User login
What’s in a Name: Defining Difficult-to-Treat axSpA and PsA
Despite an expanding arsenal of disease-modifying antirheumatic drugs (DMARDs), many patients with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) still struggle to reach remission even after trying multiple advanced treatments.
Now, international groups of experts are working to better define these “difficult-to-treat” patients to both inform care and improve selection of participants for future clinical trials.
“The idea is rather simple, and the need is relatively ubiquitous,” Denis Poddubnyy, MD, of the Charité – Universitätsmedizin Berlin and the German Rheumatism Research Center Berlin, both in Berlin, Germany, said in an interview. He is the co-primary investigator for the ongoing Assessment of SpondyloArthritis International Society (ASAS) project to develop a consensus definition of difficult-to-treat axSpA.
According to ASAS, only 40%-50% of patients with axSpA achieve a 40% improvement in ASAS response criteria (ASAS40), and few (10%-20%) achieve remission in the first 4-6 months of treatment.
“If you look into current clinical guidelines, you will see that there is no clear guidance,” on how to manage these patients, Dr. Poddubnyy continued. “In other similar recommendations for the treatment of axSpA, the only point which is clearly made with regards to nonresponders to effective anti-inflammatory treatment is to ‘check the diagnosis.’”
Multiple Reasons for Nonresponse
“While the term difficult-to-treat can refer to refractory disease, that is not the only reason why a patient might not be responding to medication. In fact, it’s likely that truly biologically refractory disease makes up only a fraction of cases that respond inadequately to treatment,” said Shikha Singla, MD, who directs the psoriatic arthritis program at the Medical College of Wisconsin in Milwaukee. She is also involved with the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) initiative to define Difficult-to-Treat and Complex-to-Manage PsA.
“Apart from the persistent articular and periarticular inflammation, there could be multiple noninflammatory factors that may be contributing to this treatment-resistant disease, including comorbid conditions such as obesity, cardiovascular disease, fibromyalgia, and even social factors such as limited access to medications,” she told this news organization. “Given these complexities, it is a matter of supreme importance to recognize and carefully delineate the elements that contribute to treatment refractory disease: Is it truly the inflammation, or are there noninflammatory components that are causing the treatment failure, or a combination of the two?”
Other contributing factors could be depression, hypersensitization, and comorbidities that prevent certain treatment approaches, added Fabian Proft, MD, also of Charité – Universitätsmedizin Berlin. Dr. Proft discussed these difficult-to-treat definition efforts at the recent Spondyloarthritis Research and Treatment Network (SPARTAN) annual meeting held in Cleveland. Patients also might not be taking their medication regularly and may be seeking alternative medicine approaches, he said.
“There is a quite clear consensus within the community” that differentiation between these two groups is needed, Dr. Proft said.
The Definitions
Terminology for these two groups can vary by professional society. The European Alliance of Associations for Rheumatology (EULAR) published a definition for “difficult-to-treat” rheumatoid arthritis (RA) that includes cases with “both inflammatory activity and/or noninflammatory complaints.”
The definition includes three criteria:
1) Treatment according to EULAR recommendation and failure of at least two biologic DMARDs (bDMARDs) or targeted synthetic DMARDs (tsDMARDs) (with different mechanisms of action) after failing conventional synthetic DMARD therapy (unless contraindicated)
2) Signs suggestive of active/progressive disease, including at least one of the following:
- Moderate disease activity (according to validated composite measures including joint counts)
- Signs (including acute phase reactants and imaging) and/or symptoms suggestive of active disease, whether joint-related or other
- Inability to taper glucocorticoid treatment
- Rapid radiographic progression (with or without signs of active disease)
- RA symptoms that are causing a reduction in quality of life
3) Symptom/sign management perceived as problematic by the rheumatologist or the patient
All three criteria must be met.
Both GRAPPA and ASAS plan to use the term “difficult-to-treat” or “treatment refractory” to describe true biologically refractory inflammatory disease and are categorizing the larger, heterogeneous group of nonresponders as “difficult-to-manage” (ASAS) or “complex-to-manage” (GRAPPA).
According to Dr. Poddubnyy, the agreed ASAS definition of difficult-to-manage has several similarities with EULAR’s RA definition, including three pillars:
- Treatment according to existing recommendations and failure of at least two different bDMARDs or tsDMARDs with different mechanisms
- Having signs and symptoms of disease (measured by high disease activity by certain disease activity indexes, persistently elevated C-reactive protein, inflammation on MRI, or rapid radiographic spinal progression)
- Symptoms/signs of disease that are considered problematic by the provider or patient
The definition was approved in January, and the manuscript is in the works, Dr. Poddubnyy said.
The GRAPPA project on PsA is still in its early stages, which so far has included a comprehensive literature review as well as a survey of GRAPPA members across 47 countries. The group is generally in agreement that two separate definitions for nonresponse to treatment are necessary, and that the “difficult-to-treat” definition — which identifies true refractory disease — should include objective signs of inflammation, Dr. Singla said.
Looking Forward
The next step of the ASAS project is to “define the pathway” from difficult-to-manage axSpA to treatment refractory disease, Dr. Poddubnyy said.
“What should be ruled out in order to exclude so-called noninflammatory causes of pain?” he continued. “It will require some Delphi exercises and [a] consensus approach.”
Proft anticipates that this treatment refractory definition in both axSpA and PsA will be most useful in research, rather than clinical practice.
“It is really important to have unified definition criteria to shape as homogeneous a cohort as possible,” he said, for future clinical trials in this population.
On the other hand, the complex/difficult-to-manage definition may be more useful for clinical practice, Dr. Proft thought.
“If you see a patient not responding to treatment, the easiest thing you can do would be to change treatment,” like swapping one biologic for another, Dr. Poddubnyy added, “but this would not be the right approach in every patient.” One goal of these initiatives is to give guidance on “what things should be looked after or excluded before you conclude this is biological [nonresponse],” he said.
Dr. Singla consults for AbbVie, Janssen, and UCB and received research funding from Eli Lilly. Dr. Poddubnyy disclosed serving as a speaker, consultant, and/or research grant recipient for multiple companies including AbbVie, Lilly, Merck Sharp and Dohme, Novartis, Pfizer, GlaxoSmithKline, Novartis, and UCB. Dr. Proft reported receiving research grants, consultant fees, or support for attending meetings and/or travel from Amgen, AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Merck Sharp and Dohme, Novartis, Pfizer, Roche, UCB, Medscape Medical News, Galapagos, and Hexal. Dr. Proft also participants on a data safety monitoring board or advisory board for AbbVie, Celgene, Janssen, Novartis, and UCB.
A version of this article appeared on Medscape.com.
Despite an expanding arsenal of disease-modifying antirheumatic drugs (DMARDs), many patients with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) still struggle to reach remission even after trying multiple advanced treatments.
Now, international groups of experts are working to better define these “difficult-to-treat” patients to both inform care and improve selection of participants for future clinical trials.
“The idea is rather simple, and the need is relatively ubiquitous,” Denis Poddubnyy, MD, of the Charité – Universitätsmedizin Berlin and the German Rheumatism Research Center Berlin, both in Berlin, Germany, said in an interview. He is the co-primary investigator for the ongoing Assessment of SpondyloArthritis International Society (ASAS) project to develop a consensus definition of difficult-to-treat axSpA.
According to ASAS, only 40%-50% of patients with axSpA achieve a 40% improvement in ASAS response criteria (ASAS40), and few (10%-20%) achieve remission in the first 4-6 months of treatment.
“If you look into current clinical guidelines, you will see that there is no clear guidance,” on how to manage these patients, Dr. Poddubnyy continued. “In other similar recommendations for the treatment of axSpA, the only point which is clearly made with regards to nonresponders to effective anti-inflammatory treatment is to ‘check the diagnosis.’”
Multiple Reasons for Nonresponse
“While the term difficult-to-treat can refer to refractory disease, that is not the only reason why a patient might not be responding to medication. In fact, it’s likely that truly biologically refractory disease makes up only a fraction of cases that respond inadequately to treatment,” said Shikha Singla, MD, who directs the psoriatic arthritis program at the Medical College of Wisconsin in Milwaukee. She is also involved with the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) initiative to define Difficult-to-Treat and Complex-to-Manage PsA.
“Apart from the persistent articular and periarticular inflammation, there could be multiple noninflammatory factors that may be contributing to this treatment-resistant disease, including comorbid conditions such as obesity, cardiovascular disease, fibromyalgia, and even social factors such as limited access to medications,” she told this news organization. “Given these complexities, it is a matter of supreme importance to recognize and carefully delineate the elements that contribute to treatment refractory disease: Is it truly the inflammation, or are there noninflammatory components that are causing the treatment failure, or a combination of the two?”
Other contributing factors could be depression, hypersensitization, and comorbidities that prevent certain treatment approaches, added Fabian Proft, MD, also of Charité – Universitätsmedizin Berlin. Dr. Proft discussed these difficult-to-treat definition efforts at the recent Spondyloarthritis Research and Treatment Network (SPARTAN) annual meeting held in Cleveland. Patients also might not be taking their medication regularly and may be seeking alternative medicine approaches, he said.
“There is a quite clear consensus within the community” that differentiation between these two groups is needed, Dr. Proft said.
The Definitions
Terminology for these two groups can vary by professional society. The European Alliance of Associations for Rheumatology (EULAR) published a definition for “difficult-to-treat” rheumatoid arthritis (RA) that includes cases with “both inflammatory activity and/or noninflammatory complaints.”
The definition includes three criteria:
1) Treatment according to EULAR recommendation and failure of at least two biologic DMARDs (bDMARDs) or targeted synthetic DMARDs (tsDMARDs) (with different mechanisms of action) after failing conventional synthetic DMARD therapy (unless contraindicated)
2) Signs suggestive of active/progressive disease, including at least one of the following:
- Moderate disease activity (according to validated composite measures including joint counts)
- Signs (including acute phase reactants and imaging) and/or symptoms suggestive of active disease, whether joint-related or other
- Inability to taper glucocorticoid treatment
- Rapid radiographic progression (with or without signs of active disease)
- RA symptoms that are causing a reduction in quality of life
3) Symptom/sign management perceived as problematic by the rheumatologist or the patient
All three criteria must be met.
Both GRAPPA and ASAS plan to use the term “difficult-to-treat” or “treatment refractory” to describe true biologically refractory inflammatory disease and are categorizing the larger, heterogeneous group of nonresponders as “difficult-to-manage” (ASAS) or “complex-to-manage” (GRAPPA).
According to Dr. Poddubnyy, the agreed ASAS definition of difficult-to-manage has several similarities with EULAR’s RA definition, including three pillars:
- Treatment according to existing recommendations and failure of at least two different bDMARDs or tsDMARDs with different mechanisms
- Having signs and symptoms of disease (measured by high disease activity by certain disease activity indexes, persistently elevated C-reactive protein, inflammation on MRI, or rapid radiographic spinal progression)
- Symptoms/signs of disease that are considered problematic by the provider or patient
The definition was approved in January, and the manuscript is in the works, Dr. Poddubnyy said.
The GRAPPA project on PsA is still in its early stages, which so far has included a comprehensive literature review as well as a survey of GRAPPA members across 47 countries. The group is generally in agreement that two separate definitions for nonresponse to treatment are necessary, and that the “difficult-to-treat” definition — which identifies true refractory disease — should include objective signs of inflammation, Dr. Singla said.
Looking Forward
The next step of the ASAS project is to “define the pathway” from difficult-to-manage axSpA to treatment refractory disease, Dr. Poddubnyy said.
“What should be ruled out in order to exclude so-called noninflammatory causes of pain?” he continued. “It will require some Delphi exercises and [a] consensus approach.”
Proft anticipates that this treatment refractory definition in both axSpA and PsA will be most useful in research, rather than clinical practice.
“It is really important to have unified definition criteria to shape as homogeneous a cohort as possible,” he said, for future clinical trials in this population.
On the other hand, the complex/difficult-to-manage definition may be more useful for clinical practice, Dr. Proft thought.
“If you see a patient not responding to treatment, the easiest thing you can do would be to change treatment,” like swapping one biologic for another, Dr. Poddubnyy added, “but this would not be the right approach in every patient.” One goal of these initiatives is to give guidance on “what things should be looked after or excluded before you conclude this is biological [nonresponse],” he said.
Dr. Singla consults for AbbVie, Janssen, and UCB and received research funding from Eli Lilly. Dr. Poddubnyy disclosed serving as a speaker, consultant, and/or research grant recipient for multiple companies including AbbVie, Lilly, Merck Sharp and Dohme, Novartis, Pfizer, GlaxoSmithKline, Novartis, and UCB. Dr. Proft reported receiving research grants, consultant fees, or support for attending meetings and/or travel from Amgen, AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Merck Sharp and Dohme, Novartis, Pfizer, Roche, UCB, Medscape Medical News, Galapagos, and Hexal. Dr. Proft also participants on a data safety monitoring board or advisory board for AbbVie, Celgene, Janssen, Novartis, and UCB.
A version of this article appeared on Medscape.com.
Despite an expanding arsenal of disease-modifying antirheumatic drugs (DMARDs), many patients with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) still struggle to reach remission even after trying multiple advanced treatments.
Now, international groups of experts are working to better define these “difficult-to-treat” patients to both inform care and improve selection of participants for future clinical trials.
“The idea is rather simple, and the need is relatively ubiquitous,” Denis Poddubnyy, MD, of the Charité – Universitätsmedizin Berlin and the German Rheumatism Research Center Berlin, both in Berlin, Germany, said in an interview. He is the co-primary investigator for the ongoing Assessment of SpondyloArthritis International Society (ASAS) project to develop a consensus definition of difficult-to-treat axSpA.
According to ASAS, only 40%-50% of patients with axSpA achieve a 40% improvement in ASAS response criteria (ASAS40), and few (10%-20%) achieve remission in the first 4-6 months of treatment.
“If you look into current clinical guidelines, you will see that there is no clear guidance,” on how to manage these patients, Dr. Poddubnyy continued. “In other similar recommendations for the treatment of axSpA, the only point which is clearly made with regards to nonresponders to effective anti-inflammatory treatment is to ‘check the diagnosis.’”
Multiple Reasons for Nonresponse
“While the term difficult-to-treat can refer to refractory disease, that is not the only reason why a patient might not be responding to medication. In fact, it’s likely that truly biologically refractory disease makes up only a fraction of cases that respond inadequately to treatment,” said Shikha Singla, MD, who directs the psoriatic arthritis program at the Medical College of Wisconsin in Milwaukee. She is also involved with the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) initiative to define Difficult-to-Treat and Complex-to-Manage PsA.
“Apart from the persistent articular and periarticular inflammation, there could be multiple noninflammatory factors that may be contributing to this treatment-resistant disease, including comorbid conditions such as obesity, cardiovascular disease, fibromyalgia, and even social factors such as limited access to medications,” she told this news organization. “Given these complexities, it is a matter of supreme importance to recognize and carefully delineate the elements that contribute to treatment refractory disease: Is it truly the inflammation, or are there noninflammatory components that are causing the treatment failure, or a combination of the two?”
Other contributing factors could be depression, hypersensitization, and comorbidities that prevent certain treatment approaches, added Fabian Proft, MD, also of Charité – Universitätsmedizin Berlin. Dr. Proft discussed these difficult-to-treat definition efforts at the recent Spondyloarthritis Research and Treatment Network (SPARTAN) annual meeting held in Cleveland. Patients also might not be taking their medication regularly and may be seeking alternative medicine approaches, he said.
“There is a quite clear consensus within the community” that differentiation between these two groups is needed, Dr. Proft said.
The Definitions
Terminology for these two groups can vary by professional society. The European Alliance of Associations for Rheumatology (EULAR) published a definition for “difficult-to-treat” rheumatoid arthritis (RA) that includes cases with “both inflammatory activity and/or noninflammatory complaints.”
The definition includes three criteria:
1) Treatment according to EULAR recommendation and failure of at least two biologic DMARDs (bDMARDs) or targeted synthetic DMARDs (tsDMARDs) (with different mechanisms of action) after failing conventional synthetic DMARD therapy (unless contraindicated)
2) Signs suggestive of active/progressive disease, including at least one of the following:
- Moderate disease activity (according to validated composite measures including joint counts)
- Signs (including acute phase reactants and imaging) and/or symptoms suggestive of active disease, whether joint-related or other
- Inability to taper glucocorticoid treatment
- Rapid radiographic progression (with or without signs of active disease)
- RA symptoms that are causing a reduction in quality of life
3) Symptom/sign management perceived as problematic by the rheumatologist or the patient
All three criteria must be met.
Both GRAPPA and ASAS plan to use the term “difficult-to-treat” or “treatment refractory” to describe true biologically refractory inflammatory disease and are categorizing the larger, heterogeneous group of nonresponders as “difficult-to-manage” (ASAS) or “complex-to-manage” (GRAPPA).
According to Dr. Poddubnyy, the agreed ASAS definition of difficult-to-manage has several similarities with EULAR’s RA definition, including three pillars:
- Treatment according to existing recommendations and failure of at least two different bDMARDs or tsDMARDs with different mechanisms
- Having signs and symptoms of disease (measured by high disease activity by certain disease activity indexes, persistently elevated C-reactive protein, inflammation on MRI, or rapid radiographic spinal progression)
- Symptoms/signs of disease that are considered problematic by the provider or patient
The definition was approved in January, and the manuscript is in the works, Dr. Poddubnyy said.
The GRAPPA project on PsA is still in its early stages, which so far has included a comprehensive literature review as well as a survey of GRAPPA members across 47 countries. The group is generally in agreement that two separate definitions for nonresponse to treatment are necessary, and that the “difficult-to-treat” definition — which identifies true refractory disease — should include objective signs of inflammation, Dr. Singla said.
Looking Forward
The next step of the ASAS project is to “define the pathway” from difficult-to-manage axSpA to treatment refractory disease, Dr. Poddubnyy said.
“What should be ruled out in order to exclude so-called noninflammatory causes of pain?” he continued. “It will require some Delphi exercises and [a] consensus approach.”
Proft anticipates that this treatment refractory definition in both axSpA and PsA will be most useful in research, rather than clinical practice.
“It is really important to have unified definition criteria to shape as homogeneous a cohort as possible,” he said, for future clinical trials in this population.
On the other hand, the complex/difficult-to-manage definition may be more useful for clinical practice, Dr. Proft thought.
“If you see a patient not responding to treatment, the easiest thing you can do would be to change treatment,” like swapping one biologic for another, Dr. Poddubnyy added, “but this would not be the right approach in every patient.” One goal of these initiatives is to give guidance on “what things should be looked after or excluded before you conclude this is biological [nonresponse],” he said.
Dr. Singla consults for AbbVie, Janssen, and UCB and received research funding from Eli Lilly. Dr. Poddubnyy disclosed serving as a speaker, consultant, and/or research grant recipient for multiple companies including AbbVie, Lilly, Merck Sharp and Dohme, Novartis, Pfizer, GlaxoSmithKline, Novartis, and UCB. Dr. Proft reported receiving research grants, consultant fees, or support for attending meetings and/or travel from Amgen, AbbVie, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, Merck Sharp and Dohme, Novartis, Pfizer, Roche, UCB, Medscape Medical News, Galapagos, and Hexal. Dr. Proft also participants on a data safety monitoring board or advisory board for AbbVie, Celgene, Janssen, Novartis, and UCB.
A version of this article appeared on Medscape.com.
FROM SPARTAN 2024
Clear Coverage Preference for Humira Over Biosimilars Seen in Most Medicare Part D Plans
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.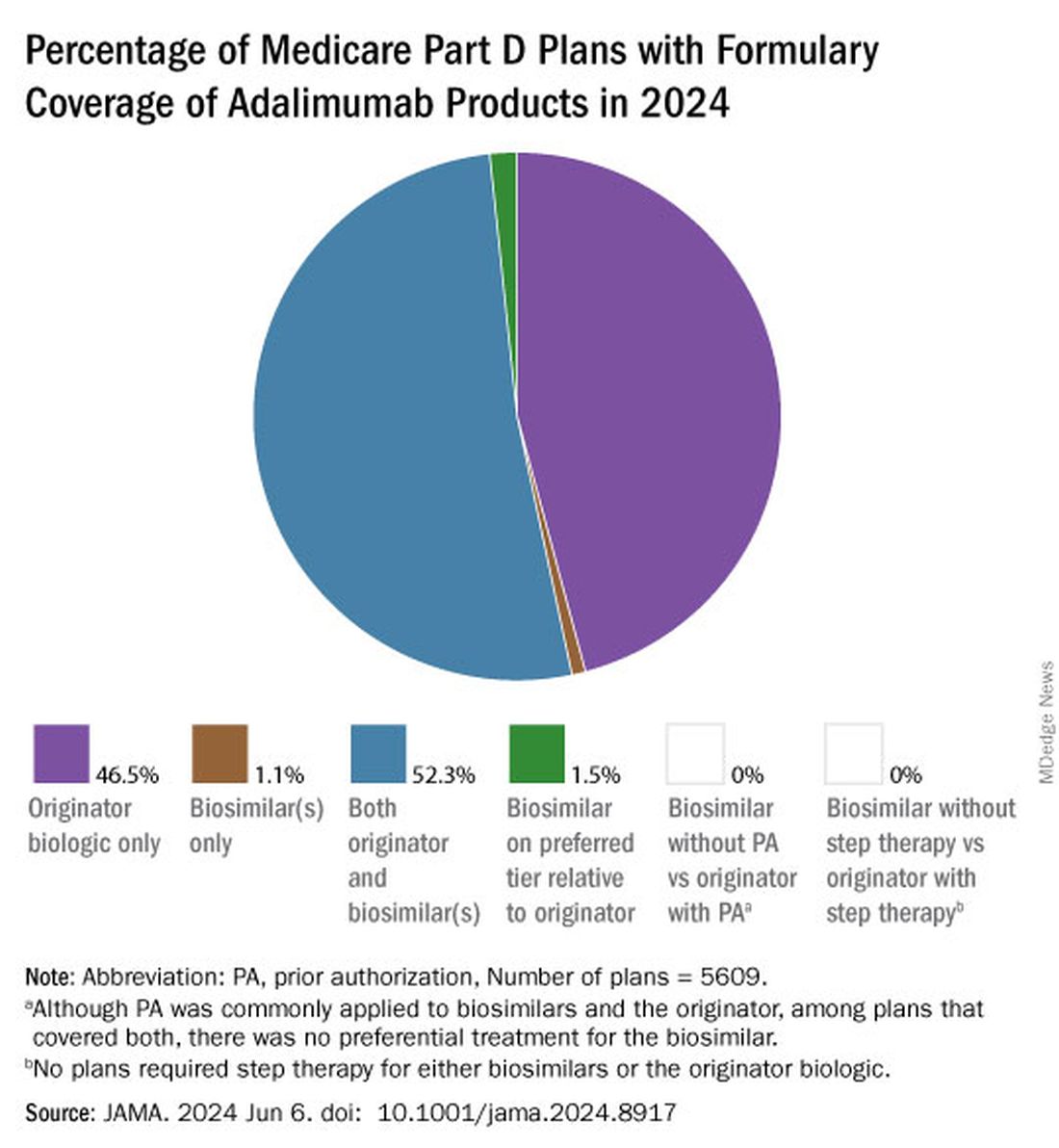
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
Despite the influx of adalimumab biosimilars entering the market in 2023, Humira remains on top.
As of January 2024, both high and low concentrations of Humira, the originator adalimumab product, are nearly universally covered by Medicare Part D plans, while only half of these plans covered adalimumab biosimilars, according to a new research letter published online on June 6, 2024, in JAMA.
Of the plans that covered both, only 1.5% had lower-tier placement for biosimilars.
“This study of formulary coverage helps explain limited uptake of adalimumab biosimilars,” wrote the authors, led by Matthew J. Klebanoff, MD, of the University of Pennsylvania, Philadelphia. “Subpar biosimilar adoption will not only undermine their potential to reduce spending but also may deter investments in biosimilar development.”
The analysis included the formulary and enrollment files for 5609 Medicare Part D plans, representing 44.4 million beneficiaries. Drug list prices and whole acquisition costs (WAC) were pulled from the Red Book database, which provides prices for prescription and over-the-counter drugs as well as medical devices and supplies.
Nearly all (98.9%) of Part D plans covered the high-concentration (100 mg/mL) version of adalimumab with a WAC of $6923. This higher concentration is the most popular formulation of the drug, making up an estimated 85% of prescriptions. By comparison, 26.8% of plans covered the high-concentration version of adalimumab-adaz (Hyrimoz), with a WAC 5% less than the reference product.
The unbranded version of adalimumab-adaz, sold at an 81% discount from the reference product, was covered by 13% of plans. Only 4.6% of plans covered high-concentration adalimumab-bwwd (Hadlima), manufactured by Samsung Bioepis.
In January 2024, no high-concentration adalimumab biosimilar had been granted interchangeability status by the US Food and Drug Administration (FDA). Adalimumab-ryvk (Simlandi) was the first biosimilar to receive this designation and was launched in late May 2024.
Coverage for the lower concentration of adalimumab was nearly universal (98.7% of plans). About half of the plans (50.7%) covered adalimumab-adbm (Cyltezo) at a 5% discount. Adalimumab-adbm (Boehringer Ingelheim) was the first interchangeable Humira biosimilar approved by the FDA, but it is only interchangeable with the less popular, lower concentration formulation of adalimumab.
All other biosimilars were covered by less than 5% of Medicare Part D plans, even with some having a WAC 86% below Humira.
Few plans (1.5%) had biosimilars on preferred tiers compared with the reference product, and no plans used prior authorization to incentivize use of biosimilars. Most plans preferred the higher-priced version of adalimumab biosimilars, which appeals to pharmacy benefit managers who can therefore receive higher rebates, the authors noted.
“Ultimately, biosimilars’ true effect on spending will depend not on their list price but rather on their net price (after rebates) and their influence on originator biologics’ net price,” they wrote. They pointed to the 38% drop in Humira’s annual net price at the end of 2023 compared with the prior year.
“Despite this price decrease, biosimilars offer far greater potential savings: Several adalimumab biosimilars have list prices that are less than half of Humira’s net price,” the authors continued, and encouraged policy makers to mandate coverage for these lower-priced options.
Dr. Klebanoff was supported by a grant from the Health Resources and Services Administration. Two coauthors were supported by a grant from the National Institute on Aging. One author reported receiving consulting fees from AbbVie, which manufactures Humira.
A version of this article appeared on Medscape.com .
FROM JAMA
Spondyloarthritis Screening Study Finds ‘High Burden of Need’ in Patients With Inflammatory Bowel Disease
More than 40% of patients with inflammatory bowel disease (IBD) screened positive for joint pain symptomatic of spondyloarthritis (SpA), according to a new study.
Of these patients, 75% did not have any history of arthritis.
“What we know is that a substantial proportion of patients with IBD do report musculoskeletal symptoms, and inflammatory back pain stands out as being one of the more frequent symptoms reported,” said Reem Jan, MBBS, a rheumatologist at the University of Chicago Medicine. She presented the study findings during the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN) in Cleveland.
“Yet a minority of these patients are evaluated by rheumatologists. So that suggests there’s a high burden of need in the IBD population to have this joint pain evaluated and addressed,” she said during her presentation.
She presented preliminary data from an ongoing project to better understand the prevalence of inflammatory arthritis in IBD — estimates range from 17% to 39%— and the risk factors for developing arthritis in this patient population.
Study Details
Researchers enrolled patients from outpatient gastroenterology clinics or procedure units at NYU Langone Health, New York City; Brigham and Women’s Hospital, Boston; University of Colorado Anschutz Medical Campus, Aurora, Colorado; Mayo Clinic, Rochester, Minnesota; University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago; and Icahn School of Medicine at Mount Sinai, New York City. Additional patients were recruited from Mercy Health, a community health system in Ohio.
Upon entry into the study, participants completed a survey documenting their history with joint pain. The survey combined questions from the DETAIL and the IBIS questionnaires.
Between January 2021 and December 2022, 669 patients joined the study. In total, 41% of patients (n = 275) screened positive.
“What really stood out to us was that of all the positive screens, only about a quarter of those patients were known to have SpA,” Dr. Jan said during her presentation. “[This] means 75% of the patients who screened positive were not known to have any type of arthritic disease.”
In addition, only 24% (n = 65) of all patients who screened positive — including those with a SpA diagnosis — had seen a rheumatologist in the previous year.
Among these patients, inflammatory back pain was the most commonly reported symptom, followed by painful swelling of peripheral joints and heel pain.
Excluding patients with a SpA diagnosis, researchers also investigated which characteristics were associated with a higher likelihood of screening positive in the questionnaire. The analysis, including 588 patients, identified the following risk factors:
- Female sex: Odds ratio (OR), 2.0; 95% CI, 1.4-2.9
- Older age: OR, 1.02; 95% CI, 1.01-1.4
- History of smoking: OR, 1.7; 95% CI, 1.1-2.6
- History of prior IBD-related surgery: OR, 1.60; 95% CI, 1.1-2.5
- History of biologic or small molecule therapy: OR, 2.3; 95% CI, 1.4-4.0
Future Directions
Commenting on the study, Mark Hwang, MD, a rheumatologist at UTHealth Houston, noted that it was “very interesting to see the fairly large, positive rates” of joint pain in patients with IBD, which certainly have clinical implications. However, it is not yet known if any of these patients went on to be diagnosed with SpA.
Jan noted that potential next steps include a follow-up analysis of patients who screened positive to see how many went on to see a rheumatologist and which patients were ultimately diagnosed with SpA or other inflammatory arthritis conditions.
These findings are a first step, Dr. Hwang said, and will likely “help further establish some of the validity of these questionnaires by testing in different patient populations,” he noted.
The ultimate goal is to “develop really good strategies to risk stratify IBD patients with the greatest need of rheumatologist consultation,” Dr. Jan said. “We certainly don’t want to see all these patients, so how can we figure out who really needs to be seen?”
Funding information was not available for this study. Dr. Hwang is conducting two clinical trials for psoriatic arthritis sponsored by Janssen and Eli Lilly. Dr. Jan reported no relevant disclosures.
A version of this article appeared on Medscape.com.
More than 40% of patients with inflammatory bowel disease (IBD) screened positive for joint pain symptomatic of spondyloarthritis (SpA), according to a new study.
Of these patients, 75% did not have any history of arthritis.
“What we know is that a substantial proportion of patients with IBD do report musculoskeletal symptoms, and inflammatory back pain stands out as being one of the more frequent symptoms reported,” said Reem Jan, MBBS, a rheumatologist at the University of Chicago Medicine. She presented the study findings during the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN) in Cleveland.
“Yet a minority of these patients are evaluated by rheumatologists. So that suggests there’s a high burden of need in the IBD population to have this joint pain evaluated and addressed,” she said during her presentation.
She presented preliminary data from an ongoing project to better understand the prevalence of inflammatory arthritis in IBD — estimates range from 17% to 39%— and the risk factors for developing arthritis in this patient population.
Study Details
Researchers enrolled patients from outpatient gastroenterology clinics or procedure units at NYU Langone Health, New York City; Brigham and Women’s Hospital, Boston; University of Colorado Anschutz Medical Campus, Aurora, Colorado; Mayo Clinic, Rochester, Minnesota; University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago; and Icahn School of Medicine at Mount Sinai, New York City. Additional patients were recruited from Mercy Health, a community health system in Ohio.
Upon entry into the study, participants completed a survey documenting their history with joint pain. The survey combined questions from the DETAIL and the IBIS questionnaires.
Between January 2021 and December 2022, 669 patients joined the study. In total, 41% of patients (n = 275) screened positive.
“What really stood out to us was that of all the positive screens, only about a quarter of those patients were known to have SpA,” Dr. Jan said during her presentation. “[This] means 75% of the patients who screened positive were not known to have any type of arthritic disease.”
In addition, only 24% (n = 65) of all patients who screened positive — including those with a SpA diagnosis — had seen a rheumatologist in the previous year.
Among these patients, inflammatory back pain was the most commonly reported symptom, followed by painful swelling of peripheral joints and heel pain.
Excluding patients with a SpA diagnosis, researchers also investigated which characteristics were associated with a higher likelihood of screening positive in the questionnaire. The analysis, including 588 patients, identified the following risk factors:
- Female sex: Odds ratio (OR), 2.0; 95% CI, 1.4-2.9
- Older age: OR, 1.02; 95% CI, 1.01-1.4
- History of smoking: OR, 1.7; 95% CI, 1.1-2.6
- History of prior IBD-related surgery: OR, 1.60; 95% CI, 1.1-2.5
- History of biologic or small molecule therapy: OR, 2.3; 95% CI, 1.4-4.0
Future Directions
Commenting on the study, Mark Hwang, MD, a rheumatologist at UTHealth Houston, noted that it was “very interesting to see the fairly large, positive rates” of joint pain in patients with IBD, which certainly have clinical implications. However, it is not yet known if any of these patients went on to be diagnosed with SpA.
Jan noted that potential next steps include a follow-up analysis of patients who screened positive to see how many went on to see a rheumatologist and which patients were ultimately diagnosed with SpA or other inflammatory arthritis conditions.
These findings are a first step, Dr. Hwang said, and will likely “help further establish some of the validity of these questionnaires by testing in different patient populations,” he noted.
The ultimate goal is to “develop really good strategies to risk stratify IBD patients with the greatest need of rheumatologist consultation,” Dr. Jan said. “We certainly don’t want to see all these patients, so how can we figure out who really needs to be seen?”
Funding information was not available for this study. Dr. Hwang is conducting two clinical trials for psoriatic arthritis sponsored by Janssen and Eli Lilly. Dr. Jan reported no relevant disclosures.
A version of this article appeared on Medscape.com.
More than 40% of patients with inflammatory bowel disease (IBD) screened positive for joint pain symptomatic of spondyloarthritis (SpA), according to a new study.
Of these patients, 75% did not have any history of arthritis.
“What we know is that a substantial proportion of patients with IBD do report musculoskeletal symptoms, and inflammatory back pain stands out as being one of the more frequent symptoms reported,” said Reem Jan, MBBS, a rheumatologist at the University of Chicago Medicine. She presented the study findings during the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN) in Cleveland.
“Yet a minority of these patients are evaluated by rheumatologists. So that suggests there’s a high burden of need in the IBD population to have this joint pain evaluated and addressed,” she said during her presentation.
She presented preliminary data from an ongoing project to better understand the prevalence of inflammatory arthritis in IBD — estimates range from 17% to 39%— and the risk factors for developing arthritis in this patient population.
Study Details
Researchers enrolled patients from outpatient gastroenterology clinics or procedure units at NYU Langone Health, New York City; Brigham and Women’s Hospital, Boston; University of Colorado Anschutz Medical Campus, Aurora, Colorado; Mayo Clinic, Rochester, Minnesota; University of Chicago Medicine Inflammatory Bowel Disease Center, Chicago; and Icahn School of Medicine at Mount Sinai, New York City. Additional patients were recruited from Mercy Health, a community health system in Ohio.
Upon entry into the study, participants completed a survey documenting their history with joint pain. The survey combined questions from the DETAIL and the IBIS questionnaires.
Between January 2021 and December 2022, 669 patients joined the study. In total, 41% of patients (n = 275) screened positive.
“What really stood out to us was that of all the positive screens, only about a quarter of those patients were known to have SpA,” Dr. Jan said during her presentation. “[This] means 75% of the patients who screened positive were not known to have any type of arthritic disease.”
In addition, only 24% (n = 65) of all patients who screened positive — including those with a SpA diagnosis — had seen a rheumatologist in the previous year.
Among these patients, inflammatory back pain was the most commonly reported symptom, followed by painful swelling of peripheral joints and heel pain.
Excluding patients with a SpA diagnosis, researchers also investigated which characteristics were associated with a higher likelihood of screening positive in the questionnaire. The analysis, including 588 patients, identified the following risk factors:
- Female sex: Odds ratio (OR), 2.0; 95% CI, 1.4-2.9
- Older age: OR, 1.02; 95% CI, 1.01-1.4
- History of smoking: OR, 1.7; 95% CI, 1.1-2.6
- History of prior IBD-related surgery: OR, 1.60; 95% CI, 1.1-2.5
- History of biologic or small molecule therapy: OR, 2.3; 95% CI, 1.4-4.0
Future Directions
Commenting on the study, Mark Hwang, MD, a rheumatologist at UTHealth Houston, noted that it was “very interesting to see the fairly large, positive rates” of joint pain in patients with IBD, which certainly have clinical implications. However, it is not yet known if any of these patients went on to be diagnosed with SpA.
Jan noted that potential next steps include a follow-up analysis of patients who screened positive to see how many went on to see a rheumatologist and which patients were ultimately diagnosed with SpA or other inflammatory arthritis conditions.
These findings are a first step, Dr. Hwang said, and will likely “help further establish some of the validity of these questionnaires by testing in different patient populations,” he noted.
The ultimate goal is to “develop really good strategies to risk stratify IBD patients with the greatest need of rheumatologist consultation,” Dr. Jan said. “We certainly don’t want to see all these patients, so how can we figure out who really needs to be seen?”
Funding information was not available for this study. Dr. Hwang is conducting two clinical trials for psoriatic arthritis sponsored by Janssen and Eli Lilly. Dr. Jan reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM SPARTAN 2024
High NSAID Use in Patients With Axial Spondyloarthritis May Not Raise Risk for Hypertension
TOPLINE:
Patients with axial spondyloarthritis (axSpA) who reported high nonsteroidal anti-inflammatory drug (NSAID) use did not have a higher risk for hypertension than those who reported low NSAID use.
METHODOLOGY:
- NSAIDs are first-line therapy for axSpA and are associated with a high risk for hypertension in the general population, but it’s unknown whether NSAID use increases the risk for hypertension in patients with axSpA, who are already at higher risk for cardiovascular disease and hypertension than the general population
- This study used the DESIR cohort, a multicenter cohort of patients with recent-onset axSpA in France, including 631 individuals aged 18-50 years who did not have hypertension at baseline and had 6 years of follow-up.
- NSAID use was evaluated at each follow-up visit, using the Assessment of Spondyloarthritis International Society NSAID index.
- A score ≥ 50 was categorized as high use, and a score < 50 was considered low use.
- The primary outcome was hypertension, defined by the use of antihypertensive medication, self-reported hypertension, and/or systolic blood pressure (BP) ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg on at least two visits.
TAKEAWAY:
- A total of 39% of patients were categorized as high NSAID users.
- Over 6 years of follow-up, 70 patients (11%) developed hypertension.
- There was no significant association between high NSAID use and the risk for hypertension.
IN PRACTICE:
The study is too preliminary to have practice application.
SOURCE:
The research was led and presented by Jose Meade-Aguilar, MD, of Boston University School of Medicine, at the Spondyloarthritis Research and Treatment Network (SPARTAN) 2024 annual meeting in Cleveland.
LIMITATIONS:
The study had a low number of hypertension events, which could be due to the younger age of participants and earlier disease stage. The study was observational, so residual or unmeasured confounding is possible.
DISCLOSURES:
The DESIR cohort study is financially supported by unrestricted grants from both the French Society for Rheumatology and Pfizer France. One coauthor reported receiving research grants and/or consultancy fees from AbbVie, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, UCB, and Sanofi. Another coauthor reported receiving research grants from UCB and consulting fees from Eli Lilly, Novartis, Pfizer, and UCB. The remaining authors had no financial, relational, or commercial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients with axial spondyloarthritis (axSpA) who reported high nonsteroidal anti-inflammatory drug (NSAID) use did not have a higher risk for hypertension than those who reported low NSAID use.
METHODOLOGY:
- NSAIDs are first-line therapy for axSpA and are associated with a high risk for hypertension in the general population, but it’s unknown whether NSAID use increases the risk for hypertension in patients with axSpA, who are already at higher risk for cardiovascular disease and hypertension than the general population
- This study used the DESIR cohort, a multicenter cohort of patients with recent-onset axSpA in France, including 631 individuals aged 18-50 years who did not have hypertension at baseline and had 6 years of follow-up.
- NSAID use was evaluated at each follow-up visit, using the Assessment of Spondyloarthritis International Society NSAID index.
- A score ≥ 50 was categorized as high use, and a score < 50 was considered low use.
- The primary outcome was hypertension, defined by the use of antihypertensive medication, self-reported hypertension, and/or systolic blood pressure (BP) ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg on at least two visits.
TAKEAWAY:
- A total of 39% of patients were categorized as high NSAID users.
- Over 6 years of follow-up, 70 patients (11%) developed hypertension.
- There was no significant association between high NSAID use and the risk for hypertension.
IN PRACTICE:
The study is too preliminary to have practice application.
SOURCE:
The research was led and presented by Jose Meade-Aguilar, MD, of Boston University School of Medicine, at the Spondyloarthritis Research and Treatment Network (SPARTAN) 2024 annual meeting in Cleveland.
LIMITATIONS:
The study had a low number of hypertension events, which could be due to the younger age of participants and earlier disease stage. The study was observational, so residual or unmeasured confounding is possible.
DISCLOSURES:
The DESIR cohort study is financially supported by unrestricted grants from both the French Society for Rheumatology and Pfizer France. One coauthor reported receiving research grants and/or consultancy fees from AbbVie, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, UCB, and Sanofi. Another coauthor reported receiving research grants from UCB and consulting fees from Eli Lilly, Novartis, Pfizer, and UCB. The remaining authors had no financial, relational, or commercial conflicts to disclose.
A version of this article appeared on Medscape.com.
TOPLINE:
Patients with axial spondyloarthritis (axSpA) who reported high nonsteroidal anti-inflammatory drug (NSAID) use did not have a higher risk for hypertension than those who reported low NSAID use.
METHODOLOGY:
- NSAIDs are first-line therapy for axSpA and are associated with a high risk for hypertension in the general population, but it’s unknown whether NSAID use increases the risk for hypertension in patients with axSpA, who are already at higher risk for cardiovascular disease and hypertension than the general population
- This study used the DESIR cohort, a multicenter cohort of patients with recent-onset axSpA in France, including 631 individuals aged 18-50 years who did not have hypertension at baseline and had 6 years of follow-up.
- NSAID use was evaluated at each follow-up visit, using the Assessment of Spondyloarthritis International Society NSAID index.
- A score ≥ 50 was categorized as high use, and a score < 50 was considered low use.
- The primary outcome was hypertension, defined by the use of antihypertensive medication, self-reported hypertension, and/or systolic blood pressure (BP) ≥ 140 mm Hg or diastolic BP ≥ 90 mm Hg on at least two visits.
TAKEAWAY:
- A total of 39% of patients were categorized as high NSAID users.
- Over 6 years of follow-up, 70 patients (11%) developed hypertension.
- There was no significant association between high NSAID use and the risk for hypertension.
IN PRACTICE:
The study is too preliminary to have practice application.
SOURCE:
The research was led and presented by Jose Meade-Aguilar, MD, of Boston University School of Medicine, at the Spondyloarthritis Research and Treatment Network (SPARTAN) 2024 annual meeting in Cleveland.
LIMITATIONS:
The study had a low number of hypertension events, which could be due to the younger age of participants and earlier disease stage. The study was observational, so residual or unmeasured confounding is possible.
DISCLOSURES:
The DESIR cohort study is financially supported by unrestricted grants from both the French Society for Rheumatology and Pfizer France. One coauthor reported receiving research grants and/or consultancy fees from AbbVie, Eli Lilly, Galapagos, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, UCB, and Sanofi. Another coauthor reported receiving research grants from UCB and consulting fees from Eli Lilly, Novartis, Pfizer, and UCB. The remaining authors had no financial, relational, or commercial conflicts to disclose.
A version of this article appeared on Medscape.com.
Specialists Are ‘Underwater’ With Some Insurance-Preferred Biosimilars
Editor’s note: This article is adapted from an explanatory statement that Dr. Feldman wrote for the Coalition of State Rheumatology Organizations (CSRO).
According to the Guinness Book of World records, the longest time someone has held their breath underwater voluntarily is 24 minutes and 37.36 seconds. While certainly an amazing feat, UnitedHealthcare, many of the Blues, and other national “payers” are expecting rheumatologists and other specialists to live “underwater” in order to take care of their patients. In other words, these insurance companies are mandating that specialists use certain provider-administered biosimilars whose acquisition cost is higher than what the insurance company is willing to reimburse them. Essentially, the insurance companies expect the rheumatologists to pay them to take care of their patients. Because of the substantial and destabilizing financial losses incurred, many practices and free-standing infusion centers have been forced to cease offering these biosimilars. Most rheumatologists will provide patients with appropriate alternatives when available and permitted by the insurer; otherwise, they must refer patients to hospital-based infusion centers. That results in delayed care and increased costs for patients and the system, because hospital-based infusion typically costs more than twice what office-based infusion costs.
Quantifying the Problem
To help quantify the magnitude of this issue, the Coalition of State Rheumatology Organizations (CSRO) recently conducted a survey of its membership. A shocking 97% of respondents reported that their practice had been affected by reimbursement rates for some biosimilars being lower than acquisition costs, with 91% of respondents stating that this issue is more pronounced for certain biosimilars than others. Across the board, respondents most frequently identified Inflectra (infliximab-dyyb) and Avsola (infliximab-axxq) as being especially affected: Over 88% and over 85% of respondents identified these two products, respectively, as being underwater. These results support the ongoing anecdotal reports CSRO continues to receive from rheumatology practices.
However, the survey results indicated that this issue is by no means confined to those two biosimilars. Truxima (rituximab-abbs) — a biosimilar for Rituxan — was frequently mentioned as well. Notably, respondents almost uniformly identified biosimilars in the infliximab and rituximab families, which illustrates that this issue is no longer confined to one or two early-to-market biosimilars but has almost become a hallmark of this particular biosimilars market. Remarkably, one respondent commented that the brand products are now cheaper to acquire than the biosimilars. Furthermore, the survey included respondents from across the country, indicating that this issue is not confined to a particular region.
How Did This Happen?
Biosimilars held promise for increasing availability and decreasing biologic costs for patients but, thus far, no patients have seen their cost go down. It appears that the only biosimilars that have made it to “preferred” status on the formulary are the ones that have made more money for the middlemen in the drug supply chain, particularly those that construct formularies. Now, we have provider-administered biosimilars whose acquisition cost exceeds the reimbursement for these drugs. This disparity was ultimately created by biosimilar manufacturers “over-rebating” their drugs to health insurance companies to gain “fail-first” status on the formulary.
For example, the manufacturer of Inflectra offered substantial rebates to health insurers for preferred formulary placement. These rebates are factored into the sales price of the medication, which then results in a rapidly declining average sales price (ASP) for the biosimilar. Unfortunately, the acquisition cost for the drug does not experience commensurate reductions, resulting in physicians being reimbursed far less for the drug than it costs to acquire. The financial losses for physicians put them underwater as a result of the acquisition costs for the preferred drugs far surpassing the reimbursement from the health insurance company that constructed the formulary.
While various factors affect ASPs and acquisition costs, this particular consequence of formulary placement based on price concessions is a major driver of the underwater situation in which physicians have found themselves with many biosimilars. Not only does that lead to a lower uptake of biosimilars, but it also results in patients being referred to the hospital outpatient infusion sites to receive this care, as freestanding infusion centers cannot treat these patients either. Hospitals incur higher costs because of facility fees and elevated rates, and this makes private rheumatology in-office infusion centers a much lower-cost option. Similarly, home infusion services, while convenient, are marginally more expensive than private practices and, in cases of biologic infusions, it is important to note that physicians’ offices have a greater safety profile than home infusion of biologics. The overall result of these “fail-first underwater drugs” is delayed and more costly care for the patient and the “system,” particularly self-insured employers.
What Is Being Done to Correct This?
Since ASPs are updated quarterly, it is possible that acquisition costs and reimbursements might stabilize over time, making the drugs affordable again to practices. However, that does not appear to be happening in the near future, so that possibility does not offer immediate relief to struggling practices. It doesn’t promise a favorable outlook for future biosimilar entries of provider-administered medications if formularies continue to prefer the highest-rebated medication.
This dynamic between ASP and acquisition cost does not happen on the pharmacy side because the price concessions on specific drug rebates and fees are proprietary. There appears to be no equivalent to a publicly known ASP on the pharmacy side, which has led to myriad pricing definitions and manipulation on the pharmacy benefit side of medications. In any event, the savings from rebates and other manufacturer price concessions on pharmacy drugs do not influence ASPs of medical benefit drugs.
The Inflation Reduction Act provided a temporary increase in the add-on payment for biosimilars from ASP+6% to ASP+8%, but as long as the biosimilar’s ASP is lower than the reference brand’s ASP, that temporary increase does not appear to make up for the large differential between ASP and acquisition cost. It should be noted that any federal attempt to artificially lower the ASP of a provider-administered drug without a pathway assuring that the acquisition cost for the provider is less than the reimbursement is going to result in loss of access for patients to those medications and/or higher hospital site of care costs.
A Few Partial Fixes, But Most Complaints Go Ignored
Considering the higher costs of hospital-based infusion, insurers should be motivated to keep patients within private practices. Perhaps through insurers’ recognition of that fact, some practices have successfully negotiated exceptions for specific patients by discussing this situation with insurers. From the feedback that CSRO has received from rheumatology practices, it appears that most insurers have been ignoring the complaints from physicians. The few who have responded have resulted in only partial fixes, with some of the biosimilars still left underwater.
Ultimate Solution?
This issue is a direct result of the “rebate game,” whereby price concessions from drug manufacturers drive formulary placement. For provider-administered medications, this results in an artificially lowered ASP, not as a consequence of free-market incentives that benefit the patient, but as a result of misaligned incentives created by Safe Harbor–protected “kickbacks,” distorting the free market and paradoxically reducing access to these medications, delaying care, and increasing prices for patients and the healthcare system.
While federal and state governments are not likely to address this particular situation in the biosimilars market, CSRO is highlighting this issue as a prime example of why the current formulary construction system urgently requires federal reform. At this time, the biosimilars most affected are Inflectra and Avsola, but if nothing changes, more and more biosimilars will fall victim to the short-sighted pricing strategy of aggressive rebating to gain formulary position, with physician purchasers and patients left to navigate the aftermath. The existing system, which necessitates drug companies purchasing formulary access from pharmacy benefit managers, has led to delayed and even denied patient access to certain provider-administered drugs. Moreover, it now appears to be hindering the adoption of biosimilars.
To address this, a multifaceted approach is required. It not only involves reevaluating the rebate system and its impact on formulary construction and ASP, but also ensuring that acquisition costs for providers are aligned with reimbursement rates. Insurers must recognize the economic and clinical value of maintaining infusions within private practices and immediately update their policies to ensure that physician in-office infusion is financially feasible for these “fail-first” biosimilars.
Ultimately, the goal should be to create a sustainable model that promotes the use of affordable biosimilars, enhances patient access to affordable care, and supports the financial viability of medical practices. Concerted efforts to reform the current formulary construction system are required to achieve a healthcare environment that is both cost effective and patient centric.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Editor’s note: This article is adapted from an explanatory statement that Dr. Feldman wrote for the Coalition of State Rheumatology Organizations (CSRO).
According to the Guinness Book of World records, the longest time someone has held their breath underwater voluntarily is 24 minutes and 37.36 seconds. While certainly an amazing feat, UnitedHealthcare, many of the Blues, and other national “payers” are expecting rheumatologists and other specialists to live “underwater” in order to take care of their patients. In other words, these insurance companies are mandating that specialists use certain provider-administered biosimilars whose acquisition cost is higher than what the insurance company is willing to reimburse them. Essentially, the insurance companies expect the rheumatologists to pay them to take care of their patients. Because of the substantial and destabilizing financial losses incurred, many practices and free-standing infusion centers have been forced to cease offering these biosimilars. Most rheumatologists will provide patients with appropriate alternatives when available and permitted by the insurer; otherwise, they must refer patients to hospital-based infusion centers. That results in delayed care and increased costs for patients and the system, because hospital-based infusion typically costs more than twice what office-based infusion costs.
Quantifying the Problem
To help quantify the magnitude of this issue, the Coalition of State Rheumatology Organizations (CSRO) recently conducted a survey of its membership. A shocking 97% of respondents reported that their practice had been affected by reimbursement rates for some biosimilars being lower than acquisition costs, with 91% of respondents stating that this issue is more pronounced for certain biosimilars than others. Across the board, respondents most frequently identified Inflectra (infliximab-dyyb) and Avsola (infliximab-axxq) as being especially affected: Over 88% and over 85% of respondents identified these two products, respectively, as being underwater. These results support the ongoing anecdotal reports CSRO continues to receive from rheumatology practices.
However, the survey results indicated that this issue is by no means confined to those two biosimilars. Truxima (rituximab-abbs) — a biosimilar for Rituxan — was frequently mentioned as well. Notably, respondents almost uniformly identified biosimilars in the infliximab and rituximab families, which illustrates that this issue is no longer confined to one or two early-to-market biosimilars but has almost become a hallmark of this particular biosimilars market. Remarkably, one respondent commented that the brand products are now cheaper to acquire than the biosimilars. Furthermore, the survey included respondents from across the country, indicating that this issue is not confined to a particular region.
How Did This Happen?
Biosimilars held promise for increasing availability and decreasing biologic costs for patients but, thus far, no patients have seen their cost go down. It appears that the only biosimilars that have made it to “preferred” status on the formulary are the ones that have made more money for the middlemen in the drug supply chain, particularly those that construct formularies. Now, we have provider-administered biosimilars whose acquisition cost exceeds the reimbursement for these drugs. This disparity was ultimately created by biosimilar manufacturers “over-rebating” their drugs to health insurance companies to gain “fail-first” status on the formulary.
For example, the manufacturer of Inflectra offered substantial rebates to health insurers for preferred formulary placement. These rebates are factored into the sales price of the medication, which then results in a rapidly declining average sales price (ASP) for the biosimilar. Unfortunately, the acquisition cost for the drug does not experience commensurate reductions, resulting in physicians being reimbursed far less for the drug than it costs to acquire. The financial losses for physicians put them underwater as a result of the acquisition costs for the preferred drugs far surpassing the reimbursement from the health insurance company that constructed the formulary.
While various factors affect ASPs and acquisition costs, this particular consequence of formulary placement based on price concessions is a major driver of the underwater situation in which physicians have found themselves with many biosimilars. Not only does that lead to a lower uptake of biosimilars, but it also results in patients being referred to the hospital outpatient infusion sites to receive this care, as freestanding infusion centers cannot treat these patients either. Hospitals incur higher costs because of facility fees and elevated rates, and this makes private rheumatology in-office infusion centers a much lower-cost option. Similarly, home infusion services, while convenient, are marginally more expensive than private practices and, in cases of biologic infusions, it is important to note that physicians’ offices have a greater safety profile than home infusion of biologics. The overall result of these “fail-first underwater drugs” is delayed and more costly care for the patient and the “system,” particularly self-insured employers.
What Is Being Done to Correct This?
Since ASPs are updated quarterly, it is possible that acquisition costs and reimbursements might stabilize over time, making the drugs affordable again to practices. However, that does not appear to be happening in the near future, so that possibility does not offer immediate relief to struggling practices. It doesn’t promise a favorable outlook for future biosimilar entries of provider-administered medications if formularies continue to prefer the highest-rebated medication.
This dynamic between ASP and acquisition cost does not happen on the pharmacy side because the price concessions on specific drug rebates and fees are proprietary. There appears to be no equivalent to a publicly known ASP on the pharmacy side, which has led to myriad pricing definitions and manipulation on the pharmacy benefit side of medications. In any event, the savings from rebates and other manufacturer price concessions on pharmacy drugs do not influence ASPs of medical benefit drugs.
The Inflation Reduction Act provided a temporary increase in the add-on payment for biosimilars from ASP+6% to ASP+8%, but as long as the biosimilar’s ASP is lower than the reference brand’s ASP, that temporary increase does not appear to make up for the large differential between ASP and acquisition cost. It should be noted that any federal attempt to artificially lower the ASP of a provider-administered drug without a pathway assuring that the acquisition cost for the provider is less than the reimbursement is going to result in loss of access for patients to those medications and/or higher hospital site of care costs.
A Few Partial Fixes, But Most Complaints Go Ignored
Considering the higher costs of hospital-based infusion, insurers should be motivated to keep patients within private practices. Perhaps through insurers’ recognition of that fact, some practices have successfully negotiated exceptions for specific patients by discussing this situation with insurers. From the feedback that CSRO has received from rheumatology practices, it appears that most insurers have been ignoring the complaints from physicians. The few who have responded have resulted in only partial fixes, with some of the biosimilars still left underwater.
Ultimate Solution?
This issue is a direct result of the “rebate game,” whereby price concessions from drug manufacturers drive formulary placement. For provider-administered medications, this results in an artificially lowered ASP, not as a consequence of free-market incentives that benefit the patient, but as a result of misaligned incentives created by Safe Harbor–protected “kickbacks,” distorting the free market and paradoxically reducing access to these medications, delaying care, and increasing prices for patients and the healthcare system.
While federal and state governments are not likely to address this particular situation in the biosimilars market, CSRO is highlighting this issue as a prime example of why the current formulary construction system urgently requires federal reform. At this time, the biosimilars most affected are Inflectra and Avsola, but if nothing changes, more and more biosimilars will fall victim to the short-sighted pricing strategy of aggressive rebating to gain formulary position, with physician purchasers and patients left to navigate the aftermath. The existing system, which necessitates drug companies purchasing formulary access from pharmacy benefit managers, has led to delayed and even denied patient access to certain provider-administered drugs. Moreover, it now appears to be hindering the adoption of biosimilars.
To address this, a multifaceted approach is required. It not only involves reevaluating the rebate system and its impact on formulary construction and ASP, but also ensuring that acquisition costs for providers are aligned with reimbursement rates. Insurers must recognize the economic and clinical value of maintaining infusions within private practices and immediately update their policies to ensure that physician in-office infusion is financially feasible for these “fail-first” biosimilars.
Ultimately, the goal should be to create a sustainable model that promotes the use of affordable biosimilars, enhances patient access to affordable care, and supports the financial viability of medical practices. Concerted efforts to reform the current formulary construction system are required to achieve a healthcare environment that is both cost effective and patient centric.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
Editor’s note: This article is adapted from an explanatory statement that Dr. Feldman wrote for the Coalition of State Rheumatology Organizations (CSRO).
According to the Guinness Book of World records, the longest time someone has held their breath underwater voluntarily is 24 minutes and 37.36 seconds. While certainly an amazing feat, UnitedHealthcare, many of the Blues, and other national “payers” are expecting rheumatologists and other specialists to live “underwater” in order to take care of their patients. In other words, these insurance companies are mandating that specialists use certain provider-administered biosimilars whose acquisition cost is higher than what the insurance company is willing to reimburse them. Essentially, the insurance companies expect the rheumatologists to pay them to take care of their patients. Because of the substantial and destabilizing financial losses incurred, many practices and free-standing infusion centers have been forced to cease offering these biosimilars. Most rheumatologists will provide patients with appropriate alternatives when available and permitted by the insurer; otherwise, they must refer patients to hospital-based infusion centers. That results in delayed care and increased costs for patients and the system, because hospital-based infusion typically costs more than twice what office-based infusion costs.
Quantifying the Problem
To help quantify the magnitude of this issue, the Coalition of State Rheumatology Organizations (CSRO) recently conducted a survey of its membership. A shocking 97% of respondents reported that their practice had been affected by reimbursement rates for some biosimilars being lower than acquisition costs, with 91% of respondents stating that this issue is more pronounced for certain biosimilars than others. Across the board, respondents most frequently identified Inflectra (infliximab-dyyb) and Avsola (infliximab-axxq) as being especially affected: Over 88% and over 85% of respondents identified these two products, respectively, as being underwater. These results support the ongoing anecdotal reports CSRO continues to receive from rheumatology practices.
However, the survey results indicated that this issue is by no means confined to those two biosimilars. Truxima (rituximab-abbs) — a biosimilar for Rituxan — was frequently mentioned as well. Notably, respondents almost uniformly identified biosimilars in the infliximab and rituximab families, which illustrates that this issue is no longer confined to one or two early-to-market biosimilars but has almost become a hallmark of this particular biosimilars market. Remarkably, one respondent commented that the brand products are now cheaper to acquire than the biosimilars. Furthermore, the survey included respondents from across the country, indicating that this issue is not confined to a particular region.
How Did This Happen?
Biosimilars held promise for increasing availability and decreasing biologic costs for patients but, thus far, no patients have seen their cost go down. It appears that the only biosimilars that have made it to “preferred” status on the formulary are the ones that have made more money for the middlemen in the drug supply chain, particularly those that construct formularies. Now, we have provider-administered biosimilars whose acquisition cost exceeds the reimbursement for these drugs. This disparity was ultimately created by biosimilar manufacturers “over-rebating” their drugs to health insurance companies to gain “fail-first” status on the formulary.
For example, the manufacturer of Inflectra offered substantial rebates to health insurers for preferred formulary placement. These rebates are factored into the sales price of the medication, which then results in a rapidly declining average sales price (ASP) for the biosimilar. Unfortunately, the acquisition cost for the drug does not experience commensurate reductions, resulting in physicians being reimbursed far less for the drug than it costs to acquire. The financial losses for physicians put them underwater as a result of the acquisition costs for the preferred drugs far surpassing the reimbursement from the health insurance company that constructed the formulary.
While various factors affect ASPs and acquisition costs, this particular consequence of formulary placement based on price concessions is a major driver of the underwater situation in which physicians have found themselves with many biosimilars. Not only does that lead to a lower uptake of biosimilars, but it also results in patients being referred to the hospital outpatient infusion sites to receive this care, as freestanding infusion centers cannot treat these patients either. Hospitals incur higher costs because of facility fees and elevated rates, and this makes private rheumatology in-office infusion centers a much lower-cost option. Similarly, home infusion services, while convenient, are marginally more expensive than private practices and, in cases of biologic infusions, it is important to note that physicians’ offices have a greater safety profile than home infusion of biologics. The overall result of these “fail-first underwater drugs” is delayed and more costly care for the patient and the “system,” particularly self-insured employers.
What Is Being Done to Correct This?
Since ASPs are updated quarterly, it is possible that acquisition costs and reimbursements might stabilize over time, making the drugs affordable again to practices. However, that does not appear to be happening in the near future, so that possibility does not offer immediate relief to struggling practices. It doesn’t promise a favorable outlook for future biosimilar entries of provider-administered medications if formularies continue to prefer the highest-rebated medication.
This dynamic between ASP and acquisition cost does not happen on the pharmacy side because the price concessions on specific drug rebates and fees are proprietary. There appears to be no equivalent to a publicly known ASP on the pharmacy side, which has led to myriad pricing definitions and manipulation on the pharmacy benefit side of medications. In any event, the savings from rebates and other manufacturer price concessions on pharmacy drugs do not influence ASPs of medical benefit drugs.
The Inflation Reduction Act provided a temporary increase in the add-on payment for biosimilars from ASP+6% to ASP+8%, but as long as the biosimilar’s ASP is lower than the reference brand’s ASP, that temporary increase does not appear to make up for the large differential between ASP and acquisition cost. It should be noted that any federal attempt to artificially lower the ASP of a provider-administered drug without a pathway assuring that the acquisition cost for the provider is less than the reimbursement is going to result in loss of access for patients to those medications and/or higher hospital site of care costs.
A Few Partial Fixes, But Most Complaints Go Ignored
Considering the higher costs of hospital-based infusion, insurers should be motivated to keep patients within private practices. Perhaps through insurers’ recognition of that fact, some practices have successfully negotiated exceptions for specific patients by discussing this situation with insurers. From the feedback that CSRO has received from rheumatology practices, it appears that most insurers have been ignoring the complaints from physicians. The few who have responded have resulted in only partial fixes, with some of the biosimilars still left underwater.
Ultimate Solution?
This issue is a direct result of the “rebate game,” whereby price concessions from drug manufacturers drive formulary placement. For provider-administered medications, this results in an artificially lowered ASP, not as a consequence of free-market incentives that benefit the patient, but as a result of misaligned incentives created by Safe Harbor–protected “kickbacks,” distorting the free market and paradoxically reducing access to these medications, delaying care, and increasing prices for patients and the healthcare system.
While federal and state governments are not likely to address this particular situation in the biosimilars market, CSRO is highlighting this issue as a prime example of why the current formulary construction system urgently requires federal reform. At this time, the biosimilars most affected are Inflectra and Avsola, but if nothing changes, more and more biosimilars will fall victim to the short-sighted pricing strategy of aggressive rebating to gain formulary position, with physician purchasers and patients left to navigate the aftermath. The existing system, which necessitates drug companies purchasing formulary access from pharmacy benefit managers, has led to delayed and even denied patient access to certain provider-administered drugs. Moreover, it now appears to be hindering the adoption of biosimilars.
To address this, a multifaceted approach is required. It not only involves reevaluating the rebate system and its impact on formulary construction and ASP, but also ensuring that acquisition costs for providers are aligned with reimbursement rates. Insurers must recognize the economic and clinical value of maintaining infusions within private practices and immediately update their policies to ensure that physician in-office infusion is financially feasible for these “fail-first” biosimilars.
Ultimately, the goal should be to create a sustainable model that promotes the use of affordable biosimilars, enhances patient access to affordable care, and supports the financial viability of medical practices. Concerted efforts to reform the current formulary construction system are required to achieve a healthcare environment that is both cost effective and patient centric.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s vice president of advocacy and government affairs and its immediate past president, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
CVD Risk Rises With Higher NSAID Doses in Ankylosing Spondylitis
TOPLINE:
Higher doses of nonsteroidal anti-inflammatory drugs (NSAIDs) increase the risk for cardiovascular diseases (CVDs) such as ischemic heart disease, stroke, and congestive heart failure in patients with ankylosing spondylitis (AS) compared with lower doses.
METHODOLOGY:
- NSAIDs can suppress inflammation and relieve pain in patients with AS, but long-term treatment with NSAIDs poses concerns regarding gastrointestinal and renal toxicities and increased CVD risk.
- This nationwide cohort study used data from the Korean National Health Insurance database to investigate the risk for CVD associated with an increasing NSAID dosage in a real-world AS cohort.
- Investigators recruited 19,775 patients (mean age, 36.1 years; 75% men) with newly diagnosed AS and without any prior CVD between January 2010 and December 2018, among whom 99.7% received NSAID treatment and 30.2% received tumor necrosis factor inhibitor treatment.
- A time-varying approach was used to assess the NSAID exposure, wherein periods of NSAID use were defined as “NSAID-exposed” and periods longer than 1 month without NSAID use were defined as “NSAID-unexposed.”
- The primary outcome was the composite outcome of ischemic heart disease, stroke, or congestive heart failure.
TAKEAWAY:
- During the follow-up period of 98,290 person-years, 1663 cases of CVD were identified, which included 1157 cases of ischemic heart disease, 301 cases of stroke, and 613 cases of congestive heart failure.
- After adjusting for confounders, each defined daily dose increase in NSAIDs raised the risk for incident CVD by 10% (adjusted hazard ratio [aHR], 1.10; 95% CI, 1.08-1.13).
- Similarly, increasing the dose of NSAIDs was associated with an increased risk for ischemic heart disease (aHR, 1.08; 95% CI, 1.05-1.11), stroke (aHR, 1.09; 95% CI, 1.04-1.15), and congestive heart failure (aHR, 1.12; 95% CI, 1.08-1.16).
- The association between increasing NSAID dose and increased CVD risk was consistent across various subgroups, with NSAIDs posing a greater threat to cardiovascular health in women than in men.
IN PRACTICE:
The authors wrote, “Taken together, these results suggest that increasing the dose of NSAIDs is associated with a higher cardiovascular risk in AS, but that the increased risk might be lower than that in the general population.”
SOURCE:
First author Ji-Won Kim, MD, PhD, of the Division of Rheumatology, Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, the Republic of Korea, and colleagues had their work published online on April 9 in Annals of the Rheumatic Diseases.
LIMITATIONS:
The study was of retrospective nature. The levels of acute phase reactants and AS disease activity could not be determined owing to a lack of data in the National Health Insurance database. The accuracy of the diagnosis of cardiovascular outcomes on the basis of the International Classification of Disease codes was also questionable.
DISCLOSURES:
The study was supported by the National Research Foundation of Korea. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher doses of nonsteroidal anti-inflammatory drugs (NSAIDs) increase the risk for cardiovascular diseases (CVDs) such as ischemic heart disease, stroke, and congestive heart failure in patients with ankylosing spondylitis (AS) compared with lower doses.
METHODOLOGY:
- NSAIDs can suppress inflammation and relieve pain in patients with AS, but long-term treatment with NSAIDs poses concerns regarding gastrointestinal and renal toxicities and increased CVD risk.
- This nationwide cohort study used data from the Korean National Health Insurance database to investigate the risk for CVD associated with an increasing NSAID dosage in a real-world AS cohort.
- Investigators recruited 19,775 patients (mean age, 36.1 years; 75% men) with newly diagnosed AS and without any prior CVD between January 2010 and December 2018, among whom 99.7% received NSAID treatment and 30.2% received tumor necrosis factor inhibitor treatment.
- A time-varying approach was used to assess the NSAID exposure, wherein periods of NSAID use were defined as “NSAID-exposed” and periods longer than 1 month without NSAID use were defined as “NSAID-unexposed.”
- The primary outcome was the composite outcome of ischemic heart disease, stroke, or congestive heart failure.
TAKEAWAY:
- During the follow-up period of 98,290 person-years, 1663 cases of CVD were identified, which included 1157 cases of ischemic heart disease, 301 cases of stroke, and 613 cases of congestive heart failure.
- After adjusting for confounders, each defined daily dose increase in NSAIDs raised the risk for incident CVD by 10% (adjusted hazard ratio [aHR], 1.10; 95% CI, 1.08-1.13).
- Similarly, increasing the dose of NSAIDs was associated with an increased risk for ischemic heart disease (aHR, 1.08; 95% CI, 1.05-1.11), stroke (aHR, 1.09; 95% CI, 1.04-1.15), and congestive heart failure (aHR, 1.12; 95% CI, 1.08-1.16).
- The association between increasing NSAID dose and increased CVD risk was consistent across various subgroups, with NSAIDs posing a greater threat to cardiovascular health in women than in men.
IN PRACTICE:
The authors wrote, “Taken together, these results suggest that increasing the dose of NSAIDs is associated with a higher cardiovascular risk in AS, but that the increased risk might be lower than that in the general population.”
SOURCE:
First author Ji-Won Kim, MD, PhD, of the Division of Rheumatology, Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, the Republic of Korea, and colleagues had their work published online on April 9 in Annals of the Rheumatic Diseases.
LIMITATIONS:
The study was of retrospective nature. The levels of acute phase reactants and AS disease activity could not be determined owing to a lack of data in the National Health Insurance database. The accuracy of the diagnosis of cardiovascular outcomes on the basis of the International Classification of Disease codes was also questionable.
DISCLOSURES:
The study was supported by the National Research Foundation of Korea. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher doses of nonsteroidal anti-inflammatory drugs (NSAIDs) increase the risk for cardiovascular diseases (CVDs) such as ischemic heart disease, stroke, and congestive heart failure in patients with ankylosing spondylitis (AS) compared with lower doses.
METHODOLOGY:
- NSAIDs can suppress inflammation and relieve pain in patients with AS, but long-term treatment with NSAIDs poses concerns regarding gastrointestinal and renal toxicities and increased CVD risk.
- This nationwide cohort study used data from the Korean National Health Insurance database to investigate the risk for CVD associated with an increasing NSAID dosage in a real-world AS cohort.
- Investigators recruited 19,775 patients (mean age, 36.1 years; 75% men) with newly diagnosed AS and without any prior CVD between January 2010 and December 2018, among whom 99.7% received NSAID treatment and 30.2% received tumor necrosis factor inhibitor treatment.
- A time-varying approach was used to assess the NSAID exposure, wherein periods of NSAID use were defined as “NSAID-exposed” and periods longer than 1 month without NSAID use were defined as “NSAID-unexposed.”
- The primary outcome was the composite outcome of ischemic heart disease, stroke, or congestive heart failure.
TAKEAWAY:
- During the follow-up period of 98,290 person-years, 1663 cases of CVD were identified, which included 1157 cases of ischemic heart disease, 301 cases of stroke, and 613 cases of congestive heart failure.
- After adjusting for confounders, each defined daily dose increase in NSAIDs raised the risk for incident CVD by 10% (adjusted hazard ratio [aHR], 1.10; 95% CI, 1.08-1.13).
- Similarly, increasing the dose of NSAIDs was associated with an increased risk for ischemic heart disease (aHR, 1.08; 95% CI, 1.05-1.11), stroke (aHR, 1.09; 95% CI, 1.04-1.15), and congestive heart failure (aHR, 1.12; 95% CI, 1.08-1.16).
- The association between increasing NSAID dose and increased CVD risk was consistent across various subgroups, with NSAIDs posing a greater threat to cardiovascular health in women than in men.
IN PRACTICE:
The authors wrote, “Taken together, these results suggest that increasing the dose of NSAIDs is associated with a higher cardiovascular risk in AS, but that the increased risk might be lower than that in the general population.”
SOURCE:
First author Ji-Won Kim, MD, PhD, of the Division of Rheumatology, Department of Internal Medicine, Daegu Catholic University School of Medicine, Daegu, the Republic of Korea, and colleagues had their work published online on April 9 in Annals of the Rheumatic Diseases.
LIMITATIONS:
The study was of retrospective nature. The levels of acute phase reactants and AS disease activity could not be determined owing to a lack of data in the National Health Insurance database. The accuracy of the diagnosis of cardiovascular outcomes on the basis of the International Classification of Disease codes was also questionable.
DISCLOSURES:
The study was supported by the National Research Foundation of Korea. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Cancer Identified Via Serum Metabolites, Lipids in Rheumatic Disease or Paraneoplasia
TOPLINE:
A diagnostic model based on the concentrations of four metabolites and one lipid ratio can reliably predict cancer in patients with rheumatic and musculoskeletal diseases (RMDs) or paraneoplasia, providing high sensitivity and specificity.
METHODOLOGY:
- The metabolome profile can differentiate between nonspecific inflammatory symptoms such as those associated with paraneoplastic conditions or RMDs, which can help accelerate cancer diagnosis and treatment.
- To assess if changes in the serum metabolome profile could indicate cancer in patients with RMD, researchers performed nuclear magnetic resonance analysis of the sera of patients with rheumatoid arthritis (RA) with a history of invasive cancer (n = 56; age, 69.9 years; 76.8% women) or without such history (n = 52; age, 56.1 years; 57.7% women).
- Blinded validation was conducted in a cohort of patients with RA or spondyloarthritis with or without a history of invasive cancer.
- Additionally, the model performance was tested in a cohort of patients having RA or spondyloarthritis with active cancer or cancer treatment, pulmonary and lymphoid type cancers, paraneoplastic syndromes, and facultative solid noninvasive precancerous lesions and nonmelanoma skin cancer; in samples prior to the development of malignancy; and in a cohort of patients with systemic lupus erythematosus (SLE).
- The final model comprised five variables. The goodness of fit of the model was described using the area under the receiver operating characteristic curve (AUC).
TAKEAWAY:
- Based on the concentrations of acetate, creatine, glycine, and formate and the L1/L6 lipid ratio, the diagnostic model yielded an excellent AUC (0.987) and high sensitivity (0.932) and specificity (0.946) for cancer diagnosis in patients with RA.
- The diagnostic model yielded an AUC of 0.937 in the blinded validation cohort of patients with RA and an AUC of 0.927 in the merged RA and spondyloarthritis cohort.
- Although the diagnostic model accurately diagnosed cancer in all the patients with paraneoplasia, it could do so accurately in only 50% of patients with noninvasive or in situ precancerous lesions and nonmelanoma skin cancers.
- The performance of the model was poor in the SLE cohort (AUC, 0.656), and it could not identify patients at risk for later invasive cancer development.
IN PRACTICE:
“This limited-invasive assay has considerable potential of high clinical value to facilitate timely diagnosis of cancer in paraneoplastic rheumatic syndromes as well as become a valuable active surveillance tool in RA and SpA [spondyloarthritis] patients with a high risk of developing cancer,” the authors wrote.
SOURCE:
The study, led by Karolina Gente, MHBA, Heidelberg University Hospital, Heidelberg, Germany, was published online on April 1, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
The limited invasiveness during sampling might account for the model’s inability to identify three early-stage, low-grade tumors and its nonreliability in identifying noninvasive facultative precancerous lesions and nonmelanoma skin cancers. Given its poor performance in the SLE cohort, the model may not be suitable for universal application in more systemic rheumatic diseases.
DISCLOSURES:
This study was supported by an unrestricted investigator-initiated grant from the Foundation Commission of the Medical Faculty, University of Heidelberg, Germany. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
A diagnostic model based on the concentrations of four metabolites and one lipid ratio can reliably predict cancer in patients with rheumatic and musculoskeletal diseases (RMDs) or paraneoplasia, providing high sensitivity and specificity.
METHODOLOGY:
- The metabolome profile can differentiate between nonspecific inflammatory symptoms such as those associated with paraneoplastic conditions or RMDs, which can help accelerate cancer diagnosis and treatment.
- To assess if changes in the serum metabolome profile could indicate cancer in patients with RMD, researchers performed nuclear magnetic resonance analysis of the sera of patients with rheumatoid arthritis (RA) with a history of invasive cancer (n = 56; age, 69.9 years; 76.8% women) or without such history (n = 52; age, 56.1 years; 57.7% women).
- Blinded validation was conducted in a cohort of patients with RA or spondyloarthritis with or without a history of invasive cancer.
- Additionally, the model performance was tested in a cohort of patients having RA or spondyloarthritis with active cancer or cancer treatment, pulmonary and lymphoid type cancers, paraneoplastic syndromes, and facultative solid noninvasive precancerous lesions and nonmelanoma skin cancer; in samples prior to the development of malignancy; and in a cohort of patients with systemic lupus erythematosus (SLE).
- The final model comprised five variables. The goodness of fit of the model was described using the area under the receiver operating characteristic curve (AUC).
TAKEAWAY:
- Based on the concentrations of acetate, creatine, glycine, and formate and the L1/L6 lipid ratio, the diagnostic model yielded an excellent AUC (0.987) and high sensitivity (0.932) and specificity (0.946) for cancer diagnosis in patients with RA.
- The diagnostic model yielded an AUC of 0.937 in the blinded validation cohort of patients with RA and an AUC of 0.927 in the merged RA and spondyloarthritis cohort.
- Although the diagnostic model accurately diagnosed cancer in all the patients with paraneoplasia, it could do so accurately in only 50% of patients with noninvasive or in situ precancerous lesions and nonmelanoma skin cancers.
- The performance of the model was poor in the SLE cohort (AUC, 0.656), and it could not identify patients at risk for later invasive cancer development.
IN PRACTICE:
“This limited-invasive assay has considerable potential of high clinical value to facilitate timely diagnosis of cancer in paraneoplastic rheumatic syndromes as well as become a valuable active surveillance tool in RA and SpA [spondyloarthritis] patients with a high risk of developing cancer,” the authors wrote.
SOURCE:
The study, led by Karolina Gente, MHBA, Heidelberg University Hospital, Heidelberg, Germany, was published online on April 1, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
The limited invasiveness during sampling might account for the model’s inability to identify three early-stage, low-grade tumors and its nonreliability in identifying noninvasive facultative precancerous lesions and nonmelanoma skin cancers. Given its poor performance in the SLE cohort, the model may not be suitable for universal application in more systemic rheumatic diseases.
DISCLOSURES:
This study was supported by an unrestricted investigator-initiated grant from the Foundation Commission of the Medical Faculty, University of Heidelberg, Germany. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
TOPLINE:
A diagnostic model based on the concentrations of four metabolites and one lipid ratio can reliably predict cancer in patients with rheumatic and musculoskeletal diseases (RMDs) or paraneoplasia, providing high sensitivity and specificity.
METHODOLOGY:
- The metabolome profile can differentiate between nonspecific inflammatory symptoms such as those associated with paraneoplastic conditions or RMDs, which can help accelerate cancer diagnosis and treatment.
- To assess if changes in the serum metabolome profile could indicate cancer in patients with RMD, researchers performed nuclear magnetic resonance analysis of the sera of patients with rheumatoid arthritis (RA) with a history of invasive cancer (n = 56; age, 69.9 years; 76.8% women) or without such history (n = 52; age, 56.1 years; 57.7% women).
- Blinded validation was conducted in a cohort of patients with RA or spondyloarthritis with or without a history of invasive cancer.
- Additionally, the model performance was tested in a cohort of patients having RA or spondyloarthritis with active cancer or cancer treatment, pulmonary and lymphoid type cancers, paraneoplastic syndromes, and facultative solid noninvasive precancerous lesions and nonmelanoma skin cancer; in samples prior to the development of malignancy; and in a cohort of patients with systemic lupus erythematosus (SLE).
- The final model comprised five variables. The goodness of fit of the model was described using the area under the receiver operating characteristic curve (AUC).
TAKEAWAY:
- Based on the concentrations of acetate, creatine, glycine, and formate and the L1/L6 lipid ratio, the diagnostic model yielded an excellent AUC (0.987) and high sensitivity (0.932) and specificity (0.946) for cancer diagnosis in patients with RA.
- The diagnostic model yielded an AUC of 0.937 in the blinded validation cohort of patients with RA and an AUC of 0.927 in the merged RA and spondyloarthritis cohort.
- Although the diagnostic model accurately diagnosed cancer in all the patients with paraneoplasia, it could do so accurately in only 50% of patients with noninvasive or in situ precancerous lesions and nonmelanoma skin cancers.
- The performance of the model was poor in the SLE cohort (AUC, 0.656), and it could not identify patients at risk for later invasive cancer development.
IN PRACTICE:
“This limited-invasive assay has considerable potential of high clinical value to facilitate timely diagnosis of cancer in paraneoplastic rheumatic syndromes as well as become a valuable active surveillance tool in RA and SpA [spondyloarthritis] patients with a high risk of developing cancer,” the authors wrote.
SOURCE:
The study, led by Karolina Gente, MHBA, Heidelberg University Hospital, Heidelberg, Germany, was published online on April 1, 2024, in Annals of the Rheumatic Diseases.
LIMITATIONS:
The limited invasiveness during sampling might account for the model’s inability to identify three early-stage, low-grade tumors and its nonreliability in identifying noninvasive facultative precancerous lesions and nonmelanoma skin cancers. Given its poor performance in the SLE cohort, the model may not be suitable for universal application in more systemic rheumatic diseases.
DISCLOSURES:
This study was supported by an unrestricted investigator-initiated grant from the Foundation Commission of the Medical Faculty, University of Heidelberg, Germany. The authors declared no conflicts of interest.
A version of this article appeared on Medscape.com.
Poor Use of ICD-10 Rheumatology Codes Suggests New Approach Needed for ICD-11 Adoption
Inflammatory arthritis codes increased 30-fold in the transition from the ninth to the 10th revision of the International Classification of Diseases (ICD-9 and -10), yet few were used in clinical practice, according to new research.
Most of the new codes for inflammatory arthritis in ICD-10 were rarely used, if at all, from 2015 to 2021.
“About 10-20 codes were comprising the majority of usage for inflammatory arthritis patients in ICD-10,” first author Justin Zhu, a researcher and medical student at Yale University in New Haven, Connecticut, told this news organization. “The other 380 or 400 codes just weren’t seeing a lot of use.”
The findings show the difficulties of transitioning to a new system, he added, and emphasize the need for additional training to improve adoption of ICD-11. The new coding system launched globally in January 2022, but it is not clear when it will be implemented in the United States.
ICD-10 was launched in the United States in 2015, with the goal of enabling greater specificity in identifying health conditions. For example, the new coding system allowed users to include information on laterality and anatomic location for the first time. The total number of codes increased from 14,500 with ICD-9 to 70,000 with ICD-10, with the number of inflammatory arthritis diagnosis codes growing from 14 to 425.
To see how these ICD-10 codes were utilized compared with ICD-9, Zhu and colleagues used national multi-insurance administrative claims data to find inflammatory arthritis diagnostic codes for over 5.1 million patients. About half were coded in ICD-9, while the remaining half were coded in ICD-10. Mr. Zhu and colleagues defined “higher-usage codes” as those that were used more than 1% of the time.
The findings were published in a research letter in JAMA Network Open on April 18.
For ICD-9, four of the available 14 codes (28.6%) were higher-usage codes. In contrast, only nine of the 425 ICD-10 codes (2.1%) were frequently used. Though ICD-10 allowed for increased granularity in diagnosis, data showed that nonspecific codes were most popular. Of the 20 most used ICD-10 arthritis codes, 65% contained “unspecified or other specified” in its wording.
The researchers also found that there was no significant change in these higher-usage codes throughout the study period from 2015 to 2021, suggesting there was not a detectable learning curve in ICD-10 usage among physicians and coders. They also found that clinician specialty did not change code usage patterns.
“The percentage of codes used was not better for rheumatologists (who might be expected to be more refined users of such codes) than primary care clinicians,” Mr. Zhu and colleagues wrote.
Moving to ICD-11 Brings Challenges as Well as Opportunities
Mr. Zhu noted that the study highlights the challenges of adopting new technological systems into daily practice, which can inform the eventual transition to ICD-11.
“There is this need to emphasize training as well as just invest more in improving adoption of ICD-11,” he said.
Michael Pine, MD, MBA, of MJP Healthcare Innovations, LLC in Evanston, Illinois, added that ICD-11 needs to be more user-friendly to be useful in practice. While ICD-10 allowed for greater granularity in coding, it did not result in “usable granularity, in terms of the things doctors really want to communicate,” he told this news organization.
And the transition to ICD-11 could pose greater challenges; rather than ICD-10’s taxonomy system, ICD-11 is formatted as an ontology.
“Although ICD-11 retains some precoordinated codes that convey multifaceted compound concepts, its structure and syntax also provide for post-coordination, a new feature to the ICD that supports the customized combination of concepts and modifier codes to capture previously inaccessible clinical nuance,” he wrote in a coauthored invited commentary.
This added clinical nuance, however, will potentially make coding more complex, he said. One solution is to automate coding, such that clinicians could input information in a natural clinical format that makes sense to them, which would then be translated into ICD-11 code by a program. (This would then be translated back to the user in the natural clinical format to ensure accuracy.)
This type of process would limit how much any one person would need to know about ICD-11 to code diagnoses effectively, while also taking full advantage of the increasing specificity of the new coding system, he said.
Such a program does not yet exist but could be possible with intensive investment in the transition to ICD-11.
The findings of this study serve as a cautionary tale for future transitions to new systems without considering the importance of user experience and usability, Dr. Pine noted. If the United States takes an approach for the adoption of ICD-11 that is similar to that used for ICD-10, it is likely to be “just another overhyped transition” that will make users unwilling to adopt any new system moving forward out of frustration.
But if the United States takes a different, innovative approach, the opposite could be true.
“In short, the US must decide whether it is time to invest considerable resources and effort into a 21st-century information system that could overcome such hindrances as asymmetric information for decision-making, faulty risk adjustment in performance evaluations and payment formulas, and burdens imposed by current coding and documentation practices,” the commentary reads.
“It will allow us to make the best of what computers do and the best of what clinicians do,” Dr. Pine added, “and get them to work together in ways which would not have been conceivable 50 years ago.”
No information on study funding was provided. Mr. Zhu and Dr. Pine did not disclose any competing interests.
A version of this article appeared on Medscape.com.
Inflammatory arthritis codes increased 30-fold in the transition from the ninth to the 10th revision of the International Classification of Diseases (ICD-9 and -10), yet few were used in clinical practice, according to new research.
Most of the new codes for inflammatory arthritis in ICD-10 were rarely used, if at all, from 2015 to 2021.
“About 10-20 codes were comprising the majority of usage for inflammatory arthritis patients in ICD-10,” first author Justin Zhu, a researcher and medical student at Yale University in New Haven, Connecticut, told this news organization. “The other 380 or 400 codes just weren’t seeing a lot of use.”
The findings show the difficulties of transitioning to a new system, he added, and emphasize the need for additional training to improve adoption of ICD-11. The new coding system launched globally in January 2022, but it is not clear when it will be implemented in the United States.
ICD-10 was launched in the United States in 2015, with the goal of enabling greater specificity in identifying health conditions. For example, the new coding system allowed users to include information on laterality and anatomic location for the first time. The total number of codes increased from 14,500 with ICD-9 to 70,000 with ICD-10, with the number of inflammatory arthritis diagnosis codes growing from 14 to 425.
To see how these ICD-10 codes were utilized compared with ICD-9, Zhu and colleagues used national multi-insurance administrative claims data to find inflammatory arthritis diagnostic codes for over 5.1 million patients. About half were coded in ICD-9, while the remaining half were coded in ICD-10. Mr. Zhu and colleagues defined “higher-usage codes” as those that were used more than 1% of the time.
The findings were published in a research letter in JAMA Network Open on April 18.
For ICD-9, four of the available 14 codes (28.6%) were higher-usage codes. In contrast, only nine of the 425 ICD-10 codes (2.1%) were frequently used. Though ICD-10 allowed for increased granularity in diagnosis, data showed that nonspecific codes were most popular. Of the 20 most used ICD-10 arthritis codes, 65% contained “unspecified or other specified” in its wording.
The researchers also found that there was no significant change in these higher-usage codes throughout the study period from 2015 to 2021, suggesting there was not a detectable learning curve in ICD-10 usage among physicians and coders. They also found that clinician specialty did not change code usage patterns.
“The percentage of codes used was not better for rheumatologists (who might be expected to be more refined users of such codes) than primary care clinicians,” Mr. Zhu and colleagues wrote.
Moving to ICD-11 Brings Challenges as Well as Opportunities
Mr. Zhu noted that the study highlights the challenges of adopting new technological systems into daily practice, which can inform the eventual transition to ICD-11.
“There is this need to emphasize training as well as just invest more in improving adoption of ICD-11,” he said.
Michael Pine, MD, MBA, of MJP Healthcare Innovations, LLC in Evanston, Illinois, added that ICD-11 needs to be more user-friendly to be useful in practice. While ICD-10 allowed for greater granularity in coding, it did not result in “usable granularity, in terms of the things doctors really want to communicate,” he told this news organization.
And the transition to ICD-11 could pose greater challenges; rather than ICD-10’s taxonomy system, ICD-11 is formatted as an ontology.
“Although ICD-11 retains some precoordinated codes that convey multifaceted compound concepts, its structure and syntax also provide for post-coordination, a new feature to the ICD that supports the customized combination of concepts and modifier codes to capture previously inaccessible clinical nuance,” he wrote in a coauthored invited commentary.
This added clinical nuance, however, will potentially make coding more complex, he said. One solution is to automate coding, such that clinicians could input information in a natural clinical format that makes sense to them, which would then be translated into ICD-11 code by a program. (This would then be translated back to the user in the natural clinical format to ensure accuracy.)
This type of process would limit how much any one person would need to know about ICD-11 to code diagnoses effectively, while also taking full advantage of the increasing specificity of the new coding system, he said.
Such a program does not yet exist but could be possible with intensive investment in the transition to ICD-11.
The findings of this study serve as a cautionary tale for future transitions to new systems without considering the importance of user experience and usability, Dr. Pine noted. If the United States takes an approach for the adoption of ICD-11 that is similar to that used for ICD-10, it is likely to be “just another overhyped transition” that will make users unwilling to adopt any new system moving forward out of frustration.
But if the United States takes a different, innovative approach, the opposite could be true.
“In short, the US must decide whether it is time to invest considerable resources and effort into a 21st-century information system that could overcome such hindrances as asymmetric information for decision-making, faulty risk adjustment in performance evaluations and payment formulas, and burdens imposed by current coding and documentation practices,” the commentary reads.
“It will allow us to make the best of what computers do and the best of what clinicians do,” Dr. Pine added, “and get them to work together in ways which would not have been conceivable 50 years ago.”
No information on study funding was provided. Mr. Zhu and Dr. Pine did not disclose any competing interests.
A version of this article appeared on Medscape.com.
Inflammatory arthritis codes increased 30-fold in the transition from the ninth to the 10th revision of the International Classification of Diseases (ICD-9 and -10), yet few were used in clinical practice, according to new research.
Most of the new codes for inflammatory arthritis in ICD-10 were rarely used, if at all, from 2015 to 2021.
“About 10-20 codes were comprising the majority of usage for inflammatory arthritis patients in ICD-10,” first author Justin Zhu, a researcher and medical student at Yale University in New Haven, Connecticut, told this news organization. “The other 380 or 400 codes just weren’t seeing a lot of use.”
The findings show the difficulties of transitioning to a new system, he added, and emphasize the need for additional training to improve adoption of ICD-11. The new coding system launched globally in January 2022, but it is not clear when it will be implemented in the United States.
ICD-10 was launched in the United States in 2015, with the goal of enabling greater specificity in identifying health conditions. For example, the new coding system allowed users to include information on laterality and anatomic location for the first time. The total number of codes increased from 14,500 with ICD-9 to 70,000 with ICD-10, with the number of inflammatory arthritis diagnosis codes growing from 14 to 425.
To see how these ICD-10 codes were utilized compared with ICD-9, Zhu and colleagues used national multi-insurance administrative claims data to find inflammatory arthritis diagnostic codes for over 5.1 million patients. About half were coded in ICD-9, while the remaining half were coded in ICD-10. Mr. Zhu and colleagues defined “higher-usage codes” as those that were used more than 1% of the time.
The findings were published in a research letter in JAMA Network Open on April 18.
For ICD-9, four of the available 14 codes (28.6%) were higher-usage codes. In contrast, only nine of the 425 ICD-10 codes (2.1%) were frequently used. Though ICD-10 allowed for increased granularity in diagnosis, data showed that nonspecific codes were most popular. Of the 20 most used ICD-10 arthritis codes, 65% contained “unspecified or other specified” in its wording.
The researchers also found that there was no significant change in these higher-usage codes throughout the study period from 2015 to 2021, suggesting there was not a detectable learning curve in ICD-10 usage among physicians and coders. They also found that clinician specialty did not change code usage patterns.
“The percentage of codes used was not better for rheumatologists (who might be expected to be more refined users of such codes) than primary care clinicians,” Mr. Zhu and colleagues wrote.
Moving to ICD-11 Brings Challenges as Well as Opportunities
Mr. Zhu noted that the study highlights the challenges of adopting new technological systems into daily practice, which can inform the eventual transition to ICD-11.
“There is this need to emphasize training as well as just invest more in improving adoption of ICD-11,” he said.
Michael Pine, MD, MBA, of MJP Healthcare Innovations, LLC in Evanston, Illinois, added that ICD-11 needs to be more user-friendly to be useful in practice. While ICD-10 allowed for greater granularity in coding, it did not result in “usable granularity, in terms of the things doctors really want to communicate,” he told this news organization.
And the transition to ICD-11 could pose greater challenges; rather than ICD-10’s taxonomy system, ICD-11 is formatted as an ontology.
“Although ICD-11 retains some precoordinated codes that convey multifaceted compound concepts, its structure and syntax also provide for post-coordination, a new feature to the ICD that supports the customized combination of concepts and modifier codes to capture previously inaccessible clinical nuance,” he wrote in a coauthored invited commentary.
This added clinical nuance, however, will potentially make coding more complex, he said. One solution is to automate coding, such that clinicians could input information in a natural clinical format that makes sense to them, which would then be translated into ICD-11 code by a program. (This would then be translated back to the user in the natural clinical format to ensure accuracy.)
This type of process would limit how much any one person would need to know about ICD-11 to code diagnoses effectively, while also taking full advantage of the increasing specificity of the new coding system, he said.
Such a program does not yet exist but could be possible with intensive investment in the transition to ICD-11.
The findings of this study serve as a cautionary tale for future transitions to new systems without considering the importance of user experience and usability, Dr. Pine noted. If the United States takes an approach for the adoption of ICD-11 that is similar to that used for ICD-10, it is likely to be “just another overhyped transition” that will make users unwilling to adopt any new system moving forward out of frustration.
But if the United States takes a different, innovative approach, the opposite could be true.
“In short, the US must decide whether it is time to invest considerable resources and effort into a 21st-century information system that could overcome such hindrances as asymmetric information for decision-making, faulty risk adjustment in performance evaluations and payment formulas, and burdens imposed by current coding and documentation practices,” the commentary reads.
“It will allow us to make the best of what computers do and the best of what clinicians do,” Dr. Pine added, “and get them to work together in ways which would not have been conceivable 50 years ago.”
No information on study funding was provided. Mr. Zhu and Dr. Pine did not disclose any competing interests.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Combined Pediatric Derm-Rheum Clinics Supported by Survey Respondents
TOPLINE:
.
METHODOLOGY:
- Combined pediatric dermatology-rheumatology clinics can improve patient outcomes and experiences, particularly for pediatric autoimmune conditions presenting with both cutaneous and systemic manifestations.
- The researchers surveyed 208 pediatric dermatologists working in combined pediatric dermatology-rheumatology clinics.
- A total of 13 member responses were recorded from three countries: 10 from the United States, two from Mexico, and one from Canada.
TAKEAWAY:
- Perceived benefits of combined clinics were improved patient care through coordinated treatment decisions and timely communication between providers.
- Patient satisfaction was favorable, and patients and families endorsed the combined clinic approach.
- Barriers to clinic establishment included differences in the pace between dermatology and rheumatology clinic flow, the need to generate more relative value units, resistance from colleagues, and limited time.
- Areas that needed improvement included more time for patient visits, dedicated research assistants, new patient referrals, additional patient rooms, resources for research, and patient care infrastructure.
IN PRACTICE:
The insights from this survey “will hopefully inspire further development of these combined clinics,” the authors wrote.
SOURCE:
The investigation, led by Olga S. Cherepakhin, BS, University of Washington, Seattle, Washington, was published in Pediatric Dermatology.
LIMITATIONS:
Limitations included the subjective nature, lack of some information, selection bias, and small number of respondents, and the survey reflected the perspective of the pediatric dermatologists only.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. One author reported full-time employment at Janssen R&D, and the other authors had no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Combined pediatric dermatology-rheumatology clinics can improve patient outcomes and experiences, particularly for pediatric autoimmune conditions presenting with both cutaneous and systemic manifestations.
- The researchers surveyed 208 pediatric dermatologists working in combined pediatric dermatology-rheumatology clinics.
- A total of 13 member responses were recorded from three countries: 10 from the United States, two from Mexico, and one from Canada.
TAKEAWAY:
- Perceived benefits of combined clinics were improved patient care through coordinated treatment decisions and timely communication between providers.
- Patient satisfaction was favorable, and patients and families endorsed the combined clinic approach.
- Barriers to clinic establishment included differences in the pace between dermatology and rheumatology clinic flow, the need to generate more relative value units, resistance from colleagues, and limited time.
- Areas that needed improvement included more time for patient visits, dedicated research assistants, new patient referrals, additional patient rooms, resources for research, and patient care infrastructure.
IN PRACTICE:
The insights from this survey “will hopefully inspire further development of these combined clinics,” the authors wrote.
SOURCE:
The investigation, led by Olga S. Cherepakhin, BS, University of Washington, Seattle, Washington, was published in Pediatric Dermatology.
LIMITATIONS:
Limitations included the subjective nature, lack of some information, selection bias, and small number of respondents, and the survey reflected the perspective of the pediatric dermatologists only.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. One author reported full-time employment at Janssen R&D, and the other authors had no disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
.
METHODOLOGY:
- Combined pediatric dermatology-rheumatology clinics can improve patient outcomes and experiences, particularly for pediatric autoimmune conditions presenting with both cutaneous and systemic manifestations.
- The researchers surveyed 208 pediatric dermatologists working in combined pediatric dermatology-rheumatology clinics.
- A total of 13 member responses were recorded from three countries: 10 from the United States, two from Mexico, and one from Canada.
TAKEAWAY:
- Perceived benefits of combined clinics were improved patient care through coordinated treatment decisions and timely communication between providers.
- Patient satisfaction was favorable, and patients and families endorsed the combined clinic approach.
- Barriers to clinic establishment included differences in the pace between dermatology and rheumatology clinic flow, the need to generate more relative value units, resistance from colleagues, and limited time.
- Areas that needed improvement included more time for patient visits, dedicated research assistants, new patient referrals, additional patient rooms, resources for research, and patient care infrastructure.
IN PRACTICE:
The insights from this survey “will hopefully inspire further development of these combined clinics,” the authors wrote.
SOURCE:
The investigation, led by Olga S. Cherepakhin, BS, University of Washington, Seattle, Washington, was published in Pediatric Dermatology.
LIMITATIONS:
Limitations included the subjective nature, lack of some information, selection bias, and small number of respondents, and the survey reflected the perspective of the pediatric dermatologists only.
DISCLOSURES:
The study was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health. One author reported full-time employment at Janssen R&D, and the other authors had no disclosures.
A version of this article appeared on Medscape.com.
Body Fat Levels Affect Physical Function in Biologic-Treated Axial Spondyloarthritis
TOPLINE:
Higher levels of body fat and visceral adipose tissue are associated with increased functional disability and reduced spinal mobility in patients with axial spondyloarthritis (axSpA) receiving biologic disease-modifying antirheumatic drugs (bDMARDs).
METHODOLOGY:
- Research showed that patients with axSpA respond poorly to tumor necrosis factor inhibitors if they have a high body mass index (BMI) or obesity; however, studies delving into the association between biologic therapy and body composition are limited.
- Researchers investigated the association between body composition evaluated by bioimpedance analysis and disease activity, physical function, and mobility in 74 patients with axSpA (mean age, 36.5; 71.6% men) at 6 months and 1 year after initiating bDMARDs.
- These participants from the German Spondyloarthritis Inception Cohort presented with high disease activity despite previous treatment with nonsteroidal anti-inflammatory drugs and initiated bDMARD therapy between 2015 and 2019.
- Bath Ankylosing Spondylitis Disease Activity Index and Axial Spondyloarthritis Disease Activity Score were used to measure disease activity, while Bath Ankylosing Spondylitis Functional Index and Bath Ankylosing Spondylitis Mobility Index assessed physical function and spinal mobility, respectively.
- BMI, fat mass, fat mass index, and visceral adipose tissue (VAT) were used to determine body composition along with other parameters.
TAKEAWAY:
- Higher BMI (parameter estimates [β], 0.081; 95% CI, 0.016-0.145), fat mass (β, 0.037; 95% CI, 0.004-0.070), and fat mass index (β, 0.125; 95% CI, 0.031-0.219) were associated with worse physical function in the overall population.
- VAT was positively associated with reduced spinal mobility (β, 0.201; 95% CI, 0.071-0.332), particularly in men.
- In women, an increase in VAT was linked to worse disease activity and functional disability.
- Treatment with bDMARDs reduced all disease activity parameters but led to an increase in BMI and fat-related parameters, indicating that lifestyle modifications are also necessary to achieve the desired outcomes with bDMARD therapy.
IN PRACTICE:
“Overall, our findings highlight the importance of maintaining a healthy body weight and body composition — characterized by adequate lean mass and reduced FM [fat mass] — to improve physical function and quality of life in patients with SpA,” the authors wrote.
SOURCE:
The study was led by Valeria Rios Rodriguez, MD, department of gastroenterology, infectiology and rheumatology, Charité Universitätsmedizin Berlin, Germany. It was published online March 20, 2024, in Rheumatology (Oxford).
LIMITATIONS:
This study lacked a control group of patients with axSpA who did not receive biologics. It also did not include dietary habits and comorbidities such as hypertension or diabetes. Additionally, bioimpedance analysis was chosen as the method to assess body composition instead of dual-energy x-ray absorptiometry.
DISCLOSURES:
The study was funded by the German Federal Ministry of Education and Research and the Berlin Institute of Health. Some of the authors declared receiving personal fees, grants, and consulting fees from various pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher levels of body fat and visceral adipose tissue are associated with increased functional disability and reduced spinal mobility in patients with axial spondyloarthritis (axSpA) receiving biologic disease-modifying antirheumatic drugs (bDMARDs).
METHODOLOGY:
- Research showed that patients with axSpA respond poorly to tumor necrosis factor inhibitors if they have a high body mass index (BMI) or obesity; however, studies delving into the association between biologic therapy and body composition are limited.
- Researchers investigated the association between body composition evaluated by bioimpedance analysis and disease activity, physical function, and mobility in 74 patients with axSpA (mean age, 36.5; 71.6% men) at 6 months and 1 year after initiating bDMARDs.
- These participants from the German Spondyloarthritis Inception Cohort presented with high disease activity despite previous treatment with nonsteroidal anti-inflammatory drugs and initiated bDMARD therapy between 2015 and 2019.
- Bath Ankylosing Spondylitis Disease Activity Index and Axial Spondyloarthritis Disease Activity Score were used to measure disease activity, while Bath Ankylosing Spondylitis Functional Index and Bath Ankylosing Spondylitis Mobility Index assessed physical function and spinal mobility, respectively.
- BMI, fat mass, fat mass index, and visceral adipose tissue (VAT) were used to determine body composition along with other parameters.
TAKEAWAY:
- Higher BMI (parameter estimates [β], 0.081; 95% CI, 0.016-0.145), fat mass (β, 0.037; 95% CI, 0.004-0.070), and fat mass index (β, 0.125; 95% CI, 0.031-0.219) were associated with worse physical function in the overall population.
- VAT was positively associated with reduced spinal mobility (β, 0.201; 95% CI, 0.071-0.332), particularly in men.
- In women, an increase in VAT was linked to worse disease activity and functional disability.
- Treatment with bDMARDs reduced all disease activity parameters but led to an increase in BMI and fat-related parameters, indicating that lifestyle modifications are also necessary to achieve the desired outcomes with bDMARD therapy.
IN PRACTICE:
“Overall, our findings highlight the importance of maintaining a healthy body weight and body composition — characterized by adequate lean mass and reduced FM [fat mass] — to improve physical function and quality of life in patients with SpA,” the authors wrote.
SOURCE:
The study was led by Valeria Rios Rodriguez, MD, department of gastroenterology, infectiology and rheumatology, Charité Universitätsmedizin Berlin, Germany. It was published online March 20, 2024, in Rheumatology (Oxford).
LIMITATIONS:
This study lacked a control group of patients with axSpA who did not receive biologics. It also did not include dietary habits and comorbidities such as hypertension or diabetes. Additionally, bioimpedance analysis was chosen as the method to assess body composition instead of dual-energy x-ray absorptiometry.
DISCLOSURES:
The study was funded by the German Federal Ministry of Education and Research and the Berlin Institute of Health. Some of the authors declared receiving personal fees, grants, and consulting fees from various pharmaceutical companies.
A version of this article appeared on Medscape.com.
TOPLINE:
Higher levels of body fat and visceral adipose tissue are associated with increased functional disability and reduced spinal mobility in patients with axial spondyloarthritis (axSpA) receiving biologic disease-modifying antirheumatic drugs (bDMARDs).
METHODOLOGY:
- Research showed that patients with axSpA respond poorly to tumor necrosis factor inhibitors if they have a high body mass index (BMI) or obesity; however, studies delving into the association between biologic therapy and body composition are limited.
- Researchers investigated the association between body composition evaluated by bioimpedance analysis and disease activity, physical function, and mobility in 74 patients with axSpA (mean age, 36.5; 71.6% men) at 6 months and 1 year after initiating bDMARDs.
- These participants from the German Spondyloarthritis Inception Cohort presented with high disease activity despite previous treatment with nonsteroidal anti-inflammatory drugs and initiated bDMARD therapy between 2015 and 2019.
- Bath Ankylosing Spondylitis Disease Activity Index and Axial Spondyloarthritis Disease Activity Score were used to measure disease activity, while Bath Ankylosing Spondylitis Functional Index and Bath Ankylosing Spondylitis Mobility Index assessed physical function and spinal mobility, respectively.
- BMI, fat mass, fat mass index, and visceral adipose tissue (VAT) were used to determine body composition along with other parameters.
TAKEAWAY:
- Higher BMI (parameter estimates [β], 0.081; 95% CI, 0.016-0.145), fat mass (β, 0.037; 95% CI, 0.004-0.070), and fat mass index (β, 0.125; 95% CI, 0.031-0.219) were associated with worse physical function in the overall population.
- VAT was positively associated with reduced spinal mobility (β, 0.201; 95% CI, 0.071-0.332), particularly in men.
- In women, an increase in VAT was linked to worse disease activity and functional disability.
- Treatment with bDMARDs reduced all disease activity parameters but led to an increase in BMI and fat-related parameters, indicating that lifestyle modifications are also necessary to achieve the desired outcomes with bDMARD therapy.
IN PRACTICE:
“Overall, our findings highlight the importance of maintaining a healthy body weight and body composition — characterized by adequate lean mass and reduced FM [fat mass] — to improve physical function and quality of life in patients with SpA,” the authors wrote.
SOURCE:
The study was led by Valeria Rios Rodriguez, MD, department of gastroenterology, infectiology and rheumatology, Charité Universitätsmedizin Berlin, Germany. It was published online March 20, 2024, in Rheumatology (Oxford).
LIMITATIONS:
This study lacked a control group of patients with axSpA who did not receive biologics. It also did not include dietary habits and comorbidities such as hypertension or diabetes. Additionally, bioimpedance analysis was chosen as the method to assess body composition instead of dual-energy x-ray absorptiometry.
DISCLOSURES:
The study was funded by the German Federal Ministry of Education and Research and the Berlin Institute of Health. Some of the authors declared receiving personal fees, grants, and consulting fees from various pharmaceutical companies.
A version of this article appeared on Medscape.com.








