User login
Dysregulated sleep is common in children with eosinophilic esophagitis
, Rasintra Siriwat, MD, and colleagues have ascertained.
Children with eosinophilic esophagitis (EoE) also were found to have a high prevalence of atopic diseases, including allergic rhinitis and eczema – findings that could be driving the breathing problems, said Dr. Siriwat, a neurology fellow at the Cleveland Clinic, and coauthors.
The retrospective study comprised 81 children with a diagnosis of EoE who were referred to sleep clinics. In this group, 46 of the children had active EoE (having gastrointestinal symptoms, including feeding difficulties, dysphagia, reflux, nausea/vomiting, or epigastric pain at presentation). The other 35 had an EoE diagnosis but no symptoms on presentation and were categorized as having inactive EoE. Most were male (71.6%) and white (92.5%). The mean age in the cohort was 10 years and the mean body mass index for all subjects was 22 kg/m2. A control group of 192 children without an EoE diagnosis who had overnight polysomnography were included in the analysis.
Allergic-type comorbidities were common among those with active EoE, including allergic rhinitis (55.5%), food allergy (39.5%), and eczema (26%). In addition, a quarter had attention-deficit/hyperactivity disorder, 22% an autism spectrum disorder, 21% a neurological disease, and 29% a psychiatric disorder.
Several sleep complaints were common in the entire EoE cohort, including snoring (76.5 %), restless sleep (66.6%), legs jerking or leg discomfort (43.2%), and daytime sleepiness (58%).
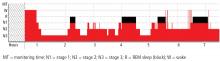
All children underwent an overnight polysomnography. Compared with controls, the children with EoE had significantly higher non-REM2 sleep, significantly lower non-REM3 sleep, lower REM, increased periodic leg movement disorder, and increased arousal index.
“Of note, we found a much higher percentage of [periodic leg movement disorder] in active EoE compared to inactive EoE,” the authors said.
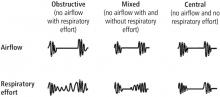
The most common sleep diagnosis for the children with EoE was sleep-disordered breathing. Of 62 children with EoE and sleep disordered breathing, 37% had obstructive sleep apnea (OSA). Two patients had central sleep apnea and five had nocturnal hypoventilation. Children with EoE also reported parasomnia symptoms such as sleep talking (35.8%), sleepwalking (16%), bruxism (23.4%), night terrors (28.4%), and nocturnal enuresis (21.2%).
Of the 59 children with leg movement, 20 had periodic limb movement disorder and 5 were diagnosed with restless leg syndrome. Two were diagnosed with narcolepsy and three with hypersomnia. Four children had a circadian rhythm disorder.
“Notably, the majority of children with EoE had symptoms of sleep-disordered breathing, and more than one-third of total subjects were diagnosed with OSA,” the authors noted. “However, most of them were mild-moderate OSA. It should be noted that the prevalence of OSA in the pediatric population is 1%-5% mostly between the ages of 2-8 years, while the mean age of our subjects was 10 years old. The high prevalence of mild-moderate OSA in the EoE population might be explained by the relationship between EoE and atopic disease.”
Dr. Siriwat had no financial disclosures. The study was supported by Cincinnati Children’s Hospital Research Fund.
SOURCE: Siriwat R et al. Sleep Med. 2019 Sep 11. doi: 10.1016/j.sleep.2019.08.018.
, Rasintra Siriwat, MD, and colleagues have ascertained.
Children with eosinophilic esophagitis (EoE) also were found to have a high prevalence of atopic diseases, including allergic rhinitis and eczema – findings that could be driving the breathing problems, said Dr. Siriwat, a neurology fellow at the Cleveland Clinic, and coauthors.
The retrospective study comprised 81 children with a diagnosis of EoE who were referred to sleep clinics. In this group, 46 of the children had active EoE (having gastrointestinal symptoms, including feeding difficulties, dysphagia, reflux, nausea/vomiting, or epigastric pain at presentation). The other 35 had an EoE diagnosis but no symptoms on presentation and were categorized as having inactive EoE. Most were male (71.6%) and white (92.5%). The mean age in the cohort was 10 years and the mean body mass index for all subjects was 22 kg/m2. A control group of 192 children without an EoE diagnosis who had overnight polysomnography were included in the analysis.
Allergic-type comorbidities were common among those with active EoE, including allergic rhinitis (55.5%), food allergy (39.5%), and eczema (26%). In addition, a quarter had attention-deficit/hyperactivity disorder, 22% an autism spectrum disorder, 21% a neurological disease, and 29% a psychiatric disorder.
Several sleep complaints were common in the entire EoE cohort, including snoring (76.5 %), restless sleep (66.6%), legs jerking or leg discomfort (43.2%), and daytime sleepiness (58%).
All children underwent an overnight polysomnography. Compared with controls, the children with EoE had significantly higher non-REM2 sleep, significantly lower non-REM3 sleep, lower REM, increased periodic leg movement disorder, and increased arousal index.
“Of note, we found a much higher percentage of [periodic leg movement disorder] in active EoE compared to inactive EoE,” the authors said.
The most common sleep diagnosis for the children with EoE was sleep-disordered breathing. Of 62 children with EoE and sleep disordered breathing, 37% had obstructive sleep apnea (OSA). Two patients had central sleep apnea and five had nocturnal hypoventilation. Children with EoE also reported parasomnia symptoms such as sleep talking (35.8%), sleepwalking (16%), bruxism (23.4%), night terrors (28.4%), and nocturnal enuresis (21.2%).
Of the 59 children with leg movement, 20 had periodic limb movement disorder and 5 were diagnosed with restless leg syndrome. Two were diagnosed with narcolepsy and three with hypersomnia. Four children had a circadian rhythm disorder.
“Notably, the majority of children with EoE had symptoms of sleep-disordered breathing, and more than one-third of total subjects were diagnosed with OSA,” the authors noted. “However, most of them were mild-moderate OSA. It should be noted that the prevalence of OSA in the pediatric population is 1%-5% mostly between the ages of 2-8 years, while the mean age of our subjects was 10 years old. The high prevalence of mild-moderate OSA in the EoE population might be explained by the relationship between EoE and atopic disease.”
Dr. Siriwat had no financial disclosures. The study was supported by Cincinnati Children’s Hospital Research Fund.
SOURCE: Siriwat R et al. Sleep Med. 2019 Sep 11. doi: 10.1016/j.sleep.2019.08.018.
, Rasintra Siriwat, MD, and colleagues have ascertained.
Children with eosinophilic esophagitis (EoE) also were found to have a high prevalence of atopic diseases, including allergic rhinitis and eczema – findings that could be driving the breathing problems, said Dr. Siriwat, a neurology fellow at the Cleveland Clinic, and coauthors.
The retrospective study comprised 81 children with a diagnosis of EoE who were referred to sleep clinics. In this group, 46 of the children had active EoE (having gastrointestinal symptoms, including feeding difficulties, dysphagia, reflux, nausea/vomiting, or epigastric pain at presentation). The other 35 had an EoE diagnosis but no symptoms on presentation and were categorized as having inactive EoE. Most were male (71.6%) and white (92.5%). The mean age in the cohort was 10 years and the mean body mass index for all subjects was 22 kg/m2. A control group of 192 children without an EoE diagnosis who had overnight polysomnography were included in the analysis.
Allergic-type comorbidities were common among those with active EoE, including allergic rhinitis (55.5%), food allergy (39.5%), and eczema (26%). In addition, a quarter had attention-deficit/hyperactivity disorder, 22% an autism spectrum disorder, 21% a neurological disease, and 29% a psychiatric disorder.
Several sleep complaints were common in the entire EoE cohort, including snoring (76.5 %), restless sleep (66.6%), legs jerking or leg discomfort (43.2%), and daytime sleepiness (58%).
All children underwent an overnight polysomnography. Compared with controls, the children with EoE had significantly higher non-REM2 sleep, significantly lower non-REM3 sleep, lower REM, increased periodic leg movement disorder, and increased arousal index.
“Of note, we found a much higher percentage of [periodic leg movement disorder] in active EoE compared to inactive EoE,” the authors said.
The most common sleep diagnosis for the children with EoE was sleep-disordered breathing. Of 62 children with EoE and sleep disordered breathing, 37% had obstructive sleep apnea (OSA). Two patients had central sleep apnea and five had nocturnal hypoventilation. Children with EoE also reported parasomnia symptoms such as sleep talking (35.8%), sleepwalking (16%), bruxism (23.4%), night terrors (28.4%), and nocturnal enuresis (21.2%).
Of the 59 children with leg movement, 20 had periodic limb movement disorder and 5 were diagnosed with restless leg syndrome. Two were diagnosed with narcolepsy and three with hypersomnia. Four children had a circadian rhythm disorder.
“Notably, the majority of children with EoE had symptoms of sleep-disordered breathing, and more than one-third of total subjects were diagnosed with OSA,” the authors noted. “However, most of them were mild-moderate OSA. It should be noted that the prevalence of OSA in the pediatric population is 1%-5% mostly between the ages of 2-8 years, while the mean age of our subjects was 10 years old. The high prevalence of mild-moderate OSA in the EoE population might be explained by the relationship between EoE and atopic disease.”
Dr. Siriwat had no financial disclosures. The study was supported by Cincinnati Children’s Hospital Research Fund.
SOURCE: Siriwat R et al. Sleep Med. 2019 Sep 11. doi: 10.1016/j.sleep.2019.08.018.
FROM SLEEP MEDICINE
Can sleep apnea be accurately diagnosed at home?
ILLUSTRATIVE CASE
A 50-year-old overweight male with a history of hypertension presents to your office for a yearly physical. On review of symptoms, he notes feeling constantly tired, despite reported good sleep hygiene practices. He scores 11 on the Epworth Sleepiness Scale, and his wife complains about his snoring. You have a high suspicion of obstructive sleep apnea. What is your next step?
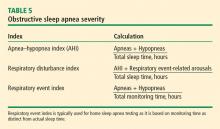
Obstructive sleep apnea (OSA) is quite common, affecting at least 2% to 4% of the general adult population.2 The gold standard for OSA diagnosis has been laboratory polysomnography (PSG) to measure the apnea-hypopnea index (AHI), which is the average number of apneas and hypopneas per hour of sleep, and the respiratory event index (REI), which is the average number of apneas, hypopneas, and respiratory effort-related arousals per hour of sleep. A minimum of 5 on the AHI or REI, along with clinical symptoms, is required for diagnosis.
Many adults go undiagnosed and untreated, however, due to barriers to diagnosis including the inconvenience of laboratory PSG.3 Sleep laboratories often have a significant wait time for evaluation, and sleeping in an unfamiliar place can be inconvenient or intolerable for some patients, making diagnosis difficult despite high clinical suspicion. Untreated sleep apnea is associated with an increased risk of hypertension, coronary artery disease, congestive heart failure, stroke, atrial fibrillation, and type 2 diabetes.4
Home sleep studies are an alternative for patients with a high risk of OSA without comorbid sleep conditions, heart failure, or chronic obstructive pulmonary disease (COPD). This study investigated the long-term effectiveness of diagnosis by home respiratory polygraphy (HRP) vs laboratory PSG in patients with an intermediate to high clinical suspicion for OSA.
STUDY SUMMARY
Home Dx is noninferior to lab Dx in all aspects studied
This multicenter, noninferiority randomized controlled trial and cost analysis study conducted in Spain randomized 430 adults referred to pulmonology for suspected OSA to receive either in-lab PSG or HRP. Patients received treatment with continuous positive airway pressure (CPAP) if their REI was ≥ 5 for HRP or their AHI was ≥ 5 for PSG with significant clinical symptoms, which is consistent with the Spanish Sleep Network guidelines.5 All patients in both arms received sleep hygiene instruction, nutrition education, and single-session auto-CPAP titration, and were evaluated at 1 and 3 months to assess for compliance. At 6 months, all patients were evaluated with PSG.
HRP was found to be non-inferior to PSG based on Epworth Sleepiness Scale (ESS) scores evaluated at baseline and at 6-month follow-up (HRP mean = -4.2 points; 95% confidence interval [CI], -4.8 to -3.6 and PSG mean -4.9; 95% CI, -5.4 to -4.3; P = .14). Both groups had similar secondary outcomes. Quality-of-life as measured by the 30-point Functional Outcomes of Sleep Questionnaire improved by an average of 6.7 (standard deviation [SD] = 16.7) in the HRP group vs 6.5 (SD = 18.1) in the PSG group (P = .92). Systolic and diastolic blood pressure improved significantly in both groups without any statistically significant difference between the groups. HRP was also found to be more cost-effective than PSG with a savings equivalent to more than half the cost of PSG, or about $450 per study (depending on the exchange rate).
WHAT’S NEW
HRP offers advantages for low-risk patients
In the majority of patients, OSA can be diagnosed at home with outcomes similar to those for lab diagnosis, decreased cost, and decreased time from suspected diagnosis to treatment. HRP is acceptable for patients with a high probability of OSA without significant comorbidities if monitoring includes at least airflow, respiratory effort, and blood oxygenation.6
Continue to: CAVEATS
CAVEATS
Recommendations are somewhat ambiguous
This study, as well as current guidelines, recommend home sleep studies for patients with a high clinical suspicion or high pre-test probability of OSA and who lack comorbid conditions that could affect sleep. The comorbid conditions are well identified: COPD, heart failure hypoventilation syndromes, insomnia, hypersomnia, parasomnia, periodic limb movement disorder, narcolepsy, and chronic opioid use.6 However, what constitutes “a high clinical suspicion” or “high pre-test probability” was not well defined in this study.
Several clinical screening tools are available and include the ESS, Berlin Questionnaire, and STOP-BANG Scoring System (Snoring, Tiredness, Observed apnea, Pressure [systemic hypertension], Body mass index > 35, Age > 50 years, Neck circumference > 16 inches, male Gender). An ESS score ≥ 10 warrants further evaluation, but is not very sensitive. Two or more positive categories on the Berlin Questionnaire indicates a high risk of OSA with a sensitivity of 76%, 77%, and 77% for mild, moderate, and severe OSA, respectively.7 A score of ≥ 3 on the STOP-BANG Scoring System has been validated and has a sensitivity of 83.6%, 92.9%, and 100% for an AHI > 5, > 15, and > 30, respectively.8
Home sleep studies should not be used to screen the general population.
CHALLENGES TO IMPLEMENTATION
Recommendations may present a challenge but insurance should not
The American Academy of Sleep Medicine recommends that portable monitoring must record airflow, respiratory effort, and blood oxygenation, and the device must be able to display the raw data to be interpreted by a board-certified sleep medicine physician according to current published standards.6 Implementation would require appropriate selection of a home monitoring device, consultation with a sleep medicine specialist, and significant patient education to ensure interpretable results.
Insurance should not be a barrier to implementation as the Centers for Medicare and Medicaid Services accept home sleep apnea testing results for CPAP prescriptions.9 However, variability currently exists regarding the extent to which private insurers provide coverage for home sleep apnea testing.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Corral J, Sánchez-Quiroga MÁ, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196:1181-1190.
2. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
3. Colten H, Abboud F, Block G, et al. Sleep disorders and sleep deprivation: an unmet public health problem. 2006. Washington, DC: National Academy of Sciences.
4. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
5. Lloberes P, Durán-Cantolla J, Martinez-Garcia MA, et al. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2011;47:143-156.
6. Rosen IM, Kirsch DB, Chervin RD; American Academy of Sleep Medicine Board of Directors. Clinical use of a home sleep apnea test: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2017;13:1205-1207.
7. Chiu HY, Chen PY, Chuang, LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP and Epworth Sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57-70.
8. Chung, F, Yegneswaran B, Lio P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
9. Centers for Medicare and Medicaid Services. Decision Memo for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (CAG-00093R2). March 13, 2008. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=204. Accessed September 6, 2019.
ILLUSTRATIVE CASE
A 50-year-old overweight male with a history of hypertension presents to your office for a yearly physical. On review of symptoms, he notes feeling constantly tired, despite reported good sleep hygiene practices. He scores 11 on the Epworth Sleepiness Scale, and his wife complains about his snoring. You have a high suspicion of obstructive sleep apnea. What is your next step?
Obstructive sleep apnea (OSA) is quite common, affecting at least 2% to 4% of the general adult population.2 The gold standard for OSA diagnosis has been laboratory polysomnography (PSG) to measure the apnea-hypopnea index (AHI), which is the average number of apneas and hypopneas per hour of sleep, and the respiratory event index (REI), which is the average number of apneas, hypopneas, and respiratory effort-related arousals per hour of sleep. A minimum of 5 on the AHI or REI, along with clinical symptoms, is required for diagnosis.
Many adults go undiagnosed and untreated, however, due to barriers to diagnosis including the inconvenience of laboratory PSG.3 Sleep laboratories often have a significant wait time for evaluation, and sleeping in an unfamiliar place can be inconvenient or intolerable for some patients, making diagnosis difficult despite high clinical suspicion. Untreated sleep apnea is associated with an increased risk of hypertension, coronary artery disease, congestive heart failure, stroke, atrial fibrillation, and type 2 diabetes.4
Home sleep studies are an alternative for patients with a high risk of OSA without comorbid sleep conditions, heart failure, or chronic obstructive pulmonary disease (COPD). This study investigated the long-term effectiveness of diagnosis by home respiratory polygraphy (HRP) vs laboratory PSG in patients with an intermediate to high clinical suspicion for OSA.
STUDY SUMMARY
Home Dx is noninferior to lab Dx in all aspects studied
This multicenter, noninferiority randomized controlled trial and cost analysis study conducted in Spain randomized 430 adults referred to pulmonology for suspected OSA to receive either in-lab PSG or HRP. Patients received treatment with continuous positive airway pressure (CPAP) if their REI was ≥ 5 for HRP or their AHI was ≥ 5 for PSG with significant clinical symptoms, which is consistent with the Spanish Sleep Network guidelines.5 All patients in both arms received sleep hygiene instruction, nutrition education, and single-session auto-CPAP titration, and were evaluated at 1 and 3 months to assess for compliance. At 6 months, all patients were evaluated with PSG.
HRP was found to be non-inferior to PSG based on Epworth Sleepiness Scale (ESS) scores evaluated at baseline and at 6-month follow-up (HRP mean = -4.2 points; 95% confidence interval [CI], -4.8 to -3.6 and PSG mean -4.9; 95% CI, -5.4 to -4.3; P = .14). Both groups had similar secondary outcomes. Quality-of-life as measured by the 30-point Functional Outcomes of Sleep Questionnaire improved by an average of 6.7 (standard deviation [SD] = 16.7) in the HRP group vs 6.5 (SD = 18.1) in the PSG group (P = .92). Systolic and diastolic blood pressure improved significantly in both groups without any statistically significant difference between the groups. HRP was also found to be more cost-effective than PSG with a savings equivalent to more than half the cost of PSG, or about $450 per study (depending on the exchange rate).
WHAT’S NEW
HRP offers advantages for low-risk patients
In the majority of patients, OSA can be diagnosed at home with outcomes similar to those for lab diagnosis, decreased cost, and decreased time from suspected diagnosis to treatment. HRP is acceptable for patients with a high probability of OSA without significant comorbidities if monitoring includes at least airflow, respiratory effort, and blood oxygenation.6
Continue to: CAVEATS
CAVEATS
Recommendations are somewhat ambiguous
This study, as well as current guidelines, recommend home sleep studies for patients with a high clinical suspicion or high pre-test probability of OSA and who lack comorbid conditions that could affect sleep. The comorbid conditions are well identified: COPD, heart failure hypoventilation syndromes, insomnia, hypersomnia, parasomnia, periodic limb movement disorder, narcolepsy, and chronic opioid use.6 However, what constitutes “a high clinical suspicion” or “high pre-test probability” was not well defined in this study.
Several clinical screening tools are available and include the ESS, Berlin Questionnaire, and STOP-BANG Scoring System (Snoring, Tiredness, Observed apnea, Pressure [systemic hypertension], Body mass index > 35, Age > 50 years, Neck circumference > 16 inches, male Gender). An ESS score ≥ 10 warrants further evaluation, but is not very sensitive. Two or more positive categories on the Berlin Questionnaire indicates a high risk of OSA with a sensitivity of 76%, 77%, and 77% for mild, moderate, and severe OSA, respectively.7 A score of ≥ 3 on the STOP-BANG Scoring System has been validated and has a sensitivity of 83.6%, 92.9%, and 100% for an AHI > 5, > 15, and > 30, respectively.8
Home sleep studies should not be used to screen the general population.
CHALLENGES TO IMPLEMENTATION
Recommendations may present a challenge but insurance should not
The American Academy of Sleep Medicine recommends that portable monitoring must record airflow, respiratory effort, and blood oxygenation, and the device must be able to display the raw data to be interpreted by a board-certified sleep medicine physician according to current published standards.6 Implementation would require appropriate selection of a home monitoring device, consultation with a sleep medicine specialist, and significant patient education to ensure interpretable results.
Insurance should not be a barrier to implementation as the Centers for Medicare and Medicaid Services accept home sleep apnea testing results for CPAP prescriptions.9 However, variability currently exists regarding the extent to which private insurers provide coverage for home sleep apnea testing.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 50-year-old overweight male with a history of hypertension presents to your office for a yearly physical. On review of symptoms, he notes feeling constantly tired, despite reported good sleep hygiene practices. He scores 11 on the Epworth Sleepiness Scale, and his wife complains about his snoring. You have a high suspicion of obstructive sleep apnea. What is your next step?
Obstructive sleep apnea (OSA) is quite common, affecting at least 2% to 4% of the general adult population.2 The gold standard for OSA diagnosis has been laboratory polysomnography (PSG) to measure the apnea-hypopnea index (AHI), which is the average number of apneas and hypopneas per hour of sleep, and the respiratory event index (REI), which is the average number of apneas, hypopneas, and respiratory effort-related arousals per hour of sleep. A minimum of 5 on the AHI or REI, along with clinical symptoms, is required for diagnosis.
Many adults go undiagnosed and untreated, however, due to barriers to diagnosis including the inconvenience of laboratory PSG.3 Sleep laboratories often have a significant wait time for evaluation, and sleeping in an unfamiliar place can be inconvenient or intolerable for some patients, making diagnosis difficult despite high clinical suspicion. Untreated sleep apnea is associated with an increased risk of hypertension, coronary artery disease, congestive heart failure, stroke, atrial fibrillation, and type 2 diabetes.4
Home sleep studies are an alternative for patients with a high risk of OSA without comorbid sleep conditions, heart failure, or chronic obstructive pulmonary disease (COPD). This study investigated the long-term effectiveness of diagnosis by home respiratory polygraphy (HRP) vs laboratory PSG in patients with an intermediate to high clinical suspicion for OSA.
STUDY SUMMARY
Home Dx is noninferior to lab Dx in all aspects studied
This multicenter, noninferiority randomized controlled trial and cost analysis study conducted in Spain randomized 430 adults referred to pulmonology for suspected OSA to receive either in-lab PSG or HRP. Patients received treatment with continuous positive airway pressure (CPAP) if their REI was ≥ 5 for HRP or their AHI was ≥ 5 for PSG with significant clinical symptoms, which is consistent with the Spanish Sleep Network guidelines.5 All patients in both arms received sleep hygiene instruction, nutrition education, and single-session auto-CPAP titration, and were evaluated at 1 and 3 months to assess for compliance. At 6 months, all patients were evaluated with PSG.
HRP was found to be non-inferior to PSG based on Epworth Sleepiness Scale (ESS) scores evaluated at baseline and at 6-month follow-up (HRP mean = -4.2 points; 95% confidence interval [CI], -4.8 to -3.6 and PSG mean -4.9; 95% CI, -5.4 to -4.3; P = .14). Both groups had similar secondary outcomes. Quality-of-life as measured by the 30-point Functional Outcomes of Sleep Questionnaire improved by an average of 6.7 (standard deviation [SD] = 16.7) in the HRP group vs 6.5 (SD = 18.1) in the PSG group (P = .92). Systolic and diastolic blood pressure improved significantly in both groups without any statistically significant difference between the groups. HRP was also found to be more cost-effective than PSG with a savings equivalent to more than half the cost of PSG, or about $450 per study (depending on the exchange rate).
WHAT’S NEW
HRP offers advantages for low-risk patients
In the majority of patients, OSA can be diagnosed at home with outcomes similar to those for lab diagnosis, decreased cost, and decreased time from suspected diagnosis to treatment. HRP is acceptable for patients with a high probability of OSA without significant comorbidities if monitoring includes at least airflow, respiratory effort, and blood oxygenation.6
Continue to: CAVEATS
CAVEATS
Recommendations are somewhat ambiguous
This study, as well as current guidelines, recommend home sleep studies for patients with a high clinical suspicion or high pre-test probability of OSA and who lack comorbid conditions that could affect sleep. The comorbid conditions are well identified: COPD, heart failure hypoventilation syndromes, insomnia, hypersomnia, parasomnia, periodic limb movement disorder, narcolepsy, and chronic opioid use.6 However, what constitutes “a high clinical suspicion” or “high pre-test probability” was not well defined in this study.
Several clinical screening tools are available and include the ESS, Berlin Questionnaire, and STOP-BANG Scoring System (Snoring, Tiredness, Observed apnea, Pressure [systemic hypertension], Body mass index > 35, Age > 50 years, Neck circumference > 16 inches, male Gender). An ESS score ≥ 10 warrants further evaluation, but is not very sensitive. Two or more positive categories on the Berlin Questionnaire indicates a high risk of OSA with a sensitivity of 76%, 77%, and 77% for mild, moderate, and severe OSA, respectively.7 A score of ≥ 3 on the STOP-BANG Scoring System has been validated and has a sensitivity of 83.6%, 92.9%, and 100% for an AHI > 5, > 15, and > 30, respectively.8
Home sleep studies should not be used to screen the general population.
CHALLENGES TO IMPLEMENTATION
Recommendations may present a challenge but insurance should not
The American Academy of Sleep Medicine recommends that portable monitoring must record airflow, respiratory effort, and blood oxygenation, and the device must be able to display the raw data to be interpreted by a board-certified sleep medicine physician according to current published standards.6 Implementation would require appropriate selection of a home monitoring device, consultation with a sleep medicine specialist, and significant patient education to ensure interpretable results.
Insurance should not be a barrier to implementation as the Centers for Medicare and Medicaid Services accept home sleep apnea testing results for CPAP prescriptions.9 However, variability currently exists regarding the extent to which private insurers provide coverage for home sleep apnea testing.
ACKNOWLEDGMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Corral J, Sánchez-Quiroga MÁ, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196:1181-1190.
2. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
3. Colten H, Abboud F, Block G, et al. Sleep disorders and sleep deprivation: an unmet public health problem. 2006. Washington, DC: National Academy of Sciences.
4. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
5. Lloberes P, Durán-Cantolla J, Martinez-Garcia MA, et al. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2011;47:143-156.
6. Rosen IM, Kirsch DB, Chervin RD; American Academy of Sleep Medicine Board of Directors. Clinical use of a home sleep apnea test: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2017;13:1205-1207.
7. Chiu HY, Chen PY, Chuang, LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP and Epworth Sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57-70.
8. Chung, F, Yegneswaran B, Lio P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
9. Centers for Medicare and Medicaid Services. Decision Memo for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (CAG-00093R2). March 13, 2008. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=204. Accessed September 6, 2019.
1. Corral J, Sánchez-Quiroga MÁ, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196:1181-1190.
2. Epstein LJ, Kristo D, Strollo PJ, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263-276.
3. Colten H, Abboud F, Block G, et al. Sleep disorders and sleep deprivation: an unmet public health problem. 2006. Washington, DC: National Academy of Sciences.
4. Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136-143.
5. Lloberes P, Durán-Cantolla J, Martinez-Garcia MA, et al. Diagnosis and treatment of sleep apnea-hypopnea syndrome. Spanish Society of Pulmonology and Thoracic Surgery. Arch Bronconeumol. 2011;47:143-156.
6. Rosen IM, Kirsch DB, Chervin RD; American Academy of Sleep Medicine Board of Directors. Clinical use of a home sleep apnea test: an American Academy of Sleep Medicine position statement. J Clin Sleep Med. 2017;13:1205-1207.
7. Chiu HY, Chen PY, Chuang, LP, et al. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP and Epworth Sleepiness scale in detecting obstructive sleep apnea: a bivariate meta-analysis. Sleep Med Rev. 2017;36:57-70.
8. Chung, F, Yegneswaran B, Lio P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108:812-821.
9. Centers for Medicare and Medicaid Services. Decision Memo for Continuous Positive Airway Pressure (CPAP) Therapy for Obstructive Sleep Apnea (OSA) (CAG-00093R2). March 13, 2008. https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=204. Accessed September 6, 2019.
PRACTICE CHANGER
Consider ordering home respiratory polygraphy vs laboratory sleep studies for patients suspected of having obstructive sleep apnea.1
Corral J, Sánchez-Quiroga MÁ, Carmona-Bernal C, et al. Conventional polysomnography is not necessary for the management of most patients with suspected obstructive sleep apnea. Noninferiority, randomized controlled trial. Am J Respir Crit Care Med. 2017;196:1181-1190.
STRENGTH OF RECOMMENDATION
B: Based on a multicenter, noninferiority randomized controlled trial and cost analysis study.
What are the risks to inpatients during hospital construction or renovation?
Hospital-acquired infections related to construction and renovation activities account for more than 5,000 deaths per year across the United States.1
Hospital construction, renovation, and demolition projects ultimately serve the interests of patients, but they also can put inpatients at risk of mold infection, Legionnaires disease, sleep deprivation, exacerbation of lung disease, and in rare cases, physical injury.
Hospitals are in a continuous state of transformation to meet the needs of medical and technologic advances and an increasing patient population,1 and in the last 10 years, more than $200 billion has been spent on construction projects at US healthcare facilities. Therefore, constant attention is needed to reduce the risks to the health of hospitalized patients during these projects.
HOSPITAL-ACQUIRED INFECTIONS
Mold infections
Construction can cause substantial dust contamination and scatter large amounts of fungal spores. An analysis conducted during a period of excavation at a hospital campus showed a significant association between excavation activities and hospital-acquired mold infections (hazard ratio [HR] 2.8, P = .01) but not yeast infections (HR 0.75, P = .78).2
Aspergillus species have been the organisms most commonly involved in hospital-acquired mold infection. In a review of 53 studies including 458 patients,3 A fumigatus was identified in 154 patients, and A flavus was identified in 101 patients. A niger, A terreus, A nidulans, Zygomycetes, and other fungi were also identified, but to a much lesser extent. Hematologic malignancies were the predominant underlying morbidity in 299 patients. Half of the sources of healthcare-associated Aspergillus outbreaks were estimated to result from construction and renovation activities within or surrounding the hospital.3
Heavy demolition and transportation of wreckage have been found to cause the greatest concentrations of Aspergillus species,1 but even small concentrations may be sufficient to cause infection in high-risk hospitalized patients.3 Invasive pulmonary aspergillosis is the mold infection most commonly associated with these activities, particularly in immunocompromised and critically ill patients. It is characterized by invasion of lung tissue by Aspergillus hyphae. Hematogenous dissemination occurs in about 25% of patients, and the death rate often exceeds 50%.4
A review of cases of fungal infection during hospital construction, renovation, and demolition projects from 1976 to 2014 identified 372 infected patients, of whom 180 died.5 The majority of infections were due to Aspergillus. Other fungi included Rhizopus, Candida, and Fusarium. Infections occurred mainly in patients with hematologic malignancies and patients who had undergone stem cell transplant (76%), followed by patients with other malignancies or transplant (19%). Rarely affected were patients in the intensive care unit or patients with rheumatologic diseases or on hemodialysis.5
Legionnaires disease
Legionnaires disease is a form of atypical pneumonia caused by the bacterium Legionella, often associated with differing degrees of gastrointestinal symptoms. Legionella species are the bacteria most often associated with construction in hospitals, as construction and demolition often result in collections of stagnant water.
The primary mode of transmission is inhalation of contaminated mist or aerosols. Legionella species can also colonize newly constructed hospital buildings within weeks of installation of water fixtures.
In a large university-affiliated hospital, 2 cases of nosocomial legionellosis were identified during a period of major construction.6 An epidemiologic investigation traced the source to a widespread contamination of potable water within the hospital. One patient’s isolate was similar to that of a water sample from the faucet in his room, and an association between Legionnaires disease and construction was postulated.
Another institution’s newly constructed hematology-oncology unit identified 10 cases of Legionnaires disease over a 12-week period in patients and visitors with exposure to the unit during and within the incubation period.7 A clinical and environmental assessment found 3 clinical isolates of Legionella identical to environmental isolates found from the unit, strongly implicating the potable water system as the likely source.7
In Ohio, 11 cases of hospital-acquired Legionnaires disease were identified in patients moved to a newly constructed 12-story addition to a hospital, and 1 of those died.8
Legionella infections appear to be less common than mold infections when reviewing the available literature on patients exposed to hospital construction, renovation, or demolition activities. Yet unlike mold infections, which occur mostly in immunocompromised patients, Legionella also affects people with normal immunity.1
NONCOMMUNICABLE ILLNESSES
Sleep deprivation
Noise in hospitals has been linked to sleep disturbances in inpatients. A study using noise dosimeters in a university hospital found a mean continuous noise level of 63.5 dBA (A-weighting of decibels indicates risk of hearing loss) over a 24-hour period, a level more than 2 times higher than the recommended 30 dBA.9 The same study also found a significant correlation between sleep disturbance in inpatients and increasing noise levels, in a dose-response manner.
Common sources of noise during construction may include power generators, welding and cutting equipment, and transport of materials. While construction activities themselves have yet to be directly linked to sleep deprivation in patients, construction is inevitably accompanied by noise.
Noise is the most common factor interfering with sleep reported by hospitalized patients. Other effects of noise on patients include a rise in heart rate and blood pressure, increased cholesterol and triglyceride levels, increased use of sedatives, and longer length of stay.9,10 Although construction is rarely done at night, patients generally take naps during the day, so the noise is disruptive.
Physical injuries
Hospitalized patients rarely suffer injuries related to hospital construction. However, these incidents may be underreported. Few cases of physical injury in patients exposed to construction or renovation in healthcare facilities can be found through a Web search.11,12
Exacerbation of lung disease
Inhalation of indoor air pollutants exposed during renovation can directly trigger an inflammatory response and cause exacerbation in patients with chronic lung diseases such as asthma and chronic obstructive pulmonary disease. No study has specifically examined the effect of hospital construction or renovation on exacerbation of chronic lung diseases in hospitalized patients. Nevertheless, dust and indoor air pollutants from building renovation have often been reported as agents associated with work-related asthma.13
THE MESSAGE
Although the risks to inpatients during hospital construction projects appear minimal, their effect can at times be detrimental, especially to the immunocompromised. Hospitals should adhere to infection control risk assessment protocols during construction events. The small number of outbreaks of construction-related infections can make the diagnosis of nosocomial origin of these infections challenging; a high index of suspicion is needed.
Currently in the United States, there is no standard regarding acceptable levels of airborne mold concentrations, and data to support routine hospital air sampling or validation of available air samplers are inadequate. This remains an area for future research.14,15
Certain measures have been shown to significantly decrease the risk of mold infections and other nosocomial infections during construction projects, including16:
- Effective dust control through containment units and barriers
- Consistent use of high-efficiency particulate air filters in hospital units that care for immunocompromised and critically ill patients
- Routine surveillance.
Noise and vibration can be reduced by temporary walls and careful tool selection and scheduling. Similarly, temporary walls and other barriers help protect healthcare employees and patients from the risk of direct physical injury.
Preconstruction risk assessments that address infection control, safety, noise, and air quality are crucial, and the Joint Commission generally requires such assessments. Further, education of hospital staff and members of the construction team about the potential detrimental effects of hospital construction and renovation is essential to secure a safe environment.
- Clair JD, Colatrella S. Opening Pandora’s (tool) box: health care construction and associated risk for nosocomial infection. Infect Disord Drug Targets 2013; 13(3):177–183. pmid:23961740
- Pokala HR, Leonard D, Cox J, et al. Association of hospital construction with the development of healthcare associated environmental mold infections (HAEMI) in pediatric patients with leukemia. Pediatr Blood Cancer 2014; 61(2):276–280. doi:10.1002/pbc.24685
- Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect 2006; 63(3):246–254. doi:10.1016/j.jhin.2006.02.014
- Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med 2018; 141:121–131. doi:10.1016/j.rmed.2018.06.029
- Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 2015; 61(3):433–444. doi:10.1093/cid/civ297
- Perola O, Kauppinen J, Kusnetsov J, Heikkinen J, Jokinen C, Katila ML. Nosocomial Legionella pneumophila serogroup 5 outbreak associated with persistent colonization of a hospital water system. APMIS 2002; 110(12):863–868. pmid:12645664
- Francois Watkins LK, Toews KE, Harris AM, et al. Lessons from an outbreak of Legionnaires disease on a hematology-oncology unit. Infect Control Hosp Epidemiol 2017; 38(3):306–313. doi:10.1017/ice.2016.281
- Lin YE, Stout JE, Yu VL. Prevention of hospital-acquired legionellosis. Curr Opin Infect Dis 2011; 24(4):350–356. doi:10.1097/QCO.0b013e3283486c6e
- Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol 2014; 29:e2014006. doi:10.5620/eht.2014.29.e2014006
- Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med 2012; 157(3):170–179. doi:10.7326/0003-4819-157-3-201208070-00472
- Heldt D; The Gazette. Accident will delay University of Iowa Hospitals construction work for several days. www.thegazette.com/2013/03/08/university-of-iowa-hospitals-patient-injured-by-falling-construction-debris. Accessed July 22, 2019.
- Darrah N; Fox News. Texas hospital explosion kills 1, leaves 12 injured. www.foxnews.com/us/texas-hospital-explosion-kills-1-leaves-12-injured. Accessed July 22, 2019.
- Centers for Disease Control and Prevention (CDC). Work-related asthma: most frequently reported agents associated with work-related asthma cases by state, 2009–2012. wwwn.cdc.gov/eworld/Data/926. Accessed July 22, 2019.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63(4):e1–e60. doi:10.1093/cid/ciw326
- Chang CC, Athan E, Morrissey CO, Slavin MA. Preventing invasive fungal infection during hospital building works. Intern Med J 2008; 38(6b):538–541. doi:10.1111/j.1445-5994.2008.01727.x
- Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001; 66(4):257–262. doi:10.1002/ajh.1054
Hospital-acquired infections related to construction and renovation activities account for more than 5,000 deaths per year across the United States.1
Hospital construction, renovation, and demolition projects ultimately serve the interests of patients, but they also can put inpatients at risk of mold infection, Legionnaires disease, sleep deprivation, exacerbation of lung disease, and in rare cases, physical injury.
Hospitals are in a continuous state of transformation to meet the needs of medical and technologic advances and an increasing patient population,1 and in the last 10 years, more than $200 billion has been spent on construction projects at US healthcare facilities. Therefore, constant attention is needed to reduce the risks to the health of hospitalized patients during these projects.
HOSPITAL-ACQUIRED INFECTIONS
Mold infections
Construction can cause substantial dust contamination and scatter large amounts of fungal spores. An analysis conducted during a period of excavation at a hospital campus showed a significant association between excavation activities and hospital-acquired mold infections (hazard ratio [HR] 2.8, P = .01) but not yeast infections (HR 0.75, P = .78).2
Aspergillus species have been the organisms most commonly involved in hospital-acquired mold infection. In a review of 53 studies including 458 patients,3 A fumigatus was identified in 154 patients, and A flavus was identified in 101 patients. A niger, A terreus, A nidulans, Zygomycetes, and other fungi were also identified, but to a much lesser extent. Hematologic malignancies were the predominant underlying morbidity in 299 patients. Half of the sources of healthcare-associated Aspergillus outbreaks were estimated to result from construction and renovation activities within or surrounding the hospital.3
Heavy demolition and transportation of wreckage have been found to cause the greatest concentrations of Aspergillus species,1 but even small concentrations may be sufficient to cause infection in high-risk hospitalized patients.3 Invasive pulmonary aspergillosis is the mold infection most commonly associated with these activities, particularly in immunocompromised and critically ill patients. It is characterized by invasion of lung tissue by Aspergillus hyphae. Hematogenous dissemination occurs in about 25% of patients, and the death rate often exceeds 50%.4
A review of cases of fungal infection during hospital construction, renovation, and demolition projects from 1976 to 2014 identified 372 infected patients, of whom 180 died.5 The majority of infections were due to Aspergillus. Other fungi included Rhizopus, Candida, and Fusarium. Infections occurred mainly in patients with hematologic malignancies and patients who had undergone stem cell transplant (76%), followed by patients with other malignancies or transplant (19%). Rarely affected were patients in the intensive care unit or patients with rheumatologic diseases or on hemodialysis.5
Legionnaires disease
Legionnaires disease is a form of atypical pneumonia caused by the bacterium Legionella, often associated with differing degrees of gastrointestinal symptoms. Legionella species are the bacteria most often associated with construction in hospitals, as construction and demolition often result in collections of stagnant water.
The primary mode of transmission is inhalation of contaminated mist or aerosols. Legionella species can also colonize newly constructed hospital buildings within weeks of installation of water fixtures.
In a large university-affiliated hospital, 2 cases of nosocomial legionellosis were identified during a period of major construction.6 An epidemiologic investigation traced the source to a widespread contamination of potable water within the hospital. One patient’s isolate was similar to that of a water sample from the faucet in his room, and an association between Legionnaires disease and construction was postulated.
Another institution’s newly constructed hematology-oncology unit identified 10 cases of Legionnaires disease over a 12-week period in patients and visitors with exposure to the unit during and within the incubation period.7 A clinical and environmental assessment found 3 clinical isolates of Legionella identical to environmental isolates found from the unit, strongly implicating the potable water system as the likely source.7
In Ohio, 11 cases of hospital-acquired Legionnaires disease were identified in patients moved to a newly constructed 12-story addition to a hospital, and 1 of those died.8
Legionella infections appear to be less common than mold infections when reviewing the available literature on patients exposed to hospital construction, renovation, or demolition activities. Yet unlike mold infections, which occur mostly in immunocompromised patients, Legionella also affects people with normal immunity.1
NONCOMMUNICABLE ILLNESSES
Sleep deprivation
Noise in hospitals has been linked to sleep disturbances in inpatients. A study using noise dosimeters in a university hospital found a mean continuous noise level of 63.5 dBA (A-weighting of decibels indicates risk of hearing loss) over a 24-hour period, a level more than 2 times higher than the recommended 30 dBA.9 The same study also found a significant correlation between sleep disturbance in inpatients and increasing noise levels, in a dose-response manner.
Common sources of noise during construction may include power generators, welding and cutting equipment, and transport of materials. While construction activities themselves have yet to be directly linked to sleep deprivation in patients, construction is inevitably accompanied by noise.
Noise is the most common factor interfering with sleep reported by hospitalized patients. Other effects of noise on patients include a rise in heart rate and blood pressure, increased cholesterol and triglyceride levels, increased use of sedatives, and longer length of stay.9,10 Although construction is rarely done at night, patients generally take naps during the day, so the noise is disruptive.
Physical injuries
Hospitalized patients rarely suffer injuries related to hospital construction. However, these incidents may be underreported. Few cases of physical injury in patients exposed to construction or renovation in healthcare facilities can be found through a Web search.11,12
Exacerbation of lung disease
Inhalation of indoor air pollutants exposed during renovation can directly trigger an inflammatory response and cause exacerbation in patients with chronic lung diseases such as asthma and chronic obstructive pulmonary disease. No study has specifically examined the effect of hospital construction or renovation on exacerbation of chronic lung diseases in hospitalized patients. Nevertheless, dust and indoor air pollutants from building renovation have often been reported as agents associated with work-related asthma.13
THE MESSAGE
Although the risks to inpatients during hospital construction projects appear minimal, their effect can at times be detrimental, especially to the immunocompromised. Hospitals should adhere to infection control risk assessment protocols during construction events. The small number of outbreaks of construction-related infections can make the diagnosis of nosocomial origin of these infections challenging; a high index of suspicion is needed.
Currently in the United States, there is no standard regarding acceptable levels of airborne mold concentrations, and data to support routine hospital air sampling or validation of available air samplers are inadequate. This remains an area for future research.14,15
Certain measures have been shown to significantly decrease the risk of mold infections and other nosocomial infections during construction projects, including16:
- Effective dust control through containment units and barriers
- Consistent use of high-efficiency particulate air filters in hospital units that care for immunocompromised and critically ill patients
- Routine surveillance.
Noise and vibration can be reduced by temporary walls and careful tool selection and scheduling. Similarly, temporary walls and other barriers help protect healthcare employees and patients from the risk of direct physical injury.
Preconstruction risk assessments that address infection control, safety, noise, and air quality are crucial, and the Joint Commission generally requires such assessments. Further, education of hospital staff and members of the construction team about the potential detrimental effects of hospital construction and renovation is essential to secure a safe environment.
Hospital-acquired infections related to construction and renovation activities account for more than 5,000 deaths per year across the United States.1
Hospital construction, renovation, and demolition projects ultimately serve the interests of patients, but they also can put inpatients at risk of mold infection, Legionnaires disease, sleep deprivation, exacerbation of lung disease, and in rare cases, physical injury.
Hospitals are in a continuous state of transformation to meet the needs of medical and technologic advances and an increasing patient population,1 and in the last 10 years, more than $200 billion has been spent on construction projects at US healthcare facilities. Therefore, constant attention is needed to reduce the risks to the health of hospitalized patients during these projects.
HOSPITAL-ACQUIRED INFECTIONS
Mold infections
Construction can cause substantial dust contamination and scatter large amounts of fungal spores. An analysis conducted during a period of excavation at a hospital campus showed a significant association between excavation activities and hospital-acquired mold infections (hazard ratio [HR] 2.8, P = .01) but not yeast infections (HR 0.75, P = .78).2
Aspergillus species have been the organisms most commonly involved in hospital-acquired mold infection. In a review of 53 studies including 458 patients,3 A fumigatus was identified in 154 patients, and A flavus was identified in 101 patients. A niger, A terreus, A nidulans, Zygomycetes, and other fungi were also identified, but to a much lesser extent. Hematologic malignancies were the predominant underlying morbidity in 299 patients. Half of the sources of healthcare-associated Aspergillus outbreaks were estimated to result from construction and renovation activities within or surrounding the hospital.3
Heavy demolition and transportation of wreckage have been found to cause the greatest concentrations of Aspergillus species,1 but even small concentrations may be sufficient to cause infection in high-risk hospitalized patients.3 Invasive pulmonary aspergillosis is the mold infection most commonly associated with these activities, particularly in immunocompromised and critically ill patients. It is characterized by invasion of lung tissue by Aspergillus hyphae. Hematogenous dissemination occurs in about 25% of patients, and the death rate often exceeds 50%.4
A review of cases of fungal infection during hospital construction, renovation, and demolition projects from 1976 to 2014 identified 372 infected patients, of whom 180 died.5 The majority of infections were due to Aspergillus. Other fungi included Rhizopus, Candida, and Fusarium. Infections occurred mainly in patients with hematologic malignancies and patients who had undergone stem cell transplant (76%), followed by patients with other malignancies or transplant (19%). Rarely affected were patients in the intensive care unit or patients with rheumatologic diseases or on hemodialysis.5
Legionnaires disease
Legionnaires disease is a form of atypical pneumonia caused by the bacterium Legionella, often associated with differing degrees of gastrointestinal symptoms. Legionella species are the bacteria most often associated with construction in hospitals, as construction and demolition often result in collections of stagnant water.
The primary mode of transmission is inhalation of contaminated mist or aerosols. Legionella species can also colonize newly constructed hospital buildings within weeks of installation of water fixtures.
In a large university-affiliated hospital, 2 cases of nosocomial legionellosis were identified during a period of major construction.6 An epidemiologic investigation traced the source to a widespread contamination of potable water within the hospital. One patient’s isolate was similar to that of a water sample from the faucet in his room, and an association between Legionnaires disease and construction was postulated.
Another institution’s newly constructed hematology-oncology unit identified 10 cases of Legionnaires disease over a 12-week period in patients and visitors with exposure to the unit during and within the incubation period.7 A clinical and environmental assessment found 3 clinical isolates of Legionella identical to environmental isolates found from the unit, strongly implicating the potable water system as the likely source.7
In Ohio, 11 cases of hospital-acquired Legionnaires disease were identified in patients moved to a newly constructed 12-story addition to a hospital, and 1 of those died.8
Legionella infections appear to be less common than mold infections when reviewing the available literature on patients exposed to hospital construction, renovation, or demolition activities. Yet unlike mold infections, which occur mostly in immunocompromised patients, Legionella also affects people with normal immunity.1
NONCOMMUNICABLE ILLNESSES
Sleep deprivation
Noise in hospitals has been linked to sleep disturbances in inpatients. A study using noise dosimeters in a university hospital found a mean continuous noise level of 63.5 dBA (A-weighting of decibels indicates risk of hearing loss) over a 24-hour period, a level more than 2 times higher than the recommended 30 dBA.9 The same study also found a significant correlation between sleep disturbance in inpatients and increasing noise levels, in a dose-response manner.
Common sources of noise during construction may include power generators, welding and cutting equipment, and transport of materials. While construction activities themselves have yet to be directly linked to sleep deprivation in patients, construction is inevitably accompanied by noise.
Noise is the most common factor interfering with sleep reported by hospitalized patients. Other effects of noise on patients include a rise in heart rate and blood pressure, increased cholesterol and triglyceride levels, increased use of sedatives, and longer length of stay.9,10 Although construction is rarely done at night, patients generally take naps during the day, so the noise is disruptive.
Physical injuries
Hospitalized patients rarely suffer injuries related to hospital construction. However, these incidents may be underreported. Few cases of physical injury in patients exposed to construction or renovation in healthcare facilities can be found through a Web search.11,12
Exacerbation of lung disease
Inhalation of indoor air pollutants exposed during renovation can directly trigger an inflammatory response and cause exacerbation in patients with chronic lung diseases such as asthma and chronic obstructive pulmonary disease. No study has specifically examined the effect of hospital construction or renovation on exacerbation of chronic lung diseases in hospitalized patients. Nevertheless, dust and indoor air pollutants from building renovation have often been reported as agents associated with work-related asthma.13
THE MESSAGE
Although the risks to inpatients during hospital construction projects appear minimal, their effect can at times be detrimental, especially to the immunocompromised. Hospitals should adhere to infection control risk assessment protocols during construction events. The small number of outbreaks of construction-related infections can make the diagnosis of nosocomial origin of these infections challenging; a high index of suspicion is needed.
Currently in the United States, there is no standard regarding acceptable levels of airborne mold concentrations, and data to support routine hospital air sampling or validation of available air samplers are inadequate. This remains an area for future research.14,15
Certain measures have been shown to significantly decrease the risk of mold infections and other nosocomial infections during construction projects, including16:
- Effective dust control through containment units and barriers
- Consistent use of high-efficiency particulate air filters in hospital units that care for immunocompromised and critically ill patients
- Routine surveillance.
Noise and vibration can be reduced by temporary walls and careful tool selection and scheduling. Similarly, temporary walls and other barriers help protect healthcare employees and patients from the risk of direct physical injury.
Preconstruction risk assessments that address infection control, safety, noise, and air quality are crucial, and the Joint Commission generally requires such assessments. Further, education of hospital staff and members of the construction team about the potential detrimental effects of hospital construction and renovation is essential to secure a safe environment.
- Clair JD, Colatrella S. Opening Pandora’s (tool) box: health care construction and associated risk for nosocomial infection. Infect Disord Drug Targets 2013; 13(3):177–183. pmid:23961740
- Pokala HR, Leonard D, Cox J, et al. Association of hospital construction with the development of healthcare associated environmental mold infections (HAEMI) in pediatric patients with leukemia. Pediatr Blood Cancer 2014; 61(2):276–280. doi:10.1002/pbc.24685
- Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect 2006; 63(3):246–254. doi:10.1016/j.jhin.2006.02.014
- Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med 2018; 141:121–131. doi:10.1016/j.rmed.2018.06.029
- Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 2015; 61(3):433–444. doi:10.1093/cid/civ297
- Perola O, Kauppinen J, Kusnetsov J, Heikkinen J, Jokinen C, Katila ML. Nosocomial Legionella pneumophila serogroup 5 outbreak associated with persistent colonization of a hospital water system. APMIS 2002; 110(12):863–868. pmid:12645664
- Francois Watkins LK, Toews KE, Harris AM, et al. Lessons from an outbreak of Legionnaires disease on a hematology-oncology unit. Infect Control Hosp Epidemiol 2017; 38(3):306–313. doi:10.1017/ice.2016.281
- Lin YE, Stout JE, Yu VL. Prevention of hospital-acquired legionellosis. Curr Opin Infect Dis 2011; 24(4):350–356. doi:10.1097/QCO.0b013e3283486c6e
- Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol 2014; 29:e2014006. doi:10.5620/eht.2014.29.e2014006
- Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med 2012; 157(3):170–179. doi:10.7326/0003-4819-157-3-201208070-00472
- Heldt D; The Gazette. Accident will delay University of Iowa Hospitals construction work for several days. www.thegazette.com/2013/03/08/university-of-iowa-hospitals-patient-injured-by-falling-construction-debris. Accessed July 22, 2019.
- Darrah N; Fox News. Texas hospital explosion kills 1, leaves 12 injured. www.foxnews.com/us/texas-hospital-explosion-kills-1-leaves-12-injured. Accessed July 22, 2019.
- Centers for Disease Control and Prevention (CDC). Work-related asthma: most frequently reported agents associated with work-related asthma cases by state, 2009–2012. wwwn.cdc.gov/eworld/Data/926. Accessed July 22, 2019.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63(4):e1–e60. doi:10.1093/cid/ciw326
- Chang CC, Athan E, Morrissey CO, Slavin MA. Preventing invasive fungal infection during hospital building works. Intern Med J 2008; 38(6b):538–541. doi:10.1111/j.1445-5994.2008.01727.x
- Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001; 66(4):257–262. doi:10.1002/ajh.1054
- Clair JD, Colatrella S. Opening Pandora’s (tool) box: health care construction and associated risk for nosocomial infection. Infect Disord Drug Targets 2013; 13(3):177–183. pmid:23961740
- Pokala HR, Leonard D, Cox J, et al. Association of hospital construction with the development of healthcare associated environmental mold infections (HAEMI) in pediatric patients with leukemia. Pediatr Blood Cancer 2014; 61(2):276–280. doi:10.1002/pbc.24685
- Vonberg RP, Gastmeier P. Nosocomial aspergillosis in outbreak settings. J Hosp Infect 2006; 63(3):246–254. doi:10.1016/j.jhin.2006.02.014
- Kanj A, Abdallah N, Soubani AO. The spectrum of pulmonary aspergillosis. Respir Med 2018; 141:121–131. doi:10.1016/j.rmed.2018.06.029
- Kanamori H, Rutala WA, Sickbert-Bennett EE, Weber DJ. Review of fungal outbreaks and infection prevention in healthcare settings during construction and renovation. Clin Infect Dis 2015; 61(3):433–444. doi:10.1093/cid/civ297
- Perola O, Kauppinen J, Kusnetsov J, Heikkinen J, Jokinen C, Katila ML. Nosocomial Legionella pneumophila serogroup 5 outbreak associated with persistent colonization of a hospital water system. APMIS 2002; 110(12):863–868. pmid:12645664
- Francois Watkins LK, Toews KE, Harris AM, et al. Lessons from an outbreak of Legionnaires disease on a hematology-oncology unit. Infect Control Hosp Epidemiol 2017; 38(3):306–313. doi:10.1017/ice.2016.281
- Lin YE, Stout JE, Yu VL. Prevention of hospital-acquired legionellosis. Curr Opin Infect Dis 2011; 24(4):350–356. doi:10.1097/QCO.0b013e3283486c6e
- Park MJ, Yoo JH, Cho BW, Kim KT, Jeong WC, Ha M. Noise in hospital rooms and sleep disturbance in hospitalized medical patients. Environ Health Toxicol 2014; 29:e2014006. doi:10.5620/eht.2014.29.e2014006
- Buxton OM, Ellenbogen JM, Wang W, et al. Sleep disruption due to hospital noises: a prospective evaluation. Ann Intern Med 2012; 157(3):170–179. doi:10.7326/0003-4819-157-3-201208070-00472
- Heldt D; The Gazette. Accident will delay University of Iowa Hospitals construction work for several days. www.thegazette.com/2013/03/08/university-of-iowa-hospitals-patient-injured-by-falling-construction-debris. Accessed July 22, 2019.
- Darrah N; Fox News. Texas hospital explosion kills 1, leaves 12 injured. www.foxnews.com/us/texas-hospital-explosion-kills-1-leaves-12-injured. Accessed July 22, 2019.
- Centers for Disease Control and Prevention (CDC). Work-related asthma: most frequently reported agents associated with work-related asthma cases by state, 2009–2012. wwwn.cdc.gov/eworld/Data/926. Accessed July 22, 2019.
- Patterson TF, Thompson GR 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of Aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis 2016; 63(4):e1–e60. doi:10.1093/cid/ciw326
- Chang CC, Athan E, Morrissey CO, Slavin MA. Preventing invasive fungal infection during hospital building works. Intern Med J 2008; 38(6b):538–541. doi:10.1111/j.1445-5994.2008.01727.x
- Oren I, Haddad N, Finkelstein R, Rowe JM. Invasive pulmonary aspergillosis in neutropenic patients during hospital construction: before and after chemoprophylaxis and institution of HEPA filters. Am J Hematol 2001; 66(4):257–262. doi:10.1002/ajh.1054
Adult insomnia associated with childhood behavioral problems
Yohannes Adama Melaku, MPH, PhD, of the Adelaide (Australia) Institute for Sleep Health at Flinders University and coauthors drew data from the 1970 UK Birth Cohort Study. This study followed an initial cohort of 16,571 babies who were born during a single week, with follow-up at ages 5, 10, 16, 26, 30, 38, 42, and 46 years. For the purposes of this study, the investigators looked at participants who, at 42 years of age, were alive and not lost to follow-up and who responded to an invitation to be interviewed; the sample sizes in the analysis were 8,050 participants aged 5 years, 9,090 participants aged 10 years, 9,653 participants aged 16 years, and 9,841 participants aged 42 years.
Behavior was measured at ages 5 years and 16 years using the Rutter Behavioral Scale (RBS) and at age 10 years using a visual analog scale, and insomnia symptoms were assessed through interviewing participants in adulthood about duration of sleep, difficulty initiating sleep, difficulty maintaining sleep, and not feeling rested on waking. Participants were organized into normal behavior (less than or equal to 80th percentile on RBS), moderate behavioral problems (greater than the 80th percentile but less than or equal to the 95th percentile), and severe behavioral problems (above 95th percentile). The investigators then devised two models for their analysis: Model 1 adjusted for sex, parent’s social class and educational level, marital status, educational status, and social class, and model 2 adjusted for physical activity level and body mass index (BMI) trajectory (from 10 to 42 years), perceived health status, and number of noncommunicable diseases, although this latter model yielded fewer statistically significant results in some analyses.
Odds for difficulty initiating or maintaining sleep as an adult was increased among participants with severe behavioral problems at age 5 years in model 1 (adjusted odds ratio, 1.50; 95% confidence interval, 1.14-1.96; P = .004), as well as for those with severe problems at 10 years (aOR, 1.30; 95% CI, 1.14-1.63; P = .001), and at 16 years (aOR, 2.17; 95% CI, 1.59-2.91; P less than .001). The aORs also were higher individually for difficulty initiating sleep and for difficulty maintaining sleep in all age groups.
The association with adulthood insomnia was stronger in participants with externalizing behavioral problems such as lying, bullying, restlessness, and fighting than it was in those with internalizing behavioral problems such as worry, fearfulness, and solitariness.
“Although early sleep problems should be identified, we should additionally identify children with moderate to severe behavioral problems that persist throughout childhood as potential beneficiaries of early intervention with a sleep health focus,” the authors wrote.
One of the study’s limitations was a lack of standardized insomnia measures in the cohort study; however, the researchers suggested that the symptoms included reflect those of standardized measures and diagnostic criteria.
“This study is the first, to our knowledge, to suggest an unfavorable association of early-life behavioral problems with adulthood sleep health, underlining the importance of treating behavioral problems in children and addressing insomnia from a life-course perspective,” they concluded.
No study sponsor was identified. The authors reported no relevant financial disclosures.
SOURCE: Melaku YA et al. JAMA Netw Open. 2019 Sep 6. doi: 10.1001/jamanetworkopen.2019.10861.
Yohannes Adama Melaku, MPH, PhD, of the Adelaide (Australia) Institute for Sleep Health at Flinders University and coauthors drew data from the 1970 UK Birth Cohort Study. This study followed an initial cohort of 16,571 babies who were born during a single week, with follow-up at ages 5, 10, 16, 26, 30, 38, 42, and 46 years. For the purposes of this study, the investigators looked at participants who, at 42 years of age, were alive and not lost to follow-up and who responded to an invitation to be interviewed; the sample sizes in the analysis were 8,050 participants aged 5 years, 9,090 participants aged 10 years, 9,653 participants aged 16 years, and 9,841 participants aged 42 years.
Behavior was measured at ages 5 years and 16 years using the Rutter Behavioral Scale (RBS) and at age 10 years using a visual analog scale, and insomnia symptoms were assessed through interviewing participants in adulthood about duration of sleep, difficulty initiating sleep, difficulty maintaining sleep, and not feeling rested on waking. Participants were organized into normal behavior (less than or equal to 80th percentile on RBS), moderate behavioral problems (greater than the 80th percentile but less than or equal to the 95th percentile), and severe behavioral problems (above 95th percentile). The investigators then devised two models for their analysis: Model 1 adjusted for sex, parent’s social class and educational level, marital status, educational status, and social class, and model 2 adjusted for physical activity level and body mass index (BMI) trajectory (from 10 to 42 years), perceived health status, and number of noncommunicable diseases, although this latter model yielded fewer statistically significant results in some analyses.
Odds for difficulty initiating or maintaining sleep as an adult was increased among participants with severe behavioral problems at age 5 years in model 1 (adjusted odds ratio, 1.50; 95% confidence interval, 1.14-1.96; P = .004), as well as for those with severe problems at 10 years (aOR, 1.30; 95% CI, 1.14-1.63; P = .001), and at 16 years (aOR, 2.17; 95% CI, 1.59-2.91; P less than .001). The aORs also were higher individually for difficulty initiating sleep and for difficulty maintaining sleep in all age groups.
The association with adulthood insomnia was stronger in participants with externalizing behavioral problems such as lying, bullying, restlessness, and fighting than it was in those with internalizing behavioral problems such as worry, fearfulness, and solitariness.
“Although early sleep problems should be identified, we should additionally identify children with moderate to severe behavioral problems that persist throughout childhood as potential beneficiaries of early intervention with a sleep health focus,” the authors wrote.
One of the study’s limitations was a lack of standardized insomnia measures in the cohort study; however, the researchers suggested that the symptoms included reflect those of standardized measures and diagnostic criteria.
“This study is the first, to our knowledge, to suggest an unfavorable association of early-life behavioral problems with adulthood sleep health, underlining the importance of treating behavioral problems in children and addressing insomnia from a life-course perspective,” they concluded.
No study sponsor was identified. The authors reported no relevant financial disclosures.
SOURCE: Melaku YA et al. JAMA Netw Open. 2019 Sep 6. doi: 10.1001/jamanetworkopen.2019.10861.
Yohannes Adama Melaku, MPH, PhD, of the Adelaide (Australia) Institute for Sleep Health at Flinders University and coauthors drew data from the 1970 UK Birth Cohort Study. This study followed an initial cohort of 16,571 babies who were born during a single week, with follow-up at ages 5, 10, 16, 26, 30, 38, 42, and 46 years. For the purposes of this study, the investigators looked at participants who, at 42 years of age, were alive and not lost to follow-up and who responded to an invitation to be interviewed; the sample sizes in the analysis were 8,050 participants aged 5 years, 9,090 participants aged 10 years, 9,653 participants aged 16 years, and 9,841 participants aged 42 years.
Behavior was measured at ages 5 years and 16 years using the Rutter Behavioral Scale (RBS) and at age 10 years using a visual analog scale, and insomnia symptoms were assessed through interviewing participants in adulthood about duration of sleep, difficulty initiating sleep, difficulty maintaining sleep, and not feeling rested on waking. Participants were organized into normal behavior (less than or equal to 80th percentile on RBS), moderate behavioral problems (greater than the 80th percentile but less than or equal to the 95th percentile), and severe behavioral problems (above 95th percentile). The investigators then devised two models for their analysis: Model 1 adjusted for sex, parent’s social class and educational level, marital status, educational status, and social class, and model 2 adjusted for physical activity level and body mass index (BMI) trajectory (from 10 to 42 years), perceived health status, and number of noncommunicable diseases, although this latter model yielded fewer statistically significant results in some analyses.
Odds for difficulty initiating or maintaining sleep as an adult was increased among participants with severe behavioral problems at age 5 years in model 1 (adjusted odds ratio, 1.50; 95% confidence interval, 1.14-1.96; P = .004), as well as for those with severe problems at 10 years (aOR, 1.30; 95% CI, 1.14-1.63; P = .001), and at 16 years (aOR, 2.17; 95% CI, 1.59-2.91; P less than .001). The aORs also were higher individually for difficulty initiating sleep and for difficulty maintaining sleep in all age groups.
The association with adulthood insomnia was stronger in participants with externalizing behavioral problems such as lying, bullying, restlessness, and fighting than it was in those with internalizing behavioral problems such as worry, fearfulness, and solitariness.
“Although early sleep problems should be identified, we should additionally identify children with moderate to severe behavioral problems that persist throughout childhood as potential beneficiaries of early intervention with a sleep health focus,” the authors wrote.
One of the study’s limitations was a lack of standardized insomnia measures in the cohort study; however, the researchers suggested that the symptoms included reflect those of standardized measures and diagnostic criteria.
“This study is the first, to our knowledge, to suggest an unfavorable association of early-life behavioral problems with adulthood sleep health, underlining the importance of treating behavioral problems in children and addressing insomnia from a life-course perspective,” they concluded.
No study sponsor was identified. The authors reported no relevant financial disclosures.
SOURCE: Melaku YA et al. JAMA Netw Open. 2019 Sep 6. doi: 10.1001/jamanetworkopen.2019.10861.
FROM JAMA NETWORK OPEN
No link found between sleep position, pregnancy outcomes
such as stillbirth, small-for-gestational-age (SGA) newborns, and gestational hypertensive disorders. The finding, published in the October issue of Obstetrics & Gynecology, conflicts with previous retrospective case-control studies that suggested left-side sleeping may lead to reduced risk. The disagreement may be due to the prospective nature of the new study, which followed 8,706 women over 4 years.
Right-side or back sleeping had attracted suspicion because of its potential to compress uterine blood vessels and decrease uterine blood flow, and various public health campaigns urge pregnant women to sleep on their left side. Although case-control studies backed up those worries, those retrospective approaches can suffer from limitations including recall bias, in which grieving mothers overreport suspect sleeping behaviors, perhaps in search of an explanation for their loss.
Prospective analyses can counter some of those limitations. The researchers, led by Robert Silver, MD, of the University of Utah, Salt Lake City, conducted a secondary analysis of the nuMoM2b study, examining adverse pregnancy outcomes and risk factors. It was a multicenter observational cohort study of 8,706 nulliparous women with singleton gestations who completed two sleep questionnaires: one between 6 and 13 weeks of gestation, and one between 22 and 29 weeks.
Adverse outcomes occurred in 1,903 women, including 178 cases of both SGA and hypertensive disorders, 8 with SGA plus stillbirth, 3 with hypertensive disorders plus stillbirth, and 2 cases with all three complications.
The researchers found no association between any adverse outcomes and sleep position either at the first visit in early pregnancy (adjusted odds ratio, 1.00; 95% confidence interval, 0.89-1.14) or the third visit in midpregnancy (aOR, 0.99; 95% CI, 0.89-1.11). Propensity score matching to adjust non-left lateral positioning to the composite outcome also showed no association.
In midpregnancy, there was an association between non-left lateral sleeping and reduced risk of stillbirth (aOR, 0.27; 95% CI, 0.09-0.75). “This observation is likely spurious owing to small numbers of stillbirths, Dr. Silver and associates said.
A post hoc analysis indicated that the study was sufficiently powered to detect clinically meaningful risks; ORs of 1.2 for hypertensive disorders, 1.23 for SGA, 2.4 for stillbirth, and 1.2 for the composite outcome.
Let sleeping mothers lie
Pregnant women have enough on their minds. They shouldn’t have to worry about sleep position as well, according to Nathan Fox, MD, professor of obstetrics, gynecology, and reproductive science at Icahn School of Medicine at Mount Sinai, New York, and Emily Oster, PhD, economist at Brown University, Providence, R.I., who wrote an accompanying editorial.
It may seem harmless to direct women to sleep on their left side even if it has no benefit, but restricting a woman’s sleep options may leave her less well rested at a time when she is about to enter a period of sleep deprivation, as well as contribute to general discomfort, in their opinion.
Also, in the rare cases of a bad outcome, advice based on limited or poor quality evidence contributes to “devastating and unwarranted feelings of responsibility and guilt, and this harm to women already suffering from sadness and despair must not be minimized,” they said.
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and grants from multiple universities. One author received research funding from Kyndermed through her institution. Another receives royalties from UpToDate.com. Dr. Fox and Dr. Oster have no relevant financial disclosures.
SOURCES: Silver RM et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003458; Fox N and Oster E. Obstet. Gynecol. 2019 Oct. doi: 10.1097/AOG.0000000000003466
such as stillbirth, small-for-gestational-age (SGA) newborns, and gestational hypertensive disorders. The finding, published in the October issue of Obstetrics & Gynecology, conflicts with previous retrospective case-control studies that suggested left-side sleeping may lead to reduced risk. The disagreement may be due to the prospective nature of the new study, which followed 8,706 women over 4 years.
Right-side or back sleeping had attracted suspicion because of its potential to compress uterine blood vessels and decrease uterine blood flow, and various public health campaigns urge pregnant women to sleep on their left side. Although case-control studies backed up those worries, those retrospective approaches can suffer from limitations including recall bias, in which grieving mothers overreport suspect sleeping behaviors, perhaps in search of an explanation for their loss.
Prospective analyses can counter some of those limitations. The researchers, led by Robert Silver, MD, of the University of Utah, Salt Lake City, conducted a secondary analysis of the nuMoM2b study, examining adverse pregnancy outcomes and risk factors. It was a multicenter observational cohort study of 8,706 nulliparous women with singleton gestations who completed two sleep questionnaires: one between 6 and 13 weeks of gestation, and one between 22 and 29 weeks.
Adverse outcomes occurred in 1,903 women, including 178 cases of both SGA and hypertensive disorders, 8 with SGA plus stillbirth, 3 with hypertensive disorders plus stillbirth, and 2 cases with all three complications.
The researchers found no association between any adverse outcomes and sleep position either at the first visit in early pregnancy (adjusted odds ratio, 1.00; 95% confidence interval, 0.89-1.14) or the third visit in midpregnancy (aOR, 0.99; 95% CI, 0.89-1.11). Propensity score matching to adjust non-left lateral positioning to the composite outcome also showed no association.
In midpregnancy, there was an association between non-left lateral sleeping and reduced risk of stillbirth (aOR, 0.27; 95% CI, 0.09-0.75). “This observation is likely spurious owing to small numbers of stillbirths, Dr. Silver and associates said.
A post hoc analysis indicated that the study was sufficiently powered to detect clinically meaningful risks; ORs of 1.2 for hypertensive disorders, 1.23 for SGA, 2.4 for stillbirth, and 1.2 for the composite outcome.
Let sleeping mothers lie
Pregnant women have enough on their minds. They shouldn’t have to worry about sleep position as well, according to Nathan Fox, MD, professor of obstetrics, gynecology, and reproductive science at Icahn School of Medicine at Mount Sinai, New York, and Emily Oster, PhD, economist at Brown University, Providence, R.I., who wrote an accompanying editorial.
It may seem harmless to direct women to sleep on their left side even if it has no benefit, but restricting a woman’s sleep options may leave her less well rested at a time when she is about to enter a period of sleep deprivation, as well as contribute to general discomfort, in their opinion.
Also, in the rare cases of a bad outcome, advice based on limited or poor quality evidence contributes to “devastating and unwarranted feelings of responsibility and guilt, and this harm to women already suffering from sadness and despair must not be minimized,” they said.
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and grants from multiple universities. One author received research funding from Kyndermed through her institution. Another receives royalties from UpToDate.com. Dr. Fox and Dr. Oster have no relevant financial disclosures.
SOURCES: Silver RM et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003458; Fox N and Oster E. Obstet. Gynecol. 2019 Oct. doi: 10.1097/AOG.0000000000003466
such as stillbirth, small-for-gestational-age (SGA) newborns, and gestational hypertensive disorders. The finding, published in the October issue of Obstetrics & Gynecology, conflicts with previous retrospective case-control studies that suggested left-side sleeping may lead to reduced risk. The disagreement may be due to the prospective nature of the new study, which followed 8,706 women over 4 years.
Right-side or back sleeping had attracted suspicion because of its potential to compress uterine blood vessels and decrease uterine blood flow, and various public health campaigns urge pregnant women to sleep on their left side. Although case-control studies backed up those worries, those retrospective approaches can suffer from limitations including recall bias, in which grieving mothers overreport suspect sleeping behaviors, perhaps in search of an explanation for their loss.
Prospective analyses can counter some of those limitations. The researchers, led by Robert Silver, MD, of the University of Utah, Salt Lake City, conducted a secondary analysis of the nuMoM2b study, examining adverse pregnancy outcomes and risk factors. It was a multicenter observational cohort study of 8,706 nulliparous women with singleton gestations who completed two sleep questionnaires: one between 6 and 13 weeks of gestation, and one between 22 and 29 weeks.
Adverse outcomes occurred in 1,903 women, including 178 cases of both SGA and hypertensive disorders, 8 with SGA plus stillbirth, 3 with hypertensive disorders plus stillbirth, and 2 cases with all three complications.
The researchers found no association between any adverse outcomes and sleep position either at the first visit in early pregnancy (adjusted odds ratio, 1.00; 95% confidence interval, 0.89-1.14) or the third visit in midpregnancy (aOR, 0.99; 95% CI, 0.89-1.11). Propensity score matching to adjust non-left lateral positioning to the composite outcome also showed no association.
In midpregnancy, there was an association between non-left lateral sleeping and reduced risk of stillbirth (aOR, 0.27; 95% CI, 0.09-0.75). “This observation is likely spurious owing to small numbers of stillbirths, Dr. Silver and associates said.
A post hoc analysis indicated that the study was sufficiently powered to detect clinically meaningful risks; ORs of 1.2 for hypertensive disorders, 1.23 for SGA, 2.4 for stillbirth, and 1.2 for the composite outcome.
Let sleeping mothers lie
Pregnant women have enough on their minds. They shouldn’t have to worry about sleep position as well, according to Nathan Fox, MD, professor of obstetrics, gynecology, and reproductive science at Icahn School of Medicine at Mount Sinai, New York, and Emily Oster, PhD, economist at Brown University, Providence, R.I., who wrote an accompanying editorial.
It may seem harmless to direct women to sleep on their left side even if it has no benefit, but restricting a woman’s sleep options may leave her less well rested at a time when she is about to enter a period of sleep deprivation, as well as contribute to general discomfort, in their opinion.
Also, in the rare cases of a bad outcome, advice based on limited or poor quality evidence contributes to “devastating and unwarranted feelings of responsibility and guilt, and this harm to women already suffering from sadness and despair must not be minimized,” they said.
The study received funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and grants from multiple universities. One author received research funding from Kyndermed through her institution. Another receives royalties from UpToDate.com. Dr. Fox and Dr. Oster have no relevant financial disclosures.
SOURCES: Silver RM et al. Obstet Gynecol. 2019. doi: 10.1097/AOG.0000000000003458; Fox N and Oster E. Obstet. Gynecol. 2019 Oct. doi: 10.1097/AOG.0000000000003466
FROM OBSTETRICS & GYNECOLOGY
Less CPAP time linked to exacerbation in COPD/OSA overlap syndrome
and all‐cause mortality, according to a retrospective cohort study.
“These factors should be taken into account when considering the management and prognosis of these patients,” the researchers said in the Clinical Respiratory Journal.
Prior studies have found that patients with COPD and OSA – that is, with overlap syndrome – “have a substantially greater risk of morbidity and mortality, compared to those with either COPD or OSA alone,” said Philippe E. Jaoude, MD, and Ali A. El Solh, MD, both of the Veterans Affairs Western New York Healthcare System in Buffalo and the University at Buffalo.
To identify factors associated with COPD exacerbation and all‐cause mortality in patients with overlap syndrome, Dr. Jaoude and Dr. El Solh reviewed the electronic health records of patients with simultaneous COPD and OSA. They compared patients with overlap syndrome who had an acute exacerbation of COPD during a 42-month period with a control group of patients with overlap syndrome who did not have exacerbations during that time. Patients with exacerbations and controls were matched 1:1 by age and body mass index.
Eligible patients were aged 42-90 years, had objectively confirmed COPD, and had documented OSA by in-laboratory polysomnography (that is, at least five obstructive apneas and hypopneas per hour). The investigators defined a COPD exacerbation as a sustained worsening of a patient’s respiratory condition that warranted additional treatment.
Of 225 eligible patients, 92 had at least one COPD exacerbation between March 2014 and September 2017. Patients with COPD exacerbation and controls had a mean age of about 68 years. The group of patients with exacerbation had a higher percentage of active smokers (21% vs. 9%) and had poorer lung function (mean forced expiratory volume in 1 second percent predicted: 55.2% vs. 64.5%).
“Although the rate of CPAP adherence between the two groups was not significantly different, the average time of CPAP use was significantly higher in patients with no recorded exacerbation,” the researchers reported – 285.4 min/night versus 238.2 min/night.
In all, 146 patients (79.4%) survived, and 38 patients (20.6%) died during the study period. The crude mortality rate was significantly higher in the group with COPD exacerbations (14% vs. 7%).
“Multivariate logistic regression analysis identified the independent risk factors associated with COPD exacerbations as active smoking, worse airflow limitation, and lower CPAP utilization,” they said. “As for all-cause mortality, a higher burden of comorbidities, worse airflow limitation, and lower time of CPAP use were independently associated with poor outcome.”
The researchers noted that they cannot rule out the possibility that patients who were adherent to CPAP were systematically different from those who were not.
The authors had no conflicts of interest.
SOURCE: Jaoude P et al. Clin Respir J. 2019 Aug 22. doi: 10.1111/crj.13079.
and all‐cause mortality, according to a retrospective cohort study.
“These factors should be taken into account when considering the management and prognosis of these patients,” the researchers said in the Clinical Respiratory Journal.
Prior studies have found that patients with COPD and OSA – that is, with overlap syndrome – “have a substantially greater risk of morbidity and mortality, compared to those with either COPD or OSA alone,” said Philippe E. Jaoude, MD, and Ali A. El Solh, MD, both of the Veterans Affairs Western New York Healthcare System in Buffalo and the University at Buffalo.
To identify factors associated with COPD exacerbation and all‐cause mortality in patients with overlap syndrome, Dr. Jaoude and Dr. El Solh reviewed the electronic health records of patients with simultaneous COPD and OSA. They compared patients with overlap syndrome who had an acute exacerbation of COPD during a 42-month period with a control group of patients with overlap syndrome who did not have exacerbations during that time. Patients with exacerbations and controls were matched 1:1 by age and body mass index.
Eligible patients were aged 42-90 years, had objectively confirmed COPD, and had documented OSA by in-laboratory polysomnography (that is, at least five obstructive apneas and hypopneas per hour). The investigators defined a COPD exacerbation as a sustained worsening of a patient’s respiratory condition that warranted additional treatment.
Of 225 eligible patients, 92 had at least one COPD exacerbation between March 2014 and September 2017. Patients with COPD exacerbation and controls had a mean age of about 68 years. The group of patients with exacerbation had a higher percentage of active smokers (21% vs. 9%) and had poorer lung function (mean forced expiratory volume in 1 second percent predicted: 55.2% vs. 64.5%).
“Although the rate of CPAP adherence between the two groups was not significantly different, the average time of CPAP use was significantly higher in patients with no recorded exacerbation,” the researchers reported – 285.4 min/night versus 238.2 min/night.
In all, 146 patients (79.4%) survived, and 38 patients (20.6%) died during the study period. The crude mortality rate was significantly higher in the group with COPD exacerbations (14% vs. 7%).
“Multivariate logistic regression analysis identified the independent risk factors associated with COPD exacerbations as active smoking, worse airflow limitation, and lower CPAP utilization,” they said. “As for all-cause mortality, a higher burden of comorbidities, worse airflow limitation, and lower time of CPAP use were independently associated with poor outcome.”
The researchers noted that they cannot rule out the possibility that patients who were adherent to CPAP were systematically different from those who were not.
The authors had no conflicts of interest.
SOURCE: Jaoude P et al. Clin Respir J. 2019 Aug 22. doi: 10.1111/crj.13079.
and all‐cause mortality, according to a retrospective cohort study.
“These factors should be taken into account when considering the management and prognosis of these patients,” the researchers said in the Clinical Respiratory Journal.
Prior studies have found that patients with COPD and OSA – that is, with overlap syndrome – “have a substantially greater risk of morbidity and mortality, compared to those with either COPD or OSA alone,” said Philippe E. Jaoude, MD, and Ali A. El Solh, MD, both of the Veterans Affairs Western New York Healthcare System in Buffalo and the University at Buffalo.
To identify factors associated with COPD exacerbation and all‐cause mortality in patients with overlap syndrome, Dr. Jaoude and Dr. El Solh reviewed the electronic health records of patients with simultaneous COPD and OSA. They compared patients with overlap syndrome who had an acute exacerbation of COPD during a 42-month period with a control group of patients with overlap syndrome who did not have exacerbations during that time. Patients with exacerbations and controls were matched 1:1 by age and body mass index.
Eligible patients were aged 42-90 years, had objectively confirmed COPD, and had documented OSA by in-laboratory polysomnography (that is, at least five obstructive apneas and hypopneas per hour). The investigators defined a COPD exacerbation as a sustained worsening of a patient’s respiratory condition that warranted additional treatment.
Of 225 eligible patients, 92 had at least one COPD exacerbation between March 2014 and September 2017. Patients with COPD exacerbation and controls had a mean age of about 68 years. The group of patients with exacerbation had a higher percentage of active smokers (21% vs. 9%) and had poorer lung function (mean forced expiratory volume in 1 second percent predicted: 55.2% vs. 64.5%).
“Although the rate of CPAP adherence between the two groups was not significantly different, the average time of CPAP use was significantly higher in patients with no recorded exacerbation,” the researchers reported – 285.4 min/night versus 238.2 min/night.
In all, 146 patients (79.4%) survived, and 38 patients (20.6%) died during the study period. The crude mortality rate was significantly higher in the group with COPD exacerbations (14% vs. 7%).
“Multivariate logistic regression analysis identified the independent risk factors associated with COPD exacerbations as active smoking, worse airflow limitation, and lower CPAP utilization,” they said. “As for all-cause mortality, a higher burden of comorbidities, worse airflow limitation, and lower time of CPAP use were independently associated with poor outcome.”
The researchers noted that they cannot rule out the possibility that patients who were adherent to CPAP were systematically different from those who were not.
The authors had no conflicts of interest.
SOURCE: Jaoude P et al. Clin Respir J. 2019 Aug 22. doi: 10.1111/crj.13079.
FROM CLINICAL RESPIRATORY JOURNAL
Guideline: Blood CO2 can be used to screen for OHS
A blood test for elevated carbon dioxide may be used in screening adults for obesity hypoventilation syndrome, according to new guidelines.
Obese adults with sleep-disordered breathing and increased blood carbon dioxide levels during the day are likely to have obesity hypoventilation syndrome (OHS), a result of shallow or slow breathing that can lead to respiratory failure, heart failure, pulmonary hypertension, and death. Pulmonologists and sleep specialists may be the first to see and diagnose patients with OHS in the outpatient setting, while other cases are diagnosed in the hospital when patients present with hypercapnic respiratory failure.
Screening for OHS usually involves measuring arterial blood gases, which is not standard practice in outpatient clinics. Patients often remain undiagnosed and untreated until late in the course of the disease, according to the American Thoracic Society, which in August published a new diagnosis and management guideline aiming to boost early diagnosis and reduce variability in treatment (Am J Respir Crit Care Med. 2019;200:3,e6–e24).
The guideline authors, led by Babak Mokhlesi, MD, of the University of Chicago, recommend a simpler screening method – measuring serum bicarbonate only – to rule out OHS in obese patients with nighttime breathing problems.
Serum bicarbonate should be measured in obese patients with sleep-disordered breathing and a low likelihood of OHS, Dr. Mokhlesi and colleagues recommend in the guideline. If serum bicarbonate is below 27 mmol/L, it is not necessary to conduct further testing as the patient is unlikely to have OHS.
In patients whose serum bicarbonate is higher than 27 mmol/L, or who are strongly suspected of having OHS at presentation because of severe obesity or other symptoms, arterial blood gases should be measured and a sleep study conducted. The guideline authors said that there is insufficient evidence to recommend that pulse oximetry be used in the diagnostic pathway for OHS.
First-line treatment for stable, ambulatory patients with OHS should be positive airway pressure during sleep, rather than noninvasive ventilation, Dr. Mokhlesi and colleagues concluded. For patients with comorbid obstructive sleep apnea – as many as 70% of OHS patients also have OSA – the first-line treatment should be continuous positive airway pressure (CPAP) at night, the guideline states.
Patients hospitalized with respiratory failure and suspected of having OHS should be discharged with noninvasive ventilation until diagnostic procedures can be performed, along with PAP titration in a sleep lab.
In patients initially treated with CPAP who remain symptomatic or whose blood carbon dioxide does not improve, noninvasive ventilation can be tried, the guidelines say. Finally, patients diagnosed with OHS should be guided to weight loss interventions with the aim of reducing body weight by 25%-30%. This can include referral for bariatric surgery in patients without contraindications.
Dr. Mokhlesi and colleagues acknowledged that all of the recommendations contained in the guideline are classed as “conditional,” based on the quality of evidence assessed.
The American Thoracic Society funded the study. Dr. Mokhlesi and 7 coauthors disclosed financial conflicts of interest, while an additional 13 coauthors had none. Disclosures can be found on the AJRCCM website.
SOURCE: Mokhlesi B et al. Am J Respir Crit Care Med. 2019;200:3,e6-e24
A blood test for elevated carbon dioxide may be used in screening adults for obesity hypoventilation syndrome, according to new guidelines.
Obese adults with sleep-disordered breathing and increased blood carbon dioxide levels during the day are likely to have obesity hypoventilation syndrome (OHS), a result of shallow or slow breathing that can lead to respiratory failure, heart failure, pulmonary hypertension, and death. Pulmonologists and sleep specialists may be the first to see and diagnose patients with OHS in the outpatient setting, while other cases are diagnosed in the hospital when patients present with hypercapnic respiratory failure.
Screening for OHS usually involves measuring arterial blood gases, which is not standard practice in outpatient clinics. Patients often remain undiagnosed and untreated until late in the course of the disease, according to the American Thoracic Society, which in August published a new diagnosis and management guideline aiming to boost early diagnosis and reduce variability in treatment (Am J Respir Crit Care Med. 2019;200:3,e6–e24).
The guideline authors, led by Babak Mokhlesi, MD, of the University of Chicago, recommend a simpler screening method – measuring serum bicarbonate only – to rule out OHS in obese patients with nighttime breathing problems.
Serum bicarbonate should be measured in obese patients with sleep-disordered breathing and a low likelihood of OHS, Dr. Mokhlesi and colleagues recommend in the guideline. If serum bicarbonate is below 27 mmol/L, it is not necessary to conduct further testing as the patient is unlikely to have OHS.
In patients whose serum bicarbonate is higher than 27 mmol/L, or who are strongly suspected of having OHS at presentation because of severe obesity or other symptoms, arterial blood gases should be measured and a sleep study conducted. The guideline authors said that there is insufficient evidence to recommend that pulse oximetry be used in the diagnostic pathway for OHS.
First-line treatment for stable, ambulatory patients with OHS should be positive airway pressure during sleep, rather than noninvasive ventilation, Dr. Mokhlesi and colleagues concluded. For patients with comorbid obstructive sleep apnea – as many as 70% of OHS patients also have OSA – the first-line treatment should be continuous positive airway pressure (CPAP) at night, the guideline states.
Patients hospitalized with respiratory failure and suspected of having OHS should be discharged with noninvasive ventilation until diagnostic procedures can be performed, along with PAP titration in a sleep lab.
In patients initially treated with CPAP who remain symptomatic or whose blood carbon dioxide does not improve, noninvasive ventilation can be tried, the guidelines say. Finally, patients diagnosed with OHS should be guided to weight loss interventions with the aim of reducing body weight by 25%-30%. This can include referral for bariatric surgery in patients without contraindications.
Dr. Mokhlesi and colleagues acknowledged that all of the recommendations contained in the guideline are classed as “conditional,” based on the quality of evidence assessed.
The American Thoracic Society funded the study. Dr. Mokhlesi and 7 coauthors disclosed financial conflicts of interest, while an additional 13 coauthors had none. Disclosures can be found on the AJRCCM website.
SOURCE: Mokhlesi B et al. Am J Respir Crit Care Med. 2019;200:3,e6-e24
A blood test for elevated carbon dioxide may be used in screening adults for obesity hypoventilation syndrome, according to new guidelines.
Obese adults with sleep-disordered breathing and increased blood carbon dioxide levels during the day are likely to have obesity hypoventilation syndrome (OHS), a result of shallow or slow breathing that can lead to respiratory failure, heart failure, pulmonary hypertension, and death. Pulmonologists and sleep specialists may be the first to see and diagnose patients with OHS in the outpatient setting, while other cases are diagnosed in the hospital when patients present with hypercapnic respiratory failure.
Screening for OHS usually involves measuring arterial blood gases, which is not standard practice in outpatient clinics. Patients often remain undiagnosed and untreated until late in the course of the disease, according to the American Thoracic Society, which in August published a new diagnosis and management guideline aiming to boost early diagnosis and reduce variability in treatment (Am J Respir Crit Care Med. 2019;200:3,e6–e24).
The guideline authors, led by Babak Mokhlesi, MD, of the University of Chicago, recommend a simpler screening method – measuring serum bicarbonate only – to rule out OHS in obese patients with nighttime breathing problems.
Serum bicarbonate should be measured in obese patients with sleep-disordered breathing and a low likelihood of OHS, Dr. Mokhlesi and colleagues recommend in the guideline. If serum bicarbonate is below 27 mmol/L, it is not necessary to conduct further testing as the patient is unlikely to have OHS.
In patients whose serum bicarbonate is higher than 27 mmol/L, or who are strongly suspected of having OHS at presentation because of severe obesity or other symptoms, arterial blood gases should be measured and a sleep study conducted. The guideline authors said that there is insufficient evidence to recommend that pulse oximetry be used in the diagnostic pathway for OHS.
First-line treatment for stable, ambulatory patients with OHS should be positive airway pressure during sleep, rather than noninvasive ventilation, Dr. Mokhlesi and colleagues concluded. For patients with comorbid obstructive sleep apnea – as many as 70% of OHS patients also have OSA – the first-line treatment should be continuous positive airway pressure (CPAP) at night, the guideline states.
Patients hospitalized with respiratory failure and suspected of having OHS should be discharged with noninvasive ventilation until diagnostic procedures can be performed, along with PAP titration in a sleep lab.
In patients initially treated with CPAP who remain symptomatic or whose blood carbon dioxide does not improve, noninvasive ventilation can be tried, the guidelines say. Finally, patients diagnosed with OHS should be guided to weight loss interventions with the aim of reducing body weight by 25%-30%. This can include referral for bariatric surgery in patients without contraindications.
Dr. Mokhlesi and colleagues acknowledged that all of the recommendations contained in the guideline are classed as “conditional,” based on the quality of evidence assessed.
The American Thoracic Society funded the study. Dr. Mokhlesi and 7 coauthors disclosed financial conflicts of interest, while an additional 13 coauthors had none. Disclosures can be found on the AJRCCM website.
SOURCE: Mokhlesi B et al. Am J Respir Crit Care Med. 2019;200:3,e6-e24
FROM THE AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
Noninvasive ventilation: Redefining insurance guidelines
Noninvasive ventilation (NIV) supports patient’s breathing without the immediate need for tracheotomy or intubation. The Center for Medicare and Medicaid Services (CMS) defines respiratory assist devices (RAD) as bi-level devices with back-up respiratory rate capability, which provide noninvasive modes of ventilation for respiratory insufficiency or sleep-related respiratory disorders in a home or hospital setting (21 CFR 868.5895). These devices are smaller in size with provision of the external battery (if needed) but limited by inability to offer daytime ventilatory mode (ie, mouthpiece ventilation). Currently, respiratory assist devices have been in DMEPOS Competitive Bidding Program since 2011, (similar to PAP devices for sleep apnea syndromes), which puts a 13-month capped rental in which the patient gets the device, supplies, and services for 13 months subsequent to which patient owns the device and supplies are paid separately by CMS (https://www.dmecompetitivebid.com/cbic/cbic.nsf/DocsCat/Home).
On the other hand, CMS defines home mechanical ventilators (HMV) as life supporting/sustaining devices for patients of all age groups used in various settings, included but not limited to home, hospital, institutional setting, transportation, or wherever portability is needed. The ventilators have increased portability due to external and internal battery, provision of mouthpiece ventilation, and at least six pressure modes and three volumes modes. Currently, the ventilators are under the frequently and substantially serviced act [42 U.S.C. § 1395m(a)(3)]. Under this act, the patient never owns the device but the device, ancillary supplies, clinical support (trained respiratory therapists), and servicing of the device are included in the monthly payments, which can last indefinitely. Thus, ventilators have both higher reimbursement rates and uncapped rental periods; beneficiaries not only pay higher monthly co-payments for these devices but also pay over a longer rental period. Nonetheless, these services are vital in keeping a certain subset of patients comfortable at home and out of higher cost settings. The current populations that directly benefit from this service are patients with polio, amyotrophic lateral sclerosis, muscular dystrophies, spinal muscle atrophy, thoracic restrictive disorder, and chronic hypercapnic respiratory failure due to COPD, to name a few. Thus, HMV has been vital in “freeing” these frail and vulnerable patient populations from their hospital beds, improving the quality of life, as well as mortality.
With the advent of technologic advancements, HMV, especially the noninvasive pressure support ventilator, is now capable of doing multiple modes, including CPAP, RAD modes, and ventilator modes. This could create a potential of abuse when the durable medical equipment supplier bills CMS for the ventilator but clinically, a lower cost CPAP, auto bi-level PAP, or RAD is indicated. The 2016 report from the Office of Inspector General (OIG) noted that CMS paid 85 times more claims for noninvasive pressure support ventilators in 2015 than in 2009 (from $3.8 million to $340 million). [https://tinyurl.com/y3ckskrb]. Expenditure increased from 2014 to 2015 alone accounted for 47% of the entire $337 million increase from 2009 to 2015. But, the report could not implicate reduced prices for CPAP devices and RADs under the Competitive Bidding Program to be driving increased billing for ventilators. They did find that the diagnoses used for these claims have shifted dramatically from neuromuscular diseases to other chronic respiratory conditions.
Since then, in January 2016, CMS consolidated billing codes for ventilators, and also reduced the reimbursement amount for noninvasive pressure support ventilators. After this change, between 2015 and 2016, median monthly rental rate of products decreased from $1,561 to $1,055; a reduction of 32% [https://tinyurl.com/y3ckskrb]. CMS presently is proposing to include HMV in the competitive bidding program to help with misuse and cost reduction. But proposed addition of the home ventilators in competitive bidding risks elimination of the vital services that are so important to keep a very “vulnerable and frail” population out of higher cost facilities. Because of this, CMS would see increased costs due to frequent emergency rooms visits, frequent intubations, intensive care unit stays, and admissions to long-term care at skilled nursing on one hand, but negatively impacting the quality of life of these patients on the other hand. This addition would have serious unintended consequences on Medicaid recipients, especially the pediatric population.
As a clinical guide, RADs are used for similar clinical conditions as HMV, but are meant for less severe respiratory conditions. Ideally, getting a RAD device for a patient should be governed by the physician’s clinical judgment rather than rigorous qualification criteria, nonetheless current RAD coverage policy in not only difficult but includes unnecessary qualification criteria, and as a result pushing the patient towards more costly ventilators. Unfortunately, CMS policies have not kept up with the technological advances of noninvasive ventilation. This has led to increased costs and utilization of noninvasive ventilators. In our opinion, including noninvasive ventilators in competitive bidding to reduce cost utilization is not the solution.
CMS needs to work with medical providers, beneficiaries, and various stakeholders to revise the current respiratory assist device and home mechanical ventilator guidelines in order to ensure that the appropriate patient is eligible for the correct device, without putting a very vulnerable patient population at risk.
Dr. Sahni is Clinical Assistant Professor, Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Illinois at Chicago; Dr. Wolfe is Associate Professor of Medicine (Pulmonary & Critical Care) and Neurology (Sleep Medicine), Northwestern University, Chicago, Illinois.
Noninvasive ventilation (NIV) supports patient’s breathing without the immediate need for tracheotomy or intubation. The Center for Medicare and Medicaid Services (CMS) defines respiratory assist devices (RAD) as bi-level devices with back-up respiratory rate capability, which provide noninvasive modes of ventilation for respiratory insufficiency or sleep-related respiratory disorders in a home or hospital setting (21 CFR 868.5895). These devices are smaller in size with provision of the external battery (if needed) but limited by inability to offer daytime ventilatory mode (ie, mouthpiece ventilation). Currently, respiratory assist devices have been in DMEPOS Competitive Bidding Program since 2011, (similar to PAP devices for sleep apnea syndromes), which puts a 13-month capped rental in which the patient gets the device, supplies, and services for 13 months subsequent to which patient owns the device and supplies are paid separately by CMS (https://www.dmecompetitivebid.com/cbic/cbic.nsf/DocsCat/Home).
On the other hand, CMS defines home mechanical ventilators (HMV) as life supporting/sustaining devices for patients of all age groups used in various settings, included but not limited to home, hospital, institutional setting, transportation, or wherever portability is needed. The ventilators have increased portability due to external and internal battery, provision of mouthpiece ventilation, and at least six pressure modes and three volumes modes. Currently, the ventilators are under the frequently and substantially serviced act [42 U.S.C. § 1395m(a)(3)]. Under this act, the patient never owns the device but the device, ancillary supplies, clinical support (trained respiratory therapists), and servicing of the device are included in the monthly payments, which can last indefinitely. Thus, ventilators have both higher reimbursement rates and uncapped rental periods; beneficiaries not only pay higher monthly co-payments for these devices but also pay over a longer rental period. Nonetheless, these services are vital in keeping a certain subset of patients comfortable at home and out of higher cost settings. The current populations that directly benefit from this service are patients with polio, amyotrophic lateral sclerosis, muscular dystrophies, spinal muscle atrophy, thoracic restrictive disorder, and chronic hypercapnic respiratory failure due to COPD, to name a few. Thus, HMV has been vital in “freeing” these frail and vulnerable patient populations from their hospital beds, improving the quality of life, as well as mortality.
With the advent of technologic advancements, HMV, especially the noninvasive pressure support ventilator, is now capable of doing multiple modes, including CPAP, RAD modes, and ventilator modes. This could create a potential of abuse when the durable medical equipment supplier bills CMS for the ventilator but clinically, a lower cost CPAP, auto bi-level PAP, or RAD is indicated. The 2016 report from the Office of Inspector General (OIG) noted that CMS paid 85 times more claims for noninvasive pressure support ventilators in 2015 than in 2009 (from $3.8 million to $340 million). [https://tinyurl.com/y3ckskrb]. Expenditure increased from 2014 to 2015 alone accounted for 47% of the entire $337 million increase from 2009 to 2015. But, the report could not implicate reduced prices for CPAP devices and RADs under the Competitive Bidding Program to be driving increased billing for ventilators. They did find that the diagnoses used for these claims have shifted dramatically from neuromuscular diseases to other chronic respiratory conditions.
Since then, in January 2016, CMS consolidated billing codes for ventilators, and also reduced the reimbursement amount for noninvasive pressure support ventilators. After this change, between 2015 and 2016, median monthly rental rate of products decreased from $1,561 to $1,055; a reduction of 32% [https://tinyurl.com/y3ckskrb]. CMS presently is proposing to include HMV in the competitive bidding program to help with misuse and cost reduction. But proposed addition of the home ventilators in competitive bidding risks elimination of the vital services that are so important to keep a very “vulnerable and frail” population out of higher cost facilities. Because of this, CMS would see increased costs due to frequent emergency rooms visits, frequent intubations, intensive care unit stays, and admissions to long-term care at skilled nursing on one hand, but negatively impacting the quality of life of these patients on the other hand. This addition would have serious unintended consequences on Medicaid recipients, especially the pediatric population.
As a clinical guide, RADs are used for similar clinical conditions as HMV, but are meant for less severe respiratory conditions. Ideally, getting a RAD device for a patient should be governed by the physician’s clinical judgment rather than rigorous qualification criteria, nonetheless current RAD coverage policy in not only difficult but includes unnecessary qualification criteria, and as a result pushing the patient towards more costly ventilators. Unfortunately, CMS policies have not kept up with the technological advances of noninvasive ventilation. This has led to increased costs and utilization of noninvasive ventilators. In our opinion, including noninvasive ventilators in competitive bidding to reduce cost utilization is not the solution.
CMS needs to work with medical providers, beneficiaries, and various stakeholders to revise the current respiratory assist device and home mechanical ventilator guidelines in order to ensure that the appropriate patient is eligible for the correct device, without putting a very vulnerable patient population at risk.
Dr. Sahni is Clinical Assistant Professor, Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Illinois at Chicago; Dr. Wolfe is Associate Professor of Medicine (Pulmonary & Critical Care) and Neurology (Sleep Medicine), Northwestern University, Chicago, Illinois.
Noninvasive ventilation (NIV) supports patient’s breathing without the immediate need for tracheotomy or intubation. The Center for Medicare and Medicaid Services (CMS) defines respiratory assist devices (RAD) as bi-level devices with back-up respiratory rate capability, which provide noninvasive modes of ventilation for respiratory insufficiency or sleep-related respiratory disorders in a home or hospital setting (21 CFR 868.5895). These devices are smaller in size with provision of the external battery (if needed) but limited by inability to offer daytime ventilatory mode (ie, mouthpiece ventilation). Currently, respiratory assist devices have been in DMEPOS Competitive Bidding Program since 2011, (similar to PAP devices for sleep apnea syndromes), which puts a 13-month capped rental in which the patient gets the device, supplies, and services for 13 months subsequent to which patient owns the device and supplies are paid separately by CMS (https://www.dmecompetitivebid.com/cbic/cbic.nsf/DocsCat/Home).
On the other hand, CMS defines home mechanical ventilators (HMV) as life supporting/sustaining devices for patients of all age groups used in various settings, included but not limited to home, hospital, institutional setting, transportation, or wherever portability is needed. The ventilators have increased portability due to external and internal battery, provision of mouthpiece ventilation, and at least six pressure modes and three volumes modes. Currently, the ventilators are under the frequently and substantially serviced act [42 U.S.C. § 1395m(a)(3)]. Under this act, the patient never owns the device but the device, ancillary supplies, clinical support (trained respiratory therapists), and servicing of the device are included in the monthly payments, which can last indefinitely. Thus, ventilators have both higher reimbursement rates and uncapped rental periods; beneficiaries not only pay higher monthly co-payments for these devices but also pay over a longer rental period. Nonetheless, these services are vital in keeping a certain subset of patients comfortable at home and out of higher cost settings. The current populations that directly benefit from this service are patients with polio, amyotrophic lateral sclerosis, muscular dystrophies, spinal muscle atrophy, thoracic restrictive disorder, and chronic hypercapnic respiratory failure due to COPD, to name a few. Thus, HMV has been vital in “freeing” these frail and vulnerable patient populations from their hospital beds, improving the quality of life, as well as mortality.
With the advent of technologic advancements, HMV, especially the noninvasive pressure support ventilator, is now capable of doing multiple modes, including CPAP, RAD modes, and ventilator modes. This could create a potential of abuse when the durable medical equipment supplier bills CMS for the ventilator but clinically, a lower cost CPAP, auto bi-level PAP, or RAD is indicated. The 2016 report from the Office of Inspector General (OIG) noted that CMS paid 85 times more claims for noninvasive pressure support ventilators in 2015 than in 2009 (from $3.8 million to $340 million). [https://tinyurl.com/y3ckskrb]. Expenditure increased from 2014 to 2015 alone accounted for 47% of the entire $337 million increase from 2009 to 2015. But, the report could not implicate reduced prices for CPAP devices and RADs under the Competitive Bidding Program to be driving increased billing for ventilators. They did find that the diagnoses used for these claims have shifted dramatically from neuromuscular diseases to other chronic respiratory conditions.
Since then, in January 2016, CMS consolidated billing codes for ventilators, and also reduced the reimbursement amount for noninvasive pressure support ventilators. After this change, between 2015 and 2016, median monthly rental rate of products decreased from $1,561 to $1,055; a reduction of 32% [https://tinyurl.com/y3ckskrb]. CMS presently is proposing to include HMV in the competitive bidding program to help with misuse and cost reduction. But proposed addition of the home ventilators in competitive bidding risks elimination of the vital services that are so important to keep a very “vulnerable and frail” population out of higher cost facilities. Because of this, CMS would see increased costs due to frequent emergency rooms visits, frequent intubations, intensive care unit stays, and admissions to long-term care at skilled nursing on one hand, but negatively impacting the quality of life of these patients on the other hand. This addition would have serious unintended consequences on Medicaid recipients, especially the pediatric population.
As a clinical guide, RADs are used for similar clinical conditions as HMV, but are meant for less severe respiratory conditions. Ideally, getting a RAD device for a patient should be governed by the physician’s clinical judgment rather than rigorous qualification criteria, nonetheless current RAD coverage policy in not only difficult but includes unnecessary qualification criteria, and as a result pushing the patient towards more costly ventilators. Unfortunately, CMS policies have not kept up with the technological advances of noninvasive ventilation. This has led to increased costs and utilization of noninvasive ventilators. In our opinion, including noninvasive ventilators in competitive bidding to reduce cost utilization is not the solution.
CMS needs to work with medical providers, beneficiaries, and various stakeholders to revise the current respiratory assist device and home mechanical ventilator guidelines in order to ensure that the appropriate patient is eligible for the correct device, without putting a very vulnerable patient population at risk.
Dr. Sahni is Clinical Assistant Professor, Division of Pulmonary, Critical Care, and Sleep Medicine at the University of Illinois at Chicago; Dr. Wolfe is Associate Professor of Medicine (Pulmonary & Critical Care) and Neurology (Sleep Medicine), Northwestern University, Chicago, Illinois.
Obstructive sleep apnea: A wake-up call for better outcomes
For too many of us, a good night’s sleep is a rare occurrence. Lack of quality sleep has profound negative effects on our health, safety, and wellbeing. An estimated 50 to 70 million Americans have sleep disturbances, including 10% to 17% of men and 3% to 9% of women with moderate to severe obstructive sleep apnea (OSA).1 Not only is OSA highly prevalent, 82% to 93% of individuals with moderate to severe OSA are unaware they have it, and it remains undiagnosed.2
OSA is a potentially serious medical disorder affecting the heart, brain, and metabolism. These physiological changes negatively impact public safety, occupational and academic achievement, and even mortality.
This Cleveland Clinic Journal of Medicine supplement presents a state-of-the-art review of OSA, including the health and societal consequences of OSA and current treatment options. The goal of this publication is to inform and educate healthcare providers from all backgrounds and levels of care who are interested in improving patient outcomes through attention to sleep medicine.
Because OSA is prevalent and underdiagnosed, Jessica Vensel Rundo, MD, MS, reviews the symptoms of OSA, clinical presentation, and the readily available, effective screening tools for detecting sleep apnea. Greater awareness and screening for sleep disturbances informs the need for further diagnostic tests such as laboratory polysomnography and home sleep apnea testing.
The link between OSA and the heart is presented by Reena Mehra, MD, MS, with an overview of the physiology of sleep-heart interactions and the association of OSA and cardiovascular health. Dr. Mehra also reviews central sleep apnea and discusses 2 newer therapies for it: adaptive servoventilation and phrenic nerve stimulation.
Beyond heart health, OSA also adversely affects quality of life, safety, and other important health factors. Harneet Walia, MD, discusses consequences of sleep apnea such as daytime sleepiness, fatigue, drowsy driving, depression, metabolic diseases, and cognitive impairment.
Several treatment options exist for patients diagnosed with OSA. Positive airway pressure (PAP) therapy is the gold standard for treatment of OSA. Colleen G. Lance, MD, reviews and presents case scenarios about the efficacy of PAP therapy, features of continuous PAP therapy, and innovative strategies to improve adherence to therapy.
In addition to PAP therapy, there are alternative treatments for OSA that may benefit some patients. Tina Waters, MD, considers alternatives to PAP therapy, such as lifestyle changes, expiratory PAP therapy, oral appliances, upper airway surgery, and hypoglossal nerve stimulation.
I hope you enjoy this supplement and find it useful to improving the health and quality-of-life outcomes of patients in your care.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20(9):705706.
For too many of us, a good night’s sleep is a rare occurrence. Lack of quality sleep has profound negative effects on our health, safety, and wellbeing. An estimated 50 to 70 million Americans have sleep disturbances, including 10% to 17% of men and 3% to 9% of women with moderate to severe obstructive sleep apnea (OSA).1 Not only is OSA highly prevalent, 82% to 93% of individuals with moderate to severe OSA are unaware they have it, and it remains undiagnosed.2
OSA is a potentially serious medical disorder affecting the heart, brain, and metabolism. These physiological changes negatively impact public safety, occupational and academic achievement, and even mortality.
This Cleveland Clinic Journal of Medicine supplement presents a state-of-the-art review of OSA, including the health and societal consequences of OSA and current treatment options. The goal of this publication is to inform and educate healthcare providers from all backgrounds and levels of care who are interested in improving patient outcomes through attention to sleep medicine.
Because OSA is prevalent and underdiagnosed, Jessica Vensel Rundo, MD, MS, reviews the symptoms of OSA, clinical presentation, and the readily available, effective screening tools for detecting sleep apnea. Greater awareness and screening for sleep disturbances informs the need for further diagnostic tests such as laboratory polysomnography and home sleep apnea testing.
The link between OSA and the heart is presented by Reena Mehra, MD, MS, with an overview of the physiology of sleep-heart interactions and the association of OSA and cardiovascular health. Dr. Mehra also reviews central sleep apnea and discusses 2 newer therapies for it: adaptive servoventilation and phrenic nerve stimulation.
Beyond heart health, OSA also adversely affects quality of life, safety, and other important health factors. Harneet Walia, MD, discusses consequences of sleep apnea such as daytime sleepiness, fatigue, drowsy driving, depression, metabolic diseases, and cognitive impairment.
Several treatment options exist for patients diagnosed with OSA. Positive airway pressure (PAP) therapy is the gold standard for treatment of OSA. Colleen G. Lance, MD, reviews and presents case scenarios about the efficacy of PAP therapy, features of continuous PAP therapy, and innovative strategies to improve adherence to therapy.
In addition to PAP therapy, there are alternative treatments for OSA that may benefit some patients. Tina Waters, MD, considers alternatives to PAP therapy, such as lifestyle changes, expiratory PAP therapy, oral appliances, upper airway surgery, and hypoglossal nerve stimulation.
I hope you enjoy this supplement and find it useful to improving the health and quality-of-life outcomes of patients in your care.
For too many of us, a good night’s sleep is a rare occurrence. Lack of quality sleep has profound negative effects on our health, safety, and wellbeing. An estimated 50 to 70 million Americans have sleep disturbances, including 10% to 17% of men and 3% to 9% of women with moderate to severe obstructive sleep apnea (OSA).1 Not only is OSA highly prevalent, 82% to 93% of individuals with moderate to severe OSA are unaware they have it, and it remains undiagnosed.2
OSA is a potentially serious medical disorder affecting the heart, brain, and metabolism. These physiological changes negatively impact public safety, occupational and academic achievement, and even mortality.
This Cleveland Clinic Journal of Medicine supplement presents a state-of-the-art review of OSA, including the health and societal consequences of OSA and current treatment options. The goal of this publication is to inform and educate healthcare providers from all backgrounds and levels of care who are interested in improving patient outcomes through attention to sleep medicine.
Because OSA is prevalent and underdiagnosed, Jessica Vensel Rundo, MD, MS, reviews the symptoms of OSA, clinical presentation, and the readily available, effective screening tools for detecting sleep apnea. Greater awareness and screening for sleep disturbances informs the need for further diagnostic tests such as laboratory polysomnography and home sleep apnea testing.
The link between OSA and the heart is presented by Reena Mehra, MD, MS, with an overview of the physiology of sleep-heart interactions and the association of OSA and cardiovascular health. Dr. Mehra also reviews central sleep apnea and discusses 2 newer therapies for it: adaptive servoventilation and phrenic nerve stimulation.
Beyond heart health, OSA also adversely affects quality of life, safety, and other important health factors. Harneet Walia, MD, discusses consequences of sleep apnea such as daytime sleepiness, fatigue, drowsy driving, depression, metabolic diseases, and cognitive impairment.
Several treatment options exist for patients diagnosed with OSA. Positive airway pressure (PAP) therapy is the gold standard for treatment of OSA. Colleen G. Lance, MD, reviews and presents case scenarios about the efficacy of PAP therapy, features of continuous PAP therapy, and innovative strategies to improve adherence to therapy.
In addition to PAP therapy, there are alternative treatments for OSA that may benefit some patients. Tina Waters, MD, considers alternatives to PAP therapy, such as lifestyle changes, expiratory PAP therapy, oral appliances, upper airway surgery, and hypoglossal nerve stimulation.
I hope you enjoy this supplement and find it useful to improving the health and quality-of-life outcomes of patients in your care.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20(9):705706.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Young T, Evans L, Finn L, Palta M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20(9):705706.
Obstructive sleep apnea basics
DEFINITION
Obstructive sleep apnea (OSA) occurs when there are recurrent episodes of upper airway collapse and obstruction during sleep associated with arousals with or without oxygen desaturations. The oropharynx in the back of the throat collapses during OSA events to cause arousal or oxygen desaturation or both resulting in fragmented sleep.
PREVALENCE
Studies reveal OSA is prevalent. A 2015 study in Switzerland reported 50% of men and 23% of women had at least moderate OSA.1 In 2002, the Sleep Heart Health study found that 24% of men and 9% of women have at least mild OSA.2 In the Wisconsin Sleep Study Cohort, it was reported that 10% of men and 3% of women age 30 to 49 have at least moderate OSA, while 17% of men and 9% of women age 50 to 70 have at least moderate OSA.3 OSA is highly underrecognized and it is estimated that 82% of men and 93% of women in the United States with OSA are undiagnosed.4
SYMPTOMS
RISK FACTORS
The risk of OSA is influenced by unmodifiable and modifiable factors. Unmodifiable risk factors include male sex, age, and race. Genetic predisposition or a family history of OSA as well as cranial facial anatomy resulting in narrow airways may impart higher risk of OSA. Modifiable risk factors include obesity, medications that cause muscle relaxation and narrowing of the airway (opiates, benzodiazepines, alcohol), endocrine disorders (hypothyroidism, polycystic ovarian syndrome), smoking, and nasal congestion or obstruction.6
Sex
Men are at higher risk for OSA than women although once women reach menopause they have a risk similar to men. Postmenopausal women on hormone replacement therapy were found to have lower rates of OSA, suggesting that loss of hormones results in greater risk of OSA.7,8 Women also have more OSA during rapid eye movement (REM) sleep and less OSA when sleeping supine, whereas most men have OSA when sleeping supine.9,10 OSA is less severe in women compared with men of similar body mass index (BMI).11 Symptoms vary in men and women: snoring and witnessed apneas are more common in men whereas insomnia and excessive daytime sleepiness are more common in women.11 This may account for delayed diagnosis and the higher mortality in women compared with men.
Age
The risk of OSA increases with age. In a study of men 65 or older, the prevalence of moderate OSA was 23% in men younger than 72 and 30% in men older than 80.12 By comparison, the prevalence of moderate OSA in men 30 to 40 years was 10%.3 Increased risk of OSA with age may be due to age-related reduction in slow wave sleep (ie, deep sleep), which is protective against sleep-disordered breathing and airway collapse.13 Older adults are also less symptomatic, reporting less daytime sleepiness and fatigue.14
Race
The Sleep Heart Health Study found a slightly increased risk of moderate to severe OSA in blacks (20%) and American Indians (23%) compared with whites (17%).2 Another study showed the prevalence of OSA was 30% in whites, 32% in blacks, 38% in Hispanics, and 39% in Chinese individuals.15 A higher prevalence of OSA in young blacks (≤ 25 years) compared with whites was reported,16 although another study found no differences based on race in older patients.17 These differences among racial groups may be due to variations in craniofacial anatomy.
Obesity
There is a correlation between increased risk of OSA and obesity (BMI > 30 kg/m2) and its correlates of greater waist-to-hip ratio and neck circumference.2 A 10% increase in body weight results in a sixfold increase in moderate to severe OSA and increases the apnea–hypopnea index (AHI; number of breath pauses or respiratory events per hour) by 32% whereas a 10% decrease in weight decreases the AHI by 26%.18
COMORBIDITIES
OSA is associated with a number of comorbid conditions including stroke, myocardial infarction, hypertension, hyperlipidemia, glucose intolerance, diabetes, arrhythmias including atrial fibrillation, pulmonary hypertension, congestive heart failure, and depression. Patients with moderate or severe OSA are at higher risk of these comorbid conditions.19
Patients with cardiovascular disease have a very high prevalence of OSA: hypertension (83% mild to 30% moderate to severe OSA), heart failure (55% to 12%), arrhythmias (50% to 20%), stroke (75% to 57%), and coronary heart disease (65% to 38%).20 Increased awareness and early diagnosis of OSA is critical to reducing cardiovascular disease burden.
SCREENING
Sleep history
A sleep history starts with determining the patient’s total sleep time, based on time to bed, time to fall asleep, and time of wake up, including any difficulty falling asleep, staying asleep, or daytime naps.
Symptoms. Daytime naps generally indicate a sleep deficit or sleep that is not refreshing. A review of sleep and daytime symptoms associated with OSA (Table 1) helps determine if excessive daytime sleepiness or unrefreshing sleep is out of proportion with the amount of sleep the patient is getting at night.
Some patients with OSA may have memory or concentration issues or feel like they have attention deficit disorder. In fact some patients are diagnosed with attention deficit disorder because of their insufficient sleep or unrefreshing sleep.
Drowsy driving is a special concern in patients with untreated OSA and sleep deprivation. Many patients have drowsy driving episodes or difficulty staying awake during long-distance driving. Caffeine use is also important information as excessive caffeine may be used to combat sleepiness during the day.
The Epworth Sleepiness Scale is a clinical screening tool that presents 8 situations for patients to consider and indicate their level of sleepiness and likelihood of falling asleep (never = 0; slight = 1; moderate = 2, high = 3).21,22 A total score ≥ 10 is considered abnormal in that the patient is excessively sleepy compared with most people.
Risk factors and comorbid conditions. OSA risk factors and comorbidities, including a BMI obesity assessment, should be reviewed with patients. Nasal congestion or mouth breathing especially at night could be due to airway obstruction increasing the risk of OSA. Family history of OSA, tobacco, alcohol use, other medical conditions, and medications should also be discussed.
Physical examination
- Neck circumference greater than 17 inches for men or greater than 16 inches for women
- BMI greater than 30
- Friedman class tongue position class 3 or greater (Figure 1)
- Mouth features (present/enlarged tonsils, macroglossia, jaw misalignment)
- Nasal abnormalities (turbinate hypertrophy, deviated septum).5
Patients with Friedman palate positions class 3 and 4 have a higher risk of OSA due to airway crowding during sleep when the airway naturally collapses a little and is even more restricted.
Narrow airways or oropharyngeal crowding can also be due to a swollen, enlarged, or elongated uvula; present or enlarged tonsils; or lateral wall narrowing. Alone or in combination, these features can contribute to airway obstruction.
Other signs in the mouth suggestive of obstruction are macroglossia (enlarged tongue) and tongue ridging. Tongue ridging or scalloping impressions typically occur during sleep and are caused by the tongue moving forward to open the airway and pressing against the teeth.
Retrognathia (lower jaw offset behind upper jaw) can narrow the airway and increase the risk of OSA as can a high arch palate, overbite (upper teeth forward), or overjet (upper teeth over the top of lower teeth).
A nasal examination for nasal valve collapse (ie, nostril collapses with inhalation), deviated septum, and inferior turbinate hypertrophy impart an increased risk of OSA.
Screening tools
In addition to the Epworth Sleepiness Scale, the STOP-BANG questionnaire can help determine if a patient should be tested further for OSA. The STOP-BANG questionnaire consists of 8 yes-no questions where more than 2 yes responses indicate the patient is at higher risk for moderate to severe OSA (93% sensitivity): Snore, Tired, Observed stopped breathing, high blood Pressure, BMI > 35 kg/m2, Age > 50, Neck > 15.75 inches, Gender = male).23
SLEEP STUDIES
Polysomnography
Home sleep apnea test
HSATs record 4 to 7 parameters including airflow (thermal and nasal pressure), effort (inductive plethysmography), and oximetry. No electroencephalogram is used, so sleep is not recorded; it is assumed the patient is sleeping for the duration of the test. As such, respiratory events are based on oxygen desaturations and reduced airflow and pressure as well as chest and abdomen effort. The raw data are edited and manually scored and reviewed by a sleep specialist.25
Although the HSAT is convenient for many patients, it underestimates the severity of sleep-related breathing disorders. HSAT is intended to confirm OSA in patients with a high likelihood of OSA based on their sleep history.26 It is ideally employed for adult patients with no major medical problems or other sleep problems who are at high risk for moderate to severe OSA based on the STOP-BANG questionnaire or those with daytime sleepiness and 2 of the 3 symptoms of snoring, witnessed apnea, or hypertension.27
A negative or inconclusive HSAT warrants a PSG to ensure the patient does not have OSA. Use of HSAT is contraindicated in patients with
- Significant cardiopulmonary disease
- Potential weakness due to a neuromuscular condition
- Awake hypoventilation or high risk for sleep-related hypoventilation (severe obesity)
- History of stroke
- Chronic opioid use
- Severe insomnia
- Symptoms of other significant sleep disorders
- Environmental/personal factors that would preclude adequate acquisition and interpretation of data (disruptions from children, pets, other factors).27
DIAGNOSTIC CRITERIA
Respiratory events captured on a PSG or HSAT
The OSA diagnostic criteria are based on the occurrence of obstructive respiratory events recorded during sleep such as apneas, hypopneas, and respiratory event-related arousals.
Hypopneas. A hypopnea is a respiratory event resulting in reduced airflow. The America Association of Sleep Medicine’s preferred definition is a reduction in nasal pressure of at least 30% for 10 seconds or longer with 3% or greater oxygen desaturation or an electroencephalogram arousal. Another acceptable definition is at least 30% reduction in thoracoabdominal movement or airflow with 4% or greater oxygen desaturation, which is used by the Centers for Medicare and Medicaid Services and other insurers.29,30 Hypopnea requires greater oxygen desaturation and is not dependent on arousals, which can sometimes make it more challenging to identify OSA (Figure 2).
Respiratory event-related arousals. Respiratory event-related arousals are respiratory events not meeting apnea or hypopnea criteria. They are measured as a sequence of breaths of 10 or more seconds with increasing respiratory effort or flattening of the nasal pressure waveform leading to arousal (Figure 2).29 Respiratory event-related arousals are disruptive to sleep and have many of the same consequences as apneas and hypopneas.
Severity
SUMMARY
OSA results from airway collapse and obstruction during sleep, often causing arousal from sleep with or without oxygen desaturation. The prevalence of OSA is underestimated and it is underdiagnosed despite known risk factors and comorbid conditions. Screening for OSA with a sleep history, simple upper airway examination, and quick validated screening tool like the STOP-BANG or Epworth Sleepiness Scale aid in identifying the need for testing for OSA. A laboratory sleep study with a PSG can confirm the diagnosis and severity of OSA. HSATs are available to confirm the diagnosis of OSA in patients at high risk for moderate to severe OSA.
- Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015; 3(4):310–318.
- Young T, Shahar E, Nieto FJ, et al; for the Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults. Arch Intern Med 2002; 162(8):893–900.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Young T, Evans L, Finn L, Palta M. Estimation of clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20(9):705–706.
- Epstein LJ, Kristo D, Strollo Jr, PJ, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5(3):263–276.
- Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA 2004; 291(16):2013–2016.
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2003; 167(9):1181–1185.
- Shahar E, Redline S, Young T, et al; for the Sleep Heart Health Study Research Group. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003; 167(9):1186–1192.
- O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 2000; 161(5):1465–1472.
- Collop NA, Adkins D, Phillips BA. Gender differences in sleep and sleep-disordered breathing. Clin Chest Med 2004; 25(2):257–268.
- Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 1994; 149(3 Pt 1):722–726.
- Mehra R, Stone KL, Blackwell T, et al; for the Osteoporotic Fractures in Men Study. Prevalence and correlates of sleep-disordered breathing in older men: Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 2007; 55(9):1356–1364.
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 2000; 284(7):861–868.
- Groth M. Sleep apnea in the elderly. Clin Geriatr Med 2005; 21:701–712.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997; 155(1):186–192.
- Song Y, Ancoli-Israel S, Lewis CE, Redline S, Harrison SL, Stone KL. The association of race/ethnicity with objectively measured sleep characteristics in older men. Behav Sleep Med 2011; 10(1):54–69.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284(23):3015–3021.
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69(7):841–858.
- Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. Chest 1993; 103(1):30–36.
- Chervin RD, Aldrich MS. The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology 1999; 52(1):125–131.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108(5):812–821.
- Iber C, Ancoli-Israel S, Chesson A, Quan SF; for the American Academy of Sleep and Medicine. The ASSM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Winchester, IL: American Academy of Sleep Medicine; 2007.
- Centers for Medicare and Medicaid Services. Medicare Learning Network. Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/mm6048.pdf. Accessed August 19, 2019.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med 2007; 3(7):737–747.
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13(3):479–504.
- Sateia MJ. International classification of sleep disorders—3rd ed: highlights and modifications. Chest 2014; 146(5):1387–1394.
- AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.5. American Academy of Sleep Medicine; April 2018.
- Centers for Medicare and Medicaid Services. Medicare Coverage Database. www.cms.gov/medicare-coverage-database. Accessed August 19, 2019.
DEFINITION
Obstructive sleep apnea (OSA) occurs when there are recurrent episodes of upper airway collapse and obstruction during sleep associated with arousals with or without oxygen desaturations. The oropharynx in the back of the throat collapses during OSA events to cause arousal or oxygen desaturation or both resulting in fragmented sleep.
PREVALENCE
Studies reveal OSA is prevalent. A 2015 study in Switzerland reported 50% of men and 23% of women had at least moderate OSA.1 In 2002, the Sleep Heart Health study found that 24% of men and 9% of women have at least mild OSA.2 In the Wisconsin Sleep Study Cohort, it was reported that 10% of men and 3% of women age 30 to 49 have at least moderate OSA, while 17% of men and 9% of women age 50 to 70 have at least moderate OSA.3 OSA is highly underrecognized and it is estimated that 82% of men and 93% of women in the United States with OSA are undiagnosed.4
SYMPTOMS
RISK FACTORS
The risk of OSA is influenced by unmodifiable and modifiable factors. Unmodifiable risk factors include male sex, age, and race. Genetic predisposition or a family history of OSA as well as cranial facial anatomy resulting in narrow airways may impart higher risk of OSA. Modifiable risk factors include obesity, medications that cause muscle relaxation and narrowing of the airway (opiates, benzodiazepines, alcohol), endocrine disorders (hypothyroidism, polycystic ovarian syndrome), smoking, and nasal congestion or obstruction.6
Sex
Men are at higher risk for OSA than women although once women reach menopause they have a risk similar to men. Postmenopausal women on hormone replacement therapy were found to have lower rates of OSA, suggesting that loss of hormones results in greater risk of OSA.7,8 Women also have more OSA during rapid eye movement (REM) sleep and less OSA when sleeping supine, whereas most men have OSA when sleeping supine.9,10 OSA is less severe in women compared with men of similar body mass index (BMI).11 Symptoms vary in men and women: snoring and witnessed apneas are more common in men whereas insomnia and excessive daytime sleepiness are more common in women.11 This may account for delayed diagnosis and the higher mortality in women compared with men.
Age
The risk of OSA increases with age. In a study of men 65 or older, the prevalence of moderate OSA was 23% in men younger than 72 and 30% in men older than 80.12 By comparison, the prevalence of moderate OSA in men 30 to 40 years was 10%.3 Increased risk of OSA with age may be due to age-related reduction in slow wave sleep (ie, deep sleep), which is protective against sleep-disordered breathing and airway collapse.13 Older adults are also less symptomatic, reporting less daytime sleepiness and fatigue.14
Race
The Sleep Heart Health Study found a slightly increased risk of moderate to severe OSA in blacks (20%) and American Indians (23%) compared with whites (17%).2 Another study showed the prevalence of OSA was 30% in whites, 32% in blacks, 38% in Hispanics, and 39% in Chinese individuals.15 A higher prevalence of OSA in young blacks (≤ 25 years) compared with whites was reported,16 although another study found no differences based on race in older patients.17 These differences among racial groups may be due to variations in craniofacial anatomy.
Obesity
There is a correlation between increased risk of OSA and obesity (BMI > 30 kg/m2) and its correlates of greater waist-to-hip ratio and neck circumference.2 A 10% increase in body weight results in a sixfold increase in moderate to severe OSA and increases the apnea–hypopnea index (AHI; number of breath pauses or respiratory events per hour) by 32% whereas a 10% decrease in weight decreases the AHI by 26%.18
COMORBIDITIES
OSA is associated with a number of comorbid conditions including stroke, myocardial infarction, hypertension, hyperlipidemia, glucose intolerance, diabetes, arrhythmias including atrial fibrillation, pulmonary hypertension, congestive heart failure, and depression. Patients with moderate or severe OSA are at higher risk of these comorbid conditions.19
Patients with cardiovascular disease have a very high prevalence of OSA: hypertension (83% mild to 30% moderate to severe OSA), heart failure (55% to 12%), arrhythmias (50% to 20%), stroke (75% to 57%), and coronary heart disease (65% to 38%).20 Increased awareness and early diagnosis of OSA is critical to reducing cardiovascular disease burden.
SCREENING
Sleep history
A sleep history starts with determining the patient’s total sleep time, based on time to bed, time to fall asleep, and time of wake up, including any difficulty falling asleep, staying asleep, or daytime naps.
Symptoms. Daytime naps generally indicate a sleep deficit or sleep that is not refreshing. A review of sleep and daytime symptoms associated with OSA (Table 1) helps determine if excessive daytime sleepiness or unrefreshing sleep is out of proportion with the amount of sleep the patient is getting at night.
Some patients with OSA may have memory or concentration issues or feel like they have attention deficit disorder. In fact some patients are diagnosed with attention deficit disorder because of their insufficient sleep or unrefreshing sleep.
Drowsy driving is a special concern in patients with untreated OSA and sleep deprivation. Many patients have drowsy driving episodes or difficulty staying awake during long-distance driving. Caffeine use is also important information as excessive caffeine may be used to combat sleepiness during the day.
The Epworth Sleepiness Scale is a clinical screening tool that presents 8 situations for patients to consider and indicate their level of sleepiness and likelihood of falling asleep (never = 0; slight = 1; moderate = 2, high = 3).21,22 A total score ≥ 10 is considered abnormal in that the patient is excessively sleepy compared with most people.
Risk factors and comorbid conditions. OSA risk factors and comorbidities, including a BMI obesity assessment, should be reviewed with patients. Nasal congestion or mouth breathing especially at night could be due to airway obstruction increasing the risk of OSA. Family history of OSA, tobacco, alcohol use, other medical conditions, and medications should also be discussed.
Physical examination
- Neck circumference greater than 17 inches for men or greater than 16 inches for women
- BMI greater than 30
- Friedman class tongue position class 3 or greater (Figure 1)
- Mouth features (present/enlarged tonsils, macroglossia, jaw misalignment)
- Nasal abnormalities (turbinate hypertrophy, deviated septum).5
Patients with Friedman palate positions class 3 and 4 have a higher risk of OSA due to airway crowding during sleep when the airway naturally collapses a little and is even more restricted.
Narrow airways or oropharyngeal crowding can also be due to a swollen, enlarged, or elongated uvula; present or enlarged tonsils; or lateral wall narrowing. Alone or in combination, these features can contribute to airway obstruction.
Other signs in the mouth suggestive of obstruction are macroglossia (enlarged tongue) and tongue ridging. Tongue ridging or scalloping impressions typically occur during sleep and are caused by the tongue moving forward to open the airway and pressing against the teeth.
Retrognathia (lower jaw offset behind upper jaw) can narrow the airway and increase the risk of OSA as can a high arch palate, overbite (upper teeth forward), or overjet (upper teeth over the top of lower teeth).
A nasal examination for nasal valve collapse (ie, nostril collapses with inhalation), deviated septum, and inferior turbinate hypertrophy impart an increased risk of OSA.
Screening tools
In addition to the Epworth Sleepiness Scale, the STOP-BANG questionnaire can help determine if a patient should be tested further for OSA. The STOP-BANG questionnaire consists of 8 yes-no questions where more than 2 yes responses indicate the patient is at higher risk for moderate to severe OSA (93% sensitivity): Snore, Tired, Observed stopped breathing, high blood Pressure, BMI > 35 kg/m2, Age > 50, Neck > 15.75 inches, Gender = male).23
SLEEP STUDIES
Polysomnography
Home sleep apnea test
HSATs record 4 to 7 parameters including airflow (thermal and nasal pressure), effort (inductive plethysmography), and oximetry. No electroencephalogram is used, so sleep is not recorded; it is assumed the patient is sleeping for the duration of the test. As such, respiratory events are based on oxygen desaturations and reduced airflow and pressure as well as chest and abdomen effort. The raw data are edited and manually scored and reviewed by a sleep specialist.25
Although the HSAT is convenient for many patients, it underestimates the severity of sleep-related breathing disorders. HSAT is intended to confirm OSA in patients with a high likelihood of OSA based on their sleep history.26 It is ideally employed for adult patients with no major medical problems or other sleep problems who are at high risk for moderate to severe OSA based on the STOP-BANG questionnaire or those with daytime sleepiness and 2 of the 3 symptoms of snoring, witnessed apnea, or hypertension.27
A negative or inconclusive HSAT warrants a PSG to ensure the patient does not have OSA. Use of HSAT is contraindicated in patients with
- Significant cardiopulmonary disease
- Potential weakness due to a neuromuscular condition
- Awake hypoventilation or high risk for sleep-related hypoventilation (severe obesity)
- History of stroke
- Chronic opioid use
- Severe insomnia
- Symptoms of other significant sleep disorders
- Environmental/personal factors that would preclude adequate acquisition and interpretation of data (disruptions from children, pets, other factors).27
DIAGNOSTIC CRITERIA
Respiratory events captured on a PSG or HSAT
The OSA diagnostic criteria are based on the occurrence of obstructive respiratory events recorded during sleep such as apneas, hypopneas, and respiratory event-related arousals.
Hypopneas. A hypopnea is a respiratory event resulting in reduced airflow. The America Association of Sleep Medicine’s preferred definition is a reduction in nasal pressure of at least 30% for 10 seconds or longer with 3% or greater oxygen desaturation or an electroencephalogram arousal. Another acceptable definition is at least 30% reduction in thoracoabdominal movement or airflow with 4% or greater oxygen desaturation, which is used by the Centers for Medicare and Medicaid Services and other insurers.29,30 Hypopnea requires greater oxygen desaturation and is not dependent on arousals, which can sometimes make it more challenging to identify OSA (Figure 2).
Respiratory event-related arousals. Respiratory event-related arousals are respiratory events not meeting apnea or hypopnea criteria. They are measured as a sequence of breaths of 10 or more seconds with increasing respiratory effort or flattening of the nasal pressure waveform leading to arousal (Figure 2).29 Respiratory event-related arousals are disruptive to sleep and have many of the same consequences as apneas and hypopneas.
Severity
SUMMARY
OSA results from airway collapse and obstruction during sleep, often causing arousal from sleep with or without oxygen desaturation. The prevalence of OSA is underestimated and it is underdiagnosed despite known risk factors and comorbid conditions. Screening for OSA with a sleep history, simple upper airway examination, and quick validated screening tool like the STOP-BANG or Epworth Sleepiness Scale aid in identifying the need for testing for OSA. A laboratory sleep study with a PSG can confirm the diagnosis and severity of OSA. HSATs are available to confirm the diagnosis of OSA in patients at high risk for moderate to severe OSA.
DEFINITION
Obstructive sleep apnea (OSA) occurs when there are recurrent episodes of upper airway collapse and obstruction during sleep associated with arousals with or without oxygen desaturations. The oropharynx in the back of the throat collapses during OSA events to cause arousal or oxygen desaturation or both resulting in fragmented sleep.
PREVALENCE
Studies reveal OSA is prevalent. A 2015 study in Switzerland reported 50% of men and 23% of women had at least moderate OSA.1 In 2002, the Sleep Heart Health study found that 24% of men and 9% of women have at least mild OSA.2 In the Wisconsin Sleep Study Cohort, it was reported that 10% of men and 3% of women age 30 to 49 have at least moderate OSA, while 17% of men and 9% of women age 50 to 70 have at least moderate OSA.3 OSA is highly underrecognized and it is estimated that 82% of men and 93% of women in the United States with OSA are undiagnosed.4
SYMPTOMS
RISK FACTORS
The risk of OSA is influenced by unmodifiable and modifiable factors. Unmodifiable risk factors include male sex, age, and race. Genetic predisposition or a family history of OSA as well as cranial facial anatomy resulting in narrow airways may impart higher risk of OSA. Modifiable risk factors include obesity, medications that cause muscle relaxation and narrowing of the airway (opiates, benzodiazepines, alcohol), endocrine disorders (hypothyroidism, polycystic ovarian syndrome), smoking, and nasal congestion or obstruction.6
Sex
Men are at higher risk for OSA than women although once women reach menopause they have a risk similar to men. Postmenopausal women on hormone replacement therapy were found to have lower rates of OSA, suggesting that loss of hormones results in greater risk of OSA.7,8 Women also have more OSA during rapid eye movement (REM) sleep and less OSA when sleeping supine, whereas most men have OSA when sleeping supine.9,10 OSA is less severe in women compared with men of similar body mass index (BMI).11 Symptoms vary in men and women: snoring and witnessed apneas are more common in men whereas insomnia and excessive daytime sleepiness are more common in women.11 This may account for delayed diagnosis and the higher mortality in women compared with men.
Age
The risk of OSA increases with age. In a study of men 65 or older, the prevalence of moderate OSA was 23% in men younger than 72 and 30% in men older than 80.12 By comparison, the prevalence of moderate OSA in men 30 to 40 years was 10%.3 Increased risk of OSA with age may be due to age-related reduction in slow wave sleep (ie, deep sleep), which is protective against sleep-disordered breathing and airway collapse.13 Older adults are also less symptomatic, reporting less daytime sleepiness and fatigue.14
Race
The Sleep Heart Health Study found a slightly increased risk of moderate to severe OSA in blacks (20%) and American Indians (23%) compared with whites (17%).2 Another study showed the prevalence of OSA was 30% in whites, 32% in blacks, 38% in Hispanics, and 39% in Chinese individuals.15 A higher prevalence of OSA in young blacks (≤ 25 years) compared with whites was reported,16 although another study found no differences based on race in older patients.17 These differences among racial groups may be due to variations in craniofacial anatomy.
Obesity
There is a correlation between increased risk of OSA and obesity (BMI > 30 kg/m2) and its correlates of greater waist-to-hip ratio and neck circumference.2 A 10% increase in body weight results in a sixfold increase in moderate to severe OSA and increases the apnea–hypopnea index (AHI; number of breath pauses or respiratory events per hour) by 32% whereas a 10% decrease in weight decreases the AHI by 26%.18
COMORBIDITIES
OSA is associated with a number of comorbid conditions including stroke, myocardial infarction, hypertension, hyperlipidemia, glucose intolerance, diabetes, arrhythmias including atrial fibrillation, pulmonary hypertension, congestive heart failure, and depression. Patients with moderate or severe OSA are at higher risk of these comorbid conditions.19
Patients with cardiovascular disease have a very high prevalence of OSA: hypertension (83% mild to 30% moderate to severe OSA), heart failure (55% to 12%), arrhythmias (50% to 20%), stroke (75% to 57%), and coronary heart disease (65% to 38%).20 Increased awareness and early diagnosis of OSA is critical to reducing cardiovascular disease burden.
SCREENING
Sleep history
A sleep history starts with determining the patient’s total sleep time, based on time to bed, time to fall asleep, and time of wake up, including any difficulty falling asleep, staying asleep, or daytime naps.
Symptoms. Daytime naps generally indicate a sleep deficit or sleep that is not refreshing. A review of sleep and daytime symptoms associated with OSA (Table 1) helps determine if excessive daytime sleepiness or unrefreshing sleep is out of proportion with the amount of sleep the patient is getting at night.
Some patients with OSA may have memory or concentration issues or feel like they have attention deficit disorder. In fact some patients are diagnosed with attention deficit disorder because of their insufficient sleep or unrefreshing sleep.
Drowsy driving is a special concern in patients with untreated OSA and sleep deprivation. Many patients have drowsy driving episodes or difficulty staying awake during long-distance driving. Caffeine use is also important information as excessive caffeine may be used to combat sleepiness during the day.
The Epworth Sleepiness Scale is a clinical screening tool that presents 8 situations for patients to consider and indicate their level of sleepiness and likelihood of falling asleep (never = 0; slight = 1; moderate = 2, high = 3).21,22 A total score ≥ 10 is considered abnormal in that the patient is excessively sleepy compared with most people.
Risk factors and comorbid conditions. OSA risk factors and comorbidities, including a BMI obesity assessment, should be reviewed with patients. Nasal congestion or mouth breathing especially at night could be due to airway obstruction increasing the risk of OSA. Family history of OSA, tobacco, alcohol use, other medical conditions, and medications should also be discussed.
Physical examination
- Neck circumference greater than 17 inches for men or greater than 16 inches for women
- BMI greater than 30
- Friedman class tongue position class 3 or greater (Figure 1)
- Mouth features (present/enlarged tonsils, macroglossia, jaw misalignment)
- Nasal abnormalities (turbinate hypertrophy, deviated septum).5
Patients with Friedman palate positions class 3 and 4 have a higher risk of OSA due to airway crowding during sleep when the airway naturally collapses a little and is even more restricted.
Narrow airways or oropharyngeal crowding can also be due to a swollen, enlarged, or elongated uvula; present or enlarged tonsils; or lateral wall narrowing. Alone or in combination, these features can contribute to airway obstruction.
Other signs in the mouth suggestive of obstruction are macroglossia (enlarged tongue) and tongue ridging. Tongue ridging or scalloping impressions typically occur during sleep and are caused by the tongue moving forward to open the airway and pressing against the teeth.
Retrognathia (lower jaw offset behind upper jaw) can narrow the airway and increase the risk of OSA as can a high arch palate, overbite (upper teeth forward), or overjet (upper teeth over the top of lower teeth).
A nasal examination for nasal valve collapse (ie, nostril collapses with inhalation), deviated septum, and inferior turbinate hypertrophy impart an increased risk of OSA.
Screening tools
In addition to the Epworth Sleepiness Scale, the STOP-BANG questionnaire can help determine if a patient should be tested further for OSA. The STOP-BANG questionnaire consists of 8 yes-no questions where more than 2 yes responses indicate the patient is at higher risk for moderate to severe OSA (93% sensitivity): Snore, Tired, Observed stopped breathing, high blood Pressure, BMI > 35 kg/m2, Age > 50, Neck > 15.75 inches, Gender = male).23
SLEEP STUDIES
Polysomnography
Home sleep apnea test
HSATs record 4 to 7 parameters including airflow (thermal and nasal pressure), effort (inductive plethysmography), and oximetry. No electroencephalogram is used, so sleep is not recorded; it is assumed the patient is sleeping for the duration of the test. As such, respiratory events are based on oxygen desaturations and reduced airflow and pressure as well as chest and abdomen effort. The raw data are edited and manually scored and reviewed by a sleep specialist.25
Although the HSAT is convenient for many patients, it underestimates the severity of sleep-related breathing disorders. HSAT is intended to confirm OSA in patients with a high likelihood of OSA based on their sleep history.26 It is ideally employed for adult patients with no major medical problems or other sleep problems who are at high risk for moderate to severe OSA based on the STOP-BANG questionnaire or those with daytime sleepiness and 2 of the 3 symptoms of snoring, witnessed apnea, or hypertension.27
A negative or inconclusive HSAT warrants a PSG to ensure the patient does not have OSA. Use of HSAT is contraindicated in patients with
- Significant cardiopulmonary disease
- Potential weakness due to a neuromuscular condition
- Awake hypoventilation or high risk for sleep-related hypoventilation (severe obesity)
- History of stroke
- Chronic opioid use
- Severe insomnia
- Symptoms of other significant sleep disorders
- Environmental/personal factors that would preclude adequate acquisition and interpretation of data (disruptions from children, pets, other factors).27
DIAGNOSTIC CRITERIA
Respiratory events captured on a PSG or HSAT
The OSA diagnostic criteria are based on the occurrence of obstructive respiratory events recorded during sleep such as apneas, hypopneas, and respiratory event-related arousals.
Hypopneas. A hypopnea is a respiratory event resulting in reduced airflow. The America Association of Sleep Medicine’s preferred definition is a reduction in nasal pressure of at least 30% for 10 seconds or longer with 3% or greater oxygen desaturation or an electroencephalogram arousal. Another acceptable definition is at least 30% reduction in thoracoabdominal movement or airflow with 4% or greater oxygen desaturation, which is used by the Centers for Medicare and Medicaid Services and other insurers.29,30 Hypopnea requires greater oxygen desaturation and is not dependent on arousals, which can sometimes make it more challenging to identify OSA (Figure 2).
Respiratory event-related arousals. Respiratory event-related arousals are respiratory events not meeting apnea or hypopnea criteria. They are measured as a sequence of breaths of 10 or more seconds with increasing respiratory effort or flattening of the nasal pressure waveform leading to arousal (Figure 2).29 Respiratory event-related arousals are disruptive to sleep and have many of the same consequences as apneas and hypopneas.
Severity
SUMMARY
OSA results from airway collapse and obstruction during sleep, often causing arousal from sleep with or without oxygen desaturation. The prevalence of OSA is underestimated and it is underdiagnosed despite known risk factors and comorbid conditions. Screening for OSA with a sleep history, simple upper airway examination, and quick validated screening tool like the STOP-BANG or Epworth Sleepiness Scale aid in identifying the need for testing for OSA. A laboratory sleep study with a PSG can confirm the diagnosis and severity of OSA. HSATs are available to confirm the diagnosis of OSA in patients at high risk for moderate to severe OSA.
- Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015; 3(4):310–318.
- Young T, Shahar E, Nieto FJ, et al; for the Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults. Arch Intern Med 2002; 162(8):893–900.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Young T, Evans L, Finn L, Palta M. Estimation of clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20(9):705–706.
- Epstein LJ, Kristo D, Strollo Jr, PJ, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5(3):263–276.
- Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA 2004; 291(16):2013–2016.
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2003; 167(9):1181–1185.
- Shahar E, Redline S, Young T, et al; for the Sleep Heart Health Study Research Group. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003; 167(9):1186–1192.
- O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 2000; 161(5):1465–1472.
- Collop NA, Adkins D, Phillips BA. Gender differences in sleep and sleep-disordered breathing. Clin Chest Med 2004; 25(2):257–268.
- Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 1994; 149(3 Pt 1):722–726.
- Mehra R, Stone KL, Blackwell T, et al; for the Osteoporotic Fractures in Men Study. Prevalence and correlates of sleep-disordered breathing in older men: Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 2007; 55(9):1356–1364.
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 2000; 284(7):861–868.
- Groth M. Sleep apnea in the elderly. Clin Geriatr Med 2005; 21:701–712.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997; 155(1):186–192.
- Song Y, Ancoli-Israel S, Lewis CE, Redline S, Harrison SL, Stone KL. The association of race/ethnicity with objectively measured sleep characteristics in older men. Behav Sleep Med 2011; 10(1):54–69.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284(23):3015–3021.
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69(7):841–858.
- Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. Chest 1993; 103(1):30–36.
- Chervin RD, Aldrich MS. The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology 1999; 52(1):125–131.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108(5):812–821.
- Iber C, Ancoli-Israel S, Chesson A, Quan SF; for the American Academy of Sleep and Medicine. The ASSM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Winchester, IL: American Academy of Sleep Medicine; 2007.
- Centers for Medicare and Medicaid Services. Medicare Learning Network. Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/mm6048.pdf. Accessed August 19, 2019.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med 2007; 3(7):737–747.
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13(3):479–504.
- Sateia MJ. International classification of sleep disorders—3rd ed: highlights and modifications. Chest 2014; 146(5):1387–1394.
- AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.5. American Academy of Sleep Medicine; April 2018.
- Centers for Medicare and Medicaid Services. Medicare Coverage Database. www.cms.gov/medicare-coverage-database. Accessed August 19, 2019.
- Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: the HypnoLaus study. Lancet Respir Med 2015; 3(4):310–318.
- Young T, Shahar E, Nieto FJ, et al; for the Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults. Arch Intern Med 2002; 162(8):893–900.
- Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 2013; 177(9):1006–1014.
- Young T, Evans L, Finn L, Palta M. Estimation of clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997; 20(9):705–706.
- Epstein LJ, Kristo D, Strollo Jr, PJ, et al; Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med 2009; 5(3):263–276.
- Young T, Skatrud J, Peppard PE. Risk factors for obstructive sleep apnea in adults. JAMA 2004; 291(16):2013–2016.
- Young T, Finn L, Austin D, Peterson A. Menopausal status and sleep-disordered breathing in the Wisconsin Sleep Cohort Study. Am J Respir Crit Care Med 2003; 167(9):1181–1185.
- Shahar E, Redline S, Young T, et al; for the Sleep Heart Health Study Research Group. Hormone replacement therapy and sleep-disordered breathing. Am J Respir Crit Care Med 2003; 167(9):1186–1192.
- O’Connor C, Thornley KS, Hanly PJ. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med 2000; 161(5):1465–1472.
- Collop NA, Adkins D, Phillips BA. Gender differences in sleep and sleep-disordered breathing. Clin Chest Med 2004; 25(2):257–268.
- Redline S, Kump K, Tishler PV, Browner I, Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med 1994; 149(3 Pt 1):722–726.
- Mehra R, Stone KL, Blackwell T, et al; for the Osteoporotic Fractures in Men Study. Prevalence and correlates of sleep-disordered breathing in older men: Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 2007; 55(9):1356–1364.
- Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA 2000; 284(7):861–868.
- Groth M. Sleep apnea in the elderly. Clin Geriatr Med 2005; 21:701–712.
- Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38(6):877–888.
- Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997; 155(1):186–192.
- Song Y, Ancoli-Israel S, Lewis CE, Redline S, Harrison SL, Stone KL. The association of race/ethnicity with objectively measured sleep characteristics in older men. Behav Sleep Med 2011; 10(1):54–69.
- Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000; 284(23):3015–3021.
- Shahar E, Whitney CW, Redline S, et al. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med. 2001;163:19–25
- Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol 2017; 69(7):841–858.
- Johns MW. Daytime sleepiness, snoring, and obstructive sleep apnea. Chest 1993; 103(1):30–36.
- Chervin RD, Aldrich MS. The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology 1999; 52(1):125–131.
- Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology 2008; 108(5):812–821.
- Iber C, Ancoli-Israel S, Chesson A, Quan SF; for the American Academy of Sleep and Medicine. The ASSM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Winchester, IL: American Academy of Sleep Medicine; 2007.
- Centers for Medicare and Medicaid Services. Medicare Learning Network. Continuous positive airway pressure (CPAP) therapy for obstructive sleep apnea (OSA). www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/downloads/mm6048.pdf. Accessed August 19, 2019.
- Collop NA, Anderson WM, Boehlecke B, et al; Portable Monitoring Task Force of the American Academy of Sleep Medicine. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. J Clin Sleep Med 2007; 3(7):737–747.
- Kapur VK, Auckley DH, Chowdhuri S, et al. Clinical practice guideline for diagnostic testing for adult obstructive sleep apnea: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med 2017; 13(3):479–504.
- Sateia MJ. International classification of sleep disorders—3rd ed: highlights and modifications. Chest 2014; 146(5):1387–1394.
- AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Version 2.5. American Academy of Sleep Medicine; April 2018.
- Centers for Medicare and Medicaid Services. Medicare Coverage Database. www.cms.gov/medicare-coverage-database. Accessed August 19, 2019.
KEY POINTS
- OSA is characterized by repeated episodes of complete or partial obstruction of the airway during sleep.
- The prevalence of OSA is underestimated and underdiagnosed.
- A sleep history, simple upper airway examination, and quick validated screening tool like the STOP-BANG or Epworth Sleepiness Scale aid in identifying the need for testing for OSA.
- Polysomnogram is the gold standard for evaluation of OSA. Home sleep apnea tests can be used to confirm a diagnosis of OSA in patients at high risk for moderate to severe OSA.