User login
Will AI or robotics steal your job?
NEW YORK – Artificial intelligence is currently linked to specific problem solving and is not some form of Terminator model capable of handling multiple tasks with autonomy. In other words, each time you hear the term “AI,” it is a computer solving a specific problem or task using algorithms “and not ‘thinking’ like you and me,” said Ido Weinberg, MD, assistant professor, Harvard Medical School, Boston.
AI is present in daily life – everything from cellphones to the Alexa voice interface on a smart speaker. That AI system, however, is amassing data, learning about you, and using that data intelligently, Dr. Weinberg said at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
AI in health care make sense, he said, because the health sector is a vast consumer market with potential for financial gain. Repetition, which is common in the health sector, is one of the foundations required for using AI and robotics. If a task can be repeated, then it means a machine can do it, said Dr. Weinberg.
The spread of AI and robotics one day may improve health care accessibility in remote areas where physicians with the appropriate training may not be available.
AI is already at work in the health care industry. “Pulmonary nodule detection can be done better with machines than by people, pathological identification and scanning of various slides can be done better by a machine than by a humans,” he said.
Artificial intelligence also can be designed to detect emotion by assessing various cues in phrasing, key words, and tone. These AI functions already are being used by sales reps on the phone to defuse and control interactions with customers and complainants. AI also can be implemented in interactions with people, which is an important part of dealing with patients, Dr. Weinberg said. Drug discovery is a key area where AI is flourishing, as well.
Luckily, in terms of physicians keeping their jobs, there are barriers to the use of AI to replace clinicians, Dr. Weinberg pointed out. Health care is not a monolith, and every specialty is different, meaning AI would have to be tailored to each task and specialty for each unique field. Quick proliferation of AI across the board is unlikely, especially when the varying roles of nurses and physician assistants are included.
Although robots in science fiction stories and films often are capable of multitasking a variety of needs, robots at present are much more limited in real life. In surgical situations, for example, they can perform specifically tailored tasks but cannot extend beyond those defined parameters as a real surgeon can, according to Dr. Weinberg, and this lack of flexibility is a severe limitation on the expansion of AI into health care.
Despite these limitations, Dr. Weinberg urged attendees to consider how AI can be used to facilitate their work.
“Believe in the roadblocks, but be a fast adopter – an early adopter – and understand where AI can currently augment you and make you better and more productive,” he said. “And keep doing procedures; AI and robotics currently have a problem with most of those,” Dr. Weinberg concluded.
Dr. Weinberg reported no conflicts relevant to his talk.
NEW YORK – Artificial intelligence is currently linked to specific problem solving and is not some form of Terminator model capable of handling multiple tasks with autonomy. In other words, each time you hear the term “AI,” it is a computer solving a specific problem or task using algorithms “and not ‘thinking’ like you and me,” said Ido Weinberg, MD, assistant professor, Harvard Medical School, Boston.
AI is present in daily life – everything from cellphones to the Alexa voice interface on a smart speaker. That AI system, however, is amassing data, learning about you, and using that data intelligently, Dr. Weinberg said at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
AI in health care make sense, he said, because the health sector is a vast consumer market with potential for financial gain. Repetition, which is common in the health sector, is one of the foundations required for using AI and robotics. If a task can be repeated, then it means a machine can do it, said Dr. Weinberg.
The spread of AI and robotics one day may improve health care accessibility in remote areas where physicians with the appropriate training may not be available.
AI is already at work in the health care industry. “Pulmonary nodule detection can be done better with machines than by people, pathological identification and scanning of various slides can be done better by a machine than by a humans,” he said.
Artificial intelligence also can be designed to detect emotion by assessing various cues in phrasing, key words, and tone. These AI functions already are being used by sales reps on the phone to defuse and control interactions with customers and complainants. AI also can be implemented in interactions with people, which is an important part of dealing with patients, Dr. Weinberg said. Drug discovery is a key area where AI is flourishing, as well.
Luckily, in terms of physicians keeping their jobs, there are barriers to the use of AI to replace clinicians, Dr. Weinberg pointed out. Health care is not a monolith, and every specialty is different, meaning AI would have to be tailored to each task and specialty for each unique field. Quick proliferation of AI across the board is unlikely, especially when the varying roles of nurses and physician assistants are included.
Although robots in science fiction stories and films often are capable of multitasking a variety of needs, robots at present are much more limited in real life. In surgical situations, for example, they can perform specifically tailored tasks but cannot extend beyond those defined parameters as a real surgeon can, according to Dr. Weinberg, and this lack of flexibility is a severe limitation on the expansion of AI into health care.
Despite these limitations, Dr. Weinberg urged attendees to consider how AI can be used to facilitate their work.
“Believe in the roadblocks, but be a fast adopter – an early adopter – and understand where AI can currently augment you and make you better and more productive,” he said. “And keep doing procedures; AI and robotics currently have a problem with most of those,” Dr. Weinberg concluded.
Dr. Weinberg reported no conflicts relevant to his talk.
NEW YORK – Artificial intelligence is currently linked to specific problem solving and is not some form of Terminator model capable of handling multiple tasks with autonomy. In other words, each time you hear the term “AI,” it is a computer solving a specific problem or task using algorithms “and not ‘thinking’ like you and me,” said Ido Weinberg, MD, assistant professor, Harvard Medical School, Boston.
AI is present in daily life – everything from cellphones to the Alexa voice interface on a smart speaker. That AI system, however, is amassing data, learning about you, and using that data intelligently, Dr. Weinberg said at a symposium on vascular and endovascular issues sponsored by the Cleveland Clinic Foundation.
AI in health care make sense, he said, because the health sector is a vast consumer market with potential for financial gain. Repetition, which is common in the health sector, is one of the foundations required for using AI and robotics. If a task can be repeated, then it means a machine can do it, said Dr. Weinberg.
The spread of AI and robotics one day may improve health care accessibility in remote areas where physicians with the appropriate training may not be available.
AI is already at work in the health care industry. “Pulmonary nodule detection can be done better with machines than by people, pathological identification and scanning of various slides can be done better by a machine than by a humans,” he said.
Artificial intelligence also can be designed to detect emotion by assessing various cues in phrasing, key words, and tone. These AI functions already are being used by sales reps on the phone to defuse and control interactions with customers and complainants. AI also can be implemented in interactions with people, which is an important part of dealing with patients, Dr. Weinberg said. Drug discovery is a key area where AI is flourishing, as well.
Luckily, in terms of physicians keeping their jobs, there are barriers to the use of AI to replace clinicians, Dr. Weinberg pointed out. Health care is not a monolith, and every specialty is different, meaning AI would have to be tailored to each task and specialty for each unique field. Quick proliferation of AI across the board is unlikely, especially when the varying roles of nurses and physician assistants are included.
Although robots in science fiction stories and films often are capable of multitasking a variety of needs, robots at present are much more limited in real life. In surgical situations, for example, they can perform specifically tailored tasks but cannot extend beyond those defined parameters as a real surgeon can, according to Dr. Weinberg, and this lack of flexibility is a severe limitation on the expansion of AI into health care.
Despite these limitations, Dr. Weinberg urged attendees to consider how AI can be used to facilitate their work.
“Believe in the roadblocks, but be a fast adopter – an early adopter – and understand where AI can currently augment you and make you better and more productive,” he said. “And keep doing procedures; AI and robotics currently have a problem with most of those,” Dr. Weinberg concluded.
Dr. Weinberg reported no conflicts relevant to his talk.
REPORTING FROM THE VEITHSYMPOSIUM
Single-port robotic sacrocolpopexy has same learning curve as multiport
LAS VEGAS – according to Israeli investigators.
Multiport sacrocolpopexy is common, but the single-port approach hasn’t really caught on yet. That’s likely to change, however, with ongoing development of the da Vinci robotic platform, said lead investigator Emad Matanes, MD, of the Rambam Medical Center, Haifa, Israel.
The medical center recently has been switching over to the single-port approach, and Dr. Matanes and his colleagues wanted to share their experience with surgeons considering doing the same.
They compared their first 52 multiport cases during Dec. 2011-Dec. 2012 to their first 52 single-port cases during Aug. 2015-Aug. 2017.
It took about 15 cases with either approach for operative times to stabilize, dropping from an average of 222 minutes to 161 minutes after the first 15 single-port cases, and from 224 to 198 minutes after the first 15 multiport cases (J Minim Invasive Gynecol. 2018 Nov-Dec;25[7]:S47-S8).
With both, “we reached our steady state after the first 15. Both approaches are feasible, and for both, surgery times improve after 15 cases,” Dr. Matanes said at the American Association of Gynecologic Laparoscopists Global Congress.
Overall, the single-port approach proved about 20 minutes quicker, due to shorter docking and anesthesia times.
There wasn’t a single prolapse recurrence in either group, even after the first few cases. There was slightly less postoperative pain with single-port surgery (visual analogue scale sore 1.5 vs. 2 points), and slightly less estimated blood loss (38 mL vs. 54 mL), but neither difference was statistically significant.
There were no statistically significant differences between women in the two groups. Their average age was 58 years, mean body mass index was 28 kg/m2, and most women had a preoperative Pelvic Organ Prolapse Quantification score of 3.
There was no outside funding, and Dr. Matanes didn’t disclose any relevant financial disclosures.
LAS VEGAS – according to Israeli investigators.
Multiport sacrocolpopexy is common, but the single-port approach hasn’t really caught on yet. That’s likely to change, however, with ongoing development of the da Vinci robotic platform, said lead investigator Emad Matanes, MD, of the Rambam Medical Center, Haifa, Israel.
The medical center recently has been switching over to the single-port approach, and Dr. Matanes and his colleagues wanted to share their experience with surgeons considering doing the same.
They compared their first 52 multiport cases during Dec. 2011-Dec. 2012 to their first 52 single-port cases during Aug. 2015-Aug. 2017.
It took about 15 cases with either approach for operative times to stabilize, dropping from an average of 222 minutes to 161 minutes after the first 15 single-port cases, and from 224 to 198 minutes after the first 15 multiport cases (J Minim Invasive Gynecol. 2018 Nov-Dec;25[7]:S47-S8).
With both, “we reached our steady state after the first 15. Both approaches are feasible, and for both, surgery times improve after 15 cases,” Dr. Matanes said at the American Association of Gynecologic Laparoscopists Global Congress.
Overall, the single-port approach proved about 20 minutes quicker, due to shorter docking and anesthesia times.
There wasn’t a single prolapse recurrence in either group, even after the first few cases. There was slightly less postoperative pain with single-port surgery (visual analogue scale sore 1.5 vs. 2 points), and slightly less estimated blood loss (38 mL vs. 54 mL), but neither difference was statistically significant.
There were no statistically significant differences between women in the two groups. Their average age was 58 years, mean body mass index was 28 kg/m2, and most women had a preoperative Pelvic Organ Prolapse Quantification score of 3.
There was no outside funding, and Dr. Matanes didn’t disclose any relevant financial disclosures.
LAS VEGAS – according to Israeli investigators.
Multiport sacrocolpopexy is common, but the single-port approach hasn’t really caught on yet. That’s likely to change, however, with ongoing development of the da Vinci robotic platform, said lead investigator Emad Matanes, MD, of the Rambam Medical Center, Haifa, Israel.
The medical center recently has been switching over to the single-port approach, and Dr. Matanes and his colleagues wanted to share their experience with surgeons considering doing the same.
They compared their first 52 multiport cases during Dec. 2011-Dec. 2012 to their first 52 single-port cases during Aug. 2015-Aug. 2017.
It took about 15 cases with either approach for operative times to stabilize, dropping from an average of 222 minutes to 161 minutes after the first 15 single-port cases, and from 224 to 198 minutes after the first 15 multiport cases (J Minim Invasive Gynecol. 2018 Nov-Dec;25[7]:S47-S8).
With both, “we reached our steady state after the first 15. Both approaches are feasible, and for both, surgery times improve after 15 cases,” Dr. Matanes said at the American Association of Gynecologic Laparoscopists Global Congress.
Overall, the single-port approach proved about 20 minutes quicker, due to shorter docking and anesthesia times.
There wasn’t a single prolapse recurrence in either group, even after the first few cases. There was slightly less postoperative pain with single-port surgery (visual analogue scale sore 1.5 vs. 2 points), and slightly less estimated blood loss (38 mL vs. 54 mL), but neither difference was statistically significant.
There were no statistically significant differences between women in the two groups. Their average age was 58 years, mean body mass index was 28 kg/m2, and most women had a preoperative Pelvic Organ Prolapse Quantification score of 3.
There was no outside funding, and Dr. Matanes didn’t disclose any relevant financial disclosures.
REPORTING FROM THE AAGL GLOBAL CONGRESS
MIS for cervical cancer: Is it not for anyone or not for everyone?
Shock waves moved through the gynecologic oncology world on Oct. 31, 2018, when the New England Journal of Medicine published two papers on survival outcomes for women undergoing surgery for early stage cervical cancer.
The first was a randomized controlled trial of laparotomy and minimally invasive surgery (MIS) for radical hysterectomy called the LACC trial.1 In the multicenter, international trial of 631 women, the primary objective was disease-specific survival (cervical cancer–related deaths) and was powered to detect noninferiority of the MIS approach when compared with laparotomy. The trial was closed early when investigators noted a lower than expected rate of 3-year, disease-free survival (91% vs. 97%) from cervical cancer in the MIS group, which was made up of 84% laparoscopic and 16% robotic approaches, versus laparotomy. There were 19 deaths in the MIS group observed versus three in the laparotomy group. The conclusions of the trial were that MIS surgery is associated with inferior cervical cancer survival.
In the second study, authors analyzed data from large U.S. databases – the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) Program – to collect all-cause mortality for patients with early-stage cervical cancer who had undergone radical hysterectomy during 2010-2013.2 Among 2,461 observed results, 1,225 had undergone MIS surgery with the majority (79.8%) via robotic-assistance. Women undergoing MIS approaches had smaller, lower grade tumors; were more likely to be white, privately insured, and of a higher income; and had surgery later in the cohort and by nonacademic centers. The researchers adjusted for risk factors with an analytic process called propensity-score weighting, which matched the groups more closely in an attempt to minimize confounders. They identified higher all-cause mortality among women who were treated with an MIS approach, compared with those treated with laparotomy (hazard ratio, 1.65). They also observed a significant decline in the survival from cervical cancer annually that corresponded to the uptake of MIS radical hysterectomies.
In the wake of these publications, many concluded that gynecologic oncologists should no longer offer a minimally invasive approach for radical hysterectomy. Certainly level I evidence published in a highly influential journal is compelling, and the consistency in findings over two studies adds further weight to the results. However, was this the correct conclusion to draw from these results? Surgeons who had been performing MIS radical hysterectomies for many years with favorable outcomes are challenging this and are raising questions about external generalizability and whether these findings were driven by the surgery itself or by the surgeon.
The studies’ authors proposed hypotheses for their results that implicate the surgical route rather than the surgeon; however, these seem ad hoc and not well supported by data, including the authors’ own data. The first was the hypothesis that cervical tumors were being disrupted and disseminated through the use of uterine manipulators in MIS approaches. However, cervical cancers are fairly routinely “disrupted” by preoperative cone biopsies, loop electrosurgical excision procedures (LEEP), and sharp biopsies, which are arguably more invasive than placement of a manipulator. Uterine manipulators routinely are used in endometrial cancer surgeries, in which the manipulator is embedded within the tumor, without an associated negative survival effect in randomized trials.3 Additionally, not all surgeons utilize manipulators for radical hysterectomies, and these studies did not measure or report on their use; therefore, it is impossible to know whether, and by what magnitude, manipulators played a role. Finally, if uterine manipulators are the explanation for inferior survival, surely the recommendation should be to discourage their use, rather than abandon the MIS approach all together.
The other explanation offered was exposure of the tumor to CO2 gas. This seems an even less plausible explanation because CO2 gas is routinely used in MIS cancer surgeries for endometrial, prostate, gastric, and colorectal surgeries and is used as insufflation for malignant interventional endoscopies without a significant deleterious effect. Additionally, the cervix is not exposed to CO2 until colpotomy at the procedure’s end – and only briefly. The in vitro studies implicating a negative effect of simulated CO2 pneumoperitoneum are neither compelling nor consistent.4,5
I would like to propose another hypothesis for the results: surgical proficiency. Surgery, unlike medical interventions, is not a simple variable that is dichotomous – performed or not. Surgeons do not randomly select operative approaches for patients. We select surgical approaches based on patients’ circumstances and surgeon factors, including our own mastery of the various techniques. and any surgeon recognizes this if he or she has observed more than one surgeon or has attempted a procedure via different routes. While some procedures, such as extrafascial hysterectomy for endometrial cancer, are relatively straightforward and surgeon capabilities are more equitable across different approaches, cervical cancer surgery is quite different.
Early-stage cervical cancer primarily exerts radial growth into the cervical stroma and parametria. Curative surgical excision requires broadly negative margins through this tissue, a so called “radical hysterectomy.” The radicality of hysterectomy has been categorized in stages, acknowledging that different sized lesions require different volumes of parametrial resection to achieve adequate clearance from the tumor.6 In doing so, the surgeon must skeletonize and mobilize the distal ureters, cardinal ligament webs, and uterosacral ligaments. These structures are in close proximity to major vascular and neural structures. Hence, the radical hysterectomy is, without dispute, a technically challenging procedure.
Minimally invasive surgery further handicaps the surgeon by eliminating manual contact with tissue, and relying on complex instrumentation, electrosurgical modalities, and loss of haptics. The learning curve for MIS radical hysterectomy is further attenuated by their relative infrequency. Therefore, it makes sense that, when the MIS approach is randomly assigned to surgeons (such as in the LACC trial) or broadly and independently applied (as in the retrospective series), one might see variations in skill, quality, and outcomes, including oncologic outcomes.
The retrospective study by Melamed et al. acknowledged that surgeon skill and volume may contribute to their findings but stated that, because of the nature of their source data, they were unable to explain why they observed their results. The LACC trial attempted to overcome the issue of surgeon skill by ensuring all surgeons were from high-volume sites and had videos reviewed of their cases. However, the videos were chosen by the surgeons themselves and not available for audit in the study’s supplemental material. The LACC trial was conducted over a 9-year period across 33 sites and enrolled a total of 631 subjects. This equates to an enrollment of approximately two patients per site per year and either reflects extremely low-volume sites or highly selective patient enrollment. If the latter, what was different about the unenrolled patients and what was the preferred chosen route of surgery for them?
All 34 recurrences occurred in patients from just 14 of the 33 sites in the LACC trial. That means that less than half of the sites contributed to all of the recurrences. The authors provided no details on the specific sites, surgeons, or accrual rates in their manuscript or supplemental materials. Therefore, readers are unable to know what was different about those sites; whether they contributed the most patients and, therefore, the most recurrences; or whether they were low-volume sites with lower quality.
While margin status, positive or negative, was reported, there was no data captured regarding volume of resected parametrial tissue, or relative distance from tumor to margin, both of which might provide the reader with a better appraisal of surgeon proficiency and consistency in radicality of the two approaches. The incidence of locoregional (pelvic) recurrences were higher in the MIS arm, which is expected if there were inadequate margins around the laparoscopically resected tumors.
Finally, the authors of the LACC trial observed equivalent rates of postoperative complications between the laparotomy and MIS groups. The main virtue for MIS approaches is the reduction in perioperative morbidity. To observe no perioperative morbidity benefit in the MIS group is a red flag suggesting that these surgeons may not have achieved proficiency with the MIS approach.
Despite these arguments, the results of these studies should be taken seriously. Clearly, it is apparent that preservation of oncologic outcomes is not guaranteed with MIS radical hysterectomy, and it should not be the chosen approach for all patients and all surgeons. However, rather than entirely abandoning this less morbid approach, I would argue that it is a call to arms for gynecologic oncologists to self-evaluate. We should know our own data with respect to case volumes, perioperative complications, and cancer-related recurrence and death.
Perhaps MIS radical hysterectomies should be consolidated among high-volume surgeons with demonstrated good outcomes? Just as has been done for rectal cancer surgery with positive effect, we should establish accredited centers of excellence.7 We also need to improve the training of surgeons in novel, difficult techniques, as well as enhance the sophistication of MIS equipment such as improved instrumentation, haptics, and vision-guided surgery (for example, real-time intraoperative assessment of the tumor margins).
Let’s not take a wholesale step backwards to the surgical approaches of a 100 years ago just because they are more straightforward. Let’s do a better job of advancing the quality of what we do for our patients in the future.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
2. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923.
3. J Clin Oncol. 2012 Mar 1;30(7):695-700.
4. Med Sci Monit. 2014 Dec 1;20:2497-503.
5. Surg Endosc. 2006 Oct;20(10):1556-9.
6. Gynecol Oncol. 2011 Aug;122(2):264-8.
7. Surgery. 2016 Mar;159(3):736-48.
Shock waves moved through the gynecologic oncology world on Oct. 31, 2018, when the New England Journal of Medicine published two papers on survival outcomes for women undergoing surgery for early stage cervical cancer.
The first was a randomized controlled trial of laparotomy and minimally invasive surgery (MIS) for radical hysterectomy called the LACC trial.1 In the multicenter, international trial of 631 women, the primary objective was disease-specific survival (cervical cancer–related deaths) and was powered to detect noninferiority of the MIS approach when compared with laparotomy. The trial was closed early when investigators noted a lower than expected rate of 3-year, disease-free survival (91% vs. 97%) from cervical cancer in the MIS group, which was made up of 84% laparoscopic and 16% robotic approaches, versus laparotomy. There were 19 deaths in the MIS group observed versus three in the laparotomy group. The conclusions of the trial were that MIS surgery is associated with inferior cervical cancer survival.
In the second study, authors analyzed data from large U.S. databases – the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) Program – to collect all-cause mortality for patients with early-stage cervical cancer who had undergone radical hysterectomy during 2010-2013.2 Among 2,461 observed results, 1,225 had undergone MIS surgery with the majority (79.8%) via robotic-assistance. Women undergoing MIS approaches had smaller, lower grade tumors; were more likely to be white, privately insured, and of a higher income; and had surgery later in the cohort and by nonacademic centers. The researchers adjusted for risk factors with an analytic process called propensity-score weighting, which matched the groups more closely in an attempt to minimize confounders. They identified higher all-cause mortality among women who were treated with an MIS approach, compared with those treated with laparotomy (hazard ratio, 1.65). They also observed a significant decline in the survival from cervical cancer annually that corresponded to the uptake of MIS radical hysterectomies.
In the wake of these publications, many concluded that gynecologic oncologists should no longer offer a minimally invasive approach for radical hysterectomy. Certainly level I evidence published in a highly influential journal is compelling, and the consistency in findings over two studies adds further weight to the results. However, was this the correct conclusion to draw from these results? Surgeons who had been performing MIS radical hysterectomies for many years with favorable outcomes are challenging this and are raising questions about external generalizability and whether these findings were driven by the surgery itself or by the surgeon.
The studies’ authors proposed hypotheses for their results that implicate the surgical route rather than the surgeon; however, these seem ad hoc and not well supported by data, including the authors’ own data. The first was the hypothesis that cervical tumors were being disrupted and disseminated through the use of uterine manipulators in MIS approaches. However, cervical cancers are fairly routinely “disrupted” by preoperative cone biopsies, loop electrosurgical excision procedures (LEEP), and sharp biopsies, which are arguably more invasive than placement of a manipulator. Uterine manipulators routinely are used in endometrial cancer surgeries, in which the manipulator is embedded within the tumor, without an associated negative survival effect in randomized trials.3 Additionally, not all surgeons utilize manipulators for radical hysterectomies, and these studies did not measure or report on their use; therefore, it is impossible to know whether, and by what magnitude, manipulators played a role. Finally, if uterine manipulators are the explanation for inferior survival, surely the recommendation should be to discourage their use, rather than abandon the MIS approach all together.
The other explanation offered was exposure of the tumor to CO2 gas. This seems an even less plausible explanation because CO2 gas is routinely used in MIS cancer surgeries for endometrial, prostate, gastric, and colorectal surgeries and is used as insufflation for malignant interventional endoscopies without a significant deleterious effect. Additionally, the cervix is not exposed to CO2 until colpotomy at the procedure’s end – and only briefly. The in vitro studies implicating a negative effect of simulated CO2 pneumoperitoneum are neither compelling nor consistent.4,5
I would like to propose another hypothesis for the results: surgical proficiency. Surgery, unlike medical interventions, is not a simple variable that is dichotomous – performed or not. Surgeons do not randomly select operative approaches for patients. We select surgical approaches based on patients’ circumstances and surgeon factors, including our own mastery of the various techniques. and any surgeon recognizes this if he or she has observed more than one surgeon or has attempted a procedure via different routes. While some procedures, such as extrafascial hysterectomy for endometrial cancer, are relatively straightforward and surgeon capabilities are more equitable across different approaches, cervical cancer surgery is quite different.
Early-stage cervical cancer primarily exerts radial growth into the cervical stroma and parametria. Curative surgical excision requires broadly negative margins through this tissue, a so called “radical hysterectomy.” The radicality of hysterectomy has been categorized in stages, acknowledging that different sized lesions require different volumes of parametrial resection to achieve adequate clearance from the tumor.6 In doing so, the surgeon must skeletonize and mobilize the distal ureters, cardinal ligament webs, and uterosacral ligaments. These structures are in close proximity to major vascular and neural structures. Hence, the radical hysterectomy is, without dispute, a technically challenging procedure.
Minimally invasive surgery further handicaps the surgeon by eliminating manual contact with tissue, and relying on complex instrumentation, electrosurgical modalities, and loss of haptics. The learning curve for MIS radical hysterectomy is further attenuated by their relative infrequency. Therefore, it makes sense that, when the MIS approach is randomly assigned to surgeons (such as in the LACC trial) or broadly and independently applied (as in the retrospective series), one might see variations in skill, quality, and outcomes, including oncologic outcomes.
The retrospective study by Melamed et al. acknowledged that surgeon skill and volume may contribute to their findings but stated that, because of the nature of their source data, they were unable to explain why they observed their results. The LACC trial attempted to overcome the issue of surgeon skill by ensuring all surgeons were from high-volume sites and had videos reviewed of their cases. However, the videos were chosen by the surgeons themselves and not available for audit in the study’s supplemental material. The LACC trial was conducted over a 9-year period across 33 sites and enrolled a total of 631 subjects. This equates to an enrollment of approximately two patients per site per year and either reflects extremely low-volume sites or highly selective patient enrollment. If the latter, what was different about the unenrolled patients and what was the preferred chosen route of surgery for them?
All 34 recurrences occurred in patients from just 14 of the 33 sites in the LACC trial. That means that less than half of the sites contributed to all of the recurrences. The authors provided no details on the specific sites, surgeons, or accrual rates in their manuscript or supplemental materials. Therefore, readers are unable to know what was different about those sites; whether they contributed the most patients and, therefore, the most recurrences; or whether they were low-volume sites with lower quality.
While margin status, positive or negative, was reported, there was no data captured regarding volume of resected parametrial tissue, or relative distance from tumor to margin, both of which might provide the reader with a better appraisal of surgeon proficiency and consistency in radicality of the two approaches. The incidence of locoregional (pelvic) recurrences were higher in the MIS arm, which is expected if there were inadequate margins around the laparoscopically resected tumors.
Finally, the authors of the LACC trial observed equivalent rates of postoperative complications between the laparotomy and MIS groups. The main virtue for MIS approaches is the reduction in perioperative morbidity. To observe no perioperative morbidity benefit in the MIS group is a red flag suggesting that these surgeons may not have achieved proficiency with the MIS approach.
Despite these arguments, the results of these studies should be taken seriously. Clearly, it is apparent that preservation of oncologic outcomes is not guaranteed with MIS radical hysterectomy, and it should not be the chosen approach for all patients and all surgeons. However, rather than entirely abandoning this less morbid approach, I would argue that it is a call to arms for gynecologic oncologists to self-evaluate. We should know our own data with respect to case volumes, perioperative complications, and cancer-related recurrence and death.
Perhaps MIS radical hysterectomies should be consolidated among high-volume surgeons with demonstrated good outcomes? Just as has been done for rectal cancer surgery with positive effect, we should establish accredited centers of excellence.7 We also need to improve the training of surgeons in novel, difficult techniques, as well as enhance the sophistication of MIS equipment such as improved instrumentation, haptics, and vision-guided surgery (for example, real-time intraoperative assessment of the tumor margins).
Let’s not take a wholesale step backwards to the surgical approaches of a 100 years ago just because they are more straightforward. Let’s do a better job of advancing the quality of what we do for our patients in the future.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
2. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923.
3. J Clin Oncol. 2012 Mar 1;30(7):695-700.
4. Med Sci Monit. 2014 Dec 1;20:2497-503.
5. Surg Endosc. 2006 Oct;20(10):1556-9.
6. Gynecol Oncol. 2011 Aug;122(2):264-8.
7. Surgery. 2016 Mar;159(3):736-48.
Shock waves moved through the gynecologic oncology world on Oct. 31, 2018, when the New England Journal of Medicine published two papers on survival outcomes for women undergoing surgery for early stage cervical cancer.
The first was a randomized controlled trial of laparotomy and minimally invasive surgery (MIS) for radical hysterectomy called the LACC trial.1 In the multicenter, international trial of 631 women, the primary objective was disease-specific survival (cervical cancer–related deaths) and was powered to detect noninferiority of the MIS approach when compared with laparotomy. The trial was closed early when investigators noted a lower than expected rate of 3-year, disease-free survival (91% vs. 97%) from cervical cancer in the MIS group, which was made up of 84% laparoscopic and 16% robotic approaches, versus laparotomy. There were 19 deaths in the MIS group observed versus three in the laparotomy group. The conclusions of the trial were that MIS surgery is associated with inferior cervical cancer survival.
In the second study, authors analyzed data from large U.S. databases – the National Cancer Database (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) Program – to collect all-cause mortality for patients with early-stage cervical cancer who had undergone radical hysterectomy during 2010-2013.2 Among 2,461 observed results, 1,225 had undergone MIS surgery with the majority (79.8%) via robotic-assistance. Women undergoing MIS approaches had smaller, lower grade tumors; were more likely to be white, privately insured, and of a higher income; and had surgery later in the cohort and by nonacademic centers. The researchers adjusted for risk factors with an analytic process called propensity-score weighting, which matched the groups more closely in an attempt to minimize confounders. They identified higher all-cause mortality among women who were treated with an MIS approach, compared with those treated with laparotomy (hazard ratio, 1.65). They also observed a significant decline in the survival from cervical cancer annually that corresponded to the uptake of MIS radical hysterectomies.
In the wake of these publications, many concluded that gynecologic oncologists should no longer offer a minimally invasive approach for radical hysterectomy. Certainly level I evidence published in a highly influential journal is compelling, and the consistency in findings over two studies adds further weight to the results. However, was this the correct conclusion to draw from these results? Surgeons who had been performing MIS radical hysterectomies for many years with favorable outcomes are challenging this and are raising questions about external generalizability and whether these findings were driven by the surgery itself or by the surgeon.
The studies’ authors proposed hypotheses for their results that implicate the surgical route rather than the surgeon; however, these seem ad hoc and not well supported by data, including the authors’ own data. The first was the hypothesis that cervical tumors were being disrupted and disseminated through the use of uterine manipulators in MIS approaches. However, cervical cancers are fairly routinely “disrupted” by preoperative cone biopsies, loop electrosurgical excision procedures (LEEP), and sharp biopsies, which are arguably more invasive than placement of a manipulator. Uterine manipulators routinely are used in endometrial cancer surgeries, in which the manipulator is embedded within the tumor, without an associated negative survival effect in randomized trials.3 Additionally, not all surgeons utilize manipulators for radical hysterectomies, and these studies did not measure or report on their use; therefore, it is impossible to know whether, and by what magnitude, manipulators played a role. Finally, if uterine manipulators are the explanation for inferior survival, surely the recommendation should be to discourage their use, rather than abandon the MIS approach all together.
The other explanation offered was exposure of the tumor to CO2 gas. This seems an even less plausible explanation because CO2 gas is routinely used in MIS cancer surgeries for endometrial, prostate, gastric, and colorectal surgeries and is used as insufflation for malignant interventional endoscopies without a significant deleterious effect. Additionally, the cervix is not exposed to CO2 until colpotomy at the procedure’s end – and only briefly. The in vitro studies implicating a negative effect of simulated CO2 pneumoperitoneum are neither compelling nor consistent.4,5
I would like to propose another hypothesis for the results: surgical proficiency. Surgery, unlike medical interventions, is not a simple variable that is dichotomous – performed or not. Surgeons do not randomly select operative approaches for patients. We select surgical approaches based on patients’ circumstances and surgeon factors, including our own mastery of the various techniques. and any surgeon recognizes this if he or she has observed more than one surgeon or has attempted a procedure via different routes. While some procedures, such as extrafascial hysterectomy for endometrial cancer, are relatively straightforward and surgeon capabilities are more equitable across different approaches, cervical cancer surgery is quite different.
Early-stage cervical cancer primarily exerts radial growth into the cervical stroma and parametria. Curative surgical excision requires broadly negative margins through this tissue, a so called “radical hysterectomy.” The radicality of hysterectomy has been categorized in stages, acknowledging that different sized lesions require different volumes of parametrial resection to achieve adequate clearance from the tumor.6 In doing so, the surgeon must skeletonize and mobilize the distal ureters, cardinal ligament webs, and uterosacral ligaments. These structures are in close proximity to major vascular and neural structures. Hence, the radical hysterectomy is, without dispute, a technically challenging procedure.
Minimally invasive surgery further handicaps the surgeon by eliminating manual contact with tissue, and relying on complex instrumentation, electrosurgical modalities, and loss of haptics. The learning curve for MIS radical hysterectomy is further attenuated by their relative infrequency. Therefore, it makes sense that, when the MIS approach is randomly assigned to surgeons (such as in the LACC trial) or broadly and independently applied (as in the retrospective series), one might see variations in skill, quality, and outcomes, including oncologic outcomes.
The retrospective study by Melamed et al. acknowledged that surgeon skill and volume may contribute to their findings but stated that, because of the nature of their source data, they were unable to explain why they observed their results. The LACC trial attempted to overcome the issue of surgeon skill by ensuring all surgeons were from high-volume sites and had videos reviewed of their cases. However, the videos were chosen by the surgeons themselves and not available for audit in the study’s supplemental material. The LACC trial was conducted over a 9-year period across 33 sites and enrolled a total of 631 subjects. This equates to an enrollment of approximately two patients per site per year and either reflects extremely low-volume sites or highly selective patient enrollment. If the latter, what was different about the unenrolled patients and what was the preferred chosen route of surgery for them?
All 34 recurrences occurred in patients from just 14 of the 33 sites in the LACC trial. That means that less than half of the sites contributed to all of the recurrences. The authors provided no details on the specific sites, surgeons, or accrual rates in their manuscript or supplemental materials. Therefore, readers are unable to know what was different about those sites; whether they contributed the most patients and, therefore, the most recurrences; or whether they were low-volume sites with lower quality.
While margin status, positive or negative, was reported, there was no data captured regarding volume of resected parametrial tissue, or relative distance from tumor to margin, both of which might provide the reader with a better appraisal of surgeon proficiency and consistency in radicality of the two approaches. The incidence of locoregional (pelvic) recurrences were higher in the MIS arm, which is expected if there were inadequate margins around the laparoscopically resected tumors.
Finally, the authors of the LACC trial observed equivalent rates of postoperative complications between the laparotomy and MIS groups. The main virtue for MIS approaches is the reduction in perioperative morbidity. To observe no perioperative morbidity benefit in the MIS group is a red flag suggesting that these surgeons may not have achieved proficiency with the MIS approach.
Despite these arguments, the results of these studies should be taken seriously. Clearly, it is apparent that preservation of oncologic outcomes is not guaranteed with MIS radical hysterectomy, and it should not be the chosen approach for all patients and all surgeons. However, rather than entirely abandoning this less morbid approach, I would argue that it is a call to arms for gynecologic oncologists to self-evaluate. We should know our own data with respect to case volumes, perioperative complications, and cancer-related recurrence and death.
Perhaps MIS radical hysterectomies should be consolidated among high-volume surgeons with demonstrated good outcomes? Just as has been done for rectal cancer surgery with positive effect, we should establish accredited centers of excellence.7 We also need to improve the training of surgeons in novel, difficult techniques, as well as enhance the sophistication of MIS equipment such as improved instrumentation, haptics, and vision-guided surgery (for example, real-time intraoperative assessment of the tumor margins).
Let’s not take a wholesale step backwards to the surgical approaches of a 100 years ago just because they are more straightforward. Let’s do a better job of advancing the quality of what we do for our patients in the future.
Dr. Rossi is an assistant professor in the division of gynecologic oncology at the University of North Carolina at Chapel Hill. She said she had no conflicts of interest. Email Dr. Rossi at [email protected].
References
1. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
2. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923.
3. J Clin Oncol. 2012 Mar 1;30(7):695-700.
4. Med Sci Monit. 2014 Dec 1;20:2497-503.
5. Surg Endosc. 2006 Oct;20(10):1556-9.
6. Gynecol Oncol. 2011 Aug;122(2):264-8.
7. Surgery. 2016 Mar;159(3):736-48.
Cervical cancer survival higher with open surgery in LACC trial
based on findings from the randomized, controlled phase 3 Laparoscopic Approach to Cervical Cancer (LACC) trial of more than 600 women.

The alarming findings, which led to early study termination, also were supported by results from a second population-based study. Both studies were published concurrently in the Oct. 31 issue of the New England Journal of Medicine.
The disease-free survival at 4.5 years among 319 patients who underwent minimally invasive surgery in the LACC trial was 86.0% vs. 96.5% in 312 patients who underwent open surgery, Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395).
At 3 years, the disease-free survival rates were 91.2% in the minimally invasive surgery group and 97.1% in open surgery group (hazard ratio for disease recurrence or death from cervical cancer, 3.74).
The differences between the groups persisted after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. In the minimally invasive surgery group, the findings were comparable for those who underwent laparoscopic vs. robot-assisted surgery, the investigators found.
Further, at 3 years, overall survival was 93.8% vs. 99.0% (HR for death from any cause, 6.00), death from cervical cancer was 4.4% vs. 0.6% (HR, 6.56), and the rate of locoregional recurrence-free survival was 94.3 vs. 98.3 (HR, 4.26) in the minimally invasive and open surgery groups, respectively.
Study participants were women with a mean age of 46 years with stage IA1, IA2, or IB1 cervical cancer, with most (91.9%) having IB1 disease, and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. They were recruited from 33 centers worldwide between June 2008 and June 2017. Most of those assigned to minimally invasive surgery underwent laparoscopic surgery (84.4%), and the remaining patients underwent robot-assisted surgery.
The treatment groups were balanced with respect to baseline characteristics, they noted.
The minimally invasive approach is widely used given that guidelines from the National Comprehensive Cancer Network and European Society of Gynecological Oncology consider both surgical approaches acceptable, and since retrospective studies suggest laparoscopic radical hysterectomy is associated with lower complication rates and comparable outcomes. However, there are limited prospective data regarding survival outcomes in early stage disease with the two approaches, the researchers said.
“Our results call into question the findings in the literature suggesting that minimally invasive radical hysterectomy is associated with no difference in oncologic outcomes as compared with the open approach,” they wrote, noting that a number of factors may explain the differences, such as concurrent vs. sequential analyses in the current studies vs. prior studies (in sequential analyses, earlier procedures may have been performed under broader indications and less clearly defined radiotherapy guidelines), and the possibility that “routine use of a uterine manipulator might increase the propensity for tumor spillage” in minimally invasive surgery.
Strengths of the study include its prospective, randomized, international multicenter design and inclusion of a per-protocol analysis that was consistent with the intention-to-treat analysis, and limitations include the fact that intended enrollment wasn’t reached because of the “safety alert raised by the data and safety monitoring committee on the basis of the higher recurrence and death in the minimally invasive surgery groups,” as well as the inability to generalize the results to patients with low-risk disease as there was lack of power to evaluate outcomes in that context.
Even though the trial was initially powered on the assumption that there would be a 4.5 year follow-up for all patients, only 59.7% reached that length of follow-up. However, the trial still reached 84% power to detect noninferiority of the primary outcome (disease-free survival) with minimally invasive surgery, which was not found, they noted.
Similarly, in the population-based cohort study of 2,461 women who underwent radical hysterectomy for stage IA2 of IB1 cervical cancer between 2010 and 2013, 4-year mortality was 9.1% among 1,225 patients who underwent minimally invasive surgery vs. 5.3% among the 1,236 patients who underwent open surgery (HR, 1.65), Alexander Melamed, MD, of Harvard Medical School, Boston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923).
Of note, the 4-year relative survival rate following radical hysterectomy for cervical cancer remained stable prior to the widespread adoption of minimally invasive approaches; an interrupted time-series analysis involving women who underwent surgery during 2000-2010, which was also conducted as part of the study, showed a decline in 4-year survival of 0.8% per year after 2006, coinciding with increased use of minimally invasive surgery, the investigators said.
For the main patient-level analysis, the researchers used the National Cancer Database, and for the time-series analysis they used information from the Surveillance, Epidemiology, and End Results program database.
“Our findings suggest that minimally invasive surgery was associated with a higher risk of death than open surgery among women who underwent radical hysterectomy for early-stage cervical cancer. This association was apparent regardless of laparoscopic approach, tumor size, or histologic type,” they concluded.
The findings are unexpected, eye-opening, and should inform practice, according to Ritu Salani, MD, of the Ohio State University, Columbus.
“This is something we have to discuss with patients,” she said in an interview, noting that while these aren’t perfect studies, they “are the best information we have.
Data reported in September at a meeting of the International Gynecologic Cancer Society show that surgical complications and quality of life outcomes are similar with minimally invasive and open surgery, therefore the findings from these two new studies suggest a need to shift back toward open surgery for patients with cervical cancer, she said.
One “catch” is that survival in the open surgery group in the LACC trial was unusually high and recurrence rates unusually low, compared with what might be expected, and the explanation for this observation is unclear.
“There may be some missing pieces that they haven’t been able to explain, but it’s not clear that they would change the outcome,” she said.
Justin Chura, MD, director of gynecologic oncology and robotic surgery at Cancer Treatment Center of America’s Eastern Regional Medical Center in Philadelphia, said in an interview, “The results of the study by Ramirez et al. are certainly disappointing for those among us who are advocates of minimally invasive surgery (MIS). In my own practice, I transitioned to minimally invasive radical hysterectomy approximately 10 years ago. Now that approach has to be reconsidered. While there are likely subsets of patients who will still benefit from a MIS approach without worsening oncologic outcomes, we do not have robust data to reliably identify those patients.
“One factor that warrants further investigation is the use of a uterine manipulator. While I do not use a manipulator out of personal preference (one less step in the operating room), the idea of placing a device through the tumor or adjacent to it, has biologic plausibility in terms of displacing tumor cells into lymphatic channels,” he said. “Until we have more data, an open approach appears to be preferred.”*
Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani and Dr. Chura are members of the Ob.Gyn. News editorial board, but reported having no other relevant disclosures.*
SOURCE: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
*This article was updated 11/9/2018.
The findings by Ramirez et al. and Melamed et al. are striking in part because previous studies focused more on surgical than clinical outcomes.
They are powerful, but scientific scrutiny demands consideration of potential study-design or study-conduct issues. For example, all cancer recurrences in the LACC trial were clustered at 14 of 33 participating centers, raising questions about factors that contributed to recurrence at those centers .
Still, the findings are alarming and deal a blow to the use of minimally invasive surgical approaches in cervical cancer patients. They don’t necessarily “signal the death knell” of such approaches.
Select patients may still benefit from a less invasive approach; none of the patients with stage lA2 disease, and only one with stage lB1, grade 1 disease had a recurrence in the LACC trial.
Further, patients with tumors smaller than 2 cm also did not have worse outcomes with minimally invasive surgery in either study. However, until further details are known, surgeons should proceed cautiously and counsel patients regarding these study results.
Amanda N. Fader, MD , made her comments in an accompanying editorial (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395 ). Dr. Fader is with the Johns Hopkins University, Baltimore. She reported having no relevant disclosures.
The findings by Ramirez et al. and Melamed et al. are striking in part because previous studies focused more on surgical than clinical outcomes.
They are powerful, but scientific scrutiny demands consideration of potential study-design or study-conduct issues. For example, all cancer recurrences in the LACC trial were clustered at 14 of 33 participating centers, raising questions about factors that contributed to recurrence at those centers .
Still, the findings are alarming and deal a blow to the use of minimally invasive surgical approaches in cervical cancer patients. They don’t necessarily “signal the death knell” of such approaches.
Select patients may still benefit from a less invasive approach; none of the patients with stage lA2 disease, and only one with stage lB1, grade 1 disease had a recurrence in the LACC trial.
Further, patients with tumors smaller than 2 cm also did not have worse outcomes with minimally invasive surgery in either study. However, until further details are known, surgeons should proceed cautiously and counsel patients regarding these study results.
Amanda N. Fader, MD , made her comments in an accompanying editorial (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395 ). Dr. Fader is with the Johns Hopkins University, Baltimore. She reported having no relevant disclosures.
The findings by Ramirez et al. and Melamed et al. are striking in part because previous studies focused more on surgical than clinical outcomes.
They are powerful, but scientific scrutiny demands consideration of potential study-design or study-conduct issues. For example, all cancer recurrences in the LACC trial were clustered at 14 of 33 participating centers, raising questions about factors that contributed to recurrence at those centers .
Still, the findings are alarming and deal a blow to the use of minimally invasive surgical approaches in cervical cancer patients. They don’t necessarily “signal the death knell” of such approaches.
Select patients may still benefit from a less invasive approach; none of the patients with stage lA2 disease, and only one with stage lB1, grade 1 disease had a recurrence in the LACC trial.
Further, patients with tumors smaller than 2 cm also did not have worse outcomes with minimally invasive surgery in either study. However, until further details are known, surgeons should proceed cautiously and counsel patients regarding these study results.
Amanda N. Fader, MD , made her comments in an accompanying editorial (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395 ). Dr. Fader is with the Johns Hopkins University, Baltimore. She reported having no relevant disclosures.
based on findings from the randomized, controlled phase 3 Laparoscopic Approach to Cervical Cancer (LACC) trial of more than 600 women.

The alarming findings, which led to early study termination, also were supported by results from a second population-based study. Both studies were published concurrently in the Oct. 31 issue of the New England Journal of Medicine.
The disease-free survival at 4.5 years among 319 patients who underwent minimally invasive surgery in the LACC trial was 86.0% vs. 96.5% in 312 patients who underwent open surgery, Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395).
At 3 years, the disease-free survival rates were 91.2% in the minimally invasive surgery group and 97.1% in open surgery group (hazard ratio for disease recurrence or death from cervical cancer, 3.74).
The differences between the groups persisted after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. In the minimally invasive surgery group, the findings were comparable for those who underwent laparoscopic vs. robot-assisted surgery, the investigators found.
Further, at 3 years, overall survival was 93.8% vs. 99.0% (HR for death from any cause, 6.00), death from cervical cancer was 4.4% vs. 0.6% (HR, 6.56), and the rate of locoregional recurrence-free survival was 94.3 vs. 98.3 (HR, 4.26) in the minimally invasive and open surgery groups, respectively.
Study participants were women with a mean age of 46 years with stage IA1, IA2, or IB1 cervical cancer, with most (91.9%) having IB1 disease, and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. They were recruited from 33 centers worldwide between June 2008 and June 2017. Most of those assigned to minimally invasive surgery underwent laparoscopic surgery (84.4%), and the remaining patients underwent robot-assisted surgery.
The treatment groups were balanced with respect to baseline characteristics, they noted.
The minimally invasive approach is widely used given that guidelines from the National Comprehensive Cancer Network and European Society of Gynecological Oncology consider both surgical approaches acceptable, and since retrospective studies suggest laparoscopic radical hysterectomy is associated with lower complication rates and comparable outcomes. However, there are limited prospective data regarding survival outcomes in early stage disease with the two approaches, the researchers said.
“Our results call into question the findings in the literature suggesting that minimally invasive radical hysterectomy is associated with no difference in oncologic outcomes as compared with the open approach,” they wrote, noting that a number of factors may explain the differences, such as concurrent vs. sequential analyses in the current studies vs. prior studies (in sequential analyses, earlier procedures may have been performed under broader indications and less clearly defined radiotherapy guidelines), and the possibility that “routine use of a uterine manipulator might increase the propensity for tumor spillage” in minimally invasive surgery.
Strengths of the study include its prospective, randomized, international multicenter design and inclusion of a per-protocol analysis that was consistent with the intention-to-treat analysis, and limitations include the fact that intended enrollment wasn’t reached because of the “safety alert raised by the data and safety monitoring committee on the basis of the higher recurrence and death in the minimally invasive surgery groups,” as well as the inability to generalize the results to patients with low-risk disease as there was lack of power to evaluate outcomes in that context.
Even though the trial was initially powered on the assumption that there would be a 4.5 year follow-up for all patients, only 59.7% reached that length of follow-up. However, the trial still reached 84% power to detect noninferiority of the primary outcome (disease-free survival) with minimally invasive surgery, which was not found, they noted.
Similarly, in the population-based cohort study of 2,461 women who underwent radical hysterectomy for stage IA2 of IB1 cervical cancer between 2010 and 2013, 4-year mortality was 9.1% among 1,225 patients who underwent minimally invasive surgery vs. 5.3% among the 1,236 patients who underwent open surgery (HR, 1.65), Alexander Melamed, MD, of Harvard Medical School, Boston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923).
Of note, the 4-year relative survival rate following radical hysterectomy for cervical cancer remained stable prior to the widespread adoption of minimally invasive approaches; an interrupted time-series analysis involving women who underwent surgery during 2000-2010, which was also conducted as part of the study, showed a decline in 4-year survival of 0.8% per year after 2006, coinciding with increased use of minimally invasive surgery, the investigators said.
For the main patient-level analysis, the researchers used the National Cancer Database, and for the time-series analysis they used information from the Surveillance, Epidemiology, and End Results program database.
“Our findings suggest that minimally invasive surgery was associated with a higher risk of death than open surgery among women who underwent radical hysterectomy for early-stage cervical cancer. This association was apparent regardless of laparoscopic approach, tumor size, or histologic type,” they concluded.
The findings are unexpected, eye-opening, and should inform practice, according to Ritu Salani, MD, of the Ohio State University, Columbus.
“This is something we have to discuss with patients,” she said in an interview, noting that while these aren’t perfect studies, they “are the best information we have.
Data reported in September at a meeting of the International Gynecologic Cancer Society show that surgical complications and quality of life outcomes are similar with minimally invasive and open surgery, therefore the findings from these two new studies suggest a need to shift back toward open surgery for patients with cervical cancer, she said.
One “catch” is that survival in the open surgery group in the LACC trial was unusually high and recurrence rates unusually low, compared with what might be expected, and the explanation for this observation is unclear.
“There may be some missing pieces that they haven’t been able to explain, but it’s not clear that they would change the outcome,” she said.
Justin Chura, MD, director of gynecologic oncology and robotic surgery at Cancer Treatment Center of America’s Eastern Regional Medical Center in Philadelphia, said in an interview, “The results of the study by Ramirez et al. are certainly disappointing for those among us who are advocates of minimally invasive surgery (MIS). In my own practice, I transitioned to minimally invasive radical hysterectomy approximately 10 years ago. Now that approach has to be reconsidered. While there are likely subsets of patients who will still benefit from a MIS approach without worsening oncologic outcomes, we do not have robust data to reliably identify those patients.
“One factor that warrants further investigation is the use of a uterine manipulator. While I do not use a manipulator out of personal preference (one less step in the operating room), the idea of placing a device through the tumor or adjacent to it, has biologic plausibility in terms of displacing tumor cells into lymphatic channels,” he said. “Until we have more data, an open approach appears to be preferred.”*
Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani and Dr. Chura are members of the Ob.Gyn. News editorial board, but reported having no other relevant disclosures.*
SOURCE: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
*This article was updated 11/9/2018.
based on findings from the randomized, controlled phase 3 Laparoscopic Approach to Cervical Cancer (LACC) trial of more than 600 women.

The alarming findings, which led to early study termination, also were supported by results from a second population-based study. Both studies were published concurrently in the Oct. 31 issue of the New England Journal of Medicine.
The disease-free survival at 4.5 years among 319 patients who underwent minimally invasive surgery in the LACC trial was 86.0% vs. 96.5% in 312 patients who underwent open surgery, Pedro T. Ramirez, MD, of the University of Texas MD Anderson Cancer Center, Houston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395).
At 3 years, the disease-free survival rates were 91.2% in the minimally invasive surgery group and 97.1% in open surgery group (hazard ratio for disease recurrence or death from cervical cancer, 3.74).
The differences between the groups persisted after adjustment for age, body mass index, disease stage, lymphovascular invasion, and lymph-node involvement. In the minimally invasive surgery group, the findings were comparable for those who underwent laparoscopic vs. robot-assisted surgery, the investigators found.
Further, at 3 years, overall survival was 93.8% vs. 99.0% (HR for death from any cause, 6.00), death from cervical cancer was 4.4% vs. 0.6% (HR, 6.56), and the rate of locoregional recurrence-free survival was 94.3 vs. 98.3 (HR, 4.26) in the minimally invasive and open surgery groups, respectively.
Study participants were women with a mean age of 46 years with stage IA1, IA2, or IB1 cervical cancer, with most (91.9%) having IB1 disease, and either squamous-cell carcinoma, adenocarcinoma, or adenosquamous carcinoma. They were recruited from 33 centers worldwide between June 2008 and June 2017. Most of those assigned to minimally invasive surgery underwent laparoscopic surgery (84.4%), and the remaining patients underwent robot-assisted surgery.
The treatment groups were balanced with respect to baseline characteristics, they noted.
The minimally invasive approach is widely used given that guidelines from the National Comprehensive Cancer Network and European Society of Gynecological Oncology consider both surgical approaches acceptable, and since retrospective studies suggest laparoscopic radical hysterectomy is associated with lower complication rates and comparable outcomes. However, there are limited prospective data regarding survival outcomes in early stage disease with the two approaches, the researchers said.
“Our results call into question the findings in the literature suggesting that minimally invasive radical hysterectomy is associated with no difference in oncologic outcomes as compared with the open approach,” they wrote, noting that a number of factors may explain the differences, such as concurrent vs. sequential analyses in the current studies vs. prior studies (in sequential analyses, earlier procedures may have been performed under broader indications and less clearly defined radiotherapy guidelines), and the possibility that “routine use of a uterine manipulator might increase the propensity for tumor spillage” in minimally invasive surgery.
Strengths of the study include its prospective, randomized, international multicenter design and inclusion of a per-protocol analysis that was consistent with the intention-to-treat analysis, and limitations include the fact that intended enrollment wasn’t reached because of the “safety alert raised by the data and safety monitoring committee on the basis of the higher recurrence and death in the minimally invasive surgery groups,” as well as the inability to generalize the results to patients with low-risk disease as there was lack of power to evaluate outcomes in that context.
Even though the trial was initially powered on the assumption that there would be a 4.5 year follow-up for all patients, only 59.7% reached that length of follow-up. However, the trial still reached 84% power to detect noninferiority of the primary outcome (disease-free survival) with minimally invasive surgery, which was not found, they noted.
Similarly, in the population-based cohort study of 2,461 women who underwent radical hysterectomy for stage IA2 of IB1 cervical cancer between 2010 and 2013, 4-year mortality was 9.1% among 1,225 patients who underwent minimally invasive surgery vs. 5.3% among the 1,236 patients who underwent open surgery (HR, 1.65), Alexander Melamed, MD, of Harvard Medical School, Boston, and his colleagues reported (N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1804923).
Of note, the 4-year relative survival rate following radical hysterectomy for cervical cancer remained stable prior to the widespread adoption of minimally invasive approaches; an interrupted time-series analysis involving women who underwent surgery during 2000-2010, which was also conducted as part of the study, showed a decline in 4-year survival of 0.8% per year after 2006, coinciding with increased use of minimally invasive surgery, the investigators said.
For the main patient-level analysis, the researchers used the National Cancer Database, and for the time-series analysis they used information from the Surveillance, Epidemiology, and End Results program database.
“Our findings suggest that minimally invasive surgery was associated with a higher risk of death than open surgery among women who underwent radical hysterectomy for early-stage cervical cancer. This association was apparent regardless of laparoscopic approach, tumor size, or histologic type,” they concluded.
The findings are unexpected, eye-opening, and should inform practice, according to Ritu Salani, MD, of the Ohio State University, Columbus.
“This is something we have to discuss with patients,” she said in an interview, noting that while these aren’t perfect studies, they “are the best information we have.
Data reported in September at a meeting of the International Gynecologic Cancer Society show that surgical complications and quality of life outcomes are similar with minimally invasive and open surgery, therefore the findings from these two new studies suggest a need to shift back toward open surgery for patients with cervical cancer, she said.
One “catch” is that survival in the open surgery group in the LACC trial was unusually high and recurrence rates unusually low, compared with what might be expected, and the explanation for this observation is unclear.
“There may be some missing pieces that they haven’t been able to explain, but it’s not clear that they would change the outcome,” she said.
Justin Chura, MD, director of gynecologic oncology and robotic surgery at Cancer Treatment Center of America’s Eastern Regional Medical Center in Philadelphia, said in an interview, “The results of the study by Ramirez et al. are certainly disappointing for those among us who are advocates of minimally invasive surgery (MIS). In my own practice, I transitioned to minimally invasive radical hysterectomy approximately 10 years ago. Now that approach has to be reconsidered. While there are likely subsets of patients who will still benefit from a MIS approach without worsening oncologic outcomes, we do not have robust data to reliably identify those patients.
“One factor that warrants further investigation is the use of a uterine manipulator. While I do not use a manipulator out of personal preference (one less step in the operating room), the idea of placing a device through the tumor or adjacent to it, has biologic plausibility in terms of displacing tumor cells into lymphatic channels,” he said. “Until we have more data, an open approach appears to be preferred.”*
Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani and Dr. Chura are members of the Ob.Gyn. News editorial board, but reported having no other relevant disclosures.*
SOURCE: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
*This article was updated 11/9/2018.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Cervical cancer recurrence and survival rates were worse with minimally invasive vs. open surgery in a prospective study.
Major finding: Disease-free survival at 4.5 years was 86% with minimally invasive vs. 96.5% with open surgery.
Study details: The phase 3 LACC trial of more than 600 women with cervical cancer, and a population based study of nearly 2,500 women with cervical cancer.
Disclosures: Dr. Ramirez and Dr. Melamed each reported having no relevant disclosures. Dr. Salani is a member of the OB.GYN. News editorial board, but reported having no other relevant disclosures.
Source: Ramirez P. N Engl J Med. 2018 Oct 31. doi: 10.1056/NEJMoa1806395.
Robot-assisted laparoscopic tubal anastomosis following sterilization

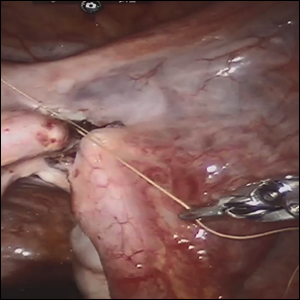
Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.

Female sterilization is the most common method of contraception worldwide, and the second most common contraceptive method used in the United States. Approximately 643,000 sterilization procedures are performed annually.1 Approximately 1% to 3% of women who undergo sterilization will subsequently undergo a sterilization reversal.2 Although multiple variables have been identified, change in marital status is the most commonly cited reason for desiring a tubal reversal.3,4 Tubal anastomosis can be a technically challenging surgical procedure when done by laparoscopy, especially given the microsurgical elements that are required. Several modifications, including limiting the number of sutures, have evolved as a result of its tedious nature.5 By leveraging 3D magnification, articulating instruments, and tremor filtration, it is only natural that robotic surgery has been applied to tubal anastomosis.
In this video, we review some background information surrounding a tubal reversal, followed by demonstration of a robotic interpretation of a 2-stitch anastomosis technique in a patient who successfully conceived and delivered.6 Overall robot-assisted laparoscopic tubal anastomosis is a feasible and safe option for women who desire reversal of surgical sterilization, with pregnancy and live-birth rates comparable to those observed when an open technique is utilized.7 I hope that you will find this video beneficial to your clinical practice.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.
- Chan LM, Westhoff CL. Tubal sterilization trends in the United States. Fertil Steril. 2010;94:1-6.
- Moss CC. Sterilization: a review and update. Obstet Gynecol Clin North Am. 2015-12-01;42:713-724.
- Gordts S, Campo R, Puttemans P, Gordts S. Clinical factors determining pregnancy outcome after microsurgical tubal anastomosis. Fertil Steril. 2009;92:1198-1202.
- Chi I-C, Jones DB. Incidence, risk factors, and prevention of poststerilization regret in women. Obstet Gynecol Surv. 1994;49:722-732.
- Dubuisson JB, Swolin K. Laparoscopic tubal anastomosis (the one stitch technique): preliminary results. Human Reprod. 1995;10:2044-2046.
- Bissonnette FCA, Lapensee L, Bouzayen R. Outpatient laparoscopic tubal anastomosis and subsequent fertility. Fertil Steril. 1999;72:549-552.
- Caillet M, Vandromme J, Rozenberg S, Paesmans M, Germay O, Degueldre M. Robotically assisted laparoscopic microsurgical tubal anastomosis: a retrospective study. Fertil Steril. 2010;94:1844-1847.
Clinical trial: Robotic versus laparoscopic ventral hernia repair
The Robotic Versus Laparoscopic Ventral Hernia Repair study is an interventional trial recruiting patients undergoing elective ventral hernia repair appropriate for minimally invasive surgery.
The trial will compare outcomes of laparoscopic and robotic approaches to ventral hernia repair. compared with the laparoscopic approach, and has been endorsed by the American Hernia Society. However, this evidence is based on database and cohort studies, and more randomized, controlled trials are needed to assess the true effects of the robotic approach.
Patients will be included if they are scheduled for a ventral hernia repair that has been deemed appropriate for minimally invasive surgery. Exclusion criteria include being unlikely to survive for 2 years post surgery, being unlikely to follow up, having advanced chronic obstructive pulmonary disease or congestive heart failure, having a history of open abdomen or extensive lysis of adhesions, having ascites caused by cirrhosis or malignancy, having an active infection, and having a hernia defect size larger than 12 cm.
The primary outcome measure is total number of days spent in the hospital. Secondary outcomes include rates of surgical site infection, rates of surgical site occurrence, rates of hernia reoccurrence, patient-centered outcomes collected using HerQLes (a hernia quality of life measuring instrument), patient-centered outcomes collected using the EQ-5D questionnaire, and cost from a health care perspective.
The primary completion date is April 30, 2020, and the study completion date is April 30, 2023. About 120 people are expected to be recruited.
Find more information on the study page at Clinicaltrials.gov.
The Robotic Versus Laparoscopic Ventral Hernia Repair study is an interventional trial recruiting patients undergoing elective ventral hernia repair appropriate for minimally invasive surgery.
The trial will compare outcomes of laparoscopic and robotic approaches to ventral hernia repair. compared with the laparoscopic approach, and has been endorsed by the American Hernia Society. However, this evidence is based on database and cohort studies, and more randomized, controlled trials are needed to assess the true effects of the robotic approach.
Patients will be included if they are scheduled for a ventral hernia repair that has been deemed appropriate for minimally invasive surgery. Exclusion criteria include being unlikely to survive for 2 years post surgery, being unlikely to follow up, having advanced chronic obstructive pulmonary disease or congestive heart failure, having a history of open abdomen or extensive lysis of adhesions, having ascites caused by cirrhosis or malignancy, having an active infection, and having a hernia defect size larger than 12 cm.
The primary outcome measure is total number of days spent in the hospital. Secondary outcomes include rates of surgical site infection, rates of surgical site occurrence, rates of hernia reoccurrence, patient-centered outcomes collected using HerQLes (a hernia quality of life measuring instrument), patient-centered outcomes collected using the EQ-5D questionnaire, and cost from a health care perspective.
The primary completion date is April 30, 2020, and the study completion date is April 30, 2023. About 120 people are expected to be recruited.
Find more information on the study page at Clinicaltrials.gov.
The Robotic Versus Laparoscopic Ventral Hernia Repair study is an interventional trial recruiting patients undergoing elective ventral hernia repair appropriate for minimally invasive surgery.
The trial will compare outcomes of laparoscopic and robotic approaches to ventral hernia repair. compared with the laparoscopic approach, and has been endorsed by the American Hernia Society. However, this evidence is based on database and cohort studies, and more randomized, controlled trials are needed to assess the true effects of the robotic approach.
Patients will be included if they are scheduled for a ventral hernia repair that has been deemed appropriate for minimally invasive surgery. Exclusion criteria include being unlikely to survive for 2 years post surgery, being unlikely to follow up, having advanced chronic obstructive pulmonary disease or congestive heart failure, having a history of open abdomen or extensive lysis of adhesions, having ascites caused by cirrhosis or malignancy, having an active infection, and having a hernia defect size larger than 12 cm.
The primary outcome measure is total number of days spent in the hospital. Secondary outcomes include rates of surgical site infection, rates of surgical site occurrence, rates of hernia reoccurrence, patient-centered outcomes collected using HerQLes (a hernia quality of life measuring instrument), patient-centered outcomes collected using the EQ-5D questionnaire, and cost from a health care perspective.
The primary completion date is April 30, 2020, and the study completion date is April 30, 2023. About 120 people are expected to be recruited.
Find more information on the study page at Clinicaltrials.gov.
Robotic approach falls short for sleeve gastrectomy
SEATTLE – according to a review of 86,953 cases in the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database.
“Robotic sleeve gastrectomy increases [use of] hospital resources. ... These findings may explain the low utilization rate of the robotic approach to sleeve gastrectomy,” said lead investigator Reza Alizadeh, MD, a surgery resident at the University of California, Irvine.
Sleeve gastrectomy has eclipsed gastric bypass as the most common weight loss surgery in United States. While most are done laparoscopically, the use of robots is becoming more common, so the investigators wanted to compare outcomes in a large number of cases. They turned to the metabolic and bariatric surgery database, which is jointly maintained by the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery. Emergent, converted, and revision cases were excluded from the analysis to avoid confounding.
Almost 94% of the cases were done laparoscopically, with the rest done robotically. Mean operative time was 101 min in the robotic arm, and 1.5% of patients developed anastomotic leaks. Mean operative time in the laparoscopic group was 74 minutes, and 0.5% of patients developed leaks. After adjustment for potential confounders, leaks were 3.4 times more likely with the robotic approach (95% confidence interval, 2.47-4.0; P less than .01). It wasn’t possible to determine whether there were any differences in the type of stapling done in the two groups.
Meanwhile, 0.8% of robotic surgery patients developed surgical site infections versus 0.6% of the laparoscopic cases. After adjustment, infections were 38% more likely with the robot (95% CI, 1.01-1.89; P = 0.03). Dr. Alizadeh noted that the database only goes out to 30 days, so “the true complication rates may be underestimated.”
The findings are consistent with previous investigations. It’s unclear whether there’s something inherently riskier about robotic sleeve gastrectomy itself or whether surgeons haven’t quite got the knack of it yet. The higher leak rate with robotic surgery, “I believe, is mostly related to the small number of [robotic] cases being done. We are still in the beginning stages of utilizing the robotic approach. Maybe there’s a learning curve, and we need more experience and more practice,” Dr. Alizadeh said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
Indeed, others have reported that it takes more than two dozen cases to become proficient in another procedure, robotic esophagectomy.
The mean length of stay in the study was slightly, but not statistically significantly, longer in the robotic arm (1.8 vs. 1.7 days; P = 0.17). There was no statistically significant difference in in-hospital mortality.
The laparoscopic group had more men than did the robotic group (21.4% vs. 19.7%, respectively) and more chronic steroid use (1.7% vs. 1.3%), plus more patients were dependent on oxygen (0.7% vs. 0.3%). The robotic group had more obstructive sleep apnea than did the laparoscopic group (37.3% vs. 36% of cases) and a higher incidence of hypoalbuminemia (8.4% vs. 7%). The analysis adjusted for the differences.
The findings were pretty much the same when the team repeated their analysis with the 2016 database numbers, which were released while the SAGES presentation was being prepared. The only big difference was an increase in the number of robotic cases, up from 6.1% in 2015 to 6.6% of cases in 2016.
The was no external funding for the work, and the investigators had no relevant disclosures.
SOURCE: Alizadeh RF et al. SAGES 2018, Abstract S024.
SEATTLE – according to a review of 86,953 cases in the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database.
“Robotic sleeve gastrectomy increases [use of] hospital resources. ... These findings may explain the low utilization rate of the robotic approach to sleeve gastrectomy,” said lead investigator Reza Alizadeh, MD, a surgery resident at the University of California, Irvine.
Sleeve gastrectomy has eclipsed gastric bypass as the most common weight loss surgery in United States. While most are done laparoscopically, the use of robots is becoming more common, so the investigators wanted to compare outcomes in a large number of cases. They turned to the metabolic and bariatric surgery database, which is jointly maintained by the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery. Emergent, converted, and revision cases were excluded from the analysis to avoid confounding.
Almost 94% of the cases were done laparoscopically, with the rest done robotically. Mean operative time was 101 min in the robotic arm, and 1.5% of patients developed anastomotic leaks. Mean operative time in the laparoscopic group was 74 minutes, and 0.5% of patients developed leaks. After adjustment for potential confounders, leaks were 3.4 times more likely with the robotic approach (95% confidence interval, 2.47-4.0; P less than .01). It wasn’t possible to determine whether there were any differences in the type of stapling done in the two groups.
Meanwhile, 0.8% of robotic surgery patients developed surgical site infections versus 0.6% of the laparoscopic cases. After adjustment, infections were 38% more likely with the robot (95% CI, 1.01-1.89; P = 0.03). Dr. Alizadeh noted that the database only goes out to 30 days, so “the true complication rates may be underestimated.”
The findings are consistent with previous investigations. It’s unclear whether there’s something inherently riskier about robotic sleeve gastrectomy itself or whether surgeons haven’t quite got the knack of it yet. The higher leak rate with robotic surgery, “I believe, is mostly related to the small number of [robotic] cases being done. We are still in the beginning stages of utilizing the robotic approach. Maybe there’s a learning curve, and we need more experience and more practice,” Dr. Alizadeh said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
Indeed, others have reported that it takes more than two dozen cases to become proficient in another procedure, robotic esophagectomy.
The mean length of stay in the study was slightly, but not statistically significantly, longer in the robotic arm (1.8 vs. 1.7 days; P = 0.17). There was no statistically significant difference in in-hospital mortality.
The laparoscopic group had more men than did the robotic group (21.4% vs. 19.7%, respectively) and more chronic steroid use (1.7% vs. 1.3%), plus more patients were dependent on oxygen (0.7% vs. 0.3%). The robotic group had more obstructive sleep apnea than did the laparoscopic group (37.3% vs. 36% of cases) and a higher incidence of hypoalbuminemia (8.4% vs. 7%). The analysis adjusted for the differences.
The findings were pretty much the same when the team repeated their analysis with the 2016 database numbers, which were released while the SAGES presentation was being prepared. The only big difference was an increase in the number of robotic cases, up from 6.1% in 2015 to 6.6% of cases in 2016.
The was no external funding for the work, and the investigators had no relevant disclosures.
SOURCE: Alizadeh RF et al. SAGES 2018, Abstract S024.
SEATTLE – according to a review of 86,953 cases in the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database.
“Robotic sleeve gastrectomy increases [use of] hospital resources. ... These findings may explain the low utilization rate of the robotic approach to sleeve gastrectomy,” said lead investigator Reza Alizadeh, MD, a surgery resident at the University of California, Irvine.
Sleeve gastrectomy has eclipsed gastric bypass as the most common weight loss surgery in United States. While most are done laparoscopically, the use of robots is becoming more common, so the investigators wanted to compare outcomes in a large number of cases. They turned to the metabolic and bariatric surgery database, which is jointly maintained by the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery. Emergent, converted, and revision cases were excluded from the analysis to avoid confounding.
Almost 94% of the cases were done laparoscopically, with the rest done robotically. Mean operative time was 101 min in the robotic arm, and 1.5% of patients developed anastomotic leaks. Mean operative time in the laparoscopic group was 74 minutes, and 0.5% of patients developed leaks. After adjustment for potential confounders, leaks were 3.4 times more likely with the robotic approach (95% confidence interval, 2.47-4.0; P less than .01). It wasn’t possible to determine whether there were any differences in the type of stapling done in the two groups.
Meanwhile, 0.8% of robotic surgery patients developed surgical site infections versus 0.6% of the laparoscopic cases. After adjustment, infections were 38% more likely with the robot (95% CI, 1.01-1.89; P = 0.03). Dr. Alizadeh noted that the database only goes out to 30 days, so “the true complication rates may be underestimated.”
The findings are consistent with previous investigations. It’s unclear whether there’s something inherently riskier about robotic sleeve gastrectomy itself or whether surgeons haven’t quite got the knack of it yet. The higher leak rate with robotic surgery, “I believe, is mostly related to the small number of [robotic] cases being done. We are still in the beginning stages of utilizing the robotic approach. Maybe there’s a learning curve, and we need more experience and more practice,” Dr. Alizadeh said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
Indeed, others have reported that it takes more than two dozen cases to become proficient in another procedure, robotic esophagectomy.
The mean length of stay in the study was slightly, but not statistically significantly, longer in the robotic arm (1.8 vs. 1.7 days; P = 0.17). There was no statistically significant difference in in-hospital mortality.
The laparoscopic group had more men than did the robotic group (21.4% vs. 19.7%, respectively) and more chronic steroid use (1.7% vs. 1.3%), plus more patients were dependent on oxygen (0.7% vs. 0.3%). The robotic group had more obstructive sleep apnea than did the laparoscopic group (37.3% vs. 36% of cases) and a higher incidence of hypoalbuminemia (8.4% vs. 7%). The analysis adjusted for the differences.
The findings were pretty much the same when the team repeated their analysis with the 2016 database numbers, which were released while the SAGES presentation was being prepared. The only big difference was an increase in the number of robotic cases, up from 6.1% in 2015 to 6.6% of cases in 2016.
The was no external funding for the work, and the investigators had no relevant disclosures.
SOURCE: Alizadeh RF et al. SAGES 2018, Abstract S024.
REPORTING FROM SAGES 2018
Key clinical point: Operative times are longer, and leaks and surgical site infections more common, when surgeons opt for robotic instead of laparoscopic sleeve gastrectomy.
Major finding: Anastomotic leaks were 3.4 times more likely with the robotic approach (95% CI 2.47-4.0; P less than .01).
Study details: Review of 86,953 cases in the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database
Disclosures: The was no external funding for the project, and the investigators had no relevant disclosures.
Source: Alizadeh RF et al. SAGES 2018, Abstract S024
The case for closing robotic surgery port sites
JACKSONVILLE, FLA. – Findings from a retrospective chart review of robotic operations performed over 6 years has identified situations in which surgeons may consider closing 8-mm port sites after robotic surgery, according to a presentation at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.

“Although the incidence of hernia through the 8-mm port sites was low, it’s still important because it’s a significant cause of morbidity in these patients,” Dr. Diez-Barroso said. Two of the three 8-mm port-site hernias required emergency surgery for small bowel incarceration.
“Both of the hernias occurred in the left lower quadrant in the lateral most port, near the anterior superior iliac spine,” he said. “The nearest site of muscle insertions was where the abdominal wall muscle layers have a limited ability to slide over one another during insufflation and desufflation and therefore have a lack of ability to seal off the port site correctly.”
These results have caused surgeons in his group to take a closer look at their own practices, Dr. Diez-Barroso said. “In our practice, now we’re considering closure of the ports in that location in the presence of known risk factors for hernia formation,” he said.
Dr. Diez-Barroso noted other scenarios when surgeons might consider closing 8-mm port sites, for example, after a prolonged operation, when significant torque has been placed on the port site, and in obese patients. The two cases of emergency surgery for port-site hernias involved obese patients: a female with a body mass index of 33 kg/m2 who had an abdominoperineal resection and a male with a BMI of 34 kg/m2 who had a right-sided ventral hernia repair.
The study had a number of limitations, Dr. Diez-Barroso said: its small sample size, retrospective nature, and short follow-up. “Moving forward, to understand better the true incidence of port-site hernias, we want further investigation with longer follow-up times and a larger sample size,” he said.
During questions, moderator Lesly Ann Dossett, MD, FACS, of the University of Michigan, Ann Arbor, asked whether there were other steps surgeons could take, such as where to place the ports or how much torque they apply, besides closing the ports.
“We’ve always placed ports with the standard approach: inserting them perpendicular to the abdominal wall,” Dr. Diez-Barroso said. “Others have theorized that the lateral sites undergo more torque, but I think that also needs further investigation.”
Dr. Diez-Barroso and coauthors reported having no financial disclosures.
Source: Diez-Barroso R. Academic Surgical Congress 2018.
JACKSONVILLE, FLA. – Findings from a retrospective chart review of robotic operations performed over 6 years has identified situations in which surgeons may consider closing 8-mm port sites after robotic surgery, according to a presentation at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.

“Although the incidence of hernia through the 8-mm port sites was low, it’s still important because it’s a significant cause of morbidity in these patients,” Dr. Diez-Barroso said. Two of the three 8-mm port-site hernias required emergency surgery for small bowel incarceration.
“Both of the hernias occurred in the left lower quadrant in the lateral most port, near the anterior superior iliac spine,” he said. “The nearest site of muscle insertions was where the abdominal wall muscle layers have a limited ability to slide over one another during insufflation and desufflation and therefore have a lack of ability to seal off the port site correctly.”
These results have caused surgeons in his group to take a closer look at their own practices, Dr. Diez-Barroso said. “In our practice, now we’re considering closure of the ports in that location in the presence of known risk factors for hernia formation,” he said.
Dr. Diez-Barroso noted other scenarios when surgeons might consider closing 8-mm port sites, for example, after a prolonged operation, when significant torque has been placed on the port site, and in obese patients. The two cases of emergency surgery for port-site hernias involved obese patients: a female with a body mass index of 33 kg/m2 who had an abdominoperineal resection and a male with a BMI of 34 kg/m2 who had a right-sided ventral hernia repair.
The study had a number of limitations, Dr. Diez-Barroso said: its small sample size, retrospective nature, and short follow-up. “Moving forward, to understand better the true incidence of port-site hernias, we want further investigation with longer follow-up times and a larger sample size,” he said.
During questions, moderator Lesly Ann Dossett, MD, FACS, of the University of Michigan, Ann Arbor, asked whether there were other steps surgeons could take, such as where to place the ports or how much torque they apply, besides closing the ports.
“We’ve always placed ports with the standard approach: inserting them perpendicular to the abdominal wall,” Dr. Diez-Barroso said. “Others have theorized that the lateral sites undergo more torque, but I think that also needs further investigation.”
Dr. Diez-Barroso and coauthors reported having no financial disclosures.
Source: Diez-Barroso R. Academic Surgical Congress 2018.
JACKSONVILLE, FLA. – Findings from a retrospective chart review of robotic operations performed over 6 years has identified situations in which surgeons may consider closing 8-mm port sites after robotic surgery, according to a presentation at the Association for Academic Surgery/Society of University Surgeons Academic Surgical Congress.

“Although the incidence of hernia through the 8-mm port sites was low, it’s still important because it’s a significant cause of morbidity in these patients,” Dr. Diez-Barroso said. Two of the three 8-mm port-site hernias required emergency surgery for small bowel incarceration.
“Both of the hernias occurred in the left lower quadrant in the lateral most port, near the anterior superior iliac spine,” he said. “The nearest site of muscle insertions was where the abdominal wall muscle layers have a limited ability to slide over one another during insufflation and desufflation and therefore have a lack of ability to seal off the port site correctly.”
These results have caused surgeons in his group to take a closer look at their own practices, Dr. Diez-Barroso said. “In our practice, now we’re considering closure of the ports in that location in the presence of known risk factors for hernia formation,” he said.
Dr. Diez-Barroso noted other scenarios when surgeons might consider closing 8-mm port sites, for example, after a prolonged operation, when significant torque has been placed on the port site, and in obese patients. The two cases of emergency surgery for port-site hernias involved obese patients: a female with a body mass index of 33 kg/m2 who had an abdominoperineal resection and a male with a BMI of 34 kg/m2 who had a right-sided ventral hernia repair.
The study had a number of limitations, Dr. Diez-Barroso said: its small sample size, retrospective nature, and short follow-up. “Moving forward, to understand better the true incidence of port-site hernias, we want further investigation with longer follow-up times and a larger sample size,” he said.
During questions, moderator Lesly Ann Dossett, MD, FACS, of the University of Michigan, Ann Arbor, asked whether there were other steps surgeons could take, such as where to place the ports or how much torque they apply, besides closing the ports.
“We’ve always placed ports with the standard approach: inserting them perpendicular to the abdominal wall,” Dr. Diez-Barroso said. “Others have theorized that the lateral sites undergo more torque, but I think that also needs further investigation.”
Dr. Diez-Barroso and coauthors reported having no financial disclosures.
Source: Diez-Barroso R. Academic Surgical Congress 2018.
AT THE ACADEMIC SURGICAL CONGRESS
Key clinical point: Some 8-mm robotic port sites may warrant closure under certain circumstances.
Major finding: Of 178 patients, 3 had complications caused by 8-mm robotic port sites that were not closed, 2 of which required emergency reoperation for small bowel incarceration.
Data source: Retrospective chart review of 178 patients who had robotic general and oncologic surgical procedures between July 2010 and December 2016.
Disclosures: Dr. Diez-Barroso and coauthors reported having no financial disclosures.
Source: Diez-Barroso R. Academic Surgical Congress 2018.
ROBOT trial compares surgical approaches to esophagectomy
SAN FRANCISCO – Patients undergoing had less morbidity and pain and similarly good oncologic outcomes, when the surgery was performed by robot-assisted laparoscopy instead of by the open technique, a phase 3 clinical trial has found.
Investigators of the ROBOT (Robot-assisted Thoracolaparoscopic Esophagectomy vs. Open Transthoracic Esophagectomy) trial, led by Pieter C. van der Sluis, MD, a surgeon at the University Medical Center Utrecht, the Netherlands, randomized 112 patients with resectable esophageal cancer to open transthoracic esophagectomy – considered to be the gold standard – or robot-assisted minimally invasive thoracolaparoscopic esophagectomy.
“Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy improves postoperative outcome. There were no differences in oncologic outcomes, and our oncologic outcomes were in concordance with the highest standards nowadays,” Dr. van der Sluis summarized. “This trial provides evidence for the minimally invasive approach over the open approach, and especially the robot-assisted minimally invasive esophagectomy.”
The investigators will report a full cost comparison separately. “We see that costs are lower, though not significantly lower, with the robot,” he said, giving a preview. “We are going to show that the real costs of the operation are in the complications. When you have complications that involve the ICU and reoperations, some patients are in the hospital for months after the surgery. So by investing a little extra money in the surgical procedure, you might actually get it back by reducing the complications.”
When asked by an attendee why the trial did not compare robotic esophagectomy with thoracoscopic esophagectomy, Dr. van der Sluis noted that such comparison is complicated by many factors; for example, the challenge of finding surgeons skilled in both techniques, and the likelihood of small differences in outcomes, potentially requiring enrollment of thousands of patients to have adequate study power. “We concluded that such a trial might not be feasible,” he said.
Parsing the findings
“The complication rates [in this trial] are very high in the robotic and open groups, much higher than reported in some well-controlled prospective and retrospective studies,” commented session attendee Kenneth Meredith, MD, FACS, professor at Florida State University, Sarasota, and director of gastrointestinal oncology, Sarasota Memorial Institute for Cancer Care.
He wondered how extensive the investigators’ experience with robotics was and how many cases they had done on their learning curve. Data from his group suggest that surgeons must perform 29 cases of robotic esophagectomy before the complication rate drops (Dis Esophagus. 2017;30:1-7).
“That’s more then half of the patients in the robotic arm of their study,” he noted in an interview. “I find this needs to be explained. If the authors are past their learning curve, why were the complication rates so high?” Additionally, the 80% rate in the open group “is among the highest I’ve seen in many years.”
The lack of significant differences in complete resection rate and in lymph node harvest was also surprising, as he and other robotics users have found that this technique can improve these outcomes, Dr. Meredith added. This could likewise be a learning curve phenomenon.
Although ROBOT’s comparison of robotic with open esophagectomy is relevant, “it would have been more relevant to compare robotic to minimally invasive esophagectomy [MIE],” he maintained, as MIE has been shown to improve outcomes relative to open surgery (Lancet. 2012;379:1887-92).
“There are many high-volume centers in MIE but not necessarily robotics. The two are often mutually exclusive, and a multicenter trial in which each center performs high volumes of their respective technique, rather then mandating each center perform an operation they may not be facile in,” would be practical, Dr. Meredith concluded.
Study details
“The main objective in our trial was to reduce surgical trauma and reduce the percentage of complications,” Dr. van der Sluis told attendees of the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Results showed that compared with peers in the open surgery group, patients in the robotic-assisted surgery group specifically had a lower rate of pulmonary complications (32% vs. 58%, P = .005), largely due to a reduction in rate of pneumonia (28% vs. 55%, P = .005), and a lower rate of cardiac complications (22% vs. 47%, P = .006), almost entirely due to a reduction in rate of atrial fibrillation (22% vs. 46%, P = .01).
There was a trend toward fewer wound infections with robotics (4% vs. 14%, P = .09), with a large difference in thoracic wound infections (0% vs. 9%, P = .06).
The two groups were statistically indistinguishable on rates of anastomotic leakage (24% and 20%) and recurrent laryngeal nerve injury (9% and 11%). The fairly high rate of anastomotic leakage was likely due to the center’s use of cervical anastomosis at the time of the trial, according to Dr. van der Sluis; they have since started using thoracic anastomosis, and will report results with that technique soon.
There was also no significant difference between groups in the rate of in-hospital mortality (4% with robotic surgery and 2% with open surgery), median hospital length of stay (14 and 16 days), and ICU length of stay (1 day in each group).
Patients in the robotics group more commonly had functional recovery within 2 weeks (70% vs. 51%, P = .04). And on the Quality of Life Questionnaire Core 30, they had better scores for health-related quality of life at discharge (57.9 vs 44.6, P = .02) and at 6 weeks (68.7 vs. 57.6, P = .03), and for physical functioning at discharge (54.5 vs. 41.0, P = .03) and 6 weeks (69.3 vs. 58.6, P = .049).
The two groups were similar on rates of R0 resection (93% and 96%) and median number of lymph nodes retrieved (27 and 25), reported Dr. van der Sluis. Pain during the first 14 days after surgery was lower for the robotics group (P = .003).
With a median follow-up of 40 months, the robotics and open groups did not differ significantly on disease-free survival (median, 26 and 28 months) and overall survival (not reached in either group).
Dr. van der Sluis disclosed no relevant conflicts of interest.
SOURCE: van der Sluis PC et al. 2018 GI Cancer Symposium, Abstract 156148
SAN FRANCISCO – Patients undergoing had less morbidity and pain and similarly good oncologic outcomes, when the surgery was performed by robot-assisted laparoscopy instead of by the open technique, a phase 3 clinical trial has found.
Investigators of the ROBOT (Robot-assisted Thoracolaparoscopic Esophagectomy vs. Open Transthoracic Esophagectomy) trial, led by Pieter C. van der Sluis, MD, a surgeon at the University Medical Center Utrecht, the Netherlands, randomized 112 patients with resectable esophageal cancer to open transthoracic esophagectomy – considered to be the gold standard – or robot-assisted minimally invasive thoracolaparoscopic esophagectomy.
“Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy improves postoperative outcome. There were no differences in oncologic outcomes, and our oncologic outcomes were in concordance with the highest standards nowadays,” Dr. van der Sluis summarized. “This trial provides evidence for the minimally invasive approach over the open approach, and especially the robot-assisted minimally invasive esophagectomy.”
The investigators will report a full cost comparison separately. “We see that costs are lower, though not significantly lower, with the robot,” he said, giving a preview. “We are going to show that the real costs of the operation are in the complications. When you have complications that involve the ICU and reoperations, some patients are in the hospital for months after the surgery. So by investing a little extra money in the surgical procedure, you might actually get it back by reducing the complications.”
When asked by an attendee why the trial did not compare robotic esophagectomy with thoracoscopic esophagectomy, Dr. van der Sluis noted that such comparison is complicated by many factors; for example, the challenge of finding surgeons skilled in both techniques, and the likelihood of small differences in outcomes, potentially requiring enrollment of thousands of patients to have adequate study power. “We concluded that such a trial might not be feasible,” he said.
Parsing the findings
“The complication rates [in this trial] are very high in the robotic and open groups, much higher than reported in some well-controlled prospective and retrospective studies,” commented session attendee Kenneth Meredith, MD, FACS, professor at Florida State University, Sarasota, and director of gastrointestinal oncology, Sarasota Memorial Institute for Cancer Care.
He wondered how extensive the investigators’ experience with robotics was and how many cases they had done on their learning curve. Data from his group suggest that surgeons must perform 29 cases of robotic esophagectomy before the complication rate drops (Dis Esophagus. 2017;30:1-7).
“That’s more then half of the patients in the robotic arm of their study,” he noted in an interview. “I find this needs to be explained. If the authors are past their learning curve, why were the complication rates so high?” Additionally, the 80% rate in the open group “is among the highest I’ve seen in many years.”
The lack of significant differences in complete resection rate and in lymph node harvest was also surprising, as he and other robotics users have found that this technique can improve these outcomes, Dr. Meredith added. This could likewise be a learning curve phenomenon.
Although ROBOT’s comparison of robotic with open esophagectomy is relevant, “it would have been more relevant to compare robotic to minimally invasive esophagectomy [MIE],” he maintained, as MIE has been shown to improve outcomes relative to open surgery (Lancet. 2012;379:1887-92).
“There are many high-volume centers in MIE but not necessarily robotics. The two are often mutually exclusive, and a multicenter trial in which each center performs high volumes of their respective technique, rather then mandating each center perform an operation they may not be facile in,” would be practical, Dr. Meredith concluded.
Study details
“The main objective in our trial was to reduce surgical trauma and reduce the percentage of complications,” Dr. van der Sluis told attendees of the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Results showed that compared with peers in the open surgery group, patients in the robotic-assisted surgery group specifically had a lower rate of pulmonary complications (32% vs. 58%, P = .005), largely due to a reduction in rate of pneumonia (28% vs. 55%, P = .005), and a lower rate of cardiac complications (22% vs. 47%, P = .006), almost entirely due to a reduction in rate of atrial fibrillation (22% vs. 46%, P = .01).
There was a trend toward fewer wound infections with robotics (4% vs. 14%, P = .09), with a large difference in thoracic wound infections (0% vs. 9%, P = .06).
The two groups were statistically indistinguishable on rates of anastomotic leakage (24% and 20%) and recurrent laryngeal nerve injury (9% and 11%). The fairly high rate of anastomotic leakage was likely due to the center’s use of cervical anastomosis at the time of the trial, according to Dr. van der Sluis; they have since started using thoracic anastomosis, and will report results with that technique soon.
There was also no significant difference between groups in the rate of in-hospital mortality (4% with robotic surgery and 2% with open surgery), median hospital length of stay (14 and 16 days), and ICU length of stay (1 day in each group).
Patients in the robotics group more commonly had functional recovery within 2 weeks (70% vs. 51%, P = .04). And on the Quality of Life Questionnaire Core 30, they had better scores for health-related quality of life at discharge (57.9 vs 44.6, P = .02) and at 6 weeks (68.7 vs. 57.6, P = .03), and for physical functioning at discharge (54.5 vs. 41.0, P = .03) and 6 weeks (69.3 vs. 58.6, P = .049).
The two groups were similar on rates of R0 resection (93% and 96%) and median number of lymph nodes retrieved (27 and 25), reported Dr. van der Sluis. Pain during the first 14 days after surgery was lower for the robotics group (P = .003).
With a median follow-up of 40 months, the robotics and open groups did not differ significantly on disease-free survival (median, 26 and 28 months) and overall survival (not reached in either group).
Dr. van der Sluis disclosed no relevant conflicts of interest.
SOURCE: van der Sluis PC et al. 2018 GI Cancer Symposium, Abstract 156148
SAN FRANCISCO – Patients undergoing had less morbidity and pain and similarly good oncologic outcomes, when the surgery was performed by robot-assisted laparoscopy instead of by the open technique, a phase 3 clinical trial has found.
Investigators of the ROBOT (Robot-assisted Thoracolaparoscopic Esophagectomy vs. Open Transthoracic Esophagectomy) trial, led by Pieter C. van der Sluis, MD, a surgeon at the University Medical Center Utrecht, the Netherlands, randomized 112 patients with resectable esophageal cancer to open transthoracic esophagectomy – considered to be the gold standard – or robot-assisted minimally invasive thoracolaparoscopic esophagectomy.
“Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy improves postoperative outcome. There were no differences in oncologic outcomes, and our oncologic outcomes were in concordance with the highest standards nowadays,” Dr. van der Sluis summarized. “This trial provides evidence for the minimally invasive approach over the open approach, and especially the robot-assisted minimally invasive esophagectomy.”
The investigators will report a full cost comparison separately. “We see that costs are lower, though not significantly lower, with the robot,” he said, giving a preview. “We are going to show that the real costs of the operation are in the complications. When you have complications that involve the ICU and reoperations, some patients are in the hospital for months after the surgery. So by investing a little extra money in the surgical procedure, you might actually get it back by reducing the complications.”
When asked by an attendee why the trial did not compare robotic esophagectomy with thoracoscopic esophagectomy, Dr. van der Sluis noted that such comparison is complicated by many factors; for example, the challenge of finding surgeons skilled in both techniques, and the likelihood of small differences in outcomes, potentially requiring enrollment of thousands of patients to have adequate study power. “We concluded that such a trial might not be feasible,” he said.
Parsing the findings
“The complication rates [in this trial] are very high in the robotic and open groups, much higher than reported in some well-controlled prospective and retrospective studies,” commented session attendee Kenneth Meredith, MD, FACS, professor at Florida State University, Sarasota, and director of gastrointestinal oncology, Sarasota Memorial Institute for Cancer Care.
He wondered how extensive the investigators’ experience with robotics was and how many cases they had done on their learning curve. Data from his group suggest that surgeons must perform 29 cases of robotic esophagectomy before the complication rate drops (Dis Esophagus. 2017;30:1-7).
“That’s more then half of the patients in the robotic arm of their study,” he noted in an interview. “I find this needs to be explained. If the authors are past their learning curve, why were the complication rates so high?” Additionally, the 80% rate in the open group “is among the highest I’ve seen in many years.”
The lack of significant differences in complete resection rate and in lymph node harvest was also surprising, as he and other robotics users have found that this technique can improve these outcomes, Dr. Meredith added. This could likewise be a learning curve phenomenon.
Although ROBOT’s comparison of robotic with open esophagectomy is relevant, “it would have been more relevant to compare robotic to minimally invasive esophagectomy [MIE],” he maintained, as MIE has been shown to improve outcomes relative to open surgery (Lancet. 2012;379:1887-92).
“There are many high-volume centers in MIE but not necessarily robotics. The two are often mutually exclusive, and a multicenter trial in which each center performs high volumes of their respective technique, rather then mandating each center perform an operation they may not be facile in,” would be practical, Dr. Meredith concluded.
Study details
“The main objective in our trial was to reduce surgical trauma and reduce the percentage of complications,” Dr. van der Sluis told attendees of the symposium, sponsored by the American Gastroenterological Association, the American Society for Clinical Oncology, the American Society for Radiation Oncology, and the Society of Surgical Oncology.
Results showed that compared with peers in the open surgery group, patients in the robotic-assisted surgery group specifically had a lower rate of pulmonary complications (32% vs. 58%, P = .005), largely due to a reduction in rate of pneumonia (28% vs. 55%, P = .005), and a lower rate of cardiac complications (22% vs. 47%, P = .006), almost entirely due to a reduction in rate of atrial fibrillation (22% vs. 46%, P = .01).
There was a trend toward fewer wound infections with robotics (4% vs. 14%, P = .09), with a large difference in thoracic wound infections (0% vs. 9%, P = .06).
The two groups were statistically indistinguishable on rates of anastomotic leakage (24% and 20%) and recurrent laryngeal nerve injury (9% and 11%). The fairly high rate of anastomotic leakage was likely due to the center’s use of cervical anastomosis at the time of the trial, according to Dr. van der Sluis; they have since started using thoracic anastomosis, and will report results with that technique soon.
There was also no significant difference between groups in the rate of in-hospital mortality (4% with robotic surgery and 2% with open surgery), median hospital length of stay (14 and 16 days), and ICU length of stay (1 day in each group).
Patients in the robotics group more commonly had functional recovery within 2 weeks (70% vs. 51%, P = .04). And on the Quality of Life Questionnaire Core 30, they had better scores for health-related quality of life at discharge (57.9 vs 44.6, P = .02) and at 6 weeks (68.7 vs. 57.6, P = .03), and for physical functioning at discharge (54.5 vs. 41.0, P = .03) and 6 weeks (69.3 vs. 58.6, P = .049).
The two groups were similar on rates of R0 resection (93% and 96%) and median number of lymph nodes retrieved (27 and 25), reported Dr. van der Sluis. Pain during the first 14 days after surgery was lower for the robotics group (P = .003).
With a median follow-up of 40 months, the robotics and open groups did not differ significantly on disease-free survival (median, 26 and 28 months) and overall survival (not reached in either group).
Dr. van der Sluis disclosed no relevant conflicts of interest.
SOURCE: van der Sluis PC et al. 2018 GI Cancer Symposium, Abstract 156148
REPORTING FROM THE 2018 GI CANCERS SYMPOSIUM
Key clinical point: Patients with esophageal cancer undergoing esophagectomy are less likely to experience complications when the surgery is performed robotically.
Major finding: Compared with open transthoracic esophagectomy, robot-assisted minimally invasive thoracolaparoscopic esophagectomy had a lower rate of MCDC grade 2 or higher surgery-related postoperative complications (59% vs. 80%).
Data source: A single-center phase 3 randomized controlled trial among 112 patients with resectable esophageal cancer.
Disclosures: Dr. van der Sluis disclosed no relevant conflicts of interest.
Source: van der Sluis PC et al. 2018 GI Cancer Symposium, Abstract 156148
Surgery residents cite time challenges to robotics training
Although a majority of surgical residents plan to incorporate robotics in practice, 80% cited time commitment as a barrier to completing a nonmandatory robotics curriculum, according to a survey published online in the American Journal of Surgery.
Most surgery residents agree that robotics training is important, but most academic institutions have not yet established a mandatory training program, wrote Vernissia Tam, MD, of the University of Pittsburgh and her colleagues (Am J Surg. 2017. doi: 10.1016/j.amjsurg.2017.08.051).
To determine resident attitudes about robotics and the impact of a robotics curriculum, the researchers surveyed 48 general surgery residents in 2014-2015 and 49 residents in 2016-2017 at a single academic center. Overall, 98% and 96% of the two groups, respectively, reported high interest improving robotic skills, and more than two-thirds reported plans to use robotics in their practices.
The introduction of a voluntary, structured robotics program yielded significant improvements in the percentage of residents using both a robotic backpack simulator (from 18% to 39%) and an inanimate box trainer (increased from 20% to 41%).
However, of 60 unique residents between the two survey time points, only 24 began the robotics curriculum (40%) and only 11 (18%) completed it. In a follow-up survey of residents who had not yet completed the robotics training, 80% said that “time away from clinical responsibilities and/or research was the most commonly cited barrier to curriculum completion,” Dr. Tam and her associates noted.
The study was limited in part by the use of data from a single center over a short period of time, but “we believe these results provide a broad needs assessment for a structured robotics program and identify barriers to implementing a novel curriculum,” the researchers wrote. Many health professionals argue that a competence-based program, rather than time-based, would be more effective and accessible to students, so “development of an inanimate deliberate practice system with weekly opportunities is a viable avenue to increase technical skills and learn surgical procedures,” they said.
The researchers had no financial conflicts to disclose. The study was supported in part by an Intuitive Surgical Education Grant.
SOURCE: Tam V et al. Am J Surg. 2017. doi: 10.1016/j.amjsurg.2017.08.051.
Although a majority of surgical residents plan to incorporate robotics in practice, 80% cited time commitment as a barrier to completing a nonmandatory robotics curriculum, according to a survey published online in the American Journal of Surgery.
Most surgery residents agree that robotics training is important, but most academic institutions have not yet established a mandatory training program, wrote Vernissia Tam, MD, of the University of Pittsburgh and her colleagues (Am J Surg. 2017. doi: 10.1016/j.amjsurg.2017.08.051).
To determine resident attitudes about robotics and the impact of a robotics curriculum, the researchers surveyed 48 general surgery residents in 2014-2015 and 49 residents in 2016-2017 at a single academic center. Overall, 98% and 96% of the two groups, respectively, reported high interest improving robotic skills, and more than two-thirds reported plans to use robotics in their practices.
The introduction of a voluntary, structured robotics program yielded significant improvements in the percentage of residents using both a robotic backpack simulator (from 18% to 39%) and an inanimate box trainer (increased from 20% to 41%).
However, of 60 unique residents between the two survey time points, only 24 began the robotics curriculum (40%) and only 11 (18%) completed it. In a follow-up survey of residents who had not yet completed the robotics training, 80% said that “time away from clinical responsibilities and/or research was the most commonly cited barrier to curriculum completion,” Dr. Tam and her associates noted.
The study was limited in part by the use of data from a single center over a short period of time, but “we believe these results provide a broad needs assessment for a structured robotics program and identify barriers to implementing a novel curriculum,” the researchers wrote. Many health professionals argue that a competence-based program, rather than time-based, would be more effective and accessible to students, so “development of an inanimate deliberate practice system with weekly opportunities is a viable avenue to increase technical skills and learn surgical procedures,” they said.
The researchers had no financial conflicts to disclose. The study was supported in part by an Intuitive Surgical Education Grant.
SOURCE: Tam V et al. Am J Surg. 2017. doi: 10.1016/j.amjsurg.2017.08.051.
Although a majority of surgical residents plan to incorporate robotics in practice, 80% cited time commitment as a barrier to completing a nonmandatory robotics curriculum, according to a survey published online in the American Journal of Surgery.
Most surgery residents agree that robotics training is important, but most academic institutions have not yet established a mandatory training program, wrote Vernissia Tam, MD, of the University of Pittsburgh and her colleagues (Am J Surg. 2017. doi: 10.1016/j.amjsurg.2017.08.051).
To determine resident attitudes about robotics and the impact of a robotics curriculum, the researchers surveyed 48 general surgery residents in 2014-2015 and 49 residents in 2016-2017 at a single academic center. Overall, 98% and 96% of the two groups, respectively, reported high interest improving robotic skills, and more than two-thirds reported plans to use robotics in their practices.
The introduction of a voluntary, structured robotics program yielded significant improvements in the percentage of residents using both a robotic backpack simulator (from 18% to 39%) and an inanimate box trainer (increased from 20% to 41%).
However, of 60 unique residents between the two survey time points, only 24 began the robotics curriculum (40%) and only 11 (18%) completed it. In a follow-up survey of residents who had not yet completed the robotics training, 80% said that “time away from clinical responsibilities and/or research was the most commonly cited barrier to curriculum completion,” Dr. Tam and her associates noted.
The study was limited in part by the use of data from a single center over a short period of time, but “we believe these results provide a broad needs assessment for a structured robotics program and identify barriers to implementing a novel curriculum,” the researchers wrote. Many health professionals argue that a competence-based program, rather than time-based, would be more effective and accessible to students, so “development of an inanimate deliberate practice system with weekly opportunities is a viable avenue to increase technical skills and learn surgical procedures,” they said.
The researchers had no financial conflicts to disclose. The study was supported in part by an Intuitive Surgical Education Grant.
SOURCE: Tam V et al. Am J Surg. 2017. doi: 10.1016/j.amjsurg.2017.08.051.
FROM THE AMERICAN JOURNAL OF SURGERY
Key clinical point: .
Major finding: 80% of surgical residents said that the length of time needed to complete a robotics curriculum was a barrier to doing so.
Data source: Survey of 97 general surgery residents conducted in 2014-2015 and 2016-2017.
Disclosures: The researchers had no financial conflicts to disclose. The study was supported in part by an Intuitive Surgical Education Grant.
Source: Tam V et al. Am J Surg. 2017. doi: 10.1016/j.amjsurg.2017.08.051.