User login
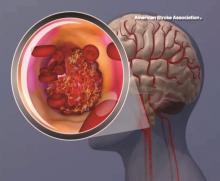
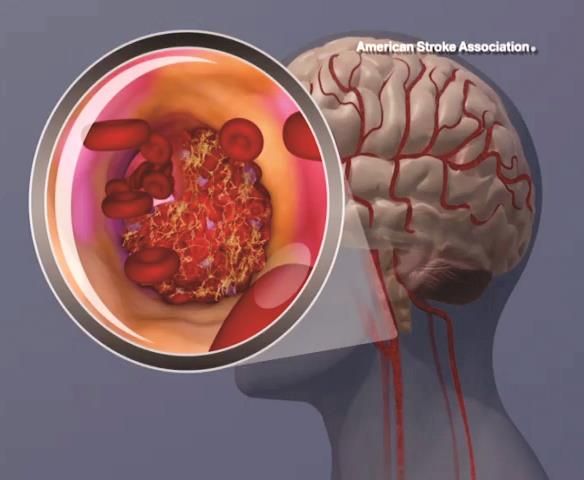
Stroke risk tied to diabetic retinopathy may not be modifiable
LOS ANGELES – Evidence continues to mount that diabetic retinopathy predicts elevated risk for stroke.
In a new study with nearly 3,000 people, those with diabetic retinopathy were 60% more likely than others with diabetes to develop an incident stroke over time. Investigators also found that addressing glucose, lipids, and blood pressure levels did not mitigate this risk in this secondary analysis of the ACCORD Eye Study.
“We are not surprised with the finding that diabetic retinopathy increases the risk of stroke — as diabetic retinopathy is common microvascular disease that is an established risk factor for cardiovascular disease,” lead author Ka-Ho Wong, BS, MBA, said in an interview.
However, “we were surprised that none of the trial interventions mitigated this risk, in particular the intensive blood pressure reduction, because hypertension is the most important cause of microvascular disease,” he said. Mr. Wong is clinical research coordinator and lab manager of the de Havenon Lab at the University of Utah Health Hospitals and Clinics in Salt Lake City.
The study findings were released Feb. 12, 2020, in advance of formal presentation at the International Stroke Conference sponsored by the American Heart Association.
Common predictor of vascular disease
Diabetic retinopathy is the most common complication of diabetes mellitus, affecting up to 50% of people living with type 1 and type 2 diabetes. In addition, previous research suggests that macrovascular diabetes complications, including stroke, could share a common or synergistic pathway.
This small vessel damage in the eye also has been linked to an increased risk of adverse cardiac events, including heart failure, as previously reported by Medscape Medical News.
To find out more, Mr. Wong and colleagues analyzed 2,828 participants in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. They compared the stroke risk between 874 people with diabetic retinopathy and another 1,954 diabetics without this complication. The average age was 62 years and 62% were men.
Diabetic neuropathy at baseline was diagnosed using the Early Treatment Diabetic Retinopathy Study Severity Scale using seven-field stereoscopic fundus photographs.
A total of 117 participants experienced a stroke during a mean follow-up of 5.4 years.
The investigators found that diabetic retinopathy was more common among patients who had a stroke (41%) versus 31% of those without a stroke (P = .016). The link between diabetic retinopathy and stroke remained in an analysis adjusted for multiple factors, including baseline age, gender, race, total cholesterol, A1c, smoking, and more. Risk remained elevated, with a hazard ratio of 1.60 (95% confidence interval, 1.10-2.32; P = .015).
Regarding the potential for modifying this risk, the association was unaffected among participants randomly assigned to the ACCORD glucose intervention (P = .305), lipid intervention (P = .546), or blood pressure intervention (P = .422).
The study was a secondary analysis, so information on stroke type and location were unavailable.
The big picture
“Diabetic retinopathy is associated with an increased risk of stroke, which suggests that the microvascular pathology inherent to diabetic retinopathy has larger cardiovascular implications,” the researchers noted.
Despite these findings, the researchers suggest that patients with diabetic retinopathy receive aggressive medical management to try to reduce their stroke risk.
“It’s important for everyone with diabetes to maintain good blood glucose control, and those with established diabetic retinopathy should pay particular attention to meeting all the stroke prevention guidelines that are established by the American Stroke Association,” said Mr. Wong.
“Patients with established diabetic retinopathy should pay particular attention to meeting all stroke prevention guidelines established by the [American Heart Association],” he added.
Mr. Wong and colleagues would like to expand on these findings. Pending grant application and funding support, they propose conducting a prospective, observational trial in stroke patients with baseline diabetic retinopathy. One aim would be to identify the most common mechanisms leading to stroke in this population, “which would have important implications for prevention efforts,” he said.
Consistent Findings
“The results of the study showing that having diabetic retinopathy is also associated with an increase in stroke really isn’t surprising. There have been other studies, population-based studies, done in the past, that have found a similar relationship,” Larry B. Goldstein, MD, said in a video commentary on the findings.
“The results are actually quite consistent with several other studies that have evaluated the same relationship,” added Dr. Goldstein, who is chair of the department of neurology and codirector of the Kentucky Neuroscience Institute, University of Kentucky HealthCare, Lexington.
Mr. Wong and Dr. Goldstein have disclosed no relevant financial relationships. The NIH’s National Institute of Neurological Disorders and Stroke funded the study.
This article first appeared on Medscape.com.
LOS ANGELES – Evidence continues to mount that diabetic retinopathy predicts elevated risk for stroke.
In a new study with nearly 3,000 people, those with diabetic retinopathy were 60% more likely than others with diabetes to develop an incident stroke over time. Investigators also found that addressing glucose, lipids, and blood pressure levels did not mitigate this risk in this secondary analysis of the ACCORD Eye Study.
“We are not surprised with the finding that diabetic retinopathy increases the risk of stroke — as diabetic retinopathy is common microvascular disease that is an established risk factor for cardiovascular disease,” lead author Ka-Ho Wong, BS, MBA, said in an interview.
However, “we were surprised that none of the trial interventions mitigated this risk, in particular the intensive blood pressure reduction, because hypertension is the most important cause of microvascular disease,” he said. Mr. Wong is clinical research coordinator and lab manager of the de Havenon Lab at the University of Utah Health Hospitals and Clinics in Salt Lake City.
The study findings were released Feb. 12, 2020, in advance of formal presentation at the International Stroke Conference sponsored by the American Heart Association.
Common predictor of vascular disease
Diabetic retinopathy is the most common complication of diabetes mellitus, affecting up to 50% of people living with type 1 and type 2 diabetes. In addition, previous research suggests that macrovascular diabetes complications, including stroke, could share a common or synergistic pathway.
This small vessel damage in the eye also has been linked to an increased risk of adverse cardiac events, including heart failure, as previously reported by Medscape Medical News.
To find out more, Mr. Wong and colleagues analyzed 2,828 participants in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. They compared the stroke risk between 874 people with diabetic retinopathy and another 1,954 diabetics without this complication. The average age was 62 years and 62% were men.
Diabetic neuropathy at baseline was diagnosed using the Early Treatment Diabetic Retinopathy Study Severity Scale using seven-field stereoscopic fundus photographs.
A total of 117 participants experienced a stroke during a mean follow-up of 5.4 years.
The investigators found that diabetic retinopathy was more common among patients who had a stroke (41%) versus 31% of those without a stroke (P = .016). The link between diabetic retinopathy and stroke remained in an analysis adjusted for multiple factors, including baseline age, gender, race, total cholesterol, A1c, smoking, and more. Risk remained elevated, with a hazard ratio of 1.60 (95% confidence interval, 1.10-2.32; P = .015).
Regarding the potential for modifying this risk, the association was unaffected among participants randomly assigned to the ACCORD glucose intervention (P = .305), lipid intervention (P = .546), or blood pressure intervention (P = .422).
The study was a secondary analysis, so information on stroke type and location were unavailable.
The big picture
“Diabetic retinopathy is associated with an increased risk of stroke, which suggests that the microvascular pathology inherent to diabetic retinopathy has larger cardiovascular implications,” the researchers noted.
Despite these findings, the researchers suggest that patients with diabetic retinopathy receive aggressive medical management to try to reduce their stroke risk.
“It’s important for everyone with diabetes to maintain good blood glucose control, and those with established diabetic retinopathy should pay particular attention to meeting all the stroke prevention guidelines that are established by the American Stroke Association,” said Mr. Wong.
“Patients with established diabetic retinopathy should pay particular attention to meeting all stroke prevention guidelines established by the [American Heart Association],” he added.
Mr. Wong and colleagues would like to expand on these findings. Pending grant application and funding support, they propose conducting a prospective, observational trial in stroke patients with baseline diabetic retinopathy. One aim would be to identify the most common mechanisms leading to stroke in this population, “which would have important implications for prevention efforts,” he said.
Consistent Findings
“The results of the study showing that having diabetic retinopathy is also associated with an increase in stroke really isn’t surprising. There have been other studies, population-based studies, done in the past, that have found a similar relationship,” Larry B. Goldstein, MD, said in a video commentary on the findings.
“The results are actually quite consistent with several other studies that have evaluated the same relationship,” added Dr. Goldstein, who is chair of the department of neurology and codirector of the Kentucky Neuroscience Institute, University of Kentucky HealthCare, Lexington.
Mr. Wong and Dr. Goldstein have disclosed no relevant financial relationships. The NIH’s National Institute of Neurological Disorders and Stroke funded the study.
This article first appeared on Medscape.com.
LOS ANGELES – Evidence continues to mount that diabetic retinopathy predicts elevated risk for stroke.
In a new study with nearly 3,000 people, those with diabetic retinopathy were 60% more likely than others with diabetes to develop an incident stroke over time. Investigators also found that addressing glucose, lipids, and blood pressure levels did not mitigate this risk in this secondary analysis of the ACCORD Eye Study.
“We are not surprised with the finding that diabetic retinopathy increases the risk of stroke — as diabetic retinopathy is common microvascular disease that is an established risk factor for cardiovascular disease,” lead author Ka-Ho Wong, BS, MBA, said in an interview.
However, “we were surprised that none of the trial interventions mitigated this risk, in particular the intensive blood pressure reduction, because hypertension is the most important cause of microvascular disease,” he said. Mr. Wong is clinical research coordinator and lab manager of the de Havenon Lab at the University of Utah Health Hospitals and Clinics in Salt Lake City.
The study findings were released Feb. 12, 2020, in advance of formal presentation at the International Stroke Conference sponsored by the American Heart Association.
Common predictor of vascular disease
Diabetic retinopathy is the most common complication of diabetes mellitus, affecting up to 50% of people living with type 1 and type 2 diabetes. In addition, previous research suggests that macrovascular diabetes complications, including stroke, could share a common or synergistic pathway.
This small vessel damage in the eye also has been linked to an increased risk of adverse cardiac events, including heart failure, as previously reported by Medscape Medical News.
To find out more, Mr. Wong and colleagues analyzed 2,828 participants in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study. They compared the stroke risk between 874 people with diabetic retinopathy and another 1,954 diabetics without this complication. The average age was 62 years and 62% were men.
Diabetic neuropathy at baseline was diagnosed using the Early Treatment Diabetic Retinopathy Study Severity Scale using seven-field stereoscopic fundus photographs.
A total of 117 participants experienced a stroke during a mean follow-up of 5.4 years.
The investigators found that diabetic retinopathy was more common among patients who had a stroke (41%) versus 31% of those without a stroke (P = .016). The link between diabetic retinopathy and stroke remained in an analysis adjusted for multiple factors, including baseline age, gender, race, total cholesterol, A1c, smoking, and more. Risk remained elevated, with a hazard ratio of 1.60 (95% confidence interval, 1.10-2.32; P = .015).
Regarding the potential for modifying this risk, the association was unaffected among participants randomly assigned to the ACCORD glucose intervention (P = .305), lipid intervention (P = .546), or blood pressure intervention (P = .422).
The study was a secondary analysis, so information on stroke type and location were unavailable.
The big picture
“Diabetic retinopathy is associated with an increased risk of stroke, which suggests that the microvascular pathology inherent to diabetic retinopathy has larger cardiovascular implications,” the researchers noted.
Despite these findings, the researchers suggest that patients with diabetic retinopathy receive aggressive medical management to try to reduce their stroke risk.
“It’s important for everyone with diabetes to maintain good blood glucose control, and those with established diabetic retinopathy should pay particular attention to meeting all the stroke prevention guidelines that are established by the American Stroke Association,” said Mr. Wong.
“Patients with established diabetic retinopathy should pay particular attention to meeting all stroke prevention guidelines established by the [American Heart Association],” he added.
Mr. Wong and colleagues would like to expand on these findings. Pending grant application and funding support, they propose conducting a prospective, observational trial in stroke patients with baseline diabetic retinopathy. One aim would be to identify the most common mechanisms leading to stroke in this population, “which would have important implications for prevention efforts,” he said.
Consistent Findings
“The results of the study showing that having diabetic retinopathy is also associated with an increase in stroke really isn’t surprising. There have been other studies, population-based studies, done in the past, that have found a similar relationship,” Larry B. Goldstein, MD, said in a video commentary on the findings.
“The results are actually quite consistent with several other studies that have evaluated the same relationship,” added Dr. Goldstein, who is chair of the department of neurology and codirector of the Kentucky Neuroscience Institute, University of Kentucky HealthCare, Lexington.
Mr. Wong and Dr. Goldstein have disclosed no relevant financial relationships. The NIH’s National Institute of Neurological Disorders and Stroke funded the study.
This article first appeared on Medscape.com.
REPORTING FROM ISC 2020
Carotid endarterectomy surpasses stenting in elderly, asymptomatic patients
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
The role for carotid intervention in asymptomatic patients with severe carotid stenosis, usually defined as a stenosis that obstructs at least 70% of the carotid lumen, is controversial right now because intensive medical management has not been compared with invasive treatments, such as carotid endarterectomy and carotid stenting, for well over a decade. New drugs and new regimens have become treatment options for patients with advanced atherosclerotic carotid artery disease, and this has returned us to a state of equipoise for medical versus interventional management. That’s the premise behind CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial), which is comparing medical treatment against endarterectomy and against carotid stenting in a randomized study. The results may be available in 2021.
The new findings are very important for helping patients and their families make informed decisions. CAS is often perceived as the safer option for older patients because it is less traumatic and invasive than CEA. The data that Dr. Voeks reported show once again that this intuitive impression about CAS in the elderly is belied by the evidence. But the findings also require cautious interpretation because they came from a post hoc, subgroup analysis.
Mai N. Nguyen-Huynh, MD , is a vascular neurologist with Kaiser Permanente Northern California in Oakland. She had no relevant disclosures. She made these comments in an interview.
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
LOS ANGELES – Carotid artery stenting in older, asymptomatic patients with severe carotid artery stenosis is, in general, as bad an idea as it has already proven to be in symptomatic patients, with a multifold increase in adverse short- and mid-term outcomes, compared with similar older, asymptomatic patients who underwent endarterectomy, according to a combined-study analysis with more than 2,500 patients.
The risk for poor outcomes in patients with severe but asymptomatic carotid artery disease who underwent carotid artery stenting (CAS), compared with patients who instead underwent carotid endarterectomy (CEA) “abruptly increased around age 75,” in an analysis that combined data from the two major, published, randomized trials that compared these two interventions in this patient population, Jenifer H. Voeks, PhD said at the International Stroke Conference sponsored by the American Heart Association.
These results “largely mirror” the findings from a similar combined analysis of data from four major, randomized trials that compared CEA and CAS in patients with symptomatic carotid disease, she noted (Lancet. 2016 Mar 26;387[10025]:1305-11). The new findings in an expanded population of asymptomatic patients derived from two separate studies showed that, in patients aged 70 years or less, “CAS appears to be a reasonable alternative to CEA, but above age 70, and certainly above age 75, age-related risk factors such as cerebrovascular anatomy and underlying cerebral pathology should be carefully considered before selecting patients for CAS,” said Dr. Voeks, a neurology researcher at the Medical University of South Carolina, Charleston. Many experts also believe that, for asymptomatic patients, intensive medical management may have returned as an alternative to either of these invasive approaches for treating severe carotid stenosis and has achieved a level of equipoise that led to the launch of CREST 2 (Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis Trial). CREST 2 is comparing CEA and CAS with medical management, and is scheduled to report results in 2021.
The data for this analysis in asymptomatic patients came from the first CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial; N Engl J Med. 2010 Jul 1;363[1]:11-23), which included 1,181 asymptomatic patients (nearly half the total enrollment, with symptomatic patients making up the balance) and had no age ceiling, as well as all 1,453 patients from the ACT 1 trial, which enrolled exclusively asymptomatic patients and limited enrollment to patients aged 79 years or less (N Engl J Med. 2016 Mar 17;374[11]: 1011-20). Because the maximum age of patients in ACT 1 was 79 years, for this analysis Dr. Voeks and associates only included the 1,091 asymptomatic CREST patients who also were within the same age ceiling. The resulting cohort of 2,544 included 1,637 patients who underwent CAS and 907 who underwent CEA (because of a 3:1 randomization ratio in ACT 1), creating the largest data set to compare CAS and CEA by age in asymptomatic patients, Dr. Voeks noted. When subdivided by age, 30% of the cohort was younger that 65 years, 54% were 65-74, and 16% were 75-79.
The primary outcome the researchers used for their analysis was the combined incidence of periprocedural stroke, MI, or death, plus the incidence of ipsilateral stroke during 4 years of follow-up post procedure. Among patients who underwent CAS, this outcome occurred in roughly 9% of patients aged 75-79 years and in about 3% of those younger than 65 years, a hazard ratio of 2.9 that was statistically significant. In contrast, the incidence of the primary outcome among patients aged 65-74 years was just 30% higher, compared with patients aged less than 65 years, a difference that was not statistically significant.
Patients who underwent CEA showed no similar relationship between age and outcome. The incidence of the primary outcome among the CEA patients was roughly the same, about 3.5%, regardless of their age.
A second analysis that considered age as a continuous variable showed a sharply spiked increase in the risk for CAS patients, compared with CEA patients once they reached about age 73-75 years. Until about age 72, the rate of the primary outcome was nearly the same regardless of whether patients underwent CAS or CEA, but the risk for adverse outcomes rose “steeply” starting at about age 75 so that by age 79 the rate of the primary outcome approached 300% higher among the CAS patients compared with CEA patients, Dr. Voeks said.
She cautioned that the analysis included just 115 total primary-outcome events, which makes the incidence rate estimates somewhat imprecise, and that the data reflect outcomes in patients who were treated more than a decade ago, but these data remain the only reported results from large randomized trials that compared CAS and CEA in asymptomatic patients.
Dr. Voeks reported no disclosures.
SOURCE: Voeks JH al. Stroke. 2020 Feb 12;51[suppl 1], Abstract 70.
REPORTING FROM ISC 2020
‘A glimmer of hope’ for stroke/mortality benefit with AFib catheter ablation
SNOWMASS, COLO. – stroke, major bleeding, or cardiac arrest, compared with rhythm and/or rate control drugs in a propensity score–weighted, retrospective, observational study.
Findings of the investigation, which included more than 183,000 real-world patients in routine clinical practice, were reported by Peter S. Noseworthy, MD, during the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The results breathe new life into the controversy created by the previously reported CABANA trial (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation), a 10-country study in which 2,204 patients with atrial fibrillation (AFib) were randomized to catheter ablation or antiarrhythmic and/or rhythm control medications and followed for a mean of about 4 years. CABANA yielded a negative result (JAMA. 2019 Apr 2;321[13]:1261-74), with the prespecified intent-to-treat analysis indicating no significant between-group difference in the primary composite endpoint – the very same one that was positive in the large observational study.
However, CABANA was marred by major problems arising from protocol deviations: Nearly 28% of patients assigned to medical therapy crossed over to catheter ablation, typically because their antiarrhythmic drugs failed, and 10% of patients randomized to catheter ablation never got it. This muddies the waters when trying to identify a true stroke/mortality benefit for catheter ablation, if indeed any such benefit was actually present.
Here’s where the controversy arose: While CABANA must be called a negative trial based upon the disappointing results of the intent-to-treat analysis, a prespecified post hoc analysis of patients as actually treated showed a statistically significant 27% relative risk reduction for the primary composite endpoint in the catheter ablation group. That’s strikingly similar to the 30% relative risk reduction for catheter ablation seen in the huge observational study, where the CABANA-type primary outcome occurred in 22.5% of the medically managed patients and 16.8% of those who underwent catheter ablation, noted Dr. Noseworthy, professor of medicine and director of heart rhythm and physiology at the Mayo Clinic in Rochester, Minn.
He ought to know: He was both an investigator in CABANA and first author of the published observational study (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
In the observational study, Dr. Noseworthy and coinvestigators utilized a huge U.S. administrative health claims database in order to identify a nationally representative group of 183,760 AFib patients, 12,032 of whom were treated with catheter ablation and the rest with antiarrhythmic and/or rhythm control drugs during the same years the CABANA trial was enrolling patients. The two groups were balanced using propensity score weighting to adjust for baseline differences in 90 variables.
The investigators sought to learn if the CABANA study population was representative of real-world AFib patients, and whether the observational experience could help resolve the CABANA controversy. It turned out that most AFib patients seen in daily clinical practice were CABANA like; that is, 74% of them would have been eligible for the clinical trial because they were symptomatic, over age 65, or younger than 65 with at least one CHADS2 stroke risk factor. About 22% of the large real-world sample would have been excluded from CABANA because they’d failed on amiodarone and other antiarrhythmic agents or had previously undergone ablation. About 4% of patients failed to meet the CABANA inclusion criteria.
The risk reduction for the composite endpoint associated with catheter ablation in the large retrospective study was greatest in the CABANA-like patients, at 30%. It was less robust but still statistically significant at 15% in patients who met at least one of the exclusion criteria for the trial.
The sheer size of this study provides greater statistical power than in CABANA. Of course, a nonrandomized, propensity score–based comparison such as this is always susceptible to confounding, even after adjustment for 90 variables. But the observational study does offer “a glimmer of hope” that catheter ablation, done in the right patients, might confer a stroke risk reduction and mortality benefit, he said.
The 33% relative risk reduction in the small group of real-world patients who failed to meet the CABANA inclusion criteria, while numerically impressive, wasn’t close to statistical significance, probably because event rates in that population were so low.
“Even if you could reduce stroke risk with ablation in that low-risk group, it would be a very inefficient way to reduce the population burden of stroke,” Dr. Noseworthy observed.
Putting together the results of CABANA and the large observational study to sum up his view of where catheter ablation for AF[ib] stands today, Dr. Noseworthy commented, “Ablation is reasonable for symptom control in many patients, basically anyone who is either breaking through on drugs or doesn’t want to take the drugs and is highly symptomatic. And there may be a small stroke and/or mortality benefit for people who are in the sweet spot – and those are people who look a lot like the patients enrolled in CABANA.”
Patients who met the exclusion criteria for CABANA are too advanced in their AFib to be likely to derive a stroke or mortality benefit from catheter ablation. “It’s very hard to move the needle in these patients with either a drug or catheter ablation approach. I wouldn’t try to reduce the risk of stroke here with an expensive and invasive procedure,” the electrophysiologist concluded.
He reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – stroke, major bleeding, or cardiac arrest, compared with rhythm and/or rate control drugs in a propensity score–weighted, retrospective, observational study.
Findings of the investigation, which included more than 183,000 real-world patients in routine clinical practice, were reported by Peter S. Noseworthy, MD, during the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The results breathe new life into the controversy created by the previously reported CABANA trial (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation), a 10-country study in which 2,204 patients with atrial fibrillation (AFib) were randomized to catheter ablation or antiarrhythmic and/or rhythm control medications and followed for a mean of about 4 years. CABANA yielded a negative result (JAMA. 2019 Apr 2;321[13]:1261-74), with the prespecified intent-to-treat analysis indicating no significant between-group difference in the primary composite endpoint – the very same one that was positive in the large observational study.
However, CABANA was marred by major problems arising from protocol deviations: Nearly 28% of patients assigned to medical therapy crossed over to catheter ablation, typically because their antiarrhythmic drugs failed, and 10% of patients randomized to catheter ablation never got it. This muddies the waters when trying to identify a true stroke/mortality benefit for catheter ablation, if indeed any such benefit was actually present.
Here’s where the controversy arose: While CABANA must be called a negative trial based upon the disappointing results of the intent-to-treat analysis, a prespecified post hoc analysis of patients as actually treated showed a statistically significant 27% relative risk reduction for the primary composite endpoint in the catheter ablation group. That’s strikingly similar to the 30% relative risk reduction for catheter ablation seen in the huge observational study, where the CABANA-type primary outcome occurred in 22.5% of the medically managed patients and 16.8% of those who underwent catheter ablation, noted Dr. Noseworthy, professor of medicine and director of heart rhythm and physiology at the Mayo Clinic in Rochester, Minn.
He ought to know: He was both an investigator in CABANA and first author of the published observational study (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
In the observational study, Dr. Noseworthy and coinvestigators utilized a huge U.S. administrative health claims database in order to identify a nationally representative group of 183,760 AFib patients, 12,032 of whom were treated with catheter ablation and the rest with antiarrhythmic and/or rhythm control drugs during the same years the CABANA trial was enrolling patients. The two groups were balanced using propensity score weighting to adjust for baseline differences in 90 variables.
The investigators sought to learn if the CABANA study population was representative of real-world AFib patients, and whether the observational experience could help resolve the CABANA controversy. It turned out that most AFib patients seen in daily clinical practice were CABANA like; that is, 74% of them would have been eligible for the clinical trial because they were symptomatic, over age 65, or younger than 65 with at least one CHADS2 stroke risk factor. About 22% of the large real-world sample would have been excluded from CABANA because they’d failed on amiodarone and other antiarrhythmic agents or had previously undergone ablation. About 4% of patients failed to meet the CABANA inclusion criteria.
The risk reduction for the composite endpoint associated with catheter ablation in the large retrospective study was greatest in the CABANA-like patients, at 30%. It was less robust but still statistically significant at 15% in patients who met at least one of the exclusion criteria for the trial.
The sheer size of this study provides greater statistical power than in CABANA. Of course, a nonrandomized, propensity score–based comparison such as this is always susceptible to confounding, even after adjustment for 90 variables. But the observational study does offer “a glimmer of hope” that catheter ablation, done in the right patients, might confer a stroke risk reduction and mortality benefit, he said.
The 33% relative risk reduction in the small group of real-world patients who failed to meet the CABANA inclusion criteria, while numerically impressive, wasn’t close to statistical significance, probably because event rates in that population were so low.
“Even if you could reduce stroke risk with ablation in that low-risk group, it would be a very inefficient way to reduce the population burden of stroke,” Dr. Noseworthy observed.
Putting together the results of CABANA and the large observational study to sum up his view of where catheter ablation for AF[ib] stands today, Dr. Noseworthy commented, “Ablation is reasonable for symptom control in many patients, basically anyone who is either breaking through on drugs or doesn’t want to take the drugs and is highly symptomatic. And there may be a small stroke and/or mortality benefit for people who are in the sweet spot – and those are people who look a lot like the patients enrolled in CABANA.”
Patients who met the exclusion criteria for CABANA are too advanced in their AFib to be likely to derive a stroke or mortality benefit from catheter ablation. “It’s very hard to move the needle in these patients with either a drug or catheter ablation approach. I wouldn’t try to reduce the risk of stroke here with an expensive and invasive procedure,” the electrophysiologist concluded.
He reported having no financial conflicts regarding his presentation.
SNOWMASS, COLO. – stroke, major bleeding, or cardiac arrest, compared with rhythm and/or rate control drugs in a propensity score–weighted, retrospective, observational study.
Findings of the investigation, which included more than 183,000 real-world patients in routine clinical practice, were reported by Peter S. Noseworthy, MD, during the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
The results breathe new life into the controversy created by the previously reported CABANA trial (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation), a 10-country study in which 2,204 patients with atrial fibrillation (AFib) were randomized to catheter ablation or antiarrhythmic and/or rhythm control medications and followed for a mean of about 4 years. CABANA yielded a negative result (JAMA. 2019 Apr 2;321[13]:1261-74), with the prespecified intent-to-treat analysis indicating no significant between-group difference in the primary composite endpoint – the very same one that was positive in the large observational study.
However, CABANA was marred by major problems arising from protocol deviations: Nearly 28% of patients assigned to medical therapy crossed over to catheter ablation, typically because their antiarrhythmic drugs failed, and 10% of patients randomized to catheter ablation never got it. This muddies the waters when trying to identify a true stroke/mortality benefit for catheter ablation, if indeed any such benefit was actually present.
Here’s where the controversy arose: While CABANA must be called a negative trial based upon the disappointing results of the intent-to-treat analysis, a prespecified post hoc analysis of patients as actually treated showed a statistically significant 27% relative risk reduction for the primary composite endpoint in the catheter ablation group. That’s strikingly similar to the 30% relative risk reduction for catheter ablation seen in the huge observational study, where the CABANA-type primary outcome occurred in 22.5% of the medically managed patients and 16.8% of those who underwent catheter ablation, noted Dr. Noseworthy, professor of medicine and director of heart rhythm and physiology at the Mayo Clinic in Rochester, Minn.
He ought to know: He was both an investigator in CABANA and first author of the published observational study (Eur Heart J. 2019 Apr 21;40[16]:1257-64).
In the observational study, Dr. Noseworthy and coinvestigators utilized a huge U.S. administrative health claims database in order to identify a nationally representative group of 183,760 AFib patients, 12,032 of whom were treated with catheter ablation and the rest with antiarrhythmic and/or rhythm control drugs during the same years the CABANA trial was enrolling patients. The two groups were balanced using propensity score weighting to adjust for baseline differences in 90 variables.
The investigators sought to learn if the CABANA study population was representative of real-world AFib patients, and whether the observational experience could help resolve the CABANA controversy. It turned out that most AFib patients seen in daily clinical practice were CABANA like; that is, 74% of them would have been eligible for the clinical trial because they were symptomatic, over age 65, or younger than 65 with at least one CHADS2 stroke risk factor. About 22% of the large real-world sample would have been excluded from CABANA because they’d failed on amiodarone and other antiarrhythmic agents or had previously undergone ablation. About 4% of patients failed to meet the CABANA inclusion criteria.
The risk reduction for the composite endpoint associated with catheter ablation in the large retrospective study was greatest in the CABANA-like patients, at 30%. It was less robust but still statistically significant at 15% in patients who met at least one of the exclusion criteria for the trial.
The sheer size of this study provides greater statistical power than in CABANA. Of course, a nonrandomized, propensity score–based comparison such as this is always susceptible to confounding, even after adjustment for 90 variables. But the observational study does offer “a glimmer of hope” that catheter ablation, done in the right patients, might confer a stroke risk reduction and mortality benefit, he said.
The 33% relative risk reduction in the small group of real-world patients who failed to meet the CABANA inclusion criteria, while numerically impressive, wasn’t close to statistical significance, probably because event rates in that population were so low.
“Even if you could reduce stroke risk with ablation in that low-risk group, it would be a very inefficient way to reduce the population burden of stroke,” Dr. Noseworthy observed.
Putting together the results of CABANA and the large observational study to sum up his view of where catheter ablation for AF[ib] stands today, Dr. Noseworthy commented, “Ablation is reasonable for symptom control in many patients, basically anyone who is either breaking through on drugs or doesn’t want to take the drugs and is highly symptomatic. And there may be a small stroke and/or mortality benefit for people who are in the sweet spot – and those are people who look a lot like the patients enrolled in CABANA.”
Patients who met the exclusion criteria for CABANA are too advanced in their AFib to be likely to derive a stroke or mortality benefit from catheter ablation. “It’s very hard to move the needle in these patients with either a drug or catheter ablation approach. I wouldn’t try to reduce the risk of stroke here with an expensive and invasive procedure,” the electrophysiologist concluded.
He reported having no financial conflicts regarding his presentation.
REPORTING FROM ACC SNOWMASS 2020
Thrombectomy access lags for U.S. stroke patients
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
In 2017, roughly 3 years after evidence from several studies made endovascular thrombectomy first-line treatment for selected acute ischemic stroke patients, the treatment was available at barely more than one-third of all U.S. stroke centers, available within 30-minute access to just over 30% of Americans, and available within 15-minute access to one-fifth of U.S. residents, based on information in a comprehensive U.S. database.
These numbers showed that “current direct EVT [endovascular thrombectomy] access in the United States is suboptimal under predominate EMS routing protocols,” Amrou Sarraj, MD, and his associates wrote in an article published online in Stroke on Feb. 12. “Only in eight states did the coverage exceed 25% of the population, and nine states had coverage for less than 10% of the population. These results reflect limited access to an effective treatment modality that would improve clinical outcomes in patients with large strokes and prevent potentially devastating disability,” wrote Dr. Sarraj, chief of the general neurology service at Memorial-Hermann Hospital in Houston and coauthors.
Their analysis of data collected in 2017 by the Medicare Provider Analysis and Review (MEDPAR) database, maintained by the Centers for Medicare & Medicaid Services, identified two apparently effective ways to improve EVT access for acute ischemic stroke patients: First, systematically divert patients to a nearby center that offers EVT even when it means bypassing a closer stroke center that does not perform EVT when the added travel time is less than 15 minutes. Second, convert selected stroke centers that currently do not perform EVT into centers that do. Between these two approaches, the strategy of having ambulances bypass stroke centers that do not perform EVT and continuing to ones that do generally has the greater potential to boost access, the authors found. They based their analysis exclusively on their calculations of expected consequences rather than actual experience.
The calculations showed that bypassing non-EVT centers when the added bypass time computed to less than 15 minutes linked with an anticipated overall U.S. gain in access of about 17%, or 52 million people, extending the ability of acute ischemic stroke patients able to quickly reach an EVT center to about 37% of the American public. The second approach to boost access, converting the top 10% of stroke centers based on case volume that currently do not provide EVT to centers that do offer it, would result in expanded access for about 23 million additional Americans, raising the total with access to about 27% of the public, the new report said.
As part of this analysis, the MEDPAR data identified 1,941 U.S. centers providing stroke services during 2017, of which 713 (37%) had performed at least one EVT procedure. By comparison, 2015 MEDPAR data showed 577 U.S. stroke centers performing EVT, indicating that during the 2-3 years following several reports in early 2015 on the net benefits of EVT for acute ischemic stroke patients, the number of U.S. stroke centers offering this treatment had grown by a relative 24%. Based on the locations of the stroke centers that made EVT available in 2017, Dr. Sarraj and coauthors calculated that the 713 EVT-capable stroke centers provided emergency access within a 15-minute ground-ambulance trip for 61 million Americans (20% of the U.S. population), and within a 30-minute ground-transport trip to 95 million residents (31%).
Boosting these numbers by implementing a systematic bypass of stroke patients past non-EVT stroke centers to nearby centers that are EVT capable “has the benefit of ease of implementation and requires less time and resources,” the authors said. However, they also noted the heterogeneity of circumstances based on variables like population density and stroke center distribution, which means that in some locations the most effective way to boost access would be by increasing the number of stroke centers that provide EVT.
In 2018, Dr. Sarraj and associates reported results from a similar analysis of MEDPAR data that used 30-minute and 60-minute ground-transport times as the criteria for their calculations.
The study received no commercial funding. Dr. Sarraj reported receiving research funding from Stryker Neurovascular outside of this work. One coauthor reported serving in roles for the University of Texas Health System for which the institution has been funded via various industry and government grants, and another coauthor reported receiving research funding from the Patient-Centered Outcomes Research Institute, the National Institutes of Health, Genentech, and CSL Behring, as well as consulting fees from Frazer Ltd.
SOURCE: Sarraj A et al. Stroke. 2020 Feb 12. doi: 10.1161/STROKEAHA.120.028850.
FROM STROKE
Mobile stroke unit had clinical impact on EVT
In its first year of operation, a mobile stroke unit in Melbourne demonstrated substantial savings in time to commencement of both thrombolysis and endovascular thrombectomy (EVT), results from a prospective study showed.
“While previously published data from MSU [mobile stroke unit] services in Europe and North America show substantial reductions in time to thrombolysis of approximately 30-45 minutes, little is known about the clinical impact on EVT,” first author Henry Zhao, MBBS, and colleagues wrote in a study published in Stroke.
Launched in November 2017, the Melbourne MSU is based at a large comprehensive stroke center and operates with a 20-km radius, servicing about 1.7 million people within the city of Melbourne. It is staffed with an onboard neurologist or senior stroke fellow who provides primary assessment and treatment decisions, a stroke advanced practice nurse who provides clinical support and treatment administration, a clinician who provides CT imaging, and advanced life support and mobile intensive care paramedics who provide transport logistics and paramedicine support. For the current analysis, MSU patients who received reperfusion therapy were compared with control patients presenting to metropolitan Melbourne stroke units via standard ambulance within MSU operating hours. The primary outcome was median time difference in first ambulance dispatch to treatment, which the researchers used quantile regression analysis to determine. Time savings were subsequently converted to disability-adjusted life years (DALY) avoiding using published estimates.
Dr. Zhao of the Melbourne Brain Centre and department of neurology at Royal Melbourne Hospital and his colleagues reported that, in its first year of operation, the Melbourne MSU administered prehospital thrombolysis to 100 patients with a mean age of nearly 74 years. More than half of the patients (62%) were male. Compared with controls, the median time savings per MSU patient was 26 minutes for dispatch to hospital arrival and 15 minutes for hospital arrival to thrombolysis (P less than .0010 for both associations). The calculated overall time saving from dispatch to thrombolysis was 42.5 minutes.
Over the same time period, 41 MSU patients with a mean age of 76 years received EVT dispatch-to-treatment time saving of 51 minutes (P less than 0.001). This included a median time saving of 17 minutes for EVT hospital arrival to arterial puncture for MSU patients (P = .001). Overall estimated median DALYs saved through earlier provision of reperfusion therapies were 20.9 for thrombolysis and 24.6 for EVT.
“The benefit in EVT patients was primarily driven by prehospital MSU diagnosis of large vessel occlusion, which enabled bypass of a local non-EVT center directly to a comprehensive stroke center in almost 50% of patients with large vessel occlusion,” the researchers wrote. “Even when patients were located close to an EVT center, MSU pre-notification and facilitated workflows achieved a reduction in hospital arrival to arterial puncture by one-third. Furthermore, the time saving was seen despite the majority of EVT patients receiving repeat imaging in hospital to visualize the extracranial circulation.”
The study is scheduled to be presented at the International Stroke Conference on Feb. 20.
The Melbourne MSU received funding from the Australian Commonwealth Government, Victorian State Government, Royal Melbourne Hospital Neurosciences Foundation, Stroke Foundation, the Florey Institute of Neurosciences and Mental Health, the University of Melbourne, Boehringer Ingelheim, and private donation. Dr. Zhao disclosed that he has received grants from the Australian Commonwealth Government and the University of Melbourne and personal fees from Boehringer Ingelheim.
SOURCE: Zhao H et al. Stroke. 2020 Feb 12. doi: 10.1161/strokeaha.119.027843.
In its first year of operation, a mobile stroke unit in Melbourne demonstrated substantial savings in time to commencement of both thrombolysis and endovascular thrombectomy (EVT), results from a prospective study showed.
“While previously published data from MSU [mobile stroke unit] services in Europe and North America show substantial reductions in time to thrombolysis of approximately 30-45 minutes, little is known about the clinical impact on EVT,” first author Henry Zhao, MBBS, and colleagues wrote in a study published in Stroke.
Launched in November 2017, the Melbourne MSU is based at a large comprehensive stroke center and operates with a 20-km radius, servicing about 1.7 million people within the city of Melbourne. It is staffed with an onboard neurologist or senior stroke fellow who provides primary assessment and treatment decisions, a stroke advanced practice nurse who provides clinical support and treatment administration, a clinician who provides CT imaging, and advanced life support and mobile intensive care paramedics who provide transport logistics and paramedicine support. For the current analysis, MSU patients who received reperfusion therapy were compared with control patients presenting to metropolitan Melbourne stroke units via standard ambulance within MSU operating hours. The primary outcome was median time difference in first ambulance dispatch to treatment, which the researchers used quantile regression analysis to determine. Time savings were subsequently converted to disability-adjusted life years (DALY) avoiding using published estimates.
Dr. Zhao of the Melbourne Brain Centre and department of neurology at Royal Melbourne Hospital and his colleagues reported that, in its first year of operation, the Melbourne MSU administered prehospital thrombolysis to 100 patients with a mean age of nearly 74 years. More than half of the patients (62%) were male. Compared with controls, the median time savings per MSU patient was 26 minutes for dispatch to hospital arrival and 15 minutes for hospital arrival to thrombolysis (P less than .0010 for both associations). The calculated overall time saving from dispatch to thrombolysis was 42.5 minutes.
Over the same time period, 41 MSU patients with a mean age of 76 years received EVT dispatch-to-treatment time saving of 51 minutes (P less than 0.001). This included a median time saving of 17 minutes for EVT hospital arrival to arterial puncture for MSU patients (P = .001). Overall estimated median DALYs saved through earlier provision of reperfusion therapies were 20.9 for thrombolysis and 24.6 for EVT.
“The benefit in EVT patients was primarily driven by prehospital MSU diagnosis of large vessel occlusion, which enabled bypass of a local non-EVT center directly to a comprehensive stroke center in almost 50% of patients with large vessel occlusion,” the researchers wrote. “Even when patients were located close to an EVT center, MSU pre-notification and facilitated workflows achieved a reduction in hospital arrival to arterial puncture by one-third. Furthermore, the time saving was seen despite the majority of EVT patients receiving repeat imaging in hospital to visualize the extracranial circulation.”
The study is scheduled to be presented at the International Stroke Conference on Feb. 20.
The Melbourne MSU received funding from the Australian Commonwealth Government, Victorian State Government, Royal Melbourne Hospital Neurosciences Foundation, Stroke Foundation, the Florey Institute of Neurosciences and Mental Health, the University of Melbourne, Boehringer Ingelheim, and private donation. Dr. Zhao disclosed that he has received grants from the Australian Commonwealth Government and the University of Melbourne and personal fees from Boehringer Ingelheim.
SOURCE: Zhao H et al. Stroke. 2020 Feb 12. doi: 10.1161/strokeaha.119.027843.
In its first year of operation, a mobile stroke unit in Melbourne demonstrated substantial savings in time to commencement of both thrombolysis and endovascular thrombectomy (EVT), results from a prospective study showed.
“While previously published data from MSU [mobile stroke unit] services in Europe and North America show substantial reductions in time to thrombolysis of approximately 30-45 minutes, little is known about the clinical impact on EVT,” first author Henry Zhao, MBBS, and colleagues wrote in a study published in Stroke.
Launched in November 2017, the Melbourne MSU is based at a large comprehensive stroke center and operates with a 20-km radius, servicing about 1.7 million people within the city of Melbourne. It is staffed with an onboard neurologist or senior stroke fellow who provides primary assessment and treatment decisions, a stroke advanced practice nurse who provides clinical support and treatment administration, a clinician who provides CT imaging, and advanced life support and mobile intensive care paramedics who provide transport logistics and paramedicine support. For the current analysis, MSU patients who received reperfusion therapy were compared with control patients presenting to metropolitan Melbourne stroke units via standard ambulance within MSU operating hours. The primary outcome was median time difference in first ambulance dispatch to treatment, which the researchers used quantile regression analysis to determine. Time savings were subsequently converted to disability-adjusted life years (DALY) avoiding using published estimates.
Dr. Zhao of the Melbourne Brain Centre and department of neurology at Royal Melbourne Hospital and his colleagues reported that, in its first year of operation, the Melbourne MSU administered prehospital thrombolysis to 100 patients with a mean age of nearly 74 years. More than half of the patients (62%) were male. Compared with controls, the median time savings per MSU patient was 26 minutes for dispatch to hospital arrival and 15 minutes for hospital arrival to thrombolysis (P less than .0010 for both associations). The calculated overall time saving from dispatch to thrombolysis was 42.5 minutes.
Over the same time period, 41 MSU patients with a mean age of 76 years received EVT dispatch-to-treatment time saving of 51 minutes (P less than 0.001). This included a median time saving of 17 minutes for EVT hospital arrival to arterial puncture for MSU patients (P = .001). Overall estimated median DALYs saved through earlier provision of reperfusion therapies were 20.9 for thrombolysis and 24.6 for EVT.
“The benefit in EVT patients was primarily driven by prehospital MSU diagnosis of large vessel occlusion, which enabled bypass of a local non-EVT center directly to a comprehensive stroke center in almost 50% of patients with large vessel occlusion,” the researchers wrote. “Even when patients were located close to an EVT center, MSU pre-notification and facilitated workflows achieved a reduction in hospital arrival to arterial puncture by one-third. Furthermore, the time saving was seen despite the majority of EVT patients receiving repeat imaging in hospital to visualize the extracranial circulation.”
The study is scheduled to be presented at the International Stroke Conference on Feb. 20.
The Melbourne MSU received funding from the Australian Commonwealth Government, Victorian State Government, Royal Melbourne Hospital Neurosciences Foundation, Stroke Foundation, the Florey Institute of Neurosciences and Mental Health, the University of Melbourne, Boehringer Ingelheim, and private donation. Dr. Zhao disclosed that he has received grants from the Australian Commonwealth Government and the University of Melbourne and personal fees from Boehringer Ingelheim.
SOURCE: Zhao H et al. Stroke. 2020 Feb 12. doi: 10.1161/strokeaha.119.027843.
FROM STROKE
Key clinical point: A mobile stroke unit (MSU) substantially reduced time to reperfusion therapies.
Major finding: Compared with controls, the median time savings per MSU patient was 26 minutes for dispatch to hospital arrival and 15 minutes for hospital arrival to thrombolysis (P less than .0010 for both associations).
Study details: A prospective study of 100 stroke patients.
Disclosures: The Melbourne MSU received funding from the Australian Commonwealth Government, Victorian State Government, Royal Melbourne Hospital Neurosciences Foundation, Stroke Foundation, the Florey Institute of Neurosciences and Mental Health, the University of Melbourne, Boehringer Ingelheim, and private donation. Dr. Zhao disclosed that he has received grants from the Australian Commonwealth Government and the University of Melbourne and personal fees from Boehringer Ingelheim.
Source: Zhao H et al. Stroke. 2020 Feb 12. doi: 10.1161/strokeaha.119.027843.
BP levels during endovascular stroke therapy affect neurologic outcomes
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
FROM JAMA NEUROLOGY
Vigilance safely keeps AFib patients off anticoagulants post ablation
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
REPORTING FROM THE AF SYMPOSIUM 2020
Race, ethnicity may influence outcomes after supratentorial intracerebral hemorrhage
researchers reported Jan. 22 in Neurology.
“There has been considerable research on stroke in older people, but there is still much to be learned about stroke in younger people and how it affects people of different races and ethnicities,” study author Daniel Woo, MD, professor of neurology at the University of Cincinnati, said in a news release. “Our study found that, even when you account for factors that affect outcomes, such as how big the stroke is, race and ethnicity were still independent predictors of how well people would recover.”
A subset of ERICH participants
To examine predictors of functional outcome in young patients with ICH, researchers analyzed data from a subset of patients in the Ethnic/Racial Variations in Intracerebral Hemorrhage (ERICH) study. ERICH enrolled patients with nontraumatic ICHs at 42 sites in the United States. It included 1,000 non-Hispanic black patients, 1,000 non-Hispanic white patients, and 1,000 Hispanic patients. Participants self-reported race and ethnicity.
Lead author Laura C. Miyares from Yale School of Medicine in New Haven, Conn., and colleagues analyzed data from 418 patients in ERICH who were aged 18-49 years and had supratentorial ICH. The cohort had an average age of 43 years, and 69% were male. In this subset, 41% were black, 12% were white, and 47% were Hispanic.
The primary outcome was modified Rankin Scale (mRS) score 3 months after the ICH, and the investigators defined a poor outcome as a score of 4 or greater. At 3 months, 35% had a poor functional outcome. Approximately 18% were unable to walk without assistance and attend to their bodily needs (mRS 4); 8% were bedridden, incontinent, and required nursing care (mRS 5); and 10% had died (mRS 6).
The percentage of patients with a poor functional outcome was 52% among white patients, 35% among black patients, and 31% among Hispanic patients. In a univariable analysis, black patients had a 51% reduction in odds of a poor outcome, compared with white patients, and Hispanic patients had a 59% reduction.
“The association between race/ethnicity and 3-month post-ICH functional outcome remained significant after adjusting for age, sex, premorbid disability, ICH location, ICH volume, [intraventricular hemorrhage] extension, systolic blood pressure, and [Glasgow Coma Scale] score on admission,” the researchers said. “In multivariable analysis, using white patients as the reference category, black patients had a 58% reduction in the odds of poor functional outcome at 3 months and Hispanic patients had a 66% reduction in the same odds.”
Their analysis identified the importance of other risk factors as well. “The volume of the hematoma, the most powerful predictor of outcome in older patients with ICH, was also found to be the most significant predictor of poor outcome in young patients,” they said.
Vascular risks and oral anticoagulants
About 80% of the young adults with ICH had a history of diagnosed hypertension. In nearly half, the condition was untreated. “After hypertension, the most common stroke risk factors in the young were diabetes, high cholesterol, smoking, and alcohol abuse,” the authors said. “In combination, these results indicate that vascular risk factors, especially untreated, could explain a large proportion of cases of ICH in the young.”
“Our results also point to treatment with oral anticoagulants before hospitalization as a potential mediator of the effect of race/ethnicity on short-term functional outcomes,” they said. About 8% of the white patients used oral anticoagulants, compared with 4% of the black patients and 1% of the Hispanic patients. Oral anticoagulant treatment “is a known risk factor for ICH and an established predictor of poor outcome in this condition. However, because only a small proportion of enrolled young patients with ICH were on [oral anticoagulants] prior to presentation, these results should be further validated by future studies.”
The study’s limitations include the broad categorization of racial and ethnic groups, the fact that younger patients with supratentorial ICH were more likely to be black or Hispanic and less likely to be white, and the exclusion of a significant proportion of cases of young white patients with smaller ICH volumes because of missing data, the researchers noted. Although the cohort was large, researchers may need to study more patients to capture differences among racial and ethnic groups, the investigators said.
The association between race/ethnicity and functional outcome could relate to “distinct pathophysiologies of the initial bleed or unique mechanisms of secondary injury,” the researchers suggested. “Future studies are necessary to probe the potential biological and social mediators of these findings to elucidate the role of race/ethnicity in ICH severity and functional recovery, and to develop improved prognostication for a racially varied population.”
ERICH is supported by the National Institute of Neurological Disorders and Stroke. Authors disclosed grants from the government, professional societies, and a university.
SOURCE: Miyares LC et al. Neurology. 2020 Jan 22. doi: 10.1212/WNL.0000000000008930.
researchers reported Jan. 22 in Neurology.
“There has been considerable research on stroke in older people, but there is still much to be learned about stroke in younger people and how it affects people of different races and ethnicities,” study author Daniel Woo, MD, professor of neurology at the University of Cincinnati, said in a news release. “Our study found that, even when you account for factors that affect outcomes, such as how big the stroke is, race and ethnicity were still independent predictors of how well people would recover.”
A subset of ERICH participants
To examine predictors of functional outcome in young patients with ICH, researchers analyzed data from a subset of patients in the Ethnic/Racial Variations in Intracerebral Hemorrhage (ERICH) study. ERICH enrolled patients with nontraumatic ICHs at 42 sites in the United States. It included 1,000 non-Hispanic black patients, 1,000 non-Hispanic white patients, and 1,000 Hispanic patients. Participants self-reported race and ethnicity.
Lead author Laura C. Miyares from Yale School of Medicine in New Haven, Conn., and colleagues analyzed data from 418 patients in ERICH who were aged 18-49 years and had supratentorial ICH. The cohort had an average age of 43 years, and 69% were male. In this subset, 41% were black, 12% were white, and 47% were Hispanic.
The primary outcome was modified Rankin Scale (mRS) score 3 months after the ICH, and the investigators defined a poor outcome as a score of 4 or greater. At 3 months, 35% had a poor functional outcome. Approximately 18% were unable to walk without assistance and attend to their bodily needs (mRS 4); 8% were bedridden, incontinent, and required nursing care (mRS 5); and 10% had died (mRS 6).
The percentage of patients with a poor functional outcome was 52% among white patients, 35% among black patients, and 31% among Hispanic patients. In a univariable analysis, black patients had a 51% reduction in odds of a poor outcome, compared with white patients, and Hispanic patients had a 59% reduction.
“The association between race/ethnicity and 3-month post-ICH functional outcome remained significant after adjusting for age, sex, premorbid disability, ICH location, ICH volume, [intraventricular hemorrhage] extension, systolic blood pressure, and [Glasgow Coma Scale] score on admission,” the researchers said. “In multivariable analysis, using white patients as the reference category, black patients had a 58% reduction in the odds of poor functional outcome at 3 months and Hispanic patients had a 66% reduction in the same odds.”
Their analysis identified the importance of other risk factors as well. “The volume of the hematoma, the most powerful predictor of outcome in older patients with ICH, was also found to be the most significant predictor of poor outcome in young patients,” they said.
Vascular risks and oral anticoagulants
About 80% of the young adults with ICH had a history of diagnosed hypertension. In nearly half, the condition was untreated. “After hypertension, the most common stroke risk factors in the young were diabetes, high cholesterol, smoking, and alcohol abuse,” the authors said. “In combination, these results indicate that vascular risk factors, especially untreated, could explain a large proportion of cases of ICH in the young.”
“Our results also point to treatment with oral anticoagulants before hospitalization as a potential mediator of the effect of race/ethnicity on short-term functional outcomes,” they said. About 8% of the white patients used oral anticoagulants, compared with 4% of the black patients and 1% of the Hispanic patients. Oral anticoagulant treatment “is a known risk factor for ICH and an established predictor of poor outcome in this condition. However, because only a small proportion of enrolled young patients with ICH were on [oral anticoagulants] prior to presentation, these results should be further validated by future studies.”
The study’s limitations include the broad categorization of racial and ethnic groups, the fact that younger patients with supratentorial ICH were more likely to be black or Hispanic and less likely to be white, and the exclusion of a significant proportion of cases of young white patients with smaller ICH volumes because of missing data, the researchers noted. Although the cohort was large, researchers may need to study more patients to capture differences among racial and ethnic groups, the investigators said.
The association between race/ethnicity and functional outcome could relate to “distinct pathophysiologies of the initial bleed or unique mechanisms of secondary injury,” the researchers suggested. “Future studies are necessary to probe the potential biological and social mediators of these findings to elucidate the role of race/ethnicity in ICH severity and functional recovery, and to develop improved prognostication for a racially varied population.”
ERICH is supported by the National Institute of Neurological Disorders and Stroke. Authors disclosed grants from the government, professional societies, and a university.
SOURCE: Miyares LC et al. Neurology. 2020 Jan 22. doi: 10.1212/WNL.0000000000008930.
researchers reported Jan. 22 in Neurology.
“There has been considerable research on stroke in older people, but there is still much to be learned about stroke in younger people and how it affects people of different races and ethnicities,” study author Daniel Woo, MD, professor of neurology at the University of Cincinnati, said in a news release. “Our study found that, even when you account for factors that affect outcomes, such as how big the stroke is, race and ethnicity were still independent predictors of how well people would recover.”
A subset of ERICH participants
To examine predictors of functional outcome in young patients with ICH, researchers analyzed data from a subset of patients in the Ethnic/Racial Variations in Intracerebral Hemorrhage (ERICH) study. ERICH enrolled patients with nontraumatic ICHs at 42 sites in the United States. It included 1,000 non-Hispanic black patients, 1,000 non-Hispanic white patients, and 1,000 Hispanic patients. Participants self-reported race and ethnicity.
Lead author Laura C. Miyares from Yale School of Medicine in New Haven, Conn., and colleagues analyzed data from 418 patients in ERICH who were aged 18-49 years and had supratentorial ICH. The cohort had an average age of 43 years, and 69% were male. In this subset, 41% were black, 12% were white, and 47% were Hispanic.
The primary outcome was modified Rankin Scale (mRS) score 3 months after the ICH, and the investigators defined a poor outcome as a score of 4 or greater. At 3 months, 35% had a poor functional outcome. Approximately 18% were unable to walk without assistance and attend to their bodily needs (mRS 4); 8% were bedridden, incontinent, and required nursing care (mRS 5); and 10% had died (mRS 6).
The percentage of patients with a poor functional outcome was 52% among white patients, 35% among black patients, and 31% among Hispanic patients. In a univariable analysis, black patients had a 51% reduction in odds of a poor outcome, compared with white patients, and Hispanic patients had a 59% reduction.
“The association between race/ethnicity and 3-month post-ICH functional outcome remained significant after adjusting for age, sex, premorbid disability, ICH location, ICH volume, [intraventricular hemorrhage] extension, systolic blood pressure, and [Glasgow Coma Scale] score on admission,” the researchers said. “In multivariable analysis, using white patients as the reference category, black patients had a 58% reduction in the odds of poor functional outcome at 3 months and Hispanic patients had a 66% reduction in the same odds.”
Their analysis identified the importance of other risk factors as well. “The volume of the hematoma, the most powerful predictor of outcome in older patients with ICH, was also found to be the most significant predictor of poor outcome in young patients,” they said.
Vascular risks and oral anticoagulants
About 80% of the young adults with ICH had a history of diagnosed hypertension. In nearly half, the condition was untreated. “After hypertension, the most common stroke risk factors in the young were diabetes, high cholesterol, smoking, and alcohol abuse,” the authors said. “In combination, these results indicate that vascular risk factors, especially untreated, could explain a large proportion of cases of ICH in the young.”
“Our results also point to treatment with oral anticoagulants before hospitalization as a potential mediator of the effect of race/ethnicity on short-term functional outcomes,” they said. About 8% of the white patients used oral anticoagulants, compared with 4% of the black patients and 1% of the Hispanic patients. Oral anticoagulant treatment “is a known risk factor for ICH and an established predictor of poor outcome in this condition. However, because only a small proportion of enrolled young patients with ICH were on [oral anticoagulants] prior to presentation, these results should be further validated by future studies.”
The study’s limitations include the broad categorization of racial and ethnic groups, the fact that younger patients with supratentorial ICH were more likely to be black or Hispanic and less likely to be white, and the exclusion of a significant proportion of cases of young white patients with smaller ICH volumes because of missing data, the researchers noted. Although the cohort was large, researchers may need to study more patients to capture differences among racial and ethnic groups, the investigators said.
The association between race/ethnicity and functional outcome could relate to “distinct pathophysiologies of the initial bleed or unique mechanisms of secondary injury,” the researchers suggested. “Future studies are necessary to probe the potential biological and social mediators of these findings to elucidate the role of race/ethnicity in ICH severity and functional recovery, and to develop improved prognostication for a racially varied population.”
ERICH is supported by the National Institute of Neurological Disorders and Stroke. Authors disclosed grants from the government, professional societies, and a university.
SOURCE: Miyares LC et al. Neurology. 2020 Jan 22. doi: 10.1212/WNL.0000000000008930.
FROM NEUROLOGY
Key clinical point: Among young adults with supratentorial intracerebral hemorrhage (ICH), black race and Hispanic ethnicity are associated with better functional outcomes, compared with white race.
Major finding: In multivariable analysis, black patients had a 58% reduction in the odds of poor functional outcome at 3 months, compared with white patients, and Hispanic patients had a 66% reduction.
Study details: An analysis of data from a subset of 418 patients in the Ethnic/Racial Variations in Intracerebral Hemorrhage (ERICH) study.
Disclosures: ERICH is supported by the National Institute of Neurological Disorders and Stroke. Authors disclosed grants from the government, professional societies, and a university.
Source: Miyares LC et al. Neurology. 2020 Jan 22. doi: 10.1212/WNL.0000000000008930.
Cardiovascular risks associated with cannabis use
Researchers are recommending routine screening of marijuana use in cardiovascular care settings.
A review of current evidence suggests an association between marijuana use and adverse cardiovascular effects, as well as interactions between marijuana and cardiovascular medications.
Although more research is needed, the review authors suggested patients may benefit from marijuana screening and testing as well as discussions about the potential risks of marijuana use in the setting of cardiovascular disease.
Ersilia M. DeFilippis, MD, of Columbia University Irving Medical Center in New York and colleagues conducted this review, which was published in the Journal of the American College of Cardiology.
The authors noted that research on marijuana use and cardiovascular disease is limited. The different forms of cannabis and various routes of administration have made it difficult to draw concrete conclusions about marijuana products. Additionally, there have been no randomized, controlled trials of marijuana products in the United States because such trials are illegal; however, there are observational studies linking marijuana use and adverse cardiovascular effects.
Snapshot of available evidence
One study showed that smoking marijuana produces many of the same cardiotoxic chemicals produced by smoking tobacco (BMJ. 2003 May 3;326[7396]:942-3). Another study suggested marijuana smokers may have greater exposure to harmful chemicals (J Psychoactive Drugs. 1988 Jan-Mar;20[1]:43-6).
More specifically, a meta-analysis suggested that smoking marijuana was one of the top three triggers of myocardial infarction (Lancet. 2011 Feb 26;377[9767]:732-40). And in a systematic analysis, 28 of 33 studies linked marijuana use to an increased risk of acute coronary syndromes (Clin Toxicol [Phila]. 2019 Oct;57[10]:831-41).
Furthermore, a study of 2.5 million marijuana users showed that 3% experienced arrhythmias (Int J Cardiol. 2018 Aug 1;264:91-2). A population survey showed that people who smoked marijuana in the past year experienced a 3.3-fold higher rate of cerebrovascular events (Aust N Z J Public Health. 2016 Jun;40[3]:226-30).
Studies have also indicated that cannabinoids can affect cardiovascular medications, including antiarrhythmics, calcium-channel blockers, isosorbide dinitrate/mononitrate, statins, beta-blockers, warfarin, theophylline, and nonsteroidal anti-inflammatory drugs (Medicines [Basel]. 2018 Dec 23;6[1] pii: E3; Curr Top Behav Neurosci. 2017;32:249-62; Pharmacogenet Genomics. 2009 Jul;19[7]:559-62; Ann Pharmacother. 2009 Jul;43[7]:1347-53; Pharmacol Ther. 2019 Sep;201:25-38).
Reviewer recommendations
Cardiovascular specialists should be informed about regulations governing marijuana products, as well as “potential health consequences of marijuana and its derivatives,” according to Dr. DeFilippis and colleagues.
The authors recommend routinely screening patients for marijuana use, perhaps using the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (PLoS One. 2017 May 26;12[5]:e0178194) or the Cannabis Abuse Screening Test (Int J Methods Psychiatr Res. 2018 Jun;27[2]:e1597).
The authors say urine toxicology “may be reasonable” for patients with myocardial infarction or new-onset heart failure. Such testing is required for patients undergoing a heart transplant because marijuana use may affect their candidacy.
Dr. DeFilippis and colleagues say cardiovascular specialists should inform patients about the risks associated with marijuana use. The authors recommend shared decision making for patients who use marijuana for symptom management or palliative purposes.
Three review authors disclosed relationships with many different pharmaceutical companies. One author disclosed relationships with Medscape Cardiology and WebMD, which are owned by the same parent company as MDedge.
SOURCE: J Am Coll Cardiol. 2020 Jan 20. doi: 10.1016/j.jacc.2019.11.025.
Researchers are recommending routine screening of marijuana use in cardiovascular care settings.
A review of current evidence suggests an association between marijuana use and adverse cardiovascular effects, as well as interactions between marijuana and cardiovascular medications.
Although more research is needed, the review authors suggested patients may benefit from marijuana screening and testing as well as discussions about the potential risks of marijuana use in the setting of cardiovascular disease.
Ersilia M. DeFilippis, MD, of Columbia University Irving Medical Center in New York and colleagues conducted this review, which was published in the Journal of the American College of Cardiology.
The authors noted that research on marijuana use and cardiovascular disease is limited. The different forms of cannabis and various routes of administration have made it difficult to draw concrete conclusions about marijuana products. Additionally, there have been no randomized, controlled trials of marijuana products in the United States because such trials are illegal; however, there are observational studies linking marijuana use and adverse cardiovascular effects.
Snapshot of available evidence
One study showed that smoking marijuana produces many of the same cardiotoxic chemicals produced by smoking tobacco (BMJ. 2003 May 3;326[7396]:942-3). Another study suggested marijuana smokers may have greater exposure to harmful chemicals (J Psychoactive Drugs. 1988 Jan-Mar;20[1]:43-6).
More specifically, a meta-analysis suggested that smoking marijuana was one of the top three triggers of myocardial infarction (Lancet. 2011 Feb 26;377[9767]:732-40). And in a systematic analysis, 28 of 33 studies linked marijuana use to an increased risk of acute coronary syndromes (Clin Toxicol [Phila]. 2019 Oct;57[10]:831-41).
Furthermore, a study of 2.5 million marijuana users showed that 3% experienced arrhythmias (Int J Cardiol. 2018 Aug 1;264:91-2). A population survey showed that people who smoked marijuana in the past year experienced a 3.3-fold higher rate of cerebrovascular events (Aust N Z J Public Health. 2016 Jun;40[3]:226-30).
Studies have also indicated that cannabinoids can affect cardiovascular medications, including antiarrhythmics, calcium-channel blockers, isosorbide dinitrate/mononitrate, statins, beta-blockers, warfarin, theophylline, and nonsteroidal anti-inflammatory drugs (Medicines [Basel]. 2018 Dec 23;6[1] pii: E3; Curr Top Behav Neurosci. 2017;32:249-62; Pharmacogenet Genomics. 2009 Jul;19[7]:559-62; Ann Pharmacother. 2009 Jul;43[7]:1347-53; Pharmacol Ther. 2019 Sep;201:25-38).
Reviewer recommendations
Cardiovascular specialists should be informed about regulations governing marijuana products, as well as “potential health consequences of marijuana and its derivatives,” according to Dr. DeFilippis and colleagues.
The authors recommend routinely screening patients for marijuana use, perhaps using the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (PLoS One. 2017 May 26;12[5]:e0178194) or the Cannabis Abuse Screening Test (Int J Methods Psychiatr Res. 2018 Jun;27[2]:e1597).
The authors say urine toxicology “may be reasonable” for patients with myocardial infarction or new-onset heart failure. Such testing is required for patients undergoing a heart transplant because marijuana use may affect their candidacy.
Dr. DeFilippis and colleagues say cardiovascular specialists should inform patients about the risks associated with marijuana use. The authors recommend shared decision making for patients who use marijuana for symptom management or palliative purposes.
Three review authors disclosed relationships with many different pharmaceutical companies. One author disclosed relationships with Medscape Cardiology and WebMD, which are owned by the same parent company as MDedge.
SOURCE: J Am Coll Cardiol. 2020 Jan 20. doi: 10.1016/j.jacc.2019.11.025.
Researchers are recommending routine screening of marijuana use in cardiovascular care settings.
A review of current evidence suggests an association between marijuana use and adverse cardiovascular effects, as well as interactions between marijuana and cardiovascular medications.
Although more research is needed, the review authors suggested patients may benefit from marijuana screening and testing as well as discussions about the potential risks of marijuana use in the setting of cardiovascular disease.
Ersilia M. DeFilippis, MD, of Columbia University Irving Medical Center in New York and colleagues conducted this review, which was published in the Journal of the American College of Cardiology.
The authors noted that research on marijuana use and cardiovascular disease is limited. The different forms of cannabis and various routes of administration have made it difficult to draw concrete conclusions about marijuana products. Additionally, there have been no randomized, controlled trials of marijuana products in the United States because such trials are illegal; however, there are observational studies linking marijuana use and adverse cardiovascular effects.
Snapshot of available evidence
One study showed that smoking marijuana produces many of the same cardiotoxic chemicals produced by smoking tobacco (BMJ. 2003 May 3;326[7396]:942-3). Another study suggested marijuana smokers may have greater exposure to harmful chemicals (J Psychoactive Drugs. 1988 Jan-Mar;20[1]:43-6).
More specifically, a meta-analysis suggested that smoking marijuana was one of the top three triggers of myocardial infarction (Lancet. 2011 Feb 26;377[9767]:732-40). And in a systematic analysis, 28 of 33 studies linked marijuana use to an increased risk of acute coronary syndromes (Clin Toxicol [Phila]. 2019 Oct;57[10]:831-41).
Furthermore, a study of 2.5 million marijuana users showed that 3% experienced arrhythmias (Int J Cardiol. 2018 Aug 1;264:91-2). A population survey showed that people who smoked marijuana in the past year experienced a 3.3-fold higher rate of cerebrovascular events (Aust N Z J Public Health. 2016 Jun;40[3]:226-30).
Studies have also indicated that cannabinoids can affect cardiovascular medications, including antiarrhythmics, calcium-channel blockers, isosorbide dinitrate/mononitrate, statins, beta-blockers, warfarin, theophylline, and nonsteroidal anti-inflammatory drugs (Medicines [Basel]. 2018 Dec 23;6[1] pii: E3; Curr Top Behav Neurosci. 2017;32:249-62; Pharmacogenet Genomics. 2009 Jul;19[7]:559-62; Ann Pharmacother. 2009 Jul;43[7]:1347-53; Pharmacol Ther. 2019 Sep;201:25-38).
Reviewer recommendations
Cardiovascular specialists should be informed about regulations governing marijuana products, as well as “potential health consequences of marijuana and its derivatives,” according to Dr. DeFilippis and colleagues.
The authors recommend routinely screening patients for marijuana use, perhaps using the Daily Sessions, Frequency, Age of Onset, and Quantity of Cannabis Use Inventory (PLoS One. 2017 May 26;12[5]:e0178194) or the Cannabis Abuse Screening Test (Int J Methods Psychiatr Res. 2018 Jun;27[2]:e1597).
The authors say urine toxicology “may be reasonable” for patients with myocardial infarction or new-onset heart failure. Such testing is required for patients undergoing a heart transplant because marijuana use may affect their candidacy.
Dr. DeFilippis and colleagues say cardiovascular specialists should inform patients about the risks associated with marijuana use. The authors recommend shared decision making for patients who use marijuana for symptom management or palliative purposes.
Three review authors disclosed relationships with many different pharmaceutical companies. One author disclosed relationships with Medscape Cardiology and WebMD, which are owned by the same parent company as MDedge.
SOURCE: J Am Coll Cardiol. 2020 Jan 20. doi: 10.1016/j.jacc.2019.11.025.
FROM JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
ODYSSEY Outcomes: Alirocumab cut stroke, PAD, VTE
PHILADELPHIA – Treatment with the PCSK9 inhibitor alirocumab linked with a significant cut in the rates of peripheral artery disease events and ischemic strokes without increasing the rate of hemorrhagic strokes, and alirocumab treatment also showed a trend toward an association with a reduced rate of venous thromboembolic events in prespecified, ancillary analyses of data collected from more than 18,000 patients in the ODYSSEY Outcomes trial.
The analyses that looked at peripheral artery disease (PAD) events and venous thromboembolism (VTE) events also suggested that the apparent ability of alirocumab to reduce their incidence may have been largely mediated through a reduction in Lp(a) lipoprotein, with less of a contribution from the drug’s primary action of reducing LDL cholesterol, Gregory G. Schwartz, MD, said at the American Heart Association scientific sessions.
When used on top of intensive statin treatment, as in the ODYSSEY Outcomes trial, treatment with the PCSK9 inhibitor alirocumab “may be useful to prevent PAD events, particularly in patients with high levels of Lp(a),” said Dr. Schwartz, professor of medicine at the University of Colorado Denver in Aurora. In the analysis he reported, patients treated with alirocumab for a median of 2.8 years had a statistically significant 31% reduced rate of PAD or VTE event and a significant 31% reduced rate of PAD events alone, compared with control patients who received placebo, he reported. Alirocumab treatment was also associated with a 33% lower rate of VTE events only, but the overall rate of these events was low, and this difference just missed statistical significance with a P value of .06.
“Levels of Lp(a), but not LDL cholesterol, predicted the risk of PAD events,” and in patients on alirocumab treatment “the magnitude of Lp(a) reduction, but not LDL-cholesterol reduction, was associated with a reduction in PAD events and VTE.” The reduction in PAD events linked with alirocumab treatment “may be related to Lp(a) lowering,” Dr. Schwartz suggested.
The link between alirocumab treatment and a reduction in ischemic stroke with no increase in hemorrhagic strokes appeared in a separate prespecified analysis from ODYSSEY Outcomes that looked at the rates of ischemic stroke, hemorrhagic stroke, and their combined incidence during the median 2.8 year of study follow-up. Patients treated with alirocumab had a statistically significant 27% reduction in their rate of ischemic strokes compared with patients on placebo, and a statistically significant 28% relative reduction in the rate of any stroke with alirocumab treatment, J. Wouter Jukema, MD, said in a separate report at the meeting. The rate of hemorrhagic strokes was small, and showed a nominal 17% reduction in patients treated with alirocumab, compared with controls, a difference that was not statistically significant.
Further analysis of the stroke outcomes also showed that these reductions in total strokes occurred with alirocumab treatment at roughly similar rates regardless of baseline level of LDL cholesterol or history of a prior cerebrovascular event. Analysis also showed that the rate of hemorrhagic strokes was consistently low regardless of the on-treatment level of LDL cholesterol. Even among patients whose LDL cholesterol level fell below 25 mg/dL on alirocumab treatment, the incidence of hemorrhagic strokes during follow-up was 0.1%, “a very reassuring finding,” said Dr. Jukema, professor of cardiology at Leiden (The Netherlands) University. The stroke analyses did not examine possible linkages of these effects with changes in level of Lp(a).
ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) included 18,924 patients who had experienced an acute coronary syndrome event within the prior 12 months and had an LDL cholesterol level of at least 70 mg/dL despite maximally tolerated statin treatment and randomized them to treatment with alirocumab or placebo. The primary endpoint was the combination of coronary heart disease death, nonfatal MI, ischemic stroke, and hospitalization for unstable angina, which alirocumab effectively reduced compared with placebo (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
The PAD analysis tallied the combined rate of acute limb ischemia, revascularization, or amputation related to PAD, and the VTE cases included patients who developed deep vein thrombosis or pulmonary embolism. All cases were nonadjudicated reports from participating investigators. Because Lp(a) makes up a portion of LDL cholesterol, Dr. Schwartz and associates calculated adjusted values for LDL cholesterol that were independent of Lp(a).
In a multivariable analysis that adjusted for demographic and clinical characteristics as well as baseline Lp(a) and the calculated level of LDL cholesterol, every 1 mg/dL decrease in Lp(a) linked with a statistically significant, nearly 1% decrease in the rate of either a PAD or VTE event, while the change in LDL cholesterol had no significant relationship with this endpoint, said Dr. Schwartz.
The impact of Lp(a) lowering was most dramatic among the subgroups of patients who entered the study with the highest levels of Lp(a). “In the lowest quartile [for baseline level of Lp(a)] the effect of treatment [with alirocumab] was inconsequential; all of the action was in the upper two quartiles,” he said. Dr. Schwartz highlighted that 90% of patients in the study were on an “intense” statin dosage, and 97% received some statin treatment. Against that treatment background, the findings showed that patients still had residual cardiovascular disease risk that did not appear to respond to changes in LDL cholesterol but which did appear to respond to a reduction in Lp(a) produced by alirocumab. Dr. Schwartz further suggested that alirocumab’s reduction of Lp(a) might also mediate the drug’s apparent effect on reducing VTE incidence, possibly because Lp(a) is structurally similar to plasminogen and hence can have prothrombotic effects.
ODYSSEY Outcomes was sponsored by Sanofi and Regeneron, the companies that market alirocumab (Praluent). Dr. Schwartz has received research support from Sanofi and from Resverlogix, Roche, and The Medicines Company. Dr. Jukema has been a speaker for and received research support from Sanofi Regeneron, and has also been a speaker for Amgen, MSD, and Roche and has also received research support from Biotronik
SOURCE: Schwartz GG et al. AHA 2019, Abstract 309; Jukema JW et al. AHA 2019, Abstract 334.
PHILADELPHIA – Treatment with the PCSK9 inhibitor alirocumab linked with a significant cut in the rates of peripheral artery disease events and ischemic strokes without increasing the rate of hemorrhagic strokes, and alirocumab treatment also showed a trend toward an association with a reduced rate of venous thromboembolic events in prespecified, ancillary analyses of data collected from more than 18,000 patients in the ODYSSEY Outcomes trial.
The analyses that looked at peripheral artery disease (PAD) events and venous thromboembolism (VTE) events also suggested that the apparent ability of alirocumab to reduce their incidence may have been largely mediated through a reduction in Lp(a) lipoprotein, with less of a contribution from the drug’s primary action of reducing LDL cholesterol, Gregory G. Schwartz, MD, said at the American Heart Association scientific sessions.
When used on top of intensive statin treatment, as in the ODYSSEY Outcomes trial, treatment with the PCSK9 inhibitor alirocumab “may be useful to prevent PAD events, particularly in patients with high levels of Lp(a),” said Dr. Schwartz, professor of medicine at the University of Colorado Denver in Aurora. In the analysis he reported, patients treated with alirocumab for a median of 2.8 years had a statistically significant 31% reduced rate of PAD or VTE event and a significant 31% reduced rate of PAD events alone, compared with control patients who received placebo, he reported. Alirocumab treatment was also associated with a 33% lower rate of VTE events only, but the overall rate of these events was low, and this difference just missed statistical significance with a P value of .06.
“Levels of Lp(a), but not LDL cholesterol, predicted the risk of PAD events,” and in patients on alirocumab treatment “the magnitude of Lp(a) reduction, but not LDL-cholesterol reduction, was associated with a reduction in PAD events and VTE.” The reduction in PAD events linked with alirocumab treatment “may be related to Lp(a) lowering,” Dr. Schwartz suggested.
The link between alirocumab treatment and a reduction in ischemic stroke with no increase in hemorrhagic strokes appeared in a separate prespecified analysis from ODYSSEY Outcomes that looked at the rates of ischemic stroke, hemorrhagic stroke, and their combined incidence during the median 2.8 year of study follow-up. Patients treated with alirocumab had a statistically significant 27% reduction in their rate of ischemic strokes compared with patients on placebo, and a statistically significant 28% relative reduction in the rate of any stroke with alirocumab treatment, J. Wouter Jukema, MD, said in a separate report at the meeting. The rate of hemorrhagic strokes was small, and showed a nominal 17% reduction in patients treated with alirocumab, compared with controls, a difference that was not statistically significant.
Further analysis of the stroke outcomes also showed that these reductions in total strokes occurred with alirocumab treatment at roughly similar rates regardless of baseline level of LDL cholesterol or history of a prior cerebrovascular event. Analysis also showed that the rate of hemorrhagic strokes was consistently low regardless of the on-treatment level of LDL cholesterol. Even among patients whose LDL cholesterol level fell below 25 mg/dL on alirocumab treatment, the incidence of hemorrhagic strokes during follow-up was 0.1%, “a very reassuring finding,” said Dr. Jukema, professor of cardiology at Leiden (The Netherlands) University. The stroke analyses did not examine possible linkages of these effects with changes in level of Lp(a).
ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) included 18,924 patients who had experienced an acute coronary syndrome event within the prior 12 months and had an LDL cholesterol level of at least 70 mg/dL despite maximally tolerated statin treatment and randomized them to treatment with alirocumab or placebo. The primary endpoint was the combination of coronary heart disease death, nonfatal MI, ischemic stroke, and hospitalization for unstable angina, which alirocumab effectively reduced compared with placebo (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
The PAD analysis tallied the combined rate of acute limb ischemia, revascularization, or amputation related to PAD, and the VTE cases included patients who developed deep vein thrombosis or pulmonary embolism. All cases were nonadjudicated reports from participating investigators. Because Lp(a) makes up a portion of LDL cholesterol, Dr. Schwartz and associates calculated adjusted values for LDL cholesterol that were independent of Lp(a).
In a multivariable analysis that adjusted for demographic and clinical characteristics as well as baseline Lp(a) and the calculated level of LDL cholesterol, every 1 mg/dL decrease in Lp(a) linked with a statistically significant, nearly 1% decrease in the rate of either a PAD or VTE event, while the change in LDL cholesterol had no significant relationship with this endpoint, said Dr. Schwartz.
The impact of Lp(a) lowering was most dramatic among the subgroups of patients who entered the study with the highest levels of Lp(a). “In the lowest quartile [for baseline level of Lp(a)] the effect of treatment [with alirocumab] was inconsequential; all of the action was in the upper two quartiles,” he said. Dr. Schwartz highlighted that 90% of patients in the study were on an “intense” statin dosage, and 97% received some statin treatment. Against that treatment background, the findings showed that patients still had residual cardiovascular disease risk that did not appear to respond to changes in LDL cholesterol but which did appear to respond to a reduction in Lp(a) produced by alirocumab. Dr. Schwartz further suggested that alirocumab’s reduction of Lp(a) might also mediate the drug’s apparent effect on reducing VTE incidence, possibly because Lp(a) is structurally similar to plasminogen and hence can have prothrombotic effects.
ODYSSEY Outcomes was sponsored by Sanofi and Regeneron, the companies that market alirocumab (Praluent). Dr. Schwartz has received research support from Sanofi and from Resverlogix, Roche, and The Medicines Company. Dr. Jukema has been a speaker for and received research support from Sanofi Regeneron, and has also been a speaker for Amgen, MSD, and Roche and has also received research support from Biotronik
SOURCE: Schwartz GG et al. AHA 2019, Abstract 309; Jukema JW et al. AHA 2019, Abstract 334.
PHILADELPHIA – Treatment with the PCSK9 inhibitor alirocumab linked with a significant cut in the rates of peripheral artery disease events and ischemic strokes without increasing the rate of hemorrhagic strokes, and alirocumab treatment also showed a trend toward an association with a reduced rate of venous thromboembolic events in prespecified, ancillary analyses of data collected from more than 18,000 patients in the ODYSSEY Outcomes trial.
The analyses that looked at peripheral artery disease (PAD) events and venous thromboembolism (VTE) events also suggested that the apparent ability of alirocumab to reduce their incidence may have been largely mediated through a reduction in Lp(a) lipoprotein, with less of a contribution from the drug’s primary action of reducing LDL cholesterol, Gregory G. Schwartz, MD, said at the American Heart Association scientific sessions.
When used on top of intensive statin treatment, as in the ODYSSEY Outcomes trial, treatment with the PCSK9 inhibitor alirocumab “may be useful to prevent PAD events, particularly in patients with high levels of Lp(a),” said Dr. Schwartz, professor of medicine at the University of Colorado Denver in Aurora. In the analysis he reported, patients treated with alirocumab for a median of 2.8 years had a statistically significant 31% reduced rate of PAD or VTE event and a significant 31% reduced rate of PAD events alone, compared with control patients who received placebo, he reported. Alirocumab treatment was also associated with a 33% lower rate of VTE events only, but the overall rate of these events was low, and this difference just missed statistical significance with a P value of .06.
“Levels of Lp(a), but not LDL cholesterol, predicted the risk of PAD events,” and in patients on alirocumab treatment “the magnitude of Lp(a) reduction, but not LDL-cholesterol reduction, was associated with a reduction in PAD events and VTE.” The reduction in PAD events linked with alirocumab treatment “may be related to Lp(a) lowering,” Dr. Schwartz suggested.
The link between alirocumab treatment and a reduction in ischemic stroke with no increase in hemorrhagic strokes appeared in a separate prespecified analysis from ODYSSEY Outcomes that looked at the rates of ischemic stroke, hemorrhagic stroke, and their combined incidence during the median 2.8 year of study follow-up. Patients treated with alirocumab had a statistically significant 27% reduction in their rate of ischemic strokes compared with patients on placebo, and a statistically significant 28% relative reduction in the rate of any stroke with alirocumab treatment, J. Wouter Jukema, MD, said in a separate report at the meeting. The rate of hemorrhagic strokes was small, and showed a nominal 17% reduction in patients treated with alirocumab, compared with controls, a difference that was not statistically significant.
Further analysis of the stroke outcomes also showed that these reductions in total strokes occurred with alirocumab treatment at roughly similar rates regardless of baseline level of LDL cholesterol or history of a prior cerebrovascular event. Analysis also showed that the rate of hemorrhagic strokes was consistently low regardless of the on-treatment level of LDL cholesterol. Even among patients whose LDL cholesterol level fell below 25 mg/dL on alirocumab treatment, the incidence of hemorrhagic strokes during follow-up was 0.1%, “a very reassuring finding,” said Dr. Jukema, professor of cardiology at Leiden (The Netherlands) University. The stroke analyses did not examine possible linkages of these effects with changes in level of Lp(a).
ODYSSEY Outcomes (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) included 18,924 patients who had experienced an acute coronary syndrome event within the prior 12 months and had an LDL cholesterol level of at least 70 mg/dL despite maximally tolerated statin treatment and randomized them to treatment with alirocumab or placebo. The primary endpoint was the combination of coronary heart disease death, nonfatal MI, ischemic stroke, and hospitalization for unstable angina, which alirocumab effectively reduced compared with placebo (N Engl J Med. 2018 Nov 29;379[22]:2097-107).
The PAD analysis tallied the combined rate of acute limb ischemia, revascularization, or amputation related to PAD, and the VTE cases included patients who developed deep vein thrombosis or pulmonary embolism. All cases were nonadjudicated reports from participating investigators. Because Lp(a) makes up a portion of LDL cholesterol, Dr. Schwartz and associates calculated adjusted values for LDL cholesterol that were independent of Lp(a).
In a multivariable analysis that adjusted for demographic and clinical characteristics as well as baseline Lp(a) and the calculated level of LDL cholesterol, every 1 mg/dL decrease in Lp(a) linked with a statistically significant, nearly 1% decrease in the rate of either a PAD or VTE event, while the change in LDL cholesterol had no significant relationship with this endpoint, said Dr. Schwartz.
The impact of Lp(a) lowering was most dramatic among the subgroups of patients who entered the study with the highest levels of Lp(a). “In the lowest quartile [for baseline level of Lp(a)] the effect of treatment [with alirocumab] was inconsequential; all of the action was in the upper two quartiles,” he said. Dr. Schwartz highlighted that 90% of patients in the study were on an “intense” statin dosage, and 97% received some statin treatment. Against that treatment background, the findings showed that patients still had residual cardiovascular disease risk that did not appear to respond to changes in LDL cholesterol but which did appear to respond to a reduction in Lp(a) produced by alirocumab. Dr. Schwartz further suggested that alirocumab’s reduction of Lp(a) might also mediate the drug’s apparent effect on reducing VTE incidence, possibly because Lp(a) is structurally similar to plasminogen and hence can have prothrombotic effects.
ODYSSEY Outcomes was sponsored by Sanofi and Regeneron, the companies that market alirocumab (Praluent). Dr. Schwartz has received research support from Sanofi and from Resverlogix, Roche, and The Medicines Company. Dr. Jukema has been a speaker for and received research support from Sanofi Regeneron, and has also been a speaker for Amgen, MSD, and Roche and has also received research support from Biotronik
SOURCE: Schwartz GG et al. AHA 2019, Abstract 309; Jukema JW et al. AHA 2019, Abstract 334.
REPORTING FROM THE AHA 2019