User login
Antimalarials prove protective against long-term lupus damage
SAN DIEGO – Potentially modifiable risk factors for future irreversible organ damage in lupus patients include hypertension, the use of corticosteroids, and higher levels of inflammation early on, according to findings from the SLICC (Systemic Lupus International Collaborating Clinics) Inception Cohort Study.
In addition, the study identified the use of antimalarial drugs as the one significant protective factor against steady accrual of irreversible organ damage in lupus patients.
"These findings help us pave the way to consider whether, firstly, one could use damage as a primary endpoint in future clinical trials in lupus – somewhat akin to how the erosion score is used in rheumatoid arthritis – and secondly, the results suggest particular interventions that might be important in reducing the risk of damage over time," Dr. Ian N. Bruce said at the annual meeting of the American College of Rheumatology.
The study also identified several fixed and unmodifiable risk factors for irreversible damage in lupus patients: older age at diagnosis, male gender, and being black or white Americans, added Dr. Bruce, professor of rheumatology at the University of Manchester (U.K.) and chair of the SLICC research group.
The SLICC Inception Cohort Study involves 1,722 patients at 31 centers in 11 countries in North America, Europe, and Asia who enrolled within 15 months after being formally diagnosed with systemic lupus erythematosus based upon the 1997 ACR criteria. They averaged 35 years of age and had an average of 4.25 comprehensive annual follow-up visits during the study period.
Irreversible organ damage was assessed using the SLICC/ACR Damage Index, or SDI. At baseline, 35% of patients had at least one item of damage as indicated by an SDI score of 1 or more. Over time, damage rates slowly and steadily increased such that by 6 years of follow-up 51% of participants had an SDI of at least 1.
In a multivariate analysis, patients with an SDI score of 1 at baseline had a highly significant 37% reduction in the risk of increasing their score during follow-up if they were taking antimalarials, compared with those not taking antimalarials.
On the other hand, patients with a baseline SDI of 1 were 61% more likely to experience an increase in their damage score during follow-up if they had hypertension and 43% more likely to do so if they were on corticosteroids than if they weren’t. Moreover, their risk of going from an SDI of 1 to a higher SDI indicative of mounting damage increased by 10% for every 3-point increase on the SLE Disease Activity Index (SLEDAI).
Patients with a baseline SDI of 0 were 64% more likely to progress to a score of 1 or more during follow-up if they were taking corticosteroids and 71% more likely to do so if they were hypertensive. Their risk also increased by 17% for each 3-point increase in SLEDAI. Men had a 48% greater risk of going from an SDI of 0 to 1 or more than women. Asians were 40% less likely to develop irreversible damage.
Each 1-point increase in SDI score was associated with a 46% increased risk of mortality, as well as with poorer health-related quality of life, especially as reflected in SF-36 physical component scores.
Session chair Dr. Roberto Caricchio of Temple University, Philadelphia, called the SLICC study "very important work."
"It teaches us to be aggressive up-front with our lupus patients, which we often aren’t. We tend to spare ourselves because it’s a chronic disease, and we know we’ll see these patients for the next 20 years, so we try to spare them from certain therapies," said Dr. Caricchio.
Dr. Bruce concurred. "I think a concerted effort to switch the disease off in almost a treat-to-target way, getting people into remission, may well be very important with regard to avoiding long-term damage. If we could do that without using steroids, that would be ideal," he commented.
"SLICC is interested in the fact that most clinical trials in lupus to date have taken a very small subsection of the population, those with high disease activity, and used a particular biologic agent or new molecule to show that it improved disease activity. But actually the majority of people with lupus – around 60% have low-grade, grumbling disease and are on low-dose steroids. And those are the ones who accumulate damage. I think we need to have a paradigm shift in how we do clinical trials in lupus and think about doing lupus trials against a damage endpoint," the rheumatologist continued.
Power calculations based upon the SLICC Inception Cohort Study suggest such trials could be relatively modest in size, he added.
SLICC receives financial support from GlaxoSmithKline, Bristol-Myers Squibb, and Human Genome Sciences. Dr. Bruce reported receiving research funding from GlaxoSmithKline, Bristol-Myers Squibb, Roche, and UCB.
SAN DIEGO – Potentially modifiable risk factors for future irreversible organ damage in lupus patients include hypertension, the use of corticosteroids, and higher levels of inflammation early on, according to findings from the SLICC (Systemic Lupus International Collaborating Clinics) Inception Cohort Study.
In addition, the study identified the use of antimalarial drugs as the one significant protective factor against steady accrual of irreversible organ damage in lupus patients.
"These findings help us pave the way to consider whether, firstly, one could use damage as a primary endpoint in future clinical trials in lupus – somewhat akin to how the erosion score is used in rheumatoid arthritis – and secondly, the results suggest particular interventions that might be important in reducing the risk of damage over time," Dr. Ian N. Bruce said at the annual meeting of the American College of Rheumatology.
The study also identified several fixed and unmodifiable risk factors for irreversible damage in lupus patients: older age at diagnosis, male gender, and being black or white Americans, added Dr. Bruce, professor of rheumatology at the University of Manchester (U.K.) and chair of the SLICC research group.
The SLICC Inception Cohort Study involves 1,722 patients at 31 centers in 11 countries in North America, Europe, and Asia who enrolled within 15 months after being formally diagnosed with systemic lupus erythematosus based upon the 1997 ACR criteria. They averaged 35 years of age and had an average of 4.25 comprehensive annual follow-up visits during the study period.
Irreversible organ damage was assessed using the SLICC/ACR Damage Index, or SDI. At baseline, 35% of patients had at least one item of damage as indicated by an SDI score of 1 or more. Over time, damage rates slowly and steadily increased such that by 6 years of follow-up 51% of participants had an SDI of at least 1.
In a multivariate analysis, patients with an SDI score of 1 at baseline had a highly significant 37% reduction in the risk of increasing their score during follow-up if they were taking antimalarials, compared with those not taking antimalarials.
On the other hand, patients with a baseline SDI of 1 were 61% more likely to experience an increase in their damage score during follow-up if they had hypertension and 43% more likely to do so if they were on corticosteroids than if they weren’t. Moreover, their risk of going from an SDI of 1 to a higher SDI indicative of mounting damage increased by 10% for every 3-point increase on the SLE Disease Activity Index (SLEDAI).
Patients with a baseline SDI of 0 were 64% more likely to progress to a score of 1 or more during follow-up if they were taking corticosteroids and 71% more likely to do so if they were hypertensive. Their risk also increased by 17% for each 3-point increase in SLEDAI. Men had a 48% greater risk of going from an SDI of 0 to 1 or more than women. Asians were 40% less likely to develop irreversible damage.
Each 1-point increase in SDI score was associated with a 46% increased risk of mortality, as well as with poorer health-related quality of life, especially as reflected in SF-36 physical component scores.
Session chair Dr. Roberto Caricchio of Temple University, Philadelphia, called the SLICC study "very important work."
"It teaches us to be aggressive up-front with our lupus patients, which we often aren’t. We tend to spare ourselves because it’s a chronic disease, and we know we’ll see these patients for the next 20 years, so we try to spare them from certain therapies," said Dr. Caricchio.
Dr. Bruce concurred. "I think a concerted effort to switch the disease off in almost a treat-to-target way, getting people into remission, may well be very important with regard to avoiding long-term damage. If we could do that without using steroids, that would be ideal," he commented.
"SLICC is interested in the fact that most clinical trials in lupus to date have taken a very small subsection of the population, those with high disease activity, and used a particular biologic agent or new molecule to show that it improved disease activity. But actually the majority of people with lupus – around 60% have low-grade, grumbling disease and are on low-dose steroids. And those are the ones who accumulate damage. I think we need to have a paradigm shift in how we do clinical trials in lupus and think about doing lupus trials against a damage endpoint," the rheumatologist continued.
Power calculations based upon the SLICC Inception Cohort Study suggest such trials could be relatively modest in size, he added.
SLICC receives financial support from GlaxoSmithKline, Bristol-Myers Squibb, and Human Genome Sciences. Dr. Bruce reported receiving research funding from GlaxoSmithKline, Bristol-Myers Squibb, Roche, and UCB.
SAN DIEGO – Potentially modifiable risk factors for future irreversible organ damage in lupus patients include hypertension, the use of corticosteroids, and higher levels of inflammation early on, according to findings from the SLICC (Systemic Lupus International Collaborating Clinics) Inception Cohort Study.
In addition, the study identified the use of antimalarial drugs as the one significant protective factor against steady accrual of irreversible organ damage in lupus patients.
"These findings help us pave the way to consider whether, firstly, one could use damage as a primary endpoint in future clinical trials in lupus – somewhat akin to how the erosion score is used in rheumatoid arthritis – and secondly, the results suggest particular interventions that might be important in reducing the risk of damage over time," Dr. Ian N. Bruce said at the annual meeting of the American College of Rheumatology.
The study also identified several fixed and unmodifiable risk factors for irreversible damage in lupus patients: older age at diagnosis, male gender, and being black or white Americans, added Dr. Bruce, professor of rheumatology at the University of Manchester (U.K.) and chair of the SLICC research group.
The SLICC Inception Cohort Study involves 1,722 patients at 31 centers in 11 countries in North America, Europe, and Asia who enrolled within 15 months after being formally diagnosed with systemic lupus erythematosus based upon the 1997 ACR criteria. They averaged 35 years of age and had an average of 4.25 comprehensive annual follow-up visits during the study period.
Irreversible organ damage was assessed using the SLICC/ACR Damage Index, or SDI. At baseline, 35% of patients had at least one item of damage as indicated by an SDI score of 1 or more. Over time, damage rates slowly and steadily increased such that by 6 years of follow-up 51% of participants had an SDI of at least 1.
In a multivariate analysis, patients with an SDI score of 1 at baseline had a highly significant 37% reduction in the risk of increasing their score during follow-up if they were taking antimalarials, compared with those not taking antimalarials.
On the other hand, patients with a baseline SDI of 1 were 61% more likely to experience an increase in their damage score during follow-up if they had hypertension and 43% more likely to do so if they were on corticosteroids than if they weren’t. Moreover, their risk of going from an SDI of 1 to a higher SDI indicative of mounting damage increased by 10% for every 3-point increase on the SLE Disease Activity Index (SLEDAI).
Patients with a baseline SDI of 0 were 64% more likely to progress to a score of 1 or more during follow-up if they were taking corticosteroids and 71% more likely to do so if they were hypertensive. Their risk also increased by 17% for each 3-point increase in SLEDAI. Men had a 48% greater risk of going from an SDI of 0 to 1 or more than women. Asians were 40% less likely to develop irreversible damage.
Each 1-point increase in SDI score was associated with a 46% increased risk of mortality, as well as with poorer health-related quality of life, especially as reflected in SF-36 physical component scores.
Session chair Dr. Roberto Caricchio of Temple University, Philadelphia, called the SLICC study "very important work."
"It teaches us to be aggressive up-front with our lupus patients, which we often aren’t. We tend to spare ourselves because it’s a chronic disease, and we know we’ll see these patients for the next 20 years, so we try to spare them from certain therapies," said Dr. Caricchio.
Dr. Bruce concurred. "I think a concerted effort to switch the disease off in almost a treat-to-target way, getting people into remission, may well be very important with regard to avoiding long-term damage. If we could do that without using steroids, that would be ideal," he commented.
"SLICC is interested in the fact that most clinical trials in lupus to date have taken a very small subsection of the population, those with high disease activity, and used a particular biologic agent or new molecule to show that it improved disease activity. But actually the majority of people with lupus – around 60% have low-grade, grumbling disease and are on low-dose steroids. And those are the ones who accumulate damage. I think we need to have a paradigm shift in how we do clinical trials in lupus and think about doing lupus trials against a damage endpoint," the rheumatologist continued.
Power calculations based upon the SLICC Inception Cohort Study suggest such trials could be relatively modest in size, he added.
SLICC receives financial support from GlaxoSmithKline, Bristol-Myers Squibb, and Human Genome Sciences. Dr. Bruce reported receiving research funding from GlaxoSmithKline, Bristol-Myers Squibb, Roche, and UCB.
AT THE ACR ANNUAL MEETING
Major finding: Patients with recently diagnosed SLE and no significant organ damage at baseline were 71% more likely to develop irreversible organ damage during follow-up if they were hypertensive and 64% more likely to do so if they were taking corticosteroids. Antimalarial drugs showed a protective effect against accrual of damage.
Data source: The Systemic Lupus International Collaborating Clinics Inception Cohort Study involves 1,722 patients at 31 centers in 11 countries, all recruited within 15 months after diagnosis.
Disclosures: The study group receives funding from GlaxoSmithKline, Bristol-Myers Squibb, and Human Genome Sciences. The presenter receives research grants from GlaxoSmithKline, Bristol-Myers Squibb, and several other pharmaceutical companies.
Depression accounts for psoriatics’ increased MI risk
SAN DIEGO – Depression is an independent risk factor for acute myocardial infarction in patients with psoriasis or psoriatic arthritis, a large population-based cohort study indicates.
In this study of more than 10,000 British Columbians with psoriasis and/or psoriatic arthritis, the increased risk of MI was confined to the patient subset having comorbid depression, Lindsay C. Burns, Ph.D., reported at the annual meeting of the American College of Rheumatology.
"These data underscore the need to actively screen for depression among psoriasis and psoriatic arthritis patients and closely monitor cardiovascular health in this high-risk group to improve long-term survival," declared Dr. Burns of the University of British Columbia, Vancouver.
She and her coinvestigators mined the comprehensive health records available for 4.1 million adults through British Columbia’s universal medical insurance coverage system in order to identify all 10,041 patients who were diagnosed with psoriasis by a dermatologist or psoriatic arthritis by a rheumatologist during 1996-2006, and who at that time had no history of MI. The patients were matched by age, gender, and years of follow-up with 47,415 controls.
Acute MI occurred in 268 patients with psoriasis or psoriatic arthritis, for an incidence rate of 5.8 cases per 1,000 person-years. The incidence rate of physician-diagnosed depression was 3.4 per 1,000 person-years in the psoriatic group, with a 10-year prevalence of 21.6%.
In a multivariate regression analysis adjusted for comorbid conditions, socioeconomic status, health resource utilization, age, and gender, individuals with psoriasis or psoriatic arthritis were 26% more likely to be depressed than controls. Diagnosis of depression in psoriatic patients during the follow-up period increased their risk of having an MI by an adjusted 80% compared with psoriatic patients without the psychiatric diagnosis.
Psoriatic subjects without depression had a statistically nonsignificant 10% increased risk of MI compared with nonpsoriatic controls. In contrast, psoriatic patients with diagnosed depression had a 60% greater MI risk than controls, according to Dr. Burns.
The study was funded by the Arthritis Research Center of Canada. Dr. Burns reported having no financial conflicts of interest.
SAN DIEGO – Depression is an independent risk factor for acute myocardial infarction in patients with psoriasis or psoriatic arthritis, a large population-based cohort study indicates.
In this study of more than 10,000 British Columbians with psoriasis and/or psoriatic arthritis, the increased risk of MI was confined to the patient subset having comorbid depression, Lindsay C. Burns, Ph.D., reported at the annual meeting of the American College of Rheumatology.
"These data underscore the need to actively screen for depression among psoriasis and psoriatic arthritis patients and closely monitor cardiovascular health in this high-risk group to improve long-term survival," declared Dr. Burns of the University of British Columbia, Vancouver.
She and her coinvestigators mined the comprehensive health records available for 4.1 million adults through British Columbia’s universal medical insurance coverage system in order to identify all 10,041 patients who were diagnosed with psoriasis by a dermatologist or psoriatic arthritis by a rheumatologist during 1996-2006, and who at that time had no history of MI. The patients were matched by age, gender, and years of follow-up with 47,415 controls.
Acute MI occurred in 268 patients with psoriasis or psoriatic arthritis, for an incidence rate of 5.8 cases per 1,000 person-years. The incidence rate of physician-diagnosed depression was 3.4 per 1,000 person-years in the psoriatic group, with a 10-year prevalence of 21.6%.
In a multivariate regression analysis adjusted for comorbid conditions, socioeconomic status, health resource utilization, age, and gender, individuals with psoriasis or psoriatic arthritis were 26% more likely to be depressed than controls. Diagnosis of depression in psoriatic patients during the follow-up period increased their risk of having an MI by an adjusted 80% compared with psoriatic patients without the psychiatric diagnosis.
Psoriatic subjects without depression had a statistically nonsignificant 10% increased risk of MI compared with nonpsoriatic controls. In contrast, psoriatic patients with diagnosed depression had a 60% greater MI risk than controls, according to Dr. Burns.
The study was funded by the Arthritis Research Center of Canada. Dr. Burns reported having no financial conflicts of interest.
SAN DIEGO – Depression is an independent risk factor for acute myocardial infarction in patients with psoriasis or psoriatic arthritis, a large population-based cohort study indicates.
In this study of more than 10,000 British Columbians with psoriasis and/or psoriatic arthritis, the increased risk of MI was confined to the patient subset having comorbid depression, Lindsay C. Burns, Ph.D., reported at the annual meeting of the American College of Rheumatology.
"These data underscore the need to actively screen for depression among psoriasis and psoriatic arthritis patients and closely monitor cardiovascular health in this high-risk group to improve long-term survival," declared Dr. Burns of the University of British Columbia, Vancouver.
She and her coinvestigators mined the comprehensive health records available for 4.1 million adults through British Columbia’s universal medical insurance coverage system in order to identify all 10,041 patients who were diagnosed with psoriasis by a dermatologist or psoriatic arthritis by a rheumatologist during 1996-2006, and who at that time had no history of MI. The patients were matched by age, gender, and years of follow-up with 47,415 controls.
Acute MI occurred in 268 patients with psoriasis or psoriatic arthritis, for an incidence rate of 5.8 cases per 1,000 person-years. The incidence rate of physician-diagnosed depression was 3.4 per 1,000 person-years in the psoriatic group, with a 10-year prevalence of 21.6%.
In a multivariate regression analysis adjusted for comorbid conditions, socioeconomic status, health resource utilization, age, and gender, individuals with psoriasis or psoriatic arthritis were 26% more likely to be depressed than controls. Diagnosis of depression in psoriatic patients during the follow-up period increased their risk of having an MI by an adjusted 80% compared with psoriatic patients without the psychiatric diagnosis.
Psoriatic subjects without depression had a statistically nonsignificant 10% increased risk of MI compared with nonpsoriatic controls. In contrast, psoriatic patients with diagnosed depression had a 60% greater MI risk than controls, according to Dr. Burns.
The study was funded by the Arthritis Research Center of Canada. Dr. Burns reported having no financial conflicts of interest.
AT THE ACR ANNUAL MEETING
Major finding: Patients with psoriasis or psoriatic arthritis who developed comorbid depression had a highly significant 80% increased risk of acute MI during follow-up, compared with those without depression.
Data source: This was a population-based cohort study involving 10,041 adults diagnosed with psoriasis or psoriatic arthritis in British Columbia during 1996-2006 and more than 47,000 controls.
Disclosures: The study was funded by the Arthritis Research Center of Canada. Dr. Burns reported having no financial conflicts of interest.
Apremilast’s positive study results made it the talk of ACR 2013
SAN DIEGO – The novel oral phosphodiesterase-4 inhibitor apremilast cut an impressively wide swath through the annual meeting of the American College of Rheumatology on the strength of positive results in three separate pivotal phase III trials for psoriatic arthritis and a favorable phase II study in Behçet’s syndrome.
Dr. Alvin F. Wells reported that apremilast resulted in clinically meaningful improvement in psoriatic arthritis symptoms, physical function, and associated skin psoriasis at 16 weeks in the PALACE 4 trial. Moreover, the improvement remained durable through 52 weeks in the phase III clinical trial.
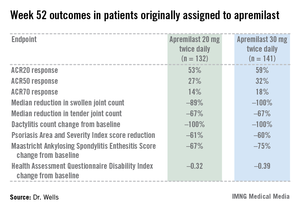
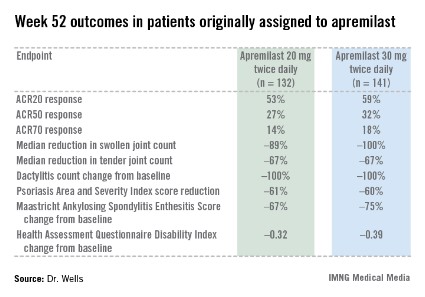
PALACE 4 compared apremilast against placebo as a first-line treatment in 527 patients with psoriatic arthritis not previously treated with a disease-modifying antirheumatic drug. This was a population with active disease: a mean baseline 11 swollen and 20 tender joints; a Health Assessment Questionnaire Disability Index score averaging 1.07; and a 50% prevalence of dactylitis, with a mean severity score of 2.0. Sixty-five percent of patients had enthesitis, with a median Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) of 3.0. Participants had a mean 16-year history of psoriasis and a 3.4-year duration of psoriatic arthritis.
The primary study endpoint was attainment of an American College of Rheumatology 20% improvement (ACR20) response at 16 weeks. This was achieved in 29.2% of patients randomized to apremilast at 20 mg twice daily, 32.3% on 30 mg twice daily, and 16.9% on placebo. At week 16, patients on placebo were re-randomized to apremilast at 20 mg or 30 mg twice weekly. By week 52, an ACR20 response was achieved by 53% of patients in the apremilast 20 mg twice-daily group and 59% of those on the higher dose, according to Dr. Wells, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc.
Apremilast also scored well on numerous secondary endpoints (see chart). For example, the swollen joint count decreased by a median of 89% over the course of 52 weeks in patients on apremilast at 20 mg twice daily.
"The way I like to think of that result is at least 50% of patients had an 89% improvement in their swollen joint count," he explained.
During the first 16 weeks of PALACE 4, there were fewer serious adverse events in apremilast-treated patients than with placebo-treated patients. The most frequent side effects of apremilast were nausea, diarrhea, and headache, affecting 16%, 12%, and 8%, respectively, of patients in the higher-dose arm. These adverse events were almost exclusively mild or moderate, they began within the first 2 weeks of treatment, and they resolved in 4 weeks despite continued treatment. Less than 2% of apremilast-treated patients dropped out of the study due to nausea or diarrhea through week 52.
PALACE 4 differed from the PALACE 2 and 3 trials, also presented at the ACR meeting, in that PALACE 2 and 3 involved only patients with psoriatic arthritis previously treated with biologic agents or other disease-modifying antirheumatic drugs. All three studies had the same design, and the results in terms of both efficacy and safety were consistent across the full clinical trial program. No clinically meaningful changes in laboratory values were seen in any of the PALACE trials, suggesting ongoing lab monitoring may not be necessary.
Also at the ACR annual meeting, Dr. Gülen Hatemi of Istanbul University, Turkey, in a reprise of her report several weeks earlier at the annual congress of the European Academy of Dermatology and Venereology, presented a phase II study showing apremilast to be highly effective in treating the oral ulcers that are the cardinal feature of Behçet’s syndrome.
Celgene filed for approval of apremilast for psoriatic arthritis with the Food and Drug Administration earlier this year and anticipates a decision in March 2014. The company also plans to apply to the European regulatory agency for the same indication before year’s end, and to petition the FDA for approval in psoriasis within the same time frame. A 500-patient phase III study of apremilast in ankylosing spondylitis, known as POSTURE, is well underway.
PALACE 4 was sponsored by Celgene. Dr. Wells reported receiving a research grant from the company.
SAN DIEGO – The novel oral phosphodiesterase-4 inhibitor apremilast cut an impressively wide swath through the annual meeting of the American College of Rheumatology on the strength of positive results in three separate pivotal phase III trials for psoriatic arthritis and a favorable phase II study in Behçet’s syndrome.
Dr. Alvin F. Wells reported that apremilast resulted in clinically meaningful improvement in psoriatic arthritis symptoms, physical function, and associated skin psoriasis at 16 weeks in the PALACE 4 trial. Moreover, the improvement remained durable through 52 weeks in the phase III clinical trial.

PALACE 4 compared apremilast against placebo as a first-line treatment in 527 patients with psoriatic arthritis not previously treated with a disease-modifying antirheumatic drug. This was a population with active disease: a mean baseline 11 swollen and 20 tender joints; a Health Assessment Questionnaire Disability Index score averaging 1.07; and a 50% prevalence of dactylitis, with a mean severity score of 2.0. Sixty-five percent of patients had enthesitis, with a median Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) of 3.0. Participants had a mean 16-year history of psoriasis and a 3.4-year duration of psoriatic arthritis.
The primary study endpoint was attainment of an American College of Rheumatology 20% improvement (ACR20) response at 16 weeks. This was achieved in 29.2% of patients randomized to apremilast at 20 mg twice daily, 32.3% on 30 mg twice daily, and 16.9% on placebo. At week 16, patients on placebo were re-randomized to apremilast at 20 mg or 30 mg twice weekly. By week 52, an ACR20 response was achieved by 53% of patients in the apremilast 20 mg twice-daily group and 59% of those on the higher dose, according to Dr. Wells, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc.
Apremilast also scored well on numerous secondary endpoints (see chart). For example, the swollen joint count decreased by a median of 89% over the course of 52 weeks in patients on apremilast at 20 mg twice daily.
"The way I like to think of that result is at least 50% of patients had an 89% improvement in their swollen joint count," he explained.
During the first 16 weeks of PALACE 4, there were fewer serious adverse events in apremilast-treated patients than with placebo-treated patients. The most frequent side effects of apremilast were nausea, diarrhea, and headache, affecting 16%, 12%, and 8%, respectively, of patients in the higher-dose arm. These adverse events were almost exclusively mild or moderate, they began within the first 2 weeks of treatment, and they resolved in 4 weeks despite continued treatment. Less than 2% of apremilast-treated patients dropped out of the study due to nausea or diarrhea through week 52.
PALACE 4 differed from the PALACE 2 and 3 trials, also presented at the ACR meeting, in that PALACE 2 and 3 involved only patients with psoriatic arthritis previously treated with biologic agents or other disease-modifying antirheumatic drugs. All three studies had the same design, and the results in terms of both efficacy and safety were consistent across the full clinical trial program. No clinically meaningful changes in laboratory values were seen in any of the PALACE trials, suggesting ongoing lab monitoring may not be necessary.
Also at the ACR annual meeting, Dr. Gülen Hatemi of Istanbul University, Turkey, in a reprise of her report several weeks earlier at the annual congress of the European Academy of Dermatology and Venereology, presented a phase II study showing apremilast to be highly effective in treating the oral ulcers that are the cardinal feature of Behçet’s syndrome.
Celgene filed for approval of apremilast for psoriatic arthritis with the Food and Drug Administration earlier this year and anticipates a decision in March 2014. The company also plans to apply to the European regulatory agency for the same indication before year’s end, and to petition the FDA for approval in psoriasis within the same time frame. A 500-patient phase III study of apremilast in ankylosing spondylitis, known as POSTURE, is well underway.
PALACE 4 was sponsored by Celgene. Dr. Wells reported receiving a research grant from the company.
SAN DIEGO – The novel oral phosphodiesterase-4 inhibitor apremilast cut an impressively wide swath through the annual meeting of the American College of Rheumatology on the strength of positive results in three separate pivotal phase III trials for psoriatic arthritis and a favorable phase II study in Behçet’s syndrome.
Dr. Alvin F. Wells reported that apremilast resulted in clinically meaningful improvement in psoriatic arthritis symptoms, physical function, and associated skin psoriasis at 16 weeks in the PALACE 4 trial. Moreover, the improvement remained durable through 52 weeks in the phase III clinical trial.

PALACE 4 compared apremilast against placebo as a first-line treatment in 527 patients with psoriatic arthritis not previously treated with a disease-modifying antirheumatic drug. This was a population with active disease: a mean baseline 11 swollen and 20 tender joints; a Health Assessment Questionnaire Disability Index score averaging 1.07; and a 50% prevalence of dactylitis, with a mean severity score of 2.0. Sixty-five percent of patients had enthesitis, with a median Maastricht Ankylosing Spondylitis Enthesitis Score (MASES) of 3.0. Participants had a mean 16-year history of psoriasis and a 3.4-year duration of psoriatic arthritis.
The primary study endpoint was attainment of an American College of Rheumatology 20% improvement (ACR20) response at 16 weeks. This was achieved in 29.2% of patients randomized to apremilast at 20 mg twice daily, 32.3% on 30 mg twice daily, and 16.9% on placebo. At week 16, patients on placebo were re-randomized to apremilast at 20 mg or 30 mg twice weekly. By week 52, an ACR20 response was achieved by 53% of patients in the apremilast 20 mg twice-daily group and 59% of those on the higher dose, according to Dr. Wells, director of the Rheumatology and Immunotherapy Center in Franklin, Wisc.
Apremilast also scored well on numerous secondary endpoints (see chart). For example, the swollen joint count decreased by a median of 89% over the course of 52 weeks in patients on apremilast at 20 mg twice daily.
"The way I like to think of that result is at least 50% of patients had an 89% improvement in their swollen joint count," he explained.
During the first 16 weeks of PALACE 4, there were fewer serious adverse events in apremilast-treated patients than with placebo-treated patients. The most frequent side effects of apremilast were nausea, diarrhea, and headache, affecting 16%, 12%, and 8%, respectively, of patients in the higher-dose arm. These adverse events were almost exclusively mild or moderate, they began within the first 2 weeks of treatment, and they resolved in 4 weeks despite continued treatment. Less than 2% of apremilast-treated patients dropped out of the study due to nausea or diarrhea through week 52.
PALACE 4 differed from the PALACE 2 and 3 trials, also presented at the ACR meeting, in that PALACE 2 and 3 involved only patients with psoriatic arthritis previously treated with biologic agents or other disease-modifying antirheumatic drugs. All three studies had the same design, and the results in terms of both efficacy and safety were consistent across the full clinical trial program. No clinically meaningful changes in laboratory values were seen in any of the PALACE trials, suggesting ongoing lab monitoring may not be necessary.
Also at the ACR annual meeting, Dr. Gülen Hatemi of Istanbul University, Turkey, in a reprise of her report several weeks earlier at the annual congress of the European Academy of Dermatology and Venereology, presented a phase II study showing apremilast to be highly effective in treating the oral ulcers that are the cardinal feature of Behçet’s syndrome.
Celgene filed for approval of apremilast for psoriatic arthritis with the Food and Drug Administration earlier this year and anticipates a decision in March 2014. The company also plans to apply to the European regulatory agency for the same indication before year’s end, and to petition the FDA for approval in psoriasis within the same time frame. A 500-patient phase III study of apremilast in ankylosing spondylitis, known as POSTURE, is well underway.
PALACE 4 was sponsored by Celgene. Dr. Wells reported receiving a research grant from the company.
AT THE ACR ANNUAL MEETING
Major finding: After 16 weeks of treatment, the investigational drug apremilast achieved an ACR20 response of 29.2% at 20 mg twice daily as first-line therapy in patients with psoriatic arthritis and 32.3% at 30 mg twice daily, whereas placebo-treated controls achieved a rate of 16.9%.
Data source: The PALACE 4 study was a pivotal phase III, randomized, placebo-controlled trial including 527 patients with active psoriatic arthritis.
Disclosures: PALACE 4 was sponsored by Celgene. Dr. Wells reported receiving a research grant from the company.
Cyclophosphamide and rituximab combo reduced severe lupus flares
SAN DIEGO – The combination of intravenous cyclophosphamide and rituximab shows promise for the reduction of lupus flares, both renal and nonrenal, in patients with severe systemic lupus erythematosus, according to Dr. Ali Shahzad.
Moreover, this benefit did not come at the cost of a significant increase in infections, as compared with intravenous cyclophosphamide monotherapy, Dr. Shahzad reported at the annual meeting of the American College of Rheumatology.
He reported on 43 patients with severe, recurrent SLE. Thirty-one were placed on intravenous cyclophosphamide monotherapy administered according to the National Institutes of Health standard protocol for the treatment of lupus nephritis. The other 12 got cyclophosphamide plus two 1,000-mg doses of rituximab (Rituxan) given 15 days apart. The combination regimen was given at the physician’s discretion for recalcitrant or recurrent flares of lupus nephritis in 10 of 12 cases, and for treatment-resistant CNS lupus or other extrarenal lupus in the other 2. Eight of the 12 recipients of combination therapy had previously been treated with intravenous cyclophosphamide, in most cases for lupus nephritis.
In the combination therapy group, the median duration of follow-up prior to dual therapy was 36 months, with an additional 21 months of follow-up after receiving the combination. Prior to combination therapy, these 12 patients collectively had 38 lupus flares, 26 of which featured renal involvement. In contrast, post treatment they developed just 13 flares, only 3 of which were renal, according to Dr. Shahzad of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md.
The 43 SLE patients had a total of 15 bacterial, 6 fungal, and 18 viral infections. And although there was a consistent trend toward higher rates in the combination therapy group, the differences fell far short of statistical significance.
Dr. Shahzad acknowledged the small sample size and retrospective design as important study limitations. However, based upon these encouraging, albeit preliminary, study findings, he and his NIH colleagues said they are planning a prospective clinical trial examining the efficacy and tolerability of the intravenous cyclophosphamide/rituximab combination in patients with severe, recurrent SLE.
Dr. Shahzad reported having no financial conflicts regarding this NIH-sponsored study.
SAN DIEGO – The combination of intravenous cyclophosphamide and rituximab shows promise for the reduction of lupus flares, both renal and nonrenal, in patients with severe systemic lupus erythematosus, according to Dr. Ali Shahzad.
Moreover, this benefit did not come at the cost of a significant increase in infections, as compared with intravenous cyclophosphamide monotherapy, Dr. Shahzad reported at the annual meeting of the American College of Rheumatology.
He reported on 43 patients with severe, recurrent SLE. Thirty-one were placed on intravenous cyclophosphamide monotherapy administered according to the National Institutes of Health standard protocol for the treatment of lupus nephritis. The other 12 got cyclophosphamide plus two 1,000-mg doses of rituximab (Rituxan) given 15 days apart. The combination regimen was given at the physician’s discretion for recalcitrant or recurrent flares of lupus nephritis in 10 of 12 cases, and for treatment-resistant CNS lupus or other extrarenal lupus in the other 2. Eight of the 12 recipients of combination therapy had previously been treated with intravenous cyclophosphamide, in most cases for lupus nephritis.
In the combination therapy group, the median duration of follow-up prior to dual therapy was 36 months, with an additional 21 months of follow-up after receiving the combination. Prior to combination therapy, these 12 patients collectively had 38 lupus flares, 26 of which featured renal involvement. In contrast, post treatment they developed just 13 flares, only 3 of which were renal, according to Dr. Shahzad of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md.
The 43 SLE patients had a total of 15 bacterial, 6 fungal, and 18 viral infections. And although there was a consistent trend toward higher rates in the combination therapy group, the differences fell far short of statistical significance.
Dr. Shahzad acknowledged the small sample size and retrospective design as important study limitations. However, based upon these encouraging, albeit preliminary, study findings, he and his NIH colleagues said they are planning a prospective clinical trial examining the efficacy and tolerability of the intravenous cyclophosphamide/rituximab combination in patients with severe, recurrent SLE.
Dr. Shahzad reported having no financial conflicts regarding this NIH-sponsored study.
SAN DIEGO – The combination of intravenous cyclophosphamide and rituximab shows promise for the reduction of lupus flares, both renal and nonrenal, in patients with severe systemic lupus erythematosus, according to Dr. Ali Shahzad.
Moreover, this benefit did not come at the cost of a significant increase in infections, as compared with intravenous cyclophosphamide monotherapy, Dr. Shahzad reported at the annual meeting of the American College of Rheumatology.
He reported on 43 patients with severe, recurrent SLE. Thirty-one were placed on intravenous cyclophosphamide monotherapy administered according to the National Institutes of Health standard protocol for the treatment of lupus nephritis. The other 12 got cyclophosphamide plus two 1,000-mg doses of rituximab (Rituxan) given 15 days apart. The combination regimen was given at the physician’s discretion for recalcitrant or recurrent flares of lupus nephritis in 10 of 12 cases, and for treatment-resistant CNS lupus or other extrarenal lupus in the other 2. Eight of the 12 recipients of combination therapy had previously been treated with intravenous cyclophosphamide, in most cases for lupus nephritis.
In the combination therapy group, the median duration of follow-up prior to dual therapy was 36 months, with an additional 21 months of follow-up after receiving the combination. Prior to combination therapy, these 12 patients collectively had 38 lupus flares, 26 of which featured renal involvement. In contrast, post treatment they developed just 13 flares, only 3 of which were renal, according to Dr. Shahzad of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, Md.
The 43 SLE patients had a total of 15 bacterial, 6 fungal, and 18 viral infections. And although there was a consistent trend toward higher rates in the combination therapy group, the differences fell far short of statistical significance.
Dr. Shahzad acknowledged the small sample size and retrospective design as important study limitations. However, based upon these encouraging, albeit preliminary, study findings, he and his NIH colleagues said they are planning a prospective clinical trial examining the efficacy and tolerability of the intravenous cyclophosphamide/rituximab combination in patients with severe, recurrent SLE.
Dr. Shahzad reported having no financial conflicts regarding this NIH-sponsored study.
AT THE ACR ANNUAL MEETING
Major finding: Patients with severe recurrent systemic lupus erythematosus collectively had 38 lupus flares, including 26 with renal involvement, during a median 36-month period before undergoing combination therapy with intravenous cyclophosphamide and rituximab; they had 13 flares, only 3 of which featured renal involvement, during 21 months afterward.
Data source: A retrospective study including 43 patients with severe recurrent SLE, 31 of whom were placed on intravenous cyclophosphamide monotherapy, while 12 received combination therapy with cyclophosphamide plus rituximab.
Disclosures: This study was sponsored by the National Institutes of Health. The presenter, an employee of the National Institute of Arthritis and Musculoskeletal and Skin Diseases, reported having no relevant financial conflicts.
Psoriasis drug research aimed at severe disease
ISTANBUL, TURKEY – The current intensive period of new drug development in psoriasis is cause for enthusiastic celebration by patients and physicians alike. That is, except for one glaring research gap.
Almost all of the promising drugs now in phase-II or -III clinical trials are systemic agents aimed at the minority of psoriasis patients with more severe disease. There’s not nearly as much in the pipeline for the many patients with mild psoriasis who are most appropriately treated with topical therapy, Dr. Hervé Bachelez observed at the annual congress of the European Academy of Dermatology and Venereology.
Indeed, he identified only two promising topical agents advancing through the developmental pipeline: Pfizer’s tofacitinib, a small-molecule Janus kinase inhibitor under study in both topical and oral formulations, and Anacor’s AN2728, a boron-containing molecule that inhibits phosphodiesterase-4 (PDE-4).
Industry’s lack of enthusiasm for developing new topical agents is difficult to understand, given that 84% of all U.S. prescriptions written for psoriasis in 2009 were for topical agents, according to IMS. Moreover, patients clearly believe there is a major unmet need for new and better topicals: a recent survey of 2,151 European psoriasis patients and their dermatologists found that only 45% of patients on topical therapy were satisfied with their treatment. And a mere 35% of dermatologists rated current topical treatments as satisfactory (J. Dermatolog. Treat. 2013;24:193-8).
The great bulk of the action in psoriasis drug development now is focused on injectable biologics and oral small molecules. Among them are three interleukin-17 inhibitors: Novartis’ secukinumab, Amgen’s brodalumab, and Eli Lilly’s ixekizumab. Celgene is developing apremilast as an oral PDE-4 inhibitor. Merck and Janssen Biotech are developing MK-3222 and CNTO 1959, respectively, as interleukin-23 inhibitors.
Dr. Bachelez, professor of dermatology and head of the inflammatory skin diseases unit at Saint Louis University Hospital, Paris, reported serving on the advisory boards of 10 pharmaceutical companies.
ISTANBUL, TURKEY – The current intensive period of new drug development in psoriasis is cause for enthusiastic celebration by patients and physicians alike. That is, except for one glaring research gap.
Almost all of the promising drugs now in phase-II or -III clinical trials are systemic agents aimed at the minority of psoriasis patients with more severe disease. There’s not nearly as much in the pipeline for the many patients with mild psoriasis who are most appropriately treated with topical therapy, Dr. Hervé Bachelez observed at the annual congress of the European Academy of Dermatology and Venereology.
Indeed, he identified only two promising topical agents advancing through the developmental pipeline: Pfizer’s tofacitinib, a small-molecule Janus kinase inhibitor under study in both topical and oral formulations, and Anacor’s AN2728, a boron-containing molecule that inhibits phosphodiesterase-4 (PDE-4).
Industry’s lack of enthusiasm for developing new topical agents is difficult to understand, given that 84% of all U.S. prescriptions written for psoriasis in 2009 were for topical agents, according to IMS. Moreover, patients clearly believe there is a major unmet need for new and better topicals: a recent survey of 2,151 European psoriasis patients and their dermatologists found that only 45% of patients on topical therapy were satisfied with their treatment. And a mere 35% of dermatologists rated current topical treatments as satisfactory (J. Dermatolog. Treat. 2013;24:193-8).
The great bulk of the action in psoriasis drug development now is focused on injectable biologics and oral small molecules. Among them are three interleukin-17 inhibitors: Novartis’ secukinumab, Amgen’s brodalumab, and Eli Lilly’s ixekizumab. Celgene is developing apremilast as an oral PDE-4 inhibitor. Merck and Janssen Biotech are developing MK-3222 and CNTO 1959, respectively, as interleukin-23 inhibitors.
Dr. Bachelez, professor of dermatology and head of the inflammatory skin diseases unit at Saint Louis University Hospital, Paris, reported serving on the advisory boards of 10 pharmaceutical companies.
ISTANBUL, TURKEY – The current intensive period of new drug development in psoriasis is cause for enthusiastic celebration by patients and physicians alike. That is, except for one glaring research gap.
Almost all of the promising drugs now in phase-II or -III clinical trials are systemic agents aimed at the minority of psoriasis patients with more severe disease. There’s not nearly as much in the pipeline for the many patients with mild psoriasis who are most appropriately treated with topical therapy, Dr. Hervé Bachelez observed at the annual congress of the European Academy of Dermatology and Venereology.
Indeed, he identified only two promising topical agents advancing through the developmental pipeline: Pfizer’s tofacitinib, a small-molecule Janus kinase inhibitor under study in both topical and oral formulations, and Anacor’s AN2728, a boron-containing molecule that inhibits phosphodiesterase-4 (PDE-4).
Industry’s lack of enthusiasm for developing new topical agents is difficult to understand, given that 84% of all U.S. prescriptions written for psoriasis in 2009 were for topical agents, according to IMS. Moreover, patients clearly believe there is a major unmet need for new and better topicals: a recent survey of 2,151 European psoriasis patients and their dermatologists found that only 45% of patients on topical therapy were satisfied with their treatment. And a mere 35% of dermatologists rated current topical treatments as satisfactory (J. Dermatolog. Treat. 2013;24:193-8).
The great bulk of the action in psoriasis drug development now is focused on injectable biologics and oral small molecules. Among them are three interleukin-17 inhibitors: Novartis’ secukinumab, Amgen’s brodalumab, and Eli Lilly’s ixekizumab. Celgene is developing apremilast as an oral PDE-4 inhibitor. Merck and Janssen Biotech are developing MK-3222 and CNTO 1959, respectively, as interleukin-23 inhibitors.
Dr. Bachelez, professor of dermatology and head of the inflammatory skin diseases unit at Saint Louis University Hospital, Paris, reported serving on the advisory boards of 10 pharmaceutical companies.
EXPERT ANALYSIS FROM THE EADV CONGRESS
Abatacept may reduce maintenance immunosuppression need in lupus nephritis
ATLANTA – Adding abatacept to low-dose intravenous cyclophosphamide did not improve the rate of complete renal response at 1 year, compared with IV cyclophosphamide alone, in patients with class III or IV lupus nephritis in the randomized, controlled ACCESS Trial.
The phase II findings suggested, however, that abatacept reduced the need for maintenance immunosuppression, and they also demonstrated for the first time that low-dose IV cyclophosphamide may have applicability in racially and ethnically diverse patients with lupus nephritis, Dr. Brad H. Rovin reported at Kidney Week 2013.
The overall complete renal response rates were similar at 33% and 31%, respectively, in 134 patients with class III or IV lupus nephritis who were enrolled in the trial, treated with a steroid taper and 500 mg of IV cyclophosphamide every 2 weeks for 6 weeks followed by 2 mg/kg of azathioprine daily. Patients then were randomized to receive abatacept or placebo at week 0, 2, and 4 and then monthly.
Complete plus partial responses occurred in 59% of patients in each arm, Dr. Rovin said at the conference, which was sponsored by the American Society of Nephrology.
Patients in the study had a urine protein-to-creatinine ratio (UPCR) greater than 1. Complete renal response was defined as a UPCR of less than 0.5, serum creatinine at normal or within 25% of baseline, and prednisone dose tapered to 10 mg daily or less. African Americans comprised 39% of the cohort, and Hispanic/Mestizo patients comprised 40%.
Among the African American patients, 33% in the treatment group, compared with 16% in the placebo group, achieved complete renal response. This difference was not statistically significant.
This finding is important, because while low-dose cyclophosphamide – the "Euro-Lupus regimen" – has been shown to be as effective as standard and more toxic higher dosing, studies had primarily included a European Caucasian population. It was unclear whether the low-dose regimen would be as effective in a multiracial and multiethnic population, said Dr. Rovin of Ohio State University Medical Center.
No differences were seen between the treatment and control groups with respect to anti-dsDNA or complement levels and/or the frequency of serious or infectious adverse events or study withdrawals.
Of note, azathioprine was stopped in treatment group patients who achieved complete renal response – but not control group patients who achieved complete renal response. These patients were followed to 52 weeks. At that time, 50% and 62% of the treatment group and control group patients, respectively, who were complete responders at 24 weeks still met the criteria for complete response, Dr. Rovin said.
While abatacept did not increase complete response rates, the treatment was associated with maintenance of complete renal response to 1 year despite discontinuation of maintenance immunosuppression in the treated patients and continued azathioprine treatment in the control group. This suggests that abatacept may reduce the need for maintenance immunosuppression, he said.
While it was disappointing that the addition of abatacept to background induction therapy with low-dose IV cyclophosphamide and corticosteroids did not improve the complete renal response rate in this study – despite murine models suggesting that the use of abatacept and IV cyclophosphamide act synergistically to arrest lupus nephritis progression – the findings offer two very important lessons for clinical practice, he said.
"We can take the low-dose cyclophosphamide regimen and potentially use that with good results in our [multiethnic, multiracial] patient population that we see here in this country, and I think that’s a huge step forward. It gives us another option to use besides mycophenolate mofetil and high-dose cyclophosphamide," he said, adding that the low-dose IV cyclophosphamide regimen is well tolerated and delivers a total dose of only 3 g of the drug – a dose that generally does not predispose to premature ovarian failure.
This is particularly important, because women of childbearing years comprise a majority of the patient population with lupus nephritis, he explained.
"We know from the Euro-Lupus trial that the low-dose regimen, over 10 years, provided the same maintenance of renal function as the high-dose regimen, so we have very good long-term follow-up. We don’t quite have that yet with mycophenolate mofetil, so I think that for those of us who are very frequent users of cyclophosphamide, this is a very good alternative to the high-dose, highly toxic regimen that has for so long been the standard of care," he said.
This study was funded by the National Institute of Allergy and Infectious Diseases via the Immune Tolerance Network. Bristol Myers Squibb provided the study drug. Dr. Rovin reported ties to Genentech, Questcor, TEVA, Biogen-IDEC, HGS, Johnson & Johnson, Roche, BMS, and TG Therapeutics.
ATLANTA – Adding abatacept to low-dose intravenous cyclophosphamide did not improve the rate of complete renal response at 1 year, compared with IV cyclophosphamide alone, in patients with class III or IV lupus nephritis in the randomized, controlled ACCESS Trial.
The phase II findings suggested, however, that abatacept reduced the need for maintenance immunosuppression, and they also demonstrated for the first time that low-dose IV cyclophosphamide may have applicability in racially and ethnically diverse patients with lupus nephritis, Dr. Brad H. Rovin reported at Kidney Week 2013.
The overall complete renal response rates were similar at 33% and 31%, respectively, in 134 patients with class III or IV lupus nephritis who were enrolled in the trial, treated with a steroid taper and 500 mg of IV cyclophosphamide every 2 weeks for 6 weeks followed by 2 mg/kg of azathioprine daily. Patients then were randomized to receive abatacept or placebo at week 0, 2, and 4 and then monthly.
Complete plus partial responses occurred in 59% of patients in each arm, Dr. Rovin said at the conference, which was sponsored by the American Society of Nephrology.
Patients in the study had a urine protein-to-creatinine ratio (UPCR) greater than 1. Complete renal response was defined as a UPCR of less than 0.5, serum creatinine at normal or within 25% of baseline, and prednisone dose tapered to 10 mg daily or less. African Americans comprised 39% of the cohort, and Hispanic/Mestizo patients comprised 40%.
Among the African American patients, 33% in the treatment group, compared with 16% in the placebo group, achieved complete renal response. This difference was not statistically significant.
This finding is important, because while low-dose cyclophosphamide – the "Euro-Lupus regimen" – has been shown to be as effective as standard and more toxic higher dosing, studies had primarily included a European Caucasian population. It was unclear whether the low-dose regimen would be as effective in a multiracial and multiethnic population, said Dr. Rovin of Ohio State University Medical Center.
No differences were seen between the treatment and control groups with respect to anti-dsDNA or complement levels and/or the frequency of serious or infectious adverse events or study withdrawals.
Of note, azathioprine was stopped in treatment group patients who achieved complete renal response – but not control group patients who achieved complete renal response. These patients were followed to 52 weeks. At that time, 50% and 62% of the treatment group and control group patients, respectively, who were complete responders at 24 weeks still met the criteria for complete response, Dr. Rovin said.
While abatacept did not increase complete response rates, the treatment was associated with maintenance of complete renal response to 1 year despite discontinuation of maintenance immunosuppression in the treated patients and continued azathioprine treatment in the control group. This suggests that abatacept may reduce the need for maintenance immunosuppression, he said.
While it was disappointing that the addition of abatacept to background induction therapy with low-dose IV cyclophosphamide and corticosteroids did not improve the complete renal response rate in this study – despite murine models suggesting that the use of abatacept and IV cyclophosphamide act synergistically to arrest lupus nephritis progression – the findings offer two very important lessons for clinical practice, he said.
"We can take the low-dose cyclophosphamide regimen and potentially use that with good results in our [multiethnic, multiracial] patient population that we see here in this country, and I think that’s a huge step forward. It gives us another option to use besides mycophenolate mofetil and high-dose cyclophosphamide," he said, adding that the low-dose IV cyclophosphamide regimen is well tolerated and delivers a total dose of only 3 g of the drug – a dose that generally does not predispose to premature ovarian failure.
This is particularly important, because women of childbearing years comprise a majority of the patient population with lupus nephritis, he explained.
"We know from the Euro-Lupus trial that the low-dose regimen, over 10 years, provided the same maintenance of renal function as the high-dose regimen, so we have very good long-term follow-up. We don’t quite have that yet with mycophenolate mofetil, so I think that for those of us who are very frequent users of cyclophosphamide, this is a very good alternative to the high-dose, highly toxic regimen that has for so long been the standard of care," he said.
This study was funded by the National Institute of Allergy and Infectious Diseases via the Immune Tolerance Network. Bristol Myers Squibb provided the study drug. Dr. Rovin reported ties to Genentech, Questcor, TEVA, Biogen-IDEC, HGS, Johnson & Johnson, Roche, BMS, and TG Therapeutics.
ATLANTA – Adding abatacept to low-dose intravenous cyclophosphamide did not improve the rate of complete renal response at 1 year, compared with IV cyclophosphamide alone, in patients with class III or IV lupus nephritis in the randomized, controlled ACCESS Trial.
The phase II findings suggested, however, that abatacept reduced the need for maintenance immunosuppression, and they also demonstrated for the first time that low-dose IV cyclophosphamide may have applicability in racially and ethnically diverse patients with lupus nephritis, Dr. Brad H. Rovin reported at Kidney Week 2013.
The overall complete renal response rates were similar at 33% and 31%, respectively, in 134 patients with class III or IV lupus nephritis who were enrolled in the trial, treated with a steroid taper and 500 mg of IV cyclophosphamide every 2 weeks for 6 weeks followed by 2 mg/kg of azathioprine daily. Patients then were randomized to receive abatacept or placebo at week 0, 2, and 4 and then monthly.
Complete plus partial responses occurred in 59% of patients in each arm, Dr. Rovin said at the conference, which was sponsored by the American Society of Nephrology.
Patients in the study had a urine protein-to-creatinine ratio (UPCR) greater than 1. Complete renal response was defined as a UPCR of less than 0.5, serum creatinine at normal or within 25% of baseline, and prednisone dose tapered to 10 mg daily or less. African Americans comprised 39% of the cohort, and Hispanic/Mestizo patients comprised 40%.
Among the African American patients, 33% in the treatment group, compared with 16% in the placebo group, achieved complete renal response. This difference was not statistically significant.
This finding is important, because while low-dose cyclophosphamide – the "Euro-Lupus regimen" – has been shown to be as effective as standard and more toxic higher dosing, studies had primarily included a European Caucasian population. It was unclear whether the low-dose regimen would be as effective in a multiracial and multiethnic population, said Dr. Rovin of Ohio State University Medical Center.
No differences were seen between the treatment and control groups with respect to anti-dsDNA or complement levels and/or the frequency of serious or infectious adverse events or study withdrawals.
Of note, azathioprine was stopped in treatment group patients who achieved complete renal response – but not control group patients who achieved complete renal response. These patients were followed to 52 weeks. At that time, 50% and 62% of the treatment group and control group patients, respectively, who were complete responders at 24 weeks still met the criteria for complete response, Dr. Rovin said.
While abatacept did not increase complete response rates, the treatment was associated with maintenance of complete renal response to 1 year despite discontinuation of maintenance immunosuppression in the treated patients and continued azathioprine treatment in the control group. This suggests that abatacept may reduce the need for maintenance immunosuppression, he said.
While it was disappointing that the addition of abatacept to background induction therapy with low-dose IV cyclophosphamide and corticosteroids did not improve the complete renal response rate in this study – despite murine models suggesting that the use of abatacept and IV cyclophosphamide act synergistically to arrest lupus nephritis progression – the findings offer two very important lessons for clinical practice, he said.
"We can take the low-dose cyclophosphamide regimen and potentially use that with good results in our [multiethnic, multiracial] patient population that we see here in this country, and I think that’s a huge step forward. It gives us another option to use besides mycophenolate mofetil and high-dose cyclophosphamide," he said, adding that the low-dose IV cyclophosphamide regimen is well tolerated and delivers a total dose of only 3 g of the drug – a dose that generally does not predispose to premature ovarian failure.
This is particularly important, because women of childbearing years comprise a majority of the patient population with lupus nephritis, he explained.
"We know from the Euro-Lupus trial that the low-dose regimen, over 10 years, provided the same maintenance of renal function as the high-dose regimen, so we have very good long-term follow-up. We don’t quite have that yet with mycophenolate mofetil, so I think that for those of us who are very frequent users of cyclophosphamide, this is a very good alternative to the high-dose, highly toxic regimen that has for so long been the standard of care," he said.
This study was funded by the National Institute of Allergy and Infectious Diseases via the Immune Tolerance Network. Bristol Myers Squibb provided the study drug. Dr. Rovin reported ties to Genentech, Questcor, TEVA, Biogen-IDEC, HGS, Johnson & Johnson, Roche, BMS, and TG Therapeutics.
AT KIDNEY WEEK 2013
Major finding: Complete renal response rates were similar at 33% and 31% in treatment vs. placebo group patients, respectively.
Data source: Phase II of the randomized, placebo-controlled ACCESS trial involving 134 patients.
Disclosures: This study was funded by the National Institute of Allergy and Infectious Diseases via the Immune Tolerance Network. Bristol Myers Squibb provided the study drug. Dr. Rovin reported ties to Genentech, Questcor, TEVA, Biogen-IDEC, HGS, Johnson & Johnson, Roche, BMS, and TG Therapeutics.
Novel predictor of poor outcomes in lupus pregnancies
SAN DIEGO – Alteration in the balance of placentally secreted angiogenic factors early in pregnancy provides a potent new predictor of subsequent preeclampsia and other poor outcomes in pregnant women with systemic lupus erythematosus and/or antiphospholipid antibody syndrome.
Patients with systemic lupus erythematosus (SLE) and/or antiphospholipid antibody syndrome (APS) who had an elevated ratio of a splice variant of vascular endothelial growth factor R1 called sFLT1 to placental growth factor (PlGF) when measured at 16-19 weeks’ gestation were at 13.8-fold increased relative risk of preeclampsia before 34 weeks, compared with patients with an sFLT1/PlGF ratio below that cut-point in the large, multicenter, observational PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and SLE) study, Dr. Jane E. Salmon reported at the annual meeting of the American College of Rheumatology.
"Nearly half of the patients with an SFLT1/PlGF ratio greater than 3.45 when measured at 16-19 weeks’ gestation will develop early preeclampsia. On the other hand, a low ratio, as well as low levels of sFLT1 or high levels of PlGF, can reassure physicians and patients that preterm preeclampsia is unlikely: a 3% chance," said Dr. Salmon, professor of medicine and of ob.gyn. at Cornell University and a rheumatologist at the Hospital for Special Surgery, both in New York.
Pregnancy in patients with lupus is associated with obstetric complications placing both mother and fetus at great risk. Yet, until now it hasn’t been possible to predict which patients will have poor outcomes.
The key to identifying those at high risk lies in a recognition that preeclampsia and other poor outcomes are dramatic manifestations of placental insufficiency, which actually begins, initially silently, early in pregnancy. The maternal hypertension, proteinuria, thrombocytopenia, and other end-organ manifestations of preeclampsia are caused by maternal endothelial dysfunction mediated by placental secretion of antiangiogenic factors. Angiogenic growth factors, such as PIGF and vascular endothelial growth factor (VEGF), are essential to a healthy endothelium. But placentally secreted sFLT1 binds to these two angiogenic growth factors, rendering them unavailable to the endothelium, she explained.
Overexpression of sFLT1 in multiple animal models results in hypertension and proteinuria, the hallmarks of preeclampsia. Moreover, cancer patients treated with VEGF inhibitors often develop these two conditions. Based in part on these observations, Dr. Salmon and her coinvestigators turned to the PROMISSE study to test their hypothesis that elevated levels of antiangiogenic factors early in pregnancy predict poor outcomes in patients with SLE and/or APS. The prospective study involved 503 pregnant women with SLE and/or APS and 204 healthy controls, all with monthly blood draws starting before 12 weeks’ gestation.
The composite outcome of preeclampsia, small for gestational age, indicated preterm delivery, and other adverse events occurred in 37% of SLE patients who also had APS. The rate was 16% in patients with SLE alone, 26% in those with APS alone, and 3% in controls.
Subjects with SLE and/or APS who developed preeclampsia and other pregnancy complications displayed significantly higher levels of sFLT1 beginning at 12 weeks and sustained through 31 weeks’ gestation, compared with those with normal pregnancies. Moreover, PlGF levels were significantly lower during weeks 16-31 in the patients with pregnancy complications. The investigators determined that the best predictor of pregnancy complications was the ratio of antiangiogenic sFLT1 to angiogenic PlGF. And the optimal cut-point was 3.45.
Audience members said that while a predictive test for preeclampsia is most welcome, the fact remains that physicians don’t have a lot to offer in terms of prevention or treatment of this feared pregnancy complication. Dr. Salmon responded that the SFLT1/PlGF ratio can be used to risk-stratify pregnant lupus patients for future interventional trials with new drugs looking at new pathways. Already, for example, other investigators have reported some success using a strategy targeting sFLT1 itself. In a small study, they found that women with severe preeclampsia who had their blood run through a heparin column that binds and removes sFLT1 were able to maintain their pregnancies for up to 2 weeks.
"It’s a tiny, open-label trial involving a device, but I think that will move forward," she predicted.
The PROMISSE study was funded by the National Institutes of Health, the Alliance for Lupus Research, and the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery. Dr. Salmon reported having received research grants from and/or serving as a consultant to Alexion, Novartis, and Roche.
SLE, APS, vascular endothelial growth factor R1, sFLT1, placental growth factor, PlGF, preeclampsia, PROMISSE study, Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and SLE study, Dr. Jane E. Salmon, annual meeting of the American College of Rheumatology, early preeclampsia,
SAN DIEGO – Alteration in the balance of placentally secreted angiogenic factors early in pregnancy provides a potent new predictor of subsequent preeclampsia and other poor outcomes in pregnant women with systemic lupus erythematosus and/or antiphospholipid antibody syndrome.
Patients with systemic lupus erythematosus (SLE) and/or antiphospholipid antibody syndrome (APS) who had an elevated ratio of a splice variant of vascular endothelial growth factor R1 called sFLT1 to placental growth factor (PlGF) when measured at 16-19 weeks’ gestation were at 13.8-fold increased relative risk of preeclampsia before 34 weeks, compared with patients with an sFLT1/PlGF ratio below that cut-point in the large, multicenter, observational PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and SLE) study, Dr. Jane E. Salmon reported at the annual meeting of the American College of Rheumatology.
"Nearly half of the patients with an SFLT1/PlGF ratio greater than 3.45 when measured at 16-19 weeks’ gestation will develop early preeclampsia. On the other hand, a low ratio, as well as low levels of sFLT1 or high levels of PlGF, can reassure physicians and patients that preterm preeclampsia is unlikely: a 3% chance," said Dr. Salmon, professor of medicine and of ob.gyn. at Cornell University and a rheumatologist at the Hospital for Special Surgery, both in New York.
Pregnancy in patients with lupus is associated with obstetric complications placing both mother and fetus at great risk. Yet, until now it hasn’t been possible to predict which patients will have poor outcomes.
The key to identifying those at high risk lies in a recognition that preeclampsia and other poor outcomes are dramatic manifestations of placental insufficiency, which actually begins, initially silently, early in pregnancy. The maternal hypertension, proteinuria, thrombocytopenia, and other end-organ manifestations of preeclampsia are caused by maternal endothelial dysfunction mediated by placental secretion of antiangiogenic factors. Angiogenic growth factors, such as PIGF and vascular endothelial growth factor (VEGF), are essential to a healthy endothelium. But placentally secreted sFLT1 binds to these two angiogenic growth factors, rendering them unavailable to the endothelium, she explained.
Overexpression of sFLT1 in multiple animal models results in hypertension and proteinuria, the hallmarks of preeclampsia. Moreover, cancer patients treated with VEGF inhibitors often develop these two conditions. Based in part on these observations, Dr. Salmon and her coinvestigators turned to the PROMISSE study to test their hypothesis that elevated levels of antiangiogenic factors early in pregnancy predict poor outcomes in patients with SLE and/or APS. The prospective study involved 503 pregnant women with SLE and/or APS and 204 healthy controls, all with monthly blood draws starting before 12 weeks’ gestation.
The composite outcome of preeclampsia, small for gestational age, indicated preterm delivery, and other adverse events occurred in 37% of SLE patients who also had APS. The rate was 16% in patients with SLE alone, 26% in those with APS alone, and 3% in controls.
Subjects with SLE and/or APS who developed preeclampsia and other pregnancy complications displayed significantly higher levels of sFLT1 beginning at 12 weeks and sustained through 31 weeks’ gestation, compared with those with normal pregnancies. Moreover, PlGF levels were significantly lower during weeks 16-31 in the patients with pregnancy complications. The investigators determined that the best predictor of pregnancy complications was the ratio of antiangiogenic sFLT1 to angiogenic PlGF. And the optimal cut-point was 3.45.
Audience members said that while a predictive test for preeclampsia is most welcome, the fact remains that physicians don’t have a lot to offer in terms of prevention or treatment of this feared pregnancy complication. Dr. Salmon responded that the SFLT1/PlGF ratio can be used to risk-stratify pregnant lupus patients for future interventional trials with new drugs looking at new pathways. Already, for example, other investigators have reported some success using a strategy targeting sFLT1 itself. In a small study, they found that women with severe preeclampsia who had their blood run through a heparin column that binds and removes sFLT1 were able to maintain their pregnancies for up to 2 weeks.
"It’s a tiny, open-label trial involving a device, but I think that will move forward," she predicted.
The PROMISSE study was funded by the National Institutes of Health, the Alliance for Lupus Research, and the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery. Dr. Salmon reported having received research grants from and/or serving as a consultant to Alexion, Novartis, and Roche.
SAN DIEGO – Alteration in the balance of placentally secreted angiogenic factors early in pregnancy provides a potent new predictor of subsequent preeclampsia and other poor outcomes in pregnant women with systemic lupus erythematosus and/or antiphospholipid antibody syndrome.
Patients with systemic lupus erythematosus (SLE) and/or antiphospholipid antibody syndrome (APS) who had an elevated ratio of a splice variant of vascular endothelial growth factor R1 called sFLT1 to placental growth factor (PlGF) when measured at 16-19 weeks’ gestation were at 13.8-fold increased relative risk of preeclampsia before 34 weeks, compared with patients with an sFLT1/PlGF ratio below that cut-point in the large, multicenter, observational PROMISSE (Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and SLE) study, Dr. Jane E. Salmon reported at the annual meeting of the American College of Rheumatology.
"Nearly half of the patients with an SFLT1/PlGF ratio greater than 3.45 when measured at 16-19 weeks’ gestation will develop early preeclampsia. On the other hand, a low ratio, as well as low levels of sFLT1 or high levels of PlGF, can reassure physicians and patients that preterm preeclampsia is unlikely: a 3% chance," said Dr. Salmon, professor of medicine and of ob.gyn. at Cornell University and a rheumatologist at the Hospital for Special Surgery, both in New York.
Pregnancy in patients with lupus is associated with obstetric complications placing both mother and fetus at great risk. Yet, until now it hasn’t been possible to predict which patients will have poor outcomes.
The key to identifying those at high risk lies in a recognition that preeclampsia and other poor outcomes are dramatic manifestations of placental insufficiency, which actually begins, initially silently, early in pregnancy. The maternal hypertension, proteinuria, thrombocytopenia, and other end-organ manifestations of preeclampsia are caused by maternal endothelial dysfunction mediated by placental secretion of antiangiogenic factors. Angiogenic growth factors, such as PIGF and vascular endothelial growth factor (VEGF), are essential to a healthy endothelium. But placentally secreted sFLT1 binds to these two angiogenic growth factors, rendering them unavailable to the endothelium, she explained.
Overexpression of sFLT1 in multiple animal models results in hypertension and proteinuria, the hallmarks of preeclampsia. Moreover, cancer patients treated with VEGF inhibitors often develop these two conditions. Based in part on these observations, Dr. Salmon and her coinvestigators turned to the PROMISSE study to test their hypothesis that elevated levels of antiangiogenic factors early in pregnancy predict poor outcomes in patients with SLE and/or APS. The prospective study involved 503 pregnant women with SLE and/or APS and 204 healthy controls, all with monthly blood draws starting before 12 weeks’ gestation.
The composite outcome of preeclampsia, small for gestational age, indicated preterm delivery, and other adverse events occurred in 37% of SLE patients who also had APS. The rate was 16% in patients with SLE alone, 26% in those with APS alone, and 3% in controls.
Subjects with SLE and/or APS who developed preeclampsia and other pregnancy complications displayed significantly higher levels of sFLT1 beginning at 12 weeks and sustained through 31 weeks’ gestation, compared with those with normal pregnancies. Moreover, PlGF levels were significantly lower during weeks 16-31 in the patients with pregnancy complications. The investigators determined that the best predictor of pregnancy complications was the ratio of antiangiogenic sFLT1 to angiogenic PlGF. And the optimal cut-point was 3.45.
Audience members said that while a predictive test for preeclampsia is most welcome, the fact remains that physicians don’t have a lot to offer in terms of prevention or treatment of this feared pregnancy complication. Dr. Salmon responded that the SFLT1/PlGF ratio can be used to risk-stratify pregnant lupus patients for future interventional trials with new drugs looking at new pathways. Already, for example, other investigators have reported some success using a strategy targeting sFLT1 itself. In a small study, they found that women with severe preeclampsia who had their blood run through a heparin column that binds and removes sFLT1 were able to maintain their pregnancies for up to 2 weeks.
"It’s a tiny, open-label trial involving a device, but I think that will move forward," she predicted.
The PROMISSE study was funded by the National Institutes of Health, the Alliance for Lupus Research, and the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery. Dr. Salmon reported having received research grants from and/or serving as a consultant to Alexion, Novartis, and Roche.
SLE, APS, vascular endothelial growth factor R1, sFLT1, placental growth factor, PlGF, preeclampsia, PROMISSE study, Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and SLE study, Dr. Jane E. Salmon, annual meeting of the American College of Rheumatology, early preeclampsia,
SLE, APS, vascular endothelial growth factor R1, sFLT1, placental growth factor, PlGF, preeclampsia, PROMISSE study, Predictors of Pregnancy Outcome: Biomarkers in Antiphospholipid Antibody Syndrome and SLE study, Dr. Jane E. Salmon, annual meeting of the American College of Rheumatology, early preeclampsia,
AT THE ACR ANNUAL MEETING
Major finding: Pregnant patients with SLE and/or APS had nearly a 14-fold increased risk of developing preeclampsia before 34 weeks’ gestation when they displayed an abnormal ratio of two key placentally derived angiogenic factors at 16-19 weeks’ gestation.
Data source: This analysis included 503 pregnant women with SLE and/or APS and 204 pregnant controls participating in the prospective, observational, multicenter PROMISSE study.
Disclosures: The study was funded by the National Institutes of Health, the Alliance for Lupus Research, and the Mary Kirkland Center for Lupus Research at the Hospital for Special Surgery. The presenter reported no relevant financial interests.
Impact of psoriasis on sexual activity
One third of a group of women with psoriasis reported that the pain associated with their condition interfered with their sexual activity, according to findings from a survey presented by Dr. Jennifer C. Cather.
Based on responses from a survey of 60 women with moderate to severe psoriasis, the specific complaints that were the most common ways in which psoriasis interfered with sexual activity were itchiness (19%), the need to adjust sexual position (10%), and bleeding (9%), Dr. Cather reported at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. The survey was part of an effort to determine the impact of psoriasis on women’s sexual activity, desires, and relationships.
The data were previously presented in a poster at the annual congress of the European Academy of Dermatology and Venereology (Istanbul.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Cather disclosed that she is a consultant, speaker, or researcher for AbbVie, Novartis, Leo, Janssen, Amgen, Celgene, Merck, and Pfizer.
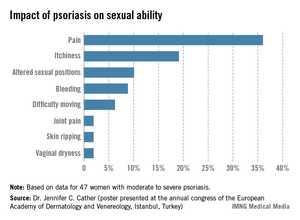
One third of a group of women with psoriasis reported that the pain associated with their condition interfered with their sexual activity, according to findings from a survey presented by Dr. Jennifer C. Cather.
Based on responses from a survey of 60 women with moderate to severe psoriasis, the specific complaints that were the most common ways in which psoriasis interfered with sexual activity were itchiness (19%), the need to adjust sexual position (10%), and bleeding (9%), Dr. Cather reported at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. The survey was part of an effort to determine the impact of psoriasis on women’s sexual activity, desires, and relationships.
The data were previously presented in a poster at the annual congress of the European Academy of Dermatology and Venereology (Istanbul.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Cather disclosed that she is a consultant, speaker, or researcher for AbbVie, Novartis, Leo, Janssen, Amgen, Celgene, Merck, and Pfizer.

One third of a group of women with psoriasis reported that the pain associated with their condition interfered with their sexual activity, according to findings from a survey presented by Dr. Jennifer C. Cather.
Based on responses from a survey of 60 women with moderate to severe psoriasis, the specific complaints that were the most common ways in which psoriasis interfered with sexual activity were itchiness (19%), the need to adjust sexual position (10%), and bleeding (9%), Dr. Cather reported at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. The survey was part of an effort to determine the impact of psoriasis on women’s sexual activity, desires, and relationships.
The data were previously presented in a poster at the annual congress of the European Academy of Dermatology and Venereology (Istanbul.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Cather disclosed that she is a consultant, speaker, or researcher for AbbVie, Novartis, Leo, Janssen, Amgen, Celgene, Merck, and Pfizer.

EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR
Treat-to-target approach for psoriatic arthritis found beneficial
SAN DIEGO – Compared with standard care, intensive management of psoriatic arthritis using a treat-to-target approach significantly improved joint and skin outcomes for patients newly diagnosed with the disease, results from a multicenter, randomized controlled trial showed.
"Treating to target works in this disease, and it’s going to result in better long-term outcomes," lead investigator Dr. Philip Helliwell said during a press briefing at the annual meeting of the American College of Rheumatology.
Dr. Helliwell and his associates at eight centers in the United Kingdom randomized 206 patients with early psoriatic arthritis to standard care or intensive management, and followed them for 48 weeks. Patients in the standard care group were treated by a rheumatologist with no set protocol and no limitations, while those in the intensive management group followed a strict treatment protocol with escalation of therapy if minimal disease activity criteria were not met.
Patients in the intensive management group were started on methotrexate with rapid escalation to a dose of *25 mg/week after 6 weeks if they tolerated the drug. If they did not meet minimal disease criteria after 12 weeks, they received a more powerful combination of disease-modifying antirheumatic drugs (DMARDs).
After another 12 weeks, patients in the intensive management group were given anti–tumor necrosis factor therapy if they had three or more tender joints. If they had fewer than three tender or swollen joints but did not meet the minimal disease activity criteria, they were given methotrexate and an alternative DMARD. Patients in the standard care group were treated with DMARDs, but with no set time limits for drug therapy escalation or measurements to reach.
The primary outcome measures were the proportion of patients in both groups who achieved ACR20, ACR50, and ACR70 criteria for disease activity, which represent disease improvement of 20%, 50%, and 70%, respectively. Dr. Helliwell reported that compared with the standard care group, a higher proportion of patients in the intensive management group achieved ACR20 (62% vs. 45%, respectively), ACR50 (51% vs. 25%), and ACR70 (38% vs. 17%). A higher proportion of patients in the intensive management group also achieved a Psoriasis Area and Severity Index 75 compared with their counterparts in the standard care group (59% vs. 33%).
Research in psoriatic arthritis has "lagged behind that of rheumatoid arthritis for years in terms of pathogenesis and treatment paradigms," noted Dr. Helliwell, a senior lecturer in rheumatology at the Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England). "I think the study we’ve reported brings psoriatic arthritis right up to date alongside RA."
He said that up to one-third of people with psoriasis will develop psoriatic arthritis, "so it’s not an insignificant arthritis. It’s often not recognized. One survey we did of people with psoriasis in the community in the U.K. found that up to half of the people with psoriatic arthritis didn’t know they had it. They’d seen their doctor for various reasons and had been told they’d had another form of arthritis, or they were fobbed off with other diagnoses."
The researchers have yet to perform a cost analysis comparing the two treatment groups.
The study was funded by Arthritis Research UK and Pfizer. Dr. Helliwell disclosed that he has received consulting fees from Pfizer.
*Correction 11/11/13: A previous version of this story misstated the methotrexate dosage used in the study. This version has been updated to reflect the correct dosage.
SAN DIEGO – Compared with standard care, intensive management of psoriatic arthritis using a treat-to-target approach significantly improved joint and skin outcomes for patients newly diagnosed with the disease, results from a multicenter, randomized controlled trial showed.
"Treating to target works in this disease, and it’s going to result in better long-term outcomes," lead investigator Dr. Philip Helliwell said during a press briefing at the annual meeting of the American College of Rheumatology.
Dr. Helliwell and his associates at eight centers in the United Kingdom randomized 206 patients with early psoriatic arthritis to standard care or intensive management, and followed them for 48 weeks. Patients in the standard care group were treated by a rheumatologist with no set protocol and no limitations, while those in the intensive management group followed a strict treatment protocol with escalation of therapy if minimal disease activity criteria were not met.
Patients in the intensive management group were started on methotrexate with rapid escalation to a dose of *25 mg/week after 6 weeks if they tolerated the drug. If they did not meet minimal disease criteria after 12 weeks, they received a more powerful combination of disease-modifying antirheumatic drugs (DMARDs).
After another 12 weeks, patients in the intensive management group were given anti–tumor necrosis factor therapy if they had three or more tender joints. If they had fewer than three tender or swollen joints but did not meet the minimal disease activity criteria, they were given methotrexate and an alternative DMARD. Patients in the standard care group were treated with DMARDs, but with no set time limits for drug therapy escalation or measurements to reach.
The primary outcome measures were the proportion of patients in both groups who achieved ACR20, ACR50, and ACR70 criteria for disease activity, which represent disease improvement of 20%, 50%, and 70%, respectively. Dr. Helliwell reported that compared with the standard care group, a higher proportion of patients in the intensive management group achieved ACR20 (62% vs. 45%, respectively), ACR50 (51% vs. 25%), and ACR70 (38% vs. 17%). A higher proportion of patients in the intensive management group also achieved a Psoriasis Area and Severity Index 75 compared with their counterparts in the standard care group (59% vs. 33%).
Research in psoriatic arthritis has "lagged behind that of rheumatoid arthritis for years in terms of pathogenesis and treatment paradigms," noted Dr. Helliwell, a senior lecturer in rheumatology at the Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England). "I think the study we’ve reported brings psoriatic arthritis right up to date alongside RA."
He said that up to one-third of people with psoriasis will develop psoriatic arthritis, "so it’s not an insignificant arthritis. It’s often not recognized. One survey we did of people with psoriasis in the community in the U.K. found that up to half of the people with psoriatic arthritis didn’t know they had it. They’d seen their doctor for various reasons and had been told they’d had another form of arthritis, or they were fobbed off with other diagnoses."
The researchers have yet to perform a cost analysis comparing the two treatment groups.
The study was funded by Arthritis Research UK and Pfizer. Dr. Helliwell disclosed that he has received consulting fees from Pfizer.
*Correction 11/11/13: A previous version of this story misstated the methotrexate dosage used in the study. This version has been updated to reflect the correct dosage.
SAN DIEGO – Compared with standard care, intensive management of psoriatic arthritis using a treat-to-target approach significantly improved joint and skin outcomes for patients newly diagnosed with the disease, results from a multicenter, randomized controlled trial showed.
"Treating to target works in this disease, and it’s going to result in better long-term outcomes," lead investigator Dr. Philip Helliwell said during a press briefing at the annual meeting of the American College of Rheumatology.
Dr. Helliwell and his associates at eight centers in the United Kingdom randomized 206 patients with early psoriatic arthritis to standard care or intensive management, and followed them for 48 weeks. Patients in the standard care group were treated by a rheumatologist with no set protocol and no limitations, while those in the intensive management group followed a strict treatment protocol with escalation of therapy if minimal disease activity criteria were not met.
Patients in the intensive management group were started on methotrexate with rapid escalation to a dose of *25 mg/week after 6 weeks if they tolerated the drug. If they did not meet minimal disease criteria after 12 weeks, they received a more powerful combination of disease-modifying antirheumatic drugs (DMARDs).
After another 12 weeks, patients in the intensive management group were given anti–tumor necrosis factor therapy if they had three or more tender joints. If they had fewer than three tender or swollen joints but did not meet the minimal disease activity criteria, they were given methotrexate and an alternative DMARD. Patients in the standard care group were treated with DMARDs, but with no set time limits for drug therapy escalation or measurements to reach.
The primary outcome measures were the proportion of patients in both groups who achieved ACR20, ACR50, and ACR70 criteria for disease activity, which represent disease improvement of 20%, 50%, and 70%, respectively. Dr. Helliwell reported that compared with the standard care group, a higher proportion of patients in the intensive management group achieved ACR20 (62% vs. 45%, respectively), ACR50 (51% vs. 25%), and ACR70 (38% vs. 17%). A higher proportion of patients in the intensive management group also achieved a Psoriasis Area and Severity Index 75 compared with their counterparts in the standard care group (59% vs. 33%).
Research in psoriatic arthritis has "lagged behind that of rheumatoid arthritis for years in terms of pathogenesis and treatment paradigms," noted Dr. Helliwell, a senior lecturer in rheumatology at the Institute of Rheumatic and Musculoskeletal Medicine at the University of Leeds (England). "I think the study we’ve reported brings psoriatic arthritis right up to date alongside RA."
He said that up to one-third of people with psoriasis will develop psoriatic arthritis, "so it’s not an insignificant arthritis. It’s often not recognized. One survey we did of people with psoriasis in the community in the U.K. found that up to half of the people with psoriatic arthritis didn’t know they had it. They’d seen their doctor for various reasons and had been told they’d had another form of arthritis, or they were fobbed off with other diagnoses."
The researchers have yet to perform a cost analysis comparing the two treatment groups.
The study was funded by Arthritis Research UK and Pfizer. Dr. Helliwell disclosed that he has received consulting fees from Pfizer.
*Correction 11/11/13: A previous version of this story misstated the methotrexate dosage used in the study. This version has been updated to reflect the correct dosage.
AT THE ACR ANNUAL MEETING
Major finding: At 48 weeks, a higher proportion of psoriatic arthritis patients in the intensive management group achieved ACR70, compared with those in the standard care group (38% vs. 17%, respectively).
Data source: A multicenter trial of 206 patients with early psoriatic arthritis who were randomized to standard care or intensive management and followed for 48 weeks.
Disclosures: The study was funded by Arthritis Research UK and Pfizer. Dr. Helliwell disclosed that he has received consulting fees from Pfizer.
Women’s sexuality diminished by psoriasis
LAS VEGAS – Psoriasis has a negative impact on women’s sexual desire, sexual ability, and sexual relationships, according to Dr. Jennifer Cather of the Modern Dermatology-Aesthetics Center in Dallas.
Dr. Cather and her colleagues are developing a clinical tool to assess the disease’s sexual impact; as part of those efforts, they conducted 60 interviews with moderately to severely psoriatic women with a mean age of 41 years.
The survey results showed that sexuality was clearly another reason why it’s important to keep psoriasis in check. Pain and itchiness during sex, self-consciousness and embarrassment, and avoidance of dating and intimate relationships were among the most common problems the women reported.
"We really didn’t appreciate the impact psoriasis had on relationships" before the survey, Dr. Cather said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. "The sexual impact has been underappreciated," she said.
Those problems might help explain why women with psoriasis, especially those aged younger than 35 years, tend to have fewer babies, she added. Psoriatic women also have higher rates of induced and spontaneous abortions and are more likely to have preterm and underweight births.
Some of the women surveyed said that they worried about passing psoriasis to their children. "Maybe there’s voluntary childlessness," Dr. Cather said. But when women in her practice mention they don’t want kids for fear of passing on the disease, "I answer right back, ‘You don’t have to have what you have. I can help you with it,’ " she said.
Methotrexate, acitretin, and psoralen photochemotherapy (PUVA) are contraindicated during pregnancy, but ultraviolet B (UVB) treatments are safe, said Dr. Cather. She also uses tumor necrosis factor inhibitors, which are FDA pregnancy category B agents; she prefers etanercept for its short half-life (just over 4 days) and because ob.gyns. are usually familiar with it. The Organization of Teratology Information Specialists (OTIS) keeps the etanercept and adalimumab pregnancy registries, and "there’s [been] no signal to date" for those drugs, she said.
Psoriatic women should know that the odds are with them for having a normal pregnancy, and that pregnancy is likely to help clear their skin, Dr. Cather said.
But because half of the pregnancies in the United States are unplanned, it’s important to discuss pregnancy – and psoriasis treatment during pregnancy – as part of routine care. "It’s difficult if a psoriasis patient calls you in a panic because they’re not sure their drug is safe, and not sure their doctor will let them continue it,’ she said. Some ob.gyns. are comfortable with letting women stay on their psoriasis therapy, while others want them to quit everything, even topical steroids.
"In my clinic, when a psoriasis patient gets pregnant, usually we’ve planned for it and are excited about it, and we’ve had some dialogue with the ob.gyn.," she explained.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Cather is a consultant, speaker, or researcher for AbbVie, Novartis, Leo, Janssen, Amgen, Celgene, Merck, and Pfizer.
LAS VEGAS – Psoriasis has a negative impact on women’s sexual desire, sexual ability, and sexual relationships, according to Dr. Jennifer Cather of the Modern Dermatology-Aesthetics Center in Dallas.
Dr. Cather and her colleagues are developing a clinical tool to assess the disease’s sexual impact; as part of those efforts, they conducted 60 interviews with moderately to severely psoriatic women with a mean age of 41 years.
The survey results showed that sexuality was clearly another reason why it’s important to keep psoriasis in check. Pain and itchiness during sex, self-consciousness and embarrassment, and avoidance of dating and intimate relationships were among the most common problems the women reported.
"We really didn’t appreciate the impact psoriasis had on relationships" before the survey, Dr. Cather said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. "The sexual impact has been underappreciated," she said.
Those problems might help explain why women with psoriasis, especially those aged younger than 35 years, tend to have fewer babies, she added. Psoriatic women also have higher rates of induced and spontaneous abortions and are more likely to have preterm and underweight births.
Some of the women surveyed said that they worried about passing psoriasis to their children. "Maybe there’s voluntary childlessness," Dr. Cather said. But when women in her practice mention they don’t want kids for fear of passing on the disease, "I answer right back, ‘You don’t have to have what you have. I can help you with it,’ " she said.
Methotrexate, acitretin, and psoralen photochemotherapy (PUVA) are contraindicated during pregnancy, but ultraviolet B (UVB) treatments are safe, said Dr. Cather. She also uses tumor necrosis factor inhibitors, which are FDA pregnancy category B agents; she prefers etanercept for its short half-life (just over 4 days) and because ob.gyns. are usually familiar with it. The Organization of Teratology Information Specialists (OTIS) keeps the etanercept and adalimumab pregnancy registries, and "there’s [been] no signal to date" for those drugs, she said.
Psoriatic women should know that the odds are with them for having a normal pregnancy, and that pregnancy is likely to help clear their skin, Dr. Cather said.
But because half of the pregnancies in the United States are unplanned, it’s important to discuss pregnancy – and psoriasis treatment during pregnancy – as part of routine care. "It’s difficult if a psoriasis patient calls you in a panic because they’re not sure their drug is safe, and not sure their doctor will let them continue it,’ she said. Some ob.gyns. are comfortable with letting women stay on their psoriasis therapy, while others want them to quit everything, even topical steroids.
"In my clinic, when a psoriasis patient gets pregnant, usually we’ve planned for it and are excited about it, and we’ve had some dialogue with the ob.gyn.," she explained.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Cather is a consultant, speaker, or researcher for AbbVie, Novartis, Leo, Janssen, Amgen, Celgene, Merck, and Pfizer.
LAS VEGAS – Psoriasis has a negative impact on women’s sexual desire, sexual ability, and sexual relationships, according to Dr. Jennifer Cather of the Modern Dermatology-Aesthetics Center in Dallas.
Dr. Cather and her colleagues are developing a clinical tool to assess the disease’s sexual impact; as part of those efforts, they conducted 60 interviews with moderately to severely psoriatic women with a mean age of 41 years.
The survey results showed that sexuality was clearly another reason why it’s important to keep psoriasis in check. Pain and itchiness during sex, self-consciousness and embarrassment, and avoidance of dating and intimate relationships were among the most common problems the women reported.
"We really didn’t appreciate the impact psoriasis had on relationships" before the survey, Dr. Cather said at the Skin Disease Education Foundation’s annual Las Vegas dermatology seminar. "The sexual impact has been underappreciated," she said.
Those problems might help explain why women with psoriasis, especially those aged younger than 35 years, tend to have fewer babies, she added. Psoriatic women also have higher rates of induced and spontaneous abortions and are more likely to have preterm and underweight births.
Some of the women surveyed said that they worried about passing psoriasis to their children. "Maybe there’s voluntary childlessness," Dr. Cather said. But when women in her practice mention they don’t want kids for fear of passing on the disease, "I answer right back, ‘You don’t have to have what you have. I can help you with it,’ " she said.
Methotrexate, acitretin, and psoralen photochemotherapy (PUVA) are contraindicated during pregnancy, but ultraviolet B (UVB) treatments are safe, said Dr. Cather. She also uses tumor necrosis factor inhibitors, which are FDA pregnancy category B agents; she prefers etanercept for its short half-life (just over 4 days) and because ob.gyns. are usually familiar with it. The Organization of Teratology Information Specialists (OTIS) keeps the etanercept and adalimumab pregnancy registries, and "there’s [been] no signal to date" for those drugs, she said.
Psoriatic women should know that the odds are with them for having a normal pregnancy, and that pregnancy is likely to help clear their skin, Dr. Cather said.
But because half of the pregnancies in the United States are unplanned, it’s important to discuss pregnancy – and psoriasis treatment during pregnancy – as part of routine care. "It’s difficult if a psoriasis patient calls you in a panic because they’re not sure their drug is safe, and not sure their doctor will let them continue it,’ she said. Some ob.gyns. are comfortable with letting women stay on their psoriasis therapy, while others want them to quit everything, even topical steroids.
"In my clinic, when a psoriasis patient gets pregnant, usually we’ve planned for it and are excited about it, and we’ve had some dialogue with the ob.gyn.," she explained.
SDEF and this news organization are owned by Frontline Medical Communications. Dr. Cather is a consultant, speaker, or researcher for AbbVie, Novartis, Leo, Janssen, Amgen, Celgene, Merck, and Pfizer.
EXPERT ANALYSIS FROM SDEF LAS VEGAS DERMATOLOGY SEMINAR