User login
Flu activity down from its third peak of the season, COVID-19 still a factor
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Influenza activity measures dropped during the week ending March 28, but the percentage of deaths attributed to pneumonia and influenza (P&I) has risen into epidemic territory, according to the Centers for Disease Control and Prevention.
This influenza news, however, needs to be viewed through a COVID-19 lens.
The P&I mortality data are reported together and are always a week behind the other measures, in this case covering the week ending March 21, but they show influenza deaths dropping to 0.8% as the overall P&I rate rose from 7.4% to 8.2%, a pneumonia-fueled increase that was “likely associated with COVID-19 rather than influenza,” the CDC’s influenza division noted.
The two main activity measures, at least, are on the same page for the first time since the end of February.
The rate of outpatient visits for influenza-like illness (ILI) had been dropping up to that point but then rose for an unprecedented third time this season, a change probably brought about by COVID-related health care–seeking behavior, the influenza division reported in its weekly FluView report.
This corresponding third drop in ILI activity brought the rate down to 5.4% this week from 6.2% the previous week, the CDC reported. The two previous high points occurred during the weeks ending Dec. 28 (7.0%) and Feb. 8 (6.7%)
The COVID-related changes, such as increased use of telemedicine and social distancing, “impact data from [the Outpatient Influenza-Like Illness Surveillance Network] in ways that are difficult to differentiate from changes in illness levels and should be interpreted with caution,” the CDC investigators noted.
The other activity measure, positive tests of respiratory specimens for influenza at clinical laboratories, continued the decline that started in mid-February by falling from 7.3% to 2.1%, its lowest rate since October, CDC data show.
Overall flu-related deaths may be down, but mortality in children continued at a near-record level. Seven such deaths were reported this past week, which brings the total for the 2019-2020 season to 162. “This number is higher than recorded at the same time in every season since reporting began in 2004-05, except for the 2009 pandemic,” the CDC noted.
Vascular biomarkers predict pulmonary hypertension in systemic sclerosis
Levels of three vascular biomarkers – hepatocyte growth factor, soluble Flt-1, and platelet-derived growth factor – were elevated a mean of 3 years before systemic sclerosis (SSc) patients developed pulmonary hypertension (PH) in a prospective cohort of 300 subjects.
However, the associations with PH were not very robust. For instance, above an optimal cut point of 9.89 pg/mL for platelet-derived growth factor (PlGF), the sensitivity for future PH was 82%, specificity 56%, and area under the curve (AUC) 0.69. An elevation above the optimal cut point for soluble Flt-1 (sFlt1) – 93.8 pg/mL – was 71% specific and 51% sensitive, with an AUC of 0.61.
Adding PlGF and sFlt1 elevations to carbon monoxide diffusing capacity, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) level, and percent forced vital capacity to predict PH increased the AUC modestly, from 0.72 to 0.77.
The data suggest, perhaps, an early warning system for PH. “Once vascular biomarkers are observed to be elevated, the frequency of other screening tests (e.g., NT-proBNP, DLCO) may be increased in a more cost-effective approach,” wrote investigators led by rheumatologist Christopher Mecoli, MD, an assistant professor at Johns Hopkins University, Baltimore, in Arthritis & Rheumatology.
“In the end, the authors did not overstate the case and cautiously recommended that using biomarkers might be useful in the future. The finding that when there are increased numbers of abnormalities of vascular markers, there would be an increased probability of pulmonary hypertension, makes sense.” However, “this was a major fishing expedition, and the data are certainly not sufficient to suggest anything clinical but are of some interest with respect to the general hypothesis,” said rheumatologist Daniel Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, when asked for comment.
The subjects were followed for at least 5 years and had no evidence of PH at study entry. Levels of P1GF, sFlt-1, hepatocyte growth factor (HGF), soluble endoglin, and endostatin were assessed at baseline and at regular intervals thereafter. A total of 46 patients (15%) developed PH after a mean of 3 years.
Risk of PH was associated with baseline elevations of HGF (hazard ratio, 1.99; 95% CI, 1.24-3.17; P = .004); sFlt1 (HR, 3.04; 95% CI, 1.29-7.14; P = .011); and PlGF (HR, 2.74; 95% CI, 1.32-5.69; P = .007).
Just 2 of 25 patients (8%) with no biomarkers elevated at baseline developed PH versus 12 of 29 (42%) with all five elevated. That translated to a dose-response relationship, with each additional elevated biomarker increasing the risk of PH by 78% (95% CI, 1.2-2.6; P = .004).
“There [was] no consistent trend of increasing biomarker levels over time as patients approach[ed] a diagnosis of [PH]. ... Serial testing may have value in patients with early disease to first detect elevations in biomarkers,” but “once elevated, the utility of serially monitoring appears low,” the investigators wrote.
It’s not surprising that “a higher number of elevated biomarkers relating to vascular dysfunction would correspond to a higher risk of PH,” the team wrote. However, “while these biomarkers hold promise in the risk stratification of SSc patients, many more vascular molecules exist which may have similar or greater value.”
There was no substantial correlation between any biomarker and disease duration, age at enrollment, or age at diagnosis, and no significant difference in biomarker level based on patient comorbidities. No biomarker was significantly associated with medication use at cohort entry, and none were significantly associated with the risk of ischemic digital lesions.
The majority of patients were white women. At enrollment, the average age was 52 years, and subjects had SSc for a mean of 10 years.
The work was funded by the National Institutes of Health, among others. Investigator disclosures were not reported.
SOURCE: Mecoli C et al. Arthritis Rheumatol. 2020 Mar 21. doi: 10.1002/art.41265.
Levels of three vascular biomarkers – hepatocyte growth factor, soluble Flt-1, and platelet-derived growth factor – were elevated a mean of 3 years before systemic sclerosis (SSc) patients developed pulmonary hypertension (PH) in a prospective cohort of 300 subjects.
However, the associations with PH were not very robust. For instance, above an optimal cut point of 9.89 pg/mL for platelet-derived growth factor (PlGF), the sensitivity for future PH was 82%, specificity 56%, and area under the curve (AUC) 0.69. An elevation above the optimal cut point for soluble Flt-1 (sFlt1) – 93.8 pg/mL – was 71% specific and 51% sensitive, with an AUC of 0.61.
Adding PlGF and sFlt1 elevations to carbon monoxide diffusing capacity, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) level, and percent forced vital capacity to predict PH increased the AUC modestly, from 0.72 to 0.77.
The data suggest, perhaps, an early warning system for PH. “Once vascular biomarkers are observed to be elevated, the frequency of other screening tests (e.g., NT-proBNP, DLCO) may be increased in a more cost-effective approach,” wrote investigators led by rheumatologist Christopher Mecoli, MD, an assistant professor at Johns Hopkins University, Baltimore, in Arthritis & Rheumatology.
“In the end, the authors did not overstate the case and cautiously recommended that using biomarkers might be useful in the future. The finding that when there are increased numbers of abnormalities of vascular markers, there would be an increased probability of pulmonary hypertension, makes sense.” However, “this was a major fishing expedition, and the data are certainly not sufficient to suggest anything clinical but are of some interest with respect to the general hypothesis,” said rheumatologist Daniel Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, when asked for comment.
The subjects were followed for at least 5 years and had no evidence of PH at study entry. Levels of P1GF, sFlt-1, hepatocyte growth factor (HGF), soluble endoglin, and endostatin were assessed at baseline and at regular intervals thereafter. A total of 46 patients (15%) developed PH after a mean of 3 years.
Risk of PH was associated with baseline elevations of HGF (hazard ratio, 1.99; 95% CI, 1.24-3.17; P = .004); sFlt1 (HR, 3.04; 95% CI, 1.29-7.14; P = .011); and PlGF (HR, 2.74; 95% CI, 1.32-5.69; P = .007).
Just 2 of 25 patients (8%) with no biomarkers elevated at baseline developed PH versus 12 of 29 (42%) with all five elevated. That translated to a dose-response relationship, with each additional elevated biomarker increasing the risk of PH by 78% (95% CI, 1.2-2.6; P = .004).
“There [was] no consistent trend of increasing biomarker levels over time as patients approach[ed] a diagnosis of [PH]. ... Serial testing may have value in patients with early disease to first detect elevations in biomarkers,” but “once elevated, the utility of serially monitoring appears low,” the investigators wrote.
It’s not surprising that “a higher number of elevated biomarkers relating to vascular dysfunction would correspond to a higher risk of PH,” the team wrote. However, “while these biomarkers hold promise in the risk stratification of SSc patients, many more vascular molecules exist which may have similar or greater value.”
There was no substantial correlation between any biomarker and disease duration, age at enrollment, or age at diagnosis, and no significant difference in biomarker level based on patient comorbidities. No biomarker was significantly associated with medication use at cohort entry, and none were significantly associated with the risk of ischemic digital lesions.
The majority of patients were white women. At enrollment, the average age was 52 years, and subjects had SSc for a mean of 10 years.
The work was funded by the National Institutes of Health, among others. Investigator disclosures were not reported.
SOURCE: Mecoli C et al. Arthritis Rheumatol. 2020 Mar 21. doi: 10.1002/art.41265.
Levels of three vascular biomarkers – hepatocyte growth factor, soluble Flt-1, and platelet-derived growth factor – were elevated a mean of 3 years before systemic sclerosis (SSc) patients developed pulmonary hypertension (PH) in a prospective cohort of 300 subjects.
However, the associations with PH were not very robust. For instance, above an optimal cut point of 9.89 pg/mL for platelet-derived growth factor (PlGF), the sensitivity for future PH was 82%, specificity 56%, and area under the curve (AUC) 0.69. An elevation above the optimal cut point for soluble Flt-1 (sFlt1) – 93.8 pg/mL – was 71% specific and 51% sensitive, with an AUC of 0.61.
Adding PlGF and sFlt1 elevations to carbon monoxide diffusing capacity, N-terminal of the prohormone brain natriuretic peptide (NT-proBNP) level, and percent forced vital capacity to predict PH increased the AUC modestly, from 0.72 to 0.77.
The data suggest, perhaps, an early warning system for PH. “Once vascular biomarkers are observed to be elevated, the frequency of other screening tests (e.g., NT-proBNP, DLCO) may be increased in a more cost-effective approach,” wrote investigators led by rheumatologist Christopher Mecoli, MD, an assistant professor at Johns Hopkins University, Baltimore, in Arthritis & Rheumatology.
“In the end, the authors did not overstate the case and cautiously recommended that using biomarkers might be useful in the future. The finding that when there are increased numbers of abnormalities of vascular markers, there would be an increased probability of pulmonary hypertension, makes sense.” However, “this was a major fishing expedition, and the data are certainly not sufficient to suggest anything clinical but are of some interest with respect to the general hypothesis,” said rheumatologist Daniel Furst, MD, professor of medicine (emeritus) at the University of California, Los Angeles, when asked for comment.
The subjects were followed for at least 5 years and had no evidence of PH at study entry. Levels of P1GF, sFlt-1, hepatocyte growth factor (HGF), soluble endoglin, and endostatin were assessed at baseline and at regular intervals thereafter. A total of 46 patients (15%) developed PH after a mean of 3 years.
Risk of PH was associated with baseline elevations of HGF (hazard ratio, 1.99; 95% CI, 1.24-3.17; P = .004); sFlt1 (HR, 3.04; 95% CI, 1.29-7.14; P = .011); and PlGF (HR, 2.74; 95% CI, 1.32-5.69; P = .007).
Just 2 of 25 patients (8%) with no biomarkers elevated at baseline developed PH versus 12 of 29 (42%) with all five elevated. That translated to a dose-response relationship, with each additional elevated biomarker increasing the risk of PH by 78% (95% CI, 1.2-2.6; P = .004).
“There [was] no consistent trend of increasing biomarker levels over time as patients approach[ed] a diagnosis of [PH]. ... Serial testing may have value in patients with early disease to first detect elevations in biomarkers,” but “once elevated, the utility of serially monitoring appears low,” the investigators wrote.
It’s not surprising that “a higher number of elevated biomarkers relating to vascular dysfunction would correspond to a higher risk of PH,” the team wrote. However, “while these biomarkers hold promise in the risk stratification of SSc patients, many more vascular molecules exist which may have similar or greater value.”
There was no substantial correlation between any biomarker and disease duration, age at enrollment, or age at diagnosis, and no significant difference in biomarker level based on patient comorbidities. No biomarker was significantly associated with medication use at cohort entry, and none were significantly associated with the risk of ischemic digital lesions.
The majority of patients were white women. At enrollment, the average age was 52 years, and subjects had SSc for a mean of 10 years.
The work was funded by the National Institutes of Health, among others. Investigator disclosures were not reported.
SOURCE: Mecoli C et al. Arthritis Rheumatol. 2020 Mar 21. doi: 10.1002/art.41265.
FROM ARTHRITIS & RHEUMATOLOGY
Key clinical point: Levels of three vascular biomarkers – hepatocyte growth factor, soluble Flt-1, and platelet-derived growth factor – were elevated a mean of 3 years before systemic sclerosis patients developed pulmonary hypertension.
Major finding: The associations with pulmonary hypertension were not very robust. For instance, above an optimal cut point of 9.89 pg/mL for platelet-derived growth factor, the sensitivity for future pulmonary hypertension was 82%, specificity 56%, and area under the curve 0.69. An elevation above the optimal cut point for soluble Flt-1 – 93.8 pg/mL – was 71% specific and 51% sensitive, with an area under the curve of 0.61.
Study details: A prospective cohort of 300 patients
Disclosures: The work was funded by the National Institutes of Health, among others. Investigator disclosures weren’t reported.
Source: Mecoli C et al. Arthritis Rheumatol. 2020 Mar 21. doi: 10.1002/art.41265.
Peanut OIT-induced eosinophilia may eventually resolve
Almost all patients who develop gastrointestinal side effects from oral immunotherapy for severe food allergies develop some degree of esophageal eosinophilia, but that eventually resolves in most of them after a year of treatment, according to results of a pilot study that was to be presented at the American Academy of Allergy, Asthma & Immunology annual meeting. The AAAAI canceled the meeting and provided abstracts and access to presenters for press coverage.
The findings may help identify biomarkers of persistent eosinophilia despite oral immunotherapy.
In January of this year the Food and Drug Administration approved oral immunotherapy (OIT), known as peanut allergen powder-dnfp, or peanut OIT (POIT), for severe food allergies. In an interview, lead study author Benjamin Wright, MD, of the Mayo Clinic, Phoenix, said OIT is a “promising proactive” treatment for food allergies. “But questions regarding the safety of immunotherapy remain,” he said. “About 30% of patients can develop GI side effects, including abdominal pain and vomiting; most concerning is that some patients develop eosinophilic esophagitis (EoE).”
The pilot study was a mechanistic substudy of 20 adult patients with immunoglobulin E–mediated peanut allergies enrolled in the phase 2 Peanut Oral Immunotherapy Safety, Efficacy and Discovery trial (POISED), with 15 randomized to treatment and the remainder to placebo. They had serial gastrointestinal biopsies at baseline (n = 20), 1 year (n = 7 treatment, 3 placebo) and 2 years (n = 7 treatment, 4 placebo) to evaluate eosinophils per high-power field (eos/hpf).
Baseline characteristics between the treatment and placebo groups were similar, with some having signs of preexisting disease. About 14% of them had clinically significant EoE, represented as a measure of more than 15 eos/hpf, Dr. Wright said. “One of the findings that was really fascinating to us was that all of the subjects had evidence of dilated intercellular spaces at baseline,” he said. “This indicates that all the subjects have some degree of epithelial barrier dysfunction before they start OIT.” Dilated intercellular spaces are a marker of inflammation.
Four patients in the treatment group had mild endoscopic findings at weeks 52 and 104, as did one patient on placebo, Dr. Wright said. A plot of eosinophil counts showed a peak at 52 weeks but near resolution at 104 weeks for all but one patient on OIT. “One of the most interesting trends that we noted was that, for most of patients, OIT-induced eosinophilia was transient and not fixed,” he said. “We noted a triangle pattern where tissue eosinophilia peaks and then resolves with the continuation of therapy.” EoE Histologic scoring system results followed a similar pattern in these patients, he added.
Also, results of the comprehensive GI Symptom Questionnaire, which assessed symptoms such as abdominal pain, difficulty swallowing, refusal to eat, and vomiting, showed that patient-reported GI symptoms did not correlate with tissue eosinophilia, Dr. Wright said. “To us, that suggests that perhaps eosinophils are not central to disease pathology or symptom development in these patients,” he said.
However, the findings validate that, in a small number of patients, OIT induces EoE, Dr. Wright said. He used a treadmill analogy to explain how OIT influences epithelial remodeling in some patients. “We’re constantly renewing our esophageal epithelium every 2 weeks, and when you challenge it with an antigen (i.e., OIT), the treadmill speeds up,” he said. “There may be some patients who will fall if the treadmill gets too fast, and they develop disease.”
He added, “Distinguishing someone’s fitness before they get on the treadmill is really going be a key moving forward in determining which subjects are good participants for OIT or how to dose OIT.”
Dr. Wright reported receiving grants from the Arizona Biomedical Research Consortium and Phoenix Children’s Hospital Foundation. Coauthors reported receiving grants from the National Institutes of Health and the Consortium for Food Allergy Research, as well as relationships with Aimmune Therapeutics, Regeneron Pharmaceuticals, Sanofi, Consortium for Food Allergy Research, DBV Technologies, Astellas, AnaptysBio, and Novartis.
SOURCE: Wright B et al. AAAAI, Session 2605, Abstract No. 259.
Almost all patients who develop gastrointestinal side effects from oral immunotherapy for severe food allergies develop some degree of esophageal eosinophilia, but that eventually resolves in most of them after a year of treatment, according to results of a pilot study that was to be presented at the American Academy of Allergy, Asthma & Immunology annual meeting. The AAAAI canceled the meeting and provided abstracts and access to presenters for press coverage.
The findings may help identify biomarkers of persistent eosinophilia despite oral immunotherapy.
In January of this year the Food and Drug Administration approved oral immunotherapy (OIT), known as peanut allergen powder-dnfp, or peanut OIT (POIT), for severe food allergies. In an interview, lead study author Benjamin Wright, MD, of the Mayo Clinic, Phoenix, said OIT is a “promising proactive” treatment for food allergies. “But questions regarding the safety of immunotherapy remain,” he said. “About 30% of patients can develop GI side effects, including abdominal pain and vomiting; most concerning is that some patients develop eosinophilic esophagitis (EoE).”
The pilot study was a mechanistic substudy of 20 adult patients with immunoglobulin E–mediated peanut allergies enrolled in the phase 2 Peanut Oral Immunotherapy Safety, Efficacy and Discovery trial (POISED), with 15 randomized to treatment and the remainder to placebo. They had serial gastrointestinal biopsies at baseline (n = 20), 1 year (n = 7 treatment, 3 placebo) and 2 years (n = 7 treatment, 4 placebo) to evaluate eosinophils per high-power field (eos/hpf).
Baseline characteristics between the treatment and placebo groups were similar, with some having signs of preexisting disease. About 14% of them had clinically significant EoE, represented as a measure of more than 15 eos/hpf, Dr. Wright said. “One of the findings that was really fascinating to us was that all of the subjects had evidence of dilated intercellular spaces at baseline,” he said. “This indicates that all the subjects have some degree of epithelial barrier dysfunction before they start OIT.” Dilated intercellular spaces are a marker of inflammation.
Four patients in the treatment group had mild endoscopic findings at weeks 52 and 104, as did one patient on placebo, Dr. Wright said. A plot of eosinophil counts showed a peak at 52 weeks but near resolution at 104 weeks for all but one patient on OIT. “One of the most interesting trends that we noted was that, for most of patients, OIT-induced eosinophilia was transient and not fixed,” he said. “We noted a triangle pattern where tissue eosinophilia peaks and then resolves with the continuation of therapy.” EoE Histologic scoring system results followed a similar pattern in these patients, he added.
Also, results of the comprehensive GI Symptom Questionnaire, which assessed symptoms such as abdominal pain, difficulty swallowing, refusal to eat, and vomiting, showed that patient-reported GI symptoms did not correlate with tissue eosinophilia, Dr. Wright said. “To us, that suggests that perhaps eosinophils are not central to disease pathology or symptom development in these patients,” he said.
However, the findings validate that, in a small number of patients, OIT induces EoE, Dr. Wright said. He used a treadmill analogy to explain how OIT influences epithelial remodeling in some patients. “We’re constantly renewing our esophageal epithelium every 2 weeks, and when you challenge it with an antigen (i.e., OIT), the treadmill speeds up,” he said. “There may be some patients who will fall if the treadmill gets too fast, and they develop disease.”
He added, “Distinguishing someone’s fitness before they get on the treadmill is really going be a key moving forward in determining which subjects are good participants for OIT or how to dose OIT.”
Dr. Wright reported receiving grants from the Arizona Biomedical Research Consortium and Phoenix Children’s Hospital Foundation. Coauthors reported receiving grants from the National Institutes of Health and the Consortium for Food Allergy Research, as well as relationships with Aimmune Therapeutics, Regeneron Pharmaceuticals, Sanofi, Consortium for Food Allergy Research, DBV Technologies, Astellas, AnaptysBio, and Novartis.
SOURCE: Wright B et al. AAAAI, Session 2605, Abstract No. 259.
Almost all patients who develop gastrointestinal side effects from oral immunotherapy for severe food allergies develop some degree of esophageal eosinophilia, but that eventually resolves in most of them after a year of treatment, according to results of a pilot study that was to be presented at the American Academy of Allergy, Asthma & Immunology annual meeting. The AAAAI canceled the meeting and provided abstracts and access to presenters for press coverage.
The findings may help identify biomarkers of persistent eosinophilia despite oral immunotherapy.
In January of this year the Food and Drug Administration approved oral immunotherapy (OIT), known as peanut allergen powder-dnfp, or peanut OIT (POIT), for severe food allergies. In an interview, lead study author Benjamin Wright, MD, of the Mayo Clinic, Phoenix, said OIT is a “promising proactive” treatment for food allergies. “But questions regarding the safety of immunotherapy remain,” he said. “About 30% of patients can develop GI side effects, including abdominal pain and vomiting; most concerning is that some patients develop eosinophilic esophagitis (EoE).”
The pilot study was a mechanistic substudy of 20 adult patients with immunoglobulin E–mediated peanut allergies enrolled in the phase 2 Peanut Oral Immunotherapy Safety, Efficacy and Discovery trial (POISED), with 15 randomized to treatment and the remainder to placebo. They had serial gastrointestinal biopsies at baseline (n = 20), 1 year (n = 7 treatment, 3 placebo) and 2 years (n = 7 treatment, 4 placebo) to evaluate eosinophils per high-power field (eos/hpf).
Baseline characteristics between the treatment and placebo groups were similar, with some having signs of preexisting disease. About 14% of them had clinically significant EoE, represented as a measure of more than 15 eos/hpf, Dr. Wright said. “One of the findings that was really fascinating to us was that all of the subjects had evidence of dilated intercellular spaces at baseline,” he said. “This indicates that all the subjects have some degree of epithelial barrier dysfunction before they start OIT.” Dilated intercellular spaces are a marker of inflammation.
Four patients in the treatment group had mild endoscopic findings at weeks 52 and 104, as did one patient on placebo, Dr. Wright said. A plot of eosinophil counts showed a peak at 52 weeks but near resolution at 104 weeks for all but one patient on OIT. “One of the most interesting trends that we noted was that, for most of patients, OIT-induced eosinophilia was transient and not fixed,” he said. “We noted a triangle pattern where tissue eosinophilia peaks and then resolves with the continuation of therapy.” EoE Histologic scoring system results followed a similar pattern in these patients, he added.
Also, results of the comprehensive GI Symptom Questionnaire, which assessed symptoms such as abdominal pain, difficulty swallowing, refusal to eat, and vomiting, showed that patient-reported GI symptoms did not correlate with tissue eosinophilia, Dr. Wright said. “To us, that suggests that perhaps eosinophils are not central to disease pathology or symptom development in these patients,” he said.
However, the findings validate that, in a small number of patients, OIT induces EoE, Dr. Wright said. He used a treadmill analogy to explain how OIT influences epithelial remodeling in some patients. “We’re constantly renewing our esophageal epithelium every 2 weeks, and when you challenge it with an antigen (i.e., OIT), the treadmill speeds up,” he said. “There may be some patients who will fall if the treadmill gets too fast, and they develop disease.”
He added, “Distinguishing someone’s fitness before they get on the treadmill is really going be a key moving forward in determining which subjects are good participants for OIT or how to dose OIT.”
Dr. Wright reported receiving grants from the Arizona Biomedical Research Consortium and Phoenix Children’s Hospital Foundation. Coauthors reported receiving grants from the National Institutes of Health and the Consortium for Food Allergy Research, as well as relationships with Aimmune Therapeutics, Regeneron Pharmaceuticals, Sanofi, Consortium for Food Allergy Research, DBV Technologies, Astellas, AnaptysBio, and Novartis.
SOURCE: Wright B et al. AAAAI, Session 2605, Abstract No. 259.
FROM AAAAI
Predictors of bacteremia in children hospitalized with community-acquired pneumonia
Children with bacteremia had longer lengths of stay
Clinical question: Are blood cultures warranted in specific subsets of children hospitalized with community-acquired pneumonia (CAP)?
Background: Guidelines from the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America recommend obtaining blood cultures in children hospitalized with moderate to severe community-acquired pneumonia. This group of authors recently published a study showing the prevalence of bacteremia of 2.5% in a cohort of generally healthy children hospitalized with CAP who had blood cultures obtained, with only 0.4% harboring a pathogen not susceptible to penicillin. They found low yield for blood cultures in children hospitalized with CAP.
Study design: Retrospective Cohort Study.
Setting: Pediatric Health Information System Plus (PHIS+) database (six institutions).
Synopsis: Secondary analysis of prior study of children aged 3 months to 18 years hospitalized with CAP between 2007 to 2011. For the secondary analysis only children in whom a blood culture was obtained on the initial or second day of hospitalization were studied. CAP was defined by a primary ICD-9 discharge diagnosis code for pneumonia or a primary ICD-9 discharge diagnosis code for pleural effusion with a secondary diagnosis code for pneumonia. Children transferred into the study institution and children with complex chronic conditions were excluded from the study. The primary outcome was the presence of bacteremia based on pathogen detection in the initial blood culture. Bacteria were labeled as pathogens or contaminants.
A total of 7,509 children were included in the initial study. Of them, 2,568 (34.2%) had a blood culture obtained on the initial or second day of hospitalization; 65 (2.5%) of the children with blood cultures obtained on admission had bacteremia. The most common penicillin-susceptible blood pathogen isolated was Streptococcus pneumoniae (n = 47). Eleven children (0.4%) had bacteremia with a pathogen not susceptible to penicillin. Children with bacteremia had a higher median admission white blood cell (WBC) count than did those without bacteremia (17.5 × 103 cells per mcL vs. 12.4 × 103 cells per mcL; P < .01) and definite radiographic pneumonia on admission chest radiograph (P < .01). C-reactive protein and erythrocyte sedimentation rate were also higher in children with bacteremia but were only obtained in 35% and 15% of patients, respectively. Children with bacteremia had a higher prevalence of complicated pneumonia on admission (P = .06) than did children without bacteremia. Children with bacteremia had longer lengths of stay (4 days vs. 2 days; P < .01) and were more likely to be admitted to an ICU (P < .01) than were children without bacteremia.
This study is limited by its sample because all of the patients were cared for at tertiary care hospitals. It is also limited by its timing; the PHIS+ data set spans the introduction of the 13-valent pneumococcal vaccine, and so the current prevalence of bacteremia in CAP may be lower than that found in the study.
Bottom line: The prevalence of bacteremia was low among a cohort of generally healthy children hospitalized with CAP, and no features strongly predicted the presence of bacteremia. The authors recommend that blood cultures in children with CAP should be limited to patients admitted to the ICU.
Citation: Lipsett SC et al. Predictors of Bacteremia in Children Hospitalized With Community-Acquired Pneumonia. Hosp Pediatr. 2019 Oct;9(10):770-8.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the Pediatrics Editor for The Hospitalist.
Children with bacteremia had longer lengths of stay
Children with bacteremia had longer lengths of stay
Clinical question: Are blood cultures warranted in specific subsets of children hospitalized with community-acquired pneumonia (CAP)?
Background: Guidelines from the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America recommend obtaining blood cultures in children hospitalized with moderate to severe community-acquired pneumonia. This group of authors recently published a study showing the prevalence of bacteremia of 2.5% in a cohort of generally healthy children hospitalized with CAP who had blood cultures obtained, with only 0.4% harboring a pathogen not susceptible to penicillin. They found low yield for blood cultures in children hospitalized with CAP.
Study design: Retrospective Cohort Study.
Setting: Pediatric Health Information System Plus (PHIS+) database (six institutions).
Synopsis: Secondary analysis of prior study of children aged 3 months to 18 years hospitalized with CAP between 2007 to 2011. For the secondary analysis only children in whom a blood culture was obtained on the initial or second day of hospitalization were studied. CAP was defined by a primary ICD-9 discharge diagnosis code for pneumonia or a primary ICD-9 discharge diagnosis code for pleural effusion with a secondary diagnosis code for pneumonia. Children transferred into the study institution and children with complex chronic conditions were excluded from the study. The primary outcome was the presence of bacteremia based on pathogen detection in the initial blood culture. Bacteria were labeled as pathogens or contaminants.
A total of 7,509 children were included in the initial study. Of them, 2,568 (34.2%) had a blood culture obtained on the initial or second day of hospitalization; 65 (2.5%) of the children with blood cultures obtained on admission had bacteremia. The most common penicillin-susceptible blood pathogen isolated was Streptococcus pneumoniae (n = 47). Eleven children (0.4%) had bacteremia with a pathogen not susceptible to penicillin. Children with bacteremia had a higher median admission white blood cell (WBC) count than did those without bacteremia (17.5 × 103 cells per mcL vs. 12.4 × 103 cells per mcL; P < .01) and definite radiographic pneumonia on admission chest radiograph (P < .01). C-reactive protein and erythrocyte sedimentation rate were also higher in children with bacteremia but were only obtained in 35% and 15% of patients, respectively. Children with bacteremia had a higher prevalence of complicated pneumonia on admission (P = .06) than did children without bacteremia. Children with bacteremia had longer lengths of stay (4 days vs. 2 days; P < .01) and were more likely to be admitted to an ICU (P < .01) than were children without bacteremia.
This study is limited by its sample because all of the patients were cared for at tertiary care hospitals. It is also limited by its timing; the PHIS+ data set spans the introduction of the 13-valent pneumococcal vaccine, and so the current prevalence of bacteremia in CAP may be lower than that found in the study.
Bottom line: The prevalence of bacteremia was low among a cohort of generally healthy children hospitalized with CAP, and no features strongly predicted the presence of bacteremia. The authors recommend that blood cultures in children with CAP should be limited to patients admitted to the ICU.
Citation: Lipsett SC et al. Predictors of Bacteremia in Children Hospitalized With Community-Acquired Pneumonia. Hosp Pediatr. 2019 Oct;9(10):770-8.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the Pediatrics Editor for The Hospitalist.
Clinical question: Are blood cultures warranted in specific subsets of children hospitalized with community-acquired pneumonia (CAP)?
Background: Guidelines from the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America recommend obtaining blood cultures in children hospitalized with moderate to severe community-acquired pneumonia. This group of authors recently published a study showing the prevalence of bacteremia of 2.5% in a cohort of generally healthy children hospitalized with CAP who had blood cultures obtained, with only 0.4% harboring a pathogen not susceptible to penicillin. They found low yield for blood cultures in children hospitalized with CAP.
Study design: Retrospective Cohort Study.
Setting: Pediatric Health Information System Plus (PHIS+) database (six institutions).
Synopsis: Secondary analysis of prior study of children aged 3 months to 18 years hospitalized with CAP between 2007 to 2011. For the secondary analysis only children in whom a blood culture was obtained on the initial or second day of hospitalization were studied. CAP was defined by a primary ICD-9 discharge diagnosis code for pneumonia or a primary ICD-9 discharge diagnosis code for pleural effusion with a secondary diagnosis code for pneumonia. Children transferred into the study institution and children with complex chronic conditions were excluded from the study. The primary outcome was the presence of bacteremia based on pathogen detection in the initial blood culture. Bacteria were labeled as pathogens or contaminants.
A total of 7,509 children were included in the initial study. Of them, 2,568 (34.2%) had a blood culture obtained on the initial or second day of hospitalization; 65 (2.5%) of the children with blood cultures obtained on admission had bacteremia. The most common penicillin-susceptible blood pathogen isolated was Streptococcus pneumoniae (n = 47). Eleven children (0.4%) had bacteremia with a pathogen not susceptible to penicillin. Children with bacteremia had a higher median admission white blood cell (WBC) count than did those without bacteremia (17.5 × 103 cells per mcL vs. 12.4 × 103 cells per mcL; P < .01) and definite radiographic pneumonia on admission chest radiograph (P < .01). C-reactive protein and erythrocyte sedimentation rate were also higher in children with bacteremia but were only obtained in 35% and 15% of patients, respectively. Children with bacteremia had a higher prevalence of complicated pneumonia on admission (P = .06) than did children without bacteremia. Children with bacteremia had longer lengths of stay (4 days vs. 2 days; P < .01) and were more likely to be admitted to an ICU (P < .01) than were children without bacteremia.
This study is limited by its sample because all of the patients were cared for at tertiary care hospitals. It is also limited by its timing; the PHIS+ data set spans the introduction of the 13-valent pneumococcal vaccine, and so the current prevalence of bacteremia in CAP may be lower than that found in the study.
Bottom line: The prevalence of bacteremia was low among a cohort of generally healthy children hospitalized with CAP, and no features strongly predicted the presence of bacteremia. The authors recommend that blood cultures in children with CAP should be limited to patients admitted to the ICU.
Citation: Lipsett SC et al. Predictors of Bacteremia in Children Hospitalized With Community-Acquired Pneumonia. Hosp Pediatr. 2019 Oct;9(10):770-8.
Dr. Kumar is a pediatric hospitalist at Cleveland Clinic Children’s. She is a clinical assistant professor of pediatrics at Case Western Reserve University, Cleveland, and serves as the Pediatrics Editor for The Hospitalist.
Gene-targeting therapy shown to reduce mastocytosis symptoms
by about 30%, according to early results of a clinical trial scheduled to be presented at the American Academy of Allergy, Asthma, and Immunology annual meeting. The AAAAI canceled the meeting and provided abstracts and access to presenters for press coverage.
“This correlates with reduction from very severe to moderate or from moderate to mild category, and all the reductions in symptoms were statistically significant,” Cem Akin, MD, of the University of Michigan, Ann Arbor, said in an interview. He reported on part 1 of the phase 2 PIONEER trial of the kinase inhibitor avapritinib, described as a potent and highly selective inhibitor of the KIT D816V mutation that affects 90% of patients with systemic mastocytosis.
Currently, Dr. Akin noted, patients with indolent or smoldering systemic mastocytosis must rely on over-the-counter antihistamines used for seasonal allergies. “These patients use antihistamines in higher doses because mastocytosis patients have higher counts of mast cells that release histamines that cause a variety of symptoms,” he said. Symptoms, which can occur suddenly, include flushing and reactions that resemble allergic or anaphylactic reactions.
The purpose of the part 1 study was to evaluate three different dosing levels of avapritinib vs. placebo: 25, 50, and 100 mg. Ten patients were in each dosing group and nine were in the placebo group. The primary outcome was reduction in total symptom scores at 16 weeks as measured by the Indolent SM Symptom Assessment Form. “All three dose groups showed significant reductions in total symptom scores as well as specific symptoms that were most bothersome to the patient, whether skin symptoms or GI or neurocognitive symptoms,” Dr. Akin said. “All three doses were effective; the average reduction was about 30% compared to baseline.” Specifically, 25-mg dosing showed an average 30% reduction, 50-mg dosing showed an average 19% reduction, and 100-mg dosing showed an average 35% reduction.
The researchers determined that the 25-mg daily dose was the most effective and safest, with no patients on the dose reporting grade 3 adverse events, Dr. Akin said. In total 20% and 40% of the 50- and 100-mg dose groups, respectively, reported grade 3 AEs, according to study results.* The 25-mg daily dose will be evaluated in part 2 of the trial. The trial is estimated to enroll 112 total patients, according to the ClinicalTrials.gov filing. In part 3, patients who complete parts 1 or 2, including those initially randomized to placebo, may participate in a long-term open-label extension, receiving 25 mg avapritinib plus best supportive care.
“This is targeting a population whose symptoms are not controlled by antihistamines, based on a minimum total symptom score according to diaries they fill out, and they have to be on at least two different systemic medications – antihistamine or proton-pump inhibitor and leukotriene inhibitor – and they still have significant symptoms,” Dr. Akin said. He estimated that this describes about two-thirds of his patients with indolent or smoldering systematic mastocytosis.
“This is a disease that also takes a psychological toll,” he said. “This is a problem that starts in the bone marrow; it is similar to a hematological stem-cell disorder that affects the mast cell progenitor and it’s caused by a mutation that has not been particularly targeted until this drug,” he said. While most of these patients live with a benign mastocytosis their entire lives, the symptoms can be debilitating to the point where the disease disrupts and restricts social activities and comprises their quality of life, he said.
“This is a groundbreaking therapy that will change the way we think about mastocytosis treatment going forward,” Dr. Akin said. “It’s the first time we are actually targeting the underlying mutation that’s causing the disease, in terms of reducing directly that mutation as opposed to just treating the symptoms in indolent disease.”
Scheduled session moderator Anil Nanda, MD, of the Asthma and Allergy Center in Lewisville, Texas, said the findings are encouraging. “As a practicing allergist and immunologist in the community, it is very exciting to have a potential new treatment option for indolent or smoldering systemic mastocytosis,” he said via email. “Patients appreciate new options in therapy.”
Dr. Akin, the primary investigator, receives funding from and serves as a consultant for Blueprint Medicines, which sponsored the trial. He also disclosed a financial relationship with Novartis.
SOURCE: Akin C et al. AAAAI 2020, Presentation L5.
*Correction, 4/6/2020: An earlier version of this story misstated the percentage of grade 3 adverse events. In total 20% and 40% of the 50- and 100-mg dose groups, respectively, reported grade 3 adverse events.
by about 30%, according to early results of a clinical trial scheduled to be presented at the American Academy of Allergy, Asthma, and Immunology annual meeting. The AAAAI canceled the meeting and provided abstracts and access to presenters for press coverage.
“This correlates with reduction from very severe to moderate or from moderate to mild category, and all the reductions in symptoms were statistically significant,” Cem Akin, MD, of the University of Michigan, Ann Arbor, said in an interview. He reported on part 1 of the phase 2 PIONEER trial of the kinase inhibitor avapritinib, described as a potent and highly selective inhibitor of the KIT D816V mutation that affects 90% of patients with systemic mastocytosis.
Currently, Dr. Akin noted, patients with indolent or smoldering systemic mastocytosis must rely on over-the-counter antihistamines used for seasonal allergies. “These patients use antihistamines in higher doses because mastocytosis patients have higher counts of mast cells that release histamines that cause a variety of symptoms,” he said. Symptoms, which can occur suddenly, include flushing and reactions that resemble allergic or anaphylactic reactions.
The purpose of the part 1 study was to evaluate three different dosing levels of avapritinib vs. placebo: 25, 50, and 100 mg. Ten patients were in each dosing group and nine were in the placebo group. The primary outcome was reduction in total symptom scores at 16 weeks as measured by the Indolent SM Symptom Assessment Form. “All three dose groups showed significant reductions in total symptom scores as well as specific symptoms that were most bothersome to the patient, whether skin symptoms or GI or neurocognitive symptoms,” Dr. Akin said. “All three doses were effective; the average reduction was about 30% compared to baseline.” Specifically, 25-mg dosing showed an average 30% reduction, 50-mg dosing showed an average 19% reduction, and 100-mg dosing showed an average 35% reduction.
The researchers determined that the 25-mg daily dose was the most effective and safest, with no patients on the dose reporting grade 3 adverse events, Dr. Akin said. In total 20% and 40% of the 50- and 100-mg dose groups, respectively, reported grade 3 AEs, according to study results.* The 25-mg daily dose will be evaluated in part 2 of the trial. The trial is estimated to enroll 112 total patients, according to the ClinicalTrials.gov filing. In part 3, patients who complete parts 1 or 2, including those initially randomized to placebo, may participate in a long-term open-label extension, receiving 25 mg avapritinib plus best supportive care.
“This is targeting a population whose symptoms are not controlled by antihistamines, based on a minimum total symptom score according to diaries they fill out, and they have to be on at least two different systemic medications – antihistamine or proton-pump inhibitor and leukotriene inhibitor – and they still have significant symptoms,” Dr. Akin said. He estimated that this describes about two-thirds of his patients with indolent or smoldering systematic mastocytosis.
“This is a disease that also takes a psychological toll,” he said. “This is a problem that starts in the bone marrow; it is similar to a hematological stem-cell disorder that affects the mast cell progenitor and it’s caused by a mutation that has not been particularly targeted until this drug,” he said. While most of these patients live with a benign mastocytosis their entire lives, the symptoms can be debilitating to the point where the disease disrupts and restricts social activities and comprises their quality of life, he said.
“This is a groundbreaking therapy that will change the way we think about mastocytosis treatment going forward,” Dr. Akin said. “It’s the first time we are actually targeting the underlying mutation that’s causing the disease, in terms of reducing directly that mutation as opposed to just treating the symptoms in indolent disease.”
Scheduled session moderator Anil Nanda, MD, of the Asthma and Allergy Center in Lewisville, Texas, said the findings are encouraging. “As a practicing allergist and immunologist in the community, it is very exciting to have a potential new treatment option for indolent or smoldering systemic mastocytosis,” he said via email. “Patients appreciate new options in therapy.”
Dr. Akin, the primary investigator, receives funding from and serves as a consultant for Blueprint Medicines, which sponsored the trial. He also disclosed a financial relationship with Novartis.
SOURCE: Akin C et al. AAAAI 2020, Presentation L5.
*Correction, 4/6/2020: An earlier version of this story misstated the percentage of grade 3 adverse events. In total 20% and 40% of the 50- and 100-mg dose groups, respectively, reported grade 3 adverse events.
by about 30%, according to early results of a clinical trial scheduled to be presented at the American Academy of Allergy, Asthma, and Immunology annual meeting. The AAAAI canceled the meeting and provided abstracts and access to presenters for press coverage.
“This correlates with reduction from very severe to moderate or from moderate to mild category, and all the reductions in symptoms were statistically significant,” Cem Akin, MD, of the University of Michigan, Ann Arbor, said in an interview. He reported on part 1 of the phase 2 PIONEER trial of the kinase inhibitor avapritinib, described as a potent and highly selective inhibitor of the KIT D816V mutation that affects 90% of patients with systemic mastocytosis.
Currently, Dr. Akin noted, patients with indolent or smoldering systemic mastocytosis must rely on over-the-counter antihistamines used for seasonal allergies. “These patients use antihistamines in higher doses because mastocytosis patients have higher counts of mast cells that release histamines that cause a variety of symptoms,” he said. Symptoms, which can occur suddenly, include flushing and reactions that resemble allergic or anaphylactic reactions.
The purpose of the part 1 study was to evaluate three different dosing levels of avapritinib vs. placebo: 25, 50, and 100 mg. Ten patients were in each dosing group and nine were in the placebo group. The primary outcome was reduction in total symptom scores at 16 weeks as measured by the Indolent SM Symptom Assessment Form. “All three dose groups showed significant reductions in total symptom scores as well as specific symptoms that were most bothersome to the patient, whether skin symptoms or GI or neurocognitive symptoms,” Dr. Akin said. “All three doses were effective; the average reduction was about 30% compared to baseline.” Specifically, 25-mg dosing showed an average 30% reduction, 50-mg dosing showed an average 19% reduction, and 100-mg dosing showed an average 35% reduction.
The researchers determined that the 25-mg daily dose was the most effective and safest, with no patients on the dose reporting grade 3 adverse events, Dr. Akin said. In total 20% and 40% of the 50- and 100-mg dose groups, respectively, reported grade 3 AEs, according to study results.* The 25-mg daily dose will be evaluated in part 2 of the trial. The trial is estimated to enroll 112 total patients, according to the ClinicalTrials.gov filing. In part 3, patients who complete parts 1 or 2, including those initially randomized to placebo, may participate in a long-term open-label extension, receiving 25 mg avapritinib plus best supportive care.
“This is targeting a population whose symptoms are not controlled by antihistamines, based on a minimum total symptom score according to diaries they fill out, and they have to be on at least two different systemic medications – antihistamine or proton-pump inhibitor and leukotriene inhibitor – and they still have significant symptoms,” Dr. Akin said. He estimated that this describes about two-thirds of his patients with indolent or smoldering systematic mastocytosis.
“This is a disease that also takes a psychological toll,” he said. “This is a problem that starts in the bone marrow; it is similar to a hematological stem-cell disorder that affects the mast cell progenitor and it’s caused by a mutation that has not been particularly targeted until this drug,” he said. While most of these patients live with a benign mastocytosis their entire lives, the symptoms can be debilitating to the point where the disease disrupts and restricts social activities and comprises their quality of life, he said.
“This is a groundbreaking therapy that will change the way we think about mastocytosis treatment going forward,” Dr. Akin said. “It’s the first time we are actually targeting the underlying mutation that’s causing the disease, in terms of reducing directly that mutation as opposed to just treating the symptoms in indolent disease.”
Scheduled session moderator Anil Nanda, MD, of the Asthma and Allergy Center in Lewisville, Texas, said the findings are encouraging. “As a practicing allergist and immunologist in the community, it is very exciting to have a potential new treatment option for indolent or smoldering systemic mastocytosis,” he said via email. “Patients appreciate new options in therapy.”
Dr. Akin, the primary investigator, receives funding from and serves as a consultant for Blueprint Medicines, which sponsored the trial. He also disclosed a financial relationship with Novartis.
SOURCE: Akin C et al. AAAAI 2020, Presentation L5.
*Correction, 4/6/2020: An earlier version of this story misstated the percentage of grade 3 adverse events. In total 20% and 40% of the 50- and 100-mg dose groups, respectively, reported grade 3 adverse events.
FROM AAAAI
Acid-suppressant medications in infants with bronchiolitis raises later allergy risk
Infants who are hospitalized for severe bronchiolitis and receive acid-suppressant medications may be at risk of developing allergic disease by age 3 years, according to recent research released as an abstract for the American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage
“Among children with a history of severe bronchiolitis during infancy, exposure to acid-suppressant medications during infancy further increases the risk of developing recurrent wheeze by age 3 years,” Lacey B. Robinson, MD, of the division of rheumatology, allergy, and immunology in the department of medicine at Massachusetts General Hospital in Boston, said in an interview.
Bronchiolitis is a risk factor in infants for developing conditions such as recurrent wheeze and childhood asthma in early childhood. Acid-suppressant medications like proton pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2RAs) may further increase the risk of allergic disease. One study by Mitre et al. published in JAMA Pediatrics showed use of acid-suppressant medications in infants up to 6 months raised the risk of allergic disease (JAMA Pediatr. 2018;172[6]:e180315). Some studies suggest between 30% and 50% of infants diagnosed with bronchiolitis requiring hospitalization will develop asthma by age 5 years (J Allergy Clin Immunol Pract. 2017 Jan - Feb;5[1]:92-6).
“Children with severe bronchiolitis during infancy are at a high risk of developing recurrent wheeze and subsequent asthma. There is limited evidence to suggest that exposure to acid suppressant medications [such as proton pump inhibitors and histamine-2 receptor antagonists] prenatally and during early childhood increases the risk of childhood asthma,” Dr. Robinson said. “It is not known if exposure to acid suppressant medications during infancy further increases the risk of developing recurrent wheeze among high-risk children, such as in those with a history of severe bronchiolitis during infancy.”
Dr. Robinson and colleagues performed a multicenter, prospective cohort study of 921 infants who were hospitalized for severe bronchiolitis between 2011 and 2014. The investigators reviewed the medical records of the infants for acid suppressant medication use, as well as parent report of acid suppressant medication use, during an infant’s first 12 months. Overall, 879 children were analyzed after excluding for patients who developed recurrent wheeze prior to receiving acid suppressant medications, as well as patients with incomplete data. The investigators used the National Institutes of Health Guidelines for the Diagnosis and Management of Asthma (EPR-3) to define recurrent wheeze. A Cox-proportional hazard model was used to analyze the time to event, which was stratified by age and adjusted for confounders.
Infants with a history of severe bronchiolitis were at greater risk of developing recurrent wheeze by age 3 years after being exposed to acid-suppressant medications, compared with infants who were not exposed, Dr. Robinson said. Of the 879 infants in the final analysis, 159 (18%) received acid-suppressant medications, and 68 of 159 patients (43%) went on to develop recurrent wheeze, compared with 206 of 720 infants (29%) who were not exposed (unadjusted hazard ratio, 1.63; 95% confidence interval, 1.24-2.14).
After adjustment for confounders such as gender, race and ethnicity; gestational age; delivery type; severity of bronchiolitis; respiratory syncytial virus (RSV) infection status; maternal atopy; use of acid-suppressant medications during pregnancy; median household income; and insurance status, the association between recurrent wheeze and acid-suppressant medication use during infancy remained (adjusted HR, 1.54; 95% CI, 1.15-2.07).
“More research is needed on this important topic including studies in other populations,” such as in healthy children, Dr. Robinson said. “We encourage future research on this important and understudied topic, including further research on the potential underlying mechanisms of this association.”
Dr. Robinson reported no relevant financial disclosures.
SOURCE: Robinson L. AAAAI 2020, Abstract L1
Infants who are hospitalized for severe bronchiolitis and receive acid-suppressant medications may be at risk of developing allergic disease by age 3 years, according to recent research released as an abstract for the American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage
“Among children with a history of severe bronchiolitis during infancy, exposure to acid-suppressant medications during infancy further increases the risk of developing recurrent wheeze by age 3 years,” Lacey B. Robinson, MD, of the division of rheumatology, allergy, and immunology in the department of medicine at Massachusetts General Hospital in Boston, said in an interview.
Bronchiolitis is a risk factor in infants for developing conditions such as recurrent wheeze and childhood asthma in early childhood. Acid-suppressant medications like proton pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2RAs) may further increase the risk of allergic disease. One study by Mitre et al. published in JAMA Pediatrics showed use of acid-suppressant medications in infants up to 6 months raised the risk of allergic disease (JAMA Pediatr. 2018;172[6]:e180315). Some studies suggest between 30% and 50% of infants diagnosed with bronchiolitis requiring hospitalization will develop asthma by age 5 years (J Allergy Clin Immunol Pract. 2017 Jan - Feb;5[1]:92-6).
“Children with severe bronchiolitis during infancy are at a high risk of developing recurrent wheeze and subsequent asthma. There is limited evidence to suggest that exposure to acid suppressant medications [such as proton pump inhibitors and histamine-2 receptor antagonists] prenatally and during early childhood increases the risk of childhood asthma,” Dr. Robinson said. “It is not known if exposure to acid suppressant medications during infancy further increases the risk of developing recurrent wheeze among high-risk children, such as in those with a history of severe bronchiolitis during infancy.”
Dr. Robinson and colleagues performed a multicenter, prospective cohort study of 921 infants who were hospitalized for severe bronchiolitis between 2011 and 2014. The investigators reviewed the medical records of the infants for acid suppressant medication use, as well as parent report of acid suppressant medication use, during an infant’s first 12 months. Overall, 879 children were analyzed after excluding for patients who developed recurrent wheeze prior to receiving acid suppressant medications, as well as patients with incomplete data. The investigators used the National Institutes of Health Guidelines for the Diagnosis and Management of Asthma (EPR-3) to define recurrent wheeze. A Cox-proportional hazard model was used to analyze the time to event, which was stratified by age and adjusted for confounders.
Infants with a history of severe bronchiolitis were at greater risk of developing recurrent wheeze by age 3 years after being exposed to acid-suppressant medications, compared with infants who were not exposed, Dr. Robinson said. Of the 879 infants in the final analysis, 159 (18%) received acid-suppressant medications, and 68 of 159 patients (43%) went on to develop recurrent wheeze, compared with 206 of 720 infants (29%) who were not exposed (unadjusted hazard ratio, 1.63; 95% confidence interval, 1.24-2.14).
After adjustment for confounders such as gender, race and ethnicity; gestational age; delivery type; severity of bronchiolitis; respiratory syncytial virus (RSV) infection status; maternal atopy; use of acid-suppressant medications during pregnancy; median household income; and insurance status, the association between recurrent wheeze and acid-suppressant medication use during infancy remained (adjusted HR, 1.54; 95% CI, 1.15-2.07).
“More research is needed on this important topic including studies in other populations,” such as in healthy children, Dr. Robinson said. “We encourage future research on this important and understudied topic, including further research on the potential underlying mechanisms of this association.”
Dr. Robinson reported no relevant financial disclosures.
SOURCE: Robinson L. AAAAI 2020, Abstract L1
Infants who are hospitalized for severe bronchiolitis and receive acid-suppressant medications may be at risk of developing allergic disease by age 3 years, according to recent research released as an abstract for the American Academy of Allergy, Asthma & Immunology (AAAAI) Annual Meeting.
The AAAAI canceled their annual meeting and provided abstracts and access to presenters for press coverage
“Among children with a history of severe bronchiolitis during infancy, exposure to acid-suppressant medications during infancy further increases the risk of developing recurrent wheeze by age 3 years,” Lacey B. Robinson, MD, of the division of rheumatology, allergy, and immunology in the department of medicine at Massachusetts General Hospital in Boston, said in an interview.
Bronchiolitis is a risk factor in infants for developing conditions such as recurrent wheeze and childhood asthma in early childhood. Acid-suppressant medications like proton pump inhibitors (PPIs) and histamine-2 receptor antagonists (H2RAs) may further increase the risk of allergic disease. One study by Mitre et al. published in JAMA Pediatrics showed use of acid-suppressant medications in infants up to 6 months raised the risk of allergic disease (JAMA Pediatr. 2018;172[6]:e180315). Some studies suggest between 30% and 50% of infants diagnosed with bronchiolitis requiring hospitalization will develop asthma by age 5 years (J Allergy Clin Immunol Pract. 2017 Jan - Feb;5[1]:92-6).
“Children with severe bronchiolitis during infancy are at a high risk of developing recurrent wheeze and subsequent asthma. There is limited evidence to suggest that exposure to acid suppressant medications [such as proton pump inhibitors and histamine-2 receptor antagonists] prenatally and during early childhood increases the risk of childhood asthma,” Dr. Robinson said. “It is not known if exposure to acid suppressant medications during infancy further increases the risk of developing recurrent wheeze among high-risk children, such as in those with a history of severe bronchiolitis during infancy.”
Dr. Robinson and colleagues performed a multicenter, prospective cohort study of 921 infants who were hospitalized for severe bronchiolitis between 2011 and 2014. The investigators reviewed the medical records of the infants for acid suppressant medication use, as well as parent report of acid suppressant medication use, during an infant’s first 12 months. Overall, 879 children were analyzed after excluding for patients who developed recurrent wheeze prior to receiving acid suppressant medications, as well as patients with incomplete data. The investigators used the National Institutes of Health Guidelines for the Diagnosis and Management of Asthma (EPR-3) to define recurrent wheeze. A Cox-proportional hazard model was used to analyze the time to event, which was stratified by age and adjusted for confounders.
Infants with a history of severe bronchiolitis were at greater risk of developing recurrent wheeze by age 3 years after being exposed to acid-suppressant medications, compared with infants who were not exposed, Dr. Robinson said. Of the 879 infants in the final analysis, 159 (18%) received acid-suppressant medications, and 68 of 159 patients (43%) went on to develop recurrent wheeze, compared with 206 of 720 infants (29%) who were not exposed (unadjusted hazard ratio, 1.63; 95% confidence interval, 1.24-2.14).
After adjustment for confounders such as gender, race and ethnicity; gestational age; delivery type; severity of bronchiolitis; respiratory syncytial virus (RSV) infection status; maternal atopy; use of acid-suppressant medications during pregnancy; median household income; and insurance status, the association between recurrent wheeze and acid-suppressant medication use during infancy remained (adjusted HR, 1.54; 95% CI, 1.15-2.07).
“More research is needed on this important topic including studies in other populations,” such as in healthy children, Dr. Robinson said. “We encourage future research on this important and understudied topic, including further research on the potential underlying mechanisms of this association.”
Dr. Robinson reported no relevant financial disclosures.
SOURCE: Robinson L. AAAAI 2020, Abstract L1
REPORTING FROM AAAAI 2020
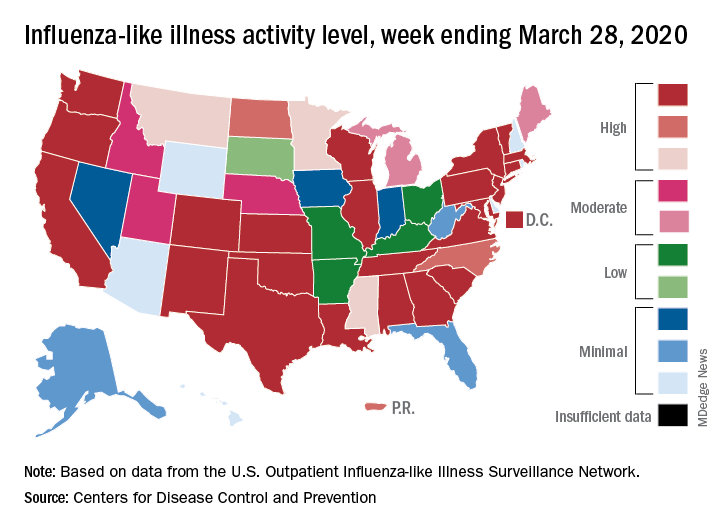
Flu activity measures continue COVID-19–related divergence
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
The 2019-2020 flu paradox continues in the United States: Fewer respiratory samples are testing positive for influenza, but more people are seeking care for respiratory symptoms because of COVID-19, according to the Centers for Disease Control and Prevention.
compared with 14.9% the week before, but outpatient visits for influenza-like illness (ILI) rose from 5.6% of all visits to 6.2% for third week of March, the CDC’s influenza division reported.
The CDC defines ILI as “fever (temperature of 100°F [37.8°C] or greater) and a cough and/or a sore throat without a known cause other than influenza.” The outpatient ILI visit rate needs to get below the national baseline of 2.4% for the CDC to call the end of the 2019-2020 flu season.
This week’s map shows that fewer states are at the highest level of ILI activity on the CDC’s 1-10 scale: 33 states plus Puerto Rico for the week ending March 21, compared with 35 and Puerto Rico the previous week. The number of states at level 10 had risen the two previous weeks, CDC data show.
“Influenza severity indicators remain moderate to low overall, but hospitalization rates differ by age group, with high rates among children and young adults,” the influenza division said.
Overall mortality also has not been high, but 155 children have died from the flu so far in 2019-2020, which is more than any season since the 2009 pandemic, the CDC noted.
Study identifies risk factors for infection after transbronchial biopsy
Among patients who undergo endobronchial ultrasound-guided transbronchial biopsy using a guide sheath (EBUS-GS-TBB) for diagnosing lung cancer, cavitation and low-density areas inside the target lesion on CT and stenosis of the responsible bronchus are risk factors for infection after the procedure, according to a study published in CHEST.
“Infectious complications after [transbronchial biopsy] constitute a serious clinical problem because they might delay the start of treatment or cause the intended treatment to be modified to a milder one,” said Tomohide Souma, MD, of the department of respiratory medicine at Fujita Health University in Toyoake, Japan, and colleagues. “The precise mechanism of such complications is still unclear, and effective prophylaxis procedures have not been established. ... Thus, it is very important to identify the risk factors for infectious complications after TBB if and when these complications are to be avoided.”
To evaluate potential risk factors for infectious complications after EBUS-GS-TBB in a large sample of patients, Dr. Souma and colleagues retrospectively studied the medical records of 1,045 consecutive patients (median age, 72; 68% male) who underwent EBUS-GS-TBB between January 2013 and December 2017 at Fujita Health University Hospital.
In all, 47 patients developed infections, a cumulative incidence of about 4.5%. Infections included pneumonia (51.1%), intratumoral infection (29.8%), and three cases each of lung abscess, pleurisy, and empyema. Three patients, two with empyema and one with lung abscess, died within 1 month before administration of anticancer treatment. “In total, more than 40% of patients with post–EBUS-GS-TBB infection were unable to receive preplanned anticancer treatment,” the researchers said.
On multivariate analysis, cavitation in the lesion (odds ratio, 3.63), low-density areas in the lesion (OR, 13.26), and bronchoscopic findings of responsible bronchus stenosis (OR, 7.82) were significantly associated with development of infections post biopsy.
An analysis that matched 89 patients who received prophylactic antibiotics with controls who did not receive prophylactic antibiotics did not find that prophylactic antibiotics significantly reduced the likelihood of post–EBUS-GS-TBB infection.
“Notably, three risk factors found in our study indicate that the inflammation-prone status of lesions may be the most important factor for developing post–EBUS-TBB infection,” Dr. Souma and colleagues said. “Although our study does not rebuff the role of antibiotics in postbronchoscopy infection therapy, clinicians should notify patients that post-TBB infection may occur despite the use of prophylactic antibiotics. We recommend that careful and frequent follow-up be applied to patients undergoing diagnostic EBUS-GS-TBB with reference to the risk factors identified in our study.”
A. Christine Argento, MD, FCCP, assistant professor of medicine and thoracic surgery and director of the interventional pulmonary fellowship program at Northwestern University, Chicago, noted that this is an important study on a topic that has not been well described in the past.
“This paper ... identifies three factors that were associated with infectious complications – namely, cavitation, low density areas, and a visibly stenosed bronchus leading to the lesion,” she said. “When planning bronchoscopy to sample lesions that fit one of these three criteria, I will likely be more cautious in the future meaning that in these cases, I would limit biopsies to 6-8 pieces which is typically sufficient and I would minimize any trauma to the bronchus leading to the lesion, as if the bronchus is already stenosed on bronchoscopic inspection it is likely inflamed and will only be exacerbated by repeated manipulation and insertions with the bronchoscope and guide sheath leading to a postobstructive phenomenon that was observed in this cohort.
“As far as pleurisy and empyema, it is not described if [the investigators] used fluoroscopy, but this would be an important aspect,” she added. “Ideally, one would not cause disruption of the pleural surface as contamination from the lung to the pleural space can have serious and prolonged infectious consequences as was reported in this study. Fluoroscopy would help the operator to avoid taking samples that would be too close to the pleural surface and could potentially decrease this complication.
“In the United States, it is not always standard practice to see patients 5-7 days following bronchoscopy to assess for complications. Although some of these patients would have presented for evaluation with symptoms, presumably several of these patients would not have. Also pre- and postbronchoscopy labs are not commonly drawn in the United States and so a rise in white blood cells or C-reactive protein would not be known.
“Finally, [the investigators] point out that prophylactic antibiotics do not seem to be effective, and I would agree based on their results. I would only consider using antibiotics as a directed measure if the patient develops infectious complications and the antibiotic choice and duration of therapy would be tailored to the specific complication encountered,” she said.
The researchers had no disclosures.
SOURCE: Souma T et al. CHEST. 2020 Mar 4. doi: 10.1016/j.chest.2020.02.025.
Among patients who undergo endobronchial ultrasound-guided transbronchial biopsy using a guide sheath (EBUS-GS-TBB) for diagnosing lung cancer, cavitation and low-density areas inside the target lesion on CT and stenosis of the responsible bronchus are risk factors for infection after the procedure, according to a study published in CHEST.
“Infectious complications after [transbronchial biopsy] constitute a serious clinical problem because they might delay the start of treatment or cause the intended treatment to be modified to a milder one,” said Tomohide Souma, MD, of the department of respiratory medicine at Fujita Health University in Toyoake, Japan, and colleagues. “The precise mechanism of such complications is still unclear, and effective prophylaxis procedures have not been established. ... Thus, it is very important to identify the risk factors for infectious complications after TBB if and when these complications are to be avoided.”
To evaluate potential risk factors for infectious complications after EBUS-GS-TBB in a large sample of patients, Dr. Souma and colleagues retrospectively studied the medical records of 1,045 consecutive patients (median age, 72; 68% male) who underwent EBUS-GS-TBB between January 2013 and December 2017 at Fujita Health University Hospital.
In all, 47 patients developed infections, a cumulative incidence of about 4.5%. Infections included pneumonia (51.1%), intratumoral infection (29.8%), and three cases each of lung abscess, pleurisy, and empyema. Three patients, two with empyema and one with lung abscess, died within 1 month before administration of anticancer treatment. “In total, more than 40% of patients with post–EBUS-GS-TBB infection were unable to receive preplanned anticancer treatment,” the researchers said.
On multivariate analysis, cavitation in the lesion (odds ratio, 3.63), low-density areas in the lesion (OR, 13.26), and bronchoscopic findings of responsible bronchus stenosis (OR, 7.82) were significantly associated with development of infections post biopsy.
An analysis that matched 89 patients who received prophylactic antibiotics with controls who did not receive prophylactic antibiotics did not find that prophylactic antibiotics significantly reduced the likelihood of post–EBUS-GS-TBB infection.
“Notably, three risk factors found in our study indicate that the inflammation-prone status of lesions may be the most important factor for developing post–EBUS-TBB infection,” Dr. Souma and colleagues said. “Although our study does not rebuff the role of antibiotics in postbronchoscopy infection therapy, clinicians should notify patients that post-TBB infection may occur despite the use of prophylactic antibiotics. We recommend that careful and frequent follow-up be applied to patients undergoing diagnostic EBUS-GS-TBB with reference to the risk factors identified in our study.”
A. Christine Argento, MD, FCCP, assistant professor of medicine and thoracic surgery and director of the interventional pulmonary fellowship program at Northwestern University, Chicago, noted that this is an important study on a topic that has not been well described in the past.
“This paper ... identifies three factors that were associated with infectious complications – namely, cavitation, low density areas, and a visibly stenosed bronchus leading to the lesion,” she said. “When planning bronchoscopy to sample lesions that fit one of these three criteria, I will likely be more cautious in the future meaning that in these cases, I would limit biopsies to 6-8 pieces which is typically sufficient and I would minimize any trauma to the bronchus leading to the lesion, as if the bronchus is already stenosed on bronchoscopic inspection it is likely inflamed and will only be exacerbated by repeated manipulation and insertions with the bronchoscope and guide sheath leading to a postobstructive phenomenon that was observed in this cohort.
“As far as pleurisy and empyema, it is not described if [the investigators] used fluoroscopy, but this would be an important aspect,” she added. “Ideally, one would not cause disruption of the pleural surface as contamination from the lung to the pleural space can have serious and prolonged infectious consequences as was reported in this study. Fluoroscopy would help the operator to avoid taking samples that would be too close to the pleural surface and could potentially decrease this complication.
“In the United States, it is not always standard practice to see patients 5-7 days following bronchoscopy to assess for complications. Although some of these patients would have presented for evaluation with symptoms, presumably several of these patients would not have. Also pre- and postbronchoscopy labs are not commonly drawn in the United States and so a rise in white blood cells or C-reactive protein would not be known.
“Finally, [the investigators] point out that prophylactic antibiotics do not seem to be effective, and I would agree based on their results. I would only consider using antibiotics as a directed measure if the patient develops infectious complications and the antibiotic choice and duration of therapy would be tailored to the specific complication encountered,” she said.
The researchers had no disclosures.
SOURCE: Souma T et al. CHEST. 2020 Mar 4. doi: 10.1016/j.chest.2020.02.025.
Among patients who undergo endobronchial ultrasound-guided transbronchial biopsy using a guide sheath (EBUS-GS-TBB) for diagnosing lung cancer, cavitation and low-density areas inside the target lesion on CT and stenosis of the responsible bronchus are risk factors for infection after the procedure, according to a study published in CHEST.
“Infectious complications after [transbronchial biopsy] constitute a serious clinical problem because they might delay the start of treatment or cause the intended treatment to be modified to a milder one,” said Tomohide Souma, MD, of the department of respiratory medicine at Fujita Health University in Toyoake, Japan, and colleagues. “The precise mechanism of such complications is still unclear, and effective prophylaxis procedures have not been established. ... Thus, it is very important to identify the risk factors for infectious complications after TBB if and when these complications are to be avoided.”
To evaluate potential risk factors for infectious complications after EBUS-GS-TBB in a large sample of patients, Dr. Souma and colleagues retrospectively studied the medical records of 1,045 consecutive patients (median age, 72; 68% male) who underwent EBUS-GS-TBB between January 2013 and December 2017 at Fujita Health University Hospital.
In all, 47 patients developed infections, a cumulative incidence of about 4.5%. Infections included pneumonia (51.1%), intratumoral infection (29.8%), and three cases each of lung abscess, pleurisy, and empyema. Three patients, two with empyema and one with lung abscess, died within 1 month before administration of anticancer treatment. “In total, more than 40% of patients with post–EBUS-GS-TBB infection were unable to receive preplanned anticancer treatment,” the researchers said.
On multivariate analysis, cavitation in the lesion (odds ratio, 3.63), low-density areas in the lesion (OR, 13.26), and bronchoscopic findings of responsible bronchus stenosis (OR, 7.82) were significantly associated with development of infections post biopsy.
An analysis that matched 89 patients who received prophylactic antibiotics with controls who did not receive prophylactic antibiotics did not find that prophylactic antibiotics significantly reduced the likelihood of post–EBUS-GS-TBB infection.
“Notably, three risk factors found in our study indicate that the inflammation-prone status of lesions may be the most important factor for developing post–EBUS-TBB infection,” Dr. Souma and colleagues said. “Although our study does not rebuff the role of antibiotics in postbronchoscopy infection therapy, clinicians should notify patients that post-TBB infection may occur despite the use of prophylactic antibiotics. We recommend that careful and frequent follow-up be applied to patients undergoing diagnostic EBUS-GS-TBB with reference to the risk factors identified in our study.”
A. Christine Argento, MD, FCCP, assistant professor of medicine and thoracic surgery and director of the interventional pulmonary fellowship program at Northwestern University, Chicago, noted that this is an important study on a topic that has not been well described in the past.
“This paper ... identifies three factors that were associated with infectious complications – namely, cavitation, low density areas, and a visibly stenosed bronchus leading to the lesion,” she said. “When planning bronchoscopy to sample lesions that fit one of these three criteria, I will likely be more cautious in the future meaning that in these cases, I would limit biopsies to 6-8 pieces which is typically sufficient and I would minimize any trauma to the bronchus leading to the lesion, as if the bronchus is already stenosed on bronchoscopic inspection it is likely inflamed and will only be exacerbated by repeated manipulation and insertions with the bronchoscope and guide sheath leading to a postobstructive phenomenon that was observed in this cohort.
“As far as pleurisy and empyema, it is not described if [the investigators] used fluoroscopy, but this would be an important aspect,” she added. “Ideally, one would not cause disruption of the pleural surface as contamination from the lung to the pleural space can have serious and prolonged infectious consequences as was reported in this study. Fluoroscopy would help the operator to avoid taking samples that would be too close to the pleural surface and could potentially decrease this complication.
“In the United States, it is not always standard practice to see patients 5-7 days following bronchoscopy to assess for complications. Although some of these patients would have presented for evaluation with symptoms, presumably several of these patients would not have. Also pre- and postbronchoscopy labs are not commonly drawn in the United States and so a rise in white blood cells or C-reactive protein would not be known.
“Finally, [the investigators] point out that prophylactic antibiotics do not seem to be effective, and I would agree based on their results. I would only consider using antibiotics as a directed measure if the patient develops infectious complications and the antibiotic choice and duration of therapy would be tailored to the specific complication encountered,” she said.
The researchers had no disclosures.
SOURCE: Souma T et al. CHEST. 2020 Mar 4. doi: 10.1016/j.chest.2020.02.025.
FROM CHEST
Reports suggest possible in utero transmission of novel coronavirus 2019
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
Reports of three neonates with elevated IgM antibody concentrations whose mothers had COVID-19 in two articles raise questions about whether the infants may have been infected with the virus in utero.
The data, while provocative, “are not conclusive and do not prove in utero transmission” of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), editorialists cautioned.
“The suggestion of in utero transmission rests on IgM detection in these 3 neonates, and IgM is a challenging way to diagnose many congenital infections,” David W. Kimberlin, MD, and Sergio Stagno, MD, of the division of pediatric infectious diseases at University of Alabama at Birmingham, wrote in their editorial. “IgM antibodies are too large to cross the placenta and so detection in a newborn reasonably could be assumed to reflect fetal production following in utero infection. However, most congenital infections are not diagnosed based on IgM detection because IgM assays can be prone to false-positive and false-negative results, along with cross-reactivity and testing challenges.”
None of the three infants had a positive reverse transcriptase–polymerase chain reaction (RT-PCR) test result, “so there is not virologic evidence for congenital infection in these cases to support the serologic suggestion of in utero transmission,” the editorialists noted.
Examining the possibility of vertical transmission
A prior case series of nine pregnant women found no transmission of the virus from mother to child, but the question of in utero transmission is not settled, said Lan Dong, MD, of the department of obstetrics and gynecology at Renmin Hospital of Wuhan University in China and colleagues. In their research letter, the investigators described a newborn with elevated IgM antibodies to novel coronavirus 2019 born to a mother with COVID-19. The infant was delivered by cesarean section February 22, 2020, at Renmin Hospital in a negative-pressure isolation room.
“The mother wore an N95 mask and did not hold the infant,” the researchers said. “The neonate had no symptoms and was immediately quarantined in the neonatal intensive care unit. At 2 hours of age, the SARS-CoV-2 IgG level was 140.32 AU/mL and the IgM level was 45.83 AU/mL.” Although the infant may have been infected at delivery, IgM antibodies usually take days to appear, Dr. Dong and colleagues wrote. “The infant’s repeatedly negative RT-PCR test results on nasopharyngeal swabs are difficult to explain, although these tests are not always positive with infection. ... Additional examination of maternal and newborn samples should be done to confirm this preliminary observation.”
A review of infants’ serologic characteristics
Hui Zeng, MD, of the department of laboratory medicine at Zhongnan Hospital of Wuhan University in China and colleagues retrospectively reviewed clinical records and laboratory results for six pregnant women with COVID-19, according to a study in JAMA. The women had mild clinical manifestations and were admitted to Zhongnan Hospital between February 16 and March 6. “All had cesarean deliveries in their third trimester in negative pressure isolation rooms,” the investigators said. “All mothers wore masks, and all medical staff wore protective suits and double masks. The infants were isolated from their mothers immediately after delivery.”
Two of the infants had elevated IgG and IgM concentrations. IgM “is not usually transferred from mother to fetus because of its larger macromolecular structure. ... Whether the placentas of women in this study were damaged and abnormal is unknown,” Dr. Zeng and colleagues said. “Alternatively, IgM could have been produced by the infant if the virus crossed the placenta.”
“Although these 2 studies deserve careful evaluation, more definitive evidence is needed” before physicians can “counsel pregnant women that their fetuses are at risk from congenital infection with SARS-CoV-2,” Dr. Kimberlin and Dr. Stagno concluded.
Dr. Dong and associates had no conflicts of interest. Their work was supported by the National Key Research and Development Project and others. Dr. Zeng and colleagues had no relevant financial disclosures. Their study was supported by grants from the National Natural Science Foundation of China and Zhongnan Hospital. Dr. Kimberlin and Dr. Stagno had no conflicts of interest.
SOURCE: Dong L et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4621; Zeng H et al. JAMA. 2020 Mar 26. doi: 10.1001/jama.2020.4861.
FROM JAMA
Despite strict controls, some infants born to mothers with COVID-19 appear infected
Despite implementation of strict infection control and prevention procedures in a hospital in Wuhan, China, according to Lingkong Zeng, MD, of the department of neonatology at Wuhan Children’s Hospital, and associates.
Thirty-three neonates born to mothers with COVID-19 were included in the study, published as a research letter in JAMA Pediatrics. Of this group, three neonates (9%) were confirmed to be infected with the novel coronavirus 2019 at 2 and 4 days of life through nasopharyngeal and anal swabs.
Of the three infected neonates, two were born at 40 weeks’ gestation and the third was born at 31 weeks. The two full-term infants had mild symptoms such as lethargy and fever and were negative for the virus at 6 days of life. The preterm infant had somewhat worse symptoms, but the investigators acknowledged that “the most seriously ill neonate may have been symptomatic from prematurity, asphyxia, and sepsis, rather than [the novel coronavirus 2019] infection.” They added that outcomes for all three neonates were favorable, consistent with past research.
“Because strict infection control and prevention procedures were implemented during the delivery, it is likely that the sources of [novel coronavirus 2019] in the neonates’ upper respiratory tracts or anuses were maternal in origin,” Dr. Zeng and associates surmised.
While previous studies have shown no evidence of COVID-19 transmission between mothers and neonates, and all samples, including amniotic fluid, cord blood, and breast milk, were negative for the novel coronavirus 2019, “vertical maternal-fetal transmission cannot be ruled out in the current cohort. Therefore, it is crucial to screen pregnant women and implement strict infection control measures, quarantine of infected mothers, and close monitoring of neonates at risk of COVID-19,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Zeng L et al. JAMA Pediatrics. 2020 Mar 26. doi: 10.1001/jamapediatrics.2020.0878.
Despite implementation of strict infection control and prevention procedures in a hospital in Wuhan, China, according to Lingkong Zeng, MD, of the department of neonatology at Wuhan Children’s Hospital, and associates.
Thirty-three neonates born to mothers with COVID-19 were included in the study, published as a research letter in JAMA Pediatrics. Of this group, three neonates (9%) were confirmed to be infected with the novel coronavirus 2019 at 2 and 4 days of life through nasopharyngeal and anal swabs.
Of the three infected neonates, two were born at 40 weeks’ gestation and the third was born at 31 weeks. The two full-term infants had mild symptoms such as lethargy and fever and were negative for the virus at 6 days of life. The preterm infant had somewhat worse symptoms, but the investigators acknowledged that “the most seriously ill neonate may have been symptomatic from prematurity, asphyxia, and sepsis, rather than [the novel coronavirus 2019] infection.” They added that outcomes for all three neonates were favorable, consistent with past research.
“Because strict infection control and prevention procedures were implemented during the delivery, it is likely that the sources of [novel coronavirus 2019] in the neonates’ upper respiratory tracts or anuses were maternal in origin,” Dr. Zeng and associates surmised.
While previous studies have shown no evidence of COVID-19 transmission between mothers and neonates, and all samples, including amniotic fluid, cord blood, and breast milk, were negative for the novel coronavirus 2019, “vertical maternal-fetal transmission cannot be ruled out in the current cohort. Therefore, it is crucial to screen pregnant women and implement strict infection control measures, quarantine of infected mothers, and close monitoring of neonates at risk of COVID-19,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Zeng L et al. JAMA Pediatrics. 2020 Mar 26. doi: 10.1001/jamapediatrics.2020.0878.
Despite implementation of strict infection control and prevention procedures in a hospital in Wuhan, China, according to Lingkong Zeng, MD, of the department of neonatology at Wuhan Children’s Hospital, and associates.
Thirty-three neonates born to mothers with COVID-19 were included in the study, published as a research letter in JAMA Pediatrics. Of this group, three neonates (9%) were confirmed to be infected with the novel coronavirus 2019 at 2 and 4 days of life through nasopharyngeal and anal swabs.
Of the three infected neonates, two were born at 40 weeks’ gestation and the third was born at 31 weeks. The two full-term infants had mild symptoms such as lethargy and fever and were negative for the virus at 6 days of life. The preterm infant had somewhat worse symptoms, but the investigators acknowledged that “the most seriously ill neonate may have been symptomatic from prematurity, asphyxia, and sepsis, rather than [the novel coronavirus 2019] infection.” They added that outcomes for all three neonates were favorable, consistent with past research.
“Because strict infection control and prevention procedures were implemented during the delivery, it is likely that the sources of [novel coronavirus 2019] in the neonates’ upper respiratory tracts or anuses were maternal in origin,” Dr. Zeng and associates surmised.
While previous studies have shown no evidence of COVID-19 transmission between mothers and neonates, and all samples, including amniotic fluid, cord blood, and breast milk, were negative for the novel coronavirus 2019, “vertical maternal-fetal transmission cannot be ruled out in the current cohort. Therefore, it is crucial to screen pregnant women and implement strict infection control measures, quarantine of infected mothers, and close monitoring of neonates at risk of COVID-19,” the investigators concluded.
The study authors reported that they had no conflicts of interest.
SOURCE: Zeng L et al. JAMA Pediatrics. 2020 Mar 26. doi: 10.1001/jamapediatrics.2020.0878.
FROM JAMA PEDIATRICS