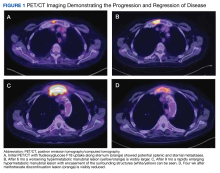
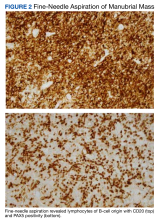
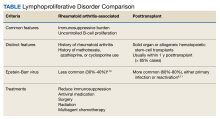
User login
For MD-IQ only
Impact of Stewardship Assistance Pilot Program for Veterans on Adherence and Persistence to Oral mCRPC Therapies
Background
Given the poor prognosis of patients with metastatic castration-resistant prostate cancer (mCRPC), interventions aimed at increasing adherence to oral treatments have the potential to improve patient outcomes. This study evaluates the impact of a patient stewardship assistance pilot program (stewardship program) on the adherence and persistence to oral treatments among patients with mCRPC at VA medical centers (VAMCs).
Methods
A non-randomized controlled study design and data from the VA Corporate Data Warehouse were used. The study included patients treated with an oral mCRPC therapy (i.e., abiraterone acetate or enzalutamide) between 08/2018 and 12/2019. Patients participating in the stewardship program formed the intervention arm and patients not participating the controls. Control patients were selected and matched 1:3 based on age, race and index year. The index date was the date of initiation of abiraterone acetate or enzalutamide. Outcomes included persistence (no gap >60 days of supply) and adherence (proportion of days covered [PDC] ≥80%) to oral mCRPC treatment post-index. Persistence and adherence were compared between the two arms using a Cox proportional hazard model and logistic regression model, respectively, adjusted for baseline characteristics.
Results
The study included 108 intervention patients (mean age: 74.6, 19.4% Black or African American, 44.4% from South, mean Quan-CCI: 6.7) and 324 control patients (mean age: 74.6, 19.4% Black or African American, 31.5% from South, mean Quan-CCI: 6.2). There was no statistically significant difference in persistence between the intervention and control arms (hazard ratio [95% confidence interval]: 0.84 [0.66-1.10], p-value: 0.211), with respective median times to discontinuation of 18 and 19 months. Over the first 12 months post-index, the proportion of adherent patients was not significantly different between the intervention arm and the control arm (50.6% vs. 50.9%; odds ratio [95% confidence interval]: 1.05 [0.80-1.38], p-value: 0.729).
Conclusions
In this racially diverse study of patients treated at VAMCs, high levels of persistence and adherence to oral mCRPC therapy were observed. The absence of any significant difference in adherence and persistence from the study intervention suggests that a stewardship assistance program aimed at improving adherence and persistence of patients with mCRPC may not be required at VAMCs.
Background
Given the poor prognosis of patients with metastatic castration-resistant prostate cancer (mCRPC), interventions aimed at increasing adherence to oral treatments have the potential to improve patient outcomes. This study evaluates the impact of a patient stewardship assistance pilot program (stewardship program) on the adherence and persistence to oral treatments among patients with mCRPC at VA medical centers (VAMCs).
Methods
A non-randomized controlled study design and data from the VA Corporate Data Warehouse were used. The study included patients treated with an oral mCRPC therapy (i.e., abiraterone acetate or enzalutamide) between 08/2018 and 12/2019. Patients participating in the stewardship program formed the intervention arm and patients not participating the controls. Control patients were selected and matched 1:3 based on age, race and index year. The index date was the date of initiation of abiraterone acetate or enzalutamide. Outcomes included persistence (no gap >60 days of supply) and adherence (proportion of days covered [PDC] ≥80%) to oral mCRPC treatment post-index. Persistence and adherence were compared between the two arms using a Cox proportional hazard model and logistic regression model, respectively, adjusted for baseline characteristics.
Results
The study included 108 intervention patients (mean age: 74.6, 19.4% Black or African American, 44.4% from South, mean Quan-CCI: 6.7) and 324 control patients (mean age: 74.6, 19.4% Black or African American, 31.5% from South, mean Quan-CCI: 6.2). There was no statistically significant difference in persistence between the intervention and control arms (hazard ratio [95% confidence interval]: 0.84 [0.66-1.10], p-value: 0.211), with respective median times to discontinuation of 18 and 19 months. Over the first 12 months post-index, the proportion of adherent patients was not significantly different between the intervention arm and the control arm (50.6% vs. 50.9%; odds ratio [95% confidence interval]: 1.05 [0.80-1.38], p-value: 0.729).
Conclusions
In this racially diverse study of patients treated at VAMCs, high levels of persistence and adherence to oral mCRPC therapy were observed. The absence of any significant difference in adherence and persistence from the study intervention suggests that a stewardship assistance program aimed at improving adherence and persistence of patients with mCRPC may not be required at VAMCs.
Background
Given the poor prognosis of patients with metastatic castration-resistant prostate cancer (mCRPC), interventions aimed at increasing adherence to oral treatments have the potential to improve patient outcomes. This study evaluates the impact of a patient stewardship assistance pilot program (stewardship program) on the adherence and persistence to oral treatments among patients with mCRPC at VA medical centers (VAMCs).
Methods
A non-randomized controlled study design and data from the VA Corporate Data Warehouse were used. The study included patients treated with an oral mCRPC therapy (i.e., abiraterone acetate or enzalutamide) between 08/2018 and 12/2019. Patients participating in the stewardship program formed the intervention arm and patients not participating the controls. Control patients were selected and matched 1:3 based on age, race and index year. The index date was the date of initiation of abiraterone acetate or enzalutamide. Outcomes included persistence (no gap >60 days of supply) and adherence (proportion of days covered [PDC] ≥80%) to oral mCRPC treatment post-index. Persistence and adherence were compared between the two arms using a Cox proportional hazard model and logistic regression model, respectively, adjusted for baseline characteristics.
Results
The study included 108 intervention patients (mean age: 74.6, 19.4% Black or African American, 44.4% from South, mean Quan-CCI: 6.7) and 324 control patients (mean age: 74.6, 19.4% Black or African American, 31.5% from South, mean Quan-CCI: 6.2). There was no statistically significant difference in persistence between the intervention and control arms (hazard ratio [95% confidence interval]: 0.84 [0.66-1.10], p-value: 0.211), with respective median times to discontinuation of 18 and 19 months. Over the first 12 months post-index, the proportion of adherent patients was not significantly different between the intervention arm and the control arm (50.6% vs. 50.9%; odds ratio [95% confidence interval]: 1.05 [0.80-1.38], p-value: 0.729).
Conclusions
In this racially diverse study of patients treated at VAMCs, high levels of persistence and adherence to oral mCRPC therapy were observed. The absence of any significant difference in adherence and persistence from the study intervention suggests that a stewardship assistance program aimed at improving adherence and persistence of patients with mCRPC may not be required at VAMCs.
In Prostate Cancer, Most Roads Lead to VA Pathway
The newly updated US Department of Veterans Affairs (VA) prostate cancer clinical pathway looks like a set of guidelines, but it’s really something unique. As attendees learned at an Association of VA Hematology/Oncology (AVAHO) regional meeting in Detroit in June, the clinical pathways are designed to point the way toward a standard ideal treatment for the majority of cases, not just to suggest a number of possible options.
“Pathways will always offer one scenario. They try to get oncologists to practice in a similar fashion so things can be managed more uniformly,” Michael M. Goodman, MD, told Federal Practitioner prior to the AVAHO meeting that was focused on prostate cancer care. Goodman is an associate professor of medicine with Atrium Health Wake Forest Baptist Medical Center and helped develop the VA genitourinary oncology pathways.
“The overall goal is not just to standardize care as much as possible but also to synthesize the best and most cost-effective practices,” Goodman said. For example, “If you have 5 different therapies, and they all have about the same efficacy and safety, and 1 is less costly than the other 4, then it would make sense to choose that.”
The VA has offered pathways for multiple types of cancer since 2021, and the pathway for prostate cancer is among the most comprehensive. The VA system updated the pathway in March 2024, is available online both via SharePoint and externally.
“It goes through the entire gamut from screening, diagnosis, and management to end of life,” Goodman explained. Multiple disciplines, from primary care and surgery to genetics and imaging, can rely on the pathway to assist decision-making.
In terms of screening, the pathway offers a flow map guiding the screening choices. In patients aged ≤ 54 years, only certain high-risk groups, such as African Americans and those with a family history of prostate cancer, should be screened. From ages 54 to 69 years, patients should be consulted as part of a shared decision making process, while screening is not recommended for patients aged ≥ 70 years.
Pathway flow maps also provide information about diagnostic standards, evaluation of the newly diagnosed, risk stratification, molecular testing, and end-of-life care.
Goodman says the pathway is now integrated into the VA electronic health record system via a template so clinicians can easily document pathway use. This allows the VA to track the use of the pathways locally, regionally, and nationally track the use of the pathways.
Clinicians are not mandated to follow every step in the pathway, but Goodman said the goal is > 80% adherence. If clinicians follow the standards, he said, “you’re considering efficacy, safety, and cost for that veteran.”
Prospective data suggests that adherence to the pathway eliminates certain disparities. African American veterans, for example, are as well-represented or even better represented than White veterans in prostate cancer care when pathways are followed.
Why might clinicians veer from the pathway? “If you’re seeing a patient who was treated in the community with drug X, but drug Y is chosen by the pathway, you can carry on with the previous care.” Alternatively, in some cases, patients may not tolerate the pathway standard, Goodman noted.
Goodman reports that he consults the pathway every day. “It’s helped standardize the care I provide to ensure there’s no gaps in how I’m treating patients.”
The newly updated US Department of Veterans Affairs (VA) prostate cancer clinical pathway looks like a set of guidelines, but it’s really something unique. As attendees learned at an Association of VA Hematology/Oncology (AVAHO) regional meeting in Detroit in June, the clinical pathways are designed to point the way toward a standard ideal treatment for the majority of cases, not just to suggest a number of possible options.
“Pathways will always offer one scenario. They try to get oncologists to practice in a similar fashion so things can be managed more uniformly,” Michael M. Goodman, MD, told Federal Practitioner prior to the AVAHO meeting that was focused on prostate cancer care. Goodman is an associate professor of medicine with Atrium Health Wake Forest Baptist Medical Center and helped develop the VA genitourinary oncology pathways.
“The overall goal is not just to standardize care as much as possible but also to synthesize the best and most cost-effective practices,” Goodman said. For example, “If you have 5 different therapies, and they all have about the same efficacy and safety, and 1 is less costly than the other 4, then it would make sense to choose that.”
The VA has offered pathways for multiple types of cancer since 2021, and the pathway for prostate cancer is among the most comprehensive. The VA system updated the pathway in March 2024, is available online both via SharePoint and externally.
“It goes through the entire gamut from screening, diagnosis, and management to end of life,” Goodman explained. Multiple disciplines, from primary care and surgery to genetics and imaging, can rely on the pathway to assist decision-making.
In terms of screening, the pathway offers a flow map guiding the screening choices. In patients aged ≤ 54 years, only certain high-risk groups, such as African Americans and those with a family history of prostate cancer, should be screened. From ages 54 to 69 years, patients should be consulted as part of a shared decision making process, while screening is not recommended for patients aged ≥ 70 years.
Pathway flow maps also provide information about diagnostic standards, evaluation of the newly diagnosed, risk stratification, molecular testing, and end-of-life care.
Goodman says the pathway is now integrated into the VA electronic health record system via a template so clinicians can easily document pathway use. This allows the VA to track the use of the pathways locally, regionally, and nationally track the use of the pathways.
Clinicians are not mandated to follow every step in the pathway, but Goodman said the goal is > 80% adherence. If clinicians follow the standards, he said, “you’re considering efficacy, safety, and cost for that veteran.”
Prospective data suggests that adherence to the pathway eliminates certain disparities. African American veterans, for example, are as well-represented or even better represented than White veterans in prostate cancer care when pathways are followed.
Why might clinicians veer from the pathway? “If you’re seeing a patient who was treated in the community with drug X, but drug Y is chosen by the pathway, you can carry on with the previous care.” Alternatively, in some cases, patients may not tolerate the pathway standard, Goodman noted.
Goodman reports that he consults the pathway every day. “It’s helped standardize the care I provide to ensure there’s no gaps in how I’m treating patients.”
The newly updated US Department of Veterans Affairs (VA) prostate cancer clinical pathway looks like a set of guidelines, but it’s really something unique. As attendees learned at an Association of VA Hematology/Oncology (AVAHO) regional meeting in Detroit in June, the clinical pathways are designed to point the way toward a standard ideal treatment for the majority of cases, not just to suggest a number of possible options.
“Pathways will always offer one scenario. They try to get oncologists to practice in a similar fashion so things can be managed more uniformly,” Michael M. Goodman, MD, told Federal Practitioner prior to the AVAHO meeting that was focused on prostate cancer care. Goodman is an associate professor of medicine with Atrium Health Wake Forest Baptist Medical Center and helped develop the VA genitourinary oncology pathways.
“The overall goal is not just to standardize care as much as possible but also to synthesize the best and most cost-effective practices,” Goodman said. For example, “If you have 5 different therapies, and they all have about the same efficacy and safety, and 1 is less costly than the other 4, then it would make sense to choose that.”
The VA has offered pathways for multiple types of cancer since 2021, and the pathway for prostate cancer is among the most comprehensive. The VA system updated the pathway in March 2024, is available online both via SharePoint and externally.
“It goes through the entire gamut from screening, diagnosis, and management to end of life,” Goodman explained. Multiple disciplines, from primary care and surgery to genetics and imaging, can rely on the pathway to assist decision-making.
In terms of screening, the pathway offers a flow map guiding the screening choices. In patients aged ≤ 54 years, only certain high-risk groups, such as African Americans and those with a family history of prostate cancer, should be screened. From ages 54 to 69 years, patients should be consulted as part of a shared decision making process, while screening is not recommended for patients aged ≥ 70 years.
Pathway flow maps also provide information about diagnostic standards, evaluation of the newly diagnosed, risk stratification, molecular testing, and end-of-life care.
Goodman says the pathway is now integrated into the VA electronic health record system via a template so clinicians can easily document pathway use. This allows the VA to track the use of the pathways locally, regionally, and nationally track the use of the pathways.
Clinicians are not mandated to follow every step in the pathway, but Goodman said the goal is > 80% adherence. If clinicians follow the standards, he said, “you’re considering efficacy, safety, and cost for that veteran.”
Prospective data suggests that adherence to the pathway eliminates certain disparities. African American veterans, for example, are as well-represented or even better represented than White veterans in prostate cancer care when pathways are followed.
Why might clinicians veer from the pathway? “If you’re seeing a patient who was treated in the community with drug X, but drug Y is chosen by the pathway, you can carry on with the previous care.” Alternatively, in some cases, patients may not tolerate the pathway standard, Goodman noted.
Goodman reports that he consults the pathway every day. “It’s helped standardize the care I provide to ensure there’s no gaps in how I’m treating patients.”
Experts Focus on Quality-of-Life Data in Prostate Cancer
A central aim of prostate cancer treatment is to prolong survival, but trials often overlook another key goal: Improving — or at least maintaining — quality of life (QoL).
The trials explored the effects of treatment suspension or intensification on health-related QoL as well as interventions to manage side effects in different patient populations.
The first presentation focused on a post hoc analysis of the phase 3 EMBARK trial, which looked at the effect of suspending treatment on health-related QoL in men with nonmetastatic disease at a high risk for biochemical recurrence.
Earlier findings from the trial, presented at ESMO in 2023, showed enzalutamide alone or in combination with androgen deprivation therapy (ADT) was associated with a significant improvement in metastasis-free survival vs placebo plus leuprolide.
The initial trial randomized 1068 patients at a high risk for biochemical recurrence to these three treatment groups and suspended therapy at week 37 if prostate-specific antigen (PSA) levels fell below 0.2 ng/mL. Patients, however, were not randomized into the treatment suspension groups. Treatment resumed if PSA levels rose to ≥ 2.0 ng/mL in patients who had undergone radical prostatectomy or ≥ 5.0 ng/mL in those who had not had surgery.
The post hoc analysis, which assessed patient-reported QoL outcomes following treatment suspension at baseline and every 12 weeks until progression, found no meaningful changes in the worst pain in the past 24 hours, as measured by the Brief Pain Inventory–Short Form.
Patients also reported no meaningful changes in total and physical well-being scores on the Functional Assessment of Cancer Therapy–Prostate (FACT-P) and on the European Quality of Life Five-Dimensions (EuroQol-5D) visual analog scale score, as well as no meaningful changes in sexual activity and urinary and bowel symptoms, based on scores from the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Prostate 25 (QLQ-PR25).
Hormone treatment-related symptoms on the QLQ-PR25, however, “quickly improved but eventually began to worsen after week 97,” explained lead author Stephen J. Freedland, MD, from Cedars-Sinai Medical Center, Los Angeles, California, who presented the new findings at ASCO.
Dr. Freedland concluded that the EMBARK results show that enzalutamide, with or without ADT, improves metastasis-free survival vs leuprolide alone, without affecting global health-related QoL during treatment or after treatment suspension.
However, Channing J. Paller, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Medicine, Baltimore, Maryland, who was not involved in the research, pointed out that “patient selection is key” when choosing therapies, given that ADT has distinct adverse effects. Comorbidities and adverse effects “must be taken into consideration to help the doctor and patient make more personalized treatment choices.”
Treatment Intensification and QoL
Another presentation explored health-related QoL outcomes from the phase 3 PRESTO trial.
The study examined ADT intensification in 504 patients who had high-risk biochemically relapsed nonmetastatic hormone-sensitive prostate cancer and a PSA doubling time of 9 months or less. Patients were randomized to ADT monotherapy with degarelix or leuprolide, ADT plus apalutamide, or ADT plus apalutamide, abiraterone acetate, and prednisone.
In previous data from PRESTO, the combination therapy groups both had significantly longer median PSA progression-free survival than the ADT monotherapy arm.
The latest data looked at the health-related QoL outcomes in the PRESTO population, measured using the Expanded Prostate Cancer Index Composite, the PROMIS Fatigue tool, the Hot Flash Related Daily Interference Scale, and the EuroQol-5D.
Ronald C. Chen, MD, MPH, of the University of Kansas Medical Center, Kansas City, who presented the new findings at ASCO, reported that ADT plus apalutamide improved PSA progression-free survival over ADT alone and did not meaningfully increase common treatment-related symptoms, such as hormonal symptoms, sexual dysfunction, hot flash interference, and fatigue.
However, treatment intensification with triple androgen regimen did not lead to further improvements in PSA progression-free survival but did increase the rate of serious adverse events, the time to testosterone recover, and increased hot flash interference.
PRESTO as well as EMBARK “provide a strong rationale for intensification of androgen blockade in men with high-risk biochemical recurrence after completing primary local therapy” and could even “reduce the need for subsequent treatment,” concluded Dr. Chen.
CBT for Managing ADT Side Effects
Up to 80% of men receiving ADT to treat prostate cancer experience night sweats and hot flashes, which are associated with sleep disturbance, anxiety, low mood, and cognitive impairments.
A third trial presented during the session looked at the impact of cognitive-behavioral therapy (CBT) on these side effects of ADT treatment.
Initial findings from the MANCAN study found that CBT delivered by a psychologist reduced the impact of hot flashes and night sweats at 6 weeks.
The MANCAN2 study assessed QoL at 6 months among 162 patients with localized or advanced prostate cancer who underwent at least 6 months of continuous ADT and who experienced more severe hot flashes and night sweats, defined as a score of ≥ 2 on the hot flashes and night sweats problem rating scale.
Study participants were randomized to CBT plus treatment as usual, or treatment as usual alone, with the intervention consisting of two CBT group sessions 4 weeks apart. Between CBT sessions, patients could refer to a booklet and CD, alongside exercises and CBT strategies.
MANCAN2 confirmed that CBT was associated with a significantly greater reduction in hot flash and night sweat scores over standard care alone at 6 weeks. Patients receiving CBT also reported better QoL, sleep, and functional status but those differences did not reach statistical significance.
By 6 months, those in the CBT group still reported better outcomes in each category, but no differences were statistically significant at this time point. Overall, however, 14% of treatment as usual alone patients discontinued ADT at 6 months vs none in the CBT arm.
“Further research is therefore needed to determine whether or not you can make this effect more durable” and to look at “the potential for CBT to support treatment compliance,” said study presenter Simon J. Crabb, PhD, MBBS, from the University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton, England.
QoL With Radioligand Crossover
Finally, the phase 3 PSMAfore study compared 177Lu-PSMA-617 with abiraterone or enzalutamide in 468 taxane-naive patients with metastatic castration-resistant prostate cancer who had progressed on a previous androgen receptor pathway inhibitor.
In earlier analyses, Karim Fizazi, MD, PhD, Institut Gustave Roussy, Université Paris-Saclay, Paris, France, reported that 177Lu-PSMA-617 improved radiographic progression-free survival by 59% over androgen receptor pathway inhibitor therapy but did not lead to significant differences in overall survival.
In a new interim analysis, Dr. Fizazi and colleagues explored outcomes in patients eligible to cross over to 177Lu-PSMA-617 following androgen receptor pathway inhibitor therapy. Assessments of health-related QoL revealed that 177Lu-PSMA-617 led to about a 40% improvement in scores on two QoL tools — 41% with FACT-P and 39% with EuroQol-5D.
On subscales of FACT-P, Dr. Fizazi reported that 177Lu-PSMA-617 was also associated with a significantly longer time to worsening in physical, functional, and emotional well-being over standard therapy. A pain inventory score indicated that 177Lu-PSMA-617 led to a 31% improvement in the time to worsening pain intensity, as well as a 33% increase in the time to worsening pain interference.
With the treatment having a “favorable safety profile,” Dr. Fizazi said the results suggest 177Lu-PSMA-617 is a “treatment option” for patients with metastatic castration-resistant prostate cancer who have undergone androgen receptor pathway inhibitor treatment.
MANCAN2 was funded by the UK National Institute for Health and Care Research. EMBARK was funded by Astellas Pharma and Pfizer, the codevelopers of enzalutamide. PRESTO was funded by Alliance Foundation Trials and Johnson & Johnson. PSMAfore was funded by Novartis. Dr. Freedland declared relationships with Pfizer and Astellas Pharma, among others. Paller declared relationships with AstraZeneca, Dendreon, Exelixis, Janssen Oncology, Omnitura, Lilly, and Bayer. Dr. Chen declared relationships with Astellas Pharma, Pfizer, and others. Dr. Crabb declared relationships with AstraZeneca, Bristol-Myers Squibb, Ipsen, Merck, Amgen, Amphista Therapeutics, Bayer, Janssen, MSD, Pfizer, Astex Pharmaceuticals, Clovis Oncology, and Roche. Dr. Fizazi reported relationships with Novartis, AstraZeneca, and a dozen other companies.
A version of this article first appeared on Medscape.com.
A central aim of prostate cancer treatment is to prolong survival, but trials often overlook another key goal: Improving — or at least maintaining — quality of life (QoL).
The trials explored the effects of treatment suspension or intensification on health-related QoL as well as interventions to manage side effects in different patient populations.
The first presentation focused on a post hoc analysis of the phase 3 EMBARK trial, which looked at the effect of suspending treatment on health-related QoL in men with nonmetastatic disease at a high risk for biochemical recurrence.
Earlier findings from the trial, presented at ESMO in 2023, showed enzalutamide alone or in combination with androgen deprivation therapy (ADT) was associated with a significant improvement in metastasis-free survival vs placebo plus leuprolide.
The initial trial randomized 1068 patients at a high risk for biochemical recurrence to these three treatment groups and suspended therapy at week 37 if prostate-specific antigen (PSA) levels fell below 0.2 ng/mL. Patients, however, were not randomized into the treatment suspension groups. Treatment resumed if PSA levels rose to ≥ 2.0 ng/mL in patients who had undergone radical prostatectomy or ≥ 5.0 ng/mL in those who had not had surgery.
The post hoc analysis, which assessed patient-reported QoL outcomes following treatment suspension at baseline and every 12 weeks until progression, found no meaningful changes in the worst pain in the past 24 hours, as measured by the Brief Pain Inventory–Short Form.
Patients also reported no meaningful changes in total and physical well-being scores on the Functional Assessment of Cancer Therapy–Prostate (FACT-P) and on the European Quality of Life Five-Dimensions (EuroQol-5D) visual analog scale score, as well as no meaningful changes in sexual activity and urinary and bowel symptoms, based on scores from the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Prostate 25 (QLQ-PR25).
Hormone treatment-related symptoms on the QLQ-PR25, however, “quickly improved but eventually began to worsen after week 97,” explained lead author Stephen J. Freedland, MD, from Cedars-Sinai Medical Center, Los Angeles, California, who presented the new findings at ASCO.
Dr. Freedland concluded that the EMBARK results show that enzalutamide, with or without ADT, improves metastasis-free survival vs leuprolide alone, without affecting global health-related QoL during treatment or after treatment suspension.
However, Channing J. Paller, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Medicine, Baltimore, Maryland, who was not involved in the research, pointed out that “patient selection is key” when choosing therapies, given that ADT has distinct adverse effects. Comorbidities and adverse effects “must be taken into consideration to help the doctor and patient make more personalized treatment choices.”
Treatment Intensification and QoL
Another presentation explored health-related QoL outcomes from the phase 3 PRESTO trial.
The study examined ADT intensification in 504 patients who had high-risk biochemically relapsed nonmetastatic hormone-sensitive prostate cancer and a PSA doubling time of 9 months or less. Patients were randomized to ADT monotherapy with degarelix or leuprolide, ADT plus apalutamide, or ADT plus apalutamide, abiraterone acetate, and prednisone.
In previous data from PRESTO, the combination therapy groups both had significantly longer median PSA progression-free survival than the ADT monotherapy arm.
The latest data looked at the health-related QoL outcomes in the PRESTO population, measured using the Expanded Prostate Cancer Index Composite, the PROMIS Fatigue tool, the Hot Flash Related Daily Interference Scale, and the EuroQol-5D.
Ronald C. Chen, MD, MPH, of the University of Kansas Medical Center, Kansas City, who presented the new findings at ASCO, reported that ADT plus apalutamide improved PSA progression-free survival over ADT alone and did not meaningfully increase common treatment-related symptoms, such as hormonal symptoms, sexual dysfunction, hot flash interference, and fatigue.
However, treatment intensification with triple androgen regimen did not lead to further improvements in PSA progression-free survival but did increase the rate of serious adverse events, the time to testosterone recover, and increased hot flash interference.
PRESTO as well as EMBARK “provide a strong rationale for intensification of androgen blockade in men with high-risk biochemical recurrence after completing primary local therapy” and could even “reduce the need for subsequent treatment,” concluded Dr. Chen.
CBT for Managing ADT Side Effects
Up to 80% of men receiving ADT to treat prostate cancer experience night sweats and hot flashes, which are associated with sleep disturbance, anxiety, low mood, and cognitive impairments.
A third trial presented during the session looked at the impact of cognitive-behavioral therapy (CBT) on these side effects of ADT treatment.
Initial findings from the MANCAN study found that CBT delivered by a psychologist reduced the impact of hot flashes and night sweats at 6 weeks.
The MANCAN2 study assessed QoL at 6 months among 162 patients with localized or advanced prostate cancer who underwent at least 6 months of continuous ADT and who experienced more severe hot flashes and night sweats, defined as a score of ≥ 2 on the hot flashes and night sweats problem rating scale.
Study participants were randomized to CBT plus treatment as usual, or treatment as usual alone, with the intervention consisting of two CBT group sessions 4 weeks apart. Between CBT sessions, patients could refer to a booklet and CD, alongside exercises and CBT strategies.
MANCAN2 confirmed that CBT was associated with a significantly greater reduction in hot flash and night sweat scores over standard care alone at 6 weeks. Patients receiving CBT also reported better QoL, sleep, and functional status but those differences did not reach statistical significance.
By 6 months, those in the CBT group still reported better outcomes in each category, but no differences were statistically significant at this time point. Overall, however, 14% of treatment as usual alone patients discontinued ADT at 6 months vs none in the CBT arm.
“Further research is therefore needed to determine whether or not you can make this effect more durable” and to look at “the potential for CBT to support treatment compliance,” said study presenter Simon J. Crabb, PhD, MBBS, from the University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton, England.
QoL With Radioligand Crossover
Finally, the phase 3 PSMAfore study compared 177Lu-PSMA-617 with abiraterone or enzalutamide in 468 taxane-naive patients with metastatic castration-resistant prostate cancer who had progressed on a previous androgen receptor pathway inhibitor.
In earlier analyses, Karim Fizazi, MD, PhD, Institut Gustave Roussy, Université Paris-Saclay, Paris, France, reported that 177Lu-PSMA-617 improved radiographic progression-free survival by 59% over androgen receptor pathway inhibitor therapy but did not lead to significant differences in overall survival.
In a new interim analysis, Dr. Fizazi and colleagues explored outcomes in patients eligible to cross over to 177Lu-PSMA-617 following androgen receptor pathway inhibitor therapy. Assessments of health-related QoL revealed that 177Lu-PSMA-617 led to about a 40% improvement in scores on two QoL tools — 41% with FACT-P and 39% with EuroQol-5D.
On subscales of FACT-P, Dr. Fizazi reported that 177Lu-PSMA-617 was also associated with a significantly longer time to worsening in physical, functional, and emotional well-being over standard therapy. A pain inventory score indicated that 177Lu-PSMA-617 led to a 31% improvement in the time to worsening pain intensity, as well as a 33% increase in the time to worsening pain interference.
With the treatment having a “favorable safety profile,” Dr. Fizazi said the results suggest 177Lu-PSMA-617 is a “treatment option” for patients with metastatic castration-resistant prostate cancer who have undergone androgen receptor pathway inhibitor treatment.
MANCAN2 was funded by the UK National Institute for Health and Care Research. EMBARK was funded by Astellas Pharma and Pfizer, the codevelopers of enzalutamide. PRESTO was funded by Alliance Foundation Trials and Johnson & Johnson. PSMAfore was funded by Novartis. Dr. Freedland declared relationships with Pfizer and Astellas Pharma, among others. Paller declared relationships with AstraZeneca, Dendreon, Exelixis, Janssen Oncology, Omnitura, Lilly, and Bayer. Dr. Chen declared relationships with Astellas Pharma, Pfizer, and others. Dr. Crabb declared relationships with AstraZeneca, Bristol-Myers Squibb, Ipsen, Merck, Amgen, Amphista Therapeutics, Bayer, Janssen, MSD, Pfizer, Astex Pharmaceuticals, Clovis Oncology, and Roche. Dr. Fizazi reported relationships with Novartis, AstraZeneca, and a dozen other companies.
A version of this article first appeared on Medscape.com.
A central aim of prostate cancer treatment is to prolong survival, but trials often overlook another key goal: Improving — or at least maintaining — quality of life (QoL).
The trials explored the effects of treatment suspension or intensification on health-related QoL as well as interventions to manage side effects in different patient populations.
The first presentation focused on a post hoc analysis of the phase 3 EMBARK trial, which looked at the effect of suspending treatment on health-related QoL in men with nonmetastatic disease at a high risk for biochemical recurrence.
Earlier findings from the trial, presented at ESMO in 2023, showed enzalutamide alone or in combination with androgen deprivation therapy (ADT) was associated with a significant improvement in metastasis-free survival vs placebo plus leuprolide.
The initial trial randomized 1068 patients at a high risk for biochemical recurrence to these three treatment groups and suspended therapy at week 37 if prostate-specific antigen (PSA) levels fell below 0.2 ng/mL. Patients, however, were not randomized into the treatment suspension groups. Treatment resumed if PSA levels rose to ≥ 2.0 ng/mL in patients who had undergone radical prostatectomy or ≥ 5.0 ng/mL in those who had not had surgery.
The post hoc analysis, which assessed patient-reported QoL outcomes following treatment suspension at baseline and every 12 weeks until progression, found no meaningful changes in the worst pain in the past 24 hours, as measured by the Brief Pain Inventory–Short Form.
Patients also reported no meaningful changes in total and physical well-being scores on the Functional Assessment of Cancer Therapy–Prostate (FACT-P) and on the European Quality of Life Five-Dimensions (EuroQol-5D) visual analog scale score, as well as no meaningful changes in sexual activity and urinary and bowel symptoms, based on scores from the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire–Prostate 25 (QLQ-PR25).
Hormone treatment-related symptoms on the QLQ-PR25, however, “quickly improved but eventually began to worsen after week 97,” explained lead author Stephen J. Freedland, MD, from Cedars-Sinai Medical Center, Los Angeles, California, who presented the new findings at ASCO.
Dr. Freedland concluded that the EMBARK results show that enzalutamide, with or without ADT, improves metastasis-free survival vs leuprolide alone, without affecting global health-related QoL during treatment or after treatment suspension.
However, Channing J. Paller, MD, of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins Medicine, Baltimore, Maryland, who was not involved in the research, pointed out that “patient selection is key” when choosing therapies, given that ADT has distinct adverse effects. Comorbidities and adverse effects “must be taken into consideration to help the doctor and patient make more personalized treatment choices.”
Treatment Intensification and QoL
Another presentation explored health-related QoL outcomes from the phase 3 PRESTO trial.
The study examined ADT intensification in 504 patients who had high-risk biochemically relapsed nonmetastatic hormone-sensitive prostate cancer and a PSA doubling time of 9 months or less. Patients were randomized to ADT monotherapy with degarelix or leuprolide, ADT plus apalutamide, or ADT plus apalutamide, abiraterone acetate, and prednisone.
In previous data from PRESTO, the combination therapy groups both had significantly longer median PSA progression-free survival than the ADT monotherapy arm.
The latest data looked at the health-related QoL outcomes in the PRESTO population, measured using the Expanded Prostate Cancer Index Composite, the PROMIS Fatigue tool, the Hot Flash Related Daily Interference Scale, and the EuroQol-5D.
Ronald C. Chen, MD, MPH, of the University of Kansas Medical Center, Kansas City, who presented the new findings at ASCO, reported that ADT plus apalutamide improved PSA progression-free survival over ADT alone and did not meaningfully increase common treatment-related symptoms, such as hormonal symptoms, sexual dysfunction, hot flash interference, and fatigue.
However, treatment intensification with triple androgen regimen did not lead to further improvements in PSA progression-free survival but did increase the rate of serious adverse events, the time to testosterone recover, and increased hot flash interference.
PRESTO as well as EMBARK “provide a strong rationale for intensification of androgen blockade in men with high-risk biochemical recurrence after completing primary local therapy” and could even “reduce the need for subsequent treatment,” concluded Dr. Chen.
CBT for Managing ADT Side Effects
Up to 80% of men receiving ADT to treat prostate cancer experience night sweats and hot flashes, which are associated with sleep disturbance, anxiety, low mood, and cognitive impairments.
A third trial presented during the session looked at the impact of cognitive-behavioral therapy (CBT) on these side effects of ADT treatment.
Initial findings from the MANCAN study found that CBT delivered by a psychologist reduced the impact of hot flashes and night sweats at 6 weeks.
The MANCAN2 study assessed QoL at 6 months among 162 patients with localized or advanced prostate cancer who underwent at least 6 months of continuous ADT and who experienced more severe hot flashes and night sweats, defined as a score of ≥ 2 on the hot flashes and night sweats problem rating scale.
Study participants were randomized to CBT plus treatment as usual, or treatment as usual alone, with the intervention consisting of two CBT group sessions 4 weeks apart. Between CBT sessions, patients could refer to a booklet and CD, alongside exercises and CBT strategies.
MANCAN2 confirmed that CBT was associated with a significantly greater reduction in hot flash and night sweat scores over standard care alone at 6 weeks. Patients receiving CBT also reported better QoL, sleep, and functional status but those differences did not reach statistical significance.
By 6 months, those in the CBT group still reported better outcomes in each category, but no differences were statistically significant at this time point. Overall, however, 14% of treatment as usual alone patients discontinued ADT at 6 months vs none in the CBT arm.
“Further research is therefore needed to determine whether or not you can make this effect more durable” and to look at “the potential for CBT to support treatment compliance,” said study presenter Simon J. Crabb, PhD, MBBS, from the University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton, England.
QoL With Radioligand Crossover
Finally, the phase 3 PSMAfore study compared 177Lu-PSMA-617 with abiraterone or enzalutamide in 468 taxane-naive patients with metastatic castration-resistant prostate cancer who had progressed on a previous androgen receptor pathway inhibitor.
In earlier analyses, Karim Fizazi, MD, PhD, Institut Gustave Roussy, Université Paris-Saclay, Paris, France, reported that 177Lu-PSMA-617 improved radiographic progression-free survival by 59% over androgen receptor pathway inhibitor therapy but did not lead to significant differences in overall survival.
In a new interim analysis, Dr. Fizazi and colleagues explored outcomes in patients eligible to cross over to 177Lu-PSMA-617 following androgen receptor pathway inhibitor therapy. Assessments of health-related QoL revealed that 177Lu-PSMA-617 led to about a 40% improvement in scores on two QoL tools — 41% with FACT-P and 39% with EuroQol-5D.
On subscales of FACT-P, Dr. Fizazi reported that 177Lu-PSMA-617 was also associated with a significantly longer time to worsening in physical, functional, and emotional well-being over standard therapy. A pain inventory score indicated that 177Lu-PSMA-617 led to a 31% improvement in the time to worsening pain intensity, as well as a 33% increase in the time to worsening pain interference.
With the treatment having a “favorable safety profile,” Dr. Fizazi said the results suggest 177Lu-PSMA-617 is a “treatment option” for patients with metastatic castration-resistant prostate cancer who have undergone androgen receptor pathway inhibitor treatment.
MANCAN2 was funded by the UK National Institute for Health and Care Research. EMBARK was funded by Astellas Pharma and Pfizer, the codevelopers of enzalutamide. PRESTO was funded by Alliance Foundation Trials and Johnson & Johnson. PSMAfore was funded by Novartis. Dr. Freedland declared relationships with Pfizer and Astellas Pharma, among others. Paller declared relationships with AstraZeneca, Dendreon, Exelixis, Janssen Oncology, Omnitura, Lilly, and Bayer. Dr. Chen declared relationships with Astellas Pharma, Pfizer, and others. Dr. Crabb declared relationships with AstraZeneca, Bristol-Myers Squibb, Ipsen, Merck, Amgen, Amphista Therapeutics, Bayer, Janssen, MSD, Pfizer, Astex Pharmaceuticals, Clovis Oncology, and Roche. Dr. Fizazi reported relationships with Novartis, AstraZeneca, and a dozen other companies.
A version of this article first appeared on Medscape.com.
FROM ASCO 2024
AVAHO Mtg: Germline Testing Key for Vets With High-Risk PC
Not too long ago, prostate-cancer genetics didn’t mean much to patient care. But in recent years, the landscape of therapy has transformed as researchers have discovered links between multiple genes and aggressive tumors.
Now, as a hematologist-oncologist explained to attendees at an Association of VA Hematology/Oncology regional meeting in Detroit, genetic tests can guide treatment for some—but not all—men with prostate cancer.
For patients with mutations, appropriate supplemental medications “can improve overall outcomes and have a long-standing impact on patients” said Scott J. Dawsey, MD, of the John D. Dingell Veterans Affairs Medical Center in Detroit in an interview following the AVAHO meeting, which focused on the management of prostate cancer.
As Dawsey explained, about 10% of patients with prostate cancer appear to have genetic mutations, although the exact percentage is unclear. The mutations are especially common in metastatic forms of prostate cancer. They’re estimated to be present in 11.8%-16.2% of those cases.
While these proportions are relatively small, the number of overall prostate-cancer cases with mutations is large due to the high burden of the disease, Dawsey said. Prostate cancer is by far the most common cancer in men, and estimated 299,010 cases will be diagnosed in the United States this year.
According to Dawsey, genetic mutations seem to boost the risk of more aggressive disease—and the risk of other malignancies—by disrupting DNA repair. This process can lead to even more mutations that may “make the cancer behave and grow more aggressively.”
But not all prostate cancer patients need to undergo genetic testing. Dawsey urged colleagues to figure out which patients should be tested by consulting National Comprehensive Cancer Network (NCCN) guidelines and the newly updated US Department of Veterans Affairs (VA) prostate cancer clinical pathway.
The two sets of recommendations agree on germline testing in patients with cases that are metastatic, very high risk, and high risk. Lower-risk cases should only be tested if patients meet family history criteria. The sets of guidelines also recommend somatic testing in patients with metastatic cancer.
In addition to providing guidance about treatment, genetic test results can have implications regarding other potential malignancies that may affect patients, Dawsey said. The results may also have implications for cancer risk in family members.
Several drugs are now available for patients with genetic mutations, including checkpoint inhibitors and PARP inhibitors. The drugs, which have unique mechanisms of action, are given in addition to standard prostate cancer treatments, he said.
“If a patient doesn’t have one of these genetic changes,” he said, “these drugs aren’t an option.”
A long list of drugs or combinations of drugs are in clinical trials, including the poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors olaparib, abiraterone, and niraparib and the checkpoint inhibitors nivolumab and cemiplimab.
The drugs generally improve response rates and progression-free survival, Dawsey said, and patients are generally able to tolerate them. In regard to which drugs to choose, he suggested consulting the and NCCN guidelines and the VA oncology clinical pathway for prostate cancer.
Not too long ago, prostate-cancer genetics didn’t mean much to patient care. But in recent years, the landscape of therapy has transformed as researchers have discovered links between multiple genes and aggressive tumors.
Now, as a hematologist-oncologist explained to attendees at an Association of VA Hematology/Oncology regional meeting in Detroit, genetic tests can guide treatment for some—but not all—men with prostate cancer.
For patients with mutations, appropriate supplemental medications “can improve overall outcomes and have a long-standing impact on patients” said Scott J. Dawsey, MD, of the John D. Dingell Veterans Affairs Medical Center in Detroit in an interview following the AVAHO meeting, which focused on the management of prostate cancer.
As Dawsey explained, about 10% of patients with prostate cancer appear to have genetic mutations, although the exact percentage is unclear. The mutations are especially common in metastatic forms of prostate cancer. They’re estimated to be present in 11.8%-16.2% of those cases.
While these proportions are relatively small, the number of overall prostate-cancer cases with mutations is large due to the high burden of the disease, Dawsey said. Prostate cancer is by far the most common cancer in men, and estimated 299,010 cases will be diagnosed in the United States this year.
According to Dawsey, genetic mutations seem to boost the risk of more aggressive disease—and the risk of other malignancies—by disrupting DNA repair. This process can lead to even more mutations that may “make the cancer behave and grow more aggressively.”
But not all prostate cancer patients need to undergo genetic testing. Dawsey urged colleagues to figure out which patients should be tested by consulting National Comprehensive Cancer Network (NCCN) guidelines and the newly updated US Department of Veterans Affairs (VA) prostate cancer clinical pathway.
The two sets of recommendations agree on germline testing in patients with cases that are metastatic, very high risk, and high risk. Lower-risk cases should only be tested if patients meet family history criteria. The sets of guidelines also recommend somatic testing in patients with metastatic cancer.
In addition to providing guidance about treatment, genetic test results can have implications regarding other potential malignancies that may affect patients, Dawsey said. The results may also have implications for cancer risk in family members.
Several drugs are now available for patients with genetic mutations, including checkpoint inhibitors and PARP inhibitors. The drugs, which have unique mechanisms of action, are given in addition to standard prostate cancer treatments, he said.
“If a patient doesn’t have one of these genetic changes,” he said, “these drugs aren’t an option.”
A long list of drugs or combinations of drugs are in clinical trials, including the poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors olaparib, abiraterone, and niraparib and the checkpoint inhibitors nivolumab and cemiplimab.
The drugs generally improve response rates and progression-free survival, Dawsey said, and patients are generally able to tolerate them. In regard to which drugs to choose, he suggested consulting the and NCCN guidelines and the VA oncology clinical pathway for prostate cancer.
Not too long ago, prostate-cancer genetics didn’t mean much to patient care. But in recent years, the landscape of therapy has transformed as researchers have discovered links between multiple genes and aggressive tumors.
Now, as a hematologist-oncologist explained to attendees at an Association of VA Hematology/Oncology regional meeting in Detroit, genetic tests can guide treatment for some—but not all—men with prostate cancer.
For patients with mutations, appropriate supplemental medications “can improve overall outcomes and have a long-standing impact on patients” said Scott J. Dawsey, MD, of the John D. Dingell Veterans Affairs Medical Center in Detroit in an interview following the AVAHO meeting, which focused on the management of prostate cancer.
As Dawsey explained, about 10% of patients with prostate cancer appear to have genetic mutations, although the exact percentage is unclear. The mutations are especially common in metastatic forms of prostate cancer. They’re estimated to be present in 11.8%-16.2% of those cases.
While these proportions are relatively small, the number of overall prostate-cancer cases with mutations is large due to the high burden of the disease, Dawsey said. Prostate cancer is by far the most common cancer in men, and estimated 299,010 cases will be diagnosed in the United States this year.
According to Dawsey, genetic mutations seem to boost the risk of more aggressive disease—and the risk of other malignancies—by disrupting DNA repair. This process can lead to even more mutations that may “make the cancer behave and grow more aggressively.”
But not all prostate cancer patients need to undergo genetic testing. Dawsey urged colleagues to figure out which patients should be tested by consulting National Comprehensive Cancer Network (NCCN) guidelines and the newly updated US Department of Veterans Affairs (VA) prostate cancer clinical pathway.
The two sets of recommendations agree on germline testing in patients with cases that are metastatic, very high risk, and high risk. Lower-risk cases should only be tested if patients meet family history criteria. The sets of guidelines also recommend somatic testing in patients with metastatic cancer.
In addition to providing guidance about treatment, genetic test results can have implications regarding other potential malignancies that may affect patients, Dawsey said. The results may also have implications for cancer risk in family members.
Several drugs are now available for patients with genetic mutations, including checkpoint inhibitors and PARP inhibitors. The drugs, which have unique mechanisms of action, are given in addition to standard prostate cancer treatments, he said.
“If a patient doesn’t have one of these genetic changes,” he said, “these drugs aren’t an option.”
A long list of drugs or combinations of drugs are in clinical trials, including the poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors olaparib, abiraterone, and niraparib and the checkpoint inhibitors nivolumab and cemiplimab.
The drugs generally improve response rates and progression-free survival, Dawsey said, and patients are generally able to tolerate them. In regard to which drugs to choose, he suggested consulting the and NCCN guidelines and the VA oncology clinical pathway for prostate cancer.
New Screening Protocol May Improve Prostate Cancer Detection
TOPLINE:
according to preliminary findings from the Finnish ProScreen randomized clinical trial.
METHODOLOGY:
- Prostate-specific antigen (PSA) screening is currently recommended for men in the United States starting at age 55. However, the test is controversial, in large part because it often detects prostate cancer that is not clinically relevant and may lead to overtreatment of men with low-grade disease.
- The current ProScreen trial assessed a screening intervention that aims to reduce unnecessary diagnoses of prostate cancer but still catch relevant cancers and reduce prostate cancer mortality.
- The researchers randomized 60,745 eligible men aged 50-63 years to be invited to a three-phase screening intervention (n = 15,201) or to be part of a control group that was not invited to screen (n = 45,544).
- The screening group who agreed to participate (n = 7744) first underwent a PSA test. Those with a PSA of ≥ 3.0 ng/mL then underwent a four-kallikrein panel to identify high-grade prostate cancer. Those with a kallikrein panel risk score of 7.5% or higher underwent an MRI of the prostate gland.
- Targeted biopsies were performed in those with abnormal prostate gland findings on MRI. Most patients with a negative MRI were not recommended for systematic biopsy unless they had a PSA density of ≥ 0.15 ng/mL.
TAKEAWAY:
- Among the 7744 invited men who agreed to the three-phase screening protocol (51%), ultimately 209 (2.7% of all screened participants) had a targeted transrectal prostate biopsy. Overall, 136 of the biopsies (65%) detected cancer — 32 low-grade and 128 high-grade prostate cancers, for cumulative incidence rates of 0.41% and 1.65%, respectively.
- Over a 3.2-year median follow-up among the 7457 invited men who refused screening, seven low-grade and 44 high-grade prostate cancers were detected (cumulative incidence rates, 0.1% and 0.6%, respectively).
- Among the entire invited screening group, 39 low-grade (cumulative incidence, 0.26%) and 172 high-grade prostate cancers (cumulative incidence, 1.13%) were detected.
- Among men in the control group, 65 low-grade prostate cancers were ultimately identified and 282 high-grade. The risk difference between the invited screening group and control group was 0.11% for low-grade disease and 0.51% for high-grade disease. Compared with the control group, the intervention led to the detection of one additional low-grade prostate cancer per 909 men invited to screen and one additional high-grade prostate cancer per 196 men invited.
IN PRACTICE:
The three-phase screening approach used in this study detected additional cancers, compared with a control group not invited for screening, but “these results are descriptive and should be interpreted provisionally pending results from the trial on the primary outcomes of prostate cancer mortality,” the investigators said.
SOURCE:
This study was conducted by the ProScreen Trial Investigators, including first author Anssi Auvinen, MD, PhD, of Tampere University, Tampere, Finland, and was published online in JAMAalongside an accompanying editorial.
LIMITATIONS:
Absolute differences between the two randomized groups in this study were small and had unclear clinical importance. Prior screening was reported by several participants and may have reduced cancer detection. The results are based on a single invitation for screening, meaning some high-grade cancers were likely missed; subsequent screening invitations may identify missed cancers. No data were available on cancers missed at screening, and interval cancer incidence is needed to assess sensitivity of the screening protocol used in the study.
DISCLOSURES:
The ProScreen trial is funded by grants from the Academy of Finland, the Finnish Cancer Foundation, the Jane and Aatos Erkko Foundation, the Finland State Research Funding, Helsinki University Hospital, the Sigrid Jusélius Foundation, and the Päivikki and Sakari Sohlberg Foundation. Dr. Auvinen reported having no disclosures. Multiple co-authors reported associations outside the submitted work. The full list of author disclosures is included with the full text of the article.
A version of this article appeared on Medscape.com.
TOPLINE:
according to preliminary findings from the Finnish ProScreen randomized clinical trial.
METHODOLOGY:
- Prostate-specific antigen (PSA) screening is currently recommended for men in the United States starting at age 55. However, the test is controversial, in large part because it often detects prostate cancer that is not clinically relevant and may lead to overtreatment of men with low-grade disease.
- The current ProScreen trial assessed a screening intervention that aims to reduce unnecessary diagnoses of prostate cancer but still catch relevant cancers and reduce prostate cancer mortality.
- The researchers randomized 60,745 eligible men aged 50-63 years to be invited to a three-phase screening intervention (n = 15,201) or to be part of a control group that was not invited to screen (n = 45,544).
- The screening group who agreed to participate (n = 7744) first underwent a PSA test. Those with a PSA of ≥ 3.0 ng/mL then underwent a four-kallikrein panel to identify high-grade prostate cancer. Those with a kallikrein panel risk score of 7.5% or higher underwent an MRI of the prostate gland.
- Targeted biopsies were performed in those with abnormal prostate gland findings on MRI. Most patients with a negative MRI were not recommended for systematic biopsy unless they had a PSA density of ≥ 0.15 ng/mL.
TAKEAWAY:
- Among the 7744 invited men who agreed to the three-phase screening protocol (51%), ultimately 209 (2.7% of all screened participants) had a targeted transrectal prostate biopsy. Overall, 136 of the biopsies (65%) detected cancer — 32 low-grade and 128 high-grade prostate cancers, for cumulative incidence rates of 0.41% and 1.65%, respectively.
- Over a 3.2-year median follow-up among the 7457 invited men who refused screening, seven low-grade and 44 high-grade prostate cancers were detected (cumulative incidence rates, 0.1% and 0.6%, respectively).
- Among the entire invited screening group, 39 low-grade (cumulative incidence, 0.26%) and 172 high-grade prostate cancers (cumulative incidence, 1.13%) were detected.
- Among men in the control group, 65 low-grade prostate cancers were ultimately identified and 282 high-grade. The risk difference between the invited screening group and control group was 0.11% for low-grade disease and 0.51% for high-grade disease. Compared with the control group, the intervention led to the detection of one additional low-grade prostate cancer per 909 men invited to screen and one additional high-grade prostate cancer per 196 men invited.
IN PRACTICE:
The three-phase screening approach used in this study detected additional cancers, compared with a control group not invited for screening, but “these results are descriptive and should be interpreted provisionally pending results from the trial on the primary outcomes of prostate cancer mortality,” the investigators said.
SOURCE:
This study was conducted by the ProScreen Trial Investigators, including first author Anssi Auvinen, MD, PhD, of Tampere University, Tampere, Finland, and was published online in JAMAalongside an accompanying editorial.
LIMITATIONS:
Absolute differences between the two randomized groups in this study were small and had unclear clinical importance. Prior screening was reported by several participants and may have reduced cancer detection. The results are based on a single invitation for screening, meaning some high-grade cancers were likely missed; subsequent screening invitations may identify missed cancers. No data were available on cancers missed at screening, and interval cancer incidence is needed to assess sensitivity of the screening protocol used in the study.
DISCLOSURES:
The ProScreen trial is funded by grants from the Academy of Finland, the Finnish Cancer Foundation, the Jane and Aatos Erkko Foundation, the Finland State Research Funding, Helsinki University Hospital, the Sigrid Jusélius Foundation, and the Päivikki and Sakari Sohlberg Foundation. Dr. Auvinen reported having no disclosures. Multiple co-authors reported associations outside the submitted work. The full list of author disclosures is included with the full text of the article.
A version of this article appeared on Medscape.com.
TOPLINE:
according to preliminary findings from the Finnish ProScreen randomized clinical trial.
METHODOLOGY:
- Prostate-specific antigen (PSA) screening is currently recommended for men in the United States starting at age 55. However, the test is controversial, in large part because it often detects prostate cancer that is not clinically relevant and may lead to overtreatment of men with low-grade disease.
- The current ProScreen trial assessed a screening intervention that aims to reduce unnecessary diagnoses of prostate cancer but still catch relevant cancers and reduce prostate cancer mortality.
- The researchers randomized 60,745 eligible men aged 50-63 years to be invited to a three-phase screening intervention (n = 15,201) or to be part of a control group that was not invited to screen (n = 45,544).
- The screening group who agreed to participate (n = 7744) first underwent a PSA test. Those with a PSA of ≥ 3.0 ng/mL then underwent a four-kallikrein panel to identify high-grade prostate cancer. Those with a kallikrein panel risk score of 7.5% or higher underwent an MRI of the prostate gland.
- Targeted biopsies were performed in those with abnormal prostate gland findings on MRI. Most patients with a negative MRI were not recommended for systematic biopsy unless they had a PSA density of ≥ 0.15 ng/mL.
TAKEAWAY:
- Among the 7744 invited men who agreed to the three-phase screening protocol (51%), ultimately 209 (2.7% of all screened participants) had a targeted transrectal prostate biopsy. Overall, 136 of the biopsies (65%) detected cancer — 32 low-grade and 128 high-grade prostate cancers, for cumulative incidence rates of 0.41% and 1.65%, respectively.
- Over a 3.2-year median follow-up among the 7457 invited men who refused screening, seven low-grade and 44 high-grade prostate cancers were detected (cumulative incidence rates, 0.1% and 0.6%, respectively).
- Among the entire invited screening group, 39 low-grade (cumulative incidence, 0.26%) and 172 high-grade prostate cancers (cumulative incidence, 1.13%) were detected.
- Among men in the control group, 65 low-grade prostate cancers were ultimately identified and 282 high-grade. The risk difference between the invited screening group and control group was 0.11% for low-grade disease and 0.51% for high-grade disease. Compared with the control group, the intervention led to the detection of one additional low-grade prostate cancer per 909 men invited to screen and one additional high-grade prostate cancer per 196 men invited.
IN PRACTICE:
The three-phase screening approach used in this study detected additional cancers, compared with a control group not invited for screening, but “these results are descriptive and should be interpreted provisionally pending results from the trial on the primary outcomes of prostate cancer mortality,” the investigators said.
SOURCE:
This study was conducted by the ProScreen Trial Investigators, including first author Anssi Auvinen, MD, PhD, of Tampere University, Tampere, Finland, and was published online in JAMAalongside an accompanying editorial.
LIMITATIONS:
Absolute differences between the two randomized groups in this study were small and had unclear clinical importance. Prior screening was reported by several participants and may have reduced cancer detection. The results are based on a single invitation for screening, meaning some high-grade cancers were likely missed; subsequent screening invitations may identify missed cancers. No data were available on cancers missed at screening, and interval cancer incidence is needed to assess sensitivity of the screening protocol used in the study.
DISCLOSURES:
The ProScreen trial is funded by grants from the Academy of Finland, the Finnish Cancer Foundation, the Jane and Aatos Erkko Foundation, the Finland State Research Funding, Helsinki University Hospital, the Sigrid Jusélius Foundation, and the Päivikki and Sakari Sohlberg Foundation. Dr. Auvinen reported having no disclosures. Multiple co-authors reported associations outside the submitted work. The full list of author disclosures is included with the full text of the article.
A version of this article appeared on Medscape.com.
How Long Should Active Surveillance Last?
Previous studies have shown that active surveillance continued for 15 years is appropriate to identify men who progress and need treatment, but now data out to 25 years “suggest that meticulous follow-up is needed over a longer time if the chance for cure is not to be missed,” said Emmeli Palmstedt, PhD, a research student in the Department of Urology at the Sahlgrenska Academy at the University of Gothenburg, Sweden. “These data are crucial, given the long current life expectancy” of men in otherwise good health.
Dr. Palmstedt presented the findings at the 2024 annual meeting of the European Association of Urology.
At many centers, active surveillance is a standard of care for men with low-risk prostate cancer based on a benefit-to-risk ratio that favors delayed intervention, according to Palmstedt. Several studies, including the Göteburg-1 active surveillance trial initiated at her institution, have supported follow-up for 15 years. A new set of data from Göteborg now extends to 25 years.
Long-Life Expectancy Justifies Extended Surveillance
The prospective Göteborg study began enrolling men with very low- or low-risk (78%) or intermediate-risk (22%) prostate cancer in 1995. In the active surveillance program, prostate-specific antigen (PSA) was measured routinely with biopsies ordered for PSA levels ≥ 2.5 ng/mL.
In an analysis published in 2016 when 202 (43%) of 474 patients managed with active surveillance had discontinued surveillance to start treatment, the median follow-up period was 8 years. The rate of mortality associated with prostate cancer at 15 years was estimated to be 0% for men in the very low-risk group, 4% for men in the low-risk group, and 10% for those with intermediate-risk tumors. The estimates for failure-free survival at 15 years were 88%, 77%, and 40% for the very low-, low-, and intermediate-risk groups, respectively.
In the most recent follow-up, when the median age in the Göteburg-1 study was 80 years (the median age at diagnosis was 66 years), the median follow-up period was 15.1 years with a range of up to 28.1 years. In this analysis, which focused on patients with low-risk prostate cancer at baseline, discontinuations from active surveillance had climbed to 47%. Most of these men discontinued to initiate treatment, but 79 (16%) had failed acute surveillance, meaning their progression was not caught in time for curative-intent treatment, and 2% had died from prostate cancer.
Treatment-Free Survival Falls to 31%
The rate of treatment-free survival, which was estimated to be 65% in the 15-year analysis published in 2016, had declined to 31%. The rate of failure-free survival was 59%, and prostate cancer-specific survival was 92%, according to the researchers.
While Dr. Palmstedt did not separate out her data for very low- and low-risk patients, she noted that deaths from prostate cancer among all low-risk patients climbed fourfold (8% vs 2%) since the 2016 figures were published. The proportion of men no longer failure-free climbed from 10% to more than 40%.
“These are non-negligible numbers,” said Dr. Palmstedt, who added that overall survival fell from 69% at 15 years to 37% at 25 years.
Although some men between the 15-year and 25-year timepoints were switched to watchful waiting, these data have not yet been analyzed.
The low rate of deaths from prostate cancer over the extended period is reassuring, Dr. Palmstedt said, but the main message from the new study is that active surveillance permits curative-intent treatment to be offered even after late follow-up. She emphasized that patients without progression by 15 years cannot be considered “safe.”
Based on these data, “men with a long remaining life expectancy should be informed that active surveillance is still viable after 15 years,” Dr. Palmstedt said.
Active Surveillance Now More Common
Over the past decade, the proportion of men with prostate cancer managed with active surveillance has been rising steadily, according to Matthew R. Cooperberg, MD, MPH, professor of urology at the University of California, San Francisco. In a study published last year in JAMA Network Open, Dr. Cooperberg and his colleagues reported that rates of active surveillance rose from 26.5% in 2014 to 59.6% in 2021. However, given the value of the approach for avoiding overtreatment of men with low-risk prostate cancers, even that increase is not enough, he said.
“The window of opportunity for cure is typically very wide,” Dr. Cooperberg said. Although many men “will never need treatment ... long-term surveillance is definitely important” for those that do, he said. The data from trials like Göteborg-1 support the principle that this strategy still preserves the option of treatment when it is needed.
“Treatment for cure at age 70 is generally far preferable to treatment at 55, and surveillance should absolutely be preferred treatment for the vast majority of men with low-grade disease at diagnosis,” he explained.
Dr. Palmstedt reported no potential conflicts of interest. Dr. Cooperberg reported financial relationships with Astellas, AstraZeneca, Bayer, Dendreon, Exact Sciences, Janssen, Merck, Pfizer, and Verana Health.
A version of this article appeared on Medscape.com.
Previous studies have shown that active surveillance continued for 15 years is appropriate to identify men who progress and need treatment, but now data out to 25 years “suggest that meticulous follow-up is needed over a longer time if the chance for cure is not to be missed,” said Emmeli Palmstedt, PhD, a research student in the Department of Urology at the Sahlgrenska Academy at the University of Gothenburg, Sweden. “These data are crucial, given the long current life expectancy” of men in otherwise good health.
Dr. Palmstedt presented the findings at the 2024 annual meeting of the European Association of Urology.
At many centers, active surveillance is a standard of care for men with low-risk prostate cancer based on a benefit-to-risk ratio that favors delayed intervention, according to Palmstedt. Several studies, including the Göteburg-1 active surveillance trial initiated at her institution, have supported follow-up for 15 years. A new set of data from Göteborg now extends to 25 years.
Long-Life Expectancy Justifies Extended Surveillance
The prospective Göteborg study began enrolling men with very low- or low-risk (78%) or intermediate-risk (22%) prostate cancer in 1995. In the active surveillance program, prostate-specific antigen (PSA) was measured routinely with biopsies ordered for PSA levels ≥ 2.5 ng/mL.
In an analysis published in 2016 when 202 (43%) of 474 patients managed with active surveillance had discontinued surveillance to start treatment, the median follow-up period was 8 years. The rate of mortality associated with prostate cancer at 15 years was estimated to be 0% for men in the very low-risk group, 4% for men in the low-risk group, and 10% for those with intermediate-risk tumors. The estimates for failure-free survival at 15 years were 88%, 77%, and 40% for the very low-, low-, and intermediate-risk groups, respectively.
In the most recent follow-up, when the median age in the Göteburg-1 study was 80 years (the median age at diagnosis was 66 years), the median follow-up period was 15.1 years with a range of up to 28.1 years. In this analysis, which focused on patients with low-risk prostate cancer at baseline, discontinuations from active surveillance had climbed to 47%. Most of these men discontinued to initiate treatment, but 79 (16%) had failed acute surveillance, meaning their progression was not caught in time for curative-intent treatment, and 2% had died from prostate cancer.
Treatment-Free Survival Falls to 31%
The rate of treatment-free survival, which was estimated to be 65% in the 15-year analysis published in 2016, had declined to 31%. The rate of failure-free survival was 59%, and prostate cancer-specific survival was 92%, according to the researchers.
While Dr. Palmstedt did not separate out her data for very low- and low-risk patients, she noted that deaths from prostate cancer among all low-risk patients climbed fourfold (8% vs 2%) since the 2016 figures were published. The proportion of men no longer failure-free climbed from 10% to more than 40%.
“These are non-negligible numbers,” said Dr. Palmstedt, who added that overall survival fell from 69% at 15 years to 37% at 25 years.
Although some men between the 15-year and 25-year timepoints were switched to watchful waiting, these data have not yet been analyzed.
The low rate of deaths from prostate cancer over the extended period is reassuring, Dr. Palmstedt said, but the main message from the new study is that active surveillance permits curative-intent treatment to be offered even after late follow-up. She emphasized that patients without progression by 15 years cannot be considered “safe.”
Based on these data, “men with a long remaining life expectancy should be informed that active surveillance is still viable after 15 years,” Dr. Palmstedt said.
Active Surveillance Now More Common
Over the past decade, the proportion of men with prostate cancer managed with active surveillance has been rising steadily, according to Matthew R. Cooperberg, MD, MPH, professor of urology at the University of California, San Francisco. In a study published last year in JAMA Network Open, Dr. Cooperberg and his colleagues reported that rates of active surveillance rose from 26.5% in 2014 to 59.6% in 2021. However, given the value of the approach for avoiding overtreatment of men with low-risk prostate cancers, even that increase is not enough, he said.
“The window of opportunity for cure is typically very wide,” Dr. Cooperberg said. Although many men “will never need treatment ... long-term surveillance is definitely important” for those that do, he said. The data from trials like Göteborg-1 support the principle that this strategy still preserves the option of treatment when it is needed.
“Treatment for cure at age 70 is generally far preferable to treatment at 55, and surveillance should absolutely be preferred treatment for the vast majority of men with low-grade disease at diagnosis,” he explained.
Dr. Palmstedt reported no potential conflicts of interest. Dr. Cooperberg reported financial relationships with Astellas, AstraZeneca, Bayer, Dendreon, Exact Sciences, Janssen, Merck, Pfizer, and Verana Health.
A version of this article appeared on Medscape.com.
Previous studies have shown that active surveillance continued for 15 years is appropriate to identify men who progress and need treatment, but now data out to 25 years “suggest that meticulous follow-up is needed over a longer time if the chance for cure is not to be missed,” said Emmeli Palmstedt, PhD, a research student in the Department of Urology at the Sahlgrenska Academy at the University of Gothenburg, Sweden. “These data are crucial, given the long current life expectancy” of men in otherwise good health.
Dr. Palmstedt presented the findings at the 2024 annual meeting of the European Association of Urology.
At many centers, active surveillance is a standard of care for men with low-risk prostate cancer based on a benefit-to-risk ratio that favors delayed intervention, according to Palmstedt. Several studies, including the Göteburg-1 active surveillance trial initiated at her institution, have supported follow-up for 15 years. A new set of data from Göteborg now extends to 25 years.
Long-Life Expectancy Justifies Extended Surveillance
The prospective Göteborg study began enrolling men with very low- or low-risk (78%) or intermediate-risk (22%) prostate cancer in 1995. In the active surveillance program, prostate-specific antigen (PSA) was measured routinely with biopsies ordered for PSA levels ≥ 2.5 ng/mL.
In an analysis published in 2016 when 202 (43%) of 474 patients managed with active surveillance had discontinued surveillance to start treatment, the median follow-up period was 8 years. The rate of mortality associated with prostate cancer at 15 years was estimated to be 0% for men in the very low-risk group, 4% for men in the low-risk group, and 10% for those with intermediate-risk tumors. The estimates for failure-free survival at 15 years were 88%, 77%, and 40% for the very low-, low-, and intermediate-risk groups, respectively.
In the most recent follow-up, when the median age in the Göteburg-1 study was 80 years (the median age at diagnosis was 66 years), the median follow-up period was 15.1 years with a range of up to 28.1 years. In this analysis, which focused on patients with low-risk prostate cancer at baseline, discontinuations from active surveillance had climbed to 47%. Most of these men discontinued to initiate treatment, but 79 (16%) had failed acute surveillance, meaning their progression was not caught in time for curative-intent treatment, and 2% had died from prostate cancer.
Treatment-Free Survival Falls to 31%
The rate of treatment-free survival, which was estimated to be 65% in the 15-year analysis published in 2016, had declined to 31%. The rate of failure-free survival was 59%, and prostate cancer-specific survival was 92%, according to the researchers.
While Dr. Palmstedt did not separate out her data for very low- and low-risk patients, she noted that deaths from prostate cancer among all low-risk patients climbed fourfold (8% vs 2%) since the 2016 figures were published. The proportion of men no longer failure-free climbed from 10% to more than 40%.
“These are non-negligible numbers,” said Dr. Palmstedt, who added that overall survival fell from 69% at 15 years to 37% at 25 years.
Although some men between the 15-year and 25-year timepoints were switched to watchful waiting, these data have not yet been analyzed.
The low rate of deaths from prostate cancer over the extended period is reassuring, Dr. Palmstedt said, but the main message from the new study is that active surveillance permits curative-intent treatment to be offered even after late follow-up. She emphasized that patients without progression by 15 years cannot be considered “safe.”
Based on these data, “men with a long remaining life expectancy should be informed that active surveillance is still viable after 15 years,” Dr. Palmstedt said.
Active Surveillance Now More Common
Over the past decade, the proportion of men with prostate cancer managed with active surveillance has been rising steadily, according to Matthew R. Cooperberg, MD, MPH, professor of urology at the University of California, San Francisco. In a study published last year in JAMA Network Open, Dr. Cooperberg and his colleagues reported that rates of active surveillance rose from 26.5% in 2014 to 59.6% in 2021. However, given the value of the approach for avoiding overtreatment of men with low-risk prostate cancers, even that increase is not enough, he said.
“The window of opportunity for cure is typically very wide,” Dr. Cooperberg said. Although many men “will never need treatment ... long-term surveillance is definitely important” for those that do, he said. The data from trials like Göteborg-1 support the principle that this strategy still preserves the option of treatment when it is needed.
“Treatment for cure at age 70 is generally far preferable to treatment at 55, and surveillance should absolutely be preferred treatment for the vast majority of men with low-grade disease at diagnosis,” he explained.
Dr. Palmstedt reported no potential conflicts of interest. Dr. Cooperberg reported financial relationships with Astellas, AstraZeneca, Bayer, Dendreon, Exact Sciences, Janssen, Merck, Pfizer, and Verana Health.
A version of this article appeared on Medscape.com.
Meat Linked to Higher Erectile Dysfunction Risk
Rachel S. Rubin, MD: Welcome to another episode of Sex Matters. I’m Dr. Rachel Rubin. I’m a urologist and sexual medicine specialist based in the Washington, DC area, and I interview amazingly cool people doing research in sexual medicine.
I heard an incredible lecture while I was at the Mayo Clinic urology conference by Dr. Stacy Loeb, who is a wonderful researcher of all things prostate cancer and men’s health, who is now talking more plant-based diets. Her lecture was so good, I begged her to join me for this discussion.
Dr. Loeb, I would love for you to introduce yourself.
Stacy Loeb, MD: I’m Dr. Loeb. I’m a urologist at New York University in the Manhattan VA, and I recently became board certified in lifestyle medicine because it’s so important for sexual health and, really, everything that we do.
Dr. Rubin: You recently became very interested in studying plant-based diets. How did that start, and how has the research evolved over time?
Dr. Loeb: It’s really amazing. For one thing, more of our patients with prostate cancer die of heart disease than of prostate cancer. And erectile dysfunction is really an early warning sign of cardiovascular disease. We felt like it was incumbent upon us, even within urology and sexual medicine, to better understand the basis for lifestyle modification that can help with these issues.
Dr. Rubin: Tell us more about what you found for erectile dysfunction. How much benefit do people get by switching to a plant-based diet?
Dr. Loeb: First we looked at erectile function in men without prostate cancer in the health professionals follow-up study, a very large cohort study out of Harvard University. We found that among omnivorous people, those who ate more plant-based and less animal-based food were less likely to have incident erectile dysfunction. Then, we published a new paper looking at patients with prostate cancer. These men have extra challenges for sexual function because in addition to the standard cardiovascular changes with aging, prostate cancer treatment can affect the nerves that are involved in erections. But amazingly, even in that population, we found that the men who ate more plant-based and less animal-based food had better scores for erectile function.
That was really good news, and it’s a win-win. There is no reason not to counsel our patients to eat more plant-based foods. Meat is not masculine. Meat is associated with a higher risk for erectile dysfunction and is considered carcinogenic. It’s just something that we should try to stay away from.
Dr. Rubin: How do you counsel patients who might not be ready to go fully plant-based? Is a little better than nothing? How do you even start these conversations with people? Do you have any tips for primary care doctors?
Dr. Loeb: Great question. A little bit is very much better than nothing. In fact, in the health professionals follow-up study, we actually looked at quintiles of people who ate the most meat and animal-based foods and the least plant-based foods all the way up to the most plant-based and the least animal-based diets. Along that spectrum, it really does make a big difference. Anywhere that patients can start from is definitely better than nothing.
Simple things such as Meatless Monday or choosing a few days that they will give up animal-based foods will help. For some people, trying new things is easier than cutting things out, for example, trying a milk substitute such as oat, almond, or soy milk instead of dairy milk. That could be a great first step, or trying some dishes that don’t include meat — maybe a tofu stir fry or a taco or burrito without the meat.
There are many great options out there. In terms of resources for doctors, the Physicians Committee for Responsible Medicine has a great website. They have fact sheets for a lot of the common questions that people ask such as how can I get enough protein or calcium on a plant-based diet? This isn’t a problem at all. In fact, Novak Djokovic and many other elite athletes eat plant-based diets, and they get enough protein with a much higher requirement than most of us who are not elite athletes. These fact sheets explain which plant foods are the best
I also like Nutritionfacts.org. They also have all kinds of great videos and resources. Both of these websites have recipes that were created by doctors and nutritionists.
We can suggest that our patients work with a nutritionist or join a virtual program. For example, Plant Powered here in New York has virtual plant-based jumpstart programs. People around the country can get in on programs that have nutritionists and health coaches — for people who want a boost.
Dr. Rubin: The data are really compelling. When you were speaking, not a person in the room was interested in having a steak that night for dinner, even with a steakhouse in the hotel.
What do you say to men who have prostate cancer or suffer from erectile dysfunction? Do any data show that by going plant-based you may show improvements? We have recent studies that show that regular exercise might be as good as Viagra.
Dr. Loeb: It’s definitely not too late, even if you’ve already been diagnosed with these conditions. In my own practice, I have seen changes in patients. In fact, one of the case scenarios that I submitted for the lifestyle medicine boards was a patient who adopted a whole food, plant-based diet and no longer uses Viagra. This is definitely something that’s possible to do with intensive lifestyle modification.
Dr. Rubin: Maybe vegetables are the new sexual health aide. How can people find out more? I know you have a Sirius XM radio show.
Dr. Loeb: It’s the Men’s Health Show on Sirius XM channel 110. It’s on Wednesdays from 6:00 to 8:00 PM ET, or you can listen to it on demand anytime through the Sirius XM app.
Dr. Rubin: You have done an enormous amount of research in prostate cancer and sexual medicine. You are an all-star in the field. Thank you for sharing all of your knowledge about plant-based diets. You’ve given us all a lot to think about today.
Dr. Rubin has disclosed the following relevant financial relationships: Serve(d) as a speaker for Sprout; received research grant from Maternal Medical; received income in an amount equal to or greater than $250 from Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
Rachel S. Rubin, MD: Welcome to another episode of Sex Matters. I’m Dr. Rachel Rubin. I’m a urologist and sexual medicine specialist based in the Washington, DC area, and I interview amazingly cool people doing research in sexual medicine.
I heard an incredible lecture while I was at the Mayo Clinic urology conference by Dr. Stacy Loeb, who is a wonderful researcher of all things prostate cancer and men’s health, who is now talking more plant-based diets. Her lecture was so good, I begged her to join me for this discussion.
Dr. Loeb, I would love for you to introduce yourself.
Stacy Loeb, MD: I’m Dr. Loeb. I’m a urologist at New York University in the Manhattan VA, and I recently became board certified in lifestyle medicine because it’s so important for sexual health and, really, everything that we do.
Dr. Rubin: You recently became very interested in studying plant-based diets. How did that start, and how has the research evolved over time?
Dr. Loeb: It’s really amazing. For one thing, more of our patients with prostate cancer die of heart disease than of prostate cancer. And erectile dysfunction is really an early warning sign of cardiovascular disease. We felt like it was incumbent upon us, even within urology and sexual medicine, to better understand the basis for lifestyle modification that can help with these issues.
Dr. Rubin: Tell us more about what you found for erectile dysfunction. How much benefit do people get by switching to a plant-based diet?
Dr. Loeb: First we looked at erectile function in men without prostate cancer in the health professionals follow-up study, a very large cohort study out of Harvard University. We found that among omnivorous people, those who ate more plant-based and less animal-based food were less likely to have incident erectile dysfunction. Then, we published a new paper looking at patients with prostate cancer. These men have extra challenges for sexual function because in addition to the standard cardiovascular changes with aging, prostate cancer treatment can affect the nerves that are involved in erections. But amazingly, even in that population, we found that the men who ate more plant-based and less animal-based food had better scores for erectile function.
That was really good news, and it’s a win-win. There is no reason not to counsel our patients to eat more plant-based foods. Meat is not masculine. Meat is associated with a higher risk for erectile dysfunction and is considered carcinogenic. It’s just something that we should try to stay away from.
Dr. Rubin: How do you counsel patients who might not be ready to go fully plant-based? Is a little better than nothing? How do you even start these conversations with people? Do you have any tips for primary care doctors?
Dr. Loeb: Great question. A little bit is very much better than nothing. In fact, in the health professionals follow-up study, we actually looked at quintiles of people who ate the most meat and animal-based foods and the least plant-based foods all the way up to the most plant-based and the least animal-based diets. Along that spectrum, it really does make a big difference. Anywhere that patients can start from is definitely better than nothing.
Simple things such as Meatless Monday or choosing a few days that they will give up animal-based foods will help. For some people, trying new things is easier than cutting things out, for example, trying a milk substitute such as oat, almond, or soy milk instead of dairy milk. That could be a great first step, or trying some dishes that don’t include meat — maybe a tofu stir fry or a taco or burrito without the meat.
There are many great options out there. In terms of resources for doctors, the Physicians Committee for Responsible Medicine has a great website. They have fact sheets for a lot of the common questions that people ask such as how can I get enough protein or calcium on a plant-based diet? This isn’t a problem at all. In fact, Novak Djokovic and many other elite athletes eat plant-based diets, and they get enough protein with a much higher requirement than most of us who are not elite athletes. These fact sheets explain which plant foods are the best
I also like Nutritionfacts.org. They also have all kinds of great videos and resources. Both of these websites have recipes that were created by doctors and nutritionists.
We can suggest that our patients work with a nutritionist or join a virtual program. For example, Plant Powered here in New York has virtual plant-based jumpstart programs. People around the country can get in on programs that have nutritionists and health coaches — for people who want a boost.
Dr. Rubin: The data are really compelling. When you were speaking, not a person in the room was interested in having a steak that night for dinner, even with a steakhouse in the hotel.
What do you say to men who have prostate cancer or suffer from erectile dysfunction? Do any data show that by going plant-based you may show improvements? We have recent studies that show that regular exercise might be as good as Viagra.
Dr. Loeb: It’s definitely not too late, even if you’ve already been diagnosed with these conditions. In my own practice, I have seen changes in patients. In fact, one of the case scenarios that I submitted for the lifestyle medicine boards was a patient who adopted a whole food, plant-based diet and no longer uses Viagra. This is definitely something that’s possible to do with intensive lifestyle modification.
Dr. Rubin: Maybe vegetables are the new sexual health aide. How can people find out more? I know you have a Sirius XM radio show.
Dr. Loeb: It’s the Men’s Health Show on Sirius XM channel 110. It’s on Wednesdays from 6:00 to 8:00 PM ET, or you can listen to it on demand anytime through the Sirius XM app.
Dr. Rubin: You have done an enormous amount of research in prostate cancer and sexual medicine. You are an all-star in the field. Thank you for sharing all of your knowledge about plant-based diets. You’ve given us all a lot to think about today.
Dr. Rubin has disclosed the following relevant financial relationships: Serve(d) as a speaker for Sprout; received research grant from Maternal Medical; received income in an amount equal to or greater than $250 from Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
Rachel S. Rubin, MD: Welcome to another episode of Sex Matters. I’m Dr. Rachel Rubin. I’m a urologist and sexual medicine specialist based in the Washington, DC area, and I interview amazingly cool people doing research in sexual medicine.
I heard an incredible lecture while I was at the Mayo Clinic urology conference by Dr. Stacy Loeb, who is a wonderful researcher of all things prostate cancer and men’s health, who is now talking more plant-based diets. Her lecture was so good, I begged her to join me for this discussion.
Dr. Loeb, I would love for you to introduce yourself.
Stacy Loeb, MD: I’m Dr. Loeb. I’m a urologist at New York University in the Manhattan VA, and I recently became board certified in lifestyle medicine because it’s so important for sexual health and, really, everything that we do.
Dr. Rubin: You recently became very interested in studying plant-based diets. How did that start, and how has the research evolved over time?
Dr. Loeb: It’s really amazing. For one thing, more of our patients with prostate cancer die of heart disease than of prostate cancer. And erectile dysfunction is really an early warning sign of cardiovascular disease. We felt like it was incumbent upon us, even within urology and sexual medicine, to better understand the basis for lifestyle modification that can help with these issues.
Dr. Rubin: Tell us more about what you found for erectile dysfunction. How much benefit do people get by switching to a plant-based diet?
Dr. Loeb: First we looked at erectile function in men without prostate cancer in the health professionals follow-up study, a very large cohort study out of Harvard University. We found that among omnivorous people, those who ate more plant-based and less animal-based food were less likely to have incident erectile dysfunction. Then, we published a new paper looking at patients with prostate cancer. These men have extra challenges for sexual function because in addition to the standard cardiovascular changes with aging, prostate cancer treatment can affect the nerves that are involved in erections. But amazingly, even in that population, we found that the men who ate more plant-based and less animal-based food had better scores for erectile function.
That was really good news, and it’s a win-win. There is no reason not to counsel our patients to eat more plant-based foods. Meat is not masculine. Meat is associated with a higher risk for erectile dysfunction and is considered carcinogenic. It’s just something that we should try to stay away from.
Dr. Rubin: How do you counsel patients who might not be ready to go fully plant-based? Is a little better than nothing? How do you even start these conversations with people? Do you have any tips for primary care doctors?
Dr. Loeb: Great question. A little bit is very much better than nothing. In fact, in the health professionals follow-up study, we actually looked at quintiles of people who ate the most meat and animal-based foods and the least plant-based foods all the way up to the most plant-based and the least animal-based diets. Along that spectrum, it really does make a big difference. Anywhere that patients can start from is definitely better than nothing.
Simple things such as Meatless Monday or choosing a few days that they will give up animal-based foods will help. For some people, trying new things is easier than cutting things out, for example, trying a milk substitute such as oat, almond, or soy milk instead of dairy milk. That could be a great first step, or trying some dishes that don’t include meat — maybe a tofu stir fry or a taco or burrito without the meat.
There are many great options out there. In terms of resources for doctors, the Physicians Committee for Responsible Medicine has a great website. They have fact sheets for a lot of the common questions that people ask such as how can I get enough protein or calcium on a plant-based diet? This isn’t a problem at all. In fact, Novak Djokovic and many other elite athletes eat plant-based diets, and they get enough protein with a much higher requirement than most of us who are not elite athletes. These fact sheets explain which plant foods are the best
I also like Nutritionfacts.org. They also have all kinds of great videos and resources. Both of these websites have recipes that were created by doctors and nutritionists.
We can suggest that our patients work with a nutritionist or join a virtual program. For example, Plant Powered here in New York has virtual plant-based jumpstart programs. People around the country can get in on programs that have nutritionists and health coaches — for people who want a boost.
Dr. Rubin: The data are really compelling. When you were speaking, not a person in the room was interested in having a steak that night for dinner, even with a steakhouse in the hotel.
What do you say to men who have prostate cancer or suffer from erectile dysfunction? Do any data show that by going plant-based you may show improvements? We have recent studies that show that regular exercise might be as good as Viagra.
Dr. Loeb: It’s definitely not too late, even if you’ve already been diagnosed with these conditions. In my own practice, I have seen changes in patients. In fact, one of the case scenarios that I submitted for the lifestyle medicine boards was a patient who adopted a whole food, plant-based diet and no longer uses Viagra. This is definitely something that’s possible to do with intensive lifestyle modification.
Dr. Rubin: Maybe vegetables are the new sexual health aide. How can people find out more? I know you have a Sirius XM radio show.
Dr. Loeb: It’s the Men’s Health Show on Sirius XM channel 110. It’s on Wednesdays from 6:00 to 8:00 PM ET, or you can listen to it on demand anytime through the Sirius XM app.
Dr. Rubin: You have done an enormous amount of research in prostate cancer and sexual medicine. You are an all-star in the field. Thank you for sharing all of your knowledge about plant-based diets. You’ve given us all a lot to think about today.
Dr. Rubin has disclosed the following relevant financial relationships: Serve(d) as a speaker for Sprout; received research grant from Maternal Medical; received income in an amount equal to or greater than $250 from Absorption Pharmaceuticals, GSK, and Endo.
A version of this article appeared on Medscape.com.
Repeat MCED Testing May ID Early-Stage and Unscreened Cancers
This was the conclusion of recent data presented by Ora Karp Gordon, MD, MS, during a session at the American Association for Cancer Research annual meeting.
The MCED test, known as Galleri, was made clinically available in the United States in April 2021. Developed by GRAIL LLC, the test analyzes cell-free DNA in the blood using targeted methylation analysis and machine learning to detect the presence of a cancer signal and determine its organ of origin or cancer signal origin. The initial screening of over 53,000 individuals with the Galleri test detected a cancer signal in 1.1% of participants.
The new real-world analysis examines the outcomes of repeat MCED testing in 5,794 individuals.
The study looked at individuals who initially received a ‘no cancer signal detected’ result and then underwent a second Galleri test. Over 80% of participants received their follow-up test 10-18 months after the first, with a median interval between blood draws of 12.9 months.
“The repeat tests detect those cancer cases that have reached the detection threshold since their last MCED test, which should be less than one year of incidence,” Dr. Gordon, professor at Saint John’s Cancer Institute, Santa Monica, California, said in an interview. “We are just now starting to see results from patients who get their second and even third round of screening.”
“Galleri is recommended to be used annually in addition to USPSTF [US Preventive Services Task Force]–recommended cancer screening tests, like mammography and colonoscopy,” she said.
This recommendation is based on a modeling study suggesting that annual screening would improve stage shift, diagnostic yield, and potentially mortality when compared to biennial screening, although biennial screening was still favorable compared with no screening, she explained.
Early Real-World Evidence of Repeat Testing
Among the cohort of 5,794 individuals who received repeat testing, 26 received a positive cancer signal on their second test, yielding a cancer signal detection rate of 0.45% (95% CI: 0.31%-0.66%). The cancer signal detection rate was slightly higher in men. The rate was 0.50% (95% CI: 0.32%-0.81%; 17 of 3367) in men versus 0.37% (95% CI: 0.2%-0.7%; 9 of 2427) in women.
During her presentation, Dr. Gordon highlighted that the repeat testing signal detection rate was lower than the initial 0.95% rate (95% CI: 0.87-1.0; 510 of 53,744) seen in the previous larger cohort of patients who were retested at 1 year.
She acknowledged that the lower cancer signal detection rate of repeat testing may indicate some degree of ‘early adopter’ bias, where those who return for a second test are systematically different from the general screening population. This could suggest that broader population-level screening may yield different results, she continued.
Shift Toward Unscreened Cancers
The top cancer types identified in the second round of testing were lymphoid, head and neck, bladder/urothelial, colorectal, and anal cancers. Clinicians were able to confirm clinical outcomes in 12 of 26 cases, in which cancer signals were detected. Of those 12 cases, 8 individuals received a cancer diagnosis and 4 did not have cancer. The remaining 14 of 26 cases in which cancer signals were detected are still under investigation.
“We found a shift away from USPSTF screen-detected cancers, like breast, lung, and prostate, and relative increase in unscreened urinary, head and neck, and lymphoid cancers, with 75% of cancers being those without any screening guidelines,” Dr. Gordon said in an interview.
She added that patients who choose to retest may have different cancer rates for several reasons, including bias toward a population that is health conscious and adhered to all recommended cancer screening.
“So the shift toward unscreened cancers is not unexpected and highlights the value of Galleri,” she said, but also acknowledged that “continued monitoring is needed to see if this translates in a persistent finding over time and tests.”
Shift Toward Early-Stage Cancers
Staging information was available for five cases, and Dr. Gordon highlighted in her talk that four of these confirmed cancers were stage I, including cancers of the anus, head and neck, bladder, and lymphoma. The fifth confirmed cancer with staging information was stage IV ovarian cancer.
“It is still early, and the numbers are very small, but the detection of early-stage cancers with second annual testing is very encouraging as these are the cases where MCED testing could have the greatest impact in improving outcomes through earlier treatment,” Dr. Gordon told this publication.
During an interview after the talk, Kenneth L. Kehl, MD, MPH, echoed that data must be confirmed in larger cohorts.
“The shift toward earlier stage cancers that are less detectable by standard screening methods is an interesting result, but we need to be cautious since the numbers were relatively small, and we do not have data on cancers that were diagnosed among patients whose second MCED test was also negative,” said Dr. Kehl, a medical oncologist at Dana-Farber Cancer Institute, Boston.
MCED Results Could Help Direct Diagnostic Workup
The test’s ability to predict the organ of origin was highly accurate, correctly identifying the cancer type in all eight confirmed cases. Among the eight cases with a confirmed cancer diagnosis, the accuracy of the first prediction was 100%, and diagnoses included invasive cancers across multiple tissues and organs, including anus, colon, head and neck, urothelial tract, ovary, and the lymphatic system.
“The fact that the site of origin for 100% of confirmed cancers was accurately predicted with GRAIL’s CSO by Galleri test confirms the promise that this can guide workup when a cancer signal is detected,” Dr. Gordon noted in the interview.
Looking Ahead
Dr. Kehl, who was not involved in the MCED study, noted in an interview that “further data on test characteristics beyond positive predictive value, including the sensitivity, specificity, and negative predictive value, as well as demonstration of clinical benefit — ideally in a randomized trial — will likely be required for MCED testing to become a standard public health recommendation.”
He added that challenges associated with implementing annual screening with MCED tests include the risks of both false positives and false negatives as testing becomes more widely available.
“False positives cause anxiety and lead to additional testing that may carry its own risks, and we need to understand if potentially false negative tests will be associated with less uptake of established screening strategies,” Dr. Kehl said in an interview. However, he noted that serial testing could lead to more frequent diagnoses of early-stage cancers that may be less detectable by standard methods.
Dr. Gordon reported financial relationships with GRAIL LLC and Genetic Technologies Corporation. Dr. Kehl reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing healthcare products used by or on patients.
This was the conclusion of recent data presented by Ora Karp Gordon, MD, MS, during a session at the American Association for Cancer Research annual meeting.
The MCED test, known as Galleri, was made clinically available in the United States in April 2021. Developed by GRAIL LLC, the test analyzes cell-free DNA in the blood using targeted methylation analysis and machine learning to detect the presence of a cancer signal and determine its organ of origin or cancer signal origin. The initial screening of over 53,000 individuals with the Galleri test detected a cancer signal in 1.1% of participants.
The new real-world analysis examines the outcomes of repeat MCED testing in 5,794 individuals.
The study looked at individuals who initially received a ‘no cancer signal detected’ result and then underwent a second Galleri test. Over 80% of participants received their follow-up test 10-18 months after the first, with a median interval between blood draws of 12.9 months.
“The repeat tests detect those cancer cases that have reached the detection threshold since their last MCED test, which should be less than one year of incidence,” Dr. Gordon, professor at Saint John’s Cancer Institute, Santa Monica, California, said in an interview. “We are just now starting to see results from patients who get their second and even third round of screening.”
“Galleri is recommended to be used annually in addition to USPSTF [US Preventive Services Task Force]–recommended cancer screening tests, like mammography and colonoscopy,” she said.
This recommendation is based on a modeling study suggesting that annual screening would improve stage shift, diagnostic yield, and potentially mortality when compared to biennial screening, although biennial screening was still favorable compared with no screening, she explained.
Early Real-World Evidence of Repeat Testing
Among the cohort of 5,794 individuals who received repeat testing, 26 received a positive cancer signal on their second test, yielding a cancer signal detection rate of 0.45% (95% CI: 0.31%-0.66%). The cancer signal detection rate was slightly higher in men. The rate was 0.50% (95% CI: 0.32%-0.81%; 17 of 3367) in men versus 0.37% (95% CI: 0.2%-0.7%; 9 of 2427) in women.
During her presentation, Dr. Gordon highlighted that the repeat testing signal detection rate was lower than the initial 0.95% rate (95% CI: 0.87-1.0; 510 of 53,744) seen in the previous larger cohort of patients who were retested at 1 year.
She acknowledged that the lower cancer signal detection rate of repeat testing may indicate some degree of ‘early adopter’ bias, where those who return for a second test are systematically different from the general screening population. This could suggest that broader population-level screening may yield different results, she continued.
Shift Toward Unscreened Cancers
The top cancer types identified in the second round of testing were lymphoid, head and neck, bladder/urothelial, colorectal, and anal cancers. Clinicians were able to confirm clinical outcomes in 12 of 26 cases, in which cancer signals were detected. Of those 12 cases, 8 individuals received a cancer diagnosis and 4 did not have cancer. The remaining 14 of 26 cases in which cancer signals were detected are still under investigation.
“We found a shift away from USPSTF screen-detected cancers, like breast, lung, and prostate, and relative increase in unscreened urinary, head and neck, and lymphoid cancers, with 75% of cancers being those without any screening guidelines,” Dr. Gordon said in an interview.
She added that patients who choose to retest may have different cancer rates for several reasons, including bias toward a population that is health conscious and adhered to all recommended cancer screening.
“So the shift toward unscreened cancers is not unexpected and highlights the value of Galleri,” she said, but also acknowledged that “continued monitoring is needed to see if this translates in a persistent finding over time and tests.”
Shift Toward Early-Stage Cancers
Staging information was available for five cases, and Dr. Gordon highlighted in her talk that four of these confirmed cancers were stage I, including cancers of the anus, head and neck, bladder, and lymphoma. The fifth confirmed cancer with staging information was stage IV ovarian cancer.
“It is still early, and the numbers are very small, but the detection of early-stage cancers with second annual testing is very encouraging as these are the cases where MCED testing could have the greatest impact in improving outcomes through earlier treatment,” Dr. Gordon told this publication.
During an interview after the talk, Kenneth L. Kehl, MD, MPH, echoed that data must be confirmed in larger cohorts.
“The shift toward earlier stage cancers that are less detectable by standard screening methods is an interesting result, but we need to be cautious since the numbers were relatively small, and we do not have data on cancers that were diagnosed among patients whose second MCED test was also negative,” said Dr. Kehl, a medical oncologist at Dana-Farber Cancer Institute, Boston.
MCED Results Could Help Direct Diagnostic Workup
The test’s ability to predict the organ of origin was highly accurate, correctly identifying the cancer type in all eight confirmed cases. Among the eight cases with a confirmed cancer diagnosis, the accuracy of the first prediction was 100%, and diagnoses included invasive cancers across multiple tissues and organs, including anus, colon, head and neck, urothelial tract, ovary, and the lymphatic system.
“The fact that the site of origin for 100% of confirmed cancers was accurately predicted with GRAIL’s CSO by Galleri test confirms the promise that this can guide workup when a cancer signal is detected,” Dr. Gordon noted in the interview.
Looking Ahead
Dr. Kehl, who was not involved in the MCED study, noted in an interview that “further data on test characteristics beyond positive predictive value, including the sensitivity, specificity, and negative predictive value, as well as demonstration of clinical benefit — ideally in a randomized trial — will likely be required for MCED testing to become a standard public health recommendation.”
He added that challenges associated with implementing annual screening with MCED tests include the risks of both false positives and false negatives as testing becomes more widely available.
“False positives cause anxiety and lead to additional testing that may carry its own risks, and we need to understand if potentially false negative tests will be associated with less uptake of established screening strategies,” Dr. Kehl said in an interview. However, he noted that serial testing could lead to more frequent diagnoses of early-stage cancers that may be less detectable by standard methods.
Dr. Gordon reported financial relationships with GRAIL LLC and Genetic Technologies Corporation. Dr. Kehl reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing healthcare products used by or on patients.
This was the conclusion of recent data presented by Ora Karp Gordon, MD, MS, during a session at the American Association for Cancer Research annual meeting.
The MCED test, known as Galleri, was made clinically available in the United States in April 2021. Developed by GRAIL LLC, the test analyzes cell-free DNA in the blood using targeted methylation analysis and machine learning to detect the presence of a cancer signal and determine its organ of origin or cancer signal origin. The initial screening of over 53,000 individuals with the Galleri test detected a cancer signal in 1.1% of participants.
The new real-world analysis examines the outcomes of repeat MCED testing in 5,794 individuals.
The study looked at individuals who initially received a ‘no cancer signal detected’ result and then underwent a second Galleri test. Over 80% of participants received their follow-up test 10-18 months after the first, with a median interval between blood draws of 12.9 months.
“The repeat tests detect those cancer cases that have reached the detection threshold since their last MCED test, which should be less than one year of incidence,” Dr. Gordon, professor at Saint John’s Cancer Institute, Santa Monica, California, said in an interview. “We are just now starting to see results from patients who get their second and even third round of screening.”
“Galleri is recommended to be used annually in addition to USPSTF [US Preventive Services Task Force]–recommended cancer screening tests, like mammography and colonoscopy,” she said.
This recommendation is based on a modeling study suggesting that annual screening would improve stage shift, diagnostic yield, and potentially mortality when compared to biennial screening, although biennial screening was still favorable compared with no screening, she explained.
Early Real-World Evidence of Repeat Testing
Among the cohort of 5,794 individuals who received repeat testing, 26 received a positive cancer signal on their second test, yielding a cancer signal detection rate of 0.45% (95% CI: 0.31%-0.66%). The cancer signal detection rate was slightly higher in men. The rate was 0.50% (95% CI: 0.32%-0.81%; 17 of 3367) in men versus 0.37% (95% CI: 0.2%-0.7%; 9 of 2427) in women.
During her presentation, Dr. Gordon highlighted that the repeat testing signal detection rate was lower than the initial 0.95% rate (95% CI: 0.87-1.0; 510 of 53,744) seen in the previous larger cohort of patients who were retested at 1 year.
She acknowledged that the lower cancer signal detection rate of repeat testing may indicate some degree of ‘early adopter’ bias, where those who return for a second test are systematically different from the general screening population. This could suggest that broader population-level screening may yield different results, she continued.
Shift Toward Unscreened Cancers
The top cancer types identified in the second round of testing were lymphoid, head and neck, bladder/urothelial, colorectal, and anal cancers. Clinicians were able to confirm clinical outcomes in 12 of 26 cases, in which cancer signals were detected. Of those 12 cases, 8 individuals received a cancer diagnosis and 4 did not have cancer. The remaining 14 of 26 cases in which cancer signals were detected are still under investigation.
“We found a shift away from USPSTF screen-detected cancers, like breast, lung, and prostate, and relative increase in unscreened urinary, head and neck, and lymphoid cancers, with 75% of cancers being those without any screening guidelines,” Dr. Gordon said in an interview.
She added that patients who choose to retest may have different cancer rates for several reasons, including bias toward a population that is health conscious and adhered to all recommended cancer screening.
“So the shift toward unscreened cancers is not unexpected and highlights the value of Galleri,” she said, but also acknowledged that “continued monitoring is needed to see if this translates in a persistent finding over time and tests.”
Shift Toward Early-Stage Cancers
Staging information was available for five cases, and Dr. Gordon highlighted in her talk that four of these confirmed cancers were stage I, including cancers of the anus, head and neck, bladder, and lymphoma. The fifth confirmed cancer with staging information was stage IV ovarian cancer.
“It is still early, and the numbers are very small, but the detection of early-stage cancers with second annual testing is very encouraging as these are the cases where MCED testing could have the greatest impact in improving outcomes through earlier treatment,” Dr. Gordon told this publication.
During an interview after the talk, Kenneth L. Kehl, MD, MPH, echoed that data must be confirmed in larger cohorts.
“The shift toward earlier stage cancers that are less detectable by standard screening methods is an interesting result, but we need to be cautious since the numbers were relatively small, and we do not have data on cancers that were diagnosed among patients whose second MCED test was also negative,” said Dr. Kehl, a medical oncologist at Dana-Farber Cancer Institute, Boston.
MCED Results Could Help Direct Diagnostic Workup
The test’s ability to predict the organ of origin was highly accurate, correctly identifying the cancer type in all eight confirmed cases. Among the eight cases with a confirmed cancer diagnosis, the accuracy of the first prediction was 100%, and diagnoses included invasive cancers across multiple tissues and organs, including anus, colon, head and neck, urothelial tract, ovary, and the lymphatic system.
“The fact that the site of origin for 100% of confirmed cancers was accurately predicted with GRAIL’s CSO by Galleri test confirms the promise that this can guide workup when a cancer signal is detected,” Dr. Gordon noted in the interview.
Looking Ahead
Dr. Kehl, who was not involved in the MCED study, noted in an interview that “further data on test characteristics beyond positive predictive value, including the sensitivity, specificity, and negative predictive value, as well as demonstration of clinical benefit — ideally in a randomized trial — will likely be required for MCED testing to become a standard public health recommendation.”
He added that challenges associated with implementing annual screening with MCED tests include the risks of both false positives and false negatives as testing becomes more widely available.
“False positives cause anxiety and lead to additional testing that may carry its own risks, and we need to understand if potentially false negative tests will be associated with less uptake of established screening strategies,” Dr. Kehl said in an interview. However, he noted that serial testing could lead to more frequent diagnoses of early-stage cancers that may be less detectable by standard methods.
Dr. Gordon reported financial relationships with GRAIL LLC and Genetic Technologies Corporation. Dr. Kehl reported no relationships with entities whose primary business is producing, marketing, selling, reselling, or distributing healthcare products used by or on patients.
FROM AACR 2024
EBER-Negative, Double-Hit High-Grade B-Cell Lymphoma Responding to Methotrexate Discontinuation
High-grade B-cell lymphomas (HGBCLs) are aggressive lymphoproliferative disorders (LPDs) that require fluorescence in-situ hybridization to identify gene rearrangements within MYC and BCL2 and/or BCL6 oncogenes. Traditionally referred to as double-hit or triple-hit lymphomas, HGBCL is a newer entity in the 2016 updated World Health Organization classification of lymphoid neoplasms.1 More than 90% of patients with HGBCL present with advanced clinical features, such as central nervous system involvement, leukocytosis, or lactose dehydrogenase (LDH) greater than 3 times the upper limit of normal. Treatment outcomes with aggressive multiagent chemotherapy combined with anti-CD20–targeted therapy are generally worse for patients with double-hit disease, especially among frail patients with advanced age. Patients with underlying autoimmune and rheumatologic conditions, such as rheumatoid arthritis (RA), are at higher risk for developing LPDs. These include highly aggressive subtypes of non-Hodgkin lymphoma, such as HGBCL, likely due to cascading events secondary to chronic inflammation and/or immunosuppressive medications. These immunodeficiency-associated LPDs often express positivity for Epstein-Barr virus-encoded small RNA (EBER).
We present a case of double-hit HGBCL that was EBER negative with MYC and BCL6 rearrangements in an older veteran with RA managed with methotrexate. An excellent sustained response was observed for the patient’s stage IV double-hit HGBCL disease within 4 weeks of methotrexate discontinuation. To our knowledge, this is the first reported response to methotrexate discontinuation for a patient with HGBCL.
CASE PRESENTATION
A male veteran aged 81 years presented to the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, with an unintentional 25-pound weight loss over 18 months. Pertinent history included RA managed with methotrexate 15 mg weekly for 6 years and a previous remote seizure. The patients prior prostate cancer was treated with radiation at the time of diagnosis and ongoing androgen deprivation therapy. Initial workup with chest X-ray and chest computed tomography (CT) indicated loculated left pleural fluid collection with a suspected splenic tumor.
A positron-emission tomography (PET)/CT was ordered given his history of prostate cancer, which showed potential splenic and sternal metastases with corresponding fludeoxyglucose F18 uptake (Figure 1A). Biopsy was not pursued due to the potential for splenic hemorrhage. Based on the patient’s RA and methotrexate use, the collection of findings was initially thought to represent a non-Hodgkin lymphoma, with knowledge that metastatic prostate cancer refractory to androgen deprivation therapy was possible. Because he was unable to undergo a splenic biopsy, an observation strategy involving repeat PET/CT every 6 months was started.
The surveillance PET/CT 6 months later conveyed worsened disease burden with increased avidity in the manubrium (Figure 1B). The patient’s case was discussed at the RGMVAMC tumor board, and the recommendation was to continue with surveillance follow-up imaging because image-guided biopsy might not definitively yield a diagnosis. Repeat PET/CT3 months later indicated continued worsening of disease (Figure 1C) with a rapidly enlarging hypermetabolic mass in the manubrium that extended anteriorly into the subcutaneous tissues and encased the bilateral anterior jugular veins. On physical examination, this sternal mass had become painful and was clearly evident. Additionally, increased avidity in multiple upper abdominal and retroperitoneal lymph nodes was observed.
Interventional radiology was consulted to assist with a percutaneous fine-needle aspiration of the manubrial mass, which revealed a dense aggregate of large, atypical lymphocytes confirmed to be of B-cell origin (CD20 and PAX5 positive) (Figure 2). The atypical B cells demonstrated co-expression of BCL6, BCL2, MUM1, and MYC but were negative for CD30 and EBER by in situ hybridization. The overall morphologic and immunophenotypic findings were consistent with a large B-cell lymphoma. Fluorescent in-situ hybridization identified the presence of MYC and BCL6 gene rearrangements, and the mass was consequently best classified as a double-hit HGBCL.
Given the patient’s history of long-term methotrexate use, we thought the HGBCL may have reflected an immunodeficiency-associated LPD, although the immunophenotype was not classic because of the CD30 and EBER negativity. With the known toxicity and poor treatment outcomes of aggressive multiagent chemotherapy for patients with double-hit HGBCL—particularly in the older adult population—methotrexate was discontinued on a trial basis.
A PET/CT was completed 4 weeks after methotrexate was discontinued due to concerns about managing an HGBCL without chemotherapy or anti-CD20–directed therapy. The updated PET/CT showed significant improvement with marked reduction in avidity of his manubrial lesion (Figure 1D). Three months after methotrexate discontinuation, the patient remained in partial remission for his double-hit HGBCL, as evidenced by no findings of sternal mass on repeat examinations with continued decrease in hypermetabolic findings on PET/CT. The patient's RA symptoms rebounded, and rheumatology colleagues prescribed sulfasalazine and periodic steroid tapers to help control his inflammatory arthritis. Fourteen months after discontinuation of methotrexate, the patient died after developing pneumonia, which led to multisystemic organ failure.
DISCUSSION
HGBCL with MYC and BCL2 and/or BCL6 rearrangements is an aggressive LPD.1 A definitive diagnosis requires collection of morphologic and immunophenotypic evaluations of suspicious tissue. Approximately 60% of patients with HGBCL have translocations in MYC and BCL2, 20% have MYC and BCL6 translocations, and the remaining 20% have MYC, BCL2 and BCL6 translocations (triple-hit disease).1
The MYC and BCL gene rearrangements are thought to synergistically drive tumorigenesis, leading to accelerated lymphoma progression and a lesser response to standard multiagent chemotherapy than seen in diffuse large B-cell lymphoma.1-3 Consequently, there have been several attempts to increase treatment efficacy with intense chemotherapy regimens, namely DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), or by adding targeted agents, such as ibrutinib and venetoclax to a standard R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone) backbone.4-7 Though the standard choice of therapy for fit patients harboring HGBCL remains controversial, these aggressive regimens at standard doses are typically difficult to tolerate for patients aged > 80 years.
Patients with immunosuppression are at higher risk for developing LPDs, including aggressive B-cell non-Hodgkin lymphomas such as diffuse large B-cell lymphoma. These patients are frequently classified into 2 groups: those with underlying autoimmune conditions (RA-associated LPDs), or those who have undergone solid-organ or allogeneic hematopoietic stem-cell transplants, which drives the development of posttransplant LPDs (Table).8-11 Both types of LPDs are often EBER positive, indicating some association with Epstein-Barr virus infection driven by ongoing immunosuppression, with knowledge that this finding is not absolute and is less frequent among patients with autoimmune conditions than those with posttransplant LPD.8,12
For indolent and early-stage aggressive LPDs, reduction of immunosuppression is a reasonable frontline treatment. In fact, Tokuyama and colleagues reported a previous case in which an methotrexate-associated EBER-positive early-stage diffuse large B-cell lymphoma responded well to methotrexate withdrawal.13 For advanced, aggressive LPDs associated with immunosuppression, a combination strategy of reducing immunosuppression and initiating a standard multiagent systemic therapy such as with R-CHOP is more common. Reducing immunosuppression without adding systemic anticancer therapy can certainly be considered in patients with EBER-negative LPDs; however, there is less evidence supporting this approach in the literature.
A case series of patients with EBER-positive double-hit HGBCL has been described previously, and response rates were low despite aggressive treatment.14 The current case differs from that case series in 2 ways. First, our patient did not have EBER-positive disease despite having an HGBCL associated with RA and methotrexate use. Second, our patient had a very rapid and excellent partial response simply with methotrexate discontinuation. Aggressive treatment was considered initially; however, given the patient’s age and performance status, reduction of immunosuppression alone was considered the frontline approach.
This case indicates that methotrexate withdrawal may lead to remission in patients with double-hit lymphoma, even without clear signs of Epstein-Barr virus infection being present. We are not sure why our patient with EBER-negative HGBCL responded differently to methotrexate withdrawal than the patients in the aforementioned case series with EBER-positive disease; nevertheless, a short trial of methotrexate withdrawal with repeat imaging 4 to 8 weeks after discontinuation seems reasonable for patients who are older, frail, and seemingly not fit for more aggressive treatment.
CONCLUSIONS
For our older patient with RA and biopsy-proven, stage IV EBER-negative HGBCL bearing MYC and BCL6 rearrangements (double hit), discontinuation of methotrexate led to a rapid and sustained marked response. Reducing immunosuppression should be considered for patients with LPDs associated with autoimmune conditions or immunosuppressive medications, regardless of additional multiagent systemic therapy administration. In older patients who are frail with aggressive B-cell lymphomas, a short trial of methotrexate withdrawal with quick interval imaging is a reasonable frontline option, regardless of EBER status.
1. Sesques P, Johnson NA. Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood. 2017;129(3):280-288. doi:10.1182/blood-2016-02-636316
2. Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319-2331. doi:10.1182/blood-2010-09-297879
3. Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131(18):2060-2064. doi:10.1182/blood-2017-12-820605
4. Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018;5(12):e609-e617. doi:10.1016/S2352-3026(18)30177-7
5. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285-1295. doi:10.1200/JCO.18.02403
6. Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600-609. doi:10.1182/blood.2020006578
7. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). B-cell lymphomas. Version 2.2024. January 18, 2024. Accessed January 24, 2024. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
8. Abbas F, Kossi ME, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches. World J Transplant. 2020;10(2):29-46. doi:10.5500/wjt.v10.i2.29
9. Hoshida Y, Xu JX, Fujita S, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34(2):322-331.
10. Salloum E, Cooper DL, Howe G, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14(6):1943-1949. doi:10.1200/JCO.1996.14.6.1943
11. Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, Ten Berge IJM. Epstein-Barr virus–positive posttransplant lymphoproliferative disease after solid organ transplantation: pathogenesis, clinical manifestations, diagnosis, and management. Transplantation Direct. 2015;2(1):e48. doi:10.1097/txd.0000000000000557
12. Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029-4038. doi:10.1182/blood-2007-10-11997413. Tokuyama K, Okada F, Matsumoto S, et al. EBV-positive methotrexate-diffuse large B cell lymphoma in a rheumatoid arthritis patient. Jpn J Radiol. 2014;32(3):183-187. doi:10.1007/s11604-013-0280-y
14. Liu H, Xu-Monette ZY, Tang G, et al. EBV+ high-grade B cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements: a multi-institutional study. Histopathology. 2022;80(3):575-588. doi:10.1111/his.14585
High-grade B-cell lymphomas (HGBCLs) are aggressive lymphoproliferative disorders (LPDs) that require fluorescence in-situ hybridization to identify gene rearrangements within MYC and BCL2 and/or BCL6 oncogenes. Traditionally referred to as double-hit or triple-hit lymphomas, HGBCL is a newer entity in the 2016 updated World Health Organization classification of lymphoid neoplasms.1 More than 90% of patients with HGBCL present with advanced clinical features, such as central nervous system involvement, leukocytosis, or lactose dehydrogenase (LDH) greater than 3 times the upper limit of normal. Treatment outcomes with aggressive multiagent chemotherapy combined with anti-CD20–targeted therapy are generally worse for patients with double-hit disease, especially among frail patients with advanced age. Patients with underlying autoimmune and rheumatologic conditions, such as rheumatoid arthritis (RA), are at higher risk for developing LPDs. These include highly aggressive subtypes of non-Hodgkin lymphoma, such as HGBCL, likely due to cascading events secondary to chronic inflammation and/or immunosuppressive medications. These immunodeficiency-associated LPDs often express positivity for Epstein-Barr virus-encoded small RNA (EBER).
We present a case of double-hit HGBCL that was EBER negative with MYC and BCL6 rearrangements in an older veteran with RA managed with methotrexate. An excellent sustained response was observed for the patient’s stage IV double-hit HGBCL disease within 4 weeks of methotrexate discontinuation. To our knowledge, this is the first reported response to methotrexate discontinuation for a patient with HGBCL.
CASE PRESENTATION
A male veteran aged 81 years presented to the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, with an unintentional 25-pound weight loss over 18 months. Pertinent history included RA managed with methotrexate 15 mg weekly for 6 years and a previous remote seizure. The patients prior prostate cancer was treated with radiation at the time of diagnosis and ongoing androgen deprivation therapy. Initial workup with chest X-ray and chest computed tomography (CT) indicated loculated left pleural fluid collection with a suspected splenic tumor.
A positron-emission tomography (PET)/CT was ordered given his history of prostate cancer, which showed potential splenic and sternal metastases with corresponding fludeoxyglucose F18 uptake (Figure 1A). Biopsy was not pursued due to the potential for splenic hemorrhage. Based on the patient’s RA and methotrexate use, the collection of findings was initially thought to represent a non-Hodgkin lymphoma, with knowledge that metastatic prostate cancer refractory to androgen deprivation therapy was possible. Because he was unable to undergo a splenic biopsy, an observation strategy involving repeat PET/CT every 6 months was started.
The surveillance PET/CT 6 months later conveyed worsened disease burden with increased avidity in the manubrium (Figure 1B). The patient’s case was discussed at the RGMVAMC tumor board, and the recommendation was to continue with surveillance follow-up imaging because image-guided biopsy might not definitively yield a diagnosis. Repeat PET/CT3 months later indicated continued worsening of disease (Figure 1C) with a rapidly enlarging hypermetabolic mass in the manubrium that extended anteriorly into the subcutaneous tissues and encased the bilateral anterior jugular veins. On physical examination, this sternal mass had become painful and was clearly evident. Additionally, increased avidity in multiple upper abdominal and retroperitoneal lymph nodes was observed.
Interventional radiology was consulted to assist with a percutaneous fine-needle aspiration of the manubrial mass, which revealed a dense aggregate of large, atypical lymphocytes confirmed to be of B-cell origin (CD20 and PAX5 positive) (Figure 2). The atypical B cells demonstrated co-expression of BCL6, BCL2, MUM1, and MYC but were negative for CD30 and EBER by in situ hybridization. The overall morphologic and immunophenotypic findings were consistent with a large B-cell lymphoma. Fluorescent in-situ hybridization identified the presence of MYC and BCL6 gene rearrangements, and the mass was consequently best classified as a double-hit HGBCL.
Given the patient’s history of long-term methotrexate use, we thought the HGBCL may have reflected an immunodeficiency-associated LPD, although the immunophenotype was not classic because of the CD30 and EBER negativity. With the known toxicity and poor treatment outcomes of aggressive multiagent chemotherapy for patients with double-hit HGBCL—particularly in the older adult population—methotrexate was discontinued on a trial basis.
A PET/CT was completed 4 weeks after methotrexate was discontinued due to concerns about managing an HGBCL without chemotherapy or anti-CD20–directed therapy. The updated PET/CT showed significant improvement with marked reduction in avidity of his manubrial lesion (Figure 1D). Three months after methotrexate discontinuation, the patient remained in partial remission for his double-hit HGBCL, as evidenced by no findings of sternal mass on repeat examinations with continued decrease in hypermetabolic findings on PET/CT. The patient's RA symptoms rebounded, and rheumatology colleagues prescribed sulfasalazine and periodic steroid tapers to help control his inflammatory arthritis. Fourteen months after discontinuation of methotrexate, the patient died after developing pneumonia, which led to multisystemic organ failure.
DISCUSSION
HGBCL with MYC and BCL2 and/or BCL6 rearrangements is an aggressive LPD.1 A definitive diagnosis requires collection of morphologic and immunophenotypic evaluations of suspicious tissue. Approximately 60% of patients with HGBCL have translocations in MYC and BCL2, 20% have MYC and BCL6 translocations, and the remaining 20% have MYC, BCL2 and BCL6 translocations (triple-hit disease).1
The MYC and BCL gene rearrangements are thought to synergistically drive tumorigenesis, leading to accelerated lymphoma progression and a lesser response to standard multiagent chemotherapy than seen in diffuse large B-cell lymphoma.1-3 Consequently, there have been several attempts to increase treatment efficacy with intense chemotherapy regimens, namely DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), or by adding targeted agents, such as ibrutinib and venetoclax to a standard R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone) backbone.4-7 Though the standard choice of therapy for fit patients harboring HGBCL remains controversial, these aggressive regimens at standard doses are typically difficult to tolerate for patients aged > 80 years.
Patients with immunosuppression are at higher risk for developing LPDs, including aggressive B-cell non-Hodgkin lymphomas such as diffuse large B-cell lymphoma. These patients are frequently classified into 2 groups: those with underlying autoimmune conditions (RA-associated LPDs), or those who have undergone solid-organ or allogeneic hematopoietic stem-cell transplants, which drives the development of posttransplant LPDs (Table).8-11 Both types of LPDs are often EBER positive, indicating some association with Epstein-Barr virus infection driven by ongoing immunosuppression, with knowledge that this finding is not absolute and is less frequent among patients with autoimmune conditions than those with posttransplant LPD.8,12
For indolent and early-stage aggressive LPDs, reduction of immunosuppression is a reasonable frontline treatment. In fact, Tokuyama and colleagues reported a previous case in which an methotrexate-associated EBER-positive early-stage diffuse large B-cell lymphoma responded well to methotrexate withdrawal.13 For advanced, aggressive LPDs associated with immunosuppression, a combination strategy of reducing immunosuppression and initiating a standard multiagent systemic therapy such as with R-CHOP is more common. Reducing immunosuppression without adding systemic anticancer therapy can certainly be considered in patients with EBER-negative LPDs; however, there is less evidence supporting this approach in the literature.
A case series of patients with EBER-positive double-hit HGBCL has been described previously, and response rates were low despite aggressive treatment.14 The current case differs from that case series in 2 ways. First, our patient did not have EBER-positive disease despite having an HGBCL associated with RA and methotrexate use. Second, our patient had a very rapid and excellent partial response simply with methotrexate discontinuation. Aggressive treatment was considered initially; however, given the patient’s age and performance status, reduction of immunosuppression alone was considered the frontline approach.
This case indicates that methotrexate withdrawal may lead to remission in patients with double-hit lymphoma, even without clear signs of Epstein-Barr virus infection being present. We are not sure why our patient with EBER-negative HGBCL responded differently to methotrexate withdrawal than the patients in the aforementioned case series with EBER-positive disease; nevertheless, a short trial of methotrexate withdrawal with repeat imaging 4 to 8 weeks after discontinuation seems reasonable for patients who are older, frail, and seemingly not fit for more aggressive treatment.
CONCLUSIONS
For our older patient with RA and biopsy-proven, stage IV EBER-negative HGBCL bearing MYC and BCL6 rearrangements (double hit), discontinuation of methotrexate led to a rapid and sustained marked response. Reducing immunosuppression should be considered for patients with LPDs associated with autoimmune conditions or immunosuppressive medications, regardless of additional multiagent systemic therapy administration. In older patients who are frail with aggressive B-cell lymphomas, a short trial of methotrexate withdrawal with quick interval imaging is a reasonable frontline option, regardless of EBER status.
High-grade B-cell lymphomas (HGBCLs) are aggressive lymphoproliferative disorders (LPDs) that require fluorescence in-situ hybridization to identify gene rearrangements within MYC and BCL2 and/or BCL6 oncogenes. Traditionally referred to as double-hit or triple-hit lymphomas, HGBCL is a newer entity in the 2016 updated World Health Organization classification of lymphoid neoplasms.1 More than 90% of patients with HGBCL present with advanced clinical features, such as central nervous system involvement, leukocytosis, or lactose dehydrogenase (LDH) greater than 3 times the upper limit of normal. Treatment outcomes with aggressive multiagent chemotherapy combined with anti-CD20–targeted therapy are generally worse for patients with double-hit disease, especially among frail patients with advanced age. Patients with underlying autoimmune and rheumatologic conditions, such as rheumatoid arthritis (RA), are at higher risk for developing LPDs. These include highly aggressive subtypes of non-Hodgkin lymphoma, such as HGBCL, likely due to cascading events secondary to chronic inflammation and/or immunosuppressive medications. These immunodeficiency-associated LPDs often express positivity for Epstein-Barr virus-encoded small RNA (EBER).
We present a case of double-hit HGBCL that was EBER negative with MYC and BCL6 rearrangements in an older veteran with RA managed with methotrexate. An excellent sustained response was observed for the patient’s stage IV double-hit HGBCL disease within 4 weeks of methotrexate discontinuation. To our knowledge, this is the first reported response to methotrexate discontinuation for a patient with HGBCL.
CASE PRESENTATION
A male veteran aged 81 years presented to the Raymond G. Murphy Veterans Affairs Medical Center (RGMVAMC) in Albuquerque, New Mexico, with an unintentional 25-pound weight loss over 18 months. Pertinent history included RA managed with methotrexate 15 mg weekly for 6 years and a previous remote seizure. The patients prior prostate cancer was treated with radiation at the time of diagnosis and ongoing androgen deprivation therapy. Initial workup with chest X-ray and chest computed tomography (CT) indicated loculated left pleural fluid collection with a suspected splenic tumor.
A positron-emission tomography (PET)/CT was ordered given his history of prostate cancer, which showed potential splenic and sternal metastases with corresponding fludeoxyglucose F18 uptake (Figure 1A). Biopsy was not pursued due to the potential for splenic hemorrhage. Based on the patient’s RA and methotrexate use, the collection of findings was initially thought to represent a non-Hodgkin lymphoma, with knowledge that metastatic prostate cancer refractory to androgen deprivation therapy was possible. Because he was unable to undergo a splenic biopsy, an observation strategy involving repeat PET/CT every 6 months was started.
The surveillance PET/CT 6 months later conveyed worsened disease burden with increased avidity in the manubrium (Figure 1B). The patient’s case was discussed at the RGMVAMC tumor board, and the recommendation was to continue with surveillance follow-up imaging because image-guided biopsy might not definitively yield a diagnosis. Repeat PET/CT3 months later indicated continued worsening of disease (Figure 1C) with a rapidly enlarging hypermetabolic mass in the manubrium that extended anteriorly into the subcutaneous tissues and encased the bilateral anterior jugular veins. On physical examination, this sternal mass had become painful and was clearly evident. Additionally, increased avidity in multiple upper abdominal and retroperitoneal lymph nodes was observed.
Interventional radiology was consulted to assist with a percutaneous fine-needle aspiration of the manubrial mass, which revealed a dense aggregate of large, atypical lymphocytes confirmed to be of B-cell origin (CD20 and PAX5 positive) (Figure 2). The atypical B cells demonstrated co-expression of BCL6, BCL2, MUM1, and MYC but were negative for CD30 and EBER by in situ hybridization. The overall morphologic and immunophenotypic findings were consistent with a large B-cell lymphoma. Fluorescent in-situ hybridization identified the presence of MYC and BCL6 gene rearrangements, and the mass was consequently best classified as a double-hit HGBCL.
Given the patient’s history of long-term methotrexate use, we thought the HGBCL may have reflected an immunodeficiency-associated LPD, although the immunophenotype was not classic because of the CD30 and EBER negativity. With the known toxicity and poor treatment outcomes of aggressive multiagent chemotherapy for patients with double-hit HGBCL—particularly in the older adult population—methotrexate was discontinued on a trial basis.
A PET/CT was completed 4 weeks after methotrexate was discontinued due to concerns about managing an HGBCL without chemotherapy or anti-CD20–directed therapy. The updated PET/CT showed significant improvement with marked reduction in avidity of his manubrial lesion (Figure 1D). Three months after methotrexate discontinuation, the patient remained in partial remission for his double-hit HGBCL, as evidenced by no findings of sternal mass on repeat examinations with continued decrease in hypermetabolic findings on PET/CT. The patient's RA symptoms rebounded, and rheumatology colleagues prescribed sulfasalazine and periodic steroid tapers to help control his inflammatory arthritis. Fourteen months after discontinuation of methotrexate, the patient died after developing pneumonia, which led to multisystemic organ failure.
DISCUSSION
HGBCL with MYC and BCL2 and/or BCL6 rearrangements is an aggressive LPD.1 A definitive diagnosis requires collection of morphologic and immunophenotypic evaluations of suspicious tissue. Approximately 60% of patients with HGBCL have translocations in MYC and BCL2, 20% have MYC and BCL6 translocations, and the remaining 20% have MYC, BCL2 and BCL6 translocations (triple-hit disease).1
The MYC and BCL gene rearrangements are thought to synergistically drive tumorigenesis, leading to accelerated lymphoma progression and a lesser response to standard multiagent chemotherapy than seen in diffuse large B-cell lymphoma.1-3 Consequently, there have been several attempts to increase treatment efficacy with intense chemotherapy regimens, namely DA-EPOCH-R (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab), or by adding targeted agents, such as ibrutinib and venetoclax to a standard R-CHOP (rituximab with reduced cyclophosphamide, doxorubicin, vincristine, and prednisone) backbone.4-7 Though the standard choice of therapy for fit patients harboring HGBCL remains controversial, these aggressive regimens at standard doses are typically difficult to tolerate for patients aged > 80 years.
Patients with immunosuppression are at higher risk for developing LPDs, including aggressive B-cell non-Hodgkin lymphomas such as diffuse large B-cell lymphoma. These patients are frequently classified into 2 groups: those with underlying autoimmune conditions (RA-associated LPDs), or those who have undergone solid-organ or allogeneic hematopoietic stem-cell transplants, which drives the development of posttransplant LPDs (Table).8-11 Both types of LPDs are often EBER positive, indicating some association with Epstein-Barr virus infection driven by ongoing immunosuppression, with knowledge that this finding is not absolute and is less frequent among patients with autoimmune conditions than those with posttransplant LPD.8,12
For indolent and early-stage aggressive LPDs, reduction of immunosuppression is a reasonable frontline treatment. In fact, Tokuyama and colleagues reported a previous case in which an methotrexate-associated EBER-positive early-stage diffuse large B-cell lymphoma responded well to methotrexate withdrawal.13 For advanced, aggressive LPDs associated with immunosuppression, a combination strategy of reducing immunosuppression and initiating a standard multiagent systemic therapy such as with R-CHOP is more common. Reducing immunosuppression without adding systemic anticancer therapy can certainly be considered in patients with EBER-negative LPDs; however, there is less evidence supporting this approach in the literature.
A case series of patients with EBER-positive double-hit HGBCL has been described previously, and response rates were low despite aggressive treatment.14 The current case differs from that case series in 2 ways. First, our patient did not have EBER-positive disease despite having an HGBCL associated with RA and methotrexate use. Second, our patient had a very rapid and excellent partial response simply with methotrexate discontinuation. Aggressive treatment was considered initially; however, given the patient’s age and performance status, reduction of immunosuppression alone was considered the frontline approach.
This case indicates that methotrexate withdrawal may lead to remission in patients with double-hit lymphoma, even without clear signs of Epstein-Barr virus infection being present. We are not sure why our patient with EBER-negative HGBCL responded differently to methotrexate withdrawal than the patients in the aforementioned case series with EBER-positive disease; nevertheless, a short trial of methotrexate withdrawal with repeat imaging 4 to 8 weeks after discontinuation seems reasonable for patients who are older, frail, and seemingly not fit for more aggressive treatment.
CONCLUSIONS
For our older patient with RA and biopsy-proven, stage IV EBER-negative HGBCL bearing MYC and BCL6 rearrangements (double hit), discontinuation of methotrexate led to a rapid and sustained marked response. Reducing immunosuppression should be considered for patients with LPDs associated with autoimmune conditions or immunosuppressive medications, regardless of additional multiagent systemic therapy administration. In older patients who are frail with aggressive B-cell lymphomas, a short trial of methotrexate withdrawal with quick interval imaging is a reasonable frontline option, regardless of EBER status.
1. Sesques P, Johnson NA. Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood. 2017;129(3):280-288. doi:10.1182/blood-2016-02-636316
2. Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319-2331. doi:10.1182/blood-2010-09-297879
3. Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131(18):2060-2064. doi:10.1182/blood-2017-12-820605
4. Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018;5(12):e609-e617. doi:10.1016/S2352-3026(18)30177-7
5. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285-1295. doi:10.1200/JCO.18.02403
6. Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600-609. doi:10.1182/blood.2020006578
7. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). B-cell lymphomas. Version 2.2024. January 18, 2024. Accessed January 24, 2024. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
8. Abbas F, Kossi ME, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches. World J Transplant. 2020;10(2):29-46. doi:10.5500/wjt.v10.i2.29
9. Hoshida Y, Xu JX, Fujita S, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34(2):322-331.
10. Salloum E, Cooper DL, Howe G, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14(6):1943-1949. doi:10.1200/JCO.1996.14.6.1943
11. Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, Ten Berge IJM. Epstein-Barr virus–positive posttransplant lymphoproliferative disease after solid organ transplantation: pathogenesis, clinical manifestations, diagnosis, and management. Transplantation Direct. 2015;2(1):e48. doi:10.1097/txd.0000000000000557
12. Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029-4038. doi:10.1182/blood-2007-10-11997413. Tokuyama K, Okada F, Matsumoto S, et al. EBV-positive methotrexate-diffuse large B cell lymphoma in a rheumatoid arthritis patient. Jpn J Radiol. 2014;32(3):183-187. doi:10.1007/s11604-013-0280-y
14. Liu H, Xu-Monette ZY, Tang G, et al. EBV+ high-grade B cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements: a multi-institutional study. Histopathology. 2022;80(3):575-588. doi:10.1111/his.14585
1. Sesques P, Johnson NA. Approach to the diagnosis and treatment of high-grade B-cell lymphomas with MYC and BCL2 and/or BCL6 rearrangements. Blood. 2017;129(3):280-288. doi:10.1182/blood-2016-02-636316
2. Aukema SM, Siebert R, Schuuring E, et al. Double-hit B-cell lymphomas. Blood. 2011;117(8):2319-2331. doi:10.1182/blood-2010-09-297879
3. Scott DW, King RL, Staiger AM, et al. High-grade B-cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements with diffuse large B-cell lymphoma morphology. Blood. 2018;131(18):2060-2064. doi:10.1182/blood-2017-12-820605
4. Dunleavy K, Fanale MA, Abramson JS, et al. Dose-adjusted EPOCH-R (etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) in untreated aggressive diffuse large B-cell lymphoma with MYC rearrangement: a prospective, multicentre, single-arm phase 2 study. Lancet Haematol. 2018;5(12):e609-e617. doi:10.1016/S2352-3026(18)30177-7
5. Younes A, Sehn LH, Johnson P, et al. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285-1295. doi:10.1200/JCO.18.02403
6. Morschhauser F, Feugier P, Flinn IW, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood. 2021;137(5):600-609. doi:10.1182/blood.2020006578
7. National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). B-cell lymphomas. Version 2.2024. January 18, 2024. Accessed January 24, 2024. https://www.nccn.org/professionals/physician_gls/pdf/b-cell.pdf
8. Abbas F, Kossi ME, Shaheen IS, Sharma A, Halawa A. Post-transplantation lymphoproliferative disorders: current concepts and future therapeutic approaches. World J Transplant. 2020;10(2):29-46. doi:10.5500/wjt.v10.i2.29
9. Hoshida Y, Xu JX, Fujita S, et al. Lymphoproliferative disorders in rheumatoid arthritis: clinicopathological analysis of 76 cases in relation to methotrexate medication. J Rheumatol. 2007;34(2):322-331.
10. Salloum E, Cooper DL, Howe G, et al. Spontaneous regression of lymphoproliferative disorders in patients treated with methotrexate for rheumatoid arthritis and other rheumatic diseases. J Clin Oncol. 1996;14(6):1943-1949. doi:10.1200/JCO.1996.14.6.1943
11. Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, Ten Berge IJM. Epstein-Barr virus–positive posttransplant lymphoproliferative disease after solid organ transplantation: pathogenesis, clinical manifestations, diagnosis, and management. Transplantation Direct. 2015;2(1):e48. doi:10.1097/txd.0000000000000557
12. Ekström Smedby K, Vajdic CM, Falster M, et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111(8):4029-4038. doi:10.1182/blood-2007-10-11997413. Tokuyama K, Okada F, Matsumoto S, et al. EBV-positive methotrexate-diffuse large B cell lymphoma in a rheumatoid arthritis patient. Jpn J Radiol. 2014;32(3):183-187. doi:10.1007/s11604-013-0280-y
14. Liu H, Xu-Monette ZY, Tang G, et al. EBV+ high-grade B cell lymphoma with MYC and BCL2 and/or BCL6 rearrangements: a multi-institutional study. Histopathology. 2022;80(3):575-588. doi:10.1111/his.14585
New Trials in Prostate Cancer: Could Your Patient Benefit?
Metastatic castration-sensitive prostate cancer
Adults with this diagnosis may be interested in a randomized, double-blind, phase 3 study examining whether an experimental poly (ADP-ribose) polymerase (PARP) inhibitor called saruparib can further delay disease progression when added to a next-generation hormonal agent such as abiraterone (Zytiga), darolutamide (Nubeqa), or enzalutamide (Xtandi).
One group of participants will take daily oral doses of saruparib plus physician’s choice of a next-generation hormonal agent until disease progression or another reason for stopping therapy. The other group will add a placebo to a next-generation hormonal agent.
Sites in Rhode Island, Arkansas, California, Michigan, Australia, Canada, Japan, Taiwan, Thailand, the United Kingdom, and South Korea began seeking the trial’s 1800 participants in November 2023. Research centers in 31 other US states and 18 other countries are gearing up. The primary endpoint is radiographic progression-free survival. Overall survival and quality of life (QoL) are secondary endpoints. More details at clinicaltrials.gov.
This news organization asked Marc Garnick, MD, professor of medicine, Harvard Medical School and Beth Israel Deaconess Medical Center, Boston, for his take on the trial. “The study is interesting since it is adding to the evaluations of continued intensification for first-line therapy and will help further elucidate the role of PARP inhibition regardless of homologous repair status,” Dr. Garnick said. “Plus, saruparib is supposedly more selective on PARP1, which in-and-of-itself is of potential benefit.”
Metastatic castration-resistant prostate cancer
People with this type of cancer who have progressed on a next-generation hormonal agent may be eligible for a randomized, open-label, phase 3 trial testing an investigational oral treatment called MK-5684 to see if it increases survival more effectively than switching to an alternative next-generation hormonal agent.
MK-5684 is designed to inhibit the CYP11A1 enzyme, thereby disrupting the androgen-receptor signaling pathway.
One group will take twice-daily tablets of MK-5684 plus hormone replacement therapy, oral dexamethasone, and oral fludrocortisone acetate (Florinef), with rescue hydrocortisone as needed. The other participants will take daily tablets of a next-generation hormonal agent: Either enzalutamide or abiraterone. Patients assigned to abiraterone will also be given prednisone tablets.
US-based sites in nine states and Puerto Rico started looking for the trial’s 1500 participants in December 2023 in partnership with study centers in Australia, Israel, South Korea, and Taiwan. The primary endpoints are radiographic progression-free survival and overall survival. QoL will not be tracked. More details at clinicaltrials.gov.
Metastatic castration-resistant prostate cancer
Patients in this situation who have progressed on taxane-based chemotherapy as well as a next-generation hormonal agent have the option to enroll in another phase 3 MK-5684 study.
Like the trial described above, all patients will remain on their respective therapy until disease progression. In this trial, one group will take twice-daily tablets of MK-5684 without hormone replacement therapy but the same mix of oral dexamethasone and fludrocortisone. Rescue hydrocortisone will also be available. The second group will be assigned either enzalutamide or abiraterone plus prednisone.
Sites in Puerto Rico, Colorado, Nevada, and Virginia, and five other countries outside the United States, opened their doors to the first of 1200 patients in December 2023. The primary endpoints are radiographic progression-free survival and overall survival, analyzed separately for patients with and without an androgen receptor ligand-binding domain mutation. QoL will not be measured. More details at clinicaltrials.gov.
High-risk prostate cancer
People with this diagnosis can join a randomized, open-label, phase 3 National Cancer Institute study to test whether stereotactic body radiation therapy (SBRT) is as effective as conventional external beam radiation therapy (EBRT) at preventing metastasis.
SBRT delivers radiation to tumors with higher precision than EBRT. The advantage of SBRT is the ability to deliver fewer doses over a shorter duration with less collateral damage to surrounding tissues.
In the trial, half of participants will undergo five treatments of SBRT over 2 weeks, while the other half will receive 20-45 treatments of EBRT over 4-9 weeks. Study sites in 14 US states began recruiting the trial’s 1209 participants in November 2023. Metastasis-free survival over 15 years is the primary endpoint, overall survival is a secondary endpoint, and QoL measures, apart from fatigue, will not be tracked. More details at clinicaltrials.gov.
Dr. Garnick viewed this study as “problematic because patient accrual ends in 2036 with a readout in 2041.” He added, “What its relevance will be at that time is unlikely to provide practice changes, since in that interval there will undoubtedly be multiple advances in place.”
Newly diagnosed favorable intermediate risk prostate cancer
People with this type of cancer are eligible for an open-label, phase 4 real-world study of a radioactive diagnostic agent called piflufolastat F 18 (Pylarify) that targets prostate-specific membrane antigen (PSMA)–positive lesions. Piflufolastat is designed to enhance detection of metastases during PSMA-targeted PET.
Participants will receive a single injection of piflufolastat followed 1-2 hours later by a single whole-body PET-CT or PET-MRI scan. A study site at the Hoag Cancer Center in Irvine, California, welcomed the first of the trial’s 274 participants in February 2024. Sites in Tower Urology, Los Angeles, and the Cleveland Clinic, Ohio, are gearing up. Detection rate is the primary endpoint. Overall survival and QoL are not measured. More details at clinicaltrials.gov
Stages I-IV prostate cancer without bone metastases. People 60 years or older with this type of prostate cancer who are just starting androgen deprivation therapy are eligible for a phase 3, placebo-controlled trial investigating whether high-dose vitamin D can prevent or reduce androgen-deprivation therapy-induced bone loss.
For 1 year, participants will take tablets of high-dose vitamin D or a placebo and then undergo dual x-ray absorptiometry. The Ochsner Medical Center in Jefferson, Louisiana, started recruiting 366 trial participants in December 2023. Reduction in bone mineral density loss in the hip and spine over 1 year is the primary objective. QoL is a secondary objective, and overall survival will not be measured. More details at clinicaltrials.gov
Dr. Garnick expressed some concerns with the trial design so far, including that “the dose of vitamin D is not delineated nor is the target vitamin D level.”
All trial information is from the National Institutes of Health’s National Library of Medicine (online at clinicaltrials.gov). Dr. Garnick did not report conflicts with any of the trials.
A version of this article appeared on Medscape.com.
Metastatic castration-sensitive prostate cancer
Adults with this diagnosis may be interested in a randomized, double-blind, phase 3 study examining whether an experimental poly (ADP-ribose) polymerase (PARP) inhibitor called saruparib can further delay disease progression when added to a next-generation hormonal agent such as abiraterone (Zytiga), darolutamide (Nubeqa), or enzalutamide (Xtandi).
One group of participants will take daily oral doses of saruparib plus physician’s choice of a next-generation hormonal agent until disease progression or another reason for stopping therapy. The other group will add a placebo to a next-generation hormonal agent.
Sites in Rhode Island, Arkansas, California, Michigan, Australia, Canada, Japan, Taiwan, Thailand, the United Kingdom, and South Korea began seeking the trial’s 1800 participants in November 2023. Research centers in 31 other US states and 18 other countries are gearing up. The primary endpoint is radiographic progression-free survival. Overall survival and quality of life (QoL) are secondary endpoints. More details at clinicaltrials.gov.
This news organization asked Marc Garnick, MD, professor of medicine, Harvard Medical School and Beth Israel Deaconess Medical Center, Boston, for his take on the trial. “The study is interesting since it is adding to the evaluations of continued intensification for first-line therapy and will help further elucidate the role of PARP inhibition regardless of homologous repair status,” Dr. Garnick said. “Plus, saruparib is supposedly more selective on PARP1, which in-and-of-itself is of potential benefit.”
Metastatic castration-resistant prostate cancer
People with this type of cancer who have progressed on a next-generation hormonal agent may be eligible for a randomized, open-label, phase 3 trial testing an investigational oral treatment called MK-5684 to see if it increases survival more effectively than switching to an alternative next-generation hormonal agent.
MK-5684 is designed to inhibit the CYP11A1 enzyme, thereby disrupting the androgen-receptor signaling pathway.
One group will take twice-daily tablets of MK-5684 plus hormone replacement therapy, oral dexamethasone, and oral fludrocortisone acetate (Florinef), with rescue hydrocortisone as needed. The other participants will take daily tablets of a next-generation hormonal agent: Either enzalutamide or abiraterone. Patients assigned to abiraterone will also be given prednisone tablets.
US-based sites in nine states and Puerto Rico started looking for the trial’s 1500 participants in December 2023 in partnership with study centers in Australia, Israel, South Korea, and Taiwan. The primary endpoints are radiographic progression-free survival and overall survival. QoL will not be tracked. More details at clinicaltrials.gov.
Metastatic castration-resistant prostate cancer
Patients in this situation who have progressed on taxane-based chemotherapy as well as a next-generation hormonal agent have the option to enroll in another phase 3 MK-5684 study.
Like the trial described above, all patients will remain on their respective therapy until disease progression. In this trial, one group will take twice-daily tablets of MK-5684 without hormone replacement therapy but the same mix of oral dexamethasone and fludrocortisone. Rescue hydrocortisone will also be available. The second group will be assigned either enzalutamide or abiraterone plus prednisone.
Sites in Puerto Rico, Colorado, Nevada, and Virginia, and five other countries outside the United States, opened their doors to the first of 1200 patients in December 2023. The primary endpoints are radiographic progression-free survival and overall survival, analyzed separately for patients with and without an androgen receptor ligand-binding domain mutation. QoL will not be measured. More details at clinicaltrials.gov.
High-risk prostate cancer
People with this diagnosis can join a randomized, open-label, phase 3 National Cancer Institute study to test whether stereotactic body radiation therapy (SBRT) is as effective as conventional external beam radiation therapy (EBRT) at preventing metastasis.
SBRT delivers radiation to tumors with higher precision than EBRT. The advantage of SBRT is the ability to deliver fewer doses over a shorter duration with less collateral damage to surrounding tissues.
In the trial, half of participants will undergo five treatments of SBRT over 2 weeks, while the other half will receive 20-45 treatments of EBRT over 4-9 weeks. Study sites in 14 US states began recruiting the trial’s 1209 participants in November 2023. Metastasis-free survival over 15 years is the primary endpoint, overall survival is a secondary endpoint, and QoL measures, apart from fatigue, will not be tracked. More details at clinicaltrials.gov.
Dr. Garnick viewed this study as “problematic because patient accrual ends in 2036 with a readout in 2041.” He added, “What its relevance will be at that time is unlikely to provide practice changes, since in that interval there will undoubtedly be multiple advances in place.”
Newly diagnosed favorable intermediate risk prostate cancer
People with this type of cancer are eligible for an open-label, phase 4 real-world study of a radioactive diagnostic agent called piflufolastat F 18 (Pylarify) that targets prostate-specific membrane antigen (PSMA)–positive lesions. Piflufolastat is designed to enhance detection of metastases during PSMA-targeted PET.
Participants will receive a single injection of piflufolastat followed 1-2 hours later by a single whole-body PET-CT or PET-MRI scan. A study site at the Hoag Cancer Center in Irvine, California, welcomed the first of the trial’s 274 participants in February 2024. Sites in Tower Urology, Los Angeles, and the Cleveland Clinic, Ohio, are gearing up. Detection rate is the primary endpoint. Overall survival and QoL are not measured. More details at clinicaltrials.gov
Stages I-IV prostate cancer without bone metastases. People 60 years or older with this type of prostate cancer who are just starting androgen deprivation therapy are eligible for a phase 3, placebo-controlled trial investigating whether high-dose vitamin D can prevent or reduce androgen-deprivation therapy-induced bone loss.
For 1 year, participants will take tablets of high-dose vitamin D or a placebo and then undergo dual x-ray absorptiometry. The Ochsner Medical Center in Jefferson, Louisiana, started recruiting 366 trial participants in December 2023. Reduction in bone mineral density loss in the hip and spine over 1 year is the primary objective. QoL is a secondary objective, and overall survival will not be measured. More details at clinicaltrials.gov
Dr. Garnick expressed some concerns with the trial design so far, including that “the dose of vitamin D is not delineated nor is the target vitamin D level.”
All trial information is from the National Institutes of Health’s National Library of Medicine (online at clinicaltrials.gov). Dr. Garnick did not report conflicts with any of the trials.
A version of this article appeared on Medscape.com.
Metastatic castration-sensitive prostate cancer
Adults with this diagnosis may be interested in a randomized, double-blind, phase 3 study examining whether an experimental poly (ADP-ribose) polymerase (PARP) inhibitor called saruparib can further delay disease progression when added to a next-generation hormonal agent such as abiraterone (Zytiga), darolutamide (Nubeqa), or enzalutamide (Xtandi).
One group of participants will take daily oral doses of saruparib plus physician’s choice of a next-generation hormonal agent until disease progression or another reason for stopping therapy. The other group will add a placebo to a next-generation hormonal agent.
Sites in Rhode Island, Arkansas, California, Michigan, Australia, Canada, Japan, Taiwan, Thailand, the United Kingdom, and South Korea began seeking the trial’s 1800 participants in November 2023. Research centers in 31 other US states and 18 other countries are gearing up. The primary endpoint is radiographic progression-free survival. Overall survival and quality of life (QoL) are secondary endpoints. More details at clinicaltrials.gov.
This news organization asked Marc Garnick, MD, professor of medicine, Harvard Medical School and Beth Israel Deaconess Medical Center, Boston, for his take on the trial. “The study is interesting since it is adding to the evaluations of continued intensification for first-line therapy and will help further elucidate the role of PARP inhibition regardless of homologous repair status,” Dr. Garnick said. “Plus, saruparib is supposedly more selective on PARP1, which in-and-of-itself is of potential benefit.”
Metastatic castration-resistant prostate cancer
People with this type of cancer who have progressed on a next-generation hormonal agent may be eligible for a randomized, open-label, phase 3 trial testing an investigational oral treatment called MK-5684 to see if it increases survival more effectively than switching to an alternative next-generation hormonal agent.
MK-5684 is designed to inhibit the CYP11A1 enzyme, thereby disrupting the androgen-receptor signaling pathway.
One group will take twice-daily tablets of MK-5684 plus hormone replacement therapy, oral dexamethasone, and oral fludrocortisone acetate (Florinef), with rescue hydrocortisone as needed. The other participants will take daily tablets of a next-generation hormonal agent: Either enzalutamide or abiraterone. Patients assigned to abiraterone will also be given prednisone tablets.
US-based sites in nine states and Puerto Rico started looking for the trial’s 1500 participants in December 2023 in partnership with study centers in Australia, Israel, South Korea, and Taiwan. The primary endpoints are radiographic progression-free survival and overall survival. QoL will not be tracked. More details at clinicaltrials.gov.
Metastatic castration-resistant prostate cancer
Patients in this situation who have progressed on taxane-based chemotherapy as well as a next-generation hormonal agent have the option to enroll in another phase 3 MK-5684 study.
Like the trial described above, all patients will remain on their respective therapy until disease progression. In this trial, one group will take twice-daily tablets of MK-5684 without hormone replacement therapy but the same mix of oral dexamethasone and fludrocortisone. Rescue hydrocortisone will also be available. The second group will be assigned either enzalutamide or abiraterone plus prednisone.
Sites in Puerto Rico, Colorado, Nevada, and Virginia, and five other countries outside the United States, opened their doors to the first of 1200 patients in December 2023. The primary endpoints are radiographic progression-free survival and overall survival, analyzed separately for patients with and without an androgen receptor ligand-binding domain mutation. QoL will not be measured. More details at clinicaltrials.gov.
High-risk prostate cancer
People with this diagnosis can join a randomized, open-label, phase 3 National Cancer Institute study to test whether stereotactic body radiation therapy (SBRT) is as effective as conventional external beam radiation therapy (EBRT) at preventing metastasis.
SBRT delivers radiation to tumors with higher precision than EBRT. The advantage of SBRT is the ability to deliver fewer doses over a shorter duration with less collateral damage to surrounding tissues.
In the trial, half of participants will undergo five treatments of SBRT over 2 weeks, while the other half will receive 20-45 treatments of EBRT over 4-9 weeks. Study sites in 14 US states began recruiting the trial’s 1209 participants in November 2023. Metastasis-free survival over 15 years is the primary endpoint, overall survival is a secondary endpoint, and QoL measures, apart from fatigue, will not be tracked. More details at clinicaltrials.gov.
Dr. Garnick viewed this study as “problematic because patient accrual ends in 2036 with a readout in 2041.” He added, “What its relevance will be at that time is unlikely to provide practice changes, since in that interval there will undoubtedly be multiple advances in place.”
Newly diagnosed favorable intermediate risk prostate cancer
People with this type of cancer are eligible for an open-label, phase 4 real-world study of a radioactive diagnostic agent called piflufolastat F 18 (Pylarify) that targets prostate-specific membrane antigen (PSMA)–positive lesions. Piflufolastat is designed to enhance detection of metastases during PSMA-targeted PET.
Participants will receive a single injection of piflufolastat followed 1-2 hours later by a single whole-body PET-CT or PET-MRI scan. A study site at the Hoag Cancer Center in Irvine, California, welcomed the first of the trial’s 274 participants in February 2024. Sites in Tower Urology, Los Angeles, and the Cleveland Clinic, Ohio, are gearing up. Detection rate is the primary endpoint. Overall survival and QoL are not measured. More details at clinicaltrials.gov
Stages I-IV prostate cancer without bone metastases. People 60 years or older with this type of prostate cancer who are just starting androgen deprivation therapy are eligible for a phase 3, placebo-controlled trial investigating whether high-dose vitamin D can prevent or reduce androgen-deprivation therapy-induced bone loss.
For 1 year, participants will take tablets of high-dose vitamin D or a placebo and then undergo dual x-ray absorptiometry. The Ochsner Medical Center in Jefferson, Louisiana, started recruiting 366 trial participants in December 2023. Reduction in bone mineral density loss in the hip and spine over 1 year is the primary objective. QoL is a secondary objective, and overall survival will not be measured. More details at clinicaltrials.gov
Dr. Garnick expressed some concerns with the trial design so far, including that “the dose of vitamin D is not delineated nor is the target vitamin D level.”
All trial information is from the National Institutes of Health’s National Library of Medicine (online at clinicaltrials.gov). Dr. Garnick did not report conflicts with any of the trials.
A version of this article appeared on Medscape.com.