User login
Bimekizumab Eases Disease Impact in bDMARD-naive, TNFi-IR Patients with PsA
Key clinical point: Bimekizumab improved disease impact in a rapid and sustained manner in patients with psoriatic arthritis (PsA) who were naive to biologic disease-modifying antirheumatic drugs (bDMARD-naive) or had prior inadequate response to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: A numerically higher proportion of bDMARD-naive patients receiving bimekizumab vs placebo achieved a clinically meaningful improvement in disease impact at week 4 (20.3% vs 2.5%) and 16 (36.8% vs 10.1%). These improvements were sustained till week 52 in patients who received bimekizumab continuously (49.0%) and in those who switched from placebo to bimekizumab (44.4%). Results were similar in the TNFi-IR subgroup.
Study details: Findings are from two phase 3 studies including 1112 patients with PsA who were bDMARD-naive or TNFi-IR and were randomly assigned to receive 160 mg bimekizumab every 4 weeks (n = 698) or placebo with crossover to bimekizumab at week 16 (n = 414).
Disclosures: This study was sponsored by UCB Pharma. Four authors declared being employees or shareholders of UCB Pharma. Other authors declared various ties with various sources, including UCB Pharma.
Source: Gossec L, Orbai AM, de Wit M, et al. Effect of bimekizumab on patient-reported disease impact in patients with psoriatic arthritis: 1-year results from two phase 3 studies. Rheumatology (Oxford). 2024 (May 16). doi: 10.1093/rheumatology/keae277 Source
Key clinical point: Bimekizumab improved disease impact in a rapid and sustained manner in patients with psoriatic arthritis (PsA) who were naive to biologic disease-modifying antirheumatic drugs (bDMARD-naive) or had prior inadequate response to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: A numerically higher proportion of bDMARD-naive patients receiving bimekizumab vs placebo achieved a clinically meaningful improvement in disease impact at week 4 (20.3% vs 2.5%) and 16 (36.8% vs 10.1%). These improvements were sustained till week 52 in patients who received bimekizumab continuously (49.0%) and in those who switched from placebo to bimekizumab (44.4%). Results were similar in the TNFi-IR subgroup.
Study details: Findings are from two phase 3 studies including 1112 patients with PsA who were bDMARD-naive or TNFi-IR and were randomly assigned to receive 160 mg bimekizumab every 4 weeks (n = 698) or placebo with crossover to bimekizumab at week 16 (n = 414).
Disclosures: This study was sponsored by UCB Pharma. Four authors declared being employees or shareholders of UCB Pharma. Other authors declared various ties with various sources, including UCB Pharma.
Source: Gossec L, Orbai AM, de Wit M, et al. Effect of bimekizumab on patient-reported disease impact in patients with psoriatic arthritis: 1-year results from two phase 3 studies. Rheumatology (Oxford). 2024 (May 16). doi: 10.1093/rheumatology/keae277 Source
Key clinical point: Bimekizumab improved disease impact in a rapid and sustained manner in patients with psoriatic arthritis (PsA) who were naive to biologic disease-modifying antirheumatic drugs (bDMARD-naive) or had prior inadequate response to tumor necrosis factor inhibitors (TNFi-IR).
Major finding: A numerically higher proportion of bDMARD-naive patients receiving bimekizumab vs placebo achieved a clinically meaningful improvement in disease impact at week 4 (20.3% vs 2.5%) and 16 (36.8% vs 10.1%). These improvements were sustained till week 52 in patients who received bimekizumab continuously (49.0%) and in those who switched from placebo to bimekizumab (44.4%). Results were similar in the TNFi-IR subgroup.
Study details: Findings are from two phase 3 studies including 1112 patients with PsA who were bDMARD-naive or TNFi-IR and were randomly assigned to receive 160 mg bimekizumab every 4 weeks (n = 698) or placebo with crossover to bimekizumab at week 16 (n = 414).
Disclosures: This study was sponsored by UCB Pharma. Four authors declared being employees or shareholders of UCB Pharma. Other authors declared various ties with various sources, including UCB Pharma.
Source: Gossec L, Orbai AM, de Wit M, et al. Effect of bimekizumab on patient-reported disease impact in patients with psoriatic arthritis: 1-year results from two phase 3 studies. Rheumatology (Oxford). 2024 (May 16). doi: 10.1093/rheumatology/keae277 Source
Risankizumab Effective in Resolving Enthesitis and Dactylitis in PsA
Key clinical point: Risankizumab vs placebo led to higher resolution rates for enthesitis and dactylitis at 24 weeks in patients with active psoriatic arthritis (PsA), which were sustained through 52 weeks.
Major finding: At week 24, a higher proportion of risankizumab- vs placebo-treated patients achieved resolution of enthesitis (48.4% vs 34.8%; P < .001), dactylitis (68.1% vs 51.0%; P < .001), and enthesitis + dactylitis (42.2% vs 28.6%; P < .05). More than 50% of patients who continuously received risankizumab or switched from placebo to risankizumab at week 24 achieved resolution of enthesitis, dactylitis, or both.
Study details: This integrated post hoc analysis of the KEEPsAKE 1 and KEEPsAKE 2 trials included 1407 patients with PsA and previous inadequate response or intolerance to conventional synthetic or biologic disease-modifying antirheumatic drugs who received risankizumab or placebo with crossover to risankizumab at week 24.
Disclosures: This study was funded by AbbVie. Four authors declared being employees or holding stocks, stock options, or patents of AbbVie. Five authors declared ties with various sources, including AbbVie.
Source: Kwatra SG, Khattri S, Amin AZ, et al. Enthesitis and dactylitis resolution with risankizumab for active psoriatic arthritis: Integrated analysis of the randomized KEEPsAKE 1 and 2 trials. Dermatol Ther (Heidelb). 2024;14:1517-1530 (May 13). doi: 10.1007/s13555-024-01174-4 Source
Key clinical point: Risankizumab vs placebo led to higher resolution rates for enthesitis and dactylitis at 24 weeks in patients with active psoriatic arthritis (PsA), which were sustained through 52 weeks.
Major finding: At week 24, a higher proportion of risankizumab- vs placebo-treated patients achieved resolution of enthesitis (48.4% vs 34.8%; P < .001), dactylitis (68.1% vs 51.0%; P < .001), and enthesitis + dactylitis (42.2% vs 28.6%; P < .05). More than 50% of patients who continuously received risankizumab or switched from placebo to risankizumab at week 24 achieved resolution of enthesitis, dactylitis, or both.
Study details: This integrated post hoc analysis of the KEEPsAKE 1 and KEEPsAKE 2 trials included 1407 patients with PsA and previous inadequate response or intolerance to conventional synthetic or biologic disease-modifying antirheumatic drugs who received risankizumab or placebo with crossover to risankizumab at week 24.
Disclosures: This study was funded by AbbVie. Four authors declared being employees or holding stocks, stock options, or patents of AbbVie. Five authors declared ties with various sources, including AbbVie.
Source: Kwatra SG, Khattri S, Amin AZ, et al. Enthesitis and dactylitis resolution with risankizumab for active psoriatic arthritis: Integrated analysis of the randomized KEEPsAKE 1 and 2 trials. Dermatol Ther (Heidelb). 2024;14:1517-1530 (May 13). doi: 10.1007/s13555-024-01174-4 Source
Key clinical point: Risankizumab vs placebo led to higher resolution rates for enthesitis and dactylitis at 24 weeks in patients with active psoriatic arthritis (PsA), which were sustained through 52 weeks.
Major finding: At week 24, a higher proportion of risankizumab- vs placebo-treated patients achieved resolution of enthesitis (48.4% vs 34.8%; P < .001), dactylitis (68.1% vs 51.0%; P < .001), and enthesitis + dactylitis (42.2% vs 28.6%; P < .05). More than 50% of patients who continuously received risankizumab or switched from placebo to risankizumab at week 24 achieved resolution of enthesitis, dactylitis, or both.
Study details: This integrated post hoc analysis of the KEEPsAKE 1 and KEEPsAKE 2 trials included 1407 patients with PsA and previous inadequate response or intolerance to conventional synthetic or biologic disease-modifying antirheumatic drugs who received risankizumab or placebo with crossover to risankizumab at week 24.
Disclosures: This study was funded by AbbVie. Four authors declared being employees or holding stocks, stock options, or patents of AbbVie. Five authors declared ties with various sources, including AbbVie.
Source: Kwatra SG, Khattri S, Amin AZ, et al. Enthesitis and dactylitis resolution with risankizumab for active psoriatic arthritis: Integrated analysis of the randomized KEEPsAKE 1 and 2 trials. Dermatol Ther (Heidelb). 2024;14:1517-1530 (May 13). doi: 10.1007/s13555-024-01174-4 Source
Real-World Prevalence and Clinical Characteristics of Difficult-To-Treat PsA
Key clinical point: This real-world study showed that almost 1 in 6 patients with psoriatic arthritis (PsA) had potentially difficult-to-treat (D2T) disease, which was associated with extensive psoriasis, higher body mass index (BMI), and a history of inflammatory bowel disease (IBD).
Major finding: Of 467 patients, 16.5% had D2T PsA. Compared to non-D2T patients, those with D2T disease were more likely to have extensive psoriasis at diagnosis (odds ratio [OR] 5.05; P < .0001), higher BMI (OR 1.07; P = .023), and a history of IBD (OR 1.22; P = .026).
Study details: This study analyzed 467 patients with PsA from a Greek registry who had ≥6-months of disease duration, progressed on disease modifying anti-rheumatic drugs with different mechanisms of actions, and had disease activity index for PsA > 14 or were not at minimal disease activity.
Disclosures: The registry was funded by the Greek (Hellenic) Rheumatology Society. The authors declared no conflicts of interest.
Source: Vassilakis KD, Papagoras C, Fytanidis N, et al. Identification and characteristics of patients with potential difficult-to-treat Psoriatic Arthritis: Exploratory analyses of the Greek PsA registry. Rheumatology (Oxford). 2024 (May 17). doi: 10.1093/rheumatology/keae263 Source
Key clinical point: This real-world study showed that almost 1 in 6 patients with psoriatic arthritis (PsA) had potentially difficult-to-treat (D2T) disease, which was associated with extensive psoriasis, higher body mass index (BMI), and a history of inflammatory bowel disease (IBD).
Major finding: Of 467 patients, 16.5% had D2T PsA. Compared to non-D2T patients, those with D2T disease were more likely to have extensive psoriasis at diagnosis (odds ratio [OR] 5.05; P < .0001), higher BMI (OR 1.07; P = .023), and a history of IBD (OR 1.22; P = .026).
Study details: This study analyzed 467 patients with PsA from a Greek registry who had ≥6-months of disease duration, progressed on disease modifying anti-rheumatic drugs with different mechanisms of actions, and had disease activity index for PsA > 14 or were not at minimal disease activity.
Disclosures: The registry was funded by the Greek (Hellenic) Rheumatology Society. The authors declared no conflicts of interest.
Source: Vassilakis KD, Papagoras C, Fytanidis N, et al. Identification and characteristics of patients with potential difficult-to-treat Psoriatic Arthritis: Exploratory analyses of the Greek PsA registry. Rheumatology (Oxford). 2024 (May 17). doi: 10.1093/rheumatology/keae263 Source
Key clinical point: This real-world study showed that almost 1 in 6 patients with psoriatic arthritis (PsA) had potentially difficult-to-treat (D2T) disease, which was associated with extensive psoriasis, higher body mass index (BMI), and a history of inflammatory bowel disease (IBD).
Major finding: Of 467 patients, 16.5% had D2T PsA. Compared to non-D2T patients, those with D2T disease were more likely to have extensive psoriasis at diagnosis (odds ratio [OR] 5.05; P < .0001), higher BMI (OR 1.07; P = .023), and a history of IBD (OR 1.22; P = .026).
Study details: This study analyzed 467 patients with PsA from a Greek registry who had ≥6-months of disease duration, progressed on disease modifying anti-rheumatic drugs with different mechanisms of actions, and had disease activity index for PsA > 14 or were not at minimal disease activity.
Disclosures: The registry was funded by the Greek (Hellenic) Rheumatology Society. The authors declared no conflicts of interest.
Source: Vassilakis KD, Papagoras C, Fytanidis N, et al. Identification and characteristics of patients with potential difficult-to-treat Psoriatic Arthritis: Exploratory analyses of the Greek PsA registry. Rheumatology (Oxford). 2024 (May 17). doi: 10.1093/rheumatology/keae263 Source
Low Stress Resilience in Adolescence Raises Risk for Psoriatic Arthritis
Key clinical point: Low stress resilience during adolescence increased the risk of developing psoriatic arthritis (PsA) later in life in a cohort of >1.6 million men who were followed up for up to 51 years.
Major finding: Over nearly 51 years of follow-up, 9433 (0.6%) men developed first onset PsA. Low vs high stress resilience increased the risk for new-onset PsA by 23% in the overall cohort (adjusted hazard ratio [aHR] 1.23; 95% CI 1.15-1.32) and 53% in the subgroup of patients who were hospitalized due to severe PsA (aHR 1.53; 95% CI 1.32-1.77).
Study details: This prospective cohort study included 1,669,422 men from the Swedish Military Service Conscription Register, of whom 20.4%, 58.0%, and 21.5% had low, medium, and high stress resilience levels, respectively.
Disclosures: This study was supported by the Swedish Research Council for Health and other sources. One author declared receiving honoraria as consultant or speaker from various sources. Other authors declared no conflicts of interest.
Source: Laskowski M, Schiöler L, Åberg M, et al. Influence of stress resilience in adolescence on long-term risk of psoriasis and psoriatic arthritis among men: A prospective register-based cohort study in Sweden. J Eur Acad Dermatol Venereol. 2024 (May 20). doi: 10.1111/jdv.20069 Source
Key clinical point: Low stress resilience during adolescence increased the risk of developing psoriatic arthritis (PsA) later in life in a cohort of >1.6 million men who were followed up for up to 51 years.
Major finding: Over nearly 51 years of follow-up, 9433 (0.6%) men developed first onset PsA. Low vs high stress resilience increased the risk for new-onset PsA by 23% in the overall cohort (adjusted hazard ratio [aHR] 1.23; 95% CI 1.15-1.32) and 53% in the subgroup of patients who were hospitalized due to severe PsA (aHR 1.53; 95% CI 1.32-1.77).
Study details: This prospective cohort study included 1,669,422 men from the Swedish Military Service Conscription Register, of whom 20.4%, 58.0%, and 21.5% had low, medium, and high stress resilience levels, respectively.
Disclosures: This study was supported by the Swedish Research Council for Health and other sources. One author declared receiving honoraria as consultant or speaker from various sources. Other authors declared no conflicts of interest.
Source: Laskowski M, Schiöler L, Åberg M, et al. Influence of stress resilience in adolescence on long-term risk of psoriasis and psoriatic arthritis among men: A prospective register-based cohort study in Sweden. J Eur Acad Dermatol Venereol. 2024 (May 20). doi: 10.1111/jdv.20069 Source
Key clinical point: Low stress resilience during adolescence increased the risk of developing psoriatic arthritis (PsA) later in life in a cohort of >1.6 million men who were followed up for up to 51 years.
Major finding: Over nearly 51 years of follow-up, 9433 (0.6%) men developed first onset PsA. Low vs high stress resilience increased the risk for new-onset PsA by 23% in the overall cohort (adjusted hazard ratio [aHR] 1.23; 95% CI 1.15-1.32) and 53% in the subgroup of patients who were hospitalized due to severe PsA (aHR 1.53; 95% CI 1.32-1.77).
Study details: This prospective cohort study included 1,669,422 men from the Swedish Military Service Conscription Register, of whom 20.4%, 58.0%, and 21.5% had low, medium, and high stress resilience levels, respectively.
Disclosures: This study was supported by the Swedish Research Council for Health and other sources. One author declared receiving honoraria as consultant or speaker from various sources. Other authors declared no conflicts of interest.
Source: Laskowski M, Schiöler L, Åberg M, et al. Influence of stress resilience in adolescence on long-term risk of psoriasis and psoriatic arthritis among men: A prospective register-based cohort study in Sweden. J Eur Acad Dermatol Venereol. 2024 (May 20). doi: 10.1111/jdv.20069 Source
EULAR 2024 Preview: Therapeutics in Development Take Center Stage
The European Alliance of Associations for Rheumatology (EULAR) 2024 European Congress of Rheumatology annual meeting is about to take place in Vienna, Austria. From June 12 to 15, some of the world’s leading researchers and clinicians will convene to present and learn about data on some of the new and innovative treatments for people with rheumatic and musculoskeletal diseases (RMDs) as well as to discuss how to use and optimize existing approaches.
Ahead of the Congress, this news organization asked the Congress Committee’s Scientific Programme Chair Caroline Ospelt, MD, PhD, and Abstract Chair Christian Dejaco, MD, PhD, MBA, to discuss some of their highlights of this year’s meeting.
From Bench to Bedside
“For me, the beauty at EULAR is really that you have the latest on basic research, how this can be translated in clinical trials, and then the last step would be how EULAR recommends it to be used in clinical practice,” Dr. Ospelt, professor of experimental rheumatology at University Hospital Zurich, said in an interview.
“So, if you go to EULAR continuously, you can actually follow the whole story of how novelty comes into clinical practice,” she added.
In a separate interview, Dr. Dejaco, a consultant rheumatologist and associate professor at the Medical University of Graz in Austria, said: “There are several new drug trials that are going to be presented.”
One of his highlights on the use of new drugs for the treatment of giant cell arteritis will be the phase 3 SELECT-GCA trial of the Janus kinase (JAK) inhibitor upadacitinib (LBA0001).
“It’s a trial that hopefully will lead to the approval of this drug in this indication,” Dr. Dejaco said.
Late-Breaking Abstracts
Dr. Ospelt noted: “We had a lot of good late-breaking abstracts this year.”
Some of these include:
- Real-world data on the comparative effectiveness of five different classes of drugs used to treat psoriatic arthritis (PsA; LBA0002)
- The 16-week results of a phase 2b/3 study with the novel interleukin (IL)–17A inhibitor izokibep in people with PsA (LBA0005)
- Data from the COSPIRIT-JIA trial on the efficacy and safety of ixekizumab (Taltz) in juvenile idiopathic arthritis (LBA0009)
- Phase 2 data on the safety and efficacy of the CD38-targeting monoclonal antibody daratumumab in systemic lupus erythematosus (LBA0007)
- Results of the phase 2 DAHLIAS study of the anti–neonatal Fc receptor monoclonal antibody nipocalimab in people with primary Sjögren disease (LBA0010)
- Safety and immunogenicity data from a phase 1 study of an active anti–IL-6 immunotherapy in people with knee osteoarthritis (LBA0011)
The latter is “really interesting,” Dr. Ospelt said. As of now, there is no approved treatment for osteoarthritis, and there is no immunotherapy, “so this would be the first.”
But it’s not just the late-breaker abstracts to look out for. Dr. Dejaco highlighted two abstracts that will be presented during the Abstract Plenary:
- A phase 3 study of a new selective JAK1 inhibitor, SHR0302, in rheumatoid arthritis (OP0037)
- A multi-omics analysis and targeted gene-editing study in people with , which causes inflammatory and hematologic changes (OP0073)
Of the latter, he said, “this disease is still incompletely understood, and this abstract really helps to better understand the mechanisms underlying this disease.”
One to Watch: CAR T-Cell Therapy
Dr. Ospelt said that the scientific program is about 80% clinical and 20% basic science overall. However, more sessions are being held jointly because data are starting to move from the bench to bedside.
One of the basic science areas that has had “a real buzz” around it and is now producing results in the clinic is the use of chimeric antigen receptor (CAR) T cells. In one of the first, and perhaps aptly titled What Is New, or WIN, sessions of the congress, Georg Schett, MD, vice president of research at Friedrich-Alexander-Universität Erlangen-Nüremberg in Germany, will discuss the use of CAR T-cell therapy for inflammatory RMDs. There are also multiple abstract presentations on this topic.
In-depth tissue analysis and prediction of treatment response is another interesting approach, Dr. Ospelt said. “I think that’s the way to go, that we come from the blood, we go into the tissue.” A “very nice” example of this approach will be presented during the Abstract Plenary session on Wednesday, June 12, looking at how synovial tissue macrophages may be able to give information on likely treatment response in treatment-naive rheumatoid arthritis (OP0062). There are also some further findings related to the tissue biopsy–driven treatment trial R4RA that are being presented at the meeting (OP0218, OP0242, and POS0351).
EULAR Highlighted Sessions
Among the highlighted sessions on the EULAR 2024 website is one on axial involvement in PsA and spondyloarthritis (SpA).
“Axial involvement in psoriatic arthritis and peripheral involvement in axial spondyloarthritis is quite a hot topic at the moment,” Dr. Ospelt said. There are lots of questions: “How connected are they? How different are they? Do we need different treatment for axial involvement compared to peripheral involvement?”
Another EULAR highlighted session is the 75th anniversary of glucocorticoid treatment, during which Past President of EULAR and Emeritus Professor of Rheumatology Josef S. Smolen, MD, will overview the “past, present, and future” of glucocorticoids in RMDs. Consultant rheumatologist Frank Buttgereit, MD, from the German Rheumatism Research Center in Berlin, will discuss the practicalities of using these drugs in clinical practice.
Dr. Dejaco noted: “Glucocorticoids have been one of the most important treatments for a very long time, and they’re still the most important treatment for the acute treatment of systemic inflammatory diseases.”
For a long time, there was no alternative to using steroids, he added, but steroid-sparing options now exist, and there will be data presented on a new type of drug that could potentially be used to control cortisol levels in the body (OP0335).
Recommendations and More
Dr. Ospelt and Dr. Dejaco both pointed out other sessions that are likely to be very popular, such as the first and second EULAR Recommendations sessions, a session on rheumatoid arthritis prevention, as well as the many presentations and sessions on digital health and nonpharmacologic interventions such as exercise.
With over 5242 submitted abstracts, there is going to be no shortage of data being presented at EULAR 2024. Alongside the traditional abstract submission categories, this year there is a new clinical case reports category.
“We had about 578 submissions for that category,” Dr. Dejaco said. There were 3315 abstracts submitted for the clinical research category, 812 for the basic and translational research category, 283 from health professionals in rheumatology, 152 from patient groups, and 102 in the field of pediatric rheumatology.
Join in On-Site, Watch on Demand
EULAR 2024 reverts to an on-site–only meeting this year. Some of the more lighthearted yet educational elements of the program for those attending include the second edition of the EMEUNET Rheumatology Quiz and, new for this year, two escape rooms. These rooms will provide an interactive experience where small teams will have to solve rheumatologic conundrums in order to escape the room within the hour, Dr. Dejaco explained. There will also be a morning run on Friday, June 14. “It’s not a race, it’s simply to meet and run together,” Dr. Dejaco said.
But if you cannot make the congress in person, the EULAR 2024 Livestream will be broadcasting throughout the congress. Anyone registered by June 30 will have on-demand access to the recorded content from June 17 until December 31, 2024.
Abstracts for the meeting will be published as a supplement to Annals of the Rheumatic Diseases, the official journal of EULAR.
Dr. Ospelt reported no relevant financial relationships. Dr. Dejaco has received consulting/speaker fees from AbbVie, Eli Lilly, Janssen, Sparrow, Novartis, Pfizer, Roche, Galapagos, and Sanofi.
A version of this article appeared on Medscape.com.
The European Alliance of Associations for Rheumatology (EULAR) 2024 European Congress of Rheumatology annual meeting is about to take place in Vienna, Austria. From June 12 to 15, some of the world’s leading researchers and clinicians will convene to present and learn about data on some of the new and innovative treatments for people with rheumatic and musculoskeletal diseases (RMDs) as well as to discuss how to use and optimize existing approaches.
Ahead of the Congress, this news organization asked the Congress Committee’s Scientific Programme Chair Caroline Ospelt, MD, PhD, and Abstract Chair Christian Dejaco, MD, PhD, MBA, to discuss some of their highlights of this year’s meeting.
From Bench to Bedside
“For me, the beauty at EULAR is really that you have the latest on basic research, how this can be translated in clinical trials, and then the last step would be how EULAR recommends it to be used in clinical practice,” Dr. Ospelt, professor of experimental rheumatology at University Hospital Zurich, said in an interview.
“So, if you go to EULAR continuously, you can actually follow the whole story of how novelty comes into clinical practice,” she added.
In a separate interview, Dr. Dejaco, a consultant rheumatologist and associate professor at the Medical University of Graz in Austria, said: “There are several new drug trials that are going to be presented.”
One of his highlights on the use of new drugs for the treatment of giant cell arteritis will be the phase 3 SELECT-GCA trial of the Janus kinase (JAK) inhibitor upadacitinib (LBA0001).
“It’s a trial that hopefully will lead to the approval of this drug in this indication,” Dr. Dejaco said.
Late-Breaking Abstracts
Dr. Ospelt noted: “We had a lot of good late-breaking abstracts this year.”
Some of these include:
- Real-world data on the comparative effectiveness of five different classes of drugs used to treat psoriatic arthritis (PsA; LBA0002)
- The 16-week results of a phase 2b/3 study with the novel interleukin (IL)–17A inhibitor izokibep in people with PsA (LBA0005)
- Data from the COSPIRIT-JIA trial on the efficacy and safety of ixekizumab (Taltz) in juvenile idiopathic arthritis (LBA0009)
- Phase 2 data on the safety and efficacy of the CD38-targeting monoclonal antibody daratumumab in systemic lupus erythematosus (LBA0007)
- Results of the phase 2 DAHLIAS study of the anti–neonatal Fc receptor monoclonal antibody nipocalimab in people with primary Sjögren disease (LBA0010)
- Safety and immunogenicity data from a phase 1 study of an active anti–IL-6 immunotherapy in people with knee osteoarthritis (LBA0011)
The latter is “really interesting,” Dr. Ospelt said. As of now, there is no approved treatment for osteoarthritis, and there is no immunotherapy, “so this would be the first.”
But it’s not just the late-breaker abstracts to look out for. Dr. Dejaco highlighted two abstracts that will be presented during the Abstract Plenary:
- A phase 3 study of a new selective JAK1 inhibitor, SHR0302, in rheumatoid arthritis (OP0037)
- A multi-omics analysis and targeted gene-editing study in people with , which causes inflammatory and hematologic changes (OP0073)
Of the latter, he said, “this disease is still incompletely understood, and this abstract really helps to better understand the mechanisms underlying this disease.”
One to Watch: CAR T-Cell Therapy
Dr. Ospelt said that the scientific program is about 80% clinical and 20% basic science overall. However, more sessions are being held jointly because data are starting to move from the bench to bedside.
One of the basic science areas that has had “a real buzz” around it and is now producing results in the clinic is the use of chimeric antigen receptor (CAR) T cells. In one of the first, and perhaps aptly titled What Is New, or WIN, sessions of the congress, Georg Schett, MD, vice president of research at Friedrich-Alexander-Universität Erlangen-Nüremberg in Germany, will discuss the use of CAR T-cell therapy for inflammatory RMDs. There are also multiple abstract presentations on this topic.
In-depth tissue analysis and prediction of treatment response is another interesting approach, Dr. Ospelt said. “I think that’s the way to go, that we come from the blood, we go into the tissue.” A “very nice” example of this approach will be presented during the Abstract Plenary session on Wednesday, June 12, looking at how synovial tissue macrophages may be able to give information on likely treatment response in treatment-naive rheumatoid arthritis (OP0062). There are also some further findings related to the tissue biopsy–driven treatment trial R4RA that are being presented at the meeting (OP0218, OP0242, and POS0351).
EULAR Highlighted Sessions
Among the highlighted sessions on the EULAR 2024 website is one on axial involvement in PsA and spondyloarthritis (SpA).
“Axial involvement in psoriatic arthritis and peripheral involvement in axial spondyloarthritis is quite a hot topic at the moment,” Dr. Ospelt said. There are lots of questions: “How connected are they? How different are they? Do we need different treatment for axial involvement compared to peripheral involvement?”
Another EULAR highlighted session is the 75th anniversary of glucocorticoid treatment, during which Past President of EULAR and Emeritus Professor of Rheumatology Josef S. Smolen, MD, will overview the “past, present, and future” of glucocorticoids in RMDs. Consultant rheumatologist Frank Buttgereit, MD, from the German Rheumatism Research Center in Berlin, will discuss the practicalities of using these drugs in clinical practice.
Dr. Dejaco noted: “Glucocorticoids have been one of the most important treatments for a very long time, and they’re still the most important treatment for the acute treatment of systemic inflammatory diseases.”
For a long time, there was no alternative to using steroids, he added, but steroid-sparing options now exist, and there will be data presented on a new type of drug that could potentially be used to control cortisol levels in the body (OP0335).
Recommendations and More
Dr. Ospelt and Dr. Dejaco both pointed out other sessions that are likely to be very popular, such as the first and second EULAR Recommendations sessions, a session on rheumatoid arthritis prevention, as well as the many presentations and sessions on digital health and nonpharmacologic interventions such as exercise.
With over 5242 submitted abstracts, there is going to be no shortage of data being presented at EULAR 2024. Alongside the traditional abstract submission categories, this year there is a new clinical case reports category.
“We had about 578 submissions for that category,” Dr. Dejaco said. There were 3315 abstracts submitted for the clinical research category, 812 for the basic and translational research category, 283 from health professionals in rheumatology, 152 from patient groups, and 102 in the field of pediatric rheumatology.
Join in On-Site, Watch on Demand
EULAR 2024 reverts to an on-site–only meeting this year. Some of the more lighthearted yet educational elements of the program for those attending include the second edition of the EMEUNET Rheumatology Quiz and, new for this year, two escape rooms. These rooms will provide an interactive experience where small teams will have to solve rheumatologic conundrums in order to escape the room within the hour, Dr. Dejaco explained. There will also be a morning run on Friday, June 14. “It’s not a race, it’s simply to meet and run together,” Dr. Dejaco said.
But if you cannot make the congress in person, the EULAR 2024 Livestream will be broadcasting throughout the congress. Anyone registered by June 30 will have on-demand access to the recorded content from June 17 until December 31, 2024.
Abstracts for the meeting will be published as a supplement to Annals of the Rheumatic Diseases, the official journal of EULAR.
Dr. Ospelt reported no relevant financial relationships. Dr. Dejaco has received consulting/speaker fees from AbbVie, Eli Lilly, Janssen, Sparrow, Novartis, Pfizer, Roche, Galapagos, and Sanofi.
A version of this article appeared on Medscape.com.
The European Alliance of Associations for Rheumatology (EULAR) 2024 European Congress of Rheumatology annual meeting is about to take place in Vienna, Austria. From June 12 to 15, some of the world’s leading researchers and clinicians will convene to present and learn about data on some of the new and innovative treatments for people with rheumatic and musculoskeletal diseases (RMDs) as well as to discuss how to use and optimize existing approaches.
Ahead of the Congress, this news organization asked the Congress Committee’s Scientific Programme Chair Caroline Ospelt, MD, PhD, and Abstract Chair Christian Dejaco, MD, PhD, MBA, to discuss some of their highlights of this year’s meeting.
From Bench to Bedside
“For me, the beauty at EULAR is really that you have the latest on basic research, how this can be translated in clinical trials, and then the last step would be how EULAR recommends it to be used in clinical practice,” Dr. Ospelt, professor of experimental rheumatology at University Hospital Zurich, said in an interview.
“So, if you go to EULAR continuously, you can actually follow the whole story of how novelty comes into clinical practice,” she added.
In a separate interview, Dr. Dejaco, a consultant rheumatologist and associate professor at the Medical University of Graz in Austria, said: “There are several new drug trials that are going to be presented.”
One of his highlights on the use of new drugs for the treatment of giant cell arteritis will be the phase 3 SELECT-GCA trial of the Janus kinase (JAK) inhibitor upadacitinib (LBA0001).
“It’s a trial that hopefully will lead to the approval of this drug in this indication,” Dr. Dejaco said.
Late-Breaking Abstracts
Dr. Ospelt noted: “We had a lot of good late-breaking abstracts this year.”
Some of these include:
- Real-world data on the comparative effectiveness of five different classes of drugs used to treat psoriatic arthritis (PsA; LBA0002)
- The 16-week results of a phase 2b/3 study with the novel interleukin (IL)–17A inhibitor izokibep in people with PsA (LBA0005)
- Data from the COSPIRIT-JIA trial on the efficacy and safety of ixekizumab (Taltz) in juvenile idiopathic arthritis (LBA0009)
- Phase 2 data on the safety and efficacy of the CD38-targeting monoclonal antibody daratumumab in systemic lupus erythematosus (LBA0007)
- Results of the phase 2 DAHLIAS study of the anti–neonatal Fc receptor monoclonal antibody nipocalimab in people with primary Sjögren disease (LBA0010)
- Safety and immunogenicity data from a phase 1 study of an active anti–IL-6 immunotherapy in people with knee osteoarthritis (LBA0011)
The latter is “really interesting,” Dr. Ospelt said. As of now, there is no approved treatment for osteoarthritis, and there is no immunotherapy, “so this would be the first.”
But it’s not just the late-breaker abstracts to look out for. Dr. Dejaco highlighted two abstracts that will be presented during the Abstract Plenary:
- A phase 3 study of a new selective JAK1 inhibitor, SHR0302, in rheumatoid arthritis (OP0037)
- A multi-omics analysis and targeted gene-editing study in people with , which causes inflammatory and hematologic changes (OP0073)
Of the latter, he said, “this disease is still incompletely understood, and this abstract really helps to better understand the mechanisms underlying this disease.”
One to Watch: CAR T-Cell Therapy
Dr. Ospelt said that the scientific program is about 80% clinical and 20% basic science overall. However, more sessions are being held jointly because data are starting to move from the bench to bedside.
One of the basic science areas that has had “a real buzz” around it and is now producing results in the clinic is the use of chimeric antigen receptor (CAR) T cells. In one of the first, and perhaps aptly titled What Is New, or WIN, sessions of the congress, Georg Schett, MD, vice president of research at Friedrich-Alexander-Universität Erlangen-Nüremberg in Germany, will discuss the use of CAR T-cell therapy for inflammatory RMDs. There are also multiple abstract presentations on this topic.
In-depth tissue analysis and prediction of treatment response is another interesting approach, Dr. Ospelt said. “I think that’s the way to go, that we come from the blood, we go into the tissue.” A “very nice” example of this approach will be presented during the Abstract Plenary session on Wednesday, June 12, looking at how synovial tissue macrophages may be able to give information on likely treatment response in treatment-naive rheumatoid arthritis (OP0062). There are also some further findings related to the tissue biopsy–driven treatment trial R4RA that are being presented at the meeting (OP0218, OP0242, and POS0351).
EULAR Highlighted Sessions
Among the highlighted sessions on the EULAR 2024 website is one on axial involvement in PsA and spondyloarthritis (SpA).
“Axial involvement in psoriatic arthritis and peripheral involvement in axial spondyloarthritis is quite a hot topic at the moment,” Dr. Ospelt said. There are lots of questions: “How connected are they? How different are they? Do we need different treatment for axial involvement compared to peripheral involvement?”
Another EULAR highlighted session is the 75th anniversary of glucocorticoid treatment, during which Past President of EULAR and Emeritus Professor of Rheumatology Josef S. Smolen, MD, will overview the “past, present, and future” of glucocorticoids in RMDs. Consultant rheumatologist Frank Buttgereit, MD, from the German Rheumatism Research Center in Berlin, will discuss the practicalities of using these drugs in clinical practice.
Dr. Dejaco noted: “Glucocorticoids have been one of the most important treatments for a very long time, and they’re still the most important treatment for the acute treatment of systemic inflammatory diseases.”
For a long time, there was no alternative to using steroids, he added, but steroid-sparing options now exist, and there will be data presented on a new type of drug that could potentially be used to control cortisol levels in the body (OP0335).
Recommendations and More
Dr. Ospelt and Dr. Dejaco both pointed out other sessions that are likely to be very popular, such as the first and second EULAR Recommendations sessions, a session on rheumatoid arthritis prevention, as well as the many presentations and sessions on digital health and nonpharmacologic interventions such as exercise.
With over 5242 submitted abstracts, there is going to be no shortage of data being presented at EULAR 2024. Alongside the traditional abstract submission categories, this year there is a new clinical case reports category.
“We had about 578 submissions for that category,” Dr. Dejaco said. There were 3315 abstracts submitted for the clinical research category, 812 for the basic and translational research category, 283 from health professionals in rheumatology, 152 from patient groups, and 102 in the field of pediatric rheumatology.
Join in On-Site, Watch on Demand
EULAR 2024 reverts to an on-site–only meeting this year. Some of the more lighthearted yet educational elements of the program for those attending include the second edition of the EMEUNET Rheumatology Quiz and, new for this year, two escape rooms. These rooms will provide an interactive experience where small teams will have to solve rheumatologic conundrums in order to escape the room within the hour, Dr. Dejaco explained. There will also be a morning run on Friday, June 14. “It’s not a race, it’s simply to meet and run together,” Dr. Dejaco said.
But if you cannot make the congress in person, the EULAR 2024 Livestream will be broadcasting throughout the congress. Anyone registered by June 30 will have on-demand access to the recorded content from June 17 until December 31, 2024.
Abstracts for the meeting will be published as a supplement to Annals of the Rheumatic Diseases, the official journal of EULAR.
Dr. Ospelt reported no relevant financial relationships. Dr. Dejaco has received consulting/speaker fees from AbbVie, Eli Lilly, Janssen, Sparrow, Novartis, Pfizer, Roche, Galapagos, and Sanofi.
A version of this article appeared on Medscape.com.
Clear Coverage Preference for Humira Over Biosimilars Seen in Most Medicare Part D Plans
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.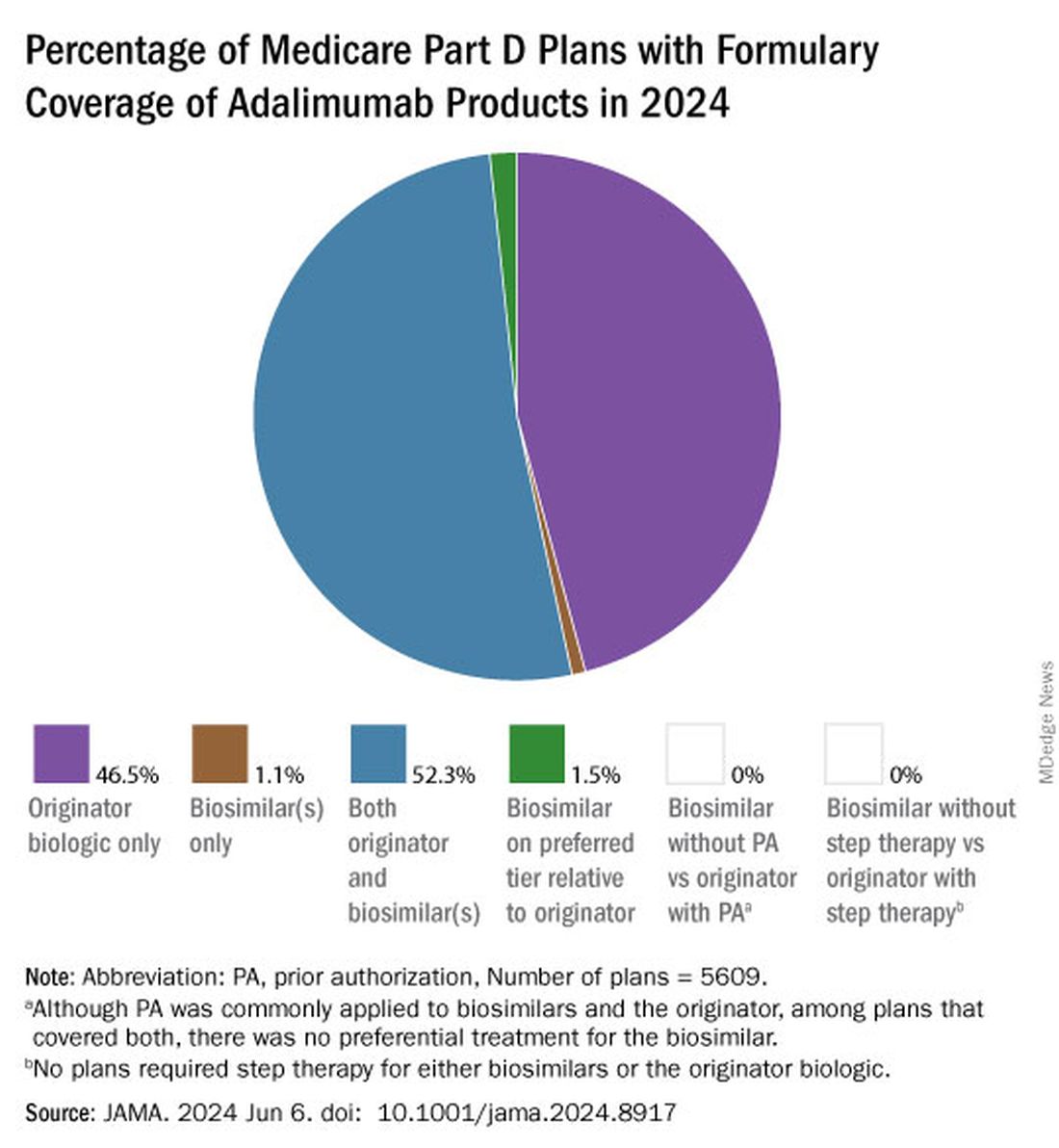
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
FROM JAMA
Over-the-Counter Arthritis Supplements Pose Adrenal Danger
BOSTON —
Patients who have been taking these supplements for prolonged periods must slowly taper off them with corticosteroid replacement, because abruptly stopping the supplement can precipitate AI, Kevin S. Wei, MD, said in a presentation of 12 cases — the largest such series to date of the phenomenon — at the annual meeting of the Endocrine Society.
The specific supplements used were Artri King in eight of the patients, Ardosons in two, and Ajo Rey in one. In April 2022, the US Food and Drug Administration issued a warning that Artri King contains diclofenac and dexamethasone not listed on the product label. In July 2023, the agency issued an expanded warning about that product and others including Ajo Rey.
The supplements are not believed to be sold in the United States, but they are available in Mexico and can be ordered online, said Dr. Wei, a second-year resident at the Keck School of Medicine at the University of California, Los Angeles.
“We found that quite a lot of patients after they’ve been on the Artri King or some other over the counter arthritis supplement, started developing these cushingoid features seen in the physical exam, such as rounded facial features or stretch marks of their abdomen,” he said.
And “when patients are abruptly taken off those supplements … sometimes this can cause them to go into signs or symptoms of adrenal insufficiency. That can occasionally be life-threatening if it’s not addressed in an inpatient setting,” Dr. Wei said.
In an interview, session moderator Sharon L. Wardlaw, MD, professor of medicine at Columbia University Irving Medical Center, New York, explained that when a person takes these drugs containing hidden glucocorticoids, “they won’t be picked up in a cortisol assay, but they’ll suppress the [adrenocorticotropic hormone] and the person’s own cortisol production. They look like they have Cushing, but when you measure their hormone levels, they’re undetectable. And then people wonder what’s going on. Well, their [hypothalamic-pituitary-adrenal] axis is suppressed.”
But if the product is suddenly stopped without cortisol replacement “If they get an infection they can die because they can’t mount a cortisol response.”
The takeaway message, she said, is “always ask patients to show you their supplements and look at them. In many cases, that’s why they work so well for pain relief because they have ingredients that people shouldn’t be taking.”
Twelve Patients Seen During 2022-2023
The 12 patients were seen during 2022-2023 at an endocrinology consult service in an urban safety net hospital. Their median age was 52 years, and one third were women. All had started using the supplements for joint pain, with a median of about 6 months of use prior to cessation.
Presenting symptoms included nausea/vomiting in 42%, fatigue in 42%, abdominal pain in 33%, and dizziness in 17%. Physical exam findings included moon facies in 66%, central adiposity in 66%, abdominal striae in 50%, dorsocervical fat pad in 33%, and bruising in 33%. Three required intensive care admission.
Cortisol testing was performed in 11 of the patients and was normal (≥ 16 mcg/dL) in just one. AI (≤ 3 mcg/dL) was found in three, while the rest had indeterminate results. Of those seven patients, subsequent cosyntropin-stimulation testing suggested AI (cortisol < 16 mcg/dL at 60 minutes post stimulation) in four patients, while the other two showed reduced but normal responses (cortisol 18.2-18.4 mcg/dL).
Ten of the 12 patients were prescribed glucocorticoid tapering replacements to avoid precipitating adrenal crisis, most commonly twice-daily hydrocortisone. Of those ten, eight continued to take the replacement steroids 1-2 years later, Dr. Wei said.
Dr. Wei and Dr. Wardlaw had no disclosures.
A version of this article appeared on Medscape.com.
BOSTON —
Patients who have been taking these supplements for prolonged periods must slowly taper off them with corticosteroid replacement, because abruptly stopping the supplement can precipitate AI, Kevin S. Wei, MD, said in a presentation of 12 cases — the largest such series to date of the phenomenon — at the annual meeting of the Endocrine Society.
The specific supplements used were Artri King in eight of the patients, Ardosons in two, and Ajo Rey in one. In April 2022, the US Food and Drug Administration issued a warning that Artri King contains diclofenac and dexamethasone not listed on the product label. In July 2023, the agency issued an expanded warning about that product and others including Ajo Rey.
The supplements are not believed to be sold in the United States, but they are available in Mexico and can be ordered online, said Dr. Wei, a second-year resident at the Keck School of Medicine at the University of California, Los Angeles.
“We found that quite a lot of patients after they’ve been on the Artri King or some other over the counter arthritis supplement, started developing these cushingoid features seen in the physical exam, such as rounded facial features or stretch marks of their abdomen,” he said.
And “when patients are abruptly taken off those supplements … sometimes this can cause them to go into signs or symptoms of adrenal insufficiency. That can occasionally be life-threatening if it’s not addressed in an inpatient setting,” Dr. Wei said.
In an interview, session moderator Sharon L. Wardlaw, MD, professor of medicine at Columbia University Irving Medical Center, New York, explained that when a person takes these drugs containing hidden glucocorticoids, “they won’t be picked up in a cortisol assay, but they’ll suppress the [adrenocorticotropic hormone] and the person’s own cortisol production. They look like they have Cushing, but when you measure their hormone levels, they’re undetectable. And then people wonder what’s going on. Well, their [hypothalamic-pituitary-adrenal] axis is suppressed.”
But if the product is suddenly stopped without cortisol replacement “If they get an infection they can die because they can’t mount a cortisol response.”
The takeaway message, she said, is “always ask patients to show you their supplements and look at them. In many cases, that’s why they work so well for pain relief because they have ingredients that people shouldn’t be taking.”
Twelve Patients Seen During 2022-2023
The 12 patients were seen during 2022-2023 at an endocrinology consult service in an urban safety net hospital. Their median age was 52 years, and one third were women. All had started using the supplements for joint pain, with a median of about 6 months of use prior to cessation.
Presenting symptoms included nausea/vomiting in 42%, fatigue in 42%, abdominal pain in 33%, and dizziness in 17%. Physical exam findings included moon facies in 66%, central adiposity in 66%, abdominal striae in 50%, dorsocervical fat pad in 33%, and bruising in 33%. Three required intensive care admission.
Cortisol testing was performed in 11 of the patients and was normal (≥ 16 mcg/dL) in just one. AI (≤ 3 mcg/dL) was found in three, while the rest had indeterminate results. Of those seven patients, subsequent cosyntropin-stimulation testing suggested AI (cortisol < 16 mcg/dL at 60 minutes post stimulation) in four patients, while the other two showed reduced but normal responses (cortisol 18.2-18.4 mcg/dL).
Ten of the 12 patients were prescribed glucocorticoid tapering replacements to avoid precipitating adrenal crisis, most commonly twice-daily hydrocortisone. Of those ten, eight continued to take the replacement steroids 1-2 years later, Dr. Wei said.
Dr. Wei and Dr. Wardlaw had no disclosures.
A version of this article appeared on Medscape.com.
BOSTON —
Patients who have been taking these supplements for prolonged periods must slowly taper off them with corticosteroid replacement, because abruptly stopping the supplement can precipitate AI, Kevin S. Wei, MD, said in a presentation of 12 cases — the largest such series to date of the phenomenon — at the annual meeting of the Endocrine Society.
The specific supplements used were Artri King in eight of the patients, Ardosons in two, and Ajo Rey in one. In April 2022, the US Food and Drug Administration issued a warning that Artri King contains diclofenac and dexamethasone not listed on the product label. In July 2023, the agency issued an expanded warning about that product and others including Ajo Rey.
The supplements are not believed to be sold in the United States, but they are available in Mexico and can be ordered online, said Dr. Wei, a second-year resident at the Keck School of Medicine at the University of California, Los Angeles.
“We found that quite a lot of patients after they’ve been on the Artri King or some other over the counter arthritis supplement, started developing these cushingoid features seen in the physical exam, such as rounded facial features or stretch marks of their abdomen,” he said.
And “when patients are abruptly taken off those supplements … sometimes this can cause them to go into signs or symptoms of adrenal insufficiency. That can occasionally be life-threatening if it’s not addressed in an inpatient setting,” Dr. Wei said.
In an interview, session moderator Sharon L. Wardlaw, MD, professor of medicine at Columbia University Irving Medical Center, New York, explained that when a person takes these drugs containing hidden glucocorticoids, “they won’t be picked up in a cortisol assay, but they’ll suppress the [adrenocorticotropic hormone] and the person’s own cortisol production. They look like they have Cushing, but when you measure their hormone levels, they’re undetectable. And then people wonder what’s going on. Well, their [hypothalamic-pituitary-adrenal] axis is suppressed.”
But if the product is suddenly stopped without cortisol replacement “If they get an infection they can die because they can’t mount a cortisol response.”
The takeaway message, she said, is “always ask patients to show you their supplements and look at them. In many cases, that’s why they work so well for pain relief because they have ingredients that people shouldn’t be taking.”
Twelve Patients Seen During 2022-2023
The 12 patients were seen during 2022-2023 at an endocrinology consult service in an urban safety net hospital. Their median age was 52 years, and one third were women. All had started using the supplements for joint pain, with a median of about 6 months of use prior to cessation.
Presenting symptoms included nausea/vomiting in 42%, fatigue in 42%, abdominal pain in 33%, and dizziness in 17%. Physical exam findings included moon facies in 66%, central adiposity in 66%, abdominal striae in 50%, dorsocervical fat pad in 33%, and bruising in 33%. Three required intensive care admission.
Cortisol testing was performed in 11 of the patients and was normal (≥ 16 mcg/dL) in just one. AI (≤ 3 mcg/dL) was found in three, while the rest had indeterminate results. Of those seven patients, subsequent cosyntropin-stimulation testing suggested AI (cortisol < 16 mcg/dL at 60 minutes post stimulation) in four patients, while the other two showed reduced but normal responses (cortisol 18.2-18.4 mcg/dL).
Ten of the 12 patients were prescribed glucocorticoid tapering replacements to avoid precipitating adrenal crisis, most commonly twice-daily hydrocortisone. Of those ten, eight continued to take the replacement steroids 1-2 years later, Dr. Wei said.
Dr. Wei and Dr. Wardlaw had no disclosures.
A version of this article appeared on Medscape.com.
Study Finds Mace Risk Remains High in Patients with Psoriasis, Dyslipidemia
Over a period of 5 years, the, even after adjusting for covariates, results from a large retrospective study showed.
“It is well-established that psoriasis is an independent risk factor for the development of MACE, with cardiometabolic risk factors being more prevalent and incident among patients with psoriasis,” the study’s first author Ana Ormaza Vera, MD, a dermatology research fellow at Eastern Virginia Medical School, Norfolk, said in an interview after the annual meeting of the Society for Investigational Dermatology, where the study was presented during a late-breaking abstract session.
Current guidelines from the joint American Academy of Dermatology/National Psoriasis Foundation and the American Academy of Cardiology/American Heart Association Task Force recommend statins, a lipid-lowering and anti-inflammatory therapy, “for patients with psoriasis who have additional risk-enhancing factors, similar to recommendations made for the general population without psoriasis,” she noted. But how the incidence of MACE differs between patients with and without psoriasis while on statin therapy “has not been explored in real-world settings,” she added.
To address this question, the researchers used real-world data from the TriNetX health research network to identify individuals aged 18-90 years with a diagnosis of both psoriasis and lipid disorders who were undergoing treatment with statins. Those with a prior history of MACE were excluded from the analysis. Patients with lipid disorders on statin therapy, but without psoriatic disease, were matched 1:1 by age, sex, race, ethnicity, common risk factors for MACE, and medications shown to reduce MACE risk. The researchers then assessed the cohorts 5 years following their first statin prescription and used the TriNetX analytics tool to calculate the odds ratio (OR) with 95% CI to evaluate the likelihood of MACE in the presence of statin therapy.
Dr. Ormaza Vera and colleagues identified 20,660 patients with psoriasis and 2,768,429 patients without psoriasis who met the criteria for analysis. After propensity score matching, each cohort included 20,660 patients with a mean age of 60 years. During the 5-year observation period, 2725 patients in the psoriasis cohort experienced MACE compared with 2203 patients in the non-psoriasis cohort (OR, 1.40; 95% CI, 1.317-1.488).
“This was an unexpected outcome that challenges the current understanding and highlights the need for further research into tailored treatments for cardiovascular risk in psoriasis patients,” Dr. Ormaza Vera told this news organization.
She acknowledged certain limitations of the study, including its retrospective design, the inherent limitations of an observational study, and the use of electronic medical record data.
Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, who was asked to comment on the study results, said that the findings imply that there is more than statin use alone to protect someone with psoriasis from having an increased risk for MACE. “This is not really surprising because statin use alone is only part of a prevention strategy in someone with psoriasis who usually has multiple comorbidities,” Dr. Green said. “On the other hand, the study only went out for 5 years and cardiovascular disease is a long accumulating process, so it could also be too early to demonstrate MACE prevention.”
The study was funded by a grant from the American Skin Association. Dr. Ormaza Vera and her coauthors reported having no relevant disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
A version of this article appeared on Medscape.com .
Over a period of 5 years, the, even after adjusting for covariates, results from a large retrospective study showed.
“It is well-established that psoriasis is an independent risk factor for the development of MACE, with cardiometabolic risk factors being more prevalent and incident among patients with psoriasis,” the study’s first author Ana Ormaza Vera, MD, a dermatology research fellow at Eastern Virginia Medical School, Norfolk, said in an interview after the annual meeting of the Society for Investigational Dermatology, where the study was presented during a late-breaking abstract session.
Current guidelines from the joint American Academy of Dermatology/National Psoriasis Foundation and the American Academy of Cardiology/American Heart Association Task Force recommend statins, a lipid-lowering and anti-inflammatory therapy, “for patients with psoriasis who have additional risk-enhancing factors, similar to recommendations made for the general population without psoriasis,” she noted. But how the incidence of MACE differs between patients with and without psoriasis while on statin therapy “has not been explored in real-world settings,” she added.
To address this question, the researchers used real-world data from the TriNetX health research network to identify individuals aged 18-90 years with a diagnosis of both psoriasis and lipid disorders who were undergoing treatment with statins. Those with a prior history of MACE were excluded from the analysis. Patients with lipid disorders on statin therapy, but without psoriatic disease, were matched 1:1 by age, sex, race, ethnicity, common risk factors for MACE, and medications shown to reduce MACE risk. The researchers then assessed the cohorts 5 years following their first statin prescription and used the TriNetX analytics tool to calculate the odds ratio (OR) with 95% CI to evaluate the likelihood of MACE in the presence of statin therapy.
Dr. Ormaza Vera and colleagues identified 20,660 patients with psoriasis and 2,768,429 patients without psoriasis who met the criteria for analysis. After propensity score matching, each cohort included 20,660 patients with a mean age of 60 years. During the 5-year observation period, 2725 patients in the psoriasis cohort experienced MACE compared with 2203 patients in the non-psoriasis cohort (OR, 1.40; 95% CI, 1.317-1.488).
“This was an unexpected outcome that challenges the current understanding and highlights the need for further research into tailored treatments for cardiovascular risk in psoriasis patients,” Dr. Ormaza Vera told this news organization.
She acknowledged certain limitations of the study, including its retrospective design, the inherent limitations of an observational study, and the use of electronic medical record data.
Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, who was asked to comment on the study results, said that the findings imply that there is more than statin use alone to protect someone with psoriasis from having an increased risk for MACE. “This is not really surprising because statin use alone is only part of a prevention strategy in someone with psoriasis who usually has multiple comorbidities,” Dr. Green said. “On the other hand, the study only went out for 5 years and cardiovascular disease is a long accumulating process, so it could also be too early to demonstrate MACE prevention.”
The study was funded by a grant from the American Skin Association. Dr. Ormaza Vera and her coauthors reported having no relevant disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
A version of this article appeared on Medscape.com .
Over a period of 5 years, the, even after adjusting for covariates, results from a large retrospective study showed.
“It is well-established that psoriasis is an independent risk factor for the development of MACE, with cardiometabolic risk factors being more prevalent and incident among patients with psoriasis,” the study’s first author Ana Ormaza Vera, MD, a dermatology research fellow at Eastern Virginia Medical School, Norfolk, said in an interview after the annual meeting of the Society for Investigational Dermatology, where the study was presented during a late-breaking abstract session.
Current guidelines from the joint American Academy of Dermatology/National Psoriasis Foundation and the American Academy of Cardiology/American Heart Association Task Force recommend statins, a lipid-lowering and anti-inflammatory therapy, “for patients with psoriasis who have additional risk-enhancing factors, similar to recommendations made for the general population without psoriasis,” she noted. But how the incidence of MACE differs between patients with and without psoriasis while on statin therapy “has not been explored in real-world settings,” she added.
To address this question, the researchers used real-world data from the TriNetX health research network to identify individuals aged 18-90 years with a diagnosis of both psoriasis and lipid disorders who were undergoing treatment with statins. Those with a prior history of MACE were excluded from the analysis. Patients with lipid disorders on statin therapy, but without psoriatic disease, were matched 1:1 by age, sex, race, ethnicity, common risk factors for MACE, and medications shown to reduce MACE risk. The researchers then assessed the cohorts 5 years following their first statin prescription and used the TriNetX analytics tool to calculate the odds ratio (OR) with 95% CI to evaluate the likelihood of MACE in the presence of statin therapy.
Dr. Ormaza Vera and colleagues identified 20,660 patients with psoriasis and 2,768,429 patients without psoriasis who met the criteria for analysis. After propensity score matching, each cohort included 20,660 patients with a mean age of 60 years. During the 5-year observation period, 2725 patients in the psoriasis cohort experienced MACE compared with 2203 patients in the non-psoriasis cohort (OR, 1.40; 95% CI, 1.317-1.488).
“This was an unexpected outcome that challenges the current understanding and highlights the need for further research into tailored treatments for cardiovascular risk in psoriasis patients,” Dr. Ormaza Vera told this news organization.
She acknowledged certain limitations of the study, including its retrospective design, the inherent limitations of an observational study, and the use of electronic medical record data.
Lawrence J. Green, MD, clinical professor of dermatology, George Washington University, Washington, who was asked to comment on the study results, said that the findings imply that there is more than statin use alone to protect someone with psoriasis from having an increased risk for MACE. “This is not really surprising because statin use alone is only part of a prevention strategy in someone with psoriasis who usually has multiple comorbidities,” Dr. Green said. “On the other hand, the study only went out for 5 years and cardiovascular disease is a long accumulating process, so it could also be too early to demonstrate MACE prevention.”
The study was funded by a grant from the American Skin Association. Dr. Ormaza Vera and her coauthors reported having no relevant disclosures. Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies.
A version of this article appeared on Medscape.com .
FROM SID 2024
Commentary: Transition from Psoriasis to PsA and New Drug Analyses, June 2024
In regard to treatment, bimekizumab is a new monoclonal antibody that dually targets interleukin (IL)-17A and IL-17F and is highly efficacious for the treatment of psoriasis. In a meta-analysis of four placebo-controlled randomized clinical trials that included 1323 patients with PsA (age 18 years or older), of whom 853 received bimekizumab, Su and colleagues demonstrated that bimekizumab led to a significantly higher response rate for minimal disease activity (risk ratio [RR] 4.188; P < .001) and a 70% or greater improvement in the American College of Rheumatology (ACR) criteria (RR 7.932; P < .0001) when compared with placebo. Bimekizumab was superior to placebo in achieving ACR20/50/70 response at a dose of 160 mg. The risk for treatment-emergent adverse events was modestly higher with bimekizumab vs placebo (RR 1.423; P = .023), whereas the risk for serious cancers, upper respiratory tract infection, injection site reactions, and pharyngitis was similar for both. Thus, bimekizumab is an efficacious agent for the treatment of PsA. Future head-to-head studies will help clinicians determine the role of this drug in the management of PsA.
Not all patients respond equally well to targeted therapies, and the so-called challenging-to-treat patients are being increasingly described. Kivitz and colleagues recently described the efficacy of secukinumab, a monoclonal antibody targeting IL-17A, in these challenging-to-treat patients from the United States. In a post hoc subgroup analysis of four phase 3 studies that included 279 patients, they demonstrated that patients receiving 300 mg secukinumab and 150 mg with a loading dose had a higher rate of achieving the ACR20 response (59.7% and 43.4%, respectively) vs 15.6% for placebo (both P < .0001). The Psoriasis Area and Severity Index 90 response was 47.1% and 22.2%, respectively, vs 5.3% (both P < .05). Thus, secukinumab is efficacious in more challenging-to-treat patients. However, such patients need to be better characterized so that effective treatment strategies to achieve a state of low disease activity may be implemented.
In regard to treatment, bimekizumab is a new monoclonal antibody that dually targets interleukin (IL)-17A and IL-17F and is highly efficacious for the treatment of psoriasis. In a meta-analysis of four placebo-controlled randomized clinical trials that included 1323 patients with PsA (age 18 years or older), of whom 853 received bimekizumab, Su and colleagues demonstrated that bimekizumab led to a significantly higher response rate for minimal disease activity (risk ratio [RR] 4.188; P < .001) and a 70% or greater improvement in the American College of Rheumatology (ACR) criteria (RR 7.932; P < .0001) when compared with placebo. Bimekizumab was superior to placebo in achieving ACR20/50/70 response at a dose of 160 mg. The risk for treatment-emergent adverse events was modestly higher with bimekizumab vs placebo (RR 1.423; P = .023), whereas the risk for serious cancers, upper respiratory tract infection, injection site reactions, and pharyngitis was similar for both. Thus, bimekizumab is an efficacious agent for the treatment of PsA. Future head-to-head studies will help clinicians determine the role of this drug in the management of PsA.
Not all patients respond equally well to targeted therapies, and the so-called challenging-to-treat patients are being increasingly described. Kivitz and colleagues recently described the efficacy of secukinumab, a monoclonal antibody targeting IL-17A, in these challenging-to-treat patients from the United States. In a post hoc subgroup analysis of four phase 3 studies that included 279 patients, they demonstrated that patients receiving 300 mg secukinumab and 150 mg with a loading dose had a higher rate of achieving the ACR20 response (59.7% and 43.4%, respectively) vs 15.6% for placebo (both P < .0001). The Psoriasis Area and Severity Index 90 response was 47.1% and 22.2%, respectively, vs 5.3% (both P < .05). Thus, secukinumab is efficacious in more challenging-to-treat patients. However, such patients need to be better characterized so that effective treatment strategies to achieve a state of low disease activity may be implemented.
In regard to treatment, bimekizumab is a new monoclonal antibody that dually targets interleukin (IL)-17A and IL-17F and is highly efficacious for the treatment of psoriasis. In a meta-analysis of four placebo-controlled randomized clinical trials that included 1323 patients with PsA (age 18 years or older), of whom 853 received bimekizumab, Su and colleagues demonstrated that bimekizumab led to a significantly higher response rate for minimal disease activity (risk ratio [RR] 4.188; P < .001) and a 70% or greater improvement in the American College of Rheumatology (ACR) criteria (RR 7.932; P < .0001) when compared with placebo. Bimekizumab was superior to placebo in achieving ACR20/50/70 response at a dose of 160 mg. The risk for treatment-emergent adverse events was modestly higher with bimekizumab vs placebo (RR 1.423; P = .023), whereas the risk for serious cancers, upper respiratory tract infection, injection site reactions, and pharyngitis was similar for both. Thus, bimekizumab is an efficacious agent for the treatment of PsA. Future head-to-head studies will help clinicians determine the role of this drug in the management of PsA.
Not all patients respond equally well to targeted therapies, and the so-called challenging-to-treat patients are being increasingly described. Kivitz and colleagues recently described the efficacy of secukinumab, a monoclonal antibody targeting IL-17A, in these challenging-to-treat patients from the United States. In a post hoc subgroup analysis of four phase 3 studies that included 279 patients, they demonstrated that patients receiving 300 mg secukinumab and 150 mg with a loading dose had a higher rate of achieving the ACR20 response (59.7% and 43.4%, respectively) vs 15.6% for placebo (both P < .0001). The Psoriasis Area and Severity Index 90 response was 47.1% and 22.2%, respectively, vs 5.3% (both P < .05). Thus, secukinumab is efficacious in more challenging-to-treat patients. However, such patients need to be better characterized so that effective treatment strategies to achieve a state of low disease activity may be implemented.
Cortisol Test Confirms HPA Axis Recovery from Steroid Use
TOPLINE:
An early serum cortisol concentration of > 237 nmol/L (> 8.6 μg/dL) has been validated as a safe and useful screening test with 100% specificity for predicting recovery of the hypothalamic-pituitary-adrenal (HPA) axis in patients on tapering regimes from long‐term chronic glucocorticoid therapy (CGT).
METHODOLOGY:
- A retrospective review of 250-µg Synacthen test (SST) results performed in patients on tapering CGT doses from a single-center rheumatology department over 12 months.
- A total of 60 SSTs were performed in 58 patients, all in the morning (7-12 AM) after withholding CGT for 48 hours.
- Peripheral blood was sampled for cortisol at baseline, 30 minutes, and 60 minutes.
- Adrenal insufficiency (AI) was defined as a peak serum cortisol concentration.
TAKEAWAY:
- The mean duration of CGT (all prednisolone) was 63 months, prescribed primarily for giant cell arteritis/polymyalgia rheumatica (48%) and inflammatory arthritis (18%), with a mean daily dose of 3.4 mg at the time of SST.
- With the investigators’ previously reported basal serum cortisol concentration of > 237 nmol/L (> 8.6 μg/dL) used to confirm an intact HPA axis, no patient with AI would have been missed, but 37 of 51 (73%) unnecessary SSTs in euadrenal patients would have been avoided.
- A basal serum cortisol concentration of > 227 nmol/L had a specificity of 100% for predicting passing the SST, while a basal serum cortisol concentration of ≤ 55 nmol/L had a 100% sensitivity for predicting failure.
- A mean daily prednisolone dosing at the time of SST in patients with AI was significantly higher than that with normal SSTs (5.7 vs 2.9 mg, respectively; P = .01).
IN PRACTICE:
“This offers a more rapid, convenient, and cost‐effective screening method for patients requiring biochemical assessment of the HPA axis with the potential for significant resource savings without any adverse impact on patient safety,” the authors wrote.
SOURCE:
The study was conducted by Ella Sharma, of the Department of Endocrinology, Royal Victoria Infirmary, Newcastle upon Tyne, UK, and colleagues and published online on May 19, 2024, as a letter in Clinical Endocrinology.
LIMITATIONS:
Not provided.
DISCLOSURES:
Not provided.
A version of this article appeared on Medscape.com.
TOPLINE:
An early serum cortisol concentration of > 237 nmol/L (> 8.6 μg/dL) has been validated as a safe and useful screening test with 100% specificity for predicting recovery of the hypothalamic-pituitary-adrenal (HPA) axis in patients on tapering regimes from long‐term chronic glucocorticoid therapy (CGT).
METHODOLOGY:
- A retrospective review of 250-µg Synacthen test (SST) results performed in patients on tapering CGT doses from a single-center rheumatology department over 12 months.
- A total of 60 SSTs were performed in 58 patients, all in the morning (7-12 AM) after withholding CGT for 48 hours.
- Peripheral blood was sampled for cortisol at baseline, 30 minutes, and 60 minutes.
- Adrenal insufficiency (AI) was defined as a peak serum cortisol concentration.
TAKEAWAY:
- The mean duration of CGT (all prednisolone) was 63 months, prescribed primarily for giant cell arteritis/polymyalgia rheumatica (48%) and inflammatory arthritis (18%), with a mean daily dose of 3.4 mg at the time of SST.
- With the investigators’ previously reported basal serum cortisol concentration of > 237 nmol/L (> 8.6 μg/dL) used to confirm an intact HPA axis, no patient with AI would have been missed, but 37 of 51 (73%) unnecessary SSTs in euadrenal patients would have been avoided.
- A basal serum cortisol concentration of > 227 nmol/L had a specificity of 100% for predicting passing the SST, while a basal serum cortisol concentration of ≤ 55 nmol/L had a 100% sensitivity for predicting failure.
- A mean daily prednisolone dosing at the time of SST in patients with AI was significantly higher than that with normal SSTs (5.7 vs 2.9 mg, respectively; P = .01).
IN PRACTICE:
“This offers a more rapid, convenient, and cost‐effective screening method for patients requiring biochemical assessment of the HPA axis with the potential for significant resource savings without any adverse impact on patient safety,” the authors wrote.
SOURCE:
The study was conducted by Ella Sharma, of the Department of Endocrinology, Royal Victoria Infirmary, Newcastle upon Tyne, UK, and colleagues and published online on May 19, 2024, as a letter in Clinical Endocrinology.
LIMITATIONS:
Not provided.
DISCLOSURES:
Not provided.
A version of this article appeared on Medscape.com.
TOPLINE:
An early serum cortisol concentration of > 237 nmol/L (> 8.6 μg/dL) has been validated as a safe and useful screening test with 100% specificity for predicting recovery of the hypothalamic-pituitary-adrenal (HPA) axis in patients on tapering regimes from long‐term chronic glucocorticoid therapy (CGT).
METHODOLOGY:
- A retrospective review of 250-µg Synacthen test (SST) results performed in patients on tapering CGT doses from a single-center rheumatology department over 12 months.
- A total of 60 SSTs were performed in 58 patients, all in the morning (7-12 AM) after withholding CGT for 48 hours.
- Peripheral blood was sampled for cortisol at baseline, 30 minutes, and 60 minutes.
- Adrenal insufficiency (AI) was defined as a peak serum cortisol concentration.
TAKEAWAY:
- The mean duration of CGT (all prednisolone) was 63 months, prescribed primarily for giant cell arteritis/polymyalgia rheumatica (48%) and inflammatory arthritis (18%), with a mean daily dose of 3.4 mg at the time of SST.
- With the investigators’ previously reported basal serum cortisol concentration of > 237 nmol/L (> 8.6 μg/dL) used to confirm an intact HPA axis, no patient with AI would have been missed, but 37 of 51 (73%) unnecessary SSTs in euadrenal patients would have been avoided.
- A basal serum cortisol concentration of > 227 nmol/L had a specificity of 100% for predicting passing the SST, while a basal serum cortisol concentration of ≤ 55 nmol/L had a 100% sensitivity for predicting failure.
- A mean daily prednisolone dosing at the time of SST in patients with AI was significantly higher than that with normal SSTs (5.7 vs 2.9 mg, respectively; P = .01).
IN PRACTICE:
“This offers a more rapid, convenient, and cost‐effective screening method for patients requiring biochemical assessment of the HPA axis with the potential for significant resource savings without any adverse impact on patient safety,” the authors wrote.
SOURCE:
The study was conducted by Ella Sharma, of the Department of Endocrinology, Royal Victoria Infirmary, Newcastle upon Tyne, UK, and colleagues and published online on May 19, 2024, as a letter in Clinical Endocrinology.
LIMITATIONS:
Not provided.
DISCLOSURES:
Not provided.
A version of this article appeared on Medscape.com.





