User login
Guideline: Diagnosis and treatment of adults with community-acquired pneumonia
A new guideline has been published to update the 2007 guidelines for the management of adults with community-acquired pneumonia (CAP).
The practice guideline was jointly written by an ad hoc committee of the American Thoracic Society and Infectious Diseases Society of America. CAP refers to a pneumonia infection that was acquired by a patient in his or her community. Decisions about which antibiotics to use to treat this kind of infection are based on risk factors for resistant organisms and the severity of illness.
Pathogens
Traditionally, CAP is caused by common bacterial pathogens that include Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella species, Chlamydia pneumonia, and Moraxella catarrhalis. Risk factors for multidrug resistant pathogens such as methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa include previous infection with MRSA or P. aeruginosa, recent hospitalization, and requiring parenteral antibiotics in the last 90 days.
Defining severe community-acquired pneumonia
The health care–associated pneumonia, or HCAP, classification should no longer be used to determine empiric treatment. The recommendations for which antibiotics to use are linked to the severity of illness. Previously the site of treatment drove antibiotic selection, but since decision about the site of care can be affected by many considerations, the guidelines recommend using the CAP severity criteria. Severe CAP includes either one major or at least three minor criteria.
Major criteria are:
- Septic shock requiring vasopressors.
- Respiratory failure requiring mechanical ventilation.
Minor criteria are:
- Respiratory rate greater than or equal to 30 breaths/min.
- Ratio of arterial O2 partial pressure to fractional inspired O2 less than or equal to 250.
- Multilobar infiltrates.
- Confusion/disorientation.
- Uremia (blood urea nitrogen level greater than or equal to 20 mg/dL).
- Leukopenia (white blood cell count less than 4,000 cells/mcL).
- Thrombocytopenia (platelet count less than 100,000 mcL)
- Hypothermia (core temperature less than 36º C).
- Hypotension requiring aggressive fluid resuscitation.
Management and diagnostic testing
Clinicians should use the Pneumonia Severity Index (PSI) and clinical judgment to guide the site of treatment for patients. Gram stain, sputum, and blood culture should not be routinely obtained in an outpatient setting. Legionella antigen should not be routinely obtained unless indicated by epidemiological factors. During influenza season, a rapid influenza assay, preferably a nucleic acid amplification test, should be obtained to help guide treatment.
For patients with severe CAP or risk factors for MRSA or P. aeruginosa, gram stain and culture and Legionella antigen should be obtained to manage antibiotic choices. Also, blood cultures should be obtained for these patients.
Empiric antibiotic therapy should be initiated based on clinical judgment and radiographic confirmation of CAP. Serum procalcitonin should not be used to assess initiation of antibiotic therapy.
Empiric antibiotic therapy
Healthy adults without comorbidities should be treated with monotherapy of either:
- Amoxicillin 1 g three times daily.
- OR doxycycline 100 mg twice daily.
- OR a macrolide (azithromycin 500 mg on first day then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended release 1,000 mg daily) only in areas with pneumococcal resistance to macrolides less than 25%.
Adults with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; or asplenia should be treated with:
- Amoxicillin/clavulanate 500 mg/125 mg three times daily, or amoxicillin/ clavulanate 875 mg/125 mg twice daily, or 2,000 mg/125 mg twice daily, or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily); and a macrolide (azithromycin 500 mg on first day then 250 mg daily, clarithromycin [500 mg twice daily or extended release 1,000 mg once daily]), or doxycycline 100 mg twice daily. (Some experts recommend that the first dose of doxycycline should be 200 mg.)
- OR monotherapy with respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily).
Inpatient pneumonia that is not severe, without risk factors for resistant organisms should be treated with:
- Beta-lactam (ampicillin 1 sulbactam 1.5-3 g every 6 h, cefotaxime 1-2 g every 8 h, ceftriaxone 1-2 g daily, or ceftaroline 600 mg every 12 h) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily).
- OR monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily).
If there is a contraindication for the use of both a macrolide and a fluoroquinolone, then doxycycline can be used instead.
Severe inpatient pneumonia without risk factors for resistant organisms should be treated with combination therapy of either (agents and doses the same as above):
- Beta-lactam and macrolide.
- OR fluoroquinolone and beta-lactam.
It is recommended to not routinely add anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected. Clinicians should identify risk factors for MRSA or P. aeruginosa before adding additional agents.
Duration of antibiotic therapy is determined by the patient achieving clinical stability with no less than 5 days of antibiotics. In adults with symptom resolution within 5-7 days, no additional follow-up chest imaging is recommended. If patients test positive for influenza, then anti-influenza treatment such as oseltamivir should be used in addition to antibiotics regardless of length of influenza symptoms before presentation.
The bottom line
CAP treatment should be based on severity of illness and risk factors for resistant organisms. Blood and sputum cultures are recommended only for patients with severe pneumonia. There have been important changes in the recommendations for antibiotic treatment of CAP, with high-dose amoxicillin recommended for most patients with CAP who are treated as outpatients. Patients who exhibit clinical stability should be treated for at least 5 days and do not require follow up imaging studies.
For a podcast of this guideline, go to iTunes and download the Infectious Diseases Society of America guideline podcast.
Reference
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
Tina Chuong, DO, is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
A new guideline has been published to update the 2007 guidelines for the management of adults with community-acquired pneumonia (CAP).
The practice guideline was jointly written by an ad hoc committee of the American Thoracic Society and Infectious Diseases Society of America. CAP refers to a pneumonia infection that was acquired by a patient in his or her community. Decisions about which antibiotics to use to treat this kind of infection are based on risk factors for resistant organisms and the severity of illness.
Pathogens
Traditionally, CAP is caused by common bacterial pathogens that include Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella species, Chlamydia pneumonia, and Moraxella catarrhalis. Risk factors for multidrug resistant pathogens such as methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa include previous infection with MRSA or P. aeruginosa, recent hospitalization, and requiring parenteral antibiotics in the last 90 days.
Defining severe community-acquired pneumonia
The health care–associated pneumonia, or HCAP, classification should no longer be used to determine empiric treatment. The recommendations for which antibiotics to use are linked to the severity of illness. Previously the site of treatment drove antibiotic selection, but since decision about the site of care can be affected by many considerations, the guidelines recommend using the CAP severity criteria. Severe CAP includes either one major or at least three minor criteria.
Major criteria are:
- Septic shock requiring vasopressors.
- Respiratory failure requiring mechanical ventilation.
Minor criteria are:
- Respiratory rate greater than or equal to 30 breaths/min.
- Ratio of arterial O2 partial pressure to fractional inspired O2 less than or equal to 250.
- Multilobar infiltrates.
- Confusion/disorientation.
- Uremia (blood urea nitrogen level greater than or equal to 20 mg/dL).
- Leukopenia (white blood cell count less than 4,000 cells/mcL).
- Thrombocytopenia (platelet count less than 100,000 mcL)
- Hypothermia (core temperature less than 36º C).
- Hypotension requiring aggressive fluid resuscitation.
Management and diagnostic testing
Clinicians should use the Pneumonia Severity Index (PSI) and clinical judgment to guide the site of treatment for patients. Gram stain, sputum, and blood culture should not be routinely obtained in an outpatient setting. Legionella antigen should not be routinely obtained unless indicated by epidemiological factors. During influenza season, a rapid influenza assay, preferably a nucleic acid amplification test, should be obtained to help guide treatment.
For patients with severe CAP or risk factors for MRSA or P. aeruginosa, gram stain and culture and Legionella antigen should be obtained to manage antibiotic choices. Also, blood cultures should be obtained for these patients.
Empiric antibiotic therapy should be initiated based on clinical judgment and radiographic confirmation of CAP. Serum procalcitonin should not be used to assess initiation of antibiotic therapy.
Empiric antibiotic therapy
Healthy adults without comorbidities should be treated with monotherapy of either:
- Amoxicillin 1 g three times daily.
- OR doxycycline 100 mg twice daily.
- OR a macrolide (azithromycin 500 mg on first day then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended release 1,000 mg daily) only in areas with pneumococcal resistance to macrolides less than 25%.
Adults with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; or asplenia should be treated with:
- Amoxicillin/clavulanate 500 mg/125 mg three times daily, or amoxicillin/ clavulanate 875 mg/125 mg twice daily, or 2,000 mg/125 mg twice daily, or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily); and a macrolide (azithromycin 500 mg on first day then 250 mg daily, clarithromycin [500 mg twice daily or extended release 1,000 mg once daily]), or doxycycline 100 mg twice daily. (Some experts recommend that the first dose of doxycycline should be 200 mg.)
- OR monotherapy with respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily).
Inpatient pneumonia that is not severe, without risk factors for resistant organisms should be treated with:
- Beta-lactam (ampicillin 1 sulbactam 1.5-3 g every 6 h, cefotaxime 1-2 g every 8 h, ceftriaxone 1-2 g daily, or ceftaroline 600 mg every 12 h) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily).
- OR monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily).
If there is a contraindication for the use of both a macrolide and a fluoroquinolone, then doxycycline can be used instead.
Severe inpatient pneumonia without risk factors for resistant organisms should be treated with combination therapy of either (agents and doses the same as above):
- Beta-lactam and macrolide.
- OR fluoroquinolone and beta-lactam.
It is recommended to not routinely add anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected. Clinicians should identify risk factors for MRSA or P. aeruginosa before adding additional agents.
Duration of antibiotic therapy is determined by the patient achieving clinical stability with no less than 5 days of antibiotics. In adults with symptom resolution within 5-7 days, no additional follow-up chest imaging is recommended. If patients test positive for influenza, then anti-influenza treatment such as oseltamivir should be used in addition to antibiotics regardless of length of influenza symptoms before presentation.
The bottom line
CAP treatment should be based on severity of illness and risk factors for resistant organisms. Blood and sputum cultures are recommended only for patients with severe pneumonia. There have been important changes in the recommendations for antibiotic treatment of CAP, with high-dose amoxicillin recommended for most patients with CAP who are treated as outpatients. Patients who exhibit clinical stability should be treated for at least 5 days and do not require follow up imaging studies.
For a podcast of this guideline, go to iTunes and download the Infectious Diseases Society of America guideline podcast.
Reference
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
Tina Chuong, DO, is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
A new guideline has been published to update the 2007 guidelines for the management of adults with community-acquired pneumonia (CAP).
The practice guideline was jointly written by an ad hoc committee of the American Thoracic Society and Infectious Diseases Society of America. CAP refers to a pneumonia infection that was acquired by a patient in his or her community. Decisions about which antibiotics to use to treat this kind of infection are based on risk factors for resistant organisms and the severity of illness.
Pathogens
Traditionally, CAP is caused by common bacterial pathogens that include Streptococcus pneumoniae, Haemophilus influenzae, Mycoplasma pneumoniae, Staphylococcus aureus, Legionella species, Chlamydia pneumonia, and Moraxella catarrhalis. Risk factors for multidrug resistant pathogens such as methicillin-resistant S. aureus (MRSA) and Pseudomonas aeruginosa include previous infection with MRSA or P. aeruginosa, recent hospitalization, and requiring parenteral antibiotics in the last 90 days.
Defining severe community-acquired pneumonia
The health care–associated pneumonia, or HCAP, classification should no longer be used to determine empiric treatment. The recommendations for which antibiotics to use are linked to the severity of illness. Previously the site of treatment drove antibiotic selection, but since decision about the site of care can be affected by many considerations, the guidelines recommend using the CAP severity criteria. Severe CAP includes either one major or at least three minor criteria.
Major criteria are:
- Septic shock requiring vasopressors.
- Respiratory failure requiring mechanical ventilation.
Minor criteria are:
- Respiratory rate greater than or equal to 30 breaths/min.
- Ratio of arterial O2 partial pressure to fractional inspired O2 less than or equal to 250.
- Multilobar infiltrates.
- Confusion/disorientation.
- Uremia (blood urea nitrogen level greater than or equal to 20 mg/dL).
- Leukopenia (white blood cell count less than 4,000 cells/mcL).
- Thrombocytopenia (platelet count less than 100,000 mcL)
- Hypothermia (core temperature less than 36º C).
- Hypotension requiring aggressive fluid resuscitation.
Management and diagnostic testing
Clinicians should use the Pneumonia Severity Index (PSI) and clinical judgment to guide the site of treatment for patients. Gram stain, sputum, and blood culture should not be routinely obtained in an outpatient setting. Legionella antigen should not be routinely obtained unless indicated by epidemiological factors. During influenza season, a rapid influenza assay, preferably a nucleic acid amplification test, should be obtained to help guide treatment.
For patients with severe CAP or risk factors for MRSA or P. aeruginosa, gram stain and culture and Legionella antigen should be obtained to manage antibiotic choices. Also, blood cultures should be obtained for these patients.
Empiric antibiotic therapy should be initiated based on clinical judgment and radiographic confirmation of CAP. Serum procalcitonin should not be used to assess initiation of antibiotic therapy.
Empiric antibiotic therapy
Healthy adults without comorbidities should be treated with monotherapy of either:
- Amoxicillin 1 g three times daily.
- OR doxycycline 100 mg twice daily.
- OR a macrolide (azithromycin 500 mg on first day then 250 mg daily or clarithromycin 500 mg twice daily or clarithromycin extended release 1,000 mg daily) only in areas with pneumococcal resistance to macrolides less than 25%.
Adults with comorbidities such as chronic heart, lung, liver, or renal disease; diabetes mellitus; alcoholism; malignancy; or asplenia should be treated with:
- Amoxicillin/clavulanate 500 mg/125 mg three times daily, or amoxicillin/ clavulanate 875 mg/125 mg twice daily, or 2,000 mg/125 mg twice daily, or a cephalosporin (cefpodoxime 200 mg twice daily or cefuroxime 500 mg twice daily); and a macrolide (azithromycin 500 mg on first day then 250 mg daily, clarithromycin [500 mg twice daily or extended release 1,000 mg once daily]), or doxycycline 100 mg twice daily. (Some experts recommend that the first dose of doxycycline should be 200 mg.)
- OR monotherapy with respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily, or gemifloxacin 320 mg daily).
Inpatient pneumonia that is not severe, without risk factors for resistant organisms should be treated with:
- Beta-lactam (ampicillin 1 sulbactam 1.5-3 g every 6 h, cefotaxime 1-2 g every 8 h, ceftriaxone 1-2 g daily, or ceftaroline 600 mg every 12 h) and a macrolide (azithromycin 500 mg daily or clarithromycin 500 mg twice daily).
- OR monotherapy with a respiratory fluoroquinolone (levofloxacin 750 mg daily, moxifloxacin 400 mg daily).
If there is a contraindication for the use of both a macrolide and a fluoroquinolone, then doxycycline can be used instead.
Severe inpatient pneumonia without risk factors for resistant organisms should be treated with combination therapy of either (agents and doses the same as above):
- Beta-lactam and macrolide.
- OR fluoroquinolone and beta-lactam.
It is recommended to not routinely add anaerobic coverage for suspected aspiration pneumonia unless lung abscess or empyema is suspected. Clinicians should identify risk factors for MRSA or P. aeruginosa before adding additional agents.
Duration of antibiotic therapy is determined by the patient achieving clinical stability with no less than 5 days of antibiotics. In adults with symptom resolution within 5-7 days, no additional follow-up chest imaging is recommended. If patients test positive for influenza, then anti-influenza treatment such as oseltamivir should be used in addition to antibiotics regardless of length of influenza symptoms before presentation.
The bottom line
CAP treatment should be based on severity of illness and risk factors for resistant organisms. Blood and sputum cultures are recommended only for patients with severe pneumonia. There have been important changes in the recommendations for antibiotic treatment of CAP, with high-dose amoxicillin recommended for most patients with CAP who are treated as outpatients. Patients who exhibit clinical stability should be treated for at least 5 days and do not require follow up imaging studies.
For a podcast of this guideline, go to iTunes and download the Infectious Diseases Society of America guideline podcast.
Reference
Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019 Oct 1;200(7):e45-e67.
Tina Chuong, DO, is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. Dr. Skolnik is professor of family and community medicine at Jefferson Medical College, Philadelphia, and an associate director of the family medicine residency program at Abington Jefferson Health.
Tide beginning to turn on vaccine hesitancy
NEW ORLEANS –
The shift began with the measles outbreak in Southern California in late 2014, he said. According to the Centers for Disease Control and Prevention, 125 measles cases with rash that occurred between Dec. 28, 2014, and Feb. 8, 2015, were confirmed in U.S. residents. Of these, 100 were California residents (MMWR. 2015 Feb 20;64[06];153-4).
“This outbreak spread ultimately to 25 states and involved 189 people,” Dr. Offit said at the annual meeting of the American Academy of Pediatrics. “It was in the news almost every day. As a consequence, there were measles outbreaks in New York, New Jersey, Florida, Oregon, and Texas, and Washington, which began to turn the public sentiment against the antivaccine movement.”
Even longstanding skeptics are changing their tune. Dr. Offit, professor of pediatrics in the division of infectious diseases at the Children’s Hospital of Philadelphia, cited a recent study from the Autism Science Foundation which found that 85% of parents of children with autism spectrum disorder don’t believe that vaccines cause the condition. “Although there will be parents who continue to believe that vaccines cause autism, most parents of children with autism don’t believe that,” he said. “Also, it’s a little hard to make your case that vaccines are dangerous and that you shouldn’t get them in the midst of outbreaks.”
Perhaps the greatest pushback against antivaccination efforts has been made in the legal arena. In 2019 alone, legislators in California banned parents from not vaccinating their kids because of personal beliefs, while lawmakers in New York repealed the religious exemption to vaccinate, those in Maine repealed the religious and philosophical exemption, those in New Jersey required detailed written explanation for religious exemption, and those in Washington State repealed the philosophical exemption for the MMR vaccine.
Pushback also is apparent on various social media platforms. For example, Dr. Offit said, Pinterest restricts vaccine search results to curb the spread of misinformation, YouTube removes ads from antivaccine channels, Amazon Prime has pulled antivaccination documentaries from its video service, and Facebook has taken steps to curb misinformation about vaccines. “With outbreaks and with children suffering, the media and public sentiment has largely turned against those who are vehemently against vaccines,” he said. “I’m talking about an angry, politically connected, lawyer-backed group of people who are conspiracy theorists, [those] who no matter what you say, they’re going to believe there’s a conspiracy theory to hurt their children and not believe you. When that group becomes big enough and you start to see outbreaks like we’ve seen, then it becomes an issue. That’s where it comes down to legislation. Is it your inalienable right as a U.S. citizen to allow your child to catch and transmit a potentially fatal infection? That’s what we’re struggling with now.”
When meeting with parents who are skeptical about vaccines or refuse their children to have them, Dr. Offit advises clinicians to “go down swinging” in favor of vaccination. He shared how his wife, Bonnie, a pediatrician who practices in suburban Philadelphia, counsels parents who raise such concerns. “The way she handled it initially was to do the best she could to eventually get people vaccinated,” he said. “She was successful about one-quarter of the time. Then she drew a line. She started saying to parents, ‘Look; don’t put me in a position where you are asking me to practice substandard care. I can’t send them out of this room knowing that there’s more measles out there, knowing that there’s mumps out there, knowing that there’s whooping cough out there, knowing that there’s pneumococcus and varicella out there. If this child leaves this office and is hurt by any of those viruses or bacteria and I knew I could have done something to prevent it, I couldn’t live with myself. If you’re going to let this child out without being vaccinated I can’t see you anymore because I’m responsible for the health of this child.’ With that [approach], she has been far more successful. Because at some level, if you continue to see that patient, you’re tacitly agreeing that it’s okay to [not vaccinate].”
In 2000, Dr. Offit and colleagues created the Vaccine Education Center at Children’s Hospital of Philadelphia, which provides complete, up-to-date, and reliable information about vaccines to parents and clinicians. It summarizes the purpose of each vaccine, and the relative risks and benefits in easy-to-read language. The CDC also maintains updated information about vaccines and immunizations on its web site. For his part, Dr. Offit tells parents that passing on an opportunity to vaccinate their child is not a risk-free choice. “If you choose not to get a vaccine you probably will get away with it, but you might not,” he said. “You are playing a game of Russian roulette. It may not be five empty chambers and one bullet, but maybe it’s 100,000 empty chambers and one bullet. There’s a bullet there.”
Dr. Offit reported having no relevant financial disclosures.
NEW ORLEANS –
The shift began with the measles outbreak in Southern California in late 2014, he said. According to the Centers for Disease Control and Prevention, 125 measles cases with rash that occurred between Dec. 28, 2014, and Feb. 8, 2015, were confirmed in U.S. residents. Of these, 100 were California residents (MMWR. 2015 Feb 20;64[06];153-4).
“This outbreak spread ultimately to 25 states and involved 189 people,” Dr. Offit said at the annual meeting of the American Academy of Pediatrics. “It was in the news almost every day. As a consequence, there were measles outbreaks in New York, New Jersey, Florida, Oregon, and Texas, and Washington, which began to turn the public sentiment against the antivaccine movement.”
Even longstanding skeptics are changing their tune. Dr. Offit, professor of pediatrics in the division of infectious diseases at the Children’s Hospital of Philadelphia, cited a recent study from the Autism Science Foundation which found that 85% of parents of children with autism spectrum disorder don’t believe that vaccines cause the condition. “Although there will be parents who continue to believe that vaccines cause autism, most parents of children with autism don’t believe that,” he said. “Also, it’s a little hard to make your case that vaccines are dangerous and that you shouldn’t get them in the midst of outbreaks.”
Perhaps the greatest pushback against antivaccination efforts has been made in the legal arena. In 2019 alone, legislators in California banned parents from not vaccinating their kids because of personal beliefs, while lawmakers in New York repealed the religious exemption to vaccinate, those in Maine repealed the religious and philosophical exemption, those in New Jersey required detailed written explanation for religious exemption, and those in Washington State repealed the philosophical exemption for the MMR vaccine.
Pushback also is apparent on various social media platforms. For example, Dr. Offit said, Pinterest restricts vaccine search results to curb the spread of misinformation, YouTube removes ads from antivaccine channels, Amazon Prime has pulled antivaccination documentaries from its video service, and Facebook has taken steps to curb misinformation about vaccines. “With outbreaks and with children suffering, the media and public sentiment has largely turned against those who are vehemently against vaccines,” he said. “I’m talking about an angry, politically connected, lawyer-backed group of people who are conspiracy theorists, [those] who no matter what you say, they’re going to believe there’s a conspiracy theory to hurt their children and not believe you. When that group becomes big enough and you start to see outbreaks like we’ve seen, then it becomes an issue. That’s where it comes down to legislation. Is it your inalienable right as a U.S. citizen to allow your child to catch and transmit a potentially fatal infection? That’s what we’re struggling with now.”
When meeting with parents who are skeptical about vaccines or refuse their children to have them, Dr. Offit advises clinicians to “go down swinging” in favor of vaccination. He shared how his wife, Bonnie, a pediatrician who practices in suburban Philadelphia, counsels parents who raise such concerns. “The way she handled it initially was to do the best she could to eventually get people vaccinated,” he said. “She was successful about one-quarter of the time. Then she drew a line. She started saying to parents, ‘Look; don’t put me in a position where you are asking me to practice substandard care. I can’t send them out of this room knowing that there’s more measles out there, knowing that there’s mumps out there, knowing that there’s whooping cough out there, knowing that there’s pneumococcus and varicella out there. If this child leaves this office and is hurt by any of those viruses or bacteria and I knew I could have done something to prevent it, I couldn’t live with myself. If you’re going to let this child out without being vaccinated I can’t see you anymore because I’m responsible for the health of this child.’ With that [approach], she has been far more successful. Because at some level, if you continue to see that patient, you’re tacitly agreeing that it’s okay to [not vaccinate].”
In 2000, Dr. Offit and colleagues created the Vaccine Education Center at Children’s Hospital of Philadelphia, which provides complete, up-to-date, and reliable information about vaccines to parents and clinicians. It summarizes the purpose of each vaccine, and the relative risks and benefits in easy-to-read language. The CDC also maintains updated information about vaccines and immunizations on its web site. For his part, Dr. Offit tells parents that passing on an opportunity to vaccinate their child is not a risk-free choice. “If you choose not to get a vaccine you probably will get away with it, but you might not,” he said. “You are playing a game of Russian roulette. It may not be five empty chambers and one bullet, but maybe it’s 100,000 empty chambers and one bullet. There’s a bullet there.”
Dr. Offit reported having no relevant financial disclosures.
NEW ORLEANS –
The shift began with the measles outbreak in Southern California in late 2014, he said. According to the Centers for Disease Control and Prevention, 125 measles cases with rash that occurred between Dec. 28, 2014, and Feb. 8, 2015, were confirmed in U.S. residents. Of these, 100 were California residents (MMWR. 2015 Feb 20;64[06];153-4).
“This outbreak spread ultimately to 25 states and involved 189 people,” Dr. Offit said at the annual meeting of the American Academy of Pediatrics. “It was in the news almost every day. As a consequence, there were measles outbreaks in New York, New Jersey, Florida, Oregon, and Texas, and Washington, which began to turn the public sentiment against the antivaccine movement.”
Even longstanding skeptics are changing their tune. Dr. Offit, professor of pediatrics in the division of infectious diseases at the Children’s Hospital of Philadelphia, cited a recent study from the Autism Science Foundation which found that 85% of parents of children with autism spectrum disorder don’t believe that vaccines cause the condition. “Although there will be parents who continue to believe that vaccines cause autism, most parents of children with autism don’t believe that,” he said. “Also, it’s a little hard to make your case that vaccines are dangerous and that you shouldn’t get them in the midst of outbreaks.”
Perhaps the greatest pushback against antivaccination efforts has been made in the legal arena. In 2019 alone, legislators in California banned parents from not vaccinating their kids because of personal beliefs, while lawmakers in New York repealed the religious exemption to vaccinate, those in Maine repealed the religious and philosophical exemption, those in New Jersey required detailed written explanation for religious exemption, and those in Washington State repealed the philosophical exemption for the MMR vaccine.
Pushback also is apparent on various social media platforms. For example, Dr. Offit said, Pinterest restricts vaccine search results to curb the spread of misinformation, YouTube removes ads from antivaccine channels, Amazon Prime has pulled antivaccination documentaries from its video service, and Facebook has taken steps to curb misinformation about vaccines. “With outbreaks and with children suffering, the media and public sentiment has largely turned against those who are vehemently against vaccines,” he said. “I’m talking about an angry, politically connected, lawyer-backed group of people who are conspiracy theorists, [those] who no matter what you say, they’re going to believe there’s a conspiracy theory to hurt their children and not believe you. When that group becomes big enough and you start to see outbreaks like we’ve seen, then it becomes an issue. That’s where it comes down to legislation. Is it your inalienable right as a U.S. citizen to allow your child to catch and transmit a potentially fatal infection? That’s what we’re struggling with now.”
When meeting with parents who are skeptical about vaccines or refuse their children to have them, Dr. Offit advises clinicians to “go down swinging” in favor of vaccination. He shared how his wife, Bonnie, a pediatrician who practices in suburban Philadelphia, counsels parents who raise such concerns. “The way she handled it initially was to do the best she could to eventually get people vaccinated,” he said. “She was successful about one-quarter of the time. Then she drew a line. She started saying to parents, ‘Look; don’t put me in a position where you are asking me to practice substandard care. I can’t send them out of this room knowing that there’s more measles out there, knowing that there’s mumps out there, knowing that there’s whooping cough out there, knowing that there’s pneumococcus and varicella out there. If this child leaves this office and is hurt by any of those viruses or bacteria and I knew I could have done something to prevent it, I couldn’t live with myself. If you’re going to let this child out without being vaccinated I can’t see you anymore because I’m responsible for the health of this child.’ With that [approach], she has been far more successful. Because at some level, if you continue to see that patient, you’re tacitly agreeing that it’s okay to [not vaccinate].”
In 2000, Dr. Offit and colleagues created the Vaccine Education Center at Children’s Hospital of Philadelphia, which provides complete, up-to-date, and reliable information about vaccines to parents and clinicians. It summarizes the purpose of each vaccine, and the relative risks and benefits in easy-to-read language. The CDC also maintains updated information about vaccines and immunizations on its web site. For his part, Dr. Offit tells parents that passing on an opportunity to vaccinate their child is not a risk-free choice. “If you choose not to get a vaccine you probably will get away with it, but you might not,” he said. “You are playing a game of Russian roulette. It may not be five empty chambers and one bullet, but maybe it’s 100,000 empty chambers and one bullet. There’s a bullet there.”
Dr. Offit reported having no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 2019
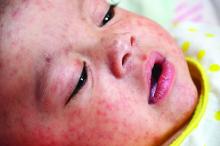
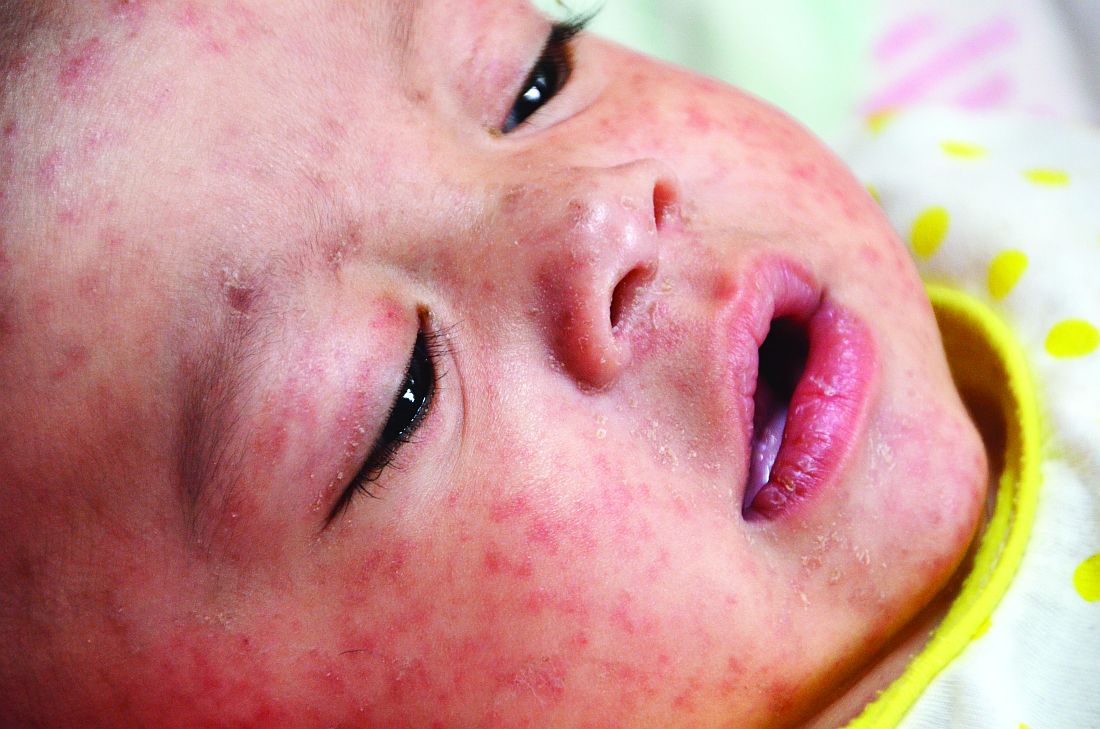
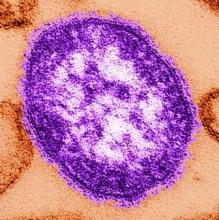
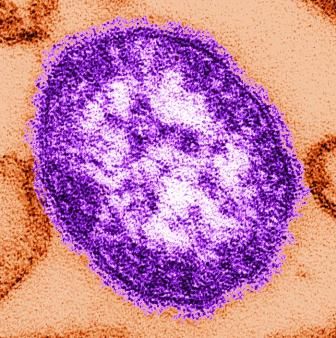
Measles infection linked to impaired ‘immune memory’
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science.
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 the children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
The study did find increases in measles virus–specific antigens in children both after measles infection and MMR vaccination. However the authors did not detect any changes in total IgG, IgA, or IgM levels.
Dr. Mina and associates wrote.
They also noted that controls who received the MMR vaccine showed a marked increase in overall antibody repertoire.
In a separate investigation reported in Science Immunology, Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, and coauthors investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles to determine if B-cell impairment can lead to measles-associated immunosuppression. Their antibody genes were sequenced before any symptoms of measles developed and roughly 40 days after rash. Two control groups also were sequenced accordingly: vaccinated adults and three unvaccinated children from the same community who were not infected with measles.
Naive B cells from individuals in the vaccinated and uninfected control groups showed high correlation of immunoglobulin heavy chain (IgVH-J) gene frequencies across time periods (R2 = 0.96 and 0.92, respectively) but no significant differences in gene expression (P greater than .05). At the same time, although B-cell frequencies in measles patients recovered to levels before infection, they had significant changes in IgVH-J gene frequencies (P = .01) and decreased correlation in gene expression (R2 = 0.78).
In addition, individuals in the control groups had “a stable genetic composition of B memory cells” but no significant changes in the third complementarity-determining region (CDR3) lengths or mutational frequency of IgVH-J genes (P greater than .05). B memory cells in measles patients, however, showed increases in mutational frequency (P = .0008) and a reduction in CDR3 length (P = .017) of IgVH genes, Dr. Petrova and associates reported.
The study by Mina et al. was supported by grants from various U.S., European, and Finnish foundations and national organizations. Some of the coauthors had relationships with biotechnology and pharmaceutical companies, and three reported a patent holding related to technology used in the study. The study by Petrova et al. was funded by grants to the investigators from various Indonesian and German organizations and the Wellcome Trust. The authors reported no conflicts of interest.
SOURCES: Mina M et al. Science. 2019 Nov 1;366:599-606; Petrova VN et al. Sci Immunol. 2019 Nov 1. doi: 10.1126/sciimmunol.aay6125.
As a result of reduced vaccination, after decades of decline, the number of worldwide cases of measles has increased by nearly 300% since 2018. Epidemiologic evidence has associated measles infections with increases in morbidity and mortality for as long as 5 years after the infection and suggests that, in the prevaccine era, measles virus may have been associated with up to 50% of all childhood deaths, mostly because of nonmeasles infections. Measles replication in immune cells has been hypothesized to impair immune memory, potentially causing what some scientists call “immunological amnesia.”A measles virus receptor, called CD150/ SLAMF1, is highly expressed on memory T, B, and plasma cells. Measles virus gains entry to these immune memory cells using that receptor and kills the cells.
The scientists stated that it could take months or years to return the immune repertoire back to baseline. During the rebuilding process, children would be at increased risk for infectious diseases they had previously experienced.
In a second outstanding paper, Petrova et al. in Science Immunology studied B cells before and after measles infection, and identified two immunologic consequences: The naive B-cell pool was depleted, leading to a return to immunologic immaturity, and the memory B-cell pool was depleted, resulting in compromised immune memory to previously encountered pathogens.
Thus, the link between measles infections and increased susceptibility to other infections and increased deaths from nonmeasles infectious diseases in the aftermath of measles has been revealed. This information adds new data to share with parents who consider refusing measles vaccination. The risks are far greater than getting measles.
Michael E. Pichichero, MD, is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He was asked to comment on the articles. Dr. Pichichero has no conflicts to declare.
As a result of reduced vaccination, after decades of decline, the number of worldwide cases of measles has increased by nearly 300% since 2018. Epidemiologic evidence has associated measles infections with increases in morbidity and mortality for as long as 5 years after the infection and suggests that, in the prevaccine era, measles virus may have been associated with up to 50% of all childhood deaths, mostly because of nonmeasles infections. Measles replication in immune cells has been hypothesized to impair immune memory, potentially causing what some scientists call “immunological amnesia.”A measles virus receptor, called CD150/ SLAMF1, is highly expressed on memory T, B, and plasma cells. Measles virus gains entry to these immune memory cells using that receptor and kills the cells.
The scientists stated that it could take months or years to return the immune repertoire back to baseline. During the rebuilding process, children would be at increased risk for infectious diseases they had previously experienced.
In a second outstanding paper, Petrova et al. in Science Immunology studied B cells before and after measles infection, and identified two immunologic consequences: The naive B-cell pool was depleted, leading to a return to immunologic immaturity, and the memory B-cell pool was depleted, resulting in compromised immune memory to previously encountered pathogens.
Thus, the link between measles infections and increased susceptibility to other infections and increased deaths from nonmeasles infectious diseases in the aftermath of measles has been revealed. This information adds new data to share with parents who consider refusing measles vaccination. The risks are far greater than getting measles.
Michael E. Pichichero, MD, is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He was asked to comment on the articles. Dr. Pichichero has no conflicts to declare.
As a result of reduced vaccination, after decades of decline, the number of worldwide cases of measles has increased by nearly 300% since 2018. Epidemiologic evidence has associated measles infections with increases in morbidity and mortality for as long as 5 years after the infection and suggests that, in the prevaccine era, measles virus may have been associated with up to 50% of all childhood deaths, mostly because of nonmeasles infections. Measles replication in immune cells has been hypothesized to impair immune memory, potentially causing what some scientists call “immunological amnesia.”A measles virus receptor, called CD150/ SLAMF1, is highly expressed on memory T, B, and plasma cells. Measles virus gains entry to these immune memory cells using that receptor and kills the cells.
The scientists stated that it could take months or years to return the immune repertoire back to baseline. During the rebuilding process, children would be at increased risk for infectious diseases they had previously experienced.
In a second outstanding paper, Petrova et al. in Science Immunology studied B cells before and after measles infection, and identified two immunologic consequences: The naive B-cell pool was depleted, leading to a return to immunologic immaturity, and the memory B-cell pool was depleted, resulting in compromised immune memory to previously encountered pathogens.
Thus, the link between measles infections and increased susceptibility to other infections and increased deaths from nonmeasles infectious diseases in the aftermath of measles has been revealed. This information adds new data to share with parents who consider refusing measles vaccination. The risks are far greater than getting measles.
Michael E. Pichichero, MD, is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. He was asked to comment on the articles. Dr. Pichichero has no conflicts to declare.
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science.
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 the children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
The study did find increases in measles virus–specific antigens in children both after measles infection and MMR vaccination. However the authors did not detect any changes in total IgG, IgA, or IgM levels.
Dr. Mina and associates wrote.
They also noted that controls who received the MMR vaccine showed a marked increase in overall antibody repertoire.
In a separate investigation reported in Science Immunology, Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, and coauthors investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles to determine if B-cell impairment can lead to measles-associated immunosuppression. Their antibody genes were sequenced before any symptoms of measles developed and roughly 40 days after rash. Two control groups also were sequenced accordingly: vaccinated adults and three unvaccinated children from the same community who were not infected with measles.
Naive B cells from individuals in the vaccinated and uninfected control groups showed high correlation of immunoglobulin heavy chain (IgVH-J) gene frequencies across time periods (R2 = 0.96 and 0.92, respectively) but no significant differences in gene expression (P greater than .05). At the same time, although B-cell frequencies in measles patients recovered to levels before infection, they had significant changes in IgVH-J gene frequencies (P = .01) and decreased correlation in gene expression (R2 = 0.78).
In addition, individuals in the control groups had “a stable genetic composition of B memory cells” but no significant changes in the third complementarity-determining region (CDR3) lengths or mutational frequency of IgVH-J genes (P greater than .05). B memory cells in measles patients, however, showed increases in mutational frequency (P = .0008) and a reduction in CDR3 length (P = .017) of IgVH genes, Dr. Petrova and associates reported.
The study by Mina et al. was supported by grants from various U.S., European, and Finnish foundations and national organizations. Some of the coauthors had relationships with biotechnology and pharmaceutical companies, and three reported a patent holding related to technology used in the study. The study by Petrova et al. was funded by grants to the investigators from various Indonesian and German organizations and the Wellcome Trust. The authors reported no conflicts of interest.
SOURCES: Mina M et al. Science. 2019 Nov 1;366:599-606; Petrova VN et al. Sci Immunol. 2019 Nov 1. doi: 10.1126/sciimmunol.aay6125.
Infection with the measles virus appears to reduce immunity to other pathogens, according to a paper published in Science.
The hypothesis that the measles virus could cause “immunological amnesia” by impairing immune memory is supported by early research showing children with measles had negative cutaneous tuberculin reactions after having previously tested positive.
“Subsequent studies have shown decreased interferon signaling, skewed cytokine responses, lymphopenia, and suppression of lymphocyte proliferation shortly after infection,” wrote Michael Mina, MD, from Brigham and Women’s Hospital in Boston, and coauthors.
“Given the variation in the degree of immune repertoire modulation we observed, we anticipate that future risk of morbidity and mortality after measles would not be homogeneous but would be skewed toward individuals with the most severe elimination of immunological memory,” they wrote. “These findings underscore the crucial need for continued widespread vaccination.”
In this study, researchers compared the levels of around 400 pathogen-specific antibodies in blood samples from 77 unvaccinated children, taken before and 2 months after natural measles infection, with 5 unvaccinated children who did not contract measles. A total of 34 the children experienced mild measles, and 43 had severe measles.
They found that the samples taken after measles infection showed “substantial” reductions in the number of pathogen epitopes, compared with the samples from children who did not get infected with measles.
This amounted to approximately a 20% mean reduction in overall diversity or size of the antibody repertoire. However, in children who experienced severe measles, there was a median loss of 40% (range, 11%-62%) of antibody repertoire, compared with a median of 33% (range, 12%-73%) range in children who experienced mild infection. Meanwhile, the control subjects retained approximately 90% of their antibody repertoire over a similar or longer time period. Some children lost up to 70% of antibodies for specific pathogens.
The study did find increases in measles virus–specific antigens in children both after measles infection and MMR vaccination. However the authors did not detect any changes in total IgG, IgA, or IgM levels.
Dr. Mina and associates wrote.
They also noted that controls who received the MMR vaccine showed a marked increase in overall antibody repertoire.
In a separate investigation reported in Science Immunology, Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, and coauthors investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles to determine if B-cell impairment can lead to measles-associated immunosuppression. Their antibody genes were sequenced before any symptoms of measles developed and roughly 40 days after rash. Two control groups also were sequenced accordingly: vaccinated adults and three unvaccinated children from the same community who were not infected with measles.
Naive B cells from individuals in the vaccinated and uninfected control groups showed high correlation of immunoglobulin heavy chain (IgVH-J) gene frequencies across time periods (R2 = 0.96 and 0.92, respectively) but no significant differences in gene expression (P greater than .05). At the same time, although B-cell frequencies in measles patients recovered to levels before infection, they had significant changes in IgVH-J gene frequencies (P = .01) and decreased correlation in gene expression (R2 = 0.78).
In addition, individuals in the control groups had “a stable genetic composition of B memory cells” but no significant changes in the third complementarity-determining region (CDR3) lengths or mutational frequency of IgVH-J genes (P greater than .05). B memory cells in measles patients, however, showed increases in mutational frequency (P = .0008) and a reduction in CDR3 length (P = .017) of IgVH genes, Dr. Petrova and associates reported.
The study by Mina et al. was supported by grants from various U.S., European, and Finnish foundations and national organizations. Some of the coauthors had relationships with biotechnology and pharmaceutical companies, and three reported a patent holding related to technology used in the study. The study by Petrova et al. was funded by grants to the investigators from various Indonesian and German organizations and the Wellcome Trust. The authors reported no conflicts of interest.
SOURCES: Mina M et al. Science. 2019 Nov 1;366:599-606; Petrova VN et al. Sci Immunol. 2019 Nov 1. doi: 10.1126/sciimmunol.aay6125.
FROM SCIENCE
Measles causes B-cell changes, leading to ‘immune amnesia’
“Our findings provide a biological explanation for the observed increase in childhood mortality and secondary infections several years after an episode of measles,” said Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, and coauthors. The study was published in Science Immunology.
To determine if B-cell impairment can lead to measles-associated immunosuppression, the researchers investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. Their antibody genes were sequenced before any symptoms of measles developed and roughly 40 days after rash. Two control groups also were sequenced accordingly: vaccinated adults and three unvaccinated children from the same community who were not infected with measles.
Naive B cells from individuals in the vaccinated and uninfected control groups showed high correlation of immunoglobulin heavy chain (IGHV-J) gene frequencies across time periods (R2 = 0.96 and 0.92, respectively) but no significant differences in gene expression (P greater than .05). At the same time, although B cell frequencies in measles patients recovered to levels before infection, they had significant changes in IGHV-J gene frequencies (P = .01) and decreased correlation in gene expression (R2 = 0.78).
In addition, individuals in the control groups had “a stable genetic composition of B memory cells” but no significant changes in the third complementarity-determining region (CDR3) lengths or mutational frequency of IGHV genes (P greater than .05). B memory cells in measles patients, however, showed increases in mutational frequency (P = .0008) and a reduction in CDR3 length (P = .017) of IGHV genes, Dr. Petrova and associates said.
Finally, the researchers confirmed a hypothesis about the depletion of B memory cell clones during measles and a repopulation of new cells with less clonal expansion. The frequency of individual IGHV-J gene combinations before infection was correlated with a reduction after infection, “with the most frequent combinations undergoing the most marked depletion” and the result being an increase in genetic diversity.
To further test their findings, the researchers vaccinated two groups of four ferrets with live-attenuated influenza vaccine (LAIV) and at 4 weeks infected one of the groups with canine distemper virus (CDV), a surrogate for MeV. At 14 weeks after vaccination, the uninfected group maintained high levels of influenza-specific neutralizing antibodies while the infected group saw impaired B cells and a subsequent reduction in neutralizing antibodies.
Understanding the impact of measles on the immune system
“How measles infection has such a long-lasting deleterious effect on the immune system while allowing robust immunity against itself has been a burning immunological question,” Duane R. Wesemann, MD, PhD, of Brigham and Women’s Hospital in Boston, said in an accompanying editorial. The research from Petrova et al. begins to answer that question.
Among the observations he found most interesting was how “post-measles memory cells were more diverse than the pre-measles memory pool,” despite expectations that measles immunity would be dominant. He speculated that the void in memory cells is filled by a set of clones binding to unidentified or nonnative antigens, which may bring polyclonal diversity into B memory cells.
More research is needed to determine just what these findings mean, including looking beyond memory cell depletion and focusing on the impact of immature immunoglobulin repertoires in naive cells. But his broad takeaway is that measles remains both a public health concern and an opportunity to understand how the human body counters disease.
“The unique relationship measles has with the human immune system,” he said, “can illuminate aspects of its inner workings.”
The study was funded by grants to the investigators the Indonesian Endowment Fund for Education, the Wellcome Trust, the German Centre for Infection Research, the Collaborative Research Centre of the German Research Foundation, the German Ministry of Health, and the Royal Society. The authors declared no conflicts of interest. Dr. Wesemann reported receiving support from National Institutes of Health grants and an award from the Burroughs Wellcome Fund; he also reports being a consultant for OpenBiome.
SOURCE: Petrova VN et al. Sci Immunol. 2019 Nov 1. doi: 10.1126/sciimmunol.aay6125; Wesemann DR. Sci Immunol. 2019 Nov 1. doi: 10.1126/sciimmunol.aaz4195.
“Our findings provide a biological explanation for the observed increase in childhood mortality and secondary infections several years after an episode of measles,” said Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, and coauthors. The study was published in Science Immunology.
To determine if B-cell impairment can lead to measles-associated immunosuppression, the researchers investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. Their antibody genes were sequenced before any symptoms of measles developed and roughly 40 days after rash. Two control groups also were sequenced accordingly: vaccinated adults and three unvaccinated children from the same community who were not infected with measles.
Naive B cells from individuals in the vaccinated and uninfected control groups showed high correlation of immunoglobulin heavy chain (IGHV-J) gene frequencies across time periods (R2 = 0.96 and 0.92, respectively) but no significant differences in gene expression (P greater than .05). At the same time, although B cell frequencies in measles patients recovered to levels before infection, they had significant changes in IGHV-J gene frequencies (P = .01) and decreased correlation in gene expression (R2 = 0.78).
In addition, individuals in the control groups had “a stable genetic composition of B memory cells” but no significant changes in the third complementarity-determining region (CDR3) lengths or mutational frequency of IGHV genes (P greater than .05). B memory cells in measles patients, however, showed increases in mutational frequency (P = .0008) and a reduction in CDR3 length (P = .017) of IGHV genes, Dr. Petrova and associates said.
Finally, the researchers confirmed a hypothesis about the depletion of B memory cell clones during measles and a repopulation of new cells with less clonal expansion. The frequency of individual IGHV-J gene combinations before infection was correlated with a reduction after infection, “with the most frequent combinations undergoing the most marked depletion” and the result being an increase in genetic diversity.
To further test their findings, the researchers vaccinated two groups of four ferrets with live-attenuated influenza vaccine (LAIV) and at 4 weeks infected one of the groups with canine distemper virus (CDV), a surrogate for MeV. At 14 weeks after vaccination, the uninfected group maintained high levels of influenza-specific neutralizing antibodies while the infected group saw impaired B cells and a subsequent reduction in neutralizing antibodies.
Understanding the impact of measles on the immune system
“How measles infection has such a long-lasting deleterious effect on the immune system while allowing robust immunity against itself has been a burning immunological question,” Duane R. Wesemann, MD, PhD, of Brigham and Women’s Hospital in Boston, said in an accompanying editorial. The research from Petrova et al. begins to answer that question.
Among the observations he found most interesting was how “post-measles memory cells were more diverse than the pre-measles memory pool,” despite expectations that measles immunity would be dominant. He speculated that the void in memory cells is filled by a set of clones binding to unidentified or nonnative antigens, which may bring polyclonal diversity into B memory cells.
More research is needed to determine just what these findings mean, including looking beyond memory cell depletion and focusing on the impact of immature immunoglobulin repertoires in naive cells. But his broad takeaway is that measles remains both a public health concern and an opportunity to understand how the human body counters disease.
“The unique relationship measles has with the human immune system,” he said, “can illuminate aspects of its inner workings.”
The study was funded by grants to the investigators the Indonesian Endowment Fund for Education, the Wellcome Trust, the German Centre for Infection Research, the Collaborative Research Centre of the German Research Foundation, the German Ministry of Health, and the Royal Society. The authors declared no conflicts of interest. Dr. Wesemann reported receiving support from National Institutes of Health grants and an award from the Burroughs Wellcome Fund; he also reports being a consultant for OpenBiome.
SOURCE: Petrova VN et al. Sci Immunol. 2019 Nov 1. doi: 10.1126/sciimmunol.aay6125; Wesemann DR. Sci Immunol. 2019 Nov 1. doi: 10.1126/sciimmunol.aaz4195.
“Our findings provide a biological explanation for the observed increase in childhood mortality and secondary infections several years after an episode of measles,” said Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, and coauthors. The study was published in Science Immunology.
To determine if B-cell impairment can lead to measles-associated immunosuppression, the researchers investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. Their antibody genes were sequenced before any symptoms of measles developed and roughly 40 days after rash. Two control groups also were sequenced accordingly: vaccinated adults and three unvaccinated children from the same community who were not infected with measles.
Naive B cells from individuals in the vaccinated and uninfected control groups showed high correlation of immunoglobulin heavy chain (IGHV-J) gene frequencies across time periods (R2 = 0.96 and 0.92, respectively) but no significant differences in gene expression (P greater than .05). At the same time, although B cell frequencies in measles patients recovered to levels before infection, they had significant changes in IGHV-J gene frequencies (P = .01) and decreased correlation in gene expression (R2 = 0.78).
In addition, individuals in the control groups had “a stable genetic composition of B memory cells” but no significant changes in the third complementarity-determining region (CDR3) lengths or mutational frequency of IGHV genes (P greater than .05). B memory cells in measles patients, however, showed increases in mutational frequency (P = .0008) and a reduction in CDR3 length (P = .017) of IGHV genes, Dr. Petrova and associates said.
Finally, the researchers confirmed a hypothesis about the depletion of B memory cell clones during measles and a repopulation of new cells with less clonal expansion. The frequency of individual IGHV-J gene combinations before infection was correlated with a reduction after infection, “with the most frequent combinations undergoing the most marked depletion” and the result being an increase in genetic diversity.
To further test their findings, the researchers vaccinated two groups of four ferrets with live-attenuated influenza vaccine (LAIV) and at 4 weeks infected one of the groups with canine distemper virus (CDV), a surrogate for MeV. At 14 weeks after vaccination, the uninfected group maintained high levels of influenza-specific neutralizing antibodies while the infected group saw impaired B cells and a subsequent reduction in neutralizing antibodies.
Understanding the impact of measles on the immune system
“How measles infection has such a long-lasting deleterious effect on the immune system while allowing robust immunity against itself has been a burning immunological question,” Duane R. Wesemann, MD, PhD, of Brigham and Women’s Hospital in Boston, said in an accompanying editorial. The research from Petrova et al. begins to answer that question.
Among the observations he found most interesting was how “post-measles memory cells were more diverse than the pre-measles memory pool,” despite expectations that measles immunity would be dominant. He speculated that the void in memory cells is filled by a set of clones binding to unidentified or nonnative antigens, which may bring polyclonal diversity into B memory cells.
More research is needed to determine just what these findings mean, including looking beyond memory cell depletion and focusing on the impact of immature immunoglobulin repertoires in naive cells. But his broad takeaway is that measles remains both a public health concern and an opportunity to understand how the human body counters disease.
“The unique relationship measles has with the human immune system,” he said, “can illuminate aspects of its inner workings.”
The study was funded by grants to the investigators the Indonesian Endowment Fund for Education, the Wellcome Trust, the German Centre for Infection Research, the Collaborative Research Centre of the German Research Foundation, the German Ministry of Health, and the Royal Society. The authors declared no conflicts of interest. Dr. Wesemann reported receiving support from National Institutes of Health grants and an award from the Burroughs Wellcome Fund; he also reports being a consultant for OpenBiome.
SOURCE: Petrova VN et al. Sci Immunol. 2019 Nov 1. doi: 10.1126/sciimmunol.aay6125; Wesemann DR. Sci Immunol. 2019 Nov 1. doi: 10.1126/sciimmunol.aaz4195.
FROM SCIENCE IMMUNOTHERAPY
Brain abscess with lung infection? Think Nocardia
ST. LOUIS – according to University of California, San Francisco, investigators.
Nocardia – an ubiquitous gram-positive rod normally found in standing water, decaying plants, and soil, that can cause problems when it is inhaled as dust or introduced through a nick in the skin – is an underappreciated cause of brain abscess that is not covered by standard empiric therapy targeting the more common causes: Staphylococcus and Streptococcus bacteria, said senior investigator Megan Richie, MD, an assistant neurology professor at UCSF.
“Patients that have a lung infection with a new brain abscess should be started on empiric therapy not just for pyogenic organisms, but also for Nocardia pending biopsy and operative culture data, especially given that empiric therapy of high-dose Bactrim for Nocardia is relatively benign,” she said at the annual meeting of the American Neurological Association.
The advice comes from a comparison of 14 Nocardia cases with 42 randomly selected Staph/Strep cases in a university radiologic database. Nine Nocardia cases were confirmed by operative specimen culture, the rest by lung, blood, or other tissue cultures.
Dr. Richie and colleagues suspected an association with lung infection, which has been reported anecdotally in the literature. The researchers wanted to take a quantitative look to see if it held up statistically after pushback on a brain abscess patient with a lung infection. “We were concerned this patient had Nocardia, but it took quite some time to convince other doctors that we really needed to start [Bactrim]. The patient was not immunocompromised and the infectious disease team said ‘Nocardia brain infections don’t happen in immunocompetent patients,’” Dr. Richie said,
The man did, however, turn out to have Nocardia, and of the 14 cases in the series, four patients (29%) were not immunosuppressed. “I think this would surprise [physicians] who have a little bit less experience with this organism,” Dr. Richie said.Patients with a Nocardia brain abscess were far more likely to have a concomitant lung infection (86% vs. 2%; odds ratio, 246; 95% confidence interval, 21-2953; P less than .0001). Staph/Strep brain abscess patients were more likely to have concomitant ear or sinus infections (40% versus 0%; P = .005). Immunosuppression did turn out to be more common in the Nocardia group, as well (71% vs. 19%; OR, 11; 95% CI, 3-43; P = .001), as did diabetes (36% vs. 10%; P = .03).
Nocardia patients were older (median age, 61 yrs vs. 46 yrs: P = .01) and more likely to be Hispanic (36% vs. 10%; P = .04). There were no differences in sex; neurosurgery history; intravenous drug use; or endocarditis.
On imaging, Nocardia brain abscesses were poorly circumscribed and tended to have multiple lobes, “often two in a figure-eight pattern,” Dr. Richie said. Nocardia diagnosis took longer (median, 7 vs. 4 days; P = .04), “which makes sense because it is a harder diagnosis to make,” she said.
Operative specimen culture was the most potent diagnostic tool. Blood cultures were positive in just one Nocardia patient and a few controls.
There was no external funding, and the investigators did not have any relevant disclosures.
ST. LOUIS – according to University of California, San Francisco, investigators.
Nocardia – an ubiquitous gram-positive rod normally found in standing water, decaying plants, and soil, that can cause problems when it is inhaled as dust or introduced through a nick in the skin – is an underappreciated cause of brain abscess that is not covered by standard empiric therapy targeting the more common causes: Staphylococcus and Streptococcus bacteria, said senior investigator Megan Richie, MD, an assistant neurology professor at UCSF.
“Patients that have a lung infection with a new brain abscess should be started on empiric therapy not just for pyogenic organisms, but also for Nocardia pending biopsy and operative culture data, especially given that empiric therapy of high-dose Bactrim for Nocardia is relatively benign,” she said at the annual meeting of the American Neurological Association.
The advice comes from a comparison of 14 Nocardia cases with 42 randomly selected Staph/Strep cases in a university radiologic database. Nine Nocardia cases were confirmed by operative specimen culture, the rest by lung, blood, or other tissue cultures.
Dr. Richie and colleagues suspected an association with lung infection, which has been reported anecdotally in the literature. The researchers wanted to take a quantitative look to see if it held up statistically after pushback on a brain abscess patient with a lung infection. “We were concerned this patient had Nocardia, but it took quite some time to convince other doctors that we really needed to start [Bactrim]. The patient was not immunocompromised and the infectious disease team said ‘Nocardia brain infections don’t happen in immunocompetent patients,’” Dr. Richie said,
The man did, however, turn out to have Nocardia, and of the 14 cases in the series, four patients (29%) were not immunosuppressed. “I think this would surprise [physicians] who have a little bit less experience with this organism,” Dr. Richie said.Patients with a Nocardia brain abscess were far more likely to have a concomitant lung infection (86% vs. 2%; odds ratio, 246; 95% confidence interval, 21-2953; P less than .0001). Staph/Strep brain abscess patients were more likely to have concomitant ear or sinus infections (40% versus 0%; P = .005). Immunosuppression did turn out to be more common in the Nocardia group, as well (71% vs. 19%; OR, 11; 95% CI, 3-43; P = .001), as did diabetes (36% vs. 10%; P = .03).
Nocardia patients were older (median age, 61 yrs vs. 46 yrs: P = .01) and more likely to be Hispanic (36% vs. 10%; P = .04). There were no differences in sex; neurosurgery history; intravenous drug use; or endocarditis.
On imaging, Nocardia brain abscesses were poorly circumscribed and tended to have multiple lobes, “often two in a figure-eight pattern,” Dr. Richie said. Nocardia diagnosis took longer (median, 7 vs. 4 days; P = .04), “which makes sense because it is a harder diagnosis to make,” she said.
Operative specimen culture was the most potent diagnostic tool. Blood cultures were positive in just one Nocardia patient and a few controls.
There was no external funding, and the investigators did not have any relevant disclosures.
ST. LOUIS – according to University of California, San Francisco, investigators.
Nocardia – an ubiquitous gram-positive rod normally found in standing water, decaying plants, and soil, that can cause problems when it is inhaled as dust or introduced through a nick in the skin – is an underappreciated cause of brain abscess that is not covered by standard empiric therapy targeting the more common causes: Staphylococcus and Streptococcus bacteria, said senior investigator Megan Richie, MD, an assistant neurology professor at UCSF.
“Patients that have a lung infection with a new brain abscess should be started on empiric therapy not just for pyogenic organisms, but also for Nocardia pending biopsy and operative culture data, especially given that empiric therapy of high-dose Bactrim for Nocardia is relatively benign,” she said at the annual meeting of the American Neurological Association.
The advice comes from a comparison of 14 Nocardia cases with 42 randomly selected Staph/Strep cases in a university radiologic database. Nine Nocardia cases were confirmed by operative specimen culture, the rest by lung, blood, or other tissue cultures.
Dr. Richie and colleagues suspected an association with lung infection, which has been reported anecdotally in the literature. The researchers wanted to take a quantitative look to see if it held up statistically after pushback on a brain abscess patient with a lung infection. “We were concerned this patient had Nocardia, but it took quite some time to convince other doctors that we really needed to start [Bactrim]. The patient was not immunocompromised and the infectious disease team said ‘Nocardia brain infections don’t happen in immunocompetent patients,’” Dr. Richie said,
The man did, however, turn out to have Nocardia, and of the 14 cases in the series, four patients (29%) were not immunosuppressed. “I think this would surprise [physicians] who have a little bit less experience with this organism,” Dr. Richie said.Patients with a Nocardia brain abscess were far more likely to have a concomitant lung infection (86% vs. 2%; odds ratio, 246; 95% confidence interval, 21-2953; P less than .0001). Staph/Strep brain abscess patients were more likely to have concomitant ear or sinus infections (40% versus 0%; P = .005). Immunosuppression did turn out to be more common in the Nocardia group, as well (71% vs. 19%; OR, 11; 95% CI, 3-43; P = .001), as did diabetes (36% vs. 10%; P = .03).
Nocardia patients were older (median age, 61 yrs vs. 46 yrs: P = .01) and more likely to be Hispanic (36% vs. 10%; P = .04). There were no differences in sex; neurosurgery history; intravenous drug use; or endocarditis.
On imaging, Nocardia brain abscesses were poorly circumscribed and tended to have multiple lobes, “often two in a figure-eight pattern,” Dr. Richie said. Nocardia diagnosis took longer (median, 7 vs. 4 days; P = .04), “which makes sense because it is a harder diagnosis to make,” she said.
Operative specimen culture was the most potent diagnostic tool. Blood cultures were positive in just one Nocardia patient and a few controls.
There was no external funding, and the investigators did not have any relevant disclosures.
REPORTING FROM ANA 2019
ACIP approves child and adolescent vaccination schedule for 2020
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the child and adolescent immunization schedule for 2020.
by busy providers,” Candice Robinson, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases, said at the CDC’s October meeting of ACIP. Updates reflect changes in language in the adult vaccination schedule, notably the change in the definition of “contraindication.” The updated wording in the Notes substitutes “not recommended or contraindicated” instead of the word “contraindicated” only.
Another notable change was the addition of information on adolescent vaccination of children who received the meningococcal ACWY vaccine before 10 years of age. For “children in whom boosters are not recommended due to an ongoing or increased risk of meningococcal disease” (such as a healthy child traveling to an endemic area), they should receive MenACWY according to the recommended adolescent schedule. But those children for whom boosters are recommended because of increased disease risk from conditions including complement deficiency, HIV, or asplenia should “follow the booster schedule for persons at increased risk.”
Other changes include restructuring of the notes for the live attenuated influenza vaccine (LAIV) in special situations. The schedule now uses a bulleted list to show that LAIV should not be used in the following circumstances:
- Having history of severe allergic reaction to a previous vaccine or vaccine component.
- Using aspirin or a salicylate-containing medication.
- Being aged 2-4 years with a history of asthma or wheezing.
- Having immunocompromised conditions.
- Having anatomic or functional asplenia.
- Having cochlear implants.
- Experiencing cerebrospinal fluid–oropharyngeal communication.
- Having immunocompromised close contacts or caregivers.
- Being pregnant.
- Having received flu antivirals within the previous 48 hours.
In addition, language on shared clinical decision-making was added to the notes on the meningococcal B vaccine for adolescents and young adults aged 18-23 years not at increased risk. Based on shared clinical decision making, the recommendation is a “two-dose series of Bexsero at least 1 month apart” or “two-dose series of Trumenba at least 6 months apart; if dose two is administered earlier than 6 months, administer a third dose at least 4 months after dose two.”
Several vaccines’ Notes sections, including hepatitis B and meningococcal disease, added links to detailed recommendations in the corresponding issues of the CDC’s Morbidity and Mortality Weekly Report, to allow clinicians easy access to additional information.
View the current Child & Adolescent Vaccination Schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the child and adolescent immunization schedule for 2020.
by busy providers,” Candice Robinson, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases, said at the CDC’s October meeting of ACIP. Updates reflect changes in language in the adult vaccination schedule, notably the change in the definition of “contraindication.” The updated wording in the Notes substitutes “not recommended or contraindicated” instead of the word “contraindicated” only.
Another notable change was the addition of information on adolescent vaccination of children who received the meningococcal ACWY vaccine before 10 years of age. For “children in whom boosters are not recommended due to an ongoing or increased risk of meningococcal disease” (such as a healthy child traveling to an endemic area), they should receive MenACWY according to the recommended adolescent schedule. But those children for whom boosters are recommended because of increased disease risk from conditions including complement deficiency, HIV, or asplenia should “follow the booster schedule for persons at increased risk.”
Other changes include restructuring of the notes for the live attenuated influenza vaccine (LAIV) in special situations. The schedule now uses a bulleted list to show that LAIV should not be used in the following circumstances:
- Having history of severe allergic reaction to a previous vaccine or vaccine component.
- Using aspirin or a salicylate-containing medication.
- Being aged 2-4 years with a history of asthma or wheezing.
- Having immunocompromised conditions.
- Having anatomic or functional asplenia.
- Having cochlear implants.
- Experiencing cerebrospinal fluid–oropharyngeal communication.
- Having immunocompromised close contacts or caregivers.
- Being pregnant.
- Having received flu antivirals within the previous 48 hours.
In addition, language on shared clinical decision-making was added to the notes on the meningococcal B vaccine for adolescents and young adults aged 18-23 years not at increased risk. Based on shared clinical decision making, the recommendation is a “two-dose series of Bexsero at least 1 month apart” or “two-dose series of Trumenba at least 6 months apart; if dose two is administered earlier than 6 months, administer a third dose at least 4 months after dose two.”
Several vaccines’ Notes sections, including hepatitis B and meningococcal disease, added links to detailed recommendations in the corresponding issues of the CDC’s Morbidity and Mortality Weekly Report, to allow clinicians easy access to additional information.
View the current Child & Adolescent Vaccination Schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the child and adolescent immunization schedule for 2020.
by busy providers,” Candice Robinson, MD, MPH, of the CDC’s National Center for Immunization and Respiratory Diseases, said at the CDC’s October meeting of ACIP. Updates reflect changes in language in the adult vaccination schedule, notably the change in the definition of “contraindication.” The updated wording in the Notes substitutes “not recommended or contraindicated” instead of the word “contraindicated” only.
Another notable change was the addition of information on adolescent vaccination of children who received the meningococcal ACWY vaccine before 10 years of age. For “children in whom boosters are not recommended due to an ongoing or increased risk of meningococcal disease” (such as a healthy child traveling to an endemic area), they should receive MenACWY according to the recommended adolescent schedule. But those children for whom boosters are recommended because of increased disease risk from conditions including complement deficiency, HIV, or asplenia should “follow the booster schedule for persons at increased risk.”
Other changes include restructuring of the notes for the live attenuated influenza vaccine (LAIV) in special situations. The schedule now uses a bulleted list to show that LAIV should not be used in the following circumstances:
- Having history of severe allergic reaction to a previous vaccine or vaccine component.
- Using aspirin or a salicylate-containing medication.
- Being aged 2-4 years with a history of asthma or wheezing.
- Having immunocompromised conditions.
- Having anatomic or functional asplenia.
- Having cochlear implants.
- Experiencing cerebrospinal fluid–oropharyngeal communication.
- Having immunocompromised close contacts or caregivers.
- Being pregnant.
- Having received flu antivirals within the previous 48 hours.
In addition, language on shared clinical decision-making was added to the notes on the meningococcal B vaccine for adolescents and young adults aged 18-23 years not at increased risk. Based on shared clinical decision making, the recommendation is a “two-dose series of Bexsero at least 1 month apart” or “two-dose series of Trumenba at least 6 months apart; if dose two is administered earlier than 6 months, administer a third dose at least 4 months after dose two.”
Several vaccines’ Notes sections, including hepatitis B and meningococcal disease, added links to detailed recommendations in the corresponding issues of the CDC’s Morbidity and Mortality Weekly Report, to allow clinicians easy access to additional information.
View the current Child & Adolescent Vaccination Schedule here.
The ACIP members had no financial conflicts to disclose.
FROM AN ACIP MEETING
ACIP approves 2020 adult vaccination schedule
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the adult immunization schedule for 2020, although some fine-tuning may occur before publication.
“Some of the wordsmithing may be done later,” ACIP executive secretary Amanda Cohn, MD, said at the ACIP October meeting.
Key updates to the schedule included a change in wording for the definition of the red bars on the table to include “not recommended or contraindicated” instead of only the word “contraindicated.” Committee members were especially interested in changing this wording to guide clinicians in use of the live attenuated influenza vaccine because of its potential value in vaccinating health care personnel.
Other updates include language that vaccination of adolescents and young adults aged 16-23 years who are not at increased risk for meningococcal disease should be vaccinated as follows: “Based on shared clinical decision making, 2-dose series MenB-4C at least 1 month apart or 2-dose series MenB-FHbp at 0, 6 months.”
Similarly, clinical decision-making language was added to the notes for the pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13).
The routine vaccination calls for only one dose of PPSV23 given on or after the individual’s 65th birthday. Then, based on shared clinical decision making, a dose of PCV13 is recommended for immunocompetent individuals aged 65 years and older. The notes also state that, based on shared clinical decision making, PCV13 and PPSV23 should not be given in the same visit and, if both will be given, PCV13 should be first and should be given 1 year before PPSV23. In addition, “PPSV23 should be given at least 5 years after any previous PPSV23 dose.”
The schedule also adds shared clinical decision making to the notes on human papillomavirus vaccination for adults aged 27-45 years.
The committee members acknowledged the increasing complexity of the adult vaccination schedule, but several members agreed that it is accessible to many clinicians.
“We can’t let the perfect be the enemy of the good” said Jason Goldman, MD, liaison representing the American College of Physicians. “Those who want to learn the schedule will learn it; the health system will learn it,” even if not every specialist does.
The table “is something to draw you in,” said Sandra Fryhofer, MD, an internist who is liaison for the American Medical Association. The notes provide more details.
More specific information about contraindications for patients with cochlear implants, which also came up in the discussion, may be added to the schedule at a later date.
View the current adult vaccination schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the adult immunization schedule for 2020, although some fine-tuning may occur before publication.
“Some of the wordsmithing may be done later,” ACIP executive secretary Amanda Cohn, MD, said at the ACIP October meeting.
Key updates to the schedule included a change in wording for the definition of the red bars on the table to include “not recommended or contraindicated” instead of only the word “contraindicated.” Committee members were especially interested in changing this wording to guide clinicians in use of the live attenuated influenza vaccine because of its potential value in vaccinating health care personnel.
Other updates include language that vaccination of adolescents and young adults aged 16-23 years who are not at increased risk for meningococcal disease should be vaccinated as follows: “Based on shared clinical decision making, 2-dose series MenB-4C at least 1 month apart or 2-dose series MenB-FHbp at 0, 6 months.”
Similarly, clinical decision-making language was added to the notes for the pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13).
The routine vaccination calls for only one dose of PPSV23 given on or after the individual’s 65th birthday. Then, based on shared clinical decision making, a dose of PCV13 is recommended for immunocompetent individuals aged 65 years and older. The notes also state that, based on shared clinical decision making, PCV13 and PPSV23 should not be given in the same visit and, if both will be given, PCV13 should be first and should be given 1 year before PPSV23. In addition, “PPSV23 should be given at least 5 years after any previous PPSV23 dose.”
The schedule also adds shared clinical decision making to the notes on human papillomavirus vaccination for adults aged 27-45 years.
The committee members acknowledged the increasing complexity of the adult vaccination schedule, but several members agreed that it is accessible to many clinicians.
“We can’t let the perfect be the enemy of the good” said Jason Goldman, MD, liaison representing the American College of Physicians. “Those who want to learn the schedule will learn it; the health system will learn it,” even if not every specialist does.
The table “is something to draw you in,” said Sandra Fryhofer, MD, an internist who is liaison for the American Medical Association. The notes provide more details.
More specific information about contraindications for patients with cochlear implants, which also came up in the discussion, may be added to the schedule at a later date.
View the current adult vaccination schedule here.
The ACIP members had no financial conflicts to disclose.
The Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices voted unanimously to approve the adult immunization schedule for 2020, although some fine-tuning may occur before publication.
“Some of the wordsmithing may be done later,” ACIP executive secretary Amanda Cohn, MD, said at the ACIP October meeting.
Key updates to the schedule included a change in wording for the definition of the red bars on the table to include “not recommended or contraindicated” instead of only the word “contraindicated.” Committee members were especially interested in changing this wording to guide clinicians in use of the live attenuated influenza vaccine because of its potential value in vaccinating health care personnel.
Other updates include language that vaccination of adolescents and young adults aged 16-23 years who are not at increased risk for meningococcal disease should be vaccinated as follows: “Based on shared clinical decision making, 2-dose series MenB-4C at least 1 month apart or 2-dose series MenB-FHbp at 0, 6 months.”
Similarly, clinical decision-making language was added to the notes for the pneumococcal polysaccharide vaccine (PPSV23) and the 13-valent pneumococcal conjugate vaccine (PCV13).
The routine vaccination calls for only one dose of PPSV23 given on or after the individual’s 65th birthday. Then, based on shared clinical decision making, a dose of PCV13 is recommended for immunocompetent individuals aged 65 years and older. The notes also state that, based on shared clinical decision making, PCV13 and PPSV23 should not be given in the same visit and, if both will be given, PCV13 should be first and should be given 1 year before PPSV23. In addition, “PPSV23 should be given at least 5 years after any previous PPSV23 dose.”
The schedule also adds shared clinical decision making to the notes on human papillomavirus vaccination for adults aged 27-45 years.
The committee members acknowledged the increasing complexity of the adult vaccination schedule, but several members agreed that it is accessible to many clinicians.
“We can’t let the perfect be the enemy of the good” said Jason Goldman, MD, liaison representing the American College of Physicians. “Those who want to learn the schedule will learn it; the health system will learn it,” even if not every specialist does.
The table “is something to draw you in,” said Sandra Fryhofer, MD, an internist who is liaison for the American Medical Association. The notes provide more details.
More specific information about contraindications for patients with cochlear implants, which also came up in the discussion, may be added to the schedule at a later date.
View the current adult vaccination schedule here.
The ACIP members had no financial conflicts to disclose.
ACIP recommends two options for pertussis vaccination
Either the Tdap or Td vaccine is an acceptable option for pertussis vaccination in most situations, recommended the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 14-0 vote at the October meeting, based on the immunization schedule for persons aged 7 years and older.
Safety data showed no differences in safety concerns between Tdap and Td, including data from pregnant women, said Fiona Havers, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), Atlanta.
Several of the ACIP members noted that the revised language to include both Tdap and Td reflects the increased use of Tdap and allows for maximum flexibility in clinical settings.
The revised language advises that booster doses of “either Td or Tdap” every 10 years throughout life are recommended for continued protection against tetanus and diphtheria. In addition, either Td or Tdap should be used if a tetanus toxoid–containing vaccine is indicated for prophylaxis in nonpregnant individuals.
For catch-up recommendations, which also apply to pregnant women, the committee approved the following wording for a series of three doses for individuals aged 7-18 years and 19 years and older who have never been vaccinated, that “the preferred schedule is a dose of Tdap (preferably the first dose), followed by either Tdap or Td at least 4 weeks afterward and another dose of either Td or Tdap 6-12 months later.” Individuals in these same age groups who are not fully vaccinated should receive one dose of Tdap, and a dose of either Td or Tdap if additional doses are needed.
The committee also voted unanimously 14-0 to accept the updated wording for pertussis vaccination in the Vaccines for Children program.
The ACIP members had no financial conflicts to disclose.
Either the Tdap or Td vaccine is an acceptable option for pertussis vaccination in most situations, recommended the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 14-0 vote at the October meeting, based on the immunization schedule for persons aged 7 years and older.
Safety data showed no differences in safety concerns between Tdap and Td, including data from pregnant women, said Fiona Havers, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), Atlanta.
Several of the ACIP members noted that the revised language to include both Tdap and Td reflects the increased use of Tdap and allows for maximum flexibility in clinical settings.
The revised language advises that booster doses of “either Td or Tdap” every 10 years throughout life are recommended for continued protection against tetanus and diphtheria. In addition, either Td or Tdap should be used if a tetanus toxoid–containing vaccine is indicated for prophylaxis in nonpregnant individuals.
For catch-up recommendations, which also apply to pregnant women, the committee approved the following wording for a series of three doses for individuals aged 7-18 years and 19 years and older who have never been vaccinated, that “the preferred schedule is a dose of Tdap (preferably the first dose), followed by either Tdap or Td at least 4 weeks afterward and another dose of either Td or Tdap 6-12 months later.” Individuals in these same age groups who are not fully vaccinated should receive one dose of Tdap, and a dose of either Td or Tdap if additional doses are needed.
The committee also voted unanimously 14-0 to accept the updated wording for pertussis vaccination in the Vaccines for Children program.
The ACIP members had no financial conflicts to disclose.
Either the Tdap or Td vaccine is an acceptable option for pertussis vaccination in most situations, recommended the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices.
In a unanimous 14-0 vote at the October meeting, based on the immunization schedule for persons aged 7 years and older.
Safety data showed no differences in safety concerns between Tdap and Td, including data from pregnant women, said Fiona Havers, MD, of the CDC’s National Center for Immunization and Respiratory Diseases (NCIRD), Atlanta.
Several of the ACIP members noted that the revised language to include both Tdap and Td reflects the increased use of Tdap and allows for maximum flexibility in clinical settings.
The revised language advises that booster doses of “either Td or Tdap” every 10 years throughout life are recommended for continued protection against tetanus and diphtheria. In addition, either Td or Tdap should be used if a tetanus toxoid–containing vaccine is indicated for prophylaxis in nonpregnant individuals.
For catch-up recommendations, which also apply to pregnant women, the committee approved the following wording for a series of three doses for individuals aged 7-18 years and 19 years and older who have never been vaccinated, that “the preferred schedule is a dose of Tdap (preferably the first dose), followed by either Tdap or Td at least 4 weeks afterward and another dose of either Td or Tdap 6-12 months later.” Individuals in these same age groups who are not fully vaccinated should receive one dose of Tdap, and a dose of either Td or Tdap if additional doses are needed.
The committee also voted unanimously 14-0 to accept the updated wording for pertussis vaccination in the Vaccines for Children program.
The ACIP members had no financial conflicts to disclose.
FROM AN ACIP MEETING
New test edges closer to rapid, accurate ID of active TB
A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection, according to investigators.
, reported lead author Rushdy Ahmad, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Mass., and colleagues. When fully developed, such a test could improve interventions for the most vulnerable patients, such as those with HIV, among whom TB often goes undiagnosed.
“Rapid and accurate diagnosis of active TB with current sputum-based diagnostic tools remains challenging in high-burden, resource-limited settings,” the investigators wrote. Their report is in Science Translational Medicine.
They went on to explain the gap that currently exists between microscopy, which is operator dependent and insensitive, and newer technologies, such as nucleic acid amplification, which are more sensitive but heavily resource dependent. “Furthermore, two of the most vulnerable and highly affected groups – young children and adults with HIV infection – are unlikely to be diagnosed using sputum because of difficulty obtaining sputum and low bacillary loads in the sample.”
To look for a more practical option, the investigators drew blood from 406 patients with chronic cough. Then, using a bead-based immunoassay with machine learning, the investigators identified four blood proteins associated with active TB infection: interleukin-6 (IL-6), IL-8, IL-18, and vascular endothelial growth factor (VEGF). Blind validation of 317 samples from patients with chronic cough in Asia, Africa, and South America showed that the four biomarkers offered a sensitivity of 80% and a specificity of 65%. By adding a fifth biomarker, an antibody against TB antigen Ag85B, the investigators were able to raise accuracy figures to 86% sensitivity and 69% specificity.
Adding even more biomarkers could theoretically raise accuracy even further, according to the investigators. The WHO minimal performance thresholds are 90% sensitivity and 70% specificity, with optimal targets slightly higher, at 95% sensitivity and 80% specificity. Although these standards have not yet been met, the investigators plan on testing the existing assay in real-world scenarios while simultaneously aiming to make it better.
“A near-term goal is ... to incrementally improve the marker panel up to an anticipated 6- to 10-plex assay,” the investigators wrote. “However, given the urgency of the problem, the possibility of incremental improvements will not delay platform refinement and field testing.”
The Bill and Melinda Gates Foundation funded the study. The investigators reported additional relationships with Quanterix Corporation and FIND.
SOURCE: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection, according to investigators.
, reported lead author Rushdy Ahmad, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Mass., and colleagues. When fully developed, such a test could improve interventions for the most vulnerable patients, such as those with HIV, among whom TB often goes undiagnosed.
“Rapid and accurate diagnosis of active TB with current sputum-based diagnostic tools remains challenging in high-burden, resource-limited settings,” the investigators wrote. Their report is in Science Translational Medicine.
They went on to explain the gap that currently exists between microscopy, which is operator dependent and insensitive, and newer technologies, such as nucleic acid amplification, which are more sensitive but heavily resource dependent. “Furthermore, two of the most vulnerable and highly affected groups – young children and adults with HIV infection – are unlikely to be diagnosed using sputum because of difficulty obtaining sputum and low bacillary loads in the sample.”
To look for a more practical option, the investigators drew blood from 406 patients with chronic cough. Then, using a bead-based immunoassay with machine learning, the investigators identified four blood proteins associated with active TB infection: interleukin-6 (IL-6), IL-8, IL-18, and vascular endothelial growth factor (VEGF). Blind validation of 317 samples from patients with chronic cough in Asia, Africa, and South America showed that the four biomarkers offered a sensitivity of 80% and a specificity of 65%. By adding a fifth biomarker, an antibody against TB antigen Ag85B, the investigators were able to raise accuracy figures to 86% sensitivity and 69% specificity.
Adding even more biomarkers could theoretically raise accuracy even further, according to the investigators. The WHO minimal performance thresholds are 90% sensitivity and 70% specificity, with optimal targets slightly higher, at 95% sensitivity and 80% specificity. Although these standards have not yet been met, the investigators plan on testing the existing assay in real-world scenarios while simultaneously aiming to make it better.
“A near-term goal is ... to incrementally improve the marker panel up to an anticipated 6- to 10-plex assay,” the investigators wrote. “However, given the urgency of the problem, the possibility of incremental improvements will not delay platform refinement and field testing.”
The Bill and Melinda Gates Foundation funded the study. The investigators reported additional relationships with Quanterix Corporation and FIND.
SOURCE: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection, according to investigators.
, reported lead author Rushdy Ahmad, PhD, of the Broad Institute of MIT and Harvard in Cambridge, Mass., and colleagues. When fully developed, such a test could improve interventions for the most vulnerable patients, such as those with HIV, among whom TB often goes undiagnosed.
“Rapid and accurate diagnosis of active TB with current sputum-based diagnostic tools remains challenging in high-burden, resource-limited settings,” the investigators wrote. Their report is in Science Translational Medicine.
They went on to explain the gap that currently exists between microscopy, which is operator dependent and insensitive, and newer technologies, such as nucleic acid amplification, which are more sensitive but heavily resource dependent. “Furthermore, two of the most vulnerable and highly affected groups – young children and adults with HIV infection – are unlikely to be diagnosed using sputum because of difficulty obtaining sputum and low bacillary loads in the sample.”
To look for a more practical option, the investigators drew blood from 406 patients with chronic cough. Then, using a bead-based immunoassay with machine learning, the investigators identified four blood proteins associated with active TB infection: interleukin-6 (IL-6), IL-8, IL-18, and vascular endothelial growth factor (VEGF). Blind validation of 317 samples from patients with chronic cough in Asia, Africa, and South America showed that the four biomarkers offered a sensitivity of 80% and a specificity of 65%. By adding a fifth biomarker, an antibody against TB antigen Ag85B, the investigators were able to raise accuracy figures to 86% sensitivity and 69% specificity.
Adding even more biomarkers could theoretically raise accuracy even further, according to the investigators. The WHO minimal performance thresholds are 90% sensitivity and 70% specificity, with optimal targets slightly higher, at 95% sensitivity and 80% specificity. Although these standards have not yet been met, the investigators plan on testing the existing assay in real-world scenarios while simultaneously aiming to make it better.
“A near-term goal is ... to incrementally improve the marker panel up to an anticipated 6- to 10-plex assay,” the investigators wrote. “However, given the urgency of the problem, the possibility of incremental improvements will not delay platform refinement and field testing.”
The Bill and Melinda Gates Foundation funded the study. The investigators reported additional relationships with Quanterix Corporation and FIND.
SOURCE: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
FROM SCIENCE TRANSLATIONAL MEDICINE
Key clinical point: A new point-of-care assay designed with machine learning offers improved accuracy for rapid identification of active tuberculosis (TB) infection.
Major finding: The assay had a sensitivity of 86%.
Study details: A machine learning and validation study involving patients with chronic cough from multiple countries.
Disclosures: The Bill and Melinda Gates Foundation funded the study. The investigators reported relationships with Quanterix Corporation and FIND.
Source: Ahmad et al. Sci Transl Med. 2019 Oct 23. doi: 10.1126/scitranslmed.aaw8287.
Amoxicillin/clavulanate emerges as best antibiotic for childhood bronchiectasis
MADRID – A placebo-controlled trial has confirmed that amoxicillin/clavulanate is beneficial for resolution of acute exacerbations in nonsevere bronchiectasis while also demonstrating a greater relative effect than azithromycin, based on data presented at the annual congress of the European Respiratory Society.
“We now have robust data with which to support our guidelines,” reported Vikas Goyal, MD, of the Children’s Health Clinical Unit, University of Queensland, Brisbane, Australia.
The study addresses a knowledge gap. Antibiotics are already recommended by many guidelines for treatment of acute exacerbations in children with bronchiectasis, but Dr. Goyal said that no controlled trials have ever been performed in this age group to confirm superiority to placebo.
In this multicenter study, called BEST-1, 197 children with bronchiectasis were randomized at the start of an exacerbation to placebo, 45 mg/kg per day of amoxicillin/clavulanate, or 5 mg/kg per day of azithromycin. To maintain blinding, patients in the active treatment groups received a dummy for the opposite antibiotic while patients on placebo received dummies for both active agents.
For the primary outcome, 65% of children randomized to amoxicillin/clavulanate had resolution of their exacerbation by day 14 versus 61% of those randomized to azithromycin and 43% of those randomized to placebo. On the basis of relative risk for reaching this end point, the outcome was superior to placebo for amoxicillin/clavulanate (RR, 1.5; P = .015).
Although the relative risk for azithromycin (RR, 1.4; P = .042) was only slightly lower, it did not reach a prespecified level of significance set at P = .025. Dr. Goyal did report that the resolution rate at 14 days in the placebo group was “higher than expected.”
In this trial, 53% of the 154 children who were tested for respiratory viruses with nasal swabs on day 1 of the exacerbation were found to have respiratory viruses. Of these viruses, rhinovirus was the most common, according to Dr. Goyal, whose data were published just prior to his presentation (Lancet Respir Med. 2019;7:791-801).
The median durations of the exacerbations were 7 days, 8 days, and 10 days for those treated with amoxicillin/clavulanate, azithromycin, and placebo, respectively. The difference between amoxicillin/clavulanate and placebo, but not that between azithromycin and placebo, reached statistical significance, Dr. Goyal said.
There were no between group differences in the time to next exacerbation.
In discussing limitations of this study, Dr. Goyal pointed out that the optimal doses of amoxicillin/clavulanate or azithromycin have never been established for the treatment of exacerbations in children with bronchiectasis. He noted that some infectious disease specialists have advocated higher doses of both than those employed in this trial, but dose-ranging studies have never been conducted in this age group.
In this study, adverse events were less common on azithromycin than amoxicillin/clavulanate (21% vs. 30%), but none were severe, according to Dr. Goyal. He said treatment with azithromycin was associated with increased macrolide-resistant bacteria.
On the basis of these data, Dr. Goyal concluded that amoxicillin/clavulanate should remain, as already specified in some guidelines, the standard first-line therapy for nonsevere exacerbations in nonhospitalized children with bronchiectasis. He recommended reserving azithromycin as an alternative therapy.
Dr. Goyal reports no potential conflicts of interest.
SOURCE: Goyal V et al. Lancet Respir Med. 2019;7:791-801.
MADRID – A placebo-controlled trial has confirmed that amoxicillin/clavulanate is beneficial for resolution of acute exacerbations in nonsevere bronchiectasis while also demonstrating a greater relative effect than azithromycin, based on data presented at the annual congress of the European Respiratory Society.
“We now have robust data with which to support our guidelines,” reported Vikas Goyal, MD, of the Children’s Health Clinical Unit, University of Queensland, Brisbane, Australia.
The study addresses a knowledge gap. Antibiotics are already recommended by many guidelines for treatment of acute exacerbations in children with bronchiectasis, but Dr. Goyal said that no controlled trials have ever been performed in this age group to confirm superiority to placebo.
In this multicenter study, called BEST-1, 197 children with bronchiectasis were randomized at the start of an exacerbation to placebo, 45 mg/kg per day of amoxicillin/clavulanate, or 5 mg/kg per day of azithromycin. To maintain blinding, patients in the active treatment groups received a dummy for the opposite antibiotic while patients on placebo received dummies for both active agents.
For the primary outcome, 65% of children randomized to amoxicillin/clavulanate had resolution of their exacerbation by day 14 versus 61% of those randomized to azithromycin and 43% of those randomized to placebo. On the basis of relative risk for reaching this end point, the outcome was superior to placebo for amoxicillin/clavulanate (RR, 1.5; P = .015).
Although the relative risk for azithromycin (RR, 1.4; P = .042) was only slightly lower, it did not reach a prespecified level of significance set at P = .025. Dr. Goyal did report that the resolution rate at 14 days in the placebo group was “higher than expected.”
In this trial, 53% of the 154 children who were tested for respiratory viruses with nasal swabs on day 1 of the exacerbation were found to have respiratory viruses. Of these viruses, rhinovirus was the most common, according to Dr. Goyal, whose data were published just prior to his presentation (Lancet Respir Med. 2019;7:791-801).
The median durations of the exacerbations were 7 days, 8 days, and 10 days for those treated with amoxicillin/clavulanate, azithromycin, and placebo, respectively. The difference between amoxicillin/clavulanate and placebo, but not that between azithromycin and placebo, reached statistical significance, Dr. Goyal said.
There were no between group differences in the time to next exacerbation.
In discussing limitations of this study, Dr. Goyal pointed out that the optimal doses of amoxicillin/clavulanate or azithromycin have never been established for the treatment of exacerbations in children with bronchiectasis. He noted that some infectious disease specialists have advocated higher doses of both than those employed in this trial, but dose-ranging studies have never been conducted in this age group.
In this study, adverse events were less common on azithromycin than amoxicillin/clavulanate (21% vs. 30%), but none were severe, according to Dr. Goyal. He said treatment with azithromycin was associated with increased macrolide-resistant bacteria.
On the basis of these data, Dr. Goyal concluded that amoxicillin/clavulanate should remain, as already specified in some guidelines, the standard first-line therapy for nonsevere exacerbations in nonhospitalized children with bronchiectasis. He recommended reserving azithromycin as an alternative therapy.
Dr. Goyal reports no potential conflicts of interest.
SOURCE: Goyal V et al. Lancet Respir Med. 2019;7:791-801.
MADRID – A placebo-controlled trial has confirmed that amoxicillin/clavulanate is beneficial for resolution of acute exacerbations in nonsevere bronchiectasis while also demonstrating a greater relative effect than azithromycin, based on data presented at the annual congress of the European Respiratory Society.
“We now have robust data with which to support our guidelines,” reported Vikas Goyal, MD, of the Children’s Health Clinical Unit, University of Queensland, Brisbane, Australia.
The study addresses a knowledge gap. Antibiotics are already recommended by many guidelines for treatment of acute exacerbations in children with bronchiectasis, but Dr. Goyal said that no controlled trials have ever been performed in this age group to confirm superiority to placebo.
In this multicenter study, called BEST-1, 197 children with bronchiectasis were randomized at the start of an exacerbation to placebo, 45 mg/kg per day of amoxicillin/clavulanate, or 5 mg/kg per day of azithromycin. To maintain blinding, patients in the active treatment groups received a dummy for the opposite antibiotic while patients on placebo received dummies for both active agents.
For the primary outcome, 65% of children randomized to amoxicillin/clavulanate had resolution of their exacerbation by day 14 versus 61% of those randomized to azithromycin and 43% of those randomized to placebo. On the basis of relative risk for reaching this end point, the outcome was superior to placebo for amoxicillin/clavulanate (RR, 1.5; P = .015).
Although the relative risk for azithromycin (RR, 1.4; P = .042) was only slightly lower, it did not reach a prespecified level of significance set at P = .025. Dr. Goyal did report that the resolution rate at 14 days in the placebo group was “higher than expected.”
In this trial, 53% of the 154 children who were tested for respiratory viruses with nasal swabs on day 1 of the exacerbation were found to have respiratory viruses. Of these viruses, rhinovirus was the most common, according to Dr. Goyal, whose data were published just prior to his presentation (Lancet Respir Med. 2019;7:791-801).
The median durations of the exacerbations were 7 days, 8 days, and 10 days for those treated with amoxicillin/clavulanate, azithromycin, and placebo, respectively. The difference between amoxicillin/clavulanate and placebo, but not that between azithromycin and placebo, reached statistical significance, Dr. Goyal said.
There were no between group differences in the time to next exacerbation.
In discussing limitations of this study, Dr. Goyal pointed out that the optimal doses of amoxicillin/clavulanate or azithromycin have never been established for the treatment of exacerbations in children with bronchiectasis. He noted that some infectious disease specialists have advocated higher doses of both than those employed in this trial, but dose-ranging studies have never been conducted in this age group.
In this study, adverse events were less common on azithromycin than amoxicillin/clavulanate (21% vs. 30%), but none were severe, according to Dr. Goyal. He said treatment with azithromycin was associated with increased macrolide-resistant bacteria.
On the basis of these data, Dr. Goyal concluded that amoxicillin/clavulanate should remain, as already specified in some guidelines, the standard first-line therapy for nonsevere exacerbations in nonhospitalized children with bronchiectasis. He recommended reserving azithromycin as an alternative therapy.
Dr. Goyal reports no potential conflicts of interest.
SOURCE: Goyal V et al. Lancet Respir Med. 2019;7:791-801.
REPORTING FROM ERS 2019