User login
Which GI Side Effects Should GLP-1 Prescribers Worry About?
Several recent studies have sought to expound upon what role, if any, GLP-1 RAs may have in increasing the risk for specific gastrointestinal (GI) adverse events.
Herein is a summary of the most current information on this topic, as well as my best guidance for clinicians on integrating it into the clinical care of their patients.
Aspiration Risks
Albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, and tirzepatide are among the class of medications known as GLP-1 RAs. These medications all work by mimicking the action of hormonal incretins, which are released postprandially. Incretins affect the pancreatic glucose-dependent release of insulin, inhibit release of glucagon, stimulate satiety, and reduce gastric emptying. This last effect has raised concerns that patients taking GLP-1 RAs might be at an elevated risk for endoscopy-related aspiration.
In June 2023, the American Society of Anesthesiologists released recommendations asking providers to consider holding back GLP-1 RAs in patients with scheduled elective procedures.
In August 2023, five national GI societies — the American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition — issued their own joint statement on the issue.
In the absence of sufficient evidence, these groups suggested that healthcare providers “exercise best practices when performing endoscopy on these patients on GLP-1 [RAs].” They called for more data and encouraged key stakeholders to work together to develop the necessary evidence to provide guidance for these patients prior to elective endoscopy. A rapid clinical update issued by the American Gastroenterological Association in 2024 was consistent with these earlier multisociety recommendations.
Two studies presented at 2024’s Digestive Disease Week provided additional reassurance that concerns about aspiration with these medications were perhaps unwarranted.
The first (since published in The American Journal of Gastroenterology ) was a case-control study of 16,295 patients undergoing upper endoscopy, among whom 306 were taking GLP-1 RAs. It showed a higher rate of solid gastric residue among those taking GLP-1 RAs compared with controls (14% vs 4%, respectively). Patients who had prolonged fasting and clear liquids for concurrent colonoscopy had lower residue rates (2% vs 11%, respectively). However, there were no recorded incidents of procedural complications or aspiration.
The second was a retrospective cohort study using TriNetX, a federated cloud-based network pulling millions of data points from multiple US healthcare organizations. It found that the incidence of aspiration pneumonitis and emergent intubation during or immediately after esophagogastroduodenoscopy and colonoscopy among those taking GLP-1 RAs was not increased compared with those not taking these medications.
These were followed in June 2024 by a systematic review and meta-analysis published by Hiramoto and colleagues, which included 15 studies. The researchers showed a 36-minute prolongation for solid-food emptying and no delay in liquid emptying for patients taking GLP-1 RAs vs controls. The authors concluded that the minimal delay in solid-food emptying would be offset by standard preprocedural fasting periods.
There is concern that patients with complicated type 2 diabetes may have a bit more of a risk for aspiration. However, this was not supported by an analysis from Barlowe and colleagues, who used a national claims database to identify 15,119 patients with type 2 diabetes on GLP-1 RAs. They found no increased events of pulmonary complications (ie, aspiration, pneumonia, respiratory failure) within 14 days following esophagogastroduodenoscopy. Additional evidence suggests that the risk for aspiration in these patients seems to be offset by prolonged fasting and intake of clear liquids.
Although physicians clearly need to use clinical judgment when performing endoscopic procedures on these patients, the emerging evidence on safety has been encouraging.
Association With GI Adverse Events
A recent retrospective analysis of real-world data from 10,328 new users of GLP-1 RAs with diabetes/obesity reported that the most common GI adverse events in this cohort were abdominal pain (57.6%), constipation (30.4%), diarrhea (32.7%), nausea and vomiting (23.4%), GI bleeding (15.9%), gastroparesis (5.1%), and pancreatitis (3.4%).
Notably, dulaglutide and liraglutide had higher rates of abdominal pain, constipation, diarrhea, and nausea and vomiting than did semaglutide and exenatide. Compared with semaglutide, dulaglutide and liraglutide had slightly higher odds of abdominal pain, gastroparesis, and nausea and vomiting. There were no significant differences between the GLP-1 RAs in the risk for GI bleeding or pancreatitis.
A 2023 report in JAMA observed that the risk for bowel obstruction is also elevated among patients using these agents for weight loss. Possible reasons for this are currently unknown.
Studies are needed to analyze possible variations in safety profiles between GLP-1 RAs to better guide selection of these drugs, particularly in patients with GI risk factors. Furthermore, the causal relationship between GLP-1 RAs with other concomitant medications requires further investigation.
Although relatively infrequent, the risk for GI adverse events should be given special consideration by providers when prescribing them for weight loss, because the risk/benefit ratios may be different from those in patients with diabetes.
A Lack of Hepatic Concerns
GLP-1 RAs have demonstrated a significant impact on body weight and glycemic control, as well as beneficial effects on clinical, biochemical, and histologic markers in patients with metabolic dysfunction–associated steatotic liver disease (MASLD). These favorable changes are evident by reductions in the hepatic cytolysis markers (ie, aspartate aminotransferase and alanine aminotransferase).
GLP-1 RAs may provide a protective function by reducing the accumulation of hepatic triglycerides and expression of several collagen genes. Some preclinical data suggest a risk reduction for progression to hepatocellular carcinoma, and animal studies indicate that complete suppression of hepatic carcinogenesis is achieved with liraglutide.
The most recent assessment of risk reduction for MASLD progression comes from a Scandinavian cohort analysis of national registries. In looking at 91,479 patients using GLP-1 RAs, investigators demonstrated this treatment was associated with a significant reduction in the composite primary endpoint of hepatocellular carcinoma, as well as both compensated and decompensated cirrhosis.
Given the various favorable hepatic effects of GLP-1 RAs, it is likely that the composite benefit on MASLD is multifactorial. The current literature is clear that it is safe to use these agents across the spectrum of MASLD with or without fibrosis, although it must be noted that GLP-1 RAs are not approved by the Food and Drug Administration for this indication.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Several recent studies have sought to expound upon what role, if any, GLP-1 RAs may have in increasing the risk for specific gastrointestinal (GI) adverse events.
Herein is a summary of the most current information on this topic, as well as my best guidance for clinicians on integrating it into the clinical care of their patients.
Aspiration Risks
Albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, and tirzepatide are among the class of medications known as GLP-1 RAs. These medications all work by mimicking the action of hormonal incretins, which are released postprandially. Incretins affect the pancreatic glucose-dependent release of insulin, inhibit release of glucagon, stimulate satiety, and reduce gastric emptying. This last effect has raised concerns that patients taking GLP-1 RAs might be at an elevated risk for endoscopy-related aspiration.
In June 2023, the American Society of Anesthesiologists released recommendations asking providers to consider holding back GLP-1 RAs in patients with scheduled elective procedures.
In August 2023, five national GI societies — the American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition — issued their own joint statement on the issue.
In the absence of sufficient evidence, these groups suggested that healthcare providers “exercise best practices when performing endoscopy on these patients on GLP-1 [RAs].” They called for more data and encouraged key stakeholders to work together to develop the necessary evidence to provide guidance for these patients prior to elective endoscopy. A rapid clinical update issued by the American Gastroenterological Association in 2024 was consistent with these earlier multisociety recommendations.
Two studies presented at 2024’s Digestive Disease Week provided additional reassurance that concerns about aspiration with these medications were perhaps unwarranted.
The first (since published in The American Journal of Gastroenterology ) was a case-control study of 16,295 patients undergoing upper endoscopy, among whom 306 were taking GLP-1 RAs. It showed a higher rate of solid gastric residue among those taking GLP-1 RAs compared with controls (14% vs 4%, respectively). Patients who had prolonged fasting and clear liquids for concurrent colonoscopy had lower residue rates (2% vs 11%, respectively). However, there were no recorded incidents of procedural complications or aspiration.
The second was a retrospective cohort study using TriNetX, a federated cloud-based network pulling millions of data points from multiple US healthcare organizations. It found that the incidence of aspiration pneumonitis and emergent intubation during or immediately after esophagogastroduodenoscopy and colonoscopy among those taking GLP-1 RAs was not increased compared with those not taking these medications.
These were followed in June 2024 by a systematic review and meta-analysis published by Hiramoto and colleagues, which included 15 studies. The researchers showed a 36-minute prolongation for solid-food emptying and no delay in liquid emptying for patients taking GLP-1 RAs vs controls. The authors concluded that the minimal delay in solid-food emptying would be offset by standard preprocedural fasting periods.
There is concern that patients with complicated type 2 diabetes may have a bit more of a risk for aspiration. However, this was not supported by an analysis from Barlowe and colleagues, who used a national claims database to identify 15,119 patients with type 2 diabetes on GLP-1 RAs. They found no increased events of pulmonary complications (ie, aspiration, pneumonia, respiratory failure) within 14 days following esophagogastroduodenoscopy. Additional evidence suggests that the risk for aspiration in these patients seems to be offset by prolonged fasting and intake of clear liquids.
Although physicians clearly need to use clinical judgment when performing endoscopic procedures on these patients, the emerging evidence on safety has been encouraging.
Association With GI Adverse Events
A recent retrospective analysis of real-world data from 10,328 new users of GLP-1 RAs with diabetes/obesity reported that the most common GI adverse events in this cohort were abdominal pain (57.6%), constipation (30.4%), diarrhea (32.7%), nausea and vomiting (23.4%), GI bleeding (15.9%), gastroparesis (5.1%), and pancreatitis (3.4%).
Notably, dulaglutide and liraglutide had higher rates of abdominal pain, constipation, diarrhea, and nausea and vomiting than did semaglutide and exenatide. Compared with semaglutide, dulaglutide and liraglutide had slightly higher odds of abdominal pain, gastroparesis, and nausea and vomiting. There were no significant differences between the GLP-1 RAs in the risk for GI bleeding or pancreatitis.
A 2023 report in JAMA observed that the risk for bowel obstruction is also elevated among patients using these agents for weight loss. Possible reasons for this are currently unknown.
Studies are needed to analyze possible variations in safety profiles between GLP-1 RAs to better guide selection of these drugs, particularly in patients with GI risk factors. Furthermore, the causal relationship between GLP-1 RAs with other concomitant medications requires further investigation.
Although relatively infrequent, the risk for GI adverse events should be given special consideration by providers when prescribing them for weight loss, because the risk/benefit ratios may be different from those in patients with diabetes.
A Lack of Hepatic Concerns
GLP-1 RAs have demonstrated a significant impact on body weight and glycemic control, as well as beneficial effects on clinical, biochemical, and histologic markers in patients with metabolic dysfunction–associated steatotic liver disease (MASLD). These favorable changes are evident by reductions in the hepatic cytolysis markers (ie, aspartate aminotransferase and alanine aminotransferase).
GLP-1 RAs may provide a protective function by reducing the accumulation of hepatic triglycerides and expression of several collagen genes. Some preclinical data suggest a risk reduction for progression to hepatocellular carcinoma, and animal studies indicate that complete suppression of hepatic carcinogenesis is achieved with liraglutide.
The most recent assessment of risk reduction for MASLD progression comes from a Scandinavian cohort analysis of national registries. In looking at 91,479 patients using GLP-1 RAs, investigators demonstrated this treatment was associated with a significant reduction in the composite primary endpoint of hepatocellular carcinoma, as well as both compensated and decompensated cirrhosis.
Given the various favorable hepatic effects of GLP-1 RAs, it is likely that the composite benefit on MASLD is multifactorial. The current literature is clear that it is safe to use these agents across the spectrum of MASLD with or without fibrosis, although it must be noted that GLP-1 RAs are not approved by the Food and Drug Administration for this indication.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Several recent studies have sought to expound upon what role, if any, GLP-1 RAs may have in increasing the risk for specific gastrointestinal (GI) adverse events.
Herein is a summary of the most current information on this topic, as well as my best guidance for clinicians on integrating it into the clinical care of their patients.
Aspiration Risks
Albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, semaglutide, and tirzepatide are among the class of medications known as GLP-1 RAs. These medications all work by mimicking the action of hormonal incretins, which are released postprandially. Incretins affect the pancreatic glucose-dependent release of insulin, inhibit release of glucagon, stimulate satiety, and reduce gastric emptying. This last effect has raised concerns that patients taking GLP-1 RAs might be at an elevated risk for endoscopy-related aspiration.
In June 2023, the American Society of Anesthesiologists released recommendations asking providers to consider holding back GLP-1 RAs in patients with scheduled elective procedures.
In August 2023, five national GI societies — the American Gastroenterological Association, American Association for the Study of Liver Diseases, American College of Gastroenterology, American Society for Gastrointestinal Endoscopy, and North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition — issued their own joint statement on the issue.
In the absence of sufficient evidence, these groups suggested that healthcare providers “exercise best practices when performing endoscopy on these patients on GLP-1 [RAs].” They called for more data and encouraged key stakeholders to work together to develop the necessary evidence to provide guidance for these patients prior to elective endoscopy. A rapid clinical update issued by the American Gastroenterological Association in 2024 was consistent with these earlier multisociety recommendations.
Two studies presented at 2024’s Digestive Disease Week provided additional reassurance that concerns about aspiration with these medications were perhaps unwarranted.
The first (since published in The American Journal of Gastroenterology ) was a case-control study of 16,295 patients undergoing upper endoscopy, among whom 306 were taking GLP-1 RAs. It showed a higher rate of solid gastric residue among those taking GLP-1 RAs compared with controls (14% vs 4%, respectively). Patients who had prolonged fasting and clear liquids for concurrent colonoscopy had lower residue rates (2% vs 11%, respectively). However, there were no recorded incidents of procedural complications or aspiration.
The second was a retrospective cohort study using TriNetX, a federated cloud-based network pulling millions of data points from multiple US healthcare organizations. It found that the incidence of aspiration pneumonitis and emergent intubation during or immediately after esophagogastroduodenoscopy and colonoscopy among those taking GLP-1 RAs was not increased compared with those not taking these medications.
These were followed in June 2024 by a systematic review and meta-analysis published by Hiramoto and colleagues, which included 15 studies. The researchers showed a 36-minute prolongation for solid-food emptying and no delay in liquid emptying for patients taking GLP-1 RAs vs controls. The authors concluded that the minimal delay in solid-food emptying would be offset by standard preprocedural fasting periods.
There is concern that patients with complicated type 2 diabetes may have a bit more of a risk for aspiration. However, this was not supported by an analysis from Barlowe and colleagues, who used a national claims database to identify 15,119 patients with type 2 diabetes on GLP-1 RAs. They found no increased events of pulmonary complications (ie, aspiration, pneumonia, respiratory failure) within 14 days following esophagogastroduodenoscopy. Additional evidence suggests that the risk for aspiration in these patients seems to be offset by prolonged fasting and intake of clear liquids.
Although physicians clearly need to use clinical judgment when performing endoscopic procedures on these patients, the emerging evidence on safety has been encouraging.
Association With GI Adverse Events
A recent retrospective analysis of real-world data from 10,328 new users of GLP-1 RAs with diabetes/obesity reported that the most common GI adverse events in this cohort were abdominal pain (57.6%), constipation (30.4%), diarrhea (32.7%), nausea and vomiting (23.4%), GI bleeding (15.9%), gastroparesis (5.1%), and pancreatitis (3.4%).
Notably, dulaglutide and liraglutide had higher rates of abdominal pain, constipation, diarrhea, and nausea and vomiting than did semaglutide and exenatide. Compared with semaglutide, dulaglutide and liraglutide had slightly higher odds of abdominal pain, gastroparesis, and nausea and vomiting. There were no significant differences between the GLP-1 RAs in the risk for GI bleeding or pancreatitis.
A 2023 report in JAMA observed that the risk for bowel obstruction is also elevated among patients using these agents for weight loss. Possible reasons for this are currently unknown.
Studies are needed to analyze possible variations in safety profiles between GLP-1 RAs to better guide selection of these drugs, particularly in patients with GI risk factors. Furthermore, the causal relationship between GLP-1 RAs with other concomitant medications requires further investigation.
Although relatively infrequent, the risk for GI adverse events should be given special consideration by providers when prescribing them for weight loss, because the risk/benefit ratios may be different from those in patients with diabetes.
A Lack of Hepatic Concerns
GLP-1 RAs have demonstrated a significant impact on body weight and glycemic control, as well as beneficial effects on clinical, biochemical, and histologic markers in patients with metabolic dysfunction–associated steatotic liver disease (MASLD). These favorable changes are evident by reductions in the hepatic cytolysis markers (ie, aspartate aminotransferase and alanine aminotransferase).
GLP-1 RAs may provide a protective function by reducing the accumulation of hepatic triglycerides and expression of several collagen genes. Some preclinical data suggest a risk reduction for progression to hepatocellular carcinoma, and animal studies indicate that complete suppression of hepatic carcinogenesis is achieved with liraglutide.
The most recent assessment of risk reduction for MASLD progression comes from a Scandinavian cohort analysis of national registries. In looking at 91,479 patients using GLP-1 RAs, investigators demonstrated this treatment was associated with a significant reduction in the composite primary endpoint of hepatocellular carcinoma, as well as both compensated and decompensated cirrhosis.
Given the various favorable hepatic effects of GLP-1 RAs, it is likely that the composite benefit on MASLD is multifactorial. The current literature is clear that it is safe to use these agents across the spectrum of MASLD with or without fibrosis, although it must be noted that GLP-1 RAs are not approved by the Food and Drug Administration for this indication.
Dr. Johnson is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, Virginia, and a past president of the American College of Gastroenterology. He disclosed ties with ISOTHRIVE and Johnson & Johnson.
A version of this article appeared on Medscape.com.
Esophageal Cancer Risk Unchanged After Helicobacter Eradication
This finding suggests that eradication of H pylori is safe with regard to esophageal cancer risk, and eradication campaigns are not contributing to the rising incidence of esophageal adenocarcinoma (EAC) over the past four decades, reported lead author Anna-Klara Wiklund, MD, of Karolinska Institutet, Stockholm, Sweden, and colleagues.“The decreased risk of esophageal adenocarcinoma seen in individuals with H pylori infection is probably explained by the H pylori–induced gastric atrophy, which reduces gastric acid production and thus acidic gastroesophageal reflux, the main risk factor for this tumor,” the investigators wrote in Gastroenterology. “It seems plausible that eradication of H pylori would increase the risk of EAC, although the answer to this question is unknown with the only study on the topic (from our group) having too few cases and too short follow-up.”
That study involved only 11 cases of EAC.
For the present study, Dr. Wiklund and colleagues aggregated data from all individuals who had undergone H pylori eradication in Finland, Denmark, Iceland, Norway, and Sweden from 1995 to 2019. The dataset comprised 661,987 such individuals with more than 5 million person-years after eradication therapy, including 550 cases of EAC. Median follow-up time was approximately 8 years, ranging from 1 to 24 years.
Analyzing these data revealed that standardized incidence ratio (SIR) of EAC was not increased after eradication therapy (0.89; 95% CI, 0.82-0.97). In fact, SIR decreased over time after eradication, reaching as low as 0.73 (95% CI, 0.61-0.86) during the follow-up period of 11-24 years. These findings were maintained regardless of age or sex, and within country-by-country analyses.
SIR for esophageal squamous cell carcinoma, which was calculated for comparison, showed no association with eradication therapy (0.99; 95% CI, 0.89-1.11).
“This study found no evidence supporting the hypothesis of a gradually increasing risk of esophageal adenocarcinoma over time after H pylori eradication treatment,” the investigators wrote.
Other risks were detected, including an overall increased SIR of EAC observed among participants with gastroesophageal reflux disease (GERD) and those using long-term proton pump inhibitors (PPIs). These were expected, however, “considering the strong and well-established association with EAC.”
Dr. Wiklund and colleagues suggested that more studies are needed to confirm their findings, although the present data provide confidence that H pylori eradication does not raise risk of EAC.
“This is valuable knowledge when considering eradication treatment for individual patients and eradication programs in high-risk populations of gastric cancer,” they wrote. “The results should be generalizable to other high-income countries with low prevalence of H pylori and high incidence of EAC, but studies from other regions with different patterns of these conditions are warranted.”
They also called for more basic research to understand why eradicating H pylori does not lead to an increased risk of EAC.The study was supported by Sjoberg Foundation, Nordic Cancer Union, Stockholm County Council, Stockholm Cancer Society. Investigators disclosed no conflicts of interest.
Understanding the demographic and biomarker risk predictors of esophageal cancer continues to be a research priority. Many esophageal cancer patients fall outside of current screening guidelines. Updated recommendations have suggested including high risk-women, driven by higher quality datasets, emerging biomarkers, and cost effective non-endoscopic screening devices.
In this article, Wiklund et al. challenge another dogma that Helicobacter pylori infection offers protection against esophageal cancer. More specifically that overtreatment of H pylori is associated with increased incidence of esophageal adenocarcinoma. Their Nordic data set identified 550 cases of esophageal cancer in the 661,987 patients treated for H pylori from 1995–2018 who were followed >5 million person-years. Interestingly, standardized incidence ratio of esophageal adenocarcinoma decreased over time.
This large dataset continues to encourage us to treat H pylori in patients at risk of progressing to gastric cancer. This parallels a growing fund of literature encouraging us to move away from the linear pathophysiologic logic that eliminating H pylori-induced gastric atrophy provokes gastroesophageal reflux disease and esophageal cancer. Instead we should factor in other parameters, including the complex interaction between the esophageal microbiome and gastric H pylori. Some postulated mechanisms include an extension of the gastric inflammatory milieu into the esophagus, and potential crosstalk with the esophageal microbiome.
Such studies underscore the need to personalize both foregut cancer screening criteria and treatment of inflammatory conditions at a patient and population level, so that we can make meaningful impacts in disease prevalence and cancer survival.
Fouad Otaki, MD, is associate professor in the Division of Gastroenterology & Hepatology at Oregon Health & Science University, Portland.
Understanding the demographic and biomarker risk predictors of esophageal cancer continues to be a research priority. Many esophageal cancer patients fall outside of current screening guidelines. Updated recommendations have suggested including high risk-women, driven by higher quality datasets, emerging biomarkers, and cost effective non-endoscopic screening devices.
In this article, Wiklund et al. challenge another dogma that Helicobacter pylori infection offers protection against esophageal cancer. More specifically that overtreatment of H pylori is associated with increased incidence of esophageal adenocarcinoma. Their Nordic data set identified 550 cases of esophageal cancer in the 661,987 patients treated for H pylori from 1995–2018 who were followed >5 million person-years. Interestingly, standardized incidence ratio of esophageal adenocarcinoma decreased over time.
This large dataset continues to encourage us to treat H pylori in patients at risk of progressing to gastric cancer. This parallels a growing fund of literature encouraging us to move away from the linear pathophysiologic logic that eliminating H pylori-induced gastric atrophy provokes gastroesophageal reflux disease and esophageal cancer. Instead we should factor in other parameters, including the complex interaction between the esophageal microbiome and gastric H pylori. Some postulated mechanisms include an extension of the gastric inflammatory milieu into the esophagus, and potential crosstalk with the esophageal microbiome.
Such studies underscore the need to personalize both foregut cancer screening criteria and treatment of inflammatory conditions at a patient and population level, so that we can make meaningful impacts in disease prevalence and cancer survival.
Fouad Otaki, MD, is associate professor in the Division of Gastroenterology & Hepatology at Oregon Health & Science University, Portland.
Understanding the demographic and biomarker risk predictors of esophageal cancer continues to be a research priority. Many esophageal cancer patients fall outside of current screening guidelines. Updated recommendations have suggested including high risk-women, driven by higher quality datasets, emerging biomarkers, and cost effective non-endoscopic screening devices.
In this article, Wiklund et al. challenge another dogma that Helicobacter pylori infection offers protection against esophageal cancer. More specifically that overtreatment of H pylori is associated with increased incidence of esophageal adenocarcinoma. Their Nordic data set identified 550 cases of esophageal cancer in the 661,987 patients treated for H pylori from 1995–2018 who were followed >5 million person-years. Interestingly, standardized incidence ratio of esophageal adenocarcinoma decreased over time.
This large dataset continues to encourage us to treat H pylori in patients at risk of progressing to gastric cancer. This parallels a growing fund of literature encouraging us to move away from the linear pathophysiologic logic that eliminating H pylori-induced gastric atrophy provokes gastroesophageal reflux disease and esophageal cancer. Instead we should factor in other parameters, including the complex interaction between the esophageal microbiome and gastric H pylori. Some postulated mechanisms include an extension of the gastric inflammatory milieu into the esophagus, and potential crosstalk with the esophageal microbiome.
Such studies underscore the need to personalize both foregut cancer screening criteria and treatment of inflammatory conditions at a patient and population level, so that we can make meaningful impacts in disease prevalence and cancer survival.
Fouad Otaki, MD, is associate professor in the Division of Gastroenterology & Hepatology at Oregon Health & Science University, Portland.
This finding suggests that eradication of H pylori is safe with regard to esophageal cancer risk, and eradication campaigns are not contributing to the rising incidence of esophageal adenocarcinoma (EAC) over the past four decades, reported lead author Anna-Klara Wiklund, MD, of Karolinska Institutet, Stockholm, Sweden, and colleagues.“The decreased risk of esophageal adenocarcinoma seen in individuals with H pylori infection is probably explained by the H pylori–induced gastric atrophy, which reduces gastric acid production and thus acidic gastroesophageal reflux, the main risk factor for this tumor,” the investigators wrote in Gastroenterology. “It seems plausible that eradication of H pylori would increase the risk of EAC, although the answer to this question is unknown with the only study on the topic (from our group) having too few cases and too short follow-up.”
That study involved only 11 cases of EAC.
For the present study, Dr. Wiklund and colleagues aggregated data from all individuals who had undergone H pylori eradication in Finland, Denmark, Iceland, Norway, and Sweden from 1995 to 2019. The dataset comprised 661,987 such individuals with more than 5 million person-years after eradication therapy, including 550 cases of EAC. Median follow-up time was approximately 8 years, ranging from 1 to 24 years.
Analyzing these data revealed that standardized incidence ratio (SIR) of EAC was not increased after eradication therapy (0.89; 95% CI, 0.82-0.97). In fact, SIR decreased over time after eradication, reaching as low as 0.73 (95% CI, 0.61-0.86) during the follow-up period of 11-24 years. These findings were maintained regardless of age or sex, and within country-by-country analyses.
SIR for esophageal squamous cell carcinoma, which was calculated for comparison, showed no association with eradication therapy (0.99; 95% CI, 0.89-1.11).
“This study found no evidence supporting the hypothesis of a gradually increasing risk of esophageal adenocarcinoma over time after H pylori eradication treatment,” the investigators wrote.
Other risks were detected, including an overall increased SIR of EAC observed among participants with gastroesophageal reflux disease (GERD) and those using long-term proton pump inhibitors (PPIs). These were expected, however, “considering the strong and well-established association with EAC.”
Dr. Wiklund and colleagues suggested that more studies are needed to confirm their findings, although the present data provide confidence that H pylori eradication does not raise risk of EAC.
“This is valuable knowledge when considering eradication treatment for individual patients and eradication programs in high-risk populations of gastric cancer,” they wrote. “The results should be generalizable to other high-income countries with low prevalence of H pylori and high incidence of EAC, but studies from other regions with different patterns of these conditions are warranted.”
They also called for more basic research to understand why eradicating H pylori does not lead to an increased risk of EAC.The study was supported by Sjoberg Foundation, Nordic Cancer Union, Stockholm County Council, Stockholm Cancer Society. Investigators disclosed no conflicts of interest.
This finding suggests that eradication of H pylori is safe with regard to esophageal cancer risk, and eradication campaigns are not contributing to the rising incidence of esophageal adenocarcinoma (EAC) over the past four decades, reported lead author Anna-Klara Wiklund, MD, of Karolinska Institutet, Stockholm, Sweden, and colleagues.“The decreased risk of esophageal adenocarcinoma seen in individuals with H pylori infection is probably explained by the H pylori–induced gastric atrophy, which reduces gastric acid production and thus acidic gastroesophageal reflux, the main risk factor for this tumor,” the investigators wrote in Gastroenterology. “It seems plausible that eradication of H pylori would increase the risk of EAC, although the answer to this question is unknown with the only study on the topic (from our group) having too few cases and too short follow-up.”
That study involved only 11 cases of EAC.
For the present study, Dr. Wiklund and colleagues aggregated data from all individuals who had undergone H pylori eradication in Finland, Denmark, Iceland, Norway, and Sweden from 1995 to 2019. The dataset comprised 661,987 such individuals with more than 5 million person-years after eradication therapy, including 550 cases of EAC. Median follow-up time was approximately 8 years, ranging from 1 to 24 years.
Analyzing these data revealed that standardized incidence ratio (SIR) of EAC was not increased after eradication therapy (0.89; 95% CI, 0.82-0.97). In fact, SIR decreased over time after eradication, reaching as low as 0.73 (95% CI, 0.61-0.86) during the follow-up period of 11-24 years. These findings were maintained regardless of age or sex, and within country-by-country analyses.
SIR for esophageal squamous cell carcinoma, which was calculated for comparison, showed no association with eradication therapy (0.99; 95% CI, 0.89-1.11).
“This study found no evidence supporting the hypothesis of a gradually increasing risk of esophageal adenocarcinoma over time after H pylori eradication treatment,” the investigators wrote.
Other risks were detected, including an overall increased SIR of EAC observed among participants with gastroesophageal reflux disease (GERD) and those using long-term proton pump inhibitors (PPIs). These were expected, however, “considering the strong and well-established association with EAC.”
Dr. Wiklund and colleagues suggested that more studies are needed to confirm their findings, although the present data provide confidence that H pylori eradication does not raise risk of EAC.
“This is valuable knowledge when considering eradication treatment for individual patients and eradication programs in high-risk populations of gastric cancer,” they wrote. “The results should be generalizable to other high-income countries with low prevalence of H pylori and high incidence of EAC, but studies from other regions with different patterns of these conditions are warranted.”
They also called for more basic research to understand why eradicating H pylori does not lead to an increased risk of EAC.The study was supported by Sjoberg Foundation, Nordic Cancer Union, Stockholm County Council, Stockholm Cancer Society. Investigators disclosed no conflicts of interest.
FROM GASTROENTEROLOGY
Eosinophilic Esophagitis Often Persists Despite Treatment
based on a recent retrospective study.
Challenging patient journeys were common across age groups, with a range of ongoing symptoms and histological abnormalities supporting high unmet need among patients with EoE, lead author Olulade Ayodele, MBBS, MPH, of Takeda Development Center Americas and colleagues reported.
“Recent studies have found that patients with EoE experience a complicated journey to diagnosis and a substantial disease burden, which requires significant healthcare resource utilization,” the investigators wrote in Gastro Hep Advances . “Reasons for this may include delays in diagnosis owing to nonspecific symptoms, adaptive behaviors, progression of silent disease, lack of adequate follow-up or referral, or suboptimal treatment after diagnosis.”Two medications are currently Food and Drug administration approved for EoE: dupilumab, a biologic for patients aged 1 year and older, and budesonide oral suspension, a topical corticosteroid for patients aged 11 years and older.
The investigators noted that “biologic therapies may not always be selected as first-line treatment, and are often associated with high costs”; however, the effects of real-world treatment decisions like these are poorly documented, prompting the present study.
The final dataset comprised 613 patients with newly diagnosed EoE treated in a rural integrated healthcare system, all of whom had at least 12 months of data before and after a predetermined index date. Individuals were stratified by age, including 182 children, 146 adolescents, 244 adults, and 41 older adults.
Signs and symptoms of EoE frequently worsened after the index date, including dysphagia (34.6% before, 49.9% after), abdominal pain (33.0% before, 48.1% after), and nausea/vomiting (20.1% before, 31.5% after).
At baseline, 80.5% of endoscopies were abnormal and 87.9% of patients had more than 15 eosinophils/high-power field. These parameters improved post index; however, 3 years later, 62.3% of patients still had abnormal endoscopic appearance and 51.2% had abnormal histologic activity.
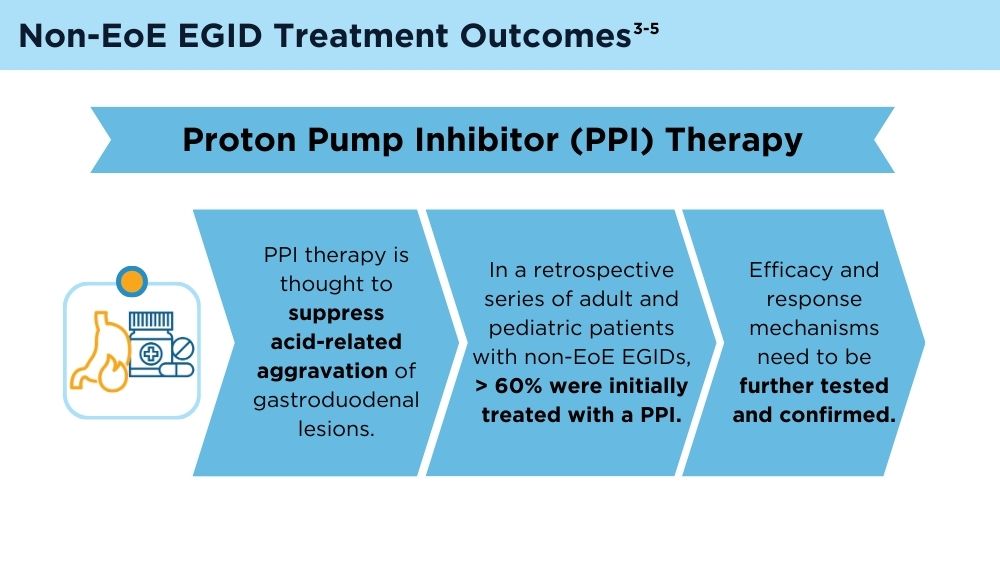
Before and after index, the most prescribed treatments were corticosteroids (47.3% before, 87.9% after) and proton pump inhibitors (51.1% before, 96.1% after).
After index, 44.0% of patients discontinued their first-line treatment, and 13.9% experienced disease progression.
“We found that a substantial portion of patients with EoE received variable medical treatments, and did not report undergoing follow-up care, consulting with specialists, or routinely undergoing endoscopy with biopsy after diagnosis; the reasons for this are unknown, but experiences do not appear to be consistent with current guideline recommendations,” Dr. Ayodele and colleagues wrote.
They also noted substantial healthcare resource utilization; more than half of the patients visited emergency departments, and nearly one in five were admitted as inpatients.
“Our findings outline the persistent disease activity and difficult therapeutic journeys faced by patients with EoE irrespective of their age, as well as the substantial disease burden,” the investigators concluded. “These data highlight the potential unmet medical need of patients with EoE in the United States.”The study was funded by Shire Human Genetic Therapies, a member of the Takeda group of companies. The investigators disclosed additional relationships with RTI Health Solutions and Receptos/Celgene.
In a large, retrospective, real-world cohort study, investigators examined the patient journey in 613 child, adolescent, and adult patients with eosinophilic esophagitis (EoE) via healthcare claims database and electronic medical record data. As we enter into an exciting era in novel biologic therapies in EoE, the article provides comprehensive and reliable information in several critical and actionable areas with respect to EoE diagnosis and management.
The study highlights the heterogeneity of the patient experience in EoE and suggests that improvements in the reliability and precision of EoE care models will impact healthcare utilization. In particular, the findings support the need for structured and systematic mechanisms for appropriate follow-up after the index diagnosis and increased use and continued development of novel therapies.
Anand Jain, MD, is assistant professor in the Division of Digestive Diseases at Emory University School of Medicine, Atlanta, Georgia. Ravinder Mittal, MD, AGAF, is professor in the Division of Gastroenterology at the University of California, San Diego, and staff physician at the San Diego VA Hospital. They report no conflicts of interest.
In a large, retrospective, real-world cohort study, investigators examined the patient journey in 613 child, adolescent, and adult patients with eosinophilic esophagitis (EoE) via healthcare claims database and electronic medical record data. As we enter into an exciting era in novel biologic therapies in EoE, the article provides comprehensive and reliable information in several critical and actionable areas with respect to EoE diagnosis and management.
The study highlights the heterogeneity of the patient experience in EoE and suggests that improvements in the reliability and precision of EoE care models will impact healthcare utilization. In particular, the findings support the need for structured and systematic mechanisms for appropriate follow-up after the index diagnosis and increased use and continued development of novel therapies.
Anand Jain, MD, is assistant professor in the Division of Digestive Diseases at Emory University School of Medicine, Atlanta, Georgia. Ravinder Mittal, MD, AGAF, is professor in the Division of Gastroenterology at the University of California, San Diego, and staff physician at the San Diego VA Hospital. They report no conflicts of interest.
In a large, retrospective, real-world cohort study, investigators examined the patient journey in 613 child, adolescent, and adult patients with eosinophilic esophagitis (EoE) via healthcare claims database and electronic medical record data. As we enter into an exciting era in novel biologic therapies in EoE, the article provides comprehensive and reliable information in several critical and actionable areas with respect to EoE diagnosis and management.
The study highlights the heterogeneity of the patient experience in EoE and suggests that improvements in the reliability and precision of EoE care models will impact healthcare utilization. In particular, the findings support the need for structured and systematic mechanisms for appropriate follow-up after the index diagnosis and increased use and continued development of novel therapies.
Anand Jain, MD, is assistant professor in the Division of Digestive Diseases at Emory University School of Medicine, Atlanta, Georgia. Ravinder Mittal, MD, AGAF, is professor in the Division of Gastroenterology at the University of California, San Diego, and staff physician at the San Diego VA Hospital. They report no conflicts of interest.
based on a recent retrospective study.
Challenging patient journeys were common across age groups, with a range of ongoing symptoms and histological abnormalities supporting high unmet need among patients with EoE, lead author Olulade Ayodele, MBBS, MPH, of Takeda Development Center Americas and colleagues reported.
“Recent studies have found that patients with EoE experience a complicated journey to diagnosis and a substantial disease burden, which requires significant healthcare resource utilization,” the investigators wrote in Gastro Hep Advances . “Reasons for this may include delays in diagnosis owing to nonspecific symptoms, adaptive behaviors, progression of silent disease, lack of adequate follow-up or referral, or suboptimal treatment after diagnosis.”Two medications are currently Food and Drug administration approved for EoE: dupilumab, a biologic for patients aged 1 year and older, and budesonide oral suspension, a topical corticosteroid for patients aged 11 years and older.
The investigators noted that “biologic therapies may not always be selected as first-line treatment, and are often associated with high costs”; however, the effects of real-world treatment decisions like these are poorly documented, prompting the present study.
The final dataset comprised 613 patients with newly diagnosed EoE treated in a rural integrated healthcare system, all of whom had at least 12 months of data before and after a predetermined index date. Individuals were stratified by age, including 182 children, 146 adolescents, 244 adults, and 41 older adults.
Signs and symptoms of EoE frequently worsened after the index date, including dysphagia (34.6% before, 49.9% after), abdominal pain (33.0% before, 48.1% after), and nausea/vomiting (20.1% before, 31.5% after).
At baseline, 80.5% of endoscopies were abnormal and 87.9% of patients had more than 15 eosinophils/high-power field. These parameters improved post index; however, 3 years later, 62.3% of patients still had abnormal endoscopic appearance and 51.2% had abnormal histologic activity.
Before and after index, the most prescribed treatments were corticosteroids (47.3% before, 87.9% after) and proton pump inhibitors (51.1% before, 96.1% after).
After index, 44.0% of patients discontinued their first-line treatment, and 13.9% experienced disease progression.
“We found that a substantial portion of patients with EoE received variable medical treatments, and did not report undergoing follow-up care, consulting with specialists, or routinely undergoing endoscopy with biopsy after diagnosis; the reasons for this are unknown, but experiences do not appear to be consistent with current guideline recommendations,” Dr. Ayodele and colleagues wrote.
They also noted substantial healthcare resource utilization; more than half of the patients visited emergency departments, and nearly one in five were admitted as inpatients.
“Our findings outline the persistent disease activity and difficult therapeutic journeys faced by patients with EoE irrespective of their age, as well as the substantial disease burden,” the investigators concluded. “These data highlight the potential unmet medical need of patients with EoE in the United States.”The study was funded by Shire Human Genetic Therapies, a member of the Takeda group of companies. The investigators disclosed additional relationships with RTI Health Solutions and Receptos/Celgene.
based on a recent retrospective study.
Challenging patient journeys were common across age groups, with a range of ongoing symptoms and histological abnormalities supporting high unmet need among patients with EoE, lead author Olulade Ayodele, MBBS, MPH, of Takeda Development Center Americas and colleagues reported.
“Recent studies have found that patients with EoE experience a complicated journey to diagnosis and a substantial disease burden, which requires significant healthcare resource utilization,” the investigators wrote in Gastro Hep Advances . “Reasons for this may include delays in diagnosis owing to nonspecific symptoms, adaptive behaviors, progression of silent disease, lack of adequate follow-up or referral, or suboptimal treatment after diagnosis.”Two medications are currently Food and Drug administration approved for EoE: dupilumab, a biologic for patients aged 1 year and older, and budesonide oral suspension, a topical corticosteroid for patients aged 11 years and older.
The investigators noted that “biologic therapies may not always be selected as first-line treatment, and are often associated with high costs”; however, the effects of real-world treatment decisions like these are poorly documented, prompting the present study.
The final dataset comprised 613 patients with newly diagnosed EoE treated in a rural integrated healthcare system, all of whom had at least 12 months of data before and after a predetermined index date. Individuals were stratified by age, including 182 children, 146 adolescents, 244 adults, and 41 older adults.
Signs and symptoms of EoE frequently worsened after the index date, including dysphagia (34.6% before, 49.9% after), abdominal pain (33.0% before, 48.1% after), and nausea/vomiting (20.1% before, 31.5% after).
At baseline, 80.5% of endoscopies were abnormal and 87.9% of patients had more than 15 eosinophils/high-power field. These parameters improved post index; however, 3 years later, 62.3% of patients still had abnormal endoscopic appearance and 51.2% had abnormal histologic activity.
Before and after index, the most prescribed treatments were corticosteroids (47.3% before, 87.9% after) and proton pump inhibitors (51.1% before, 96.1% after).
After index, 44.0% of patients discontinued their first-line treatment, and 13.9% experienced disease progression.
“We found that a substantial portion of patients with EoE received variable medical treatments, and did not report undergoing follow-up care, consulting with specialists, or routinely undergoing endoscopy with biopsy after diagnosis; the reasons for this are unknown, but experiences do not appear to be consistent with current guideline recommendations,” Dr. Ayodele and colleagues wrote.
They also noted substantial healthcare resource utilization; more than half of the patients visited emergency departments, and nearly one in five were admitted as inpatients.
“Our findings outline the persistent disease activity and difficult therapeutic journeys faced by patients with EoE irrespective of their age, as well as the substantial disease burden,” the investigators concluded. “These data highlight the potential unmet medical need of patients with EoE in the United States.”The study was funded by Shire Human Genetic Therapies, a member of the Takeda group of companies. The investigators disclosed additional relationships with RTI Health Solutions and Receptos/Celgene.
FROM GASTRO HEP ADVANCES
Dupilumab Effective in PPI-Refractory Pediatric EoE
Good news for younger children suffering from the uncommon but debilitating gastrointestinal condition eosinophilic esophagitis (EoE): Data from this trial led to a January US Food and Drug Administration (FDA) approval of the anti-inflammatory biologic for patients aged 1-11 years weighing at least 15 kg.
In addition, the trial, published in The New England Journal of Medicine, found that a higher-exposure dupilumab regimen (approximating the trough concentration of a 300-mg dose administered once weekly versus every 2 weeks) improved key secondary end points, according to gastroenterologist Mirna Chehade, MD, MPH, AGAF, a professor of pediatrics at Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital in New York City, and colleagues.
In 2022, the FDA approved the drug for those aged 12 or older weighing at least 40 kg.
“Left untreated or inadequately treated, EoE can progress to esophageal narrowing and strictures, leading to increased risk of food impactions and the need for esophageal dilations,” Dr. Chehade said in an interview. “Therefore, it’s important that children with EoE have the FDA-approved treatment option based on our study that can address their underlying disease starting at a young age.”
She added that dupilumab has the exciting potential to transform the standard of care for many young children living with EoE. “There are, however, factors to consider before switching a child to dupilumab — all related to the child’s specific medical history and therefore the perceived potential benefits from the drug.”
Commenting on the study but not involved in it, Toni Webster, DO, a pediatric gastroenterologist at Cohen Children’s Medical Center in Queens, New York, and an assistant professor at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, said, “Like many allergic diseases, EoE is on the rise and, unfortunately, is affecting our children at alarming rates and at earlier ages. Given its efficacy and side-effect profile, dupilumab will vastly change our ability to treat EoE, especially for families who find diet and daily medication to be a challenge.”
Dr. Webster noted that an elimination diet is a rigorous choice that is often difficult to navigate. And the oral administration of off-label choices, proton pump inhibitors, and swallowed topical steroids, as well as the newly FDA-approved oral budesonide therapy (Eohilia), may also be challenging because many children have precluding aversions to oral therapy. “Regardless of age, treatment choice for EoE should be a good fit that is a plausible addition to a family’s lifestyle,” she said.
Blocking interleukin 4 and interleukin 13 inflammatory pathways, dupilumab has shown efficacy in other atopic diseases such as eczema. It broadly inhibits most aspects of type 2 inflammation and that action is reflected in its histologic and transcriptomic effects in affected tissues, Dr. Chehade and associates explained.
The Trial
Conducted at one Canadian and 26 US sites, the two-part phase 3 study randomly assigned 102 EoE patients aged 1-11 years who were refractory to proton pump inhibition in a 2:2:1:1 ratio.
Part A enrolled 102 patients and evaluated dupilumab at a weight-tiered higher-dose or lower-dose regimen vs placebo (two groups) for 16 weeks.
Part B was a 36-week extended active treatment period in which eligible dupilumab recipients from part A maintained their weight-tiered higher- or lower-dose regimen, whereas those in the placebo groups switched to weight-tiered higher- or lower-dose dupilumab.
The primary end point was histologic remission (peak esophageal intraepithelial eosinophil count, ≤ 6 per high-power field) at week 16. Continued dupilumab treatment appeared to maintain its effect through week 52.
During part A, histologic remission occurred in 25 of the 37 higher-exposure patients (68%), 18 of the 31 lower-exposure patients (58%), and one of the 34 placebo patients (3%).
The difference between the higher-exposure regimen and placebo was 65 percentage points (95% confidence interval [CI], 48-81; P < .001), whereas that between the lower-exposure regimen and placebo was 55 percentage points (95% CI, 37-73; P < .001).
Higher exposure led to significant improvements in histologic, endoscopic, and transcriptomic measures over placebo. Improvements between baseline and week 52 in all patients were generally similar to those between baseline and week 16 in patients who received dupilumab in part A.
As for adverse events, in part A, the incidence of coronavirus disease, nausea, injection-site pain, and headache was at least 10 percentage points higher among dupilumab recipients at either dose than among placebo recipients. Serious adverse events were reported in three dupilumab patients during part A and in six patients overall during part B.
A Balanced Approach
On a cautionary note, Eric H. Chiou, MD, an assistant professor of pediatrics at Baylor College of Medicine and a pediatric gastroenterologist at Texas Children’s Hospital in Houston, said that while dupilumab shows great promise, further research is needed on its cost-effectiveness in EoE.
“The cost of treatment will need to be compared relative to potential long-term savings from reduced hospitalizations, fewer complications, and improved quality of life,” said Dr. Chiou, who was not involved in the study. “A balanced approach that considers clinical efficacy, patient well-being, cost-effectiveness, and equity is essential.”
He added that despite the study’s encouraging results, long-term safety and efficacy data are needed to fully understand the impact of dupilumab on pediatric patients with EoE. “Dupilumab will need to be compared with existing treatments for EoE such as dietary management and swallowed topical corticosteroids in terms of efficacy, safety, and quality of life improvements.”
Additionally, further research is required to identify which patients are most likely to benefit from this therapy and to explore any potential complications associated with its long-term use. “Understanding the optimal dosing and duration of treatment will also be crucial for maximizing benefits while minimizing risks,” Dr. Chiou said.
Dr. Chehade agreed. “While it’s that great that young children finally have an FDA-approved drug to treat their EoE, more research is needed to learn which patient subsets would derive maximum benefit from dupilumab and at which specific steps in their medical management journey should dupilumab be used.”
This study was supported by Sanofi and Regeneron Pharmaceuticals. Dr. Chehade disclosed research funding from and consulting for numerous private sector companies, among others, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Shire-Takeda, and Bristol-Myers Squibb. Multiple study coauthors disclosed various relationships with private-sector companies, including Sanofi and Regeneron Pharmaceuticals, for research funding, consulting, travel, employment, and stock or intellectual ownership. Dr. Webster and Dr. Chiou disclosed no competing interests relevant to their comments.
A version of this article first appeared on Medscape.com.
Good news for younger children suffering from the uncommon but debilitating gastrointestinal condition eosinophilic esophagitis (EoE): Data from this trial led to a January US Food and Drug Administration (FDA) approval of the anti-inflammatory biologic for patients aged 1-11 years weighing at least 15 kg.
In addition, the trial, published in The New England Journal of Medicine, found that a higher-exposure dupilumab regimen (approximating the trough concentration of a 300-mg dose administered once weekly versus every 2 weeks) improved key secondary end points, according to gastroenterologist Mirna Chehade, MD, MPH, AGAF, a professor of pediatrics at Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital in New York City, and colleagues.
In 2022, the FDA approved the drug for those aged 12 or older weighing at least 40 kg.
“Left untreated or inadequately treated, EoE can progress to esophageal narrowing and strictures, leading to increased risk of food impactions and the need for esophageal dilations,” Dr. Chehade said in an interview. “Therefore, it’s important that children with EoE have the FDA-approved treatment option based on our study that can address their underlying disease starting at a young age.”
She added that dupilumab has the exciting potential to transform the standard of care for many young children living with EoE. “There are, however, factors to consider before switching a child to dupilumab — all related to the child’s specific medical history and therefore the perceived potential benefits from the drug.”
Commenting on the study but not involved in it, Toni Webster, DO, a pediatric gastroenterologist at Cohen Children’s Medical Center in Queens, New York, and an assistant professor at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, said, “Like many allergic diseases, EoE is on the rise and, unfortunately, is affecting our children at alarming rates and at earlier ages. Given its efficacy and side-effect profile, dupilumab will vastly change our ability to treat EoE, especially for families who find diet and daily medication to be a challenge.”
Dr. Webster noted that an elimination diet is a rigorous choice that is often difficult to navigate. And the oral administration of off-label choices, proton pump inhibitors, and swallowed topical steroids, as well as the newly FDA-approved oral budesonide therapy (Eohilia), may also be challenging because many children have precluding aversions to oral therapy. “Regardless of age, treatment choice for EoE should be a good fit that is a plausible addition to a family’s lifestyle,” she said.
Blocking interleukin 4 and interleukin 13 inflammatory pathways, dupilumab has shown efficacy in other atopic diseases such as eczema. It broadly inhibits most aspects of type 2 inflammation and that action is reflected in its histologic and transcriptomic effects in affected tissues, Dr. Chehade and associates explained.
The Trial
Conducted at one Canadian and 26 US sites, the two-part phase 3 study randomly assigned 102 EoE patients aged 1-11 years who were refractory to proton pump inhibition in a 2:2:1:1 ratio.
Part A enrolled 102 patients and evaluated dupilumab at a weight-tiered higher-dose or lower-dose regimen vs placebo (two groups) for 16 weeks.
Part B was a 36-week extended active treatment period in which eligible dupilumab recipients from part A maintained their weight-tiered higher- or lower-dose regimen, whereas those in the placebo groups switched to weight-tiered higher- or lower-dose dupilumab.
The primary end point was histologic remission (peak esophageal intraepithelial eosinophil count, ≤ 6 per high-power field) at week 16. Continued dupilumab treatment appeared to maintain its effect through week 52.
During part A, histologic remission occurred in 25 of the 37 higher-exposure patients (68%), 18 of the 31 lower-exposure patients (58%), and one of the 34 placebo patients (3%).
The difference between the higher-exposure regimen and placebo was 65 percentage points (95% confidence interval [CI], 48-81; P < .001), whereas that between the lower-exposure regimen and placebo was 55 percentage points (95% CI, 37-73; P < .001).
Higher exposure led to significant improvements in histologic, endoscopic, and transcriptomic measures over placebo. Improvements between baseline and week 52 in all patients were generally similar to those between baseline and week 16 in patients who received dupilumab in part A.
As for adverse events, in part A, the incidence of coronavirus disease, nausea, injection-site pain, and headache was at least 10 percentage points higher among dupilumab recipients at either dose than among placebo recipients. Serious adverse events were reported in three dupilumab patients during part A and in six patients overall during part B.
A Balanced Approach
On a cautionary note, Eric H. Chiou, MD, an assistant professor of pediatrics at Baylor College of Medicine and a pediatric gastroenterologist at Texas Children’s Hospital in Houston, said that while dupilumab shows great promise, further research is needed on its cost-effectiveness in EoE.
“The cost of treatment will need to be compared relative to potential long-term savings from reduced hospitalizations, fewer complications, and improved quality of life,” said Dr. Chiou, who was not involved in the study. “A balanced approach that considers clinical efficacy, patient well-being, cost-effectiveness, and equity is essential.”
He added that despite the study’s encouraging results, long-term safety and efficacy data are needed to fully understand the impact of dupilumab on pediatric patients with EoE. “Dupilumab will need to be compared with existing treatments for EoE such as dietary management and swallowed topical corticosteroids in terms of efficacy, safety, and quality of life improvements.”
Additionally, further research is required to identify which patients are most likely to benefit from this therapy and to explore any potential complications associated with its long-term use. “Understanding the optimal dosing and duration of treatment will also be crucial for maximizing benefits while minimizing risks,” Dr. Chiou said.
Dr. Chehade agreed. “While it’s that great that young children finally have an FDA-approved drug to treat their EoE, more research is needed to learn which patient subsets would derive maximum benefit from dupilumab and at which specific steps in their medical management journey should dupilumab be used.”
This study was supported by Sanofi and Regeneron Pharmaceuticals. Dr. Chehade disclosed research funding from and consulting for numerous private sector companies, among others, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Shire-Takeda, and Bristol-Myers Squibb. Multiple study coauthors disclosed various relationships with private-sector companies, including Sanofi and Regeneron Pharmaceuticals, for research funding, consulting, travel, employment, and stock or intellectual ownership. Dr. Webster and Dr. Chiou disclosed no competing interests relevant to their comments.
A version of this article first appeared on Medscape.com.
Good news for younger children suffering from the uncommon but debilitating gastrointestinal condition eosinophilic esophagitis (EoE): Data from this trial led to a January US Food and Drug Administration (FDA) approval of the anti-inflammatory biologic for patients aged 1-11 years weighing at least 15 kg.
In addition, the trial, published in The New England Journal of Medicine, found that a higher-exposure dupilumab regimen (approximating the trough concentration of a 300-mg dose administered once weekly versus every 2 weeks) improved key secondary end points, according to gastroenterologist Mirna Chehade, MD, MPH, AGAF, a professor of pediatrics at Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital in New York City, and colleagues.
In 2022, the FDA approved the drug for those aged 12 or older weighing at least 40 kg.
“Left untreated or inadequately treated, EoE can progress to esophageal narrowing and strictures, leading to increased risk of food impactions and the need for esophageal dilations,” Dr. Chehade said in an interview. “Therefore, it’s important that children with EoE have the FDA-approved treatment option based on our study that can address their underlying disease starting at a young age.”
She added that dupilumab has the exciting potential to transform the standard of care for many young children living with EoE. “There are, however, factors to consider before switching a child to dupilumab — all related to the child’s specific medical history and therefore the perceived potential benefits from the drug.”
Commenting on the study but not involved in it, Toni Webster, DO, a pediatric gastroenterologist at Cohen Children’s Medical Center in Queens, New York, and an assistant professor at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, said, “Like many allergic diseases, EoE is on the rise and, unfortunately, is affecting our children at alarming rates and at earlier ages. Given its efficacy and side-effect profile, dupilumab will vastly change our ability to treat EoE, especially for families who find diet and daily medication to be a challenge.”
Dr. Webster noted that an elimination diet is a rigorous choice that is often difficult to navigate. And the oral administration of off-label choices, proton pump inhibitors, and swallowed topical steroids, as well as the newly FDA-approved oral budesonide therapy (Eohilia), may also be challenging because many children have precluding aversions to oral therapy. “Regardless of age, treatment choice for EoE should be a good fit that is a plausible addition to a family’s lifestyle,” she said.
Blocking interleukin 4 and interleukin 13 inflammatory pathways, dupilumab has shown efficacy in other atopic diseases such as eczema. It broadly inhibits most aspects of type 2 inflammation and that action is reflected in its histologic and transcriptomic effects in affected tissues, Dr. Chehade and associates explained.
The Trial
Conducted at one Canadian and 26 US sites, the two-part phase 3 study randomly assigned 102 EoE patients aged 1-11 years who were refractory to proton pump inhibition in a 2:2:1:1 ratio.
Part A enrolled 102 patients and evaluated dupilumab at a weight-tiered higher-dose or lower-dose regimen vs placebo (two groups) for 16 weeks.
Part B was a 36-week extended active treatment period in which eligible dupilumab recipients from part A maintained their weight-tiered higher- or lower-dose regimen, whereas those in the placebo groups switched to weight-tiered higher- or lower-dose dupilumab.
The primary end point was histologic remission (peak esophageal intraepithelial eosinophil count, ≤ 6 per high-power field) at week 16. Continued dupilumab treatment appeared to maintain its effect through week 52.
During part A, histologic remission occurred in 25 of the 37 higher-exposure patients (68%), 18 of the 31 lower-exposure patients (58%), and one of the 34 placebo patients (3%).
The difference between the higher-exposure regimen and placebo was 65 percentage points (95% confidence interval [CI], 48-81; P < .001), whereas that between the lower-exposure regimen and placebo was 55 percentage points (95% CI, 37-73; P < .001).
Higher exposure led to significant improvements in histologic, endoscopic, and transcriptomic measures over placebo. Improvements between baseline and week 52 in all patients were generally similar to those between baseline and week 16 in patients who received dupilumab in part A.
As for adverse events, in part A, the incidence of coronavirus disease, nausea, injection-site pain, and headache was at least 10 percentage points higher among dupilumab recipients at either dose than among placebo recipients. Serious adverse events were reported in three dupilumab patients during part A and in six patients overall during part B.
A Balanced Approach
On a cautionary note, Eric H. Chiou, MD, an assistant professor of pediatrics at Baylor College of Medicine and a pediatric gastroenterologist at Texas Children’s Hospital in Houston, said that while dupilumab shows great promise, further research is needed on its cost-effectiveness in EoE.
“The cost of treatment will need to be compared relative to potential long-term savings from reduced hospitalizations, fewer complications, and improved quality of life,” said Dr. Chiou, who was not involved in the study. “A balanced approach that considers clinical efficacy, patient well-being, cost-effectiveness, and equity is essential.”
He added that despite the study’s encouraging results, long-term safety and efficacy data are needed to fully understand the impact of dupilumab on pediatric patients with EoE. “Dupilumab will need to be compared with existing treatments for EoE such as dietary management and swallowed topical corticosteroids in terms of efficacy, safety, and quality of life improvements.”
Additionally, further research is required to identify which patients are most likely to benefit from this therapy and to explore any potential complications associated with its long-term use. “Understanding the optimal dosing and duration of treatment will also be crucial for maximizing benefits while minimizing risks,” Dr. Chiou said.
Dr. Chehade agreed. “While it’s that great that young children finally have an FDA-approved drug to treat their EoE, more research is needed to learn which patient subsets would derive maximum benefit from dupilumab and at which specific steps in their medical management journey should dupilumab be used.”
This study was supported by Sanofi and Regeneron Pharmaceuticals. Dr. Chehade disclosed research funding from and consulting for numerous private sector companies, among others, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Shire-Takeda, and Bristol-Myers Squibb. Multiple study coauthors disclosed various relationships with private-sector companies, including Sanofi and Regeneron Pharmaceuticals, for research funding, consulting, travel, employment, and stock or intellectual ownership. Dr. Webster and Dr. Chiou disclosed no competing interests relevant to their comments.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
GLP-1 Receptor Agonists in Endoscopy
Dear colleagues,
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are revolutionizing the field of obesity management and are now common medication in patients presenting for endoscopy. With their effect on gastric emptying, the American Society of Anesthesiologists has recommended cessation of such agents prior to endoscopy. However, is this necessary in patients who have been on a clear liquid diet in preparation for a colonoscopy or who are undergoing moderate sedation? Additionally, there are risks to holding GLP-1 RAs, especially for those taking them for glycemic control.
In this issue of Perspectives, Dr. Thomas Hickey and Dr. Ryan Pouliot discuss the nuances of pre-procedure cessation from an anesthesiologist’s perspective. Dr. Jana Al Hashash provides a gastroenterologist’s view, also highlighting the current paucity of evidence guiding management strategies. We hope these pieces will help your discussions in managing GLP-1 RAs prior to endoscopy in your own practice. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Connecticut, and chief of endoscopy at West Haven (Connecticut) VA Medical Center. He is an associate editor for GI & Hepatology News.
GLP-1 Receptor Agonists in Endoscopy
BY THOMAS R. HICKEY, MD; RYAN C. POULIOT, MD
In response to the recent dramatic increase in GLP-1 receptor agonist (GLP-1RA) prescribing and at the urging of its membership, the American Society of Anesthesiologists issued guidance on the preoperative management of these medications. The big takeaways were recommendations that patients on daily dosing should hold their dose on the day of a procedure, and that patients on weekly dosing should hold their dose a week prior.
The ASA guidance recognizes the sparse available evidence base and makes its recommendations in the spirit of patient safety, presuming that a more conservative approach will mitigate risk of rare but potentially devastating pulmonary aspiration, until prospective evidence informs the ideal approach. Until that approach is defined, whether more or less conservative, it is expected that anesthesiologists will adhere to their professional society’s recommendations.
Meanwhile, the American Gastroenterological Association Institute Rapid Clinical Practice Update (CPU) makes little distinction in the management of the endoscopy patient on GLP-1RA. A key refrain throughout the CPU is that there is no actionable data to justify the harms that may come to patients from stopping these medications (e.g., withdrawal of benefit to glycemic control and cardiovascular health) and in delaying or canceling procedures, which could lead to further stress on an overburdened workforce and add complexity to periprocedural processes.
Anesthesiologists should rightly consider themselves leaders in patient safety. As such, when a serious safety concern emerges they should be compelled to caution despite the possibility of other harms, until their concerns are mitigated by robust clinical evidence. Thankfully these questions are quite amenable to research, and prospective trials are already reporting compelling data that residual gastric contents, clearly a risk factor for aspiration, are increased in GLP-1RA groups compared to controls. This is evident even while following recommended fasting times and abstinences from these medications, and adjusting for confounders (e.g., age, diabetes, body mass index).1,2 It logically follows that large studies are likely to find an increased aspiration risk in GLP-1RA populations. Indeed, this increased risk has already been identified in a large retrospective study of endoscopy patients.3 These findings support the ASA’s caution. Additional data indicate that standard fasting guidelines in this patient population may be inadequate.4
The ASA guidance does not differentiate between patients undergoing surgery in the operating room and procedures in the endoscopy suite. Part of our task is to provide perspective on whether GLP-1RA management deserves different treatment for endoscopy patients. We can only speculate pending further data. For example, a prolonged fasting period including a full day of clears, with or without a bowel prep, intuitively protects against pulmonary aspiration. However, this is unlikely to mitigate an anesthesiologist’s concern that administration of propofol, frequently to a state of general anesthesia with an unsecured airway and resulting in a patient devoid of airway protection reflexes, is an inherently higher risk scenario for aspiration compared to surgery in the operating room with a secured airway. We also expect prospective trials will confirm retrospective findings that both propofol and procedures including upper endoscopy confer a higher risk for aspiration compared with conscious sedation and colonoscopy.3
We suggest a reasonable approach based on society guidance and existing evidence, pending additional data. Endoscopists and anesthesiologists should continue this important conversation with a specific focus on risks and benefits in order to decrease conflict and achieve consensus. If anesthesia care is desired, the patient instructions should be updated to reflect ASA guidance. Special attention should be paid to the “gray area,” for example those who did not hold the GLP-1 agonist as recommended.
This category of patients can be considered on a case-by-case basis by the anesthesiologist, proceduralist, and patient, with a range of options including: proceeding with endoscopist-directed sedation, proceeding with anesthesiology-administered conscious sedation, rescheduling the procedure, and proceeding with general anesthesia with rapid-sequence intubation. In addition to patient factors (e.g., GI symptoms, urgency of procedure), this consideration would vary based on local resources (e.g., presence or absence of anesthesia support staff, emergency airway equipment, nursing staff to comfort recovering patients after general endotracheal anesthesia), and aspiration risk inherent to the procedure (e.g., upper and or combination upper and lower endoscopy vs colonoscopy alone). Proficiency and availability of point-of-care ultrasound are rapidly increasing; adoption of a pre-procedure gastric ultrasound to assess for solids, thick liquids, or large volume of clear liquids may provide a less nuanced, more objective means to address this question.
While the question of periprocedural management of these medications has generated intense interest among anesthesiologists and endoscopists alike, it is worth noting the net positive health effects these drugs are likely to have on our patients, including improved glycemic control, significant weight loss, and decreased cardiovascular risk. We are eager to see whether these benefits translate into an overall improvement in periprocedural outcomes, including in our endoscopy patients.
Dr. Hickey is assistant professor of anesthesiology at the Yale University School of Medicine, New Haven, Connecticut, and the VA Connecticut Healthcare System. Dr. Pouliot is assistant professor of anesthesiology at the Geisel School of Medicine at Dartmouth, Hanover, New Hampshire, and Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire.
References
1. Sherwin M et al. Influence of semaglutide use on the presence of residual gastric solids on gastric ultrasound: A prospective observational study in volunteers without obesity recently started on semaglutide. Can J Anaesth. 2023 Aug. doi:10.1007/s12630-023-02549-5.
2. Wu F et al. Association of glucagon-like peptide receptor 1 agonist therapy with the presence of gastric contents in fasting patients undergoing endoscopy under anesthesia care: A historical cohort study. Can J Anaesth. 2024 Mar 14. doi:10.1007/s12630-024-02719-z.
3. Yeo YH et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use. Gastroenterology. 2024 Mar 27. doi:10.1053/j.gastro.2024.03.015.
4. Sen S et al. Glucagon-like peptide-1 receptor agonist use and residual gastric content before anesthesia. JAMA Surg. 2024 Mar 6. doi:10.1001/jamasurg.2024.0111.
The Impact of GLP-1 Receptor Agonists On Endoscopy
BY JANA G. AL HASHASH, MD, MSc, AGAF
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have been approved for the treatment of type 2 diabetes mellitus since 2005. They have become more widely used over the last couple of years for weight loss in individuals who suffer from adiposity-based chronic disease.
The remarkable positive effects that GLP-1 RAs have had on weight loss as well as other medical conditions such as heart disease, hypertension, metabolic dysfunction–associated steatotic liver disease, among many others, have gained these drugs more traction. Even in situations when insurance companies deny coverage of GLP-1 RAs, many patients have been resorting to other routes to obtain these medications, commonly by purchasing them from online compounding pharmacies.
As such, more and more of our patients who present to endoscopy suites across the country are on one of the available GLP-1 RAs. This has necessitated endoscopists and anesthesiologists to become more familiar with the impact of GLP-1 RAs on patients undergoing endoscopic procedures.
Similar to narcotics, GLP-1 RAs affect gastrointestinal motility and delay gastric emptying. Common side effects of patients receiving GLP-1 RAs include nausea, vomiting, and increased satiety. Patients on GLP-1 RAs for weight loss may also have other contributing risk factors for gastroparesis such as diabetes mellitus which may further delay gastric emptying.
For endoscopists, our goals are to achieve the highest quality examination in the safest way possible. As such, being on a GLP-1 RAs could compromise both goals; but to date, the exact impact of these drugs on exam quality and patient safety is yet to be determined.
Studies have shown that patients on GLP-1 RAs have increased gastric residue on upper endoscopy compared with patients not on GLP-1 RAs. The effect of this increased residue on aspiration risk and clinically meaningful patient outcomes is being investigated, and the available published data are conflicting. Additionally, other published cases have shown that GLP-1 RAs are associated with increased solid gastric residue but not liquids, and that symptoms of dyspepsia and abdominal bloating are associated with an increased probability of residual gastric content.
Given the valid concern for increased gastric content residue, anesthesia specialists became more strict about which GLP-1 RA users they would agree to sedate, which ones they would intubate, and which procedures they would cancel. As one would imagine, cancellation and intubation rates have been increasing, and these have affected the schedules of patients, their families, and physicians.
The concern with GLP-1 RAs does not only apply to upper endoscopies, but also impacts colonoscopies. In addition to the concerns of aspiration and pneumonia, studies have shown that the use of GLP-1 RAs may be associated with a lower quality of bowel preparation and higher need for repeat colonoscopy. A study, which I believe is critical, showed that patients on GLP-1 RAs who were scheduled for upper endoscopy and colonoscopy were found to have less gastric residue and less risk of complications when compared with patients who were only having an upper endoscopy. This study sets the stage for a modified prep for patients on GLP-1 RAs prior to their procedures, since patients who received a modified/extended liquid diet on the day prior to their procedure (those preparing for a colonoscopy), had a protective effect against retained gastric content.
Clearly, there is a knowledge gap and a need for guidance. In our recently published AGA Rapid CPU, we advised an individualized approach to managing patients on GLP-1 RAs in the pre-endoscopic setting. Factors to consider are the indication for the GLP-1 RAs, the dose being used, duration of use, and indication and urgency of the procedure, as well as the presence of symptoms in the preoperative area (i.e., do patients have any nausea, vomiting, dyspepsia, etc.). Also an important factor is the facility in which the endoscopy will be taking place, as certain centers have the capacity to act fast and prevent complications or address them in a timely manner while other centers may not be prepared.
We proposed that a modified liquid diet be considered in patients prior to their endoscopies by advising patients to adhere to a clear liquid diet the day before the procedure, as this may help decrease gastric residue and be the safest and best approach for patients on GLP-1 RAs. Of course, it is important to note that more prospective studies are needed to inform clinical practice, and until then, we will have to individualize our approach and continue to put patient safety first.
Dr. Al Hashash is a gastroenterologist and associate professor of medicine at Mayo Clinic, Jacksonville, Florida.
Dear colleagues,
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are revolutionizing the field of obesity management and are now common medication in patients presenting for endoscopy. With their effect on gastric emptying, the American Society of Anesthesiologists has recommended cessation of such agents prior to endoscopy. However, is this necessary in patients who have been on a clear liquid diet in preparation for a colonoscopy or who are undergoing moderate sedation? Additionally, there are risks to holding GLP-1 RAs, especially for those taking them for glycemic control.
In this issue of Perspectives, Dr. Thomas Hickey and Dr. Ryan Pouliot discuss the nuances of pre-procedure cessation from an anesthesiologist’s perspective. Dr. Jana Al Hashash provides a gastroenterologist’s view, also highlighting the current paucity of evidence guiding management strategies. We hope these pieces will help your discussions in managing GLP-1 RAs prior to endoscopy in your own practice. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Connecticut, and chief of endoscopy at West Haven (Connecticut) VA Medical Center. He is an associate editor for GI & Hepatology News.
GLP-1 Receptor Agonists in Endoscopy
BY THOMAS R. HICKEY, MD; RYAN C. POULIOT, MD
In response to the recent dramatic increase in GLP-1 receptor agonist (GLP-1RA) prescribing and at the urging of its membership, the American Society of Anesthesiologists issued guidance on the preoperative management of these medications. The big takeaways were recommendations that patients on daily dosing should hold their dose on the day of a procedure, and that patients on weekly dosing should hold their dose a week prior.
The ASA guidance recognizes the sparse available evidence base and makes its recommendations in the spirit of patient safety, presuming that a more conservative approach will mitigate risk of rare but potentially devastating pulmonary aspiration, until prospective evidence informs the ideal approach. Until that approach is defined, whether more or less conservative, it is expected that anesthesiologists will adhere to their professional society’s recommendations.
Meanwhile, the American Gastroenterological Association Institute Rapid Clinical Practice Update (CPU) makes little distinction in the management of the endoscopy patient on GLP-1RA. A key refrain throughout the CPU is that there is no actionable data to justify the harms that may come to patients from stopping these medications (e.g., withdrawal of benefit to glycemic control and cardiovascular health) and in delaying or canceling procedures, which could lead to further stress on an overburdened workforce and add complexity to periprocedural processes.
Anesthesiologists should rightly consider themselves leaders in patient safety. As such, when a serious safety concern emerges they should be compelled to caution despite the possibility of other harms, until their concerns are mitigated by robust clinical evidence. Thankfully these questions are quite amenable to research, and prospective trials are already reporting compelling data that residual gastric contents, clearly a risk factor for aspiration, are increased in GLP-1RA groups compared to controls. This is evident even while following recommended fasting times and abstinences from these medications, and adjusting for confounders (e.g., age, diabetes, body mass index).1,2 It logically follows that large studies are likely to find an increased aspiration risk in GLP-1RA populations. Indeed, this increased risk has already been identified in a large retrospective study of endoscopy patients.3 These findings support the ASA’s caution. Additional data indicate that standard fasting guidelines in this patient population may be inadequate.4
The ASA guidance does not differentiate between patients undergoing surgery in the operating room and procedures in the endoscopy suite. Part of our task is to provide perspective on whether GLP-1RA management deserves different treatment for endoscopy patients. We can only speculate pending further data. For example, a prolonged fasting period including a full day of clears, with or without a bowel prep, intuitively protects against pulmonary aspiration. However, this is unlikely to mitigate an anesthesiologist’s concern that administration of propofol, frequently to a state of general anesthesia with an unsecured airway and resulting in a patient devoid of airway protection reflexes, is an inherently higher risk scenario for aspiration compared to surgery in the operating room with a secured airway. We also expect prospective trials will confirm retrospective findings that both propofol and procedures including upper endoscopy confer a higher risk for aspiration compared with conscious sedation and colonoscopy.3
We suggest a reasonable approach based on society guidance and existing evidence, pending additional data. Endoscopists and anesthesiologists should continue this important conversation with a specific focus on risks and benefits in order to decrease conflict and achieve consensus. If anesthesia care is desired, the patient instructions should be updated to reflect ASA guidance. Special attention should be paid to the “gray area,” for example those who did not hold the GLP-1 agonist as recommended.
This category of patients can be considered on a case-by-case basis by the anesthesiologist, proceduralist, and patient, with a range of options including: proceeding with endoscopist-directed sedation, proceeding with anesthesiology-administered conscious sedation, rescheduling the procedure, and proceeding with general anesthesia with rapid-sequence intubation. In addition to patient factors (e.g., GI symptoms, urgency of procedure), this consideration would vary based on local resources (e.g., presence or absence of anesthesia support staff, emergency airway equipment, nursing staff to comfort recovering patients after general endotracheal anesthesia), and aspiration risk inherent to the procedure (e.g., upper and or combination upper and lower endoscopy vs colonoscopy alone). Proficiency and availability of point-of-care ultrasound are rapidly increasing; adoption of a pre-procedure gastric ultrasound to assess for solids, thick liquids, or large volume of clear liquids may provide a less nuanced, more objective means to address this question.
While the question of periprocedural management of these medications has generated intense interest among anesthesiologists and endoscopists alike, it is worth noting the net positive health effects these drugs are likely to have on our patients, including improved glycemic control, significant weight loss, and decreased cardiovascular risk. We are eager to see whether these benefits translate into an overall improvement in periprocedural outcomes, including in our endoscopy patients.
Dr. Hickey is assistant professor of anesthesiology at the Yale University School of Medicine, New Haven, Connecticut, and the VA Connecticut Healthcare System. Dr. Pouliot is assistant professor of anesthesiology at the Geisel School of Medicine at Dartmouth, Hanover, New Hampshire, and Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire.
References
1. Sherwin M et al. Influence of semaglutide use on the presence of residual gastric solids on gastric ultrasound: A prospective observational study in volunteers without obesity recently started on semaglutide. Can J Anaesth. 2023 Aug. doi:10.1007/s12630-023-02549-5.
2. Wu F et al. Association of glucagon-like peptide receptor 1 agonist therapy with the presence of gastric contents in fasting patients undergoing endoscopy under anesthesia care: A historical cohort study. Can J Anaesth. 2024 Mar 14. doi:10.1007/s12630-024-02719-z.
3. Yeo YH et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use. Gastroenterology. 2024 Mar 27. doi:10.1053/j.gastro.2024.03.015.
4. Sen S et al. Glucagon-like peptide-1 receptor agonist use and residual gastric content before anesthesia. JAMA Surg. 2024 Mar 6. doi:10.1001/jamasurg.2024.0111.
The Impact of GLP-1 Receptor Agonists On Endoscopy
BY JANA G. AL HASHASH, MD, MSc, AGAF
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have been approved for the treatment of type 2 diabetes mellitus since 2005. They have become more widely used over the last couple of years for weight loss in individuals who suffer from adiposity-based chronic disease.
The remarkable positive effects that GLP-1 RAs have had on weight loss as well as other medical conditions such as heart disease, hypertension, metabolic dysfunction–associated steatotic liver disease, among many others, have gained these drugs more traction. Even in situations when insurance companies deny coverage of GLP-1 RAs, many patients have been resorting to other routes to obtain these medications, commonly by purchasing them from online compounding pharmacies.
As such, more and more of our patients who present to endoscopy suites across the country are on one of the available GLP-1 RAs. This has necessitated endoscopists and anesthesiologists to become more familiar with the impact of GLP-1 RAs on patients undergoing endoscopic procedures.
Similar to narcotics, GLP-1 RAs affect gastrointestinal motility and delay gastric emptying. Common side effects of patients receiving GLP-1 RAs include nausea, vomiting, and increased satiety. Patients on GLP-1 RAs for weight loss may also have other contributing risk factors for gastroparesis such as diabetes mellitus which may further delay gastric emptying.
For endoscopists, our goals are to achieve the highest quality examination in the safest way possible. As such, being on a GLP-1 RAs could compromise both goals; but to date, the exact impact of these drugs on exam quality and patient safety is yet to be determined.
Studies have shown that patients on GLP-1 RAs have increased gastric residue on upper endoscopy compared with patients not on GLP-1 RAs. The effect of this increased residue on aspiration risk and clinically meaningful patient outcomes is being investigated, and the available published data are conflicting. Additionally, other published cases have shown that GLP-1 RAs are associated with increased solid gastric residue but not liquids, and that symptoms of dyspepsia and abdominal bloating are associated with an increased probability of residual gastric content.
Given the valid concern for increased gastric content residue, anesthesia specialists became more strict about which GLP-1 RA users they would agree to sedate, which ones they would intubate, and which procedures they would cancel. As one would imagine, cancellation and intubation rates have been increasing, and these have affected the schedules of patients, their families, and physicians.
The concern with GLP-1 RAs does not only apply to upper endoscopies, but also impacts colonoscopies. In addition to the concerns of aspiration and pneumonia, studies have shown that the use of GLP-1 RAs may be associated with a lower quality of bowel preparation and higher need for repeat colonoscopy. A study, which I believe is critical, showed that patients on GLP-1 RAs who were scheduled for upper endoscopy and colonoscopy were found to have less gastric residue and less risk of complications when compared with patients who were only having an upper endoscopy. This study sets the stage for a modified prep for patients on GLP-1 RAs prior to their procedures, since patients who received a modified/extended liquid diet on the day prior to their procedure (those preparing for a colonoscopy), had a protective effect against retained gastric content.
Clearly, there is a knowledge gap and a need for guidance. In our recently published AGA Rapid CPU, we advised an individualized approach to managing patients on GLP-1 RAs in the pre-endoscopic setting. Factors to consider are the indication for the GLP-1 RAs, the dose being used, duration of use, and indication and urgency of the procedure, as well as the presence of symptoms in the preoperative area (i.e., do patients have any nausea, vomiting, dyspepsia, etc.). Also an important factor is the facility in which the endoscopy will be taking place, as certain centers have the capacity to act fast and prevent complications or address them in a timely manner while other centers may not be prepared.
We proposed that a modified liquid diet be considered in patients prior to their endoscopies by advising patients to adhere to a clear liquid diet the day before the procedure, as this may help decrease gastric residue and be the safest and best approach for patients on GLP-1 RAs. Of course, it is important to note that more prospective studies are needed to inform clinical practice, and until then, we will have to individualize our approach and continue to put patient safety first.
Dr. Al Hashash is a gastroenterologist and associate professor of medicine at Mayo Clinic, Jacksonville, Florida.
Dear colleagues,
Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are revolutionizing the field of obesity management and are now common medication in patients presenting for endoscopy. With their effect on gastric emptying, the American Society of Anesthesiologists has recommended cessation of such agents prior to endoscopy. However, is this necessary in patients who have been on a clear liquid diet in preparation for a colonoscopy or who are undergoing moderate sedation? Additionally, there are risks to holding GLP-1 RAs, especially for those taking them for glycemic control.
In this issue of Perspectives, Dr. Thomas Hickey and Dr. Ryan Pouliot discuss the nuances of pre-procedure cessation from an anesthesiologist’s perspective. Dr. Jana Al Hashash provides a gastroenterologist’s view, also highlighting the current paucity of evidence guiding management strategies. We hope these pieces will help your discussions in managing GLP-1 RAs prior to endoscopy in your own practice. We welcome your thoughts on this issue on X @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Connecticut, and chief of endoscopy at West Haven (Connecticut) VA Medical Center. He is an associate editor for GI & Hepatology News.
GLP-1 Receptor Agonists in Endoscopy
BY THOMAS R. HICKEY, MD; RYAN C. POULIOT, MD
In response to the recent dramatic increase in GLP-1 receptor agonist (GLP-1RA) prescribing and at the urging of its membership, the American Society of Anesthesiologists issued guidance on the preoperative management of these medications. The big takeaways were recommendations that patients on daily dosing should hold their dose on the day of a procedure, and that patients on weekly dosing should hold their dose a week prior.
The ASA guidance recognizes the sparse available evidence base and makes its recommendations in the spirit of patient safety, presuming that a more conservative approach will mitigate risk of rare but potentially devastating pulmonary aspiration, until prospective evidence informs the ideal approach. Until that approach is defined, whether more or less conservative, it is expected that anesthesiologists will adhere to their professional society’s recommendations.
Meanwhile, the American Gastroenterological Association Institute Rapid Clinical Practice Update (CPU) makes little distinction in the management of the endoscopy patient on GLP-1RA. A key refrain throughout the CPU is that there is no actionable data to justify the harms that may come to patients from stopping these medications (e.g., withdrawal of benefit to glycemic control and cardiovascular health) and in delaying or canceling procedures, which could lead to further stress on an overburdened workforce and add complexity to periprocedural processes.
Anesthesiologists should rightly consider themselves leaders in patient safety. As such, when a serious safety concern emerges they should be compelled to caution despite the possibility of other harms, until their concerns are mitigated by robust clinical evidence. Thankfully these questions are quite amenable to research, and prospective trials are already reporting compelling data that residual gastric contents, clearly a risk factor for aspiration, are increased in GLP-1RA groups compared to controls. This is evident even while following recommended fasting times and abstinences from these medications, and adjusting for confounders (e.g., age, diabetes, body mass index).1,2 It logically follows that large studies are likely to find an increased aspiration risk in GLP-1RA populations. Indeed, this increased risk has already been identified in a large retrospective study of endoscopy patients.3 These findings support the ASA’s caution. Additional data indicate that standard fasting guidelines in this patient population may be inadequate.4
The ASA guidance does not differentiate between patients undergoing surgery in the operating room and procedures in the endoscopy suite. Part of our task is to provide perspective on whether GLP-1RA management deserves different treatment for endoscopy patients. We can only speculate pending further data. For example, a prolonged fasting period including a full day of clears, with or without a bowel prep, intuitively protects against pulmonary aspiration. However, this is unlikely to mitigate an anesthesiologist’s concern that administration of propofol, frequently to a state of general anesthesia with an unsecured airway and resulting in a patient devoid of airway protection reflexes, is an inherently higher risk scenario for aspiration compared to surgery in the operating room with a secured airway. We also expect prospective trials will confirm retrospective findings that both propofol and procedures including upper endoscopy confer a higher risk for aspiration compared with conscious sedation and colonoscopy.3
We suggest a reasonable approach based on society guidance and existing evidence, pending additional data. Endoscopists and anesthesiologists should continue this important conversation with a specific focus on risks and benefits in order to decrease conflict and achieve consensus. If anesthesia care is desired, the patient instructions should be updated to reflect ASA guidance. Special attention should be paid to the “gray area,” for example those who did not hold the GLP-1 agonist as recommended.
This category of patients can be considered on a case-by-case basis by the anesthesiologist, proceduralist, and patient, with a range of options including: proceeding with endoscopist-directed sedation, proceeding with anesthesiology-administered conscious sedation, rescheduling the procedure, and proceeding with general anesthesia with rapid-sequence intubation. In addition to patient factors (e.g., GI symptoms, urgency of procedure), this consideration would vary based on local resources (e.g., presence or absence of anesthesia support staff, emergency airway equipment, nursing staff to comfort recovering patients after general endotracheal anesthesia), and aspiration risk inherent to the procedure (e.g., upper and or combination upper and lower endoscopy vs colonoscopy alone). Proficiency and availability of point-of-care ultrasound are rapidly increasing; adoption of a pre-procedure gastric ultrasound to assess for solids, thick liquids, or large volume of clear liquids may provide a less nuanced, more objective means to address this question.
While the question of periprocedural management of these medications has generated intense interest among anesthesiologists and endoscopists alike, it is worth noting the net positive health effects these drugs are likely to have on our patients, including improved glycemic control, significant weight loss, and decreased cardiovascular risk. We are eager to see whether these benefits translate into an overall improvement in periprocedural outcomes, including in our endoscopy patients.
Dr. Hickey is assistant professor of anesthesiology at the Yale University School of Medicine, New Haven, Connecticut, and the VA Connecticut Healthcare System. Dr. Pouliot is assistant professor of anesthesiology at the Geisel School of Medicine at Dartmouth, Hanover, New Hampshire, and Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire.
References
1. Sherwin M et al. Influence of semaglutide use on the presence of residual gastric solids on gastric ultrasound: A prospective observational study in volunteers without obesity recently started on semaglutide. Can J Anaesth. 2023 Aug. doi:10.1007/s12630-023-02549-5.
2. Wu F et al. Association of glucagon-like peptide receptor 1 agonist therapy with the presence of gastric contents in fasting patients undergoing endoscopy under anesthesia care: A historical cohort study. Can J Anaesth. 2024 Mar 14. doi:10.1007/s12630-024-02719-z.
3. Yeo YH et al. Increased risk of aspiration pneumonia associated with endoscopic procedures among patients with glucagon-like peptide 1 receptor agonist use. Gastroenterology. 2024 Mar 27. doi:10.1053/j.gastro.2024.03.015.
4. Sen S et al. Glucagon-like peptide-1 receptor agonist use and residual gastric content before anesthesia. JAMA Surg. 2024 Mar 6. doi:10.1001/jamasurg.2024.0111.
The Impact of GLP-1 Receptor Agonists On Endoscopy
BY JANA G. AL HASHASH, MD, MSc, AGAF
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) have been approved for the treatment of type 2 diabetes mellitus since 2005. They have become more widely used over the last couple of years for weight loss in individuals who suffer from adiposity-based chronic disease.
The remarkable positive effects that GLP-1 RAs have had on weight loss as well as other medical conditions such as heart disease, hypertension, metabolic dysfunction–associated steatotic liver disease, among many others, have gained these drugs more traction. Even in situations when insurance companies deny coverage of GLP-1 RAs, many patients have been resorting to other routes to obtain these medications, commonly by purchasing them from online compounding pharmacies.
As such, more and more of our patients who present to endoscopy suites across the country are on one of the available GLP-1 RAs. This has necessitated endoscopists and anesthesiologists to become more familiar with the impact of GLP-1 RAs on patients undergoing endoscopic procedures.
Similar to narcotics, GLP-1 RAs affect gastrointestinal motility and delay gastric emptying. Common side effects of patients receiving GLP-1 RAs include nausea, vomiting, and increased satiety. Patients on GLP-1 RAs for weight loss may also have other contributing risk factors for gastroparesis such as diabetes mellitus which may further delay gastric emptying.
For endoscopists, our goals are to achieve the highest quality examination in the safest way possible. As such, being on a GLP-1 RAs could compromise both goals; but to date, the exact impact of these drugs on exam quality and patient safety is yet to be determined.
Studies have shown that patients on GLP-1 RAs have increased gastric residue on upper endoscopy compared with patients not on GLP-1 RAs. The effect of this increased residue on aspiration risk and clinically meaningful patient outcomes is being investigated, and the available published data are conflicting. Additionally, other published cases have shown that GLP-1 RAs are associated with increased solid gastric residue but not liquids, and that symptoms of dyspepsia and abdominal bloating are associated with an increased probability of residual gastric content.
Given the valid concern for increased gastric content residue, anesthesia specialists became more strict about which GLP-1 RA users they would agree to sedate, which ones they would intubate, and which procedures they would cancel. As one would imagine, cancellation and intubation rates have been increasing, and these have affected the schedules of patients, their families, and physicians.
The concern with GLP-1 RAs does not only apply to upper endoscopies, but also impacts colonoscopies. In addition to the concerns of aspiration and pneumonia, studies have shown that the use of GLP-1 RAs may be associated with a lower quality of bowel preparation and higher need for repeat colonoscopy. A study, which I believe is critical, showed that patients on GLP-1 RAs who were scheduled for upper endoscopy and colonoscopy were found to have less gastric residue and less risk of complications when compared with patients who were only having an upper endoscopy. This study sets the stage for a modified prep for patients on GLP-1 RAs prior to their procedures, since patients who received a modified/extended liquid diet on the day prior to their procedure (those preparing for a colonoscopy), had a protective effect against retained gastric content.
Clearly, there is a knowledge gap and a need for guidance. In our recently published AGA Rapid CPU, we advised an individualized approach to managing patients on GLP-1 RAs in the pre-endoscopic setting. Factors to consider are the indication for the GLP-1 RAs, the dose being used, duration of use, and indication and urgency of the procedure, as well as the presence of symptoms in the preoperative area (i.e., do patients have any nausea, vomiting, dyspepsia, etc.). Also an important factor is the facility in which the endoscopy will be taking place, as certain centers have the capacity to act fast and prevent complications or address them in a timely manner while other centers may not be prepared.
We proposed that a modified liquid diet be considered in patients prior to their endoscopies by advising patients to adhere to a clear liquid diet the day before the procedure, as this may help decrease gastric residue and be the safest and best approach for patients on GLP-1 RAs. Of course, it is important to note that more prospective studies are needed to inform clinical practice, and until then, we will have to individualize our approach and continue to put patient safety first.
Dr. Al Hashash is a gastroenterologist and associate professor of medicine at Mayo Clinic, Jacksonville, Florida.
Eosinophilic Gastrointestinal Diseases: Beyond EoE
- Dellon ES, Gonsalves N, Abonia JP, et al. International consensus recommendations for eosinophilic gastrointestinal disease nomenclature. Clin Gastroenterol Hepatol. 2022;20(11):2474-2484.e3. doi:10.1016/j.cgh.2022.02.017
- Naramore S, Gupta SK. Nonesophageal eosinophilic gastrointestinal disorders: clinical care and future directions. J Pediatr Gastroenterol Nutr. 2018;67(3):318-321. doi:10.1097/MPG.0000000000002040
- Kinoshita Y, Sanuki T. Review of non-eosinophilic esophagitis-eosinophilic gastrointestinal disease (non-EoE-EGID) and a case series of twenty-eight affected patients. Biomolecules. 2023;13(9):1417. doi:10.3390/biom13091417
- Gonsalves N, Doerfler B, Zalewski A, et al. Prospective study of an amino acid-based elemental diet in an eosinophilic gastritis and gastroenteritis nutrition trial. J Allergy Clin Immunol. 2023;152(3):676-688. doi:10.1016/j.jaci.2023.05.024
- Oshima T. Biologic therapies targeting eosinophilic gastrointestinal diseases. Intern Med. 2023;62(23):3429-3430. doi:10.2169/internalmedicine.1911-23
- Pineton de Chambrun G, Gonzalez F, Canva JY, et al. Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2011;9(11):950-956.e1. doi:10.1016/j.cgh.2011.07.017
- Hirano I, Collins MH, King E, et al; CEGIR Investigators. Prospective endoscopic activity assessment for eosinophilic gastritis in a multi-site cohort. Am J Gastroenterol. 2022;117(3):413-423. doi:10.14309/ajg.0000000000001625
- Pesek RD, Reed CC, Muir AB, et al; Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). Increasing rates of diagnosis, substantial co-occurrence, and variable treatment patterns of eosinophilic gastritis, gastroenteritis, and colitis based on 10-year data across a multicenter consortium. Am J Gastroenterol. 2019;114(6):984-994. doi:10.14309/ajg.0000000000000228
- Dellon ES, Gonsalves N, Abonia JP, et al. International consensus recommendations for eosinophilic gastrointestinal disease nomenclature. Clin Gastroenterol Hepatol. 2022;20(11):2474-2484.e3. doi:10.1016/j.cgh.2022.02.017
- Naramore S, Gupta SK. Nonesophageal eosinophilic gastrointestinal disorders: clinical care and future directions. J Pediatr Gastroenterol Nutr. 2018;67(3):318-321. doi:10.1097/MPG.0000000000002040
- Kinoshita Y, Sanuki T. Review of non-eosinophilic esophagitis-eosinophilic gastrointestinal disease (non-EoE-EGID) and a case series of twenty-eight affected patients. Biomolecules. 2023;13(9):1417. doi:10.3390/biom13091417
- Gonsalves N, Doerfler B, Zalewski A, et al. Prospective study of an amino acid-based elemental diet in an eosinophilic gastritis and gastroenteritis nutrition trial. J Allergy Clin Immunol. 2023;152(3):676-688. doi:10.1016/j.jaci.2023.05.024
- Oshima T. Biologic therapies targeting eosinophilic gastrointestinal diseases. Intern Med. 2023;62(23):3429-3430. doi:10.2169/internalmedicine.1911-23
- Pineton de Chambrun G, Gonzalez F, Canva JY, et al. Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2011;9(11):950-956.e1. doi:10.1016/j.cgh.2011.07.017
- Hirano I, Collins MH, King E, et al; CEGIR Investigators. Prospective endoscopic activity assessment for eosinophilic gastritis in a multi-site cohort. Am J Gastroenterol. 2022;117(3):413-423. doi:10.14309/ajg.0000000000001625
- Pesek RD, Reed CC, Muir AB, et al; Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). Increasing rates of diagnosis, substantial co-occurrence, and variable treatment patterns of eosinophilic gastritis, gastroenteritis, and colitis based on 10-year data across a multicenter consortium. Am J Gastroenterol. 2019;114(6):984-994. doi:10.14309/ajg.0000000000000228
- Dellon ES, Gonsalves N, Abonia JP, et al. International consensus recommendations for eosinophilic gastrointestinal disease nomenclature. Clin Gastroenterol Hepatol. 2022;20(11):2474-2484.e3. doi:10.1016/j.cgh.2022.02.017
- Naramore S, Gupta SK. Nonesophageal eosinophilic gastrointestinal disorders: clinical care and future directions. J Pediatr Gastroenterol Nutr. 2018;67(3):318-321. doi:10.1097/MPG.0000000000002040
- Kinoshita Y, Sanuki T. Review of non-eosinophilic esophagitis-eosinophilic gastrointestinal disease (non-EoE-EGID) and a case series of twenty-eight affected patients. Biomolecules. 2023;13(9):1417. doi:10.3390/biom13091417
- Gonsalves N, Doerfler B, Zalewski A, et al. Prospective study of an amino acid-based elemental diet in an eosinophilic gastritis and gastroenteritis nutrition trial. J Allergy Clin Immunol. 2023;152(3):676-688. doi:10.1016/j.jaci.2023.05.024
- Oshima T. Biologic therapies targeting eosinophilic gastrointestinal diseases. Intern Med. 2023;62(23):3429-3430. doi:10.2169/internalmedicine.1911-23
- Pineton de Chambrun G, Gonzalez F, Canva JY, et al. Natural history of eosinophilic gastroenteritis. Clin Gastroenterol Hepatol. 2011;9(11):950-956.e1. doi:10.1016/j.cgh.2011.07.017
- Hirano I, Collins MH, King E, et al; CEGIR Investigators. Prospective endoscopic activity assessment for eosinophilic gastritis in a multi-site cohort. Am J Gastroenterol. 2022;117(3):413-423. doi:10.14309/ajg.0000000000001625
- Pesek RD, Reed CC, Muir AB, et al; Consortium of Eosinophilic Gastrointestinal Disease Researchers (CEGIR). Increasing rates of diagnosis, substantial co-occurrence, and variable treatment patterns of eosinophilic gastritis, gastroenteritis, and colitis based on 10-year data across a multicenter consortium. Am J Gastroenterol. 2019;114(6):984-994. doi:10.14309/ajg.0000000000000228
Healthy Sleep Linked to Lower Odds for Digestive Diseases
TOPLINE:
Healthier sleep is associated with lower odds of developing a wide range of gastrointestinal conditions, regardless of genetic susceptibility, new research revealed.
METHODOLOGY:
- Due to the widespread prevalence of sleep issues and a growing burden of digestive diseases globally, researchers investigated the association between sleep quality and digestive disorders in a prospective cohort study of 410,586 people in the UK Biobank.
- Five individual sleep behaviors were assessed: sleep duration, insomnia, snoring, daytime sleepiness, and chronotype.
- A healthy sleep was defined as a morning chronotype, 7-8 hours of sleep duration, no self-reported snoring, never or rare insomnia, and a low frequency of daytime sleepiness, for a score of 5/5.
- The study investigators tracked the development of 16 digestive diseases over a mean period of 13.2 years.
- As well as looking at healthy sleep scores, researchers considered genetic susceptibility to gastrointestinal conditions.
TAKEAWAY:
- Of the 16 digestive diseases looked at, the reduction of risk was highest for irritable bowel syndrome at 50% (HR, 0.50; 95% CI, 0.45-0.57).
- A healthy sleep score was also associated with 37% reduced odds for metabolic dysfunction–associated steatotic liver disease (formerly known as nonalcoholic fatty liver disease; HR, 0.63; 95% CI, 0.55-0.71), 35% lower chance for peptic ulcer (HR, 0.65; 95% CI, 0.058-0.74), 34% reduced chance for dyspepsia (HR, 0.66; 95% CI, 0.58-0.75), and a 25% lower risk for diverticulosis (HR, 0.75; 95% CI, 0.71-0.80).
- High genetic risk and poor sleep scores were also associated with increased odds (53% to > 200%) of developing digestive diseases.
- However, healthy sleep reduced the risk for digestive diseases regardless of genetic susceptibility.
IN PRACTICE:
“Our findings underscore the potential holistic impact of different sleep behaviors in mitigating the risk of digestive diseases in clinical practice,” wrote Shiyi Yu, MD, of Guangdong Provincial People’s Hospital, Guangzhou, Guangdong, China, and colleagues.
Poor sleep can also change our gut microbiome, Dr. Yu told this news organization. If you don’t sleep well, the repair of the gut lining cannot be finished during the night.
SOURCE:
The study was presented at the Digestive Disease Week® (DDW), 2024, annual meeting.
DISCLOSURES:
Dr. Yu had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Healthier sleep is associated with lower odds of developing a wide range of gastrointestinal conditions, regardless of genetic susceptibility, new research revealed.
METHODOLOGY:
- Due to the widespread prevalence of sleep issues and a growing burden of digestive diseases globally, researchers investigated the association between sleep quality and digestive disorders in a prospective cohort study of 410,586 people in the UK Biobank.
- Five individual sleep behaviors were assessed: sleep duration, insomnia, snoring, daytime sleepiness, and chronotype.
- A healthy sleep was defined as a morning chronotype, 7-8 hours of sleep duration, no self-reported snoring, never or rare insomnia, and a low frequency of daytime sleepiness, for a score of 5/5.
- The study investigators tracked the development of 16 digestive diseases over a mean period of 13.2 years.
- As well as looking at healthy sleep scores, researchers considered genetic susceptibility to gastrointestinal conditions.
TAKEAWAY:
- Of the 16 digestive diseases looked at, the reduction of risk was highest for irritable bowel syndrome at 50% (HR, 0.50; 95% CI, 0.45-0.57).
- A healthy sleep score was also associated with 37% reduced odds for metabolic dysfunction–associated steatotic liver disease (formerly known as nonalcoholic fatty liver disease; HR, 0.63; 95% CI, 0.55-0.71), 35% lower chance for peptic ulcer (HR, 0.65; 95% CI, 0.058-0.74), 34% reduced chance for dyspepsia (HR, 0.66; 95% CI, 0.58-0.75), and a 25% lower risk for diverticulosis (HR, 0.75; 95% CI, 0.71-0.80).
- High genetic risk and poor sleep scores were also associated with increased odds (53% to > 200%) of developing digestive diseases.
- However, healthy sleep reduced the risk for digestive diseases regardless of genetic susceptibility.
IN PRACTICE:
“Our findings underscore the potential holistic impact of different sleep behaviors in mitigating the risk of digestive diseases in clinical practice,” wrote Shiyi Yu, MD, of Guangdong Provincial People’s Hospital, Guangzhou, Guangdong, China, and colleagues.
Poor sleep can also change our gut microbiome, Dr. Yu told this news organization. If you don’t sleep well, the repair of the gut lining cannot be finished during the night.
SOURCE:
The study was presented at the Digestive Disease Week® (DDW), 2024, annual meeting.
DISCLOSURES:
Dr. Yu had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
TOPLINE:
Healthier sleep is associated with lower odds of developing a wide range of gastrointestinal conditions, regardless of genetic susceptibility, new research revealed.
METHODOLOGY:
- Due to the widespread prevalence of sleep issues and a growing burden of digestive diseases globally, researchers investigated the association between sleep quality and digestive disorders in a prospective cohort study of 410,586 people in the UK Biobank.
- Five individual sleep behaviors were assessed: sleep duration, insomnia, snoring, daytime sleepiness, and chronotype.
- A healthy sleep was defined as a morning chronotype, 7-8 hours of sleep duration, no self-reported snoring, never or rare insomnia, and a low frequency of daytime sleepiness, for a score of 5/5.
- The study investigators tracked the development of 16 digestive diseases over a mean period of 13.2 years.
- As well as looking at healthy sleep scores, researchers considered genetic susceptibility to gastrointestinal conditions.
TAKEAWAY:
- Of the 16 digestive diseases looked at, the reduction of risk was highest for irritable bowel syndrome at 50% (HR, 0.50; 95% CI, 0.45-0.57).
- A healthy sleep score was also associated with 37% reduced odds for metabolic dysfunction–associated steatotic liver disease (formerly known as nonalcoholic fatty liver disease; HR, 0.63; 95% CI, 0.55-0.71), 35% lower chance for peptic ulcer (HR, 0.65; 95% CI, 0.058-0.74), 34% reduced chance for dyspepsia (HR, 0.66; 95% CI, 0.58-0.75), and a 25% lower risk for diverticulosis (HR, 0.75; 95% CI, 0.71-0.80).
- High genetic risk and poor sleep scores were also associated with increased odds (53% to > 200%) of developing digestive diseases.
- However, healthy sleep reduced the risk for digestive diseases regardless of genetic susceptibility.
IN PRACTICE:
“Our findings underscore the potential holistic impact of different sleep behaviors in mitigating the risk of digestive diseases in clinical practice,” wrote Shiyi Yu, MD, of Guangdong Provincial People’s Hospital, Guangzhou, Guangdong, China, and colleagues.
Poor sleep can also change our gut microbiome, Dr. Yu told this news organization. If you don’t sleep well, the repair of the gut lining cannot be finished during the night.
SOURCE:
The study was presented at the Digestive Disease Week® (DDW), 2024, annual meeting.
DISCLOSURES:
Dr. Yu had no relevant financial disclosures.
A version of this article appeared on Medscape.com.
Endoscopic Procedure Targets ‘Hunger Hormone’ for Weight Loss
WASHINGTON — .
“Patients reported a decrease in hunger, appetite, and cravings and an increase in control over [their] eating,” said senior study investigator Christopher McGowan, MD, AGAF, a gastroenterologist in private practice and medical director of True You Weight Loss in Cary, North Carolina.
“They generally described that their relationship with food had changed,” Dr. McGowan said at a May 8 press briefing during which his research (Abstract 516) was previewed for Digestive Disease Week® (DDW).
Researchers targeted the gastric fundus because its mucosal lining contains 80%-90% of the cells that produce ghrelin. When a person diets and/or loses weight, ghrelin levels increase, making the person hungrier and preventing sustained weight loss, Dr. McGowan said.
Previously, the only proven way to reduce ghrelin was to surgically remove or bypass the fundus. Weight-loss medications like Wegovy, Zepbound, and Ozempic target a different hormonal pathway, glucagon-like peptide 1 (GLP-1).
“What we’ve learned from the GLP-1 medications is the profound impact of reducing hunger,” Dr. McGowan said. “That’s what patients describe quite often — that it really changes their life and their quality of life. That’s really, really important.”
Major Findings
In the trial, 10 women (mean age, 38 years; mean body mass index, 40.2) underwent endoscopic fundic mucosal ablation via hybrid argon plasma coagulation in an ambulatory setting under general anesthesia from November 1, 2022, to April 14, 2023. The procedure took less than an hour on average, and the technique gave them easy access to the fundus, Dr. McGowan said.
Compared with baseline, there were multiple beneficial outcomes at 6 months:
- 45% less circulating ghrelin in the blood.
- 53% drop in ghrelin-producing cells in the fundus.
- 42% reduction in stomach capacity.
- 43% decrease in hunger, appetite, and cravings.
- 7.7% body weight loss.
Over the 6 months of the study, mean ghrelin concentrations dropped from 461.6 pg/mL at baseline to 254.8 pg/mL (P = .006).
It is fascinating that the hormone ghrelin decreased just by ablating, said Loren Laine, MD, AGAF, professor of medicine (digestive diseases) at Yale School of Medicine and chair of DDW 2024. “They used the same device that we use to treat bleeding ulcers or lesions in the stomach and applied it broadly over the whole upper part of the stomach.”
In a standard nutrient drink test, the maximum tolerated volume among participants dropped from a mean 27.3 oz at baseline to 15.8 oz at 6-month follow-up (P = .004).
Participants also completed three questionnaires. From baseline to 6 months, their DAILY EATS mean hunger score decreased from 6.2 to 4 (P = .002), mean Eating Drivers Index score dropped from 7 to 4 (P < .001), and WEL-SF score improved from 47.7 to 62.4 (P = .001).
Repeat endoscopy at 6 months showed that the gastric fundus contracted and healed. An unexpected and beneficial finding was fibrotic tissue, which made the fundus less able to expand, Dr. McGowan said. A smaller fundus “is critical for feeling full.”
No serious adverse events were reported. Participants described gas pressure, mild nausea, and cramping, all of which lasted 1-3 days, he said.
“The key here is preserving safety. This is why we use the technique of injecting a fluid cushion prior to ablating, so we’re not entering any deeper layers of the stomach,” Dr. McGowan said. “Importantly, there are no nerve receptors within the lining of the stomach, so there’s no pain from this procedure.”
Another Anti-Obesity Tool?
“We’re all familiar with the epidemic that is obesity affecting nearly one in two adults, and the profound impact that it has on patients’ health, their quality of life, as well as the healthcare system,” Dr. McGowan said. “It’s clear that we need every tool possible to address this because we know that obesity is not a matter of willpower. It’s a disease.”
Gastric fundus ablation “may represent, and frankly should represent, a treatment option for the greater than 100 million US adults with obesity,” he added.
Not every patient wants to or can access GLP-1 medications, Dr. McGowan said. Also, “there’s a difference between taking a medication long-term, requiring an injection every week, vs a single intervention in time that carries forward.”
Ablation could also help people transition after they stop GLP-1 medications to help them maintain their weight loss, he said.
Weight loss is the endpoint you care about the most, said Dr. Laine, who co-moderated the press briefing.
Though the weight loss of 7.7% was not a large percentage, it was only 10 patients. We will have to see whether the total body weight loss is different when they do the procedure in more patients or if they can combine different mechanisms, Dr. Laine said.
It remains unclear whether gastric fundus ablation would be a stand-alone procedure or used in combination with another endoscopic weight-management intervention, bariatric surgery, or medication.
The endoscopic sleeve, which is a stomach-reducing procedure, is very effective, but it doesn’t diminish hunger, Dr. McGowan said. Combining it with ablation may be “a best-of-both-worlds scenario.”
Dr. Laine added that another open question is whether the gastric fundal accommodation will be associated with any side effects such as dyspepsia.
Dr. McGowan reported consulting for Boston Scientific and Apollo Endosurgery. Dr. Laine reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON — .
“Patients reported a decrease in hunger, appetite, and cravings and an increase in control over [their] eating,” said senior study investigator Christopher McGowan, MD, AGAF, a gastroenterologist in private practice and medical director of True You Weight Loss in Cary, North Carolina.
“They generally described that their relationship with food had changed,” Dr. McGowan said at a May 8 press briefing during which his research (Abstract 516) was previewed for Digestive Disease Week® (DDW).
Researchers targeted the gastric fundus because its mucosal lining contains 80%-90% of the cells that produce ghrelin. When a person diets and/or loses weight, ghrelin levels increase, making the person hungrier and preventing sustained weight loss, Dr. McGowan said.
Previously, the only proven way to reduce ghrelin was to surgically remove or bypass the fundus. Weight-loss medications like Wegovy, Zepbound, and Ozempic target a different hormonal pathway, glucagon-like peptide 1 (GLP-1).
“What we’ve learned from the GLP-1 medications is the profound impact of reducing hunger,” Dr. McGowan said. “That’s what patients describe quite often — that it really changes their life and their quality of life. That’s really, really important.”
Major Findings
In the trial, 10 women (mean age, 38 years; mean body mass index, 40.2) underwent endoscopic fundic mucosal ablation via hybrid argon plasma coagulation in an ambulatory setting under general anesthesia from November 1, 2022, to April 14, 2023. The procedure took less than an hour on average, and the technique gave them easy access to the fundus, Dr. McGowan said.
Compared with baseline, there were multiple beneficial outcomes at 6 months:
- 45% less circulating ghrelin in the blood.
- 53% drop in ghrelin-producing cells in the fundus.
- 42% reduction in stomach capacity.
- 43% decrease in hunger, appetite, and cravings.
- 7.7% body weight loss.
Over the 6 months of the study, mean ghrelin concentrations dropped from 461.6 pg/mL at baseline to 254.8 pg/mL (P = .006).
It is fascinating that the hormone ghrelin decreased just by ablating, said Loren Laine, MD, AGAF, professor of medicine (digestive diseases) at Yale School of Medicine and chair of DDW 2024. “They used the same device that we use to treat bleeding ulcers or lesions in the stomach and applied it broadly over the whole upper part of the stomach.”
In a standard nutrient drink test, the maximum tolerated volume among participants dropped from a mean 27.3 oz at baseline to 15.8 oz at 6-month follow-up (P = .004).
Participants also completed three questionnaires. From baseline to 6 months, their DAILY EATS mean hunger score decreased from 6.2 to 4 (P = .002), mean Eating Drivers Index score dropped from 7 to 4 (P < .001), and WEL-SF score improved from 47.7 to 62.4 (P = .001).
Repeat endoscopy at 6 months showed that the gastric fundus contracted and healed. An unexpected and beneficial finding was fibrotic tissue, which made the fundus less able to expand, Dr. McGowan said. A smaller fundus “is critical for feeling full.”
No serious adverse events were reported. Participants described gas pressure, mild nausea, and cramping, all of which lasted 1-3 days, he said.
“The key here is preserving safety. This is why we use the technique of injecting a fluid cushion prior to ablating, so we’re not entering any deeper layers of the stomach,” Dr. McGowan said. “Importantly, there are no nerve receptors within the lining of the stomach, so there’s no pain from this procedure.”
Another Anti-Obesity Tool?
“We’re all familiar with the epidemic that is obesity affecting nearly one in two adults, and the profound impact that it has on patients’ health, their quality of life, as well as the healthcare system,” Dr. McGowan said. “It’s clear that we need every tool possible to address this because we know that obesity is not a matter of willpower. It’s a disease.”
Gastric fundus ablation “may represent, and frankly should represent, a treatment option for the greater than 100 million US adults with obesity,” he added.
Not every patient wants to or can access GLP-1 medications, Dr. McGowan said. Also, “there’s a difference between taking a medication long-term, requiring an injection every week, vs a single intervention in time that carries forward.”
Ablation could also help people transition after they stop GLP-1 medications to help them maintain their weight loss, he said.
Weight loss is the endpoint you care about the most, said Dr. Laine, who co-moderated the press briefing.
Though the weight loss of 7.7% was not a large percentage, it was only 10 patients. We will have to see whether the total body weight loss is different when they do the procedure in more patients or if they can combine different mechanisms, Dr. Laine said.
It remains unclear whether gastric fundus ablation would be a stand-alone procedure or used in combination with another endoscopic weight-management intervention, bariatric surgery, or medication.
The endoscopic sleeve, which is a stomach-reducing procedure, is very effective, but it doesn’t diminish hunger, Dr. McGowan said. Combining it with ablation may be “a best-of-both-worlds scenario.”
Dr. Laine added that another open question is whether the gastric fundal accommodation will be associated with any side effects such as dyspepsia.
Dr. McGowan reported consulting for Boston Scientific and Apollo Endosurgery. Dr. Laine reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
WASHINGTON — .
“Patients reported a decrease in hunger, appetite, and cravings and an increase in control over [their] eating,” said senior study investigator Christopher McGowan, MD, AGAF, a gastroenterologist in private practice and medical director of True You Weight Loss in Cary, North Carolina.
“They generally described that their relationship with food had changed,” Dr. McGowan said at a May 8 press briefing during which his research (Abstract 516) was previewed for Digestive Disease Week® (DDW).
Researchers targeted the gastric fundus because its mucosal lining contains 80%-90% of the cells that produce ghrelin. When a person diets and/or loses weight, ghrelin levels increase, making the person hungrier and preventing sustained weight loss, Dr. McGowan said.
Previously, the only proven way to reduce ghrelin was to surgically remove or bypass the fundus. Weight-loss medications like Wegovy, Zepbound, and Ozempic target a different hormonal pathway, glucagon-like peptide 1 (GLP-1).
“What we’ve learned from the GLP-1 medications is the profound impact of reducing hunger,” Dr. McGowan said. “That’s what patients describe quite often — that it really changes their life and their quality of life. That’s really, really important.”
Major Findings
In the trial, 10 women (mean age, 38 years; mean body mass index, 40.2) underwent endoscopic fundic mucosal ablation via hybrid argon plasma coagulation in an ambulatory setting under general anesthesia from November 1, 2022, to April 14, 2023. The procedure took less than an hour on average, and the technique gave them easy access to the fundus, Dr. McGowan said.
Compared with baseline, there were multiple beneficial outcomes at 6 months:
- 45% less circulating ghrelin in the blood.
- 53% drop in ghrelin-producing cells in the fundus.
- 42% reduction in stomach capacity.
- 43% decrease in hunger, appetite, and cravings.
- 7.7% body weight loss.
Over the 6 months of the study, mean ghrelin concentrations dropped from 461.6 pg/mL at baseline to 254.8 pg/mL (P = .006).
It is fascinating that the hormone ghrelin decreased just by ablating, said Loren Laine, MD, AGAF, professor of medicine (digestive diseases) at Yale School of Medicine and chair of DDW 2024. “They used the same device that we use to treat bleeding ulcers or lesions in the stomach and applied it broadly over the whole upper part of the stomach.”
In a standard nutrient drink test, the maximum tolerated volume among participants dropped from a mean 27.3 oz at baseline to 15.8 oz at 6-month follow-up (P = .004).
Participants also completed three questionnaires. From baseline to 6 months, their DAILY EATS mean hunger score decreased from 6.2 to 4 (P = .002), mean Eating Drivers Index score dropped from 7 to 4 (P < .001), and WEL-SF score improved from 47.7 to 62.4 (P = .001).
Repeat endoscopy at 6 months showed that the gastric fundus contracted and healed. An unexpected and beneficial finding was fibrotic tissue, which made the fundus less able to expand, Dr. McGowan said. A smaller fundus “is critical for feeling full.”
No serious adverse events were reported. Participants described gas pressure, mild nausea, and cramping, all of which lasted 1-3 days, he said.
“The key here is preserving safety. This is why we use the technique of injecting a fluid cushion prior to ablating, so we’re not entering any deeper layers of the stomach,” Dr. McGowan said. “Importantly, there are no nerve receptors within the lining of the stomach, so there’s no pain from this procedure.”
Another Anti-Obesity Tool?
“We’re all familiar with the epidemic that is obesity affecting nearly one in two adults, and the profound impact that it has on patients’ health, their quality of life, as well as the healthcare system,” Dr. McGowan said. “It’s clear that we need every tool possible to address this because we know that obesity is not a matter of willpower. It’s a disease.”
Gastric fundus ablation “may represent, and frankly should represent, a treatment option for the greater than 100 million US adults with obesity,” he added.
Not every patient wants to or can access GLP-1 medications, Dr. McGowan said. Also, “there’s a difference between taking a medication long-term, requiring an injection every week, vs a single intervention in time that carries forward.”
Ablation could also help people transition after they stop GLP-1 medications to help them maintain their weight loss, he said.
Weight loss is the endpoint you care about the most, said Dr. Laine, who co-moderated the press briefing.
Though the weight loss of 7.7% was not a large percentage, it was only 10 patients. We will have to see whether the total body weight loss is different when they do the procedure in more patients or if they can combine different mechanisms, Dr. Laine said.
It remains unclear whether gastric fundus ablation would be a stand-alone procedure or used in combination with another endoscopic weight-management intervention, bariatric surgery, or medication.
The endoscopic sleeve, which is a stomach-reducing procedure, is very effective, but it doesn’t diminish hunger, Dr. McGowan said. Combining it with ablation may be “a best-of-both-worlds scenario.”
Dr. Laine added that another open question is whether the gastric fundal accommodation will be associated with any side effects such as dyspepsia.
Dr. McGowan reported consulting for Boston Scientific and Apollo Endosurgery. Dr. Laine reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DDW 2024
AGA Clinical Guideline Stresses Patient Preferences in Barrett’s Treatment
Published in Gastroenterology , the clinical practice guideline makes five main recommendations — one strong and four conditional — based on very low to moderate evidence. It also stresses that providers should practice shared decision making according to patient preferences and risk perception.
For the most part, the new guideline is not a significant departure from the way expert endoscopists are currently practicing EET for BE and related neoplasia, gastroenterologist Joel H. Rubenstein, MD, MSc, AGAF, of the Barrett’s Esophagus Program in the Division of Gastroenterology at University of Michigan Medical School at Ann Arbor, said in an interview. One of three first authors of the guideline, Dr. Rubenstein added, “There is, however, considerable variability in how endoscopists practice, and we hope this guidance will serve as a useful resource to refer to for best practices.”
Added gastroenterologist Tarek Sawas, MD, MPH, assistant professor of internal medicine at UT Southwestern Medical Center in Dallas, “We hope the update will provide some clarity for practice and for implementation, while allowing gastroenterologists the freedom to decide what is best for patients based on lesion characteristics.”
Dr. Sawas added that one of the differences in the new guideline relates to the approach to low-grade dysplasia. While earlier guidance favored treatment over surveillance, patient preferences should now be factored into management. “Some patients are risk-averse and prefer to wait and watch, while others place more value on treatment and just want to get on with it,” he said.
When this guideline was circulated for public comment, “the areas prompting the most feedback was on our current suggestions against the routine use of EET in non-dysplastic BE and for the use of either endoscopic mucosal resection [EMR] or endoscopic submucosal dissection [ESD] for resection — with the expectation that the vast majority may be managed with EMR,” Dr. Rubenstein said.
“We felt that ESD would work best for larger lesions,” explained Dr. Sawas. “There aren’t a lot data in this area, just some observational studies, but we should have more data for comparison in the next few years.”
The incidence of esophageal adenocarcinoma continues to rise and an update was deemed in order since the AGA’s last formal guidance on this subject using the systematic GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology was issued in 2011. “In the following time span, there’s been a lot of research, particularly with regard to management of low-grade dysplasia and endoscopic resection techniques,” Dr. Rubenstein said.
Key Recommendations
The 14 guideline panelists made the following suggestions for treatment and implementation based on different levels of certainty of evidence (CoE):
1. If high-grade dysplasia (HGD) is present, EET is recommended over surveillance, with subsequent surveillance performed at 3, 6, and 12 months, and annually thereafter. (Strong recommendation, moderate CoE).
Surveillance endoscopies should obtain targeted tissue samples of visible lesions and random biopsies of the cardia and distal 2 cm of the tubular esophagus.
2. In patients with low-grade dysplasia, EET is also preferred to surveillance. But for those placing a higher value on the certain harms and a lower value on the uncertain benefits of EET for reducing mortality, surveillance endoscopy is a reasonable option. (Conditional recommendation, low CoE).
Following EET, clinicians should perform surveillance at years 1 and 3 after complete eradication of intestinal metaplasia, then revert to the surveillance intervals used in non-dysplastic BE.
3. For non-dysplastic BE, the AGA advises against the routine use of EET. (Conditional recommendation, low CoE).
4. Patients undergoing EET should have resection of visible lesions followed by ablation of the remaining BE segment rather than resection of the entire segment.
In patients with only a small area of BE beyond the visible lesion, endoscopic resection is acceptable and may be preferred over repeated ablation. Radiofrequency ablation is the preferred ablative modality. (Conditional recommendation, very low CoE).
5. For treating visible neoplastic lesions the AGA suggests either EMR or ESD based on lesion characteristics. (Conditional recommendation, very low CoE).
Patients with suspected T1 esophageal adenocarcinoma (EAC) should be considered for EET. Endoscopic resection is recommended over endoscopic ultrasound for distinguishing EAC from HGD and for staging depth of invasion.
The vast majority of neoplastic lesions may be managed with EMR rather than ESD. Patients who have bulky lesions, or lesions highly suspicious of at least T1b invasion and are deemed candidates for endoscopic resection might benefit from ESD over EMR. Those with previously failed EMR might benefit from ESD.
As to the generally low quality of the supporting evidence, Dr. Rubenstein said, “Unfortunately, very few decisions we make in medicine are supported by high certainty of evidence, but we still have to make a decision.” He pointed out that the guideline highlights areas for future research that could help strengthen or change the guideline’s recommendations.
Considering benefits and harms, the panelists concluded that overall CoE across critical desirable outcomes of disease progression to EAC was moderate. Patient-important outcomes informing the harms were strictures, major bleeding perforation, and serious adverse events.
Lifestyle
The guidance also urges providers to counsel BE patients on tobacco cessation and weight loss if needed, and notes the specter of cancer may incentivize patients to make lifestyle changes.
The most common causes of death in EET patients are cardiovascular disease and other cancers, for which tobacco use and obesity are also major risk factors, and tobacco is associated with strictures, the panelists wrote. “The prospect of progression to cancer in patients with dysplastic BE often holds greater valence than prior counseling attempts, and patients may re-commit to such efforts following consultation for EET.”
Going Forward
Areas for future attention include:
- Identifying populations with non-dysplastic BE whose risk warrants EET
- Balancing risk and benefit of EET in low-grade dysplasia
- Randomized controlled trials comparing EMR and ESD in higher-risk lesions
- Optimal management of post-EET pain
- Stricture prevention and control
- Managing resistant/recurrent disease beyond reflux control
- Optimal surveillance and biopsy strategies following EETThis guideline was supported by the National Institutes of Health, the Department of Defense, the Veterans Administration Health Services and Research Division, and the Katy O. and Paul M. Rady Endowed Chair in Esophageal Cancer Research at the University of Colorado.
Dr. Sawas had no competing interests to disclose. Dr. Rubenstein reported research funding from Lucid Diagnostics.
Several other panelists reported research funding or consultation fees from various pharmaceutical and biotechnology companies.
Published in Gastroenterology , the clinical practice guideline makes five main recommendations — one strong and four conditional — based on very low to moderate evidence. It also stresses that providers should practice shared decision making according to patient preferences and risk perception.
For the most part, the new guideline is not a significant departure from the way expert endoscopists are currently practicing EET for BE and related neoplasia, gastroenterologist Joel H. Rubenstein, MD, MSc, AGAF, of the Barrett’s Esophagus Program in the Division of Gastroenterology at University of Michigan Medical School at Ann Arbor, said in an interview. One of three first authors of the guideline, Dr. Rubenstein added, “There is, however, considerable variability in how endoscopists practice, and we hope this guidance will serve as a useful resource to refer to for best practices.”
Added gastroenterologist Tarek Sawas, MD, MPH, assistant professor of internal medicine at UT Southwestern Medical Center in Dallas, “We hope the update will provide some clarity for practice and for implementation, while allowing gastroenterologists the freedom to decide what is best for patients based on lesion characteristics.”
Dr. Sawas added that one of the differences in the new guideline relates to the approach to low-grade dysplasia. While earlier guidance favored treatment over surveillance, patient preferences should now be factored into management. “Some patients are risk-averse and prefer to wait and watch, while others place more value on treatment and just want to get on with it,” he said.
When this guideline was circulated for public comment, “the areas prompting the most feedback was on our current suggestions against the routine use of EET in non-dysplastic BE and for the use of either endoscopic mucosal resection [EMR] or endoscopic submucosal dissection [ESD] for resection — with the expectation that the vast majority may be managed with EMR,” Dr. Rubenstein said.
“We felt that ESD would work best for larger lesions,” explained Dr. Sawas. “There aren’t a lot data in this area, just some observational studies, but we should have more data for comparison in the next few years.”
The incidence of esophageal adenocarcinoma continues to rise and an update was deemed in order since the AGA’s last formal guidance on this subject using the systematic GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology was issued in 2011. “In the following time span, there’s been a lot of research, particularly with regard to management of low-grade dysplasia and endoscopic resection techniques,” Dr. Rubenstein said.
Key Recommendations
The 14 guideline panelists made the following suggestions for treatment and implementation based on different levels of certainty of evidence (CoE):
1. If high-grade dysplasia (HGD) is present, EET is recommended over surveillance, with subsequent surveillance performed at 3, 6, and 12 months, and annually thereafter. (Strong recommendation, moderate CoE).
Surveillance endoscopies should obtain targeted tissue samples of visible lesions and random biopsies of the cardia and distal 2 cm of the tubular esophagus.
2. In patients with low-grade dysplasia, EET is also preferred to surveillance. But for those placing a higher value on the certain harms and a lower value on the uncertain benefits of EET for reducing mortality, surveillance endoscopy is a reasonable option. (Conditional recommendation, low CoE).
Following EET, clinicians should perform surveillance at years 1 and 3 after complete eradication of intestinal metaplasia, then revert to the surveillance intervals used in non-dysplastic BE.
3. For non-dysplastic BE, the AGA advises against the routine use of EET. (Conditional recommendation, low CoE).
4. Patients undergoing EET should have resection of visible lesions followed by ablation of the remaining BE segment rather than resection of the entire segment.
In patients with only a small area of BE beyond the visible lesion, endoscopic resection is acceptable and may be preferred over repeated ablation. Radiofrequency ablation is the preferred ablative modality. (Conditional recommendation, very low CoE).
5. For treating visible neoplastic lesions the AGA suggests either EMR or ESD based on lesion characteristics. (Conditional recommendation, very low CoE).
Patients with suspected T1 esophageal adenocarcinoma (EAC) should be considered for EET. Endoscopic resection is recommended over endoscopic ultrasound for distinguishing EAC from HGD and for staging depth of invasion.
The vast majority of neoplastic lesions may be managed with EMR rather than ESD. Patients who have bulky lesions, or lesions highly suspicious of at least T1b invasion and are deemed candidates for endoscopic resection might benefit from ESD over EMR. Those with previously failed EMR might benefit from ESD.
As to the generally low quality of the supporting evidence, Dr. Rubenstein said, “Unfortunately, very few decisions we make in medicine are supported by high certainty of evidence, but we still have to make a decision.” He pointed out that the guideline highlights areas for future research that could help strengthen or change the guideline’s recommendations.
Considering benefits and harms, the panelists concluded that overall CoE across critical desirable outcomes of disease progression to EAC was moderate. Patient-important outcomes informing the harms were strictures, major bleeding perforation, and serious adverse events.
Lifestyle
The guidance also urges providers to counsel BE patients on tobacco cessation and weight loss if needed, and notes the specter of cancer may incentivize patients to make lifestyle changes.
The most common causes of death in EET patients are cardiovascular disease and other cancers, for which tobacco use and obesity are also major risk factors, and tobacco is associated with strictures, the panelists wrote. “The prospect of progression to cancer in patients with dysplastic BE often holds greater valence than prior counseling attempts, and patients may re-commit to such efforts following consultation for EET.”
Going Forward
Areas for future attention include:
- Identifying populations with non-dysplastic BE whose risk warrants EET
- Balancing risk and benefit of EET in low-grade dysplasia
- Randomized controlled trials comparing EMR and ESD in higher-risk lesions
- Optimal management of post-EET pain
- Stricture prevention and control
- Managing resistant/recurrent disease beyond reflux control
- Optimal surveillance and biopsy strategies following EETThis guideline was supported by the National Institutes of Health, the Department of Defense, the Veterans Administration Health Services and Research Division, and the Katy O. and Paul M. Rady Endowed Chair in Esophageal Cancer Research at the University of Colorado.
Dr. Sawas had no competing interests to disclose. Dr. Rubenstein reported research funding from Lucid Diagnostics.
Several other panelists reported research funding or consultation fees from various pharmaceutical and biotechnology companies.
Published in Gastroenterology , the clinical practice guideline makes five main recommendations — one strong and four conditional — based on very low to moderate evidence. It also stresses that providers should practice shared decision making according to patient preferences and risk perception.
For the most part, the new guideline is not a significant departure from the way expert endoscopists are currently practicing EET for BE and related neoplasia, gastroenterologist Joel H. Rubenstein, MD, MSc, AGAF, of the Barrett’s Esophagus Program in the Division of Gastroenterology at University of Michigan Medical School at Ann Arbor, said in an interview. One of three first authors of the guideline, Dr. Rubenstein added, “There is, however, considerable variability in how endoscopists practice, and we hope this guidance will serve as a useful resource to refer to for best practices.”
Added gastroenterologist Tarek Sawas, MD, MPH, assistant professor of internal medicine at UT Southwestern Medical Center in Dallas, “We hope the update will provide some clarity for practice and for implementation, while allowing gastroenterologists the freedom to decide what is best for patients based on lesion characteristics.”
Dr. Sawas added that one of the differences in the new guideline relates to the approach to low-grade dysplasia. While earlier guidance favored treatment over surveillance, patient preferences should now be factored into management. “Some patients are risk-averse and prefer to wait and watch, while others place more value on treatment and just want to get on with it,” he said.
When this guideline was circulated for public comment, “the areas prompting the most feedback was on our current suggestions against the routine use of EET in non-dysplastic BE and for the use of either endoscopic mucosal resection [EMR] or endoscopic submucosal dissection [ESD] for resection — with the expectation that the vast majority may be managed with EMR,” Dr. Rubenstein said.
“We felt that ESD would work best for larger lesions,” explained Dr. Sawas. “There aren’t a lot data in this area, just some observational studies, but we should have more data for comparison in the next few years.”
The incidence of esophageal adenocarcinoma continues to rise and an update was deemed in order since the AGA’s last formal guidance on this subject using the systematic GRADE (Grading of Recommendations Assessment, Development, and Evaluation) methodology was issued in 2011. “In the following time span, there’s been a lot of research, particularly with regard to management of low-grade dysplasia and endoscopic resection techniques,” Dr. Rubenstein said.
Key Recommendations
The 14 guideline panelists made the following suggestions for treatment and implementation based on different levels of certainty of evidence (CoE):
1. If high-grade dysplasia (HGD) is present, EET is recommended over surveillance, with subsequent surveillance performed at 3, 6, and 12 months, and annually thereafter. (Strong recommendation, moderate CoE).
Surveillance endoscopies should obtain targeted tissue samples of visible lesions and random biopsies of the cardia and distal 2 cm of the tubular esophagus.
2. In patients with low-grade dysplasia, EET is also preferred to surveillance. But for those placing a higher value on the certain harms and a lower value on the uncertain benefits of EET for reducing mortality, surveillance endoscopy is a reasonable option. (Conditional recommendation, low CoE).
Following EET, clinicians should perform surveillance at years 1 and 3 after complete eradication of intestinal metaplasia, then revert to the surveillance intervals used in non-dysplastic BE.
3. For non-dysplastic BE, the AGA advises against the routine use of EET. (Conditional recommendation, low CoE).
4. Patients undergoing EET should have resection of visible lesions followed by ablation of the remaining BE segment rather than resection of the entire segment.
In patients with only a small area of BE beyond the visible lesion, endoscopic resection is acceptable and may be preferred over repeated ablation. Radiofrequency ablation is the preferred ablative modality. (Conditional recommendation, very low CoE).
5. For treating visible neoplastic lesions the AGA suggests either EMR or ESD based on lesion characteristics. (Conditional recommendation, very low CoE).
Patients with suspected T1 esophageal adenocarcinoma (EAC) should be considered for EET. Endoscopic resection is recommended over endoscopic ultrasound for distinguishing EAC from HGD and for staging depth of invasion.
The vast majority of neoplastic lesions may be managed with EMR rather than ESD. Patients who have bulky lesions, or lesions highly suspicious of at least T1b invasion and are deemed candidates for endoscopic resection might benefit from ESD over EMR. Those with previously failed EMR might benefit from ESD.
As to the generally low quality of the supporting evidence, Dr. Rubenstein said, “Unfortunately, very few decisions we make in medicine are supported by high certainty of evidence, but we still have to make a decision.” He pointed out that the guideline highlights areas for future research that could help strengthen or change the guideline’s recommendations.
Considering benefits and harms, the panelists concluded that overall CoE across critical desirable outcomes of disease progression to EAC was moderate. Patient-important outcomes informing the harms were strictures, major bleeding perforation, and serious adverse events.
Lifestyle
The guidance also urges providers to counsel BE patients on tobacco cessation and weight loss if needed, and notes the specter of cancer may incentivize patients to make lifestyle changes.
The most common causes of death in EET patients are cardiovascular disease and other cancers, for which tobacco use and obesity are also major risk factors, and tobacco is associated with strictures, the panelists wrote. “The prospect of progression to cancer in patients with dysplastic BE often holds greater valence than prior counseling attempts, and patients may re-commit to such efforts following consultation for EET.”
Going Forward
Areas for future attention include:
- Identifying populations with non-dysplastic BE whose risk warrants EET
- Balancing risk and benefit of EET in low-grade dysplasia
- Randomized controlled trials comparing EMR and ESD in higher-risk lesions
- Optimal management of post-EET pain
- Stricture prevention and control
- Managing resistant/recurrent disease beyond reflux control
- Optimal surveillance and biopsy strategies following EETThis guideline was supported by the National Institutes of Health, the Department of Defense, the Veterans Administration Health Services and Research Division, and the Katy O. and Paul M. Rady Endowed Chair in Esophageal Cancer Research at the University of Colorado.
Dr. Sawas had no competing interests to disclose. Dr. Rubenstein reported research funding from Lucid Diagnostics.
Several other panelists reported research funding or consultation fees from various pharmaceutical and biotechnology companies.
FROM GASTROENTEROLOGY
Endoscopic Management of Barrett’s Esophagus
Introduction
Barrett’s esophagus (BE) is characterized by the replacement of squamous epithelium by columnar metaplasia of the distal esophagus (>1 cm length). It is a precancerous condition, with 3%-5% of patients with BE developing esophageal adenocarcinoma (EAC) in their lifetime. EAC is one of the cancers with high morbidity and mortality (5-year survival < 20%), and its incidence has been on the rise. Studies examining the natural history of BE have demonstrated that the progression happens through a metaplasia-dysplasia-neoplasia sequence. Therefore, early detection of BE and timely management to prevent progression to EAC is crucial.
Grades of Dysplasia
The current gold standard for the diagnosis of BE neoplasia includes a high-quality endoscopic evaluation and biopsies. Biopsies should be obtained from any visible lesions (nodules, ulcers) followed by a random 4-quadrant fashion (Seattle protocol) interval of the entire length of the BE segment. It is essential to pay attention to the results of the biopsy that have been obtained since it will not only determine the surveillance interval but is crucial in planning any necessary endoscopic therapy. The possible results of the biopsy and its implications are:
- No intestinal metaplasia (IM): This would rule out Barrett’s esophagus and no further surveillance would be necessary. A recent population-based study of over 1 million patients showed a 55% and 61% reduced risk of upper gastrointestinal (UGI) cancer and deaths respectively after a negative endoscopy.1
- Intestinal metaplasia with no dysplasia (non-dysplastic BE): Biopsies confirm presence of intestinal metaplasia in the biopsies without any evidence of dysplasia. While the rate of progression to EAC is low (0.07%-0.25%), it is not absent and thus surveillance would be indicated. Current guidelines suggest repeating an endoscopy with biopsy in 5 years if the length of BE is < 3 cm or 3 years if length of BE ≥ 3 cm.2
- Indeterminate for dysplasia (BE-IND): Biopsies confirm IM but are not able to definitively rule out dysplasia. This can be seen in about 4%-8% of the biopsies obtained. The progression rates to EAC are reported to be comparable or lower to low-grade dysplasia (LGD), so the current recommendation is to intensify acid reduction therapy and repeat endoscopy in 6 months. If repeat endoscopy downgrades to non-dysplastic, then can follow surveillance according to NDBE protocol; otherwise recommend continuing surveillance every 12 months.
- Low-grade dysplasia (BE-LGD): Biopsies confirm IM but also show tightly packed overlapping basal nuclei with hyperchromasia and irregular contours, basal stratification of nuclei, and diminished goblet and columnar cell mucus. There is significant inter-observer variability reported,3 and thus the slides must be reviewed by a second pathologist with experience in BE to confirm the findings. Once confirmed, based on risk factors such as presence of multifocal LGD, persistence of LGD, presence of visible lesions, etc., the patient can be offered Barrett’s endoscopic therapy (BET) or undergo continued surveillance. The decision of pursuing one or the other would be dependent on patient preference and shared decision-making between the patient and the provider.
- High-grade dysplasia (BE-HGD): Biopsies confirm IM with cells showing greater degree of cytologic and architectural alterations of dysplasia than LGD but without overt neoplastic features. Over 40% of the patients would progress to EAC and thus the current recommendations would be to recommend BET in these patients.4
- Esophageal adenocarcinoma (EAC): Biopsies demonstrate neoplasia. If the neoplastic changes are limited to the mucosa (T1a) on endoscopic ultrasound or cross-sectional imaging, then BET is suggested. If there is involvement of submucosa, then depending on the depth of invasion, absence of high-risk features (poor differentiation, lymphovascular invasion), BET can be considered as an alternative to esophagectomy.
Lesion Detection on Endoscopy
Data from large population-based studies with at least 3 years of follow-up reported that 58%-66% of EAC detected during endoscopy were diagnosed within 1 year of an index Barrett’s esophagus screening endoscopy, or post-endoscopy Barrett’s neoplasia, and were considered likely to have been missed during index endoscopy.5 This underscores the importance of careful and systematic endoscopic examination during an upper endoscopy.
Studies have also demonstrated that longer examination time was associated with significantly higher detection of HGD/EAC.6,7 Careful examination of the tubular esophagus and gastroesophageal junction (GEJ) should be performed in forward and retroflexed views looking for any subtle areas of nodularity, loop distortion, variability in vascular patterns, mucosal changes concerning for dysplasia or neoplasia. Use of high-definition white light endoscopy (HD-WLE) and virtual chromoendoscopy techniques such as narrow banding imaging (NBI) or blue laser imaging (BLI) are currently recommended in the guidelines.2 Spray chromoendoscopy using acetic acid can also be utilized. Another exciting development is the use of artificial intelligence (AI) in detecting and diagnosing BE associated lesions and neoplasia.
Barrett’s Endoscopic Therapy (BET)
Patients with visible lesions, dysplasia, or early EAC are candidates for BET (Table 1). 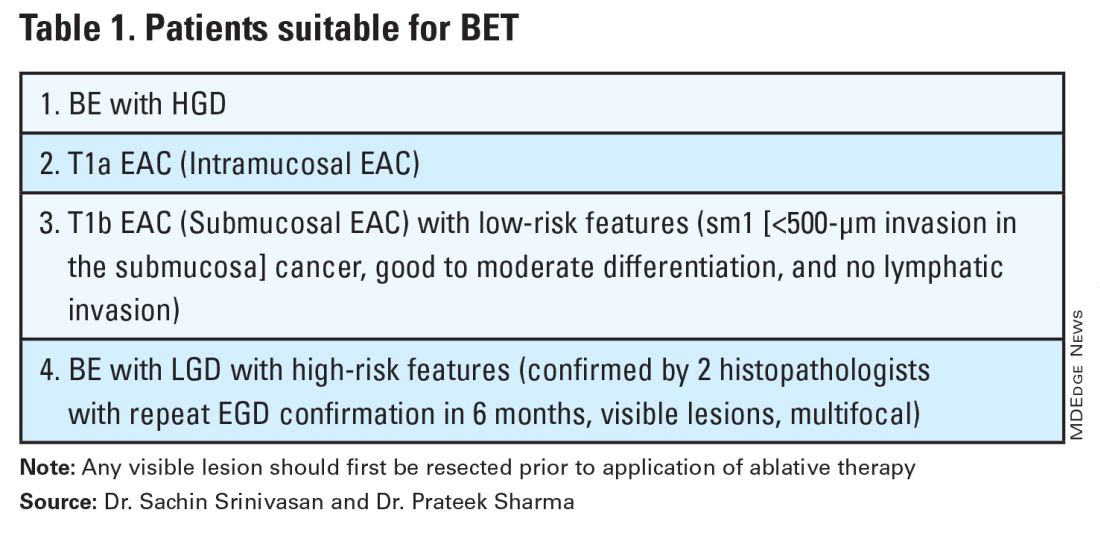
BET involves resective and ablative modalities. The resective modalities include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) and are the modalities of choice for nodular or raised lesions.
EMR involves endoscopic resection of abnormal mucosa using either lift-assisted technique or multi-band ligation (Figure 1). 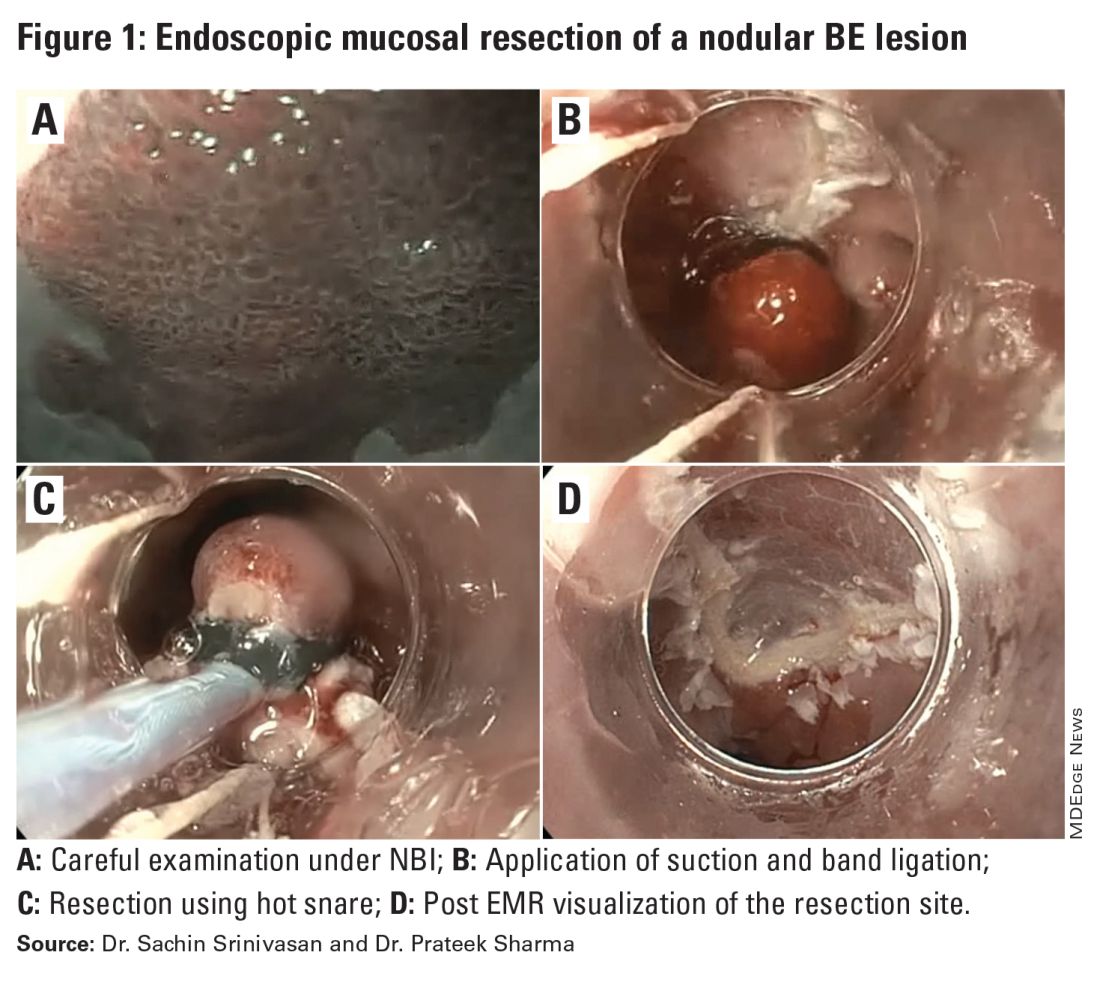
ESD, on the other hand, involves submucosal dissection and perimeter resection of the lesion, thus providing the advantage of an en-bloc resection. In a recent randomized controlled trial (RCT) of 40 patients undergoing ESD vs EMR for HGD/EAC, ESD was better for curative resection (R0) (58%) compared with EMR (12%); however, the remission rates at 3 months were comparable with two perforations reported in the ESD group while there were no complications in the EMR group.8
There is an apparent learning curve when it comes to these advanced techniques, and with more experience, we are seeing comparable results for both these modalities. However, given the complexity and time required for the procedure, current practices typically involve preserving ESD for lesions > 2 cm, those having a likelihood of cancer in the superficial submucosa, or those that EMR cannot remove due to underlying fibrosis or post-EMR recurrence.
The ablative modalities include radiofrequency ablation (RFA), cryotherapy, and hybrid argon plasma coagulation (hybrid APC). These modalities are used for flat lesions, and as therapy following endoscopic resection of nodular lesions to treat residual flat segment of BE. RFA, one of the earliest introduced endoscopic modalities, involves applying directed and controlled heat energy to ablate lesions. Current devices allow circumferential or focal application of RFA. It is a safe and effective modality with good complete eradication of IM (CE-IM) (71%-93%) and complete eradication of dysplasia (CE-D) (91%-100%) rates. These results have been sustained even at 2 years, with the most recent long-term data from a registry study showing a relapse rate of 6% for dysplasia and 19% for IM after 8 years, suggesting durability of this treatment.9
Cryotherapy involves the application of liquid nitrogen or rapidly expanding CO2 to the abnormal mucosa, leading to the rapid freezing and thawing that leads to the death of the cells. Cryogen can be applied as a spray or using a balloon with the spray nozzle in the center. This modality can be used to treat focal lesions and/or larger segments. While it has not been systematically compared with RFA, rates of CE-IM up to 81% and CE-D up to 97% are reported. Hybrid APC involves the use of submucosal saline injection to provide a protective cushion before APC is applied. It has CE-IM rate of 69% and CE-D rate of 67%-86%.10 In a recent RCT of 101 patients randomized to RFA or hybrid APC, CE-IM rates were similar (RFA:74.2% vs hAPC: 82.9%).11
Recently, another technique called radiofrequency vapor ablation (RFVA) is being evaluated, which involves ablating BE segment using vapor at 100° C generated with an RF electrode. A proof-of-concept study of 15 patients showed median squamous conversion of 55% (IQR 33-74) and 98% (IQR 56-99) for 1- and 3-second applications, respectively, with no reported adverse events.12
Barrett’s Refractory to Endoscopic Therapy
Failure of BET is defined as persistent columnar lined epithelium (intestinal metaplasia) with inadequate response, after adequate attempts at endoscopic ablation therapy (after resection) with at least four ablation sessions.13 If encountered, special attention must be given to check compliance with proton pump inhibitors (PPIs), previous incomplete resection, and presence of large hiatal hernia. If CE-IM is not achieved after multiple sessions, change of ablative modality is typically considered. In addition, careful examination for visible lesions should be performed and even if a small one is noted, this should be first resected prior to application of any ablative therapy.
Currently there are no guideline recommendations regarding the preference of one endoscopic modality over another or consideration of potential endoscopic or surgical fundoplication. These modalities primarily rely on technologies available at an institution and the preference of a provider based on their training and experience. Most studies indicate 1-3 sessions (~ 3 months apart) of ablative treatment before achieving CE-IM.
Success and Adverse Events of BET
In a recent real-world study of over 27,000 patients with dysplastic BE, 5295 underwent BET. Analysis showed that patients with HGD/EAC who had BET had a significantly lower 3-year mortality (HGD: RR, 0.59; 95%CI, 0.49-0.71; EAC: RR, 0.53; 95% CI, 0.44-0.65) compared with those who did not undergo BET. Esophageal strictures were the most common adverse event and were noted in 6.5%, followed by chest pain (1.8%), upper GI bleeding (0.47%), and esophageal perforation (0.2%).14
In general, adverse events can be divided into immediate and delayed adverse events. Immediate adverse events typically involve bleeding and perforation that can occur during or shortly after the procedure. These are reported at higher rates with resective modalities compared with ablative therapies. Standard endoscopic techniques involving coagulation grasper or clips can be used to achieve hemostasis. Endoscopic suturing devices offer the ability to contain any perforation. The need for surgical intervention is small and limited to adverse events not detected during the procedure.
Delayed adverse events such as stricture and stenosis are higher for resective modalities (up to 30%), especially when involving more significant than 75% of the esophageal circumference. Post-procedural pain/dysphagia is most common after ablative therapies. Dysphagia reported after any endoscopic therapy should be promptly evaluated, and sequential dilation until the goal esophageal lumen is achieved should be performed every 2-4 weeks.
Recurrences and Surveillance After BET
What is established is that recurrences can occur and may be subtle, therefore detailed endoscopic surveillance is required. In a prospective study, recurrence rates of 15%-16% for IM and 3%-5% for any dysplasia were reported, with the majority being in the first 2 years after achieving CE-IM.15 A systematic review of 21 studies looking at the location of recurrences suggested that the majority (56%) occur in the distal esophagus. Of those that occur in the esophagus, about 80% of them were in the distal 2 cm of the esophagus and only 50% of the recurrences were visible recurrences, thus reiterating the importance of meticulous examination and systematic biopsies.16
On the contrary, a recent single-center study of 217 patients who had achieved CE-IM with 5.5 years of follow-up demonstrated a 26% and 8% recurrence of IM and dysplasia, respectively. One hundred percent of the recurrence in the esophagus was reported as visible.17 Therefore, follow-up endoscopy surveillance protocol after CE-IM should still involve meticulous examination, biopsy of visible lesions, and systematic biopsies for non-visible lesions from the original BE segment, similar to those patients who have not needed BET.
Current guidelines based on expert consensus and evidence recommend surveillance after CE-IM based on original most advanced histology:2
1. LGD: 1 year, 3 years, and every 2 years after that.
2. HGD/EAC: 3 months, 6 months, 12 months, and annually after that.
There is no clear guideline on when to stop surveillance since the longest available follow-up is around 10 years, and recurrences are still detected. A potential surveillance endpoint may be based on age and comorbidities, especially those that would preclude a patient from being a candidate for BET.
When Should a Patient Be Referred?
BE patients with visible lesions and/or dysplastic changes in the biopsy who would require BET should be considered for referral to high-volume centers. Studies have shown higher success for CE-IM and lower rates of adverse events and recurrences in these patients managed at expert centers. The presence of a multidisciplinary team involving pathologists, surgeons, and oncologists is critical and offers a timely opportunity in case of need for a high-risk patient.
Conclusion
BE is a precursor to EAC, with rising incidence and poor 5-year survival. Endoscopic diagnosis is the gold standard and requires a high-quality examination and biopsies. Based on histopathology, a systematic surveillance and BET plan should be performed to achieve CE-IM in patients with dysplasia. Once CE-IM is achieved, regular surveillance should be performed with careful attention to recurrences and complications from the BET modalities.
Dr. Srinivasan and Dr. Sharma are based at the University of Kansas Medical Center, Kansas City, Kansas, and the Kansas City Veterans Affairs Medical Center, Kansas City, Missouri. Dr. Srinivasan has no relevant disclosures. Dr. Sharma disclosed research grants from ERBE, Ironwood Pharmaceuticals, Olympus, and Medtronic. He has served as a consultant for Takeda, Samsung Bioepis, Olympus, and Lumendi, and reports other funding from Medtronic, Fujifilm Medical Systems USA, and Salix.
References
1. Holmberg D, et al. Incidence and mortality in upper gastrointestinal cancer after negative endoscopy for gastroesophageal reflux disease. Gastroenterology. 2022;162(2):431-438.e4.
2. Shaheen NJ, et al. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am J Gastroenterol. 2022 Apr;117(4):559-587.
3. Pech O, et al. Inter-observer variability in the diagnosis of low-grade dysplasia in pathologists: A comparison between experienced and inexperienced pathologists. Gastrointest Endosc. 2006 Apr;63(5):AB130.
4. Krishnamoorthi R, et al. Factors associated with progression of Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1046-1055.e8.
5. Visrodia K, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology. 2016 Mar;150(3):599-607.e7; quiz e14-5.
6. Perisetti A, Sharma P. Tips for improving the identification of neoplastic visible lesions in Barrett’s esophagus. Gastrointest Endosc. 2023 Feb;97(2):248-250.
7. Gupta N, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012 Sep;76(3):531-538.
8. Terheggen G, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017 May;66(5):783-793.
9. Wolfson P, et al. Endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: A final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc. 2022 Aug;96(2):223-233.
10. Rösch T, et al. 1151 Multicenter feasibility study of combined injection and argon plasma coagulation (hybrid-APC) in the ablation therapy of neoplastic Barrett esophagus. Gastrointest Endosc. 2017;85(5):AB154.
11. Knabe M, et al. Radiofrequency ablation versus hybrid argon plasma coagulation in Barrett’s esophagus: A prospective randomised trial. Surg Endosc. 2023;37(10):7803-7811.
12. Van Munster SN, et al. Radiofrequency vapor ablation for Barrett’s esophagus: Feasibility, safety, and proof of concept in a stepwise study with in vitro, animal, and the first in-human application. Endoscopy. 2021 Nov;53(11):1162-1168.
13. Emura F, et al. Rio de Janeiro global consensus on landmarks, definitions, and classifications in Barrett’s esophagus: World Endoscopy Organization Delphi study. Gastroenterology. 2022 Jul;163(1):84-96.e2.
14. Singh RR, et al. Real-world evidence of safety and effectiveness of Barrett’s endoscopic therapy. Gastrointest Endosc. 2023 Aug;98(2):155-161.e1.
15. Wani S, et al. Recurrence Is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol. 2020 Oct;18(11):2609-2617.e2.
16. Duvvuri A, et al. Mo1273 Location and pattern of recurrences in patients with Barrett’s esophagus after endoscopic therapy: A systematic review and critical analysis of the published literature. Gastrointest Endosc. 2020;91(6):AB410-1.
17. He T, et al. Location and appearance of dysplastic Barrett’s esophagus recurrence after endoscopic eradication therapy: No additional yield from random biopsy sampling neosquamous mucosa. Gastrointest Endosc. 2023 Nov;98(5):722-732.
Introduction
Barrett’s esophagus (BE) is characterized by the replacement of squamous epithelium by columnar metaplasia of the distal esophagus (>1 cm length). It is a precancerous condition, with 3%-5% of patients with BE developing esophageal adenocarcinoma (EAC) in their lifetime. EAC is one of the cancers with high morbidity and mortality (5-year survival < 20%), and its incidence has been on the rise. Studies examining the natural history of BE have demonstrated that the progression happens through a metaplasia-dysplasia-neoplasia sequence. Therefore, early detection of BE and timely management to prevent progression to EAC is crucial.
Grades of Dysplasia
The current gold standard for the diagnosis of BE neoplasia includes a high-quality endoscopic evaluation and biopsies. Biopsies should be obtained from any visible lesions (nodules, ulcers) followed by a random 4-quadrant fashion (Seattle protocol) interval of the entire length of the BE segment. It is essential to pay attention to the results of the biopsy that have been obtained since it will not only determine the surveillance interval but is crucial in planning any necessary endoscopic therapy. The possible results of the biopsy and its implications are:
- No intestinal metaplasia (IM): This would rule out Barrett’s esophagus and no further surveillance would be necessary. A recent population-based study of over 1 million patients showed a 55% and 61% reduced risk of upper gastrointestinal (UGI) cancer and deaths respectively after a negative endoscopy.1
- Intestinal metaplasia with no dysplasia (non-dysplastic BE): Biopsies confirm presence of intestinal metaplasia in the biopsies without any evidence of dysplasia. While the rate of progression to EAC is low (0.07%-0.25%), it is not absent and thus surveillance would be indicated. Current guidelines suggest repeating an endoscopy with biopsy in 5 years if the length of BE is < 3 cm or 3 years if length of BE ≥ 3 cm.2
- Indeterminate for dysplasia (BE-IND): Biopsies confirm IM but are not able to definitively rule out dysplasia. This can be seen in about 4%-8% of the biopsies obtained. The progression rates to EAC are reported to be comparable or lower to low-grade dysplasia (LGD), so the current recommendation is to intensify acid reduction therapy and repeat endoscopy in 6 months. If repeat endoscopy downgrades to non-dysplastic, then can follow surveillance according to NDBE protocol; otherwise recommend continuing surveillance every 12 months.
- Low-grade dysplasia (BE-LGD): Biopsies confirm IM but also show tightly packed overlapping basal nuclei with hyperchromasia and irregular contours, basal stratification of nuclei, and diminished goblet and columnar cell mucus. There is significant inter-observer variability reported,3 and thus the slides must be reviewed by a second pathologist with experience in BE to confirm the findings. Once confirmed, based on risk factors such as presence of multifocal LGD, persistence of LGD, presence of visible lesions, etc., the patient can be offered Barrett’s endoscopic therapy (BET) or undergo continued surveillance. The decision of pursuing one or the other would be dependent on patient preference and shared decision-making between the patient and the provider.
- High-grade dysplasia (BE-HGD): Biopsies confirm IM with cells showing greater degree of cytologic and architectural alterations of dysplasia than LGD but without overt neoplastic features. Over 40% of the patients would progress to EAC and thus the current recommendations would be to recommend BET in these patients.4
- Esophageal adenocarcinoma (EAC): Biopsies demonstrate neoplasia. If the neoplastic changes are limited to the mucosa (T1a) on endoscopic ultrasound or cross-sectional imaging, then BET is suggested. If there is involvement of submucosa, then depending on the depth of invasion, absence of high-risk features (poor differentiation, lymphovascular invasion), BET can be considered as an alternative to esophagectomy.
Lesion Detection on Endoscopy
Data from large population-based studies with at least 3 years of follow-up reported that 58%-66% of EAC detected during endoscopy were diagnosed within 1 year of an index Barrett’s esophagus screening endoscopy, or post-endoscopy Barrett’s neoplasia, and were considered likely to have been missed during index endoscopy.5 This underscores the importance of careful and systematic endoscopic examination during an upper endoscopy.
Studies have also demonstrated that longer examination time was associated with significantly higher detection of HGD/EAC.6,7 Careful examination of the tubular esophagus and gastroesophageal junction (GEJ) should be performed in forward and retroflexed views looking for any subtle areas of nodularity, loop distortion, variability in vascular patterns, mucosal changes concerning for dysplasia or neoplasia. Use of high-definition white light endoscopy (HD-WLE) and virtual chromoendoscopy techniques such as narrow banding imaging (NBI) or blue laser imaging (BLI) are currently recommended in the guidelines.2 Spray chromoendoscopy using acetic acid can also be utilized. Another exciting development is the use of artificial intelligence (AI) in detecting and diagnosing BE associated lesions and neoplasia.
Barrett’s Endoscopic Therapy (BET)
Patients with visible lesions, dysplasia, or early EAC are candidates for BET (Table 1). 
BET involves resective and ablative modalities. The resective modalities include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) and are the modalities of choice for nodular or raised lesions.
EMR involves endoscopic resection of abnormal mucosa using either lift-assisted technique or multi-band ligation (Figure 1). 
ESD, on the other hand, involves submucosal dissection and perimeter resection of the lesion, thus providing the advantage of an en-bloc resection. In a recent randomized controlled trial (RCT) of 40 patients undergoing ESD vs EMR for HGD/EAC, ESD was better for curative resection (R0) (58%) compared with EMR (12%); however, the remission rates at 3 months were comparable with two perforations reported in the ESD group while there were no complications in the EMR group.8
There is an apparent learning curve when it comes to these advanced techniques, and with more experience, we are seeing comparable results for both these modalities. However, given the complexity and time required for the procedure, current practices typically involve preserving ESD for lesions > 2 cm, those having a likelihood of cancer in the superficial submucosa, or those that EMR cannot remove due to underlying fibrosis or post-EMR recurrence.
The ablative modalities include radiofrequency ablation (RFA), cryotherapy, and hybrid argon plasma coagulation (hybrid APC). These modalities are used for flat lesions, and as therapy following endoscopic resection of nodular lesions to treat residual flat segment of BE. RFA, one of the earliest introduced endoscopic modalities, involves applying directed and controlled heat energy to ablate lesions. Current devices allow circumferential or focal application of RFA. It is a safe and effective modality with good complete eradication of IM (CE-IM) (71%-93%) and complete eradication of dysplasia (CE-D) (91%-100%) rates. These results have been sustained even at 2 years, with the most recent long-term data from a registry study showing a relapse rate of 6% for dysplasia and 19% for IM after 8 years, suggesting durability of this treatment.9
Cryotherapy involves the application of liquid nitrogen or rapidly expanding CO2 to the abnormal mucosa, leading to the rapid freezing and thawing that leads to the death of the cells. Cryogen can be applied as a spray or using a balloon with the spray nozzle in the center. This modality can be used to treat focal lesions and/or larger segments. While it has not been systematically compared with RFA, rates of CE-IM up to 81% and CE-D up to 97% are reported. Hybrid APC involves the use of submucosal saline injection to provide a protective cushion before APC is applied. It has CE-IM rate of 69% and CE-D rate of 67%-86%.10 In a recent RCT of 101 patients randomized to RFA or hybrid APC, CE-IM rates were similar (RFA:74.2% vs hAPC: 82.9%).11
Recently, another technique called radiofrequency vapor ablation (RFVA) is being evaluated, which involves ablating BE segment using vapor at 100° C generated with an RF electrode. A proof-of-concept study of 15 patients showed median squamous conversion of 55% (IQR 33-74) and 98% (IQR 56-99) for 1- and 3-second applications, respectively, with no reported adverse events.12
Barrett’s Refractory to Endoscopic Therapy
Failure of BET is defined as persistent columnar lined epithelium (intestinal metaplasia) with inadequate response, after adequate attempts at endoscopic ablation therapy (after resection) with at least four ablation sessions.13 If encountered, special attention must be given to check compliance with proton pump inhibitors (PPIs), previous incomplete resection, and presence of large hiatal hernia. If CE-IM is not achieved after multiple sessions, change of ablative modality is typically considered. In addition, careful examination for visible lesions should be performed and even if a small one is noted, this should be first resected prior to application of any ablative therapy.
Currently there are no guideline recommendations regarding the preference of one endoscopic modality over another or consideration of potential endoscopic or surgical fundoplication. These modalities primarily rely on technologies available at an institution and the preference of a provider based on their training and experience. Most studies indicate 1-3 sessions (~ 3 months apart) of ablative treatment before achieving CE-IM.
Success and Adverse Events of BET
In a recent real-world study of over 27,000 patients with dysplastic BE, 5295 underwent BET. Analysis showed that patients with HGD/EAC who had BET had a significantly lower 3-year mortality (HGD: RR, 0.59; 95%CI, 0.49-0.71; EAC: RR, 0.53; 95% CI, 0.44-0.65) compared with those who did not undergo BET. Esophageal strictures were the most common adverse event and were noted in 6.5%, followed by chest pain (1.8%), upper GI bleeding (0.47%), and esophageal perforation (0.2%).14
In general, adverse events can be divided into immediate and delayed adverse events. Immediate adverse events typically involve bleeding and perforation that can occur during or shortly after the procedure. These are reported at higher rates with resective modalities compared with ablative therapies. Standard endoscopic techniques involving coagulation grasper or clips can be used to achieve hemostasis. Endoscopic suturing devices offer the ability to contain any perforation. The need for surgical intervention is small and limited to adverse events not detected during the procedure.
Delayed adverse events such as stricture and stenosis are higher for resective modalities (up to 30%), especially when involving more significant than 75% of the esophageal circumference. Post-procedural pain/dysphagia is most common after ablative therapies. Dysphagia reported after any endoscopic therapy should be promptly evaluated, and sequential dilation until the goal esophageal lumen is achieved should be performed every 2-4 weeks.
Recurrences and Surveillance After BET
What is established is that recurrences can occur and may be subtle, therefore detailed endoscopic surveillance is required. In a prospective study, recurrence rates of 15%-16% for IM and 3%-5% for any dysplasia were reported, with the majority being in the first 2 years after achieving CE-IM.15 A systematic review of 21 studies looking at the location of recurrences suggested that the majority (56%) occur in the distal esophagus. Of those that occur in the esophagus, about 80% of them were in the distal 2 cm of the esophagus and only 50% of the recurrences were visible recurrences, thus reiterating the importance of meticulous examination and systematic biopsies.16
On the contrary, a recent single-center study of 217 patients who had achieved CE-IM with 5.5 years of follow-up demonstrated a 26% and 8% recurrence of IM and dysplasia, respectively. One hundred percent of the recurrence in the esophagus was reported as visible.17 Therefore, follow-up endoscopy surveillance protocol after CE-IM should still involve meticulous examination, biopsy of visible lesions, and systematic biopsies for non-visible lesions from the original BE segment, similar to those patients who have not needed BET.
Current guidelines based on expert consensus and evidence recommend surveillance after CE-IM based on original most advanced histology:2
1. LGD: 1 year, 3 years, and every 2 years after that.
2. HGD/EAC: 3 months, 6 months, 12 months, and annually after that.
There is no clear guideline on when to stop surveillance since the longest available follow-up is around 10 years, and recurrences are still detected. A potential surveillance endpoint may be based on age and comorbidities, especially those that would preclude a patient from being a candidate for BET.
When Should a Patient Be Referred?
BE patients with visible lesions and/or dysplastic changes in the biopsy who would require BET should be considered for referral to high-volume centers. Studies have shown higher success for CE-IM and lower rates of adverse events and recurrences in these patients managed at expert centers. The presence of a multidisciplinary team involving pathologists, surgeons, and oncologists is critical and offers a timely opportunity in case of need for a high-risk patient.
Conclusion
BE is a precursor to EAC, with rising incidence and poor 5-year survival. Endoscopic diagnosis is the gold standard and requires a high-quality examination and biopsies. Based on histopathology, a systematic surveillance and BET plan should be performed to achieve CE-IM in patients with dysplasia. Once CE-IM is achieved, regular surveillance should be performed with careful attention to recurrences and complications from the BET modalities.
Dr. Srinivasan and Dr. Sharma are based at the University of Kansas Medical Center, Kansas City, Kansas, and the Kansas City Veterans Affairs Medical Center, Kansas City, Missouri. Dr. Srinivasan has no relevant disclosures. Dr. Sharma disclosed research grants from ERBE, Ironwood Pharmaceuticals, Olympus, and Medtronic. He has served as a consultant for Takeda, Samsung Bioepis, Olympus, and Lumendi, and reports other funding from Medtronic, Fujifilm Medical Systems USA, and Salix.
References
1. Holmberg D, et al. Incidence and mortality in upper gastrointestinal cancer after negative endoscopy for gastroesophageal reflux disease. Gastroenterology. 2022;162(2):431-438.e4.
2. Shaheen NJ, et al. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am J Gastroenterol. 2022 Apr;117(4):559-587.
3. Pech O, et al. Inter-observer variability in the diagnosis of low-grade dysplasia in pathologists: A comparison between experienced and inexperienced pathologists. Gastrointest Endosc. 2006 Apr;63(5):AB130.
4. Krishnamoorthi R, et al. Factors associated with progression of Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1046-1055.e8.
5. Visrodia K, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology. 2016 Mar;150(3):599-607.e7; quiz e14-5.
6. Perisetti A, Sharma P. Tips for improving the identification of neoplastic visible lesions in Barrett’s esophagus. Gastrointest Endosc. 2023 Feb;97(2):248-250.
7. Gupta N, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012 Sep;76(3):531-538.
8. Terheggen G, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017 May;66(5):783-793.
9. Wolfson P, et al. Endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: A final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc. 2022 Aug;96(2):223-233.
10. Rösch T, et al. 1151 Multicenter feasibility study of combined injection and argon plasma coagulation (hybrid-APC) in the ablation therapy of neoplastic Barrett esophagus. Gastrointest Endosc. 2017;85(5):AB154.
11. Knabe M, et al. Radiofrequency ablation versus hybrid argon plasma coagulation in Barrett’s esophagus: A prospective randomised trial. Surg Endosc. 2023;37(10):7803-7811.
12. Van Munster SN, et al. Radiofrequency vapor ablation for Barrett’s esophagus: Feasibility, safety, and proof of concept in a stepwise study with in vitro, animal, and the first in-human application. Endoscopy. 2021 Nov;53(11):1162-1168.
13. Emura F, et al. Rio de Janeiro global consensus on landmarks, definitions, and classifications in Barrett’s esophagus: World Endoscopy Organization Delphi study. Gastroenterology. 2022 Jul;163(1):84-96.e2.
14. Singh RR, et al. Real-world evidence of safety and effectiveness of Barrett’s endoscopic therapy. Gastrointest Endosc. 2023 Aug;98(2):155-161.e1.
15. Wani S, et al. Recurrence Is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol. 2020 Oct;18(11):2609-2617.e2.
16. Duvvuri A, et al. Mo1273 Location and pattern of recurrences in patients with Barrett’s esophagus after endoscopic therapy: A systematic review and critical analysis of the published literature. Gastrointest Endosc. 2020;91(6):AB410-1.
17. He T, et al. Location and appearance of dysplastic Barrett’s esophagus recurrence after endoscopic eradication therapy: No additional yield from random biopsy sampling neosquamous mucosa. Gastrointest Endosc. 2023 Nov;98(5):722-732.
Introduction
Barrett’s esophagus (BE) is characterized by the replacement of squamous epithelium by columnar metaplasia of the distal esophagus (>1 cm length). It is a precancerous condition, with 3%-5% of patients with BE developing esophageal adenocarcinoma (EAC) in their lifetime. EAC is one of the cancers with high morbidity and mortality (5-year survival < 20%), and its incidence has been on the rise. Studies examining the natural history of BE have demonstrated that the progression happens through a metaplasia-dysplasia-neoplasia sequence. Therefore, early detection of BE and timely management to prevent progression to EAC is crucial.
Grades of Dysplasia
The current gold standard for the diagnosis of BE neoplasia includes a high-quality endoscopic evaluation and biopsies. Biopsies should be obtained from any visible lesions (nodules, ulcers) followed by a random 4-quadrant fashion (Seattle protocol) interval of the entire length of the BE segment. It is essential to pay attention to the results of the biopsy that have been obtained since it will not only determine the surveillance interval but is crucial in planning any necessary endoscopic therapy. The possible results of the biopsy and its implications are:
- No intestinal metaplasia (IM): This would rule out Barrett’s esophagus and no further surveillance would be necessary. A recent population-based study of over 1 million patients showed a 55% and 61% reduced risk of upper gastrointestinal (UGI) cancer and deaths respectively after a negative endoscopy.1
- Intestinal metaplasia with no dysplasia (non-dysplastic BE): Biopsies confirm presence of intestinal metaplasia in the biopsies without any evidence of dysplasia. While the rate of progression to EAC is low (0.07%-0.25%), it is not absent and thus surveillance would be indicated. Current guidelines suggest repeating an endoscopy with biopsy in 5 years if the length of BE is < 3 cm or 3 years if length of BE ≥ 3 cm.2
- Indeterminate for dysplasia (BE-IND): Biopsies confirm IM but are not able to definitively rule out dysplasia. This can be seen in about 4%-8% of the biopsies obtained. The progression rates to EAC are reported to be comparable or lower to low-grade dysplasia (LGD), so the current recommendation is to intensify acid reduction therapy and repeat endoscopy in 6 months. If repeat endoscopy downgrades to non-dysplastic, then can follow surveillance according to NDBE protocol; otherwise recommend continuing surveillance every 12 months.
- Low-grade dysplasia (BE-LGD): Biopsies confirm IM but also show tightly packed overlapping basal nuclei with hyperchromasia and irregular contours, basal stratification of nuclei, and diminished goblet and columnar cell mucus. There is significant inter-observer variability reported,3 and thus the slides must be reviewed by a second pathologist with experience in BE to confirm the findings. Once confirmed, based on risk factors such as presence of multifocal LGD, persistence of LGD, presence of visible lesions, etc., the patient can be offered Barrett’s endoscopic therapy (BET) or undergo continued surveillance. The decision of pursuing one or the other would be dependent on patient preference and shared decision-making between the patient and the provider.
- High-grade dysplasia (BE-HGD): Biopsies confirm IM with cells showing greater degree of cytologic and architectural alterations of dysplasia than LGD but without overt neoplastic features. Over 40% of the patients would progress to EAC and thus the current recommendations would be to recommend BET in these patients.4
- Esophageal adenocarcinoma (EAC): Biopsies demonstrate neoplasia. If the neoplastic changes are limited to the mucosa (T1a) on endoscopic ultrasound or cross-sectional imaging, then BET is suggested. If there is involvement of submucosa, then depending on the depth of invasion, absence of high-risk features (poor differentiation, lymphovascular invasion), BET can be considered as an alternative to esophagectomy.
Lesion Detection on Endoscopy
Data from large population-based studies with at least 3 years of follow-up reported that 58%-66% of EAC detected during endoscopy were diagnosed within 1 year of an index Barrett’s esophagus screening endoscopy, or post-endoscopy Barrett’s neoplasia, and were considered likely to have been missed during index endoscopy.5 This underscores the importance of careful and systematic endoscopic examination during an upper endoscopy.
Studies have also demonstrated that longer examination time was associated with significantly higher detection of HGD/EAC.6,7 Careful examination of the tubular esophagus and gastroesophageal junction (GEJ) should be performed in forward and retroflexed views looking for any subtle areas of nodularity, loop distortion, variability in vascular patterns, mucosal changes concerning for dysplasia or neoplasia. Use of high-definition white light endoscopy (HD-WLE) and virtual chromoendoscopy techniques such as narrow banding imaging (NBI) or blue laser imaging (BLI) are currently recommended in the guidelines.2 Spray chromoendoscopy using acetic acid can also be utilized. Another exciting development is the use of artificial intelligence (AI) in detecting and diagnosing BE associated lesions and neoplasia.
Barrett’s Endoscopic Therapy (BET)
Patients with visible lesions, dysplasia, or early EAC are candidates for BET (Table 1). 
BET involves resective and ablative modalities. The resective modalities include endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) and are the modalities of choice for nodular or raised lesions.
EMR involves endoscopic resection of abnormal mucosa using either lift-assisted technique or multi-band ligation (Figure 1). 
ESD, on the other hand, involves submucosal dissection and perimeter resection of the lesion, thus providing the advantage of an en-bloc resection. In a recent randomized controlled trial (RCT) of 40 patients undergoing ESD vs EMR for HGD/EAC, ESD was better for curative resection (R0) (58%) compared with EMR (12%); however, the remission rates at 3 months were comparable with two perforations reported in the ESD group while there were no complications in the EMR group.8
There is an apparent learning curve when it comes to these advanced techniques, and with more experience, we are seeing comparable results for both these modalities. However, given the complexity and time required for the procedure, current practices typically involve preserving ESD for lesions > 2 cm, those having a likelihood of cancer in the superficial submucosa, or those that EMR cannot remove due to underlying fibrosis or post-EMR recurrence.
The ablative modalities include radiofrequency ablation (RFA), cryotherapy, and hybrid argon plasma coagulation (hybrid APC). These modalities are used for flat lesions, and as therapy following endoscopic resection of nodular lesions to treat residual flat segment of BE. RFA, one of the earliest introduced endoscopic modalities, involves applying directed and controlled heat energy to ablate lesions. Current devices allow circumferential or focal application of RFA. It is a safe and effective modality with good complete eradication of IM (CE-IM) (71%-93%) and complete eradication of dysplasia (CE-D) (91%-100%) rates. These results have been sustained even at 2 years, with the most recent long-term data from a registry study showing a relapse rate of 6% for dysplasia and 19% for IM after 8 years, suggesting durability of this treatment.9
Cryotherapy involves the application of liquid nitrogen or rapidly expanding CO2 to the abnormal mucosa, leading to the rapid freezing and thawing that leads to the death of the cells. Cryogen can be applied as a spray or using a balloon with the spray nozzle in the center. This modality can be used to treat focal lesions and/or larger segments. While it has not been systematically compared with RFA, rates of CE-IM up to 81% and CE-D up to 97% are reported. Hybrid APC involves the use of submucosal saline injection to provide a protective cushion before APC is applied. It has CE-IM rate of 69% and CE-D rate of 67%-86%.10 In a recent RCT of 101 patients randomized to RFA or hybrid APC, CE-IM rates were similar (RFA:74.2% vs hAPC: 82.9%).11
Recently, another technique called radiofrequency vapor ablation (RFVA) is being evaluated, which involves ablating BE segment using vapor at 100° C generated with an RF electrode. A proof-of-concept study of 15 patients showed median squamous conversion of 55% (IQR 33-74) and 98% (IQR 56-99) for 1- and 3-second applications, respectively, with no reported adverse events.12
Barrett’s Refractory to Endoscopic Therapy
Failure of BET is defined as persistent columnar lined epithelium (intestinal metaplasia) with inadequate response, after adequate attempts at endoscopic ablation therapy (after resection) with at least four ablation sessions.13 If encountered, special attention must be given to check compliance with proton pump inhibitors (PPIs), previous incomplete resection, and presence of large hiatal hernia. If CE-IM is not achieved after multiple sessions, change of ablative modality is typically considered. In addition, careful examination for visible lesions should be performed and even if a small one is noted, this should be first resected prior to application of any ablative therapy.
Currently there are no guideline recommendations regarding the preference of one endoscopic modality over another or consideration of potential endoscopic or surgical fundoplication. These modalities primarily rely on technologies available at an institution and the preference of a provider based on their training and experience. Most studies indicate 1-3 sessions (~ 3 months apart) of ablative treatment before achieving CE-IM.
Success and Adverse Events of BET
In a recent real-world study of over 27,000 patients with dysplastic BE, 5295 underwent BET. Analysis showed that patients with HGD/EAC who had BET had a significantly lower 3-year mortality (HGD: RR, 0.59; 95%CI, 0.49-0.71; EAC: RR, 0.53; 95% CI, 0.44-0.65) compared with those who did not undergo BET. Esophageal strictures were the most common adverse event and were noted in 6.5%, followed by chest pain (1.8%), upper GI bleeding (0.47%), and esophageal perforation (0.2%).14
In general, adverse events can be divided into immediate and delayed adverse events. Immediate adverse events typically involve bleeding and perforation that can occur during or shortly after the procedure. These are reported at higher rates with resective modalities compared with ablative therapies. Standard endoscopic techniques involving coagulation grasper or clips can be used to achieve hemostasis. Endoscopic suturing devices offer the ability to contain any perforation. The need for surgical intervention is small and limited to adverse events not detected during the procedure.
Delayed adverse events such as stricture and stenosis are higher for resective modalities (up to 30%), especially when involving more significant than 75% of the esophageal circumference. Post-procedural pain/dysphagia is most common after ablative therapies. Dysphagia reported after any endoscopic therapy should be promptly evaluated, and sequential dilation until the goal esophageal lumen is achieved should be performed every 2-4 weeks.
Recurrences and Surveillance After BET
What is established is that recurrences can occur and may be subtle, therefore detailed endoscopic surveillance is required. In a prospective study, recurrence rates of 15%-16% for IM and 3%-5% for any dysplasia were reported, with the majority being in the first 2 years after achieving CE-IM.15 A systematic review of 21 studies looking at the location of recurrences suggested that the majority (56%) occur in the distal esophagus. Of those that occur in the esophagus, about 80% of them were in the distal 2 cm of the esophagus and only 50% of the recurrences were visible recurrences, thus reiterating the importance of meticulous examination and systematic biopsies.16
On the contrary, a recent single-center study of 217 patients who had achieved CE-IM with 5.5 years of follow-up demonstrated a 26% and 8% recurrence of IM and dysplasia, respectively. One hundred percent of the recurrence in the esophagus was reported as visible.17 Therefore, follow-up endoscopy surveillance protocol after CE-IM should still involve meticulous examination, biopsy of visible lesions, and systematic biopsies for non-visible lesions from the original BE segment, similar to those patients who have not needed BET.
Current guidelines based on expert consensus and evidence recommend surveillance after CE-IM based on original most advanced histology:2
1. LGD: 1 year, 3 years, and every 2 years after that.
2. HGD/EAC: 3 months, 6 months, 12 months, and annually after that.
There is no clear guideline on when to stop surveillance since the longest available follow-up is around 10 years, and recurrences are still detected. A potential surveillance endpoint may be based on age and comorbidities, especially those that would preclude a patient from being a candidate for BET.
When Should a Patient Be Referred?
BE patients with visible lesions and/or dysplastic changes in the biopsy who would require BET should be considered for referral to high-volume centers. Studies have shown higher success for CE-IM and lower rates of adverse events and recurrences in these patients managed at expert centers. The presence of a multidisciplinary team involving pathologists, surgeons, and oncologists is critical and offers a timely opportunity in case of need for a high-risk patient.
Conclusion
BE is a precursor to EAC, with rising incidence and poor 5-year survival. Endoscopic diagnosis is the gold standard and requires a high-quality examination and biopsies. Based on histopathology, a systematic surveillance and BET plan should be performed to achieve CE-IM in patients with dysplasia. Once CE-IM is achieved, regular surveillance should be performed with careful attention to recurrences and complications from the BET modalities.
Dr. Srinivasan and Dr. Sharma are based at the University of Kansas Medical Center, Kansas City, Kansas, and the Kansas City Veterans Affairs Medical Center, Kansas City, Missouri. Dr. Srinivasan has no relevant disclosures. Dr. Sharma disclosed research grants from ERBE, Ironwood Pharmaceuticals, Olympus, and Medtronic. He has served as a consultant for Takeda, Samsung Bioepis, Olympus, and Lumendi, and reports other funding from Medtronic, Fujifilm Medical Systems USA, and Salix.
References
1. Holmberg D, et al. Incidence and mortality in upper gastrointestinal cancer after negative endoscopy for gastroesophageal reflux disease. Gastroenterology. 2022;162(2):431-438.e4.
2. Shaheen NJ, et al. Diagnosis and management of Barrett’s esophagus: An updated ACG guideline. Am J Gastroenterol. 2022 Apr;117(4):559-587.
3. Pech O, et al. Inter-observer variability in the diagnosis of low-grade dysplasia in pathologists: A comparison between experienced and inexperienced pathologists. Gastrointest Endosc. 2006 Apr;63(5):AB130.
4. Krishnamoorthi R, et al. Factors associated with progression of Barrett’s esophagus: A systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018 Jul;16(7):1046-1055.e8.
5. Visrodia K, et al. Magnitude of missed esophageal adenocarcinoma after Barrett’s esophagus diagnosis: A systematic review and meta-analysis. Gastroenterology. 2016 Mar;150(3):599-607.e7; quiz e14-5.
6. Perisetti A, Sharma P. Tips for improving the identification of neoplastic visible lesions in Barrett’s esophagus. Gastrointest Endosc. 2023 Feb;97(2):248-250.
7. Gupta N, et al. Longer inspection time is associated with increased detection of high-grade dysplasia and esophageal adenocarcinoma in Barrett’s esophagus. Gastrointest Endosc. 2012 Sep;76(3):531-538.
8. Terheggen G, et al. A randomised trial of endoscopic submucosal dissection versus endoscopic mucosal resection for early Barrett’s neoplasia. Gut. 2017 May;66(5):783-793.
9. Wolfson P, et al. Endoscopic eradication therapy for Barrett’s esophagus-related neoplasia: A final 10-year report from the UK National HALO Radiofrequency Ablation Registry. Gastrointest Endosc. 2022 Aug;96(2):223-233.
10. Rösch T, et al. 1151 Multicenter feasibility study of combined injection and argon plasma coagulation (hybrid-APC) in the ablation therapy of neoplastic Barrett esophagus. Gastrointest Endosc. 2017;85(5):AB154.
11. Knabe M, et al. Radiofrequency ablation versus hybrid argon plasma coagulation in Barrett’s esophagus: A prospective randomised trial. Surg Endosc. 2023;37(10):7803-7811.
12. Van Munster SN, et al. Radiofrequency vapor ablation for Barrett’s esophagus: Feasibility, safety, and proof of concept in a stepwise study with in vitro, animal, and the first in-human application. Endoscopy. 2021 Nov;53(11):1162-1168.
13. Emura F, et al. Rio de Janeiro global consensus on landmarks, definitions, and classifications in Barrett’s esophagus: World Endoscopy Organization Delphi study. Gastroenterology. 2022 Jul;163(1):84-96.e2.
14. Singh RR, et al. Real-world evidence of safety and effectiveness of Barrett’s endoscopic therapy. Gastrointest Endosc. 2023 Aug;98(2):155-161.e1.
15. Wani S, et al. Recurrence Is rare following complete eradication of intestinal metaplasia in patients with Barrett’s esophagus and peaks at 18 months. Clin Gastroenterol Hepatol. 2020 Oct;18(11):2609-2617.e2.
16. Duvvuri A, et al. Mo1273 Location and pattern of recurrences in patients with Barrett’s esophagus after endoscopic therapy: A systematic review and critical analysis of the published literature. Gastrointest Endosc. 2020;91(6):AB410-1.
17. He T, et al. Location and appearance of dysplastic Barrett’s esophagus recurrence after endoscopic eradication therapy: No additional yield from random biopsy sampling neosquamous mucosa. Gastrointest Endosc. 2023 Nov;98(5):722-732.