User login
New guidelines for laser treatment of cutaneous vascular anomalies
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
A new practice guideline is setting a standard for doctors who use lasers to treat cutaneous vascular anomalies.
Poor treatment has been an issue in this field because no uniform guidelines existed to inform practice, according to a press release from the American Society for Laser Medicine and Surgery.
The laser treatment settings can vary based on the type and location of the birthmark and also the patient’s skin type, which has resulted in an inconsistent approach from clinicians, according to the release.
“For decades, I have observed adverse outcomes from the improper laser treatment of vascular birthmarks,” Linda Rozell-Shannon, PhD, president and founder of the Vascular Birthmarks Foundation said in a statement from ASLMS. “As a result of these guidelines, patient outcomes will be improved.”
The guideline, published on the ASLMS website along with supporting videos, was jointly developed by ASLMS, VBF, and an international group of clinicians, marking the first consensus guideline on laser treatments for cutaneous vascular anomalies. It details 32 best practice directives for various scenarios, including advice on safety considerations, additional testing, and when to refer.
“It is important to realize that just because someone is board certified does not mean they are skilled in treating all conditions or using all lasers,” Paul Friedman, MD, a dermatologist in Houston, and former president of ASLMS, said in the ASLMS statement.
Vascular birthmarks are a common condition affecting up to 14% of children, according to VBF. Most are hemangiomas, a buildup of blood vessels that usually appears at birth or within a month after birth. Laser therapy reduces the size and color of the anomalies.
Support for this initiative was provided by Candela Medical.
A version of this article first appeared on Medscape.com.
Surgeon in the C-suite

“If you don’t have a seat at the table, you are probably on the menu.” I first heard this quote in 2013, and it launched my interest in health care leadership and influenced me countless times over the last 10 years.
As Chief of Staff at Cleveland Clinic, I oversee nearly 5,000 physicians and scientists across the globe. I am involved in the physician life cycle: recruiting, hiring, privileging and credentialing, talent development, promotion, professionalism, and career transitions. I also sit at the intersection of medical care and the business of medicine. This means leading 18 clinical service lines responsible for 5.6 million visits, 161,000 surgeries, and billions of dollars in operating revenue per year. How I spend most of my time is a far cry from what I spent 11 years’ training to do—gynecologic surgery. This shift in my career was not because I changed my mind about caring for patients or that I tired of being a full-time surgeon. Nothing could be further from the truth. Women’s health remains my “why,” and my leadership journey has taught me that it is critical to have a seat at the table for the sake of ObGyns and women everywhere.
Women’s health on the menu
I will start with a concrete example of when we, as women and ObGyns, were on the menu. In late 2019, the Ohio state House of Representatives introduced a bill that subjected doctors to potential murder charges if they did not try everything to save the life of a mother and fetus, “including attempting to reimplant an ectopic pregnancy into the woman’s uterus.”1 This bill was based on 2 case reports—one from 1915 and one from 1980—which were both low quality, and the latter case was deemed to be fraudulent.2 How did this happen?
An Ohio state representative developed the bill with help from a lobbyist and without input from physicians or content experts. When asked, the representative shared that “he never researched whether re-implanting an ectopic pregnancy into a woman’s uterus was a viable medical procedure before including it in the bill.”3 He added, “I heard about it over the years. I never questioned it or gave it a lot of thought.”3
This example resonates deeply with many of us; it inspires us to speak up and act. As ObGyns, we clearly understand the consequences of legal and regulatory change in women’s health and how it directly impacts our patients and each of us as physicians. Let’s shift to something that you may feel less passion about, but I believe is equally important. This is where obstetrician-gynecologists sit in the intersection of medical care and business. This is the space where I spend most of my time, and from this vantage point, I worry about our field.
The business of medicine
Starting at the macroeconomic level, let’s think about how we as physicians are reimbursed and who makes these decisions. Looking at the national health care expenditure data, Medicare and Medicaid spending makes up nearly 40% of the total spend, and it is growing.4 Additionally, private health insurance tends to follow Centers for Medicare and Medicaid Services (CMS) decision making, further compounding its influence.4 In simple terms, CMS decides what is covered and how much we are paid. Whether you are in a solo private practice, an employer health care organization, or an academic medical center, physician reimbursement is declining.
In fact, Congress passed its year-end omnibus legislation in the final days of 2022, including a 2% Medicare physician payment cut for 2023,5 at a time when expenses to practice medicine, including nonphysician staff and supplies, are at an all-time high and we are living in a 6% inflationary state. This translates into being asked to serve more patients and cut costs. Our day-to-day feels much tighter, and this is why: Medicare physician pay increased just 11% over the past 20 years6 (2001–2021) in comparison to the cost of running a medical practice, which increased nearly 40% during that time. In other words, adjusting for inflation in practice costs, Medicare physician payment has fallen 22% over the last 20 years.7
Depending on your employment model, you may feel insulated from these changes as increases in reimbursement have occurred in other areas, such as hospitals and ambulatory surgery centers.8 In the short term, these increases help, as organizations will see additional funds. But there are 2 main issues: First, it is not nearly enough when you consider the soaring costs of running a hospital. And second, looking at our national population, we rely tremendously on self-employed doctors to serve our patients.
More than 80% of US counties lack adequate health care infrastructure.9 More than a third of the US population has less-than-adequate access to pharmacies, primary care physicians, hospitals, trauma centers, and low-cost health centers.9 To put things into perspective, more than 20% of counties in the United States are hospital deserts, where most people must drive more than 30 minutes to reach the closest hospital.9
There is good reason for this. Operating a hospital is a challenging endeavor. Even before the COVID-19 pandemic and the most recent health care financial challenges, most health care systems and large hospitals operated with very low operating margins (2%–3%). Businesses with similar margins include grocery stores and car dealerships. These low-margin businesses, including health care, rely on high volume for sustainability. High patient volumes distribute expensive hospital costs over many encounters. If physicians cannot sustain practices across the country, it is challenging to have sufficient admission and surgical volumes to justify the cost base of hospitals.
To tie this together, we have very little influence on what we are paid for our services. Reimbursement is declining, which makes it hard to have financially sustainable practices. As hospitals struggle, there is more pressure to prioritize highly profitable service lines, like orthopedics and urology, which are associated with favorable technical revenue. As hospitals are threatened, health care deserts widen, which leaves our entire health care system in jeopardy. Not surprisingly, this most likely affects those who face additional barriers to access, such as those with lower income, limited internet access, and lack of insurance. Together, these barriers further widen disparities in health care outcomes, including outcomes for women. Additionally, this death by a thousand cuts has eroded morale and increased physician burnout.
Transforming how we practice medicine is the only viable solution. I have good news: You are the leaders you have been waiting for.
Continue to: Physicians make good managers...
Physicians make good managers
To successfully transform how we practice medicine, it is critical that those leading the transformation deeply understand how medicine is practiced. The level of understanding required can be achieved only through years of medical practice, as a doctor. We understand how medical teams interact and that different sectors of our health care system are interdependent. Also, because physicians drive patient activity and ultimately reimbursement, having a seat at the table is crucial.
Some health care systems are run by businesspeople—people with finance backgrounds—and others are led by physicians. In 2017, Becker’s Hospital Review listed the chief executive officers (CEOs) of 183 nonprofit hospital and health systems.10 Of these, only 25% were led by individuals with an MD. Looking at the 115 largest hospitals in the United States, 30% are physician led.10 Considering the top 10 hospitals ranked by U.S. News & World Report for 2022, 8 of 10 have a physician at the helm.
Beyond raters and rankers, physician-led hospitals do better. Goodall compared CEOs in the top 100 best hospitals in U.S. News & World Report in 3 key medical specialties: cancer, digestive disorders, and cardiac care.11 The study explored the question: “Are hospitals’ quality ranked more highly when they are led by a medically trained doctor or non-MD professional managers?”11 Analysis revealed that hospital quality scores are about 25% higher in physician-run hospitals than in manager-run hospitals.11 Additional research shows that good management practices correlate with hospital performance, and that “the proportion of managers with a clinical degree has the largest positive effect.”12
Several theories exist as to why doctors make good managers in the health care setting.13,14 Doctors may create a more sympathetic and productive work environment for other clinicians because they are one of them. They have peer-to-peer credibility—because they have walked the walk, they have insight and perspective into how medicine is practiced.
Physicians serve as effective change agents for their organizations in several ways:
- First, physicians take a clinical approach in their leadership roles13 and focus on patient care at the center of their decisions. We see the people behind the numbers. Simply put, we humanize the operational side of health care.
- As physicians, we understand the interconnectivity in the practice of medicine. While closing certain service lines may be financially beneficial, these services are often closely linked to profitable service lines.
- Beyond physicians taking a clinical approach to leadership, we emphasize quality.13 Because we all have experienced complications and lived through bad outcomes alongside our patients, we understand deeply how important patient safety and quality is, and we are not willing to sacrifice that for financial gain. For us, this is personal. We don’t see our solution to health care challenges as an “or” situation, instead we view it as an “and” situation.
- Physician leaders often can improve medical staff engagement.13 A 2018 national survey of physicians found that those who are satisfied with their leadership are more engaged at work, have greater job satisfaction, and are less likely to experience signs of burnout.15 Physician administrators add value here.
Continue to: Surgeons as leaders...
Surgeons as leaders
What do we know about surgeons as physician leaders? Looking at the previously mentioned lists of physician leaders, surgeons are relatively absent. In the Becker’s Hospital Review study of nonprofit hospitals, only 9% of CEOs were surgeons.10 In addition, when reviewing data that associated physician leaders and hospital performance, only 3 of the CEOs were surgeons.11 Given that surgeons make up approximately 19% of US physicians, we are underrepresented.
The omission of surgeons as leaders seems inappropriate given that most hospitals are financially reliant on revenue related to surgical care and optimizing this space is an enormous opportunity. Berger and colleagues offered 3 theories as to why there are fewer surgeon leaders16:
- The relative pay of surgeons exceeds that of most other specialties, and there may be less incentive to accept the challenges presented by leadership roles. (I will add that surgeon leadership is more costly to a system.)
- The craftsmanship nature of surgery discourages the development of other career interests beginning at the trainee level.
- Surgeons have been perceived stereotypically to exhibit arrogance, a characteristic that others may not warm to.
This last observation stings. Successful leadership takes social skill and teamwork.14 Although medical care is one of the few disciplines in which lack of teamwork might cost lives, physicians are not trained to be team players. We recognize how our training has led us to be lone wolves or gunners, situations where we as individuals had to beat others to secure our spot. We have been trained in command-and-control environments, in stepping up as a leader in highly stressful situations. This part of surgical culture may handicap surgeons in their quest to be health care leaders.
Other traits, however, make us particularly great leaders in health care. Our desire to succeed, willingness to push ourselves to extremes, ability to laser focus on a task, acceptance of delayed gratification, and aptitude for making timely decisions on limited data help us succeed in leadership roles. Seven years of surgical training helped me develop the grit I use every day in the C-suite.
We need more physician and surgeon leadership to thrive in the challenging health care landscape. Berger and colleagues proposed 3 potential solutions to increase the number of surgeons in hospital leadership positions16:
Nurture future surgical leaders through exposure to management training. Given the contribution to both expense in support services and resources and revenue related to surgical care, each organization needs a content expert to guide these decisions.
Recognize the important contributions that surgeons already make regarding quality, safety, and operational efficiency. An excellent example of this is the American College of Surgeons National Surgical Quality Improvement Program. Because surgeons are content experts in this area, we are primed to lead.
Hospitals, medical schools, and academic departments of surgery should recognize administrative efforts as an important part of the overall academic mission. As the adage states, “No margin, no mission.” We need bright minds to preserve and grow our margins so that we can further invest in our missions.
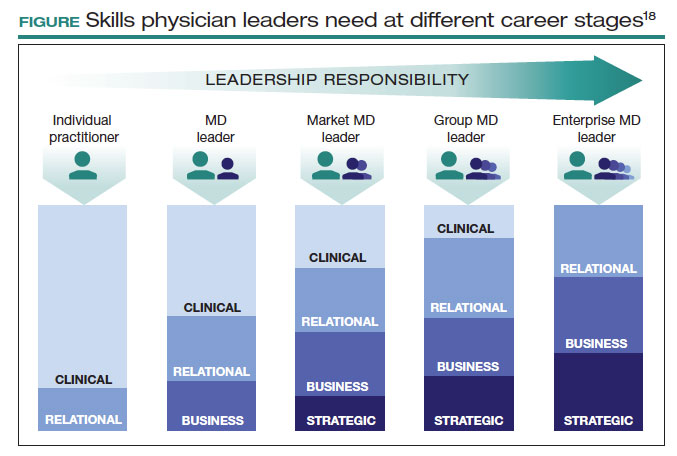
This is not easy. Given the barriers, this will not happen organically. Charan and colleagues provided an outline for a leadership pathway adapted for physicians (FIGURE).17,18 It starts with the individual practitioner who is a practicing physician and spends most of their time focused on patient care. As a physician becomes more interested in leadership, they develop new skills and take on more and more responsibility. As they increase in leadership responsibility, they tend to reduce clinical time and increase time spent on strategic and business management. This framework creates a pipeline so that physicians and surgeons can be developed strategically and given increasing responsibility as they develop their capabilities and expand their skill sets.
The leadership challenge
To thrive, we must transform health care by changing how we practice medicine. As ObGyns, we are the leaders we have been waiting for. As you ponder your future, think of your current career and the opportunities you might have. Do you have a seat at the table? What table is that? How are you using your knowledge, expertise, and privilege to advance health care and medicine? I challenge you to critically evaluate this—and lead. ●
- Law T. Ohio bill suggests doctors who perform abortions could face jail, unless they perform a non-existent treatment. December 1, 2019. Time. Accessed June 12, 2023. https://time.com/5742053 /ectopic-pregnancy-ohio-abortion-bill/
- Grossman D. Ohio abortion, ectopic pregnancy bill: ‘it’s both bad medicine and bad law-making.’ May 21, 2019. Cincinnati.com–The Enquirer. Accessed June 12, 2023. https://www .cincinnati.com/story/opinion/2019/05/21/ohio-abortion-bill -john-becker-daniel-grossman-ectopic-pregnancy-false-medicine /3753610002/
- Lobbyist had hand in bill sparking ectopic pregnancy flap. December 11, 2019. Associated Press. Accessed June 12, 2023. https://apnews .com/article/03216e44405fa184ae0ab80fa85089f8
- NHE fact sheet. CMS.gov. Updated February 17, 2023. Accessed June 12, 2023. https://www.cms.gov/research-statistics-data-and -systems/statistics-trends-and-reports/nationalhealthexpenddata /nhe-fact-sheet
- Senate passes omnibus spending bill with health provisions. December 23, 2022. American Hospital Association. Accessed June 12, 2023. https://www.aha.org/special-bulletin/2022-12-20-appropriations -committees-release-omnibus-spending-bill-health-provisions
- Medicare updates compared to inflation (2001-2021). October 2021. American Medical Association. Accessed June 12, 2023. https://www .ama-assn.org/system/files/medicare-pay-chart-2021.pdf
- Resneck Jr J. Medicare physician payment reform is long overdue. October 3, 2022. American Medical Association. Accessed June 7, 2023. https://www.ama-assn.org/about/leadership /medicare-physician-payment-reform-long-overdue
- Isenberg M. The stark reality of physician reimbursement. August 24, 2022. Zotec Partners. Accessed June 13, 2023. https://zotecpartners. com/advocacy-zpac/test-1/
- Nguyen A. Mapping healthcare deserts: 80% of the country lacks adequate access to healthcare. September 9, 2021. GoodRx Health. Accessed June 13, 2023. https://www.goodrx.com/healthcare -access/research/healthcare-deserts-80-percent-of-country-lacks -adequate-healthcare-access
- 183 nonprofit hospital and health system CEOs to know–2017. Updated June 20, 2018. Becker’s Hospital Review. Accessed June 7, 2023. https://www.beckershospitalreview.com/lists/188-nonprofit -hospital-and-health-system-ceos-to-know-2017.html
- Goodall AH. Physician-leaders and hospital performance: is there an association? Soc Sci Med. 2011;73:535-539. doi:10.1016 /j.socscimed.2011.06.025
- Bloom N, Sadun R, Van Reenen J. Does Management Matter in Healthcare? Center for Economic Performance and Harvard Business School; 2014.
- Turner J. Why healthcare C-suites should include physicians. September 3, 2019. Managed Healthcare Executive. Accessed June 13, 2023. https://www.managedhealthcareexecutive.com /view/why-healthcare-c-suites-should-include-physicians
- Stoller JK, Goodall A, Baker A. Why the best hospitals are managed by doctors. December 27, 2016. Harvard Business Review. Accessed June 13, 2023. https://hbr.org/2016/12/why-the-best-hospitals -are-managed-by-doctors
- Hayhurst C. Data confirms: leaders, physician burnout is on you. April 3, 2019. Aetnahealth. Accessed June 13, 2023. https://www .athenahealth.com/knowledge-hub/practice-management /research-confirms-leaders-burnout-you
- Berger DH, Goodall A, Tsai AY. The importance of increasing surgeon participation in hospital leadership. JAMA Surg. 2019;154:281-282. doi:10.1001/jamasurg.2018.5080
- Charan R, Drotter S, Noel J. The Leadership Pipeline: How to Build the Leadership-Powered Company. Jossey-Bass; 2001.
- Perry J, Mobley F, Brubaker M. Most doctors have little or no management training, and that’s a problem. December 15, 2017. Harvard Business Review. Accessed June 7, 2023. https://hbr.org/2017/12 /most-doctors-have-little-or-no-management-training-and-thats -a-problem

“If you don’t have a seat at the table, you are probably on the menu.” I first heard this quote in 2013, and it launched my interest in health care leadership and influenced me countless times over the last 10 years.
As Chief of Staff at Cleveland Clinic, I oversee nearly 5,000 physicians and scientists across the globe. I am involved in the physician life cycle: recruiting, hiring, privileging and credentialing, talent development, promotion, professionalism, and career transitions. I also sit at the intersection of medical care and the business of medicine. This means leading 18 clinical service lines responsible for 5.6 million visits, 161,000 surgeries, and billions of dollars in operating revenue per year. How I spend most of my time is a far cry from what I spent 11 years’ training to do—gynecologic surgery. This shift in my career was not because I changed my mind about caring for patients or that I tired of being a full-time surgeon. Nothing could be further from the truth. Women’s health remains my “why,” and my leadership journey has taught me that it is critical to have a seat at the table for the sake of ObGyns and women everywhere.
Women’s health on the menu
I will start with a concrete example of when we, as women and ObGyns, were on the menu. In late 2019, the Ohio state House of Representatives introduced a bill that subjected doctors to potential murder charges if they did not try everything to save the life of a mother and fetus, “including attempting to reimplant an ectopic pregnancy into the woman’s uterus.”1 This bill was based on 2 case reports—one from 1915 and one from 1980—which were both low quality, and the latter case was deemed to be fraudulent.2 How did this happen?
An Ohio state representative developed the bill with help from a lobbyist and without input from physicians or content experts. When asked, the representative shared that “he never researched whether re-implanting an ectopic pregnancy into a woman’s uterus was a viable medical procedure before including it in the bill.”3 He added, “I heard about it over the years. I never questioned it or gave it a lot of thought.”3
This example resonates deeply with many of us; it inspires us to speak up and act. As ObGyns, we clearly understand the consequences of legal and regulatory change in women’s health and how it directly impacts our patients and each of us as physicians. Let’s shift to something that you may feel less passion about, but I believe is equally important. This is where obstetrician-gynecologists sit in the intersection of medical care and business. This is the space where I spend most of my time, and from this vantage point, I worry about our field.
The business of medicine
Starting at the macroeconomic level, let’s think about how we as physicians are reimbursed and who makes these decisions. Looking at the national health care expenditure data, Medicare and Medicaid spending makes up nearly 40% of the total spend, and it is growing.4 Additionally, private health insurance tends to follow Centers for Medicare and Medicaid Services (CMS) decision making, further compounding its influence.4 In simple terms, CMS decides what is covered and how much we are paid. Whether you are in a solo private practice, an employer health care organization, or an academic medical center, physician reimbursement is declining.
In fact, Congress passed its year-end omnibus legislation in the final days of 2022, including a 2% Medicare physician payment cut for 2023,5 at a time when expenses to practice medicine, including nonphysician staff and supplies, are at an all-time high and we are living in a 6% inflationary state. This translates into being asked to serve more patients and cut costs. Our day-to-day feels much tighter, and this is why: Medicare physician pay increased just 11% over the past 20 years6 (2001–2021) in comparison to the cost of running a medical practice, which increased nearly 40% during that time. In other words, adjusting for inflation in practice costs, Medicare physician payment has fallen 22% over the last 20 years.7
Depending on your employment model, you may feel insulated from these changes as increases in reimbursement have occurred in other areas, such as hospitals and ambulatory surgery centers.8 In the short term, these increases help, as organizations will see additional funds. But there are 2 main issues: First, it is not nearly enough when you consider the soaring costs of running a hospital. And second, looking at our national population, we rely tremendously on self-employed doctors to serve our patients.
More than 80% of US counties lack adequate health care infrastructure.9 More than a third of the US population has less-than-adequate access to pharmacies, primary care physicians, hospitals, trauma centers, and low-cost health centers.9 To put things into perspective, more than 20% of counties in the United States are hospital deserts, where most people must drive more than 30 minutes to reach the closest hospital.9
There is good reason for this. Operating a hospital is a challenging endeavor. Even before the COVID-19 pandemic and the most recent health care financial challenges, most health care systems and large hospitals operated with very low operating margins (2%–3%). Businesses with similar margins include grocery stores and car dealerships. These low-margin businesses, including health care, rely on high volume for sustainability. High patient volumes distribute expensive hospital costs over many encounters. If physicians cannot sustain practices across the country, it is challenging to have sufficient admission and surgical volumes to justify the cost base of hospitals.
To tie this together, we have very little influence on what we are paid for our services. Reimbursement is declining, which makes it hard to have financially sustainable practices. As hospitals struggle, there is more pressure to prioritize highly profitable service lines, like orthopedics and urology, which are associated with favorable technical revenue. As hospitals are threatened, health care deserts widen, which leaves our entire health care system in jeopardy. Not surprisingly, this most likely affects those who face additional barriers to access, such as those with lower income, limited internet access, and lack of insurance. Together, these barriers further widen disparities in health care outcomes, including outcomes for women. Additionally, this death by a thousand cuts has eroded morale and increased physician burnout.
Transforming how we practice medicine is the only viable solution. I have good news: You are the leaders you have been waiting for.
Continue to: Physicians make good managers...
Physicians make good managers
To successfully transform how we practice medicine, it is critical that those leading the transformation deeply understand how medicine is practiced. The level of understanding required can be achieved only through years of medical practice, as a doctor. We understand how medical teams interact and that different sectors of our health care system are interdependent. Also, because physicians drive patient activity and ultimately reimbursement, having a seat at the table is crucial.
Some health care systems are run by businesspeople—people with finance backgrounds—and others are led by physicians. In 2017, Becker’s Hospital Review listed the chief executive officers (CEOs) of 183 nonprofit hospital and health systems.10 Of these, only 25% were led by individuals with an MD. Looking at the 115 largest hospitals in the United States, 30% are physician led.10 Considering the top 10 hospitals ranked by U.S. News & World Report for 2022, 8 of 10 have a physician at the helm.
Beyond raters and rankers, physician-led hospitals do better. Goodall compared CEOs in the top 100 best hospitals in U.S. News & World Report in 3 key medical specialties: cancer, digestive disorders, and cardiac care.11 The study explored the question: “Are hospitals’ quality ranked more highly when they are led by a medically trained doctor or non-MD professional managers?”11 Analysis revealed that hospital quality scores are about 25% higher in physician-run hospitals than in manager-run hospitals.11 Additional research shows that good management practices correlate with hospital performance, and that “the proportion of managers with a clinical degree has the largest positive effect.”12
Several theories exist as to why doctors make good managers in the health care setting.13,14 Doctors may create a more sympathetic and productive work environment for other clinicians because they are one of them. They have peer-to-peer credibility—because they have walked the walk, they have insight and perspective into how medicine is practiced.
Physicians serve as effective change agents for their organizations in several ways:
- First, physicians take a clinical approach in their leadership roles13 and focus on patient care at the center of their decisions. We see the people behind the numbers. Simply put, we humanize the operational side of health care.
- As physicians, we understand the interconnectivity in the practice of medicine. While closing certain service lines may be financially beneficial, these services are often closely linked to profitable service lines.
- Beyond physicians taking a clinical approach to leadership, we emphasize quality.13 Because we all have experienced complications and lived through bad outcomes alongside our patients, we understand deeply how important patient safety and quality is, and we are not willing to sacrifice that for financial gain. For us, this is personal. We don’t see our solution to health care challenges as an “or” situation, instead we view it as an “and” situation.
- Physician leaders often can improve medical staff engagement.13 A 2018 national survey of physicians found that those who are satisfied with their leadership are more engaged at work, have greater job satisfaction, and are less likely to experience signs of burnout.15 Physician administrators add value here.
Continue to: Surgeons as leaders...
Surgeons as leaders
What do we know about surgeons as physician leaders? Looking at the previously mentioned lists of physician leaders, surgeons are relatively absent. In the Becker’s Hospital Review study of nonprofit hospitals, only 9% of CEOs were surgeons.10 In addition, when reviewing data that associated physician leaders and hospital performance, only 3 of the CEOs were surgeons.11 Given that surgeons make up approximately 19% of US physicians, we are underrepresented.
The omission of surgeons as leaders seems inappropriate given that most hospitals are financially reliant on revenue related to surgical care and optimizing this space is an enormous opportunity. Berger and colleagues offered 3 theories as to why there are fewer surgeon leaders16:
- The relative pay of surgeons exceeds that of most other specialties, and there may be less incentive to accept the challenges presented by leadership roles. (I will add that surgeon leadership is more costly to a system.)
- The craftsmanship nature of surgery discourages the development of other career interests beginning at the trainee level.
- Surgeons have been perceived stereotypically to exhibit arrogance, a characteristic that others may not warm to.
This last observation stings. Successful leadership takes social skill and teamwork.14 Although medical care is one of the few disciplines in which lack of teamwork might cost lives, physicians are not trained to be team players. We recognize how our training has led us to be lone wolves or gunners, situations where we as individuals had to beat others to secure our spot. We have been trained in command-and-control environments, in stepping up as a leader in highly stressful situations. This part of surgical culture may handicap surgeons in their quest to be health care leaders.
Other traits, however, make us particularly great leaders in health care. Our desire to succeed, willingness to push ourselves to extremes, ability to laser focus on a task, acceptance of delayed gratification, and aptitude for making timely decisions on limited data help us succeed in leadership roles. Seven years of surgical training helped me develop the grit I use every day in the C-suite.
We need more physician and surgeon leadership to thrive in the challenging health care landscape. Berger and colleagues proposed 3 potential solutions to increase the number of surgeons in hospital leadership positions16:
Nurture future surgical leaders through exposure to management training. Given the contribution to both expense in support services and resources and revenue related to surgical care, each organization needs a content expert to guide these decisions.
Recognize the important contributions that surgeons already make regarding quality, safety, and operational efficiency. An excellent example of this is the American College of Surgeons National Surgical Quality Improvement Program. Because surgeons are content experts in this area, we are primed to lead.
Hospitals, medical schools, and academic departments of surgery should recognize administrative efforts as an important part of the overall academic mission. As the adage states, “No margin, no mission.” We need bright minds to preserve and grow our margins so that we can further invest in our missions.
This is not easy. Given the barriers, this will not happen organically. Charan and colleagues provided an outline for a leadership pathway adapted for physicians (FIGURE).17,18 It starts with the individual practitioner who is a practicing physician and spends most of their time focused on patient care. As a physician becomes more interested in leadership, they develop new skills and take on more and more responsibility. As they increase in leadership responsibility, they tend to reduce clinical time and increase time spent on strategic and business management. This framework creates a pipeline so that physicians and surgeons can be developed strategically and given increasing responsibility as they develop their capabilities and expand their skill sets.

The leadership challenge
To thrive, we must transform health care by changing how we practice medicine. As ObGyns, we are the leaders we have been waiting for. As you ponder your future, think of your current career and the opportunities you might have. Do you have a seat at the table? What table is that? How are you using your knowledge, expertise, and privilege to advance health care and medicine? I challenge you to critically evaluate this—and lead. ●

“If you don’t have a seat at the table, you are probably on the menu.” I first heard this quote in 2013, and it launched my interest in health care leadership and influenced me countless times over the last 10 years.
As Chief of Staff at Cleveland Clinic, I oversee nearly 5,000 physicians and scientists across the globe. I am involved in the physician life cycle: recruiting, hiring, privileging and credentialing, talent development, promotion, professionalism, and career transitions. I also sit at the intersection of medical care and the business of medicine. This means leading 18 clinical service lines responsible for 5.6 million visits, 161,000 surgeries, and billions of dollars in operating revenue per year. How I spend most of my time is a far cry from what I spent 11 years’ training to do—gynecologic surgery. This shift in my career was not because I changed my mind about caring for patients or that I tired of being a full-time surgeon. Nothing could be further from the truth. Women’s health remains my “why,” and my leadership journey has taught me that it is critical to have a seat at the table for the sake of ObGyns and women everywhere.
Women’s health on the menu
I will start with a concrete example of when we, as women and ObGyns, were on the menu. In late 2019, the Ohio state House of Representatives introduced a bill that subjected doctors to potential murder charges if they did not try everything to save the life of a mother and fetus, “including attempting to reimplant an ectopic pregnancy into the woman’s uterus.”1 This bill was based on 2 case reports—one from 1915 and one from 1980—which were both low quality, and the latter case was deemed to be fraudulent.2 How did this happen?
An Ohio state representative developed the bill with help from a lobbyist and without input from physicians or content experts. When asked, the representative shared that “he never researched whether re-implanting an ectopic pregnancy into a woman’s uterus was a viable medical procedure before including it in the bill.”3 He added, “I heard about it over the years. I never questioned it or gave it a lot of thought.”3
This example resonates deeply with many of us; it inspires us to speak up and act. As ObGyns, we clearly understand the consequences of legal and regulatory change in women’s health and how it directly impacts our patients and each of us as physicians. Let’s shift to something that you may feel less passion about, but I believe is equally important. This is where obstetrician-gynecologists sit in the intersection of medical care and business. This is the space where I spend most of my time, and from this vantage point, I worry about our field.
The business of medicine
Starting at the macroeconomic level, let’s think about how we as physicians are reimbursed and who makes these decisions. Looking at the national health care expenditure data, Medicare and Medicaid spending makes up nearly 40% of the total spend, and it is growing.4 Additionally, private health insurance tends to follow Centers for Medicare and Medicaid Services (CMS) decision making, further compounding its influence.4 In simple terms, CMS decides what is covered and how much we are paid. Whether you are in a solo private practice, an employer health care organization, or an academic medical center, physician reimbursement is declining.
In fact, Congress passed its year-end omnibus legislation in the final days of 2022, including a 2% Medicare physician payment cut for 2023,5 at a time when expenses to practice medicine, including nonphysician staff and supplies, are at an all-time high and we are living in a 6% inflationary state. This translates into being asked to serve more patients and cut costs. Our day-to-day feels much tighter, and this is why: Medicare physician pay increased just 11% over the past 20 years6 (2001–2021) in comparison to the cost of running a medical practice, which increased nearly 40% during that time. In other words, adjusting for inflation in practice costs, Medicare physician payment has fallen 22% over the last 20 years.7
Depending on your employment model, you may feel insulated from these changes as increases in reimbursement have occurred in other areas, such as hospitals and ambulatory surgery centers.8 In the short term, these increases help, as organizations will see additional funds. But there are 2 main issues: First, it is not nearly enough when you consider the soaring costs of running a hospital. And second, looking at our national population, we rely tremendously on self-employed doctors to serve our patients.
More than 80% of US counties lack adequate health care infrastructure.9 More than a third of the US population has less-than-adequate access to pharmacies, primary care physicians, hospitals, trauma centers, and low-cost health centers.9 To put things into perspective, more than 20% of counties in the United States are hospital deserts, where most people must drive more than 30 minutes to reach the closest hospital.9
There is good reason for this. Operating a hospital is a challenging endeavor. Even before the COVID-19 pandemic and the most recent health care financial challenges, most health care systems and large hospitals operated with very low operating margins (2%–3%). Businesses with similar margins include grocery stores and car dealerships. These low-margin businesses, including health care, rely on high volume for sustainability. High patient volumes distribute expensive hospital costs over many encounters. If physicians cannot sustain practices across the country, it is challenging to have sufficient admission and surgical volumes to justify the cost base of hospitals.
To tie this together, we have very little influence on what we are paid for our services. Reimbursement is declining, which makes it hard to have financially sustainable practices. As hospitals struggle, there is more pressure to prioritize highly profitable service lines, like orthopedics and urology, which are associated with favorable technical revenue. As hospitals are threatened, health care deserts widen, which leaves our entire health care system in jeopardy. Not surprisingly, this most likely affects those who face additional barriers to access, such as those with lower income, limited internet access, and lack of insurance. Together, these barriers further widen disparities in health care outcomes, including outcomes for women. Additionally, this death by a thousand cuts has eroded morale and increased physician burnout.
Transforming how we practice medicine is the only viable solution. I have good news: You are the leaders you have been waiting for.
Continue to: Physicians make good managers...
Physicians make good managers
To successfully transform how we practice medicine, it is critical that those leading the transformation deeply understand how medicine is practiced. The level of understanding required can be achieved only through years of medical practice, as a doctor. We understand how medical teams interact and that different sectors of our health care system are interdependent. Also, because physicians drive patient activity and ultimately reimbursement, having a seat at the table is crucial.
Some health care systems are run by businesspeople—people with finance backgrounds—and others are led by physicians. In 2017, Becker’s Hospital Review listed the chief executive officers (CEOs) of 183 nonprofit hospital and health systems.10 Of these, only 25% were led by individuals with an MD. Looking at the 115 largest hospitals in the United States, 30% are physician led.10 Considering the top 10 hospitals ranked by U.S. News & World Report for 2022, 8 of 10 have a physician at the helm.
Beyond raters and rankers, physician-led hospitals do better. Goodall compared CEOs in the top 100 best hospitals in U.S. News & World Report in 3 key medical specialties: cancer, digestive disorders, and cardiac care.11 The study explored the question: “Are hospitals’ quality ranked more highly when they are led by a medically trained doctor or non-MD professional managers?”11 Analysis revealed that hospital quality scores are about 25% higher in physician-run hospitals than in manager-run hospitals.11 Additional research shows that good management practices correlate with hospital performance, and that “the proportion of managers with a clinical degree has the largest positive effect.”12
Several theories exist as to why doctors make good managers in the health care setting.13,14 Doctors may create a more sympathetic and productive work environment for other clinicians because they are one of them. They have peer-to-peer credibility—because they have walked the walk, they have insight and perspective into how medicine is practiced.
Physicians serve as effective change agents for their organizations in several ways:
- First, physicians take a clinical approach in their leadership roles13 and focus on patient care at the center of their decisions. We see the people behind the numbers. Simply put, we humanize the operational side of health care.
- As physicians, we understand the interconnectivity in the practice of medicine. While closing certain service lines may be financially beneficial, these services are often closely linked to profitable service lines.
- Beyond physicians taking a clinical approach to leadership, we emphasize quality.13 Because we all have experienced complications and lived through bad outcomes alongside our patients, we understand deeply how important patient safety and quality is, and we are not willing to sacrifice that for financial gain. For us, this is personal. We don’t see our solution to health care challenges as an “or” situation, instead we view it as an “and” situation.
- Physician leaders often can improve medical staff engagement.13 A 2018 national survey of physicians found that those who are satisfied with their leadership are more engaged at work, have greater job satisfaction, and are less likely to experience signs of burnout.15 Physician administrators add value here.
Continue to: Surgeons as leaders...
Surgeons as leaders
What do we know about surgeons as physician leaders? Looking at the previously mentioned lists of physician leaders, surgeons are relatively absent. In the Becker’s Hospital Review study of nonprofit hospitals, only 9% of CEOs were surgeons.10 In addition, when reviewing data that associated physician leaders and hospital performance, only 3 of the CEOs were surgeons.11 Given that surgeons make up approximately 19% of US physicians, we are underrepresented.
The omission of surgeons as leaders seems inappropriate given that most hospitals are financially reliant on revenue related to surgical care and optimizing this space is an enormous opportunity. Berger and colleagues offered 3 theories as to why there are fewer surgeon leaders16:
- The relative pay of surgeons exceeds that of most other specialties, and there may be less incentive to accept the challenges presented by leadership roles. (I will add that surgeon leadership is more costly to a system.)
- The craftsmanship nature of surgery discourages the development of other career interests beginning at the trainee level.
- Surgeons have been perceived stereotypically to exhibit arrogance, a characteristic that others may not warm to.
This last observation stings. Successful leadership takes social skill and teamwork.14 Although medical care is one of the few disciplines in which lack of teamwork might cost lives, physicians are not trained to be team players. We recognize how our training has led us to be lone wolves or gunners, situations where we as individuals had to beat others to secure our spot. We have been trained in command-and-control environments, in stepping up as a leader in highly stressful situations. This part of surgical culture may handicap surgeons in their quest to be health care leaders.
Other traits, however, make us particularly great leaders in health care. Our desire to succeed, willingness to push ourselves to extremes, ability to laser focus on a task, acceptance of delayed gratification, and aptitude for making timely decisions on limited data help us succeed in leadership roles. Seven years of surgical training helped me develop the grit I use every day in the C-suite.
We need more physician and surgeon leadership to thrive in the challenging health care landscape. Berger and colleagues proposed 3 potential solutions to increase the number of surgeons in hospital leadership positions16:
Nurture future surgical leaders through exposure to management training. Given the contribution to both expense in support services and resources and revenue related to surgical care, each organization needs a content expert to guide these decisions.
Recognize the important contributions that surgeons already make regarding quality, safety, and operational efficiency. An excellent example of this is the American College of Surgeons National Surgical Quality Improvement Program. Because surgeons are content experts in this area, we are primed to lead.
Hospitals, medical schools, and academic departments of surgery should recognize administrative efforts as an important part of the overall academic mission. As the adage states, “No margin, no mission.” We need bright minds to preserve and grow our margins so that we can further invest in our missions.
This is not easy. Given the barriers, this will not happen organically. Charan and colleagues provided an outline for a leadership pathway adapted for physicians (FIGURE).17,18 It starts with the individual practitioner who is a practicing physician and spends most of their time focused on patient care. As a physician becomes more interested in leadership, they develop new skills and take on more and more responsibility. As they increase in leadership responsibility, they tend to reduce clinical time and increase time spent on strategic and business management. This framework creates a pipeline so that physicians and surgeons can be developed strategically and given increasing responsibility as they develop their capabilities and expand their skill sets.

The leadership challenge
To thrive, we must transform health care by changing how we practice medicine. As ObGyns, we are the leaders we have been waiting for. As you ponder your future, think of your current career and the opportunities you might have. Do you have a seat at the table? What table is that? How are you using your knowledge, expertise, and privilege to advance health care and medicine? I challenge you to critically evaluate this—and lead. ●
- Law T. Ohio bill suggests doctors who perform abortions could face jail, unless they perform a non-existent treatment. December 1, 2019. Time. Accessed June 12, 2023. https://time.com/5742053 /ectopic-pregnancy-ohio-abortion-bill/
- Grossman D. Ohio abortion, ectopic pregnancy bill: ‘it’s both bad medicine and bad law-making.’ May 21, 2019. Cincinnati.com–The Enquirer. Accessed June 12, 2023. https://www .cincinnati.com/story/opinion/2019/05/21/ohio-abortion-bill -john-becker-daniel-grossman-ectopic-pregnancy-false-medicine /3753610002/
- Lobbyist had hand in bill sparking ectopic pregnancy flap. December 11, 2019. Associated Press. Accessed June 12, 2023. https://apnews .com/article/03216e44405fa184ae0ab80fa85089f8
- NHE fact sheet. CMS.gov. Updated February 17, 2023. Accessed June 12, 2023. https://www.cms.gov/research-statistics-data-and -systems/statistics-trends-and-reports/nationalhealthexpenddata /nhe-fact-sheet
- Senate passes omnibus spending bill with health provisions. December 23, 2022. American Hospital Association. Accessed June 12, 2023. https://www.aha.org/special-bulletin/2022-12-20-appropriations -committees-release-omnibus-spending-bill-health-provisions
- Medicare updates compared to inflation (2001-2021). October 2021. American Medical Association. Accessed June 12, 2023. https://www .ama-assn.org/system/files/medicare-pay-chart-2021.pdf
- Resneck Jr J. Medicare physician payment reform is long overdue. October 3, 2022. American Medical Association. Accessed June 7, 2023. https://www.ama-assn.org/about/leadership /medicare-physician-payment-reform-long-overdue
- Isenberg M. The stark reality of physician reimbursement. August 24, 2022. Zotec Partners. Accessed June 13, 2023. https://zotecpartners. com/advocacy-zpac/test-1/
- Nguyen A. Mapping healthcare deserts: 80% of the country lacks adequate access to healthcare. September 9, 2021. GoodRx Health. Accessed June 13, 2023. https://www.goodrx.com/healthcare -access/research/healthcare-deserts-80-percent-of-country-lacks -adequate-healthcare-access
- 183 nonprofit hospital and health system CEOs to know–2017. Updated June 20, 2018. Becker’s Hospital Review. Accessed June 7, 2023. https://www.beckershospitalreview.com/lists/188-nonprofit -hospital-and-health-system-ceos-to-know-2017.html
- Goodall AH. Physician-leaders and hospital performance: is there an association? Soc Sci Med. 2011;73:535-539. doi:10.1016 /j.socscimed.2011.06.025
- Bloom N, Sadun R, Van Reenen J. Does Management Matter in Healthcare? Center for Economic Performance and Harvard Business School; 2014.
- Turner J. Why healthcare C-suites should include physicians. September 3, 2019. Managed Healthcare Executive. Accessed June 13, 2023. https://www.managedhealthcareexecutive.com /view/why-healthcare-c-suites-should-include-physicians
- Stoller JK, Goodall A, Baker A. Why the best hospitals are managed by doctors. December 27, 2016. Harvard Business Review. Accessed June 13, 2023. https://hbr.org/2016/12/why-the-best-hospitals -are-managed-by-doctors
- Hayhurst C. Data confirms: leaders, physician burnout is on you. April 3, 2019. Aetnahealth. Accessed June 13, 2023. https://www .athenahealth.com/knowledge-hub/practice-management /research-confirms-leaders-burnout-you
- Berger DH, Goodall A, Tsai AY. The importance of increasing surgeon participation in hospital leadership. JAMA Surg. 2019;154:281-282. doi:10.1001/jamasurg.2018.5080
- Charan R, Drotter S, Noel J. The Leadership Pipeline: How to Build the Leadership-Powered Company. Jossey-Bass; 2001.
- Perry J, Mobley F, Brubaker M. Most doctors have little or no management training, and that’s a problem. December 15, 2017. Harvard Business Review. Accessed June 7, 2023. https://hbr.org/2017/12 /most-doctors-have-little-or-no-management-training-and-thats -a-problem
- Law T. Ohio bill suggests doctors who perform abortions could face jail, unless they perform a non-existent treatment. December 1, 2019. Time. Accessed June 12, 2023. https://time.com/5742053 /ectopic-pregnancy-ohio-abortion-bill/
- Grossman D. Ohio abortion, ectopic pregnancy bill: ‘it’s both bad medicine and bad law-making.’ May 21, 2019. Cincinnati.com–The Enquirer. Accessed June 12, 2023. https://www .cincinnati.com/story/opinion/2019/05/21/ohio-abortion-bill -john-becker-daniel-grossman-ectopic-pregnancy-false-medicine /3753610002/
- Lobbyist had hand in bill sparking ectopic pregnancy flap. December 11, 2019. Associated Press. Accessed June 12, 2023. https://apnews .com/article/03216e44405fa184ae0ab80fa85089f8
- NHE fact sheet. CMS.gov. Updated February 17, 2023. Accessed June 12, 2023. https://www.cms.gov/research-statistics-data-and -systems/statistics-trends-and-reports/nationalhealthexpenddata /nhe-fact-sheet
- Senate passes omnibus spending bill with health provisions. December 23, 2022. American Hospital Association. Accessed June 12, 2023. https://www.aha.org/special-bulletin/2022-12-20-appropriations -committees-release-omnibus-spending-bill-health-provisions
- Medicare updates compared to inflation (2001-2021). October 2021. American Medical Association. Accessed June 12, 2023. https://www .ama-assn.org/system/files/medicare-pay-chart-2021.pdf
- Resneck Jr J. Medicare physician payment reform is long overdue. October 3, 2022. American Medical Association. Accessed June 7, 2023. https://www.ama-assn.org/about/leadership /medicare-physician-payment-reform-long-overdue
- Isenberg M. The stark reality of physician reimbursement. August 24, 2022. Zotec Partners. Accessed June 13, 2023. https://zotecpartners. com/advocacy-zpac/test-1/
- Nguyen A. Mapping healthcare deserts: 80% of the country lacks adequate access to healthcare. September 9, 2021. GoodRx Health. Accessed June 13, 2023. https://www.goodrx.com/healthcare -access/research/healthcare-deserts-80-percent-of-country-lacks -adequate-healthcare-access
- 183 nonprofit hospital and health system CEOs to know–2017. Updated June 20, 2018. Becker’s Hospital Review. Accessed June 7, 2023. https://www.beckershospitalreview.com/lists/188-nonprofit -hospital-and-health-system-ceos-to-know-2017.html
- Goodall AH. Physician-leaders and hospital performance: is there an association? Soc Sci Med. 2011;73:535-539. doi:10.1016 /j.socscimed.2011.06.025
- Bloom N, Sadun R, Van Reenen J. Does Management Matter in Healthcare? Center for Economic Performance and Harvard Business School; 2014.
- Turner J. Why healthcare C-suites should include physicians. September 3, 2019. Managed Healthcare Executive. Accessed June 13, 2023. https://www.managedhealthcareexecutive.com /view/why-healthcare-c-suites-should-include-physicians
- Stoller JK, Goodall A, Baker A. Why the best hospitals are managed by doctors. December 27, 2016. Harvard Business Review. Accessed June 13, 2023. https://hbr.org/2016/12/why-the-best-hospitals -are-managed-by-doctors
- Hayhurst C. Data confirms: leaders, physician burnout is on you. April 3, 2019. Aetnahealth. Accessed June 13, 2023. https://www .athenahealth.com/knowledge-hub/practice-management /research-confirms-leaders-burnout-you
- Berger DH, Goodall A, Tsai AY. The importance of increasing surgeon participation in hospital leadership. JAMA Surg. 2019;154:281-282. doi:10.1001/jamasurg.2018.5080
- Charan R, Drotter S, Noel J. The Leadership Pipeline: How to Build the Leadership-Powered Company. Jossey-Bass; 2001.
- Perry J, Mobley F, Brubaker M. Most doctors have little or no management training, and that’s a problem. December 15, 2017. Harvard Business Review. Accessed June 7, 2023. https://hbr.org/2017/12 /most-doctors-have-little-or-no-management-training-and-thats -a-problem
Surgical volume and outcomes for gynecologic surgery: Is more always better?
Over the last 3 decades, abundant evidence has demonstrated the association between surgical volume and outcomes. Patients operated on by high-volume surgeons and at high-volume hospitals have superior outcomes.
Surgical volume in gynecology
The association between both hospital and surgeon volume and outcomes has been explored across a number of gynecologic procedures.3 A meta-analysis that included 741,000 patients found that low-volume surgeons had an increased rate of complications overall, a higher rate of intraoperative complications, and a higher rate of postoperative complications compared with high-volume surgeons. While there was no association between volume and mortality overall, when limited to gynecologic oncology studies, low surgeon volume was associated with increased perioperative mortality.3
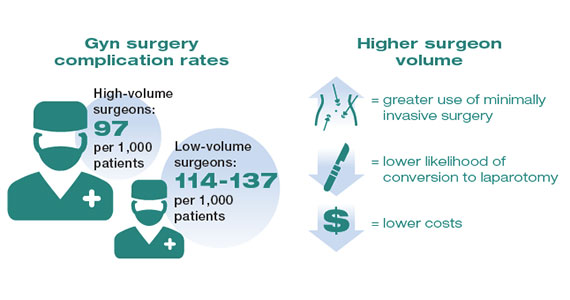
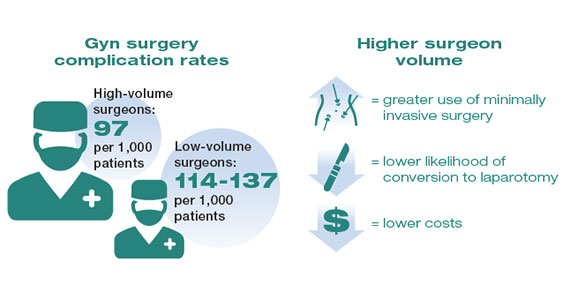
While these studies demonstrated a statistically significant association between surgeon volume and perioperative outcomes, the magnitude of the effect is modest compared with other higher-risk procedures associated with greater perioperative morbidity. For example, in a large study that examined oncologic and cardiovascular surgery, perioperative mortality in patients who underwent pancreatic resection was reduced from 15% for low-volume surgeons to 5% for high-volume surgeons.1 By contrast, for gynecologic surgery, complications occurred in 97 per 1,000 patients operated on by high-volume surgeons compared with between 114 and 137 per 1,000 for low-volume surgeons. Thus, to avoid 1 in-hospital complication, 30 surgeries performed by low-volume surgeons would need to be moved to high-volume surgeons. For intraoperative complications, 38 patients would need to be moved from low- to high-volume surgeons to prevent 1 such complication.3 In addition to morbidity and mortality, higher surgeon volume is associated with greater use of minimally invasive surgery, a lower likelihood of conversion to laparotomy, and lower costs.3
Similarly, hospital volume also has been associated with outcomes for gynecologic surgery.4 In a report of patients who underwent laparoscopic hysterectomy, the authors found that the complication rate was 18% lower for patients at high- versus low-volume hospitals. In addition, cost was lower at the high-volume centers.4 Like surgeon volume, the magnitude of the differential in outcomes between high- and low-volume hospitals is often modest.4
While most studies have focused on short-term outcomes, surgical volume appears also to be associated with longer-term outcomes. For gynecologic cancer, studies have demonstrated an association between hospital volume and survival for ovarian and cervical cancer.5-7 A large report of centers across the United States found that the 5-year survival rate increased from 39% for patients treated at low-volume centers to 51% at the highest-volume hospitals.5 In urogynecology, surgeon volume has been associated with midurethral sling revision. One study noted that after an individual surgeon performed 50 procedures a year, each additional case was associated with a decline in the rate of sling revision.8 One could argue that these longer-term end points may be the measures that matter most to patients.
Although the magnitude of the association between surgical volume and outcomes in gynecology appears to be relatively modest, outcomes for very-low-volume (VLV) surgeons are substantially worse. An analysis of more than 430,000 patients who underwent hysterectomy compared outcomes between VLV surgeons (characterized as surgeons who performed only 1 hysterectomy in the prior year) and other gynecologic surgeons. The overall complication rate was 32% in VLV surgeons compared with 10% among other surgeons, while the perioperative mortality rate was 2.5% versus 0.2% in the 2 groups, respectively. Likely reflecting changing practice patterns in gynecology, a sizable number of surgeons were classified as VLV physicians.9
Continue to: Public health applications of gynecologic surgical volume...
Public health applications of gynecologic surgical volume
The large body of literature on volume and outcomes has led to a number of public health initiatives aimed at reducing perioperative morbidity and mortality. Broadly, these efforts focus on regionalization of care, targeted quality improvement, and the development of minimum volume standards. Each strategy holds promise but also the potential to lead to unwanted consequences.
Regionalization of care
Recognition of the volume-outcomes paradigm has led to efforts to regionalize care for complex procedures to high-volume surgeons and centers.10 A cohort study of surgical patterns of care for Medicare recipients who underwent cancer resections or abdominal aortic aneurysm repair from 1999 to 2008 demonstrated these shifting practice patterns. For example, in 1999–2000, pancreatectomy was performed in 1,308 hospitals, with a median case volume of 5 procedures per year. By 2007–2008, the number of hospitals in which pancreatectomy was performed declined to 978, and the median case volume rose to 16 procedures per year. Importantly, over this time period, risk-adjusted mortality for pancreatectomy declined by 19%, and increased hospital volume was responsible for more than two-thirds of the decline in mortality.10
There has similarly been a gradual concentration of some gynecologic procedures to higher-volume surgeons and centers.11,12 Among patients undergoing hysterectomy for endometrial cancer in New York State, 845 surgeons with a mean case volume of 3 procedures per year treated patients in 2000. By 2014, the number of surgeons who performed these operations declined to 317 while mean annual case volume rose to 10 procedures per year. The number of hospitals in which women with endometrial cancer were treated declined from 182 to 98 over the same time period.11 Similar trends were noted for patients undergoing ovarian cancer resection.12 While patterns of gynecologic care for some surgical procedures have clearly changed, it has been more difficult to link these changes to improvements in outcomes.11,12
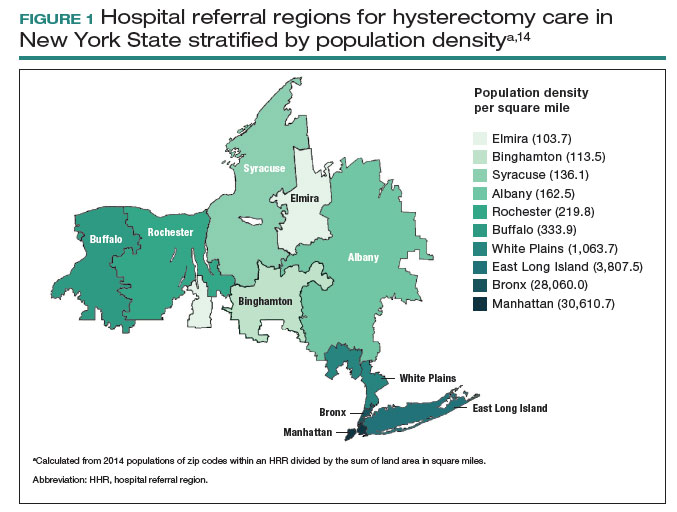
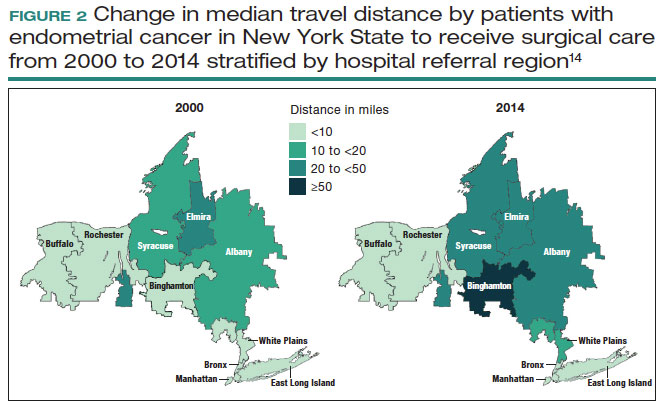
Despite the intuitive appeal of regionalization of surgical care, such a strategy has a number of limitations and practical challenges. Not surprisingly, limiting the number of surgeons and hospitals that perform a given procedure necessitates that patients travel a greater distance to obtain necessary surgical care.13,14 An analysis of endometrial cancer patients in New York State stratified patients based on their area of residence into 10 hospital referral regions (HRRs), which represent health care markets for tertiary medical care. From 2000 to 2014, the distance patients traveled to receive their surgical care increased in all of the HRRs studied. This was most pronounced in 1 of the HRRs in which the median travel distance rose by 47 miles over the 15-year period (FIGURE 1; FIGURE 2).14
Whether patients are willing to travel for care remains a matter of debate and depends on the disease, the surgical procedure, and the anticipated benefit associated with a longer travel distance.15,16 In a discrete choice experiment, 100 participants were given a hypothetical scenario in which they had potentially resectable pancreatic cancer; they were queried on their willingness to travel for care based on varying differences in mortality between a local and regional hospital.15 When mortality at the local hospital was double that of the regional hospital (6% vs 3%), 45% of patients chose to remain at the local hospital. When the differential increased to a 4 times greater mortality at the local hospital (12% vs 3%), 23% of patients still chose to remain at the local hospital.15
A similar study asked patients with ovarian neoplasms whether they would travel 50 miles to a regional center for surgery based on some degree of increased 5-year survival.16 Overall, 79% of patients would travel for a 4% improvement in survival while 97% would travel for a 12% improvement in survival.16
Lastly, a number of studies have shown that regionalization of surgical care disproportionately affects Black and Hispanic patients and those with low socioeconomic status.12,13,17 A simulation study on the effect of regionalizing care for pancreatectomy noted that using a hospital volume threshold of 20 procedures per year, a higher percentage of Black and Hispanic patients than White patients would be required to travel to a higher-volume center.13 Similarly, Medicaid recipients were more likely to be affected.13 Despite the inequities in who must travel for regionalized care, prior work has suggested that regionalization of cancer care to high-volume centers may reduce racial and socioeconomic disparities in survival for some cancers.18
Targeted quality improvement
Realizing the practical limitations of regionalization of care, an alternative strategy is to improve the quality of care at low-volume hospitals.5,19 Quality of care and surgical volume often are correlated, and the delivery of high-quality care can mitigate some of the influence of surgical volume on outcomes.
These principles were demonstrated in a study of more than 100,000 patients with ovarian cancer that stratified treating hospitals into volume quintiles.5 As expected, survival (both 2- and 5-year) was highest in the highest-volume quintile hospitals (FIGURE 3).5 Similarly, quality of care, measured through adherence to various process measures, was also highest in the highest-volume quintile hospitals. Interestingly, in the second-fourth volume quintile hospitals, there was substantial variation in adherence to quality metrics. Among hospitals with higher quality care, an improved survival was noted compared with lower quality care hospitals within the same volume quintile. Survival at high-quality, intermediate-volume hospitals approached that of the high-volume quintile hospitals.5
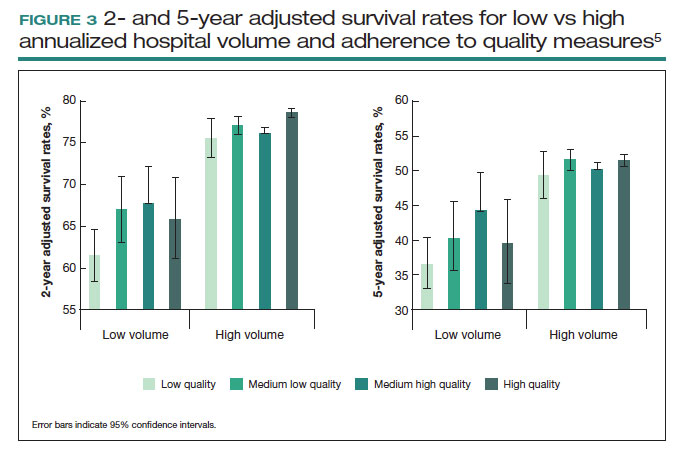
These findings highlight the importance of quality of care as well as the complex interplay of surgical volume and other factors.20 Many have argued that it may be more appropriate to measure quality of care and past performance and outcomes rather than surgical volume.21
Continue to: Minimum volume standards...
Minimum volume standards
While efforts to regionalize surgical care have gradually evolved, calls have been growing to formalize policies that limit the performance of some procedures to surgeons and centers that meet a minimum volume threshold or standard.21 One such effort, based on consensus from 3 academic hospital systems, was a campaign for hospitals to “Take the Volume Pledge.”21 The campaign’s goal is to encourage health care systems to restrict the performance of 10 procedures to surgeons and hospitals within their systems that meet a minimum volume standard for the given operations.21 In essence, procedures would be restricted for low-volume providers and centers and triaged to higher-volume surgeons and hospitals within a given health care system.21
Proponents of the Volume Pledge argue that it is a relatively straightforward way to align patients and providers to optimize outcomes. The Volume Pledge focuses on larger hospital systems and encourages referral within the given system, thus mitigating competitive and financial concerns about referring patients to outside providers. Those who have argued against the Volume Pledge point out that the volume cut points chosen are somewhat arbitrary, that these policies have the potential to negatively impact rural hospitals and those serving smaller communities, and that quality is a more appropriate metric than volume.22 The Volume Pledge does not include any gynecologic procedures, and to date it has met with only limited success.23
Perhaps more directly applicable to gynecologic surgeons are ongoing national trends to base hospital credentialing on surgical volume. In essence, individual surgeons must demonstrate that they have performed a minimum number of procedures to obtain or retain privileges.24,25 While there is strong evidence of the association between volume and outcomes for some complex surgical procedures, linking volume to credentialing has a number of potential pitfalls. Studies of surgical outcomes based on volume represent average performance, and many low-volume providers have better-than-expected outcomes. Volume measures typically represent recent performance; it is difficult to measure the overall experience of individual surgeons. Similarly, surgical outcomes depend on both the surgeon and the system in which the surgeon operates. It is difficult, if not impossible, to account for differences in the environment in which a surgeon works.25
A study of gynecologic surgeons who performed hysterectomy in New York State demonstrates many of the complexities of volume-based credentialing.26 In a cohort of more than55,000 patients who underwent abdominal hysterectomy, there was a strong association between low surgeon volume and a higher-than-expected rate of complications. If one were to consider limiting privileges to even the lowest-volume providers, there would be a significant impact on the surgical workforce. In this cohort, limiting credentialing to the lowest-volume providers, those who performed only 1 abdominal hysterectomy in the prior year would restrict the privileges of 17.5% of the surgeons in the cohort. Further, in this low-volume cohort that performed only 1 abdominal hysterectomy in the prior year, 69% of the surgeons actually had outcomes that were better than predicted.26 These data highlight not only the difficulty of applying averages to individual surgeons but also the profound impact that policy changes could have on the practice of gynecologic surgery.
Volume-outcomes paradigm discussions continue
The association between higher surgeon and hospital procedural volume for gynecologic surgeries and improved outcomes now has been convincingly demonstrated. With this knowledge, over the last decade the patterns of care for patients undergoing gynecologic surgery have clearly shifted, and these operations are now more commonly being performed by a smaller number of physicians and at fewer hospitals.
While efforts to improve quality are clearly important, many policy interventions, such as regionalization of care, have untoward consequences that must be considered. As we move forward, it will be essential to ensure that there is a robust debate among patients, providers, and policymakers on the merits of public health policies based on the volume-outcomes paradigm. ●
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:11281137.
- Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:21-33.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130:545-553.
- Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11-17.
- Matsuo K, Shimada M, Yamaguchi S, et al. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol. 2019;133:1086-1098.
- Brennand EA, Quan H. Evaluation of the effect of surgeon’s operative volume and specialty on likelihood of revision after mesh midurethral sling placement. Obstet Gynecol. 2019;133:1099-1108.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:21282137.
- Wright JD, Ruiz MP, Chen L, et al. Changes in surgical volume and outcomes over time for women undergoing hysterectomy for endometrial cancer. Obstet Gynecol. 2018;132:59-69.
- Wright JD, Chen L, Buskwofie A, et al. Regionalization of care for women with ovarian cancer. Gynecol Oncol. 2019;154:394-400.
- Fong ZV, Hashimoto DA, Jin G, et al. Simulated volume-based regionalization of complex procedures: impact on spatial access to care. Ann Surg. 2021;274:312-318.
- Knisely A, Huang Y, Melamed A, et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am J Obstet Gynecol. 2020;222:58.e1-58.e10.
- Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204-209.
- Shalowitz DI, Nivasch E, Burger RA, et al. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol. 2018;148:42-48.
- Rehmani SS, Liu B, Al-Ayoubi AM, et al. Racial disparity in utilization of high-volume hospitals for surgical treatment of esophageal cancer. Ann Thorac Surg. 2018;106:346-353.
- Nattinger AB, Rademacher N, McGinley EL, et al. Can regionalization of care reduce socioeconomic disparities in breast cancer survival? Med Care. 2021;59:77-81.
- Auerbach AD, Hilton JF, Maselli J, et al. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696-704.
- Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg. 2012;143:287-293.
- Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388-1390.
- Blanco BA, Kothari AN, Blackwell RH, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161:837-845.
- Farjah F, Grau-Sepulveda MV, Gaissert H, et al. Volume Pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38:3518-3527.
- Tracy EE, Zephyrin LC, Rosman DA, et al. Credentialing based on surgical volume, physician workforce challenges, and patient access. Obstet Gynecol. 2013;122:947-951.
- Statement on credentialing and privileging and volume performance issues. April 1, 2018. American College of Surgeons. Accessed April 10, 2023. https://facs.org/about-acs/statements/credentialing-andprivileging-and-volume-performance-issues/
- Ruiz MP, Chen L, Hou JY, et al. Effect of minimum-volume standards on patient outcomes and surgical practice patterns for hysterectomy. Obstet Gynecol. 2018;132:1229-1237.
Over the last 3 decades, abundant evidence has demonstrated the association between surgical volume and outcomes. Patients operated on by high-volume surgeons and at high-volume hospitals have superior outcomes.
Surgical volume in gynecology
The association between both hospital and surgeon volume and outcomes has been explored across a number of gynecologic procedures.3 A meta-analysis that included 741,000 patients found that low-volume surgeons had an increased rate of complications overall, a higher rate of intraoperative complications, and a higher rate of postoperative complications compared with high-volume surgeons. While there was no association between volume and mortality overall, when limited to gynecologic oncology studies, low surgeon volume was associated with increased perioperative mortality.3
While these studies demonstrated a statistically significant association between surgeon volume and perioperative outcomes, the magnitude of the effect is modest compared with other higher-risk procedures associated with greater perioperative morbidity. For example, in a large study that examined oncologic and cardiovascular surgery, perioperative mortality in patients who underwent pancreatic resection was reduced from 15% for low-volume surgeons to 5% for high-volume surgeons.1 By contrast, for gynecologic surgery, complications occurred in 97 per 1,000 patients operated on by high-volume surgeons compared with between 114 and 137 per 1,000 for low-volume surgeons. Thus, to avoid 1 in-hospital complication, 30 surgeries performed by low-volume surgeons would need to be moved to high-volume surgeons. For intraoperative complications, 38 patients would need to be moved from low- to high-volume surgeons to prevent 1 such complication.3 In addition to morbidity and mortality, higher surgeon volume is associated with greater use of minimally invasive surgery, a lower likelihood of conversion to laparotomy, and lower costs.3

Similarly, hospital volume also has been associated with outcomes for gynecologic surgery.4 In a report of patients who underwent laparoscopic hysterectomy, the authors found that the complication rate was 18% lower for patients at high- versus low-volume hospitals. In addition, cost was lower at the high-volume centers.4 Like surgeon volume, the magnitude of the differential in outcomes between high- and low-volume hospitals is often modest.4
While most studies have focused on short-term outcomes, surgical volume appears also to be associated with longer-term outcomes. For gynecologic cancer, studies have demonstrated an association between hospital volume and survival for ovarian and cervical cancer.5-7 A large report of centers across the United States found that the 5-year survival rate increased from 39% for patients treated at low-volume centers to 51% at the highest-volume hospitals.5 In urogynecology, surgeon volume has been associated with midurethral sling revision. One study noted that after an individual surgeon performed 50 procedures a year, each additional case was associated with a decline in the rate of sling revision.8 One could argue that these longer-term end points may be the measures that matter most to patients.
Although the magnitude of the association between surgical volume and outcomes in gynecology appears to be relatively modest, outcomes for very-low-volume (VLV) surgeons are substantially worse. An analysis of more than 430,000 patients who underwent hysterectomy compared outcomes between VLV surgeons (characterized as surgeons who performed only 1 hysterectomy in the prior year) and other gynecologic surgeons. The overall complication rate was 32% in VLV surgeons compared with 10% among other surgeons, while the perioperative mortality rate was 2.5% versus 0.2% in the 2 groups, respectively. Likely reflecting changing practice patterns in gynecology, a sizable number of surgeons were classified as VLV physicians.9
Continue to: Public health applications of gynecologic surgical volume...
Public health applications of gynecologic surgical volume
The large body of literature on volume and outcomes has led to a number of public health initiatives aimed at reducing perioperative morbidity and mortality. Broadly, these efforts focus on regionalization of care, targeted quality improvement, and the development of minimum volume standards. Each strategy holds promise but also the potential to lead to unwanted consequences.
Regionalization of care
Recognition of the volume-outcomes paradigm has led to efforts to regionalize care for complex procedures to high-volume surgeons and centers.10 A cohort study of surgical patterns of care for Medicare recipients who underwent cancer resections or abdominal aortic aneurysm repair from 1999 to 2008 demonstrated these shifting practice patterns. For example, in 1999–2000, pancreatectomy was performed in 1,308 hospitals, with a median case volume of 5 procedures per year. By 2007–2008, the number of hospitals in which pancreatectomy was performed declined to 978, and the median case volume rose to 16 procedures per year. Importantly, over this time period, risk-adjusted mortality for pancreatectomy declined by 19%, and increased hospital volume was responsible for more than two-thirds of the decline in mortality.10
There has similarly been a gradual concentration of some gynecologic procedures to higher-volume surgeons and centers.11,12 Among patients undergoing hysterectomy for endometrial cancer in New York State, 845 surgeons with a mean case volume of 3 procedures per year treated patients in 2000. By 2014, the number of surgeons who performed these operations declined to 317 while mean annual case volume rose to 10 procedures per year. The number of hospitals in which women with endometrial cancer were treated declined from 182 to 98 over the same time period.11 Similar trends were noted for patients undergoing ovarian cancer resection.12 While patterns of gynecologic care for some surgical procedures have clearly changed, it has been more difficult to link these changes to improvements in outcomes.11,12
Despite the intuitive appeal of regionalization of surgical care, such a strategy has a number of limitations and practical challenges. Not surprisingly, limiting the number of surgeons and hospitals that perform a given procedure necessitates that patients travel a greater distance to obtain necessary surgical care.13,14 An analysis of endometrial cancer patients in New York State stratified patients based on their area of residence into 10 hospital referral regions (HRRs), which represent health care markets for tertiary medical care. From 2000 to 2014, the distance patients traveled to receive their surgical care increased in all of the HRRs studied. This was most pronounced in 1 of the HRRs in which the median travel distance rose by 47 miles over the 15-year period (FIGURE 1; FIGURE 2).14
Whether patients are willing to travel for care remains a matter of debate and depends on the disease, the surgical procedure, and the anticipated benefit associated with a longer travel distance.15,16 In a discrete choice experiment, 100 participants were given a hypothetical scenario in which they had potentially resectable pancreatic cancer; they were queried on their willingness to travel for care based on varying differences in mortality between a local and regional hospital.15 When mortality at the local hospital was double that of the regional hospital (6% vs 3%), 45% of patients chose to remain at the local hospital. When the differential increased to a 4 times greater mortality at the local hospital (12% vs 3%), 23% of patients still chose to remain at the local hospital.15


A similar study asked patients with ovarian neoplasms whether they would travel 50 miles to a regional center for surgery based on some degree of increased 5-year survival.16 Overall, 79% of patients would travel for a 4% improvement in survival while 97% would travel for a 12% improvement in survival.16
Lastly, a number of studies have shown that regionalization of surgical care disproportionately affects Black and Hispanic patients and those with low socioeconomic status.12,13,17 A simulation study on the effect of regionalizing care for pancreatectomy noted that using a hospital volume threshold of 20 procedures per year, a higher percentage of Black and Hispanic patients than White patients would be required to travel to a higher-volume center.13 Similarly, Medicaid recipients were more likely to be affected.13 Despite the inequities in who must travel for regionalized care, prior work has suggested that regionalization of cancer care to high-volume centers may reduce racial and socioeconomic disparities in survival for some cancers.18
Targeted quality improvement
Realizing the practical limitations of regionalization of care, an alternative strategy is to improve the quality of care at low-volume hospitals.5,19 Quality of care and surgical volume often are correlated, and the delivery of high-quality care can mitigate some of the influence of surgical volume on outcomes.
These principles were demonstrated in a study of more than 100,000 patients with ovarian cancer that stratified treating hospitals into volume quintiles.5 As expected, survival (both 2- and 5-year) was highest in the highest-volume quintile hospitals (FIGURE 3).5 Similarly, quality of care, measured through adherence to various process measures, was also highest in the highest-volume quintile hospitals. Interestingly, in the second-fourth volume quintile hospitals, there was substantial variation in adherence to quality metrics. Among hospitals with higher quality care, an improved survival was noted compared with lower quality care hospitals within the same volume quintile. Survival at high-quality, intermediate-volume hospitals approached that of the high-volume quintile hospitals.5

These findings highlight the importance of quality of care as well as the complex interplay of surgical volume and other factors.20 Many have argued that it may be more appropriate to measure quality of care and past performance and outcomes rather than surgical volume.21
Continue to: Minimum volume standards...
Minimum volume standards
While efforts to regionalize surgical care have gradually evolved, calls have been growing to formalize policies that limit the performance of some procedures to surgeons and centers that meet a minimum volume threshold or standard.21 One such effort, based on consensus from 3 academic hospital systems, was a campaign for hospitals to “Take the Volume Pledge.”21 The campaign’s goal is to encourage health care systems to restrict the performance of 10 procedures to surgeons and hospitals within their systems that meet a minimum volume standard for the given operations.21 In essence, procedures would be restricted for low-volume providers and centers and triaged to higher-volume surgeons and hospitals within a given health care system.21
Proponents of the Volume Pledge argue that it is a relatively straightforward way to align patients and providers to optimize outcomes. The Volume Pledge focuses on larger hospital systems and encourages referral within the given system, thus mitigating competitive and financial concerns about referring patients to outside providers. Those who have argued against the Volume Pledge point out that the volume cut points chosen are somewhat arbitrary, that these policies have the potential to negatively impact rural hospitals and those serving smaller communities, and that quality is a more appropriate metric than volume.22 The Volume Pledge does not include any gynecologic procedures, and to date it has met with only limited success.23
Perhaps more directly applicable to gynecologic surgeons are ongoing national trends to base hospital credentialing on surgical volume. In essence, individual surgeons must demonstrate that they have performed a minimum number of procedures to obtain or retain privileges.24,25 While there is strong evidence of the association between volume and outcomes for some complex surgical procedures, linking volume to credentialing has a number of potential pitfalls. Studies of surgical outcomes based on volume represent average performance, and many low-volume providers have better-than-expected outcomes. Volume measures typically represent recent performance; it is difficult to measure the overall experience of individual surgeons. Similarly, surgical outcomes depend on both the surgeon and the system in which the surgeon operates. It is difficult, if not impossible, to account for differences in the environment in which a surgeon works.25
A study of gynecologic surgeons who performed hysterectomy in New York State demonstrates many of the complexities of volume-based credentialing.26 In a cohort of more than55,000 patients who underwent abdominal hysterectomy, there was a strong association between low surgeon volume and a higher-than-expected rate of complications. If one were to consider limiting privileges to even the lowest-volume providers, there would be a significant impact on the surgical workforce. In this cohort, limiting credentialing to the lowest-volume providers, those who performed only 1 abdominal hysterectomy in the prior year would restrict the privileges of 17.5% of the surgeons in the cohort. Further, in this low-volume cohort that performed only 1 abdominal hysterectomy in the prior year, 69% of the surgeons actually had outcomes that were better than predicted.26 These data highlight not only the difficulty of applying averages to individual surgeons but also the profound impact that policy changes could have on the practice of gynecologic surgery.
Volume-outcomes paradigm discussions continue
The association between higher surgeon and hospital procedural volume for gynecologic surgeries and improved outcomes now has been convincingly demonstrated. With this knowledge, over the last decade the patterns of care for patients undergoing gynecologic surgery have clearly shifted, and these operations are now more commonly being performed by a smaller number of physicians and at fewer hospitals.
While efforts to improve quality are clearly important, many policy interventions, such as regionalization of care, have untoward consequences that must be considered. As we move forward, it will be essential to ensure that there is a robust debate among patients, providers, and policymakers on the merits of public health policies based on the volume-outcomes paradigm. ●
Over the last 3 decades, abundant evidence has demonstrated the association between surgical volume and outcomes. Patients operated on by high-volume surgeons and at high-volume hospitals have superior outcomes.
Surgical volume in gynecology
The association between both hospital and surgeon volume and outcomes has been explored across a number of gynecologic procedures.3 A meta-analysis that included 741,000 patients found that low-volume surgeons had an increased rate of complications overall, a higher rate of intraoperative complications, and a higher rate of postoperative complications compared with high-volume surgeons. While there was no association between volume and mortality overall, when limited to gynecologic oncology studies, low surgeon volume was associated with increased perioperative mortality.3
While these studies demonstrated a statistically significant association between surgeon volume and perioperative outcomes, the magnitude of the effect is modest compared with other higher-risk procedures associated with greater perioperative morbidity. For example, in a large study that examined oncologic and cardiovascular surgery, perioperative mortality in patients who underwent pancreatic resection was reduced from 15% for low-volume surgeons to 5% for high-volume surgeons.1 By contrast, for gynecologic surgery, complications occurred in 97 per 1,000 patients operated on by high-volume surgeons compared with between 114 and 137 per 1,000 for low-volume surgeons. Thus, to avoid 1 in-hospital complication, 30 surgeries performed by low-volume surgeons would need to be moved to high-volume surgeons. For intraoperative complications, 38 patients would need to be moved from low- to high-volume surgeons to prevent 1 such complication.3 In addition to morbidity and mortality, higher surgeon volume is associated with greater use of minimally invasive surgery, a lower likelihood of conversion to laparotomy, and lower costs.3

Similarly, hospital volume also has been associated with outcomes for gynecologic surgery.4 In a report of patients who underwent laparoscopic hysterectomy, the authors found that the complication rate was 18% lower for patients at high- versus low-volume hospitals. In addition, cost was lower at the high-volume centers.4 Like surgeon volume, the magnitude of the differential in outcomes between high- and low-volume hospitals is often modest.4
While most studies have focused on short-term outcomes, surgical volume appears also to be associated with longer-term outcomes. For gynecologic cancer, studies have demonstrated an association between hospital volume and survival for ovarian and cervical cancer.5-7 A large report of centers across the United States found that the 5-year survival rate increased from 39% for patients treated at low-volume centers to 51% at the highest-volume hospitals.5 In urogynecology, surgeon volume has been associated with midurethral sling revision. One study noted that after an individual surgeon performed 50 procedures a year, each additional case was associated with a decline in the rate of sling revision.8 One could argue that these longer-term end points may be the measures that matter most to patients.
Although the magnitude of the association between surgical volume and outcomes in gynecology appears to be relatively modest, outcomes for very-low-volume (VLV) surgeons are substantially worse. An analysis of more than 430,000 patients who underwent hysterectomy compared outcomes between VLV surgeons (characterized as surgeons who performed only 1 hysterectomy in the prior year) and other gynecologic surgeons. The overall complication rate was 32% in VLV surgeons compared with 10% among other surgeons, while the perioperative mortality rate was 2.5% versus 0.2% in the 2 groups, respectively. Likely reflecting changing practice patterns in gynecology, a sizable number of surgeons were classified as VLV physicians.9
Continue to: Public health applications of gynecologic surgical volume...
Public health applications of gynecologic surgical volume
The large body of literature on volume and outcomes has led to a number of public health initiatives aimed at reducing perioperative morbidity and mortality. Broadly, these efforts focus on regionalization of care, targeted quality improvement, and the development of minimum volume standards. Each strategy holds promise but also the potential to lead to unwanted consequences.
Regionalization of care
Recognition of the volume-outcomes paradigm has led to efforts to regionalize care for complex procedures to high-volume surgeons and centers.10 A cohort study of surgical patterns of care for Medicare recipients who underwent cancer resections or abdominal aortic aneurysm repair from 1999 to 2008 demonstrated these shifting practice patterns. For example, in 1999–2000, pancreatectomy was performed in 1,308 hospitals, with a median case volume of 5 procedures per year. By 2007–2008, the number of hospitals in which pancreatectomy was performed declined to 978, and the median case volume rose to 16 procedures per year. Importantly, over this time period, risk-adjusted mortality for pancreatectomy declined by 19%, and increased hospital volume was responsible for more than two-thirds of the decline in mortality.10
There has similarly been a gradual concentration of some gynecologic procedures to higher-volume surgeons and centers.11,12 Among patients undergoing hysterectomy for endometrial cancer in New York State, 845 surgeons with a mean case volume of 3 procedures per year treated patients in 2000. By 2014, the number of surgeons who performed these operations declined to 317 while mean annual case volume rose to 10 procedures per year. The number of hospitals in which women with endometrial cancer were treated declined from 182 to 98 over the same time period.11 Similar trends were noted for patients undergoing ovarian cancer resection.12 While patterns of gynecologic care for some surgical procedures have clearly changed, it has been more difficult to link these changes to improvements in outcomes.11,12
Despite the intuitive appeal of regionalization of surgical care, such a strategy has a number of limitations and practical challenges. Not surprisingly, limiting the number of surgeons and hospitals that perform a given procedure necessitates that patients travel a greater distance to obtain necessary surgical care.13,14 An analysis of endometrial cancer patients in New York State stratified patients based on their area of residence into 10 hospital referral regions (HRRs), which represent health care markets for tertiary medical care. From 2000 to 2014, the distance patients traveled to receive their surgical care increased in all of the HRRs studied. This was most pronounced in 1 of the HRRs in which the median travel distance rose by 47 miles over the 15-year period (FIGURE 1; FIGURE 2).14
Whether patients are willing to travel for care remains a matter of debate and depends on the disease, the surgical procedure, and the anticipated benefit associated with a longer travel distance.15,16 In a discrete choice experiment, 100 participants were given a hypothetical scenario in which they had potentially resectable pancreatic cancer; they were queried on their willingness to travel for care based on varying differences in mortality between a local and regional hospital.15 When mortality at the local hospital was double that of the regional hospital (6% vs 3%), 45% of patients chose to remain at the local hospital. When the differential increased to a 4 times greater mortality at the local hospital (12% vs 3%), 23% of patients still chose to remain at the local hospital.15


A similar study asked patients with ovarian neoplasms whether they would travel 50 miles to a regional center for surgery based on some degree of increased 5-year survival.16 Overall, 79% of patients would travel for a 4% improvement in survival while 97% would travel for a 12% improvement in survival.16
Lastly, a number of studies have shown that regionalization of surgical care disproportionately affects Black and Hispanic patients and those with low socioeconomic status.12,13,17 A simulation study on the effect of regionalizing care for pancreatectomy noted that using a hospital volume threshold of 20 procedures per year, a higher percentage of Black and Hispanic patients than White patients would be required to travel to a higher-volume center.13 Similarly, Medicaid recipients were more likely to be affected.13 Despite the inequities in who must travel for regionalized care, prior work has suggested that regionalization of cancer care to high-volume centers may reduce racial and socioeconomic disparities in survival for some cancers.18
Targeted quality improvement
Realizing the practical limitations of regionalization of care, an alternative strategy is to improve the quality of care at low-volume hospitals.5,19 Quality of care and surgical volume often are correlated, and the delivery of high-quality care can mitigate some of the influence of surgical volume on outcomes.
These principles were demonstrated in a study of more than 100,000 patients with ovarian cancer that stratified treating hospitals into volume quintiles.5 As expected, survival (both 2- and 5-year) was highest in the highest-volume quintile hospitals (FIGURE 3).5 Similarly, quality of care, measured through adherence to various process measures, was also highest in the highest-volume quintile hospitals. Interestingly, in the second-fourth volume quintile hospitals, there was substantial variation in adherence to quality metrics. Among hospitals with higher quality care, an improved survival was noted compared with lower quality care hospitals within the same volume quintile. Survival at high-quality, intermediate-volume hospitals approached that of the high-volume quintile hospitals.5

These findings highlight the importance of quality of care as well as the complex interplay of surgical volume and other factors.20 Many have argued that it may be more appropriate to measure quality of care and past performance and outcomes rather than surgical volume.21
Continue to: Minimum volume standards...
Minimum volume standards
While efforts to regionalize surgical care have gradually evolved, calls have been growing to formalize policies that limit the performance of some procedures to surgeons and centers that meet a minimum volume threshold or standard.21 One such effort, based on consensus from 3 academic hospital systems, was a campaign for hospitals to “Take the Volume Pledge.”21 The campaign’s goal is to encourage health care systems to restrict the performance of 10 procedures to surgeons and hospitals within their systems that meet a minimum volume standard for the given operations.21 In essence, procedures would be restricted for low-volume providers and centers and triaged to higher-volume surgeons and hospitals within a given health care system.21
Proponents of the Volume Pledge argue that it is a relatively straightforward way to align patients and providers to optimize outcomes. The Volume Pledge focuses on larger hospital systems and encourages referral within the given system, thus mitigating competitive and financial concerns about referring patients to outside providers. Those who have argued against the Volume Pledge point out that the volume cut points chosen are somewhat arbitrary, that these policies have the potential to negatively impact rural hospitals and those serving smaller communities, and that quality is a more appropriate metric than volume.22 The Volume Pledge does not include any gynecologic procedures, and to date it has met with only limited success.23
Perhaps more directly applicable to gynecologic surgeons are ongoing national trends to base hospital credentialing on surgical volume. In essence, individual surgeons must demonstrate that they have performed a minimum number of procedures to obtain or retain privileges.24,25 While there is strong evidence of the association between volume and outcomes for some complex surgical procedures, linking volume to credentialing has a number of potential pitfalls. Studies of surgical outcomes based on volume represent average performance, and many low-volume providers have better-than-expected outcomes. Volume measures typically represent recent performance; it is difficult to measure the overall experience of individual surgeons. Similarly, surgical outcomes depend on both the surgeon and the system in which the surgeon operates. It is difficult, if not impossible, to account for differences in the environment in which a surgeon works.25
A study of gynecologic surgeons who performed hysterectomy in New York State demonstrates many of the complexities of volume-based credentialing.26 In a cohort of more than55,000 patients who underwent abdominal hysterectomy, there was a strong association between low surgeon volume and a higher-than-expected rate of complications. If one were to consider limiting privileges to even the lowest-volume providers, there would be a significant impact on the surgical workforce. In this cohort, limiting credentialing to the lowest-volume providers, those who performed only 1 abdominal hysterectomy in the prior year would restrict the privileges of 17.5% of the surgeons in the cohort. Further, in this low-volume cohort that performed only 1 abdominal hysterectomy in the prior year, 69% of the surgeons actually had outcomes that were better than predicted.26 These data highlight not only the difficulty of applying averages to individual surgeons but also the profound impact that policy changes could have on the practice of gynecologic surgery.
Volume-outcomes paradigm discussions continue
The association between higher surgeon and hospital procedural volume for gynecologic surgeries and improved outcomes now has been convincingly demonstrated. With this knowledge, over the last decade the patterns of care for patients undergoing gynecologic surgery have clearly shifted, and these operations are now more commonly being performed by a smaller number of physicians and at fewer hospitals.
While efforts to improve quality are clearly important, many policy interventions, such as regionalization of care, have untoward consequences that must be considered. As we move forward, it will be essential to ensure that there is a robust debate among patients, providers, and policymakers on the merits of public health policies based on the volume-outcomes paradigm. ●
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:11281137.
- Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:21-33.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130:545-553.
- Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11-17.
- Matsuo K, Shimada M, Yamaguchi S, et al. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol. 2019;133:1086-1098.
- Brennand EA, Quan H. Evaluation of the effect of surgeon’s operative volume and specialty on likelihood of revision after mesh midurethral sling placement. Obstet Gynecol. 2019;133:1099-1108.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:21282137.
- Wright JD, Ruiz MP, Chen L, et al. Changes in surgical volume and outcomes over time for women undergoing hysterectomy for endometrial cancer. Obstet Gynecol. 2018;132:59-69.
- Wright JD, Chen L, Buskwofie A, et al. Regionalization of care for women with ovarian cancer. Gynecol Oncol. 2019;154:394-400.
- Fong ZV, Hashimoto DA, Jin G, et al. Simulated volume-based regionalization of complex procedures: impact on spatial access to care. Ann Surg. 2021;274:312-318.
- Knisely A, Huang Y, Melamed A, et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am J Obstet Gynecol. 2020;222:58.e1-58.e10.
- Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204-209.
- Shalowitz DI, Nivasch E, Burger RA, et al. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol. 2018;148:42-48.
- Rehmani SS, Liu B, Al-Ayoubi AM, et al. Racial disparity in utilization of high-volume hospitals for surgical treatment of esophageal cancer. Ann Thorac Surg. 2018;106:346-353.
- Nattinger AB, Rademacher N, McGinley EL, et al. Can regionalization of care reduce socioeconomic disparities in breast cancer survival? Med Care. 2021;59:77-81.
- Auerbach AD, Hilton JF, Maselli J, et al. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696-704.
- Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg. 2012;143:287-293.
- Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388-1390.
- Blanco BA, Kothari AN, Blackwell RH, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161:837-845.
- Farjah F, Grau-Sepulveda MV, Gaissert H, et al. Volume Pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38:3518-3527.
- Tracy EE, Zephyrin LC, Rosman DA, et al. Credentialing based on surgical volume, physician workforce challenges, and patient access. Obstet Gynecol. 2013;122:947-951.
- Statement on credentialing and privileging and volume performance issues. April 1, 2018. American College of Surgeons. Accessed April 10, 2023. https://facs.org/about-acs/statements/credentialing-andprivileging-and-volume-performance-issues/
- Ruiz MP, Chen L, Hou JY, et al. Effect of minimum-volume standards on patient outcomes and surgical practice patterns for hysterectomy. Obstet Gynecol. 2018;132:1229-1237.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:11281137.
- Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:21-33.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130:545-553.
- Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11-17.
- Matsuo K, Shimada M, Yamaguchi S, et al. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol. 2019;133:1086-1098.
- Brennand EA, Quan H. Evaluation of the effect of surgeon’s operative volume and specialty on likelihood of revision after mesh midurethral sling placement. Obstet Gynecol. 2019;133:1099-1108.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:21282137.
- Wright JD, Ruiz MP, Chen L, et al. Changes in surgical volume and outcomes over time for women undergoing hysterectomy for endometrial cancer. Obstet Gynecol. 2018;132:59-69.
- Wright JD, Chen L, Buskwofie A, et al. Regionalization of care for women with ovarian cancer. Gynecol Oncol. 2019;154:394-400.
- Fong ZV, Hashimoto DA, Jin G, et al. Simulated volume-based regionalization of complex procedures: impact on spatial access to care. Ann Surg. 2021;274:312-318.
- Knisely A, Huang Y, Melamed A, et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am J Obstet Gynecol. 2020;222:58.e1-58.e10.
- Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204-209.
- Shalowitz DI, Nivasch E, Burger RA, et al. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol. 2018;148:42-48.
- Rehmani SS, Liu B, Al-Ayoubi AM, et al. Racial disparity in utilization of high-volume hospitals for surgical treatment of esophageal cancer. Ann Thorac Surg. 2018;106:346-353.
- Nattinger AB, Rademacher N, McGinley EL, et al. Can regionalization of care reduce socioeconomic disparities in breast cancer survival? Med Care. 2021;59:77-81.
- Auerbach AD, Hilton JF, Maselli J, et al. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696-704.
- Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg. 2012;143:287-293.
- Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388-1390.
- Blanco BA, Kothari AN, Blackwell RH, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161:837-845.
- Farjah F, Grau-Sepulveda MV, Gaissert H, et al. Volume Pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38:3518-3527.
- Tracy EE, Zephyrin LC, Rosman DA, et al. Credentialing based on surgical volume, physician workforce challenges, and patient access. Obstet Gynecol. 2013;122:947-951.
- Statement on credentialing and privileging and volume performance issues. April 1, 2018. American College of Surgeons. Accessed April 10, 2023. https://facs.org/about-acs/statements/credentialing-andprivileging-and-volume-performance-issues/
- Ruiz MP, Chen L, Hou JY, et al. Effect of minimum-volume standards on patient outcomes and surgical practice patterns for hysterectomy. Obstet Gynecol. 2018;132:1229-1237.
AAP issues guidance on inguinal hernias
Faraz A. Khan, MD, an adjunct associate professor in the division of pediatric surgery at Loma Linda (Calif.) University Children’s Hospital, led the AAP’s Committee on Fetus and Newborn, sections on surgery and urology, in writing the guidance, published in Pediatrics.
An inguinal hernia, a common pediatric surgical condition (90% are in boys, the authors wrote), appears as a bulge in the groin or scrotum and requires surgical repair to prevent a more severe incarcerated hernia, which occurs when organs from the abdomen become trapped in the hernia.
The risk of that incarceration drives the preference and timing of surgical repair, the authors wrote.
The incidence of inguinal hernias is about 8-50 per 1,000 live births in term infants and is much higher in extremely low-birth-weight infants.
Ankush Gosain, MD, PhD, chief of pediatric surgery at Children’s Hospital Colorado, Aurora, who was not involved in the AAP clinical report, said in an interview that the best timing for the surgery on a premature infant has been an unanswered question and this guidance is helpful.
Inguinal hernias in preterm infants are especially common. The incidence is reported to be as high as 20%.
Repair can wait until babies have left NICU
The authors concluded that there was moderate-quality evidence supporting deferring hernia repair until after discharge from the neonatal intensive care unit because this may reduce the risk of respiratory problems without increasing risk of incarceration or another operation.
But Dr. Gosain noted that the authors left the door open for data from a study that recently finished enrolling patients. That trial (Dr. Gosain is a site investigator) is expected to help determine whether an early- or late-term approach is best in preterm infants.
“There are pluses and minuses that we and the neonatologists and the anesthesiologists recognize,” he said.
Laparoscopic approach as good, sometimes better
Dr. Gosain also said he was glad to see the authors addressed the merits of the laparoscopic approach and when it is preferred.
The authors noted that a laparoscopic approach is increasingly popular – rates have grown fivefold between 2009 and 2018 – and they found it is “at least as effective as, if not better than,” the current preferred method, traditional open high ligation of the hernia sac.
Laparoscopy also appears to be a feasible option in managing recurrent hernias.
Dr. Gosain said that, when the laparoscopic approach was developed, there was concern that it would lead to higher recurrence of the hernias. “That concern has diminished over time,” he added. The paper helps give surgeons and pediatricians peace of mind that this is a safe approach.
Who should perform the surgeries?
The authors concluded that, ideally, pediatric surgical specialists, pediatric urologists, or general surgeons with a significant yearly case volume should perform the surgeries.
They found a significant inverse relationship between recurrence rates and general surgeon case volume: general surgeons who completed fewer than 10 pediatric inguinal hernias per year had the highest recurrence rates and the highest-volume general surgeons had recurrence rates similar to pediatric surgical specialists.
Pediatric surgical specialists trained in fellowships had the lowest rate of hernia recurrences.
Dr. Gosain said he was glad the authors pointed out that both the surgeon and the anesthesiologist ideally should have that specialty training.
No evidence that anesthetic exposure affects neurodevelopment
The researchers found no conclusive evidence that otherwise-healthy children’s exposure to a single relatively short duration of anesthetic adds any significant risk to neurodevelopment or academic performance, or increases risk of ADHD or autism spectrum disorder.
Contralateral exploration with unilateral hernia
Providers continue to debate contralateral exploration among patients with unilateral inguinal hernia. Proponents of exploration cite a 10%-15% rate of developing of a hernia at a later time. Therefore, routine exploration and, if identified, ligation of a patent processus vaginalis (PPV) may avoid a subsequent anesthetic.
Opponents counter that not all PPVs will become clinically significant inguinal hernias, and doing routine exploration exposes the patient to potentially unnecessary complications.
The authors wrote: “In the absence of strong data for or against repair of incidentally discovered contralateral PPV, family values related to the risks and benefits of each approach from a nuanced preoperative discussion should be considered.”
Dr. Gosain said that, with all of the guidance points, “you need to have a true conversation between the surgeon and the parents with pluses and minuses of the different approaches because one is not necessarily absolutely better than the other.”
The authors and Dr. Gosain declare no relevant financial relationships.
Faraz A. Khan, MD, an adjunct associate professor in the division of pediatric surgery at Loma Linda (Calif.) University Children’s Hospital, led the AAP’s Committee on Fetus and Newborn, sections on surgery and urology, in writing the guidance, published in Pediatrics.
An inguinal hernia, a common pediatric surgical condition (90% are in boys, the authors wrote), appears as a bulge in the groin or scrotum and requires surgical repair to prevent a more severe incarcerated hernia, which occurs when organs from the abdomen become trapped in the hernia.
The risk of that incarceration drives the preference and timing of surgical repair, the authors wrote.
The incidence of inguinal hernias is about 8-50 per 1,000 live births in term infants and is much higher in extremely low-birth-weight infants.
Ankush Gosain, MD, PhD, chief of pediatric surgery at Children’s Hospital Colorado, Aurora, who was not involved in the AAP clinical report, said in an interview that the best timing for the surgery on a premature infant has been an unanswered question and this guidance is helpful.
Inguinal hernias in preterm infants are especially common. The incidence is reported to be as high as 20%.
Repair can wait until babies have left NICU
The authors concluded that there was moderate-quality evidence supporting deferring hernia repair until after discharge from the neonatal intensive care unit because this may reduce the risk of respiratory problems without increasing risk of incarceration or another operation.
But Dr. Gosain noted that the authors left the door open for data from a study that recently finished enrolling patients. That trial (Dr. Gosain is a site investigator) is expected to help determine whether an early- or late-term approach is best in preterm infants.
“There are pluses and minuses that we and the neonatologists and the anesthesiologists recognize,” he said.
Laparoscopic approach as good, sometimes better
Dr. Gosain also said he was glad to see the authors addressed the merits of the laparoscopic approach and when it is preferred.
The authors noted that a laparoscopic approach is increasingly popular – rates have grown fivefold between 2009 and 2018 – and they found it is “at least as effective as, if not better than,” the current preferred method, traditional open high ligation of the hernia sac.
Laparoscopy also appears to be a feasible option in managing recurrent hernias.
Dr. Gosain said that, when the laparoscopic approach was developed, there was concern that it would lead to higher recurrence of the hernias. “That concern has diminished over time,” he added. The paper helps give surgeons and pediatricians peace of mind that this is a safe approach.
Who should perform the surgeries?
The authors concluded that, ideally, pediatric surgical specialists, pediatric urologists, or general surgeons with a significant yearly case volume should perform the surgeries.
They found a significant inverse relationship between recurrence rates and general surgeon case volume: general surgeons who completed fewer than 10 pediatric inguinal hernias per year had the highest recurrence rates and the highest-volume general surgeons had recurrence rates similar to pediatric surgical specialists.
Pediatric surgical specialists trained in fellowships had the lowest rate of hernia recurrences.
Dr. Gosain said he was glad the authors pointed out that both the surgeon and the anesthesiologist ideally should have that specialty training.
No evidence that anesthetic exposure affects neurodevelopment
The researchers found no conclusive evidence that otherwise-healthy children’s exposure to a single relatively short duration of anesthetic adds any significant risk to neurodevelopment or academic performance, or increases risk of ADHD or autism spectrum disorder.
Contralateral exploration with unilateral hernia
Providers continue to debate contralateral exploration among patients with unilateral inguinal hernia. Proponents of exploration cite a 10%-15% rate of developing of a hernia at a later time. Therefore, routine exploration and, if identified, ligation of a patent processus vaginalis (PPV) may avoid a subsequent anesthetic.
Opponents counter that not all PPVs will become clinically significant inguinal hernias, and doing routine exploration exposes the patient to potentially unnecessary complications.
The authors wrote: “In the absence of strong data for or against repair of incidentally discovered contralateral PPV, family values related to the risks and benefits of each approach from a nuanced preoperative discussion should be considered.”
Dr. Gosain said that, with all of the guidance points, “you need to have a true conversation between the surgeon and the parents with pluses and minuses of the different approaches because one is not necessarily absolutely better than the other.”
The authors and Dr. Gosain declare no relevant financial relationships.
Faraz A. Khan, MD, an adjunct associate professor in the division of pediatric surgery at Loma Linda (Calif.) University Children’s Hospital, led the AAP’s Committee on Fetus and Newborn, sections on surgery and urology, in writing the guidance, published in Pediatrics.
An inguinal hernia, a common pediatric surgical condition (90% are in boys, the authors wrote), appears as a bulge in the groin or scrotum and requires surgical repair to prevent a more severe incarcerated hernia, which occurs when organs from the abdomen become trapped in the hernia.
The risk of that incarceration drives the preference and timing of surgical repair, the authors wrote.
The incidence of inguinal hernias is about 8-50 per 1,000 live births in term infants and is much higher in extremely low-birth-weight infants.
Ankush Gosain, MD, PhD, chief of pediatric surgery at Children’s Hospital Colorado, Aurora, who was not involved in the AAP clinical report, said in an interview that the best timing for the surgery on a premature infant has been an unanswered question and this guidance is helpful.
Inguinal hernias in preterm infants are especially common. The incidence is reported to be as high as 20%.
Repair can wait until babies have left NICU
The authors concluded that there was moderate-quality evidence supporting deferring hernia repair until after discharge from the neonatal intensive care unit because this may reduce the risk of respiratory problems without increasing risk of incarceration or another operation.
But Dr. Gosain noted that the authors left the door open for data from a study that recently finished enrolling patients. That trial (Dr. Gosain is a site investigator) is expected to help determine whether an early- or late-term approach is best in preterm infants.
“There are pluses and minuses that we and the neonatologists and the anesthesiologists recognize,” he said.
Laparoscopic approach as good, sometimes better
Dr. Gosain also said he was glad to see the authors addressed the merits of the laparoscopic approach and when it is preferred.
The authors noted that a laparoscopic approach is increasingly popular – rates have grown fivefold between 2009 and 2018 – and they found it is “at least as effective as, if not better than,” the current preferred method, traditional open high ligation of the hernia sac.
Laparoscopy also appears to be a feasible option in managing recurrent hernias.
Dr. Gosain said that, when the laparoscopic approach was developed, there was concern that it would lead to higher recurrence of the hernias. “That concern has diminished over time,” he added. The paper helps give surgeons and pediatricians peace of mind that this is a safe approach.
Who should perform the surgeries?
The authors concluded that, ideally, pediatric surgical specialists, pediatric urologists, or general surgeons with a significant yearly case volume should perform the surgeries.
They found a significant inverse relationship between recurrence rates and general surgeon case volume: general surgeons who completed fewer than 10 pediatric inguinal hernias per year had the highest recurrence rates and the highest-volume general surgeons had recurrence rates similar to pediatric surgical specialists.
Pediatric surgical specialists trained in fellowships had the lowest rate of hernia recurrences.
Dr. Gosain said he was glad the authors pointed out that both the surgeon and the anesthesiologist ideally should have that specialty training.
No evidence that anesthetic exposure affects neurodevelopment
The researchers found no conclusive evidence that otherwise-healthy children’s exposure to a single relatively short duration of anesthetic adds any significant risk to neurodevelopment or academic performance, or increases risk of ADHD or autism spectrum disorder.
Contralateral exploration with unilateral hernia
Providers continue to debate contralateral exploration among patients with unilateral inguinal hernia. Proponents of exploration cite a 10%-15% rate of developing of a hernia at a later time. Therefore, routine exploration and, if identified, ligation of a patent processus vaginalis (PPV) may avoid a subsequent anesthetic.
Opponents counter that not all PPVs will become clinically significant inguinal hernias, and doing routine exploration exposes the patient to potentially unnecessary complications.
The authors wrote: “In the absence of strong data for or against repair of incidentally discovered contralateral PPV, family values related to the risks and benefits of each approach from a nuanced preoperative discussion should be considered.”
Dr. Gosain said that, with all of the guidance points, “you need to have a true conversation between the surgeon and the parents with pluses and minuses of the different approaches because one is not necessarily absolutely better than the other.”
The authors and Dr. Gosain declare no relevant financial relationships.
FROM PEDIATRICS
Girls with congenital diaphragmatic hernia have lower survival rates
TOPLINE:
, a study published in the Journal of Pediatrics has found.
METHODOLOGY:
- The retrospective cohort study used demographic, clinical, and outcomes data from the registry to investigate the role of biological sex for babies with CDH.
- The study included more than 7,200 newborns at 105 hospitals across 17 countries from January 2007 to December 2018.
- The primary outcomes were 30-day, 60-day, and in-hospital mortality.
- The secondary outcomes included weight gain during admission, feeding, and oxygen status at 30 days and at discharge.
TAKEAWAY:
- After controlling for markers of disease severity, girls with CDH had a 32% increased risk for death at 30-days (adjusted hazard ratio, 1.32; P = .02).
- Girls had lower survival rates at 30 days (77.3%), compared with boys (80.1%).
- The disparity remained at 60 days, with the survival rate for girls with CDH (73.5%) modestly lower than that for boys (77.2%), and also at discharge – 70.2%, compared with 74.2%, respectively.
- The survival differences between boys and girls primarily affected babies who did not undergo cannulation; in this group, the survival rate for girls was 77.5%, compared with 82.1% for boys.
- Girls with CDH weighed less at birth (2.8 kg) than boys with the condition (3 kg); birth weight is a for babies with CDH, .
IN PRACTICE:
“Although racial and ethnic outcome disparities have been documented in CDH, disparities between males and females are not well known,” Shaun Kunisaki, MD, MSc, a senior author of the study and professor of surgery at Johns Hopkins University in Baltimore, said in a press release about the findings. “It is really important to understand if those disparities exist, because it may change how we can better manage these patients.”
STUDY DETAILS:
The authors include seven from the division of general pediatric surgery and the division of pediatric respiratory sciences at Johns Hopkins University. Two authors are from the department of pediatric surgery at the University of Texas and Children’s Memorial Hermann Hospital, both in Houston.
LIMITATIONS:
The authors point to potential challenges with data collection, including diagnosis miscoding and missing data. They acknowledge that MRI fetal lung volumes, degree of liver herniation, and emerging markers of socioeconomic status were not available in the database. The study also could not examine long-term outcomes because data were limited to in-hospital variables.
DISCLOSURES:
The authors report no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a study published in the Journal of Pediatrics has found.
METHODOLOGY:
- The retrospective cohort study used demographic, clinical, and outcomes data from the registry to investigate the role of biological sex for babies with CDH.
- The study included more than 7,200 newborns at 105 hospitals across 17 countries from January 2007 to December 2018.
- The primary outcomes were 30-day, 60-day, and in-hospital mortality.
- The secondary outcomes included weight gain during admission, feeding, and oxygen status at 30 days and at discharge.
TAKEAWAY:
- After controlling for markers of disease severity, girls with CDH had a 32% increased risk for death at 30-days (adjusted hazard ratio, 1.32; P = .02).
- Girls had lower survival rates at 30 days (77.3%), compared with boys (80.1%).
- The disparity remained at 60 days, with the survival rate for girls with CDH (73.5%) modestly lower than that for boys (77.2%), and also at discharge – 70.2%, compared with 74.2%, respectively.
- The survival differences between boys and girls primarily affected babies who did not undergo cannulation; in this group, the survival rate for girls was 77.5%, compared with 82.1% for boys.
- Girls with CDH weighed less at birth (2.8 kg) than boys with the condition (3 kg); birth weight is a for babies with CDH, .
IN PRACTICE:
“Although racial and ethnic outcome disparities have been documented in CDH, disparities between males and females are not well known,” Shaun Kunisaki, MD, MSc, a senior author of the study and professor of surgery at Johns Hopkins University in Baltimore, said in a press release about the findings. “It is really important to understand if those disparities exist, because it may change how we can better manage these patients.”
STUDY DETAILS:
The authors include seven from the division of general pediatric surgery and the division of pediatric respiratory sciences at Johns Hopkins University. Two authors are from the department of pediatric surgery at the University of Texas and Children’s Memorial Hermann Hospital, both in Houston.
LIMITATIONS:
The authors point to potential challenges with data collection, including diagnosis miscoding and missing data. They acknowledge that MRI fetal lung volumes, degree of liver herniation, and emerging markers of socioeconomic status were not available in the database. The study also could not examine long-term outcomes because data were limited to in-hospital variables.
DISCLOSURES:
The authors report no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
, a study published in the Journal of Pediatrics has found.
METHODOLOGY:
- The retrospective cohort study used demographic, clinical, and outcomes data from the registry to investigate the role of biological sex for babies with CDH.
- The study included more than 7,200 newborns at 105 hospitals across 17 countries from January 2007 to December 2018.
- The primary outcomes were 30-day, 60-day, and in-hospital mortality.
- The secondary outcomes included weight gain during admission, feeding, and oxygen status at 30 days and at discharge.
TAKEAWAY:
- After controlling for markers of disease severity, girls with CDH had a 32% increased risk for death at 30-days (adjusted hazard ratio, 1.32; P = .02).
- Girls had lower survival rates at 30 days (77.3%), compared with boys (80.1%).
- The disparity remained at 60 days, with the survival rate for girls with CDH (73.5%) modestly lower than that for boys (77.2%), and also at discharge – 70.2%, compared with 74.2%, respectively.
- The survival differences between boys and girls primarily affected babies who did not undergo cannulation; in this group, the survival rate for girls was 77.5%, compared with 82.1% for boys.
- Girls with CDH weighed less at birth (2.8 kg) than boys with the condition (3 kg); birth weight is a for babies with CDH, .
IN PRACTICE:
“Although racial and ethnic outcome disparities have been documented in CDH, disparities between males and females are not well known,” Shaun Kunisaki, MD, MSc, a senior author of the study and professor of surgery at Johns Hopkins University in Baltimore, said in a press release about the findings. “It is really important to understand if those disparities exist, because it may change how we can better manage these patients.”
STUDY DETAILS:
The authors include seven from the division of general pediatric surgery and the division of pediatric respiratory sciences at Johns Hopkins University. Two authors are from the department of pediatric surgery at the University of Texas and Children’s Memorial Hermann Hospital, both in Houston.
LIMITATIONS:
The authors point to potential challenges with data collection, including diagnosis miscoding and missing data. They acknowledge that MRI fetal lung volumes, degree of liver herniation, and emerging markers of socioeconomic status were not available in the database. The study also could not examine long-term outcomes because data were limited to in-hospital variables.
DISCLOSURES:
The authors report no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Raising the bar (and the OR table):Ergonomics in MIGS
Work-related musculoskeletal disorders (WMSDs) are “musculoskeletal disorders (injuries or disorders of the muscles, nerves, tendons, joints, cartilage, and spinal discs) in which the work environment and performance of work contribute significantly to the condition; and/or the condition is made worse or persists longer due to work conditions.”1 The health care industry has one of the highest rates of WMSDs, even when compared with traditional labor-intensive occupations, such as coal mining. In 2017, the health care industry reported more than a half million incidents of work-related injury and illness.2,3 In particular, surgeons are at increased risk for WMSDs, since they repetitively perform the classic tenets of poor ergonomics, including operating in static, extreme, and awkward positions and for prolonged periods of time.3
Gynecologic surgeons face unique ergonomic challenges. Operating in the pelvis requires an oblique approach that adds complexity and inhibits appropriate ergonomic positioning.4 All modalities of surgery incur their own challenges and risks to the surgeon, including minimally invasive gynecologic surgery (MIGS), which has become the standard of care for most conditions. Although MIGS has several benefits for the patient, a survey of gynecologic oncologists found that 88% of respondents reported discomfort related to MIGS.5 Several factors contribute to the development of WMSDs in surgery, including lack of ergonomic awareness, suboptimal ergonomic education and training,5,6 and ergonomically poor operating room (OR) equipment and instrument design.7 Furthermore, surgical culture does not generally prioritize ergonomics in the OR or requests for ergonomic accommodations.7,8
Within 5 years, a physician workforce shortage is projected for the United States.9 WMSDs contribute to workforce issues as they are associated with decreased productivity; time off needed for pain and treatment, including short-term disability; and possibly early retirement (as those who are older and have more work experience may be more likely to seek medical attention).10 In a 2013 study of vaginal surgeons, 14% missed work; 21% modified their work hours, work type, or amount of surgery; and 29% modified their surgical technique because of injury.10 Work-related pain also can negatively affect mental health, sleep, relationships, and quality of life.6
Recently, awareness has increased regarding WMSDs and their consequences, which has led to significant strides in the study of ergonomics among surgeons, a growing body of research on the topic, and guidance for optimizing ergonomics in the OR.
Risk factors for ergonomic strain
Several factors contribute to ergonomic strain and, subsequently, the development of WMSDs. Recognizing these factors can direct strategies for injury prevention.
Patient factors
The prevalence of obesity in the United States increased from 30.5% in 1999–2000 to 41.9% between 2017 and 2020.11 As the average patient’s body mass index (BMI) has increased, there is concern for a parallel increase in the ergonomic strain on laparoscopic surgeons.
A study of simulated laparoscopic tasks at varying model BMI levels demonstrated increased surgeon postural stress and workload at higher model BMIs (50 kg/m2) when compared with lower model BMIs (20 and 30 kg/m2).11 This result was supported in another study, which demonstrated both increased muscle activity and increased time needed to complete a surgical task with laparoscopic surgery; interestingly, when the same study measured these parameters for robotic surgery, this association was not seen.12 This suggests that a robotic rather than a laparoscopic approach may avoid some of the ergonomic strain associated with increased patient BMI.
Continue to: Surgeon factors...
Surgeon factors
Various surgeon characteristics have been shown to influence ergonomics in the OR. Surgeons with smaller hand sizes, for example, reported greater physical discomfort and demonstrated greater ergonomic workload when operating laparoscopically.13-15 In particular, those with a glove size of 6.5 or smaller have more difficulty using laparoscopic instruments, and those with a glove size smaller than 7 demonstrate a larger decline in grip strength when using laparoscopic instruments repeatedly.14,16
Surgeon height also can affect the amount of time spent in high-risk, nonergonomic positions. In a study that evaluated video recordings of surgeon posture during gynecologic laparoscopy, shorter surgeons were noted to use greater degrees of neck rotation to look at the monitor.17 Furthermore, surgeons with shorter arm lengths experienced more “extreme positions” of the nondominant shoulder and elbow.17 This trend also was seen in open and robotic surgery, where surgeons with a height of 66 cm or less reported increased pain scores after operating.18
Surgical instruments and OR setup
Surgical instrument characteristics can contribute to ergonomic strain, especially when the instruments have been designed with a one-size-fits-all mentality.8,19 In an examination of the anthropometric measurements of surgeon hand sizes and their correlation with difficulty when using a “standard” laparoscopic instrument, surgeons with smaller finger and hand spans had trouble using these instruments.19 Another study compared surgeon grip strength and ergonomic workloads after using 3 laparoscopic advanced bipolar instruments.16 Gender and hand size aside, the authors found that use of several of the laparoscopic devices led to greater decline in grip strength.16
The setup of the OR also can have a profound effect on the surgeon’s ergonomics. Monitor placement, for example, is crucial to ergonomic success. One study found that positioning the monitor directly in front of the surgeon at eye level was associated with the lowest neck muscle activity during a simulated task.20
Route of surgery
Each surgical approach has intrinsic ergonomic risks. With laparoscopy, surgeons often remain in straight head and back positions without much trunk motion, especially when compared with open surgery.21 In one study, laparoscopic surgeons spent more than 60% of a case in a static position and more than 80% of a case in a high-risk, “demanding” neck position.22
Robotic surgery, in contrast to laparoscopy, often has been cited as being more “ergonomic.” While robotic surgery has less of an effect on the neck, shoulders, arms, and legs than laparoscopy23 and often is associated with less physical discomfort than either open or laparoscopic surgery,23,24 robotic surgery still maintains its own innate ergonomic risks. Of robotic surgeons surveyed, 56.1% reported neck stiffness, finger fatigue, and eye symptoms in one study.25 In another survey study, more robotic surgeons (72%) reported physical symptoms than laparoscopic (57%) and open (49%) surgeons.26Vaginal surgery also puts surgeons at ergonomic risk. A majority of surgeons (87.2%) who completed more than 50% of their cases vaginally reported a history of WMSDs.10 Vaginal surgery places surgeons in awkward positions of the neck, shoulder, and trunk frequently and for longer durations.27
Continue to: Strategies for preventing WMSDs...
Strategies for preventing WMSDs
As factors that contribute to the development of WMSDs are identified, preventive strategies can be targeted to these individual factors. Research has focused on appropriate setup of the OR, surgeon posture, intraoperative microbreaks, and stretching both in and outside of the OR.
1. OR setup and positioning of the surgeon by MIGS route
The route of MIGS affects OR setup and surgeon posture. Ergonomic recommendations for laparoscopy, robotic surgery, and vaginal surgery are all unique to the risks posed by each particular approach.
Laparoscopic surgery. Laparoscopic monitors should face the surgeon directly, with the screen just below eye level to maintain the surgeon’s neck in a neutral position.28 The table height should be set for the tallest surgeon, and shorter surgeons should stand on steps as needed.28 The table height also should allow for the surgeon’s hands to be at elbow height, with the elbows bent at 90 degrees with the wrists straight.29 Foot pedals should be placed at the surgeons’ foot level and should be reached easily.28 Additionally, the patient’s arms should be tucked at their sides to allow surgeons a larger operative space.29 When using laparoscopic instruments, locking and ratcheting features should be used whenever possible to reduce prolonged grip or squeeze forces.28 The laparoscopic camera should be held in the palm with the wrist in a neutral position.29
Robotic surgery. Positioning and setup of the robotic console is a main focus of ergonomic recommendations. The surgeon’s chair should be brought as close to the console as possible, and the knees positioned in a 90-degree angle.30 The foot pedals should be brought toward the surgeon to maintain this angle of the knees.30 The console should be rotated toward the surgeon and then the height adjusted so that the surgeon can look through the eyepiece while sitting upright and can maintain the neck in a neutral position.28,30 The surgeon’s forehead should rest comfortably on the headrest.29 The forearms should rest on the armrest while the arms are maintained in a neutral position and the shoulders remain relaxed while the surgeon holds the robotic controls.30 It is important to utilize the armrest often to relieve stress on the arm while operating.28 Frequent use of the clutch function can keep the robotic controls in the center of the workspace.28
Vaginal surgery. Both seated and standing positions are associated with high-risk positioning of the trunk and bilateral shoulders, respectively, in vaginal surgery.31 However, surgeons who stand while operating vaginally reported more discomfort in the bilateral wrists, thighs, and lower legs than those who operated while seated.31 This suggests a potential ergonomic advantage to the seated position for vaginal surgery. Chair height should be adjusted so the surgeon can look straight ahead with the neck in a neutral position.32 Surgeons should consider using a headlamp, as this may prevent repetitive awkward movements to adjust overhead lights.32 For standing surgery, the table height should be adjusted for the tallest surgeon, and shorter surgeons or assistants should use steps as needed.3
Surgical assistants should switch sides during the course of the case to avoid excessive unilateral upper-extremity strain.32 The addition of a table-mounted vaginal retractor system may be useful in relieving physical strain for surgical assistants, but data currently are lacking to demonstrate this ergonomic benefit.33 Further studies are needed, especially since many surgeons take on the role of surgical assist in the teaching environment and subsequently report more WMSDs than their colleagues who do not work in teaching environments.10,34
2. Pain relief from individual ergonomic positioning devices
Apart from adjusting how the OR equipment is arranged or how the surgeons adjust their positioning, several devices that assist with surgeon positioning—including gel mats or insoles, exoskeletons, and “augmented reality” glasses—are being studied.
The use of gel mats or insoles in the OR has mixed evidence in the literature.35-37
Exoskeletons, external devices that support a surgeon’s posture and positioning, have been studied thus far in simulated nonsterile surgical environments. Preliminarily, it appears that use of an exoskeleton can decrease muscle activity and time spent in static positions, with a reported decrease in post-task user discomfort.38,39 More data are needed to determine if exoskeletons can be used in the sterile setting and for longer durations as may occur in actual OR cases.
Augmented reality glasses project the laparoscopic monitor image to the glasses, which frees the surgeon to place the “monitor” in a more neutral, ergonomic position. In one study, use of augmented reality glasses was associated with decreased muscle activity and a reduction in Rapid Entire Body Assessment (REBA) scores when compared with use of the conventional laparoscopic monitor.40More data are needed on these emerging technologies to determine whether adverse effects occur with prolonged use.
Continue to: 3. Implementing intraoperative microbreaks and stretching...
3. Implementing intraoperative microbreaks and stretching
The American College of Surgeons (ACS) recommends that surgeons avoid prolonged static postures during procedures.28 One strategy for preventing sustained positioning is to incorporate breaks with associated stretching routinely during surgery.28
Microbreaks. In a landmark study by Park and colleagues in 2017, 120-second long targeted stretching microbreaks (TSMBs) were completed every 20 to 40 minutes during a surgery, and results demonstrated improved postoperative surgeon pain scores without an associated increase in the length of the case.41 These surgeons reported improved pain in the neck, bilateral shoulders, bilateral hands, and lower back. Eighty-eight percent of surgeons reported either improvement or “no change” in their mental focus, and 100% reported improvement or “no change” in their physical performance after TSMBs were implemented.42 Of surveyed surgeons, 87% wanted TSMBs incorporated routinely.41,42
Stretches. Multiple resources, such as the ACS and the Mayo Clinic, for intraoperative stretches are available. The ACS recommends performing neck and shoulder stretches during intraoperative microbreaks, including a range-of-movement neck exercise, deep cervical flexor training, and standing scapular retraction.28 The ACS also demonstrates lumbrical stretches for the fingers and passive wrist extension exercises to be used intraoperatively (or between cases) (FIGURE 1).28 The Mayo Clinic Hallbeck Human Factors Engineering Laboratories has a publicly available “OR Stretch Instructional Video” in which the surgeon is guided through several different short stretches, including shoulder shrugging and side bends, that can be used during surgery.43
Both the ACS and the Mayo Clinic provide examples of pertinent stretch exercises for use when not in the sterile environment, between cases or after cases are complete. The ACS recommends several neck and shoulder stretches for the trapezius, levator scapulae, and pectoralis and recommends the use of a foam roller to improve thoracic mobility (FIGURE 2).28 As above, the Mayo Clinic Hallbeck Human Factors Engineering Laboratories has a publicly available “OR-Stretch Between Surgery Stretches Video” in which the surgeon is guided through several short stretches that are done in a seated position, including stretches for the hamstring, lower back, and arms (FIGURE 3).43
Many of the above-mentioned stretches were designed for use in the context of open, laparoscopic, or robotic surgery. For the vaginal surgeon, the intraoperative ergonomic stressors differ from those of other routes of surgery, and thus stretches tailored to the positioning during vaginal surgery are necessary. In a video recently published by the Society of Gynecologic Surgeons, several stretches are reviewed that target high-risk positions often held by the surgeon or assistant when operating vaginally.44 These stretches include cervical retraction, thoracic extension, external arm rotation, cervical side bending, and lumbar extension (FIGURE 4).44 The recommendation is to complete these exercises 2 times per day, with 8 to 10 repetitions per set.44
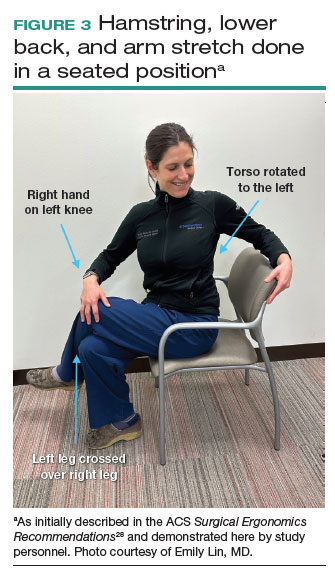
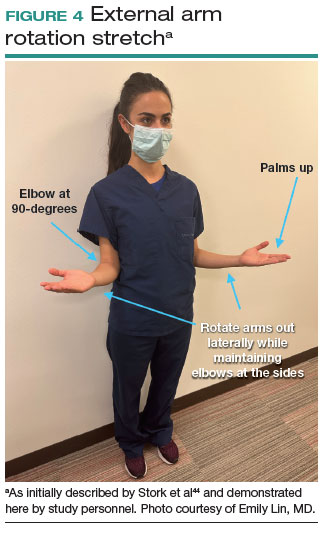
Prioritizing ergonomic awareness and training
As caregivers, it is not uncommon for us to prioritize the needs of others before those of ourselves. However, WMSDs are prevalent, and their downstream effects may cause catastrophic professional and personal losses. Cumulatively, the global impact of WMSDs is a significant issue for the health care workforce and its longevity.
To prevent WMSDs, it is imperative that surgeons are aware of the factors that contribute to injury development and the appropriate, accessible modifications for these factors. While each surgical modality confers its own ergonomic challenges, these risks can be mitigated through increased awareness of OR setup, surgeon positioning, and incorporation of microbreaks and stretching exercises during and after surgical procedures.
Formal training in surgical ergonomics is lacking across specialties, including gynecology.45 Multiple educational interventions have been proposed and studied to help fill this training gap.30,46-49When used, these interventions have been associated with increased knowledge of surgical ergonomic principles or reduction in surgeon pain scores, including trainees.50 As we become more cognizant of WMSDs, standardized resident curricula should be developed in an effort to reduce the prevalence of these potentially career-ending injuries.
In addition to education, cultivating a culture in which ergonomics is prioritized is essential. Although most surgeons report work-related pain, very few report their injuries to occupational health. For example, while 29% of gynecologic oncologists reported seeking treatment for a WMSD, only 1% had reported their injury to their employer.5 In a study of ACS members, only 19% of injuries were reported, 30% of surgeons stated that they did not know how to report an injury, and 21% felt that the resources for surgeons during and after an injury were inadequate.6
As we prioritize the health and safety of our patients, we also need to promote ergonomic awareness in the OR, respect the need for accommodations, encourage injury reporting, support surgeons who need to take time away for medical treatment, and partner with industry to develop new instruments and technology with effective ergonomic features. ●
- Workplace health glossary. Reviewed February 12, 2020. Centers for Disease Control and Prevention. Accessed May 18, 2023. https://www.cdc.gov/workplacehealthpromotion/tools-resources /glossary/glossary.html#W
- Epstein S, Sparer EH, Tran BN, et al. Prevalence of work-related musculoskeletal disorders among surgeons and interventionalists: a systematic review and meta-analysis. JAMA Surg. 2018;153:e174947.
- Yurteri-Kaplan LA, Park AJ. Surgical ergonomics and preventing workrelated musculoskeletal disorders. Obstet Gynecol. 2023;141:455-462.
- Symer MM, Keller DS. Human factors in pelvic surgery. Eur J Surg Oncol. 2022;48:2346-2351.
- Franasiak J, Ko EM, Kidd J, et al. Physical strain and urgent need for ergonomic training among gynecologic oncologists who perform minimally invasive surgery. Gynecol Oncol. 2012;126:437-442.
- Davis WT, Fletcher SA, Guillamondegui OD. Musculoskeletal occupational injury among surgeons: effects for patients, providers, and institutions. J Surg Res. 2014;189:207-212.e6.
- Fox M. Surgeons face unique ergonomic challenges. American College of Surgeons. September 1, 2022. Accessed May 22, 2023. https://www.facs.org/for-medical-professionals/news-publications /news-and-articles/bulletin/september-2022-volume-107-issue-9 /surgeons-face-unique-ergonomic-challenges/
- Wong JMK, Carey ET, King C, et al. A call to action for ergonomic surgical devices designed for diverse surgeon end users. Obstet Gynecol. 2023;141:463-466.
- IHS Inc. The Complexities of Physician Supply and Demand: Projections from 2014 to 2025. Association of American Medical Colleges. April 5, 2016.
- Kim-Fine S, Woolley SM, Weaver AL, et al. Work-related musculoskeletal disorders among vaginal surgeons. Int Urogynecol J. 2013;24:1191-1200.
- Sers R, Forrester S, Zecca M, et al. The ergonomic impact of patient body mass index on surgeon posture during simulated laparoscopy. Appl Ergon. 2021;97:103501.
- Moss EL, Sarhanis P, Ind T, et al. Impact of obesity on surgeon ergonomics in robotic and straight-stick laparoscopic surgery. J Minim Invasive Gynecol. 2020;27:1063-1069.
- Sutton E, Irvin M, Zeigler C, et al. The ergonomics of women in surgery. Surg Endosc. 2014;28:1051-1055.
- Berguer R, Hreljac A. The relationship between hand size and difficulty using surgical instruments: a survey of 726 laparoscopic surgeons. Surg Endosc. 2004;18:508-512.
- Bellini MI, Amabile MI, Saullo P, et al. A woman’s place is in theatre, but are theatres designed with women in mind? A systematic review of ergonomics for women in surgery. J Clin Med. 2022;11:3496.
- Wong JMK, Moore KJ, Lewis P, et al. Ergonomic assessment of surgeon characteristics and laparoscopic device strain in gynecologic surgery. J Minim Invasive Gynecol. 2022;29:1357-1363.
- Aitchison LP, Cui CK, Arnold A, et al. The ergonomics of laparoscopic surgery: a quantitative study of the time and motion of laparoscopic surgeons in live surgical environments. Surg Endosc. 2016;30:5068-5076.
- Stewart C, Raoof M, Fong Y, et al. Who is hurting? A prospective study of surgeon ergonomics. Surg Endosc. 2022;36:292-299.
- Green SV, Morris DE, Naumann DN, et al. One size does not fit all: impact of hand size on ease of use of instruments for minimally invasive surgery. Surgeon. 2022;S1479-666X(22)00131-7.
- Matern U, Faist M, Kehl K, et al. Monitor position in laparoscopic surgery. Surg Endosc. 2005;19:436-440.
- Berguer R, Rab GT, Abu-Ghaida H, et al. A comparison of surgeons’ posture during laparoscopic and open surgical procedures. Surg Endosc. 1997;11:139-142.
- Athanasiadis DI, Monfared S, Asadi H, et al. An analysis of the ergonomic risk of surgical trainees and experienced surgeons during laparoscopic procedures. Surgery. 2021;169:496-501.
- Hotton J, Bogart E, Le Deley MC, et al. Ergonomic assessment of the surgeon’s physical workload during robot-assisted versus standard laparoscopy in a French multicenter randomized trial (ROBOGYN-1004 Trial). Ann Surg Oncol. 2023;30:916-923.
- Plerhoples TA, Hernandez-Boussard T, Wren SM. The aching surgeon: a survey of physical discomfort and symptoms following open, laparoscopic, and robotic surgery. J Robot Surg. 2012;6:65-72.
- Lee GI, Lee MR, Green I, et al. Surgeons’ physical discomfort and symptoms during robotic surgery: a comprehensive ergonomic survey study. Surg Endosc. 2017;31:1697-1706.
- McDonald ME, Ramirez PT, Munsell MF, et al. Physician pain and discomfort during minimally invasive gynecologic cancer surgery. Gynecol Oncol. 2014;134:243-247.
- Zhu X, Yurteri-Kaplan LA, Gutman RE, et al. Postural stress experienced by vaginal surgeons. Proc Hum Factors Ergonomics Soc Annu Meet. 2014;58:763-767.
- American College of Surgeons Division of Education and Surgical Ergonomics Committee. Surgical Ergonomics Recommendations. ACS Education. 2022.
- Cardenas-Trowers O, Kjellsson K, Hatch K. Ergonomics: making the OR a comfortable place. Int Urogynecol J. 2018;29:1065-1066.
- Hokenstad ED, Hallbeck MS, Lowndes BR, et al. Ergonomic robotic console configuration in gynecologic surgery: an interventional study. J Minim Invasive Gynecol. 2021;28:850-859.
- Singh R, Yurteri-Kaplan LA, Morrow MM, et al. Sitting versus standing makes a difference in musculoskeletal discomfort and postural load for surgeons performing vaginal surgery. Int Urogynecol J. 2019;30:231-237.
- Hullfish KL, Trowbridge ER, Bodine G. Ergonomics and gynecologic surgery: “surgeon protect thyself.” J Pelvic Med Surg. 2009;15:435-439.
- Woodburn KL, Kho RM. Vaginal surgery: don’t get bent out of shape. Am J Obstet Gynecol. 2020;223:762-763.
- Hobson DTG, Meriwether KV, Gaskins JT, et al. Learner satisfaction and experience with a high-definition telescopic camera during vaginal procedures: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2021;27:105-111.
- Speed G, Harris K, Keegel T. The effect of cushioning materials on musculoskeletal discomfort and fatigue during prolonged standing at work: a systematic review. Appl Ergon. 2018;70:300-334.
- Haramis G, Rosales JC, Palacios JM, et al. Prospective randomized evaluation of FOOT gel pads for operating room staff COMFORT during laparoscopic renal surgery. Urology. 2010;76:1405-1408.
- Voss RK, Chiang YJ, Cromwell KD, et al. Do no harm, except to ourselves? A survey of symptoms and injuries in oncologic surgeons and pilot study of an intraoperative ergonomic intervention. J Am Coll Surg. 2017;224:16-25.e1.
- Marquetand J, Gabriel J, Seibt R, et al. Ergonomics for surgeons—prototype of an external surgeon support system reduces muscular activity and fatigue. J Electromyogr Kinesiol. 2021;60:102586.
- Tetteh E, Hallbeck MS, Mirka GA. Effects of passive exoskeleton support on EMG measures of the neck, shoulder and trunk muscles while holding simulated surgical postures and performing a simulated surgical procedure. Appl Ergon. 2022;100:103646.
- Lim AK, Ryu J, Yoon HM, et al. Ergonomic effects of medical augmented reality glasses in video-assisted surgery. Surg Endosc. 2022;36:988-998.
- Park AE, Zahiri HR, Hallbeck MS, et al. Intraoperative “micro breaks” with targeted stretching enhance surgeon physical function and mental focus: a multicenter cohort study. Ann Surg. 2017;265:340-346.
- Hallbeck MS, Lowndes BR, Bingener J, et al. The impact of intraoperative microbreaks with exercises on surgeons: a multi-center cohort study. Appl Ergon. 2017;60:334-341.
- Hallbeck Human Factors Engineering Laboratories. OR Stretch Videos. Mayo Clinic, 2018. Accessed May 19, 2023. https://www.mayo .edu/research/labs/human-factors-engineering/or-stretch /or-stretch-videos
- Stork A, Bacon T, Corton M. Prevention of Work-Related Musculoskeletal Disorders in Vaginal Surgery. Video presentation at: Society of Gynecologic Surgeons’ Annual Scientific Meeting 2023, Tucson, AZ. Accessed April 3, 2023. https://sgs.eng.us/category.php?cat=2023 -video-presentations
- Aaron KA, Vaughan J, Gupta R, et al. The risk of ergonomic injury across surgical specialties. PLoS One. 2021;16:e0244868.
- Smith TG, Lowndes BR, Schmida E, et al. Course design and learning outcomes of a practical online ergonomics course for surgical residents. J Surg Educ. 2022;79:1489-1499.
- Franasiak J, Craven R, Mosaly P, et al. Feasibility and acceptance of a robotic surgery ergonomic training program. JSLS. 2014;18:e2014.00166.
- Cerier E, Hu A, Goldring A, et al. Ergonomics workshop improves musculoskeletal symptoms in general surgery residents. J Surg Res. 2022;280:567-574.
- Giagio S, Volpe G, Pillastrini P, et al. A preventive program for workrelated musculoskeletal disorders among surgeons: outcomes of a randomized controlled clinical trial. Ann Surg. 2019;270:969-975.
- Jensen MJ, Liao J, Van Gorp B, et al. Incorporating surgical ergonomics education into surgical residency curriculum. J Surg Educ. 2021;78:1209-1215.
Work-related musculoskeletal disorders (WMSDs) are “musculoskeletal disorders (injuries or disorders of the muscles, nerves, tendons, joints, cartilage, and spinal discs) in which the work environment and performance of work contribute significantly to the condition; and/or the condition is made worse or persists longer due to work conditions.”1 The health care industry has one of the highest rates of WMSDs, even when compared with traditional labor-intensive occupations, such as coal mining. In 2017, the health care industry reported more than a half million incidents of work-related injury and illness.2,3 In particular, surgeons are at increased risk for WMSDs, since they repetitively perform the classic tenets of poor ergonomics, including operating in static, extreme, and awkward positions and for prolonged periods of time.3
Gynecologic surgeons face unique ergonomic challenges. Operating in the pelvis requires an oblique approach that adds complexity and inhibits appropriate ergonomic positioning.4 All modalities of surgery incur their own challenges and risks to the surgeon, including minimally invasive gynecologic surgery (MIGS), which has become the standard of care for most conditions. Although MIGS has several benefits for the patient, a survey of gynecologic oncologists found that 88% of respondents reported discomfort related to MIGS.5 Several factors contribute to the development of WMSDs in surgery, including lack of ergonomic awareness, suboptimal ergonomic education and training,5,6 and ergonomically poor operating room (OR) equipment and instrument design.7 Furthermore, surgical culture does not generally prioritize ergonomics in the OR or requests for ergonomic accommodations.7,8
Within 5 years, a physician workforce shortage is projected for the United States.9 WMSDs contribute to workforce issues as they are associated with decreased productivity; time off needed for pain and treatment, including short-term disability; and possibly early retirement (as those who are older and have more work experience may be more likely to seek medical attention).10 In a 2013 study of vaginal surgeons, 14% missed work; 21% modified their work hours, work type, or amount of surgery; and 29% modified their surgical technique because of injury.10 Work-related pain also can negatively affect mental health, sleep, relationships, and quality of life.6
Recently, awareness has increased regarding WMSDs and their consequences, which has led to significant strides in the study of ergonomics among surgeons, a growing body of research on the topic, and guidance for optimizing ergonomics in the OR.
Risk factors for ergonomic strain
Several factors contribute to ergonomic strain and, subsequently, the development of WMSDs. Recognizing these factors can direct strategies for injury prevention.
Patient factors
The prevalence of obesity in the United States increased from 30.5% in 1999–2000 to 41.9% between 2017 and 2020.11 As the average patient’s body mass index (BMI) has increased, there is concern for a parallel increase in the ergonomic strain on laparoscopic surgeons.
A study of simulated laparoscopic tasks at varying model BMI levels demonstrated increased surgeon postural stress and workload at higher model BMIs (50 kg/m2) when compared with lower model BMIs (20 and 30 kg/m2).11 This result was supported in another study, which demonstrated both increased muscle activity and increased time needed to complete a surgical task with laparoscopic surgery; interestingly, when the same study measured these parameters for robotic surgery, this association was not seen.12 This suggests that a robotic rather than a laparoscopic approach may avoid some of the ergonomic strain associated with increased patient BMI.
Continue to: Surgeon factors...
Surgeon factors
Various surgeon characteristics have been shown to influence ergonomics in the OR. Surgeons with smaller hand sizes, for example, reported greater physical discomfort and demonstrated greater ergonomic workload when operating laparoscopically.13-15 In particular, those with a glove size of 6.5 or smaller have more difficulty using laparoscopic instruments, and those with a glove size smaller than 7 demonstrate a larger decline in grip strength when using laparoscopic instruments repeatedly.14,16
Surgeon height also can affect the amount of time spent in high-risk, nonergonomic positions. In a study that evaluated video recordings of surgeon posture during gynecologic laparoscopy, shorter surgeons were noted to use greater degrees of neck rotation to look at the monitor.17 Furthermore, surgeons with shorter arm lengths experienced more “extreme positions” of the nondominant shoulder and elbow.17 This trend also was seen in open and robotic surgery, where surgeons with a height of 66 cm or less reported increased pain scores after operating.18
Surgical instruments and OR setup
Surgical instrument characteristics can contribute to ergonomic strain, especially when the instruments have been designed with a one-size-fits-all mentality.8,19 In an examination of the anthropometric measurements of surgeon hand sizes and their correlation with difficulty when using a “standard” laparoscopic instrument, surgeons with smaller finger and hand spans had trouble using these instruments.19 Another study compared surgeon grip strength and ergonomic workloads after using 3 laparoscopic advanced bipolar instruments.16 Gender and hand size aside, the authors found that use of several of the laparoscopic devices led to greater decline in grip strength.16
The setup of the OR also can have a profound effect on the surgeon’s ergonomics. Monitor placement, for example, is crucial to ergonomic success. One study found that positioning the monitor directly in front of the surgeon at eye level was associated with the lowest neck muscle activity during a simulated task.20
Route of surgery
Each surgical approach has intrinsic ergonomic risks. With laparoscopy, surgeons often remain in straight head and back positions without much trunk motion, especially when compared with open surgery.21 In one study, laparoscopic surgeons spent more than 60% of a case in a static position and more than 80% of a case in a high-risk, “demanding” neck position.22
Robotic surgery, in contrast to laparoscopy, often has been cited as being more “ergonomic.” While robotic surgery has less of an effect on the neck, shoulders, arms, and legs than laparoscopy23 and often is associated with less physical discomfort than either open or laparoscopic surgery,23,24 robotic surgery still maintains its own innate ergonomic risks. Of robotic surgeons surveyed, 56.1% reported neck stiffness, finger fatigue, and eye symptoms in one study.25 In another survey study, more robotic surgeons (72%) reported physical symptoms than laparoscopic (57%) and open (49%) surgeons.26Vaginal surgery also puts surgeons at ergonomic risk. A majority of surgeons (87.2%) who completed more than 50% of their cases vaginally reported a history of WMSDs.10 Vaginal surgery places surgeons in awkward positions of the neck, shoulder, and trunk frequently and for longer durations.27
Continue to: Strategies for preventing WMSDs...
Strategies for preventing WMSDs
As factors that contribute to the development of WMSDs are identified, preventive strategies can be targeted to these individual factors. Research has focused on appropriate setup of the OR, surgeon posture, intraoperative microbreaks, and stretching both in and outside of the OR.
1. OR setup and positioning of the surgeon by MIGS route
The route of MIGS affects OR setup and surgeon posture. Ergonomic recommendations for laparoscopy, robotic surgery, and vaginal surgery are all unique to the risks posed by each particular approach.
Laparoscopic surgery. Laparoscopic monitors should face the surgeon directly, with the screen just below eye level to maintain the surgeon’s neck in a neutral position.28 The table height should be set for the tallest surgeon, and shorter surgeons should stand on steps as needed.28 The table height also should allow for the surgeon’s hands to be at elbow height, with the elbows bent at 90 degrees with the wrists straight.29 Foot pedals should be placed at the surgeons’ foot level and should be reached easily.28 Additionally, the patient’s arms should be tucked at their sides to allow surgeons a larger operative space.29 When using laparoscopic instruments, locking and ratcheting features should be used whenever possible to reduce prolonged grip or squeeze forces.28 The laparoscopic camera should be held in the palm with the wrist in a neutral position.29
Robotic surgery. Positioning and setup of the robotic console is a main focus of ergonomic recommendations. The surgeon’s chair should be brought as close to the console as possible, and the knees positioned in a 90-degree angle.30 The foot pedals should be brought toward the surgeon to maintain this angle of the knees.30 The console should be rotated toward the surgeon and then the height adjusted so that the surgeon can look through the eyepiece while sitting upright and can maintain the neck in a neutral position.28,30 The surgeon’s forehead should rest comfortably on the headrest.29 The forearms should rest on the armrest while the arms are maintained in a neutral position and the shoulders remain relaxed while the surgeon holds the robotic controls.30 It is important to utilize the armrest often to relieve stress on the arm while operating.28 Frequent use of the clutch function can keep the robotic controls in the center of the workspace.28
Vaginal surgery. Both seated and standing positions are associated with high-risk positioning of the trunk and bilateral shoulders, respectively, in vaginal surgery.31 However, surgeons who stand while operating vaginally reported more discomfort in the bilateral wrists, thighs, and lower legs than those who operated while seated.31 This suggests a potential ergonomic advantage to the seated position for vaginal surgery. Chair height should be adjusted so the surgeon can look straight ahead with the neck in a neutral position.32 Surgeons should consider using a headlamp, as this may prevent repetitive awkward movements to adjust overhead lights.32 For standing surgery, the table height should be adjusted for the tallest surgeon, and shorter surgeons or assistants should use steps as needed.3
Surgical assistants should switch sides during the course of the case to avoid excessive unilateral upper-extremity strain.32 The addition of a table-mounted vaginal retractor system may be useful in relieving physical strain for surgical assistants, but data currently are lacking to demonstrate this ergonomic benefit.33 Further studies are needed, especially since many surgeons take on the role of surgical assist in the teaching environment and subsequently report more WMSDs than their colleagues who do not work in teaching environments.10,34
2. Pain relief from individual ergonomic positioning devices
Apart from adjusting how the OR equipment is arranged or how the surgeons adjust their positioning, several devices that assist with surgeon positioning—including gel mats or insoles, exoskeletons, and “augmented reality” glasses—are being studied.
The use of gel mats or insoles in the OR has mixed evidence in the literature.35-37
Exoskeletons, external devices that support a surgeon’s posture and positioning, have been studied thus far in simulated nonsterile surgical environments. Preliminarily, it appears that use of an exoskeleton can decrease muscle activity and time spent in static positions, with a reported decrease in post-task user discomfort.38,39 More data are needed to determine if exoskeletons can be used in the sterile setting and for longer durations as may occur in actual OR cases.
Augmented reality glasses project the laparoscopic monitor image to the glasses, which frees the surgeon to place the “monitor” in a more neutral, ergonomic position. In one study, use of augmented reality glasses was associated with decreased muscle activity and a reduction in Rapid Entire Body Assessment (REBA) scores when compared with use of the conventional laparoscopic monitor.40More data are needed on these emerging technologies to determine whether adverse effects occur with prolonged use.
Continue to: 3. Implementing intraoperative microbreaks and stretching...
3. Implementing intraoperative microbreaks and stretching
The American College of Surgeons (ACS) recommends that surgeons avoid prolonged static postures during procedures.28 One strategy for preventing sustained positioning is to incorporate breaks with associated stretching routinely during surgery.28
Microbreaks. In a landmark study by Park and colleagues in 2017, 120-second long targeted stretching microbreaks (TSMBs) were completed every 20 to 40 minutes during a surgery, and results demonstrated improved postoperative surgeon pain scores without an associated increase in the length of the case.41 These surgeons reported improved pain in the neck, bilateral shoulders, bilateral hands, and lower back. Eighty-eight percent of surgeons reported either improvement or “no change” in their mental focus, and 100% reported improvement or “no change” in their physical performance after TSMBs were implemented.42 Of surveyed surgeons, 87% wanted TSMBs incorporated routinely.41,42
Stretches. Multiple resources, such as the ACS and the Mayo Clinic, for intraoperative stretches are available. The ACS recommends performing neck and shoulder stretches during intraoperative microbreaks, including a range-of-movement neck exercise, deep cervical flexor training, and standing scapular retraction.28 The ACS also demonstrates lumbrical stretches for the fingers and passive wrist extension exercises to be used intraoperatively (or between cases) (FIGURE 1).28 The Mayo Clinic Hallbeck Human Factors Engineering Laboratories has a publicly available “OR Stretch Instructional Video” in which the surgeon is guided through several different short stretches, including shoulder shrugging and side bends, that can be used during surgery.43

Both the ACS and the Mayo Clinic provide examples of pertinent stretch exercises for use when not in the sterile environment, between cases or after cases are complete. The ACS recommends several neck and shoulder stretches for the trapezius, levator scapulae, and pectoralis and recommends the use of a foam roller to improve thoracic mobility (FIGURE 2).28 As above, the Mayo Clinic Hallbeck Human Factors Engineering Laboratories has a publicly available “OR-Stretch Between Surgery Stretches Video” in which the surgeon is guided through several short stretches that are done in a seated position, including stretches for the hamstring, lower back, and arms (FIGURE 3).43
Many of the above-mentioned stretches were designed for use in the context of open, laparoscopic, or robotic surgery. For the vaginal surgeon, the intraoperative ergonomic stressors differ from those of other routes of surgery, and thus stretches tailored to the positioning during vaginal surgery are necessary. In a video recently published by the Society of Gynecologic Surgeons, several stretches are reviewed that target high-risk positions often held by the surgeon or assistant when operating vaginally.44 These stretches include cervical retraction, thoracic extension, external arm rotation, cervical side bending, and lumbar extension (FIGURE 4).44 The recommendation is to complete these exercises 2 times per day, with 8 to 10 repetitions per set.44



Prioritizing ergonomic awareness and training
As caregivers, it is not uncommon for us to prioritize the needs of others before those of ourselves. However, WMSDs are prevalent, and their downstream effects may cause catastrophic professional and personal losses. Cumulatively, the global impact of WMSDs is a significant issue for the health care workforce and its longevity.
To prevent WMSDs, it is imperative that surgeons are aware of the factors that contribute to injury development and the appropriate, accessible modifications for these factors. While each surgical modality confers its own ergonomic challenges, these risks can be mitigated through increased awareness of OR setup, surgeon positioning, and incorporation of microbreaks and stretching exercises during and after surgical procedures.
Formal training in surgical ergonomics is lacking across specialties, including gynecology.45 Multiple educational interventions have been proposed and studied to help fill this training gap.30,46-49When used, these interventions have been associated with increased knowledge of surgical ergonomic principles or reduction in surgeon pain scores, including trainees.50 As we become more cognizant of WMSDs, standardized resident curricula should be developed in an effort to reduce the prevalence of these potentially career-ending injuries.
In addition to education, cultivating a culture in which ergonomics is prioritized is essential. Although most surgeons report work-related pain, very few report their injuries to occupational health. For example, while 29% of gynecologic oncologists reported seeking treatment for a WMSD, only 1% had reported their injury to their employer.5 In a study of ACS members, only 19% of injuries were reported, 30% of surgeons stated that they did not know how to report an injury, and 21% felt that the resources for surgeons during and after an injury were inadequate.6
As we prioritize the health and safety of our patients, we also need to promote ergonomic awareness in the OR, respect the need for accommodations, encourage injury reporting, support surgeons who need to take time away for medical treatment, and partner with industry to develop new instruments and technology with effective ergonomic features. ●
Work-related musculoskeletal disorders (WMSDs) are “musculoskeletal disorders (injuries or disorders of the muscles, nerves, tendons, joints, cartilage, and spinal discs) in which the work environment and performance of work contribute significantly to the condition; and/or the condition is made worse or persists longer due to work conditions.”1 The health care industry has one of the highest rates of WMSDs, even when compared with traditional labor-intensive occupations, such as coal mining. In 2017, the health care industry reported more than a half million incidents of work-related injury and illness.2,3 In particular, surgeons are at increased risk for WMSDs, since they repetitively perform the classic tenets of poor ergonomics, including operating in static, extreme, and awkward positions and for prolonged periods of time.3
Gynecologic surgeons face unique ergonomic challenges. Operating in the pelvis requires an oblique approach that adds complexity and inhibits appropriate ergonomic positioning.4 All modalities of surgery incur their own challenges and risks to the surgeon, including minimally invasive gynecologic surgery (MIGS), which has become the standard of care for most conditions. Although MIGS has several benefits for the patient, a survey of gynecologic oncologists found that 88% of respondents reported discomfort related to MIGS.5 Several factors contribute to the development of WMSDs in surgery, including lack of ergonomic awareness, suboptimal ergonomic education and training,5,6 and ergonomically poor operating room (OR) equipment and instrument design.7 Furthermore, surgical culture does not generally prioritize ergonomics in the OR or requests for ergonomic accommodations.7,8
Within 5 years, a physician workforce shortage is projected for the United States.9 WMSDs contribute to workforce issues as they are associated with decreased productivity; time off needed for pain and treatment, including short-term disability; and possibly early retirement (as those who are older and have more work experience may be more likely to seek medical attention).10 In a 2013 study of vaginal surgeons, 14% missed work; 21% modified their work hours, work type, or amount of surgery; and 29% modified their surgical technique because of injury.10 Work-related pain also can negatively affect mental health, sleep, relationships, and quality of life.6
Recently, awareness has increased regarding WMSDs and their consequences, which has led to significant strides in the study of ergonomics among surgeons, a growing body of research on the topic, and guidance for optimizing ergonomics in the OR.
Risk factors for ergonomic strain
Several factors contribute to ergonomic strain and, subsequently, the development of WMSDs. Recognizing these factors can direct strategies for injury prevention.
Patient factors
The prevalence of obesity in the United States increased from 30.5% in 1999–2000 to 41.9% between 2017 and 2020.11 As the average patient’s body mass index (BMI) has increased, there is concern for a parallel increase in the ergonomic strain on laparoscopic surgeons.
A study of simulated laparoscopic tasks at varying model BMI levels demonstrated increased surgeon postural stress and workload at higher model BMIs (50 kg/m2) when compared with lower model BMIs (20 and 30 kg/m2).11 This result was supported in another study, which demonstrated both increased muscle activity and increased time needed to complete a surgical task with laparoscopic surgery; interestingly, when the same study measured these parameters for robotic surgery, this association was not seen.12 This suggests that a robotic rather than a laparoscopic approach may avoid some of the ergonomic strain associated with increased patient BMI.
Continue to: Surgeon factors...
Surgeon factors
Various surgeon characteristics have been shown to influence ergonomics in the OR. Surgeons with smaller hand sizes, for example, reported greater physical discomfort and demonstrated greater ergonomic workload when operating laparoscopically.13-15 In particular, those with a glove size of 6.5 or smaller have more difficulty using laparoscopic instruments, and those with a glove size smaller than 7 demonstrate a larger decline in grip strength when using laparoscopic instruments repeatedly.14,16
Surgeon height also can affect the amount of time spent in high-risk, nonergonomic positions. In a study that evaluated video recordings of surgeon posture during gynecologic laparoscopy, shorter surgeons were noted to use greater degrees of neck rotation to look at the monitor.17 Furthermore, surgeons with shorter arm lengths experienced more “extreme positions” of the nondominant shoulder and elbow.17 This trend also was seen in open and robotic surgery, where surgeons with a height of 66 cm or less reported increased pain scores after operating.18
Surgical instruments and OR setup
Surgical instrument characteristics can contribute to ergonomic strain, especially when the instruments have been designed with a one-size-fits-all mentality.8,19 In an examination of the anthropometric measurements of surgeon hand sizes and their correlation with difficulty when using a “standard” laparoscopic instrument, surgeons with smaller finger and hand spans had trouble using these instruments.19 Another study compared surgeon grip strength and ergonomic workloads after using 3 laparoscopic advanced bipolar instruments.16 Gender and hand size aside, the authors found that use of several of the laparoscopic devices led to greater decline in grip strength.16
The setup of the OR also can have a profound effect on the surgeon’s ergonomics. Monitor placement, for example, is crucial to ergonomic success. One study found that positioning the monitor directly in front of the surgeon at eye level was associated with the lowest neck muscle activity during a simulated task.20
Route of surgery
Each surgical approach has intrinsic ergonomic risks. With laparoscopy, surgeons often remain in straight head and back positions without much trunk motion, especially when compared with open surgery.21 In one study, laparoscopic surgeons spent more than 60% of a case in a static position and more than 80% of a case in a high-risk, “demanding” neck position.22
Robotic surgery, in contrast to laparoscopy, often has been cited as being more “ergonomic.” While robotic surgery has less of an effect on the neck, shoulders, arms, and legs than laparoscopy23 and often is associated with less physical discomfort than either open or laparoscopic surgery,23,24 robotic surgery still maintains its own innate ergonomic risks. Of robotic surgeons surveyed, 56.1% reported neck stiffness, finger fatigue, and eye symptoms in one study.25 In another survey study, more robotic surgeons (72%) reported physical symptoms than laparoscopic (57%) and open (49%) surgeons.26Vaginal surgery also puts surgeons at ergonomic risk. A majority of surgeons (87.2%) who completed more than 50% of their cases vaginally reported a history of WMSDs.10 Vaginal surgery places surgeons in awkward positions of the neck, shoulder, and trunk frequently and for longer durations.27
Continue to: Strategies for preventing WMSDs...
Strategies for preventing WMSDs
As factors that contribute to the development of WMSDs are identified, preventive strategies can be targeted to these individual factors. Research has focused on appropriate setup of the OR, surgeon posture, intraoperative microbreaks, and stretching both in and outside of the OR.
1. OR setup and positioning of the surgeon by MIGS route
The route of MIGS affects OR setup and surgeon posture. Ergonomic recommendations for laparoscopy, robotic surgery, and vaginal surgery are all unique to the risks posed by each particular approach.
Laparoscopic surgery. Laparoscopic monitors should face the surgeon directly, with the screen just below eye level to maintain the surgeon’s neck in a neutral position.28 The table height should be set for the tallest surgeon, and shorter surgeons should stand on steps as needed.28 The table height also should allow for the surgeon’s hands to be at elbow height, with the elbows bent at 90 degrees with the wrists straight.29 Foot pedals should be placed at the surgeons’ foot level and should be reached easily.28 Additionally, the patient’s arms should be tucked at their sides to allow surgeons a larger operative space.29 When using laparoscopic instruments, locking and ratcheting features should be used whenever possible to reduce prolonged grip or squeeze forces.28 The laparoscopic camera should be held in the palm with the wrist in a neutral position.29
Robotic surgery. Positioning and setup of the robotic console is a main focus of ergonomic recommendations. The surgeon’s chair should be brought as close to the console as possible, and the knees positioned in a 90-degree angle.30 The foot pedals should be brought toward the surgeon to maintain this angle of the knees.30 The console should be rotated toward the surgeon and then the height adjusted so that the surgeon can look through the eyepiece while sitting upright and can maintain the neck in a neutral position.28,30 The surgeon’s forehead should rest comfortably on the headrest.29 The forearms should rest on the armrest while the arms are maintained in a neutral position and the shoulders remain relaxed while the surgeon holds the robotic controls.30 It is important to utilize the armrest often to relieve stress on the arm while operating.28 Frequent use of the clutch function can keep the robotic controls in the center of the workspace.28
Vaginal surgery. Both seated and standing positions are associated with high-risk positioning of the trunk and bilateral shoulders, respectively, in vaginal surgery.31 However, surgeons who stand while operating vaginally reported more discomfort in the bilateral wrists, thighs, and lower legs than those who operated while seated.31 This suggests a potential ergonomic advantage to the seated position for vaginal surgery. Chair height should be adjusted so the surgeon can look straight ahead with the neck in a neutral position.32 Surgeons should consider using a headlamp, as this may prevent repetitive awkward movements to adjust overhead lights.32 For standing surgery, the table height should be adjusted for the tallest surgeon, and shorter surgeons or assistants should use steps as needed.3
Surgical assistants should switch sides during the course of the case to avoid excessive unilateral upper-extremity strain.32 The addition of a table-mounted vaginal retractor system may be useful in relieving physical strain for surgical assistants, but data currently are lacking to demonstrate this ergonomic benefit.33 Further studies are needed, especially since many surgeons take on the role of surgical assist in the teaching environment and subsequently report more WMSDs than their colleagues who do not work in teaching environments.10,34
2. Pain relief from individual ergonomic positioning devices
Apart from adjusting how the OR equipment is arranged or how the surgeons adjust their positioning, several devices that assist with surgeon positioning—including gel mats or insoles, exoskeletons, and “augmented reality” glasses—are being studied.
The use of gel mats or insoles in the OR has mixed evidence in the literature.35-37
Exoskeletons, external devices that support a surgeon’s posture and positioning, have been studied thus far in simulated nonsterile surgical environments. Preliminarily, it appears that use of an exoskeleton can decrease muscle activity and time spent in static positions, with a reported decrease in post-task user discomfort.38,39 More data are needed to determine if exoskeletons can be used in the sterile setting and for longer durations as may occur in actual OR cases.
Augmented reality glasses project the laparoscopic monitor image to the glasses, which frees the surgeon to place the “monitor” in a more neutral, ergonomic position. In one study, use of augmented reality glasses was associated with decreased muscle activity and a reduction in Rapid Entire Body Assessment (REBA) scores when compared with use of the conventional laparoscopic monitor.40More data are needed on these emerging technologies to determine whether adverse effects occur with prolonged use.
Continue to: 3. Implementing intraoperative microbreaks and stretching...
3. Implementing intraoperative microbreaks and stretching
The American College of Surgeons (ACS) recommends that surgeons avoid prolonged static postures during procedures.28 One strategy for preventing sustained positioning is to incorporate breaks with associated stretching routinely during surgery.28
Microbreaks. In a landmark study by Park and colleagues in 2017, 120-second long targeted stretching microbreaks (TSMBs) were completed every 20 to 40 minutes during a surgery, and results demonstrated improved postoperative surgeon pain scores without an associated increase in the length of the case.41 These surgeons reported improved pain in the neck, bilateral shoulders, bilateral hands, and lower back. Eighty-eight percent of surgeons reported either improvement or “no change” in their mental focus, and 100% reported improvement or “no change” in their physical performance after TSMBs were implemented.42 Of surveyed surgeons, 87% wanted TSMBs incorporated routinely.41,42
Stretches. Multiple resources, such as the ACS and the Mayo Clinic, for intraoperative stretches are available. The ACS recommends performing neck and shoulder stretches during intraoperative microbreaks, including a range-of-movement neck exercise, deep cervical flexor training, and standing scapular retraction.28 The ACS also demonstrates lumbrical stretches for the fingers and passive wrist extension exercises to be used intraoperatively (or between cases) (FIGURE 1).28 The Mayo Clinic Hallbeck Human Factors Engineering Laboratories has a publicly available “OR Stretch Instructional Video” in which the surgeon is guided through several different short stretches, including shoulder shrugging and side bends, that can be used during surgery.43

Both the ACS and the Mayo Clinic provide examples of pertinent stretch exercises for use when not in the sterile environment, between cases or after cases are complete. The ACS recommends several neck and shoulder stretches for the trapezius, levator scapulae, and pectoralis and recommends the use of a foam roller to improve thoracic mobility (FIGURE 2).28 As above, the Mayo Clinic Hallbeck Human Factors Engineering Laboratories has a publicly available “OR-Stretch Between Surgery Stretches Video” in which the surgeon is guided through several short stretches that are done in a seated position, including stretches for the hamstring, lower back, and arms (FIGURE 3).43
Many of the above-mentioned stretches were designed for use in the context of open, laparoscopic, or robotic surgery. For the vaginal surgeon, the intraoperative ergonomic stressors differ from those of other routes of surgery, and thus stretches tailored to the positioning during vaginal surgery are necessary. In a video recently published by the Society of Gynecologic Surgeons, several stretches are reviewed that target high-risk positions often held by the surgeon or assistant when operating vaginally.44 These stretches include cervical retraction, thoracic extension, external arm rotation, cervical side bending, and lumbar extension (FIGURE 4).44 The recommendation is to complete these exercises 2 times per day, with 8 to 10 repetitions per set.44



Prioritizing ergonomic awareness and training
As caregivers, it is not uncommon for us to prioritize the needs of others before those of ourselves. However, WMSDs are prevalent, and their downstream effects may cause catastrophic professional and personal losses. Cumulatively, the global impact of WMSDs is a significant issue for the health care workforce and its longevity.
To prevent WMSDs, it is imperative that surgeons are aware of the factors that contribute to injury development and the appropriate, accessible modifications for these factors. While each surgical modality confers its own ergonomic challenges, these risks can be mitigated through increased awareness of OR setup, surgeon positioning, and incorporation of microbreaks and stretching exercises during and after surgical procedures.
Formal training in surgical ergonomics is lacking across specialties, including gynecology.45 Multiple educational interventions have been proposed and studied to help fill this training gap.30,46-49When used, these interventions have been associated with increased knowledge of surgical ergonomic principles or reduction in surgeon pain scores, including trainees.50 As we become more cognizant of WMSDs, standardized resident curricula should be developed in an effort to reduce the prevalence of these potentially career-ending injuries.
In addition to education, cultivating a culture in which ergonomics is prioritized is essential. Although most surgeons report work-related pain, very few report their injuries to occupational health. For example, while 29% of gynecologic oncologists reported seeking treatment for a WMSD, only 1% had reported their injury to their employer.5 In a study of ACS members, only 19% of injuries were reported, 30% of surgeons stated that they did not know how to report an injury, and 21% felt that the resources for surgeons during and after an injury were inadequate.6
As we prioritize the health and safety of our patients, we also need to promote ergonomic awareness in the OR, respect the need for accommodations, encourage injury reporting, support surgeons who need to take time away for medical treatment, and partner with industry to develop new instruments and technology with effective ergonomic features. ●
- Workplace health glossary. Reviewed February 12, 2020. Centers for Disease Control and Prevention. Accessed May 18, 2023. https://www.cdc.gov/workplacehealthpromotion/tools-resources /glossary/glossary.html#W
- Epstein S, Sparer EH, Tran BN, et al. Prevalence of work-related musculoskeletal disorders among surgeons and interventionalists: a systematic review and meta-analysis. JAMA Surg. 2018;153:e174947.
- Yurteri-Kaplan LA, Park AJ. Surgical ergonomics and preventing workrelated musculoskeletal disorders. Obstet Gynecol. 2023;141:455-462.
- Symer MM, Keller DS. Human factors in pelvic surgery. Eur J Surg Oncol. 2022;48:2346-2351.
- Franasiak J, Ko EM, Kidd J, et al. Physical strain and urgent need for ergonomic training among gynecologic oncologists who perform minimally invasive surgery. Gynecol Oncol. 2012;126:437-442.
- Davis WT, Fletcher SA, Guillamondegui OD. Musculoskeletal occupational injury among surgeons: effects for patients, providers, and institutions. J Surg Res. 2014;189:207-212.e6.
- Fox M. Surgeons face unique ergonomic challenges. American College of Surgeons. September 1, 2022. Accessed May 22, 2023. https://www.facs.org/for-medical-professionals/news-publications /news-and-articles/bulletin/september-2022-volume-107-issue-9 /surgeons-face-unique-ergonomic-challenges/
- Wong JMK, Carey ET, King C, et al. A call to action for ergonomic surgical devices designed for diverse surgeon end users. Obstet Gynecol. 2023;141:463-466.
- IHS Inc. The Complexities of Physician Supply and Demand: Projections from 2014 to 2025. Association of American Medical Colleges. April 5, 2016.
- Kim-Fine S, Woolley SM, Weaver AL, et al. Work-related musculoskeletal disorders among vaginal surgeons. Int Urogynecol J. 2013;24:1191-1200.
- Sers R, Forrester S, Zecca M, et al. The ergonomic impact of patient body mass index on surgeon posture during simulated laparoscopy. Appl Ergon. 2021;97:103501.
- Moss EL, Sarhanis P, Ind T, et al. Impact of obesity on surgeon ergonomics in robotic and straight-stick laparoscopic surgery. J Minim Invasive Gynecol. 2020;27:1063-1069.
- Sutton E, Irvin M, Zeigler C, et al. The ergonomics of women in surgery. Surg Endosc. 2014;28:1051-1055.
- Berguer R, Hreljac A. The relationship between hand size and difficulty using surgical instruments: a survey of 726 laparoscopic surgeons. Surg Endosc. 2004;18:508-512.
- Bellini MI, Amabile MI, Saullo P, et al. A woman’s place is in theatre, but are theatres designed with women in mind? A systematic review of ergonomics for women in surgery. J Clin Med. 2022;11:3496.
- Wong JMK, Moore KJ, Lewis P, et al. Ergonomic assessment of surgeon characteristics and laparoscopic device strain in gynecologic surgery. J Minim Invasive Gynecol. 2022;29:1357-1363.
- Aitchison LP, Cui CK, Arnold A, et al. The ergonomics of laparoscopic surgery: a quantitative study of the time and motion of laparoscopic surgeons in live surgical environments. Surg Endosc. 2016;30:5068-5076.
- Stewart C, Raoof M, Fong Y, et al. Who is hurting? A prospective study of surgeon ergonomics. Surg Endosc. 2022;36:292-299.
- Green SV, Morris DE, Naumann DN, et al. One size does not fit all: impact of hand size on ease of use of instruments for minimally invasive surgery. Surgeon. 2022;S1479-666X(22)00131-7.
- Matern U, Faist M, Kehl K, et al. Monitor position in laparoscopic surgery. Surg Endosc. 2005;19:436-440.
- Berguer R, Rab GT, Abu-Ghaida H, et al. A comparison of surgeons’ posture during laparoscopic and open surgical procedures. Surg Endosc. 1997;11:139-142.
- Athanasiadis DI, Monfared S, Asadi H, et al. An analysis of the ergonomic risk of surgical trainees and experienced surgeons during laparoscopic procedures. Surgery. 2021;169:496-501.
- Hotton J, Bogart E, Le Deley MC, et al. Ergonomic assessment of the surgeon’s physical workload during robot-assisted versus standard laparoscopy in a French multicenter randomized trial (ROBOGYN-1004 Trial). Ann Surg Oncol. 2023;30:916-923.
- Plerhoples TA, Hernandez-Boussard T, Wren SM. The aching surgeon: a survey of physical discomfort and symptoms following open, laparoscopic, and robotic surgery. J Robot Surg. 2012;6:65-72.
- Lee GI, Lee MR, Green I, et al. Surgeons’ physical discomfort and symptoms during robotic surgery: a comprehensive ergonomic survey study. Surg Endosc. 2017;31:1697-1706.
- McDonald ME, Ramirez PT, Munsell MF, et al. Physician pain and discomfort during minimally invasive gynecologic cancer surgery. Gynecol Oncol. 2014;134:243-247.
- Zhu X, Yurteri-Kaplan LA, Gutman RE, et al. Postural stress experienced by vaginal surgeons. Proc Hum Factors Ergonomics Soc Annu Meet. 2014;58:763-767.
- American College of Surgeons Division of Education and Surgical Ergonomics Committee. Surgical Ergonomics Recommendations. ACS Education. 2022.
- Cardenas-Trowers O, Kjellsson K, Hatch K. Ergonomics: making the OR a comfortable place. Int Urogynecol J. 2018;29:1065-1066.
- Hokenstad ED, Hallbeck MS, Lowndes BR, et al. Ergonomic robotic console configuration in gynecologic surgery: an interventional study. J Minim Invasive Gynecol. 2021;28:850-859.
- Singh R, Yurteri-Kaplan LA, Morrow MM, et al. Sitting versus standing makes a difference in musculoskeletal discomfort and postural load for surgeons performing vaginal surgery. Int Urogynecol J. 2019;30:231-237.
- Hullfish KL, Trowbridge ER, Bodine G. Ergonomics and gynecologic surgery: “surgeon protect thyself.” J Pelvic Med Surg. 2009;15:435-439.
- Woodburn KL, Kho RM. Vaginal surgery: don’t get bent out of shape. Am J Obstet Gynecol. 2020;223:762-763.
- Hobson DTG, Meriwether KV, Gaskins JT, et al. Learner satisfaction and experience with a high-definition telescopic camera during vaginal procedures: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2021;27:105-111.
- Speed G, Harris K, Keegel T. The effect of cushioning materials on musculoskeletal discomfort and fatigue during prolonged standing at work: a systematic review. Appl Ergon. 2018;70:300-334.
- Haramis G, Rosales JC, Palacios JM, et al. Prospective randomized evaluation of FOOT gel pads for operating room staff COMFORT during laparoscopic renal surgery. Urology. 2010;76:1405-1408.
- Voss RK, Chiang YJ, Cromwell KD, et al. Do no harm, except to ourselves? A survey of symptoms and injuries in oncologic surgeons and pilot study of an intraoperative ergonomic intervention. J Am Coll Surg. 2017;224:16-25.e1.
- Marquetand J, Gabriel J, Seibt R, et al. Ergonomics for surgeons—prototype of an external surgeon support system reduces muscular activity and fatigue. J Electromyogr Kinesiol. 2021;60:102586.
- Tetteh E, Hallbeck MS, Mirka GA. Effects of passive exoskeleton support on EMG measures of the neck, shoulder and trunk muscles while holding simulated surgical postures and performing a simulated surgical procedure. Appl Ergon. 2022;100:103646.
- Lim AK, Ryu J, Yoon HM, et al. Ergonomic effects of medical augmented reality glasses in video-assisted surgery. Surg Endosc. 2022;36:988-998.
- Park AE, Zahiri HR, Hallbeck MS, et al. Intraoperative “micro breaks” with targeted stretching enhance surgeon physical function and mental focus: a multicenter cohort study. Ann Surg. 2017;265:340-346.
- Hallbeck MS, Lowndes BR, Bingener J, et al. The impact of intraoperative microbreaks with exercises on surgeons: a multi-center cohort study. Appl Ergon. 2017;60:334-341.
- Hallbeck Human Factors Engineering Laboratories. OR Stretch Videos. Mayo Clinic, 2018. Accessed May 19, 2023. https://www.mayo .edu/research/labs/human-factors-engineering/or-stretch /or-stretch-videos
- Stork A, Bacon T, Corton M. Prevention of Work-Related Musculoskeletal Disorders in Vaginal Surgery. Video presentation at: Society of Gynecologic Surgeons’ Annual Scientific Meeting 2023, Tucson, AZ. Accessed April 3, 2023. https://sgs.eng.us/category.php?cat=2023 -video-presentations
- Aaron KA, Vaughan J, Gupta R, et al. The risk of ergonomic injury across surgical specialties. PLoS One. 2021;16:e0244868.
- Smith TG, Lowndes BR, Schmida E, et al. Course design and learning outcomes of a practical online ergonomics course for surgical residents. J Surg Educ. 2022;79:1489-1499.
- Franasiak J, Craven R, Mosaly P, et al. Feasibility and acceptance of a robotic surgery ergonomic training program. JSLS. 2014;18:e2014.00166.
- Cerier E, Hu A, Goldring A, et al. Ergonomics workshop improves musculoskeletal symptoms in general surgery residents. J Surg Res. 2022;280:567-574.
- Giagio S, Volpe G, Pillastrini P, et al. A preventive program for workrelated musculoskeletal disorders among surgeons: outcomes of a randomized controlled clinical trial. Ann Surg. 2019;270:969-975.
- Jensen MJ, Liao J, Van Gorp B, et al. Incorporating surgical ergonomics education into surgical residency curriculum. J Surg Educ. 2021;78:1209-1215.
- Workplace health glossary. Reviewed February 12, 2020. Centers for Disease Control and Prevention. Accessed May 18, 2023. https://www.cdc.gov/workplacehealthpromotion/tools-resources /glossary/glossary.html#W
- Epstein S, Sparer EH, Tran BN, et al. Prevalence of work-related musculoskeletal disorders among surgeons and interventionalists: a systematic review and meta-analysis. JAMA Surg. 2018;153:e174947.
- Yurteri-Kaplan LA, Park AJ. Surgical ergonomics and preventing workrelated musculoskeletal disorders. Obstet Gynecol. 2023;141:455-462.
- Symer MM, Keller DS. Human factors in pelvic surgery. Eur J Surg Oncol. 2022;48:2346-2351.
- Franasiak J, Ko EM, Kidd J, et al. Physical strain and urgent need for ergonomic training among gynecologic oncologists who perform minimally invasive surgery. Gynecol Oncol. 2012;126:437-442.
- Davis WT, Fletcher SA, Guillamondegui OD. Musculoskeletal occupational injury among surgeons: effects for patients, providers, and institutions. J Surg Res. 2014;189:207-212.e6.
- Fox M. Surgeons face unique ergonomic challenges. American College of Surgeons. September 1, 2022. Accessed May 22, 2023. https://www.facs.org/for-medical-professionals/news-publications /news-and-articles/bulletin/september-2022-volume-107-issue-9 /surgeons-face-unique-ergonomic-challenges/
- Wong JMK, Carey ET, King C, et al. A call to action for ergonomic surgical devices designed for diverse surgeon end users. Obstet Gynecol. 2023;141:463-466.
- IHS Inc. The Complexities of Physician Supply and Demand: Projections from 2014 to 2025. Association of American Medical Colleges. April 5, 2016.
- Kim-Fine S, Woolley SM, Weaver AL, et al. Work-related musculoskeletal disorders among vaginal surgeons. Int Urogynecol J. 2013;24:1191-1200.
- Sers R, Forrester S, Zecca M, et al. The ergonomic impact of patient body mass index on surgeon posture during simulated laparoscopy. Appl Ergon. 2021;97:103501.
- Moss EL, Sarhanis P, Ind T, et al. Impact of obesity on surgeon ergonomics in robotic and straight-stick laparoscopic surgery. J Minim Invasive Gynecol. 2020;27:1063-1069.
- Sutton E, Irvin M, Zeigler C, et al. The ergonomics of women in surgery. Surg Endosc. 2014;28:1051-1055.
- Berguer R, Hreljac A. The relationship between hand size and difficulty using surgical instruments: a survey of 726 laparoscopic surgeons. Surg Endosc. 2004;18:508-512.
- Bellini MI, Amabile MI, Saullo P, et al. A woman’s place is in theatre, but are theatres designed with women in mind? A systematic review of ergonomics for women in surgery. J Clin Med. 2022;11:3496.
- Wong JMK, Moore KJ, Lewis P, et al. Ergonomic assessment of surgeon characteristics and laparoscopic device strain in gynecologic surgery. J Minim Invasive Gynecol. 2022;29:1357-1363.
- Aitchison LP, Cui CK, Arnold A, et al. The ergonomics of laparoscopic surgery: a quantitative study of the time and motion of laparoscopic surgeons in live surgical environments. Surg Endosc. 2016;30:5068-5076.
- Stewart C, Raoof M, Fong Y, et al. Who is hurting? A prospective study of surgeon ergonomics. Surg Endosc. 2022;36:292-299.
- Green SV, Morris DE, Naumann DN, et al. One size does not fit all: impact of hand size on ease of use of instruments for minimally invasive surgery. Surgeon. 2022;S1479-666X(22)00131-7.
- Matern U, Faist M, Kehl K, et al. Monitor position in laparoscopic surgery. Surg Endosc. 2005;19:436-440.
- Berguer R, Rab GT, Abu-Ghaida H, et al. A comparison of surgeons’ posture during laparoscopic and open surgical procedures. Surg Endosc. 1997;11:139-142.
- Athanasiadis DI, Monfared S, Asadi H, et al. An analysis of the ergonomic risk of surgical trainees and experienced surgeons during laparoscopic procedures. Surgery. 2021;169:496-501.
- Hotton J, Bogart E, Le Deley MC, et al. Ergonomic assessment of the surgeon’s physical workload during robot-assisted versus standard laparoscopy in a French multicenter randomized trial (ROBOGYN-1004 Trial). Ann Surg Oncol. 2023;30:916-923.
- Plerhoples TA, Hernandez-Boussard T, Wren SM. The aching surgeon: a survey of physical discomfort and symptoms following open, laparoscopic, and robotic surgery. J Robot Surg. 2012;6:65-72.
- Lee GI, Lee MR, Green I, et al. Surgeons’ physical discomfort and symptoms during robotic surgery: a comprehensive ergonomic survey study. Surg Endosc. 2017;31:1697-1706.
- McDonald ME, Ramirez PT, Munsell MF, et al. Physician pain and discomfort during minimally invasive gynecologic cancer surgery. Gynecol Oncol. 2014;134:243-247.
- Zhu X, Yurteri-Kaplan LA, Gutman RE, et al. Postural stress experienced by vaginal surgeons. Proc Hum Factors Ergonomics Soc Annu Meet. 2014;58:763-767.
- American College of Surgeons Division of Education and Surgical Ergonomics Committee. Surgical Ergonomics Recommendations. ACS Education. 2022.
- Cardenas-Trowers O, Kjellsson K, Hatch K. Ergonomics: making the OR a comfortable place. Int Urogynecol J. 2018;29:1065-1066.
- Hokenstad ED, Hallbeck MS, Lowndes BR, et al. Ergonomic robotic console configuration in gynecologic surgery: an interventional study. J Minim Invasive Gynecol. 2021;28:850-859.
- Singh R, Yurteri-Kaplan LA, Morrow MM, et al. Sitting versus standing makes a difference in musculoskeletal discomfort and postural load for surgeons performing vaginal surgery. Int Urogynecol J. 2019;30:231-237.
- Hullfish KL, Trowbridge ER, Bodine G. Ergonomics and gynecologic surgery: “surgeon protect thyself.” J Pelvic Med Surg. 2009;15:435-439.
- Woodburn KL, Kho RM. Vaginal surgery: don’t get bent out of shape. Am J Obstet Gynecol. 2020;223:762-763.
- Hobson DTG, Meriwether KV, Gaskins JT, et al. Learner satisfaction and experience with a high-definition telescopic camera during vaginal procedures: a randomized controlled trial. Female Pelvic Med Reconstr Surg. 2021;27:105-111.
- Speed G, Harris K, Keegel T. The effect of cushioning materials on musculoskeletal discomfort and fatigue during prolonged standing at work: a systematic review. Appl Ergon. 2018;70:300-334.
- Haramis G, Rosales JC, Palacios JM, et al. Prospective randomized evaluation of FOOT gel pads for operating room staff COMFORT during laparoscopic renal surgery. Urology. 2010;76:1405-1408.
- Voss RK, Chiang YJ, Cromwell KD, et al. Do no harm, except to ourselves? A survey of symptoms and injuries in oncologic surgeons and pilot study of an intraoperative ergonomic intervention. J Am Coll Surg. 2017;224:16-25.e1.
- Marquetand J, Gabriel J, Seibt R, et al. Ergonomics for surgeons—prototype of an external surgeon support system reduces muscular activity and fatigue. J Electromyogr Kinesiol. 2021;60:102586.
- Tetteh E, Hallbeck MS, Mirka GA. Effects of passive exoskeleton support on EMG measures of the neck, shoulder and trunk muscles while holding simulated surgical postures and performing a simulated surgical procedure. Appl Ergon. 2022;100:103646.
- Lim AK, Ryu J, Yoon HM, et al. Ergonomic effects of medical augmented reality glasses in video-assisted surgery. Surg Endosc. 2022;36:988-998.
- Park AE, Zahiri HR, Hallbeck MS, et al. Intraoperative “micro breaks” with targeted stretching enhance surgeon physical function and mental focus: a multicenter cohort study. Ann Surg. 2017;265:340-346.
- Hallbeck MS, Lowndes BR, Bingener J, et al. The impact of intraoperative microbreaks with exercises on surgeons: a multi-center cohort study. Appl Ergon. 2017;60:334-341.
- Hallbeck Human Factors Engineering Laboratories. OR Stretch Videos. Mayo Clinic, 2018. Accessed May 19, 2023. https://www.mayo .edu/research/labs/human-factors-engineering/or-stretch /or-stretch-videos
- Stork A, Bacon T, Corton M. Prevention of Work-Related Musculoskeletal Disorders in Vaginal Surgery. Video presentation at: Society of Gynecologic Surgeons’ Annual Scientific Meeting 2023, Tucson, AZ. Accessed April 3, 2023. https://sgs.eng.us/category.php?cat=2023 -video-presentations
- Aaron KA, Vaughan J, Gupta R, et al. The risk of ergonomic injury across surgical specialties. PLoS One. 2021;16:e0244868.
- Smith TG, Lowndes BR, Schmida E, et al. Course design and learning outcomes of a practical online ergonomics course for surgical residents. J Surg Educ. 2022;79:1489-1499.
- Franasiak J, Craven R, Mosaly P, et al. Feasibility and acceptance of a robotic surgery ergonomic training program. JSLS. 2014;18:e2014.00166.
- Cerier E, Hu A, Goldring A, et al. Ergonomics workshop improves musculoskeletal symptoms in general surgery residents. J Surg Res. 2022;280:567-574.
- Giagio S, Volpe G, Pillastrini P, et al. A preventive program for workrelated musculoskeletal disorders among surgeons: outcomes of a randomized controlled clinical trial. Ann Surg. 2019;270:969-975.
- Jensen MJ, Liao J, Van Gorp B, et al. Incorporating surgical ergonomics education into surgical residency curriculum. J Surg Educ. 2021;78:1209-1215.
SGS showcases gyn surgeons’ impact on innovation, education, equity, and enterprise
The theme of the 49th Annual Scientific Meeting of the Society of Gynecologic Surgeons was Impact Factor—an allusion to scientific journal impact factor, as well as how we as gynecologic surgeons have a societal impact through our innovation, education, equity, and enterprise-level efforts. This theme and the diverse roster of speakers and presentations on contemporary and controversial issues impacting today’s gynecologic surgeons clearly resonated, breaking the prior registration record with more than 200 additional attendees than the previous year.
As always, the preconference postgraduate courses delivered relevant content that spanned the educational and surgical spectrum, including: “Innovations in training gynecologic surgeons”; “Urologic surgery for the gynecologic surgeon”; the social media workshop “Gynfluencing: Using social media to find your digital voice”; and “The sim factor: Making an impact in surgical education.” This also marked the first year of offering a specific SGS Fellows/Young Attendings’ course. The featured speaker of the SGS Equity Council was Patty Brisben, philanthropist, CEO, and founder of Pure Romance.
Dr. Beri Ridgeway, Cleveland Clinic Chief of Staff, delivered the Mark D. Walters Lecture, “Surgeon in the C-suite,” on leading approximately 5,000 physicians and the importance of surgeons and specifically ObGyns having a seat at the table. The TeLinde lecturer, Dr. Pam Moalli, Professor and Division Director for Urogynecology at the University of Pittsburgh Magee Womens Hospital, spoke on “Biomaterials for gynecologic surgeons: Toward bioinspired biomimetic devices.” The panel on the “Ergonomics of gynecologic surgery” was moderated by Dr. Amanda Fader and Dr. Kim Kho, who shared their experiences with work-related musculoskeletal injury, and featured esteemed panelists Dr. Noor Abu-Alnadi from UNC, Dr. Sue Hallbeck from Mayo Clinic, and Dr. Ladin Yurteri-Kaplan from Columbia University.
The conference also featured a new format of Ted Med Talks:
- Dr. Jason Wright, Editor-in-Chief, Obstetrics & Gynecology, and Division Director of Gynecologic Oncology at Columbia University, who spoke on “Surgical volume and outcomes for gynecologic surgery: Is more always better?”
- Dr. Kelly Wright, Division Director, Minimally Invasive Gynecologic Surgery, Cedars Sinai, on “Climate change starts at 7:15”
- Dr. Ebony Carter, Associate Editor, Equity, Obstetrics & Gynecology, and Division Director, Maternal Fetal Medicine, Washington University, on “Centering equity in reproductive health research.”
In this special section, several of these talks are presented. Additionally, Dr. Laura Homewood and her coauthors will discuss gender and racial biases in a large multi-institutional sample of more than 15,000 Press Ganey patient satisfaction surveys.
Dr. Cheryl Iglesia, SGS former president, and I hope that you will consider attending #SGS2024 in Orlando, Florida, led by Dr. Suzie As-Sanie, program chair, and Dr. Rosanne Kho, current SGS president, which promises to be another exciting meeting. ●
The theme of the 49th Annual Scientific Meeting of the Society of Gynecologic Surgeons was Impact Factor—an allusion to scientific journal impact factor, as well as how we as gynecologic surgeons have a societal impact through our innovation, education, equity, and enterprise-level efforts. This theme and the diverse roster of speakers and presentations on contemporary and controversial issues impacting today’s gynecologic surgeons clearly resonated, breaking the prior registration record with more than 200 additional attendees than the previous year.
As always, the preconference postgraduate courses delivered relevant content that spanned the educational and surgical spectrum, including: “Innovations in training gynecologic surgeons”; “Urologic surgery for the gynecologic surgeon”; the social media workshop “Gynfluencing: Using social media to find your digital voice”; and “The sim factor: Making an impact in surgical education.” This also marked the first year of offering a specific SGS Fellows/Young Attendings’ course. The featured speaker of the SGS Equity Council was Patty Brisben, philanthropist, CEO, and founder of Pure Romance.
Dr. Beri Ridgeway, Cleveland Clinic Chief of Staff, delivered the Mark D. Walters Lecture, “Surgeon in the C-suite,” on leading approximately 5,000 physicians and the importance of surgeons and specifically ObGyns having a seat at the table. The TeLinde lecturer, Dr. Pam Moalli, Professor and Division Director for Urogynecology at the University of Pittsburgh Magee Womens Hospital, spoke on “Biomaterials for gynecologic surgeons: Toward bioinspired biomimetic devices.” The panel on the “Ergonomics of gynecologic surgery” was moderated by Dr. Amanda Fader and Dr. Kim Kho, who shared their experiences with work-related musculoskeletal injury, and featured esteemed panelists Dr. Noor Abu-Alnadi from UNC, Dr. Sue Hallbeck from Mayo Clinic, and Dr. Ladin Yurteri-Kaplan from Columbia University.
The conference also featured a new format of Ted Med Talks:
- Dr. Jason Wright, Editor-in-Chief, Obstetrics & Gynecology, and Division Director of Gynecologic Oncology at Columbia University, who spoke on “Surgical volume and outcomes for gynecologic surgery: Is more always better?”
- Dr. Kelly Wright, Division Director, Minimally Invasive Gynecologic Surgery, Cedars Sinai, on “Climate change starts at 7:15”
- Dr. Ebony Carter, Associate Editor, Equity, Obstetrics & Gynecology, and Division Director, Maternal Fetal Medicine, Washington University, on “Centering equity in reproductive health research.”
In this special section, several of these talks are presented. Additionally, Dr. Laura Homewood and her coauthors will discuss gender and racial biases in a large multi-institutional sample of more than 15,000 Press Ganey patient satisfaction surveys.
Dr. Cheryl Iglesia, SGS former president, and I hope that you will consider attending #SGS2024 in Orlando, Florida, led by Dr. Suzie As-Sanie, program chair, and Dr. Rosanne Kho, current SGS president, which promises to be another exciting meeting. ●
The theme of the 49th Annual Scientific Meeting of the Society of Gynecologic Surgeons was Impact Factor—an allusion to scientific journal impact factor, as well as how we as gynecologic surgeons have a societal impact through our innovation, education, equity, and enterprise-level efforts. This theme and the diverse roster of speakers and presentations on contemporary and controversial issues impacting today’s gynecologic surgeons clearly resonated, breaking the prior registration record with more than 200 additional attendees than the previous year.
As always, the preconference postgraduate courses delivered relevant content that spanned the educational and surgical spectrum, including: “Innovations in training gynecologic surgeons”; “Urologic surgery for the gynecologic surgeon”; the social media workshop “Gynfluencing: Using social media to find your digital voice”; and “The sim factor: Making an impact in surgical education.” This also marked the first year of offering a specific SGS Fellows/Young Attendings’ course. The featured speaker of the SGS Equity Council was Patty Brisben, philanthropist, CEO, and founder of Pure Romance.
Dr. Beri Ridgeway, Cleveland Clinic Chief of Staff, delivered the Mark D. Walters Lecture, “Surgeon in the C-suite,” on leading approximately 5,000 physicians and the importance of surgeons and specifically ObGyns having a seat at the table. The TeLinde lecturer, Dr. Pam Moalli, Professor and Division Director for Urogynecology at the University of Pittsburgh Magee Womens Hospital, spoke on “Biomaterials for gynecologic surgeons: Toward bioinspired biomimetic devices.” The panel on the “Ergonomics of gynecologic surgery” was moderated by Dr. Amanda Fader and Dr. Kim Kho, who shared their experiences with work-related musculoskeletal injury, and featured esteemed panelists Dr. Noor Abu-Alnadi from UNC, Dr. Sue Hallbeck from Mayo Clinic, and Dr. Ladin Yurteri-Kaplan from Columbia University.
The conference also featured a new format of Ted Med Talks:
- Dr. Jason Wright, Editor-in-Chief, Obstetrics & Gynecology, and Division Director of Gynecologic Oncology at Columbia University, who spoke on “Surgical volume and outcomes for gynecologic surgery: Is more always better?”
- Dr. Kelly Wright, Division Director, Minimally Invasive Gynecologic Surgery, Cedars Sinai, on “Climate change starts at 7:15”
- Dr. Ebony Carter, Associate Editor, Equity, Obstetrics & Gynecology, and Division Director, Maternal Fetal Medicine, Washington University, on “Centering equity in reproductive health research.”
In this special section, several of these talks are presented. Additionally, Dr. Laura Homewood and her coauthors will discuss gender and racial biases in a large multi-institutional sample of more than 15,000 Press Ganey patient satisfaction surveys.
Dr. Cheryl Iglesia, SGS former president, and I hope that you will consider attending #SGS2024 in Orlando, Florida, led by Dr. Suzie As-Sanie, program chair, and Dr. Rosanne Kho, current SGS president, which promises to be another exciting meeting. ●
Hold Ozempic before surgery to optimize patient safety?
Semaglutide and related drugs for weight loss have co-opted bariatric medicine in recent months. They have also raised serious questions for hospital-based clinicians who wonder whether the drugs may pose risks to surgery patients undergoing anesthesia.
at this point. Official guidance on best practices has not yet caught up to surging popularity of this and other glucagon-like peptide-1 (GLP-1) agonists for weight loss.
Ozempic is indicated for treating type 2 diabetes but also is prescribed off-label for weight loss. Other GLP-1 agents from Novo Nordisk, Wegovy (semaglutide) and Saxenda (liraglutide) injections, are Food and Drug Administration–approved for weight loss. These medications work by decreasing hunger and lowering how much people eat. Semaglutide also is available as a once-daily tablet for type 2 diabetes (Rybelsus).
The American Society of Anesthesiologists (ASA) has been working on guidance on the drugs. “It’s a really hot issue now. We are getting emails from our members looking for guidance,” ASA president Michael Champeau, MD, said in an interview.
But despite the interest in how the medications might affect surgery patients and interact with anesthesia, relatively little evidence exists in the literature beyond case studies. So the society is not issuing official recommendations at this point.
“We’re going to just be calling it ‘guidance’ for right now because of the paucity of the scientific literature,” said Dr. Champeau, adjunct clinical professor of anesthesiology, perioperative, and pain medicine at Stanford (Calif.) University. “It’s probably not going to have words like ‘must; it will probably have words like ‘should’ or ‘should consider.’ “
The ASA guidance could be out in written form soon, Dr. Champeau added.
Meanwhile, whether physicians should advise stopping these medications 24 hours, 48 hours, or up to 2 weeks before surgery remains unknown.
In search of some consensus, John Shields, MD, an orthopedic surgeon at Atrium Health Wake Forest Baptist Davie Medical Center in Bermuda Run, N.C., asked colleagues on #MedTwitter: “Anyone have guidelines for ozempic around time of surgery? – holding med? – how long NPO?”
Because a full stomach can interfere with anesthesia, clinicians often advise people to stop eating and drinking 12-24 hours before elective procedures (NPO). In the case of once-weekly GLP-1 injections, which can slow gastric emptying, the optimal timeframe remains an open question. The main concern is aspiration, where a patient actively vomits while under anesthesia or their stomach contents passively come back up.
Dr. Shields’ Twitter post garnered significant reaction and comments. Within 4 days, the post was retweeted 30 times and received 72 replies and comments. Dr. Shields noted the general consensus was to hold semaglutide for 1-2 weeks before a procedure. Other suggestions included recommending a liquid diet only for 24-48 hours before surgery, recommending an NPO protocol 24-36 hours in advance, or adjusting the weekly injection so the last dose is taken 5-6 days before surgery.
Anesthesiologist Cliff Gevirtz, MD, has encountered only a few surgical patients so far taking a GLP-1 for weight loss. “And thankfully no aspiration,” added Dr. Gevirtz, clinical director of office-based ambulatory anesthesia services at Somnia Anesthesia in Harrison, N.Y.
To minimize risk, some physicians will perform an ultrasound scan to assess the contents of the stomach. If surgery is elective in a patient with a full stomach, the procedure can get postponed. Another option is to proceed with the case but treat the patient as anesthesiologists approach an emergency procedure. To be safe, many will treat the case as if the patient has a full stomach.
Dr. Gevirtz said he would treat the patient as a ‘full stomach’ and perform a rapid sequence induction with cricoid pressure. He would then extubate the patient once laryngeal reflexes return.
A rapid-sequence induction involves giving the medicine that makes a patient go to sleep, giving another medicine that paralyzes them quickly, then inserting a breathing tube – all within about 30 seconds. Cricoid pressure involves pushing on the neck during intubation to try to seal off the top of the esophagus and again minimize the chances of food coming back up.
Giving metoclopramide 30 minutes before surgery is another option, Dr. Gevirtz said. Metoclopramide can hasten the emptying of stomach contents. Administration in advance is important because waiting for the drug to work can prolong time in the operating room.
Is holding semaglutide before surgery a relevant clinical question? “Yes, very much so,” said Ronnie Fass, MD, division director of gastroenterology and hepatology and the medical director of the Digestive Health Center at The MetroHealth System in Cleveland.
Dr. Fass recommended different strategies based on the semaglutide indication. Currently, clinicians at MetroHealth instruct patients to discontinue diabetic medications the day of surgery. For those who take semaglutide for diabetes, and because the medication is taken once a week, “there is growing discussion among surgeons that the medication should not be stopped prior to surgery. This is to ensure that patients’ diabetes is well controlled before and during surgery,” Dr. Fass said.
In patients taking semaglutide for weight loss only, “there is no clear answer at this point,” he said.
Dr. Fass said the question is complicated by the fact that the medication is taken once a week. “It brings up important questions about the use of the medication during surgery, which may increase the likelihood of side effects in general and for certain types of surgery. Personally, if a patient is taking [semaglutide] for weight loss only, I would consider stopping the medication before surgery.”
The ASA was able to act quickly because it already had an expert task force review how long people should fast before surgery last year – before the explosion in popularity of the GLP-1 agonists.
Although it is still a work in progress, Dr. Champeau offered “a peek” at the recommendations. “The guidance is going to look at how far in advance the drugs should be stopped, rather than looking at making people fast even longer” before surgery, he said. “There’s just no data on that latter question.”
A version of this article originally appeared on Medscape.com.
Semaglutide and related drugs for weight loss have co-opted bariatric medicine in recent months. They have also raised serious questions for hospital-based clinicians who wonder whether the drugs may pose risks to surgery patients undergoing anesthesia.
at this point. Official guidance on best practices has not yet caught up to surging popularity of this and other glucagon-like peptide-1 (GLP-1) agonists for weight loss.
Ozempic is indicated for treating type 2 diabetes but also is prescribed off-label for weight loss. Other GLP-1 agents from Novo Nordisk, Wegovy (semaglutide) and Saxenda (liraglutide) injections, are Food and Drug Administration–approved for weight loss. These medications work by decreasing hunger and lowering how much people eat. Semaglutide also is available as a once-daily tablet for type 2 diabetes (Rybelsus).
The American Society of Anesthesiologists (ASA) has been working on guidance on the drugs. “It’s a really hot issue now. We are getting emails from our members looking for guidance,” ASA president Michael Champeau, MD, said in an interview.
But despite the interest in how the medications might affect surgery patients and interact with anesthesia, relatively little evidence exists in the literature beyond case studies. So the society is not issuing official recommendations at this point.
“We’re going to just be calling it ‘guidance’ for right now because of the paucity of the scientific literature,” said Dr. Champeau, adjunct clinical professor of anesthesiology, perioperative, and pain medicine at Stanford (Calif.) University. “It’s probably not going to have words like ‘must; it will probably have words like ‘should’ or ‘should consider.’ “
The ASA guidance could be out in written form soon, Dr. Champeau added.
Meanwhile, whether physicians should advise stopping these medications 24 hours, 48 hours, or up to 2 weeks before surgery remains unknown.
In search of some consensus, John Shields, MD, an orthopedic surgeon at Atrium Health Wake Forest Baptist Davie Medical Center in Bermuda Run, N.C., asked colleagues on #MedTwitter: “Anyone have guidelines for ozempic around time of surgery? – holding med? – how long NPO?”
Because a full stomach can interfere with anesthesia, clinicians often advise people to stop eating and drinking 12-24 hours before elective procedures (NPO). In the case of once-weekly GLP-1 injections, which can slow gastric emptying, the optimal timeframe remains an open question. The main concern is aspiration, where a patient actively vomits while under anesthesia or their stomach contents passively come back up.
Dr. Shields’ Twitter post garnered significant reaction and comments. Within 4 days, the post was retweeted 30 times and received 72 replies and comments. Dr. Shields noted the general consensus was to hold semaglutide for 1-2 weeks before a procedure. Other suggestions included recommending a liquid diet only for 24-48 hours before surgery, recommending an NPO protocol 24-36 hours in advance, or adjusting the weekly injection so the last dose is taken 5-6 days before surgery.
Anesthesiologist Cliff Gevirtz, MD, has encountered only a few surgical patients so far taking a GLP-1 for weight loss. “And thankfully no aspiration,” added Dr. Gevirtz, clinical director of office-based ambulatory anesthesia services at Somnia Anesthesia in Harrison, N.Y.
To minimize risk, some physicians will perform an ultrasound scan to assess the contents of the stomach. If surgery is elective in a patient with a full stomach, the procedure can get postponed. Another option is to proceed with the case but treat the patient as anesthesiologists approach an emergency procedure. To be safe, many will treat the case as if the patient has a full stomach.
Dr. Gevirtz said he would treat the patient as a ‘full stomach’ and perform a rapid sequence induction with cricoid pressure. He would then extubate the patient once laryngeal reflexes return.
A rapid-sequence induction involves giving the medicine that makes a patient go to sleep, giving another medicine that paralyzes them quickly, then inserting a breathing tube – all within about 30 seconds. Cricoid pressure involves pushing on the neck during intubation to try to seal off the top of the esophagus and again minimize the chances of food coming back up.
Giving metoclopramide 30 minutes before surgery is another option, Dr. Gevirtz said. Metoclopramide can hasten the emptying of stomach contents. Administration in advance is important because waiting for the drug to work can prolong time in the operating room.
Is holding semaglutide before surgery a relevant clinical question? “Yes, very much so,” said Ronnie Fass, MD, division director of gastroenterology and hepatology and the medical director of the Digestive Health Center at The MetroHealth System in Cleveland.
Dr. Fass recommended different strategies based on the semaglutide indication. Currently, clinicians at MetroHealth instruct patients to discontinue diabetic medications the day of surgery. For those who take semaglutide for diabetes, and because the medication is taken once a week, “there is growing discussion among surgeons that the medication should not be stopped prior to surgery. This is to ensure that patients’ diabetes is well controlled before and during surgery,” Dr. Fass said.
In patients taking semaglutide for weight loss only, “there is no clear answer at this point,” he said.
Dr. Fass said the question is complicated by the fact that the medication is taken once a week. “It brings up important questions about the use of the medication during surgery, which may increase the likelihood of side effects in general and for certain types of surgery. Personally, if a patient is taking [semaglutide] for weight loss only, I would consider stopping the medication before surgery.”
The ASA was able to act quickly because it already had an expert task force review how long people should fast before surgery last year – before the explosion in popularity of the GLP-1 agonists.
Although it is still a work in progress, Dr. Champeau offered “a peek” at the recommendations. “The guidance is going to look at how far in advance the drugs should be stopped, rather than looking at making people fast even longer” before surgery, he said. “There’s just no data on that latter question.”
A version of this article originally appeared on Medscape.com.
Semaglutide and related drugs for weight loss have co-opted bariatric medicine in recent months. They have also raised serious questions for hospital-based clinicians who wonder whether the drugs may pose risks to surgery patients undergoing anesthesia.
at this point. Official guidance on best practices has not yet caught up to surging popularity of this and other glucagon-like peptide-1 (GLP-1) agonists for weight loss.
Ozempic is indicated for treating type 2 diabetes but also is prescribed off-label for weight loss. Other GLP-1 agents from Novo Nordisk, Wegovy (semaglutide) and Saxenda (liraglutide) injections, are Food and Drug Administration–approved for weight loss. These medications work by decreasing hunger and lowering how much people eat. Semaglutide also is available as a once-daily tablet for type 2 diabetes (Rybelsus).
The American Society of Anesthesiologists (ASA) has been working on guidance on the drugs. “It’s a really hot issue now. We are getting emails from our members looking for guidance,” ASA president Michael Champeau, MD, said in an interview.
But despite the interest in how the medications might affect surgery patients and interact with anesthesia, relatively little evidence exists in the literature beyond case studies. So the society is not issuing official recommendations at this point.
“We’re going to just be calling it ‘guidance’ for right now because of the paucity of the scientific literature,” said Dr. Champeau, adjunct clinical professor of anesthesiology, perioperative, and pain medicine at Stanford (Calif.) University. “It’s probably not going to have words like ‘must; it will probably have words like ‘should’ or ‘should consider.’ “
The ASA guidance could be out in written form soon, Dr. Champeau added.
Meanwhile, whether physicians should advise stopping these medications 24 hours, 48 hours, or up to 2 weeks before surgery remains unknown.
In search of some consensus, John Shields, MD, an orthopedic surgeon at Atrium Health Wake Forest Baptist Davie Medical Center in Bermuda Run, N.C., asked colleagues on #MedTwitter: “Anyone have guidelines for ozempic around time of surgery? – holding med? – how long NPO?”
Because a full stomach can interfere with anesthesia, clinicians often advise people to stop eating and drinking 12-24 hours before elective procedures (NPO). In the case of once-weekly GLP-1 injections, which can slow gastric emptying, the optimal timeframe remains an open question. The main concern is aspiration, where a patient actively vomits while under anesthesia or their stomach contents passively come back up.
Dr. Shields’ Twitter post garnered significant reaction and comments. Within 4 days, the post was retweeted 30 times and received 72 replies and comments. Dr. Shields noted the general consensus was to hold semaglutide for 1-2 weeks before a procedure. Other suggestions included recommending a liquid diet only for 24-48 hours before surgery, recommending an NPO protocol 24-36 hours in advance, or adjusting the weekly injection so the last dose is taken 5-6 days before surgery.
Anesthesiologist Cliff Gevirtz, MD, has encountered only a few surgical patients so far taking a GLP-1 for weight loss. “And thankfully no aspiration,” added Dr. Gevirtz, clinical director of office-based ambulatory anesthesia services at Somnia Anesthesia in Harrison, N.Y.
To minimize risk, some physicians will perform an ultrasound scan to assess the contents of the stomach. If surgery is elective in a patient with a full stomach, the procedure can get postponed. Another option is to proceed with the case but treat the patient as anesthesiologists approach an emergency procedure. To be safe, many will treat the case as if the patient has a full stomach.
Dr. Gevirtz said he would treat the patient as a ‘full stomach’ and perform a rapid sequence induction with cricoid pressure. He would then extubate the patient once laryngeal reflexes return.
A rapid-sequence induction involves giving the medicine that makes a patient go to sleep, giving another medicine that paralyzes them quickly, then inserting a breathing tube – all within about 30 seconds. Cricoid pressure involves pushing on the neck during intubation to try to seal off the top of the esophagus and again minimize the chances of food coming back up.
Giving metoclopramide 30 minutes before surgery is another option, Dr. Gevirtz said. Metoclopramide can hasten the emptying of stomach contents. Administration in advance is important because waiting for the drug to work can prolong time in the operating room.
Is holding semaglutide before surgery a relevant clinical question? “Yes, very much so,” said Ronnie Fass, MD, division director of gastroenterology and hepatology and the medical director of the Digestive Health Center at The MetroHealth System in Cleveland.
Dr. Fass recommended different strategies based on the semaglutide indication. Currently, clinicians at MetroHealth instruct patients to discontinue diabetic medications the day of surgery. For those who take semaglutide for diabetes, and because the medication is taken once a week, “there is growing discussion among surgeons that the medication should not be stopped prior to surgery. This is to ensure that patients’ diabetes is well controlled before and during surgery,” Dr. Fass said.
In patients taking semaglutide for weight loss only, “there is no clear answer at this point,” he said.
Dr. Fass said the question is complicated by the fact that the medication is taken once a week. “It brings up important questions about the use of the medication during surgery, which may increase the likelihood of side effects in general and for certain types of surgery. Personally, if a patient is taking [semaglutide] for weight loss only, I would consider stopping the medication before surgery.”
The ASA was able to act quickly because it already had an expert task force review how long people should fast before surgery last year – before the explosion in popularity of the GLP-1 agonists.
Although it is still a work in progress, Dr. Champeau offered “a peek” at the recommendations. “The guidance is going to look at how far in advance the drugs should be stopped, rather than looking at making people fast even longer” before surgery, he said. “There’s just no data on that latter question.”
A version of this article originally appeared on Medscape.com.
Hospital patient catches on fire, highlighting need for prevention
On Thanksgiving Day 2022, Kathy Stark watched as her husband of 35 years, Bobby Ray Stark, caught fire at a Nashville hospital. According to Clint Kelly, Kathy Stark’s attorney, the hospital staff was performing cardioversion to restore Bobby Ray’s heart rhythm when a spark ignited the oxygen and set the patient aflame.
Mr. Stark, 64, died of “a combination of cardiovascular disease and thermal burns,” according to a local news report. In May, Kathy Stark filed a malpractice lawsuit in U.S. District Court. Mr. Kelly hopes that the lawsuit will help improve patient safety. Meanwhile, Kathy Stark “goes to bed at night and sees her husband on fire,” Mr. Kelly says. A similar incident occurred last December in the operating room at Oregon Health & Science University, resulting in minor injuries to a patient.
Underreported, but likely dropping
Reliable data on the incidence of surgical fires is lacking because incidents may go unreported over litigation fears, says Jeffrey Feldman, MD, MSE, anesthesiologist at Children’s Hospital of Philadelphia and chair of the Anesthesia Patient Safety Foundation’s Committee on Technology.
The Pennsylvania Patient Safety Authority has been tracking surgical fires for decades, however, and experts have used the agency’s data to extrapolate how often they occur in the United States.
In 2005, nationwide incidence was estimated to be somewhere in the neighborhood of 550-600 fires annually, says Barbara G. Malanga, acting director of health care incident investigation and technology consulting at ECRI (formerly the Emergency Care Research Institute). By 2011, that number appeared to have dropped to 200-240 incidents per year.
A similar analysis in 2018 found the incidence may now be as low as 88-105 a year. The drop is likely a result of increased awareness because of educational efforts on the part of the ECRI and the APSF, including a widely disseminated video on fire safety.
The decline of surgical fires “sounds great,” says Dr. Feldman, “except that it’s a 100% preventable complication, and they’re still happening.”
Accidents waiting to happen
How do these fires happen? It comes down to the ‘fire triangle’ often taught in grade school. Fire requires three things: an ignition source, fuel, and oxygen or an oxidizing agent. Ignition sources are plentiful in a surgical suite, including any of a variety of electrical devices commonly used in surgical procedures, including defibrillators. Gowns, gauze, drapes, sponges, oxygen masks, nasal cannulae, a patient’s hair or their clothing – all provide the necessary fuel.
But the key factor for surgical fire risk is the presence of high concentrations of oxygen.
Safety protocols
The best and most obvious way to mitigate risk is to reduce the amount of supplemental oxygen, explains Dr. Feldman.
“Many patients do not require a high concentration of oxygen during sedation,” he says.
When a patient does require a higher concentration for their safety, the APSF and ECRI recommend placing an endotracheal tube or supraglottic airway rather than using an oxygen mask or a nasal cannula. “You want to deliver the oxygen in such a way that high concentration doesn’t exist in the surgical field,” Dr. Feldman says. In cases where supplemental oxygen is necessary, ECRI and APSF recommend reducing the oxygen concentration to less than 30%.
In addition, safety protocols include giving flammable prep solutions time to dry before applying towels or drapes and beginning the procedure. These precautions to ensure the safety of patients take just a moment, says Chester H. Lake Jr, MD, MS, of the department of anesthesiology at the University of Mississippi Medical Center, Jackson.
Making fire safety part of the preop routine
These safety protocols are straightforward but not always observed, experts say. Part of the reason is a matter of culture. Both anesthesiologists and surgeons have absorbed the attitude that placing an airway escalates the procedure beyond what the patient needs, says Dr. Feldman. And indeed, according to a 2013 analysis of the American Society of Anesthesiologists closed claims database, 85% of surgical fires occur in outpatient settings where airways are less likely to be placed, and 81% of those claims were for procedures that used monitored anesthesia care.
In an article on prevention of surgical fires, Dr. Lake and colleagues recommend in-house education on preventing and responding to fires at least once a year. But it shouldn’t stop there. Because these fires – horrific as they are – are fairly rare, it’s important to maintain awareness. Making fire safety a regular part of the surgical “time-out” can help further reduce incidents, he says. ECRI and the APSF have teamed up to create a poster that can help surgical teams make fire safety a regular part of their routines.
Although the national decline in surgical fires is encouraging, the problem remains serious. “You can classify these incidents as low, but it’s not low if it happens to you or a family member,” says Dr. Lake. “One is too many.”
ECRI’s Ms. Malanga agrees. “I do like to emphasize that it’s rare,” she says. “But I’d like to see us reduce this until it’s zero.”
A version of this article originally appeared on Medscape.com.
On Thanksgiving Day 2022, Kathy Stark watched as her husband of 35 years, Bobby Ray Stark, caught fire at a Nashville hospital. According to Clint Kelly, Kathy Stark’s attorney, the hospital staff was performing cardioversion to restore Bobby Ray’s heart rhythm when a spark ignited the oxygen and set the patient aflame.
Mr. Stark, 64, died of “a combination of cardiovascular disease and thermal burns,” according to a local news report. In May, Kathy Stark filed a malpractice lawsuit in U.S. District Court. Mr. Kelly hopes that the lawsuit will help improve patient safety. Meanwhile, Kathy Stark “goes to bed at night and sees her husband on fire,” Mr. Kelly says. A similar incident occurred last December in the operating room at Oregon Health & Science University, resulting in minor injuries to a patient.
Underreported, but likely dropping
Reliable data on the incidence of surgical fires is lacking because incidents may go unreported over litigation fears, says Jeffrey Feldman, MD, MSE, anesthesiologist at Children’s Hospital of Philadelphia and chair of the Anesthesia Patient Safety Foundation’s Committee on Technology.
The Pennsylvania Patient Safety Authority has been tracking surgical fires for decades, however, and experts have used the agency’s data to extrapolate how often they occur in the United States.
In 2005, nationwide incidence was estimated to be somewhere in the neighborhood of 550-600 fires annually, says Barbara G. Malanga, acting director of health care incident investigation and technology consulting at ECRI (formerly the Emergency Care Research Institute). By 2011, that number appeared to have dropped to 200-240 incidents per year.
A similar analysis in 2018 found the incidence may now be as low as 88-105 a year. The drop is likely a result of increased awareness because of educational efforts on the part of the ECRI and the APSF, including a widely disseminated video on fire safety.
The decline of surgical fires “sounds great,” says Dr. Feldman, “except that it’s a 100% preventable complication, and they’re still happening.”
Accidents waiting to happen
How do these fires happen? It comes down to the ‘fire triangle’ often taught in grade school. Fire requires three things: an ignition source, fuel, and oxygen or an oxidizing agent. Ignition sources are plentiful in a surgical suite, including any of a variety of electrical devices commonly used in surgical procedures, including defibrillators. Gowns, gauze, drapes, sponges, oxygen masks, nasal cannulae, a patient’s hair or their clothing – all provide the necessary fuel.
But the key factor for surgical fire risk is the presence of high concentrations of oxygen.
Safety protocols
The best and most obvious way to mitigate risk is to reduce the amount of supplemental oxygen, explains Dr. Feldman.
“Many patients do not require a high concentration of oxygen during sedation,” he says.
When a patient does require a higher concentration for their safety, the APSF and ECRI recommend placing an endotracheal tube or supraglottic airway rather than using an oxygen mask or a nasal cannula. “You want to deliver the oxygen in such a way that high concentration doesn’t exist in the surgical field,” Dr. Feldman says. In cases where supplemental oxygen is necessary, ECRI and APSF recommend reducing the oxygen concentration to less than 30%.
In addition, safety protocols include giving flammable prep solutions time to dry before applying towels or drapes and beginning the procedure. These precautions to ensure the safety of patients take just a moment, says Chester H. Lake Jr, MD, MS, of the department of anesthesiology at the University of Mississippi Medical Center, Jackson.
Making fire safety part of the preop routine
These safety protocols are straightforward but not always observed, experts say. Part of the reason is a matter of culture. Both anesthesiologists and surgeons have absorbed the attitude that placing an airway escalates the procedure beyond what the patient needs, says Dr. Feldman. And indeed, according to a 2013 analysis of the American Society of Anesthesiologists closed claims database, 85% of surgical fires occur in outpatient settings where airways are less likely to be placed, and 81% of those claims were for procedures that used monitored anesthesia care.
In an article on prevention of surgical fires, Dr. Lake and colleagues recommend in-house education on preventing and responding to fires at least once a year. But it shouldn’t stop there. Because these fires – horrific as they are – are fairly rare, it’s important to maintain awareness. Making fire safety a regular part of the surgical “time-out” can help further reduce incidents, he says. ECRI and the APSF have teamed up to create a poster that can help surgical teams make fire safety a regular part of their routines.
Although the national decline in surgical fires is encouraging, the problem remains serious. “You can classify these incidents as low, but it’s not low if it happens to you or a family member,” says Dr. Lake. “One is too many.”
ECRI’s Ms. Malanga agrees. “I do like to emphasize that it’s rare,” she says. “But I’d like to see us reduce this until it’s zero.”
A version of this article originally appeared on Medscape.com.
On Thanksgiving Day 2022, Kathy Stark watched as her husband of 35 years, Bobby Ray Stark, caught fire at a Nashville hospital. According to Clint Kelly, Kathy Stark’s attorney, the hospital staff was performing cardioversion to restore Bobby Ray’s heart rhythm when a spark ignited the oxygen and set the patient aflame.
Mr. Stark, 64, died of “a combination of cardiovascular disease and thermal burns,” according to a local news report. In May, Kathy Stark filed a malpractice lawsuit in U.S. District Court. Mr. Kelly hopes that the lawsuit will help improve patient safety. Meanwhile, Kathy Stark “goes to bed at night and sees her husband on fire,” Mr. Kelly says. A similar incident occurred last December in the operating room at Oregon Health & Science University, resulting in minor injuries to a patient.
Underreported, but likely dropping
Reliable data on the incidence of surgical fires is lacking because incidents may go unreported over litigation fears, says Jeffrey Feldman, MD, MSE, anesthesiologist at Children’s Hospital of Philadelphia and chair of the Anesthesia Patient Safety Foundation’s Committee on Technology.
The Pennsylvania Patient Safety Authority has been tracking surgical fires for decades, however, and experts have used the agency’s data to extrapolate how often they occur in the United States.
In 2005, nationwide incidence was estimated to be somewhere in the neighborhood of 550-600 fires annually, says Barbara G. Malanga, acting director of health care incident investigation and technology consulting at ECRI (formerly the Emergency Care Research Institute). By 2011, that number appeared to have dropped to 200-240 incidents per year.
A similar analysis in 2018 found the incidence may now be as low as 88-105 a year. The drop is likely a result of increased awareness because of educational efforts on the part of the ECRI and the APSF, including a widely disseminated video on fire safety.
The decline of surgical fires “sounds great,” says Dr. Feldman, “except that it’s a 100% preventable complication, and they’re still happening.”
Accidents waiting to happen
How do these fires happen? It comes down to the ‘fire triangle’ often taught in grade school. Fire requires three things: an ignition source, fuel, and oxygen or an oxidizing agent. Ignition sources are plentiful in a surgical suite, including any of a variety of electrical devices commonly used in surgical procedures, including defibrillators. Gowns, gauze, drapes, sponges, oxygen masks, nasal cannulae, a patient’s hair or their clothing – all provide the necessary fuel.
But the key factor for surgical fire risk is the presence of high concentrations of oxygen.
Safety protocols
The best and most obvious way to mitigate risk is to reduce the amount of supplemental oxygen, explains Dr. Feldman.
“Many patients do not require a high concentration of oxygen during sedation,” he says.
When a patient does require a higher concentration for their safety, the APSF and ECRI recommend placing an endotracheal tube or supraglottic airway rather than using an oxygen mask or a nasal cannula. “You want to deliver the oxygen in such a way that high concentration doesn’t exist in the surgical field,” Dr. Feldman says. In cases where supplemental oxygen is necessary, ECRI and APSF recommend reducing the oxygen concentration to less than 30%.
In addition, safety protocols include giving flammable prep solutions time to dry before applying towels or drapes and beginning the procedure. These precautions to ensure the safety of patients take just a moment, says Chester H. Lake Jr, MD, MS, of the department of anesthesiology at the University of Mississippi Medical Center, Jackson.
Making fire safety part of the preop routine
These safety protocols are straightforward but not always observed, experts say. Part of the reason is a matter of culture. Both anesthesiologists and surgeons have absorbed the attitude that placing an airway escalates the procedure beyond what the patient needs, says Dr. Feldman. And indeed, according to a 2013 analysis of the American Society of Anesthesiologists closed claims database, 85% of surgical fires occur in outpatient settings where airways are less likely to be placed, and 81% of those claims were for procedures that used monitored anesthesia care.
In an article on prevention of surgical fires, Dr. Lake and colleagues recommend in-house education on preventing and responding to fires at least once a year. But it shouldn’t stop there. Because these fires – horrific as they are – are fairly rare, it’s important to maintain awareness. Making fire safety a regular part of the surgical “time-out” can help further reduce incidents, he says. ECRI and the APSF have teamed up to create a poster that can help surgical teams make fire safety a regular part of their routines.
Although the national decline in surgical fires is encouraging, the problem remains serious. “You can classify these incidents as low, but it’s not low if it happens to you or a family member,” says Dr. Lake. “One is too many.”
ECRI’s Ms. Malanga agrees. “I do like to emphasize that it’s rare,” she says. “But I’d like to see us reduce this until it’s zero.”
A version of this article originally appeared on Medscape.com.
Early hysterectomy linked to higher CVD, stroke risk
TOPLINE:
METHODOLOGY:
- Risk of CVD rapidly increases after menopause, possibly owing to loss of protective effects of female sex hormones and hemorheologic changes.
- Results of previous studies of the association between hysterectomy and CVD were mixed.
- Using national health insurance data, this cohort study included 55,539 South Korean women (median age, 45 years) who underwent a hysterectomy and a propensity-matched group of women.
- The primary outcome was CVD, including myocardial infarction (MI), coronary artery revascularization, and stroke.
TAKEAWAY:
- During follow-up of just under 8 years, the hysterectomy group had an increased risk of CVD compared with the non-hysterectomy group (hazard ratio [HR] 1.25; 95% confidence interval [CI], 1.09-1.44; P = .002)
- The incidence of MI and coronary revascularization was comparable between groups, but the risk of stroke was significantly higher among those who had had a hysterectomy (HR, 1.31; 95% CI, 1.12-1.53; P < .001)
- This increase in risk was similar after excluding patients who also underwent adnexal surgery.
IN PRACTICE:
Early hysterectomy was linked to higher CVD risk, especially stroke, but since the CVD incidence wasn’t high, a change in clinical practice may not be needed, said the authors.
STUDY DETAILS:
The study was conducted by Jin-Sung Yuk, MD, PhD, Department of Obstetrics and Gynecology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Republic of Korea, and colleagues. It was published online June 12 in JAMA Network Open.
LIMITATIONS:
The study was retrospective and observational and used administrative databases that may be prone to inaccurate coding. The findings may not be generalizable outside Korea.
DISCLOSURES:
The study was supported by a National Research Foundation of Korea grant funded by the Korea government. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Risk of CVD rapidly increases after menopause, possibly owing to loss of protective effects of female sex hormones and hemorheologic changes.
- Results of previous studies of the association between hysterectomy and CVD were mixed.
- Using national health insurance data, this cohort study included 55,539 South Korean women (median age, 45 years) who underwent a hysterectomy and a propensity-matched group of women.
- The primary outcome was CVD, including myocardial infarction (MI), coronary artery revascularization, and stroke.
TAKEAWAY:
- During follow-up of just under 8 years, the hysterectomy group had an increased risk of CVD compared with the non-hysterectomy group (hazard ratio [HR] 1.25; 95% confidence interval [CI], 1.09-1.44; P = .002)
- The incidence of MI and coronary revascularization was comparable between groups, but the risk of stroke was significantly higher among those who had had a hysterectomy (HR, 1.31; 95% CI, 1.12-1.53; P < .001)
- This increase in risk was similar after excluding patients who also underwent adnexal surgery.
IN PRACTICE:
Early hysterectomy was linked to higher CVD risk, especially stroke, but since the CVD incidence wasn’t high, a change in clinical practice may not be needed, said the authors.
STUDY DETAILS:
The study was conducted by Jin-Sung Yuk, MD, PhD, Department of Obstetrics and Gynecology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Republic of Korea, and colleagues. It was published online June 12 in JAMA Network Open.
LIMITATIONS:
The study was retrospective and observational and used administrative databases that may be prone to inaccurate coding. The findings may not be generalizable outside Korea.
DISCLOSURES:
The study was supported by a National Research Foundation of Korea grant funded by the Korea government. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Risk of CVD rapidly increases after menopause, possibly owing to loss of protective effects of female sex hormones and hemorheologic changes.
- Results of previous studies of the association between hysterectomy and CVD were mixed.
- Using national health insurance data, this cohort study included 55,539 South Korean women (median age, 45 years) who underwent a hysterectomy and a propensity-matched group of women.
- The primary outcome was CVD, including myocardial infarction (MI), coronary artery revascularization, and stroke.
TAKEAWAY:
- During follow-up of just under 8 years, the hysterectomy group had an increased risk of CVD compared with the non-hysterectomy group (hazard ratio [HR] 1.25; 95% confidence interval [CI], 1.09-1.44; P = .002)
- The incidence of MI and coronary revascularization was comparable between groups, but the risk of stroke was significantly higher among those who had had a hysterectomy (HR, 1.31; 95% CI, 1.12-1.53; P < .001)
- This increase in risk was similar after excluding patients who also underwent adnexal surgery.
IN PRACTICE:
Early hysterectomy was linked to higher CVD risk, especially stroke, but since the CVD incidence wasn’t high, a change in clinical practice may not be needed, said the authors.
STUDY DETAILS:
The study was conducted by Jin-Sung Yuk, MD, PhD, Department of Obstetrics and Gynecology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Republic of Korea, and colleagues. It was published online June 12 in JAMA Network Open.
LIMITATIONS:
The study was retrospective and observational and used administrative databases that may be prone to inaccurate coding. The findings may not be generalizable outside Korea.
DISCLOSURES:
The study was supported by a National Research Foundation of Korea grant funded by the Korea government. The authors report no conflicts of interest.
A version of this article first appeared on Medscape.com.