User login
First protocol on how to use lung ultrasound to triage COVID-19
The first protocol for the use of lung ultrasound to quantitatively and reproducibly assess the degree of lung involvement in patients suspected of having COVID-19 infection has been published by a team of Italian experts with experience using the technology on the front line.
Particularly in Spain and Italy — where the pandemic has struck hardest in Europe — hard-pressed clinicians seeking to quickly understand whether patients with seemingly mild disease could be harboring more serious lung involvement have increasingly relied upon lung ultrasound in the emergency room.
Now Libertario Demi, PhD, head of the ultrasound laboratory, University of Trento, Italy, and colleagues have developed a protocol, published online March 30 in the Journal of Ultrasound Medicine, to standardize practice.
Their research, which builds on previous work by the team, offers broad agreement with industry-led algorithms and emphasizes the use of wireless, handheld ultrasound devices, ideally consisting of a separate probe and tablet, to make sterilization easy.
Firms such as the Butterfly Network, Phillips, Clarius, GE Healthcare, and Siemens are among numerous companies that produce one or more such devices, including some that are completely integrated.
Not Universally Accepted
However, lung ultrasound is not yet universally accepted as a tool for diagnosing pneumonia in the context of COVID-19 and triaging patients.
The National Health Service in England does not even mention lung ultrasound in its radiology decision tool for suspected COVID-19, specifying instead chest X-ray as the first-line diagnostic imaging tool, with CT scanning in equivocal cases.
But Giovanni Volpicelli, MD, University Hospital San Luigi Gonzaga, Turin, Italy, who has previously described his experience to Medscape Medical News, says many patients with COVID-19 in his hospital presented with a negative chest X-ray but were found to have interstitial pneumonia on lung ultrasound.
Moreover, while CT scan remains the gold standard, the risk of nosocomial infection is more easily controlled if patients do not have to be transported to the radiology department but remain in the emergency room and instead undergo lung ultrasound there, he stressed.
Experts Share Experience of Lung Ultrasound in COVID-19
In developing and publishing their protocol, Demi, senior author of the article, and other colleagues from the heavily affected cities of Northern Italy, say their aim is “to share our experience and to propose a standardization with respect to the use of lung ultrasound in the management of COVID-19 patients.”
They reviewed an anonymized database of around 60,000 ultrasound images of confirmed COVID-19 cases and reviewers were blinded to patients’ clinical backgrounds.
For image acquisition, the authors recommend scanning 14 areas in each patient for 10 seconds, making the scans intercostal to cover the widest possible surface area.
They advise the use of a single focal point on the pleural line, which they write, optimizes the beam shape for observing the lung surface.
The authors also urge that the mechanical index (MI) be kept low because high MIs sustained for long periods “may result in damaging the lung.”
They also stress that cosmetic filters and modalities such as harmonic imaging, contrast, doppler, and compounding should be avoided, alongside saturation phenomena.
What Constitutes Intermediate Disease?
Once the images have been taken, they are scored on a 0-3 scale for each of the 14 areas, with no weighting on any individual area.
A score of 0 is given when the pleural line is continuous and regular, with the presence of A-lines, denoting that the lungs are unaffected.
An area is given a score of 3 when the scan shows dense and largely extended white lung tissue, with or without consolidations, indicating severe disease.
At both ends of this spectrum, there is agreement between the Italian protocol and an algorithm developed by the Butterfly Network.
However, the two differ when it comes to scoring intermediate cases. On the Butterfly algorithm, the suggestion is to look for B-lines, caused by fluid and cellular infiltration into the interstitium, and to weigh that against the need for supplementary oxygen.
The Italian team, in contrast, says a score of 1 is given when the pleural line is indented, with vertical areas of white visible below.
A score of 2 is given when the pleural line is broken, with small to large areas of consolidation and associated areas of white below.
Demi told Medscape Medical News that they did not refer to B-lines in their protocol as their visibility depends entirely on the imaging frequency and the probe used.
“This means that scoring on B-lines, people with different machines would give completely different scores for the same patient.”
He continued: “We prefer to refer to horizontal and vertical artifacts, and provide an analysis of the patterns, which is related to the physics of the interactions between the ultrasound waves and lung surface.”
In response, Mike Stone, MD, Legacy Emanuel Medical Center, Portland, Oregon, and director of education at Butterfly, said there appears to be wide variation in lung findings that “may or may not correlate with the severity of symptoms.”
He told Medscape Medical News it is “hard to know exactly if someone with pure B-lines will progress to serious illness or if someone with some subpleural consolidations will do well.”
A Negative Ultrasound Is the Most Useful
Volpicelli believes that, in any case, any patient with an intermediate pattern will require further diagnosis, such as other imaging modalities and blood exams, and the real role of lung ultrasound is in assessing patients at either end of the spectrum.
“In other words, there are situations where lung ultrasound can be considered definitive,” he told Medscape Medical News. “For instance, if I see a patient with mild signs of the disease, just fever, and I perform lung ultrasound and see nothing, lung ultrasound rules out pneumonia.”
“This patient may have COVID-19 of course, but they do not have pneumonia, and they can be treated at home, awaiting the result of the swab test. And this is useful because you can reduce the burden in the emergency department.”
Volpicelli continued: “On the other hand, there are patients with acute respiratory failure in respiratory distress. If the lung ultrasound is normal, you can rule out COVID-19 and you need to use other diagnostic procedures to understand the problem.”
“This is also very important for us because it’s crucial to be able to remove the patient from the isolation area and perform CT scan, chest radiography, and all the other diagnostic tools that we need.”
Are Wireless Machines Needed? Not Necessarily
With regard to the use of wireless technology, the Italian team says that “in the setting of COVID-19, wireless probes and tablets represent the most appropriate ultrasound equipment” because they can “easily be wrapped in single-use plastic covers, reducing the risk of contamination,” and making sterilization easy.
Stone suggests that integrated portable devices, however, are no more likely to cause cross-contamination than separate probes and tablets, as they can fit within a sterile sheath as a single unit.
Volpicelli, for his part, doesn’t like what he sees as undue focus on wireless devices for lung ultrasound in the COVID-19 protocols.
He is concerned that recommending them as the best approach may be sending out the wrong message, which could be very “dangerous” as people may then think they cannot perform this screening with standard ultrasound machines.
For him, the issue of cross contamination with standard lung ultrasound machines is “nonexistent. Cleaning the machine is quite easy and I do it hundreds of times per week.”
He does acknowledge, however, that if the lung ultrasound is performed under certain circumstances, for example when a patient is using a continuous positive airway pressure (CPAP) machine, “the risk of having the machine contaminated is a little bit higher.”
“In these situations...we have a more intensive cleaning procedure to avoid cross-contamination.”
He stressed: “Not all centers have wireless machines, whereas a normal machine is usually in all hospitals.”
“The advantages of using lung ultrasound [in COVID-19] are too great to be limited by something that is not important in my opinion,” he concluded.
Stone is director of education at the Butterfly Network. No other conflicts of interest were declared.
This article first appeared on Medscape.com.
The first protocol for the use of lung ultrasound to quantitatively and reproducibly assess the degree of lung involvement in patients suspected of having COVID-19 infection has been published by a team of Italian experts with experience using the technology on the front line.
Particularly in Spain and Italy — where the pandemic has struck hardest in Europe — hard-pressed clinicians seeking to quickly understand whether patients with seemingly mild disease could be harboring more serious lung involvement have increasingly relied upon lung ultrasound in the emergency room.
Now Libertario Demi, PhD, head of the ultrasound laboratory, University of Trento, Italy, and colleagues have developed a protocol, published online March 30 in the Journal of Ultrasound Medicine, to standardize practice.
Their research, which builds on previous work by the team, offers broad agreement with industry-led algorithms and emphasizes the use of wireless, handheld ultrasound devices, ideally consisting of a separate probe and tablet, to make sterilization easy.
Firms such as the Butterfly Network, Phillips, Clarius, GE Healthcare, and Siemens are among numerous companies that produce one or more such devices, including some that are completely integrated.
Not Universally Accepted
However, lung ultrasound is not yet universally accepted as a tool for diagnosing pneumonia in the context of COVID-19 and triaging patients.
The National Health Service in England does not even mention lung ultrasound in its radiology decision tool for suspected COVID-19, specifying instead chest X-ray as the first-line diagnostic imaging tool, with CT scanning in equivocal cases.
But Giovanni Volpicelli, MD, University Hospital San Luigi Gonzaga, Turin, Italy, who has previously described his experience to Medscape Medical News, says many patients with COVID-19 in his hospital presented with a negative chest X-ray but were found to have interstitial pneumonia on lung ultrasound.
Moreover, while CT scan remains the gold standard, the risk of nosocomial infection is more easily controlled if patients do not have to be transported to the radiology department but remain in the emergency room and instead undergo lung ultrasound there, he stressed.
Experts Share Experience of Lung Ultrasound in COVID-19
In developing and publishing their protocol, Demi, senior author of the article, and other colleagues from the heavily affected cities of Northern Italy, say their aim is “to share our experience and to propose a standardization with respect to the use of lung ultrasound in the management of COVID-19 patients.”
They reviewed an anonymized database of around 60,000 ultrasound images of confirmed COVID-19 cases and reviewers were blinded to patients’ clinical backgrounds.
For image acquisition, the authors recommend scanning 14 areas in each patient for 10 seconds, making the scans intercostal to cover the widest possible surface area.
They advise the use of a single focal point on the pleural line, which they write, optimizes the beam shape for observing the lung surface.
The authors also urge that the mechanical index (MI) be kept low because high MIs sustained for long periods “may result in damaging the lung.”
They also stress that cosmetic filters and modalities such as harmonic imaging, contrast, doppler, and compounding should be avoided, alongside saturation phenomena.
What Constitutes Intermediate Disease?
Once the images have been taken, they are scored on a 0-3 scale for each of the 14 areas, with no weighting on any individual area.
A score of 0 is given when the pleural line is continuous and regular, with the presence of A-lines, denoting that the lungs are unaffected.
An area is given a score of 3 when the scan shows dense and largely extended white lung tissue, with or without consolidations, indicating severe disease.
At both ends of this spectrum, there is agreement between the Italian protocol and an algorithm developed by the Butterfly Network.
However, the two differ when it comes to scoring intermediate cases. On the Butterfly algorithm, the suggestion is to look for B-lines, caused by fluid and cellular infiltration into the interstitium, and to weigh that against the need for supplementary oxygen.
The Italian team, in contrast, says a score of 1 is given when the pleural line is indented, with vertical areas of white visible below.
A score of 2 is given when the pleural line is broken, with small to large areas of consolidation and associated areas of white below.
Demi told Medscape Medical News that they did not refer to B-lines in their protocol as their visibility depends entirely on the imaging frequency and the probe used.
“This means that scoring on B-lines, people with different machines would give completely different scores for the same patient.”
He continued: “We prefer to refer to horizontal and vertical artifacts, and provide an analysis of the patterns, which is related to the physics of the interactions between the ultrasound waves and lung surface.”
In response, Mike Stone, MD, Legacy Emanuel Medical Center, Portland, Oregon, and director of education at Butterfly, said there appears to be wide variation in lung findings that “may or may not correlate with the severity of symptoms.”
He told Medscape Medical News it is “hard to know exactly if someone with pure B-lines will progress to serious illness or if someone with some subpleural consolidations will do well.”
A Negative Ultrasound Is the Most Useful
Volpicelli believes that, in any case, any patient with an intermediate pattern will require further diagnosis, such as other imaging modalities and blood exams, and the real role of lung ultrasound is in assessing patients at either end of the spectrum.
“In other words, there are situations where lung ultrasound can be considered definitive,” he told Medscape Medical News. “For instance, if I see a patient with mild signs of the disease, just fever, and I perform lung ultrasound and see nothing, lung ultrasound rules out pneumonia.”
“This patient may have COVID-19 of course, but they do not have pneumonia, and they can be treated at home, awaiting the result of the swab test. And this is useful because you can reduce the burden in the emergency department.”
Volpicelli continued: “On the other hand, there are patients with acute respiratory failure in respiratory distress. If the lung ultrasound is normal, you can rule out COVID-19 and you need to use other diagnostic procedures to understand the problem.”
“This is also very important for us because it’s crucial to be able to remove the patient from the isolation area and perform CT scan, chest radiography, and all the other diagnostic tools that we need.”
Are Wireless Machines Needed? Not Necessarily
With regard to the use of wireless technology, the Italian team says that “in the setting of COVID-19, wireless probes and tablets represent the most appropriate ultrasound equipment” because they can “easily be wrapped in single-use plastic covers, reducing the risk of contamination,” and making sterilization easy.
Stone suggests that integrated portable devices, however, are no more likely to cause cross-contamination than separate probes and tablets, as they can fit within a sterile sheath as a single unit.
Volpicelli, for his part, doesn’t like what he sees as undue focus on wireless devices for lung ultrasound in the COVID-19 protocols.
He is concerned that recommending them as the best approach may be sending out the wrong message, which could be very “dangerous” as people may then think they cannot perform this screening with standard ultrasound machines.
For him, the issue of cross contamination with standard lung ultrasound machines is “nonexistent. Cleaning the machine is quite easy and I do it hundreds of times per week.”
He does acknowledge, however, that if the lung ultrasound is performed under certain circumstances, for example when a patient is using a continuous positive airway pressure (CPAP) machine, “the risk of having the machine contaminated is a little bit higher.”
“In these situations...we have a more intensive cleaning procedure to avoid cross-contamination.”
He stressed: “Not all centers have wireless machines, whereas a normal machine is usually in all hospitals.”
“The advantages of using lung ultrasound [in COVID-19] are too great to be limited by something that is not important in my opinion,” he concluded.
Stone is director of education at the Butterfly Network. No other conflicts of interest were declared.
This article first appeared on Medscape.com.
The first protocol for the use of lung ultrasound to quantitatively and reproducibly assess the degree of lung involvement in patients suspected of having COVID-19 infection has been published by a team of Italian experts with experience using the technology on the front line.
Particularly in Spain and Italy — where the pandemic has struck hardest in Europe — hard-pressed clinicians seeking to quickly understand whether patients with seemingly mild disease could be harboring more serious lung involvement have increasingly relied upon lung ultrasound in the emergency room.
Now Libertario Demi, PhD, head of the ultrasound laboratory, University of Trento, Italy, and colleagues have developed a protocol, published online March 30 in the Journal of Ultrasound Medicine, to standardize practice.
Their research, which builds on previous work by the team, offers broad agreement with industry-led algorithms and emphasizes the use of wireless, handheld ultrasound devices, ideally consisting of a separate probe and tablet, to make sterilization easy.
Firms such as the Butterfly Network, Phillips, Clarius, GE Healthcare, and Siemens are among numerous companies that produce one or more such devices, including some that are completely integrated.
Not Universally Accepted
However, lung ultrasound is not yet universally accepted as a tool for diagnosing pneumonia in the context of COVID-19 and triaging patients.
The National Health Service in England does not even mention lung ultrasound in its radiology decision tool for suspected COVID-19, specifying instead chest X-ray as the first-line diagnostic imaging tool, with CT scanning in equivocal cases.
But Giovanni Volpicelli, MD, University Hospital San Luigi Gonzaga, Turin, Italy, who has previously described his experience to Medscape Medical News, says many patients with COVID-19 in his hospital presented with a negative chest X-ray but were found to have interstitial pneumonia on lung ultrasound.
Moreover, while CT scan remains the gold standard, the risk of nosocomial infection is more easily controlled if patients do not have to be transported to the radiology department but remain in the emergency room and instead undergo lung ultrasound there, he stressed.
Experts Share Experience of Lung Ultrasound in COVID-19
In developing and publishing their protocol, Demi, senior author of the article, and other colleagues from the heavily affected cities of Northern Italy, say their aim is “to share our experience and to propose a standardization with respect to the use of lung ultrasound in the management of COVID-19 patients.”
They reviewed an anonymized database of around 60,000 ultrasound images of confirmed COVID-19 cases and reviewers were blinded to patients’ clinical backgrounds.
For image acquisition, the authors recommend scanning 14 areas in each patient for 10 seconds, making the scans intercostal to cover the widest possible surface area.
They advise the use of a single focal point on the pleural line, which they write, optimizes the beam shape for observing the lung surface.
The authors also urge that the mechanical index (MI) be kept low because high MIs sustained for long periods “may result in damaging the lung.”
They also stress that cosmetic filters and modalities such as harmonic imaging, contrast, doppler, and compounding should be avoided, alongside saturation phenomena.
What Constitutes Intermediate Disease?
Once the images have been taken, they are scored on a 0-3 scale for each of the 14 areas, with no weighting on any individual area.
A score of 0 is given when the pleural line is continuous and regular, with the presence of A-lines, denoting that the lungs are unaffected.
An area is given a score of 3 when the scan shows dense and largely extended white lung tissue, with or without consolidations, indicating severe disease.
At both ends of this spectrum, there is agreement between the Italian protocol and an algorithm developed by the Butterfly Network.
However, the two differ when it comes to scoring intermediate cases. On the Butterfly algorithm, the suggestion is to look for B-lines, caused by fluid and cellular infiltration into the interstitium, and to weigh that against the need for supplementary oxygen.
The Italian team, in contrast, says a score of 1 is given when the pleural line is indented, with vertical areas of white visible below.
A score of 2 is given when the pleural line is broken, with small to large areas of consolidation and associated areas of white below.
Demi told Medscape Medical News that they did not refer to B-lines in their protocol as their visibility depends entirely on the imaging frequency and the probe used.
“This means that scoring on B-lines, people with different machines would give completely different scores for the same patient.”
He continued: “We prefer to refer to horizontal and vertical artifacts, and provide an analysis of the patterns, which is related to the physics of the interactions between the ultrasound waves and lung surface.”
In response, Mike Stone, MD, Legacy Emanuel Medical Center, Portland, Oregon, and director of education at Butterfly, said there appears to be wide variation in lung findings that “may or may not correlate with the severity of symptoms.”
He told Medscape Medical News it is “hard to know exactly if someone with pure B-lines will progress to serious illness or if someone with some subpleural consolidations will do well.”
A Negative Ultrasound Is the Most Useful
Volpicelli believes that, in any case, any patient with an intermediate pattern will require further diagnosis, such as other imaging modalities and blood exams, and the real role of lung ultrasound is in assessing patients at either end of the spectrum.
“In other words, there are situations where lung ultrasound can be considered definitive,” he told Medscape Medical News. “For instance, if I see a patient with mild signs of the disease, just fever, and I perform lung ultrasound and see nothing, lung ultrasound rules out pneumonia.”
“This patient may have COVID-19 of course, but they do not have pneumonia, and they can be treated at home, awaiting the result of the swab test. And this is useful because you can reduce the burden in the emergency department.”
Volpicelli continued: “On the other hand, there are patients with acute respiratory failure in respiratory distress. If the lung ultrasound is normal, you can rule out COVID-19 and you need to use other diagnostic procedures to understand the problem.”
“This is also very important for us because it’s crucial to be able to remove the patient from the isolation area and perform CT scan, chest radiography, and all the other diagnostic tools that we need.”
Are Wireless Machines Needed? Not Necessarily
With regard to the use of wireless technology, the Italian team says that “in the setting of COVID-19, wireless probes and tablets represent the most appropriate ultrasound equipment” because they can “easily be wrapped in single-use plastic covers, reducing the risk of contamination,” and making sterilization easy.
Stone suggests that integrated portable devices, however, are no more likely to cause cross-contamination than separate probes and tablets, as they can fit within a sterile sheath as a single unit.
Volpicelli, for his part, doesn’t like what he sees as undue focus on wireless devices for lung ultrasound in the COVID-19 protocols.
He is concerned that recommending them as the best approach may be sending out the wrong message, which could be very “dangerous” as people may then think they cannot perform this screening with standard ultrasound machines.
For him, the issue of cross contamination with standard lung ultrasound machines is “nonexistent. Cleaning the machine is quite easy and I do it hundreds of times per week.”
He does acknowledge, however, that if the lung ultrasound is performed under certain circumstances, for example when a patient is using a continuous positive airway pressure (CPAP) machine, “the risk of having the machine contaminated is a little bit higher.”
“In these situations...we have a more intensive cleaning procedure to avoid cross-contamination.”
He stressed: “Not all centers have wireless machines, whereas a normal machine is usually in all hospitals.”
“The advantages of using lung ultrasound [in COVID-19] are too great to be limited by something that is not important in my opinion,” he concluded.
Stone is director of education at the Butterfly Network. No other conflicts of interest were declared.
This article first appeared on Medscape.com.
FDA approves first generic albuterol inhaler
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
The Food and Drug Administration has approved the first generic of Proventil HFA (albuterol sulfate) metered-dose inhaler, 90 mcg/inhalation, according to a release from the agency. This inhaler is indicated for prevention of bronchospasm in patients aged 4 years and older. Specifically, these are patients with reversible obstructive airway disease or exercise-induced bronchospasm.
“The FDA recognizes the increased demand for albuterol products during the novel coronavirus pandemic,” said FDA Commissioner Stephen M. Hahn, MD.
The most common side effects include upper respiratory tract infection, rhinitis, nausea, vomiting, rapid heart rate, tremor, and nervousness.
This approval comes as part of FDA’s efforts to guide industry through the development process of generic products, according to the release. Complex combination products – such as this inhaler, which comprises both medication and a delivery system – can be more challenging to develop than solid oral dosage forms, such as tablets.
The FDA released a draft guidance in March 2020 specific to proposed generic albuterol sulfate metered-dose inhalers, including drug products referencing Proventil HFA. As with other similar guidances, it details the steps companies need to take in developing generics in order to submit complete applications for those products. The full news release regarding this approval is available on the FDA website.
This article was updated 4/8/20.
Comorbidities the rule in New York’s COVID-19 deaths
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.
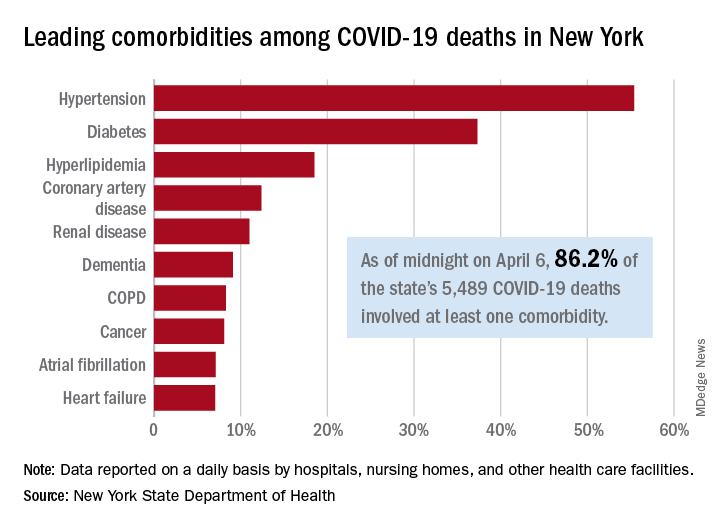
As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.

As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.

As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
Superior turbinate eosinophilia predicts olfactory decline in patients with CRS
Olfactory decline in patients with chronic rhinosinusitis (CRS) after endoscopic sinus surgery is linked to superior turbinate eosinophilia, according to recent research released as an abstract from the American Academy of Allergy, Asthma, and Immunology annual meeting. The AAAAI canceled the meeting and provided abstracts and access to presenters for press coverage.
“,” Dawei Wu, MD, of Beijing Anzhen Hospital, Capital Medical University in Beijing, China, said in an interview.
There has been some research in the literature pointing to the link between CRS-associated olfactory dysfunction and superior turbinate eosinophilia. In a 2017 study, Lavin et al. found eosinophil markers in the superior turbinate tissue were elevated in patients with CRS with nasal polyps. One of the gene expressions of the eosinophil marker Charcot Leyden crystal protein (CLC) was inversely associated with olfactory threshold, which led the researchers to believe there was a link between olfactory decline and superior turbinate eosinophilia in these patients (Laryngoscope. 2017 Oct;127[10]:2210-2218).
Olfactory decline associated with CRS is the most common reason for loss of smell in ear, nose, and throat clinics, Dr. Wu said, but predicting this olfactory decline after endoscopic sinus surgery can be clinically challenging.
“The distinct feature of this smell disorder is the fluctuation in olfactory dysfunction which is mainly due to the recurrence of inflammation within the olfactory cleft. Notably, the level of eosinophils within the olfactory cleft significantly and positively correlated with the degree of olfactory dysfunction in patients with CRS both pre- and postoperatively,” he said.
Dr. Wu and colleagues conducted a prospective study to determine whether there was a link in CRS patients between preoperative superior turbinate eosinophilia and olfactory dysfunction after endoscopic sinus surgery. “We aimed to explore potential predictors of postoperative olfactory decline,” Dr. Wu said.
Overall, the investigators enrolled 78 patients with CRS in the study, where they received an olfactory assessment prior to and 3 months after endoscopic sinus surgery. The investigators used Sniffin’ Sticks (Burghardt; Wedel, Germany), a 12-item psychophysical smell test that uses everyday odors to conduct the olfactory assessment. If patients had a decrease in their threshold-discrimination-identification (TDI) score after surgery, they were determined to have olfactory deterioration. Prior to surgery, investigators measured olfactory cleft opacification using CT, with the olfactory cleft endoscopy scale used after surgery. The investigators also sampled patients’ superior turbinates at the time of surgery.
The results showed 23 of 78 patients (29.49%) had olfactory decline at 3 months after endoscopic sinus surgery. Those patients with olfactory decline had significantly higher tissue, blood eosinophil levels, and TDI scores before surgery, compared with patients with CRS who did not have any loss of smell. Patients with olfactory decline also had olfactory cleft opacification and olfactory cleft endoscopy scale scores, compared with patients who had no loss of smell after surgery.
One factor that predicted olfactory decline in these patients was an absolute count of 23.5 eosinophils per high-power field in the superior turbinate, researchers said (area under the ROC curve, 0.901). “Continuous elimination of the eosinophilic inflammation within the olfactory cleft in CRS patients with olfactory dysfunction may prevent the olfactory fluctuation after endoscopic sinus surgery,” Dr. Wu said.
Dr. Wu reports no relevant conflicts of interest.
SOURCE: Wu D et al. AAAAI. Abstract L7.
Olfactory decline in patients with chronic rhinosinusitis (CRS) after endoscopic sinus surgery is linked to superior turbinate eosinophilia, according to recent research released as an abstract from the American Academy of Allergy, Asthma, and Immunology annual meeting. The AAAAI canceled the meeting and provided abstracts and access to presenters for press coverage.
“,” Dawei Wu, MD, of Beijing Anzhen Hospital, Capital Medical University in Beijing, China, said in an interview.
There has been some research in the literature pointing to the link between CRS-associated olfactory dysfunction and superior turbinate eosinophilia. In a 2017 study, Lavin et al. found eosinophil markers in the superior turbinate tissue were elevated in patients with CRS with nasal polyps. One of the gene expressions of the eosinophil marker Charcot Leyden crystal protein (CLC) was inversely associated with olfactory threshold, which led the researchers to believe there was a link between olfactory decline and superior turbinate eosinophilia in these patients (Laryngoscope. 2017 Oct;127[10]:2210-2218).
Olfactory decline associated with CRS is the most common reason for loss of smell in ear, nose, and throat clinics, Dr. Wu said, but predicting this olfactory decline after endoscopic sinus surgery can be clinically challenging.
“The distinct feature of this smell disorder is the fluctuation in olfactory dysfunction which is mainly due to the recurrence of inflammation within the olfactory cleft. Notably, the level of eosinophils within the olfactory cleft significantly and positively correlated with the degree of olfactory dysfunction in patients with CRS both pre- and postoperatively,” he said.
Dr. Wu and colleagues conducted a prospective study to determine whether there was a link in CRS patients between preoperative superior turbinate eosinophilia and olfactory dysfunction after endoscopic sinus surgery. “We aimed to explore potential predictors of postoperative olfactory decline,” Dr. Wu said.
Overall, the investigators enrolled 78 patients with CRS in the study, where they received an olfactory assessment prior to and 3 months after endoscopic sinus surgery. The investigators used Sniffin’ Sticks (Burghardt; Wedel, Germany), a 12-item psychophysical smell test that uses everyday odors to conduct the olfactory assessment. If patients had a decrease in their threshold-discrimination-identification (TDI) score after surgery, they were determined to have olfactory deterioration. Prior to surgery, investigators measured olfactory cleft opacification using CT, with the olfactory cleft endoscopy scale used after surgery. The investigators also sampled patients’ superior turbinates at the time of surgery.
The results showed 23 of 78 patients (29.49%) had olfactory decline at 3 months after endoscopic sinus surgery. Those patients with olfactory decline had significantly higher tissue, blood eosinophil levels, and TDI scores before surgery, compared with patients with CRS who did not have any loss of smell. Patients with olfactory decline also had olfactory cleft opacification and olfactory cleft endoscopy scale scores, compared with patients who had no loss of smell after surgery.
One factor that predicted olfactory decline in these patients was an absolute count of 23.5 eosinophils per high-power field in the superior turbinate, researchers said (area under the ROC curve, 0.901). “Continuous elimination of the eosinophilic inflammation within the olfactory cleft in CRS patients with olfactory dysfunction may prevent the olfactory fluctuation after endoscopic sinus surgery,” Dr. Wu said.
Dr. Wu reports no relevant conflicts of interest.
SOURCE: Wu D et al. AAAAI. Abstract L7.
Olfactory decline in patients with chronic rhinosinusitis (CRS) after endoscopic sinus surgery is linked to superior turbinate eosinophilia, according to recent research released as an abstract from the American Academy of Allergy, Asthma, and Immunology annual meeting. The AAAAI canceled the meeting and provided abstracts and access to presenters for press coverage.
“,” Dawei Wu, MD, of Beijing Anzhen Hospital, Capital Medical University in Beijing, China, said in an interview.
There has been some research in the literature pointing to the link between CRS-associated olfactory dysfunction and superior turbinate eosinophilia. In a 2017 study, Lavin et al. found eosinophil markers in the superior turbinate tissue were elevated in patients with CRS with nasal polyps. One of the gene expressions of the eosinophil marker Charcot Leyden crystal protein (CLC) was inversely associated with olfactory threshold, which led the researchers to believe there was a link between olfactory decline and superior turbinate eosinophilia in these patients (Laryngoscope. 2017 Oct;127[10]:2210-2218).
Olfactory decline associated with CRS is the most common reason for loss of smell in ear, nose, and throat clinics, Dr. Wu said, but predicting this olfactory decline after endoscopic sinus surgery can be clinically challenging.
“The distinct feature of this smell disorder is the fluctuation in olfactory dysfunction which is mainly due to the recurrence of inflammation within the olfactory cleft. Notably, the level of eosinophils within the olfactory cleft significantly and positively correlated with the degree of olfactory dysfunction in patients with CRS both pre- and postoperatively,” he said.
Dr. Wu and colleagues conducted a prospective study to determine whether there was a link in CRS patients between preoperative superior turbinate eosinophilia and olfactory dysfunction after endoscopic sinus surgery. “We aimed to explore potential predictors of postoperative olfactory decline,” Dr. Wu said.
Overall, the investigators enrolled 78 patients with CRS in the study, where they received an olfactory assessment prior to and 3 months after endoscopic sinus surgery. The investigators used Sniffin’ Sticks (Burghardt; Wedel, Germany), a 12-item psychophysical smell test that uses everyday odors to conduct the olfactory assessment. If patients had a decrease in their threshold-discrimination-identification (TDI) score after surgery, they were determined to have olfactory deterioration. Prior to surgery, investigators measured olfactory cleft opacification using CT, with the olfactory cleft endoscopy scale used after surgery. The investigators also sampled patients’ superior turbinates at the time of surgery.
The results showed 23 of 78 patients (29.49%) had olfactory decline at 3 months after endoscopic sinus surgery. Those patients with olfactory decline had significantly higher tissue, blood eosinophil levels, and TDI scores before surgery, compared with patients with CRS who did not have any loss of smell. Patients with olfactory decline also had olfactory cleft opacification and olfactory cleft endoscopy scale scores, compared with patients who had no loss of smell after surgery.
One factor that predicted olfactory decline in these patients was an absolute count of 23.5 eosinophils per high-power field in the superior turbinate, researchers said (area under the ROC curve, 0.901). “Continuous elimination of the eosinophilic inflammation within the olfactory cleft in CRS patients with olfactory dysfunction may prevent the olfactory fluctuation after endoscopic sinus surgery,” Dr. Wu said.
Dr. Wu reports no relevant conflicts of interest.
SOURCE: Wu D et al. AAAAI. Abstract L7.
FROM AAAAI
A Veteran Presenting With Altered Mental Status and Clonus
►Zachary Reese, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC):Dr. Weller, the differential diagnosis for altered mental status is quite broad. How does the presence of clonus change or focus your approach to altered mental status?
►Jason Weller, MD, Instructor of Neurology, Boston Medical Center (BMC) and VABHS:The presence of clonus does not significantly narrow the differential. It does, however, suggest a central component to the patient’s altered mental status. Specifically, it implies that the underlying process, whether systemic or neurologic, interferes with central nervous system (CNS) control of the neuromuscular system.1 The differential is still quite broad and includes metabolic derangements (eg, uremia, electrolyte disturbances, hypercarbia, and thyroid dysfunction), medication toxicity from olanzapine or duloxetine, and vascular processes (eg, CNS vasculitis). Infectious etiologies, both within the CNS and systemically, can cause encephalopathy, as can autoimmune processes, such as immune-mediated encephalitis. Finally, primary neurologic conditions such as myoclonic epilepsy can be considered. Given the patient’s medical history, serotonin syndrome must be considered.
►Dr. Reese: Given the concern for serotonin syndrome, the admitting medical team discontinued the patient’s duloxetine. Dr. Weller, what is the pathophysiology of serotonin syndrome, and how is it diagnosed?
►Dr. Weller: Serotonin is ubiquitous throughout the body and brain. Serotonin syndrome is caused by excess endogenous or exogenous serotonin, and this is usually caused by a variety of medications. The symptoms range from tachycardia, agitation, and diaphoresis to sustained clonus, hyperthermia, and shock.2,3 The extent of serotonin syndrome is typically thought to reflect the degree of serotonergic activity.4
Serotonin syndrome is a clinical diagnosis. While there are no tests that can confirm the diagnosis, the Hunter criteria can be used to assist with making the diagnosis.5 Per the Hunter criteria, a patient can be diagnosed with serotonin syndrome if they have taken a serotonergic agent and have at least 1 of the following: spontaneous clonus, inducible or ocular clonus with agitation or diaphoresis, tremor and hyperreflexia, or hypertonia with fever and clonus. This patient had taken duloxetine and had inducible clonus and diaphoresis, thus suggesting a diagnosis of serotonin syndrome.
►Dr. Reese: Aside from selective serotonin reuptake inhibitors (SSRIs), are there other medications that we typically prescribe that can cause serotonin syndrome?
►Dr. Weller: In addition to SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs), other commonly prescribed medications that can cause serotonin syndrome are 5-HT3 antagonists (eg, ondansetron), 5-HT agonists (eg, triptans), and opioids (eg, fentanyl and tramadol). There are also case reports of atypical antipsychotics (eg, olanzapine) causing serotonin syndrome because of their antagonism of the 5-HT2 and 5-HT3 receptors.2 Additionally, linezolid is commonly overlooked as a cause of serotonin syndrome given its action as a monoamine oxidase inhibitor.4 In this patient, it would be prudent to discontinue olanzapine and duloxetine.
►Dr. Reese: Duloxetine, olanzapine, and buprenorphine/naloxone were discontinuedgiven concern for serotonin syndrome. Although there are not strong data that buprenorphine/ naloxone can cause serotonin syndrome, the team discontinued the medication in case it might be contributing to the patient’s encephalopathy, while closely monitoring the patient for withdrawal. There was a rapid improvement in the patient’s symptoms over the 24 hours after discontinuation of the 3 medications.
As part of the initial workup, the patient received a computed tomography (CT) scan of his chest to follow up pulmonary nodules identified 16 months prior. The CT scan showed interval growth of the pulmonary nodules in the right lower lobe to 2 cm with extension into the major fissure, which was concerning for malignancy. Plans were made for an outpatient positron emission tomography (PET) scan after hospital discharge.
Dr. Schlechter and Dr. Rangachari, what factors can help us determine whether or not further workup of a malignancy should occur before discharge or can be deferred to the outpatient setting?
►Benjamin Schlechter, MD, Instructor in Medicine, BIDMC; and Deepa Rangachari, MD, Assistant Professor of Medicine, BIDMC: Key considerations in this domain include rapidity of growth and any threat to critical end-organ function (ie, brain, heart, lungs, kidney, and liver). If the malignancy is bulky and/or rapidly progressing to the point that the patient has significant symptoms burden and/or end-organ dysfunction, then initiating the evaluation as an inpatient may be necessary. For suspected intrathoracic malignancies, considering whether this may be a high-grade process (ie, small cell lung cancer) is often a vital branch point. Key considerations in this regard are the following: Is it a bulky central tumor? Is there evidence of widespread metastatic disease, an obstructing mass, and/or tumor lysis? One final and critical aspect to consider is whether there are any patient- specific barriers to timely and reliable outpatient follow-up. If there is no evidence of rapid progression, bulky disease with threatened end-organ involvement, and/or issues with timely and reliable follow-up, then outpatient evaluation is often the best approach to ensure a comprehensive and well-coordinated effort on the patient’s behalf.
►Dr. Reese: Buprenorphine/naloxone was restarted without return of the symptoms. The patient was discharged home with an outpatient PET scan scheduled the following week. Unfortunately, the patient was unable to keep this appointment. Three weeks after hospital discharge, the patient presented again to the emergency department with gradually worsening altered mental status, confusion, visual hallucinations, and myoclonic jerking of the arms and legs. Medication adherence was confirmed by the patient’s wife, resulting in a low concern for serotonin syndrome. Physical examination revealed confusion, dysarthria, diffuse, arrhythmic, myoclonic jerking in all extremities, asterixis in the upper extremities, and hyperreflexia.
A CT scan of the brain did not reveal an intracranial process. A spot electroencephalograph (EEG) and magnetic resonance image (MRI) of the brain were obtained. Dr. Weller, what is the utility of spot EEG vs 24-hour EEG? When might we choose one over the other?
►Dr. Weller: If a patient is persistently altered, then a spot EEG would be sufficient to capture a seizure if that is what is causing the patient’s altered mental status. However, if the patient’s mental status is waxing and waning, then that may warrant a 24-hour EEG because the patient may need to be monitored for longer periods to capture an event that is causing intermittent alterations in mental status.6 Additionally, patients who are acutely ill may require long-term monitoring for the purpose of treatment and outcome management.
►Dr. Reese: The spot EEG showed nearly continuous generalized slowing indicative of a diffuse encephalopathy. The MRI of the brain showed scattered, nonspecific periventricular T2 hyperintense foci, suggestive of advanced chronic microvascular ischemic changes.
A PET CT was obtained and revealed mildly fluorodeoxyglucose (FDG)-avid, enlarging nodules within the right lower lobe, which was suspicious for malignancy. There were no other areas of FDG avidity on the PET scan. Valproic acid was initiated for treatment of myoclonus with transition to clonazepam when no improvement was seen. After starting clonazepam, the patient’s condition stabilized.
Dr. Weller, given the additional history, how has your differential diagnosis changed?
►Dr. Weller: Given the patient’s laboratory findings, we can be quite sure that there is not a contributing metabolic process. The findings suggestive of metastatic cancer, along with the profound neurologic changes, are most concerning for a paraneoplastic syndrome. I would suggest biopsy and consideration of a lumbar puncture. One can also send serum markers, including a paraneoplastic antibody panel.
►Dr. Reese: Biopsy of the mass in his right lower lobe revealed squamous cell lung cancer. Dr. Schlechter and Dr. Rangachari, do you have a framework for the different forms of lung cancer?
►Dr. Schlechter/Dr. Rangachari: The 2 broad categories of lung cancer are small cell and non-small cell (NSCLC). Small cell lung cancer has a tight association with tobacco exposure and is often clinically defined by rapid, bulky progression (ie, weeks to months).7,8 NSCLCs are also commonly seen in those with tobacco exposure, though not always. The main subgroups in this category are adenocarcinoma and squamous cell carcinoma. These cancers often evolve at a slower pace (ie, months to years).8 While small cell lung cancers are highgrade tumors and exquisitely sensitive to chemotherapy and radiation, NSCLCs tend to be less responsive to such therapies. The staging evaluation for either entity is the same and consists of defining localized vs metastatic disease.
►Dr. Reese: Because this patient had an MRI and PET scan that were both negative for metastatic disease, can we assume that this patient had stage I NSCLC?
►Dr. Schlechter/Dr. Rangachari: Not necessarily. While PET and MRI brain are exceptionally helpful in detecting distant metastases, they may over- or underestimate intrathoracic lymph node involvement by as much as 20%.9 As such, dedicated lymph node staging—either via bronchoscopy (endobronchial ultrasound) or surgically (mediastinoscopy) is indicated as lymph node involvement can significantly alter the stage, prognosis, and optimal therapeutic approach.10,11
►Dr. Reese: After this diagnosis was made, the teams caring for this patient attributed his altered mental status to a paraneoplastic syndrome. What is a paraneoplastic syndrome, and how does a paraneoplastic syndrome from malignancy present? Does its presence worsen a patient’s prognosis?
►Dr. Schlechter/Dr. Rangachari: A paraneoplastic syndrome is defined by an immunologic response to the cancer that ends up erroneously targeting self-antigens. Paraneoplastic syndromes are associated with a broad array of clinical findings—from endocrinopathy to encephalopathy—and certain neoplasms are more commonly associated with these syndromes than others (eg, small cell lung cancer and thymoma). Further, severity and onset of a paraneoplastic syndrome does not correlate with the burden of visible disease—and the syndrome may predate the cancer diagnosis by months to years.11 While treatment of the cancer affords the best hope of resolving the paraneoplastic syndrome, the cancer and the paraneoplastic process may have a discordant trajectory, with the paraneoplastic syndrome persisting even after the cancer is maximally treated. Although one might assume that paraneoplastic syndromes portend worse outcomes, in some cases, a presentation with the paraneoplastic syndrome may afford sooner detection of an otherwise occult/asymptomatic malignancy.
►Dr. Reese: The following week, the serum paraneoplastic antibody panel that tested for anti-Yo antibody, anti-Ri antibody,and anti-Hu antibody came back negative. Dr. Weller, what does this mean? Since we have yet to obtain a lumbar puncture, might his symptoms still be caused by a paraneoplastic syndrome?
►Dr. Weller: The negative serum test just means that he does not have antibodies to those 3 antibodies. There are now over 30 different paraneoplastic antibodies that have been discovered, and there are always more that are being discovered. So this negative test result does not exclude a paraneoplastic syndrome in the appropriate clinical context.12 Furthermore, the sensitivity and specificity for certain antibodies are different based upon source fluid, and cerebrospinal fluid testing would provide more diagnostic clarity. A negative test for paraneoplastic syndrome, by itself, would similarly not exclude a paraneoplastic syndrome. Often, empiric treatment is the best diagnostic option for paraneoplastic and autoimmune encephalopathies.
►Dr. Reese: The following week, the patient was discharged to rehabilitation with clonazepam for his symptoms and a scheduled follow-up. Given the patient’s frailty and medical comorbidities, thoracic surgery recommended consultation with radiation oncology. Dr. Schlechter and Dr. Rangachari, when do we decide to use radiation vs chemotherapy for someone with lung cancer?
►Dr. Schlechter/Dr. Rangachari: Patients with early stage, nonmetastatic NSCLC may not always be candidates for surgical resection on the basis of pulmonary function, other medical comorbidities (as in this case), anatomic considerations, and/or patient preference. In these cases, if there is lung-limited disease without lymph node involvement (ie, stage I/II NSCLC) and the patient is not felt to be an operative candidate, then alternatives to surgery include either radiation or ablation.13,14 As we care for an aging and comorbid population, evolving evidence suggests that well-selected patients with early stage disease undergoing these nonoperative approaches have roughly equivalent outcomes to those undergoing conventional surgical resection.13 In such cases, multidisciplinary consultation with a team having dedicated expertise in these various operative and nonoperative modalities is essential.
►Dr. Reese: The patient followed up with radiation oncology for consideration of radiation treatment, but his simulation CT scan showed some ground-glass opacity that were concerning for inflammation vs infection. The patient’s case was discussed at the multidisciplinary tumor board, and it was determined to treat him with antibiotics for a possible pneumonia before proceeding with radiation therapy. After he completed antibiotic treatment, he underwent 10 fractions of radiation treatment, which he tolerated well.
1. Kojovic M, Cordivari C, Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4(1):47-62.
2. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
3. Arora B, Kannikeswaran N. The serotonin syndrome-the need for physician’s awareness. Int J Emerg Med. 2010;3(4):373-377.
4. Boyer EW, Shannon M. The serotonin syndrome [published correction appears in N Engl J Med. 2007;356(23):2437 and N Engl J Med. 2009;361(17):1714]. N Engl J Med.
2005;352(11):1112-1120.
5. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM.
2003;96(9):635-642.
6. Nordli DR Jr. Usefulness of video-EEG monitoring. Epilepsia. 2006;47(suppl 1):26-30.
7. Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24(28):4526-4527.
8. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-1260.
9. Cerfolio RJ, Bryant AS, Ojha B, Eloubeidi M. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg. 2005;80(4):1207-1214.
10. El-Osta H, Jani P, Mansour A, Rascoe P, Jafri S. Endobronchial ultrasound for nodal staging of patients with non-smallcell lung cancer with radiologically normal mediastinum. A meta-analysis. Ann Am Thorac Soc. 2018;15(7):864-874.
11. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543-1554.
12. McKeon A. Autoimmune Encephalopathies and Dementias. Continuum (Minneap Minn). 2016;22(2 Dementia): 538-558.
13. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Nonsmall cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
14. Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807-821.
►Zachary Reese, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC):Dr. Weller, the differential diagnosis for altered mental status is quite broad. How does the presence of clonus change or focus your approach to altered mental status?
►Jason Weller, MD, Instructor of Neurology, Boston Medical Center (BMC) and VABHS:The presence of clonus does not significantly narrow the differential. It does, however, suggest a central component to the patient’s altered mental status. Specifically, it implies that the underlying process, whether systemic or neurologic, interferes with central nervous system (CNS) control of the neuromuscular system.1 The differential is still quite broad and includes metabolic derangements (eg, uremia, electrolyte disturbances, hypercarbia, and thyroid dysfunction), medication toxicity from olanzapine or duloxetine, and vascular processes (eg, CNS vasculitis). Infectious etiologies, both within the CNS and systemically, can cause encephalopathy, as can autoimmune processes, such as immune-mediated encephalitis. Finally, primary neurologic conditions such as myoclonic epilepsy can be considered. Given the patient’s medical history, serotonin syndrome must be considered.
►Dr. Reese: Given the concern for serotonin syndrome, the admitting medical team discontinued the patient’s duloxetine. Dr. Weller, what is the pathophysiology of serotonin syndrome, and how is it diagnosed?
►Dr. Weller: Serotonin is ubiquitous throughout the body and brain. Serotonin syndrome is caused by excess endogenous or exogenous serotonin, and this is usually caused by a variety of medications. The symptoms range from tachycardia, agitation, and diaphoresis to sustained clonus, hyperthermia, and shock.2,3 The extent of serotonin syndrome is typically thought to reflect the degree of serotonergic activity.4
Serotonin syndrome is a clinical diagnosis. While there are no tests that can confirm the diagnosis, the Hunter criteria can be used to assist with making the diagnosis.5 Per the Hunter criteria, a patient can be diagnosed with serotonin syndrome if they have taken a serotonergic agent and have at least 1 of the following: spontaneous clonus, inducible or ocular clonus with agitation or diaphoresis, tremor and hyperreflexia, or hypertonia with fever and clonus. This patient had taken duloxetine and had inducible clonus and diaphoresis, thus suggesting a diagnosis of serotonin syndrome.
►Dr. Reese: Aside from selective serotonin reuptake inhibitors (SSRIs), are there other medications that we typically prescribe that can cause serotonin syndrome?
►Dr. Weller: In addition to SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs), other commonly prescribed medications that can cause serotonin syndrome are 5-HT3 antagonists (eg, ondansetron), 5-HT agonists (eg, triptans), and opioids (eg, fentanyl and tramadol). There are also case reports of atypical antipsychotics (eg, olanzapine) causing serotonin syndrome because of their antagonism of the 5-HT2 and 5-HT3 receptors.2 Additionally, linezolid is commonly overlooked as a cause of serotonin syndrome given its action as a monoamine oxidase inhibitor.4 In this patient, it would be prudent to discontinue olanzapine and duloxetine.
►Dr. Reese: Duloxetine, olanzapine, and buprenorphine/naloxone were discontinuedgiven concern for serotonin syndrome. Although there are not strong data that buprenorphine/ naloxone can cause serotonin syndrome, the team discontinued the medication in case it might be contributing to the patient’s encephalopathy, while closely monitoring the patient for withdrawal. There was a rapid improvement in the patient’s symptoms over the 24 hours after discontinuation of the 3 medications.
As part of the initial workup, the patient received a computed tomography (CT) scan of his chest to follow up pulmonary nodules identified 16 months prior. The CT scan showed interval growth of the pulmonary nodules in the right lower lobe to 2 cm with extension into the major fissure, which was concerning for malignancy. Plans were made for an outpatient positron emission tomography (PET) scan after hospital discharge.
Dr. Schlechter and Dr. Rangachari, what factors can help us determine whether or not further workup of a malignancy should occur before discharge or can be deferred to the outpatient setting?
►Benjamin Schlechter, MD, Instructor in Medicine, BIDMC; and Deepa Rangachari, MD, Assistant Professor of Medicine, BIDMC: Key considerations in this domain include rapidity of growth and any threat to critical end-organ function (ie, brain, heart, lungs, kidney, and liver). If the malignancy is bulky and/or rapidly progressing to the point that the patient has significant symptoms burden and/or end-organ dysfunction, then initiating the evaluation as an inpatient may be necessary. For suspected intrathoracic malignancies, considering whether this may be a high-grade process (ie, small cell lung cancer) is often a vital branch point. Key considerations in this regard are the following: Is it a bulky central tumor? Is there evidence of widespread metastatic disease, an obstructing mass, and/or tumor lysis? One final and critical aspect to consider is whether there are any patient- specific barriers to timely and reliable outpatient follow-up. If there is no evidence of rapid progression, bulky disease with threatened end-organ involvement, and/or issues with timely and reliable follow-up, then outpatient evaluation is often the best approach to ensure a comprehensive and well-coordinated effort on the patient’s behalf.
►Dr. Reese: Buprenorphine/naloxone was restarted without return of the symptoms. The patient was discharged home with an outpatient PET scan scheduled the following week. Unfortunately, the patient was unable to keep this appointment. Three weeks after hospital discharge, the patient presented again to the emergency department with gradually worsening altered mental status, confusion, visual hallucinations, and myoclonic jerking of the arms and legs. Medication adherence was confirmed by the patient’s wife, resulting in a low concern for serotonin syndrome. Physical examination revealed confusion, dysarthria, diffuse, arrhythmic, myoclonic jerking in all extremities, asterixis in the upper extremities, and hyperreflexia.
A CT scan of the brain did not reveal an intracranial process. A spot electroencephalograph (EEG) and magnetic resonance image (MRI) of the brain were obtained. Dr. Weller, what is the utility of spot EEG vs 24-hour EEG? When might we choose one over the other?
►Dr. Weller: If a patient is persistently altered, then a spot EEG would be sufficient to capture a seizure if that is what is causing the patient’s altered mental status. However, if the patient’s mental status is waxing and waning, then that may warrant a 24-hour EEG because the patient may need to be monitored for longer periods to capture an event that is causing intermittent alterations in mental status.6 Additionally, patients who are acutely ill may require long-term monitoring for the purpose of treatment and outcome management.
►Dr. Reese: The spot EEG showed nearly continuous generalized slowing indicative of a diffuse encephalopathy. The MRI of the brain showed scattered, nonspecific periventricular T2 hyperintense foci, suggestive of advanced chronic microvascular ischemic changes.
A PET CT was obtained and revealed mildly fluorodeoxyglucose (FDG)-avid, enlarging nodules within the right lower lobe, which was suspicious for malignancy. There were no other areas of FDG avidity on the PET scan. Valproic acid was initiated for treatment of myoclonus with transition to clonazepam when no improvement was seen. After starting clonazepam, the patient’s condition stabilized.
Dr. Weller, given the additional history, how has your differential diagnosis changed?
►Dr. Weller: Given the patient’s laboratory findings, we can be quite sure that there is not a contributing metabolic process. The findings suggestive of metastatic cancer, along with the profound neurologic changes, are most concerning for a paraneoplastic syndrome. I would suggest biopsy and consideration of a lumbar puncture. One can also send serum markers, including a paraneoplastic antibody panel.
►Dr. Reese: Biopsy of the mass in his right lower lobe revealed squamous cell lung cancer. Dr. Schlechter and Dr. Rangachari, do you have a framework for the different forms of lung cancer?
►Dr. Schlechter/Dr. Rangachari: The 2 broad categories of lung cancer are small cell and non-small cell (NSCLC). Small cell lung cancer has a tight association with tobacco exposure and is often clinically defined by rapid, bulky progression (ie, weeks to months).7,8 NSCLCs are also commonly seen in those with tobacco exposure, though not always. The main subgroups in this category are adenocarcinoma and squamous cell carcinoma. These cancers often evolve at a slower pace (ie, months to years).8 While small cell lung cancers are highgrade tumors and exquisitely sensitive to chemotherapy and radiation, NSCLCs tend to be less responsive to such therapies. The staging evaluation for either entity is the same and consists of defining localized vs metastatic disease.
►Dr. Reese: Because this patient had an MRI and PET scan that were both negative for metastatic disease, can we assume that this patient had stage I NSCLC?
►Dr. Schlechter/Dr. Rangachari: Not necessarily. While PET and MRI brain are exceptionally helpful in detecting distant metastases, they may over- or underestimate intrathoracic lymph node involvement by as much as 20%.9 As such, dedicated lymph node staging—either via bronchoscopy (endobronchial ultrasound) or surgically (mediastinoscopy) is indicated as lymph node involvement can significantly alter the stage, prognosis, and optimal therapeutic approach.10,11
►Dr. Reese: After this diagnosis was made, the teams caring for this patient attributed his altered mental status to a paraneoplastic syndrome. What is a paraneoplastic syndrome, and how does a paraneoplastic syndrome from malignancy present? Does its presence worsen a patient’s prognosis?
►Dr. Schlechter/Dr. Rangachari: A paraneoplastic syndrome is defined by an immunologic response to the cancer that ends up erroneously targeting self-antigens. Paraneoplastic syndromes are associated with a broad array of clinical findings—from endocrinopathy to encephalopathy—and certain neoplasms are more commonly associated with these syndromes than others (eg, small cell lung cancer and thymoma). Further, severity and onset of a paraneoplastic syndrome does not correlate with the burden of visible disease—and the syndrome may predate the cancer diagnosis by months to years.11 While treatment of the cancer affords the best hope of resolving the paraneoplastic syndrome, the cancer and the paraneoplastic process may have a discordant trajectory, with the paraneoplastic syndrome persisting even after the cancer is maximally treated. Although one might assume that paraneoplastic syndromes portend worse outcomes, in some cases, a presentation with the paraneoplastic syndrome may afford sooner detection of an otherwise occult/asymptomatic malignancy.
►Dr. Reese: The following week, the serum paraneoplastic antibody panel that tested for anti-Yo antibody, anti-Ri antibody,and anti-Hu antibody came back negative. Dr. Weller, what does this mean? Since we have yet to obtain a lumbar puncture, might his symptoms still be caused by a paraneoplastic syndrome?
►Dr. Weller: The negative serum test just means that he does not have antibodies to those 3 antibodies. There are now over 30 different paraneoplastic antibodies that have been discovered, and there are always more that are being discovered. So this negative test result does not exclude a paraneoplastic syndrome in the appropriate clinical context.12 Furthermore, the sensitivity and specificity for certain antibodies are different based upon source fluid, and cerebrospinal fluid testing would provide more diagnostic clarity. A negative test for paraneoplastic syndrome, by itself, would similarly not exclude a paraneoplastic syndrome. Often, empiric treatment is the best diagnostic option for paraneoplastic and autoimmune encephalopathies.
►Dr. Reese: The following week, the patient was discharged to rehabilitation with clonazepam for his symptoms and a scheduled follow-up. Given the patient’s frailty and medical comorbidities, thoracic surgery recommended consultation with radiation oncology. Dr. Schlechter and Dr. Rangachari, when do we decide to use radiation vs chemotherapy for someone with lung cancer?
►Dr. Schlechter/Dr. Rangachari: Patients with early stage, nonmetastatic NSCLC may not always be candidates for surgical resection on the basis of pulmonary function, other medical comorbidities (as in this case), anatomic considerations, and/or patient preference. In these cases, if there is lung-limited disease without lymph node involvement (ie, stage I/II NSCLC) and the patient is not felt to be an operative candidate, then alternatives to surgery include either radiation or ablation.13,14 As we care for an aging and comorbid population, evolving evidence suggests that well-selected patients with early stage disease undergoing these nonoperative approaches have roughly equivalent outcomes to those undergoing conventional surgical resection.13 In such cases, multidisciplinary consultation with a team having dedicated expertise in these various operative and nonoperative modalities is essential.
►Dr. Reese: The patient followed up with radiation oncology for consideration of radiation treatment, but his simulation CT scan showed some ground-glass opacity that were concerning for inflammation vs infection. The patient’s case was discussed at the multidisciplinary tumor board, and it was determined to treat him with antibiotics for a possible pneumonia before proceeding with radiation therapy. After he completed antibiotic treatment, he underwent 10 fractions of radiation treatment, which he tolerated well.
►Zachary Reese, MD, Chief Medical Resident, VABHS and Beth Israel Deaconess Medical Center (BIDMC):Dr. Weller, the differential diagnosis for altered mental status is quite broad. How does the presence of clonus change or focus your approach to altered mental status?
►Jason Weller, MD, Instructor of Neurology, Boston Medical Center (BMC) and VABHS:The presence of clonus does not significantly narrow the differential. It does, however, suggest a central component to the patient’s altered mental status. Specifically, it implies that the underlying process, whether systemic or neurologic, interferes with central nervous system (CNS) control of the neuromuscular system.1 The differential is still quite broad and includes metabolic derangements (eg, uremia, electrolyte disturbances, hypercarbia, and thyroid dysfunction), medication toxicity from olanzapine or duloxetine, and vascular processes (eg, CNS vasculitis). Infectious etiologies, both within the CNS and systemically, can cause encephalopathy, as can autoimmune processes, such as immune-mediated encephalitis. Finally, primary neurologic conditions such as myoclonic epilepsy can be considered. Given the patient’s medical history, serotonin syndrome must be considered.
►Dr. Reese: Given the concern for serotonin syndrome, the admitting medical team discontinued the patient’s duloxetine. Dr. Weller, what is the pathophysiology of serotonin syndrome, and how is it diagnosed?
►Dr. Weller: Serotonin is ubiquitous throughout the body and brain. Serotonin syndrome is caused by excess endogenous or exogenous serotonin, and this is usually caused by a variety of medications. The symptoms range from tachycardia, agitation, and diaphoresis to sustained clonus, hyperthermia, and shock.2,3 The extent of serotonin syndrome is typically thought to reflect the degree of serotonergic activity.4
Serotonin syndrome is a clinical diagnosis. While there are no tests that can confirm the diagnosis, the Hunter criteria can be used to assist with making the diagnosis.5 Per the Hunter criteria, a patient can be diagnosed with serotonin syndrome if they have taken a serotonergic agent and have at least 1 of the following: spontaneous clonus, inducible or ocular clonus with agitation or diaphoresis, tremor and hyperreflexia, or hypertonia with fever and clonus. This patient had taken duloxetine and had inducible clonus and diaphoresis, thus suggesting a diagnosis of serotonin syndrome.
►Dr. Reese: Aside from selective serotonin reuptake inhibitors (SSRIs), are there other medications that we typically prescribe that can cause serotonin syndrome?
►Dr. Weller: In addition to SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs), other commonly prescribed medications that can cause serotonin syndrome are 5-HT3 antagonists (eg, ondansetron), 5-HT agonists (eg, triptans), and opioids (eg, fentanyl and tramadol). There are also case reports of atypical antipsychotics (eg, olanzapine) causing serotonin syndrome because of their antagonism of the 5-HT2 and 5-HT3 receptors.2 Additionally, linezolid is commonly overlooked as a cause of serotonin syndrome given its action as a monoamine oxidase inhibitor.4 In this patient, it would be prudent to discontinue olanzapine and duloxetine.
►Dr. Reese: Duloxetine, olanzapine, and buprenorphine/naloxone were discontinuedgiven concern for serotonin syndrome. Although there are not strong data that buprenorphine/ naloxone can cause serotonin syndrome, the team discontinued the medication in case it might be contributing to the patient’s encephalopathy, while closely monitoring the patient for withdrawal. There was a rapid improvement in the patient’s symptoms over the 24 hours after discontinuation of the 3 medications.
As part of the initial workup, the patient received a computed tomography (CT) scan of his chest to follow up pulmonary nodules identified 16 months prior. The CT scan showed interval growth of the pulmonary nodules in the right lower lobe to 2 cm with extension into the major fissure, which was concerning for malignancy. Plans were made for an outpatient positron emission tomography (PET) scan after hospital discharge.
Dr. Schlechter and Dr. Rangachari, what factors can help us determine whether or not further workup of a malignancy should occur before discharge or can be deferred to the outpatient setting?
►Benjamin Schlechter, MD, Instructor in Medicine, BIDMC; and Deepa Rangachari, MD, Assistant Professor of Medicine, BIDMC: Key considerations in this domain include rapidity of growth and any threat to critical end-organ function (ie, brain, heart, lungs, kidney, and liver). If the malignancy is bulky and/or rapidly progressing to the point that the patient has significant symptoms burden and/or end-organ dysfunction, then initiating the evaluation as an inpatient may be necessary. For suspected intrathoracic malignancies, considering whether this may be a high-grade process (ie, small cell lung cancer) is often a vital branch point. Key considerations in this regard are the following: Is it a bulky central tumor? Is there evidence of widespread metastatic disease, an obstructing mass, and/or tumor lysis? One final and critical aspect to consider is whether there are any patient- specific barriers to timely and reliable outpatient follow-up. If there is no evidence of rapid progression, bulky disease with threatened end-organ involvement, and/or issues with timely and reliable follow-up, then outpatient evaluation is often the best approach to ensure a comprehensive and well-coordinated effort on the patient’s behalf.
►Dr. Reese: Buprenorphine/naloxone was restarted without return of the symptoms. The patient was discharged home with an outpatient PET scan scheduled the following week. Unfortunately, the patient was unable to keep this appointment. Three weeks after hospital discharge, the patient presented again to the emergency department with gradually worsening altered mental status, confusion, visual hallucinations, and myoclonic jerking of the arms and legs. Medication adherence was confirmed by the patient’s wife, resulting in a low concern for serotonin syndrome. Physical examination revealed confusion, dysarthria, diffuse, arrhythmic, myoclonic jerking in all extremities, asterixis in the upper extremities, and hyperreflexia.
A CT scan of the brain did not reveal an intracranial process. A spot electroencephalograph (EEG) and magnetic resonance image (MRI) of the brain were obtained. Dr. Weller, what is the utility of spot EEG vs 24-hour EEG? When might we choose one over the other?
►Dr. Weller: If a patient is persistently altered, then a spot EEG would be sufficient to capture a seizure if that is what is causing the patient’s altered mental status. However, if the patient’s mental status is waxing and waning, then that may warrant a 24-hour EEG because the patient may need to be monitored for longer periods to capture an event that is causing intermittent alterations in mental status.6 Additionally, patients who are acutely ill may require long-term monitoring for the purpose of treatment and outcome management.
►Dr. Reese: The spot EEG showed nearly continuous generalized slowing indicative of a diffuse encephalopathy. The MRI of the brain showed scattered, nonspecific periventricular T2 hyperintense foci, suggestive of advanced chronic microvascular ischemic changes.
A PET CT was obtained and revealed mildly fluorodeoxyglucose (FDG)-avid, enlarging nodules within the right lower lobe, which was suspicious for malignancy. There were no other areas of FDG avidity on the PET scan. Valproic acid was initiated for treatment of myoclonus with transition to clonazepam when no improvement was seen. After starting clonazepam, the patient’s condition stabilized.
Dr. Weller, given the additional history, how has your differential diagnosis changed?
►Dr. Weller: Given the patient’s laboratory findings, we can be quite sure that there is not a contributing metabolic process. The findings suggestive of metastatic cancer, along with the profound neurologic changes, are most concerning for a paraneoplastic syndrome. I would suggest biopsy and consideration of a lumbar puncture. One can also send serum markers, including a paraneoplastic antibody panel.
►Dr. Reese: Biopsy of the mass in his right lower lobe revealed squamous cell lung cancer. Dr. Schlechter and Dr. Rangachari, do you have a framework for the different forms of lung cancer?
►Dr. Schlechter/Dr. Rangachari: The 2 broad categories of lung cancer are small cell and non-small cell (NSCLC). Small cell lung cancer has a tight association with tobacco exposure and is often clinically defined by rapid, bulky progression (ie, weeks to months).7,8 NSCLCs are also commonly seen in those with tobacco exposure, though not always. The main subgroups in this category are adenocarcinoma and squamous cell carcinoma. These cancers often evolve at a slower pace (ie, months to years).8 While small cell lung cancers are highgrade tumors and exquisitely sensitive to chemotherapy and radiation, NSCLCs tend to be less responsive to such therapies. The staging evaluation for either entity is the same and consists of defining localized vs metastatic disease.
►Dr. Reese: Because this patient had an MRI and PET scan that were both negative for metastatic disease, can we assume that this patient had stage I NSCLC?
►Dr. Schlechter/Dr. Rangachari: Not necessarily. While PET and MRI brain are exceptionally helpful in detecting distant metastases, they may over- or underestimate intrathoracic lymph node involvement by as much as 20%.9 As such, dedicated lymph node staging—either via bronchoscopy (endobronchial ultrasound) or surgically (mediastinoscopy) is indicated as lymph node involvement can significantly alter the stage, prognosis, and optimal therapeutic approach.10,11
►Dr. Reese: After this diagnosis was made, the teams caring for this patient attributed his altered mental status to a paraneoplastic syndrome. What is a paraneoplastic syndrome, and how does a paraneoplastic syndrome from malignancy present? Does its presence worsen a patient’s prognosis?
►Dr. Schlechter/Dr. Rangachari: A paraneoplastic syndrome is defined by an immunologic response to the cancer that ends up erroneously targeting self-antigens. Paraneoplastic syndromes are associated with a broad array of clinical findings—from endocrinopathy to encephalopathy—and certain neoplasms are more commonly associated with these syndromes than others (eg, small cell lung cancer and thymoma). Further, severity and onset of a paraneoplastic syndrome does not correlate with the burden of visible disease—and the syndrome may predate the cancer diagnosis by months to years.11 While treatment of the cancer affords the best hope of resolving the paraneoplastic syndrome, the cancer and the paraneoplastic process may have a discordant trajectory, with the paraneoplastic syndrome persisting even after the cancer is maximally treated. Although one might assume that paraneoplastic syndromes portend worse outcomes, in some cases, a presentation with the paraneoplastic syndrome may afford sooner detection of an otherwise occult/asymptomatic malignancy.
►Dr. Reese: The following week, the serum paraneoplastic antibody panel that tested for anti-Yo antibody, anti-Ri antibody,and anti-Hu antibody came back negative. Dr. Weller, what does this mean? Since we have yet to obtain a lumbar puncture, might his symptoms still be caused by a paraneoplastic syndrome?
►Dr. Weller: The negative serum test just means that he does not have antibodies to those 3 antibodies. There are now over 30 different paraneoplastic antibodies that have been discovered, and there are always more that are being discovered. So this negative test result does not exclude a paraneoplastic syndrome in the appropriate clinical context.12 Furthermore, the sensitivity and specificity for certain antibodies are different based upon source fluid, and cerebrospinal fluid testing would provide more diagnostic clarity. A negative test for paraneoplastic syndrome, by itself, would similarly not exclude a paraneoplastic syndrome. Often, empiric treatment is the best diagnostic option for paraneoplastic and autoimmune encephalopathies.
►Dr. Reese: The following week, the patient was discharged to rehabilitation with clonazepam for his symptoms and a scheduled follow-up. Given the patient’s frailty and medical comorbidities, thoracic surgery recommended consultation with radiation oncology. Dr. Schlechter and Dr. Rangachari, when do we decide to use radiation vs chemotherapy for someone with lung cancer?
►Dr. Schlechter/Dr. Rangachari: Patients with early stage, nonmetastatic NSCLC may not always be candidates for surgical resection on the basis of pulmonary function, other medical comorbidities (as in this case), anatomic considerations, and/or patient preference. In these cases, if there is lung-limited disease without lymph node involvement (ie, stage I/II NSCLC) and the patient is not felt to be an operative candidate, then alternatives to surgery include either radiation or ablation.13,14 As we care for an aging and comorbid population, evolving evidence suggests that well-selected patients with early stage disease undergoing these nonoperative approaches have roughly equivalent outcomes to those undergoing conventional surgical resection.13 In such cases, multidisciplinary consultation with a team having dedicated expertise in these various operative and nonoperative modalities is essential.
►Dr. Reese: The patient followed up with radiation oncology for consideration of radiation treatment, but his simulation CT scan showed some ground-glass opacity that were concerning for inflammation vs infection. The patient’s case was discussed at the multidisciplinary tumor board, and it was determined to treat him with antibiotics for a possible pneumonia before proceeding with radiation therapy. After he completed antibiotic treatment, he underwent 10 fractions of radiation treatment, which he tolerated well.
1. Kojovic M, Cordivari C, Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4(1):47-62.
2. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
3. Arora B, Kannikeswaran N. The serotonin syndrome-the need for physician’s awareness. Int J Emerg Med. 2010;3(4):373-377.
4. Boyer EW, Shannon M. The serotonin syndrome [published correction appears in N Engl J Med. 2007;356(23):2437 and N Engl J Med. 2009;361(17):1714]. N Engl J Med.
2005;352(11):1112-1120.
5. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM.
2003;96(9):635-642.
6. Nordli DR Jr. Usefulness of video-EEG monitoring. Epilepsia. 2006;47(suppl 1):26-30.
7. Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24(28):4526-4527.
8. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-1260.
9. Cerfolio RJ, Bryant AS, Ojha B, Eloubeidi M. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg. 2005;80(4):1207-1214.
10. El-Osta H, Jani P, Mansour A, Rascoe P, Jafri S. Endobronchial ultrasound for nodal staging of patients with non-smallcell lung cancer with radiologically normal mediastinum. A meta-analysis. Ann Am Thorac Soc. 2018;15(7):864-874.
11. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543-1554.
12. McKeon A. Autoimmune Encephalopathies and Dementias. Continuum (Minneap Minn). 2016;22(2 Dementia): 538-558.
13. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Nonsmall cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
14. Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807-821.
1. Kojovic M, Cordivari C, Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4(1):47-62.
2. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
3. Arora B, Kannikeswaran N. The serotonin syndrome-the need for physician’s awareness. Int J Emerg Med. 2010;3(4):373-377.
4. Boyer EW, Shannon M. The serotonin syndrome [published correction appears in N Engl J Med. 2007;356(23):2437 and N Engl J Med. 2009;361(17):1714]. N Engl J Med.
2005;352(11):1112-1120.
5. Dunkley EJC, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM.
2003;96(9):635-642.
6. Nordli DR Jr. Usefulness of video-EEG monitoring. Epilepsia. 2006;47(suppl 1):26-30.
7. Ettinger DS, Aisner J. Changing face of small-cell lung cancer: real and artifact. J Clin Oncol. 2006;24(28):4526-4527.
8. Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10(9):1243-1260.
9. Cerfolio RJ, Bryant AS, Ojha B, Eloubeidi M. Improving the inaccuracies of clinical staging of patients with NSCLC: a prospective trial. Ann Thorac Surg. 2005;80(4):1207-1214.
10. El-Osta H, Jani P, Mansour A, Rascoe P, Jafri S. Endobronchial ultrasound for nodal staging of patients with non-smallcell lung cancer with radiologically normal mediastinum. A meta-analysis. Ann Am Thorac Soc. 2018;15(7):864-874.
11. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543-1554.
12. McKeon A. Autoimmune Encephalopathies and Dementias. Continuum (Minneap Minn). 2016;22(2 Dementia): 538-558.
13. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Nonsmall cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83(5):584-594.
14. Ettinger DS, Aisner DL, Wood DE, et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 5.2018. J Natl Compr Canc Netw. 2018;16(7):807-821.
Asthma: Newer Tx options mean more targeted therapy
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include
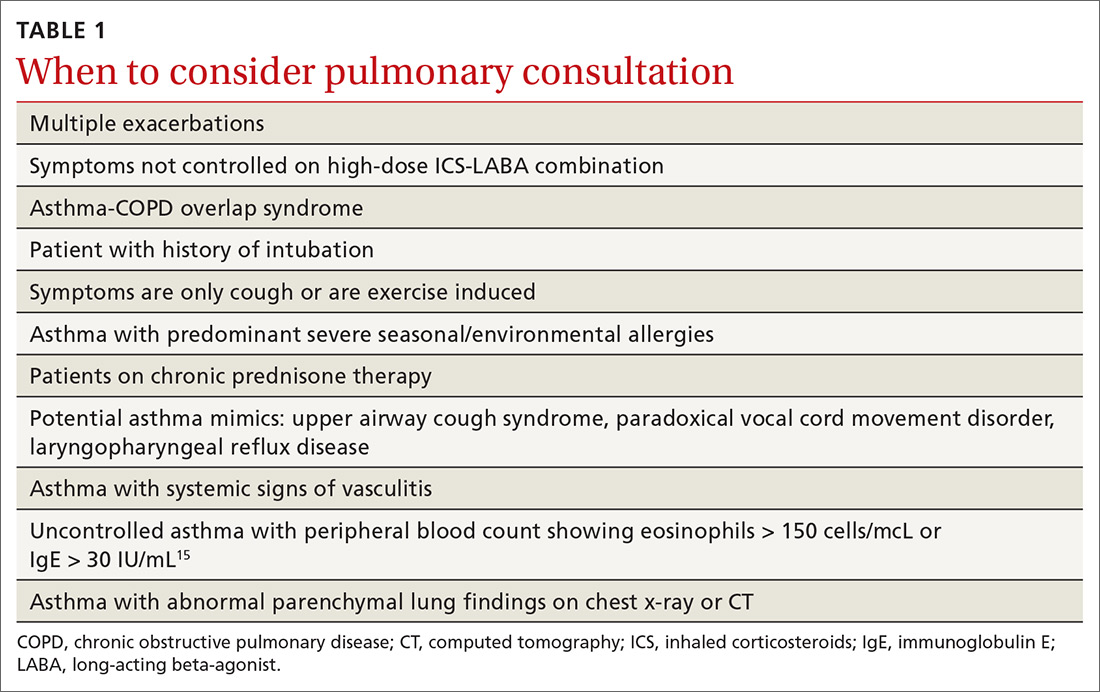
The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4
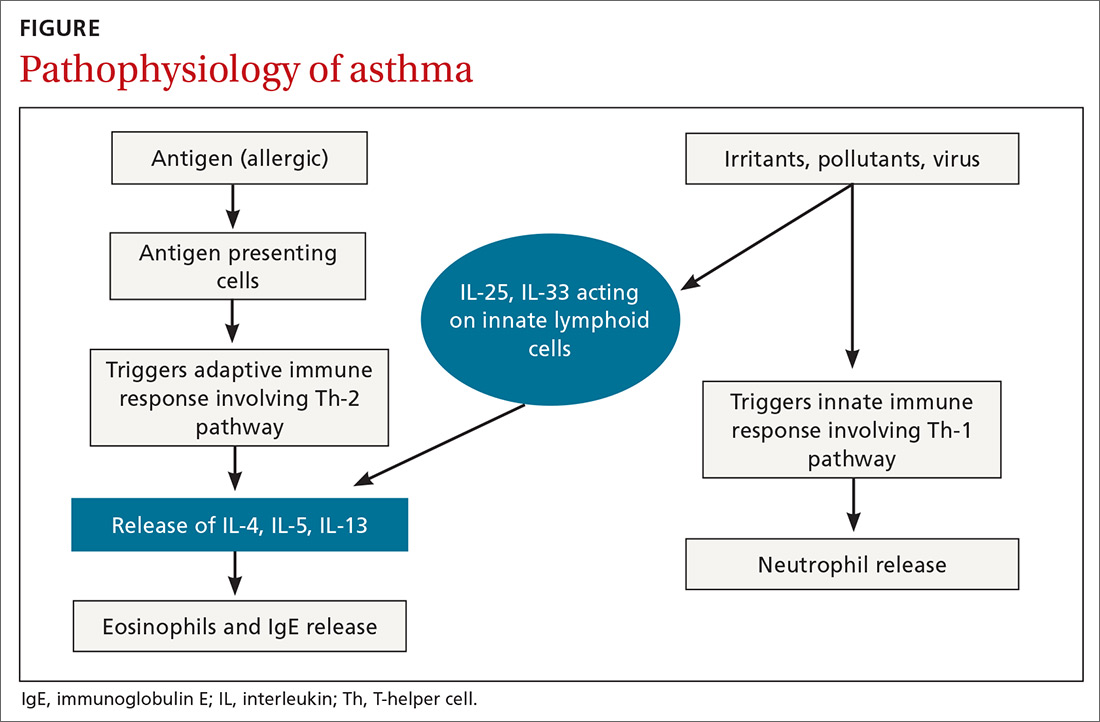
In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.
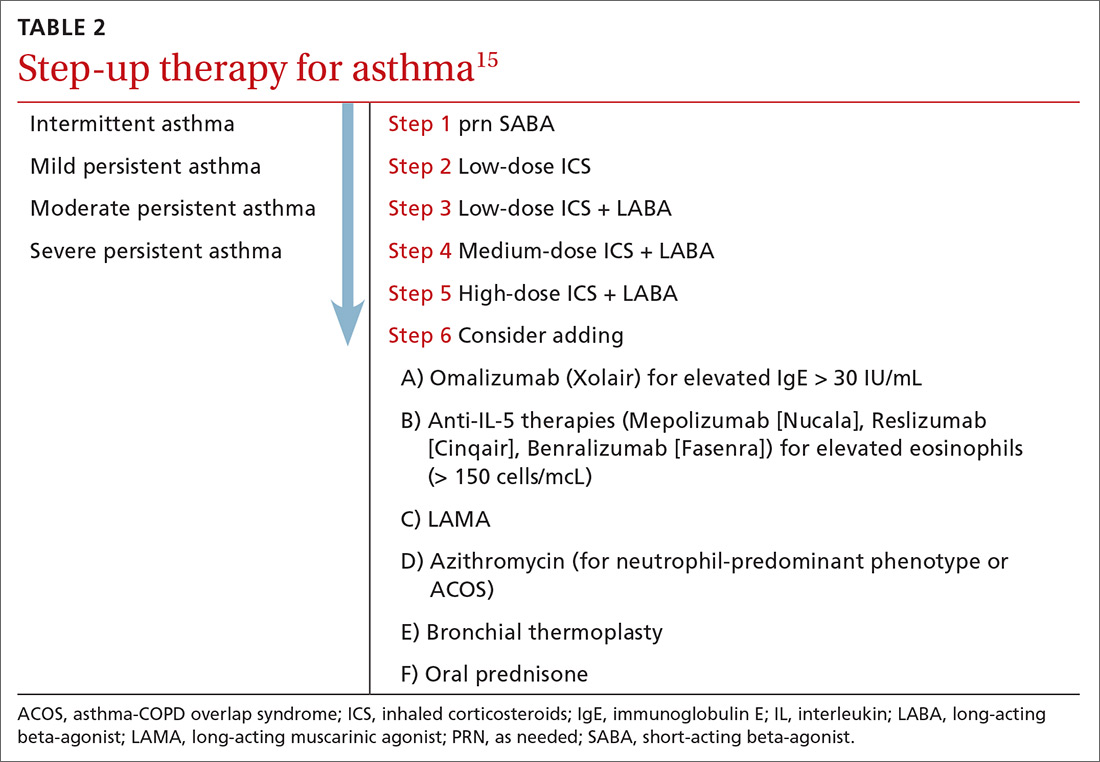
Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.
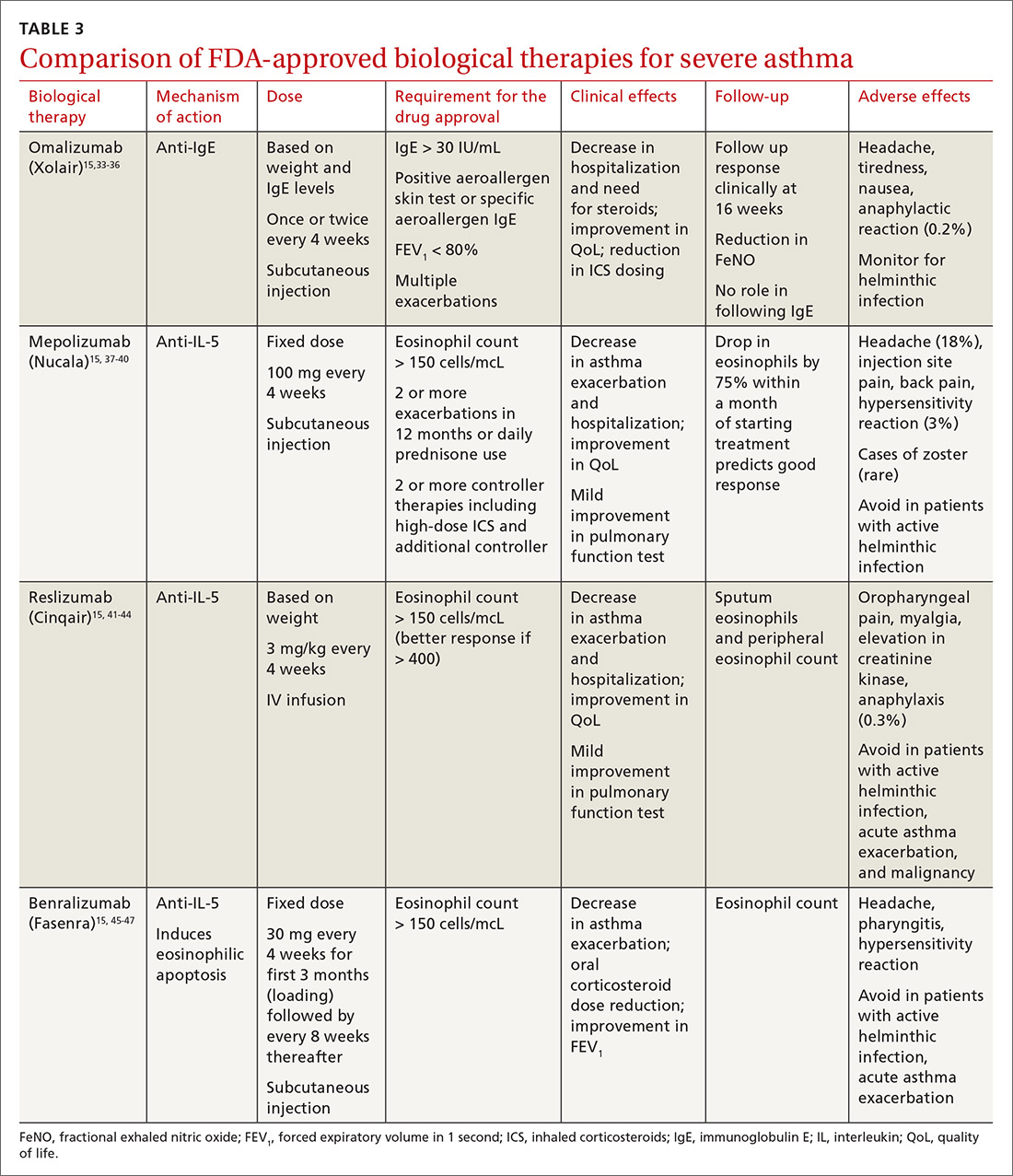
Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; [email protected]
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include

The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4

In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.

Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.

Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; [email protected]
Recent advances in our understanding of asthma pathophysiology have led to the development of new treatment approaches to this chronic respiratory condition, which affects 25 million Americans or nearly 8% of the population.1 As a result, asthma treatment options have expanded from just simple inhalers and corticosteroids to include

The pathophysiology of asthma provides key targets for therapy
There are 2 basic phenotypes of asthma—neutrophilic predominant and eosinophilic predominant—and 3 key components to its pathophysiology2:
Airway inflammation. Asthma is mediated through either a type 1 T-helper (Th-1) cell or a type 2 T-helper (Th-2) cell response, the pathways of which have a fair amount of overlap (FIGURE). In the neutrophilic-predominant phenotype, irritants, pollutants, and viruses trigger an innate Th-1 cell–mediated pathway that leads to subsequent neutrophil release. This asthma phenotype responds poorly to standard asthma therapy.2-4

In the eosinophilic-predominant phenotype, environmental allergic antigens induce a Th-2 cell–mediated response in the airways of patients with asthma.5-7 This creates a downstream effect on the release of interleukins (IL) including IL-4, IL-5, and IL-13. IL-4 triggers immunoglobulin (Ig) E release, which subsequently induces mast cells to release inflammatory cytokines, while IL-5 and IL-13 are responsible for eosinophilic response. These cytokines and eosinophils induce airway hyperresponsiveness, remodeling, and mucus production. Through repeated exposure, chronic inflammation develops and subsequently causes structural changes related to increased smooth muscle mass, goblet cell hyperplasia, and thickening of lamina reticularis.8,9 Understanding of this pathobiological pathway has led to the development of anti-IgE and anti-IL-5 drugs (to be discussed shortly).
Airway obstruction. Early asthmatic response is due to acute bronchoconstriction secondary to IgE; this is followed by airway edema occurring 6 to 24 hours after an acute event (called late asthmatic response). The obstruction is worsened by an overproduction of mucus, which may take weeks to resolve.10 Longstanding inflammation can lead to structural changes and reduced airflow reversibility.
Bronchial hyperresponsiveness is induced by various forms of allergens, pollutants, or viral upper respiratory infections. Sympathetic control in the airway is mediated via beta-2 adrenoceptors expressed on airway smooth muscle, which are responsible for the effect of bronchodilation in response to albuterol.11,12 Cholinergic pathways may further contribute to bronchial hyperresponsiveness and form the basis for the efficacy of anticholinergic therapy.12,13
What we’ve learned about asthma can inform treatment decisions
Presentation may vary, as asthma has many forms including cough-variant asthma and exercise-induced asthma. Airflow limitation is typically identified through spirometry and characterized by reduced (< 70% in adults) forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) or bronchodilator response positivity (an increase in post-bronchodilator FEV1 > 12% or FVC > 200 mL from baseline).2 If spirometry is not diagnostic but suspicion for asthma remains, bronchial provocation testing or exercise challenge testing may be needed.
Continue to: Additional diagnostic considerations...
Additional diagnostic considerations may impact the treatment plan for patients with asthma:
Asthma and COPD. A history of smoking is a key factor in the diagnosis of chronic obstructive pulmonary disease (COPD)—but many patients with asthma are also smokers. This subgroup may have asthma-COPD overlap syndrome (ACOS). It is important to determine whether these patients are asthma predominant or COPD predominant, because appropriate first-line treatment will differ. Patients who are COPD predominant demonstrate reduced diffusion capacity (DLCO) and abnormal PaCO2 on arterial blood gas. They also may show more structural damage on chest computed tomography (CT) than patients with asthma do. Asthma-predominant patients are more likely to have eosinophilia.14
Patients with severe persistent asthma or frequent exacerbations, or those receiving step-up therapy, may require additional serologic testing. Specialized testing for IgE and eosinophil count, as well as a sensitized allergy panel, may help clinicians in selecting specific biological therapies for treatment of severe asthma (further discussion to follow). We recommend using a serum allergy panel, as it is a quick and easy way to identify patients with extrinsic allergies, whereas skin-based testing is often time consuming and may require referral to a specialist.2,5,15
Aspergillus. An additional consideration is testing for Aspergillus antibodies. Aspergillus is a ubiquitous fungus found in the airways of humans. In patients with asthma, however, it can trigger an intense inflammatory response known as allergic bronchopulmonary aspergillosis. ABPA is not an infection. It should be considered in patients who have lived in a damp, old housing environment with possible mold exposure. Treatment of ABPA involves oral corticosteroids; there are varying reports of efficacy with voriconazole or itraconazole as suppressive therapy or steroid-sparing treatment.16-18
Getting a handle on an ever-expanding asthma Tx arsenal
The goals of asthma treatment are symptom control and risk minimization. Treatment choices are dictated in part by disease severity (mild, moderate, severe) and classification (intermittent, persistent). Asthma therapy is traditionally described as step-up and step-down; TABLE 2 summarizes available pharmacotherapy for asthma and provides a framework for add-on therapy as the disease advances.

Continue to: Over the past decade...
Over the past decade, a number of therapeutic options have been introduced or added to the pantheon of asthma treatment.
Inhaled medications
This category includes inhaled corticosteroids (ICS), which are recommended for use alone or in combination with long-acting beta-agonists (LABA) or with long-acting
ICS is the first choice for long-term control of persistent asthma.2 Its molecular effects include activating anti-inflammatory genes, switching off inflammatory genes, and inhibiting inflammatory cells, combined with enhancement of beta-2-adrenergic receptor expression. The cumulative effect is reduction in airway responsiveness in asthma patients.19-22
LABAs are next in line in the step-up, step-down model of symptom management. LABAs should not be prescribed as stand-alone therapy in patients with asthma, as they have received a black box warning from the US Food and Drug Administration (FDA) for an increase in asthma-related death23—a concern that has not been demonstrated with the combination of ICS-LABA.
LABAs cause smooth muscle relaxation in the lungs.24 There are 3 combination products currently available: once-daily fluticasone furoate/vilanterol (Breo), twice-daily fluticasone propionate/salmeterol (Advair), and twice-daily budesonide/formoterol (Symbicort).
Continue to: Once-daily fluticasone furoate/vilanterol...
Once-daily fluticasone furoate/vilanterol has been shown to improve mean FEV1.25 In a 24-week, open-label, multicenter randomized controlled trial to evaluate the efficacy and safety of all 3 combination ICS-LABAs, preliminary results indicated that—at least in a tightly controlled setting—once-daily fluticasone furoate/vilanterol provides asthma control similar to the twice-daily combinations and is well tolerated.26
Two ultra-long-acting (24-hour) LABAs, olodaterol (Striverdi Respimat) and indacaterol (Arcapta Neohaler), are being studied for possible use in asthma treatment. In a phase 2 trial investigating therapy for moderate-to-severe persistent asthma, 24-hour FEV1 improved with olodeaterol when compared to placebo.27
Another ongoing clinical trial is studying the effects of ultra-long-acting bronchodilator therapy (olodaterol vs combination olodaterol/tiotropium) in asthma patients who smoke and who are already using ICS (ClinicalTrials.gov NCT02682862). Indacaterol has been shown to be effective in the treatment of moderate-to-severe asthma in a once-a-day dosing regimen.28 However, when compared to mometasone alone, a combination of indacaterol and mometasone demonstrated no statistically significant reduction in time to serious exacerbation.29
The LAMA tiotropium is recommended as add-on therapy for patients whose asthma is uncontrolled despite use of low-dose ICS-LABA or as an alternative to high-dose ICS-LABA, per Global Initiative for Asthma (GINA) 2019 guidelines.15
Tiotropium induces bronchodilation by selectively inhibiting the action of acetylcholine at muscarinic (M) receptors in bronchial smooth muscles; it has a longer duration of action because of its slower dissociation from receptor types M1 and M3.30 Tiotropium respimat (Spiriva, Tiova) has been approved for COPD for many years; in 2013, it was shown to prevent worsening of symptomatic asthma and increase time to first severe exacerbation.13 The FDA subsequently approved tiotropium as an add-on treatment for patients with uncontrolled asthma despite use of ICS-LABA.
Continue to: Glycopyrronium bromide...
Glycopyrronium bromide (glycopyrrolate, multiple brand names) and umeclidinium (Incruse Ellipta) are LAMAs that are approved for COPD treatment but have not yet been approved for patients who have asthma only.31
Biological therapies
In the past few years, improved understanding of asthma’s pathophysiology has led to the development of biological therapy for severe asthma. This therapy is directed at Th-2 inflammatory pathways (FIGURE) and targets various inflammatory markers, such as IgE, IL-5, and eosinophils.
Biologicals are not the first-line therapy for the management of severe asthma. Ideal candidates for this therapy are patients who have exhausted other forms of severe asthma treatment, including ICS-LABA, LAMA, leukotriene receptor antagonists, and mucus-clearing agents. Patients with frequent exacerbations who need continuous steroids or need steroids at least twice a year should be considered for biologicals.32
All biological therapies must be administered in a clinical setting, as they carry risk for anaphylaxis. TABLE 315,33-47 summarizes all approved biologicals for the management of severe asthma.

Anti-IgE therapy. Omalizumab (Xolair) was the first approved biological therapy for severe asthma (in 2003). It is a recombinant humanized IgG1 monoclonal antibody that binds to free IgE and down regulates the inflammatory cascade. It is therefore best suited for patients with early-onset allergic asthma with a high IgE count. The dose and frequency (once or twice per month) of omalizumab are based on IgE levels and patient weight. Omalizumab reduces asthma exacerbation (up to 45%) and hospitalization (up to 85%).34 Omalizumab also reduces the need for high-dose ICS-LABA therapy and improves quality of life (QoL).33,34
Continue to: Its efficacy and safety...
Its efficacy and safety have been proven outside the clinical trial setting. Treatment response should be assessed over a 3- to 4-month period, using fractional exhalation of nitric oxide (FeNO); serial measurement of IgE levels is not recommended for this purpose. Once started, treatment should be considered long term, as discontinuation of treatment has been shown to lead to recurrence of symptoms and exacerbation.35,36 Of note, the GINA guidelines recommend omalizumab over prednisone as add-on therapy for severe persistent asthma.15
Anti-IL-5 therapy. IL-5 is the main cytokine for growth, differentiation, and activation of eosinophils in the Th-2-mediated inflammatory cascade. Mepolizumab, reslizumab, and benralizumab are 3 FDA-approved anti-IL-5 monoclonal antibody therapies for severe eosinophilic asthma. Mepolizumab has been the most commonly studied anti-IL-5 therapy, while benralizumab, the latest of the 3, has a unique property of inducing eosinophilic apoptosis. There has been no direct comparison of the different anti-IL-5 therapies.
Mepolizumab (Nucala) is a mouse anti-human monoclonal antibody that binds to IL-5 and prevents it from binding to IL-5 receptors on the eosinophil surface. Mepolizumab should be considered in patients with a peripheral eosinophil count > 150 cells/mcL; it has shown a trend of greater benefit in patients with a very high eosinophil count (75% reduction in exacerbation with blood eosinophil count > 500 cells/mcL compared to 56% exacerbation reduction with blood eosinophil count > 150 cells/mcL).37
Mepolizumab has consistently been shown to reduce asthma exacerbation (by about 50%) and emergency department (ED) visits and hospitalization (60%), when compared with placebo in clinical trials.37,38 It also reduces the need for oral corticosteroids, an effect sustained for up to 52 weeks.39,40 The Mepolizumab adjUnctive therapy in subjects with Severe eosinophiliC Asthma (MUSCA) study showed that mepolizumab was associated with significant improvement of health-related QoL, lung function, and asthma symptoms in patients with severe eosinophilic asthma.38
GINA guidelines recommend mepolizumab as an add-on therapy for severe asthma. Mepolizumab is given as a fixed dose of 100 mg every 4 weeks. A 300-mg dose has also been approved for eosinophilic granulomatosis with polyangiitis. Monitoring with serial eosinophils might be of value in determining the efficacy of the drug. Mepolizumab is currently in clinical trials for a broad spectrum of diseases, including COPD, hyper-eosinophilic syndrome, and ABPA.
Continue to: Reslizumab (Cinqair)...
Reslizumab (Cinqair) is a rat anti-human monoclonal antibody of the IgG4κ subtype that binds to a small region of IL-5 and subsequently blocks IL-5 from binding to the IL-5 receptor complex on the cell surface of eosinophils. It is currently approved for use as a 3-mg/kg IV infusion every 4 weeks. In large clinical trials,41-43 reslizumab decreased asthma exacerbation and improved QoL, asthma control, and lung function. Most of the study populations had an eosinophil count > 400 cells/mcL. A small study also suggested patients with severe eosinophilic asthma with prednisone dependency (10 mg/d) had better sputum eosinophilia suppression and asthma control with reslizumab when compared with mepolizumab.44
Benralizumab (Fasenra) is a humanized IgG1 anti-IL-5 receptor α monoclonal antibody derived from mice. It induces apoptosis of eosinophils and, to a lesser extent, of basophils.45 In clinical trials, it demonstrated a reduction in asthma exacerbation rate and improvement in prebronchodilator FEV1 and asthma symptoms.46,47 It does not need reconstitution, as the drug is dispensed as prefilled syringes with fixed non-weight-based dosing. Another potential advantage to benralizumab is that after the loading dose, subsequent doses are given every 8 weeks.
Bronchial thermoplasty
Bronchial thermoplasty (BT) is a novel nonpharmacologic intervention that entails the delivery of controlled radiofrequency-generated heat via a catheter inserted into the bronchial tree of the lungs through a flexible bronchoscope. The potential mechanism of action is reduction in airway smooth muscle mass and inflammatory markers.
Evidence for BT started with the Asthma Intervention Research (AIR) and Research in Severe Asthma (RISA) trials.48,49 In the AIR study, BT was shown to reduce the rate of mild exacerbations and improve morning peak expiratory flow and asthma scores at 12 months.48 In the RISA trial, BT resulted in improvements in Asthma Quality of Life Questionnaire (AQLQ) score and need for rescue medication at 52 weeks, as well as a trend toward decrease in steroid use.49
However, these studies were criticized for not having a placebo group—an issue addressed in the AIR2 trial, which compared bronchial thermoplasty with a sham procedure. AIR2 demonstrated improvements in AQLQ score and a 32% reduction in severe exacerbations and 84% fewer ED visits in the post-treatment period (up to 1 year post treatment).50
Continue to: Both treatment groups...
Both treatment groups experienced an increase in respiratory adverse events: during the treatment period (up to 6 weeks post procedure), 16 subjects (8.4%) in the BT group required 19 hospitalizations for respiratory symptoms and 2 subjects (2%) in the sham group required 2 hospitalizations. A follow-up observational study involving a cohort of AIR2 patients demonstrated long-lasting effects of BT in asthma exacerbation frequency, ED visits, and stabilization of FEV1 for up to 5 years.51
The Post-market Post-FDA Approval Clinical Trial Evaluating Bronchial Thermoplasty in Severe Persistent Asthma (PAS2) showed similar beneficial effects of BT on asthma control despite enrolling subjects who may have had poorer asthma control in the “real world” setting.52
In summary, BT results in modest improvements in AQLQ scores and clinically worthwhile reductions in severe exacerbations and ED visits in the year post treatment, which may persist for up to 5 years. BT causes short-term increases in asthma-related morbidity, including hospital admissions. While there is encouraging data and the scope is increasing, BT remains limited to carefully selected (by a specialist) patients with severe asthma that is poorly controlled despite maximal inhaled therapy.
Immunotherapy
Immunotherapy for allergic disease is aimed at inducing immune tolerance to an allergen and alleviating allergic symptoms. This is done by administration of the allergen to which the patient is sensitive. There are 2 approaches: subcutaneous immunotherapy (SCIT) and sublingual immunotherapy (SLIT; a dissolvable tablet under the tongue or an aqueous or liquid extract).
Immunotherapy is generally reserved for patients who have allergic symptoms with exposure to a trigger and evidence (through skin or serum testing) of specific IgE to that trigger, especially if there is poor response to pharmacotherapy and allergen avoidance. Overall, evidence in this field is limited: Most studies have included patients with mild asthma, and few studies have compared immunotherapy with pharmacologic therapy or used standardized outcomes, such as exacerbations.
Continue to: SCIT
SCIT. A 2010 Cochrane review concluded that SCIT reduces asthma symptoms and use of asthma medications and improves bronchial hyperreactivity. Adverse effects include uncommon anaphylactic reactions, which may be life-threatening.53
SLIT has advantages over SCIT as it can be administered by patients or caregivers, does not require injections, and carries a much lower risk for anaphylaxis. Modest benefits have been seen in adults and children, but there is concern about the design of many early studies.
A 2015 Cochrane review of SLIT in asthma recommended further research using validated scales and important outcomes for patients and decision makers so that SLIT can be properly assessed as a clinical treatment for asthma.54 A subsequently published study of SLIT for house dust mites (HDM) in patients with asthma and HDM allergic rhinitis demonstrated a modest reduction in use of ICS with high-dose SLIT.55
In another recent study, among adults with HDM allergy-related asthma not well controlled by ICS, the addition of HDM SLIT to maintenance medications improved time to first moderate-or-severe asthma exacerbation during ICS reduction.56 Additional studies are needed to assess long-term efficacy and safety. However, for patients who experience exacerbations despite use of a low-dose or medium-dose ICS-LABA combination, SLIT can now be considered as an add-on therapy.
Per the GINA guidelines, the potential benefits of allergen immunotherapy must be weighed against the risk for adverse effects, including anaphylaxis, and the inconvenience and cost of the prolonged course of therapy.15
Continue to: Azithromycin
Azithromycin
Macrolides have immunomodulatory and anti-inflammatory effects in addition to their antibacterial effects. Maintenance treatment with macrolides such as azithromycin has been proven to be effective in chronic neutrophilic airway diseases (FIGURE). There have been attempts to assess whether this therapy can be useful in asthma management, as well. Some randomized controlled trials and meta-analyses have shown conflicting results, and early studies were limited by lack of data, heterogeneous results, and inadequate study designs.
The AZithromycin Against pLacebo in Exacerbations of Asthma (AZALEA) study was a randomized, multicenter, double-blind, placebo-controlled clinical trial in the United Kingdom among patients requiring emergency care for acute asthma exacerbations. Azithromycin added to standard care for asthma attacks did not result in clinical benefit.57 While azithromycin in acute exacerbation is not currently recommended, recent trials in outpatient settings have shown promise.
The AZIthromycin in Severe ASThma study (AZISAST) was a randomized, double-blind, placebo-controlled trial in subjects with exacerbation-prone severe asthma in Belgium. Low-dose azithromycin (250 mg 3 times a week) as an add-on treatment to combination ICS-LABA therapy for 6 months did not reduce the rate of severe asthma exacerbations or lower respiratory tract infection (LRTI). However, subjects with a non-eosinophilic variant (neutrophilic phenotype) experienced significant reduction in the rate of exacerbation and LRTI.58
The recently published Asthma and Macrolides: the AZithromycin Efficacy and Safety Study (AMAZES) shows promise for chronic azithromycin therapy as an add-on to medium-to-high-dose inhaled steroids and a long-acting bronchodilator in adults with uncontrolled persistent asthma. This was a large multicenter, randomized, double-blind, placebo-controlled, parallel group trial in New Zealand and Australia. Patients were excluded if they had hearing impairment or abnormally prolonged QTc. Azithromycin at a dose of 500 mg 3 times a week for 48 months reduced asthma exacerbations and improved QoL compared to placebo. The effect was sustained between subgroups based on phenotypes (eosinophilic vs noneosinophilic; frequent exacerbators vs nonfrequent exacerbators) and even among those with symptom differences at baseline (eg, cough or sputum positivity). The rate of antibiotic courses for respiratory infectious episodes was significantly reduced in the azithromycin-treated group.59
The take-away: Chronic azithromycin might prove to be a useful agent in the long-term management of asthma patients whose disease is not well controlled on inhaled therapy. Further studies on mechanism and effects of prolonged antibiotic use will shed more light. For more information, see When guideline treatment of asthma fails, consider a macrolide antibiotic; http://bit.ly/2vDAWc6.
Continue to: A new era
A new era
We have entered an exciting era of asthma management, with the introduction of several novel modalities, such as biological therapy and bronchial thermoplasty, as well as use of known drugs such as macrolides, immunotherapy, and LAMA. This was made possible through a better understanding of the biological pathways of asthma. Asthma management has moved toward more personalized, targeted therapy based on asthma phenotypes.
It’s important to remember, however, that pharmacological and nonpharmacological aspects of management—including inhaler techniques, adherence to inhaler therapy, vaccinations, control of asthma triggers, and smoking cessation—remain the foundation of optimal asthma management and need to be aggressively addressed before embarking on advanced treatment options. Patients whose asthma is not well controlled with inhaled medications or who have frequent exacerbations (requiring use of steroids) should be comanaged by an expert asthma specialist to explore all possible therapies.
CORRESPONDENCE
Mayur Rali, MD, 995 Newbridge Road, Bellmore, NY 11710; [email protected]
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
1. Centers for Disease Control and Prevention. Most recent national asthma data. Updated May 2019. www.cdc.gov/asthma/most_recent_national_asthma_data.htm. Accessed March 6, 2020.
2. National Asthma Education and Prevention Program. Expert panel report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—summary report 2007. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma [published correction appears in Am J Respir Crit Care Med. 2009;180(8):796]. Am J Respir Crit Care Med. 2009;180:388–395.
4. Fahy JV. Type 2 inflammation in asthma—present in most, absent in many. Nat Rev Immunol. 2015;15:57–65.
5. Busse WW. Inflammation in asthma: the cornerstone of the disease and target of therapy. J Allergy Clin Immunol. 1998;102(4 pt 2):S17-S22.
6. Lane SJ, Lee TH. Mast cell effector mechanisms. J Allergy Clin Immunol. 1996;98(5 pt 2):S67-S71.
7. Robinson DS, Bentley AM, Hartnell A, et al. Activated memory T helper cells in bronchoalveolar lavage fluid from patients with atopic asthma: relation to asthma symptoms, lung function, and bronchial responsiveness. Thorax. 1993;48:26-32.
8. Grigoraş A, Grigoraş CC, Giuşcă SE, et al. Remodeling of basement membrane in patients with asthma. Rom J Morphol Embryol. 2016;57:115-119.
9. Huang SK, Xiao HQ, Kleine-Tebbe J, et al. IL-13 expression at the sites of allergen challenge in patients with asthma. J Immunol. 1995;155:2688-2694.
10. Hansbro PM, Starkey MR, Mattes J, et al. Pulmonary immunity during respiratory infections in early life and the development of severe asthma. Ann Am Thorac Soc. 2014;11 suppl 5:S297-S302.
11. Apter AJ, Reisine ST, Willard A, et al. The effect of inhaled albuterol in moderate to severe asthma. J Allergy Clin Immunol. 1996;98:295-301.
12. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
13. Kerstjens HA, O’Byrne PM. Tiotropium for the treatment of asthma: a drug safety evaluation. Expert Opin Drug Saf. 2016;15:1115-1124.
14. Global Initiative for Asthma. Diagnosis of diseases of chronic air flow limitation: asthma, COPD and asthma-COPD overlap syndrome (ACOS) 2014. https://ginasthma.org/wp-content/uploads/2019/11/GINA_GOLD_ACOS_2014-wms.pdf. Accessed March 12, 2020.
15. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. Updated 2019. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 12, 2020.
16. Khanbabaee G, Enayat J, Chavoshzadeh Z, et al. Serum level of specific IgG antibody for aspergillus and its association with severity of asthma in asthmatic children. Acta Microbiol Immunol Hung. 2012;59:43-50.
17. Agbetile J, Bourne M, Fairs A, et al. Effectiveness of voriconazole in the treatment of aspergillus fumigatus-associated asthma (EVITA3 study). J Allergy Clin Immunol. 2014;134:33-39.
18. Stevens DA, Schwartz HJ, Lee JY, et al. A randomized trial of itraconazole in allergic bronchopulmonary aspergillosis. N Engl J Med. 2000;342:756-762.
19. Barnes PJ. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011;163:29-43.
20. Oakley RH, Cidlowski JA. The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol. 2013;132:1033-1044.
21. Barnes PJ. Scientific rationale for inhaled combination therapy with long-acting beta2-agonists and corticosteroids. Eur Respir J. 2002;19:182-191.
22. Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma—an update. Br J Pharmacol. 2016;173:3405-3430.
23. Wijesinghe M, Perrin K, Harwood M, et al. The risk of asthma mortality with inhaled long acting beta-agonists. Postgrad Med J. 2008;84:467-472.
24. Cazzola M, Page CP, Rogliani P, et al. β2-agonist therapy in lung disease. Am J Respir Crit Care Med. 2013;187:690-696.
25. Bernstein DI, Bateman ED, Woodcock A, et al. Fluticasone furoate (FF)/vilanterol (100/25 mcg or 200/25 mcg) or FF (100 mcg) in persistent asthma. J Asthma. 2015;52:1073-1083.
26. Devillier P, Humbert M, Boye A, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respir Med. 2018;141:111-120.
27. Beeh KM, LaForce C, Gahlemann M, et al. Randomised, double-blind, placebo-controlled crossover study to investigate different dosing regimens of olodaterol delivered via Respimat(R) in patients with moderate to severe persistent asthma. Respir Res. 2015;16:87.
28. LaForce C, Alexander M, Deckelmann R, et al. Indacaterol provides sustained 24 h bronchodilation on once-daily dosing in asthma: a 7-day dose-ranging study. Allergy. 2008;63:103-111.
29. Beasley RW, Donohue JF, Mehta R, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open. 2015;5:e006131.
30. Aalbers R, Park HS. Positioning of long-acting muscarinic antagonists in the management of asthma. Allergy Asthma Immunol Res. 2017;9:386-393.
31. Lee LA, Briggs A, Edwards LD, et al. A randomized, three-period crossover study of umeclidinium as monotherapy in adult patients with asthma. Respir Med. 2015;109:63-73.
32. Israel E, Reddel HK. Severe and difficult-to-treat asthma in adults. N Engl J Med. 2017;377:965-976.
33. Normansell R, Walker S, Milan SJ, et al. Omalizumab for asthma in adults and children. Cochrane Database Syst Rev. 2014;(1):CD003559.
34. Hanania NA, Wenzel S, Rosen K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187:804-811.
35. Slavin RG, Ferioli C, Tannenbaum SJ, et al. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107-113.e3.
36. Ledford D, Busse W, Trzaskoma B, et al. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140:162-169.e2.
37. Ortega HG, Liu MC, Pavord ID, et al. Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med. 2014;371:1198-1207.
38. Chupp GL, Bradford ES, Albers FC, et al. Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med. 2017;5:390-400.
39. Lugogo N, Domingo C, Chanez P, et al. Long-term efficacy and safety of mepolizumab in patients with severe eosinophilic asthma: a multi-center, open-label, phase IIIb study. Clin Ther. 2016;38:2058-2070.e1.
40. Bel EH, Wenzel SE, Thompson PJ, et al. Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med. 2014;371:1189-1197.
41. Castro M, Zangrilli J, Wechsler ME. Corrections. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med. 2015;3:e15.
42. Bjermer L, Lemiere C, Maspero J, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest. 2016;150:789-798.
43. Corren J, Weinstein S, Janka L, et al. Phase 3 study of reslizumab in patients with poorly controlled asthma: Effects across a broad range of eosinophil counts. Chest. 2016;150:799-810.
44. Mukherjee M, Aleman Paramo F, Kjarsgaard M, et al. Weight-adjusted intravenous reslizumab in severe asthma with inadequate response to fixed-dose subcutaneous mepolizumab. Am J Respir Crit Care Med. 2018;197:38-46.
45. Kolbeck R, Kozhich A, Koike M, et al. MEDI-563, a humanized anti-IL-5 receptor alpha mAb with enhanced antibody-dependent cell-mediated cytotoxicity function. J Allergy Clin Immunol. 2010;125:1344-1353.e2.
46. Bleecker ER, FitzGerald JM, Chanez P, et al. Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet. 2016;388:2115-2127.
47. FitzGerald JM, Bleecker ER, Nair P, et al. Benralizumab, an anti-interleukin-5 receptor alpha monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;388:2128-2141.
48. Cox G, Thomson NC, Rubin AS, et al. Asthma control during the year after bronchial thermoplasty. N Engl J Med. 2007;356:1327-1337.
49. Pavord ID, Cox G, Thomson NC, et al. Safety and efficacy of bronchial thermoplasty in symptomatic, severe asthma. Am J Respir Crit Care Med. 2007;176:1185-1191.
50. Castro M, Rubin AS, Laviolette M, et al. Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med. 2010;181:116-124.
51. Wechsler ME, Laviolette M, Rubin AS, et al. Bronchial thermoplasty: Long-term safety and effectiveness in patients with severe persistent asthma. J Allergy Clin Immunol. 2013;132:1295-1302.
52. Chupp G, Laviolette M, Cohn L, et al. Long-term outcomes of bronchial thermoplasty in subjects with severe asthma: A comparison of 3-year follow-up results from two prospective multicentre studies. Eur Respir J. 2017;50:1700017.
53. Abramson MJ, Puy RM, Weiner JM. Injection allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2010;(8):CD001186.
54. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015;(8):CD011293.
55. Mosbech H, Deckelmann R, de Blay F, et al. Standardized quality (SQ) house dust mite sublingual immunotherapy tablet (ALK) reduces inhaled corticosteroid use while maintaining asthma control: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2014;134:568575.e7.
56. Virchow JC, Backer V, Kuna P, et al. Efficacy of a house dust mite sublingual allergen immunotherapy tablet in adults with allergic asthma: a randomized clinical trial. JAMA. 2016;315:1715-1725.
57. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma : the AZALEA randomized clinical trial. JAMA Intern Med. 2016;176:1630-1637.
58. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
59. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
PRACTICE RECOMMENDATIONS
› Consider inhaled corticosteroids (ICS) as your first choice for a long-term control agent to treat asthma; add a long-acting beta agonist (LABA) when needed. A
› Use long-acting muscarinic antagonists (LAMA) as add-on therapy for patients whose asthma is uncontrolled despite the use of low-dose ICS-LABA, or as an alternative to high-dose ICS-LABA. A
› Consider biological therapies for patients with asthma exacerbations that require steroids at least twice a year. B
› Use azithromycin as an add-on therapy to ICS-LABA for a select group of patients with uncontrolled persistent asthma (neutrophilic phenotype). C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Infected Bronchogenic Cyst With Left Atrial, Pulmonary Artery, and Esophageal Compression
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
COVID-19 linked to multiple cardiovascular presentations
It’s becoming clear that COVID-19 infection can involve the cardiovascular system in many different ways, and this has “evolving” potential implications for treatment, say a team of cardiologists on the frontlines of the COVID-19 battle in New York City.
In an article published online April 3 in Circulation, Justin Fried, MD, Division of Cardiology, Columbia University, New York City, and colleagues present four case studies of COVID-19 patients with various cardiovascular presentations.
Case 1 is a 64-year-old woman whose predominant symptoms on admission were cardiac in nature, including chest pain and ST elevation, but without fever, cough, or other symptoms suggestive of COVID-19.
“In patients presenting with what appears to be a typical cardiac syndrome, COVID-19 infection should be in the differential during the current pandemic, even in the absence of fever or cough,” the clinicians advise.
Case 2 is a 38-year-old man with cardiogenic shock and acute respiratory distress with profound hypoxia who was rescued with veno-arterial-venous extracorporeal membrane oxygenation (VV ECMO).
The initial presentation of this patient was more characteristic of severe COVID-19 disease, and cardiac involvement only became apparent after the initiation of ECMO, Fried and colleagues report.
Based on this case, they advise a “low threshold” to assess for cardiogenic shock in patients with acute systolic heart failure related to COVID-19. If inotropic support fails in these patients, intra-aortic balloon pump should be considered first for mechanical circulatory support because it requires the least maintenance from medical support staff.
In addition, in their experience, when a patient on VV ECMO develops superimposed cardiogenic shock, adding an arterial conduit at a relatively low blood flow rate may provide the necessary circulatory support without inducing left ventricular distension, they note.
“Our experience confirms that rescue of patients even with profound cardiogenic or mixed shock may be possible with temporary hemodynamic support at centers with availability of such devices,” Fried and colleagues report.
Case 3 is a 64-year-old woman with underlying cardiac disease who developed profound decompensation with COVID-19 infection.
This case demonstrates that the infection can cause decompensation of underlying heart failure and may lead to mixed shock, the clinicians say.
“Invasive hemodynamic monitoring, if feasible, may be helpful to manage the cardiac component of shock in such cases. Medications that prolong the QT interval are being considered for COVID-19 patients and may require closer monitoring in patients with underlying structural heart disease,” they note.
Case 4 is a 51-year-old man who underwent a heart transplant in 2007 and a kidney transplant in 2010. He had COVID-19 symptoms akin to those seen in nonimmunosuppressed patients with COVID-19.
The COVID-19 pandemic presents a “unique challenge” for solid organ transplant recipients, with only “limited” data on how to adjust immunosuppression during COVID-19 infection, Fried and colleagues say.
The pandemic also creates a challenge for the management of heart failure patients on the heart transplant wait list; the risks of delaying a transplant need to be balanced against the risks of donor infection and uncertainty regarding the impact of post-transplant immunosuppression protocols, they note.
As reported by Medscape Medical News, the American Heart Association has developed a COVID-19 patient registry to collect data on cardiovascular conditions and outcomes related to COVID-19 infection.
To participate in the registry, contact [email protected].
This article first appeared on Medscape.com.
It’s becoming clear that COVID-19 infection can involve the cardiovascular system in many different ways, and this has “evolving” potential implications for treatment, say a team of cardiologists on the frontlines of the COVID-19 battle in New York City.
In an article published online April 3 in Circulation, Justin Fried, MD, Division of Cardiology, Columbia University, New York City, and colleagues present four case studies of COVID-19 patients with various cardiovascular presentations.
Case 1 is a 64-year-old woman whose predominant symptoms on admission were cardiac in nature, including chest pain and ST elevation, but without fever, cough, or other symptoms suggestive of COVID-19.
“In patients presenting with what appears to be a typical cardiac syndrome, COVID-19 infection should be in the differential during the current pandemic, even in the absence of fever or cough,” the clinicians advise.
Case 2 is a 38-year-old man with cardiogenic shock and acute respiratory distress with profound hypoxia who was rescued with veno-arterial-venous extracorporeal membrane oxygenation (VV ECMO).
The initial presentation of this patient was more characteristic of severe COVID-19 disease, and cardiac involvement only became apparent after the initiation of ECMO, Fried and colleagues report.
Based on this case, they advise a “low threshold” to assess for cardiogenic shock in patients with acute systolic heart failure related to COVID-19. If inotropic support fails in these patients, intra-aortic balloon pump should be considered first for mechanical circulatory support because it requires the least maintenance from medical support staff.
In addition, in their experience, when a patient on VV ECMO develops superimposed cardiogenic shock, adding an arterial conduit at a relatively low blood flow rate may provide the necessary circulatory support without inducing left ventricular distension, they note.
“Our experience confirms that rescue of patients even with profound cardiogenic or mixed shock may be possible with temporary hemodynamic support at centers with availability of such devices,” Fried and colleagues report.
Case 3 is a 64-year-old woman with underlying cardiac disease who developed profound decompensation with COVID-19 infection.
This case demonstrates that the infection can cause decompensation of underlying heart failure and may lead to mixed shock, the clinicians say.
“Invasive hemodynamic monitoring, if feasible, may be helpful to manage the cardiac component of shock in such cases. Medications that prolong the QT interval are being considered for COVID-19 patients and may require closer monitoring in patients with underlying structural heart disease,” they note.
Case 4 is a 51-year-old man who underwent a heart transplant in 2007 and a kidney transplant in 2010. He had COVID-19 symptoms akin to those seen in nonimmunosuppressed patients with COVID-19.
The COVID-19 pandemic presents a “unique challenge” for solid organ transplant recipients, with only “limited” data on how to adjust immunosuppression during COVID-19 infection, Fried and colleagues say.
The pandemic also creates a challenge for the management of heart failure patients on the heart transplant wait list; the risks of delaying a transplant need to be balanced against the risks of donor infection and uncertainty regarding the impact of post-transplant immunosuppression protocols, they note.
As reported by Medscape Medical News, the American Heart Association has developed a COVID-19 patient registry to collect data on cardiovascular conditions and outcomes related to COVID-19 infection.
To participate in the registry, contact [email protected].
This article first appeared on Medscape.com.
It’s becoming clear that COVID-19 infection can involve the cardiovascular system in many different ways, and this has “evolving” potential implications for treatment, say a team of cardiologists on the frontlines of the COVID-19 battle in New York City.
In an article published online April 3 in Circulation, Justin Fried, MD, Division of Cardiology, Columbia University, New York City, and colleagues present four case studies of COVID-19 patients with various cardiovascular presentations.
Case 1 is a 64-year-old woman whose predominant symptoms on admission were cardiac in nature, including chest pain and ST elevation, but without fever, cough, or other symptoms suggestive of COVID-19.
“In patients presenting with what appears to be a typical cardiac syndrome, COVID-19 infection should be in the differential during the current pandemic, even in the absence of fever or cough,” the clinicians advise.
Case 2 is a 38-year-old man with cardiogenic shock and acute respiratory distress with profound hypoxia who was rescued with veno-arterial-venous extracorporeal membrane oxygenation (VV ECMO).
The initial presentation of this patient was more characteristic of severe COVID-19 disease, and cardiac involvement only became apparent after the initiation of ECMO, Fried and colleagues report.
Based on this case, they advise a “low threshold” to assess for cardiogenic shock in patients with acute systolic heart failure related to COVID-19. If inotropic support fails in these patients, intra-aortic balloon pump should be considered first for mechanical circulatory support because it requires the least maintenance from medical support staff.
In addition, in their experience, when a patient on VV ECMO develops superimposed cardiogenic shock, adding an arterial conduit at a relatively low blood flow rate may provide the necessary circulatory support without inducing left ventricular distension, they note.
“Our experience confirms that rescue of patients even with profound cardiogenic or mixed shock may be possible with temporary hemodynamic support at centers with availability of such devices,” Fried and colleagues report.
Case 3 is a 64-year-old woman with underlying cardiac disease who developed profound decompensation with COVID-19 infection.
This case demonstrates that the infection can cause decompensation of underlying heart failure and may lead to mixed shock, the clinicians say.
“Invasive hemodynamic monitoring, if feasible, may be helpful to manage the cardiac component of shock in such cases. Medications that prolong the QT interval are being considered for COVID-19 patients and may require closer monitoring in patients with underlying structural heart disease,” they note.
Case 4 is a 51-year-old man who underwent a heart transplant in 2007 and a kidney transplant in 2010. He had COVID-19 symptoms akin to those seen in nonimmunosuppressed patients with COVID-19.
The COVID-19 pandemic presents a “unique challenge” for solid organ transplant recipients, with only “limited” data on how to adjust immunosuppression during COVID-19 infection, Fried and colleagues say.
The pandemic also creates a challenge for the management of heart failure patients on the heart transplant wait list; the risks of delaying a transplant need to be balanced against the risks of donor infection and uncertainty regarding the impact of post-transplant immunosuppression protocols, they note.
As reported by Medscape Medical News, the American Heart Association has developed a COVID-19 patient registry to collect data on cardiovascular conditions and outcomes related to COVID-19 infection.
To participate in the registry, contact [email protected].
This article first appeared on Medscape.com.
Aerosolization of COVID-19 and Contamination Risks During Respiratory Treatments
Beyond asthma and chronic obstructive pulmonary disease (COPD), inhalation therapy is a mainstay in the management of bronchiectasis, cystic fibrosis, and pulmonary artery hypertension. Several US Food and Drug Administration off-label indications for inhalational medications include hypoxia secondary to acute respiratory distress syndrome (ARDS) and intraoperative and postoperative pulmonary hypertension during and following cardiac surgery, respectively.1-11 Therapeutic delivery of aerosols to the lung may be provided via nebulization, pressurized metered-dose inhalers (pMDI), and other devices (eg, dry powder inhalers, soft-mist inhalers, and smart inhalers).12 The most common aerosolized medications given in the clinical setting are bronchodilators.12
Product selection is often guided by practice guidelines (Table 1), consideration of the formulation’s advantages and disadvantages (Table 2), and/or formulary considerations. For example, current guidelines for COPD state that there is no evidence for superiority of nebulized bronchodilator therapy over handheld devices in patients who can use them properly.2 Due to equivalence, nebulized formulations are commonly used in hospitals, emergency departments (EDs) and ambulatory clinics based on the drug’s unit cost. In contrast, a pMDI is often more cost-effective for use in ambulatory patients who are administering multiple doses from the same canister.
The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommend droplet and contact precautions for all patients suspected or diagnosed with novel coronavirus-19 (COVID-19).13,14 Airborne precautions must be applied when performing aerosol-generating medical procedures (AGMPs), including but not limited to, open suctioning of the respiratory tract, intubation, bronchoscopy, and cardiopulmonary resuscitation (CPR). Data from the severe acute respiratory syndrome (SARS-CoV) epidemic suggest that nebulization of medication is also an AGMP.15-17
Institutions must ensure that their health care workers (HCWs) are wearing appropriate personal protective equipment (PPE) including gloves, long-sleeved gowns, eye protection, and fit-tested particulate respirators (N95 mask) for airborne procedures and are carefully discarding PPE after use.13,14 Due to severe shortages in available respirators in the US supply chain, the CDC has temporarily modified WHO recommendations. Face masks are now an acceptable alternative to protect HCWs from splashes and sprays from procedures not likely to generate aerosols and for cleaning of rooms, although there is no evidence to support this decision.
Internationally, HCWs are falling ill with COVID-19. Data from Italy and Spain show that about 9% to 13% of these countries’ cases are HCWs.18,19 Within the US, the Ohio health department reports approximately 16% of cases are HCWs.20 It is possible that 20% of frontline HCWs will become infected.21 Evolving laboratory research shows that COVID-19 remains viable in aerosols for up to 3 hours postaerosolization, thus making aerosol transmission plausible.22 Nebulizers convert liquids into aerosols and during dispersal may potentially cause secondary inhalation of fugitive emissions.23 Since interim CDC infection control guidance is to allow only essential personnel to enter the room of patients with COVID-19, many facilities will rely on their frontline nursing staff to clean and disinfect high-touch surfaces following routine care activities.24
Achieving adequate fomite disinfection following viral aerosolization may pose a significant problem for any patient receiving scheduled doses of nebulized medications. Additionally, for personnel who clean rooms following intermittent drug nebulization while wearing PPE that includes a face mask, protection from aerosolized virus may be inadequate. Subsequently, fugitive emissions from nebulized medications may potentially contribute to both nosocomial COVID-19 transmission and viral infections in the medical staff until proven otherwise by studies conducted outside of the laboratory. Prevention of infection in the medical staff is imperative since federal health care systems cannot sustain a significant loss of its workforce.
Recommendations
We recommend that health care systems stop business as usual and adopt public health recommendations issued by Canadian and Hong Kong health care authorities for the management of suspected or confirmed COVID-19 disease.25-28 We have further clarified and expanded on these interventions. During viral pandemics, prescribers and health care systems should:
- Deprescribe nebulized therapies on medical wards and intensive care units as an infection control measure. Also avoid use in any outpatient health care setting (eg, community-based clinics, EDs, triage).
- Avoid initiation of nebulized unproven therapies (eg, n-acetylcysteine, hypertonic saline).1
- Use alternative bronchodilator formulations as appropriate (eg, oral β-2 agonist, recognizing its slower onset) before prescribing nebulized agents to patients who are uncooperative or unable to follow directions needed to use a pMDI with a spacer or have experienced a prior poor response to a pMDI with spacer (eg, OptiChamber Diamond, Philips).25,27
- Limit nebulized drug utilization (eg, bronchodilators, epoprostenol) to patients who are on mechanical ventilation and will receive nebulized therapies via a closed system or to patients housed in negative pressure hospital rooms.22 Use a viral filter (eg, Salter Labs system) to decrease the spread of infection for those receiving epoprostenol via face mask.25
- Adjust procurement practices (eg, pharmacy, logistics) to address the transition from nebulized drugs to alternatives.
- Add a safety net to the drug-ordering process by restricting new orders for nebulized therapies to the prior authorization process.27 Apply the exclusion criterion of suspected or definite COVID-19.
- Add a safety net to environmental service practices. Nursing staff should track patients who received ≥ 1 nebulizations via open (before diagnosis) or closed systems so that staff wear suitable PPE to include a N-95 mask while cleaning the room.
Conclusions
To implement the aggressive infection control guidance promulgated here, we recommend collaboration with infection control, pharmacy service (eg, prior authorization team, clinical pharmacy team, and procurement team), respiratory therapy, pulmonary and other critical care physicians, EDs, CPR committee, and other stakeholders. When making significant transitions in clinical care during a viral pandemic, guidelines must be timely, use imperative wording, and consist of easily identifiable education and/or instructions for the affected frontline staff in order to change attitudes.29 Additionally, when transitioning from nebulized bronchodilators to pMDI, educational in-services should be provided to frontline staff to avoid misconceptions regarding pMDI treatment efficacy and patients’ ability to use their pMDI with spacer.30
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville.
1. Strickland SL, Rubin BK, Haas CF, Volsko TA, Drescher GS, O’Malley CA. AARC Clinical Practice Guideline: effectiveness of pharmacologic airway clearance therapies in hospitalized patients. Respir Care. 2015;60(7):1071-1077.
2. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2020 GOLD Report. https://goldcopd.org/gold-reports/. Accessed March 26, 2020.
3. Van Geffen WH, Douma WR, Slebos DJ, Kerstjens HAM. Bronchodilators delivered by nebulizer versus pMDI with spacer or DPI for exacerbations of COPD (Review). Cochrane Database Syst Rev. 2016;8:CD011826.
4. Global Initiative for Asthma. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 26, 2020.
5. Global Initiative for Asthma. Difficult-to-treat and severe asthma in adolescent and adult patients: diagnosis and management. https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf. Accessed March 26, 2020.
6. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulizers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
7. Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2015;7:CD010337.
8. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST Guideline and Expert Panel Report. CHEST. 2014;146(2):449-475.
9. Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Resp Res. 2019;6(1):e000420.
10. McGinn K, Reichert M. A comparison of inhaled nitric oxide versus inhaled epoprostenol for acute pulmonary hypertension following cardiac surgery. Ann Pharmacother. 2016;50(1):22-26.
11. Dzierba AL, Abel EE, Buckley MS, Lat I. A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or acute respiratory distress syndrome. Pharmacotherapy. 2014;34(3):279-290.
12. Pleasants RA, Hess DR. Aerosol delivery devices for obstructive lung diseases. Respir Care. 2018;63(6):708-733.
13. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Accessed March 26, 2020.
14. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Revised March 7, 2020. Accessed March 26, 2020.
15. Wong RSM, Hui DS. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10(2):339-341.
16. Wong T-W, Lee C-K, Tam W, et al; Outbreak Study Group. Emerg Infect Dis. 2004;10(2):269-276.
17. Seto WH, Tsang D, Yung RWH, et al; Advisors of Expert SARS group of Hospital Authority. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet. 2003;361(9368):1519-1520.
18. Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. https://jamanetwork.com/journals/jama/fullarticle/2763401?resultClick=1. Published March 17, 2020. Accessed March 26, 2020.
19. Jones S. Spain: doctors struggle to cope as 514 die from coronavirus in a day. The Guardian. March 24, 2020. https://www.theguardian.com/world/2020/mar/24/spain-doctors-lack-protection-coronavirus-covid-19. Accessed March 27, 2020.
20. 16% of Ohio’s diagnosed COVID-19 cases are healthcare workers. https://www.wlwt.com/article/16-of-ohio-s-diagnosed-covid-19-cases-are-healthcare-workers/31930566#. Updated March 25, 2020. Accessed March 27, 2020.
21. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30627-9/fulltext. Accessed March 27, 2020.
22. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as Compared with SARS-CoV-1 [published online ahead of print, 2020 Mar 17]. N Engl J Med. 2020;10.1056/NEJMc2004973.
23. McGrath JA, O’Sullivan A, Bennett G, et al. Investigation of the quantity of exhaled aerosol released into the environment during nebulization. Pharmaceutics. 2019;11(2):75.
24. Centers for Disease Control and Prevention. Healthcare Infection prevention and control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/infection-prevention-control-faq.html. Revised March 24, 2020. Accessed March 26, 2020.
25. Practice standards of respiratory procedures: post SARS era. Use of aerosolized medications. December 2003. http://www.hkresp.com/hkts.php?page=page/hkts/detail&meid=93742. Accessed March 26, 2020.
26. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anesth. 2020. [ePub ahead of print.]
27. Newhouse MT. RE: transmission of coronavirus by nebulizer- as serious, underappreciated risk! https://www.cmaj.ca/content/re-transmission-corona-virus-nebulizer-serious-underappreciated-risk. Accessed March 26, 2020. [ePub ahead of print.]
28. Moira C-Y. Severe acute respiratory syndrome (SARS) and healthcare workers. Int J Occup Environ Health. 2004;10(4):421-427.
29. Timen A, Hulscher MEJL, Rust L, et al. Barriers to implementing infection prevention and control guidelines during crises: experiences of health care professionals. Am J Infect Control. 2010;38(9):726-733.
30. Khoo SM, Tan LK, Said N, Lim TK. Metered-dose inhaler with spacer instead of nebulizer during the outbreak of severe acute respiratory syndrome in Singapore. Respir Care. 2009;54(7):855-860.
Beyond asthma and chronic obstructive pulmonary disease (COPD), inhalation therapy is a mainstay in the management of bronchiectasis, cystic fibrosis, and pulmonary artery hypertension. Several US Food and Drug Administration off-label indications for inhalational medications include hypoxia secondary to acute respiratory distress syndrome (ARDS) and intraoperative and postoperative pulmonary hypertension during and following cardiac surgery, respectively.1-11 Therapeutic delivery of aerosols to the lung may be provided via nebulization, pressurized metered-dose inhalers (pMDI), and other devices (eg, dry powder inhalers, soft-mist inhalers, and smart inhalers).12 The most common aerosolized medications given in the clinical setting are bronchodilators.12
Product selection is often guided by practice guidelines (Table 1), consideration of the formulation’s advantages and disadvantages (Table 2), and/or formulary considerations. For example, current guidelines for COPD state that there is no evidence for superiority of nebulized bronchodilator therapy over handheld devices in patients who can use them properly.2 Due to equivalence, nebulized formulations are commonly used in hospitals, emergency departments (EDs) and ambulatory clinics based on the drug’s unit cost. In contrast, a pMDI is often more cost-effective for use in ambulatory patients who are administering multiple doses from the same canister.
The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommend droplet and contact precautions for all patients suspected or diagnosed with novel coronavirus-19 (COVID-19).13,14 Airborne precautions must be applied when performing aerosol-generating medical procedures (AGMPs), including but not limited to, open suctioning of the respiratory tract, intubation, bronchoscopy, and cardiopulmonary resuscitation (CPR). Data from the severe acute respiratory syndrome (SARS-CoV) epidemic suggest that nebulization of medication is also an AGMP.15-17
Institutions must ensure that their health care workers (HCWs) are wearing appropriate personal protective equipment (PPE) including gloves, long-sleeved gowns, eye protection, and fit-tested particulate respirators (N95 mask) for airborne procedures and are carefully discarding PPE after use.13,14 Due to severe shortages in available respirators in the US supply chain, the CDC has temporarily modified WHO recommendations. Face masks are now an acceptable alternative to protect HCWs from splashes and sprays from procedures not likely to generate aerosols and for cleaning of rooms, although there is no evidence to support this decision.
Internationally, HCWs are falling ill with COVID-19. Data from Italy and Spain show that about 9% to 13% of these countries’ cases are HCWs.18,19 Within the US, the Ohio health department reports approximately 16% of cases are HCWs.20 It is possible that 20% of frontline HCWs will become infected.21 Evolving laboratory research shows that COVID-19 remains viable in aerosols for up to 3 hours postaerosolization, thus making aerosol transmission plausible.22 Nebulizers convert liquids into aerosols and during dispersal may potentially cause secondary inhalation of fugitive emissions.23 Since interim CDC infection control guidance is to allow only essential personnel to enter the room of patients with COVID-19, many facilities will rely on their frontline nursing staff to clean and disinfect high-touch surfaces following routine care activities.24
Achieving adequate fomite disinfection following viral aerosolization may pose a significant problem for any patient receiving scheduled doses of nebulized medications. Additionally, for personnel who clean rooms following intermittent drug nebulization while wearing PPE that includes a face mask, protection from aerosolized virus may be inadequate. Subsequently, fugitive emissions from nebulized medications may potentially contribute to both nosocomial COVID-19 transmission and viral infections in the medical staff until proven otherwise by studies conducted outside of the laboratory. Prevention of infection in the medical staff is imperative since federal health care systems cannot sustain a significant loss of its workforce.
Recommendations
We recommend that health care systems stop business as usual and adopt public health recommendations issued by Canadian and Hong Kong health care authorities for the management of suspected or confirmed COVID-19 disease.25-28 We have further clarified and expanded on these interventions. During viral pandemics, prescribers and health care systems should:
- Deprescribe nebulized therapies on medical wards and intensive care units as an infection control measure. Also avoid use in any outpatient health care setting (eg, community-based clinics, EDs, triage).
- Avoid initiation of nebulized unproven therapies (eg, n-acetylcysteine, hypertonic saline).1
- Use alternative bronchodilator formulations as appropriate (eg, oral β-2 agonist, recognizing its slower onset) before prescribing nebulized agents to patients who are uncooperative or unable to follow directions needed to use a pMDI with a spacer or have experienced a prior poor response to a pMDI with spacer (eg, OptiChamber Diamond, Philips).25,27
- Limit nebulized drug utilization (eg, bronchodilators, epoprostenol) to patients who are on mechanical ventilation and will receive nebulized therapies via a closed system or to patients housed in negative pressure hospital rooms.22 Use a viral filter (eg, Salter Labs system) to decrease the spread of infection for those receiving epoprostenol via face mask.25
- Adjust procurement practices (eg, pharmacy, logistics) to address the transition from nebulized drugs to alternatives.
- Add a safety net to the drug-ordering process by restricting new orders for nebulized therapies to the prior authorization process.27 Apply the exclusion criterion of suspected or definite COVID-19.
- Add a safety net to environmental service practices. Nursing staff should track patients who received ≥ 1 nebulizations via open (before diagnosis) or closed systems so that staff wear suitable PPE to include a N-95 mask while cleaning the room.
Conclusions
To implement the aggressive infection control guidance promulgated here, we recommend collaboration with infection control, pharmacy service (eg, prior authorization team, clinical pharmacy team, and procurement team), respiratory therapy, pulmonary and other critical care physicians, EDs, CPR committee, and other stakeholders. When making significant transitions in clinical care during a viral pandemic, guidelines must be timely, use imperative wording, and consist of easily identifiable education and/or instructions for the affected frontline staff in order to change attitudes.29 Additionally, when transitioning from nebulized bronchodilators to pMDI, educational in-services should be provided to frontline staff to avoid misconceptions regarding pMDI treatment efficacy and patients’ ability to use their pMDI with spacer.30
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville.
Beyond asthma and chronic obstructive pulmonary disease (COPD), inhalation therapy is a mainstay in the management of bronchiectasis, cystic fibrosis, and pulmonary artery hypertension. Several US Food and Drug Administration off-label indications for inhalational medications include hypoxia secondary to acute respiratory distress syndrome (ARDS) and intraoperative and postoperative pulmonary hypertension during and following cardiac surgery, respectively.1-11 Therapeutic delivery of aerosols to the lung may be provided via nebulization, pressurized metered-dose inhalers (pMDI), and other devices (eg, dry powder inhalers, soft-mist inhalers, and smart inhalers).12 The most common aerosolized medications given in the clinical setting are bronchodilators.12
Product selection is often guided by practice guidelines (Table 1), consideration of the formulation’s advantages and disadvantages (Table 2), and/or formulary considerations. For example, current guidelines for COPD state that there is no evidence for superiority of nebulized bronchodilator therapy over handheld devices in patients who can use them properly.2 Due to equivalence, nebulized formulations are commonly used in hospitals, emergency departments (EDs) and ambulatory clinics based on the drug’s unit cost. In contrast, a pMDI is often more cost-effective for use in ambulatory patients who are administering multiple doses from the same canister.
The World Health Organization (WHO) and the Centers for Disease Control and Prevention (CDC) recommend droplet and contact precautions for all patients suspected or diagnosed with novel coronavirus-19 (COVID-19).13,14 Airborne precautions must be applied when performing aerosol-generating medical procedures (AGMPs), including but not limited to, open suctioning of the respiratory tract, intubation, bronchoscopy, and cardiopulmonary resuscitation (CPR). Data from the severe acute respiratory syndrome (SARS-CoV) epidemic suggest that nebulization of medication is also an AGMP.15-17
Institutions must ensure that their health care workers (HCWs) are wearing appropriate personal protective equipment (PPE) including gloves, long-sleeved gowns, eye protection, and fit-tested particulate respirators (N95 mask) for airborne procedures and are carefully discarding PPE after use.13,14 Due to severe shortages in available respirators in the US supply chain, the CDC has temporarily modified WHO recommendations. Face masks are now an acceptable alternative to protect HCWs from splashes and sprays from procedures not likely to generate aerosols and for cleaning of rooms, although there is no evidence to support this decision.
Internationally, HCWs are falling ill with COVID-19. Data from Italy and Spain show that about 9% to 13% of these countries’ cases are HCWs.18,19 Within the US, the Ohio health department reports approximately 16% of cases are HCWs.20 It is possible that 20% of frontline HCWs will become infected.21 Evolving laboratory research shows that COVID-19 remains viable in aerosols for up to 3 hours postaerosolization, thus making aerosol transmission plausible.22 Nebulizers convert liquids into aerosols and during dispersal may potentially cause secondary inhalation of fugitive emissions.23 Since interim CDC infection control guidance is to allow only essential personnel to enter the room of patients with COVID-19, many facilities will rely on their frontline nursing staff to clean and disinfect high-touch surfaces following routine care activities.24
Achieving adequate fomite disinfection following viral aerosolization may pose a significant problem for any patient receiving scheduled doses of nebulized medications. Additionally, for personnel who clean rooms following intermittent drug nebulization while wearing PPE that includes a face mask, protection from aerosolized virus may be inadequate. Subsequently, fugitive emissions from nebulized medications may potentially contribute to both nosocomial COVID-19 transmission and viral infections in the medical staff until proven otherwise by studies conducted outside of the laboratory. Prevention of infection in the medical staff is imperative since federal health care systems cannot sustain a significant loss of its workforce.
Recommendations
We recommend that health care systems stop business as usual and adopt public health recommendations issued by Canadian and Hong Kong health care authorities for the management of suspected or confirmed COVID-19 disease.25-28 We have further clarified and expanded on these interventions. During viral pandemics, prescribers and health care systems should:
- Deprescribe nebulized therapies on medical wards and intensive care units as an infection control measure. Also avoid use in any outpatient health care setting (eg, community-based clinics, EDs, triage).
- Avoid initiation of nebulized unproven therapies (eg, n-acetylcysteine, hypertonic saline).1
- Use alternative bronchodilator formulations as appropriate (eg, oral β-2 agonist, recognizing its slower onset) before prescribing nebulized agents to patients who are uncooperative or unable to follow directions needed to use a pMDI with a spacer or have experienced a prior poor response to a pMDI with spacer (eg, OptiChamber Diamond, Philips).25,27
- Limit nebulized drug utilization (eg, bronchodilators, epoprostenol) to patients who are on mechanical ventilation and will receive nebulized therapies via a closed system or to patients housed in negative pressure hospital rooms.22 Use a viral filter (eg, Salter Labs system) to decrease the spread of infection for those receiving epoprostenol via face mask.25
- Adjust procurement practices (eg, pharmacy, logistics) to address the transition from nebulized drugs to alternatives.
- Add a safety net to the drug-ordering process by restricting new orders for nebulized therapies to the prior authorization process.27 Apply the exclusion criterion of suspected or definite COVID-19.
- Add a safety net to environmental service practices. Nursing staff should track patients who received ≥ 1 nebulizations via open (before diagnosis) or closed systems so that staff wear suitable PPE to include a N-95 mask while cleaning the room.
Conclusions
To implement the aggressive infection control guidance promulgated here, we recommend collaboration with infection control, pharmacy service (eg, prior authorization team, clinical pharmacy team, and procurement team), respiratory therapy, pulmonary and other critical care physicians, EDs, CPR committee, and other stakeholders. When making significant transitions in clinical care during a viral pandemic, guidelines must be timely, use imperative wording, and consist of easily identifiable education and/or instructions for the affected frontline staff in order to change attitudes.29 Additionally, when transitioning from nebulized bronchodilators to pMDI, educational in-services should be provided to frontline staff to avoid misconceptions regarding pMDI treatment efficacy and patients’ ability to use their pMDI with spacer.30
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville.
1. Strickland SL, Rubin BK, Haas CF, Volsko TA, Drescher GS, O’Malley CA. AARC Clinical Practice Guideline: effectiveness of pharmacologic airway clearance therapies in hospitalized patients. Respir Care. 2015;60(7):1071-1077.
2. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2020 GOLD Report. https://goldcopd.org/gold-reports/. Accessed March 26, 2020.
3. Van Geffen WH, Douma WR, Slebos DJ, Kerstjens HAM. Bronchodilators delivered by nebulizer versus pMDI with spacer or DPI for exacerbations of COPD (Review). Cochrane Database Syst Rev. 2016;8:CD011826.
4. Global Initiative for Asthma. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 26, 2020.
5. Global Initiative for Asthma. Difficult-to-treat and severe asthma in adolescent and adult patients: diagnosis and management. https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf. Accessed March 26, 2020.
6. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulizers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
7. Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2015;7:CD010337.
8. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST Guideline and Expert Panel Report. CHEST. 2014;146(2):449-475.
9. Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Resp Res. 2019;6(1):e000420.
10. McGinn K, Reichert M. A comparison of inhaled nitric oxide versus inhaled epoprostenol for acute pulmonary hypertension following cardiac surgery. Ann Pharmacother. 2016;50(1):22-26.
11. Dzierba AL, Abel EE, Buckley MS, Lat I. A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or acute respiratory distress syndrome. Pharmacotherapy. 2014;34(3):279-290.
12. Pleasants RA, Hess DR. Aerosol delivery devices for obstructive lung diseases. Respir Care. 2018;63(6):708-733.
13. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Accessed March 26, 2020.
14. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Revised March 7, 2020. Accessed March 26, 2020.
15. Wong RSM, Hui DS. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10(2):339-341.
16. Wong T-W, Lee C-K, Tam W, et al; Outbreak Study Group. Emerg Infect Dis. 2004;10(2):269-276.
17. Seto WH, Tsang D, Yung RWH, et al; Advisors of Expert SARS group of Hospital Authority. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet. 2003;361(9368):1519-1520.
18. Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. https://jamanetwork.com/journals/jama/fullarticle/2763401?resultClick=1. Published March 17, 2020. Accessed March 26, 2020.
19. Jones S. Spain: doctors struggle to cope as 514 die from coronavirus in a day. The Guardian. March 24, 2020. https://www.theguardian.com/world/2020/mar/24/spain-doctors-lack-protection-coronavirus-covid-19. Accessed March 27, 2020.
20. 16% of Ohio’s diagnosed COVID-19 cases are healthcare workers. https://www.wlwt.com/article/16-of-ohio-s-diagnosed-covid-19-cases-are-healthcare-workers/31930566#. Updated March 25, 2020. Accessed March 27, 2020.
21. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30627-9/fulltext. Accessed March 27, 2020.
22. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as Compared with SARS-CoV-1 [published online ahead of print, 2020 Mar 17]. N Engl J Med. 2020;10.1056/NEJMc2004973.
23. McGrath JA, O’Sullivan A, Bennett G, et al. Investigation of the quantity of exhaled aerosol released into the environment during nebulization. Pharmaceutics. 2019;11(2):75.
24. Centers for Disease Control and Prevention. Healthcare Infection prevention and control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/infection-prevention-control-faq.html. Revised March 24, 2020. Accessed March 26, 2020.
25. Practice standards of respiratory procedures: post SARS era. Use of aerosolized medications. December 2003. http://www.hkresp.com/hkts.php?page=page/hkts/detail&meid=93742. Accessed March 26, 2020.
26. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anesth. 2020. [ePub ahead of print.]
27. Newhouse MT. RE: transmission of coronavirus by nebulizer- as serious, underappreciated risk! https://www.cmaj.ca/content/re-transmission-corona-virus-nebulizer-serious-underappreciated-risk. Accessed March 26, 2020. [ePub ahead of print.]
28. Moira C-Y. Severe acute respiratory syndrome (SARS) and healthcare workers. Int J Occup Environ Health. 2004;10(4):421-427.
29. Timen A, Hulscher MEJL, Rust L, et al. Barriers to implementing infection prevention and control guidelines during crises: experiences of health care professionals. Am J Infect Control. 2010;38(9):726-733.
30. Khoo SM, Tan LK, Said N, Lim TK. Metered-dose inhaler with spacer instead of nebulizer during the outbreak of severe acute respiratory syndrome in Singapore. Respir Care. 2009;54(7):855-860.
1. Strickland SL, Rubin BK, Haas CF, Volsko TA, Drescher GS, O’Malley CA. AARC Clinical Practice Guideline: effectiveness of pharmacologic airway clearance therapies in hospitalized patients. Respir Care. 2015;60(7):1071-1077.
2. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2020 GOLD Report. https://goldcopd.org/gold-reports/. Accessed March 26, 2020.
3. Van Geffen WH, Douma WR, Slebos DJ, Kerstjens HAM. Bronchodilators delivered by nebulizer versus pMDI with spacer or DPI for exacerbations of COPD (Review). Cochrane Database Syst Rev. 2016;8:CD011826.
4. Global Initiative for Asthma. https://ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019-wms.pdf. Accessed March 26, 2020.
5. Global Initiative for Asthma. Difficult-to-treat and severe asthma in adolescent and adult patients: diagnosis and management. https://ginasthma.org/wp-content/uploads/2019/04/GINA-Severe-asthma-Pocket-Guide-v2.0-wms-1.pdf. Accessed March 26, 2020.
6. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulizers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
7. Welsh EJ, Evans DJ, Fowler SJ, Spencer S. Interventions for bronchiectasis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2015;7:CD010337.
8. Taichman DB, Ornelas J, Chung L, et al. Pharmacologic therapy for pulmonary arterial hypertension in adults: CHEST Guideline and Expert Panel Report. CHEST. 2014;146(2):449-475.
9. Griffiths MJD, McAuley DF, Perkins GD, et al. Guidelines on the management of acute respiratory distress syndrome. BMJ Open Resp Res. 2019;6(1):e000420.
10. McGinn K, Reichert M. A comparison of inhaled nitric oxide versus inhaled epoprostenol for acute pulmonary hypertension following cardiac surgery. Ann Pharmacother. 2016;50(1):22-26.
11. Dzierba AL, Abel EE, Buckley MS, Lat I. A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or acute respiratory distress syndrome. Pharmacotherapy. 2014;34(3):279-290.
12. Pleasants RA, Hess DR. Aerosol delivery devices for obstructive lung diseases. Respir Care. 2018;63(6):708-733.
13. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (nCoV) infection is suspected. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected Accessed March 26, 2020.
14. Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Revised March 7, 2020. Accessed March 26, 2020.
15. Wong RSM, Hui DS. Index patient and SARS outbreak in Hong Kong. Emerg Infect Dis. 2004;10(2):339-341.
16. Wong T-W, Lee C-K, Tam W, et al; Outbreak Study Group. Emerg Infect Dis. 2004;10(2):269-276.
17. Seto WH, Tsang D, Yung RWH, et al; Advisors of Expert SARS group of Hospital Authority. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS). Lancet. 2003;361(9368):1519-1520.
18. Livingston E, Bucher K. Coronavirus Disease 2019 (COVID-19) in Italy. https://jamanetwork.com/journals/jama/fullarticle/2763401?resultClick=1. Published March 17, 2020. Accessed March 26, 2020.
19. Jones S. Spain: doctors struggle to cope as 514 die from coronavirus in a day. The Guardian. March 24, 2020. https://www.theguardian.com/world/2020/mar/24/spain-doctors-lack-protection-coronavirus-covid-19. Accessed March 27, 2020.
20. 16% of Ohio’s diagnosed COVID-19 cases are healthcare workers. https://www.wlwt.com/article/16-of-ohio-s-diagnosed-covid-19-cases-are-healthcare-workers/31930566#. Updated March 25, 2020. Accessed March 27, 2020.
21. Remuzzi A, Remuzzi G. COVID-19 and Italy: what next? Lancet. http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30627-9/fulltext. Accessed March 27, 2020.
22. van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as Compared with SARS-CoV-1 [published online ahead of print, 2020 Mar 17]. N Engl J Med. 2020;10.1056/NEJMc2004973.
23. McGrath JA, O’Sullivan A, Bennett G, et al. Investigation of the quantity of exhaled aerosol released into the environment during nebulization. Pharmaceutics. 2019;11(2):75.
24. Centers for Disease Control and Prevention. Healthcare Infection prevention and control FAQs for COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/infection-control/infection-prevention-control-faq.html. Revised March 24, 2020. Accessed March 26, 2020.
25. Practice standards of respiratory procedures: post SARS era. Use of aerosolized medications. December 2003. http://www.hkresp.com/hkts.php?page=page/hkts/detail&meid=93742. Accessed March 26, 2020.
26. Wax RS, Christian MD. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anesth. 2020. [ePub ahead of print.]
27. Newhouse MT. RE: transmission of coronavirus by nebulizer- as serious, underappreciated risk! https://www.cmaj.ca/content/re-transmission-corona-virus-nebulizer-serious-underappreciated-risk. Accessed March 26, 2020. [ePub ahead of print.]
28. Moira C-Y. Severe acute respiratory syndrome (SARS) and healthcare workers. Int J Occup Environ Health. 2004;10(4):421-427.
29. Timen A, Hulscher MEJL, Rust L, et al. Barriers to implementing infection prevention and control guidelines during crises: experiences of health care professionals. Am J Infect Control. 2010;38(9):726-733.
30. Khoo SM, Tan LK, Said N, Lim TK. Metered-dose inhaler with spacer instead of nebulizer during the outbreak of severe acute respiratory syndrome in Singapore. Respir Care. 2009;54(7):855-860.
AAP issues guidance on managing infants born to mothers with COVID-19
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.
“Pediatric cases of COVID-19 are so far reported as less severe than disease occurring among older individuals,” Karen M. Puopolo, MD, PhD, a neonatologist and chief of the section on newborn pediatrics at Pennsylvania Hospital, Philadelphia, and coauthors wrote in the 18-page document, which was released on April 2, 2020, along with an abbreviated “Frequently Asked Questions” summary. However, one study of children with COVID-19 in China found that 12% of confirmed cases occurred among 731 infants aged less than 1 year; 24% of those 86 infants “suffered severe or critical illness” (Pediatrics. 2020 March. doi: 10.1542/peds.2020-0702). There were no deaths reported among these infants. Other case reports have documented COVID-19 in children aged as young as 2 days.
The document, which was assembled by members of the AAP Committee on Fetus and Newborn, Section on Neonatal Perinatal Medicine, and Committee on Infectious Diseases, pointed out that “considerable uncertainty” exists about the possibility for vertical transmission of SARS-CoV-2 from infected pregnant women to their newborns. “Evidence-based guidelines for managing antenatal, intrapartum, and neonatal care around COVID-19 would require an understanding of whether the virus can be transmitted transplacentally; a determination of which maternal body fluids may be infectious; and data of adequate statistical power that describe which maternal, intrapartum, and neonatal factors influence perinatal transmission,” according to the document. “In the midst of the pandemic these data do not exist, with only limited information currently available to address these issues.”
Based on the best available evidence, the guidance authors recommend that clinicians temporarily separate newborns from affected mothers to minimize the risk of postnatal infant infection from maternal respiratory secretions. “Newborns should be bathed as soon as reasonably possible after birth to remove virus potentially present on skin surfaces,” they wrote. “Clinical staff should use airborne, droplet, and contact precautions until newborn virologic status is known to be negative by SARS-CoV-2 [polymerase chain reaction] testing.”
While SARS-CoV-2 has not been detected in breast milk to date, the authors noted that mothers with COVID-19 can express breast milk to be fed to their infants by uninfected caregivers until specific maternal criteria are met. In addition, infants born to mothers with COVID-19 should be tested for SARS-CoV-2 at 24 hours and, if still in the birth facility, at 48 hours after birth. Centers with limited resources for testing may make individual risk/benefit decisions regarding testing.
For infants infected with SARS-CoV-2 but have no symptoms of the disease, they “may be discharged home on a case-by-case basis with appropriate precautions and plans for frequent outpatient follow-up contacts (either by phone, telemedicine, or in office) through 14 days after birth,” according to the document.
If both infant and mother are discharged from the hospital and the mother still has COVID-19 symptoms, she should maintain at least 6 feet of distance from the baby; if she is in closer proximity she should use a mask and hand hygiene. The mother can stop such precautions until she is afebrile without the use of antipyretics for at least 72 hours, and it is at least 7 days since her symptoms first occurred.
In cases where infants require ongoing neonatal intensive care, mothers infected with COVID-19 should not visit their newborn until she is afebrile without the use of antipyretics for at least 72 hours, her respiratory symptoms are improved, and she has negative results of a molecular assay for detection of SARS-CoV-2 from at least two consecutive nasopharyngeal swab specimens collected at least 24 hours apart.