User login
Redosing Rituximab to Maintain ANCA Vasculitis Remission: When Is Best?
Maintaining remission in patients with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis who have kept their autoantibodies in check after at least 2 years on rituximab therapy has proved challenging, but a team of nephrologists in Boston have reported that a longer-term strategy that uses a rise in B-cell levels as a threshold for rituximab infusions may be the better of two strategies at reducing relapse risks.
“The bottom line is with the B-cell strategy, which is that rituximab was redosed when the B cells recovered or started to recover, we only have a 6% rate in relapses by 3 years,” senior study author John L. Niles, MD, assistant professor of medicine at the Harvard Medical School and director of the Vasculitis and Glomerulonephritis Center at Massachusetts General Hospital in Boston, Massachusetts, told Medscape Medical News.
“Whereas in the other strategy, we were waiting for a serologic relapse and hoping we could prevent clinical relapses, but we still have about 30% rate of relapse by 3 years.”
Dr. Niles and his associates reported their findings from the MAINTANCVAS study (for MAINTenance of ANCA VASculitis) December 11, 2023, in Annals of the Rheumatic Diseases. Their single-center study compared two different treatment strategies in patients with ANCA-associated vasculitis in remission after completing at least 2 years of fixed-schedule rituximab therapy: an approach that reinfused rituximab upon B-cell repopulation, called the B-cell arm and a strategy that reinfused rituximab when serologic levels of ANCA increased significantly, which they called the ANCA arm. A total of 115 patients were randomly assigned to either arm.
Study Results
Median follow-up was 4.1 years from study entry. Throughout the study, 5 of 58 patients in the B-cell arm and 14 of 57 in the ANCA arm had relapses. According to Kaplan-Meier analysis, at 3 years after study entry, 4.1% of patients in the B-cell arm had a relapse vs 20.5% of patients in the ANCA arm. At 5 years, the respective relapse rates were 11.3% and 27.7%. Overall, four major relapses occurred in the B-cell arm and seven in the ANCA arm.
The COVID-19 pandemic caused the researchers to halt the study before it was fully enrolled, Dr. Niles said. The study also attributed high rates of serious adverse events (SAEs) in the B-cell arm to cases of COVID-19 in that study population. The overall number of SAEs was identical in both arms: 22 (P = .95). But the B-cell arm had six cases of COVID-19 vs one in the ANCA arm, including two deaths because of COVID-19.
The study findings provided insight into how to best individualize treatment in patients with ANCA-associated vasculitis, Dr. Niles said. “We will typically start with the B-cell strategy after 2 years, but to the extent that people have infections or hypogammaglobulinemia, we’ll start stretching a little longer on the B cells, and if the level is too high in terms of infection, we’ll stop and switch to the ANCA strategy,” he said.
He added, “Relapsers get a more strict B-cell strategy, and people with infections get much longer intervals or even switch entirely to the ANCA strategy.”
Because the study ended before it was fully enrolled, it was underpowered for subgroup analyses, Dr. Niles noted. One such potential subgroup was relapsing patients with interstitial lung disease as the primary clinical finding. “The interstitial lung disease doesn’t seem to respond as well to therapy as the other classic features of ANCA disease,” Dr. Niles said. “It’s the one part that’s the most problematic for the long run. It behaves differently, and there’s going to need to be more research on ILD. Fortunately, it’s a fairly small percentage of the total group, but it’s the most difficult part of this disease.”
Findings in Context
This study brings clarity on how to best manage patients with ANCA-associated vasculitis, Robert Hylland, MD, an assistant clinical professor of rheumatology at Michigan State University College of Osteopathic Medicine, told this news organization.
“Most of us have tried to discern from the literature that exists how to manage [ANCA-associated vasculitis]. There have been a number of different approaches, and they have changed over the course of time,” Dr. Hylland said. “But now this article helps us to understand how to proceed with this disease after we have induced remission. The ability to determine the validity of serology vs B-cell depletion was brought out very nicely in this article.”
The size of the study population was a strength of the study, Dr. Hylland said.
He credited the study authors for providing insight into using positive myeloperoxidase (MPO)- or proteinase 3 (PR3)-ANCA readings to guide treatment for relapses. The study defined a serologic ANCA flare in the ANCA arm as a fivefold increase in MPO and a fourfold rise in PR3.
“Many of us wouldn’t have recognized that a less than fivefold increase, for example, in the MPO could be watched for a while, where most of us would have been treating that serologic flare,” Hylland said.
The study also highlighted the difficulty of evaluating a patient who has neither a positive ANCA nor a significant increase in their B-cell counts and yet still has clinical signs and symptoms of a relapse, such as with granulomatosis with polyangiitis, also known as Wegener’s granulomatosis.
“A lot of physicians tend to feel a little more relaxed when they see their patient is serologically doing well and yet, when they come in, some of the subtle symptoms of Wegener’s could be ignored if you don’t recognize that there’s a considerable number who will come to you with having had treatment and still have negative serology,” Hylland said.
The study had no specific outside funding source. Dr. Niles and Dr. Hylland report no relevant financial relationships. Two co-authors report financial relationships with pharmaceutical companies.
A version of this article appeared on Medscape.com.
Maintaining remission in patients with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis who have kept their autoantibodies in check after at least 2 years on rituximab therapy has proved challenging, but a team of nephrologists in Boston have reported that a longer-term strategy that uses a rise in B-cell levels as a threshold for rituximab infusions may be the better of two strategies at reducing relapse risks.
“The bottom line is with the B-cell strategy, which is that rituximab was redosed when the B cells recovered or started to recover, we only have a 6% rate in relapses by 3 years,” senior study author John L. Niles, MD, assistant professor of medicine at the Harvard Medical School and director of the Vasculitis and Glomerulonephritis Center at Massachusetts General Hospital in Boston, Massachusetts, told Medscape Medical News.
“Whereas in the other strategy, we were waiting for a serologic relapse and hoping we could prevent clinical relapses, but we still have about 30% rate of relapse by 3 years.”
Dr. Niles and his associates reported their findings from the MAINTANCVAS study (for MAINTenance of ANCA VASculitis) December 11, 2023, in Annals of the Rheumatic Diseases. Their single-center study compared two different treatment strategies in patients with ANCA-associated vasculitis in remission after completing at least 2 years of fixed-schedule rituximab therapy: an approach that reinfused rituximab upon B-cell repopulation, called the B-cell arm and a strategy that reinfused rituximab when serologic levels of ANCA increased significantly, which they called the ANCA arm. A total of 115 patients were randomly assigned to either arm.
Study Results
Median follow-up was 4.1 years from study entry. Throughout the study, 5 of 58 patients in the B-cell arm and 14 of 57 in the ANCA arm had relapses. According to Kaplan-Meier analysis, at 3 years after study entry, 4.1% of patients in the B-cell arm had a relapse vs 20.5% of patients in the ANCA arm. At 5 years, the respective relapse rates were 11.3% and 27.7%. Overall, four major relapses occurred in the B-cell arm and seven in the ANCA arm.
The COVID-19 pandemic caused the researchers to halt the study before it was fully enrolled, Dr. Niles said. The study also attributed high rates of serious adverse events (SAEs) in the B-cell arm to cases of COVID-19 in that study population. The overall number of SAEs was identical in both arms: 22 (P = .95). But the B-cell arm had six cases of COVID-19 vs one in the ANCA arm, including two deaths because of COVID-19.
The study findings provided insight into how to best individualize treatment in patients with ANCA-associated vasculitis, Dr. Niles said. “We will typically start with the B-cell strategy after 2 years, but to the extent that people have infections or hypogammaglobulinemia, we’ll start stretching a little longer on the B cells, and if the level is too high in terms of infection, we’ll stop and switch to the ANCA strategy,” he said.
He added, “Relapsers get a more strict B-cell strategy, and people with infections get much longer intervals or even switch entirely to the ANCA strategy.”
Because the study ended before it was fully enrolled, it was underpowered for subgroup analyses, Dr. Niles noted. One such potential subgroup was relapsing patients with interstitial lung disease as the primary clinical finding. “The interstitial lung disease doesn’t seem to respond as well to therapy as the other classic features of ANCA disease,” Dr. Niles said. “It’s the one part that’s the most problematic for the long run. It behaves differently, and there’s going to need to be more research on ILD. Fortunately, it’s a fairly small percentage of the total group, but it’s the most difficult part of this disease.”
Findings in Context
This study brings clarity on how to best manage patients with ANCA-associated vasculitis, Robert Hylland, MD, an assistant clinical professor of rheumatology at Michigan State University College of Osteopathic Medicine, told this news organization.
“Most of us have tried to discern from the literature that exists how to manage [ANCA-associated vasculitis]. There have been a number of different approaches, and they have changed over the course of time,” Dr. Hylland said. “But now this article helps us to understand how to proceed with this disease after we have induced remission. The ability to determine the validity of serology vs B-cell depletion was brought out very nicely in this article.”
The size of the study population was a strength of the study, Dr. Hylland said.
He credited the study authors for providing insight into using positive myeloperoxidase (MPO)- or proteinase 3 (PR3)-ANCA readings to guide treatment for relapses. The study defined a serologic ANCA flare in the ANCA arm as a fivefold increase in MPO and a fourfold rise in PR3.
“Many of us wouldn’t have recognized that a less than fivefold increase, for example, in the MPO could be watched for a while, where most of us would have been treating that serologic flare,” Hylland said.
The study also highlighted the difficulty of evaluating a patient who has neither a positive ANCA nor a significant increase in their B-cell counts and yet still has clinical signs and symptoms of a relapse, such as with granulomatosis with polyangiitis, also known as Wegener’s granulomatosis.
“A lot of physicians tend to feel a little more relaxed when they see their patient is serologically doing well and yet, when they come in, some of the subtle symptoms of Wegener’s could be ignored if you don’t recognize that there’s a considerable number who will come to you with having had treatment and still have negative serology,” Hylland said.
The study had no specific outside funding source. Dr. Niles and Dr. Hylland report no relevant financial relationships. Two co-authors report financial relationships with pharmaceutical companies.
A version of this article appeared on Medscape.com.
Maintaining remission in patients with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis who have kept their autoantibodies in check after at least 2 years on rituximab therapy has proved challenging, but a team of nephrologists in Boston have reported that a longer-term strategy that uses a rise in B-cell levels as a threshold for rituximab infusions may be the better of two strategies at reducing relapse risks.
“The bottom line is with the B-cell strategy, which is that rituximab was redosed when the B cells recovered or started to recover, we only have a 6% rate in relapses by 3 years,” senior study author John L. Niles, MD, assistant professor of medicine at the Harvard Medical School and director of the Vasculitis and Glomerulonephritis Center at Massachusetts General Hospital in Boston, Massachusetts, told Medscape Medical News.
“Whereas in the other strategy, we were waiting for a serologic relapse and hoping we could prevent clinical relapses, but we still have about 30% rate of relapse by 3 years.”
Dr. Niles and his associates reported their findings from the MAINTANCVAS study (for MAINTenance of ANCA VASculitis) December 11, 2023, in Annals of the Rheumatic Diseases. Their single-center study compared two different treatment strategies in patients with ANCA-associated vasculitis in remission after completing at least 2 years of fixed-schedule rituximab therapy: an approach that reinfused rituximab upon B-cell repopulation, called the B-cell arm and a strategy that reinfused rituximab when serologic levels of ANCA increased significantly, which they called the ANCA arm. A total of 115 patients were randomly assigned to either arm.
Study Results
Median follow-up was 4.1 years from study entry. Throughout the study, 5 of 58 patients in the B-cell arm and 14 of 57 in the ANCA arm had relapses. According to Kaplan-Meier analysis, at 3 years after study entry, 4.1% of patients in the B-cell arm had a relapse vs 20.5% of patients in the ANCA arm. At 5 years, the respective relapse rates were 11.3% and 27.7%. Overall, four major relapses occurred in the B-cell arm and seven in the ANCA arm.
The COVID-19 pandemic caused the researchers to halt the study before it was fully enrolled, Dr. Niles said. The study also attributed high rates of serious adverse events (SAEs) in the B-cell arm to cases of COVID-19 in that study population. The overall number of SAEs was identical in both arms: 22 (P = .95). But the B-cell arm had six cases of COVID-19 vs one in the ANCA arm, including two deaths because of COVID-19.
The study findings provided insight into how to best individualize treatment in patients with ANCA-associated vasculitis, Dr. Niles said. “We will typically start with the B-cell strategy after 2 years, but to the extent that people have infections or hypogammaglobulinemia, we’ll start stretching a little longer on the B cells, and if the level is too high in terms of infection, we’ll stop and switch to the ANCA strategy,” he said.
He added, “Relapsers get a more strict B-cell strategy, and people with infections get much longer intervals or even switch entirely to the ANCA strategy.”
Because the study ended before it was fully enrolled, it was underpowered for subgroup analyses, Dr. Niles noted. One such potential subgroup was relapsing patients with interstitial lung disease as the primary clinical finding. “The interstitial lung disease doesn’t seem to respond as well to therapy as the other classic features of ANCA disease,” Dr. Niles said. “It’s the one part that’s the most problematic for the long run. It behaves differently, and there’s going to need to be more research on ILD. Fortunately, it’s a fairly small percentage of the total group, but it’s the most difficult part of this disease.”
Findings in Context
This study brings clarity on how to best manage patients with ANCA-associated vasculitis, Robert Hylland, MD, an assistant clinical professor of rheumatology at Michigan State University College of Osteopathic Medicine, told this news organization.
“Most of us have tried to discern from the literature that exists how to manage [ANCA-associated vasculitis]. There have been a number of different approaches, and they have changed over the course of time,” Dr. Hylland said. “But now this article helps us to understand how to proceed with this disease after we have induced remission. The ability to determine the validity of serology vs B-cell depletion was brought out very nicely in this article.”
The size of the study population was a strength of the study, Dr. Hylland said.
He credited the study authors for providing insight into using positive myeloperoxidase (MPO)- or proteinase 3 (PR3)-ANCA readings to guide treatment for relapses. The study defined a serologic ANCA flare in the ANCA arm as a fivefold increase in MPO and a fourfold rise in PR3.
“Many of us wouldn’t have recognized that a less than fivefold increase, for example, in the MPO could be watched for a while, where most of us would have been treating that serologic flare,” Hylland said.
The study also highlighted the difficulty of evaluating a patient who has neither a positive ANCA nor a significant increase in their B-cell counts and yet still has clinical signs and symptoms of a relapse, such as with granulomatosis with polyangiitis, also known as Wegener’s granulomatosis.
“A lot of physicians tend to feel a little more relaxed when they see their patient is serologically doing well and yet, when they come in, some of the subtle symptoms of Wegener’s could be ignored if you don’t recognize that there’s a considerable number who will come to you with having had treatment and still have negative serology,” Hylland said.
The study had no specific outside funding source. Dr. Niles and Dr. Hylland report no relevant financial relationships. Two co-authors report financial relationships with pharmaceutical companies.
A version of this article appeared on Medscape.com.
FROM ANNALS OF THE RHEUMATIC DISEASES
How to Reduce Cardiovascular Morbidity and Mortality in Psoriasis and PsA
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.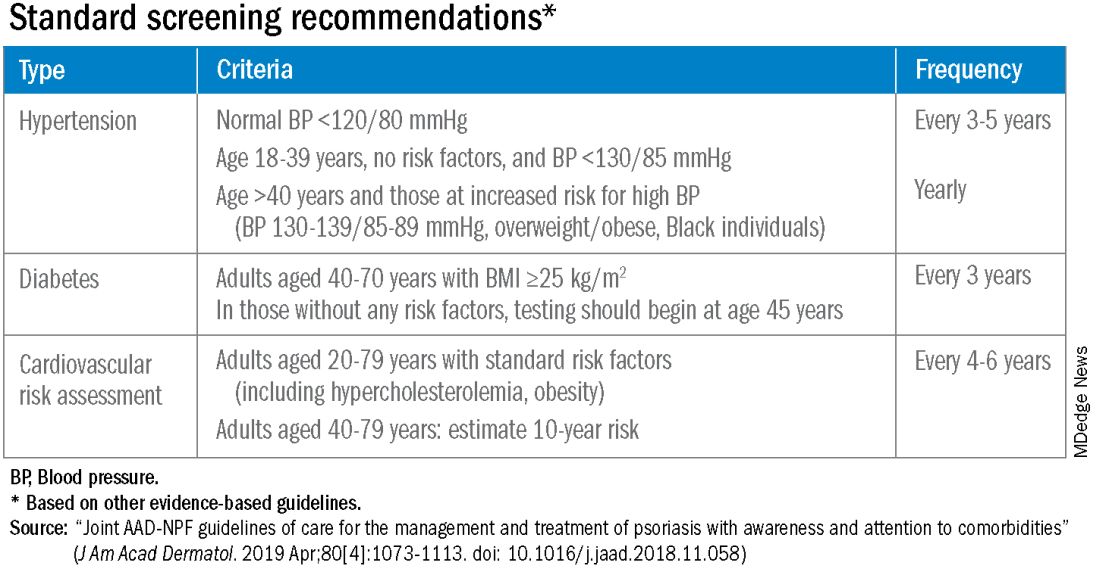
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Patients with psoriatic disease have significantly higher risks of myocardial infarction, stroke, and cardiovascular mortality than does the general population, yet research consistently paints what dermatologist Joel M. Gelfand, MD, calls an “abysmal” picture: Only a minority of patients with psoriatic disease know about their increased risks, only a minority of dermatologists and rheumatologists screen for cardiovascular risk factors like lipid levels and blood pressure, and only a minority of patients diagnosed with hyperlipidemia are adequately treated with statin therapy.
In the literature and at medical meetings, Dr. Gelfand and others who have studied cardiovascular disease (CVD) comorbidity and physician practices have been urging dermatologists and rheumatologists to play a more consistent and active role in primary cardiovascular prevention for patients with psoriatic disease, who are up to 50% more likely than patients without it to develop CVD and who tend to have atherosclerosis at earlier ages.
According to the 2019 joint American Academy of Dermatology (AAD)–National Psoriasis Foundation (NPF) guidelines for managing psoriasis “with awareness and attention to comorbidities,” this means not only ensuring that all patients with psoriasis receive standard CV risk assessment (screening for hypertension, diabetes, and hyperlipidemia), but also recognizing that patients who are candidates for systemic therapy or phototherapy — or who have psoriasis involving > 10% of body surface area — may benefit from earlier and more frequent screening.
CV risk and premature mortality rises with the severity of skin disease, and patients with psoriatic arthritis (PsA) are believed to have risk levels similar to patients with moderate-severe psoriasis, cardiologist Michael S. Garshick, MD, director of the cardio-rheumatology program at New York University Langone Health, said in an interview.
In a recent survey study of 100 patients seen at NYU Langone Health’s psoriasis specialty clinic, only one-third indicated they had been advised by their physicians to be screened for CV risk factors, and only one-third reported having been told of the connection between psoriasis and CVD risk. Dr. Garshick shared the unpublished findings at the annual research symposium of the NPF in October.
Similarly, data from the National Ambulatory Medical Care Survey shows that just 16% of psoriasis-related visits to dermatology providers from 2007 to 2016 involved screening for CV risk factors. Screening rates were 11% for body mass index, 7.4% for blood pressure, 2.9% for cholesterol, and 1.7% for glucose, Dr. Gelfand and coauthors reported in 2023. .
Such findings are concerning because research shows that fewer than a quarter of patients with psoriasis have a primary care visit within a year of establishing care with their physicians, and that, overall, fewer than half of commercially insured adults under age 65 visit a primary care physician each year, according to John S. Barbieri, MD, of the department of dermatology at Brigham and Women’s Hospital in Boston. He included these findings when reporting in 2022 on a survey study on CVD screening.
In many cases, dermatologists and rheumatologists may be the primary providers for patients with psoriatic disease. So, “the question is, how can the dermatologist or rheumatologist use their interactions as a touchpoint to improve the patient’s well-being?” Dr. Barbieri said in an interview.
For the dermatologist, educating patients about the higher CVD risk fits well into conversations about “how there may be inflammation inside the body as well as in the skin,” he said. “Talk about cardiovascular risk just as you talk about PsA risk.” Both specialists, he added, can incorporate blood pressure readings and look for opportunities to measure lipid levels and hemoglobin A1c (HbA1c). These labs can easily be integrated into a biologic work-up.
“The hard part — and this needs to be individualized — is how do you want to handle [abnormal readings]? Do you want to take on a lot of the ownership and calculate [10-year CVD] risk scores and then counsel patients accordingly?” Dr. Barbieri said. “Or do you want to try to refer, and encourage them to work with their PCP? There a high-touch version and a low-touch version of how you can turn screening into action, into a care plan.”
Beyond traditional risk elevation, the primary care hand-off
Rheumatologists “in general may be more apt to screen for cardiovascular disease” as a result of their internal medicine residency training, and “we’re generally more comfortable prescribing ... if we need to,” said Alexis R. Ogdie, MD, a rheumatologist at the Hospital of the University of Pennsylvania, Philadelphia, and director of the Penn Psoriatic Arthritis Clinic.
Referral to a preventive cardiologist for management of abnormal lab results or ongoing monitoring and prevention is ideal, but when hand-offs to primary care physicians are made — the more common scenario — education is important. “A common problem is that there is underrecognition of the cardiovascular risk being elevated in our patients,” she said, above and beyond risk posed by traditional risk factors such as dyslipidemia, hypertension, metabolic syndrome, and obesity, all of which have been shown to occur more frequently in patients with psoriatic disease than in the general population.
Risk stratification guides CVD prevention in the general population, and “if you use typical scores for cardiovascular risk, they may underestimate risk for our patients with PsA,” said Dr. Ogdie, who has reported on CV risk in patients with PsA. “Relative to what the patient’s perceived risk is, they may be treated similarly (to the general population). But relative to their actual risk, they’re undertreated.”
The 2019 AAD-NPF psoriasis guidelines recommend utilizing a 1.5 multiplication factor in risk score models, such as the American College of Cardiology’s Atherosclerotic Cardiovascular Disease (ASCVD) Risk Estimator, when the patient has a body surface area >10% or is a candidate for systemic therapy or phototherapy.
Similarly, the 2018 American Heart Association (AHA)-ACC Guideline on the Management of Blood Cholesterol defines psoriasis, along with RA, metabolic syndrome, HIV, and other diseases, as a “cardiovascular risk enhancer” that should be factored into assessments of ASCVD risk. (The guideline does not specify a psoriasis severity threshold.)
“It’s the first time the specialty [of cardiology] has said, ‘pay attention to a skin disease,’ ” Dr. Gelfand said at the NPF meeting.
Using the 1.5 multiplication factor, a patient who otherwise would be classified in the AHA/ACC guideline as “borderline risk,” with a 10-year ASCVD risk of 5% to <7.5%, would instead have an “intermediate” 10-year ASCVD risk of ≥7.5% to <20%. Application of the AHA-ACC “risk enhancer” would have a similar effect.
For management, the main impact of psoriasis being considered a risk enhancer is that “it lowers the threshold for treatment with standard cardiovascular prevention medications such as statins.”
In general, “we should be taking a more aggressive approach to the management of traditional cardiovascular risk factors” in patients with psoriatic disease, he said. Instead of telling a patient with mildly elevated blood pressure, ‘I’ll see you in a year or two,’ or a patient entering a prediabetic stage to “watch what you eat, and I’ll see you in a couple of years,” clinicians need to be more vigilant.
“It’s about recognizing that these traditional cardiometabolic risk factors, synergistically with psoriasis, can start enhancing CV risk at an earlier age than we might expect,” said Dr. Garshick, whose 2021 review of CV risk in psoriasis describes how the inflammatory milieu in psoriasis is linked to atherosclerosis development.
Cardiologists are aware of this, but “many primary care physicians are not. It takes time for medical knowledge to diffuse,” Dr. Gelfand said. “Tell the PCP, in notes or in a form letter, that there is a higher risk of CV disease, and reference the AHA/ACC guidelines,” he advised. “You don’t want your patient to go to their doctor and the doctor to [be uninformed].”
‘Patients trust us’
Dr. Gelfand has been at the forefront of research on psoriasis and heart disease. A study he coauthored in 2006, for instance, documented an independent risk of MI, with adjusted relative risks of 1.29 and 3.10 for a 30-year-old patient with mild or severe disease, respectively, and higher risks for a 60-year-old. In 2010, he and coinvestigators found that severe psoriasis was an independent risk factor for CV mortality (HR, 1.57) after adjusting for age, sex, smoking, diabetes, hypertension, and hyperlipidemia.
Today, along with Dr. Barbieri, Dr. Ogdie, and others, he is studying the feasibility and efficacy of a proposed national, “centralized care coordinator” model of care whereby dermatologists and rheumatologists would educate the patient, order lipid and HbA1c measurements as medically appropriate, and then refer patients as needed to a care coordinator. The care coordinator would calculate a 10-year CVD risk score and counsel the patient on possible next steps.
In a pilot study of 85 patients at four sites, 92% of patients followed through on their physician’s recommendations to have labs drawn, and 86% indicated the model was acceptable and feasible. A total of 27% of patients had “newly identified, previously undiagnosed, elevated cardiovascular disease risk,” and exploratory effectiveness results indicated a successful reduction in predicted CVD risk in patients who started statins, Dr. Gelfand reported at the NPF meeting.
With funding from the NPF, a larger, single-arm, pragmatic “CP3” trial (NCT05908240) is enrolling 525 patients with psoriasis at 10-20 academic and nonacademic dermatology sites across the United States to further test the model. The primary endpoint will be the change in LDL cholesterol measured at 6 months among people with a 10-year risk ≥5%. Secondary endpoints will cover improvement in disease severity and quality of life, behavior modification, patient experience, and other issues.
“We have only 10-15 minutes [with patients] ... a care coordinator who is empathetic and understanding and [informed] could make a big difference,” Dr. Gelfand said at the NPF meeting. If findings are positive, the model would be tested in rheumatology sites as well. The hope, he said, is that the NPF would be able to fund an in-house care coordinator(s) for the long-term.
Notably, a patient survey conducted as part of exploratory research leading up to the care coordinator project showed that patients trust their dermatologist or rheumatologist for CVD education and screening. Among 160 patients with psoriasis and 162 patients with PsA, 76% and 90% agreed that “I would like it if my dermatologist/rheumatologist educated me about my risk of heart disease,” and 60% and 75%, respectively, agree that “it would be convenient for me to have my cholesterol checked by my dermatologist/rheumatologist.”
“Patients trust us,” Dr. Gelfand said at the NPF meeting. “And the pilot study shows us that patients are motivated.”
Taking an individualized, holistic, longitudinal approach
“Sometimes you do have to triage bit,” Dr. Gelfand said in an interview. “For a young person with normal body weight who doesn’t smoke and has mild psoriasis, one could just educate and advise that they see their primary care physician” for monitoring.
“But for the same patient who is obese, maybe smokes, and doesn’t have a primary care physician, I’d order labs,” he said. “You don’t want a patient walking out the door with an [undiagnosed] LDL of 160 or hypertension.”
Age is also an important consideration, as excess CVD risk associated with autoimmune diseases like psoriasis rises with age, Dr. Gelfand said during a seminar on psoriasis and PsA held at NYU Langone in December. For a young person, typically, “I need to focus on education and lifestyle … setting them on a healthy lifestyle trajectory,” he said. “Once they get to 40, from 40 to 75 or so, that’s a sweet spot for medical intervention to lower cardiovascular risk.”
Even at older ages, however, lipid management is not the be-all and end-all, he said in the interview. “We have to be holistic.”
One advantage of having highly successful therapies for psoriasis, and to a lesser extent PsA, is the time that becomes available during follow-up visits — once disease is under control — to “focus on other things,” he said. Waiting until disease is under control to discuss diet, exercise, or smoking, for instance, makes sense anyway, he said. “You don’t want to overwhelm patients with too much to do at once.”
Indeed, said dermatologist Robert E. Kalb, MD, of the Buffalo Medical Group in Buffalo, NY, “patients have an open mind [about discussing cardiovascular disease risk], but it is not high on their radar. Most of them just want to get their skin clear.” (Dr. Kalb participated in the care coordinator pilot study, and said in an interview that since its completion, he has been more routinely ordering relevant labs.)
Rheumatologists are less fortunate with highly successful therapies, but “over the continuum of care, we do have time in office visits” to discuss issues like smoking, exercise, and lifestyle, Dr. Ogdie said. “I think of each of those pieces as part of our job.”
In the future, as researchers learn more about the impact of psoriasis and PsA treatments on CVD risk, it may be possible to tailor treatments or to prescribe treatments knowing that the therapies could reduce risk. Observational and epidemiologic data suggest that tumor necrosis factor-alpha inhibitor therapy over 3 years reduces the risk of MI, and that patients whose psoriasis is treated have reduced aortic inflammation, improved myocardial strain, and reduced coronary plaque burden, Dr. Garshick said at the NPF meeting.
“But when we look at the randomized controlled trials, they’re actually inconclusive that targeting inflammation in psoriatic disease reduces surrogates of cardiovascular disease,” he said. Dr. Garshick’s own research focuses on platelet and endothelial biology in psoriasis.
Dr. Barbieri reported he had no relevant disclosures. Dr. Garshick reported consulting fees from Bristol-Myers Squibb, Kiniksa, Horizon Therapeutics, and Agepha. Dr. Ogdie reported financial relationships with AbbVie, Amgen, Bristol-Myers Squibb, Celgene, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Novartis, Pfizer, Takeda, and UCB. Dr. Gelfand reported serving as a consultant for AbbVie, Artax, Bristol-Myers Squibb, GlaxoSmithKline, and other companies.
Slow-to-moderate weight loss better than rapid with antiobesity drugs in OA
TOPLINE:
Individuals with overweight or obesity and knee or hip osteoarthritis (OA) who used antiobesity medications and achieved slow-to-moderate weight loss had a lower risk for all-cause mortality than did those with weight gain or stable weight in a population-based cohort study emulating a randomized controlled trial. Patients who rapidly lost weight had mortality similar to those with weight gain or stable weight.
METHODOLOGY:
- The researchers used the IQVIA Medical Research Database to identify overweight or obese individuals with knee or hip OA; they conducted a hypothetical trial comparing the effects of slow-to-moderate weight loss (defined as 2%-10% of body weight) and rapid weight loss (defined as 5% or more of body weight) within 1 year of starting antiobesity medications.
- The final analysis included patients with a mean age of 60.9 years who met the criteria for treatment adherence to orlistat (n = 3028), sibutramine (n = 2919), or rimonabant (n = 797).
- The primary outcome was all-cause mortality over a 5-year follow-up period; secondary outcomes included hypertension, type 2 diabetes, and venous thromboembolism.
TAKEAWAY:
- All-cause mortality at 5 years was 5.3% with weight gain or stable weight, 4.0% with slow to moderate weight loss, and 5.4% with rapid weight loss.
- Hazard ratios for all-cause mortality were 0.72 (95% CI, 0.56-0.92) for slow to moderate weight loss and 0.99 (95% CI, 0.67-1.44) for the rapid weight loss group.
- Weight loss was associated with the secondary outcomes of reduced hypertension, type 2 diabetes, and venous thromboembolism in a dose-dependent manner.
- A slightly increased risk for cardiovascular disease occurred in the rapid weight loss group, compared with the weight gain or stable group, but this difference was not significant.
IN PRACTICE:
“Our finding that gradual weight loss by antiobesity medications lowers all-cause mortality, if confirmed by future studies, could guide policy-making and improve the well-being of patients with overweight or obesity and knee or hip OA,” the researchers wrote.
SOURCE:
The lead author on the study was Jie Wei, MD, of Central South University, Changsha, China. The study was published online in Arthritis & Rheumatology.
LIMITATIONS:
Study limitations included the inability to control for factors such as exercise, diet, and disease severity; the inability to assess the risk for cause-specific mortality; and the inability to account for the impact of pain reduction and improved function as a result of weight loss.
DISCLOSURES:
The study was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, the Natural Science Foundation of Hunan Province, the Central South University Innovation-Driven Research Programme, and the Science and Technology Innovation Program of Hunan Province. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Individuals with overweight or obesity and knee or hip osteoarthritis (OA) who used antiobesity medications and achieved slow-to-moderate weight loss had a lower risk for all-cause mortality than did those with weight gain or stable weight in a population-based cohort study emulating a randomized controlled trial. Patients who rapidly lost weight had mortality similar to those with weight gain or stable weight.
METHODOLOGY:
- The researchers used the IQVIA Medical Research Database to identify overweight or obese individuals with knee or hip OA; they conducted a hypothetical trial comparing the effects of slow-to-moderate weight loss (defined as 2%-10% of body weight) and rapid weight loss (defined as 5% or more of body weight) within 1 year of starting antiobesity medications.
- The final analysis included patients with a mean age of 60.9 years who met the criteria for treatment adherence to orlistat (n = 3028), sibutramine (n = 2919), or rimonabant (n = 797).
- The primary outcome was all-cause mortality over a 5-year follow-up period; secondary outcomes included hypertension, type 2 diabetes, and venous thromboembolism.
TAKEAWAY:
- All-cause mortality at 5 years was 5.3% with weight gain or stable weight, 4.0% with slow to moderate weight loss, and 5.4% with rapid weight loss.
- Hazard ratios for all-cause mortality were 0.72 (95% CI, 0.56-0.92) for slow to moderate weight loss and 0.99 (95% CI, 0.67-1.44) for the rapid weight loss group.
- Weight loss was associated with the secondary outcomes of reduced hypertension, type 2 diabetes, and venous thromboembolism in a dose-dependent manner.
- A slightly increased risk for cardiovascular disease occurred in the rapid weight loss group, compared with the weight gain or stable group, but this difference was not significant.
IN PRACTICE:
“Our finding that gradual weight loss by antiobesity medications lowers all-cause mortality, if confirmed by future studies, could guide policy-making and improve the well-being of patients with overweight or obesity and knee or hip OA,” the researchers wrote.
SOURCE:
The lead author on the study was Jie Wei, MD, of Central South University, Changsha, China. The study was published online in Arthritis & Rheumatology.
LIMITATIONS:
Study limitations included the inability to control for factors such as exercise, diet, and disease severity; the inability to assess the risk for cause-specific mortality; and the inability to account for the impact of pain reduction and improved function as a result of weight loss.
DISCLOSURES:
The study was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, the Natural Science Foundation of Hunan Province, the Central South University Innovation-Driven Research Programme, and the Science and Technology Innovation Program of Hunan Province. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Individuals with overweight or obesity and knee or hip osteoarthritis (OA) who used antiobesity medications and achieved slow-to-moderate weight loss had a lower risk for all-cause mortality than did those with weight gain or stable weight in a population-based cohort study emulating a randomized controlled trial. Patients who rapidly lost weight had mortality similar to those with weight gain or stable weight.
METHODOLOGY:
- The researchers used the IQVIA Medical Research Database to identify overweight or obese individuals with knee or hip OA; they conducted a hypothetical trial comparing the effects of slow-to-moderate weight loss (defined as 2%-10% of body weight) and rapid weight loss (defined as 5% or more of body weight) within 1 year of starting antiobesity medications.
- The final analysis included patients with a mean age of 60.9 years who met the criteria for treatment adherence to orlistat (n = 3028), sibutramine (n = 2919), or rimonabant (n = 797).
- The primary outcome was all-cause mortality over a 5-year follow-up period; secondary outcomes included hypertension, type 2 diabetes, and venous thromboembolism.
TAKEAWAY:
- All-cause mortality at 5 years was 5.3% with weight gain or stable weight, 4.0% with slow to moderate weight loss, and 5.4% with rapid weight loss.
- Hazard ratios for all-cause mortality were 0.72 (95% CI, 0.56-0.92) for slow to moderate weight loss and 0.99 (95% CI, 0.67-1.44) for the rapid weight loss group.
- Weight loss was associated with the secondary outcomes of reduced hypertension, type 2 diabetes, and venous thromboembolism in a dose-dependent manner.
- A slightly increased risk for cardiovascular disease occurred in the rapid weight loss group, compared with the weight gain or stable group, but this difference was not significant.
IN PRACTICE:
“Our finding that gradual weight loss by antiobesity medications lowers all-cause mortality, if confirmed by future studies, could guide policy-making and improve the well-being of patients with overweight or obesity and knee or hip OA,” the researchers wrote.
SOURCE:
The lead author on the study was Jie Wei, MD, of Central South University, Changsha, China. The study was published online in Arthritis & Rheumatology.
LIMITATIONS:
Study limitations included the inability to control for factors such as exercise, diet, and disease severity; the inability to assess the risk for cause-specific mortality; and the inability to account for the impact of pain reduction and improved function as a result of weight loss.
DISCLOSURES:
The study was supported by the National Key Research and Development Plan, the National Natural Science Foundation of China, the Project Program of National Clinical Research Center for Geriatric Disorders, the Natural Science Foundation of Hunan Province, the Central South University Innovation-Driven Research Programme, and the Science and Technology Innovation Program of Hunan Province. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Researchers making strides to better understand RA-associated interstitial lung disease
SAN DIEGO — Clinically significant interstitial lung disease (ILD) is believed to occur in 5%-10% of patients with rheumatoid arthritis (RA), but robust data are lacking on how to best predict which patients face the highest risk for RA-associated ILD. However, the results of several studies presented at the American College of Rheumatology annual meeting indicate that researchers are making strides in this field of rheumatologic care.
Adding Genetic Factors Improves ILD Risk Prediction
In the realm of risk stratification, Austin M. Wheeler, MD, a rheumatology fellow at the University of Nebraska Medical Center, Omaha, discussed the development and validation of a combined clinical and genetic risk score for ILD. “There is clear and well documented phenotypic and genetic overlap of ILD with idiopathic pulmonary fibrosis (IPF),” Dr. Wheeler said. “A number of clinical risk factors have been described for RA-ILD, including older age, male sex, smoking history, higher disease activity, and seropositivity. There are also well-documented genetic risk factors for RA-ILD. The MUC5B genetic variant is the strongest risk factor for IPF, and it’s been described in RA-ILD as well.”
A recently published study indicated that a genetic risk score without the MUC5B variant improved predictive ability for IPF and interstitial lung abnormalities better than using the MUC5B variant alone, “but no prior attempts have been made at developing a composite genetic risk score in RA-ILD” using both genetic and clinical risk factors, he said.
For the current study, Dr. Wheeler and colleagues drew from 2,386 participants in the Veterans Affairs Rheumatoid Arthritis (VARA) Registry, a multicenter, prospective cohort of US veterans with rheumatologist-diagnosed RA and who fulfilled the 1987 ACR classification criteria. The researchers validated ILD through a systematic review of medical records, including clinical diagnosis of ILD plus either imaging or lung biopsy findings, and collected whole genome data that included 12 single nucleotide polymorphisms (SNPs) previously identified to be associated with risk for RA-ILD. They then used a meta-analytic approach to create pooled associations for each of those respective SNPs using data from the VARA registry participants as well as participants from the past study where the SNPs were first identified. “Those pooled associations were what we used for our effects size within the genetic risk score,” which ended up using five of the SNPs, Dr. Wheeler explained. Next, he and his colleagues combined the genetic risk score with clinical risk factors including age, sex, smoking history, disease activity, and rheumatoid factor (RF) positivity to create their combined risk score.
The mean age of the cohort was 70 years, 89% were male, 78% had a smoking history, and 78% were anti–cyclic citrullinated peptide (CCP) antibody positive. Of the 2,386 participants, 224 (9.4%) had RA-ILD. The full composite risk score had the highest area under the receiver operating curve (AUC) of 0.67, compared with an AUC of 0.623 using the clinical factors alone, 0.651 using the clinical factors plus only the MUC5B variant, and 0.654 using the composite score minus only the MUC5B variant. These AUCs show that “the combined risk score performs better than clinical factors even without the inclusion of the MUC5B variant in the score, which is notable because it supports the importance of further investigation into polygenic risk scores in RA-ILD as there is clearly more at play in a patient’s overall genetic risk,” Dr. Wheeler said.
As an example of the composite score’s ability to discriminate between people with and without RA-ILD, a cutpoint of 0.05 gave a sensitivity of 90.2% and would have eliminated about 25% of the cohort from unnecessary high-resolution CT scans and pulmonary function tests, he said.
“This study demonstrates the potential utility of genetic risk scores in RA-ILD identification and supports further investigation into individual risk stratification and screening,” he concluded. “This isn’t ready for clinical applicability by any means, but I think it serves as a proof of concept of the idea of a genetic risk score in RA-ILD.”
Biomarker Score Investigated
In a separate abstract, Brent Luedders, MD, assistant professor of rheumatology and immunology at the University of Nebraska Medical Center, and colleagues set out to determine if a previously derived biomarker score is associated with prevalent and incident ILD in the same VARA Registry cohort. An abstract presented at the ACR 2022 annual meeting found that a panel derived from IPF peripheral biomarkers was significantly associated with RA-ILD, including matrix metalloproteinase (MMP)-2, -7, and -9, eotaxin, macrophage-derived chemokine (MDC), monocyte chemoattractant protein-1 (MCP-1), fms-like tyrosine kinase 3 ligand (Flt3L) and interleukin-8 (IL-8). For the current analysis, Dr. Luedders and colleagues measured the concentrations of seven biomarkers (MMP-7, MMP-9, eotaxin, MDC, MCP-1, Flt3L, IL-8) from serum/plasma samples collected from VARA’s participants at enrollment to develop a score based on the concentrations of each biomarker.
Baseline characteristics were similar between the groups, although those with prevalent RA-ILD were slightly older than those without ILD, and those who developed incident ILD during follow-up had slightly higher RA disease activity at the time of enrollment. When the researchers examined the association of the biomarker score with prevalent RA-ILD as a continuous measure, they found an adjusted OR of 1.08 for prevalent RA-ILD for each 1-point increase in the biomarker score. “When this was divided into quartiles, we found that the highest quartile of the biomarker score was associated with an adjusted odds ratio of 2.31 for prevalent RA-ILD,” Dr. Luedders said. “We saw a significant P for trend of < .001, suggesting a dose-response relationship, in which higher scores had higher risk.” Similar associations were observed for incident RA-ILD, in which participants with the highest quartile had an adjusted hazard ratio of 2.26 for incident RA-ILD.
The AUC of 0.653 that was obtained with clinical factors did not significantly improve with inclusion of the biomarker score, rising to only 0.669. “In receiver operating characteristic analysis, the addition of the biomarker score to clinical variables (age, sex, race, smoking status, anti-CCP positivity, and RA disease activity by DAS28) did not lead to a significant increase in the area under the curve. Therefore, further work is needed to identify combinations of clinical, biomarker, and other factors to accurately predict which people with RA will develop ILD,” he said.
Dr. Luedders acknowledged certain limitations of the results, including the fact that MMP-2 was not measured in this cohort and thus not included in the score. “This was an observational study with usual care; therefore, the absence of systemic evaluation for ILD may miss early or mild RA-ILD cases,” he added. “Similarly, a male predominance may limit the generalizability, and we have limited information on the RA-ILD pattern.” He concluded that the study results “support the shared pathogenesis of IPF and RA-ILD. However, we found that this score has limited discriminative performance, compared to clinical risk factors alone.”
Drilling Down on ILD Subtypes
In a poster abstract presentation at the meeting, Gregory Campbell McDermott, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, highlighted results from a study that investigated differences in demographic, serologic, and lifestyle factors for RA-ILD and the major subtypes of RA-ILD: usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP). “Historically, RA-ILD has been studied as a single entity, even though we increasingly recognized that there are lots of different subtypes that fall under the umbrella of RA-ILD,” Dr. McDermott said in an interview. “We are also learning that the different subtypes probably have both prognostic and potentially therapeutic implications. For example, the UIP subtype, which is the most fibrotic subtype, has the worst prognosis but also may be a potential target for antifibrotic therapies. We’ve been trying to see if we can identify factors that are associated with specific subtypes, in particular the UIP subtype which has the worst prognosis.”
He and his colleagues examined 208 patients with RA-ILD with a mean age of 51 years and 547 patients with RA but no ILD with a mean age of 49 years from two RA cohorts comprising 3,328 patients: the Mass General Brigham Biobank RA Cohort and the Brigham RA Sequential Study (BRASS). Of the 208 RA-ILD cases, nearly half (48%) were RA-UIP, 18% were RA-NSIP, 8% were organizing pneumonia, 3% were respiratory bronchiolitis-ILD, and 23% were other/indeterminate. After conducting multivariable adjusted analyses, the researchers found that RA-ILD was associated with male sex (OR, 1.58; 95% CI, 1.09-2.23), seropositivity for RF and/or anti-CCP (OR, 2.22; 95% CI, 1.51-3.24) and being an ever smoker (OR, 1.70; 95% CI, 1.13-2.54). Having all three of these risk factors was strongly associated with RA-ILD (OR, 6.04; 95% CI, 2.92-12.47) and with RA-UIP in particular (OR, 7.1). “We found that a lot of the traditional RA-ILD risk factors like male sex, history of smoking, and seropositive status were most strongly associated with a UIP pattern,” Dr. McDermott said. “We think this is a first step in trying to understand how these different ILD subtypes may have different risk factors, pathogenesis, and potentially different treatments, prevention, and screening strategies.”
While clinicians wait for guidelines on systemic autoimmune rheumatic disease-associated ILD that are expected to be published by the ACR in 2024, he added that “we probably shouldn’t screen every single person with RA for ILD, but we need to identify people who have symptoms or findings on clinical exam. This study wasn’t designed to look specifically at who is at high risk, but I think we are moving toward that question: Who is high risk, and who’s asymptomatic [but] may need more screening?”
He pointed out limitations of the study, including its retrospective design and the fact that imaging was done for clinical purposes, “so it’s probably a higher risk group to begin with than the whole RA population,” he said. “We also didn’t have data on RA disease activity or erosions, some of these other measures that we think are important for understanding the full RA disease phenotype in these patients.”
Dr. Wheeler reported having no disclosures. Dr. Luedders reported that his study was supported by the VA, the Rheumatology Research Foundation, and the University of Nebraska Medical Center Mentored Scholars Program. Dr. McDermott reported that his study was supported by the Rheumatology Research Foundation.
SAN DIEGO — Clinically significant interstitial lung disease (ILD) is believed to occur in 5%-10% of patients with rheumatoid arthritis (RA), but robust data are lacking on how to best predict which patients face the highest risk for RA-associated ILD. However, the results of several studies presented at the American College of Rheumatology annual meeting indicate that researchers are making strides in this field of rheumatologic care.
Adding Genetic Factors Improves ILD Risk Prediction
In the realm of risk stratification, Austin M. Wheeler, MD, a rheumatology fellow at the University of Nebraska Medical Center, Omaha, discussed the development and validation of a combined clinical and genetic risk score for ILD. “There is clear and well documented phenotypic and genetic overlap of ILD with idiopathic pulmonary fibrosis (IPF),” Dr. Wheeler said. “A number of clinical risk factors have been described for RA-ILD, including older age, male sex, smoking history, higher disease activity, and seropositivity. There are also well-documented genetic risk factors for RA-ILD. The MUC5B genetic variant is the strongest risk factor for IPF, and it’s been described in RA-ILD as well.”
A recently published study indicated that a genetic risk score without the MUC5B variant improved predictive ability for IPF and interstitial lung abnormalities better than using the MUC5B variant alone, “but no prior attempts have been made at developing a composite genetic risk score in RA-ILD” using both genetic and clinical risk factors, he said.
For the current study, Dr. Wheeler and colleagues drew from 2,386 participants in the Veterans Affairs Rheumatoid Arthritis (VARA) Registry, a multicenter, prospective cohort of US veterans with rheumatologist-diagnosed RA and who fulfilled the 1987 ACR classification criteria. The researchers validated ILD through a systematic review of medical records, including clinical diagnosis of ILD plus either imaging or lung biopsy findings, and collected whole genome data that included 12 single nucleotide polymorphisms (SNPs) previously identified to be associated with risk for RA-ILD. They then used a meta-analytic approach to create pooled associations for each of those respective SNPs using data from the VARA registry participants as well as participants from the past study where the SNPs were first identified. “Those pooled associations were what we used for our effects size within the genetic risk score,” which ended up using five of the SNPs, Dr. Wheeler explained. Next, he and his colleagues combined the genetic risk score with clinical risk factors including age, sex, smoking history, disease activity, and rheumatoid factor (RF) positivity to create their combined risk score.
The mean age of the cohort was 70 years, 89% were male, 78% had a smoking history, and 78% were anti–cyclic citrullinated peptide (CCP) antibody positive. Of the 2,386 participants, 224 (9.4%) had RA-ILD. The full composite risk score had the highest area under the receiver operating curve (AUC) of 0.67, compared with an AUC of 0.623 using the clinical factors alone, 0.651 using the clinical factors plus only the MUC5B variant, and 0.654 using the composite score minus only the MUC5B variant. These AUCs show that “the combined risk score performs better than clinical factors even without the inclusion of the MUC5B variant in the score, which is notable because it supports the importance of further investigation into polygenic risk scores in RA-ILD as there is clearly more at play in a patient’s overall genetic risk,” Dr. Wheeler said.
As an example of the composite score’s ability to discriminate between people with and without RA-ILD, a cutpoint of 0.05 gave a sensitivity of 90.2% and would have eliminated about 25% of the cohort from unnecessary high-resolution CT scans and pulmonary function tests, he said.
“This study demonstrates the potential utility of genetic risk scores in RA-ILD identification and supports further investigation into individual risk stratification and screening,” he concluded. “This isn’t ready for clinical applicability by any means, but I think it serves as a proof of concept of the idea of a genetic risk score in RA-ILD.”
Biomarker Score Investigated
In a separate abstract, Brent Luedders, MD, assistant professor of rheumatology and immunology at the University of Nebraska Medical Center, and colleagues set out to determine if a previously derived biomarker score is associated with prevalent and incident ILD in the same VARA Registry cohort. An abstract presented at the ACR 2022 annual meeting found that a panel derived from IPF peripheral biomarkers was significantly associated with RA-ILD, including matrix metalloproteinase (MMP)-2, -7, and -9, eotaxin, macrophage-derived chemokine (MDC), monocyte chemoattractant protein-1 (MCP-1), fms-like tyrosine kinase 3 ligand (Flt3L) and interleukin-8 (IL-8). For the current analysis, Dr. Luedders and colleagues measured the concentrations of seven biomarkers (MMP-7, MMP-9, eotaxin, MDC, MCP-1, Flt3L, IL-8) from serum/plasma samples collected from VARA’s participants at enrollment to develop a score based on the concentrations of each biomarker.
Baseline characteristics were similar between the groups, although those with prevalent RA-ILD were slightly older than those without ILD, and those who developed incident ILD during follow-up had slightly higher RA disease activity at the time of enrollment. When the researchers examined the association of the biomarker score with prevalent RA-ILD as a continuous measure, they found an adjusted OR of 1.08 for prevalent RA-ILD for each 1-point increase in the biomarker score. “When this was divided into quartiles, we found that the highest quartile of the biomarker score was associated with an adjusted odds ratio of 2.31 for prevalent RA-ILD,” Dr. Luedders said. “We saw a significant P for trend of < .001, suggesting a dose-response relationship, in which higher scores had higher risk.” Similar associations were observed for incident RA-ILD, in which participants with the highest quartile had an adjusted hazard ratio of 2.26 for incident RA-ILD.
The AUC of 0.653 that was obtained with clinical factors did not significantly improve with inclusion of the biomarker score, rising to only 0.669. “In receiver operating characteristic analysis, the addition of the biomarker score to clinical variables (age, sex, race, smoking status, anti-CCP positivity, and RA disease activity by DAS28) did not lead to a significant increase in the area under the curve. Therefore, further work is needed to identify combinations of clinical, biomarker, and other factors to accurately predict which people with RA will develop ILD,” he said.
Dr. Luedders acknowledged certain limitations of the results, including the fact that MMP-2 was not measured in this cohort and thus not included in the score. “This was an observational study with usual care; therefore, the absence of systemic evaluation for ILD may miss early or mild RA-ILD cases,” he added. “Similarly, a male predominance may limit the generalizability, and we have limited information on the RA-ILD pattern.” He concluded that the study results “support the shared pathogenesis of IPF and RA-ILD. However, we found that this score has limited discriminative performance, compared to clinical risk factors alone.”
Drilling Down on ILD Subtypes
In a poster abstract presentation at the meeting, Gregory Campbell McDermott, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, highlighted results from a study that investigated differences in demographic, serologic, and lifestyle factors for RA-ILD and the major subtypes of RA-ILD: usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP). “Historically, RA-ILD has been studied as a single entity, even though we increasingly recognized that there are lots of different subtypes that fall under the umbrella of RA-ILD,” Dr. McDermott said in an interview. “We are also learning that the different subtypes probably have both prognostic and potentially therapeutic implications. For example, the UIP subtype, which is the most fibrotic subtype, has the worst prognosis but also may be a potential target for antifibrotic therapies. We’ve been trying to see if we can identify factors that are associated with specific subtypes, in particular the UIP subtype which has the worst prognosis.”
He and his colleagues examined 208 patients with RA-ILD with a mean age of 51 years and 547 patients with RA but no ILD with a mean age of 49 years from two RA cohorts comprising 3,328 patients: the Mass General Brigham Biobank RA Cohort and the Brigham RA Sequential Study (BRASS). Of the 208 RA-ILD cases, nearly half (48%) were RA-UIP, 18% were RA-NSIP, 8% were organizing pneumonia, 3% were respiratory bronchiolitis-ILD, and 23% were other/indeterminate. After conducting multivariable adjusted analyses, the researchers found that RA-ILD was associated with male sex (OR, 1.58; 95% CI, 1.09-2.23), seropositivity for RF and/or anti-CCP (OR, 2.22; 95% CI, 1.51-3.24) and being an ever smoker (OR, 1.70; 95% CI, 1.13-2.54). Having all three of these risk factors was strongly associated with RA-ILD (OR, 6.04; 95% CI, 2.92-12.47) and with RA-UIP in particular (OR, 7.1). “We found that a lot of the traditional RA-ILD risk factors like male sex, history of smoking, and seropositive status were most strongly associated with a UIP pattern,” Dr. McDermott said. “We think this is a first step in trying to understand how these different ILD subtypes may have different risk factors, pathogenesis, and potentially different treatments, prevention, and screening strategies.”
While clinicians wait for guidelines on systemic autoimmune rheumatic disease-associated ILD that are expected to be published by the ACR in 2024, he added that “we probably shouldn’t screen every single person with RA for ILD, but we need to identify people who have symptoms or findings on clinical exam. This study wasn’t designed to look specifically at who is at high risk, but I think we are moving toward that question: Who is high risk, and who’s asymptomatic [but] may need more screening?”
He pointed out limitations of the study, including its retrospective design and the fact that imaging was done for clinical purposes, “so it’s probably a higher risk group to begin with than the whole RA population,” he said. “We also didn’t have data on RA disease activity or erosions, some of these other measures that we think are important for understanding the full RA disease phenotype in these patients.”
Dr. Wheeler reported having no disclosures. Dr. Luedders reported that his study was supported by the VA, the Rheumatology Research Foundation, and the University of Nebraska Medical Center Mentored Scholars Program. Dr. McDermott reported that his study was supported by the Rheumatology Research Foundation.
SAN DIEGO — Clinically significant interstitial lung disease (ILD) is believed to occur in 5%-10% of patients with rheumatoid arthritis (RA), but robust data are lacking on how to best predict which patients face the highest risk for RA-associated ILD. However, the results of several studies presented at the American College of Rheumatology annual meeting indicate that researchers are making strides in this field of rheumatologic care.
Adding Genetic Factors Improves ILD Risk Prediction
In the realm of risk stratification, Austin M. Wheeler, MD, a rheumatology fellow at the University of Nebraska Medical Center, Omaha, discussed the development and validation of a combined clinical and genetic risk score for ILD. “There is clear and well documented phenotypic and genetic overlap of ILD with idiopathic pulmonary fibrosis (IPF),” Dr. Wheeler said. “A number of clinical risk factors have been described for RA-ILD, including older age, male sex, smoking history, higher disease activity, and seropositivity. There are also well-documented genetic risk factors for RA-ILD. The MUC5B genetic variant is the strongest risk factor for IPF, and it’s been described in RA-ILD as well.”
A recently published study indicated that a genetic risk score without the MUC5B variant improved predictive ability for IPF and interstitial lung abnormalities better than using the MUC5B variant alone, “but no prior attempts have been made at developing a composite genetic risk score in RA-ILD” using both genetic and clinical risk factors, he said.
For the current study, Dr. Wheeler and colleagues drew from 2,386 participants in the Veterans Affairs Rheumatoid Arthritis (VARA) Registry, a multicenter, prospective cohort of US veterans with rheumatologist-diagnosed RA and who fulfilled the 1987 ACR classification criteria. The researchers validated ILD through a systematic review of medical records, including clinical diagnosis of ILD plus either imaging or lung biopsy findings, and collected whole genome data that included 12 single nucleotide polymorphisms (SNPs) previously identified to be associated with risk for RA-ILD. They then used a meta-analytic approach to create pooled associations for each of those respective SNPs using data from the VARA registry participants as well as participants from the past study where the SNPs were first identified. “Those pooled associations were what we used for our effects size within the genetic risk score,” which ended up using five of the SNPs, Dr. Wheeler explained. Next, he and his colleagues combined the genetic risk score with clinical risk factors including age, sex, smoking history, disease activity, and rheumatoid factor (RF) positivity to create their combined risk score.
The mean age of the cohort was 70 years, 89% were male, 78% had a smoking history, and 78% were anti–cyclic citrullinated peptide (CCP) antibody positive. Of the 2,386 participants, 224 (9.4%) had RA-ILD. The full composite risk score had the highest area under the receiver operating curve (AUC) of 0.67, compared with an AUC of 0.623 using the clinical factors alone, 0.651 using the clinical factors plus only the MUC5B variant, and 0.654 using the composite score minus only the MUC5B variant. These AUCs show that “the combined risk score performs better than clinical factors even without the inclusion of the MUC5B variant in the score, which is notable because it supports the importance of further investigation into polygenic risk scores in RA-ILD as there is clearly more at play in a patient’s overall genetic risk,” Dr. Wheeler said.
As an example of the composite score’s ability to discriminate between people with and without RA-ILD, a cutpoint of 0.05 gave a sensitivity of 90.2% and would have eliminated about 25% of the cohort from unnecessary high-resolution CT scans and pulmonary function tests, he said.
“This study demonstrates the potential utility of genetic risk scores in RA-ILD identification and supports further investigation into individual risk stratification and screening,” he concluded. “This isn’t ready for clinical applicability by any means, but I think it serves as a proof of concept of the idea of a genetic risk score in RA-ILD.”
Biomarker Score Investigated
In a separate abstract, Brent Luedders, MD, assistant professor of rheumatology and immunology at the University of Nebraska Medical Center, and colleagues set out to determine if a previously derived biomarker score is associated with prevalent and incident ILD in the same VARA Registry cohort. An abstract presented at the ACR 2022 annual meeting found that a panel derived from IPF peripheral biomarkers was significantly associated with RA-ILD, including matrix metalloproteinase (MMP)-2, -7, and -9, eotaxin, macrophage-derived chemokine (MDC), monocyte chemoattractant protein-1 (MCP-1), fms-like tyrosine kinase 3 ligand (Flt3L) and interleukin-8 (IL-8). For the current analysis, Dr. Luedders and colleagues measured the concentrations of seven biomarkers (MMP-7, MMP-9, eotaxin, MDC, MCP-1, Flt3L, IL-8) from serum/plasma samples collected from VARA’s participants at enrollment to develop a score based on the concentrations of each biomarker.
Baseline characteristics were similar between the groups, although those with prevalent RA-ILD were slightly older than those without ILD, and those who developed incident ILD during follow-up had slightly higher RA disease activity at the time of enrollment. When the researchers examined the association of the biomarker score with prevalent RA-ILD as a continuous measure, they found an adjusted OR of 1.08 for prevalent RA-ILD for each 1-point increase in the biomarker score. “When this was divided into quartiles, we found that the highest quartile of the biomarker score was associated with an adjusted odds ratio of 2.31 for prevalent RA-ILD,” Dr. Luedders said. “We saw a significant P for trend of < .001, suggesting a dose-response relationship, in which higher scores had higher risk.” Similar associations were observed for incident RA-ILD, in which participants with the highest quartile had an adjusted hazard ratio of 2.26 for incident RA-ILD.
The AUC of 0.653 that was obtained with clinical factors did not significantly improve with inclusion of the biomarker score, rising to only 0.669. “In receiver operating characteristic analysis, the addition of the biomarker score to clinical variables (age, sex, race, smoking status, anti-CCP positivity, and RA disease activity by DAS28) did not lead to a significant increase in the area under the curve. Therefore, further work is needed to identify combinations of clinical, biomarker, and other factors to accurately predict which people with RA will develop ILD,” he said.
Dr. Luedders acknowledged certain limitations of the results, including the fact that MMP-2 was not measured in this cohort and thus not included in the score. “This was an observational study with usual care; therefore, the absence of systemic evaluation for ILD may miss early or mild RA-ILD cases,” he added. “Similarly, a male predominance may limit the generalizability, and we have limited information on the RA-ILD pattern.” He concluded that the study results “support the shared pathogenesis of IPF and RA-ILD. However, we found that this score has limited discriminative performance, compared to clinical risk factors alone.”
Drilling Down on ILD Subtypes
In a poster abstract presentation at the meeting, Gregory Campbell McDermott, MD, MPH, a rheumatologist at Brigham and Women’s Hospital, Boston, highlighted results from a study that investigated differences in demographic, serologic, and lifestyle factors for RA-ILD and the major subtypes of RA-ILD: usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP). “Historically, RA-ILD has been studied as a single entity, even though we increasingly recognized that there are lots of different subtypes that fall under the umbrella of RA-ILD,” Dr. McDermott said in an interview. “We are also learning that the different subtypes probably have both prognostic and potentially therapeutic implications. For example, the UIP subtype, which is the most fibrotic subtype, has the worst prognosis but also may be a potential target for antifibrotic therapies. We’ve been trying to see if we can identify factors that are associated with specific subtypes, in particular the UIP subtype which has the worst prognosis.”
He and his colleagues examined 208 patients with RA-ILD with a mean age of 51 years and 547 patients with RA but no ILD with a mean age of 49 years from two RA cohorts comprising 3,328 patients: the Mass General Brigham Biobank RA Cohort and the Brigham RA Sequential Study (BRASS). Of the 208 RA-ILD cases, nearly half (48%) were RA-UIP, 18% were RA-NSIP, 8% were organizing pneumonia, 3% were respiratory bronchiolitis-ILD, and 23% were other/indeterminate. After conducting multivariable adjusted analyses, the researchers found that RA-ILD was associated with male sex (OR, 1.58; 95% CI, 1.09-2.23), seropositivity for RF and/or anti-CCP (OR, 2.22; 95% CI, 1.51-3.24) and being an ever smoker (OR, 1.70; 95% CI, 1.13-2.54). Having all three of these risk factors was strongly associated with RA-ILD (OR, 6.04; 95% CI, 2.92-12.47) and with RA-UIP in particular (OR, 7.1). “We found that a lot of the traditional RA-ILD risk factors like male sex, history of smoking, and seropositive status were most strongly associated with a UIP pattern,” Dr. McDermott said. “We think this is a first step in trying to understand how these different ILD subtypes may have different risk factors, pathogenesis, and potentially different treatments, prevention, and screening strategies.”
While clinicians wait for guidelines on systemic autoimmune rheumatic disease-associated ILD that are expected to be published by the ACR in 2024, he added that “we probably shouldn’t screen every single person with RA for ILD, but we need to identify people who have symptoms or findings on clinical exam. This study wasn’t designed to look specifically at who is at high risk, but I think we are moving toward that question: Who is high risk, and who’s asymptomatic [but] may need more screening?”
He pointed out limitations of the study, including its retrospective design and the fact that imaging was done for clinical purposes, “so it’s probably a higher risk group to begin with than the whole RA population,” he said. “We also didn’t have data on RA disease activity or erosions, some of these other measures that we think are important for understanding the full RA disease phenotype in these patients.”
Dr. Wheeler reported having no disclosures. Dr. Luedders reported that his study was supported by the VA, the Rheumatology Research Foundation, and the University of Nebraska Medical Center Mentored Scholars Program. Dr. McDermott reported that his study was supported by the Rheumatology Research Foundation.
FROM ACR 2023
Anti-Rheumatic Drugs Linked to Reduced Thyroid Disease Incidence
TOPLINE:
Patients with rheumatoid arthritis (RA) in a large Swedish population cohort show a reduced incidence of autoimmune thyroid diseases, such as hypothyroidism or hyperthyroidism, after being diagnosed with RA, with the effect being more pronounced among those treated with disease-modifying anti-rheumatic drugs (DMARDs), particularly TNF-inhibitors.
Although DMARDs are commonly used in the treatment of RA, the drugs are rarely used to treat autoimmune thyroid diseases. The new results support theories raised in previous smaller studies that DMARDs could have a protective effect against thyroid disease.
METHODOLOGY:
- The study involved 13,731 patients with new-onset RA who were listed in the Swedish Rheumatology Quality Register between 2006 and 2018.
- The patients were matched for sex, age, and residential area with up to five reference individuals in the general population of 63,201 comparators.
- Overall, patients with RA were 64.7% female, with a mean age of 59. They were followed up with their matched comparators until the development of autoimmune thyroid disease, death, emigration, or the end of the study period, December 2019.
- The relative risks of autoimmune thyroid disease following a diagnosis of RA and with treatment with DMARDs were compared with those risks in the general population.
- Participants with a non-autoimmune cause for thyroxine prescription were excluded, as were those with an autoimmune thyroid disease at the time of RA diagnosis.
TAKEAWAY:
- Following their RA diagnosis, 321 (2.3%) of patients developed an autoimmune thyroid disease, compared with 1838 (2.9%) in the general population comparators, representing an incidence of 3.7 vs 4.6 per 1000 person-years (hazard ratio [HR], 0.81).
- The lower incidence of autoimmune thyroid disease was more pronounced with longer RA duration. For instance, at 10-14 years after an RA diagnosis, the incidence was 2.9 vs. 4.5 autoimmune thyroid disease events per 1000 person-years, respectively (HR, 0.64).
- The decreased risk of incident autoimmune thyroid disease among RA patients compared with the general population was strongest among patients treated with biologic DMARDs (bDMARD), with an HR of 0.54.
- The reduced incidence of autoimmune thyroid disease with bDMARD use was most pronounced among users of TNF-inhibitors (HR, 0.67).
- The lower incidence of autoimmune thyroid disease following a diagnosis of RA contrasts with previous studies showing an increased risk for thyroid disease associated with RA.
- However, the decreased risk of thyroid disease following bDMARD treatment supports the theory that immunomodulatory treatment could also have an effect of blunting the inflammatory processes that can lead to overt clinical autoimmune thyroid disease.
IN PRACTICE:
“To our knowledge, no previous study has investigated whether the risk of new-onset autoimmune thyroid disease is affected by RA treatment in early RA,” the authors report.
“Our results demonstrate that compared to the general population, patients with RA treated with bDMARDs, TNF-inhibitors in particular, are at decreased risk of developing autoimmune thyroid disease, a finding that calls for replication and may open for drug-repurposing studies,” they note.
SOURCE:
The study was conducted by first author Kristin Waldenlind, PhD, of the Department of Medicine, Solna, Division of Clinical Epidemiology, Karolinska Institutet, Stockholm, Sweden, and colleagues. It was published online November 27 in the Journal of Internal Medicine.
LIMITATIONS:
The study lacked details on participants’ thyroid autoantibody and hormone levels.
The presence of autoimmune thyroid disease was determined based on prescriptions for thyroxine, hence the authors cannot exclude the possibility of a lower threshold for thyroxine prescription among patients treated with DMARDs.
Information was not available on potential risk factors for RA and autoimmune thyroid disease that might have introduced confounding, such as smoking or obesity.
DISCLOSURES:
The study received funding from the Swedish Research Council, the Swedish Heart Lung Foundation, Vinnova, and Region Stockholm/Karolinska Institutet (ALF). The authors’ disclosures are detailed in the published study.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients with rheumatoid arthritis (RA) in a large Swedish population cohort show a reduced incidence of autoimmune thyroid diseases, such as hypothyroidism or hyperthyroidism, after being diagnosed with RA, with the effect being more pronounced among those treated with disease-modifying anti-rheumatic drugs (DMARDs), particularly TNF-inhibitors.
Although DMARDs are commonly used in the treatment of RA, the drugs are rarely used to treat autoimmune thyroid diseases. The new results support theories raised in previous smaller studies that DMARDs could have a protective effect against thyroid disease.
METHODOLOGY:
- The study involved 13,731 patients with new-onset RA who were listed in the Swedish Rheumatology Quality Register between 2006 and 2018.
- The patients were matched for sex, age, and residential area with up to five reference individuals in the general population of 63,201 comparators.
- Overall, patients with RA were 64.7% female, with a mean age of 59. They were followed up with their matched comparators until the development of autoimmune thyroid disease, death, emigration, or the end of the study period, December 2019.
- The relative risks of autoimmune thyroid disease following a diagnosis of RA and with treatment with DMARDs were compared with those risks in the general population.
- Participants with a non-autoimmune cause for thyroxine prescription were excluded, as were those with an autoimmune thyroid disease at the time of RA diagnosis.
TAKEAWAY:
- Following their RA diagnosis, 321 (2.3%) of patients developed an autoimmune thyroid disease, compared with 1838 (2.9%) in the general population comparators, representing an incidence of 3.7 vs 4.6 per 1000 person-years (hazard ratio [HR], 0.81).
- The lower incidence of autoimmune thyroid disease was more pronounced with longer RA duration. For instance, at 10-14 years after an RA diagnosis, the incidence was 2.9 vs. 4.5 autoimmune thyroid disease events per 1000 person-years, respectively (HR, 0.64).
- The decreased risk of incident autoimmune thyroid disease among RA patients compared with the general population was strongest among patients treated with biologic DMARDs (bDMARD), with an HR of 0.54.
- The reduced incidence of autoimmune thyroid disease with bDMARD use was most pronounced among users of TNF-inhibitors (HR, 0.67).
- The lower incidence of autoimmune thyroid disease following a diagnosis of RA contrasts with previous studies showing an increased risk for thyroid disease associated with RA.
- However, the decreased risk of thyroid disease following bDMARD treatment supports the theory that immunomodulatory treatment could also have an effect of blunting the inflammatory processes that can lead to overt clinical autoimmune thyroid disease.
IN PRACTICE:
“To our knowledge, no previous study has investigated whether the risk of new-onset autoimmune thyroid disease is affected by RA treatment in early RA,” the authors report.
“Our results demonstrate that compared to the general population, patients with RA treated with bDMARDs, TNF-inhibitors in particular, are at decreased risk of developing autoimmune thyroid disease, a finding that calls for replication and may open for drug-repurposing studies,” they note.
SOURCE:
The study was conducted by first author Kristin Waldenlind, PhD, of the Department of Medicine, Solna, Division of Clinical Epidemiology, Karolinska Institutet, Stockholm, Sweden, and colleagues. It was published online November 27 in the Journal of Internal Medicine.
LIMITATIONS:
The study lacked details on participants’ thyroid autoantibody and hormone levels.
The presence of autoimmune thyroid disease was determined based on prescriptions for thyroxine, hence the authors cannot exclude the possibility of a lower threshold for thyroxine prescription among patients treated with DMARDs.
Information was not available on potential risk factors for RA and autoimmune thyroid disease that might have introduced confounding, such as smoking or obesity.
DISCLOSURES:
The study received funding from the Swedish Research Council, the Swedish Heart Lung Foundation, Vinnova, and Region Stockholm/Karolinska Institutet (ALF). The authors’ disclosures are detailed in the published study.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients with rheumatoid arthritis (RA) in a large Swedish population cohort show a reduced incidence of autoimmune thyroid diseases, such as hypothyroidism or hyperthyroidism, after being diagnosed with RA, with the effect being more pronounced among those treated with disease-modifying anti-rheumatic drugs (DMARDs), particularly TNF-inhibitors.
Although DMARDs are commonly used in the treatment of RA, the drugs are rarely used to treat autoimmune thyroid diseases. The new results support theories raised in previous smaller studies that DMARDs could have a protective effect against thyroid disease.
METHODOLOGY:
- The study involved 13,731 patients with new-onset RA who were listed in the Swedish Rheumatology Quality Register between 2006 and 2018.
- The patients were matched for sex, age, and residential area with up to five reference individuals in the general population of 63,201 comparators.
- Overall, patients with RA were 64.7% female, with a mean age of 59. They were followed up with their matched comparators until the development of autoimmune thyroid disease, death, emigration, or the end of the study period, December 2019.
- The relative risks of autoimmune thyroid disease following a diagnosis of RA and with treatment with DMARDs were compared with those risks in the general population.
- Participants with a non-autoimmune cause for thyroxine prescription were excluded, as were those with an autoimmune thyroid disease at the time of RA diagnosis.
TAKEAWAY:
- Following their RA diagnosis, 321 (2.3%) of patients developed an autoimmune thyroid disease, compared with 1838 (2.9%) in the general population comparators, representing an incidence of 3.7 vs 4.6 per 1000 person-years (hazard ratio [HR], 0.81).
- The lower incidence of autoimmune thyroid disease was more pronounced with longer RA duration. For instance, at 10-14 years after an RA diagnosis, the incidence was 2.9 vs. 4.5 autoimmune thyroid disease events per 1000 person-years, respectively (HR, 0.64).
- The decreased risk of incident autoimmune thyroid disease among RA patients compared with the general population was strongest among patients treated with biologic DMARDs (bDMARD), with an HR of 0.54.
- The reduced incidence of autoimmune thyroid disease with bDMARD use was most pronounced among users of TNF-inhibitors (HR, 0.67).
- The lower incidence of autoimmune thyroid disease following a diagnosis of RA contrasts with previous studies showing an increased risk for thyroid disease associated with RA.
- However, the decreased risk of thyroid disease following bDMARD treatment supports the theory that immunomodulatory treatment could also have an effect of blunting the inflammatory processes that can lead to overt clinical autoimmune thyroid disease.
IN PRACTICE:
“To our knowledge, no previous study has investigated whether the risk of new-onset autoimmune thyroid disease is affected by RA treatment in early RA,” the authors report.
“Our results demonstrate that compared to the general population, patients with RA treated with bDMARDs, TNF-inhibitors in particular, are at decreased risk of developing autoimmune thyroid disease, a finding that calls for replication and may open for drug-repurposing studies,” they note.
SOURCE:
The study was conducted by first author Kristin Waldenlind, PhD, of the Department of Medicine, Solna, Division of Clinical Epidemiology, Karolinska Institutet, Stockholm, Sweden, and colleagues. It was published online November 27 in the Journal of Internal Medicine.
LIMITATIONS:
The study lacked details on participants’ thyroid autoantibody and hormone levels.
The presence of autoimmune thyroid disease was determined based on prescriptions for thyroxine, hence the authors cannot exclude the possibility of a lower threshold for thyroxine prescription among patients treated with DMARDs.
Information was not available on potential risk factors for RA and autoimmune thyroid disease that might have introduced confounding, such as smoking or obesity.
DISCLOSURES:
The study received funding from the Swedish Research Council, the Swedish Heart Lung Foundation, Vinnova, and Region Stockholm/Karolinska Institutet (ALF). The authors’ disclosures are detailed in the published study.
A version of this article appeared on Medscape.com.
Low-dose naltrexone falls short for fibromyalgia
TOPLINE:
Women with fibromyalgia who received low-dose naltrexone showed no significant improvement in pain at 12 weeks, compared with those who received placebo in a randomized trial.
METHODOLOGY:
- The researchers randomly assigned 99 women with fibromyalgia at a single center in Denmark to a daily dose of 6 mg of naltrexone or a placebo for 12 weeks.
- The primary outcome was within-group change in pain intensity from baseline to 12 weeks, measured using an 11-point numeric rating scale (NRS) and the Fibromyalgia Impact Questionnaire-Revised (FIQR); outcomes were measured at 4, 8, and 12 weeks.
- Secondary outcomes included the global impact of FIQR total scores, as well as FIQR scores for tenderness, fatigue, , , anxiety, memory, stiffness, and physical function.
TAKEAWAY:
- The patients ranged in age from 18 to 64 years, with a mean age of 50.6 years, and all but one was White.
- At 12 weeks, the mean change in pain intensity was greater in the naltrexone group compared with the placebo group (−1.3 points vs −0.9 points, respectively), but the difference was not statistically significant.
- Of the secondary outcomes, only memory problems related to fibromyalgia showed significant improvement with naltrexone compared with placebo (−0.93 vs −0.30; P = .004), although the significance was lost after adjusting for multiplicity.
- Adverse events were infrequent and similar between the groups; four of 49 patients in the naltrexone group and three of 50 in the placebo group discontinued their assigned treatments because of side effects, and no safety concerns appeared related to the 6-mg dose.
IN PRACTICE:
“At this time we recommend that off-label treatment of patients who have responded to low-dose naltrexone should not be terminated, but we recommend against initiating low-dose naltrexone for low-dose naltrexone-naive patients with fibromyalgia pending the results of additional adequately powered studies with distinctive inflammatory and autoantibody patient profiles,” Winfried Häuser, MD, of the Center for Pain Medicine and Mental Health, Saarbrücken, Germany, and Mary-Ann Fitzcharles, MD, of McGill University Health Centre, Montreal, Canada, wrote in an accompanying editorial.
SOURCE:
The lead author of the study was Karin Due Bruun, MD, of Odense University Hospital, Denmark. The full study and accompanying editorial were published online in The Lancet Rheumatology.
LIMITATIONS:
The study was only powered to detect a difference of 1.0 NRS points for pain intensity; other limitations include the homogenous population that prevents generalizability to other groups and the relatively short follow-up period.
DISCLOSURES:
The study was supported by the Danish Rheumatism Association, Odense University Hospital, Danielsen’s Foundation, and the Oak Foundation. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Women with fibromyalgia who received low-dose naltrexone showed no significant improvement in pain at 12 weeks, compared with those who received placebo in a randomized trial.
METHODOLOGY:
- The researchers randomly assigned 99 women with fibromyalgia at a single center in Denmark to a daily dose of 6 mg of naltrexone or a placebo for 12 weeks.
- The primary outcome was within-group change in pain intensity from baseline to 12 weeks, measured using an 11-point numeric rating scale (NRS) and the Fibromyalgia Impact Questionnaire-Revised (FIQR); outcomes were measured at 4, 8, and 12 weeks.
- Secondary outcomes included the global impact of FIQR total scores, as well as FIQR scores for tenderness, fatigue, , , anxiety, memory, stiffness, and physical function.
TAKEAWAY:
- The patients ranged in age from 18 to 64 years, with a mean age of 50.6 years, and all but one was White.
- At 12 weeks, the mean change in pain intensity was greater in the naltrexone group compared with the placebo group (−1.3 points vs −0.9 points, respectively), but the difference was not statistically significant.
- Of the secondary outcomes, only memory problems related to fibromyalgia showed significant improvement with naltrexone compared with placebo (−0.93 vs −0.30; P = .004), although the significance was lost after adjusting for multiplicity.
- Adverse events were infrequent and similar between the groups; four of 49 patients in the naltrexone group and three of 50 in the placebo group discontinued their assigned treatments because of side effects, and no safety concerns appeared related to the 6-mg dose.
IN PRACTICE:
“At this time we recommend that off-label treatment of patients who have responded to low-dose naltrexone should not be terminated, but we recommend against initiating low-dose naltrexone for low-dose naltrexone-naive patients with fibromyalgia pending the results of additional adequately powered studies with distinctive inflammatory and autoantibody patient profiles,” Winfried Häuser, MD, of the Center for Pain Medicine and Mental Health, Saarbrücken, Germany, and Mary-Ann Fitzcharles, MD, of McGill University Health Centre, Montreal, Canada, wrote in an accompanying editorial.
SOURCE:
The lead author of the study was Karin Due Bruun, MD, of Odense University Hospital, Denmark. The full study and accompanying editorial were published online in The Lancet Rheumatology.
LIMITATIONS:
The study was only powered to detect a difference of 1.0 NRS points for pain intensity; other limitations include the homogenous population that prevents generalizability to other groups and the relatively short follow-up period.
DISCLOSURES:
The study was supported by the Danish Rheumatism Association, Odense University Hospital, Danielsen’s Foundation, and the Oak Foundation. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Women with fibromyalgia who received low-dose naltrexone showed no significant improvement in pain at 12 weeks, compared with those who received placebo in a randomized trial.
METHODOLOGY:
- The researchers randomly assigned 99 women with fibromyalgia at a single center in Denmark to a daily dose of 6 mg of naltrexone or a placebo for 12 weeks.
- The primary outcome was within-group change in pain intensity from baseline to 12 weeks, measured using an 11-point numeric rating scale (NRS) and the Fibromyalgia Impact Questionnaire-Revised (FIQR); outcomes were measured at 4, 8, and 12 weeks.
- Secondary outcomes included the global impact of FIQR total scores, as well as FIQR scores for tenderness, fatigue, , , anxiety, memory, stiffness, and physical function.
TAKEAWAY:
- The patients ranged in age from 18 to 64 years, with a mean age of 50.6 years, and all but one was White.
- At 12 weeks, the mean change in pain intensity was greater in the naltrexone group compared with the placebo group (−1.3 points vs −0.9 points, respectively), but the difference was not statistically significant.
- Of the secondary outcomes, only memory problems related to fibromyalgia showed significant improvement with naltrexone compared with placebo (−0.93 vs −0.30; P = .004), although the significance was lost after adjusting for multiplicity.
- Adverse events were infrequent and similar between the groups; four of 49 patients in the naltrexone group and three of 50 in the placebo group discontinued their assigned treatments because of side effects, and no safety concerns appeared related to the 6-mg dose.
IN PRACTICE:
“At this time we recommend that off-label treatment of patients who have responded to low-dose naltrexone should not be terminated, but we recommend against initiating low-dose naltrexone for low-dose naltrexone-naive patients with fibromyalgia pending the results of additional adequately powered studies with distinctive inflammatory and autoantibody patient profiles,” Winfried Häuser, MD, of the Center for Pain Medicine and Mental Health, Saarbrücken, Germany, and Mary-Ann Fitzcharles, MD, of McGill University Health Centre, Montreal, Canada, wrote in an accompanying editorial.
SOURCE:
The lead author of the study was Karin Due Bruun, MD, of Odense University Hospital, Denmark. The full study and accompanying editorial were published online in The Lancet Rheumatology.
LIMITATIONS:
The study was only powered to detect a difference of 1.0 NRS points for pain intensity; other limitations include the homogenous population that prevents generalizability to other groups and the relatively short follow-up period.
DISCLOSURES:
The study was supported by the Danish Rheumatism Association, Odense University Hospital, Danielsen’s Foundation, and the Oak Foundation. The researchers had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
Autoimmune Skin Diseases Linked To Risk Of Adverse Pregnancy Outcomes
SAN DIEGO — , results from a large case-control study suggest.
Patients with systemic autoimmune conditions are known to have an increased risk for adverse pregnancy outcomes, “but we weren’t sure if that was the case for patients with autoimmune skin conditions,” presenting study author Heejo Keum, a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas, said in an interview during a poster session at the American College of Rheumatology (ACR) 2023 annual meeting. “There are case reports or nationwide population-based studies on patients with alopecia areata and vitiligo, but those were outside of the US, so we wanted to see if these outcomes could be studied in a larger population-based study in the US.”
Drawing from the TriNetX US Collaborative Network, a database of electronic medical records of 94 million patients in the United States, the researchers identified pregnant patients aged 15-44 years between January 1, 2016, and December 31, 2021. Cases were defined as patients diagnosed with at least one autoimmune skin disease (ASD) prior to the end of pregnancy, including alopecia areata, bullous pemphigoid, cicatricial pemphigoid, dermatitis herpetiformis, cutaneous lupus erythematosus, epidermolysis bullosa acquisita, morphea, pemphigus foliaceus, pemphigus vulgaris, vitiligo, and amyopathic DM. There were two control groups: healthy controls (those without ASDs, systemic lupus erythematosus or rheumatoid arthritis) and disease controls (those with SLE or RA). The researchers used ICD-10 codes to identify pregnancy endpoints, including live births, spontaneous abortion, and stillbirth. Patients with a history of hidradenitis suppurative were excluded from the analysis, as were those with common autoimmune disease such as Hashimoto’s thyroiditis, Grave’s disease, and type 1 diabetes.
The primary outcomes were adverse pregnancy outcomes defined as spontaneous abortion, gestational hypertension, preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction (IUGR), preterm premature rupture of membranes (PPROM), and preterm birth. The researchers used 1:1 propensity scoring to match patients with ASDs to controls by age, race, ethnicity, comorbidities, obesity, and substance use, and used odds ratio (OR) analysis with a 95% confidence interval (CI) to calculate each outcome.
Ms. Keum reported results from 3,654 women with ASDs, 3,654 healthy controls, 2,147 women with SLE, and 889 women with RA.
The three most common ASDs were vitiligo (30%), alopecia areata (30%), and cutaneous lupus erythematosus (27%). Compared with healthy controls, patients with ASDs were more likely to have spontaneous abortions (OR=1.5 [1.4-1.7], P<.001), and preeclampsia/eclampsia (OR=1.2 [1.0-1.3], P=.04). Compared with women with SLE, women with ASDs were less likely to have preeclampsia/eclampsia (OR=0.7 [0.6-0.9, P=.001); preterm birth (OR= 0.5 [0.4-0.7], P<.001); PPROM (OR=0.6 [0.4-0.9], P=.004), or an infant with IUGR (OR=0.6 [0.5-0.8], P<.001), but they were more likely to have a spontaneous abortion (OR=1.2 [1.1-1.3], P=.003). Overall, patients with ASDs had similar risks for adverse pregnancy outcomes as patients with RA.
“We found that patients with cutaneous lupus and vitiligo had higher rates of spontaneous abortion, which is interesting because we didn’t expect that,” Ms. Keum told this news organization. “Studies have shown that vitiligo patients might have an increased risk of pregnancy loss, so I think it’s important to have that discussion with those patients. It might benefit them to talk to a maternal-fetal medicine specialist. As for next steps, we want to look at how medication use and disease flare or disease severity play a role in APOs.”
In their poster, the researchers acknowledged limitations of the study, including the inability to verify diagnoses or assess disease severity. Also, while medication use and concomitant antiphospholipid syndrome were evaluated as risk factors for advanced pregnancy outcomes, the number of patients per group was too small for analysis.
Karl Saardi, MD, director of the inpatient dermatology service at George Washington University Hospital, Washington, who was asked to comment on the study, said that in his view, the choice of disease states included in the analysis “is a bit arbitrary.” He added that “it would have been more helpful to compare controls versus discoid lupus versus systemic lupus or controls versus amyopathic dermatomyositis versus dermatomyositis with myopathy.”
The study received funding support from the Rheumatology Research Foundation and the UT Southwestern Dean’s Research Scholar program. Neither Ms. Keum nor Dr. Saardi reported having relevant disclosures.
SAN DIEGO — , results from a large case-control study suggest.
Patients with systemic autoimmune conditions are known to have an increased risk for adverse pregnancy outcomes, “but we weren’t sure if that was the case for patients with autoimmune skin conditions,” presenting study author Heejo Keum, a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas, said in an interview during a poster session at the American College of Rheumatology (ACR) 2023 annual meeting. “There are case reports or nationwide population-based studies on patients with alopecia areata and vitiligo, but those were outside of the US, so we wanted to see if these outcomes could be studied in a larger population-based study in the US.”
Drawing from the TriNetX US Collaborative Network, a database of electronic medical records of 94 million patients in the United States, the researchers identified pregnant patients aged 15-44 years between January 1, 2016, and December 31, 2021. Cases were defined as patients diagnosed with at least one autoimmune skin disease (ASD) prior to the end of pregnancy, including alopecia areata, bullous pemphigoid, cicatricial pemphigoid, dermatitis herpetiformis, cutaneous lupus erythematosus, epidermolysis bullosa acquisita, morphea, pemphigus foliaceus, pemphigus vulgaris, vitiligo, and amyopathic DM. There were two control groups: healthy controls (those without ASDs, systemic lupus erythematosus or rheumatoid arthritis) and disease controls (those with SLE or RA). The researchers used ICD-10 codes to identify pregnancy endpoints, including live births, spontaneous abortion, and stillbirth. Patients with a history of hidradenitis suppurative were excluded from the analysis, as were those with common autoimmune disease such as Hashimoto’s thyroiditis, Grave’s disease, and type 1 diabetes.
The primary outcomes were adverse pregnancy outcomes defined as spontaneous abortion, gestational hypertension, preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction (IUGR), preterm premature rupture of membranes (PPROM), and preterm birth. The researchers used 1:1 propensity scoring to match patients with ASDs to controls by age, race, ethnicity, comorbidities, obesity, and substance use, and used odds ratio (OR) analysis with a 95% confidence interval (CI) to calculate each outcome.
Ms. Keum reported results from 3,654 women with ASDs, 3,654 healthy controls, 2,147 women with SLE, and 889 women with RA.
The three most common ASDs were vitiligo (30%), alopecia areata (30%), and cutaneous lupus erythematosus (27%). Compared with healthy controls, patients with ASDs were more likely to have spontaneous abortions (OR=1.5 [1.4-1.7], P<.001), and preeclampsia/eclampsia (OR=1.2 [1.0-1.3], P=.04). Compared with women with SLE, women with ASDs were less likely to have preeclampsia/eclampsia (OR=0.7 [0.6-0.9, P=.001); preterm birth (OR= 0.5 [0.4-0.7], P<.001); PPROM (OR=0.6 [0.4-0.9], P=.004), or an infant with IUGR (OR=0.6 [0.5-0.8], P<.001), but they were more likely to have a spontaneous abortion (OR=1.2 [1.1-1.3], P=.003). Overall, patients with ASDs had similar risks for adverse pregnancy outcomes as patients with RA.
“We found that patients with cutaneous lupus and vitiligo had higher rates of spontaneous abortion, which is interesting because we didn’t expect that,” Ms. Keum told this news organization. “Studies have shown that vitiligo patients might have an increased risk of pregnancy loss, so I think it’s important to have that discussion with those patients. It might benefit them to talk to a maternal-fetal medicine specialist. As for next steps, we want to look at how medication use and disease flare or disease severity play a role in APOs.”
In their poster, the researchers acknowledged limitations of the study, including the inability to verify diagnoses or assess disease severity. Also, while medication use and concomitant antiphospholipid syndrome were evaluated as risk factors for advanced pregnancy outcomes, the number of patients per group was too small for analysis.
Karl Saardi, MD, director of the inpatient dermatology service at George Washington University Hospital, Washington, who was asked to comment on the study, said that in his view, the choice of disease states included in the analysis “is a bit arbitrary.” He added that “it would have been more helpful to compare controls versus discoid lupus versus systemic lupus or controls versus amyopathic dermatomyositis versus dermatomyositis with myopathy.”
The study received funding support from the Rheumatology Research Foundation and the UT Southwestern Dean’s Research Scholar program. Neither Ms. Keum nor Dr. Saardi reported having relevant disclosures.
SAN DIEGO — , results from a large case-control study suggest.
Patients with systemic autoimmune conditions are known to have an increased risk for adverse pregnancy outcomes, “but we weren’t sure if that was the case for patients with autoimmune skin conditions,” presenting study author Heejo Keum, a fourth-year medical student at the University of Texas Southwestern Medical Center, Dallas, said in an interview during a poster session at the American College of Rheumatology (ACR) 2023 annual meeting. “There are case reports or nationwide population-based studies on patients with alopecia areata and vitiligo, but those were outside of the US, so we wanted to see if these outcomes could be studied in a larger population-based study in the US.”
Drawing from the TriNetX US Collaborative Network, a database of electronic medical records of 94 million patients in the United States, the researchers identified pregnant patients aged 15-44 years between January 1, 2016, and December 31, 2021. Cases were defined as patients diagnosed with at least one autoimmune skin disease (ASD) prior to the end of pregnancy, including alopecia areata, bullous pemphigoid, cicatricial pemphigoid, dermatitis herpetiformis, cutaneous lupus erythematosus, epidermolysis bullosa acquisita, morphea, pemphigus foliaceus, pemphigus vulgaris, vitiligo, and amyopathic DM. There were two control groups: healthy controls (those without ASDs, systemic lupus erythematosus or rheumatoid arthritis) and disease controls (those with SLE or RA). The researchers used ICD-10 codes to identify pregnancy endpoints, including live births, spontaneous abortion, and stillbirth. Patients with a history of hidradenitis suppurative were excluded from the analysis, as were those with common autoimmune disease such as Hashimoto’s thyroiditis, Grave’s disease, and type 1 diabetes.
The primary outcomes were adverse pregnancy outcomes defined as spontaneous abortion, gestational hypertension, preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction (IUGR), preterm premature rupture of membranes (PPROM), and preterm birth. The researchers used 1:1 propensity scoring to match patients with ASDs to controls by age, race, ethnicity, comorbidities, obesity, and substance use, and used odds ratio (OR) analysis with a 95% confidence interval (CI) to calculate each outcome.
Ms. Keum reported results from 3,654 women with ASDs, 3,654 healthy controls, 2,147 women with SLE, and 889 women with RA.
The three most common ASDs were vitiligo (30%), alopecia areata (30%), and cutaneous lupus erythematosus (27%). Compared with healthy controls, patients with ASDs were more likely to have spontaneous abortions (OR=1.5 [1.4-1.7], P<.001), and preeclampsia/eclampsia (OR=1.2 [1.0-1.3], P=.04). Compared with women with SLE, women with ASDs were less likely to have preeclampsia/eclampsia (OR=0.7 [0.6-0.9, P=.001); preterm birth (OR= 0.5 [0.4-0.7], P<.001); PPROM (OR=0.6 [0.4-0.9], P=.004), or an infant with IUGR (OR=0.6 [0.5-0.8], P<.001), but they were more likely to have a spontaneous abortion (OR=1.2 [1.1-1.3], P=.003). Overall, patients with ASDs had similar risks for adverse pregnancy outcomes as patients with RA.
“We found that patients with cutaneous lupus and vitiligo had higher rates of spontaneous abortion, which is interesting because we didn’t expect that,” Ms. Keum told this news organization. “Studies have shown that vitiligo patients might have an increased risk of pregnancy loss, so I think it’s important to have that discussion with those patients. It might benefit them to talk to a maternal-fetal medicine specialist. As for next steps, we want to look at how medication use and disease flare or disease severity play a role in APOs.”
In their poster, the researchers acknowledged limitations of the study, including the inability to verify diagnoses or assess disease severity. Also, while medication use and concomitant antiphospholipid syndrome were evaluated as risk factors for advanced pregnancy outcomes, the number of patients per group was too small for analysis.
Karl Saardi, MD, director of the inpatient dermatology service at George Washington University Hospital, Washington, who was asked to comment on the study, said that in his view, the choice of disease states included in the analysis “is a bit arbitrary.” He added that “it would have been more helpful to compare controls versus discoid lupus versus systemic lupus or controls versus amyopathic dermatomyositis versus dermatomyositis with myopathy.”
The study received funding support from the Rheumatology Research Foundation and the UT Southwestern Dean’s Research Scholar program. Neither Ms. Keum nor Dr. Saardi reported having relevant disclosures.
FROM ACR 2023
COVID vaccination protects B cell–deficient patients through T-cell responses
TOPLINE:
In individuals with low B-cell counts, T cells have enhanced responses to COVID-19 vaccination and may help prevent severe disease after infection.
METHODOLOGY:
- How the immune systems of B cell–deficient patients respond to SARS-CoV-2 infection and vaccination is not fully understood.
- Researchers evaluated anti–SARS-CoV-2 T-cell responses in 33 patients treated with rituximab (RTX), 12 patients with common variable immune deficiency, and 44 controls.
- The study analyzed effector and memory CD4+ and CD8+ T-cell responses to SARS-CoV-2 after infection and vaccination.
TAKEAWAY:
- All B cell–deficient individuals (those treated with RTX or those with a diagnosis of common variable immune deficiency) had increased effector and memory T-cell responses after SARS-CoV-2 vaccination, compared with controls.
- Patients treated with RTX who were vaccinated against COVID-19 had 4.8-fold reduced odds of moderate or severe disease. (These data were not available for patients with common variable immune deficiency.)
- RTX treatment was associated with a decrease in preexisting T-cell immunity in unvaccinated patients, regardless of prior infection with SARS-CoV-2.
- This association was not found in vaccinated patients treated with RTX.
IN PRACTICE:
“[These findings] provide support for vaccination in this vulnerable population and demonstrate the potential benefit of vaccine-induced CD8+ T-cell responses on reducing disease severity from SARS-CoV-2 infection in the absence of spike protein–specific antibodies,” the authors wrote.
SOURCE:
The study was published online on November 29 in Science Translational Medicine. The first author is Reza Zonozi, MD, who conducted the research while at Massachusetts General Hospital, Boston, and is now in private practice in northern Virginia.
LIMITATIONS:
Researchers did not obtain specimens from patients with common variable immune deficiency after SARS-CoV-2 infection. Only a small subset of immunophenotyped participants had subsequent SARS-CoV-2 infection.
DISCLOSURES:
The research was supported by grants from the National Institutes of Health, the Centers for Disease Control and Prevention, the Howard Hughes Medical Institute, the Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard Medical School, the Mark and Lisa Schwartz Foundation and E. Schwartz; the Lambertus Family Foundation; and S. Edgerly and P. Edgerly. Four authors reported relationships with pharmaceutical companies including AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Merck, and Pfizer.
A version of this article first appeared on Medscape.com.
TOPLINE:
In individuals with low B-cell counts, T cells have enhanced responses to COVID-19 vaccination and may help prevent severe disease after infection.
METHODOLOGY:
- How the immune systems of B cell–deficient patients respond to SARS-CoV-2 infection and vaccination is not fully understood.
- Researchers evaluated anti–SARS-CoV-2 T-cell responses in 33 patients treated with rituximab (RTX), 12 patients with common variable immune deficiency, and 44 controls.
- The study analyzed effector and memory CD4+ and CD8+ T-cell responses to SARS-CoV-2 after infection and vaccination.
TAKEAWAY:
- All B cell–deficient individuals (those treated with RTX or those with a diagnosis of common variable immune deficiency) had increased effector and memory T-cell responses after SARS-CoV-2 vaccination, compared with controls.
- Patients treated with RTX who were vaccinated against COVID-19 had 4.8-fold reduced odds of moderate or severe disease. (These data were not available for patients with common variable immune deficiency.)
- RTX treatment was associated with a decrease in preexisting T-cell immunity in unvaccinated patients, regardless of prior infection with SARS-CoV-2.
- This association was not found in vaccinated patients treated with RTX.
IN PRACTICE:
“[These findings] provide support for vaccination in this vulnerable population and demonstrate the potential benefit of vaccine-induced CD8+ T-cell responses on reducing disease severity from SARS-CoV-2 infection in the absence of spike protein–specific antibodies,” the authors wrote.
SOURCE:
The study was published online on November 29 in Science Translational Medicine. The first author is Reza Zonozi, MD, who conducted the research while at Massachusetts General Hospital, Boston, and is now in private practice in northern Virginia.
LIMITATIONS:
Researchers did not obtain specimens from patients with common variable immune deficiency after SARS-CoV-2 infection. Only a small subset of immunophenotyped participants had subsequent SARS-CoV-2 infection.
DISCLOSURES:
The research was supported by grants from the National Institutes of Health, the Centers for Disease Control and Prevention, the Howard Hughes Medical Institute, the Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard Medical School, the Mark and Lisa Schwartz Foundation and E. Schwartz; the Lambertus Family Foundation; and S. Edgerly and P. Edgerly. Four authors reported relationships with pharmaceutical companies including AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Merck, and Pfizer.
A version of this article first appeared on Medscape.com.
TOPLINE:
In individuals with low B-cell counts, T cells have enhanced responses to COVID-19 vaccination and may help prevent severe disease after infection.
METHODOLOGY:
- How the immune systems of B cell–deficient patients respond to SARS-CoV-2 infection and vaccination is not fully understood.
- Researchers evaluated anti–SARS-CoV-2 T-cell responses in 33 patients treated with rituximab (RTX), 12 patients with common variable immune deficiency, and 44 controls.
- The study analyzed effector and memory CD4+ and CD8+ T-cell responses to SARS-CoV-2 after infection and vaccination.
TAKEAWAY:
- All B cell–deficient individuals (those treated with RTX or those with a diagnosis of common variable immune deficiency) had increased effector and memory T-cell responses after SARS-CoV-2 vaccination, compared with controls.
- Patients treated with RTX who were vaccinated against COVID-19 had 4.8-fold reduced odds of moderate or severe disease. (These data were not available for patients with common variable immune deficiency.)
- RTX treatment was associated with a decrease in preexisting T-cell immunity in unvaccinated patients, regardless of prior infection with SARS-CoV-2.
- This association was not found in vaccinated patients treated with RTX.
IN PRACTICE:
“[These findings] provide support for vaccination in this vulnerable population and demonstrate the potential benefit of vaccine-induced CD8+ T-cell responses on reducing disease severity from SARS-CoV-2 infection in the absence of spike protein–specific antibodies,” the authors wrote.
SOURCE:
The study was published online on November 29 in Science Translational Medicine. The first author is Reza Zonozi, MD, who conducted the research while at Massachusetts General Hospital, Boston, and is now in private practice in northern Virginia.
LIMITATIONS:
Researchers did not obtain specimens from patients with common variable immune deficiency after SARS-CoV-2 infection. Only a small subset of immunophenotyped participants had subsequent SARS-CoV-2 infection.
DISCLOSURES:
The research was supported by grants from the National Institutes of Health, the Centers for Disease Control and Prevention, the Howard Hughes Medical Institute, the Ragon Institute of Massachusetts General Hospital, Massachusetts Institute of Technology, and Harvard Medical School, the Mark and Lisa Schwartz Foundation and E. Schwartz; the Lambertus Family Foundation; and S. Edgerly and P. Edgerly. Four authors reported relationships with pharmaceutical companies including AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Gilead Sciences, Merck, and Pfizer.
A version of this article first appeared on Medscape.com.
Rheumatology Match Day results for 2024 follow trends of past years
While adult rheumatology programs continue to have high match rates, pediatric rheumatology programs remain less popular.
The National Residency Matching Program (NRMP) reported on Nov. 29 that rheumatology filled 124 of 127 programs (97.6%), with 273 (98.9%) of 276 positions filled. Comparatively, pediatric rheumatology filled 21 out of 38 programs (55%) and 32 (61.5%) of 52 positions.
This year, the number of programs and positions across all specialties rose by 3%, whereas the number of applications only rose by 0.4% (35 additional applicants).
“The growth of fellowship programs and positions in the Match reflect training opportunities and the future workforce trends of medical subspecialties,” said NRMP President Donna Lamb, DHSc, MBA, BSN, in a statement. “While the increase in applicant numbers did not keep pace with the increase in positions this year, the Match rate for applicants remains strong at 82%.”
In adult rheumatology, matched applicants included 117 MD graduates, 86 foreign applicants, 38 DO graduates, and 32 U.S. citizen international medical graduates. A total of 348 applicants preferred the specialty, and 78% matched to rheumatology, whereas 2% matched to a different specialty. Another 70 applicants (20%) did not match to any program.
In pediatric rheumatology, matched applicants included 23 MD graduates, 6 DO graduates, and 3 foreign applicants. All applicants who preferred pediatric rheumatology matched to a program.
Adult rheumatology was one of several specialties that filled over 95% of positions. The other specialties that matched at that rate were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, critical care medicine, gastroenterology, hematology and oncology, and pulmonary/critical care. Interventional Pulmonology and Oncology was the only specialty to achieve a 100% fill rate.
A version of this article first appeared on Medscape.com.
While adult rheumatology programs continue to have high match rates, pediatric rheumatology programs remain less popular.
The National Residency Matching Program (NRMP) reported on Nov. 29 that rheumatology filled 124 of 127 programs (97.6%), with 273 (98.9%) of 276 positions filled. Comparatively, pediatric rheumatology filled 21 out of 38 programs (55%) and 32 (61.5%) of 52 positions.
This year, the number of programs and positions across all specialties rose by 3%, whereas the number of applications only rose by 0.4% (35 additional applicants).
“The growth of fellowship programs and positions in the Match reflect training opportunities and the future workforce trends of medical subspecialties,” said NRMP President Donna Lamb, DHSc, MBA, BSN, in a statement. “While the increase in applicant numbers did not keep pace with the increase in positions this year, the Match rate for applicants remains strong at 82%.”
In adult rheumatology, matched applicants included 117 MD graduates, 86 foreign applicants, 38 DO graduates, and 32 U.S. citizen international medical graduates. A total of 348 applicants preferred the specialty, and 78% matched to rheumatology, whereas 2% matched to a different specialty. Another 70 applicants (20%) did not match to any program.
In pediatric rheumatology, matched applicants included 23 MD graduates, 6 DO graduates, and 3 foreign applicants. All applicants who preferred pediatric rheumatology matched to a program.
Adult rheumatology was one of several specialties that filled over 95% of positions. The other specialties that matched at that rate were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, critical care medicine, gastroenterology, hematology and oncology, and pulmonary/critical care. Interventional Pulmonology and Oncology was the only specialty to achieve a 100% fill rate.
A version of this article first appeared on Medscape.com.
While adult rheumatology programs continue to have high match rates, pediatric rheumatology programs remain less popular.
The National Residency Matching Program (NRMP) reported on Nov. 29 that rheumatology filled 124 of 127 programs (97.6%), with 273 (98.9%) of 276 positions filled. Comparatively, pediatric rheumatology filled 21 out of 38 programs (55%) and 32 (61.5%) of 52 positions.
This year, the number of programs and positions across all specialties rose by 3%, whereas the number of applications only rose by 0.4% (35 additional applicants).
“The growth of fellowship programs and positions in the Match reflect training opportunities and the future workforce trends of medical subspecialties,” said NRMP President Donna Lamb, DHSc, MBA, BSN, in a statement. “While the increase in applicant numbers did not keep pace with the increase in positions this year, the Match rate for applicants remains strong at 82%.”
In adult rheumatology, matched applicants included 117 MD graduates, 86 foreign applicants, 38 DO graduates, and 32 U.S. citizen international medical graduates. A total of 348 applicants preferred the specialty, and 78% matched to rheumatology, whereas 2% matched to a different specialty. Another 70 applicants (20%) did not match to any program.
In pediatric rheumatology, matched applicants included 23 MD graduates, 6 DO graduates, and 3 foreign applicants. All applicants who preferred pediatric rheumatology matched to a program.
Adult rheumatology was one of several specialties that filled over 95% of positions. The other specialties that matched at that rate were allergy and immunology, cardiovascular disease, clinical cardiac electrophysiology, critical care medicine, gastroenterology, hematology and oncology, and pulmonary/critical care. Interventional Pulmonology and Oncology was the only specialty to achieve a 100% fill rate.
A version of this article first appeared on Medscape.com.
Low-dose methotrexate carries higher risk for older patients with CKD
TOPLINE:
The use of low-dose methotrexate among older adults with chronic kidney disease (CKD) was associated with a significantly increased risk at 90 days for serious adverse events requiring a hospital visit, compared with starting treatment with hydroxychloroquine.
METHODOLOGY:
- In a retrospective, population-based cohort study conducted in Ontario, researchers used linked administrative healthcare data to identify adults aged 66 years and older with CKD who were not undergoing dialysis and were new to medication; CKD was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2.
- The study population included 2,309 individuals who began treatment with low-dose methotrexate (5-35 mg/week); they were matched with 2,309 individuals who began treatment with hydroxychloroquine (200-400 mg/day). The median age was 76 years, 69% were women, and rheumatoid arthritis was the most common diagnosis (56%).
- The primary outcome was the risk of a hospital visit at 90 days for a composite of serious adverse events that included myelosuppression, sepsis, pneumotoxic effects, or hepatoxic effects.
TAKEAWAY:
- Overall, 3.55% of methotrexate patients and 1.73% of hydroxychloroquine patients met the primary outcome (risk ratio, 2.05); these events occurred at a median of 49 days and 43 days after starting the medications for the two groups, respectively.
- In an analysis by eGFR category, the risk of serious adverse events at 90 days increased among patients with eGFR levels less than 45 mL/min per 1.73 m2 (RR, 2.79).
- In a secondary comparison, the 90-day risk of serious adverse events was higher among methotrexate patients who began treatment with doses of 15-35 mg/week in comparison with those whose initial doses were 5 to less than 15 mg/week.
IN PRACTICE:
“Patients with CKD starting low-dose methotrexate should have active surveillance, including blood tests and chest radiographs performed regularly to monitor for signs of myelosuppression, infection, hepatotoxic effects, and pneumotoxic effects,” the researchers wrote.
SOURCE:
The lead author on the study was Flory T. Muanda, MD, of Western University, London, Ont. The study was published online in JAMA Network Open.
LIMITATIONS:
The observational design and lack of data on patients’ adherence to medications were among the limiting factors, as were the focus on older adults with CKD and the lack of assessment of the risk-benefit ratio of low-dose methotrexate.
DISCLOSURES:
The study was supported by the Institute for Clinical Evaluative Sciences. Dr. Muanda had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
The use of low-dose methotrexate among older adults with chronic kidney disease (CKD) was associated with a significantly increased risk at 90 days for serious adverse events requiring a hospital visit, compared with starting treatment with hydroxychloroquine.
METHODOLOGY:
- In a retrospective, population-based cohort study conducted in Ontario, researchers used linked administrative healthcare data to identify adults aged 66 years and older with CKD who were not undergoing dialysis and were new to medication; CKD was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2.
- The study population included 2,309 individuals who began treatment with low-dose methotrexate (5-35 mg/week); they were matched with 2,309 individuals who began treatment with hydroxychloroquine (200-400 mg/day). The median age was 76 years, 69% were women, and rheumatoid arthritis was the most common diagnosis (56%).
- The primary outcome was the risk of a hospital visit at 90 days for a composite of serious adverse events that included myelosuppression, sepsis, pneumotoxic effects, or hepatoxic effects.
TAKEAWAY:
- Overall, 3.55% of methotrexate patients and 1.73% of hydroxychloroquine patients met the primary outcome (risk ratio, 2.05); these events occurred at a median of 49 days and 43 days after starting the medications for the two groups, respectively.
- In an analysis by eGFR category, the risk of serious adverse events at 90 days increased among patients with eGFR levels less than 45 mL/min per 1.73 m2 (RR, 2.79).
- In a secondary comparison, the 90-day risk of serious adverse events was higher among methotrexate patients who began treatment with doses of 15-35 mg/week in comparison with those whose initial doses were 5 to less than 15 mg/week.
IN PRACTICE:
“Patients with CKD starting low-dose methotrexate should have active surveillance, including blood tests and chest radiographs performed regularly to monitor for signs of myelosuppression, infection, hepatotoxic effects, and pneumotoxic effects,” the researchers wrote.
SOURCE:
The lead author on the study was Flory T. Muanda, MD, of Western University, London, Ont. The study was published online in JAMA Network Open.
LIMITATIONS:
The observational design and lack of data on patients’ adherence to medications were among the limiting factors, as were the focus on older adults with CKD and the lack of assessment of the risk-benefit ratio of low-dose methotrexate.
DISCLOSURES:
The study was supported by the Institute for Clinical Evaluative Sciences. Dr. Muanda had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
The use of low-dose methotrexate among older adults with chronic kidney disease (CKD) was associated with a significantly increased risk at 90 days for serious adverse events requiring a hospital visit, compared with starting treatment with hydroxychloroquine.
METHODOLOGY:
- In a retrospective, population-based cohort study conducted in Ontario, researchers used linked administrative healthcare data to identify adults aged 66 years and older with CKD who were not undergoing dialysis and were new to medication; CKD was defined as an estimated glomerular filtration rate (eGFR) of less than 60 mL/min per 1.73 m2.
- The study population included 2,309 individuals who began treatment with low-dose methotrexate (5-35 mg/week); they were matched with 2,309 individuals who began treatment with hydroxychloroquine (200-400 mg/day). The median age was 76 years, 69% were women, and rheumatoid arthritis was the most common diagnosis (56%).
- The primary outcome was the risk of a hospital visit at 90 days for a composite of serious adverse events that included myelosuppression, sepsis, pneumotoxic effects, or hepatoxic effects.
TAKEAWAY:
- Overall, 3.55% of methotrexate patients and 1.73% of hydroxychloroquine patients met the primary outcome (risk ratio, 2.05); these events occurred at a median of 49 days and 43 days after starting the medications for the two groups, respectively.
- In an analysis by eGFR category, the risk of serious adverse events at 90 days increased among patients with eGFR levels less than 45 mL/min per 1.73 m2 (RR, 2.79).
- In a secondary comparison, the 90-day risk of serious adverse events was higher among methotrexate patients who began treatment with doses of 15-35 mg/week in comparison with those whose initial doses were 5 to less than 15 mg/week.
IN PRACTICE:
“Patients with CKD starting low-dose methotrexate should have active surveillance, including blood tests and chest radiographs performed regularly to monitor for signs of myelosuppression, infection, hepatotoxic effects, and pneumotoxic effects,” the researchers wrote.
SOURCE:
The lead author on the study was Flory T. Muanda, MD, of Western University, London, Ont. The study was published online in JAMA Network Open.
LIMITATIONS:
The observational design and lack of data on patients’ adherence to medications were among the limiting factors, as were the focus on older adults with CKD and the lack of assessment of the risk-benefit ratio of low-dose methotrexate.
DISCLOSURES:
The study was supported by the Institute for Clinical Evaluative Sciences. Dr. Muanda had no financial conflicts to disclose.
A version of this article appeared on Medscape.com.













