User login
VIDEO: When to use MRI in breast cancer
CHICAGO – For most women, there’s little role for MRI in screening for and treating breast cancer
However, there are important exceptions. In an interview at the American College of Surgeons Clinical Congress, Dr. Monica Morrow, chief of breast surgery at Memorial Sloan-Kettering Cancer Center in Manhattan, explained what those exceptions are, and how she uses MRI in her practice.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – For most women, there’s little role for MRI in screening for and treating breast cancer
However, there are important exceptions. In an interview at the American College of Surgeons Clinical Congress, Dr. Monica Morrow, chief of breast surgery at Memorial Sloan-Kettering Cancer Center in Manhattan, explained what those exceptions are, and how she uses MRI in her practice.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – For most women, there’s little role for MRI in screening for and treating breast cancer
However, there are important exceptions. In an interview at the American College of Surgeons Clinical Congress, Dr. Monica Morrow, chief of breast surgery at Memorial Sloan-Kettering Cancer Center in Manhattan, explained what those exceptions are, and how she uses MRI in her practice.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
EXPERT ANALYSIS FROM THE AMERICAN COLLEGE OF SURGEONS CLINICAL CONGRESS
PD-L1 status of NSCLC consistent across primary, nodes, and metastases
DENVER – In patients with non–small cell lung cancer (NSCLC), the programmed death-ligand 1 (PD-L1) status of the primary tumor is generally a reliable predictor of the status of tumor elsewhere the body, suggest a pair of retrospective cohort studies presented at a world conference on lung cancer.
Results showed a high rate of concordance of PD-L1 immunohistochemical (IHC) staining, whether comparing primary with nodes (81%-89%) or primary with metastasis (77%), investigators reported.
These findings are relevant in that some studies have suggested that high PD-L1 expression is a biomarker for benefit from agents that inhibit the programmed cell death 1 (PD-1) signaling pathway. Thus, being able to use archival primary tumor to assess PD-L1 status might help guide decisions about treatment options in the metastatic setting.
“This is pretty good concordance, especially considering that our assays only have about 75% concordance [among them], depending on how you measure it and what cutpoints you use and which antibodies you use,” said invited discussant Dr. David Rimm, professor of pathology and of medicine, director of pathology tissue services, and director of translational pathology at Yale University in New Haven, Conn.
“The take-home message is, is the glass half full or is the glass half empty? And the real message is that maybe the glass is twice as big as it needs to be,” he said. “That is, what we really need to do is come up with a uniform assay here that’s standardized so that we can actually do studies like this a little more carefully.”
In the first study, Dr. Brandon S. Sheffield, of the pathology office at BC Cancer Agency, Vancouver, studied 78 patients who underwent resection of a primary nonsquamous NSCLC and were found to have nodal involvement.
For each patient, they compared PD-L1 staining between the primary tumor and the matched nodal tumor, simultaneously testing various antibodies; Bristol-Myers Squibb Canada performed some of the IHC. Tumors were considered positive if at least 1% of cells stained with any intensity.
Results showed an 81% rate of concordance of PD-L1 staining between the primary and node; in about 8% of cases, only the primary was positive, and in about 9%, only the node was positive.
Study results also showed good concordance across the three different antibodies: SP142 (Spring Bioscience), E1L3N (Cell Signaling Technology), and 28-8 (Dako). In 76% of cases, all three antibodies were in agreement.
In some cases, clusters of infiltrating macrophages stained for PD-L1. “This could represent a possible pitfall, especially using a cutoff as low as 1%, although with some practice, one can appreciate that the staining of macrophages is somewhat different than the crisp membranous staining seen in tumor cells,” Dr. Sheffield.
“PD-L1 IHC is feasible and it can be done in your laboratory. There are small but very relevant differences in testing primary tumor tissue and lymph node metastasis, and that will need to be explored with a bias toward testing more, not less, tissue,” he concluded. “Multiple methods for PD-L1 IHC appear to be equivalent, and we should be able to have some freedom in choosing the best PD-L1 IHC assay for our own laboratories.”
In the second study, Dr. Paul Mitchell of Austin Health, Melbourne, and colleagues assessed PD-L1 staining among 433 sequential patients who underwent resection of primary NSCLC between 1992 and 2010. Bristol-Myers Squibb performed the IHC staining and some of the scoring.
In one set of assays, the investigators used the 28.8 antibody and considered tumors to be positive if at least 5% of cells showed membranous staining of any intensity.
Results here showed that 28% of primaries were PD-L1 positive. The rate was similar for men and women, but higher in squamous tumors than in adenocarcinomas (39% vs. 18%). It was merely 14% in patients having an epidermal growth factor receptor (EGFR) mutation. The rate of positivity increased with the number of pack-years of smoking and, starting 5 years after cessation, decreased over time.
A total of 57 patients had paired primary tumor and metastatic tumor (most commonly from brain metastases). The median time from primary to metastasis was 1.3 years; in eight patients, metastasis was synchronous.
In this cohort, there was a 77% rate of concordance of PD-L1 staining between the primary and metastasis (r = 0.37, P = .0049); in 11% of cases, only the primary was positive, and in 12%, only the metastasis was positive. Also, among the eight patients having multiple metastases, all samples were concordant in six patients.
In another set of assays, the investigators used the E11340 XP antibody (Cell Signaling Technology) and considered tumors to be strongly positive if more than 50% of cells stained with intensity of 2 or higher (ASCO 2015, abstract 11051).
Here, 24% of primaries were strongly positive. In a multivariate analysis, patients with stage III disease who had high PD-L1 expression in both the primary and node had better disease-free survival (hazard ratio, 0.49; P = .031) and overall survival (HR, 0.46; P = .006).
Among the 53 patients who had paired primary and nodal tumor, PD-L1 staining was concordant in these sites in 89% of patients.
“These data do suggest that PD-L1 status in general in the primary NSCLC predicts the PD-L1 status in metastases as well as in the nodes,” Dr. Mitchell said. “However, if the PD-L1 expression status is critical in the decision to treat metastatic NSCLC with a PD-1 pathway inhibitor, then rebiopsy of a metastasis may be warranted,” he added.
Dr. Sheffield reported that he had no relevant disclosures; Bristol-Myers Squibb Canada performed some of the IHC staining. Dr. Mitchell disclosed ties with AstraZeneca, Roche, Boehringer-Ingelheim, Bristol-Myers Squibb, and MSD. Bristol-Myers Squibb performed IHC staining and some of the scoring.
The conference was sponsored by the International Association for the Study of Lung Cancer.
DENVER – In patients with non–small cell lung cancer (NSCLC), the programmed death-ligand 1 (PD-L1) status of the primary tumor is generally a reliable predictor of the status of tumor elsewhere the body, suggest a pair of retrospective cohort studies presented at a world conference on lung cancer.
Results showed a high rate of concordance of PD-L1 immunohistochemical (IHC) staining, whether comparing primary with nodes (81%-89%) or primary with metastasis (77%), investigators reported.
These findings are relevant in that some studies have suggested that high PD-L1 expression is a biomarker for benefit from agents that inhibit the programmed cell death 1 (PD-1) signaling pathway. Thus, being able to use archival primary tumor to assess PD-L1 status might help guide decisions about treatment options in the metastatic setting.
“This is pretty good concordance, especially considering that our assays only have about 75% concordance [among them], depending on how you measure it and what cutpoints you use and which antibodies you use,” said invited discussant Dr. David Rimm, professor of pathology and of medicine, director of pathology tissue services, and director of translational pathology at Yale University in New Haven, Conn.
“The take-home message is, is the glass half full or is the glass half empty? And the real message is that maybe the glass is twice as big as it needs to be,” he said. “That is, what we really need to do is come up with a uniform assay here that’s standardized so that we can actually do studies like this a little more carefully.”
In the first study, Dr. Brandon S. Sheffield, of the pathology office at BC Cancer Agency, Vancouver, studied 78 patients who underwent resection of a primary nonsquamous NSCLC and were found to have nodal involvement.
For each patient, they compared PD-L1 staining between the primary tumor and the matched nodal tumor, simultaneously testing various antibodies; Bristol-Myers Squibb Canada performed some of the IHC. Tumors were considered positive if at least 1% of cells stained with any intensity.
Results showed an 81% rate of concordance of PD-L1 staining between the primary and node; in about 8% of cases, only the primary was positive, and in about 9%, only the node was positive.
Study results also showed good concordance across the three different antibodies: SP142 (Spring Bioscience), E1L3N (Cell Signaling Technology), and 28-8 (Dako). In 76% of cases, all three antibodies were in agreement.
In some cases, clusters of infiltrating macrophages stained for PD-L1. “This could represent a possible pitfall, especially using a cutoff as low as 1%, although with some practice, one can appreciate that the staining of macrophages is somewhat different than the crisp membranous staining seen in tumor cells,” Dr. Sheffield.
“PD-L1 IHC is feasible and it can be done in your laboratory. There are small but very relevant differences in testing primary tumor tissue and lymph node metastasis, and that will need to be explored with a bias toward testing more, not less, tissue,” he concluded. “Multiple methods for PD-L1 IHC appear to be equivalent, and we should be able to have some freedom in choosing the best PD-L1 IHC assay for our own laboratories.”
In the second study, Dr. Paul Mitchell of Austin Health, Melbourne, and colleagues assessed PD-L1 staining among 433 sequential patients who underwent resection of primary NSCLC between 1992 and 2010. Bristol-Myers Squibb performed the IHC staining and some of the scoring.
In one set of assays, the investigators used the 28.8 antibody and considered tumors to be positive if at least 5% of cells showed membranous staining of any intensity.
Results here showed that 28% of primaries were PD-L1 positive. The rate was similar for men and women, but higher in squamous tumors than in adenocarcinomas (39% vs. 18%). It was merely 14% in patients having an epidermal growth factor receptor (EGFR) mutation. The rate of positivity increased with the number of pack-years of smoking and, starting 5 years after cessation, decreased over time.
A total of 57 patients had paired primary tumor and metastatic tumor (most commonly from brain metastases). The median time from primary to metastasis was 1.3 years; in eight patients, metastasis was synchronous.
In this cohort, there was a 77% rate of concordance of PD-L1 staining between the primary and metastasis (r = 0.37, P = .0049); in 11% of cases, only the primary was positive, and in 12%, only the metastasis was positive. Also, among the eight patients having multiple metastases, all samples were concordant in six patients.
In another set of assays, the investigators used the E11340 XP antibody (Cell Signaling Technology) and considered tumors to be strongly positive if more than 50% of cells stained with intensity of 2 or higher (ASCO 2015, abstract 11051).
Here, 24% of primaries were strongly positive. In a multivariate analysis, patients with stage III disease who had high PD-L1 expression in both the primary and node had better disease-free survival (hazard ratio, 0.49; P = .031) and overall survival (HR, 0.46; P = .006).
Among the 53 patients who had paired primary and nodal tumor, PD-L1 staining was concordant in these sites in 89% of patients.
“These data do suggest that PD-L1 status in general in the primary NSCLC predicts the PD-L1 status in metastases as well as in the nodes,” Dr. Mitchell said. “However, if the PD-L1 expression status is critical in the decision to treat metastatic NSCLC with a PD-1 pathway inhibitor, then rebiopsy of a metastasis may be warranted,” he added.
Dr. Sheffield reported that he had no relevant disclosures; Bristol-Myers Squibb Canada performed some of the IHC staining. Dr. Mitchell disclosed ties with AstraZeneca, Roche, Boehringer-Ingelheim, Bristol-Myers Squibb, and MSD. Bristol-Myers Squibb performed IHC staining and some of the scoring.
The conference was sponsored by the International Association for the Study of Lung Cancer.
DENVER – In patients with non–small cell lung cancer (NSCLC), the programmed death-ligand 1 (PD-L1) status of the primary tumor is generally a reliable predictor of the status of tumor elsewhere the body, suggest a pair of retrospective cohort studies presented at a world conference on lung cancer.
Results showed a high rate of concordance of PD-L1 immunohistochemical (IHC) staining, whether comparing primary with nodes (81%-89%) or primary with metastasis (77%), investigators reported.
These findings are relevant in that some studies have suggested that high PD-L1 expression is a biomarker for benefit from agents that inhibit the programmed cell death 1 (PD-1) signaling pathway. Thus, being able to use archival primary tumor to assess PD-L1 status might help guide decisions about treatment options in the metastatic setting.
“This is pretty good concordance, especially considering that our assays only have about 75% concordance [among them], depending on how you measure it and what cutpoints you use and which antibodies you use,” said invited discussant Dr. David Rimm, professor of pathology and of medicine, director of pathology tissue services, and director of translational pathology at Yale University in New Haven, Conn.
“The take-home message is, is the glass half full or is the glass half empty? And the real message is that maybe the glass is twice as big as it needs to be,” he said. “That is, what we really need to do is come up with a uniform assay here that’s standardized so that we can actually do studies like this a little more carefully.”
In the first study, Dr. Brandon S. Sheffield, of the pathology office at BC Cancer Agency, Vancouver, studied 78 patients who underwent resection of a primary nonsquamous NSCLC and were found to have nodal involvement.
For each patient, they compared PD-L1 staining between the primary tumor and the matched nodal tumor, simultaneously testing various antibodies; Bristol-Myers Squibb Canada performed some of the IHC. Tumors were considered positive if at least 1% of cells stained with any intensity.
Results showed an 81% rate of concordance of PD-L1 staining between the primary and node; in about 8% of cases, only the primary was positive, and in about 9%, only the node was positive.
Study results also showed good concordance across the three different antibodies: SP142 (Spring Bioscience), E1L3N (Cell Signaling Technology), and 28-8 (Dako). In 76% of cases, all three antibodies were in agreement.
In some cases, clusters of infiltrating macrophages stained for PD-L1. “This could represent a possible pitfall, especially using a cutoff as low as 1%, although with some practice, one can appreciate that the staining of macrophages is somewhat different than the crisp membranous staining seen in tumor cells,” Dr. Sheffield.
“PD-L1 IHC is feasible and it can be done in your laboratory. There are small but very relevant differences in testing primary tumor tissue and lymph node metastasis, and that will need to be explored with a bias toward testing more, not less, tissue,” he concluded. “Multiple methods for PD-L1 IHC appear to be equivalent, and we should be able to have some freedom in choosing the best PD-L1 IHC assay for our own laboratories.”
In the second study, Dr. Paul Mitchell of Austin Health, Melbourne, and colleagues assessed PD-L1 staining among 433 sequential patients who underwent resection of primary NSCLC between 1992 and 2010. Bristol-Myers Squibb performed the IHC staining and some of the scoring.
In one set of assays, the investigators used the 28.8 antibody and considered tumors to be positive if at least 5% of cells showed membranous staining of any intensity.
Results here showed that 28% of primaries were PD-L1 positive. The rate was similar for men and women, but higher in squamous tumors than in adenocarcinomas (39% vs. 18%). It was merely 14% in patients having an epidermal growth factor receptor (EGFR) mutation. The rate of positivity increased with the number of pack-years of smoking and, starting 5 years after cessation, decreased over time.
A total of 57 patients had paired primary tumor and metastatic tumor (most commonly from brain metastases). The median time from primary to metastasis was 1.3 years; in eight patients, metastasis was synchronous.
In this cohort, there was a 77% rate of concordance of PD-L1 staining between the primary and metastasis (r = 0.37, P = .0049); in 11% of cases, only the primary was positive, and in 12%, only the metastasis was positive. Also, among the eight patients having multiple metastases, all samples were concordant in six patients.
In another set of assays, the investigators used the E11340 XP antibody (Cell Signaling Technology) and considered tumors to be strongly positive if more than 50% of cells stained with intensity of 2 or higher (ASCO 2015, abstract 11051).
Here, 24% of primaries were strongly positive. In a multivariate analysis, patients with stage III disease who had high PD-L1 expression in both the primary and node had better disease-free survival (hazard ratio, 0.49; P = .031) and overall survival (HR, 0.46; P = .006).
Among the 53 patients who had paired primary and nodal tumor, PD-L1 staining was concordant in these sites in 89% of patients.
“These data do suggest that PD-L1 status in general in the primary NSCLC predicts the PD-L1 status in metastases as well as in the nodes,” Dr. Mitchell said. “However, if the PD-L1 expression status is critical in the decision to treat metastatic NSCLC with a PD-1 pathway inhibitor, then rebiopsy of a metastasis may be warranted,” he added.
Dr. Sheffield reported that he had no relevant disclosures; Bristol-Myers Squibb Canada performed some of the IHC staining. Dr. Mitchell disclosed ties with AstraZeneca, Roche, Boehringer-Ingelheim, Bristol-Myers Squibb, and MSD. Bristol-Myers Squibb performed IHC staining and some of the scoring.
The conference was sponsored by the International Association for the Study of Lung Cancer.
AT THE IASLC WORLD CONFERENCE
Key clinical point: PD-L1 status of the primary in NSCLC generally predicts that of nodal tumor and metastases.
Major finding: The rate of concordance for PD-L1 staining comparing primary with lymph nodes was 81%-89% and comparing primary with metastasis was 77%.
Data source: A pair of retrospective cohort studies of 78 patients with nonsquamous NSCLC and 433 patients with NSCLC.
Disclosures: Dr. Sheffield reported that he had no relevant disclosures; Bristol-Myers Squibb Canada performed some of the IHC staining. Dr. Mitchell disclosed ties with AstraZeneca, Roche, Boehringer-Ingelheim, Bristol-Myers Squibb, and MSD. Bristol-Myers Squibb performed IHC staining and some of the scoring.
Nipple-sparing mastectomy feasible in N+ early breast cancer
SAN FRANCISCO – Nipple-sparing mastectomy can be a safe surgical option for carefully selected patients with node-positive breast cancers, investigators reported at the 2015 ASCO Breast Cancer Symposium.
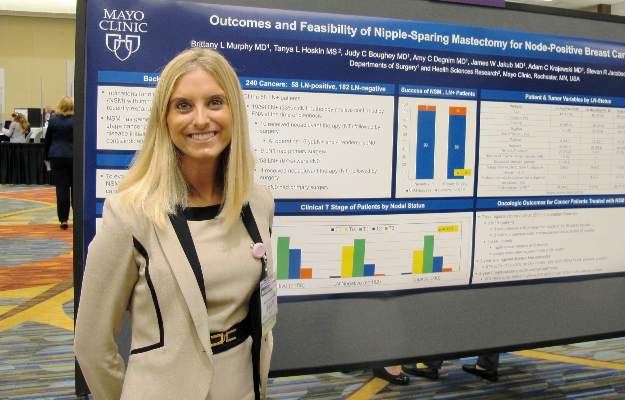
In a series of 226 patients with a total of 240 breast tumors, there was no significant difference between patients with node-positive or node-negative disease in the rate of conversion from a planned nipple-sparing procedure to a skin-sparing procedure, reported Dr. Brittany L. Murphy, a general surgery resident, and her colleagues at the Mayo Clinic in Rochester, Minn.
“Among women with node-positive breast cancer, nipple-sparing surgery may be appropriate for patients who do not have T4 or inflammatory carcinoma, patients that do not have multifocal disease, and who clinically and on imaging modalities do not appear to have nipple involvement,” Dr. Murphy said in an interview at the symposium.
In general, surgeons consider nipple-sparing mastectomies to be most appropriate for patients with early-stage disease; nodal involvement is often considered a contraindication, she said.
To see whether nipple-sparing surgery could be safely performed in patients with node-positive disease, the researchers took a retrospective look at data on 226 patients (14 with bilateral cancers) scheduled for nipple-sparing mastectomy at their center from 2009 through 2014.
In all, 182 of the cancers were lymph-node negative, and 58 were positive. Of the 58 node-positive cases, 27 (47%) were T2/T3 tumors, compared with 31 of 182 (17%) node-negative cases (P less than .0001). There were no significant differences between the groups in either estrogen receptor or HER2 receptor positivity, however.
Of the node-positive cases, 19 (33%) had cN1 (clinical) nodal involvement with pathology confirmed by fine-needle aspiration at the time of diagnosis, and 10 of these patients underwent neoadjuvant therapy followed by surgery. At the time of surgery, 6 of the 10 had pathologically confirmed positivity, and 4 were found to have ypN0 status. The remaining 9 patients in this group went on to primary surgery without neoadjuvant therapy.
Of the 39 patients who were clinically node negative, 4 had neoadjuvant therapy followed by surgery, and 35 went on to primary surgery.
The nipple-sparing procedure was successfully performed in 13 of the 14 node-positive patients who received neoadjuvant therapy and in 39 of 44 node-positive patients who went on to primary surgery. Six of the node-positive patients required conversion to a skin-sparing technique, either at the time of initial surgery based on frozen section pathology (five patients) or at a second procedure (one patient).
There were a total of seven locoregional recurrences among all patients treated with nipple-sparing mastectomy, including five in node-positive patients and two in node-negative patients. This difference was not significant.
Among the node-positive patients, three of the recurrences were in subcutaneous flaps away from the nipple at 13, 30, and 46 months of follow-up. In two cases, involved ipsilateral supraclavicular and mediastinal lymph nodes were detected at 24 and 32 months.
In the node-negative patients, one recurrence was in the nipple-areolar complex at 82 months, and one was in axillary nodes after negative sentinel lymph node biopsy at 20 months.
The 3-year locoregional disease-free estimates were 87% for lymph node positive patients compared with 99% for node negative patients (P = .007).
There were no differences between the groups regarding 3-year breast cancer–specific survival estimates, at 97% for lymph-node positive patients, and 99% for node-negative patients.
“Short-term oncologic outcomes were satisfactory. These data suggest that nipple-sparing mastectomy may be appropriate for carefully selected lymph node-positive breast cancer patients,” Dr, Murphy and colleagues wrote in a poster presented at the symposium.
SAN FRANCISCO – Nipple-sparing mastectomy can be a safe surgical option for carefully selected patients with node-positive breast cancers, investigators reported at the 2015 ASCO Breast Cancer Symposium.
In a series of 226 patients with a total of 240 breast tumors, there was no significant difference between patients with node-positive or node-negative disease in the rate of conversion from a planned nipple-sparing procedure to a skin-sparing procedure, reported Dr. Brittany L. Murphy, a general surgery resident, and her colleagues at the Mayo Clinic in Rochester, Minn.
“Among women with node-positive breast cancer, nipple-sparing surgery may be appropriate for patients who do not have T4 or inflammatory carcinoma, patients that do not have multifocal disease, and who clinically and on imaging modalities do not appear to have nipple involvement,” Dr. Murphy said in an interview at the symposium.
In general, surgeons consider nipple-sparing mastectomies to be most appropriate for patients with early-stage disease; nodal involvement is often considered a contraindication, she said.
To see whether nipple-sparing surgery could be safely performed in patients with node-positive disease, the researchers took a retrospective look at data on 226 patients (14 with bilateral cancers) scheduled for nipple-sparing mastectomy at their center from 2009 through 2014.
In all, 182 of the cancers were lymph-node negative, and 58 were positive. Of the 58 node-positive cases, 27 (47%) were T2/T3 tumors, compared with 31 of 182 (17%) node-negative cases (P less than .0001). There were no significant differences between the groups in either estrogen receptor or HER2 receptor positivity, however.
Of the node-positive cases, 19 (33%) had cN1 (clinical) nodal involvement with pathology confirmed by fine-needle aspiration at the time of diagnosis, and 10 of these patients underwent neoadjuvant therapy followed by surgery. At the time of surgery, 6 of the 10 had pathologically confirmed positivity, and 4 were found to have ypN0 status. The remaining 9 patients in this group went on to primary surgery without neoadjuvant therapy.
Of the 39 patients who were clinically node negative, 4 had neoadjuvant therapy followed by surgery, and 35 went on to primary surgery.
The nipple-sparing procedure was successfully performed in 13 of the 14 node-positive patients who received neoadjuvant therapy and in 39 of 44 node-positive patients who went on to primary surgery. Six of the node-positive patients required conversion to a skin-sparing technique, either at the time of initial surgery based on frozen section pathology (five patients) or at a second procedure (one patient).
There were a total of seven locoregional recurrences among all patients treated with nipple-sparing mastectomy, including five in node-positive patients and two in node-negative patients. This difference was not significant.
Among the node-positive patients, three of the recurrences were in subcutaneous flaps away from the nipple at 13, 30, and 46 months of follow-up. In two cases, involved ipsilateral supraclavicular and mediastinal lymph nodes were detected at 24 and 32 months.
In the node-negative patients, one recurrence was in the nipple-areolar complex at 82 months, and one was in axillary nodes after negative sentinel lymph node biopsy at 20 months.
The 3-year locoregional disease-free estimates were 87% for lymph node positive patients compared with 99% for node negative patients (P = .007).
There were no differences between the groups regarding 3-year breast cancer–specific survival estimates, at 97% for lymph-node positive patients, and 99% for node-negative patients.
“Short-term oncologic outcomes were satisfactory. These data suggest that nipple-sparing mastectomy may be appropriate for carefully selected lymph node-positive breast cancer patients,” Dr, Murphy and colleagues wrote in a poster presented at the symposium.
SAN FRANCISCO – Nipple-sparing mastectomy can be a safe surgical option for carefully selected patients with node-positive breast cancers, investigators reported at the 2015 ASCO Breast Cancer Symposium.
In a series of 226 patients with a total of 240 breast tumors, there was no significant difference between patients with node-positive or node-negative disease in the rate of conversion from a planned nipple-sparing procedure to a skin-sparing procedure, reported Dr. Brittany L. Murphy, a general surgery resident, and her colleagues at the Mayo Clinic in Rochester, Minn.
“Among women with node-positive breast cancer, nipple-sparing surgery may be appropriate for patients who do not have T4 or inflammatory carcinoma, patients that do not have multifocal disease, and who clinically and on imaging modalities do not appear to have nipple involvement,” Dr. Murphy said in an interview at the symposium.
In general, surgeons consider nipple-sparing mastectomies to be most appropriate for patients with early-stage disease; nodal involvement is often considered a contraindication, she said.
To see whether nipple-sparing surgery could be safely performed in patients with node-positive disease, the researchers took a retrospective look at data on 226 patients (14 with bilateral cancers) scheduled for nipple-sparing mastectomy at their center from 2009 through 2014.
In all, 182 of the cancers were lymph-node negative, and 58 were positive. Of the 58 node-positive cases, 27 (47%) were T2/T3 tumors, compared with 31 of 182 (17%) node-negative cases (P less than .0001). There were no significant differences between the groups in either estrogen receptor or HER2 receptor positivity, however.
Of the node-positive cases, 19 (33%) had cN1 (clinical) nodal involvement with pathology confirmed by fine-needle aspiration at the time of diagnosis, and 10 of these patients underwent neoadjuvant therapy followed by surgery. At the time of surgery, 6 of the 10 had pathologically confirmed positivity, and 4 were found to have ypN0 status. The remaining 9 patients in this group went on to primary surgery without neoadjuvant therapy.
Of the 39 patients who were clinically node negative, 4 had neoadjuvant therapy followed by surgery, and 35 went on to primary surgery.
The nipple-sparing procedure was successfully performed in 13 of the 14 node-positive patients who received neoadjuvant therapy and in 39 of 44 node-positive patients who went on to primary surgery. Six of the node-positive patients required conversion to a skin-sparing technique, either at the time of initial surgery based on frozen section pathology (five patients) or at a second procedure (one patient).
There were a total of seven locoregional recurrences among all patients treated with nipple-sparing mastectomy, including five in node-positive patients and two in node-negative patients. This difference was not significant.
Among the node-positive patients, three of the recurrences were in subcutaneous flaps away from the nipple at 13, 30, and 46 months of follow-up. In two cases, involved ipsilateral supraclavicular and mediastinal lymph nodes were detected at 24 and 32 months.
In the node-negative patients, one recurrence was in the nipple-areolar complex at 82 months, and one was in axillary nodes after negative sentinel lymph node biopsy at 20 months.
The 3-year locoregional disease-free estimates were 87% for lymph node positive patients compared with 99% for node negative patients (P = .007).
There were no differences between the groups regarding 3-year breast cancer–specific survival estimates, at 97% for lymph-node positive patients, and 99% for node-negative patients.
“Short-term oncologic outcomes were satisfactory. These data suggest that nipple-sparing mastectomy may be appropriate for carefully selected lymph node-positive breast cancer patients,” Dr, Murphy and colleagues wrote in a poster presented at the symposium.
AT THE 2015 ASCO BREAST CANCER SYMPOSIUM
Key clinical point: Some women with lymph-node positive early breast cancer may safely undergo nipple-sparing mastectomy.
Major finding: Three-year breast cancer-specific survival estimates were 97% for node positive patients and 99% for node-negative patients.
Data source: Retrospective review of data on 226 women with 240 early breast cancers.
Disclosures: The study was institutionally supported. Dr. Murphy reported no conflicts of interest.
Positive lumpectomy margin risk rises with breast density
SAN FRANCISCO – Breast density is an independent risk factor for positive lumpectomy margins, pointing to a need for better methods of intraoperative margin assessment, researchers contend.
Data from a randomized clinical trial evaluating intraoperative tumor margin detection techniques indicate that for every increase in breast density category, the risk of positive margins on the main lumpectomy specimen increases by 46%, reported Dr. Tanir Allweis of Hebrew University Medical Center in Jerusalem, Israel.
“The use of newer technology and advanced techniques for intraoperative margin assessment, more extensive preop evaluation of these patients with MRI, or more liberal reshaving during the time of initial lumpectomy might be able to decrease the rate of reoperations in women with dense breasts,” Dr. Allweis said in an interview at the 2015 ASCO Breast Cancer Symposium.
She and colleague Dr. Freya Schnabel of New York University Langone Medical Center in New York City reviewed data on women enrolled in a clinical trial in which patients were randomized to lumpectomy with standard margin assessment or the use of an intraoperative radiofrequency spectroscopy device (MarginProbe). Of the 664 women enrolled in the trial, information on breast density was available for 450, and these women were included in the current study.The authors looked at data on breast density, patient and tumor characteristics, and the margin status of the primary lumpectomy specimen prior to randomization (that is, before the use of the device or the surgeon’s customary margin assessment technique).
They defined positive margins as ink on tumor. Breast density was rated on a scale of 1 (mostly fatty) to 4 (extremely dense) according to the American College of Radiology BI-RADS breast density descriptors.
Higher breast density was associated with younger age at diagnosis, lower body mass index, smaller breasts, and smaller specimen volume. Women with dense breasts were more likely to have had preoperative MRI (odds ratio [OR] 2. P less than .0001).
Each increase in breast density category was associated with an OR of 1.46 for positive margins in the main lumpectomy specimen. Thus, while women with mostly fatty breasts had a 14% risk for positive margins, women with extremely dense breasts had a 40% risk for positive margins.
The association between breast density and margin positivity remained significant after the researchers controlled for age, BMI, breast volume, and specimen volume (adjusted OR 1.39-1.52, P less than .036).
The investigators plan to explore whether the tumors in denser breasts may be larger than initially suspected because of the documented difficulties in imaging extremely dense tissues.
“These results suggest that the use of adjunctive methods for intraoperative margin assessment may be particularly helpful in this patient population. Further research will be important to clarify the benefit of various methods to decrease the rate for reexcision procedures in patients with increased breast density,” the investigators wrote in a poster presented at the symposium.
SAN FRANCISCO – Breast density is an independent risk factor for positive lumpectomy margins, pointing to a need for better methods of intraoperative margin assessment, researchers contend.
Data from a randomized clinical trial evaluating intraoperative tumor margin detection techniques indicate that for every increase in breast density category, the risk of positive margins on the main lumpectomy specimen increases by 46%, reported Dr. Tanir Allweis of Hebrew University Medical Center in Jerusalem, Israel.
“The use of newer technology and advanced techniques for intraoperative margin assessment, more extensive preop evaluation of these patients with MRI, or more liberal reshaving during the time of initial lumpectomy might be able to decrease the rate of reoperations in women with dense breasts,” Dr. Allweis said in an interview at the 2015 ASCO Breast Cancer Symposium.
She and colleague Dr. Freya Schnabel of New York University Langone Medical Center in New York City reviewed data on women enrolled in a clinical trial in which patients were randomized to lumpectomy with standard margin assessment or the use of an intraoperative radiofrequency spectroscopy device (MarginProbe). Of the 664 women enrolled in the trial, information on breast density was available for 450, and these women were included in the current study.The authors looked at data on breast density, patient and tumor characteristics, and the margin status of the primary lumpectomy specimen prior to randomization (that is, before the use of the device or the surgeon’s customary margin assessment technique).
They defined positive margins as ink on tumor. Breast density was rated on a scale of 1 (mostly fatty) to 4 (extremely dense) according to the American College of Radiology BI-RADS breast density descriptors.
Higher breast density was associated with younger age at diagnosis, lower body mass index, smaller breasts, and smaller specimen volume. Women with dense breasts were more likely to have had preoperative MRI (odds ratio [OR] 2. P less than .0001).
Each increase in breast density category was associated with an OR of 1.46 for positive margins in the main lumpectomy specimen. Thus, while women with mostly fatty breasts had a 14% risk for positive margins, women with extremely dense breasts had a 40% risk for positive margins.
The association between breast density and margin positivity remained significant after the researchers controlled for age, BMI, breast volume, and specimen volume (adjusted OR 1.39-1.52, P less than .036).
The investigators plan to explore whether the tumors in denser breasts may be larger than initially suspected because of the documented difficulties in imaging extremely dense tissues.
“These results suggest that the use of adjunctive methods for intraoperative margin assessment may be particularly helpful in this patient population. Further research will be important to clarify the benefit of various methods to decrease the rate for reexcision procedures in patients with increased breast density,” the investigators wrote in a poster presented at the symposium.
SAN FRANCISCO – Breast density is an independent risk factor for positive lumpectomy margins, pointing to a need for better methods of intraoperative margin assessment, researchers contend.
Data from a randomized clinical trial evaluating intraoperative tumor margin detection techniques indicate that for every increase in breast density category, the risk of positive margins on the main lumpectomy specimen increases by 46%, reported Dr. Tanir Allweis of Hebrew University Medical Center in Jerusalem, Israel.
“The use of newer technology and advanced techniques for intraoperative margin assessment, more extensive preop evaluation of these patients with MRI, or more liberal reshaving during the time of initial lumpectomy might be able to decrease the rate of reoperations in women with dense breasts,” Dr. Allweis said in an interview at the 2015 ASCO Breast Cancer Symposium.
She and colleague Dr. Freya Schnabel of New York University Langone Medical Center in New York City reviewed data on women enrolled in a clinical trial in which patients were randomized to lumpectomy with standard margin assessment or the use of an intraoperative radiofrequency spectroscopy device (MarginProbe). Of the 664 women enrolled in the trial, information on breast density was available for 450, and these women were included in the current study.The authors looked at data on breast density, patient and tumor characteristics, and the margin status of the primary lumpectomy specimen prior to randomization (that is, before the use of the device or the surgeon’s customary margin assessment technique).
They defined positive margins as ink on tumor. Breast density was rated on a scale of 1 (mostly fatty) to 4 (extremely dense) according to the American College of Radiology BI-RADS breast density descriptors.
Higher breast density was associated with younger age at diagnosis, lower body mass index, smaller breasts, and smaller specimen volume. Women with dense breasts were more likely to have had preoperative MRI (odds ratio [OR] 2. P less than .0001).
Each increase in breast density category was associated with an OR of 1.46 for positive margins in the main lumpectomy specimen. Thus, while women with mostly fatty breasts had a 14% risk for positive margins, women with extremely dense breasts had a 40% risk for positive margins.
The association between breast density and margin positivity remained significant after the researchers controlled for age, BMI, breast volume, and specimen volume (adjusted OR 1.39-1.52, P less than .036).
The investigators plan to explore whether the tumors in denser breasts may be larger than initially suspected because of the documented difficulties in imaging extremely dense tissues.
“These results suggest that the use of adjunctive methods for intraoperative margin assessment may be particularly helpful in this patient population. Further research will be important to clarify the benefit of various methods to decrease the rate for reexcision procedures in patients with increased breast density,” the investigators wrote in a poster presented at the symposium.
AT THE 2015 ASCO BREAST CANCER SYMPOSIUM
Key clinical point: The risk for positive lumpectomy margins rises as breast density increases.
Major finding: .Women with extremely dense breasts had a 40% risk for ink on tumor in the main lumpectomy specimen.
Data source: Review of data on 450 of 664 women enrolled in a randomized clinical trial.
Disclosures: Dune Medical Devices supported the trial. Dr. Allweis and Dr. Schnabel reported no conflicts of interest.
Two heads plus four hands equal safe, shorter bilateral mastectomies
SAN FRANCISCO – Two surgeons working in tandem, one on each side of the patient, can significantly reduce the total operative time for bilateral mastectomies without compromising safety or quality of results, according to a study presented at the 2015 ASCO Breast Cancer Symposium.
A review of records on consecutive cases of bilateral mastectomy with tissue expander reconstruction (BMTR) showed that mean overall surgery time (start of incision to closure) was about 23 minutes shorter when two surgeons were working at once, and mean general surgery time (start of incision to end of mastectomy procedure) was 41 minutes shorter with two surgeons vs. a solo operator, reported Dr. Suniti Nimbkar of the department of surgery at Brigham and Women’s Hospital, Boston.
“We did find that there was a significant reduction in time when two surgeons work together, and that reduction is particularly emphasized when you have a patient who is larger, perhaps a heavier patient, and when you have to do more extensive surgery,” she said in an interview at the symposium.
Dual surgeons did take slightly longer to perform the reconstructive surgery portion of the procedure, however.
Effective cosurgery is like a healthy marriage or a successful restaurant kitchen, with partners learning how to anticipate each other’s needs, knowing when to lend a hand, and intuitively grasping when to get out of the way, Dr. Nimbkar suggested.
“As two surgeons work together more and more, they just inevitably grow together in how they operate. I know for example that my direct partner and I operate very similarly, whereas sometimes if I do a cosurgery with a second surgeon that I don’t always operate with we have to work together to understand each other’s approach,” she said.
The investigators scanned the charts of 116 consecutive women who underwent BMTR done by eight breast surgeons at their center, looking for potential differences in operative time and 30-day postoperative complications. In all, 67 of the procedures were cosurgeries, and 49 were done by a single surgeon.
They found that in bivariate analysis, mean general surgery time was 75.8 minutes for the surgical duos, compared with 116.8 minutes for the solo surgeons (P less than .0001). Overall surgery time was also shorter, at 255.2 minutes vs. 278.3 minutes, respectively (P = .005).
Although there were numerically more complications among patients of cosurgeons, there was no statistically significant difference in postoperative complications rates between the twin and singleton surgeons.
A linear regression model showed that factors significantly associated with general surgery time were cosurgeries (P less than .0001), total breast weight (P =.03) and axillary dissection (P = .0003).
The authors noted that although two surgeons cut operative times significantly, the amount of time savings was not proportional to what they expected.
“We also want to know if the plastic surgeons are happy with the results if there are two different surgeons. Do they feel that there is too much of a difference in the way the mastectomy comes out, or is it something they’re fine with? So one of the next steps we’re going to take is to survey the plastic surgeons as to whether they’re comfortable with what we’re doing or if they have ideas about how we can be more uniform,” Dr. Nimbkar said.
SAN FRANCISCO – Two surgeons working in tandem, one on each side of the patient, can significantly reduce the total operative time for bilateral mastectomies without compromising safety or quality of results, according to a study presented at the 2015 ASCO Breast Cancer Symposium.
A review of records on consecutive cases of bilateral mastectomy with tissue expander reconstruction (BMTR) showed that mean overall surgery time (start of incision to closure) was about 23 minutes shorter when two surgeons were working at once, and mean general surgery time (start of incision to end of mastectomy procedure) was 41 minutes shorter with two surgeons vs. a solo operator, reported Dr. Suniti Nimbkar of the department of surgery at Brigham and Women’s Hospital, Boston.
“We did find that there was a significant reduction in time when two surgeons work together, and that reduction is particularly emphasized when you have a patient who is larger, perhaps a heavier patient, and when you have to do more extensive surgery,” she said in an interview at the symposium.
Dual surgeons did take slightly longer to perform the reconstructive surgery portion of the procedure, however.
Effective cosurgery is like a healthy marriage or a successful restaurant kitchen, with partners learning how to anticipate each other’s needs, knowing when to lend a hand, and intuitively grasping when to get out of the way, Dr. Nimbkar suggested.
“As two surgeons work together more and more, they just inevitably grow together in how they operate. I know for example that my direct partner and I operate very similarly, whereas sometimes if I do a cosurgery with a second surgeon that I don’t always operate with we have to work together to understand each other’s approach,” she said.
The investigators scanned the charts of 116 consecutive women who underwent BMTR done by eight breast surgeons at their center, looking for potential differences in operative time and 30-day postoperative complications. In all, 67 of the procedures were cosurgeries, and 49 were done by a single surgeon.
They found that in bivariate analysis, mean general surgery time was 75.8 minutes for the surgical duos, compared with 116.8 minutes for the solo surgeons (P less than .0001). Overall surgery time was also shorter, at 255.2 minutes vs. 278.3 minutes, respectively (P = .005).
Although there were numerically more complications among patients of cosurgeons, there was no statistically significant difference in postoperative complications rates between the twin and singleton surgeons.
A linear regression model showed that factors significantly associated with general surgery time were cosurgeries (P less than .0001), total breast weight (P =.03) and axillary dissection (P = .0003).
The authors noted that although two surgeons cut operative times significantly, the amount of time savings was not proportional to what they expected.
“We also want to know if the plastic surgeons are happy with the results if there are two different surgeons. Do they feel that there is too much of a difference in the way the mastectomy comes out, or is it something they’re fine with? So one of the next steps we’re going to take is to survey the plastic surgeons as to whether they’re comfortable with what we’re doing or if they have ideas about how we can be more uniform,” Dr. Nimbkar said.
SAN FRANCISCO – Two surgeons working in tandem, one on each side of the patient, can significantly reduce the total operative time for bilateral mastectomies without compromising safety or quality of results, according to a study presented at the 2015 ASCO Breast Cancer Symposium.
A review of records on consecutive cases of bilateral mastectomy with tissue expander reconstruction (BMTR) showed that mean overall surgery time (start of incision to closure) was about 23 minutes shorter when two surgeons were working at once, and mean general surgery time (start of incision to end of mastectomy procedure) was 41 minutes shorter with two surgeons vs. a solo operator, reported Dr. Suniti Nimbkar of the department of surgery at Brigham and Women’s Hospital, Boston.
“We did find that there was a significant reduction in time when two surgeons work together, and that reduction is particularly emphasized when you have a patient who is larger, perhaps a heavier patient, and when you have to do more extensive surgery,” she said in an interview at the symposium.
Dual surgeons did take slightly longer to perform the reconstructive surgery portion of the procedure, however.
Effective cosurgery is like a healthy marriage or a successful restaurant kitchen, with partners learning how to anticipate each other’s needs, knowing when to lend a hand, and intuitively grasping when to get out of the way, Dr. Nimbkar suggested.
“As two surgeons work together more and more, they just inevitably grow together in how they operate. I know for example that my direct partner and I operate very similarly, whereas sometimes if I do a cosurgery with a second surgeon that I don’t always operate with we have to work together to understand each other’s approach,” she said.
The investigators scanned the charts of 116 consecutive women who underwent BMTR done by eight breast surgeons at their center, looking for potential differences in operative time and 30-day postoperative complications. In all, 67 of the procedures were cosurgeries, and 49 were done by a single surgeon.
They found that in bivariate analysis, mean general surgery time was 75.8 minutes for the surgical duos, compared with 116.8 minutes for the solo surgeons (P less than .0001). Overall surgery time was also shorter, at 255.2 minutes vs. 278.3 minutes, respectively (P = .005).
Although there were numerically more complications among patients of cosurgeons, there was no statistically significant difference in postoperative complications rates between the twin and singleton surgeons.
A linear regression model showed that factors significantly associated with general surgery time were cosurgeries (P less than .0001), total breast weight (P =.03) and axillary dissection (P = .0003).
The authors noted that although two surgeons cut operative times significantly, the amount of time savings was not proportional to what they expected.
“We also want to know if the plastic surgeons are happy with the results if there are two different surgeons. Do they feel that there is too much of a difference in the way the mastectomy comes out, or is it something they’re fine with? So one of the next steps we’re going to take is to survey the plastic surgeons as to whether they’re comfortable with what we’re doing or if they have ideas about how we can be more uniform,” Dr. Nimbkar said.
AT THE 2015 ASCO BREAST CANCER SYMPOSIUM
Key clinical point:Bilateral mastectomy performed by two surgeons shortens operative time without increasing complications.
Major finding: Mean general surgery time was 75.8 minutes for two surgeons vs. 116.8 minutes for solo surgeons (P less than .0001).
Data source: Retrospective review of records on 116 consecutive patients undergoing bilateral mastectomies.
Disclosures: The study was internally funded. The authors reported no conflicts of interest.
ASCO: Neoadjuvant chemo does not increase breast cancer surgery complications
SAN FRANCISCO – Breast cancer surgery within 30 days of neoadjuvant chemotherapy appears to be safe, with no significant increase in risk of postoperative complications compared with surgery performed in women who did not receive chemotherapy.
An analysis of data on more than 3,500 patients who underwent surgery for invasive breast cancer after receiving neoadjuvant chemotherapy showed that in an analysis adjusted to better balance patient characteristics, there were no significant differences in postoperative complication rates for patients who received chemotherapy and those who did not, reported Dr. Erin Cordeiro from the Ottawa Hospital in Ontario, Canada, and associates, at a breast cancer symposium sponsored jointly by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology.
“I really think this should put to rest the concern that surgeons might have about neoadjuvant chemotherapy particularly because this [study] compressed people within 30 days of the surgery date,” commented Dr. Charles E. Geyer, Jr., from the Virginia Commonwealth University School of Medicine in Richmond. Dr. Geyer was the invited discussant.
The use of neoadjuvant chemotherapy in the treatment of patients with breast cancer continues to increase, growing from 14% in 2006, to 20% in 2011, Dr. Cordeiro said.
But because myelosuppression, particularly neutropenia, is a common side of chemotherapy, surgeons are often concerned that operating too soon after a patient completes a course of chemotherapy could result in increased post-operative complications, she said.
To determine whether neoadjuvant chemotherapy would increase the proportion of overall 30-day postoperative complications among patients undergoing surgery for invasive breast cancer, Dr. Cordeiro drew on the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which contains validated, risk-adjusted surgical outcomes data from more than 600 hospitals in 49 states, Canada, and Europe.
She identified data on 67,685 patients who underwent surgery for invasive breast cancer from 2005 through 2012. Of this group, 3,624 (5.5%) had received neoadjuvant chemotherapy. Investigators excludedpPatients with high-risk comorbidities and those who underwent other surgery concurrent with breast surgery (e.g., oophorectomy).
In unadjusted analysis, the rate of a composite of overall 30-day post-operative complications, the primary outcome, was 4.9% for patients who had undergone neoadjuvant chemotherapy, compared with 3.7% for patients who did not and this difference was significant (P= .0003). The higher rate among patient who had received chemotherapy recently was largely due to infections, Dr. Cordeiro said.
However, in a multivariate analysis adjusted for propensity score – a measure of the probability of receiving neoeadjuvant chemotherapy by age, diabetes, chronic obstructive pulmonary disease, hypertension, bilateral surgery, and/or year of surgery – the differences between patients who underwent neoadjuvant chemo and those who did not vanished (odds ratio 1.16; 95% confidence interval, 0.98-1.36).
Dr. Cordeiro acknowledged that the study was limited by the use of retrospective data, a lack of knowledge of exactly when chemotherapy was stopped in relation to the surgery data, lack of data on which regimens were used, and no information about prophylatic antibiotics or thromboprophylaxis.
The investigators plan to perform additional subgroup analyses to determine whether there might be differences in complication rates in patients who did or did not receive neoadjuvant chemo by type of breast surgery (breast-conserving vs. mastectomy), type of axillary surgery (sentinel node biopsy vs. axillary lymph node dissection) or among patients who undergo immediate reconstruction.
The study was presented as a poster and in an oral abstract session.
SAN FRANCISCO – Breast cancer surgery within 30 days of neoadjuvant chemotherapy appears to be safe, with no significant increase in risk of postoperative complications compared with surgery performed in women who did not receive chemotherapy.
An analysis of data on more than 3,500 patients who underwent surgery for invasive breast cancer after receiving neoadjuvant chemotherapy showed that in an analysis adjusted to better balance patient characteristics, there were no significant differences in postoperative complication rates for patients who received chemotherapy and those who did not, reported Dr. Erin Cordeiro from the Ottawa Hospital in Ontario, Canada, and associates, at a breast cancer symposium sponsored jointly by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology.
“I really think this should put to rest the concern that surgeons might have about neoadjuvant chemotherapy particularly because this [study] compressed people within 30 days of the surgery date,” commented Dr. Charles E. Geyer, Jr., from the Virginia Commonwealth University School of Medicine in Richmond. Dr. Geyer was the invited discussant.
The use of neoadjuvant chemotherapy in the treatment of patients with breast cancer continues to increase, growing from 14% in 2006, to 20% in 2011, Dr. Cordeiro said.
But because myelosuppression, particularly neutropenia, is a common side of chemotherapy, surgeons are often concerned that operating too soon after a patient completes a course of chemotherapy could result in increased post-operative complications, she said.
To determine whether neoadjuvant chemotherapy would increase the proportion of overall 30-day postoperative complications among patients undergoing surgery for invasive breast cancer, Dr. Cordeiro drew on the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which contains validated, risk-adjusted surgical outcomes data from more than 600 hospitals in 49 states, Canada, and Europe.
She identified data on 67,685 patients who underwent surgery for invasive breast cancer from 2005 through 2012. Of this group, 3,624 (5.5%) had received neoadjuvant chemotherapy. Investigators excludedpPatients with high-risk comorbidities and those who underwent other surgery concurrent with breast surgery (e.g., oophorectomy).
In unadjusted analysis, the rate of a composite of overall 30-day post-operative complications, the primary outcome, was 4.9% for patients who had undergone neoadjuvant chemotherapy, compared with 3.7% for patients who did not and this difference was significant (P= .0003). The higher rate among patient who had received chemotherapy recently was largely due to infections, Dr. Cordeiro said.
However, in a multivariate analysis adjusted for propensity score – a measure of the probability of receiving neoeadjuvant chemotherapy by age, diabetes, chronic obstructive pulmonary disease, hypertension, bilateral surgery, and/or year of surgery – the differences between patients who underwent neoadjuvant chemo and those who did not vanished (odds ratio 1.16; 95% confidence interval, 0.98-1.36).
Dr. Cordeiro acknowledged that the study was limited by the use of retrospective data, a lack of knowledge of exactly when chemotherapy was stopped in relation to the surgery data, lack of data on which regimens were used, and no information about prophylatic antibiotics or thromboprophylaxis.
The investigators plan to perform additional subgroup analyses to determine whether there might be differences in complication rates in patients who did or did not receive neoadjuvant chemo by type of breast surgery (breast-conserving vs. mastectomy), type of axillary surgery (sentinel node biopsy vs. axillary lymph node dissection) or among patients who undergo immediate reconstruction.
The study was presented as a poster and in an oral abstract session.
SAN FRANCISCO – Breast cancer surgery within 30 days of neoadjuvant chemotherapy appears to be safe, with no significant increase in risk of postoperative complications compared with surgery performed in women who did not receive chemotherapy.
An analysis of data on more than 3,500 patients who underwent surgery for invasive breast cancer after receiving neoadjuvant chemotherapy showed that in an analysis adjusted to better balance patient characteristics, there were no significant differences in postoperative complication rates for patients who received chemotherapy and those who did not, reported Dr. Erin Cordeiro from the Ottawa Hospital in Ontario, Canada, and associates, at a breast cancer symposium sponsored jointly by the American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology.
“I really think this should put to rest the concern that surgeons might have about neoadjuvant chemotherapy particularly because this [study] compressed people within 30 days of the surgery date,” commented Dr. Charles E. Geyer, Jr., from the Virginia Commonwealth University School of Medicine in Richmond. Dr. Geyer was the invited discussant.
The use of neoadjuvant chemotherapy in the treatment of patients with breast cancer continues to increase, growing from 14% in 2006, to 20% in 2011, Dr. Cordeiro said.
But because myelosuppression, particularly neutropenia, is a common side of chemotherapy, surgeons are often concerned that operating too soon after a patient completes a course of chemotherapy could result in increased post-operative complications, she said.
To determine whether neoadjuvant chemotherapy would increase the proportion of overall 30-day postoperative complications among patients undergoing surgery for invasive breast cancer, Dr. Cordeiro drew on the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP) database, which contains validated, risk-adjusted surgical outcomes data from more than 600 hospitals in 49 states, Canada, and Europe.
She identified data on 67,685 patients who underwent surgery for invasive breast cancer from 2005 through 2012. Of this group, 3,624 (5.5%) had received neoadjuvant chemotherapy. Investigators excludedpPatients with high-risk comorbidities and those who underwent other surgery concurrent with breast surgery (e.g., oophorectomy).
In unadjusted analysis, the rate of a composite of overall 30-day post-operative complications, the primary outcome, was 4.9% for patients who had undergone neoadjuvant chemotherapy, compared with 3.7% for patients who did not and this difference was significant (P= .0003). The higher rate among patient who had received chemotherapy recently was largely due to infections, Dr. Cordeiro said.
However, in a multivariate analysis adjusted for propensity score – a measure of the probability of receiving neoeadjuvant chemotherapy by age, diabetes, chronic obstructive pulmonary disease, hypertension, bilateral surgery, and/or year of surgery – the differences between patients who underwent neoadjuvant chemo and those who did not vanished (odds ratio 1.16; 95% confidence interval, 0.98-1.36).
Dr. Cordeiro acknowledged that the study was limited by the use of retrospective data, a lack of knowledge of exactly when chemotherapy was stopped in relation to the surgery data, lack of data on which regimens were used, and no information about prophylatic antibiotics or thromboprophylaxis.
The investigators plan to perform additional subgroup analyses to determine whether there might be differences in complication rates in patients who did or did not receive neoadjuvant chemo by type of breast surgery (breast-conserving vs. mastectomy), type of axillary surgery (sentinel node biopsy vs. axillary lymph node dissection) or among patients who undergo immediate reconstruction.
The study was presented as a poster and in an oral abstract session.
AT THE ASCO BREAST CANCER SYMPOSIUM
Key clinical point: Neoadjuvant chemotherapy does not appear to increase the risk for 30-day post-operative complications following surgery for invasive breast cancer.
Major finding: The odds ratio for postoperative complications among patients who underwent chemotherapy was 1.16 but was not statistically significant.
Data source: Retrospective review from a prospective surgical outcomes database on 67,685 patients, 3,624 of whom had neoadjuvant chemotherapy.
Disclosures: Funding for the study was not disclosed. Dr. Cordeiro reported having no conflicts of interest. Dr. Geyer reported a consulting or advisory role with Stennion, research funding from Incyte, and travel expenses from Abbvie, AstraZeneca, and Genetech/Roche.
Is Skip N2 metastasis its own category?
So-called “skip metastasis” of lung cancer to the lymph nodes – when the cancer “skips” over the N1 bronchopulmonary or hilar stage to N2 ipsilateral mediastinal metastasis – may be associated with distinct histological characteristics that can further help understand its association with longer survival and better prognosis in advanced resectable lung adenocarcinoma, according to a small study from China.
Researchers at Fudan (Shanghai ) University Cancer Center published their findings online ahead of print for the October issue of the Journal of Thoracic and Cardiovascular Surgery (2015 July 6 [doi: 10.1016/j.jtcvs.2015.03.067]). In all, they enrolled 177 patients with N2 adenocarcinoma, 45 (25.4%) of whom had skip N2 metastasis.
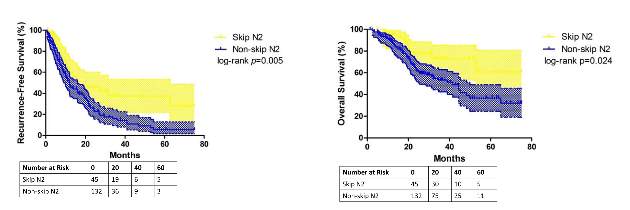
They reported that patients with skip metastasis had considerably better 5-year recurrence-free survival rates of 37.4% vs. 5.7% and better overall survival rates of 60.7% vs. 32.1% when compared with those with non-skip involvement.
“There are distinct differences in clinicopathological features and prognosis in patients with or without skip N2 metastasis,” Dr. Haiquan Chen and his colleagues said. “Considering the results of our study, subclassifications of mediastinal lymph nodes metastases would have potential clinical significance for patients with lung adenocarcinoma.”
Dr. Chen and his colleagues sought to identify specific histological features that characterized the association between skip N2 metastasis and adenocarcinoma subtypes and prognosis. “Skip N2 patients have more cases that are acinar adenocarcinoma subtype, well differentiated and located in the right lung than [do] non-skip patients,” they said.
In fact, they found the predictive value of skip N2 was more significant in patients with right-lung disease, with 5-year recurrence-free survival of 36.6% vs. 0% and overall survival of 57.2% vs. 28% in non–right-lung lesions. They also reported that tumor size of 3 cm or smaller in skip N2 was associated with significantly improved survival rates – 43% vs. 6.7% recurrence-free survival and 74.6% vs. 27.6% for overall survival, compared with patients with larger tumors.
The skip N2 lung adenocarcinoma patients had “remarkably lower incidence” of vascular invasion of the lymph nodes, Dr. Chen and his coauthors wrote. Skip N2 patients also had lower, but not statistically significant, rates of pleural invasion. The Fudan University researchers also reported that the incidence of non-skip N2 metastasis was “significantly high” in patients with papillary-predominant subtype.
“Considering our results, skip N2 should not be recognized as [a] predictor for better survival in all lung adenocarcinoma cases, but in [a] more specific group of patients,” Dr. Chen and his coauthors said.
A multivariate analysis confirmed the predictive significance of skip N2 for recurrence-free survival, but not so much for overall survival. Single N2 metastasis was also an independent predictor for better recurrence-free and overall survival, Dr. Chen and his colleagues said.
The study received funding from the Key Construction Program of the National “985” Project, Ministry of Science and Technology of China; the National Natural Science Foundation of China; the Science and Technology Commission of Shanghai Municipality; and Shanghai Hospital Development Center.
The authors had no disclosures.
“Perhaps the most interesting aspect of the study by Chen and colleagues is the novel observation that skip metastases seem to correlate with acinar histological subtype of lung adenocarcinoma,” Dr. Valerie Rusch of Memorial Sloan Kettering Cancer Center, New York, said in her invited commentary (J Thorac Cardiovasc Surg. 2015 May 8 [doi: 10.1016/j.jtcvs.2015.04.051]) .
“This nicely performed study adds to the evidence that [non–small cell lung cancer) with skip metastases are a distinct subset of stage IIIa disease,” she said.
Dr. Rusch noted that when the International Association for the Study of Lung Cancer (IASLC) revised its lung cancer staging system in 2007 (J Thorac Oncol. 2007;2:603-12), a report for which she served as lead author, it considered giving non–small cell lung cancer with skip metastases its own category. However, the authors decided not to do so because of the small numbers of patients who fall into the category.
In the updated histological classification for adenocarcinoma in 2011 from IASLC, along with the American Thoracic Society and European Respiratory Society (J Thorac Oncol. 2011;6[2]:244-85) , papillary and acinar-predominant adenocarcinomas appear to be associated with similar outcomes. However, the Fudan (Shanghai) University researchers suggest “that there may be some important differences between the two subtypes,” Dr. Rusch said.
Because the study population was so small, the results cannot be considered “definitive,” Dr. Rusch said. “In this era of increasingly high throughput molecular medicine, future, much larger-scale analyses are needed to prove or refute these initial results.”
“Perhaps the most interesting aspect of the study by Chen and colleagues is the novel observation that skip metastases seem to correlate with acinar histological subtype of lung adenocarcinoma,” Dr. Valerie Rusch of Memorial Sloan Kettering Cancer Center, New York, said in her invited commentary (J Thorac Cardiovasc Surg. 2015 May 8 [doi: 10.1016/j.jtcvs.2015.04.051]) .
“This nicely performed study adds to the evidence that [non–small cell lung cancer) with skip metastases are a distinct subset of stage IIIa disease,” she said.
Dr. Rusch noted that when the International Association for the Study of Lung Cancer (IASLC) revised its lung cancer staging system in 2007 (J Thorac Oncol. 2007;2:603-12), a report for which she served as lead author, it considered giving non–small cell lung cancer with skip metastases its own category. However, the authors decided not to do so because of the small numbers of patients who fall into the category.
In the updated histological classification for adenocarcinoma in 2011 from IASLC, along with the American Thoracic Society and European Respiratory Society (J Thorac Oncol. 2011;6[2]:244-85) , papillary and acinar-predominant adenocarcinomas appear to be associated with similar outcomes. However, the Fudan (Shanghai) University researchers suggest “that there may be some important differences between the two subtypes,” Dr. Rusch said.
Because the study population was so small, the results cannot be considered “definitive,” Dr. Rusch said. “In this era of increasingly high throughput molecular medicine, future, much larger-scale analyses are needed to prove or refute these initial results.”
“Perhaps the most interesting aspect of the study by Chen and colleagues is the novel observation that skip metastases seem to correlate with acinar histological subtype of lung adenocarcinoma,” Dr. Valerie Rusch of Memorial Sloan Kettering Cancer Center, New York, said in her invited commentary (J Thorac Cardiovasc Surg. 2015 May 8 [doi: 10.1016/j.jtcvs.2015.04.051]) .
“This nicely performed study adds to the evidence that [non–small cell lung cancer) with skip metastases are a distinct subset of stage IIIa disease,” she said.
Dr. Rusch noted that when the International Association for the Study of Lung Cancer (IASLC) revised its lung cancer staging system in 2007 (J Thorac Oncol. 2007;2:603-12), a report for which she served as lead author, it considered giving non–small cell lung cancer with skip metastases its own category. However, the authors decided not to do so because of the small numbers of patients who fall into the category.
In the updated histological classification for adenocarcinoma in 2011 from IASLC, along with the American Thoracic Society and European Respiratory Society (J Thorac Oncol. 2011;6[2]:244-85) , papillary and acinar-predominant adenocarcinomas appear to be associated with similar outcomes. However, the Fudan (Shanghai) University researchers suggest “that there may be some important differences between the two subtypes,” Dr. Rusch said.
Because the study population was so small, the results cannot be considered “definitive,” Dr. Rusch said. “In this era of increasingly high throughput molecular medicine, future, much larger-scale analyses are needed to prove or refute these initial results.”
So-called “skip metastasis” of lung cancer to the lymph nodes – when the cancer “skips” over the N1 bronchopulmonary or hilar stage to N2 ipsilateral mediastinal metastasis – may be associated with distinct histological characteristics that can further help understand its association with longer survival and better prognosis in advanced resectable lung adenocarcinoma, according to a small study from China.
Researchers at Fudan (Shanghai ) University Cancer Center published their findings online ahead of print for the October issue of the Journal of Thoracic and Cardiovascular Surgery (2015 July 6 [doi: 10.1016/j.jtcvs.2015.03.067]). In all, they enrolled 177 patients with N2 adenocarcinoma, 45 (25.4%) of whom had skip N2 metastasis.
They reported that patients with skip metastasis had considerably better 5-year recurrence-free survival rates of 37.4% vs. 5.7% and better overall survival rates of 60.7% vs. 32.1% when compared with those with non-skip involvement.
“There are distinct differences in clinicopathological features and prognosis in patients with or without skip N2 metastasis,” Dr. Haiquan Chen and his colleagues said. “Considering the results of our study, subclassifications of mediastinal lymph nodes metastases would have potential clinical significance for patients with lung adenocarcinoma.”
Dr. Chen and his colleagues sought to identify specific histological features that characterized the association between skip N2 metastasis and adenocarcinoma subtypes and prognosis. “Skip N2 patients have more cases that are acinar adenocarcinoma subtype, well differentiated and located in the right lung than [do] non-skip patients,” they said.
In fact, they found the predictive value of skip N2 was more significant in patients with right-lung disease, with 5-year recurrence-free survival of 36.6% vs. 0% and overall survival of 57.2% vs. 28% in non–right-lung lesions. They also reported that tumor size of 3 cm or smaller in skip N2 was associated with significantly improved survival rates – 43% vs. 6.7% recurrence-free survival and 74.6% vs. 27.6% for overall survival, compared with patients with larger tumors.
The skip N2 lung adenocarcinoma patients had “remarkably lower incidence” of vascular invasion of the lymph nodes, Dr. Chen and his coauthors wrote. Skip N2 patients also had lower, but not statistically significant, rates of pleural invasion. The Fudan University researchers also reported that the incidence of non-skip N2 metastasis was “significantly high” in patients with papillary-predominant subtype.
“Considering our results, skip N2 should not be recognized as [a] predictor for better survival in all lung adenocarcinoma cases, but in [a] more specific group of patients,” Dr. Chen and his coauthors said.
A multivariate analysis confirmed the predictive significance of skip N2 for recurrence-free survival, but not so much for overall survival. Single N2 metastasis was also an independent predictor for better recurrence-free and overall survival, Dr. Chen and his colleagues said.
The study received funding from the Key Construction Program of the National “985” Project, Ministry of Science and Technology of China; the National Natural Science Foundation of China; the Science and Technology Commission of Shanghai Municipality; and Shanghai Hospital Development Center.
The authors had no disclosures.
So-called “skip metastasis” of lung cancer to the lymph nodes – when the cancer “skips” over the N1 bronchopulmonary or hilar stage to N2 ipsilateral mediastinal metastasis – may be associated with distinct histological characteristics that can further help understand its association with longer survival and better prognosis in advanced resectable lung adenocarcinoma, according to a small study from China.
Researchers at Fudan (Shanghai ) University Cancer Center published their findings online ahead of print for the October issue of the Journal of Thoracic and Cardiovascular Surgery (2015 July 6 [doi: 10.1016/j.jtcvs.2015.03.067]). In all, they enrolled 177 patients with N2 adenocarcinoma, 45 (25.4%) of whom had skip N2 metastasis.
They reported that patients with skip metastasis had considerably better 5-year recurrence-free survival rates of 37.4% vs. 5.7% and better overall survival rates of 60.7% vs. 32.1% when compared with those with non-skip involvement.
“There are distinct differences in clinicopathological features and prognosis in patients with or without skip N2 metastasis,” Dr. Haiquan Chen and his colleagues said. “Considering the results of our study, subclassifications of mediastinal lymph nodes metastases would have potential clinical significance for patients with lung adenocarcinoma.”
Dr. Chen and his colleagues sought to identify specific histological features that characterized the association between skip N2 metastasis and adenocarcinoma subtypes and prognosis. “Skip N2 patients have more cases that are acinar adenocarcinoma subtype, well differentiated and located in the right lung than [do] non-skip patients,” they said.
In fact, they found the predictive value of skip N2 was more significant in patients with right-lung disease, with 5-year recurrence-free survival of 36.6% vs. 0% and overall survival of 57.2% vs. 28% in non–right-lung lesions. They also reported that tumor size of 3 cm or smaller in skip N2 was associated with significantly improved survival rates – 43% vs. 6.7% recurrence-free survival and 74.6% vs. 27.6% for overall survival, compared with patients with larger tumors.
The skip N2 lung adenocarcinoma patients had “remarkably lower incidence” of vascular invasion of the lymph nodes, Dr. Chen and his coauthors wrote. Skip N2 patients also had lower, but not statistically significant, rates of pleural invasion. The Fudan University researchers also reported that the incidence of non-skip N2 metastasis was “significantly high” in patients with papillary-predominant subtype.
“Considering our results, skip N2 should not be recognized as [a] predictor for better survival in all lung adenocarcinoma cases, but in [a] more specific group of patients,” Dr. Chen and his coauthors said.
A multivariate analysis confirmed the predictive significance of skip N2 for recurrence-free survival, but not so much for overall survival. Single N2 metastasis was also an independent predictor for better recurrence-free and overall survival, Dr. Chen and his colleagues said.
The study received funding from the Key Construction Program of the National “985” Project, Ministry of Science and Technology of China; the National Natural Science Foundation of China; the Science and Technology Commission of Shanghai Municipality; and Shanghai Hospital Development Center.
The authors had no disclosures.
Key clinical point: Skip N2 metastases in resectable lung cancer have distinct histological characteristics from non-skip N2 disease.
Major finding: A subset of patients with skip N2 metastasis had higher rates of acinar adenocarcinoma subtype and right-lung disease.
Data source: Retrospective analysis of 177 patients with lung adenocarcinoma and N2 metastasis
Disclosures: The study received funding from the government of China and Shanghai Municipality as well as Shanghai Hospital Development Center. The authors have no relationships to disclose.
Radiation + lumpectomy tied to increased survival in older women with TNBC
Older women with triple-negative breast cancer appear to get an overall survival and disease-specific survival benefit with the addition of radiation to breast-conserving surgery, authors of a retrospective study said.
Among 974 women aged 70 and above with T1-2, N0, M0 triple-negative breast cancer (TNBC; lacking the Her2-neu, estrogen, and progesterone receptors), the addition of radiation to lumpectomy was associated at 23 months’ follow-up with an overall survival (OS) rate of 98.2%, compared with 85.6% for women who received lumpectomy alone (P less than .001). Respective rates of disease-specific survival (DSS) were 99% and 94% (P = .003).
“The use of adjuvant radiation therapy after lumpectomy for elderly women with early-stage TNBC was associated with improved OS and DSS. Noting the potential for selection bias in this study, future prospective study is required to define the management of early-stage triple-negative breast cancer,” wrote Dr. Sean Szjea and colleagues at the University of Texas, Galveston, in a meeting abstract. The study will be presented at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
It’s known that older women with estrogen-receptor positive disease can have good clinical outcomes with lumpectomy and adjuvant therapy alone, but whether adding radiation to breast-conserving surgery in older women with TNBC offers clinical benefit is less certain, the investigators said, prompting them to dive for data into the Surveillance, Epidemiology, and End Results database.
They collected information on 974 women aged 70 or older who underwent lumpectomy for early-stage TNBC with no nodal invasion or metastatic disease from 2010 through 2011. Of this group, 662 (68%) also received radiation therapy.
In addition to determining the OS and DSS rates in the overall population, the investigators conducted multivariate regression modeling controlling for confounding variables, including the use of neoadjuvant chemotherapy, the number of lymph nodes sampled, age, laterality, grade, T stage, the extent of surgery, and the existence of other cancers. They found that the survival benefit for radiation held for both OS (hazard ratio [HR], 0.14; P less than .001) and DSS (HR, 0.14; P = .01).
The authors reported having no relevant financial disclosures.
Older women with triple-negative breast cancer appear to get an overall survival and disease-specific survival benefit with the addition of radiation to breast-conserving surgery, authors of a retrospective study said.
Among 974 women aged 70 and above with T1-2, N0, M0 triple-negative breast cancer (TNBC; lacking the Her2-neu, estrogen, and progesterone receptors), the addition of radiation to lumpectomy was associated at 23 months’ follow-up with an overall survival (OS) rate of 98.2%, compared with 85.6% for women who received lumpectomy alone (P less than .001). Respective rates of disease-specific survival (DSS) were 99% and 94% (P = .003).
“The use of adjuvant radiation therapy after lumpectomy for elderly women with early-stage TNBC was associated with improved OS and DSS. Noting the potential for selection bias in this study, future prospective study is required to define the management of early-stage triple-negative breast cancer,” wrote Dr. Sean Szjea and colleagues at the University of Texas, Galveston, in a meeting abstract. The study will be presented at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
It’s known that older women with estrogen-receptor positive disease can have good clinical outcomes with lumpectomy and adjuvant therapy alone, but whether adding radiation to breast-conserving surgery in older women with TNBC offers clinical benefit is less certain, the investigators said, prompting them to dive for data into the Surveillance, Epidemiology, and End Results database.
They collected information on 974 women aged 70 or older who underwent lumpectomy for early-stage TNBC with no nodal invasion or metastatic disease from 2010 through 2011. Of this group, 662 (68%) also received radiation therapy.
In addition to determining the OS and DSS rates in the overall population, the investigators conducted multivariate regression modeling controlling for confounding variables, including the use of neoadjuvant chemotherapy, the number of lymph nodes sampled, age, laterality, grade, T stage, the extent of surgery, and the existence of other cancers. They found that the survival benefit for radiation held for both OS (hazard ratio [HR], 0.14; P less than .001) and DSS (HR, 0.14; P = .01).
The authors reported having no relevant financial disclosures.
Older women with triple-negative breast cancer appear to get an overall survival and disease-specific survival benefit with the addition of radiation to breast-conserving surgery, authors of a retrospective study said.
Among 974 women aged 70 and above with T1-2, N0, M0 triple-negative breast cancer (TNBC; lacking the Her2-neu, estrogen, and progesterone receptors), the addition of radiation to lumpectomy was associated at 23 months’ follow-up with an overall survival (OS) rate of 98.2%, compared with 85.6% for women who received lumpectomy alone (P less than .001). Respective rates of disease-specific survival (DSS) were 99% and 94% (P = .003).
“The use of adjuvant radiation therapy after lumpectomy for elderly women with early-stage TNBC was associated with improved OS and DSS. Noting the potential for selection bias in this study, future prospective study is required to define the management of early-stage triple-negative breast cancer,” wrote Dr. Sean Szjea and colleagues at the University of Texas, Galveston, in a meeting abstract. The study will be presented at a breast cancer symposium sponsored by the American Society of Clinical Oncology.
It’s known that older women with estrogen-receptor positive disease can have good clinical outcomes with lumpectomy and adjuvant therapy alone, but whether adding radiation to breast-conserving surgery in older women with TNBC offers clinical benefit is less certain, the investigators said, prompting them to dive for data into the Surveillance, Epidemiology, and End Results database.
They collected information on 974 women aged 70 or older who underwent lumpectomy for early-stage TNBC with no nodal invasion or metastatic disease from 2010 through 2011. Of this group, 662 (68%) also received radiation therapy.
In addition to determining the OS and DSS rates in the overall population, the investigators conducted multivariate regression modeling controlling for confounding variables, including the use of neoadjuvant chemotherapy, the number of lymph nodes sampled, age, laterality, grade, T stage, the extent of surgery, and the existence of other cancers. They found that the survival benefit for radiation held for both OS (hazard ratio [HR], 0.14; P less than .001) and DSS (HR, 0.14; P = .01).
The authors reported having no relevant financial disclosures.
FROM THE 2015 ASCO BREAST CANCER SYMPOSIUM
Key clinical point: Radiation added to breast-conserving surgery in women 70 and older with early-stage triple-negative breast cancer was associated with improved survival.
Major finding: Overall survival with radiation and lumpectomy was 98.2%, compared with 85.6% for women who received lumpectomy alone (P less than .001)
Data source: A prospective database review of 974 women aged 70 and older treated from 2010 through 2011.
Disclosures: The authors reported having no relevant financial disclosures.
DCIS recurrences have declined significantly
Recurrence rates of ductal carcinoma in situ (DCIS ) have declined significantly over 3 decades, with the improvements likely because of better screening and pathologic assessment, investigators say.
A retrospective review of 2,996 women who underwent breast-conserving surgery (BCS) for DCIS at Memorial Sloan Kettering Cancer Center in New York from 1978 through 2010 showed that there were 363 recurrences within 5 years of treatment (12%). The 5-year recurrence rate for women treated from 1978 through 1998 was 13.6%, compared with 6.6% for women treated from 1999 through 2010.
The hazard ratio (HR) for more recent treatment was 0.62 (P less than .0001), report Dr. Kimberly J. Van Zee and colleagues at MSKCC in an oral abstract to be presented at the symposium sponsored by the American Society of Clinical Oncology.
The period of treatment remained a significant predictor of lower recurrence after multivariate analysis adjusting for age, family history, radiologic vs. clinical presentation, nuclear grade (nonhigh vs. high), necrosis, number of excisions (two or few vs. three or more), margin status (positive or close margins vs. negative), radiation, and use of endocrine therapy (HR 0.74, P = 0.02).
When the investigators stratified patients by whether they received radiation in addition to BCS, they found that recurrence rates dropped only for those patients who did not receive radiation (HR 0.62, P = 0.003). Among patients who received radiation, recurrence rates did not decline over time (HR 1.13, P = 0.6).
The authors conclude that the lower risk of recurrence over time is only partially explained by changes in clinical practice, such as screening, emphasis on negative surgical margins, and use of adjuvant therapies. The fact that the decline was seen only for women who did not receive radiation suggests that the improvements were not attributable to improvement in radiation therapy techniques. Rather, they may be due to improvements in mammography and in pathologic assessment, the investigators maintain.
“The lower recurrence risk observed for DCIS patients treated in more recent years is important for patient education, especially in view of the widely reported recent increase in use of mastectomy,” they write in the study abstract.
Recurrence rates of ductal carcinoma in situ (DCIS ) have declined significantly over 3 decades, with the improvements likely because of better screening and pathologic assessment, investigators say.
A retrospective review of 2,996 women who underwent breast-conserving surgery (BCS) for DCIS at Memorial Sloan Kettering Cancer Center in New York from 1978 through 2010 showed that there were 363 recurrences within 5 years of treatment (12%). The 5-year recurrence rate for women treated from 1978 through 1998 was 13.6%, compared with 6.6% for women treated from 1999 through 2010.
The hazard ratio (HR) for more recent treatment was 0.62 (P less than .0001), report Dr. Kimberly J. Van Zee and colleagues at MSKCC in an oral abstract to be presented at the symposium sponsored by the American Society of Clinical Oncology.
The period of treatment remained a significant predictor of lower recurrence after multivariate analysis adjusting for age, family history, radiologic vs. clinical presentation, nuclear grade (nonhigh vs. high), necrosis, number of excisions (two or few vs. three or more), margin status (positive or close margins vs. negative), radiation, and use of endocrine therapy (HR 0.74, P = 0.02).
When the investigators stratified patients by whether they received radiation in addition to BCS, they found that recurrence rates dropped only for those patients who did not receive radiation (HR 0.62, P = 0.003). Among patients who received radiation, recurrence rates did not decline over time (HR 1.13, P = 0.6).
The authors conclude that the lower risk of recurrence over time is only partially explained by changes in clinical practice, such as screening, emphasis on negative surgical margins, and use of adjuvant therapies. The fact that the decline was seen only for women who did not receive radiation suggests that the improvements were not attributable to improvement in radiation therapy techniques. Rather, they may be due to improvements in mammography and in pathologic assessment, the investigators maintain.
“The lower recurrence risk observed for DCIS patients treated in more recent years is important for patient education, especially in view of the widely reported recent increase in use of mastectomy,” they write in the study abstract.
Recurrence rates of ductal carcinoma in situ (DCIS ) have declined significantly over 3 decades, with the improvements likely because of better screening and pathologic assessment, investigators say.
A retrospective review of 2,996 women who underwent breast-conserving surgery (BCS) for DCIS at Memorial Sloan Kettering Cancer Center in New York from 1978 through 2010 showed that there were 363 recurrences within 5 years of treatment (12%). The 5-year recurrence rate for women treated from 1978 through 1998 was 13.6%, compared with 6.6% for women treated from 1999 through 2010.
The hazard ratio (HR) for more recent treatment was 0.62 (P less than .0001), report Dr. Kimberly J. Van Zee and colleagues at MSKCC in an oral abstract to be presented at the symposium sponsored by the American Society of Clinical Oncology.
The period of treatment remained a significant predictor of lower recurrence after multivariate analysis adjusting for age, family history, radiologic vs. clinical presentation, nuclear grade (nonhigh vs. high), necrosis, number of excisions (two or few vs. three or more), margin status (positive or close margins vs. negative), radiation, and use of endocrine therapy (HR 0.74, P = 0.02).
When the investigators stratified patients by whether they received radiation in addition to BCS, they found that recurrence rates dropped only for those patients who did not receive radiation (HR 0.62, P = 0.003). Among patients who received radiation, recurrence rates did not decline over time (HR 1.13, P = 0.6).
The authors conclude that the lower risk of recurrence over time is only partially explained by changes in clinical practice, such as screening, emphasis on negative surgical margins, and use of adjuvant therapies. The fact that the decline was seen only for women who did not receive radiation suggests that the improvements were not attributable to improvement in radiation therapy techniques. Rather, they may be due to improvements in mammography and in pathologic assessment, the investigators maintain.
“The lower recurrence risk observed for DCIS patients treated in more recent years is important for patient education, especially in view of the widely reported recent increase in use of mastectomy,” they write in the study abstract.
Key clinical point: Recurrence rates of DCIS in patients treated with breast-conserving surgery declined significantly from 1978 through 2010.
Major finding: The 5-year recurrence rate for women treated from 1978 through 1998 was 13.6%, compared with 6.6% for women treated from 1999 through 2010.
Data source: Retrospective case review of 2,996 women with DCIS treated with breast-conserving surgery with or without radiation.
Disclosures: The funding source was not disclosed. Coauthor Dr. Monica Morrow disclosed receiving honoraria from Genomic Health.
Clinical advances drive lung cancer staging, classification changes
DENVER – The term “precision medicine” can be applied to both clinical care and to pathology, as newly updated staging and classification systems for lung cancer show.
The proposed revised (8th) edition of the TNM staging system for lung cancer gives more weight to tumor size as a prognostic factor, reclassifies some primary tumor (T) descriptors, validates current nodal status (N) descriptors, modifies the definition of some types of metastases (M), and includes additional stages for better prognostic stratification, reported Dr. Ramón Rami-Porta from the Universitari Mútua Terrassa in Barcelona, at a world conference on lung cancer sponsored by the International Association for the Study of Lung Cancer.
Similarly, the updated World Health Organization (WHO) Classification of Lung Tumors, described by Dr. William D. Travis from the Memorial Sloan Kettering Cancer Center in New York, incorporates knowledge gained from immunohistochemistry and molecular testing for common genetic mutations into recommendations for treating the specific clinical circumstances of patients with lung cancer.
WHO’s Next
“The 2015 WHO Classification captures a remarkable decade of advances in every lung cancer specialty, from pathology – including histology, cytology, immunohistochemistry, genetics – to oncology, surgery, radiology, and epidemiology. The rapid expansion of immunohistochemical and molecular tools has had a profound impact on how we were able to reclassify a number of tumors, in addition to how we were able to contribute to improvement of subtyping of lung cancers, particularly non–small cell lung cancer,” Dr. Travis said at a media briefing following his discussion of the new classification at a plenary session.
The changes are expected to improve clinical management of patients with advanced lung cancer by clarifying criteria and terminology for small biopsies and cytology, establishing more accurate histologic subtyping, suggesting strategic management of small tissues, and streamlining the work flow for molecular testing. The classification also emphasizes the need for multidisciplinary cooperation among myriad clinicians, he said.
For surgically resected patients, the classification officially recognizes for the first time subsets of non–small cell lung cancer of adenocarcinoma histology with survival rates of 100% (adenocarcinoma in situ), or nearly 100% (minimally invasive adenocarcinoma).
Among the major changes that will affect the diagnosis of surgically resected patients are the adoption of the 2011 IASLC/ATS/ERS Lung Adenocarcinoma Classification, restriction of a diagnosis of large cell carcinoma to tumors lacking clear differentiation by both immunohistochemistry and morphology, reclassifying of squamous cancers into keratinizing, nonkeratinizing, and basaloid subtypes with elimination of clear cell, small cell, and papillary subtypes. Neuroendocrine subtypes are grouped together, but their classification otherwise remains largely unchanged.
The revised classification is expected to improve prediction of survival and recurrence, predict whether a patient is likely to have a survival benefit with platinum-based chemotherapy, allow radiologic pathologic correlations, and affects TNM staging by emphasizing solid tumor size (vs. whole tumor size), Dr. Travis said.
TNM Changes
The proposed changes to the TNM tumor staging have been submitted for approval to the American Joint Committee on Cancer and the Union for International Cancer Control.
If adopted, they would represent the first significant changes since the 7th edition’s publication in 2009. The changes are based on data on more than 77,000 patients diagnosed with lung cancer from 1999 through 2010.
The proposed changes are not intended, however, to alter clinical practice, and instead “imply a taxonomic refinement rather than new indications of already established treatment protocols,” Dr. Rami-Porta said.
In some cases, the proposed changes would result in an upgrading of the T stage, while others would result in downgrading. For example, tumors that range in size between 1 and 2 cm, designated T1a in the 7th edition, would be T1b in the 8th edition. Similarly, tumors larger than 2 cm and up to 3 cm would be upgraded from T1b to T1c, those larger than 4 up to 5 would go from T2a to T2b, those larger 5 and up to 7 cm would rise from T2b to T3, and those larger than 7 cm would be reclassified from T3 to T4. Tumors invading the diaphragm would also be upgraded from T3 to T4 under the proposed revisions.
In contrast, tumors with limited invasion of the trachea (bronchus less than 2 cm from the carina) would be downgraded from T3 to T2, as would tumors associated with total atelectasis and/or pneumonitis.
The current N descriptors are adequate for predicting prognosis, the investigators determined, prompting the recommendation to retain them in the new edition.
The investigators propose slight changes to the M descriptors of metastases. Although they found no significant differences in survival found among patients with M1a (metastases within the chest cavity) descriptors, when distant metastases outside the chest cavity (M1b) were assessed by to the number of metastases, they found that patients with tumors with one metastasis in one organ had significantly better outcomes than those who had multiple metastases in one or more organs.
The proposed revision would continue to group in the M1a category cases with pleural/pericardial effusions, contralateral/bilateral lung nodules, contralateral/bilateral pleural nodules, or a combination of multiple parameters. However, single metastatic lesions in a single distant organ would be reclassified as M1b, and multiple lesions in a single organ or multiple lesions in multiple organs would be reclassified as M1c.
DENVER – The term “precision medicine” can be applied to both clinical care and to pathology, as newly updated staging and classification systems for lung cancer show.
The proposed revised (8th) edition of the TNM staging system for lung cancer gives more weight to tumor size as a prognostic factor, reclassifies some primary tumor (T) descriptors, validates current nodal status (N) descriptors, modifies the definition of some types of metastases (M), and includes additional stages for better prognostic stratification, reported Dr. Ramón Rami-Porta from the Universitari Mútua Terrassa in Barcelona, at a world conference on lung cancer sponsored by the International Association for the Study of Lung Cancer.
Similarly, the updated World Health Organization (WHO) Classification of Lung Tumors, described by Dr. William D. Travis from the Memorial Sloan Kettering Cancer Center in New York, incorporates knowledge gained from immunohistochemistry and molecular testing for common genetic mutations into recommendations for treating the specific clinical circumstances of patients with lung cancer.
WHO’s Next
“The 2015 WHO Classification captures a remarkable decade of advances in every lung cancer specialty, from pathology – including histology, cytology, immunohistochemistry, genetics – to oncology, surgery, radiology, and epidemiology. The rapid expansion of immunohistochemical and molecular tools has had a profound impact on how we were able to reclassify a number of tumors, in addition to how we were able to contribute to improvement of subtyping of lung cancers, particularly non–small cell lung cancer,” Dr. Travis said at a media briefing following his discussion of the new classification at a plenary session.
The changes are expected to improve clinical management of patients with advanced lung cancer by clarifying criteria and terminology for small biopsies and cytology, establishing more accurate histologic subtyping, suggesting strategic management of small tissues, and streamlining the work flow for molecular testing. The classification also emphasizes the need for multidisciplinary cooperation among myriad clinicians, he said.
For surgically resected patients, the classification officially recognizes for the first time subsets of non–small cell lung cancer of adenocarcinoma histology with survival rates of 100% (adenocarcinoma in situ), or nearly 100% (minimally invasive adenocarcinoma).
Among the major changes that will affect the diagnosis of surgically resected patients are the adoption of the 2011 IASLC/ATS/ERS Lung Adenocarcinoma Classification, restriction of a diagnosis of large cell carcinoma to tumors lacking clear differentiation by both immunohistochemistry and morphology, reclassifying of squamous cancers into keratinizing, nonkeratinizing, and basaloid subtypes with elimination of clear cell, small cell, and papillary subtypes. Neuroendocrine subtypes are grouped together, but their classification otherwise remains largely unchanged.
The revised classification is expected to improve prediction of survival and recurrence, predict whether a patient is likely to have a survival benefit with platinum-based chemotherapy, allow radiologic pathologic correlations, and affects TNM staging by emphasizing solid tumor size (vs. whole tumor size), Dr. Travis said.
TNM Changes
The proposed changes to the TNM tumor staging have been submitted for approval to the American Joint Committee on Cancer and the Union for International Cancer Control.
If adopted, they would represent the first significant changes since the 7th edition’s publication in 2009. The changes are based on data on more than 77,000 patients diagnosed with lung cancer from 1999 through 2010.
The proposed changes are not intended, however, to alter clinical practice, and instead “imply a taxonomic refinement rather than new indications of already established treatment protocols,” Dr. Rami-Porta said.
In some cases, the proposed changes would result in an upgrading of the T stage, while others would result in downgrading. For example, tumors that range in size between 1 and 2 cm, designated T1a in the 7th edition, would be T1b in the 8th edition. Similarly, tumors larger than 2 cm and up to 3 cm would be upgraded from T1b to T1c, those larger than 4 up to 5 would go from T2a to T2b, those larger 5 and up to 7 cm would rise from T2b to T3, and those larger than 7 cm would be reclassified from T3 to T4. Tumors invading the diaphragm would also be upgraded from T3 to T4 under the proposed revisions.
In contrast, tumors with limited invasion of the trachea (bronchus less than 2 cm from the carina) would be downgraded from T3 to T2, as would tumors associated with total atelectasis and/or pneumonitis.
The current N descriptors are adequate for predicting prognosis, the investigators determined, prompting the recommendation to retain them in the new edition.
The investigators propose slight changes to the M descriptors of metastases. Although they found no significant differences in survival found among patients with M1a (metastases within the chest cavity) descriptors, when distant metastases outside the chest cavity (M1b) were assessed by to the number of metastases, they found that patients with tumors with one metastasis in one organ had significantly better outcomes than those who had multiple metastases in one or more organs.
The proposed revision would continue to group in the M1a category cases with pleural/pericardial effusions, contralateral/bilateral lung nodules, contralateral/bilateral pleural nodules, or a combination of multiple parameters. However, single metastatic lesions in a single distant organ would be reclassified as M1b, and multiple lesions in a single organ or multiple lesions in multiple organs would be reclassified as M1c.
DENVER – The term “precision medicine” can be applied to both clinical care and to pathology, as newly updated staging and classification systems for lung cancer show.
The proposed revised (8th) edition of the TNM staging system for lung cancer gives more weight to tumor size as a prognostic factor, reclassifies some primary tumor (T) descriptors, validates current nodal status (N) descriptors, modifies the definition of some types of metastases (M), and includes additional stages for better prognostic stratification, reported Dr. Ramón Rami-Porta from the Universitari Mútua Terrassa in Barcelona, at a world conference on lung cancer sponsored by the International Association for the Study of Lung Cancer.
Similarly, the updated World Health Organization (WHO) Classification of Lung Tumors, described by Dr. William D. Travis from the Memorial Sloan Kettering Cancer Center in New York, incorporates knowledge gained from immunohistochemistry and molecular testing for common genetic mutations into recommendations for treating the specific clinical circumstances of patients with lung cancer.
WHO’s Next
“The 2015 WHO Classification captures a remarkable decade of advances in every lung cancer specialty, from pathology – including histology, cytology, immunohistochemistry, genetics – to oncology, surgery, radiology, and epidemiology. The rapid expansion of immunohistochemical and molecular tools has had a profound impact on how we were able to reclassify a number of tumors, in addition to how we were able to contribute to improvement of subtyping of lung cancers, particularly non–small cell lung cancer,” Dr. Travis said at a media briefing following his discussion of the new classification at a plenary session.
The changes are expected to improve clinical management of patients with advanced lung cancer by clarifying criteria and terminology for small biopsies and cytology, establishing more accurate histologic subtyping, suggesting strategic management of small tissues, and streamlining the work flow for molecular testing. The classification also emphasizes the need for multidisciplinary cooperation among myriad clinicians, he said.
For surgically resected patients, the classification officially recognizes for the first time subsets of non–small cell lung cancer of adenocarcinoma histology with survival rates of 100% (adenocarcinoma in situ), or nearly 100% (minimally invasive adenocarcinoma).
Among the major changes that will affect the diagnosis of surgically resected patients are the adoption of the 2011 IASLC/ATS/ERS Lung Adenocarcinoma Classification, restriction of a diagnosis of large cell carcinoma to tumors lacking clear differentiation by both immunohistochemistry and morphology, reclassifying of squamous cancers into keratinizing, nonkeratinizing, and basaloid subtypes with elimination of clear cell, small cell, and papillary subtypes. Neuroendocrine subtypes are grouped together, but their classification otherwise remains largely unchanged.
The revised classification is expected to improve prediction of survival and recurrence, predict whether a patient is likely to have a survival benefit with platinum-based chemotherapy, allow radiologic pathologic correlations, and affects TNM staging by emphasizing solid tumor size (vs. whole tumor size), Dr. Travis said.
TNM Changes
The proposed changes to the TNM tumor staging have been submitted for approval to the American Joint Committee on Cancer and the Union for International Cancer Control.
If adopted, they would represent the first significant changes since the 7th edition’s publication in 2009. The changes are based on data on more than 77,000 patients diagnosed with lung cancer from 1999 through 2010.
The proposed changes are not intended, however, to alter clinical practice, and instead “imply a taxonomic refinement rather than new indications of already established treatment protocols,” Dr. Rami-Porta said.
In some cases, the proposed changes would result in an upgrading of the T stage, while others would result in downgrading. For example, tumors that range in size between 1 and 2 cm, designated T1a in the 7th edition, would be T1b in the 8th edition. Similarly, tumors larger than 2 cm and up to 3 cm would be upgraded from T1b to T1c, those larger than 4 up to 5 would go from T2a to T2b, those larger 5 and up to 7 cm would rise from T2b to T3, and those larger than 7 cm would be reclassified from T3 to T4. Tumors invading the diaphragm would also be upgraded from T3 to T4 under the proposed revisions.
In contrast, tumors with limited invasion of the trachea (bronchus less than 2 cm from the carina) would be downgraded from T3 to T2, as would tumors associated with total atelectasis and/or pneumonitis.
The current N descriptors are adequate for predicting prognosis, the investigators determined, prompting the recommendation to retain them in the new edition.
The investigators propose slight changes to the M descriptors of metastases. Although they found no significant differences in survival found among patients with M1a (metastases within the chest cavity) descriptors, when distant metastases outside the chest cavity (M1b) were assessed by to the number of metastases, they found that patients with tumors with one metastasis in one organ had significantly better outcomes than those who had multiple metastases in one or more organs.
The proposed revision would continue to group in the M1a category cases with pleural/pericardial effusions, contralateral/bilateral lung nodules, contralateral/bilateral pleural nodules, or a combination of multiple parameters. However, single metastatic lesions in a single distant organ would be reclassified as M1b, and multiple lesions in a single organ or multiple lesions in multiple organs would be reclassified as M1c.
AT THE IASLC WORLD CONFERENCE
Key clinical point: Improved understanding of lung cancer over the last decade has prompted updates to international tumor staging and classification systems.
Major finding: The WHO 2015 Classification of Lung Tumors is expected to aid clinical practice.
Data source: Conference presentation of key changes to the WHO Classification and TNM staging system.
Disclosures: Dr. Travis and Dr. Rami-Porta reported having no disclosures relevant to their presentations.