User login
Advancing the fight against drug-resistant malaria

Image by Ute Frevert
and Margaret Shear
Researchers say they have designed a compound that kills malaria parasites—even those resistant to current antimalarial therapy—but
avoids harming human cells.
The compound exploits tiny structural differences between the parasitic and human versions of the proteasome.
In preclinical experiments, this proteasome inhibitor was able to kill artemisinin-resistant malaria parasites and further sensitize parasites to artemisinin.
Matthew Bogyo, PhD, of Stanford University School of Medicine in California, and his colleagues conducted this research and recounted the results in a letter to Nature.
Previous research has shown that proteasome inhibitors can be toxic to the malaria parasite Plasmodium falciparum. But the drugs have tended to inhibit the human version of the proteasome too, resulting in toxicity that would be unacceptable in a malaria drug.
Dr Bogyo and his colleagues wanted to overcome this problem, so they produced highly purified preparations of both human and P falciparum proteasomes. The team then “fed” those 2 preparations a set of protein fragments containing a variety of amino-acid linkages to see which amino-acid linkages the proteasomes would cleave.
The researchers identified 113 amino-acid linkages that are readily cleaved by P falciparum proteasomes but not so well by human proteasomes, and 153 amino-acid linkages where the reverse is the case.
The team used this information to design tiny protein snippets that failed to interact with human proteasomes but inhibited parts of the P falciparum proteasomes responsible for cleaving certain amino-acid links.
The researchers investigated the basis for this selectivity by using high-resolution electron microscopy to map the detailed structure of the parasite and human proteasomes. This allowed them to optimize the protein snippets they were using as parasite-selective proteasome inhibitors.
The 3-amino-acid snippet they ultimately focused on, called WLL, was able to inhibit 2 different catalytic regions in P falciparum proteasomes without having any effect on those of cultured human cells. There was a 600-fold difference in WLL’s potency at killing the parasitic cells over the human cells.
In experiments with mice, the researchers saw a nearly complete reduction of malaria parasites with both single and multiple doses of WLL.
Other tests, performed on artemisinin-resistant parasites infecting human red blood cells, suggested the WLL compound was equally effective at killing artemisinin-resistant parasites and artemisinin-sensitive parasites.
Dr Bogyo pointed out that the artemisinin family of drugs work by modifying proteins in the parasite. Resistance occurs when the parasites’ proteasomes are able to recycle those modified proteins. But this means that artemisinin-treated parasites are particularly sensitive to disruption of normal protein function.
“The compounds we’ve derived can kill artemisinin-resistant parasites because those parasites have an increased need for highly efficient proteasomes,” he said.
“So combining the proteasome inhibitor with artemisinin should make it possible to block the onset of resistance. That will, in turn, allow the continued use of that front-line malaria treatment, which has been so effective up until now.”
Clinical trials of compounds derived from this research remain several years away, Dr Bogyo added.
Study author Leann Tilley, PhD, of the University of Melbourne in Victoria, Australia, and her team are working with experts from Takeda Pharmaceutical Company Limited and Medicines for Malaria Venture to identify additional classes of parasite-specific proteasome inhibitors that could be advanced to clinical trials.
“The next step is screening the Takeda libraries to find a similar drug that doesn’t affect the human proteasome,” Dr Tilley said. “The current drug is a good start, but it’s not yet suitable for humans. It needs to be able to be administered orally and needs to last a long time in the blood stream.”
Dr Tilley said if they can find an existing drug in Takeda’s libraries that matches the structure of the new malaria drug, they could move it toward human trials very quickly. ![]()

Image by Ute Frevert
and Margaret Shear
Researchers say they have designed a compound that kills malaria parasites—even those resistant to current antimalarial therapy—but
avoids harming human cells.
The compound exploits tiny structural differences between the parasitic and human versions of the proteasome.
In preclinical experiments, this proteasome inhibitor was able to kill artemisinin-resistant malaria parasites and further sensitize parasites to artemisinin.
Matthew Bogyo, PhD, of Stanford University School of Medicine in California, and his colleagues conducted this research and recounted the results in a letter to Nature.
Previous research has shown that proteasome inhibitors can be toxic to the malaria parasite Plasmodium falciparum. But the drugs have tended to inhibit the human version of the proteasome too, resulting in toxicity that would be unacceptable in a malaria drug.
Dr Bogyo and his colleagues wanted to overcome this problem, so they produced highly purified preparations of both human and P falciparum proteasomes. The team then “fed” those 2 preparations a set of protein fragments containing a variety of amino-acid linkages to see which amino-acid linkages the proteasomes would cleave.
The researchers identified 113 amino-acid linkages that are readily cleaved by P falciparum proteasomes but not so well by human proteasomes, and 153 amino-acid linkages where the reverse is the case.
The team used this information to design tiny protein snippets that failed to interact with human proteasomes but inhibited parts of the P falciparum proteasomes responsible for cleaving certain amino-acid links.
The researchers investigated the basis for this selectivity by using high-resolution electron microscopy to map the detailed structure of the parasite and human proteasomes. This allowed them to optimize the protein snippets they were using as parasite-selective proteasome inhibitors.
The 3-amino-acid snippet they ultimately focused on, called WLL, was able to inhibit 2 different catalytic regions in P falciparum proteasomes without having any effect on those of cultured human cells. There was a 600-fold difference in WLL’s potency at killing the parasitic cells over the human cells.
In experiments with mice, the researchers saw a nearly complete reduction of malaria parasites with both single and multiple doses of WLL.
Other tests, performed on artemisinin-resistant parasites infecting human red blood cells, suggested the WLL compound was equally effective at killing artemisinin-resistant parasites and artemisinin-sensitive parasites.
Dr Bogyo pointed out that the artemisinin family of drugs work by modifying proteins in the parasite. Resistance occurs when the parasites’ proteasomes are able to recycle those modified proteins. But this means that artemisinin-treated parasites are particularly sensitive to disruption of normal protein function.
“The compounds we’ve derived can kill artemisinin-resistant parasites because those parasites have an increased need for highly efficient proteasomes,” he said.
“So combining the proteasome inhibitor with artemisinin should make it possible to block the onset of resistance. That will, in turn, allow the continued use of that front-line malaria treatment, which has been so effective up until now.”
Clinical trials of compounds derived from this research remain several years away, Dr Bogyo added.
Study author Leann Tilley, PhD, of the University of Melbourne in Victoria, Australia, and her team are working with experts from Takeda Pharmaceutical Company Limited and Medicines for Malaria Venture to identify additional classes of parasite-specific proteasome inhibitors that could be advanced to clinical trials.
“The next step is screening the Takeda libraries to find a similar drug that doesn’t affect the human proteasome,” Dr Tilley said. “The current drug is a good start, but it’s not yet suitable for humans. It needs to be able to be administered orally and needs to last a long time in the blood stream.”
Dr Tilley said if they can find an existing drug in Takeda’s libraries that matches the structure of the new malaria drug, they could move it toward human trials very quickly. ![]()

Image by Ute Frevert
and Margaret Shear
Researchers say they have designed a compound that kills malaria parasites—even those resistant to current antimalarial therapy—but
avoids harming human cells.
The compound exploits tiny structural differences between the parasitic and human versions of the proteasome.
In preclinical experiments, this proteasome inhibitor was able to kill artemisinin-resistant malaria parasites and further sensitize parasites to artemisinin.
Matthew Bogyo, PhD, of Stanford University School of Medicine in California, and his colleagues conducted this research and recounted the results in a letter to Nature.
Previous research has shown that proteasome inhibitors can be toxic to the malaria parasite Plasmodium falciparum. But the drugs have tended to inhibit the human version of the proteasome too, resulting in toxicity that would be unacceptable in a malaria drug.
Dr Bogyo and his colleagues wanted to overcome this problem, so they produced highly purified preparations of both human and P falciparum proteasomes. The team then “fed” those 2 preparations a set of protein fragments containing a variety of amino-acid linkages to see which amino-acid linkages the proteasomes would cleave.
The researchers identified 113 amino-acid linkages that are readily cleaved by P falciparum proteasomes but not so well by human proteasomes, and 153 amino-acid linkages where the reverse is the case.
The team used this information to design tiny protein snippets that failed to interact with human proteasomes but inhibited parts of the P falciparum proteasomes responsible for cleaving certain amino-acid links.
The researchers investigated the basis for this selectivity by using high-resolution electron microscopy to map the detailed structure of the parasite and human proteasomes. This allowed them to optimize the protein snippets they were using as parasite-selective proteasome inhibitors.
The 3-amino-acid snippet they ultimately focused on, called WLL, was able to inhibit 2 different catalytic regions in P falciparum proteasomes without having any effect on those of cultured human cells. There was a 600-fold difference in WLL’s potency at killing the parasitic cells over the human cells.
In experiments with mice, the researchers saw a nearly complete reduction of malaria parasites with both single and multiple doses of WLL.
Other tests, performed on artemisinin-resistant parasites infecting human red blood cells, suggested the WLL compound was equally effective at killing artemisinin-resistant parasites and artemisinin-sensitive parasites.
Dr Bogyo pointed out that the artemisinin family of drugs work by modifying proteins in the parasite. Resistance occurs when the parasites’ proteasomes are able to recycle those modified proteins. But this means that artemisinin-treated parasites are particularly sensitive to disruption of normal protein function.
“The compounds we’ve derived can kill artemisinin-resistant parasites because those parasites have an increased need for highly efficient proteasomes,” he said.
“So combining the proteasome inhibitor with artemisinin should make it possible to block the onset of resistance. That will, in turn, allow the continued use of that front-line malaria treatment, which has been so effective up until now.”
Clinical trials of compounds derived from this research remain several years away, Dr Bogyo added.
Study author Leann Tilley, PhD, of the University of Melbourne in Victoria, Australia, and her team are working with experts from Takeda Pharmaceutical Company Limited and Medicines for Malaria Venture to identify additional classes of parasite-specific proteasome inhibitors that could be advanced to clinical trials.
“The next step is screening the Takeda libraries to find a similar drug that doesn’t affect the human proteasome,” Dr Tilley said. “The current drug is a good start, but it’s not yet suitable for humans. It needs to be able to be administered orally and needs to last a long time in the blood stream.”
Dr Tilley said if they can find an existing drug in Takeda’s libraries that matches the structure of the new malaria drug, they could move it toward human trials very quickly. ![]()
Low doses of iron may cause cell damage
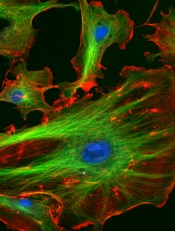
Image courtesy of NIH
Low doses of iron can modify the vascular endothelium and induce a DNA-damage response, researchers have reported in PLOS ONE.
The team observed these phenomena in vitro and said the results must be confirmed via additional research.
However, the findings suggest a need to assess the amount of iron given in standard treatments and the effects this may have on the body, according to Claire Shovlin, PhD, of Imperial College London in the UK.
Dr Shovlin and her colleagues studied human endothelial cells, adding either placebo or an iron solution of 10 micromolar, which is a similar concentration to that seen in the blood after taking an iron tablet.
Within 10 minutes, cells treated with the iron solution had activated DNA repair systems, and these were still activated 6 hours later.
“We already knew that iron could be damaging to cells in very high doses,” Dr Shovlin said. “However, in this study, we found that when we applied the kinds of levels of iron you would find in the bloodstream after taking an iron tablet, this also seemed to be able to trigger cell damage—at least in the laboratory. In other words, cells seem more sensitive to iron than we previously thought.”
“This is very early stage research, and we need more work to confirm these findings and investigate what effects this may have on the body. We are still not sure how these laboratory findings translate to blood vessels in the body.”
“However, this study helps to open the conversation about how much iron people take. At the moment, each standard iron tablet contains almost 10 times the amount of iron men are recommended to eat each day, and these dosages haven’t changed for more than 50 years. This research suggests we may need to think more carefully about how much iron we give to people, and try and tailor the dose to the patient.”
Dr Shovlin and her colleagues initially started researching this area after finding that a small proportion of people using iron tablets for hereditary hemorrhagic telangiectasia, which causes abnormalities in the blood vessels, reported their nose bleeds worsened after iron treatment. ![]()

Image courtesy of NIH
Low doses of iron can modify the vascular endothelium and induce a DNA-damage response, researchers have reported in PLOS ONE.
The team observed these phenomena in vitro and said the results must be confirmed via additional research.
However, the findings suggest a need to assess the amount of iron given in standard treatments and the effects this may have on the body, according to Claire Shovlin, PhD, of Imperial College London in the UK.
Dr Shovlin and her colleagues studied human endothelial cells, adding either placebo or an iron solution of 10 micromolar, which is a similar concentration to that seen in the blood after taking an iron tablet.
Within 10 minutes, cells treated with the iron solution had activated DNA repair systems, and these were still activated 6 hours later.
“We already knew that iron could be damaging to cells in very high doses,” Dr Shovlin said. “However, in this study, we found that when we applied the kinds of levels of iron you would find in the bloodstream after taking an iron tablet, this also seemed to be able to trigger cell damage—at least in the laboratory. In other words, cells seem more sensitive to iron than we previously thought.”
“This is very early stage research, and we need more work to confirm these findings and investigate what effects this may have on the body. We are still not sure how these laboratory findings translate to blood vessels in the body.”
“However, this study helps to open the conversation about how much iron people take. At the moment, each standard iron tablet contains almost 10 times the amount of iron men are recommended to eat each day, and these dosages haven’t changed for more than 50 years. This research suggests we may need to think more carefully about how much iron we give to people, and try and tailor the dose to the patient.”
Dr Shovlin and her colleagues initially started researching this area after finding that a small proportion of people using iron tablets for hereditary hemorrhagic telangiectasia, which causes abnormalities in the blood vessels, reported their nose bleeds worsened after iron treatment. ![]()

Image courtesy of NIH
Low doses of iron can modify the vascular endothelium and induce a DNA-damage response, researchers have reported in PLOS ONE.
The team observed these phenomena in vitro and said the results must be confirmed via additional research.
However, the findings suggest a need to assess the amount of iron given in standard treatments and the effects this may have on the body, according to Claire Shovlin, PhD, of Imperial College London in the UK.
Dr Shovlin and her colleagues studied human endothelial cells, adding either placebo or an iron solution of 10 micromolar, which is a similar concentration to that seen in the blood after taking an iron tablet.
Within 10 minutes, cells treated with the iron solution had activated DNA repair systems, and these were still activated 6 hours later.
“We already knew that iron could be damaging to cells in very high doses,” Dr Shovlin said. “However, in this study, we found that when we applied the kinds of levels of iron you would find in the bloodstream after taking an iron tablet, this also seemed to be able to trigger cell damage—at least in the laboratory. In other words, cells seem more sensitive to iron than we previously thought.”
“This is very early stage research, and we need more work to confirm these findings and investigate what effects this may have on the body. We are still not sure how these laboratory findings translate to blood vessels in the body.”
“However, this study helps to open the conversation about how much iron people take. At the moment, each standard iron tablet contains almost 10 times the amount of iron men are recommended to eat each day, and these dosages haven’t changed for more than 50 years. This research suggests we may need to think more carefully about how much iron we give to people, and try and tailor the dose to the patient.”
Dr Shovlin and her colleagues initially started researching this area after finding that a small proportion of people using iron tablets for hereditary hemorrhagic telangiectasia, which causes abnormalities in the blood vessels, reported their nose bleeds worsened after iron treatment. ![]()
Method may reduce toxicity of anticancer agents

Researchers believe they have found a way to make certain anticancer agents safer without compromising their efficacy.
The team noted that Aurora B kinase inhibitors and other agents targeting the cell cycle have proven effective but highly toxic in clinical trials.
In an attempt to solve this problem, the researchers turned to nanotechnology. They encapsulated the Aurora B kinase inhibitor AZD2811 in polymeric nanoparticles called Accurins.
Susan Ashton, of AstraZeneca in Macclesfield, Cheshire, UK, and her colleagues developed the Accurins and described the work in Science Translational Medicine. The work was funded by AstraZeneca.
The Accurins consist of block copolymers of poly-D,L-lactide and poly(ethylene glycol). The researchers used an ion pairing approach to efficiently encapsulate AZD2811 and control release of the drug.
They found the Accurins could release AZD2811 continuously for more than 1 week in vitro. The nanoparticles also reduced tumor phosphorylated histone H3 levels in vivo for up to 96 hours after a single administration.
The researchers tested the AZD2811 Accurins in mice with diffuse large B-cell lymphoma and rats with colorectal tumors. The nanoparticles accumulated specifically in tumors, where they slowly released AZD2811 to cancer cells.
Compared to AZD1152 (a water-soluble prodrug of AZD2811), the AZD2811 Accurins blocked tumor growth more effectively at one-half the drug dose and caused fewer side effects in the rodents.
Based on these results, the researchers said Accurins could provide efficacy and tolerability using a more convenient dosing regimen, which may extend the utility of Aurora B kinase inhibition to a broader range of hematologic and solid tumor malignancies.
A phase 1 study (NCT02579226) testing AZD2811 Accurins in advanced solid tumors is currently recruiting patients.
A related Focus article published in Science Translational Medicine offers more insights on how Accurin nanoparticles may help enhance the safety and antitumor activity of Aurora kinase inhibitors and other molecularly targeted drugs. ![]()

Researchers believe they have found a way to make certain anticancer agents safer without compromising their efficacy.
The team noted that Aurora B kinase inhibitors and other agents targeting the cell cycle have proven effective but highly toxic in clinical trials.
In an attempt to solve this problem, the researchers turned to nanotechnology. They encapsulated the Aurora B kinase inhibitor AZD2811 in polymeric nanoparticles called Accurins.
Susan Ashton, of AstraZeneca in Macclesfield, Cheshire, UK, and her colleagues developed the Accurins and described the work in Science Translational Medicine. The work was funded by AstraZeneca.
The Accurins consist of block copolymers of poly-D,L-lactide and poly(ethylene glycol). The researchers used an ion pairing approach to efficiently encapsulate AZD2811 and control release of the drug.
They found the Accurins could release AZD2811 continuously for more than 1 week in vitro. The nanoparticles also reduced tumor phosphorylated histone H3 levels in vivo for up to 96 hours after a single administration.
The researchers tested the AZD2811 Accurins in mice with diffuse large B-cell lymphoma and rats with colorectal tumors. The nanoparticles accumulated specifically in tumors, where they slowly released AZD2811 to cancer cells.
Compared to AZD1152 (a water-soluble prodrug of AZD2811), the AZD2811 Accurins blocked tumor growth more effectively at one-half the drug dose and caused fewer side effects in the rodents.
Based on these results, the researchers said Accurins could provide efficacy and tolerability using a more convenient dosing regimen, which may extend the utility of Aurora B kinase inhibition to a broader range of hematologic and solid tumor malignancies.
A phase 1 study (NCT02579226) testing AZD2811 Accurins in advanced solid tumors is currently recruiting patients.
A related Focus article published in Science Translational Medicine offers more insights on how Accurin nanoparticles may help enhance the safety and antitumor activity of Aurora kinase inhibitors and other molecularly targeted drugs. ![]()

Researchers believe they have found a way to make certain anticancer agents safer without compromising their efficacy.
The team noted that Aurora B kinase inhibitors and other agents targeting the cell cycle have proven effective but highly toxic in clinical trials.
In an attempt to solve this problem, the researchers turned to nanotechnology. They encapsulated the Aurora B kinase inhibitor AZD2811 in polymeric nanoparticles called Accurins.
Susan Ashton, of AstraZeneca in Macclesfield, Cheshire, UK, and her colleagues developed the Accurins and described the work in Science Translational Medicine. The work was funded by AstraZeneca.
The Accurins consist of block copolymers of poly-D,L-lactide and poly(ethylene glycol). The researchers used an ion pairing approach to efficiently encapsulate AZD2811 and control release of the drug.
They found the Accurins could release AZD2811 continuously for more than 1 week in vitro. The nanoparticles also reduced tumor phosphorylated histone H3 levels in vivo for up to 96 hours after a single administration.
The researchers tested the AZD2811 Accurins in mice with diffuse large B-cell lymphoma and rats with colorectal tumors. The nanoparticles accumulated specifically in tumors, where they slowly released AZD2811 to cancer cells.
Compared to AZD1152 (a water-soluble prodrug of AZD2811), the AZD2811 Accurins blocked tumor growth more effectively at one-half the drug dose and caused fewer side effects in the rodents.
Based on these results, the researchers said Accurins could provide efficacy and tolerability using a more convenient dosing regimen, which may extend the utility of Aurora B kinase inhibition to a broader range of hematologic and solid tumor malignancies.
A phase 1 study (NCT02579226) testing AZD2811 Accurins in advanced solid tumors is currently recruiting patients.
A related Focus article published in Science Translational Medicine offers more insights on how Accurin nanoparticles may help enhance the safety and antitumor activity of Aurora kinase inhibitors and other molecularly targeted drugs. ![]()
Gut microbiota linked to severity of malaria
Microorganisms in the gut play a role in the severity of malaria, according to a study published in PNAS.
Investigators examined the gut microbiomes of mice and found evidence to suggest that malaria severity is not only a function of the parasite or the host. It is also influenced by the microbes in the infected organism.
And 2 types of bacteria—Bifidobacterium and Lactobacillus—were associated with reduced malaria severity.
“The research provides a potential new avenue to investigate factors that control the severity of malaria,” said study author Steven Wilhelm, PhD, of the University of Tennessee at Knoxville.
“With 1 million people dying [of malaria] each year, many of whom are young children, any approach that may save even a few lives is worth following up on.”
With this study, Dr Wilhelm and his colleagues found that genetically similar mice acquired from different vendors had differences in pathology after malaria infection. There were significant differences in both parasite burden and mortality after infection with multiple Plasmodium species.
The investigators measured gut microbiomes in the mice by sequencing bacteria in the digestive tract and noted significant differences within the different mouse populations.
So the team transferred cecal content from the first set of mice into germ-free mice and found that differences in malaria severity were transferred.
The mice that received transplants from donors that were more resistant to malaria had low parasite burdens. And mice that received transplants from donors that were more susceptible to malaria had high parasite burdens.
The investigators also observed an increased abundance of Bifidobacterium and Lactobacillus bacteria in the mice that exhibited reduced malaria pathology.
So the team took mice that were more susceptible to malaria, treated them with antibiotics, and fed them yogurt containing Bifidobacterium and Lactobacillus. As expected, the severity of malaria in these mice decreased.
“These results demonstrate the possibility of modifying the gut microbiome to prevent severe malaria,” said study author Nathan Schmidt, PhD, of the University of Louisville in Kentucky.
Dr Wilhelm noted that, although the research interventions lessened the severity of malaria in mice, it did not prevent or cure it. And the investigators are a long way from perfecting similar treatments in humans but are working on understanding the mechanism.
“A way to help people who are infected—and especially a simple and cheap way, as much of the infection occurs in the developing world—would be a great service to society,” Dr Wilhelm said. ![]()

Microorganisms in the gut play a role in the severity of malaria, according to a study published in PNAS.
Investigators examined the gut microbiomes of mice and found evidence to suggest that malaria severity is not only a function of the parasite or the host. It is also influenced by the microbes in the infected organism.
And 2 types of bacteria—Bifidobacterium and Lactobacillus—were associated with reduced malaria severity.
“The research provides a potential new avenue to investigate factors that control the severity of malaria,” said study author Steven Wilhelm, PhD, of the University of Tennessee at Knoxville.
“With 1 million people dying [of malaria] each year, many of whom are young children, any approach that may save even a few lives is worth following up on.”
With this study, Dr Wilhelm and his colleagues found that genetically similar mice acquired from different vendors had differences in pathology after malaria infection. There were significant differences in both parasite burden and mortality after infection with multiple Plasmodium species.
The investigators measured gut microbiomes in the mice by sequencing bacteria in the digestive tract and noted significant differences within the different mouse populations.
So the team transferred cecal content from the first set of mice into germ-free mice and found that differences in malaria severity were transferred.
The mice that received transplants from donors that were more resistant to malaria had low parasite burdens. And mice that received transplants from donors that were more susceptible to malaria had high parasite burdens.
The investigators also observed an increased abundance of Bifidobacterium and Lactobacillus bacteria in the mice that exhibited reduced malaria pathology.
So the team took mice that were more susceptible to malaria, treated them with antibiotics, and fed them yogurt containing Bifidobacterium and Lactobacillus. As expected, the severity of malaria in these mice decreased.
“These results demonstrate the possibility of modifying the gut microbiome to prevent severe malaria,” said study author Nathan Schmidt, PhD, of the University of Louisville in Kentucky.
Dr Wilhelm noted that, although the research interventions lessened the severity of malaria in mice, it did not prevent or cure it. And the investigators are a long way from perfecting similar treatments in humans but are working on understanding the mechanism.
“A way to help people who are infected—and especially a simple and cheap way, as much of the infection occurs in the developing world—would be a great service to society,” Dr Wilhelm said. ![]()

Microorganisms in the gut play a role in the severity of malaria, according to a study published in PNAS.
Investigators examined the gut microbiomes of mice and found evidence to suggest that malaria severity is not only a function of the parasite or the host. It is also influenced by the microbes in the infected organism.
And 2 types of bacteria—Bifidobacterium and Lactobacillus—were associated with reduced malaria severity.
“The research provides a potential new avenue to investigate factors that control the severity of malaria,” said study author Steven Wilhelm, PhD, of the University of Tennessee at Knoxville.
“With 1 million people dying [of malaria] each year, many of whom are young children, any approach that may save even a few lives is worth following up on.”
With this study, Dr Wilhelm and his colleagues found that genetically similar mice acquired from different vendors had differences in pathology after malaria infection. There were significant differences in both parasite burden and mortality after infection with multiple Plasmodium species.
The investigators measured gut microbiomes in the mice by sequencing bacteria in the digestive tract and noted significant differences within the different mouse populations.
So the team transferred cecal content from the first set of mice into germ-free mice and found that differences in malaria severity were transferred.
The mice that received transplants from donors that were more resistant to malaria had low parasite burdens. And mice that received transplants from donors that were more susceptible to malaria had high parasite burdens.
The investigators also observed an increased abundance of Bifidobacterium and Lactobacillus bacteria in the mice that exhibited reduced malaria pathology.
So the team took mice that were more susceptible to malaria, treated them with antibiotics, and fed them yogurt containing Bifidobacterium and Lactobacillus. As expected, the severity of malaria in these mice decreased.
“These results demonstrate the possibility of modifying the gut microbiome to prevent severe malaria,” said study author Nathan Schmidt, PhD, of the University of Louisville in Kentucky.
Dr Wilhelm noted that, although the research interventions lessened the severity of malaria in mice, it did not prevent or cure it. And the investigators are a long way from perfecting similar treatments in humans but are working on understanding the mechanism.
“A way to help people who are infected—and especially a simple and cheap way, as much of the infection occurs in the developing world—would be a great service to society,” Dr Wilhelm said. ![]()
FDA approves drug for patients receiving MEC

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved a supplemental new drug application for single-dose fosaprepitant dimeglumine (Emend) for injection.
The agency approved the substance P/neurokinin-1 (NK1) receptor antagonist for use in combination with other anti-emetic medicines to prevent delayed nausea and vomiting in adults receiving initial and repeat courses of moderately emetogenic chemotherapy (MEC).
This makes fosaprepitant dimeglumine the first intravenous NK1 receptor antagonist approved in the US for patients receiving either highly emetogenic chemotherapy or MEC.
Fosaprepitant dimeglumine has not been studied for the treatment of established nausea and vomiting.
The FDA’s latest approval of fosaprepitant dimeglumine is supported by data from a phase 3 study published in the Annals of Oncology.
Patients receiving MEC were given ondansetron and dexamethasone (n=498) or ondansetron and dexamethasone plus a single intravenous infusion of fosaprepitant dimeglumine (n=502).
The primary endpoint was complete response (CR)—defined as no vomiting and no use of rescue therapy—in the delayed phase of chemotherapy-induced nausea and vomiting, which is 25 to 120 hours after the initiation of chemotherapy.
Secondary endpoints included CR in the overall and acute phases—0 to 120 and 0 to 24 hours after MEC initiation, respectively—and no vomiting in the overall phase.
The fosaprepitant regimen improved CR significantly in the delayed and overall phases but not in the acute phase.
In the delayed phase, the CR rate was 78.9% with the fosaprepitant regimen and 68.5% with the control regimen (P<0.001). In the acute phase, the CR rate was 93.2% and 91.0%, respectively (P=0.184). Overall, the CR rate was 77.1% and 66.9%, respectively (P<0.001).
In the overall phase, the proportion of subjects with no vomiting was 82.7% with the fosaprepitant regimen and 72.9% with the control regimen (P<0.001). The proportion of patients with no significant nausea was 83.2% and 77.9%, respectively (P=0.030).
The most common adverse events reported in the fosaprepitant and control arms, respectively, were fatigue (15% vs 13%), diarrhea (13% vs 11%), neutropenia (8% vs 7%), asthenia (4% vs 3%), anemia (3% vs 2%), peripheral neuropathy (3% vs 2%), leukopenia (2% vs 1%), dyspepsia (2% vs 1%), urinary tract infection (2% vs 1%), and pain in extremity (2% vs 1%).
Fosaprepitant dimeglumine is a product of Merck. For more details on the drug, see the prescribing information. ![]()

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved a supplemental new drug application for single-dose fosaprepitant dimeglumine (Emend) for injection.
The agency approved the substance P/neurokinin-1 (NK1) receptor antagonist for use in combination with other anti-emetic medicines to prevent delayed nausea and vomiting in adults receiving initial and repeat courses of moderately emetogenic chemotherapy (MEC).
This makes fosaprepitant dimeglumine the first intravenous NK1 receptor antagonist approved in the US for patients receiving either highly emetogenic chemotherapy or MEC.
Fosaprepitant dimeglumine has not been studied for the treatment of established nausea and vomiting.
The FDA’s latest approval of fosaprepitant dimeglumine is supported by data from a phase 3 study published in the Annals of Oncology.
Patients receiving MEC were given ondansetron and dexamethasone (n=498) or ondansetron and dexamethasone plus a single intravenous infusion of fosaprepitant dimeglumine (n=502).
The primary endpoint was complete response (CR)—defined as no vomiting and no use of rescue therapy—in the delayed phase of chemotherapy-induced nausea and vomiting, which is 25 to 120 hours after the initiation of chemotherapy.
Secondary endpoints included CR in the overall and acute phases—0 to 120 and 0 to 24 hours after MEC initiation, respectively—and no vomiting in the overall phase.
The fosaprepitant regimen improved CR significantly in the delayed and overall phases but not in the acute phase.
In the delayed phase, the CR rate was 78.9% with the fosaprepitant regimen and 68.5% with the control regimen (P<0.001). In the acute phase, the CR rate was 93.2% and 91.0%, respectively (P=0.184). Overall, the CR rate was 77.1% and 66.9%, respectively (P<0.001).
In the overall phase, the proportion of subjects with no vomiting was 82.7% with the fosaprepitant regimen and 72.9% with the control regimen (P<0.001). The proportion of patients with no significant nausea was 83.2% and 77.9%, respectively (P=0.030).
The most common adverse events reported in the fosaprepitant and control arms, respectively, were fatigue (15% vs 13%), diarrhea (13% vs 11%), neutropenia (8% vs 7%), asthenia (4% vs 3%), anemia (3% vs 2%), peripheral neuropathy (3% vs 2%), leukopenia (2% vs 1%), dyspepsia (2% vs 1%), urinary tract infection (2% vs 1%), and pain in extremity (2% vs 1%).
Fosaprepitant dimeglumine is a product of Merck. For more details on the drug, see the prescribing information. ![]()

Photo by Rhoda Baer
The US Food and Drug Administration (FDA) has approved a supplemental new drug application for single-dose fosaprepitant dimeglumine (Emend) for injection.
The agency approved the substance P/neurokinin-1 (NK1) receptor antagonist for use in combination with other anti-emetic medicines to prevent delayed nausea and vomiting in adults receiving initial and repeat courses of moderately emetogenic chemotherapy (MEC).
This makes fosaprepitant dimeglumine the first intravenous NK1 receptor antagonist approved in the US for patients receiving either highly emetogenic chemotherapy or MEC.
Fosaprepitant dimeglumine has not been studied for the treatment of established nausea and vomiting.
The FDA’s latest approval of fosaprepitant dimeglumine is supported by data from a phase 3 study published in the Annals of Oncology.
Patients receiving MEC were given ondansetron and dexamethasone (n=498) or ondansetron and dexamethasone plus a single intravenous infusion of fosaprepitant dimeglumine (n=502).
The primary endpoint was complete response (CR)—defined as no vomiting and no use of rescue therapy—in the delayed phase of chemotherapy-induced nausea and vomiting, which is 25 to 120 hours after the initiation of chemotherapy.
Secondary endpoints included CR in the overall and acute phases—0 to 120 and 0 to 24 hours after MEC initiation, respectively—and no vomiting in the overall phase.
The fosaprepitant regimen improved CR significantly in the delayed and overall phases but not in the acute phase.
In the delayed phase, the CR rate was 78.9% with the fosaprepitant regimen and 68.5% with the control regimen (P<0.001). In the acute phase, the CR rate was 93.2% and 91.0%, respectively (P=0.184). Overall, the CR rate was 77.1% and 66.9%, respectively (P<0.001).
In the overall phase, the proportion of subjects with no vomiting was 82.7% with the fosaprepitant regimen and 72.9% with the control regimen (P<0.001). The proportion of patients with no significant nausea was 83.2% and 77.9%, respectively (P=0.030).
The most common adverse events reported in the fosaprepitant and control arms, respectively, were fatigue (15% vs 13%), diarrhea (13% vs 11%), neutropenia (8% vs 7%), asthenia (4% vs 3%), anemia (3% vs 2%), peripheral neuropathy (3% vs 2%), leukopenia (2% vs 1%), dyspepsia (2% vs 1%), urinary tract infection (2% vs 1%), and pain in extremity (2% vs 1%).
Fosaprepitant dimeglumine is a product of Merck. For more details on the drug, see the prescribing information. ![]()
EHA creates ‘roadmap’ for hematology research

Photo by Daniel Sone
The European Hematology Association (EHA) has created a “roadmap” for hematology research in Europe.
This guidance document summarizes the current status of basic, translational, and clinical hematology research and identifies areas of unmet scientific and medical need in Europe.
It is intended to help European and national policy makers, funding agencies, charities, research institutes, and researchers make decisions on initiating, funding, or developing research.
The guidance, “The European Hematology Association Roadmap for European Hematology Research: A Consensus Document,” is published in this month’s issue of haematologica.
“For the first time, hematologists in Europe came together to develop a roadmap to guide hematology research in Europe” said Andreas Engert, MD, chair of the EHA Research Roadmap Task Force.
“Hematology in Europe has achieved a lot, but the discipline must focus and collaborate to be efficient and remain successful in improving patient outcomes. The roadmap does just that and will determine the research agenda in Europe in the coming years.”
Roughly 300 experts from more than 20 countries—including clinicians, basic researchers, and patients—contributed to the roadmap. Stakeholders such as national hematology societies, patient organizations, hematology trial groups, and other European organizations were consulted to comment on the final draft version.
The final roadmap has 9 sections: normal hematopoiesis, malignant lymphoid and myeloid diseases, anemias and related diseases, platelet disorders, blood coagulation and hemostatic disorders, transfusion medicine, infections in hematology, and hematopoietic stem cell transplantation.
The roadmap lists priorities and needs in these areas, including the need for targeted therapies based on genomic profiling and chemical biology, the need to eradicate minimal residual disease, and the need for treatments that are better tolerated by elderly patients.
“Now’s the time for Europe to pay attention,” said Ulrich Jäger, MD, chair of the EHA European Affairs Committee.
“With an aging population, the slow recovery from the financial and Euro crises, costly medical breakthroughs and innovations—quite a few of which involve hematology researchers—Europe faces increased health expenditures while budgets are limited.”
“Policy makers are rightfully cautious when spending the taxpayers’ money. So it is our responsibility to provide the policy makers with the information and evidence they need to decide where their support impacts knowledge and health most efficiently, to the benefit of patients and society. The Research Roadmap delivers on that. Now, it is up to the policy makers in the EU to deliver too.” ![]()

Photo by Daniel Sone
The European Hematology Association (EHA) has created a “roadmap” for hematology research in Europe.
This guidance document summarizes the current status of basic, translational, and clinical hematology research and identifies areas of unmet scientific and medical need in Europe.
It is intended to help European and national policy makers, funding agencies, charities, research institutes, and researchers make decisions on initiating, funding, or developing research.
The guidance, “The European Hematology Association Roadmap for European Hematology Research: A Consensus Document,” is published in this month’s issue of haematologica.
“For the first time, hematologists in Europe came together to develop a roadmap to guide hematology research in Europe” said Andreas Engert, MD, chair of the EHA Research Roadmap Task Force.
“Hematology in Europe has achieved a lot, but the discipline must focus and collaborate to be efficient and remain successful in improving patient outcomes. The roadmap does just that and will determine the research agenda in Europe in the coming years.”
Roughly 300 experts from more than 20 countries—including clinicians, basic researchers, and patients—contributed to the roadmap. Stakeholders such as national hematology societies, patient organizations, hematology trial groups, and other European organizations were consulted to comment on the final draft version.
The final roadmap has 9 sections: normal hematopoiesis, malignant lymphoid and myeloid diseases, anemias and related diseases, platelet disorders, blood coagulation and hemostatic disorders, transfusion medicine, infections in hematology, and hematopoietic stem cell transplantation.
The roadmap lists priorities and needs in these areas, including the need for targeted therapies based on genomic profiling and chemical biology, the need to eradicate minimal residual disease, and the need for treatments that are better tolerated by elderly patients.
“Now’s the time for Europe to pay attention,” said Ulrich Jäger, MD, chair of the EHA European Affairs Committee.
“With an aging population, the slow recovery from the financial and Euro crises, costly medical breakthroughs and innovations—quite a few of which involve hematology researchers—Europe faces increased health expenditures while budgets are limited.”
“Policy makers are rightfully cautious when spending the taxpayers’ money. So it is our responsibility to provide the policy makers with the information and evidence they need to decide where their support impacts knowledge and health most efficiently, to the benefit of patients and society. The Research Roadmap delivers on that. Now, it is up to the policy makers in the EU to deliver too.” ![]()

Photo by Daniel Sone
The European Hematology Association (EHA) has created a “roadmap” for hematology research in Europe.
This guidance document summarizes the current status of basic, translational, and clinical hematology research and identifies areas of unmet scientific and medical need in Europe.
It is intended to help European and national policy makers, funding agencies, charities, research institutes, and researchers make decisions on initiating, funding, or developing research.
The guidance, “The European Hematology Association Roadmap for European Hematology Research: A Consensus Document,” is published in this month’s issue of haematologica.
“For the first time, hematologists in Europe came together to develop a roadmap to guide hematology research in Europe” said Andreas Engert, MD, chair of the EHA Research Roadmap Task Force.
“Hematology in Europe has achieved a lot, but the discipline must focus and collaborate to be efficient and remain successful in improving patient outcomes. The roadmap does just that and will determine the research agenda in Europe in the coming years.”
Roughly 300 experts from more than 20 countries—including clinicians, basic researchers, and patients—contributed to the roadmap. Stakeholders such as national hematology societies, patient organizations, hematology trial groups, and other European organizations were consulted to comment on the final draft version.
The final roadmap has 9 sections: normal hematopoiesis, malignant lymphoid and myeloid diseases, anemias and related diseases, platelet disorders, blood coagulation and hemostatic disorders, transfusion medicine, infections in hematology, and hematopoietic stem cell transplantation.
The roadmap lists priorities and needs in these areas, including the need for targeted therapies based on genomic profiling and chemical biology, the need to eradicate minimal residual disease, and the need for treatments that are better tolerated by elderly patients.
“Now’s the time for Europe to pay attention,” said Ulrich Jäger, MD, chair of the EHA European Affairs Committee.
“With an aging population, the slow recovery from the financial and Euro crises, costly medical breakthroughs and innovations—quite a few of which involve hematology researchers—Europe faces increased health expenditures while budgets are limited.”
“Policy makers are rightfully cautious when spending the taxpayers’ money. So it is our responsibility to provide the policy makers with the information and evidence they need to decide where their support impacts knowledge and health most efficiently, to the benefit of patients and society. The Research Roadmap delivers on that. Now, it is up to the policy makers in the EU to deliver too.” ![]()
When loved ones get cancer, people turn to the Web

chemotherapy
Photo by Rhoda Baer
Loved ones of cancer patients often turn to the Internet for further information about the disease, but they are less inclined to seek emotional support from social media forums, according to a study published in Computers, Informatics, Nursing.
It is fairly common for loved ones of cancer patients to develop depression or anxiety disorders as a result of the diagnosis, but there aren’t many studies focusing specifically on cancer patients’ caregivers and family members, said study author Carolyn Lauckner, PhD, of the University of Georgia in Athens.
“I think, sometimes, the loved ones and caregivers get forgotten about,” she said. “And that’s why I wanted to research this population to see if there are ways that we can better support these individuals.”
Dr Lauckner surveyed 191 people whose loved ones were diagnosed with cancer in the past year or who were currently acting as caregivers to someone with cancer. The motivation behind the research was personal for Dr Lauckner.
“I went through a period of time where I had 3 loved ones diagnosed within a short amount of time,” she said. “I had these experiences where I heard about the diagnosis and I would go online to look it up, and then I would immediately become terrified and freak out about all the stuff I read online.”
Like Dr Lauckner, more than 75% of the subjects she surveyed searched online for information on a loved one’s disease. Most looked for treatment options, prevention strategies and risk factors, and prognosis information.
“I was pleasantly surprised by the amount of people who said that they were looking for prevention information online and detection information,” Dr Lauckner said. “[T]hat shows that not only are they concerned for their loved one but they’re also concerned about how they themselves can avoid cancer, which, from a public health perspective, is great.”
Respondents were less inclined to view blogs or go online to hear about others’ cancer experiences. These kinds of sites were linked to negative emotions for participants, such as fear, sadness, and anger.
“A lot of people, especially in the cancer realm, they will use blogs or discussion posts to vent and to talk about the harsh realities of living with an illness,” Dr Lauckner said.
“And while I think that that is beneficial for both the person who is writing it and potentially for some people who want an idea of what to expect, when someone is dealing with the prospect of their loved one having to go through that experience, it can be extremely distressing.”
The most commonly visited websites were those of charitable organizations like the American Cancer Society, which were associated with positive emotions. Dr Lauckner said she found this information encouraging because it shows that participants were consulting reliable sources of information and not being swayed by personal accounts as much.
Dr Lauckner ultimately wants to build on the information gleaned in this study to determine the most effective use of social media and technology to distribute cancer prevention and risk reduction messages to the public. ![]()

chemotherapy
Photo by Rhoda Baer
Loved ones of cancer patients often turn to the Internet for further information about the disease, but they are less inclined to seek emotional support from social media forums, according to a study published in Computers, Informatics, Nursing.
It is fairly common for loved ones of cancer patients to develop depression or anxiety disorders as a result of the diagnosis, but there aren’t many studies focusing specifically on cancer patients’ caregivers and family members, said study author Carolyn Lauckner, PhD, of the University of Georgia in Athens.
“I think, sometimes, the loved ones and caregivers get forgotten about,” she said. “And that’s why I wanted to research this population to see if there are ways that we can better support these individuals.”
Dr Lauckner surveyed 191 people whose loved ones were diagnosed with cancer in the past year or who were currently acting as caregivers to someone with cancer. The motivation behind the research was personal for Dr Lauckner.
“I went through a period of time where I had 3 loved ones diagnosed within a short amount of time,” she said. “I had these experiences where I heard about the diagnosis and I would go online to look it up, and then I would immediately become terrified and freak out about all the stuff I read online.”
Like Dr Lauckner, more than 75% of the subjects she surveyed searched online for information on a loved one’s disease. Most looked for treatment options, prevention strategies and risk factors, and prognosis information.
“I was pleasantly surprised by the amount of people who said that they were looking for prevention information online and detection information,” Dr Lauckner said. “[T]hat shows that not only are they concerned for their loved one but they’re also concerned about how they themselves can avoid cancer, which, from a public health perspective, is great.”
Respondents were less inclined to view blogs or go online to hear about others’ cancer experiences. These kinds of sites were linked to negative emotions for participants, such as fear, sadness, and anger.
“A lot of people, especially in the cancer realm, they will use blogs or discussion posts to vent and to talk about the harsh realities of living with an illness,” Dr Lauckner said.
“And while I think that that is beneficial for both the person who is writing it and potentially for some people who want an idea of what to expect, when someone is dealing with the prospect of their loved one having to go through that experience, it can be extremely distressing.”
The most commonly visited websites were those of charitable organizations like the American Cancer Society, which were associated with positive emotions. Dr Lauckner said she found this information encouraging because it shows that participants were consulting reliable sources of information and not being swayed by personal accounts as much.
Dr Lauckner ultimately wants to build on the information gleaned in this study to determine the most effective use of social media and technology to distribute cancer prevention and risk reduction messages to the public. ![]()

chemotherapy
Photo by Rhoda Baer
Loved ones of cancer patients often turn to the Internet for further information about the disease, but they are less inclined to seek emotional support from social media forums, according to a study published in Computers, Informatics, Nursing.
It is fairly common for loved ones of cancer patients to develop depression or anxiety disorders as a result of the diagnosis, but there aren’t many studies focusing specifically on cancer patients’ caregivers and family members, said study author Carolyn Lauckner, PhD, of the University of Georgia in Athens.
“I think, sometimes, the loved ones and caregivers get forgotten about,” she said. “And that’s why I wanted to research this population to see if there are ways that we can better support these individuals.”
Dr Lauckner surveyed 191 people whose loved ones were diagnosed with cancer in the past year or who were currently acting as caregivers to someone with cancer. The motivation behind the research was personal for Dr Lauckner.
“I went through a period of time where I had 3 loved ones diagnosed within a short amount of time,” she said. “I had these experiences where I heard about the diagnosis and I would go online to look it up, and then I would immediately become terrified and freak out about all the stuff I read online.”
Like Dr Lauckner, more than 75% of the subjects she surveyed searched online for information on a loved one’s disease. Most looked for treatment options, prevention strategies and risk factors, and prognosis information.
“I was pleasantly surprised by the amount of people who said that they were looking for prevention information online and detection information,” Dr Lauckner said. “[T]hat shows that not only are they concerned for their loved one but they’re also concerned about how they themselves can avoid cancer, which, from a public health perspective, is great.”
Respondents were less inclined to view blogs or go online to hear about others’ cancer experiences. These kinds of sites were linked to negative emotions for participants, such as fear, sadness, and anger.
“A lot of people, especially in the cancer realm, they will use blogs or discussion posts to vent and to talk about the harsh realities of living with an illness,” Dr Lauckner said.
“And while I think that that is beneficial for both the person who is writing it and potentially for some people who want an idea of what to expect, when someone is dealing with the prospect of their loved one having to go through that experience, it can be extremely distressing.”
The most commonly visited websites were those of charitable organizations like the American Cancer Society, which were associated with positive emotions. Dr Lauckner said she found this information encouraging because it shows that participants were consulting reliable sources of information and not being swayed by personal accounts as much.
Dr Lauckner ultimately wants to build on the information gleaned in this study to determine the most effective use of social media and technology to distribute cancer prevention and risk reduction messages to the public.
Bile acid supports production of HSCs, team says
Photo by Åsa Hansdotter
Experiments in pregnant mice indicate that bile acid supports the production of hematopoietic stem cells (HSCs) in fetuses.
The work revealed large amounts of bile acids inside mouse fetuses and suggested these acids are transferred from the mothers via the placenta to help the fetuses produce HSCs.
“Fetuses produce small amounts of bile acids on their own, but here we are talking about much larger quantities,” said study author Kenichi Miharada, PhD, of Lund University in Sweden.
“The bile acid appears to be produced by the mother and then transferred to the fetus via the placenta.”
Dr Miharada and his colleagues detailed this discovery in Cell Stem Cell.
The investigators already knew that bile acid is produced in the fetal liver, but they did not know why.
With this study, they discovered that bile acid supports the production of HSCs in the fetal liver and enables them to develop normally. The additional contribution from the mother is important for the fetus to develop normally.
And although a large part of bile acid is toxic for cells, it undergoes a purification process when transferred through the placenta, letting only harmless bile acid through to the fetus.
“Our hypothesis is that the consequence of a damaged placenta, which, for various reasons, is unable to transfer bile acids to the fetus, can lead to leukemia or other blood diseases later in life, and we will continue our research to see if this hypothesis holds up,” Dr Miharada said.
He and his colleagues also said this work has implications for producing HSCs that could treat these blood diseases.
The problem with making HSCs proliferate outside the body is that the artificial growth gives rise to an accumulation of abnormal proteins in the endoplasmic reticulum (ER). This ER stress, if severe and chronic, causes cell death.
Dr Miharada and his colleagues previously showed it is possible to reduce ER stress chemically by adding bile acids to the cell culture. Bile acids, which are produced naturally in the liver and stored in the gallbladder, support protein production during the cell division process.
“Compared to other ways of trying to develop stem cells to treat blood diseases, this method is safer and quicker because it does not involve using any artificial substances or any genetic modifications, merely a substance that already exists inside the body,” Dr Miharada explained.

Photo by Åsa Hansdotter
Experiments in pregnant mice indicate that bile acid supports the production of hematopoietic stem cells (HSCs) in fetuses.
The work revealed large amounts of bile acids inside mouse fetuses and suggested these acids are transferred from the mothers via the placenta to help the fetuses produce HSCs.
“Fetuses produce small amounts of bile acids on their own, but here we are talking about much larger quantities,” said study author Kenichi Miharada, PhD, of Lund University in Sweden.
“The bile acid appears to be produced by the mother and then transferred to the fetus via the placenta.”
Dr Miharada and his colleagues detailed this discovery in Cell Stem Cell.
The investigators already knew that bile acid is produced in the fetal liver, but they did not know why.
With this study, they discovered that bile acid supports the production of HSCs in the fetal liver and enables them to develop normally. The additional contribution from the mother is important for the fetus to develop normally.
And although a large part of bile acid is toxic for cells, it undergoes a purification process when transferred through the placenta, letting only harmless bile acid through to the fetus.
“Our hypothesis is that the consequence of a damaged placenta, which, for various reasons, is unable to transfer bile acids to the fetus, can lead to leukemia or other blood diseases later in life, and we will continue our research to see if this hypothesis holds up,” Dr Miharada said.
He and his colleagues also said this work has implications for producing HSCs that could treat these blood diseases.
The problem with making HSCs proliferate outside the body is that the artificial growth gives rise to an accumulation of abnormal proteins in the endoplasmic reticulum (ER). This ER stress, if severe and chronic, causes cell death.
Dr Miharada and his colleagues previously showed it is possible to reduce ER stress chemically by adding bile acids to the cell culture. Bile acids, which are produced naturally in the liver and stored in the gallbladder, support protein production during the cell division process.
“Compared to other ways of trying to develop stem cells to treat blood diseases, this method is safer and quicker because it does not involve using any artificial substances or any genetic modifications, merely a substance that already exists inside the body,” Dr Miharada explained.

Photo by Åsa Hansdotter
Experiments in pregnant mice indicate that bile acid supports the production of hematopoietic stem cells (HSCs) in fetuses.
The work revealed large amounts of bile acids inside mouse fetuses and suggested these acids are transferred from the mothers via the placenta to help the fetuses produce HSCs.
“Fetuses produce small amounts of bile acids on their own, but here we are talking about much larger quantities,” said study author Kenichi Miharada, PhD, of Lund University in Sweden.
“The bile acid appears to be produced by the mother and then transferred to the fetus via the placenta.”
Dr Miharada and his colleagues detailed this discovery in Cell Stem Cell.
The investigators already knew that bile acid is produced in the fetal liver, but they did not know why.
With this study, they discovered that bile acid supports the production of HSCs in the fetal liver and enables them to develop normally. The additional contribution from the mother is important for the fetus to develop normally.
And although a large part of bile acid is toxic for cells, it undergoes a purification process when transferred through the placenta, letting only harmless bile acid through to the fetus.
“Our hypothesis is that the consequence of a damaged placenta, which, for various reasons, is unable to transfer bile acids to the fetus, can lead to leukemia or other blood diseases later in life, and we will continue our research to see if this hypothesis holds up,” Dr Miharada said.
He and his colleagues also said this work has implications for producing HSCs that could treat these blood diseases.
The problem with making HSCs proliferate outside the body is that the artificial growth gives rise to an accumulation of abnormal proteins in the endoplasmic reticulum (ER). This ER stress, if severe and chronic, causes cell death.
Dr Miharada and his colleagues previously showed it is possible to reduce ER stress chemically by adding bile acids to the cell culture. Bile acids, which are produced naturally in the liver and stored in the gallbladder, support protein production during the cell division process.
“Compared to other ways of trying to develop stem cells to treat blood diseases, this method is safer and quicker because it does not involve using any artificial substances or any genetic modifications, merely a substance that already exists inside the body,” Dr Miharada explained.
FDA clears new iteration of blood-draw device
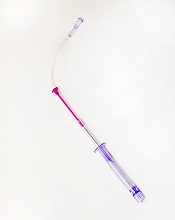
Photo courtesy of
Velano Vascular
The US Food and Drug Administration (FDA) has granted 510(k) clearance for a new iteration of a needle-free blood-draw device produced by Velano Vascular.
The device was originally cleared by the FDA last year.
It resembles a common syringe and allows peripheral intravenous catheters to be repurposed to draw blood from patients.
The goal of this is to reduce the need for additional needle sticks among patients receiving medications and hydration via intravenous delivery.
The new clearance for the blood-draw device covers a pair of modifications aimed at inpatient blood draws.
The first modification is a clamp for use with syringe draws. And the second is a revised indication for use that removes a limitation on when the device can be used with in-dwelling peripheral intravenous catheters.
“We rapidly implemented and pursued FDA clearance for these modifications based on input from patients and medical professionals who are using and systematically assessing our blood-draw technology,” said Eric Stone, co-founder & CEO of Velano Vascular.
Velano Vascular said it is working closely with clinical and non-profit partners, including Brigham and Women’s Hospital, Intermountain Healthcare, Griffin Health, The University of Pennsylvania Health System, The Children’s Hospital of Philadelphia, Children’s National Hospital, and Planetree, to capture patient and practitioner input regarding today’s approaches to inpatient blood draws and how the Velano technology could eventually become a standard of care.

Photo courtesy of
Velano Vascular
The US Food and Drug Administration (FDA) has granted 510(k) clearance for a new iteration of a needle-free blood-draw device produced by Velano Vascular.
The device was originally cleared by the FDA last year.
It resembles a common syringe and allows peripheral intravenous catheters to be repurposed to draw blood from patients.
The goal of this is to reduce the need for additional needle sticks among patients receiving medications and hydration via intravenous delivery.
The new clearance for the blood-draw device covers a pair of modifications aimed at inpatient blood draws.
The first modification is a clamp for use with syringe draws. And the second is a revised indication for use that removes a limitation on when the device can be used with in-dwelling peripheral intravenous catheters.
“We rapidly implemented and pursued FDA clearance for these modifications based on input from patients and medical professionals who are using and systematically assessing our blood-draw technology,” said Eric Stone, co-founder & CEO of Velano Vascular.
Velano Vascular said it is working closely with clinical and non-profit partners, including Brigham and Women’s Hospital, Intermountain Healthcare, Griffin Health, The University of Pennsylvania Health System, The Children’s Hospital of Philadelphia, Children’s National Hospital, and Planetree, to capture patient and practitioner input regarding today’s approaches to inpatient blood draws and how the Velano technology could eventually become a standard of care.

Photo courtesy of
Velano Vascular
The US Food and Drug Administration (FDA) has granted 510(k) clearance for a new iteration of a needle-free blood-draw device produced by Velano Vascular.
The device was originally cleared by the FDA last year.
It resembles a common syringe and allows peripheral intravenous catheters to be repurposed to draw blood from patients.
The goal of this is to reduce the need for additional needle sticks among patients receiving medications and hydration via intravenous delivery.
The new clearance for the blood-draw device covers a pair of modifications aimed at inpatient blood draws.
The first modification is a clamp for use with syringe draws. And the second is a revised indication for use that removes a limitation on when the device can be used with in-dwelling peripheral intravenous catheters.
“We rapidly implemented and pursued FDA clearance for these modifications based on input from patients and medical professionals who are using and systematically assessing our blood-draw technology,” said Eric Stone, co-founder & CEO of Velano Vascular.
Velano Vascular said it is working closely with clinical and non-profit partners, including Brigham and Women’s Hospital, Intermountain Healthcare, Griffin Health, The University of Pennsylvania Health System, The Children’s Hospital of Philadelphia, Children’s National Hospital, and Planetree, to capture patient and practitioner input regarding today’s approaches to inpatient blood draws and how the Velano technology could eventually become a standard of care.
Incorporating cultural beliefs into cancer care

Photo by Daniel Sone
Understanding and integrating patients’ cultural beliefs into cancer treatment plans may help improve their acceptance of and adherence to treatment in multicultural settings, according to research published in the Journal of Global Oncology.
Researchers examined traditional Maya healers’ understanding of cancer and found that, although there are key differences between Maya and Western medicine perspectives, they also share many fundamental concepts.
“Maya healers understand cancer in remarkably similar ways to Western doctors,” said lead study author Mónica Berger-González, PhD, of the Institute for Environmental Decisions at ETH Zurich in Switzerland.
“Recognizing this is the first step to bridging the gap between cultures and ultimately providing better, more effective services for indigenous populations.”
Nearly half of the population in Guatemala (approximately 5.4 million people) relies on Maya medicine. Traditional healers have practiced in Guatemala for more than 2000 years, with the healing tradition passed down orally and through apprenticeship.
According to the authors, this is one of the first studies to explore the subject of Maya healers and cancer across several ethno-linguistic groups, and limited data exist on survival outcomes.
Dr Berger-González and her colleagues conducted in-depth interviews with 67 healers across various ethnic and language groups in Guatemala, exploring how its indigenous people define and treat cancer.
Of the Maya healers interviewed, 46% were illiterate. Although only 36% were able to define the word cancer, most (85%) were familiar with the term and identified malignancy as a core characteristic of the disease, explaining the concept of metastasis clearly.
The analysis also revealed that Maya healers understand the origins of cancer in ways that align closely with Western medical concepts.
When asked to identify the physical causes of cancer, 10 of 17 causes provided correlated directly with cancer risk factors as understood in Western medicine. Healers cited causes such as the consumption of harmful foods (46.3%), hereditary conditions (29.6%), and lifestyle factors such as smoking or working with toxic substances (29.6%).
One notable difference identified between the 2 perspectives is that Maya healers’ view of cancer is not limited to the physical body, but rather includes a complex imbalance of the emotions, mind, and spirit.
The Maya treatment of cancer is consequently holistic and seeks to restore that balance. This is achieved through a combination of methods—such as regulating diet, plant therapy, detoxifying baths—as well as social, psychological, and spiritual methods, the latter of which, the authors note, is harder to grasp in Western medicine.
“If healthcare professionals do not understand indigenous peoples’ conception of cancer, these patients are far less likely to accept and adhere to treatment in the public healthcare system,” Dr Berger-González said.
Many indigenous people in Guatemala do not have access to Western medicine services, cannot afford them, or prefer Maya medicine even when Western medical treatment is available. Yet Western medicine practitioners have little to no training in multicultural management or traditional indigenous medicine.
The authors offer 3 key recommendations to help address the challenges of providing care in multicultural settings:
- Adequate training of healthcare professionals on cultural and social perceptions of cancer
- Increasing evidence-based research on traditional medicine
- Establishing national regulations on integrating traditional and Western medicine—following other countries like Peru, Brazil, and Ecuador, which have successfully incorporated these aspects of care.
The authors plan to continue their transdisciplinary research with the goal of providing biomedical evidence that advances different aspects of Maya medicine.

Photo by Daniel Sone
Understanding and integrating patients’ cultural beliefs into cancer treatment plans may help improve their acceptance of and adherence to treatment in multicultural settings, according to research published in the Journal of Global Oncology.
Researchers examined traditional Maya healers’ understanding of cancer and found that, although there are key differences between Maya and Western medicine perspectives, they also share many fundamental concepts.
“Maya healers understand cancer in remarkably similar ways to Western doctors,” said lead study author Mónica Berger-González, PhD, of the Institute for Environmental Decisions at ETH Zurich in Switzerland.
“Recognizing this is the first step to bridging the gap between cultures and ultimately providing better, more effective services for indigenous populations.”
Nearly half of the population in Guatemala (approximately 5.4 million people) relies on Maya medicine. Traditional healers have practiced in Guatemala for more than 2000 years, with the healing tradition passed down orally and through apprenticeship.
According to the authors, this is one of the first studies to explore the subject of Maya healers and cancer across several ethno-linguistic groups, and limited data exist on survival outcomes.
Dr Berger-González and her colleagues conducted in-depth interviews with 67 healers across various ethnic and language groups in Guatemala, exploring how its indigenous people define and treat cancer.
Of the Maya healers interviewed, 46% were illiterate. Although only 36% were able to define the word cancer, most (85%) were familiar with the term and identified malignancy as a core characteristic of the disease, explaining the concept of metastasis clearly.
The analysis also revealed that Maya healers understand the origins of cancer in ways that align closely with Western medical concepts.
When asked to identify the physical causes of cancer, 10 of 17 causes provided correlated directly with cancer risk factors as understood in Western medicine. Healers cited causes such as the consumption of harmful foods (46.3%), hereditary conditions (29.6%), and lifestyle factors such as smoking or working with toxic substances (29.6%).
One notable difference identified between the 2 perspectives is that Maya healers’ view of cancer is not limited to the physical body, but rather includes a complex imbalance of the emotions, mind, and spirit.
The Maya treatment of cancer is consequently holistic and seeks to restore that balance. This is achieved through a combination of methods—such as regulating diet, plant therapy, detoxifying baths—as well as social, psychological, and spiritual methods, the latter of which, the authors note, is harder to grasp in Western medicine.
“If healthcare professionals do not understand indigenous peoples’ conception of cancer, these patients are far less likely to accept and adhere to treatment in the public healthcare system,” Dr Berger-González said.
Many indigenous people in Guatemala do not have access to Western medicine services, cannot afford them, or prefer Maya medicine even when Western medical treatment is available. Yet Western medicine practitioners have little to no training in multicultural management or traditional indigenous medicine.
The authors offer 3 key recommendations to help address the challenges of providing care in multicultural settings:
- Adequate training of healthcare professionals on cultural and social perceptions of cancer
- Increasing evidence-based research on traditional medicine
- Establishing national regulations on integrating traditional and Western medicine—following other countries like Peru, Brazil, and Ecuador, which have successfully incorporated these aspects of care.
The authors plan to continue their transdisciplinary research with the goal of providing biomedical evidence that advances different aspects of Maya medicine.

Photo by Daniel Sone
Understanding and integrating patients’ cultural beliefs into cancer treatment plans may help improve their acceptance of and adherence to treatment in multicultural settings, according to research published in the Journal of Global Oncology.
Researchers examined traditional Maya healers’ understanding of cancer and found that, although there are key differences between Maya and Western medicine perspectives, they also share many fundamental concepts.
“Maya healers understand cancer in remarkably similar ways to Western doctors,” said lead study author Mónica Berger-González, PhD, of the Institute for Environmental Decisions at ETH Zurich in Switzerland.
“Recognizing this is the first step to bridging the gap between cultures and ultimately providing better, more effective services for indigenous populations.”
Nearly half of the population in Guatemala (approximately 5.4 million people) relies on Maya medicine. Traditional healers have practiced in Guatemala for more than 2000 years, with the healing tradition passed down orally and through apprenticeship.
According to the authors, this is one of the first studies to explore the subject of Maya healers and cancer across several ethno-linguistic groups, and limited data exist on survival outcomes.
Dr Berger-González and her colleagues conducted in-depth interviews with 67 healers across various ethnic and language groups in Guatemala, exploring how its indigenous people define and treat cancer.
Of the Maya healers interviewed, 46% were illiterate. Although only 36% were able to define the word cancer, most (85%) were familiar with the term and identified malignancy as a core characteristic of the disease, explaining the concept of metastasis clearly.
The analysis also revealed that Maya healers understand the origins of cancer in ways that align closely with Western medical concepts.
When asked to identify the physical causes of cancer, 10 of 17 causes provided correlated directly with cancer risk factors as understood in Western medicine. Healers cited causes such as the consumption of harmful foods (46.3%), hereditary conditions (29.6%), and lifestyle factors such as smoking or working with toxic substances (29.6%).
One notable difference identified between the 2 perspectives is that Maya healers’ view of cancer is not limited to the physical body, but rather includes a complex imbalance of the emotions, mind, and spirit.
The Maya treatment of cancer is consequently holistic and seeks to restore that balance. This is achieved through a combination of methods—such as regulating diet, plant therapy, detoxifying baths—as well as social, psychological, and spiritual methods, the latter of which, the authors note, is harder to grasp in Western medicine.
“If healthcare professionals do not understand indigenous peoples’ conception of cancer, these patients are far less likely to accept and adhere to treatment in the public healthcare system,” Dr Berger-González said.
Many indigenous people in Guatemala do not have access to Western medicine services, cannot afford them, or prefer Maya medicine even when Western medical treatment is available. Yet Western medicine practitioners have little to no training in multicultural management or traditional indigenous medicine.
The authors offer 3 key recommendations to help address the challenges of providing care in multicultural settings:
- Adequate training of healthcare professionals on cultural and social perceptions of cancer
- Increasing evidence-based research on traditional medicine
- Establishing national regulations on integrating traditional and Western medicine—following other countries like Peru, Brazil, and Ecuador, which have successfully incorporated these aspects of care.
The authors plan to continue their transdisciplinary research with the goal of providing biomedical evidence that advances different aspects of Maya medicine.