User login
Esophageal motility issues may promote respiratory disease
Individuals with esophageal dysmotility had significantly higher scores on measures of airway reflux symptoms, based on data from 441 patients.
Many patients with chronic respiratory diseases experience persistent symptoms despite optimal treatment, and the reason is often unclear and frustrating for clinicians and patients, Dominic L. Sykes, MD, of Hull (England) University Teaching Hospitals NHS Trust, and colleagues wrote.
Although more studies in recent years have explored the association between gastroesophageal reflux and respiratory diseases such as asthma and chronic obstructive pulmonary disease, data on a potential link between esophageal motility and respiratory disease in adults are limited, they noted.
In a study published in Respiratory Medicine, the researchers reviewed data from 441 adults with refractory respiratory symptoms who were treated at a single center between Jan. 1, 2011, and Dec. 1, 2021. Symptoms included persistent cough and breathlessness despite optimal medication. The participants underwent examination with high-resolution esophageal manometry (HROM). Airway reflux was measured using the Hull Airways Reflux Questionnaire (HARQ). The mean age of the patients was 56.5 years, and 64% were women.
Overall, the most common diagnoses were chronic cough (77%), asthma (10%), and interstitial lung disease (7%). The prevalence of esophageal dysmotility was 66%. Patients with esophageal dysmotility had significantly higher HARQ scores than those with normal motility (40.6 vs. 35.3; P < .001). Approximately one-third of the patients had normal motility (34.5%) on HROM, 54% had ineffective esophageal motility, 7.3% had absent contractility, 3.2% had esophageal-gastric junction outflow obstruction, 0.5% had distal esophageal spasm, 0.5% has achalasia, and one patient had hypercontractile esophagus.
No significant differences in manometric diagnoses appeared between men and women. In addition, HARQ scores showed a significant inverse correlation with esophageal contractility as measured by distal contractile integral (DCI).
“The proportion of patients with esophageal dysmotility is consistently high over a range of respiratory diseases, including interstitial lung disease (72%), airways disease (57%), and chronic cough (68%),” and the findings suggest that esophageal disease may play a role in patients with persistent respiratory symptoms, they noted.
The study authors proposed that “impaired peristaltic activity of the esophagus, leading to aspiration of gaseous nonacidic refluxate into the airways, may be a contributor in the development and progression of respiratory disease.” They added that the HARQ offers clinicians a useful screening tool for assessing the need for esophageal study in patients with persistent respiratory symptoms that should be used before considering antireflux surgery.
The study findings were limited by several factors including the lack of lung function data for patients with airway disease and ILD and the inability to show causality between esophageal dysmotility and refractory respiratory symptoms, the researchers noted. Other limitations include the retrospective design, and the lack of data on symptom severity and the subsequent impact on outcomes.
However, the results support the need for additional research into the relationship between esophageal dysmotility, lung function, and symptom burden in chronic respiratory disease, and may inform investigations of therapeutic targets, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Individuals with esophageal dysmotility had significantly higher scores on measures of airway reflux symptoms, based on data from 441 patients.
Many patients with chronic respiratory diseases experience persistent symptoms despite optimal treatment, and the reason is often unclear and frustrating for clinicians and patients, Dominic L. Sykes, MD, of Hull (England) University Teaching Hospitals NHS Trust, and colleagues wrote.
Although more studies in recent years have explored the association between gastroesophageal reflux and respiratory diseases such as asthma and chronic obstructive pulmonary disease, data on a potential link between esophageal motility and respiratory disease in adults are limited, they noted.
In a study published in Respiratory Medicine, the researchers reviewed data from 441 adults with refractory respiratory symptoms who were treated at a single center between Jan. 1, 2011, and Dec. 1, 2021. Symptoms included persistent cough and breathlessness despite optimal medication. The participants underwent examination with high-resolution esophageal manometry (HROM). Airway reflux was measured using the Hull Airways Reflux Questionnaire (HARQ). The mean age of the patients was 56.5 years, and 64% were women.
Overall, the most common diagnoses were chronic cough (77%), asthma (10%), and interstitial lung disease (7%). The prevalence of esophageal dysmotility was 66%. Patients with esophageal dysmotility had significantly higher HARQ scores than those with normal motility (40.6 vs. 35.3; P < .001). Approximately one-third of the patients had normal motility (34.5%) on HROM, 54% had ineffective esophageal motility, 7.3% had absent contractility, 3.2% had esophageal-gastric junction outflow obstruction, 0.5% had distal esophageal spasm, 0.5% has achalasia, and one patient had hypercontractile esophagus.
No significant differences in manometric diagnoses appeared between men and women. In addition, HARQ scores showed a significant inverse correlation with esophageal contractility as measured by distal contractile integral (DCI).
“The proportion of patients with esophageal dysmotility is consistently high over a range of respiratory diseases, including interstitial lung disease (72%), airways disease (57%), and chronic cough (68%),” and the findings suggest that esophageal disease may play a role in patients with persistent respiratory symptoms, they noted.
The study authors proposed that “impaired peristaltic activity of the esophagus, leading to aspiration of gaseous nonacidic refluxate into the airways, may be a contributor in the development and progression of respiratory disease.” They added that the HARQ offers clinicians a useful screening tool for assessing the need for esophageal study in patients with persistent respiratory symptoms that should be used before considering antireflux surgery.
The study findings were limited by several factors including the lack of lung function data for patients with airway disease and ILD and the inability to show causality between esophageal dysmotility and refractory respiratory symptoms, the researchers noted. Other limitations include the retrospective design, and the lack of data on symptom severity and the subsequent impact on outcomes.
However, the results support the need for additional research into the relationship between esophageal dysmotility, lung function, and symptom burden in chronic respiratory disease, and may inform investigations of therapeutic targets, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Individuals with esophageal dysmotility had significantly higher scores on measures of airway reflux symptoms, based on data from 441 patients.
Many patients with chronic respiratory diseases experience persistent symptoms despite optimal treatment, and the reason is often unclear and frustrating for clinicians and patients, Dominic L. Sykes, MD, of Hull (England) University Teaching Hospitals NHS Trust, and colleagues wrote.
Although more studies in recent years have explored the association between gastroesophageal reflux and respiratory diseases such as asthma and chronic obstructive pulmonary disease, data on a potential link between esophageal motility and respiratory disease in adults are limited, they noted.
In a study published in Respiratory Medicine, the researchers reviewed data from 441 adults with refractory respiratory symptoms who were treated at a single center between Jan. 1, 2011, and Dec. 1, 2021. Symptoms included persistent cough and breathlessness despite optimal medication. The participants underwent examination with high-resolution esophageal manometry (HROM). Airway reflux was measured using the Hull Airways Reflux Questionnaire (HARQ). The mean age of the patients was 56.5 years, and 64% were women.
Overall, the most common diagnoses were chronic cough (77%), asthma (10%), and interstitial lung disease (7%). The prevalence of esophageal dysmotility was 66%. Patients with esophageal dysmotility had significantly higher HARQ scores than those with normal motility (40.6 vs. 35.3; P < .001). Approximately one-third of the patients had normal motility (34.5%) on HROM, 54% had ineffective esophageal motility, 7.3% had absent contractility, 3.2% had esophageal-gastric junction outflow obstruction, 0.5% had distal esophageal spasm, 0.5% has achalasia, and one patient had hypercontractile esophagus.
No significant differences in manometric diagnoses appeared between men and women. In addition, HARQ scores showed a significant inverse correlation with esophageal contractility as measured by distal contractile integral (DCI).
“The proportion of patients with esophageal dysmotility is consistently high over a range of respiratory diseases, including interstitial lung disease (72%), airways disease (57%), and chronic cough (68%),” and the findings suggest that esophageal disease may play a role in patients with persistent respiratory symptoms, they noted.
The study authors proposed that “impaired peristaltic activity of the esophagus, leading to aspiration of gaseous nonacidic refluxate into the airways, may be a contributor in the development and progression of respiratory disease.” They added that the HARQ offers clinicians a useful screening tool for assessing the need for esophageal study in patients with persistent respiratory symptoms that should be used before considering antireflux surgery.
The study findings were limited by several factors including the lack of lung function data for patients with airway disease and ILD and the inability to show causality between esophageal dysmotility and refractory respiratory symptoms, the researchers noted. Other limitations include the retrospective design, and the lack of data on symptom severity and the subsequent impact on outcomes.
However, the results support the need for additional research into the relationship between esophageal dysmotility, lung function, and symptom burden in chronic respiratory disease, and may inform investigations of therapeutic targets, they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM RESPIRATORY MEDICINE
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Lung volume reduction methods show similar results for emphysema
BARCELONA – For patients with emphysema who are suitable candidates for lung volume reduction surgery, in a randomized trial.
Among patients with emphysema amenable to surgery, there were similar improvements between the treatment groups at 12-month follow-up as assessed by the iBODE score, a composite disease severity measure incorporating body mass index, airflow obstruction, dyspnea, and exercise capacity (incremental shuttle walk test), reported Sara Buttery, BSc, a research physiotherapist and PhD candidate at the National Heart and Lung Institute at Imperial College London.
“Until now there had been no direct comparison of the two to inform decision-making when a person seems to be suitable for either. Bronchoscopic lung volume reduction is a less invasive option and is thought to be ‘less risky’ but, until now, there has not been substantial research to support this,” she said at the annual congress of the European Respiratory Society.
Ms. Buttery and colleagues conducted a randomized, controlled, single-blinded superiority trial to see whether LVRS could be superior to BLVR with valves. They enrolled 88 patients (52% male) with a mean age of 64, and randomly assigned them to receive either LVRS (41 patients) or the less-invasive BLVR (47 patients).
As noted before, there were no significant differences in outcomes at 1 year, with similar degrees of improvement between the surgical techniques for both the composite iBODE score (–1.10 for LVRS vs. –0.82 for BLVR, nonsignificant), and for the individual components of the score.
In addition, the treatments were associated with similar reductions in gas trapping, with residual volume percentage predicted –36.1 with LVRS versus –30.5 with BLVR (nonsignificant).
One patient in each group died during the 12 months of follow-up. The death of the patient in the BLVR group was deemed to be treatment related; the death of the patient in the LVRS group was related to a noninfective exacerbation of chronic obstructive pulmonary disease.
Invited discussant Isabelle Opitz, MD, from University Hospital Zürich told Ms. Buttery: “I have to congratulate you for this very first randomized controlled trial comparing both procedures in a superiority design.”
She pointed out, however, that the number of patients lost to follow-up and crossover of some patients randomized to bronchoscopy raised questions about the powering of the study.
“We did a sensitivity analysis to have a look to see if there was any difference between the patients who did return and the ones who didn’t, and there was no difference at baseline between those patients.” Ms. Buttery said.
She noted that follow-up visits were hampered by the COVID-19 pandemic and the inability of many patients to come into the clinic.
Dr. Opitz also asked about COPD Assessment Test (CAT) scores that were included in the trial design but not reported in the presentation. Ms. Buttery said that the CAT results favored the LVRS group, and that the results would be included in a future economic analysis.
“The results from this first randomized controlled trial suggest that BLVR may be a good therapeutic option for those patients for whom either procedure is suitable,” said Alexander Mathioudakis, MD, PhD, from the University of Manchester (England), who was not involved with this study but commented on it in a press statement. “Lung volume reduction surgery is an invasive operation as it requires a small incision to be made in the chest, which is stitched up after the procedure. As such, it has risks associated with surgery and it takes longer to recover from than bronchoscopic lung volume reduction. On the other hand, endobronchial valves placement is also associated with side effects, such as pneumonia, or valve displacement. Therefore, both the safety and effectiveness of the two procedures need to be investigated further, in larger groups of patients, but the results from this trial are very encouraging.”
The study is supported by the U.K. National Institute of Health Research. Ms. Buttery, Dr. Opitz, and Dr. Mathioudakis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BARCELONA – For patients with emphysema who are suitable candidates for lung volume reduction surgery, in a randomized trial.
Among patients with emphysema amenable to surgery, there were similar improvements between the treatment groups at 12-month follow-up as assessed by the iBODE score, a composite disease severity measure incorporating body mass index, airflow obstruction, dyspnea, and exercise capacity (incremental shuttle walk test), reported Sara Buttery, BSc, a research physiotherapist and PhD candidate at the National Heart and Lung Institute at Imperial College London.
“Until now there had been no direct comparison of the two to inform decision-making when a person seems to be suitable for either. Bronchoscopic lung volume reduction is a less invasive option and is thought to be ‘less risky’ but, until now, there has not been substantial research to support this,” she said at the annual congress of the European Respiratory Society.
Ms. Buttery and colleagues conducted a randomized, controlled, single-blinded superiority trial to see whether LVRS could be superior to BLVR with valves. They enrolled 88 patients (52% male) with a mean age of 64, and randomly assigned them to receive either LVRS (41 patients) or the less-invasive BLVR (47 patients).
As noted before, there were no significant differences in outcomes at 1 year, with similar degrees of improvement between the surgical techniques for both the composite iBODE score (–1.10 for LVRS vs. –0.82 for BLVR, nonsignificant), and for the individual components of the score.
In addition, the treatments were associated with similar reductions in gas trapping, with residual volume percentage predicted –36.1 with LVRS versus –30.5 with BLVR (nonsignificant).
One patient in each group died during the 12 months of follow-up. The death of the patient in the BLVR group was deemed to be treatment related; the death of the patient in the LVRS group was related to a noninfective exacerbation of chronic obstructive pulmonary disease.
Invited discussant Isabelle Opitz, MD, from University Hospital Zürich told Ms. Buttery: “I have to congratulate you for this very first randomized controlled trial comparing both procedures in a superiority design.”
She pointed out, however, that the number of patients lost to follow-up and crossover of some patients randomized to bronchoscopy raised questions about the powering of the study.
“We did a sensitivity analysis to have a look to see if there was any difference between the patients who did return and the ones who didn’t, and there was no difference at baseline between those patients.” Ms. Buttery said.
She noted that follow-up visits were hampered by the COVID-19 pandemic and the inability of many patients to come into the clinic.
Dr. Opitz also asked about COPD Assessment Test (CAT) scores that were included in the trial design but not reported in the presentation. Ms. Buttery said that the CAT results favored the LVRS group, and that the results would be included in a future economic analysis.
“The results from this first randomized controlled trial suggest that BLVR may be a good therapeutic option for those patients for whom either procedure is suitable,” said Alexander Mathioudakis, MD, PhD, from the University of Manchester (England), who was not involved with this study but commented on it in a press statement. “Lung volume reduction surgery is an invasive operation as it requires a small incision to be made in the chest, which is stitched up after the procedure. As such, it has risks associated with surgery and it takes longer to recover from than bronchoscopic lung volume reduction. On the other hand, endobronchial valves placement is also associated with side effects, such as pneumonia, or valve displacement. Therefore, both the safety and effectiveness of the two procedures need to be investigated further, in larger groups of patients, but the results from this trial are very encouraging.”
The study is supported by the U.K. National Institute of Health Research. Ms. Buttery, Dr. Opitz, and Dr. Mathioudakis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
BARCELONA – For patients with emphysema who are suitable candidates for lung volume reduction surgery, in a randomized trial.
Among patients with emphysema amenable to surgery, there were similar improvements between the treatment groups at 12-month follow-up as assessed by the iBODE score, a composite disease severity measure incorporating body mass index, airflow obstruction, dyspnea, and exercise capacity (incremental shuttle walk test), reported Sara Buttery, BSc, a research physiotherapist and PhD candidate at the National Heart and Lung Institute at Imperial College London.
“Until now there had been no direct comparison of the two to inform decision-making when a person seems to be suitable for either. Bronchoscopic lung volume reduction is a less invasive option and is thought to be ‘less risky’ but, until now, there has not been substantial research to support this,” she said at the annual congress of the European Respiratory Society.
Ms. Buttery and colleagues conducted a randomized, controlled, single-blinded superiority trial to see whether LVRS could be superior to BLVR with valves. They enrolled 88 patients (52% male) with a mean age of 64, and randomly assigned them to receive either LVRS (41 patients) or the less-invasive BLVR (47 patients).
As noted before, there were no significant differences in outcomes at 1 year, with similar degrees of improvement between the surgical techniques for both the composite iBODE score (–1.10 for LVRS vs. –0.82 for BLVR, nonsignificant), and for the individual components of the score.
In addition, the treatments were associated with similar reductions in gas trapping, with residual volume percentage predicted –36.1 with LVRS versus –30.5 with BLVR (nonsignificant).
One patient in each group died during the 12 months of follow-up. The death of the patient in the BLVR group was deemed to be treatment related; the death of the patient in the LVRS group was related to a noninfective exacerbation of chronic obstructive pulmonary disease.
Invited discussant Isabelle Opitz, MD, from University Hospital Zürich told Ms. Buttery: “I have to congratulate you for this very first randomized controlled trial comparing both procedures in a superiority design.”
She pointed out, however, that the number of patients lost to follow-up and crossover of some patients randomized to bronchoscopy raised questions about the powering of the study.
“We did a sensitivity analysis to have a look to see if there was any difference between the patients who did return and the ones who didn’t, and there was no difference at baseline between those patients.” Ms. Buttery said.
She noted that follow-up visits were hampered by the COVID-19 pandemic and the inability of many patients to come into the clinic.
Dr. Opitz also asked about COPD Assessment Test (CAT) scores that were included in the trial design but not reported in the presentation. Ms. Buttery said that the CAT results favored the LVRS group, and that the results would be included in a future economic analysis.
“The results from this first randomized controlled trial suggest that BLVR may be a good therapeutic option for those patients for whom either procedure is suitable,” said Alexander Mathioudakis, MD, PhD, from the University of Manchester (England), who was not involved with this study but commented on it in a press statement. “Lung volume reduction surgery is an invasive operation as it requires a small incision to be made in the chest, which is stitched up after the procedure. As such, it has risks associated with surgery and it takes longer to recover from than bronchoscopic lung volume reduction. On the other hand, endobronchial valves placement is also associated with side effects, such as pneumonia, or valve displacement. Therefore, both the safety and effectiveness of the two procedures need to be investigated further, in larger groups of patients, but the results from this trial are very encouraging.”
The study is supported by the U.K. National Institute of Health Research. Ms. Buttery, Dr. Opitz, and Dr. Mathioudakis reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ERS 2022 CONGRESS
CDC warns of enterovirus strain linked to polio-like condition
, according to a Health Network Alert advisory by the Centers for Disease Control and Prevention.
In August, health care providers and hospitals notified the CDC of an increase in severe respiratory illness in children who also tested positive for rhinovirus (RV) or enterovirus (EV). Additional testing revealed that some children were positive for EV-D68, which primarily causes acute respiratory illness. However, the virus has been associated with acute flaccid myelitis (AFM), a rare neurologic condition involving muscle weakness.
Also, in July and August 2022, surveillance networks reported an increase in EV-D68 activity compared with the same months in 2019, 2020, and 2021, the agency said in the alert. As of Aug. 30, the CDC has not received any reports of AFM beginning this year; however, spikes in EV-D68 typically come before cases of AFM, they said.
“Something we are always on the lookout for in the late summer and fall is AFM cases,” said Rick Malley, MD, of the division of infectious disease at Boston Children’s Hospital, in an interview with this news organization. “Unfortunately, we kind of expect them during enterovirus season,” he said. That season is thought to peak in the late summer and early fall.
Since the CDC began tracking AFM in August 2014, there have been 692 confirmed cases in the United States. AFM cases spiked in 2014, 2016, and 2018, mostly in young children. In 2021, there were 28 confirmed cases across 15 states. The CDC did not specify the age of those cases, but in 2018 – when EV-D68 most recently circulated at high levels – the median age of children who visited the emergency department or were hospitalized for EV-D68–associated respiratory illness was 3 years.
“[AFM] can be very severe and it can be very scary for the parents of children who have it,” Dr. Malley said, “but given the prevalence of enteroviruses in the community, you have to conclude it’s a relatively rare event in susceptible individuals. Why some get it and others don’t is unfortunately unclear at this moment.”
The CDC recommends that providers consider EV-D68 as a possible cause for acute, severe respiratory illness in children. If the cause of a respiratory illness in a severely ill patient is not clear, health professionals should test for RVs and EVs, if this is not already part of a typical diagnostic workflow, the agency said. Currently, there are no vaccines or specific treatments for RV or EV, and the CDC recommends supportive clinical management.
The advisory also urged providers to “strongly consider AFM in patients with acute flaccid limb weakness, especially after respiratory illness or fever, and between the months of August and November 2022.”
For any patient presenting with possible AFM, clinicians should collect samples from multiple sources, including cerebrospinal fluid, serum, stool, and a nasopharyngeal or oropharyngeal swab. Samples should be taken “as early as possible and preferably on the day of onset of limb weakness,” the alert said. There is currently no specific medicine for AFM, the agency said, though recommended interventions may vary for each patient.
A version of this article first appeared on Medscape.com.
, according to a Health Network Alert advisory by the Centers for Disease Control and Prevention.
In August, health care providers and hospitals notified the CDC of an increase in severe respiratory illness in children who also tested positive for rhinovirus (RV) or enterovirus (EV). Additional testing revealed that some children were positive for EV-D68, which primarily causes acute respiratory illness. However, the virus has been associated with acute flaccid myelitis (AFM), a rare neurologic condition involving muscle weakness.
Also, in July and August 2022, surveillance networks reported an increase in EV-D68 activity compared with the same months in 2019, 2020, and 2021, the agency said in the alert. As of Aug. 30, the CDC has not received any reports of AFM beginning this year; however, spikes in EV-D68 typically come before cases of AFM, they said.
“Something we are always on the lookout for in the late summer and fall is AFM cases,” said Rick Malley, MD, of the division of infectious disease at Boston Children’s Hospital, in an interview with this news organization. “Unfortunately, we kind of expect them during enterovirus season,” he said. That season is thought to peak in the late summer and early fall.
Since the CDC began tracking AFM in August 2014, there have been 692 confirmed cases in the United States. AFM cases spiked in 2014, 2016, and 2018, mostly in young children. In 2021, there were 28 confirmed cases across 15 states. The CDC did not specify the age of those cases, but in 2018 – when EV-D68 most recently circulated at high levels – the median age of children who visited the emergency department or were hospitalized for EV-D68–associated respiratory illness was 3 years.
“[AFM] can be very severe and it can be very scary for the parents of children who have it,” Dr. Malley said, “but given the prevalence of enteroviruses in the community, you have to conclude it’s a relatively rare event in susceptible individuals. Why some get it and others don’t is unfortunately unclear at this moment.”
The CDC recommends that providers consider EV-D68 as a possible cause for acute, severe respiratory illness in children. If the cause of a respiratory illness in a severely ill patient is not clear, health professionals should test for RVs and EVs, if this is not already part of a typical diagnostic workflow, the agency said. Currently, there are no vaccines or specific treatments for RV or EV, and the CDC recommends supportive clinical management.
The advisory also urged providers to “strongly consider AFM in patients with acute flaccid limb weakness, especially after respiratory illness or fever, and between the months of August and November 2022.”
For any patient presenting with possible AFM, clinicians should collect samples from multiple sources, including cerebrospinal fluid, serum, stool, and a nasopharyngeal or oropharyngeal swab. Samples should be taken “as early as possible and preferably on the day of onset of limb weakness,” the alert said. There is currently no specific medicine for AFM, the agency said, though recommended interventions may vary for each patient.
A version of this article first appeared on Medscape.com.
, according to a Health Network Alert advisory by the Centers for Disease Control and Prevention.
In August, health care providers and hospitals notified the CDC of an increase in severe respiratory illness in children who also tested positive for rhinovirus (RV) or enterovirus (EV). Additional testing revealed that some children were positive for EV-D68, which primarily causes acute respiratory illness. However, the virus has been associated with acute flaccid myelitis (AFM), a rare neurologic condition involving muscle weakness.
Also, in July and August 2022, surveillance networks reported an increase in EV-D68 activity compared with the same months in 2019, 2020, and 2021, the agency said in the alert. As of Aug. 30, the CDC has not received any reports of AFM beginning this year; however, spikes in EV-D68 typically come before cases of AFM, they said.
“Something we are always on the lookout for in the late summer and fall is AFM cases,” said Rick Malley, MD, of the division of infectious disease at Boston Children’s Hospital, in an interview with this news organization. “Unfortunately, we kind of expect them during enterovirus season,” he said. That season is thought to peak in the late summer and early fall.
Since the CDC began tracking AFM in August 2014, there have been 692 confirmed cases in the United States. AFM cases spiked in 2014, 2016, and 2018, mostly in young children. In 2021, there were 28 confirmed cases across 15 states. The CDC did not specify the age of those cases, but in 2018 – when EV-D68 most recently circulated at high levels – the median age of children who visited the emergency department or were hospitalized for EV-D68–associated respiratory illness was 3 years.
“[AFM] can be very severe and it can be very scary for the parents of children who have it,” Dr. Malley said, “but given the prevalence of enteroviruses in the community, you have to conclude it’s a relatively rare event in susceptible individuals. Why some get it and others don’t is unfortunately unclear at this moment.”
The CDC recommends that providers consider EV-D68 as a possible cause for acute, severe respiratory illness in children. If the cause of a respiratory illness in a severely ill patient is not clear, health professionals should test for RVs and EVs, if this is not already part of a typical diagnostic workflow, the agency said. Currently, there are no vaccines or specific treatments for RV or EV, and the CDC recommends supportive clinical management.
The advisory also urged providers to “strongly consider AFM in patients with acute flaccid limb weakness, especially after respiratory illness or fever, and between the months of August and November 2022.”
For any patient presenting with possible AFM, clinicians should collect samples from multiple sources, including cerebrospinal fluid, serum, stool, and a nasopharyngeal or oropharyngeal swab. Samples should be taken “as early as possible and preferably on the day of onset of limb weakness,” the alert said. There is currently no specific medicine for AFM, the agency said, though recommended interventions may vary for each patient.
A version of this article first appeared on Medscape.com.
ILD on the rise: Doctors offer tips for diagnosing deadly disease
“There is definitely a delay from the time of symptom onset to the time that they are even evaluated for ILD,” said Dr. Kulkarni of the department of pulmonary, allergy and critical care medicine at the University of Alabama, Birmingham. “Some patients have had a significant loss of lung function by the time they come to see us. By that point we are limited by what treatment options we can offer.”
Interstitial lung disease is an umbrella term for a group of disorders involving progressive scarring of the lungs – typically irreversible – usually caused by long-term exposure to hazardous materials or by autoimmune effects. It includes idiopathic pulmonary fibrosis (IPF), a disease that is fairly rare but which has therapy options that can be effective if caught early enough. The term pulmonary fibrosis refers to lung scarring. Another type of ILD is pulmonary sarcoidosis, in which small clumps of immune cells form in the lungs in an immune response sometimes following an environmental trigger, and can lead to lung scarring if it doesn’t resolve.
Cases of ILD appear to be on the rise, and COVID-19 has made diagnosing it more complicated. One study found the prevalence of ILD and pulmonary sarcoidosis in high-income countries was about 122 of every 100,000 people in 1990 and rose to about 198 of every 100,000 people in 2017. The data were pulled from the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Globally, the researchers found a prevalence of 62 per 100,000 in 1990, compared with 82 per 100,000 in 2017.
If all of a patient’s symptoms have appeared post COVID and a physician is seeing a patient within 4-6 weeks of COVID symptoms, it is likely that the symptoms are COVID related. But a full work-up is recommended if a patient has lung crackles, which are an indicator of lung scarring, she said.
“The patterns that are seen on CT scan for COVID pneumonia are very distinct from what we expect to see with idiopathic pulmonary fibrosis,” Dr. Kulkarni said. “Putting all this information together is what is important to differentiate it from COVID pneumonia, as well as other types of ILD.”
A study published earlier this year found similarities between COVID-19 and IPF in gene expression, their IL-15-heavy cytokine storms, and the type of damage to alveolar cells. Both might be driven by endoplasmic reticulum stress, they found.
“COVID-19 resembles IPF at a fundamental level,” they wrote.
Jeffrey Horowitz, MD, a pulmonologist and professor of medicine at the Ohio State University, said the need for early diagnosis is in part a function of the therapies available for ILD.
“They don’t make the lung function better,” he said. “So delays in diagnosis mean that there’s the possibility of underlying progression for months, or sometimes years, before the diagnosis is recognized.”
In an area in which diagnosis is delayed and the prognosis is dire – 3-5 years in untreated patients after diagnosis – “there’s a tremendous amount of nihilism out there” among patients, he said.
He said patients with long-term shortness of breath and unexplained cough are often told they have asthma and are prescribed inhalers, but then further assessment isn’t performed when those don’t work.
Diagnosing ILD in primary care
Many primary care physicians feel ill-equipped to discuss IPF. More than a dozen physicians contacted for this piece to talk about ILD either did not respond, or said they felt unqualified to respond to questions on the disease.
“Not my area of expertise” and “I don’t think I’m the right person for this discussion” were two of the responses provided to this news organization.
“For some reason, in the world of primary care, it seems like there’s an impediment to getting pulmonary function studies,” Dr. Horowitz said. “Anybody who has a persistent ongoing prolonged unexplained shortness of breath and cough should have pulmonary function studies done.”
Listening to the lungs alone might not be enough, he said. There might be no clear sign in the case of early pulmonary fibrosis, he said.
“There’s the textbook description of these Velcro-sounding crackles, but sometimes it’s very subtle,” he said. “And unless you’re listening very carefully it can easily be missed by somebody who has a busy practice, or it’s loud.”
William E. Golden, MD, professor of medicine and public health at the University of Arkansas, Little Rock, is the sole primary care physician contacted for this piece who spoke with authority on ILD.
For cases of suspected ILD, internist Dr. Golden, who also serves on the editorial advisory board of Internal Medicine News, suggested ordering a test for diffusing capacity for carbon monoxide (DLCO), which will be low in the case of IPF, along with a fine-cut lung CT scan to assess ongoing fibrotic changes.
It’s “not that difficult, but you need to have an index of suspicion for the diagnosis,” he said.
New initiative for helping diagnose ILD
Dr. Kulkarni is a committee member for a new effort under way to try to get patients with ILD diagnosed earlier.
The initiative, called Bridging Specialties: Timely Diagnosis for ILD Patients, has already produced an introductory podcast and a white paper on the effort, and its rationale is expected to be released soon, according to Dr. Kulkarni and her fellow committee members.
The American College of Chest Physicians and the Three Lakes Foundation – a foundation dedicated to pulmonary fibrosis awareness and research – are working together on this initiative. They plan to put together a suite of resources, to be gradually rolled out on the college’s website, to raise awareness about the importance of early diagnosis of ILD.
The full toolkit, expected to be rolled out over the next 12 months, will include a series of podcasts and resources on how to get patients diagnosed earlier and steps to take in cases of suspected ILD, Dr. Kulkarni said.
“The goal would be to try to increase awareness about the disease so that people start thinking more about it up front – and not after we’ve ruled out everything else,” she said. The main audience will be primary care providers, but patients and community pulmonologists would likely also benefit from the resources, the committee members said.
The urgency of the initiative stems from the way ILD treatments work. They are antifibrotic, meaning they help prevent scar tissue from forming, but they can’t reverse scar tissue that has already formed. If scarring is severe, the only option might be a lung transplant, and, since the average age at ILD diagnosis is in the 60s, many patients have comorbidities that make them ineligible for transplant. According to the Global Burden of Disease Study mentioned earlier, the death rate per 100,000 people with ILD was 1.93 in 2017.
“The longer we take to diagnose it, the more chance that inflammation will become scar tissue,” Dr. Kularni explained.
William Lago, MD, another member of the committee and a family physician, said identifying ILD early is not a straightforward matter .
“When they first present, it’s hard to pick up,” said Dr. Lago, who is also a staff physician at Cleveland Clinic’s Wooster Family Health Center and medical director of the COVID Recover Clinic there. “Many of them, even themselves, will discount the symptoms.”
Dr. Lago said that patients might resist having a work-up even when a primary care physician identifies symptoms as possible ILD. In rural settings, they might have to travel quite a distance for a CT scan or other necessary evaluations, or they might just not think the symptoms are serious enough.
“Most of the time when I’ve picked up some of my pulmonary fibrosis patients, it’s been incidentally while they’re in the office for other things,” he said. He often has to “push the issue” for further work-up, he said.
The overlap of shortness of breath and cough with other, much more common disorders, such as heart disease or chronic obstructive pulmonary disease (COPD), make ILD diagnosis a challenge, he said.
“For most of us, we’ve got sometimes 10 or 15 minutes with a patient who’s presenting with 5-6 different problems. And the shortness of breath or the occasional cough – that they think is nothing – is probably the least of those,” Dr. Lago said.
Dr. Golden said he suspected a tool like the one being developed by CHEST to be useful for some and not useful for others. He added that “no one has the time to spend on that kind of thing.”
Instead, he suggested just reinforcing what the core symptoms are and what the core testing is, “to make people think about it.”
Dr. Horowitiz seemed more optimistic about the likelihood of the CHEST tool being utilized to diagnose ILD.
Whether and how he would use the CHEST resource will depend on the final form it takes, Dr. Horowitz said. It’s encouraging that it’s being put together by a credible source, he added.
Dr. Kulkarni reported financial relationships with Boehringer Ingelheim, Aluda Pharmaceuticals and PureTech Lyt-100 Inc. Dr. Lago, Dr. Horowitz, and Dr. Golden reported no relevant disclosures.
Katie Lennon contributed to this report.
“There is definitely a delay from the time of symptom onset to the time that they are even evaluated for ILD,” said Dr. Kulkarni of the department of pulmonary, allergy and critical care medicine at the University of Alabama, Birmingham. “Some patients have had a significant loss of lung function by the time they come to see us. By that point we are limited by what treatment options we can offer.”
Interstitial lung disease is an umbrella term for a group of disorders involving progressive scarring of the lungs – typically irreversible – usually caused by long-term exposure to hazardous materials or by autoimmune effects. It includes idiopathic pulmonary fibrosis (IPF), a disease that is fairly rare but which has therapy options that can be effective if caught early enough. The term pulmonary fibrosis refers to lung scarring. Another type of ILD is pulmonary sarcoidosis, in which small clumps of immune cells form in the lungs in an immune response sometimes following an environmental trigger, and can lead to lung scarring if it doesn’t resolve.
Cases of ILD appear to be on the rise, and COVID-19 has made diagnosing it more complicated. One study found the prevalence of ILD and pulmonary sarcoidosis in high-income countries was about 122 of every 100,000 people in 1990 and rose to about 198 of every 100,000 people in 2017. The data were pulled from the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Globally, the researchers found a prevalence of 62 per 100,000 in 1990, compared with 82 per 100,000 in 2017.
If all of a patient’s symptoms have appeared post COVID and a physician is seeing a patient within 4-6 weeks of COVID symptoms, it is likely that the symptoms are COVID related. But a full work-up is recommended if a patient has lung crackles, which are an indicator of lung scarring, she said.
“The patterns that are seen on CT scan for COVID pneumonia are very distinct from what we expect to see with idiopathic pulmonary fibrosis,” Dr. Kulkarni said. “Putting all this information together is what is important to differentiate it from COVID pneumonia, as well as other types of ILD.”
A study published earlier this year found similarities between COVID-19 and IPF in gene expression, their IL-15-heavy cytokine storms, and the type of damage to alveolar cells. Both might be driven by endoplasmic reticulum stress, they found.
“COVID-19 resembles IPF at a fundamental level,” they wrote.
Jeffrey Horowitz, MD, a pulmonologist and professor of medicine at the Ohio State University, said the need for early diagnosis is in part a function of the therapies available for ILD.
“They don’t make the lung function better,” he said. “So delays in diagnosis mean that there’s the possibility of underlying progression for months, or sometimes years, before the diagnosis is recognized.”
In an area in which diagnosis is delayed and the prognosis is dire – 3-5 years in untreated patients after diagnosis – “there’s a tremendous amount of nihilism out there” among patients, he said.
He said patients with long-term shortness of breath and unexplained cough are often told they have asthma and are prescribed inhalers, but then further assessment isn’t performed when those don’t work.
Diagnosing ILD in primary care
Many primary care physicians feel ill-equipped to discuss IPF. More than a dozen physicians contacted for this piece to talk about ILD either did not respond, or said they felt unqualified to respond to questions on the disease.
“Not my area of expertise” and “I don’t think I’m the right person for this discussion” were two of the responses provided to this news organization.
“For some reason, in the world of primary care, it seems like there’s an impediment to getting pulmonary function studies,” Dr. Horowitz said. “Anybody who has a persistent ongoing prolonged unexplained shortness of breath and cough should have pulmonary function studies done.”
Listening to the lungs alone might not be enough, he said. There might be no clear sign in the case of early pulmonary fibrosis, he said.
“There’s the textbook description of these Velcro-sounding crackles, but sometimes it’s very subtle,” he said. “And unless you’re listening very carefully it can easily be missed by somebody who has a busy practice, or it’s loud.”
William E. Golden, MD, professor of medicine and public health at the University of Arkansas, Little Rock, is the sole primary care physician contacted for this piece who spoke with authority on ILD.
For cases of suspected ILD, internist Dr. Golden, who also serves on the editorial advisory board of Internal Medicine News, suggested ordering a test for diffusing capacity for carbon monoxide (DLCO), which will be low in the case of IPF, along with a fine-cut lung CT scan to assess ongoing fibrotic changes.
It’s “not that difficult, but you need to have an index of suspicion for the diagnosis,” he said.
New initiative for helping diagnose ILD
Dr. Kulkarni is a committee member for a new effort under way to try to get patients with ILD diagnosed earlier.
The initiative, called Bridging Specialties: Timely Diagnosis for ILD Patients, has already produced an introductory podcast and a white paper on the effort, and its rationale is expected to be released soon, according to Dr. Kulkarni and her fellow committee members.
The American College of Chest Physicians and the Three Lakes Foundation – a foundation dedicated to pulmonary fibrosis awareness and research – are working together on this initiative. They plan to put together a suite of resources, to be gradually rolled out on the college’s website, to raise awareness about the importance of early diagnosis of ILD.
The full toolkit, expected to be rolled out over the next 12 months, will include a series of podcasts and resources on how to get patients diagnosed earlier and steps to take in cases of suspected ILD, Dr. Kulkarni said.
“The goal would be to try to increase awareness about the disease so that people start thinking more about it up front – and not after we’ve ruled out everything else,” she said. The main audience will be primary care providers, but patients and community pulmonologists would likely also benefit from the resources, the committee members said.
The urgency of the initiative stems from the way ILD treatments work. They are antifibrotic, meaning they help prevent scar tissue from forming, but they can’t reverse scar tissue that has already formed. If scarring is severe, the only option might be a lung transplant, and, since the average age at ILD diagnosis is in the 60s, many patients have comorbidities that make them ineligible for transplant. According to the Global Burden of Disease Study mentioned earlier, the death rate per 100,000 people with ILD was 1.93 in 2017.
“The longer we take to diagnose it, the more chance that inflammation will become scar tissue,” Dr. Kularni explained.
William Lago, MD, another member of the committee and a family physician, said identifying ILD early is not a straightforward matter .
“When they first present, it’s hard to pick up,” said Dr. Lago, who is also a staff physician at Cleveland Clinic’s Wooster Family Health Center and medical director of the COVID Recover Clinic there. “Many of them, even themselves, will discount the symptoms.”
Dr. Lago said that patients might resist having a work-up even when a primary care physician identifies symptoms as possible ILD. In rural settings, they might have to travel quite a distance for a CT scan or other necessary evaluations, or they might just not think the symptoms are serious enough.
“Most of the time when I’ve picked up some of my pulmonary fibrosis patients, it’s been incidentally while they’re in the office for other things,” he said. He often has to “push the issue” for further work-up, he said.
The overlap of shortness of breath and cough with other, much more common disorders, such as heart disease or chronic obstructive pulmonary disease (COPD), make ILD diagnosis a challenge, he said.
“For most of us, we’ve got sometimes 10 or 15 minutes with a patient who’s presenting with 5-6 different problems. And the shortness of breath or the occasional cough – that they think is nothing – is probably the least of those,” Dr. Lago said.
Dr. Golden said he suspected a tool like the one being developed by CHEST to be useful for some and not useful for others. He added that “no one has the time to spend on that kind of thing.”
Instead, he suggested just reinforcing what the core symptoms are and what the core testing is, “to make people think about it.”
Dr. Horowitiz seemed more optimistic about the likelihood of the CHEST tool being utilized to diagnose ILD.
Whether and how he would use the CHEST resource will depend on the final form it takes, Dr. Horowitz said. It’s encouraging that it’s being put together by a credible source, he added.
Dr. Kulkarni reported financial relationships with Boehringer Ingelheim, Aluda Pharmaceuticals and PureTech Lyt-100 Inc. Dr. Lago, Dr. Horowitz, and Dr. Golden reported no relevant disclosures.
Katie Lennon contributed to this report.
“There is definitely a delay from the time of symptom onset to the time that they are even evaluated for ILD,” said Dr. Kulkarni of the department of pulmonary, allergy and critical care medicine at the University of Alabama, Birmingham. “Some patients have had a significant loss of lung function by the time they come to see us. By that point we are limited by what treatment options we can offer.”
Interstitial lung disease is an umbrella term for a group of disorders involving progressive scarring of the lungs – typically irreversible – usually caused by long-term exposure to hazardous materials or by autoimmune effects. It includes idiopathic pulmonary fibrosis (IPF), a disease that is fairly rare but which has therapy options that can be effective if caught early enough. The term pulmonary fibrosis refers to lung scarring. Another type of ILD is pulmonary sarcoidosis, in which small clumps of immune cells form in the lungs in an immune response sometimes following an environmental trigger, and can lead to lung scarring if it doesn’t resolve.
Cases of ILD appear to be on the rise, and COVID-19 has made diagnosing it more complicated. One study found the prevalence of ILD and pulmonary sarcoidosis in high-income countries was about 122 of every 100,000 people in 1990 and rose to about 198 of every 100,000 people in 2017. The data were pulled from the Global Burden of Diseases, Injuries, and Risk Factors Study 2017. Globally, the researchers found a prevalence of 62 per 100,000 in 1990, compared with 82 per 100,000 in 2017.
If all of a patient’s symptoms have appeared post COVID and a physician is seeing a patient within 4-6 weeks of COVID symptoms, it is likely that the symptoms are COVID related. But a full work-up is recommended if a patient has lung crackles, which are an indicator of lung scarring, she said.
“The patterns that are seen on CT scan for COVID pneumonia are very distinct from what we expect to see with idiopathic pulmonary fibrosis,” Dr. Kulkarni said. “Putting all this information together is what is important to differentiate it from COVID pneumonia, as well as other types of ILD.”
A study published earlier this year found similarities between COVID-19 and IPF in gene expression, their IL-15-heavy cytokine storms, and the type of damage to alveolar cells. Both might be driven by endoplasmic reticulum stress, they found.
“COVID-19 resembles IPF at a fundamental level,” they wrote.
Jeffrey Horowitz, MD, a pulmonologist and professor of medicine at the Ohio State University, said the need for early diagnosis is in part a function of the therapies available for ILD.
“They don’t make the lung function better,” he said. “So delays in diagnosis mean that there’s the possibility of underlying progression for months, or sometimes years, before the diagnosis is recognized.”
In an area in which diagnosis is delayed and the prognosis is dire – 3-5 years in untreated patients after diagnosis – “there’s a tremendous amount of nihilism out there” among patients, he said.
He said patients with long-term shortness of breath and unexplained cough are often told they have asthma and are prescribed inhalers, but then further assessment isn’t performed when those don’t work.
Diagnosing ILD in primary care
Many primary care physicians feel ill-equipped to discuss IPF. More than a dozen physicians contacted for this piece to talk about ILD either did not respond, or said they felt unqualified to respond to questions on the disease.
“Not my area of expertise” and “I don’t think I’m the right person for this discussion” were two of the responses provided to this news organization.
“For some reason, in the world of primary care, it seems like there’s an impediment to getting pulmonary function studies,” Dr. Horowitz said. “Anybody who has a persistent ongoing prolonged unexplained shortness of breath and cough should have pulmonary function studies done.”
Listening to the lungs alone might not be enough, he said. There might be no clear sign in the case of early pulmonary fibrosis, he said.
“There’s the textbook description of these Velcro-sounding crackles, but sometimes it’s very subtle,” he said. “And unless you’re listening very carefully it can easily be missed by somebody who has a busy practice, or it’s loud.”
William E. Golden, MD, professor of medicine and public health at the University of Arkansas, Little Rock, is the sole primary care physician contacted for this piece who spoke with authority on ILD.
For cases of suspected ILD, internist Dr. Golden, who also serves on the editorial advisory board of Internal Medicine News, suggested ordering a test for diffusing capacity for carbon monoxide (DLCO), which will be low in the case of IPF, along with a fine-cut lung CT scan to assess ongoing fibrotic changes.
It’s “not that difficult, but you need to have an index of suspicion for the diagnosis,” he said.
New initiative for helping diagnose ILD
Dr. Kulkarni is a committee member for a new effort under way to try to get patients with ILD diagnosed earlier.
The initiative, called Bridging Specialties: Timely Diagnosis for ILD Patients, has already produced an introductory podcast and a white paper on the effort, and its rationale is expected to be released soon, according to Dr. Kulkarni and her fellow committee members.
The American College of Chest Physicians and the Three Lakes Foundation – a foundation dedicated to pulmonary fibrosis awareness and research – are working together on this initiative. They plan to put together a suite of resources, to be gradually rolled out on the college’s website, to raise awareness about the importance of early diagnosis of ILD.
The full toolkit, expected to be rolled out over the next 12 months, will include a series of podcasts and resources on how to get patients diagnosed earlier and steps to take in cases of suspected ILD, Dr. Kulkarni said.
“The goal would be to try to increase awareness about the disease so that people start thinking more about it up front – and not after we’ve ruled out everything else,” she said. The main audience will be primary care providers, but patients and community pulmonologists would likely also benefit from the resources, the committee members said.
The urgency of the initiative stems from the way ILD treatments work. They are antifibrotic, meaning they help prevent scar tissue from forming, but they can’t reverse scar tissue that has already formed. If scarring is severe, the only option might be a lung transplant, and, since the average age at ILD diagnosis is in the 60s, many patients have comorbidities that make them ineligible for transplant. According to the Global Burden of Disease Study mentioned earlier, the death rate per 100,000 people with ILD was 1.93 in 2017.
“The longer we take to diagnose it, the more chance that inflammation will become scar tissue,” Dr. Kularni explained.
William Lago, MD, another member of the committee and a family physician, said identifying ILD early is not a straightforward matter .
“When they first present, it’s hard to pick up,” said Dr. Lago, who is also a staff physician at Cleveland Clinic’s Wooster Family Health Center and medical director of the COVID Recover Clinic there. “Many of them, even themselves, will discount the symptoms.”
Dr. Lago said that patients might resist having a work-up even when a primary care physician identifies symptoms as possible ILD. In rural settings, they might have to travel quite a distance for a CT scan or other necessary evaluations, or they might just not think the symptoms are serious enough.
“Most of the time when I’ve picked up some of my pulmonary fibrosis patients, it’s been incidentally while they’re in the office for other things,” he said. He often has to “push the issue” for further work-up, he said.
The overlap of shortness of breath and cough with other, much more common disorders, such as heart disease or chronic obstructive pulmonary disease (COPD), make ILD diagnosis a challenge, he said.
“For most of us, we’ve got sometimes 10 or 15 minutes with a patient who’s presenting with 5-6 different problems. And the shortness of breath or the occasional cough – that they think is nothing – is probably the least of those,” Dr. Lago said.
Dr. Golden said he suspected a tool like the one being developed by CHEST to be useful for some and not useful for others. He added that “no one has the time to spend on that kind of thing.”
Instead, he suggested just reinforcing what the core symptoms are and what the core testing is, “to make people think about it.”
Dr. Horowitiz seemed more optimistic about the likelihood of the CHEST tool being utilized to diagnose ILD.
Whether and how he would use the CHEST resource will depend on the final form it takes, Dr. Horowitz said. It’s encouraging that it’s being put together by a credible source, he added.
Dr. Kulkarni reported financial relationships with Boehringer Ingelheim, Aluda Pharmaceuticals and PureTech Lyt-100 Inc. Dr. Lago, Dr. Horowitz, and Dr. Golden reported no relevant disclosures.
Katie Lennon contributed to this report.
COPD inhaler therapy: A path to success
Managing chronic obstructive pulmonary disease (COPD) presents a significant challenge to busy clinicians in many ways, especially when one is approaching the long list of inhaled pharmaceutical agents with an eye toward a cost-effective, patient-centered regimen. Inhaled agents remain expensive, with few available in generic form.
Our primary goal in this article is to detail these agents’ utility, limitations, and relative cost. Specifically, we review why the following considerations are important:
- Choose the right delivery device and drug while considering patient factors.
- Provide patient education through allied health professionals.
- Reduce environmental exposures.
- Rethink the use of inhaled corticosteroids (ICS).
- Understand the role of dual therapy and triple therapy.
There are numerous other treatment modalities for COPD that are recommended in national and international practice guidelines, including vaccination, pulmonary rehabilitation, home visits, phosphodiesterase-4 inhibitors, oral glucocorticoids, supplemental oxygen, and ventilatory support.1 Discussion of those modalities is beyond the scope of this review.
Pathophysiology and pharmacotherapy targets
COPD is characterized by persistent respiratory symptoms and airflow limitation, usually due to airway or alveolar abnormalities, or both, caused by environmental and host factors.2 Sustained lung parenchymal irritation results from exposure to noxious fumes generated by tobacco, pollution, chemicals, and cleaning agents. Host factors include lung immaturity at birth; genetic mutations, such as alpha-1 antitrypsin deficiency and dysregulation of elastase; and increased reactivity of bronchial smooth muscles, similar to what is seen in asthma.1

Improving ventilation with the intention of relieving dyspnea is the goal of inhaler pharmacotherapy; targets include muscarinic receptors and beta 2-adrenergic receptors that act on bronchial smooth muscle and the autonomic nervous system. Immune modulators, such as corticosteroids, help reduce inflammation around airways.1 Recent pharmacotherapeutic developments include combinations of inhaled medications and expanding options for devices that deliver drugs.
Delivery devices: Options and optimizing their use
Three principal types of inhaler devices are available: pressurized metered-dose inhalers (MDIs), dry-powder inhalers (DPIs), and soft-mist inhalers (SMIs). These devices, and nebulizers, facilitate medication delivery into the lungs (TABLE 13-9).
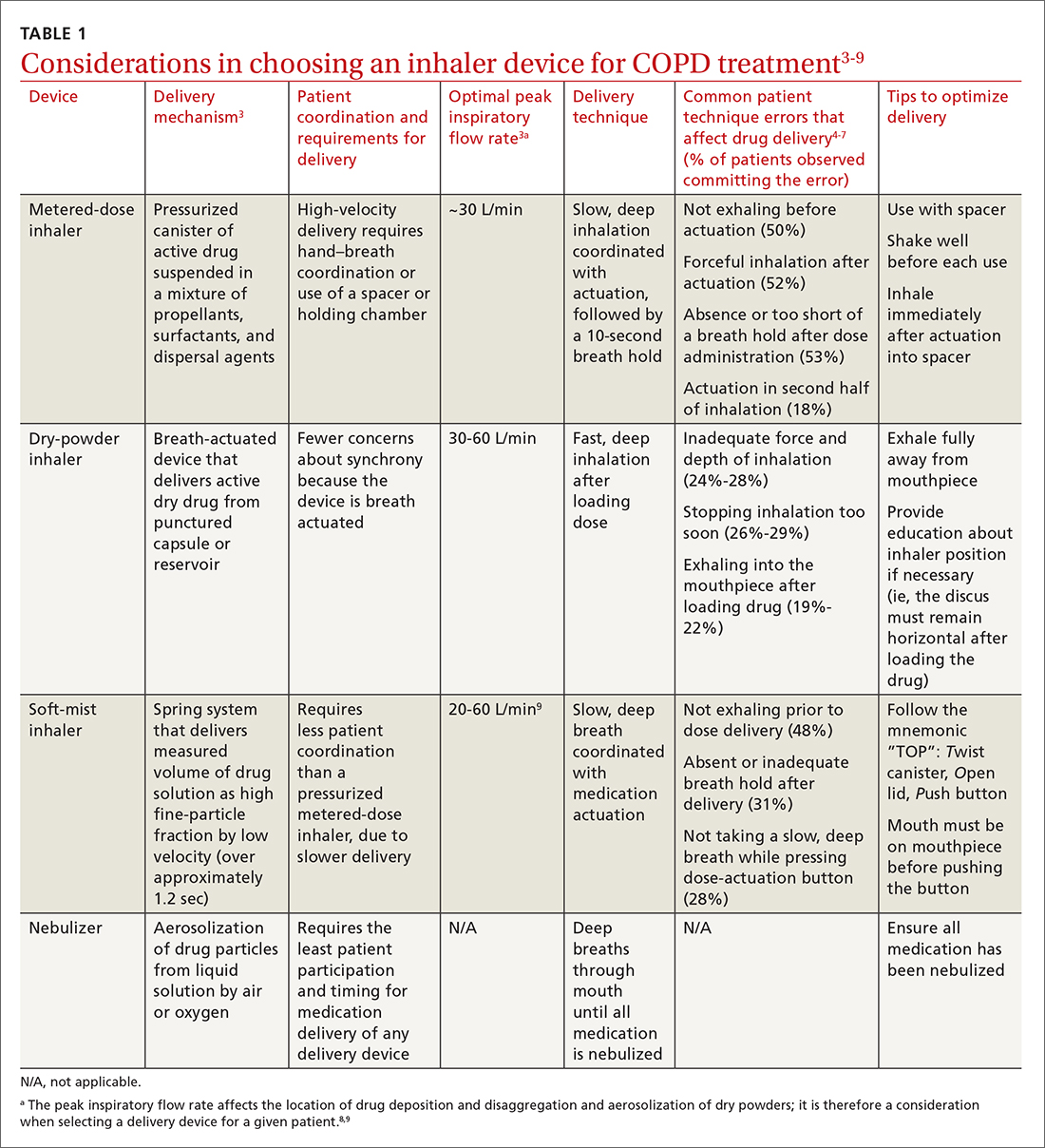
Errors in using inhalers affect outcome. Correct inhaler technique is essential for optimal delivery of inhaled medications. Errors in technique when using an inhaled delivery device lead to inadequate drug delivery and are associated with poor outcomes: 90% of patients make errors that are classified as critical (ie, those that reduce drug delivery) or noncritical.2 Critical inhaler errors increase the risk of hospitalization and emergency department visits, and can necessitate a course of oral corticosteroids.10 Many critical errors are device specific; several such errors are described in TABLE 1.3-9
Continue to: Patient education
Patient education is necessary to ensure that drug is delivered to the patient consistently, with the same expectation of effect seen in efficacy studies (which usually provide rigorous inhaler technique training and require demonstration of proficiency).1,2,10 For the busy clinician, a multidisciplinary approach, discussed shortly, can help. Guidelines developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that inhaler technique be reassessed at every visit and when evaluating treatment response.1TABLE 13-9 provides information on each device type, patient requirements for use, proper technique, common errors in use, and tips for optimizing delivery.
Inhaler education and assessment of technique that is provided to patients in collaboration with a clinical pharmacist, nursing staff, and a respiratory therapist can help alleviate the pressure on a time-constrained primary care physician. Furthermore, pharmacist involvement in the COPD management team meaningfully improves inhaler technique and medication adherence.6,7 Intervention by a pharmacist correlates with a significant reduction in number of exacerbations; an increased likelihood that the patient has a COPD care plan and has received the pneumococcal vaccine; and an improvement in the mean health-related quality of life.11,12
In primary care practices that lack robust multidisciplinary resources, we recommend utilizing virtual resources, such as educational videos, to allow face-to-face or virtual education. A free source of such resources is the COPD Foundation,a a not-for-profit organization funded partly by industry.
Short- and long-acting inhaled medications for COPD
Each class of inhaled medication for treating COPD is discussed broadly in the following sections. TABLE 21 provides details about individual drugs, devices available to deliver them, and starting dosages.
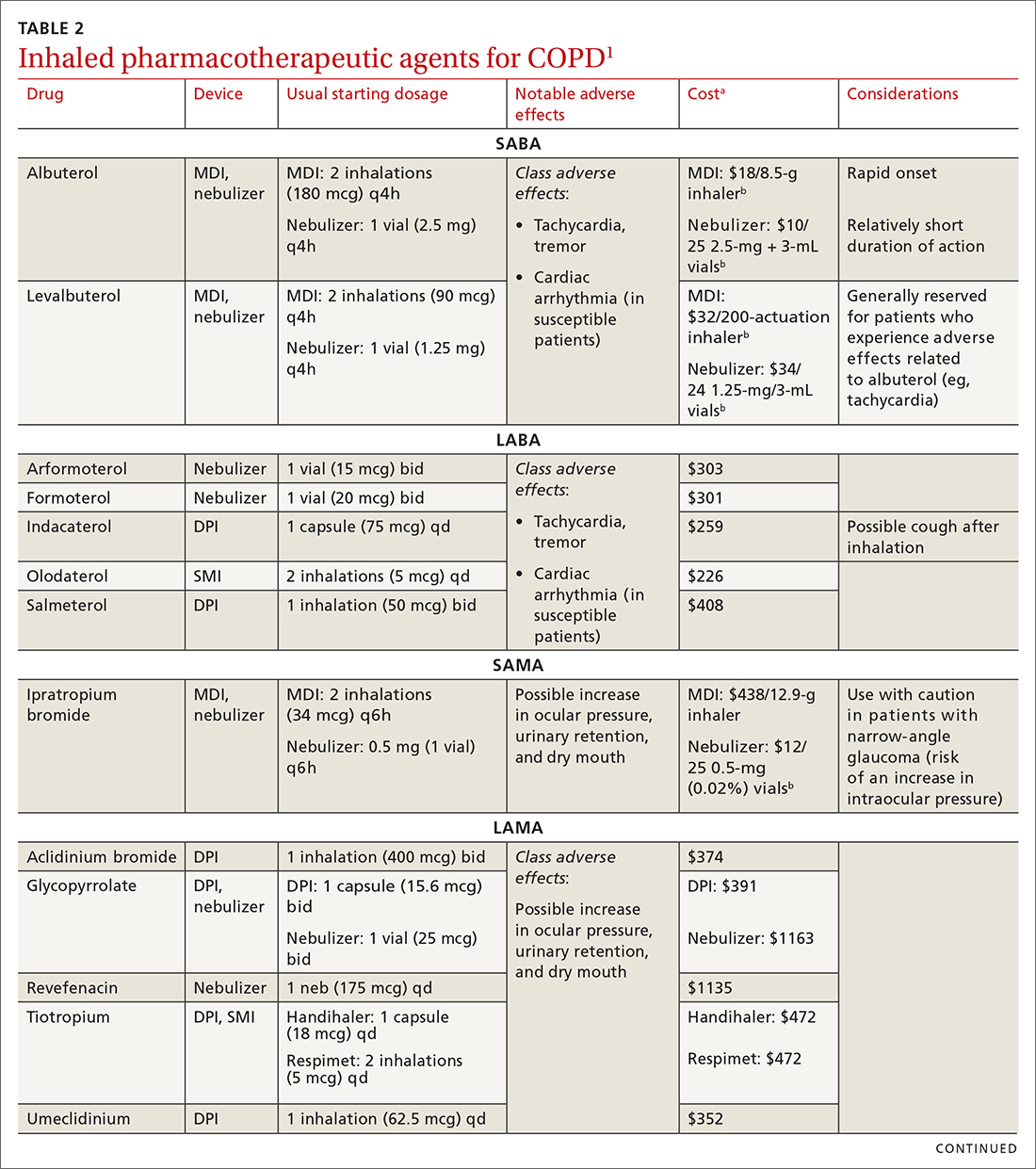
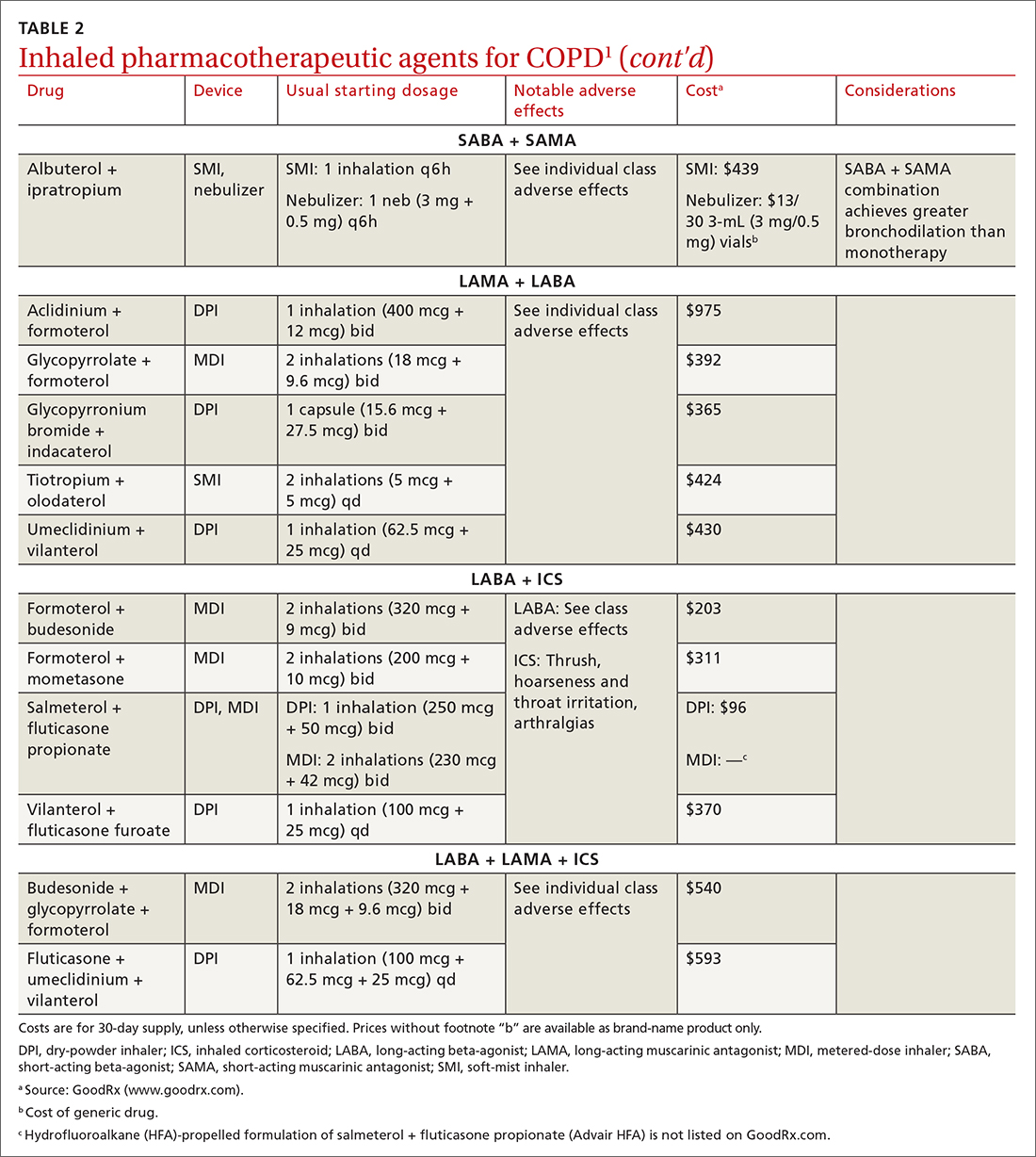
Short-acting agents
These drugs are available in MDI, SMI, and nebulizer delivery devices. When portability and equipment burden are important to the patient, we recommend an MDI over a nebulizer; an MDI is as efficacious as a nebulizer in improving forced expiratory volume in 1 second (FEV1) and reducing the length of hospital stay for exacerbations.4
Continue to: SABAs
Short-acting beta 2-adrenergic agonists (or beta-agonists [SABAs]). Beta-agonists are typically used to treat exacerbations. They facilitate bronchodilation by upregulating cyclic adenosine monophosphate, preventing smooth-muscle contraction, and reducing dynamic hyperinflation. The effect of a SABA lasts 4 to 6 hours.
In general, SABAs are not recommended for daily use in stable COPD. However, they can be useful, and appropriate, for treating occasional dyspnea and can confer additional symptom improvement when used occasionally along with a long-acting beta 2-adrenergic agonist (or beta-agonist [LABA]; discussed later).1
Albuterol, a commonly used SABA, is less expensive than, and just as effective as, same-class levalbuterol for decreasing breathlessness associated with acute exacerbations. There is no significant difference between the 2 drugs in regard to the incidence of tachycardia or palpitations in patients with cardiovascular disease.13
Although no significant differences have been observed in outcomes when a nebulizer or an MDI is used to administer a SABA, it’s wise to avoid continuous SABA nebulizer therapy, due to the increased risk of disease transmission through the generation of droplets.1,4 Instead, it’s appropriate to use an MDI regimen of 1 to 3 puffs every hour for 2 to 3 hours, followed by 1 to 3 puffs every 2 to 4 hours thereafter, based on the patient’s response.1,4
Short-acting muscarinic antagonists (SAMAs). Muscarinic antagonists achieve bronchodilation by blocking acetylcholine on muscarinic receptors. We do not specifically recommend SAMAs over SABAs for treating COPD exacerbations in our patients: There is no difference in improvement in FEV1 during an acute exacerbation. Nebulized delivery of a SAMA raises concern for an increase in the risk of acute narrow-angle glaucoma, a risk that can be reduced by using a mask during administration.1,14
Continue to: SABA + SAMA
SABA + SAMA. One combination formulation of the 2 short-term classes of drugs (albuterol [SABA] + ipratropium [SAMA]), US Food and Drug Administration (FDA)–approved for every-6-hour dosing, is available for SMI delivery devices and nebulizers. In the setting of a hospitalized patient who requires more frequent bronchodilator dosing, we use albuterol and ipratropium delivered separately (ie, dosed independently), with ipratropium dosed no more frequently than every 4 hours.
Long-acting agents
The mechanisms of long-acting agents are similar to those of their short-acting counterparts. The recommendation is to continue use of a long-acting bronchodilator during exacerbations, when feasible.1
LABA monotherapy reduces exacerbations that result in hospitalization (number needed to treat [NNT] = 39, to prevent 1 hospitalization in an 8-month period).15 Specifically, formoterol at higher dosages reduces exacerbations requiring hospitalization (NNT = 23, to prevent 1 exacerbation in a 6-month to 3-year period).15 Evidence supports better control of symptoms when a LABA is combined with a long-acting muscarinic antagonist (LAMA; discussed shortly).1,15
Adverse effects of LABAs include sinus tachycardia, tachyphylaxis, somatic tremors, and, less commonly, hypokalemia—the latter specific to the LABA dosage and concomitant use of a thiazide diuretic. Other adverse effects include a mild decrease in the partial pressure of O2 and, in patients with heart failure, increased oxygen consumption. Although higher dosages are not associated with an increased incidence of nonfatal adverse events, there appears to be no additional benefit to higher dosages in regard to mortality, particularly in patients with stable COPD.1,15
LAMA. Monotherapy with a LAMA reduces the severity of COPD symptoms and reduces the risk of exacerbations and hospitalization (NNT = 58, to prevent 1 hospitalization in a 3 to 48–month period).16 Tiotropium is superior to LABA as monotherapy in (1) reducing exacerbations (NNT = 33, to prevent 1 exacerbation in a 3 to 12–month period) and (2) being associated with a lower rate of all adverse events.17 LAMAs also confer additional benefit when used in combination with agents of other classes, which we discuss in a bit.
Continue to: The most commonly...
The most commonly reported adverse effect of a LAMA is dry mouth. Some patients report developing a bitter metallic taste in the mouth.1
ICSs are not recommended as monotherapy in COPD.1 However, an ICS can be combined with a LABA to reduce the risk of exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 However, this combination increases the risk of pneumonia in this population (number needed to harm [NNH] = 36, to cause 1 case of nonfatal pneumonia per year).18
ICSs increase the incidence of oropharyngeal candidiasis and hoarseness. In addition, ICSs increase the risk of pneumonia in some patients with COPD18—in particular, current smokers, patients ≥ 55 years of age, and patients with a history of pneumonia or exacerbations, a body mass index < 25, or severe COPD symptoms.1,18 ICS therapy does reduce the risk of COPD exacerbations in patients with a history of asthma or with eosinophilia > 300 cells/μL and in those who have a history of hospitalization for COPD exacerbations.19,20
The risk of pneumonia is not equal across all ICS agents. Fluticasone increases the risk of pneumonia (NNH = 23, to cause 1 case of pneumonia in a 22-month period).21 Budesonide showed no statistically significant increase in risk of pneumonia.22 However, further studies on the risk of pneumonia with budesonide are needed because those cited in the Cochrane review21 were much smaller trials, compared to trials of fluticasone, and of low-to-moderate quality. Furthermore, evidence is mixed whether ICS monotherapy in COPD worsens mortality during an 18-month study period.21-23
For these reasons, it’s reasonable to (1) exercise caution when considering the addition of an ICS to LABA therapy and (2) limit such a combination to the setting of severe disease (as discussed already).
Continue to: LABA + LAMA
LABA + LAMA. In a trial of patients with moderate-to-severe COPD, combining a LABA and a LAMA did not reduce the risk of exacerbations or hospitalizations, compared to LABA or LAMA monotherapy, but did improve subjects’ reported daily symptoms and quality of life scores (using the St. George’s Respiratory Questionnaireb; NNT = 14 [LAMA monotherapy] and NNT = 9 [LABA monotherapy], both in a 3 to 12–month period).24 However, another study that looked at patients with moderate-to-severe COPD found that combining a LABA and a LAMA led to fewer exacerbations (NNT = 22, to prevent 1 exacerbation in a 3 to 12–month period) and a lower risk of pneumonia (NNT = 93, to prevent 1 case of pneumonia in a 3 to 12–month period) than LABA + ICS.25
LABA + ICS. This dual therapy is falling out of favor, compared to treatment with LABA + LAMA, because LABA + ICS formulations are less effective at reducing exacerbations and increase the risk of pneumonia in patients with moderate-to-severe COPD.1,25 However, LABA + ICS therapy still has a role in a subset of patients with COPD (discussed in the section on ICS). A LABA combined with an ICS does reduce exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 Expect that the reported rates of candidiasis, hoarseness, and pneumonia associated with an ICS will be similar with LABA + ICS.18
LABA + LAMA + ICS. These are the newest combination inhaled agents approved for clinical use. It is recommended that escalation to such triple therapy be reserved for patients with persistent dyspnea on LAMA + LABA therapy and who have the factors (previously described) that suggest benefit from adding an ICS.1 Several clinical trials have provided guidance:
- In the 2018 TRIBUTE trial,26 beclometasone (ICS) + formoterol (LABA) + glycopyrronium (LAMA) c outperformed indacaterol (LABA) + glycopyrronium for preventing moderate-to-severe exacerbations (NNT = 11, to prevent 1 exacerbation per year) in patients with symptomatic COPD who have severe or very severe airflow resistance and a history of a moderate-to-severe exacerbation during the previous year.
- In the 2017 TRINITY trial,27 beclometasone + formoterol + glycopyrroniumc outperformed tiotropium (LAMA) in preventing moderate-to-severe exacerbations (NNT = 9, to prevent 1 exacerbation per year) in patients with an FEV1 < 50% and a history of ≥ 1 moderate-to-severe exacerbation during the previous year.
- In the 2020 ETHOS trial,28 budesonide + formoterol + glycopyrronium (approved by the FDA in 2020 under the brand name Breztri) outperformed both glycopyrrolate + formoterol (LABA) and budesonide (ICS) + formoterol in preventing moderate-to-severe exacerbations (NNT = 56 and 34, respectively, to prevent 1 exacerbation per year) in patients with moderate-to-severe COPD who had a history of ≥ 1 exacerbation in the previous year. Additionally, higher-dose budesonide + formoterol + glycopyrronium reduced 1-year mortality to a modest degree compared to glycopyrrolate + formoterol (NNT = 100, to prevent 1 death in a 12-month period).
- A 2016 Cochrane review that compared tiotropium + LABA + ICS to tiotropium monotherapy29 showed improvement in FEV1 and patient-reported symptoms and quality of life scores. However, the review showed no difference in exacerbations or hospitalizations over a 1-year period.
Mitigating environmental exposures that affect inhaler medication efficacy
Tobacco smoke. Emphasizing smoking cessation is highly relevant in patients who are still smoking. Smoking impedes the efficacy of ICSs in reducing exacerbations of COPD.30 Along with improved lung function, former smokers with COPD experience fewer exacerbations (NNT = 73, to prevent 1 exacerbation in a 4-year period for all former smokers; NNT = 33, to do so for smokers who quit > 10 years ago).31,32
A 2005 Veterans Health Administration study showed reduced mortality in smokers who were enrolled in a 10-week smoking cessation program, had access to nicotine replacement therapy, and received strong physician messaging.33 Despite a 20% to 25% quit rate, the NNT was 56 to prevent 1 death in 14.5 years across the entire group. It is worth having patients take advantage of this 3-pronged approach if it is available in your community or health system.
Continue to: Exposure to air pollution
Exposure to air pollution. Air pollutants other than tobacco smoke remain important modifiable factors that impact COPD. These include organic and inorganic dusts, chemical agents and fumes, and burning of solid biomass (eg, wood, coal) indoors in open fires or poorly functioning stoves.1 With this risk in mind, counsel patients regarding efficient home ventilation, use of nonpolluting cooking stoves, and the reduction of occupational exposure to these potential irritants.
GOLD approach to starting and adjusting inhaled therapy
Initiating inhaled therapy
A good resource for family physicians is the GOLD refined ABCD assessment scheme for initiating inhaler therapy that integrates symptoms and exacerbations (TABLE 31). To assess the severity of dyspnea, either the Modified Medical Research Council (mMRC) Questionnaire or COPD Assessment Test (CAT) can be used. A moderate exacerbation requires an oral corticosteroid or antibiotic, or both; a severe exacerbation requires an emergency department visit or hospitalization, or both. TABLE 31 offers a guide to choosing initial therapy based on these factors.1
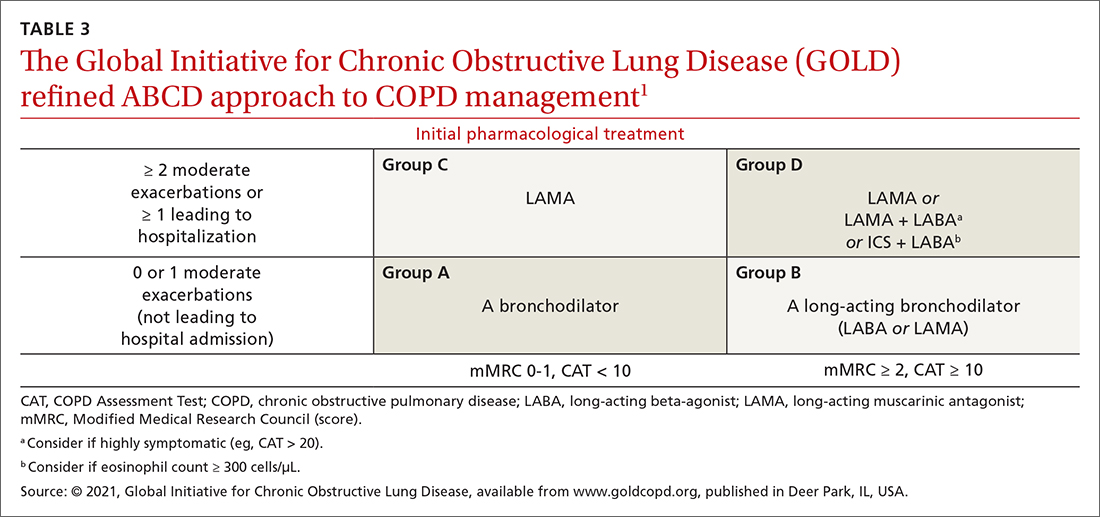
Following up on and adjusting an inhaler regimen
Adjust inhaler pharmacotherapy based on whether exacerbations or daily symptoms of dyspnea are more bothersome to the patient. Escalation of therapy involves adding other long-acting agents and is warranted for patients with exacerbations or severe or worsening dyspnea. Before escalating therapy with additional agents, reassess the appropriateness of the delivery device that the patient has been using and assess their adherence to the prescribed regimen.1
Dyspnea predominates. Escalate with LABA + LAMA. For a patient already taking an ICS, consider removing that ICS if the original indication was inappropriate, no response to treatment has been noted, or pneumonia develops.1
Exacerbations predominate. Escalate with LABA + LAMA or with LABA + ICS. Consider adding an ICS in patients who have a history of asthma, eosinophilia > 300 cells/uL, or eosinophilia > 100 cells/uL and 2 moderate exacerbations or 1 severe (ie, hospitalizing) exacerbation. This addition of an ICS results in dual or triple therapy (ie, either LABA + ICS or LABA + LAMA + ICS).1
Continue to: Unclear what predominates?
Unclear what predominates? Follow the exacerbation predominance pathway.1
Additional decision-making might be necessary in several circumstances:
- For the patient who requires further titration beyond these pathways, consider triple therapy as LABA + LAMA + ICS, unless the eosinophil count is < 100 cell/μL.1
- Consider de-escalating ICS therapy if the patient develops pneumonia, there is a lack of demonstrated benefit, or the initial indication was uncertain or inappropriate.
- For the patient who continues to have significant dyspnea despite dual or triple therapy, consider investigating and treating other causes of dyspnea.1
Last, keep in mind that evidence is limited regarding escalating the dosage of these agents (1) beyond what is listed in TABLE 21 and (2) in specific instances mentioned in the discussion of each inhaler class.
a www.copdfoundation.org/Learn-More/EducationalMaterials-Resources/Educational-Video-Series.aspx
b www.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
CORRESPONDENCE
Michael Arnold, DO, FAAFP, Carl R. Darnall Army Medical Center, Uniformed Service University, 36065 Santa Fe Avenue, Fort Hood, TX 76544; [email protected]
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2022 Report. Accessed August 15, 2022. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
2. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. doi:10.1186/s12931-017-0710-y
3. Haidl P, Heindl S, Siemon K, et al. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75. doi: 10.1016/j.rmed.2016.07.013
4. van Geffen WH, Douma WR, Slebos DJ, et al. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;2016:CD011826. doi:10.1002/14651858.CD011826.pub2
5. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30:381-387. doi:10.1089/jamp.2017.1416
6. Iwanaga T, Tohda Y, Nakamura S, et al. The Respimat soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39:1021-1030. doi:10.1007/s40261-019-00835-z
7. Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234. doi:10.1177/1479973119901234
8. Sharma G, Mahler DA, Mayorga VM, et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4:217-224. doi: 10.15326/jcopdf.4.3.2017.0183
9. Chen SY, Huang CK, Peng HC, et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12:704316. doi: 10.3389/fphar.2021.704316
10. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930-938. doi:10.1016/j.rmed.2011.01.005
11. Fathima M, Bawa Z, Mitchell B, et al. COPD management in community pharmacy results in improved inhaler use, immunization rate, COPD action plan ownership, COPD knowledge, and reductions in exacerbation rates. Int J Chron Obstruct Pulmon Dis. 2021;16:519-533. doi: 10.2147/COPD.S288792
12. van der Molen T, van Boven JF, Maguire T, et al. Optimizing identification and management of COPD patients – reviewing the role of the community pharmacist. Br J Clin Pharmacol. 2017;83:192-201. doi: 10.1111/bcp.13087
13. Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm. 2015;72:1026-1035. doi:10.2146/ajhp140551
14. Brown CD, McCrory DC, White J. Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2001;2001:CD002984. doi: 10.1002/14651858.CD002984
15. Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD010177. doi: 10.1002/14651858.CD010177.pub2
16. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;2014:CD009285. doi:10.1002/14651858.CD009285.pub3
17. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD009157. doi:10.1002/14651858.CD009157.pub2
18. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD006829. doi: 10.1002/14651858.CD006829.pub2
19. Yun JH, Lamb A, Chase R, et al; . Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
20. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52:1801219. doi:10.1183/13993003.01219-2018
21. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014:CD010115. doi: 10.1002/14651858.CD010115.pub2
22. Calverley PMA, Anderson JA, Celli B, et al; TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789. doi:10.1056/NEJMoa063070
23. Vestbo J, Anderson JA, Brook RD, et al; SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817-1826. doi:10.1016/S0140-6736(16)30069-1
24. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989. doi:10.1002/14651858.CD008989.pub3
25. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066. doi:10.1002/14651858.CD012066.pub2
26. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 208;391:1076-1084. doi:10.1016/S0140-6736(18)30206-X
27. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919-1929. doi: 10.1016/S0140-6736(17)30188-5
28. Rabe KF, Martinez FJ, Ferguson GT, et al; ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35-48. doi:10.1056/NEJMoa1916046
29. Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta2-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 20162016:CD008532. doi: 10.1002/14651858.CD008532.pub3
30. Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10:e037509. doi:10.1136/bmjopen-2020-037509
31. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675-679. doi:10.1164/rccm.2112096
32. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457-463. doi:10.1007/s11606-009-0907-y
33. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239. doi: 10.7326/0003-4819-142-4-200502150-00005
Managing chronic obstructive pulmonary disease (COPD) presents a significant challenge to busy clinicians in many ways, especially when one is approaching the long list of inhaled pharmaceutical agents with an eye toward a cost-effective, patient-centered regimen. Inhaled agents remain expensive, with few available in generic form.
Our primary goal in this article is to detail these agents’ utility, limitations, and relative cost. Specifically, we review why the following considerations are important:
- Choose the right delivery device and drug while considering patient factors.
- Provide patient education through allied health professionals.
- Reduce environmental exposures.
- Rethink the use of inhaled corticosteroids (ICS).
- Understand the role of dual therapy and triple therapy.
There are numerous other treatment modalities for COPD that are recommended in national and international practice guidelines, including vaccination, pulmonary rehabilitation, home visits, phosphodiesterase-4 inhibitors, oral glucocorticoids, supplemental oxygen, and ventilatory support.1 Discussion of those modalities is beyond the scope of this review.
Pathophysiology and pharmacotherapy targets
COPD is characterized by persistent respiratory symptoms and airflow limitation, usually due to airway or alveolar abnormalities, or both, caused by environmental and host factors.2 Sustained lung parenchymal irritation results from exposure to noxious fumes generated by tobacco, pollution, chemicals, and cleaning agents. Host factors include lung immaturity at birth; genetic mutations, such as alpha-1 antitrypsin deficiency and dysregulation of elastase; and increased reactivity of bronchial smooth muscles, similar to what is seen in asthma.1

Improving ventilation with the intention of relieving dyspnea is the goal of inhaler pharmacotherapy; targets include muscarinic receptors and beta 2-adrenergic receptors that act on bronchial smooth muscle and the autonomic nervous system. Immune modulators, such as corticosteroids, help reduce inflammation around airways.1 Recent pharmacotherapeutic developments include combinations of inhaled medications and expanding options for devices that deliver drugs.
Delivery devices: Options and optimizing their use
Three principal types of inhaler devices are available: pressurized metered-dose inhalers (MDIs), dry-powder inhalers (DPIs), and soft-mist inhalers (SMIs). These devices, and nebulizers, facilitate medication delivery into the lungs (TABLE 13-9).

Errors in using inhalers affect outcome. Correct inhaler technique is essential for optimal delivery of inhaled medications. Errors in technique when using an inhaled delivery device lead to inadequate drug delivery and are associated with poor outcomes: 90% of patients make errors that are classified as critical (ie, those that reduce drug delivery) or noncritical.2 Critical inhaler errors increase the risk of hospitalization and emergency department visits, and can necessitate a course of oral corticosteroids.10 Many critical errors are device specific; several such errors are described in TABLE 1.3-9
Continue to: Patient education
Patient education is necessary to ensure that drug is delivered to the patient consistently, with the same expectation of effect seen in efficacy studies (which usually provide rigorous inhaler technique training and require demonstration of proficiency).1,2,10 For the busy clinician, a multidisciplinary approach, discussed shortly, can help. Guidelines developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that inhaler technique be reassessed at every visit and when evaluating treatment response.1TABLE 13-9 provides information on each device type, patient requirements for use, proper technique, common errors in use, and tips for optimizing delivery.
Inhaler education and assessment of technique that is provided to patients in collaboration with a clinical pharmacist, nursing staff, and a respiratory therapist can help alleviate the pressure on a time-constrained primary care physician. Furthermore, pharmacist involvement in the COPD management team meaningfully improves inhaler technique and medication adherence.6,7 Intervention by a pharmacist correlates with a significant reduction in number of exacerbations; an increased likelihood that the patient has a COPD care plan and has received the pneumococcal vaccine; and an improvement in the mean health-related quality of life.11,12
In primary care practices that lack robust multidisciplinary resources, we recommend utilizing virtual resources, such as educational videos, to allow face-to-face or virtual education. A free source of such resources is the COPD Foundation,a a not-for-profit organization funded partly by industry.
Short- and long-acting inhaled medications for COPD
Each class of inhaled medication for treating COPD is discussed broadly in the following sections. TABLE 21 provides details about individual drugs, devices available to deliver them, and starting dosages.


Short-acting agents
These drugs are available in MDI, SMI, and nebulizer delivery devices. When portability and equipment burden are important to the patient, we recommend an MDI over a nebulizer; an MDI is as efficacious as a nebulizer in improving forced expiratory volume in 1 second (FEV1) and reducing the length of hospital stay for exacerbations.4
Continue to: SABAs
Short-acting beta 2-adrenergic agonists (or beta-agonists [SABAs]). Beta-agonists are typically used to treat exacerbations. They facilitate bronchodilation by upregulating cyclic adenosine monophosphate, preventing smooth-muscle contraction, and reducing dynamic hyperinflation. The effect of a SABA lasts 4 to 6 hours.
In general, SABAs are not recommended for daily use in stable COPD. However, they can be useful, and appropriate, for treating occasional dyspnea and can confer additional symptom improvement when used occasionally along with a long-acting beta 2-adrenergic agonist (or beta-agonist [LABA]; discussed later).1
Albuterol, a commonly used SABA, is less expensive than, and just as effective as, same-class levalbuterol for decreasing breathlessness associated with acute exacerbations. There is no significant difference between the 2 drugs in regard to the incidence of tachycardia or palpitations in patients with cardiovascular disease.13
Although no significant differences have been observed in outcomes when a nebulizer or an MDI is used to administer a SABA, it’s wise to avoid continuous SABA nebulizer therapy, due to the increased risk of disease transmission through the generation of droplets.1,4 Instead, it’s appropriate to use an MDI regimen of 1 to 3 puffs every hour for 2 to 3 hours, followed by 1 to 3 puffs every 2 to 4 hours thereafter, based on the patient’s response.1,4
Short-acting muscarinic antagonists (SAMAs). Muscarinic antagonists achieve bronchodilation by blocking acetylcholine on muscarinic receptors. We do not specifically recommend SAMAs over SABAs for treating COPD exacerbations in our patients: There is no difference in improvement in FEV1 during an acute exacerbation. Nebulized delivery of a SAMA raises concern for an increase in the risk of acute narrow-angle glaucoma, a risk that can be reduced by using a mask during administration.1,14
Continue to: SABA + SAMA
SABA + SAMA. One combination formulation of the 2 short-term classes of drugs (albuterol [SABA] + ipratropium [SAMA]), US Food and Drug Administration (FDA)–approved for every-6-hour dosing, is available for SMI delivery devices and nebulizers. In the setting of a hospitalized patient who requires more frequent bronchodilator dosing, we use albuterol and ipratropium delivered separately (ie, dosed independently), with ipratropium dosed no more frequently than every 4 hours.
Long-acting agents
The mechanisms of long-acting agents are similar to those of their short-acting counterparts. The recommendation is to continue use of a long-acting bronchodilator during exacerbations, when feasible.1
LABA monotherapy reduces exacerbations that result in hospitalization (number needed to treat [NNT] = 39, to prevent 1 hospitalization in an 8-month period).15 Specifically, formoterol at higher dosages reduces exacerbations requiring hospitalization (NNT = 23, to prevent 1 exacerbation in a 6-month to 3-year period).15 Evidence supports better control of symptoms when a LABA is combined with a long-acting muscarinic antagonist (LAMA; discussed shortly).1,15
Adverse effects of LABAs include sinus tachycardia, tachyphylaxis, somatic tremors, and, less commonly, hypokalemia—the latter specific to the LABA dosage and concomitant use of a thiazide diuretic. Other adverse effects include a mild decrease in the partial pressure of O2 and, in patients with heart failure, increased oxygen consumption. Although higher dosages are not associated with an increased incidence of nonfatal adverse events, there appears to be no additional benefit to higher dosages in regard to mortality, particularly in patients with stable COPD.1,15
LAMA. Monotherapy with a LAMA reduces the severity of COPD symptoms and reduces the risk of exacerbations and hospitalization (NNT = 58, to prevent 1 hospitalization in a 3 to 48–month period).16 Tiotropium is superior to LABA as monotherapy in (1) reducing exacerbations (NNT = 33, to prevent 1 exacerbation in a 3 to 12–month period) and (2) being associated with a lower rate of all adverse events.17 LAMAs also confer additional benefit when used in combination with agents of other classes, which we discuss in a bit.
Continue to: The most commonly...
The most commonly reported adverse effect of a LAMA is dry mouth. Some patients report developing a bitter metallic taste in the mouth.1
ICSs are not recommended as monotherapy in COPD.1 However, an ICS can be combined with a LABA to reduce the risk of exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 However, this combination increases the risk of pneumonia in this population (number needed to harm [NNH] = 36, to cause 1 case of nonfatal pneumonia per year).18
ICSs increase the incidence of oropharyngeal candidiasis and hoarseness. In addition, ICSs increase the risk of pneumonia in some patients with COPD18—in particular, current smokers, patients ≥ 55 years of age, and patients with a history of pneumonia or exacerbations, a body mass index < 25, or severe COPD symptoms.1,18 ICS therapy does reduce the risk of COPD exacerbations in patients with a history of asthma or with eosinophilia > 300 cells/μL and in those who have a history of hospitalization for COPD exacerbations.19,20
The risk of pneumonia is not equal across all ICS agents. Fluticasone increases the risk of pneumonia (NNH = 23, to cause 1 case of pneumonia in a 22-month period).21 Budesonide showed no statistically significant increase in risk of pneumonia.22 However, further studies on the risk of pneumonia with budesonide are needed because those cited in the Cochrane review21 were much smaller trials, compared to trials of fluticasone, and of low-to-moderate quality. Furthermore, evidence is mixed whether ICS monotherapy in COPD worsens mortality during an 18-month study period.21-23
For these reasons, it’s reasonable to (1) exercise caution when considering the addition of an ICS to LABA therapy and (2) limit such a combination to the setting of severe disease (as discussed already).
Continue to: LABA + LAMA
LABA + LAMA. In a trial of patients with moderate-to-severe COPD, combining a LABA and a LAMA did not reduce the risk of exacerbations or hospitalizations, compared to LABA or LAMA monotherapy, but did improve subjects’ reported daily symptoms and quality of life scores (using the St. George’s Respiratory Questionnaireb; NNT = 14 [LAMA monotherapy] and NNT = 9 [LABA monotherapy], both in a 3 to 12–month period).24 However, another study that looked at patients with moderate-to-severe COPD found that combining a LABA and a LAMA led to fewer exacerbations (NNT = 22, to prevent 1 exacerbation in a 3 to 12–month period) and a lower risk of pneumonia (NNT = 93, to prevent 1 case of pneumonia in a 3 to 12–month period) than LABA + ICS.25
LABA + ICS. This dual therapy is falling out of favor, compared to treatment with LABA + LAMA, because LABA + ICS formulations are less effective at reducing exacerbations and increase the risk of pneumonia in patients with moderate-to-severe COPD.1,25 However, LABA + ICS therapy still has a role in a subset of patients with COPD (discussed in the section on ICS). A LABA combined with an ICS does reduce exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 Expect that the reported rates of candidiasis, hoarseness, and pneumonia associated with an ICS will be similar with LABA + ICS.18
LABA + LAMA + ICS. These are the newest combination inhaled agents approved for clinical use. It is recommended that escalation to such triple therapy be reserved for patients with persistent dyspnea on LAMA + LABA therapy and who have the factors (previously described) that suggest benefit from adding an ICS.1 Several clinical trials have provided guidance:
- In the 2018 TRIBUTE trial,26 beclometasone (ICS) + formoterol (LABA) + glycopyrronium (LAMA) c outperformed indacaterol (LABA) + glycopyrronium for preventing moderate-to-severe exacerbations (NNT = 11, to prevent 1 exacerbation per year) in patients with symptomatic COPD who have severe or very severe airflow resistance and a history of a moderate-to-severe exacerbation during the previous year.
- In the 2017 TRINITY trial,27 beclometasone + formoterol + glycopyrroniumc outperformed tiotropium (LAMA) in preventing moderate-to-severe exacerbations (NNT = 9, to prevent 1 exacerbation per year) in patients with an FEV1 < 50% and a history of ≥ 1 moderate-to-severe exacerbation during the previous year.
- In the 2020 ETHOS trial,28 budesonide + formoterol + glycopyrronium (approved by the FDA in 2020 under the brand name Breztri) outperformed both glycopyrrolate + formoterol (LABA) and budesonide (ICS) + formoterol in preventing moderate-to-severe exacerbations (NNT = 56 and 34, respectively, to prevent 1 exacerbation per year) in patients with moderate-to-severe COPD who had a history of ≥ 1 exacerbation in the previous year. Additionally, higher-dose budesonide + formoterol + glycopyrronium reduced 1-year mortality to a modest degree compared to glycopyrrolate + formoterol (NNT = 100, to prevent 1 death in a 12-month period).
- A 2016 Cochrane review that compared tiotropium + LABA + ICS to tiotropium monotherapy29 showed improvement in FEV1 and patient-reported symptoms and quality of life scores. However, the review showed no difference in exacerbations or hospitalizations over a 1-year period.
Mitigating environmental exposures that affect inhaler medication efficacy
Tobacco smoke. Emphasizing smoking cessation is highly relevant in patients who are still smoking. Smoking impedes the efficacy of ICSs in reducing exacerbations of COPD.30 Along with improved lung function, former smokers with COPD experience fewer exacerbations (NNT = 73, to prevent 1 exacerbation in a 4-year period for all former smokers; NNT = 33, to do so for smokers who quit > 10 years ago).31,32
A 2005 Veterans Health Administration study showed reduced mortality in smokers who were enrolled in a 10-week smoking cessation program, had access to nicotine replacement therapy, and received strong physician messaging.33 Despite a 20% to 25% quit rate, the NNT was 56 to prevent 1 death in 14.5 years across the entire group. It is worth having patients take advantage of this 3-pronged approach if it is available in your community or health system.
Continue to: Exposure to air pollution
Exposure to air pollution. Air pollutants other than tobacco smoke remain important modifiable factors that impact COPD. These include organic and inorganic dusts, chemical agents and fumes, and burning of solid biomass (eg, wood, coal) indoors in open fires or poorly functioning stoves.1 With this risk in mind, counsel patients regarding efficient home ventilation, use of nonpolluting cooking stoves, and the reduction of occupational exposure to these potential irritants.
GOLD approach to starting and adjusting inhaled therapy
Initiating inhaled therapy
A good resource for family physicians is the GOLD refined ABCD assessment scheme for initiating inhaler therapy that integrates symptoms and exacerbations (TABLE 31). To assess the severity of dyspnea, either the Modified Medical Research Council (mMRC) Questionnaire or COPD Assessment Test (CAT) can be used. A moderate exacerbation requires an oral corticosteroid or antibiotic, or both; a severe exacerbation requires an emergency department visit or hospitalization, or both. TABLE 31 offers a guide to choosing initial therapy based on these factors.1

Following up on and adjusting an inhaler regimen
Adjust inhaler pharmacotherapy based on whether exacerbations or daily symptoms of dyspnea are more bothersome to the patient. Escalation of therapy involves adding other long-acting agents and is warranted for patients with exacerbations or severe or worsening dyspnea. Before escalating therapy with additional agents, reassess the appropriateness of the delivery device that the patient has been using and assess their adherence to the prescribed regimen.1
Dyspnea predominates. Escalate with LABA + LAMA. For a patient already taking an ICS, consider removing that ICS if the original indication was inappropriate, no response to treatment has been noted, or pneumonia develops.1
Exacerbations predominate. Escalate with LABA + LAMA or with LABA + ICS. Consider adding an ICS in patients who have a history of asthma, eosinophilia > 300 cells/uL, or eosinophilia > 100 cells/uL and 2 moderate exacerbations or 1 severe (ie, hospitalizing) exacerbation. This addition of an ICS results in dual or triple therapy (ie, either LABA + ICS or LABA + LAMA + ICS).1
Continue to: Unclear what predominates?
Unclear what predominates? Follow the exacerbation predominance pathway.1
Additional decision-making might be necessary in several circumstances:
- For the patient who requires further titration beyond these pathways, consider triple therapy as LABA + LAMA + ICS, unless the eosinophil count is < 100 cell/μL.1
- Consider de-escalating ICS therapy if the patient develops pneumonia, there is a lack of demonstrated benefit, or the initial indication was uncertain or inappropriate.
- For the patient who continues to have significant dyspnea despite dual or triple therapy, consider investigating and treating other causes of dyspnea.1
Last, keep in mind that evidence is limited regarding escalating the dosage of these agents (1) beyond what is listed in TABLE 21 and (2) in specific instances mentioned in the discussion of each inhaler class.
a www.copdfoundation.org/Learn-More/EducationalMaterials-Resources/Educational-Video-Series.aspx
b www.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
CORRESPONDENCE
Michael Arnold, DO, FAAFP, Carl R. Darnall Army Medical Center, Uniformed Service University, 36065 Santa Fe Avenue, Fort Hood, TX 76544; [email protected]
Managing chronic obstructive pulmonary disease (COPD) presents a significant challenge to busy clinicians in many ways, especially when one is approaching the long list of inhaled pharmaceutical agents with an eye toward a cost-effective, patient-centered regimen. Inhaled agents remain expensive, with few available in generic form.
Our primary goal in this article is to detail these agents’ utility, limitations, and relative cost. Specifically, we review why the following considerations are important:
- Choose the right delivery device and drug while considering patient factors.
- Provide patient education through allied health professionals.
- Reduce environmental exposures.
- Rethink the use of inhaled corticosteroids (ICS).
- Understand the role of dual therapy and triple therapy.
There are numerous other treatment modalities for COPD that are recommended in national and international practice guidelines, including vaccination, pulmonary rehabilitation, home visits, phosphodiesterase-4 inhibitors, oral glucocorticoids, supplemental oxygen, and ventilatory support.1 Discussion of those modalities is beyond the scope of this review.
Pathophysiology and pharmacotherapy targets
COPD is characterized by persistent respiratory symptoms and airflow limitation, usually due to airway or alveolar abnormalities, or both, caused by environmental and host factors.2 Sustained lung parenchymal irritation results from exposure to noxious fumes generated by tobacco, pollution, chemicals, and cleaning agents. Host factors include lung immaturity at birth; genetic mutations, such as alpha-1 antitrypsin deficiency and dysregulation of elastase; and increased reactivity of bronchial smooth muscles, similar to what is seen in asthma.1

Improving ventilation with the intention of relieving dyspnea is the goal of inhaler pharmacotherapy; targets include muscarinic receptors and beta 2-adrenergic receptors that act on bronchial smooth muscle and the autonomic nervous system. Immune modulators, such as corticosteroids, help reduce inflammation around airways.1 Recent pharmacotherapeutic developments include combinations of inhaled medications and expanding options for devices that deliver drugs.
Delivery devices: Options and optimizing their use
Three principal types of inhaler devices are available: pressurized metered-dose inhalers (MDIs), dry-powder inhalers (DPIs), and soft-mist inhalers (SMIs). These devices, and nebulizers, facilitate medication delivery into the lungs (TABLE 13-9).

Errors in using inhalers affect outcome. Correct inhaler technique is essential for optimal delivery of inhaled medications. Errors in technique when using an inhaled delivery device lead to inadequate drug delivery and are associated with poor outcomes: 90% of patients make errors that are classified as critical (ie, those that reduce drug delivery) or noncritical.2 Critical inhaler errors increase the risk of hospitalization and emergency department visits, and can necessitate a course of oral corticosteroids.10 Many critical errors are device specific; several such errors are described in TABLE 1.3-9
Continue to: Patient education
Patient education is necessary to ensure that drug is delivered to the patient consistently, with the same expectation of effect seen in efficacy studies (which usually provide rigorous inhaler technique training and require demonstration of proficiency).1,2,10 For the busy clinician, a multidisciplinary approach, discussed shortly, can help. Guidelines developed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that inhaler technique be reassessed at every visit and when evaluating treatment response.1TABLE 13-9 provides information on each device type, patient requirements for use, proper technique, common errors in use, and tips for optimizing delivery.
Inhaler education and assessment of technique that is provided to patients in collaboration with a clinical pharmacist, nursing staff, and a respiratory therapist can help alleviate the pressure on a time-constrained primary care physician. Furthermore, pharmacist involvement in the COPD management team meaningfully improves inhaler technique and medication adherence.6,7 Intervention by a pharmacist correlates with a significant reduction in number of exacerbations; an increased likelihood that the patient has a COPD care plan and has received the pneumococcal vaccine; and an improvement in the mean health-related quality of life.11,12
In primary care practices that lack robust multidisciplinary resources, we recommend utilizing virtual resources, such as educational videos, to allow face-to-face or virtual education. A free source of such resources is the COPD Foundation,a a not-for-profit organization funded partly by industry.
Short- and long-acting inhaled medications for COPD
Each class of inhaled medication for treating COPD is discussed broadly in the following sections. TABLE 21 provides details about individual drugs, devices available to deliver them, and starting dosages.


Short-acting agents
These drugs are available in MDI, SMI, and nebulizer delivery devices. When portability and equipment burden are important to the patient, we recommend an MDI over a nebulizer; an MDI is as efficacious as a nebulizer in improving forced expiratory volume in 1 second (FEV1) and reducing the length of hospital stay for exacerbations.4
Continue to: SABAs
Short-acting beta 2-adrenergic agonists (or beta-agonists [SABAs]). Beta-agonists are typically used to treat exacerbations. They facilitate bronchodilation by upregulating cyclic adenosine monophosphate, preventing smooth-muscle contraction, and reducing dynamic hyperinflation. The effect of a SABA lasts 4 to 6 hours.
In general, SABAs are not recommended for daily use in stable COPD. However, they can be useful, and appropriate, for treating occasional dyspnea and can confer additional symptom improvement when used occasionally along with a long-acting beta 2-adrenergic agonist (or beta-agonist [LABA]; discussed later).1
Albuterol, a commonly used SABA, is less expensive than, and just as effective as, same-class levalbuterol for decreasing breathlessness associated with acute exacerbations. There is no significant difference between the 2 drugs in regard to the incidence of tachycardia or palpitations in patients with cardiovascular disease.13
Although no significant differences have been observed in outcomes when a nebulizer or an MDI is used to administer a SABA, it’s wise to avoid continuous SABA nebulizer therapy, due to the increased risk of disease transmission through the generation of droplets.1,4 Instead, it’s appropriate to use an MDI regimen of 1 to 3 puffs every hour for 2 to 3 hours, followed by 1 to 3 puffs every 2 to 4 hours thereafter, based on the patient’s response.1,4
Short-acting muscarinic antagonists (SAMAs). Muscarinic antagonists achieve bronchodilation by blocking acetylcholine on muscarinic receptors. We do not specifically recommend SAMAs over SABAs for treating COPD exacerbations in our patients: There is no difference in improvement in FEV1 during an acute exacerbation. Nebulized delivery of a SAMA raises concern for an increase in the risk of acute narrow-angle glaucoma, a risk that can be reduced by using a mask during administration.1,14
Continue to: SABA + SAMA
SABA + SAMA. One combination formulation of the 2 short-term classes of drugs (albuterol [SABA] + ipratropium [SAMA]), US Food and Drug Administration (FDA)–approved for every-6-hour dosing, is available for SMI delivery devices and nebulizers. In the setting of a hospitalized patient who requires more frequent bronchodilator dosing, we use albuterol and ipratropium delivered separately (ie, dosed independently), with ipratropium dosed no more frequently than every 4 hours.
Long-acting agents
The mechanisms of long-acting agents are similar to those of their short-acting counterparts. The recommendation is to continue use of a long-acting bronchodilator during exacerbations, when feasible.1
LABA monotherapy reduces exacerbations that result in hospitalization (number needed to treat [NNT] = 39, to prevent 1 hospitalization in an 8-month period).15 Specifically, formoterol at higher dosages reduces exacerbations requiring hospitalization (NNT = 23, to prevent 1 exacerbation in a 6-month to 3-year period).15 Evidence supports better control of symptoms when a LABA is combined with a long-acting muscarinic antagonist (LAMA; discussed shortly).1,15
Adverse effects of LABAs include sinus tachycardia, tachyphylaxis, somatic tremors, and, less commonly, hypokalemia—the latter specific to the LABA dosage and concomitant use of a thiazide diuretic. Other adverse effects include a mild decrease in the partial pressure of O2 and, in patients with heart failure, increased oxygen consumption. Although higher dosages are not associated with an increased incidence of nonfatal adverse events, there appears to be no additional benefit to higher dosages in regard to mortality, particularly in patients with stable COPD.1,15
LAMA. Monotherapy with a LAMA reduces the severity of COPD symptoms and reduces the risk of exacerbations and hospitalization (NNT = 58, to prevent 1 hospitalization in a 3 to 48–month period).16 Tiotropium is superior to LABA as monotherapy in (1) reducing exacerbations (NNT = 33, to prevent 1 exacerbation in a 3 to 12–month period) and (2) being associated with a lower rate of all adverse events.17 LAMAs also confer additional benefit when used in combination with agents of other classes, which we discuss in a bit.
Continue to: The most commonly...
The most commonly reported adverse effect of a LAMA is dry mouth. Some patients report developing a bitter metallic taste in the mouth.1
ICSs are not recommended as monotherapy in COPD.1 However, an ICS can be combined with a LABA to reduce the risk of exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 However, this combination increases the risk of pneumonia in this population (number needed to harm [NNH] = 36, to cause 1 case of nonfatal pneumonia per year).18
ICSs increase the incidence of oropharyngeal candidiasis and hoarseness. In addition, ICSs increase the risk of pneumonia in some patients with COPD18—in particular, current smokers, patients ≥ 55 years of age, and patients with a history of pneumonia or exacerbations, a body mass index < 25, or severe COPD symptoms.1,18 ICS therapy does reduce the risk of COPD exacerbations in patients with a history of asthma or with eosinophilia > 300 cells/μL and in those who have a history of hospitalization for COPD exacerbations.19,20
The risk of pneumonia is not equal across all ICS agents. Fluticasone increases the risk of pneumonia (NNH = 23, to cause 1 case of pneumonia in a 22-month period).21 Budesonide showed no statistically significant increase in risk of pneumonia.22 However, further studies on the risk of pneumonia with budesonide are needed because those cited in the Cochrane review21 were much smaller trials, compared to trials of fluticasone, and of low-to-moderate quality. Furthermore, evidence is mixed whether ICS monotherapy in COPD worsens mortality during an 18-month study period.21-23
For these reasons, it’s reasonable to (1) exercise caution when considering the addition of an ICS to LABA therapy and (2) limit such a combination to the setting of severe disease (as discussed already).
Continue to: LABA + LAMA
LABA + LAMA. In a trial of patients with moderate-to-severe COPD, combining a LABA and a LAMA did not reduce the risk of exacerbations or hospitalizations, compared to LABA or LAMA monotherapy, but did improve subjects’ reported daily symptoms and quality of life scores (using the St. George’s Respiratory Questionnaireb; NNT = 14 [LAMA monotherapy] and NNT = 9 [LABA monotherapy], both in a 3 to 12–month period).24 However, another study that looked at patients with moderate-to-severe COPD found that combining a LABA and a LAMA led to fewer exacerbations (NNT = 22, to prevent 1 exacerbation in a 3 to 12–month period) and a lower risk of pneumonia (NNT = 93, to prevent 1 case of pneumonia in a 3 to 12–month period) than LABA + ICS.25
LABA + ICS. This dual therapy is falling out of favor, compared to treatment with LABA + LAMA, because LABA + ICS formulations are less effective at reducing exacerbations and increase the risk of pneumonia in patients with moderate-to-severe COPD.1,25 However, LABA + ICS therapy still has a role in a subset of patients with COPD (discussed in the section on ICS). A LABA combined with an ICS does reduce exacerbations in patients with severe COPD (NNT = 22, to prevent 1 exacerbation per year).18 Expect that the reported rates of candidiasis, hoarseness, and pneumonia associated with an ICS will be similar with LABA + ICS.18
LABA + LAMA + ICS. These are the newest combination inhaled agents approved for clinical use. It is recommended that escalation to such triple therapy be reserved for patients with persistent dyspnea on LAMA + LABA therapy and who have the factors (previously described) that suggest benefit from adding an ICS.1 Several clinical trials have provided guidance:
- In the 2018 TRIBUTE trial,26 beclometasone (ICS) + formoterol (LABA) + glycopyrronium (LAMA) c outperformed indacaterol (LABA) + glycopyrronium for preventing moderate-to-severe exacerbations (NNT = 11, to prevent 1 exacerbation per year) in patients with symptomatic COPD who have severe or very severe airflow resistance and a history of a moderate-to-severe exacerbation during the previous year.
- In the 2017 TRINITY trial,27 beclometasone + formoterol + glycopyrroniumc outperformed tiotropium (LAMA) in preventing moderate-to-severe exacerbations (NNT = 9, to prevent 1 exacerbation per year) in patients with an FEV1 < 50% and a history of ≥ 1 moderate-to-severe exacerbation during the previous year.
- In the 2020 ETHOS trial,28 budesonide + formoterol + glycopyrronium (approved by the FDA in 2020 under the brand name Breztri) outperformed both glycopyrrolate + formoterol (LABA) and budesonide (ICS) + formoterol in preventing moderate-to-severe exacerbations (NNT = 56 and 34, respectively, to prevent 1 exacerbation per year) in patients with moderate-to-severe COPD who had a history of ≥ 1 exacerbation in the previous year. Additionally, higher-dose budesonide + formoterol + glycopyrronium reduced 1-year mortality to a modest degree compared to glycopyrrolate + formoterol (NNT = 100, to prevent 1 death in a 12-month period).
- A 2016 Cochrane review that compared tiotropium + LABA + ICS to tiotropium monotherapy29 showed improvement in FEV1 and patient-reported symptoms and quality of life scores. However, the review showed no difference in exacerbations or hospitalizations over a 1-year period.
Mitigating environmental exposures that affect inhaler medication efficacy
Tobacco smoke. Emphasizing smoking cessation is highly relevant in patients who are still smoking. Smoking impedes the efficacy of ICSs in reducing exacerbations of COPD.30 Along with improved lung function, former smokers with COPD experience fewer exacerbations (NNT = 73, to prevent 1 exacerbation in a 4-year period for all former smokers; NNT = 33, to do so for smokers who quit > 10 years ago).31,32
A 2005 Veterans Health Administration study showed reduced mortality in smokers who were enrolled in a 10-week smoking cessation program, had access to nicotine replacement therapy, and received strong physician messaging.33 Despite a 20% to 25% quit rate, the NNT was 56 to prevent 1 death in 14.5 years across the entire group. It is worth having patients take advantage of this 3-pronged approach if it is available in your community or health system.
Continue to: Exposure to air pollution
Exposure to air pollution. Air pollutants other than tobacco smoke remain important modifiable factors that impact COPD. These include organic and inorganic dusts, chemical agents and fumes, and burning of solid biomass (eg, wood, coal) indoors in open fires or poorly functioning stoves.1 With this risk in mind, counsel patients regarding efficient home ventilation, use of nonpolluting cooking stoves, and the reduction of occupational exposure to these potential irritants.
GOLD approach to starting and adjusting inhaled therapy
Initiating inhaled therapy
A good resource for family physicians is the GOLD refined ABCD assessment scheme for initiating inhaler therapy that integrates symptoms and exacerbations (TABLE 31). To assess the severity of dyspnea, either the Modified Medical Research Council (mMRC) Questionnaire or COPD Assessment Test (CAT) can be used. A moderate exacerbation requires an oral corticosteroid or antibiotic, or both; a severe exacerbation requires an emergency department visit or hospitalization, or both. TABLE 31 offers a guide to choosing initial therapy based on these factors.1

Following up on and adjusting an inhaler regimen
Adjust inhaler pharmacotherapy based on whether exacerbations or daily symptoms of dyspnea are more bothersome to the patient. Escalation of therapy involves adding other long-acting agents and is warranted for patients with exacerbations or severe or worsening dyspnea. Before escalating therapy with additional agents, reassess the appropriateness of the delivery device that the patient has been using and assess their adherence to the prescribed regimen.1
Dyspnea predominates. Escalate with LABA + LAMA. For a patient already taking an ICS, consider removing that ICS if the original indication was inappropriate, no response to treatment has been noted, or pneumonia develops.1
Exacerbations predominate. Escalate with LABA + LAMA or with LABA + ICS. Consider adding an ICS in patients who have a history of asthma, eosinophilia > 300 cells/uL, or eosinophilia > 100 cells/uL and 2 moderate exacerbations or 1 severe (ie, hospitalizing) exacerbation. This addition of an ICS results in dual or triple therapy (ie, either LABA + ICS or LABA + LAMA + ICS).1
Continue to: Unclear what predominates?
Unclear what predominates? Follow the exacerbation predominance pathway.1
Additional decision-making might be necessary in several circumstances:
- For the patient who requires further titration beyond these pathways, consider triple therapy as LABA + LAMA + ICS, unless the eosinophil count is < 100 cell/μL.1
- Consider de-escalating ICS therapy if the patient develops pneumonia, there is a lack of demonstrated benefit, or the initial indication was uncertain or inappropriate.
- For the patient who continues to have significant dyspnea despite dual or triple therapy, consider investigating and treating other causes of dyspnea.1
Last, keep in mind that evidence is limited regarding escalating the dosage of these agents (1) beyond what is listed in TABLE 21 and (2) in specific instances mentioned in the discussion of each inhaler class.
a www.copdfoundation.org/Learn-More/EducationalMaterials-Resources/Educational-Video-Series.aspx
b www.thoracic.org/members/assemblies/assemblies/srn/questionaires/sgrq.php
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
c Not an FDA-approved combination inhaled-agent treatment; approved in the European Union, under various brand names, by the European Medicines Agency.
CORRESPONDENCE
Michael Arnold, DO, FAAFP, Carl R. Darnall Army Medical Center, Uniformed Service University, 36065 Santa Fe Avenue, Fort Hood, TX 76544; [email protected]
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2022 Report. Accessed August 15, 2022. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
2. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. doi:10.1186/s12931-017-0710-y
3. Haidl P, Heindl S, Siemon K, et al. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75. doi: 10.1016/j.rmed.2016.07.013
4. van Geffen WH, Douma WR, Slebos DJ, et al. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;2016:CD011826. doi:10.1002/14651858.CD011826.pub2
5. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30:381-387. doi:10.1089/jamp.2017.1416
6. Iwanaga T, Tohda Y, Nakamura S, et al. The Respimat soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39:1021-1030. doi:10.1007/s40261-019-00835-z
7. Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234. doi:10.1177/1479973119901234
8. Sharma G, Mahler DA, Mayorga VM, et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4:217-224. doi: 10.15326/jcopdf.4.3.2017.0183
9. Chen SY, Huang CK, Peng HC, et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12:704316. doi: 10.3389/fphar.2021.704316
10. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930-938. doi:10.1016/j.rmed.2011.01.005
11. Fathima M, Bawa Z, Mitchell B, et al. COPD management in community pharmacy results in improved inhaler use, immunization rate, COPD action plan ownership, COPD knowledge, and reductions in exacerbation rates. Int J Chron Obstruct Pulmon Dis. 2021;16:519-533. doi: 10.2147/COPD.S288792
12. van der Molen T, van Boven JF, Maguire T, et al. Optimizing identification and management of COPD patients – reviewing the role of the community pharmacist. Br J Clin Pharmacol. 2017;83:192-201. doi: 10.1111/bcp.13087
13. Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm. 2015;72:1026-1035. doi:10.2146/ajhp140551
14. Brown CD, McCrory DC, White J. Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2001;2001:CD002984. doi: 10.1002/14651858.CD002984
15. Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD010177. doi: 10.1002/14651858.CD010177.pub2
16. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;2014:CD009285. doi:10.1002/14651858.CD009285.pub3
17. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD009157. doi:10.1002/14651858.CD009157.pub2
18. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD006829. doi: 10.1002/14651858.CD006829.pub2
19. Yun JH, Lamb A, Chase R, et al; . Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
20. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52:1801219. doi:10.1183/13993003.01219-2018
21. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014:CD010115. doi: 10.1002/14651858.CD010115.pub2
22. Calverley PMA, Anderson JA, Celli B, et al; TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789. doi:10.1056/NEJMoa063070
23. Vestbo J, Anderson JA, Brook RD, et al; SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817-1826. doi:10.1016/S0140-6736(16)30069-1
24. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989. doi:10.1002/14651858.CD008989.pub3
25. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066. doi:10.1002/14651858.CD012066.pub2
26. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 208;391:1076-1084. doi:10.1016/S0140-6736(18)30206-X
27. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919-1929. doi: 10.1016/S0140-6736(17)30188-5
28. Rabe KF, Martinez FJ, Ferguson GT, et al; ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35-48. doi:10.1056/NEJMoa1916046
29. Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta2-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 20162016:CD008532. doi: 10.1002/14651858.CD008532.pub3
30. Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10:e037509. doi:10.1136/bmjopen-2020-037509
31. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675-679. doi:10.1164/rccm.2112096
32. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457-463. doi:10.1007/s11606-009-0907-y
33. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239. doi: 10.7326/0003-4819-142-4-200502150-00005
1. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: 2022 Report. Accessed August 15, 2022. https://goldcopd.org/wp-content/uploads/2021/12/GOLD-REPORT-2022-v1.1-22Nov2021_WMV.pdf
2. Usmani OS, Lavorini F, Marshall J, et al. Critical inhaler errors in asthma and COPD: a systematic review of impact on health outcomes. Respir Res. 2018;19:10. doi:10.1186/s12931-017-0710-y
3. Haidl P, Heindl S, Siemon K, et al. Inhalation device requirements for patients’ inhalation maneuvers. Respir Med. 2016;118:65-75. doi: 10.1016/j.rmed.2016.07.013
4. van Geffen WH, Douma WR, Slebos DJ, et al. Bronchodilators delivered by nebuliser versus pMDI with spacer or DPI for exacerbations of COPD. Cochrane Database Syst Rev. 2016;2016:CD011826. doi:10.1002/14651858.CD011826.pub2
5. Ghosh S, Ohar JA, Drummond MB. Peak inspiratory flow rate in chronic obstructive pulmonary disease: implications for dry powder inhalers. J Aerosol Med Pulm Drug Deliv. 2017;30:381-387. doi:10.1089/jamp.2017.1416
6. Iwanaga T, Tohda Y, Nakamura S, et al. The Respimat soft mist inhaler: implications of drug delivery characteristics for patients. Clin Drug Investig. 2019;39:1021-1030. doi:10.1007/s40261-019-00835-z
7. Navaie M, Dembek C, Cho-Reyes S, et al. Device use errors with soft mist inhalers: a global systematic literature review and meta-analysis. Chron Respir Dis. 2020;17:1479973119901234. doi:10.1177/1479973119901234
8. Sharma G, Mahler DA, Mayorga VM, et al. Prevalence of low peak inspiratory flow rate at discharge in patients hospitalized for COPD exacerbation. Chronic Obstr Pulm Dis. 2017;4:217-224. doi: 10.15326/jcopdf.4.3.2017.0183
9. Chen SY, Huang CK, Peng HC, et al. Peak-inspiratory-flow-rate guided inhalation therapy reduce severe exacerbation of COPD. Front Pharmacol. 2021;12:704316. doi: 10.3389/fphar.2021.704316
10. Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105:930-938. doi:10.1016/j.rmed.2011.01.005
11. Fathima M, Bawa Z, Mitchell B, et al. COPD management in community pharmacy results in improved inhaler use, immunization rate, COPD action plan ownership, COPD knowledge, and reductions in exacerbation rates. Int J Chron Obstruct Pulmon Dis. 2021;16:519-533. doi: 10.2147/COPD.S288792
12. van der Molen T, van Boven JF, Maguire T, et al. Optimizing identification and management of COPD patients – reviewing the role of the community pharmacist. Br J Clin Pharmacol. 2017;83:192-201. doi: 10.1111/bcp.13087
13. Brunetti L, Poiani G, Dhanaliwala F, et al. Clinical outcomes and treatment cost comparison of levalbuterol versus albuterol in hospitalized adults with chronic obstructive pulmonary disease or asthma. Am J Health Syst Pharm. 2015;72:1026-1035. doi:10.2146/ajhp140551
14. Brown CD, McCrory DC, White J. Inhaled short-acting beta2-agonists versus ipratropium for acute exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2001;2001:CD002984. doi: 10.1002/14651858.CD002984
15. Kew KM, Mavergames C, Walters JAE. Long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;(10):CD010177. doi: 10.1002/14651858.CD010177.pub2
16. Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;2014:CD009285. doi:10.1002/14651858.CD009285.pub3
17. Chong J, Karner C, Poole P. Tiotropium versus long-acting beta-agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD009157. doi:10.1002/14651858.CD009157.pub2
18. Nannini LJ, Lasserson TJ, Poole P. Combined corticosteroid and long-acting beta2-agonist in one inhaler versus long-acting beta2-agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;2012:CD006829. doi: 10.1002/14651858.CD006829.pub2
19. Yun JH, Lamb A, Chase R, et al; . Blood eosinophil count thresholds and exacerbations in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2018;141:2037-2047.e10. doi:10.1016/j.jaci.2018.04.010
20. Agusti A, Fabbri LM, Singh D, et al. Inhaled corticosteroids in COPD: friend or foe? Eur Respir J. 2018;52:1801219. doi:10.1183/13993003.01219-2018
21. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014:CD010115. doi: 10.1002/14651858.CD010115.pub2
22. Calverley PMA, Anderson JA, Celli B, et al; TORCH Investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775-789. doi:10.1056/NEJMoa063070
23. Vestbo J, Anderson JA, Brook RD, et al; SUMMIT Investigators. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387:1817-1826. doi:10.1016/S0140-6736(16)30069-1
24. Farne HA, Cates CJ. Long-acting beta2-agonist in addition to tiotropium versus either tiotropium or long-acting beta2-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015:CD008989. doi:10.1002/14651858.CD008989.pub3
25. Horita N, Goto A, Shibata Y, et al. Long-acting muscarinic antagonist (LAMA) plus long-acting beta-agonist (LABA) versus LABA plus inhaled corticosteroid (ICS) for stable chronic obstructive pulmonary disease (COPD). Cochrane Database Syst Rev. 2017;2:CD012066. doi:10.1002/14651858.CD012066.pub2
26. Papi A, Vestbo J, Fabbri L, et al. Extrafine inhaled triple therapy versus dual bronchodilator therapy in chronic obstructive pulmonary disease (TRIBUTE): a double-blind, parallel group, randomised controlled trial. Lancet. 208;391:1076-1084. doi:10.1016/S0140-6736(18)30206-X
27. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919-1929. doi: 10.1016/S0140-6736(17)30188-5
28. Rabe KF, Martinez FJ, Ferguson GT, et al; ETHOS Investigators. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383:35-48. doi:10.1056/NEJMoa1916046
29. Rojas-Reyes MX, García Morales OM, Dennis RJ, et al. Combination inhaled steroid and long-acting beta2-agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 20162016:CD008532. doi: 10.1002/14651858.CD008532.pub3
30. Sonnex K, Alleemudder H, Knaggs R. Impact of smoking status on the efficacy of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review. BMJ Open. 2020;10:e037509. doi:10.1136/bmjopen-2020-037509
31. Anthonisen NR, Connett JE, Murray RP. Smoking and lung function of Lung Health Study participants after 11 years. Am J Respir Crit Care Med. 2002;166:675-679. doi:10.1164/rccm.2112096
32. Au DH, Bryson CL, Chien JW, et al. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457-463. doi:10.1007/s11606-009-0907-y
33. Anthonisen NR, Skeans MA, Wise RA, et al. The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233-239. doi: 10.7326/0003-4819-142-4-200502150-00005
PRACTICE RECOMMENDATIONS
› Follow guideline advice that (1) in general, short-acting beta-agonists (SABAs) are not for daily use in stable chronic obstructive pulmonary disease (COPD) but (2) agents in this class of drugs might have a role in relieving occasional COPD-associated dyspnea. C
› Prescribe albuterol over levalbuterol when a SABA is indicated because of the lower cost of albuterol, its comparative efficacy, and its lower incidence of tachycardia and palpitations, even in patients with cardiovascular disease. B
› Avoid the use of an inhaled corticosteroid, or consider withdrawing inhaled corticosteroid therapy, in patients with COPD whose blood eosinophil count is < 100 cells/μL or who have repeated bouts of pneumonia or a history of mycobacterial infection. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Influenza vaccine may offer much more than flu prevention
in new findings that suggest the vaccine itself, and not just avoidance of the virus, may be beneficial.
“We postulate that influenza vaccination may have a protective effect against stroke that may be partly independent of influenza prevention,” study investigator Francisco J. de Abajo, MD, PhD, MPH, of the University of Alcalá, Madrid, said in an interview.
“Although the study is observational and this finding can also be explained by unmeasured confounding factors, we feel that a direct biological effect of vaccine cannot be ruled out and this finding opens new avenues for investigation.”
The study was published online in Neurology.
‘Not a spurious association’
While there is a well-established link between seasonal influenza and increased ischemic stroke risk, the role of flu vaccination in stroke prevention is unclear.
In the nested case-control study, researchers evaluated data from primary care practices in Spain between 2001 and 2015. They identified 14,322 patients with first-time ischemic stroke. Of these, 9,542 had noncardioembolic stroke and 4,780 had cardioembolic stroke.
Each case was matched with five controls from the population of age- and sex-matched controls without stroke (n = 71,610).
Those in the stroke group had a slightly higher rate of flu vaccination than controls, at 41.4% versus 40.5% (odds ratio, 1.05).
Adjusted analysis revealed those who received flu vaccination were less likely to experience ischemic stroke within 15-30 days of vaccination (OR, 0.79) and, to a lesser degree, over up to 150 days (OR, 0.92).
The reduced risk associated with the flu vaccine was observed with both types of ischemic stroke and appeared to offer stroke protection outside of flu season.
The reduced risk was also found in subgroup comparisons in men, women, those aged over and under 65 years, and those with intermediate and high vascular risk.
Importantly, a separate analysis of pneumococcal vaccination did not show a similar reduction in stroke risk (adjusted OR, 1.08).
“The lack of protection found with the pneumococcal vaccine actually reinforces the hypothesis that the protection of influenza vaccine is not a spurious association, as both vaccines might share the same biases and confounding factors,” Dr. de Abajo said.
Anti-inflammatory effect?
Influenza infection is known to induce a systemic inflammatory response that “can precipitate atheroma plaque rupture mediated by elevated concentrations of reactive proteins and cytokines,” the investigators noted, and so, avoiding infection could prevent those effects.
The results are consistent with other studies that have shown similar findings, including recent data from the INTERSTROKE trial. However, the reduced risk observed in the current study even in years without a flu epidemic expands on previous findings.
“This finding suggests that other mechanisms different from the prevention of influenza infection – e.g., a direct biological effect – could account for the risk reduction found,” the investigators wrote.
In terms of the nature of that effect, Dr. de Abajo noted that, “at this stage, we can only speculate.
“Having said that, there are some pieces of evidence that suggest influenza vaccination may release anti-inflammatory mediators that can stabilize the atheroma plaque. This is an interesting hypothesis that should be addressed in the near future,” he added.
‘More than just flu prevention’
In an accompanying editorial, Dixon Yang, MD, and Mitchell S.V. Elkind, MD, agree that the findings point to intriguing potential unexpected benefits of the vaccine.
“This case-control study ... importantly suggests the influenza vaccine is more than just about preventing the flu,” they wrote.
Dr. Elkind said in an interview that the mechanism could indeed involve an anti-inflammatory effect.
“There is some evidence that antibiotics also have anti-inflammatory properties that might reduce risk of stroke or the brain damage from a stroke,” he noted. “So, it is plausible that some of the effect of the vaccine on reducing risk of stroke may be through a reduction in inflammation.”
Dr. Elkind noted that the magnitude of the reduction observed with the vaccine, though not substantial, is important. “The magnitude of effect for any one individual may be modest, but it is in the ballpark of the effect of other commonly used approaches to stroke prevention, such as taking an aspirin a day, which reduces risk of stroke by about 20%. But because influenza is so common, the impact of even a small effect for an individual can have a large impact at the population level. So, the results are of public health significance.”
The study received support from the Biomedical Research Foundation of the Prince of Asturias University Hospital and the Institute of Health Carlos III in Madrid. Dr. Elkind has reported receiving ancillary funding but no personal compensation from Roche for a federally funded trial of stroke prevention.
A version of this article first appeared on Medscape.com.
in new findings that suggest the vaccine itself, and not just avoidance of the virus, may be beneficial.
“We postulate that influenza vaccination may have a protective effect against stroke that may be partly independent of influenza prevention,” study investigator Francisco J. de Abajo, MD, PhD, MPH, of the University of Alcalá, Madrid, said in an interview.
“Although the study is observational and this finding can also be explained by unmeasured confounding factors, we feel that a direct biological effect of vaccine cannot be ruled out and this finding opens new avenues for investigation.”
The study was published online in Neurology.
‘Not a spurious association’
While there is a well-established link between seasonal influenza and increased ischemic stroke risk, the role of flu vaccination in stroke prevention is unclear.
In the nested case-control study, researchers evaluated data from primary care practices in Spain between 2001 and 2015. They identified 14,322 patients with first-time ischemic stroke. Of these, 9,542 had noncardioembolic stroke and 4,780 had cardioembolic stroke.
Each case was matched with five controls from the population of age- and sex-matched controls without stroke (n = 71,610).
Those in the stroke group had a slightly higher rate of flu vaccination than controls, at 41.4% versus 40.5% (odds ratio, 1.05).
Adjusted analysis revealed those who received flu vaccination were less likely to experience ischemic stroke within 15-30 days of vaccination (OR, 0.79) and, to a lesser degree, over up to 150 days (OR, 0.92).
The reduced risk associated with the flu vaccine was observed with both types of ischemic stroke and appeared to offer stroke protection outside of flu season.
The reduced risk was also found in subgroup comparisons in men, women, those aged over and under 65 years, and those with intermediate and high vascular risk.
Importantly, a separate analysis of pneumococcal vaccination did not show a similar reduction in stroke risk (adjusted OR, 1.08).
“The lack of protection found with the pneumococcal vaccine actually reinforces the hypothesis that the protection of influenza vaccine is not a spurious association, as both vaccines might share the same biases and confounding factors,” Dr. de Abajo said.
Anti-inflammatory effect?
Influenza infection is known to induce a systemic inflammatory response that “can precipitate atheroma plaque rupture mediated by elevated concentrations of reactive proteins and cytokines,” the investigators noted, and so, avoiding infection could prevent those effects.
The results are consistent with other studies that have shown similar findings, including recent data from the INTERSTROKE trial. However, the reduced risk observed in the current study even in years without a flu epidemic expands on previous findings.
“This finding suggests that other mechanisms different from the prevention of influenza infection – e.g., a direct biological effect – could account for the risk reduction found,” the investigators wrote.
In terms of the nature of that effect, Dr. de Abajo noted that, “at this stage, we can only speculate.
“Having said that, there are some pieces of evidence that suggest influenza vaccination may release anti-inflammatory mediators that can stabilize the atheroma plaque. This is an interesting hypothesis that should be addressed in the near future,” he added.
‘More than just flu prevention’
In an accompanying editorial, Dixon Yang, MD, and Mitchell S.V. Elkind, MD, agree that the findings point to intriguing potential unexpected benefits of the vaccine.
“This case-control study ... importantly suggests the influenza vaccine is more than just about preventing the flu,” they wrote.
Dr. Elkind said in an interview that the mechanism could indeed involve an anti-inflammatory effect.
“There is some evidence that antibiotics also have anti-inflammatory properties that might reduce risk of stroke or the brain damage from a stroke,” he noted. “So, it is plausible that some of the effect of the vaccine on reducing risk of stroke may be through a reduction in inflammation.”
Dr. Elkind noted that the magnitude of the reduction observed with the vaccine, though not substantial, is important. “The magnitude of effect for any one individual may be modest, but it is in the ballpark of the effect of other commonly used approaches to stroke prevention, such as taking an aspirin a day, which reduces risk of stroke by about 20%. But because influenza is so common, the impact of even a small effect for an individual can have a large impact at the population level. So, the results are of public health significance.”
The study received support from the Biomedical Research Foundation of the Prince of Asturias University Hospital and the Institute of Health Carlos III in Madrid. Dr. Elkind has reported receiving ancillary funding but no personal compensation from Roche for a federally funded trial of stroke prevention.
A version of this article first appeared on Medscape.com.
in new findings that suggest the vaccine itself, and not just avoidance of the virus, may be beneficial.
“We postulate that influenza vaccination may have a protective effect against stroke that may be partly independent of influenza prevention,” study investigator Francisco J. de Abajo, MD, PhD, MPH, of the University of Alcalá, Madrid, said in an interview.
“Although the study is observational and this finding can also be explained by unmeasured confounding factors, we feel that a direct biological effect of vaccine cannot be ruled out and this finding opens new avenues for investigation.”
The study was published online in Neurology.
‘Not a spurious association’
While there is a well-established link between seasonal influenza and increased ischemic stroke risk, the role of flu vaccination in stroke prevention is unclear.
In the nested case-control study, researchers evaluated data from primary care practices in Spain between 2001 and 2015. They identified 14,322 patients with first-time ischemic stroke. Of these, 9,542 had noncardioembolic stroke and 4,780 had cardioembolic stroke.
Each case was matched with five controls from the population of age- and sex-matched controls without stroke (n = 71,610).
Those in the stroke group had a slightly higher rate of flu vaccination than controls, at 41.4% versus 40.5% (odds ratio, 1.05).
Adjusted analysis revealed those who received flu vaccination were less likely to experience ischemic stroke within 15-30 days of vaccination (OR, 0.79) and, to a lesser degree, over up to 150 days (OR, 0.92).
The reduced risk associated with the flu vaccine was observed with both types of ischemic stroke and appeared to offer stroke protection outside of flu season.
The reduced risk was also found in subgroup comparisons in men, women, those aged over and under 65 years, and those with intermediate and high vascular risk.
Importantly, a separate analysis of pneumococcal vaccination did not show a similar reduction in stroke risk (adjusted OR, 1.08).
“The lack of protection found with the pneumococcal vaccine actually reinforces the hypothesis that the protection of influenza vaccine is not a spurious association, as both vaccines might share the same biases and confounding factors,” Dr. de Abajo said.
Anti-inflammatory effect?
Influenza infection is known to induce a systemic inflammatory response that “can precipitate atheroma plaque rupture mediated by elevated concentrations of reactive proteins and cytokines,” the investigators noted, and so, avoiding infection could prevent those effects.
The results are consistent with other studies that have shown similar findings, including recent data from the INTERSTROKE trial. However, the reduced risk observed in the current study even in years without a flu epidemic expands on previous findings.
“This finding suggests that other mechanisms different from the prevention of influenza infection – e.g., a direct biological effect – could account for the risk reduction found,” the investigators wrote.
In terms of the nature of that effect, Dr. de Abajo noted that, “at this stage, we can only speculate.
“Having said that, there are some pieces of evidence that suggest influenza vaccination may release anti-inflammatory mediators that can stabilize the atheroma plaque. This is an interesting hypothesis that should be addressed in the near future,” he added.
‘More than just flu prevention’
In an accompanying editorial, Dixon Yang, MD, and Mitchell S.V. Elkind, MD, agree that the findings point to intriguing potential unexpected benefits of the vaccine.
“This case-control study ... importantly suggests the influenza vaccine is more than just about preventing the flu,” they wrote.
Dr. Elkind said in an interview that the mechanism could indeed involve an anti-inflammatory effect.
“There is some evidence that antibiotics also have anti-inflammatory properties that might reduce risk of stroke or the brain damage from a stroke,” he noted. “So, it is plausible that some of the effect of the vaccine on reducing risk of stroke may be through a reduction in inflammation.”
Dr. Elkind noted that the magnitude of the reduction observed with the vaccine, though not substantial, is important. “The magnitude of effect for any one individual may be modest, but it is in the ballpark of the effect of other commonly used approaches to stroke prevention, such as taking an aspirin a day, which reduces risk of stroke by about 20%. But because influenza is so common, the impact of even a small effect for an individual can have a large impact at the population level. So, the results are of public health significance.”
The study received support from the Biomedical Research Foundation of the Prince of Asturias University Hospital and the Institute of Health Carlos III in Madrid. Dr. Elkind has reported receiving ancillary funding but no personal compensation from Roche for a federally funded trial of stroke prevention.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
New study supports safety of COVID-19 boosters during pregnancy
Doctors and health professionals continue to recommend COVID-19 vaccine boosters or third doses for adolescents and adults more than 5 months after their initial vaccinations with the Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 primary vaccine series or more than 2 months after receiving the Janssen JNJ-78436735 vaccine, Alisa Kachikis, MD, of the University of Washington, Seattle, and colleagues wrote in JAMA Network Open.
Although multiple studies have shown that the COVID-19 primary series is safe and well tolerated in pregnant and lactating women, information on the safety and tolerability of boosters are lacking, the researchers noted.
“COVID-19 will be with us for a while, and it is important to continue to provide data on COVID-19 vaccines in these groups, particularly because there still are many questions about the vaccine, and because pregnant individuals have been, understandably, more hesitant to receive COVID-19 vaccines,” Dr. Kachikis said in an interview. “The findings of this study that COVID-19 booster doses are well tolerated among pregnant and lactating individuals are especially pertinent with the new COVID-19 boosters available this fall.”
In the new study, the researchers reviewed data from 17,014 participants who were part of an ongoing online prospective study of COVID-19 vaccines in pregnant and lactating individuals. Data were collected between October 2021 and April 2022 through an online survey.
The study population included 2,009 participants (11.8%) who were pregnant at the time of their booster or third dose, 10,279 (60.4%) who were lactating, and 4,726 (27.8%) who were neither pregnant nor lactating. The mean age of the participants was 33.3 years; 92.1% self-identified as White, 94.5% self-identified as non-Hispanic, and 99.7% self-identified as female.
The receipt of a booster was similar across trimesters; 26.4%, 36.5%, and 37.1% of participants received boosters or third doses in the first, second, and third trimester, respectively. The primary outcome was self-reported vaccine reactions within 24 hours of the dose.
Overall, 82.8% of the respondents reported a reaction at the site of the injection, such as redness, pain, or swelling, and 67.9% reported at least one systemic symptom, such as aches and pains, headache, chills, or fever. The most frequently reported symptoms across all groups were injection-site pain (82.2%) and fatigue (54.4%).
The pregnant women were significantly more likely than nonpregnant or nonlactating individuals to report any local reaction at the injection site (adjusted odds ratio, 1.2; P = .01), but less likely to report any systemic reaction (aOR, 0.7; P < .001).
The majority (97.6%) of the pregnant respondents and 96.0% of those lactating reported no obstetric or lactation concerns after vaccination.
Overall, a majority of the respondents reported that recommendations from public health authorities were helpful in their decision to receive a COVID-19 booster or third dose (90.0% of pregnant respondents, 89.9% of lactating respondents, and 88.1% of those neither pregnant nor lactating).
Although vaccine uptake in the current study population was high (91.1% overall and 95.0% of those pregnant), “the importance of the health care professional’s recommendation is pertinent given the ongoing increased vaccine hesitancy among pregnant individuals in the context of the COVID-19 vaccine,” the researchers emphasized.
The study findings were limited by several factors including the reliance on self-reports and a convenience sample composed mainly of health care workers because of their vaccine eligibility at the time the study started, which limits generalizability, the researchers noted. Analyses on the pregnancy outcomes of those who were pregnant when vaccinated are in progress.
The results were strengthened by the large study population that included participants from all 50 states and several territories, and ability to compare results between pregnant and lactating individuals with those who were neither pregnant nor lactating, but were of childbearing age, they said.
The results support the safety of COVID-19 boosters for pregnant and breastfeeding individuals, and these data are important to inform discussions between patients and clinicians to boost vaccine uptake and acceptance in this population, they concluded.
“Our earlier data analysis showed that pregnant and lactating individuals did very well with the initial COVID-19 vaccine series, so it was not very surprising that they also did well with COVID-19 booster or third doses,” Dr. Kachikis said in an interview.
There are two takeaway messages for clinicians, she said: “First, pregnant and lactating individuals tolerated the COVID-19 booster well. The second is that clinicians are very important when it comes to vaccine acceptance.”
“In our study, we found that, while pregnant participants were more likely to report that they were hesitant to receive the booster, they also were more likely to have discussed the COVID-19 booster with their health care provider, and to have received a recommendation to receive the booster. So, spending a little bit of extra time with patients discussing COVID-19 boosters and recommending them can make a significant difference,” she said.
The message of the study is highly reassuring for pregnant and lactating individuals, Dr. Kachikis added. “Most of the participants reported that they had fewer symptoms with the COVID-19 booster compared to the primary vaccine series, which is good news, especially since a new COVID-19 booster is being recommended for the fall.”
Reassuring findings for doctors and patients
The current study is especially timely, as updated COVID-19 boosters have now been recommended for most individuals by the Centers for Disease Control and Prevention, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
The findings support previous studies on the tolerability of COVID-19 vaccinations in pregnant and lactating persons, said Dr. Badell, who was not involved in the study.
The reassuring message for clinicians is that COVID-19 booster vaccinations are similarly well tolerated in pregnancy and lactation as they are in nonpregnant individuals, said Dr. Badell. “Given the risks of COVID infections in pregnancy and neonates, reassuring data on the tolerability and safety of vaccination in this population is very important.” Also, the researchers found that all three cohorts reported that recommendations from public or medical health authorities helped them make a decision about vaccination; “thus the more data to support these recommendations, the better,” she emphasized.
If you are pregnant or breastfeeding, the message from the study is that COVID-19 booster vaccinations are similarly well tolerated by those who are pregnant or breastfeeding and those who are not, said Dr. Badell.
“This study provides additional support for the strong recommendation to encourage not only COVID-19 vaccination in pregnancy and lactation, but booster vaccinations specifically,” and pregnant and breastfeeding individuals should not be excluded from the new CDC recommendations for COVID-19 boosters, she said.
Future research suggestions
Next steps for research include evaluating the obstetrical and neonatal outcomes in pregnancy and lactation following COVID- 19 boosters, Dr. Badell added.
Dr. Kachikis suggested studies try to answer the remaining questions about COVID-19 vaccines and the immunity of pregnant and lactating persons, particularly since they were excluded from the early clinical trials in 2020.
The study was supported by the National Institute of Allergy and Infectious Diseases, a Women’s Reproductive Health Research Award, and the National Center for Advancing Translational Sciences of the National Institutes of Health. \Dr. Kachikis disclosed serving as a research consultant for Pfizer and GlaxoSmithKline and as an unpaid consultant for GlaxoSmithKline unrelated to the current study, as well as grant support from Merck and Pfizer unrelated to the current study. Dr. Badell had no financial conflicts to disclose.
Doctors and health professionals continue to recommend COVID-19 vaccine boosters or third doses for adolescents and adults more than 5 months after their initial vaccinations with the Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 primary vaccine series or more than 2 months after receiving the Janssen JNJ-78436735 vaccine, Alisa Kachikis, MD, of the University of Washington, Seattle, and colleagues wrote in JAMA Network Open.
Although multiple studies have shown that the COVID-19 primary series is safe and well tolerated in pregnant and lactating women, information on the safety and tolerability of boosters are lacking, the researchers noted.
“COVID-19 will be with us for a while, and it is important to continue to provide data on COVID-19 vaccines in these groups, particularly because there still are many questions about the vaccine, and because pregnant individuals have been, understandably, more hesitant to receive COVID-19 vaccines,” Dr. Kachikis said in an interview. “The findings of this study that COVID-19 booster doses are well tolerated among pregnant and lactating individuals are especially pertinent with the new COVID-19 boosters available this fall.”
In the new study, the researchers reviewed data from 17,014 participants who were part of an ongoing online prospective study of COVID-19 vaccines in pregnant and lactating individuals. Data were collected between October 2021 and April 2022 through an online survey.
The study population included 2,009 participants (11.8%) who were pregnant at the time of their booster or third dose, 10,279 (60.4%) who were lactating, and 4,726 (27.8%) who were neither pregnant nor lactating. The mean age of the participants was 33.3 years; 92.1% self-identified as White, 94.5% self-identified as non-Hispanic, and 99.7% self-identified as female.
The receipt of a booster was similar across trimesters; 26.4%, 36.5%, and 37.1% of participants received boosters or third doses in the first, second, and third trimester, respectively. The primary outcome was self-reported vaccine reactions within 24 hours of the dose.
Overall, 82.8% of the respondents reported a reaction at the site of the injection, such as redness, pain, or swelling, and 67.9% reported at least one systemic symptom, such as aches and pains, headache, chills, or fever. The most frequently reported symptoms across all groups were injection-site pain (82.2%) and fatigue (54.4%).
The pregnant women were significantly more likely than nonpregnant or nonlactating individuals to report any local reaction at the injection site (adjusted odds ratio, 1.2; P = .01), but less likely to report any systemic reaction (aOR, 0.7; P < .001).
The majority (97.6%) of the pregnant respondents and 96.0% of those lactating reported no obstetric or lactation concerns after vaccination.
Overall, a majority of the respondents reported that recommendations from public health authorities were helpful in their decision to receive a COVID-19 booster or third dose (90.0% of pregnant respondents, 89.9% of lactating respondents, and 88.1% of those neither pregnant nor lactating).
Although vaccine uptake in the current study population was high (91.1% overall and 95.0% of those pregnant), “the importance of the health care professional’s recommendation is pertinent given the ongoing increased vaccine hesitancy among pregnant individuals in the context of the COVID-19 vaccine,” the researchers emphasized.
The study findings were limited by several factors including the reliance on self-reports and a convenience sample composed mainly of health care workers because of their vaccine eligibility at the time the study started, which limits generalizability, the researchers noted. Analyses on the pregnancy outcomes of those who were pregnant when vaccinated are in progress.
The results were strengthened by the large study population that included participants from all 50 states and several territories, and ability to compare results between pregnant and lactating individuals with those who were neither pregnant nor lactating, but were of childbearing age, they said.
The results support the safety of COVID-19 boosters for pregnant and breastfeeding individuals, and these data are important to inform discussions between patients and clinicians to boost vaccine uptake and acceptance in this population, they concluded.
“Our earlier data analysis showed that pregnant and lactating individuals did very well with the initial COVID-19 vaccine series, so it was not very surprising that they also did well with COVID-19 booster or third doses,” Dr. Kachikis said in an interview.
There are two takeaway messages for clinicians, she said: “First, pregnant and lactating individuals tolerated the COVID-19 booster well. The second is that clinicians are very important when it comes to vaccine acceptance.”
“In our study, we found that, while pregnant participants were more likely to report that they were hesitant to receive the booster, they also were more likely to have discussed the COVID-19 booster with their health care provider, and to have received a recommendation to receive the booster. So, spending a little bit of extra time with patients discussing COVID-19 boosters and recommending them can make a significant difference,” she said.
The message of the study is highly reassuring for pregnant and lactating individuals, Dr. Kachikis added. “Most of the participants reported that they had fewer symptoms with the COVID-19 booster compared to the primary vaccine series, which is good news, especially since a new COVID-19 booster is being recommended for the fall.”
Reassuring findings for doctors and patients
The current study is especially timely, as updated COVID-19 boosters have now been recommended for most individuals by the Centers for Disease Control and Prevention, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
The findings support previous studies on the tolerability of COVID-19 vaccinations in pregnant and lactating persons, said Dr. Badell, who was not involved in the study.
The reassuring message for clinicians is that COVID-19 booster vaccinations are similarly well tolerated in pregnancy and lactation as they are in nonpregnant individuals, said Dr. Badell. “Given the risks of COVID infections in pregnancy and neonates, reassuring data on the tolerability and safety of vaccination in this population is very important.” Also, the researchers found that all three cohorts reported that recommendations from public or medical health authorities helped them make a decision about vaccination; “thus the more data to support these recommendations, the better,” she emphasized.
If you are pregnant or breastfeeding, the message from the study is that COVID-19 booster vaccinations are similarly well tolerated by those who are pregnant or breastfeeding and those who are not, said Dr. Badell.
“This study provides additional support for the strong recommendation to encourage not only COVID-19 vaccination in pregnancy and lactation, but booster vaccinations specifically,” and pregnant and breastfeeding individuals should not be excluded from the new CDC recommendations for COVID-19 boosters, she said.
Future research suggestions
Next steps for research include evaluating the obstetrical and neonatal outcomes in pregnancy and lactation following COVID- 19 boosters, Dr. Badell added.
Dr. Kachikis suggested studies try to answer the remaining questions about COVID-19 vaccines and the immunity of pregnant and lactating persons, particularly since they were excluded from the early clinical trials in 2020.
The study was supported by the National Institute of Allergy and Infectious Diseases, a Women’s Reproductive Health Research Award, and the National Center for Advancing Translational Sciences of the National Institutes of Health. \Dr. Kachikis disclosed serving as a research consultant for Pfizer and GlaxoSmithKline and as an unpaid consultant for GlaxoSmithKline unrelated to the current study, as well as grant support from Merck and Pfizer unrelated to the current study. Dr. Badell had no financial conflicts to disclose.
Doctors and health professionals continue to recommend COVID-19 vaccine boosters or third doses for adolescents and adults more than 5 months after their initial vaccinations with the Pfizer-BioNTech BNT162b2 or Moderna mRNA-1273 primary vaccine series or more than 2 months after receiving the Janssen JNJ-78436735 vaccine, Alisa Kachikis, MD, of the University of Washington, Seattle, and colleagues wrote in JAMA Network Open.
Although multiple studies have shown that the COVID-19 primary series is safe and well tolerated in pregnant and lactating women, information on the safety and tolerability of boosters are lacking, the researchers noted.
“COVID-19 will be with us for a while, and it is important to continue to provide data on COVID-19 vaccines in these groups, particularly because there still are many questions about the vaccine, and because pregnant individuals have been, understandably, more hesitant to receive COVID-19 vaccines,” Dr. Kachikis said in an interview. “The findings of this study that COVID-19 booster doses are well tolerated among pregnant and lactating individuals are especially pertinent with the new COVID-19 boosters available this fall.”
In the new study, the researchers reviewed data from 17,014 participants who were part of an ongoing online prospective study of COVID-19 vaccines in pregnant and lactating individuals. Data were collected between October 2021 and April 2022 through an online survey.
The study population included 2,009 participants (11.8%) who were pregnant at the time of their booster or third dose, 10,279 (60.4%) who were lactating, and 4,726 (27.8%) who were neither pregnant nor lactating. The mean age of the participants was 33.3 years; 92.1% self-identified as White, 94.5% self-identified as non-Hispanic, and 99.7% self-identified as female.
The receipt of a booster was similar across trimesters; 26.4%, 36.5%, and 37.1% of participants received boosters or third doses in the first, second, and third trimester, respectively. The primary outcome was self-reported vaccine reactions within 24 hours of the dose.
Overall, 82.8% of the respondents reported a reaction at the site of the injection, such as redness, pain, or swelling, and 67.9% reported at least one systemic symptom, such as aches and pains, headache, chills, or fever. The most frequently reported symptoms across all groups were injection-site pain (82.2%) and fatigue (54.4%).
The pregnant women were significantly more likely than nonpregnant or nonlactating individuals to report any local reaction at the injection site (adjusted odds ratio, 1.2; P = .01), but less likely to report any systemic reaction (aOR, 0.7; P < .001).
The majority (97.6%) of the pregnant respondents and 96.0% of those lactating reported no obstetric or lactation concerns after vaccination.
Overall, a majority of the respondents reported that recommendations from public health authorities were helpful in their decision to receive a COVID-19 booster or third dose (90.0% of pregnant respondents, 89.9% of lactating respondents, and 88.1% of those neither pregnant nor lactating).
Although vaccine uptake in the current study population was high (91.1% overall and 95.0% of those pregnant), “the importance of the health care professional’s recommendation is pertinent given the ongoing increased vaccine hesitancy among pregnant individuals in the context of the COVID-19 vaccine,” the researchers emphasized.
The study findings were limited by several factors including the reliance on self-reports and a convenience sample composed mainly of health care workers because of their vaccine eligibility at the time the study started, which limits generalizability, the researchers noted. Analyses on the pregnancy outcomes of those who were pregnant when vaccinated are in progress.
The results were strengthened by the large study population that included participants from all 50 states and several territories, and ability to compare results between pregnant and lactating individuals with those who were neither pregnant nor lactating, but were of childbearing age, they said.
The results support the safety of COVID-19 boosters for pregnant and breastfeeding individuals, and these data are important to inform discussions between patients and clinicians to boost vaccine uptake and acceptance in this population, they concluded.
“Our earlier data analysis showed that pregnant and lactating individuals did very well with the initial COVID-19 vaccine series, so it was not very surprising that they also did well with COVID-19 booster or third doses,” Dr. Kachikis said in an interview.
There are two takeaway messages for clinicians, she said: “First, pregnant and lactating individuals tolerated the COVID-19 booster well. The second is that clinicians are very important when it comes to vaccine acceptance.”
“In our study, we found that, while pregnant participants were more likely to report that they were hesitant to receive the booster, they also were more likely to have discussed the COVID-19 booster with their health care provider, and to have received a recommendation to receive the booster. So, spending a little bit of extra time with patients discussing COVID-19 boosters and recommending them can make a significant difference,” she said.
The message of the study is highly reassuring for pregnant and lactating individuals, Dr. Kachikis added. “Most of the participants reported that they had fewer symptoms with the COVID-19 booster compared to the primary vaccine series, which is good news, especially since a new COVID-19 booster is being recommended for the fall.”
Reassuring findings for doctors and patients
The current study is especially timely, as updated COVID-19 boosters have now been recommended for most individuals by the Centers for Disease Control and Prevention, Martina L. Badell, MD, a maternal-fetal medicine specialist at Emory University, Atlanta, said in an interview.
The findings support previous studies on the tolerability of COVID-19 vaccinations in pregnant and lactating persons, said Dr. Badell, who was not involved in the study.
The reassuring message for clinicians is that COVID-19 booster vaccinations are similarly well tolerated in pregnancy and lactation as they are in nonpregnant individuals, said Dr. Badell. “Given the risks of COVID infections in pregnancy and neonates, reassuring data on the tolerability and safety of vaccination in this population is very important.” Also, the researchers found that all three cohorts reported that recommendations from public or medical health authorities helped them make a decision about vaccination; “thus the more data to support these recommendations, the better,” she emphasized.
If you are pregnant or breastfeeding, the message from the study is that COVID-19 booster vaccinations are similarly well tolerated by those who are pregnant or breastfeeding and those who are not, said Dr. Badell.
“This study provides additional support for the strong recommendation to encourage not only COVID-19 vaccination in pregnancy and lactation, but booster vaccinations specifically,” and pregnant and breastfeeding individuals should not be excluded from the new CDC recommendations for COVID-19 boosters, she said.
Future research suggestions
Next steps for research include evaluating the obstetrical and neonatal outcomes in pregnancy and lactation following COVID- 19 boosters, Dr. Badell added.
Dr. Kachikis suggested studies try to answer the remaining questions about COVID-19 vaccines and the immunity of pregnant and lactating persons, particularly since they were excluded from the early clinical trials in 2020.
The study was supported by the National Institute of Allergy and Infectious Diseases, a Women’s Reproductive Health Research Award, and the National Center for Advancing Translational Sciences of the National Institutes of Health. \Dr. Kachikis disclosed serving as a research consultant for Pfizer and GlaxoSmithKline and as an unpaid consultant for GlaxoSmithKline unrelated to the current study, as well as grant support from Merck and Pfizer unrelated to the current study. Dr. Badell had no financial conflicts to disclose.
FROM JAMA NETWORK OPEN
Unvaccinated 10 times more likely to be hospitalized for Omicron
The data, which included almost 200,000 COVID-19–associated hospitalizations across 13 states, also showed that vaccinated, hospitalized patients were more often older and already dealing with other health conditions, compared with unvaccinated, hospitalized patients, reported lead author Fiona P. Havers, MD, of the CDC, Atlanta.
“Unlike previously published reports and web pages … this study reports hospitalization rates by vaccination status and clinical and demographic characteristics of hospitalized patients, beginning with the period when vaccines first became available, and includes comparisons of unvaccinated persons, persons vaccinated with a primary series without a booster dose, and those vaccinated with a primary series and at least 1 booster dose,” the investigators wrote in JAMA Internal Medicine.
In total, the investigators reviewed 192,509 hospitalizations involving patients 18 years and older. The study period spanned from Jan. 1, 2021, to April 30, 2022. Data were reported month by month, showing that the relative monthly hospitalization rate peaked in May 2021, when it was 17.7 times higher for unvaccinated versus vaccinated individuals (with or without a booster).
To account for differences in clinical course between Delta and Omicron, the investigators also analyzed data sorted into two time periods: July-December 2021 (Delta predominant) and January-April 2022 (Omicron BA.1 predominant). These analyses revealed the greater hospitalization risk presented by Delta. Specifically, unvaccinated people were 12.2 times more likely to be hospitalized for Delta than vaccinated people, with or without a booster, versus 6.8 times for Omicron BA.1.
Study shows power of the booster
A closer look at the Omicron BA.1 data showed the power of a booster dose. From January to April 2022, individuals who were fully vaccinated with a booster dose were 10.5 times less likely than unvaccinated individuals to be hospitalized for Omicron BA.1. Plus, boosted people were 2.5 times less likely to be hospitalized for Omicron BA.1 than people who got vaccinated but skipped the booster.
“The high hospitalization rates in unvaccinated compared with vaccinated persons with and without a booster dose underscores the importance of COVID-19 vaccinations in preventing hospitalizations and suggests that increasing vaccination coverage, including booster dose coverage, can prevent hospitalizations, serious illness, and death,” the investigators wrote.
The study also revealed that vaccinated hospitalized patients were significantly older, on average, than unvaccinated hospitalized patients (median, 70 vs. 58 years; P < .001). They were also significantly more likely to have three or more underlying medical conditions (77.8% vs. 51.6%; P < .001)
“A greater proportion of hospitalized cases among vaccinated persons occurred in individuals with medical fragility who were older, more likely to reside in long-term care facilities, and have three or more underlying medical conditions, including immunosuppressive conditions,” the investigators wrote.
New variants outpacing data, vaccines remain essential
While data from April 2022 alone showed a 3.5-fold higher rate of hospitalization among unvaccinated versus vaccinated individuals with or without a booster, newer data suggest that emerging strains of Omicron are putting more people in the hospital.
A recent report by the CDC showed weekly hospitalization rates climbing from March 20 to May 31, 2022, which coincided with predominance of the newer Omicron BA.2 variant. While unvaccinated people were still around 3.5 times more likely to be hospitalized than vaccinated people, overall hospitalization rates jumped 3-fold for people 65 years and older, and 1.7-fold for adults younger than 65. Adding further complexity to this constantly evolving situation is that Omicron BA.2 has since been joined by the BA.4 and BA.5 lineages, for which vaccines are now available.
In the paper published in JAMA Internal Medicine, the CDC report, and in a comment for this article, the CDC offered the same take-home message: Get vaccinated.
“These findings reinforce previous research illustrating how vaccination provides protection from hospitalization due to COVID-19,” a CDC spokesperson said. “COVID-19 vaccines are proven to help prevent serious COVID-19 illness, and everyone ages 6 months and older should stay up to date with COVID-19 vaccines.”
The study published in JAMA Internal Medicine was supported by the CDC. The investigators disclosed additional relationships with Sanofi, GSK, MedImmune, and others.
The data, which included almost 200,000 COVID-19–associated hospitalizations across 13 states, also showed that vaccinated, hospitalized patients were more often older and already dealing with other health conditions, compared with unvaccinated, hospitalized patients, reported lead author Fiona P. Havers, MD, of the CDC, Atlanta.
“Unlike previously published reports and web pages … this study reports hospitalization rates by vaccination status and clinical and demographic characteristics of hospitalized patients, beginning with the period when vaccines first became available, and includes comparisons of unvaccinated persons, persons vaccinated with a primary series without a booster dose, and those vaccinated with a primary series and at least 1 booster dose,” the investigators wrote in JAMA Internal Medicine.
In total, the investigators reviewed 192,509 hospitalizations involving patients 18 years and older. The study period spanned from Jan. 1, 2021, to April 30, 2022. Data were reported month by month, showing that the relative monthly hospitalization rate peaked in May 2021, when it was 17.7 times higher for unvaccinated versus vaccinated individuals (with or without a booster).
To account for differences in clinical course between Delta and Omicron, the investigators also analyzed data sorted into two time periods: July-December 2021 (Delta predominant) and January-April 2022 (Omicron BA.1 predominant). These analyses revealed the greater hospitalization risk presented by Delta. Specifically, unvaccinated people were 12.2 times more likely to be hospitalized for Delta than vaccinated people, with or without a booster, versus 6.8 times for Omicron BA.1.
Study shows power of the booster
A closer look at the Omicron BA.1 data showed the power of a booster dose. From January to April 2022, individuals who were fully vaccinated with a booster dose were 10.5 times less likely than unvaccinated individuals to be hospitalized for Omicron BA.1. Plus, boosted people were 2.5 times less likely to be hospitalized for Omicron BA.1 than people who got vaccinated but skipped the booster.
“The high hospitalization rates in unvaccinated compared with vaccinated persons with and without a booster dose underscores the importance of COVID-19 vaccinations in preventing hospitalizations and suggests that increasing vaccination coverage, including booster dose coverage, can prevent hospitalizations, serious illness, and death,” the investigators wrote.
The study also revealed that vaccinated hospitalized patients were significantly older, on average, than unvaccinated hospitalized patients (median, 70 vs. 58 years; P < .001). They were also significantly more likely to have three or more underlying medical conditions (77.8% vs. 51.6%; P < .001)
“A greater proportion of hospitalized cases among vaccinated persons occurred in individuals with medical fragility who were older, more likely to reside in long-term care facilities, and have three or more underlying medical conditions, including immunosuppressive conditions,” the investigators wrote.
New variants outpacing data, vaccines remain essential
While data from April 2022 alone showed a 3.5-fold higher rate of hospitalization among unvaccinated versus vaccinated individuals with or without a booster, newer data suggest that emerging strains of Omicron are putting more people in the hospital.
A recent report by the CDC showed weekly hospitalization rates climbing from March 20 to May 31, 2022, which coincided with predominance of the newer Omicron BA.2 variant. While unvaccinated people were still around 3.5 times more likely to be hospitalized than vaccinated people, overall hospitalization rates jumped 3-fold for people 65 years and older, and 1.7-fold for adults younger than 65. Adding further complexity to this constantly evolving situation is that Omicron BA.2 has since been joined by the BA.4 and BA.5 lineages, for which vaccines are now available.
In the paper published in JAMA Internal Medicine, the CDC report, and in a comment for this article, the CDC offered the same take-home message: Get vaccinated.
“These findings reinforce previous research illustrating how vaccination provides protection from hospitalization due to COVID-19,” a CDC spokesperson said. “COVID-19 vaccines are proven to help prevent serious COVID-19 illness, and everyone ages 6 months and older should stay up to date with COVID-19 vaccines.”
The study published in JAMA Internal Medicine was supported by the CDC. The investigators disclosed additional relationships with Sanofi, GSK, MedImmune, and others.
The data, which included almost 200,000 COVID-19–associated hospitalizations across 13 states, also showed that vaccinated, hospitalized patients were more often older and already dealing with other health conditions, compared with unvaccinated, hospitalized patients, reported lead author Fiona P. Havers, MD, of the CDC, Atlanta.
“Unlike previously published reports and web pages … this study reports hospitalization rates by vaccination status and clinical and demographic characteristics of hospitalized patients, beginning with the period when vaccines first became available, and includes comparisons of unvaccinated persons, persons vaccinated with a primary series without a booster dose, and those vaccinated with a primary series and at least 1 booster dose,” the investigators wrote in JAMA Internal Medicine.
In total, the investigators reviewed 192,509 hospitalizations involving patients 18 years and older. The study period spanned from Jan. 1, 2021, to April 30, 2022. Data were reported month by month, showing that the relative monthly hospitalization rate peaked in May 2021, when it was 17.7 times higher for unvaccinated versus vaccinated individuals (with or without a booster).
To account for differences in clinical course between Delta and Omicron, the investigators also analyzed data sorted into two time periods: July-December 2021 (Delta predominant) and January-April 2022 (Omicron BA.1 predominant). These analyses revealed the greater hospitalization risk presented by Delta. Specifically, unvaccinated people were 12.2 times more likely to be hospitalized for Delta than vaccinated people, with or without a booster, versus 6.8 times for Omicron BA.1.
Study shows power of the booster
A closer look at the Omicron BA.1 data showed the power of a booster dose. From January to April 2022, individuals who were fully vaccinated with a booster dose were 10.5 times less likely than unvaccinated individuals to be hospitalized for Omicron BA.1. Plus, boosted people were 2.5 times less likely to be hospitalized for Omicron BA.1 than people who got vaccinated but skipped the booster.
“The high hospitalization rates in unvaccinated compared with vaccinated persons with and without a booster dose underscores the importance of COVID-19 vaccinations in preventing hospitalizations and suggests that increasing vaccination coverage, including booster dose coverage, can prevent hospitalizations, serious illness, and death,” the investigators wrote.
The study also revealed that vaccinated hospitalized patients were significantly older, on average, than unvaccinated hospitalized patients (median, 70 vs. 58 years; P < .001). They were also significantly more likely to have three or more underlying medical conditions (77.8% vs. 51.6%; P < .001)
“A greater proportion of hospitalized cases among vaccinated persons occurred in individuals with medical fragility who were older, more likely to reside in long-term care facilities, and have three or more underlying medical conditions, including immunosuppressive conditions,” the investigators wrote.
New variants outpacing data, vaccines remain essential
While data from April 2022 alone showed a 3.5-fold higher rate of hospitalization among unvaccinated versus vaccinated individuals with or without a booster, newer data suggest that emerging strains of Omicron are putting more people in the hospital.
A recent report by the CDC showed weekly hospitalization rates climbing from March 20 to May 31, 2022, which coincided with predominance of the newer Omicron BA.2 variant. While unvaccinated people were still around 3.5 times more likely to be hospitalized than vaccinated people, overall hospitalization rates jumped 3-fold for people 65 years and older, and 1.7-fold for adults younger than 65. Adding further complexity to this constantly evolving situation is that Omicron BA.2 has since been joined by the BA.4 and BA.5 lineages, for which vaccines are now available.
In the paper published in JAMA Internal Medicine, the CDC report, and in a comment for this article, the CDC offered the same take-home message: Get vaccinated.
“These findings reinforce previous research illustrating how vaccination provides protection from hospitalization due to COVID-19,” a CDC spokesperson said. “COVID-19 vaccines are proven to help prevent serious COVID-19 illness, and everyone ages 6 months and older should stay up to date with COVID-19 vaccines.”
The study published in JAMA Internal Medicine was supported by the CDC. The investigators disclosed additional relationships with Sanofi, GSK, MedImmune, and others.
FROM JAMA INTERNAL MEDICINE
Biomarker-guided steroid therapy shown safe for COPD
Eosinophil-guided corticosteroid therapy for patients with chronic obstructive pulmonary disease (COPD) is equivalent in efficacy to standard of care therapy, but the eosinophil-guided therapy may help mitigate the harmful side effects associated with even short courses of corticosteroids, investigators said in a primary care–based randomized trial.
Among patients in 14 primary care practices in the United Kingdom who experienced COPD exacerbations, the proportion of patients who experienced treatment failure at day 28 was 27% for those who were randomized to receive prednisolone only when blood eosinophil counts on a point-of-care assay equaled or exceeded 2%, compared with 34% of all patients randomized to standard of care.
The relative risk for treatment failure using the eosinophil-guided approach was 0.82, which did not reach statistical significance, but indicated noninferiority for the biomarker-based dosing method, Mona Bafadhel, MD, of King’s College London, reported on behalf of colleagues in the Stratified Treatment to Reduce Risk in COPD (STARR2) trial.
This is the largest primary care multicenter trial, and probably adds another 20% to the literature base for exacerbations in COPD,” she said in an oral abstract presentation at the European Respiratory Society 2022 Congress.
“A personalized endotype-based treatment with oral prednisolone is possible in patients with COPD and I think should be now part of clinical guidelines,” she added.
Too much of a good thing
Although systemic corticosteroids are the universal treatment for COPD exacerbations, the drugs are also known to increase harm, with studies showing that cumulative doses of oral corticosteroids in COPD patients is associated with an increased risk for death. In addition, systemic corticosteroids are the third most common cause of adverse events leading to hospitalization, behind only chemotherapy and antibiotic use leading to Clostridioides difficile infections, Dr. Bafadhel said.
“And of course, corticosteroids are associated with significant harmful effects, including a five-times increased risk of sepsis, three-times increased risk of [venous thromboembolism], and a twice-increased risk of fracture,” she said.
Dr. Bafadhel and colleagues had previously shown in the single-center BEAT-COPD study that peripheral blood eosinophils at the time of a moderate COPD exacerbation could be used to safely direct oral corticosteroid therapy. She also pointed to a 2019 multicenter open-label study showing that eosinophil-guided care was noninferior to standard prescribing of oral corticosteroids for patients with severe exacerbations.
Primary care study
The investigators conducted the current study to test whether eosinophil-guided therapy at the point of care in a primary practice setting was efficacious, with the ultimate goal of encouraging changes in guidelines.
They recruited patients with COPD exacerbations from 14 general practices in Oxfordshire and Buckinghamshire in the Thames Valley.
The patients were randomly assigned to receive either standard of care or the biomarker-guided intervention for 14 days. In this arm, patients with eosinophil counts of 2% or greater received matched prednisolone, while patients with counts below 2% received placebo. The patients were blinded to the assigned drug.
A total of 203 exacerbations among 152 patients were evenly allocated to treatment or control groups. The mean patient age was 71. Of the 102 exacerbations allocated to eosinophil-guided therapy, 34 were treated with placebo.
As noted before, in the intention-to-treat analysis the primary outcome of the treatment failure rate, defined as any need for antibiotics and/or steroids at one month, was 27% in the biomarker-guided arm and 34% in the standard care arm.
“In the per-protocol analysis we also demonstrated that there was a suggestion that there is possible superiority of using blood eosinophil-directed oral corticosteroid prescriptions at the time of acute exacerbation using the point-of-care eosinophil test,” Dr. Bafadhel said.
There were no significant differences in the secondary outcomes of mean change in forced expiratory volume in 1 second (FEV1), COPD Assessment Test scores from exacerbation to follow-up, and symptoms according to a visual analog scale.
Invited discussant Dave Singh, MD, of the University of Manchester, England, asked Dr. Bafadhel how the data she presented supported her conclusions about the potential benefits of eosinophil-guided therapy, given that the P values were nonsignificant.
“The primary outcome was powered on noninferiority, and of course what we’ve shown is that it’s not any worse, it’s not any better, but of course it’s the effect of how many courses of steroids you can reduce in that population,” Dr. Bafadhel replied.
She noted that although the investigators have not performed an economic analysis to determine how many adverse events might be avoided using the biomarker-guided approach, “we do know that some of these patients who are given prednisolone, their comorbidities of diabetes worsened, for example.”
In the online Q&A for the presentation, Sohail Ansari, MD, from the Mid and South Essex NHS Foundation Trust in the United Kingdom, said that many patients in primary care practices receive “rescue packs” containing antibiotics and steroids, but may not be equipped to know when they should use the steroids and therefore may overuse them.
“Perhaps community-based, adequately resourced respiratory teams [may] be a way forward, but it will need adequate investment and commitment,” he wrote.
The trial was supported by the University of Oxford and National Institute for Health and Care Research, UK. Dr. Bafadhel reported grant and research support from the National Institute for Health and Care Research, Asthma & Lung UK, AstraZeneca, and Roche, and honoraria or fees from others. Dr. Singh reported speaking fees, honoraria, and research grants from multiple companies. Dr. Ansari reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Eosinophil-guided corticosteroid therapy for patients with chronic obstructive pulmonary disease (COPD) is equivalent in efficacy to standard of care therapy, but the eosinophil-guided therapy may help mitigate the harmful side effects associated with even short courses of corticosteroids, investigators said in a primary care–based randomized trial.
Among patients in 14 primary care practices in the United Kingdom who experienced COPD exacerbations, the proportion of patients who experienced treatment failure at day 28 was 27% for those who were randomized to receive prednisolone only when blood eosinophil counts on a point-of-care assay equaled or exceeded 2%, compared with 34% of all patients randomized to standard of care.
The relative risk for treatment failure using the eosinophil-guided approach was 0.82, which did not reach statistical significance, but indicated noninferiority for the biomarker-based dosing method, Mona Bafadhel, MD, of King’s College London, reported on behalf of colleagues in the Stratified Treatment to Reduce Risk in COPD (STARR2) trial.
This is the largest primary care multicenter trial, and probably adds another 20% to the literature base for exacerbations in COPD,” she said in an oral abstract presentation at the European Respiratory Society 2022 Congress.
“A personalized endotype-based treatment with oral prednisolone is possible in patients with COPD and I think should be now part of clinical guidelines,” she added.
Too much of a good thing
Although systemic corticosteroids are the universal treatment for COPD exacerbations, the drugs are also known to increase harm, with studies showing that cumulative doses of oral corticosteroids in COPD patients is associated with an increased risk for death. In addition, systemic corticosteroids are the third most common cause of adverse events leading to hospitalization, behind only chemotherapy and antibiotic use leading to Clostridioides difficile infections, Dr. Bafadhel said.
“And of course, corticosteroids are associated with significant harmful effects, including a five-times increased risk of sepsis, three-times increased risk of [venous thromboembolism], and a twice-increased risk of fracture,” she said.
Dr. Bafadhel and colleagues had previously shown in the single-center BEAT-COPD study that peripheral blood eosinophils at the time of a moderate COPD exacerbation could be used to safely direct oral corticosteroid therapy. She also pointed to a 2019 multicenter open-label study showing that eosinophil-guided care was noninferior to standard prescribing of oral corticosteroids for patients with severe exacerbations.
Primary care study
The investigators conducted the current study to test whether eosinophil-guided therapy at the point of care in a primary practice setting was efficacious, with the ultimate goal of encouraging changes in guidelines.
They recruited patients with COPD exacerbations from 14 general practices in Oxfordshire and Buckinghamshire in the Thames Valley.
The patients were randomly assigned to receive either standard of care or the biomarker-guided intervention for 14 days. In this arm, patients with eosinophil counts of 2% or greater received matched prednisolone, while patients with counts below 2% received placebo. The patients were blinded to the assigned drug.
A total of 203 exacerbations among 152 patients were evenly allocated to treatment or control groups. The mean patient age was 71. Of the 102 exacerbations allocated to eosinophil-guided therapy, 34 were treated with placebo.
As noted before, in the intention-to-treat analysis the primary outcome of the treatment failure rate, defined as any need for antibiotics and/or steroids at one month, was 27% in the biomarker-guided arm and 34% in the standard care arm.
“In the per-protocol analysis we also demonstrated that there was a suggestion that there is possible superiority of using blood eosinophil-directed oral corticosteroid prescriptions at the time of acute exacerbation using the point-of-care eosinophil test,” Dr. Bafadhel said.
There were no significant differences in the secondary outcomes of mean change in forced expiratory volume in 1 second (FEV1), COPD Assessment Test scores from exacerbation to follow-up, and symptoms according to a visual analog scale.
Invited discussant Dave Singh, MD, of the University of Manchester, England, asked Dr. Bafadhel how the data she presented supported her conclusions about the potential benefits of eosinophil-guided therapy, given that the P values were nonsignificant.
“The primary outcome was powered on noninferiority, and of course what we’ve shown is that it’s not any worse, it’s not any better, but of course it’s the effect of how many courses of steroids you can reduce in that population,” Dr. Bafadhel replied.
She noted that although the investigators have not performed an economic analysis to determine how many adverse events might be avoided using the biomarker-guided approach, “we do know that some of these patients who are given prednisolone, their comorbidities of diabetes worsened, for example.”
In the online Q&A for the presentation, Sohail Ansari, MD, from the Mid and South Essex NHS Foundation Trust in the United Kingdom, said that many patients in primary care practices receive “rescue packs” containing antibiotics and steroids, but may not be equipped to know when they should use the steroids and therefore may overuse them.
“Perhaps community-based, adequately resourced respiratory teams [may] be a way forward, but it will need adequate investment and commitment,” he wrote.
The trial was supported by the University of Oxford and National Institute for Health and Care Research, UK. Dr. Bafadhel reported grant and research support from the National Institute for Health and Care Research, Asthma & Lung UK, AstraZeneca, and Roche, and honoraria or fees from others. Dr. Singh reported speaking fees, honoraria, and research grants from multiple companies. Dr. Ansari reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Eosinophil-guided corticosteroid therapy for patients with chronic obstructive pulmonary disease (COPD) is equivalent in efficacy to standard of care therapy, but the eosinophil-guided therapy may help mitigate the harmful side effects associated with even short courses of corticosteroids, investigators said in a primary care–based randomized trial.
Among patients in 14 primary care practices in the United Kingdom who experienced COPD exacerbations, the proportion of patients who experienced treatment failure at day 28 was 27% for those who were randomized to receive prednisolone only when blood eosinophil counts on a point-of-care assay equaled or exceeded 2%, compared with 34% of all patients randomized to standard of care.
The relative risk for treatment failure using the eosinophil-guided approach was 0.82, which did not reach statistical significance, but indicated noninferiority for the biomarker-based dosing method, Mona Bafadhel, MD, of King’s College London, reported on behalf of colleagues in the Stratified Treatment to Reduce Risk in COPD (STARR2) trial.
This is the largest primary care multicenter trial, and probably adds another 20% to the literature base for exacerbations in COPD,” she said in an oral abstract presentation at the European Respiratory Society 2022 Congress.
“A personalized endotype-based treatment with oral prednisolone is possible in patients with COPD and I think should be now part of clinical guidelines,” she added.
Too much of a good thing
Although systemic corticosteroids are the universal treatment for COPD exacerbations, the drugs are also known to increase harm, with studies showing that cumulative doses of oral corticosteroids in COPD patients is associated with an increased risk for death. In addition, systemic corticosteroids are the third most common cause of adverse events leading to hospitalization, behind only chemotherapy and antibiotic use leading to Clostridioides difficile infections, Dr. Bafadhel said.
“And of course, corticosteroids are associated with significant harmful effects, including a five-times increased risk of sepsis, three-times increased risk of [venous thromboembolism], and a twice-increased risk of fracture,” she said.
Dr. Bafadhel and colleagues had previously shown in the single-center BEAT-COPD study that peripheral blood eosinophils at the time of a moderate COPD exacerbation could be used to safely direct oral corticosteroid therapy. She also pointed to a 2019 multicenter open-label study showing that eosinophil-guided care was noninferior to standard prescribing of oral corticosteroids for patients with severe exacerbations.
Primary care study
The investigators conducted the current study to test whether eosinophil-guided therapy at the point of care in a primary practice setting was efficacious, with the ultimate goal of encouraging changes in guidelines.
They recruited patients with COPD exacerbations from 14 general practices in Oxfordshire and Buckinghamshire in the Thames Valley.
The patients were randomly assigned to receive either standard of care or the biomarker-guided intervention for 14 days. In this arm, patients with eosinophil counts of 2% or greater received matched prednisolone, while patients with counts below 2% received placebo. The patients were blinded to the assigned drug.
A total of 203 exacerbations among 152 patients were evenly allocated to treatment or control groups. The mean patient age was 71. Of the 102 exacerbations allocated to eosinophil-guided therapy, 34 were treated with placebo.
As noted before, in the intention-to-treat analysis the primary outcome of the treatment failure rate, defined as any need for antibiotics and/or steroids at one month, was 27% in the biomarker-guided arm and 34% in the standard care arm.
“In the per-protocol analysis we also demonstrated that there was a suggestion that there is possible superiority of using blood eosinophil-directed oral corticosteroid prescriptions at the time of acute exacerbation using the point-of-care eosinophil test,” Dr. Bafadhel said.
There were no significant differences in the secondary outcomes of mean change in forced expiratory volume in 1 second (FEV1), COPD Assessment Test scores from exacerbation to follow-up, and symptoms according to a visual analog scale.
Invited discussant Dave Singh, MD, of the University of Manchester, England, asked Dr. Bafadhel how the data she presented supported her conclusions about the potential benefits of eosinophil-guided therapy, given that the P values were nonsignificant.
“The primary outcome was powered on noninferiority, and of course what we’ve shown is that it’s not any worse, it’s not any better, but of course it’s the effect of how many courses of steroids you can reduce in that population,” Dr. Bafadhel replied.
She noted that although the investigators have not performed an economic analysis to determine how many adverse events might be avoided using the biomarker-guided approach, “we do know that some of these patients who are given prednisolone, their comorbidities of diabetes worsened, for example.”
In the online Q&A for the presentation, Sohail Ansari, MD, from the Mid and South Essex NHS Foundation Trust in the United Kingdom, said that many patients in primary care practices receive “rescue packs” containing antibiotics and steroids, but may not be equipped to know when they should use the steroids and therefore may overuse them.
“Perhaps community-based, adequately resourced respiratory teams [may] be a way forward, but it will need adequate investment and commitment,” he wrote.
The trial was supported by the University of Oxford and National Institute for Health and Care Research, UK. Dr. Bafadhel reported grant and research support from the National Institute for Health and Care Research, Asthma & Lung UK, AstraZeneca, and Roche, and honoraria or fees from others. Dr. Singh reported speaking fees, honoraria, and research grants from multiple companies. Dr. Ansari reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM ERS 2022 CONGRESS