User login
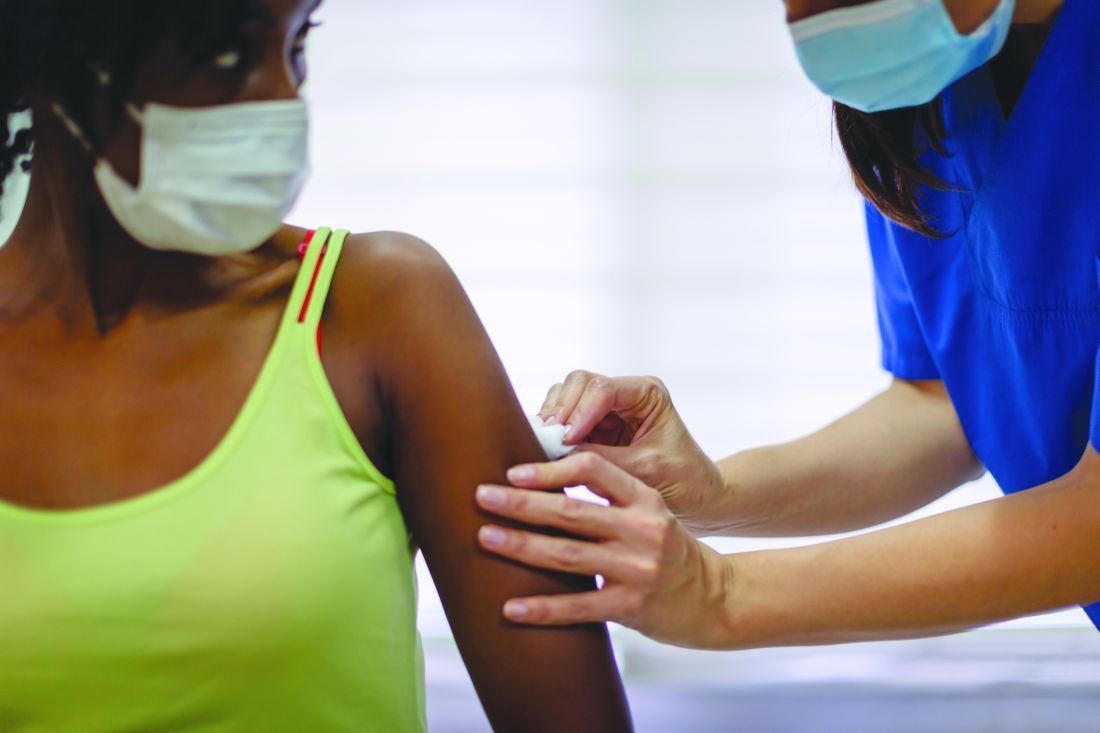
More signs COVID shots are safe for pregnant women
As the U.S. races to vaccinate millions of people against the coronavirus, pregnant women face the extra challenge of not knowing whether the vaccines are safe for them or their unborn babies.
None of the recent COVID-19 vaccine trials, including those for Pfizer, Moderna, and Johnson & Johnson, enrolled pregnant or breastfeeding women because they consider them a high-risk group.
That was despite the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists asking that pregnant and breastfeeding women be included in trials. The Food and Drug Administration even included pregnant women in the COVID-19 vaccine emergency use authorization (EUA) because of their higher risk of having a more severe disease.
Despite that lack of clinical trial data, more and more smaller studies are suggesting that the vaccines are safe for both mother and child.
Pfizer is now studying its two-dose vaccine in 4,000 pregnant and breastfeeding women to see how safe, tolerated, and robust their immune response is. Researchers will also look at how safe the vaccine is for infants and whether mothers pass along antibodies to children. But the preliminary results won’t be available until the end of the year, a Pfizer spokesperson says.
Without that information, pregnant women are less likely to get vaccinated, according to a large international survey. Less than 45% of pregnant women in the United States said they intended to get vaccinated even when they were told the vaccine was safe and 90% effective. That figure rises to 52% of pregnant women in 16 countries, including the United States, compared with 74% of nonpregnant women willing to be vaccinated. The findings were published online March 1, 2021, in the European Journal of Epidemiology.
The vaccine-hesitant pregnant women in the international study were most concerned that the COVID-19 vaccine could harm their developing fetuses, a worry related to the lack of clinical evidence in pregnant women, said lead researcher Julia Wu, ScD, an epidemiologist at the Harvard School of Public Health’s Human Immunomics Initiative in Boston.
The information vacuum also increases the chances that “people will fall victim to misinformation campaigns like the one on social media that claims that the COVID-19 vaccine causes infertility,” Dr. Wu said. This unfounded claim has deterred some women of childbearing age from getting the vaccine.
Deciding to get vaccinated
Frontline health care professionals were in the first group eligible to receive the vaccine in December 2020. “All of us who were pregnant ... had to decide whether to wait for the data, because we don’t know what the risks are, or go ahead and get it [the vaccine]. We had been dealing with the pandemic for months and were afraid of being exposed to the virus and infecting family members,” said Jacqueline Parchem, MD, a maternal-fetal medicine specialist at the University of Texas Health Science Center, Houston.
Given the lack of safety data, the CDC guidance to pregnant women has been to consult with their doctors and that it’s a personal choice. The Center for Disease Control and Prevention’s latest vaccine guidance said that “there is no evidence that antibodies formed from COVID-19 vaccination cause any problem with pregnancy, including the development of the placenta.”
The CDC is monitoring vaccinated people through its v-safe program and reported on April 12 that more than 86,000 v-safe participants said they were pregnant when they were vaccinated.
Health care workers who were nursing their infants when they were eligible for the vaccine faced a similar dilemma as pregnant women – they lacked the data on them to make a truly informed decision.
“I was nervous about the vaccine side effects for myself and whether my son Bennett, who was about a year old, would experience any of these himself,” said Christa Carrig, a labor and delivery nurse at Massachusetts General Hospital in Boston, who was breastfeeding at the time.
She and Dr. Parchem know that pregnant women with COVID-19 are more likely to have severe illness and complications such as high blood pressure and preterm delivery. “Pregnancy takes a toll on the body. When a woman gets COVID-19 and that insult is added, women who were otherwise young and healthy get much sicker than you would expect,” said Ms. Carrig.
“As a high-risk pregnancy specialist, I know that, with COVID, that babies don’t do well when moms are sick,” said Dr. Parchem.
Pregnant women accounted for more than 84,629 cases of COVID-19 and 95 deaths in the United States between Jan. 22 last year and April 12 this year, according to the CDC COVID data tracker.
Dr. Parchem and Ms. Carrig decided to get vaccinated because of their high risk of exposure to COVID-19 at work. After the second dose, Ms. Carrig reported chills but Bennett had no side effects from breastfeeding. Dr. Parchem, who delivered a healthy baby boy in February, reported no side effects other than a sore arm.
“There’s also a psychological benefit to returning to some sense of normalcy,” said Dr. Parchem. “My mother was finally able to visit us to see the new baby after we were all vaccinated. This was the first visit in more than a year.”
New study results
Ms. Carrig was one of 131 vaccinated hospital workers in the Boston area who took part in the first study to profile the immune response in pregnant and breastfeeding women and compare it with both nonpregnant and pregnant women who had COVID-19.
The study was not designed to evaluate the safety of the vaccines or whether they prevent COVID-19 illness and hospitalizations. That is the role of the large vaccine trials, the authors said.
The participants were aged 18-45 years and received both doses of either Pfizer or Moderna vaccines during one of their trimesters. They provided blood and/or breast milk samples after each vaccine dose, 2-6 weeks after the last dose, and at delivery for the 10 who gave birth during the study.
The vaccines produced a similar strong antibody response among the pregnant/breastfeeding women and nonpregnant women. Their antibody levels were much higher than those found in the pregnant women who had COVID-19, the researchers reported on March 25, 2021, in the American Journal of Obstetrics and Gynecology.
“This is important because a lot of people tend to think once they’ve had COVID-19, they are protected from the virus. This finding suggests that the vaccines produce a stronger antibody response than the infection itself, and this might be important for long-lasting protection against COVID-19,” said Dr. Parchem.
The study also addressed whether newborns benefit from the antibodies produced by their mothers. “In the 10 women who delivered, we detected antibodies in their umbilical cords and breast milk,” says Andrea Edlow, MD, lead researcher and a maternal-fetal medicine specialist at Massachusetts General Hospital.
Newborns are particularly vulnerable to respiratory infections because they have small airways and their immune systems are underdeveloped. These infections can be lethal early in life.
“The public health strategy is to vaccinate mothers against respiratory viruses, bacteria, and parasites that neonates up to 6 months are exposed to. Influenza and pertussis (whooping cough) are two examples of vaccines that we give mothers that we know transfer [antibodies] across the umbilical cord,” said Dr. Edlow.
But this “passive transfer immunity” is different from active immunity, when the body produces its own antibody immune response, she explains.
A different study, also published in March, confirmed that antibodies were transferred from 27 vaccinated pregnant mothers to their infants when they delivered. A new finding was that the women who were vaccinated with both doses and earlier in their third semester passed on more antibodies than the women who were vaccinated later or with only one dose.
Impact of the studies
The Society for Maternal-Fetal Medicine updated its guidance on counseling pregnant and lactating patients about the COVID-19 vaccines to include Dr. Edlow’s study.
“We were struck by how much pregnant and breastfeeding women want to participate in research and to help others in the same situation make decisions. I hope this will be an example to drug companies doing research on new vaccines in the future – that they should not be left behind and can make decisions themselves whether to participate after weighing the risks and benefits,” said Dr. Edlow.
She continues to enroll more vaccinated women in her study in the Boston area, including non–health care workers who have asked to take part.
“It was worth getting vaccinated and participating in the study. I know that I have antibodies and it worked and that I passed them on to Bennett. Also, I know that all the information is available for other women who are questioning whether to get vaccinated or not,” said Ms. Carrig.
Dr. Parchem is also taking part in the CDC’s v-safe pregnancy registry, which is collecting health and safety data on vaccinated pregnant women.
Before she was vaccinated, Dr. Parchem said, “my advice was very measured because we lacked data either saying that it definitely works or showing that it was unsafe. Now that we have this data supporting the benefits, I feel more confident in recommending the vaccines.”
A version of this article first appeared on Medscape.com.
As the U.S. races to vaccinate millions of people against the coronavirus, pregnant women face the extra challenge of not knowing whether the vaccines are safe for them or their unborn babies.
None of the recent COVID-19 vaccine trials, including those for Pfizer, Moderna, and Johnson & Johnson, enrolled pregnant or breastfeeding women because they consider them a high-risk group.
That was despite the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists asking that pregnant and breastfeeding women be included in trials. The Food and Drug Administration even included pregnant women in the COVID-19 vaccine emergency use authorization (EUA) because of their higher risk of having a more severe disease.
Despite that lack of clinical trial data, more and more smaller studies are suggesting that the vaccines are safe for both mother and child.
Pfizer is now studying its two-dose vaccine in 4,000 pregnant and breastfeeding women to see how safe, tolerated, and robust their immune response is. Researchers will also look at how safe the vaccine is for infants and whether mothers pass along antibodies to children. But the preliminary results won’t be available until the end of the year, a Pfizer spokesperson says.
Without that information, pregnant women are less likely to get vaccinated, according to a large international survey. Less than 45% of pregnant women in the United States said they intended to get vaccinated even when they were told the vaccine was safe and 90% effective. That figure rises to 52% of pregnant women in 16 countries, including the United States, compared with 74% of nonpregnant women willing to be vaccinated. The findings were published online March 1, 2021, in the European Journal of Epidemiology.
The vaccine-hesitant pregnant women in the international study were most concerned that the COVID-19 vaccine could harm their developing fetuses, a worry related to the lack of clinical evidence in pregnant women, said lead researcher Julia Wu, ScD, an epidemiologist at the Harvard School of Public Health’s Human Immunomics Initiative in Boston.
The information vacuum also increases the chances that “people will fall victim to misinformation campaigns like the one on social media that claims that the COVID-19 vaccine causes infertility,” Dr. Wu said. This unfounded claim has deterred some women of childbearing age from getting the vaccine.
Deciding to get vaccinated
Frontline health care professionals were in the first group eligible to receive the vaccine in December 2020. “All of us who were pregnant ... had to decide whether to wait for the data, because we don’t know what the risks are, or go ahead and get it [the vaccine]. We had been dealing with the pandemic for months and were afraid of being exposed to the virus and infecting family members,” said Jacqueline Parchem, MD, a maternal-fetal medicine specialist at the University of Texas Health Science Center, Houston.
Given the lack of safety data, the CDC guidance to pregnant women has been to consult with their doctors and that it’s a personal choice. The Center for Disease Control and Prevention’s latest vaccine guidance said that “there is no evidence that antibodies formed from COVID-19 vaccination cause any problem with pregnancy, including the development of the placenta.”
The CDC is monitoring vaccinated people through its v-safe program and reported on April 12 that more than 86,000 v-safe participants said they were pregnant when they were vaccinated.
Health care workers who were nursing their infants when they were eligible for the vaccine faced a similar dilemma as pregnant women – they lacked the data on them to make a truly informed decision.
“I was nervous about the vaccine side effects for myself and whether my son Bennett, who was about a year old, would experience any of these himself,” said Christa Carrig, a labor and delivery nurse at Massachusetts General Hospital in Boston, who was breastfeeding at the time.
She and Dr. Parchem know that pregnant women with COVID-19 are more likely to have severe illness and complications such as high blood pressure and preterm delivery. “Pregnancy takes a toll on the body. When a woman gets COVID-19 and that insult is added, women who were otherwise young and healthy get much sicker than you would expect,” said Ms. Carrig.
“As a high-risk pregnancy specialist, I know that, with COVID, that babies don’t do well when moms are sick,” said Dr. Parchem.
Pregnant women accounted for more than 84,629 cases of COVID-19 and 95 deaths in the United States between Jan. 22 last year and April 12 this year, according to the CDC COVID data tracker.
Dr. Parchem and Ms. Carrig decided to get vaccinated because of their high risk of exposure to COVID-19 at work. After the second dose, Ms. Carrig reported chills but Bennett had no side effects from breastfeeding. Dr. Parchem, who delivered a healthy baby boy in February, reported no side effects other than a sore arm.
“There’s also a psychological benefit to returning to some sense of normalcy,” said Dr. Parchem. “My mother was finally able to visit us to see the new baby after we were all vaccinated. This was the first visit in more than a year.”
New study results
Ms. Carrig was one of 131 vaccinated hospital workers in the Boston area who took part in the first study to profile the immune response in pregnant and breastfeeding women and compare it with both nonpregnant and pregnant women who had COVID-19.
The study was not designed to evaluate the safety of the vaccines or whether they prevent COVID-19 illness and hospitalizations. That is the role of the large vaccine trials, the authors said.
The participants were aged 18-45 years and received both doses of either Pfizer or Moderna vaccines during one of their trimesters. They provided blood and/or breast milk samples after each vaccine dose, 2-6 weeks after the last dose, and at delivery for the 10 who gave birth during the study.
The vaccines produced a similar strong antibody response among the pregnant/breastfeeding women and nonpregnant women. Their antibody levels were much higher than those found in the pregnant women who had COVID-19, the researchers reported on March 25, 2021, in the American Journal of Obstetrics and Gynecology.
“This is important because a lot of people tend to think once they’ve had COVID-19, they are protected from the virus. This finding suggests that the vaccines produce a stronger antibody response than the infection itself, and this might be important for long-lasting protection against COVID-19,” said Dr. Parchem.
The study also addressed whether newborns benefit from the antibodies produced by their mothers. “In the 10 women who delivered, we detected antibodies in their umbilical cords and breast milk,” says Andrea Edlow, MD, lead researcher and a maternal-fetal medicine specialist at Massachusetts General Hospital.
Newborns are particularly vulnerable to respiratory infections because they have small airways and their immune systems are underdeveloped. These infections can be lethal early in life.
“The public health strategy is to vaccinate mothers against respiratory viruses, bacteria, and parasites that neonates up to 6 months are exposed to. Influenza and pertussis (whooping cough) are two examples of vaccines that we give mothers that we know transfer [antibodies] across the umbilical cord,” said Dr. Edlow.
But this “passive transfer immunity” is different from active immunity, when the body produces its own antibody immune response, she explains.
A different study, also published in March, confirmed that antibodies were transferred from 27 vaccinated pregnant mothers to their infants when they delivered. A new finding was that the women who were vaccinated with both doses and earlier in their third semester passed on more antibodies than the women who were vaccinated later or with only one dose.
Impact of the studies
The Society for Maternal-Fetal Medicine updated its guidance on counseling pregnant and lactating patients about the COVID-19 vaccines to include Dr. Edlow’s study.
“We were struck by how much pregnant and breastfeeding women want to participate in research and to help others in the same situation make decisions. I hope this will be an example to drug companies doing research on new vaccines in the future – that they should not be left behind and can make decisions themselves whether to participate after weighing the risks and benefits,” said Dr. Edlow.
She continues to enroll more vaccinated women in her study in the Boston area, including non–health care workers who have asked to take part.
“It was worth getting vaccinated and participating in the study. I know that I have antibodies and it worked and that I passed them on to Bennett. Also, I know that all the information is available for other women who are questioning whether to get vaccinated or not,” said Ms. Carrig.
Dr. Parchem is also taking part in the CDC’s v-safe pregnancy registry, which is collecting health and safety data on vaccinated pregnant women.
Before she was vaccinated, Dr. Parchem said, “my advice was very measured because we lacked data either saying that it definitely works or showing that it was unsafe. Now that we have this data supporting the benefits, I feel more confident in recommending the vaccines.”
A version of this article first appeared on Medscape.com.
As the U.S. races to vaccinate millions of people against the coronavirus, pregnant women face the extra challenge of not knowing whether the vaccines are safe for them or their unborn babies.
None of the recent COVID-19 vaccine trials, including those for Pfizer, Moderna, and Johnson & Johnson, enrolled pregnant or breastfeeding women because they consider them a high-risk group.
That was despite the Society for Maternal-Fetal Medicine and the American College of Obstetricians and Gynecologists asking that pregnant and breastfeeding women be included in trials. The Food and Drug Administration even included pregnant women in the COVID-19 vaccine emergency use authorization (EUA) because of their higher risk of having a more severe disease.
Despite that lack of clinical trial data, more and more smaller studies are suggesting that the vaccines are safe for both mother and child.
Pfizer is now studying its two-dose vaccine in 4,000 pregnant and breastfeeding women to see how safe, tolerated, and robust their immune response is. Researchers will also look at how safe the vaccine is for infants and whether mothers pass along antibodies to children. But the preliminary results won’t be available until the end of the year, a Pfizer spokesperson says.
Without that information, pregnant women are less likely to get vaccinated, according to a large international survey. Less than 45% of pregnant women in the United States said they intended to get vaccinated even when they were told the vaccine was safe and 90% effective. That figure rises to 52% of pregnant women in 16 countries, including the United States, compared with 74% of nonpregnant women willing to be vaccinated. The findings were published online March 1, 2021, in the European Journal of Epidemiology.
The vaccine-hesitant pregnant women in the international study were most concerned that the COVID-19 vaccine could harm their developing fetuses, a worry related to the lack of clinical evidence in pregnant women, said lead researcher Julia Wu, ScD, an epidemiologist at the Harvard School of Public Health’s Human Immunomics Initiative in Boston.
The information vacuum also increases the chances that “people will fall victim to misinformation campaigns like the one on social media that claims that the COVID-19 vaccine causes infertility,” Dr. Wu said. This unfounded claim has deterred some women of childbearing age from getting the vaccine.
Deciding to get vaccinated
Frontline health care professionals were in the first group eligible to receive the vaccine in December 2020. “All of us who were pregnant ... had to decide whether to wait for the data, because we don’t know what the risks are, or go ahead and get it [the vaccine]. We had been dealing with the pandemic for months and were afraid of being exposed to the virus and infecting family members,” said Jacqueline Parchem, MD, a maternal-fetal medicine specialist at the University of Texas Health Science Center, Houston.
Given the lack of safety data, the CDC guidance to pregnant women has been to consult with their doctors and that it’s a personal choice. The Center for Disease Control and Prevention’s latest vaccine guidance said that “there is no evidence that antibodies formed from COVID-19 vaccination cause any problem with pregnancy, including the development of the placenta.”
The CDC is monitoring vaccinated people through its v-safe program and reported on April 12 that more than 86,000 v-safe participants said they were pregnant when they were vaccinated.
Health care workers who were nursing their infants when they were eligible for the vaccine faced a similar dilemma as pregnant women – they lacked the data on them to make a truly informed decision.
“I was nervous about the vaccine side effects for myself and whether my son Bennett, who was about a year old, would experience any of these himself,” said Christa Carrig, a labor and delivery nurse at Massachusetts General Hospital in Boston, who was breastfeeding at the time.
She and Dr. Parchem know that pregnant women with COVID-19 are more likely to have severe illness and complications such as high blood pressure and preterm delivery. “Pregnancy takes a toll on the body. When a woman gets COVID-19 and that insult is added, women who were otherwise young and healthy get much sicker than you would expect,” said Ms. Carrig.
“As a high-risk pregnancy specialist, I know that, with COVID, that babies don’t do well when moms are sick,” said Dr. Parchem.
Pregnant women accounted for more than 84,629 cases of COVID-19 and 95 deaths in the United States between Jan. 22 last year and April 12 this year, according to the CDC COVID data tracker.
Dr. Parchem and Ms. Carrig decided to get vaccinated because of their high risk of exposure to COVID-19 at work. After the second dose, Ms. Carrig reported chills but Bennett had no side effects from breastfeeding. Dr. Parchem, who delivered a healthy baby boy in February, reported no side effects other than a sore arm.
“There’s also a psychological benefit to returning to some sense of normalcy,” said Dr. Parchem. “My mother was finally able to visit us to see the new baby after we were all vaccinated. This was the first visit in more than a year.”
New study results
Ms. Carrig was one of 131 vaccinated hospital workers in the Boston area who took part in the first study to profile the immune response in pregnant and breastfeeding women and compare it with both nonpregnant and pregnant women who had COVID-19.
The study was not designed to evaluate the safety of the vaccines or whether they prevent COVID-19 illness and hospitalizations. That is the role of the large vaccine trials, the authors said.
The participants were aged 18-45 years and received both doses of either Pfizer or Moderna vaccines during one of their trimesters. They provided blood and/or breast milk samples after each vaccine dose, 2-6 weeks after the last dose, and at delivery for the 10 who gave birth during the study.
The vaccines produced a similar strong antibody response among the pregnant/breastfeeding women and nonpregnant women. Their antibody levels were much higher than those found in the pregnant women who had COVID-19, the researchers reported on March 25, 2021, in the American Journal of Obstetrics and Gynecology.
“This is important because a lot of people tend to think once they’ve had COVID-19, they are protected from the virus. This finding suggests that the vaccines produce a stronger antibody response than the infection itself, and this might be important for long-lasting protection against COVID-19,” said Dr. Parchem.
The study also addressed whether newborns benefit from the antibodies produced by their mothers. “In the 10 women who delivered, we detected antibodies in their umbilical cords and breast milk,” says Andrea Edlow, MD, lead researcher and a maternal-fetal medicine specialist at Massachusetts General Hospital.
Newborns are particularly vulnerable to respiratory infections because they have small airways and their immune systems are underdeveloped. These infections can be lethal early in life.
“The public health strategy is to vaccinate mothers against respiratory viruses, bacteria, and parasites that neonates up to 6 months are exposed to. Influenza and pertussis (whooping cough) are two examples of vaccines that we give mothers that we know transfer [antibodies] across the umbilical cord,” said Dr. Edlow.
But this “passive transfer immunity” is different from active immunity, when the body produces its own antibody immune response, she explains.
A different study, also published in March, confirmed that antibodies were transferred from 27 vaccinated pregnant mothers to their infants when they delivered. A new finding was that the women who were vaccinated with both doses and earlier in their third semester passed on more antibodies than the women who were vaccinated later or with only one dose.
Impact of the studies
The Society for Maternal-Fetal Medicine updated its guidance on counseling pregnant and lactating patients about the COVID-19 vaccines to include Dr. Edlow’s study.
“We were struck by how much pregnant and breastfeeding women want to participate in research and to help others in the same situation make decisions. I hope this will be an example to drug companies doing research on new vaccines in the future – that they should not be left behind and can make decisions themselves whether to participate after weighing the risks and benefits,” said Dr. Edlow.
She continues to enroll more vaccinated women in her study in the Boston area, including non–health care workers who have asked to take part.
“It was worth getting vaccinated and participating in the study. I know that I have antibodies and it worked and that I passed them on to Bennett. Also, I know that all the information is available for other women who are questioning whether to get vaccinated or not,” said Ms. Carrig.
Dr. Parchem is also taking part in the CDC’s v-safe pregnancy registry, which is collecting health and safety data on vaccinated pregnant women.
Before she was vaccinated, Dr. Parchem said, “my advice was very measured because we lacked data either saying that it definitely works or showing that it was unsafe. Now that we have this data supporting the benefits, I feel more confident in recommending the vaccines.”
A version of this article first appeared on Medscape.com.
Most patients with chronic inflammatory diseases have sufficient response to COVID-19 vaccination
Glucocorticoids and B-cell–depleting therapies are trouble spots
Although most patients with chronic inflammatory diseases mounted immune responses after two doses of mRNA-based COVID-19 vaccines, glucocorticoids and B-cell–depleting therapies markedly reduced the response, according to a recently published preprint of a new study.
The study, published on MedRxiv and not yet peer reviewed, involved a prospective look at 133 patients with chronic inflammatory disease (CID) and 53 patients with healthy immune systems at Washington University, St. Louis, and the University of California, San Francisco. It is regarded as the largest and most detailed study yet in how vaccines perform in people with immune-mediated inflammatory disease. The patients were enrolled between December 2020 and March 2021, and the most common diseases were inflammatory bowel disease (32%), rheumatoid arthritis (29%), spondyloarthritis (15%), and systemic lupus erythematosus (11%).
A ‘modest’ reduction in antibody response
Senior author Alfred Kim, MD, PhD, of the department of medicine at Washington University, said the overall results so far are encouraging.
“Most patients with an autoimmune disease that are on immunosuppression can mount antibody responses,” he said. “We’re seeing the majority of our subjects respond.”
The immune-healthy controls and most of the patients with CID had a robust immune response against the spike protein, although the CID group had a mean reduction in antibody titers that was three times lower than the controls (P = .0092). The CID group similarly had a 2.7-fold reduction in preventing neutralization, or halting the virus’ ability to infect (P < .0001), researchers reported.
This reduction in response is “modest,” he said.
“Is the level of reduction going to be detrimental for protection? Time will tell,” he said, adding that researchers anticipate that it won’t have a critical effect on protection because responses tended to be within the range of the immunocompetent controls, who themselves had wildly varied antibody titers across a 20-fold range. “ ‘Optimal’ isn’t necessarily the same as ‘sufficient.’ ”
Type of medication has big impact on antibody titers
But there was a wide variety of effects on the immune response depending on the medication. Glucorticoids resulted in a response that was 10 times lower than the immune-healthy controls, as well as fewer circulating plasmablasts after vaccination. Researchers found that 98% of controls were seropositive for antibody, compared with 92% of those with CID who were not taking prednisone, and 65% of CID patients on prednisone (P = .0006 and .0115, respectively). Prevention of neutralization of the virus was similarly reduced in those groups, compared with the controls. Dr. Kim noted this was a small sample size, with about 15 patients. These effects were seen regardless of the dose.
“We would’ve anticipated this would have been dose dependent, so this was a little bit surprising,” Dr. Kim said.
B-cell–depleting therapies, such as rituximab (Rituxan) and ocrelizumab (Ocrevus), reduced antibody titers by 36 times, compared with controls (P < .0001), with a similar reduction in preventing infection (P = .0066), the researchers found. The reduction in antibody titers was the most pronounced among those who had received B-cell–depleting therapies within the previous 6 months. Dr. Kim noted this was a small sample size, with about 10 patients.
CID study subjects taking an antimetabolite, including methotrexate, had an average of a two- to threefold reduction in antibody titers and in neutralization (P = .0006). This reduction was greatest with methotrexate, researchers found (P = .0027).
JAK inhibitors also significantly reduced antibody titers (P = .0066), but the reduction in neutralization of the virus was not significant. In addition, researchers found a reduction in antibody titers, the prevention of viral infection, and circulating plasmablasts among those on tumor necrosis factor (TNF) inhibitors, compared with controls, but these were insignificant statistically except for virus neutralization.
Dr. Kim said he hopes the glucocorticoid data spur physicians to try harder to wean patients off the drugs, when possible, in keeping with recommendations already in place.
“The general culture in rheumatology has been very lax about the need to reduce glucocorticoids,” he said. “This reinvigorates that call.” Questions about possible drug holidays from glucocorticoids remain, regarding how long a holiday would be needed, he said. He noted that many patients on glucocorticoids nonetheless mounted responses.
Those on B-cell–depleting therapies present a “much more difficult” question, he said. Some patients possibly could wait a bit longer than their normal, every-6-month schedule, but it’s an individual decision, he said. Since a booster of influenza vaccine has been found to enhance the response even within the 6-month window among ocrelizumab patients, a booster of COVID-19 vaccine might also help, although this remains to be studied.
The study group has already increased its sample size and is looking at adverse reactions and long-term immune responses, Dr. Kim said.
Encouraging, rather than discouraging, results
Leonard Calabrese, DO, professor of medicine at the Cleveland Clinic in Ohio, said the findings shouldn’t discourage clinicians from encouraging vaccination.
“There’s still a preponderance of people who will develop a robust antibody vaccine response,” he said.
He cautioned that the findings look only at antibodies to the spike protein and at plasmablasts. The reduction in these titers is “of concern,” he said, but “we don’t really know with certainty what are the effects of these drugs, and these data are on the overall biologic protective effect of the vaccine. There’s much more to a vaccine response than anti–spike protein and plasmablasts,” including cell-mediated immune response.
For an individual patient, the findings “mean a lot,” he said.
“I think that people who are on significant prednisone and B-cell–depleting agents, I think you have to share with them that there’s a reasonable chance that you’re not going to be making a response similar to healthy people,” he said. “Thus, even with your vaccine, we’re not going to cut you loose to do things that are violating social distancing and group settings. … Should you be hugging your grandchildren if you’re a rituximab vaccine recipient? I think I would wait until we have a little bit more data.”
Kevin Winthrop, MD, MPH, professor of ophthalmology at Oregon Health & Science University, Portland, where he studies vaccinations in the immunocompromised, said that glucocorticoids tend to have little effect on vaccinations generally at low doses.
When effects are seen they can be difficult to interpret, he said.
“It’s hard to extricate that from the effect of the underlying disease,” he said. The drug can be a proxy for worse disease control.
Although it’s a small study, it’s reassuring that overall the responses were similar to healthy controls.
For B-cell–depleting therapies, his usual guidance is to not give vaccine until a patient is at least 3 months out from their last dose, and not to restart until at least 2 weeks after vaccination.
“It’s not surprising that some of these DMARDs [disease-modifying antirheumatic drugs] do negatively affect vaccine response, particularly B-cell–depletion therapy. We need to do some studies to find a way to overcome that, or optimize delivery of the vaccine.”
Dr. Kim reported participating in consulting, advisory board, or speaker’s bureau for Alexion, Aurinia, Annexon Biosciences, Exagen Diagnostics, and GlaxoSmithKline, and receiving funding under a sponsored research agreement unrelated to the data in the paper from GlaxoSmithKline. Dr. Winthrop reported receiving consulting fees from Pfizer, AbbVie, UCB, Eli Lilly, Galapagos, GlaxoSmithKline, Roche, Gilead, Bristol-Myers Squibb, Regeneron, Sanofi, AstraZeneca, Novartis, and research grants from Bristol-Myers Squibb and Pfizer. Dr. Calabrese reported no relevant disclosures.
Glucocorticoids and B-cell–depleting therapies are trouble spots
Glucocorticoids and B-cell–depleting therapies are trouble spots
Although most patients with chronic inflammatory diseases mounted immune responses after two doses of mRNA-based COVID-19 vaccines, glucocorticoids and B-cell–depleting therapies markedly reduced the response, according to a recently published preprint of a new study.
The study, published on MedRxiv and not yet peer reviewed, involved a prospective look at 133 patients with chronic inflammatory disease (CID) and 53 patients with healthy immune systems at Washington University, St. Louis, and the University of California, San Francisco. It is regarded as the largest and most detailed study yet in how vaccines perform in people with immune-mediated inflammatory disease. The patients were enrolled between December 2020 and March 2021, and the most common diseases were inflammatory bowel disease (32%), rheumatoid arthritis (29%), spondyloarthritis (15%), and systemic lupus erythematosus (11%).
A ‘modest’ reduction in antibody response
Senior author Alfred Kim, MD, PhD, of the department of medicine at Washington University, said the overall results so far are encouraging.
“Most patients with an autoimmune disease that are on immunosuppression can mount antibody responses,” he said. “We’re seeing the majority of our subjects respond.”
The immune-healthy controls and most of the patients with CID had a robust immune response against the spike protein, although the CID group had a mean reduction in antibody titers that was three times lower than the controls (P = .0092). The CID group similarly had a 2.7-fold reduction in preventing neutralization, or halting the virus’ ability to infect (P < .0001), researchers reported.
This reduction in response is “modest,” he said.
“Is the level of reduction going to be detrimental for protection? Time will tell,” he said, adding that researchers anticipate that it won’t have a critical effect on protection because responses tended to be within the range of the immunocompetent controls, who themselves had wildly varied antibody titers across a 20-fold range. “ ‘Optimal’ isn’t necessarily the same as ‘sufficient.’ ”
Type of medication has big impact on antibody titers
But there was a wide variety of effects on the immune response depending on the medication. Glucorticoids resulted in a response that was 10 times lower than the immune-healthy controls, as well as fewer circulating plasmablasts after vaccination. Researchers found that 98% of controls were seropositive for antibody, compared with 92% of those with CID who were not taking prednisone, and 65% of CID patients on prednisone (P = .0006 and .0115, respectively). Prevention of neutralization of the virus was similarly reduced in those groups, compared with the controls. Dr. Kim noted this was a small sample size, with about 15 patients. These effects were seen regardless of the dose.
“We would’ve anticipated this would have been dose dependent, so this was a little bit surprising,” Dr. Kim said.
B-cell–depleting therapies, such as rituximab (Rituxan) and ocrelizumab (Ocrevus), reduced antibody titers by 36 times, compared with controls (P < .0001), with a similar reduction in preventing infection (P = .0066), the researchers found. The reduction in antibody titers was the most pronounced among those who had received B-cell–depleting therapies within the previous 6 months. Dr. Kim noted this was a small sample size, with about 10 patients.
CID study subjects taking an antimetabolite, including methotrexate, had an average of a two- to threefold reduction in antibody titers and in neutralization (P = .0006). This reduction was greatest with methotrexate, researchers found (P = .0027).
JAK inhibitors also significantly reduced antibody titers (P = .0066), but the reduction in neutralization of the virus was not significant. In addition, researchers found a reduction in antibody titers, the prevention of viral infection, and circulating plasmablasts among those on tumor necrosis factor (TNF) inhibitors, compared with controls, but these were insignificant statistically except for virus neutralization.
Dr. Kim said he hopes the glucocorticoid data spur physicians to try harder to wean patients off the drugs, when possible, in keeping with recommendations already in place.
“The general culture in rheumatology has been very lax about the need to reduce glucocorticoids,” he said. “This reinvigorates that call.” Questions about possible drug holidays from glucocorticoids remain, regarding how long a holiday would be needed, he said. He noted that many patients on glucocorticoids nonetheless mounted responses.
Those on B-cell–depleting therapies present a “much more difficult” question, he said. Some patients possibly could wait a bit longer than their normal, every-6-month schedule, but it’s an individual decision, he said. Since a booster of influenza vaccine has been found to enhance the response even within the 6-month window among ocrelizumab patients, a booster of COVID-19 vaccine might also help, although this remains to be studied.
The study group has already increased its sample size and is looking at adverse reactions and long-term immune responses, Dr. Kim said.
Encouraging, rather than discouraging, results
Leonard Calabrese, DO, professor of medicine at the Cleveland Clinic in Ohio, said the findings shouldn’t discourage clinicians from encouraging vaccination.
“There’s still a preponderance of people who will develop a robust antibody vaccine response,” he said.
He cautioned that the findings look only at antibodies to the spike protein and at plasmablasts. The reduction in these titers is “of concern,” he said, but “we don’t really know with certainty what are the effects of these drugs, and these data are on the overall biologic protective effect of the vaccine. There’s much more to a vaccine response than anti–spike protein and plasmablasts,” including cell-mediated immune response.
For an individual patient, the findings “mean a lot,” he said.
“I think that people who are on significant prednisone and B-cell–depleting agents, I think you have to share with them that there’s a reasonable chance that you’re not going to be making a response similar to healthy people,” he said. “Thus, even with your vaccine, we’re not going to cut you loose to do things that are violating social distancing and group settings. … Should you be hugging your grandchildren if you’re a rituximab vaccine recipient? I think I would wait until we have a little bit more data.”
Kevin Winthrop, MD, MPH, professor of ophthalmology at Oregon Health & Science University, Portland, where he studies vaccinations in the immunocompromised, said that glucocorticoids tend to have little effect on vaccinations generally at low doses.
When effects are seen they can be difficult to interpret, he said.
“It’s hard to extricate that from the effect of the underlying disease,” he said. The drug can be a proxy for worse disease control.
Although it’s a small study, it’s reassuring that overall the responses were similar to healthy controls.
For B-cell–depleting therapies, his usual guidance is to not give vaccine until a patient is at least 3 months out from their last dose, and not to restart until at least 2 weeks after vaccination.
“It’s not surprising that some of these DMARDs [disease-modifying antirheumatic drugs] do negatively affect vaccine response, particularly B-cell–depletion therapy. We need to do some studies to find a way to overcome that, or optimize delivery of the vaccine.”
Dr. Kim reported participating in consulting, advisory board, or speaker’s bureau for Alexion, Aurinia, Annexon Biosciences, Exagen Diagnostics, and GlaxoSmithKline, and receiving funding under a sponsored research agreement unrelated to the data in the paper from GlaxoSmithKline. Dr. Winthrop reported receiving consulting fees from Pfizer, AbbVie, UCB, Eli Lilly, Galapagos, GlaxoSmithKline, Roche, Gilead, Bristol-Myers Squibb, Regeneron, Sanofi, AstraZeneca, Novartis, and research grants from Bristol-Myers Squibb and Pfizer. Dr. Calabrese reported no relevant disclosures.
Although most patients with chronic inflammatory diseases mounted immune responses after two doses of mRNA-based COVID-19 vaccines, glucocorticoids and B-cell–depleting therapies markedly reduced the response, according to a recently published preprint of a new study.
The study, published on MedRxiv and not yet peer reviewed, involved a prospective look at 133 patients with chronic inflammatory disease (CID) and 53 patients with healthy immune systems at Washington University, St. Louis, and the University of California, San Francisco. It is regarded as the largest and most detailed study yet in how vaccines perform in people with immune-mediated inflammatory disease. The patients were enrolled between December 2020 and March 2021, and the most common diseases were inflammatory bowel disease (32%), rheumatoid arthritis (29%), spondyloarthritis (15%), and systemic lupus erythematosus (11%).
A ‘modest’ reduction in antibody response
Senior author Alfred Kim, MD, PhD, of the department of medicine at Washington University, said the overall results so far are encouraging.
“Most patients with an autoimmune disease that are on immunosuppression can mount antibody responses,” he said. “We’re seeing the majority of our subjects respond.”
The immune-healthy controls and most of the patients with CID had a robust immune response against the spike protein, although the CID group had a mean reduction in antibody titers that was three times lower than the controls (P = .0092). The CID group similarly had a 2.7-fold reduction in preventing neutralization, or halting the virus’ ability to infect (P < .0001), researchers reported.
This reduction in response is “modest,” he said.
“Is the level of reduction going to be detrimental for protection? Time will tell,” he said, adding that researchers anticipate that it won’t have a critical effect on protection because responses tended to be within the range of the immunocompetent controls, who themselves had wildly varied antibody titers across a 20-fold range. “ ‘Optimal’ isn’t necessarily the same as ‘sufficient.’ ”
Type of medication has big impact on antibody titers
But there was a wide variety of effects on the immune response depending on the medication. Glucorticoids resulted in a response that was 10 times lower than the immune-healthy controls, as well as fewer circulating plasmablasts after vaccination. Researchers found that 98% of controls were seropositive for antibody, compared with 92% of those with CID who were not taking prednisone, and 65% of CID patients on prednisone (P = .0006 and .0115, respectively). Prevention of neutralization of the virus was similarly reduced in those groups, compared with the controls. Dr. Kim noted this was a small sample size, with about 15 patients. These effects were seen regardless of the dose.
“We would’ve anticipated this would have been dose dependent, so this was a little bit surprising,” Dr. Kim said.
B-cell–depleting therapies, such as rituximab (Rituxan) and ocrelizumab (Ocrevus), reduced antibody titers by 36 times, compared with controls (P < .0001), with a similar reduction in preventing infection (P = .0066), the researchers found. The reduction in antibody titers was the most pronounced among those who had received B-cell–depleting therapies within the previous 6 months. Dr. Kim noted this was a small sample size, with about 10 patients.
CID study subjects taking an antimetabolite, including methotrexate, had an average of a two- to threefold reduction in antibody titers and in neutralization (P = .0006). This reduction was greatest with methotrexate, researchers found (P = .0027).
JAK inhibitors also significantly reduced antibody titers (P = .0066), but the reduction in neutralization of the virus was not significant. In addition, researchers found a reduction in antibody titers, the prevention of viral infection, and circulating plasmablasts among those on tumor necrosis factor (TNF) inhibitors, compared with controls, but these were insignificant statistically except for virus neutralization.
Dr. Kim said he hopes the glucocorticoid data spur physicians to try harder to wean patients off the drugs, when possible, in keeping with recommendations already in place.
“The general culture in rheumatology has been very lax about the need to reduce glucocorticoids,” he said. “This reinvigorates that call.” Questions about possible drug holidays from glucocorticoids remain, regarding how long a holiday would be needed, he said. He noted that many patients on glucocorticoids nonetheless mounted responses.
Those on B-cell–depleting therapies present a “much more difficult” question, he said. Some patients possibly could wait a bit longer than their normal, every-6-month schedule, but it’s an individual decision, he said. Since a booster of influenza vaccine has been found to enhance the response even within the 6-month window among ocrelizumab patients, a booster of COVID-19 vaccine might also help, although this remains to be studied.
The study group has already increased its sample size and is looking at adverse reactions and long-term immune responses, Dr. Kim said.
Encouraging, rather than discouraging, results
Leonard Calabrese, DO, professor of medicine at the Cleveland Clinic in Ohio, said the findings shouldn’t discourage clinicians from encouraging vaccination.
“There’s still a preponderance of people who will develop a robust antibody vaccine response,” he said.
He cautioned that the findings look only at antibodies to the spike protein and at plasmablasts. The reduction in these titers is “of concern,” he said, but “we don’t really know with certainty what are the effects of these drugs, and these data are on the overall biologic protective effect of the vaccine. There’s much more to a vaccine response than anti–spike protein and plasmablasts,” including cell-mediated immune response.
For an individual patient, the findings “mean a lot,” he said.
“I think that people who are on significant prednisone and B-cell–depleting agents, I think you have to share with them that there’s a reasonable chance that you’re not going to be making a response similar to healthy people,” he said. “Thus, even with your vaccine, we’re not going to cut you loose to do things that are violating social distancing and group settings. … Should you be hugging your grandchildren if you’re a rituximab vaccine recipient? I think I would wait until we have a little bit more data.”
Kevin Winthrop, MD, MPH, professor of ophthalmology at Oregon Health & Science University, Portland, where he studies vaccinations in the immunocompromised, said that glucocorticoids tend to have little effect on vaccinations generally at low doses.
When effects are seen they can be difficult to interpret, he said.
“It’s hard to extricate that from the effect of the underlying disease,” he said. The drug can be a proxy for worse disease control.
Although it’s a small study, it’s reassuring that overall the responses were similar to healthy controls.
For B-cell–depleting therapies, his usual guidance is to not give vaccine until a patient is at least 3 months out from their last dose, and not to restart until at least 2 weeks after vaccination.
“It’s not surprising that some of these DMARDs [disease-modifying antirheumatic drugs] do negatively affect vaccine response, particularly B-cell–depletion therapy. We need to do some studies to find a way to overcome that, or optimize delivery of the vaccine.”
Dr. Kim reported participating in consulting, advisory board, or speaker’s bureau for Alexion, Aurinia, Annexon Biosciences, Exagen Diagnostics, and GlaxoSmithKline, and receiving funding under a sponsored research agreement unrelated to the data in the paper from GlaxoSmithKline. Dr. Winthrop reported receiving consulting fees from Pfizer, AbbVie, UCB, Eli Lilly, Galapagos, GlaxoSmithKline, Roche, Gilead, Bristol-Myers Squibb, Regeneron, Sanofi, AstraZeneca, Novartis, and research grants from Bristol-Myers Squibb and Pfizer. Dr. Calabrese reported no relevant disclosures.
FROM MEDRXIV
Addressing women’s concerns about the J&J vaccine
A rare form of venous thromboembolism (VTE) has developed in premenopausal women who have received the Johnson & Johnson (J&J) SARS-CoV-2 vaccine.
This week we learned that of the more than 6.8 million individuals in the United States who received the single-dose J&J vaccine, six women aged 18-48 years have been diagnosed with cerebral venous sinus thrombosis, and all had thrombocytopenia. In each case, symptoms were first noted 1-2 weeks after vaccination. The Food and Drug Administration and Centers for Disease Control and Prevention have recommended a pause in the administration of this vaccine.
Women’s health clinicians are already hearing from concerned patients, who understandably have questions about what this news means for them.
If they have already received the J&J vaccine within the past 3 weeks, I advise them that, although risks for any vaccine-related problems are extremely low, they should be mindful of new-onset leg or abdominal pain, or an unusual or severe headache. Such patients should contact their physician as soon as possible, and if they cannot be seen quickly, it would be appropriate to visit a hospital ED. When seeking medical care, patients should specify details of their vaccination history. Depending on the individual issues present, women with suggestive symptoms should receive blood work, Doppler venous studies (if there is a suspicion of lower-extremity deep vein thrombosis), and appropriate imaging (if there is concern for cerebral venous sinus thrombosis or pulmonary embolism).
As physicians and scientists at the CDC and FDA dig into this issue, I assume they are asking questions to determine whether the affected women have any factors that might increase their baseline risk for VTE, such as:
- A body mass index of at least 30 kg/m2
- Use of combination estrogen-progestin contraceptives (pill, ring, or patch)
- Known or suspected chronic inflammatory conditions such as rheumatoid arthritis, systemic lupus erythematosus, or
- Known familial or other thrombophilic conditions or chronic
- Recent prolonged immobility, such as a long airplane or automobile trip, which might increase risk for VTE
Experts say that the risk for a serious adverse event following receipt of the J&J vaccine is outweighed by the benefits of vaccination against COVID disease. However, that may not be enough to allay concerns among some premenopausal women.
Even if the “pause” in the administration of the vaccine is lifted, some women may be asking whether they should receive J&J’s viral vector vaccine or request one of the messenger RNA vaccines. I will be looking to the expert opinions of Anthony S. Fauci, MD, and advice from the CDC and FDA for guidance here. However, it may be reasonable to steer high-risk reproductive-age women away from the J&J vaccine in favor of the Moderna and Pfizer vaccines, if these options are available.
A version of this article first appeared on Medscape.com.
A rare form of venous thromboembolism (VTE) has developed in premenopausal women who have received the Johnson & Johnson (J&J) SARS-CoV-2 vaccine.
This week we learned that of the more than 6.8 million individuals in the United States who received the single-dose J&J vaccine, six women aged 18-48 years have been diagnosed with cerebral venous sinus thrombosis, and all had thrombocytopenia. In each case, symptoms were first noted 1-2 weeks after vaccination. The Food and Drug Administration and Centers for Disease Control and Prevention have recommended a pause in the administration of this vaccine.
Women’s health clinicians are already hearing from concerned patients, who understandably have questions about what this news means for them.
If they have already received the J&J vaccine within the past 3 weeks, I advise them that, although risks for any vaccine-related problems are extremely low, they should be mindful of new-onset leg or abdominal pain, or an unusual or severe headache. Such patients should contact their physician as soon as possible, and if they cannot be seen quickly, it would be appropriate to visit a hospital ED. When seeking medical care, patients should specify details of their vaccination history. Depending on the individual issues present, women with suggestive symptoms should receive blood work, Doppler venous studies (if there is a suspicion of lower-extremity deep vein thrombosis), and appropriate imaging (if there is concern for cerebral venous sinus thrombosis or pulmonary embolism).
As physicians and scientists at the CDC and FDA dig into this issue, I assume they are asking questions to determine whether the affected women have any factors that might increase their baseline risk for VTE, such as:
- A body mass index of at least 30 kg/m2
- Use of combination estrogen-progestin contraceptives (pill, ring, or patch)
- Known or suspected chronic inflammatory conditions such as rheumatoid arthritis, systemic lupus erythematosus, or
- Known familial or other thrombophilic conditions or chronic
- Recent prolonged immobility, such as a long airplane or automobile trip, which might increase risk for VTE
Experts say that the risk for a serious adverse event following receipt of the J&J vaccine is outweighed by the benefits of vaccination against COVID disease. However, that may not be enough to allay concerns among some premenopausal women.
Even if the “pause” in the administration of the vaccine is lifted, some women may be asking whether they should receive J&J’s viral vector vaccine or request one of the messenger RNA vaccines. I will be looking to the expert opinions of Anthony S. Fauci, MD, and advice from the CDC and FDA for guidance here. However, it may be reasonable to steer high-risk reproductive-age women away from the J&J vaccine in favor of the Moderna and Pfizer vaccines, if these options are available.
A version of this article first appeared on Medscape.com.
A rare form of venous thromboembolism (VTE) has developed in premenopausal women who have received the Johnson & Johnson (J&J) SARS-CoV-2 vaccine.
This week we learned that of the more than 6.8 million individuals in the United States who received the single-dose J&J vaccine, six women aged 18-48 years have been diagnosed with cerebral venous sinus thrombosis, and all had thrombocytopenia. In each case, symptoms were first noted 1-2 weeks after vaccination. The Food and Drug Administration and Centers for Disease Control and Prevention have recommended a pause in the administration of this vaccine.
Women’s health clinicians are already hearing from concerned patients, who understandably have questions about what this news means for them.
If they have already received the J&J vaccine within the past 3 weeks, I advise them that, although risks for any vaccine-related problems are extremely low, they should be mindful of new-onset leg or abdominal pain, or an unusual or severe headache. Such patients should contact their physician as soon as possible, and if they cannot be seen quickly, it would be appropriate to visit a hospital ED. When seeking medical care, patients should specify details of their vaccination history. Depending on the individual issues present, women with suggestive symptoms should receive blood work, Doppler venous studies (if there is a suspicion of lower-extremity deep vein thrombosis), and appropriate imaging (if there is concern for cerebral venous sinus thrombosis or pulmonary embolism).
As physicians and scientists at the CDC and FDA dig into this issue, I assume they are asking questions to determine whether the affected women have any factors that might increase their baseline risk for VTE, such as:
- A body mass index of at least 30 kg/m2
- Use of combination estrogen-progestin contraceptives (pill, ring, or patch)
- Known or suspected chronic inflammatory conditions such as rheumatoid arthritis, systemic lupus erythematosus, or
- Known familial or other thrombophilic conditions or chronic
- Recent prolonged immobility, such as a long airplane or automobile trip, which might increase risk for VTE
Experts say that the risk for a serious adverse event following receipt of the J&J vaccine is outweighed by the benefits of vaccination against COVID disease. However, that may not be enough to allay concerns among some premenopausal women.
Even if the “pause” in the administration of the vaccine is lifted, some women may be asking whether they should receive J&J’s viral vector vaccine or request one of the messenger RNA vaccines. I will be looking to the expert opinions of Anthony S. Fauci, MD, and advice from the CDC and FDA for guidance here. However, it may be reasonable to steer high-risk reproductive-age women away from the J&J vaccine in favor of the Moderna and Pfizer vaccines, if these options are available.
A version of this article first appeared on Medscape.com.
COVID-19 vaccine response lower in kidney dialysis patients
the first study of its kind shows.
“It is well known that patients on dialysis may have a reduced response to vaccination,” Ayelet Grupper, MD, of Tel Aviv Medical Center, and colleagues observe. Their study was published online April 6 in the Clinical Journal of the American Society of Nephrology.
“I believe our findings should encourage patients with kidney failure treated with dialysis to be vaccinated as soon as vaccination becomes available for them, while we as caregivers should explore ways to enhance its efficacy in our patients,” senior author Moshe Shashar, MD, noted in a statement from the American Society of Nephrology.
Asked to comment, Peter Blake, MD, professor of medicine, University of Western Ontario, London, pointed out that COVID-19 is very common among hemodialysis patients and that the likelihood of these patients dying from it is very high. Indeed, 1.5% of approximately 12,500 patients receiving dialysis in the province of Ontario have died of COVID-19 – “a horrifying statistic and one that only long-term care home residents can compare with,” he told this news organization.
In the Israeli study, almost all dialysis patients mounted a serologic response to the Pfizer-BioNTech vaccine, which is “good news” overall, Dr. Blake said.
Also commenting on the study, Anushree Shirali, MD, of Yale University, New Haven, Conn., said she was impressed by the fact that most of the dialysis patients in the study mounted at least some IgG response to vaccination, which she said was good “in and of itself,” because that is not always the case with other vaccines.
Study compared dialysis patients with health care workers
The Israeli study included 56 patients who were receiving maintenance hemodialysis and 95 health care workers, who served as control persons.
“All participants had been previously vaccinated with the [Pfizer-BioNTech] vaccine, with the recommended dosing interval of 21 days between the first and second doses,” the investigators note. Immunogenicity was assessed using a dedicated immunoassay to quantify the level of IgG antibodies from participants’ plasma.
A cutoff for a positive antibody response was greater than or equal to 50 arbitrary units per milliliter (AU/mL). “All subjects in the control group developed a positive antibody response (≥50 AU/mL) as compared with 96% (54 of 56) in the dialysis group,” Dr. Shashar and colleagues report.
The median IgG level in the dialysis group was 2,900 AU/mL, which is significantly lower than the median of 7,401 AU/mL in the control group (P < .001), they report.
The investigators also observed a significant inverse correlation between older age and antibody levels in both groups.
The odds of being in the lower quartile were significantly higher for older individuals (odds ratio, 1.11 per year of age; P = .004) and for the dialysis group compared with the control group (OR, 2.7; P = .05).
Among the dialysis patients, older age and lower lymphocyte count were associated with antibody response in the lower quartile (OR, 1.22 per 1 year older; P = .03; and OR, 0.83 per 10-e3/mL-higher lymphocyte count; P = .05).
Among recipients older than 70 years, there was little difference in antibody response between the dialysis patients and the control group. Thus, age is clearly an important contributor to a robust humoral response, the authors observe.
For more than 90% of the patients receiving dialysis, the antibody response was well above 50 AU/mL, which was the cutoff for having a positive response.
Nevertheless, the authors suggest that their findings should prompt clinicians to consider either changing the dose or the schedule of COVID-19 vaccination for dialysis patients, as was done, for example, with the hepatitis B vaccine Engerix-B.
Dialysis patients now receive double doses of the hepatitis B vaccine, which is given in a four-series vaccine schedule rather than a three-series vaccine schedule, as is given to healthy individuals.
The authors also call for studies to assess the longevity of vaccine efficacy for dialysis patients and whether current vaccines are effective against variant strains among patients undergoing dialysis.
Some suggestion COVID-19 vaccines also elicit T-cell responses
Dr. Shirali said the news regarding the COVID-19 vaccine for dialysis patients is good, given the fact that such patients exhibit a poor response to the hepatitis B vaccine.
“There isn’t a large percentage of dialysis patients who mount a humoral response to the hepatitis B vaccine, even with the change in dosing that we use that is different than it is for the general population,” she told this news organization.
Dr. Shirali also noted that preliminary evidence suggests that COVID-19 vaccines elicit nonantibody and antibody T-cell responses and that such immunity is going to be just as important for protecting dialysis patients against COVID-19 as it is for protecting patients who are not receiving dialysis.
“Antibody responses are just one arm of vaccination,” she explained. “People can form memory T-cell responses with vaccination, and while this has not been well studied with COVID-19, there are preliminary data to suggest that T-cell responses are likely to be effective in the fight against COVID-19.” There is also the possibility that this type of response “may even be more durable than antibody responses,” she said.
The study received no funding. The authors, Dr. Blake and Dr. Shirali, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
the first study of its kind shows.
“It is well known that patients on dialysis may have a reduced response to vaccination,” Ayelet Grupper, MD, of Tel Aviv Medical Center, and colleagues observe. Their study was published online April 6 in the Clinical Journal of the American Society of Nephrology.
“I believe our findings should encourage patients with kidney failure treated with dialysis to be vaccinated as soon as vaccination becomes available for them, while we as caregivers should explore ways to enhance its efficacy in our patients,” senior author Moshe Shashar, MD, noted in a statement from the American Society of Nephrology.
Asked to comment, Peter Blake, MD, professor of medicine, University of Western Ontario, London, pointed out that COVID-19 is very common among hemodialysis patients and that the likelihood of these patients dying from it is very high. Indeed, 1.5% of approximately 12,500 patients receiving dialysis in the province of Ontario have died of COVID-19 – “a horrifying statistic and one that only long-term care home residents can compare with,” he told this news organization.
In the Israeli study, almost all dialysis patients mounted a serologic response to the Pfizer-BioNTech vaccine, which is “good news” overall, Dr. Blake said.
Also commenting on the study, Anushree Shirali, MD, of Yale University, New Haven, Conn., said she was impressed by the fact that most of the dialysis patients in the study mounted at least some IgG response to vaccination, which she said was good “in and of itself,” because that is not always the case with other vaccines.
Study compared dialysis patients with health care workers
The Israeli study included 56 patients who were receiving maintenance hemodialysis and 95 health care workers, who served as control persons.
“All participants had been previously vaccinated with the [Pfizer-BioNTech] vaccine, with the recommended dosing interval of 21 days between the first and second doses,” the investigators note. Immunogenicity was assessed using a dedicated immunoassay to quantify the level of IgG antibodies from participants’ plasma.
A cutoff for a positive antibody response was greater than or equal to 50 arbitrary units per milliliter (AU/mL). “All subjects in the control group developed a positive antibody response (≥50 AU/mL) as compared with 96% (54 of 56) in the dialysis group,” Dr. Shashar and colleagues report.
The median IgG level in the dialysis group was 2,900 AU/mL, which is significantly lower than the median of 7,401 AU/mL in the control group (P < .001), they report.
The investigators also observed a significant inverse correlation between older age and antibody levels in both groups.
The odds of being in the lower quartile were significantly higher for older individuals (odds ratio, 1.11 per year of age; P = .004) and for the dialysis group compared with the control group (OR, 2.7; P = .05).
Among the dialysis patients, older age and lower lymphocyte count were associated with antibody response in the lower quartile (OR, 1.22 per 1 year older; P = .03; and OR, 0.83 per 10-e3/mL-higher lymphocyte count; P = .05).
Among recipients older than 70 years, there was little difference in antibody response between the dialysis patients and the control group. Thus, age is clearly an important contributor to a robust humoral response, the authors observe.
For more than 90% of the patients receiving dialysis, the antibody response was well above 50 AU/mL, which was the cutoff for having a positive response.
Nevertheless, the authors suggest that their findings should prompt clinicians to consider either changing the dose or the schedule of COVID-19 vaccination for dialysis patients, as was done, for example, with the hepatitis B vaccine Engerix-B.
Dialysis patients now receive double doses of the hepatitis B vaccine, which is given in a four-series vaccine schedule rather than a three-series vaccine schedule, as is given to healthy individuals.
The authors also call for studies to assess the longevity of vaccine efficacy for dialysis patients and whether current vaccines are effective against variant strains among patients undergoing dialysis.
Some suggestion COVID-19 vaccines also elicit T-cell responses
Dr. Shirali said the news regarding the COVID-19 vaccine for dialysis patients is good, given the fact that such patients exhibit a poor response to the hepatitis B vaccine.
“There isn’t a large percentage of dialysis patients who mount a humoral response to the hepatitis B vaccine, even with the change in dosing that we use that is different than it is for the general population,” she told this news organization.
Dr. Shirali also noted that preliminary evidence suggests that COVID-19 vaccines elicit nonantibody and antibody T-cell responses and that such immunity is going to be just as important for protecting dialysis patients against COVID-19 as it is for protecting patients who are not receiving dialysis.
“Antibody responses are just one arm of vaccination,” she explained. “People can form memory T-cell responses with vaccination, and while this has not been well studied with COVID-19, there are preliminary data to suggest that T-cell responses are likely to be effective in the fight against COVID-19.” There is also the possibility that this type of response “may even be more durable than antibody responses,” she said.
The study received no funding. The authors, Dr. Blake and Dr. Shirali, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
the first study of its kind shows.
“It is well known that patients on dialysis may have a reduced response to vaccination,” Ayelet Grupper, MD, of Tel Aviv Medical Center, and colleagues observe. Their study was published online April 6 in the Clinical Journal of the American Society of Nephrology.
“I believe our findings should encourage patients with kidney failure treated with dialysis to be vaccinated as soon as vaccination becomes available for them, while we as caregivers should explore ways to enhance its efficacy in our patients,” senior author Moshe Shashar, MD, noted in a statement from the American Society of Nephrology.
Asked to comment, Peter Blake, MD, professor of medicine, University of Western Ontario, London, pointed out that COVID-19 is very common among hemodialysis patients and that the likelihood of these patients dying from it is very high. Indeed, 1.5% of approximately 12,500 patients receiving dialysis in the province of Ontario have died of COVID-19 – “a horrifying statistic and one that only long-term care home residents can compare with,” he told this news organization.
In the Israeli study, almost all dialysis patients mounted a serologic response to the Pfizer-BioNTech vaccine, which is “good news” overall, Dr. Blake said.
Also commenting on the study, Anushree Shirali, MD, of Yale University, New Haven, Conn., said she was impressed by the fact that most of the dialysis patients in the study mounted at least some IgG response to vaccination, which she said was good “in and of itself,” because that is not always the case with other vaccines.
Study compared dialysis patients with health care workers
The Israeli study included 56 patients who were receiving maintenance hemodialysis and 95 health care workers, who served as control persons.
“All participants had been previously vaccinated with the [Pfizer-BioNTech] vaccine, with the recommended dosing interval of 21 days between the first and second doses,” the investigators note. Immunogenicity was assessed using a dedicated immunoassay to quantify the level of IgG antibodies from participants’ plasma.
A cutoff for a positive antibody response was greater than or equal to 50 arbitrary units per milliliter (AU/mL). “All subjects in the control group developed a positive antibody response (≥50 AU/mL) as compared with 96% (54 of 56) in the dialysis group,” Dr. Shashar and colleagues report.
The median IgG level in the dialysis group was 2,900 AU/mL, which is significantly lower than the median of 7,401 AU/mL in the control group (P < .001), they report.
The investigators also observed a significant inverse correlation between older age and antibody levels in both groups.
The odds of being in the lower quartile were significantly higher for older individuals (odds ratio, 1.11 per year of age; P = .004) and for the dialysis group compared with the control group (OR, 2.7; P = .05).
Among the dialysis patients, older age and lower lymphocyte count were associated with antibody response in the lower quartile (OR, 1.22 per 1 year older; P = .03; and OR, 0.83 per 10-e3/mL-higher lymphocyte count; P = .05).
Among recipients older than 70 years, there was little difference in antibody response between the dialysis patients and the control group. Thus, age is clearly an important contributor to a robust humoral response, the authors observe.
For more than 90% of the patients receiving dialysis, the antibody response was well above 50 AU/mL, which was the cutoff for having a positive response.
Nevertheless, the authors suggest that their findings should prompt clinicians to consider either changing the dose or the schedule of COVID-19 vaccination for dialysis patients, as was done, for example, with the hepatitis B vaccine Engerix-B.
Dialysis patients now receive double doses of the hepatitis B vaccine, which is given in a four-series vaccine schedule rather than a three-series vaccine schedule, as is given to healthy individuals.
The authors also call for studies to assess the longevity of vaccine efficacy for dialysis patients and whether current vaccines are effective against variant strains among patients undergoing dialysis.
Some suggestion COVID-19 vaccines also elicit T-cell responses
Dr. Shirali said the news regarding the COVID-19 vaccine for dialysis patients is good, given the fact that such patients exhibit a poor response to the hepatitis B vaccine.
“There isn’t a large percentage of dialysis patients who mount a humoral response to the hepatitis B vaccine, even with the change in dosing that we use that is different than it is for the general population,” she told this news organization.
Dr. Shirali also noted that preliminary evidence suggests that COVID-19 vaccines elicit nonantibody and antibody T-cell responses and that such immunity is going to be just as important for protecting dialysis patients against COVID-19 as it is for protecting patients who are not receiving dialysis.
“Antibody responses are just one arm of vaccination,” she explained. “People can form memory T-cell responses with vaccination, and while this has not been well studied with COVID-19, there are preliminary data to suggest that T-cell responses are likely to be effective in the fight against COVID-19.” There is also the possibility that this type of response “may even be more durable than antibody responses,” she said.
The study received no funding. The authors, Dr. Blake and Dr. Shirali, have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
How some COVID-19 vaccines could cause rare blood clots
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
on April 14, 2021, after the CDC and Food and Drug Administration recommended that states hold off on using it pending a detailed review of six cases of the same kind of rare but serious event – a blood clot in the vessels that drain blood from the brain combined with a large drop in platelets, which increases the risk for bleeding.
This combination can lead to severe strokes that can lead to brain damage or death. Among the six cases reported, which came to light over the past 3 weeks, one person died, according to the CDC. All six were women and ranged in age from 18 to 48 years.
According to a report from the Vaccine Adverse Event Reporting System (VAERS), which is maintained by the Department of Health & Human Services, the woman who died was 45. She developed a gradually worsening headache about a week after receiving the Johnson & Johnson vaccine.
On March 17, the day she came to the hospital, she was dry heaving. Her headache had suddenly gotten much worse, and the left side of her body was weak, which are signs of a stroke. A CT scan revealed both bleeding in her brain and a clot in her cortical vein. She died the following day.
In addition to VAERS, which accepts reports from anyone, the CDC and FDA are monitoring at least eight other safety systems maintained by hospitals, research centers, long-term care facilities, and insurance companies for signs of trouble with the vaccines. VAERS data is searchable and open to the public. Most of these systems are not publicly available to protect patient privacy. It’s unclear which systems detected the six cases cited by federal regulators.
“These are very serious and potentially fatal problems occurring in a healthy young adult. It’s serious and we need to get to the bottom of it,” said Ed Belongia, MD, director of the Center for Clinical Epidemiology and Population Health at the Marshfield (Wis.) Clinic Research Institute. Dr. Belongia leads a research team that helps the CDC monitor vaccine safety and effectiveness.
“Safety is always the highest priority, and I think what we’ve seen here in the past 24 hours is our vaccine safety monitoring system is working,” he said.
Others agree. “I think what CDC and FDA have detected is a rare, but likely real adverse event associated with this vaccine,” said Paul Offit, MD, director of vaccine education at Children’s Hospital of Philadelphia.
Although much is still unknown about these events, they follow a similar pattern of blood clots reported with the AstraZeneca vaccine in Europe. That vaccine is now sold under the brand name Vaxzevria.
This has experts questioning whether all vaccines of this type may cause these rare clots.
“I think it’s likely a class effect,” said Dr. Offit, who was a member of the FDA advisory committee that reviewed clinical trial data on the J&J vaccine before it was authorized for use.
Adenovirus vaccines scrutinized
Both the Johnson & Johnson and Vaxzevria vaccines use an adenovirus to ferry genetic instructions for making the coronaviruses spike protein into our cells.
Adenoviruses are common, relatively simple viruses that normally cause mild cold or flu symptoms. The ones used in the vaccine are disabled so they can’t make us sick. They’re more like Trojan horses.
Once inside our cells, they release the DNA instructions they carry to make the spike protein of the new coronavirus. Those cells then crank out copies of the spike protein, which then get displayed on the outer surface of the cell membrane where they are recognized by the immune system.
The immune system then makes antibodies and other defenses against the spike so that, when the real coronavirus comes along, our bodies are ready to fight the infection.
There’s no question the vaccine works. In clinical trials, the Johnson & Johnson vaccine was 66% percent effective at preventing against moderate to severe COVID-19 infection, and none of the patients who got COVID-19 after vaccination had to be admitted to the hospital or died.
The idea behind using adenoviruses in vaccines isn’t a new one. In a kind of fight-fire-with-fire approach, the idea is to use a virus, which is good at infecting us, to fight a different kind of virus.
Researchers have been working on the concept for about 10 years, but the COVID-19 vaccines that use this technology are some of the first adenovirus-vector vaccines deployed in humans.
Only one other adenovirus vaccine, for Ebola, has been approved for use in humans. It was approved in Europe last year. Before the Johnson & Johnson vaccine, no other adenovirus vector has been available for use in humans in the United States.
There are six adenovirus-vector vaccines for COVID-19. In addition to AstraZeneca and Johnson & Johnson, there’s the Russian-developed vaccine Sputnik V, along with CanSino from China, and the Covishield vaccine in India.
Adenovirus vaccines are more stable than the mRNA vaccines. That makes them easier to store and transport.
But they have a significant downside, too. Because adenoviruses infect humans out in the world, we already make antibodies against them. So there’s always a danger that our immune systems might recognize and react to the vaccine, rendering it ineffective. For that reason, scientists try to carefully select the adenovirus vectors, or carriers, they use.
The two vaccines under investigation for blood clots are slightly different. The Johnson & Johnson vaccine uses the vector AD26, because most of the population lacks preexisting immunity to it. Vaxzevria uses an adenovirus that infects chimpanzees, called ChAdOx1.
Vaxzevria has been widely used in Europe but has not yet been authorized in the United States.
On April 7, the European Medicines Agency, Europe’s counterpart to the FDA, ruled that unusual blood clots with low blood platelets should be listed as rare side effects on the Vaxzevria vaccine.
The decision came after reviewing 62 cases of cerebral venous sinus thrombosis (CVST) linked to the vaccine and 25 cases of another rare type of clot, called a splanchnic vein thrombosis. Splanchnic veins drain blood from the major organs in the digestive system, including the stomach, liver, and intestines; 18 of those events were fatal.
The reports were culled from reporting in Europe and the United Kingdom, where around 25 million people have received the Vaxzevria vaccine, making these clots exceptionally rare, but serious.
So far, six cases of CVST have been reported in the United States, after more than 7 million doses of the Johnson & Johnson vaccines have been administered.
A key question for U.S. regulators will be the background rate for these types of rare combinations of clots and deplenished platelets. The background rate is the number of events that would be expected to occur naturally in a population of unvaccinated people. On a press call on April 13, Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, was asked about the frequency of this dangerous combination. He said the combination of low platelets and clots was so rare that it was hard to pinpoint, but might be somewhere between 2 and 14 cases per million people over the course of a year.
The first Johnson & Johnson doses were given in early March. That means the six cases came to light within the first few weeks of use of the vaccine in the United States, a very short amount of time.
“These were six cases per million people for 2 weeks, which is the same thing as 25 million per year, so it’s clearly above the background rate,” Dr. Offit said.
Studies suggest possible mechanism
On April 9, the New England Journal of Medicine published a detailed evaluation of the 11 patients in Germany and Austria who developed the rare clots after their Vaxzevria vaccines.
The study detected rare antibodies to a signaling protein called platelet factor 4, which helps to coordinate clot formation.
These same type of antibodies form in some people given the blood thinning drug heparin. In those reactions, which are also exceptionally rare, the same type of syndrome develops, leading to large, devastating clots that consume circulating platelets.
It’s not yet clear whether people who develop reactions to the vaccines already have some platelet factor 4 antibodies before they are vaccinated, or whether the vaccines somehow spur the body to make these antibodies, which then launch a kind of autoimmune attack.
The researchers on the paper gave the syndrome a name, vaccine-induced thrombotic thrombocytopenia (VITT).
It’s also not clear why more cases seem to be in women than in men. Andrew Eisenberger, MD, an associate professor of hematology and oncology at Columbia University, New York, said the most common causes of cerebral venous sinus thrombosis have to do with conditions that raise estrogen levels, like pregnancy and hormonal contraception.
“Estrogen naturally leads to changes in several clotting proteins in the blood that may predispose to abnormal blood clotting in a few different sites in the body,” he said. “The clotting changes we are encountering with some of COVID-19 vaccines are likely to be synergistic with the effects of estrogen on the blood.”
No matter the cause, the CDC on April 13 alerted doctors to keep a high index of suspicion for VITT in patients who have received the Johnson & Johnson vaccination within the last 2 weeks. In those patients, the usual course of treatment with blood thinning drugs like heparin may be harmful.
Symptoms to watch for include severe headache or backache, new neurologic symptoms, severe abdominal pain, shortness of breath, leg swelling, tiny red spots on the skin, or easy bruising.
Grappling with evidence
The CDC’s Advisory Committee on Immunization Practices will meet today in an emergency session to review the cases and see if any changes are needed to use of the J&J vaccine in the United States.
Last week, for example, the United Kingdom restricted the use of the AstraZeneca vaccine in people aged younger than 30 years, saying the risks and benefits of vaccination are “more finely balanced” for this age group.
With cases of COVID-19 rising again in the United States, and the Johnson & Johnson vaccine currently the most convenient form of protection against the virus, the committee will have to weigh the risks of that infection against the risk of rare clots caused by vaccination.
They will also likely have to rule out whether any of the cases had COVID. At least one study has reported CVST clots in three patients with confirmed COVID infections. In Europe, COVID infection did not seem to play a role in the formation of the clots with low platelets.
Hilda Bastian, PhD, a clinical trials expert who cofounded the Cochrane Collaboration, said it won’t be an easy task. Much will depend on how certain the committee members feel they know about all the events linked to the vaccine.
“That’s the really, really hard issue from my point of view for them right this moment. Have we missed any? Or how many are we likely to have missed?” asked Dr. Bastian, who lives in Australia.
“In a country that size with that fragmented [of] a health care system, how sure can you be that you know them all? That’s going to be a really difficult situation for them to grapple with, the quality of information that they’ve got,” she said.
A version of this article first appeared on Medscape.com.
Researchers stress importance of second COVID-19 vaccine dose for infliximab users
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
Patients being treated with infliximab had weakened immune responses to the first dose of the ChAdOx1 nCoV-19 (Oxford/AstraZeneca) and BNT162b2 (Pfizer/BioNTech) vaccines, compared with patients on vedolizumab (Entyvio), although a very significant number of patients from both groups seroconverted after their second dose, according to a new U.K. study of patients with inflammatory bowel disease (IBD).
“Antibody testing and adapted vaccine schedules should be considered to protect these at-risk patients,” Nicholas A. Kennedy, PhD, MBBS, of the University of Exeter (England) and colleagues wrote in a preprint published March 29 on MedRxiv.
Infliximab is an anti–tumor necrosis factor (anti-TNF) monoclonal antibody that’s approved to treat adult and pediatric Crohn’s disease and ulcerative colitis, as well as rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, and plaque psoriasis, whereas vedolizumab, a gut selective anti-integrin alpha4beta7 monoclonal antibody that is not associated with impaired systemic immune responses, is approved to treat Crohn’s disease and ulcerative colitis in adults.
A previous study from Kennedy and colleagues revealed that IBD patients on infliximab showed a weakened COVID-19 antibody response compared with patients on vedolizumab. To determine if treatment with anti-TNF drugs impacted the efficacy of the first shot of these two-dose COVID-19 vaccines, the researchers used data from the CLARITY IBD study to assess 865 infliximab- and 428 vedolizumab-treated participants without evidence of prior SARS-CoV-2 infection who had received uninterrupted biologic therapy since being recruited between Sept. 22 and Dec. 23, 2020.
In the 3-10 weeks after initial vaccination, geometric mean concentrations for SARS-CoV-2 anti-spike protein receptor-binding protein antibodies were lower in patients on infliximab, compared with patients on vedolizumab for both the Pfizer (6.0 U/mL [5.9] versus 28.8 U/mL [5.4], P < .0001) and AstraZeneca (4.7 U/mL [4.9] versus 13.8 U/mL [5.9]; P < .0001) vaccines. The researchers’ multivariable models reinforced those findings, with antibody concentrations lower in infliximab-treated patients for both the Pfizer (fold change, 0.29; 95% confidence interval, 0.21-0.40; P < .0001) and AstraZeneca (FC, 0.39; 95% CI, 0.30-0.51; P < .0001) vaccines.
After second doses of the two-dose Pfizer vaccine, 85% of patients on infliximab and 86% of patients on vedolizumab seroconverted (P = .68); similarly high seroconversion rates were seen in patients who had been infected with SARS-CoV-2 prior to receiving either vaccine. Several patient characteristics were associated with lower antibody concentrations regardless of vaccine type: being 60 years or older, use of immunomodulators, having Crohn’s disease, and being a smoker. Alternatively, non-White ethnicity was associated with higher antibody concentrations.
Evidence has ‘unclear clinical significance’
“These data, which require peer review, do not change my opinion on the safety and efficacy of COVID-19 vaccines in patients taking TNF inhibitors such as infliximab as monotherapy for the treatment of psoriatic disease,” Joel M. Gelfand MD, director of the psoriasis and phototherapy treatment center at the University of Pennsylvania, Philadelphia, said in an interview.
“First, two peer-reviewed studies found good antibody response in patients on TNF inhibitors receiving COVID-19 vaccines (doi: 10.1136/annrheumdis-2021-220289; 10.1136/annrheumdis-2021-220272). Second, antibody responses were robust in the small cohort that received the second dose of a COVID-19 vaccine. We already know that, for the two messenger RNA-based vaccines available under emergency use authorization in the U.S., a second dose is required for optimal efficacy. Thus, evidence of a reduced antibody response after just one dose is of unclear clinical significance. Third, antibody responses are only a surrogate marker, and a low antibody response doesn’t necessarily mean the patient will not be protected by the vaccine.”
Focus on the second dose of a two-dose regimen
“Tell me about the response in people who got both doses of a vaccine that you’re supposed to get both doses of,” Jeffrey Curtis, MD, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham, said in an interview. “The number of patients in that subset was small [n = 27] but in my opinion that’s the most clinically relevant analysis and the one that patients and clinicians want answered.”
He also emphasized the uncertainty around what ‘protection’ means in these early days of studying COVID-19 vaccine responses. “You can define seroprotection or seroconversion as some absolute level of an antibody response, but if you want to say ‘Mrs. Smith, your antibody level was X,’ on whatever arbitrary scale with whoever’s arbitrary lab test, nobody actually knows that Mrs. Smith is now protected from SARS-CoV-2, or how protected,” he said.
“What is not terribly controversial is: If you can’t detect antibodies, the vaccine didn’t ‘take,’ if you will. But if I tell you that the mean antibody level was X with one drug and then 2X with another drug, does that mean that you’re twice as protected? We don’t know that. I’m fearful that people are looking at these studies and thinking that more is better. It might be, but we don’t know that to be true.”
Debating the cause of weakened immune responses
“The biological plausibility of being on an anti-TNF affecting your immune reaction to a messenger RNA or even a replication-deficient viral vector vaccine doesn’t make sense,” David T. Rubin, MD, professor of medicine at the University of Chicago and chair of the National Scientific Advisory Committee of the Crohn’s and Colitis Foundation, said in an interview.
“I’m sure immunologists may differ with me on this, but given what we have come to appreciate about these vaccine mechanisms, this finding doesn’t make intuitive sense. So we need to make sure that, when this happens, we look to the next studies and try to understand, was there any other confounder that may have resulted in these findings that was not adequately adjusted for or addressed in some other way?
“When you have a study of this size, you argue, ‘Because it’s so large, any effect that was seen must be real,’ ” he added. “Alternatively, to have a study of this size, by its very nature you are limited in being able to control for certain other factors or differences between the groups.”
That said, he commended the authors for their study and acknowledged the potential questions it raises about the single-shot Johnson & Johnson vaccine. “If you only get one and you’re on infliximab, this study implies that maybe that’s not enough,” he said. “Despite the fact that Johnson & Johnson was approved as a single dose, it may be necessary to think about it as the first of two, or maybe it’s not the preferred vaccine in this group of patients.”
The study was supported by the Royal Devon and Exeter and Hull University Hospital Foundation NHS Trusts and unrestricted educational grants from Biogen (Switzerland), Celltrion Healthcare (South Korea), Galapagos NV (Belgium), and F. Hoffmann-La Roche (Switzerland). The authors acknowledged numerous potential conflicts of interest, including receiving grants, personal fees, and nonfinancial support from various pharmaceutical companies.
FROM MEDRXIV
Children could become eligible for a COVID-19 vaccine by fall, expert predicts
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
FROM THE SPD PRE-AAD MEETING
Pfizer: Vaccine shown 100% effective in children aged 12-15
The study enrolled 2,260 adolescents aged 12-15. No infections were reported in the group given the vaccine produced by Pfizer and its European partner, BioNTech, the release said. The placebo group reported 18 cases of COVID-19.
The vaccinated children showed a strong antibody response with no serious side effects.
Albert Bourla, PhD, chairman and CEO of Pfizer, said the company plans to seek Food and Drug Administration emergency use authorization, which could allow this age group to be vaccinated before the start of the next school year. Pfizer will also seek authorization from the European Medicines Agency.
“We share the urgency to expand the authorization of our vaccine to use in younger populations and are encouraged by the clinical trial data from adolescents between the ages of 12 and 15,” Dr. Bourla said in the release.
The clinical trials showed a stronger response in children aged 12-15 than the 95% effectiveness reported in clinical trials in adults. The Pfizer vaccine is now authorized to be given to people aged 16 and up in the United States.
Health experts said the clinical trials – while not peer-reviewed – amounted to very good news.
“The sooner that we can get vaccines into as many people as possible, regardless of their age, the sooner we will be able to really feel like we’re ending this pandemic for good,” Angela Rasmussen, PhD, a virologist affiliated with Georgetown University in Washington, told The New York Times.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, recently said that getting children vaccinated is an important step toward achieving herd immunity.
“We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape,” he said earlier in March during a hearing of the Senate Health, Education, Labor, and Pensions Committee. “We ultimately would like to get and have to get children into that mix.”
Pfizer said it started clinical trials during the week of March 23 with children aged 5-11 and will next start trials with children aged 2-5, followed by children aged 6 months to 2 years. Vaccine makers Moderna and AstraZeneca also have started clinical trials in younger children.
A version of this article first appeared on WebMD.com.
The study enrolled 2,260 adolescents aged 12-15. No infections were reported in the group given the vaccine produced by Pfizer and its European partner, BioNTech, the release said. The placebo group reported 18 cases of COVID-19.
The vaccinated children showed a strong antibody response with no serious side effects.
Albert Bourla, PhD, chairman and CEO of Pfizer, said the company plans to seek Food and Drug Administration emergency use authorization, which could allow this age group to be vaccinated before the start of the next school year. Pfizer will also seek authorization from the European Medicines Agency.
“We share the urgency to expand the authorization of our vaccine to use in younger populations and are encouraged by the clinical trial data from adolescents between the ages of 12 and 15,” Dr. Bourla said in the release.
The clinical trials showed a stronger response in children aged 12-15 than the 95% effectiveness reported in clinical trials in adults. The Pfizer vaccine is now authorized to be given to people aged 16 and up in the United States.
Health experts said the clinical trials – while not peer-reviewed – amounted to very good news.
“The sooner that we can get vaccines into as many people as possible, regardless of their age, the sooner we will be able to really feel like we’re ending this pandemic for good,” Angela Rasmussen, PhD, a virologist affiliated with Georgetown University in Washington, told The New York Times.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, recently said that getting children vaccinated is an important step toward achieving herd immunity.
“We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape,” he said earlier in March during a hearing of the Senate Health, Education, Labor, and Pensions Committee. “We ultimately would like to get and have to get children into that mix.”
Pfizer said it started clinical trials during the week of March 23 with children aged 5-11 and will next start trials with children aged 2-5, followed by children aged 6 months to 2 years. Vaccine makers Moderna and AstraZeneca also have started clinical trials in younger children.
A version of this article first appeared on WebMD.com.
The study enrolled 2,260 adolescents aged 12-15. No infections were reported in the group given the vaccine produced by Pfizer and its European partner, BioNTech, the release said. The placebo group reported 18 cases of COVID-19.
The vaccinated children showed a strong antibody response with no serious side effects.
Albert Bourla, PhD, chairman and CEO of Pfizer, said the company plans to seek Food and Drug Administration emergency use authorization, which could allow this age group to be vaccinated before the start of the next school year. Pfizer will also seek authorization from the European Medicines Agency.
“We share the urgency to expand the authorization of our vaccine to use in younger populations and are encouraged by the clinical trial data from adolescents between the ages of 12 and 15,” Dr. Bourla said in the release.
The clinical trials showed a stronger response in children aged 12-15 than the 95% effectiveness reported in clinical trials in adults. The Pfizer vaccine is now authorized to be given to people aged 16 and up in the United States.
Health experts said the clinical trials – while not peer-reviewed – amounted to very good news.
“The sooner that we can get vaccines into as many people as possible, regardless of their age, the sooner we will be able to really feel like we’re ending this pandemic for good,” Angela Rasmussen, PhD, a virologist affiliated with Georgetown University in Washington, told The New York Times.
Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, recently said that getting children vaccinated is an important step toward achieving herd immunity.
“We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape,” he said earlier in March during a hearing of the Senate Health, Education, Labor, and Pensions Committee. “We ultimately would like to get and have to get children into that mix.”
Pfizer said it started clinical trials during the week of March 23 with children aged 5-11 and will next start trials with children aged 2-5, followed by children aged 6 months to 2 years. Vaccine makers Moderna and AstraZeneca also have started clinical trials in younger children.
A version of this article first appeared on WebMD.com.
COVID-19 vaccination in RMD patients: Safety data “reassuring”
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
Two reports support the safety and immunogenicity of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal diseases (RMDs) and represent the first available data on such patients.
In an observational cohort study published in Annals of the Rheumatic Diseases, Caoilfhionn M. Connolly, MD, of Johns Hopkins University, Baltimore, and colleagues reviewed data from 325 adults with RMDs who received the first dose of SARS-CoV-2 mRNA vaccine during the period of Dec. 17, 2020, to Feb. 11, 2021. Of these, 51% received the Pfizer/BioNTech vaccine and 49% received the Moderna vaccine.
The patients, who were invited to participate on social media, were aged 34-54 years, 96% were women, and 89% were White. Inflammatory arthritis was the most common RMD condition (38%), followed by systemic lupus erythematosus (28%) and overlap connective tissue disease (19%). The patients were using a range of immunomodulatory treatment regimens, including nonbiologic disease modifying antirheumatic drugs (DMARDs) in 44%, biologics in 19%, and combination therapy in 37%.
Overall, 89% of patients reported localized symptoms of pain, swelling, and erythema, and 69% reported systemic symptoms. Fatigue was the most common systemic symptom, and 7.4% reported severe fatigue.
None of the patients experienced allergic reactions requiring epinephrine, and 3% reported new infections that required treatment.
“These early, reassuring results may ameliorate concern among patients and provide guidance for rheumatology providers in critical discussions regarding vaccine hesitancy or refusal,” they concluded.
Antibody responses
In another study published in Annals of the Rheumatic Diseases by the same group of researchers, antibody responses against the receptor binding domain of the SARS-CoV-2 spike protein were seen in 74% of 123 adults with an RMD at 18-26 days after receiving a first dose of SARS-CoV-2 mRNA vaccine (52% Pfizer vaccine and 48% Moderna) between Jan. 8, 2021, and Feb. 12, 2021.
The most common diagnoses in these patients were inflammatory arthritis (28%), systemic lupus erythematosus (20%), and Sjögren’s syndrome (13%). A total of 28% of participants reported taking no immunomodulatory agents, 19% reported nonbiologic DMARDs, 14% reported biologic DMARDs, and 19% reported combination therapy.
Although no differences appeared based on disease groups or overall categories of immunomodulatory therapies, patients whose treatment included mycophenolate or rituximab were significantly less likely to develop antibody responses than were patients not taking these medications (P = .001 and P = .04, respectively). Although rituximab and methotrexate have been associated with reduced responses to vaccines such as the flu vaccine, methotrexate was not associated with reduced vaccine response in this study. A total of 94% of patients taking a tumor necrosis factor inhibitor had detectable antibodies.
The studies’ findings were limited by several factors including a lack of longer-term safety data; the small, nonrandomized sample of mainly white women; limited information on immunomodulatory drug dosage and timing; lack of serial antibody measurements; use of an enzyme immunoassay designed to detect antibody response after natural infection; and the inclusion of data only on the first dose of a two-dose vaccine series, the researchers noted. However, the data should provide additional reassurance to RMD patients and their health care teams about vaccination against COVID-19, they said.
Both studies were supported by the Ben-Dov family. In addition, the studies were supported by grants to various study authors from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, and the Transplantation and Immunology Research Network of the American Society of Transplantation. One author disclosed financial relationships with Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, and Thermo Fisher Scientific. The other researchers had no financial conflicts to disclose.
FROM ANNALS OF THE RHEUMATIC DISEASES
COVID-19 maternal antibodies transferred to fetus, newborn from pregnant and lactating vaccine recipients
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
, according to a prospective cohort study published March 25 in the American Journal of Obstetrics and Gynecology.
The findings revealed that the antibody response to vaccination in this cohort was greater than that from a COVID-19 infection during pregnancy. Though the researchers detected SARS-CoV-2 antibodies in umbilical cord blood and breast milk, it’s not yet known how much protection these antibodies might provide to newborns.
“The presence of neutralizing antibody transfer in nearly all cords, and improved transfer with increased time from vaccination, points to the promise of mRNA vaccine–induced delivery of immunity to neonates,” wrote Kathryn J. Gray, MD, PhD, of Harvard Medical School and Brigham and Women’s Hospital’s department of obstetrics and gynecology, and colleagues. “Transfer would perhaps be optimized if vaccination is administered earlier during gestation, though this needs to be directly examined in future studies.”
The researchers tracked 84 pregnant women, 31 lactating women, and 16 nonpregnant women who received the COVID-19 vaccine. The titers of IgG, IgA, and IgM antibodies against the SARS-CoV-2 spike, receptor binding domain (RBD), and S1 and S2 components of the spike were measured in the 131 participants’ blood and in the lactating women’s breast milk four times: at baseline, when they received their second vaccine dose, at 2-6 weeks after their second dose, and at delivery for the 13 women who delivered during the study period.
The study population included health care workers and was predominantly White and non-Hispanic. In addition, two pregnant women, two lactating women, and one nonpregnant woman in the study had a previous SARS-CoV-2 infection.
Most of the pregnant women received the vaccine in their second (46%) or third (40%) trimester. The women across all three groups – pregnant, lactating, and nonpregnant – experienced similar side effects from the each dose of the vaccine, including fever/chills in 32% of the pregnant women and half the nonpregnant women after the second dose.
Titers induced by the vaccine were similar across the pregnant, lactating, and nonpregnant women, and titers did not differ based on the trimester when women received the vaccine. The researchers then compared the titers from the vaccine recipients to titers of 37 pregnant women drawn 4-12 weeks after a natural SARS-CoV-2 infection. Vaccine-induced titers were significantly greater than those measured in the women who had a natural infection during pregnancy (P < .001).
The researchers identified IgG, IgA, and IgM antibodies in the breast milk samples, including a boost in IgG antibodies after the second vaccine dose from baseline. “However, whether these antibodies were transferred efficiently to infants remained unclear,” the authors noted.
The researchers found vaccine-induced antibodies in all 10 umbilical cord blood samples tested, all but one of which had been exposed to two doses of the vaccine.
“The cord with the lowest spike- and RBD-specific IgG belonged to a mother who delivered between the first and second vaccine doses and had received her first vaccine dose 17 days prior to delivery, suggesting that 2 doses may be essential to optimize humoral immune transfer to the neonate,” the authors wrote. “Based on what is known about other vaccines, the amount of maternal IgG transferred across the placenta to the cord is likely to differ by trimester of vaccination.”
Although umbilical cord sera had lower titers of neutralizing antibodies than found in maternal sera, the difference was not significant (median interquartile range 52.3 vs. 104.7, P = .05). The two cord blood samples without neutralizing antibodies came from a woman who had not had the second dose and a woman who received the second dose 1 week before delivery.
“These data provide a compelling argument that COVID-19 mRNA vaccines induce similar humoral immunity in pregnant and lactating women as in the nonpregnant population,” the authors wrote. “These data do not elucidate potential risks to the fetus.”
While the study provides evidence about the immune response induced by the COVID-19 mRNA vaccines during pregnant, it leaves other questions unanswered, said Kevin A. Ault, MD, professor of ob.gyn. at The University of Kansas Medical Center in Kansas City.
“The important thing about these findings is that the COVID vaccines are immunogenic in pregnant women. There may be a benefit to the newborns because antibodies are passed on through the placenta,” Dr. Ault said in an interview. “The main questions that remain are safety of the vaccine during pregnancy and effectiveness of the vaccine during pregnancy.”
He said he expects to see more studies on the safety and effectiveness of COVID-19 vaccines during pregnancy. Despite more than 73,600 infections and 80 deaths from COVID-19 in people who were pregnant, none of the initial COVID-19 vaccine trials included pregnant or lactating participants.
“This is an important initial study to confirm the antibody generation from mRNA vaccination in pregnant women, and the passage of antibody via cord blood and breast milk,” said Linda Eckert, MD, a professor of ob.gyn. at The University of Washington, Seattle, who specializes in maternal immunization. “Further studies are important to look at the timing of vaccination in pregnancy and whether it influences the level of antibody passed to the fetus.”
Though this study is not a safety study, it “does not show increased expected vaccine reactions, such as aches, pains, and fever, in pregnant versus nonpregnant patients,” Dr. Eckert said in an interview. “It is not able to evaluate pregnancy outcome data, but it does allow pregnant women being vaccinated with the mRNA vaccines to know that the vaccine is generating protection for them, and the protection is being passed to the fetus in utero via cordblood and to the infant via breast milk.”
The research was funded by the National Institutes of Health along with the Gates Foundation, the Massachusetts Consortium on Pathogen Readiness (MassCPR), the Musk Foundation, the Ragon Institute of MGH and MIT, and Massachusetts General Hospital and Brigham and Women’s Hospital.
Lead author Dr. Gray has consulted for Illumina, BillionToOne, and Aetion, and three other authors have financial or scientific/medical advising connections to Alba Therapeutics, NextCure, Viome, Systems Seromyx, and Mirvie. Dr. Ault and Dr. Eckert had no disclosures.
FROM AMERICAN JOURNAL OF OBSTETRICS AND GYNECOLOGY