User login
Impaired clot lysis associated with mild bleeding symptoms
Patients with self-reported mild bleeding symptoms may have impaired clot lysis, according to investigators. This finding is remarkable because it contrasts with known bleeding disorders, such as hemophilia, which are associated with enhanced clot lysis, reported lead author Minka J.A. Vries, MD, of the Cardiovascular Research Institute Maastricht (CARIM) at Maastricht (the Netherlands) University and her colleagues.
The observational study, which included 335 patients undergoing elective surgery at Maastricht University Medical Center, was conducted to better understand lysis capacity, which is challenging to assess in a clinical setting. Although the Euglobulin Lysis Time (ELT) is often used in the clinic, it cannot determine the influence of hemostatic proteins or formation of a fibrin clot under physiological conditions.
“In the more recently developed lysis assays,” the investigators wrote in Thrombosis Research, “the turbidity lysis assay and the tissue plasminogen activator–rotational thromboelastometry (tPA-ROTEM) [assay], all plasma proteins are present and fibrin is formed under more physiological conditions for the measurement of fibrinolysis.” These two tests were used in the present study.
Of the 335 adult patients, 240 had self-reported mild bleeding symptoms, and 95 did not. Patients with bleeding disorders, thrombocytopenia, or anemia were excluded, as were pregnant women and those taking blood thinners or NSAIDs. Along with assessing time parameters of fibrinolysis, clot-associated proteins were measured for possible imbalances.
“We hypothesized that clot lysis capacity is enhanced in patients with mild bleeding symptoms,” the investigators wrote, based on other bleeding disorders. Surprisingly, the results told a different story.
After adjusting for sex, BMI, and age, patients with bleeding symptoms had lower tPA-ROTEM lysis speed (beta −0.35; P = .007) and longer tPA-ROTEM lysis time (beta 0.29; P = .022) than did patients without bleeding symptoms. The investigators found that tPA-ROTEM measurements depended on factor II, factor XII, alpha2-antiplasmin, plasminogen, thrombin activatable fibrinolysis inhibitor (TAFI), and plasminogen activator inhibitor–1 (PAI-1) level. In contrast, turbidity lysis assay measurements were not significantly different between groups. This latter assay was influenced by alpha2-antiplasmin, TAFI, and PAI-1.
“We did not find evidence for systemic hyperfibrinolytic capacity in patients reporting mild bleeding symptoms in comparison to patients not reporting bleeding symptoms,” the investigators concluded. “tPA-ROTEM even suggested a slower clot lysis in these patients. Though this may appear counterintuitive, our results are in line with two papers assessing systemic clot lysis in mild bleeders.”
While this phenomenon gains supporting evidence, it remains poorly understood.
“We have no good explanation for these findings,” the investigators noted.
This study was funded by the Sint Annadal Foundation Maastricht, Maastricht University Medical Centre, CTMM INCOAG Maastricht, Cardiovascular Research Institute Maastricht, and the British Heart Foundation. No conflicts of interest were reported.
SOURCE: Vries MJA et al. Thromb Res. 2018 Dec 4. doi: 10.1016/j.thromres.2018.12.004.
Patients with self-reported mild bleeding symptoms may have impaired clot lysis, according to investigators. This finding is remarkable because it contrasts with known bleeding disorders, such as hemophilia, which are associated with enhanced clot lysis, reported lead author Minka J.A. Vries, MD, of the Cardiovascular Research Institute Maastricht (CARIM) at Maastricht (the Netherlands) University and her colleagues.
The observational study, which included 335 patients undergoing elective surgery at Maastricht University Medical Center, was conducted to better understand lysis capacity, which is challenging to assess in a clinical setting. Although the Euglobulin Lysis Time (ELT) is often used in the clinic, it cannot determine the influence of hemostatic proteins or formation of a fibrin clot under physiological conditions.
“In the more recently developed lysis assays,” the investigators wrote in Thrombosis Research, “the turbidity lysis assay and the tissue plasminogen activator–rotational thromboelastometry (tPA-ROTEM) [assay], all plasma proteins are present and fibrin is formed under more physiological conditions for the measurement of fibrinolysis.” These two tests were used in the present study.
Of the 335 adult patients, 240 had self-reported mild bleeding symptoms, and 95 did not. Patients with bleeding disorders, thrombocytopenia, or anemia were excluded, as were pregnant women and those taking blood thinners or NSAIDs. Along with assessing time parameters of fibrinolysis, clot-associated proteins were measured for possible imbalances.
“We hypothesized that clot lysis capacity is enhanced in patients with mild bleeding symptoms,” the investigators wrote, based on other bleeding disorders. Surprisingly, the results told a different story.
After adjusting for sex, BMI, and age, patients with bleeding symptoms had lower tPA-ROTEM lysis speed (beta −0.35; P = .007) and longer tPA-ROTEM lysis time (beta 0.29; P = .022) than did patients without bleeding symptoms. The investigators found that tPA-ROTEM measurements depended on factor II, factor XII, alpha2-antiplasmin, plasminogen, thrombin activatable fibrinolysis inhibitor (TAFI), and plasminogen activator inhibitor–1 (PAI-1) level. In contrast, turbidity lysis assay measurements were not significantly different between groups. This latter assay was influenced by alpha2-antiplasmin, TAFI, and PAI-1.
“We did not find evidence for systemic hyperfibrinolytic capacity in patients reporting mild bleeding symptoms in comparison to patients not reporting bleeding symptoms,” the investigators concluded. “tPA-ROTEM even suggested a slower clot lysis in these patients. Though this may appear counterintuitive, our results are in line with two papers assessing systemic clot lysis in mild bleeders.”
While this phenomenon gains supporting evidence, it remains poorly understood.
“We have no good explanation for these findings,” the investigators noted.
This study was funded by the Sint Annadal Foundation Maastricht, Maastricht University Medical Centre, CTMM INCOAG Maastricht, Cardiovascular Research Institute Maastricht, and the British Heart Foundation. No conflicts of interest were reported.
SOURCE: Vries MJA et al. Thromb Res. 2018 Dec 4. doi: 10.1016/j.thromres.2018.12.004.
Patients with self-reported mild bleeding symptoms may have impaired clot lysis, according to investigators. This finding is remarkable because it contrasts with known bleeding disorders, such as hemophilia, which are associated with enhanced clot lysis, reported lead author Minka J.A. Vries, MD, of the Cardiovascular Research Institute Maastricht (CARIM) at Maastricht (the Netherlands) University and her colleagues.
The observational study, which included 335 patients undergoing elective surgery at Maastricht University Medical Center, was conducted to better understand lysis capacity, which is challenging to assess in a clinical setting. Although the Euglobulin Lysis Time (ELT) is often used in the clinic, it cannot determine the influence of hemostatic proteins or formation of a fibrin clot under physiological conditions.
“In the more recently developed lysis assays,” the investigators wrote in Thrombosis Research, “the turbidity lysis assay and the tissue plasminogen activator–rotational thromboelastometry (tPA-ROTEM) [assay], all plasma proteins are present and fibrin is formed under more physiological conditions for the measurement of fibrinolysis.” These two tests were used in the present study.
Of the 335 adult patients, 240 had self-reported mild bleeding symptoms, and 95 did not. Patients with bleeding disorders, thrombocytopenia, or anemia were excluded, as were pregnant women and those taking blood thinners or NSAIDs. Along with assessing time parameters of fibrinolysis, clot-associated proteins were measured for possible imbalances.
“We hypothesized that clot lysis capacity is enhanced in patients with mild bleeding symptoms,” the investigators wrote, based on other bleeding disorders. Surprisingly, the results told a different story.
After adjusting for sex, BMI, and age, patients with bleeding symptoms had lower tPA-ROTEM lysis speed (beta −0.35; P = .007) and longer tPA-ROTEM lysis time (beta 0.29; P = .022) than did patients without bleeding symptoms. The investigators found that tPA-ROTEM measurements depended on factor II, factor XII, alpha2-antiplasmin, plasminogen, thrombin activatable fibrinolysis inhibitor (TAFI), and plasminogen activator inhibitor–1 (PAI-1) level. In contrast, turbidity lysis assay measurements were not significantly different between groups. This latter assay was influenced by alpha2-antiplasmin, TAFI, and PAI-1.
“We did not find evidence for systemic hyperfibrinolytic capacity in patients reporting mild bleeding symptoms in comparison to patients not reporting bleeding symptoms,” the investigators concluded. “tPA-ROTEM even suggested a slower clot lysis in these patients. Though this may appear counterintuitive, our results are in line with two papers assessing systemic clot lysis in mild bleeders.”
While this phenomenon gains supporting evidence, it remains poorly understood.
“We have no good explanation for these findings,” the investigators noted.
This study was funded by the Sint Annadal Foundation Maastricht, Maastricht University Medical Centre, CTMM INCOAG Maastricht, Cardiovascular Research Institute Maastricht, and the British Heart Foundation. No conflicts of interest were reported.
SOURCE: Vries MJA et al. Thromb Res. 2018 Dec 4. doi: 10.1016/j.thromres.2018.12.004.
FROM THROMBOSIS RESEARCH
Key clinical point: Patients with self-reported mild bleeding symptoms may have impaired clot lysis, in contrast with known bleeding disorders.
Major finding: Patients with mild bleeding had longer whole blood tissue plasminogen activator-rotational thromboelastometry lysis times (P = .022) than did patients without symptoms.
Study details: An observational study of 335 adult patients undergoing elective surgery.
Disclosures: This study was funded by the Sint Annadal Foundation, Maastricht University Medical Center, CTMM INCOAG Maastricht, Cardiovascular Research Institute Maastricht, and the British Heart Foundation. No conflicts of interest were reported.
Source: Vries MJA et al. Thromb Res. 2018 Dec 4. doi: 10.1016/j.thromres.2018.12.004.
LAA closure safely treated AF patients with prior ICH
BOSTON – Sixty-three patients with atrial fibrillation (AF) and a history of intracranial hemorrhage have safely received a Watchman device for left atrial appendage (LAA) closure followed by 6 months of treatment with an individualized drug regimen to prevent thrombus formation at three U.S. centers. The 6-month outcomes of these patients were as good as the outcomes of 95 similar patients who did not have a history of intracranial hemorrhage, Moussa C. Mansour, MD, said at the annual International AF Symposium.
“Individualized, postprocedural antithrombotic management ranging from dual antiplatelet therapy to oral anticoagulation plus aspirin can be used short term,” said Dr. Mansour, director of the atrial AF program at Massachusetts General Hospital. He and his associates devised this approach “out of necessity,” he said. The findings “show that you can get these patients through the initial postprocedural period” by individualizing their antiplatelet or anticoagulant treatment.
Not many U.S. operators are currently placing LAA closure devices in patients with a history of intracranial hemorrhage (ICH). “No one knows what to do with them. There is no standard approach; it’s an evolving field,” he said in an interview.
Dr. Mansour reviewed his group’s experience at Massachusetts General with LAA closure using the Watchman device combined with the experience of teams at the Texas Cardiac Arrhythmia Institute in Austin and the HCA Midwest Health in Overland Park, Kan. This included 63 AF patients with an ICH history and 95 similar AF patients with no hemorrhagic history. Nearly half of the patients with a prior ICH also had a history of at least one ischemic stroke or transient ischemic attack, compared with just over a third of the patients without a prior ICH. The two groups had nearly identical CHA2DS2-VASc scores, 4.9 and 4.7, and also very similar HAS-BLED scores.
The patients with a prior ICH included 57% with a prior intracerebral hemorrhage, 29% with a prior subdural hemorrhage, 10% with a subarachnoid hemorrhage, and the remainder unspecified.
Thirty-two of the 63 patients with an ICH history received postprocedural anticoagulation with a direct-acting oral anticoagulant, 18 received warfarin, and 13 received dual antiplatelet therapy but no anticoagulant. Among the 45 patients treated with an oral anticoagulant, 27 also received aspirin. Among the 95 patients reviewed without an ICH history, 93 received an oral anticoagulant, and 2 received dual antiplatelet therapy.
During 180 days of follow-up, the combined incidence of death, stroke, or a major bleed was about 5% among the patients with an ICH history and about 10% among those without this history, a difference that was not statistically significant, Dr. Mansour said.
At Massachusetts General, the decision on how to treat AF patients with an ICH history after they undergo LAA closure is made by a multidisciplinary team of clinicians from the arrhythmia, neurology, radiology, anesthesia, and cardiovascular surgery services. A key factor when determining the drug regimen these patients will receive is the location and size of the ICH when imaged with CT or MRI, Dr. Mansour said. The team also assessed the current likelihood that the patient will bleed. “One size does not fit all” patients, he explained.
A randomized trial, ASAP-TOO (Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation) is now in progress and was designed to compare the safety and efficacy of LAA closure using Watchman with medical management in 888 patients deemed ineligible for anticoagulation, but enrollment into the trial has been slow. As of early 2019, the study’s organizers posted an estimated completion date of late 2023. Until that study finishes, the field will have no definitive data on how to manage these patients, Dr. Mansour said. He noted that a handful of other reports have presented evidence for the safety of LAA closure in these patients, including a review from the Cleveland Clinic on 38 AF patients who received the Watchman device after an ICH (Heart Rhythm. 2018 Dec 2. doi: 10.1016/j.hrthm.2018.11.022); a review of the Amplatzer Cardiac Plug registry, which included 198 patients with an ICH history (Int J Cardiol. 2017 June 1;236[1]:232-6); and a single-center review of 46 French patients who underwent LAA closure after intracerebral hemorrhage (J Stroke Cerebrovasc Dis. 2017 March;26[3]:545-51).
BOSTON – Sixty-three patients with atrial fibrillation (AF) and a history of intracranial hemorrhage have safely received a Watchman device for left atrial appendage (LAA) closure followed by 6 months of treatment with an individualized drug regimen to prevent thrombus formation at three U.S. centers. The 6-month outcomes of these patients were as good as the outcomes of 95 similar patients who did not have a history of intracranial hemorrhage, Moussa C. Mansour, MD, said at the annual International AF Symposium.
“Individualized, postprocedural antithrombotic management ranging from dual antiplatelet therapy to oral anticoagulation plus aspirin can be used short term,” said Dr. Mansour, director of the atrial AF program at Massachusetts General Hospital. He and his associates devised this approach “out of necessity,” he said. The findings “show that you can get these patients through the initial postprocedural period” by individualizing their antiplatelet or anticoagulant treatment.
Not many U.S. operators are currently placing LAA closure devices in patients with a history of intracranial hemorrhage (ICH). “No one knows what to do with them. There is no standard approach; it’s an evolving field,” he said in an interview.
Dr. Mansour reviewed his group’s experience at Massachusetts General with LAA closure using the Watchman device combined with the experience of teams at the Texas Cardiac Arrhythmia Institute in Austin and the HCA Midwest Health in Overland Park, Kan. This included 63 AF patients with an ICH history and 95 similar AF patients with no hemorrhagic history. Nearly half of the patients with a prior ICH also had a history of at least one ischemic stroke or transient ischemic attack, compared with just over a third of the patients without a prior ICH. The two groups had nearly identical CHA2DS2-VASc scores, 4.9 and 4.7, and also very similar HAS-BLED scores.
The patients with a prior ICH included 57% with a prior intracerebral hemorrhage, 29% with a prior subdural hemorrhage, 10% with a subarachnoid hemorrhage, and the remainder unspecified.
Thirty-two of the 63 patients with an ICH history received postprocedural anticoagulation with a direct-acting oral anticoagulant, 18 received warfarin, and 13 received dual antiplatelet therapy but no anticoagulant. Among the 45 patients treated with an oral anticoagulant, 27 also received aspirin. Among the 95 patients reviewed without an ICH history, 93 received an oral anticoagulant, and 2 received dual antiplatelet therapy.
During 180 days of follow-up, the combined incidence of death, stroke, or a major bleed was about 5% among the patients with an ICH history and about 10% among those without this history, a difference that was not statistically significant, Dr. Mansour said.
At Massachusetts General, the decision on how to treat AF patients with an ICH history after they undergo LAA closure is made by a multidisciplinary team of clinicians from the arrhythmia, neurology, radiology, anesthesia, and cardiovascular surgery services. A key factor when determining the drug regimen these patients will receive is the location and size of the ICH when imaged with CT or MRI, Dr. Mansour said. The team also assessed the current likelihood that the patient will bleed. “One size does not fit all” patients, he explained.
A randomized trial, ASAP-TOO (Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation) is now in progress and was designed to compare the safety and efficacy of LAA closure using Watchman with medical management in 888 patients deemed ineligible for anticoagulation, but enrollment into the trial has been slow. As of early 2019, the study’s organizers posted an estimated completion date of late 2023. Until that study finishes, the field will have no definitive data on how to manage these patients, Dr. Mansour said. He noted that a handful of other reports have presented evidence for the safety of LAA closure in these patients, including a review from the Cleveland Clinic on 38 AF patients who received the Watchman device after an ICH (Heart Rhythm. 2018 Dec 2. doi: 10.1016/j.hrthm.2018.11.022); a review of the Amplatzer Cardiac Plug registry, which included 198 patients with an ICH history (Int J Cardiol. 2017 June 1;236[1]:232-6); and a single-center review of 46 French patients who underwent LAA closure after intracerebral hemorrhage (J Stroke Cerebrovasc Dis. 2017 March;26[3]:545-51).
BOSTON – Sixty-three patients with atrial fibrillation (AF) and a history of intracranial hemorrhage have safely received a Watchman device for left atrial appendage (LAA) closure followed by 6 months of treatment with an individualized drug regimen to prevent thrombus formation at three U.S. centers. The 6-month outcomes of these patients were as good as the outcomes of 95 similar patients who did not have a history of intracranial hemorrhage, Moussa C. Mansour, MD, said at the annual International AF Symposium.
“Individualized, postprocedural antithrombotic management ranging from dual antiplatelet therapy to oral anticoagulation plus aspirin can be used short term,” said Dr. Mansour, director of the atrial AF program at Massachusetts General Hospital. He and his associates devised this approach “out of necessity,” he said. The findings “show that you can get these patients through the initial postprocedural period” by individualizing their antiplatelet or anticoagulant treatment.
Not many U.S. operators are currently placing LAA closure devices in patients with a history of intracranial hemorrhage (ICH). “No one knows what to do with them. There is no standard approach; it’s an evolving field,” he said in an interview.
Dr. Mansour reviewed his group’s experience at Massachusetts General with LAA closure using the Watchman device combined with the experience of teams at the Texas Cardiac Arrhythmia Institute in Austin and the HCA Midwest Health in Overland Park, Kan. This included 63 AF patients with an ICH history and 95 similar AF patients with no hemorrhagic history. Nearly half of the patients with a prior ICH also had a history of at least one ischemic stroke or transient ischemic attack, compared with just over a third of the patients without a prior ICH. The two groups had nearly identical CHA2DS2-VASc scores, 4.9 and 4.7, and also very similar HAS-BLED scores.
The patients with a prior ICH included 57% with a prior intracerebral hemorrhage, 29% with a prior subdural hemorrhage, 10% with a subarachnoid hemorrhage, and the remainder unspecified.
Thirty-two of the 63 patients with an ICH history received postprocedural anticoagulation with a direct-acting oral anticoagulant, 18 received warfarin, and 13 received dual antiplatelet therapy but no anticoagulant. Among the 45 patients treated with an oral anticoagulant, 27 also received aspirin. Among the 95 patients reviewed without an ICH history, 93 received an oral anticoagulant, and 2 received dual antiplatelet therapy.
During 180 days of follow-up, the combined incidence of death, stroke, or a major bleed was about 5% among the patients with an ICH history and about 10% among those without this history, a difference that was not statistically significant, Dr. Mansour said.
At Massachusetts General, the decision on how to treat AF patients with an ICH history after they undergo LAA closure is made by a multidisciplinary team of clinicians from the arrhythmia, neurology, radiology, anesthesia, and cardiovascular surgery services. A key factor when determining the drug regimen these patients will receive is the location and size of the ICH when imaged with CT or MRI, Dr. Mansour said. The team also assessed the current likelihood that the patient will bleed. “One size does not fit all” patients, he explained.
A randomized trial, ASAP-TOO (Assessment of the Watchman Device in Patients Unsuitable for Oral Anticoagulation) is now in progress and was designed to compare the safety and efficacy of LAA closure using Watchman with medical management in 888 patients deemed ineligible for anticoagulation, but enrollment into the trial has been slow. As of early 2019, the study’s organizers posted an estimated completion date of late 2023. Until that study finishes, the field will have no definitive data on how to manage these patients, Dr. Mansour said. He noted that a handful of other reports have presented evidence for the safety of LAA closure in these patients, including a review from the Cleveland Clinic on 38 AF patients who received the Watchman device after an ICH (Heart Rhythm. 2018 Dec 2. doi: 10.1016/j.hrthm.2018.11.022); a review of the Amplatzer Cardiac Plug registry, which included 198 patients with an ICH history (Int J Cardiol. 2017 June 1;236[1]:232-6); and a single-center review of 46 French patients who underwent LAA closure after intracerebral hemorrhage (J Stroke Cerebrovasc Dis. 2017 March;26[3]:545-51).
REPORTING FROM THE AF SYMPOSIUM 2019
Key clinical point: Tailoring the anticlotting regimen atrial fibrillation (AF) patients received after left atrial appendage (LAA) closure led to good outcomes despite prior intracranial hemorrhages.
Major finding: The 6-month rate of death, stroke, or major bleed was about 5% in AF patients who underwent LAA closure after intracranial hemorrhage.
Study details: Retrospective review of 158 atrial fibrillation patients who underwent LAA closure at any of three U.S. centers.
Disclosures: The study received no commercial funding. Dr. Mansour has been a consultant to Abbott, Biosense Webster, Boston Scientific, and Medtronic; he has received research funding from Abbott, Biosense Webster, Boehringer Ingelheim, Boston Scientific, and Pfizer; and he has an equity interest in EPD Solutions and NewPace.
CABANA: Ablation surpassed drugs for raising AF quality of life
BOSTON – Ablation of atrial fibrillation led to significantly better quality of life improvements, compared with antiarrhythmic drug therapy, in a prespecified, secondary analysis of data from the CABANA multicenter, randomized trial with 2,204 patients.
The improvements in quality of life measures in atrial fibrillation (AF) patients following ablation were “clinically meaningful” relative to drug therapy and were sustained for 5 years of follow-up, said Douglas L. Packer, MD, at the annual International AF Symposium.
The apparent incremental benefit in quality of life after ablation, compared with patients treated with drug therapy, added to the benefits previously reported from CABANA (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial) using ablation that included a highly significant reduction in recurrent AF and a significant reduction in the combined endpoint of mortality or cardiovascular hospitalization. However, the trial’s results were neutral for the study’s prespecified primary endpoint, a combination of the rate of total mortality, disabling stroke, serious bleeding, or cardiac arrest in an intention-to-treat analysis, a result first reported by Dr. Packer at the annual scientific sessions of the Heart Rhythm Society in May 2018.
When Dr. Packer spoke at the AF Symposium in late January 2019, the CABANA results had still not been published. He said that he expected an article with the main findings to appear online sometime in February 2019.
The quality of life analysis, focused on two measures, the Mayo AF-Specific Symptoms Inventory (MAFSI) (Circulation. 2008 Oct 28;118[suppl 18]:S589) and the Atrial Fibrillation Affect on Quality of Life (AFEQT) (Circ Arrhythm Electrophysiol. 2011 Feb;4[1]:15-25). The patients enrolled in the two treatment arms of CABANA had essentially identical baseline MAFSI frequency scores, but starting 3 months after entry patients randomized to the ablation arm had an average, statistically significant 1.6-point improvement in their adjusted MAFSI frequency score, compared with patients in the drug-therapy arm; this reduction persisted at about the same statistically significant level through 5 years, said Dr. Packer, an electrophysiologist and professor of medicine at the Mayo Clinic in Rochester, Minn., and lead investigator of CABANA. Patients who remained in follow-up throughout the study’s entire 60 months underwent a total of seven MAFSI assessments after their initial treatment; the average, adjusted difference in MAFSI frequency scores throughout CABANA was a statistically significant 1.4 points lower among ablated patients, compared with those randomized to drug therapy, said Dr. Packer, who presented data first reported by the CABANA researchers in 2018.
The pattern of change in the AFEQT scores was very similar. The average, adjusted AFEQT summary scores were virtually identical at baseline for the two treatment arms, but at 3 months after the study began patients in the ablation arm had an average, adjusted, statistically significant 3-point improvement in their AFEQT summary scores, compared with drug-treated patients; this incremental increase in AFEQT scores remained consistent and statistically significant through 5 years of follow-up. Throughout the study, the average, adjusted, incremental improvement in AFEQT after ablation was a statistically significant 3.4 points, said Dr. Packer.
But these quality of life measures in patients who received unblinded assignment to an ablation procedure or drug treatment in CABANA are likely unreliable, commented Peter R. Kowey, MD, an electrophysiologist at the Lankenau Heart Institute in Wynnewood, Penn., and professor of medicine at Jefferson Medical College, Philadelphia.
“Assessing quality of life in an unblinded trial seems to me to be pretty shallow,” said Dr. Kowey, a member of the CABANA steering committee and a participant in a panel at the Symposium that discussed the results. Patients who have just gone through a 6-hour procedure will often say they now feel a whole lot better, he suggested. “They’re concerned what will get done to them if they don’t say they feel better.” The same confounding also applies to other “soft” secondary endpoints used in CABANA, such as hospitalization rates.
The reliability of these more subjective outcome measures is reduced in an unblinded trial, Dr. Kowey maintained. The CABANA results “don’t change the fundamental principal of using hard endpoints,” such as those that made up the primary endpoint of the study, he said in an interview. “We put secondary endpoints into the study because they are legitimate things to look at, but they have questionable reliability,” as measures of ablation’s efficacy.
Dr. Kowey also cautioned against focusing on the per-protocol and treatment-received analyses of the CABANA results, prespecified analyses that Dr. Packer highlighted because of the high number of crossovers in the trial: 9% of patients assigned to ablation never received it and 28% of patients assigned to drug therapy actually received ablation.
The CABANA steering committee anticipated a high crossover rate, but still set the intention-to-treat analysis as primary because “per protocol introduces many biases and can’t be relied on for a study of this magnitude,” Dr. Kowey said. He also cited the statistical pitfalls of looking at multiple secondary endpoints in the CABANA results and the danger of reading too much into subgroup analyses, all from a trial with a neutral primary endpoint.
Another concern he raised centered on the generalizability of CABANA’s safety findings, which showed roughly similar adverse event rates between the two treatment arms – about 9% with ablation, 4% with drugs – although the distribution of complications types differed between the two arms. The “ ‘remarkably safe’ performance of ablation in CABANA can be attributed to ‘cherry-picked’ investigators” who performed the ablation procedures at high-volume centers, said Dr. Kowey. “The safety of ablation was predictable” in CABANA because of the selection of participating centers. “Seventy percent of U.S. ablations are being done at centers that do fewer than 25 ablations annually,” Dr. Kowey said. “We don’t know what goes on” at lower-volume centers; “ablation can’t be considered as safe as we presume” when done at centers outside the 118 sites that participated in CABANA, he maintained.
CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to these four companies as well as to CyberHeart, nContact, Sanofi-Aventis, and Toray. He has received research funding from AG, Biosense Webster, Boston Scientific, CryoCath, EP Limited, and Medtronic, and has a financial interest in AF mapping technology. Dr. Kowey has been a consultant to several companies that market antiarrhythmic drugs or market arrhythmia devices and has an equity interest in BioTelemetry.
BOSTON – Ablation of atrial fibrillation led to significantly better quality of life improvements, compared with antiarrhythmic drug therapy, in a prespecified, secondary analysis of data from the CABANA multicenter, randomized trial with 2,204 patients.
The improvements in quality of life measures in atrial fibrillation (AF) patients following ablation were “clinically meaningful” relative to drug therapy and were sustained for 5 years of follow-up, said Douglas L. Packer, MD, at the annual International AF Symposium.
The apparent incremental benefit in quality of life after ablation, compared with patients treated with drug therapy, added to the benefits previously reported from CABANA (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial) using ablation that included a highly significant reduction in recurrent AF and a significant reduction in the combined endpoint of mortality or cardiovascular hospitalization. However, the trial’s results were neutral for the study’s prespecified primary endpoint, a combination of the rate of total mortality, disabling stroke, serious bleeding, or cardiac arrest in an intention-to-treat analysis, a result first reported by Dr. Packer at the annual scientific sessions of the Heart Rhythm Society in May 2018.
When Dr. Packer spoke at the AF Symposium in late January 2019, the CABANA results had still not been published. He said that he expected an article with the main findings to appear online sometime in February 2019.
The quality of life analysis, focused on two measures, the Mayo AF-Specific Symptoms Inventory (MAFSI) (Circulation. 2008 Oct 28;118[suppl 18]:S589) and the Atrial Fibrillation Affect on Quality of Life (AFEQT) (Circ Arrhythm Electrophysiol. 2011 Feb;4[1]:15-25). The patients enrolled in the two treatment arms of CABANA had essentially identical baseline MAFSI frequency scores, but starting 3 months after entry patients randomized to the ablation arm had an average, statistically significant 1.6-point improvement in their adjusted MAFSI frequency score, compared with patients in the drug-therapy arm; this reduction persisted at about the same statistically significant level through 5 years, said Dr. Packer, an electrophysiologist and professor of medicine at the Mayo Clinic in Rochester, Minn., and lead investigator of CABANA. Patients who remained in follow-up throughout the study’s entire 60 months underwent a total of seven MAFSI assessments after their initial treatment; the average, adjusted difference in MAFSI frequency scores throughout CABANA was a statistically significant 1.4 points lower among ablated patients, compared with those randomized to drug therapy, said Dr. Packer, who presented data first reported by the CABANA researchers in 2018.
The pattern of change in the AFEQT scores was very similar. The average, adjusted AFEQT summary scores were virtually identical at baseline for the two treatment arms, but at 3 months after the study began patients in the ablation arm had an average, adjusted, statistically significant 3-point improvement in their AFEQT summary scores, compared with drug-treated patients; this incremental increase in AFEQT scores remained consistent and statistically significant through 5 years of follow-up. Throughout the study, the average, adjusted, incremental improvement in AFEQT after ablation was a statistically significant 3.4 points, said Dr. Packer.
But these quality of life measures in patients who received unblinded assignment to an ablation procedure or drug treatment in CABANA are likely unreliable, commented Peter R. Kowey, MD, an electrophysiologist at the Lankenau Heart Institute in Wynnewood, Penn., and professor of medicine at Jefferson Medical College, Philadelphia.
“Assessing quality of life in an unblinded trial seems to me to be pretty shallow,” said Dr. Kowey, a member of the CABANA steering committee and a participant in a panel at the Symposium that discussed the results. Patients who have just gone through a 6-hour procedure will often say they now feel a whole lot better, he suggested. “They’re concerned what will get done to them if they don’t say they feel better.” The same confounding also applies to other “soft” secondary endpoints used in CABANA, such as hospitalization rates.
The reliability of these more subjective outcome measures is reduced in an unblinded trial, Dr. Kowey maintained. The CABANA results “don’t change the fundamental principal of using hard endpoints,” such as those that made up the primary endpoint of the study, he said in an interview. “We put secondary endpoints into the study because they are legitimate things to look at, but they have questionable reliability,” as measures of ablation’s efficacy.
Dr. Kowey also cautioned against focusing on the per-protocol and treatment-received analyses of the CABANA results, prespecified analyses that Dr. Packer highlighted because of the high number of crossovers in the trial: 9% of patients assigned to ablation never received it and 28% of patients assigned to drug therapy actually received ablation.
The CABANA steering committee anticipated a high crossover rate, but still set the intention-to-treat analysis as primary because “per protocol introduces many biases and can’t be relied on for a study of this magnitude,” Dr. Kowey said. He also cited the statistical pitfalls of looking at multiple secondary endpoints in the CABANA results and the danger of reading too much into subgroup analyses, all from a trial with a neutral primary endpoint.
Another concern he raised centered on the generalizability of CABANA’s safety findings, which showed roughly similar adverse event rates between the two treatment arms – about 9% with ablation, 4% with drugs – although the distribution of complications types differed between the two arms. The “ ‘remarkably safe’ performance of ablation in CABANA can be attributed to ‘cherry-picked’ investigators” who performed the ablation procedures at high-volume centers, said Dr. Kowey. “The safety of ablation was predictable” in CABANA because of the selection of participating centers. “Seventy percent of U.S. ablations are being done at centers that do fewer than 25 ablations annually,” Dr. Kowey said. “We don’t know what goes on” at lower-volume centers; “ablation can’t be considered as safe as we presume” when done at centers outside the 118 sites that participated in CABANA, he maintained.
CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to these four companies as well as to CyberHeart, nContact, Sanofi-Aventis, and Toray. He has received research funding from AG, Biosense Webster, Boston Scientific, CryoCath, EP Limited, and Medtronic, and has a financial interest in AF mapping technology. Dr. Kowey has been a consultant to several companies that market antiarrhythmic drugs or market arrhythmia devices and has an equity interest in BioTelemetry.
BOSTON – Ablation of atrial fibrillation led to significantly better quality of life improvements, compared with antiarrhythmic drug therapy, in a prespecified, secondary analysis of data from the CABANA multicenter, randomized trial with 2,204 patients.
The improvements in quality of life measures in atrial fibrillation (AF) patients following ablation were “clinically meaningful” relative to drug therapy and were sustained for 5 years of follow-up, said Douglas L. Packer, MD, at the annual International AF Symposium.
The apparent incremental benefit in quality of life after ablation, compared with patients treated with drug therapy, added to the benefits previously reported from CABANA (Catheter Ablation vs. Antiarrhythmic Drug Therapy for Atrial Fibrillation Trial) using ablation that included a highly significant reduction in recurrent AF and a significant reduction in the combined endpoint of mortality or cardiovascular hospitalization. However, the trial’s results were neutral for the study’s prespecified primary endpoint, a combination of the rate of total mortality, disabling stroke, serious bleeding, or cardiac arrest in an intention-to-treat analysis, a result first reported by Dr. Packer at the annual scientific sessions of the Heart Rhythm Society in May 2018.
When Dr. Packer spoke at the AF Symposium in late January 2019, the CABANA results had still not been published. He said that he expected an article with the main findings to appear online sometime in February 2019.
The quality of life analysis, focused on two measures, the Mayo AF-Specific Symptoms Inventory (MAFSI) (Circulation. 2008 Oct 28;118[suppl 18]:S589) and the Atrial Fibrillation Affect on Quality of Life (AFEQT) (Circ Arrhythm Electrophysiol. 2011 Feb;4[1]:15-25). The patients enrolled in the two treatment arms of CABANA had essentially identical baseline MAFSI frequency scores, but starting 3 months after entry patients randomized to the ablation arm had an average, statistically significant 1.6-point improvement in their adjusted MAFSI frequency score, compared with patients in the drug-therapy arm; this reduction persisted at about the same statistically significant level through 5 years, said Dr. Packer, an electrophysiologist and professor of medicine at the Mayo Clinic in Rochester, Minn., and lead investigator of CABANA. Patients who remained in follow-up throughout the study’s entire 60 months underwent a total of seven MAFSI assessments after their initial treatment; the average, adjusted difference in MAFSI frequency scores throughout CABANA was a statistically significant 1.4 points lower among ablated patients, compared with those randomized to drug therapy, said Dr. Packer, who presented data first reported by the CABANA researchers in 2018.
The pattern of change in the AFEQT scores was very similar. The average, adjusted AFEQT summary scores were virtually identical at baseline for the two treatment arms, but at 3 months after the study began patients in the ablation arm had an average, adjusted, statistically significant 3-point improvement in their AFEQT summary scores, compared with drug-treated patients; this incremental increase in AFEQT scores remained consistent and statistically significant through 5 years of follow-up. Throughout the study, the average, adjusted, incremental improvement in AFEQT after ablation was a statistically significant 3.4 points, said Dr. Packer.
But these quality of life measures in patients who received unblinded assignment to an ablation procedure or drug treatment in CABANA are likely unreliable, commented Peter R. Kowey, MD, an electrophysiologist at the Lankenau Heart Institute in Wynnewood, Penn., and professor of medicine at Jefferson Medical College, Philadelphia.
“Assessing quality of life in an unblinded trial seems to me to be pretty shallow,” said Dr. Kowey, a member of the CABANA steering committee and a participant in a panel at the Symposium that discussed the results. Patients who have just gone through a 6-hour procedure will often say they now feel a whole lot better, he suggested. “They’re concerned what will get done to them if they don’t say they feel better.” The same confounding also applies to other “soft” secondary endpoints used in CABANA, such as hospitalization rates.
The reliability of these more subjective outcome measures is reduced in an unblinded trial, Dr. Kowey maintained. The CABANA results “don’t change the fundamental principal of using hard endpoints,” such as those that made up the primary endpoint of the study, he said in an interview. “We put secondary endpoints into the study because they are legitimate things to look at, but they have questionable reliability,” as measures of ablation’s efficacy.
Dr. Kowey also cautioned against focusing on the per-protocol and treatment-received analyses of the CABANA results, prespecified analyses that Dr. Packer highlighted because of the high number of crossovers in the trial: 9% of patients assigned to ablation never received it and 28% of patients assigned to drug therapy actually received ablation.
The CABANA steering committee anticipated a high crossover rate, but still set the intention-to-treat analysis as primary because “per protocol introduces many biases and can’t be relied on for a study of this magnitude,” Dr. Kowey said. He also cited the statistical pitfalls of looking at multiple secondary endpoints in the CABANA results and the danger of reading too much into subgroup analyses, all from a trial with a neutral primary endpoint.
Another concern he raised centered on the generalizability of CABANA’s safety findings, which showed roughly similar adverse event rates between the two treatment arms – about 9% with ablation, 4% with drugs – although the distribution of complications types differed between the two arms. The “ ‘remarkably safe’ performance of ablation in CABANA can be attributed to ‘cherry-picked’ investigators” who performed the ablation procedures at high-volume centers, said Dr. Kowey. “The safety of ablation was predictable” in CABANA because of the selection of participating centers. “Seventy percent of U.S. ablations are being done at centers that do fewer than 25 ablations annually,” Dr. Kowey said. “We don’t know what goes on” at lower-volume centers; “ablation can’t be considered as safe as we presume” when done at centers outside the 118 sites that participated in CABANA, he maintained.
CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to these four companies as well as to CyberHeart, nContact, Sanofi-Aventis, and Toray. He has received research funding from AG, Biosense Webster, Boston Scientific, CryoCath, EP Limited, and Medtronic, and has a financial interest in AF mapping technology. Dr. Kowey has been a consultant to several companies that market antiarrhythmic drugs or market arrhythmia devices and has an equity interest in BioTelemetry.
REPORTING FROM THE AF SYMPOSIUM 2019
Key clinical point: In CABANA, atrial fibrillation ablation led to a clinically meaningful improvement of quality of life measures, compared with drug therapy.
Major finding: After ablation, the MAFSI score averaged a 1.4-point improvement; the AFEQT score averaged a 3.4-point improvement over drug therapy.
Study details: CABANA, a multicenter, randomized trial with 2,204 atrial fibrillation patients.
Disclosures: CABANA received partial funding from Biosense Webster, Boston Scientific, Medtronic, and St. Jude. Dr. Packer has been a consultant to these four companies as well as to CyberHeart, nContact, Sanofi-Aventis, and Toray. He has received research funding from AG, Biosense Webster, Boston Scientific, CryoCath, EP Limited, and Medtronic, and has a financial interest in atrial fibrillation mapping technology. Dr. Kowey has been a consultant to several companies that market antiarrhythmic drugs or market arrhythmia devices and has an equity interest in BioTelemetry.
Age of migraine onset may affect stroke risk
The age at which a patient develops migraine with aura may be an important factor in assessing stroke risk, according to a prospective cohort study published in Headache.
Patients who had onset of migraine with visual aura after age 50 years had an increased risk of ischemic stroke, compared with patients with no headache, the researchers found. Patients with longer exposure to migraine with visual aura – that is, onset before age 50 years – did not have significantly increased ischemic stroke risk, said X. Michelle Androulakis, MD, of the department of neurology at the University of South Carolina in Columbia, and her colleagues.
“Migraine, especially migraine with aura, is associated with increased risk of ischemic stroke,” but whether age of migraine onset affects the risk of cardiovascular disease has been unclear, the researchers said.
To examine the risk of ischemic stroke in migraineurs with and without aura with onset before and after age 50 years, the investigators conducted a post hoc analysis of data from the ongoing Atherosclerosis Risk in Communities (ARIC) study. The researchers adjusted for potential confounders, including diabetes, body mass index, hypertension, and hyperlipidemia.
In ARIC, participants completed a questionnaire about their migraine history at their third study visit (1993-1995) and were followed for ischemic stroke incidence over 20 years.
Of the 11,592 ARIC participants included in the analysis (mean age, 61 years; 76.5% white; and 55.3% female), 447 had migraine with aura, and 1,128 had migraine without aura. Onset of migraine with aura at age 50 years or older (average duration, 4.75 years) was associated with more than twofold greater risk of ischemic stroke, compared with no headache (multivariable adjusted hazard ratio = 2.17). Onset of migraine with aura before age 50 years (average duration, 28.17 years) was not significantly associated with stroke. A logistic regression model yielded consistent results.
In addition, patients with migraine without aura did not have an increased risk of stroke, regardless of the age of onset. The absolute risk for stroke in migraine with aura was 8.27%, and the absolute risk in migraine without aura was 4.25%.
“We found unexpected results suggesting that the onset of migraine with aura before age 50 is not associated with ischemic stroke. ... These results are specific to first-time ischemic stroke incidents that occurred in mid- to late life; therefore, it cannot be generalized to stroke in younger patients,” the authors wrote.
It could be that migraine with aura symptoms that start at a later age are a red flag for paradoxical emboli from a patent foramen ovale or microemboli, Dr. Androulakis and her colleagues noted. It also is possible that the degree of cortical spreading depression required to induce migraine with aura symptoms is different later in life versus earlier in life.
“This study underscores the importance of MA symptoms onset in evaluation of ischemic stroke risk in late life,” the researchers concluded.
The authors had no relevant conflicts of interest. ARIC has been funded by the National Heart, Lung, and Blood Institute.
SOURCE: Androulakis XM et al. Headache. 2019 Jan 21. doi: 10.1111/head.13468.
The age at which a patient develops migraine with aura may be an important factor in assessing stroke risk, according to a prospective cohort study published in Headache.
Patients who had onset of migraine with visual aura after age 50 years had an increased risk of ischemic stroke, compared with patients with no headache, the researchers found. Patients with longer exposure to migraine with visual aura – that is, onset before age 50 years – did not have significantly increased ischemic stroke risk, said X. Michelle Androulakis, MD, of the department of neurology at the University of South Carolina in Columbia, and her colleagues.
“Migraine, especially migraine with aura, is associated with increased risk of ischemic stroke,” but whether age of migraine onset affects the risk of cardiovascular disease has been unclear, the researchers said.
To examine the risk of ischemic stroke in migraineurs with and without aura with onset before and after age 50 years, the investigators conducted a post hoc analysis of data from the ongoing Atherosclerosis Risk in Communities (ARIC) study. The researchers adjusted for potential confounders, including diabetes, body mass index, hypertension, and hyperlipidemia.
In ARIC, participants completed a questionnaire about their migraine history at their third study visit (1993-1995) and were followed for ischemic stroke incidence over 20 years.
Of the 11,592 ARIC participants included in the analysis (mean age, 61 years; 76.5% white; and 55.3% female), 447 had migraine with aura, and 1,128 had migraine without aura. Onset of migraine with aura at age 50 years or older (average duration, 4.75 years) was associated with more than twofold greater risk of ischemic stroke, compared with no headache (multivariable adjusted hazard ratio = 2.17). Onset of migraine with aura before age 50 years (average duration, 28.17 years) was not significantly associated with stroke. A logistic regression model yielded consistent results.
In addition, patients with migraine without aura did not have an increased risk of stroke, regardless of the age of onset. The absolute risk for stroke in migraine with aura was 8.27%, and the absolute risk in migraine without aura was 4.25%.
“We found unexpected results suggesting that the onset of migraine with aura before age 50 is not associated with ischemic stroke. ... These results are specific to first-time ischemic stroke incidents that occurred in mid- to late life; therefore, it cannot be generalized to stroke in younger patients,” the authors wrote.
It could be that migraine with aura symptoms that start at a later age are a red flag for paradoxical emboli from a patent foramen ovale or microemboli, Dr. Androulakis and her colleagues noted. It also is possible that the degree of cortical spreading depression required to induce migraine with aura symptoms is different later in life versus earlier in life.
“This study underscores the importance of MA symptoms onset in evaluation of ischemic stroke risk in late life,” the researchers concluded.
The authors had no relevant conflicts of interest. ARIC has been funded by the National Heart, Lung, and Blood Institute.
SOURCE: Androulakis XM et al. Headache. 2019 Jan 21. doi: 10.1111/head.13468.
The age at which a patient develops migraine with aura may be an important factor in assessing stroke risk, according to a prospective cohort study published in Headache.
Patients who had onset of migraine with visual aura after age 50 years had an increased risk of ischemic stroke, compared with patients with no headache, the researchers found. Patients with longer exposure to migraine with visual aura – that is, onset before age 50 years – did not have significantly increased ischemic stroke risk, said X. Michelle Androulakis, MD, of the department of neurology at the University of South Carolina in Columbia, and her colleagues.
“Migraine, especially migraine with aura, is associated with increased risk of ischemic stroke,” but whether age of migraine onset affects the risk of cardiovascular disease has been unclear, the researchers said.
To examine the risk of ischemic stroke in migraineurs with and without aura with onset before and after age 50 years, the investigators conducted a post hoc analysis of data from the ongoing Atherosclerosis Risk in Communities (ARIC) study. The researchers adjusted for potential confounders, including diabetes, body mass index, hypertension, and hyperlipidemia.
In ARIC, participants completed a questionnaire about their migraine history at their third study visit (1993-1995) and were followed for ischemic stroke incidence over 20 years.
Of the 11,592 ARIC participants included in the analysis (mean age, 61 years; 76.5% white; and 55.3% female), 447 had migraine with aura, and 1,128 had migraine without aura. Onset of migraine with aura at age 50 years or older (average duration, 4.75 years) was associated with more than twofold greater risk of ischemic stroke, compared with no headache (multivariable adjusted hazard ratio = 2.17). Onset of migraine with aura before age 50 years (average duration, 28.17 years) was not significantly associated with stroke. A logistic regression model yielded consistent results.
In addition, patients with migraine without aura did not have an increased risk of stroke, regardless of the age of onset. The absolute risk for stroke in migraine with aura was 8.27%, and the absolute risk in migraine without aura was 4.25%.
“We found unexpected results suggesting that the onset of migraine with aura before age 50 is not associated with ischemic stroke. ... These results are specific to first-time ischemic stroke incidents that occurred in mid- to late life; therefore, it cannot be generalized to stroke in younger patients,” the authors wrote.
It could be that migraine with aura symptoms that start at a later age are a red flag for paradoxical emboli from a patent foramen ovale or microemboli, Dr. Androulakis and her colleagues noted. It also is possible that the degree of cortical spreading depression required to induce migraine with aura symptoms is different later in life versus earlier in life.
“This study underscores the importance of MA symptoms onset in evaluation of ischemic stroke risk in late life,” the researchers concluded.
The authors had no relevant conflicts of interest. ARIC has been funded by the National Heart, Lung, and Blood Institute.
SOURCE: Androulakis XM et al. Headache. 2019 Jan 21. doi: 10.1111/head.13468.
FROM HEADACHE
Key clinical point: Age of migraine onset may be an important factor in assessing stroke risk.
Major finding: (multivariable adjusted hazard ratio = 2.17).
Study details: A post hoc analysis of data from more than 11,500 participants in the Atherosclerosis Risk in Communities (ARIC) study.
Disclosures: The authors had no relevant conflicts of interest. ARIC has been funded by the National Heart, Lung, and Blood Institute.
Source: Androulakis XM et al. Headache. 2019 Jan 21. doi: 10.1111/head.13468.
Meta-analysis supports aspirin to reduce cardiovascular events
Aspirin use is associated with a reduced risk of cardiovascular events among adults without cardiovascular disease, but this protection comes with a similarly increased risk for bleeding, according to data from a meta-analysis that included more than 1 million participant-years of follow-up.
“The uncertain role of aspirin in primary prevention of cardiovascular events is reflected in contrasting recommendations offered by guideline bodies,” and has led to a decline in prescribing aspirin for primary prevention of such events, wrote Sean L. Zheng, MRCP, of Imperial College London (England) and his colleagues.
In a systematic review and meta-analysis published in JAMA, the researchers examined 13 randomized trials altogether including 164,225 participants and 1,050,511 participant-years of follow-up.
Overall, aspirin use significantly reduced a composite of cardiovascular outcomes, compared with no aspirin (hazard ratio, 0.89). The composite outcome included cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke, and it occurred in 57.1 per 10,000 participant-years in aspirin users versus 61.4 per 10,000 participant-years among individuals who did not use aspirin. The absolute risk reduction was 0.38%.
The median age of the study participants was 62 years, and roughly half (47%) were male.
However, the risk of major bleeding events was significantly higher among aspirin users, compared with nonusers (23.1 per 10,000 participant-years and 16.4 per 10,000 participant-years, respectively), with a HR of 1.43 and an absolute risk increase of 0.47%.
Aspirin use was not associated with several secondary outcomes, including reductions in all-cause mortality or cardiovascular mortality, compared with no aspirin, but it was associated with a reduced risk specifically of myocardial infarction and ischemic stroke. Few deaths related to bleeding were reported.
The number needed to treat (265) and the number needed to harm (210) were similar, which emphasizes the need for an individual approach to treatment, the researchers noted.
“Consequently, the decision to use aspirin for primary prevention may need to be made on an individual basis, accounting for the patient’s risk of bleeding and their views on the balance of risk vs. benefit,” they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Zheng SL et al. JAMA. 2019;321(3):277-87.
Aspirin remains an important tool for the primary prevention of atherothrombotic vascular events, wrote J. Michael Gaziano, MD, in an accompanying editorial.
Historically, some guidelines have recommended against using aspirin for primary prevention of cardiovascular events because of the potential for harm, Dr. Gaziano noted, so a personalized approach to aspirin based on risk assessment is warranted. Dr. Gaziano also commented that risk is fluid; for example, patients who take action to improve their health and reduce risk by stopping smoking, eating differently, or exercising can reduce their risk for future CVD events.
“Because weighing the risks and benefits of aspirin in primary prevention is complicated, it should involve a shared decision-making discussion between the patient and the clinician,” he wrote. The current meta-analysis shows the consistency of recent trials with older studies, he remarked, noting that aspirin could be even more important as a cost-effective intervention in certain parts of the world where cardiovascular disease is on the rise and other treatments for CVD may be limited.
“Aspirin remains an important medication for acute management of vascular events; for use after certain procedures; for secondary prevention; and, after careful selection of the right patients, for primary prevention,” he concluded (JAMA. 2019;321[3]:253-5).
Dr. Gaziano is affiliated with Brigham and Women’s Hospital in Boston. He disclosed serving on the executive committee of the ARRIVE trial and serving as a consultant and receiving honoraria for speaking for Bayer.
Aspirin remains an important tool for the primary prevention of atherothrombotic vascular events, wrote J. Michael Gaziano, MD, in an accompanying editorial.
Historically, some guidelines have recommended against using aspirin for primary prevention of cardiovascular events because of the potential for harm, Dr. Gaziano noted, so a personalized approach to aspirin based on risk assessment is warranted. Dr. Gaziano also commented that risk is fluid; for example, patients who take action to improve their health and reduce risk by stopping smoking, eating differently, or exercising can reduce their risk for future CVD events.
“Because weighing the risks and benefits of aspirin in primary prevention is complicated, it should involve a shared decision-making discussion between the patient and the clinician,” he wrote. The current meta-analysis shows the consistency of recent trials with older studies, he remarked, noting that aspirin could be even more important as a cost-effective intervention in certain parts of the world where cardiovascular disease is on the rise and other treatments for CVD may be limited.
“Aspirin remains an important medication for acute management of vascular events; for use after certain procedures; for secondary prevention; and, after careful selection of the right patients, for primary prevention,” he concluded (JAMA. 2019;321[3]:253-5).
Dr. Gaziano is affiliated with Brigham and Women’s Hospital in Boston. He disclosed serving on the executive committee of the ARRIVE trial and serving as a consultant and receiving honoraria for speaking for Bayer.
Aspirin remains an important tool for the primary prevention of atherothrombotic vascular events, wrote J. Michael Gaziano, MD, in an accompanying editorial.
Historically, some guidelines have recommended against using aspirin for primary prevention of cardiovascular events because of the potential for harm, Dr. Gaziano noted, so a personalized approach to aspirin based on risk assessment is warranted. Dr. Gaziano also commented that risk is fluid; for example, patients who take action to improve their health and reduce risk by stopping smoking, eating differently, or exercising can reduce their risk for future CVD events.
“Because weighing the risks and benefits of aspirin in primary prevention is complicated, it should involve a shared decision-making discussion between the patient and the clinician,” he wrote. The current meta-analysis shows the consistency of recent trials with older studies, he remarked, noting that aspirin could be even more important as a cost-effective intervention in certain parts of the world where cardiovascular disease is on the rise and other treatments for CVD may be limited.
“Aspirin remains an important medication for acute management of vascular events; for use after certain procedures; for secondary prevention; and, after careful selection of the right patients, for primary prevention,” he concluded (JAMA. 2019;321[3]:253-5).
Dr. Gaziano is affiliated with Brigham and Women’s Hospital in Boston. He disclosed serving on the executive committee of the ARRIVE trial and serving as a consultant and receiving honoraria for speaking for Bayer.
Aspirin use is associated with a reduced risk of cardiovascular events among adults without cardiovascular disease, but this protection comes with a similarly increased risk for bleeding, according to data from a meta-analysis that included more than 1 million participant-years of follow-up.
“The uncertain role of aspirin in primary prevention of cardiovascular events is reflected in contrasting recommendations offered by guideline bodies,” and has led to a decline in prescribing aspirin for primary prevention of such events, wrote Sean L. Zheng, MRCP, of Imperial College London (England) and his colleagues.
In a systematic review and meta-analysis published in JAMA, the researchers examined 13 randomized trials altogether including 164,225 participants and 1,050,511 participant-years of follow-up.
Overall, aspirin use significantly reduced a composite of cardiovascular outcomes, compared with no aspirin (hazard ratio, 0.89). The composite outcome included cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke, and it occurred in 57.1 per 10,000 participant-years in aspirin users versus 61.4 per 10,000 participant-years among individuals who did not use aspirin. The absolute risk reduction was 0.38%.
The median age of the study participants was 62 years, and roughly half (47%) were male.
However, the risk of major bleeding events was significantly higher among aspirin users, compared with nonusers (23.1 per 10,000 participant-years and 16.4 per 10,000 participant-years, respectively), with a HR of 1.43 and an absolute risk increase of 0.47%.
Aspirin use was not associated with several secondary outcomes, including reductions in all-cause mortality or cardiovascular mortality, compared with no aspirin, but it was associated with a reduced risk specifically of myocardial infarction and ischemic stroke. Few deaths related to bleeding were reported.
The number needed to treat (265) and the number needed to harm (210) were similar, which emphasizes the need for an individual approach to treatment, the researchers noted.
“Consequently, the decision to use aspirin for primary prevention may need to be made on an individual basis, accounting for the patient’s risk of bleeding and their views on the balance of risk vs. benefit,” they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Zheng SL et al. JAMA. 2019;321(3):277-87.
Aspirin use is associated with a reduced risk of cardiovascular events among adults without cardiovascular disease, but this protection comes with a similarly increased risk for bleeding, according to data from a meta-analysis that included more than 1 million participant-years of follow-up.
“The uncertain role of aspirin in primary prevention of cardiovascular events is reflected in contrasting recommendations offered by guideline bodies,” and has led to a decline in prescribing aspirin for primary prevention of such events, wrote Sean L. Zheng, MRCP, of Imperial College London (England) and his colleagues.
In a systematic review and meta-analysis published in JAMA, the researchers examined 13 randomized trials altogether including 164,225 participants and 1,050,511 participant-years of follow-up.
Overall, aspirin use significantly reduced a composite of cardiovascular outcomes, compared with no aspirin (hazard ratio, 0.89). The composite outcome included cardiovascular mortality, nonfatal myocardial infarction, and nonfatal stroke, and it occurred in 57.1 per 10,000 participant-years in aspirin users versus 61.4 per 10,000 participant-years among individuals who did not use aspirin. The absolute risk reduction was 0.38%.
The median age of the study participants was 62 years, and roughly half (47%) were male.
However, the risk of major bleeding events was significantly higher among aspirin users, compared with nonusers (23.1 per 10,000 participant-years and 16.4 per 10,000 participant-years, respectively), with a HR of 1.43 and an absolute risk increase of 0.47%.
Aspirin use was not associated with several secondary outcomes, including reductions in all-cause mortality or cardiovascular mortality, compared with no aspirin, but it was associated with a reduced risk specifically of myocardial infarction and ischemic stroke. Few deaths related to bleeding were reported.
The number needed to treat (265) and the number needed to harm (210) were similar, which emphasizes the need for an individual approach to treatment, the researchers noted.
“Consequently, the decision to use aspirin for primary prevention may need to be made on an individual basis, accounting for the patient’s risk of bleeding and their views on the balance of risk vs. benefit,” they concluded.
The researchers had no financial conflicts to disclose.
SOURCE: Zheng SL et al. JAMA. 2019;321(3):277-87.
FROM JAMA
Key clinical point:
Major finding: The absolute risk reduction was 0.38% for a composite of cardiovascular events among aspirin users versus nonusers.
Study details: The data come from a meta-analysis of 13 randomized trials altogether including 1,050,511 participants-years of follow-up.
Disclosures: The researchers had no financial conflicts to disclose.
Source: Zheng SL et al. JAMA. 2019;321(3):277-87.
Obesity paradox applies to post-stroke mortality
CHICAGO – Overweight and obese military veterans who experienced an in-hospital stroke had a lower 30-day and 1-year all-cause mortality than did those who were normal weight in a large national study, Lauren Costa reported at the American Heart Association scientific sessions.
Underweight patients had a significantly increased mortality risk, added Ms. Costa of the VA Boston Healthcare System.
It’s yet another instance of what is known as the obesity paradox, which has also been described in patients with heart failure, acute coronary syndrome, MI, chronic obstructive pulmonary disease, and other conditions.
Ms. Costa presented a retrospective study of 26,267 patients in the Veterans Health Administration database who had a first stroke in-hospital during 2002-2012. There were subsequently 14,166 deaths, including 2,473 within the first 30 days and 5,854 in the first year post stroke.
Each patient’s body mass index was calculated based on the average of all BMI measurements obtained 1-24 months prior to the stroke. The analysis of the relationship between BMI and poststroke mortality included extensive statistical adjustment for potential confounders, including age, sex, smoking, cancer, dementia, peripheral artery disease, diabetes, coronary heart disease, atrial fibrillation, chronic kidney disease, use of statins, and antihypertensive therapy.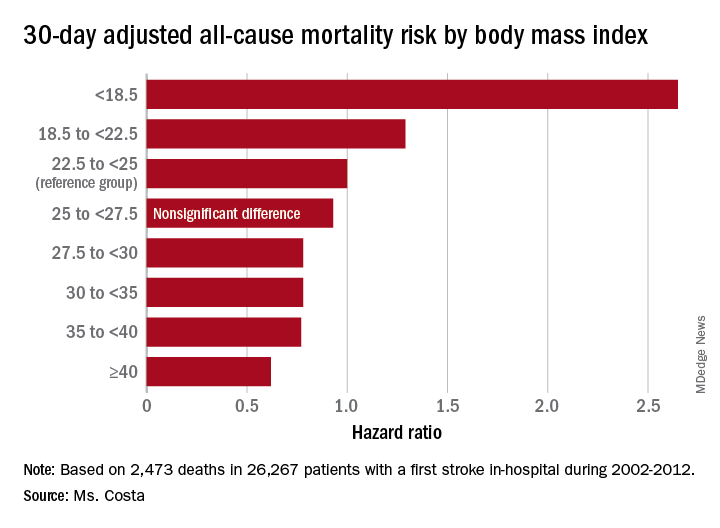
Breaking down the study population into eight BMI categories, Ms. Costa found that the adjusted risk of 30-day all-cause mortality post stroke was reduced by 22%-38% in patients in the overweight or obese groupings, compared with the reference population with a normal-weight BMI of 22.5 to less than 25 kg/m2.
One-year, all-cause mortality showed the same pattern of BMI-based significant differences.
Of deaths within 30 days post stroke, 34% were stroke-related. In an analysis restricted to that group, the evidence of an obesity paradox was attenuated. Indeed, the only BMI group with an adjusted 30-day stroke-related mortality significantly different from the normal-weight reference group were patients with Class III obesity, defined as a BMI of 40 or more. Their risk was reduced by 45%.
The obesity paradox remains a controversial issue among epidemiologists. The increased mortality associated with being underweight among patients with diseases where the obesity paradox has been documented is widely thought to be caused by frailty and/or an underlying illness not adjusted for in analyses. But the mechanism for the reduced mortality risk in overweight and obese patients seen in the VA stroke study and other studies remains unknown despite much speculation.
Ms. Costa reported having no financial conflicts regarding her study, which was supported by the Department of Veterans Affairs.
SOURCE: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
CHICAGO – Overweight and obese military veterans who experienced an in-hospital stroke had a lower 30-day and 1-year all-cause mortality than did those who were normal weight in a large national study, Lauren Costa reported at the American Heart Association scientific sessions.
Underweight patients had a significantly increased mortality risk, added Ms. Costa of the VA Boston Healthcare System.
It’s yet another instance of what is known as the obesity paradox, which has also been described in patients with heart failure, acute coronary syndrome, MI, chronic obstructive pulmonary disease, and other conditions.
Ms. Costa presented a retrospective study of 26,267 patients in the Veterans Health Administration database who had a first stroke in-hospital during 2002-2012. There were subsequently 14,166 deaths, including 2,473 within the first 30 days and 5,854 in the first year post stroke.
Each patient’s body mass index was calculated based on the average of all BMI measurements obtained 1-24 months prior to the stroke. The analysis of the relationship between BMI and poststroke mortality included extensive statistical adjustment for potential confounders, including age, sex, smoking, cancer, dementia, peripheral artery disease, diabetes, coronary heart disease, atrial fibrillation, chronic kidney disease, use of statins, and antihypertensive therapy.
Breaking down the study population into eight BMI categories, Ms. Costa found that the adjusted risk of 30-day all-cause mortality post stroke was reduced by 22%-38% in patients in the overweight or obese groupings, compared with the reference population with a normal-weight BMI of 22.5 to less than 25 kg/m2.
One-year, all-cause mortality showed the same pattern of BMI-based significant differences.
Of deaths within 30 days post stroke, 34% were stroke-related. In an analysis restricted to that group, the evidence of an obesity paradox was attenuated. Indeed, the only BMI group with an adjusted 30-day stroke-related mortality significantly different from the normal-weight reference group were patients with Class III obesity, defined as a BMI of 40 or more. Their risk was reduced by 45%.
The obesity paradox remains a controversial issue among epidemiologists. The increased mortality associated with being underweight among patients with diseases where the obesity paradox has been documented is widely thought to be caused by frailty and/or an underlying illness not adjusted for in analyses. But the mechanism for the reduced mortality risk in overweight and obese patients seen in the VA stroke study and other studies remains unknown despite much speculation.
Ms. Costa reported having no financial conflicts regarding her study, which was supported by the Department of Veterans Affairs.
SOURCE: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
CHICAGO – Overweight and obese military veterans who experienced an in-hospital stroke had a lower 30-day and 1-year all-cause mortality than did those who were normal weight in a large national study, Lauren Costa reported at the American Heart Association scientific sessions.
Underweight patients had a significantly increased mortality risk, added Ms. Costa of the VA Boston Healthcare System.
It’s yet another instance of what is known as the obesity paradox, which has also been described in patients with heart failure, acute coronary syndrome, MI, chronic obstructive pulmonary disease, and other conditions.
Ms. Costa presented a retrospective study of 26,267 patients in the Veterans Health Administration database who had a first stroke in-hospital during 2002-2012. There were subsequently 14,166 deaths, including 2,473 within the first 30 days and 5,854 in the first year post stroke.
Each patient’s body mass index was calculated based on the average of all BMI measurements obtained 1-24 months prior to the stroke. The analysis of the relationship between BMI and poststroke mortality included extensive statistical adjustment for potential confounders, including age, sex, smoking, cancer, dementia, peripheral artery disease, diabetes, coronary heart disease, atrial fibrillation, chronic kidney disease, use of statins, and antihypertensive therapy.
Breaking down the study population into eight BMI categories, Ms. Costa found that the adjusted risk of 30-day all-cause mortality post stroke was reduced by 22%-38% in patients in the overweight or obese groupings, compared with the reference population with a normal-weight BMI of 22.5 to less than 25 kg/m2.
One-year, all-cause mortality showed the same pattern of BMI-based significant differences.
Of deaths within 30 days post stroke, 34% were stroke-related. In an analysis restricted to that group, the evidence of an obesity paradox was attenuated. Indeed, the only BMI group with an adjusted 30-day stroke-related mortality significantly different from the normal-weight reference group were patients with Class III obesity, defined as a BMI of 40 or more. Their risk was reduced by 45%.
The obesity paradox remains a controversial issue among epidemiologists. The increased mortality associated with being underweight among patients with diseases where the obesity paradox has been documented is widely thought to be caused by frailty and/or an underlying illness not adjusted for in analyses. But the mechanism for the reduced mortality risk in overweight and obese patients seen in the VA stroke study and other studies remains unknown despite much speculation.
Ms. Costa reported having no financial conflicts regarding her study, which was supported by the Department of Veterans Affairs.
SOURCE: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
REPORTING FROM THE AHA SCIENTIFIC SESSIONS
Key clinical point: Heavier stroke patients have lower 30-day and 1-year all-cause mortality.
Major finding: The 30-day stroke-related mortality rate after in-hospital stroke was reduced by 45% in VA patients with Class III obesity.
Study details: This retrospective study looked at the relationship between body mass index and post-stroke mortality in more than 26,000 veterans who had an inpatient stroke, with extensive adjustments made for potential confounders.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was sponsored by the Department of Veterans Affairs.
Source: Costa L. Circulation. 2018;138(suppl 1): Abstract 14288.
ATTRACT trial shouldn’t detract from pharmacomechanical thrombolysis
CHICAGO – A closer look at the landmark ATTRACT trial of pharmacomechanical catheter-directed thrombolysis for acute deep vein thrombosis (DVT) shows multiple benefits for the intervention versus standard anticoagulation alone in the subset of participants with iliofemoral DVT, Kush R. Desai, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
ATTRACT, a National Institutes of Health–sponsored, phase 3, multicenter, open-label, assessor-blinded study, was the first-ever randomized trial of pharmacomechanical catheter-directed thrombolysis (PCDT) for acute DVT.
The results caused a major stir because, despite a sound therapeutic rationale for the procedure, the incidence of chronic postthrombotic syndrome (PTS) at 24 months of follow-up was 47% in the PCDT plus anticoagulation group and 48% in controls on anticoagulation alone (N Engl J Med. 2017 Dec 7;377[23]:2240-52). Since then, that overall negative trial has been one of the hottest topics in DVT.
“This is the first thing your educated patients who come to the emergency department with DVT will ask about. It’s the first thing they’ll see when they go online and type in ‘thrombolysis DVT,’ ” noted Dr. Desai, an interventional radiologist at Northwestern University, Chicago.
But the trial has several major flaws, he cautioned. And contrary to popular opinion, ATTRACT is not the death knell for PCDT. Far from it.
“I don’t think the story stops with ATTRACT. This isn’t the end for PCDT in patients with iliofemoral DVT,” he asserted.
That’s in part because 301 of the 692 participants in ATTRACT had DVT of the femoropopliteal segment. That’s a population in which Dr. Desai and other interventionalists wouldn’t have anticipated seeing a benefit for PCDT, because their risk of PTS is so low.
“We know through historical data that patients with iliofemoral DVT are much more likely to develop PTS and to have recurrent DVT, so this is probably one of the major shortcomings of the trial,” he explained. “It’s through no fault of the trial investigators, because the study was planned years ago when we just didn’t know as much about PTS as we do now.
“The way I look at it is, I don’t practice in the way that ATTRACT was designed,” Dr. Desai said. “I don’t typically lyse or get referrals for lysis or thrombectomy in patients who have isolated femoropopliteal DVT. It has to involve at least the common femoral vein and frequently goes up to the iliac vein.”
The ATTRACT investigators’ recent subanalysis of the 391 participants with iliofemoral DVT showed that, although there was no difference between the two study arms in the occurrence of PTS through the first 24 months of follow-up, PCDT led to a 35% reduction in the incidence of moderate or severe PTS – by a margin of 18% versus 28% in controls.
Patients in the PCDT arm also experienced significantly greater improvement in venous disease-specific quality of life through 24 months, and a greater reduction in leg pain and swelling at 10 and 30 days (Circulation. 2018 Dec 4. doi: 10.1161/CIRCULATIONAHA.118.037425).
And moderate to severe PTS is a key outcome, Dr. Desai continued. Multiple studies have shown that patients with PTS have a worse quality of life than those with chronic lung disease, arthritis, or diabetes. Moreover, the 5%-10% of patients with symptomatic DVT who develop the most-severe form of PTS – characterized by severe pain, chronic ulcerations, stasis dermatitis, venous claudication, and intractable edema – have a quality of life comparable with patients with cancer or heart failure.
The 1.5% incidence of major bleeding within 10 days in the PCDT group was 200% higher than in controls, but none of it was life threatening.
“This is reassuring: Nobody had intracranial hemorrhage; nobody had a GUSTO 5 bleed,” Dr. Desai said.
Another limitation of the ATTRACT trial is that all but one of the devices utilized for PCDT were used off label. They weren’t designed for venous application. Several on-label rheolytic, rotational thrombectomy, or clot aspiration devices have been approved since enrollment in ATTRACT was closed. Future randomized trials will utilize on-label devices in patients with acute iliofemoral DVT to clarify the role of PCDT.
It’s noteworthy that nearly half of ATTRACT participants developed PTS within 24 months of their DVT despite being on optimal anticoagulation. It’s a finding that underscores the need for improved therapies. That was the impetus for development of first-generation catheter-directed thrombolysis utilizing a percutaneously inserted catheter to infuse a fibrinolytic drug directly to the thrombus to dissolve it rapidly.
But that form of catheter-directed thrombolysis has major disadvantages, Dr. Desai explained: It’s a multiday procedure requiring ICU-level care and prolonged exposure to powerful lytic agents.
“This is where things have changed with PCDT,” he said. “We can now, with on-label devices, accelerate the thrombolysis time, reduce lytic exposure, and I think also reduce the bleeding risk, although that hasn’t been shown in a trial yet. PCDT also reduces the necessity for ICU-level care and prolonged hospitalization.”
Dr. Desai no longer performs multiday lytic procedures. “In fact, with the introduction of the newer on-label devices, I haven’t done a multiday unilateral limb lytic procedure in a couple years. I think we’ve gotten to the point where we don’t need to do that anymore.”
Indeed, PCDT makes recanalization possible as a single-day, single-session procedure.
Dr. Desai views the recent ATTRACT subanalysis as hypothesis generating.
“Should PCDT be the first-line treatment in all proximal DVT patients? No it should not – and that’s not what I would have advocated even before ATTRACT came out,” he explained. “It’s sort of a salvage procedure for patients with iliofemoral DVT and moderate to severe symptoms. And there are a significant number of such patients.”
Current understanding of the pathophysiology of PTS is that a nondissolved thrombus at the valve leaflets becomes inflammatory, with resultant valvular dysfunction leading to venous reflux and venous hypertension. PCDT is consistent with the open-vein hypothesis, which posits that, by eliminating thrombus much faster than achievable via anticoagulation, valve integrity is maintained and PTS is prevented.
Dr. Desai reported receiving consulting fees from AngioDynamics, Boston Scientific, Cook Medical, and Spectranetics.
CHICAGO – A closer look at the landmark ATTRACT trial of pharmacomechanical catheter-directed thrombolysis for acute deep vein thrombosis (DVT) shows multiple benefits for the intervention versus standard anticoagulation alone in the subset of participants with iliofemoral DVT, Kush R. Desai, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
ATTRACT, a National Institutes of Health–sponsored, phase 3, multicenter, open-label, assessor-blinded study, was the first-ever randomized trial of pharmacomechanical catheter-directed thrombolysis (PCDT) for acute DVT.
The results caused a major stir because, despite a sound therapeutic rationale for the procedure, the incidence of chronic postthrombotic syndrome (PTS) at 24 months of follow-up was 47% in the PCDT plus anticoagulation group and 48% in controls on anticoagulation alone (N Engl J Med. 2017 Dec 7;377[23]:2240-52). Since then, that overall negative trial has been one of the hottest topics in DVT.
“This is the first thing your educated patients who come to the emergency department with DVT will ask about. It’s the first thing they’ll see when they go online and type in ‘thrombolysis DVT,’ ” noted Dr. Desai, an interventional radiologist at Northwestern University, Chicago.
But the trial has several major flaws, he cautioned. And contrary to popular opinion, ATTRACT is not the death knell for PCDT. Far from it.
“I don’t think the story stops with ATTRACT. This isn’t the end for PCDT in patients with iliofemoral DVT,” he asserted.
That’s in part because 301 of the 692 participants in ATTRACT had DVT of the femoropopliteal segment. That’s a population in which Dr. Desai and other interventionalists wouldn’t have anticipated seeing a benefit for PCDT, because their risk of PTS is so low.
“We know through historical data that patients with iliofemoral DVT are much more likely to develop PTS and to have recurrent DVT, so this is probably one of the major shortcomings of the trial,” he explained. “It’s through no fault of the trial investigators, because the study was planned years ago when we just didn’t know as much about PTS as we do now.
“The way I look at it is, I don’t practice in the way that ATTRACT was designed,” Dr. Desai said. “I don’t typically lyse or get referrals for lysis or thrombectomy in patients who have isolated femoropopliteal DVT. It has to involve at least the common femoral vein and frequently goes up to the iliac vein.”
The ATTRACT investigators’ recent subanalysis of the 391 participants with iliofemoral DVT showed that, although there was no difference between the two study arms in the occurrence of PTS through the first 24 months of follow-up, PCDT led to a 35% reduction in the incidence of moderate or severe PTS – by a margin of 18% versus 28% in controls.
Patients in the PCDT arm also experienced significantly greater improvement in venous disease-specific quality of life through 24 months, and a greater reduction in leg pain and swelling at 10 and 30 days (Circulation. 2018 Dec 4. doi: 10.1161/CIRCULATIONAHA.118.037425).
And moderate to severe PTS is a key outcome, Dr. Desai continued. Multiple studies have shown that patients with PTS have a worse quality of life than those with chronic lung disease, arthritis, or diabetes. Moreover, the 5%-10% of patients with symptomatic DVT who develop the most-severe form of PTS – characterized by severe pain, chronic ulcerations, stasis dermatitis, venous claudication, and intractable edema – have a quality of life comparable with patients with cancer or heart failure.
The 1.5% incidence of major bleeding within 10 days in the PCDT group was 200% higher than in controls, but none of it was life threatening.
“This is reassuring: Nobody had intracranial hemorrhage; nobody had a GUSTO 5 bleed,” Dr. Desai said.
Another limitation of the ATTRACT trial is that all but one of the devices utilized for PCDT were used off label. They weren’t designed for venous application. Several on-label rheolytic, rotational thrombectomy, or clot aspiration devices have been approved since enrollment in ATTRACT was closed. Future randomized trials will utilize on-label devices in patients with acute iliofemoral DVT to clarify the role of PCDT.
It’s noteworthy that nearly half of ATTRACT participants developed PTS within 24 months of their DVT despite being on optimal anticoagulation. It’s a finding that underscores the need for improved therapies. That was the impetus for development of first-generation catheter-directed thrombolysis utilizing a percutaneously inserted catheter to infuse a fibrinolytic drug directly to the thrombus to dissolve it rapidly.
But that form of catheter-directed thrombolysis has major disadvantages, Dr. Desai explained: It’s a multiday procedure requiring ICU-level care and prolonged exposure to powerful lytic agents.
“This is where things have changed with PCDT,” he said. “We can now, with on-label devices, accelerate the thrombolysis time, reduce lytic exposure, and I think also reduce the bleeding risk, although that hasn’t been shown in a trial yet. PCDT also reduces the necessity for ICU-level care and prolonged hospitalization.”
Dr. Desai no longer performs multiday lytic procedures. “In fact, with the introduction of the newer on-label devices, I haven’t done a multiday unilateral limb lytic procedure in a couple years. I think we’ve gotten to the point where we don’t need to do that anymore.”
Indeed, PCDT makes recanalization possible as a single-day, single-session procedure.
Dr. Desai views the recent ATTRACT subanalysis as hypothesis generating.
“Should PCDT be the first-line treatment in all proximal DVT patients? No it should not – and that’s not what I would have advocated even before ATTRACT came out,” he explained. “It’s sort of a salvage procedure for patients with iliofemoral DVT and moderate to severe symptoms. And there are a significant number of such patients.”
Current understanding of the pathophysiology of PTS is that a nondissolved thrombus at the valve leaflets becomes inflammatory, with resultant valvular dysfunction leading to venous reflux and venous hypertension. PCDT is consistent with the open-vein hypothesis, which posits that, by eliminating thrombus much faster than achievable via anticoagulation, valve integrity is maintained and PTS is prevented.
Dr. Desai reported receiving consulting fees from AngioDynamics, Boston Scientific, Cook Medical, and Spectranetics.
CHICAGO – A closer look at the landmark ATTRACT trial of pharmacomechanical catheter-directed thrombolysis for acute deep vein thrombosis (DVT) shows multiple benefits for the intervention versus standard anticoagulation alone in the subset of participants with iliofemoral DVT, Kush R. Desai, MD, said at a symposium on vascular surgery sponsored by Northwestern University.
ATTRACT, a National Institutes of Health–sponsored, phase 3, multicenter, open-label, assessor-blinded study, was the first-ever randomized trial of pharmacomechanical catheter-directed thrombolysis (PCDT) for acute DVT.
The results caused a major stir because, despite a sound therapeutic rationale for the procedure, the incidence of chronic postthrombotic syndrome (PTS) at 24 months of follow-up was 47% in the PCDT plus anticoagulation group and 48% in controls on anticoagulation alone (N Engl J Med. 2017 Dec 7;377[23]:2240-52). Since then, that overall negative trial has been one of the hottest topics in DVT.
“This is the first thing your educated patients who come to the emergency department with DVT will ask about. It’s the first thing they’ll see when they go online and type in ‘thrombolysis DVT,’ ” noted Dr. Desai, an interventional radiologist at Northwestern University, Chicago.
But the trial has several major flaws, he cautioned. And contrary to popular opinion, ATTRACT is not the death knell for PCDT. Far from it.
“I don’t think the story stops with ATTRACT. This isn’t the end for PCDT in patients with iliofemoral DVT,” he asserted.
That’s in part because 301 of the 692 participants in ATTRACT had DVT of the femoropopliteal segment. That’s a population in which Dr. Desai and other interventionalists wouldn’t have anticipated seeing a benefit for PCDT, because their risk of PTS is so low.
“We know through historical data that patients with iliofemoral DVT are much more likely to develop PTS and to have recurrent DVT, so this is probably one of the major shortcomings of the trial,” he explained. “It’s through no fault of the trial investigators, because the study was planned years ago when we just didn’t know as much about PTS as we do now.
“The way I look at it is, I don’t practice in the way that ATTRACT was designed,” Dr. Desai said. “I don’t typically lyse or get referrals for lysis or thrombectomy in patients who have isolated femoropopliteal DVT. It has to involve at least the common femoral vein and frequently goes up to the iliac vein.”
The ATTRACT investigators’ recent subanalysis of the 391 participants with iliofemoral DVT showed that, although there was no difference between the two study arms in the occurrence of PTS through the first 24 months of follow-up, PCDT led to a 35% reduction in the incidence of moderate or severe PTS – by a margin of 18% versus 28% in controls.
Patients in the PCDT arm also experienced significantly greater improvement in venous disease-specific quality of life through 24 months, and a greater reduction in leg pain and swelling at 10 and 30 days (Circulation. 2018 Dec 4. doi: 10.1161/CIRCULATIONAHA.118.037425).
And moderate to severe PTS is a key outcome, Dr. Desai continued. Multiple studies have shown that patients with PTS have a worse quality of life than those with chronic lung disease, arthritis, or diabetes. Moreover, the 5%-10% of patients with symptomatic DVT who develop the most-severe form of PTS – characterized by severe pain, chronic ulcerations, stasis dermatitis, venous claudication, and intractable edema – have a quality of life comparable with patients with cancer or heart failure.
The 1.5% incidence of major bleeding within 10 days in the PCDT group was 200% higher than in controls, but none of it was life threatening.
“This is reassuring: Nobody had intracranial hemorrhage; nobody had a GUSTO 5 bleed,” Dr. Desai said.
Another limitation of the ATTRACT trial is that all but one of the devices utilized for PCDT were used off label. They weren’t designed for venous application. Several on-label rheolytic, rotational thrombectomy, or clot aspiration devices have been approved since enrollment in ATTRACT was closed. Future randomized trials will utilize on-label devices in patients with acute iliofemoral DVT to clarify the role of PCDT.
It’s noteworthy that nearly half of ATTRACT participants developed PTS within 24 months of their DVT despite being on optimal anticoagulation. It’s a finding that underscores the need for improved therapies. That was the impetus for development of first-generation catheter-directed thrombolysis utilizing a percutaneously inserted catheter to infuse a fibrinolytic drug directly to the thrombus to dissolve it rapidly.
But that form of catheter-directed thrombolysis has major disadvantages, Dr. Desai explained: It’s a multiday procedure requiring ICU-level care and prolonged exposure to powerful lytic agents.
“This is where things have changed with PCDT,” he said. “We can now, with on-label devices, accelerate the thrombolysis time, reduce lytic exposure, and I think also reduce the bleeding risk, although that hasn’t been shown in a trial yet. PCDT also reduces the necessity for ICU-level care and prolonged hospitalization.”
Dr. Desai no longer performs multiday lytic procedures. “In fact, with the introduction of the newer on-label devices, I haven’t done a multiday unilateral limb lytic procedure in a couple years. I think we’ve gotten to the point where we don’t need to do that anymore.”
Indeed, PCDT makes recanalization possible as a single-day, single-session procedure.
Dr. Desai views the recent ATTRACT subanalysis as hypothesis generating.
“Should PCDT be the first-line treatment in all proximal DVT patients? No it should not – and that’s not what I would have advocated even before ATTRACT came out,” he explained. “It’s sort of a salvage procedure for patients with iliofemoral DVT and moderate to severe symptoms. And there are a significant number of such patients.”
Current understanding of the pathophysiology of PTS is that a nondissolved thrombus at the valve leaflets becomes inflammatory, with resultant valvular dysfunction leading to venous reflux and venous hypertension. PCDT is consistent with the open-vein hypothesis, which posits that, by eliminating thrombus much faster than achievable via anticoagulation, valve integrity is maintained and PTS is prevented.
Dr. Desai reported receiving consulting fees from AngioDynamics, Boston Scientific, Cook Medical, and Spectranetics.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Deep sleep decreases, Alzheimer’s increases
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, physician groups are pushing back on Part B of the drug reimbursement proposal, dabigatran matches aspirin for second stroke prevention, and reassurance for pregnancy in atopic dermatitis.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Most oral HRT linked to increased VTE risk
Transdermal hormone replacement therapy is associated with the lowest risk of venous thromboembolism, yet still is relatively underused compared to oral preparations, researchers say.
Writing in the BMJ, Yana Vinogradova, PhD, of the University of Nottingham (England) and her associates reported the results of two nested case-control studies of hormone replacement therapy (HRT) and venous thromboembolism (VTE) from Jan. 1998 to Feb. 2017 that altogether included 80,396 women aged 40-79 years with a primary diagnosis of VTE matched to 391,494 female controls.
Overall, 7% of the women with VTE had been exposed to HRT in the 90 days before the index date versus 5.5% of controls.
The greatest increase in risk of VTE, compared with no exposure, was seen with oral conjugated equine estrogen with medroxyprogesterone acetate, which was associated with a more than twofold increase in risk (odds ratio, 2.10; 95% confidence interval, 1.92-2.31; P less than .01).
However transdermal HRT use was not associated with any increase in risk, compared with no HRT exposure. The data even pointed to a slight decrease in risk, which the authors suggested may be the result of some residual confounding or indication bias.
Oral HRT generally was associated with a 58% increased risk of VTE, which amounted to a number needed to harm of 1,076 and nine extra cases of VTE per 10,000 women taking oral HRT.
Dr. Vinogradova and her colleagues noted that the vast majority of women in the study were being prescribed oral HRT for menopausal symptoms despite previous studies showing transdermal HRT has much lower risk.
“When women with menopausal symptoms already have an increased VTE risk because of comorbidities or obesity, these women and their doctors should give greater consideration to transdermal HRT,” they wrote.
Lubna Pal, MBBS, director of the menopause program and professor of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., commented in an interview, “These data are tremendously reassuring. The reported findings are: 1) reaffirm what we have already known , i.e. that advancing age, higher body mass index, and higher doses of exogenous systemic estrogen therapy are associated with increased risk for VTE; 2) offer greater granularity in risk for VTE with different formulations of estrogens and progestins and different regimens than understood thus far, 3) reaffirm that, unlike oral estrogen, transdermal estrogen formulations in doses commonly utilized in clinical practice are not associated with VTE risk, and 4) provide reassurance that the absolute risk, while exaggerated with oral estrogen or combination estrogen and progestin use, is nonetheless small as reflected in the number needed to harm with oral hormone therapy being 1,076, and the number of extra VTE cases attributable to oral HT being 9 per 10,000 woman years.
“The authors are to be commended on this massive analytic undertaking that allows an improved understanding of HRT-related risk for VTE and offers meaningful guidance to the practitioner,” said Dr. Pal, who was not involved in the study.*
Estrogen-only preparations had a 40% higher risk and combined preparations had a 73% higher risk, compared with no exposure.
In estrogen-only preparations, the lowest risk was seen with estradiol, compared with conjugated equine estrogens or combined preparations.
The lowest risk of VTE among oral preparations was seen with estradiol plus dydrogesterone, which only showed a nonsignificant 18% increase in risk.
In an attempt to account for possible increased risk of VTE, the authors conducted a sensitivity analysis in a subgroup of women who had not previously used anticoagulants, but they found similar results to the main analysis.
“This sensitivity analysis indicates that most of the excluded women had probably used anticoagulants because of atrial fibrillation or hip replacement operations rather than an earlier unrecorded VTE,” they wrote.
One author declared directorship of a clinical software company, but no other conflicts of interest were declared. There was no external funding. Dr. Pal reported that she was a coinvestigator in the Kronos Early Estrogen Prevention Study and on an AMAG Pharmaceuticals advisory board and member of their speaker’s bureau.
SOURCE: Vinogradova Y et al. BMJ. 2019 Jan 9. doi: 10.1136/bmj.k4810.
*This article was updated 1/11/19.
Transdermal hormone replacement therapy is associated with the lowest risk of venous thromboembolism, yet still is relatively underused compared to oral preparations, researchers say.
Writing in the BMJ, Yana Vinogradova, PhD, of the University of Nottingham (England) and her associates reported the results of two nested case-control studies of hormone replacement therapy (HRT) and venous thromboembolism (VTE) from Jan. 1998 to Feb. 2017 that altogether included 80,396 women aged 40-79 years with a primary diagnosis of VTE matched to 391,494 female controls.
Overall, 7% of the women with VTE had been exposed to HRT in the 90 days before the index date versus 5.5% of controls.
The greatest increase in risk of VTE, compared with no exposure, was seen with oral conjugated equine estrogen with medroxyprogesterone acetate, which was associated with a more than twofold increase in risk (odds ratio, 2.10; 95% confidence interval, 1.92-2.31; P less than .01).
However transdermal HRT use was not associated with any increase in risk, compared with no HRT exposure. The data even pointed to a slight decrease in risk, which the authors suggested may be the result of some residual confounding or indication bias.
Oral HRT generally was associated with a 58% increased risk of VTE, which amounted to a number needed to harm of 1,076 and nine extra cases of VTE per 10,000 women taking oral HRT.
Dr. Vinogradova and her colleagues noted that the vast majority of women in the study were being prescribed oral HRT for menopausal symptoms despite previous studies showing transdermal HRT has much lower risk.
“When women with menopausal symptoms already have an increased VTE risk because of comorbidities or obesity, these women and their doctors should give greater consideration to transdermal HRT,” they wrote.
Lubna Pal, MBBS, director of the menopause program and professor of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., commented in an interview, “These data are tremendously reassuring. The reported findings are: 1) reaffirm what we have already known , i.e. that advancing age, higher body mass index, and higher doses of exogenous systemic estrogen therapy are associated with increased risk for VTE; 2) offer greater granularity in risk for VTE with different formulations of estrogens and progestins and different regimens than understood thus far, 3) reaffirm that, unlike oral estrogen, transdermal estrogen formulations in doses commonly utilized in clinical practice are not associated with VTE risk, and 4) provide reassurance that the absolute risk, while exaggerated with oral estrogen or combination estrogen and progestin use, is nonetheless small as reflected in the number needed to harm with oral hormone therapy being 1,076, and the number of extra VTE cases attributable to oral HT being 9 per 10,000 woman years.
“The authors are to be commended on this massive analytic undertaking that allows an improved understanding of HRT-related risk for VTE and offers meaningful guidance to the practitioner,” said Dr. Pal, who was not involved in the study.*
Estrogen-only preparations had a 40% higher risk and combined preparations had a 73% higher risk, compared with no exposure.
In estrogen-only preparations, the lowest risk was seen with estradiol, compared with conjugated equine estrogens or combined preparations.
The lowest risk of VTE among oral preparations was seen with estradiol plus dydrogesterone, which only showed a nonsignificant 18% increase in risk.
In an attempt to account for possible increased risk of VTE, the authors conducted a sensitivity analysis in a subgroup of women who had not previously used anticoagulants, but they found similar results to the main analysis.
“This sensitivity analysis indicates that most of the excluded women had probably used anticoagulants because of atrial fibrillation or hip replacement operations rather than an earlier unrecorded VTE,” they wrote.
One author declared directorship of a clinical software company, but no other conflicts of interest were declared. There was no external funding. Dr. Pal reported that she was a coinvestigator in the Kronos Early Estrogen Prevention Study and on an AMAG Pharmaceuticals advisory board and member of their speaker’s bureau.
SOURCE: Vinogradova Y et al. BMJ. 2019 Jan 9. doi: 10.1136/bmj.k4810.
*This article was updated 1/11/19.
Transdermal hormone replacement therapy is associated with the lowest risk of venous thromboembolism, yet still is relatively underused compared to oral preparations, researchers say.
Writing in the BMJ, Yana Vinogradova, PhD, of the University of Nottingham (England) and her associates reported the results of two nested case-control studies of hormone replacement therapy (HRT) and venous thromboembolism (VTE) from Jan. 1998 to Feb. 2017 that altogether included 80,396 women aged 40-79 years with a primary diagnosis of VTE matched to 391,494 female controls.
Overall, 7% of the women with VTE had been exposed to HRT in the 90 days before the index date versus 5.5% of controls.
The greatest increase in risk of VTE, compared with no exposure, was seen with oral conjugated equine estrogen with medroxyprogesterone acetate, which was associated with a more than twofold increase in risk (odds ratio, 2.10; 95% confidence interval, 1.92-2.31; P less than .01).
However transdermal HRT use was not associated with any increase in risk, compared with no HRT exposure. The data even pointed to a slight decrease in risk, which the authors suggested may be the result of some residual confounding or indication bias.
Oral HRT generally was associated with a 58% increased risk of VTE, which amounted to a number needed to harm of 1,076 and nine extra cases of VTE per 10,000 women taking oral HRT.
Dr. Vinogradova and her colleagues noted that the vast majority of women in the study were being prescribed oral HRT for menopausal symptoms despite previous studies showing transdermal HRT has much lower risk.
“When women with menopausal symptoms already have an increased VTE risk because of comorbidities or obesity, these women and their doctors should give greater consideration to transdermal HRT,” they wrote.
Lubna Pal, MBBS, director of the menopause program and professor of obstetrics, gynecology, and reproductive sciences at Yale University, New Haven, Conn., commented in an interview, “These data are tremendously reassuring. The reported findings are: 1) reaffirm what we have already known , i.e. that advancing age, higher body mass index, and higher doses of exogenous systemic estrogen therapy are associated with increased risk for VTE; 2) offer greater granularity in risk for VTE with different formulations of estrogens and progestins and different regimens than understood thus far, 3) reaffirm that, unlike oral estrogen, transdermal estrogen formulations in doses commonly utilized in clinical practice are not associated with VTE risk, and 4) provide reassurance that the absolute risk, while exaggerated with oral estrogen or combination estrogen and progestin use, is nonetheless small as reflected in the number needed to harm with oral hormone therapy being 1,076, and the number of extra VTE cases attributable to oral HT being 9 per 10,000 woman years.
“The authors are to be commended on this massive analytic undertaking that allows an improved understanding of HRT-related risk for VTE and offers meaningful guidance to the practitioner,” said Dr. Pal, who was not involved in the study.*
Estrogen-only preparations had a 40% higher risk and combined preparations had a 73% higher risk, compared with no exposure.
In estrogen-only preparations, the lowest risk was seen with estradiol, compared with conjugated equine estrogens or combined preparations.
The lowest risk of VTE among oral preparations was seen with estradiol plus dydrogesterone, which only showed a nonsignificant 18% increase in risk.
In an attempt to account for possible increased risk of VTE, the authors conducted a sensitivity analysis in a subgroup of women who had not previously used anticoagulants, but they found similar results to the main analysis.
“This sensitivity analysis indicates that most of the excluded women had probably used anticoagulants because of atrial fibrillation or hip replacement operations rather than an earlier unrecorded VTE,” they wrote.
One author declared directorship of a clinical software company, but no other conflicts of interest were declared. There was no external funding. Dr. Pal reported that she was a coinvestigator in the Kronos Early Estrogen Prevention Study and on an AMAG Pharmaceuticals advisory board and member of their speaker’s bureau.
SOURCE: Vinogradova Y et al. BMJ. 2019 Jan 9. doi: 10.1136/bmj.k4810.
*This article was updated 1/11/19.
FROM THE BMJ
Key clinical point: Transdermal HRT is not associated with any increase in VTE risk.
Major finding: Conjugated equine estrogen with medroxyprogesterone shows a twofold increase in VTE risk.
Study details: Nested case-control study in 80,396 women and 391,494 female controls.
Disclosures: One author declared directorship of a clinical software company, but no other conflicts of interest were declared. There was no external funding. Dr. Pal reported that she was a coinvestigator in the Kronos Early Estrogen Prevention Study and on an AMAG Pharmaceuticals advisory board and member of their speaker’s bureau.
Source: Vinogradova Y et al. BMJ. 2019 Jan 9. doi: 10.1136/bmj.k4810
RE-SPECT ESUS: Dabigatran matched aspirin for second stroke prevention
MONTREAL – For the second time in the past year, an anticoagulant failed to show superiority when it was compared with aspirin for preventing a second stroke in patients who had had an index embolic stroke of undetermined source (ESUS). But the most recent results gave a tantalizing suggestion that the anticoagulant approach might be effective for older patients, those at least 75 years old, possibly because these patients have the highest incidence of atrial fibrillation.
“The fact that we saw a treatment benefit in patients 75 and older [in a post hoc, subgroup analysis] means that development of atrial fibrillation (AF) is probably the most important factor,” Hans-Christoph Diener, MD, said at the World Stroke Congress. Another clue that incident AF drove a treatment benefit hidden in the new trial’s overall neutral result was that a post hoc, landmark analysis showed that, while the rate of second strokes was identical during the first year of follow-up in patients on either aspirin or the anticoagulant dabigatran (Pradaxa) after an index ESUS, patients on dabigatran had significantly fewer second strokes during subsequent follow-up.
More follow-up time was needed to see a benefit from anticoagulation because “it takes time for AF to develop, and then once a patient has AF, it takes time for a stroke to occur,” explained Dr. Diener, professor of neurology at the University of Duisburg-Essen in Essen, Germany.
The RE-SPECT ESUS (Dabigatran Etexilate for Secondary Stroke Prevention in Patients With Embolic Stroke of Undetermined Source) trial randomized 5,390 patients at more than 500 sites in 41 countries, including the United States, within 6 months of an index ESUS who had no history of AF and no severe renal impairment. All enrollees had to have less than 6 minutes of AF episodes during at least 20 hours of cardiac monitoring, and they had to be free of flow-limiting stenoses (50% or more) in arteries supplying their stroke region. Patients received either 150 mg or 110 mg of dabigatran twice daily depending on their age and renal function or 100 mg of aspirin daily. About a quarter of patients randomized to dabigatran received the lower dosage. The enrolled patients averaged 66 years old, almost two-thirds were men, and they started treatment a median of 44 days after their index stroke.
During a median 19 months’ follow-up, the incidence of a second stroke of any type was 4.1%/year among the patients on dabigatran and 4.8%/year among those on aspirin, a difference that was not statistically significant. However, the post hoc landmark analysis showed a significant reduction in second strokes with dabigatran treatment after the first year. In addition, a post hoc subgroup analysis showed that, among patients aged at least 75 years old, treatment with dabigatran linked with a statistically significant 37% reduction in second strokes, compared with treatment with aspirin, Dr. Diener reported.
The primary safety endpoint was major bleeds, as defined by the International Society on Thrombosis and Haemostasis, which occurred in 1.7%/year of patients on dabigatran and 1.4%/year of those on aspirin, a difference that was not statistically significant. Patients on dabigatran had a significant excess of major bleeds combined with clinically significant nonmajor bleeds: 3.3%/year versus 2.3%/year among those on aspirin.
A little over 4 months before Dr. Diener’s report, a separate research group published primary results from the NAVIGATE ESUS (Rivaroxaban Versus Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source) trial, which compared the anticoagulant rivaroxaban (Xarelto) with aspirin for prevention of a second stroke in 7,213 ESUS patients. The results showed no significant efficacy difference between rivaroxaban and aspirin (N Engl J Med. 2018 June 7;378[23]:2191-2201).
RE-SPECT ESUS was funded by Boehringer Ingelheim, the company that markets dabigatran (Pradaxa). Dr. Diener has been a consultant to and has received research funding from Boehringer Ingelheim, as well as several other companies.
SOURCE: Diener H-C et al. Int J Stroke. 2018;13(2_suppl):27. Abstract 100.
MONTREAL – For the second time in the past year, an anticoagulant failed to show superiority when it was compared with aspirin for preventing a second stroke in patients who had had an index embolic stroke of undetermined source (ESUS). But the most recent results gave a tantalizing suggestion that the anticoagulant approach might be effective for older patients, those at least 75 years old, possibly because these patients have the highest incidence of atrial fibrillation.
“The fact that we saw a treatment benefit in patients 75 and older [in a post hoc, subgroup analysis] means that development of atrial fibrillation (AF) is probably the most important factor,” Hans-Christoph Diener, MD, said at the World Stroke Congress. Another clue that incident AF drove a treatment benefit hidden in the new trial’s overall neutral result was that a post hoc, landmark analysis showed that, while the rate of second strokes was identical during the first year of follow-up in patients on either aspirin or the anticoagulant dabigatran (Pradaxa) after an index ESUS, patients on dabigatran had significantly fewer second strokes during subsequent follow-up.
More follow-up time was needed to see a benefit from anticoagulation because “it takes time for AF to develop, and then once a patient has AF, it takes time for a stroke to occur,” explained Dr. Diener, professor of neurology at the University of Duisburg-Essen in Essen, Germany.
The RE-SPECT ESUS (Dabigatran Etexilate for Secondary Stroke Prevention in Patients With Embolic Stroke of Undetermined Source) trial randomized 5,390 patients at more than 500 sites in 41 countries, including the United States, within 6 months of an index ESUS who had no history of AF and no severe renal impairment. All enrollees had to have less than 6 minutes of AF episodes during at least 20 hours of cardiac monitoring, and they had to be free of flow-limiting stenoses (50% or more) in arteries supplying their stroke region. Patients received either 150 mg or 110 mg of dabigatran twice daily depending on their age and renal function or 100 mg of aspirin daily. About a quarter of patients randomized to dabigatran received the lower dosage. The enrolled patients averaged 66 years old, almost two-thirds were men, and they started treatment a median of 44 days after their index stroke.
During a median 19 months’ follow-up, the incidence of a second stroke of any type was 4.1%/year among the patients on dabigatran and 4.8%/year among those on aspirin, a difference that was not statistically significant. However, the post hoc landmark analysis showed a significant reduction in second strokes with dabigatran treatment after the first year. In addition, a post hoc subgroup analysis showed that, among patients aged at least 75 years old, treatment with dabigatran linked with a statistically significant 37% reduction in second strokes, compared with treatment with aspirin, Dr. Diener reported.
The primary safety endpoint was major bleeds, as defined by the International Society on Thrombosis and Haemostasis, which occurred in 1.7%/year of patients on dabigatran and 1.4%/year of those on aspirin, a difference that was not statistically significant. Patients on dabigatran had a significant excess of major bleeds combined with clinically significant nonmajor bleeds: 3.3%/year versus 2.3%/year among those on aspirin.
A little over 4 months before Dr. Diener’s report, a separate research group published primary results from the NAVIGATE ESUS (Rivaroxaban Versus Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source) trial, which compared the anticoagulant rivaroxaban (Xarelto) with aspirin for prevention of a second stroke in 7,213 ESUS patients. The results showed no significant efficacy difference between rivaroxaban and aspirin (N Engl J Med. 2018 June 7;378[23]:2191-2201).
RE-SPECT ESUS was funded by Boehringer Ingelheim, the company that markets dabigatran (Pradaxa). Dr. Diener has been a consultant to and has received research funding from Boehringer Ingelheim, as well as several other companies.
SOURCE: Diener H-C et al. Int J Stroke. 2018;13(2_suppl):27. Abstract 100.
MONTREAL – For the second time in the past year, an anticoagulant failed to show superiority when it was compared with aspirin for preventing a second stroke in patients who had had an index embolic stroke of undetermined source (ESUS). But the most recent results gave a tantalizing suggestion that the anticoagulant approach might be effective for older patients, those at least 75 years old, possibly because these patients have the highest incidence of atrial fibrillation.
“The fact that we saw a treatment benefit in patients 75 and older [in a post hoc, subgroup analysis] means that development of atrial fibrillation (AF) is probably the most important factor,” Hans-Christoph Diener, MD, said at the World Stroke Congress. Another clue that incident AF drove a treatment benefit hidden in the new trial’s overall neutral result was that a post hoc, landmark analysis showed that, while the rate of second strokes was identical during the first year of follow-up in patients on either aspirin or the anticoagulant dabigatran (Pradaxa) after an index ESUS, patients on dabigatran had significantly fewer second strokes during subsequent follow-up.
More follow-up time was needed to see a benefit from anticoagulation because “it takes time for AF to develop, and then once a patient has AF, it takes time for a stroke to occur,” explained Dr. Diener, professor of neurology at the University of Duisburg-Essen in Essen, Germany.
The RE-SPECT ESUS (Dabigatran Etexilate for Secondary Stroke Prevention in Patients With Embolic Stroke of Undetermined Source) trial randomized 5,390 patients at more than 500 sites in 41 countries, including the United States, within 6 months of an index ESUS who had no history of AF and no severe renal impairment. All enrollees had to have less than 6 minutes of AF episodes during at least 20 hours of cardiac monitoring, and they had to be free of flow-limiting stenoses (50% or more) in arteries supplying their stroke region. Patients received either 150 mg or 110 mg of dabigatran twice daily depending on their age and renal function or 100 mg of aspirin daily. About a quarter of patients randomized to dabigatran received the lower dosage. The enrolled patients averaged 66 years old, almost two-thirds were men, and they started treatment a median of 44 days after their index stroke.
During a median 19 months’ follow-up, the incidence of a second stroke of any type was 4.1%/year among the patients on dabigatran and 4.8%/year among those on aspirin, a difference that was not statistically significant. However, the post hoc landmark analysis showed a significant reduction in second strokes with dabigatran treatment after the first year. In addition, a post hoc subgroup analysis showed that, among patients aged at least 75 years old, treatment with dabigatran linked with a statistically significant 37% reduction in second strokes, compared with treatment with aspirin, Dr. Diener reported.
The primary safety endpoint was major bleeds, as defined by the International Society on Thrombosis and Haemostasis, which occurred in 1.7%/year of patients on dabigatran and 1.4%/year of those on aspirin, a difference that was not statistically significant. Patients on dabigatran had a significant excess of major bleeds combined with clinically significant nonmajor bleeds: 3.3%/year versus 2.3%/year among those on aspirin.
A little over 4 months before Dr. Diener’s report, a separate research group published primary results from the NAVIGATE ESUS (Rivaroxaban Versus Aspirin in Secondary Prevention of Stroke and Prevention of Systemic Embolism in Patients With Recent Embolic Stroke of Undetermined Source) trial, which compared the anticoagulant rivaroxaban (Xarelto) with aspirin for prevention of a second stroke in 7,213 ESUS patients. The results showed no significant efficacy difference between rivaroxaban and aspirin (N Engl J Med. 2018 June 7;378[23]:2191-2201).
RE-SPECT ESUS was funded by Boehringer Ingelheim, the company that markets dabigatran (Pradaxa). Dr. Diener has been a consultant to and has received research funding from Boehringer Ingelheim, as well as several other companies.
SOURCE: Diener H-C et al. Int J Stroke. 2018;13(2_suppl):27. Abstract 100.
REPORTING FROM THE WORLD STROKE CONGRESS
Key clinical point:
Major finding: A second stroke occurred at 4.1%/year with dabigatran and 4.8%/year with aspirin, not a statistically significant difference.
Study details: RE-SPECT ESUS, an international randomized trial with 5,390 ESUS patients.
Disclosures: RE-SPECT ESUS was funded by Boehringer Ingelheim, the company that markets dabigatran (Pradaxa). Dr. Diener has been a consultant to and has received research funding from Boehringer Ingelheim, as well as several other companies.
Source: Diener H-C et al. Int J Stroke. 2018;13(2_suppl):27. Abstract 100.