User login
Vaccination and antiviral treatment do not affect stroke risk following shingles
HONOLULU – according to findings from a retrospective study of Medicare beneficiaries with shingles and ischemic stroke.
The findings suggest that primary prevention of shingles through vaccination might be the most effective approach to prevent shingles-associated acute ischemic stroke, said the researchers, who presented the study at the International Stroke Conference sponsored by the American Heart Association.
Almost one in three people in the United States will develop shingles, also known as herpes zoster, in their lifetime, according to the Centers for Disease Control and Prevention. Previous research has not simultaneously examined the effect of shingles vaccination and antiviral treatment following shingles onset on the risk of acute ischemic stroke.
Quanhe Yang, PhD, a senior scientist at the CDC, and his colleagues examined data for 35,186 Medicare fee-for-service beneficiaries who were 66 years or older, diagnosed with shingles during 2008-2014, and diagnosed with acute ischemic stroke within a year of shingles diagnosis. Using a self-controlled case series design, the investigators analyzed the association between shingles and stroke. Dr. Yang and his colleagues estimated the incident rate ratio (IRR) by comparing the incidence of stroke during risk periods (i.e., periods following shingles), compared with control periods. To minimize confounding by age, they restricted their analyses to approximately 365 days from the shingles index date.
To investigate how vaccination against shingles with Zostavax and antiviral treatment following shingles affected stroke risk, the researchers classified beneficiaries into the following four groups: Group 1 had no vaccination and no antiviral treatment (49% of beneficiaries), Group 2 had vaccination only (9%), Group 3 had antiviral treatment only (34%), and Group 4 had vaccination and antiviral treatment (8%). The researchers tested for interaction to examine the changes in IRRs across the four groups.
IRRs for stroke progressively declined as time passed from the index shingles date, from 1.61 at 0-14 days following shingles to 1.35 at 15-30 days, 1.16 at 31-90 days, and 1.05 at 91-180 days. The researchers found no evidence that shingles vaccination and antiviral treatment modified the risk of acute ischemic stroke. The association between shingles and risk for acute ischemic stroke was consistent across age groups (i.e., 66-74 years, 75-84 years, and 85 years or older), sex, and race (i.e., non-Hispanic white, non-Hispanic black, and Hispanic, other).
One of the study’s strengths was that its sample was a large national cohort of Medicare fee-for-service beneficiaries, Dr. Yang said. In addition, the study design eliminated all fixed confounding effects. Potential weaknesses, however, included the fact that herpes zoster diagnosis was based on administrative data and that the vaccine’s efficacy declines over time.
The findings suggest that the importance of following the recommended shingles vaccination protocol in the prevention of shingles, Dr. Yang said. Shingrix, a vaccine that the Food and Drug Administration approved in 2017, prevents shingles with an efficacy greater than 90%, he added.
The investigators reported no funding source or disclosures for this study.
SOURCE: Yang Q et al. Circulation. 2019;50(Suppl_1): Abstract 39
HONOLULU – according to findings from a retrospective study of Medicare beneficiaries with shingles and ischemic stroke.
The findings suggest that primary prevention of shingles through vaccination might be the most effective approach to prevent shingles-associated acute ischemic stroke, said the researchers, who presented the study at the International Stroke Conference sponsored by the American Heart Association.
Almost one in three people in the United States will develop shingles, also known as herpes zoster, in their lifetime, according to the Centers for Disease Control and Prevention. Previous research has not simultaneously examined the effect of shingles vaccination and antiviral treatment following shingles onset on the risk of acute ischemic stroke.
Quanhe Yang, PhD, a senior scientist at the CDC, and his colleagues examined data for 35,186 Medicare fee-for-service beneficiaries who were 66 years or older, diagnosed with shingles during 2008-2014, and diagnosed with acute ischemic stroke within a year of shingles diagnosis. Using a self-controlled case series design, the investigators analyzed the association between shingles and stroke. Dr. Yang and his colleagues estimated the incident rate ratio (IRR) by comparing the incidence of stroke during risk periods (i.e., periods following shingles), compared with control periods. To minimize confounding by age, they restricted their analyses to approximately 365 days from the shingles index date.
To investigate how vaccination against shingles with Zostavax and antiviral treatment following shingles affected stroke risk, the researchers classified beneficiaries into the following four groups: Group 1 had no vaccination and no antiviral treatment (49% of beneficiaries), Group 2 had vaccination only (9%), Group 3 had antiviral treatment only (34%), and Group 4 had vaccination and antiviral treatment (8%). The researchers tested for interaction to examine the changes in IRRs across the four groups.
IRRs for stroke progressively declined as time passed from the index shingles date, from 1.61 at 0-14 days following shingles to 1.35 at 15-30 days, 1.16 at 31-90 days, and 1.05 at 91-180 days. The researchers found no evidence that shingles vaccination and antiviral treatment modified the risk of acute ischemic stroke. The association between shingles and risk for acute ischemic stroke was consistent across age groups (i.e., 66-74 years, 75-84 years, and 85 years or older), sex, and race (i.e., non-Hispanic white, non-Hispanic black, and Hispanic, other).
One of the study’s strengths was that its sample was a large national cohort of Medicare fee-for-service beneficiaries, Dr. Yang said. In addition, the study design eliminated all fixed confounding effects. Potential weaknesses, however, included the fact that herpes zoster diagnosis was based on administrative data and that the vaccine’s efficacy declines over time.
The findings suggest that the importance of following the recommended shingles vaccination protocol in the prevention of shingles, Dr. Yang said. Shingrix, a vaccine that the Food and Drug Administration approved in 2017, prevents shingles with an efficacy greater than 90%, he added.
The investigators reported no funding source or disclosures for this study.
SOURCE: Yang Q et al. Circulation. 2019;50(Suppl_1): Abstract 39
HONOLULU – according to findings from a retrospective study of Medicare beneficiaries with shingles and ischemic stroke.
The findings suggest that primary prevention of shingles through vaccination might be the most effective approach to prevent shingles-associated acute ischemic stroke, said the researchers, who presented the study at the International Stroke Conference sponsored by the American Heart Association.
Almost one in three people in the United States will develop shingles, also known as herpes zoster, in their lifetime, according to the Centers for Disease Control and Prevention. Previous research has not simultaneously examined the effect of shingles vaccination and antiviral treatment following shingles onset on the risk of acute ischemic stroke.
Quanhe Yang, PhD, a senior scientist at the CDC, and his colleagues examined data for 35,186 Medicare fee-for-service beneficiaries who were 66 years or older, diagnosed with shingles during 2008-2014, and diagnosed with acute ischemic stroke within a year of shingles diagnosis. Using a self-controlled case series design, the investigators analyzed the association between shingles and stroke. Dr. Yang and his colleagues estimated the incident rate ratio (IRR) by comparing the incidence of stroke during risk periods (i.e., periods following shingles), compared with control periods. To minimize confounding by age, they restricted their analyses to approximately 365 days from the shingles index date.
To investigate how vaccination against shingles with Zostavax and antiviral treatment following shingles affected stroke risk, the researchers classified beneficiaries into the following four groups: Group 1 had no vaccination and no antiviral treatment (49% of beneficiaries), Group 2 had vaccination only (9%), Group 3 had antiviral treatment only (34%), and Group 4 had vaccination and antiviral treatment (8%). The researchers tested for interaction to examine the changes in IRRs across the four groups.
IRRs for stroke progressively declined as time passed from the index shingles date, from 1.61 at 0-14 days following shingles to 1.35 at 15-30 days, 1.16 at 31-90 days, and 1.05 at 91-180 days. The researchers found no evidence that shingles vaccination and antiviral treatment modified the risk of acute ischemic stroke. The association between shingles and risk for acute ischemic stroke was consistent across age groups (i.e., 66-74 years, 75-84 years, and 85 years or older), sex, and race (i.e., non-Hispanic white, non-Hispanic black, and Hispanic, other).
One of the study’s strengths was that its sample was a large national cohort of Medicare fee-for-service beneficiaries, Dr. Yang said. In addition, the study design eliminated all fixed confounding effects. Potential weaknesses, however, included the fact that herpes zoster diagnosis was based on administrative data and that the vaccine’s efficacy declines over time.
The findings suggest that the importance of following the recommended shingles vaccination protocol in the prevention of shingles, Dr. Yang said. Shingrix, a vaccine that the Food and Drug Administration approved in 2017, prevents shingles with an efficacy greater than 90%, he added.
The investigators reported no funding source or disclosures for this study.
SOURCE: Yang Q et al. Circulation. 2019;50(Suppl_1): Abstract 39
REPORTING FROM ISC 2019
Key clinical point: After a patient develops shingles, prior vaccination or treatment with antiviral medication does not change the risk of acute ischemic stroke.
Major finding: Stroke incidence increased by 61% within 14 days after shingles onset.
Study details: A self-controlled case series of 35,186 Medicare beneficiaries with shingles and acute ischemic stroke.
Disclosures: The authors reported no funding source or disclosures for this study.
Source: Yang Q et al. Circulation. 2019;50(Suppl_1), Abstract 39
Most U.S. tPA-eligible stroke patients now get treated within an hour
HONOLULU – The speed at which eligible U.S. patients with acute ischemic stroke receive thrombolytic therapy has surged in recent years, and .
By the second half of last year, 75% of acute ischemic stroke patients treated at any of the 913 U.S. hospitals in the Get With The Guidelines-Stroke program received intravenous tissue plasminogen activator (tPA; Alteplase) within 60 minutes of their hospital arrival (their door-to-needle time (DTN), and 52% received tPA with a DTN time of 45 minutes or less. These levels met the treatment-speed goals set by the second phase of the Target: Stroke program, which called for delivering tPA to 75% of appropriate stroke patients within a DTN time of 60 minutes, and within 45 minutes in at least 50% of patients, Gregg C. Fonarow, MD, and his associates reported at the International Stroke Conference, sponsored by the American Heart Association.
The analyses they reported also documented how these most recent gains in thrombolytic speed played out in improved patient outcomes. During phase 2 of Target: Stroke, which ran from January 2014 to September 2018, 85,078 U.S. patients received tPA at one of the participating hospitals. During those 4 years, the rate of in-hospital mortality was 6.0%, half the patients were discharged home, 53% could ambulate independently, and the rate of intracerebral hemorrhage (ICH) was 3.5%. The researchers compared these clinical event rates with the rates from 24,603 tPA-treated patients during 2003-2009, before the Target: Stroke campaign began. After adjustment for many potential confounders, the more recently treated cohort had a 31% relative risk reduction in in-hospital mortality, a 43% relative increase in being discharged home, a 40% relative increase in independent ambulation, and a 32% relative risk reduction in the rate of symptomatic ICH. All these between-group differences were statistically significant.
“We were hoping that, by improving DTN times we could achieve improved outcomes, but often in quality-improvement research – even when the process of care improves – the gains in outcomes don’t necessarily match expectations. Fortunately, with Target: Stroke, the remarkable improvements in timely treatment translated to remarkable improvements in clinical outcomes,” Dr. Fonarow said in an interview. “These are substantial, clinically relevant improvements in clinical outcomes for patients with acute ischemic stroke. As a result of the program, more than 100,000 acute ischemic stroke patients received much more timely acute ischemic stroke care and achieved far better clinical outcomes.”
During the 2003-2018 period reviewed, the percentage of presenting acute ischemic stroke patients who received tPA treatment at the 913 Get With The Guidelines hospitals that participated in the Target: Stroke program (and so had reviewable data) throughout all three periods rose from 6% during 2003-2009 (prestudy) to 8% during 2010-2013 (phase 1), and to 12% during 2014-2018 (phase 2). The percentages of these patients who received the drug within 60 minutes were 27% during 2003-2009, 43% during 2010-2013, and 68% during the entire 2014-2018 period, culminating in the 75% rate during July-September 2018, reported Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
Dr. Fonarow attributed the drop in the rate of ICH – from 5.7% during 2003-2009, to 4.4% during 2010-2013, and down to 3.5% during 2014-2018 – to the faster delivery of tPA. “With faster treatment, there is less ischemic brain and vascular damage and thus a lower likelihood of ICH as a complication of tPA,” he explained.
The Target: Stroke program achieved these gains in speedier thrombolytic treatment (and better recognition of eligible patients) through educational and promotional activities including dissemination of best practices. Notable best practices have included EMS prenotification of hospitals before they arrive with a stroke patient, direct transport of patients to a brain imaging scanner, premix of tPA, initiation of tPA in the brain imaging suite, and prompt data feedback, Dr. Fonarow said.
The Get With The Guidelines-Stroke and Target: Stroke programs now involve more than 2,100 U.S. hospitals, and they are able to deliver emergency care to roughly 70% of U.S. acute ischemic stroke patients, he noted.
With achievement of Target: Stroke’s phase 2 goals, the program announced its launch of a third phase, with new treatment goals: Initiation of thrombolytic treatment to 85% of eligible patients within 60 minutes, to 75% within 45 minutes, and to 50% within 30 minutes. The phase 3 Target: Stroke program also for the first time includes treatment goals for delivery of endovascular thrombectomy treatment.
SOURCE: Fonarow GC et al. ISC 2019, Abstract LBP9.
The Target: Stroke and Get With The Guidelines-Stroke programs should be commended for the very impressive achievements they have made in improved delivery of thrombolytic therapy to acute ischemic stroke patients. What’s happened over the past decade in the speed of delivery of tissue plasminogen activator for treating U.S. stroke patients has been a real success story.
Programs like Get With The Guidelines and Target: Stroke have proven their value, but a significant barrier remains to bringing this program to all U.S. stroke patients and to all U.S. hospitals that treat stroke patients. That barrier is resources. Participating hospitals need to meet certain data-collection standards, but some U.S. hospitals do not have the resources to do this.
Bruce Ovbiagele, MD , is a neurologist and chief of staff for the San Francisco Veterans Affairs Health Care System. He had no disclosures. He made these comments in an interview.
The Target: Stroke and Get With The Guidelines-Stroke programs should be commended for the very impressive achievements they have made in improved delivery of thrombolytic therapy to acute ischemic stroke patients. What’s happened over the past decade in the speed of delivery of tissue plasminogen activator for treating U.S. stroke patients has been a real success story.
Programs like Get With The Guidelines and Target: Stroke have proven their value, but a significant barrier remains to bringing this program to all U.S. stroke patients and to all U.S. hospitals that treat stroke patients. That barrier is resources. Participating hospitals need to meet certain data-collection standards, but some U.S. hospitals do not have the resources to do this.
Bruce Ovbiagele, MD , is a neurologist and chief of staff for the San Francisco Veterans Affairs Health Care System. He had no disclosures. He made these comments in an interview.
The Target: Stroke and Get With The Guidelines-Stroke programs should be commended for the very impressive achievements they have made in improved delivery of thrombolytic therapy to acute ischemic stroke patients. What’s happened over the past decade in the speed of delivery of tissue plasminogen activator for treating U.S. stroke patients has been a real success story.
Programs like Get With The Guidelines and Target: Stroke have proven their value, but a significant barrier remains to bringing this program to all U.S. stroke patients and to all U.S. hospitals that treat stroke patients. That barrier is resources. Participating hospitals need to meet certain data-collection standards, but some U.S. hospitals do not have the resources to do this.
Bruce Ovbiagele, MD , is a neurologist and chief of staff for the San Francisco Veterans Affairs Health Care System. He had no disclosures. He made these comments in an interview.
HONOLULU – The speed at which eligible U.S. patients with acute ischemic stroke receive thrombolytic therapy has surged in recent years, and .
By the second half of last year, 75% of acute ischemic stroke patients treated at any of the 913 U.S. hospitals in the Get With The Guidelines-Stroke program received intravenous tissue plasminogen activator (tPA; Alteplase) within 60 minutes of their hospital arrival (their door-to-needle time (DTN), and 52% received tPA with a DTN time of 45 minutes or less. These levels met the treatment-speed goals set by the second phase of the Target: Stroke program, which called for delivering tPA to 75% of appropriate stroke patients within a DTN time of 60 minutes, and within 45 minutes in at least 50% of patients, Gregg C. Fonarow, MD, and his associates reported at the International Stroke Conference, sponsored by the American Heart Association.
The analyses they reported also documented how these most recent gains in thrombolytic speed played out in improved patient outcomes. During phase 2 of Target: Stroke, which ran from January 2014 to September 2018, 85,078 U.S. patients received tPA at one of the participating hospitals. During those 4 years, the rate of in-hospital mortality was 6.0%, half the patients were discharged home, 53% could ambulate independently, and the rate of intracerebral hemorrhage (ICH) was 3.5%. The researchers compared these clinical event rates with the rates from 24,603 tPA-treated patients during 2003-2009, before the Target: Stroke campaign began. After adjustment for many potential confounders, the more recently treated cohort had a 31% relative risk reduction in in-hospital mortality, a 43% relative increase in being discharged home, a 40% relative increase in independent ambulation, and a 32% relative risk reduction in the rate of symptomatic ICH. All these between-group differences were statistically significant.
“We were hoping that, by improving DTN times we could achieve improved outcomes, but often in quality-improvement research – even when the process of care improves – the gains in outcomes don’t necessarily match expectations. Fortunately, with Target: Stroke, the remarkable improvements in timely treatment translated to remarkable improvements in clinical outcomes,” Dr. Fonarow said in an interview. “These are substantial, clinically relevant improvements in clinical outcomes for patients with acute ischemic stroke. As a result of the program, more than 100,000 acute ischemic stroke patients received much more timely acute ischemic stroke care and achieved far better clinical outcomes.”
During the 2003-2018 period reviewed, the percentage of presenting acute ischemic stroke patients who received tPA treatment at the 913 Get With The Guidelines hospitals that participated in the Target: Stroke program (and so had reviewable data) throughout all three periods rose from 6% during 2003-2009 (prestudy) to 8% during 2010-2013 (phase 1), and to 12% during 2014-2018 (phase 2). The percentages of these patients who received the drug within 60 minutes were 27% during 2003-2009, 43% during 2010-2013, and 68% during the entire 2014-2018 period, culminating in the 75% rate during July-September 2018, reported Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
Dr. Fonarow attributed the drop in the rate of ICH – from 5.7% during 2003-2009, to 4.4% during 2010-2013, and down to 3.5% during 2014-2018 – to the faster delivery of tPA. “With faster treatment, there is less ischemic brain and vascular damage and thus a lower likelihood of ICH as a complication of tPA,” he explained.
The Target: Stroke program achieved these gains in speedier thrombolytic treatment (and better recognition of eligible patients) through educational and promotional activities including dissemination of best practices. Notable best practices have included EMS prenotification of hospitals before they arrive with a stroke patient, direct transport of patients to a brain imaging scanner, premix of tPA, initiation of tPA in the brain imaging suite, and prompt data feedback, Dr. Fonarow said.
The Get With The Guidelines-Stroke and Target: Stroke programs now involve more than 2,100 U.S. hospitals, and they are able to deliver emergency care to roughly 70% of U.S. acute ischemic stroke patients, he noted.
With achievement of Target: Stroke’s phase 2 goals, the program announced its launch of a third phase, with new treatment goals: Initiation of thrombolytic treatment to 85% of eligible patients within 60 minutes, to 75% within 45 minutes, and to 50% within 30 minutes. The phase 3 Target: Stroke program also for the first time includes treatment goals for delivery of endovascular thrombectomy treatment.
SOURCE: Fonarow GC et al. ISC 2019, Abstract LBP9.
HONOLULU – The speed at which eligible U.S. patients with acute ischemic stroke receive thrombolytic therapy has surged in recent years, and .
By the second half of last year, 75% of acute ischemic stroke patients treated at any of the 913 U.S. hospitals in the Get With The Guidelines-Stroke program received intravenous tissue plasminogen activator (tPA; Alteplase) within 60 minutes of their hospital arrival (their door-to-needle time (DTN), and 52% received tPA with a DTN time of 45 minutes or less. These levels met the treatment-speed goals set by the second phase of the Target: Stroke program, which called for delivering tPA to 75% of appropriate stroke patients within a DTN time of 60 minutes, and within 45 minutes in at least 50% of patients, Gregg C. Fonarow, MD, and his associates reported at the International Stroke Conference, sponsored by the American Heart Association.
The analyses they reported also documented how these most recent gains in thrombolytic speed played out in improved patient outcomes. During phase 2 of Target: Stroke, which ran from January 2014 to September 2018, 85,078 U.S. patients received tPA at one of the participating hospitals. During those 4 years, the rate of in-hospital mortality was 6.0%, half the patients were discharged home, 53% could ambulate independently, and the rate of intracerebral hemorrhage (ICH) was 3.5%. The researchers compared these clinical event rates with the rates from 24,603 tPA-treated patients during 2003-2009, before the Target: Stroke campaign began. After adjustment for many potential confounders, the more recently treated cohort had a 31% relative risk reduction in in-hospital mortality, a 43% relative increase in being discharged home, a 40% relative increase in independent ambulation, and a 32% relative risk reduction in the rate of symptomatic ICH. All these between-group differences were statistically significant.
“We were hoping that, by improving DTN times we could achieve improved outcomes, but often in quality-improvement research – even when the process of care improves – the gains in outcomes don’t necessarily match expectations. Fortunately, with Target: Stroke, the remarkable improvements in timely treatment translated to remarkable improvements in clinical outcomes,” Dr. Fonarow said in an interview. “These are substantial, clinically relevant improvements in clinical outcomes for patients with acute ischemic stroke. As a result of the program, more than 100,000 acute ischemic stroke patients received much more timely acute ischemic stroke care and achieved far better clinical outcomes.”
During the 2003-2018 period reviewed, the percentage of presenting acute ischemic stroke patients who received tPA treatment at the 913 Get With The Guidelines hospitals that participated in the Target: Stroke program (and so had reviewable data) throughout all three periods rose from 6% during 2003-2009 (prestudy) to 8% during 2010-2013 (phase 1), and to 12% during 2014-2018 (phase 2). The percentages of these patients who received the drug within 60 minutes were 27% during 2003-2009, 43% during 2010-2013, and 68% during the entire 2014-2018 period, culminating in the 75% rate during July-September 2018, reported Dr. Fonarow, professor of medicine and cochief of cardiology at the University of California, Los Angeles.
Dr. Fonarow attributed the drop in the rate of ICH – from 5.7% during 2003-2009, to 4.4% during 2010-2013, and down to 3.5% during 2014-2018 – to the faster delivery of tPA. “With faster treatment, there is less ischemic brain and vascular damage and thus a lower likelihood of ICH as a complication of tPA,” he explained.
The Target: Stroke program achieved these gains in speedier thrombolytic treatment (and better recognition of eligible patients) through educational and promotional activities including dissemination of best practices. Notable best practices have included EMS prenotification of hospitals before they arrive with a stroke patient, direct transport of patients to a brain imaging scanner, premix of tPA, initiation of tPA in the brain imaging suite, and prompt data feedback, Dr. Fonarow said.
The Get With The Guidelines-Stroke and Target: Stroke programs now involve more than 2,100 U.S. hospitals, and they are able to deliver emergency care to roughly 70% of U.S. acute ischemic stroke patients, he noted.
With achievement of Target: Stroke’s phase 2 goals, the program announced its launch of a third phase, with new treatment goals: Initiation of thrombolytic treatment to 85% of eligible patients within 60 minutes, to 75% within 45 minutes, and to 50% within 30 minutes. The phase 3 Target: Stroke program also for the first time includes treatment goals for delivery of endovascular thrombectomy treatment.
SOURCE: Fonarow GC et al. ISC 2019, Abstract LBP9.
REPORTING FROM ISC 2019
Key clinical point: In late 2018, the Target: Stroke program met its phase 2 goal for timely delivery of thrombolytic therapy to acute ischemic stroke patients.
Major finding: In September 2018, 75% of eligible stroke patients underwent thrombolysis within 60 minutes of hospital arrival, and 52% within 45 minutes.
Study details: Review of data collected from 154,221 U.S. stroke patients treated with thrombolysis during 2003-2018.
Disclosures: Target: Stroke has received funding from Boehringer Ingelheim, Janssen, Bristol-Myers Squibb/Sanofi, and Merck. Dr. Fonarow had no relevant disclosures.
Source: Fonarow GC et al. ISC 2019, Abstract LBP9.
Cilostazol plus aspirin or clopidogrel reduces the risk of recurrent stroke
HONOLULU – The combination also entails a similar risk of major bleeding, compared with aspirin and clopidogrel alone, according to results from the Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com).
Dual-antiplatelet therapy with aspirin and clopidogrel reduced the rate of recurrent stroke in previous studies. The benefit of this drug combination is relatively short-lived, however, and long-term concomitant use of aspirin and clopidogrel entails a risk of major bleeding. Other data have indicated that cilostazol, which is approved by the Food and Drug Administration to alleviate intermittent claudication in patients with peripheral vascular disease, prevents stroke recurrence without increasing the incidence of serious bleeding, compared with aspirin, said Kazunori Toyoda, MD, PhD, who presented the results of the CSPS.com trial at the International Stroke Conference sponsored by the American Heart Association.
Dr. Toyoda of the National Cerebral and Cardiovascular Center in Osaka, Japan, and his colleagues randomized 1,879 high-risk patients at 8-180 days after the onset of noncardioembolic ischemic stroke identified on MRI to receive 81 or 100 mg aspirin or 50 or 75 mg clopidogrel alone, or a combination of cilostazol 100 mg twice daily with aspirin or clopidogrel. They conducted their open-label, parallel-group trial at 292 sites in Japan from December 2013 through March 2017.
To be considered at high risk, participants had to meet one or more of the following criteria: 50% or greater stenosis of a major intracranial artery, 50% or greater stenosis of an extracranial artery, and two or more vascular risk factors. The trial’s primary efficacy outcome was the first recurrence of ischemic stroke. Safety outcomes included severe or life-threatening bleeding.
The investigators ended the trial early because of a delay in recruiting patients. They enrolled 1,884 and randomized 1,879 of an anticipated 4,000 patients. At randomization, 41% in the dual-therapy group received aspirin and 59% clopidogrel, and in the monotherapy group, 40% received aspirin and 60% clopidogrel. Baseline characteristics were similar between the treatment groups. The population’s mean age was 70. Approximately 30% of patients were women.
During a median follow-up period of 17 months, ischemic stroke recurred in 29 of 932 patients receiving dual therapy including cilostazol for an annual rate of 2.2% and in 64 of 947 patients receiving monotherapy for an annual rate of 4.5% (hazard ratio, 0.49; 95% confidence interval, 0.31-0.76; P = .001). Severe or life-threatening bleeding occurred in 8 patients (0.6% per year) receiving dual therapy and 13 patients (0.9% per year) receiving monotherapy (HR, 0.66; 95% CI, 0.27-1.60; P = .354).
The study was funded by Otsuka Pharmaceutical, which manufactures cilostazol. Dr. Toyoda reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
SOURCE: Toyoda K et al. ISC 2019, Abstract LB3.
HONOLULU – The combination also entails a similar risk of major bleeding, compared with aspirin and clopidogrel alone, according to results from the Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com).
Dual-antiplatelet therapy with aspirin and clopidogrel reduced the rate of recurrent stroke in previous studies. The benefit of this drug combination is relatively short-lived, however, and long-term concomitant use of aspirin and clopidogrel entails a risk of major bleeding. Other data have indicated that cilostazol, which is approved by the Food and Drug Administration to alleviate intermittent claudication in patients with peripheral vascular disease, prevents stroke recurrence without increasing the incidence of serious bleeding, compared with aspirin, said Kazunori Toyoda, MD, PhD, who presented the results of the CSPS.com trial at the International Stroke Conference sponsored by the American Heart Association.
Dr. Toyoda of the National Cerebral and Cardiovascular Center in Osaka, Japan, and his colleagues randomized 1,879 high-risk patients at 8-180 days after the onset of noncardioembolic ischemic stroke identified on MRI to receive 81 or 100 mg aspirin or 50 or 75 mg clopidogrel alone, or a combination of cilostazol 100 mg twice daily with aspirin or clopidogrel. They conducted their open-label, parallel-group trial at 292 sites in Japan from December 2013 through March 2017.
To be considered at high risk, participants had to meet one or more of the following criteria: 50% or greater stenosis of a major intracranial artery, 50% or greater stenosis of an extracranial artery, and two or more vascular risk factors. The trial’s primary efficacy outcome was the first recurrence of ischemic stroke. Safety outcomes included severe or life-threatening bleeding.
The investigators ended the trial early because of a delay in recruiting patients. They enrolled 1,884 and randomized 1,879 of an anticipated 4,000 patients. At randomization, 41% in the dual-therapy group received aspirin and 59% clopidogrel, and in the monotherapy group, 40% received aspirin and 60% clopidogrel. Baseline characteristics were similar between the treatment groups. The population’s mean age was 70. Approximately 30% of patients were women.
During a median follow-up period of 17 months, ischemic stroke recurred in 29 of 932 patients receiving dual therapy including cilostazol for an annual rate of 2.2% and in 64 of 947 patients receiving monotherapy for an annual rate of 4.5% (hazard ratio, 0.49; 95% confidence interval, 0.31-0.76; P = .001). Severe or life-threatening bleeding occurred in 8 patients (0.6% per year) receiving dual therapy and 13 patients (0.9% per year) receiving monotherapy (HR, 0.66; 95% CI, 0.27-1.60; P = .354).
The study was funded by Otsuka Pharmaceutical, which manufactures cilostazol. Dr. Toyoda reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
SOURCE: Toyoda K et al. ISC 2019, Abstract LB3.
HONOLULU – The combination also entails a similar risk of major bleeding, compared with aspirin and clopidogrel alone, according to results from the Cilostazol Stroke Prevention Study for Antiplatelet Combination (CSPS.com).
Dual-antiplatelet therapy with aspirin and clopidogrel reduced the rate of recurrent stroke in previous studies. The benefit of this drug combination is relatively short-lived, however, and long-term concomitant use of aspirin and clopidogrel entails a risk of major bleeding. Other data have indicated that cilostazol, which is approved by the Food and Drug Administration to alleviate intermittent claudication in patients with peripheral vascular disease, prevents stroke recurrence without increasing the incidence of serious bleeding, compared with aspirin, said Kazunori Toyoda, MD, PhD, who presented the results of the CSPS.com trial at the International Stroke Conference sponsored by the American Heart Association.
Dr. Toyoda of the National Cerebral and Cardiovascular Center in Osaka, Japan, and his colleagues randomized 1,879 high-risk patients at 8-180 days after the onset of noncardioembolic ischemic stroke identified on MRI to receive 81 or 100 mg aspirin or 50 or 75 mg clopidogrel alone, or a combination of cilostazol 100 mg twice daily with aspirin or clopidogrel. They conducted their open-label, parallel-group trial at 292 sites in Japan from December 2013 through March 2017.
To be considered at high risk, participants had to meet one or more of the following criteria: 50% or greater stenosis of a major intracranial artery, 50% or greater stenosis of an extracranial artery, and two or more vascular risk factors. The trial’s primary efficacy outcome was the first recurrence of ischemic stroke. Safety outcomes included severe or life-threatening bleeding.
The investigators ended the trial early because of a delay in recruiting patients. They enrolled 1,884 and randomized 1,879 of an anticipated 4,000 patients. At randomization, 41% in the dual-therapy group received aspirin and 59% clopidogrel, and in the monotherapy group, 40% received aspirin and 60% clopidogrel. Baseline characteristics were similar between the treatment groups. The population’s mean age was 70. Approximately 30% of patients were women.
During a median follow-up period of 17 months, ischemic stroke recurred in 29 of 932 patients receiving dual therapy including cilostazol for an annual rate of 2.2% and in 64 of 947 patients receiving monotherapy for an annual rate of 4.5% (hazard ratio, 0.49; 95% confidence interval, 0.31-0.76; P = .001). Severe or life-threatening bleeding occurred in 8 patients (0.6% per year) receiving dual therapy and 13 patients (0.9% per year) receiving monotherapy (HR, 0.66; 95% CI, 0.27-1.60; P = .354).
The study was funded by Otsuka Pharmaceutical, which manufactures cilostazol. Dr. Toyoda reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
SOURCE: Toyoda K et al. ISC 2019, Abstract LB3.
REPORTING FROM ISC
Key clinical point: Treating patients at high risk of recurrent stroke with cilostazol and aspirin or clopidogrel reduced the risk of recurrent stroke more than either aspirin or clopidogrel alone and was just as safe.
Major finding: Dual therapy with cilostazol and aspirin or clopidogrel reduced the risk of recurrent stroke by approximately half, compared with aspirin or clopidogrel alone.
Study details: A multicenter, randomized, open-label, parallel-group trial including 1,879 patients at high risk of recurrent stroke.
Disclosures: Otsuka Pharmaceutical funded the study. The presenter reported receiving support from Bayer Yakuhin, Daiichi Sankyo, Bristol-Myers Squibb, and Nippon Boehringer Ingelheim.
Source: Toyoda K et al. ISC 2019, Abstract LB3.
Intensive insulin added no benefit for hyperglycemia after ischemic stroke
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
SHINE was a well-designed trial that was run with a high degree of rigor, and its results advance the field. The results left no doubt that the result was neutral, that , while resulting in an excess of severe hypoglycemia episodes.
Using a less intensive insulin regimen that does not require a continuous drip is easier. The question of how aggressive treatment needs to be when managing glucose in acute ischemic stroke patients is something that U.S. clinicians who care for stroke patients argue about virtually daily. At my center, Cedars-Sinai in Los Angeles, we have recently used an approach that blended standard insulin treatment with more aggressive treatment. The SHINE results may not be practice changing, but they will be argument changing. The new results will make a difference. We will now stop arguing. We now know what we need to do.
Patrick D. Lyden, MD , is professor and chair of neurology at Cedars-Sinai Medical Center in Los Angeles. He had no relevant disclosures. He made these comments in an interview.
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
HONOLULU – In patients who were hyperglycemic following an acute ischemic stroke, intensive insulin control using a continuous insulin drip and an aggressive blood glucose target of 80-130 mg/dL provided no incremental benefit in clinical outcome, compared with a more standard approach of serial, subcutaneous insulin injections and a moderate blood glucose target in a multicenter, U.S. trial with more than 1,100 patients.
The results also highlighted the potential downside to aggressive insulin treatment, with an associated 2.6% incidence of severe hypoglycemia, defined as blood glucose falling below 40 mg/dL, Karen C. Johnston, MD, said at the International Stroke Conference sponsored by the American Heart Association.
“Our data suggest that subcutaneously administered insulin with a target blood glucose level of less than 180 mg/dL is the preferred treatment” because it produces similar efficacy without causing any episodes of severe hypoglycemia, concluded Dr. Johnston, professor and chair of neurology at the University of Virginia in Charlottesville. “There should be no further debate” over the potential superiority of a glucose target substantially below 180 mg/dL, she added in an interview.
Continuing to use a glucose target of less than 180 mg/dL and treating patients with subcutaneous insulin injections every 6 hours to achieve this will mean substantially less resource use and precludes the need for keeping patients in intensive care beds as is needed with an insulin drip, Dr. Johnston noted. A treatment target of less than 180 mg/dL is also consistent with the most recent American Heart Association stroke treatment guidelines, which listed a blood glucose target of 140-180 mg/dL as a class IIa recommendation (Stroke. 2018 March;49[3]:e66-99).
The SHINE (Stroke Hyperglycemia Insulin Network Effort) trial enrolled 1,151 adults diagnosed with an acute ischemic stroke at 63 U.S. centers during 2012-2018, excluding patients with type 1 diabetes. Patients had to enter the study within 12 hours of their last known well time, and with an elevated blood glucose level, above 110 mg/dL in patients with type 2 diabetes or at or above 150 mg/dL in other patients. The median glucose level of enrolled patients was about 188 mg/dL. Enrolled patients averaged 66 years old, and about 80% had type 2 diabetes. The median time from last known well to randomization was just over 7 hours. Almost two-thirds of the patients received thrombolytic treatment, and about 13% underwent thrombectomy.
During up to 72 hours of treatment following enrollment the patients in the standard-treatment arm showed a fairly steady average blood glucose level of 179 mg/dL; patients in the intensive arm showed a steady average of 118 mg/dL.
The study’s primary end point was the percentage of patients with a favorable outcome 90 days after enrollment based on their modified Rankin scale score at that time, with the scores that qualified for this end point varying depending on stroke severity at baseline. The percentage of patients achieving this was 20.5% among the intensive patients and 21.6% among those who received standard insulin treatment, a difference that was not statistically significant.
The findings left open the question of how to better manage acute ischemic stroke patients who present with hyperglycemia.
“Hyperglycemic stroke patients have worse outcomes than stroke patients without hyperglycemia. More aggressively treating the hyperglycemia did not help these patients, We need to figure out what will help them,” Dr. Johnson said.
SOURCE: Johnston KC et al. ISC 2019, Abstract LB1.
REPORTING FROM ISC 2019
Key clinical point: Aggressive insulin management of hyperglycemia following an ischemic stroke gave no clinical benefit, compared with a standard approach.
Major finding: After 90 days, favorable outcomes occurred in 21% of patients on aggressive insulin treatment and 22% on standard treatment.
Study details: SHINE, a multicenter, randomized trial with 1,151 acute ischemic stroke patients.
Disclosures: SHINE received no commercial funding. Dr. Johnston had no disclosures.
Source: Johnston KC et al. ISC 2019, Abstract LB1.
Statins cut vascular events in elderly patients
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy is often discontinued for older patients who have concomitant disease or other considerations, but it should still be considered in older patients when the benefits outweigh the risks, Bernard M.Y. Cheung, PhD, and Karen S.L. Lam, MD, wrote in a related editorial.
“Even if the relative risk reduction in people older than 75 years is less than expected, statin therapy might still be justified by a high baseline cardiovascular risk, which is usually present in older people,” they said.
One explanation for the decreased relative risk reduction among older patients from the results by Fulcher et al. in the Cholesterol Treatment Trialists’ (CTT) Collaboration trial could have been the inclusion of older patients with cardiac and renal failure, and treating patients with lower cardiac risk or lowering LDL cholesterol in patients at risk of cardiovascular events can help prevent major vascular events later.
Ultimately, no drug is harmless and the risk and benefits must be weighed before making a decision to use statins with older patients just as they would in any other patient population. “The challenge for the health-care profession and the media is to convey risks and benefits in ways that patients can understand, enabling them to make an informed choice,” the authors wrote.
Dr. Cheung and Dr. Lam are from the department of medicine at Queen Mary Hospital, University of Hong Kong in Hong Kong Special Administrative Region, China. They had no relevant disclosures.
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
Statin therapy appears to reduce the risk of major vascular events for patients of all age groups, but there is less evidence that older patients with evidence of occlusive vascular disease benefit from the treatment, according to a recent meta-analysis of 28 trials from the Cholesterol Treatment Trialists’ Collaboration published in The Lancet.
Statins are “useful and affordable drug[s] that reduce heart attacks and strokes in older patients. Until now there has been an evidence gap and we wanted to look at their efficacy and safety in older people,” Jordan Fulcher, BSc (Med), MBBS, from the Cholesterol Treatment Trialists’ (CTT) Collaboration and the University of Sydney stated in a press release. “Our analysis indicates that major cardiovascular events were reduced by about a fifth, per mmol/L lower LDL cholesterol, by statin therapy across all age groups. Despite previous concerns, we found no adverse effect on cancer or nonvascular mortality in any age group.”
The researchers examined 186,854 participants from 28 CTT trials undergoing statin therapy, of whom 14,483 (8%) were older than 75 years. Patients were divided into six groups based on age and examined the risk of major cardiovascular events such as stroke, coronary revascularization and major coronary events, as well as the incidence of cancer and vascular mortality.
Among all age groups, there was a significant reduction in major vascular events, with a 21% proportional per 1.0-mmol/L reduction in LDL cholesterol (risk ratio, 0.79; 95% confidence interval, 0.77-0.81) among patients receiving statin therapy or a more intensive statin regimen, and there was a 24% proportional reduction (RR, 0.76; 95% CI, 0.73-0.79) of major coronary events per 1.0-mmol/L reduction in LDL cholesterol, with older age resulting in a lower proportional reduction of major coronary events (P = .009). The researchers also found a proportional reduction of coronary revascularization procedures by 25% (RR, 0.75; 95% CI, 0.73-0.78) and stroke by 16% (RR, 0.84; 95% CI, 0.80-0.89) among patients of any age group receiving statin therapy or more intensive statin regimen, with no significant differences between age groups.
There was a 12% proportional reduction in vascular mortality per 1.0-mmol/L reduction in LDL cholesterol (RR, 0.88; 95% CI, 0.85-0.91), but this statistic did not remain significant after the researchers excluded four trials that included patients with heart failure or who were receiving renal dialysis. After excluding these trials from the overall analysis, the researchers found the smaller proportional reductions persisted for older patients for major coronary events (P = .01) but was no longer significant for major vascular events.
The researchers noted their study was limited by the highly selected patient population, low percentage of patients older than 75 years, including trials with efficacy endpoints where some nonserious adverse events may not have been recorded, and not including some trials in the meta-analysis if they were not part of the CTT.
This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
SOURCE: Fulcher J et al. Lancet. 2019;393:407-15.
FROM THE LANCET
Key clinical point: but patients older than 75 years with occlusive vascular disease have a smaller reduction in major coronary events.
Major finding: Major vascular coronary events were reduced by 24% (risk ratio, 0.76; 95% confidence interval, 0.73-0.79) with a decrease in the reduction of coronary events among patients older than 75 years. Study details: A meta-analysis of 28 trials with 186,854 individuals undergoing statin therapy from the Cholesterol Treatment Trialists’ Collaboration.
Disclosures: This study was funded by Australian National Health and Medical Research Council, National Institute for Health Research Oxford Biomedical Research Centre, UK Medical Research Council, and British Heart Foundation. The authors have reported personal fees, grants, and consulting fees from Abbott, Aegerion, Amgen, Arisaph, AstraZeneca, Bayer, Beckmann, Berlin-Chemie, Boehringer Ingelheim, Daiichi Sankyo, Dalcor, DuPont, Esperion, GlaxoSmithKline, ISIS Pharmaceuticals, Kowa, Mylan, Pfizer, Roche, Sanofi, Singulex, The Medicines Company, and Vatera Capital, as well as the British Heart Foundation, Cancer Research UK, National Institute for Health Research Oxford Biomedical Research Centre, Medical Research Council, Nuffield Department of Population Health, Weill Cornell Medicine, and UK Biobank.
Source: Fulcher J et al. Lancet. 2019;393:407-15.
AHA report highlights CVD burden, declines in smoking, sleep importance
Almost half of U.S. adults now have some form of cardiovascular disease, according to the latest annual statistical update from the American Heart Association.
The prevalence is driven in part by the recently changed definition of hypertension, from 140/90 to 130/80 mm Hg, said authors of the American Heart Association Heart Disease and Stroke Statistics–2019 Update.
Cardiovascular disease (CVD) deaths are up, though smoking rates continue to decline, and adults are getting more exercise (Circulation. 2019;139. doi: 10.1161/CIR.0000000000000659).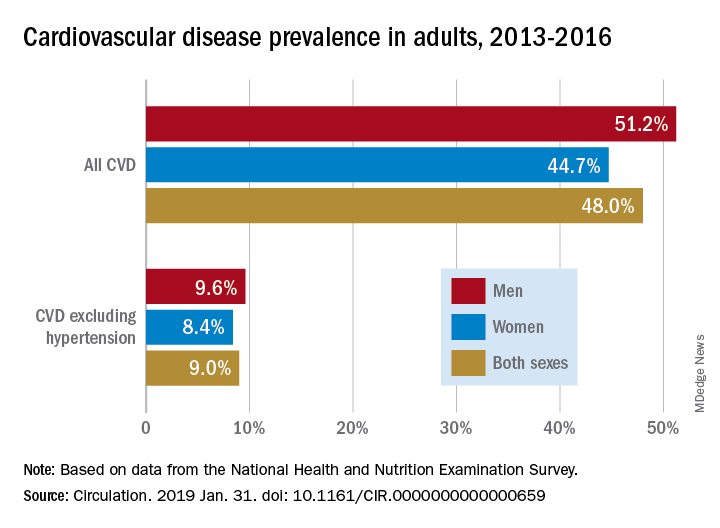
The update includes a new section on sleep and cardiovascular health, an enhanced focus on social determinants of health, and further evidence-based approaches to behavior change, according to the update’s authors, led by chair Emelia J. Benjamin, MD, professor of medicine and epidemiology at Boston University, and vice chair Paul Muntner, PhD, professor of epidemiology at the University of Alabama, Birmingham.
High blood pressure is an “overwhelming presence” that drives heart disease and stroke and can’t be dismissed in the fight against cardiovascular disease, AHA President Ivor J. Benjamin, MD, said in a statement. “Eliminating high blood pressure could have a larger impact on CVD deaths than the elimination of all other risk factors among women, and all except smoking among men.”
Using data from 2013 to 2016, 46% of adults in the United States had hypertension, and in 2016 there were 82,735 deaths attributable primarily to high blood pressure, according to the update.
Total direct costs of hypertension could approach $221 billion by 2035, according to projections in the report.
After decades of decline, U.S. cardiovascular disease deaths increased to 840,678 in 2016, up from 836,546 in 2015, the report says.
Smoking rate declines represent some of the most significant improvements outlined in the report, according to an AHA news release.
Ninety-four percent of adolescents were nonsmokers in the 2015-2016 period, which is up from 76% in 1999-2000, according to the report. The proportion of adult nonsmokers increased to 79% in 2015-2016, up from 73% in 1999-2000.
The new chapter on the importance of sleep cites data from the Centers for Disease Control and Prevention that only 65.2% of Americans have a healthy sleep duration (at least 7 hours), with even lower rates among non-Hispanic blacks, native Hawaiians and Pacific Islanders, and multiracial non-Hispanic individuals.
Short sleep duration is associated with a higher risk of all-cause mortality, total CVD, and coronary heart disease, according to a meta-analysis cited in the report. Long sleep duration, defined as greater than 8 hours, also was associated with higher risk of all-cause mortality, total CVD, coronary heart disease, and stroke.
Members of the statistical update writing group reported disclosures related to the American Heart Association, National Institutes of Health, Amgen, Sanofi, Roche, Abbott, Biogen, Medtronic, and others.
SOURCE: Benjamin EJ et al. Circulation. 2019 Jan 31.
The latest statistics on heart disease and stroke include some metrics that indicate progress, and others that suggest opportunities for improvement.
Tobacco use continues to decline; however, among high school students, e-cigarette use is up to 11.3%, which is concerning.
One bright spot is that the proportion of inactive adults has dropped to 30% in 2016, down from 40% in 2007. Despite that improvement, however, the prevalence of obesity increased significantly over the decade, to the point where nearly 40% of adults are obese and 7.7% are severely obese.
Although 48% of U.S. adults now have cardiovascular disease, according to this latest update, the number drops to just 9% when hypertension is excluded. Even so, 9% represents more than 24.3 million Americans who have coronary artery disease, stroke, or heart failure.
The cost of cardiovascular disease is astronomical, exceeding $351 billion in 2014-1205, with costs projected to increase sharply for older adults over the next few decades.
Starting in 2020, the AHA will begin charting progress in CVD using a metric called health-adjusted life expectancy (HALE), which relies on morbidity and mortality patterns to reflect the number of years a person can expect to live. Patients and the general public may find this metric more understandable than statistics about death rates and cardiovascular risk factors.
Mariell Jessup, MD, is chief science and medical officer for the American Heart Association. Her view on the latest statistical update was derived from a commentary that accompanied the update.
The latest statistics on heart disease and stroke include some metrics that indicate progress, and others that suggest opportunities for improvement.
Tobacco use continues to decline; however, among high school students, e-cigarette use is up to 11.3%, which is concerning.
One bright spot is that the proportion of inactive adults has dropped to 30% in 2016, down from 40% in 2007. Despite that improvement, however, the prevalence of obesity increased significantly over the decade, to the point where nearly 40% of adults are obese and 7.7% are severely obese.
Although 48% of U.S. adults now have cardiovascular disease, according to this latest update, the number drops to just 9% when hypertension is excluded. Even so, 9% represents more than 24.3 million Americans who have coronary artery disease, stroke, or heart failure.
The cost of cardiovascular disease is astronomical, exceeding $351 billion in 2014-1205, with costs projected to increase sharply for older adults over the next few decades.
Starting in 2020, the AHA will begin charting progress in CVD using a metric called health-adjusted life expectancy (HALE), which relies on morbidity and mortality patterns to reflect the number of years a person can expect to live. Patients and the general public may find this metric more understandable than statistics about death rates and cardiovascular risk factors.
Mariell Jessup, MD, is chief science and medical officer for the American Heart Association. Her view on the latest statistical update was derived from a commentary that accompanied the update.
The latest statistics on heart disease and stroke include some metrics that indicate progress, and others that suggest opportunities for improvement.
Tobacco use continues to decline; however, among high school students, e-cigarette use is up to 11.3%, which is concerning.
One bright spot is that the proportion of inactive adults has dropped to 30% in 2016, down from 40% in 2007. Despite that improvement, however, the prevalence of obesity increased significantly over the decade, to the point where nearly 40% of adults are obese and 7.7% are severely obese.
Although 48% of U.S. adults now have cardiovascular disease, according to this latest update, the number drops to just 9% when hypertension is excluded. Even so, 9% represents more than 24.3 million Americans who have coronary artery disease, stroke, or heart failure.
The cost of cardiovascular disease is astronomical, exceeding $351 billion in 2014-1205, with costs projected to increase sharply for older adults over the next few decades.
Starting in 2020, the AHA will begin charting progress in CVD using a metric called health-adjusted life expectancy (HALE), which relies on morbidity and mortality patterns to reflect the number of years a person can expect to live. Patients and the general public may find this metric more understandable than statistics about death rates and cardiovascular risk factors.
Mariell Jessup, MD, is chief science and medical officer for the American Heart Association. Her view on the latest statistical update was derived from a commentary that accompanied the update.
Almost half of U.S. adults now have some form of cardiovascular disease, according to the latest annual statistical update from the American Heart Association.
The prevalence is driven in part by the recently changed definition of hypertension, from 140/90 to 130/80 mm Hg, said authors of the American Heart Association Heart Disease and Stroke Statistics–2019 Update.
Cardiovascular disease (CVD) deaths are up, though smoking rates continue to decline, and adults are getting more exercise (Circulation. 2019;139. doi: 10.1161/CIR.0000000000000659).
The update includes a new section on sleep and cardiovascular health, an enhanced focus on social determinants of health, and further evidence-based approaches to behavior change, according to the update’s authors, led by chair Emelia J. Benjamin, MD, professor of medicine and epidemiology at Boston University, and vice chair Paul Muntner, PhD, professor of epidemiology at the University of Alabama, Birmingham.
High blood pressure is an “overwhelming presence” that drives heart disease and stroke and can’t be dismissed in the fight against cardiovascular disease, AHA President Ivor J. Benjamin, MD, said in a statement. “Eliminating high blood pressure could have a larger impact on CVD deaths than the elimination of all other risk factors among women, and all except smoking among men.”
Using data from 2013 to 2016, 46% of adults in the United States had hypertension, and in 2016 there were 82,735 deaths attributable primarily to high blood pressure, according to the update.
Total direct costs of hypertension could approach $221 billion by 2035, according to projections in the report.
After decades of decline, U.S. cardiovascular disease deaths increased to 840,678 in 2016, up from 836,546 in 2015, the report says.
Smoking rate declines represent some of the most significant improvements outlined in the report, according to an AHA news release.
Ninety-four percent of adolescents were nonsmokers in the 2015-2016 period, which is up from 76% in 1999-2000, according to the report. The proportion of adult nonsmokers increased to 79% in 2015-2016, up from 73% in 1999-2000.
The new chapter on the importance of sleep cites data from the Centers for Disease Control and Prevention that only 65.2% of Americans have a healthy sleep duration (at least 7 hours), with even lower rates among non-Hispanic blacks, native Hawaiians and Pacific Islanders, and multiracial non-Hispanic individuals.
Short sleep duration is associated with a higher risk of all-cause mortality, total CVD, and coronary heart disease, according to a meta-analysis cited in the report. Long sleep duration, defined as greater than 8 hours, also was associated with higher risk of all-cause mortality, total CVD, coronary heart disease, and stroke.
Members of the statistical update writing group reported disclosures related to the American Heart Association, National Institutes of Health, Amgen, Sanofi, Roche, Abbott, Biogen, Medtronic, and others.
SOURCE: Benjamin EJ et al. Circulation. 2019 Jan 31.
Almost half of U.S. adults now have some form of cardiovascular disease, according to the latest annual statistical update from the American Heart Association.
The prevalence is driven in part by the recently changed definition of hypertension, from 140/90 to 130/80 mm Hg, said authors of the American Heart Association Heart Disease and Stroke Statistics–2019 Update.
Cardiovascular disease (CVD) deaths are up, though smoking rates continue to decline, and adults are getting more exercise (Circulation. 2019;139. doi: 10.1161/CIR.0000000000000659).
The update includes a new section on sleep and cardiovascular health, an enhanced focus on social determinants of health, and further evidence-based approaches to behavior change, according to the update’s authors, led by chair Emelia J. Benjamin, MD, professor of medicine and epidemiology at Boston University, and vice chair Paul Muntner, PhD, professor of epidemiology at the University of Alabama, Birmingham.
High blood pressure is an “overwhelming presence” that drives heart disease and stroke and can’t be dismissed in the fight against cardiovascular disease, AHA President Ivor J. Benjamin, MD, said in a statement. “Eliminating high blood pressure could have a larger impact on CVD deaths than the elimination of all other risk factors among women, and all except smoking among men.”
Using data from 2013 to 2016, 46% of adults in the United States had hypertension, and in 2016 there were 82,735 deaths attributable primarily to high blood pressure, according to the update.
Total direct costs of hypertension could approach $221 billion by 2035, according to projections in the report.
After decades of decline, U.S. cardiovascular disease deaths increased to 840,678 in 2016, up from 836,546 in 2015, the report says.
Smoking rate declines represent some of the most significant improvements outlined in the report, according to an AHA news release.
Ninety-four percent of adolescents were nonsmokers in the 2015-2016 period, which is up from 76% in 1999-2000, according to the report. The proportion of adult nonsmokers increased to 79% in 2015-2016, up from 73% in 1999-2000.
The new chapter on the importance of sleep cites data from the Centers for Disease Control and Prevention that only 65.2% of Americans have a healthy sleep duration (at least 7 hours), with even lower rates among non-Hispanic blacks, native Hawaiians and Pacific Islanders, and multiracial non-Hispanic individuals.
Short sleep duration is associated with a higher risk of all-cause mortality, total CVD, and coronary heart disease, according to a meta-analysis cited in the report. Long sleep duration, defined as greater than 8 hours, also was associated with higher risk of all-cause mortality, total CVD, coronary heart disease, and stroke.
Members of the statistical update writing group reported disclosures related to the American Heart Association, National Institutes of Health, Amgen, Sanofi, Roche, Abbott, Biogen, Medtronic, and others.
SOURCE: Benjamin EJ et al. Circulation. 2019 Jan 31.
FROM CIRCULATION
Revised U.S. A fib guidelines revamp anticoagulation
The first update to U.S. medical-society guidelines for managing atrial fibrillation since 2014 raised the threshold for starting anticoagulant therapy in women, pegged the direct-acting oral anticoagulants (DOACs) as preferred over warfarin, and introduced for the first time weight loss as an important intervention tool for treating patients with an atrial arrhythmia.

On January 28, the American College of Cardiology, American Heart Association, and Heart Rhythm Society posted online a 2019 focused update (Circulation. 2019 Jan 28. doi: 10.1161/CIR.0000000000000665) to the 2014 atrial fibrillation (AF) management guidelines that the groups had previously published (J Am Coll Cardiol. 2014 Dec 2;64[21]:2246-80).
Perhaps the two most important changes, as well as the two that lead off the new document, were a pair of class I recommendations on using oral anticoagulation in AF patients.
This brought U.S. guidelines in line with European guidelines, set by the European Society of Cardiology in 2016 (Eur Heart J. 2016 Oct 7;37[38]:2893-962). It will now also mean that, because of the way the CHA2DS2-VASc score is calculated, women with AF who are at least 65 years old will no longer automatically get flagged as needing oral anticoagulant therapy.
“This is a really important shift. It’s recognition that female sex is not as important a risk factor [for AF-associated stroke] as once was thought,” commented Hugh Calkins, MD, professor of medicine at Johns Hopkins Medicine in Baltimore and a member of the panel that wrote the update. “This will change the number of women with AF who go on anticoagulation,” predicted Dr. Calkins, who directs the cardiac arrhythmia service at his center. “We have been struggling with the notion that all women 65 or older with AF had to be on an anticoagulant. Now a clinician has more leeway. In general, patients with AF remain underanticoagulated, but this clarifies practice and brings us in line with the European guidelines.”
The second important change to the anticoagulation recommendations was to specify the DOACs as recommended over warfarin in AF patients eligible for oral anticoagulation and without moderate to severe mitral stenosis or a mechanical heart valve, which also matches the 2016 European guidelines and updates the prior, 2014, U.S. guidelines, which didn’t even mention DOACs.
Prescribing a DOAC preferentially to AF patients has already become routine among electrophysiologists, but possibly not as routine among primary care physicians, so this change has the potential to shift practice, said Dr. Calkins. But the higher price for DOACs, compared with warfarin, can pose problems. “The cost of DOACs remains an issue that can be a serious limitation to some patients,” said Craig T. January, MD, professor of medicine at the University of Wisconsin in Madison and chair of the guideline-writing panel. He also bemoaned the absence of head-to-head comparisons of individual DOACs that could inform selecting among apixaban, dabigatran, edoxaban, and rivaroxaban.
Another notable change in the 2019 update was inclusion for the first time of weight loss as a recommended intervention, along with other risk factor modification, an addition that Dr. Calkins called “long overdue.”

“This is a new recommendation, and it will potentially be important,” said Dr. January, although the guidelines do not spell out how aggressive clinicians should be about having patients achieve weight loss, how much loss patients should achieve, or how they should do it. “There are a lot of observational data and basic science data suggesting the importance of weight loss. Most electrophysiologists already address weight loss. The problem is how to get patients to do it,” commented Vivek Reddy, MD, professor of medicine and director of cardiac arrhythmia services at Mount Sinai Hospital in New York.
Dr. Reddy expressed surprise over two other features of the updated guidelines. For the first time, the guidelines now address percutaneous left atrial appendage (LAA) occlusion and say: “Percutaneous LAA occlusion may be considered in patients with AF at increased risk of stroke who have contraindications to long-term anticoagulation.” The guidelines’ text acknowledges that this runs counter to the Food and Drug Administration labeling for the Watchman LAA occlusion device, which restricts the device to patients “deemed suitable for long-term warfarin (mirroring the inclusion criteria for enrollment in the clinical trials) but had an appropriate rationale to seek a nonpharmacological alternative to warfarin.”
“We do not take a position on the FDA’s” actions, Dr. January said in an interview.
“The ACC, AHA, and HRS guidelines should reflect what the FDA decided,” Dr. Reddy said in an interview. “I’m a little surprised the guidelines said that anticoagulation had to be contraindicated.
The 2019 update also added a class IIb, “may be reasonable” recommendation for catheter ablation of AF in patients with heart failure with reduced ejection fraction.
“I think a IIb recommendation is unfair; I think it should be a IIa recommendation because there have been positive results from two large, randomized, multicenter trials – CASTLE-AF [Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF; N Engl J Med. 2018 Feb 1;378(5):417-27] and AATAC [Ablation vs Amiodarone for Treatment of AFib in Patients With CHF and an ICD; Circulation. 2016 Apr 26;133(7):1637-44], as well as positive results from several smaller randomized studies,” Dr. Reddy said. “I’m really surprised” that the recommendation was not stronger.
Dr. Calkins has been a consultant to Abbott, Altathera, AtriCare, Boehringer-Ingelheim, King, Medtronic, and St. Jude and has received research funding from Boehringer-Ingelheim, Boston Scientific, and St. Jude. Dr. January had no disclosures. Dr. Reddy has been a consultant to, received research funding from, or has an equity interest in more than three dozen companies.
The first update to U.S. medical-society guidelines for managing atrial fibrillation since 2014 raised the threshold for starting anticoagulant therapy in women, pegged the direct-acting oral anticoagulants (DOACs) as preferred over warfarin, and introduced for the first time weight loss as an important intervention tool for treating patients with an atrial arrhythmia.

On January 28, the American College of Cardiology, American Heart Association, and Heart Rhythm Society posted online a 2019 focused update (Circulation. 2019 Jan 28. doi: 10.1161/CIR.0000000000000665) to the 2014 atrial fibrillation (AF) management guidelines that the groups had previously published (J Am Coll Cardiol. 2014 Dec 2;64[21]:2246-80).
Perhaps the two most important changes, as well as the two that lead off the new document, were a pair of class I recommendations on using oral anticoagulation in AF patients.
This brought U.S. guidelines in line with European guidelines, set by the European Society of Cardiology in 2016 (Eur Heart J. 2016 Oct 7;37[38]:2893-962). It will now also mean that, because of the way the CHA2DS2-VASc score is calculated, women with AF who are at least 65 years old will no longer automatically get flagged as needing oral anticoagulant therapy.
“This is a really important shift. It’s recognition that female sex is not as important a risk factor [for AF-associated stroke] as once was thought,” commented Hugh Calkins, MD, professor of medicine at Johns Hopkins Medicine in Baltimore and a member of the panel that wrote the update. “This will change the number of women with AF who go on anticoagulation,” predicted Dr. Calkins, who directs the cardiac arrhythmia service at his center. “We have been struggling with the notion that all women 65 or older with AF had to be on an anticoagulant. Now a clinician has more leeway. In general, patients with AF remain underanticoagulated, but this clarifies practice and brings us in line with the European guidelines.”
The second important change to the anticoagulation recommendations was to specify the DOACs as recommended over warfarin in AF patients eligible for oral anticoagulation and without moderate to severe mitral stenosis or a mechanical heart valve, which also matches the 2016 European guidelines and updates the prior, 2014, U.S. guidelines, which didn’t even mention DOACs.
Prescribing a DOAC preferentially to AF patients has already become routine among electrophysiologists, but possibly not as routine among primary care physicians, so this change has the potential to shift practice, said Dr. Calkins. But the higher price for DOACs, compared with warfarin, can pose problems. “The cost of DOACs remains an issue that can be a serious limitation to some patients,” said Craig T. January, MD, professor of medicine at the University of Wisconsin in Madison and chair of the guideline-writing panel. He also bemoaned the absence of head-to-head comparisons of individual DOACs that could inform selecting among apixaban, dabigatran, edoxaban, and rivaroxaban.
Another notable change in the 2019 update was inclusion for the first time of weight loss as a recommended intervention, along with other risk factor modification, an addition that Dr. Calkins called “long overdue.”

“This is a new recommendation, and it will potentially be important,” said Dr. January, although the guidelines do not spell out how aggressive clinicians should be about having patients achieve weight loss, how much loss patients should achieve, or how they should do it. “There are a lot of observational data and basic science data suggesting the importance of weight loss. Most electrophysiologists already address weight loss. The problem is how to get patients to do it,” commented Vivek Reddy, MD, professor of medicine and director of cardiac arrhythmia services at Mount Sinai Hospital in New York.
Dr. Reddy expressed surprise over two other features of the updated guidelines. For the first time, the guidelines now address percutaneous left atrial appendage (LAA) occlusion and say: “Percutaneous LAA occlusion may be considered in patients with AF at increased risk of stroke who have contraindications to long-term anticoagulation.” The guidelines’ text acknowledges that this runs counter to the Food and Drug Administration labeling for the Watchman LAA occlusion device, which restricts the device to patients “deemed suitable for long-term warfarin (mirroring the inclusion criteria for enrollment in the clinical trials) but had an appropriate rationale to seek a nonpharmacological alternative to warfarin.”
“We do not take a position on the FDA’s” actions, Dr. January said in an interview.
“The ACC, AHA, and HRS guidelines should reflect what the FDA decided,” Dr. Reddy said in an interview. “I’m a little surprised the guidelines said that anticoagulation had to be contraindicated.
The 2019 update also added a class IIb, “may be reasonable” recommendation for catheter ablation of AF in patients with heart failure with reduced ejection fraction.
“I think a IIb recommendation is unfair; I think it should be a IIa recommendation because there have been positive results from two large, randomized, multicenter trials – CASTLE-AF [Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF; N Engl J Med. 2018 Feb 1;378(5):417-27] and AATAC [Ablation vs Amiodarone for Treatment of AFib in Patients With CHF and an ICD; Circulation. 2016 Apr 26;133(7):1637-44], as well as positive results from several smaller randomized studies,” Dr. Reddy said. “I’m really surprised” that the recommendation was not stronger.
Dr. Calkins has been a consultant to Abbott, Altathera, AtriCare, Boehringer-Ingelheim, King, Medtronic, and St. Jude and has received research funding from Boehringer-Ingelheim, Boston Scientific, and St. Jude. Dr. January had no disclosures. Dr. Reddy has been a consultant to, received research funding from, or has an equity interest in more than three dozen companies.
The first update to U.S. medical-society guidelines for managing atrial fibrillation since 2014 raised the threshold for starting anticoagulant therapy in women, pegged the direct-acting oral anticoagulants (DOACs) as preferred over warfarin, and introduced for the first time weight loss as an important intervention tool for treating patients with an atrial arrhythmia.

On January 28, the American College of Cardiology, American Heart Association, and Heart Rhythm Society posted online a 2019 focused update (Circulation. 2019 Jan 28. doi: 10.1161/CIR.0000000000000665) to the 2014 atrial fibrillation (AF) management guidelines that the groups had previously published (J Am Coll Cardiol. 2014 Dec 2;64[21]:2246-80).
Perhaps the two most important changes, as well as the two that lead off the new document, were a pair of class I recommendations on using oral anticoagulation in AF patients.
This brought U.S. guidelines in line with European guidelines, set by the European Society of Cardiology in 2016 (Eur Heart J. 2016 Oct 7;37[38]:2893-962). It will now also mean that, because of the way the CHA2DS2-VASc score is calculated, women with AF who are at least 65 years old will no longer automatically get flagged as needing oral anticoagulant therapy.
“This is a really important shift. It’s recognition that female sex is not as important a risk factor [for AF-associated stroke] as once was thought,” commented Hugh Calkins, MD, professor of medicine at Johns Hopkins Medicine in Baltimore and a member of the panel that wrote the update. “This will change the number of women with AF who go on anticoagulation,” predicted Dr. Calkins, who directs the cardiac arrhythmia service at his center. “We have been struggling with the notion that all women 65 or older with AF had to be on an anticoagulant. Now a clinician has more leeway. In general, patients with AF remain underanticoagulated, but this clarifies practice and brings us in line with the European guidelines.”
The second important change to the anticoagulation recommendations was to specify the DOACs as recommended over warfarin in AF patients eligible for oral anticoagulation and without moderate to severe mitral stenosis or a mechanical heart valve, which also matches the 2016 European guidelines and updates the prior, 2014, U.S. guidelines, which didn’t even mention DOACs.
Prescribing a DOAC preferentially to AF patients has already become routine among electrophysiologists, but possibly not as routine among primary care physicians, so this change has the potential to shift practice, said Dr. Calkins. But the higher price for DOACs, compared with warfarin, can pose problems. “The cost of DOACs remains an issue that can be a serious limitation to some patients,” said Craig T. January, MD, professor of medicine at the University of Wisconsin in Madison and chair of the guideline-writing panel. He also bemoaned the absence of head-to-head comparisons of individual DOACs that could inform selecting among apixaban, dabigatran, edoxaban, and rivaroxaban.
Another notable change in the 2019 update was inclusion for the first time of weight loss as a recommended intervention, along with other risk factor modification, an addition that Dr. Calkins called “long overdue.”

“This is a new recommendation, and it will potentially be important,” said Dr. January, although the guidelines do not spell out how aggressive clinicians should be about having patients achieve weight loss, how much loss patients should achieve, or how they should do it. “There are a lot of observational data and basic science data suggesting the importance of weight loss. Most electrophysiologists already address weight loss. The problem is how to get patients to do it,” commented Vivek Reddy, MD, professor of medicine and director of cardiac arrhythmia services at Mount Sinai Hospital in New York.
Dr. Reddy expressed surprise over two other features of the updated guidelines. For the first time, the guidelines now address percutaneous left atrial appendage (LAA) occlusion and say: “Percutaneous LAA occlusion may be considered in patients with AF at increased risk of stroke who have contraindications to long-term anticoagulation.” The guidelines’ text acknowledges that this runs counter to the Food and Drug Administration labeling for the Watchman LAA occlusion device, which restricts the device to patients “deemed suitable for long-term warfarin (mirroring the inclusion criteria for enrollment in the clinical trials) but had an appropriate rationale to seek a nonpharmacological alternative to warfarin.”
“We do not take a position on the FDA’s” actions, Dr. January said in an interview.
“The ACC, AHA, and HRS guidelines should reflect what the FDA decided,” Dr. Reddy said in an interview. “I’m a little surprised the guidelines said that anticoagulation had to be contraindicated.
The 2019 update also added a class IIb, “may be reasonable” recommendation for catheter ablation of AF in patients with heart failure with reduced ejection fraction.
“I think a IIb recommendation is unfair; I think it should be a IIa recommendation because there have been positive results from two large, randomized, multicenter trials – CASTLE-AF [Catheter Ablation vs. Standard Conventional Treatment in Patients With LV Dysfunction and AF; N Engl J Med. 2018 Feb 1;378(5):417-27] and AATAC [Ablation vs Amiodarone for Treatment of AFib in Patients With CHF and an ICD; Circulation. 2016 Apr 26;133(7):1637-44], as well as positive results from several smaller randomized studies,” Dr. Reddy said. “I’m really surprised” that the recommendation was not stronger.
Dr. Calkins has been a consultant to Abbott, Altathera, AtriCare, Boehringer-Ingelheim, King, Medtronic, and St. Jude and has received research funding from Boehringer-Ingelheim, Boston Scientific, and St. Jude. Dr. January had no disclosures. Dr. Reddy has been a consultant to, received research funding from, or has an equity interest in more than three dozen companies.
SPRINT MIND published: Extension trial to add 2 years’ follow-up
A new iteration of the SPRINT MIND hypertension trial will seek to prove conclusively the original study’s tantalizing suggestion: that intensive blood pressure control decreases the risk of developing mild cognitive impairment (MCI) and, eventually, dementia.
SPRINT MIND 2.0 will re-recruit SPRINT MIND subjects and enable another follow-up cognitive assessment and other clinical tests as they remain on their standard of care blood pressure regimen. It is largely funded by an $800,000 grant from the Alzheimer’s Association.
Initially released last July at the Alzheimer’s Association International Conference, the results of the SPRINT MIND have now appeared online in JAMA. Although it failed to meet its primary endpoint of reducing dementia incidence, the study did score on two secondary endpoints. Patients who reduced their systolic blood pressure to less than 120 mm Hg were 19% less likely to develop MCI and 17% less likely to be diagnosed with all-cause dementia than were those who achieved a hypertension target of less than 140 mm Hg.
The secondary results, and positive movement in the primary results, sparked excitement in the dementia research community last summer. They have suggested that the median 5-year follow-up just wasn’t long enough to show any significant effects on dementia, which can take years to fully manifest. Adding 2 more years with SPRINT MIND 2.0 should be long enough to discern those benefits, if indeed they exist.
“SPRINT MIND 2.0 and the work leading up to it offers genuine, concrete hope,” Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said in a press statement. “MCI is a known risk factor for dementia, and everyone who experiences dementia passes through MCI. When you prevent new cases of MCI, you are preventing new cases of dementia. The Alzheimer’s Association finds these data to be compelling and is committed to getting clarity and certainty on the dementia outcome by following participants for a longer period of time.”
The study strengthens the new and energetic push to find ways to prevent dementia, which has proven itself intractable in every drug study to date.
“This study is in line with where the field of dementia research is going: preventing memory loss earlier,” said Laurie Ryan, PhD, chief of the dementias of aging branch in the National Institute on Aging. “Much like we have research-based interventions for heart health and cancer prevention, we hope to have guidance based on this and subsequent studies that will more definitively show how to slow or even stop dementia well before symptoms appear.”
NIA director Richard J. Hodes, MD, agreed.
“Dementia continues to be a large public health challenge, and based on the primary results of this study, we still have yet to find an intervention strategy proven to reduce the risk of dementia,” he said in a press statement. “Nevertheless, the secondary results showing that intensive lowering of blood pressure may reduce risk for MCI, a known risk factor for dementia, gives us additional avenues to explore on the path to prevention.”
SPRINT MIND was a substudy of the Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target of less than 120 mm Hg, while standard care had a target of less than 140 mm Hg. SPRINT showed that more intensive blood pressure control produced a 25% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death. The intensive arm was so successful that SPRINT helped inform the 2017 high blood pressure clinical guidelines from the American Heart Association and American College of Cardiology.
The SPRINT MIND substudy, headed by Jeff D. Williamson, MD, of Wake Forest University, Winston-Salem, NC, asked whether intensive management had any effect on probable all-cause dementia or MCI, as well as imaging evidence of changes in white matter lesions and brain volume. It followed patients for up to 7 years and comprised 9,361 SPRINT subjects at least 50 years old (mean, 68 years) with at least one cardiovascular risk factor. Nearly a third (30%) were black, and 10% Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and/or probable dementia. About a third had a SBP of 132 mm Hg or less, another third had a systolic pressure of 132-145 mm Hg, and the remainder had a systolic pressure greater than 145 mm Hg.
Physicians could use their choice of antihypertensive treatments. The study protocol encouraged, but did not mandate, thiazide-type diuretics as a first-line agent, followed by loop diuretics and beta-adrenergic blockers. Chlorthalidone was encouraged as the primary thiazide-type diuretic, and amlodipine as the preferred calcium-channel blocker.
The interventions did successfully control blood pressure, with a significant difference between the treatment groups. The mean SBP was 121.6 mm Hg in the intensive therapy group and 134.8 mm Hg in the standard group – a statistically significant difference of 13.3 mm Hg.
Dementia developed in 149 in the aggressive control group and 176 in the standard group – a nonsignificant difference of 17% (hazard ratio, 0.83). MCI developed in 287 in the intensive group and 353 in the standard treatment group. This amounted to a statistically significant 19% reduction. There was also a significant 15% reduction in the composite outcome of MCI or probable dementia in favor of intensive treatment.
As evidenced by the Alzheimer’s Association grant, dementia researchers chose to focus on SPRINT MIND’s positive secondary endpoints. At the AAIC meeting, Dr. Williamson even suggested that antihypertensive medications could be seen as disease-modifying agents for cognitive decline. Data support his claim: No dementia intervention yet tested has approached this level of success.
“I think we can say this is the first disease-modifying strategy to reduce the risk of MCI,” Dr. Williamson said during a press briefing. And although the primary endpoint – the 17% relative risk reduction for probable all-cause dementia – didn’t meet statistical significance, “It’s comforting to see that the benefit went in the same direction and was of the same magnitude..”
SOURCE: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
SPRINT MIND offers hope that a very achievable blood pressure goal can dramatically alter the trajectory from mild cognitive impairment to dementia, Kristine Yaffe, MD, wrote in an accompanying editorial. But at this point, it’s impossible to make specific clinical recommendations.
Additionally it is not possible, right now, to know which hypertension treatment regimens were most effective in improved cognitive outcomes.
“Information necessary to compare the effects of classes of antihypertensive agents on cognitive outcomes is also not provided. SPRINT used a quasi-pragmatic approach with suggestions for treatment choice, but practitioners approached SBP control individually, and most participants were taking multiple drugs.”
Nevertheless, the positive secondary findings and the encouraging trajectory on dementia risk should fix blood pressure management squarely into a cornerstone of dementia prevention algorithms.
“The SPRINT MIND study may not be the final approach for prevention of AD or other cognitive impairment, but it represents a major leap forward in what has emerged as a marathon journey.”
Dr. Kristine Yaffe is professor of psychiatry, neurology and epidemiology and the Roy and Marie Scola Endowed Chair at the University of California, San Francisco.
SPRINT MIND offers hope that a very achievable blood pressure goal can dramatically alter the trajectory from mild cognitive impairment to dementia, Kristine Yaffe, MD, wrote in an accompanying editorial. But at this point, it’s impossible to make specific clinical recommendations.
Additionally it is not possible, right now, to know which hypertension treatment regimens were most effective in improved cognitive outcomes.
“Information necessary to compare the effects of classes of antihypertensive agents on cognitive outcomes is also not provided. SPRINT used a quasi-pragmatic approach with suggestions for treatment choice, but practitioners approached SBP control individually, and most participants were taking multiple drugs.”
Nevertheless, the positive secondary findings and the encouraging trajectory on dementia risk should fix blood pressure management squarely into a cornerstone of dementia prevention algorithms.
“The SPRINT MIND study may not be the final approach for prevention of AD or other cognitive impairment, but it represents a major leap forward in what has emerged as a marathon journey.”
Dr. Kristine Yaffe is professor of psychiatry, neurology and epidemiology and the Roy and Marie Scola Endowed Chair at the University of California, San Francisco.
SPRINT MIND offers hope that a very achievable blood pressure goal can dramatically alter the trajectory from mild cognitive impairment to dementia, Kristine Yaffe, MD, wrote in an accompanying editorial. But at this point, it’s impossible to make specific clinical recommendations.
Additionally it is not possible, right now, to know which hypertension treatment regimens were most effective in improved cognitive outcomes.
“Information necessary to compare the effects of classes of antihypertensive agents on cognitive outcomes is also not provided. SPRINT used a quasi-pragmatic approach with suggestions for treatment choice, but practitioners approached SBP control individually, and most participants were taking multiple drugs.”
Nevertheless, the positive secondary findings and the encouraging trajectory on dementia risk should fix blood pressure management squarely into a cornerstone of dementia prevention algorithms.
“The SPRINT MIND study may not be the final approach for prevention of AD or other cognitive impairment, but it represents a major leap forward in what has emerged as a marathon journey.”
Dr. Kristine Yaffe is professor of psychiatry, neurology and epidemiology and the Roy and Marie Scola Endowed Chair at the University of California, San Francisco.
A new iteration of the SPRINT MIND hypertension trial will seek to prove conclusively the original study’s tantalizing suggestion: that intensive blood pressure control decreases the risk of developing mild cognitive impairment (MCI) and, eventually, dementia.
SPRINT MIND 2.0 will re-recruit SPRINT MIND subjects and enable another follow-up cognitive assessment and other clinical tests as they remain on their standard of care blood pressure regimen. It is largely funded by an $800,000 grant from the Alzheimer’s Association.
Initially released last July at the Alzheimer’s Association International Conference, the results of the SPRINT MIND have now appeared online in JAMA. Although it failed to meet its primary endpoint of reducing dementia incidence, the study did score on two secondary endpoints. Patients who reduced their systolic blood pressure to less than 120 mm Hg were 19% less likely to develop MCI and 17% less likely to be diagnosed with all-cause dementia than were those who achieved a hypertension target of less than 140 mm Hg.
The secondary results, and positive movement in the primary results, sparked excitement in the dementia research community last summer. They have suggested that the median 5-year follow-up just wasn’t long enough to show any significant effects on dementia, which can take years to fully manifest. Adding 2 more years with SPRINT MIND 2.0 should be long enough to discern those benefits, if indeed they exist.
“SPRINT MIND 2.0 and the work leading up to it offers genuine, concrete hope,” Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said in a press statement. “MCI is a known risk factor for dementia, and everyone who experiences dementia passes through MCI. When you prevent new cases of MCI, you are preventing new cases of dementia. The Alzheimer’s Association finds these data to be compelling and is committed to getting clarity and certainty on the dementia outcome by following participants for a longer period of time.”
The study strengthens the new and energetic push to find ways to prevent dementia, which has proven itself intractable in every drug study to date.
“This study is in line with where the field of dementia research is going: preventing memory loss earlier,” said Laurie Ryan, PhD, chief of the dementias of aging branch in the National Institute on Aging. “Much like we have research-based interventions for heart health and cancer prevention, we hope to have guidance based on this and subsequent studies that will more definitively show how to slow or even stop dementia well before symptoms appear.”
NIA director Richard J. Hodes, MD, agreed.
“Dementia continues to be a large public health challenge, and based on the primary results of this study, we still have yet to find an intervention strategy proven to reduce the risk of dementia,” he said in a press statement. “Nevertheless, the secondary results showing that intensive lowering of blood pressure may reduce risk for MCI, a known risk factor for dementia, gives us additional avenues to explore on the path to prevention.”
SPRINT MIND was a substudy of the Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target of less than 120 mm Hg, while standard care had a target of less than 140 mm Hg. SPRINT showed that more intensive blood pressure control produced a 25% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death. The intensive arm was so successful that SPRINT helped inform the 2017 high blood pressure clinical guidelines from the American Heart Association and American College of Cardiology.
The SPRINT MIND substudy, headed by Jeff D. Williamson, MD, of Wake Forest University, Winston-Salem, NC, asked whether intensive management had any effect on probable all-cause dementia or MCI, as well as imaging evidence of changes in white matter lesions and brain volume. It followed patients for up to 7 years and comprised 9,361 SPRINT subjects at least 50 years old (mean, 68 years) with at least one cardiovascular risk factor. Nearly a third (30%) were black, and 10% Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and/or probable dementia. About a third had a SBP of 132 mm Hg or less, another third had a systolic pressure of 132-145 mm Hg, and the remainder had a systolic pressure greater than 145 mm Hg.
Physicians could use their choice of antihypertensive treatments. The study protocol encouraged, but did not mandate, thiazide-type diuretics as a first-line agent, followed by loop diuretics and beta-adrenergic blockers. Chlorthalidone was encouraged as the primary thiazide-type diuretic, and amlodipine as the preferred calcium-channel blocker.
The interventions did successfully control blood pressure, with a significant difference between the treatment groups. The mean SBP was 121.6 mm Hg in the intensive therapy group and 134.8 mm Hg in the standard group – a statistically significant difference of 13.3 mm Hg.
Dementia developed in 149 in the aggressive control group and 176 in the standard group – a nonsignificant difference of 17% (hazard ratio, 0.83). MCI developed in 287 in the intensive group and 353 in the standard treatment group. This amounted to a statistically significant 19% reduction. There was also a significant 15% reduction in the composite outcome of MCI or probable dementia in favor of intensive treatment.
As evidenced by the Alzheimer’s Association grant, dementia researchers chose to focus on SPRINT MIND’s positive secondary endpoints. At the AAIC meeting, Dr. Williamson even suggested that antihypertensive medications could be seen as disease-modifying agents for cognitive decline. Data support his claim: No dementia intervention yet tested has approached this level of success.
“I think we can say this is the first disease-modifying strategy to reduce the risk of MCI,” Dr. Williamson said during a press briefing. And although the primary endpoint – the 17% relative risk reduction for probable all-cause dementia – didn’t meet statistical significance, “It’s comforting to see that the benefit went in the same direction and was of the same magnitude..”
SOURCE: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
A new iteration of the SPRINT MIND hypertension trial will seek to prove conclusively the original study’s tantalizing suggestion: that intensive blood pressure control decreases the risk of developing mild cognitive impairment (MCI) and, eventually, dementia.
SPRINT MIND 2.0 will re-recruit SPRINT MIND subjects and enable another follow-up cognitive assessment and other clinical tests as they remain on their standard of care blood pressure regimen. It is largely funded by an $800,000 grant from the Alzheimer’s Association.
Initially released last July at the Alzheimer’s Association International Conference, the results of the SPRINT MIND have now appeared online in JAMA. Although it failed to meet its primary endpoint of reducing dementia incidence, the study did score on two secondary endpoints. Patients who reduced their systolic blood pressure to less than 120 mm Hg were 19% less likely to develop MCI and 17% less likely to be diagnosed with all-cause dementia than were those who achieved a hypertension target of less than 140 mm Hg.
The secondary results, and positive movement in the primary results, sparked excitement in the dementia research community last summer. They have suggested that the median 5-year follow-up just wasn’t long enough to show any significant effects on dementia, which can take years to fully manifest. Adding 2 more years with SPRINT MIND 2.0 should be long enough to discern those benefits, if indeed they exist.
“SPRINT MIND 2.0 and the work leading up to it offers genuine, concrete hope,” Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, said in a press statement. “MCI is a known risk factor for dementia, and everyone who experiences dementia passes through MCI. When you prevent new cases of MCI, you are preventing new cases of dementia. The Alzheimer’s Association finds these data to be compelling and is committed to getting clarity and certainty on the dementia outcome by following participants for a longer period of time.”
The study strengthens the new and energetic push to find ways to prevent dementia, which has proven itself intractable in every drug study to date.
“This study is in line with where the field of dementia research is going: preventing memory loss earlier,” said Laurie Ryan, PhD, chief of the dementias of aging branch in the National Institute on Aging. “Much like we have research-based interventions for heart health and cancer prevention, we hope to have guidance based on this and subsequent studies that will more definitively show how to slow or even stop dementia well before symptoms appear.”
NIA director Richard J. Hodes, MD, agreed.
“Dementia continues to be a large public health challenge, and based on the primary results of this study, we still have yet to find an intervention strategy proven to reduce the risk of dementia,” he said in a press statement. “Nevertheless, the secondary results showing that intensive lowering of blood pressure may reduce risk for MCI, a known risk factor for dementia, gives us additional avenues to explore on the path to prevention.”
SPRINT MIND was a substudy of the Systolic Blood Pressure Intervention Trial (SPRINT). It compared two strategies for managing hypertension in older adults. The intensive strategy had a target of less than 120 mm Hg, while standard care had a target of less than 140 mm Hg. SPRINT showed that more intensive blood pressure control produced a 25% reduction in the composite primary composite endpoint of cardiovascular events, stroke, and cardiovascular death. The intensive arm was so successful that SPRINT helped inform the 2017 high blood pressure clinical guidelines from the American Heart Association and American College of Cardiology.
The SPRINT MIND substudy, headed by Jeff D. Williamson, MD, of Wake Forest University, Winston-Salem, NC, asked whether intensive management had any effect on probable all-cause dementia or MCI, as well as imaging evidence of changes in white matter lesions and brain volume. It followed patients for up to 7 years and comprised 9,361 SPRINT subjects at least 50 years old (mean, 68 years) with at least one cardiovascular risk factor. Nearly a third (30%) were black, and 10% Hispanic. The primary outcome was incident probable dementia. Secondary outcomes were MCI and a composite of MCI and/or probable dementia. About a third had a SBP of 132 mm Hg or less, another third had a systolic pressure of 132-145 mm Hg, and the remainder had a systolic pressure greater than 145 mm Hg.
Physicians could use their choice of antihypertensive treatments. The study protocol encouraged, but did not mandate, thiazide-type diuretics as a first-line agent, followed by loop diuretics and beta-adrenergic blockers. Chlorthalidone was encouraged as the primary thiazide-type diuretic, and amlodipine as the preferred calcium-channel blocker.
The interventions did successfully control blood pressure, with a significant difference between the treatment groups. The mean SBP was 121.6 mm Hg in the intensive therapy group and 134.8 mm Hg in the standard group – a statistically significant difference of 13.3 mm Hg.
Dementia developed in 149 in the aggressive control group and 176 in the standard group – a nonsignificant difference of 17% (hazard ratio, 0.83). MCI developed in 287 in the intensive group and 353 in the standard treatment group. This amounted to a statistically significant 19% reduction. There was also a significant 15% reduction in the composite outcome of MCI or probable dementia in favor of intensive treatment.
As evidenced by the Alzheimer’s Association grant, dementia researchers chose to focus on SPRINT MIND’s positive secondary endpoints. At the AAIC meeting, Dr. Williamson even suggested that antihypertensive medications could be seen as disease-modifying agents for cognitive decline. Data support his claim: No dementia intervention yet tested has approached this level of success.
“I think we can say this is the first disease-modifying strategy to reduce the risk of MCI,” Dr. Williamson said during a press briefing. And although the primary endpoint – the 17% relative risk reduction for probable all-cause dementia – didn’t meet statistical significance, “It’s comforting to see that the benefit went in the same direction and was of the same magnitude..”
SOURCE: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
FROM JAMA
Key clinical point: Keeping systolic blood pressure lower than 120 mm Hg did not significantly reduce the risk of all-cause dementia in patients with hypertension, but it did lower the risk of mild cognitive impairment and probable dementia.
Major finding: The intensively treated group had a nonsignificant 17% lower risk of dementia, and significant reductions in the risk of MCI (19%) and probable dementia (15%).
Study details: SPRINT MIND was a substudy of the SPRINT antihypertension trial.
Source: Williamson JD et al. JAMA 2019 Jan 28. doi:10.1001/jama.2018.21442.
Benefit of thrombectomy may be universal
The location of the arterial occlusive lesion and the imaging technique used to select patients for the procedure also do not influence the therapy’s benefits, the researchers said. Although the proportional benefit of thrombectomy plus medical management is uniform across subgroups, compared with medical management alone, patients may have different amounts of absolute benefit.
The results of the DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) trial, which were published in 2018, indicated that endovascular thrombectomy provided clinical benefits for patients with acute ischemic stroke if administered at 6-16 hours after stroke onset. As part of the trial’s prespecified analyses, Maarten G. Lansberg, MD, PhD, associate professor of neurology and neurological sciences at Stanford (Calif.) University Medical Center in California, and his colleagues sought to determine whether thrombectomy had uniform benefit among various patient subgroups (e.g., elderly people, patients with mild symptoms, and those who present late after onset).
A total of 296 patients were enrolled in the randomized, open-label, blinded-endpoint DEFUSE 3 trial at 38 sites in the United States. Eligible participants had acute ischemic stroke resulting from an occlusion of the internal carotid artery or middle cerebral artery and evidence of salvageable tissue on perfusion CT or MRI. In all, 182 patients met these criteria and were randomized and included in the intention-to-treat analysis. The researchers stopped DEFUSE 3 early because of efficacy.
The study’s primary endpoint was functional outcome at day 90, as measured with the modified Rankin Scale. Dr. Lansberg and his colleagues performed multivariate ordinal logistic regression to calculate the adjusted proportional association between endovascular treatment and clinical outcome among participants of various ages, baseline stroke severities, periods between onset and treatment, locations of the arterial occlusion, and imaging modalities, such as CT or MRI, used to identify salvageable tissue.
The population’s median age was 70 years, and 51% of participants were women. The median National Institutes of Health Stroke Scale score was 16. When the researchers considered the whole sample, they found that younger age, lower baseline NIHSS score, and lower serum glucose level independently predicted better functional outcome. The common odds ratio for improved functional outcome with endovascular therapy, adjusted for these variables, was 3.1. Age, NIHSS score, time to randomization, imaging modality, and location of the arterial occlusion did not interact significantly with treatment effect.
“Our results indicate that advanced age, up to 90 years, should not be considered a contraindication to thrombectomy, provided that the patient is fully independent prior to stroke onset,” said the researchers. “Although age did not modify the treatment effect, it was a strong independent predictor of 90-day disability, which is consistent with prior studies of both tissue plasminogen activator and endovascular therapy.”
The trial’s small sample size may have allowed small differences between groups to pass unnoticed, said the reseachers. Other trials of late-window thrombectomy will be required to validate these results, they concluded.
The National Institute for Neurological Disorders and Stroke supported the study through grants. Several investigators received consulting fees from and hold shares in iSchemaView, which manufactures the software that the investigators used for postprocessing of CT and MRI perfusion studies. Other authors received consulting fees from various pharmaceutical and medical device companies, including Genentech, Medtronic, Pfizer, and Stryker Neurovascular.
SOURCE: Lansberg MG et al. JAMA Neurol. 2019 Jan 28. doi: 10.1001/jamaneurol.2018.4587.
The location of the arterial occlusive lesion and the imaging technique used to select patients for the procedure also do not influence the therapy’s benefits, the researchers said. Although the proportional benefit of thrombectomy plus medical management is uniform across subgroups, compared with medical management alone, patients may have different amounts of absolute benefit.
The results of the DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) trial, which were published in 2018, indicated that endovascular thrombectomy provided clinical benefits for patients with acute ischemic stroke if administered at 6-16 hours after stroke onset. As part of the trial’s prespecified analyses, Maarten G. Lansberg, MD, PhD, associate professor of neurology and neurological sciences at Stanford (Calif.) University Medical Center in California, and his colleagues sought to determine whether thrombectomy had uniform benefit among various patient subgroups (e.g., elderly people, patients with mild symptoms, and those who present late after onset).
A total of 296 patients were enrolled in the randomized, open-label, blinded-endpoint DEFUSE 3 trial at 38 sites in the United States. Eligible participants had acute ischemic stroke resulting from an occlusion of the internal carotid artery or middle cerebral artery and evidence of salvageable tissue on perfusion CT or MRI. In all, 182 patients met these criteria and were randomized and included in the intention-to-treat analysis. The researchers stopped DEFUSE 3 early because of efficacy.
The study’s primary endpoint was functional outcome at day 90, as measured with the modified Rankin Scale. Dr. Lansberg and his colleagues performed multivariate ordinal logistic regression to calculate the adjusted proportional association between endovascular treatment and clinical outcome among participants of various ages, baseline stroke severities, periods between onset and treatment, locations of the arterial occlusion, and imaging modalities, such as CT or MRI, used to identify salvageable tissue.
The population’s median age was 70 years, and 51% of participants were women. The median National Institutes of Health Stroke Scale score was 16. When the researchers considered the whole sample, they found that younger age, lower baseline NIHSS score, and lower serum glucose level independently predicted better functional outcome. The common odds ratio for improved functional outcome with endovascular therapy, adjusted for these variables, was 3.1. Age, NIHSS score, time to randomization, imaging modality, and location of the arterial occlusion did not interact significantly with treatment effect.
“Our results indicate that advanced age, up to 90 years, should not be considered a contraindication to thrombectomy, provided that the patient is fully independent prior to stroke onset,” said the researchers. “Although age did not modify the treatment effect, it was a strong independent predictor of 90-day disability, which is consistent with prior studies of both tissue plasminogen activator and endovascular therapy.”
The trial’s small sample size may have allowed small differences between groups to pass unnoticed, said the reseachers. Other trials of late-window thrombectomy will be required to validate these results, they concluded.
The National Institute for Neurological Disorders and Stroke supported the study through grants. Several investigators received consulting fees from and hold shares in iSchemaView, which manufactures the software that the investigators used for postprocessing of CT and MRI perfusion studies. Other authors received consulting fees from various pharmaceutical and medical device companies, including Genentech, Medtronic, Pfizer, and Stryker Neurovascular.
SOURCE: Lansberg MG et al. JAMA Neurol. 2019 Jan 28. doi: 10.1001/jamaneurol.2018.4587.
The location of the arterial occlusive lesion and the imaging technique used to select patients for the procedure also do not influence the therapy’s benefits, the researchers said. Although the proportional benefit of thrombectomy plus medical management is uniform across subgroups, compared with medical management alone, patients may have different amounts of absolute benefit.
The results of the DEFUSE 3 (Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke) trial, which were published in 2018, indicated that endovascular thrombectomy provided clinical benefits for patients with acute ischemic stroke if administered at 6-16 hours after stroke onset. As part of the trial’s prespecified analyses, Maarten G. Lansberg, MD, PhD, associate professor of neurology and neurological sciences at Stanford (Calif.) University Medical Center in California, and his colleagues sought to determine whether thrombectomy had uniform benefit among various patient subgroups (e.g., elderly people, patients with mild symptoms, and those who present late after onset).
A total of 296 patients were enrolled in the randomized, open-label, blinded-endpoint DEFUSE 3 trial at 38 sites in the United States. Eligible participants had acute ischemic stroke resulting from an occlusion of the internal carotid artery or middle cerebral artery and evidence of salvageable tissue on perfusion CT or MRI. In all, 182 patients met these criteria and were randomized and included in the intention-to-treat analysis. The researchers stopped DEFUSE 3 early because of efficacy.
The study’s primary endpoint was functional outcome at day 90, as measured with the modified Rankin Scale. Dr. Lansberg and his colleagues performed multivariate ordinal logistic regression to calculate the adjusted proportional association between endovascular treatment and clinical outcome among participants of various ages, baseline stroke severities, periods between onset and treatment, locations of the arterial occlusion, and imaging modalities, such as CT or MRI, used to identify salvageable tissue.
The population’s median age was 70 years, and 51% of participants were women. The median National Institutes of Health Stroke Scale score was 16. When the researchers considered the whole sample, they found that younger age, lower baseline NIHSS score, and lower serum glucose level independently predicted better functional outcome. The common odds ratio for improved functional outcome with endovascular therapy, adjusted for these variables, was 3.1. Age, NIHSS score, time to randomization, imaging modality, and location of the arterial occlusion did not interact significantly with treatment effect.
“Our results indicate that advanced age, up to 90 years, should not be considered a contraindication to thrombectomy, provided that the patient is fully independent prior to stroke onset,” said the researchers. “Although age did not modify the treatment effect, it was a strong independent predictor of 90-day disability, which is consistent with prior studies of both tissue plasminogen activator and endovascular therapy.”
The trial’s small sample size may have allowed small differences between groups to pass unnoticed, said the reseachers. Other trials of late-window thrombectomy will be required to validate these results, they concluded.
The National Institute for Neurological Disorders and Stroke supported the study through grants. Several investigators received consulting fees from and hold shares in iSchemaView, which manufactures the software that the investigators used for postprocessing of CT and MRI perfusion studies. Other authors received consulting fees from various pharmaceutical and medical device companies, including Genentech, Medtronic, Pfizer, and Stryker Neurovascular.
SOURCE: Lansberg MG et al. JAMA Neurol. 2019 Jan 28. doi: 10.1001/jamaneurol.2018.4587.
FROM JAMA NEUROLOGY
Key clinical point: Age, symptom severity, and serum glucose do not influence the benefit of thrombectomy for acute ischemic stroke.
Major finding: The adjusted common odds ratio for improved functional outcome with endovascular therapy was 3.1.
Study details: The randomized, open-label, blinded-end-point DEFUSE 3 trial included 182 patients.
Disclosures: The National Institute for Neurological Disorders and Stroke funded the study through grants.
Source: Lansberg MG et al. JAMA Neurol. 2019 Jan 28. doi: 10.1001/jamaneurol.2018.4587.
Before you refer for AF ablation
SNOWMASS, COLO. – Appropriate in the way of benefit, along with instilling awareness of the warning signals heralding serious late complications, Samuel J. Asirvatham, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Who to steer toward ablation? You have to have a symptomatic patient – that’s a given. For the ones who are paroxysmal, the ones with a relatively normal heart, there’s a much better chance that you’ll help manage their symptoms with ablation than if they have persistent or permanent A-fib. Notice I do not use the word ‘cure’ for A-fib. We talk about controlling symptoms and decreasing frequency, because the longer follow-up you have with intensive monitoring, the more you realize that patients still tend to have some A-fib,” explained Dr. Asirvatham, an electrophysiologist who is professor of medicine and pediatrics at the Mayo Clinic in Rochester, Minn.
The rationale for early atrial fibrillation (AF) ablation in younger patients with troublesome symptoms of paroxysmal AF despite pharmacologic attempts at rate or rhythm control is that it will arrest the progression from an atrial arrhythmia that has just a few triggers readily neutralized by pulmonary vein isolation to persistent AF with a diseased heart and a multitude of arrhythmia trigger points coming from many directions.
A solid candidate for ablation of paroxysmal AF has about a 75% likelihood of having a successful first ablation procedure, with substantial improvement in symptoms and no need for medication. Another 9%-10% will achieve marked reduction in symptom burden upon addition of antiarrhythmic agents that weren’t effective before ablation.
Late complications can be deceptive
Periprocedural stroke/transient ischemic attack, tamponade, or bleeding on the table are infrequent complications readily recognized by the interventionalist. More problematic are several late complications which are often misinterpreted, with the resultant delay causing major harm.
- Pulmonary vein stenosis. This complication of inadvertent ablation inside the pulmonary vein manifests as shortness of breath, typically beginning about 4 weeks post ablation.
“This is very different from the shortness of breath they had with atrial fibrillation. They almost always have a cough that they didn’t have before, and they may have hemoptysis. It’s very important to recognize this promptly, because before it closes completely we can do an angioplasty and stent the vein with good results. But once it closes completely, it becomes an extremely complicated procedure to try to reopen that vein,” according to Dr. Asirvatham.
Very often the patient’s general cardiologist, chest physician, or primary care physician fails to recognize what’s happening. He cited an example: He recently had a patient with a cough who was first referred to an infectious disease specialist, who ordered a bronchoalveolar lavage. The specimen grew atypical actinomycetes. That prompted a referral to thoracic surgery for an open-lung biopsy. But that procedure required cardiac clearance beforehand. It was a cardiologist who said, ‘Wait – all this started after you had an ablation?’
“That patient had pulmonary vein stenosis. And, unfortunately, that complication has not gone away. Being a referral center for pulmonary vein isolation, we see just as many cases of pulmonary vein stenosis today as we did a few years ago,” he said.
- Atrial esophageal fistula. The hallmark of this complication is onset of a plethora of what Dr. Asirvatham called “funny symptoms” more than a month post ablation. These include fever, transient ischemic attacks (TIAs), sepsislike symptoms, discomfort in swallowing, and in some cases hemoptysis.
“The predominant picture is endocarditis/TIA/stroke. If you see this, and the patient has had ablation, immediately refer to surgery to have the fistula between the esophagus and heart fixed. This is not a patient where you say, ‘Nothing by mouth, give some antibiotics, and see what happens.’ I can tell you what will happen: The patient will die,” the cardiologist said.
- Atrial stiffness. This typically occurs about a month after a second or third ablation procedure, when the patient develops shortness of breath that keeps worsening.
“You think ‘pulmonary vein stenosis,’ but the CT scan shows the veins are wide open. Many of these patients will get misdiagnosed as having heart failure with preserved ejection fraction even though they never had it before. The problem here is the atrium has become too stiff from the ablation, and this stiff atrium causes increased pressure, resulting in the shortness of breath. Sometimes patients feel better over time, but sometimes it’s very difficult to treat. But it’s important to recognize atrial stiffness and exclude other causes like pulmonary vein stenosis,” Dr. Asirvatham continued.
- Gastroparesis. This occurs because of injury to the vagus nerve branches located at the top of the esophagus, with resultant delayed gastric emptying.
“It’s an uncomfortable feeling of fullness all the time. The patient will say, ‘It seems like I just ate, even though I ate 8 hours ago,” the electrophysiologist said. “Most of these patients will recover in about 6 months. They may feel better on a gastric motility agent, like a macrolide antibiotic. I personally have not seen a patient who did not feel better within 6-8 months.”
Novel treatment approaches: “A-fib may be an autonomic epilepsy of the heart”
“Patients sometimes will ask you, ‘What is this ablation? What does that mean?’ You have to be truthful and tell them that it’s just a fancy word for burning,” the electrophysiologist said.
Achievement of AF ablation without radiofrequency or cryoablation, instead utilizing nonthermal direct-current pulsed electrical fields, is “the hottest topic in the field of electrophysiology,” according to Dr. Asirvatham.
These electrical fields result in irreversible electroporation of targeted myocardial cell membranes, leading to cell death. It is a tissue-specific intervention, so it’s much less likely than conventional ablation to cause collateral damage to the esophagus and other structures.
“Direct current electroporation has transitioned from proof-of-concept studies to three relatively large patient trials. This is potentially an important breakthrough because if we don’t heat, a lot of the complications of A-fib ablation will probably decrease,” he explained.
Two other promising outside-the-box approaches to the treatment of AF are autonomic nervous system modulation at sites distant from the heart and particle beam ablation without need for cardiac catheters.
“If you put electrodes everywhere in the body to see where A-fib starts, it’s not in the atrium, not in the pulmonary veins, it’s in the nerves behind the pulmonary veins, and before those nerves it’s in some other area of the autonomic nervous system. This has given rise to the notion that A-fib may be an autonomic epilepsy of the heart,” according to the electrophysiologist.
This concept has given rise to a completely different approach to treatment of AF through neurostimulation. That’s how acupuncture works. Also, headphones have been used successfully to terminate and prevent AF by stimulating autonomic nerve centers near the ears. Low-level electrical stimulation of the vagus nerve in order to reduce stellate ganglion activity is under study. So is the application of botulinum toxin at key points in the autonomic nervous system.
“Catheters, drugs, and devices that target these areas, maybe without any ablation in the heart itself, is an exciting area of future management of A-fib,” he said.
Another promising approach is borrowed from radiation oncology: particulate ablation using beams of carbon atoms, protons, or photons.
“The first patients have now been treated for ventricular tachycardia and A-fib. It really is quite amazing how precise the lesion formation is. And with no catheters in the heart, clot can’t form on catheters,” he observed.
Dr. Asirvatham reported having no financial conflicts regarding his presentation, although he serves as a consultant to a handful of medical startup companies and holds patents on intellectual property, the royalties for which go directly to the Mayo Clinic.
SNOWMASS, COLO. – Appropriate in the way of benefit, along with instilling awareness of the warning signals heralding serious late complications, Samuel J. Asirvatham, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Who to steer toward ablation? You have to have a symptomatic patient – that’s a given. For the ones who are paroxysmal, the ones with a relatively normal heart, there’s a much better chance that you’ll help manage their symptoms with ablation than if they have persistent or permanent A-fib. Notice I do not use the word ‘cure’ for A-fib. We talk about controlling symptoms and decreasing frequency, because the longer follow-up you have with intensive monitoring, the more you realize that patients still tend to have some A-fib,” explained Dr. Asirvatham, an electrophysiologist who is professor of medicine and pediatrics at the Mayo Clinic in Rochester, Minn.
The rationale for early atrial fibrillation (AF) ablation in younger patients with troublesome symptoms of paroxysmal AF despite pharmacologic attempts at rate or rhythm control is that it will arrest the progression from an atrial arrhythmia that has just a few triggers readily neutralized by pulmonary vein isolation to persistent AF with a diseased heart and a multitude of arrhythmia trigger points coming from many directions.
A solid candidate for ablation of paroxysmal AF has about a 75% likelihood of having a successful first ablation procedure, with substantial improvement in symptoms and no need for medication. Another 9%-10% will achieve marked reduction in symptom burden upon addition of antiarrhythmic agents that weren’t effective before ablation.
Late complications can be deceptive
Periprocedural stroke/transient ischemic attack, tamponade, or bleeding on the table are infrequent complications readily recognized by the interventionalist. More problematic are several late complications which are often misinterpreted, with the resultant delay causing major harm.
- Pulmonary vein stenosis. This complication of inadvertent ablation inside the pulmonary vein manifests as shortness of breath, typically beginning about 4 weeks post ablation.
“This is very different from the shortness of breath they had with atrial fibrillation. They almost always have a cough that they didn’t have before, and they may have hemoptysis. It’s very important to recognize this promptly, because before it closes completely we can do an angioplasty and stent the vein with good results. But once it closes completely, it becomes an extremely complicated procedure to try to reopen that vein,” according to Dr. Asirvatham.
Very often the patient’s general cardiologist, chest physician, or primary care physician fails to recognize what’s happening. He cited an example: He recently had a patient with a cough who was first referred to an infectious disease specialist, who ordered a bronchoalveolar lavage. The specimen grew atypical actinomycetes. That prompted a referral to thoracic surgery for an open-lung biopsy. But that procedure required cardiac clearance beforehand. It was a cardiologist who said, ‘Wait – all this started after you had an ablation?’
“That patient had pulmonary vein stenosis. And, unfortunately, that complication has not gone away. Being a referral center for pulmonary vein isolation, we see just as many cases of pulmonary vein stenosis today as we did a few years ago,” he said.
- Atrial esophageal fistula. The hallmark of this complication is onset of a plethora of what Dr. Asirvatham called “funny symptoms” more than a month post ablation. These include fever, transient ischemic attacks (TIAs), sepsislike symptoms, discomfort in swallowing, and in some cases hemoptysis.
“The predominant picture is endocarditis/TIA/stroke. If you see this, and the patient has had ablation, immediately refer to surgery to have the fistula between the esophagus and heart fixed. This is not a patient where you say, ‘Nothing by mouth, give some antibiotics, and see what happens.’ I can tell you what will happen: The patient will die,” the cardiologist said.
- Atrial stiffness. This typically occurs about a month after a second or third ablation procedure, when the patient develops shortness of breath that keeps worsening.
“You think ‘pulmonary vein stenosis,’ but the CT scan shows the veins are wide open. Many of these patients will get misdiagnosed as having heart failure with preserved ejection fraction even though they never had it before. The problem here is the atrium has become too stiff from the ablation, and this stiff atrium causes increased pressure, resulting in the shortness of breath. Sometimes patients feel better over time, but sometimes it’s very difficult to treat. But it’s important to recognize atrial stiffness and exclude other causes like pulmonary vein stenosis,” Dr. Asirvatham continued.
- Gastroparesis. This occurs because of injury to the vagus nerve branches located at the top of the esophagus, with resultant delayed gastric emptying.
“It’s an uncomfortable feeling of fullness all the time. The patient will say, ‘It seems like I just ate, even though I ate 8 hours ago,” the electrophysiologist said. “Most of these patients will recover in about 6 months. They may feel better on a gastric motility agent, like a macrolide antibiotic. I personally have not seen a patient who did not feel better within 6-8 months.”
Novel treatment approaches: “A-fib may be an autonomic epilepsy of the heart”
“Patients sometimes will ask you, ‘What is this ablation? What does that mean?’ You have to be truthful and tell them that it’s just a fancy word for burning,” the electrophysiologist said.
Achievement of AF ablation without radiofrequency or cryoablation, instead utilizing nonthermal direct-current pulsed electrical fields, is “the hottest topic in the field of electrophysiology,” according to Dr. Asirvatham.
These electrical fields result in irreversible electroporation of targeted myocardial cell membranes, leading to cell death. It is a tissue-specific intervention, so it’s much less likely than conventional ablation to cause collateral damage to the esophagus and other structures.
“Direct current electroporation has transitioned from proof-of-concept studies to three relatively large patient trials. This is potentially an important breakthrough because if we don’t heat, a lot of the complications of A-fib ablation will probably decrease,” he explained.
Two other promising outside-the-box approaches to the treatment of AF are autonomic nervous system modulation at sites distant from the heart and particle beam ablation without need for cardiac catheters.
“If you put electrodes everywhere in the body to see where A-fib starts, it’s not in the atrium, not in the pulmonary veins, it’s in the nerves behind the pulmonary veins, and before those nerves it’s in some other area of the autonomic nervous system. This has given rise to the notion that A-fib may be an autonomic epilepsy of the heart,” according to the electrophysiologist.
This concept has given rise to a completely different approach to treatment of AF through neurostimulation. That’s how acupuncture works. Also, headphones have been used successfully to terminate and prevent AF by stimulating autonomic nerve centers near the ears. Low-level electrical stimulation of the vagus nerve in order to reduce stellate ganglion activity is under study. So is the application of botulinum toxin at key points in the autonomic nervous system.
“Catheters, drugs, and devices that target these areas, maybe without any ablation in the heart itself, is an exciting area of future management of A-fib,” he said.
Another promising approach is borrowed from radiation oncology: particulate ablation using beams of carbon atoms, protons, or photons.
“The first patients have now been treated for ventricular tachycardia and A-fib. It really is quite amazing how precise the lesion formation is. And with no catheters in the heart, clot can’t form on catheters,” he observed.
Dr. Asirvatham reported having no financial conflicts regarding his presentation, although he serves as a consultant to a handful of medical startup companies and holds patents on intellectual property, the royalties for which go directly to the Mayo Clinic.
SNOWMASS, COLO. – Appropriate in the way of benefit, along with instilling awareness of the warning signals heralding serious late complications, Samuel J. Asirvatham, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Who to steer toward ablation? You have to have a symptomatic patient – that’s a given. For the ones who are paroxysmal, the ones with a relatively normal heart, there’s a much better chance that you’ll help manage their symptoms with ablation than if they have persistent or permanent A-fib. Notice I do not use the word ‘cure’ for A-fib. We talk about controlling symptoms and decreasing frequency, because the longer follow-up you have with intensive monitoring, the more you realize that patients still tend to have some A-fib,” explained Dr. Asirvatham, an electrophysiologist who is professor of medicine and pediatrics at the Mayo Clinic in Rochester, Minn.
The rationale for early atrial fibrillation (AF) ablation in younger patients with troublesome symptoms of paroxysmal AF despite pharmacologic attempts at rate or rhythm control is that it will arrest the progression from an atrial arrhythmia that has just a few triggers readily neutralized by pulmonary vein isolation to persistent AF with a diseased heart and a multitude of arrhythmia trigger points coming from many directions.
A solid candidate for ablation of paroxysmal AF has about a 75% likelihood of having a successful first ablation procedure, with substantial improvement in symptoms and no need for medication. Another 9%-10% will achieve marked reduction in symptom burden upon addition of antiarrhythmic agents that weren’t effective before ablation.
Late complications can be deceptive
Periprocedural stroke/transient ischemic attack, tamponade, or bleeding on the table are infrequent complications readily recognized by the interventionalist. More problematic are several late complications which are often misinterpreted, with the resultant delay causing major harm.
- Pulmonary vein stenosis. This complication of inadvertent ablation inside the pulmonary vein manifests as shortness of breath, typically beginning about 4 weeks post ablation.
“This is very different from the shortness of breath they had with atrial fibrillation. They almost always have a cough that they didn’t have before, and they may have hemoptysis. It’s very important to recognize this promptly, because before it closes completely we can do an angioplasty and stent the vein with good results. But once it closes completely, it becomes an extremely complicated procedure to try to reopen that vein,” according to Dr. Asirvatham.
Very often the patient’s general cardiologist, chest physician, or primary care physician fails to recognize what’s happening. He cited an example: He recently had a patient with a cough who was first referred to an infectious disease specialist, who ordered a bronchoalveolar lavage. The specimen grew atypical actinomycetes. That prompted a referral to thoracic surgery for an open-lung biopsy. But that procedure required cardiac clearance beforehand. It was a cardiologist who said, ‘Wait – all this started after you had an ablation?’
“That patient had pulmonary vein stenosis. And, unfortunately, that complication has not gone away. Being a referral center for pulmonary vein isolation, we see just as many cases of pulmonary vein stenosis today as we did a few years ago,” he said.
- Atrial esophageal fistula. The hallmark of this complication is onset of a plethora of what Dr. Asirvatham called “funny symptoms” more than a month post ablation. These include fever, transient ischemic attacks (TIAs), sepsislike symptoms, discomfort in swallowing, and in some cases hemoptysis.
“The predominant picture is endocarditis/TIA/stroke. If you see this, and the patient has had ablation, immediately refer to surgery to have the fistula between the esophagus and heart fixed. This is not a patient where you say, ‘Nothing by mouth, give some antibiotics, and see what happens.’ I can tell you what will happen: The patient will die,” the cardiologist said.
- Atrial stiffness. This typically occurs about a month after a second or third ablation procedure, when the patient develops shortness of breath that keeps worsening.
“You think ‘pulmonary vein stenosis,’ but the CT scan shows the veins are wide open. Many of these patients will get misdiagnosed as having heart failure with preserved ejection fraction even though they never had it before. The problem here is the atrium has become too stiff from the ablation, and this stiff atrium causes increased pressure, resulting in the shortness of breath. Sometimes patients feel better over time, but sometimes it’s very difficult to treat. But it’s important to recognize atrial stiffness and exclude other causes like pulmonary vein stenosis,” Dr. Asirvatham continued.
- Gastroparesis. This occurs because of injury to the vagus nerve branches located at the top of the esophagus, with resultant delayed gastric emptying.
“It’s an uncomfortable feeling of fullness all the time. The patient will say, ‘It seems like I just ate, even though I ate 8 hours ago,” the electrophysiologist said. “Most of these patients will recover in about 6 months. They may feel better on a gastric motility agent, like a macrolide antibiotic. I personally have not seen a patient who did not feel better within 6-8 months.”
Novel treatment approaches: “A-fib may be an autonomic epilepsy of the heart”
“Patients sometimes will ask you, ‘What is this ablation? What does that mean?’ You have to be truthful and tell them that it’s just a fancy word for burning,” the electrophysiologist said.
Achievement of AF ablation without radiofrequency or cryoablation, instead utilizing nonthermal direct-current pulsed electrical fields, is “the hottest topic in the field of electrophysiology,” according to Dr. Asirvatham.
These electrical fields result in irreversible electroporation of targeted myocardial cell membranes, leading to cell death. It is a tissue-specific intervention, so it’s much less likely than conventional ablation to cause collateral damage to the esophagus and other structures.
“Direct current electroporation has transitioned from proof-of-concept studies to three relatively large patient trials. This is potentially an important breakthrough because if we don’t heat, a lot of the complications of A-fib ablation will probably decrease,” he explained.
Two other promising outside-the-box approaches to the treatment of AF are autonomic nervous system modulation at sites distant from the heart and particle beam ablation without need for cardiac catheters.
“If you put electrodes everywhere in the body to see where A-fib starts, it’s not in the atrium, not in the pulmonary veins, it’s in the nerves behind the pulmonary veins, and before those nerves it’s in some other area of the autonomic nervous system. This has given rise to the notion that A-fib may be an autonomic epilepsy of the heart,” according to the electrophysiologist.
This concept has given rise to a completely different approach to treatment of AF through neurostimulation. That’s how acupuncture works. Also, headphones have been used successfully to terminate and prevent AF by stimulating autonomic nerve centers near the ears. Low-level electrical stimulation of the vagus nerve in order to reduce stellate ganglion activity is under study. So is the application of botulinum toxin at key points in the autonomic nervous system.
“Catheters, drugs, and devices that target these areas, maybe without any ablation in the heart itself, is an exciting area of future management of A-fib,” he said.
Another promising approach is borrowed from radiation oncology: particulate ablation using beams of carbon atoms, protons, or photons.
“The first patients have now been treated for ventricular tachycardia and A-fib. It really is quite amazing how precise the lesion formation is. And with no catheters in the heart, clot can’t form on catheters,” he observed.
Dr. Asirvatham reported having no financial conflicts regarding his presentation, although he serves as a consultant to a handful of medical startup companies and holds patents on intellectual property, the royalties for which go directly to the Mayo Clinic.
REPORTING FROM ACC SNOWMASS 2019