User login
Biologics show signs of delaying arthritis in psoriasis patients
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
Patients with psoriasis treated with interleukin-12/23 inhibitors or IL-23 inhibitors were less likely to develop inflammatory arthritis, compared with those treated with tumor necrosis factor (TNF) inhibitors, according to findings from a large retrospective study.
While previous retrospective cohort studies have found biologic therapies for psoriasis can reduce the risk of developing psoriatic arthritis when compared with other treatments such as phototherapy and oral nonbiologic disease-modifying antirheumatic drugs, this analysis is the first to compare classes of biologics, Shikha Singla, MD, of the Medical College of Wisconsin, Milwaukee, and colleagues wrote in The Lancet Rheumatology.
In the analysis, researchers used the TriNetX database, which contains deidentified data from electronic medical health records from health care organizations across the United States. The study included adults diagnosed with psoriasis who were newly prescribed a biologic approved by the Food and Drug Administration for the treatment of psoriasis. Biologics were defined by drug class: anti-TNF, anti-IL-17, anti-IL-23, and anti–IL-12/23. Any patient with a diagnosis of psoriatic arthritis or other inflammatory arthritis prior to receiving a biologic prescription or within 2 weeks of receiving the prescription were excluded.
The researchers identified 15,501 eligible patients diagnosed with psoriasis during Jan. 1, 2014, to June 1, 2022, with an average follow-up time of 2.4 years. The researchers chose to start the study period in 2014 because the first non–anti-TNF drug for psoriatic arthritis was approved by the FDA in 2013 – the anti–IL-12/23 drug ustekinumab. During the study period, 976 patients developed inflammatory arthritis and were diagnosed on average 528 days after their biologic prescription.
In a multivariable analysis, the researchers found that patients prescribed IL-23 inhibitors (guselkumab [Tremfya], risankizumab [Skyrizi], tildrakizumab [Ilumya]) were nearly 60% less likely (adjusted hazard ratio, 0.41; 95% confidence interval, 0.17–0.95) to develop inflammatory arthritis than were patients taking TNF inhibitors (infliximab [Remicade], adalimumab [Humira], etanercept [Enbrel], golimumab [Simponi], certolizumab pegol [Cimzia]). The risk of developing arthritis was 42% lower (aHR, 0.58; 95% CI, 0.43-0.76) with the IL-12/23 inhibitor ustekinumab (Stelara), but there was no difference in outcomes among patients taking with IL-17 inhibitors (secukinumab [Cosentyx], ixekizumab [Taltz], or brodalumab [Siliq]), compared with TNF inhibitors. For the IL-12/23 inhibitor ustekinumab, all sensitivity analyses did not change this association. For IL-23 inhibitors, the results persisted when excluding patients who developed arthritis within 3 or 6 months after first biologic prescription and when using a higher diagnostic threshold for incident arthritis.
“There is a lot of interest in understanding if treatment of psoriasis will prevent onset of psoriatic arthritis,” said Joel M. Gelfand, MD, MSCE, director of the Psoriasis and Phototherapy Treatment Center at the University of Pennsylvania, Philadelphia, who was asked to comment on the results.
“To date, the literature is inconclusive with some studies suggesting biologics reduce risk of PsA, whereas others suggest biologic use is associated with an increased risk of PsA,” he said. “The current study is unique in that it compares biologic classes to one another and suggests that IL-12/23 and IL-23 biologics are associated with a reduced risk of PsA compared to psoriasis patients treated with TNF inhibitors and no difference was found between TNF inhibitors and IL-17 inhibitors.”
While the study posed an interesting research question, “I wouldn’t use these results to actually change treatment patterns,” Alexis R. Ogdie-Beatty, MD, an associate professor of medicine at the University of Pennsylvania, Philadelphia, said in an interview. She coauthored a commentary on the analysis. Dr. Gelfand also emphasized that this bias may have influenced the results and that these findings “should not impact clinical practice at this time.”
Although the analyses were strong, Dr. Ogdie-Beatty noted, there are inherent biases in this type of observational data that cannot be overcome. For example, if a patient comes into a dermatologist’s office with psoriasis and also has joint pain, the dermatologist may suspect that a patient could also have psoriatic arthritis and would be more likely to choose a drug that will work well for both of these conditions.
“The drugs that are known to work best for psoriatic arthritis are the TNF inhibitors and the IL-17 inhibitors,” she said. So, while the analysis found these medications were associated with higher incidence of PsA, the dermatologist was possibly treating presumptive arthritis and the patient had yet to be referred to a rheumatologist to confirm the diagnosis.
The researchers noted that they attempted to mitigate these issues by requiring that patients have at least 1 year of follow-up before receiving biologic prescription “to capture only the patients with no previous codes for any type of arthritis,” as well as conducting six sensitivity analyses.
The authors, and Dr. Ogdie-Beatty and Dr. Gelfand agreed that more research is necessary to confirm these findings. A large randomized trial may be “prohibitively expensive,” the authors noted, but pooled analyses from previous clinical trials may help with this issue. “We identified 14 published randomized trials that did head-to-head comparisons of different biologic classes with regard to effect on psoriasis, and these trials collectively contained data on more than 13,000 patients. Pooled analyses of these data could confirm the findings of the present study and would be adequately powered.”
But that approach also has limitations, as psoriatic arthritis was not assessed an outcome in these studies, Dr. Ogdie-Beatty noted. Randomizing patients who are already at a higher risk of developing PsA to different biologics could be one approach to address these questions without needing such a large patient population.
The study was conducted without outside funding or industry involvement. Dr. Singla reported no relevant financial relationships with industry, but several coauthors reported financial relationships with pharmaceutical companies that market biologics for psoriasis and psoriatic arthritis. Dr. Ogdie-Beatty reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, CorEvitas, Gilead, Happify Health, Janssen, Lilly, Novartis, Pfizer, and UCB. Dr. Gelfand reported financial relationships with Abbvie, Amgen, BMS, Boehringer Ingelheim, FIDE, Lilly, Leo, Janssen Biologics, Novartis, Pfizer, and UCB. Dr. Gelfand is a deputy editor for the Journal of Investigative Dermatology.
This article was updated 3/15/23.
FROM LANCET RHEUMATOLOGY
Psoriatic arthritis: An independent risk factor for reduced bone density and fractures
Key clinical point: Regular assessment of bone mineral density and initiation of primary prevention should be considered in patients with psoriatic arthritis (PsA) as they are predisposed to falls and fractures because of reduced bone density, particularly those with late-onset psoriasis involving scalp.
Major finding: Patients with PsA were at a significantly higher risk for osteopenia or osteoporosis (odds ratio [OR] 21.9; CI 7.1-67.7) and prevalent fractures (OR 3.42; P = .002) compared with control individuals, with scalp involvement (P = .0049) and late onset of psoriasis (P = .029) being significantly associated with greater number of prevalent fractures.
Study details: Findings are from an observational cohort study including 61 patients with PsA and 69 age-matched control individuals.
Disclosures: This study did not report the source of funding. The authors reported no conflicts of interest.
Source: Halasi A et al. Psoriatic arthritis and its special features predispose not only for osteoporosis but also for fractures and falls. J Dermatol. 2023 (Jan 17). Doi: 10.1111/1346-8138.16710
Key clinical point: Regular assessment of bone mineral density and initiation of primary prevention should be considered in patients with psoriatic arthritis (PsA) as they are predisposed to falls and fractures because of reduced bone density, particularly those with late-onset psoriasis involving scalp.
Major finding: Patients with PsA were at a significantly higher risk for osteopenia or osteoporosis (odds ratio [OR] 21.9; CI 7.1-67.7) and prevalent fractures (OR 3.42; P = .002) compared with control individuals, with scalp involvement (P = .0049) and late onset of psoriasis (P = .029) being significantly associated with greater number of prevalent fractures.
Study details: Findings are from an observational cohort study including 61 patients with PsA and 69 age-matched control individuals.
Disclosures: This study did not report the source of funding. The authors reported no conflicts of interest.
Source: Halasi A et al. Psoriatic arthritis and its special features predispose not only for osteoporosis but also for fractures and falls. J Dermatol. 2023 (Jan 17). Doi: 10.1111/1346-8138.16710
Key clinical point: Regular assessment of bone mineral density and initiation of primary prevention should be considered in patients with psoriatic arthritis (PsA) as they are predisposed to falls and fractures because of reduced bone density, particularly those with late-onset psoriasis involving scalp.
Major finding: Patients with PsA were at a significantly higher risk for osteopenia or osteoporosis (odds ratio [OR] 21.9; CI 7.1-67.7) and prevalent fractures (OR 3.42; P = .002) compared with control individuals, with scalp involvement (P = .0049) and late onset of psoriasis (P = .029) being significantly associated with greater number of prevalent fractures.
Study details: Findings are from an observational cohort study including 61 patients with PsA and 69 age-matched control individuals.
Disclosures: This study did not report the source of funding. The authors reported no conflicts of interest.
Source: Halasi A et al. Psoriatic arthritis and its special features predispose not only for osteoporosis but also for fractures and falls. J Dermatol. 2023 (Jan 17). Doi: 10.1111/1346-8138.16710
PsA: Guselkumab demonstrates consistent safety profile irrespective of prior TNFi exposure
Key clinical point: A dose of 100 mg guselkumab every 4 or 8 weeks (Q4W/Q8W) demonstrated a favorable and consistent safety profile for up to 2 years in both tumor necrosis factor-α inhibitor (TNFi)-naive and TNFi-experienced patients with active psoriatic arthritis (PsA).
Major finding: In TNFi-naive vs TNFi-experienced patients receiving guselkumab, adverse events rates were consistent through 24 weeks (220.8/100 person-years [PY] vs 251.6/100 PY) and remained low through 2 years (139.69/100 PY vs 174.0/100 PY).
Study details: This pooled safety analysis of four phase 2/3 trials included 1554 TNFi-naive and TNFi-experienced patients with active PsA who were randomly assigned to receive 100 mg guselkumab Q4W or Q8W for ≤2 years or placebo with a crossover at week 24 to guselkumab Q4W or Q8W.
Disclosures: The four trials were funded by Janssen Research & Development, LLC. Seven authors declared being current or former employees of Janssen or owning stock or stock options in Johnson & Johnson. Several authors reported ties with Janssen and other sources.
Source: Rahman P et al. Safety of guselkumab with and without prior TNF-α inhibitor treatment: Pooled results across four studies in patients with psoriatic arthritis. J Rheumatol. 2023 (Jan 15). Doi: 10.3899/jrheum.220928
Key clinical point: A dose of 100 mg guselkumab every 4 or 8 weeks (Q4W/Q8W) demonstrated a favorable and consistent safety profile for up to 2 years in both tumor necrosis factor-α inhibitor (TNFi)-naive and TNFi-experienced patients with active psoriatic arthritis (PsA).
Major finding: In TNFi-naive vs TNFi-experienced patients receiving guselkumab, adverse events rates were consistent through 24 weeks (220.8/100 person-years [PY] vs 251.6/100 PY) and remained low through 2 years (139.69/100 PY vs 174.0/100 PY).
Study details: This pooled safety analysis of four phase 2/3 trials included 1554 TNFi-naive and TNFi-experienced patients with active PsA who were randomly assigned to receive 100 mg guselkumab Q4W or Q8W for ≤2 years or placebo with a crossover at week 24 to guselkumab Q4W or Q8W.
Disclosures: The four trials were funded by Janssen Research & Development, LLC. Seven authors declared being current or former employees of Janssen or owning stock or stock options in Johnson & Johnson. Several authors reported ties with Janssen and other sources.
Source: Rahman P et al. Safety of guselkumab with and without prior TNF-α inhibitor treatment: Pooled results across four studies in patients with psoriatic arthritis. J Rheumatol. 2023 (Jan 15). Doi: 10.3899/jrheum.220928
Key clinical point: A dose of 100 mg guselkumab every 4 or 8 weeks (Q4W/Q8W) demonstrated a favorable and consistent safety profile for up to 2 years in both tumor necrosis factor-α inhibitor (TNFi)-naive and TNFi-experienced patients with active psoriatic arthritis (PsA).
Major finding: In TNFi-naive vs TNFi-experienced patients receiving guselkumab, adverse events rates were consistent through 24 weeks (220.8/100 person-years [PY] vs 251.6/100 PY) and remained low through 2 years (139.69/100 PY vs 174.0/100 PY).
Study details: This pooled safety analysis of four phase 2/3 trials included 1554 TNFi-naive and TNFi-experienced patients with active PsA who were randomly assigned to receive 100 mg guselkumab Q4W or Q8W for ≤2 years or placebo with a crossover at week 24 to guselkumab Q4W or Q8W.
Disclosures: The four trials were funded by Janssen Research & Development, LLC. Seven authors declared being current or former employees of Janssen or owning stock or stock options in Johnson & Johnson. Several authors reported ties with Janssen and other sources.
Source: Rahman P et al. Safety of guselkumab with and without prior TNF-α inhibitor treatment: Pooled results across four studies in patients with psoriatic arthritis. J Rheumatol. 2023 (Jan 15). Doi: 10.3899/jrheum.220928
Commentary: Concerning PsA treatments and comorbidities, March 2023
With regard to advanced targeted therapies, there is concern about the side effects of Janus kinase (JAK) inhibitors, especially in patients with comorbidities. To address safety concerns with upadacitinib, a selective JAK1 inhibitor, Burmester and colleagues conducted an integrated safety analysis of 12 phase 3 trials that included 6991 patients (PsA n = 907; rheumatoid arthritis [RA] n = 3209; ankylosing spondylitis n = 182; and atopic dermatitis n = 2693) who received upadacitinib (15 or 30 mg once daily). Some trials included active comparators; therefore, safety among 1008 patients (RA n = 579; PsA n = 429) who received 40-mg adalimumab every other week and 314 patients with RA who received methotrexate were compared with those treated with upadacitinib. Overall, patients with PsA receiving 15-mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and death (0.8/100 PY). Patients with PsA treated with upadacitinib had higher rates of herpes zoster, nonmelanoma skin cancer, and elevations in creatine phosphokinase when compared with patients treated with adalimumab. Although these results are reassuring to clinicians treating PsA, continued surveillance regarding the risks for venous thrombosis, cardiovascular events, and cancer are required.
In a post hoc analysis of 10 clinical trials that included patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib, Kristensen and colleagues reported that the risk for major adverse cardiac events was higher among patients with PsA and a high 10-year atherosclerotic cardiovascular disease (ASCVD) risk vs patients with a low ASCVD risk. The incidence of cancer was highest in patients with PsA and an intermediate 10-year ASCVD risk. Although these studies are reassuring, the assessment and risk stratification of adverse events with JAK inhibitors and therapies in PsA will require longer-term comparative clinical trials as well as an evaluation of observational data from disease registries.
Comorbidities also have an impact on treatment persistence in PsA. Tillett and colleagues conducted a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs. They demonstrated that among patients receiving ustekinumab, those with concomitant PsA had a higher comorbidity burden, including diabetes, hypertension, and obesity, and a shorter time to ustekinumab discontinuation when compared with those with psoriasis alone. Secondary failure of advanced therapies is increasingly noted in the management of psoriatic disease. Female sex, depression, previous exposure to biologics, and the presence of comorbidities are important risk factors. Comprehensive management of psoriatic disease should include appropriate management of comorbidities for better long-term treatment persistence and outcomes.
With regard to advanced targeted therapies, there is concern about the side effects of Janus kinase (JAK) inhibitors, especially in patients with comorbidities. To address safety concerns with upadacitinib, a selective JAK1 inhibitor, Burmester and colleagues conducted an integrated safety analysis of 12 phase 3 trials that included 6991 patients (PsA n = 907; rheumatoid arthritis [RA] n = 3209; ankylosing spondylitis n = 182; and atopic dermatitis n = 2693) who received upadacitinib (15 or 30 mg once daily). Some trials included active comparators; therefore, safety among 1008 patients (RA n = 579; PsA n = 429) who received 40-mg adalimumab every other week and 314 patients with RA who received methotrexate were compared with those treated with upadacitinib. Overall, patients with PsA receiving 15-mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and death (0.8/100 PY). Patients with PsA treated with upadacitinib had higher rates of herpes zoster, nonmelanoma skin cancer, and elevations in creatine phosphokinase when compared with patients treated with adalimumab. Although these results are reassuring to clinicians treating PsA, continued surveillance regarding the risks for venous thrombosis, cardiovascular events, and cancer are required.
In a post hoc analysis of 10 clinical trials that included patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib, Kristensen and colleagues reported that the risk for major adverse cardiac events was higher among patients with PsA and a high 10-year atherosclerotic cardiovascular disease (ASCVD) risk vs patients with a low ASCVD risk. The incidence of cancer was highest in patients with PsA and an intermediate 10-year ASCVD risk. Although these studies are reassuring, the assessment and risk stratification of adverse events with JAK inhibitors and therapies in PsA will require longer-term comparative clinical trials as well as an evaluation of observational data from disease registries.
Comorbidities also have an impact on treatment persistence in PsA. Tillett and colleagues conducted a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs. They demonstrated that among patients receiving ustekinumab, those with concomitant PsA had a higher comorbidity burden, including diabetes, hypertension, and obesity, and a shorter time to ustekinumab discontinuation when compared with those with psoriasis alone. Secondary failure of advanced therapies is increasingly noted in the management of psoriatic disease. Female sex, depression, previous exposure to biologics, and the presence of comorbidities are important risk factors. Comprehensive management of psoriatic disease should include appropriate management of comorbidities for better long-term treatment persistence and outcomes.
With regard to advanced targeted therapies, there is concern about the side effects of Janus kinase (JAK) inhibitors, especially in patients with comorbidities. To address safety concerns with upadacitinib, a selective JAK1 inhibitor, Burmester and colleagues conducted an integrated safety analysis of 12 phase 3 trials that included 6991 patients (PsA n = 907; rheumatoid arthritis [RA] n = 3209; ankylosing spondylitis n = 182; and atopic dermatitis n = 2693) who received upadacitinib (15 or 30 mg once daily). Some trials included active comparators; therefore, safety among 1008 patients (RA n = 579; PsA n = 429) who received 40-mg adalimumab every other week and 314 patients with RA who received methotrexate were compared with those treated with upadacitinib. Overall, patients with PsA receiving 15-mg upadacitinib once daily had acceptable rates of treatment-emergent adverse events (TEAE; 244.8/100 patient-years [PY]), serious TEAE (11.1/100 PY), TEAE leading to discontinuation (5.4/100 PY), and death (0.8/100 PY). Patients with PsA treated with upadacitinib had higher rates of herpes zoster, nonmelanoma skin cancer, and elevations in creatine phosphokinase when compared with patients treated with adalimumab. Although these results are reassuring to clinicians treating PsA, continued surveillance regarding the risks for venous thrombosis, cardiovascular events, and cancer are required.
In a post hoc analysis of 10 clinical trials that included patients with PsA (n = 783) and psoriasis (n = 3663) who received tofacitinib, Kristensen and colleagues reported that the risk for major adverse cardiac events was higher among patients with PsA and a high 10-year atherosclerotic cardiovascular disease (ASCVD) risk vs patients with a low ASCVD risk. The incidence of cancer was highest in patients with PsA and an intermediate 10-year ASCVD risk. Although these studies are reassuring, the assessment and risk stratification of adverse events with JAK inhibitors and therapies in PsA will require longer-term comparative clinical trials as well as an evaluation of observational data from disease registries.
Comorbidities also have an impact on treatment persistence in PsA. Tillett and colleagues conducted a retrospective study including 9057 patients with plaque psoriasis alone or with concomitant PsA who received either ustekinumab or conventional systemic disease-modifying antirheumatic drugs. They demonstrated that among patients receiving ustekinumab, those with concomitant PsA had a higher comorbidity burden, including diabetes, hypertension, and obesity, and a shorter time to ustekinumab discontinuation when compared with those with psoriasis alone. Secondary failure of advanced therapies is increasingly noted in the management of psoriatic disease. Female sex, depression, previous exposure to biologics, and the presence of comorbidities are important risk factors. Comprehensive management of psoriatic disease should include appropriate management of comorbidities for better long-term treatment persistence and outcomes.
Isolated nail psoriasis may bring arthritis into play
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.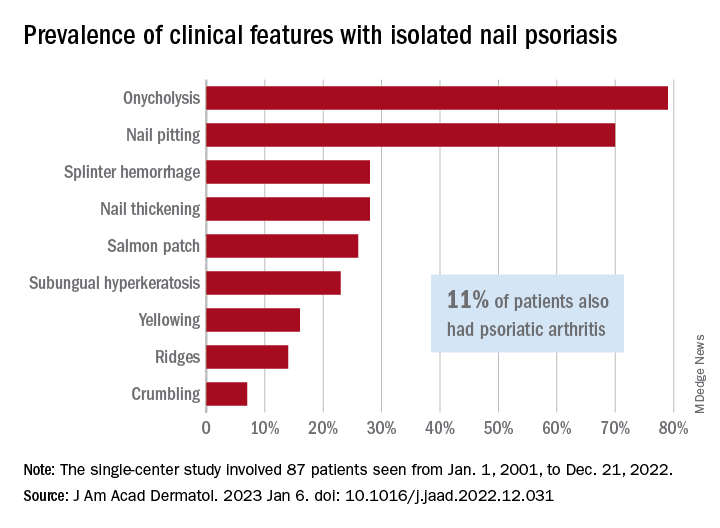
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
for dermatologists to improve their diagnostic accuracy,” investigators said in a research letter.
Diagnosis of isolated NP was delayed by almost 3 years among the 87 cases recorded and “arthritis was most often diagnosed concurrently with NP,” at a major nail referral center between Jan. 1, 2001, and Dec. 21, 2022, Michelle J. Chang of Drexel University, Philadelphia, and associates reported.
In what the authors say is, “the largest study documenting clinical and histologic features in patients with isolated NP,” the two most common clinical features were onycholysis and nail plate pitting, seen in 79% and 70% of cases, respectively. No other single feature had a prevalence higher than 28%.
The most frequent clinical dyad was onycholysis and pitting in 66% of patients, followed by onycholysis/nail thickening in 33% and onycholysis/splinter hemorrhage in 32%. The most common histologic features were parakeratosis in 79% and neutrophil infiltration in 48%, the investigators said.
Psoriatic arthritis (PsA), a focus of the study, occurred in 10 (11%) of the 87 individuals with isolated NP. Considering this finding, and “the close proximity between the nail apparatus and joint, we hypothesize a reciprocal relationship, with nail unit inflammation precipitating PsA,” Ms. Chang and associates wrote.
Senior author, Shari Lipner, MD, PhD, of the department of dermatology, Weill Cornell Medicine, New York, is a consultant for Ortho-Dermatologics, Hoth Therapeutics, and BelleTorus. Ms. Chang and the two other investigators had no conflicts of interest to declare.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Treating nail psoriasis: Intralesional injections and biologics
HONOLULU – combined with systemic therapy.
One might think of intralesional injections “as a torture method from the medieval days,” she said at the Hawaii Dermatology Seminar provided by MedscapeLIVE!, but intramatricial corticosteroid injections have been performed for many years as a treatment for nail psoriasis, typically with triamcinolone acetonide.
According to Dr. Armstrong, professor of dermatology and associate dean of clinical research at the University of Southern California, Los Angeles, nail matrix psoriasis can present as pitting, leukonychia, red macules in the lunula, crumbling, or trachyonychia. Nail bed psoriasis can present as splinter hemorrhages and onycholysis, hyperkeratosis and splinter hemorrhages, salmon patch or oil spot dyschromia, or onycholysis and salmon patch dyschromia.
In a German cross-sectional study of patients with psoriasis, nails were one of the body sites that have the greatest impact on quality of life – especially those in younger age groups.
While topical treatments are generally considered first for limited disease involving special areas such as the nails, systemic therapy is warranted in patients with moderate-to-severe involvement of specific sites or in those refractory to topical therapy, Dr. Armstrong said.
In 2018, Indian researchers published results from an open-label study of 17 patients, with nail psoriasis, comparing three treatments . Patients were assigned to three groups of 30 nails each and treated with intramatricial injections of triamcinolone acetonide (10 mg/mL), methotrexate (25 mg/mL), and cyclosporine (50 mg/mL), respectively. Each nail was treated with two injections at 6-week intervals and graded at 24 weeks using the Nail Psoriasis Severity Index (NAPSI). In the triamcinolone acetonide and methotrexate groups, 50% of treated nails showed a greater than 75% improvement at 24 weeks, compared with 33% of those in the cyclosporine group. The most side effects occurred in the nails treated with cyclosporine.
When Dr. Armstrong performs intramatricial injections, she uses triamcinolone acetonide at 10 mg/mL. However, she said, “my favorite way of treating severe nail psoriasis is with biologics.”
In an early study of patients with moderate to severe psoriasis treated with the tumor necrosis factor blocker adalimumab 80 mg subcutaneously at week 0, followed by 40 mg subcutaneously every other week from weeks 1 to 15, a post hoc analysis on the effects on nail psoriasis showed a 10-point decrease in the median NAPSI score through week 16 – from 21 to 11 .
In VOYAGE 2, which compared the interleukin-23 blocker guselkumab and adalimumab in patients with moderate to severe psoriasis, the mean percent improvement from baseline in the NAPSI score was similar in patients treated with adalimumab or guselkumab at week 16 (39.6% vs. 46.9%, respectively) and at week 24 (55% vs. 53.7%).
In another study of patients with nail psoriasis, researchers evaluated the efficacy of the IL-17A antagonist secukinumab 150 mg, 300 mg, or placebo at weeks 0, 1, 2, 3, and 4, and every 4 weeks thereafter for 2.5 years. At 2.5 years, the mean reduction in NAPSI score was 63.6% in the secukinumab 150 mg group and 73.3% in the secukinumab 300 mg group.
“I do have to tell my patients what to expect, because the nails grow out slowly, but over time we do see this increase in efficacy,” Dr. Armstrong said.
Studies of another IL-17A antagonist, ixekizumab, have yielded positive results as well, she noted. In 2021, Taiwanese researchers published a systematic review and network meta-analysis to evaluate the efficacy of small molecule inhibitors and biologics in treating nail psoriasis. They drew from 39 studies involving 15,673 patients with nail psoriasis and found that the oral Janus kinase inhibitor tofacitinib and ixekizumab had the best efficacy for treating nail psoriasis in 10-16 weeks and 24-26 weeks, respectively.
“They found that overall, the biologics have a good effect on nail psoriasis and that the treatment effects are overall quite similar,” Dr. Armstrong said.
Dr. Armstrong disclosed that she is a consultant or adviser for numerous pharmaceutical companies. She has also received research funding from Bristol-Myers Squibb, Dermavant, Dermira, Leo, Lilly, Pfizer, and UCB Pharma.
HONOLULU – combined with systemic therapy.
One might think of intralesional injections “as a torture method from the medieval days,” she said at the Hawaii Dermatology Seminar provided by MedscapeLIVE!, but intramatricial corticosteroid injections have been performed for many years as a treatment for nail psoriasis, typically with triamcinolone acetonide.
According to Dr. Armstrong, professor of dermatology and associate dean of clinical research at the University of Southern California, Los Angeles, nail matrix psoriasis can present as pitting, leukonychia, red macules in the lunula, crumbling, or trachyonychia. Nail bed psoriasis can present as splinter hemorrhages and onycholysis, hyperkeratosis and splinter hemorrhages, salmon patch or oil spot dyschromia, or onycholysis and salmon patch dyschromia.
In a German cross-sectional study of patients with psoriasis, nails were one of the body sites that have the greatest impact on quality of life – especially those in younger age groups.
While topical treatments are generally considered first for limited disease involving special areas such as the nails, systemic therapy is warranted in patients with moderate-to-severe involvement of specific sites or in those refractory to topical therapy, Dr. Armstrong said.
In 2018, Indian researchers published results from an open-label study of 17 patients, with nail psoriasis, comparing three treatments . Patients were assigned to three groups of 30 nails each and treated with intramatricial injections of triamcinolone acetonide (10 mg/mL), methotrexate (25 mg/mL), and cyclosporine (50 mg/mL), respectively. Each nail was treated with two injections at 6-week intervals and graded at 24 weeks using the Nail Psoriasis Severity Index (NAPSI). In the triamcinolone acetonide and methotrexate groups, 50% of treated nails showed a greater than 75% improvement at 24 weeks, compared with 33% of those in the cyclosporine group. The most side effects occurred in the nails treated with cyclosporine.
When Dr. Armstrong performs intramatricial injections, she uses triamcinolone acetonide at 10 mg/mL. However, she said, “my favorite way of treating severe nail psoriasis is with biologics.”
In an early study of patients with moderate to severe psoriasis treated with the tumor necrosis factor blocker adalimumab 80 mg subcutaneously at week 0, followed by 40 mg subcutaneously every other week from weeks 1 to 15, a post hoc analysis on the effects on nail psoriasis showed a 10-point decrease in the median NAPSI score through week 16 – from 21 to 11 .
In VOYAGE 2, which compared the interleukin-23 blocker guselkumab and adalimumab in patients with moderate to severe psoriasis, the mean percent improvement from baseline in the NAPSI score was similar in patients treated with adalimumab or guselkumab at week 16 (39.6% vs. 46.9%, respectively) and at week 24 (55% vs. 53.7%).
In another study of patients with nail psoriasis, researchers evaluated the efficacy of the IL-17A antagonist secukinumab 150 mg, 300 mg, or placebo at weeks 0, 1, 2, 3, and 4, and every 4 weeks thereafter for 2.5 years. At 2.5 years, the mean reduction in NAPSI score was 63.6% in the secukinumab 150 mg group and 73.3% in the secukinumab 300 mg group.
“I do have to tell my patients what to expect, because the nails grow out slowly, but over time we do see this increase in efficacy,” Dr. Armstrong said.
Studies of another IL-17A antagonist, ixekizumab, have yielded positive results as well, she noted. In 2021, Taiwanese researchers published a systematic review and network meta-analysis to evaluate the efficacy of small molecule inhibitors and biologics in treating nail psoriasis. They drew from 39 studies involving 15,673 patients with nail psoriasis and found that the oral Janus kinase inhibitor tofacitinib and ixekizumab had the best efficacy for treating nail psoriasis in 10-16 weeks and 24-26 weeks, respectively.
“They found that overall, the biologics have a good effect on nail psoriasis and that the treatment effects are overall quite similar,” Dr. Armstrong said.
Dr. Armstrong disclosed that she is a consultant or adviser for numerous pharmaceutical companies. She has also received research funding from Bristol-Myers Squibb, Dermavant, Dermira, Leo, Lilly, Pfizer, and UCB Pharma.
HONOLULU – combined with systemic therapy.
One might think of intralesional injections “as a torture method from the medieval days,” she said at the Hawaii Dermatology Seminar provided by MedscapeLIVE!, but intramatricial corticosteroid injections have been performed for many years as a treatment for nail psoriasis, typically with triamcinolone acetonide.
According to Dr. Armstrong, professor of dermatology and associate dean of clinical research at the University of Southern California, Los Angeles, nail matrix psoriasis can present as pitting, leukonychia, red macules in the lunula, crumbling, or trachyonychia. Nail bed psoriasis can present as splinter hemorrhages and onycholysis, hyperkeratosis and splinter hemorrhages, salmon patch or oil spot dyschromia, or onycholysis and salmon patch dyschromia.
In a German cross-sectional study of patients with psoriasis, nails were one of the body sites that have the greatest impact on quality of life – especially those in younger age groups.
While topical treatments are generally considered first for limited disease involving special areas such as the nails, systemic therapy is warranted in patients with moderate-to-severe involvement of specific sites or in those refractory to topical therapy, Dr. Armstrong said.
In 2018, Indian researchers published results from an open-label study of 17 patients, with nail psoriasis, comparing three treatments . Patients were assigned to three groups of 30 nails each and treated with intramatricial injections of triamcinolone acetonide (10 mg/mL), methotrexate (25 mg/mL), and cyclosporine (50 mg/mL), respectively. Each nail was treated with two injections at 6-week intervals and graded at 24 weeks using the Nail Psoriasis Severity Index (NAPSI). In the triamcinolone acetonide and methotrexate groups, 50% of treated nails showed a greater than 75% improvement at 24 weeks, compared with 33% of those in the cyclosporine group. The most side effects occurred in the nails treated with cyclosporine.
When Dr. Armstrong performs intramatricial injections, she uses triamcinolone acetonide at 10 mg/mL. However, she said, “my favorite way of treating severe nail psoriasis is with biologics.”
In an early study of patients with moderate to severe psoriasis treated with the tumor necrosis factor blocker adalimumab 80 mg subcutaneously at week 0, followed by 40 mg subcutaneously every other week from weeks 1 to 15, a post hoc analysis on the effects on nail psoriasis showed a 10-point decrease in the median NAPSI score through week 16 – from 21 to 11 .
In VOYAGE 2, which compared the interleukin-23 blocker guselkumab and adalimumab in patients with moderate to severe psoriasis, the mean percent improvement from baseline in the NAPSI score was similar in patients treated with adalimumab or guselkumab at week 16 (39.6% vs. 46.9%, respectively) and at week 24 (55% vs. 53.7%).
In another study of patients with nail psoriasis, researchers evaluated the efficacy of the IL-17A antagonist secukinumab 150 mg, 300 mg, or placebo at weeks 0, 1, 2, 3, and 4, and every 4 weeks thereafter for 2.5 years. At 2.5 years, the mean reduction in NAPSI score was 63.6% in the secukinumab 150 mg group and 73.3% in the secukinumab 300 mg group.
“I do have to tell my patients what to expect, because the nails grow out slowly, but over time we do see this increase in efficacy,” Dr. Armstrong said.
Studies of another IL-17A antagonist, ixekizumab, have yielded positive results as well, she noted. In 2021, Taiwanese researchers published a systematic review and network meta-analysis to evaluate the efficacy of small molecule inhibitors and biologics in treating nail psoriasis. They drew from 39 studies involving 15,673 patients with nail psoriasis and found that the oral Janus kinase inhibitor tofacitinib and ixekizumab had the best efficacy for treating nail psoriasis in 10-16 weeks and 24-26 weeks, respectively.
“They found that overall, the biologics have a good effect on nail psoriasis and that the treatment effects are overall quite similar,” Dr. Armstrong said.
Dr. Armstrong disclosed that she is a consultant or adviser for numerous pharmaceutical companies. She has also received research funding from Bristol-Myers Squibb, Dermavant, Dermira, Leo, Lilly, Pfizer, and UCB Pharma.
AT THE MEDSCAPELIVE! HAWAII DERMATOLOGY SEMINAR
What’s holding back physicians from prescribing biosimilars? Four specialties weigh in
While most providers think that biosimilars will positively impact care, few feel that the economic benefits of biosimilars to date are enough to motivate switching.
In a new survey of over 350 dermatologists, gastroenterologists, ophthalmologists, and rheumatologists, clinicians shared their opinions on the rapidly evolving landscape of biosimilars, detailing top concerns about prescribing these medications and how they presently use biosimilars in clinical practice. Across all specialties, providers said they would be most likely to prescribe biosimilars to new patients or if a patient’s health plan mandated the switch. Most providers listed concerns about biosimilar efficacy and lack of economic benefit as the main barriers to adoption of biosimilars in clinical practice.
Cardinal Health, a health care services company based in Dublin, Ohio, conducted the surveys from July through October 2022.
Rheumatologists want cost-savings for patients
2023 is gearing up to be a big year for biosimilars for inflammatory diseases, with at least eight adalimumab biosimilars entering the market in the United States. Amjevita, manufactured by Amgen, was the first to become commercially available on Jan. 31. Out of 103 surveyed rheumatologists, 62% said they were very comfortable prescribing biosimilars to patients, and 32% said they were somewhat comfortable. Providers said they would be most likely to prescribe a biosimilar to new patients (40%) or if biosimilars were mandated by a patient’s health plan (41%). Nearly one-third (31%) of rheumatologists said that a discount of 21%-30% from a reference product would be necessary to consider switching a patient to a biosimilar.
There are several reasons why a rheumatologist might be wary of switching patients to biosimilars, said Marcus Snow, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “Rheumatologists will always express concern about changing medications that work well for their patients. It is not ideal to ‘force switch’ to a different product, even if it is almost identical,” he told this news organization in an email. “Also, we must remember that a patient on a biologic has failed traditional medications, which speaks to the struggle a patient must endure to get their disease under control. Fail-first situations can cause a rheumatologist to be initially resistant or hesitant to any changes.”
The top concerns among rheumatologists about prescribing biosimilars were medication efficacy (36%), lack of economic benefit (24%), and evaluating when to prescribe a biosimilar versus a reference product (17%). For adalimumab biosimilars, rheumatologists said that interchangeability – a regulatory designation where a biosimilar can be automatically substituted for its reference product at the pharmacy – and citrate-free formulation were the most important product attributes. Sixty-four percent of providers also noted that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
“There needs to be a true reduction in price, to change providers’ opinions on the economic benefits of biosimilars – in the system generally and for the patient,” Dr. Snow said. “Things will get there eventually, but it is not there yet, based on the list prices we see for some biosimilars.”
Gastroenterologists emphasize patient education
Gastroenterology is another specialty to be affected by the influx of adalimumab biosimilars. Out of 72 surveyed gastroenterologists, 86% said they were very comfortable prescribing biosimilars. About half (49%) said they would be most likely to prescribe a biosimilar to patients with health plans mandating a biosimilar. More than 60% of surveyed gastroenterologists said that biosimilars would positively impact care; providers were divided on the current economic benefits of biosimilars, with 36% saying that the current discounts on biosimilars versus reference products were not favorable enough to motivate switching, and 35% stating that they were. A total of 40% of surveyed providers said that savings of 21%-30%, compared with savings of a reference product, would motivate them to switch patients to a biosimilar, with all other clinical factors being equal.
Gastroenterologists said that, along with the efficacy and cost savings of biosimilars, providing patient education (18%) was a top concern when prescribing biosimilars. Eighty-four percent of respondents said that educating patients about biosimilars as safe and effective treatment options was at least somewhat important. Nearly all participants (99%) cited device ease-of-use as at least somewhat important when considering prescribing adalimumab biosimilars, in addition to interchangeability (97%) and citrate-free formulation (93%).
“Despite general acceptance of biosimilars, there remains some uncertainty regarding their place in the current gastroenterology landscape,” wrote Vivek Kaul, MD, a professor of medicine at the University of Rochester (N.Y.) Medical Center, in the report. “This is likely because only half of the survey respondents believed that biosimilars will positively impact gastroenterology care, further highlighting the ongoing need for real-world data and incorporation of biosimilar use and interchangeability into clinical guidelines.”
Few dermatologists currently prescribe biosimilars
Eight out of ten dermatologists reported being at least somewhat comfortable prescribing biosimilars to patients, though fewer than 20% said they had prescribed a biosimilar in the past year. This indicates limited adoption of infliximab biosimilars, which were the only biosimilars with a dermatologic indication available in 2022, Alex Gross, MD, a dermatologist in Cumming, Ga., noted in his featured commentary in the report. Just 15% of respondents disagreed that biosimilars would have a positive impact on care, and 41% said they were excited about new biosimilars becoming available.
About half (47%) of dermatologists thought the economic benefits of biosimilars were not strong enough to motivate switching patients from reference products. Twenty-nine percent of respondents said that discounts of 21%-30% from a reference product would motivate them to switch patients to a biosimilar, with all other clinical factors being equal, while 20% said they were not likely to prescribe a biosimilar regardless of savings.
Dermatologists may be concerned that these cost savings may not be passed onto patients, said Alison Ehrlich, MD, a dermatologist in Washington, in an email to this news organization. Patient out-of-pocket cost savings would need to be “both significant and transparent” to begin to change providers’ minds, she noted.
Biosimilar efficacy was a top concern for 48% of dermatologists, while 13% said their main concern around prescribing biosimilars was lack of payer adoption. At least 95% of providers said that device ease-of-use and interchangeability were the most important attributes when considering adalimumab biosimilars. Nearly two-thirds (65%) reported that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
If both patients and providers are informed on biosimilar use and there are cost benefits, dermatologists’ opinions may become more favorable toward biosimilars, but that will take time, Dr. Ehrlich said. “We are very early in the game for biosimilar use in dermatology,” she added.
Ophthalmologists remain wary
Biosimilars have been relatively new to ophthalmology, with the first ranibizumab biosimilar becoming commercially available in July 2022. In the survey, 64 retina specialists were asked different questions than participants from other specialties to gauge ophthalmologists› familiarity with the biosimilars approval process and their overall comfort prescribing these medications. The primary concerns with prescribing biosimilars among respondents was payer coverage (52%), being uncomfortable with biosimilars from a clinical standpoint (48%), and administrative barriers (45%), such as prior authorization. Despite this lack of comfort with biosimilars, two-thirds of participants thought the U.S. Food and Drug Administration approval process for these medications was sufficient to evaluate their efficacy and safety. Still, fewer than half (48%) of providers said they do or would prescribe biosimilars.
George Williams, MD, a spokesperson for the American Academy of Ophthalmology, noted that the FDA approval process for biosimilars was not as rigorous as for the respective reference product, and fewer patients are followed over a shorter time period. “Since anti–[vascular endothelial growth factor (VEGF)] therapy for indications such as neovascular age-related macular degeneration continues indefinitely over years, ophthalmologists may have concerns about the long-term efficacy and safety when applied to larger real-world populations. Ophthalmologists are well aware of safety issues with VEGF inhibitors arising after FDA approval,” he told this news organization in an email.
When asked about the likelihood of using either aflibercept or ranibizumab biosimilars in their clinical practice once commercially available, 70% of ophthalmologists said they would be at least somewhat likely to prescribe aflibercept biosimilars, and 64% said they would be at least somewhat likely to prescribe ranibizumab biosimilars. About half of respondents said they would not likely switch a currently stable patient on either aflibercept or ranibizumab to the corresponding biosimilar. More than half of ophthalmologists (56%) said they would prescribe a biosimilar only if it had an interchangeability designation.
Out of all four specialties, ophthalmologists more frequently reported that higher discounts from a reference product would be necessary to consider switching a patient to a biosimilar. Currently, many ophthalmologists are comfortable with the off-label use of bevacizumab (Avastin) for treating wet age-related macular degeneration, which also offers more cost savings than any currently available biosimilar on the market, Dr. Williams said.
While the limited number of respondents makes it difficult to draw concrete conclusions, Dr. Williams emphasized that the AAO supported the use of biosimilars. “We believe that with clinical experience ophthalmic biosimilars will become useful therapeutic agents,” he noted.
A version of this article first appeared on Medscape.com.
While most providers think that biosimilars will positively impact care, few feel that the economic benefits of biosimilars to date are enough to motivate switching.
In a new survey of over 350 dermatologists, gastroenterologists, ophthalmologists, and rheumatologists, clinicians shared their opinions on the rapidly evolving landscape of biosimilars, detailing top concerns about prescribing these medications and how they presently use biosimilars in clinical practice. Across all specialties, providers said they would be most likely to prescribe biosimilars to new patients or if a patient’s health plan mandated the switch. Most providers listed concerns about biosimilar efficacy and lack of economic benefit as the main barriers to adoption of biosimilars in clinical practice.
Cardinal Health, a health care services company based in Dublin, Ohio, conducted the surveys from July through October 2022.
Rheumatologists want cost-savings for patients
2023 is gearing up to be a big year for biosimilars for inflammatory diseases, with at least eight adalimumab biosimilars entering the market in the United States. Amjevita, manufactured by Amgen, was the first to become commercially available on Jan. 31. Out of 103 surveyed rheumatologists, 62% said they were very comfortable prescribing biosimilars to patients, and 32% said they were somewhat comfortable. Providers said they would be most likely to prescribe a biosimilar to new patients (40%) or if biosimilars were mandated by a patient’s health plan (41%). Nearly one-third (31%) of rheumatologists said that a discount of 21%-30% from a reference product would be necessary to consider switching a patient to a biosimilar.
There are several reasons why a rheumatologist might be wary of switching patients to biosimilars, said Marcus Snow, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “Rheumatologists will always express concern about changing medications that work well for their patients. It is not ideal to ‘force switch’ to a different product, even if it is almost identical,” he told this news organization in an email. “Also, we must remember that a patient on a biologic has failed traditional medications, which speaks to the struggle a patient must endure to get their disease under control. Fail-first situations can cause a rheumatologist to be initially resistant or hesitant to any changes.”
The top concerns among rheumatologists about prescribing biosimilars were medication efficacy (36%), lack of economic benefit (24%), and evaluating when to prescribe a biosimilar versus a reference product (17%). For adalimumab biosimilars, rheumatologists said that interchangeability – a regulatory designation where a biosimilar can be automatically substituted for its reference product at the pharmacy – and citrate-free formulation were the most important product attributes. Sixty-four percent of providers also noted that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
“There needs to be a true reduction in price, to change providers’ opinions on the economic benefits of biosimilars – in the system generally and for the patient,” Dr. Snow said. “Things will get there eventually, but it is not there yet, based on the list prices we see for some biosimilars.”
Gastroenterologists emphasize patient education
Gastroenterology is another specialty to be affected by the influx of adalimumab biosimilars. Out of 72 surveyed gastroenterologists, 86% said they were very comfortable prescribing biosimilars. About half (49%) said they would be most likely to prescribe a biosimilar to patients with health plans mandating a biosimilar. More than 60% of surveyed gastroenterologists said that biosimilars would positively impact care; providers were divided on the current economic benefits of biosimilars, with 36% saying that the current discounts on biosimilars versus reference products were not favorable enough to motivate switching, and 35% stating that they were. A total of 40% of surveyed providers said that savings of 21%-30%, compared with savings of a reference product, would motivate them to switch patients to a biosimilar, with all other clinical factors being equal.
Gastroenterologists said that, along with the efficacy and cost savings of biosimilars, providing patient education (18%) was a top concern when prescribing biosimilars. Eighty-four percent of respondents said that educating patients about biosimilars as safe and effective treatment options was at least somewhat important. Nearly all participants (99%) cited device ease-of-use as at least somewhat important when considering prescribing adalimumab biosimilars, in addition to interchangeability (97%) and citrate-free formulation (93%).
“Despite general acceptance of biosimilars, there remains some uncertainty regarding their place in the current gastroenterology landscape,” wrote Vivek Kaul, MD, a professor of medicine at the University of Rochester (N.Y.) Medical Center, in the report. “This is likely because only half of the survey respondents believed that biosimilars will positively impact gastroenterology care, further highlighting the ongoing need for real-world data and incorporation of biosimilar use and interchangeability into clinical guidelines.”
Few dermatologists currently prescribe biosimilars
Eight out of ten dermatologists reported being at least somewhat comfortable prescribing biosimilars to patients, though fewer than 20% said they had prescribed a biosimilar in the past year. This indicates limited adoption of infliximab biosimilars, which were the only biosimilars with a dermatologic indication available in 2022, Alex Gross, MD, a dermatologist in Cumming, Ga., noted in his featured commentary in the report. Just 15% of respondents disagreed that biosimilars would have a positive impact on care, and 41% said they were excited about new biosimilars becoming available.
About half (47%) of dermatologists thought the economic benefits of biosimilars were not strong enough to motivate switching patients from reference products. Twenty-nine percent of respondents said that discounts of 21%-30% from a reference product would motivate them to switch patients to a biosimilar, with all other clinical factors being equal, while 20% said they were not likely to prescribe a biosimilar regardless of savings.
Dermatologists may be concerned that these cost savings may not be passed onto patients, said Alison Ehrlich, MD, a dermatologist in Washington, in an email to this news organization. Patient out-of-pocket cost savings would need to be “both significant and transparent” to begin to change providers’ minds, she noted.
Biosimilar efficacy was a top concern for 48% of dermatologists, while 13% said their main concern around prescribing biosimilars was lack of payer adoption. At least 95% of providers said that device ease-of-use and interchangeability were the most important attributes when considering adalimumab biosimilars. Nearly two-thirds (65%) reported that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
If both patients and providers are informed on biosimilar use and there are cost benefits, dermatologists’ opinions may become more favorable toward biosimilars, but that will take time, Dr. Ehrlich said. “We are very early in the game for biosimilar use in dermatology,” she added.
Ophthalmologists remain wary
Biosimilars have been relatively new to ophthalmology, with the first ranibizumab biosimilar becoming commercially available in July 2022. In the survey, 64 retina specialists were asked different questions than participants from other specialties to gauge ophthalmologists› familiarity with the biosimilars approval process and their overall comfort prescribing these medications. The primary concerns with prescribing biosimilars among respondents was payer coverage (52%), being uncomfortable with biosimilars from a clinical standpoint (48%), and administrative barriers (45%), such as prior authorization. Despite this lack of comfort with biosimilars, two-thirds of participants thought the U.S. Food and Drug Administration approval process for these medications was sufficient to evaluate their efficacy and safety. Still, fewer than half (48%) of providers said they do or would prescribe biosimilars.
George Williams, MD, a spokesperson for the American Academy of Ophthalmology, noted that the FDA approval process for biosimilars was not as rigorous as for the respective reference product, and fewer patients are followed over a shorter time period. “Since anti–[vascular endothelial growth factor (VEGF)] therapy for indications such as neovascular age-related macular degeneration continues indefinitely over years, ophthalmologists may have concerns about the long-term efficacy and safety when applied to larger real-world populations. Ophthalmologists are well aware of safety issues with VEGF inhibitors arising after FDA approval,” he told this news organization in an email.
When asked about the likelihood of using either aflibercept or ranibizumab biosimilars in their clinical practice once commercially available, 70% of ophthalmologists said they would be at least somewhat likely to prescribe aflibercept biosimilars, and 64% said they would be at least somewhat likely to prescribe ranibizumab biosimilars. About half of respondents said they would not likely switch a currently stable patient on either aflibercept or ranibizumab to the corresponding biosimilar. More than half of ophthalmologists (56%) said they would prescribe a biosimilar only if it had an interchangeability designation.
Out of all four specialties, ophthalmologists more frequently reported that higher discounts from a reference product would be necessary to consider switching a patient to a biosimilar. Currently, many ophthalmologists are comfortable with the off-label use of bevacizumab (Avastin) for treating wet age-related macular degeneration, which also offers more cost savings than any currently available biosimilar on the market, Dr. Williams said.
While the limited number of respondents makes it difficult to draw concrete conclusions, Dr. Williams emphasized that the AAO supported the use of biosimilars. “We believe that with clinical experience ophthalmic biosimilars will become useful therapeutic agents,” he noted.
A version of this article first appeared on Medscape.com.
While most providers think that biosimilars will positively impact care, few feel that the economic benefits of biosimilars to date are enough to motivate switching.
In a new survey of over 350 dermatologists, gastroenterologists, ophthalmologists, and rheumatologists, clinicians shared their opinions on the rapidly evolving landscape of biosimilars, detailing top concerns about prescribing these medications and how they presently use biosimilars in clinical practice. Across all specialties, providers said they would be most likely to prescribe biosimilars to new patients or if a patient’s health plan mandated the switch. Most providers listed concerns about biosimilar efficacy and lack of economic benefit as the main barriers to adoption of biosimilars in clinical practice.
Cardinal Health, a health care services company based in Dublin, Ohio, conducted the surveys from July through October 2022.
Rheumatologists want cost-savings for patients
2023 is gearing up to be a big year for biosimilars for inflammatory diseases, with at least eight adalimumab biosimilars entering the market in the United States. Amjevita, manufactured by Amgen, was the first to become commercially available on Jan. 31. Out of 103 surveyed rheumatologists, 62% said they were very comfortable prescribing biosimilars to patients, and 32% said they were somewhat comfortable. Providers said they would be most likely to prescribe a biosimilar to new patients (40%) or if biosimilars were mandated by a patient’s health plan (41%). Nearly one-third (31%) of rheumatologists said that a discount of 21%-30% from a reference product would be necessary to consider switching a patient to a biosimilar.
There are several reasons why a rheumatologist might be wary of switching patients to biosimilars, said Marcus Snow, MD, chair of the American College of Rheumatology’s Committee on Rheumatologic Care. “Rheumatologists will always express concern about changing medications that work well for their patients. It is not ideal to ‘force switch’ to a different product, even if it is almost identical,” he told this news organization in an email. “Also, we must remember that a patient on a biologic has failed traditional medications, which speaks to the struggle a patient must endure to get their disease under control. Fail-first situations can cause a rheumatologist to be initially resistant or hesitant to any changes.”
The top concerns among rheumatologists about prescribing biosimilars were medication efficacy (36%), lack of economic benefit (24%), and evaluating when to prescribe a biosimilar versus a reference product (17%). For adalimumab biosimilars, rheumatologists said that interchangeability – a regulatory designation where a biosimilar can be automatically substituted for its reference product at the pharmacy – and citrate-free formulation were the most important product attributes. Sixty-four percent of providers also noted that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
“There needs to be a true reduction in price, to change providers’ opinions on the economic benefits of biosimilars – in the system generally and for the patient,” Dr. Snow said. “Things will get there eventually, but it is not there yet, based on the list prices we see for some biosimilars.”
Gastroenterologists emphasize patient education
Gastroenterology is another specialty to be affected by the influx of adalimumab biosimilars. Out of 72 surveyed gastroenterologists, 86% said they were very comfortable prescribing biosimilars. About half (49%) said they would be most likely to prescribe a biosimilar to patients with health plans mandating a biosimilar. More than 60% of surveyed gastroenterologists said that biosimilars would positively impact care; providers were divided on the current economic benefits of biosimilars, with 36% saying that the current discounts on biosimilars versus reference products were not favorable enough to motivate switching, and 35% stating that they were. A total of 40% of surveyed providers said that savings of 21%-30%, compared with savings of a reference product, would motivate them to switch patients to a biosimilar, with all other clinical factors being equal.
Gastroenterologists said that, along with the efficacy and cost savings of biosimilars, providing patient education (18%) was a top concern when prescribing biosimilars. Eighty-four percent of respondents said that educating patients about biosimilars as safe and effective treatment options was at least somewhat important. Nearly all participants (99%) cited device ease-of-use as at least somewhat important when considering prescribing adalimumab biosimilars, in addition to interchangeability (97%) and citrate-free formulation (93%).
“Despite general acceptance of biosimilars, there remains some uncertainty regarding their place in the current gastroenterology landscape,” wrote Vivek Kaul, MD, a professor of medicine at the University of Rochester (N.Y.) Medical Center, in the report. “This is likely because only half of the survey respondents believed that biosimilars will positively impact gastroenterology care, further highlighting the ongoing need for real-world data and incorporation of biosimilar use and interchangeability into clinical guidelines.”
Few dermatologists currently prescribe biosimilars
Eight out of ten dermatologists reported being at least somewhat comfortable prescribing biosimilars to patients, though fewer than 20% said they had prescribed a biosimilar in the past year. This indicates limited adoption of infliximab biosimilars, which were the only biosimilars with a dermatologic indication available in 2022, Alex Gross, MD, a dermatologist in Cumming, Ga., noted in his featured commentary in the report. Just 15% of respondents disagreed that biosimilars would have a positive impact on care, and 41% said they were excited about new biosimilars becoming available.
About half (47%) of dermatologists thought the economic benefits of biosimilars were not strong enough to motivate switching patients from reference products. Twenty-nine percent of respondents said that discounts of 21%-30% from a reference product would motivate them to switch patients to a biosimilar, with all other clinical factors being equal, while 20% said they were not likely to prescribe a biosimilar regardless of savings.
Dermatologists may be concerned that these cost savings may not be passed onto patients, said Alison Ehrlich, MD, a dermatologist in Washington, in an email to this news organization. Patient out-of-pocket cost savings would need to be “both significant and transparent” to begin to change providers’ minds, she noted.
Biosimilar efficacy was a top concern for 48% of dermatologists, while 13% said their main concern around prescribing biosimilars was lack of payer adoption. At least 95% of providers said that device ease-of-use and interchangeability were the most important attributes when considering adalimumab biosimilars. Nearly two-thirds (65%) reported that patient out-of-pocket cost would be key when deciding to prescribe an adalimumab biosimilar.
If both patients and providers are informed on biosimilar use and there are cost benefits, dermatologists’ opinions may become more favorable toward biosimilars, but that will take time, Dr. Ehrlich said. “We are very early in the game for biosimilar use in dermatology,” she added.
Ophthalmologists remain wary
Biosimilars have been relatively new to ophthalmology, with the first ranibizumab biosimilar becoming commercially available in July 2022. In the survey, 64 retina specialists were asked different questions than participants from other specialties to gauge ophthalmologists› familiarity with the biosimilars approval process and their overall comfort prescribing these medications. The primary concerns with prescribing biosimilars among respondents was payer coverage (52%), being uncomfortable with biosimilars from a clinical standpoint (48%), and administrative barriers (45%), such as prior authorization. Despite this lack of comfort with biosimilars, two-thirds of participants thought the U.S. Food and Drug Administration approval process for these medications was sufficient to evaluate their efficacy and safety. Still, fewer than half (48%) of providers said they do or would prescribe biosimilars.
George Williams, MD, a spokesperson for the American Academy of Ophthalmology, noted that the FDA approval process for biosimilars was not as rigorous as for the respective reference product, and fewer patients are followed over a shorter time period. “Since anti–[vascular endothelial growth factor (VEGF)] therapy for indications such as neovascular age-related macular degeneration continues indefinitely over years, ophthalmologists may have concerns about the long-term efficacy and safety when applied to larger real-world populations. Ophthalmologists are well aware of safety issues with VEGF inhibitors arising after FDA approval,” he told this news organization in an email.
When asked about the likelihood of using either aflibercept or ranibizumab biosimilars in their clinical practice once commercially available, 70% of ophthalmologists said they would be at least somewhat likely to prescribe aflibercept biosimilars, and 64% said they would be at least somewhat likely to prescribe ranibizumab biosimilars. About half of respondents said they would not likely switch a currently stable patient on either aflibercept or ranibizumab to the corresponding biosimilar. More than half of ophthalmologists (56%) said they would prescribe a biosimilar only if it had an interchangeability designation.
Out of all four specialties, ophthalmologists more frequently reported that higher discounts from a reference product would be necessary to consider switching a patient to a biosimilar. Currently, many ophthalmologists are comfortable with the off-label use of bevacizumab (Avastin) for treating wet age-related macular degeneration, which also offers more cost savings than any currently available biosimilar on the market, Dr. Williams said.
While the limited number of respondents makes it difficult to draw concrete conclusions, Dr. Williams emphasized that the AAO supported the use of biosimilars. “We believe that with clinical experience ophthalmic biosimilars will become useful therapeutic agents,” he noted.
A version of this article first appeared on Medscape.com.
Expert offers caveats to perioperative antirheumatic drug guideline
The latest guideline for perioperative management of antirheumatic medication in patients undergoing total hip (THA) and total knee arthroplasty (TKA) offers recommendations based on the latest evidence, but many of those recommendations are based on a low level of evidence, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Martin Bergman, MD, clinical professor of medicine at Drexel University, Philadelphia, said the development of the American College of Rheumatology/American Association of Hip and Knee Surgeons guideline was necessary because there was a lack of consensus on when to stop treatments prior to patients with rheumatologic disease undergoing THA and TKA, and when it was appropriate to restart those treatments.
“We all were having the same problem, and I think everybody recognized that just stopping medicines forever didn’t make sense, but maybe continuing medicines also didn’t make sense,” Dr. Bergman said.
While the 2017 ACR/AAHKS perioperative management guideline contained good recommendations, the “explosion” of new medications in rheumatology made it necessary to update the guideline with the latest data on new medications such as immunosuppressants.
2022 guideline recommendations
In the 2022 guideline, which covers disease-modifying treatments taken by patients with rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis, the authors reaffirmed their recommendations to continue methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, and apremilast through total joint arthroplasty.
Where the 2022 guideline differs from the 2017 guideline is in which biologics are covered and under what circumstances they should be withheld and restarted around surgery. The 2022 guideline includes recommendations for abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, guselkumab, infliximab, ixekizumab, rituximab, secukinumab, tocilizumab, and ustekinumab. Each biologic has its own recommended stop and restart times based around the dosing interval and respective method of administration. Dr. Bergman said a general rule with biologics under the new guideline is that the timing of surgery should occur approximately 1 week after the first missed dose of the medication. The only biologic that does not follow this pattern is rituximab, where surgery should be planned for 1 month after the last missed dose.
Dr. Bergman noted that how the guidelines handle interval dosing with infliximab may present a problem. The guideline provides recommendations for patients receiving infliximab every 4 weeks, every 6 weeks, and every 8 weeks. However, Dr. Bergman said this can create a scenario where a patient receiving infliximab at a dose of 3 mg/kg every 8 weeks has surgery at 9 weeks, a patient receiving 5 mg/kg every 6 weeks has surgery at 7 weeks, and a patient receiving 10 mg/kg every 4 weeks has surgery at 5 weeks. “There is some intellectual problem with it,” he said.
Another change from the 2017 guideline is how long to wait for surgery after stopping Janus kinase inhibitors. While the 2017 guideline recommended withholding JAK inhibitors 7 days before surgery, the 2022 guideline lowered that waiting period to 3 days, Dr. Bergman explained.
Concerning use of steroids around THA and TKA surgery, “the days of stress steroid dosing are done,” Dr. Bergman said. “You don’t have to stress dose them. You just follow them, and you keep them on their steroid dose.”
The new guideline recommends restarting therapy once the wound is healed and there is no physical evidence of infection at approximately 2 weeks. “There’s no data to support this,” he said, and his concern is that patients who have stopped a tumor necrosis factor inhibitor may flare if they don’t restart their medication.
While the guideline also covered recommendations for systemic lupus erythematosus, they are “very similar” to the recommendations for inflammatory arthritis, Dr. Bergman noted. “If you have somebody who is not very sick, you stop the medications,” he said, “but try to stop anything else about a week before the surgery. If they’re sick, you basically have to keep them on their medications.”
Caveats in guideline
The recommendations in the 2022 guideline come with a number of caveats, Dr. Bergman noted. For instance, the authors acknowledged limitations in the guideline regarding providing recommendations for only THA and TKA, the “paucity of evidence” around direct infection risk resulting from medications in the perioperative period for THA and TKA, the nonseparation of biologics when assessing infection risk, and the use of dosing interval as a metric for stopping the drug without considering the drug’s half-life.
A “crucial caveat,” Dr. Bergman said, was that the guideline focused on infection risk based on a statement from a panel of patients prior to the development of the 2017 guideline, which “stated very clearly any risk of infection, while rare, was more significant to them than the possibility of postoperative flares, despite flares being reported in over 60% of patients after surgery.
“For the patients, the paramount question was infection, infection, infection, infection. That’s all they cared about, and that is the basis behind a lot of the decision-making here,” Dr. Bergman said.
Another caveat came from a communication Dr. Bergman received from one of the panel members. The panel member noted there were no conclusions or recommendations provided in the guideline for how to manage perioperative flares, such as restarting a corticosteroid or biologic agent. “There was a lot of discussion about what to do with steroids if patients flare, or what to do with [other] medications if they flare, and they just couldn’t come to a consensus,” Dr. Bergman said. “It’s just not discussed.”
Dr. Bergman said he is “somewhat critical” of the ACR/AAHKS guideline, but noted it is an “ambitious project” given the lack of evidence for the recommendations. “The alternative was stop the medications forever and having people really flare, or at least try to get some semblance of rationality behind what we’re going to do,” he said.
Response from attendees
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, took issue with the new recommendations surrounding stopping infliximab. When giving a patient infliximab every 8 weeks at 3 mg/kg, “you’re giving [it] at the nadir of the drug,” he said.
Rather than drug half-life, “it’s about inflammation,” he emphasized. “Inflammation is dominant in causing infection. It drives risk more than anything. The worst thing you can do is wash someone out.
“If you’re going beyond 8 weeks on infliximab, you’re getting closer to washing them out,” he pointed out. “I think it’s a really bad idea.”
Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at Hospital for Special Surgery, both in New York, explained that the guideline is not standard of care, which would be subject to malpractice if not implemented properly.
“When you have guidelines, you follow them unless there are clinical situations which would necessitate another approach to the patient,” he said. “Professional institutions and associations will never put forth rules, they will put forth guidelines so you have the opportunity to deviate from them when the appropriate clinical situation dictates.”
Dr. Bergman reported being a speaker and consultant for AbbVie, Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and Regeneron; he holds stock in Johnson & Johnson and Merck.
The latest guideline for perioperative management of antirheumatic medication in patients undergoing total hip (THA) and total knee arthroplasty (TKA) offers recommendations based on the latest evidence, but many of those recommendations are based on a low level of evidence, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Martin Bergman, MD, clinical professor of medicine at Drexel University, Philadelphia, said the development of the American College of Rheumatology/American Association of Hip and Knee Surgeons guideline was necessary because there was a lack of consensus on when to stop treatments prior to patients with rheumatologic disease undergoing THA and TKA, and when it was appropriate to restart those treatments.
“We all were having the same problem, and I think everybody recognized that just stopping medicines forever didn’t make sense, but maybe continuing medicines also didn’t make sense,” Dr. Bergman said.
While the 2017 ACR/AAHKS perioperative management guideline contained good recommendations, the “explosion” of new medications in rheumatology made it necessary to update the guideline with the latest data on new medications such as immunosuppressants.
2022 guideline recommendations
In the 2022 guideline, which covers disease-modifying treatments taken by patients with rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis, the authors reaffirmed their recommendations to continue methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, and apremilast through total joint arthroplasty.
Where the 2022 guideline differs from the 2017 guideline is in which biologics are covered and under what circumstances they should be withheld and restarted around surgery. The 2022 guideline includes recommendations for abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, guselkumab, infliximab, ixekizumab, rituximab, secukinumab, tocilizumab, and ustekinumab. Each biologic has its own recommended stop and restart times based around the dosing interval and respective method of administration. Dr. Bergman said a general rule with biologics under the new guideline is that the timing of surgery should occur approximately 1 week after the first missed dose of the medication. The only biologic that does not follow this pattern is rituximab, where surgery should be planned for 1 month after the last missed dose.
Dr. Bergman noted that how the guidelines handle interval dosing with infliximab may present a problem. The guideline provides recommendations for patients receiving infliximab every 4 weeks, every 6 weeks, and every 8 weeks. However, Dr. Bergman said this can create a scenario where a patient receiving infliximab at a dose of 3 mg/kg every 8 weeks has surgery at 9 weeks, a patient receiving 5 mg/kg every 6 weeks has surgery at 7 weeks, and a patient receiving 10 mg/kg every 4 weeks has surgery at 5 weeks. “There is some intellectual problem with it,” he said.
Another change from the 2017 guideline is how long to wait for surgery after stopping Janus kinase inhibitors. While the 2017 guideline recommended withholding JAK inhibitors 7 days before surgery, the 2022 guideline lowered that waiting period to 3 days, Dr. Bergman explained.
Concerning use of steroids around THA and TKA surgery, “the days of stress steroid dosing are done,” Dr. Bergman said. “You don’t have to stress dose them. You just follow them, and you keep them on their steroid dose.”
The new guideline recommends restarting therapy once the wound is healed and there is no physical evidence of infection at approximately 2 weeks. “There’s no data to support this,” he said, and his concern is that patients who have stopped a tumor necrosis factor inhibitor may flare if they don’t restart their medication.
While the guideline also covered recommendations for systemic lupus erythematosus, they are “very similar” to the recommendations for inflammatory arthritis, Dr. Bergman noted. “If you have somebody who is not very sick, you stop the medications,” he said, “but try to stop anything else about a week before the surgery. If they’re sick, you basically have to keep them on their medications.”
Caveats in guideline
The recommendations in the 2022 guideline come with a number of caveats, Dr. Bergman noted. For instance, the authors acknowledged limitations in the guideline regarding providing recommendations for only THA and TKA, the “paucity of evidence” around direct infection risk resulting from medications in the perioperative period for THA and TKA, the nonseparation of biologics when assessing infection risk, and the use of dosing interval as a metric for stopping the drug without considering the drug’s half-life.
A “crucial caveat,” Dr. Bergman said, was that the guideline focused on infection risk based on a statement from a panel of patients prior to the development of the 2017 guideline, which “stated very clearly any risk of infection, while rare, was more significant to them than the possibility of postoperative flares, despite flares being reported in over 60% of patients after surgery.
“For the patients, the paramount question was infection, infection, infection, infection. That’s all they cared about, and that is the basis behind a lot of the decision-making here,” Dr. Bergman said.
Another caveat came from a communication Dr. Bergman received from one of the panel members. The panel member noted there were no conclusions or recommendations provided in the guideline for how to manage perioperative flares, such as restarting a corticosteroid or biologic agent. “There was a lot of discussion about what to do with steroids if patients flare, or what to do with [other] medications if they flare, and they just couldn’t come to a consensus,” Dr. Bergman said. “It’s just not discussed.”
Dr. Bergman said he is “somewhat critical” of the ACR/AAHKS guideline, but noted it is an “ambitious project” given the lack of evidence for the recommendations. “The alternative was stop the medications forever and having people really flare, or at least try to get some semblance of rationality behind what we’re going to do,” he said.
Response from attendees
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, took issue with the new recommendations surrounding stopping infliximab. When giving a patient infliximab every 8 weeks at 3 mg/kg, “you’re giving [it] at the nadir of the drug,” he said.
Rather than drug half-life, “it’s about inflammation,” he emphasized. “Inflammation is dominant in causing infection. It drives risk more than anything. The worst thing you can do is wash someone out.
“If you’re going beyond 8 weeks on infliximab, you’re getting closer to washing them out,” he pointed out. “I think it’s a really bad idea.”
Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at Hospital for Special Surgery, both in New York, explained that the guideline is not standard of care, which would be subject to malpractice if not implemented properly.
“When you have guidelines, you follow them unless there are clinical situations which would necessitate another approach to the patient,” he said. “Professional institutions and associations will never put forth rules, they will put forth guidelines so you have the opportunity to deviate from them when the appropriate clinical situation dictates.”
Dr. Bergman reported being a speaker and consultant for AbbVie, Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and Regeneron; he holds stock in Johnson & Johnson and Merck.
The latest guideline for perioperative management of antirheumatic medication in patients undergoing total hip (THA) and total knee arthroplasty (TKA) offers recommendations based on the latest evidence, but many of those recommendations are based on a low level of evidence, according to a speaker at the 2023 Rheumatology Winter Clinical Symposium.
Martin Bergman, MD, clinical professor of medicine at Drexel University, Philadelphia, said the development of the American College of Rheumatology/American Association of Hip and Knee Surgeons guideline was necessary because there was a lack of consensus on when to stop treatments prior to patients with rheumatologic disease undergoing THA and TKA, and when it was appropriate to restart those treatments.
“We all were having the same problem, and I think everybody recognized that just stopping medicines forever didn’t make sense, but maybe continuing medicines also didn’t make sense,” Dr. Bergman said.
While the 2017 ACR/AAHKS perioperative management guideline contained good recommendations, the “explosion” of new medications in rheumatology made it necessary to update the guideline with the latest data on new medications such as immunosuppressants.
2022 guideline recommendations
In the 2022 guideline, which covers disease-modifying treatments taken by patients with rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis, the authors reaffirmed their recommendations to continue methotrexate, sulfasalazine, hydroxychloroquine, leflunomide, and apremilast through total joint arthroplasty.
Where the 2022 guideline differs from the 2017 guideline is in which biologics are covered and under what circumstances they should be withheld and restarted around surgery. The 2022 guideline includes recommendations for abatacept, adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, guselkumab, infliximab, ixekizumab, rituximab, secukinumab, tocilizumab, and ustekinumab. Each biologic has its own recommended stop and restart times based around the dosing interval and respective method of administration. Dr. Bergman said a general rule with biologics under the new guideline is that the timing of surgery should occur approximately 1 week after the first missed dose of the medication. The only biologic that does not follow this pattern is rituximab, where surgery should be planned for 1 month after the last missed dose.
Dr. Bergman noted that how the guidelines handle interval dosing with infliximab may present a problem. The guideline provides recommendations for patients receiving infliximab every 4 weeks, every 6 weeks, and every 8 weeks. However, Dr. Bergman said this can create a scenario where a patient receiving infliximab at a dose of 3 mg/kg every 8 weeks has surgery at 9 weeks, a patient receiving 5 mg/kg every 6 weeks has surgery at 7 weeks, and a patient receiving 10 mg/kg every 4 weeks has surgery at 5 weeks. “There is some intellectual problem with it,” he said.
Another change from the 2017 guideline is how long to wait for surgery after stopping Janus kinase inhibitors. While the 2017 guideline recommended withholding JAK inhibitors 7 days before surgery, the 2022 guideline lowered that waiting period to 3 days, Dr. Bergman explained.
Concerning use of steroids around THA and TKA surgery, “the days of stress steroid dosing are done,” Dr. Bergman said. “You don’t have to stress dose them. You just follow them, and you keep them on their steroid dose.”
The new guideline recommends restarting therapy once the wound is healed and there is no physical evidence of infection at approximately 2 weeks. “There’s no data to support this,” he said, and his concern is that patients who have stopped a tumor necrosis factor inhibitor may flare if they don’t restart their medication.
While the guideline also covered recommendations for systemic lupus erythematosus, they are “very similar” to the recommendations for inflammatory arthritis, Dr. Bergman noted. “If you have somebody who is not very sick, you stop the medications,” he said, “but try to stop anything else about a week before the surgery. If they’re sick, you basically have to keep them on their medications.”
Caveats in guideline
The recommendations in the 2022 guideline come with a number of caveats, Dr. Bergman noted. For instance, the authors acknowledged limitations in the guideline regarding providing recommendations for only THA and TKA, the “paucity of evidence” around direct infection risk resulting from medications in the perioperative period for THA and TKA, the nonseparation of biologics when assessing infection risk, and the use of dosing interval as a metric for stopping the drug without considering the drug’s half-life.
A “crucial caveat,” Dr. Bergman said, was that the guideline focused on infection risk based on a statement from a panel of patients prior to the development of the 2017 guideline, which “stated very clearly any risk of infection, while rare, was more significant to them than the possibility of postoperative flares, despite flares being reported in over 60% of patients after surgery.
“For the patients, the paramount question was infection, infection, infection, infection. That’s all they cared about, and that is the basis behind a lot of the decision-making here,” Dr. Bergman said.
Another caveat came from a communication Dr. Bergman received from one of the panel members. The panel member noted there were no conclusions or recommendations provided in the guideline for how to manage perioperative flares, such as restarting a corticosteroid or biologic agent. “There was a lot of discussion about what to do with steroids if patients flare, or what to do with [other] medications if they flare, and they just couldn’t come to a consensus,” Dr. Bergman said. “It’s just not discussed.”
Dr. Bergman said he is “somewhat critical” of the ACR/AAHKS guideline, but noted it is an “ambitious project” given the lack of evidence for the recommendations. “The alternative was stop the medications forever and having people really flare, or at least try to get some semblance of rationality behind what we’re going to do,” he said.
Response from attendees
Jack Cush, MD, a rheumatologist based in Dallas and executive editor of RheumNow.com, took issue with the new recommendations surrounding stopping infliximab. When giving a patient infliximab every 8 weeks at 3 mg/kg, “you’re giving [it] at the nadir of the drug,” he said.
Rather than drug half-life, “it’s about inflammation,” he emphasized. “Inflammation is dominant in causing infection. It drives risk more than anything. The worst thing you can do is wash someone out.
“If you’re going beyond 8 weeks on infliximab, you’re getting closer to washing them out,” he pointed out. “I think it’s a really bad idea.”
Allan Gibofsky, MD, JD, professor of medicine at Weill Cornell Medicine and codirector of the Clinic for Inflammatory Arthritis and Biologic Therapy at Hospital for Special Surgery, both in New York, explained that the guideline is not standard of care, which would be subject to malpractice if not implemented properly.
“When you have guidelines, you follow them unless there are clinical situations which would necessitate another approach to the patient,” he said. “Professional institutions and associations will never put forth rules, they will put forth guidelines so you have the opportunity to deviate from them when the appropriate clinical situation dictates.”
Dr. Bergman reported being a speaker and consultant for AbbVie, Amgen, Bristol-Myers Squibb, GlaxoSmithKline, Novartis, Pfizer, and Regeneron; he holds stock in Johnson & Johnson and Merck.
FROM RWCS 2023
New influx of Humira biosimilars may not drive immediate change
Gastroenterologists in 2023 will have more tools in their arsenal to treat patients with Crohn’s disease or ulcerative colitis. As many as 8-10 adalimumab biosimilars are anticipated to come on the market this year, giving mainstay drug Humira some vigorous competition.
Three scenarios will drive adalimumab biosimilar initiation: Insurance preference for the initial treatment of a newly diagnosed condition, a change in a patient’s insurance plan, or an insurance-mandated switch, said Edward C. Oldfield IV, MD, assistant professor at Eastern Virginia Medical School’s division of gastroenterology in Norfolk.
“Outside of these scenarios, I would encourage patients to remain on their current biologic so long as cost and accessibility remain stable,” said Dr. Oldfield.
Many factors will contribute to the success of biosimilars. Will physicians be prescribing them? How are biosimilars placed on formularies and will they be given preferred status? How will manufacturers price their biosimilars? “We have to wait and see to get the answers to these questions,” said Steven Newmark, JD, MPA, chief legal officer and director of policy, Global Healthy Living Foundation/CreakyJoints, a nonprofit advocacy organization based in New York.
Prescribing biosimilars is no different than prescribing originator biologics, so providers should know how to use them, said Mr. Newmark. “Most important will be the availability of patient-friendly resources that providers can share with their patients to provide education about and confidence in using biosimilars,” he added.
Overall, biosimilars are a good thing, said Dr. Oldfield. “In the long run they should bring down costs and increase access to medications for our patients.”
Others are skeptical that the adalimumab biosimilars will save patients much money.
Biosimilar laws were created to lower costs. However, if a patient with insurance pays only $5 a month out of pocket for Humira – a drug that normally costs $7,000 without coverage – it’s unlikely they would want to switch unless there’s comparable savings from the biosimilar, said Stephen B. Hanauer, MD, medical director of the Digestive Health Center and professor of medicine at Northwestern Medicine, Northwestern University, Evanston, Ill.
Like generics, Humira biosimilars may face some initial backlash, said Dr. Hanauer.
2023 broadens scope of adalimumab treatments
The American Gastroenterological Association describes a biosimilar as something that’s “highly similar to, but not an exact copy of, a biologic reference product already approved” by the Food and Drug Administration. Congress under the 2010 Affordable Care Act created a special, abbreviated pathway to approval for biosimilars.
AbbVie’s Humira, the global revenue for which exceeded $20 billion in 2021, has long dominated the U.S. market on injectable treatments for autoimmune diseases. The popular drug faces some competition in 2023, however, following a series of legal settlements that allowed AbbVie competitors to release their own adalimumab biosimilars.
“So far, we haven’t seen biosimilars live up to their potential in the U.S. in the inflammatory space,” said Mr. Newmark. This may change, however. Previously, biosimilars have required infusion, which demanded more time, commitment, and travel from patients. “The new set of forthcoming Humira biosimilars are injectables, an administration method preferred by patients,” he said.
The FDA will approve a biosimilar if it determines that the biological product is highly similar to the reference product, and that there are no clinically meaningful differences between the biological and reference product in terms of the safety, purity, and potency of the product.
The agency to date has approved 8 adalimumab biosimilars. These include: Idacio (adalimumab-aacf, Fresenius Kabi); Amjevita (adalimumab-atto, Amgen); Hadlima (adalimumab-bwwd, Organon); Cyltezo (adalimumab-adbm, Boehringer Ingelheim); Yusimry (adalimumab-aqvh from Coherus BioSciences); Hulio (adalimumab-fkjp; Mylan/Fujifilm Kyowa Kirin Biologics); Hyrimoz (adalimumab-adaz, Sandoz), and Abrilada (adalimumab-afzb, Pfizer).
“While FDA doesn’t formally track when products come to market, we know based on published reports that application holders for many of the currently FDA-approved biosimilars plan to market this year, starting with Amjevita being the first adalimumab biosimilar launched” in January, said Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars at the agency.
At press time, two other companies (Celltrion and Alvotech/Teva) were awaiting FDA approval for their adalimumab biosimilar drugs.
Among the eight approved drugs, Cyltezo is the only one that has a designation for interchangeability with Humira.
An interchangeable biosimilar may be substituted at the pharmacy without the intervention of the prescriber – much like generics are substituted, depending on state laws, said Dr. Yim. “However, in terms of safety and effectiveness, FDA’s standards for approval mean that biosimilar or interchangeable biosimilar products can be used in place of the reference product they were compared to.”
FDA-approved biosimilars undergo a rigorous evaluation for safety, effectiveness, and quality for their approved conditions of use, she continued. “Therefore, patients and health care providers can rely on a biosimilar to be as safe and effective for its approved uses as the original biological product.”
Remicade as a yard stick
Gastroenterologists dealt with this situation once before, when Remicade (infliximab) biosimilars came on the market in 2016, noted Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at the Cleveland Clinic.
Remicade and Humira are both tumor necrosis factor inhibitors with the same mechanism of action and many of the same indications. “We already had that experience with Remicade and biosimilar switch 2 or 3 years ago. Now we’re talking about Humira,” said Dr. Regueiro.
Most GI doctors have prescribed one of the more common infliximab biosimilars (Inflectra or Renflexis), noted Dr. Oldfield.
Cardinal Health, which recently surveyed 300 gastroenterologists, rheumatologists, and dermatologists about adalimumab biosimilars, found that gastroenterologists had the highest comfort level in prescribing them. Their top concern, however, was changing a patient from adalimumab to an adalimumab biosimilar.
For most patients, Dr. Oldfield sees the Humira reference biologic and biosimilar as equivalent.
However, he said he would change a patient’s drug only if there were a good reason or if his hand was forced by insurance. He would not make the change for a patient who recently began induction with the reference biologic or a patient with highly active clinical disease.
“While there is limited data to support this, I would also have some qualms about changing a patient from reference biologic to a biosimilar if they previously had immune-mediated pharmacokinetic failure due to antibody development with a biologic and were currently doing well on their new biologic,” he said.
Those with a new ulcerative colitis or Crohn’s diagnosis who are initiating a biologic for the first time might consider a biosimilar. If a patient is transitioning from a reference biologic to a biosimilar, “I would want to make that change during a time of stable remission and with the recognition that the switch is not a temporary switch, but a long-term switch,” he continued.
A paper that reviewed 23 observational studies of adalimumab and other biosimilars found that switching biosimilars was safe and effective. But if possible, patients should minimize the number of switches until more robust long-term data are available, added Dr. Oldfield.
If a patient is apprehensive about switching to a new therapy, “one may need to be cognizant of the ‘nocebo’ effect in which there is an unexplained or unfavorable therapeutic effect after switching,” he said.
Other gastroenterologists voiced similar reservations about switching. “I won’t use an adalimumab biosimilar unless the patient requests it, the insurance requires it, or there is a cost advantage for the patient such that they prefer it,” said Doug Wolf, MD, an Atlanta gastroenterologist.
“There is no medical treatment advantage to a biosimilar, especially if switching from Humira,” added Dr. Wolf.
Insurance will guide treatment
Once a drug is approved for use by the FDA, that drug will be available in all 50 states. “Different private insurance formularies, as well as state Medicaid formularies, might affect the actual ability of patients to receive such drugs,” said Mr. Newmark.
Patients should consult with their providers and insurance companies to see what therapies are available, he advised.
Dr. Hanauer anticipates some headaches arising for patients and doctors alike when negotiating for a specific drug.
Cyltezo may be the only biosimilar interchangeable with Humira, but the third-party pharmacy benefit manager (PBM) could negotiate for one of the noninterchangeable ones. “On a yearly basis they could switch their preference,” said Dr. Hanauer.
In the Cardinal Health survey, more than 60% of respondents said they would feel comfortable prescribing an adalimumab biosimilar only with an interchangeability designation.
A PBM may offer a patient Cyltezo if it’s cheaper than Humira. If the patient insists on staying on Humira, then they’ll have to pay more for that drug on their payer’s formulary, said Dr. Hanauer. In a worst-case scenario, a physician may have to appeal on a patient’s behalf to get Humira if the insurer offers only the biosimilar.
Taking that step to appeal is a major hassle for the physician, and leads to extra back door costs as well, said Dr. Hanauer.
Humira manufacturer AbbVie, in turn, may offer discounts and rebates to the PBMs to put Humira on their formulary. “That’s the AbbVie negotiating power. It’s not that the cost is going to be that much different. It’s going to be that there are rebates and discounts that are going to make the cost different,” he added.
As a community physician, Dr. Oldfield has specific concerns about accessibility.
The ever-increasing burden of insurance documentation and prior authorization means it can take weeks or months to get these medications approved. “The addition of new biosimilars is a welcome entrance if it can get patients the medications they need when they need it,” he said.
When it comes to prescribing biologics, many physicians rely on ancillary staff for assistance. It’s a team effort to sift through all the paperwork, observed Dr. Oldfield.
“While many community GI practices have specialized staff to deal with prior authorizations, they are still a far cry from the IBD [inflammatory bowel disease] academic centers where there are often pharmacists, nursing specialists, and home-monitoring programs to check in on patients,” he explained.
Landscape on cost is uncertain
At present, little is known about the cost of the biosimilars and impact on future drug pricing, said Dr. Oldfield.
At least for Medicare, Humira biosimilars will be considered Medicare Part D drugs if used for a medically accepted indication, said a spokesperson for the Centers for Medicare and Medicaid Services.
Part D sponsors (pharmacy and therapeutic committees) “will make the determination as to whether Amjevita and other products will be added to their formularies,” said the spokesperson.
Patients never saw a significant cost savings with Remicade biosimilars. “I imagine the same would be true with biosimilars for Humira,” said Dr. Regueiro. Patients may see greater access to these drugs, however, because the insurance plan or the pharmacy plan will make them more readily available, he added.
The hope is that, as biosimilars are introduced, the price of the originator biologic will go down, said Mr. Newmark. “Therefore, we can expect Humira to be offered at a lower price as it faces competition. Where it will sit in comparison to the forthcoming biosimilars will depend on how much biosimilar companies drop their price and how much pressure will be on PBMs and insurers to cover the lowest list price drug,” he said.
AbbVie did not respond to several requests for comment.
Charitable patient assistance programs for biosimilars or biologics can help offset the price of copayments, Mr. Newmark offered.
Ideally, insurers will offer designated biosimilars at a reduced or even no out-of-pocket expense on their formularies. This should lead to a decreased administrative burden for approval with streamlined (or even removal) of prior authorizations for certain medications, said Dr. Oldfield.
Without insurance or medication assistance programs, the cost of biosimilars is prohibitively expensive, he added.
“Biosimilars have higher research, development, and manufacturing costs than what people conventionally think of [for] a generic medication.”
Educating, advising patients
Dr. Oldfield advised that gastroenterologists refer to biologics by the generic name rather than branded name when initiating therapy unless there is a very specific reason not to. “This approach should make the process more streamlined and less subjected to quick denials for brand-only requests as biosimilars start to assume a larger market share,” he said.
Uptake of the Humira biosimilars also will depend on proper education of physicians and patients and their comfort level with the biosimilars, said Dr. Regueiro. Cleveland Clinic uses a team approach to educate on this topic, relying on pharmacists, clinicians, and nurses to explain that there’s no real difference between the reference drug and its biosimilars, based on efficacy and safety data.
Physicians can also direct patients to patient-friendly resources, said Mr. Newmark. “By starting the conversation early, it ensures that when/if the time comes that your patient is switched to or chooses a biosimilar they will feel more confident because they have the knowledge to make decisions about their care.”
The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars , is a free resource for patients, he added.
It’s important that doctors also understand these products so they can explain to their patients what to expect, said the FDA’s Dr. Yim. The FDA provides educational materials on its website, including a comprehensive curriculum toolkit.
Dr. Hanauer has served as a consultant for AbbVie, Amgen, American College of Gastroenterology, GlaxoSmithKline, American Gastroenterological Association, Pfizer, and a host of other companies . Dr. Regueiro has served on advisory boards and as a consultant for Abbvie, Janssen, UCB, Takeda, Pfizer, BMS, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET Pharma Solutions,Trellis, and Boehringer Ingelheim Pharmaceuticals. Dr. Wolf, Dr. Yim, Dr. Oldfield, and Mr. Newmark have no financial conflicts of interest.
Gastroenterologists in 2023 will have more tools in their arsenal to treat patients with Crohn’s disease or ulcerative colitis. As many as 8-10 adalimumab biosimilars are anticipated to come on the market this year, giving mainstay drug Humira some vigorous competition.
Three scenarios will drive adalimumab biosimilar initiation: Insurance preference for the initial treatment of a newly diagnosed condition, a change in a patient’s insurance plan, or an insurance-mandated switch, said Edward C. Oldfield IV, MD, assistant professor at Eastern Virginia Medical School’s division of gastroenterology in Norfolk.
“Outside of these scenarios, I would encourage patients to remain on their current biologic so long as cost and accessibility remain stable,” said Dr. Oldfield.
Many factors will contribute to the success of biosimilars. Will physicians be prescribing them? How are biosimilars placed on formularies and will they be given preferred status? How will manufacturers price their biosimilars? “We have to wait and see to get the answers to these questions,” said Steven Newmark, JD, MPA, chief legal officer and director of policy, Global Healthy Living Foundation/CreakyJoints, a nonprofit advocacy organization based in New York.
Prescribing biosimilars is no different than prescribing originator biologics, so providers should know how to use them, said Mr. Newmark. “Most important will be the availability of patient-friendly resources that providers can share with their patients to provide education about and confidence in using biosimilars,” he added.
Overall, biosimilars are a good thing, said Dr. Oldfield. “In the long run they should bring down costs and increase access to medications for our patients.”
Others are skeptical that the adalimumab biosimilars will save patients much money.
Biosimilar laws were created to lower costs. However, if a patient with insurance pays only $5 a month out of pocket for Humira – a drug that normally costs $7,000 without coverage – it’s unlikely they would want to switch unless there’s comparable savings from the biosimilar, said Stephen B. Hanauer, MD, medical director of the Digestive Health Center and professor of medicine at Northwestern Medicine, Northwestern University, Evanston, Ill.
Like generics, Humira biosimilars may face some initial backlash, said Dr. Hanauer.
2023 broadens scope of adalimumab treatments
The American Gastroenterological Association describes a biosimilar as something that’s “highly similar to, but not an exact copy of, a biologic reference product already approved” by the Food and Drug Administration. Congress under the 2010 Affordable Care Act created a special, abbreviated pathway to approval for biosimilars.
AbbVie’s Humira, the global revenue for which exceeded $20 billion in 2021, has long dominated the U.S. market on injectable treatments for autoimmune diseases. The popular drug faces some competition in 2023, however, following a series of legal settlements that allowed AbbVie competitors to release their own adalimumab biosimilars.
“So far, we haven’t seen biosimilars live up to their potential in the U.S. in the inflammatory space,” said Mr. Newmark. This may change, however. Previously, biosimilars have required infusion, which demanded more time, commitment, and travel from patients. “The new set of forthcoming Humira biosimilars are injectables, an administration method preferred by patients,” he said.
The FDA will approve a biosimilar if it determines that the biological product is highly similar to the reference product, and that there are no clinically meaningful differences between the biological and reference product in terms of the safety, purity, and potency of the product.
The agency to date has approved 8 adalimumab biosimilars. These include: Idacio (adalimumab-aacf, Fresenius Kabi); Amjevita (adalimumab-atto, Amgen); Hadlima (adalimumab-bwwd, Organon); Cyltezo (adalimumab-adbm, Boehringer Ingelheim); Yusimry (adalimumab-aqvh from Coherus BioSciences); Hulio (adalimumab-fkjp; Mylan/Fujifilm Kyowa Kirin Biologics); Hyrimoz (adalimumab-adaz, Sandoz), and Abrilada (adalimumab-afzb, Pfizer).
“While FDA doesn’t formally track when products come to market, we know based on published reports that application holders for many of the currently FDA-approved biosimilars plan to market this year, starting with Amjevita being the first adalimumab biosimilar launched” in January, said Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars at the agency.
At press time, two other companies (Celltrion and Alvotech/Teva) were awaiting FDA approval for their adalimumab biosimilar drugs.
Among the eight approved drugs, Cyltezo is the only one that has a designation for interchangeability with Humira.
An interchangeable biosimilar may be substituted at the pharmacy without the intervention of the prescriber – much like generics are substituted, depending on state laws, said Dr. Yim. “However, in terms of safety and effectiveness, FDA’s standards for approval mean that biosimilar or interchangeable biosimilar products can be used in place of the reference product they were compared to.”
FDA-approved biosimilars undergo a rigorous evaluation for safety, effectiveness, and quality for their approved conditions of use, she continued. “Therefore, patients and health care providers can rely on a biosimilar to be as safe and effective for its approved uses as the original biological product.”
Remicade as a yard stick
Gastroenterologists dealt with this situation once before, when Remicade (infliximab) biosimilars came on the market in 2016, noted Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at the Cleveland Clinic.
Remicade and Humira are both tumor necrosis factor inhibitors with the same mechanism of action and many of the same indications. “We already had that experience with Remicade and biosimilar switch 2 or 3 years ago. Now we’re talking about Humira,” said Dr. Regueiro.
Most GI doctors have prescribed one of the more common infliximab biosimilars (Inflectra or Renflexis), noted Dr. Oldfield.
Cardinal Health, which recently surveyed 300 gastroenterologists, rheumatologists, and dermatologists about adalimumab biosimilars, found that gastroenterologists had the highest comfort level in prescribing them. Their top concern, however, was changing a patient from adalimumab to an adalimumab biosimilar.
For most patients, Dr. Oldfield sees the Humira reference biologic and biosimilar as equivalent.
However, he said he would change a patient’s drug only if there were a good reason or if his hand was forced by insurance. He would not make the change for a patient who recently began induction with the reference biologic or a patient with highly active clinical disease.
“While there is limited data to support this, I would also have some qualms about changing a patient from reference biologic to a biosimilar if they previously had immune-mediated pharmacokinetic failure due to antibody development with a biologic and were currently doing well on their new biologic,” he said.
Those with a new ulcerative colitis or Crohn’s diagnosis who are initiating a biologic for the first time might consider a biosimilar. If a patient is transitioning from a reference biologic to a biosimilar, “I would want to make that change during a time of stable remission and with the recognition that the switch is not a temporary switch, but a long-term switch,” he continued.
A paper that reviewed 23 observational studies of adalimumab and other biosimilars found that switching biosimilars was safe and effective. But if possible, patients should minimize the number of switches until more robust long-term data are available, added Dr. Oldfield.
If a patient is apprehensive about switching to a new therapy, “one may need to be cognizant of the ‘nocebo’ effect in which there is an unexplained or unfavorable therapeutic effect after switching,” he said.
Other gastroenterologists voiced similar reservations about switching. “I won’t use an adalimumab biosimilar unless the patient requests it, the insurance requires it, or there is a cost advantage for the patient such that they prefer it,” said Doug Wolf, MD, an Atlanta gastroenterologist.
“There is no medical treatment advantage to a biosimilar, especially if switching from Humira,” added Dr. Wolf.
Insurance will guide treatment
Once a drug is approved for use by the FDA, that drug will be available in all 50 states. “Different private insurance formularies, as well as state Medicaid formularies, might affect the actual ability of patients to receive such drugs,” said Mr. Newmark.
Patients should consult with their providers and insurance companies to see what therapies are available, he advised.
Dr. Hanauer anticipates some headaches arising for patients and doctors alike when negotiating for a specific drug.
Cyltezo may be the only biosimilar interchangeable with Humira, but the third-party pharmacy benefit manager (PBM) could negotiate for one of the noninterchangeable ones. “On a yearly basis they could switch their preference,” said Dr. Hanauer.
In the Cardinal Health survey, more than 60% of respondents said they would feel comfortable prescribing an adalimumab biosimilar only with an interchangeability designation.
A PBM may offer a patient Cyltezo if it’s cheaper than Humira. If the patient insists on staying on Humira, then they’ll have to pay more for that drug on their payer’s formulary, said Dr. Hanauer. In a worst-case scenario, a physician may have to appeal on a patient’s behalf to get Humira if the insurer offers only the biosimilar.
Taking that step to appeal is a major hassle for the physician, and leads to extra back door costs as well, said Dr. Hanauer.
Humira manufacturer AbbVie, in turn, may offer discounts and rebates to the PBMs to put Humira on their formulary. “That’s the AbbVie negotiating power. It’s not that the cost is going to be that much different. It’s going to be that there are rebates and discounts that are going to make the cost different,” he added.
As a community physician, Dr. Oldfield has specific concerns about accessibility.
The ever-increasing burden of insurance documentation and prior authorization means it can take weeks or months to get these medications approved. “The addition of new biosimilars is a welcome entrance if it can get patients the medications they need when they need it,” he said.
When it comes to prescribing biologics, many physicians rely on ancillary staff for assistance. It’s a team effort to sift through all the paperwork, observed Dr. Oldfield.
“While many community GI practices have specialized staff to deal with prior authorizations, they are still a far cry from the IBD [inflammatory bowel disease] academic centers where there are often pharmacists, nursing specialists, and home-monitoring programs to check in on patients,” he explained.
Landscape on cost is uncertain
At present, little is known about the cost of the biosimilars and impact on future drug pricing, said Dr. Oldfield.
At least for Medicare, Humira biosimilars will be considered Medicare Part D drugs if used for a medically accepted indication, said a spokesperson for the Centers for Medicare and Medicaid Services.
Part D sponsors (pharmacy and therapeutic committees) “will make the determination as to whether Amjevita and other products will be added to their formularies,” said the spokesperson.
Patients never saw a significant cost savings with Remicade biosimilars. “I imagine the same would be true with biosimilars for Humira,” said Dr. Regueiro. Patients may see greater access to these drugs, however, because the insurance plan or the pharmacy plan will make them more readily available, he added.
The hope is that, as biosimilars are introduced, the price of the originator biologic will go down, said Mr. Newmark. “Therefore, we can expect Humira to be offered at a lower price as it faces competition. Where it will sit in comparison to the forthcoming biosimilars will depend on how much biosimilar companies drop their price and how much pressure will be on PBMs and insurers to cover the lowest list price drug,” he said.
AbbVie did not respond to several requests for comment.
Charitable patient assistance programs for biosimilars or biologics can help offset the price of copayments, Mr. Newmark offered.
Ideally, insurers will offer designated biosimilars at a reduced or even no out-of-pocket expense on their formularies. This should lead to a decreased administrative burden for approval with streamlined (or even removal) of prior authorizations for certain medications, said Dr. Oldfield.
Without insurance or medication assistance programs, the cost of biosimilars is prohibitively expensive, he added.
“Biosimilars have higher research, development, and manufacturing costs than what people conventionally think of [for] a generic medication.”
Educating, advising patients
Dr. Oldfield advised that gastroenterologists refer to biologics by the generic name rather than branded name when initiating therapy unless there is a very specific reason not to. “This approach should make the process more streamlined and less subjected to quick denials for brand-only requests as biosimilars start to assume a larger market share,” he said.
Uptake of the Humira biosimilars also will depend on proper education of physicians and patients and their comfort level with the biosimilars, said Dr. Regueiro. Cleveland Clinic uses a team approach to educate on this topic, relying on pharmacists, clinicians, and nurses to explain that there’s no real difference between the reference drug and its biosimilars, based on efficacy and safety data.
Physicians can also direct patients to patient-friendly resources, said Mr. Newmark. “By starting the conversation early, it ensures that when/if the time comes that your patient is switched to or chooses a biosimilar they will feel more confident because they have the knowledge to make decisions about their care.”
The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars , is a free resource for patients, he added.
It’s important that doctors also understand these products so they can explain to their patients what to expect, said the FDA’s Dr. Yim. The FDA provides educational materials on its website, including a comprehensive curriculum toolkit.
Dr. Hanauer has served as a consultant for AbbVie, Amgen, American College of Gastroenterology, GlaxoSmithKline, American Gastroenterological Association, Pfizer, and a host of other companies . Dr. Regueiro has served on advisory boards and as a consultant for Abbvie, Janssen, UCB, Takeda, Pfizer, BMS, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET Pharma Solutions,Trellis, and Boehringer Ingelheim Pharmaceuticals. Dr. Wolf, Dr. Yim, Dr. Oldfield, and Mr. Newmark have no financial conflicts of interest.
Gastroenterologists in 2023 will have more tools in their arsenal to treat patients with Crohn’s disease or ulcerative colitis. As many as 8-10 adalimumab biosimilars are anticipated to come on the market this year, giving mainstay drug Humira some vigorous competition.
Three scenarios will drive adalimumab biosimilar initiation: Insurance preference for the initial treatment of a newly diagnosed condition, a change in a patient’s insurance plan, or an insurance-mandated switch, said Edward C. Oldfield IV, MD, assistant professor at Eastern Virginia Medical School’s division of gastroenterology in Norfolk.
“Outside of these scenarios, I would encourage patients to remain on their current biologic so long as cost and accessibility remain stable,” said Dr. Oldfield.
Many factors will contribute to the success of biosimilars. Will physicians be prescribing them? How are biosimilars placed on formularies and will they be given preferred status? How will manufacturers price their biosimilars? “We have to wait and see to get the answers to these questions,” said Steven Newmark, JD, MPA, chief legal officer and director of policy, Global Healthy Living Foundation/CreakyJoints, a nonprofit advocacy organization based in New York.
Prescribing biosimilars is no different than prescribing originator biologics, so providers should know how to use them, said Mr. Newmark. “Most important will be the availability of patient-friendly resources that providers can share with their patients to provide education about and confidence in using biosimilars,” he added.
Overall, biosimilars are a good thing, said Dr. Oldfield. “In the long run they should bring down costs and increase access to medications for our patients.”
Others are skeptical that the adalimumab biosimilars will save patients much money.
Biosimilar laws were created to lower costs. However, if a patient with insurance pays only $5 a month out of pocket for Humira – a drug that normally costs $7,000 without coverage – it’s unlikely they would want to switch unless there’s comparable savings from the biosimilar, said Stephen B. Hanauer, MD, medical director of the Digestive Health Center and professor of medicine at Northwestern Medicine, Northwestern University, Evanston, Ill.
Like generics, Humira biosimilars may face some initial backlash, said Dr. Hanauer.
2023 broadens scope of adalimumab treatments
The American Gastroenterological Association describes a biosimilar as something that’s “highly similar to, but not an exact copy of, a biologic reference product already approved” by the Food and Drug Administration. Congress under the 2010 Affordable Care Act created a special, abbreviated pathway to approval for biosimilars.
AbbVie’s Humira, the global revenue for which exceeded $20 billion in 2021, has long dominated the U.S. market on injectable treatments for autoimmune diseases. The popular drug faces some competition in 2023, however, following a series of legal settlements that allowed AbbVie competitors to release their own adalimumab biosimilars.
“So far, we haven’t seen biosimilars live up to their potential in the U.S. in the inflammatory space,” said Mr. Newmark. This may change, however. Previously, biosimilars have required infusion, which demanded more time, commitment, and travel from patients. “The new set of forthcoming Humira biosimilars are injectables, an administration method preferred by patients,” he said.
The FDA will approve a biosimilar if it determines that the biological product is highly similar to the reference product, and that there are no clinically meaningful differences between the biological and reference product in terms of the safety, purity, and potency of the product.
The agency to date has approved 8 adalimumab biosimilars. These include: Idacio (adalimumab-aacf, Fresenius Kabi); Amjevita (adalimumab-atto, Amgen); Hadlima (adalimumab-bwwd, Organon); Cyltezo (adalimumab-adbm, Boehringer Ingelheim); Yusimry (adalimumab-aqvh from Coherus BioSciences); Hulio (adalimumab-fkjp; Mylan/Fujifilm Kyowa Kirin Biologics); Hyrimoz (adalimumab-adaz, Sandoz), and Abrilada (adalimumab-afzb, Pfizer).
“While FDA doesn’t formally track when products come to market, we know based on published reports that application holders for many of the currently FDA-approved biosimilars plan to market this year, starting with Amjevita being the first adalimumab biosimilar launched” in January, said Sarah Yim, MD, director of the Office of Therapeutic Biologics and Biosimilars at the agency.
At press time, two other companies (Celltrion and Alvotech/Teva) were awaiting FDA approval for their adalimumab biosimilar drugs.
Among the eight approved drugs, Cyltezo is the only one that has a designation for interchangeability with Humira.
An interchangeable biosimilar may be substituted at the pharmacy without the intervention of the prescriber – much like generics are substituted, depending on state laws, said Dr. Yim. “However, in terms of safety and effectiveness, FDA’s standards for approval mean that biosimilar or interchangeable biosimilar products can be used in place of the reference product they were compared to.”
FDA-approved biosimilars undergo a rigorous evaluation for safety, effectiveness, and quality for their approved conditions of use, she continued. “Therefore, patients and health care providers can rely on a biosimilar to be as safe and effective for its approved uses as the original biological product.”
Remicade as a yard stick
Gastroenterologists dealt with this situation once before, when Remicade (infliximab) biosimilars came on the market in 2016, noted Miguel Regueiro, MD, chair of the Digestive Disease and Surgery Institute at the Cleveland Clinic.
Remicade and Humira are both tumor necrosis factor inhibitors with the same mechanism of action and many of the same indications. “We already had that experience with Remicade and biosimilar switch 2 or 3 years ago. Now we’re talking about Humira,” said Dr. Regueiro.
Most GI doctors have prescribed one of the more common infliximab biosimilars (Inflectra or Renflexis), noted Dr. Oldfield.
Cardinal Health, which recently surveyed 300 gastroenterologists, rheumatologists, and dermatologists about adalimumab biosimilars, found that gastroenterologists had the highest comfort level in prescribing them. Their top concern, however, was changing a patient from adalimumab to an adalimumab biosimilar.
For most patients, Dr. Oldfield sees the Humira reference biologic and biosimilar as equivalent.
However, he said he would change a patient’s drug only if there were a good reason or if his hand was forced by insurance. He would not make the change for a patient who recently began induction with the reference biologic or a patient with highly active clinical disease.
“While there is limited data to support this, I would also have some qualms about changing a patient from reference biologic to a biosimilar if they previously had immune-mediated pharmacokinetic failure due to antibody development with a biologic and were currently doing well on their new biologic,” he said.
Those with a new ulcerative colitis or Crohn’s diagnosis who are initiating a biologic for the first time might consider a biosimilar. If a patient is transitioning from a reference biologic to a biosimilar, “I would want to make that change during a time of stable remission and with the recognition that the switch is not a temporary switch, but a long-term switch,” he continued.
A paper that reviewed 23 observational studies of adalimumab and other biosimilars found that switching biosimilars was safe and effective. But if possible, patients should minimize the number of switches until more robust long-term data are available, added Dr. Oldfield.
If a patient is apprehensive about switching to a new therapy, “one may need to be cognizant of the ‘nocebo’ effect in which there is an unexplained or unfavorable therapeutic effect after switching,” he said.
Other gastroenterologists voiced similar reservations about switching. “I won’t use an adalimumab biosimilar unless the patient requests it, the insurance requires it, or there is a cost advantage for the patient such that they prefer it,” said Doug Wolf, MD, an Atlanta gastroenterologist.
“There is no medical treatment advantage to a biosimilar, especially if switching from Humira,” added Dr. Wolf.
Insurance will guide treatment
Once a drug is approved for use by the FDA, that drug will be available in all 50 states. “Different private insurance formularies, as well as state Medicaid formularies, might affect the actual ability of patients to receive such drugs,” said Mr. Newmark.
Patients should consult with their providers and insurance companies to see what therapies are available, he advised.
Dr. Hanauer anticipates some headaches arising for patients and doctors alike when negotiating for a specific drug.
Cyltezo may be the only biosimilar interchangeable with Humira, but the third-party pharmacy benefit manager (PBM) could negotiate for one of the noninterchangeable ones. “On a yearly basis they could switch their preference,” said Dr. Hanauer.
In the Cardinal Health survey, more than 60% of respondents said they would feel comfortable prescribing an adalimumab biosimilar only with an interchangeability designation.
A PBM may offer a patient Cyltezo if it’s cheaper than Humira. If the patient insists on staying on Humira, then they’ll have to pay more for that drug on their payer’s formulary, said Dr. Hanauer. In a worst-case scenario, a physician may have to appeal on a patient’s behalf to get Humira if the insurer offers only the biosimilar.
Taking that step to appeal is a major hassle for the physician, and leads to extra back door costs as well, said Dr. Hanauer.
Humira manufacturer AbbVie, in turn, may offer discounts and rebates to the PBMs to put Humira on their formulary. “That’s the AbbVie negotiating power. It’s not that the cost is going to be that much different. It’s going to be that there are rebates and discounts that are going to make the cost different,” he added.
As a community physician, Dr. Oldfield has specific concerns about accessibility.
The ever-increasing burden of insurance documentation and prior authorization means it can take weeks or months to get these medications approved. “The addition of new biosimilars is a welcome entrance if it can get patients the medications they need when they need it,” he said.
When it comes to prescribing biologics, many physicians rely on ancillary staff for assistance. It’s a team effort to sift through all the paperwork, observed Dr. Oldfield.
“While many community GI practices have specialized staff to deal with prior authorizations, they are still a far cry from the IBD [inflammatory bowel disease] academic centers where there are often pharmacists, nursing specialists, and home-monitoring programs to check in on patients,” he explained.
Landscape on cost is uncertain
At present, little is known about the cost of the biosimilars and impact on future drug pricing, said Dr. Oldfield.
At least for Medicare, Humira biosimilars will be considered Medicare Part D drugs if used for a medically accepted indication, said a spokesperson for the Centers for Medicare and Medicaid Services.
Part D sponsors (pharmacy and therapeutic committees) “will make the determination as to whether Amjevita and other products will be added to their formularies,” said the spokesperson.
Patients never saw a significant cost savings with Remicade biosimilars. “I imagine the same would be true with biosimilars for Humira,” said Dr. Regueiro. Patients may see greater access to these drugs, however, because the insurance plan or the pharmacy plan will make them more readily available, he added.
The hope is that, as biosimilars are introduced, the price of the originator biologic will go down, said Mr. Newmark. “Therefore, we can expect Humira to be offered at a lower price as it faces competition. Where it will sit in comparison to the forthcoming biosimilars will depend on how much biosimilar companies drop their price and how much pressure will be on PBMs and insurers to cover the lowest list price drug,” he said.
AbbVie did not respond to several requests for comment.
Charitable patient assistance programs for biosimilars or biologics can help offset the price of copayments, Mr. Newmark offered.
Ideally, insurers will offer designated biosimilars at a reduced or even no out-of-pocket expense on their formularies. This should lead to a decreased administrative burden for approval with streamlined (or even removal) of prior authorizations for certain medications, said Dr. Oldfield.
Without insurance or medication assistance programs, the cost of biosimilars is prohibitively expensive, he added.
“Biosimilars have higher research, development, and manufacturing costs than what people conventionally think of [for] a generic medication.”
Educating, advising patients
Dr. Oldfield advised that gastroenterologists refer to biologics by the generic name rather than branded name when initiating therapy unless there is a very specific reason not to. “This approach should make the process more streamlined and less subjected to quick denials for brand-only requests as biosimilars start to assume a larger market share,” he said.
Uptake of the Humira biosimilars also will depend on proper education of physicians and patients and their comfort level with the biosimilars, said Dr. Regueiro. Cleveland Clinic uses a team approach to educate on this topic, relying on pharmacists, clinicians, and nurses to explain that there’s no real difference between the reference drug and its biosimilars, based on efficacy and safety data.
Physicians can also direct patients to patient-friendly resources, said Mr. Newmark. “By starting the conversation early, it ensures that when/if the time comes that your patient is switched to or chooses a biosimilar they will feel more confident because they have the knowledge to make decisions about their care.”
The Global Healthy Living Foundation’s podcast, Breaking Down Biosimilars , is a free resource for patients, he added.
It’s important that doctors also understand these products so they can explain to their patients what to expect, said the FDA’s Dr. Yim. The FDA provides educational materials on its website, including a comprehensive curriculum toolkit.
Dr. Hanauer has served as a consultant for AbbVie, Amgen, American College of Gastroenterology, GlaxoSmithKline, American Gastroenterological Association, Pfizer, and a host of other companies . Dr. Regueiro has served on advisory boards and as a consultant for Abbvie, Janssen, UCB, Takeda, Pfizer, BMS, Organon, Amgen, Genentech, Gilead, Salix, Prometheus, Lilly, Celgene, TARGET Pharma Solutions,Trellis, and Boehringer Ingelheim Pharmaceuticals. Dr. Wolf, Dr. Yim, Dr. Oldfield, and Mr. Newmark have no financial conflicts of interest.
What does the future of psoriasis treatment look like?
HONOLULU – During office visits with Andrew Blauvelt, MD, MBA, many patients well controlled on biologic therapy for their moderate to severe psoriasis often ask him when their scheduled injections can stop.
The most common question he hears is, “ ‘Why do I have to keep doing this? I’ve been clear for 2 or 3 years,’ ” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “We have terrific drugs for psoriasis, but how can we do better?”
Development of oral biologics. At least two companies are developing a peptide-type small molecule that blocks interleukin (IL)-17 or IL-23 signaling, but would be given as a pill, he said. Another concept in the works is a robotic pill for drug delivery. The pill, which is being developed by Rani Therapeutics, protects the biotherapeutic drug payload from digestion in the GI tract and auto-injects it into the wall of the small intestine, according to a report of two studies that demonstrated the safety and tolerability of the robotic pill in healthy humans.
In an animal study, the same researchers showed that delivering monoclonal antibodies with the robotic pill achieved bioavailability on par with that obtained by standard subcutaneous injections.
Identifying “super responders” who require less frequent dosing of medication. “There’s data to suggest that we can kind of back off treatment in these patients,” Dr. Blauvelt said.
Hitting treatment hard and early. “There’s a concept in medicine of hitting disease hard and hitting it early, before the disease can establish itself and cause damage,” he said.
Targeting tissue resident memory T cells. In psoriasis, the idea is that if you treat earlier, when patients are just diagnosed, “perhaps you might be able to decrease resident memory T cells that set up shop in the skin and are responsible for disease recurrences,” Dr. Blauvelt said. “Research has shown that IL-23 blockers decrease tissue resident memory T cells, and IL-17 blockers don’t. This could explain why we see long remissions in this class of drug because we’re getting at these resident memory T cells and knocking them down,” he explained. “Our hypothesis is that hitting hard and early in the treatment course with high-dose IL-23 blockade may be an effective strategy to induce long-term remissions and possible cure, what we call ‘knock-out therapy.’ ”
In a pilot study of 20 patients, Dr. Blauvelt and colleagues are evaluating whether higher initial doses of the IL-23 antagonist risankizumab (300 mg and 600 mg, 2 times and 4 times the standard initial doses for plaque psoriasis) can more effectively target resident memory T cells. “This involves dosing at weeks 0, 4, and 16, then stopping and measuring resident T cells in the tissue to see how long we can induce psoriasis remissions,” Dr. Blauvelt said.
“I have no data to share, but I think we have the potential for unprecedented PASI-100 numbers with no added safety concerns, and the potential to break away from established regular dosing patterns,” such as the possibility of yearly dosing, the possibility of long-term remissions, and the possibility of cure in some patients, he noted.
Inducing tolerance. This refers to efforts aimed at increasing regulatory T cells, which are natural T cells that calm inflammation. He described it as “revving up our natural anti-inflammatory T cells to help balance the immune system.”
Gene editing. This involves using CRISPR gene editing technology to cut genes as a way to cure disease. “What if we cut the IL-23 receptor?” Dr. Blauvelt asked. “You would get rid of that whole signaling pathway. Would the patient be fine?”
In an interview a the meeting, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said that Dr. Blauvelt “has a very exciting view” of the future of psoriasis treatments. “I think that some of it will come true; we’ll have to see which,” Dr. Stein Gold said. “The idea that we might be able to change the trajectory of disease by being aggressive upfront, and possibly modify the course, is exciting. That would be a wonderful new treatment approach.”
Dr. Blauvelt disclosed ties with AbbVie, Abcentra, Affibody, Aligos, Almirall, Alumis, Amgen, AnaptysBio, Arcutis, Arena, ASLAN Pharma, Athenex, Bluefin, Boehringer Ingelheim, Bristol Myers Squibb, Cara, Dermavant, EcoR1, Escient, Evelo, Evommune, Forte, Galderma, Highlightll, Incyte, Innovent Bio, Janssen, Landos, Leo, Lilly, Merck, Novartis, Pfizer, Rapt, Regeneron, Sanofi-Genzyme, Spherix, Sun Pharmaceuticals Industries, TLL Pharmaceutical, TrialSpark, UCB Pharma, Vibliome, and Xencor.
Dr. Stein Gold disclosed ties with Almirall, Cutera, Dermata, Galderma, Novartis, Ortho Dermatologics, and Sun Pharmaceutical Industries, Ltd.
Medscape and this news organization are owned by the same parent company.
HONOLULU – During office visits with Andrew Blauvelt, MD, MBA, many patients well controlled on biologic therapy for their moderate to severe psoriasis often ask him when their scheduled injections can stop.
The most common question he hears is, “ ‘Why do I have to keep doing this? I’ve been clear for 2 or 3 years,’ ” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “We have terrific drugs for psoriasis, but how can we do better?”
Development of oral biologics. At least two companies are developing a peptide-type small molecule that blocks interleukin (IL)-17 or IL-23 signaling, but would be given as a pill, he said. Another concept in the works is a robotic pill for drug delivery. The pill, which is being developed by Rani Therapeutics, protects the biotherapeutic drug payload from digestion in the GI tract and auto-injects it into the wall of the small intestine, according to a report of two studies that demonstrated the safety and tolerability of the robotic pill in healthy humans.
In an animal study, the same researchers showed that delivering monoclonal antibodies with the robotic pill achieved bioavailability on par with that obtained by standard subcutaneous injections.
Identifying “super responders” who require less frequent dosing of medication. “There’s data to suggest that we can kind of back off treatment in these patients,” Dr. Blauvelt said.
Hitting treatment hard and early. “There’s a concept in medicine of hitting disease hard and hitting it early, before the disease can establish itself and cause damage,” he said.
Targeting tissue resident memory T cells. In psoriasis, the idea is that if you treat earlier, when patients are just diagnosed, “perhaps you might be able to decrease resident memory T cells that set up shop in the skin and are responsible for disease recurrences,” Dr. Blauvelt said. “Research has shown that IL-23 blockers decrease tissue resident memory T cells, and IL-17 blockers don’t. This could explain why we see long remissions in this class of drug because we’re getting at these resident memory T cells and knocking them down,” he explained. “Our hypothesis is that hitting hard and early in the treatment course with high-dose IL-23 blockade may be an effective strategy to induce long-term remissions and possible cure, what we call ‘knock-out therapy.’ ”
In a pilot study of 20 patients, Dr. Blauvelt and colleagues are evaluating whether higher initial doses of the IL-23 antagonist risankizumab (300 mg and 600 mg, 2 times and 4 times the standard initial doses for plaque psoriasis) can more effectively target resident memory T cells. “This involves dosing at weeks 0, 4, and 16, then stopping and measuring resident T cells in the tissue to see how long we can induce psoriasis remissions,” Dr. Blauvelt said.
“I have no data to share, but I think we have the potential for unprecedented PASI-100 numbers with no added safety concerns, and the potential to break away from established regular dosing patterns,” such as the possibility of yearly dosing, the possibility of long-term remissions, and the possibility of cure in some patients, he noted.
Inducing tolerance. This refers to efforts aimed at increasing regulatory T cells, which are natural T cells that calm inflammation. He described it as “revving up our natural anti-inflammatory T cells to help balance the immune system.”
Gene editing. This involves using CRISPR gene editing technology to cut genes as a way to cure disease. “What if we cut the IL-23 receptor?” Dr. Blauvelt asked. “You would get rid of that whole signaling pathway. Would the patient be fine?”
In an interview a the meeting, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said that Dr. Blauvelt “has a very exciting view” of the future of psoriasis treatments. “I think that some of it will come true; we’ll have to see which,” Dr. Stein Gold said. “The idea that we might be able to change the trajectory of disease by being aggressive upfront, and possibly modify the course, is exciting. That would be a wonderful new treatment approach.”
Dr. Blauvelt disclosed ties with AbbVie, Abcentra, Affibody, Aligos, Almirall, Alumis, Amgen, AnaptysBio, Arcutis, Arena, ASLAN Pharma, Athenex, Bluefin, Boehringer Ingelheim, Bristol Myers Squibb, Cara, Dermavant, EcoR1, Escient, Evelo, Evommune, Forte, Galderma, Highlightll, Incyte, Innovent Bio, Janssen, Landos, Leo, Lilly, Merck, Novartis, Pfizer, Rapt, Regeneron, Sanofi-Genzyme, Spherix, Sun Pharmaceuticals Industries, TLL Pharmaceutical, TrialSpark, UCB Pharma, Vibliome, and Xencor.
Dr. Stein Gold disclosed ties with Almirall, Cutera, Dermata, Galderma, Novartis, Ortho Dermatologics, and Sun Pharmaceutical Industries, Ltd.
Medscape and this news organization are owned by the same parent company.
HONOLULU – During office visits with Andrew Blauvelt, MD, MBA, many patients well controlled on biologic therapy for their moderate to severe psoriasis often ask him when their scheduled injections can stop.
The most common question he hears is, “ ‘Why do I have to keep doing this? I’ve been clear for 2 or 3 years,’ ” Dr. Blauvelt, president of Oregon Medical Research Center, Portland, said at the Hawaii Dermatology Seminar provided by MedscapeLIVE! “We have terrific drugs for psoriasis, but how can we do better?”
Development of oral biologics. At least two companies are developing a peptide-type small molecule that blocks interleukin (IL)-17 or IL-23 signaling, but would be given as a pill, he said. Another concept in the works is a robotic pill for drug delivery. The pill, which is being developed by Rani Therapeutics, protects the biotherapeutic drug payload from digestion in the GI tract and auto-injects it into the wall of the small intestine, according to a report of two studies that demonstrated the safety and tolerability of the robotic pill in healthy humans.
In an animal study, the same researchers showed that delivering monoclonal antibodies with the robotic pill achieved bioavailability on par with that obtained by standard subcutaneous injections.
Identifying “super responders” who require less frequent dosing of medication. “There’s data to suggest that we can kind of back off treatment in these patients,” Dr. Blauvelt said.
Hitting treatment hard and early. “There’s a concept in medicine of hitting disease hard and hitting it early, before the disease can establish itself and cause damage,” he said.
Targeting tissue resident memory T cells. In psoriasis, the idea is that if you treat earlier, when patients are just diagnosed, “perhaps you might be able to decrease resident memory T cells that set up shop in the skin and are responsible for disease recurrences,” Dr. Blauvelt said. “Research has shown that IL-23 blockers decrease tissue resident memory T cells, and IL-17 blockers don’t. This could explain why we see long remissions in this class of drug because we’re getting at these resident memory T cells and knocking them down,” he explained. “Our hypothesis is that hitting hard and early in the treatment course with high-dose IL-23 blockade may be an effective strategy to induce long-term remissions and possible cure, what we call ‘knock-out therapy.’ ”
In a pilot study of 20 patients, Dr. Blauvelt and colleagues are evaluating whether higher initial doses of the IL-23 antagonist risankizumab (300 mg and 600 mg, 2 times and 4 times the standard initial doses for plaque psoriasis) can more effectively target resident memory T cells. “This involves dosing at weeks 0, 4, and 16, then stopping and measuring resident T cells in the tissue to see how long we can induce psoriasis remissions,” Dr. Blauvelt said.
“I have no data to share, but I think we have the potential for unprecedented PASI-100 numbers with no added safety concerns, and the potential to break away from established regular dosing patterns,” such as the possibility of yearly dosing, the possibility of long-term remissions, and the possibility of cure in some patients, he noted.
Inducing tolerance. This refers to efforts aimed at increasing regulatory T cells, which are natural T cells that calm inflammation. He described it as “revving up our natural anti-inflammatory T cells to help balance the immune system.”
Gene editing. This involves using CRISPR gene editing technology to cut genes as a way to cure disease. “What if we cut the IL-23 receptor?” Dr. Blauvelt asked. “You would get rid of that whole signaling pathway. Would the patient be fine?”
In an interview a the meeting, Linda Stein Gold, MD, director of clinical research and division head of dermatology at the Henry Ford Health System, Detroit, said that Dr. Blauvelt “has a very exciting view” of the future of psoriasis treatments. “I think that some of it will come true; we’ll have to see which,” Dr. Stein Gold said. “The idea that we might be able to change the trajectory of disease by being aggressive upfront, and possibly modify the course, is exciting. That would be a wonderful new treatment approach.”
Dr. Blauvelt disclosed ties with AbbVie, Abcentra, Affibody, Aligos, Almirall, Alumis, Amgen, AnaptysBio, Arcutis, Arena, ASLAN Pharma, Athenex, Bluefin, Boehringer Ingelheim, Bristol Myers Squibb, Cara, Dermavant, EcoR1, Escient, Evelo, Evommune, Forte, Galderma, Highlightll, Incyte, Innovent Bio, Janssen, Landos, Leo, Lilly, Merck, Novartis, Pfizer, Rapt, Regeneron, Sanofi-Genzyme, Spherix, Sun Pharmaceuticals Industries, TLL Pharmaceutical, TrialSpark, UCB Pharma, Vibliome, and Xencor.
Dr. Stein Gold disclosed ties with Almirall, Cutera, Dermata, Galderma, Novartis, Ortho Dermatologics, and Sun Pharmaceutical Industries, Ltd.
Medscape and this news organization are owned by the same parent company.
AT THE MEDSCAPE LIVE! HAWAII DERMATOLOGY SEMINAR












