User login
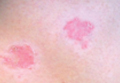
Woronoff Ring Associated With Adalimumab Therapy for Psoriasis
Striking trends emerge in SLE joint replacement
SNOWMASS, COLO. – Total knee replacement surgery rates in patients with systemic lupus erythematosus jumped sixfold nationally during a recent 15-year span – and buried within this statistic is some very good news.
The sharp rise in total knee replacement (TKR) among systemic lupus erythematosus (SLE) patients has been driven by a hefty increase in operations performed for osteoarthritis, while TKR for active SLE in the knee has declined. These trends reflect the increased longevity of patients with SLE resulting from improved medical management. For the first time, large numbers of SLE patients are surviving to an age when they, like other Americans, are more vulnerable to osteoarthritis, Dr. Susan M. Goodman explained at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
"Lupus is no longer the highly mortal disease that it was," she observed. "Clearly, patients are surviving and – I don’t know how else to put it – they’ve become middle-aged women who are having knee replacements, which is kind of a success."
She presented highlights of a soon-to-be-published study involving analysis of 10 state databases including nearly 2.8 million arthroplasties performed during 1991-2005. The rate of TKR in SLE patients climbed sixfold from 0.03 per 100,000 population in 1991 to 0.18 per 100,000 in 2005. Meanwhile, total hip replacement (THR) in SLE patients showed a modest but statistically significant increase from 0.11 to 0.18 cases per 100,000.
The proportion of lupus patients undergoing arthroplasty for avascular necrosis fell from 53% in 1991 to 24% in 2005, while the proportion undergoing arthroplasty because they developed osteoarthritis went from 23% to 61%.
Virtually all of the increase in total arthroplasties among SLE patients occurred in women aged 45 years and older. Their rate more than tripled during the study years, going from 0.076 to 0.271 cases per 100,000. Rates in female SLE patients aged 44 years and younger actually took a significant drop from 0.073 to 0.067 per 100,000. Rates in males aged 44 and younger remained flat over time, while men aged 45 and up showed a modest increase from a low baseline rate of 0.009 cases per 100,000 in 1991 to 0.034 per 100,000 in 2005, according to Dr. Goodman, a rheumatologist at the Hospital for Special Surgery and Cornell University, New York.
The proportion of TKRs among all arthroplasties performed in SLE patients increased from 16% in 1991 to 48% in 2005. Meanwhile, THRs decreased from 66% of all arthroplasties to 40%. Other joint replacements didn’t change much over time.
A particularly striking finding in the study was that the mean age at the time of arthroplasty in SLE patients increased by nearly a decade – 47.3 years in 1991 to 56.8 years in 2005. In contrast, the mean age at arthroplasty for osteoarthritis patients without SLE decreased from 71.5 to 69.0 years.
Dr. Goodman turned to additional studies by her research group and other investigators to provide a picture of arthroplasty outcomes in SLE patients.
In-hospital postoperative mortality was found to be increased in SLE patients, compared with rheumatoid arthritis patients or controls undergoing TKR or THR in a study of more than 1.5 million TKRs and THRs included in the Nationwide Inpatient Sample for 1993-2006. The Nationwide Inpatient Sample is the largest all-payer inpatient health care database in the United States.
In a multivariate logistic regression analysis adjusted for comorbidities, hospital type, and other potential confounders, investigators at Stanford (Calif.) University found that SLE patients undergoing THR had a 3.5-fold increased risk of death, compared with controls. This was driven by a 4.9-fold increased death risk in SLE patients undergoing nonelective THR or TKR, typically because of a fracture. In contrast, the rate of in-hospital death following TKR or THR in rheumatoid arthritis patients wasn’t significantly different from controls. The one comorbidity present on admission that was associated with markedly increased postoperative mortality was renal disease in SLE patients (J. Rheumatol. 2010;37:1467-72).
"Baseline renal dysfunction really seems to be a marker in lupus patients for bad perioperative outcome," Dr. Goodman observed, adding that there is a need for "increased vigilance" regarding this comorbidity.
A study of 57 SLE patients and 107 age-matched osteoarthritis patients who underwent THR at the Hospital for Special Surgery demonstrated that the lupus patients had more baseline comorbidities as reflected in their mean Charlson Comorbidity Index of 1.9, compared with 0.3 in the osteoarthritis group. Seventy-nine percent of the SLE patients, but none of the osteoarthritis patients, were on immunosuppressant therapy. The lupus patients had a 19% incidence of postoperative major adverse events, including deep vein thrombosis, arrhythmia, acute renal insufficiency, or additional surgery, compared with a 6% rate in the osteoarthritis group. The 6-day mean length of stay in the SLE group was a full day longer than that of the osteoarthritis patients, according to Dr. Goodman.
Another Hospital for Special Surgery study included 56 SLE patients undergoing THR and 45 with TKR, as well as 108 age-matched controls undergoing THR and 89 with TKR. The SLE patients had significantly worse baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and function scores than did the osteoarthritis patients. At 2 years of follow-up, however, all four groups ended up with very good pain and function outcomes, with WOMAC scores in the 80-92 range.
Yet despite these excellent outcomes, the SLE patients still felt more limited by their chronic disease. This was reflected in their lower health-related quality of life on the SF-36 physical component summary score at 2 years of follow-up: 39 in the SLE THR patients, compared with 50.1 in the osteoarthritis THR group, and 38 in the SLE TKR patients, compared with 48.4 in the osteoarthritis controls.
This and other studies paint a picture of contemporary SLE patients undergoing TKR as more closely resembling osteoarthritis patients with TKR than SLE patients undergoing THR. The average age of the SLE TKR patients, at 62.4 years, was 8 years older than the SLE THR group. The SLE TKR group’s mean body mass index of 31.5 kg/m2 was 5 kg/m2 greater than in the SLE THR group. And none of the SLE TKR patients had avascular necrosis, compared with one-third of those undergoing THR.
Dr. Goodman reported having no relevant financial relationships.
SNOWMASS, COLO. – Total knee replacement surgery rates in patients with systemic lupus erythematosus jumped sixfold nationally during a recent 15-year span – and buried within this statistic is some very good news.
The sharp rise in total knee replacement (TKR) among systemic lupus erythematosus (SLE) patients has been driven by a hefty increase in operations performed for osteoarthritis, while TKR for active SLE in the knee has declined. These trends reflect the increased longevity of patients with SLE resulting from improved medical management. For the first time, large numbers of SLE patients are surviving to an age when they, like other Americans, are more vulnerable to osteoarthritis, Dr. Susan M. Goodman explained at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
"Lupus is no longer the highly mortal disease that it was," she observed. "Clearly, patients are surviving and – I don’t know how else to put it – they’ve become middle-aged women who are having knee replacements, which is kind of a success."
She presented highlights of a soon-to-be-published study involving analysis of 10 state databases including nearly 2.8 million arthroplasties performed during 1991-2005. The rate of TKR in SLE patients climbed sixfold from 0.03 per 100,000 population in 1991 to 0.18 per 100,000 in 2005. Meanwhile, total hip replacement (THR) in SLE patients showed a modest but statistically significant increase from 0.11 to 0.18 cases per 100,000.
The proportion of lupus patients undergoing arthroplasty for avascular necrosis fell from 53% in 1991 to 24% in 2005, while the proportion undergoing arthroplasty because they developed osteoarthritis went from 23% to 61%.
Virtually all of the increase in total arthroplasties among SLE patients occurred in women aged 45 years and older. Their rate more than tripled during the study years, going from 0.076 to 0.271 cases per 100,000. Rates in female SLE patients aged 44 years and younger actually took a significant drop from 0.073 to 0.067 per 100,000. Rates in males aged 44 and younger remained flat over time, while men aged 45 and up showed a modest increase from a low baseline rate of 0.009 cases per 100,000 in 1991 to 0.034 per 100,000 in 2005, according to Dr. Goodman, a rheumatologist at the Hospital for Special Surgery and Cornell University, New York.
The proportion of TKRs among all arthroplasties performed in SLE patients increased from 16% in 1991 to 48% in 2005. Meanwhile, THRs decreased from 66% of all arthroplasties to 40%. Other joint replacements didn’t change much over time.
A particularly striking finding in the study was that the mean age at the time of arthroplasty in SLE patients increased by nearly a decade – 47.3 years in 1991 to 56.8 years in 2005. In contrast, the mean age at arthroplasty for osteoarthritis patients without SLE decreased from 71.5 to 69.0 years.
Dr. Goodman turned to additional studies by her research group and other investigators to provide a picture of arthroplasty outcomes in SLE patients.
In-hospital postoperative mortality was found to be increased in SLE patients, compared with rheumatoid arthritis patients or controls undergoing TKR or THR in a study of more than 1.5 million TKRs and THRs included in the Nationwide Inpatient Sample for 1993-2006. The Nationwide Inpatient Sample is the largest all-payer inpatient health care database in the United States.
In a multivariate logistic regression analysis adjusted for comorbidities, hospital type, and other potential confounders, investigators at Stanford (Calif.) University found that SLE patients undergoing THR had a 3.5-fold increased risk of death, compared with controls. This was driven by a 4.9-fold increased death risk in SLE patients undergoing nonelective THR or TKR, typically because of a fracture. In contrast, the rate of in-hospital death following TKR or THR in rheumatoid arthritis patients wasn’t significantly different from controls. The one comorbidity present on admission that was associated with markedly increased postoperative mortality was renal disease in SLE patients (J. Rheumatol. 2010;37:1467-72).
"Baseline renal dysfunction really seems to be a marker in lupus patients for bad perioperative outcome," Dr. Goodman observed, adding that there is a need for "increased vigilance" regarding this comorbidity.
A study of 57 SLE patients and 107 age-matched osteoarthritis patients who underwent THR at the Hospital for Special Surgery demonstrated that the lupus patients had more baseline comorbidities as reflected in their mean Charlson Comorbidity Index of 1.9, compared with 0.3 in the osteoarthritis group. Seventy-nine percent of the SLE patients, but none of the osteoarthritis patients, were on immunosuppressant therapy. The lupus patients had a 19% incidence of postoperative major adverse events, including deep vein thrombosis, arrhythmia, acute renal insufficiency, or additional surgery, compared with a 6% rate in the osteoarthritis group. The 6-day mean length of stay in the SLE group was a full day longer than that of the osteoarthritis patients, according to Dr. Goodman.
Another Hospital for Special Surgery study included 56 SLE patients undergoing THR and 45 with TKR, as well as 108 age-matched controls undergoing THR and 89 with TKR. The SLE patients had significantly worse baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and function scores than did the osteoarthritis patients. At 2 years of follow-up, however, all four groups ended up with very good pain and function outcomes, with WOMAC scores in the 80-92 range.
Yet despite these excellent outcomes, the SLE patients still felt more limited by their chronic disease. This was reflected in their lower health-related quality of life on the SF-36 physical component summary score at 2 years of follow-up: 39 in the SLE THR patients, compared with 50.1 in the osteoarthritis THR group, and 38 in the SLE TKR patients, compared with 48.4 in the osteoarthritis controls.
This and other studies paint a picture of contemporary SLE patients undergoing TKR as more closely resembling osteoarthritis patients with TKR than SLE patients undergoing THR. The average age of the SLE TKR patients, at 62.4 years, was 8 years older than the SLE THR group. The SLE TKR group’s mean body mass index of 31.5 kg/m2 was 5 kg/m2 greater than in the SLE THR group. And none of the SLE TKR patients had avascular necrosis, compared with one-third of those undergoing THR.
Dr. Goodman reported having no relevant financial relationships.
SNOWMASS, COLO. – Total knee replacement surgery rates in patients with systemic lupus erythematosus jumped sixfold nationally during a recent 15-year span – and buried within this statistic is some very good news.
The sharp rise in total knee replacement (TKR) among systemic lupus erythematosus (SLE) patients has been driven by a hefty increase in operations performed for osteoarthritis, while TKR for active SLE in the knee has declined. These trends reflect the increased longevity of patients with SLE resulting from improved medical management. For the first time, large numbers of SLE patients are surviving to an age when they, like other Americans, are more vulnerable to osteoarthritis, Dr. Susan M. Goodman explained at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
"Lupus is no longer the highly mortal disease that it was," she observed. "Clearly, patients are surviving and – I don’t know how else to put it – they’ve become middle-aged women who are having knee replacements, which is kind of a success."
She presented highlights of a soon-to-be-published study involving analysis of 10 state databases including nearly 2.8 million arthroplasties performed during 1991-2005. The rate of TKR in SLE patients climbed sixfold from 0.03 per 100,000 population in 1991 to 0.18 per 100,000 in 2005. Meanwhile, total hip replacement (THR) in SLE patients showed a modest but statistically significant increase from 0.11 to 0.18 cases per 100,000.
The proportion of lupus patients undergoing arthroplasty for avascular necrosis fell from 53% in 1991 to 24% in 2005, while the proportion undergoing arthroplasty because they developed osteoarthritis went from 23% to 61%.
Virtually all of the increase in total arthroplasties among SLE patients occurred in women aged 45 years and older. Their rate more than tripled during the study years, going from 0.076 to 0.271 cases per 100,000. Rates in female SLE patients aged 44 years and younger actually took a significant drop from 0.073 to 0.067 per 100,000. Rates in males aged 44 and younger remained flat over time, while men aged 45 and up showed a modest increase from a low baseline rate of 0.009 cases per 100,000 in 1991 to 0.034 per 100,000 in 2005, according to Dr. Goodman, a rheumatologist at the Hospital for Special Surgery and Cornell University, New York.
The proportion of TKRs among all arthroplasties performed in SLE patients increased from 16% in 1991 to 48% in 2005. Meanwhile, THRs decreased from 66% of all arthroplasties to 40%. Other joint replacements didn’t change much over time.
A particularly striking finding in the study was that the mean age at the time of arthroplasty in SLE patients increased by nearly a decade – 47.3 years in 1991 to 56.8 years in 2005. In contrast, the mean age at arthroplasty for osteoarthritis patients without SLE decreased from 71.5 to 69.0 years.
Dr. Goodman turned to additional studies by her research group and other investigators to provide a picture of arthroplasty outcomes in SLE patients.
In-hospital postoperative mortality was found to be increased in SLE patients, compared with rheumatoid arthritis patients or controls undergoing TKR or THR in a study of more than 1.5 million TKRs and THRs included in the Nationwide Inpatient Sample for 1993-2006. The Nationwide Inpatient Sample is the largest all-payer inpatient health care database in the United States.
In a multivariate logistic regression analysis adjusted for comorbidities, hospital type, and other potential confounders, investigators at Stanford (Calif.) University found that SLE patients undergoing THR had a 3.5-fold increased risk of death, compared with controls. This was driven by a 4.9-fold increased death risk in SLE patients undergoing nonelective THR or TKR, typically because of a fracture. In contrast, the rate of in-hospital death following TKR or THR in rheumatoid arthritis patients wasn’t significantly different from controls. The one comorbidity present on admission that was associated with markedly increased postoperative mortality was renal disease in SLE patients (J. Rheumatol. 2010;37:1467-72).
"Baseline renal dysfunction really seems to be a marker in lupus patients for bad perioperative outcome," Dr. Goodman observed, adding that there is a need for "increased vigilance" regarding this comorbidity.
A study of 57 SLE patients and 107 age-matched osteoarthritis patients who underwent THR at the Hospital for Special Surgery demonstrated that the lupus patients had more baseline comorbidities as reflected in their mean Charlson Comorbidity Index of 1.9, compared with 0.3 in the osteoarthritis group. Seventy-nine percent of the SLE patients, but none of the osteoarthritis patients, were on immunosuppressant therapy. The lupus patients had a 19% incidence of postoperative major adverse events, including deep vein thrombosis, arrhythmia, acute renal insufficiency, or additional surgery, compared with a 6% rate in the osteoarthritis group. The 6-day mean length of stay in the SLE group was a full day longer than that of the osteoarthritis patients, according to Dr. Goodman.
Another Hospital for Special Surgery study included 56 SLE patients undergoing THR and 45 with TKR, as well as 108 age-matched controls undergoing THR and 89 with TKR. The SLE patients had significantly worse baseline Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain and function scores than did the osteoarthritis patients. At 2 years of follow-up, however, all four groups ended up with very good pain and function outcomes, with WOMAC scores in the 80-92 range.
Yet despite these excellent outcomes, the SLE patients still felt more limited by their chronic disease. This was reflected in their lower health-related quality of life on the SF-36 physical component summary score at 2 years of follow-up: 39 in the SLE THR patients, compared with 50.1 in the osteoarthritis THR group, and 38 in the SLE TKR patients, compared with 48.4 in the osteoarthritis controls.
This and other studies paint a picture of contemporary SLE patients undergoing TKR as more closely resembling osteoarthritis patients with TKR than SLE patients undergoing THR. The average age of the SLE TKR patients, at 62.4 years, was 8 years older than the SLE THR group. The SLE TKR group’s mean body mass index of 31.5 kg/m2 was 5 kg/m2 greater than in the SLE THR group. And none of the SLE TKR patients had avascular necrosis, compared with one-third of those undergoing THR.
Dr. Goodman reported having no relevant financial relationships.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Biosimilar Dilemma
The passage of the Biologics Price Competition and Innovation Act of 2009 (BPCI Act) allowed for the creation of a regulatory pathway for new, safe, and effective biosimilar agents. Although the medical community always needs new and affordable treatments, patients and physicians are aware of and concerned about the risks associated with biologics and the lack of long-term safety data for new treatments.
In contrast to generic drugs, which are chemically identical to their branded counterparts, biosimilar agents are not chemically identical to their branded biologic counterparts because as large complex molecules derived from living cells using recombinant DNA technology, biologics can never be exactly replicated due to their inherent variability.
Because of these substantial differences, the National Psoriasis Foundation released a policy in July 2013 to ensure patient safety.
The National Psoriasis Foundation recommends that the patient-provider relationship remain at the center of all treatment planning and supports a prohibition on biosimilar substitution unless all of the following minimal thresholds are met:
- the biosimilar has been designated by the Food and Drug Administration as interchangeable with the prescribed biologic for the specified indicated use;
- the biosimilar has a unique nonproprietary name to eliminate confusion, to allow providers to accurately track the therapeutic agent in a patient's permanent record, and to allow for the collection of adverse event information;
- the biosimilar product follows the same route of administration and dosage form as the reference product;
- the pharmacist notifies the prescriber in writing or electronic communication of the intention to substitute at least 24 hours prior to the substitution;
- if explicit permission from prescribing physician and patient is not obtained within 24 hours, then the original prescription must be filled;
- the patient (or patient's authorized representative) must be informed and educated about a biosimilar substitution at the point of sale; and
- upon notification of a substitution, the pharmacy and the prescribing physician are to retain a permanent record in the patient's medical record of the biosimilar substitution.
What’s the issue?
The tension between lowering costs and using trusted therapeutic options will be at the center of the debate over biosimilar agents. The National Psoriasis Foundation policy protects physicians and patients and helps maintain their autonomy. As these drugs are developed and studied, we will have more information to inform our decisions. How will you and your patients respond to the availability of biosimilar agents?
The passage of the Biologics Price Competition and Innovation Act of 2009 (BPCI Act) allowed for the creation of a regulatory pathway for new, safe, and effective biosimilar agents. Although the medical community always needs new and affordable treatments, patients and physicians are aware of and concerned about the risks associated with biologics and the lack of long-term safety data for new treatments.
In contrast to generic drugs, which are chemically identical to their branded counterparts, biosimilar agents are not chemically identical to their branded biologic counterparts because as large complex molecules derived from living cells using recombinant DNA technology, biologics can never be exactly replicated due to their inherent variability.
Because of these substantial differences, the National Psoriasis Foundation released a policy in July 2013 to ensure patient safety.
The National Psoriasis Foundation recommends that the patient-provider relationship remain at the center of all treatment planning and supports a prohibition on biosimilar substitution unless all of the following minimal thresholds are met:
- the biosimilar has been designated by the Food and Drug Administration as interchangeable with the prescribed biologic for the specified indicated use;
- the biosimilar has a unique nonproprietary name to eliminate confusion, to allow providers to accurately track the therapeutic agent in a patient's permanent record, and to allow for the collection of adverse event information;
- the biosimilar product follows the same route of administration and dosage form as the reference product;
- the pharmacist notifies the prescriber in writing or electronic communication of the intention to substitute at least 24 hours prior to the substitution;
- if explicit permission from prescribing physician and patient is not obtained within 24 hours, then the original prescription must be filled;
- the patient (or patient's authorized representative) must be informed and educated about a biosimilar substitution at the point of sale; and
- upon notification of a substitution, the pharmacy and the prescribing physician are to retain a permanent record in the patient's medical record of the biosimilar substitution.
What’s the issue?
The tension between lowering costs and using trusted therapeutic options will be at the center of the debate over biosimilar agents. The National Psoriasis Foundation policy protects physicians and patients and helps maintain their autonomy. As these drugs are developed and studied, we will have more information to inform our decisions. How will you and your patients respond to the availability of biosimilar agents?
The passage of the Biologics Price Competition and Innovation Act of 2009 (BPCI Act) allowed for the creation of a regulatory pathway for new, safe, and effective biosimilar agents. Although the medical community always needs new and affordable treatments, patients and physicians are aware of and concerned about the risks associated with biologics and the lack of long-term safety data for new treatments.
In contrast to generic drugs, which are chemically identical to their branded counterparts, biosimilar agents are not chemically identical to their branded biologic counterparts because as large complex molecules derived from living cells using recombinant DNA technology, biologics can never be exactly replicated due to their inherent variability.
Because of these substantial differences, the National Psoriasis Foundation released a policy in July 2013 to ensure patient safety.
The National Psoriasis Foundation recommends that the patient-provider relationship remain at the center of all treatment planning and supports a prohibition on biosimilar substitution unless all of the following minimal thresholds are met:
- the biosimilar has been designated by the Food and Drug Administration as interchangeable with the prescribed biologic for the specified indicated use;
- the biosimilar has a unique nonproprietary name to eliminate confusion, to allow providers to accurately track the therapeutic agent in a patient's permanent record, and to allow for the collection of adverse event information;
- the biosimilar product follows the same route of administration and dosage form as the reference product;
- the pharmacist notifies the prescriber in writing or electronic communication of the intention to substitute at least 24 hours prior to the substitution;
- if explicit permission from prescribing physician and patient is not obtained within 24 hours, then the original prescription must be filled;
- the patient (or patient's authorized representative) must be informed and educated about a biosimilar substitution at the point of sale; and
- upon notification of a substitution, the pharmacy and the prescribing physician are to retain a permanent record in the patient's medical record of the biosimilar substitution.
What’s the issue?
The tension between lowering costs and using trusted therapeutic options will be at the center of the debate over biosimilar agents. The National Psoriasis Foundation policy protects physicians and patients and helps maintain their autonomy. As these drugs are developed and studied, we will have more information to inform our decisions. How will you and your patients respond to the availability of biosimilar agents?
AAD identifies important knowledge gaps for psoriasis
The three most important gaps in current understanding of psoriasis are the need to fully elucidate the genetic underpinnings of the disease, delineate in more detail its natural history and prognostic factors, and better characterize and treat its associated comorbidities, according to an article in the Journal of the American Academy of Dermatology.
The academy recently issued guidelines for the management of psoriasis and psoriatic arthritis, which included a section concerning the most prominent deficiencies in knowledge about the disease. In the current article (J. Am. Acad. Dermatol. 2014;70:146-67), the expert panel that wrote the guidelines now details some of those gaps and suggests how future research should address them.
Advances in genetic research have allowed considerable progress in our understanding of the pathogenesis of psoriasis, including the identification of genetic susceptibility regions and nucleotide polymorphisms that are significantly associated with immune pathways, skin barrier function, and epidermal proliferation. Future research should "explore whether the genetic basis of psoriasis stems from a primary defect in immunologic function, a defect in keratinocyte function, or a complex interaction of both," wrote Dr. Caitriona Ryan of the Psoriasis Research Center, Baylor University Medical Center, Dallas, and her associates on the expert panel.
In particular, genetic research should help elucidate the correlation between psoriasis genotype and phenotype, determining whether psoriasis and psoriatic arthritis are genetically distinct diseases, as well as the genetic differences underlying guttate, erythrodermic, inverse, palmoplantar, and pustular forms of the disease.
Immunologic studies are needed to define the roles of various inflammatory cells in patients’ skin and blood, and to assist the development of targeted immunotherapies that will correct immunologic dysfunction without causing adverse immunosuppression. Similarly, the role of angiogenesis and the vascular bed in the pathogenesis of psoriasis requires more research attention, because topical or systemic inhibition of this process appears to be beneficial.
Additionally, the environmental factors that predispose to the development of psoriasis or contribute to disease exacerbations are still poorly defined. Research into lifestyle factors and the role of bacterial and viral infections should be especially revealing, Dr. Ryan and her associates said.
Research to determine the prevalence and natural history of psoriasis across different populations also is critical. Robust, long-term, prospective epidemiology studies at the population level are key.
Several subpopulations of psoriasis patients also deserve special attention. There is a dearth of both clinical and basic scientific data on pediatric psoriasis, when children are the very patients most likely to suffer the adverse psychosocial effects of psoriasis and the most vulnerable to the adverse physical and developmental effects of treatments, the investigators noted.
Women who are pregnant or breastfeeding constitute another important subgroup of psoriasis patients. Studies are urgently needed to examine the effects of the disease itself and of its therapies on pregnancy outcomes. Similarly, the elderly are a subgroup of patients that requires special attention to adverse medication effects, because they have a high prevalence of comorbid conditions, greater use of concomitant medications, diminished renal function, and age-related changes in pharmacokinetics and pharmacodynamics.
Finally, future research must more closely examine several comorbidities now known to accompany psoriasis, including diabetes, obesity, the metabolic syndrome, and, most importantly, cardiovascular disease.
Severe psoriasis now should be considered an independent cardiovascular risk factor. "As physicians, it is our responsibility to counsel patients with psoriasis regarding the increased risk of cardiometabolic conditions and the need for lifestyle modifications, including smoking cessation, weight reduction, and regular screening for diabetes and hypertension," Dr. Ryan and her colleagues said.
Determining whether systemic treatment of psoriasis mitigates cardiometabolic risk, especially if it entails newer biologic agents, also is important, they added.
Psychological comorbidities should not be overlooked. Patients with psoriasis are known to have high rates of anxiety, depression, and alexithymia—the inability to recognize or discuss feelings or emotional states. Relaxation techniques, meditation, and cognitive-behavioral therapy have shown efficacy and need further assessment.
Sexual functioning also should be studied in greater detail, since psoriasis can have a significant impact on sexuality, particularly if the disease affects the genitals.
Dr. Ryan reported ties to Janssen-Cilag, Pfizer, Galderma, and Abbott, and her associates reported ties to numerous industry sources.
The three most important gaps in current understanding of psoriasis are the need to fully elucidate the genetic underpinnings of the disease, delineate in more detail its natural history and prognostic factors, and better characterize and treat its associated comorbidities, according to an article in the Journal of the American Academy of Dermatology.
The academy recently issued guidelines for the management of psoriasis and psoriatic arthritis, which included a section concerning the most prominent deficiencies in knowledge about the disease. In the current article (J. Am. Acad. Dermatol. 2014;70:146-67), the expert panel that wrote the guidelines now details some of those gaps and suggests how future research should address them.
Advances in genetic research have allowed considerable progress in our understanding of the pathogenesis of psoriasis, including the identification of genetic susceptibility regions and nucleotide polymorphisms that are significantly associated with immune pathways, skin barrier function, and epidermal proliferation. Future research should "explore whether the genetic basis of psoriasis stems from a primary defect in immunologic function, a defect in keratinocyte function, or a complex interaction of both," wrote Dr. Caitriona Ryan of the Psoriasis Research Center, Baylor University Medical Center, Dallas, and her associates on the expert panel.
In particular, genetic research should help elucidate the correlation between psoriasis genotype and phenotype, determining whether psoriasis and psoriatic arthritis are genetically distinct diseases, as well as the genetic differences underlying guttate, erythrodermic, inverse, palmoplantar, and pustular forms of the disease.
Immunologic studies are needed to define the roles of various inflammatory cells in patients’ skin and blood, and to assist the development of targeted immunotherapies that will correct immunologic dysfunction without causing adverse immunosuppression. Similarly, the role of angiogenesis and the vascular bed in the pathogenesis of psoriasis requires more research attention, because topical or systemic inhibition of this process appears to be beneficial.
Additionally, the environmental factors that predispose to the development of psoriasis or contribute to disease exacerbations are still poorly defined. Research into lifestyle factors and the role of bacterial and viral infections should be especially revealing, Dr. Ryan and her associates said.
Research to determine the prevalence and natural history of psoriasis across different populations also is critical. Robust, long-term, prospective epidemiology studies at the population level are key.
Several subpopulations of psoriasis patients also deserve special attention. There is a dearth of both clinical and basic scientific data on pediatric psoriasis, when children are the very patients most likely to suffer the adverse psychosocial effects of psoriasis and the most vulnerable to the adverse physical and developmental effects of treatments, the investigators noted.
Women who are pregnant or breastfeeding constitute another important subgroup of psoriasis patients. Studies are urgently needed to examine the effects of the disease itself and of its therapies on pregnancy outcomes. Similarly, the elderly are a subgroup of patients that requires special attention to adverse medication effects, because they have a high prevalence of comorbid conditions, greater use of concomitant medications, diminished renal function, and age-related changes in pharmacokinetics and pharmacodynamics.
Finally, future research must more closely examine several comorbidities now known to accompany psoriasis, including diabetes, obesity, the metabolic syndrome, and, most importantly, cardiovascular disease.
Severe psoriasis now should be considered an independent cardiovascular risk factor. "As physicians, it is our responsibility to counsel patients with psoriasis regarding the increased risk of cardiometabolic conditions and the need for lifestyle modifications, including smoking cessation, weight reduction, and regular screening for diabetes and hypertension," Dr. Ryan and her colleagues said.
Determining whether systemic treatment of psoriasis mitigates cardiometabolic risk, especially if it entails newer biologic agents, also is important, they added.
Psychological comorbidities should not be overlooked. Patients with psoriasis are known to have high rates of anxiety, depression, and alexithymia—the inability to recognize or discuss feelings or emotional states. Relaxation techniques, meditation, and cognitive-behavioral therapy have shown efficacy and need further assessment.
Sexual functioning also should be studied in greater detail, since psoriasis can have a significant impact on sexuality, particularly if the disease affects the genitals.
Dr. Ryan reported ties to Janssen-Cilag, Pfizer, Galderma, and Abbott, and her associates reported ties to numerous industry sources.
The three most important gaps in current understanding of psoriasis are the need to fully elucidate the genetic underpinnings of the disease, delineate in more detail its natural history and prognostic factors, and better characterize and treat its associated comorbidities, according to an article in the Journal of the American Academy of Dermatology.
The academy recently issued guidelines for the management of psoriasis and psoriatic arthritis, which included a section concerning the most prominent deficiencies in knowledge about the disease. In the current article (J. Am. Acad. Dermatol. 2014;70:146-67), the expert panel that wrote the guidelines now details some of those gaps and suggests how future research should address them.
Advances in genetic research have allowed considerable progress in our understanding of the pathogenesis of psoriasis, including the identification of genetic susceptibility regions and nucleotide polymorphisms that are significantly associated with immune pathways, skin barrier function, and epidermal proliferation. Future research should "explore whether the genetic basis of psoriasis stems from a primary defect in immunologic function, a defect in keratinocyte function, or a complex interaction of both," wrote Dr. Caitriona Ryan of the Psoriasis Research Center, Baylor University Medical Center, Dallas, and her associates on the expert panel.
In particular, genetic research should help elucidate the correlation between psoriasis genotype and phenotype, determining whether psoriasis and psoriatic arthritis are genetically distinct diseases, as well as the genetic differences underlying guttate, erythrodermic, inverse, palmoplantar, and pustular forms of the disease.
Immunologic studies are needed to define the roles of various inflammatory cells in patients’ skin and blood, and to assist the development of targeted immunotherapies that will correct immunologic dysfunction without causing adverse immunosuppression. Similarly, the role of angiogenesis and the vascular bed in the pathogenesis of psoriasis requires more research attention, because topical or systemic inhibition of this process appears to be beneficial.
Additionally, the environmental factors that predispose to the development of psoriasis or contribute to disease exacerbations are still poorly defined. Research into lifestyle factors and the role of bacterial and viral infections should be especially revealing, Dr. Ryan and her associates said.
Research to determine the prevalence and natural history of psoriasis across different populations also is critical. Robust, long-term, prospective epidemiology studies at the population level are key.
Several subpopulations of psoriasis patients also deserve special attention. There is a dearth of both clinical and basic scientific data on pediatric psoriasis, when children are the very patients most likely to suffer the adverse psychosocial effects of psoriasis and the most vulnerable to the adverse physical and developmental effects of treatments, the investigators noted.
Women who are pregnant or breastfeeding constitute another important subgroup of psoriasis patients. Studies are urgently needed to examine the effects of the disease itself and of its therapies on pregnancy outcomes. Similarly, the elderly are a subgroup of patients that requires special attention to adverse medication effects, because they have a high prevalence of comorbid conditions, greater use of concomitant medications, diminished renal function, and age-related changes in pharmacokinetics and pharmacodynamics.
Finally, future research must more closely examine several comorbidities now known to accompany psoriasis, including diabetes, obesity, the metabolic syndrome, and, most importantly, cardiovascular disease.
Severe psoriasis now should be considered an independent cardiovascular risk factor. "As physicians, it is our responsibility to counsel patients with psoriasis regarding the increased risk of cardiometabolic conditions and the need for lifestyle modifications, including smoking cessation, weight reduction, and regular screening for diabetes and hypertension," Dr. Ryan and her colleagues said.
Determining whether systemic treatment of psoriasis mitigates cardiometabolic risk, especially if it entails newer biologic agents, also is important, they added.
Psychological comorbidities should not be overlooked. Patients with psoriasis are known to have high rates of anxiety, depression, and alexithymia—the inability to recognize or discuss feelings or emotional states. Relaxation techniques, meditation, and cognitive-behavioral therapy have shown efficacy and need further assessment.
Sexual functioning also should be studied in greater detail, since psoriasis can have a significant impact on sexuality, particularly if the disease affects the genitals.
Dr. Ryan reported ties to Janssen-Cilag, Pfizer, Galderma, and Abbott, and her associates reported ties to numerous industry sources.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Desoximetasone spray succeeds in psoriasis studies
Significantly more psoriasis patients randomized to a 0.25% desoximetasone spray showed clinical success and treatment success, compared with those who used a placebo vehicle, based on data from a pair of phase III studies. The findings were published in the December issue of Journal of Drugs in Dermatology by Dr. Leon Kircik of Indiana University School of Medicine, Indianapolis, and his colleagues.
The study population included adults with moderate to severe plaque psoriasis. The patients used the spray twice daily for 28 days, and they were assessed at baseline and during the study using the Physician Global Assessment score and the Total Lesion Severity Score. No significant differences in adverse events were reported between the treatment and placebo groups, and no patients reported stinging or burning from the spray formulation (J. Drugs Dermatol. 2013;12:1404-10).
Significantly more psoriasis patients randomized to a 0.25% desoximetasone spray showed clinical success and treatment success, compared with those who used a placebo vehicle, based on data from a pair of phase III studies. The findings were published in the December issue of Journal of Drugs in Dermatology by Dr. Leon Kircik of Indiana University School of Medicine, Indianapolis, and his colleagues.
The study population included adults with moderate to severe plaque psoriasis. The patients used the spray twice daily for 28 days, and they were assessed at baseline and during the study using the Physician Global Assessment score and the Total Lesion Severity Score. No significant differences in adverse events were reported between the treatment and placebo groups, and no patients reported stinging or burning from the spray formulation (J. Drugs Dermatol. 2013;12:1404-10).
Significantly more psoriasis patients randomized to a 0.25% desoximetasone spray showed clinical success and treatment success, compared with those who used a placebo vehicle, based on data from a pair of phase III studies. The findings were published in the December issue of Journal of Drugs in Dermatology by Dr. Leon Kircik of Indiana University School of Medicine, Indianapolis, and his colleagues.
The study population included adults with moderate to severe plaque psoriasis. The patients used the spray twice daily for 28 days, and they were assessed at baseline and during the study using the Physician Global Assessment score and the Total Lesion Severity Score. No significant differences in adverse events were reported between the treatment and placebo groups, and no patients reported stinging or burning from the spray formulation (J. Drugs Dermatol. 2013;12:1404-10).
FROM JOURNAL OF DRUGS IN DERMATOLOGY
Consider small-fiber neuropathies in systemic lupus erythematosus
Small-fiber neuropathy is one of the most common types of peripheral neuropathy affecting patients with systemic lupus erythematosus, but it isn’t even mentioned in the American College of Rheumatology neuropsychiatric case definitions of manifestations of the disorder, according to a retrospective analysis of cohort of 2,097 patients with SLE.
Other types of peripheral neuropathy, such as acute inflammatory demyelinating neuropathies (for example, Guillain-Barré syndrome), plexopathies, and mononeuritis multiplex, are well described in the ACR-NPSLE case definitions but occur much less frequently. This, combined with the fact that small-fiber neuropathies often present as "unorthodox" pain patterns, indicates that they are underdiagnosed, said Dr. Amin Oomatia of the University of Cambridge, England, and his coinvestigators at John Hopkins University, Baltimore.
Small-fiber neuropathies arise through mechanisms that are distinct from those of other neuropathies and require different diagnostic strategies to be properly identified. In particular, small-fiber neuropathies do not always conform to the "stocking-and-glove" pattern of pain that is typical of other neuropathies in SLE, so it is likely that many affected patients "may be regarded in routine clinical care as having a ‘nonorganic’ pain disorder.
"Our findings suggest that rheumatologists and other clinicians who confront SLE patients with seemingly improbable pain patterns should consider the diagnosis of a small-fiber neuropathy," the investigators wrote, especially since it may occur in the face of normal electrodiagnostic studies.
Dr. Oomatia and his colleagues based these conclusions on their retrospective study of one medical center’s 25-year experience treating 2,097 SLE patients – the Johns Hopkins Lupus Cohort. Using details in a database of patients’ electronic medical records, they identified 82 patients who had peripheral neuropathies related to SLE.
Only one patient had peripheral neuropathy attributable to Guillain-Barré syndrome, only one patient had a plexopathy, and only six patients had mononeuritis multiplex, demonstrating that these are very infrequent complications of SLE even though they are included in ACR case definitions.
In contrast, 14 patients (17% of those with peripheral neuropathy) had biopsy-proven small-fiber neuropathies, and most of them presented with "an entirely different and unorthodox pain distribution" characterized as patchy, asymmetric, or proximal.
In particular, nine patients had pain affecting the face, torso, and/or proximal extremities. Three had burning pain over their entire bodies, the investigators said (Arthritis Rheum. 2013 Dec. 10 [doi:10.1002/art.38302]).
In these cases, punch skin biopsy showed abnormalities that disproportionately affected the proximal thigh, "which is considered a surrogate indicator of proximal-most dorsal root ganglia neuronal cell loss," they wrote. In contrast, other patients who had the typical distal pattern of neuropathic pain showed decreased intraepidermal nerve-fiber densities in the distal leg, a surrogate indicator of distal-most axonal degeneration.
Another distinguishing feature of small-fiber neuropathy was its association with a history of herpes zoster virus, opportunistic infections, and osteoporotic fractures, all unrelated to corticosteroid dose, Dr. Oomatia and his associates said.
This study was supported in part by the National Institutes of Health and the National Center for Research Resources. No potential financial conflicts of interest were reported.
Small-fiber neuropathy is one of the most common types of peripheral neuropathy affecting patients with systemic lupus erythematosus, but it isn’t even mentioned in the American College of Rheumatology neuropsychiatric case definitions of manifestations of the disorder, according to a retrospective analysis of cohort of 2,097 patients with SLE.
Other types of peripheral neuropathy, such as acute inflammatory demyelinating neuropathies (for example, Guillain-Barré syndrome), plexopathies, and mononeuritis multiplex, are well described in the ACR-NPSLE case definitions but occur much less frequently. This, combined with the fact that small-fiber neuropathies often present as "unorthodox" pain patterns, indicates that they are underdiagnosed, said Dr. Amin Oomatia of the University of Cambridge, England, and his coinvestigators at John Hopkins University, Baltimore.
Small-fiber neuropathies arise through mechanisms that are distinct from those of other neuropathies and require different diagnostic strategies to be properly identified. In particular, small-fiber neuropathies do not always conform to the "stocking-and-glove" pattern of pain that is typical of other neuropathies in SLE, so it is likely that many affected patients "may be regarded in routine clinical care as having a ‘nonorganic’ pain disorder.
"Our findings suggest that rheumatologists and other clinicians who confront SLE patients with seemingly improbable pain patterns should consider the diagnosis of a small-fiber neuropathy," the investigators wrote, especially since it may occur in the face of normal electrodiagnostic studies.
Dr. Oomatia and his colleagues based these conclusions on their retrospective study of one medical center’s 25-year experience treating 2,097 SLE patients – the Johns Hopkins Lupus Cohort. Using details in a database of patients’ electronic medical records, they identified 82 patients who had peripheral neuropathies related to SLE.
Only one patient had peripheral neuropathy attributable to Guillain-Barré syndrome, only one patient had a plexopathy, and only six patients had mononeuritis multiplex, demonstrating that these are very infrequent complications of SLE even though they are included in ACR case definitions.
In contrast, 14 patients (17% of those with peripheral neuropathy) had biopsy-proven small-fiber neuropathies, and most of them presented with "an entirely different and unorthodox pain distribution" characterized as patchy, asymmetric, or proximal.
In particular, nine patients had pain affecting the face, torso, and/or proximal extremities. Three had burning pain over their entire bodies, the investigators said (Arthritis Rheum. 2013 Dec. 10 [doi:10.1002/art.38302]).
In these cases, punch skin biopsy showed abnormalities that disproportionately affected the proximal thigh, "which is considered a surrogate indicator of proximal-most dorsal root ganglia neuronal cell loss," they wrote. In contrast, other patients who had the typical distal pattern of neuropathic pain showed decreased intraepidermal nerve-fiber densities in the distal leg, a surrogate indicator of distal-most axonal degeneration.
Another distinguishing feature of small-fiber neuropathy was its association with a history of herpes zoster virus, opportunistic infections, and osteoporotic fractures, all unrelated to corticosteroid dose, Dr. Oomatia and his associates said.
This study was supported in part by the National Institutes of Health and the National Center for Research Resources. No potential financial conflicts of interest were reported.
Small-fiber neuropathy is one of the most common types of peripheral neuropathy affecting patients with systemic lupus erythematosus, but it isn’t even mentioned in the American College of Rheumatology neuropsychiatric case definitions of manifestations of the disorder, according to a retrospective analysis of cohort of 2,097 patients with SLE.
Other types of peripheral neuropathy, such as acute inflammatory demyelinating neuropathies (for example, Guillain-Barré syndrome), plexopathies, and mononeuritis multiplex, are well described in the ACR-NPSLE case definitions but occur much less frequently. This, combined with the fact that small-fiber neuropathies often present as "unorthodox" pain patterns, indicates that they are underdiagnosed, said Dr. Amin Oomatia of the University of Cambridge, England, and his coinvestigators at John Hopkins University, Baltimore.
Small-fiber neuropathies arise through mechanisms that are distinct from those of other neuropathies and require different diagnostic strategies to be properly identified. In particular, small-fiber neuropathies do not always conform to the "stocking-and-glove" pattern of pain that is typical of other neuropathies in SLE, so it is likely that many affected patients "may be regarded in routine clinical care as having a ‘nonorganic’ pain disorder.
"Our findings suggest that rheumatologists and other clinicians who confront SLE patients with seemingly improbable pain patterns should consider the diagnosis of a small-fiber neuropathy," the investigators wrote, especially since it may occur in the face of normal electrodiagnostic studies.
Dr. Oomatia and his colleagues based these conclusions on their retrospective study of one medical center’s 25-year experience treating 2,097 SLE patients – the Johns Hopkins Lupus Cohort. Using details in a database of patients’ electronic medical records, they identified 82 patients who had peripheral neuropathies related to SLE.
Only one patient had peripheral neuropathy attributable to Guillain-Barré syndrome, only one patient had a plexopathy, and only six patients had mononeuritis multiplex, demonstrating that these are very infrequent complications of SLE even though they are included in ACR case definitions.
In contrast, 14 patients (17% of those with peripheral neuropathy) had biopsy-proven small-fiber neuropathies, and most of them presented with "an entirely different and unorthodox pain distribution" characterized as patchy, asymmetric, or proximal.
In particular, nine patients had pain affecting the face, torso, and/or proximal extremities. Three had burning pain over their entire bodies, the investigators said (Arthritis Rheum. 2013 Dec. 10 [doi:10.1002/art.38302]).
In these cases, punch skin biopsy showed abnormalities that disproportionately affected the proximal thigh, "which is considered a surrogate indicator of proximal-most dorsal root ganglia neuronal cell loss," they wrote. In contrast, other patients who had the typical distal pattern of neuropathic pain showed decreased intraepidermal nerve-fiber densities in the distal leg, a surrogate indicator of distal-most axonal degeneration.
Another distinguishing feature of small-fiber neuropathy was its association with a history of herpes zoster virus, opportunistic infections, and osteoporotic fractures, all unrelated to corticosteroid dose, Dr. Oomatia and his associates said.
This study was supported in part by the National Institutes of Health and the National Center for Research Resources. No potential financial conflicts of interest were reported.
FROM ARTHRITIS AND RHEUMATISM
Major finding: A total of 14 patients, or 17% of 82 with peripheral neuropathies, had biopsy-proven small-fiber neuropathies and often presented with unorthodox patterns of pain.
Data source: A retrospective analysis of data regarding 2,097 consecutive patients with SLE registered in the Johns Hopkins Lupus Cohort during a 25-year period, including 82 who developed peripheral neuropathies related to the disease.
Disclosures: This study was supported in part by the National Institutes of Health and the National Center for Research Resources. No potential financial conflicts of interest were reported.
Maternal lupus doubled autism risk
SAN DIEGO – The risk of autism spectrum disorders is more than doubled among children born to mothers with systemic lupus erythematosus, according to the first-ever controlled study to address the question.
That being said, women with SLE can be reassured that despite this elevated relative risk, the absolute risk that their child will be diagnosed with an autism spectrum disorder (ASD) is low – less than 1 in 50 – Dr. Evelyne Vinet said at the annual meeting of the American College of Rheumatology.
The increased risk of ASD in children born to women with SLE documented in this large study was not mediated by in utero exposure to medications for SLE, including antimalarials, immunosuppressive agents, corticosteroids, and antidepressants. Use of those drugs in pregnancy wasn’t associated with any increased risk. In light of this, further research is warranted into a highly promising alternative hypothesis: that in utero exposure to SLE-related autoantibodies, such as anti-DNA and antiphospholipid antibodies, may play a causative role, said Dr. Vinet, a rheumatologist at McGill University, Montreal.
Children born to mothers with SLE also had an increased likelihood of being diagnosed with attention-deficit/hyperactivity disorder in this study. However, in contrast to the situation with ASD, the increased risk of ADHD appeared to result from exposure to medications in utero – specifically, antidepressants and possibly immunosuppressives – rather than to maternal SLE per se, she added.
Dr. Vinet presented an analysis of the OSLER (Offspring of Systemic Lupus Erythematosus Mothers Registry) database, the world’s largest cohort of children born to mothers with SLE. OSLER includes all women with a diagnosis of SLE hospitalized for childbirth in the province of Quebec since 1989. This study included 509 women who had 719 children after they had been diagnosed with SLE, as well as 5,824 controls matched for age and year of delivery along with their 8,493 children. The mean maternal age was 30.3 years. The children were followed out to a mean age of 9.1 years.
ASD was diagnosed in 1.4% of children born to mothers with SLE, compared with 0.6% of control children. The mean age at diagnosis of ASD was noticeably lower in the offspring of mothers with SLE: 3.8 years, compared with 5.7 years in the control children.
Women with SLE had higher rates of hypertension, asthma, and diabetes than did controls at the time of delivery. They also had higher rates of obstetric complications, including preterm birth, small for gestational age, and gestational diabetes. In a multivariate analysis adjusted for these variables, maternal SLE remained independently associated with a 2.3-fold increased risk of diagnosis of an ASD in offspring.
Complete and reliable records of prescription drug use during pregnancy were available only for the roughly 20% of mothers belonging to the provincial medication public assistance program. Of the 18 cases of ASD diagnosed in the children of 1,925 mothers covered by the program, only one occurred among the 155 offspring of SLE mothers; that child had been exposed to corticosteroids in utero. Of the 17 cases of ASD diagnosed in the control group, 16 had no exposures to the medications under scrutiny, and one involved in utero exposure to an anticonvulsant.
The OSLER database doesn’t include information about maternal autoantibody levels, but Dr. Vinet and her coinvestigators have made the collection of such data a top priority in light of recent studies in animal models of SLE by other investigators. Those studies showed that SLE-related autoantibodies, including n-methyl-d-aspartate receptor antibodies and antiphospholipid antibodies, as well as interleukin-6 and other cytokines, alter fetal brain development and induce behavioral anomalies suggestive of autism, such as withdrawal, in the mouse offspring.
In addition, French investigators recently reported that 3 of 36 children born to mothers with antiphospholipid syndrome developed ASD, although Dr. Vinet noted that this small but intriguing study lacked a control group (Semin. Arthritis Rheum. 2013 Aug. 1 [doi: 10.1016/j.semarthrit.2013.07.001]).
ADHD was diagnosed in 9.9% of the children of mothers with SLE in the OSLER database, compared with 6.1% of controls. It was diagnosed when the children were older: at a mean age of 12.5 years, compared with 7.8 years in the controls. In a multivariate analysis adjusted for in utero drug exposures, the association between ADHD and maternal SLE was no longer significant. However, in utero exposure to antidepressant medication, regardless of whether or not a mother had SLE, was associated with a hefty 3.7-fold increase in ADHD in the offspring. Because of the relatively small number of mothers on antidepressant medication, Dr. Vinet is now collaborating with investigators in other Canadian provinces having more comprehensive maternal medication exposure data to take a closer look at this tentative link between in utero drug exposure and ADHD.
This study of the OSLER database was funded by the Canadian Institutes of Health Research. Dr. Vinet reported having no financial conflicts.
SAN DIEGO – The risk of autism spectrum disorders is more than doubled among children born to mothers with systemic lupus erythematosus, according to the first-ever controlled study to address the question.
That being said, women with SLE can be reassured that despite this elevated relative risk, the absolute risk that their child will be diagnosed with an autism spectrum disorder (ASD) is low – less than 1 in 50 – Dr. Evelyne Vinet said at the annual meeting of the American College of Rheumatology.
The increased risk of ASD in children born to women with SLE documented in this large study was not mediated by in utero exposure to medications for SLE, including antimalarials, immunosuppressive agents, corticosteroids, and antidepressants. Use of those drugs in pregnancy wasn’t associated with any increased risk. In light of this, further research is warranted into a highly promising alternative hypothesis: that in utero exposure to SLE-related autoantibodies, such as anti-DNA and antiphospholipid antibodies, may play a causative role, said Dr. Vinet, a rheumatologist at McGill University, Montreal.
Children born to mothers with SLE also had an increased likelihood of being diagnosed with attention-deficit/hyperactivity disorder in this study. However, in contrast to the situation with ASD, the increased risk of ADHD appeared to result from exposure to medications in utero – specifically, antidepressants and possibly immunosuppressives – rather than to maternal SLE per se, she added.
Dr. Vinet presented an analysis of the OSLER (Offspring of Systemic Lupus Erythematosus Mothers Registry) database, the world’s largest cohort of children born to mothers with SLE. OSLER includes all women with a diagnosis of SLE hospitalized for childbirth in the province of Quebec since 1989. This study included 509 women who had 719 children after they had been diagnosed with SLE, as well as 5,824 controls matched for age and year of delivery along with their 8,493 children. The mean maternal age was 30.3 years. The children were followed out to a mean age of 9.1 years.
ASD was diagnosed in 1.4% of children born to mothers with SLE, compared with 0.6% of control children. The mean age at diagnosis of ASD was noticeably lower in the offspring of mothers with SLE: 3.8 years, compared with 5.7 years in the control children.
Women with SLE had higher rates of hypertension, asthma, and diabetes than did controls at the time of delivery. They also had higher rates of obstetric complications, including preterm birth, small for gestational age, and gestational diabetes. In a multivariate analysis adjusted for these variables, maternal SLE remained independently associated with a 2.3-fold increased risk of diagnosis of an ASD in offspring.
Complete and reliable records of prescription drug use during pregnancy were available only for the roughly 20% of mothers belonging to the provincial medication public assistance program. Of the 18 cases of ASD diagnosed in the children of 1,925 mothers covered by the program, only one occurred among the 155 offspring of SLE mothers; that child had been exposed to corticosteroids in utero. Of the 17 cases of ASD diagnosed in the control group, 16 had no exposures to the medications under scrutiny, and one involved in utero exposure to an anticonvulsant.
The OSLER database doesn’t include information about maternal autoantibody levels, but Dr. Vinet and her coinvestigators have made the collection of such data a top priority in light of recent studies in animal models of SLE by other investigators. Those studies showed that SLE-related autoantibodies, including n-methyl-d-aspartate receptor antibodies and antiphospholipid antibodies, as well as interleukin-6 and other cytokines, alter fetal brain development and induce behavioral anomalies suggestive of autism, such as withdrawal, in the mouse offspring.
In addition, French investigators recently reported that 3 of 36 children born to mothers with antiphospholipid syndrome developed ASD, although Dr. Vinet noted that this small but intriguing study lacked a control group (Semin. Arthritis Rheum. 2013 Aug. 1 [doi: 10.1016/j.semarthrit.2013.07.001]).
ADHD was diagnosed in 9.9% of the children of mothers with SLE in the OSLER database, compared with 6.1% of controls. It was diagnosed when the children were older: at a mean age of 12.5 years, compared with 7.8 years in the controls. In a multivariate analysis adjusted for in utero drug exposures, the association between ADHD and maternal SLE was no longer significant. However, in utero exposure to antidepressant medication, regardless of whether or not a mother had SLE, was associated with a hefty 3.7-fold increase in ADHD in the offspring. Because of the relatively small number of mothers on antidepressant medication, Dr. Vinet is now collaborating with investigators in other Canadian provinces having more comprehensive maternal medication exposure data to take a closer look at this tentative link between in utero drug exposure and ADHD.
This study of the OSLER database was funded by the Canadian Institutes of Health Research. Dr. Vinet reported having no financial conflicts.
SAN DIEGO – The risk of autism spectrum disorders is more than doubled among children born to mothers with systemic lupus erythematosus, according to the first-ever controlled study to address the question.
That being said, women with SLE can be reassured that despite this elevated relative risk, the absolute risk that their child will be diagnosed with an autism spectrum disorder (ASD) is low – less than 1 in 50 – Dr. Evelyne Vinet said at the annual meeting of the American College of Rheumatology.
The increased risk of ASD in children born to women with SLE documented in this large study was not mediated by in utero exposure to medications for SLE, including antimalarials, immunosuppressive agents, corticosteroids, and antidepressants. Use of those drugs in pregnancy wasn’t associated with any increased risk. In light of this, further research is warranted into a highly promising alternative hypothesis: that in utero exposure to SLE-related autoantibodies, such as anti-DNA and antiphospholipid antibodies, may play a causative role, said Dr. Vinet, a rheumatologist at McGill University, Montreal.
Children born to mothers with SLE also had an increased likelihood of being diagnosed with attention-deficit/hyperactivity disorder in this study. However, in contrast to the situation with ASD, the increased risk of ADHD appeared to result from exposure to medications in utero – specifically, antidepressants and possibly immunosuppressives – rather than to maternal SLE per se, she added.
Dr. Vinet presented an analysis of the OSLER (Offspring of Systemic Lupus Erythematosus Mothers Registry) database, the world’s largest cohort of children born to mothers with SLE. OSLER includes all women with a diagnosis of SLE hospitalized for childbirth in the province of Quebec since 1989. This study included 509 women who had 719 children after they had been diagnosed with SLE, as well as 5,824 controls matched for age and year of delivery along with their 8,493 children. The mean maternal age was 30.3 years. The children were followed out to a mean age of 9.1 years.
ASD was diagnosed in 1.4% of children born to mothers with SLE, compared with 0.6% of control children. The mean age at diagnosis of ASD was noticeably lower in the offspring of mothers with SLE: 3.8 years, compared with 5.7 years in the control children.
Women with SLE had higher rates of hypertension, asthma, and diabetes than did controls at the time of delivery. They also had higher rates of obstetric complications, including preterm birth, small for gestational age, and gestational diabetes. In a multivariate analysis adjusted for these variables, maternal SLE remained independently associated with a 2.3-fold increased risk of diagnosis of an ASD in offspring.
Complete and reliable records of prescription drug use during pregnancy were available only for the roughly 20% of mothers belonging to the provincial medication public assistance program. Of the 18 cases of ASD diagnosed in the children of 1,925 mothers covered by the program, only one occurred among the 155 offspring of SLE mothers; that child had been exposed to corticosteroids in utero. Of the 17 cases of ASD diagnosed in the control group, 16 had no exposures to the medications under scrutiny, and one involved in utero exposure to an anticonvulsant.
The OSLER database doesn’t include information about maternal autoantibody levels, but Dr. Vinet and her coinvestigators have made the collection of such data a top priority in light of recent studies in animal models of SLE by other investigators. Those studies showed that SLE-related autoantibodies, including n-methyl-d-aspartate receptor antibodies and antiphospholipid antibodies, as well as interleukin-6 and other cytokines, alter fetal brain development and induce behavioral anomalies suggestive of autism, such as withdrawal, in the mouse offspring.
In addition, French investigators recently reported that 3 of 36 children born to mothers with antiphospholipid syndrome developed ASD, although Dr. Vinet noted that this small but intriguing study lacked a control group (Semin. Arthritis Rheum. 2013 Aug. 1 [doi: 10.1016/j.semarthrit.2013.07.001]).
ADHD was diagnosed in 9.9% of the children of mothers with SLE in the OSLER database, compared with 6.1% of controls. It was diagnosed when the children were older: at a mean age of 12.5 years, compared with 7.8 years in the controls. In a multivariate analysis adjusted for in utero drug exposures, the association between ADHD and maternal SLE was no longer significant. However, in utero exposure to antidepressant medication, regardless of whether or not a mother had SLE, was associated with a hefty 3.7-fold increase in ADHD in the offspring. Because of the relatively small number of mothers on antidepressant medication, Dr. Vinet is now collaborating with investigators in other Canadian provinces having more comprehensive maternal medication exposure data to take a closer look at this tentative link between in utero drug exposure and ADHD.
This study of the OSLER database was funded by the Canadian Institutes of Health Research. Dr. Vinet reported having no financial conflicts.
AT THE ACR ANNUAL MEETING
Major finding: Children born to mothers with systemic lupus erythematosus had an adjusted 2.3-fold increased risk of being diagnosed with an autism spectrum disorder, compared with controls.
Data source: This study from the world’s largest cohort of children born to mothers with SLE included 509 affected mothers and their 719 children, as well as 5,824 matched control mothers and their 8,493 children.
Disclosures: This study of the OSLER database was funded by the Canadian Institutes of Health Research. Dr. Vinet reported having no financial conflicts.
Remission reinduction with rituximab a possibility for ANCA-associated vasculitis
SAN DIEGO – Retreatment of granulomatosis with polyangiitis or microscopic polyangiitis with rituximab may be safe and effective in reinducing remission, a prospective trial has shown.
"The vast majority of patients with ANCA [antineutrophil cytoplasmic antibody]–associated vasculitis are able to achieve disease remission initially," Dr. Eli Miloslavsky said at the annual meeting of the American College of Rheumatology. "However, there’s a high rate of flare, as frequent as 55% over the first 3 years. Therefore, it’s critical to determine the best remission agent in relapsing disease."
Dr. Miloslavsky presented data from 17 patients in the RAVE (Rituximab in ANCA–Associated Vasculitis) trial who received two courses of rituximab (RTX) and were followed for an average of 301 days. Patients with a severe flare were eligible to receive an open-label course of RTX between 6 and 18 months (375 mg/m2 once a week for 4 weeks). Severe flare was defined as having a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of greater than 3 or one major BVAS/WG item. Outcomes were complete remission (no disease activity and being off of steroids), complete response (no disease activity and taking 10 g of prednisone or less), remission (no disease activity regardless of the prednisone dose), limited flare (BVAS/WG of 3 or less), and severe flare (BVAS/WG of greater than 3 or one major disease activity item). At baseline, 82% of patients who received two courses of rituximab were proteinase 3 positive and 88% had granulomatosis with polyangiitis as the clinical diagnosis.
Of the 17 patients, 11 (65%) had relapsing disease at study entry. After receiving a second course of RTX, 15 patients (88%) achieved remission in an average of 2 months, 12 (71%) had at least a complete response in an average of 5 months, and 8 (47%) reached complete remission in an average of 6 months, reported Dr. Miloslavsky of the department of rheumatology at Massachusetts General Hospital, Boston.
At the 12-month time point, 13 patients (76%) had achieved complete responses and 8 (47%) had reached complete remission.
Four flares occurred during the study. "They were all limited and the time to flare was approximately 8 months after receiving RTX," he said.
Three severe adverse events occurred, including one death (a patient with diffuse alveolar hemorrhage who did not improve and died 7 weeks after the initial flare), one case of metastatic colon cancer, and one case of severe sinusitis.
Dr. Miloslavsky acknowledged certain limitations of the study, including the small sample size, the lack of a comparison group, the lack of long-term follow-up, and the limited generalizability to myeloperoxidase-ANCA–positive patients.
The trial was funded by the Immune Tolerance Network, which is supported by the National Institute of Allergy and Infectious Diseases. Partial funding was also derived from Genentech and Biogen Idec.
SAN DIEGO – Retreatment of granulomatosis with polyangiitis or microscopic polyangiitis with rituximab may be safe and effective in reinducing remission, a prospective trial has shown.
"The vast majority of patients with ANCA [antineutrophil cytoplasmic antibody]–associated vasculitis are able to achieve disease remission initially," Dr. Eli Miloslavsky said at the annual meeting of the American College of Rheumatology. "However, there’s a high rate of flare, as frequent as 55% over the first 3 years. Therefore, it’s critical to determine the best remission agent in relapsing disease."
Dr. Miloslavsky presented data from 17 patients in the RAVE (Rituximab in ANCA–Associated Vasculitis) trial who received two courses of rituximab (RTX) and were followed for an average of 301 days. Patients with a severe flare were eligible to receive an open-label course of RTX between 6 and 18 months (375 mg/m2 once a week for 4 weeks). Severe flare was defined as having a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of greater than 3 or one major BVAS/WG item. Outcomes were complete remission (no disease activity and being off of steroids), complete response (no disease activity and taking 10 g of prednisone or less), remission (no disease activity regardless of the prednisone dose), limited flare (BVAS/WG of 3 or less), and severe flare (BVAS/WG of greater than 3 or one major disease activity item). At baseline, 82% of patients who received two courses of rituximab were proteinase 3 positive and 88% had granulomatosis with polyangiitis as the clinical diagnosis.
Of the 17 patients, 11 (65%) had relapsing disease at study entry. After receiving a second course of RTX, 15 patients (88%) achieved remission in an average of 2 months, 12 (71%) had at least a complete response in an average of 5 months, and 8 (47%) reached complete remission in an average of 6 months, reported Dr. Miloslavsky of the department of rheumatology at Massachusetts General Hospital, Boston.
At the 12-month time point, 13 patients (76%) had achieved complete responses and 8 (47%) had reached complete remission.
Four flares occurred during the study. "They were all limited and the time to flare was approximately 8 months after receiving RTX," he said.
Three severe adverse events occurred, including one death (a patient with diffuse alveolar hemorrhage who did not improve and died 7 weeks after the initial flare), one case of metastatic colon cancer, and one case of severe sinusitis.
Dr. Miloslavsky acknowledged certain limitations of the study, including the small sample size, the lack of a comparison group, the lack of long-term follow-up, and the limited generalizability to myeloperoxidase-ANCA–positive patients.
The trial was funded by the Immune Tolerance Network, which is supported by the National Institute of Allergy and Infectious Diseases. Partial funding was also derived from Genentech and Biogen Idec.
SAN DIEGO – Retreatment of granulomatosis with polyangiitis or microscopic polyangiitis with rituximab may be safe and effective in reinducing remission, a prospective trial has shown.
"The vast majority of patients with ANCA [antineutrophil cytoplasmic antibody]–associated vasculitis are able to achieve disease remission initially," Dr. Eli Miloslavsky said at the annual meeting of the American College of Rheumatology. "However, there’s a high rate of flare, as frequent as 55% over the first 3 years. Therefore, it’s critical to determine the best remission agent in relapsing disease."
Dr. Miloslavsky presented data from 17 patients in the RAVE (Rituximab in ANCA–Associated Vasculitis) trial who received two courses of rituximab (RTX) and were followed for an average of 301 days. Patients with a severe flare were eligible to receive an open-label course of RTX between 6 and 18 months (375 mg/m2 once a week for 4 weeks). Severe flare was defined as having a Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG) of greater than 3 or one major BVAS/WG item. Outcomes were complete remission (no disease activity and being off of steroids), complete response (no disease activity and taking 10 g of prednisone or less), remission (no disease activity regardless of the prednisone dose), limited flare (BVAS/WG of 3 or less), and severe flare (BVAS/WG of greater than 3 or one major disease activity item). At baseline, 82% of patients who received two courses of rituximab were proteinase 3 positive and 88% had granulomatosis with polyangiitis as the clinical diagnosis.
Of the 17 patients, 11 (65%) had relapsing disease at study entry. After receiving a second course of RTX, 15 patients (88%) achieved remission in an average of 2 months, 12 (71%) had at least a complete response in an average of 5 months, and 8 (47%) reached complete remission in an average of 6 months, reported Dr. Miloslavsky of the department of rheumatology at Massachusetts General Hospital, Boston.
At the 12-month time point, 13 patients (76%) had achieved complete responses and 8 (47%) had reached complete remission.
Four flares occurred during the study. "They were all limited and the time to flare was approximately 8 months after receiving RTX," he said.
Three severe adverse events occurred, including one death (a patient with diffuse alveolar hemorrhage who did not improve and died 7 weeks after the initial flare), one case of metastatic colon cancer, and one case of severe sinusitis.
Dr. Miloslavsky acknowledged certain limitations of the study, including the small sample size, the lack of a comparison group, the lack of long-term follow-up, and the limited generalizability to myeloperoxidase-ANCA–positive patients.
The trial was funded by the Immune Tolerance Network, which is supported by the National Institute of Allergy and Infectious Diseases. Partial funding was also derived from Genentech and Biogen Idec.
AT THE ACR ANNUAL MEETING
Major finding: Six months after receiving a second course of rituximab, 47% of patients achieved complete remission.
Data source: A study of17 patients in the rituximab in ANCA-associated vasculitis trial who received two courses of rituximab and were followed for an average of 301 days.
Disclosures: The trial was funded by the Immune Tolerance Network, which is supported by the National Institute of Allergy and Infectious Diseases. Partial funding was also derived from Genentech and Biogen Idec.
Antimalarials prove protective against long-term lupus damage
SAN DIEGO – Potentially modifiable risk factors for future irreversible organ damage in lupus patients include hypertension, the use of corticosteroids, and higher levels of inflammation early on, according to findings from the SLICC (Systemic Lupus International Collaborating Clinics) Inception Cohort Study.
In addition, the study identified the use of antimalarial drugs as the one significant protective factor against steady accrual of irreversible organ damage in lupus patients.
"These findings help us pave the way to consider whether, firstly, one could use damage as a primary endpoint in future clinical trials in lupus – somewhat akin to how the erosion score is used in rheumatoid arthritis – and secondly, the results suggest particular interventions that might be important in reducing the risk of damage over time," Dr. Ian N. Bruce said at the annual meeting of the American College of Rheumatology.
The study also identified several fixed and unmodifiable risk factors for irreversible damage in lupus patients: older age at diagnosis, male gender, and being black or white Americans, added Dr. Bruce, professor of rheumatology at the University of Manchester (U.K.) and chair of the SLICC research group.
The SLICC Inception Cohort Study involves 1,722 patients at 31 centers in 11 countries in North America, Europe, and Asia who enrolled within 15 months after being formally diagnosed with systemic lupus erythematosus based upon the 1997 ACR criteria. They averaged 35 years of age and had an average of 4.25 comprehensive annual follow-up visits during the study period.
Irreversible organ damage was assessed using the SLICC/ACR Damage Index, or SDI. At baseline, 35% of patients had at least one item of damage as indicated by an SDI score of 1 or more. Over time, damage rates slowly and steadily increased such that by 6 years of follow-up 51% of participants had an SDI of at least 1.
In a multivariate analysis, patients with an SDI score of 1 at baseline had a highly significant 37% reduction in the risk of increasing their score during follow-up if they were taking antimalarials, compared with those not taking antimalarials.
On the other hand, patients with a baseline SDI of 1 were 61% more likely to experience an increase in their damage score during follow-up if they had hypertension and 43% more likely to do so if they were on corticosteroids than if they weren’t. Moreover, their risk of going from an SDI of 1 to a higher SDI indicative of mounting damage increased by 10% for every 3-point increase on the SLE Disease Activity Index (SLEDAI).
Patients with a baseline SDI of 0 were 64% more likely to progress to a score of 1 or more during follow-up if they were taking corticosteroids and 71% more likely to do so if they were hypertensive. Their risk also increased by 17% for each 3-point increase in SLEDAI. Men had a 48% greater risk of going from an SDI of 0 to 1 or more than women. Asians were 40% less likely to develop irreversible damage.
Each 1-point increase in SDI score was associated with a 46% increased risk of mortality, as well as with poorer health-related quality of life, especially as reflected in SF-36 physical component scores.
Session chair Dr. Roberto Caricchio of Temple University, Philadelphia, called the SLICC study "very important work."
"It teaches us to be aggressive up-front with our lupus patients, which we often aren’t. We tend to spare ourselves because it’s a chronic disease, and we know we’ll see these patients for the next 20 years, so we try to spare them from certain therapies," said Dr. Caricchio.
Dr. Bruce concurred. "I think a concerted effort to switch the disease off in almost a treat-to-target way, getting people into remission, may well be very important with regard to avoiding long-term damage. If we could do that without using steroids, that would be ideal," he commented.
"SLICC is interested in the fact that most clinical trials in lupus to date have taken a very small subsection of the population, those with high disease activity, and used a particular biologic agent or new molecule to show that it improved disease activity. But actually the majority of people with lupus – around 60% have low-grade, grumbling disease and are on low-dose steroids. And those are the ones who accumulate damage. I think we need to have a paradigm shift in how we do clinical trials in lupus and think about doing lupus trials against a damage endpoint," the rheumatologist continued.
Power calculations based upon the SLICC Inception Cohort Study suggest such trials could be relatively modest in size, he added.
SLICC receives financial support from GlaxoSmithKline, Bristol-Myers Squibb, and Human Genome Sciences. Dr. Bruce reported receiving research funding from GlaxoSmithKline, Bristol-Myers Squibb, Roche, and UCB.
SAN DIEGO – Potentially modifiable risk factors for future irreversible organ damage in lupus patients include hypertension, the use of corticosteroids, and higher levels of inflammation early on, according to findings from the SLICC (Systemic Lupus International Collaborating Clinics) Inception Cohort Study.
In addition, the study identified the use of antimalarial drugs as the one significant protective factor against steady accrual of irreversible organ damage in lupus patients.
"These findings help us pave the way to consider whether, firstly, one could use damage as a primary endpoint in future clinical trials in lupus – somewhat akin to how the erosion score is used in rheumatoid arthritis – and secondly, the results suggest particular interventions that might be important in reducing the risk of damage over time," Dr. Ian N. Bruce said at the annual meeting of the American College of Rheumatology.
The study also identified several fixed and unmodifiable risk factors for irreversible damage in lupus patients: older age at diagnosis, male gender, and being black or white Americans, added Dr. Bruce, professor of rheumatology at the University of Manchester (U.K.) and chair of the SLICC research group.
The SLICC Inception Cohort Study involves 1,722 patients at 31 centers in 11 countries in North America, Europe, and Asia who enrolled within 15 months after being formally diagnosed with systemic lupus erythematosus based upon the 1997 ACR criteria. They averaged 35 years of age and had an average of 4.25 comprehensive annual follow-up visits during the study period.
Irreversible organ damage was assessed using the SLICC/ACR Damage Index, or SDI. At baseline, 35% of patients had at least one item of damage as indicated by an SDI score of 1 or more. Over time, damage rates slowly and steadily increased such that by 6 years of follow-up 51% of participants had an SDI of at least 1.
In a multivariate analysis, patients with an SDI score of 1 at baseline had a highly significant 37% reduction in the risk of increasing their score during follow-up if they were taking antimalarials, compared with those not taking antimalarials.
On the other hand, patients with a baseline SDI of 1 were 61% more likely to experience an increase in their damage score during follow-up if they had hypertension and 43% more likely to do so if they were on corticosteroids than if they weren’t. Moreover, their risk of going from an SDI of 1 to a higher SDI indicative of mounting damage increased by 10% for every 3-point increase on the SLE Disease Activity Index (SLEDAI).
Patients with a baseline SDI of 0 were 64% more likely to progress to a score of 1 or more during follow-up if they were taking corticosteroids and 71% more likely to do so if they were hypertensive. Their risk also increased by 17% for each 3-point increase in SLEDAI. Men had a 48% greater risk of going from an SDI of 0 to 1 or more than women. Asians were 40% less likely to develop irreversible damage.
Each 1-point increase in SDI score was associated with a 46% increased risk of mortality, as well as with poorer health-related quality of life, especially as reflected in SF-36 physical component scores.
Session chair Dr. Roberto Caricchio of Temple University, Philadelphia, called the SLICC study "very important work."
"It teaches us to be aggressive up-front with our lupus patients, which we often aren’t. We tend to spare ourselves because it’s a chronic disease, and we know we’ll see these patients for the next 20 years, so we try to spare them from certain therapies," said Dr. Caricchio.
Dr. Bruce concurred. "I think a concerted effort to switch the disease off in almost a treat-to-target way, getting people into remission, may well be very important with regard to avoiding long-term damage. If we could do that without using steroids, that would be ideal," he commented.
"SLICC is interested in the fact that most clinical trials in lupus to date have taken a very small subsection of the population, those with high disease activity, and used a particular biologic agent or new molecule to show that it improved disease activity. But actually the majority of people with lupus – around 60% have low-grade, grumbling disease and are on low-dose steroids. And those are the ones who accumulate damage. I think we need to have a paradigm shift in how we do clinical trials in lupus and think about doing lupus trials against a damage endpoint," the rheumatologist continued.
Power calculations based upon the SLICC Inception Cohort Study suggest such trials could be relatively modest in size, he added.
SLICC receives financial support from GlaxoSmithKline, Bristol-Myers Squibb, and Human Genome Sciences. Dr. Bruce reported receiving research funding from GlaxoSmithKline, Bristol-Myers Squibb, Roche, and UCB.
SAN DIEGO – Potentially modifiable risk factors for future irreversible organ damage in lupus patients include hypertension, the use of corticosteroids, and higher levels of inflammation early on, according to findings from the SLICC (Systemic Lupus International Collaborating Clinics) Inception Cohort Study.
In addition, the study identified the use of antimalarial drugs as the one significant protective factor against steady accrual of irreversible organ damage in lupus patients.
"These findings help us pave the way to consider whether, firstly, one could use damage as a primary endpoint in future clinical trials in lupus – somewhat akin to how the erosion score is used in rheumatoid arthritis – and secondly, the results suggest particular interventions that might be important in reducing the risk of damage over time," Dr. Ian N. Bruce said at the annual meeting of the American College of Rheumatology.
The study also identified several fixed and unmodifiable risk factors for irreversible damage in lupus patients: older age at diagnosis, male gender, and being black or white Americans, added Dr. Bruce, professor of rheumatology at the University of Manchester (U.K.) and chair of the SLICC research group.
The SLICC Inception Cohort Study involves 1,722 patients at 31 centers in 11 countries in North America, Europe, and Asia who enrolled within 15 months after being formally diagnosed with systemic lupus erythematosus based upon the 1997 ACR criteria. They averaged 35 years of age and had an average of 4.25 comprehensive annual follow-up visits during the study period.
Irreversible organ damage was assessed using the SLICC/ACR Damage Index, or SDI. At baseline, 35% of patients had at least one item of damage as indicated by an SDI score of 1 or more. Over time, damage rates slowly and steadily increased such that by 6 years of follow-up 51% of participants had an SDI of at least 1.
In a multivariate analysis, patients with an SDI score of 1 at baseline had a highly significant 37% reduction in the risk of increasing their score during follow-up if they were taking antimalarials, compared with those not taking antimalarials.
On the other hand, patients with a baseline SDI of 1 were 61% more likely to experience an increase in their damage score during follow-up if they had hypertension and 43% more likely to do so if they were on corticosteroids than if they weren’t. Moreover, their risk of going from an SDI of 1 to a higher SDI indicative of mounting damage increased by 10% for every 3-point increase on the SLE Disease Activity Index (SLEDAI).
Patients with a baseline SDI of 0 were 64% more likely to progress to a score of 1 or more during follow-up if they were taking corticosteroids and 71% more likely to do so if they were hypertensive. Their risk also increased by 17% for each 3-point increase in SLEDAI. Men had a 48% greater risk of going from an SDI of 0 to 1 or more than women. Asians were 40% less likely to develop irreversible damage.
Each 1-point increase in SDI score was associated with a 46% increased risk of mortality, as well as with poorer health-related quality of life, especially as reflected in SF-36 physical component scores.
Session chair Dr. Roberto Caricchio of Temple University, Philadelphia, called the SLICC study "very important work."
"It teaches us to be aggressive up-front with our lupus patients, which we often aren’t. We tend to spare ourselves because it’s a chronic disease, and we know we’ll see these patients for the next 20 years, so we try to spare them from certain therapies," said Dr. Caricchio.
Dr. Bruce concurred. "I think a concerted effort to switch the disease off in almost a treat-to-target way, getting people into remission, may well be very important with regard to avoiding long-term damage. If we could do that without using steroids, that would be ideal," he commented.
"SLICC is interested in the fact that most clinical trials in lupus to date have taken a very small subsection of the population, those with high disease activity, and used a particular biologic agent or new molecule to show that it improved disease activity. But actually the majority of people with lupus – around 60% have low-grade, grumbling disease and are on low-dose steroids. And those are the ones who accumulate damage. I think we need to have a paradigm shift in how we do clinical trials in lupus and think about doing lupus trials against a damage endpoint," the rheumatologist continued.
Power calculations based upon the SLICC Inception Cohort Study suggest such trials could be relatively modest in size, he added.
SLICC receives financial support from GlaxoSmithKline, Bristol-Myers Squibb, and Human Genome Sciences. Dr. Bruce reported receiving research funding from GlaxoSmithKline, Bristol-Myers Squibb, Roche, and UCB.
AT THE ACR ANNUAL MEETING
Major finding: Patients with recently diagnosed SLE and no significant organ damage at baseline were 71% more likely to develop irreversible organ damage during follow-up if they were hypertensive and 64% more likely to do so if they were taking corticosteroids. Antimalarial drugs showed a protective effect against accrual of damage.
Data source: The Systemic Lupus International Collaborating Clinics Inception Cohort Study involves 1,722 patients at 31 centers in 11 countries, all recruited within 15 months after diagnosis.
Disclosures: The study group receives funding from GlaxoSmithKline, Bristol-Myers Squibb, and Human Genome Sciences. The presenter receives research grants from GlaxoSmithKline, Bristol-Myers Squibb, and several other pharmaceutical companies.
Depression accounts for psoriatics’ increased MI risk
SAN DIEGO – Depression is an independent risk factor for acute myocardial infarction in patients with psoriasis or psoriatic arthritis, a large population-based cohort study indicates.
In this study of more than 10,000 British Columbians with psoriasis and/or psoriatic arthritis, the increased risk of MI was confined to the patient subset having comorbid depression, Lindsay C. Burns, Ph.D., reported at the annual meeting of the American College of Rheumatology.
"These data underscore the need to actively screen for depression among psoriasis and psoriatic arthritis patients and closely monitor cardiovascular health in this high-risk group to improve long-term survival," declared Dr. Burns of the University of British Columbia, Vancouver.
She and her coinvestigators mined the comprehensive health records available for 4.1 million adults through British Columbia’s universal medical insurance coverage system in order to identify all 10,041 patients who were diagnosed with psoriasis by a dermatologist or psoriatic arthritis by a rheumatologist during 1996-2006, and who at that time had no history of MI. The patients were matched by age, gender, and years of follow-up with 47,415 controls.
Acute MI occurred in 268 patients with psoriasis or psoriatic arthritis, for an incidence rate of 5.8 cases per 1,000 person-years. The incidence rate of physician-diagnosed depression was 3.4 per 1,000 person-years in the psoriatic group, with a 10-year prevalence of 21.6%.
In a multivariate regression analysis adjusted for comorbid conditions, socioeconomic status, health resource utilization, age, and gender, individuals with psoriasis or psoriatic arthritis were 26% more likely to be depressed than controls. Diagnosis of depression in psoriatic patients during the follow-up period increased their risk of having an MI by an adjusted 80% compared with psoriatic patients without the psychiatric diagnosis.
Psoriatic subjects without depression had a statistically nonsignificant 10% increased risk of MI compared with nonpsoriatic controls. In contrast, psoriatic patients with diagnosed depression had a 60% greater MI risk than controls, according to Dr. Burns.
The study was funded by the Arthritis Research Center of Canada. Dr. Burns reported having no financial conflicts of interest.
SAN DIEGO – Depression is an independent risk factor for acute myocardial infarction in patients with psoriasis or psoriatic arthritis, a large population-based cohort study indicates.
In this study of more than 10,000 British Columbians with psoriasis and/or psoriatic arthritis, the increased risk of MI was confined to the patient subset having comorbid depression, Lindsay C. Burns, Ph.D., reported at the annual meeting of the American College of Rheumatology.
"These data underscore the need to actively screen for depression among psoriasis and psoriatic arthritis patients and closely monitor cardiovascular health in this high-risk group to improve long-term survival," declared Dr. Burns of the University of British Columbia, Vancouver.
She and her coinvestigators mined the comprehensive health records available for 4.1 million adults through British Columbia’s universal medical insurance coverage system in order to identify all 10,041 patients who were diagnosed with psoriasis by a dermatologist or psoriatic arthritis by a rheumatologist during 1996-2006, and who at that time had no history of MI. The patients were matched by age, gender, and years of follow-up with 47,415 controls.
Acute MI occurred in 268 patients with psoriasis or psoriatic arthritis, for an incidence rate of 5.8 cases per 1,000 person-years. The incidence rate of physician-diagnosed depression was 3.4 per 1,000 person-years in the psoriatic group, with a 10-year prevalence of 21.6%.
In a multivariate regression analysis adjusted for comorbid conditions, socioeconomic status, health resource utilization, age, and gender, individuals with psoriasis or psoriatic arthritis were 26% more likely to be depressed than controls. Diagnosis of depression in psoriatic patients during the follow-up period increased their risk of having an MI by an adjusted 80% compared with psoriatic patients without the psychiatric diagnosis.
Psoriatic subjects without depression had a statistically nonsignificant 10% increased risk of MI compared with nonpsoriatic controls. In contrast, psoriatic patients with diagnosed depression had a 60% greater MI risk than controls, according to Dr. Burns.
The study was funded by the Arthritis Research Center of Canada. Dr. Burns reported having no financial conflicts of interest.
SAN DIEGO – Depression is an independent risk factor for acute myocardial infarction in patients with psoriasis or psoriatic arthritis, a large population-based cohort study indicates.
In this study of more than 10,000 British Columbians with psoriasis and/or psoriatic arthritis, the increased risk of MI was confined to the patient subset having comorbid depression, Lindsay C. Burns, Ph.D., reported at the annual meeting of the American College of Rheumatology.
"These data underscore the need to actively screen for depression among psoriasis and psoriatic arthritis patients and closely monitor cardiovascular health in this high-risk group to improve long-term survival," declared Dr. Burns of the University of British Columbia, Vancouver.
She and her coinvestigators mined the comprehensive health records available for 4.1 million adults through British Columbia’s universal medical insurance coverage system in order to identify all 10,041 patients who were diagnosed with psoriasis by a dermatologist or psoriatic arthritis by a rheumatologist during 1996-2006, and who at that time had no history of MI. The patients were matched by age, gender, and years of follow-up with 47,415 controls.
Acute MI occurred in 268 patients with psoriasis or psoriatic arthritis, for an incidence rate of 5.8 cases per 1,000 person-years. The incidence rate of physician-diagnosed depression was 3.4 per 1,000 person-years in the psoriatic group, with a 10-year prevalence of 21.6%.
In a multivariate regression analysis adjusted for comorbid conditions, socioeconomic status, health resource utilization, age, and gender, individuals with psoriasis or psoriatic arthritis were 26% more likely to be depressed than controls. Diagnosis of depression in psoriatic patients during the follow-up period increased their risk of having an MI by an adjusted 80% compared with psoriatic patients without the psychiatric diagnosis.
Psoriatic subjects without depression had a statistically nonsignificant 10% increased risk of MI compared with nonpsoriatic controls. In contrast, psoriatic patients with diagnosed depression had a 60% greater MI risk than controls, according to Dr. Burns.
The study was funded by the Arthritis Research Center of Canada. Dr. Burns reported having no financial conflicts of interest.
AT THE ACR ANNUAL MEETING
Major finding: Patients with psoriasis or psoriatic arthritis who developed comorbid depression had a highly significant 80% increased risk of acute MI during follow-up, compared with those without depression.
Data source: This was a population-based cohort study involving 10,041 adults diagnosed with psoriasis or psoriatic arthritis in British Columbia during 1996-2006 and more than 47,000 controls.
Disclosures: The study was funded by the Arthritis Research Center of Canada. Dr. Burns reported having no financial conflicts of interest.