User login
New Guidance Recommends Metformin to Prevent Antipsychotic Weight Gain
A new evidence-based guideline recommends prescribing metformin when initiating antipsychotic treatment to help mitigate weight gain in certain instances.
There is “good evidence” that metformin can prevent weight gain caused by antipsychotics, first author Aoife Carolan, MPharm, with Saint John of God Hospital and the Royal College of Surgeons, Dublin, Ireland, said in an interview.
“While there have been some general recommendations to use metformin for this purpose, until now, clear guidance on how to prevent this side effect of treatment has been lacking,” Carolan said. “At present, it is likely that metformin is underused and when used, it is likely to be started after the weight gain occurs. Therefore, this guideline will reflect a new practice for most clinicians.”
The guideline was published online on December 9 in Schizophrenia Bulletin.
It offers three key recommendations:
- Initiate metformin when prescribing a high-risk weight-inducing antipsychotic, such as olanzapine or clozapine.
- Initiate metformin with a medium-risk antipsychotic (quetiapine, paliperidone, or risperidone) in patients with one or more cardiometabolic risk factors; in patients aged 10-25 years; or in patients with a body mass index (BMI) between 25 and 30.
- Initiate metformin with any antipsychotic if > 3% increase in baseline body weight is observed during the first 12 months of treatment.
The guideline authors noted that a recent Cochrane review of pharmacological interventions for the prevention of antipsychotic-induced weight gain showed that metformin is the only pharmacological agent that may be effective for preventing weight gain.
The review showed that starting metformin with antipsychotic medicines can reduce the extent of weight gain by 4.03 kg, compared with controls.
In terms of dose, the guideline recommends escalating from 500 mg daily to 500 mg twice daily over 2 weeks, followed by biweekly increases of 500 mg as tolerated up to 1 g twice daily at week 6.
Metformin should be discontinued if risks for lactic acidosis are present, or the condition is suspected; if BMI falls below 20; or if the antipsychotic medicine is discontinued. Metformin should be avoided where there is harmful use of alcohol.
While the guideline focused on metformin, it also recommends that, if available, glucagon-like peptide 1 (GLP-1) agonists, should be considered for patients with a BMI > 30, certain cardiometabolic diseases, or obstructive sleep apnea.
“At present, there is insufficient evidence for the risk benefit calculation for GLP-1 agonists as a preventative agent, but we will continue to monitor the evidence and update the guideline if it is needed,” Carolan said.
Experts Weigh In
This news organization asked several psychiatrists not involved in the guideline development for their thoughts on it.
Ipsit Vahia, MD, McLean Hospital, Belmont, and Harvard Medical School, Boston, both in Massachusetts, said: “There is an urgent need for evidence to guide treatments that can mitigate the metabolic side effects of antipsychotics.”
While metformin has shown some potential based on preliminary studies, this paper offers more substantial evidence to guide clinicians in using these medications and marks a significant step forward in clinical psychiatry, Vahia said.
Lynn DeLisi, MD, also with Harvard Medical School, emphasized that decisions about the use of metformin in patients taking antipsychotics should be made on an individual basis.
“It should not be used routinely with all antipsychotics, as metformin has its own set of side effects,” said DeLisi.
Xiaoduo Fan, MD, MPH, with UMass Chan Medical School, Worcester, Massachusetts, director of UMass MIND, noted that the evidence regarding metformin’s benefits to prevent or mitigate antipsychotic-induced weight gain and other metabolic disturbances is clear.
“It was somewhat controversial when psychiatrists started to prescribe metformin 15-20 years ago, but now many psychiatrists feel comfortable doing so. In many clinical settings, especially in academically affiliated hospitals, using metformin to address antipsychotic-associated metabolic concerns has become part of the routine practice,” said Fan.
“The guideline recommendations are generally consistent with what we have been doing clinically. The publication of the guideline may help promote wider use of metformin in the patient population we serve,” Fan added.
Fan also noted that a growing body of the literature has demonstrated the weight loss effect and other metabolic benefits of GLP-1 agonists. “Compared with metformin, GLP-1 agonists are more effective in inducing weight loss and mitigating cardiometabolic risks,” he said.
Fan said his group has completed a double-blind, placebo-controlled trial of 6-month weekly injection of the GLP-1 receptor agonist exenatide, as an adjunctive treatment in 70 patients with schizophrenia. “Preliminary data analysis suggests positive metabolic benefits,” he reported.
This research had no commercial funding. Carolan had no relevant disclosures. A complete list of disclosures for the guideline authors is available with the original article. DeLisi had no relevant disclosures. Fan had received research support from Alkermes, Eli Lilly, Janssen, Otsuka Pharmaceutical, Roche, Lundbeck, Boehringer Ingelheim, Neurocrine Biosciences, Intra-Cellular Therapies, Teva, and Bristol-Myers Squibb. He served on the BMJ Best Practice’s US Advisory Panel and as the contributor for the BMJ Best Practice — Schizophrenia Topic. Vahia had served as a consultant for Otsuka.
A version of this article appeared on Medscape.com.
A new evidence-based guideline recommends prescribing metformin when initiating antipsychotic treatment to help mitigate weight gain in certain instances.
There is “good evidence” that metformin can prevent weight gain caused by antipsychotics, first author Aoife Carolan, MPharm, with Saint John of God Hospital and the Royal College of Surgeons, Dublin, Ireland, said in an interview.
“While there have been some general recommendations to use metformin for this purpose, until now, clear guidance on how to prevent this side effect of treatment has been lacking,” Carolan said. “At present, it is likely that metformin is underused and when used, it is likely to be started after the weight gain occurs. Therefore, this guideline will reflect a new practice for most clinicians.”
The guideline was published online on December 9 in Schizophrenia Bulletin.
It offers three key recommendations:
- Initiate metformin when prescribing a high-risk weight-inducing antipsychotic, such as olanzapine or clozapine.
- Initiate metformin with a medium-risk antipsychotic (quetiapine, paliperidone, or risperidone) in patients with one or more cardiometabolic risk factors; in patients aged 10-25 years; or in patients with a body mass index (BMI) between 25 and 30.
- Initiate metformin with any antipsychotic if > 3% increase in baseline body weight is observed during the first 12 months of treatment.
The guideline authors noted that a recent Cochrane review of pharmacological interventions for the prevention of antipsychotic-induced weight gain showed that metformin is the only pharmacological agent that may be effective for preventing weight gain.
The review showed that starting metformin with antipsychotic medicines can reduce the extent of weight gain by 4.03 kg, compared with controls.
In terms of dose, the guideline recommends escalating from 500 mg daily to 500 mg twice daily over 2 weeks, followed by biweekly increases of 500 mg as tolerated up to 1 g twice daily at week 6.
Metformin should be discontinued if risks for lactic acidosis are present, or the condition is suspected; if BMI falls below 20; or if the antipsychotic medicine is discontinued. Metformin should be avoided where there is harmful use of alcohol.
While the guideline focused on metformin, it also recommends that, if available, glucagon-like peptide 1 (GLP-1) agonists, should be considered for patients with a BMI > 30, certain cardiometabolic diseases, or obstructive sleep apnea.
“At present, there is insufficient evidence for the risk benefit calculation for GLP-1 agonists as a preventative agent, but we will continue to monitor the evidence and update the guideline if it is needed,” Carolan said.
Experts Weigh In
This news organization asked several psychiatrists not involved in the guideline development for their thoughts on it.
Ipsit Vahia, MD, McLean Hospital, Belmont, and Harvard Medical School, Boston, both in Massachusetts, said: “There is an urgent need for evidence to guide treatments that can mitigate the metabolic side effects of antipsychotics.”
While metformin has shown some potential based on preliminary studies, this paper offers more substantial evidence to guide clinicians in using these medications and marks a significant step forward in clinical psychiatry, Vahia said.
Lynn DeLisi, MD, also with Harvard Medical School, emphasized that decisions about the use of metformin in patients taking antipsychotics should be made on an individual basis.
“It should not be used routinely with all antipsychotics, as metformin has its own set of side effects,” said DeLisi.
Xiaoduo Fan, MD, MPH, with UMass Chan Medical School, Worcester, Massachusetts, director of UMass MIND, noted that the evidence regarding metformin’s benefits to prevent or mitigate antipsychotic-induced weight gain and other metabolic disturbances is clear.
“It was somewhat controversial when psychiatrists started to prescribe metformin 15-20 years ago, but now many psychiatrists feel comfortable doing so. In many clinical settings, especially in academically affiliated hospitals, using metformin to address antipsychotic-associated metabolic concerns has become part of the routine practice,” said Fan.
“The guideline recommendations are generally consistent with what we have been doing clinically. The publication of the guideline may help promote wider use of metformin in the patient population we serve,” Fan added.
Fan also noted that a growing body of the literature has demonstrated the weight loss effect and other metabolic benefits of GLP-1 agonists. “Compared with metformin, GLP-1 agonists are more effective in inducing weight loss and mitigating cardiometabolic risks,” he said.
Fan said his group has completed a double-blind, placebo-controlled trial of 6-month weekly injection of the GLP-1 receptor agonist exenatide, as an adjunctive treatment in 70 patients with schizophrenia. “Preliminary data analysis suggests positive metabolic benefits,” he reported.
This research had no commercial funding. Carolan had no relevant disclosures. A complete list of disclosures for the guideline authors is available with the original article. DeLisi had no relevant disclosures. Fan had received research support from Alkermes, Eli Lilly, Janssen, Otsuka Pharmaceutical, Roche, Lundbeck, Boehringer Ingelheim, Neurocrine Biosciences, Intra-Cellular Therapies, Teva, and Bristol-Myers Squibb. He served on the BMJ Best Practice’s US Advisory Panel and as the contributor for the BMJ Best Practice — Schizophrenia Topic. Vahia had served as a consultant for Otsuka.
A version of this article appeared on Medscape.com.
A new evidence-based guideline recommends prescribing metformin when initiating antipsychotic treatment to help mitigate weight gain in certain instances.
There is “good evidence” that metformin can prevent weight gain caused by antipsychotics, first author Aoife Carolan, MPharm, with Saint John of God Hospital and the Royal College of Surgeons, Dublin, Ireland, said in an interview.
“While there have been some general recommendations to use metformin for this purpose, until now, clear guidance on how to prevent this side effect of treatment has been lacking,” Carolan said. “At present, it is likely that metformin is underused and when used, it is likely to be started after the weight gain occurs. Therefore, this guideline will reflect a new practice for most clinicians.”
The guideline was published online on December 9 in Schizophrenia Bulletin.
It offers three key recommendations:
- Initiate metformin when prescribing a high-risk weight-inducing antipsychotic, such as olanzapine or clozapine.
- Initiate metformin with a medium-risk antipsychotic (quetiapine, paliperidone, or risperidone) in patients with one or more cardiometabolic risk factors; in patients aged 10-25 years; or in patients with a body mass index (BMI) between 25 and 30.
- Initiate metformin with any antipsychotic if > 3% increase in baseline body weight is observed during the first 12 months of treatment.
The guideline authors noted that a recent Cochrane review of pharmacological interventions for the prevention of antipsychotic-induced weight gain showed that metformin is the only pharmacological agent that may be effective for preventing weight gain.
The review showed that starting metformin with antipsychotic medicines can reduce the extent of weight gain by 4.03 kg, compared with controls.
In terms of dose, the guideline recommends escalating from 500 mg daily to 500 mg twice daily over 2 weeks, followed by biweekly increases of 500 mg as tolerated up to 1 g twice daily at week 6.
Metformin should be discontinued if risks for lactic acidosis are present, or the condition is suspected; if BMI falls below 20; or if the antipsychotic medicine is discontinued. Metformin should be avoided where there is harmful use of alcohol.
While the guideline focused on metformin, it also recommends that, if available, glucagon-like peptide 1 (GLP-1) agonists, should be considered for patients with a BMI > 30, certain cardiometabolic diseases, or obstructive sleep apnea.
“At present, there is insufficient evidence for the risk benefit calculation for GLP-1 agonists as a preventative agent, but we will continue to monitor the evidence and update the guideline if it is needed,” Carolan said.
Experts Weigh In
This news organization asked several psychiatrists not involved in the guideline development for their thoughts on it.
Ipsit Vahia, MD, McLean Hospital, Belmont, and Harvard Medical School, Boston, both in Massachusetts, said: “There is an urgent need for evidence to guide treatments that can mitigate the metabolic side effects of antipsychotics.”
While metformin has shown some potential based on preliminary studies, this paper offers more substantial evidence to guide clinicians in using these medications and marks a significant step forward in clinical psychiatry, Vahia said.
Lynn DeLisi, MD, also with Harvard Medical School, emphasized that decisions about the use of metformin in patients taking antipsychotics should be made on an individual basis.
“It should not be used routinely with all antipsychotics, as metformin has its own set of side effects,” said DeLisi.
Xiaoduo Fan, MD, MPH, with UMass Chan Medical School, Worcester, Massachusetts, director of UMass MIND, noted that the evidence regarding metformin’s benefits to prevent or mitigate antipsychotic-induced weight gain and other metabolic disturbances is clear.
“It was somewhat controversial when psychiatrists started to prescribe metformin 15-20 years ago, but now many psychiatrists feel comfortable doing so. In many clinical settings, especially in academically affiliated hospitals, using metformin to address antipsychotic-associated metabolic concerns has become part of the routine practice,” said Fan.
“The guideline recommendations are generally consistent with what we have been doing clinically. The publication of the guideline may help promote wider use of metformin in the patient population we serve,” Fan added.
Fan also noted that a growing body of the literature has demonstrated the weight loss effect and other metabolic benefits of GLP-1 agonists. “Compared with metformin, GLP-1 agonists are more effective in inducing weight loss and mitigating cardiometabolic risks,” he said.
Fan said his group has completed a double-blind, placebo-controlled trial of 6-month weekly injection of the GLP-1 receptor agonist exenatide, as an adjunctive treatment in 70 patients with schizophrenia. “Preliminary data analysis suggests positive metabolic benefits,” he reported.
This research had no commercial funding. Carolan had no relevant disclosures. A complete list of disclosures for the guideline authors is available with the original article. DeLisi had no relevant disclosures. Fan had received research support from Alkermes, Eli Lilly, Janssen, Otsuka Pharmaceutical, Roche, Lundbeck, Boehringer Ingelheim, Neurocrine Biosciences, Intra-Cellular Therapies, Teva, and Bristol-Myers Squibb. He served on the BMJ Best Practice’s US Advisory Panel and as the contributor for the BMJ Best Practice — Schizophrenia Topic. Vahia had served as a consultant for Otsuka.
A version of this article appeared on Medscape.com.
FROM SCHIZOPHRENIA BULLETIN
Eliminate Clozapine REMS, FDA Panels Say
Two Food and Drug Administration (FDA) advisory panels are urging the agency to eliminate the risk management program for the antipsychotic drug clozapine, saying that restrictions are limiting access to a life-changing and life-saving medication for people with schizophrenia.
Members of the Drug Safety and Risk Management and Psychopharmacologic Drugs advisory committees held a joint meeting on November 19 to address whether frequently revised restrictions that have been in place since clozapine was introduced in 1989 should be changed again. Clozapine — the only FDA-approved drug for treatment-resistant schizophrenia — can cause severe neutropenia, so is subject to a Risk Evaluation and Management Strategy (REMS).
Calling the current rules overly burdensome, a majority of committee members voted against continuing a requirement that pharmacies and physicians must provide documentation of a patient’s absolute neutrophil count (ANC) results through the REMS. Monitoring should continue, as directed in the labeling, said the panel.
Panelists also voted overwhelmingly that it is not necessary to mandate physician education about clozapine’s risk of neutropenia and the need for ANC monitoring.
The panel did not vote, however, on whether the REMS should be eliminated altogether. The FDA did not pose that as a voting question for the panels’ consideration.
Following intense lobbying by the American Psychiatric Association (APA), the National Alliance on Mental Illness, and others, the FDA announced in 2022 that the agency would exercise “enforcement discretion” by allowing prescribers and pharmacists to skirt the clozapine REMS rules. But the agency doesn’t know whether the program is meeting its goals, said Tiffany R. Farchione, MD, director of the division of psychiatry at the FDA’s Center for Drug Evaluation and Research.
Among other things, the REMS requires that physicians and pharmacists be certified to prescribe and dispense the drug, that patients be enrolled, and that patient status forms be submitted monthly, showing ANC levels and appropriateness of continuing treatment.
At the meeting, FDA officials said that 148,000 outpatient clozapine prescriptions were written in 2023. But an estimated 814,000–1.2 million Americans have treatment-resistant schizophrenia, the main indication for clozapine.
“We know the drug is being underutilized,” said Farchione, adding that the agency wants to ensure that physicians and pharmacists “can use the drug, use it safely and help the patients who need it.”
REMS a ‘Hindrance’
As reported by this news organization, research presented earlier this year at the APA annual meeting showed that the risk of moderate and severe neutropenia is low to minimal in people taking clozapine for treatment-resistant schizophrenia. Those findings prompted the study’s investigators to suggest clozapine REMS should be reconsidered.
In the November 19th committee meeting, many panelists said that clozapine was no more dangerous than many antipsychotics and that the administrative requirements were preventing clinicians from prescribing.
“I have fantasized for years about abolishing the clozapine REMS,” said Jacob S. Ballon, MD, MPH, a temporary panel member and associate professor of psychiatry at Stanford University in California.
Panelists Jess Fiedorowicz, MD, PhD, professor and senior research chair in adult psychiatry at the University of Ottawa, Canada; Megan J. Ehret, PharmD, MS, a panelist and professor at the University of Maryland School of Pharmacy, Baltimore; and Rajesh Narendran, MD, a professor in radiology and psychiatry at the University of Pittsburgh School of Medicine in Pennsylvania, agreed.
“I strongly feel that the REMS at this point is just a hindrance,” Narendran said. “I think you should get rid of the REMS.”
However, panelist Walter Dunn, MD, PhD, staff psychiatrist at the VA Greater Los Angeles Healthcare System, cautioned that modifying or eliminating the REMS might not necessarily increase prescribers. If monitoring ANC levels is still recommended in labeling, clinicians will still regard it as the standard of care, said Dunn. And “there are a whole host of other issues associated with clozapine,” that he said were “more concerning.”
Many patients are accessing clozapine without going through the REMS, which is also of concern to the FDA and drug manufacturers.
“We estimate about 42,000 patients are not participating in the REMS, said James Shamp, VP of data intelligence and program analytics at United BioSource, a company that supports drug makers.
Leah Hart, PharmD, a risk management analyst with the FDA, told the panel that the agency estimates that 25%-35% of patients taking clozapine may not be participating in the REMS.
“Today, prescribers, pharmacies, and patients do not have to participate in the REMS in order patients to obtain clozapine,” Hart said.
Public Testimony Sways Panel
But psychiatrists, pharmacists, families, and patients who testified during the 90-minute open portion of the meeting disagreed with that assessment, saying the REMS program had a devastatingly chilling effect on clozapine access.
Patty Taggart of Las Vegas said her daughter had nine suicide attempts over the past 14 years, while having tried eight different antipsychotics. In August, after the most-recent attempt, Taggart begged the psychiatrist to prescribe clozapine to her daughter. The clinician refused, citing the REMS. After her daughter’s discharge, Taggart said she found another provider who would prescribe the medication.
Lisa Castellanos said her son Daniel had been treated with a variety of antipsychotics but denied clozapine until he was arrested in 2012 for assault during a psychotic break. The state used the medication to improve Daniel’s mental state so he could stand trial. But when he went to jail after accepting a plea deal, the prison stopped the clozapine. Daniel has since deteriorated and was recently ruled ineligible for parole.
Patients and families also described being rejected at pharmacies — most of which, despite the FDA’s supposed “enforcement discretion” continue to rigorously follow REMS requirements.
Many panelists said they were moved by patients and family testimony. A dozen or more members of the public were wearing black t-shirts with white writing that declared: “Clozapine is the safest antipsychotic in the world.”
‘Blood-for-Drug Program’
Brian Barnett, MD, director of the psychiatric treatment-resistance program at the Cleveland Clinic in Ohio, said during the public portion of the meeting that “many pharmacies simply refuse to dispense clozapine likely because of the administrative burden and lack of financial incentives.”
Others want faxed lab results even when the results have been filed electronically, he said. “One of the most dangerous features of the current REMS system is its inflexibility, driven by the so-called ‘no blood, no drug’ ethos which has been baked into the minds of America’s pharmacists.”
“This is a blood-for-drug program,” agreed Rachel Strieff of Tempe, Arizona, who noted that her advocacy group, Angry Moms, and others had submitted 4,000 signatures calling for the end of the REMS. “The largest category of patients harmed by the clozapine REMS have never taken a single dose,” she said, noting that millions of eligible individuals are not getting the drug.
Panel chair James Floyd, MD, professor of medicine at the University of Washington, Seattle, said the public testimony was “very moving.” Families and patients had described “the intensity of suffering that people go through prior to getting to clozapine,” he added.
“We have to listen to that,” said Floyd.
“I want you to know that we hear you,” said Farchione. “We’re here today because of you and your loved ones. And your stories are important, and your experience is important, and what you’ve shared today will have an impact on regulatory decision making.”
While the FDA typically follows its panels’ advice, it’s unclear if the agency will do so for clozapine REMS or when it will release its final decision.
A version of this article appeared on Medscape.com.
Two Food and Drug Administration (FDA) advisory panels are urging the agency to eliminate the risk management program for the antipsychotic drug clozapine, saying that restrictions are limiting access to a life-changing and life-saving medication for people with schizophrenia.
Members of the Drug Safety and Risk Management and Psychopharmacologic Drugs advisory committees held a joint meeting on November 19 to address whether frequently revised restrictions that have been in place since clozapine was introduced in 1989 should be changed again. Clozapine — the only FDA-approved drug for treatment-resistant schizophrenia — can cause severe neutropenia, so is subject to a Risk Evaluation and Management Strategy (REMS).
Calling the current rules overly burdensome, a majority of committee members voted against continuing a requirement that pharmacies and physicians must provide documentation of a patient’s absolute neutrophil count (ANC) results through the REMS. Monitoring should continue, as directed in the labeling, said the panel.
Panelists also voted overwhelmingly that it is not necessary to mandate physician education about clozapine’s risk of neutropenia and the need for ANC monitoring.
The panel did not vote, however, on whether the REMS should be eliminated altogether. The FDA did not pose that as a voting question for the panels’ consideration.
Following intense lobbying by the American Psychiatric Association (APA), the National Alliance on Mental Illness, and others, the FDA announced in 2022 that the agency would exercise “enforcement discretion” by allowing prescribers and pharmacists to skirt the clozapine REMS rules. But the agency doesn’t know whether the program is meeting its goals, said Tiffany R. Farchione, MD, director of the division of psychiatry at the FDA’s Center for Drug Evaluation and Research.
Among other things, the REMS requires that physicians and pharmacists be certified to prescribe and dispense the drug, that patients be enrolled, and that patient status forms be submitted monthly, showing ANC levels and appropriateness of continuing treatment.
At the meeting, FDA officials said that 148,000 outpatient clozapine prescriptions were written in 2023. But an estimated 814,000–1.2 million Americans have treatment-resistant schizophrenia, the main indication for clozapine.
“We know the drug is being underutilized,” said Farchione, adding that the agency wants to ensure that physicians and pharmacists “can use the drug, use it safely and help the patients who need it.”
REMS a ‘Hindrance’
As reported by this news organization, research presented earlier this year at the APA annual meeting showed that the risk of moderate and severe neutropenia is low to minimal in people taking clozapine for treatment-resistant schizophrenia. Those findings prompted the study’s investigators to suggest clozapine REMS should be reconsidered.
In the November 19th committee meeting, many panelists said that clozapine was no more dangerous than many antipsychotics and that the administrative requirements were preventing clinicians from prescribing.
“I have fantasized for years about abolishing the clozapine REMS,” said Jacob S. Ballon, MD, MPH, a temporary panel member and associate professor of psychiatry at Stanford University in California.
Panelists Jess Fiedorowicz, MD, PhD, professor and senior research chair in adult psychiatry at the University of Ottawa, Canada; Megan J. Ehret, PharmD, MS, a panelist and professor at the University of Maryland School of Pharmacy, Baltimore; and Rajesh Narendran, MD, a professor in radiology and psychiatry at the University of Pittsburgh School of Medicine in Pennsylvania, agreed.
“I strongly feel that the REMS at this point is just a hindrance,” Narendran said. “I think you should get rid of the REMS.”
However, panelist Walter Dunn, MD, PhD, staff psychiatrist at the VA Greater Los Angeles Healthcare System, cautioned that modifying or eliminating the REMS might not necessarily increase prescribers. If monitoring ANC levels is still recommended in labeling, clinicians will still regard it as the standard of care, said Dunn. And “there are a whole host of other issues associated with clozapine,” that he said were “more concerning.”
Many patients are accessing clozapine without going through the REMS, which is also of concern to the FDA and drug manufacturers.
“We estimate about 42,000 patients are not participating in the REMS, said James Shamp, VP of data intelligence and program analytics at United BioSource, a company that supports drug makers.
Leah Hart, PharmD, a risk management analyst with the FDA, told the panel that the agency estimates that 25%-35% of patients taking clozapine may not be participating in the REMS.
“Today, prescribers, pharmacies, and patients do not have to participate in the REMS in order patients to obtain clozapine,” Hart said.
Public Testimony Sways Panel
But psychiatrists, pharmacists, families, and patients who testified during the 90-minute open portion of the meeting disagreed with that assessment, saying the REMS program had a devastatingly chilling effect on clozapine access.
Patty Taggart of Las Vegas said her daughter had nine suicide attempts over the past 14 years, while having tried eight different antipsychotics. In August, after the most-recent attempt, Taggart begged the psychiatrist to prescribe clozapine to her daughter. The clinician refused, citing the REMS. After her daughter’s discharge, Taggart said she found another provider who would prescribe the medication.
Lisa Castellanos said her son Daniel had been treated with a variety of antipsychotics but denied clozapine until he was arrested in 2012 for assault during a psychotic break. The state used the medication to improve Daniel’s mental state so he could stand trial. But when he went to jail after accepting a plea deal, the prison stopped the clozapine. Daniel has since deteriorated and was recently ruled ineligible for parole.
Patients and families also described being rejected at pharmacies — most of which, despite the FDA’s supposed “enforcement discretion” continue to rigorously follow REMS requirements.
Many panelists said they were moved by patients and family testimony. A dozen or more members of the public were wearing black t-shirts with white writing that declared: “Clozapine is the safest antipsychotic in the world.”
‘Blood-for-Drug Program’
Brian Barnett, MD, director of the psychiatric treatment-resistance program at the Cleveland Clinic in Ohio, said during the public portion of the meeting that “many pharmacies simply refuse to dispense clozapine likely because of the administrative burden and lack of financial incentives.”
Others want faxed lab results even when the results have been filed electronically, he said. “One of the most dangerous features of the current REMS system is its inflexibility, driven by the so-called ‘no blood, no drug’ ethos which has been baked into the minds of America’s pharmacists.”
“This is a blood-for-drug program,” agreed Rachel Strieff of Tempe, Arizona, who noted that her advocacy group, Angry Moms, and others had submitted 4,000 signatures calling for the end of the REMS. “The largest category of patients harmed by the clozapine REMS have never taken a single dose,” she said, noting that millions of eligible individuals are not getting the drug.
Panel chair James Floyd, MD, professor of medicine at the University of Washington, Seattle, said the public testimony was “very moving.” Families and patients had described “the intensity of suffering that people go through prior to getting to clozapine,” he added.
“We have to listen to that,” said Floyd.
“I want you to know that we hear you,” said Farchione. “We’re here today because of you and your loved ones. And your stories are important, and your experience is important, and what you’ve shared today will have an impact on regulatory decision making.”
While the FDA typically follows its panels’ advice, it’s unclear if the agency will do so for clozapine REMS or when it will release its final decision.
A version of this article appeared on Medscape.com.
Two Food and Drug Administration (FDA) advisory panels are urging the agency to eliminate the risk management program for the antipsychotic drug clozapine, saying that restrictions are limiting access to a life-changing and life-saving medication for people with schizophrenia.
Members of the Drug Safety and Risk Management and Psychopharmacologic Drugs advisory committees held a joint meeting on November 19 to address whether frequently revised restrictions that have been in place since clozapine was introduced in 1989 should be changed again. Clozapine — the only FDA-approved drug for treatment-resistant schizophrenia — can cause severe neutropenia, so is subject to a Risk Evaluation and Management Strategy (REMS).
Calling the current rules overly burdensome, a majority of committee members voted against continuing a requirement that pharmacies and physicians must provide documentation of a patient’s absolute neutrophil count (ANC) results through the REMS. Monitoring should continue, as directed in the labeling, said the panel.
Panelists also voted overwhelmingly that it is not necessary to mandate physician education about clozapine’s risk of neutropenia and the need for ANC monitoring.
The panel did not vote, however, on whether the REMS should be eliminated altogether. The FDA did not pose that as a voting question for the panels’ consideration.
Following intense lobbying by the American Psychiatric Association (APA), the National Alliance on Mental Illness, and others, the FDA announced in 2022 that the agency would exercise “enforcement discretion” by allowing prescribers and pharmacists to skirt the clozapine REMS rules. But the agency doesn’t know whether the program is meeting its goals, said Tiffany R. Farchione, MD, director of the division of psychiatry at the FDA’s Center for Drug Evaluation and Research.
Among other things, the REMS requires that physicians and pharmacists be certified to prescribe and dispense the drug, that patients be enrolled, and that patient status forms be submitted monthly, showing ANC levels and appropriateness of continuing treatment.
At the meeting, FDA officials said that 148,000 outpatient clozapine prescriptions were written in 2023. But an estimated 814,000–1.2 million Americans have treatment-resistant schizophrenia, the main indication for clozapine.
“We know the drug is being underutilized,” said Farchione, adding that the agency wants to ensure that physicians and pharmacists “can use the drug, use it safely and help the patients who need it.”
REMS a ‘Hindrance’
As reported by this news organization, research presented earlier this year at the APA annual meeting showed that the risk of moderate and severe neutropenia is low to minimal in people taking clozapine for treatment-resistant schizophrenia. Those findings prompted the study’s investigators to suggest clozapine REMS should be reconsidered.
In the November 19th committee meeting, many panelists said that clozapine was no more dangerous than many antipsychotics and that the administrative requirements were preventing clinicians from prescribing.
“I have fantasized for years about abolishing the clozapine REMS,” said Jacob S. Ballon, MD, MPH, a temporary panel member and associate professor of psychiatry at Stanford University in California.
Panelists Jess Fiedorowicz, MD, PhD, professor and senior research chair in adult psychiatry at the University of Ottawa, Canada; Megan J. Ehret, PharmD, MS, a panelist and professor at the University of Maryland School of Pharmacy, Baltimore; and Rajesh Narendran, MD, a professor in radiology and psychiatry at the University of Pittsburgh School of Medicine in Pennsylvania, agreed.
“I strongly feel that the REMS at this point is just a hindrance,” Narendran said. “I think you should get rid of the REMS.”
However, panelist Walter Dunn, MD, PhD, staff psychiatrist at the VA Greater Los Angeles Healthcare System, cautioned that modifying or eliminating the REMS might not necessarily increase prescribers. If monitoring ANC levels is still recommended in labeling, clinicians will still regard it as the standard of care, said Dunn. And “there are a whole host of other issues associated with clozapine,” that he said were “more concerning.”
Many patients are accessing clozapine without going through the REMS, which is also of concern to the FDA and drug manufacturers.
“We estimate about 42,000 patients are not participating in the REMS, said James Shamp, VP of data intelligence and program analytics at United BioSource, a company that supports drug makers.
Leah Hart, PharmD, a risk management analyst with the FDA, told the panel that the agency estimates that 25%-35% of patients taking clozapine may not be participating in the REMS.
“Today, prescribers, pharmacies, and patients do not have to participate in the REMS in order patients to obtain clozapine,” Hart said.
Public Testimony Sways Panel
But psychiatrists, pharmacists, families, and patients who testified during the 90-minute open portion of the meeting disagreed with that assessment, saying the REMS program had a devastatingly chilling effect on clozapine access.
Patty Taggart of Las Vegas said her daughter had nine suicide attempts over the past 14 years, while having tried eight different antipsychotics. In August, after the most-recent attempt, Taggart begged the psychiatrist to prescribe clozapine to her daughter. The clinician refused, citing the REMS. After her daughter’s discharge, Taggart said she found another provider who would prescribe the medication.
Lisa Castellanos said her son Daniel had been treated with a variety of antipsychotics but denied clozapine until he was arrested in 2012 for assault during a psychotic break. The state used the medication to improve Daniel’s mental state so he could stand trial. But when he went to jail after accepting a plea deal, the prison stopped the clozapine. Daniel has since deteriorated and was recently ruled ineligible for parole.
Patients and families also described being rejected at pharmacies — most of which, despite the FDA’s supposed “enforcement discretion” continue to rigorously follow REMS requirements.
Many panelists said they were moved by patients and family testimony. A dozen or more members of the public were wearing black t-shirts with white writing that declared: “Clozapine is the safest antipsychotic in the world.”
‘Blood-for-Drug Program’
Brian Barnett, MD, director of the psychiatric treatment-resistance program at the Cleveland Clinic in Ohio, said during the public portion of the meeting that “many pharmacies simply refuse to dispense clozapine likely because of the administrative burden and lack of financial incentives.”
Others want faxed lab results even when the results have been filed electronically, he said. “One of the most dangerous features of the current REMS system is its inflexibility, driven by the so-called ‘no blood, no drug’ ethos which has been baked into the minds of America’s pharmacists.”
“This is a blood-for-drug program,” agreed Rachel Strieff of Tempe, Arizona, who noted that her advocacy group, Angry Moms, and others had submitted 4,000 signatures calling for the end of the REMS. “The largest category of patients harmed by the clozapine REMS have never taken a single dose,” she said, noting that millions of eligible individuals are not getting the drug.
Panel chair James Floyd, MD, professor of medicine at the University of Washington, Seattle, said the public testimony was “very moving.” Families and patients had described “the intensity of suffering that people go through prior to getting to clozapine,” he added.
“We have to listen to that,” said Floyd.
“I want you to know that we hear you,” said Farchione. “We’re here today because of you and your loved ones. And your stories are important, and your experience is important, and what you’ve shared today will have an impact on regulatory decision making.”
While the FDA typically follows its panels’ advice, it’s unclear if the agency will do so for clozapine REMS or when it will release its final decision.
A version of this article appeared on Medscape.com.
‘Round Face’: A Viral Term’s Real Diagnostic Implications
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.
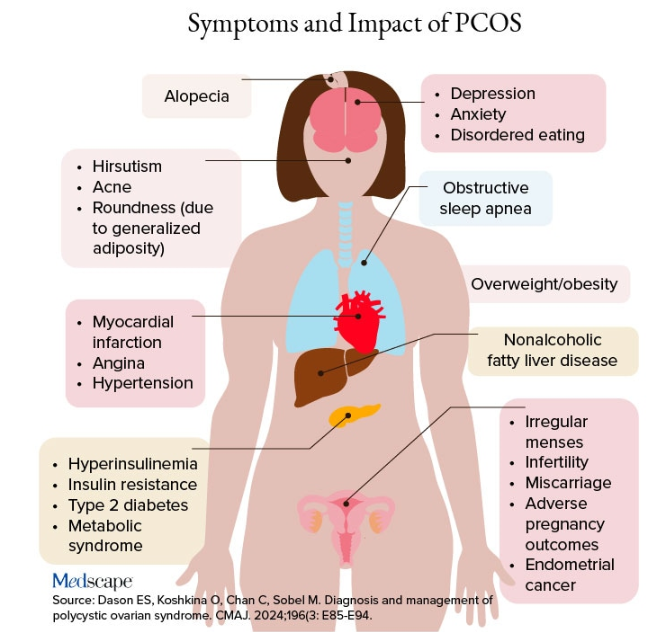
PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.

PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
“Cortisol” has become a household word, popularized by social media and tagged in videos that garnered nearly 800 million views in 2023. This is linked to the also-trending term “moon face,” which TikTok influencers and others have suggested is caused by high cortisol levels and, conversely, can be reduced through stress reduction.
“When we hear the term ‘moon face,’ we’re typically referring to Cushing syndrome [CS] or treatment with prolonged high-dose glucocorticoids,” said Anat Ben-Shlomo, MD, co-director of the Multidisciplinary Adrenal Program, Pituitary Center, Division of Endocrinology, Diabetes and Metabolism at Cedars-Sinai Medical Center, Los Angeles. Medscape Medical News previously discussed moon face in an article detailing how to diagnose CS.
Ben-Shlomo noted that the labels “moon face” and “moon facies” should be avoided for their potentially derogatory, unprofessional-sounding connotations, and that the preferred terms are “rounded face” or “round plethoric face.”
There are several disorders that can be associated with facial roundness, not all of which relate to elevated cortisol.
“It’s important for clinicians to be able distinguish between presentations due to other pathophysiologies, identify the unique constellation of Cushing-associated signs and symptoms, engage in a differential diagnosis, and treat whatever the condition is appropriately,” Katherine Sherif, MD, professor and vice chair of academic affairs, Department of Medicine, Thomas Jefferson University, Philadelphia, said in an interview.
The Unique Presentation of CS
CS results from “prolonged elevation” in plasma cortisol levels caused by either exogenous steroid use or excess endogenous steroid production.
“The shape of the face isn’t the only feature associated with CS,” Ben-Shlomo said. “There’s central obesity, particularly in the neck, supraclavicular area, chest, and abdomen. You sometimes see a posterior cervical thoracic fat pad, colloquially — but unprofessionally — called a ‘cervical hump.’ Simultaneously, the arms and legs are getting thinner.” The development of a round, plethoric face is common in long-standing significant CS, and a reddening of the skin can appear.
Additional symptoms include hirsutism and acne. “These can also be seen in other conditions, such as PCOS [polycystic ovary syndrome] but, combined with the other facial features, are more suggestive of CS,” Ben-Shlomo said.
Deep, wide purple striae appear in the trunk, breast, upper arms, and thighs, but not in the face, Ben-Shlomo advised. These appear as the fragile, thinning under-skin breaks when the patient gains weight.
Additional metabolic issues that can occur comorbidly include insulin resistance and diabetes, hypertension, osteoporosis, dyslipidemia, ecchymoses, increased susceptibility to infections, mood changes, cognitive dysfunction, low libido, infertility, weakness of muscles in the shoulders and thighs, episodes of bleeding and/or clotting, and an increased risk for heart attacks and strokes, Ben-Shlomo said.
“Not everyone presents with full-blown disease, but if you see any of these symptoms, be suspicious of CS and conduct a biochemical evaluation.” Three screening tests to use as a starting point are recommended by the Pituitary Society’s updated Consensus on Diagnosis and Management of Cushing’s Disease. The tests should be repeated to account for intra-patient variability. If two or all three tests are positive, clinicians should be suspicious of CS and move to additional testing to identify the underlying cause, Ben-Shlomo said.
‘Subclinical’ CS
Ben-Shlomo highlighted a condition called minimal autonomous cortisol secretion (formerly “subclinical CS”). “This condition is found when a person has an adrenal nodule that produces cortisol in excess, however not to levels observed in CS. An abnormal finding on the overnight 1-mg low-dose dexamethasone suppression test (LDDST) will identify this disorder, showing mildly unsuppressed morning cortisol level, while all other tests will be within normal range.”
She described minimal autonomous cortisol secretion as a form of “smoldering CS,” which has become more commonly diagnosed. “The condition needs to be treated because the patient can develop insulin resistance, metabolic syndrome, and osteoporosis over time.”
Once a cause has been determined, the optimal course of action is to take a multidisciplinary approach because CS affects multiple systems.
‘Pseudo-Cushing Syndrome’
A variety of abnormalities of the hypothalamus-pituitary adrenal (HPA) axis can be associated with hypercortisolemia and a rounder facial appearance but aren’t actually CS, Ben-Shlomo said.
Often called “pseudo-Cushing syndrome,” these conditions have recently been renamed “non-neoplastic hypercortisolism” or “physiologic non-neoplastic endogenous hypercortisolism.” They share some clinical and biochemical features of CS, but the hypercortisolemia is usually secondary to other factors. They increase the secretion of hypothalamic corticotropin-releasing hormone, which stimulates adrenocorticotropic hormone (ACTH) and adrenal cortisol secretion.
Identifying PCOS
PCOS is often associated with central obesity, Sherif noted, but not all women with PCOS have overweight or a central distribution of fat.
“Ask about menstrual periods and whether they come monthly,” Sherif advised. “If women using hormonal contraception say they have a regular cycle, ask if their cycle was regular prior to starting contraception. So many women with PCOS are undiagnosed because they started contraception in their teens to ‘regulate their periods’ and never realized they had PCOS.”
Additional symptoms of PCOS and its impact are found in the figure below.

PCOS is diagnosed when two of the following three Rotterdam criteria are met, and other diagnoses are excluded:
- Irregular menstrual cycles
- Clinical hyperandrogenism or biochemical hyperandrogenism
- Polycystic ovarian morphology on transvaginal ultrasonography or high anti-mullerian hormone (applicable only if patient is ≥ 8 years from menarche)
If PCOS is suspected, further tests can be conducted to confirm or rule out the diagnosis.
Alcohol Abuse: Alcohol abuse stimulates hypothalamic corticotropin-releasing hormone, leading to increased ACTH levels. It’s associated with a higher fasting cortisol level, particularly at 8:30 AM or so, and attributable to impaired cortisol clearance due to alcohol-related hepatic dysfunction. The LDDST will show abnormal cortisol suppression.
Sherif advised asking patients about alcohol use, recommending treatment for alcohol use disorder, and repeating clinical and biochemical workup after patients have discontinued alcohol consumption for ≥ 1 month.
Eating Disorders Mimicking CS: Eating disorders, particularly anorexia nervosa, are associated with endocrine abnormalities, amenorrhea, impaired body temperature regulation, and hypercortisolism, likely due to chronic fasting-related stress. Dysregulation of the HPA axis may linger, even after weight recovery.
It’s unlikely that patients with anorexia will display the “rounded face” associated with hypercortisolism, but some research suggests that anorexia can result in a disproportionate accumulation of central adiposity after recovery from the illness.
Neuropsychiatric Disorders: Major depressive disorder (MDD) is associated with HPA axis hyperactivity, with 20%-30% of patients with MDD showing hypercortisolemia. The post-awakening cortisol surge is more pronounced in those with MDD, and about half of patients with MDD also have high evening cortisol levels, suggesting disrupted diurnal cortisol rhythms.
Some patients with MDD have greater resistance to the feedback action of glucocorticoids on HPA axis activity, with weaker sensitivity often restored by effective pharmacotherapy of the depressive condition. Neuropsychiatric disorders are also associated with reduced activity of cortisol-deactivating enzymes. Posttraumatic stress disorder and anxiety are similarly associated with hypercortisolemia.
Addressing neuropsychiatric conditions with appropriate pharmacotherapy and psychotherapy can restore cortisol levels to normal proportions.
Diabetes, Obesity, and Metabolic Syndrome: Diabetes, obesity, and metabolic syndrome can occur comorbidly with CS, and many patients with these conditions may display both a rounder face, some central adiposity, and hypercortisolemia. For example, obesity is often related to a hyperresponsive HPA axis, with elevated cortisol secretion but normal-to-low circulatory concentrations.
Obesity is associated with increased cortisol reactivity after acute physical and/or psychosocial stressors but preserved pituitary sensitivity to feedback inhibition by the LDDST. When these conditions are appropriately managed with pharmacotherapy and lifestyle changes, cortisol levels should normalize, according to the experts.
Hypothyroidism: Hypothyroidism— Hashimoto disease as well as the subclinical variety — can be associated with weight gain, which may take the form of central obesity. Some research suggests a bidirectional relationship between hypothyroidism and obesity.
“Years ago, we didn’t conduct thyroid tests very often but now they’re easy to do, so we usually catch people with hypothyroidism at the beginning of the condition,” Sherif said. “If the patient’s thyroid hasn’t been checked in a year or so, thyroid hormone testing should be conducted.”
Thyroid disease can easily be managed with the administration of thyroid hormones.
Obstructive Sleep Apnea (OSA): OSA has an impact on HPA axis activation, especially when accompanied by obesity and hypertension. A meta-analysis of 22 studies, encompassing over 600 participants, found that continuous positive airway pressure treatment in patients with OSA reduced cortisol levels as well as blood pressure.
Treatment With Exogenous Corticosteroids: Oral corticosteroid treatment is a cornerstone of therapy in transplant, rheumatic, and autoimmune diseases. The impact of chronic exposure to exogenous glucocorticoids is similar to that with endogenous glucocorticoids.
Sherif said corticosteroid treatment can cause facial roundness in as little as 2 weeks and is characteristic in people taking these agents for longer periods. Although the effects are most pronounced with oral agents, systemic effects can be associated with inhaled corticosteroids as well.
Finding alternative anti-inflammatory treatments is advisable, if possible. The co-administration of metformin might lead to improvements in both the metabolic profile and the clinical outcomes of patients receiving glucocorticoids for inflammatory conditions.
Educating Patients: “There’s much we still don’t know about hypercortisolemia and CS, including the reasons for its impact on metabolic derangement and for the accumulation of fat in particular adipose patterns,” Ben-Shlomo said. “But experienced endocrinologists do know relatively well how to diagnose the condition, distinguish it from other conditions presenting with central obesity or a rounder face, and treat it.”
Given the casual use of the terms “moon face” and “extra cortisol” on social media, it’s important for physicians to educate patients about what elevated cortisol does and doesn’t do, and design treatment strategies accordingly.
Neither Ben-Shlomo nor Sherif reported having any disclosures.
A version of this article appeared on Medscape.com.
More Evidence Avatar Therapy Quiets Auditory Hallucinations in Psychosis
, results from the largest study of avatar therapy to date show.
The therapy allows patients to interact with a “digital embodiment” of the voice they hear, which is represented by a computer-generated face, also known as an avatar.
In the randomized, multisite, phase 2/3 AVATAR2 trial, patients who received AVATAR-Extended therapy, which included a personalized series of voiced dialogues based on their life history, plus treatment as usual (TAU) showed significantly greater improvement in distress and voice severity levels at 16 weeks vs those who received TAU only. They also had significant reductions in voice frequency at 16 and 28 weeks.
Patients in a third arm who were assigned to TAU plus AVATAR-Brief therapy, which included six sessions of a standardized version of the therapy, also showed improvements at 16 weeks, compared with TAU alone — but the clinical impact was stronger with the extended version.
“I was surprised at the extent to which the extended version seemed to be a more optimal version, and it should be the way forward with this therapy,” said study investigator Philippa A. Garety, PhD, professor emerita of clinical psychology at the Institute of Psychiatry, Psychology, and Neuroscience at King’s College, London, England.
With more than 300 participants, AVATAR2 is the largest trial to access avatar therapy to date, Garety noted.
“What’s unique about this work is that technology allows us to create safe face-to-face encounters with a representation of a person’s voice and allows them to relate to that voice in a new way,” she added.
The findings were published online in Nature Medicine.
A Decade of Research
Auditory verbal hallucinations are common in patients with schizophrenia, but currently available therapies can be ineffective, investigators wrote.
The therapy allows patients to customize how the avatar looks and sounds. Face-to-face dialogues are then conducted between the patients and avatars in order to build empowerment. A trained therapist provides support during these sessions.
As previously reported, the creator of avatar therapy, Julian Leff, MD, presented promising results from a pilot study of 26 patients at the International Congress of the Royal College of Psychiatrists in 2014.
“Opening up a dialogue between a patient and the voice they’ve been hearing is powerful,” said Leff, who was emeritus professor at the Institute of Psychiatry in London at the time.
In 2018, a randomized single-site study (AVATAR1) of 150 participants showed that the intervention was associated with a greater decrease in voice severity at 12 weeks vs supportive therapy. Past research led to the idea of incorporating personalization to better optimize the experience.
Garety noted that AVATAR2 is the largest trial to date of the therapy, as well as the first multisite trial to test the intervention, which was important in order to determine whether it could work outside of a research setting.
The study included 345 participants (61.4% men; mean age, 39.6 years) from three sites in England and one in Scotland. All were randomly assigned to receive TAU alone (n = 115), TAU plus AVATAR-Brief (n = 116), or TAU plus AVATAR-Extended (n = 114).
TAU typically included use of antipsychotics, as well as outpatient psychiatric visits and follow-up by case managers and care coordinators.
“We didn’t interfere with treatment as usual. We wanted to test whether adding this therapy to [TAU] would enhance effects and provide better treatment for their voices,” Garety noted.
AVATAR-Brief included a standardized process that focused on such things as self-esteem and assertiveness. AVATAR-Extended had two phases. In the first, participants received AVATAR-Brief therapy, whereas the second phase offered a more personalized intervention.
An ‘Unusual Finding’
The study’s primary outcome was voice-related distress at 16 and 28 weeks. Although the TAU plus AVATAR-Extended group did show a significant decrease in distress at 16 weeks vs TAU alone (–1.6 points; P = .029), the improvement was no longer significant at the 28-week follow-up (P = .175). The same was also true for the key secondary outcome of reduction in voice severity (–2.32 points; P = .009 at 16 weeks but P =.1 at 28 weeks).
The investigators noted that this might be caused by the number of dropouts in the AVATAR-Extended group by the 28-week timepoint. The completion rate for those patients was only 58%. The completion rate for the shorter, AVATAR-Brief group was 82%.
On the other hand, the other key secondary outcome of voice frequency was significantly reduced with AVATAR-Extended at both 16 weeks (–0.62 point; P = .01) and 28 weeks (–0.89 point; P = .003).
“This is an unusual finding. We’re not aware of any other psychological therapy that shows a reduction in the occurrence of the voice,” Garety said.
For TAU plus AVATAR-Brief, there were improvements at 16 weeks for distress (-1.05 points; P = .035) vs TAU alone. However, the researchers noted that this version of the therapy was just below the prespecified threshold for a clinically significant change and was at the threshold for statistical significance.
Although the shorter therapy was associated with a reduction in voice severity level at 16 weeks (–2.04 points; P = .017) vs TAU alone, there was no reduction in distress or voice severity at 28 weeks. There was no improvement in voice frequency at either timepoint.
Both the brief and the extended versions of AVATAR therapy showed improved mood and anxiety levels at 16 weeks and sustained improvement in well-being and recovery, the researchers noted.
“The short version, as expected, did deliver benefits posttreatment, but clearly the extended, optimized version outperformed the brief version. It had stronger and more lasting effects across quite a wide range of outcomes that matter to people who hear voices,” Garety said.
“In the extended version, people felt more empowered. And in just that version, the frequency of voices was reduced, which is a very important outcome,” she added.
Safety Issues?
There were 58 serious adverse events (SAEs) in total, with 51% of those occurring in the AVATAR-Extended group. Two participants in that group died; however, independent reviews deemed these events as not related to the intervention.
In addition, there were no “definitely related” SAEs and only a small number of “possibly related” SAEs, which typically included hospitalization with other contributory factors.
Garety noted during a press briefing that AVATAR therapy has now been demonstrated to be safe across two large trials.
Study limitations cited included no direct comparison between AVATAR-Brief and AVATAR-Extended or between AVATAR therapy and a different type of psychological treatment.
Overall, “we recommend that future development and provision of AVATAR therapy is primarily guided” by the AVATAR-Extended protocol, the investigators wrote.
Because the therapy was recommended by a National Institute for Health and Care Excellence Early Value Assessment, the investigators are now seeking to provide it in routine National Health Service settings to gather further real-world evidence of effectiveness over the next 3 years.
Next Steps
Although the intervention isn’t currently available to everybody who might be seeking it, “there’s a pipeline of movement from research into treatment and it’s moving towards the next stage of delivery,” Garety said.
Investigators are also looking into cultural adaptations for the therapy so it can be used in different locales, including Ethiopia and India, she added. There isn’t a US version yet, but Garety noted that investigators in Canada are looking at similar research and suspects that will also occur in the United States soon.
“We’re pioneers in this work, and it now needs to be going international and into services,” she said. “We have had many people who hear voices say what an amazing experience this has been. So, I feel very proud and excited to have been able to be part of this.”
At the press briefing, Miranda Wolpert, director of mental health at Wellcome, which funded the study, noted that it is encouraging to see the development of a new intervention that could potentially change the lives of patients across the world.
“We know that psychosis can start early in life, stopping people from having the jobs and relationships they want and from achieving the goals they want. This intervention was developed with those people to help them address an issue that really troubles them,” Wolpert said.
“For me, this represents part of a revolution we are starting to see in terms of mental health interventions and the potential impact on mental health science,” she added.
Digital Placebo Effect?
Commenting on the findings, John Torous, MD, a psychiatrist and director of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, Massachusetts, said there is a need for new treatments for schizophrenia that work with different mechanisms.
“We have a lot of medication studies but not as many innovative therapy studies. I think it’s exciting that the results, at least in the shorter-term outcome, were positive. And I think that’s something that can give people hope in using these new technologies,” said Torous, who is also an assistant professor of psychiatry at Harvard Medical School, Boston, and was not involved with the research.
Still, he did note some study limitations, including whether there could have been some type of “digital placebo effect” from the therapy.
“If you tell people they’re getting high-tech advanced digital care, that may have some effect,” he said, adding that “it’s always interesting” to tease out the benefit being delivered by the technology vs the delivery mechanism itself — or some combination of both.
Torous added, though, that it’s very difficult to have a rigorous digital control group. “It’s not necessarily a fault of their study, but it’s something to keep in mind when interpreting what the results are.”
He also noted that he would have liked to have seen a direct comparison between this new kind of psychological therapy vs standard psychological therapy, such as cognitive-behavioral therapy.
In addition, he wondered about expenses and scalability of the intervention, and whether patients would need to go to a specialized center to undergo treatment. Torous mentioned that a version involving virtual reality could perhaps make this more scalable in the future.
Overall, he said that what the investigators are currently doing is very innovative. “It’s exciting that we’re talking about the next steps. Giving people new options for psychological therapy that may be effective for their disorders is really wonderful to see,” Torous said.
The study was funded by the National Institute for Health and Care Research (NIHR), the Wellcome Trust King’s Clinical Research Facility, the NIHR Maudsley Biomedical Research Centre and Maudsley NHS Foundation Trust, King’s College London, the Manchester Biomedical Research Centre, and NHS Research Scotland, as well as by a grant from Wellcome. Garety reports being an unpaid scientific adviser to Avatar Therapy. Financial disclosures for the other investigators are fully listed in the original article. Torous reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
, results from the largest study of avatar therapy to date show.
The therapy allows patients to interact with a “digital embodiment” of the voice they hear, which is represented by a computer-generated face, also known as an avatar.
In the randomized, multisite, phase 2/3 AVATAR2 trial, patients who received AVATAR-Extended therapy, which included a personalized series of voiced dialogues based on their life history, plus treatment as usual (TAU) showed significantly greater improvement in distress and voice severity levels at 16 weeks vs those who received TAU only. They also had significant reductions in voice frequency at 16 and 28 weeks.
Patients in a third arm who were assigned to TAU plus AVATAR-Brief therapy, which included six sessions of a standardized version of the therapy, also showed improvements at 16 weeks, compared with TAU alone — but the clinical impact was stronger with the extended version.
“I was surprised at the extent to which the extended version seemed to be a more optimal version, and it should be the way forward with this therapy,” said study investigator Philippa A. Garety, PhD, professor emerita of clinical psychology at the Institute of Psychiatry, Psychology, and Neuroscience at King’s College, London, England.
With more than 300 participants, AVATAR2 is the largest trial to access avatar therapy to date, Garety noted.
“What’s unique about this work is that technology allows us to create safe face-to-face encounters with a representation of a person’s voice and allows them to relate to that voice in a new way,” she added.
The findings were published online in Nature Medicine.
A Decade of Research
Auditory verbal hallucinations are common in patients with schizophrenia, but currently available therapies can be ineffective, investigators wrote.
The therapy allows patients to customize how the avatar looks and sounds. Face-to-face dialogues are then conducted between the patients and avatars in order to build empowerment. A trained therapist provides support during these sessions.
As previously reported, the creator of avatar therapy, Julian Leff, MD, presented promising results from a pilot study of 26 patients at the International Congress of the Royal College of Psychiatrists in 2014.
“Opening up a dialogue between a patient and the voice they’ve been hearing is powerful,” said Leff, who was emeritus professor at the Institute of Psychiatry in London at the time.
In 2018, a randomized single-site study (AVATAR1) of 150 participants showed that the intervention was associated with a greater decrease in voice severity at 12 weeks vs supportive therapy. Past research led to the idea of incorporating personalization to better optimize the experience.
Garety noted that AVATAR2 is the largest trial to date of the therapy, as well as the first multisite trial to test the intervention, which was important in order to determine whether it could work outside of a research setting.
The study included 345 participants (61.4% men; mean age, 39.6 years) from three sites in England and one in Scotland. All were randomly assigned to receive TAU alone (n = 115), TAU plus AVATAR-Brief (n = 116), or TAU plus AVATAR-Extended (n = 114).
TAU typically included use of antipsychotics, as well as outpatient psychiatric visits and follow-up by case managers and care coordinators.
“We didn’t interfere with treatment as usual. We wanted to test whether adding this therapy to [TAU] would enhance effects and provide better treatment for their voices,” Garety noted.
AVATAR-Brief included a standardized process that focused on such things as self-esteem and assertiveness. AVATAR-Extended had two phases. In the first, participants received AVATAR-Brief therapy, whereas the second phase offered a more personalized intervention.
An ‘Unusual Finding’
The study’s primary outcome was voice-related distress at 16 and 28 weeks. Although the TAU plus AVATAR-Extended group did show a significant decrease in distress at 16 weeks vs TAU alone (–1.6 points; P = .029), the improvement was no longer significant at the 28-week follow-up (P = .175). The same was also true for the key secondary outcome of reduction in voice severity (–2.32 points; P = .009 at 16 weeks but P =.1 at 28 weeks).
The investigators noted that this might be caused by the number of dropouts in the AVATAR-Extended group by the 28-week timepoint. The completion rate for those patients was only 58%. The completion rate for the shorter, AVATAR-Brief group was 82%.
On the other hand, the other key secondary outcome of voice frequency was significantly reduced with AVATAR-Extended at both 16 weeks (–0.62 point; P = .01) and 28 weeks (–0.89 point; P = .003).
“This is an unusual finding. We’re not aware of any other psychological therapy that shows a reduction in the occurrence of the voice,” Garety said.
For TAU plus AVATAR-Brief, there were improvements at 16 weeks for distress (-1.05 points; P = .035) vs TAU alone. However, the researchers noted that this version of the therapy was just below the prespecified threshold for a clinically significant change and was at the threshold for statistical significance.
Although the shorter therapy was associated with a reduction in voice severity level at 16 weeks (–2.04 points; P = .017) vs TAU alone, there was no reduction in distress or voice severity at 28 weeks. There was no improvement in voice frequency at either timepoint.
Both the brief and the extended versions of AVATAR therapy showed improved mood and anxiety levels at 16 weeks and sustained improvement in well-being and recovery, the researchers noted.
“The short version, as expected, did deliver benefits posttreatment, but clearly the extended, optimized version outperformed the brief version. It had stronger and more lasting effects across quite a wide range of outcomes that matter to people who hear voices,” Garety said.
“In the extended version, people felt more empowered. And in just that version, the frequency of voices was reduced, which is a very important outcome,” she added.
Safety Issues?
There were 58 serious adverse events (SAEs) in total, with 51% of those occurring in the AVATAR-Extended group. Two participants in that group died; however, independent reviews deemed these events as not related to the intervention.
In addition, there were no “definitely related” SAEs and only a small number of “possibly related” SAEs, which typically included hospitalization with other contributory factors.
Garety noted during a press briefing that AVATAR therapy has now been demonstrated to be safe across two large trials.
Study limitations cited included no direct comparison between AVATAR-Brief and AVATAR-Extended or between AVATAR therapy and a different type of psychological treatment.
Overall, “we recommend that future development and provision of AVATAR therapy is primarily guided” by the AVATAR-Extended protocol, the investigators wrote.
Because the therapy was recommended by a National Institute for Health and Care Excellence Early Value Assessment, the investigators are now seeking to provide it in routine National Health Service settings to gather further real-world evidence of effectiveness over the next 3 years.
Next Steps
Although the intervention isn’t currently available to everybody who might be seeking it, “there’s a pipeline of movement from research into treatment and it’s moving towards the next stage of delivery,” Garety said.
Investigators are also looking into cultural adaptations for the therapy so it can be used in different locales, including Ethiopia and India, she added. There isn’t a US version yet, but Garety noted that investigators in Canada are looking at similar research and suspects that will also occur in the United States soon.
“We’re pioneers in this work, and it now needs to be going international and into services,” she said. “We have had many people who hear voices say what an amazing experience this has been. So, I feel very proud and excited to have been able to be part of this.”
At the press briefing, Miranda Wolpert, director of mental health at Wellcome, which funded the study, noted that it is encouraging to see the development of a new intervention that could potentially change the lives of patients across the world.
“We know that psychosis can start early in life, stopping people from having the jobs and relationships they want and from achieving the goals they want. This intervention was developed with those people to help them address an issue that really troubles them,” Wolpert said.
“For me, this represents part of a revolution we are starting to see in terms of mental health interventions and the potential impact on mental health science,” she added.
Digital Placebo Effect?
Commenting on the findings, John Torous, MD, a psychiatrist and director of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, Massachusetts, said there is a need for new treatments for schizophrenia that work with different mechanisms.
“We have a lot of medication studies but not as many innovative therapy studies. I think it’s exciting that the results, at least in the shorter-term outcome, were positive. And I think that’s something that can give people hope in using these new technologies,” said Torous, who is also an assistant professor of psychiatry at Harvard Medical School, Boston, and was not involved with the research.
Still, he did note some study limitations, including whether there could have been some type of “digital placebo effect” from the therapy.
“If you tell people they’re getting high-tech advanced digital care, that may have some effect,” he said, adding that “it’s always interesting” to tease out the benefit being delivered by the technology vs the delivery mechanism itself — or some combination of both.
Torous added, though, that it’s very difficult to have a rigorous digital control group. “It’s not necessarily a fault of their study, but it’s something to keep in mind when interpreting what the results are.”
He also noted that he would have liked to have seen a direct comparison between this new kind of psychological therapy vs standard psychological therapy, such as cognitive-behavioral therapy.
In addition, he wondered about expenses and scalability of the intervention, and whether patients would need to go to a specialized center to undergo treatment. Torous mentioned that a version involving virtual reality could perhaps make this more scalable in the future.
Overall, he said that what the investigators are currently doing is very innovative. “It’s exciting that we’re talking about the next steps. Giving people new options for psychological therapy that may be effective for their disorders is really wonderful to see,” Torous said.
The study was funded by the National Institute for Health and Care Research (NIHR), the Wellcome Trust King’s Clinical Research Facility, the NIHR Maudsley Biomedical Research Centre and Maudsley NHS Foundation Trust, King’s College London, the Manchester Biomedical Research Centre, and NHS Research Scotland, as well as by a grant from Wellcome. Garety reports being an unpaid scientific adviser to Avatar Therapy. Financial disclosures for the other investigators are fully listed in the original article. Torous reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
, results from the largest study of avatar therapy to date show.
The therapy allows patients to interact with a “digital embodiment” of the voice they hear, which is represented by a computer-generated face, also known as an avatar.
In the randomized, multisite, phase 2/3 AVATAR2 trial, patients who received AVATAR-Extended therapy, which included a personalized series of voiced dialogues based on their life history, plus treatment as usual (TAU) showed significantly greater improvement in distress and voice severity levels at 16 weeks vs those who received TAU only. They also had significant reductions in voice frequency at 16 and 28 weeks.
Patients in a third arm who were assigned to TAU plus AVATAR-Brief therapy, which included six sessions of a standardized version of the therapy, also showed improvements at 16 weeks, compared with TAU alone — but the clinical impact was stronger with the extended version.
“I was surprised at the extent to which the extended version seemed to be a more optimal version, and it should be the way forward with this therapy,” said study investigator Philippa A. Garety, PhD, professor emerita of clinical psychology at the Institute of Psychiatry, Psychology, and Neuroscience at King’s College, London, England.
With more than 300 participants, AVATAR2 is the largest trial to access avatar therapy to date, Garety noted.
“What’s unique about this work is that technology allows us to create safe face-to-face encounters with a representation of a person’s voice and allows them to relate to that voice in a new way,” she added.
The findings were published online in Nature Medicine.
A Decade of Research
Auditory verbal hallucinations are common in patients with schizophrenia, but currently available therapies can be ineffective, investigators wrote.
The therapy allows patients to customize how the avatar looks and sounds. Face-to-face dialogues are then conducted between the patients and avatars in order to build empowerment. A trained therapist provides support during these sessions.
As previously reported, the creator of avatar therapy, Julian Leff, MD, presented promising results from a pilot study of 26 patients at the International Congress of the Royal College of Psychiatrists in 2014.
“Opening up a dialogue between a patient and the voice they’ve been hearing is powerful,” said Leff, who was emeritus professor at the Institute of Psychiatry in London at the time.
In 2018, a randomized single-site study (AVATAR1) of 150 participants showed that the intervention was associated with a greater decrease in voice severity at 12 weeks vs supportive therapy. Past research led to the idea of incorporating personalization to better optimize the experience.
Garety noted that AVATAR2 is the largest trial to date of the therapy, as well as the first multisite trial to test the intervention, which was important in order to determine whether it could work outside of a research setting.
The study included 345 participants (61.4% men; mean age, 39.6 years) from three sites in England and one in Scotland. All were randomly assigned to receive TAU alone (n = 115), TAU plus AVATAR-Brief (n = 116), or TAU plus AVATAR-Extended (n = 114).
TAU typically included use of antipsychotics, as well as outpatient psychiatric visits and follow-up by case managers and care coordinators.
“We didn’t interfere with treatment as usual. We wanted to test whether adding this therapy to [TAU] would enhance effects and provide better treatment for their voices,” Garety noted.
AVATAR-Brief included a standardized process that focused on such things as self-esteem and assertiveness. AVATAR-Extended had two phases. In the first, participants received AVATAR-Brief therapy, whereas the second phase offered a more personalized intervention.
An ‘Unusual Finding’
The study’s primary outcome was voice-related distress at 16 and 28 weeks. Although the TAU plus AVATAR-Extended group did show a significant decrease in distress at 16 weeks vs TAU alone (–1.6 points; P = .029), the improvement was no longer significant at the 28-week follow-up (P = .175). The same was also true for the key secondary outcome of reduction in voice severity (–2.32 points; P = .009 at 16 weeks but P =.1 at 28 weeks).
The investigators noted that this might be caused by the number of dropouts in the AVATAR-Extended group by the 28-week timepoint. The completion rate for those patients was only 58%. The completion rate for the shorter, AVATAR-Brief group was 82%.
On the other hand, the other key secondary outcome of voice frequency was significantly reduced with AVATAR-Extended at both 16 weeks (–0.62 point; P = .01) and 28 weeks (–0.89 point; P = .003).
“This is an unusual finding. We’re not aware of any other psychological therapy that shows a reduction in the occurrence of the voice,” Garety said.
For TAU plus AVATAR-Brief, there were improvements at 16 weeks for distress (-1.05 points; P = .035) vs TAU alone. However, the researchers noted that this version of the therapy was just below the prespecified threshold for a clinically significant change and was at the threshold for statistical significance.
Although the shorter therapy was associated with a reduction in voice severity level at 16 weeks (–2.04 points; P = .017) vs TAU alone, there was no reduction in distress or voice severity at 28 weeks. There was no improvement in voice frequency at either timepoint.
Both the brief and the extended versions of AVATAR therapy showed improved mood and anxiety levels at 16 weeks and sustained improvement in well-being and recovery, the researchers noted.
“The short version, as expected, did deliver benefits posttreatment, but clearly the extended, optimized version outperformed the brief version. It had stronger and more lasting effects across quite a wide range of outcomes that matter to people who hear voices,” Garety said.
“In the extended version, people felt more empowered. And in just that version, the frequency of voices was reduced, which is a very important outcome,” she added.
Safety Issues?
There were 58 serious adverse events (SAEs) in total, with 51% of those occurring in the AVATAR-Extended group. Two participants in that group died; however, independent reviews deemed these events as not related to the intervention.
In addition, there were no “definitely related” SAEs and only a small number of “possibly related” SAEs, which typically included hospitalization with other contributory factors.
Garety noted during a press briefing that AVATAR therapy has now been demonstrated to be safe across two large trials.
Study limitations cited included no direct comparison between AVATAR-Brief and AVATAR-Extended or between AVATAR therapy and a different type of psychological treatment.
Overall, “we recommend that future development and provision of AVATAR therapy is primarily guided” by the AVATAR-Extended protocol, the investigators wrote.
Because the therapy was recommended by a National Institute for Health and Care Excellence Early Value Assessment, the investigators are now seeking to provide it in routine National Health Service settings to gather further real-world evidence of effectiveness over the next 3 years.
Next Steps
Although the intervention isn’t currently available to everybody who might be seeking it, “there’s a pipeline of movement from research into treatment and it’s moving towards the next stage of delivery,” Garety said.
Investigators are also looking into cultural adaptations for the therapy so it can be used in different locales, including Ethiopia and India, she added. There isn’t a US version yet, but Garety noted that investigators in Canada are looking at similar research and suspects that will also occur in the United States soon.
“We’re pioneers in this work, and it now needs to be going international and into services,” she said. “We have had many people who hear voices say what an amazing experience this has been. So, I feel very proud and excited to have been able to be part of this.”
At the press briefing, Miranda Wolpert, director of mental health at Wellcome, which funded the study, noted that it is encouraging to see the development of a new intervention that could potentially change the lives of patients across the world.
“We know that psychosis can start early in life, stopping people from having the jobs and relationships they want and from achieving the goals they want. This intervention was developed with those people to help them address an issue that really troubles them,” Wolpert said.
“For me, this represents part of a revolution we are starting to see in terms of mental health interventions and the potential impact on mental health science,” she added.
Digital Placebo Effect?
Commenting on the findings, John Torous, MD, a psychiatrist and director of digital psychiatry at Beth Israel Deaconess Medical Center, Boston, Massachusetts, said there is a need for new treatments for schizophrenia that work with different mechanisms.
“We have a lot of medication studies but not as many innovative therapy studies. I think it’s exciting that the results, at least in the shorter-term outcome, were positive. And I think that’s something that can give people hope in using these new technologies,” said Torous, who is also an assistant professor of psychiatry at Harvard Medical School, Boston, and was not involved with the research.
Still, he did note some study limitations, including whether there could have been some type of “digital placebo effect” from the therapy.
“If you tell people they’re getting high-tech advanced digital care, that may have some effect,” he said, adding that “it’s always interesting” to tease out the benefit being delivered by the technology vs the delivery mechanism itself — or some combination of both.
Torous added, though, that it’s very difficult to have a rigorous digital control group. “It’s not necessarily a fault of their study, but it’s something to keep in mind when interpreting what the results are.”
He also noted that he would have liked to have seen a direct comparison between this new kind of psychological therapy vs standard psychological therapy, such as cognitive-behavioral therapy.
In addition, he wondered about expenses and scalability of the intervention, and whether patients would need to go to a specialized center to undergo treatment. Torous mentioned that a version involving virtual reality could perhaps make this more scalable in the future.
Overall, he said that what the investigators are currently doing is very innovative. “It’s exciting that we’re talking about the next steps. Giving people new options for psychological therapy that may be effective for their disorders is really wonderful to see,” Torous said.
The study was funded by the National Institute for Health and Care Research (NIHR), the Wellcome Trust King’s Clinical Research Facility, the NIHR Maudsley Biomedical Research Centre and Maudsley NHS Foundation Trust, King’s College London, the Manchester Biomedical Research Centre, and NHS Research Scotland, as well as by a grant from Wellcome. Garety reports being an unpaid scientific adviser to Avatar Therapy. Financial disclosures for the other investigators are fully listed in the original article. Torous reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM NATURE MEDICINE
Dry Eye Linked to Increased Risk for Mental Health Disorders
TOPLINE:
Patients with dry eye disease are more than three times as likely to have mental health conditions, such as depression and anxiety, as those without the condition.
METHODOLOGY:
- Researchers used a database from the National Institutes of Health to investigate the association between dry eye disease and mental health disorders in a large and diverse nationwide population of American adults.
- They identified 18,257 patients (mean age, 64.9 years; 67% women) with dry eye disease who were propensity score–matched with 54,765 participants without the condition.
- The cases of dry eye disease were identified using Systematized Nomenclature of Medicine codes for dry eyes, meibomian gland dysfunction, and tear film insufficiency.
- The outcome measures for mental health conditions were clinical diagnoses of depressive disorders, anxiety-related disorders, bipolar disorder, and schizophrenia spectrum disorders.
TAKEAWAY:
- Patients with dry eye disease had more than triple the risk for mental health conditions than participants without the condition (adjusted odds ratio [aOR], 3.21; P < .001).
- Patients with dry eye disease had a higher risk for a depressive disorder (aOR, 3.47), anxiety-related disorder (aOR, 2.74), bipolar disorder (aOR, 2.23), and schizophrenia spectrum disorder (aOR, 2.48; P < .001 for all) than participants without the condition.
- The associations between dry eye disease and mental health conditions were significantly stronger among Black individuals than among White individuals, except for bipolar disorder.
- Dry eye disease was associated with two- to threefold higher odds of depressive disorders, anxiety-related disorders, bipolar disorder, and schizophrenia spectrum disorders even in participants who never used medications for mental health (P < .001 for all).
IN PRACTICE:
“Greater efforts should be undertaken to screen patients with DED [dry eye disease] for mental health conditions, particularly in historically medically underserved populations,” the authors of the study wrote.
SOURCE:
This study was led by Aaron T. Zhao, of the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, and was published online on October 15, 2024, in the American Journal of Ophthalmology.
LIMITATIONS:
This study relied on electronic health record data, which may have led to the inclusion of participants with undiagnosed dry eye disease as control participants. Moreover, the study did not evaluate the severity of dry eye disease or the severity and duration of mental health conditions, which may have affected the results. The database analyzed in this study may not have fully captured the complete demographic profile of the nationwide population, which may have affected the generalizability of the findings.
DISCLOSURES:
This study was supported by funding from the National Institutes of Health and Research to Prevent Blindness. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with dry eye disease are more than three times as likely to have mental health conditions, such as depression and anxiety, as those without the condition.
METHODOLOGY:
- Researchers used a database from the National Institutes of Health to investigate the association between dry eye disease and mental health disorders in a large and diverse nationwide population of American adults.
- They identified 18,257 patients (mean age, 64.9 years; 67% women) with dry eye disease who were propensity score–matched with 54,765 participants without the condition.
- The cases of dry eye disease were identified using Systematized Nomenclature of Medicine codes for dry eyes, meibomian gland dysfunction, and tear film insufficiency.
- The outcome measures for mental health conditions were clinical diagnoses of depressive disorders, anxiety-related disorders, bipolar disorder, and schizophrenia spectrum disorders.
TAKEAWAY:
- Patients with dry eye disease had more than triple the risk for mental health conditions than participants without the condition (adjusted odds ratio [aOR], 3.21; P < .001).
- Patients with dry eye disease had a higher risk for a depressive disorder (aOR, 3.47), anxiety-related disorder (aOR, 2.74), bipolar disorder (aOR, 2.23), and schizophrenia spectrum disorder (aOR, 2.48; P < .001 for all) than participants without the condition.
- The associations between dry eye disease and mental health conditions were significantly stronger among Black individuals than among White individuals, except for bipolar disorder.
- Dry eye disease was associated with two- to threefold higher odds of depressive disorders, anxiety-related disorders, bipolar disorder, and schizophrenia spectrum disorders even in participants who never used medications for mental health (P < .001 for all).
IN PRACTICE:
“Greater efforts should be undertaken to screen patients with DED [dry eye disease] for mental health conditions, particularly in historically medically underserved populations,” the authors of the study wrote.
SOURCE:
This study was led by Aaron T. Zhao, of the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, and was published online on October 15, 2024, in the American Journal of Ophthalmology.
LIMITATIONS:
This study relied on electronic health record data, which may have led to the inclusion of participants with undiagnosed dry eye disease as control participants. Moreover, the study did not evaluate the severity of dry eye disease or the severity and duration of mental health conditions, which may have affected the results. The database analyzed in this study may not have fully captured the complete demographic profile of the nationwide population, which may have affected the generalizability of the findings.
DISCLOSURES:
This study was supported by funding from the National Institutes of Health and Research to Prevent Blindness. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Patients with dry eye disease are more than three times as likely to have mental health conditions, such as depression and anxiety, as those without the condition.
METHODOLOGY:
- Researchers used a database from the National Institutes of Health to investigate the association between dry eye disease and mental health disorders in a large and diverse nationwide population of American adults.
- They identified 18,257 patients (mean age, 64.9 years; 67% women) with dry eye disease who were propensity score–matched with 54,765 participants without the condition.
- The cases of dry eye disease were identified using Systematized Nomenclature of Medicine codes for dry eyes, meibomian gland dysfunction, and tear film insufficiency.
- The outcome measures for mental health conditions were clinical diagnoses of depressive disorders, anxiety-related disorders, bipolar disorder, and schizophrenia spectrum disorders.
TAKEAWAY:
- Patients with dry eye disease had more than triple the risk for mental health conditions than participants without the condition (adjusted odds ratio [aOR], 3.21; P < .001).
- Patients with dry eye disease had a higher risk for a depressive disorder (aOR, 3.47), anxiety-related disorder (aOR, 2.74), bipolar disorder (aOR, 2.23), and schizophrenia spectrum disorder (aOR, 2.48; P < .001 for all) than participants without the condition.
- The associations between dry eye disease and mental health conditions were significantly stronger among Black individuals than among White individuals, except for bipolar disorder.
- Dry eye disease was associated with two- to threefold higher odds of depressive disorders, anxiety-related disorders, bipolar disorder, and schizophrenia spectrum disorders even in participants who never used medications for mental health (P < .001 for all).
IN PRACTICE:
“Greater efforts should be undertaken to screen patients with DED [dry eye disease] for mental health conditions, particularly in historically medically underserved populations,” the authors of the study wrote.
SOURCE:
This study was led by Aaron T. Zhao, of the Perelman School of Medicine at the University of Pennsylvania, Philadelphia, and was published online on October 15, 2024, in the American Journal of Ophthalmology.
LIMITATIONS:
This study relied on electronic health record data, which may have led to the inclusion of participants with undiagnosed dry eye disease as control participants. Moreover, the study did not evaluate the severity of dry eye disease or the severity and duration of mental health conditions, which may have affected the results. The database analyzed in this study may not have fully captured the complete demographic profile of the nationwide population, which may have affected the generalizability of the findings.
DISCLOSURES:
This study was supported by funding from the National Institutes of Health and Research to Prevent Blindness. The authors declared having no conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Clozapine and Respiratory Infection Risk: What to Know
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Clozapine is considered the drug of choice for treatment-resistant schizophrenia in guidelines globally, but it remains significantly underutilized. This is largely due to its range of side effects, particularly its increased infection risk which prompted the US Food and Drug Administration (FDA) to mandate regular blood testing to monitor neutrophil counts.
The COVID-19 pandemic raised new concerns about the care of clozapine-treated patients, leading clinicians and patients to urge the FDA to relax prescription requirements for the drug under the Risk Evaluation and Mitigation Strategy (REMS) program.
As the FDA prepares for a public hearing in November on proposed adjustments to the drug’s REMS criteria, a growing body of research is challenging the previous understanding of clozapine and infection risk.
Clarifying the Risk
Research on the link between clozapine and respiratory infections has produced conflicting results. Some studies indicate little to no increased risk for mild COVID-19 and other respiratory illnesses, while others have shown a higher likelihood of severe infection.
A recent nationwide Danish registry study of respiratory infections in people with a schizophrenia spectrum disorder could bring some clarity, Maxime Taquet, MD, a clinical lecturer at the University of Oxford, Warneford Hospital, Oxford, England, told this news organization.
By tracking periods when patients were on and off clozapine and other antipsychotics, the study offers more precise risk estimates, distinguishing the risks associated with the antipsychotic from those related to underlying schizophrenia, said Dr. Taquet, who authored an accompanying editorial on the study.
“It’s very important to try to disentangle the effects of schizophrenia, its severity, from the medication,” Dr. Taquet said. “I think that the Danish study is the first to try and really do that with as much precision as possible.”
After adjusting for key confounders including economic status and COVID-19 vaccination status, the researchers found that individuals taking antipsychotics had lower odds of testing positive for SARS-CoV-2 and similar rates of filled anti-infective prescriptions as those not taking the drugs.
Although antipsychotic use was not linked to higher rates of mild infection, it was linked to an increased risk for COVID-19 hospitalization in individuals older than 70 years, as well as hospitalization and death from other respiratory infections, mainly pneumonia, in those older than 40 years.
Notably, there was no excess risk for any outcome with clozapine vs other antipsychotics.
Strong Link to Pneumonia Risk
Results from a longitudinal Finnish study, just published in The American Journal of Psychiatry, also show an increased risk for severe outcomes from ileus and pneumonia among more than 2600 patients with schizophrenia taking clozapine.
Twenty years after initiating clozapine, the cumulative incidence estimate for ileus was 5.3% — more than sixfold higher than previously reported. The incidence of pneumonia was also high, at 29.5%.
Both illnesses were significantly associated with mortality, with odds ratios of 4.5 and 2.8, respectively.
These findings align with previous pharmacovigilance studies, with reported mortality rates for gastrointestinal hypomotility and pneumonia that were 4-10 times higher than those for agranulocytosis, the researchers said.
The study “really adds to a growing body of research suggesting a connection between clozapine use and a higher risk of developing pneumonia,” Robert O. Cotes, MD, a professor of psychiatry and behavioral sciences at Emory University, Atlanta, who specializes in the use of clozapine, told this news organization.
“Additionally, when people on clozapine do contract pneumonia, there’s concern the condition may be more dangerous,” he added.
A Closer Look at Neutropenia Risk
Neutropenia receives the lion’s share of attention among clozapine’s potential side effects, but this focus may need to be re-evaluated, Dr. Cotes said.
He pointed out that recent data suggest the risk for severe neutropenia, 2-3 years after initiating clozapine, is comparable to that of other antipsychotics.
A study of 26,630 clozapine users in Australia and New Zealand showed that most cases of severe neutropenia leading to clozapine cessation peaked within 18 weeks and was negligible after 2 years. This suggests weekly hematologic monitoring could potentially be discontinued after the 2-year mark.
Another study reported earlier this year by this news organization showed a low risk for mild or moderate neutropenia and no severe cases in nearly 1000 people taking clozapine.
“I worry that we may be missing the forest for the trees by hyperfocusing on neutropenia and not considering clozapine’s other potential serious side effects like pneumonia, myocarditis, and gastrointestinal hypermotility,” Dr. Cotes said.
Importance of Vaccines
The findings of these studies highlight the importance of vaccines in this at-risk group, said Dr. Taquet, a point emphasized by investigators of the Danish study he reviewed.
“Inspired by the experience of COVID-19 vaccine prioritization in severe mental illness and based on our findings, there is momentum for preventive action,” the authors wrote. “Our findings do not suggest the avoidance of specific antipsychotics but rather a call for increased vigilance regarding this at-risk group.”
This includes recommending pneumococcal, influenza, COVID-19, and other anti-infective vaccines in those older than 40 years treated with, or due to start, an antipsychotic.
“It’s not mandatory, but we do recommend that patients on clozapine get the regular vaccines,” Dr. Taquet said.
Pointing to the recent study on pneumonia risk, Dr. Cotes said addressing underlying risk factors, such as smoking, obesity, and possibly sedation and excessive salivation caused by clozapine, is key.
“And to make sure that vaccinations are up to date, particularly heading into this fall,” he added.
Rethinking Clozapine REMS
One of the most challenging issues facing clinicians and researchers is how to help people understand the safety profile of clozapine and to use it with more confidence, Dr. Cotes said.
“A lot of people hear about clozapine and they think about neutropenia, they think about side effects, the REMS system, and all of these factors really drive down clozapine utilization,” he said.
Treatment-resistant schizophrenia affects about a quarter of those with schizophrenia, yet only 4% of these patients receive clozapine in the United States, Dr. Cotes said. That number may be even lower for its other indication of reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder.
The clozapine REMS is viewed as a major barrier to utilization and requires certification of pharmacists and physicians and use of a central system to monitor absolute neutrophil counts for neutropenia in patients.
As previously reported by this news organization in November 2022, the FDA opted to temporarily exercise enforcement discretion for certain aspects of the drug safety program to ensure continuity of care for patients after concerns were raised by the American Psychiatric Association (APA) along with other professional organizations.
Even with that temporary enforcement discretion, “reports have shown that over half of those prescribed clozapine have trouble accessing the medication because of the REMS program,” a spokesperson for the APA told this news organization.
“Not only are patients having trouble accessing the medication, many have trouble finding a prescriber in their geographic locations and others because of the monitoring requirements have their treatment discontinued leading to negative outcomes,” the spokesperson said.
The FDA is currently reviewing the clozapine REMS and is holding a joint advisory committee meeting on November 19 to discuss the review and “possible changes to minimize burden on patients, pharmacies, and prescribers while maintaining safe use of clozapine.”
The APA plans to submit written and oral comments to the advisory committees.
“We are hopeful that the re-evaluation meeting in November will remove barriers and increase access to clozapine, which is currently highly underutilized, especially in marginalized communities,” the spokesperson said.
Dr. Cotes reported serving as a speaker and consultant for Saladax Biomedical and as a consultant for Syneos Health. Dr. Taquet reported having no competing interests.
A version of this article first appeared on Medscape.com.
Beyond One-Size-Fits-All: Precision Psychiatry Is Here
The field of psychiatry is experiencing a transformative shift toward precision medicine, a paradigm that tailors treatment to the unique characteristics of individual patients. This approach echoes advances in fields like oncology and cardiology, where precision tools have already revolutionized patient care.
But what exactly is precision psychiatry? How does it differ from traditional psychiatry? What will it look like in clinical practice? And are we there yet?
Beyond One-Size-Fits-All
The prevailing “one-size-fits-all” approach in psychiatry, which relies heavily on subjective symptom reporting, often proves ineffective due to the broad heterogeneity of diagnostic categories. This can lead to a “trial-and-error” cycle in treatment, which is time-consuming, costly, and frustrating for both doctors and patients.
In contrast, precision psychiatry has the potential to identify subtypes of psychiatric disorders and tailor treatments using measurable, objective data.
“The data supporting the use of precision psychiatry are very promising, particularly for treatment-resistant depression,” Leanne Williams, PhD, professor in the Department of Psychiatry and Behavioral Sciences at Stanford University, Stanford, and director of the Stanford Center for Precision Mental Health and Wellness, Palo Alto, California, said in an interview with this news organization.
Using functional MRI (fMRI), Dr. Williams and her team have mapped and measured patients’ brain circuitry to identify eight “biotypes” of depression that reflect combinations of dysfunction in six different circuits of the brain.
They are using these biotypes to guide treatment decisions in the clinic, matching individual patients to more targeted and effective therapies.
“We’re offering functional MRI to directly assess brain function along with other measures, so precision psychiatry is happening, and it’s really wanted by patients and their families. And the data suggest that we can double the rate of good outcomes,” said Dr. Williams.
“Neuroimaging techniques, particularly fMRI, have revolutionized our ability to map and quantify circuit abnormalities. Neural circuit measurements potentially offer the most direct window into the neural bases of psychiatric symptoms and, crucially, their modulation by treatment,” Teddy Akiki, MD, clinical scholar, Department of Psychiatry and Behavioral Sciences at Stanford, California, who works with Dr. Williams, told this news organization.
Blood-based biomarkers can complement brain imaging by providing additional information to better target treatment, help predict side effects, and guide dosage adjustments.
Precision Tools
A team led by Alexander B. Niculescu, III, MD, PhD, has found that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Dr. Niculescu is currently a professor of psychiatry and medical neuroscience at the Indiana University School of Medicine, Indianapolis. He will head west in September to direct the newly created Center for Precision Psychiatry at the University of Arizona College of Medicine–Phoenix.
MindX Sciences, the start-up company Dr. Niculescu cofounded, has been providing blood biomarker reports to “early adopting” doctors and patients.
“We are in the process of collecting and writing up the outcome data on the first 100 cases. The feedback we have received so far from the doctors and patients who have used it, as well as biopharma companies who have used it, has been very positive,” Dr. Niculescu told this news organization.
Another benefit of precision psychiatry lies in its potential to significantly accelerate drug development.
“By identifying specific neural circuits involved in subtypes of psychiatric conditions, we can repurpose or develop drugs that target these circuits more precisely. This approach allows for smaller, more focused trials with potentially higher success rates, which could speed up the typically slow and costly process of psychiatric drug development,” said Dr. Akiki.
Dr. Niculescu agreed. With precision psychiatry tools, “psychiatric drug development will become faster, cheaper, and more successful with the use of biomarkers and other precision tools,” he said.
The Future Is Already Here
The implementation and widespread adoption of precision psychiatry have several challenges.
It requires sophisticated technology and expertise, which may not be readily available in all clinical settings. Moreover, while evidence supports its use in conditions like major depression, there are fewer data on its efficacy in other psychiatric disorders, like schizophrenia.
Dr. Williams said future research should focus on expanding the evidence base for precision psychiatry across a broader range of psychiatric conditions.
Efforts to make precision tools more accessible and scalable, such as developing portable imaging technologies or more readily available biomarker tests, are also critical.
Integrating these precision tools into routine psychiatric practice will also require training and education for clinicians, as well as cost-effective solutions to make these approaches widely available.
“Mental health clinicians throughout the country are starting to employ semi-objective and objective measures in their practices, particularly self-report symptom questionnaires and pharmacogenomic assessment,” Laura Hack, MD, PhD, assistant professor, Department of Psychiatry and Behavioral Sciences, Stanford University, told this news organization.
“For precision psychiatry measures to be widely implemented, it is essential to demonstrate their reliability, clinical validity, clinical utility, and cost-effectiveness. Additionally, there is a need to develop clinical guidelines for their use, ensure that measurement tools are accessible, and educate all relevant stakeholders,” said Dr. Hack.
Right now, functional neuroimaging is used “only on a very limited basis in current clinical psychiatric practice,” Dr. Hack noted.
“We are developing standardized systems that will require less specialized expertise in functional neuroimaging and can be readily integrated into routine clinical care,” Dr. Akiki added.
Quoting William Gibson, “The future [of precision psychiatry] is already here; it’s just not evenly distributed,” said Dr. Niculescu.
Dr. Williams has disclosed relationships with One Mind PsyberGuide, Laureate Institute for Brain Research, and Et Cere Inc. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University. Dr. Akiki and Dr. Hack had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The field of psychiatry is experiencing a transformative shift toward precision medicine, a paradigm that tailors treatment to the unique characteristics of individual patients. This approach echoes advances in fields like oncology and cardiology, where precision tools have already revolutionized patient care.
But what exactly is precision psychiatry? How does it differ from traditional psychiatry? What will it look like in clinical practice? And are we there yet?
Beyond One-Size-Fits-All
The prevailing “one-size-fits-all” approach in psychiatry, which relies heavily on subjective symptom reporting, often proves ineffective due to the broad heterogeneity of diagnostic categories. This can lead to a “trial-and-error” cycle in treatment, which is time-consuming, costly, and frustrating for both doctors and patients.
In contrast, precision psychiatry has the potential to identify subtypes of psychiatric disorders and tailor treatments using measurable, objective data.
“The data supporting the use of precision psychiatry are very promising, particularly for treatment-resistant depression,” Leanne Williams, PhD, professor in the Department of Psychiatry and Behavioral Sciences at Stanford University, Stanford, and director of the Stanford Center for Precision Mental Health and Wellness, Palo Alto, California, said in an interview with this news organization.
Using functional MRI (fMRI), Dr. Williams and her team have mapped and measured patients’ brain circuitry to identify eight “biotypes” of depression that reflect combinations of dysfunction in six different circuits of the brain.
They are using these biotypes to guide treatment decisions in the clinic, matching individual patients to more targeted and effective therapies.
“We’re offering functional MRI to directly assess brain function along with other measures, so precision psychiatry is happening, and it’s really wanted by patients and their families. And the data suggest that we can double the rate of good outcomes,” said Dr. Williams.
“Neuroimaging techniques, particularly fMRI, have revolutionized our ability to map and quantify circuit abnormalities. Neural circuit measurements potentially offer the most direct window into the neural bases of psychiatric symptoms and, crucially, their modulation by treatment,” Teddy Akiki, MD, clinical scholar, Department of Psychiatry and Behavioral Sciences at Stanford, California, who works with Dr. Williams, told this news organization.
Blood-based biomarkers can complement brain imaging by providing additional information to better target treatment, help predict side effects, and guide dosage adjustments.
Precision Tools
A team led by Alexander B. Niculescu, III, MD, PhD, has found that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Dr. Niculescu is currently a professor of psychiatry and medical neuroscience at the Indiana University School of Medicine, Indianapolis. He will head west in September to direct the newly created Center for Precision Psychiatry at the University of Arizona College of Medicine–Phoenix.
MindX Sciences, the start-up company Dr. Niculescu cofounded, has been providing blood biomarker reports to “early adopting” doctors and patients.
“We are in the process of collecting and writing up the outcome data on the first 100 cases. The feedback we have received so far from the doctors and patients who have used it, as well as biopharma companies who have used it, has been very positive,” Dr. Niculescu told this news organization.
Another benefit of precision psychiatry lies in its potential to significantly accelerate drug development.
“By identifying specific neural circuits involved in subtypes of psychiatric conditions, we can repurpose or develop drugs that target these circuits more precisely. This approach allows for smaller, more focused trials with potentially higher success rates, which could speed up the typically slow and costly process of psychiatric drug development,” said Dr. Akiki.
Dr. Niculescu agreed. With precision psychiatry tools, “psychiatric drug development will become faster, cheaper, and more successful with the use of biomarkers and other precision tools,” he said.
The Future Is Already Here
The implementation and widespread adoption of precision psychiatry have several challenges.
It requires sophisticated technology and expertise, which may not be readily available in all clinical settings. Moreover, while evidence supports its use in conditions like major depression, there are fewer data on its efficacy in other psychiatric disorders, like schizophrenia.
Dr. Williams said future research should focus on expanding the evidence base for precision psychiatry across a broader range of psychiatric conditions.
Efforts to make precision tools more accessible and scalable, such as developing portable imaging technologies or more readily available biomarker tests, are also critical.
Integrating these precision tools into routine psychiatric practice will also require training and education for clinicians, as well as cost-effective solutions to make these approaches widely available.
“Mental health clinicians throughout the country are starting to employ semi-objective and objective measures in their practices, particularly self-report symptom questionnaires and pharmacogenomic assessment,” Laura Hack, MD, PhD, assistant professor, Department of Psychiatry and Behavioral Sciences, Stanford University, told this news organization.
“For precision psychiatry measures to be widely implemented, it is essential to demonstrate their reliability, clinical validity, clinical utility, and cost-effectiveness. Additionally, there is a need to develop clinical guidelines for their use, ensure that measurement tools are accessible, and educate all relevant stakeholders,” said Dr. Hack.
Right now, functional neuroimaging is used “only on a very limited basis in current clinical psychiatric practice,” Dr. Hack noted.
“We are developing standardized systems that will require less specialized expertise in functional neuroimaging and can be readily integrated into routine clinical care,” Dr. Akiki added.
Quoting William Gibson, “The future [of precision psychiatry] is already here; it’s just not evenly distributed,” said Dr. Niculescu.
Dr. Williams has disclosed relationships with One Mind PsyberGuide, Laureate Institute for Brain Research, and Et Cere Inc. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University. Dr. Akiki and Dr. Hack had no relevant disclosures.
A version of this article first appeared on Medscape.com.
The field of psychiatry is experiencing a transformative shift toward precision medicine, a paradigm that tailors treatment to the unique characteristics of individual patients. This approach echoes advances in fields like oncology and cardiology, where precision tools have already revolutionized patient care.
But what exactly is precision psychiatry? How does it differ from traditional psychiatry? What will it look like in clinical practice? And are we there yet?
Beyond One-Size-Fits-All
The prevailing “one-size-fits-all” approach in psychiatry, which relies heavily on subjective symptom reporting, often proves ineffective due to the broad heterogeneity of diagnostic categories. This can lead to a “trial-and-error” cycle in treatment, which is time-consuming, costly, and frustrating for both doctors and patients.
In contrast, precision psychiatry has the potential to identify subtypes of psychiatric disorders and tailor treatments using measurable, objective data.
“The data supporting the use of precision psychiatry are very promising, particularly for treatment-resistant depression,” Leanne Williams, PhD, professor in the Department of Psychiatry and Behavioral Sciences at Stanford University, Stanford, and director of the Stanford Center for Precision Mental Health and Wellness, Palo Alto, California, said in an interview with this news organization.
Using functional MRI (fMRI), Dr. Williams and her team have mapped and measured patients’ brain circuitry to identify eight “biotypes” of depression that reflect combinations of dysfunction in six different circuits of the brain.
They are using these biotypes to guide treatment decisions in the clinic, matching individual patients to more targeted and effective therapies.
“We’re offering functional MRI to directly assess brain function along with other measures, so precision psychiatry is happening, and it’s really wanted by patients and their families. And the data suggest that we can double the rate of good outcomes,” said Dr. Williams.
“Neuroimaging techniques, particularly fMRI, have revolutionized our ability to map and quantify circuit abnormalities. Neural circuit measurements potentially offer the most direct window into the neural bases of psychiatric symptoms and, crucially, their modulation by treatment,” Teddy Akiki, MD, clinical scholar, Department of Psychiatry and Behavioral Sciences at Stanford, California, who works with Dr. Williams, told this news organization.
Blood-based biomarkers can complement brain imaging by providing additional information to better target treatment, help predict side effects, and guide dosage adjustments.
Precision Tools
A team led by Alexander B. Niculescu, III, MD, PhD, has found that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Dr. Niculescu is currently a professor of psychiatry and medical neuroscience at the Indiana University School of Medicine, Indianapolis. He will head west in September to direct the newly created Center for Precision Psychiatry at the University of Arizona College of Medicine–Phoenix.
MindX Sciences, the start-up company Dr. Niculescu cofounded, has been providing blood biomarker reports to “early adopting” doctors and patients.
“We are in the process of collecting and writing up the outcome data on the first 100 cases. The feedback we have received so far from the doctors and patients who have used it, as well as biopharma companies who have used it, has been very positive,” Dr. Niculescu told this news organization.
Another benefit of precision psychiatry lies in its potential to significantly accelerate drug development.
“By identifying specific neural circuits involved in subtypes of psychiatric conditions, we can repurpose or develop drugs that target these circuits more precisely. This approach allows for smaller, more focused trials with potentially higher success rates, which could speed up the typically slow and costly process of psychiatric drug development,” said Dr. Akiki.
Dr. Niculescu agreed. With precision psychiatry tools, “psychiatric drug development will become faster, cheaper, and more successful with the use of biomarkers and other precision tools,” he said.
The Future Is Already Here
The implementation and widespread adoption of precision psychiatry have several challenges.
It requires sophisticated technology and expertise, which may not be readily available in all clinical settings. Moreover, while evidence supports its use in conditions like major depression, there are fewer data on its efficacy in other psychiatric disorders, like schizophrenia.
Dr. Williams said future research should focus on expanding the evidence base for precision psychiatry across a broader range of psychiatric conditions.
Efforts to make precision tools more accessible and scalable, such as developing portable imaging technologies or more readily available biomarker tests, are also critical.
Integrating these precision tools into routine psychiatric practice will also require training and education for clinicians, as well as cost-effective solutions to make these approaches widely available.
“Mental health clinicians throughout the country are starting to employ semi-objective and objective measures in their practices, particularly self-report symptom questionnaires and pharmacogenomic assessment,” Laura Hack, MD, PhD, assistant professor, Department of Psychiatry and Behavioral Sciences, Stanford University, told this news organization.
“For precision psychiatry measures to be widely implemented, it is essential to demonstrate their reliability, clinical validity, clinical utility, and cost-effectiveness. Additionally, there is a need to develop clinical guidelines for their use, ensure that measurement tools are accessible, and educate all relevant stakeholders,” said Dr. Hack.
Right now, functional neuroimaging is used “only on a very limited basis in current clinical psychiatric practice,” Dr. Hack noted.
“We are developing standardized systems that will require less specialized expertise in functional neuroimaging and can be readily integrated into routine clinical care,” Dr. Akiki added.
Quoting William Gibson, “The future [of precision psychiatry] is already here; it’s just not evenly distributed,” said Dr. Niculescu.
Dr. Williams has disclosed relationships with One Mind PsyberGuide, Laureate Institute for Brain Research, and Et Cere Inc. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University. Dr. Akiki and Dr. Hack had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Evidence Growing for Inflammation’s Role in Elevating Risk for Psychiatric Illness
New research provides more evidence that inflammation may contribute to the development of psychiatric disorders and suggests that measuring certain inflammatory biomarkers may aid in the early identification of individuals at high risk.
Using large-scale datasets, researchers found that elevated levels of certain inflammatory biomarkers, particularly leukocytes, haptoglobin, and C-reactive protein (CRP), and lower levels of anti-inflammatory immunoglobulin G (IgG) were associated with an increased risk for psychiatric disorders.
Individuals with psychiatric disorders had persistently higher levels of leukocytes and haptoglobin, as well as persistently lower levels of IgG, than controls during the 30 years before diagnosis, which suggest “long-term processes and may aid in the identification of individuals at high risk,” the researchers wrote.
In addition, a higher level of leukocytes was consistently associated with increased odds of depression across different methods of Mendelian randomization (MR) analysis, “indicating a possible causal relationship between leukocytes and depression,” they said.
The study, with first author Yu Zeng, MSc, with the Mental Health Center and West China Biomedical Big Data Center, West China Hospital, Sichuan University, Chengdu, China, was published online on August 21 in JAMA Psychiatry.
Inflammatory Phenotype
Individuals with psychiatric disorders have been found to have elevated levels of inflammatory biomarkers, but prospective evidence is limited regarding the association between inflammatory biomarkers and subsequent psychiatric disorders risk.
To investigate further, the researchers employed a “triangulation” approach consisting of an exploration dataset of 585,279 adults in the Swedish AMORIS cohort with no prior psychiatric diagnoses and a measurement of at least one inflammatory biomarker, a validation dataset of 485,620 UK Biobank participants, and genetic and MR analyses using genome-wide association study summary statistics.
In the AMORIS cohort, individuals with a higher than median level of leukocytes (hazard ratio [HR], 1.11), haptoglobin (HR, 1.13), or CRP (HR, 1.02) had an elevated risk for any psychiatric disorder. In contrast, there was an inverse association for IgG level (HR, 0.92).
“The estimates were comparable for depression, anxiety, and stress-related disorders, specifically, and these results were largely validated in the UK Biobank,” the authors reported.
In trajectory analyses, compared with controls, individuals with psychiatric disorders had higher leukocyte and haptoglobin levels and lower IgG up to three decades before being diagnosed.
The MR analysis suggested a possible causal relationship between leukocytes and depression.
The underlying mechanisms for the associations of serum leukocytes, haptoglobin, CRP, and IgG with psychiatry disorders remain unclear.
“Possible explanations mainly include blood-brain barrier disruption, microglia activation, neurotransmission impairment, and other interactions between inflammations and neuropathology,” the researchers wrote.
A related paper published online on August 21 in JAMA Psychiatry looked at trajectories of inflammation in childhood and risk for mental and cardiometabolic disorders in adulthood.
This longitudinal cohort study found that having persistently raised levels of inflammation as measured by CRP throughout childhood and adolescence, peaking at age 9 years, were associated with an increased risk of developing psychosis disorder, severe depression, and higher levels of insulin resistance.
Support for Precision Psychiatry
This study is “another strong indication that inflammation plays a role in depression,” Andrew H. Miller, MD, professor of psychiatry and behavioral sciences and director of the behavioral immunology program, Emory University School of Medicine, Atlanta, Georgia, who wasn’t involved in the study, told this news organization.
“The work adds to the mounting data that there exists an inflammatory phenotype of depression that may uniquely respond to treatment and may have a unique trajectory,” Dr. Miller said.
“Eventually the field will want to embrace this novel phenotype and better understand how to recognize it and treat it. This is our entrée into precision psychiatry where we identify the right treatment for the right patient at the right time based on an understanding of the underlying cause of their illness,” Dr. Miller added.
Also weighing in, Alexander B. Niculescu III, MD, PhD, professor of psychiatry and medical neuroscience, Indiana University School of Medicine, Indianapolis, cautioned that these biomarkers are “very nonspecific and are likely related to these subjects that go on to develop psychiatric disorders having more stressful, adverse life trajectories.”
“There are better, more specific blood biomarkers for psychiatric disorders already available,” Dr. Niculescu told this news organization.
His group recently reported that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Notably, they observed a strong circadian clock gene component to mood disorders, which helps explain why some patients’ conditions become worse with seasonal changes. It also explains the sleep alterations that occur among patients with mood disorders, they said.
This study had no commercial funding. Yu Zeng and Dr. Miller had no relevant disclosures. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University.
A version of this article first appeared on Medscape.com.
New research provides more evidence that inflammation may contribute to the development of psychiatric disorders and suggests that measuring certain inflammatory biomarkers may aid in the early identification of individuals at high risk.
Using large-scale datasets, researchers found that elevated levels of certain inflammatory biomarkers, particularly leukocytes, haptoglobin, and C-reactive protein (CRP), and lower levels of anti-inflammatory immunoglobulin G (IgG) were associated with an increased risk for psychiatric disorders.
Individuals with psychiatric disorders had persistently higher levels of leukocytes and haptoglobin, as well as persistently lower levels of IgG, than controls during the 30 years before diagnosis, which suggest “long-term processes and may aid in the identification of individuals at high risk,” the researchers wrote.
In addition, a higher level of leukocytes was consistently associated with increased odds of depression across different methods of Mendelian randomization (MR) analysis, “indicating a possible causal relationship between leukocytes and depression,” they said.
The study, with first author Yu Zeng, MSc, with the Mental Health Center and West China Biomedical Big Data Center, West China Hospital, Sichuan University, Chengdu, China, was published online on August 21 in JAMA Psychiatry.
Inflammatory Phenotype
Individuals with psychiatric disorders have been found to have elevated levels of inflammatory biomarkers, but prospective evidence is limited regarding the association between inflammatory biomarkers and subsequent psychiatric disorders risk.
To investigate further, the researchers employed a “triangulation” approach consisting of an exploration dataset of 585,279 adults in the Swedish AMORIS cohort with no prior psychiatric diagnoses and a measurement of at least one inflammatory biomarker, a validation dataset of 485,620 UK Biobank participants, and genetic and MR analyses using genome-wide association study summary statistics.
In the AMORIS cohort, individuals with a higher than median level of leukocytes (hazard ratio [HR], 1.11), haptoglobin (HR, 1.13), or CRP (HR, 1.02) had an elevated risk for any psychiatric disorder. In contrast, there was an inverse association for IgG level (HR, 0.92).
“The estimates were comparable for depression, anxiety, and stress-related disorders, specifically, and these results were largely validated in the UK Biobank,” the authors reported.
In trajectory analyses, compared with controls, individuals with psychiatric disorders had higher leukocyte and haptoglobin levels and lower IgG up to three decades before being diagnosed.
The MR analysis suggested a possible causal relationship between leukocytes and depression.
The underlying mechanisms for the associations of serum leukocytes, haptoglobin, CRP, and IgG with psychiatry disorders remain unclear.
“Possible explanations mainly include blood-brain barrier disruption, microglia activation, neurotransmission impairment, and other interactions between inflammations and neuropathology,” the researchers wrote.
A related paper published online on August 21 in JAMA Psychiatry looked at trajectories of inflammation in childhood and risk for mental and cardiometabolic disorders in adulthood.
This longitudinal cohort study found that having persistently raised levels of inflammation as measured by CRP throughout childhood and adolescence, peaking at age 9 years, were associated with an increased risk of developing psychosis disorder, severe depression, and higher levels of insulin resistance.
Support for Precision Psychiatry
This study is “another strong indication that inflammation plays a role in depression,” Andrew H. Miller, MD, professor of psychiatry and behavioral sciences and director of the behavioral immunology program, Emory University School of Medicine, Atlanta, Georgia, who wasn’t involved in the study, told this news organization.
“The work adds to the mounting data that there exists an inflammatory phenotype of depression that may uniquely respond to treatment and may have a unique trajectory,” Dr. Miller said.
“Eventually the field will want to embrace this novel phenotype and better understand how to recognize it and treat it. This is our entrée into precision psychiatry where we identify the right treatment for the right patient at the right time based on an understanding of the underlying cause of their illness,” Dr. Miller added.
Also weighing in, Alexander B. Niculescu III, MD, PhD, professor of psychiatry and medical neuroscience, Indiana University School of Medicine, Indianapolis, cautioned that these biomarkers are “very nonspecific and are likely related to these subjects that go on to develop psychiatric disorders having more stressful, adverse life trajectories.”
“There are better, more specific blood biomarkers for psychiatric disorders already available,” Dr. Niculescu told this news organization.
His group recently reported that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Notably, they observed a strong circadian clock gene component to mood disorders, which helps explain why some patients’ conditions become worse with seasonal changes. It also explains the sleep alterations that occur among patients with mood disorders, they said.
This study had no commercial funding. Yu Zeng and Dr. Miller had no relevant disclosures. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University.
A version of this article first appeared on Medscape.com.
New research provides more evidence that inflammation may contribute to the development of psychiatric disorders and suggests that measuring certain inflammatory biomarkers may aid in the early identification of individuals at high risk.
Using large-scale datasets, researchers found that elevated levels of certain inflammatory biomarkers, particularly leukocytes, haptoglobin, and C-reactive protein (CRP), and lower levels of anti-inflammatory immunoglobulin G (IgG) were associated with an increased risk for psychiatric disorders.
Individuals with psychiatric disorders had persistently higher levels of leukocytes and haptoglobin, as well as persistently lower levels of IgG, than controls during the 30 years before diagnosis, which suggest “long-term processes and may aid in the identification of individuals at high risk,” the researchers wrote.
In addition, a higher level of leukocytes was consistently associated with increased odds of depression across different methods of Mendelian randomization (MR) analysis, “indicating a possible causal relationship between leukocytes and depression,” they said.
The study, with first author Yu Zeng, MSc, with the Mental Health Center and West China Biomedical Big Data Center, West China Hospital, Sichuan University, Chengdu, China, was published online on August 21 in JAMA Psychiatry.
Inflammatory Phenotype
Individuals with psychiatric disorders have been found to have elevated levels of inflammatory biomarkers, but prospective evidence is limited regarding the association between inflammatory biomarkers and subsequent psychiatric disorders risk.
To investigate further, the researchers employed a “triangulation” approach consisting of an exploration dataset of 585,279 adults in the Swedish AMORIS cohort with no prior psychiatric diagnoses and a measurement of at least one inflammatory biomarker, a validation dataset of 485,620 UK Biobank participants, and genetic and MR analyses using genome-wide association study summary statistics.
In the AMORIS cohort, individuals with a higher than median level of leukocytes (hazard ratio [HR], 1.11), haptoglobin (HR, 1.13), or CRP (HR, 1.02) had an elevated risk for any psychiatric disorder. In contrast, there was an inverse association for IgG level (HR, 0.92).
“The estimates were comparable for depression, anxiety, and stress-related disorders, specifically, and these results were largely validated in the UK Biobank,” the authors reported.
In trajectory analyses, compared with controls, individuals with psychiatric disorders had higher leukocyte and haptoglobin levels and lower IgG up to three decades before being diagnosed.
The MR analysis suggested a possible causal relationship between leukocytes and depression.
The underlying mechanisms for the associations of serum leukocytes, haptoglobin, CRP, and IgG with psychiatry disorders remain unclear.
“Possible explanations mainly include blood-brain barrier disruption, microglia activation, neurotransmission impairment, and other interactions between inflammations and neuropathology,” the researchers wrote.
A related paper published online on August 21 in JAMA Psychiatry looked at trajectories of inflammation in childhood and risk for mental and cardiometabolic disorders in adulthood.
This longitudinal cohort study found that having persistently raised levels of inflammation as measured by CRP throughout childhood and adolescence, peaking at age 9 years, were associated with an increased risk of developing psychosis disorder, severe depression, and higher levels of insulin resistance.
Support for Precision Psychiatry
This study is “another strong indication that inflammation plays a role in depression,” Andrew H. Miller, MD, professor of psychiatry and behavioral sciences and director of the behavioral immunology program, Emory University School of Medicine, Atlanta, Georgia, who wasn’t involved in the study, told this news organization.
“The work adds to the mounting data that there exists an inflammatory phenotype of depression that may uniquely respond to treatment and may have a unique trajectory,” Dr. Miller said.
“Eventually the field will want to embrace this novel phenotype and better understand how to recognize it and treat it. This is our entrée into precision psychiatry where we identify the right treatment for the right patient at the right time based on an understanding of the underlying cause of their illness,” Dr. Miller added.
Also weighing in, Alexander B. Niculescu III, MD, PhD, professor of psychiatry and medical neuroscience, Indiana University School of Medicine, Indianapolis, cautioned that these biomarkers are “very nonspecific and are likely related to these subjects that go on to develop psychiatric disorders having more stressful, adverse life trajectories.”
“There are better, more specific blood biomarkers for psychiatric disorders already available,” Dr. Niculescu told this news organization.
His group recently reported that a panel of blood-based biomarkers can distinguish between depression and bipolar disorder, predict a person’s future risk for these disorders, and inform more tailored medication choices.
Notably, they observed a strong circadian clock gene component to mood disorders, which helps explain why some patients’ conditions become worse with seasonal changes. It also explains the sleep alterations that occur among patients with mood disorders, they said.
This study had no commercial funding. Yu Zeng and Dr. Miller had no relevant disclosures. Dr. Niculescu is a cofounder of MindX Sciences and is listed as inventor on a patent application filed by Indiana University.
A version of this article first appeared on Medscape.com.
Severe COVID-19 Tied to Increased Risk for Mental Illness
New research adds to a growing body of evidence suggesting that COVID-19 infection can be hard on mental health.
, particularly in those with severe COVID who had not been vaccinated.
Importantly, vaccination appeared to mitigate the adverse effects of COVID-19 on mental health, the investigators found.
“Our results highlight the importance COVID-19 vaccination in the general population and particularly among those with mental illnesses, who may be at higher risk of both SARS-CoV-2 infection and adverse outcomes following COVID-19,” first author Venexia Walker, PhD, with University of Bristol, United Kingdom, said in a news release.
The study was published online on August 21 in JAMA Psychiatry.
Novel Data
“Before this study, a number of papers had looked at associations of COVID diagnosis with mental ill health, and broadly speaking, they had reported associations of different magnitudes,” study author Jonathan A. C. Sterne, PhD, with University of Bristol, noted in a journal podcast.
“Some studies were restricted to patients who were hospitalized with COVID-19 and some not and the duration of follow-up varied. And importantly, the nature of COVID-19 changed profoundly as vaccination became available and there was little data on the impact of vaccination on associations of COVID-19 with subsequent mental ill health,” Dr. Sterne said.
The UK study was conducted in three cohorts — a cohort of about 18.6 million people who were diagnosed with COVID-19 before a vaccine was available, a cohort of about 14 million adults who were vaccinated, and a cohort of about 3.2 million people who were unvaccinated.
The researchers compared rates of various mental illnesses after COVID-19 with rates before or without COVID-19 and by vaccination status.
Across all cohorts, rates of most mental illnesses examined were “markedly elevated” during the first month following a COVID-19 diagnosis compared with rates before or without COVID-19.
For example, the adjusted hazard ratios for depression (the most common illness) and serious mental illness in the month after COVID-19 were 1.93 and 1.49, respectively, in the prevaccination cohort and 1.79 and 1.45, respectively, in the unvaccinated cohort compared with 1.16 and 0.91 in the vaccinated cohort.
This elevation in the rate of mental illnesses was mainly seen after severe COVID-19 that led to hospitalization and remained higher for up to a year following severe COVID-19 in unvaccinated adults.
For severe COVID-19 with hospitalization, the adjusted hazard ratio for depression in the month following admission was 16.3 in the prevaccine cohort, 15.6 in the unvaccinated cohort, and 12.9 in the vaccinated cohort.
The adjusted hazard ratios for serious mental illness in the month after COVID hospitalization was 9.71 in the prevaccine cohort, 8.75 with no vaccination, and 6.52 with vaccination.
“Incidences of other mental illnesses were broadly similar to those of depression and serious mental illness, both overall and for COVID-19 with and without hospitalization,” the authors report in their paper.
Consistent with prior research, subgroup analyzes found the association of COVID-19 and mental illness was stronger among older adults and men, with no marked differences by ethnic group.
“We should be concerned about continuing consequences in people who experienced severe COVID-19 early in the pandemic, and they may include a continuing higher incidence of mental ill health, such as depression and serious mental illness,” Dr. Sterne said in the podcast.
In terms of ongoing booster vaccinations, “people who are advised that they are under vaccinated or recommended for further COVID-19 vaccination, should take those invitations seriously, because by preventing severe COVID-19, which is what vaccination does, you can prevent consequences such as mental illness,” Dr. Sterne added.
The study was supported by the COVID-19 Longitudinal Health and Wellbeing National Core Study, which is funded by the Medical Research Council and National Institute for Health and Care Research. The authors had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research adds to a growing body of evidence suggesting that COVID-19 infection can be hard on mental health.
, particularly in those with severe COVID who had not been vaccinated.
Importantly, vaccination appeared to mitigate the adverse effects of COVID-19 on mental health, the investigators found.
“Our results highlight the importance COVID-19 vaccination in the general population and particularly among those with mental illnesses, who may be at higher risk of both SARS-CoV-2 infection and adverse outcomes following COVID-19,” first author Venexia Walker, PhD, with University of Bristol, United Kingdom, said in a news release.
The study was published online on August 21 in JAMA Psychiatry.
Novel Data
“Before this study, a number of papers had looked at associations of COVID diagnosis with mental ill health, and broadly speaking, they had reported associations of different magnitudes,” study author Jonathan A. C. Sterne, PhD, with University of Bristol, noted in a journal podcast.
“Some studies were restricted to patients who were hospitalized with COVID-19 and some not and the duration of follow-up varied. And importantly, the nature of COVID-19 changed profoundly as vaccination became available and there was little data on the impact of vaccination on associations of COVID-19 with subsequent mental ill health,” Dr. Sterne said.
The UK study was conducted in three cohorts — a cohort of about 18.6 million people who were diagnosed with COVID-19 before a vaccine was available, a cohort of about 14 million adults who were vaccinated, and a cohort of about 3.2 million people who were unvaccinated.
The researchers compared rates of various mental illnesses after COVID-19 with rates before or without COVID-19 and by vaccination status.
Across all cohorts, rates of most mental illnesses examined were “markedly elevated” during the first month following a COVID-19 diagnosis compared with rates before or without COVID-19.
For example, the adjusted hazard ratios for depression (the most common illness) and serious mental illness in the month after COVID-19 were 1.93 and 1.49, respectively, in the prevaccination cohort and 1.79 and 1.45, respectively, in the unvaccinated cohort compared with 1.16 and 0.91 in the vaccinated cohort.
This elevation in the rate of mental illnesses was mainly seen after severe COVID-19 that led to hospitalization and remained higher for up to a year following severe COVID-19 in unvaccinated adults.
For severe COVID-19 with hospitalization, the adjusted hazard ratio for depression in the month following admission was 16.3 in the prevaccine cohort, 15.6 in the unvaccinated cohort, and 12.9 in the vaccinated cohort.
The adjusted hazard ratios for serious mental illness in the month after COVID hospitalization was 9.71 in the prevaccine cohort, 8.75 with no vaccination, and 6.52 with vaccination.
“Incidences of other mental illnesses were broadly similar to those of depression and serious mental illness, both overall and for COVID-19 with and without hospitalization,” the authors report in their paper.
Consistent with prior research, subgroup analyzes found the association of COVID-19 and mental illness was stronger among older adults and men, with no marked differences by ethnic group.
“We should be concerned about continuing consequences in people who experienced severe COVID-19 early in the pandemic, and they may include a continuing higher incidence of mental ill health, such as depression and serious mental illness,” Dr. Sterne said in the podcast.
In terms of ongoing booster vaccinations, “people who are advised that they are under vaccinated or recommended for further COVID-19 vaccination, should take those invitations seriously, because by preventing severe COVID-19, which is what vaccination does, you can prevent consequences such as mental illness,” Dr. Sterne added.
The study was supported by the COVID-19 Longitudinal Health and Wellbeing National Core Study, which is funded by the Medical Research Council and National Institute for Health and Care Research. The authors had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research adds to a growing body of evidence suggesting that COVID-19 infection can be hard on mental health.
, particularly in those with severe COVID who had not been vaccinated.
Importantly, vaccination appeared to mitigate the adverse effects of COVID-19 on mental health, the investigators found.
“Our results highlight the importance COVID-19 vaccination in the general population and particularly among those with mental illnesses, who may be at higher risk of both SARS-CoV-2 infection and adverse outcomes following COVID-19,” first author Venexia Walker, PhD, with University of Bristol, United Kingdom, said in a news release.
The study was published online on August 21 in JAMA Psychiatry.
Novel Data
“Before this study, a number of papers had looked at associations of COVID diagnosis with mental ill health, and broadly speaking, they had reported associations of different magnitudes,” study author Jonathan A. C. Sterne, PhD, with University of Bristol, noted in a journal podcast.
“Some studies were restricted to patients who were hospitalized with COVID-19 and some not and the duration of follow-up varied. And importantly, the nature of COVID-19 changed profoundly as vaccination became available and there was little data on the impact of vaccination on associations of COVID-19 with subsequent mental ill health,” Dr. Sterne said.
The UK study was conducted in three cohorts — a cohort of about 18.6 million people who were diagnosed with COVID-19 before a vaccine was available, a cohort of about 14 million adults who were vaccinated, and a cohort of about 3.2 million people who were unvaccinated.
The researchers compared rates of various mental illnesses after COVID-19 with rates before or without COVID-19 and by vaccination status.
Across all cohorts, rates of most mental illnesses examined were “markedly elevated” during the first month following a COVID-19 diagnosis compared with rates before or without COVID-19.
For example, the adjusted hazard ratios for depression (the most common illness) and serious mental illness in the month after COVID-19 were 1.93 and 1.49, respectively, in the prevaccination cohort and 1.79 and 1.45, respectively, in the unvaccinated cohort compared with 1.16 and 0.91 in the vaccinated cohort.
This elevation in the rate of mental illnesses was mainly seen after severe COVID-19 that led to hospitalization and remained higher for up to a year following severe COVID-19 in unvaccinated adults.
For severe COVID-19 with hospitalization, the adjusted hazard ratio for depression in the month following admission was 16.3 in the prevaccine cohort, 15.6 in the unvaccinated cohort, and 12.9 in the vaccinated cohort.
The adjusted hazard ratios for serious mental illness in the month after COVID hospitalization was 9.71 in the prevaccine cohort, 8.75 with no vaccination, and 6.52 with vaccination.
“Incidences of other mental illnesses were broadly similar to those of depression and serious mental illness, both overall and for COVID-19 with and without hospitalization,” the authors report in their paper.
Consistent with prior research, subgroup analyzes found the association of COVID-19 and mental illness was stronger among older adults and men, with no marked differences by ethnic group.
“We should be concerned about continuing consequences in people who experienced severe COVID-19 early in the pandemic, and they may include a continuing higher incidence of mental ill health, such as depression and serious mental illness,” Dr. Sterne said in the podcast.
In terms of ongoing booster vaccinations, “people who are advised that they are under vaccinated or recommended for further COVID-19 vaccination, should take those invitations seriously, because by preventing severe COVID-19, which is what vaccination does, you can prevent consequences such as mental illness,” Dr. Sterne added.
The study was supported by the COVID-19 Longitudinal Health and Wellbeing National Core Study, which is funded by the Medical Research Council and National Institute for Health and Care Research. The authors had no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Which Medications Can Cause Edema?
Edema in the feet and legs is a common complaint in our practices. It can cause pain, weakness, heaviness, discomfort, limited movement, and a negative body image. Medications can contribute to edema, either alone or in combination with other health issues.
Therefore, it is important to know how to treat or prevent medication-induced edema.
There are four main causes of edema, and all can facilitate medication-induced edema.
- Increased capillary pressure. Conditions such as heart failure, renal dysfunction, venous insufficiency, deep vein thrombosis, and cirrhosis can increase capillary pressure, leading to edema.
- Decreased oncotic pressure. Hypoalbuminemia, a primary cause of reduced colloid oncotic pressure, can result from nephrotic syndrome, diabetic nephropathy, lupus nephropathy, amyloidosis, nephropathies, cirrhosis, chronic liver disease, and malabsorption or malnutrition.
- Increased capillary permeability. Vascular injury, often associated with diabetes, can increase capillary permeability and contribute to edema.
- Impaired lymphatic drainage. Lymphatic obstruction is common in patients with lymphedema, tumors, inflammation, fibrosis, certain infections, surgery, and congenital anomalies. Conditions such as thyroid disorders can also cause an increase in interstitial albumin and other proteins without a corresponding increase in lymphatic flow, leading to lymphedema.
Medications That Can Cause Edema
- Calcium channel blockers (CCBs). Drugs such as nifedipine and amlodipine can increase hydrostatic pressure by causing selective vasodilation of precapillary vessels, leading to increased intracapillary pressures. Newer lipophilic CCBs (eg, levamlodipine) exhibit lower rates of edema. Reducing the dose is often effective. Diuretics are not very effective for vasodilation-induced edema. Combining CCBs with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), which induce postcapillary dilation and normalize intracapillary pressure, may reduce fluid leakage into the interstitial space. This combination may be more beneficial than high-dose CCB monotherapy.
- Thiazolidinedione (eg, pioglitazone). These increase vascular permeability and hydrostatic pressure. They work by stimulating the peroxisome proliferator–activated gamma receptor, increasing vascular endothelial permeability, vascular endothelial growth factor secretion, and renal retention of sodium and fluids. Because of other adverse effects, their use is now limited.
- Agents for neuropathic pain (gabapentin and pregabalin). These drugs can induce selective vasodilation of arterioles through a mechanism similar to that of CCBs, causing increased intracapillary pressures. Edema usually begins within the first month of treatment or dose increase and often regresses after dose reduction or drug discontinuation.
- Antiparkinsonian dopamine agonists. These increase hydrostatic pressure by reducing sympathetic tone and dilating arterioles through alpha-2 adrenergic receptor activity.
- New antipsychotics. Drugs like clozapine, iloperidone, lurasidone, olanzapine, quetiapine, risperidone, and ziprasidone can increase hydrostatic pressure through antagonistic effects on alpha-1 adrenergic receptors, causing vasodilation.
- Nitrates. These drugs increase hydrostatic pressure by causing preferential venous dilation, leading to increased venous pooling.
- Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs can increase hydrostatic pressure by inhibiting vasodilation of afferent renal arterioles, decreasing the glomerular filtration rate, and stimulating the renin-angiotensin-aldosterone system, which leads to sodium and water retention. These adverse effects warrant cautious use of these agents.
- ACE inhibitors. Drugs such as enalapril and ramipril can increase vascular permeability. They reduce the metabolism and accumulation of bradykinin, which increases vascular permeability and fluid leakage. These effects are rare and are usually related to allergic responses.
- Insulin. Insulin decreases capillary oncotic pressure and increases vascular permeability. Rapid correction of hyperglycemia can cause a loss of oncotic pressure, while chronic hyperglycemia can damage vascular membranes, increasing permeability. These effects are generally benign and can be managed with careful dose titration, sodium restriction, or diuretics.
- Steroids. Steroids with mineralocorticoid activity can increase renal sodium and water retention, leading to increased blood volume. Fludrocortisone has the highest mineralocorticoid activity, while dexamethasone and methylprednisolone have negligible activity.
Implications
Understanding how these medications cause edema is important for effective management. For example, in the case of those causing edema due to reduced oncotic pressure, like insulin, slow dose titrations can help adapt to osmolarity changes. For drugs causing edema due to increased hydrostatic pressure, diuretics are more effective in acute management.
The key takeaways from this review are:
- Awareness of drug-induced edema. Many drugs besides CCBs can cause edema.
- Combination therapy. Combining ACE inhibitors or ARBs with CCBs can prevent or reduce CCB-induced edema.
- Edema management strategies. Strategies to manage or prevent edema should include dose reductions or replacement of the problematic medication, especially in severe or refractory cases.
Dr. Wajngarten, professor of cardiology, University of São Paulo, Brazil, has disclosed no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Edema in the feet and legs is a common complaint in our practices. It can cause pain, weakness, heaviness, discomfort, limited movement, and a negative body image. Medications can contribute to edema, either alone or in combination with other health issues.
Therefore, it is important to know how to treat or prevent medication-induced edema.
There are four main causes of edema, and all can facilitate medication-induced edema.
- Increased capillary pressure. Conditions such as heart failure, renal dysfunction, venous insufficiency, deep vein thrombosis, and cirrhosis can increase capillary pressure, leading to edema.
- Decreased oncotic pressure. Hypoalbuminemia, a primary cause of reduced colloid oncotic pressure, can result from nephrotic syndrome, diabetic nephropathy, lupus nephropathy, amyloidosis, nephropathies, cirrhosis, chronic liver disease, and malabsorption or malnutrition.
- Increased capillary permeability. Vascular injury, often associated with diabetes, can increase capillary permeability and contribute to edema.
- Impaired lymphatic drainage. Lymphatic obstruction is common in patients with lymphedema, tumors, inflammation, fibrosis, certain infections, surgery, and congenital anomalies. Conditions such as thyroid disorders can also cause an increase in interstitial albumin and other proteins without a corresponding increase in lymphatic flow, leading to lymphedema.
Medications That Can Cause Edema
- Calcium channel blockers (CCBs). Drugs such as nifedipine and amlodipine can increase hydrostatic pressure by causing selective vasodilation of precapillary vessels, leading to increased intracapillary pressures. Newer lipophilic CCBs (eg, levamlodipine) exhibit lower rates of edema. Reducing the dose is often effective. Diuretics are not very effective for vasodilation-induced edema. Combining CCBs with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), which induce postcapillary dilation and normalize intracapillary pressure, may reduce fluid leakage into the interstitial space. This combination may be more beneficial than high-dose CCB monotherapy.
- Thiazolidinedione (eg, pioglitazone). These increase vascular permeability and hydrostatic pressure. They work by stimulating the peroxisome proliferator–activated gamma receptor, increasing vascular endothelial permeability, vascular endothelial growth factor secretion, and renal retention of sodium and fluids. Because of other adverse effects, their use is now limited.
- Agents for neuropathic pain (gabapentin and pregabalin). These drugs can induce selective vasodilation of arterioles through a mechanism similar to that of CCBs, causing increased intracapillary pressures. Edema usually begins within the first month of treatment or dose increase and often regresses after dose reduction or drug discontinuation.
- Antiparkinsonian dopamine agonists. These increase hydrostatic pressure by reducing sympathetic tone and dilating arterioles through alpha-2 adrenergic receptor activity.
- New antipsychotics. Drugs like clozapine, iloperidone, lurasidone, olanzapine, quetiapine, risperidone, and ziprasidone can increase hydrostatic pressure through antagonistic effects on alpha-1 adrenergic receptors, causing vasodilation.
- Nitrates. These drugs increase hydrostatic pressure by causing preferential venous dilation, leading to increased venous pooling.
- Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs can increase hydrostatic pressure by inhibiting vasodilation of afferent renal arterioles, decreasing the glomerular filtration rate, and stimulating the renin-angiotensin-aldosterone system, which leads to sodium and water retention. These adverse effects warrant cautious use of these agents.
- ACE inhibitors. Drugs such as enalapril and ramipril can increase vascular permeability. They reduce the metabolism and accumulation of bradykinin, which increases vascular permeability and fluid leakage. These effects are rare and are usually related to allergic responses.
- Insulin. Insulin decreases capillary oncotic pressure and increases vascular permeability. Rapid correction of hyperglycemia can cause a loss of oncotic pressure, while chronic hyperglycemia can damage vascular membranes, increasing permeability. These effects are generally benign and can be managed with careful dose titration, sodium restriction, or diuretics.
- Steroids. Steroids with mineralocorticoid activity can increase renal sodium and water retention, leading to increased blood volume. Fludrocortisone has the highest mineralocorticoid activity, while dexamethasone and methylprednisolone have negligible activity.
Implications
Understanding how these medications cause edema is important for effective management. For example, in the case of those causing edema due to reduced oncotic pressure, like insulin, slow dose titrations can help adapt to osmolarity changes. For drugs causing edema due to increased hydrostatic pressure, diuretics are more effective in acute management.
The key takeaways from this review are:
- Awareness of drug-induced edema. Many drugs besides CCBs can cause edema.
- Combination therapy. Combining ACE inhibitors or ARBs with CCBs can prevent or reduce CCB-induced edema.
- Edema management strategies. Strategies to manage or prevent edema should include dose reductions or replacement of the problematic medication, especially in severe or refractory cases.
Dr. Wajngarten, professor of cardiology, University of São Paulo, Brazil, has disclosed no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Edema in the feet and legs is a common complaint in our practices. It can cause pain, weakness, heaviness, discomfort, limited movement, and a negative body image. Medications can contribute to edema, either alone or in combination with other health issues.
Therefore, it is important to know how to treat or prevent medication-induced edema.
There are four main causes of edema, and all can facilitate medication-induced edema.
- Increased capillary pressure. Conditions such as heart failure, renal dysfunction, venous insufficiency, deep vein thrombosis, and cirrhosis can increase capillary pressure, leading to edema.
- Decreased oncotic pressure. Hypoalbuminemia, a primary cause of reduced colloid oncotic pressure, can result from nephrotic syndrome, diabetic nephropathy, lupus nephropathy, amyloidosis, nephropathies, cirrhosis, chronic liver disease, and malabsorption or malnutrition.
- Increased capillary permeability. Vascular injury, often associated with diabetes, can increase capillary permeability and contribute to edema.
- Impaired lymphatic drainage. Lymphatic obstruction is common in patients with lymphedema, tumors, inflammation, fibrosis, certain infections, surgery, and congenital anomalies. Conditions such as thyroid disorders can also cause an increase in interstitial albumin and other proteins without a corresponding increase in lymphatic flow, leading to lymphedema.
Medications That Can Cause Edema
- Calcium channel blockers (CCBs). Drugs such as nifedipine and amlodipine can increase hydrostatic pressure by causing selective vasodilation of precapillary vessels, leading to increased intracapillary pressures. Newer lipophilic CCBs (eg, levamlodipine) exhibit lower rates of edema. Reducing the dose is often effective. Diuretics are not very effective for vasodilation-induced edema. Combining CCBs with angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), which induce postcapillary dilation and normalize intracapillary pressure, may reduce fluid leakage into the interstitial space. This combination may be more beneficial than high-dose CCB monotherapy.
- Thiazolidinedione (eg, pioglitazone). These increase vascular permeability and hydrostatic pressure. They work by stimulating the peroxisome proliferator–activated gamma receptor, increasing vascular endothelial permeability, vascular endothelial growth factor secretion, and renal retention of sodium and fluids. Because of other adverse effects, their use is now limited.
- Agents for neuropathic pain (gabapentin and pregabalin). These drugs can induce selective vasodilation of arterioles through a mechanism similar to that of CCBs, causing increased intracapillary pressures. Edema usually begins within the first month of treatment or dose increase and often regresses after dose reduction or drug discontinuation.
- Antiparkinsonian dopamine agonists. These increase hydrostatic pressure by reducing sympathetic tone and dilating arterioles through alpha-2 adrenergic receptor activity.
- New antipsychotics. Drugs like clozapine, iloperidone, lurasidone, olanzapine, quetiapine, risperidone, and ziprasidone can increase hydrostatic pressure through antagonistic effects on alpha-1 adrenergic receptors, causing vasodilation.
- Nitrates. These drugs increase hydrostatic pressure by causing preferential venous dilation, leading to increased venous pooling.
- Nonsteroidal anti-inflammatory drugs (NSAIDs). These drugs can increase hydrostatic pressure by inhibiting vasodilation of afferent renal arterioles, decreasing the glomerular filtration rate, and stimulating the renin-angiotensin-aldosterone system, which leads to sodium and water retention. These adverse effects warrant cautious use of these agents.
- ACE inhibitors. Drugs such as enalapril and ramipril can increase vascular permeability. They reduce the metabolism and accumulation of bradykinin, which increases vascular permeability and fluid leakage. These effects are rare and are usually related to allergic responses.
- Insulin. Insulin decreases capillary oncotic pressure and increases vascular permeability. Rapid correction of hyperglycemia can cause a loss of oncotic pressure, while chronic hyperglycemia can damage vascular membranes, increasing permeability. These effects are generally benign and can be managed with careful dose titration, sodium restriction, or diuretics.
- Steroids. Steroids with mineralocorticoid activity can increase renal sodium and water retention, leading to increased blood volume. Fludrocortisone has the highest mineralocorticoid activity, while dexamethasone and methylprednisolone have negligible activity.
Implications
Understanding how these medications cause edema is important for effective management. For example, in the case of those causing edema due to reduced oncotic pressure, like insulin, slow dose titrations can help adapt to osmolarity changes. For drugs causing edema due to increased hydrostatic pressure, diuretics are more effective in acute management.
The key takeaways from this review are:
- Awareness of drug-induced edema. Many drugs besides CCBs can cause edema.
- Combination therapy. Combining ACE inhibitors or ARBs with CCBs can prevent or reduce CCB-induced edema.
- Edema management strategies. Strategies to manage or prevent edema should include dose reductions or replacement of the problematic medication, especially in severe or refractory cases.
Dr. Wajngarten, professor of cardiology, University of São Paulo, Brazil, has disclosed no relevant financial relationships.
This story was translated from the Medscape Portuguese edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.