User login
Certolizumab pegol and abatacept show superiority over conventional therapy in early RA
Key clinical point: First-line therapy with biologics, including abatacept and certolizumab pegol but not tocilizumab, all in combination with methotrexate, was clinically superior to conventional therapy with bridging glucocorticoids in patients with moderate-to-severe early rheumatoid arthritis (RA).
Major finding: Compared with active conventional therapy, the Clinical Disease Activity Index-based remission rates at week 48 were significantly higher with abatacept (adjusted difference +20.1%; P < .001) and certolizumab pegol (adjusted difference +13.1%; P = .021) but not with tocilizumab, whereas the radiographic progression was low and lacked between-group differences.
Study details: Findings are from the NORD-STAR trial including 812 treatment-naive patients with moderate-to-severe early RA who were randomly assigned to receive methotrexate combined with active conventional therapy, certolizumab pegol, abatacept, or tocilizumab.
Disclosures: This study was funded by the Academy of Finland, Finska Läkaresällskapet, and other sources. Several authors declared chairing the committees of or receiving travel support, research grants, or fees for lectures, speaking, consultancy, advisory roles, or other services from various sources.
Source: Østergaard M et al, on behalf of the NORD-STAR study group. Certolizumab pegol, abatacept, tocilizumab or active conventional treatment in early rheumatoid arthritis: 48-week clinical and radiographic results of the investigator-initiated randomised controlled NORD-STAR trial. Ann Rheum Dis. 2023 (Jul 9). Doi: 10.1136/ard-2023-224116
Key clinical point: First-line therapy with biologics, including abatacept and certolizumab pegol but not tocilizumab, all in combination with methotrexate, was clinically superior to conventional therapy with bridging glucocorticoids in patients with moderate-to-severe early rheumatoid arthritis (RA).
Major finding: Compared with active conventional therapy, the Clinical Disease Activity Index-based remission rates at week 48 were significantly higher with abatacept (adjusted difference +20.1%; P < .001) and certolizumab pegol (adjusted difference +13.1%; P = .021) but not with tocilizumab, whereas the radiographic progression was low and lacked between-group differences.
Study details: Findings are from the NORD-STAR trial including 812 treatment-naive patients with moderate-to-severe early RA who were randomly assigned to receive methotrexate combined with active conventional therapy, certolizumab pegol, abatacept, or tocilizumab.
Disclosures: This study was funded by the Academy of Finland, Finska Läkaresällskapet, and other sources. Several authors declared chairing the committees of or receiving travel support, research grants, or fees for lectures, speaking, consultancy, advisory roles, or other services from various sources.
Source: Østergaard M et al, on behalf of the NORD-STAR study group. Certolizumab pegol, abatacept, tocilizumab or active conventional treatment in early rheumatoid arthritis: 48-week clinical and radiographic results of the investigator-initiated randomised controlled NORD-STAR trial. Ann Rheum Dis. 2023 (Jul 9). Doi: 10.1136/ard-2023-224116
Key clinical point: First-line therapy with biologics, including abatacept and certolizumab pegol but not tocilizumab, all in combination with methotrexate, was clinically superior to conventional therapy with bridging glucocorticoids in patients with moderate-to-severe early rheumatoid arthritis (RA).
Major finding: Compared with active conventional therapy, the Clinical Disease Activity Index-based remission rates at week 48 were significantly higher with abatacept (adjusted difference +20.1%; P < .001) and certolizumab pegol (adjusted difference +13.1%; P = .021) but not with tocilizumab, whereas the radiographic progression was low and lacked between-group differences.
Study details: Findings are from the NORD-STAR trial including 812 treatment-naive patients with moderate-to-severe early RA who were randomly assigned to receive methotrexate combined with active conventional therapy, certolizumab pegol, abatacept, or tocilizumab.
Disclosures: This study was funded by the Academy of Finland, Finska Läkaresällskapet, and other sources. Several authors declared chairing the committees of or receiving travel support, research grants, or fees for lectures, speaking, consultancy, advisory roles, or other services from various sources.
Source: Østergaard M et al, on behalf of the NORD-STAR study group. Certolizumab pegol, abatacept, tocilizumab or active conventional treatment in early rheumatoid arthritis: 48-week clinical and radiographic results of the investigator-initiated randomised controlled NORD-STAR trial. Ann Rheum Dis. 2023 (Jul 9). Doi: 10.1136/ard-2023-224116
Tofacitinib shows marginal edge over adalimumab in real-world patients with RA
Key clinical point: In patients with rheumatoid arthritis (RA), tofacitinib led to a modest yet statistically significant reduction in disease activity at 3 months compared with adalimumab; however, the reduction in disease activity was not significantly different between the treatment groups at 9 months.
Major finding: The difference in the mean Disease Activity Score in 28 Joints using C-reactive protein between patients treated with tofacitinib vs adalimumab was modest yet statistically significant at 3 months (average treatment effect [ATE] −0.2; P = .02), whereas there was no significant difference at 9 months (ATE −0.03; P = .60).
Study details: This observational study emulated a randomized controlled trial using the data of 842 biologic or targeted synthetic disease-modifying antirheumatic drug-naïve patients with RA from the OPAL dataset who initiated adalimumab (n = 569) or tofacitinib (n = 273).
Disclosures: This study did not declare any specific funding source. Four authors declared being a director of, serving on advisory boards or speakers’ bureaus for, or receiving personal fees for consultancy from various sources.
Source: Deakin CT et al, for the OPAL Rheumatology Network. Comparative effectiveness of adalimumab vs tofacitinib in patients with rheumatoid arthritis in Australia. JAMA Netw Open. 2023;6(6):e2320851 (Jun 29). Doi: 10.1001/jamanetworkopen.2023.20851
Key clinical point: In patients with rheumatoid arthritis (RA), tofacitinib led to a modest yet statistically significant reduction in disease activity at 3 months compared with adalimumab; however, the reduction in disease activity was not significantly different between the treatment groups at 9 months.
Major finding: The difference in the mean Disease Activity Score in 28 Joints using C-reactive protein between patients treated with tofacitinib vs adalimumab was modest yet statistically significant at 3 months (average treatment effect [ATE] −0.2; P = .02), whereas there was no significant difference at 9 months (ATE −0.03; P = .60).
Study details: This observational study emulated a randomized controlled trial using the data of 842 biologic or targeted synthetic disease-modifying antirheumatic drug-naïve patients with RA from the OPAL dataset who initiated adalimumab (n = 569) or tofacitinib (n = 273).
Disclosures: This study did not declare any specific funding source. Four authors declared being a director of, serving on advisory boards or speakers’ bureaus for, or receiving personal fees for consultancy from various sources.
Source: Deakin CT et al, for the OPAL Rheumatology Network. Comparative effectiveness of adalimumab vs tofacitinib in patients with rheumatoid arthritis in Australia. JAMA Netw Open. 2023;6(6):e2320851 (Jun 29). Doi: 10.1001/jamanetworkopen.2023.20851
Key clinical point: In patients with rheumatoid arthritis (RA), tofacitinib led to a modest yet statistically significant reduction in disease activity at 3 months compared with adalimumab; however, the reduction in disease activity was not significantly different between the treatment groups at 9 months.
Major finding: The difference in the mean Disease Activity Score in 28 Joints using C-reactive protein between patients treated with tofacitinib vs adalimumab was modest yet statistically significant at 3 months (average treatment effect [ATE] −0.2; P = .02), whereas there was no significant difference at 9 months (ATE −0.03; P = .60).
Study details: This observational study emulated a randomized controlled trial using the data of 842 biologic or targeted synthetic disease-modifying antirheumatic drug-naïve patients with RA from the OPAL dataset who initiated adalimumab (n = 569) or tofacitinib (n = 273).
Disclosures: This study did not declare any specific funding source. Four authors declared being a director of, serving on advisory boards or speakers’ bureaus for, or receiving personal fees for consultancy from various sources.
Source: Deakin CT et al, for the OPAL Rheumatology Network. Comparative effectiveness of adalimumab vs tofacitinib in patients with rheumatoid arthritis in Australia. JAMA Netw Open. 2023;6(6):e2320851 (Jun 29). Doi: 10.1001/jamanetworkopen.2023.20851
Humira biosimilars: Five things to know
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.
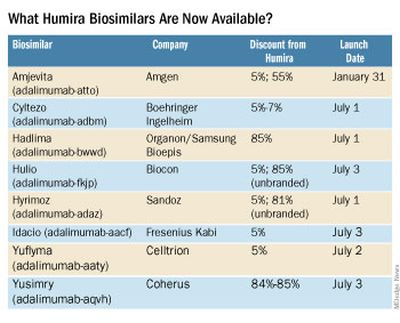
Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
The best-selling drug Humira (adalimumab) now faces competition in the United States after a 20-year monopoly. The first adalimumab biosimilar, Amjevita, launched in the United States on January 31, and in July, seven additional biosimilars became available. These drugs have the potential to lower prescription drug prices, but when and by how much remains to be seen.
Here’s what you need to know about adalimumab biosimilars.
What Humira biosimilars are now available?
Eight different biosimilars have launched in 2023 with discounts as large at 85% from Humira’s list price of $6,922. A few companies also offer two price points.

Three of these biosimilars – Hadlima, Hyrimoz, and Yuflyma – are available in high concentration formulations. This high concentration formulation makes up 85% of Humira prescriptions, according to a report from Goodroot, a collection of companies focused on lowering health care costs.
Cyltezo is currently the only adalimumab biosimilar with an interchangeability designation, meaning that a pharmacist can substitute the biosimilar for an equivalent Humira prescription without the intervention of a clinician. A total of 47 states allow for these substitutions without prior approval from a clinician, according to Goodroot, and the clinician must be notified of the switch within a certain time frame. A total of 40 states require that patients be notified of the switch before substitution.
However, it’s not clear if this interchangeability designation will prove an advantage for Cyltezo, as it is interchangeable with the lower concentration version of Humira that makes up just 15% of prescriptions.
Most of the companies behind these biosimilars are pursuing interchangeability designations for their drugs, except for Fresenius Kabi (Idacio) and Coherus (Yusimry).
A ninth biosimilar, Pfizer’s adalimumab-afzb (Abrilada), is not yet on the market and is currently awaiting an approval decision from the Food and Drug Administration to add an interchangeability designation to its prior approval for a low-concentration formulation.
Why are they priced differently?
The two price points offer different deals to payers. Pharmacy benefit managers make confidential agreements with drug manufacturers to get a discount – called a rebate – to get the drug on the PBM’s formulary. The PBM keeps a portion of that rebate, and the rest is passed on to the insurance company and patients. Biosimilars at a higher price point will likely offer larger rebates. Biosimilars offered at lower price points incorporate this discount up front in their list pricing and likely will not offer large rebates.
Will biosimilars be covered by payers?
Currently, biosimilars are being offered on formularies at parity with Humira, meaning they are on the same tier. The PBM companies OptumRx and Cigna Group’s Express Scripts will offer Amjevita (at both price points), Cyltezo, and Hyrimoz (at both price points).
“This decision allows our clients flexibility to provide access to the lower list price, so members in high-deductible plans and benefit designs with coinsurance can experience lower out-of-pocket costs,” said OptumRx spokesperson Isaac Sorensen in an email.
Mark Cuban Cost Plus Drug Company, which uses a direct-to-consumer model, will offer Yusimry for $567.27 on its website. SmithRx, a PBM based in San Francisco, announced it would partner with Cost Plus Drugs to offer Yusimry, adding that SmithRx members can use their insurance benefits to further reduce out-of-pocket costs. RxPreferred, another PBM, will also offer Yusimry through its partnership with Cuban’s company.
The news website Formulary Watch previously reported that CVS Caremark, another of the biggest PBMs, will be offering Amjevita, but as a nonpreferred brand, while Humira remains the preferred brand. CVS Caremark did not respond to a request for comment.
Will patients pay less?
Biosimilars have been touted as a potential solution to lower spending on biologic drugs, but it’s unknown if patients will ultimately benefit with lower out-of-pocket costs. It’s “impossible to predict” if the discount that third-party payers pay will be passed on to consumers, said Mark Fendrick, MD, who directs the University of Michigan Center for Value-based Insurance Design in Ann Arbor.
Generally, a consumer’s copay is a percentage of a drug’s list price, so it stands to reason that a low drug price would result in lower out-of-pocket payments. While this is mostly true, Humira has a successful copay assistance program to lower prescription costs for consumers. According to a 2022 IQVIA report, 82% of commercial prescriptions cost patients less than $10 for Humira because of this program.
To appeal to patients, biosimilar companies will need to offer similar savings, Dr. Fendrick added. “There will be some discontent if patients are actually asked to pay more out-of-pocket for a less expensive drug,” he said.
All eight companies behind these biosimilars are offering or will be launching copay saving programs, many which advertise copays as low as $0 per month for eligible patients.
How will Humira respond?
Marta Wosińska, PhD, a health care economist at the Brookings Institute, Washington, predicts payers will use these lower biosimilar prices to negotiate better deals with AbbVie, Humira’s manufacturer. “We have a lot of players coming into [the market] right now, so the competition is really fierce,” she said. In response, AbbVie will need to increase rebates on Humira and/or lower its price to compete with these biosimilars.
“The ball is in AbbVie’s court,” she said. “If [the company] is not willing to drop price sufficiently, then payers will start switching to biosimilars.”
Dr. Fendrick reported past financial relationships and consulting arrangements with AbbVie, Amgen, Arnold Ventures, Bayer, CareFirst, BlueCross BlueShield, and many other companies. Dr. Wosińska has received funding from Arnold Ventures and serves as an expert witness on antitrust cases involving generic medication.
A version of this article first appeared on Medscape.com.
Antidrug antibody effects compared across RA biologics
TOPLINE:
In patients with rheumatoid arthritis, the presence of antidrug antibodies was associated with a diminished response to biologic disease-modifying antirheumatic drugs in a prospective cohort study.
METHODOLOGY:
- Researchers prospectively analyzed data from 230 patients (mean age, 54.3 years; 77.0% women) with RA diagnosis recruited from March 3, 2014, to June 21, 2016.
- All were initiating new treatment with an anti–tumor necrosis factor (TNF) monoclonal antibody (mAb; either infliximab or adalimumab), etanercept, tocilizumab, or rituximab, according to the choice of the treating physician.
- The primary outcome was the association of antidrug antibody positivity with European Alliance of Associations for Rheumatology (EULAR) response to treatment at month 12, assessed through univariate logistic regression.
TAKEAWAY:
- At month 12, antidrug antibody positivity was 38.2% in patients who were treated with anti-TNF mAbs, 6.1% with etanercept, 50.0% with rituximab, and 20.0% with tocilizumab.
- There was an inverse association between antidrug antibody positivity directed against all biologic drugs and EULAR response at month 12 (odds ratio, 0.19; 95% confidence interval, 0.09-0.38; P < .001).
- In the multivariable analysis, antidrug antibodies, body mass index, and rheumatoid factor were independently and inversely associated with response to treatment.
- There was a significantly higher drug concentration of anti-TNF mAbs in patients with antidrug antibody–negative vs. antidrug antibody–positive status (mean difference, –9.6 mg/L; 95% CI, –12.4 to –6.9; P < .001).
IN PRACTICE:
Findings of this study suggest that antidrug antibodies are associated with nonresponse to biologic drugs and can be monitored in the management of patients with RA, particularly nonresponders.
SOURCE:
Samuel Bitouin, MD, PhD, of the rheumatology department at Paris-Saclay University, and coauthors in the ABIRISK (Anti-Biopharmaceutical Immunization: Prediction and Analysis of Clinical Relevance to Minimize the Risk) consortium reported the study in JAMA Network Open. The work was funded by a grant from the European Union Innovative Medicines Initiative.
LIMITATIONS:
Though the study demonstrated an association when all biologic drugs were analyzed together, it was not powered to demonstrate an association for each drug class.
DISCLOSURES:
Many authors reported financial relationships with pharmaceutical companies.
A version of this article first appeared on Medscape.com.
TOPLINE:
In patients with rheumatoid arthritis, the presence of antidrug antibodies was associated with a diminished response to biologic disease-modifying antirheumatic drugs in a prospective cohort study.
METHODOLOGY:
- Researchers prospectively analyzed data from 230 patients (mean age, 54.3 years; 77.0% women) with RA diagnosis recruited from March 3, 2014, to June 21, 2016.
- All were initiating new treatment with an anti–tumor necrosis factor (TNF) monoclonal antibody (mAb; either infliximab or adalimumab), etanercept, tocilizumab, or rituximab, according to the choice of the treating physician.
- The primary outcome was the association of antidrug antibody positivity with European Alliance of Associations for Rheumatology (EULAR) response to treatment at month 12, assessed through univariate logistic regression.
TAKEAWAY:
- At month 12, antidrug antibody positivity was 38.2% in patients who were treated with anti-TNF mAbs, 6.1% with etanercept, 50.0% with rituximab, and 20.0% with tocilizumab.
- There was an inverse association between antidrug antibody positivity directed against all biologic drugs and EULAR response at month 12 (odds ratio, 0.19; 95% confidence interval, 0.09-0.38; P < .001).
- In the multivariable analysis, antidrug antibodies, body mass index, and rheumatoid factor were independently and inversely associated with response to treatment.
- There was a significantly higher drug concentration of anti-TNF mAbs in patients with antidrug antibody–negative vs. antidrug antibody–positive status (mean difference, –9.6 mg/L; 95% CI, –12.4 to –6.9; P < .001).
IN PRACTICE:
Findings of this study suggest that antidrug antibodies are associated with nonresponse to biologic drugs and can be monitored in the management of patients with RA, particularly nonresponders.
SOURCE:
Samuel Bitouin, MD, PhD, of the rheumatology department at Paris-Saclay University, and coauthors in the ABIRISK (Anti-Biopharmaceutical Immunization: Prediction and Analysis of Clinical Relevance to Minimize the Risk) consortium reported the study in JAMA Network Open. The work was funded by a grant from the European Union Innovative Medicines Initiative.
LIMITATIONS:
Though the study demonstrated an association when all biologic drugs were analyzed together, it was not powered to demonstrate an association for each drug class.
DISCLOSURES:
Many authors reported financial relationships with pharmaceutical companies.
A version of this article first appeared on Medscape.com.
TOPLINE:
In patients with rheumatoid arthritis, the presence of antidrug antibodies was associated with a diminished response to biologic disease-modifying antirheumatic drugs in a prospective cohort study.
METHODOLOGY:
- Researchers prospectively analyzed data from 230 patients (mean age, 54.3 years; 77.0% women) with RA diagnosis recruited from March 3, 2014, to June 21, 2016.
- All were initiating new treatment with an anti–tumor necrosis factor (TNF) monoclonal antibody (mAb; either infliximab or adalimumab), etanercept, tocilizumab, or rituximab, according to the choice of the treating physician.
- The primary outcome was the association of antidrug antibody positivity with European Alliance of Associations for Rheumatology (EULAR) response to treatment at month 12, assessed through univariate logistic regression.
TAKEAWAY:
- At month 12, antidrug antibody positivity was 38.2% in patients who were treated with anti-TNF mAbs, 6.1% with etanercept, 50.0% with rituximab, and 20.0% with tocilizumab.
- There was an inverse association between antidrug antibody positivity directed against all biologic drugs and EULAR response at month 12 (odds ratio, 0.19; 95% confidence interval, 0.09-0.38; P < .001).
- In the multivariable analysis, antidrug antibodies, body mass index, and rheumatoid factor were independently and inversely associated with response to treatment.
- There was a significantly higher drug concentration of anti-TNF mAbs in patients with antidrug antibody–negative vs. antidrug antibody–positive status (mean difference, –9.6 mg/L; 95% CI, –12.4 to –6.9; P < .001).
IN PRACTICE:
Findings of this study suggest that antidrug antibodies are associated with nonresponse to biologic drugs and can be monitored in the management of patients with RA, particularly nonresponders.
SOURCE:
Samuel Bitouin, MD, PhD, of the rheumatology department at Paris-Saclay University, and coauthors in the ABIRISK (Anti-Biopharmaceutical Immunization: Prediction and Analysis of Clinical Relevance to Minimize the Risk) consortium reported the study in JAMA Network Open. The work was funded by a grant from the European Union Innovative Medicines Initiative.
LIMITATIONS:
Though the study demonstrated an association when all biologic drugs were analyzed together, it was not powered to demonstrate an association for each drug class.
DISCLOSURES:
Many authors reported financial relationships with pharmaceutical companies.
A version of this article first appeared on Medscape.com.
Does colchicine have a role in treating excess ASCVD risk in patients with chronic inflammatory conditions?
The recent Food and Drug Administration approval of colchicine 0.5 mg (Lodoco) for use in atherosclerotic cardiovascular disease (ASCVD) prevention will possibly create opportunities to use the drug to treat residual risk for ASCVD in some patients with immune-mediated inflammatory diseases, particularly in rheumatology.
Potential in rheumatology
The 0.5-mg dose is just a shade under the 0.6-mg, twice daily dosing rheumatologists typically prescribe for gout, Christie Bartels, MD, MS, chief of rheumatology at the University of Wisconsin–Madison, said in an interview. Clinicians also use the 0.6-mg dose off-label for pseudogout or calcium pyrophosphate deposition disease (CPPD), Dr. Bartels noted.
The new formulation opens the consideration for using colchicine more in patients with psoriatic arthritis, lupus, and rheumatoid arthritis, she said. “I think we could certainly discuss it, particularly, in secondary prevention patients who already had an event or who are at the highest risk and already on optimal traditional agents,” she said.
She cited previous comments by Paul Ridker, MD, director of the center for cardiovascular disease prevention at Brigham and Women’s Hospital in Boston, and developer of the high-sensitivity C-reactive protein (hsCRP) test for measuring inflammatory markers. “We might not know the answer because Dr. Ridker pointed out he used colchicine 0.5 mg in patients that had a high-sensitivity CRP that was high; we need patients who have had inflammation of unknown origin, so those patients presumably weren’t already on another anti-inflammatory,” she said, noting that hydroxychloroquine, methotrexate, and some biologics provide some protection from cardiovascular risks.
However, a potential role for long-term colchicine 0.5 mg in ASCVD prevention may cause consideration for changing the drug’s role in gout treatment, Dr. Bartels said. “In gout, where we do have an FDA-approved indication for colchicine, we used to use it only for the first 6 months while we were getting patients to goal on allopurinol, which was usually then monotherapy after the first 6 months,” she said. “I think this will likely change how I treat gout patients in that I may also offer to continue both medications [colchicine and allopurinol] if they are tolerating them well.
“And then in patients where I’m using it off-label in CPPD, I might again share with them that in addition to possibly helping their CPPD, there may be this added benefit to reduce inflammation just in discussing the risks and benefits of the medicine.”
However, rheumatologists must be careful in using colchicine beyond the typical 6-month cycle, Dr. Bartels said. “One of the tricky things with colchicine, and part of the reason we did not traditionally continue it specifically past the first 6 months, was that it can cause myopathies or cytopenias, so we still have to counsel patients regarding these risks and monitor that,” she said.
Additionally, colchicine can have drug interactions with statins or calcium channel blockers that can change colchicine levels. “I think the dose here is so low, the 0.5 mg, that it’s probably still safe, but again, it’s something that we have to take a look at in the patient’s whole picture and the rest of their burden of their meds in order to make a decision with them,” Dr. Bartels said.
Possibilities in dermatology
The LoDoCo2 trial one of two major randomized trials that supported approval of colchicine 0.5 mg, reported that treated patients had a 60% lower rate of gout than the placebo group (1.4% vs. 3.4%). Joel Gelfand, MD, MSCE, the James J. Leyden professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, pointed to this in evaluating the dermatologic implications of the drug’s approval. “This may be of particular interest as people with psoriasis have an increased risk of gout,” he said in emailed comments.
Colchicine’s mechanism of action to reduce inflammation parallels that of tumor necrosis factor (TNF) inhibitors used for dermatologic indications, namely by inhibiting leukocyte adhesion to disrupt the downregulation of TNF receptors, Dr. Gelfand said.
“Interestingly, observational data suggests biologics that target TNF such as adalimumab, etanercept, etc., are associated with a reduction in CV events, and in placebo-controlled trials we conducted in psoriasis patients, it reduced key inflammatory mediators of cardiovascular disease, including IL [interleukin]-6,” he said. “Randomized clinical trials to evaluate the ability of TNF inhibitors, which are now available as biosimilars, to prevent cardiovascular events in high-risk patients, should be conducted, and more work is needed to identify which additional immune-targeted treatments may lower CV risk with an acceptable safety profile.”
Colchicine currently has few indications for rare conditions in dermatology, Dr. Gelfand said, including Sweets syndrome, subcorneal pustular dermatosis, and cutaneous vasculitis. “There are some reports to suggest it may help psoriatic disease, but current data are limited and insufficient to recommend its use for psoriasis and/or psoriatic arthritis,” he said.
The approval of colchicine 0.5 mg for ASCVD could be meaningful for people with psoriasis who are also being treated for CV risk factors, Dr. Gelfand said. “Additional considerations such as signs of residual inflammation (elevated hsCRP) and CV imaging findings may be used to further guide shared decision-making for optimal use,” he said.
Another consideration he noted: “This is also a novel 0.5-mg formulation, and thus cost may be an issue.”
Would side effects bar use in gastroenterology?
Colchicine 0.5 mg may not move the needle much for expanding treatment of ASCVD in patients with inflammatory bowel disease (IBD) and potentially other gastrointestinal conditions, Edward Loftus Jr., MD, the Maxine and Jack Zarrow Family professor of gastroenterology specifically for IBD at the Mayo Clinic in Rochester, Minn., told MDEdge in emailed comments. “Given the GI side effect profile [of colchicine], I am not sure I would go there,” he said.
“Hopefully, the prescribers of this low-dose formulation are aware of the gastrointestinal side effects, such as diarrhea and nausea, and educate patients about these side effects so that a proper risk-benefit discussion can ensue,” he said.
Dr. Bartels reporting a previous financial relationship with Pfizer. Dr. Gelfand said he has financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, GlaxoSmithKline, Twill, Lilly, Leo, Moonlake, Janssen Biologics, Novartis, Pfizer, UCB, Neuroderm, and Veolia North America. Dr. Loftus disclosed relationships with AbbVie, Alvotech, Amgen, Arena, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Receptos, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Health, Janssen, KSL Diagnostics, Morphic, Ono, Pfizer, Sun, Surrozen, Takeda, Theravance, and UCB.
The recent Food and Drug Administration approval of colchicine 0.5 mg (Lodoco) for use in atherosclerotic cardiovascular disease (ASCVD) prevention will possibly create opportunities to use the drug to treat residual risk for ASCVD in some patients with immune-mediated inflammatory diseases, particularly in rheumatology.
Potential in rheumatology
The 0.5-mg dose is just a shade under the 0.6-mg, twice daily dosing rheumatologists typically prescribe for gout, Christie Bartels, MD, MS, chief of rheumatology at the University of Wisconsin–Madison, said in an interview. Clinicians also use the 0.6-mg dose off-label for pseudogout or calcium pyrophosphate deposition disease (CPPD), Dr. Bartels noted.
The new formulation opens the consideration for using colchicine more in patients with psoriatic arthritis, lupus, and rheumatoid arthritis, she said. “I think we could certainly discuss it, particularly, in secondary prevention patients who already had an event or who are at the highest risk and already on optimal traditional agents,” she said.
She cited previous comments by Paul Ridker, MD, director of the center for cardiovascular disease prevention at Brigham and Women’s Hospital in Boston, and developer of the high-sensitivity C-reactive protein (hsCRP) test for measuring inflammatory markers. “We might not know the answer because Dr. Ridker pointed out he used colchicine 0.5 mg in patients that had a high-sensitivity CRP that was high; we need patients who have had inflammation of unknown origin, so those patients presumably weren’t already on another anti-inflammatory,” she said, noting that hydroxychloroquine, methotrexate, and some biologics provide some protection from cardiovascular risks.
However, a potential role for long-term colchicine 0.5 mg in ASCVD prevention may cause consideration for changing the drug’s role in gout treatment, Dr. Bartels said. “In gout, where we do have an FDA-approved indication for colchicine, we used to use it only for the first 6 months while we were getting patients to goal on allopurinol, which was usually then monotherapy after the first 6 months,” she said. “I think this will likely change how I treat gout patients in that I may also offer to continue both medications [colchicine and allopurinol] if they are tolerating them well.
“And then in patients where I’m using it off-label in CPPD, I might again share with them that in addition to possibly helping their CPPD, there may be this added benefit to reduce inflammation just in discussing the risks and benefits of the medicine.”
However, rheumatologists must be careful in using colchicine beyond the typical 6-month cycle, Dr. Bartels said. “One of the tricky things with colchicine, and part of the reason we did not traditionally continue it specifically past the first 6 months, was that it can cause myopathies or cytopenias, so we still have to counsel patients regarding these risks and monitor that,” she said.
Additionally, colchicine can have drug interactions with statins or calcium channel blockers that can change colchicine levels. “I think the dose here is so low, the 0.5 mg, that it’s probably still safe, but again, it’s something that we have to take a look at in the patient’s whole picture and the rest of their burden of their meds in order to make a decision with them,” Dr. Bartels said.
Possibilities in dermatology
The LoDoCo2 trial one of two major randomized trials that supported approval of colchicine 0.5 mg, reported that treated patients had a 60% lower rate of gout than the placebo group (1.4% vs. 3.4%). Joel Gelfand, MD, MSCE, the James J. Leyden professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, pointed to this in evaluating the dermatologic implications of the drug’s approval. “This may be of particular interest as people with psoriasis have an increased risk of gout,” he said in emailed comments.
Colchicine’s mechanism of action to reduce inflammation parallels that of tumor necrosis factor (TNF) inhibitors used for dermatologic indications, namely by inhibiting leukocyte adhesion to disrupt the downregulation of TNF receptors, Dr. Gelfand said.
“Interestingly, observational data suggests biologics that target TNF such as adalimumab, etanercept, etc., are associated with a reduction in CV events, and in placebo-controlled trials we conducted in psoriasis patients, it reduced key inflammatory mediators of cardiovascular disease, including IL [interleukin]-6,” he said. “Randomized clinical trials to evaluate the ability of TNF inhibitors, which are now available as biosimilars, to prevent cardiovascular events in high-risk patients, should be conducted, and more work is needed to identify which additional immune-targeted treatments may lower CV risk with an acceptable safety profile.”
Colchicine currently has few indications for rare conditions in dermatology, Dr. Gelfand said, including Sweets syndrome, subcorneal pustular dermatosis, and cutaneous vasculitis. “There are some reports to suggest it may help psoriatic disease, but current data are limited and insufficient to recommend its use for psoriasis and/or psoriatic arthritis,” he said.
The approval of colchicine 0.5 mg for ASCVD could be meaningful for people with psoriasis who are also being treated for CV risk factors, Dr. Gelfand said. “Additional considerations such as signs of residual inflammation (elevated hsCRP) and CV imaging findings may be used to further guide shared decision-making for optimal use,” he said.
Another consideration he noted: “This is also a novel 0.5-mg formulation, and thus cost may be an issue.”
Would side effects bar use in gastroenterology?
Colchicine 0.5 mg may not move the needle much for expanding treatment of ASCVD in patients with inflammatory bowel disease (IBD) and potentially other gastrointestinal conditions, Edward Loftus Jr., MD, the Maxine and Jack Zarrow Family professor of gastroenterology specifically for IBD at the Mayo Clinic in Rochester, Minn., told MDEdge in emailed comments. “Given the GI side effect profile [of colchicine], I am not sure I would go there,” he said.
“Hopefully, the prescribers of this low-dose formulation are aware of the gastrointestinal side effects, such as diarrhea and nausea, and educate patients about these side effects so that a proper risk-benefit discussion can ensue,” he said.
Dr. Bartels reporting a previous financial relationship with Pfizer. Dr. Gelfand said he has financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, GlaxoSmithKline, Twill, Lilly, Leo, Moonlake, Janssen Biologics, Novartis, Pfizer, UCB, Neuroderm, and Veolia North America. Dr. Loftus disclosed relationships with AbbVie, Alvotech, Amgen, Arena, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Receptos, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Health, Janssen, KSL Diagnostics, Morphic, Ono, Pfizer, Sun, Surrozen, Takeda, Theravance, and UCB.
The recent Food and Drug Administration approval of colchicine 0.5 mg (Lodoco) for use in atherosclerotic cardiovascular disease (ASCVD) prevention will possibly create opportunities to use the drug to treat residual risk for ASCVD in some patients with immune-mediated inflammatory diseases, particularly in rheumatology.
Potential in rheumatology
The 0.5-mg dose is just a shade under the 0.6-mg, twice daily dosing rheumatologists typically prescribe for gout, Christie Bartels, MD, MS, chief of rheumatology at the University of Wisconsin–Madison, said in an interview. Clinicians also use the 0.6-mg dose off-label for pseudogout or calcium pyrophosphate deposition disease (CPPD), Dr. Bartels noted.
The new formulation opens the consideration for using colchicine more in patients with psoriatic arthritis, lupus, and rheumatoid arthritis, she said. “I think we could certainly discuss it, particularly, in secondary prevention patients who already had an event or who are at the highest risk and already on optimal traditional agents,” she said.
She cited previous comments by Paul Ridker, MD, director of the center for cardiovascular disease prevention at Brigham and Women’s Hospital in Boston, and developer of the high-sensitivity C-reactive protein (hsCRP) test for measuring inflammatory markers. “We might not know the answer because Dr. Ridker pointed out he used colchicine 0.5 mg in patients that had a high-sensitivity CRP that was high; we need patients who have had inflammation of unknown origin, so those patients presumably weren’t already on another anti-inflammatory,” she said, noting that hydroxychloroquine, methotrexate, and some biologics provide some protection from cardiovascular risks.
However, a potential role for long-term colchicine 0.5 mg in ASCVD prevention may cause consideration for changing the drug’s role in gout treatment, Dr. Bartels said. “In gout, where we do have an FDA-approved indication for colchicine, we used to use it only for the first 6 months while we were getting patients to goal on allopurinol, which was usually then monotherapy after the first 6 months,” she said. “I think this will likely change how I treat gout patients in that I may also offer to continue both medications [colchicine and allopurinol] if they are tolerating them well.
“And then in patients where I’m using it off-label in CPPD, I might again share with them that in addition to possibly helping their CPPD, there may be this added benefit to reduce inflammation just in discussing the risks and benefits of the medicine.”
However, rheumatologists must be careful in using colchicine beyond the typical 6-month cycle, Dr. Bartels said. “One of the tricky things with colchicine, and part of the reason we did not traditionally continue it specifically past the first 6 months, was that it can cause myopathies or cytopenias, so we still have to counsel patients regarding these risks and monitor that,” she said.
Additionally, colchicine can have drug interactions with statins or calcium channel blockers that can change colchicine levels. “I think the dose here is so low, the 0.5 mg, that it’s probably still safe, but again, it’s something that we have to take a look at in the patient’s whole picture and the rest of their burden of their meds in order to make a decision with them,” Dr. Bartels said.
Possibilities in dermatology
The LoDoCo2 trial one of two major randomized trials that supported approval of colchicine 0.5 mg, reported that treated patients had a 60% lower rate of gout than the placebo group (1.4% vs. 3.4%). Joel Gelfand, MD, MSCE, the James J. Leyden professor of dermatology and epidemiology at the University of Pennsylvania, Philadelphia, pointed to this in evaluating the dermatologic implications of the drug’s approval. “This may be of particular interest as people with psoriasis have an increased risk of gout,” he said in emailed comments.
Colchicine’s mechanism of action to reduce inflammation parallels that of tumor necrosis factor (TNF) inhibitors used for dermatologic indications, namely by inhibiting leukocyte adhesion to disrupt the downregulation of TNF receptors, Dr. Gelfand said.
“Interestingly, observational data suggests biologics that target TNF such as adalimumab, etanercept, etc., are associated with a reduction in CV events, and in placebo-controlled trials we conducted in psoriasis patients, it reduced key inflammatory mediators of cardiovascular disease, including IL [interleukin]-6,” he said. “Randomized clinical trials to evaluate the ability of TNF inhibitors, which are now available as biosimilars, to prevent cardiovascular events in high-risk patients, should be conducted, and more work is needed to identify which additional immune-targeted treatments may lower CV risk with an acceptable safety profile.”
Colchicine currently has few indications for rare conditions in dermatology, Dr. Gelfand said, including Sweets syndrome, subcorneal pustular dermatosis, and cutaneous vasculitis. “There are some reports to suggest it may help psoriatic disease, but current data are limited and insufficient to recommend its use for psoriasis and/or psoriatic arthritis,” he said.
The approval of colchicine 0.5 mg for ASCVD could be meaningful for people with psoriasis who are also being treated for CV risk factors, Dr. Gelfand said. “Additional considerations such as signs of residual inflammation (elevated hsCRP) and CV imaging findings may be used to further guide shared decision-making for optimal use,” he said.
Another consideration he noted: “This is also a novel 0.5-mg formulation, and thus cost may be an issue.”
Would side effects bar use in gastroenterology?
Colchicine 0.5 mg may not move the needle much for expanding treatment of ASCVD in patients with inflammatory bowel disease (IBD) and potentially other gastrointestinal conditions, Edward Loftus Jr., MD, the Maxine and Jack Zarrow Family professor of gastroenterology specifically for IBD at the Mayo Clinic in Rochester, Minn., told MDEdge in emailed comments. “Given the GI side effect profile [of colchicine], I am not sure I would go there,” he said.
“Hopefully, the prescribers of this low-dose formulation are aware of the gastrointestinal side effects, such as diarrhea and nausea, and educate patients about these side effects so that a proper risk-benefit discussion can ensue,” he said.
Dr. Bartels reporting a previous financial relationship with Pfizer. Dr. Gelfand said he has financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Boehringer Ingelheim, Celldex, GlaxoSmithKline, Twill, Lilly, Leo, Moonlake, Janssen Biologics, Novartis, Pfizer, UCB, Neuroderm, and Veolia North America. Dr. Loftus disclosed relationships with AbbVie, Alvotech, Amgen, Arena, Avalo, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene/Receptos, Celltrion Healthcare, Eli Lilly, Fresenius Kabi, Genentech, Gilead, GlaxoSmithKline, Gossamer Bio, Iterative Health, Janssen, KSL Diagnostics, Morphic, Ono, Pfizer, Sun, Surrozen, Takeda, Theravance, and UCB.
Commentary: DMARD and HCQ in RA, July 2023
Despite multiple existing conventional synthetic disease-modifying antirheumatic drug (csDMARD) and biologic DMARD (bDMARD) options, many patients with rheumatoid arthritis (RA) do not respond adequately to treatment. In an exciting development, a recent phase 2 study by Tuttle and colleagues examined a novel treatment approach in RA: stimulation of the programmed cell death protein 1 (PD-1) inhibitor pathway. PD-1 is a checkpoint inhibitor receptor whose activation reflects T-cell activation and may play a role in synovitis and extra-articular inflammation. Blocking PD-1 in cancer therapy has been associated with an increase in inflammatory arthritis. In this 12-week study, RA disease activity was analyzed in patients randomly assigned to two different monthly intravenous doses of peresolimab or placebo. Of note, a large majority of participants were seropositive for rheumatoid factor (RF) or cyclic citrullinated peptide (CCP). Patients receiving the 700-mg dose of peresolimab had a better American College of Rheumatology (ACR) 20 response than did those receiving placebo (71% vs 42%), but not a better ACR50 or ACR70 response; the 300-mg dose was not better than placebo. Although reported adverse events were similar in all three groups, with a short timeframe it would be difficult to address concerns about cancer risk. Though this novel treatment is exciting, a larger and longer-term trial is necessary to address this concern as well as potentially tease out risk factors (including age or other immunosuppression) in this susceptible group.
Two other studies examined use of a much older csDMARD therapy, hydroxychloroquine (HCQ), in Brazilian patients with RA. Bredemeier and colleagues looked at the effects of HCQ on adverse events as well as the persistence of bDMARD/targeted synthetic DMARD (tsDMARD) therapy in over 1300 patients with RA. Using the BiobadaBrasil registry of patients starting their first bDMARD or Janus kinase (JAK) inhibitor, they looked at effects of combination therapy with HCQ during the treatment course of up to six bDMARD or JAK inhibitors. At baseline, patients prescribed antimalarial therapy had shorter RA duration and began treatment earlier, perhaps due to patient or physician preferences regarding starting "milder" antimalarial medication earlier or due to use of "triple therapy" with methotrexate and sulfasalazine. Of interest, patients receiving antimalarial therapy had a lower incidence of adverse events, especially serious infections, but no effect on cardiovascular events was seen despite HCQ's perceived beneficial effects on thrombotic risk and cholesterol profile. Patients receiving HCQ were also more likely to persist in their course of bDMARD or JAK inhibitor therapy, though the effect size seems relatively small. As the focus in this study was on adverse effects, the authors' analysis of the effects on antimalarials on the persistence of therapy was not detailed.
Lin and colleagues also looked at the effects of HCQ in patients with older-onset RA with respect to mortality risk. Using data from the electronic health records of a hospital in Taiwan, mortality-associated risk factors were evaluated in 980 patients with RA diagnosed at >60 years. Male sex, current smoking status, and cancer status were all associated with mortality, whereas HCQ use was associated with reduced mortality (hazard ratio 0.30). In contrast to the registry study mentioned above, patients receiving HCQ had a lower risk for cardiovascular events, hyperlipidemia, diabetes, and chronic kidney disease. Interaction with cancer was less clear due to lower number of patients. Of interest, use of cyclosporine, leflunomide, and a bDMARD was associated with higher mortality risk. The source and true relevance of the potential risk reduction in this study is not clear because of the lack of prospective data, but combined with the information above, this study suggests that the benefits of HCQ use should not be discounted in patients with RA.
Despite multiple existing conventional synthetic disease-modifying antirheumatic drug (csDMARD) and biologic DMARD (bDMARD) options, many patients with rheumatoid arthritis (RA) do not respond adequately to treatment. In an exciting development, a recent phase 2 study by Tuttle and colleagues examined a novel treatment approach in RA: stimulation of the programmed cell death protein 1 (PD-1) inhibitor pathway. PD-1 is a checkpoint inhibitor receptor whose activation reflects T-cell activation and may play a role in synovitis and extra-articular inflammation. Blocking PD-1 in cancer therapy has been associated with an increase in inflammatory arthritis. In this 12-week study, RA disease activity was analyzed in patients randomly assigned to two different monthly intravenous doses of peresolimab or placebo. Of note, a large majority of participants were seropositive for rheumatoid factor (RF) or cyclic citrullinated peptide (CCP). Patients receiving the 700-mg dose of peresolimab had a better American College of Rheumatology (ACR) 20 response than did those receiving placebo (71% vs 42%), but not a better ACR50 or ACR70 response; the 300-mg dose was not better than placebo. Although reported adverse events were similar in all three groups, with a short timeframe it would be difficult to address concerns about cancer risk. Though this novel treatment is exciting, a larger and longer-term trial is necessary to address this concern as well as potentially tease out risk factors (including age or other immunosuppression) in this susceptible group.
Two other studies examined use of a much older csDMARD therapy, hydroxychloroquine (HCQ), in Brazilian patients with RA. Bredemeier and colleagues looked at the effects of HCQ on adverse events as well as the persistence of bDMARD/targeted synthetic DMARD (tsDMARD) therapy in over 1300 patients with RA. Using the BiobadaBrasil registry of patients starting their first bDMARD or Janus kinase (JAK) inhibitor, they looked at effects of combination therapy with HCQ during the treatment course of up to six bDMARD or JAK inhibitors. At baseline, patients prescribed antimalarial therapy had shorter RA duration and began treatment earlier, perhaps due to patient or physician preferences regarding starting "milder" antimalarial medication earlier or due to use of "triple therapy" with methotrexate and sulfasalazine. Of interest, patients receiving antimalarial therapy had a lower incidence of adverse events, especially serious infections, but no effect on cardiovascular events was seen despite HCQ's perceived beneficial effects on thrombotic risk and cholesterol profile. Patients receiving HCQ were also more likely to persist in their course of bDMARD or JAK inhibitor therapy, though the effect size seems relatively small. As the focus in this study was on adverse effects, the authors' analysis of the effects on antimalarials on the persistence of therapy was not detailed.
Lin and colleagues also looked at the effects of HCQ in patients with older-onset RA with respect to mortality risk. Using data from the electronic health records of a hospital in Taiwan, mortality-associated risk factors were evaluated in 980 patients with RA diagnosed at >60 years. Male sex, current smoking status, and cancer status were all associated with mortality, whereas HCQ use was associated with reduced mortality (hazard ratio 0.30). In contrast to the registry study mentioned above, patients receiving HCQ had a lower risk for cardiovascular events, hyperlipidemia, diabetes, and chronic kidney disease. Interaction with cancer was less clear due to lower number of patients. Of interest, use of cyclosporine, leflunomide, and a bDMARD was associated with higher mortality risk. The source and true relevance of the potential risk reduction in this study is not clear because of the lack of prospective data, but combined with the information above, this study suggests that the benefits of HCQ use should not be discounted in patients with RA.
Despite multiple existing conventional synthetic disease-modifying antirheumatic drug (csDMARD) and biologic DMARD (bDMARD) options, many patients with rheumatoid arthritis (RA) do not respond adequately to treatment. In an exciting development, a recent phase 2 study by Tuttle and colleagues examined a novel treatment approach in RA: stimulation of the programmed cell death protein 1 (PD-1) inhibitor pathway. PD-1 is a checkpoint inhibitor receptor whose activation reflects T-cell activation and may play a role in synovitis and extra-articular inflammation. Blocking PD-1 in cancer therapy has been associated with an increase in inflammatory arthritis. In this 12-week study, RA disease activity was analyzed in patients randomly assigned to two different monthly intravenous doses of peresolimab or placebo. Of note, a large majority of participants were seropositive for rheumatoid factor (RF) or cyclic citrullinated peptide (CCP). Patients receiving the 700-mg dose of peresolimab had a better American College of Rheumatology (ACR) 20 response than did those receiving placebo (71% vs 42%), but not a better ACR50 or ACR70 response; the 300-mg dose was not better than placebo. Although reported adverse events were similar in all three groups, with a short timeframe it would be difficult to address concerns about cancer risk. Though this novel treatment is exciting, a larger and longer-term trial is necessary to address this concern as well as potentially tease out risk factors (including age or other immunosuppression) in this susceptible group.
Two other studies examined use of a much older csDMARD therapy, hydroxychloroquine (HCQ), in Brazilian patients with RA. Bredemeier and colleagues looked at the effects of HCQ on adverse events as well as the persistence of bDMARD/targeted synthetic DMARD (tsDMARD) therapy in over 1300 patients with RA. Using the BiobadaBrasil registry of patients starting their first bDMARD or Janus kinase (JAK) inhibitor, they looked at effects of combination therapy with HCQ during the treatment course of up to six bDMARD or JAK inhibitors. At baseline, patients prescribed antimalarial therapy had shorter RA duration and began treatment earlier, perhaps due to patient or physician preferences regarding starting "milder" antimalarial medication earlier or due to use of "triple therapy" with methotrexate and sulfasalazine. Of interest, patients receiving antimalarial therapy had a lower incidence of adverse events, especially serious infections, but no effect on cardiovascular events was seen despite HCQ's perceived beneficial effects on thrombotic risk and cholesterol profile. Patients receiving HCQ were also more likely to persist in their course of bDMARD or JAK inhibitor therapy, though the effect size seems relatively small. As the focus in this study was on adverse effects, the authors' analysis of the effects on antimalarials on the persistence of therapy was not detailed.
Lin and colleagues also looked at the effects of HCQ in patients with older-onset RA with respect to mortality risk. Using data from the electronic health records of a hospital in Taiwan, mortality-associated risk factors were evaluated in 980 patients with RA diagnosed at >60 years. Male sex, current smoking status, and cancer status were all associated with mortality, whereas HCQ use was associated with reduced mortality (hazard ratio 0.30). In contrast to the registry study mentioned above, patients receiving HCQ had a lower risk for cardiovascular events, hyperlipidemia, diabetes, and chronic kidney disease. Interaction with cancer was less clear due to lower number of patients. Of interest, use of cyclosporine, leflunomide, and a bDMARD was associated with higher mortality risk. The source and true relevance of the potential risk reduction in this study is not clear because of the lack of prospective data, but combined with the information above, this study suggests that the benefits of HCQ use should not be discounted in patients with RA.
Methotrexate does not impair sperm quality, small study finds
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
TOPLINE:
Methotrexate (MTX) is not associated with testicular toxicity, so therapy can be safety started in men pursuing parenthood, a small study finds.
METHODOLOGY:
- Lack of evidence regarding MTX’s effect on sperm quality has resulted in inconsistent recommendations for men actively pursuing parenthood.
- Researchers enrolled 20 men aged 18 years or older with an immune-mediated inflammatory disease (IMID) who were about to begin MTX therapy and 25 healthy men as controls.
- Participants provided semen samples prior to beginning MTX therapy and 13 weeks after beginning therapy.
- Researchers tested samples in both groups for markers of testicular toxicity.
- Also evaluated whether MTX polyglutamates could be detected in sperm of seminal fluid, as a secondary outcome.
TAKEAWAY:
- Found no significant differences in conventional semen parameters, sperm DNA damage, or male reproductive endocrine axis between the MTX group and controls.
- The concentration of MTX polyglutamates is low in both sperm and seminal fluid and is particularly low in sperm.
IN PRACTICE:
“Therapy with MTX can be safely started or continued in men diagnosed with an IMID and with an active wish to become a father,” the authors write.
STUDY DETAILS:
Luis Fernando Perez-Garcia, MD, Erasmus Medical Center, Rotterdam, the Netherlands, led the research. The study was published online in Annals of the Rheumatic Diseases on June 1, 2023.
LIMITATIONS:
The small number of participants and that the study included only MTX starters and not those who have taken MTX longer term.
DISCLOSURES:
Grants from the Dutch Arthritis Foundation, The Netherlands Organization for Health Research and Development, and Consejo Nacional de Ciencia y Tecnologia funded the project. Researchers disclosed financial relationships with Galapagos NV and UCB.
A version of this article first appeared on Medscape.com.
Sarcopenia prevalence and risk in older RA patients
Key clinical point: Patients with rheumatoid arthritis (RA) who were ≥65 years old had a significantly increased risk of developing sarcopenia, particularly if they were men with poor nutritional status and long-standing disease.
Major finding: Sarcopenia was diagnosed in a higher proportion of patients with RA vs control individuals without RA (15.8% vs 3.9%; P = .014). Male sex (P = .042), longer disease duration (P = .012), and poorer nutritional status (P = .042) were significant risk factors for the development of sarcopenia in older patients with RA.
Study details: Findings are from a cross-sectional study including 76 patients age ≥ 65 years with RA and 76 age- and sex-matched control individuals without RA.
Disclosures: This study was funded by Redes de Investigación Cooperativa Orientadas a Resultados en Salud, Spain, and other sources. The authors declared no conflicts of interest.
Source: Cano-García L et al. Sarcopenia and nutrition in elderly rheumatoid arthritis patients: A cross-sectional study to determine prevalence and risk factors. Nutrients. 2023;15:2440 (May 24). doi: 10.3390/nu15112440
Key clinical point: Patients with rheumatoid arthritis (RA) who were ≥65 years old had a significantly increased risk of developing sarcopenia, particularly if they were men with poor nutritional status and long-standing disease.
Major finding: Sarcopenia was diagnosed in a higher proportion of patients with RA vs control individuals without RA (15.8% vs 3.9%; P = .014). Male sex (P = .042), longer disease duration (P = .012), and poorer nutritional status (P = .042) were significant risk factors for the development of sarcopenia in older patients with RA.
Study details: Findings are from a cross-sectional study including 76 patients age ≥ 65 years with RA and 76 age- and sex-matched control individuals without RA.
Disclosures: This study was funded by Redes de Investigación Cooperativa Orientadas a Resultados en Salud, Spain, and other sources. The authors declared no conflicts of interest.
Source: Cano-García L et al. Sarcopenia and nutrition in elderly rheumatoid arthritis patients: A cross-sectional study to determine prevalence and risk factors. Nutrients. 2023;15:2440 (May 24). doi: 10.3390/nu15112440
Key clinical point: Patients with rheumatoid arthritis (RA) who were ≥65 years old had a significantly increased risk of developing sarcopenia, particularly if they were men with poor nutritional status and long-standing disease.
Major finding: Sarcopenia was diagnosed in a higher proportion of patients with RA vs control individuals without RA (15.8% vs 3.9%; P = .014). Male sex (P = .042), longer disease duration (P = .012), and poorer nutritional status (P = .042) were significant risk factors for the development of sarcopenia in older patients with RA.
Study details: Findings are from a cross-sectional study including 76 patients age ≥ 65 years with RA and 76 age- and sex-matched control individuals without RA.
Disclosures: This study was funded by Redes de Investigación Cooperativa Orientadas a Resultados en Salud, Spain, and other sources. The authors declared no conflicts of interest.
Source: Cano-García L et al. Sarcopenia and nutrition in elderly rheumatoid arthritis patients: A cross-sectional study to determine prevalence and risk factors. Nutrients. 2023;15:2440 (May 24). doi: 10.3390/nu15112440
Progressing joint damage: An indication to consider intensive treatment in RA patients in remission or LDA
Key clinical point: Intensive treatment more effectively suppressed joint damage progression than the current treatment in patients with rheumatoid arthritis (RA) who showed joint damage progression and had low disease activity (LDA) or were in remission.
Major finding: At 1 year of treatment, intensive vs current treatment was associated with a smaller change in the van der Heijde modified total Sharp score (ΔTSS; 0.67 vs 1.79; P < .001) and joint space narrowing scores (0.57 vs 1.41; P < .001) and a larger proportion of patients achieved a ΔTSS of ≤0.5 (66.7% vs 32.4%; P = .010).
Study details: This retrospective study included 89 patients with RA in remission or with LDA who showed joint damage progression and were assigned to either receive intensive treatment or continue the current treatment.
Disclosures: This study did not declare the funding source. T Mochizuki, K Yano, and K Ikari declared receiving lecture honoraria from various sources. The other authors declared no conflicts of interest.
Source: Mochizuki T et al. Intensive treatment for the progression of joint damage in rheumatoid arthritis patients with low disease activity or remission. Mod Rheumatol. 2023 (Jun 2). doi: 10.1093/mr/road041
Key clinical point: Intensive treatment more effectively suppressed joint damage progression than the current treatment in patients with rheumatoid arthritis (RA) who showed joint damage progression and had low disease activity (LDA) or were in remission.
Major finding: At 1 year of treatment, intensive vs current treatment was associated with a smaller change in the van der Heijde modified total Sharp score (ΔTSS; 0.67 vs 1.79; P < .001) and joint space narrowing scores (0.57 vs 1.41; P < .001) and a larger proportion of patients achieved a ΔTSS of ≤0.5 (66.7% vs 32.4%; P = .010).
Study details: This retrospective study included 89 patients with RA in remission or with LDA who showed joint damage progression and were assigned to either receive intensive treatment or continue the current treatment.
Disclosures: This study did not declare the funding source. T Mochizuki, K Yano, and K Ikari declared receiving lecture honoraria from various sources. The other authors declared no conflicts of interest.
Source: Mochizuki T et al. Intensive treatment for the progression of joint damage in rheumatoid arthritis patients with low disease activity or remission. Mod Rheumatol. 2023 (Jun 2). doi: 10.1093/mr/road041
Key clinical point: Intensive treatment more effectively suppressed joint damage progression than the current treatment in patients with rheumatoid arthritis (RA) who showed joint damage progression and had low disease activity (LDA) or were in remission.
Major finding: At 1 year of treatment, intensive vs current treatment was associated with a smaller change in the van der Heijde modified total Sharp score (ΔTSS; 0.67 vs 1.79; P < .001) and joint space narrowing scores (0.57 vs 1.41; P < .001) and a larger proportion of patients achieved a ΔTSS of ≤0.5 (66.7% vs 32.4%; P = .010).
Study details: This retrospective study included 89 patients with RA in remission or with LDA who showed joint damage progression and were assigned to either receive intensive treatment or continue the current treatment.
Disclosures: This study did not declare the funding source. T Mochizuki, K Yano, and K Ikari declared receiving lecture honoraria from various sources. The other authors declared no conflicts of interest.
Source: Mochizuki T et al. Intensive treatment for the progression of joint damage in rheumatoid arthritis patients with low disease activity or remission. Mod Rheumatol. 2023 (Jun 2). doi: 10.1093/mr/road041
Ultrasound detects subclinical inflammation in RA patients with low or no disease activity
Key clinical point: Ultrasound detected subclinical inflammation in the wrist joints of most patients with rheumatoid arthritis (RA) in clinical remission or with lower disease activity, with the risk for subclinical inflammation being lower among those using biologic therapy.
Major finding: Overall, subclinical inflammation was detected in 57.4% of the patients in complete remission or with lower disease activity. Factors negatively associated with subclinical inflammation included the use of biologic therapy (odds ratio [OR] 0.59; P = .001), methotrexate (OR 0.83; P = .020), and glucocorticoids (OR 0.60; P = .001) and alcohol consumption (OR 0.55; P = .006).
Study details: This cross-sectional study included 1248 patients with RA who underwent gray scale and power Doppler ultrasound assessments of the dorsal radiolunate joints of both wrists.
Disclosures: This study was supported by the Kaohsiung Chang Gung Memorial Hospital, Taiwan, and other sources. The authors declared no conflicts of interest.
Source: Wang YW et al. Factors associated with subclinical inflammation of wrist joints in rheumatoid arthritis patients with low or no disease activity—A RA ultrasound registry study. BMC Musculoskelet Disord. 2023;24:438 (May 30). doi: 10.1186/s12891-023-06521-8
Key clinical point: Ultrasound detected subclinical inflammation in the wrist joints of most patients with rheumatoid arthritis (RA) in clinical remission or with lower disease activity, with the risk for subclinical inflammation being lower among those using biologic therapy.
Major finding: Overall, subclinical inflammation was detected in 57.4% of the patients in complete remission or with lower disease activity. Factors negatively associated with subclinical inflammation included the use of biologic therapy (odds ratio [OR] 0.59; P = .001), methotrexate (OR 0.83; P = .020), and glucocorticoids (OR 0.60; P = .001) and alcohol consumption (OR 0.55; P = .006).
Study details: This cross-sectional study included 1248 patients with RA who underwent gray scale and power Doppler ultrasound assessments of the dorsal radiolunate joints of both wrists.
Disclosures: This study was supported by the Kaohsiung Chang Gung Memorial Hospital, Taiwan, and other sources. The authors declared no conflicts of interest.
Source: Wang YW et al. Factors associated with subclinical inflammation of wrist joints in rheumatoid arthritis patients with low or no disease activity—A RA ultrasound registry study. BMC Musculoskelet Disord. 2023;24:438 (May 30). doi: 10.1186/s12891-023-06521-8
Key clinical point: Ultrasound detected subclinical inflammation in the wrist joints of most patients with rheumatoid arthritis (RA) in clinical remission or with lower disease activity, with the risk for subclinical inflammation being lower among those using biologic therapy.
Major finding: Overall, subclinical inflammation was detected in 57.4% of the patients in complete remission or with lower disease activity. Factors negatively associated with subclinical inflammation included the use of biologic therapy (odds ratio [OR] 0.59; P = .001), methotrexate (OR 0.83; P = .020), and glucocorticoids (OR 0.60; P = .001) and alcohol consumption (OR 0.55; P = .006).
Study details: This cross-sectional study included 1248 patients with RA who underwent gray scale and power Doppler ultrasound assessments of the dorsal radiolunate joints of both wrists.
Disclosures: This study was supported by the Kaohsiung Chang Gung Memorial Hospital, Taiwan, and other sources. The authors declared no conflicts of interest.
Source: Wang YW et al. Factors associated with subclinical inflammation of wrist joints in rheumatoid arthritis patients with low or no disease activity—A RA ultrasound registry study. BMC Musculoskelet Disord. 2023;24:438 (May 30). doi: 10.1186/s12891-023-06521-8




