of the cited landmark studies that demonstrated the frequency of these alterations in advanced prostate cancer.2,3
Beyond mismatch and homologous recombination repair, there are a large number of potentially targetable alterations found in advanced prostate cancer. It is thus critical that we put systems into place both to find germline and somatic alterations that will inform a veteran’s clinical care and to provide veterans access to precision oncology clinical trials.
The POPCaP Network
Because prostate cancer is such a significant issue in the VA and best practices for precision oncology can be implemented broadly once defined as successful, the PCF and the VA formed a collaboration to support a network of centers that would focus on implementing a comprehensive strategy for precision oncology in prostate cancer. There are currently 11 centers in the Precision Oncology Program for Cancer of the Prostate (POPCaP) network (Figure). These centers are tasked with comprehensively sequencing germline and somatic tissue from veterans with metastatic prostate cancer to find alterations, which could provide access to treatments that would otherwise not be available or appropriate.
The network is collaborating with NPOP, which provides clinical grade tumor gene panel sequencing for veterans with prostate cancer from > 90% of VA medical centers. POPCaP also partners with the University of Washington to use its OncoPlex gene panel and University of Michigan to use the Oncomine panel to define the best platform for defining targetable alterations for veterans with prostate cancer. Investigators participate in a monthly molecular oncology tumor board continuing medical education-accredited program, which provides guidance and education across the VA about the evidence available to assist in decision making for veterans sequenced through NPOP and the academic platforms. These efforts leverage VA’s partnership with IBM Watson for Genomics to annotate DNA sequencing results to provide clinicians with potential therapeutic options for veterans.
A clinical trials mechanism is embedded in POPCaP to broaden treatment options, improve care for men with prostate cancer, and leverage the sequencing efforts in the network. The Prostate Cancer Analysis for Therapy Choice (PATCH) clinical trials network employs an umbrella study approach whereby alterations are identified through sequencing and veterans are given access to studies embedded at sites across the network. Graff and Huang provide a detailed description of the PATCH network and its potential as a multisite clinical trials mechanism.14 For studies within the network, funds can be provided to support travel to participate in clinical trials for veterans who would be eligible for study but do not live in a catchment for a network site. POPCaP also leverages both the resources of the National Cancer Institute (NCI)-designated cancer centers that are VA academic affiliates, as well as a VA/NCI partnership (NAVIGATE) to increase veteran access to NCI cutting-edge clinical trials.
The network has regular teleconference meetings of the investigators, coordinators, and stakeholders and face-to-face meetings, which are coordinated around other national meetings. These meetings enable investigators to work collaboratively to advance current knowledge in prostate cancer through the application of complementary and synergistic research approaches. Since research plays a critical role within the learning health care system, POPCaP investigators are working to optimize the transfer of knowledge from the clinic to the bench and back to the clinic. In this regard, investigators from network sites have organized themselves into working groups to focus on multiple critical aspects of research and care within the network, including sequencing, phenotyping, health services, health disparities, and a network biorepository.
VA Office of Research and Development
With support from the VA Office of Research and Development, there are research efforts focused on the development of data analytics to identify veterans with metastatic prostate cancer within the electronic health record to ensure access to appropriate testing, treatment, and clinical trials. This will optimize tracking and continuous quality improvement in precision oncology. The Office of Research and Development also supports the use of artificial intelligence to identify predictive markers for diagnosis, prognosis, therapeutic response and patient stratification. POPCaP investigators, along with other investigators from across the VA, conduct research that continually improves the care of veterans with prostate cancer. POPCaP has a special focus on prostate cancer among African Americans, who are disproportionately affected by the disease and well represented in VA. The efforts of the working groups, the research studies and the network as a whole also serve to recruit both junior and senior investigators to the VA in order to support the VA research enterprise.
Active collaborations between the network and other elements of VA include efforts to optimize germline testing and genetic counseling in prostate cancer through the Genomic Medicine Service, which provides telehealth genetic counseling throughout the VA. POPCaP pilots innovative approaches to increase access to clinical genetics and genetic counseling services to support the volume of genetic testing of veterans with cancer. Current National Comprehensive Cancer Network (NCCN) guidelines recommend germline testing for all men with metastatic prostate cancer, which can efficiently identify the roughly 10% of veterans with metastatic disease who carry a germline alteration and provide them with access to studies, FDA-approved treatments, while also offering critical health care information to family members who may also carry a pathogenic germline alteration.
Million Veteran Program
The Million Veteran Program (MVP) has collected > 825,000 germline DNA samples from an anticipated enrollment of > 1 million veterans in one of the most ambitious genetic research efforts to correlate how germline DNA interacts with lifestyle, medications and military exposure to affect health and illness (www.research.va.gov/mvp). MVP is a racially and ethnically diverse veteran cohort that is roughly 20% African American and 7% Hispanic. More than 40,000 of the participants have had prostate cancer, one third of whom are African Americans, giving researchers unprecedented ability to discover factors that impact the development and treatment of the disease in this population. In particular, MVP will provide unique insights into the genetic mutations that drive the development of aggressive prostate cancer in all male veterans, including African Americans. These discoveries will undoubtedly lead to improved screening of and treatment for prostate cancer.
In order to demonstrate clinical utility as well as the infrastructure needs to scale up within the VHA, MVP has launched a pilot project that offers to return clinically actionable genetic results to MVP participants with metastatic prostate cancer, opening the door to new therapies to improve the length and quality of these veterans’ lives. Importantly, the pilot includes cascade testing in family members of enrolled veterans. Given that the original MVP consent did not allow for return of results, and MVP genetic testing is research grade, veterans who volunteer will provide a second consent and undergo clinical genetic testing to confirm the variants. Results from this pilot study also will inform expansion of VA precision oncology efforts for patients with other cancers such as breast cancer or ovarian cancer, where the specific genetic mutations are known to play a role, (eg, BRCA2). In addition, through an interagency agreement with the US Department of Energy (DOE), MVP is leveraging DOE expertise and high-performance computing capabilities to identify clinical and genetic risk factors for prostate cancer that will progress to metastatic disease.
This active research collaboration between POPCaP, MVP, and the Genomic Medicine Service will identify germline BRCA alterations from MVP participants with metastatic prostate cancer and give them access to therapies that may provide better outcomes and access to genetic testing for their family members.
Future Directions
The POPCaP network and its partnership with VA clinical and research efforts is anticipated to provide important insights into barriers and solutions to the implementation of precision oncology for prostate cancer across the VA. These lessons learned may also be relevant for precision oncology care in other settings. As an example, the role of germline testing and genetic counseling is growing more relevant in precision oncology, yet it is clear that the number of men and women dealing with malignancy who actually receive counseling and testing is suboptimal in most health care systems.14 Optimizing the quality and efficiency of oncogenetics within the VA system in a manner that gives access to these services for every veteran in urban or rural environments is an important goal.
The VA has done extensive work in teleoncology and the Genomic Medicine Service provides telehealth genetic counseling service to 90 VA medical facilities nationwide. Expanding on this model to create a distributed network system across the country is an opportunity that will continue to raise VA profile as a leader in this area while providing increased access to genetic services.
Finally, the clinical trials network within POPCaP already has provided valuable insights into how research efforts that originate within the VA can leverage the VA’s strengths. The use of the NPOP centralized sequencing platform to identify potentially targetable alterations across medical centers provides the potential to bring critical access to research to veterans where they live through virtual clinical trials. The VA has a centralized institutional review board that can service large multisite study participation efficiently across the VA. The promise of virtual clinical trials to interrogate relatively rare biomarkers would benefit from institution of a virtual clinical trials workflow. In theory patients with a potentially targetable biomarker could be identified through the centralized DNA sequencing platform and a clinical trial team of virtual investigators and research coordinators would work with health care providers at sites for study startup and performance. Efforts to design and implement this approach are actively being pursued.
The goal of the VA/PCF POPCaP network is to make certain that every veteran has access to appropriate genetic and genomic testing and that the results are utilized so that veterans with targetable alterations receive the best clinical care and have access to clinical trials that could benefit them individually while advancing knowledge that benefits all.
1. Montgomery B, Williams C. Prostate cancer federal health care data trends. https://www.mdedge.com/fedprac/article/208077/oncology/prostate-cancer-federal-health-care-data-trends. Published September 1, 2019. Accessed July 16, 2020.
2. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
3. Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer [published correction appears in Cell. 2018 Oct 18;175(3):889]. Cell. 2018;174(3):758-769.e9. doi:10.1016/j.cell.2018.06.039
4. Pritchard CC, Offit K, Nelson PS. DNA-repair gene mutations in metastatic prostate cancer. N Engl J Med. 2016;375(18):1804-1805. doi:10.1056/NEJMc1611137
5. Guillem JG. Molecular diagnosis of hereditary nonpolyposis colon cancer. N Engl J Med. 1998;339(13):924-925. doi:10.1056/nejm199809243391316
6. Ryan S, Jenkins MA, Win AK. Risk of prostate cancer in Lynch syndrome: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(3):437-449. doi:10.1158/1055-9965.EPI-13-1165
7. Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471-478. doi:10.1001/jamaoncol.2018.5801
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. Published 2020 May 26. doi:10.1371/journal.pone.0233260
9. Yu HA, Planchard D, Lovly CM. Sequencing therapy for genetically defined subgroups of non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2018;38:726-739. doi:10.1200/EDBK_201331
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917-921. doi:10.1038/nature03445
12. Abida W, Campbell D, Patnaik A, et al. Preliminary results from the TRITON2 study of rucaparib in patients with DNA damage repair deficiency metastatic, castration resistant prostate cancer: updated analyses. Ann Oncol. 2019;30(suppl 5): v325-v355. doi:10.1093/annonc/mdz248
13. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
14. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(suppl 4):S62-S67. doi: 10.12788/fp.0028
The US Department of Veterans Affairs (VA) is home to the Veterans Health Administration (VHA), which delivers care at 1,255 health care facilities, including 170 medical centers. The VA serves 6 million veterans each year and is the largest integrated provider of cancer care in the US. The system uses a single, enterprise-wide electronic health record. The detailed curation of clinical outcomes, laboratory results, and radiology is used in VA efforts to improve oncology outcomes for veterans. The VA also has a National Precision Oncology Program (NPOP), which offers system-wide DNA sequencing for veterans with cancer. Given its size, integration, and capabilities, the VA is an ideal setting for rapid learning cycles of testing and implementing best practices at scale.
Prostate cancer is the most common malignancy affecting men in the US. It is the most commonly-diagnosed solid tumor in the VA, and in 2014, there were 11,376 prostate cancer diagnoses in the VA.1 The clinical characteristics and treatment of veterans with prostate cancer largely parallel the broader population of men in the US.1 Although the majority of men diagnosed with prostate cancer have disease localized to the prostate, an important minority develop metastatic disease, which represents a risk for substantial morbidity and is the lethal form of the disease. Research has yielded transformative advances in the care of men with metastatic prostate cancer, including drugs targeting the testosterone/androgen signaling axis, taxane chemotherapy, the radionuclide radium-223, and a dendritic cell vaccine. Unfortunately, the magnitude and duration of response to these therapies varies widely, and determining the biology relevant to an individual patient that would better inform their treatment decisions is a critical next step. As the ability to interrogate the cancer genome has improved, relevant drivers of tumorigenesis and predictive biomarkers are being identified rapidly, and oncology care has evolved from a one-size-fits-all approach to a precision approach, which uses these biomarkers to assist in therapeutic decision making.
Precision Oncology for Prostate Cancer
A series of studies interrogating the genomics of metastatic prostate cancer have been critical to defining the relevance of precision oncology for prostate cancer. Most of what is known about the genomics of prostate cancer has been derived from analysis of samples from the prostate itself. These samples may not reflect the biology of metastasis and genetic evolution in response to treatment pressure, so the genomic alterations in metastatic disease remained incompletely characterized. Two large research teams supported by grants from the American Association for Cancer Research, Stand Up 2 Cancer, and Prostate Cancer Foundation (PCF) focused their efforts on sampling and analyzing metastatic tissue to define the most relevant genomic alterations in advanced prostate cancer.
These efforts defined a broad range of relatively common alterations in the androgen receptor, as well as the tumor suppressors TP53 and PTEN.2,3 Important subsets of less common alterations in pathways that were potentially targetable were also found, including new alterations in PIK3CA/B, BRAF/RAF1, and β-catenin. Most surprisingly, alterations of DNA repair pathways, including mismatch repair and homologous recombination were found in 20% of tumors, and half of these tumors contained germline alterations. The same groups performed a follow up analysis of germline DNA from men with metastatic prostate cancer, which confirmed that 12% of these patients carry a pathogenic germline alteration in a DNA repair pathway gene.4 These efforts immediately invigorated precision oncology clinical trials for prostate cancer and spurred an effort to find the molecular alterations that could be leveraged to improve care for men with advanced prostate cancer.
Targetable Alterations
Currently a number of genomic alterations are immediately actionable. There are several agents approved by the US Food and Drug Administration (FDA) that exploit these Achilles heels of prostate cancer. Mismatch repair deficiency occurs when any of a group of genes responsible for proofreading the fidelity of DNA replication is compromised by mutation or deletion. Imperfect reading and correction subsequently lead to many DNA mutations in a tissue (hypermutation), which then increases the risk of developing malignancy. If a defective gene in the mismatch repair pathway is inherited, a patient has a genetic predisposition to specific malignancies that are part of the Lynch syndrome.5 Prostate cancer is a relatively rare manifestation of Lynch syndrome, although it is considered one of the malignancies in the Lynch syndrome spectrum.6
Alteration of one of the mismatch repair genes also can occur spontaneously in a tumor, resulting in the same high frequency of spontaneous DNA mutations. Overall, between 3% and 5% of metastatic prostate cancers contain mismatch repair deficiency. The majority of these cases are a result of spontaneous loss or mutation of the relevant gene, but 1 in 5 of these tumors occurs as a component of Lynch syndrome.7 Identification of mismatch repair deficiency is critical because the resulting hypermutation makes these tumors particularly susceptible to intervention with immunotherapy. Up to half of patients with metastatic prostate cancer can have durable responses. This finding is consistent with the experience treating other malignancies with mismatch repair deficiency.8 Although screening for mismatch repair deficiency is standard of care for patients with malignancies such as colorectal cancer, few patients with prostate cancer may receive the mismatch repair deficiency screening (based on unpublished data). In contrast, screening is routine for patients with adenocarcinoma of the lung because their proportion of ROS1 and ALK alterations is similar to the frequency of mismatch repair deficiency when compared with patients with prostate cancer.9
Homologous recombination is another mechanism by which cells repair DNA damage and is responsible for repairing double strand breaks, the type of DNA damage most likely to lead to carcinogenesis. In advanced prostate cancer, BRCA2, ATM, BRCA1 and other members of the Fanconi Anemia/BRCA gene family are altered 20% of the time. These genes also are the most common germline alterations implicated in the development of prostate cancer.2,10 Prostate cancer is considered a BRCA-related cancer much like breast, ovarian, and pancreatic cancers. Defects in homologous recombination repair make BRCA-altered prostate cancers susceptible to DNA damaging chemotherapy, such as platinum and to the use of poly–(adenosine diphosphate–ribose) polymerase (PARP) inhibitors because cancer cells then accumulate cytotoxic and apoptotic levels of DNA.11
In May 2020, the FDA approved the use of PARP inhibitors for the treatment of prostate cancers that contain BRCA and other DNA repair alterations. Rucaparib received accelerated approval for the treatment of prostate cancers containing BRCA alterations and olaparib received full approval for treatment of prostate cancers containing an array of alterations in DNA repair genes.12,13 Both approvals were the direct result of the cited landmark studies that demonstrated the frequency of these alterations in advanced prostate cancer.2,3
Beyond mismatch and homologous recombination repair, there are a large number of potentially targetable alterations found in advanced prostate cancer. It is thus critical that we put systems into place both to find germline and somatic alterations that will inform a veteran’s clinical care and to provide veterans access to precision oncology clinical trials.
The POPCaP Network
Because prostate cancer is such a significant issue in the VA and best practices for precision oncology can be implemented broadly once defined as successful, the PCF and the VA formed a collaboration to support a network of centers that would focus on implementing a comprehensive strategy for precision oncology in prostate cancer. There are currently 11 centers in the Precision Oncology Program for Cancer of the Prostate (POPCaP) network (Figure). These centers are tasked with comprehensively sequencing germline and somatic tissue from veterans with metastatic prostate cancer to find alterations, which could provide access to treatments that would otherwise not be available or appropriate.
The network is collaborating with NPOP, which provides clinical grade tumor gene panel sequencing for veterans with prostate cancer from > 90% of VA medical centers. POPCaP also partners with the University of Washington to use its OncoPlex gene panel and University of Michigan to use the Oncomine panel to define the best platform for defining targetable alterations for veterans with prostate cancer. Investigators participate in a monthly molecular oncology tumor board continuing medical education-accredited program, which provides guidance and education across the VA about the evidence available to assist in decision making for veterans sequenced through NPOP and the academic platforms. These efforts leverage VA’s partnership with IBM Watson for Genomics to annotate DNA sequencing results to provide clinicians with potential therapeutic options for veterans.
A clinical trials mechanism is embedded in POPCaP to broaden treatment options, improve care for men with prostate cancer, and leverage the sequencing efforts in the network. The Prostate Cancer Analysis for Therapy Choice (PATCH) clinical trials network employs an umbrella study approach whereby alterations are identified through sequencing and veterans are given access to studies embedded at sites across the network. Graff and Huang provide a detailed description of the PATCH network and its potential as a multisite clinical trials mechanism.14 For studies within the network, funds can be provided to support travel to participate in clinical trials for veterans who would be eligible for study but do not live in a catchment for a network site. POPCaP also leverages both the resources of the National Cancer Institute (NCI)-designated cancer centers that are VA academic affiliates, as well as a VA/NCI partnership (NAVIGATE) to increase veteran access to NCI cutting-edge clinical trials.
The network has regular teleconference meetings of the investigators, coordinators, and stakeholders and face-to-face meetings, which are coordinated around other national meetings. These meetings enable investigators to work collaboratively to advance current knowledge in prostate cancer through the application of complementary and synergistic research approaches. Since research plays a critical role within the learning health care system, POPCaP investigators are working to optimize the transfer of knowledge from the clinic to the bench and back to the clinic. In this regard, investigators from network sites have organized themselves into working groups to focus on multiple critical aspects of research and care within the network, including sequencing, phenotyping, health services, health disparities, and a network biorepository.
VA Office of Research and Development
With support from the VA Office of Research and Development, there are research efforts focused on the development of data analytics to identify veterans with metastatic prostate cancer within the electronic health record to ensure access to appropriate testing, treatment, and clinical trials. This will optimize tracking and continuous quality improvement in precision oncology. The Office of Research and Development also supports the use of artificial intelligence to identify predictive markers for diagnosis, prognosis, therapeutic response and patient stratification. POPCaP investigators, along with other investigators from across the VA, conduct research that continually improves the care of veterans with prostate cancer. POPCaP has a special focus on prostate cancer among African Americans, who are disproportionately affected by the disease and well represented in VA. The efforts of the working groups, the research studies and the network as a whole also serve to recruit both junior and senior investigators to the VA in order to support the VA research enterprise.
Active collaborations between the network and other elements of VA include efforts to optimize germline testing and genetic counseling in prostate cancer through the Genomic Medicine Service, which provides telehealth genetic counseling throughout the VA. POPCaP pilots innovative approaches to increase access to clinical genetics and genetic counseling services to support the volume of genetic testing of veterans with cancer. Current National Comprehensive Cancer Network (NCCN) guidelines recommend germline testing for all men with metastatic prostate cancer, which can efficiently identify the roughly 10% of veterans with metastatic disease who carry a germline alteration and provide them with access to studies, FDA-approved treatments, while also offering critical health care information to family members who may also carry a pathogenic germline alteration.
Million Veteran Program
The Million Veteran Program (MVP) has collected > 825,000 germline DNA samples from an anticipated enrollment of > 1 million veterans in one of the most ambitious genetic research efforts to correlate how germline DNA interacts with lifestyle, medications and military exposure to affect health and illness (www.research.va.gov/mvp). MVP is a racially and ethnically diverse veteran cohort that is roughly 20% African American and 7% Hispanic. More than 40,000 of the participants have had prostate cancer, one third of whom are African Americans, giving researchers unprecedented ability to discover factors that impact the development and treatment of the disease in this population. In particular, MVP will provide unique insights into the genetic mutations that drive the development of aggressive prostate cancer in all male veterans, including African Americans. These discoveries will undoubtedly lead to improved screening of and treatment for prostate cancer.
In order to demonstrate clinical utility as well as the infrastructure needs to scale up within the VHA, MVP has launched a pilot project that offers to return clinically actionable genetic results to MVP participants with metastatic prostate cancer, opening the door to new therapies to improve the length and quality of these veterans’ lives. Importantly, the pilot includes cascade testing in family members of enrolled veterans. Given that the original MVP consent did not allow for return of results, and MVP genetic testing is research grade, veterans who volunteer will provide a second consent and undergo clinical genetic testing to confirm the variants. Results from this pilot study also will inform expansion of VA precision oncology efforts for patients with other cancers such as breast cancer or ovarian cancer, where the specific genetic mutations are known to play a role, (eg, BRCA2). In addition, through an interagency agreement with the US Department of Energy (DOE), MVP is leveraging DOE expertise and high-performance computing capabilities to identify clinical and genetic risk factors for prostate cancer that will progress to metastatic disease.
This active research collaboration between POPCaP, MVP, and the Genomic Medicine Service will identify germline BRCA alterations from MVP participants with metastatic prostate cancer and give them access to therapies that may provide better outcomes and access to genetic testing for their family members.
Future Directions
The POPCaP network and its partnership with VA clinical and research efforts is anticipated to provide important insights into barriers and solutions to the implementation of precision oncology for prostate cancer across the VA. These lessons learned may also be relevant for precision oncology care in other settings. As an example, the role of germline testing and genetic counseling is growing more relevant in precision oncology, yet it is clear that the number of men and women dealing with malignancy who actually receive counseling and testing is suboptimal in most health care systems.14 Optimizing the quality and efficiency of oncogenetics within the VA system in a manner that gives access to these services for every veteran in urban or rural environments is an important goal.
The VA has done extensive work in teleoncology and the Genomic Medicine Service provides telehealth genetic counseling service to 90 VA medical facilities nationwide. Expanding on this model to create a distributed network system across the country is an opportunity that will continue to raise VA profile as a leader in this area while providing increased access to genetic services.
Finally, the clinical trials network within POPCaP already has provided valuable insights into how research efforts that originate within the VA can leverage the VA’s strengths. The use of the NPOP centralized sequencing platform to identify potentially targetable alterations across medical centers provides the potential to bring critical access to research to veterans where they live through virtual clinical trials. The VA has a centralized institutional review board that can service large multisite study participation efficiently across the VA. The promise of virtual clinical trials to interrogate relatively rare biomarkers would benefit from institution of a virtual clinical trials workflow. In theory patients with a potentially targetable biomarker could be identified through the centralized DNA sequencing platform and a clinical trial team of virtual investigators and research coordinators would work with health care providers at sites for study startup and performance. Efforts to design and implement this approach are actively being pursued.
The goal of the VA/PCF POPCaP network is to make certain that every veteran has access to appropriate genetic and genomic testing and that the results are utilized so that veterans with targetable alterations receive the best clinical care and have access to clinical trials that could benefit them individually while advancing knowledge that benefits all.
The US Department of Veterans Affairs (VA) is home to the Veterans Health Administration (VHA), which delivers care at 1,255 health care facilities, including 170 medical centers. The VA serves 6 million veterans each year and is the largest integrated provider of cancer care in the US. The system uses a single, enterprise-wide electronic health record. The detailed curation of clinical outcomes, laboratory results, and radiology is used in VA efforts to improve oncology outcomes for veterans. The VA also has a National Precision Oncology Program (NPOP), which offers system-wide DNA sequencing for veterans with cancer. Given its size, integration, and capabilities, the VA is an ideal setting for rapid learning cycles of testing and implementing best practices at scale.
Prostate cancer is the most common malignancy affecting men in the US. It is the most commonly-diagnosed solid tumor in the VA, and in 2014, there were 11,376 prostate cancer diagnoses in the VA.1 The clinical characteristics and treatment of veterans with prostate cancer largely parallel the broader population of men in the US.1 Although the majority of men diagnosed with prostate cancer have disease localized to the prostate, an important minority develop metastatic disease, which represents a risk for substantial morbidity and is the lethal form of the disease. Research has yielded transformative advances in the care of men with metastatic prostate cancer, including drugs targeting the testosterone/androgen signaling axis, taxane chemotherapy, the radionuclide radium-223, and a dendritic cell vaccine. Unfortunately, the magnitude and duration of response to these therapies varies widely, and determining the biology relevant to an individual patient that would better inform their treatment decisions is a critical next step. As the ability to interrogate the cancer genome has improved, relevant drivers of tumorigenesis and predictive biomarkers are being identified rapidly, and oncology care has evolved from a one-size-fits-all approach to a precision approach, which uses these biomarkers to assist in therapeutic decision making.
Precision Oncology for Prostate Cancer
A series of studies interrogating the genomics of metastatic prostate cancer have been critical to defining the relevance of precision oncology for prostate cancer. Most of what is known about the genomics of prostate cancer has been derived from analysis of samples from the prostate itself. These samples may not reflect the biology of metastasis and genetic evolution in response to treatment pressure, so the genomic alterations in metastatic disease remained incompletely characterized. Two large research teams supported by grants from the American Association for Cancer Research, Stand Up 2 Cancer, and Prostate Cancer Foundation (PCF) focused their efforts on sampling and analyzing metastatic tissue to define the most relevant genomic alterations in advanced prostate cancer.
These efforts defined a broad range of relatively common alterations in the androgen receptor, as well as the tumor suppressors TP53 and PTEN.2,3 Important subsets of less common alterations in pathways that were potentially targetable were also found, including new alterations in PIK3CA/B, BRAF/RAF1, and β-catenin. Most surprisingly, alterations of DNA repair pathways, including mismatch repair and homologous recombination were found in 20% of tumors, and half of these tumors contained germline alterations. The same groups performed a follow up analysis of germline DNA from men with metastatic prostate cancer, which confirmed that 12% of these patients carry a pathogenic germline alteration in a DNA repair pathway gene.4 These efforts immediately invigorated precision oncology clinical trials for prostate cancer and spurred an effort to find the molecular alterations that could be leveraged to improve care for men with advanced prostate cancer.
Targetable Alterations
Currently a number of genomic alterations are immediately actionable. There are several agents approved by the US Food and Drug Administration (FDA) that exploit these Achilles heels of prostate cancer. Mismatch repair deficiency occurs when any of a group of genes responsible for proofreading the fidelity of DNA replication is compromised by mutation or deletion. Imperfect reading and correction subsequently lead to many DNA mutations in a tissue (hypermutation), which then increases the risk of developing malignancy. If a defective gene in the mismatch repair pathway is inherited, a patient has a genetic predisposition to specific malignancies that are part of the Lynch syndrome.5 Prostate cancer is a relatively rare manifestation of Lynch syndrome, although it is considered one of the malignancies in the Lynch syndrome spectrum.6
Alteration of one of the mismatch repair genes also can occur spontaneously in a tumor, resulting in the same high frequency of spontaneous DNA mutations. Overall, between 3% and 5% of metastatic prostate cancers contain mismatch repair deficiency. The majority of these cases are a result of spontaneous loss or mutation of the relevant gene, but 1 in 5 of these tumors occurs as a component of Lynch syndrome.7 Identification of mismatch repair deficiency is critical because the resulting hypermutation makes these tumors particularly susceptible to intervention with immunotherapy. Up to half of patients with metastatic prostate cancer can have durable responses. This finding is consistent with the experience treating other malignancies with mismatch repair deficiency.8 Although screening for mismatch repair deficiency is standard of care for patients with malignancies such as colorectal cancer, few patients with prostate cancer may receive the mismatch repair deficiency screening (based on unpublished data). In contrast, screening is routine for patients with adenocarcinoma of the lung because their proportion of ROS1 and ALK alterations is similar to the frequency of mismatch repair deficiency when compared with patients with prostate cancer.9
Homologous recombination is another mechanism by which cells repair DNA damage and is responsible for repairing double strand breaks, the type of DNA damage most likely to lead to carcinogenesis. In advanced prostate cancer, BRCA2, ATM, BRCA1 and other members of the Fanconi Anemia/BRCA gene family are altered 20% of the time. These genes also are the most common germline alterations implicated in the development of prostate cancer.2,10 Prostate cancer is considered a BRCA-related cancer much like breast, ovarian, and pancreatic cancers. Defects in homologous recombination repair make BRCA-altered prostate cancers susceptible to DNA damaging chemotherapy, such as platinum and to the use of poly–(adenosine diphosphate–ribose) polymerase (PARP) inhibitors because cancer cells then accumulate cytotoxic and apoptotic levels of DNA.11
In May 2020, the FDA approved the use of PARP inhibitors for the treatment of prostate cancers that contain BRCA and other DNA repair alterations. Rucaparib received accelerated approval for the treatment of prostate cancers containing BRCA alterations and olaparib received full approval for treatment of prostate cancers containing an array of alterations in DNA repair genes.12,13 Both approvals were the direct result of the cited landmark studies that demonstrated the frequency of these alterations in advanced prostate cancer.2,3
Beyond mismatch and homologous recombination repair, there are a large number of potentially targetable alterations found in advanced prostate cancer. It is thus critical that we put systems into place both to find germline and somatic alterations that will inform a veteran’s clinical care and to provide veterans access to precision oncology clinical trials.
The POPCaP Network
Because prostate cancer is such a significant issue in the VA and best practices for precision oncology can be implemented broadly once defined as successful, the PCF and the VA formed a collaboration to support a network of centers that would focus on implementing a comprehensive strategy for precision oncology in prostate cancer. There are currently 11 centers in the Precision Oncology Program for Cancer of the Prostate (POPCaP) network (Figure). These centers are tasked with comprehensively sequencing germline and somatic tissue from veterans with metastatic prostate cancer to find alterations, which could provide access to treatments that would otherwise not be available or appropriate.
The network is collaborating with NPOP, which provides clinical grade tumor gene panel sequencing for veterans with prostate cancer from > 90% of VA medical centers. POPCaP also partners with the University of Washington to use its OncoPlex gene panel and University of Michigan to use the Oncomine panel to define the best platform for defining targetable alterations for veterans with prostate cancer. Investigators participate in a monthly molecular oncology tumor board continuing medical education-accredited program, which provides guidance and education across the VA about the evidence available to assist in decision making for veterans sequenced through NPOP and the academic platforms. These efforts leverage VA’s partnership with IBM Watson for Genomics to annotate DNA sequencing results to provide clinicians with potential therapeutic options for veterans.
A clinical trials mechanism is embedded in POPCaP to broaden treatment options, improve care for men with prostate cancer, and leverage the sequencing efforts in the network. The Prostate Cancer Analysis for Therapy Choice (PATCH) clinical trials network employs an umbrella study approach whereby alterations are identified through sequencing and veterans are given access to studies embedded at sites across the network. Graff and Huang provide a detailed description of the PATCH network and its potential as a multisite clinical trials mechanism.14 For studies within the network, funds can be provided to support travel to participate in clinical trials for veterans who would be eligible for study but do not live in a catchment for a network site. POPCaP also leverages both the resources of the National Cancer Institute (NCI)-designated cancer centers that are VA academic affiliates, as well as a VA/NCI partnership (NAVIGATE) to increase veteran access to NCI cutting-edge clinical trials.
The network has regular teleconference meetings of the investigators, coordinators, and stakeholders and face-to-face meetings, which are coordinated around other national meetings. These meetings enable investigators to work collaboratively to advance current knowledge in prostate cancer through the application of complementary and synergistic research approaches. Since research plays a critical role within the learning health care system, POPCaP investigators are working to optimize the transfer of knowledge from the clinic to the bench and back to the clinic. In this regard, investigators from network sites have organized themselves into working groups to focus on multiple critical aspects of research and care within the network, including sequencing, phenotyping, health services, health disparities, and a network biorepository.
VA Office of Research and Development
With support from the VA Office of Research and Development, there are research efforts focused on the development of data analytics to identify veterans with metastatic prostate cancer within the electronic health record to ensure access to appropriate testing, treatment, and clinical trials. This will optimize tracking and continuous quality improvement in precision oncology. The Office of Research and Development also supports the use of artificial intelligence to identify predictive markers for diagnosis, prognosis, therapeutic response and patient stratification. POPCaP investigators, along with other investigators from across the VA, conduct research that continually improves the care of veterans with prostate cancer. POPCaP has a special focus on prostate cancer among African Americans, who are disproportionately affected by the disease and well represented in VA. The efforts of the working groups, the research studies and the network as a whole also serve to recruit both junior and senior investigators to the VA in order to support the VA research enterprise.
Active collaborations between the network and other elements of VA include efforts to optimize germline testing and genetic counseling in prostate cancer through the Genomic Medicine Service, which provides telehealth genetic counseling throughout the VA. POPCaP pilots innovative approaches to increase access to clinical genetics and genetic counseling services to support the volume of genetic testing of veterans with cancer. Current National Comprehensive Cancer Network (NCCN) guidelines recommend germline testing for all men with metastatic prostate cancer, which can efficiently identify the roughly 10% of veterans with metastatic disease who carry a germline alteration and provide them with access to studies, FDA-approved treatments, while also offering critical health care information to family members who may also carry a pathogenic germline alteration.
Million Veteran Program
The Million Veteran Program (MVP) has collected > 825,000 germline DNA samples from an anticipated enrollment of > 1 million veterans in one of the most ambitious genetic research efforts to correlate how germline DNA interacts with lifestyle, medications and military exposure to affect health and illness (www.research.va.gov/mvp). MVP is a racially and ethnically diverse veteran cohort that is roughly 20% African American and 7% Hispanic. More than 40,000 of the participants have had prostate cancer, one third of whom are African Americans, giving researchers unprecedented ability to discover factors that impact the development and treatment of the disease in this population. In particular, MVP will provide unique insights into the genetic mutations that drive the development of aggressive prostate cancer in all male veterans, including African Americans. These discoveries will undoubtedly lead to improved screening of and treatment for prostate cancer.
In order to demonstrate clinical utility as well as the infrastructure needs to scale up within the VHA, MVP has launched a pilot project that offers to return clinically actionable genetic results to MVP participants with metastatic prostate cancer, opening the door to new therapies to improve the length and quality of these veterans’ lives. Importantly, the pilot includes cascade testing in family members of enrolled veterans. Given that the original MVP consent did not allow for return of results, and MVP genetic testing is research grade, veterans who volunteer will provide a second consent and undergo clinical genetic testing to confirm the variants. Results from this pilot study also will inform expansion of VA precision oncology efforts for patients with other cancers such as breast cancer or ovarian cancer, where the specific genetic mutations are known to play a role, (eg, BRCA2). In addition, through an interagency agreement with the US Department of Energy (DOE), MVP is leveraging DOE expertise and high-performance computing capabilities to identify clinical and genetic risk factors for prostate cancer that will progress to metastatic disease.
This active research collaboration between POPCaP, MVP, and the Genomic Medicine Service will identify germline BRCA alterations from MVP participants with metastatic prostate cancer and give them access to therapies that may provide better outcomes and access to genetic testing for their family members.
Future Directions
The POPCaP network and its partnership with VA clinical and research efforts is anticipated to provide important insights into barriers and solutions to the implementation of precision oncology for prostate cancer across the VA. These lessons learned may also be relevant for precision oncology care in other settings. As an example, the role of germline testing and genetic counseling is growing more relevant in precision oncology, yet it is clear that the number of men and women dealing with malignancy who actually receive counseling and testing is suboptimal in most health care systems.14 Optimizing the quality and efficiency of oncogenetics within the VA system in a manner that gives access to these services for every veteran in urban or rural environments is an important goal.
The VA has done extensive work in teleoncology and the Genomic Medicine Service provides telehealth genetic counseling service to 90 VA medical facilities nationwide. Expanding on this model to create a distributed network system across the country is an opportunity that will continue to raise VA profile as a leader in this area while providing increased access to genetic services.
Finally, the clinical trials network within POPCaP already has provided valuable insights into how research efforts that originate within the VA can leverage the VA’s strengths. The use of the NPOP centralized sequencing platform to identify potentially targetable alterations across medical centers provides the potential to bring critical access to research to veterans where they live through virtual clinical trials. The VA has a centralized institutional review board that can service large multisite study participation efficiently across the VA. The promise of virtual clinical trials to interrogate relatively rare biomarkers would benefit from institution of a virtual clinical trials workflow. In theory patients with a potentially targetable biomarker could be identified through the centralized DNA sequencing platform and a clinical trial team of virtual investigators and research coordinators would work with health care providers at sites for study startup and performance. Efforts to design and implement this approach are actively being pursued.
The goal of the VA/PCF POPCaP network is to make certain that every veteran has access to appropriate genetic and genomic testing and that the results are utilized so that veterans with targetable alterations receive the best clinical care and have access to clinical trials that could benefit them individually while advancing knowledge that benefits all.
1. Montgomery B, Williams C. Prostate cancer federal health care data trends. https://www.mdedge.com/fedprac/article/208077/oncology/prostate-cancer-federal-health-care-data-trends. Published September 1, 2019. Accessed July 16, 2020.
2. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
3. Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer [published correction appears in Cell. 2018 Oct 18;175(3):889]. Cell. 2018;174(3):758-769.e9. doi:10.1016/j.cell.2018.06.039
4. Pritchard CC, Offit K, Nelson PS. DNA-repair gene mutations in metastatic prostate cancer. N Engl J Med. 2016;375(18):1804-1805. doi:10.1056/NEJMc1611137
5. Guillem JG. Molecular diagnosis of hereditary nonpolyposis colon cancer. N Engl J Med. 1998;339(13):924-925. doi:10.1056/nejm199809243391316
6. Ryan S, Jenkins MA, Win AK. Risk of prostate cancer in Lynch syndrome: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(3):437-449. doi:10.1158/1055-9965.EPI-13-1165
7. Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471-478. doi:10.1001/jamaoncol.2018.5801
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. Published 2020 May 26. doi:10.1371/journal.pone.0233260
9. Yu HA, Planchard D, Lovly CM. Sequencing therapy for genetically defined subgroups of non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2018;38:726-739. doi:10.1200/EDBK_201331
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917-921. doi:10.1038/nature03445
12. Abida W, Campbell D, Patnaik A, et al. Preliminary results from the TRITON2 study of rucaparib in patients with DNA damage repair deficiency metastatic, castration resistant prostate cancer: updated analyses. Ann Oncol. 2019;30(suppl 5): v325-v355. doi:10.1093/annonc/mdz248
13. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
14. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(suppl 4):S62-S67. doi: 10.12788/fp.0028
1. Montgomery B, Williams C. Prostate cancer federal health care data trends. https://www.mdedge.com/fedprac/article/208077/oncology/prostate-cancer-federal-health-care-data-trends. Published September 1, 2019. Accessed July 16, 2020.
2. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
3. Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer [published correction appears in Cell. 2018 Oct 18;175(3):889]. Cell. 2018;174(3):758-769.e9. doi:10.1016/j.cell.2018.06.039
4. Pritchard CC, Offit K, Nelson PS. DNA-repair gene mutations in metastatic prostate cancer. N Engl J Med. 2016;375(18):1804-1805. doi:10.1056/NEJMc1611137
5. Guillem JG. Molecular diagnosis of hereditary nonpolyposis colon cancer. N Engl J Med. 1998;339(13):924-925. doi:10.1056/nejm199809243391316
6. Ryan S, Jenkins MA, Win AK. Risk of prostate cancer in Lynch syndrome: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(3):437-449. doi:10.1158/1055-9965.EPI-13-1165
7. Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471-478. doi:10.1001/jamaoncol.2018.5801
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. Published 2020 May 26. doi:10.1371/journal.pone.0233260
9. Yu HA, Planchard D, Lovly CM. Sequencing therapy for genetically defined subgroups of non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2018;38:726-739. doi:10.1200/EDBK_201331
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917-921. doi:10.1038/nature03445
12. Abida W, Campbell D, Patnaik A, et al. Preliminary results from the TRITON2 study of rucaparib in patients with DNA damage repair deficiency metastatic, castration resistant prostate cancer: updated analyses. Ann Oncol. 2019;30(suppl 5): v325-v355. doi:10.1093/annonc/mdz248
13. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
14. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(suppl 4):S62-S67. doi: 10.12788/fp.0028
Clinical Edge Journal Scan Commentary: Prostate Cancer August 2021
Varying strategies have been evaluated to diagnose and predict risk of prostate cancer more optimally. The presence of local or systemic inflammation has been postulated to be associated with increased risk of the presence and aggressiveness of prostate cancer. Complete blood counts with differential white counts are routinely obtained, and the ratio of platelets to lymphocytes (PLR), neutrophils to lymphocytes (NLR), and monocytes to lymphocytes (MLR) have been proposed as markers of inflammation. In the study by Lee et al., the authors evaluated whether the PLR was associated with benign prostate disease, clinically insignificant prostate cancer (Gleason 6 or lower), or clinically significant prostate cancer (Gleason 7 or higher) in patients undergoing prostate biopsy. In a cohort of 1652 who underwent biopsy, the only significant finding was a lower PLR in patients with clinically significant prostate cancer and a PSA < 10 ng/mL compared with patients having benign disease or clinically insignificant disease.
Rundle et al evaluated whether NLR or MLR in the setting of cancer varied by race, as African American and Black men are at higher risk for prostate cancer than White men. In this case-control study that included 822 cases of prostate cancer and 573 controls, there were no significant differences in the trajectories of NLR or MLR over time between cases or controls in the entire cohort. The NLR and MLR were higher over time for White men without prostate cancer compared with White men with prostate cancer; however, no such difference was identified when comparing Black men with or without prostate cancer. Thus, while there may be some association between inflammation and prostate cancer diagnoses or risk of aggressiveness, the use of PLR, NLR, or MLR requires much more study to fully determine the usefulness of these ratios.
A slightly different approach to improve diagnostic strategies has been to utilize prostate MRI to identify areas of the prostate suggestive of cancer. Eklund et al evaluated the use of MRI in the setting of an organized prostate screening program. The use of MRI with targeted and standard biopsy was noninferior to standard biopsy alone in detecting clinically significant prostate cancer while fewer clinically insignificant prostate cancers were identified in the MRI with targeted and standard biopsy group compared with the standard biopsy group. This study further supports the increasing utilization of MRI as part of a biopsy approach in men at risk for prostate cancer.
Varying strategies have been evaluated to diagnose and predict risk of prostate cancer more optimally. The presence of local or systemic inflammation has been postulated to be associated with increased risk of the presence and aggressiveness of prostate cancer. Complete blood counts with differential white counts are routinely obtained, and the ratio of platelets to lymphocytes (PLR), neutrophils to lymphocytes (NLR), and monocytes to lymphocytes (MLR) have been proposed as markers of inflammation. In the study by Lee et al., the authors evaluated whether the PLR was associated with benign prostate disease, clinically insignificant prostate cancer (Gleason 6 or lower), or clinically significant prostate cancer (Gleason 7 or higher) in patients undergoing prostate biopsy. In a cohort of 1652 who underwent biopsy, the only significant finding was a lower PLR in patients with clinically significant prostate cancer and a PSA < 10 ng/mL compared with patients having benign disease or clinically insignificant disease.
Rundle et al evaluated whether NLR or MLR in the setting of cancer varied by race, as African American and Black men are at higher risk for prostate cancer than White men. In this case-control study that included 822 cases of prostate cancer and 573 controls, there were no significant differences in the trajectories of NLR or MLR over time between cases or controls in the entire cohort. The NLR and MLR were higher over time for White men without prostate cancer compared with White men with prostate cancer; however, no such difference was identified when comparing Black men with or without prostate cancer. Thus, while there may be some association between inflammation and prostate cancer diagnoses or risk of aggressiveness, the use of PLR, NLR, or MLR requires much more study to fully determine the usefulness of these ratios.
A slightly different approach to improve diagnostic strategies has been to utilize prostate MRI to identify areas of the prostate suggestive of cancer. Eklund et al evaluated the use of MRI in the setting of an organized prostate screening program. The use of MRI with targeted and standard biopsy was noninferior to standard biopsy alone in detecting clinically significant prostate cancer while fewer clinically insignificant prostate cancers were identified in the MRI with targeted and standard biopsy group compared with the standard biopsy group. This study further supports the increasing utilization of MRI as part of a biopsy approach in men at risk for prostate cancer.
Varying strategies have been evaluated to diagnose and predict risk of prostate cancer more optimally. The presence of local or systemic inflammation has been postulated to be associated with increased risk of the presence and aggressiveness of prostate cancer. Complete blood counts with differential white counts are routinely obtained, and the ratio of platelets to lymphocytes (PLR), neutrophils to lymphocytes (NLR), and monocytes to lymphocytes (MLR) have been proposed as markers of inflammation. In the study by Lee et al., the authors evaluated whether the PLR was associated with benign prostate disease, clinically insignificant prostate cancer (Gleason 6 or lower), or clinically significant prostate cancer (Gleason 7 or higher) in patients undergoing prostate biopsy. In a cohort of 1652 who underwent biopsy, the only significant finding was a lower PLR in patients with clinically significant prostate cancer and a PSA < 10 ng/mL compared with patients having benign disease or clinically insignificant disease.
Rundle et al evaluated whether NLR or MLR in the setting of cancer varied by race, as African American and Black men are at higher risk for prostate cancer than White men. In this case-control study that included 822 cases of prostate cancer and 573 controls, there were no significant differences in the trajectories of NLR or MLR over time between cases or controls in the entire cohort. The NLR and MLR were higher over time for White men without prostate cancer compared with White men with prostate cancer; however, no such difference was identified when comparing Black men with or without prostate cancer. Thus, while there may be some association between inflammation and prostate cancer diagnoses or risk of aggressiveness, the use of PLR, NLR, or MLR requires much more study to fully determine the usefulness of these ratios.
A slightly different approach to improve diagnostic strategies has been to utilize prostate MRI to identify areas of the prostate suggestive of cancer. Eklund et al evaluated the use of MRI in the setting of an organized prostate screening program. The use of MRI with targeted and standard biopsy was noninferior to standard biopsy alone in detecting clinically significant prostate cancer while fewer clinically insignificant prostate cancers were identified in the MRI with targeted and standard biopsy group compared with the standard biopsy group. This study further supports the increasing utilization of MRI as part of a biopsy approach in men at risk for prostate cancer.
Prostate Cancer: Small Cell Prostate Carcinoma-Pathology
Abdominal pain and urinary frequency
Transrectal ultrasonography (TRUS)–guided needle biopsy of the prostate confirms a diagnosis of high-grade prostate cancer, and the digital rectal exam and CT scan are concerning for extracapsular invasion. Genetic and molecular biomarker testing is recommended.
According to GLOBOCAN 2020 data, prostate cancer is the second most common type of cancer in men (second only to lung cancer) and the fifth leading cause of death globally. Compared with other races, the incidence of prostate cancer in the United States is highest in Black men, and mortality rates are more than double than those reported in White men. In its early stages, prostate cancer is often asymptomatic and has an indolent course. Locally advanced prostate cancer is a clinical scenario in which the cancer has extended beyond the prostatic capsule. It involves invasion of the pericapsular tissue, bladder neck, or seminal vesicles, without lymph node involvement or distant metastases. Biological recurrence, metastatic progression, and poor survival are associated with locally advanced prostate cancer.
In the presence of advanced disease, troublesome lower urinary tract symptoms — particularly abnormal growth of prostate cancer–induced bladder outlet obstruction — are often reported. Such symptoms have a significant impact on patients' quality of life. Other symptoms of locally advanced disease may include hematuria, pain, urinary retention, urinary incontinence, hematospermia, painful ejaculation, anejaculation, constipation, and hematochezia.
Guideline-based approaches to the management of prostate cancer begin with appropriate risk stratification based on biopsy, physical examination, and imaging evaluation. In patients with advanced prostate cancer, treatment decisions should incorporate a multidisciplinary approach and include consideration of life expectancy, comorbidities, patient preferences, and tumor characteristics. Establishing whether the patient has widely advanced disease vs locally advanced disease (clinical stage T3) is helpful for ascertaining which treatment options are available. Pain control and other supportive therapies should be optimized in cases involving advanced prostate cancer.
Androgen deprivation therapy (ADT), combined with luteinizing hormone–releasing hormone (LHRH) agonists or surgical castration, is considered first-line treatment for advanced metastatic prostate cancer. Abundant data show that ADT in advanced symptomatic metastatic prostate cancer, either in the form of surgical castration or LHRH analogues, is beneficial chiefly for palliation of symptoms. However, the combination of ADT with radical prostatectomy or radiation therapy has been shown to improve overall and cancer-specific survival in selected patients with nonmetastatic but locally advanced prostate cancer. Recently, a prospective study showed a significant improvement in urodynamic variables and International Prostate Symptom Score (IPSS) questionnaire results, including IPSS-related quality of life, in patients with advanced cancer who received ADT, although lower urinary tract symptoms persist in some patients.
For patients with metastatic hormone-sensitive prostate cancer (mHSPC), continued treatment with ADT in combination with either androgen pathway–directed therapy (abiraterone acetate plus prednisone, apalutamide, enzalutamide) or chemotherapy (docetaxel) is generally recommended. A recent meta-analysis found that the next-generation androgen receptor inhibitors abiraterone, apalutamide, and enzalutamide appear to be significantly more effective than ADT and more effective than docetaxel for mHSPC; apalutamide was the best tolerated. For selected patients with mHSPC with low-volume metastatic disease, primary radiation therapy to the prostate in combination with ADT may be offered. First-generation antiandrogens (bicalutamide, flutamide, nilutamide) in combination with LHRH agonists are not recommended for patients with mHSPC, unless needed to block testosterone flare. In addition, oral androgen pathway–directed therapy (eg, abiraterone acetate plus prednisone, apalutamide, bicalutamide, darolutamide, enzalutamide, flutamide, nilutamide) without ADT is not recommended for patients with mHSPC.
In patients with nonmetastatic castration-resistant prostate cancer (nmCRPC), darolutamide, apalutamide, and enzalutamide with continued ADT have been shown to postpone the onset of metastases and death. Unless within the context of a clinical trial, systemic chemotherapy or immunotherapy should not be offered to patients with nmCRPC.
For patients with newly diagnosed metastatic castration-resistant prostate cancer (mCRPC), continued ADT with abiraterone acetate plus prednisone, docetaxel, or enzalutamide is recommended. For patients with mCRPC who are asymptomatic or minimally symptomatic, sipuleucel-T may be offered. At present, radium-223 is the only available therapy for mCRPC that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. On the basis of results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for patients with castration-resistant prostate cancer with bone lesions. The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Complete recommendations on sequencing agents and selecting therapies for patients with advanced prostate cancer can be found in guidelines from the American Urological Association, National Comprehensive Cancer Network, and the European Association of Urology.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
Transrectal ultrasonography (TRUS)–guided needle biopsy of the prostate confirms a diagnosis of high-grade prostate cancer, and the digital rectal exam and CT scan are concerning for extracapsular invasion. Genetic and molecular biomarker testing is recommended.
According to GLOBOCAN 2020 data, prostate cancer is the second most common type of cancer in men (second only to lung cancer) and the fifth leading cause of death globally. Compared with other races, the incidence of prostate cancer in the United States is highest in Black men, and mortality rates are more than double than those reported in White men. In its early stages, prostate cancer is often asymptomatic and has an indolent course. Locally advanced prostate cancer is a clinical scenario in which the cancer has extended beyond the prostatic capsule. It involves invasion of the pericapsular tissue, bladder neck, or seminal vesicles, without lymph node involvement or distant metastases. Biological recurrence, metastatic progression, and poor survival are associated with locally advanced prostate cancer.
In the presence of advanced disease, troublesome lower urinary tract symptoms — particularly abnormal growth of prostate cancer–induced bladder outlet obstruction — are often reported. Such symptoms have a significant impact on patients' quality of life. Other symptoms of locally advanced disease may include hematuria, pain, urinary retention, urinary incontinence, hematospermia, painful ejaculation, anejaculation, constipation, and hematochezia.
Guideline-based approaches to the management of prostate cancer begin with appropriate risk stratification based on biopsy, physical examination, and imaging evaluation. In patients with advanced prostate cancer, treatment decisions should incorporate a multidisciplinary approach and include consideration of life expectancy, comorbidities, patient preferences, and tumor characteristics. Establishing whether the patient has widely advanced disease vs locally advanced disease (clinical stage T3) is helpful for ascertaining which treatment options are available. Pain control and other supportive therapies should be optimized in cases involving advanced prostate cancer.
Androgen deprivation therapy (ADT), combined with luteinizing hormone–releasing hormone (LHRH) agonists or surgical castration, is considered first-line treatment for advanced metastatic prostate cancer. Abundant data show that ADT in advanced symptomatic metastatic prostate cancer, either in the form of surgical castration or LHRH analogues, is beneficial chiefly for palliation of symptoms. However, the combination of ADT with radical prostatectomy or radiation therapy has been shown to improve overall and cancer-specific survival in selected patients with nonmetastatic but locally advanced prostate cancer. Recently, a prospective study showed a significant improvement in urodynamic variables and International Prostate Symptom Score (IPSS) questionnaire results, including IPSS-related quality of life, in patients with advanced cancer who received ADT, although lower urinary tract symptoms persist in some patients.
For patients with metastatic hormone-sensitive prostate cancer (mHSPC), continued treatment with ADT in combination with either androgen pathway–directed therapy (abiraterone acetate plus prednisone, apalutamide, enzalutamide) or chemotherapy (docetaxel) is generally recommended. A recent meta-analysis found that the next-generation androgen receptor inhibitors abiraterone, apalutamide, and enzalutamide appear to be significantly more effective than ADT and more effective than docetaxel for mHSPC; apalutamide was the best tolerated. For selected patients with mHSPC with low-volume metastatic disease, primary radiation therapy to the prostate in combination with ADT may be offered. First-generation antiandrogens (bicalutamide, flutamide, nilutamide) in combination with LHRH agonists are not recommended for patients with mHSPC, unless needed to block testosterone flare. In addition, oral androgen pathway–directed therapy (eg, abiraterone acetate plus prednisone, apalutamide, bicalutamide, darolutamide, enzalutamide, flutamide, nilutamide) without ADT is not recommended for patients with mHSPC.
In patients with nonmetastatic castration-resistant prostate cancer (nmCRPC), darolutamide, apalutamide, and enzalutamide with continued ADT have been shown to postpone the onset of metastases and death. Unless within the context of a clinical trial, systemic chemotherapy or immunotherapy should not be offered to patients with nmCRPC.
For patients with newly diagnosed metastatic castration-resistant prostate cancer (mCRPC), continued ADT with abiraterone acetate plus prednisone, docetaxel, or enzalutamide is recommended. For patients with mCRPC who are asymptomatic or minimally symptomatic, sipuleucel-T may be offered. At present, radium-223 is the only available therapy for mCRPC that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. On the basis of results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for patients with castration-resistant prostate cancer with bone lesions. The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Complete recommendations on sequencing agents and selecting therapies for patients with advanced prostate cancer can be found in guidelines from the American Urological Association, National Comprehensive Cancer Network, and the European Association of Urology.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
Transrectal ultrasonography (TRUS)–guided needle biopsy of the prostate confirms a diagnosis of high-grade prostate cancer, and the digital rectal exam and CT scan are concerning for extracapsular invasion. Genetic and molecular biomarker testing is recommended.
According to GLOBOCAN 2020 data, prostate cancer is the second most common type of cancer in men (second only to lung cancer) and the fifth leading cause of death globally. Compared with other races, the incidence of prostate cancer in the United States is highest in Black men, and mortality rates are more than double than those reported in White men. In its early stages, prostate cancer is often asymptomatic and has an indolent course. Locally advanced prostate cancer is a clinical scenario in which the cancer has extended beyond the prostatic capsule. It involves invasion of the pericapsular tissue, bladder neck, or seminal vesicles, without lymph node involvement or distant metastases. Biological recurrence, metastatic progression, and poor survival are associated with locally advanced prostate cancer.
In the presence of advanced disease, troublesome lower urinary tract symptoms — particularly abnormal growth of prostate cancer–induced bladder outlet obstruction — are often reported. Such symptoms have a significant impact on patients' quality of life. Other symptoms of locally advanced disease may include hematuria, pain, urinary retention, urinary incontinence, hematospermia, painful ejaculation, anejaculation, constipation, and hematochezia.
Guideline-based approaches to the management of prostate cancer begin with appropriate risk stratification based on biopsy, physical examination, and imaging evaluation. In patients with advanced prostate cancer, treatment decisions should incorporate a multidisciplinary approach and include consideration of life expectancy, comorbidities, patient preferences, and tumor characteristics. Establishing whether the patient has widely advanced disease vs locally advanced disease (clinical stage T3) is helpful for ascertaining which treatment options are available. Pain control and other supportive therapies should be optimized in cases involving advanced prostate cancer.
Androgen deprivation therapy (ADT), combined with luteinizing hormone–releasing hormone (LHRH) agonists or surgical castration, is considered first-line treatment for advanced metastatic prostate cancer. Abundant data show that ADT in advanced symptomatic metastatic prostate cancer, either in the form of surgical castration or LHRH analogues, is beneficial chiefly for palliation of symptoms. However, the combination of ADT with radical prostatectomy or radiation therapy has been shown to improve overall and cancer-specific survival in selected patients with nonmetastatic but locally advanced prostate cancer. Recently, a prospective study showed a significant improvement in urodynamic variables and International Prostate Symptom Score (IPSS) questionnaire results, including IPSS-related quality of life, in patients with advanced cancer who received ADT, although lower urinary tract symptoms persist in some patients.
For patients with metastatic hormone-sensitive prostate cancer (mHSPC), continued treatment with ADT in combination with either androgen pathway–directed therapy (abiraterone acetate plus prednisone, apalutamide, enzalutamide) or chemotherapy (docetaxel) is generally recommended. A recent meta-analysis found that the next-generation androgen receptor inhibitors abiraterone, apalutamide, and enzalutamide appear to be significantly more effective than ADT and more effective than docetaxel for mHSPC; apalutamide was the best tolerated. For selected patients with mHSPC with low-volume metastatic disease, primary radiation therapy to the prostate in combination with ADT may be offered. First-generation antiandrogens (bicalutamide, flutamide, nilutamide) in combination with LHRH agonists are not recommended for patients with mHSPC, unless needed to block testosterone flare. In addition, oral androgen pathway–directed therapy (eg, abiraterone acetate plus prednisone, apalutamide, bicalutamide, darolutamide, enzalutamide, flutamide, nilutamide) without ADT is not recommended for patients with mHSPC.
In patients with nonmetastatic castration-resistant prostate cancer (nmCRPC), darolutamide, apalutamide, and enzalutamide with continued ADT have been shown to postpone the onset of metastases and death. Unless within the context of a clinical trial, systemic chemotherapy or immunotherapy should not be offered to patients with nmCRPC.
For patients with newly diagnosed metastatic castration-resistant prostate cancer (mCRPC), continued ADT with abiraterone acetate plus prednisone, docetaxel, or enzalutamide is recommended. For patients with mCRPC who are asymptomatic or minimally symptomatic, sipuleucel-T may be offered. At present, radium-223 is the only available therapy for mCRPC that specifically targets bone metastases, delays development of skeletal-related events, and improves survival. On the basis of results of the ALSYMPCA study, radium-223 in combination with systemic therapies is now considered an effective, efficient, and well-tolerated therapy for patients with castration-resistant prostate cancer with bone lesions. The effects of local radiation therapy for men with metastatic prostate cancer and the optimal combination of systemic therapies in the metastatic setting are still under investigation.
Complete recommendations on sequencing agents and selecting therapies for patients with advanced prostate cancer can be found in guidelines from the American Urological Association, National Comprehensive Cancer Network, and the European Association of Urology.
Kyle A. Richards, MD, Assistant Professor, Department of Urology, University of Wisconsin-Madison; Chief of Urology, William S. Middleton Memorial VA Hospital, Madison, Wisconsin
Kyle A. Richards, MD, has disclosed no relevant financial relationships
A 58-year-old Black man presents with abdominal pain, urinary frequency and urgency, dysuria, incomplete voiding, and postmicturition dribble. The patient's medical history is unremarkable apart from stage 1 hypertension, for which he receives losartan plus amlodipine. Physical examination findings reveal an overdistended bladder with associated tenderness and a mildly enlarged prostate with a large, firm nodule on digital rectal exam. Urinalysis shows hematuria. Complete blood count and chemistry panel are normal. The total prostate-specific antigen level is 22 ng/mL.
Prostate Cancer – Diagnosis and Staging
Clinical Edge Journal Scan Commentary: Prostate Cancer July 2021
Treatment of prostate cancer presents significant challenges with respect to balancing efficacy and side effects of treatments. Thus, quality of life endpoints are included in studies of patients with prostate cancer to better understand and counsel patients with this heterogenous disease. In the 3 studies discussed here, functional or quality of life outcomes were included in the analyses.
In the first study, Hagens et al evaluated the effects of postoperative complications of robot-assisted radical prostatectomy on the health-related quality of life (HRQoL) as measured via surveys and assessment of functional outcomes. While no significant association between HRQoL at 6 months and complications postoperatively were identified, there was an association between functional outcomes and HRQoL at 6 months identified. Thus, future studies designed to identify more optimal patient selection or improved surgical techniques may benefit patients.
Studies have suggested that androgen deprivation therapy (ADT) may be associated with decreased cognitive function. However, other studies suggested that ADT via luteinizing hormone releasing hormone (LHRH) agonists or antagonists may actually have a protective effect against decreased cognitive function due to low gonadotropin exposure. Andela et al conducted a systematic review based on this hypothesis. Of the 31 studies included in the review, 16 demonstrated that ADT was not associated with decreased cognitive function, while 11 studies did support this association and 4 studies were inconclusive. Therefore, no definitive conclusions can be made based on this review, and further randomized studies are needed.
Radiation to the prostate may affect the rectum with sometimes significant effects. Hydrogel spacers injected between the prostate and rectum have been evaluated in patients with conventional radiation previously with evidence of benefit, but the benefits for patients undergoing stereotactic body radiotherapy (SBRT) are unknown. Ogita et al conducted a phase II single-arm study designed to evaluate the effects of a hydrogel spacer on gastrointestinal toxicity within 3 months of SBRT. Physician-assessed toxicity was not reduced, but patient reported toxicity was improved compared with historical controls. While this study does not support routine use in the setting of prostate SBRT, it does suggest that future larger randomized studies are worth consideration.
Treatment of prostate cancer presents significant challenges with respect to balancing efficacy and side effects of treatments. Thus, quality of life endpoints are included in studies of patients with prostate cancer to better understand and counsel patients with this heterogenous disease. In the 3 studies discussed here, functional or quality of life outcomes were included in the analyses.
In the first study, Hagens et al evaluated the effects of postoperative complications of robot-assisted radical prostatectomy on the health-related quality of life (HRQoL) as measured via surveys and assessment of functional outcomes. While no significant association between HRQoL at 6 months and complications postoperatively were identified, there was an association between functional outcomes and HRQoL at 6 months identified. Thus, future studies designed to identify more optimal patient selection or improved surgical techniques may benefit patients.
Studies have suggested that androgen deprivation therapy (ADT) may be associated with decreased cognitive function. However, other studies suggested that ADT via luteinizing hormone releasing hormone (LHRH) agonists or antagonists may actually have a protective effect against decreased cognitive function due to low gonadotropin exposure. Andela et al conducted a systematic review based on this hypothesis. Of the 31 studies included in the review, 16 demonstrated that ADT was not associated with decreased cognitive function, while 11 studies did support this association and 4 studies were inconclusive. Therefore, no definitive conclusions can be made based on this review, and further randomized studies are needed.
Radiation to the prostate may affect the rectum with sometimes significant effects. Hydrogel spacers injected between the prostate and rectum have been evaluated in patients with conventional radiation previously with evidence of benefit, but the benefits for patients undergoing stereotactic body radiotherapy (SBRT) are unknown. Ogita et al conducted a phase II single-arm study designed to evaluate the effects of a hydrogel spacer on gastrointestinal toxicity within 3 months of SBRT. Physician-assessed toxicity was not reduced, but patient reported toxicity was improved compared with historical controls. While this study does not support routine use in the setting of prostate SBRT, it does suggest that future larger randomized studies are worth consideration.
Treatment of prostate cancer presents significant challenges with respect to balancing efficacy and side effects of treatments. Thus, quality of life endpoints are included in studies of patients with prostate cancer to better understand and counsel patients with this heterogenous disease. In the 3 studies discussed here, functional or quality of life outcomes were included in the analyses.
In the first study, Hagens et al evaluated the effects of postoperative complications of robot-assisted radical prostatectomy on the health-related quality of life (HRQoL) as measured via surveys and assessment of functional outcomes. While no significant association between HRQoL at 6 months and complications postoperatively were identified, there was an association between functional outcomes and HRQoL at 6 months identified. Thus, future studies designed to identify more optimal patient selection or improved surgical techniques may benefit patients.
Studies have suggested that androgen deprivation therapy (ADT) may be associated with decreased cognitive function. However, other studies suggested that ADT via luteinizing hormone releasing hormone (LHRH) agonists or antagonists may actually have a protective effect against decreased cognitive function due to low gonadotropin exposure. Andela et al conducted a systematic review based on this hypothesis. Of the 31 studies included in the review, 16 demonstrated that ADT was not associated with decreased cognitive function, while 11 studies did support this association and 4 studies were inconclusive. Therefore, no definitive conclusions can be made based on this review, and further randomized studies are needed.
Radiation to the prostate may affect the rectum with sometimes significant effects. Hydrogel spacers injected between the prostate and rectum have been evaluated in patients with conventional radiation previously with evidence of benefit, but the benefits for patients undergoing stereotactic body radiotherapy (SBRT) are unknown. Ogita et al conducted a phase II single-arm study designed to evaluate the effects of a hydrogel spacer on gastrointestinal toxicity within 3 months of SBRT. Physician-assessed toxicity was not reduced, but patient reported toxicity was improved compared with historical controls. While this study does not support routine use in the setting of prostate SBRT, it does suggest that future larger randomized studies are worth consideration.