User login
Biosimilars perform identically to originator biologics in natural experiment
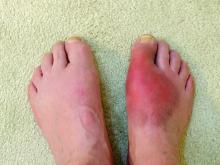
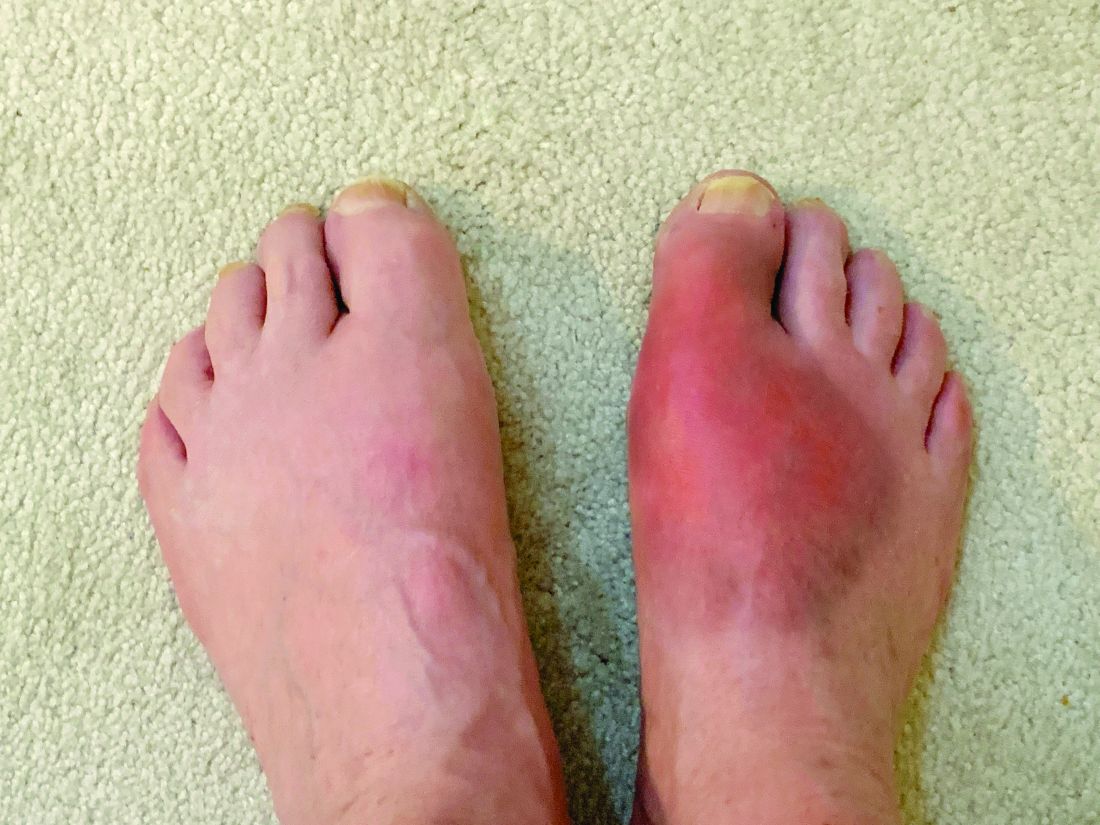
Real-world, population-based data suggest that the discontinuation rates for biosimilars prescribed to treat inflammatory rheumatic diseases are similar to those for their corresponding originator biologics, according to a study of patients in British Columbia who were required to switch to biosimilars.
“The decision to mandate use of biosimilars provided an ideal context for a natural experiment,” Diane Lacaille, MD, chair in arthritis research at the University of British Columbia, Vancouver, explained in her presentation of the study at the annual meeting of the Canadian Rheumatology Association.
On the basis of the real-world data, which was collected before and after a province-wide requirement to use biosimilars in place of originator biologics, there was no major difference in discontinuation rates, an outcome that Dr. Lacaille characterized as “a surrogate for both efficacy and safety.”
In the 2019 rheumatoid arthritis treatment guidelines from the European Alliance of Associations for Rheumatology, biosimilars are advocated for addressing the high cost of biologics based on evidence of efficacy and safety comparable with originator biologics. According to one of the coauthors of those guidelines, Tom W. J. Huizinga, MD, PhD, head of rheumatology at Leiden (Netherlands) University Medical Center, there is reasonable confidence in biosimilars as an adequate substitute for originator drugs, but real-world data are welcome.
“Real-world data provide different information than controlled trials and long-term data as well, so these [Canadian findings] are useful to support the data from [randomized controlled trials],” Dr. Huizinga said in an interview. He was not involved in the Canadian study.
Survivorship evaluated after switch to biosimilars
In British Columbia, biosimilars were mandated province-wide for new prescriptions of infliximab and etanercept in June 2017. In 2019, the mandate was extended to patients already taking originator infliximab (Remicade) and originator etanercept (Enbrel). Since that time, the mandate for biosimilars has also been applied to adalimumab (Humira). For the comparison of infliximab and etanercept originators with their biosimilars, Dr. Lacaille and associates compared survivorship for the 3 years after the policy change, when patients were on biosimilars, with the 3 years prior to the change, when patients were on the originators. They compared survivorship with originator adalimumab with its biosimilars for prior to and after the switch.
”People were followed from anti-TNF [tumor necrosis factor] initiation until discontinuation for any reason,” reported Dr. Lacaille, who said data were censored for death and moving out of the province. In British Columbia, where there is universal health care, all dispensed medications can be tracked. The definition of anti-TNF discontinuation in this study was no prescription renewal for at least 6 months.
The follow-up was censored at March 2, 2020, to avoid the potential impact of COVID-19 on antirheumatic drug use. Discontinuation was standardized for the comparison of originator with biosimilar drugs as rates per 100 person-years. Statistical adjustments were made for potential confounders.
The researchers compared 1,312 patients on etanercept and 827 on a biosimilar of it, 230 patients on infliximab and 271 on a biosimilar of it, and 1,773 on adalimumab and 2,213 on a biosimilar of it. The indication was RA in approximately 60% of those on etanercept or a biosimilar and 50% of those on infliximab or adalimumab and their biosimilars. More than half of the remaining patients had indications for psoriatic arthritis, and the rest had ankylosing spondylitis.
No differences reach statistical significance
On the basis of discontinuation rates per 100 person-years, etanercept and its biosimilars performed almost identically (37.10 vs. 37.02, respectively). Although the discontinuation rate per 100 person-years was lower on infliximab than a biosimilar of it (29.97 vs. 37.96), the difference was not statistically significant (P = .076).
For adalimumab, the discontinuation rate was also lower on the originator drug than a biosimilar of it (32.92 vs. 36.36), but, again, this difference was also insignificant (P = .56).
When the discontinuation data were evaluated on the basis of a Cox model involving a propensity weight overlap, the univariate and the multivariable analyses found that the biosimilars had similar risks for discontinuation. Univariate analysis revealed hazard ratios for discontinuation of the biosimilar relative to the originator were 0.98 (P = .783) for etanercept, 1.17 (P = .242) for infliximab, and 1.08 (P = .09) for adalimumab. In the multivariable model, adjusted HRs for discontinuation were about the same for each of the biosimilars relative to the originator: 0.98 (P = .807) for etanercept, 1.19 (P = .183) for infliximab, and 1.08 (P = .089) for adalimumab.
Relative to previously published direct comparisons, this real-world analysis and its duration of follow-up address the limitations of formal trials. In a 2020 BMJ meta-analysis of published data from 45 trials comparing biosimilar with originator drugs in patients with RA who had failed methotrexate, the authors found only “minor differences in harms and benefits,” but they cautioned that the analysis was “hampered by a lack of long-term direct comparisons.”
In an interview, Dr. Huizinga noted that a systematic review of adalimumab biosimilars that he led 2 years ago showed that they perform comparably with the originator biologics. This and other published studies have consistently shown “that there is no difference between biologics and originators.”
Dr. Lacaille disclosed financial relationships with Fresenius Kabi, Janssen, Organon, Pfizer, and Viatris. Dr. Huizinga disclosed financial relationships with Abbott, Ablynx, Biotest, Bioscience, Boehringer Ingelheim, Bristol-Myers Squibb, Crescendo Bioscience, Eli Lilly, Galapagos, Janssen, Merck, Novartis, MycoMed, Roche, Sanofi-Aventis, Takeda, and Zydus.
Real-world, population-based data suggest that the discontinuation rates for biosimilars prescribed to treat inflammatory rheumatic diseases are similar to those for their corresponding originator biologics, according to a study of patients in British Columbia who were required to switch to biosimilars.
“The decision to mandate use of biosimilars provided an ideal context for a natural experiment,” Diane Lacaille, MD, chair in arthritis research at the University of British Columbia, Vancouver, explained in her presentation of the study at the annual meeting of the Canadian Rheumatology Association.
On the basis of the real-world data, which was collected before and after a province-wide requirement to use biosimilars in place of originator biologics, there was no major difference in discontinuation rates, an outcome that Dr. Lacaille characterized as “a surrogate for both efficacy and safety.”
In the 2019 rheumatoid arthritis treatment guidelines from the European Alliance of Associations for Rheumatology, biosimilars are advocated for addressing the high cost of biologics based on evidence of efficacy and safety comparable with originator biologics. According to one of the coauthors of those guidelines, Tom W. J. Huizinga, MD, PhD, head of rheumatology at Leiden (Netherlands) University Medical Center, there is reasonable confidence in biosimilars as an adequate substitute for originator drugs, but real-world data are welcome.
“Real-world data provide different information than controlled trials and long-term data as well, so these [Canadian findings] are useful to support the data from [randomized controlled trials],” Dr. Huizinga said in an interview. He was not involved in the Canadian study.
Survivorship evaluated after switch to biosimilars
In British Columbia, biosimilars were mandated province-wide for new prescriptions of infliximab and etanercept in June 2017. In 2019, the mandate was extended to patients already taking originator infliximab (Remicade) and originator etanercept (Enbrel). Since that time, the mandate for biosimilars has also been applied to adalimumab (Humira). For the comparison of infliximab and etanercept originators with their biosimilars, Dr. Lacaille and associates compared survivorship for the 3 years after the policy change, when patients were on biosimilars, with the 3 years prior to the change, when patients were on the originators. They compared survivorship with originator adalimumab with its biosimilars for prior to and after the switch.
”People were followed from anti-TNF [tumor necrosis factor] initiation until discontinuation for any reason,” reported Dr. Lacaille, who said data were censored for death and moving out of the province. In British Columbia, where there is universal health care, all dispensed medications can be tracked. The definition of anti-TNF discontinuation in this study was no prescription renewal for at least 6 months.
The follow-up was censored at March 2, 2020, to avoid the potential impact of COVID-19 on antirheumatic drug use. Discontinuation was standardized for the comparison of originator with biosimilar drugs as rates per 100 person-years. Statistical adjustments were made for potential confounders.
The researchers compared 1,312 patients on etanercept and 827 on a biosimilar of it, 230 patients on infliximab and 271 on a biosimilar of it, and 1,773 on adalimumab and 2,213 on a biosimilar of it. The indication was RA in approximately 60% of those on etanercept or a biosimilar and 50% of those on infliximab or adalimumab and their biosimilars. More than half of the remaining patients had indications for psoriatic arthritis, and the rest had ankylosing spondylitis.
No differences reach statistical significance
On the basis of discontinuation rates per 100 person-years, etanercept and its biosimilars performed almost identically (37.10 vs. 37.02, respectively). Although the discontinuation rate per 100 person-years was lower on infliximab than a biosimilar of it (29.97 vs. 37.96), the difference was not statistically significant (P = .076).
For adalimumab, the discontinuation rate was also lower on the originator drug than a biosimilar of it (32.92 vs. 36.36), but, again, this difference was also insignificant (P = .56).
When the discontinuation data were evaluated on the basis of a Cox model involving a propensity weight overlap, the univariate and the multivariable analyses found that the biosimilars had similar risks for discontinuation. Univariate analysis revealed hazard ratios for discontinuation of the biosimilar relative to the originator were 0.98 (P = .783) for etanercept, 1.17 (P = .242) for infliximab, and 1.08 (P = .09) for adalimumab. In the multivariable model, adjusted HRs for discontinuation were about the same for each of the biosimilars relative to the originator: 0.98 (P = .807) for etanercept, 1.19 (P = .183) for infliximab, and 1.08 (P = .089) for adalimumab.
Relative to previously published direct comparisons, this real-world analysis and its duration of follow-up address the limitations of formal trials. In a 2020 BMJ meta-analysis of published data from 45 trials comparing biosimilar with originator drugs in patients with RA who had failed methotrexate, the authors found only “minor differences in harms and benefits,” but they cautioned that the analysis was “hampered by a lack of long-term direct comparisons.”
In an interview, Dr. Huizinga noted that a systematic review of adalimumab biosimilars that he led 2 years ago showed that they perform comparably with the originator biologics. This and other published studies have consistently shown “that there is no difference between biologics and originators.”
Dr. Lacaille disclosed financial relationships with Fresenius Kabi, Janssen, Organon, Pfizer, and Viatris. Dr. Huizinga disclosed financial relationships with Abbott, Ablynx, Biotest, Bioscience, Boehringer Ingelheim, Bristol-Myers Squibb, Crescendo Bioscience, Eli Lilly, Galapagos, Janssen, Merck, Novartis, MycoMed, Roche, Sanofi-Aventis, Takeda, and Zydus.
Real-world, population-based data suggest that the discontinuation rates for biosimilars prescribed to treat inflammatory rheumatic diseases are similar to those for their corresponding originator biologics, according to a study of patients in British Columbia who were required to switch to biosimilars.
“The decision to mandate use of biosimilars provided an ideal context for a natural experiment,” Diane Lacaille, MD, chair in arthritis research at the University of British Columbia, Vancouver, explained in her presentation of the study at the annual meeting of the Canadian Rheumatology Association.
On the basis of the real-world data, which was collected before and after a province-wide requirement to use biosimilars in place of originator biologics, there was no major difference in discontinuation rates, an outcome that Dr. Lacaille characterized as “a surrogate for both efficacy and safety.”
In the 2019 rheumatoid arthritis treatment guidelines from the European Alliance of Associations for Rheumatology, biosimilars are advocated for addressing the high cost of biologics based on evidence of efficacy and safety comparable with originator biologics. According to one of the coauthors of those guidelines, Tom W. J. Huizinga, MD, PhD, head of rheumatology at Leiden (Netherlands) University Medical Center, there is reasonable confidence in biosimilars as an adequate substitute for originator drugs, but real-world data are welcome.
“Real-world data provide different information than controlled trials and long-term data as well, so these [Canadian findings] are useful to support the data from [randomized controlled trials],” Dr. Huizinga said in an interview. He was not involved in the Canadian study.
Survivorship evaluated after switch to biosimilars
In British Columbia, biosimilars were mandated province-wide for new prescriptions of infliximab and etanercept in June 2017. In 2019, the mandate was extended to patients already taking originator infliximab (Remicade) and originator etanercept (Enbrel). Since that time, the mandate for biosimilars has also been applied to adalimumab (Humira). For the comparison of infliximab and etanercept originators with their biosimilars, Dr. Lacaille and associates compared survivorship for the 3 years after the policy change, when patients were on biosimilars, with the 3 years prior to the change, when patients were on the originators. They compared survivorship with originator adalimumab with its biosimilars for prior to and after the switch.
”People were followed from anti-TNF [tumor necrosis factor] initiation until discontinuation for any reason,” reported Dr. Lacaille, who said data were censored for death and moving out of the province. In British Columbia, where there is universal health care, all dispensed medications can be tracked. The definition of anti-TNF discontinuation in this study was no prescription renewal for at least 6 months.
The follow-up was censored at March 2, 2020, to avoid the potential impact of COVID-19 on antirheumatic drug use. Discontinuation was standardized for the comparison of originator with biosimilar drugs as rates per 100 person-years. Statistical adjustments were made for potential confounders.
The researchers compared 1,312 patients on etanercept and 827 on a biosimilar of it, 230 patients on infliximab and 271 on a biosimilar of it, and 1,773 on adalimumab and 2,213 on a biosimilar of it. The indication was RA in approximately 60% of those on etanercept or a biosimilar and 50% of those on infliximab or adalimumab and their biosimilars. More than half of the remaining patients had indications for psoriatic arthritis, and the rest had ankylosing spondylitis.
No differences reach statistical significance
On the basis of discontinuation rates per 100 person-years, etanercept and its biosimilars performed almost identically (37.10 vs. 37.02, respectively). Although the discontinuation rate per 100 person-years was lower on infliximab than a biosimilar of it (29.97 vs. 37.96), the difference was not statistically significant (P = .076).
For adalimumab, the discontinuation rate was also lower on the originator drug than a biosimilar of it (32.92 vs. 36.36), but, again, this difference was also insignificant (P = .56).
When the discontinuation data were evaluated on the basis of a Cox model involving a propensity weight overlap, the univariate and the multivariable analyses found that the biosimilars had similar risks for discontinuation. Univariate analysis revealed hazard ratios for discontinuation of the biosimilar relative to the originator were 0.98 (P = .783) for etanercept, 1.17 (P = .242) for infliximab, and 1.08 (P = .09) for adalimumab. In the multivariable model, adjusted HRs for discontinuation were about the same for each of the biosimilars relative to the originator: 0.98 (P = .807) for etanercept, 1.19 (P = .183) for infliximab, and 1.08 (P = .089) for adalimumab.
Relative to previously published direct comparisons, this real-world analysis and its duration of follow-up address the limitations of formal trials. In a 2020 BMJ meta-analysis of published data from 45 trials comparing biosimilar with originator drugs in patients with RA who had failed methotrexate, the authors found only “minor differences in harms and benefits,” but they cautioned that the analysis was “hampered by a lack of long-term direct comparisons.”
In an interview, Dr. Huizinga noted that a systematic review of adalimumab biosimilars that he led 2 years ago showed that they perform comparably with the originator biologics. This and other published studies have consistently shown “that there is no difference between biologics and originators.”
Dr. Lacaille disclosed financial relationships with Fresenius Kabi, Janssen, Organon, Pfizer, and Viatris. Dr. Huizinga disclosed financial relationships with Abbott, Ablynx, Biotest, Bioscience, Boehringer Ingelheim, Bristol-Myers Squibb, Crescendo Bioscience, Eli Lilly, Galapagos, Janssen, Merck, Novartis, MycoMed, Roche, Sanofi-Aventis, Takeda, and Zydus.
FROM CRA 2023
UnitedHealthcare tried to deny coverage to a chronically ill patient. He fought back, exposing the insurer’s inner workings.
In May 2021, a nurse at UnitedHealthcare called a colleague to share some welcome news about a problem the two had been grappling with for weeks.
United provided the health insurance plan for students at Penn State University. It was a large and potentially lucrative account: lots of young, healthy students paying premiums in, not too many huge medical reimbursements going out.
But Christopher McNaughton suffered from a crippling case of ulcerative colitis – an ailment that caused him to develop severe arthritis, debilitating diarrhea, numbing fatigue, and life-threatening blood clots. His medical bills were running nearly $2 million a year.
United had flagged Mr. McNaughton’s case as a “high dollar account,” and the company was reviewing whether it needed to keep paying for the expensive cocktail of drugs crafted by a Mayo Clinic specialist that had brought Mr. McNaughton’s disease under control after he’d been through years of misery.
On the 2021 phone call, which was recorded by the company, nurse Victoria Kavanaugh told her colleague that a doctor contracted by United to review the case had concluded that Mr. McNaughton’s treatment was “not medically necessary.” Her colleague, Dave Opperman, reacted to the news with a long laugh.
“I knew that was coming,” said Mr. Opperman, who heads up a United subsidiary that brokered the health insurance contract between United and Penn State. “I did too,” Ms. Kavanaugh replied.
Mr. Opperman then complained about Mr. McNaughton’s mother, whom he referred to as “this woman,” for “screaming and yelling” and “throwing tantrums” during calls with United.
The pair agreed that any appeal of the United doctor’s denial of the treatment would be a waste of the family’s time and money.
“We’re still gonna say no,” Mr. Opperman said.
More than 200 million Americans are covered by private health insurance. But data from state and federal regulators shows that insurers reject about 1 in 7 claims for treatment. Many people, faced with fighting insurance companies, simply give up: One study found that Americans file formal appeals on only 0.1% of claims denied by insurers under the Affordable Care Act.
Insurers have wide discretion in crafting what is covered by their policies, beyond some basic services mandated by federal and state law. They often deny claims for services that they deem not “medically necessary.”
When United refused to pay for Mr. McNaughton’s treatment for that reason, his family did something unusual. They fought back with a lawsuit, which uncovered a trove of materials, including internal emails and tape-recorded exchanges among company employees. Those records offer an extraordinary behind-the-scenes look at how one of America’s leading health care insurers relentlessly fought to reduce spending on care, even as its profits rose to record levels.
As United reviewed Mr. McNaughton’s treatment, he and his family were often in the dark about what was happening or their rights. Meanwhile, United employees misrepresented critical findings and ignored warnings from doctors about the risks of altering Mr. McNaughton’s drug plan.
At one point, court records show, United inaccurately reported to Penn State and the family that Mr. McNaughton’s doctor had agreed to lower the doses of his medication. Another time, a doctor paid by United concluded that denying payments for Mr. McNaughton’s treatment could put his health at risk, but the company buried his report and did not consider its findings. The insurer did, however, consider a report submitted by a company doctor who rubber-stamped the recommendation of a United nurse to reject paying for the treatment.
United declined to answer specific questions about the case, even after Mr. McNaughton signed a release provided by the insurer to allow it to discuss details of his interactions with the company. United noted that it ultimately paid for all of Mr. McNaughton’s treatments. In a written response, United spokesperson Maria Gordon Shydlo wrote that the company’s guiding concern was Mr. McNaughton’s well-being.
“Mr. McNaughton’s treatment involves medication dosages that far exceed [Food and Drug Administration] guidelines,” the statement said. “In cases like this, we review treatment plans based on current clinical guidelines to help ensure patient safety.”
But the records reviewed by ProPublica show that United had another, equally urgent goal in dealing with Mr. McNaughton. In emails, officials calculated what Mr. McNaughton was costing them to keep his crippling disease at bay and how much they would save if they forced him to undergo a cheaper treatment that had already failed him. As the family pressed the company to back down, first through Penn State and then through a lawsuit, the United officials handling the case bristled.
“This is just unbelievable,” Ms. Kavanaugh said of Mr. McNaughton’s family in one call to discuss his case. ”They’re just really pushing the envelope, and I’m surprised, like I don’t even know what to say.”
The same meal every day
Now 31, Mr. McNaughton grew up in State College, Pa., just blocks from the Penn State campus. Both of his parents are faculty members at the university.
In the winter of 2014, Mr. McNaughton was halfway through his junior year at Bard College in New York. At 6 feet, 4 inches tall, he was a guard on the basketball team and had started most of the team’s games since the start of his sophomore year. He was majoring in psychology.
When Mr. McNaughton returned to school after the winter holiday break, he started to experience frequent bouts of bloody diarrhea. After just a few days on campus, he went home to State College, where doctors diagnosed him with a severe case of ulcerative colitis.
A chronic inflammatory bowel disease that causes swelling and ulcers in the digestive tract, ulcerative colitis has no cure, and ongoing treatment is needed to alleviate symptoms and prevent serious health complications. The majority of cases produce mild to moderate symptoms. Mr. McNaughton’s case was severe.
Treatments for ulcerative colitis include steroids and special drugs known as biologics that work to reduce inflammation in the large intestine.
Mr. McNaughton, however, failed to get meaningful relief from the drugs his doctors initially prescribed. He was experiencing bloody diarrhea up to 20 times a day, with such severe stomach pain that he spent much of his day curled up on a couch. He had little appetite and lost 50 pounds. Severe anemia left him fatigued. He suffered from other conditions related to his colitis, including crippling arthritis. He was hospitalized several times to treat dangerous blood clots.
For 2 years, in an effort to help alleviate his symptoms, he ate the same meals every day: Rice Chex cereal and scrambled eggs for breakfast, a cup of white rice with plain chicken breast for lunch, and a similar meal for dinner, occasionally swapping in tilapia.
His hometown doctors referred him to a specialist at the University of Pittsburgh, who tried unsuccessfully to bring his disease under control. That doctor ended up referring Mr. McNaughton to Edward V. Loftus Jr., MD, at the Mayo Clinic in Rochester, Minn., which has been ranked as the best gastroenterology hospital in the country every year since 1990 by U.S. News & World Report.
For his first visit with Dr. Loftus in May 2015, Mr. McNaughton and his mother, Janice Light, charted hospitals along the 900-mile drive from Pennsylvania to Minnesota in case they needed medical help along the way.
Mornings were the hardest. Mr. McNaughton often spent several hours in the bathroom at the start of the day. To prepare for his meeting with Dr. Loftus, he set his alarm for 3:30 a.m. so he could be ready for the 7:30 a.m. appointment. Even with that preparation, he had to stop twice to use a bathroom on the 5-minute walk from the hotel to the clinic. When they met, Dr. Loftus looked at Mr. McNaughton and told him that he appeared incapacitated. It was, he told the student, as if Mr. McNaughton were chained to the bathroom, with no outside life. He had not been able to return to school and spent most days indoors, managing his symptoms as best he could.
Mr. McNaughton had tried a number of medications by this point, none of which worked. This pattern would repeat itself during the first couple of years that Dr. Loftus treated him.
In addition to trying to find a treatment that would bring Mr. McNaughton’s colitis into remission, Dr. Loftus wanted to wean him off the steroid prednisone, which he had been taking since his initial diagnosis in 2014. The drug is commonly prescribed to colitis patients to control inflammation, but prolonged use can lead to severe side effects including cataracts, osteoporosis, increased risk of infection, and fatigue. Mr. McNaughton also experienced “moon face,” a side effect caused by the shifting of fat deposits that results in the face becoming puffy and rounder.
In 2018, Dr. Loftus and Mr. McNaughton decided to try an unusual regimen. Many patients with inflammatory bowel diseases such as colitis take a single biologic drug as treatment. Whereas traditional drugs are chemically synthesized, biologics are manufactured in living systems, such as plant or animal cells. A year’s supply of an individual biologic drug can cost up to $500,000. They are often given through infusions in a medical facility, which adds to the cost.
Mr. McNaughton had tried individual biologics, and then two in combination, without much success. He and Dr. Loftus then agreed to try two biologic drugs together at doses well above those recommended by the Food and Drug Administration. The federal Agency for Healthcare Research and Quality estimates one in five prescriptions written today are for off-label uses.
There are drawbacks to the practice. Since some uses and doses of particular drugs have not been extensively studied, the risks and efficacy of using them off-label are not well known. Also, some drug manufacturers have improperly pushed off-label usage of their products to boost sales despite little or no evidence to support their use in those situations. Like many leading experts and researchers in his field, Dr. Loftus has been paid to do consulting related to the biologic drugs taken by Mr. McNaughton. The payments related to those drugs have ranged from a total of $1,440 in 2020 to $51,235 in 2018. Dr. Loftus said much of his work with pharmaceutical companies was related to conducting clinical trials on new drugs.
In cases of off-label prescribing, patients are depending upon their doctors’ expertise and experience with the drug. “In this case, I was comfortable that the potential benefits to Chris outweighed the risks,” Dr. Loftus said.
There was evidence that the treatment plan for Mr. McNaughton might work, including studies that had found dual biologic therapy to be efficacious and safe. The two drugs he takes, Entyvio and Remicade, have the same purpose – to reduce inflammation in the large intestine – but each works differently in the body. Remicade, marketed by Janssen Biotech, targets a protein that causes inflammation. Entyvio, made by Takeda Pharmaceuticals, works by preventing an excess of white blood cells from entering into the gastrointestinal tract.
As for any suggestion by United doctors that his treatment plan for Mr. McNaughton was out of bounds or dangerous, Dr. Loftus said “my treatment of Chris was not clinically inappropriate – as was shown by Chris’ positive outcome.”
The unusual high-dose combination of two biologic drugs produced a remarkable change in Mr. McNaughton. He no longer had blood in his stool, and his trips to the bathroom were cut from 20 times a day to 3 or 4. He was able to eat different foods and put on weight. He had more energy. He tapered off prednisone.
“If you told me in 2015 that I would be living like this, I would have asked where do I sign up,” Mr. McNaughton said of the change he experienced with the new drug regimen.
When he first started the new treatment, Mr. McNaughton was covered under his family’s plan, and all his bills were paid. Mr. McNaughton enrolled at the university in 2020. Before switching to United’s plan for students, Mr. McNaughton and his parents consulted with a health advocacy service offered to faculty members. A benefits specialist assured them the drugs taken by Mr. McNaughton would be covered by United.
Mr. McNaughton joined the student plan in July 2020, and his infusions that month and the following month were paid for by United. In September, the insurer indicated payment on his claims was “pending,” something it did for his other claims that came in during the rest of the year.
Mr. McNaughton and his family were worried. They called United to make sure there wasn’t a problem; the insurer told them, they said, that it only needed to check his medical records. When the family called again, United told them it had the documentation needed, they said. United, in a court filing last year, said it received two calls from the family and each time indicated that all of the necessary medical records had not yet been received.
In January 2021, Mr. McNaughton received a new explanation of benefits for the prior months. All of the claims for his care, beginning in September, were no longer “pending.” They were stamped “DENIED.” The total outstanding bill for his treatment was $807,086.
When Mr. McNaughton’s mother reached a United customer service representative the next day to ask why bills that had been paid in the summer were being denied for the fall, the representative told her the account was being reviewed because of “a high dollar amount on the claims,” according to a recording of the call.
Misrepresentations
With United refusing to pay, the family was terrified of being stuck with medical bills that would bankrupt them and deprive Mr. McNaughton of treatment that they considered miraculous.
They turned to Penn State for help. Ms. Light and Mr. McNaughton’s father, David McNaughton, hoped their position as faculty members would make the school more willing to intervene on their behalf.
“After more than 30 years on faculty, my husband and I know that this is not how Penn State would want its students to be treated,” Ms. Light wrote to a school official in February 2021.
In response to questions from ProPublica, Penn State spokesperson Lisa Powers wrote that “supporting the health and well-being of our students is always of primary importance” and that “our hearts go out to any student and family impacted by a serious medical condition.” The university, she wrote, does “not comment on students’ individual circumstances or disclose information from their records.” Mr. McNaughton offered to grant Penn State whatever permissions it needed to speak about his case with ProPublica. The school, however, wrote that it would not comment “even if confidentiality has been waived.”
The family appealed to school administrators. Because the effectiveness of biologics wanes in some patients if doses are skipped, Mr. McNaughton and his parents were worried about even a delay in treatment. His doctor wrote that if he missed scheduled infusions of the drugs, there was “a high likelihood they would no longer be effective.”
During a conference call arranged by Penn State officials on March 5, 2021, United agreed to pay for Mr. McNaughton’s care through the end of the plan year that August. Penn State immediately notified the family of the “wonderful news” while also apologizing for “the stress this has caused Chris and your family.”
Behind the scenes, Mr. McNaughton’s review had “gone all the way to the top” at United’s student health plan division, Ms. Kavanaugh, the nurse, said in a recorded conversation.
The family’s relief was short-lived. A month later, United started another review of Mr. McNaughton’s care, overseen by Ms. Kavanaugh, to determine if it would pay for the treatment in the upcoming plan year.
The nurse sent the Mr. McNaughton case to a company called Medical Review Institute of America. Insurers often turn to companies like MRIoA to review coverage decisions involving expensive treatments or specialized care.
Ms. Kavanaugh, who was assigned to a special investigations unit at United, let her feelings about the matter be known in a recorded telephone call with a representative of MRIoA.
“This school apparently is a big client of ours,” she said. She then shared her opinion of Mr. McNaughton’s treatment. “Really this is a case of a kid who’s getting a drug way too much, like too much of a dose,” Ms. Kavanaugh said. She said it was “insane that they would even think that this is reasonable” and “to be honest with you, they’re awfully pushy considering that we are paying through the end of this school year.”
On a call with an outside contractor, the United nurse claimed Mr. McNaughton was on a higher dose of medication than the FDA approved, which is a common practice.
MRIoA sent the case to Vikas Pabby, MD, a gastroenterologist at UCLA Health and a professor at the university’s medical school. His May 2021 review of Mr. McNaughton’s case was just one of more than 300 Dr. Pabby did for MRIoA that month, for which he was paid $23,000 in total, according to a log of his work produced in the lawsuit.
In a May 4, 2021, report, Dr. Pabby concluded Mr. McNaughton’s treatment was not medically necessary, because United’s policies for the two drugs taken by Mr. McNaughton did not support using them in combination.
Insurers spell out what services they cover in plan policies, lengthy documents that can be confusing and difficult to understand. Many policies, such as Mr. McNaughton’s, contain a provision that treatments and procedures must be “medically necessary” in order to be covered. The definition of medically necessary differs by plan. Some don’t even define the term. Mr. McNaughton’s policy contains a five-part definition, including that the treatment must be “in accordance with the standards of good medical policy” and “the most appropriate supply or level of service which can be safely provided.”
Behind the scenes at United, Mr. Opperman and Ms. Kavanaugh agreed that if Mr. McNaughton were to appeal Dr. Pabby’s decision, the insurer would simply rule against him. “I just think it’s a waste of money and time to appeal and send it to another one when we know we’re gonna get the same answer,” Mr. Opperman said, according to a recording in court files. At Mr. Opperman’s urging, United decided to skip the usual appeals process and arrange for Dr. Pabby to have a so-called “peer-to-peer” discussion with Dr. Loftus, the Mayo physician treating Mr. McNaughton. Such a conversation, in which a patient’s doctor talks with an insurance company’s doctor to advocate for the prescribed treatment, usually occurs only after a customer has appealed a denial and the appeal has been rejected.
When Ms. Kavanaugh called Dr. Loftus’ office to set up a conversation with Dr. Pabby, she explained it was an urgent matter and had been requested by Mr. McNaughton. “You know I’ve just gotten to know Christopher,” she explained, although she had never spoken with him. “We’re trying to advocate and help and get this peer-to-peer set up.”
Mr. McNaughton, meanwhile, had no idea at the time that a United doctor had decided his treatment was unnecessary and that the insurer was trying to set up a phone call with his physician.
In the peer-to-peer conversation, Dr. Loftus told Dr. Pabby that Mr. McNaughton had “a very complicated case” and that lower doses had not worked for him, according to an internal MRIoA memo.
Following his conversation with Dr. Loftus, Dr. Pabby created a second report for United. He recommended the insurer pay for both drugs, but at reduced doses. He added new language saying that the safety of using both drugs at the higher levels “is not established.”
When Ms. Kavanaugh shared the May 12 decision from Dr. Pabby with others at United, her boss responded with an email calling it “great news.”
Then Mr. Opperman sent an email that puzzled the McNaughtons.
In it, Mr. Opperman claimed that Dr. Loftus and Dr. Pabby had agreed that Mr. McNaughton should be on significantly lower doses of both drugs. He said Dr. Loftus “will work with the patient to start titrating them down to a normal dose range.” Mr. Opperman wrote that United would cover Mr. McNaughton’s treatment in the coming year, but only at the reduced doses. Mr. Opperman did not respond to emails and phone messages seeking comment.
Mr. McNaughton didn’t believe a word of it. He had already tried and failed treatment with those drugs at lower doses, and it was Dr. Loftus who had upped the doses, leading to his remission from severe colitis.
The only thing that made sense to Mr. McNaughton was that the treatment United said it would now pay for was dramatically cheaper – saving the company at least hundreds of thousands of dollars a year – than his prescribed treatment because it sliced the size of the doses by more than half.
When the family contacted Dr. Loftus for an explanation, they were outraged by what they heard. Dr. Loftus told them that he had never recommended lowering the dosage. In a letter, Dr. Loftus wrote that changing Mr. McNaughton’s treatment “would have serious detrimental effects on both his short term and long term health and could potentially involve life threatening complications. This would ultimately incur far greater medical costs. Chris was on the doses suggested by United Healthcare before, and they were not at all effective.”
It would not be until the lawsuit that it would become clear how Dr. Loftus’ conversations had been so seriously misrepresented.
Under questioning by Mr. McNaughton’s lawyers, Ms. Kavanaugh acknowledged that she was the source of the incorrect claim that Mr. McNaughton’s doctor had agreed to a change in treatment.
“I incorrectly made an assumption that they had come to some sort of agreement,” she said in a deposition last August. “It was my first peer-to-peer. I did not realize that that simply does not occur.”
Ms. Kavanaugh did not respond to emails and telephone messages seeking comment.
When the McNaughtons first learned of Mr. Opperman’s inaccurate report of the phone call with Dr. Loftus, it unnerved them. They started to question if their case would be fairly reviewed.
“When we got the denial and they lied about what Dr. Loftus said, it just hit me that none of this matters,” Mr. McNaughton said. “They will just say or do anything to get rid of me. It delegitimized the entire review process. When I got that denial, I was crushed.”
A buried report
While the family tried to sort out the inaccurate report, United continued putting the McNaughton case in front of more company doctors.
On May 21, 2021, United sent the case to one of its own doctors, Nady Cates, MD, for an additional review. The review was marked “escalated issue.” Dr. Cates is a United medical director, a title used by many insurers for physicians who review cases. It is work he has been doing as an employee of health insurers since 1989 and at United since 2010. He has not practiced medicine since the early 1990s.
Dr. Cates, in a deposition, said he stopped seeing patients because of the long hours involved and because “AIDS was coming around then. I was seeing a lot of military folks who had venereal diseases, and I guess I was concerned about being exposed.” He transitioned to reviewing paperwork for the insurance industry, he said, because “I guess I was a chicken.”
When he had practiced, Dr. Cates said, he hadn’t treated patients with ulcerative colitis and had referred those cases to a gastroenterologist.
He said his review of Mr. McNaughton’s case primarily involved reading a United nurse’s recommendation to deny his care and making sure “that there wasn’t a decimal place that was out of line.” He said he copied and pasted the nurse’s recommendation and typed “agree” on his review of Mr. McNaughton’s case.
Dr. Cates said that he does about a hundred reviews a week. He said that in his reviews he typically checks to see if any medications are prescribed in accordance with the insurer’s guidelines, and if not, he denies it. United’s policies, he said, prevented him from considering that Mr. McNaughton had failed other treatments or that Dr. Loftus was a leading expert in his field.
“You are giving zero weight to the treating doctor’s opinion on the necessity of the treatment regimen?” a lawyer asked Dr. Cates in his deposition. He responded, “Yeah.”
Attempts to contact Dr. Cates for comment were unsuccessful.
At the same time Dr. Cates was looking at Mr. McNaughton’s case, yet another review was underway at MRIoA. United said it sent the case back to MRIoA after the insurer received the letter from Dr. Loftus warning of the life-threatening complications that might occur if the dosages were reduced.
On May 24, 2021, the new report requested by MRIoA arrived. It came to a completely different conclusion than all of the previous reviews.
Nitin Kumar, MD, a gastroenterologist in Illinois, concluded that Mr. McNaughton’s established treatment plan was not only medically necessary and appropriate but that lowering his doses “can result in a lack of effective therapy of Ulcerative Colitis, with complications of uncontrolled disease (including dysplasia leading to colorectal cancer), flare, hospitalization, need for surgery, and toxic megacolon.”
Unlike other doctors who produced reports for United, Dr. Kumar discussed the harm that Mr. McNaughton might suffer if United required him to change his treatment. “His disease is significantly severe, with diagnosis at a young age,” Dr. Kumar wrote. “He has failed every biologic medication class recommended by guidelines. Therefore, guidelines can no longer be applied in this case.” He cited six studies of patients using two biologic drugs together and wrote that they revealed no significant safety issues and found the therapy to be “broadly successful.”
When Ms. Kavanaugh learned of Dr. Kumar’s report, she quickly moved to quash it and get the case returned to Dr. Pabby, according to her deposition.
In a recorded telephone call, Ms. Kavanaugh told an MRIoA representative that “I had asked that this go back through Dr. Pabby, and it went through a different doctor and they had a much different result.” After further discussion, the MRIoA representative agreed to send the case back to Dr. Pabby. “I appreciate that,” Ms. Kavanaugh replied. “I just want to make sure, because, I mean, it’s obviously a very different result than what we’ve been getting on this case.”
MRIoA case notes show that at 7:04 a.m. on May 25, 2021, Dr. Pabby was assigned to take a look at the case for the third time. At 7:27 a.m., the notes indicate, Dr. Pabby again rejected Mr. McNaughton’s treatment plan. While noting it was “difficult to control” Mr. McNaughton’s ulcerative colitis, Dr. Pabby added that his doses “far exceed what is approved by literature” and that the “safety of the requested doses is not supported by literature.”
In a deposition, Ms. Kavanaugh said that after she opened the Kumar report and read that he was supporting Mr. McNaughton’s current treatment plan, she immediately spoke to her supervisor, who told her to call MRIoA and have the case sent back to Dr. Pabby for review.
Ms. Kavanaugh said she didn’t save a copy of the Kumar report, nor did she forward it to anyone at United or to officials at Penn State who had been inquiring about the McNaughton case. “I didn’t because it shouldn’t have existed,” she said. “It should have gone back to Dr. Pabby.”
When asked if the Kumar report caused her any concerns given his warning that Mr. McNaughton risked cancer or hospitalization if his regimen were changed, Ms. Kavanaugh said she didn’t read his full report. “I saw that it was not the correct doctor, I saw the initial outcome and I was asked to send it back,” she said. Ms. Kavanaugh added, “I have a lot of empathy for this member, but it needed to go back to the peer-to-peer reviewer.”
In a court filing, United said Ms. Kavanaugh was correct in insisting that Dr. Pabby conduct the review and that MRIoA confirmed that Dr. Pabby should have been the one doing the review.
The Kumar report was not provided to Mr. McNaughton when his lawyer, Jonathan M. Gesk, first asked United and MRIoA for any reviews of the case. Mr. Gesk discovered it by accident when he was listening to a recorded telephone call produced by United in which Ms. Kavanaugh mentioned a report number Mr. Gesk had not heard before. He then called MRIoA, which confirmed the report existed and eventually provided it to him.
Dr. Pabby asked ProPublica to direct any questions about his involvement in the matter to MRIoA. The company did not respond to questions from ProPublica about the case.
A sense of hopelessness
When Mr. McNaughton enrolled at Penn State in 2020, it brought a sense of normalcy that he had lost when he was first diagnosed with colitis. He still needed monthly hours-long infusions and suffered occasional flare-ups and symptoms, but he was attending classes in person and living a life similar to the one he had before his diagnosis.
It was a striking contrast to the previous 6 years, which he had spent largely confined to his parents’ house in State College. The frequent bouts of diarrhea made it difficult to go out. He didn’t talk much to friends and spent as much time as he could studying potential treatments and reviewing ongoing clinical trials. He tried to keep up with the occasional online course, but his disease made it difficult to make any real progress toward a degree.
United, in correspondence with Mr. McNaughton, noted that its review of his care was “not a treatment decision. Treatment decisions are made between you and your physician.” But by threatening not to pay for his medications, or only to pay for a different regimen, Mr. McNaughton said, United was in fact attempting to dictate his treatment. From his perspective, the insurer was playing doctor, making decisions without ever examining him or even speaking to him.
The idea of changing his treatment or stopping it altogether caused constant worry for Mr. McNaughton, exacerbating his colitis and triggering physical symptoms, according to his doctors. Those included a large ulcer on his leg and welts under his skin on his thighs and shin that made his leg muscles stiff and painful to the point where he couldn’t bend his leg or walk properly. There were daily migraines and severe stomach pain. “I was consumed with this situation,” Mr. McNaughton said. “My path was unconventional, but I was proud of myself for fighting back and finishing school and getting my life back on track. I thought they were singling me out. My biggest fear was going back to the hell.”
Mr. McNaughton said he contemplated suicide on several occasions, dreading a return to a life where he was housebound or hospitalized.
Mr. McNaughton and his parents talked about his possibly moving to Canada where his grandmother lived and seeking treatment there under the nation’s government health plan.
Dr. Loftus connected Mr. McNaughton with a psychologist who specializes in helping patients with chronic digestive diseases.
The psychologist, Tiffany Taft, PsyD, said Mr. McNaughton was not an unusual case. About one in three patients with diseases like colitis suffer from medical trauma or PTSD related to it, she said, often the result of issues related to getting appropriate treatment approved by insurers.
“You get into hopelessness,” she said of the depression that accompanies fighting with insurance companies over care. “They feel like ‘I can’t fix that. I am screwed.’ When you can’t control things with what an insurance company is doing, anxiety, PTSD and depression get mixed together.”
In the case of Mr. McNaughton, Dr. Taft said, he was being treated by one of the best gastroenterologists in the world, was doing well with his treatment, and then was suddenly notified he might be on the hook for nearly a million dollars in medical charges without access to his medications. “It sends you immediately into panic about all these horrific things that could happen,” Dr. Taft said. The physical and mental symptoms Mr. McNaughton suffered after his care was threatened were “triggered” by the stress he experienced, she said.
In early June 2021, United informed Mr. McNaughton in a letter that it would not cover the cost of his treatment regimen in the next academic year, starting in August. The insurer said it would pay only for a treatment plan that called for a significant reduction in the doses of the drugs he took.
United wrote that the decision came after his “records have been reviewed three times and the medical reviewers have concluded that the medication as prescribed does not meet the Medical Necessity requirement of the plan.”
In August 2021, Mr. McNaughton filed a federal lawsuit accusing United of acting in bad faith and unreasonably making treatment decisions based on financial concerns and not what was the best and most effective treatment. It claims United had a duty to find information that supported Mr. McNaughton’s claim for treatment rather than looking for ways to deny coverage.
United, in a court filing, said it did not breach any duty it owed to Mr. McNaughton and acted in good faith. On Sept. 20, 2021, a month after filing the lawsuit, and with United again balking at paying for his treatment, Mr. McNaughton asked a judge to grant a temporary restraining order requiring United to pay for his care. With the looming threat of a court hearing on the motion, United quickly agreed to cover the cost of Mr. McNaughton’s treatment through the end of the 2021-2022 academic year. It also dropped a demand requiring Mr. McNaughton to settle the matter as a condition of the insurer paying for his treatment as prescribed by Dr. Loftus, according to an email sent by United’s lawyer.
The cost of treatment
It is not surprising that insurers are carefully scrutinizing the care of patients treated with biologics, which are among the most expensive medications on the market. Biologics are considered specialty drugs, a class that includes the best-selling Humira, used to treat arthritis. Specialty drug spending in the United States is expected to reach $505 billion in 2023, according to an estimate from Optum, United’s health services division. The Institute for Clinical and Economic Review, a nonprofit that analyzes the value of drugs, found in 2020 that the biologic drugs used to treat patients like Mr. McNaughton are often effective but overpriced for their therapeutic benefit. To be judged cost-effective by ICER, the biologics should sell at a steep discount to their current market price, the panel found.
A panel convened by ICER to review its analysis cautioned that insurance coverage “should be structured to prevent situations in which patients are forced to choose a treatment approach on the basis of cost.” ICER also found examples where insurance company policies failed to keep pace with updates to clinical practice guidelines based on emerging research.
United officials did not make the cost of treatment an issue when discussing Mr. McNaughton’s care with Penn State administrators or the family.
Bill Truxal, the president of UnitedHealthcare StudentResources, the company’s student health plan division, told a Penn State official that the insurer wanted the “best for the student” and it had “nothing to do with cost,” according to notes the official took of the conversation.
Behind the scenes, however, the price of Mr. McNaughton’s care was front and center at United.
In one email, Mr. Opperman asked about the cost difference if the insurer insisted on paying only for greatly reduced doses of the biologic drugs. Ms. Kavanaugh responded that the insurer had paid $1.1 million in claims for Mr. McNaughton’s care as of the middle of May 2021. If the reduced doses had been in place, the amount would have been cut to $260,218, she wrote.
United was keeping close tabs on Mr. McNaughton at the highest levels of the company. On Aug. 2, 2021, Mr. Opperman notified Mr. Truxal and a United lawyer that Mr. McNaughton “has just purchased the plan again for the 21-22 school year.”
A month later, Ms. Kavanaugh shared another calculation with United executives showing that the insurer spent over $1.7 million on Mr. McNaughton in the prior plan year.
United officials strategized about how to best explain why it was reviewing Mr. McNaughton’s drug regimen, according to an internal email. They pointed to a justification often used by health insurers when denying claims. “As the cost of healthcare continues to climb to soaring heights, it has been determined that a judicious review of these drugs should be included” in order to “make healthcare more affordable for our members,” Ms. Kavanaugh offered as a potential talking point in an April 23, 2021, email.
Three days later, UnitedHealth Group filed an annual statement with the U.S. Securities and Exchange Commission disclosing its pay for top executives in the prior year. Then-CEO David Wichmann was paid $17.9 million in salary and other compensation in 2020. Wichmann retired early the following year, and his total compensation that year exceeded $140 million, according to calculations in a compensation database maintained by the Star Tribune in Minneapolis. The newspaper said the amount was the most paid to an executive in the state since it started tracking pay more than 2 decades ago. About $110 million of that total came from Wichmann exercising stock options accumulated during his stewardship.
The McNaughtons were well aware of the financial situation at United. They looked at publicly available financial results and annual reports. Last year, United reported a profit of $20.1 billion on revenues of $324.2 billion.
When discussing the case with Penn State, Ms. Light said, she told university administrators that United could pay for a year of her son’s treatment using just minutes’ worth of profit.
‘Betrayed’
Mr. McNaughton has been able to continue receiving his infusions for now, anyway. In October, United notified him it was once again reviewing his care, although the insurer quickly reversed course when his lawyer intervened. United, in a court filing, said the review was a mistake and that it had erred in putting Mr. McNaughton’s claims into pending status.
Mr. McNaughton said he is fortunate his parents were employed at the same school he was attending, which was critical in getting the attention of administrators there. But that help had its limits.
In June 2021, just a week after United told Mr. McNaughton it would not cover his treatment plan in the upcoming plan year, Penn State essentially walked away from the matter.
In an email to the McNaughtons and United, Penn State Associate Vice President for Student Affairs Andrea Dowhower wrote that administrators “have observed an unfortunate breakdown in communication” between Mr. McNaughton and his family and the university health insurance plan, “which appears from our perspective to have resulted in a standstill between the two parties.” While she proposed some potential steps to help settle the matter, she wrote that “Penn State’s role in this process is as a resource for students like Chris who, for whatever reason, have experienced difficulty navigating the complex world of health insurance.” The university’s role “is limited,” she wrote, and the school “simply must leave” the issue of the best treatment for Mr. McNaughton to “the appropriate health care professionals.”
In a statement, a Penn State spokesperson wrote that “as a third party in this arrangement, the University’s role is limited and Penn State officials can only help a student manage an issue based on information that a student/family, medical personnel, and/or insurance provider give – with the hope that all information is accurate and that the lines of communication remain open between the insured and the insurer.”
Penn State declined to provide financial information about the plan. However, the university and United share at least one tie that they have not publicly disclosed.
When the McNaughtons first reached out to the university for help, they were referred to the school’s student health insurance coordinator. The official, Heather Klinger, wrote in an email to the family in February 2021 that “I appreciate your trusting me to resolve this for you.”
In April 2022, United began paying Ms. Klinger’s salary, an arrangement which is not noted on the university website. Ms. Klinger appears in the online staff directory on the Penn State University Health Services web page, and has a university phone number, a university address, and a Penn State email listed as her contact. The school said she has maintained a part-time status with the university to allow her to access relevant data systems at both the university and United.
The university said students “benefit” from having a United employee to handle questions about insurance coverage and that the arrangement is “not uncommon” for student health plans.
The family was dismayed to learn that Ms. Klinger was now a full-time employee of United.
“We did feel betrayed,” Ms. Light said. Ms. Klinger did not respond to an email seeking comment.
Mr. McNaughton’s fight to maintain his treatment regimen has come at a cost of time, debilitating stress, and depression. “My biggest fear is realizing I might have to do this every year of my life,” he said.
Mr. McNaughton said one motivation for his lawsuit was to expose how insurers like United make decisions about what care they will pay for and what they will not. The case remains pending, a court docket shows.
He has been accepted to Penn State’s law school. He hopes to become a health care lawyer working for patients who find themselves in situations similar to his.
He plans to re-enroll in the United health care plan when he starts school next fall.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
In May 2021, a nurse at UnitedHealthcare called a colleague to share some welcome news about a problem the two had been grappling with for weeks.
United provided the health insurance plan for students at Penn State University. It was a large and potentially lucrative account: lots of young, healthy students paying premiums in, not too many huge medical reimbursements going out.
But Christopher McNaughton suffered from a crippling case of ulcerative colitis – an ailment that caused him to develop severe arthritis, debilitating diarrhea, numbing fatigue, and life-threatening blood clots. His medical bills were running nearly $2 million a year.
United had flagged Mr. McNaughton’s case as a “high dollar account,” and the company was reviewing whether it needed to keep paying for the expensive cocktail of drugs crafted by a Mayo Clinic specialist that had brought Mr. McNaughton’s disease under control after he’d been through years of misery.
On the 2021 phone call, which was recorded by the company, nurse Victoria Kavanaugh told her colleague that a doctor contracted by United to review the case had concluded that Mr. McNaughton’s treatment was “not medically necessary.” Her colleague, Dave Opperman, reacted to the news with a long laugh.
“I knew that was coming,” said Mr. Opperman, who heads up a United subsidiary that brokered the health insurance contract between United and Penn State. “I did too,” Ms. Kavanaugh replied.
Mr. Opperman then complained about Mr. McNaughton’s mother, whom he referred to as “this woman,” for “screaming and yelling” and “throwing tantrums” during calls with United.
The pair agreed that any appeal of the United doctor’s denial of the treatment would be a waste of the family’s time and money.
“We’re still gonna say no,” Mr. Opperman said.
More than 200 million Americans are covered by private health insurance. But data from state and federal regulators shows that insurers reject about 1 in 7 claims for treatment. Many people, faced with fighting insurance companies, simply give up: One study found that Americans file formal appeals on only 0.1% of claims denied by insurers under the Affordable Care Act.
Insurers have wide discretion in crafting what is covered by their policies, beyond some basic services mandated by federal and state law. They often deny claims for services that they deem not “medically necessary.”
When United refused to pay for Mr. McNaughton’s treatment for that reason, his family did something unusual. They fought back with a lawsuit, which uncovered a trove of materials, including internal emails and tape-recorded exchanges among company employees. Those records offer an extraordinary behind-the-scenes look at how one of America’s leading health care insurers relentlessly fought to reduce spending on care, even as its profits rose to record levels.
As United reviewed Mr. McNaughton’s treatment, he and his family were often in the dark about what was happening or their rights. Meanwhile, United employees misrepresented critical findings and ignored warnings from doctors about the risks of altering Mr. McNaughton’s drug plan.
At one point, court records show, United inaccurately reported to Penn State and the family that Mr. McNaughton’s doctor had agreed to lower the doses of his medication. Another time, a doctor paid by United concluded that denying payments for Mr. McNaughton’s treatment could put his health at risk, but the company buried his report and did not consider its findings. The insurer did, however, consider a report submitted by a company doctor who rubber-stamped the recommendation of a United nurse to reject paying for the treatment.
United declined to answer specific questions about the case, even after Mr. McNaughton signed a release provided by the insurer to allow it to discuss details of his interactions with the company. United noted that it ultimately paid for all of Mr. McNaughton’s treatments. In a written response, United spokesperson Maria Gordon Shydlo wrote that the company’s guiding concern was Mr. McNaughton’s well-being.
“Mr. McNaughton’s treatment involves medication dosages that far exceed [Food and Drug Administration] guidelines,” the statement said. “In cases like this, we review treatment plans based on current clinical guidelines to help ensure patient safety.”
But the records reviewed by ProPublica show that United had another, equally urgent goal in dealing with Mr. McNaughton. In emails, officials calculated what Mr. McNaughton was costing them to keep his crippling disease at bay and how much they would save if they forced him to undergo a cheaper treatment that had already failed him. As the family pressed the company to back down, first through Penn State and then through a lawsuit, the United officials handling the case bristled.
“This is just unbelievable,” Ms. Kavanaugh said of Mr. McNaughton’s family in one call to discuss his case. ”They’re just really pushing the envelope, and I’m surprised, like I don’t even know what to say.”
The same meal every day
Now 31, Mr. McNaughton grew up in State College, Pa., just blocks from the Penn State campus. Both of his parents are faculty members at the university.
In the winter of 2014, Mr. McNaughton was halfway through his junior year at Bard College in New York. At 6 feet, 4 inches tall, he was a guard on the basketball team and had started most of the team’s games since the start of his sophomore year. He was majoring in psychology.
When Mr. McNaughton returned to school after the winter holiday break, he started to experience frequent bouts of bloody diarrhea. After just a few days on campus, he went home to State College, where doctors diagnosed him with a severe case of ulcerative colitis.
A chronic inflammatory bowel disease that causes swelling and ulcers in the digestive tract, ulcerative colitis has no cure, and ongoing treatment is needed to alleviate symptoms and prevent serious health complications. The majority of cases produce mild to moderate symptoms. Mr. McNaughton’s case was severe.
Treatments for ulcerative colitis include steroids and special drugs known as biologics that work to reduce inflammation in the large intestine.
Mr. McNaughton, however, failed to get meaningful relief from the drugs his doctors initially prescribed. He was experiencing bloody diarrhea up to 20 times a day, with such severe stomach pain that he spent much of his day curled up on a couch. He had little appetite and lost 50 pounds. Severe anemia left him fatigued. He suffered from other conditions related to his colitis, including crippling arthritis. He was hospitalized several times to treat dangerous blood clots.
For 2 years, in an effort to help alleviate his symptoms, he ate the same meals every day: Rice Chex cereal and scrambled eggs for breakfast, a cup of white rice with plain chicken breast for lunch, and a similar meal for dinner, occasionally swapping in tilapia.
His hometown doctors referred him to a specialist at the University of Pittsburgh, who tried unsuccessfully to bring his disease under control. That doctor ended up referring Mr. McNaughton to Edward V. Loftus Jr., MD, at the Mayo Clinic in Rochester, Minn., which has been ranked as the best gastroenterology hospital in the country every year since 1990 by U.S. News & World Report.
For his first visit with Dr. Loftus in May 2015, Mr. McNaughton and his mother, Janice Light, charted hospitals along the 900-mile drive from Pennsylvania to Minnesota in case they needed medical help along the way.
Mornings were the hardest. Mr. McNaughton often spent several hours in the bathroom at the start of the day. To prepare for his meeting with Dr. Loftus, he set his alarm for 3:30 a.m. so he could be ready for the 7:30 a.m. appointment. Even with that preparation, he had to stop twice to use a bathroom on the 5-minute walk from the hotel to the clinic. When they met, Dr. Loftus looked at Mr. McNaughton and told him that he appeared incapacitated. It was, he told the student, as if Mr. McNaughton were chained to the bathroom, with no outside life. He had not been able to return to school and spent most days indoors, managing his symptoms as best he could.
Mr. McNaughton had tried a number of medications by this point, none of which worked. This pattern would repeat itself during the first couple of years that Dr. Loftus treated him.
In addition to trying to find a treatment that would bring Mr. McNaughton’s colitis into remission, Dr. Loftus wanted to wean him off the steroid prednisone, which he had been taking since his initial diagnosis in 2014. The drug is commonly prescribed to colitis patients to control inflammation, but prolonged use can lead to severe side effects including cataracts, osteoporosis, increased risk of infection, and fatigue. Mr. McNaughton also experienced “moon face,” a side effect caused by the shifting of fat deposits that results in the face becoming puffy and rounder.
In 2018, Dr. Loftus and Mr. McNaughton decided to try an unusual regimen. Many patients with inflammatory bowel diseases such as colitis take a single biologic drug as treatment. Whereas traditional drugs are chemically synthesized, biologics are manufactured in living systems, such as plant or animal cells. A year’s supply of an individual biologic drug can cost up to $500,000. They are often given through infusions in a medical facility, which adds to the cost.
Mr. McNaughton had tried individual biologics, and then two in combination, without much success. He and Dr. Loftus then agreed to try two biologic drugs together at doses well above those recommended by the Food and Drug Administration. The federal Agency for Healthcare Research and Quality estimates one in five prescriptions written today are for off-label uses.
There are drawbacks to the practice. Since some uses and doses of particular drugs have not been extensively studied, the risks and efficacy of using them off-label are not well known. Also, some drug manufacturers have improperly pushed off-label usage of their products to boost sales despite little or no evidence to support their use in those situations. Like many leading experts and researchers in his field, Dr. Loftus has been paid to do consulting related to the biologic drugs taken by Mr. McNaughton. The payments related to those drugs have ranged from a total of $1,440 in 2020 to $51,235 in 2018. Dr. Loftus said much of his work with pharmaceutical companies was related to conducting clinical trials on new drugs.
In cases of off-label prescribing, patients are depending upon their doctors’ expertise and experience with the drug. “In this case, I was comfortable that the potential benefits to Chris outweighed the risks,” Dr. Loftus said.
There was evidence that the treatment plan for Mr. McNaughton might work, including studies that had found dual biologic therapy to be efficacious and safe. The two drugs he takes, Entyvio and Remicade, have the same purpose – to reduce inflammation in the large intestine – but each works differently in the body. Remicade, marketed by Janssen Biotech, targets a protein that causes inflammation. Entyvio, made by Takeda Pharmaceuticals, works by preventing an excess of white blood cells from entering into the gastrointestinal tract.
As for any suggestion by United doctors that his treatment plan for Mr. McNaughton was out of bounds or dangerous, Dr. Loftus said “my treatment of Chris was not clinically inappropriate – as was shown by Chris’ positive outcome.”
The unusual high-dose combination of two biologic drugs produced a remarkable change in Mr. McNaughton. He no longer had blood in his stool, and his trips to the bathroom were cut from 20 times a day to 3 or 4. He was able to eat different foods and put on weight. He had more energy. He tapered off prednisone.
“If you told me in 2015 that I would be living like this, I would have asked where do I sign up,” Mr. McNaughton said of the change he experienced with the new drug regimen.
When he first started the new treatment, Mr. McNaughton was covered under his family’s plan, and all his bills were paid. Mr. McNaughton enrolled at the university in 2020. Before switching to United’s plan for students, Mr. McNaughton and his parents consulted with a health advocacy service offered to faculty members. A benefits specialist assured them the drugs taken by Mr. McNaughton would be covered by United.
Mr. McNaughton joined the student plan in July 2020, and his infusions that month and the following month were paid for by United. In September, the insurer indicated payment on his claims was “pending,” something it did for his other claims that came in during the rest of the year.
Mr. McNaughton and his family were worried. They called United to make sure there wasn’t a problem; the insurer told them, they said, that it only needed to check his medical records. When the family called again, United told them it had the documentation needed, they said. United, in a court filing last year, said it received two calls from the family and each time indicated that all of the necessary medical records had not yet been received.
In January 2021, Mr. McNaughton received a new explanation of benefits for the prior months. All of the claims for his care, beginning in September, were no longer “pending.” They were stamped “DENIED.” The total outstanding bill for his treatment was $807,086.
When Mr. McNaughton’s mother reached a United customer service representative the next day to ask why bills that had been paid in the summer were being denied for the fall, the representative told her the account was being reviewed because of “a high dollar amount on the claims,” according to a recording of the call.
Misrepresentations
With United refusing to pay, the family was terrified of being stuck with medical bills that would bankrupt them and deprive Mr. McNaughton of treatment that they considered miraculous.
They turned to Penn State for help. Ms. Light and Mr. McNaughton’s father, David McNaughton, hoped their position as faculty members would make the school more willing to intervene on their behalf.
“After more than 30 years on faculty, my husband and I know that this is not how Penn State would want its students to be treated,” Ms. Light wrote to a school official in February 2021.
In response to questions from ProPublica, Penn State spokesperson Lisa Powers wrote that “supporting the health and well-being of our students is always of primary importance” and that “our hearts go out to any student and family impacted by a serious medical condition.” The university, she wrote, does “not comment on students’ individual circumstances or disclose information from their records.” Mr. McNaughton offered to grant Penn State whatever permissions it needed to speak about his case with ProPublica. The school, however, wrote that it would not comment “even if confidentiality has been waived.”
The family appealed to school administrators. Because the effectiveness of biologics wanes in some patients if doses are skipped, Mr. McNaughton and his parents were worried about even a delay in treatment. His doctor wrote that if he missed scheduled infusions of the drugs, there was “a high likelihood they would no longer be effective.”
During a conference call arranged by Penn State officials on March 5, 2021, United agreed to pay for Mr. McNaughton’s care through the end of the plan year that August. Penn State immediately notified the family of the “wonderful news” while also apologizing for “the stress this has caused Chris and your family.”
Behind the scenes, Mr. McNaughton’s review had “gone all the way to the top” at United’s student health plan division, Ms. Kavanaugh, the nurse, said in a recorded conversation.
The family’s relief was short-lived. A month later, United started another review of Mr. McNaughton’s care, overseen by Ms. Kavanaugh, to determine if it would pay for the treatment in the upcoming plan year.
The nurse sent the Mr. McNaughton case to a company called Medical Review Institute of America. Insurers often turn to companies like MRIoA to review coverage decisions involving expensive treatments or specialized care.
Ms. Kavanaugh, who was assigned to a special investigations unit at United, let her feelings about the matter be known in a recorded telephone call with a representative of MRIoA.
“This school apparently is a big client of ours,” she said. She then shared her opinion of Mr. McNaughton’s treatment. “Really this is a case of a kid who’s getting a drug way too much, like too much of a dose,” Ms. Kavanaugh said. She said it was “insane that they would even think that this is reasonable” and “to be honest with you, they’re awfully pushy considering that we are paying through the end of this school year.”
On a call with an outside contractor, the United nurse claimed Mr. McNaughton was on a higher dose of medication than the FDA approved, which is a common practice.
MRIoA sent the case to Vikas Pabby, MD, a gastroenterologist at UCLA Health and a professor at the university’s medical school. His May 2021 review of Mr. McNaughton’s case was just one of more than 300 Dr. Pabby did for MRIoA that month, for which he was paid $23,000 in total, according to a log of his work produced in the lawsuit.
In a May 4, 2021, report, Dr. Pabby concluded Mr. McNaughton’s treatment was not medically necessary, because United’s policies for the two drugs taken by Mr. McNaughton did not support using them in combination.
Insurers spell out what services they cover in plan policies, lengthy documents that can be confusing and difficult to understand. Many policies, such as Mr. McNaughton’s, contain a provision that treatments and procedures must be “medically necessary” in order to be covered. The definition of medically necessary differs by plan. Some don’t even define the term. Mr. McNaughton’s policy contains a five-part definition, including that the treatment must be “in accordance with the standards of good medical policy” and “the most appropriate supply or level of service which can be safely provided.”
Behind the scenes at United, Mr. Opperman and Ms. Kavanaugh agreed that if Mr. McNaughton were to appeal Dr. Pabby’s decision, the insurer would simply rule against him. “I just think it’s a waste of money and time to appeal and send it to another one when we know we’re gonna get the same answer,” Mr. Opperman said, according to a recording in court files. At Mr. Opperman’s urging, United decided to skip the usual appeals process and arrange for Dr. Pabby to have a so-called “peer-to-peer” discussion with Dr. Loftus, the Mayo physician treating Mr. McNaughton. Such a conversation, in which a patient’s doctor talks with an insurance company’s doctor to advocate for the prescribed treatment, usually occurs only after a customer has appealed a denial and the appeal has been rejected.
When Ms. Kavanaugh called Dr. Loftus’ office to set up a conversation with Dr. Pabby, she explained it was an urgent matter and had been requested by Mr. McNaughton. “You know I’ve just gotten to know Christopher,” she explained, although she had never spoken with him. “We’re trying to advocate and help and get this peer-to-peer set up.”
Mr. McNaughton, meanwhile, had no idea at the time that a United doctor had decided his treatment was unnecessary and that the insurer was trying to set up a phone call with his physician.
In the peer-to-peer conversation, Dr. Loftus told Dr. Pabby that Mr. McNaughton had “a very complicated case” and that lower doses had not worked for him, according to an internal MRIoA memo.
Following his conversation with Dr. Loftus, Dr. Pabby created a second report for United. He recommended the insurer pay for both drugs, but at reduced doses. He added new language saying that the safety of using both drugs at the higher levels “is not established.”
When Ms. Kavanaugh shared the May 12 decision from Dr. Pabby with others at United, her boss responded with an email calling it “great news.”
Then Mr. Opperman sent an email that puzzled the McNaughtons.
In it, Mr. Opperman claimed that Dr. Loftus and Dr. Pabby had agreed that Mr. McNaughton should be on significantly lower doses of both drugs. He said Dr. Loftus “will work with the patient to start titrating them down to a normal dose range.” Mr. Opperman wrote that United would cover Mr. McNaughton’s treatment in the coming year, but only at the reduced doses. Mr. Opperman did not respond to emails and phone messages seeking comment.
Mr. McNaughton didn’t believe a word of it. He had already tried and failed treatment with those drugs at lower doses, and it was Dr. Loftus who had upped the doses, leading to his remission from severe colitis.
The only thing that made sense to Mr. McNaughton was that the treatment United said it would now pay for was dramatically cheaper – saving the company at least hundreds of thousands of dollars a year – than his prescribed treatment because it sliced the size of the doses by more than half.
When the family contacted Dr. Loftus for an explanation, they were outraged by what they heard. Dr. Loftus told them that he had never recommended lowering the dosage. In a letter, Dr. Loftus wrote that changing Mr. McNaughton’s treatment “would have serious detrimental effects on both his short term and long term health and could potentially involve life threatening complications. This would ultimately incur far greater medical costs. Chris was on the doses suggested by United Healthcare before, and they were not at all effective.”
It would not be until the lawsuit that it would become clear how Dr. Loftus’ conversations had been so seriously misrepresented.
Under questioning by Mr. McNaughton’s lawyers, Ms. Kavanaugh acknowledged that she was the source of the incorrect claim that Mr. McNaughton’s doctor had agreed to a change in treatment.
“I incorrectly made an assumption that they had come to some sort of agreement,” she said in a deposition last August. “It was my first peer-to-peer. I did not realize that that simply does not occur.”
Ms. Kavanaugh did not respond to emails and telephone messages seeking comment.
When the McNaughtons first learned of Mr. Opperman’s inaccurate report of the phone call with Dr. Loftus, it unnerved them. They started to question if their case would be fairly reviewed.
“When we got the denial and they lied about what Dr. Loftus said, it just hit me that none of this matters,” Mr. McNaughton said. “They will just say or do anything to get rid of me. It delegitimized the entire review process. When I got that denial, I was crushed.”
A buried report
While the family tried to sort out the inaccurate report, United continued putting the McNaughton case in front of more company doctors.
On May 21, 2021, United sent the case to one of its own doctors, Nady Cates, MD, for an additional review. The review was marked “escalated issue.” Dr. Cates is a United medical director, a title used by many insurers for physicians who review cases. It is work he has been doing as an employee of health insurers since 1989 and at United since 2010. He has not practiced medicine since the early 1990s.
Dr. Cates, in a deposition, said he stopped seeing patients because of the long hours involved and because “AIDS was coming around then. I was seeing a lot of military folks who had venereal diseases, and I guess I was concerned about being exposed.” He transitioned to reviewing paperwork for the insurance industry, he said, because “I guess I was a chicken.”
When he had practiced, Dr. Cates said, he hadn’t treated patients with ulcerative colitis and had referred those cases to a gastroenterologist.
He said his review of Mr. McNaughton’s case primarily involved reading a United nurse’s recommendation to deny his care and making sure “that there wasn’t a decimal place that was out of line.” He said he copied and pasted the nurse’s recommendation and typed “agree” on his review of Mr. McNaughton’s case.
Dr. Cates said that he does about a hundred reviews a week. He said that in his reviews he typically checks to see if any medications are prescribed in accordance with the insurer’s guidelines, and if not, he denies it. United’s policies, he said, prevented him from considering that Mr. McNaughton had failed other treatments or that Dr. Loftus was a leading expert in his field.
“You are giving zero weight to the treating doctor’s opinion on the necessity of the treatment regimen?” a lawyer asked Dr. Cates in his deposition. He responded, “Yeah.”
Attempts to contact Dr. Cates for comment were unsuccessful.
At the same time Dr. Cates was looking at Mr. McNaughton’s case, yet another review was underway at MRIoA. United said it sent the case back to MRIoA after the insurer received the letter from Dr. Loftus warning of the life-threatening complications that might occur if the dosages were reduced.
On May 24, 2021, the new report requested by MRIoA arrived. It came to a completely different conclusion than all of the previous reviews.
Nitin Kumar, MD, a gastroenterologist in Illinois, concluded that Mr. McNaughton’s established treatment plan was not only medically necessary and appropriate but that lowering his doses “can result in a lack of effective therapy of Ulcerative Colitis, with complications of uncontrolled disease (including dysplasia leading to colorectal cancer), flare, hospitalization, need for surgery, and toxic megacolon.”
Unlike other doctors who produced reports for United, Dr. Kumar discussed the harm that Mr. McNaughton might suffer if United required him to change his treatment. “His disease is significantly severe, with diagnosis at a young age,” Dr. Kumar wrote. “He has failed every biologic medication class recommended by guidelines. Therefore, guidelines can no longer be applied in this case.” He cited six studies of patients using two biologic drugs together and wrote that they revealed no significant safety issues and found the therapy to be “broadly successful.”
When Ms. Kavanaugh learned of Dr. Kumar’s report, she quickly moved to quash it and get the case returned to Dr. Pabby, according to her deposition.
In a recorded telephone call, Ms. Kavanaugh told an MRIoA representative that “I had asked that this go back through Dr. Pabby, and it went through a different doctor and they had a much different result.” After further discussion, the MRIoA representative agreed to send the case back to Dr. Pabby. “I appreciate that,” Ms. Kavanaugh replied. “I just want to make sure, because, I mean, it’s obviously a very different result than what we’ve been getting on this case.”
MRIoA case notes show that at 7:04 a.m. on May 25, 2021, Dr. Pabby was assigned to take a look at the case for the third time. At 7:27 a.m., the notes indicate, Dr. Pabby again rejected Mr. McNaughton’s treatment plan. While noting it was “difficult to control” Mr. McNaughton’s ulcerative colitis, Dr. Pabby added that his doses “far exceed what is approved by literature” and that the “safety of the requested doses is not supported by literature.”
In a deposition, Ms. Kavanaugh said that after she opened the Kumar report and read that he was supporting Mr. McNaughton’s current treatment plan, she immediately spoke to her supervisor, who told her to call MRIoA and have the case sent back to Dr. Pabby for review.
Ms. Kavanaugh said she didn’t save a copy of the Kumar report, nor did she forward it to anyone at United or to officials at Penn State who had been inquiring about the McNaughton case. “I didn’t because it shouldn’t have existed,” she said. “It should have gone back to Dr. Pabby.”
When asked if the Kumar report caused her any concerns given his warning that Mr. McNaughton risked cancer or hospitalization if his regimen were changed, Ms. Kavanaugh said she didn’t read his full report. “I saw that it was not the correct doctor, I saw the initial outcome and I was asked to send it back,” she said. Ms. Kavanaugh added, “I have a lot of empathy for this member, but it needed to go back to the peer-to-peer reviewer.”
In a court filing, United said Ms. Kavanaugh was correct in insisting that Dr. Pabby conduct the review and that MRIoA confirmed that Dr. Pabby should have been the one doing the review.
The Kumar report was not provided to Mr. McNaughton when his lawyer, Jonathan M. Gesk, first asked United and MRIoA for any reviews of the case. Mr. Gesk discovered it by accident when he was listening to a recorded telephone call produced by United in which Ms. Kavanaugh mentioned a report number Mr. Gesk had not heard before. He then called MRIoA, which confirmed the report existed and eventually provided it to him.
Dr. Pabby asked ProPublica to direct any questions about his involvement in the matter to MRIoA. The company did not respond to questions from ProPublica about the case.
A sense of hopelessness
When Mr. McNaughton enrolled at Penn State in 2020, it brought a sense of normalcy that he had lost when he was first diagnosed with colitis. He still needed monthly hours-long infusions and suffered occasional flare-ups and symptoms, but he was attending classes in person and living a life similar to the one he had before his diagnosis.
It was a striking contrast to the previous 6 years, which he had spent largely confined to his parents’ house in State College. The frequent bouts of diarrhea made it difficult to go out. He didn’t talk much to friends and spent as much time as he could studying potential treatments and reviewing ongoing clinical trials. He tried to keep up with the occasional online course, but his disease made it difficult to make any real progress toward a degree.
United, in correspondence with Mr. McNaughton, noted that its review of his care was “not a treatment decision. Treatment decisions are made between you and your physician.” But by threatening not to pay for his medications, or only to pay for a different regimen, Mr. McNaughton said, United was in fact attempting to dictate his treatment. From his perspective, the insurer was playing doctor, making decisions without ever examining him or even speaking to him.
The idea of changing his treatment or stopping it altogether caused constant worry for Mr. McNaughton, exacerbating his colitis and triggering physical symptoms, according to his doctors. Those included a large ulcer on his leg and welts under his skin on his thighs and shin that made his leg muscles stiff and painful to the point where he couldn’t bend his leg or walk properly. There were daily migraines and severe stomach pain. “I was consumed with this situation,” Mr. McNaughton said. “My path was unconventional, but I was proud of myself for fighting back and finishing school and getting my life back on track. I thought they were singling me out. My biggest fear was going back to the hell.”
Mr. McNaughton said he contemplated suicide on several occasions, dreading a return to a life where he was housebound or hospitalized.
Mr. McNaughton and his parents talked about his possibly moving to Canada where his grandmother lived and seeking treatment there under the nation’s government health plan.
Dr. Loftus connected Mr. McNaughton with a psychologist who specializes in helping patients with chronic digestive diseases.
The psychologist, Tiffany Taft, PsyD, said Mr. McNaughton was not an unusual case. About one in three patients with diseases like colitis suffer from medical trauma or PTSD related to it, she said, often the result of issues related to getting appropriate treatment approved by insurers.
“You get into hopelessness,” she said of the depression that accompanies fighting with insurance companies over care. “They feel like ‘I can’t fix that. I am screwed.’ When you can’t control things with what an insurance company is doing, anxiety, PTSD and depression get mixed together.”
In the case of Mr. McNaughton, Dr. Taft said, he was being treated by one of the best gastroenterologists in the world, was doing well with his treatment, and then was suddenly notified he might be on the hook for nearly a million dollars in medical charges without access to his medications. “It sends you immediately into panic about all these horrific things that could happen,” Dr. Taft said. The physical and mental symptoms Mr. McNaughton suffered after his care was threatened were “triggered” by the stress he experienced, she said.
In early June 2021, United informed Mr. McNaughton in a letter that it would not cover the cost of his treatment regimen in the next academic year, starting in August. The insurer said it would pay only for a treatment plan that called for a significant reduction in the doses of the drugs he took.
United wrote that the decision came after his “records have been reviewed three times and the medical reviewers have concluded that the medication as prescribed does not meet the Medical Necessity requirement of the plan.”
In August 2021, Mr. McNaughton filed a federal lawsuit accusing United of acting in bad faith and unreasonably making treatment decisions based on financial concerns and not what was the best and most effective treatment. It claims United had a duty to find information that supported Mr. McNaughton’s claim for treatment rather than looking for ways to deny coverage.
United, in a court filing, said it did not breach any duty it owed to Mr. McNaughton and acted in good faith. On Sept. 20, 2021, a month after filing the lawsuit, and with United again balking at paying for his treatment, Mr. McNaughton asked a judge to grant a temporary restraining order requiring United to pay for his care. With the looming threat of a court hearing on the motion, United quickly agreed to cover the cost of Mr. McNaughton’s treatment through the end of the 2021-2022 academic year. It also dropped a demand requiring Mr. McNaughton to settle the matter as a condition of the insurer paying for his treatment as prescribed by Dr. Loftus, according to an email sent by United’s lawyer.
The cost of treatment
It is not surprising that insurers are carefully scrutinizing the care of patients treated with biologics, which are among the most expensive medications on the market. Biologics are considered specialty drugs, a class that includes the best-selling Humira, used to treat arthritis. Specialty drug spending in the United States is expected to reach $505 billion in 2023, according to an estimate from Optum, United’s health services division. The Institute for Clinical and Economic Review, a nonprofit that analyzes the value of drugs, found in 2020 that the biologic drugs used to treat patients like Mr. McNaughton are often effective but overpriced for their therapeutic benefit. To be judged cost-effective by ICER, the biologics should sell at a steep discount to their current market price, the panel found.
A panel convened by ICER to review its analysis cautioned that insurance coverage “should be structured to prevent situations in which patients are forced to choose a treatment approach on the basis of cost.” ICER also found examples where insurance company policies failed to keep pace with updates to clinical practice guidelines based on emerging research.
United officials did not make the cost of treatment an issue when discussing Mr. McNaughton’s care with Penn State administrators or the family.
Bill Truxal, the president of UnitedHealthcare StudentResources, the company’s student health plan division, told a Penn State official that the insurer wanted the “best for the student” and it had “nothing to do with cost,” according to notes the official took of the conversation.
Behind the scenes, however, the price of Mr. McNaughton’s care was front and center at United.
In one email, Mr. Opperman asked about the cost difference if the insurer insisted on paying only for greatly reduced doses of the biologic drugs. Ms. Kavanaugh responded that the insurer had paid $1.1 million in claims for Mr. McNaughton’s care as of the middle of May 2021. If the reduced doses had been in place, the amount would have been cut to $260,218, she wrote.
United was keeping close tabs on Mr. McNaughton at the highest levels of the company. On Aug. 2, 2021, Mr. Opperman notified Mr. Truxal and a United lawyer that Mr. McNaughton “has just purchased the plan again for the 21-22 school year.”
A month later, Ms. Kavanaugh shared another calculation with United executives showing that the insurer spent over $1.7 million on Mr. McNaughton in the prior plan year.
United officials strategized about how to best explain why it was reviewing Mr. McNaughton’s drug regimen, according to an internal email. They pointed to a justification often used by health insurers when denying claims. “As the cost of healthcare continues to climb to soaring heights, it has been determined that a judicious review of these drugs should be included” in order to “make healthcare more affordable for our members,” Ms. Kavanaugh offered as a potential talking point in an April 23, 2021, email.
Three days later, UnitedHealth Group filed an annual statement with the U.S. Securities and Exchange Commission disclosing its pay for top executives in the prior year. Then-CEO David Wichmann was paid $17.9 million in salary and other compensation in 2020. Wichmann retired early the following year, and his total compensation that year exceeded $140 million, according to calculations in a compensation database maintained by the Star Tribune in Minneapolis. The newspaper said the amount was the most paid to an executive in the state since it started tracking pay more than 2 decades ago. About $110 million of that total came from Wichmann exercising stock options accumulated during his stewardship.
The McNaughtons were well aware of the financial situation at United. They looked at publicly available financial results and annual reports. Last year, United reported a profit of $20.1 billion on revenues of $324.2 billion.
When discussing the case with Penn State, Ms. Light said, she told university administrators that United could pay for a year of her son’s treatment using just minutes’ worth of profit.
‘Betrayed’
Mr. McNaughton has been able to continue receiving his infusions for now, anyway. In October, United notified him it was once again reviewing his care, although the insurer quickly reversed course when his lawyer intervened. United, in a court filing, said the review was a mistake and that it had erred in putting Mr. McNaughton’s claims into pending status.
Mr. McNaughton said he is fortunate his parents were employed at the same school he was attending, which was critical in getting the attention of administrators there. But that help had its limits.
In June 2021, just a week after United told Mr. McNaughton it would not cover his treatment plan in the upcoming plan year, Penn State essentially walked away from the matter.
In an email to the McNaughtons and United, Penn State Associate Vice President for Student Affairs Andrea Dowhower wrote that administrators “have observed an unfortunate breakdown in communication” between Mr. McNaughton and his family and the university health insurance plan, “which appears from our perspective to have resulted in a standstill between the two parties.” While she proposed some potential steps to help settle the matter, she wrote that “Penn State’s role in this process is as a resource for students like Chris who, for whatever reason, have experienced difficulty navigating the complex world of health insurance.” The university’s role “is limited,” she wrote, and the school “simply must leave” the issue of the best treatment for Mr. McNaughton to “the appropriate health care professionals.”
In a statement, a Penn State spokesperson wrote that “as a third party in this arrangement, the University’s role is limited and Penn State officials can only help a student manage an issue based on information that a student/family, medical personnel, and/or insurance provider give – with the hope that all information is accurate and that the lines of communication remain open between the insured and the insurer.”
Penn State declined to provide financial information about the plan. However, the university and United share at least one tie that they have not publicly disclosed.
When the McNaughtons first reached out to the university for help, they were referred to the school’s student health insurance coordinator. The official, Heather Klinger, wrote in an email to the family in February 2021 that “I appreciate your trusting me to resolve this for you.”
In April 2022, United began paying Ms. Klinger’s salary, an arrangement which is not noted on the university website. Ms. Klinger appears in the online staff directory on the Penn State University Health Services web page, and has a university phone number, a university address, and a Penn State email listed as her contact. The school said she has maintained a part-time status with the university to allow her to access relevant data systems at both the university and United.
The university said students “benefit” from having a United employee to handle questions about insurance coverage and that the arrangement is “not uncommon” for student health plans.
The family was dismayed to learn that Ms. Klinger was now a full-time employee of United.
“We did feel betrayed,” Ms. Light said. Ms. Klinger did not respond to an email seeking comment.
Mr. McNaughton’s fight to maintain his treatment regimen has come at a cost of time, debilitating stress, and depression. “My biggest fear is realizing I might have to do this every year of my life,” he said.
Mr. McNaughton said one motivation for his lawsuit was to expose how insurers like United make decisions about what care they will pay for and what they will not. The case remains pending, a court docket shows.
He has been accepted to Penn State’s law school. He hopes to become a health care lawyer working for patients who find themselves in situations similar to his.
He plans to re-enroll in the United health care plan when he starts school next fall.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
In May 2021, a nurse at UnitedHealthcare called a colleague to share some welcome news about a problem the two had been grappling with for weeks.
United provided the health insurance plan for students at Penn State University. It was a large and potentially lucrative account: lots of young, healthy students paying premiums in, not too many huge medical reimbursements going out.
But Christopher McNaughton suffered from a crippling case of ulcerative colitis – an ailment that caused him to develop severe arthritis, debilitating diarrhea, numbing fatigue, and life-threatening blood clots. His medical bills were running nearly $2 million a year.
United had flagged Mr. McNaughton’s case as a “high dollar account,” and the company was reviewing whether it needed to keep paying for the expensive cocktail of drugs crafted by a Mayo Clinic specialist that had brought Mr. McNaughton’s disease under control after he’d been through years of misery.
On the 2021 phone call, which was recorded by the company, nurse Victoria Kavanaugh told her colleague that a doctor contracted by United to review the case had concluded that Mr. McNaughton’s treatment was “not medically necessary.” Her colleague, Dave Opperman, reacted to the news with a long laugh.
“I knew that was coming,” said Mr. Opperman, who heads up a United subsidiary that brokered the health insurance contract between United and Penn State. “I did too,” Ms. Kavanaugh replied.
Mr. Opperman then complained about Mr. McNaughton’s mother, whom he referred to as “this woman,” for “screaming and yelling” and “throwing tantrums” during calls with United.
The pair agreed that any appeal of the United doctor’s denial of the treatment would be a waste of the family’s time and money.
“We’re still gonna say no,” Mr. Opperman said.
More than 200 million Americans are covered by private health insurance. But data from state and federal regulators shows that insurers reject about 1 in 7 claims for treatment. Many people, faced with fighting insurance companies, simply give up: One study found that Americans file formal appeals on only 0.1% of claims denied by insurers under the Affordable Care Act.
Insurers have wide discretion in crafting what is covered by their policies, beyond some basic services mandated by federal and state law. They often deny claims for services that they deem not “medically necessary.”
When United refused to pay for Mr. McNaughton’s treatment for that reason, his family did something unusual. They fought back with a lawsuit, which uncovered a trove of materials, including internal emails and tape-recorded exchanges among company employees. Those records offer an extraordinary behind-the-scenes look at how one of America’s leading health care insurers relentlessly fought to reduce spending on care, even as its profits rose to record levels.
As United reviewed Mr. McNaughton’s treatment, he and his family were often in the dark about what was happening or their rights. Meanwhile, United employees misrepresented critical findings and ignored warnings from doctors about the risks of altering Mr. McNaughton’s drug plan.
At one point, court records show, United inaccurately reported to Penn State and the family that Mr. McNaughton’s doctor had agreed to lower the doses of his medication. Another time, a doctor paid by United concluded that denying payments for Mr. McNaughton’s treatment could put his health at risk, but the company buried his report and did not consider its findings. The insurer did, however, consider a report submitted by a company doctor who rubber-stamped the recommendation of a United nurse to reject paying for the treatment.
United declined to answer specific questions about the case, even after Mr. McNaughton signed a release provided by the insurer to allow it to discuss details of his interactions with the company. United noted that it ultimately paid for all of Mr. McNaughton’s treatments. In a written response, United spokesperson Maria Gordon Shydlo wrote that the company’s guiding concern was Mr. McNaughton’s well-being.
“Mr. McNaughton’s treatment involves medication dosages that far exceed [Food and Drug Administration] guidelines,” the statement said. “In cases like this, we review treatment plans based on current clinical guidelines to help ensure patient safety.”
But the records reviewed by ProPublica show that United had another, equally urgent goal in dealing with Mr. McNaughton. In emails, officials calculated what Mr. McNaughton was costing them to keep his crippling disease at bay and how much they would save if they forced him to undergo a cheaper treatment that had already failed him. As the family pressed the company to back down, first through Penn State and then through a lawsuit, the United officials handling the case bristled.
“This is just unbelievable,” Ms. Kavanaugh said of Mr. McNaughton’s family in one call to discuss his case. ”They’re just really pushing the envelope, and I’m surprised, like I don’t even know what to say.”
The same meal every day
Now 31, Mr. McNaughton grew up in State College, Pa., just blocks from the Penn State campus. Both of his parents are faculty members at the university.
In the winter of 2014, Mr. McNaughton was halfway through his junior year at Bard College in New York. At 6 feet, 4 inches tall, he was a guard on the basketball team and had started most of the team’s games since the start of his sophomore year. He was majoring in psychology.
When Mr. McNaughton returned to school after the winter holiday break, he started to experience frequent bouts of bloody diarrhea. After just a few days on campus, he went home to State College, where doctors diagnosed him with a severe case of ulcerative colitis.
A chronic inflammatory bowel disease that causes swelling and ulcers in the digestive tract, ulcerative colitis has no cure, and ongoing treatment is needed to alleviate symptoms and prevent serious health complications. The majority of cases produce mild to moderate symptoms. Mr. McNaughton’s case was severe.
Treatments for ulcerative colitis include steroids and special drugs known as biologics that work to reduce inflammation in the large intestine.
Mr. McNaughton, however, failed to get meaningful relief from the drugs his doctors initially prescribed. He was experiencing bloody diarrhea up to 20 times a day, with such severe stomach pain that he spent much of his day curled up on a couch. He had little appetite and lost 50 pounds. Severe anemia left him fatigued. He suffered from other conditions related to his colitis, including crippling arthritis. He was hospitalized several times to treat dangerous blood clots.
For 2 years, in an effort to help alleviate his symptoms, he ate the same meals every day: Rice Chex cereal and scrambled eggs for breakfast, a cup of white rice with plain chicken breast for lunch, and a similar meal for dinner, occasionally swapping in tilapia.
His hometown doctors referred him to a specialist at the University of Pittsburgh, who tried unsuccessfully to bring his disease under control. That doctor ended up referring Mr. McNaughton to Edward V. Loftus Jr., MD, at the Mayo Clinic in Rochester, Minn., which has been ranked as the best gastroenterology hospital in the country every year since 1990 by U.S. News & World Report.
For his first visit with Dr. Loftus in May 2015, Mr. McNaughton and his mother, Janice Light, charted hospitals along the 900-mile drive from Pennsylvania to Minnesota in case they needed medical help along the way.
Mornings were the hardest. Mr. McNaughton often spent several hours in the bathroom at the start of the day. To prepare for his meeting with Dr. Loftus, he set his alarm for 3:30 a.m. so he could be ready for the 7:30 a.m. appointment. Even with that preparation, he had to stop twice to use a bathroom on the 5-minute walk from the hotel to the clinic. When they met, Dr. Loftus looked at Mr. McNaughton and told him that he appeared incapacitated. It was, he told the student, as if Mr. McNaughton were chained to the bathroom, with no outside life. He had not been able to return to school and spent most days indoors, managing his symptoms as best he could.
Mr. McNaughton had tried a number of medications by this point, none of which worked. This pattern would repeat itself during the first couple of years that Dr. Loftus treated him.
In addition to trying to find a treatment that would bring Mr. McNaughton’s colitis into remission, Dr. Loftus wanted to wean him off the steroid prednisone, which he had been taking since his initial diagnosis in 2014. The drug is commonly prescribed to colitis patients to control inflammation, but prolonged use can lead to severe side effects including cataracts, osteoporosis, increased risk of infection, and fatigue. Mr. McNaughton also experienced “moon face,” a side effect caused by the shifting of fat deposits that results in the face becoming puffy and rounder.
In 2018, Dr. Loftus and Mr. McNaughton decided to try an unusual regimen. Many patients with inflammatory bowel diseases such as colitis take a single biologic drug as treatment. Whereas traditional drugs are chemically synthesized, biologics are manufactured in living systems, such as plant or animal cells. A year’s supply of an individual biologic drug can cost up to $500,000. They are often given through infusions in a medical facility, which adds to the cost.
Mr. McNaughton had tried individual biologics, and then two in combination, without much success. He and Dr. Loftus then agreed to try two biologic drugs together at doses well above those recommended by the Food and Drug Administration. The federal Agency for Healthcare Research and Quality estimates one in five prescriptions written today are for off-label uses.
There are drawbacks to the practice. Since some uses and doses of particular drugs have not been extensively studied, the risks and efficacy of using them off-label are not well known. Also, some drug manufacturers have improperly pushed off-label usage of their products to boost sales despite little or no evidence to support their use in those situations. Like many leading experts and researchers in his field, Dr. Loftus has been paid to do consulting related to the biologic drugs taken by Mr. McNaughton. The payments related to those drugs have ranged from a total of $1,440 in 2020 to $51,235 in 2018. Dr. Loftus said much of his work with pharmaceutical companies was related to conducting clinical trials on new drugs.
In cases of off-label prescribing, patients are depending upon their doctors’ expertise and experience with the drug. “In this case, I was comfortable that the potential benefits to Chris outweighed the risks,” Dr. Loftus said.
There was evidence that the treatment plan for Mr. McNaughton might work, including studies that had found dual biologic therapy to be efficacious and safe. The two drugs he takes, Entyvio and Remicade, have the same purpose – to reduce inflammation in the large intestine – but each works differently in the body. Remicade, marketed by Janssen Biotech, targets a protein that causes inflammation. Entyvio, made by Takeda Pharmaceuticals, works by preventing an excess of white blood cells from entering into the gastrointestinal tract.
As for any suggestion by United doctors that his treatment plan for Mr. McNaughton was out of bounds or dangerous, Dr. Loftus said “my treatment of Chris was not clinically inappropriate – as was shown by Chris’ positive outcome.”
The unusual high-dose combination of two biologic drugs produced a remarkable change in Mr. McNaughton. He no longer had blood in his stool, and his trips to the bathroom were cut from 20 times a day to 3 or 4. He was able to eat different foods and put on weight. He had more energy. He tapered off prednisone.
“If you told me in 2015 that I would be living like this, I would have asked where do I sign up,” Mr. McNaughton said of the change he experienced with the new drug regimen.
When he first started the new treatment, Mr. McNaughton was covered under his family’s plan, and all his bills were paid. Mr. McNaughton enrolled at the university in 2020. Before switching to United’s plan for students, Mr. McNaughton and his parents consulted with a health advocacy service offered to faculty members. A benefits specialist assured them the drugs taken by Mr. McNaughton would be covered by United.
Mr. McNaughton joined the student plan in July 2020, and his infusions that month and the following month were paid for by United. In September, the insurer indicated payment on his claims was “pending,” something it did for his other claims that came in during the rest of the year.
Mr. McNaughton and his family were worried. They called United to make sure there wasn’t a problem; the insurer told them, they said, that it only needed to check his medical records. When the family called again, United told them it had the documentation needed, they said. United, in a court filing last year, said it received two calls from the family and each time indicated that all of the necessary medical records had not yet been received.
In January 2021, Mr. McNaughton received a new explanation of benefits for the prior months. All of the claims for his care, beginning in September, were no longer “pending.” They were stamped “DENIED.” The total outstanding bill for his treatment was $807,086.
When Mr. McNaughton’s mother reached a United customer service representative the next day to ask why bills that had been paid in the summer were being denied for the fall, the representative told her the account was being reviewed because of “a high dollar amount on the claims,” according to a recording of the call.
Misrepresentations
With United refusing to pay, the family was terrified of being stuck with medical bills that would bankrupt them and deprive Mr. McNaughton of treatment that they considered miraculous.
They turned to Penn State for help. Ms. Light and Mr. McNaughton’s father, David McNaughton, hoped their position as faculty members would make the school more willing to intervene on their behalf.
“After more than 30 years on faculty, my husband and I know that this is not how Penn State would want its students to be treated,” Ms. Light wrote to a school official in February 2021.
In response to questions from ProPublica, Penn State spokesperson Lisa Powers wrote that “supporting the health and well-being of our students is always of primary importance” and that “our hearts go out to any student and family impacted by a serious medical condition.” The university, she wrote, does “not comment on students’ individual circumstances or disclose information from their records.” Mr. McNaughton offered to grant Penn State whatever permissions it needed to speak about his case with ProPublica. The school, however, wrote that it would not comment “even if confidentiality has been waived.”
The family appealed to school administrators. Because the effectiveness of biologics wanes in some patients if doses are skipped, Mr. McNaughton and his parents were worried about even a delay in treatment. His doctor wrote that if he missed scheduled infusions of the drugs, there was “a high likelihood they would no longer be effective.”
During a conference call arranged by Penn State officials on March 5, 2021, United agreed to pay for Mr. McNaughton’s care through the end of the plan year that August. Penn State immediately notified the family of the “wonderful news” while also apologizing for “the stress this has caused Chris and your family.”
Behind the scenes, Mr. McNaughton’s review had “gone all the way to the top” at United’s student health plan division, Ms. Kavanaugh, the nurse, said in a recorded conversation.
The family’s relief was short-lived. A month later, United started another review of Mr. McNaughton’s care, overseen by Ms. Kavanaugh, to determine if it would pay for the treatment in the upcoming plan year.
The nurse sent the Mr. McNaughton case to a company called Medical Review Institute of America. Insurers often turn to companies like MRIoA to review coverage decisions involving expensive treatments or specialized care.
Ms. Kavanaugh, who was assigned to a special investigations unit at United, let her feelings about the matter be known in a recorded telephone call with a representative of MRIoA.
“This school apparently is a big client of ours,” she said. She then shared her opinion of Mr. McNaughton’s treatment. “Really this is a case of a kid who’s getting a drug way too much, like too much of a dose,” Ms. Kavanaugh said. She said it was “insane that they would even think that this is reasonable” and “to be honest with you, they’re awfully pushy considering that we are paying through the end of this school year.”
On a call with an outside contractor, the United nurse claimed Mr. McNaughton was on a higher dose of medication than the FDA approved, which is a common practice.
MRIoA sent the case to Vikas Pabby, MD, a gastroenterologist at UCLA Health and a professor at the university’s medical school. His May 2021 review of Mr. McNaughton’s case was just one of more than 300 Dr. Pabby did for MRIoA that month, for which he was paid $23,000 in total, according to a log of his work produced in the lawsuit.
In a May 4, 2021, report, Dr. Pabby concluded Mr. McNaughton’s treatment was not medically necessary, because United’s policies for the two drugs taken by Mr. McNaughton did not support using them in combination.
Insurers spell out what services they cover in plan policies, lengthy documents that can be confusing and difficult to understand. Many policies, such as Mr. McNaughton’s, contain a provision that treatments and procedures must be “medically necessary” in order to be covered. The definition of medically necessary differs by plan. Some don’t even define the term. Mr. McNaughton’s policy contains a five-part definition, including that the treatment must be “in accordance with the standards of good medical policy” and “the most appropriate supply or level of service which can be safely provided.”
Behind the scenes at United, Mr. Opperman and Ms. Kavanaugh agreed that if Mr. McNaughton were to appeal Dr. Pabby’s decision, the insurer would simply rule against him. “I just think it’s a waste of money and time to appeal and send it to another one when we know we’re gonna get the same answer,” Mr. Opperman said, according to a recording in court files. At Mr. Opperman’s urging, United decided to skip the usual appeals process and arrange for Dr. Pabby to have a so-called “peer-to-peer” discussion with Dr. Loftus, the Mayo physician treating Mr. McNaughton. Such a conversation, in which a patient’s doctor talks with an insurance company’s doctor to advocate for the prescribed treatment, usually occurs only after a customer has appealed a denial and the appeal has been rejected.
When Ms. Kavanaugh called Dr. Loftus’ office to set up a conversation with Dr. Pabby, she explained it was an urgent matter and had been requested by Mr. McNaughton. “You know I’ve just gotten to know Christopher,” she explained, although she had never spoken with him. “We’re trying to advocate and help and get this peer-to-peer set up.”
Mr. McNaughton, meanwhile, had no idea at the time that a United doctor had decided his treatment was unnecessary and that the insurer was trying to set up a phone call with his physician.
In the peer-to-peer conversation, Dr. Loftus told Dr. Pabby that Mr. McNaughton had “a very complicated case” and that lower doses had not worked for him, according to an internal MRIoA memo.
Following his conversation with Dr. Loftus, Dr. Pabby created a second report for United. He recommended the insurer pay for both drugs, but at reduced doses. He added new language saying that the safety of using both drugs at the higher levels “is not established.”
When Ms. Kavanaugh shared the May 12 decision from Dr. Pabby with others at United, her boss responded with an email calling it “great news.”
Then Mr. Opperman sent an email that puzzled the McNaughtons.
In it, Mr. Opperman claimed that Dr. Loftus and Dr. Pabby had agreed that Mr. McNaughton should be on significantly lower doses of both drugs. He said Dr. Loftus “will work with the patient to start titrating them down to a normal dose range.” Mr. Opperman wrote that United would cover Mr. McNaughton’s treatment in the coming year, but only at the reduced doses. Mr. Opperman did not respond to emails and phone messages seeking comment.
Mr. McNaughton didn’t believe a word of it. He had already tried and failed treatment with those drugs at lower doses, and it was Dr. Loftus who had upped the doses, leading to his remission from severe colitis.
The only thing that made sense to Mr. McNaughton was that the treatment United said it would now pay for was dramatically cheaper – saving the company at least hundreds of thousands of dollars a year – than his prescribed treatment because it sliced the size of the doses by more than half.
When the family contacted Dr. Loftus for an explanation, they were outraged by what they heard. Dr. Loftus told them that he had never recommended lowering the dosage. In a letter, Dr. Loftus wrote that changing Mr. McNaughton’s treatment “would have serious detrimental effects on both his short term and long term health and could potentially involve life threatening complications. This would ultimately incur far greater medical costs. Chris was on the doses suggested by United Healthcare before, and they were not at all effective.”
It would not be until the lawsuit that it would become clear how Dr. Loftus’ conversations had been so seriously misrepresented.
Under questioning by Mr. McNaughton’s lawyers, Ms. Kavanaugh acknowledged that she was the source of the incorrect claim that Mr. McNaughton’s doctor had agreed to a change in treatment.
“I incorrectly made an assumption that they had come to some sort of agreement,” she said in a deposition last August. “It was my first peer-to-peer. I did not realize that that simply does not occur.”
Ms. Kavanaugh did not respond to emails and telephone messages seeking comment.
When the McNaughtons first learned of Mr. Opperman’s inaccurate report of the phone call with Dr. Loftus, it unnerved them. They started to question if their case would be fairly reviewed.
“When we got the denial and they lied about what Dr. Loftus said, it just hit me that none of this matters,” Mr. McNaughton said. “They will just say or do anything to get rid of me. It delegitimized the entire review process. When I got that denial, I was crushed.”
A buried report
While the family tried to sort out the inaccurate report, United continued putting the McNaughton case in front of more company doctors.
On May 21, 2021, United sent the case to one of its own doctors, Nady Cates, MD, for an additional review. The review was marked “escalated issue.” Dr. Cates is a United medical director, a title used by many insurers for physicians who review cases. It is work he has been doing as an employee of health insurers since 1989 and at United since 2010. He has not practiced medicine since the early 1990s.
Dr. Cates, in a deposition, said he stopped seeing patients because of the long hours involved and because “AIDS was coming around then. I was seeing a lot of military folks who had venereal diseases, and I guess I was concerned about being exposed.” He transitioned to reviewing paperwork for the insurance industry, he said, because “I guess I was a chicken.”
When he had practiced, Dr. Cates said, he hadn’t treated patients with ulcerative colitis and had referred those cases to a gastroenterologist.
He said his review of Mr. McNaughton’s case primarily involved reading a United nurse’s recommendation to deny his care and making sure “that there wasn’t a decimal place that was out of line.” He said he copied and pasted the nurse’s recommendation and typed “agree” on his review of Mr. McNaughton’s case.
Dr. Cates said that he does about a hundred reviews a week. He said that in his reviews he typically checks to see if any medications are prescribed in accordance with the insurer’s guidelines, and if not, he denies it. United’s policies, he said, prevented him from considering that Mr. McNaughton had failed other treatments or that Dr. Loftus was a leading expert in his field.
“You are giving zero weight to the treating doctor’s opinion on the necessity of the treatment regimen?” a lawyer asked Dr. Cates in his deposition. He responded, “Yeah.”
Attempts to contact Dr. Cates for comment were unsuccessful.
At the same time Dr. Cates was looking at Mr. McNaughton’s case, yet another review was underway at MRIoA. United said it sent the case back to MRIoA after the insurer received the letter from Dr. Loftus warning of the life-threatening complications that might occur if the dosages were reduced.
On May 24, 2021, the new report requested by MRIoA arrived. It came to a completely different conclusion than all of the previous reviews.
Nitin Kumar, MD, a gastroenterologist in Illinois, concluded that Mr. McNaughton’s established treatment plan was not only medically necessary and appropriate but that lowering his doses “can result in a lack of effective therapy of Ulcerative Colitis, with complications of uncontrolled disease (including dysplasia leading to colorectal cancer), flare, hospitalization, need for surgery, and toxic megacolon.”
Unlike other doctors who produced reports for United, Dr. Kumar discussed the harm that Mr. McNaughton might suffer if United required him to change his treatment. “His disease is significantly severe, with diagnosis at a young age,” Dr. Kumar wrote. “He has failed every biologic medication class recommended by guidelines. Therefore, guidelines can no longer be applied in this case.” He cited six studies of patients using two biologic drugs together and wrote that they revealed no significant safety issues and found the therapy to be “broadly successful.”
When Ms. Kavanaugh learned of Dr. Kumar’s report, she quickly moved to quash it and get the case returned to Dr. Pabby, according to her deposition.
In a recorded telephone call, Ms. Kavanaugh told an MRIoA representative that “I had asked that this go back through Dr. Pabby, and it went through a different doctor and they had a much different result.” After further discussion, the MRIoA representative agreed to send the case back to Dr. Pabby. “I appreciate that,” Ms. Kavanaugh replied. “I just want to make sure, because, I mean, it’s obviously a very different result than what we’ve been getting on this case.”
MRIoA case notes show that at 7:04 a.m. on May 25, 2021, Dr. Pabby was assigned to take a look at the case for the third time. At 7:27 a.m., the notes indicate, Dr. Pabby again rejected Mr. McNaughton’s treatment plan. While noting it was “difficult to control” Mr. McNaughton’s ulcerative colitis, Dr. Pabby added that his doses “far exceed what is approved by literature” and that the “safety of the requested doses is not supported by literature.”
In a deposition, Ms. Kavanaugh said that after she opened the Kumar report and read that he was supporting Mr. McNaughton’s current treatment plan, she immediately spoke to her supervisor, who told her to call MRIoA and have the case sent back to Dr. Pabby for review.
Ms. Kavanaugh said she didn’t save a copy of the Kumar report, nor did she forward it to anyone at United or to officials at Penn State who had been inquiring about the McNaughton case. “I didn’t because it shouldn’t have existed,” she said. “It should have gone back to Dr. Pabby.”
When asked if the Kumar report caused her any concerns given his warning that Mr. McNaughton risked cancer or hospitalization if his regimen were changed, Ms. Kavanaugh said she didn’t read his full report. “I saw that it was not the correct doctor, I saw the initial outcome and I was asked to send it back,” she said. Ms. Kavanaugh added, “I have a lot of empathy for this member, but it needed to go back to the peer-to-peer reviewer.”
In a court filing, United said Ms. Kavanaugh was correct in insisting that Dr. Pabby conduct the review and that MRIoA confirmed that Dr. Pabby should have been the one doing the review.
The Kumar report was not provided to Mr. McNaughton when his lawyer, Jonathan M. Gesk, first asked United and MRIoA for any reviews of the case. Mr. Gesk discovered it by accident when he was listening to a recorded telephone call produced by United in which Ms. Kavanaugh mentioned a report number Mr. Gesk had not heard before. He then called MRIoA, which confirmed the report existed and eventually provided it to him.
Dr. Pabby asked ProPublica to direct any questions about his involvement in the matter to MRIoA. The company did not respond to questions from ProPublica about the case.
A sense of hopelessness
When Mr. McNaughton enrolled at Penn State in 2020, it brought a sense of normalcy that he had lost when he was first diagnosed with colitis. He still needed monthly hours-long infusions and suffered occasional flare-ups and symptoms, but he was attending classes in person and living a life similar to the one he had before his diagnosis.
It was a striking contrast to the previous 6 years, which he had spent largely confined to his parents’ house in State College. The frequent bouts of diarrhea made it difficult to go out. He didn’t talk much to friends and spent as much time as he could studying potential treatments and reviewing ongoing clinical trials. He tried to keep up with the occasional online course, but his disease made it difficult to make any real progress toward a degree.
United, in correspondence with Mr. McNaughton, noted that its review of his care was “not a treatment decision. Treatment decisions are made between you and your physician.” But by threatening not to pay for his medications, or only to pay for a different regimen, Mr. McNaughton said, United was in fact attempting to dictate his treatment. From his perspective, the insurer was playing doctor, making decisions without ever examining him or even speaking to him.
The idea of changing his treatment or stopping it altogether caused constant worry for Mr. McNaughton, exacerbating his colitis and triggering physical symptoms, according to his doctors. Those included a large ulcer on his leg and welts under his skin on his thighs and shin that made his leg muscles stiff and painful to the point where he couldn’t bend his leg or walk properly. There were daily migraines and severe stomach pain. “I was consumed with this situation,” Mr. McNaughton said. “My path was unconventional, but I was proud of myself for fighting back and finishing school and getting my life back on track. I thought they were singling me out. My biggest fear was going back to the hell.”
Mr. McNaughton said he contemplated suicide on several occasions, dreading a return to a life where he was housebound or hospitalized.
Mr. McNaughton and his parents talked about his possibly moving to Canada where his grandmother lived and seeking treatment there under the nation’s government health plan.
Dr. Loftus connected Mr. McNaughton with a psychologist who specializes in helping patients with chronic digestive diseases.
The psychologist, Tiffany Taft, PsyD, said Mr. McNaughton was not an unusual case. About one in three patients with diseases like colitis suffer from medical trauma or PTSD related to it, she said, often the result of issues related to getting appropriate treatment approved by insurers.
“You get into hopelessness,” she said of the depression that accompanies fighting with insurance companies over care. “They feel like ‘I can’t fix that. I am screwed.’ When you can’t control things with what an insurance company is doing, anxiety, PTSD and depression get mixed together.”
In the case of Mr. McNaughton, Dr. Taft said, he was being treated by one of the best gastroenterologists in the world, was doing well with his treatment, and then was suddenly notified he might be on the hook for nearly a million dollars in medical charges without access to his medications. “It sends you immediately into panic about all these horrific things that could happen,” Dr. Taft said. The physical and mental symptoms Mr. McNaughton suffered after his care was threatened were “triggered” by the stress he experienced, she said.
In early June 2021, United informed Mr. McNaughton in a letter that it would not cover the cost of his treatment regimen in the next academic year, starting in August. The insurer said it would pay only for a treatment plan that called for a significant reduction in the doses of the drugs he took.
United wrote that the decision came after his “records have been reviewed three times and the medical reviewers have concluded that the medication as prescribed does not meet the Medical Necessity requirement of the plan.”
In August 2021, Mr. McNaughton filed a federal lawsuit accusing United of acting in bad faith and unreasonably making treatment decisions based on financial concerns and not what was the best and most effective treatment. It claims United had a duty to find information that supported Mr. McNaughton’s claim for treatment rather than looking for ways to deny coverage.
United, in a court filing, said it did not breach any duty it owed to Mr. McNaughton and acted in good faith. On Sept. 20, 2021, a month after filing the lawsuit, and with United again balking at paying for his treatment, Mr. McNaughton asked a judge to grant a temporary restraining order requiring United to pay for his care. With the looming threat of a court hearing on the motion, United quickly agreed to cover the cost of Mr. McNaughton’s treatment through the end of the 2021-2022 academic year. It also dropped a demand requiring Mr. McNaughton to settle the matter as a condition of the insurer paying for his treatment as prescribed by Dr. Loftus, according to an email sent by United’s lawyer.
The cost of treatment
It is not surprising that insurers are carefully scrutinizing the care of patients treated with biologics, which are among the most expensive medications on the market. Biologics are considered specialty drugs, a class that includes the best-selling Humira, used to treat arthritis. Specialty drug spending in the United States is expected to reach $505 billion in 2023, according to an estimate from Optum, United’s health services division. The Institute for Clinical and Economic Review, a nonprofit that analyzes the value of drugs, found in 2020 that the biologic drugs used to treat patients like Mr. McNaughton are often effective but overpriced for their therapeutic benefit. To be judged cost-effective by ICER, the biologics should sell at a steep discount to their current market price, the panel found.
A panel convened by ICER to review its analysis cautioned that insurance coverage “should be structured to prevent situations in which patients are forced to choose a treatment approach on the basis of cost.” ICER also found examples where insurance company policies failed to keep pace with updates to clinical practice guidelines based on emerging research.
United officials did not make the cost of treatment an issue when discussing Mr. McNaughton’s care with Penn State administrators or the family.
Bill Truxal, the president of UnitedHealthcare StudentResources, the company’s student health plan division, told a Penn State official that the insurer wanted the “best for the student” and it had “nothing to do with cost,” according to notes the official took of the conversation.
Behind the scenes, however, the price of Mr. McNaughton’s care was front and center at United.
In one email, Mr. Opperman asked about the cost difference if the insurer insisted on paying only for greatly reduced doses of the biologic drugs. Ms. Kavanaugh responded that the insurer had paid $1.1 million in claims for Mr. McNaughton’s care as of the middle of May 2021. If the reduced doses had been in place, the amount would have been cut to $260,218, she wrote.
United was keeping close tabs on Mr. McNaughton at the highest levels of the company. On Aug. 2, 2021, Mr. Opperman notified Mr. Truxal and a United lawyer that Mr. McNaughton “has just purchased the plan again for the 21-22 school year.”
A month later, Ms. Kavanaugh shared another calculation with United executives showing that the insurer spent over $1.7 million on Mr. McNaughton in the prior plan year.
United officials strategized about how to best explain why it was reviewing Mr. McNaughton’s drug regimen, according to an internal email. They pointed to a justification often used by health insurers when denying claims. “As the cost of healthcare continues to climb to soaring heights, it has been determined that a judicious review of these drugs should be included” in order to “make healthcare more affordable for our members,” Ms. Kavanaugh offered as a potential talking point in an April 23, 2021, email.
Three days later, UnitedHealth Group filed an annual statement with the U.S. Securities and Exchange Commission disclosing its pay for top executives in the prior year. Then-CEO David Wichmann was paid $17.9 million in salary and other compensation in 2020. Wichmann retired early the following year, and his total compensation that year exceeded $140 million, according to calculations in a compensation database maintained by the Star Tribune in Minneapolis. The newspaper said the amount was the most paid to an executive in the state since it started tracking pay more than 2 decades ago. About $110 million of that total came from Wichmann exercising stock options accumulated during his stewardship.
The McNaughtons were well aware of the financial situation at United. They looked at publicly available financial results and annual reports. Last year, United reported a profit of $20.1 billion on revenues of $324.2 billion.
When discussing the case with Penn State, Ms. Light said, she told university administrators that United could pay for a year of her son’s treatment using just minutes’ worth of profit.
‘Betrayed’
Mr. McNaughton has been able to continue receiving his infusions for now, anyway. In October, United notified him it was once again reviewing his care, although the insurer quickly reversed course when his lawyer intervened. United, in a court filing, said the review was a mistake and that it had erred in putting Mr. McNaughton’s claims into pending status.
Mr. McNaughton said he is fortunate his parents were employed at the same school he was attending, which was critical in getting the attention of administrators there. But that help had its limits.
In June 2021, just a week after United told Mr. McNaughton it would not cover his treatment plan in the upcoming plan year, Penn State essentially walked away from the matter.
In an email to the McNaughtons and United, Penn State Associate Vice President for Student Affairs Andrea Dowhower wrote that administrators “have observed an unfortunate breakdown in communication” between Mr. McNaughton and his family and the university health insurance plan, “which appears from our perspective to have resulted in a standstill between the two parties.” While she proposed some potential steps to help settle the matter, she wrote that “Penn State’s role in this process is as a resource for students like Chris who, for whatever reason, have experienced difficulty navigating the complex world of health insurance.” The university’s role “is limited,” she wrote, and the school “simply must leave” the issue of the best treatment for Mr. McNaughton to “the appropriate health care professionals.”
In a statement, a Penn State spokesperson wrote that “as a third party in this arrangement, the University’s role is limited and Penn State officials can only help a student manage an issue based on information that a student/family, medical personnel, and/or insurance provider give – with the hope that all information is accurate and that the lines of communication remain open between the insured and the insurer.”
Penn State declined to provide financial information about the plan. However, the university and United share at least one tie that they have not publicly disclosed.
When the McNaughtons first reached out to the university for help, they were referred to the school’s student health insurance coordinator. The official, Heather Klinger, wrote in an email to the family in February 2021 that “I appreciate your trusting me to resolve this for you.”
In April 2022, United began paying Ms. Klinger’s salary, an arrangement which is not noted on the university website. Ms. Klinger appears in the online staff directory on the Penn State University Health Services web page, and has a university phone number, a university address, and a Penn State email listed as her contact. The school said she has maintained a part-time status with the university to allow her to access relevant data systems at both the university and United.
The university said students “benefit” from having a United employee to handle questions about insurance coverage and that the arrangement is “not uncommon” for student health plans.
The family was dismayed to learn that Ms. Klinger was now a full-time employee of United.
“We did feel betrayed,” Ms. Light said. Ms. Klinger did not respond to an email seeking comment.
Mr. McNaughton’s fight to maintain his treatment regimen has come at a cost of time, debilitating stress, and depression. “My biggest fear is realizing I might have to do this every year of my life,” he said.
Mr. McNaughton said one motivation for his lawsuit was to expose how insurers like United make decisions about what care they will pay for and what they will not. The case remains pending, a court docket shows.
He has been accepted to Penn State’s law school. He hopes to become a health care lawyer working for patients who find themselves in situations similar to his.
He plans to re-enroll in the United health care plan when he starts school next fall.
This story was originally published on ProPublica. ProPublica is a nonprofit newsroom that investigates abuses of power. Sign up to receive the biggest stories as soon as they’re published.
Prehospital COVID therapy effective in rheumatic disease patients
Outpatient COVID-19 treatment with monoclonal antibodies or antiretroviral medications such as nirmatrelvir-ritonavir (Paxlovid) administered to patients with systemic autoimmune rheumatic disease led to lower odds of having severe outcomes when compared with similar patients who received no outpatient treatment in a real-world, retrospective analysis of cases.
The investigators found that there were nine hospitalizations or deaths (2.1%) among 426 patients who received outpatient treatment, compared with 49 (17.6%) among 278 who did not receive outpatient treatment, yielding an odds ratio of 0.12 (95% confidence interval, 0.05-0.25), after adjusting for age, sex, race, comorbidities, and kidney function. The study was published in Lancet Rheumatology.
“Across the board, there was a really strong association with receiving outpatient treatment and lower risk of severe COVID-19,” senior author Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine, Harvard Medical School and Brigham and Women’s Hospital, Boston, said in an interview. “It is pretty powerful evidence that, in this high-risk group, that treatment still matters related to preventing severe COVID. We found almost all patients who had severe COVID-19, either hospitalized or who had died, were in the untreated group.”
Early outpatient treatment an important tool in patients with rheumatic disease
Dr. Sparks noted that he and his coinvestigators conducted the study because the benefit of outpatient COVID-19 treatments in individuals with systemic autoimmune rheumatic disease was not adequately determined in clinical trials because they had infrequent enrollment of such patients.
The analysis included 704 patients with a mean age of 58.4 years who were seen at Mass General Brigham Integrated Health Care System, a multicenter health care system that includes 14 hospitals and primary care or specialty outpatient centers in the Boston area. A majority were female (76%) and White (84%). Nearly half had rheumatoid arthritis. Of the 704, 426 (61%) received outpatient treatment, which included nirmatrelvir-ritonavir (n = 307), monoclonal antibodies (n = 105), molnupiravir (n = 5), remdesivir (n = 3), and combination treatment (n = 6).
The findings underline the need to individualize approaches to outpatient treatment in those who test positive for SARS-CoV-2 to fend off severe COVID-19, according to Dr. Sparks. “It seems if you are vaccinated and in the general population that you are way less likely to have severe COVID-19 in the current environment, but that doesn’t necessarily apply to some high-risk groups like patients on immunosuppression. There are still patients at risk of severe COVID-19, and some of them are in this group of rheumatic patients. This should be part of the discussion related to deciding whether or not to treat.”
Dr. Sparks noted that vaccination against COVID-19 confers protection against developing severe COVID-19 in patients with rheumatic disease as it does in the general population, but patients with rheumatic diseases remain at increased risk for severe presentation. “Certainly, the vaccines really help our patients too, but there’s still a bit of a gap between the risk for our patients with rheumatic diseases and the general population” in developing severe COVID-19.
Dr. Sparks said he hopes the results represent a “call to action” that even among vaccinated patients there are still some who have poor outcomes, and that early outpatient treatment appears to be an important tool in the fight against poor outcomes from SARS-CoV-2 infection.
COVID-19 rebound
The study also reported on the phenomenon of COVID-19 rebound (recurrence of symptoms and test positivity after regimen completion) after oral outpatient SARS-CoV-2 treatment. “This [COVID-19 rebound] is a downside to treatment,” he said. COVID rebound was not infrequent: A total of 25 (8%) of 318 patients who received oral outpatient treatment had documented COVID-19 rebound.
“It was reassuring because we found no one who had rebound progressed to have severe COVID-19,” Dr. Sparks said. “On the other hand, [rebound] happened pretty frequently in our data, as 8% of patients are documented to have it.”
Dr. Sparks said he and coinvestigators speculate that more patients in the cohort may have experienced COVID-19 rebound but did not communicate this to their health care providers, and, as such, it was not documented in the medical record. The potential development of COVID-19 rebound “is something to counsel your patients about.” COVID-19 rebound is a phenomenon that is being most commonly observed with nirmatrelvir-ritonavir as outpatient treatment.
Possible confounding factors in study
Katie Bechman, MBChB, clinical lecturer in rheumatology at King’s College London, who coauthored an accompanying editorial about the study and its findings, pointed out that the study is limited by its observational design.
“With any study that looks at the efficacy of treatment, especially in an observational cohort, you’re going to have to consider the unmeasured confounding and the difference between these two groups,” Dr. Bechman said. “I know that they did try to adjust for that in this study, but there’s always going to be factors that we can’t [control for]. That is something that needs to be considered. I think that’s always something we need to consider when we’re looking at observational data.”
In lieu of a randomized, controlled trial, Dr. Bechman noted that the study and its associated findings serve as “the best data we have,” and she described the results as “very informative and positive.”
She added that the large number of patients represents a strength of the study, as does the robust method employed for identifying which patients had COVID-19.
The learnings from this study with respect to outpatient treatment can be applied to more common illnesses that patients with rheumatic disease may develop, such as the flu, according to Dr. Bechman.
“One of the positive aspects from this pandemic is that we’ve learned a huge amount about how best to treat certain viruses and prevent them in patients,” she said. “It would be worth thinking towards the future, what we can do for illnesses that we see very commonly in these populations. There may be treatment regimens that we haven’t really considered until now. You could hypothesize that in the next couple of years, if we have an influenza breakout, that we should be providing some prehospital antiviral treatment to patients, especially the ones that are at high risk.”
The study was conducted without outside funding. Dr. Sparks has received research support from Bristol-Myers Squibb and consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer unrelated to this work. Dr. Bechman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Outpatient COVID-19 treatment with monoclonal antibodies or antiretroviral medications such as nirmatrelvir-ritonavir (Paxlovid) administered to patients with systemic autoimmune rheumatic disease led to lower odds of having severe outcomes when compared with similar patients who received no outpatient treatment in a real-world, retrospective analysis of cases.
The investigators found that there were nine hospitalizations or deaths (2.1%) among 426 patients who received outpatient treatment, compared with 49 (17.6%) among 278 who did not receive outpatient treatment, yielding an odds ratio of 0.12 (95% confidence interval, 0.05-0.25), after adjusting for age, sex, race, comorbidities, and kidney function. The study was published in Lancet Rheumatology.
“Across the board, there was a really strong association with receiving outpatient treatment and lower risk of severe COVID-19,” senior author Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine, Harvard Medical School and Brigham and Women’s Hospital, Boston, said in an interview. “It is pretty powerful evidence that, in this high-risk group, that treatment still matters related to preventing severe COVID. We found almost all patients who had severe COVID-19, either hospitalized or who had died, were in the untreated group.”
Early outpatient treatment an important tool in patients with rheumatic disease
Dr. Sparks noted that he and his coinvestigators conducted the study because the benefit of outpatient COVID-19 treatments in individuals with systemic autoimmune rheumatic disease was not adequately determined in clinical trials because they had infrequent enrollment of such patients.
The analysis included 704 patients with a mean age of 58.4 years who were seen at Mass General Brigham Integrated Health Care System, a multicenter health care system that includes 14 hospitals and primary care or specialty outpatient centers in the Boston area. A majority were female (76%) and White (84%). Nearly half had rheumatoid arthritis. Of the 704, 426 (61%) received outpatient treatment, which included nirmatrelvir-ritonavir (n = 307), monoclonal antibodies (n = 105), molnupiravir (n = 5), remdesivir (n = 3), and combination treatment (n = 6).
The findings underline the need to individualize approaches to outpatient treatment in those who test positive for SARS-CoV-2 to fend off severe COVID-19, according to Dr. Sparks. “It seems if you are vaccinated and in the general population that you are way less likely to have severe COVID-19 in the current environment, but that doesn’t necessarily apply to some high-risk groups like patients on immunosuppression. There are still patients at risk of severe COVID-19, and some of them are in this group of rheumatic patients. This should be part of the discussion related to deciding whether or not to treat.”
Dr. Sparks noted that vaccination against COVID-19 confers protection against developing severe COVID-19 in patients with rheumatic disease as it does in the general population, but patients with rheumatic diseases remain at increased risk for severe presentation. “Certainly, the vaccines really help our patients too, but there’s still a bit of a gap between the risk for our patients with rheumatic diseases and the general population” in developing severe COVID-19.
Dr. Sparks said he hopes the results represent a “call to action” that even among vaccinated patients there are still some who have poor outcomes, and that early outpatient treatment appears to be an important tool in the fight against poor outcomes from SARS-CoV-2 infection.
COVID-19 rebound
The study also reported on the phenomenon of COVID-19 rebound (recurrence of symptoms and test positivity after regimen completion) after oral outpatient SARS-CoV-2 treatment. “This [COVID-19 rebound] is a downside to treatment,” he said. COVID rebound was not infrequent: A total of 25 (8%) of 318 patients who received oral outpatient treatment had documented COVID-19 rebound.
“It was reassuring because we found no one who had rebound progressed to have severe COVID-19,” Dr. Sparks said. “On the other hand, [rebound] happened pretty frequently in our data, as 8% of patients are documented to have it.”
Dr. Sparks said he and coinvestigators speculate that more patients in the cohort may have experienced COVID-19 rebound but did not communicate this to their health care providers, and, as such, it was not documented in the medical record. The potential development of COVID-19 rebound “is something to counsel your patients about.” COVID-19 rebound is a phenomenon that is being most commonly observed with nirmatrelvir-ritonavir as outpatient treatment.
Possible confounding factors in study
Katie Bechman, MBChB, clinical lecturer in rheumatology at King’s College London, who coauthored an accompanying editorial about the study and its findings, pointed out that the study is limited by its observational design.
“With any study that looks at the efficacy of treatment, especially in an observational cohort, you’re going to have to consider the unmeasured confounding and the difference between these two groups,” Dr. Bechman said. “I know that they did try to adjust for that in this study, but there’s always going to be factors that we can’t [control for]. That is something that needs to be considered. I think that’s always something we need to consider when we’re looking at observational data.”
In lieu of a randomized, controlled trial, Dr. Bechman noted that the study and its associated findings serve as “the best data we have,” and she described the results as “very informative and positive.”
She added that the large number of patients represents a strength of the study, as does the robust method employed for identifying which patients had COVID-19.
The learnings from this study with respect to outpatient treatment can be applied to more common illnesses that patients with rheumatic disease may develop, such as the flu, according to Dr. Bechman.
“One of the positive aspects from this pandemic is that we’ve learned a huge amount about how best to treat certain viruses and prevent them in patients,” she said. “It would be worth thinking towards the future, what we can do for illnesses that we see very commonly in these populations. There may be treatment regimens that we haven’t really considered until now. You could hypothesize that in the next couple of years, if we have an influenza breakout, that we should be providing some prehospital antiviral treatment to patients, especially the ones that are at high risk.”
The study was conducted without outside funding. Dr. Sparks has received research support from Bristol-Myers Squibb and consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer unrelated to this work. Dr. Bechman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Outpatient COVID-19 treatment with monoclonal antibodies or antiretroviral medications such as nirmatrelvir-ritonavir (Paxlovid) administered to patients with systemic autoimmune rheumatic disease led to lower odds of having severe outcomes when compared with similar patients who received no outpatient treatment in a real-world, retrospective analysis of cases.
The investigators found that there were nine hospitalizations or deaths (2.1%) among 426 patients who received outpatient treatment, compared with 49 (17.6%) among 278 who did not receive outpatient treatment, yielding an odds ratio of 0.12 (95% confidence interval, 0.05-0.25), after adjusting for age, sex, race, comorbidities, and kidney function. The study was published in Lancet Rheumatology.
“Across the board, there was a really strong association with receiving outpatient treatment and lower risk of severe COVID-19,” senior author Jeffrey A. Sparks, MD, MMSc, assistant professor of medicine, Harvard Medical School and Brigham and Women’s Hospital, Boston, said in an interview. “It is pretty powerful evidence that, in this high-risk group, that treatment still matters related to preventing severe COVID. We found almost all patients who had severe COVID-19, either hospitalized or who had died, were in the untreated group.”
Early outpatient treatment an important tool in patients with rheumatic disease
Dr. Sparks noted that he and his coinvestigators conducted the study because the benefit of outpatient COVID-19 treatments in individuals with systemic autoimmune rheumatic disease was not adequately determined in clinical trials because they had infrequent enrollment of such patients.
The analysis included 704 patients with a mean age of 58.4 years who were seen at Mass General Brigham Integrated Health Care System, a multicenter health care system that includes 14 hospitals and primary care or specialty outpatient centers in the Boston area. A majority were female (76%) and White (84%). Nearly half had rheumatoid arthritis. Of the 704, 426 (61%) received outpatient treatment, which included nirmatrelvir-ritonavir (n = 307), monoclonal antibodies (n = 105), molnupiravir (n = 5), remdesivir (n = 3), and combination treatment (n = 6).
The findings underline the need to individualize approaches to outpatient treatment in those who test positive for SARS-CoV-2 to fend off severe COVID-19, according to Dr. Sparks. “It seems if you are vaccinated and in the general population that you are way less likely to have severe COVID-19 in the current environment, but that doesn’t necessarily apply to some high-risk groups like patients on immunosuppression. There are still patients at risk of severe COVID-19, and some of them are in this group of rheumatic patients. This should be part of the discussion related to deciding whether or not to treat.”
Dr. Sparks noted that vaccination against COVID-19 confers protection against developing severe COVID-19 in patients with rheumatic disease as it does in the general population, but patients with rheumatic diseases remain at increased risk for severe presentation. “Certainly, the vaccines really help our patients too, but there’s still a bit of a gap between the risk for our patients with rheumatic diseases and the general population” in developing severe COVID-19.
Dr. Sparks said he hopes the results represent a “call to action” that even among vaccinated patients there are still some who have poor outcomes, and that early outpatient treatment appears to be an important tool in the fight against poor outcomes from SARS-CoV-2 infection.
COVID-19 rebound
The study also reported on the phenomenon of COVID-19 rebound (recurrence of symptoms and test positivity after regimen completion) after oral outpatient SARS-CoV-2 treatment. “This [COVID-19 rebound] is a downside to treatment,” he said. COVID rebound was not infrequent: A total of 25 (8%) of 318 patients who received oral outpatient treatment had documented COVID-19 rebound.
“It was reassuring because we found no one who had rebound progressed to have severe COVID-19,” Dr. Sparks said. “On the other hand, [rebound] happened pretty frequently in our data, as 8% of patients are documented to have it.”
Dr. Sparks said he and coinvestigators speculate that more patients in the cohort may have experienced COVID-19 rebound but did not communicate this to their health care providers, and, as such, it was not documented in the medical record. The potential development of COVID-19 rebound “is something to counsel your patients about.” COVID-19 rebound is a phenomenon that is being most commonly observed with nirmatrelvir-ritonavir as outpatient treatment.
Possible confounding factors in study
Katie Bechman, MBChB, clinical lecturer in rheumatology at King’s College London, who coauthored an accompanying editorial about the study and its findings, pointed out that the study is limited by its observational design.
“With any study that looks at the efficacy of treatment, especially in an observational cohort, you’re going to have to consider the unmeasured confounding and the difference between these two groups,” Dr. Bechman said. “I know that they did try to adjust for that in this study, but there’s always going to be factors that we can’t [control for]. That is something that needs to be considered. I think that’s always something we need to consider when we’re looking at observational data.”
In lieu of a randomized, controlled trial, Dr. Bechman noted that the study and its associated findings serve as “the best data we have,” and she described the results as “very informative and positive.”
She added that the large number of patients represents a strength of the study, as does the robust method employed for identifying which patients had COVID-19.
The learnings from this study with respect to outpatient treatment can be applied to more common illnesses that patients with rheumatic disease may develop, such as the flu, according to Dr. Bechman.
“One of the positive aspects from this pandemic is that we’ve learned a huge amount about how best to treat certain viruses and prevent them in patients,” she said. “It would be worth thinking towards the future, what we can do for illnesses that we see very commonly in these populations. There may be treatment regimens that we haven’t really considered until now. You could hypothesize that in the next couple of years, if we have an influenza breakout, that we should be providing some prehospital antiviral treatment to patients, especially the ones that are at high risk.”
The study was conducted without outside funding. Dr. Sparks has received research support from Bristol-Myers Squibb and consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer unrelated to this work. Dr. Bechman reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
In families with gout, obesity and alcohol add to personal risk
Gout-associated genetic factors increase the risk of gout by nearly two and a half times among people with a close family history of the disease. The risk is approximately three times higher among people with a family history of gout who are also heavy drinkers; for people with a family history of gout who are also overweight, the risk is four times higher, according to a large population-based study from South Korea.
The increased familial risk of gout (hazard ratio, 2.42) dropped only slightly after adjustment for lifestyle and biological risk factors (HR, 2.29), suggesting that genes are the key drivers for the risk of gout among first-degree relatives.
Risk was highest among individuals with an affected brother (HR, 3.00), followed by father (HR, 2.33), sister (HR, 1.97), and mother (HR, 1.68).
“Although the familial aggregation of gout [where a first-degree relative has the disease] is influenced by both genetic and lifestyle/biological factors, our findings suggest that a genetic predisposition is the predominant driver of familial aggregation,” first author Kyoung-Hoon Kim, PhD, from Health Insurance Review and Assessment Service, Wonju-si, South Korea, and colleagues wrote in Arthritis Care and Research.
However, lifestyle is still important, as suggested by comparisons with members of the general population who do not have a family history of gout or a high body mass index (BMI). The risk increased for persons with a family history of gout who were also overweight (HR, 4.39), and it increased further for people with obesity (HR, 6.62), suggesting a dose-response interaction, the authors wrote.
When family history was combined with heavy alcohol consumption, the risk rose (HR, 2.95) in comparison with the general population who had neither risk factor.
The study fills a gap in evidence on “familial risk of gout as opposed to hereditary risk of gout, which has long been recognized,” the researchers wrote.
In addition, the findings suggest the possibility of a dose-dependent gene-environment interaction, “as the combination of both a family history of gout and either high BMI or heavy alcohol consumption was associated with a markedly increased risk of disease, which was even further elevated among obese individuals.”
Abhishek Abhishek, MD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust, reflected on the minimal attenuation after adjustment for lifestyle and demographic factors. “This suggests that most of the familial impact is, in fact, genetic rather than due to shared environmental factors and is an important finding.”
He said in an interview that the findings also confirmed the synergistic effect of genetic and lifestyle factors in causing gout. “Lifestyle factors such as alcohol excess and obesity should be addressed more aggressively in those with a first-degree relative with gout.
“Although not directly evaluated in this study, aggressive management of excess weight and high alcohol consumption may prevent the onset of gout or improve its outcomes in those who already have this condition,” he added.
Study of over 5 million individuals with familial aggregation of gout
The researchers drew on data from the government-operated mandatory insurance service that provides for South Korea’s entire population of over 50 million people (the National Health Insurance database), as well as the National Health Screening Program database. Information on familial relationships and risk factor data were identified for 5,524,403 individuals from 2002 to 2018 who had a blood-related first-degree relative.
Familial risk was calculated by comparing the risk of individuals with and those without affected first-degree relatives. Interactions between family history and obesity or alcohol consumption were assessed using a scale that measured gout risk due to interaction of two factors.
Initially, adjustments to familial risk were made with respect to age and sex. Subsequently, possible risk factors included smoking, BMI, hypertension, and hyperglycemia.
Alcohol consumption levels were noted and categorized as nondrinker, moderate drinker, or heavy drinker, with different consumption levels for men and women. For men, heavy drinking was defined as having at least two drinks per week and at least five drinks on any day; for women, heavy drinking was defined as having at least two drinks per week and at least four drinks on any day.
Overweight and obesity were determined on the basis of BMI, using standard categories: overweight was defined as BMI of 25 to less than 30 kg/m2, and obesity was defined as BMI of 30 or higher.
Dr. Kim and coauthors noted that both high BMI and heavy drinking were associated with an increased risk of gout, regardless of whether there was a family history of the disease, and that the findings suggest “a dose-dependent interactive relationship in which genetic factors and obesity potentiate each other rather than operating independently.”
People who are both overweight and have a family history of disease had a combined risk of gout that was significantly higher than the sum of their individual risk factors (HR, 4.39 vs. 3.43). This risk was accentuated among people with obesity (HR, 6.62 vs. 4.74) and was more pronounced in men than in women.
In other risk analyses in which familial and nonfamilial gout risk groups were compared, the risk associated with obesity was higher in the familial, compared with the nonfamilial group (HR, 5.50 vs. 5.36).
Bruce Rothschild, MD, a rheumatologist with Indiana University Health, Muncie, and research associate at Carnegie Museum of Natural History, Pittsburgh, shared his thoughts on the study in an interview and noted some limitations. “The findings of this study do not conflict with what is generally believed, but there are several issues that complicate interpretation,” he began. “The first is how gout is diagnosed. Since crystal presence confirmation is rare in clinical practice, and by assumption of the database used, diagnosis is based on fulfillment of a certain number of criteria, one of which is hyperuricemia – this is not actual confirmation of diagnosis.”
He pointed out that the incidence of gout depends on who received treatment, and the study excluded those who were not receiving treatment and those who were not prescribed allopurinol or febuxostat. “Single parents were also excluded, and this may also have affected results.
“Overweight and obesity were not adjusted for age, and the interpretation is age dependent,” he added. “It really comes down to the way gout is diagnosed, and this is a worldwide problem because the diagnosis has been so dumbed down that we don’t really know what is claimed as gout.”
Dr. Kim and coauthors disclosed no relevant financial relationships. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadilla Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Rothschild disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gout-associated genetic factors increase the risk of gout by nearly two and a half times among people with a close family history of the disease. The risk is approximately three times higher among people with a family history of gout who are also heavy drinkers; for people with a family history of gout who are also overweight, the risk is four times higher, according to a large population-based study from South Korea.
The increased familial risk of gout (hazard ratio, 2.42) dropped only slightly after adjustment for lifestyle and biological risk factors (HR, 2.29), suggesting that genes are the key drivers for the risk of gout among first-degree relatives.
Risk was highest among individuals with an affected brother (HR, 3.00), followed by father (HR, 2.33), sister (HR, 1.97), and mother (HR, 1.68).
“Although the familial aggregation of gout [where a first-degree relative has the disease] is influenced by both genetic and lifestyle/biological factors, our findings suggest that a genetic predisposition is the predominant driver of familial aggregation,” first author Kyoung-Hoon Kim, PhD, from Health Insurance Review and Assessment Service, Wonju-si, South Korea, and colleagues wrote in Arthritis Care and Research.
However, lifestyle is still important, as suggested by comparisons with members of the general population who do not have a family history of gout or a high body mass index (BMI). The risk increased for persons with a family history of gout who were also overweight (HR, 4.39), and it increased further for people with obesity (HR, 6.62), suggesting a dose-response interaction, the authors wrote.
When family history was combined with heavy alcohol consumption, the risk rose (HR, 2.95) in comparison with the general population who had neither risk factor.
The study fills a gap in evidence on “familial risk of gout as opposed to hereditary risk of gout, which has long been recognized,” the researchers wrote.
In addition, the findings suggest the possibility of a dose-dependent gene-environment interaction, “as the combination of both a family history of gout and either high BMI or heavy alcohol consumption was associated with a markedly increased risk of disease, which was even further elevated among obese individuals.”
Abhishek Abhishek, MD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust, reflected on the minimal attenuation after adjustment for lifestyle and demographic factors. “This suggests that most of the familial impact is, in fact, genetic rather than due to shared environmental factors and is an important finding.”
He said in an interview that the findings also confirmed the synergistic effect of genetic and lifestyle factors in causing gout. “Lifestyle factors such as alcohol excess and obesity should be addressed more aggressively in those with a first-degree relative with gout.
“Although not directly evaluated in this study, aggressive management of excess weight and high alcohol consumption may prevent the onset of gout or improve its outcomes in those who already have this condition,” he added.
Study of over 5 million individuals with familial aggregation of gout
The researchers drew on data from the government-operated mandatory insurance service that provides for South Korea’s entire population of over 50 million people (the National Health Insurance database), as well as the National Health Screening Program database. Information on familial relationships and risk factor data were identified for 5,524,403 individuals from 2002 to 2018 who had a blood-related first-degree relative.
Familial risk was calculated by comparing the risk of individuals with and those without affected first-degree relatives. Interactions between family history and obesity or alcohol consumption were assessed using a scale that measured gout risk due to interaction of two factors.
Initially, adjustments to familial risk were made with respect to age and sex. Subsequently, possible risk factors included smoking, BMI, hypertension, and hyperglycemia.
Alcohol consumption levels were noted and categorized as nondrinker, moderate drinker, or heavy drinker, with different consumption levels for men and women. For men, heavy drinking was defined as having at least two drinks per week and at least five drinks on any day; for women, heavy drinking was defined as having at least two drinks per week and at least four drinks on any day.
Overweight and obesity were determined on the basis of BMI, using standard categories: overweight was defined as BMI of 25 to less than 30 kg/m2, and obesity was defined as BMI of 30 or higher.
Dr. Kim and coauthors noted that both high BMI and heavy drinking were associated with an increased risk of gout, regardless of whether there was a family history of the disease, and that the findings suggest “a dose-dependent interactive relationship in which genetic factors and obesity potentiate each other rather than operating independently.”
People who are both overweight and have a family history of disease had a combined risk of gout that was significantly higher than the sum of their individual risk factors (HR, 4.39 vs. 3.43). This risk was accentuated among people with obesity (HR, 6.62 vs. 4.74) and was more pronounced in men than in women.
In other risk analyses in which familial and nonfamilial gout risk groups were compared, the risk associated with obesity was higher in the familial, compared with the nonfamilial group (HR, 5.50 vs. 5.36).
Bruce Rothschild, MD, a rheumatologist with Indiana University Health, Muncie, and research associate at Carnegie Museum of Natural History, Pittsburgh, shared his thoughts on the study in an interview and noted some limitations. “The findings of this study do not conflict with what is generally believed, but there are several issues that complicate interpretation,” he began. “The first is how gout is diagnosed. Since crystal presence confirmation is rare in clinical practice, and by assumption of the database used, diagnosis is based on fulfillment of a certain number of criteria, one of which is hyperuricemia – this is not actual confirmation of diagnosis.”
He pointed out that the incidence of gout depends on who received treatment, and the study excluded those who were not receiving treatment and those who were not prescribed allopurinol or febuxostat. “Single parents were also excluded, and this may also have affected results.
“Overweight and obesity were not adjusted for age, and the interpretation is age dependent,” he added. “It really comes down to the way gout is diagnosed, and this is a worldwide problem because the diagnosis has been so dumbed down that we don’t really know what is claimed as gout.”
Dr. Kim and coauthors disclosed no relevant financial relationships. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadilla Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Rothschild disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Gout-associated genetic factors increase the risk of gout by nearly two and a half times among people with a close family history of the disease. The risk is approximately three times higher among people with a family history of gout who are also heavy drinkers; for people with a family history of gout who are also overweight, the risk is four times higher, according to a large population-based study from South Korea.
The increased familial risk of gout (hazard ratio, 2.42) dropped only slightly after adjustment for lifestyle and biological risk factors (HR, 2.29), suggesting that genes are the key drivers for the risk of gout among first-degree relatives.
Risk was highest among individuals with an affected brother (HR, 3.00), followed by father (HR, 2.33), sister (HR, 1.97), and mother (HR, 1.68).
“Although the familial aggregation of gout [where a first-degree relative has the disease] is influenced by both genetic and lifestyle/biological factors, our findings suggest that a genetic predisposition is the predominant driver of familial aggregation,” first author Kyoung-Hoon Kim, PhD, from Health Insurance Review and Assessment Service, Wonju-si, South Korea, and colleagues wrote in Arthritis Care and Research.
However, lifestyle is still important, as suggested by comparisons with members of the general population who do not have a family history of gout or a high body mass index (BMI). The risk increased for persons with a family history of gout who were also overweight (HR, 4.39), and it increased further for people with obesity (HR, 6.62), suggesting a dose-response interaction, the authors wrote.
When family history was combined with heavy alcohol consumption, the risk rose (HR, 2.95) in comparison with the general population who had neither risk factor.
The study fills a gap in evidence on “familial risk of gout as opposed to hereditary risk of gout, which has long been recognized,” the researchers wrote.
In addition, the findings suggest the possibility of a dose-dependent gene-environment interaction, “as the combination of both a family history of gout and either high BMI or heavy alcohol consumption was associated with a markedly increased risk of disease, which was even further elevated among obese individuals.”
Abhishek Abhishek, MD, professor of rheumatology and honorary consultant rheumatologist at Nottingham (England) University Hospitals NHS Trust, reflected on the minimal attenuation after adjustment for lifestyle and demographic factors. “This suggests that most of the familial impact is, in fact, genetic rather than due to shared environmental factors and is an important finding.”
He said in an interview that the findings also confirmed the synergistic effect of genetic and lifestyle factors in causing gout. “Lifestyle factors such as alcohol excess and obesity should be addressed more aggressively in those with a first-degree relative with gout.
“Although not directly evaluated in this study, aggressive management of excess weight and high alcohol consumption may prevent the onset of gout or improve its outcomes in those who already have this condition,” he added.
Study of over 5 million individuals with familial aggregation of gout
The researchers drew on data from the government-operated mandatory insurance service that provides for South Korea’s entire population of over 50 million people (the National Health Insurance database), as well as the National Health Screening Program database. Information on familial relationships and risk factor data were identified for 5,524,403 individuals from 2002 to 2018 who had a blood-related first-degree relative.
Familial risk was calculated by comparing the risk of individuals with and those without affected first-degree relatives. Interactions between family history and obesity or alcohol consumption were assessed using a scale that measured gout risk due to interaction of two factors.
Initially, adjustments to familial risk were made with respect to age and sex. Subsequently, possible risk factors included smoking, BMI, hypertension, and hyperglycemia.
Alcohol consumption levels were noted and categorized as nondrinker, moderate drinker, or heavy drinker, with different consumption levels for men and women. For men, heavy drinking was defined as having at least two drinks per week and at least five drinks on any day; for women, heavy drinking was defined as having at least two drinks per week and at least four drinks on any day.
Overweight and obesity were determined on the basis of BMI, using standard categories: overweight was defined as BMI of 25 to less than 30 kg/m2, and obesity was defined as BMI of 30 or higher.
Dr. Kim and coauthors noted that both high BMI and heavy drinking were associated with an increased risk of gout, regardless of whether there was a family history of the disease, and that the findings suggest “a dose-dependent interactive relationship in which genetic factors and obesity potentiate each other rather than operating independently.”
People who are both overweight and have a family history of disease had a combined risk of gout that was significantly higher than the sum of their individual risk factors (HR, 4.39 vs. 3.43). This risk was accentuated among people with obesity (HR, 6.62 vs. 4.74) and was more pronounced in men than in women.
In other risk analyses in which familial and nonfamilial gout risk groups were compared, the risk associated with obesity was higher in the familial, compared with the nonfamilial group (HR, 5.50 vs. 5.36).
Bruce Rothschild, MD, a rheumatologist with Indiana University Health, Muncie, and research associate at Carnegie Museum of Natural History, Pittsburgh, shared his thoughts on the study in an interview and noted some limitations. “The findings of this study do not conflict with what is generally believed, but there are several issues that complicate interpretation,” he began. “The first is how gout is diagnosed. Since crystal presence confirmation is rare in clinical practice, and by assumption of the database used, diagnosis is based on fulfillment of a certain number of criteria, one of which is hyperuricemia – this is not actual confirmation of diagnosis.”
He pointed out that the incidence of gout depends on who received treatment, and the study excluded those who were not receiving treatment and those who were not prescribed allopurinol or febuxostat. “Single parents were also excluded, and this may also have affected results.
“Overweight and obesity were not adjusted for age, and the interpretation is age dependent,” he added. “It really comes down to the way gout is diagnosed, and this is a worldwide problem because the diagnosis has been so dumbed down that we don’t really know what is claimed as gout.”
Dr. Kim and coauthors disclosed no relevant financial relationships. Dr. Abhishek has received institutional research grants from AstraZeneca and Oxford Immunotech and personal fees from UpToDate, Springer, Cadilla Pharmaceuticals, NGM Bio, Limbic, and Inflazome. Dr. Rothschild disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARTHRITIS CARE AND RESEARCH
Systemic sclerosis antibodies show link to interstitial lung disease in RA
Adults with rheumatoid arthritis or primary Sjogren’s syndrome plus interstitial lung disease had higher levels of systemic sclerosis–specific antibodies than those without lung disease, based on data from 101 individuals.
Systemic sclerosis (SSc) has been associated with the development of interstitial lung disease (ILD), but the prevalence of SSc autoantibodies in patients with rheumatoid arthritis (RA) and primary Sjogren’s syndrome (SS) has not been explored, wrote Vasilike Koulouri, MD, of Kapodistrian University of Athens, and colleagues.
In a study published in the Journal of Translational Autoimmunity, the researchers reviewed serum data from patients with RA and SS using immunoblot assays to determine the prevalence of SSc-specific and anti-Ro52 autoantibodies, both of which have been associated with ILD in SSc patients.
The study population included 28 RA patients with ILD, 32 RA patients without ILD, 9 primary SS patients with ILD, and 32 primary SS patients with no ILD. The mean age of the RA participants was 63.4 years, 70% were women, and the mean age at RA diagnosis was 50.2 years. The mean age of the primary SS group was 60.3 years, 87.8% were female, and the mean age at diagnosis was 52.7 years.
Overall, SSc-specific antibodies across all titers were detected more frequently in RA patients with ILD compared with those with no ILD, though not statistically significant (42.9% vs. 21.9%, P = .08). However, “This trend was mainly attributed to the statistically significant difference between the two groups at strong titers (25% vs. 3.1%, P = .01),” the researchers wrote. Notably, they added.
No significant differences appeared in the prevalence of SSc-specific or Ro52 autoantibodies between primary SS patients with and without ILD, which might be attributable in part to the increased prevalence of anticentromere antibodies in primary SS, the researchers said.
RA patients who were positive for SSc-specific antibodies at strong titers were significantly more likely to have respiratory abnormalities than those who were negative (87.5% vs. 47.2%, P = .04), but no such differences appeared in primary SS patients.
“Early detection of SSc antibodies could be important in clinical practice as it may mandate further diagnostic (for example, screening for pulmonary hypertension) and therapeutic approaches of these patients,” the researchers wrote in their discussion.
The study findings were limited by several factors, mainly the small sample size, but also the potential for false-positive results on antibody titers, lack of data on the clinical significance of medium autoantibody titers, and the lack of long-term follow-up data, the researchers noted.
However, the results suggest that many seropositive RA patients with evidence of ILD “may evolve to a clinically evident overlap of RA and SSc” that would benefit from targeted treatment, they concluded.
The study was supported by a grant from Novartis AG and by the Molecular Immunology and Clinical Applications Unit, Department of Physiology, School of Medicine, National and Kapodistrian University of Athens. The researchers had no financial conflicts to disclose.
Adults with rheumatoid arthritis or primary Sjogren’s syndrome plus interstitial lung disease had higher levels of systemic sclerosis–specific antibodies than those without lung disease, based on data from 101 individuals.
Systemic sclerosis (SSc) has been associated with the development of interstitial lung disease (ILD), but the prevalence of SSc autoantibodies in patients with rheumatoid arthritis (RA) and primary Sjogren’s syndrome (SS) has not been explored, wrote Vasilike Koulouri, MD, of Kapodistrian University of Athens, and colleagues.
In a study published in the Journal of Translational Autoimmunity, the researchers reviewed serum data from patients with RA and SS using immunoblot assays to determine the prevalence of SSc-specific and anti-Ro52 autoantibodies, both of which have been associated with ILD in SSc patients.
The study population included 28 RA patients with ILD, 32 RA patients without ILD, 9 primary SS patients with ILD, and 32 primary SS patients with no ILD. The mean age of the RA participants was 63.4 years, 70% were women, and the mean age at RA diagnosis was 50.2 years. The mean age of the primary SS group was 60.3 years, 87.8% were female, and the mean age at diagnosis was 52.7 years.
Overall, SSc-specific antibodies across all titers were detected more frequently in RA patients with ILD compared with those with no ILD, though not statistically significant (42.9% vs. 21.9%, P = .08). However, “This trend was mainly attributed to the statistically significant difference between the two groups at strong titers (25% vs. 3.1%, P = .01),” the researchers wrote. Notably, they added.
No significant differences appeared in the prevalence of SSc-specific or Ro52 autoantibodies between primary SS patients with and without ILD, which might be attributable in part to the increased prevalence of anticentromere antibodies in primary SS, the researchers said.
RA patients who were positive for SSc-specific antibodies at strong titers were significantly more likely to have respiratory abnormalities than those who were negative (87.5% vs. 47.2%, P = .04), but no such differences appeared in primary SS patients.
“Early detection of SSc antibodies could be important in clinical practice as it may mandate further diagnostic (for example, screening for pulmonary hypertension) and therapeutic approaches of these patients,” the researchers wrote in their discussion.
The study findings were limited by several factors, mainly the small sample size, but also the potential for false-positive results on antibody titers, lack of data on the clinical significance of medium autoantibody titers, and the lack of long-term follow-up data, the researchers noted.
However, the results suggest that many seropositive RA patients with evidence of ILD “may evolve to a clinically evident overlap of RA and SSc” that would benefit from targeted treatment, they concluded.
The study was supported by a grant from Novartis AG and by the Molecular Immunology and Clinical Applications Unit, Department of Physiology, School of Medicine, National and Kapodistrian University of Athens. The researchers had no financial conflicts to disclose.
Adults with rheumatoid arthritis or primary Sjogren’s syndrome plus interstitial lung disease had higher levels of systemic sclerosis–specific antibodies than those without lung disease, based on data from 101 individuals.
Systemic sclerosis (SSc) has been associated with the development of interstitial lung disease (ILD), but the prevalence of SSc autoantibodies in patients with rheumatoid arthritis (RA) and primary Sjogren’s syndrome (SS) has not been explored, wrote Vasilike Koulouri, MD, of Kapodistrian University of Athens, and colleagues.
In a study published in the Journal of Translational Autoimmunity, the researchers reviewed serum data from patients with RA and SS using immunoblot assays to determine the prevalence of SSc-specific and anti-Ro52 autoantibodies, both of which have been associated with ILD in SSc patients.
The study population included 28 RA patients with ILD, 32 RA patients without ILD, 9 primary SS patients with ILD, and 32 primary SS patients with no ILD. The mean age of the RA participants was 63.4 years, 70% were women, and the mean age at RA diagnosis was 50.2 years. The mean age of the primary SS group was 60.3 years, 87.8% were female, and the mean age at diagnosis was 52.7 years.
Overall, SSc-specific antibodies across all titers were detected more frequently in RA patients with ILD compared with those with no ILD, though not statistically significant (42.9% vs. 21.9%, P = .08). However, “This trend was mainly attributed to the statistically significant difference between the two groups at strong titers (25% vs. 3.1%, P = .01),” the researchers wrote. Notably, they added.
No significant differences appeared in the prevalence of SSc-specific or Ro52 autoantibodies between primary SS patients with and without ILD, which might be attributable in part to the increased prevalence of anticentromere antibodies in primary SS, the researchers said.
RA patients who were positive for SSc-specific antibodies at strong titers were significantly more likely to have respiratory abnormalities than those who were negative (87.5% vs. 47.2%, P = .04), but no such differences appeared in primary SS patients.
“Early detection of SSc antibodies could be important in clinical practice as it may mandate further diagnostic (for example, screening for pulmonary hypertension) and therapeutic approaches of these patients,” the researchers wrote in their discussion.
The study findings were limited by several factors, mainly the small sample size, but also the potential for false-positive results on antibody titers, lack of data on the clinical significance of medium autoantibody titers, and the lack of long-term follow-up data, the researchers noted.
However, the results suggest that many seropositive RA patients with evidence of ILD “may evolve to a clinically evident overlap of RA and SSc” that would benefit from targeted treatment, they concluded.
The study was supported by a grant from Novartis AG and by the Molecular Immunology and Clinical Applications Unit, Department of Physiology, School of Medicine, National and Kapodistrian University of Athens. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF TRANSLATIONAL AUTOIMMUNITY
Running does not cause lasting cartilage damage
Running does not appear to cause sustained wear and tear of healthy knee cartilage, with research suggesting that the small, short-term changes to cartilage after a run reverse within hours.
A systematic review and meta-analysis published in the most recent issue of Osteoarthritis and Cartilage presents the findings involving 396 adults, which compared the “before” and “after” state of healthy knee cartilage in runners.
Running is often thought to be detrimental to joint health, wrote Sally Coburn, PhD candidate at the La Trobe Sport & Exercise Medicine Research Centre at La Trobe University in Melbourne and coauthors, but this perception is not supported by evidence.
For the analysis, the researchers included studies that looked at either knee or hip cartilage using MRI to assess its size, shape, structure, and/or composition both in the 48 hours before a single bout of running and in the 48 hours after. The analysis aimed to include adults with or at risk of osteoarthritis, but only 57 of the 446 knees in the analysis fit these criteria.
In studies where participants underwent MRI within 20 minutes of running, there was an immediate postrun decrease in the volume of cartilage, ranging from –3.3% for weight-bearing femoral cartilage to –4.1% for tibial cartilage volume. This also revealed a decrease in T1 and T2 relaxation times, which are specialized MRI measures that reflect the composition of cartilage and which can indicate a breakdown of cartilage structure in the case of diseases such as arthritis.
Reversal of short-term cartilage changes
However, within 48 hours of the run, data from studies that repeated the MRIs more than once after the initial prerun scan suggested these changes reversed back to prerun levels.
“We were able to pool delayed T2 relaxation time measures from studies that repeated scans of the same participants 60 minutes and 91 minutes post-run and found no effect of running on tibiofemoral joint cartilage composition,” the authors write.
For example, one study in marathon runners found no difference in cartilage thickness in the tibiofemoral joint between baseline and at 2-10 hours and 12 hours after the marathon. Another showed the immediate post-run decrease in patellofemoral joint cartilage thickness had reverted back to prerun levels when the scan was repeated 24 hours after the run.
“The changes are very minimal and not inconsistent with what’s expected for your cartilage which is functioning normally,” Ms. Coburn told this news organization.
Sparse data in people with osteoarthritis
The authors said there were not enough data from individuals with osteoarthritis to be able to pool and quantify their cartilage changes. However, one study in the analysis found that cartilage lesions in people considered at risk of osteoarthritis because of prior anterior cruciate ligament reconstruction were unchanged after running.
Another suggested that the decrease in femoral cartilage volume recorded at 15 minutes persisted at 45 minutes, while a separate study found significantly increased T2 relaxation times at 45 minutes after a run in those with knee osteoarthritis but not in those without osteoarthritis.
Senior author Adam Culvenor, PhD, senior research fellow at the La Trobe Centre, said their analysis suggested running was healthy, with small changes in cartilage that resolve quickly, but “we really don’t know yet if running is safe for people with osteoarthritis,” he said. “We need much more work in that space.”
Overall, the study evidence was rated as being of low certainty, which Dr. Coburn said was related to the small numbers in each study, which in turn relates to the cost and logistical challenges of the specialized MRI scan used.
“Study of a repeated exposure over a long duration of time on a disease that has a long natural history, like osteoarthritis, is challenging in that most funding agencies will not fund studies longer than 5 years,” Grace Hsiao-Wei Lo, MD, of the department of immunology, allergy, and rheumatology at the Baylor College of Medicine in Houston, said in an email.
Dr. Lo, who was not involved with this review and meta-analysis, said there are still concerns about the effect of running on knee osteoarthritis among those with the disease, although there are some data to suggest that among those who self-select to run, there are no negative outcomes for the knee.
An accompanying editorial noted that research into the effect of running on those with osteoarthritis was still in its infancy. “This would help to guide clinical practice on how to support people with osteoarthritis, with regard to accessing the health benefits of running participation,” write Jean-Francois Esculier, PT, PhD, from the University of British Columbia, Vancouver, and Christian Barton, PhD, with the La Trobe Centre, pointing out there were a lack of evidence-based clinical recommendations for people with osteoarthritis who want to start or continue running.
It’s a question that PhD candidate Michaela Khan, MSc, is trying to answer at the University of British Columbia. “Our lab did a pilot study for my current study now, and they found that osteoarthritic cartilage took a little bit longer to recover than their healthy counterparts,” Ms. Khan said. Her research is suggesting that people with osteoarthritis not only can run, but even those with severe disease, who might be candidates for knee replacement, can run long distances.
Commenting on the analysis, Ms. Khan said the main take-home message was that healthy cartilage seems to recover after running, and that there is not an ongoing effect of ‘wear and tear.’
“That’s changing the narrative that if you keep running, it will wear away your cartilage, it’ll hurt your knees,” she said. “Now, we have a good synthesis of scientific evidence to prove maybe otherwise.”
Ms. Coburn and Dr. Culvenor report grant support from the National Health & Medical Research Council of Australia, and another author reports grant support from the U.S. National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors, as well as Dr. Lo and Ms. Khan, report relevant financial relationships.
Running does not appear to cause sustained wear and tear of healthy knee cartilage, with research suggesting that the small, short-term changes to cartilage after a run reverse within hours.
A systematic review and meta-analysis published in the most recent issue of Osteoarthritis and Cartilage presents the findings involving 396 adults, which compared the “before” and “after” state of healthy knee cartilage in runners.
Running is often thought to be detrimental to joint health, wrote Sally Coburn, PhD candidate at the La Trobe Sport & Exercise Medicine Research Centre at La Trobe University in Melbourne and coauthors, but this perception is not supported by evidence.
For the analysis, the researchers included studies that looked at either knee or hip cartilage using MRI to assess its size, shape, structure, and/or composition both in the 48 hours before a single bout of running and in the 48 hours after. The analysis aimed to include adults with or at risk of osteoarthritis, but only 57 of the 446 knees in the analysis fit these criteria.
In studies where participants underwent MRI within 20 minutes of running, there was an immediate postrun decrease in the volume of cartilage, ranging from –3.3% for weight-bearing femoral cartilage to –4.1% for tibial cartilage volume. This also revealed a decrease in T1 and T2 relaxation times, which are specialized MRI measures that reflect the composition of cartilage and which can indicate a breakdown of cartilage structure in the case of diseases such as arthritis.
Reversal of short-term cartilage changes
However, within 48 hours of the run, data from studies that repeated the MRIs more than once after the initial prerun scan suggested these changes reversed back to prerun levels.
“We were able to pool delayed T2 relaxation time measures from studies that repeated scans of the same participants 60 minutes and 91 minutes post-run and found no effect of running on tibiofemoral joint cartilage composition,” the authors write.
For example, one study in marathon runners found no difference in cartilage thickness in the tibiofemoral joint between baseline and at 2-10 hours and 12 hours after the marathon. Another showed the immediate post-run decrease in patellofemoral joint cartilage thickness had reverted back to prerun levels when the scan was repeated 24 hours after the run.
“The changes are very minimal and not inconsistent with what’s expected for your cartilage which is functioning normally,” Ms. Coburn told this news organization.
Sparse data in people with osteoarthritis
The authors said there were not enough data from individuals with osteoarthritis to be able to pool and quantify their cartilage changes. However, one study in the analysis found that cartilage lesions in people considered at risk of osteoarthritis because of prior anterior cruciate ligament reconstruction were unchanged after running.
Another suggested that the decrease in femoral cartilage volume recorded at 15 minutes persisted at 45 minutes, while a separate study found significantly increased T2 relaxation times at 45 minutes after a run in those with knee osteoarthritis but not in those without osteoarthritis.
Senior author Adam Culvenor, PhD, senior research fellow at the La Trobe Centre, said their analysis suggested running was healthy, with small changes in cartilage that resolve quickly, but “we really don’t know yet if running is safe for people with osteoarthritis,” he said. “We need much more work in that space.”
Overall, the study evidence was rated as being of low certainty, which Dr. Coburn said was related to the small numbers in each study, which in turn relates to the cost and logistical challenges of the specialized MRI scan used.
“Study of a repeated exposure over a long duration of time on a disease that has a long natural history, like osteoarthritis, is challenging in that most funding agencies will not fund studies longer than 5 years,” Grace Hsiao-Wei Lo, MD, of the department of immunology, allergy, and rheumatology at the Baylor College of Medicine in Houston, said in an email.
Dr. Lo, who was not involved with this review and meta-analysis, said there are still concerns about the effect of running on knee osteoarthritis among those with the disease, although there are some data to suggest that among those who self-select to run, there are no negative outcomes for the knee.
An accompanying editorial noted that research into the effect of running on those with osteoarthritis was still in its infancy. “This would help to guide clinical practice on how to support people with osteoarthritis, with regard to accessing the health benefits of running participation,” write Jean-Francois Esculier, PT, PhD, from the University of British Columbia, Vancouver, and Christian Barton, PhD, with the La Trobe Centre, pointing out there were a lack of evidence-based clinical recommendations for people with osteoarthritis who want to start or continue running.
It’s a question that PhD candidate Michaela Khan, MSc, is trying to answer at the University of British Columbia. “Our lab did a pilot study for my current study now, and they found that osteoarthritic cartilage took a little bit longer to recover than their healthy counterparts,” Ms. Khan said. Her research is suggesting that people with osteoarthritis not only can run, but even those with severe disease, who might be candidates for knee replacement, can run long distances.
Commenting on the analysis, Ms. Khan said the main take-home message was that healthy cartilage seems to recover after running, and that there is not an ongoing effect of ‘wear and tear.’
“That’s changing the narrative that if you keep running, it will wear away your cartilage, it’ll hurt your knees,” she said. “Now, we have a good synthesis of scientific evidence to prove maybe otherwise.”
Ms. Coburn and Dr. Culvenor report grant support from the National Health & Medical Research Council of Australia, and another author reports grant support from the U.S. National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors, as well as Dr. Lo and Ms. Khan, report relevant financial relationships.
Running does not appear to cause sustained wear and tear of healthy knee cartilage, with research suggesting that the small, short-term changes to cartilage after a run reverse within hours.
A systematic review and meta-analysis published in the most recent issue of Osteoarthritis and Cartilage presents the findings involving 396 adults, which compared the “before” and “after” state of healthy knee cartilage in runners.
Running is often thought to be detrimental to joint health, wrote Sally Coburn, PhD candidate at the La Trobe Sport & Exercise Medicine Research Centre at La Trobe University in Melbourne and coauthors, but this perception is not supported by evidence.
For the analysis, the researchers included studies that looked at either knee or hip cartilage using MRI to assess its size, shape, structure, and/or composition both in the 48 hours before a single bout of running and in the 48 hours after. The analysis aimed to include adults with or at risk of osteoarthritis, but only 57 of the 446 knees in the analysis fit these criteria.
In studies where participants underwent MRI within 20 minutes of running, there was an immediate postrun decrease in the volume of cartilage, ranging from –3.3% for weight-bearing femoral cartilage to –4.1% for tibial cartilage volume. This also revealed a decrease in T1 and T2 relaxation times, which are specialized MRI measures that reflect the composition of cartilage and which can indicate a breakdown of cartilage structure in the case of diseases such as arthritis.
Reversal of short-term cartilage changes
However, within 48 hours of the run, data from studies that repeated the MRIs more than once after the initial prerun scan suggested these changes reversed back to prerun levels.
“We were able to pool delayed T2 relaxation time measures from studies that repeated scans of the same participants 60 minutes and 91 minutes post-run and found no effect of running on tibiofemoral joint cartilage composition,” the authors write.
For example, one study in marathon runners found no difference in cartilage thickness in the tibiofemoral joint between baseline and at 2-10 hours and 12 hours after the marathon. Another showed the immediate post-run decrease in patellofemoral joint cartilage thickness had reverted back to prerun levels when the scan was repeated 24 hours after the run.
“The changes are very minimal and not inconsistent with what’s expected for your cartilage which is functioning normally,” Ms. Coburn told this news organization.
Sparse data in people with osteoarthritis
The authors said there were not enough data from individuals with osteoarthritis to be able to pool and quantify their cartilage changes. However, one study in the analysis found that cartilage lesions in people considered at risk of osteoarthritis because of prior anterior cruciate ligament reconstruction were unchanged after running.
Another suggested that the decrease in femoral cartilage volume recorded at 15 minutes persisted at 45 minutes, while a separate study found significantly increased T2 relaxation times at 45 minutes after a run in those with knee osteoarthritis but not in those without osteoarthritis.
Senior author Adam Culvenor, PhD, senior research fellow at the La Trobe Centre, said their analysis suggested running was healthy, with small changes in cartilage that resolve quickly, but “we really don’t know yet if running is safe for people with osteoarthritis,” he said. “We need much more work in that space.”
Overall, the study evidence was rated as being of low certainty, which Dr. Coburn said was related to the small numbers in each study, which in turn relates to the cost and logistical challenges of the specialized MRI scan used.
“Study of a repeated exposure over a long duration of time on a disease that has a long natural history, like osteoarthritis, is challenging in that most funding agencies will not fund studies longer than 5 years,” Grace Hsiao-Wei Lo, MD, of the department of immunology, allergy, and rheumatology at the Baylor College of Medicine in Houston, said in an email.
Dr. Lo, who was not involved with this review and meta-analysis, said there are still concerns about the effect of running on knee osteoarthritis among those with the disease, although there are some data to suggest that among those who self-select to run, there are no negative outcomes for the knee.
An accompanying editorial noted that research into the effect of running on those with osteoarthritis was still in its infancy. “This would help to guide clinical practice on how to support people with osteoarthritis, with regard to accessing the health benefits of running participation,” write Jean-Francois Esculier, PT, PhD, from the University of British Columbia, Vancouver, and Christian Barton, PhD, with the La Trobe Centre, pointing out there were a lack of evidence-based clinical recommendations for people with osteoarthritis who want to start or continue running.
It’s a question that PhD candidate Michaela Khan, MSc, is trying to answer at the University of British Columbia. “Our lab did a pilot study for my current study now, and they found that osteoarthritic cartilage took a little bit longer to recover than their healthy counterparts,” Ms. Khan said. Her research is suggesting that people with osteoarthritis not only can run, but even those with severe disease, who might be candidates for knee replacement, can run long distances.
Commenting on the analysis, Ms. Khan said the main take-home message was that healthy cartilage seems to recover after running, and that there is not an ongoing effect of ‘wear and tear.’
“That’s changing the narrative that if you keep running, it will wear away your cartilage, it’ll hurt your knees,” she said. “Now, we have a good synthesis of scientific evidence to prove maybe otherwise.”
Ms. Coburn and Dr. Culvenor report grant support from the National Health & Medical Research Council of Australia, and another author reports grant support from the U.S. National Institute of Arthritis and Musculoskeletal and Skin Diseases. The authors, as well as Dr. Lo and Ms. Khan, report relevant financial relationships.
First Humira biosimilar launches in U.S.
The first biosimilar for Humira, adalimumab-atto (Amjevita), is now available in the United States, according to an announcement on Jan. 31 by the manufacturer, Amgen. At least seven other U.S. Food and Drug Administration–approved Humira biosimilars are expected to become available later in 2023.
Amjevita was approved by the FDA in September 2016 for multiple inflammatory diseases, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The delayed launch was part of a global settlement with Humira’s manufacturer, AbbVie.
Humira (adalimumab) has been available since 2002 and is consistently one of the top-selling drugs in the United States. A single 40-mg Amjevita pen device will be available at two prices: a list price (wholesale acquisition cost) of $1,557.59, 55% below the current Humira list price, and a list price of $3,288.24, 5% below the current Humira list price, according to Amgen.
“Amgen’s goal is to provide broad access for patients by offering two options to health plans and pharmacy benefit managers,” the company said in the press release.
Patients are less likely to benefit from the more significant discount, said Marta Wosinska, PhD, a health care economist at the Brookings Institute in Washington, DC. It's expected that insurance companies will use the higher list price for Amjevita, she said, as this higher price will also likely have higher rebates. Rebates are payments to health insurance payers provided by drug manufacturers to promote use of an expensive drug. Some pharmacy benefit managers have already said that they plan to charge patients the same amount for Humira as its biosimilars, Dr. Wosinska said.
"For an existing patient, there's really no incentive for them to switch," she said in an interview.
So far only one insurance company, Kaiser Permanente, has plans to switch patients over to biosimilars, according to the health policy podcast Tradeoffs, and the insurer will stop covering Humira by the end of this year.
A version of this article first appeared on Medscape.com.
*This story was updated 2/1/2023.
The first biosimilar for Humira, adalimumab-atto (Amjevita), is now available in the United States, according to an announcement on Jan. 31 by the manufacturer, Amgen. At least seven other U.S. Food and Drug Administration–approved Humira biosimilars are expected to become available later in 2023.
Amjevita was approved by the FDA in September 2016 for multiple inflammatory diseases, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The delayed launch was part of a global settlement with Humira’s manufacturer, AbbVie.
Humira (adalimumab) has been available since 2002 and is consistently one of the top-selling drugs in the United States. A single 40-mg Amjevita pen device will be available at two prices: a list price (wholesale acquisition cost) of $1,557.59, 55% below the current Humira list price, and a list price of $3,288.24, 5% below the current Humira list price, according to Amgen.
“Amgen’s goal is to provide broad access for patients by offering two options to health plans and pharmacy benefit managers,” the company said in the press release.
Patients are less likely to benefit from the more significant discount, said Marta Wosinska, PhD, a health care economist at the Brookings Institute in Washington, DC. It's expected that insurance companies will use the higher list price for Amjevita, she said, as this higher price will also likely have higher rebates. Rebates are payments to health insurance payers provided by drug manufacturers to promote use of an expensive drug. Some pharmacy benefit managers have already said that they plan to charge patients the same amount for Humira as its biosimilars, Dr. Wosinska said.
"For an existing patient, there's really no incentive for them to switch," she said in an interview.
So far only one insurance company, Kaiser Permanente, has plans to switch patients over to biosimilars, according to the health policy podcast Tradeoffs, and the insurer will stop covering Humira by the end of this year.
A version of this article first appeared on Medscape.com.
*This story was updated 2/1/2023.
The first biosimilar for Humira, adalimumab-atto (Amjevita), is now available in the United States, according to an announcement on Jan. 31 by the manufacturer, Amgen. At least seven other U.S. Food and Drug Administration–approved Humira biosimilars are expected to become available later in 2023.
Amjevita was approved by the FDA in September 2016 for multiple inflammatory diseases, including rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and plaque psoriasis. The delayed launch was part of a global settlement with Humira’s manufacturer, AbbVie.
Humira (adalimumab) has been available since 2002 and is consistently one of the top-selling drugs in the United States. A single 40-mg Amjevita pen device will be available at two prices: a list price (wholesale acquisition cost) of $1,557.59, 55% below the current Humira list price, and a list price of $3,288.24, 5% below the current Humira list price, according to Amgen.
“Amgen’s goal is to provide broad access for patients by offering two options to health plans and pharmacy benefit managers,” the company said in the press release.
Patients are less likely to benefit from the more significant discount, said Marta Wosinska, PhD, a health care economist at the Brookings Institute in Washington, DC. It's expected that insurance companies will use the higher list price for Amjevita, she said, as this higher price will also likely have higher rebates. Rebates are payments to health insurance payers provided by drug manufacturers to promote use of an expensive drug. Some pharmacy benefit managers have already said that they plan to charge patients the same amount for Humira as its biosimilars, Dr. Wosinska said.
"For an existing patient, there's really no incentive for them to switch," she said in an interview.
So far only one insurance company, Kaiser Permanente, has plans to switch patients over to biosimilars, according to the health policy podcast Tradeoffs, and the insurer will stop covering Humira by the end of this year.
A version of this article first appeared on Medscape.com.
*This story was updated 2/1/2023.
75 years: A look back on the fascinating history of methotrexate and folate antagonists
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
If you could go back in time 75 years and tell Dr. Sidney Farber, the developer of methotrexate for cancer therapy, that 21st-century medicine would utilize his specially designed drug more in rheumatology than oncology, he might be surprised. He might scratch his head even more, hearing of his drug sparking interest in still other medical fields, like cardiology.
But drug repurposing is not so uncommon. One classic example is aspirin. Once the most common pain medication and used also in rheumatology, aspirin now finds a range of applications, from colorectal cancer to the prevention of cardiovascular and cerebrovascular thrombosis. Minoxidil is another example, developed for hypertension but used today mostly to stop hair loss. Perhaps most ironic is thalidomide, utilized today for leprosy and multiple myeloma, yet actually contraindicated for its original application, nausea of pregnancy.
Methotrexate, thus, has much in common with other medical treatments, and yet its origin story is as unique and as fascinating as the story of Dr. Farber himself. While this is a rheumatology article, it’s also a story about the origin of a particular rheumatologic treatment, and so the story of that origin will take us mostly through a discussion of hematologic malignancy and of the clinical researcher who dared search for a cure.
Born in 1903, in Buffalo, New York, third of fourteen children of Jewish immigrants from Poland, Dr. Farber grew up in a household that was crowded but academically rigorous. His father, Simon, routinely brought home textbooks, assigning each child a book to read and on which to write a report. His mother, Matilda, was as devoted as her husband to raising the children to succeed in their adopted new country. Upstairs, the children were permitted to speak Yiddish, but downstairs they were required to use only English and German.
As a teen, Dr. Farber lived through the 1918 influenza pandemic that killed at least 50 million people worldwide, including more than 2,000 Buffalonians. This probably helped motivate him to study medicine, but with antisemitism overt in the America of the early 1920s, securing admission to a U.S. medical school was close to impossible. So, in what now seems like the greatest of ironies, Dr. Farber began medical studies in Germany, then transferred for the second year to a U.S. program that seemed adequate – Harvard Medical School, from which he graduated in 1927. From there, he trained as a pathologist, focusing ultimately on pediatric pathology. But, frustrated by case after case of malignancy, whose young victims he’d often have to autopsy, Dr. Farber decided that he wanted to advance the pitiful state of cancer therapeutics, especially for hematologic malignancy.
This was a tall order in the 1930s and early 1940s, when cancer therapeutics consisted only of surgical resection and very primitive forms of radiation therapy. Applicable only to neoplasia that was localized, these options were useless against malignancies in the blood, like acute lymphoblastic leukemia (ALL), but by January 1948 there was at least one glimmer of hope. At that time, one patient with ALL, 2-year-old Robert Sandler, was too ill to join his twin brother Elliott for snow play outside their home in the Dorchester section of Boston. Diagnosed back in August, Robert had suffered multiple episodes of fever, anemia, and thrombocytopenia. His illness had enlarged his spleen dramatically and caused pathologic bone fractures with excruciating bone pain, and for a while he couldn’t walk because of pressure on his lower spinal cord. All of this was the result of uncontrolled mitosis and cell division of lymphoblasts, immature lymphocytes. By December, these out-of-control cells had elevated the boy’s white blood cell count to a peak of 70,000/mcL, more than six times the high end of the normal range (4,500-11,000/mcL). This had happened despite treatment with an experimental drug, developed at Boston Children’s Hospital by Dr. Farber and his team, working on the assumption that inhibition of folate metabolism should slow the growth of tumor cells. On Dec. 28, however, Dr. Farber had switched the child to a new drug with a chemical structure just slightly different from the other agent’s.
Merely another chemical modification in a series of attempts by the research team, the new drug, aminopterin, was not expected to do anything dramatic, but Dr. Farber and the team had come such a long way since the middle of 1947, when he’d actually done the opposite of what he was doing now. On the basis of British research from India showing folic acid deficiency as the basis of a common type of anemia in malnourished people, Dr. Farber had reasoned that children with leukemia, who also suffered from anemia, might also benefit from folic acid supplementation. Even without prior rodent testing, Dr. Farber had tried giving the nutrient to patients with ALL, a strategy made possible by the presence of a spectacular chemist working on folic acid synthesis at Farber’s own hospital to help combat folate deficiency. Born into a poor Brahmin family in India, the chemist, Dr. Yellapragada SubbaRow, had begun life with so much stacked against him as to appear even less likely during childhood than the young Dr. Farber to grow up to make major contributions to medicine. Going through childhood with death all around him, Dr. SubbaRow was motivated to study medicine, but getting into medical school had been an uphill fight, given his family’s economic difficulty. Knowing that he’d also face discrimination on account of his low status after receiving admission to a medical program, SubbaRow could have made things a bit easier for himself by living within the norms of the British Imperial system, but as a supporter of Mohandas Gandhi’s nationalist movement, he boycotted British goods. As a medical student, this meant doing things like wearing Indian-made surgical gloves, instead of the English products that were expected of the students. Such actions led Dr. SubbaRow to receive a kind of second-rate medical degree, rather than the prestigious MBBS.
The political situation also led Dr. SubbaRow to emigrate to the United States, where, ironically, his medical degree initially was taken less seriously than it had been taken in his British-occupied homeland. He thus worked in the capacity of a hospital night porter at Peter Bent Brigham Hospital (the future Brigham and Women’s Hospital), doing menial tasks like changing sheets to make ends meet. He studied, however, and made enough of an impression to gain admission to the same institution that also admitted Farber through the backdoor, Harvard Medical School. This launched him into a research career in which he not only would be instrumental in developing folate antagonists and other classes of drugs, but also would make him the codiscoverer of the role of creatine phosphate and ATP in cellular energy metabolism. Sadly, even after obtaining his top-notch American credentials and contributing through his research to what you might say is a good chunk of the biochemistry pathways that first year medical students memorize without ever learning who discovered them, Dr. SubbaRow still faced prejudice for the rest of his life, which turned out to last only until the age of 53. To add insult to injury, he is rarely remembered for his role.
Dr. Farber proceeded with the folic acid supplementation idea in patients with ALL, even though ALL caused a hypoproliferative anemia, whereas anemia from folate deficiency was megaloblastic, meaning that erythrocytes were produced but they were oversized and dysfunctional. Tragically, folic acid had accelerated the disease process in children with ALL, but the process of chemical experimentation aimed at synthesizing folate also produced some compounds that mimicked chemical precursors of folate in a way that made them antifolates, inhibitors of folate metabolism. If folic acid made lymphoblasts grow faster, Dr. Farber had reasoned that antifolates should inhibit their growth. He thus asked the chemistry lab to focus on folate inhibitors. Testing aminopterin, beginning with young Robert Sandler at the end of December, is what proved his hypothesis correct. By late January, aminopterin had brought the child’s WBC count down to the realm of 12,000, just slightly above normal, with symptoms and signs abating as well, and by February, the child could play with his twin brother. It was not a cure; malignant lymphoblasts still showed on microscopy of Robert’s blood. While he and some 15 other children whom Dr. Farber treated in this early trial would all succumb to ALL, they experienced remission lasting several months.
This was a big deal because the concept of chemotherapy was based only on serendipitous observations of WBC counts dropping in soldiers exposed to nitrogen mustard gas during World War I and during an incident in World War II, yet aminopterin had been designed from the ground up. Though difficult to synthesize in quantities, there was no reason for Dr. Farber’s team not to keep tweaking the drug, and so they did. Replacing one hydrogen atom with a methyl group, they turned it into methotrexate.
Proving easier to synthesize and less toxic, methotrexate would become a workhorse for chemotherapy over the next couple of decades, but the capability of both methotrexate and aminopterin to blunt the growth of white blood cells and other cells did not go unnoticed outside the realm of oncology. As early as the 1950s, dermatologists were using aminopterin to treat psoriasis. This led to the approval of methotrexate for psoriasis in 1972.
Meanwhile, like oncology, infectious diseases, aviation medicine, and so many other areas of practice, rheumatology had gotten a major boost from research stemming from World War II. During the war, Dr. Philip Hench of the Mayo Clinic developed cortisone, which pilots used to stay alert and energetic during trans-Atlantic flights. But it turned out that cortisone had a powerful immunosuppressive effect that dramatically improved rheumatoid arthritis, leading Dr. Hench to receive the Nobel Prize in Physiology or Medicine in 1950. By the end of the 1950s, however, the significant side effects of long-term corticosteroid therapy were very clear, so over the next few decades there was a major effort to develop different treatments for RA and other rheumatologic diseases.
Top on the list of such agents was methotrexate, developed for RA in part by Dr. Michael Weinblatt of Brigham and Women’s Hospital in Boston. In the 1980s, Dr. Weinblatt published the first clinical trial showing the benefits of methotrexate for RA patients. This has since developed into a standard treatment, noticeably different from the original malignancy application in that it is a low-dose regimen. Patients taking methotrexate for RA typically receive no more than 25 mg per week orally, and often much less. Rheumatology today includes expertise in keeping long-term methotrexate therapy safe by monitoring liver function and through other routine tests. The routine nature of the therapy has brought methotrexate to the point of beckoning in a realm that Dr. Farber might not have predicted in his wildest imagination: cardiology. This is on account of the growing appreciation of the inflammatory process in the pathophysiology of atherosclerotic heart disease.
Meanwhile, being an antimetabolite, harmful to rapidly dividing cells, the danger of methotrexate to the embryo and fetus was recognized early. This made methotrexate off-limits to pregnant women, yet it also has made the drug useful as an abortifacient. Though not as good for medication abortion in unwanted but thriving pregnancies, where mifepristone/misoprostol has become the regimen of choice, methotrexate has become a workhorse in other obstetrical settings, such as for ending ectopic pregnancy.
Looking at the present and into the future, the potential for this very old medication looks wide open, as if it could go in any direction, so let’s wind up the discussion with the thought that we may be in for some surprises. Rather than jumping deeply into any rheumatologic issue, we spent most of this article weaving through other medical issues, but does this not make today’s story fairly analogous to rheumatology itself?
Dr. Warmflash is a physician from Portland, Ore. He reported no conflicts of interest.
This story was updated 2/10/2023.
A version of this article first appeared on Medscape.com.
High HDL-C levels linked to increased fracture risk
High levels of high-density lipoprotein cholesterol (HDL-C) in older adults are associated with a higher risk of sustaining a fracture than lower HDL-C levels, a new study suggests.
“Two animal studies showing that HDL-C reduces bone mineral density by reducing osteoblast number and function provide a plausible explanation for why high HDL-C may increase the risk of fractures,” Monira Hussain, MBBS, MPH, PhD, of Monash University in Melbourne, told this news organization. “So, it was not surprising when our analyses provided evidence that amongst those in the highest quintile of HDL-C (> 74 mg/dL), there was a [33%] increased risk of fractures.”
After adjustment, one standard deviation increment in HDL-C level was associated with a 14% higher risk of fracture during a 4-year follow-up.
Based on this and other studies, Dr. Hussain said, “I believe that the finding of a very high HDL-C [should] alert clinicians to a higher risk of mortality, fractures, and possibly other threats to their patient’s health.”
The study was published online in JAMA Cardiology.
Independent risk factor
For this report, the researchers conducted a post hoc analysis of data from the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial and the ASPREE-Fracture substudy.
ASPREE was a double-blind, randomized, placebo-controlled primary prevention trial of aspirin. Participants were 16,703 community-dwelling Australians and 2,411 individuals from the United States with a mean age of 75 and without evident cardiovascular disease, dementia, physical disability, or life-limiting chronic illness.
The ASPREE-Fracture substudy collected data on fractures reported post randomization from the Australian participants. Fractures were confirmed by imaging and adjudicated by an expert panel and included both traumatic and minimal trauma fractures.
Of the 16,262 participants who had a plasma HDL-C measurement at baseline (55% women), 1,659 (10.2%) experienced at least one fracture over a median of 4 years. This included 711 minimal trauma fractures (for example, falls from standing height) and 948 other trauma fractures, mainly falls on stairs, ladders, or stools.
Higher rates of fractures occurred in the highest quintile of HDL-C level where the mean level was 89 mg/dL. At baseline, participants in that quintile had a lower BMI, a high prevalence of current/former smoking and current alcohol use, 12 years or longer of school, more physical activity, and higher use of antiosteoporosis medication. They also had less chronic kidney disease, diabetes, prefrailty/frailty, or treatment with lipid-lowering drugs.
In a fully adjusted model, each standard deviation increment in HDL-C level was associated with a 14% higher risk of fractures (hazard ratio, 1.14). When analyzed in quintiles, compared with participants in Q1, those in Q5 had a 33% higher risk for fracture (HR, 1.33).
Prevalence rates were similar between the sexes. The increase in fracture risk appeared to be independent of traditional risk factors for fractures, including age, sex, physical activity, alcohol use, frailty, BMI, smoking status, diabetes, chronic kidney disease, use of lipid-lowering or antiosteoporosis drugs, and education, the authors note.
The results persisted in sensitivity analyses in restricted subgroups of interest and in stratified analyses – including, for example, only minimal fractures; participants not taking antiosteoporosis drugs or statins; never smokers; nondrinkers; and those engaging in minimal physical activity (walking less than 30 minutes per day).
No association was observed between non–HDL-C levels and fractures.
The authors conclude that the study “provides robust evidence that higher levels of HDL-C are associated with incident fractures in both male and female individuals, independent of conventional risk factors.”
Clinically useful?
Commenting on the study for this news organization, Marilyn Tan, MD, clinic chief of the Endocrine Clinic and clinical associate professor of medicine at Stanford (Calif.) University, said, “I certainly would not recommend anyone do anything to actively lower their HDL levels. HDL levels are largely determined by genetics, diet, and lifestyle, with some effects from certain medications/supplements. Studies have demonstrated that moderately higher HDL levels may be protective for atherosclerosis.”
In the current study, she said, “Causation has not been proven, and importantly there is no evidence that reducing HDL levels reduces fracture risk. Also, this association between raised HDL levels and fracture risk has not been demonstrated consistently in other studies.”
Furthermore, she noted, the preclinical trials on which the authors based their hypothesis – that is, an association between HDL and a reduction in the number and function of osteoblasts – “has not been demonstrated widely in human subjects.”
“We have a large armamentarium of FDA-approved treatments for osteoporosis that have been clinically proven to reduce fracture risk very significantly, and these are the tools [in addition to lifestyle changes] we should use to reduce fracture risk,” Dr. Tan concluded.
John Wilkins, MD, of Northwestern University, Chicago, and Anand Rohatgi, MD, MSCS, of UT Southwestern Medical Center, Dallas, also point out some limitations of the study in a related editorial.
They note the inclusion of predominantly healthy adults with a mean age of 75, a population that could yield different findings from middle-aged cohorts with chronic illnesses, as well as a lack of clarity regarding the possible role of alcohol intake among the study participants.
Furthermore, the editorialists write, although significant associations were shown in this study, “models were not adjusted for detailed measures of exercise/activity, triglycerides, or any other lipids, including other HDL compositional measures such as HDL-P or ApoA-I levels. There was no assessment of whether HDL-C improved discrimination, reclassification, or any other validated measures of risk prediction performance.
“Taken together,” they conclude, “this study alone leaves several unanswered questions as to whether high HDL-C could be a useful biomarker to detect fracture risk.”
No commercial funding was disclosed. The authors report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
High levels of high-density lipoprotein cholesterol (HDL-C) in older adults are associated with a higher risk of sustaining a fracture than lower HDL-C levels, a new study suggests.
“Two animal studies showing that HDL-C reduces bone mineral density by reducing osteoblast number and function provide a plausible explanation for why high HDL-C may increase the risk of fractures,” Monira Hussain, MBBS, MPH, PhD, of Monash University in Melbourne, told this news organization. “So, it was not surprising when our analyses provided evidence that amongst those in the highest quintile of HDL-C (> 74 mg/dL), there was a [33%] increased risk of fractures.”
After adjustment, one standard deviation increment in HDL-C level was associated with a 14% higher risk of fracture during a 4-year follow-up.
Based on this and other studies, Dr. Hussain said, “I believe that the finding of a very high HDL-C [should] alert clinicians to a higher risk of mortality, fractures, and possibly other threats to their patient’s health.”
The study was published online in JAMA Cardiology.
Independent risk factor
For this report, the researchers conducted a post hoc analysis of data from the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial and the ASPREE-Fracture substudy.
ASPREE was a double-blind, randomized, placebo-controlled primary prevention trial of aspirin. Participants were 16,703 community-dwelling Australians and 2,411 individuals from the United States with a mean age of 75 and without evident cardiovascular disease, dementia, physical disability, or life-limiting chronic illness.
The ASPREE-Fracture substudy collected data on fractures reported post randomization from the Australian participants. Fractures were confirmed by imaging and adjudicated by an expert panel and included both traumatic and minimal trauma fractures.
Of the 16,262 participants who had a plasma HDL-C measurement at baseline (55% women), 1,659 (10.2%) experienced at least one fracture over a median of 4 years. This included 711 minimal trauma fractures (for example, falls from standing height) and 948 other trauma fractures, mainly falls on stairs, ladders, or stools.
Higher rates of fractures occurred in the highest quintile of HDL-C level where the mean level was 89 mg/dL. At baseline, participants in that quintile had a lower BMI, a high prevalence of current/former smoking and current alcohol use, 12 years or longer of school, more physical activity, and higher use of antiosteoporosis medication. They also had less chronic kidney disease, diabetes, prefrailty/frailty, or treatment with lipid-lowering drugs.
In a fully adjusted model, each standard deviation increment in HDL-C level was associated with a 14% higher risk of fractures (hazard ratio, 1.14). When analyzed in quintiles, compared with participants in Q1, those in Q5 had a 33% higher risk for fracture (HR, 1.33).
Prevalence rates were similar between the sexes. The increase in fracture risk appeared to be independent of traditional risk factors for fractures, including age, sex, physical activity, alcohol use, frailty, BMI, smoking status, diabetes, chronic kidney disease, use of lipid-lowering or antiosteoporosis drugs, and education, the authors note.
The results persisted in sensitivity analyses in restricted subgroups of interest and in stratified analyses – including, for example, only minimal fractures; participants not taking antiosteoporosis drugs or statins; never smokers; nondrinkers; and those engaging in minimal physical activity (walking less than 30 minutes per day).
No association was observed between non–HDL-C levels and fractures.
The authors conclude that the study “provides robust evidence that higher levels of HDL-C are associated with incident fractures in both male and female individuals, independent of conventional risk factors.”
Clinically useful?
Commenting on the study for this news organization, Marilyn Tan, MD, clinic chief of the Endocrine Clinic and clinical associate professor of medicine at Stanford (Calif.) University, said, “I certainly would not recommend anyone do anything to actively lower their HDL levels. HDL levels are largely determined by genetics, diet, and lifestyle, with some effects from certain medications/supplements. Studies have demonstrated that moderately higher HDL levels may be protective for atherosclerosis.”
In the current study, she said, “Causation has not been proven, and importantly there is no evidence that reducing HDL levels reduces fracture risk. Also, this association between raised HDL levels and fracture risk has not been demonstrated consistently in other studies.”
Furthermore, she noted, the preclinical trials on which the authors based their hypothesis – that is, an association between HDL and a reduction in the number and function of osteoblasts – “has not been demonstrated widely in human subjects.”
“We have a large armamentarium of FDA-approved treatments for osteoporosis that have been clinically proven to reduce fracture risk very significantly, and these are the tools [in addition to lifestyle changes] we should use to reduce fracture risk,” Dr. Tan concluded.
John Wilkins, MD, of Northwestern University, Chicago, and Anand Rohatgi, MD, MSCS, of UT Southwestern Medical Center, Dallas, also point out some limitations of the study in a related editorial.
They note the inclusion of predominantly healthy adults with a mean age of 75, a population that could yield different findings from middle-aged cohorts with chronic illnesses, as well as a lack of clarity regarding the possible role of alcohol intake among the study participants.
Furthermore, the editorialists write, although significant associations were shown in this study, “models were not adjusted for detailed measures of exercise/activity, triglycerides, or any other lipids, including other HDL compositional measures such as HDL-P or ApoA-I levels. There was no assessment of whether HDL-C improved discrimination, reclassification, or any other validated measures of risk prediction performance.
“Taken together,” they conclude, “this study alone leaves several unanswered questions as to whether high HDL-C could be a useful biomarker to detect fracture risk.”
No commercial funding was disclosed. The authors report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
High levels of high-density lipoprotein cholesterol (HDL-C) in older adults are associated with a higher risk of sustaining a fracture than lower HDL-C levels, a new study suggests.
“Two animal studies showing that HDL-C reduces bone mineral density by reducing osteoblast number and function provide a plausible explanation for why high HDL-C may increase the risk of fractures,” Monira Hussain, MBBS, MPH, PhD, of Monash University in Melbourne, told this news organization. “So, it was not surprising when our analyses provided evidence that amongst those in the highest quintile of HDL-C (> 74 mg/dL), there was a [33%] increased risk of fractures.”
After adjustment, one standard deviation increment in HDL-C level was associated with a 14% higher risk of fracture during a 4-year follow-up.
Based on this and other studies, Dr. Hussain said, “I believe that the finding of a very high HDL-C [should] alert clinicians to a higher risk of mortality, fractures, and possibly other threats to their patient’s health.”
The study was published online in JAMA Cardiology.
Independent risk factor
For this report, the researchers conducted a post hoc analysis of data from the Aspirin in Reducing Events in the Elderly (ASPREE) clinical trial and the ASPREE-Fracture substudy.
ASPREE was a double-blind, randomized, placebo-controlled primary prevention trial of aspirin. Participants were 16,703 community-dwelling Australians and 2,411 individuals from the United States with a mean age of 75 and without evident cardiovascular disease, dementia, physical disability, or life-limiting chronic illness.
The ASPREE-Fracture substudy collected data on fractures reported post randomization from the Australian participants. Fractures were confirmed by imaging and adjudicated by an expert panel and included both traumatic and minimal trauma fractures.
Of the 16,262 participants who had a plasma HDL-C measurement at baseline (55% women), 1,659 (10.2%) experienced at least one fracture over a median of 4 years. This included 711 minimal trauma fractures (for example, falls from standing height) and 948 other trauma fractures, mainly falls on stairs, ladders, or stools.
Higher rates of fractures occurred in the highest quintile of HDL-C level where the mean level was 89 mg/dL. At baseline, participants in that quintile had a lower BMI, a high prevalence of current/former smoking and current alcohol use, 12 years or longer of school, more physical activity, and higher use of antiosteoporosis medication. They also had less chronic kidney disease, diabetes, prefrailty/frailty, or treatment with lipid-lowering drugs.
In a fully adjusted model, each standard deviation increment in HDL-C level was associated with a 14% higher risk of fractures (hazard ratio, 1.14). When analyzed in quintiles, compared with participants in Q1, those in Q5 had a 33% higher risk for fracture (HR, 1.33).
Prevalence rates were similar between the sexes. The increase in fracture risk appeared to be independent of traditional risk factors for fractures, including age, sex, physical activity, alcohol use, frailty, BMI, smoking status, diabetes, chronic kidney disease, use of lipid-lowering or antiosteoporosis drugs, and education, the authors note.
The results persisted in sensitivity analyses in restricted subgroups of interest and in stratified analyses – including, for example, only minimal fractures; participants not taking antiosteoporosis drugs or statins; never smokers; nondrinkers; and those engaging in minimal physical activity (walking less than 30 minutes per day).
No association was observed between non–HDL-C levels and fractures.
The authors conclude that the study “provides robust evidence that higher levels of HDL-C are associated with incident fractures in both male and female individuals, independent of conventional risk factors.”
Clinically useful?
Commenting on the study for this news organization, Marilyn Tan, MD, clinic chief of the Endocrine Clinic and clinical associate professor of medicine at Stanford (Calif.) University, said, “I certainly would not recommend anyone do anything to actively lower their HDL levels. HDL levels are largely determined by genetics, diet, and lifestyle, with some effects from certain medications/supplements. Studies have demonstrated that moderately higher HDL levels may be protective for atherosclerosis.”
In the current study, she said, “Causation has not been proven, and importantly there is no evidence that reducing HDL levels reduces fracture risk. Also, this association between raised HDL levels and fracture risk has not been demonstrated consistently in other studies.”
Furthermore, she noted, the preclinical trials on which the authors based their hypothesis – that is, an association between HDL and a reduction in the number and function of osteoblasts – “has not been demonstrated widely in human subjects.”
“We have a large armamentarium of FDA-approved treatments for osteoporosis that have been clinically proven to reduce fracture risk very significantly, and these are the tools [in addition to lifestyle changes] we should use to reduce fracture risk,” Dr. Tan concluded.
John Wilkins, MD, of Northwestern University, Chicago, and Anand Rohatgi, MD, MSCS, of UT Southwestern Medical Center, Dallas, also point out some limitations of the study in a related editorial.
They note the inclusion of predominantly healthy adults with a mean age of 75, a population that could yield different findings from middle-aged cohorts with chronic illnesses, as well as a lack of clarity regarding the possible role of alcohol intake among the study participants.
Furthermore, the editorialists write, although significant associations were shown in this study, “models were not adjusted for detailed measures of exercise/activity, triglycerides, or any other lipids, including other HDL compositional measures such as HDL-P or ApoA-I levels. There was no assessment of whether HDL-C improved discrimination, reclassification, or any other validated measures of risk prediction performance.
“Taken together,” they conclude, “this study alone leaves several unanswered questions as to whether high HDL-C could be a useful biomarker to detect fracture risk.”
No commercial funding was disclosed. The authors report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
VEXAS syndrome: More common, variable, and severe than expected
A recently discovered inflammatory disease known as VEXAS syndrome is more common, variable, and dangerous than previously understood, according to results of a retrospective observational study of a large health care system database. The findings, published in JAMA, found that it struck 1 in 4,269 men over the age of 50 in a largely White population and caused a wide variety of symptoms.
“The disease is quite severe,” study lead author David Beck, MD, PhD, of the department of medicine at NYU Langone Health, said in an interview. Patients with the condition “have a variety of clinical symptoms affecting different parts of the body and are being managed by different medical specialties.”
Dr. Beck and colleagues first described VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome in 2020. They linked it to mutations in the UBA1 (ubiquitin-like modifier activating enzyme 1) gene. The enzyme initiates a process that identifies misfolded proteins as targets for degradation.
“VEXAS syndrome is characterized by anemia and inflammation in the skin, lungs, cartilage, and joints,” Dr. Beck said. “These symptoms are frequently mistaken for other rheumatic or hematologic diseases. However, this syndrome has a different cause, is treated differently, requires additional monitoring, and can be far more severe.”
According to him, hundreds of people have been diagnosed with the disease in the short time since it was defined. The disease is believed to be fatal in some cases. A previous report found that the median survival was 9 years among patients with a certain variant; that was significantly less than patients with two other variants.
For the new study, researchers searched for UBA1 variants in genetic data from 163,096 subjects (mean age, 52.8 years; 94% White, 61% women) who took part in the Geisinger MyCode Community Health Initiative. The 1996-2022 data comes from patients at 10 Pennsylvania hospitals.
Eleven people (9 males, 2 females) had likely UBA1 variants, and all had anemia. The cases accounted for 1 in 13,591 unrelated people (95% confidence interval, 1:7,775-1:23,758), 1 in 4,269 men older than 50 years (95% CI, 1:2,319-1:7,859), and 1 in 26,238 women older than 50 years (95% CI, 1:7,196-1:147,669).
Other common findings included macrocytosis (91%), skin problems (73%), and pulmonary disease (91%). Ten patients (91%) required transfusions.
Five of the 11 subjects didn’t meet the previously defined criteria for VEXAS syndrome. None had been diagnosed with the condition, which is not surprising considering that it hadn’t been discovered and described until recently.
Just over half of the patients – 55% – had a clinical diagnosis that was previously linked to VEXAS syndrome. “This means that slightly less than half of the patients with VEXAS syndrome had no clear associated clinical diagnosis,” Dr. Beck said. “The lack of associated clinical diagnoses may be due to the variety of nonspecific clinical characteristics that span different subspecialities in VEXAS syndrome. VEXAS syndrome represents an example of a multisystem disease where patients and their symptoms may get lost in the shuffle.”
In the future, “professionals should look out for patients with unexplained inflammation – and some combination of hematologic, rheumatologic, pulmonary, and dermatologic clinical manifestations – that either don’t carry a clinical diagnosis or don’t respond to first-line therapies,” Dr. Beck said. “These patients will also frequently be anemic, have low platelet counts, elevated markers of inflammation in the blood, and be dependent on corticosteroids.”
Diagnosis can be made via genetic testing, but the study authors note that it “is not routinely offered on standard workup for myeloid neoplasms or immune dysregulation diagnostic panels.”
As for treatment, Dr. Beck said the disease “can be partially controlled by multiple different anticytokine therapies or biologics. However, in most cases, patients still need additional steroids and/or disease-modifying antirheumatic agents [DMARDs]. In addition, bone marrow transplantation has shown signs of being a highly effective therapy.”
The study authors say more research is needed to understand the disease’s prevalence in more diverse populations.
In an interview, Matthew J. Koster, MD, a rheumatologist at Mayo Clinic in Rochester, Minn., who’s studied the disease but didn’t take part in this research project, said the findings are valid and “highly important.
“The findings of this study highlight what many academic and quaternary referral centers were wondering: Is VEXAS really more common than we think, with patients hiding in plain sight? The answer is yes,” he said. “Currently, there are less than 400 cases reported in the literature of VEXAS, but large centers are diagnosing this condition with some frequency. For example, at Mayo Clinic in Rochester, we diagnose on average one new patient with VEXAS every 7-14 days and have diagnosed 60 in the past 18 months. A national collaborative group in France has diagnosed approximately 250 patients over that same time frame when pooling patients nationwide.”
The prevalence is high enough, he said, that “clinicians should consider that some of the patients with diseases that are not responding to treatment may in fact have VEXAS rather than ‘refractory’ relapsing polychondritis or ‘recalcitrant’ rheumatoid arthritis, etc.”
The National Institute of Health funded the study. Dr. Beck, the other authors, and Dr. Koster report no disclosures.
A recently discovered inflammatory disease known as VEXAS syndrome is more common, variable, and dangerous than previously understood, according to results of a retrospective observational study of a large health care system database. The findings, published in JAMA, found that it struck 1 in 4,269 men over the age of 50 in a largely White population and caused a wide variety of symptoms.
“The disease is quite severe,” study lead author David Beck, MD, PhD, of the department of medicine at NYU Langone Health, said in an interview. Patients with the condition “have a variety of clinical symptoms affecting different parts of the body and are being managed by different medical specialties.”
Dr. Beck and colleagues first described VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome in 2020. They linked it to mutations in the UBA1 (ubiquitin-like modifier activating enzyme 1) gene. The enzyme initiates a process that identifies misfolded proteins as targets for degradation.
“VEXAS syndrome is characterized by anemia and inflammation in the skin, lungs, cartilage, and joints,” Dr. Beck said. “These symptoms are frequently mistaken for other rheumatic or hematologic diseases. However, this syndrome has a different cause, is treated differently, requires additional monitoring, and can be far more severe.”
According to him, hundreds of people have been diagnosed with the disease in the short time since it was defined. The disease is believed to be fatal in some cases. A previous report found that the median survival was 9 years among patients with a certain variant; that was significantly less than patients with two other variants.
For the new study, researchers searched for UBA1 variants in genetic data from 163,096 subjects (mean age, 52.8 years; 94% White, 61% women) who took part in the Geisinger MyCode Community Health Initiative. The 1996-2022 data comes from patients at 10 Pennsylvania hospitals.
Eleven people (9 males, 2 females) had likely UBA1 variants, and all had anemia. The cases accounted for 1 in 13,591 unrelated people (95% confidence interval, 1:7,775-1:23,758), 1 in 4,269 men older than 50 years (95% CI, 1:2,319-1:7,859), and 1 in 26,238 women older than 50 years (95% CI, 1:7,196-1:147,669).
Other common findings included macrocytosis (91%), skin problems (73%), and pulmonary disease (91%). Ten patients (91%) required transfusions.
Five of the 11 subjects didn’t meet the previously defined criteria for VEXAS syndrome. None had been diagnosed with the condition, which is not surprising considering that it hadn’t been discovered and described until recently.
Just over half of the patients – 55% – had a clinical diagnosis that was previously linked to VEXAS syndrome. “This means that slightly less than half of the patients with VEXAS syndrome had no clear associated clinical diagnosis,” Dr. Beck said. “The lack of associated clinical diagnoses may be due to the variety of nonspecific clinical characteristics that span different subspecialities in VEXAS syndrome. VEXAS syndrome represents an example of a multisystem disease where patients and their symptoms may get lost in the shuffle.”
In the future, “professionals should look out for patients with unexplained inflammation – and some combination of hematologic, rheumatologic, pulmonary, and dermatologic clinical manifestations – that either don’t carry a clinical diagnosis or don’t respond to first-line therapies,” Dr. Beck said. “These patients will also frequently be anemic, have low platelet counts, elevated markers of inflammation in the blood, and be dependent on corticosteroids.”
Diagnosis can be made via genetic testing, but the study authors note that it “is not routinely offered on standard workup for myeloid neoplasms or immune dysregulation diagnostic panels.”
As for treatment, Dr. Beck said the disease “can be partially controlled by multiple different anticytokine therapies or biologics. However, in most cases, patients still need additional steroids and/or disease-modifying antirheumatic agents [DMARDs]. In addition, bone marrow transplantation has shown signs of being a highly effective therapy.”
The study authors say more research is needed to understand the disease’s prevalence in more diverse populations.
In an interview, Matthew J. Koster, MD, a rheumatologist at Mayo Clinic in Rochester, Minn., who’s studied the disease but didn’t take part in this research project, said the findings are valid and “highly important.
“The findings of this study highlight what many academic and quaternary referral centers were wondering: Is VEXAS really more common than we think, with patients hiding in plain sight? The answer is yes,” he said. “Currently, there are less than 400 cases reported in the literature of VEXAS, but large centers are diagnosing this condition with some frequency. For example, at Mayo Clinic in Rochester, we diagnose on average one new patient with VEXAS every 7-14 days and have diagnosed 60 in the past 18 months. A national collaborative group in France has diagnosed approximately 250 patients over that same time frame when pooling patients nationwide.”
The prevalence is high enough, he said, that “clinicians should consider that some of the patients with diseases that are not responding to treatment may in fact have VEXAS rather than ‘refractory’ relapsing polychondritis or ‘recalcitrant’ rheumatoid arthritis, etc.”
The National Institute of Health funded the study. Dr. Beck, the other authors, and Dr. Koster report no disclosures.
A recently discovered inflammatory disease known as VEXAS syndrome is more common, variable, and dangerous than previously understood, according to results of a retrospective observational study of a large health care system database. The findings, published in JAMA, found that it struck 1 in 4,269 men over the age of 50 in a largely White population and caused a wide variety of symptoms.
“The disease is quite severe,” study lead author David Beck, MD, PhD, of the department of medicine at NYU Langone Health, said in an interview. Patients with the condition “have a variety of clinical symptoms affecting different parts of the body and are being managed by different medical specialties.”
Dr. Beck and colleagues first described VEXAS (vacuoles, E1-ubiquitin-activating enzyme, X-linked, autoinflammatory, somatic) syndrome in 2020. They linked it to mutations in the UBA1 (ubiquitin-like modifier activating enzyme 1) gene. The enzyme initiates a process that identifies misfolded proteins as targets for degradation.
“VEXAS syndrome is characterized by anemia and inflammation in the skin, lungs, cartilage, and joints,” Dr. Beck said. “These symptoms are frequently mistaken for other rheumatic or hematologic diseases. However, this syndrome has a different cause, is treated differently, requires additional monitoring, and can be far more severe.”
According to him, hundreds of people have been diagnosed with the disease in the short time since it was defined. The disease is believed to be fatal in some cases. A previous report found that the median survival was 9 years among patients with a certain variant; that was significantly less than patients with two other variants.
For the new study, researchers searched for UBA1 variants in genetic data from 163,096 subjects (mean age, 52.8 years; 94% White, 61% women) who took part in the Geisinger MyCode Community Health Initiative. The 1996-2022 data comes from patients at 10 Pennsylvania hospitals.
Eleven people (9 males, 2 females) had likely UBA1 variants, and all had anemia. The cases accounted for 1 in 13,591 unrelated people (95% confidence interval, 1:7,775-1:23,758), 1 in 4,269 men older than 50 years (95% CI, 1:2,319-1:7,859), and 1 in 26,238 women older than 50 years (95% CI, 1:7,196-1:147,669).
Other common findings included macrocytosis (91%), skin problems (73%), and pulmonary disease (91%). Ten patients (91%) required transfusions.
Five of the 11 subjects didn’t meet the previously defined criteria for VEXAS syndrome. None had been diagnosed with the condition, which is not surprising considering that it hadn’t been discovered and described until recently.
Just over half of the patients – 55% – had a clinical diagnosis that was previously linked to VEXAS syndrome. “This means that slightly less than half of the patients with VEXAS syndrome had no clear associated clinical diagnosis,” Dr. Beck said. “The lack of associated clinical diagnoses may be due to the variety of nonspecific clinical characteristics that span different subspecialities in VEXAS syndrome. VEXAS syndrome represents an example of a multisystem disease where patients and their symptoms may get lost in the shuffle.”
In the future, “professionals should look out for patients with unexplained inflammation – and some combination of hematologic, rheumatologic, pulmonary, and dermatologic clinical manifestations – that either don’t carry a clinical diagnosis or don’t respond to first-line therapies,” Dr. Beck said. “These patients will also frequently be anemic, have low platelet counts, elevated markers of inflammation in the blood, and be dependent on corticosteroids.”
Diagnosis can be made via genetic testing, but the study authors note that it “is not routinely offered on standard workup for myeloid neoplasms or immune dysregulation diagnostic panels.”
As for treatment, Dr. Beck said the disease “can be partially controlled by multiple different anticytokine therapies or biologics. However, in most cases, patients still need additional steroids and/or disease-modifying antirheumatic agents [DMARDs]. In addition, bone marrow transplantation has shown signs of being a highly effective therapy.”
The study authors say more research is needed to understand the disease’s prevalence in more diverse populations.
In an interview, Matthew J. Koster, MD, a rheumatologist at Mayo Clinic in Rochester, Minn., who’s studied the disease but didn’t take part in this research project, said the findings are valid and “highly important.
“The findings of this study highlight what many academic and quaternary referral centers were wondering: Is VEXAS really more common than we think, with patients hiding in plain sight? The answer is yes,” he said. “Currently, there are less than 400 cases reported in the literature of VEXAS, but large centers are diagnosing this condition with some frequency. For example, at Mayo Clinic in Rochester, we diagnose on average one new patient with VEXAS every 7-14 days and have diagnosed 60 in the past 18 months. A national collaborative group in France has diagnosed approximately 250 patients over that same time frame when pooling patients nationwide.”
The prevalence is high enough, he said, that “clinicians should consider that some of the patients with diseases that are not responding to treatment may in fact have VEXAS rather than ‘refractory’ relapsing polychondritis or ‘recalcitrant’ rheumatoid arthritis, etc.”
The National Institute of Health funded the study. Dr. Beck, the other authors, and Dr. Koster report no disclosures.
FROM JAMA