User login
CPAP adherence varies by age, geographic location, study finds
SAN ANTONIO –
However, whether the sources of variability stem from patient factors such as disease severity and socioeconomic status, provider factors, environmental factors, or selection biases in those who are diagnosed with obstructive sleep apnea and treated with CPAP remains to be understood, lead study author Sanjay R. Patel, MD, said at the annual meeting of the Associated Professional Sleep Societies.
In 2015, the American Academy of Sleep Medicine (AASM) endorsed CPAP adherence as a process measure, and the Centers for Medicare and Medicaid Services has used CPAP adherence as an outcome measure to limit long-term coverage of the therapy. It defines CPAP adherence as 4 or more hours of use on greater than 70% of nights in a consecutive 30-day period within the first 90 days. “Strengths of CPAP adherence as an outcome measure include the fact that it is easy to measure and it predicts improvement in sleepiness, quality of life, and blood pressure control,” said Dr. Patel, who directs the University of Pittsburgh’s Center for Sleep and Cardiovascular Outcomes Research. “One issue as to whether we should use CPAP adherence as an outcome-based quality of care measure is, does variability reflect performance at the provider and/or health care system?”
In an effort to describe CPAP adherence rates in general clinical practice as well as sources of variability, Dr. Patel and colleagues evaluated telemonitoring data maintained by Philips Respironics. The study population consisted of 714,270 patients initiated on CPAP therapy between November 2015 and August 2018 who had at least one usage session of CPAP or APAP.
Overall, 90-day adherence to CPAP was 72.5%. Age, sex, and state of residence were all significantly associated with adherence rates (P less than .05). Specifically, adherence rates ranged from 54.8% among those 18-30 years of age to 79.1% among those 61-70 years of age. “There was a plateauing of adherence rates among those in their 70s, and men tended to have a higher adherence level than women across all age groups (73.3% vs. 71.4%, respectively),” he said. “Also, people who got started on CPAP in January had a higher level of adherence than people who got started in May. The differences are relatively small compared to the large age differences, but there was a consistent trend.”
When the researchers carried out age- and sex-adjusted analyses, they observed that adherence rates were lowest in the Northeast and Southwest and highest in the Upper Midwest and Mountain West. Adherence rates ranged from 50.8% in the District of Columbia and 60.5% in New York up to 81.2% in Idaho and 81.9% in South Dakota.
“The question is, is this variability explained by quality measures?” Dr. Patel asked. “We tried to answer this question by seeing whether the variability in adherence by location correlated with other metrics of health care quality.” To accomplish this, they used Dartmouth Atlas, a project that uses Medicare data to understand drivers of health care spending and quality. To understand geographic variability in CPAP adherence, they mapped ZIP codes onto hospital referral regions (HRRs), which are regional health care markets for tertiary medical care. Each HRR has at least one hospital that performs major cardiovascular procedures and neurosurgery. ZIP codes were mapped to 306 HRRs where the majority of residents get their tertiary care.
The researchers observed that Medicare enrollees who saw a primary care physician in the past 12 months had higher rates of adherence, compared with those who did not. “Twenty-three percent of the variance in CPAP adherence across the country can be explained by this measure of having a primary care doctor,” Dr. Patel said. In addition, patients who received care from HRRs located in the middle of the United States had high adherence rates. Top performers were facilities located in Madison, Wis.; Wausau, Wis.; Dubuque, Iowa; and Bloomington, Ill. Poor performers included facilities located in the boroughs of Manhattan and the Bronx, in New York; Muskegon, Mich.; Miami; and Buffalo, N.Y.
“Some of the geographical variability may be due to patient factors such as race, income, and education level,” Dr. Patel said. “That will need to be appropriately addressed in developing a quality of care measure. Nevertheless, some of the geographic variability appears to be related to health care system and provider factors. This variability could be potentially reduced through implementation of a CPAP adherence quality outcome measure.”
Dr. Patel disclosed that he has received grant/research support from Bayer Pharmaceuticals and Philips Respironics, and has served as a consultant to the American Academy of Sleep Medicine.
SOURCE: Patel SR et al. SLEEP 2019, Abstract 0513.
SAN ANTONIO –
However, whether the sources of variability stem from patient factors such as disease severity and socioeconomic status, provider factors, environmental factors, or selection biases in those who are diagnosed with obstructive sleep apnea and treated with CPAP remains to be understood, lead study author Sanjay R. Patel, MD, said at the annual meeting of the Associated Professional Sleep Societies.
In 2015, the American Academy of Sleep Medicine (AASM) endorsed CPAP adherence as a process measure, and the Centers for Medicare and Medicaid Services has used CPAP adherence as an outcome measure to limit long-term coverage of the therapy. It defines CPAP adherence as 4 or more hours of use on greater than 70% of nights in a consecutive 30-day period within the first 90 days. “Strengths of CPAP adherence as an outcome measure include the fact that it is easy to measure and it predicts improvement in sleepiness, quality of life, and blood pressure control,” said Dr. Patel, who directs the University of Pittsburgh’s Center for Sleep and Cardiovascular Outcomes Research. “One issue as to whether we should use CPAP adherence as an outcome-based quality of care measure is, does variability reflect performance at the provider and/or health care system?”
In an effort to describe CPAP adherence rates in general clinical practice as well as sources of variability, Dr. Patel and colleagues evaluated telemonitoring data maintained by Philips Respironics. The study population consisted of 714,270 patients initiated on CPAP therapy between November 2015 and August 2018 who had at least one usage session of CPAP or APAP.
Overall, 90-day adherence to CPAP was 72.5%. Age, sex, and state of residence were all significantly associated with adherence rates (P less than .05). Specifically, adherence rates ranged from 54.8% among those 18-30 years of age to 79.1% among those 61-70 years of age. “There was a plateauing of adherence rates among those in their 70s, and men tended to have a higher adherence level than women across all age groups (73.3% vs. 71.4%, respectively),” he said. “Also, people who got started on CPAP in January had a higher level of adherence than people who got started in May. The differences are relatively small compared to the large age differences, but there was a consistent trend.”
When the researchers carried out age- and sex-adjusted analyses, they observed that adherence rates were lowest in the Northeast and Southwest and highest in the Upper Midwest and Mountain West. Adherence rates ranged from 50.8% in the District of Columbia and 60.5% in New York up to 81.2% in Idaho and 81.9% in South Dakota.
“The question is, is this variability explained by quality measures?” Dr. Patel asked. “We tried to answer this question by seeing whether the variability in adherence by location correlated with other metrics of health care quality.” To accomplish this, they used Dartmouth Atlas, a project that uses Medicare data to understand drivers of health care spending and quality. To understand geographic variability in CPAP adherence, they mapped ZIP codes onto hospital referral regions (HRRs), which are regional health care markets for tertiary medical care. Each HRR has at least one hospital that performs major cardiovascular procedures and neurosurgery. ZIP codes were mapped to 306 HRRs where the majority of residents get their tertiary care.
The researchers observed that Medicare enrollees who saw a primary care physician in the past 12 months had higher rates of adherence, compared with those who did not. “Twenty-three percent of the variance in CPAP adherence across the country can be explained by this measure of having a primary care doctor,” Dr. Patel said. In addition, patients who received care from HRRs located in the middle of the United States had high adherence rates. Top performers were facilities located in Madison, Wis.; Wausau, Wis.; Dubuque, Iowa; and Bloomington, Ill. Poor performers included facilities located in the boroughs of Manhattan and the Bronx, in New York; Muskegon, Mich.; Miami; and Buffalo, N.Y.
“Some of the geographical variability may be due to patient factors such as race, income, and education level,” Dr. Patel said. “That will need to be appropriately addressed in developing a quality of care measure. Nevertheless, some of the geographic variability appears to be related to health care system and provider factors. This variability could be potentially reduced through implementation of a CPAP adherence quality outcome measure.”
Dr. Patel disclosed that he has received grant/research support from Bayer Pharmaceuticals and Philips Respironics, and has served as a consultant to the American Academy of Sleep Medicine.
SOURCE: Patel SR et al. SLEEP 2019, Abstract 0513.
SAN ANTONIO –
However, whether the sources of variability stem from patient factors such as disease severity and socioeconomic status, provider factors, environmental factors, or selection biases in those who are diagnosed with obstructive sleep apnea and treated with CPAP remains to be understood, lead study author Sanjay R. Patel, MD, said at the annual meeting of the Associated Professional Sleep Societies.
In 2015, the American Academy of Sleep Medicine (AASM) endorsed CPAP adherence as a process measure, and the Centers for Medicare and Medicaid Services has used CPAP adherence as an outcome measure to limit long-term coverage of the therapy. It defines CPAP adherence as 4 or more hours of use on greater than 70% of nights in a consecutive 30-day period within the first 90 days. “Strengths of CPAP adherence as an outcome measure include the fact that it is easy to measure and it predicts improvement in sleepiness, quality of life, and blood pressure control,” said Dr. Patel, who directs the University of Pittsburgh’s Center for Sleep and Cardiovascular Outcomes Research. “One issue as to whether we should use CPAP adherence as an outcome-based quality of care measure is, does variability reflect performance at the provider and/or health care system?”
In an effort to describe CPAP adherence rates in general clinical practice as well as sources of variability, Dr. Patel and colleagues evaluated telemonitoring data maintained by Philips Respironics. The study population consisted of 714,270 patients initiated on CPAP therapy between November 2015 and August 2018 who had at least one usage session of CPAP or APAP.
Overall, 90-day adherence to CPAP was 72.5%. Age, sex, and state of residence were all significantly associated with adherence rates (P less than .05). Specifically, adherence rates ranged from 54.8% among those 18-30 years of age to 79.1% among those 61-70 years of age. “There was a plateauing of adherence rates among those in their 70s, and men tended to have a higher adherence level than women across all age groups (73.3% vs. 71.4%, respectively),” he said. “Also, people who got started on CPAP in January had a higher level of adherence than people who got started in May. The differences are relatively small compared to the large age differences, but there was a consistent trend.”
When the researchers carried out age- and sex-adjusted analyses, they observed that adherence rates were lowest in the Northeast and Southwest and highest in the Upper Midwest and Mountain West. Adherence rates ranged from 50.8% in the District of Columbia and 60.5% in New York up to 81.2% in Idaho and 81.9% in South Dakota.
“The question is, is this variability explained by quality measures?” Dr. Patel asked. “We tried to answer this question by seeing whether the variability in adherence by location correlated with other metrics of health care quality.” To accomplish this, they used Dartmouth Atlas, a project that uses Medicare data to understand drivers of health care spending and quality. To understand geographic variability in CPAP adherence, they mapped ZIP codes onto hospital referral regions (HRRs), which are regional health care markets for tertiary medical care. Each HRR has at least one hospital that performs major cardiovascular procedures and neurosurgery. ZIP codes were mapped to 306 HRRs where the majority of residents get their tertiary care.
The researchers observed that Medicare enrollees who saw a primary care physician in the past 12 months had higher rates of adherence, compared with those who did not. “Twenty-three percent of the variance in CPAP adherence across the country can be explained by this measure of having a primary care doctor,” Dr. Patel said. In addition, patients who received care from HRRs located in the middle of the United States had high adherence rates. Top performers were facilities located in Madison, Wis.; Wausau, Wis.; Dubuque, Iowa; and Bloomington, Ill. Poor performers included facilities located in the boroughs of Manhattan and the Bronx, in New York; Muskegon, Mich.; Miami; and Buffalo, N.Y.
“Some of the geographical variability may be due to patient factors such as race, income, and education level,” Dr. Patel said. “That will need to be appropriately addressed in developing a quality of care measure. Nevertheless, some of the geographic variability appears to be related to health care system and provider factors. This variability could be potentially reduced through implementation of a CPAP adherence quality outcome measure.”
Dr. Patel disclosed that he has received grant/research support from Bayer Pharmaceuticals and Philips Respironics, and has served as a consultant to the American Academy of Sleep Medicine.
SOURCE: Patel SR et al. SLEEP 2019, Abstract 0513.
REPORTING FROM SLEEP 2019
AASM hypopnea definition best for detecting OSA cases, study finds
SAN DIEGO – The prevalence of obstructive sleep apnea (OSA) is substantially lower using the Centers for Medicare & Medicaid Services apnea-hypopnea index definition of OSA than using the one recommended by the American Academy of Sleep Medicine.
In addition,
The findings come from an analysis which set out to assess the relationship between OSA and hypertension using the AASM-recommended definition and the 2018 American Heart Association/American College of Cardiology blood pressure guidelines, and to determine if there is an association between hypertension and OSA among individuals who did not meet the CMS definition of OSA.
“Given the substantial morbidity associated with hypertension, these results suggest that universal adoption of the AASM AHI definition would be a reasonable step in ensuring appropriate diagnosis and treatment of OSA,” lead study author Stuart F. Quan, MD, said at the annual meeting of the Associated Professional Sleep Societies.
Dr. Quan, of the division of sleep and circadian disorders at Brigham and Women’s Hospital in Boston, noted that a number of studies have demonstrated that OSA is a risk factor for hypertension and a variety of other medical conditions. “Rightly or wrongly, the most important metric for determining whether OSA is present and determining its severity, is the apnea-hypopnea index,” he said. “It’s the most common metric used for determining OSA severity, and mostly importantly, Medicare and some other insurers use this metric to determine whether a person is eligible for treatment. If a person falls above the line, they can get continuous positive airway pressure, for example. If they’re below the line, that’s too bad; they don’t have OSA insofar as the insurance company is concerned.”
There is no controversy as to what constitutes apnea, he continued, but some disagreement exists on the definition of hypopnea. The AASM recommends using a 3% oxygen desaturation or an arousal, while Medicare uses a definition of hypopnea requiring only a 4% oxygen desaturation. Hypertension definitions have changed recently as well. Before 2018, the definition of hypertension was greater than 140/90 mm Hg for people younger than age 65 years and 150/80 mm Hg for people age 65 years and older. In 2018, the AHA and ACC changed the hypertension guidelines, defining normal as less than 120/80 mm Hg.
“Previous studies linking OSA and hypertension used older definitions, but to my knowledge there are no current studies examining the association between OSA and hypertension using new definitions,” Dr. Quan said.
He reported on results from an analysis of 6,307 participants in the Sleep Heart Health Study who underwent home polysomnography. Their AHI defined by a 3% oxygen desaturation or an arousal was classified into four categories of OSA severity: fewer than 5 events per hour (normal sleep), 5-14 events per hour (mild sleep apnea), 15-29 events per hour (moderate sleep apnea), and 30 or more events per hour (severe sleep apnea).
The researchers used three definitions of dichotomous BP elevation: elevated (greater than 120/80 mm Hg or use of hypertension medications [meds]), stage 1 (greater than 130/80 mm Hg or meds), or stage 2 (greater than 140/90 mm Hg or meds). They used logistic regression to assess the association between elevated BP and/or hypertension and OSA severity, controlling for demographics and body mass index. Additional analyses utilized multiple linear regression to determine the relationship between natural log AHI and systolic and diastolic BP, controlling for the same covariates.
For all definitions of elevated BP, increasing OSA severity was associated with greater likelihood of an elevated or hypertensive status in fully adjusted models. Specifically, the odds ratios among those with elevated BP was 1.30 (95% confidence interval, 1.10-1.54), 1.41 (95% CI, 1.15-1.72), and 1.69 (95% CI, 1.32-2.17) for mild, moderate, and severe sleep apnea, respectively. The ORs among those with stage 1 BP was 1.27 (95% CI, 1.09-1.49), 1.36 (95% CI, 1.13-1.63), 1.58 (95% CI, 1.27-1.97) for mild, moderate, and severe sleep apnea, while the OR among those with stage 2 BP was 1.07 (95% CI, 0.92-1.26), 1.22 (95% CI, 1.02-1.45), 1.38 (95% CI, 1.12-1.69) for mild, moderate, and severe sleep apnea. Linear regression found that AHI was associated with both systolic and diastolic BP in fully adjusted models.
“Using the AASM and CMS AHI definitions, increasing severity of AHI is associated with greater likelihood of having an elevated blood pressure or hypertension,” Dr. Quan concluded. “However, the prevalence of OSA was substantially lower using the CMS definition of OSA. In fact, 218 of these individuals had moderate to severe OSA when the AASM definition was applied.”
He characterized the study as “a practical analysis, a way to help identify patients who might benefit from treatment. This is not the issue of whether the science of 3% AHI is better than 4%.”
The Sleep Heart Health Study was supported by the National Heart, Lung, and Blood Institute. Dr. Quan reported that he helped draft the AASM AHI recommendations but had no other relevant disclosures.
SOURCE: Quan SF et al. SLEEP 2019, Abstract 0501.
SAN DIEGO – The prevalence of obstructive sleep apnea (OSA) is substantially lower using the Centers for Medicare & Medicaid Services apnea-hypopnea index definition of OSA than using the one recommended by the American Academy of Sleep Medicine.
In addition,
The findings come from an analysis which set out to assess the relationship between OSA and hypertension using the AASM-recommended definition and the 2018 American Heart Association/American College of Cardiology blood pressure guidelines, and to determine if there is an association between hypertension and OSA among individuals who did not meet the CMS definition of OSA.
“Given the substantial morbidity associated with hypertension, these results suggest that universal adoption of the AASM AHI definition would be a reasonable step in ensuring appropriate diagnosis and treatment of OSA,” lead study author Stuart F. Quan, MD, said at the annual meeting of the Associated Professional Sleep Societies.
Dr. Quan, of the division of sleep and circadian disorders at Brigham and Women’s Hospital in Boston, noted that a number of studies have demonstrated that OSA is a risk factor for hypertension and a variety of other medical conditions. “Rightly or wrongly, the most important metric for determining whether OSA is present and determining its severity, is the apnea-hypopnea index,” he said. “It’s the most common metric used for determining OSA severity, and mostly importantly, Medicare and some other insurers use this metric to determine whether a person is eligible for treatment. If a person falls above the line, they can get continuous positive airway pressure, for example. If they’re below the line, that’s too bad; they don’t have OSA insofar as the insurance company is concerned.”
There is no controversy as to what constitutes apnea, he continued, but some disagreement exists on the definition of hypopnea. The AASM recommends using a 3% oxygen desaturation or an arousal, while Medicare uses a definition of hypopnea requiring only a 4% oxygen desaturation. Hypertension definitions have changed recently as well. Before 2018, the definition of hypertension was greater than 140/90 mm Hg for people younger than age 65 years and 150/80 mm Hg for people age 65 years and older. In 2018, the AHA and ACC changed the hypertension guidelines, defining normal as less than 120/80 mm Hg.
“Previous studies linking OSA and hypertension used older definitions, but to my knowledge there are no current studies examining the association between OSA and hypertension using new definitions,” Dr. Quan said.
He reported on results from an analysis of 6,307 participants in the Sleep Heart Health Study who underwent home polysomnography. Their AHI defined by a 3% oxygen desaturation or an arousal was classified into four categories of OSA severity: fewer than 5 events per hour (normal sleep), 5-14 events per hour (mild sleep apnea), 15-29 events per hour (moderate sleep apnea), and 30 or more events per hour (severe sleep apnea).
The researchers used three definitions of dichotomous BP elevation: elevated (greater than 120/80 mm Hg or use of hypertension medications [meds]), stage 1 (greater than 130/80 mm Hg or meds), or stage 2 (greater than 140/90 mm Hg or meds). They used logistic regression to assess the association between elevated BP and/or hypertension and OSA severity, controlling for demographics and body mass index. Additional analyses utilized multiple linear regression to determine the relationship between natural log AHI and systolic and diastolic BP, controlling for the same covariates.
For all definitions of elevated BP, increasing OSA severity was associated with greater likelihood of an elevated or hypertensive status in fully adjusted models. Specifically, the odds ratios among those with elevated BP was 1.30 (95% confidence interval, 1.10-1.54), 1.41 (95% CI, 1.15-1.72), and 1.69 (95% CI, 1.32-2.17) for mild, moderate, and severe sleep apnea, respectively. The ORs among those with stage 1 BP was 1.27 (95% CI, 1.09-1.49), 1.36 (95% CI, 1.13-1.63), 1.58 (95% CI, 1.27-1.97) for mild, moderate, and severe sleep apnea, while the OR among those with stage 2 BP was 1.07 (95% CI, 0.92-1.26), 1.22 (95% CI, 1.02-1.45), 1.38 (95% CI, 1.12-1.69) for mild, moderate, and severe sleep apnea. Linear regression found that AHI was associated with both systolic and diastolic BP in fully adjusted models.
“Using the AASM and CMS AHI definitions, increasing severity of AHI is associated with greater likelihood of having an elevated blood pressure or hypertension,” Dr. Quan concluded. “However, the prevalence of OSA was substantially lower using the CMS definition of OSA. In fact, 218 of these individuals had moderate to severe OSA when the AASM definition was applied.”
He characterized the study as “a practical analysis, a way to help identify patients who might benefit from treatment. This is not the issue of whether the science of 3% AHI is better than 4%.”
The Sleep Heart Health Study was supported by the National Heart, Lung, and Blood Institute. Dr. Quan reported that he helped draft the AASM AHI recommendations but had no other relevant disclosures.
SOURCE: Quan SF et al. SLEEP 2019, Abstract 0501.
SAN DIEGO – The prevalence of obstructive sleep apnea (OSA) is substantially lower using the Centers for Medicare & Medicaid Services apnea-hypopnea index definition of OSA than using the one recommended by the American Academy of Sleep Medicine.
In addition,
The findings come from an analysis which set out to assess the relationship between OSA and hypertension using the AASM-recommended definition and the 2018 American Heart Association/American College of Cardiology blood pressure guidelines, and to determine if there is an association between hypertension and OSA among individuals who did not meet the CMS definition of OSA.
“Given the substantial morbidity associated with hypertension, these results suggest that universal adoption of the AASM AHI definition would be a reasonable step in ensuring appropriate diagnosis and treatment of OSA,” lead study author Stuart F. Quan, MD, said at the annual meeting of the Associated Professional Sleep Societies.
Dr. Quan, of the division of sleep and circadian disorders at Brigham and Women’s Hospital in Boston, noted that a number of studies have demonstrated that OSA is a risk factor for hypertension and a variety of other medical conditions. “Rightly or wrongly, the most important metric for determining whether OSA is present and determining its severity, is the apnea-hypopnea index,” he said. “It’s the most common metric used for determining OSA severity, and mostly importantly, Medicare and some other insurers use this metric to determine whether a person is eligible for treatment. If a person falls above the line, they can get continuous positive airway pressure, for example. If they’re below the line, that’s too bad; they don’t have OSA insofar as the insurance company is concerned.”
There is no controversy as to what constitutes apnea, he continued, but some disagreement exists on the definition of hypopnea. The AASM recommends using a 3% oxygen desaturation or an arousal, while Medicare uses a definition of hypopnea requiring only a 4% oxygen desaturation. Hypertension definitions have changed recently as well. Before 2018, the definition of hypertension was greater than 140/90 mm Hg for people younger than age 65 years and 150/80 mm Hg for people age 65 years and older. In 2018, the AHA and ACC changed the hypertension guidelines, defining normal as less than 120/80 mm Hg.
“Previous studies linking OSA and hypertension used older definitions, but to my knowledge there are no current studies examining the association between OSA and hypertension using new definitions,” Dr. Quan said.
He reported on results from an analysis of 6,307 participants in the Sleep Heart Health Study who underwent home polysomnography. Their AHI defined by a 3% oxygen desaturation or an arousal was classified into four categories of OSA severity: fewer than 5 events per hour (normal sleep), 5-14 events per hour (mild sleep apnea), 15-29 events per hour (moderate sleep apnea), and 30 or more events per hour (severe sleep apnea).
The researchers used three definitions of dichotomous BP elevation: elevated (greater than 120/80 mm Hg or use of hypertension medications [meds]), stage 1 (greater than 130/80 mm Hg or meds), or stage 2 (greater than 140/90 mm Hg or meds). They used logistic regression to assess the association between elevated BP and/or hypertension and OSA severity, controlling for demographics and body mass index. Additional analyses utilized multiple linear regression to determine the relationship between natural log AHI and systolic and diastolic BP, controlling for the same covariates.
For all definitions of elevated BP, increasing OSA severity was associated with greater likelihood of an elevated or hypertensive status in fully adjusted models. Specifically, the odds ratios among those with elevated BP was 1.30 (95% confidence interval, 1.10-1.54), 1.41 (95% CI, 1.15-1.72), and 1.69 (95% CI, 1.32-2.17) for mild, moderate, and severe sleep apnea, respectively. The ORs among those with stage 1 BP was 1.27 (95% CI, 1.09-1.49), 1.36 (95% CI, 1.13-1.63), 1.58 (95% CI, 1.27-1.97) for mild, moderate, and severe sleep apnea, while the OR among those with stage 2 BP was 1.07 (95% CI, 0.92-1.26), 1.22 (95% CI, 1.02-1.45), 1.38 (95% CI, 1.12-1.69) for mild, moderate, and severe sleep apnea. Linear regression found that AHI was associated with both systolic and diastolic BP in fully adjusted models.
“Using the AASM and CMS AHI definitions, increasing severity of AHI is associated with greater likelihood of having an elevated blood pressure or hypertension,” Dr. Quan concluded. “However, the prevalence of OSA was substantially lower using the CMS definition of OSA. In fact, 218 of these individuals had moderate to severe OSA when the AASM definition was applied.”
He characterized the study as “a practical analysis, a way to help identify patients who might benefit from treatment. This is not the issue of whether the science of 3% AHI is better than 4%.”
The Sleep Heart Health Study was supported by the National Heart, Lung, and Blood Institute. Dr. Quan reported that he helped draft the AASM AHI recommendations but had no other relevant disclosures.
SOURCE: Quan SF et al. SLEEP 2019, Abstract 0501.
REPORTING FROM SLEEP 2019
Sleepiest OSA patients have worse CV outcomes
SAN ANTONIO – Patients with obstructive sleep apnea who complain of feeling tired when they wake up, being sleepy during the day, and have a high score on the Epworth Sleepiness Scale face an increased risk for cardiovascular disease, results from a population-based analysis suggest.
“OSA is a highly heterogeneous disease, with multiple clinical presentations and consequences,” the study’s first author, Diego R. Mazzotti, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “These patients also have diverse comorbidities, and there are arbitrary severity definitions and variable therapeutic responses. It’s difficult to lump these patients together.”
Symptom subtypes of OSA were originally described in the Icelandic Sleep Apnea Cohort, and defined as excessively sleepy, minimally symptomatic, and disturbed sleep (Eur Respir J. 2014; 44[6]:1600-7). These distinct clusters were identified based on symptom experiences and the existence of major comorbidities. “This concept is more popular today, trying to identify symptom clusters, or groups of individuals, that share similar polysomnographic data, and then compare differences in prevalence or incidence of cardiovascular disease,” said Dr. Mazzotti, a research associate at the University of Pennsylvania, Philadelphia. “That’s a concept that needs to be moving forward.”
Dr. Mazzotti and colleagues set out to determine if OSA symptom subtypes are present in the Sleep Heart Health Study, a multicenter, prospective, community-based cohort of individuals aged 40 years and older designed to assess the cardiovascular (CV) consequences of OSA. They also wanted to know if there is additional evidence of the relevance of OSA symptom subtypes, particularly with respect to cardiovascular disease .
Participant-reported symptoms, such as difficulty falling and staying asleep, snoring, fatigue, drowsy driving and daytime sleepiness, and responses to the Epworth Sleepiness Scale were used to determine the patient’s subtype. Assessments including questionnaires and in-home polysomnography were conducted at baseline (between 1995 and 1998) and follow-up (between 2001 and 2003), while CV outcomes were assessed until the end of follow-up (between 2008 and 2011).
In all, 1,207 patients from the Sleep Heart Health Study met criteria for moderate to severe OSA (apnea-hypopnea index, or AHI, of 15 or greater) and were included in the final analysis. They were followed for a mean of 12 years. Based on the clustering of symptoms, the researchers identified four OSA symptom subtypes: disturbed sleep (12%), minimally symptomatic (33%), excessively sleepy (17%), and moderately sleepy (38%) – proportions that were similar to those observed in prior studies.
The disturbed sleep subtype presented with increased prevalence of “insomnialike” symptoms, such as difficulty initiating or maintaining sleep, according to Dr. Mazzotti. “On the other hand, the excessively sleepy subtype presented with a very high prevalence of several symptoms related to excessive daytime sleepiness, while the moderately sleepy showed a moderately high prevalence of such symptoms, but not as much when compared to the excessively sleepy subtype,” he explained. “Finally, the minimally symptomatic subtype was found to have the lowest prevalence of all investigated symptoms, suggesting that these patients have low symptom burden. They do not complain as much, even though they have moderate-to-severe OSA.”
Next, Dr. Mazzotti and colleagues used Kaplan-Meier survival analysis and Cox proportional hazards models to evaluate whether subtypes were associated with incident coronary heart disease (CHD), heart failure, and CV disease, including CV mortality. Similar analyses were performed comparing each symptom subtype with 2,830 individuals without OSA (AHI less than 5).
Compared with other subtypes, the excessively sleepy group had a more than threefold increased odds of prevalent heart failure, after adjustment for other CV risk factors. They also had a 1.7- to 2.3-fold increased risk for incident CV disease (P less than .001), CHD (P = .015) and heart failure (P = 0.018), after adjustment for other CV risk factors.
“Compared to individuals without OSA, the excessively sleepy subtype is the only subtype with increased risk of incident CV disease and CHD,” Dr. Mazzotti said. “It is possible that excessively sleepy OSA patients are more likely to benefit from CPAP therapy in preventing CV disease.” These results were published online earlier this year (Am J Respir Crit Care Med. 2019 Feb 15. doi: 10.1164/rccm.201808-1509OC).
Dr. Mazzotti reported having no financial disclosures.
SOURCE: Mazzotti D et al. SLEEP 2019, Abstract 0586.
SAN ANTONIO – Patients with obstructive sleep apnea who complain of feeling tired when they wake up, being sleepy during the day, and have a high score on the Epworth Sleepiness Scale face an increased risk for cardiovascular disease, results from a population-based analysis suggest.
“OSA is a highly heterogeneous disease, with multiple clinical presentations and consequences,” the study’s first author, Diego R. Mazzotti, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “These patients also have diverse comorbidities, and there are arbitrary severity definitions and variable therapeutic responses. It’s difficult to lump these patients together.”
Symptom subtypes of OSA were originally described in the Icelandic Sleep Apnea Cohort, and defined as excessively sleepy, minimally symptomatic, and disturbed sleep (Eur Respir J. 2014; 44[6]:1600-7). These distinct clusters were identified based on symptom experiences and the existence of major comorbidities. “This concept is more popular today, trying to identify symptom clusters, or groups of individuals, that share similar polysomnographic data, and then compare differences in prevalence or incidence of cardiovascular disease,” said Dr. Mazzotti, a research associate at the University of Pennsylvania, Philadelphia. “That’s a concept that needs to be moving forward.”
Dr. Mazzotti and colleagues set out to determine if OSA symptom subtypes are present in the Sleep Heart Health Study, a multicenter, prospective, community-based cohort of individuals aged 40 years and older designed to assess the cardiovascular (CV) consequences of OSA. They also wanted to know if there is additional evidence of the relevance of OSA symptom subtypes, particularly with respect to cardiovascular disease .
Participant-reported symptoms, such as difficulty falling and staying asleep, snoring, fatigue, drowsy driving and daytime sleepiness, and responses to the Epworth Sleepiness Scale were used to determine the patient’s subtype. Assessments including questionnaires and in-home polysomnography were conducted at baseline (between 1995 and 1998) and follow-up (between 2001 and 2003), while CV outcomes were assessed until the end of follow-up (between 2008 and 2011).
In all, 1,207 patients from the Sleep Heart Health Study met criteria for moderate to severe OSA (apnea-hypopnea index, or AHI, of 15 or greater) and were included in the final analysis. They were followed for a mean of 12 years. Based on the clustering of symptoms, the researchers identified four OSA symptom subtypes: disturbed sleep (12%), minimally symptomatic (33%), excessively sleepy (17%), and moderately sleepy (38%) – proportions that were similar to those observed in prior studies.
The disturbed sleep subtype presented with increased prevalence of “insomnialike” symptoms, such as difficulty initiating or maintaining sleep, according to Dr. Mazzotti. “On the other hand, the excessively sleepy subtype presented with a very high prevalence of several symptoms related to excessive daytime sleepiness, while the moderately sleepy showed a moderately high prevalence of such symptoms, but not as much when compared to the excessively sleepy subtype,” he explained. “Finally, the minimally symptomatic subtype was found to have the lowest prevalence of all investigated symptoms, suggesting that these patients have low symptom burden. They do not complain as much, even though they have moderate-to-severe OSA.”
Next, Dr. Mazzotti and colleagues used Kaplan-Meier survival analysis and Cox proportional hazards models to evaluate whether subtypes were associated with incident coronary heart disease (CHD), heart failure, and CV disease, including CV mortality. Similar analyses were performed comparing each symptom subtype with 2,830 individuals without OSA (AHI less than 5).
Compared with other subtypes, the excessively sleepy group had a more than threefold increased odds of prevalent heart failure, after adjustment for other CV risk factors. They also had a 1.7- to 2.3-fold increased risk for incident CV disease (P less than .001), CHD (P = .015) and heart failure (P = 0.018), after adjustment for other CV risk factors.
“Compared to individuals without OSA, the excessively sleepy subtype is the only subtype with increased risk of incident CV disease and CHD,” Dr. Mazzotti said. “It is possible that excessively sleepy OSA patients are more likely to benefit from CPAP therapy in preventing CV disease.” These results were published online earlier this year (Am J Respir Crit Care Med. 2019 Feb 15. doi: 10.1164/rccm.201808-1509OC).
Dr. Mazzotti reported having no financial disclosures.
SOURCE: Mazzotti D et al. SLEEP 2019, Abstract 0586.
SAN ANTONIO – Patients with obstructive sleep apnea who complain of feeling tired when they wake up, being sleepy during the day, and have a high score on the Epworth Sleepiness Scale face an increased risk for cardiovascular disease, results from a population-based analysis suggest.
“OSA is a highly heterogeneous disease, with multiple clinical presentations and consequences,” the study’s first author, Diego R. Mazzotti, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “These patients also have diverse comorbidities, and there are arbitrary severity definitions and variable therapeutic responses. It’s difficult to lump these patients together.”
Symptom subtypes of OSA were originally described in the Icelandic Sleep Apnea Cohort, and defined as excessively sleepy, minimally symptomatic, and disturbed sleep (Eur Respir J. 2014; 44[6]:1600-7). These distinct clusters were identified based on symptom experiences and the existence of major comorbidities. “This concept is more popular today, trying to identify symptom clusters, or groups of individuals, that share similar polysomnographic data, and then compare differences in prevalence or incidence of cardiovascular disease,” said Dr. Mazzotti, a research associate at the University of Pennsylvania, Philadelphia. “That’s a concept that needs to be moving forward.”
Dr. Mazzotti and colleagues set out to determine if OSA symptom subtypes are present in the Sleep Heart Health Study, a multicenter, prospective, community-based cohort of individuals aged 40 years and older designed to assess the cardiovascular (CV) consequences of OSA. They also wanted to know if there is additional evidence of the relevance of OSA symptom subtypes, particularly with respect to cardiovascular disease .
Participant-reported symptoms, such as difficulty falling and staying asleep, snoring, fatigue, drowsy driving and daytime sleepiness, and responses to the Epworth Sleepiness Scale were used to determine the patient’s subtype. Assessments including questionnaires and in-home polysomnography were conducted at baseline (between 1995 and 1998) and follow-up (between 2001 and 2003), while CV outcomes were assessed until the end of follow-up (between 2008 and 2011).
In all, 1,207 patients from the Sleep Heart Health Study met criteria for moderate to severe OSA (apnea-hypopnea index, or AHI, of 15 or greater) and were included in the final analysis. They were followed for a mean of 12 years. Based on the clustering of symptoms, the researchers identified four OSA symptom subtypes: disturbed sleep (12%), minimally symptomatic (33%), excessively sleepy (17%), and moderately sleepy (38%) – proportions that were similar to those observed in prior studies.
The disturbed sleep subtype presented with increased prevalence of “insomnialike” symptoms, such as difficulty initiating or maintaining sleep, according to Dr. Mazzotti. “On the other hand, the excessively sleepy subtype presented with a very high prevalence of several symptoms related to excessive daytime sleepiness, while the moderately sleepy showed a moderately high prevalence of such symptoms, but not as much when compared to the excessively sleepy subtype,” he explained. “Finally, the minimally symptomatic subtype was found to have the lowest prevalence of all investigated symptoms, suggesting that these patients have low symptom burden. They do not complain as much, even though they have moderate-to-severe OSA.”
Next, Dr. Mazzotti and colleagues used Kaplan-Meier survival analysis and Cox proportional hazards models to evaluate whether subtypes were associated with incident coronary heart disease (CHD), heart failure, and CV disease, including CV mortality. Similar analyses were performed comparing each symptom subtype with 2,830 individuals without OSA (AHI less than 5).
Compared with other subtypes, the excessively sleepy group had a more than threefold increased odds of prevalent heart failure, after adjustment for other CV risk factors. They also had a 1.7- to 2.3-fold increased risk for incident CV disease (P less than .001), CHD (P = .015) and heart failure (P = 0.018), after adjustment for other CV risk factors.
“Compared to individuals without OSA, the excessively sleepy subtype is the only subtype with increased risk of incident CV disease and CHD,” Dr. Mazzotti said. “It is possible that excessively sleepy OSA patients are more likely to benefit from CPAP therapy in preventing CV disease.” These results were published online earlier this year (Am J Respir Crit Care Med. 2019 Feb 15. doi: 10.1164/rccm.201808-1509OC).
Dr. Mazzotti reported having no financial disclosures.
SOURCE: Mazzotti D et al. SLEEP 2019, Abstract 0586.
REPORTING FROM SLEEP 2019
Click for Credit: Roux-en-Y for diabetes; Exercise & fall prevention; more
Here are 5 articles from the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Cloud of inconsistency hangs over cannabis data
To take the posttest, go to: https://bit.ly/2NfjaDS
Expires February 6, 2020
2. Roux-en-Y achieves diabetes remission in majority of patients
To take the posttest, go to: https://bit.ly/2x9hLnE
Expires February 6, 2020
3. Socioeconomic status, race found to impact CPAP compliance
To take the posttest, go to: https://bit.ly/2RBpLa9
Expires February 8, 2020
4. Exercise type matters for fall prevention among elderly
To take the posttest, go to: https://bit.ly/2X26OUh
Expires February 12, 2020
5. Adult HIV patients should receive standard vaccinations, with caveats
To take the posttest, go to: https://bit.ly/2X1S7LV
Expires February 12, 2020
Here are 5 articles from the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Cloud of inconsistency hangs over cannabis data
To take the posttest, go to: https://bit.ly/2NfjaDS
Expires February 6, 2020
2. Roux-en-Y achieves diabetes remission in majority of patients
To take the posttest, go to: https://bit.ly/2x9hLnE
Expires February 6, 2020
3. Socioeconomic status, race found to impact CPAP compliance
To take the posttest, go to: https://bit.ly/2RBpLa9
Expires February 8, 2020
4. Exercise type matters for fall prevention among elderly
To take the posttest, go to: https://bit.ly/2X26OUh
Expires February 12, 2020
5. Adult HIV patients should receive standard vaccinations, with caveats
To take the posttest, go to: https://bit.ly/2X1S7LV
Expires February 12, 2020
Here are 5 articles from the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Cloud of inconsistency hangs over cannabis data
To take the posttest, go to: https://bit.ly/2NfjaDS
Expires February 6, 2020
2. Roux-en-Y achieves diabetes remission in majority of patients
To take the posttest, go to: https://bit.ly/2x9hLnE
Expires February 6, 2020
3. Socioeconomic status, race found to impact CPAP compliance
To take the posttest, go to: https://bit.ly/2RBpLa9
Expires February 8, 2020
4. Exercise type matters for fall prevention among elderly
To take the posttest, go to: https://bit.ly/2X26OUh
Expires February 12, 2020
5. Adult HIV patients should receive standard vaccinations, with caveats
To take the posttest, go to: https://bit.ly/2X1S7LV
Expires February 12, 2020
Hypersomnolence: Unraveling the causes
Establishing a diagnosis of hypersomnia—recurrent episodes of excessive daytime sleepiness (EDS) or prolonged nighttime sleep—requires a stepwise assessment. We describe a complex case of an older adult who presented with multiple potential causes of hypersomnolence.
CASE REPORT
Persistent daytime sleepiness
Mr. W, age 63, is a veteran with a medical history significant for severe obstructive sleep apnea (OSA), insomnia, restless leg syndrome, hypertension, and major depressive disorder. He reported long-standing EDS that was causing functional and social impairment. Mr. W’s EDS persisted despite the use of continuous positive airway pressure (CPAP) therapy. A download of his CPAP compliance summary revealed both optimal CPAP adherence (>7-hour usage for 95%) and control of OSA (Apnea Hypopnea Index <5). His Epworth Sleepiness Scale (ESS) score remained at 20 out of 24. Another clinician had previously prescribed modafinil to treat Mr. W’s EDS, which was presumed to be related to sleep apnea. At the time of assessment, Mr. W was taking modafinil, 200 mg every morning, without significant relief of his daytime somnolence. Laboratory results revealed normal liver function tests, electrolytes, and hormonal levels, and a urine toxicology was negative. Mr. W said he constantly rubbed his legs to ease his bilateral leg movement. He reported both sensory and motor components, and relief with movement and absence of sensations in the morning.1 Gabapentin was initiated and titrated to a therapeutic dose to stabilize these symptoms.
Further contemplation led the treating clinician to investigate sleep deprivation or insomnia as potential causes of Mr. W’s daytime somnolence. Mr. W also reported occasional insomnia symptoms. To probe for the culprit of daytime sleepiness, actigraphy wrist monitoring was performed and showed no persistent insomnia or circadian rhythm disturbances.2 Medication reconciliation revealed Mr. W was taking 2 medications (fluoxetine and modafinil) that made him alert, but because he took these in the morning, it was unlikely that they were affecting his sleep. Upon review of his sleep habits, Mr. W’s naps were rare and unrefreshing during the day and he was not drinking excessive amounts of caffeinated beverages.
The diagnostic uncertainty led the treating clinician to order a polysomnography sleep study (PSG) with Multiple Sleep Latency Test (MSLT), which revealed a mean sleep latency of 4.1 minutes with no rapid eye movement (REM) periods during his PSG nor next-day napping.3 The PSG showed sleep fragmentation with a sleep efficiency of 90%. The results indicated residual sleepiness secondary to OSA.
Next, the clinician prescribed dextroamphetamine, 25 mg/d, which lowered Mr. W’s ESS score by 2 points (18 out of 24). The clinician presumed that if the stimulant worked, the diagnosis would more likely fit the criteria for residual sleepiness from OSA, rather than idiopathic hypersomnia (IH). Due to a lack of efficacy and adverse effects, the patient was tapered off this medication.
Mr. W reported that he experienced sleepiness during his service in the military at age 23. He also said he did not feel refreshed if he napped during the day.
To address the hypersomnia, he was prescribed off-label sodium oxybate. Sodium oxybate was efficacious and well tolerated; it was slowly titrated up to 9 g/d. After taking sodium oxybate for 2 months, Mr. W’s ESS score diminished to 6. Currently, he reports no functional impairment. A repeat actigraphy showed minimal sleep fragmentation and a strong normal circadian rhythm.
Continue to: Identifying hypersomnia
Identifying hypersomnia
Idiopathic hypersomnia should be considered when a patient’s excessive sleep or EDS are not better explained by another sleep disorder, other medical or psychiatric disorders, or the use of illicit drugs or medications.4 Idiopathic hypersomnia is characterized by EDS that occurs in the absence of cataplexy and is accompanied by no more than 1 sleep-onset REM (SOREM) period on an MSLT and the preceding PSG combined. The differential diagnosis includes narcolepsy, sleep apnea, and
In IH, evidence of hypersomnia must be demonstrated by an MSLT showing a mean sleep latency of <8 minutes or by PSG or wrist actigraphy showing a total 24-hour sleep time of >660 minutes.4 A prolonged and severe form of sleep inertia, consisting of prolonged difficulty waking up with repeated returns to sleep, irritability, automatic behavior, and confusion, often occurs in IH but is not pathognomonic.4
Naps are long—often 60 minutes—and described as unrefreshing by 46% to 78% of patients.4 Sleep efficiency on polysomnography is usually high (mean 90% to 94%). Self-reported total sleep time is longer than in controls and is >10 hours in at least 30% of patients.4 Unfortunately, symptoms and certain objective findings of IH are not unique to the disorder and are considered ubiquitous.
For Mr. W, a diagnosis of narcolepsy was unlikely due to his MSLT results. Patients with narcolepsy have cataplexy (REM dissociation) and/or at least 2 SOREM periods on MLST, or at least 1 SOREM period on MLST in conjunction with a SOREM on the preceding PSG,4 which Mr. W did not exhibit. Patients with narcolepsy typically take refreshing naps lasting 15 to 30 minutes. Although not unique to narcolepsy, common findings include hypnagogic hallucinations and sleep paralysis. Patients with narcolepsy typically do not have sleep inertia but, when seemingly awake, have lapses in vigilance sometimes in combination with automatic behavior, such as writing gibberish or interrupting a conversation with a completely different topic. Another characteristic PSG finding is moderate to severe sleep fragmentation, which may be due to associated periodic limb movements or instability in sleep/wake transitions.5 Mr. W had no history of traumatic brain injury that would suggest hypersomnolence secondary to a brain injury.
Among medical conditions, OSA is the predominant cause of EDS, but this, too, was unlikely for Mr. W because the CPAP therapy reports indicated excellent chronic use and effect. His apnea/hypopnea index was low, and the lowest oxygen saturation recorded on his pre-MSLT PSG using CPAP was 93%. Subjectively, Mr. W reported no choking, gasping, or snoring while receiving CPAP therapy.
Continue to: Restless leg syndrome...
Restless leg syndrome was excluded because after receiving gabapentin, both Mr. W and his wife reported improvement in his leg movements.
Although patients with mood disorders such as depression have normal MSLT results, Mr. W reported no excessive time lying in bed awake, which patients with depression often describe as fatigue and sleepiness. In addition, Mr. W’s score on the Clinically Useful Depression Outcome Scale indicated he was not depressed.
Mr. W’s clinician prescribed off-label sodium oxybate to address his EDS. Its potential benefit in this case may be related to its activity on gamma-aminobutyric acid (GABAB) receptors and its effects in prolonging slow-wave sleep, which has restorative properties. This treatment’s effectiveness in this patient was surprising and without precedent. Because the causes of IH often are not precisely defined, we do not recommend administering a trial of this medication without stepwise exclusion of other causes of sleepiness as demonstrated in Pagel’s algorithm “Diagnosis and Management of Conditions That Cause Excessive Daytime Sleepiness,”6 available at www.aafp.org/afp/2009/0301/p391.html.
1. Kallweit U, Siccoli MM, Poryazova R, et al. Excessive daytime sleepiness in idiopathic restless legs syndrome: characteristics and evolution under dopaminergic treatment. Eur Neurol. 2009;62(3):176-179.
2. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514-1527.
3. Carskadon MA. Guidelines for the Multiple Sleep Latency Test (MSLT): a standard measure of sleepiness. Sleep. 1986;9(4):519-524.
4. American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
5. Bahammam A. Periodic leg movements in narcolepsy patients: impact on sleep architecture. Acta Neurol Scand. 2007;115(5):351-355.
6. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
Establishing a diagnosis of hypersomnia—recurrent episodes of excessive daytime sleepiness (EDS) or prolonged nighttime sleep—requires a stepwise assessment. We describe a complex case of an older adult who presented with multiple potential causes of hypersomnolence.
CASE REPORT
Persistent daytime sleepiness
Mr. W, age 63, is a veteran with a medical history significant for severe obstructive sleep apnea (OSA), insomnia, restless leg syndrome, hypertension, and major depressive disorder. He reported long-standing EDS that was causing functional and social impairment. Mr. W’s EDS persisted despite the use of continuous positive airway pressure (CPAP) therapy. A download of his CPAP compliance summary revealed both optimal CPAP adherence (>7-hour usage for 95%) and control of OSA (Apnea Hypopnea Index <5). His Epworth Sleepiness Scale (ESS) score remained at 20 out of 24. Another clinician had previously prescribed modafinil to treat Mr. W’s EDS, which was presumed to be related to sleep apnea. At the time of assessment, Mr. W was taking modafinil, 200 mg every morning, without significant relief of his daytime somnolence. Laboratory results revealed normal liver function tests, electrolytes, and hormonal levels, and a urine toxicology was negative. Mr. W said he constantly rubbed his legs to ease his bilateral leg movement. He reported both sensory and motor components, and relief with movement and absence of sensations in the morning.1 Gabapentin was initiated and titrated to a therapeutic dose to stabilize these symptoms.
Further contemplation led the treating clinician to investigate sleep deprivation or insomnia as potential causes of Mr. W’s daytime somnolence. Mr. W also reported occasional insomnia symptoms. To probe for the culprit of daytime sleepiness, actigraphy wrist monitoring was performed and showed no persistent insomnia or circadian rhythm disturbances.2 Medication reconciliation revealed Mr. W was taking 2 medications (fluoxetine and modafinil) that made him alert, but because he took these in the morning, it was unlikely that they were affecting his sleep. Upon review of his sleep habits, Mr. W’s naps were rare and unrefreshing during the day and he was not drinking excessive amounts of caffeinated beverages.
The diagnostic uncertainty led the treating clinician to order a polysomnography sleep study (PSG) with Multiple Sleep Latency Test (MSLT), which revealed a mean sleep latency of 4.1 minutes with no rapid eye movement (REM) periods during his PSG nor next-day napping.3 The PSG showed sleep fragmentation with a sleep efficiency of 90%. The results indicated residual sleepiness secondary to OSA.
Next, the clinician prescribed dextroamphetamine, 25 mg/d, which lowered Mr. W’s ESS score by 2 points (18 out of 24). The clinician presumed that if the stimulant worked, the diagnosis would more likely fit the criteria for residual sleepiness from OSA, rather than idiopathic hypersomnia (IH). Due to a lack of efficacy and adverse effects, the patient was tapered off this medication.
Mr. W reported that he experienced sleepiness during his service in the military at age 23. He also said he did not feel refreshed if he napped during the day.
To address the hypersomnia, he was prescribed off-label sodium oxybate. Sodium oxybate was efficacious and well tolerated; it was slowly titrated up to 9 g/d. After taking sodium oxybate for 2 months, Mr. W’s ESS score diminished to 6. Currently, he reports no functional impairment. A repeat actigraphy showed minimal sleep fragmentation and a strong normal circadian rhythm.
Continue to: Identifying hypersomnia
Identifying hypersomnia
Idiopathic hypersomnia should be considered when a patient’s excessive sleep or EDS are not better explained by another sleep disorder, other medical or psychiatric disorders, or the use of illicit drugs or medications.4 Idiopathic hypersomnia is characterized by EDS that occurs in the absence of cataplexy and is accompanied by no more than 1 sleep-onset REM (SOREM) period on an MSLT and the preceding PSG combined. The differential diagnosis includes narcolepsy, sleep apnea, and
In IH, evidence of hypersomnia must be demonstrated by an MSLT showing a mean sleep latency of <8 minutes or by PSG or wrist actigraphy showing a total 24-hour sleep time of >660 minutes.4 A prolonged and severe form of sleep inertia, consisting of prolonged difficulty waking up with repeated returns to sleep, irritability, automatic behavior, and confusion, often occurs in IH but is not pathognomonic.4
Naps are long—often 60 minutes—and described as unrefreshing by 46% to 78% of patients.4 Sleep efficiency on polysomnography is usually high (mean 90% to 94%). Self-reported total sleep time is longer than in controls and is >10 hours in at least 30% of patients.4 Unfortunately, symptoms and certain objective findings of IH are not unique to the disorder and are considered ubiquitous.
For Mr. W, a diagnosis of narcolepsy was unlikely due to his MSLT results. Patients with narcolepsy have cataplexy (REM dissociation) and/or at least 2 SOREM periods on MLST, or at least 1 SOREM period on MLST in conjunction with a SOREM on the preceding PSG,4 which Mr. W did not exhibit. Patients with narcolepsy typically take refreshing naps lasting 15 to 30 minutes. Although not unique to narcolepsy, common findings include hypnagogic hallucinations and sleep paralysis. Patients with narcolepsy typically do not have sleep inertia but, when seemingly awake, have lapses in vigilance sometimes in combination with automatic behavior, such as writing gibberish or interrupting a conversation with a completely different topic. Another characteristic PSG finding is moderate to severe sleep fragmentation, which may be due to associated periodic limb movements or instability in sleep/wake transitions.5 Mr. W had no history of traumatic brain injury that would suggest hypersomnolence secondary to a brain injury.
Among medical conditions, OSA is the predominant cause of EDS, but this, too, was unlikely for Mr. W because the CPAP therapy reports indicated excellent chronic use and effect. His apnea/hypopnea index was low, and the lowest oxygen saturation recorded on his pre-MSLT PSG using CPAP was 93%. Subjectively, Mr. W reported no choking, gasping, or snoring while receiving CPAP therapy.
Continue to: Restless leg syndrome...
Restless leg syndrome was excluded because after receiving gabapentin, both Mr. W and his wife reported improvement in his leg movements.
Although patients with mood disorders such as depression have normal MSLT results, Mr. W reported no excessive time lying in bed awake, which patients with depression often describe as fatigue and sleepiness. In addition, Mr. W’s score on the Clinically Useful Depression Outcome Scale indicated he was not depressed.
Mr. W’s clinician prescribed off-label sodium oxybate to address his EDS. Its potential benefit in this case may be related to its activity on gamma-aminobutyric acid (GABAB) receptors and its effects in prolonging slow-wave sleep, which has restorative properties. This treatment’s effectiveness in this patient was surprising and without precedent. Because the causes of IH often are not precisely defined, we do not recommend administering a trial of this medication without stepwise exclusion of other causes of sleepiness as demonstrated in Pagel’s algorithm “Diagnosis and Management of Conditions That Cause Excessive Daytime Sleepiness,”6 available at www.aafp.org/afp/2009/0301/p391.html.
Establishing a diagnosis of hypersomnia—recurrent episodes of excessive daytime sleepiness (EDS) or prolonged nighttime sleep—requires a stepwise assessment. We describe a complex case of an older adult who presented with multiple potential causes of hypersomnolence.
CASE REPORT
Persistent daytime sleepiness
Mr. W, age 63, is a veteran with a medical history significant for severe obstructive sleep apnea (OSA), insomnia, restless leg syndrome, hypertension, and major depressive disorder. He reported long-standing EDS that was causing functional and social impairment. Mr. W’s EDS persisted despite the use of continuous positive airway pressure (CPAP) therapy. A download of his CPAP compliance summary revealed both optimal CPAP adherence (>7-hour usage for 95%) and control of OSA (Apnea Hypopnea Index <5). His Epworth Sleepiness Scale (ESS) score remained at 20 out of 24. Another clinician had previously prescribed modafinil to treat Mr. W’s EDS, which was presumed to be related to sleep apnea. At the time of assessment, Mr. W was taking modafinil, 200 mg every morning, without significant relief of his daytime somnolence. Laboratory results revealed normal liver function tests, electrolytes, and hormonal levels, and a urine toxicology was negative. Mr. W said he constantly rubbed his legs to ease his bilateral leg movement. He reported both sensory and motor components, and relief with movement and absence of sensations in the morning.1 Gabapentin was initiated and titrated to a therapeutic dose to stabilize these symptoms.
Further contemplation led the treating clinician to investigate sleep deprivation or insomnia as potential causes of Mr. W’s daytime somnolence. Mr. W also reported occasional insomnia symptoms. To probe for the culprit of daytime sleepiness, actigraphy wrist monitoring was performed and showed no persistent insomnia or circadian rhythm disturbances.2 Medication reconciliation revealed Mr. W was taking 2 medications (fluoxetine and modafinil) that made him alert, but because he took these in the morning, it was unlikely that they were affecting his sleep. Upon review of his sleep habits, Mr. W’s naps were rare and unrefreshing during the day and he was not drinking excessive amounts of caffeinated beverages.
The diagnostic uncertainty led the treating clinician to order a polysomnography sleep study (PSG) with Multiple Sleep Latency Test (MSLT), which revealed a mean sleep latency of 4.1 minutes with no rapid eye movement (REM) periods during his PSG nor next-day napping.3 The PSG showed sleep fragmentation with a sleep efficiency of 90%. The results indicated residual sleepiness secondary to OSA.
Next, the clinician prescribed dextroamphetamine, 25 mg/d, which lowered Mr. W’s ESS score by 2 points (18 out of 24). The clinician presumed that if the stimulant worked, the diagnosis would more likely fit the criteria for residual sleepiness from OSA, rather than idiopathic hypersomnia (IH). Due to a lack of efficacy and adverse effects, the patient was tapered off this medication.
Mr. W reported that he experienced sleepiness during his service in the military at age 23. He also said he did not feel refreshed if he napped during the day.
To address the hypersomnia, he was prescribed off-label sodium oxybate. Sodium oxybate was efficacious and well tolerated; it was slowly titrated up to 9 g/d. After taking sodium oxybate for 2 months, Mr. W’s ESS score diminished to 6. Currently, he reports no functional impairment. A repeat actigraphy showed minimal sleep fragmentation and a strong normal circadian rhythm.
Continue to: Identifying hypersomnia
Identifying hypersomnia
Idiopathic hypersomnia should be considered when a patient’s excessive sleep or EDS are not better explained by another sleep disorder, other medical or psychiatric disorders, or the use of illicit drugs or medications.4 Idiopathic hypersomnia is characterized by EDS that occurs in the absence of cataplexy and is accompanied by no more than 1 sleep-onset REM (SOREM) period on an MSLT and the preceding PSG combined. The differential diagnosis includes narcolepsy, sleep apnea, and
In IH, evidence of hypersomnia must be demonstrated by an MSLT showing a mean sleep latency of <8 minutes or by PSG or wrist actigraphy showing a total 24-hour sleep time of >660 minutes.4 A prolonged and severe form of sleep inertia, consisting of prolonged difficulty waking up with repeated returns to sleep, irritability, automatic behavior, and confusion, often occurs in IH but is not pathognomonic.4
Naps are long—often 60 minutes—and described as unrefreshing by 46% to 78% of patients.4 Sleep efficiency on polysomnography is usually high (mean 90% to 94%). Self-reported total sleep time is longer than in controls and is >10 hours in at least 30% of patients.4 Unfortunately, symptoms and certain objective findings of IH are not unique to the disorder and are considered ubiquitous.
For Mr. W, a diagnosis of narcolepsy was unlikely due to his MSLT results. Patients with narcolepsy have cataplexy (REM dissociation) and/or at least 2 SOREM periods on MLST, or at least 1 SOREM period on MLST in conjunction with a SOREM on the preceding PSG,4 which Mr. W did not exhibit. Patients with narcolepsy typically take refreshing naps lasting 15 to 30 minutes. Although not unique to narcolepsy, common findings include hypnagogic hallucinations and sleep paralysis. Patients with narcolepsy typically do not have sleep inertia but, when seemingly awake, have lapses in vigilance sometimes in combination with automatic behavior, such as writing gibberish or interrupting a conversation with a completely different topic. Another characteristic PSG finding is moderate to severe sleep fragmentation, which may be due to associated periodic limb movements or instability in sleep/wake transitions.5 Mr. W had no history of traumatic brain injury that would suggest hypersomnolence secondary to a brain injury.
Among medical conditions, OSA is the predominant cause of EDS, but this, too, was unlikely for Mr. W because the CPAP therapy reports indicated excellent chronic use and effect. His apnea/hypopnea index was low, and the lowest oxygen saturation recorded on his pre-MSLT PSG using CPAP was 93%. Subjectively, Mr. W reported no choking, gasping, or snoring while receiving CPAP therapy.
Continue to: Restless leg syndrome...
Restless leg syndrome was excluded because after receiving gabapentin, both Mr. W and his wife reported improvement in his leg movements.
Although patients with mood disorders such as depression have normal MSLT results, Mr. W reported no excessive time lying in bed awake, which patients with depression often describe as fatigue and sleepiness. In addition, Mr. W’s score on the Clinically Useful Depression Outcome Scale indicated he was not depressed.
Mr. W’s clinician prescribed off-label sodium oxybate to address his EDS. Its potential benefit in this case may be related to its activity on gamma-aminobutyric acid (GABAB) receptors and its effects in prolonging slow-wave sleep, which has restorative properties. This treatment’s effectiveness in this patient was surprising and without precedent. Because the causes of IH often are not precisely defined, we do not recommend administering a trial of this medication without stepwise exclusion of other causes of sleepiness as demonstrated in Pagel’s algorithm “Diagnosis and Management of Conditions That Cause Excessive Daytime Sleepiness,”6 available at www.aafp.org/afp/2009/0301/p391.html.
1. Kallweit U, Siccoli MM, Poryazova R, et al. Excessive daytime sleepiness in idiopathic restless legs syndrome: characteristics and evolution under dopaminergic treatment. Eur Neurol. 2009;62(3):176-179.
2. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514-1527.
3. Carskadon MA. Guidelines for the Multiple Sleep Latency Test (MSLT): a standard measure of sleepiness. Sleep. 1986;9(4):519-524.
4. American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
5. Bahammam A. Periodic leg movements in narcolepsy patients: impact on sleep architecture. Acta Neurol Scand. 2007;115(5):351-355.
6. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
1. Kallweit U, Siccoli MM, Poryazova R, et al. Excessive daytime sleepiness in idiopathic restless legs syndrome: characteristics and evolution under dopaminergic treatment. Eur Neurol. 2009;62(3):176-179.
2. Martin JL, Hakim AD. Wrist actigraphy. Chest. 2011;139(6):1514-1527.
3. Carskadon MA. Guidelines for the Multiple Sleep Latency Test (MSLT): a standard measure of sleepiness. Sleep. 1986;9(4):519-524.
4. American Academy of Sleep Medicine. International Classification of Sleep Disorders, 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014.
5. Bahammam A. Periodic leg movements in narcolepsy patients: impact on sleep architecture. Acta Neurol Scand. 2007;115(5):351-355.
6. Pagel JF. Excessive daytime sleepiness. Am Fam Physician. 2009;79(5):391-396.
The jealous insomniac
CASE Anxious and jealous
Mrs. H, age 28, presents to the emergency department (ED) with pressured speech, emotional lability, loose associations, and echolalia. On physical examination, Mrs. H is noted to have hand tremors. Mrs. H says she has not slept for the past 5 days and is experiencing anxiety and heart palpitations.
She also says that for the past 2 years she has believed that her husband is having an affair with her best friend. However, her current presentation—which she attributes to the alleged affair—began a week before she came to the ED. According to her husband, Mrs. H was “perfectly fine until a week ago” and her symptoms “appeared out of nowhere.” He reports that this has never happened before.
Mrs. H is admitted to the psychiatry unit. The nursing team reports that on the first night, Mrs. H was “running and screaming on the unit, out of control,” and was “tearful, manicky, and dysphoric.”
Mrs. H has no significant medical or psychiatric history. Her family history is significant for hyperthyroidism in her mother and maternal grandmother. Mrs. H says she smokes cigarettes (1 pack/d) but denies alcohol or illicit drug use.
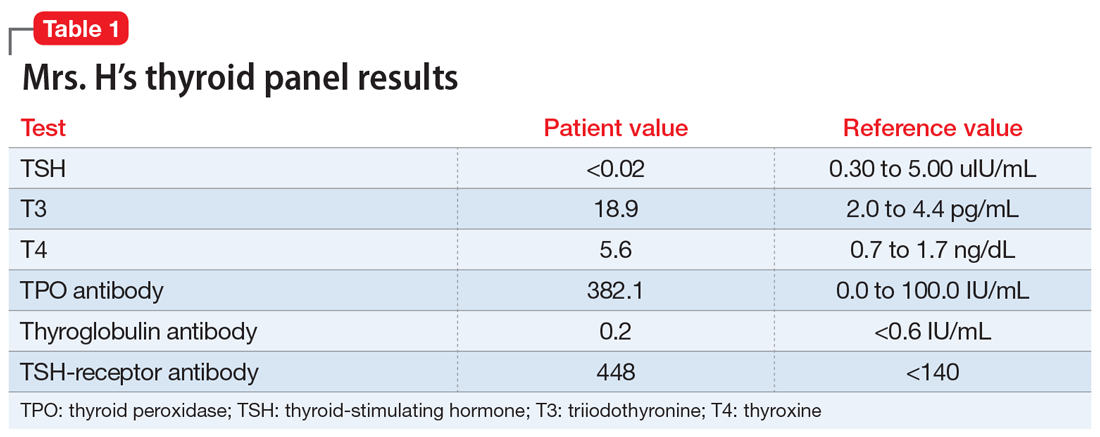
EVALUATION A telling thyroid panel
Mrs. H undergoes laboratory testing, including a complete blood count, comprehensive metabolic panel, and thyroid panel due to her family history of thyroid-related disorders. The thyroid panel shows the presence of the thyroid-stimulating hormone (TSH) receptor antibody; a low TSH level; elevated triiodothyronine (T3) and thyroxine (T4) levels, with T3 > T4; elevated thyroid peroxidase (TPO) antibody; and elevated thyroglobulin antibody (Table 1). A scan shows the thyroid gland to be normal/top-normal size and is read by radiology to be indicative of a resolving thyroiditis vs Graves’ disease. An electrocardiogram indicates a heart rate of 139 beats per minute.
[polldaddy:10352133]
The authors’ observations
Mrs. H fits the presentation of psychosis secondary to Graves’ disease. However, our differential consisted of thyroiditis, brief psychotic disorder, delusional disorder (jealous type), and bipolar mania.
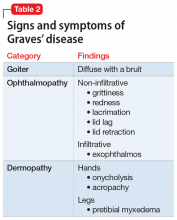
Brief psychotic disorder, bipolar mania, and delusional disorder were better explained by Graves’ disease, and Mrs. H’s jealous delusion resulted in functional impairment, which eliminated delusional disorder. Her family history of hyperthyroidism, as well as her sex and history of tobacco use, supported the diagnosis of Graves’ disease. Although Mrs. H did not experience goiter, ophthalmopathy, or dermopathy, which are common signs and symptoms of Graves’ disease (Table 2), she did present with irritability, insomnia, tachycardia, and a hand tremor. Her psychiatric symptoms included anxiety, emotional lability and, most importantly, psychosis. Her laboratory results included the presence of the TSH-receptor antibody, a low TSH level, and elevated T3 and T4 levels (T3>T4), confirming the diagnosis of early-onset Graves’ disease.
Continue to: Graves' disease
Graves’ disease
Graves’ disease is the most common cause of hyperthyroidism, representing approximately 50% to 80% of cases.1 Graves’ disease occurs most often in women, smokers, and those with a personal or family history of autoimmune disease; although patients of any age may be affected, the peak incidence occurs between age 40 and 60.1
Graves’ disease results from the production of immunoglobulin G (IgG) antibodies that activate the TSH receptor on the surface of thyroid follicular cells.1 The presence of the TSH-receptor antibody, in addition to a low TSH and elevated T3 and T4 levels (T3>T4), are common laboratory findings in patients with this disease. A thyroid scan will also show increased radiotracer accumulation.
Patients with Graves’ disease, as well as those with hyperthyroidism, tend to report weight loss, increased appetite, heat intolerance, irritability, insomnia, and palpitations. In addition to the above symptoms, the identifying signs and symptoms of Graves’ disease include a goiter, ophthalmopathy, and dermopathy (Table 2). Rarely, patients with Graves’ disease can present with psychosis, which is often complicated by thyrotoxicosis.2
[polldaddy:10352135]
TREATMENT Antipsychotic and a beta blocker
Based on her signs, symptoms, and laboratory findings, Mrs. H receives risperidone, 1 mg twice daily, for psychosis, and atenolol, 25 mg twice daily, for heart palpitations. Over 4 days, her symptoms decrease; she experiences more linear thought and decreased flight-of-ideas, and becomes unsure about the truth of her husband’s alleged affair. Her impulsive behaviors and severe mood lability cease. Her tachycardia remains controlled with atenolol.
The authors’ observations
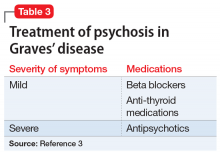
Rapid initiation of treatment is important when managing patients with Graves’ disease, because untreated patients have a higher risk of psychiatric illness, cardiac disease, arrhythmia, and sudden cardiac death.1 Patients with Graves’ disease typically are treated with thionamides, radioactive iodine, and/or surgery. When a patient presents with psychosis as a result of thyrotoxicosis, treatment focuses on improving the thyrotoxicosis through anti-thyroid medications and beta blockers (Table 33). Psychotropic medications, such as antipsychotics, are not indicated for primary treatment, but are given to patients who have severe psychosis until symptoms have resolved.3 For Mrs. H, the severity of her psychosis necessitated risperidone in addition to atenolol.
OUTCOME Continuous medical management; no ablation
Mrs. H is discharged with immediate outpatient follow-up with an endocrinology team to discuss the best long-term management of her thyroiditis. Mrs. H opts for continuous medical management (as opposed to ablation) and is administered methimazole, 15 mg/d, to treat Graves’ disease.
The authors’ observations
This case provides useful information regarding recognizing psychosis as the initial sign of Graves’ disease. Although Graves’ disease represents 50% to 80% of cases of hyperthyroidism,1 psychosis as the first clinical presentation of this disease is extremely rare. Several case reports, however, have described this phenomenon,2,3 and further studies would be helpful to determine its true prevalence.
Continue to: Bottom Line
Bottom Line
Although extremely rare, psychosis as the initial clinical presentation of Graves’ disease can occur. The early diagnosis of Graves’ disease is critical to prevent cardiovascular implications and death.
Related Resources
- Abraham P, Acharya S. Current and emerging treatment options for Graves’ hyperthyroidism. Ther Clin Risk Manag. 2010;6:29-40.
- Bunevicius R, Prange AJ Jr. Psychiatric manifestations of Graves’ hyperthyroidism: pathophysiology and treatment options. CNS Drugs. 2006;20(11):897-909.
- Ginsberg J. Diagnosis and management of Graves’ disease. CMAJ. 2003;168(5):575-585.
Drug Brand Names
Atenolol • Tenormin
Methimazole • Tapazole
Risperidone • Risperdal
1. Girgis C, Champion B, Wall J. Current concepts in Graves’ disease. Ther Adv Endocrinol Metab. 2011;2(3):135-144.
2. Urias-Uribe L, Valdez-Solis E, González-Milán C, et al. Psychosis crisis associated with thyrotoxicosis due to Graves’ disease. Case Rep Psychiatry. 2017;2017:6803682. doi: 10.1155/2017/6803682.
3. Ugwu ET, Maluze J, Onyebueke GC. Graves’ thyrotoxicosis presenting as schizophreniform psychosis: a case report and literature review. Int J Endocrinol Metab. 2017;15(1):e41977. doi: 10.5812/ijem.41977.
CASE Anxious and jealous
Mrs. H, age 28, presents to the emergency department (ED) with pressured speech, emotional lability, loose associations, and echolalia. On physical examination, Mrs. H is noted to have hand tremors. Mrs. H says she has not slept for the past 5 days and is experiencing anxiety and heart palpitations.
She also says that for the past 2 years she has believed that her husband is having an affair with her best friend. However, her current presentation—which she attributes to the alleged affair—began a week before she came to the ED. According to her husband, Mrs. H was “perfectly fine until a week ago” and her symptoms “appeared out of nowhere.” He reports that this has never happened before.
Mrs. H is admitted to the psychiatry unit. The nursing team reports that on the first night, Mrs. H was “running and screaming on the unit, out of control,” and was “tearful, manicky, and dysphoric.”
Mrs. H has no significant medical or psychiatric history. Her family history is significant for hyperthyroidism in her mother and maternal grandmother. Mrs. H says she smokes cigarettes (1 pack/d) but denies alcohol or illicit drug use.

EVALUATION A telling thyroid panel
Mrs. H undergoes laboratory testing, including a complete blood count, comprehensive metabolic panel, and thyroid panel due to her family history of thyroid-related disorders. The thyroid panel shows the presence of the thyroid-stimulating hormone (TSH) receptor antibody; a low TSH level; elevated triiodothyronine (T3) and thyroxine (T4) levels, with T3 > T4; elevated thyroid peroxidase (TPO) antibody; and elevated thyroglobulin antibody (Table 1). A scan shows the thyroid gland to be normal/top-normal size and is read by radiology to be indicative of a resolving thyroiditis vs Graves’ disease. An electrocardiogram indicates a heart rate of 139 beats per minute.
[polldaddy:10352133]
The authors’ observations
Mrs. H fits the presentation of psychosis secondary to Graves’ disease. However, our differential consisted of thyroiditis, brief psychotic disorder, delusional disorder (jealous type), and bipolar mania.
Brief psychotic disorder, bipolar mania, and delusional disorder were better explained by Graves’ disease, and Mrs. H’s jealous delusion resulted in functional impairment, which eliminated delusional disorder. Her family history of hyperthyroidism, as well as her sex and history of tobacco use, supported the diagnosis of Graves’ disease. Although Mrs. H did not experience goiter, ophthalmopathy, or dermopathy, which are common signs and symptoms of Graves’ disease (Table 2), she did present with irritability, insomnia, tachycardia, and a hand tremor. Her psychiatric symptoms included anxiety, emotional lability and, most importantly, psychosis. Her laboratory results included the presence of the TSH-receptor antibody, a low TSH level, and elevated T3 and T4 levels (T3>T4), confirming the diagnosis of early-onset Graves’ disease.
Continue to: Graves' disease
Graves’ disease
Graves’ disease is the most common cause of hyperthyroidism, representing approximately 50% to 80% of cases.1 Graves’ disease occurs most often in women, smokers, and those with a personal or family history of autoimmune disease; although patients of any age may be affected, the peak incidence occurs between age 40 and 60.1
Graves’ disease results from the production of immunoglobulin G (IgG) antibodies that activate the TSH receptor on the surface of thyroid follicular cells.1 The presence of the TSH-receptor antibody, in addition to a low TSH and elevated T3 and T4 levels (T3>T4), are common laboratory findings in patients with this disease. A thyroid scan will also show increased radiotracer accumulation.
Patients with Graves’ disease, as well as those with hyperthyroidism, tend to report weight loss, increased appetite, heat intolerance, irritability, insomnia, and palpitations. In addition to the above symptoms, the identifying signs and symptoms of Graves’ disease include a goiter, ophthalmopathy, and dermopathy (Table 2). Rarely, patients with Graves’ disease can present with psychosis, which is often complicated by thyrotoxicosis.2
[polldaddy:10352135]
TREATMENT Antipsychotic and a beta blocker
Based on her signs, symptoms, and laboratory findings, Mrs. H receives risperidone, 1 mg twice daily, for psychosis, and atenolol, 25 mg twice daily, for heart palpitations. Over 4 days, her symptoms decrease; she experiences more linear thought and decreased flight-of-ideas, and becomes unsure about the truth of her husband’s alleged affair. Her impulsive behaviors and severe mood lability cease. Her tachycardia remains controlled with atenolol.
The authors’ observations
Rapid initiation of treatment is important when managing patients with Graves’ disease, because untreated patients have a higher risk of psychiatric illness, cardiac disease, arrhythmia, and sudden cardiac death.1 Patients with Graves’ disease typically are treated with thionamides, radioactive iodine, and/or surgery. When a patient presents with psychosis as a result of thyrotoxicosis, treatment focuses on improving the thyrotoxicosis through anti-thyroid medications and beta blockers (Table 33). Psychotropic medications, such as antipsychotics, are not indicated for primary treatment, but are given to patients who have severe psychosis until symptoms have resolved.3 For Mrs. H, the severity of her psychosis necessitated risperidone in addition to atenolol.
OUTCOME Continuous medical management; no ablation
Mrs. H is discharged with immediate outpatient follow-up with an endocrinology team to discuss the best long-term management of her thyroiditis. Mrs. H opts for continuous medical management (as opposed to ablation) and is administered methimazole, 15 mg/d, to treat Graves’ disease.
The authors’ observations
This case provides useful information regarding recognizing psychosis as the initial sign of Graves’ disease. Although Graves’ disease represents 50% to 80% of cases of hyperthyroidism,1 psychosis as the first clinical presentation of this disease is extremely rare. Several case reports, however, have described this phenomenon,2,3 and further studies would be helpful to determine its true prevalence.
Continue to: Bottom Line
Bottom Line
Although extremely rare, psychosis as the initial clinical presentation of Graves’ disease can occur. The early diagnosis of Graves’ disease is critical to prevent cardiovascular implications and death.
Related Resources
- Abraham P, Acharya S. Current and emerging treatment options for Graves’ hyperthyroidism. Ther Clin Risk Manag. 2010;6:29-40.
- Bunevicius R, Prange AJ Jr. Psychiatric manifestations of Graves’ hyperthyroidism: pathophysiology and treatment options. CNS Drugs. 2006;20(11):897-909.
- Ginsberg J. Diagnosis and management of Graves’ disease. CMAJ. 2003;168(5):575-585.
Drug Brand Names
Atenolol • Tenormin
Methimazole • Tapazole
Risperidone • Risperdal
CASE Anxious and jealous
Mrs. H, age 28, presents to the emergency department (ED) with pressured speech, emotional lability, loose associations, and echolalia. On physical examination, Mrs. H is noted to have hand tremors. Mrs. H says she has not slept for the past 5 days and is experiencing anxiety and heart palpitations.
She also says that for the past 2 years she has believed that her husband is having an affair with her best friend. However, her current presentation—which she attributes to the alleged affair—began a week before she came to the ED. According to her husband, Mrs. H was “perfectly fine until a week ago” and her symptoms “appeared out of nowhere.” He reports that this has never happened before.
Mrs. H is admitted to the psychiatry unit. The nursing team reports that on the first night, Mrs. H was “running and screaming on the unit, out of control,” and was “tearful, manicky, and dysphoric.”
Mrs. H has no significant medical or psychiatric history. Her family history is significant for hyperthyroidism in her mother and maternal grandmother. Mrs. H says she smokes cigarettes (1 pack/d) but denies alcohol or illicit drug use.

EVALUATION A telling thyroid panel
Mrs. H undergoes laboratory testing, including a complete blood count, comprehensive metabolic panel, and thyroid panel due to her family history of thyroid-related disorders. The thyroid panel shows the presence of the thyroid-stimulating hormone (TSH) receptor antibody; a low TSH level; elevated triiodothyronine (T3) and thyroxine (T4) levels, with T3 > T4; elevated thyroid peroxidase (TPO) antibody; and elevated thyroglobulin antibody (Table 1). A scan shows the thyroid gland to be normal/top-normal size and is read by radiology to be indicative of a resolving thyroiditis vs Graves’ disease. An electrocardiogram indicates a heart rate of 139 beats per minute.
[polldaddy:10352133]
The authors’ observations
Mrs. H fits the presentation of psychosis secondary to Graves’ disease. However, our differential consisted of thyroiditis, brief psychotic disorder, delusional disorder (jealous type), and bipolar mania.
Brief psychotic disorder, bipolar mania, and delusional disorder were better explained by Graves’ disease, and Mrs. H’s jealous delusion resulted in functional impairment, which eliminated delusional disorder. Her family history of hyperthyroidism, as well as her sex and history of tobacco use, supported the diagnosis of Graves’ disease. Although Mrs. H did not experience goiter, ophthalmopathy, or dermopathy, which are common signs and symptoms of Graves’ disease (Table 2), she did present with irritability, insomnia, tachycardia, and a hand tremor. Her psychiatric symptoms included anxiety, emotional lability and, most importantly, psychosis. Her laboratory results included the presence of the TSH-receptor antibody, a low TSH level, and elevated T3 and T4 levels (T3>T4), confirming the diagnosis of early-onset Graves’ disease.
Continue to: Graves' disease
Graves’ disease
Graves’ disease is the most common cause of hyperthyroidism, representing approximately 50% to 80% of cases.1 Graves’ disease occurs most often in women, smokers, and those with a personal or family history of autoimmune disease; although patients of any age may be affected, the peak incidence occurs between age 40 and 60.1
Graves’ disease results from the production of immunoglobulin G (IgG) antibodies that activate the TSH receptor on the surface of thyroid follicular cells.1 The presence of the TSH-receptor antibody, in addition to a low TSH and elevated T3 and T4 levels (T3>T4), are common laboratory findings in patients with this disease. A thyroid scan will also show increased radiotracer accumulation.
Patients with Graves’ disease, as well as those with hyperthyroidism, tend to report weight loss, increased appetite, heat intolerance, irritability, insomnia, and palpitations. In addition to the above symptoms, the identifying signs and symptoms of Graves’ disease include a goiter, ophthalmopathy, and dermopathy (Table 2). Rarely, patients with Graves’ disease can present with psychosis, which is often complicated by thyrotoxicosis.2
[polldaddy:10352135]
TREATMENT Antipsychotic and a beta blocker
Based on her signs, symptoms, and laboratory findings, Mrs. H receives risperidone, 1 mg twice daily, for psychosis, and atenolol, 25 mg twice daily, for heart palpitations. Over 4 days, her symptoms decrease; she experiences more linear thought and decreased flight-of-ideas, and becomes unsure about the truth of her husband’s alleged affair. Her impulsive behaviors and severe mood lability cease. Her tachycardia remains controlled with atenolol.
The authors’ observations
Rapid initiation of treatment is important when managing patients with Graves’ disease, because untreated patients have a higher risk of psychiatric illness, cardiac disease, arrhythmia, and sudden cardiac death.1 Patients with Graves’ disease typically are treated with thionamides, radioactive iodine, and/or surgery. When a patient presents with psychosis as a result of thyrotoxicosis, treatment focuses on improving the thyrotoxicosis through anti-thyroid medications and beta blockers (Table 33). Psychotropic medications, such as antipsychotics, are not indicated for primary treatment, but are given to patients who have severe psychosis until symptoms have resolved.3 For Mrs. H, the severity of her psychosis necessitated risperidone in addition to atenolol.
OUTCOME Continuous medical management; no ablation
Mrs. H is discharged with immediate outpatient follow-up with an endocrinology team to discuss the best long-term management of her thyroiditis. Mrs. H opts for continuous medical management (as opposed to ablation) and is administered methimazole, 15 mg/d, to treat Graves’ disease.
The authors’ observations
This case provides useful information regarding recognizing psychosis as the initial sign of Graves’ disease. Although Graves’ disease represents 50% to 80% of cases of hyperthyroidism,1 psychosis as the first clinical presentation of this disease is extremely rare. Several case reports, however, have described this phenomenon,2,3 and further studies would be helpful to determine its true prevalence.
Continue to: Bottom Line
Bottom Line
Although extremely rare, psychosis as the initial clinical presentation of Graves’ disease can occur. The early diagnosis of Graves’ disease is critical to prevent cardiovascular implications and death.
Related Resources
- Abraham P, Acharya S. Current and emerging treatment options for Graves’ hyperthyroidism. Ther Clin Risk Manag. 2010;6:29-40.
- Bunevicius R, Prange AJ Jr. Psychiatric manifestations of Graves’ hyperthyroidism: pathophysiology and treatment options. CNS Drugs. 2006;20(11):897-909.
- Ginsberg J. Diagnosis and management of Graves’ disease. CMAJ. 2003;168(5):575-585.
Drug Brand Names
Atenolol • Tenormin
Methimazole • Tapazole
Risperidone • Risperdal
1. Girgis C, Champion B, Wall J. Current concepts in Graves’ disease. Ther Adv Endocrinol Metab. 2011;2(3):135-144.
2. Urias-Uribe L, Valdez-Solis E, González-Milán C, et al. Psychosis crisis associated with thyrotoxicosis due to Graves’ disease. Case Rep Psychiatry. 2017;2017:6803682. doi: 10.1155/2017/6803682.
3. Ugwu ET, Maluze J, Onyebueke GC. Graves’ thyrotoxicosis presenting as schizophreniform psychosis: a case report and literature review. Int J Endocrinol Metab. 2017;15(1):e41977. doi: 10.5812/ijem.41977.
1. Girgis C, Champion B, Wall J. Current concepts in Graves’ disease. Ther Adv Endocrinol Metab. 2011;2(3):135-144.
2. Urias-Uribe L, Valdez-Solis E, González-Milán C, et al. Psychosis crisis associated with thyrotoxicosis due to Graves’ disease. Case Rep Psychiatry. 2017;2017:6803682. doi: 10.1155/2017/6803682.
3. Ugwu ET, Maluze J, Onyebueke GC. Graves’ thyrotoxicosis presenting as schizophreniform psychosis: a case report and literature review. Int J Endocrinol Metab. 2017;15(1):e41977. doi: 10.5812/ijem.41977.
Study eyes narcolepsy’s impact on patient quality of life
SAN ANTONIO – Narcolepsy adversely impacts one’s health-related quality of life in a variety of ways, from elevated levels of depression to negative social stigma, results from a mixed methods study suggest.
“Despite established pharmacological treatments to reduce narcolepsy symptoms, health-related quality of life remains poor,” the study’s first author, Jason C. Ong, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “The impact these symptoms have on functioning, the disease burden, and psychosocial functioning in particular is very important. Psychosocial functioning is particularly poor.”
Previous research has shown that people with narcolepsy have two- to four times the rate of psychiatric comorbidities and that health-related stigma is a predictor of depression and poor functioning, said Dr. Ong, a psychologist with the Center for Circadian and Sleep Medicine at the Northwestern University Feinberg School of Medicine, Chicago. In an effort to assess current practices for addressing the psychosocial needs of persons with narcolepsy and to identify potential strategies that could be used to develop a psychosocial intervention, he and his associates conducted a mixed methods study to examine how narcolepsy symptoms impact health-related quality of life and the appropriateness of different health-related quality of life measures for the disorder. “Our long-term goal is to see if we can use this information to help inform the feasibility of a psychosocial intervention to improve health-related quality of life,” he said.
For the study, 29 adults with an established diagnosis of narcolepsy completed online versions of the Patient Health Questionnaire-9 (PHQ-9), the Patient Reported Outcomes Measurement Information System (PROMIS), the 36-item Short Form Survey (SF-36), and the Epworth Sleepiness Scale (ESS). They also participated in a focus group, which consisted of questions pertaining to quality of life for persons with narcolepsy, current practices for addressing psychosocial health of affected individuals, and suggestions for developing a psychosocial intervention. The researchers used thematic analysis to reduce the qualitative data to key themes.
Most of the study participants (93%) were female, 90% were white, their mean age was 31, and their mean time since narcolepsy diagnosis was 4.3 years. Clinically significant elevations on the PROMIS scale, defined as a t-score of greater than 60, were reported for depression (t-score of 64.8), anxiety (66.3), fatigue (68.3), and sleep impairment (66.9). Elevations in depressive symptoms were reported on the PHQ-9 (a mean of 15.79), “which corresponds to moderately severe levels,” Dr. Ong said. “The ESS was highly elevated and fit well with the scales for sleep impairment as well as fatigue on the PROMIS. Overall, there was nice congruence across these measures.”
On the SF-36, the researchers observed that there were deficits in physical and emotional aspects of role limitations, and in energy/fatigue. “One thing we did find was a significant difference in general functioning, where patients with type 1 narcolepsy were worse off than those with type 2 narcolepsy (P less than .05).”
Qualitative data from focus groups revealed several key themes, including the perception that narcolepsy is poorly understood by the public and health care providers.
“People have the perception that if you have narcolepsy, you just feel fine and then you fall asleep,” Dr. Ong said. “They don’t understand that it’s a constant thing. Negative social stigma was also common. As a result, we found a lot of negative impact on self-esteem and self-efficacy. People talked about being hesitant to tell other people about their diagnosis, feeling that they’re ashamed of having narcolepsy. They felt less capable. One person said, ‘I get tired trying to explain why I’m tired.’”
Another common theme that emerged was the challenge of optimal treatment for their narcolepsy. Most patients met with sleep doctors or clinics every 3-6 months. “They said that this was generally good for discussing medications and symptom management, but there didn’t seem to be much time to talk about psychosocial aspects,” Dr. Ong said. “That seemed to be one area of need. There was also a strong dissatisfaction with mental health providers. People talked about how their mental health provider really didn’t understand narcolepsy. It did seem to reduce rapport and the ability to trust their therapist. Some talked about the challenges of accessibility. In some cases, people said their narcolepsy symptoms created challenges with appointment attendance.”
In terms of preferences for a psychosocial intervention, respondents generally “preferred some kind of online or Internet delivery,” he said. “They prefer a team approach with a clinician who’s knowledgeable about both sleep and mental health.”
Dr. Ong acknowledged certain limitations of the study, including its small sample size and the fact that it was not adequately powered to detect differences between type 1 and type 2 narcolepsy.
The study was funded by a grant from Wake Up Narcolepsy. Dr. Ong reported having no relevant financial disclosures.
SOURCE: Ong J et al., SLEEP 2019, abstract 0624.
SAN ANTONIO – Narcolepsy adversely impacts one’s health-related quality of life in a variety of ways, from elevated levels of depression to negative social stigma, results from a mixed methods study suggest.
“Despite established pharmacological treatments to reduce narcolepsy symptoms, health-related quality of life remains poor,” the study’s first author, Jason C. Ong, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “The impact these symptoms have on functioning, the disease burden, and psychosocial functioning in particular is very important. Psychosocial functioning is particularly poor.”
Previous research has shown that people with narcolepsy have two- to four times the rate of psychiatric comorbidities and that health-related stigma is a predictor of depression and poor functioning, said Dr. Ong, a psychologist with the Center for Circadian and Sleep Medicine at the Northwestern University Feinberg School of Medicine, Chicago. In an effort to assess current practices for addressing the psychosocial needs of persons with narcolepsy and to identify potential strategies that could be used to develop a psychosocial intervention, he and his associates conducted a mixed methods study to examine how narcolepsy symptoms impact health-related quality of life and the appropriateness of different health-related quality of life measures for the disorder. “Our long-term goal is to see if we can use this information to help inform the feasibility of a psychosocial intervention to improve health-related quality of life,” he said.
For the study, 29 adults with an established diagnosis of narcolepsy completed online versions of the Patient Health Questionnaire-9 (PHQ-9), the Patient Reported Outcomes Measurement Information System (PROMIS), the 36-item Short Form Survey (SF-36), and the Epworth Sleepiness Scale (ESS). They also participated in a focus group, which consisted of questions pertaining to quality of life for persons with narcolepsy, current practices for addressing psychosocial health of affected individuals, and suggestions for developing a psychosocial intervention. The researchers used thematic analysis to reduce the qualitative data to key themes.
Most of the study participants (93%) were female, 90% were white, their mean age was 31, and their mean time since narcolepsy diagnosis was 4.3 years. Clinically significant elevations on the PROMIS scale, defined as a t-score of greater than 60, were reported for depression (t-score of 64.8), anxiety (66.3), fatigue (68.3), and sleep impairment (66.9). Elevations in depressive symptoms were reported on the PHQ-9 (a mean of 15.79), “which corresponds to moderately severe levels,” Dr. Ong said. “The ESS was highly elevated and fit well with the scales for sleep impairment as well as fatigue on the PROMIS. Overall, there was nice congruence across these measures.”
On the SF-36, the researchers observed that there were deficits in physical and emotional aspects of role limitations, and in energy/fatigue. “One thing we did find was a significant difference in general functioning, where patients with type 1 narcolepsy were worse off than those with type 2 narcolepsy (P less than .05).”
Qualitative data from focus groups revealed several key themes, including the perception that narcolepsy is poorly understood by the public and health care providers.
“People have the perception that if you have narcolepsy, you just feel fine and then you fall asleep,” Dr. Ong said. “They don’t understand that it’s a constant thing. Negative social stigma was also common. As a result, we found a lot of negative impact on self-esteem and self-efficacy. People talked about being hesitant to tell other people about their diagnosis, feeling that they’re ashamed of having narcolepsy. They felt less capable. One person said, ‘I get tired trying to explain why I’m tired.’”
Another common theme that emerged was the challenge of optimal treatment for their narcolepsy. Most patients met with sleep doctors or clinics every 3-6 months. “They said that this was generally good for discussing medications and symptom management, but there didn’t seem to be much time to talk about psychosocial aspects,” Dr. Ong said. “That seemed to be one area of need. There was also a strong dissatisfaction with mental health providers. People talked about how their mental health provider really didn’t understand narcolepsy. It did seem to reduce rapport and the ability to trust their therapist. Some talked about the challenges of accessibility. In some cases, people said their narcolepsy symptoms created challenges with appointment attendance.”
In terms of preferences for a psychosocial intervention, respondents generally “preferred some kind of online or Internet delivery,” he said. “They prefer a team approach with a clinician who’s knowledgeable about both sleep and mental health.”
Dr. Ong acknowledged certain limitations of the study, including its small sample size and the fact that it was not adequately powered to detect differences between type 1 and type 2 narcolepsy.
The study was funded by a grant from Wake Up Narcolepsy. Dr. Ong reported having no relevant financial disclosures.
SOURCE: Ong J et al., SLEEP 2019, abstract 0624.
SAN ANTONIO – Narcolepsy adversely impacts one’s health-related quality of life in a variety of ways, from elevated levels of depression to negative social stigma, results from a mixed methods study suggest.
“Despite established pharmacological treatments to reduce narcolepsy symptoms, health-related quality of life remains poor,” the study’s first author, Jason C. Ong, PhD, said at the annual meeting of the Associated Professional Sleep Societies. “The impact these symptoms have on functioning, the disease burden, and psychosocial functioning in particular is very important. Psychosocial functioning is particularly poor.”
Previous research has shown that people with narcolepsy have two- to four times the rate of psychiatric comorbidities and that health-related stigma is a predictor of depression and poor functioning, said Dr. Ong, a psychologist with the Center for Circadian and Sleep Medicine at the Northwestern University Feinberg School of Medicine, Chicago. In an effort to assess current practices for addressing the psychosocial needs of persons with narcolepsy and to identify potential strategies that could be used to develop a psychosocial intervention, he and his associates conducted a mixed methods study to examine how narcolepsy symptoms impact health-related quality of life and the appropriateness of different health-related quality of life measures for the disorder. “Our long-term goal is to see if we can use this information to help inform the feasibility of a psychosocial intervention to improve health-related quality of life,” he said.
For the study, 29 adults with an established diagnosis of narcolepsy completed online versions of the Patient Health Questionnaire-9 (PHQ-9), the Patient Reported Outcomes Measurement Information System (PROMIS), the 36-item Short Form Survey (SF-36), and the Epworth Sleepiness Scale (ESS). They also participated in a focus group, which consisted of questions pertaining to quality of life for persons with narcolepsy, current practices for addressing psychosocial health of affected individuals, and suggestions for developing a psychosocial intervention. The researchers used thematic analysis to reduce the qualitative data to key themes.
Most of the study participants (93%) were female, 90% were white, their mean age was 31, and their mean time since narcolepsy diagnosis was 4.3 years. Clinically significant elevations on the PROMIS scale, defined as a t-score of greater than 60, were reported for depression (t-score of 64.8), anxiety (66.3), fatigue (68.3), and sleep impairment (66.9). Elevations in depressive symptoms were reported on the PHQ-9 (a mean of 15.79), “which corresponds to moderately severe levels,” Dr. Ong said. “The ESS was highly elevated and fit well with the scales for sleep impairment as well as fatigue on the PROMIS. Overall, there was nice congruence across these measures.”
On the SF-36, the researchers observed that there were deficits in physical and emotional aspects of role limitations, and in energy/fatigue. “One thing we did find was a significant difference in general functioning, where patients with type 1 narcolepsy were worse off than those with type 2 narcolepsy (P less than .05).”
Qualitative data from focus groups revealed several key themes, including the perception that narcolepsy is poorly understood by the public and health care providers.
“People have the perception that if you have narcolepsy, you just feel fine and then you fall asleep,” Dr. Ong said. “They don’t understand that it’s a constant thing. Negative social stigma was also common. As a result, we found a lot of negative impact on self-esteem and self-efficacy. People talked about being hesitant to tell other people about their diagnosis, feeling that they’re ashamed of having narcolepsy. They felt less capable. One person said, ‘I get tired trying to explain why I’m tired.’”
Another common theme that emerged was the challenge of optimal treatment for their narcolepsy. Most patients met with sleep doctors or clinics every 3-6 months. “They said that this was generally good for discussing medications and symptom management, but there didn’t seem to be much time to talk about psychosocial aspects,” Dr. Ong said. “That seemed to be one area of need. There was also a strong dissatisfaction with mental health providers. People talked about how their mental health provider really didn’t understand narcolepsy. It did seem to reduce rapport and the ability to trust their therapist. Some talked about the challenges of accessibility. In some cases, people said their narcolepsy symptoms created challenges with appointment attendance.”
In terms of preferences for a psychosocial intervention, respondents generally “preferred some kind of online or Internet delivery,” he said. “They prefer a team approach with a clinician who’s knowledgeable about both sleep and mental health.”
Dr. Ong acknowledged certain limitations of the study, including its small sample size and the fact that it was not adequately powered to detect differences between type 1 and type 2 narcolepsy.
The study was funded by a grant from Wake Up Narcolepsy. Dr. Ong reported having no relevant financial disclosures.
SOURCE: Ong J et al., SLEEP 2019, abstract 0624.
REPORTING FROM SLEEP 2019
Subset of patients benefits from in-hospital sleep apnea screening
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
SAN ANTONIO – In the clinical opinion of Richard J. Schwab, MD,
“Many diseases are adversely affected by sleep apnea, including myocardial infarction, hypertension, a cerebrovascular accident, pulmonary hypertension, atrial fibrillation, diabetes, and congestive heart failure,” Dr. Schwab, interim chief of the University of Pennsylvania Perelman School of Medicine’s Division of Sleep Medicine, said at the annual meeting of the Associated Professional Sleep Societies.
“Continuous positive airway pressure [CPAP] may help heart failure patients and reduce 30-day readmission rates, which has important financial implications in the University of Pennsylvania Health system. CPAP may also decrease the rapid responses and cardiac arrests at night,” he said.
A few years ago, Dr. Schwab and his associates set out to determine whether PAP adherence in cardiac patients with sleep-disordered breathing reduced readmission rates 30 days after discharge (J Clin Sleep Med. 2014;10:1051-59). They evaluated 104 consecutive cardiovascular hospitalized patients reporting symptoms of sleep-disordered breathing (SDB) between January of 2012 and March of 2013, and collected demographic data, SDB type, PAP adherence, and data regarding 30-day hospital readmission/ED visits. Apnea was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor. Hypopnea was scored when there was at least a 50% reduction in airflow with an associated 3% or greater oxyhemoglobin desaturation. Central apnea (CSA) was scored when there was a 90% or greater cessation of airflow detected through the nasal pressure sensor and no effort in the thorax and abdomen. If more than 50% of the apneas were central, the SDB was classified as CSA. If more than 50% of apneas were obstructive in nature, it was considered obstructive sleep apnea (OSA).
The mean age of the patients was 59 years, 63% were male, their mean body mass index was 34 kg/m2, 87% had heart failure, and 82% had hypertension. Of the 104 patients, 81 had SDB and 23 did not. The 30-day readmission rate was 29% in patients who did not use PAP, 30% in partial users, and 0% in full users (P = .0246).
The researchers found that 81 patients (78%) had sleep disordered breathing. Of these, 65 (80%) had OSA while 16 (20%) had CSA. The study demonstrated that performing inpatient sleep studies was feasible. “Our study indicated that SDB is common in hospitalized cardiac patients, with the majority of patients manifesting OSA,” said Dr. Schwab, medical director of the Penn Sleep Centers. “The data suggest that hospital readmission and ED visits 30 days after discharge were significantly lower in patients with cardiac disease and SDB who adhere to PAP treatment than those who are not adherent.”
Dr. Schwab is part of a research team conducting a longer study with ResMed to examine 30-, 60-, and 90-day readmission rates in cardiac inpatients newly diagnosed with OSA and started on auto-PAP (APAP). They plan to evaluate the ejection fraction during hospitalization and in follow-up, as well as the effect of an in-laboratory sleep study at 1 month. The long-term follow-up is planned for 3 years.
Launching an inpatient sleep apnea consult service in the hospital makes sense, Dr. Schwab continued, because home sleep studies are approved for the diagnosis of sleep apnea, APAP can determine optimal CPAP settings, insurance will cover CPAP with a home or inpatient sleep study, and patients can get CPAP/APAP at or before discharge. “Sleep techs or respiratory therapists can perform these sleep studies,” he said. At Penn, a nurse practitioner (NP) runs this service using the Alice NightOne home sleep testing device and the WatchPAT portable sleep apnea diagnostic device.
The notion of performing in-hospital sleep studies should be an easy sell to cardiologists and hospital administrators, Dr. Schwab said, because the program will decrease hospital readmissions, “which is going to save the hospital a lot of money. In addition, these patients can come back for in-laboratory sleep studies. There is also increased revenue from the consults and progress notes, and the professional fee for sleep study interpretation. The most challenging part of the inpatient sleep consult service is trying to get these patients to follow up in the sleep center with the NP.”
Dr. Schwab is an investigator for the recently launched Penn Medicine Nudge Unit Project, which is funded by the National Institutes of Health. The project includes a multidisciplinary team of providers from the Hospital of the University of Pennsylvania, Penn Presbyterian Medical Center, and Penn Medicine Risk Management. If an inpatient has a BMI of 35 kg/m2 or greater, the clinician will be “nudged” via an enterprise messaging system (EMS) prompt to order an inpatient sleep oximetry. “They have to respond to that nudge,” Dr. Schwab said. “If the oximetry is consistent for sleep apnea, there will be another nudge to consult with the sleep medicine team. If the oximetry is negative, they will be nudged to get an outpatient consult with the sleep medicine team.” For patients undergoing preadmission testing for any type of surgery who score 4 or more on the STOP-Bang questionnaire (Chest 2016;149:631-38), the clinician is “nudged” to order an outpatient sleep consultation.
Benefits to such an approach, he said, include a decrease in resource allocation, shorter hospital stays, patient perceived improvement in quality of sleep, improved patient survey scores, and the fact that apnea treatment may decrease the need for rapid response. “It also reduces medical-legal concerns, improves patient outcomes, decreases readmissions, and generates revenue from inpatient and outpatient sleep studies,” Dr. Schwab said. Barriers to such an approach include the fact that there is no defined pathway at many institutions for recognizing and referring suspected OSA patients. “There is often a lack of care coordination between primary providers and sleep medicine, and sleep is viewed as ambulatory care, not as a part of inpatient care,” he said.
Last year, Dr. Schwab and his colleagues at UPenn conducted a pilot study to develop and test a pathway for identifying OSA in high-risk inpatient and preadmission patient populations. Of 389 patients admitted between Aug. 20 and Sept. 20 of 2018, 43 had a BMI of 35 kg/m2 or greater. Of these, 10 were screened with oximetry and 8 were positive for severe apnea. Of these eight cases, five inpatient consults were ordered, one outpatient consult was ordered, one patient had no consult ordered, and one patient was discharged before the consult was ordered.
Dr. Schwab also performed a pilot study in patients undergoing preoperative testing with the STOP-Bang questionnaire. “When we piloted this, there were over 200 patients who could have been sent to the outpatient sleep consult service, and we referred none,” Dr. Schwab said. “We are just starting to implement a program to screen them. We can treat these people for their sleep apnea and prevent chronic adverse sequelae associated with this disease.”
Both the inpatient and outpatient screening programs for sleep apnea are built within their electronic medical record. “Building this within your EMR requires effort, but it’s doable,” he said.
Dr. Schwab disclosed that he has received grants from the National Institutes of Health, ResMed, and Inspire Medical Systems.
EXPERT ANALYSIS FROM SLEEP 2019
Briefest flash of light can alter the human circadian system
SAN ANTONIO –
“This becomes a complementary way to help people with various kinds of circadian phase disorders,” the study’s first author, Jamie M. Zeitzer, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies. “Right now under ideal laboratory circumstances, you can change someone’s circadian timing by about 3 hours. That’s not happening in the real world; that’s what you do in a lab. That’s with very bright light for 6 hours and very dim light the rest of the time.”
In an effort to build on previous literature related to circadian phase shifting and continuous light exposure in rodents and in humans, Dr. Zeitzer, of the department of psychiatry and behavioral sciences at Stanford (Calif.) University, and colleagues enrolled 56 healthy young men and women in their 20s and 30s to take part in two parallel 16-day studies. For the first 14 days, study participants maintained a regular sleep/wake cycle at home as confirmed through actigraphy and sleep logs. They spent the final 2 days in a specialized time-isolation laboratory, during which the phase of the circadian pacemaker (salivary melatonin onset) was determined in constant routine conditions on evening one and two; light exposure occurred between these two phase determinations on night one.
Light exposure consisted of 1 hour of a sequence of light flashes delivered through a pair of modified welding goggles during enforced wake starting 2 hours after habitual bedtime. Flashes were presented every 15 seconds and varied either by duration (from 10 microseconds to 10 seconds at a fixed intensity of 2,200 lux) or intensity (a range between 3 and 9,500 lux, with a duration fixed at 2 milliseconds).
Dr. Zeitzer and colleagues observed no significant difference in the phase shift created between flashes that were given at 10 microseconds and flashes that were given at 10 seconds. “That’s a six-log unit variation,” he said during a presentation of the results at the meeting. “There are a million times more photons given in 10-second flashes over the hour than there are in the 10-microsecond flashes. Despite the fact that there are a million more photons, you get the exact same phase shift in both of these conditions. You need very little light in order to generate these phase shifts. You’re talking about less than 1 second of light stretched out over 1 hour.”
The researchers also observed that flash intensity showed a sigmoidal relationship with phase shifting, with a half-maximal shift observed at 8 lux and 90% of the maximal shift occurring after exposure to flashes as dim as 50 lux. None of the flash sequences caused acute suppression of melatonin.
“We did not anticipate the invariance, that anything from 10 microseconds to 10 seconds gives us no difference [in phase shifting],” Dr. Zeitzer said. “That was surprising. I thought that more light would be slightly less effective in terms of photons but still give a bigger [phase] shift, but that didn’t happen. In the intensity response, we see things are more sensitive to light flashes than they are to continuous light, which is also surprising. It implies that a different part of the eye is responding to light flashes than it is to continuous light. It provides more information about how to minimize the amount of light we’re using and maximize the amount of shift.”
Which photoreceptors underlie the responses remains unclear, he continued, “but given the characteristics of photoreceptors, our hypothesis is that flashes are being mediated through a cone cell response, while the response to continuous light is being primarily mediated through a melanopsin response. A future question we plan to investigate is, can selective sequential simultaneous activation of different photoreceptors create enhanced phase shifts?”
The study was supported by the Department of Defense. Dr. Zeitzer reported having no financial disclosures.
SAN ANTONIO –
“This becomes a complementary way to help people with various kinds of circadian phase disorders,” the study’s first author, Jamie M. Zeitzer, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies. “Right now under ideal laboratory circumstances, you can change someone’s circadian timing by about 3 hours. That’s not happening in the real world; that’s what you do in a lab. That’s with very bright light for 6 hours and very dim light the rest of the time.”
In an effort to build on previous literature related to circadian phase shifting and continuous light exposure in rodents and in humans, Dr. Zeitzer, of the department of psychiatry and behavioral sciences at Stanford (Calif.) University, and colleagues enrolled 56 healthy young men and women in their 20s and 30s to take part in two parallel 16-day studies. For the first 14 days, study participants maintained a regular sleep/wake cycle at home as confirmed through actigraphy and sleep logs. They spent the final 2 days in a specialized time-isolation laboratory, during which the phase of the circadian pacemaker (salivary melatonin onset) was determined in constant routine conditions on evening one and two; light exposure occurred between these two phase determinations on night one.
Light exposure consisted of 1 hour of a sequence of light flashes delivered through a pair of modified welding goggles during enforced wake starting 2 hours after habitual bedtime. Flashes were presented every 15 seconds and varied either by duration (from 10 microseconds to 10 seconds at a fixed intensity of 2,200 lux) or intensity (a range between 3 and 9,500 lux, with a duration fixed at 2 milliseconds).
Dr. Zeitzer and colleagues observed no significant difference in the phase shift created between flashes that were given at 10 microseconds and flashes that were given at 10 seconds. “That’s a six-log unit variation,” he said during a presentation of the results at the meeting. “There are a million times more photons given in 10-second flashes over the hour than there are in the 10-microsecond flashes. Despite the fact that there are a million more photons, you get the exact same phase shift in both of these conditions. You need very little light in order to generate these phase shifts. You’re talking about less than 1 second of light stretched out over 1 hour.”
The researchers also observed that flash intensity showed a sigmoidal relationship with phase shifting, with a half-maximal shift observed at 8 lux and 90% of the maximal shift occurring after exposure to flashes as dim as 50 lux. None of the flash sequences caused acute suppression of melatonin.
“We did not anticipate the invariance, that anything from 10 microseconds to 10 seconds gives us no difference [in phase shifting],” Dr. Zeitzer said. “That was surprising. I thought that more light would be slightly less effective in terms of photons but still give a bigger [phase] shift, but that didn’t happen. In the intensity response, we see things are more sensitive to light flashes than they are to continuous light, which is also surprising. It implies that a different part of the eye is responding to light flashes than it is to continuous light. It provides more information about how to minimize the amount of light we’re using and maximize the amount of shift.”
Which photoreceptors underlie the responses remains unclear, he continued, “but given the characteristics of photoreceptors, our hypothesis is that flashes are being mediated through a cone cell response, while the response to continuous light is being primarily mediated through a melanopsin response. A future question we plan to investigate is, can selective sequential simultaneous activation of different photoreceptors create enhanced phase shifts?”
The study was supported by the Department of Defense. Dr. Zeitzer reported having no financial disclosures.
SAN ANTONIO –
“This becomes a complementary way to help people with various kinds of circadian phase disorders,” the study’s first author, Jamie M. Zeitzer, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies. “Right now under ideal laboratory circumstances, you can change someone’s circadian timing by about 3 hours. That’s not happening in the real world; that’s what you do in a lab. That’s with very bright light for 6 hours and very dim light the rest of the time.”
In an effort to build on previous literature related to circadian phase shifting and continuous light exposure in rodents and in humans, Dr. Zeitzer, of the department of psychiatry and behavioral sciences at Stanford (Calif.) University, and colleagues enrolled 56 healthy young men and women in their 20s and 30s to take part in two parallel 16-day studies. For the first 14 days, study participants maintained a regular sleep/wake cycle at home as confirmed through actigraphy and sleep logs. They spent the final 2 days in a specialized time-isolation laboratory, during which the phase of the circadian pacemaker (salivary melatonin onset) was determined in constant routine conditions on evening one and two; light exposure occurred between these two phase determinations on night one.
Light exposure consisted of 1 hour of a sequence of light flashes delivered through a pair of modified welding goggles during enforced wake starting 2 hours after habitual bedtime. Flashes were presented every 15 seconds and varied either by duration (from 10 microseconds to 10 seconds at a fixed intensity of 2,200 lux) or intensity (a range between 3 and 9,500 lux, with a duration fixed at 2 milliseconds).
Dr. Zeitzer and colleagues observed no significant difference in the phase shift created between flashes that were given at 10 microseconds and flashes that were given at 10 seconds. “That’s a six-log unit variation,” he said during a presentation of the results at the meeting. “There are a million times more photons given in 10-second flashes over the hour than there are in the 10-microsecond flashes. Despite the fact that there are a million more photons, you get the exact same phase shift in both of these conditions. You need very little light in order to generate these phase shifts. You’re talking about less than 1 second of light stretched out over 1 hour.”
The researchers also observed that flash intensity showed a sigmoidal relationship with phase shifting, with a half-maximal shift observed at 8 lux and 90% of the maximal shift occurring after exposure to flashes as dim as 50 lux. None of the flash sequences caused acute suppression of melatonin.
“We did not anticipate the invariance, that anything from 10 microseconds to 10 seconds gives us no difference [in phase shifting],” Dr. Zeitzer said. “That was surprising. I thought that more light would be slightly less effective in terms of photons but still give a bigger [phase] shift, but that didn’t happen. In the intensity response, we see things are more sensitive to light flashes than they are to continuous light, which is also surprising. It implies that a different part of the eye is responding to light flashes than it is to continuous light. It provides more information about how to minimize the amount of light we’re using and maximize the amount of shift.”
Which photoreceptors underlie the responses remains unclear, he continued, “but given the characteristics of photoreceptors, our hypothesis is that flashes are being mediated through a cone cell response, while the response to continuous light is being primarily mediated through a melanopsin response. A future question we plan to investigate is, can selective sequential simultaneous activation of different photoreceptors create enhanced phase shifts?”
The study was supported by the Department of Defense. Dr. Zeitzer reported having no financial disclosures.
REPORTING FROM SLEEP 2019
Key clinical point: When distributed as flashes, the human circadian system can be phase shifted by extraordinarily brief and dim light.
Major finding: The researchers observed no significant difference in the phase shift created between flashes that were given at 10 microseconds and flashes that were given at 10 seconds.
Study details: Two parallel 16-day studies involving 56 healthy men and women in their 20s and 30s.
Disclosures: The study was supported by the Department of Defense. Dr. Zeitzer reported having no financial disclosures.
Sleep quality linked to gut microbiome biodiversity
SAN ANTONIO – , according to results from a population sample of adults.
“These findings are preliminary and very early in the growth of this field,” lead study author Erika W. Hagen, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies
According to Dr. Hagen, an epidemiologist at the University of Wisconsin–Madison, experimental studies in mice have shown that disturbed sleep is associated with gut microbiota composition, and a few small experimental studies in humans have found associations between curtailed sleep and measures of gut microbiota richness and diversity.
In an effort to examine associations of subjectively and objectively assessed sleep metrics with indices of gut microbiome richness and diversity, Dr. Hagen and colleagues assessed 482 individuals who participated in the Survey of the Health of Wisconsin and completed in-home study visits in 2016. They provided fecal samples, participated in a week-long wrist actigraphy protocol to measure sleep, and completed questionnaires about sleep, diet, and other health and sociodemographic factors, and an assessment of physical activity by waist-worn actigraphy.
Metrics of species richness included the Chao1 and the ACE, which estimate the number of species. Metrics of the diversity of the gut microbiome included the Inverse Simpson index and the Shannon index. All metrics were regressed on self-reported sleep duration, extreme daytime sleepiness, the Epworth Sleepiness Scale (ESS), and actigraphy-measured sleep duration and wake after sleep onset (WASO). Next, the researchers estimated associations between each of the sleep and diversity measures separately, adjusting for age and sex and then additionally adjusting for body mass index, moderate-vigorous physical activity, and dietary fat and fiber.
The mean age of the 482 subjects was 56 years, 57% were female, and the mean body mass index was 30 kg/m2. After the researchers adjusted for gender and age, they found that greater WASO was statistically significantly associated with lower richness and alpha diversity (P less than .05). These associations remained significant on the Chao1 measure and borderline significant on the ACE and Shannon measures after further adjustment for BMI, physical activity, and dietary fiber and fat. For example, 60 minutes greater WASO was associated with an approximate 26% population standard deviation reduction in microbial richness as measured by Chao1. In fully-adjusted models, greater daytime sleepiness was associated with lower richness and diversity on all indices (P = .01-.06). The ESS and sleep duration were not associated with microbiota richness or diversity.
“Our results suggest that sleep quality is associated with gut microbiome richness and diversity,” Dr. Hagen said. “Our results are in line with other research on this topic. What’s interesting is how your sleep over a period of time is affecting these measures of your microbiome. That’s something people can do something about with [eating] habits over time. What would be great is to collect longitudinal data so that you could characterize sleep over a longer period of time, but also so you could measure the microbiome at different time points to see what’s changing with changes in sleep. That would be interesting to untangle.”
She acknowledged certain limitations of the study, including the small sample size and the cross-sectional design. The study was supported by the University of Wisconsin School of Medicine and Public Health through the Wisconsin Partnership Program.
SOURCE: Hagen EW et al. SLEEP 2019, Abstract 0106.
SAN ANTONIO – , according to results from a population sample of adults.
“These findings are preliminary and very early in the growth of this field,” lead study author Erika W. Hagen, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies
According to Dr. Hagen, an epidemiologist at the University of Wisconsin–Madison, experimental studies in mice have shown that disturbed sleep is associated with gut microbiota composition, and a few small experimental studies in humans have found associations between curtailed sleep and measures of gut microbiota richness and diversity.
In an effort to examine associations of subjectively and objectively assessed sleep metrics with indices of gut microbiome richness and diversity, Dr. Hagen and colleagues assessed 482 individuals who participated in the Survey of the Health of Wisconsin and completed in-home study visits in 2016. They provided fecal samples, participated in a week-long wrist actigraphy protocol to measure sleep, and completed questionnaires about sleep, diet, and other health and sociodemographic factors, and an assessment of physical activity by waist-worn actigraphy.
Metrics of species richness included the Chao1 and the ACE, which estimate the number of species. Metrics of the diversity of the gut microbiome included the Inverse Simpson index and the Shannon index. All metrics were regressed on self-reported sleep duration, extreme daytime sleepiness, the Epworth Sleepiness Scale (ESS), and actigraphy-measured sleep duration and wake after sleep onset (WASO). Next, the researchers estimated associations between each of the sleep and diversity measures separately, adjusting for age and sex and then additionally adjusting for body mass index, moderate-vigorous physical activity, and dietary fat and fiber.
The mean age of the 482 subjects was 56 years, 57% were female, and the mean body mass index was 30 kg/m2. After the researchers adjusted for gender and age, they found that greater WASO was statistically significantly associated with lower richness and alpha diversity (P less than .05). These associations remained significant on the Chao1 measure and borderline significant on the ACE and Shannon measures after further adjustment for BMI, physical activity, and dietary fiber and fat. For example, 60 minutes greater WASO was associated with an approximate 26% population standard deviation reduction in microbial richness as measured by Chao1. In fully-adjusted models, greater daytime sleepiness was associated with lower richness and diversity on all indices (P = .01-.06). The ESS and sleep duration were not associated with microbiota richness or diversity.
“Our results suggest that sleep quality is associated with gut microbiome richness and diversity,” Dr. Hagen said. “Our results are in line with other research on this topic. What’s interesting is how your sleep over a period of time is affecting these measures of your microbiome. That’s something people can do something about with [eating] habits over time. What would be great is to collect longitudinal data so that you could characterize sleep over a longer period of time, but also so you could measure the microbiome at different time points to see what’s changing with changes in sleep. That would be interesting to untangle.”
She acknowledged certain limitations of the study, including the small sample size and the cross-sectional design. The study was supported by the University of Wisconsin School of Medicine and Public Health through the Wisconsin Partnership Program.
SOURCE: Hagen EW et al. SLEEP 2019, Abstract 0106.
SAN ANTONIO – , according to results from a population sample of adults.
“These findings are preliminary and very early in the growth of this field,” lead study author Erika W. Hagen, PhD, said during an interview at the annual meeting of the Associated Professional Sleep Societies
According to Dr. Hagen, an epidemiologist at the University of Wisconsin–Madison, experimental studies in mice have shown that disturbed sleep is associated with gut microbiota composition, and a few small experimental studies in humans have found associations between curtailed sleep and measures of gut microbiota richness and diversity.
In an effort to examine associations of subjectively and objectively assessed sleep metrics with indices of gut microbiome richness and diversity, Dr. Hagen and colleagues assessed 482 individuals who participated in the Survey of the Health of Wisconsin and completed in-home study visits in 2016. They provided fecal samples, participated in a week-long wrist actigraphy protocol to measure sleep, and completed questionnaires about sleep, diet, and other health and sociodemographic factors, and an assessment of physical activity by waist-worn actigraphy.
Metrics of species richness included the Chao1 and the ACE, which estimate the number of species. Metrics of the diversity of the gut microbiome included the Inverse Simpson index and the Shannon index. All metrics were regressed on self-reported sleep duration, extreme daytime sleepiness, the Epworth Sleepiness Scale (ESS), and actigraphy-measured sleep duration and wake after sleep onset (WASO). Next, the researchers estimated associations between each of the sleep and diversity measures separately, adjusting for age and sex and then additionally adjusting for body mass index, moderate-vigorous physical activity, and dietary fat and fiber.
The mean age of the 482 subjects was 56 years, 57% were female, and the mean body mass index was 30 kg/m2. After the researchers adjusted for gender and age, they found that greater WASO was statistically significantly associated with lower richness and alpha diversity (P less than .05). These associations remained significant on the Chao1 measure and borderline significant on the ACE and Shannon measures after further adjustment for BMI, physical activity, and dietary fiber and fat. For example, 60 minutes greater WASO was associated with an approximate 26% population standard deviation reduction in microbial richness as measured by Chao1. In fully-adjusted models, greater daytime sleepiness was associated with lower richness and diversity on all indices (P = .01-.06). The ESS and sleep duration were not associated with microbiota richness or diversity.
“Our results suggest that sleep quality is associated with gut microbiome richness and diversity,” Dr. Hagen said. “Our results are in line with other research on this topic. What’s interesting is how your sleep over a period of time is affecting these measures of your microbiome. That’s something people can do something about with [eating] habits over time. What would be great is to collect longitudinal data so that you could characterize sleep over a longer period of time, but also so you could measure the microbiome at different time points to see what’s changing with changes in sleep. That would be interesting to untangle.”
She acknowledged certain limitations of the study, including the small sample size and the cross-sectional design. The study was supported by the University of Wisconsin School of Medicine and Public Health through the Wisconsin Partnership Program.
SOURCE: Hagen EW et al. SLEEP 2019, Abstract 0106.
REPORTING FROM SLEEP 2019
Key clinical point: Better quality of sleep, but not duration of sleep, was associated with greater species richness and diversity of the gut microbiome.
Major finding: In fully adjusted models, greater daytime sleepiness was associated with lower richness and diversity of the gut microbiome on all indices (P = .01-.06).
Study details: An assessment of 482 individuals who participated in the Survey of the Health of Wisconsin.
Disclosures: The study was supported by the University of Wisconsin School of Medicine and Public Health through the Wisconsin Partnership Program.
Source: Hagen EW et al. SLEEP 2019, Abstract 0106.