User login
DAPT may benefit symptomatic carotid endarterectomy patients
LOS ANGELES –
The only patients who benefited from postsurgical treatment with dual antiplatelet therapy (DAPT) were those who were symptomatic (had a stroke or transient ischemic attack) prior to their carotid endarterectomy surgery, a minority of the more than 17,000 matched U.S. patients who underwent carotid endarterectomy during 2003-2018 and were part of this analysis, Nathan Belkin, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
Among patients with symptoms prior to their carotid endarterectomy, DAPT at the time of hospital discharge was associated with a 2-year follow-up rate of stroke, transient ischemic attack (TIA), or death of about 8%, compared with a rate of about 11% among similar patients discharged on aspirin only, a statistically significant difference. In contrast, among patients who were asymptomatic prior to their carotid endarterectomy, discharge treatment with aspirin only was associated with a 2-year event rate similar to the rate among patients discharged on DAPT.
Based in part on this finding, Dr. Belkin and associates at the University of Pennsylvania, Philadelphia, now start symptomatic patients scheduled for carotid endarterectomy on DAPT with aspirin plus clopidogrel (Plavix) about 2 weeks before surgery, and then continue the combined regimen long term after surgery. A prospective, randomized study is needed to fully resolve the optimal use of DAPT in patients with significant carotid artery disease scheduled for carotid endarterectomy, but until then, “we’re individualizing DAPT” to patients at high risk because of a prior stroke or TIA who also have no evidence of an elevated bleeding risk, said Dr. Belkin, a vascular surgeon.
“We hypothesize that patients with systemic carotid disease have a systemic disease process and more activated platelets,” which suggests a potential benefit from DAPT, he explained. But the data that Dr. Belkin reported also indicated that recent U.S. use of DAPT in patients undergoing carotid endarterectomy has moved beyond this subgroup. The U.S. national data set that Dr. Belkin used for the analysis, the Vascular Quality Initiative registry maintained by the Society for Vascular Surgery, included 87,074 patients who underwent carotid endarterectomy during 2003-2018. During the entire 16-year period, 30% of patients overall received a prescription for DAPT at hospital discharge, but this level went steadily up during those years. In 2003, the rate of DAPT prescriptions at discharge was below 10% of patients but then rose incrementally over the following years and by 2018 had increased to about 44% despite a prevalence of symptomatic carotid disease closer to about a third of patients.
“It’s surprising that so many patients received DAPT for carotid disease” in recent years, commented Mai N. Nguyen-Huynh, MD, a vascular neurologist with Kaiser Permanente Northern California in Oakland. “It’s been thought that DAPT, and especially clopidogrel, was more beneficial for patients with intracranial atherosclerotic disease, but not so much for patients with carotid disease,” she said in an interview. “We don’t always see systemic atherosclerotic disease in patients with carotid artery disease. It’s not standard practice to look for systemic atherosclerotic disease in patients with carotid disease,” unless something in the patient’s presentation suggests wider vascular-disease progression.
The primary analysis that Dr. Belkin and associates ran removed about 16% of the patients who underwent carotid endarterectomy from the database: those who received no antiplatelet drug, those who received only clopidogrel, and those who went home from surgery on an anticoagulant. Among the remaining 72,122 patients, 35% received DAPT at discharge and 65% received aspirin only. The patients averaged 70 years old, 61% were men, 37% had a history of stroke or TIA, and their overall 2-year incidence of stroke, TIA, or death was 7.3%. To adjust for many baseline differences between the patients discharged on DAPT and those who got only aspirin, the researchers used propensity-score sorting to identify 17,398 matched patients from the two treatment subgroups, 24% of the total population. Comparison of these DAPT and aspirin-only subgroups showed no difference in the overall, 2-year rate of stroke, TIA, or death.
However, when the analysis divided the patients into asymptomatic and symptomatic subgroups, those discharged on DAPT showed a statistically significant lower rate of stroke, TIA, or death during 2 years of follow-up. The same symptomatic subgroup also showed a statistically significant lower rate of total mortality during 5 years of follow-up when treated with DAPT compared with aspirin only, again an absolute, between-group difference of about 3 percentage points that was statistically significant, a difference not seen in the asymptomatic patients. The type of treatment that symptomatic patients received had no relationship to their 2-year incidence of stroke or TIA.
To confirm these findings, Dr. Belkin and coworkers ran a multivariate logistic regression analysis on the data collected from all 72,122 patients who underwent carotid endarterectomy and subsequently received either DAPT or aspirin only. The only statistically significant association between treatment and outcome was among the symptomatic patients who received DAPT, who had a significant reduction in their 5-year mortality, compared with symptomatic patients who received only aspirin at hospital discharge.
Ideally, a comparison of DAPT and aspirin-only treatment should also assess the incidence and severity of bleeding events associated with these treatments, but bleeding data were not available in the database, Dr. Belkin said.
Dr. Belkin and Dr. Nguyen-Huynh had no relevant disclosures.
SOURCE: Belkin N et al. Stroke. 2020 Feb;51(suppl 1): Abstract 67.
LOS ANGELES –
The only patients who benefited from postsurgical treatment with dual antiplatelet therapy (DAPT) were those who were symptomatic (had a stroke or transient ischemic attack) prior to their carotid endarterectomy surgery, a minority of the more than 17,000 matched U.S. patients who underwent carotid endarterectomy during 2003-2018 and were part of this analysis, Nathan Belkin, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
Among patients with symptoms prior to their carotid endarterectomy, DAPT at the time of hospital discharge was associated with a 2-year follow-up rate of stroke, transient ischemic attack (TIA), or death of about 8%, compared with a rate of about 11% among similar patients discharged on aspirin only, a statistically significant difference. In contrast, among patients who were asymptomatic prior to their carotid endarterectomy, discharge treatment with aspirin only was associated with a 2-year event rate similar to the rate among patients discharged on DAPT.
Based in part on this finding, Dr. Belkin and associates at the University of Pennsylvania, Philadelphia, now start symptomatic patients scheduled for carotid endarterectomy on DAPT with aspirin plus clopidogrel (Plavix) about 2 weeks before surgery, and then continue the combined regimen long term after surgery. A prospective, randomized study is needed to fully resolve the optimal use of DAPT in patients with significant carotid artery disease scheduled for carotid endarterectomy, but until then, “we’re individualizing DAPT” to patients at high risk because of a prior stroke or TIA who also have no evidence of an elevated bleeding risk, said Dr. Belkin, a vascular surgeon.
“We hypothesize that patients with systemic carotid disease have a systemic disease process and more activated platelets,” which suggests a potential benefit from DAPT, he explained. But the data that Dr. Belkin reported also indicated that recent U.S. use of DAPT in patients undergoing carotid endarterectomy has moved beyond this subgroup. The U.S. national data set that Dr. Belkin used for the analysis, the Vascular Quality Initiative registry maintained by the Society for Vascular Surgery, included 87,074 patients who underwent carotid endarterectomy during 2003-2018. During the entire 16-year period, 30% of patients overall received a prescription for DAPT at hospital discharge, but this level went steadily up during those years. In 2003, the rate of DAPT prescriptions at discharge was below 10% of patients but then rose incrementally over the following years and by 2018 had increased to about 44% despite a prevalence of symptomatic carotid disease closer to about a third of patients.
“It’s surprising that so many patients received DAPT for carotid disease” in recent years, commented Mai N. Nguyen-Huynh, MD, a vascular neurologist with Kaiser Permanente Northern California in Oakland. “It’s been thought that DAPT, and especially clopidogrel, was more beneficial for patients with intracranial atherosclerotic disease, but not so much for patients with carotid disease,” she said in an interview. “We don’t always see systemic atherosclerotic disease in patients with carotid artery disease. It’s not standard practice to look for systemic atherosclerotic disease in patients with carotid disease,” unless something in the patient’s presentation suggests wider vascular-disease progression.
The primary analysis that Dr. Belkin and associates ran removed about 16% of the patients who underwent carotid endarterectomy from the database: those who received no antiplatelet drug, those who received only clopidogrel, and those who went home from surgery on an anticoagulant. Among the remaining 72,122 patients, 35% received DAPT at discharge and 65% received aspirin only. The patients averaged 70 years old, 61% were men, 37% had a history of stroke or TIA, and their overall 2-year incidence of stroke, TIA, or death was 7.3%. To adjust for many baseline differences between the patients discharged on DAPT and those who got only aspirin, the researchers used propensity-score sorting to identify 17,398 matched patients from the two treatment subgroups, 24% of the total population. Comparison of these DAPT and aspirin-only subgroups showed no difference in the overall, 2-year rate of stroke, TIA, or death.
However, when the analysis divided the patients into asymptomatic and symptomatic subgroups, those discharged on DAPT showed a statistically significant lower rate of stroke, TIA, or death during 2 years of follow-up. The same symptomatic subgroup also showed a statistically significant lower rate of total mortality during 5 years of follow-up when treated with DAPT compared with aspirin only, again an absolute, between-group difference of about 3 percentage points that was statistically significant, a difference not seen in the asymptomatic patients. The type of treatment that symptomatic patients received had no relationship to their 2-year incidence of stroke or TIA.
To confirm these findings, Dr. Belkin and coworkers ran a multivariate logistic regression analysis on the data collected from all 72,122 patients who underwent carotid endarterectomy and subsequently received either DAPT or aspirin only. The only statistically significant association between treatment and outcome was among the symptomatic patients who received DAPT, who had a significant reduction in their 5-year mortality, compared with symptomatic patients who received only aspirin at hospital discharge.
Ideally, a comparison of DAPT and aspirin-only treatment should also assess the incidence and severity of bleeding events associated with these treatments, but bleeding data were not available in the database, Dr. Belkin said.
Dr. Belkin and Dr. Nguyen-Huynh had no relevant disclosures.
SOURCE: Belkin N et al. Stroke. 2020 Feb;51(suppl 1): Abstract 67.
LOS ANGELES –
The only patients who benefited from postsurgical treatment with dual antiplatelet therapy (DAPT) were those who were symptomatic (had a stroke or transient ischemic attack) prior to their carotid endarterectomy surgery, a minority of the more than 17,000 matched U.S. patients who underwent carotid endarterectomy during 2003-2018 and were part of this analysis, Nathan Belkin, MD, said at the International Stroke Conference, sponsored by the American Heart Association.
Among patients with symptoms prior to their carotid endarterectomy, DAPT at the time of hospital discharge was associated with a 2-year follow-up rate of stroke, transient ischemic attack (TIA), or death of about 8%, compared with a rate of about 11% among similar patients discharged on aspirin only, a statistically significant difference. In contrast, among patients who were asymptomatic prior to their carotid endarterectomy, discharge treatment with aspirin only was associated with a 2-year event rate similar to the rate among patients discharged on DAPT.
Based in part on this finding, Dr. Belkin and associates at the University of Pennsylvania, Philadelphia, now start symptomatic patients scheduled for carotid endarterectomy on DAPT with aspirin plus clopidogrel (Plavix) about 2 weeks before surgery, and then continue the combined regimen long term after surgery. A prospective, randomized study is needed to fully resolve the optimal use of DAPT in patients with significant carotid artery disease scheduled for carotid endarterectomy, but until then, “we’re individualizing DAPT” to patients at high risk because of a prior stroke or TIA who also have no evidence of an elevated bleeding risk, said Dr. Belkin, a vascular surgeon.
“We hypothesize that patients with systemic carotid disease have a systemic disease process and more activated platelets,” which suggests a potential benefit from DAPT, he explained. But the data that Dr. Belkin reported also indicated that recent U.S. use of DAPT in patients undergoing carotid endarterectomy has moved beyond this subgroup. The U.S. national data set that Dr. Belkin used for the analysis, the Vascular Quality Initiative registry maintained by the Society for Vascular Surgery, included 87,074 patients who underwent carotid endarterectomy during 2003-2018. During the entire 16-year period, 30% of patients overall received a prescription for DAPT at hospital discharge, but this level went steadily up during those years. In 2003, the rate of DAPT prescriptions at discharge was below 10% of patients but then rose incrementally over the following years and by 2018 had increased to about 44% despite a prevalence of symptomatic carotid disease closer to about a third of patients.
“It’s surprising that so many patients received DAPT for carotid disease” in recent years, commented Mai N. Nguyen-Huynh, MD, a vascular neurologist with Kaiser Permanente Northern California in Oakland. “It’s been thought that DAPT, and especially clopidogrel, was more beneficial for patients with intracranial atherosclerotic disease, but not so much for patients with carotid disease,” she said in an interview. “We don’t always see systemic atherosclerotic disease in patients with carotid artery disease. It’s not standard practice to look for systemic atherosclerotic disease in patients with carotid disease,” unless something in the patient’s presentation suggests wider vascular-disease progression.
The primary analysis that Dr. Belkin and associates ran removed about 16% of the patients who underwent carotid endarterectomy from the database: those who received no antiplatelet drug, those who received only clopidogrel, and those who went home from surgery on an anticoagulant. Among the remaining 72,122 patients, 35% received DAPT at discharge and 65% received aspirin only. The patients averaged 70 years old, 61% were men, 37% had a history of stroke or TIA, and their overall 2-year incidence of stroke, TIA, or death was 7.3%. To adjust for many baseline differences between the patients discharged on DAPT and those who got only aspirin, the researchers used propensity-score sorting to identify 17,398 matched patients from the two treatment subgroups, 24% of the total population. Comparison of these DAPT and aspirin-only subgroups showed no difference in the overall, 2-year rate of stroke, TIA, or death.
However, when the analysis divided the patients into asymptomatic and symptomatic subgroups, those discharged on DAPT showed a statistically significant lower rate of stroke, TIA, or death during 2 years of follow-up. The same symptomatic subgroup also showed a statistically significant lower rate of total mortality during 5 years of follow-up when treated with DAPT compared with aspirin only, again an absolute, between-group difference of about 3 percentage points that was statistically significant, a difference not seen in the asymptomatic patients. The type of treatment that symptomatic patients received had no relationship to their 2-year incidence of stroke or TIA.
To confirm these findings, Dr. Belkin and coworkers ran a multivariate logistic regression analysis on the data collected from all 72,122 patients who underwent carotid endarterectomy and subsequently received either DAPT or aspirin only. The only statistically significant association between treatment and outcome was among the symptomatic patients who received DAPT, who had a significant reduction in their 5-year mortality, compared with symptomatic patients who received only aspirin at hospital discharge.
Ideally, a comparison of DAPT and aspirin-only treatment should also assess the incidence and severity of bleeding events associated with these treatments, but bleeding data were not available in the database, Dr. Belkin said.
Dr. Belkin and Dr. Nguyen-Huynh had no relevant disclosures.
SOURCE: Belkin N et al. Stroke. 2020 Feb;51(suppl 1): Abstract 67.
REPORTING FROM ISC 2020
Transradial access gains converts among U.S. interventional neurologists
LOS ANGELES –
“It’s growing dramatically in U.S. practice. It may be hype, but there is big excitement. We are still in an assessment mode, but the adoption rate has been high,” Raul G. Nogueira, MD, said in an interview during the International Stroke Conference sponsored by the American Heart Association. “The big advantage [of transradial catheterization entry] is elimination of groin complications, some of which can be pretty bad. Is it safe for the brain? It’s probably okay, but that needs more study,” said Dr. Nogueira, professor of neurology at Emory University and director of the Neurovascular Service at the Grady Marcus Stroke and Neuroscience Center in Atlanta.
His uncertainty stems from the more difficult route taken to advance a catheter from the wrist into brain vessels, a maneuver that requires significant manipulation of the catheter tip, unlike the path from the right radial artery into the heart’s arteries, a “straight shot,” he explained. To reach the brain’s vasculature, the tip must execute a spin “that may scrape small emboli from the arch or arteries, so we need to look at this a little more carefully.” Ideally in a prospective, randomized study, he said. “We need to see whether the burden of [magnetic resonance] lesions is any higher when you go through the radial [artery].”
Some of the first-reported, large-scale U.S. experiences using a radial-artery approach for various neurovascular procedures, including a few thrombectomy cases, came in a series of 1,272 patients treated at any of four U.S. centers during July 2018 to June 2019, a period when the neurovascular staffs at all four centers transitioned from primarily using femoral-artery access to using radial access as their default mode. During the 12-month transition period, overall use of radial access at all four centers rose from roughly a quarter of all neurovascular interventions during July to September 2018 to closer to 80% by April to June 2019, Eyad Almallouhi, MD, reported at the conference.
During the entire 12 months, the operators ran up a 94% rate of successfully completed procedures using radial access, a rate that rose from about 88% during the first quarter to roughly 95% success during the fourth quarter tracked, said Dr. Almallouhi, a neurologist at the Medical University of South Carolina in Charleston. The rate of crossover from what began as a transradial procedure but switched to transfemoral was just under 6% overall, with a nearly 14% crossover rate during the first quarter that then dropped to around 5% for the rest of the transition year. Crossovers for interventional procedures throughout the study year occurred at a 12% rate, while crossovers for diagnostic procedures occurred at a 5% clip throughout the entire year.
None of the transradial patients had a major access-site complication, and minor complications occurred in less than 2% of the patients, including 11 with a forearm hematoma, 6 with forearm pain, and 5 with oozing at their access site. The absence of any major access-site complications among the transradial-access patients in this series contrasts with a recent report of a 1.7% rate of major complications secondary to femoral-artery access for mechanical thrombectomy in a combined analysis of data from seven published studies that included 660 thrombectomy procedures (Am J Neuroradiol. 2019 Feb. doi: 10.3174/ajnr.A6423). The other three centers that participated in the study Dr. Almallouhi presented were the University of Miami, Thomas Jefferson University in Philadelphia, and the University of Pittsburgh.
Of the 1,272 total procedures studied, 83% were diagnostic procedures, which had an overall 95% success rate, and 17% were interventional procedures, which had a success rate of 89%. The interventional transradial procedures included 62 primary coilings of aneurysms, 44 stent-assisted aneurysm coilings, 40 patients who underwent a flow diversion, 21 balloon-assisted aneurysm coilings, and 24 patients who underwent stroke thrombectomy.
The size of the devices commonly used for thrombectomy are often too large to allow for radial-artery access, noted Dr. Nogueira. For urgent interventions like thrombectomy “we use balloon-guided catheters that are large-bore and don’t fit well in the radial,” he said, although thrombectomy via the radial artery without a balloon-guided catheter is possible for clots located in the basilar artery. Last year, researchers in Germany reported using a balloon-guided catheter to perform mechanical thrombectomy via the radial artery (Interv Neuroradiol. 2019 Oct 1;25[5]:508-10). But it’s a different story for elective, diagnostic procedures. “I have moved most of these to transradial,” Dr. Nogueira said. He and his coauthors summarized the case for transradial access for cerebral angiography in a recent review; in addition to enhanced safety they cited other advantages including improved patient satisfaction and reduced cost because of a shorter length of stay (Interv Cardiol Clin. 2020 Jan;9[1]:75-86).
Despite his enthusiasm and the enthusiasm of other neurointerventionalists for the transradial approach, other stroke neurologists have been more cautious and slower to shift away from the femoral approach. “Our experience has been that for most cases it’s a bit more challenging to access the cervical vessels from the radial artery than from the traditional femoral approach. For arches with complex anatomy, however, the transradial approach can be of benefit in some cases, depending on the angles that need to be traversed,” commented Jeremy Payne, MD, director of the Banner Center for Neurovascular Medicine and medical director of the Banner—University Medical Center Phoenix Comprehensive Stroke Program. Dr. Payne highlighted that, while he is not an interventionalist himself, he and his interventional staff have regularly discussed the transradial option.
“In the cardiology literature the radial approach has been very successful, with better overall safety than the traditional femoral approach. Largely this seems to do with the anatomy of the aortic arch. It’s simply a more direct approach to the coronaries via the right radial artery; getting the wire into the correct vessel is significantly more difficult the more acute the angle it has to traverse,” such as when the target is an intracerebral vessel, Dr. Payne said in an interview.
“Our experience in the past 6 months has been about 25% transradial for some of our procedures, mainly diagnostic angiograms. We don’t find any difference in safety, however, as our transfemoral procedures are already very safe. One of the benefits of a transradial approach has been that a closure device may not be needed, with fewer vascular complications at the access site, such as fistula formation. We use ultrasound for access, and have not seen a difference in those approaches at all so far. One might argue that using ultrasound to establish access would slow us down, but so far our fastest case start-to-recanalization time in an acute stroke this year was 6 minutes, so speed does not appear to be a limiting issue. Another concern overall for transradial access is the potential limitation in the tools we may be able to deploy, given the smaller size of the vessel. It is reassuring [in the report from Dr. Almallouhi] that a variety of cases were successfully completed via this approach. However, fewer than 2% of their cases [24 patients] were apparently emergent, acute strokes, lending no specific support to that context. I do not expect that to change based on this paper,” Dr. Payne concluded.
“It is not clear to me that transradial neurointervention will change much. We have excellent safety data for the femoral approach, a proven track record of efficacy, and for most patients it seems to afford a somewhat wider range of tools that can be deployed, with simpler anatomy for accessing the cervical vessels in most arches. It is reassuring that the results reported by Dr. Almallouhi did not suggest negative outcomes, and as such I suspect the transradial approach at least gives us an additional option in a minority of patients. We have seen in the past 5-10 years an explosion of tools for the endovascular treatment of stroke; transradial access represents another potential strategy that appears so far to be safe,” Dr. Payne said.
Drs. Nogueira, Almallouhi, and Payne had no relevant disclosures.
SOURCE: Almallouhi E et al. Stroke. 2020 Feb;51(suppl 1):A64.
LOS ANGELES –
“It’s growing dramatically in U.S. practice. It may be hype, but there is big excitement. We are still in an assessment mode, but the adoption rate has been high,” Raul G. Nogueira, MD, said in an interview during the International Stroke Conference sponsored by the American Heart Association. “The big advantage [of transradial catheterization entry] is elimination of groin complications, some of which can be pretty bad. Is it safe for the brain? It’s probably okay, but that needs more study,” said Dr. Nogueira, professor of neurology at Emory University and director of the Neurovascular Service at the Grady Marcus Stroke and Neuroscience Center in Atlanta.
His uncertainty stems from the more difficult route taken to advance a catheter from the wrist into brain vessels, a maneuver that requires significant manipulation of the catheter tip, unlike the path from the right radial artery into the heart’s arteries, a “straight shot,” he explained. To reach the brain’s vasculature, the tip must execute a spin “that may scrape small emboli from the arch or arteries, so we need to look at this a little more carefully.” Ideally in a prospective, randomized study, he said. “We need to see whether the burden of [magnetic resonance] lesions is any higher when you go through the radial [artery].”
Some of the first-reported, large-scale U.S. experiences using a radial-artery approach for various neurovascular procedures, including a few thrombectomy cases, came in a series of 1,272 patients treated at any of four U.S. centers during July 2018 to June 2019, a period when the neurovascular staffs at all four centers transitioned from primarily using femoral-artery access to using radial access as their default mode. During the 12-month transition period, overall use of radial access at all four centers rose from roughly a quarter of all neurovascular interventions during July to September 2018 to closer to 80% by April to June 2019, Eyad Almallouhi, MD, reported at the conference.
During the entire 12 months, the operators ran up a 94% rate of successfully completed procedures using radial access, a rate that rose from about 88% during the first quarter to roughly 95% success during the fourth quarter tracked, said Dr. Almallouhi, a neurologist at the Medical University of South Carolina in Charleston. The rate of crossover from what began as a transradial procedure but switched to transfemoral was just under 6% overall, with a nearly 14% crossover rate during the first quarter that then dropped to around 5% for the rest of the transition year. Crossovers for interventional procedures throughout the study year occurred at a 12% rate, while crossovers for diagnostic procedures occurred at a 5% clip throughout the entire year.
None of the transradial patients had a major access-site complication, and minor complications occurred in less than 2% of the patients, including 11 with a forearm hematoma, 6 with forearm pain, and 5 with oozing at their access site. The absence of any major access-site complications among the transradial-access patients in this series contrasts with a recent report of a 1.7% rate of major complications secondary to femoral-artery access for mechanical thrombectomy in a combined analysis of data from seven published studies that included 660 thrombectomy procedures (Am J Neuroradiol. 2019 Feb. doi: 10.3174/ajnr.A6423). The other three centers that participated in the study Dr. Almallouhi presented were the University of Miami, Thomas Jefferson University in Philadelphia, and the University of Pittsburgh.
Of the 1,272 total procedures studied, 83% were diagnostic procedures, which had an overall 95% success rate, and 17% were interventional procedures, which had a success rate of 89%. The interventional transradial procedures included 62 primary coilings of aneurysms, 44 stent-assisted aneurysm coilings, 40 patients who underwent a flow diversion, 21 balloon-assisted aneurysm coilings, and 24 patients who underwent stroke thrombectomy.
The size of the devices commonly used for thrombectomy are often too large to allow for radial-artery access, noted Dr. Nogueira. For urgent interventions like thrombectomy “we use balloon-guided catheters that are large-bore and don’t fit well in the radial,” he said, although thrombectomy via the radial artery without a balloon-guided catheter is possible for clots located in the basilar artery. Last year, researchers in Germany reported using a balloon-guided catheter to perform mechanical thrombectomy via the radial artery (Interv Neuroradiol. 2019 Oct 1;25[5]:508-10). But it’s a different story for elective, diagnostic procedures. “I have moved most of these to transradial,” Dr. Nogueira said. He and his coauthors summarized the case for transradial access for cerebral angiography in a recent review; in addition to enhanced safety they cited other advantages including improved patient satisfaction and reduced cost because of a shorter length of stay (Interv Cardiol Clin. 2020 Jan;9[1]:75-86).
Despite his enthusiasm and the enthusiasm of other neurointerventionalists for the transradial approach, other stroke neurologists have been more cautious and slower to shift away from the femoral approach. “Our experience has been that for most cases it’s a bit more challenging to access the cervical vessels from the radial artery than from the traditional femoral approach. For arches with complex anatomy, however, the transradial approach can be of benefit in some cases, depending on the angles that need to be traversed,” commented Jeremy Payne, MD, director of the Banner Center for Neurovascular Medicine and medical director of the Banner—University Medical Center Phoenix Comprehensive Stroke Program. Dr. Payne highlighted that, while he is not an interventionalist himself, he and his interventional staff have regularly discussed the transradial option.
“In the cardiology literature the radial approach has been very successful, with better overall safety than the traditional femoral approach. Largely this seems to do with the anatomy of the aortic arch. It’s simply a more direct approach to the coronaries via the right radial artery; getting the wire into the correct vessel is significantly more difficult the more acute the angle it has to traverse,” such as when the target is an intracerebral vessel, Dr. Payne said in an interview.
“Our experience in the past 6 months has been about 25% transradial for some of our procedures, mainly diagnostic angiograms. We don’t find any difference in safety, however, as our transfemoral procedures are already very safe. One of the benefits of a transradial approach has been that a closure device may not be needed, with fewer vascular complications at the access site, such as fistula formation. We use ultrasound for access, and have not seen a difference in those approaches at all so far. One might argue that using ultrasound to establish access would slow us down, but so far our fastest case start-to-recanalization time in an acute stroke this year was 6 minutes, so speed does not appear to be a limiting issue. Another concern overall for transradial access is the potential limitation in the tools we may be able to deploy, given the smaller size of the vessel. It is reassuring [in the report from Dr. Almallouhi] that a variety of cases were successfully completed via this approach. However, fewer than 2% of their cases [24 patients] were apparently emergent, acute strokes, lending no specific support to that context. I do not expect that to change based on this paper,” Dr. Payne concluded.
“It is not clear to me that transradial neurointervention will change much. We have excellent safety data for the femoral approach, a proven track record of efficacy, and for most patients it seems to afford a somewhat wider range of tools that can be deployed, with simpler anatomy for accessing the cervical vessels in most arches. It is reassuring that the results reported by Dr. Almallouhi did not suggest negative outcomes, and as such I suspect the transradial approach at least gives us an additional option in a minority of patients. We have seen in the past 5-10 years an explosion of tools for the endovascular treatment of stroke; transradial access represents another potential strategy that appears so far to be safe,” Dr. Payne said.
Drs. Nogueira, Almallouhi, and Payne had no relevant disclosures.
SOURCE: Almallouhi E et al. Stroke. 2020 Feb;51(suppl 1):A64.
LOS ANGELES –
“It’s growing dramatically in U.S. practice. It may be hype, but there is big excitement. We are still in an assessment mode, but the adoption rate has been high,” Raul G. Nogueira, MD, said in an interview during the International Stroke Conference sponsored by the American Heart Association. “The big advantage [of transradial catheterization entry] is elimination of groin complications, some of which can be pretty bad. Is it safe for the brain? It’s probably okay, but that needs more study,” said Dr. Nogueira, professor of neurology at Emory University and director of the Neurovascular Service at the Grady Marcus Stroke and Neuroscience Center in Atlanta.
His uncertainty stems from the more difficult route taken to advance a catheter from the wrist into brain vessels, a maneuver that requires significant manipulation of the catheter tip, unlike the path from the right radial artery into the heart’s arteries, a “straight shot,” he explained. To reach the brain’s vasculature, the tip must execute a spin “that may scrape small emboli from the arch or arteries, so we need to look at this a little more carefully.” Ideally in a prospective, randomized study, he said. “We need to see whether the burden of [magnetic resonance] lesions is any higher when you go through the radial [artery].”
Some of the first-reported, large-scale U.S. experiences using a radial-artery approach for various neurovascular procedures, including a few thrombectomy cases, came in a series of 1,272 patients treated at any of four U.S. centers during July 2018 to June 2019, a period when the neurovascular staffs at all four centers transitioned from primarily using femoral-artery access to using radial access as their default mode. During the 12-month transition period, overall use of radial access at all four centers rose from roughly a quarter of all neurovascular interventions during July to September 2018 to closer to 80% by April to June 2019, Eyad Almallouhi, MD, reported at the conference.
During the entire 12 months, the operators ran up a 94% rate of successfully completed procedures using radial access, a rate that rose from about 88% during the first quarter to roughly 95% success during the fourth quarter tracked, said Dr. Almallouhi, a neurologist at the Medical University of South Carolina in Charleston. The rate of crossover from what began as a transradial procedure but switched to transfemoral was just under 6% overall, with a nearly 14% crossover rate during the first quarter that then dropped to around 5% for the rest of the transition year. Crossovers for interventional procedures throughout the study year occurred at a 12% rate, while crossovers for diagnostic procedures occurred at a 5% clip throughout the entire year.
None of the transradial patients had a major access-site complication, and minor complications occurred in less than 2% of the patients, including 11 with a forearm hematoma, 6 with forearm pain, and 5 with oozing at their access site. The absence of any major access-site complications among the transradial-access patients in this series contrasts with a recent report of a 1.7% rate of major complications secondary to femoral-artery access for mechanical thrombectomy in a combined analysis of data from seven published studies that included 660 thrombectomy procedures (Am J Neuroradiol. 2019 Feb. doi: 10.3174/ajnr.A6423). The other three centers that participated in the study Dr. Almallouhi presented were the University of Miami, Thomas Jefferson University in Philadelphia, and the University of Pittsburgh.
Of the 1,272 total procedures studied, 83% were diagnostic procedures, which had an overall 95% success rate, and 17% were interventional procedures, which had a success rate of 89%. The interventional transradial procedures included 62 primary coilings of aneurysms, 44 stent-assisted aneurysm coilings, 40 patients who underwent a flow diversion, 21 balloon-assisted aneurysm coilings, and 24 patients who underwent stroke thrombectomy.
The size of the devices commonly used for thrombectomy are often too large to allow for radial-artery access, noted Dr. Nogueira. For urgent interventions like thrombectomy “we use balloon-guided catheters that are large-bore and don’t fit well in the radial,” he said, although thrombectomy via the radial artery without a balloon-guided catheter is possible for clots located in the basilar artery. Last year, researchers in Germany reported using a balloon-guided catheter to perform mechanical thrombectomy via the radial artery (Interv Neuroradiol. 2019 Oct 1;25[5]:508-10). But it’s a different story for elective, diagnostic procedures. “I have moved most of these to transradial,” Dr. Nogueira said. He and his coauthors summarized the case for transradial access for cerebral angiography in a recent review; in addition to enhanced safety they cited other advantages including improved patient satisfaction and reduced cost because of a shorter length of stay (Interv Cardiol Clin. 2020 Jan;9[1]:75-86).
Despite his enthusiasm and the enthusiasm of other neurointerventionalists for the transradial approach, other stroke neurologists have been more cautious and slower to shift away from the femoral approach. “Our experience has been that for most cases it’s a bit more challenging to access the cervical vessels from the radial artery than from the traditional femoral approach. For arches with complex anatomy, however, the transradial approach can be of benefit in some cases, depending on the angles that need to be traversed,” commented Jeremy Payne, MD, director of the Banner Center for Neurovascular Medicine and medical director of the Banner—University Medical Center Phoenix Comprehensive Stroke Program. Dr. Payne highlighted that, while he is not an interventionalist himself, he and his interventional staff have regularly discussed the transradial option.
“In the cardiology literature the radial approach has been very successful, with better overall safety than the traditional femoral approach. Largely this seems to do with the anatomy of the aortic arch. It’s simply a more direct approach to the coronaries via the right radial artery; getting the wire into the correct vessel is significantly more difficult the more acute the angle it has to traverse,” such as when the target is an intracerebral vessel, Dr. Payne said in an interview.
“Our experience in the past 6 months has been about 25% transradial for some of our procedures, mainly diagnostic angiograms. We don’t find any difference in safety, however, as our transfemoral procedures are already very safe. One of the benefits of a transradial approach has been that a closure device may not be needed, with fewer vascular complications at the access site, such as fistula formation. We use ultrasound for access, and have not seen a difference in those approaches at all so far. One might argue that using ultrasound to establish access would slow us down, but so far our fastest case start-to-recanalization time in an acute stroke this year was 6 minutes, so speed does not appear to be a limiting issue. Another concern overall for transradial access is the potential limitation in the tools we may be able to deploy, given the smaller size of the vessel. It is reassuring [in the report from Dr. Almallouhi] that a variety of cases were successfully completed via this approach. However, fewer than 2% of their cases [24 patients] were apparently emergent, acute strokes, lending no specific support to that context. I do not expect that to change based on this paper,” Dr. Payne concluded.
“It is not clear to me that transradial neurointervention will change much. We have excellent safety data for the femoral approach, a proven track record of efficacy, and for most patients it seems to afford a somewhat wider range of tools that can be deployed, with simpler anatomy for accessing the cervical vessels in most arches. It is reassuring that the results reported by Dr. Almallouhi did not suggest negative outcomes, and as such I suspect the transradial approach at least gives us an additional option in a minority of patients. We have seen in the past 5-10 years an explosion of tools for the endovascular treatment of stroke; transradial access represents another potential strategy that appears so far to be safe,” Dr. Payne said.
Drs. Nogueira, Almallouhi, and Payne had no relevant disclosures.
SOURCE: Almallouhi E et al. Stroke. 2020 Feb;51(suppl 1):A64.
REPORTING FROM ISC 2020
More evidence backs LDL below 70 to reduce recurrent stroke
LOS ANGELES – In a subanalysis of the TST (Treat Stroke to Target) trial, restricting analysis to only French participants followed for an average of 5 years demonstrated an even more robust potential to reduce recurrent stroke and other major cardiovascular events by treating patients to an LDL target of below 70 mg/dL. Treating LDL to a mean of 66 mg/dL versus 96 mg/dL was associated with a 26% relative risk reduction for the composite endpoint of ischemic stroke, MI, new symptoms requiring urgent coronary or carotid revascularization, and vascular death in an adjusted analysis.
“The results are similar to the main paper but even more spectacular, with no increase in hemorrhagic stroke whatsoever, and positive results on any stroke,” study investigator Pierre Amarenco, MD, professor and chair of the department of neurology and Stroke Centre, Bichat University Hospital, Paris, said.
Dr. Amarenco presented the findings as a late-breaking abstract at the International Stroke Conference sponsored by the American Heart Association. The trial was published simultaneously in the journal Stroke.
In the full TST trial population, risk was reduced by 22% with more-aggressive LDL-lowering treatment, compared with the more lax 90-110 mg/dL target.
The TST cohort included both French and Korean participants. Dr. Amarenco and colleagues focused on the French population in the current study because the group was larger (2,148 vs. 742 Korean participants) and had a longer follow-up, an average of 5.3 years compared to 2.0 years among Korean patients. The initial study had shown “very significant results in the French patients and no apparent effect in Korean patients,” he said. The longer duration of treatment in the French cohort could have contributed to the greater risk reduction, said Dr. Amarenco.
A 2017 European Atherosclerosis Society Consensus Panel statement noted that exposure time to lipid-lowering drugs correlates with outcomes. The European Stroke Organization and the American Heart Association/American Stroke Association guidelines each recommend intensive statin treatment to lower serum lipids following an ischemic stroke of atherosclerotic origin or after a transient ischemic attack (TIA). However, the current researchers noted that the recommendations do not specify specific target numbers.
“Therefore, there is uncertainty about the target levels of LDL cholesterol,” he said.
Aiming at different targets
To learn more, Dr. Amarenco and colleagues randomly assigned 1,073 of the French patients to a target LDL treatment group of 70 mg/dL and another 1,075 to a target range of 90-110 mg/dL. They enrolled participants at 61 sites in France. Mean age was 67 years. All participants had experienced an ischemic stroke within 3 months or a TIA within 15 days of baseline. They presented either with a modified Rankin Scale poststroke score of 0-3 or a TIA that included at least arm and leg motor deficit or speech disturbance that lasted more than 10 minutes.
Investigators could use any type and any dose of statin to reach the respective targets. Statins could be prescribed as monotherapy or in combination with ezetimibe (Zetia) or other agents. The baseline mean LDL cholesterol level was 137 mg/dL in the lower target group and 138 mg/dL in the higher target group, respectively (3.5 mmol/L in both groups). Dr. Amarenco and colleagues measured LDL cholesterol levels at 3 weeks postrandomization and then every 6 months.
A smaller proportion of the lower LDL cholesterol target group experienced the adverse composite outcome, 9.6%, compared with 12.9% of the higher LDL cholesterol target group. This translated to a hazard ratio of 0.73 (95% confidence interval, 0.57-0.94; P = .015). The absolute risk reduction was 3.3% with a number needed to treat of 30.
An analysis adjusted for covariates showed a hazard ratio of 0.74 (95% CI, 0.57-0.95; P = .019).
Cerebral infarction and acute cerebral artery revascularization were reduced by 27% (HR, 0.73; 95% CI, 0.54-0.99; P = .046). Cerebral infarction or intracranial hemorrhage (all strokes) were reduced by 28% (HR, 0.72; 95% CI, 0.54-0.98; P = .023). In this case, there was an absolute risk reduction of 2.9% and a number needed to treat of 34.
In contrast, MI or urgent coronary revascularization following new symptoms were not significantly reduced (HR, 0.66; 95% CI, 0.67-1.20; P = .18). The investigators also reported nonsignificant results regarding vascular death (HR, 0.76; 95% CI, 0.44-1.32; P = .32] and all deaths (HR, 1.0; 95% CI, 0.74-1.35; P = .99).
Dr. Amarenco and colleagues also tracked adverse events. They found intracranial hemorrhage occurred in 13 (1.2%) patients assigned an LDL cholesterol below 70 mg/dL and in 11 (1%) patients assigned an LDL cholesterol of 100 ± 10 mg/dL. In this analysis, the hazard ratio was 1.17 (95% CI, 0.53-2.62; P = .70), and the absolute difference was 0.2%.
The investigators also reported that 10.3% of the lower LDL target group vs 13.6% of the higher LDL target group experienced either the primary outcome or intracranial hemorrhage. This translated to a 25% relative risk reduction (HR, 0.75; 95% CI, 0.58-0.96; P = .021), an absolute risk reduction of 3.3% and a number needed to treat of 30.
Avoiding one in four events
Assessing the French participants in the TST trial showed that targeting LDL below 70 mg/dL for more than 5 years avoided more than one in four subsequent major cardiovascular events among adults who experienced a recent ischemic stroke or TIA.
Furthermore, more intense LDL lowering also avoided more than one in four recurrent cerebral infarctions or urgent carotid revascularizations following a TIA, as well as one in four recurrent cerebral infarctions or hemorrhages (all strokes), compared with the higher LDL target.
“This was obtained without increasing the risk of intracranial hemorrhage with a number needed to treat of 30,” the researchers noted. “In the context of all randomized clinical trials with statin and other lipid-lowering drugs, there is no reason to think that Asian patients do not benefit from statin treatment and from a lower target LDL cholesterol,” the researchers added.
Therefore, they plan to continue assessing the 742 Korean participants until they reach a median of 5 years of follow-up.
Clinically validating results
“My feeling is that these data are highly supportive of a practice that many of us have been using for years without this level of evidence,” Mitchell S.V. Elkind, MD, said when asked to comment on the study.
Prior secondary analyses of studies, including research into patients with intracranial atherosclerosis, demonstrated benefit from treating to this lower LDL cholesterol target. “These studies were suggestive enough that many of us were treating patients aggressively with statins,” added Dr. Elkind, professor of neurology and epidemiology and chief of the division of neurology clinical outcomes research and population sciences at Columbia University in New York.
“But this really confirms that [fact] with clinical trial evidence,” said Dr. Elkind, “and I think will be very useful to us as clinicians.”
The results could be used to counsel patients about the potential benefits of statin therapy or to motivate primary care providers to treat patients more aggressively, said Dr. Elkind, who will begin his term as president of the American Heart Association/American Stroke Association in July.
This study was supported by a grant from the French Ministry of Health and from SOS-Attaque Cérébrale Association, with unrestricted grants from Pfizer, AstraZeneca, and Merck for French sites and from Pfizer for South Korean sites.
Dr. Amarenco receives research grant support and consulting fees from Pfizer, Merck, and AstraZeneca. Elkind had has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SOURCE: Amarenko P et al. ISC 2020. Late-breaking abstract 9.
LOS ANGELES – In a subanalysis of the TST (Treat Stroke to Target) trial, restricting analysis to only French participants followed for an average of 5 years demonstrated an even more robust potential to reduce recurrent stroke and other major cardiovascular events by treating patients to an LDL target of below 70 mg/dL. Treating LDL to a mean of 66 mg/dL versus 96 mg/dL was associated with a 26% relative risk reduction for the composite endpoint of ischemic stroke, MI, new symptoms requiring urgent coronary or carotid revascularization, and vascular death in an adjusted analysis.
“The results are similar to the main paper but even more spectacular, with no increase in hemorrhagic stroke whatsoever, and positive results on any stroke,” study investigator Pierre Amarenco, MD, professor and chair of the department of neurology and Stroke Centre, Bichat University Hospital, Paris, said.
Dr. Amarenco presented the findings as a late-breaking abstract at the International Stroke Conference sponsored by the American Heart Association. The trial was published simultaneously in the journal Stroke.
In the full TST trial population, risk was reduced by 22% with more-aggressive LDL-lowering treatment, compared with the more lax 90-110 mg/dL target.
The TST cohort included both French and Korean participants. Dr. Amarenco and colleagues focused on the French population in the current study because the group was larger (2,148 vs. 742 Korean participants) and had a longer follow-up, an average of 5.3 years compared to 2.0 years among Korean patients. The initial study had shown “very significant results in the French patients and no apparent effect in Korean patients,” he said. The longer duration of treatment in the French cohort could have contributed to the greater risk reduction, said Dr. Amarenco.
A 2017 European Atherosclerosis Society Consensus Panel statement noted that exposure time to lipid-lowering drugs correlates with outcomes. The European Stroke Organization and the American Heart Association/American Stroke Association guidelines each recommend intensive statin treatment to lower serum lipids following an ischemic stroke of atherosclerotic origin or after a transient ischemic attack (TIA). However, the current researchers noted that the recommendations do not specify specific target numbers.
“Therefore, there is uncertainty about the target levels of LDL cholesterol,” he said.
Aiming at different targets
To learn more, Dr. Amarenco and colleagues randomly assigned 1,073 of the French patients to a target LDL treatment group of 70 mg/dL and another 1,075 to a target range of 90-110 mg/dL. They enrolled participants at 61 sites in France. Mean age was 67 years. All participants had experienced an ischemic stroke within 3 months or a TIA within 15 days of baseline. They presented either with a modified Rankin Scale poststroke score of 0-3 or a TIA that included at least arm and leg motor deficit or speech disturbance that lasted more than 10 minutes.
Investigators could use any type and any dose of statin to reach the respective targets. Statins could be prescribed as monotherapy or in combination with ezetimibe (Zetia) or other agents. The baseline mean LDL cholesterol level was 137 mg/dL in the lower target group and 138 mg/dL in the higher target group, respectively (3.5 mmol/L in both groups). Dr. Amarenco and colleagues measured LDL cholesterol levels at 3 weeks postrandomization and then every 6 months.
A smaller proportion of the lower LDL cholesterol target group experienced the adverse composite outcome, 9.6%, compared with 12.9% of the higher LDL cholesterol target group. This translated to a hazard ratio of 0.73 (95% confidence interval, 0.57-0.94; P = .015). The absolute risk reduction was 3.3% with a number needed to treat of 30.
An analysis adjusted for covariates showed a hazard ratio of 0.74 (95% CI, 0.57-0.95; P = .019).
Cerebral infarction and acute cerebral artery revascularization were reduced by 27% (HR, 0.73; 95% CI, 0.54-0.99; P = .046). Cerebral infarction or intracranial hemorrhage (all strokes) were reduced by 28% (HR, 0.72; 95% CI, 0.54-0.98; P = .023). In this case, there was an absolute risk reduction of 2.9% and a number needed to treat of 34.
In contrast, MI or urgent coronary revascularization following new symptoms were not significantly reduced (HR, 0.66; 95% CI, 0.67-1.20; P = .18). The investigators also reported nonsignificant results regarding vascular death (HR, 0.76; 95% CI, 0.44-1.32; P = .32] and all deaths (HR, 1.0; 95% CI, 0.74-1.35; P = .99).
Dr. Amarenco and colleagues also tracked adverse events. They found intracranial hemorrhage occurred in 13 (1.2%) patients assigned an LDL cholesterol below 70 mg/dL and in 11 (1%) patients assigned an LDL cholesterol of 100 ± 10 mg/dL. In this analysis, the hazard ratio was 1.17 (95% CI, 0.53-2.62; P = .70), and the absolute difference was 0.2%.
The investigators also reported that 10.3% of the lower LDL target group vs 13.6% of the higher LDL target group experienced either the primary outcome or intracranial hemorrhage. This translated to a 25% relative risk reduction (HR, 0.75; 95% CI, 0.58-0.96; P = .021), an absolute risk reduction of 3.3% and a number needed to treat of 30.
Avoiding one in four events
Assessing the French participants in the TST trial showed that targeting LDL below 70 mg/dL for more than 5 years avoided more than one in four subsequent major cardiovascular events among adults who experienced a recent ischemic stroke or TIA.
Furthermore, more intense LDL lowering also avoided more than one in four recurrent cerebral infarctions or urgent carotid revascularizations following a TIA, as well as one in four recurrent cerebral infarctions or hemorrhages (all strokes), compared with the higher LDL target.
“This was obtained without increasing the risk of intracranial hemorrhage with a number needed to treat of 30,” the researchers noted. “In the context of all randomized clinical trials with statin and other lipid-lowering drugs, there is no reason to think that Asian patients do not benefit from statin treatment and from a lower target LDL cholesterol,” the researchers added.
Therefore, they plan to continue assessing the 742 Korean participants until they reach a median of 5 years of follow-up.
Clinically validating results
“My feeling is that these data are highly supportive of a practice that many of us have been using for years without this level of evidence,” Mitchell S.V. Elkind, MD, said when asked to comment on the study.
Prior secondary analyses of studies, including research into patients with intracranial atherosclerosis, demonstrated benefit from treating to this lower LDL cholesterol target. “These studies were suggestive enough that many of us were treating patients aggressively with statins,” added Dr. Elkind, professor of neurology and epidemiology and chief of the division of neurology clinical outcomes research and population sciences at Columbia University in New York.
“But this really confirms that [fact] with clinical trial evidence,” said Dr. Elkind, “and I think will be very useful to us as clinicians.”
The results could be used to counsel patients about the potential benefits of statin therapy or to motivate primary care providers to treat patients more aggressively, said Dr. Elkind, who will begin his term as president of the American Heart Association/American Stroke Association in July.
This study was supported by a grant from the French Ministry of Health and from SOS-Attaque Cérébrale Association, with unrestricted grants from Pfizer, AstraZeneca, and Merck for French sites and from Pfizer for South Korean sites.
Dr. Amarenco receives research grant support and consulting fees from Pfizer, Merck, and AstraZeneca. Elkind had has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SOURCE: Amarenko P et al. ISC 2020. Late-breaking abstract 9.
LOS ANGELES – In a subanalysis of the TST (Treat Stroke to Target) trial, restricting analysis to only French participants followed for an average of 5 years demonstrated an even more robust potential to reduce recurrent stroke and other major cardiovascular events by treating patients to an LDL target of below 70 mg/dL. Treating LDL to a mean of 66 mg/dL versus 96 mg/dL was associated with a 26% relative risk reduction for the composite endpoint of ischemic stroke, MI, new symptoms requiring urgent coronary or carotid revascularization, and vascular death in an adjusted analysis.
“The results are similar to the main paper but even more spectacular, with no increase in hemorrhagic stroke whatsoever, and positive results on any stroke,” study investigator Pierre Amarenco, MD, professor and chair of the department of neurology and Stroke Centre, Bichat University Hospital, Paris, said.
Dr. Amarenco presented the findings as a late-breaking abstract at the International Stroke Conference sponsored by the American Heart Association. The trial was published simultaneously in the journal Stroke.
In the full TST trial population, risk was reduced by 22% with more-aggressive LDL-lowering treatment, compared with the more lax 90-110 mg/dL target.
The TST cohort included both French and Korean participants. Dr. Amarenco and colleagues focused on the French population in the current study because the group was larger (2,148 vs. 742 Korean participants) and had a longer follow-up, an average of 5.3 years compared to 2.0 years among Korean patients. The initial study had shown “very significant results in the French patients and no apparent effect in Korean patients,” he said. The longer duration of treatment in the French cohort could have contributed to the greater risk reduction, said Dr. Amarenco.
A 2017 European Atherosclerosis Society Consensus Panel statement noted that exposure time to lipid-lowering drugs correlates with outcomes. The European Stroke Organization and the American Heart Association/American Stroke Association guidelines each recommend intensive statin treatment to lower serum lipids following an ischemic stroke of atherosclerotic origin or after a transient ischemic attack (TIA). However, the current researchers noted that the recommendations do not specify specific target numbers.
“Therefore, there is uncertainty about the target levels of LDL cholesterol,” he said.
Aiming at different targets
To learn more, Dr. Amarenco and colleagues randomly assigned 1,073 of the French patients to a target LDL treatment group of 70 mg/dL and another 1,075 to a target range of 90-110 mg/dL. They enrolled participants at 61 sites in France. Mean age was 67 years. All participants had experienced an ischemic stroke within 3 months or a TIA within 15 days of baseline. They presented either with a modified Rankin Scale poststroke score of 0-3 or a TIA that included at least arm and leg motor deficit or speech disturbance that lasted more than 10 minutes.
Investigators could use any type and any dose of statin to reach the respective targets. Statins could be prescribed as monotherapy or in combination with ezetimibe (Zetia) or other agents. The baseline mean LDL cholesterol level was 137 mg/dL in the lower target group and 138 mg/dL in the higher target group, respectively (3.5 mmol/L in both groups). Dr. Amarenco and colleagues measured LDL cholesterol levels at 3 weeks postrandomization and then every 6 months.
A smaller proportion of the lower LDL cholesterol target group experienced the adverse composite outcome, 9.6%, compared with 12.9% of the higher LDL cholesterol target group. This translated to a hazard ratio of 0.73 (95% confidence interval, 0.57-0.94; P = .015). The absolute risk reduction was 3.3% with a number needed to treat of 30.
An analysis adjusted for covariates showed a hazard ratio of 0.74 (95% CI, 0.57-0.95; P = .019).
Cerebral infarction and acute cerebral artery revascularization were reduced by 27% (HR, 0.73; 95% CI, 0.54-0.99; P = .046). Cerebral infarction or intracranial hemorrhage (all strokes) were reduced by 28% (HR, 0.72; 95% CI, 0.54-0.98; P = .023). In this case, there was an absolute risk reduction of 2.9% and a number needed to treat of 34.
In contrast, MI or urgent coronary revascularization following new symptoms were not significantly reduced (HR, 0.66; 95% CI, 0.67-1.20; P = .18). The investigators also reported nonsignificant results regarding vascular death (HR, 0.76; 95% CI, 0.44-1.32; P = .32] and all deaths (HR, 1.0; 95% CI, 0.74-1.35; P = .99).
Dr. Amarenco and colleagues also tracked adverse events. They found intracranial hemorrhage occurred in 13 (1.2%) patients assigned an LDL cholesterol below 70 mg/dL and in 11 (1%) patients assigned an LDL cholesterol of 100 ± 10 mg/dL. In this analysis, the hazard ratio was 1.17 (95% CI, 0.53-2.62; P = .70), and the absolute difference was 0.2%.
The investigators also reported that 10.3% of the lower LDL target group vs 13.6% of the higher LDL target group experienced either the primary outcome or intracranial hemorrhage. This translated to a 25% relative risk reduction (HR, 0.75; 95% CI, 0.58-0.96; P = .021), an absolute risk reduction of 3.3% and a number needed to treat of 30.
Avoiding one in four events
Assessing the French participants in the TST trial showed that targeting LDL below 70 mg/dL for more than 5 years avoided more than one in four subsequent major cardiovascular events among adults who experienced a recent ischemic stroke or TIA.
Furthermore, more intense LDL lowering also avoided more than one in four recurrent cerebral infarctions or urgent carotid revascularizations following a TIA, as well as one in four recurrent cerebral infarctions or hemorrhages (all strokes), compared with the higher LDL target.
“This was obtained without increasing the risk of intracranial hemorrhage with a number needed to treat of 30,” the researchers noted. “In the context of all randomized clinical trials with statin and other lipid-lowering drugs, there is no reason to think that Asian patients do not benefit from statin treatment and from a lower target LDL cholesterol,” the researchers added.
Therefore, they plan to continue assessing the 742 Korean participants until they reach a median of 5 years of follow-up.
Clinically validating results
“My feeling is that these data are highly supportive of a practice that many of us have been using for years without this level of evidence,” Mitchell S.V. Elkind, MD, said when asked to comment on the study.
Prior secondary analyses of studies, including research into patients with intracranial atherosclerosis, demonstrated benefit from treating to this lower LDL cholesterol target. “These studies were suggestive enough that many of us were treating patients aggressively with statins,” added Dr. Elkind, professor of neurology and epidemiology and chief of the division of neurology clinical outcomes research and population sciences at Columbia University in New York.
“But this really confirms that [fact] with clinical trial evidence,” said Dr. Elkind, “and I think will be very useful to us as clinicians.”
The results could be used to counsel patients about the potential benefits of statin therapy or to motivate primary care providers to treat patients more aggressively, said Dr. Elkind, who will begin his term as president of the American Heart Association/American Stroke Association in July.
This study was supported by a grant from the French Ministry of Health and from SOS-Attaque Cérébrale Association, with unrestricted grants from Pfizer, AstraZeneca, and Merck for French sites and from Pfizer for South Korean sites.
Dr. Amarenco receives research grant support and consulting fees from Pfizer, Merck, and AstraZeneca. Elkind had has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
SOURCE: Amarenko P et al. ISC 2020. Late-breaking abstract 9.
REPORTING FROM ISC 2020
BASILAR: Endovascular treatment improves outcomes in BAO stroke
LOS ANGELES – Endovascular therapy significantly improved functional outcomes and reduced mortality at 90 days, compared with standard thrombolysis alone, new evidence from a large, prospective registry study suggests.
Participants who received both interventions were almost five times more likely to be able to walk independently at 90 days compared with those who received thrombolysis alone.
Despite multiple trials supporting the potential benefits of endovascular therapy for anterior stroke, little prospective research addresses outcomes associated with an ischemic stroke caused by a posterior basilar artery occlusion (BAO).
“Basilar artery occlusion is the ‘orphan’ of the large vessel occlusions,” Raul Gomes Nogueira, MD, PhD, said here at a late-breaking abstract session at the International Stroke Conference sponsored by the American Heart Association.
“They account for about 5% of the large vessel occlusions – but have the most dismal prognosis.” Severe disability and mortality rates associated with BAO, for example, reach an estimated 68% to 78%, he said.
The results, from the EVT for Acute Basilar Artery Occlusion Study (BASILAR), were also simultaneously published in JAMA Neurology.
Prior studies in this patient population are generally single-center, retrospective studies and “the numbers tend to be small,” said Nogueira, who is affiliated with the Marcus Stroke and Neuroscience Center, Grady Memorial Hospital, Emory University School of Medicine in Atlanta, Georgia.
Nogueira and colleagues studied 829 consecutive adults who presented with an acute, symptomatic BAO. They examined a nationwide prospective registry study of people with radiologically confirmed BAO in 47 comprehensive stroke centers across 15 provinces in China.
The median age was 65 years and 74% were men. A total 182 participants received thrombolysis therapy within 6 hours of estimated BAO onset. The 647 people in the dual intervention group also received endovascular therapy within 24 hours.
Standard medical treatment included intravenous rt-PA or urokinase, antiplatelet drugs and systematic anticoagulation alone or in combination. Endovascular therapy included mechanical thrombectomy with stent retrievers and/or thromboaspiration, balloon angioplasty, stenting, intra-arterial thrombolysis, or a combination of these interventions.
Interestingly, participants were not randomly assigned, in part because of the favorable outcomes associated with endovascular therapy. “The high number of patients who received [the dual intervention] may suggest the existence of a lack of equipoise among participating centers,” the researchers note.
Key Efficacy Endpoints
A significantly higher proportion of people in the dual treatment group achieved the primary outcome, functional improvement at 90 days, at 32%, compared with 9.3% in the thrombolysis-only group. This endpoint was defined as a modified Rankin Scale (mRS) score of 3 or less, which reflects an ability to walk independently. The difference was statistically significant (P < .001).
The absolute difference between groups was 22.7% (95% confidence interval, 17.1%-28.2%) with an adjusted odds ratio of 4.70 (95% CI, 2.53-8.75; P < .001) in favor of dual intervention.
The number needed to treat for one additional patient to be able to walk unassisted was 4.4.
Other outcomes, including differences in National Institutes of Health Stroke Scale scores from baseline to 5 to 7 days or discharge, as well as propensity score matching and subgroup analyses, likewise supported the superiority of using both interventions.
Safety Outcomes
Nogueira and colleagues also assessed safety. They found that symptomatic intracerebral hemorrhage (ICH) occurred in 45 patients, or 7.1% of the endovascular treatment group. In contrast, only one patient, or 0.5%, of the standard medical treatment alone cohort experienced an ICH. This difference was statistically significant (P < .001).
Mortality at 90 days was significantly lower in the endovascular therapy plus medical therapy group, 46.2%, compared with 71.4% in the standard medical treatment alone group (P < .001).
The absolute difference in mortality was 25.2% (95% CI, 17.6%-2.8%) favoring dual treatment, with an adjusted odds ratio of 2.93 (95% CI, 1.95-4.40; P < .001).
Rates of other serious adverse events during the 90-day follow-up period were similar in the two study groups, Nogueira said.
He acknowledged that the nonrandomized design was a limitation of the registry study, adding that “sometimes in life it’s important to acknowledge the best of what can be done. It’s very hard when you have access to thrombectomy to randomize people.”
However, other researchers have attempted or are enrolling people with BAO into trials that randomly assign them to endovascular therapy and standard medical treatment or medical treatment alone.
The BEST trial in China, for example, randomly assigned 131 patients to these groups but was stopped early in September 2017. “The BEST trial was terminated prematurely because of loss of equipoise that led to a high crossover rate and drop in valid recruitment,” the current researchers note.
“The other two trials…are facing the challenge of whether they will achieve their inclusion target,” they add, “because a growing number of stroke centers are unwilling to randomize patients to standard medical treatment alone after the many positive results of trials for endovascular treatment in patients with anterior-circulation stroke.”
The BAOCHE trial from China, for example, is ongoing with approximately 110 patients enrolled so far.
Investigators for the Basilar Artery International Cooperation Study (BASICS) in the Netherlands just completed enrollment of their 300th and final patient in December 2019.
“We are hopeful BASICS trial will shed additional light,” Nogueira said. The results are expected to be presented at the European Stroke Organization Conference in Vienna in May 2020.
More Guidance From MRI?
“With the advent of the stent retrievers and successful recanalization, we know there can be better outcomes for patients. And we know the morbidity and mortality of the basilar artery occlusions are so poor that we tend to want to be aggressive in these cases,” session comoderator Shlee S. Song, MD, director of the Comprehensive Stroke Center and associate professor of neurology at Cedars-Sinai Medical Center in Los Angeles, California, told Medscape Medical News when asked to comment on the study.
“I agree that we’ve lost equipoise in this cohort – that we really cannot do a randomized trial anymore. You know if you don’t do anything, 90% of the time there will be a poor outcome,” she added.
This is an important study for showing how BAO patients fare after endovascular treatment, Song said.
One unanswered question from the study is if any of the centers in China used magnetic resonance imaging to help determine the most appropriate candidates for endovascular treatment of these posterior circulation strokes, which is a common practice in the United States, she said.
The study was supported by the National Science Fund for Distinguished Young Scholars, Chongqing Major Disease Prevention and Control Technology Research Project, Army Medical University Clinical Medical Research Talent Training Program, and Major Clinical Innovation Technology Project of the Second Affiliated Hospital of the Army Military Medical University. Sing had no relevant disclosures. Nogueira’s financial disclosures include working as a consultant for Stryker Neurovascular; as a principal investigator on the Imperative trial and the PROST trial; as a steering committee member for Biogen for the CHARM trial; as an advisory board member for Cerenovus/Neuravi, Phenox, Anaconda, Genentech, Biogen, Prolong Pharmaceuticals and Brainomix; and as an advisory board member with stock options for Viz.ai, Corindus Vascular Robotics, Vesalio, Ceretrieve, Astrocyte Pharmaceuticals, and Cerebrotech.
This article first appeared on Medscape.com.
SOURCE: Nogueira RG et al. ISC 2020. Late-breaking abstract 17.
LOS ANGELES – Endovascular therapy significantly improved functional outcomes and reduced mortality at 90 days, compared with standard thrombolysis alone, new evidence from a large, prospective registry study suggests.
Participants who received both interventions were almost five times more likely to be able to walk independently at 90 days compared with those who received thrombolysis alone.
Despite multiple trials supporting the potential benefits of endovascular therapy for anterior stroke, little prospective research addresses outcomes associated with an ischemic stroke caused by a posterior basilar artery occlusion (BAO).
“Basilar artery occlusion is the ‘orphan’ of the large vessel occlusions,” Raul Gomes Nogueira, MD, PhD, said here at a late-breaking abstract session at the International Stroke Conference sponsored by the American Heart Association.
“They account for about 5% of the large vessel occlusions – but have the most dismal prognosis.” Severe disability and mortality rates associated with BAO, for example, reach an estimated 68% to 78%, he said.
The results, from the EVT for Acute Basilar Artery Occlusion Study (BASILAR), were also simultaneously published in JAMA Neurology.
Prior studies in this patient population are generally single-center, retrospective studies and “the numbers tend to be small,” said Nogueira, who is affiliated with the Marcus Stroke and Neuroscience Center, Grady Memorial Hospital, Emory University School of Medicine in Atlanta, Georgia.
Nogueira and colleagues studied 829 consecutive adults who presented with an acute, symptomatic BAO. They examined a nationwide prospective registry study of people with radiologically confirmed BAO in 47 comprehensive stroke centers across 15 provinces in China.
The median age was 65 years and 74% were men. A total 182 participants received thrombolysis therapy within 6 hours of estimated BAO onset. The 647 people in the dual intervention group also received endovascular therapy within 24 hours.
Standard medical treatment included intravenous rt-PA or urokinase, antiplatelet drugs and systematic anticoagulation alone or in combination. Endovascular therapy included mechanical thrombectomy with stent retrievers and/or thromboaspiration, balloon angioplasty, stenting, intra-arterial thrombolysis, or a combination of these interventions.
Interestingly, participants were not randomly assigned, in part because of the favorable outcomes associated with endovascular therapy. “The high number of patients who received [the dual intervention] may suggest the existence of a lack of equipoise among participating centers,” the researchers note.
Key Efficacy Endpoints
A significantly higher proportion of people in the dual treatment group achieved the primary outcome, functional improvement at 90 days, at 32%, compared with 9.3% in the thrombolysis-only group. This endpoint was defined as a modified Rankin Scale (mRS) score of 3 or less, which reflects an ability to walk independently. The difference was statistically significant (P < .001).
The absolute difference between groups was 22.7% (95% confidence interval, 17.1%-28.2%) with an adjusted odds ratio of 4.70 (95% CI, 2.53-8.75; P < .001) in favor of dual intervention.
The number needed to treat for one additional patient to be able to walk unassisted was 4.4.
Other outcomes, including differences in National Institutes of Health Stroke Scale scores from baseline to 5 to 7 days or discharge, as well as propensity score matching and subgroup analyses, likewise supported the superiority of using both interventions.
Safety Outcomes
Nogueira and colleagues also assessed safety. They found that symptomatic intracerebral hemorrhage (ICH) occurred in 45 patients, or 7.1% of the endovascular treatment group. In contrast, only one patient, or 0.5%, of the standard medical treatment alone cohort experienced an ICH. This difference was statistically significant (P < .001).
Mortality at 90 days was significantly lower in the endovascular therapy plus medical therapy group, 46.2%, compared with 71.4% in the standard medical treatment alone group (P < .001).
The absolute difference in mortality was 25.2% (95% CI, 17.6%-2.8%) favoring dual treatment, with an adjusted odds ratio of 2.93 (95% CI, 1.95-4.40; P < .001).
Rates of other serious adverse events during the 90-day follow-up period were similar in the two study groups, Nogueira said.
He acknowledged that the nonrandomized design was a limitation of the registry study, adding that “sometimes in life it’s important to acknowledge the best of what can be done. It’s very hard when you have access to thrombectomy to randomize people.”
However, other researchers have attempted or are enrolling people with BAO into trials that randomly assign them to endovascular therapy and standard medical treatment or medical treatment alone.
The BEST trial in China, for example, randomly assigned 131 patients to these groups but was stopped early in September 2017. “The BEST trial was terminated prematurely because of loss of equipoise that led to a high crossover rate and drop in valid recruitment,” the current researchers note.
“The other two trials…are facing the challenge of whether they will achieve their inclusion target,” they add, “because a growing number of stroke centers are unwilling to randomize patients to standard medical treatment alone after the many positive results of trials for endovascular treatment in patients with anterior-circulation stroke.”
The BAOCHE trial from China, for example, is ongoing with approximately 110 patients enrolled so far.
Investigators for the Basilar Artery International Cooperation Study (BASICS) in the Netherlands just completed enrollment of their 300th and final patient in December 2019.
“We are hopeful BASICS trial will shed additional light,” Nogueira said. The results are expected to be presented at the European Stroke Organization Conference in Vienna in May 2020.
More Guidance From MRI?
“With the advent of the stent retrievers and successful recanalization, we know there can be better outcomes for patients. And we know the morbidity and mortality of the basilar artery occlusions are so poor that we tend to want to be aggressive in these cases,” session comoderator Shlee S. Song, MD, director of the Comprehensive Stroke Center and associate professor of neurology at Cedars-Sinai Medical Center in Los Angeles, California, told Medscape Medical News when asked to comment on the study.
“I agree that we’ve lost equipoise in this cohort – that we really cannot do a randomized trial anymore. You know if you don’t do anything, 90% of the time there will be a poor outcome,” she added.
This is an important study for showing how BAO patients fare after endovascular treatment, Song said.
One unanswered question from the study is if any of the centers in China used magnetic resonance imaging to help determine the most appropriate candidates for endovascular treatment of these posterior circulation strokes, which is a common practice in the United States, she said.
The study was supported by the National Science Fund for Distinguished Young Scholars, Chongqing Major Disease Prevention and Control Technology Research Project, Army Medical University Clinical Medical Research Talent Training Program, and Major Clinical Innovation Technology Project of the Second Affiliated Hospital of the Army Military Medical University. Sing had no relevant disclosures. Nogueira’s financial disclosures include working as a consultant for Stryker Neurovascular; as a principal investigator on the Imperative trial and the PROST trial; as a steering committee member for Biogen for the CHARM trial; as an advisory board member for Cerenovus/Neuravi, Phenox, Anaconda, Genentech, Biogen, Prolong Pharmaceuticals and Brainomix; and as an advisory board member with stock options for Viz.ai, Corindus Vascular Robotics, Vesalio, Ceretrieve, Astrocyte Pharmaceuticals, and Cerebrotech.
This article first appeared on Medscape.com.
SOURCE: Nogueira RG et al. ISC 2020. Late-breaking abstract 17.
LOS ANGELES – Endovascular therapy significantly improved functional outcomes and reduced mortality at 90 days, compared with standard thrombolysis alone, new evidence from a large, prospective registry study suggests.
Participants who received both interventions were almost five times more likely to be able to walk independently at 90 days compared with those who received thrombolysis alone.
Despite multiple trials supporting the potential benefits of endovascular therapy for anterior stroke, little prospective research addresses outcomes associated with an ischemic stroke caused by a posterior basilar artery occlusion (BAO).
“Basilar artery occlusion is the ‘orphan’ of the large vessel occlusions,” Raul Gomes Nogueira, MD, PhD, said here at a late-breaking abstract session at the International Stroke Conference sponsored by the American Heart Association.
“They account for about 5% of the large vessel occlusions – but have the most dismal prognosis.” Severe disability and mortality rates associated with BAO, for example, reach an estimated 68% to 78%, he said.
The results, from the EVT for Acute Basilar Artery Occlusion Study (BASILAR), were also simultaneously published in JAMA Neurology.
Prior studies in this patient population are generally single-center, retrospective studies and “the numbers tend to be small,” said Nogueira, who is affiliated with the Marcus Stroke and Neuroscience Center, Grady Memorial Hospital, Emory University School of Medicine in Atlanta, Georgia.
Nogueira and colleagues studied 829 consecutive adults who presented with an acute, symptomatic BAO. They examined a nationwide prospective registry study of people with radiologically confirmed BAO in 47 comprehensive stroke centers across 15 provinces in China.
The median age was 65 years and 74% were men. A total 182 participants received thrombolysis therapy within 6 hours of estimated BAO onset. The 647 people in the dual intervention group also received endovascular therapy within 24 hours.
Standard medical treatment included intravenous rt-PA or urokinase, antiplatelet drugs and systematic anticoagulation alone or in combination. Endovascular therapy included mechanical thrombectomy with stent retrievers and/or thromboaspiration, balloon angioplasty, stenting, intra-arterial thrombolysis, or a combination of these interventions.
Interestingly, participants were not randomly assigned, in part because of the favorable outcomes associated with endovascular therapy. “The high number of patients who received [the dual intervention] may suggest the existence of a lack of equipoise among participating centers,” the researchers note.
Key Efficacy Endpoints
A significantly higher proportion of people in the dual treatment group achieved the primary outcome, functional improvement at 90 days, at 32%, compared with 9.3% in the thrombolysis-only group. This endpoint was defined as a modified Rankin Scale (mRS) score of 3 or less, which reflects an ability to walk independently. The difference was statistically significant (P < .001).
The absolute difference between groups was 22.7% (95% confidence interval, 17.1%-28.2%) with an adjusted odds ratio of 4.70 (95% CI, 2.53-8.75; P < .001) in favor of dual intervention.
The number needed to treat for one additional patient to be able to walk unassisted was 4.4.
Other outcomes, including differences in National Institutes of Health Stroke Scale scores from baseline to 5 to 7 days or discharge, as well as propensity score matching and subgroup analyses, likewise supported the superiority of using both interventions.
Safety Outcomes
Nogueira and colleagues also assessed safety. They found that symptomatic intracerebral hemorrhage (ICH) occurred in 45 patients, or 7.1% of the endovascular treatment group. In contrast, only one patient, or 0.5%, of the standard medical treatment alone cohort experienced an ICH. This difference was statistically significant (P < .001).
Mortality at 90 days was significantly lower in the endovascular therapy plus medical therapy group, 46.2%, compared with 71.4% in the standard medical treatment alone group (P < .001).
The absolute difference in mortality was 25.2% (95% CI, 17.6%-2.8%) favoring dual treatment, with an adjusted odds ratio of 2.93 (95% CI, 1.95-4.40; P < .001).
Rates of other serious adverse events during the 90-day follow-up period were similar in the two study groups, Nogueira said.
He acknowledged that the nonrandomized design was a limitation of the registry study, adding that “sometimes in life it’s important to acknowledge the best of what can be done. It’s very hard when you have access to thrombectomy to randomize people.”
However, other researchers have attempted or are enrolling people with BAO into trials that randomly assign them to endovascular therapy and standard medical treatment or medical treatment alone.
The BEST trial in China, for example, randomly assigned 131 patients to these groups but was stopped early in September 2017. “The BEST trial was terminated prematurely because of loss of equipoise that led to a high crossover rate and drop in valid recruitment,” the current researchers note.
“The other two trials…are facing the challenge of whether they will achieve their inclusion target,” they add, “because a growing number of stroke centers are unwilling to randomize patients to standard medical treatment alone after the many positive results of trials for endovascular treatment in patients with anterior-circulation stroke.”
The BAOCHE trial from China, for example, is ongoing with approximately 110 patients enrolled so far.
Investigators for the Basilar Artery International Cooperation Study (BASICS) in the Netherlands just completed enrollment of their 300th and final patient in December 2019.
“We are hopeful BASICS trial will shed additional light,” Nogueira said. The results are expected to be presented at the European Stroke Organization Conference in Vienna in May 2020.
More Guidance From MRI?
“With the advent of the stent retrievers and successful recanalization, we know there can be better outcomes for patients. And we know the morbidity and mortality of the basilar artery occlusions are so poor that we tend to want to be aggressive in these cases,” session comoderator Shlee S. Song, MD, director of the Comprehensive Stroke Center and associate professor of neurology at Cedars-Sinai Medical Center in Los Angeles, California, told Medscape Medical News when asked to comment on the study.
“I agree that we’ve lost equipoise in this cohort – that we really cannot do a randomized trial anymore. You know if you don’t do anything, 90% of the time there will be a poor outcome,” she added.
This is an important study for showing how BAO patients fare after endovascular treatment, Song said.
One unanswered question from the study is if any of the centers in China used magnetic resonance imaging to help determine the most appropriate candidates for endovascular treatment of these posterior circulation strokes, which is a common practice in the United States, she said.
The study was supported by the National Science Fund for Distinguished Young Scholars, Chongqing Major Disease Prevention and Control Technology Research Project, Army Medical University Clinical Medical Research Talent Training Program, and Major Clinical Innovation Technology Project of the Second Affiliated Hospital of the Army Military Medical University. Sing had no relevant disclosures. Nogueira’s financial disclosures include working as a consultant for Stryker Neurovascular; as a principal investigator on the Imperative trial and the PROST trial; as a steering committee member for Biogen for the CHARM trial; as an advisory board member for Cerenovus/Neuravi, Phenox, Anaconda, Genentech, Biogen, Prolong Pharmaceuticals and Brainomix; and as an advisory board member with stock options for Viz.ai, Corindus Vascular Robotics, Vesalio, Ceretrieve, Astrocyte Pharmaceuticals, and Cerebrotech.
This article first appeared on Medscape.com.
SOURCE: Nogueira RG et al. ISC 2020. Late-breaking abstract 17.
REPORTING FROM ISC 2020
Dulaglutide OK for primary, secondary CV risk reduction in U.S.
The US Food and Drug Administration (FDA) has additionally approved dulaglutide (Trulicity) for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes with and without established cardiovascular disease (CVD) or multiple CV risk factors, the company has announced.
Dulaglutide is a once-weekly injectable glucagonlike peptide-1 (GLP-1) receptor agonist first approved in the United States in 2014 for the treatment of type 2 diabetes.
It is now the first and only type 2 diabetes medicine approved to reduce the risk of CV events for both primary and secondary prevention populations. The European Medicines Agency approved a similar indication for dulaglutide last fall.
The new US indication is based on results of the CV outcomes trial for dulaglutide, known as REWIND, which was the longest-running CV outcomes trial in the GLP-1 agonist class.
Chair of the REWIND study, Hertzel Gerstein, MD, professor of medicine at McMaster University and Hamilton Health Sciences, Ontario, Canada, said in a Lilly statement that the trial included a “broad population of people living with type 2 diabetes, reflective of those in the general population. We therefore assessed the effect of Trulicity in people with established CVD as well as those with multiple CV risk factors.”
“Globally, over 415 million people have type 2 diabetes, which is itself a CV risk factor. However, only about one third have established CVD, which is why this new indication, and the supporting evidence, is important for the millions of people in the United States living with diabetes,” he added.
Other GLP-1 agonists have been granted approvals for additional reduction of CV events in patients with type 2 diabetes, but only for secondary prevention.
Most recently the FDA expanded the indication for once-weekly semaglutide to include reducing the risk for MACE, including CV death, nonfatal myocardial infarction, or nonfatal stroke, in adults with type 2 diabetes who have established CVD.
Additional approval based on REWIND trial
The REWIND trial included primarily people with type 2 diabetes without established CVD. The full study results were presented at the 2019 American Diabetes Association Scientific Sessions.
REWIND showed a significant reduction in risk of MACE – a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or CV death – which occurred in 12.0% of patients in the dulaglutide group, compared with 13.4% of patients in the placebo group, for a risk reduction of 0.88 (95% confidence interval, 0.79-0.99; P = .026), which was consistent across subgroups.
All three components of the MACE primary endpoint showed a reduction with dulaglutide, compared with placebo, including CV death (hazard ratio, 0.91; 95% CI, 0.78-1.06) and nonfatal MI (HR, 0.96; 95% CI, 0.79-1.16), with the strongest and only significant effect seen in nonfatal stroke (HR, 0.76; 95% CI, 0.61-0.95).
No difference was seen between groups in hospital admissions for heart failure.
Dulaglutide was also found to modestly reduce weight by around 1.5 kg (P = .0001) and systolic blood pressure by 1.7 mm Hg (P = .0001).
The safety profile of dulaglutide in REWIND was consistent with other members of the GLP-1 agonist class, with gastrointestinal events being the most common adverse event leading to discontinuation.
Sherry Martin, MD, Lilly’s vice president, medical affairs, noted in the company statement: “For the first time, health care providers can prescribe a diabetes medicine proven to significantly reduce the risk of experiencing a CV event for people with type 2 diabetes with and without established CVD.”
“Trulicity can help people achieve their A1C goals and protect them from experiencing a CV event with a once-weekly, easy-to-use treatment option,” added Martin.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has additionally approved dulaglutide (Trulicity) for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes with and without established cardiovascular disease (CVD) or multiple CV risk factors, the company has announced.
Dulaglutide is a once-weekly injectable glucagonlike peptide-1 (GLP-1) receptor agonist first approved in the United States in 2014 for the treatment of type 2 diabetes.
It is now the first and only type 2 diabetes medicine approved to reduce the risk of CV events for both primary and secondary prevention populations. The European Medicines Agency approved a similar indication for dulaglutide last fall.
The new US indication is based on results of the CV outcomes trial for dulaglutide, known as REWIND, which was the longest-running CV outcomes trial in the GLP-1 agonist class.
Chair of the REWIND study, Hertzel Gerstein, MD, professor of medicine at McMaster University and Hamilton Health Sciences, Ontario, Canada, said in a Lilly statement that the trial included a “broad population of people living with type 2 diabetes, reflective of those in the general population. We therefore assessed the effect of Trulicity in people with established CVD as well as those with multiple CV risk factors.”
“Globally, over 415 million people have type 2 diabetes, which is itself a CV risk factor. However, only about one third have established CVD, which is why this new indication, and the supporting evidence, is important for the millions of people in the United States living with diabetes,” he added.
Other GLP-1 agonists have been granted approvals for additional reduction of CV events in patients with type 2 diabetes, but only for secondary prevention.
Most recently the FDA expanded the indication for once-weekly semaglutide to include reducing the risk for MACE, including CV death, nonfatal myocardial infarction, or nonfatal stroke, in adults with type 2 diabetes who have established CVD.
Additional approval based on REWIND trial
The REWIND trial included primarily people with type 2 diabetes without established CVD. The full study results were presented at the 2019 American Diabetes Association Scientific Sessions.
REWIND showed a significant reduction in risk of MACE – a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or CV death – which occurred in 12.0% of patients in the dulaglutide group, compared with 13.4% of patients in the placebo group, for a risk reduction of 0.88 (95% confidence interval, 0.79-0.99; P = .026), which was consistent across subgroups.
All three components of the MACE primary endpoint showed a reduction with dulaglutide, compared with placebo, including CV death (hazard ratio, 0.91; 95% CI, 0.78-1.06) and nonfatal MI (HR, 0.96; 95% CI, 0.79-1.16), with the strongest and only significant effect seen in nonfatal stroke (HR, 0.76; 95% CI, 0.61-0.95).
No difference was seen between groups in hospital admissions for heart failure.
Dulaglutide was also found to modestly reduce weight by around 1.5 kg (P = .0001) and systolic blood pressure by 1.7 mm Hg (P = .0001).
The safety profile of dulaglutide in REWIND was consistent with other members of the GLP-1 agonist class, with gastrointestinal events being the most common adverse event leading to discontinuation.
Sherry Martin, MD, Lilly’s vice president, medical affairs, noted in the company statement: “For the first time, health care providers can prescribe a diabetes medicine proven to significantly reduce the risk of experiencing a CV event for people with type 2 diabetes with and without established CVD.”
“Trulicity can help people achieve their A1C goals and protect them from experiencing a CV event with a once-weekly, easy-to-use treatment option,” added Martin.
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) has additionally approved dulaglutide (Trulicity) for reducing the risk of major adverse cardiovascular events (MACE) in adults with type 2 diabetes with and without established cardiovascular disease (CVD) or multiple CV risk factors, the company has announced.
Dulaglutide is a once-weekly injectable glucagonlike peptide-1 (GLP-1) receptor agonist first approved in the United States in 2014 for the treatment of type 2 diabetes.
It is now the first and only type 2 diabetes medicine approved to reduce the risk of CV events for both primary and secondary prevention populations. The European Medicines Agency approved a similar indication for dulaglutide last fall.
The new US indication is based on results of the CV outcomes trial for dulaglutide, known as REWIND, which was the longest-running CV outcomes trial in the GLP-1 agonist class.
Chair of the REWIND study, Hertzel Gerstein, MD, professor of medicine at McMaster University and Hamilton Health Sciences, Ontario, Canada, said in a Lilly statement that the trial included a “broad population of people living with type 2 diabetes, reflective of those in the general population. We therefore assessed the effect of Trulicity in people with established CVD as well as those with multiple CV risk factors.”
“Globally, over 415 million people have type 2 diabetes, which is itself a CV risk factor. However, only about one third have established CVD, which is why this new indication, and the supporting evidence, is important for the millions of people in the United States living with diabetes,” he added.
Other GLP-1 agonists have been granted approvals for additional reduction of CV events in patients with type 2 diabetes, but only for secondary prevention.
Most recently the FDA expanded the indication for once-weekly semaglutide to include reducing the risk for MACE, including CV death, nonfatal myocardial infarction, or nonfatal stroke, in adults with type 2 diabetes who have established CVD.
Additional approval based on REWIND trial
The REWIND trial included primarily people with type 2 diabetes without established CVD. The full study results were presented at the 2019 American Diabetes Association Scientific Sessions.
REWIND showed a significant reduction in risk of MACE – a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or CV death – which occurred in 12.0% of patients in the dulaglutide group, compared with 13.4% of patients in the placebo group, for a risk reduction of 0.88 (95% confidence interval, 0.79-0.99; P = .026), which was consistent across subgroups.
All three components of the MACE primary endpoint showed a reduction with dulaglutide, compared with placebo, including CV death (hazard ratio, 0.91; 95% CI, 0.78-1.06) and nonfatal MI (HR, 0.96; 95% CI, 0.79-1.16), with the strongest and only significant effect seen in nonfatal stroke (HR, 0.76; 95% CI, 0.61-0.95).
No difference was seen between groups in hospital admissions for heart failure.
Dulaglutide was also found to modestly reduce weight by around 1.5 kg (P = .0001) and systolic blood pressure by 1.7 mm Hg (P = .0001).
The safety profile of dulaglutide in REWIND was consistent with other members of the GLP-1 agonist class, with gastrointestinal events being the most common adverse event leading to discontinuation.
Sherry Martin, MD, Lilly’s vice president, medical affairs, noted in the company statement: “For the first time, health care providers can prescribe a diabetes medicine proven to significantly reduce the risk of experiencing a CV event for people with type 2 diabetes with and without established CVD.”
“Trulicity can help people achieve their A1C goals and protect them from experiencing a CV event with a once-weekly, easy-to-use treatment option,” added Martin.
This article first appeared on Medscape.com.
First clinical evidence of neuroprotection in acute stroke?
LOS ANGELES – A new potential neuroprotectant agent has been found to be beneficial for patients with acute ischemic stroke undergoing endovascular thrombectomy in a large placebo-controlled trial, but only for those patients who did not also receive thrombolysis.
There was no difference between groups on the primary outcome in the main analysis of the trial, lead author Michael Hill, MD, reported.
However, “In our study, we found a dramatic interaction of nerinetide with alteplase. There was a large benefit of nerinetide in patients not given thrombolysis, but in patients who received alteplase, this benefit was completely obliterated,” Dr. Hill said in an interview.
“In patients not treated with thrombolysis, we found a large effect size with a 9.5% absolute improvement in patients having an independent outcome (modified Rankin Score [mRS] 0-2) and a number need to treat of 10 to 11,” he said. “We also found a mortality benefit and a reduction in the size of strokes, with all other secondary outcomes going in the right direction.
“The drug works really well in patients who do not get thrombolysis, but it doesn’t work at all in patients who have had thrombolysis. The thrombolytic appears to break the peptide down so it is inactive,” he added.
“This is the first evidence that neuroprotection is possible in human stroke. This has never been shown before,” Dr. Hill noted. “Many previous clinical trials of potential neuroprotectants have been negative. We think this is a major breakthrough. This is pretty exciting stuff with really tantalizing results.”
Dr. Hill, professor of neurology at the University of Calgary (Alta.), presented results of the ESCAPE-NA1 trial on Feb. 20 at the International Stroke Conference (ISC) 2020. The trial was also simultaneously published online (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30258-0).
Endogenous nitric oxide
The new agent – known as NA1 or nerinetide – is a 20-amino-acid peptide with a novel mechanism of action; it inhibits signaling that leads to neuronal excitotoxicity. “It reduces endogenous nitric oxide generated inside the cell during ischemia, which is one of the main biochemical processes contributing to cell death,” Dr. Hill explained. In a primate model of ischemia reperfusion that was published in Nature in 2012, it was highly protective, he added.
The drug is given just once at the time of thrombectomy. It is short lived in the blood but detectable in the brain for up to 24 hours, he said.
The trial included 1,105 patients who had experienced acute ischemic stroke due to large-vessel occlusion within a 12-hour treatment window and for whom imaging results suitable for thrombectomy were available. The patients were randomly assigned to receive either intravenous nerinetide in a single dose of 2.6 mg/kg or saline placebo at the time of thrombectomy.
Patients were stratified by intravenous alteplase treatment and by declared endovascular device choice.
The primary outcome was a favorable functional outcome 90 days after randomization, defined as an mRS score of 0-2. In the main analysis of the whole population, this favorable outcome was achieved for 61.4% of the group that received nerinetide and for 59.2% of the placebo group, a nonsignificant difference. Secondary outcomes were also similar between the two groups.
But an exploratory analysis showed evidence that nerinetide’s treatment effect was modified by alteplase treatment. Among the patients who did not receive alteplase, use of nerinetide was associated with improved outcomes, whereas no benefit was found in the alteplase stratum. The difference in absolute risk slightly but not significantly favored placebo.
In the stratum that did not receive alteplase (40% of the trial population), the favorable mRS outcome was achieved by 59.3% of patients who received nerinetide, compared with 49.8% of those given placebo – a significant difference (adjusted risk ratio, 1.18; 95% confidence interval, 1.01-1.38).
There was also a 7.5% absolute risk reduction in mortality at 90 days post treatment with nerinetide for the patients who did not receive thrombolysis. This resulted in an approximate halving of the hazard of death (adjusted hazard ratio, 0.56).
In addition, infarct size was reduced in those patients who received nerinetide but not thrombolysis.
Among the patients who received alteplase, the proportion of patients who achieved an mRS of 0-2 was similar between groups, as were median infarct volumes.
The observed treatment effect modification by alteplase was supported by reductions in peak plasma nerinetide concentrations in the alteplase stratum, the researchers reported.
They said that the combination of the clinical results in the no-thrombolytic stratum and subsequent tests documenting that nerinetide is broken down by plasmin (which is generated by alteplase) “provide evidence that the clinical observation of effect modification is not a chance finding.” But they added: “This novel observation will require additional confirmation, and we cannot draw a definitive conclusion on treatment effect in this study.”
“Shaking up the field”
There is still more work to do, Dr. Hill said. “We don’t fully understand the pharmacology, and we will certainly have to do another trial, but we believe this agent is going to shake the field up. This is a totally new drug, and we have to think carefully about where it could fit in.”
“The obvious first group is those patients who do not receive thrombolysis. This is a large group, as most patients do not present in time for thrombolysis. Then we can work on the biochemistry and see if we can develop a version of nerinetide that is resistant to breakdown by thrombolysis,” he said.
Another possibility would be to withhold thrombolysis and give nerinetide instead. “It may be that thrombolysis is not needed if patients are receiving thrombectomy – this is being suggested now in initial studies,” Hill stated.
They also chose a very select group of patients – those undergoing thrombectomy, who represent only 10% to 15% of stroke patients. “We have to work out how to expand that population,” he said.
Hill noted that there have been many examples in the past of potential neuroprotectant agents that have worked in animal models of ischemia-reperfusion but that failed in humans with acute stroke.
“Until recently, we have not had a reliable ischemia-reperfusion model in humans, but now with endovascular therapy, we have a situation where the blood flow is reliably restored, which is an ideal situation to test new neuroprotectant agents. That may be another factor that has contributed to our positive findings,” he said.
In an accompanying comment in The Lancet, Graeme J. Hankey, MD, of the University of Western Australia, Perth, noted that although endovascular thrombectomy after use of intravenous alteplase improves reperfusion and clinical outcomes for a fifth of patients with ischemic stroke caused by large-artery occlusion, half of patients do not recover an independent lifestyle. Cytoprotection aims to augment the resilience of neurons, neurovascular units, and white matter during ischemia until perfusion is restored (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30316-0).
Dr. Hankey also pointed out that numerous cytoprotection strategies have been reported to reduce brain infarction in preclinical models of ischemic stroke but have not been found to improve clinical outcomes in clinical trials involving patients with ischemic stroke.
The advent of thrombectomy provides an opportunity to reassess cytoprotection as an adjunctive therapy for patients with types of temporary brain ischemia that align more closely with successful preclinical models of ischemia, cytoprotection, and reperfusion, he added.
On the results of the current study and the benefit in the no-thrombolysis group, Dr. Hankey stated: “Although this result might be a chance finding or confounded by the indication for alteplase, complementary pharmacokinetic data in a small number of patients treated with nerinetide showed that alteplase lowered plasma concentrations of nerinetide, probably by converting plasminogen to plasmin, which cleaves peptide bonds not only in fibrin but also in the eicosapeptide nerinetide.”
He said the ESCAPE-NA1 trial “informs the study of cytoprotection as an adjunct therapy to reperfusion in acute ischemic stroke” and suggested that researchers who have reported encouraging results of other cytoprotective therapies for ischemic stroke should test their compounds for interactions with concurrent thrombolytic therapies.
The ESCAPE-NA1 trial was sponsored by NoNO, the company developing nerinetide. Dr. Hill has received grants from NoNO for the conduct of the study, is named on a U.S. patent for systems and methods for assisting in decision making and triaging for acute stroke patients, and owns stock in Calgary Scientific. Other coauthors are employees of NoNO or have stock options in the company. Dr. Hankey has received personal honoraria from the American Heart Association, AC Immune, Bayer, Bristol-Myers Squibb, and Medscape outside the area of work that he commented on.
This article first appeared on Medscape.com.
LOS ANGELES – A new potential neuroprotectant agent has been found to be beneficial for patients with acute ischemic stroke undergoing endovascular thrombectomy in a large placebo-controlled trial, but only for those patients who did not also receive thrombolysis.
There was no difference between groups on the primary outcome in the main analysis of the trial, lead author Michael Hill, MD, reported.
However, “In our study, we found a dramatic interaction of nerinetide with alteplase. There was a large benefit of nerinetide in patients not given thrombolysis, but in patients who received alteplase, this benefit was completely obliterated,” Dr. Hill said in an interview.
“In patients not treated with thrombolysis, we found a large effect size with a 9.5% absolute improvement in patients having an independent outcome (modified Rankin Score [mRS] 0-2) and a number need to treat of 10 to 11,” he said. “We also found a mortality benefit and a reduction in the size of strokes, with all other secondary outcomes going in the right direction.
“The drug works really well in patients who do not get thrombolysis, but it doesn’t work at all in patients who have had thrombolysis. The thrombolytic appears to break the peptide down so it is inactive,” he added.
“This is the first evidence that neuroprotection is possible in human stroke. This has never been shown before,” Dr. Hill noted. “Many previous clinical trials of potential neuroprotectants have been negative. We think this is a major breakthrough. This is pretty exciting stuff with really tantalizing results.”
Dr. Hill, professor of neurology at the University of Calgary (Alta.), presented results of the ESCAPE-NA1 trial on Feb. 20 at the International Stroke Conference (ISC) 2020. The trial was also simultaneously published online (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30258-0).
Endogenous nitric oxide
The new agent – known as NA1 or nerinetide – is a 20-amino-acid peptide with a novel mechanism of action; it inhibits signaling that leads to neuronal excitotoxicity. “It reduces endogenous nitric oxide generated inside the cell during ischemia, which is one of the main biochemical processes contributing to cell death,” Dr. Hill explained. In a primate model of ischemia reperfusion that was published in Nature in 2012, it was highly protective, he added.
The drug is given just once at the time of thrombectomy. It is short lived in the blood but detectable in the brain for up to 24 hours, he said.
The trial included 1,105 patients who had experienced acute ischemic stroke due to large-vessel occlusion within a 12-hour treatment window and for whom imaging results suitable for thrombectomy were available. The patients were randomly assigned to receive either intravenous nerinetide in a single dose of 2.6 mg/kg or saline placebo at the time of thrombectomy.
Patients were stratified by intravenous alteplase treatment and by declared endovascular device choice.
The primary outcome was a favorable functional outcome 90 days after randomization, defined as an mRS score of 0-2. In the main analysis of the whole population, this favorable outcome was achieved for 61.4% of the group that received nerinetide and for 59.2% of the placebo group, a nonsignificant difference. Secondary outcomes were also similar between the two groups.
But an exploratory analysis showed evidence that nerinetide’s treatment effect was modified by alteplase treatment. Among the patients who did not receive alteplase, use of nerinetide was associated with improved outcomes, whereas no benefit was found in the alteplase stratum. The difference in absolute risk slightly but not significantly favored placebo.
In the stratum that did not receive alteplase (40% of the trial population), the favorable mRS outcome was achieved by 59.3% of patients who received nerinetide, compared with 49.8% of those given placebo – a significant difference (adjusted risk ratio, 1.18; 95% confidence interval, 1.01-1.38).
There was also a 7.5% absolute risk reduction in mortality at 90 days post treatment with nerinetide for the patients who did not receive thrombolysis. This resulted in an approximate halving of the hazard of death (adjusted hazard ratio, 0.56).
In addition, infarct size was reduced in those patients who received nerinetide but not thrombolysis.
Among the patients who received alteplase, the proportion of patients who achieved an mRS of 0-2 was similar between groups, as were median infarct volumes.
The observed treatment effect modification by alteplase was supported by reductions in peak plasma nerinetide concentrations in the alteplase stratum, the researchers reported.
They said that the combination of the clinical results in the no-thrombolytic stratum and subsequent tests documenting that nerinetide is broken down by plasmin (which is generated by alteplase) “provide evidence that the clinical observation of effect modification is not a chance finding.” But they added: “This novel observation will require additional confirmation, and we cannot draw a definitive conclusion on treatment effect in this study.”
“Shaking up the field”
There is still more work to do, Dr. Hill said. “We don’t fully understand the pharmacology, and we will certainly have to do another trial, but we believe this agent is going to shake the field up. This is a totally new drug, and we have to think carefully about where it could fit in.”
“The obvious first group is those patients who do not receive thrombolysis. This is a large group, as most patients do not present in time for thrombolysis. Then we can work on the biochemistry and see if we can develop a version of nerinetide that is resistant to breakdown by thrombolysis,” he said.
Another possibility would be to withhold thrombolysis and give nerinetide instead. “It may be that thrombolysis is not needed if patients are receiving thrombectomy – this is being suggested now in initial studies,” Hill stated.
They also chose a very select group of patients – those undergoing thrombectomy, who represent only 10% to 15% of stroke patients. “We have to work out how to expand that population,” he said.
Hill noted that there have been many examples in the past of potential neuroprotectant agents that have worked in animal models of ischemia-reperfusion but that failed in humans with acute stroke.
“Until recently, we have not had a reliable ischemia-reperfusion model in humans, but now with endovascular therapy, we have a situation where the blood flow is reliably restored, which is an ideal situation to test new neuroprotectant agents. That may be another factor that has contributed to our positive findings,” he said.
In an accompanying comment in The Lancet, Graeme J. Hankey, MD, of the University of Western Australia, Perth, noted that although endovascular thrombectomy after use of intravenous alteplase improves reperfusion and clinical outcomes for a fifth of patients with ischemic stroke caused by large-artery occlusion, half of patients do not recover an independent lifestyle. Cytoprotection aims to augment the resilience of neurons, neurovascular units, and white matter during ischemia until perfusion is restored (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30316-0).
Dr. Hankey also pointed out that numerous cytoprotection strategies have been reported to reduce brain infarction in preclinical models of ischemic stroke but have not been found to improve clinical outcomes in clinical trials involving patients with ischemic stroke.
The advent of thrombectomy provides an opportunity to reassess cytoprotection as an adjunctive therapy for patients with types of temporary brain ischemia that align more closely with successful preclinical models of ischemia, cytoprotection, and reperfusion, he added.
On the results of the current study and the benefit in the no-thrombolysis group, Dr. Hankey stated: “Although this result might be a chance finding or confounded by the indication for alteplase, complementary pharmacokinetic data in a small number of patients treated with nerinetide showed that alteplase lowered plasma concentrations of nerinetide, probably by converting plasminogen to plasmin, which cleaves peptide bonds not only in fibrin but also in the eicosapeptide nerinetide.”
He said the ESCAPE-NA1 trial “informs the study of cytoprotection as an adjunct therapy to reperfusion in acute ischemic stroke” and suggested that researchers who have reported encouraging results of other cytoprotective therapies for ischemic stroke should test their compounds for interactions with concurrent thrombolytic therapies.
The ESCAPE-NA1 trial was sponsored by NoNO, the company developing nerinetide. Dr. Hill has received grants from NoNO for the conduct of the study, is named on a U.S. patent for systems and methods for assisting in decision making and triaging for acute stroke patients, and owns stock in Calgary Scientific. Other coauthors are employees of NoNO or have stock options in the company. Dr. Hankey has received personal honoraria from the American Heart Association, AC Immune, Bayer, Bristol-Myers Squibb, and Medscape outside the area of work that he commented on.
This article first appeared on Medscape.com.
LOS ANGELES – A new potential neuroprotectant agent has been found to be beneficial for patients with acute ischemic stroke undergoing endovascular thrombectomy in a large placebo-controlled trial, but only for those patients who did not also receive thrombolysis.
There was no difference between groups on the primary outcome in the main analysis of the trial, lead author Michael Hill, MD, reported.
However, “In our study, we found a dramatic interaction of nerinetide with alteplase. There was a large benefit of nerinetide in patients not given thrombolysis, but in patients who received alteplase, this benefit was completely obliterated,” Dr. Hill said in an interview.
“In patients not treated with thrombolysis, we found a large effect size with a 9.5% absolute improvement in patients having an independent outcome (modified Rankin Score [mRS] 0-2) and a number need to treat of 10 to 11,” he said. “We also found a mortality benefit and a reduction in the size of strokes, with all other secondary outcomes going in the right direction.
“The drug works really well in patients who do not get thrombolysis, but it doesn’t work at all in patients who have had thrombolysis. The thrombolytic appears to break the peptide down so it is inactive,” he added.
“This is the first evidence that neuroprotection is possible in human stroke. This has never been shown before,” Dr. Hill noted. “Many previous clinical trials of potential neuroprotectants have been negative. We think this is a major breakthrough. This is pretty exciting stuff with really tantalizing results.”
Dr. Hill, professor of neurology at the University of Calgary (Alta.), presented results of the ESCAPE-NA1 trial on Feb. 20 at the International Stroke Conference (ISC) 2020. The trial was also simultaneously published online (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30258-0).
Endogenous nitric oxide
The new agent – known as NA1 or nerinetide – is a 20-amino-acid peptide with a novel mechanism of action; it inhibits signaling that leads to neuronal excitotoxicity. “It reduces endogenous nitric oxide generated inside the cell during ischemia, which is one of the main biochemical processes contributing to cell death,” Dr. Hill explained. In a primate model of ischemia reperfusion that was published in Nature in 2012, it was highly protective, he added.
The drug is given just once at the time of thrombectomy. It is short lived in the blood but detectable in the brain for up to 24 hours, he said.
The trial included 1,105 patients who had experienced acute ischemic stroke due to large-vessel occlusion within a 12-hour treatment window and for whom imaging results suitable for thrombectomy were available. The patients were randomly assigned to receive either intravenous nerinetide in a single dose of 2.6 mg/kg or saline placebo at the time of thrombectomy.
Patients were stratified by intravenous alteplase treatment and by declared endovascular device choice.
The primary outcome was a favorable functional outcome 90 days after randomization, defined as an mRS score of 0-2. In the main analysis of the whole population, this favorable outcome was achieved for 61.4% of the group that received nerinetide and for 59.2% of the placebo group, a nonsignificant difference. Secondary outcomes were also similar between the two groups.
But an exploratory analysis showed evidence that nerinetide’s treatment effect was modified by alteplase treatment. Among the patients who did not receive alteplase, use of nerinetide was associated with improved outcomes, whereas no benefit was found in the alteplase stratum. The difference in absolute risk slightly but not significantly favored placebo.
In the stratum that did not receive alteplase (40% of the trial population), the favorable mRS outcome was achieved by 59.3% of patients who received nerinetide, compared with 49.8% of those given placebo – a significant difference (adjusted risk ratio, 1.18; 95% confidence interval, 1.01-1.38).
There was also a 7.5% absolute risk reduction in mortality at 90 days post treatment with nerinetide for the patients who did not receive thrombolysis. This resulted in an approximate halving of the hazard of death (adjusted hazard ratio, 0.56).
In addition, infarct size was reduced in those patients who received nerinetide but not thrombolysis.
Among the patients who received alteplase, the proportion of patients who achieved an mRS of 0-2 was similar between groups, as were median infarct volumes.
The observed treatment effect modification by alteplase was supported by reductions in peak plasma nerinetide concentrations in the alteplase stratum, the researchers reported.
They said that the combination of the clinical results in the no-thrombolytic stratum and subsequent tests documenting that nerinetide is broken down by plasmin (which is generated by alteplase) “provide evidence that the clinical observation of effect modification is not a chance finding.” But they added: “This novel observation will require additional confirmation, and we cannot draw a definitive conclusion on treatment effect in this study.”
“Shaking up the field”
There is still more work to do, Dr. Hill said. “We don’t fully understand the pharmacology, and we will certainly have to do another trial, but we believe this agent is going to shake the field up. This is a totally new drug, and we have to think carefully about where it could fit in.”
“The obvious first group is those patients who do not receive thrombolysis. This is a large group, as most patients do not present in time for thrombolysis. Then we can work on the biochemistry and see if we can develop a version of nerinetide that is resistant to breakdown by thrombolysis,” he said.
Another possibility would be to withhold thrombolysis and give nerinetide instead. “It may be that thrombolysis is not needed if patients are receiving thrombectomy – this is being suggested now in initial studies,” Hill stated.
They also chose a very select group of patients – those undergoing thrombectomy, who represent only 10% to 15% of stroke patients. “We have to work out how to expand that population,” he said.
Hill noted that there have been many examples in the past of potential neuroprotectant agents that have worked in animal models of ischemia-reperfusion but that failed in humans with acute stroke.
“Until recently, we have not had a reliable ischemia-reperfusion model in humans, but now with endovascular therapy, we have a situation where the blood flow is reliably restored, which is an ideal situation to test new neuroprotectant agents. That may be another factor that has contributed to our positive findings,” he said.
In an accompanying comment in The Lancet, Graeme J. Hankey, MD, of the University of Western Australia, Perth, noted that although endovascular thrombectomy after use of intravenous alteplase improves reperfusion and clinical outcomes for a fifth of patients with ischemic stroke caused by large-artery occlusion, half of patients do not recover an independent lifestyle. Cytoprotection aims to augment the resilience of neurons, neurovascular units, and white matter during ischemia until perfusion is restored (Lancet. 2020 Feb 20; doi: 10.1016/S0140-6736(20)30316-0).
Dr. Hankey also pointed out that numerous cytoprotection strategies have been reported to reduce brain infarction in preclinical models of ischemic stroke but have not been found to improve clinical outcomes in clinical trials involving patients with ischemic stroke.
The advent of thrombectomy provides an opportunity to reassess cytoprotection as an adjunctive therapy for patients with types of temporary brain ischemia that align more closely with successful preclinical models of ischemia, cytoprotection, and reperfusion, he added.
On the results of the current study and the benefit in the no-thrombolysis group, Dr. Hankey stated: “Although this result might be a chance finding or confounded by the indication for alteplase, complementary pharmacokinetic data in a small number of patients treated with nerinetide showed that alteplase lowered plasma concentrations of nerinetide, probably by converting plasminogen to plasmin, which cleaves peptide bonds not only in fibrin but also in the eicosapeptide nerinetide.”
He said the ESCAPE-NA1 trial “informs the study of cytoprotection as an adjunct therapy to reperfusion in acute ischemic stroke” and suggested that researchers who have reported encouraging results of other cytoprotective therapies for ischemic stroke should test their compounds for interactions with concurrent thrombolytic therapies.
The ESCAPE-NA1 trial was sponsored by NoNO, the company developing nerinetide. Dr. Hill has received grants from NoNO for the conduct of the study, is named on a U.S. patent for systems and methods for assisting in decision making and triaging for acute stroke patients, and owns stock in Calgary Scientific. Other coauthors are employees of NoNO or have stock options in the company. Dr. Hankey has received personal honoraria from the American Heart Association, AC Immune, Bayer, Bristol-Myers Squibb, and Medscape outside the area of work that he commented on.
This article first appeared on Medscape.com.
Higher endovascular thrombectomy volumes yield better stroke outcomes
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
The results reported by Dr. Stein raise issues about balancing the access to certain therapies with the outcomes of those therapies. Having procedures like endovascular thrombectomy for acute ischemic stroke done primarily at high-volume centers might improve procedural outcomes, but having more centers offering this treatment across wider geographical areas would make this treatment more broadly available to more people.
For endovascular thrombectomy, center volume and experience may be much more important than proceduralist volume because having a smoothly functioning system in place is so important for rapid stroke assessment and treatment. It’s also important for programs to provide experienced and comprehensive postthrombectomy care. Success in endovascular thrombectomy involves much more than just taking a clot out. It means quickly and smoothly moving patients through the steps that precede thrombectomy and then following the intervention with a range of services that optimize recovery.
Ashutosh P. Jadhav, MD, PhD , is director of the comprehensive stroke center at the University of Pittsburgh. He had no relevant disclosures. He made these comments in an interview.
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
LOS ANGELES – Higher case volumes matter for getting better outcomes in acute ischemic stroke patients treated with endovascular thrombectomy, according to data from more than 13,000 Medicare patients treated during 2016 and 2017.
That’s hardly surprising, given that it’s consistent with what’s already been reported for several other types of endovascular and transcatheter procedures: The more cases a center or individual proceduralist performs, the better their patients do. Routine use of endovascular thrombectomy to treat selected acute ischemic stroke patients is a new-enough paradigm that until now few reports have come out that looked at this issue (Stroke. 2019 May;50[5]:1178-83).
The new analysis of Medicare data “is one of the first contemporary studies of the volume-outcome relationship in endovascular thrombectomy,” Laura K. Stein, MD, said at the International Stroke Conference sponsored by the American Heart Association. The analysis showed that, when the researchers adjusted the Medicare data to better reflect overall case volumes (Medicare patients represent just 59% of all endovascular thrombectomies performed on U.S. acute ischemic stroke patients), the minimum case number for a stroke center to have statistically better in-hospital survival than lower volume centers was 24 cases/year, and 29 cases/year to have a statistically significant higher rate of “good” outcomes than lower-volume centers, reported Dr. Stein, a stroke neurologist with the Mount Sinai Health System in New York. For individual proceduralists, the minimum, adjusted case number to have statistically better acute patient survival was 4 cases/year, and 19 cases/year to have a statistically better rate of good outcomes.
For this analysis, good outcomes were defined as cases when patients left the hospital following their acute care and returned home with either self care or a home health care service, and also patients discharged to rehabilitation. “Bad” outcomes for this analysis were discharges to a skilled nursing facility or hospice, as well as patients who died during their acute hospitalization.
The analyses also showed no plateau to the volume effect for any of the four parameters examined: in-hospital mortality by center and by proceduralist, and the rates of good outcomes by center and by proceduralist. For each of these measures, as case volume increased above the minimum number needed to produce statistically better outcomes, the rate of good outcomes continued to steadily rise and acute mortality continued to steadily fall.
The study run by Dr. Stein and associates used data collected by the Center for Medicare & Medicaid Services on 13,311 Medicare patients who underwent endovascular thrombectomy for acute ischemic stroke at any of 641 U.S. hospitals and received treatment from any of 2,754 thrombectomy proceduralists. Outcomes rated as good occurred in 56% of the patients. The statistical adjustments that the researchers applied to calculate the incremental effect of increasing case volume took into account the variables of patient age, sex, and comorbidities measured by the Charlson Comorbidity Index.
The analysis also showed that, during this 2-year period, the average number of endovascular thrombectomy cases among Medicare patients was just under 21 cases per center, with a range of 1-160 cases; for individual proceduralists, the average was just under 5 cases, with a range of 1-82 cases.
The 19 case/year volume minimum that the analysis identified for an individual proceduralist to have a statistically significant higher rate of good outcomes, compared with lower-volume proceduralists, came close to the 15 cases/year minimum set by the Joint Commission in 2019 for individual operators at centers seeking accreditation from the Joint Commission as either a Thrombectomy-Capable Stroke Center or a Comprehensive Stroke Center. The CMS has not yet set thrombectomy case-load requirements for centers or operators to qualify for Medicare reimbursements, although CMS has set such standards for other endovascular procedures, such as transcatheter aortic valve replacement. When setting such standards, CMS has cited its need to balance the better outcomes produced by higher-volume centers against a societal interest in facilitating access to vital medical services, a balance that Dr. Stein also highlighted in her talk.
“We want to optimize access as well as outcomes for every patient,” she said. “These data support certification volume standards,” but they are “in no way an argument for limiting access based on volume.”
Dr. Stein had no disclosures.
SOURCE: Stein LK et al. ISC 2020, Abstract LB11.
REPORTING FROM ISC 2020
TNK dose in large-vessel stroke: 0.25 mg/kg is sufficient
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
A new study suggests that the 0.25-mg/kg dose of the thrombolytic tenecteplase (TNK) is just as good at facilitating reperfusion of the blocked artery in patients with ischemic large-vessel stroke prior to planned thrombectomy as the higher 0.4-mg/kg dose.
The EXTEND-IA TNK Part 2 trial was presented today at the American Stroke Association’s International Stroke Conference (ISC) 2020 in Los Angeles and was published online simultaneously (JAMA. 2020 Feb 20. doi: 10.1001/jama.2020.1511).
“We found the 0.4-mg/kg dose was no better than 0.25 mg/kg. There was absolutely no perceptible difference, so it appears that 0.25 mg/kg is enough,” lead investigator Bruce Campbell, MBBS, PhD, said in an interview.
“Our study was conducted in patients with large-vessel occlusions heading for thrombectomy, but I think the results can be extrapolated to patients with smaller occlusions too,” he added.
The study also showed that one-fifth of patients given tenecteplase experienced reperfusion before thrombectomy was performed. The percentage rose to one-third among patients from rural areas, whose longer times in transport led to an increase in the time between thrombolysis and thrombectomy.
“I think these data are as good as we’re going to get on the optimal dose of TNK. Our endpoint was reperfusion rates – a good, solid biological marker of benefit – but if a difference in clinical outcomes is wanted, that would take a trial of several thousand patients, which is never likely to be done,” said Dr. Campbell, who is from the Department of Neurology at the Royal Melbourne Hospital, Australia.
The researchers note that tenecteplase has a practical advantage over alteplase in that it is given as a bolus injection, whereas alteplase is given as bolus followed by a 1-hour infusion.
Results from the first EXTEND-IA TNK study suggested that tenecteplase 0.25 mg/kg produced higher reperfusion rates than alteplase (N Engl J Med. 2018;378:1573-82). However, the larger NOR-TEST study found no difference in efficacy or safety between a 0.4-mg/kg dose of tenecteplase and alteplase in patients with mild stroke (Lancet Neurol. 2017 Oct;16[10]:781-8).
TNK use in stroke varies around the world. The drug is not licensed for use in stroke anywhere, which Dr. Campbell attributes to a lack of incentive for the manufacturer, Genentech/Boehringer Ingelheim. That company also markets alteplase, the main thrombolytic used in stroke.
But many countries have now included TNK in their stroke guidelines, Dr. Campbell noted. “This has only recently occurred in the U.S., where it has a 2b recommendation, and the dose recommendations are somewhat confusing, advocating 0.25 mg/kg in large-vessel occlusions [as was used in the first EXTEND IA study] and 0.4 mg/kg in non–large vessel occlusions [from the NOR-TEST trial].
“This makes no biological sense whatsoever, recommending a higher dose for smaller occlusions, but that is just a literal translation of the design of the two major studies. I’m hoping our current results will help clarify the dosage issue and that might encourage more use of TNK altogether,” he commented.
For the current study, conducted in Australia and New Zealand, 300 patients who had experienced ischemic large-vessel stroke within 4.5 hours of symptom onset and who were scheduled for endovascular thrombectomy were randomly assigned to receive open-label thrombolysis with tenecteplase 0.4 mg/kg or 0.25 mg/kg.
The primary outcome, reperfusion of greater than 50% of the involved ischemic territory prior to thrombectomy, occurred in 19.3% of both groups. There was also no difference in any of the functional-outcome secondary endpoints or all-cause mortality between the two doses.
“While we didn’t find any extra benefit of the 0.4-mg/kg dose over the 0.25-mg/kg dose, we also didn’t find any extra harm, and this gives us reassurance in the emergency situation if the weight of the patient is overestimated; then we have a window of safety,” Dr. Campbell commented. “While there was a nonsignificant numerical increase in intracranial hemorrhage in the 0.4-mg/kg group, the excess bleeds were caused by puncturing of the vessels during thrombectomy, so I don’t think we can blame the TNK dose for that.
Better reperfusion than with alteplase?
Noting that the original EXTEND-IA TNK study showed higher reperfusion rates with tenecteplase vs alteplase and a trend toward better outcomes on the mRS scale, Campbell reported that a pooled analysis of the TNK results from the current study with those from the first study confirmed these findings.
“We found a doubling in the rate of reperfusion with TNK vs. alteplase, and the [modified Rankin Scale] shift analysis remained positive,” he said.
“I think we say with confidence that TNK is at least as good as alteplase and probably better, but further studies comparing the two agents are ongoing,” he added.
Of note, for the 41 patients from rural areas in the current study, in whom the time from thrombolysis to thrombectomy was longer (152 min vs. 41 min for patients from urban areas), reperfusion rates were higher (34% vs 17%), and there was no difference in dosage between the two groups.
Commenting on these latest results in an interview, Nicola Logallo, MD, of Haukeland University Hospital, Bergen, Norway, who was part of the NOR-TEST trial, said: “There is some evidence supporting the use of TNK 0.4 mg/kg in mild stroke patients, based mainly on the results from the NOR-TEST trial, and the use of TNK 0.25 mg/kg in patients undergoing thrombectomy, based on Dr. Campbell’s previous EXTEND-TNK trial. Dr. Campbell’s new study confirms that probably the higher dose of TNK does not add any advantages in terms of clinical outcome.”
Hemorrhagic complications appear to be similar in the two groups, Dr. Logallo said. “Overall, the 0.25-mg/kg TNK dose could therefore be considered as the most convenient and sensible, at least in patients undergoing thrombectomy. When it comes to the remaining stroke patients receiving thrombolysis, it remains unclear which is the best dose, but studies such as TASTE, NOR-TEST 2, AcT, and ATTEST-2 will hopefully answer this question within the next years.”
Also commenting on the study, Michael Hill, MD, professor of neurology at University of Calgary, Alberta, Canada, said the results “confirm that a good proportion of patients given TNK reperfuse before the angiogram and clarifies the dose. This is useful information.”
Dr. Hill said TNK is used routinely in some countries – mainly in Australia and Norway, where the studies have been conducted – but there is now a movement toward use of TNK in North America, too.
“Studies so far suggest that it could be more effective than alteplase, and as it is more fibrin specific, it could be safer. It is also easier to give with a bolus dose, but perhaps the biggest driver might be that it is cheaper than alteplase. Momentum is building, and many leading investigators are now conducting new studies with TNK with several more studies coming out in the next year or so,” Dr. Hill added.
The EXTEND-IA TNK Part 2 trial was supported by grants from the National Health and Medical Research Council of Australia and the National Heart Foundation of Australia. Campbell reports receiving grants from both institutions during the conduct of the study.
This article first appeared on Medscape.com.
ARCADIA: Predicting risk of atrial cardiopathy poststroke
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.
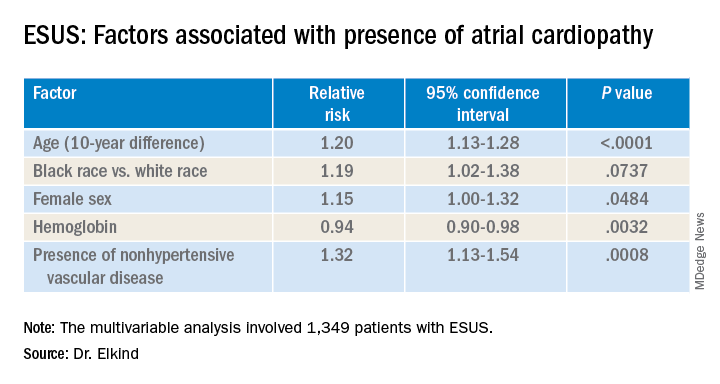
Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.

Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
LOS ANGELES – Older age, female sex, black race, relative anemia, and a history of cardiovascular disease are associated with greater risk for atrial cardiopathy among people who experienced an embolic stroke of undetermined source (ESUS), new evidence suggests.

Atrial cardiopathy is a suspected cause of ESUS independent of atrial fibrillation. However, clinical predictors to help physicians identify which ESUS patients are at increased risk remain unknown.
The risk for atrial cardiopathy was 34% higher for women versus men with ESUS in this analysis. In addition, black participants had a 29% increased risk, compared with others, and each 10 years of age increased risk for atrial cardiopathy by 30% in an univariable analysis.
“Modest effects of these associations suggest that all ESUS patients, regardless of underlying demographic and risk factors, may have atrial cardiopathy,” principal investigator Mitchell S.V. Elkind, MD, of Columbia University, New York, said when presenting results at the 2020 International Stroke Conference, sponsored by the American Heart Association.
For this reason, he added, all people with ESUS should be considered for recruitment into the ongoing ARCADIA (AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke) trial, of which he is one of the principal investigators.
ESUS is a heterogeneous condition, and some patients may be responsive to anticoagulants and some might not, Elkind said. This observation “led us to consider alternative ways for ischemic disease to lead to stroke. We would hypothesize that the underlying atrium can be a risk for stroke by itself.”
Not yet available is the primary efficacy outcome of the multicenter, randomized ARCADIA trial comparing apixaban with aspirin in reducing risk for recurrent stroke of any type. However, Dr. Elkind and colleagues have recruited 1,505 patients to date, enough to analyze factors that predict risk for recurrent stroke among people with evidence of atrial cardiopathy.
All ARCADIA participants are 45 years of age or older and have no history of atrial fibrillation. Atrial cardiopathy was defined by presence of at least one of three biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), P wave terminal force velocity, or evidence of a left atrial diameter of 3 cm/m2 or larger on echocardiography.
Of the 1,349 ARCADIA participants eligible for the current analysis, approximately one-third met one or more of these criteria for atrial cardiopathy.
Those with atrial cardiopathy were “more likely to be black and be women, and tended to have shorter time from stroke to screening,” Dr. Elkind said. In addition, heart failure, hypertension, and peripheral artery disease were more common in those with atrial cardiopathy. This group also was more likely to have an elevation in creatinine and lower hemoglobin and hematocrit levels.
“Heart disease, ischemic heart disease and non-hypertensive vascular disease were significant risk factors” for recurrent stroke in the study, Dr. Elkind added.
Elkind said that, surprisingly, there was no independent association between the time to measurement of NT-proBNP and risk, suggesting that this biomarker “does not rise simply in response to stroke, but reflects a stable condition.”
The multicenter ARCADIA trial is recruiting additional participants at 142 sites now, Dr. Elkind said, “and we are still looking for more sites.”
Which comes first?
“He is looking at what the predictors are for cardiopathy in these patients, which is fascinating for all of us,” session moderator Michelle Christina Johansen, MD, assistant professor of neurology at Johns Hopkins University, Baltimore, said in an interview when asked to comment.
There is always the conundrum of what came first — the chicken or the egg, Johansen said. Do these patients have stroke that then somehow led to a state that predisposes them to have atrial cardiopathy? Or, rather, was it an atrial cardiopathy state independent of atrial fibrillation that then led to stroke?
“That is why looking at predictors in this population is of such interest,” she said. The study could help identify a subgroup of patients at higher risk for atrial cardiopathy and guide clinical decision-making when patients present with ESUS.
“One of the things I found interesting was that he found that atrial cardiopathy patients were older [a mean 69 years]. This was amazing, because ESUS patients in general tend to be younger,” Dr. Johansen said.
“And there is about a 4-5% risk of recurrence with these patients. So. it was interesting that prior stroke or [transient ischemic attack] was not associated.”*
The National Institute of Neurological Disorders and Stroke, the BMS-Pfizer Alliance, and Roche provide funding for ARCADIA. Dr. Elkind and Dr. Johansen disclosed no relevant financial relationships.
SOURCE: Elkind M et al. ISC 2020, Abstract 26.
This article first appeared on Medscape.com.
*Correction, 4/28/20: An earlier version of this article misstated the risk of recurrence.
REPORTING FROM ISC 2020
Shingles vaccine linked to lower stroke risk
LOS ANGELES – Prevention of shingles with the Zoster Vaccine Live may reduce the risk of subsequent stroke among older adults as well, the first study to examine this association suggests. Shingles vaccination was linked to a 20% decrease in stroke risk in people younger than 80 years of age in the large Medicare cohort study. Older participants showed a 10% reduced risk, according to data released in advance of formal presentation at this week’s International Stroke Conference, sponsored by the American Heart Association.
Reductions were seen for both ischemic and hemorrhagic events.
“Our findings might encourage people age 50 or older to get vaccinated against shingles and to prevent shingles-associated stroke risk,” Quanhe Yang, PhD, lead study author and senior scientist at the Centers for Disease Control and Prevention, said in an interview.
Dr. Yang and colleagues evaluated the only shingles vaccine available at the time of the study, Zoster Vaccine Live (Zostavax). However, the CDC now calls an adjuvanted, nonlive recombinant vaccine (Shingrix) the preferred shingles vaccine for healthy adults aged 50 years and older. Shingrix was approved in 2017. Zostavax, approved in 2006, can still be used in healthy adults aged 60 years and older, the agency states.
A reduction in inflammation from Zoster Vaccine Live may be the mechanism by which stroke risk is reduced, Dr. Yang said. The newer vaccine, which the CDC notes is more than 90% effective, might provide even greater protection against stroke, although more research is needed, he added.
Interestingly, prior research suggested that, once a person develops shingles, it may be too late. Dr. Yang and colleagues showed vaccination or antiviral treatment after a shingles episode was not effective at reducing stroke risk in research presented at the 2019 International Stroke Conference.
Shingles can present as a painful reactivation of chickenpox, also known as the varicella-zoster virus. Shingles is also common; Dr. Yang estimated one in three people who had chickenpox will develop the condition at some point in their lifetime. In addition, researchers have linked shingles to an elevated risk of stroke.
To assess the vaccine’s protective effect on stroke, Dr. Yang and colleagues reviewed health records for 1.38 million Medicare recipients. All participants were aged 66 years or older, had no history of stroke at baseline, and received the Zoster Vaccine Live during 2008-2016. The investigators compared the stroke rate in this vaccinated group with the rate in a matched control group of the same number of Medicare fee-for-service beneficiaries who did not receive the vaccination. They adjusted their analysis for age, sex, race, medications, and comorbidities.
The overall decrease of 16% in stroke risk associated with vaccination included a 12% drop in hemorrhagic stroke and 18% decrease in ischemic stroke over a median follow-up of 3.9 years follow-up (interquartile range, 2.7-5.4).
The adjusted hazard ratios comparing the vaccinated with control groups were 0.84 (95% confidence interval, 0.83-0.85) for all stroke; 0.82 (95% CI, 0.81-0.83) for acute ischemic stroke; and 0.88 (95% CI, 0.84-0.91) for hemorrhagic stroke.
The vaccinated group experienced 42,267 stroke events during that time. This rate included 33,510 acute ischemic strokes and 4,318 hemorrhagic strokes. At the same time, 48,139 strokes occurred in the control group. The breakdown included 39,334 ischemic and 4,713 hemorrhagic events.
“Approximately 1 million people in the United States get shingles each year, yet there is a vaccine to help prevent it,” Dr. Yang stated in a news release. “Our study results may encourage people ages 50 and older to follow the recommendation and get vaccinated against shingles. You are reducing the risk of shingles, and at the same time, you may be reducing your risk of stroke.”
“Further studies are needed to confirm our findings of association between Zostavax vaccine and risk of stroke,” Dr. Yang said.
Because the CDC Advisory Committee on Immunization Practices recommended Shingrix vaccine only for healthy adults 50 years and older in 2017, there were insufficient data in Medicare to study the association between that vaccine and risk of stroke at the time of the current study.
“However, two doses of Shingrix are more than 90% effective at preventing shingles and postherpetic neuralgia, and higher than that of Zostavax,” Dr. Yang said.
‘Very intriguing’ research
“This is a very interesting study,” Ralph L. Sacco, MD, past president of the American Heart Association, said in a video commentary released in advance of the conference. It was a very large sample, he noted, and those older than age 60 years who had the vaccine were protected with a lower risk for both ischemic and hemorrhagic stroke.
“So it is very intriguing,” added Dr. Sacco, chairman of the department of neurology at the University of Miami. “We know things like shingles can increase inflammation and increase the risk of stroke,” Dr. Sacco said, “but this is the first time in a very large Medicare database that it was shown that those who had the vaccine had a lower risk of stroke.”
The CDC funded this study. Dr. Yang and Dr. Sacco have disclosed no relevant financial relationships.
SOURCE: Yang Q et al. ISC 2020, Abstract TP493.
This article first appeared on Medscape.com.
LOS ANGELES – Prevention of shingles with the Zoster Vaccine Live may reduce the risk of subsequent stroke among older adults as well, the first study to examine this association suggests. Shingles vaccination was linked to a 20% decrease in stroke risk in people younger than 80 years of age in the large Medicare cohort study. Older participants showed a 10% reduced risk, according to data released in advance of formal presentation at this week’s International Stroke Conference, sponsored by the American Heart Association.
Reductions were seen for both ischemic and hemorrhagic events.
“Our findings might encourage people age 50 or older to get vaccinated against shingles and to prevent shingles-associated stroke risk,” Quanhe Yang, PhD, lead study author and senior scientist at the Centers for Disease Control and Prevention, said in an interview.
Dr. Yang and colleagues evaluated the only shingles vaccine available at the time of the study, Zoster Vaccine Live (Zostavax). However, the CDC now calls an adjuvanted, nonlive recombinant vaccine (Shingrix) the preferred shingles vaccine for healthy adults aged 50 years and older. Shingrix was approved in 2017. Zostavax, approved in 2006, can still be used in healthy adults aged 60 years and older, the agency states.
A reduction in inflammation from Zoster Vaccine Live may be the mechanism by which stroke risk is reduced, Dr. Yang said. The newer vaccine, which the CDC notes is more than 90% effective, might provide even greater protection against stroke, although more research is needed, he added.
Interestingly, prior research suggested that, once a person develops shingles, it may be too late. Dr. Yang and colleagues showed vaccination or antiviral treatment after a shingles episode was not effective at reducing stroke risk in research presented at the 2019 International Stroke Conference.
Shingles can present as a painful reactivation of chickenpox, also known as the varicella-zoster virus. Shingles is also common; Dr. Yang estimated one in three people who had chickenpox will develop the condition at some point in their lifetime. In addition, researchers have linked shingles to an elevated risk of stroke.
To assess the vaccine’s protective effect on stroke, Dr. Yang and colleagues reviewed health records for 1.38 million Medicare recipients. All participants were aged 66 years or older, had no history of stroke at baseline, and received the Zoster Vaccine Live during 2008-2016. The investigators compared the stroke rate in this vaccinated group with the rate in a matched control group of the same number of Medicare fee-for-service beneficiaries who did not receive the vaccination. They adjusted their analysis for age, sex, race, medications, and comorbidities.
The overall decrease of 16% in stroke risk associated with vaccination included a 12% drop in hemorrhagic stroke and 18% decrease in ischemic stroke over a median follow-up of 3.9 years follow-up (interquartile range, 2.7-5.4).
The adjusted hazard ratios comparing the vaccinated with control groups were 0.84 (95% confidence interval, 0.83-0.85) for all stroke; 0.82 (95% CI, 0.81-0.83) for acute ischemic stroke; and 0.88 (95% CI, 0.84-0.91) for hemorrhagic stroke.
The vaccinated group experienced 42,267 stroke events during that time. This rate included 33,510 acute ischemic strokes and 4,318 hemorrhagic strokes. At the same time, 48,139 strokes occurred in the control group. The breakdown included 39,334 ischemic and 4,713 hemorrhagic events.
“Approximately 1 million people in the United States get shingles each year, yet there is a vaccine to help prevent it,” Dr. Yang stated in a news release. “Our study results may encourage people ages 50 and older to follow the recommendation and get vaccinated against shingles. You are reducing the risk of shingles, and at the same time, you may be reducing your risk of stroke.”
“Further studies are needed to confirm our findings of association between Zostavax vaccine and risk of stroke,” Dr. Yang said.
Because the CDC Advisory Committee on Immunization Practices recommended Shingrix vaccine only for healthy adults 50 years and older in 2017, there were insufficient data in Medicare to study the association between that vaccine and risk of stroke at the time of the current study.
“However, two doses of Shingrix are more than 90% effective at preventing shingles and postherpetic neuralgia, and higher than that of Zostavax,” Dr. Yang said.
‘Very intriguing’ research
“This is a very interesting study,” Ralph L. Sacco, MD, past president of the American Heart Association, said in a video commentary released in advance of the conference. It was a very large sample, he noted, and those older than age 60 years who had the vaccine were protected with a lower risk for both ischemic and hemorrhagic stroke.
“So it is very intriguing,” added Dr. Sacco, chairman of the department of neurology at the University of Miami. “We know things like shingles can increase inflammation and increase the risk of stroke,” Dr. Sacco said, “but this is the first time in a very large Medicare database that it was shown that those who had the vaccine had a lower risk of stroke.”
The CDC funded this study. Dr. Yang and Dr. Sacco have disclosed no relevant financial relationships.
SOURCE: Yang Q et al. ISC 2020, Abstract TP493.
This article first appeared on Medscape.com.
LOS ANGELES – Prevention of shingles with the Zoster Vaccine Live may reduce the risk of subsequent stroke among older adults as well, the first study to examine this association suggests. Shingles vaccination was linked to a 20% decrease in stroke risk in people younger than 80 years of age in the large Medicare cohort study. Older participants showed a 10% reduced risk, according to data released in advance of formal presentation at this week’s International Stroke Conference, sponsored by the American Heart Association.
Reductions were seen for both ischemic and hemorrhagic events.
“Our findings might encourage people age 50 or older to get vaccinated against shingles and to prevent shingles-associated stroke risk,” Quanhe Yang, PhD, lead study author and senior scientist at the Centers for Disease Control and Prevention, said in an interview.
Dr. Yang and colleagues evaluated the only shingles vaccine available at the time of the study, Zoster Vaccine Live (Zostavax). However, the CDC now calls an adjuvanted, nonlive recombinant vaccine (Shingrix) the preferred shingles vaccine for healthy adults aged 50 years and older. Shingrix was approved in 2017. Zostavax, approved in 2006, can still be used in healthy adults aged 60 years and older, the agency states.
A reduction in inflammation from Zoster Vaccine Live may be the mechanism by which stroke risk is reduced, Dr. Yang said. The newer vaccine, which the CDC notes is more than 90% effective, might provide even greater protection against stroke, although more research is needed, he added.
Interestingly, prior research suggested that, once a person develops shingles, it may be too late. Dr. Yang and colleagues showed vaccination or antiviral treatment after a shingles episode was not effective at reducing stroke risk in research presented at the 2019 International Stroke Conference.
Shingles can present as a painful reactivation of chickenpox, also known as the varicella-zoster virus. Shingles is also common; Dr. Yang estimated one in three people who had chickenpox will develop the condition at some point in their lifetime. In addition, researchers have linked shingles to an elevated risk of stroke.
To assess the vaccine’s protective effect on stroke, Dr. Yang and colleagues reviewed health records for 1.38 million Medicare recipients. All participants were aged 66 years or older, had no history of stroke at baseline, and received the Zoster Vaccine Live during 2008-2016. The investigators compared the stroke rate in this vaccinated group with the rate in a matched control group of the same number of Medicare fee-for-service beneficiaries who did not receive the vaccination. They adjusted their analysis for age, sex, race, medications, and comorbidities.
The overall decrease of 16% in stroke risk associated with vaccination included a 12% drop in hemorrhagic stroke and 18% decrease in ischemic stroke over a median follow-up of 3.9 years follow-up (interquartile range, 2.7-5.4).
The adjusted hazard ratios comparing the vaccinated with control groups were 0.84 (95% confidence interval, 0.83-0.85) for all stroke; 0.82 (95% CI, 0.81-0.83) for acute ischemic stroke; and 0.88 (95% CI, 0.84-0.91) for hemorrhagic stroke.
The vaccinated group experienced 42,267 stroke events during that time. This rate included 33,510 acute ischemic strokes and 4,318 hemorrhagic strokes. At the same time, 48,139 strokes occurred in the control group. The breakdown included 39,334 ischemic and 4,713 hemorrhagic events.
“Approximately 1 million people in the United States get shingles each year, yet there is a vaccine to help prevent it,” Dr. Yang stated in a news release. “Our study results may encourage people ages 50 and older to follow the recommendation and get vaccinated against shingles. You are reducing the risk of shingles, and at the same time, you may be reducing your risk of stroke.”
“Further studies are needed to confirm our findings of association between Zostavax vaccine and risk of stroke,” Dr. Yang said.
Because the CDC Advisory Committee on Immunization Practices recommended Shingrix vaccine only for healthy adults 50 years and older in 2017, there were insufficient data in Medicare to study the association between that vaccine and risk of stroke at the time of the current study.
“However, two doses of Shingrix are more than 90% effective at preventing shingles and postherpetic neuralgia, and higher than that of Zostavax,” Dr. Yang said.
‘Very intriguing’ research
“This is a very interesting study,” Ralph L. Sacco, MD, past president of the American Heart Association, said in a video commentary released in advance of the conference. It was a very large sample, he noted, and those older than age 60 years who had the vaccine were protected with a lower risk for both ischemic and hemorrhagic stroke.
“So it is very intriguing,” added Dr. Sacco, chairman of the department of neurology at the University of Miami. “We know things like shingles can increase inflammation and increase the risk of stroke,” Dr. Sacco said, “but this is the first time in a very large Medicare database that it was shown that those who had the vaccine had a lower risk of stroke.”
The CDC funded this study. Dr. Yang and Dr. Sacco have disclosed no relevant financial relationships.
SOURCE: Yang Q et al. ISC 2020, Abstract TP493.
This article first appeared on Medscape.com.
REPORTING FROM ISC 2020












