User login
Facts to help you keep pace with the vaccine conversation
The current increase in measles cases in the United States has sharpened the focus on antivaccine activities. While the percentage of US children who are fully vaccinated remains high (≥ 94%), the number of un- or undervaccinated children has been growing1 because of nonmedical exemptions from school vaccine requirements due to concerns about vaccine safety and an underappreciation of the benefits of vaccines. Family physicians need to be conversant with several important aspects of this matter, including the magnitude of benefits provided by childhood vaccines, as well as the systems already in place for
- assessing vaccine effectiveness and safety,
- making recommendations on the use of vaccines,
- monitoring safety after vaccine approval, and
- compensating those affected by rare but serious vaccine-related adverse events (AEs).
Familiarity with these issues will allow for informed discussions with parents who are vaccine hesitant and with those who have read or heard inaccurate information.
The benefits of vaccines are indisputable
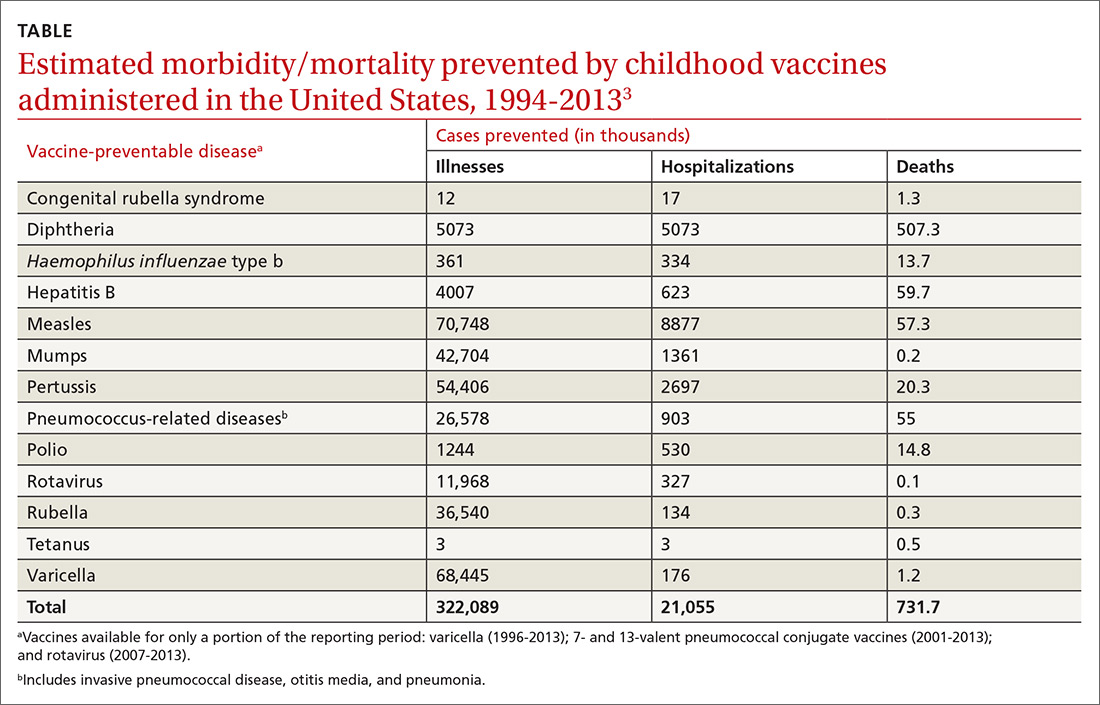
In 1999, the Centers for Disease Control and Prevention (CDC) published a list of 9 selected childhood infectious diseases and compared their incidences before and after immunization was available.2 Each of these infections causes morbidity, sequelae, and mortality at predictable rates depending on the infectious agent. The comparisons were dramatic: Measles, with a baseline annual morbidity of 503,282 cases, fell to just 89 cases; poliomyelitis decreased from 16,316 to 0; and Haemophilus influenzae type b declined from 20,000 to 54. In a 2014 analysis, the CDC stated that “among 78.6 million children born during 1994–2013, routine childhood immunization was estimated to prevent 322 million illnesses (averaging 4.1 illnesses per child) and 21 million hospitalizations (0.27 per child) over the course of their lifetimes and avert 732,000 premature deaths from vaccine-preventable illnesses” (TABLE).3
It is not unusual to hear a vaccine opponent say that childhood infectious diseases are not serious and that it is better for a child to contract the infection and let the immune system fight it naturally. Measles is often used as an example. This argument ignores some important aspects of vaccine benefits.
It is true in the United States that the average child who contracts measles will recover from it and not suffer immediate or long-term effects. However, it is also true that measles has a hospitalization rate of about 20% and a death rate of between 1/500 and 1/1000 cases.4 Mortality is much higher in developing countries. Prior to widespread use of measles vaccine, hundreds of thousands of cases of measles occurred each year. That translated into hundreds of preventable child deaths per year. An individual case does not tell the full story about the public health impact of infectious illnesses.
In addition, there are often unappreciated sequelae from child infections, such as shingles occurring years after resolution of a chickenpox infection. There are also societal consequences of child infections, such as deafness from congenital rubella and intergenerational transfer of infectious agents to family members at risk for serious consequences (influenza from a child to a grandparent). Finally, infected children pose a risk to those who cannot be vaccinated because of immune deficiencies and other medical conditions.
A multilayered US system monitors vaccine safety
Responsibility for assuring the safety of vaccines lies with the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research and with the CDC’s Immunization Safety Office (ISO). The FDA is responsible for the initial assessment of the effectiveness and safety of new vaccines and for ongoing monitoring of the manufacturing facilities where vaccines are produced. After FDA approval, safety is monitored using a multilayered system that includes the Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD) system, the Clinical Immunization Safety Assessment (CISA) Project, and periodic reviews by the National Academy of Medicine (NAM), previously the Institute of Medicine. In addition, there is a large number of studies published each year by the nation’s—and world’s—medical research community on vaccine effectiveness and safety.
Continue to: VAERS
VAERS (https://vaers.hhs.gov/) is a passive reporting system that allows patients, physicians, and other health care providers to record suspected vaccine-related adverse events.5 It was created in 1990 and is run by the FDA and the CDC. It is not intended to be a comprehensive or definitive list of proven vaccine-related harms. As a passive reporting system, it is subject to both over- and underreporting, and the data from it are often misinterpreted and used incorrectly by vaccine opponents—eg, wrongly declaring that VAERS reports of possible AEs are proven cases. It provides a sentinel system that is monitored for indications of possible serious AEs linked to a particular vaccine. When a suspected interaction is detected, it is investigated by the VSD system.
VSD is a collaboration of the CDC’s ISO and 8 geographically distributed health care organizations with complete electronic patient medical information on their members. VSD conducts studies when a question about vaccine safety arises, when new vaccines are licensed, or when there are new vaccine recommendations. A description of VSD sites, the research methods used, and a list of publications describing study results can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html#organizations. If the VSD system finds a link between serious AEs and a particular vaccine, this association is reported to the Advisory Committee on Immunization Practices (ACIP) for consideration in changing recommendations regarding that vaccine. This happens only rarely.
CISA was established in 2001 as a network of vaccine safety experts at 7 academic medical centers who collaborate with the CDC’s ISO. CISA conducts studies on specific questions related to vaccine safety and provides a consultation service to clinicians and researchers who have questions about vaccine safety. A description of the CISA sites, past publications on vaccine safety, and ongoing research priorities can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html.
NAM (https://nam.edu/) conducts periodic reviews of vaccine safety and vaccine-caused AEs. The most recent was published in 2012 and looked at possible AEs of 8 vaccines containing 12 different antigens.6 The literature search for this review found more than 12,000 articles, which speaks to the volume of scientific work on vaccine safety. These NAM reports document the rarity of severe AEs to vaccines and are used with other information to construct the table for the Vaccine Injury Compensation Program (VICP), which is described below.
Are vaccines killing children?
Vaccine opponents frequently claim that vaccines cause much more harm than is documented, including the deaths of children. A vaccine opponent made this claim in my state (Arizona) at a legislative committee hearing even though our state child mortality review committee has been investigating all child deaths for decades and has never attributed a death to a vaccine.
Continue to: One study conducted...
One study conducted using the VSD system from January 1, 2005, to December 31, 2011, identified 1100 deaths occurring within 12 months of any vaccination among 2,189,504 VSD enrollees ages 9 to 26 years.7 They found that the risk of death in this age group was not increased during the 30 days after vaccination, and no deaths were found to be causally associated with vaccination. Deaths among children do occur and, due to the number of vaccines administered, some deaths will occur within a short time period after a vaccine. This temporal association does not prove the death was vaccine-caused, but vaccine opponents have claimed that it does.
The vaccine injury compensation system
In 1986, the federal government established a no-fault system—the National Vaccine Injury Compensation Program (VICP)—to compensate those who suffer a serious AE from a vaccine covered by the program. This system is administered by the Health Resources and Services Administration (HRSA) in the Department of Health and Human Services (DHHS). HRSA maintains a table of proven AEs of specific vaccines, based in part on the NAM report mentioned earlier. Petitions for compensation—with proof of an AE following the administration of a vaccine that is included on the HRSA table—are accepted and remunerated if the AE lasted > 6 months or resulted in hospitalization. Petitions that allege AEs following administration of a vaccine not included on the table are nevertheless reviewed by the staff of HRSA, who can still recommend compensation based on the medical evidence. If HRSA declines the petition, the petitioner can appeal the case in the US Court of Federal Claims, which makes the final decision on a petition’s validity and, if warranted, the type and amount of compensation.
From 2006 to 2017, > 3.4 billion doses of vaccines covered by VICP were distributed in the United States.8 During this period, 6293 petitions were adjudicated by the court; 4311 were compensated.8 For every 1 million doses of vaccine distributed, 1 individual was compensated. Seventy percent of these compensations were awarded to petitioners despite a lack of clear evidence that the patient’s condition was caused by a vaccine.8 The rate of compensation for conditions proven to be caused by a vaccine was 1/3.33 million.8
The VICP pays for attorney fees, in some cases even if the petition is denied, but does not allow contingency fees. Since the beginning of the program, more than $4 billion has been awarded.8 The program is funded by a 75-cent tax on each vaccine antigen. Because serious AEs are so rare, the trust fund established to administer the VICP finances has a surplus of about $6 billion.
The Advisory Committee on Immunization Practices
After a vaccine is approved for use by the FDA, ACIP makes recommendations for its use in the US civilian population.9,10 ACIP, created in 1964, was chartered as a federal advisory committee to provide expert external advice to the Director of the CDC and the Secretary of DHHS on the use of vaccines
Continue to: As an official...
As an official federal advisory committee governed by the Federal Advisory Committee Act, ACIP operates under strict requirements for public notification of meetings, allowing for written and oral public comment at its meetings, and timely publication of minutes. ACIP meeting minutes are posted soon after each meeting, along with draft recommendations. ACIP meeting agendas and slide presentations are available on the ACIP Web site (https://www.cdc.gov/vaccines/acip/index.html).
ACIP consists of 15 members serving overlapping 4-year terms, appointed by the Secretary of DHHS from a list of candidates proposed by the CDC. One member is a consumer representative; the other members have expertise in vaccinology, immunology, pediatrics, internal medicine, infectious diseases, preventive medicine, and public health. In the CDC, staff support for ACIP is provided by the National Center for Immunization and Respiratory Diseases, Office of Infectious Diseases.
ACIP holds 2-day meetings 3 times a year. Much of the work occurs between meetings, by work groups via phone conferences. Work groups are chaired by an ACIP member and staffed by one or more CDC programmatic, content-expert professionals. Membership of the work groups consists of at least 2 ACIP members, representatives from relevant professional clinical and public health organizations, and other individuals with specific expertise. Work groups propose recommendations to ACIP, which can adopt, revise, or reject them.
When formulating recommendations for a particular vaccine, ACIP considers the burden of disease prevented, the effectiveness and safety of the vaccine, cost effectiveness, and practical and logistical issues of implementing recommendations. ACIP also receives frequent reports from ISO regarding the safety of vaccines previously approved. Since 2011, ACIP has used a standardized, modified GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to assess the evidence regarding effectiveness and safety of new vaccines and an evidence-to-recommendation framework to transparently explain how it arrives at recommendations.11,12
We can recommend vaccines with confidence
In the United States, we have a secure supply of safe vaccines, a transparent method of making vaccine recommendations, a robust system to monitor vaccine safety, and an efficient system to compensate those who experience a rare, serious adverse reaction to a vaccine. The US public health system has achieved a marked reduction in morbidity and mortality from childhood infectious diseases, mostly because of vaccines. Many people today have not experienced or seen children with these once-common childhood infections and may not appreciate the seriousness of childhood infectious diseases or the full value of vaccines. As family physicians, we can help address this problem and recommend vaccines to our patients with confidence.
1. Mellerson JL, Maxwell CB, Knighton CL, et al. Vaccine coverage for selected vaccines and exemption rates among children in kindergarten—United States, 2017-18 school year. MMWR Morb Mortal Wkly Rep. 2018;67:1115-1122.
2. CDC. Ten great public health achievements—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241-243.
3. Whitney CG, Zhou F, Singleton J, et al. Benefits from immunization during the Vaccines for Children Program era—United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63:352-355.
4. CDC. Complications of measles. https://www.cdc.gov/measles/symptoms/complications.html. Accessed July 16, 2019.
5. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398-4405.
6. IOM (Institute of Medicine). Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press; 2012.
7. McCarthy NL, Gee J, Sukumaran L, et al. Vaccination and 30-day mortality risk in children, adolescents, and young adults. Pediatrics. 2016;137:1-8.
8. HRSA. Data and Statistics. https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/monthly-stats-may-2019.pdf. Accessed July 16, 2019.
9. Pickering LK, Orenstein WA, Sun W, et al. FDA licensure of and ACIP recommendations for vaccines. Vaccine. 2017;37:5027-5036.
10. Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann Intern Med. 2009;150:45-49.
11. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
12. Lee G, Carr W. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018:76:1271-1272.
The current increase in measles cases in the United States has sharpened the focus on antivaccine activities. While the percentage of US children who are fully vaccinated remains high (≥ 94%), the number of un- or undervaccinated children has been growing1 because of nonmedical exemptions from school vaccine requirements due to concerns about vaccine safety and an underappreciation of the benefits of vaccines. Family physicians need to be conversant with several important aspects of this matter, including the magnitude of benefits provided by childhood vaccines, as well as the systems already in place for
- assessing vaccine effectiveness and safety,
- making recommendations on the use of vaccines,
- monitoring safety after vaccine approval, and
- compensating those affected by rare but serious vaccine-related adverse events (AEs).
Familiarity with these issues will allow for informed discussions with parents who are vaccine hesitant and with those who have read or heard inaccurate information.
The benefits of vaccines are indisputable
In 1999, the Centers for Disease Control and Prevention (CDC) published a list of 9 selected childhood infectious diseases and compared their incidences before and after immunization was available.2 Each of these infections causes morbidity, sequelae, and mortality at predictable rates depending on the infectious agent. The comparisons were dramatic: Measles, with a baseline annual morbidity of 503,282 cases, fell to just 89 cases; poliomyelitis decreased from 16,316 to 0; and Haemophilus influenzae type b declined from 20,000 to 54. In a 2014 analysis, the CDC stated that “among 78.6 million children born during 1994–2013, routine childhood immunization was estimated to prevent 322 million illnesses (averaging 4.1 illnesses per child) and 21 million hospitalizations (0.27 per child) over the course of their lifetimes and avert 732,000 premature deaths from vaccine-preventable illnesses” (TABLE).3

It is not unusual to hear a vaccine opponent say that childhood infectious diseases are not serious and that it is better for a child to contract the infection and let the immune system fight it naturally. Measles is often used as an example. This argument ignores some important aspects of vaccine benefits.
It is true in the United States that the average child who contracts measles will recover from it and not suffer immediate or long-term effects. However, it is also true that measles has a hospitalization rate of about 20% and a death rate of between 1/500 and 1/1000 cases.4 Mortality is much higher in developing countries. Prior to widespread use of measles vaccine, hundreds of thousands of cases of measles occurred each year. That translated into hundreds of preventable child deaths per year. An individual case does not tell the full story about the public health impact of infectious illnesses.
In addition, there are often unappreciated sequelae from child infections, such as shingles occurring years after resolution of a chickenpox infection. There are also societal consequences of child infections, such as deafness from congenital rubella and intergenerational transfer of infectious agents to family members at risk for serious consequences (influenza from a child to a grandparent). Finally, infected children pose a risk to those who cannot be vaccinated because of immune deficiencies and other medical conditions.
A multilayered US system monitors vaccine safety
Responsibility for assuring the safety of vaccines lies with the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research and with the CDC’s Immunization Safety Office (ISO). The FDA is responsible for the initial assessment of the effectiveness and safety of new vaccines and for ongoing monitoring of the manufacturing facilities where vaccines are produced. After FDA approval, safety is monitored using a multilayered system that includes the Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD) system, the Clinical Immunization Safety Assessment (CISA) Project, and periodic reviews by the National Academy of Medicine (NAM), previously the Institute of Medicine. In addition, there is a large number of studies published each year by the nation’s—and world’s—medical research community on vaccine effectiveness and safety.
Continue to: VAERS
VAERS (https://vaers.hhs.gov/) is a passive reporting system that allows patients, physicians, and other health care providers to record suspected vaccine-related adverse events.5 It was created in 1990 and is run by the FDA and the CDC. It is not intended to be a comprehensive or definitive list of proven vaccine-related harms. As a passive reporting system, it is subject to both over- and underreporting, and the data from it are often misinterpreted and used incorrectly by vaccine opponents—eg, wrongly declaring that VAERS reports of possible AEs are proven cases. It provides a sentinel system that is monitored for indications of possible serious AEs linked to a particular vaccine. When a suspected interaction is detected, it is investigated by the VSD system.
VSD is a collaboration of the CDC’s ISO and 8 geographically distributed health care organizations with complete electronic patient medical information on their members. VSD conducts studies when a question about vaccine safety arises, when new vaccines are licensed, or when there are new vaccine recommendations. A description of VSD sites, the research methods used, and a list of publications describing study results can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html#organizations. If the VSD system finds a link between serious AEs and a particular vaccine, this association is reported to the Advisory Committee on Immunization Practices (ACIP) for consideration in changing recommendations regarding that vaccine. This happens only rarely.
CISA was established in 2001 as a network of vaccine safety experts at 7 academic medical centers who collaborate with the CDC’s ISO. CISA conducts studies on specific questions related to vaccine safety and provides a consultation service to clinicians and researchers who have questions about vaccine safety. A description of the CISA sites, past publications on vaccine safety, and ongoing research priorities can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html.
NAM (https://nam.edu/) conducts periodic reviews of vaccine safety and vaccine-caused AEs. The most recent was published in 2012 and looked at possible AEs of 8 vaccines containing 12 different antigens.6 The literature search for this review found more than 12,000 articles, which speaks to the volume of scientific work on vaccine safety. These NAM reports document the rarity of severe AEs to vaccines and are used with other information to construct the table for the Vaccine Injury Compensation Program (VICP), which is described below.
Are vaccines killing children?
Vaccine opponents frequently claim that vaccines cause much more harm than is documented, including the deaths of children. A vaccine opponent made this claim in my state (Arizona) at a legislative committee hearing even though our state child mortality review committee has been investigating all child deaths for decades and has never attributed a death to a vaccine.
Continue to: One study conducted...
One study conducted using the VSD system from January 1, 2005, to December 31, 2011, identified 1100 deaths occurring within 12 months of any vaccination among 2,189,504 VSD enrollees ages 9 to 26 years.7 They found that the risk of death in this age group was not increased during the 30 days after vaccination, and no deaths were found to be causally associated with vaccination. Deaths among children do occur and, due to the number of vaccines administered, some deaths will occur within a short time period after a vaccine. This temporal association does not prove the death was vaccine-caused, but vaccine opponents have claimed that it does.
The vaccine injury compensation system
In 1986, the federal government established a no-fault system—the National Vaccine Injury Compensation Program (VICP)—to compensate those who suffer a serious AE from a vaccine covered by the program. This system is administered by the Health Resources and Services Administration (HRSA) in the Department of Health and Human Services (DHHS). HRSA maintains a table of proven AEs of specific vaccines, based in part on the NAM report mentioned earlier. Petitions for compensation—with proof of an AE following the administration of a vaccine that is included on the HRSA table—are accepted and remunerated if the AE lasted > 6 months or resulted in hospitalization. Petitions that allege AEs following administration of a vaccine not included on the table are nevertheless reviewed by the staff of HRSA, who can still recommend compensation based on the medical evidence. If HRSA declines the petition, the petitioner can appeal the case in the US Court of Federal Claims, which makes the final decision on a petition’s validity and, if warranted, the type and amount of compensation.
From 2006 to 2017, > 3.4 billion doses of vaccines covered by VICP were distributed in the United States.8 During this period, 6293 petitions were adjudicated by the court; 4311 were compensated.8 For every 1 million doses of vaccine distributed, 1 individual was compensated. Seventy percent of these compensations were awarded to petitioners despite a lack of clear evidence that the patient’s condition was caused by a vaccine.8 The rate of compensation for conditions proven to be caused by a vaccine was 1/3.33 million.8
The VICP pays for attorney fees, in some cases even if the petition is denied, but does not allow contingency fees. Since the beginning of the program, more than $4 billion has been awarded.8 The program is funded by a 75-cent tax on each vaccine antigen. Because serious AEs are so rare, the trust fund established to administer the VICP finances has a surplus of about $6 billion.
The Advisory Committee on Immunization Practices
After a vaccine is approved for use by the FDA, ACIP makes recommendations for its use in the US civilian population.9,10 ACIP, created in 1964, was chartered as a federal advisory committee to provide expert external advice to the Director of the CDC and the Secretary of DHHS on the use of vaccines
Continue to: As an official...
As an official federal advisory committee governed by the Federal Advisory Committee Act, ACIP operates under strict requirements for public notification of meetings, allowing for written and oral public comment at its meetings, and timely publication of minutes. ACIP meeting minutes are posted soon after each meeting, along with draft recommendations. ACIP meeting agendas and slide presentations are available on the ACIP Web site (https://www.cdc.gov/vaccines/acip/index.html).
ACIP consists of 15 members serving overlapping 4-year terms, appointed by the Secretary of DHHS from a list of candidates proposed by the CDC. One member is a consumer representative; the other members have expertise in vaccinology, immunology, pediatrics, internal medicine, infectious diseases, preventive medicine, and public health. In the CDC, staff support for ACIP is provided by the National Center for Immunization and Respiratory Diseases, Office of Infectious Diseases.
ACIP holds 2-day meetings 3 times a year. Much of the work occurs between meetings, by work groups via phone conferences. Work groups are chaired by an ACIP member and staffed by one or more CDC programmatic, content-expert professionals. Membership of the work groups consists of at least 2 ACIP members, representatives from relevant professional clinical and public health organizations, and other individuals with specific expertise. Work groups propose recommendations to ACIP, which can adopt, revise, or reject them.
When formulating recommendations for a particular vaccine, ACIP considers the burden of disease prevented, the effectiveness and safety of the vaccine, cost effectiveness, and practical and logistical issues of implementing recommendations. ACIP also receives frequent reports from ISO regarding the safety of vaccines previously approved. Since 2011, ACIP has used a standardized, modified GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to assess the evidence regarding effectiveness and safety of new vaccines and an evidence-to-recommendation framework to transparently explain how it arrives at recommendations.11,12
We can recommend vaccines with confidence
In the United States, we have a secure supply of safe vaccines, a transparent method of making vaccine recommendations, a robust system to monitor vaccine safety, and an efficient system to compensate those who experience a rare, serious adverse reaction to a vaccine. The US public health system has achieved a marked reduction in morbidity and mortality from childhood infectious diseases, mostly because of vaccines. Many people today have not experienced or seen children with these once-common childhood infections and may not appreciate the seriousness of childhood infectious diseases or the full value of vaccines. As family physicians, we can help address this problem and recommend vaccines to our patients with confidence.
The current increase in measles cases in the United States has sharpened the focus on antivaccine activities. While the percentage of US children who are fully vaccinated remains high (≥ 94%), the number of un- or undervaccinated children has been growing1 because of nonmedical exemptions from school vaccine requirements due to concerns about vaccine safety and an underappreciation of the benefits of vaccines. Family physicians need to be conversant with several important aspects of this matter, including the magnitude of benefits provided by childhood vaccines, as well as the systems already in place for
- assessing vaccine effectiveness and safety,
- making recommendations on the use of vaccines,
- monitoring safety after vaccine approval, and
- compensating those affected by rare but serious vaccine-related adverse events (AEs).
Familiarity with these issues will allow for informed discussions with parents who are vaccine hesitant and with those who have read or heard inaccurate information.
The benefits of vaccines are indisputable
In 1999, the Centers for Disease Control and Prevention (CDC) published a list of 9 selected childhood infectious diseases and compared their incidences before and after immunization was available.2 Each of these infections causes morbidity, sequelae, and mortality at predictable rates depending on the infectious agent. The comparisons were dramatic: Measles, with a baseline annual morbidity of 503,282 cases, fell to just 89 cases; poliomyelitis decreased from 16,316 to 0; and Haemophilus influenzae type b declined from 20,000 to 54. In a 2014 analysis, the CDC stated that “among 78.6 million children born during 1994–2013, routine childhood immunization was estimated to prevent 322 million illnesses (averaging 4.1 illnesses per child) and 21 million hospitalizations (0.27 per child) over the course of their lifetimes and avert 732,000 premature deaths from vaccine-preventable illnesses” (TABLE).3

It is not unusual to hear a vaccine opponent say that childhood infectious diseases are not serious and that it is better for a child to contract the infection and let the immune system fight it naturally. Measles is often used as an example. This argument ignores some important aspects of vaccine benefits.
It is true in the United States that the average child who contracts measles will recover from it and not suffer immediate or long-term effects. However, it is also true that measles has a hospitalization rate of about 20% and a death rate of between 1/500 and 1/1000 cases.4 Mortality is much higher in developing countries. Prior to widespread use of measles vaccine, hundreds of thousands of cases of measles occurred each year. That translated into hundreds of preventable child deaths per year. An individual case does not tell the full story about the public health impact of infectious illnesses.
In addition, there are often unappreciated sequelae from child infections, such as shingles occurring years after resolution of a chickenpox infection. There are also societal consequences of child infections, such as deafness from congenital rubella and intergenerational transfer of infectious agents to family members at risk for serious consequences (influenza from a child to a grandparent). Finally, infected children pose a risk to those who cannot be vaccinated because of immune deficiencies and other medical conditions.
A multilayered US system monitors vaccine safety
Responsibility for assuring the safety of vaccines lies with the US Food and Drug Administration (FDA) Center for Biologics Evaluation and Research and with the CDC’s Immunization Safety Office (ISO). The FDA is responsible for the initial assessment of the effectiveness and safety of new vaccines and for ongoing monitoring of the manufacturing facilities where vaccines are produced. After FDA approval, safety is monitored using a multilayered system that includes the Vaccine Adverse Event Reporting System (VAERS), the Vaccine Safety Datalink (VSD) system, the Clinical Immunization Safety Assessment (CISA) Project, and periodic reviews by the National Academy of Medicine (NAM), previously the Institute of Medicine. In addition, there is a large number of studies published each year by the nation’s—and world’s—medical research community on vaccine effectiveness and safety.
Continue to: VAERS
VAERS (https://vaers.hhs.gov/) is a passive reporting system that allows patients, physicians, and other health care providers to record suspected vaccine-related adverse events.5 It was created in 1990 and is run by the FDA and the CDC. It is not intended to be a comprehensive or definitive list of proven vaccine-related harms. As a passive reporting system, it is subject to both over- and underreporting, and the data from it are often misinterpreted and used incorrectly by vaccine opponents—eg, wrongly declaring that VAERS reports of possible AEs are proven cases. It provides a sentinel system that is monitored for indications of possible serious AEs linked to a particular vaccine. When a suspected interaction is detected, it is investigated by the VSD system.
VSD is a collaboration of the CDC’s ISO and 8 geographically distributed health care organizations with complete electronic patient medical information on their members. VSD conducts studies when a question about vaccine safety arises, when new vaccines are licensed, or when there are new vaccine recommendations. A description of VSD sites, the research methods used, and a list of publications describing study results can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vsd/index.html#organizations. If the VSD system finds a link between serious AEs and a particular vaccine, this association is reported to the Advisory Committee on Immunization Practices (ACIP) for consideration in changing recommendations regarding that vaccine. This happens only rarely.
CISA was established in 2001 as a network of vaccine safety experts at 7 academic medical centers who collaborate with the CDC’s ISO. CISA conducts studies on specific questions related to vaccine safety and provides a consultation service to clinicians and researchers who have questions about vaccine safety. A description of the CISA sites, past publications on vaccine safety, and ongoing research priorities can be found at https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/cisa/index.html.
NAM (https://nam.edu/) conducts periodic reviews of vaccine safety and vaccine-caused AEs. The most recent was published in 2012 and looked at possible AEs of 8 vaccines containing 12 different antigens.6 The literature search for this review found more than 12,000 articles, which speaks to the volume of scientific work on vaccine safety. These NAM reports document the rarity of severe AEs to vaccines and are used with other information to construct the table for the Vaccine Injury Compensation Program (VICP), which is described below.
Are vaccines killing children?
Vaccine opponents frequently claim that vaccines cause much more harm than is documented, including the deaths of children. A vaccine opponent made this claim in my state (Arizona) at a legislative committee hearing even though our state child mortality review committee has been investigating all child deaths for decades and has never attributed a death to a vaccine.
Continue to: One study conducted...
One study conducted using the VSD system from January 1, 2005, to December 31, 2011, identified 1100 deaths occurring within 12 months of any vaccination among 2,189,504 VSD enrollees ages 9 to 26 years.7 They found that the risk of death in this age group was not increased during the 30 days after vaccination, and no deaths were found to be causally associated with vaccination. Deaths among children do occur and, due to the number of vaccines administered, some deaths will occur within a short time period after a vaccine. This temporal association does not prove the death was vaccine-caused, but vaccine opponents have claimed that it does.
The vaccine injury compensation system
In 1986, the federal government established a no-fault system—the National Vaccine Injury Compensation Program (VICP)—to compensate those who suffer a serious AE from a vaccine covered by the program. This system is administered by the Health Resources and Services Administration (HRSA) in the Department of Health and Human Services (DHHS). HRSA maintains a table of proven AEs of specific vaccines, based in part on the NAM report mentioned earlier. Petitions for compensation—with proof of an AE following the administration of a vaccine that is included on the HRSA table—are accepted and remunerated if the AE lasted > 6 months or resulted in hospitalization. Petitions that allege AEs following administration of a vaccine not included on the table are nevertheless reviewed by the staff of HRSA, who can still recommend compensation based on the medical evidence. If HRSA declines the petition, the petitioner can appeal the case in the US Court of Federal Claims, which makes the final decision on a petition’s validity and, if warranted, the type and amount of compensation.
From 2006 to 2017, > 3.4 billion doses of vaccines covered by VICP were distributed in the United States.8 During this period, 6293 petitions were adjudicated by the court; 4311 were compensated.8 For every 1 million doses of vaccine distributed, 1 individual was compensated. Seventy percent of these compensations were awarded to petitioners despite a lack of clear evidence that the patient’s condition was caused by a vaccine.8 The rate of compensation for conditions proven to be caused by a vaccine was 1/3.33 million.8
The VICP pays for attorney fees, in some cases even if the petition is denied, but does not allow contingency fees. Since the beginning of the program, more than $4 billion has been awarded.8 The program is funded by a 75-cent tax on each vaccine antigen. Because serious AEs are so rare, the trust fund established to administer the VICP finances has a surplus of about $6 billion.
The Advisory Committee on Immunization Practices
After a vaccine is approved for use by the FDA, ACIP makes recommendations for its use in the US civilian population.9,10 ACIP, created in 1964, was chartered as a federal advisory committee to provide expert external advice to the Director of the CDC and the Secretary of DHHS on the use of vaccines
Continue to: As an official...
As an official federal advisory committee governed by the Federal Advisory Committee Act, ACIP operates under strict requirements for public notification of meetings, allowing for written and oral public comment at its meetings, and timely publication of minutes. ACIP meeting minutes are posted soon after each meeting, along with draft recommendations. ACIP meeting agendas and slide presentations are available on the ACIP Web site (https://www.cdc.gov/vaccines/acip/index.html).
ACIP consists of 15 members serving overlapping 4-year terms, appointed by the Secretary of DHHS from a list of candidates proposed by the CDC. One member is a consumer representative; the other members have expertise in vaccinology, immunology, pediatrics, internal medicine, infectious diseases, preventive medicine, and public health. In the CDC, staff support for ACIP is provided by the National Center for Immunization and Respiratory Diseases, Office of Infectious Diseases.
ACIP holds 2-day meetings 3 times a year. Much of the work occurs between meetings, by work groups via phone conferences. Work groups are chaired by an ACIP member and staffed by one or more CDC programmatic, content-expert professionals. Membership of the work groups consists of at least 2 ACIP members, representatives from relevant professional clinical and public health organizations, and other individuals with specific expertise. Work groups propose recommendations to ACIP, which can adopt, revise, or reject them.
When formulating recommendations for a particular vaccine, ACIP considers the burden of disease prevented, the effectiveness and safety of the vaccine, cost effectiveness, and practical and logistical issues of implementing recommendations. ACIP also receives frequent reports from ISO regarding the safety of vaccines previously approved. Since 2011, ACIP has used a standardized, modified GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) system to assess the evidence regarding effectiveness and safety of new vaccines and an evidence-to-recommendation framework to transparently explain how it arrives at recommendations.11,12
We can recommend vaccines with confidence
In the United States, we have a secure supply of safe vaccines, a transparent method of making vaccine recommendations, a robust system to monitor vaccine safety, and an efficient system to compensate those who experience a rare, serious adverse reaction to a vaccine. The US public health system has achieved a marked reduction in morbidity and mortality from childhood infectious diseases, mostly because of vaccines. Many people today have not experienced or seen children with these once-common childhood infections and may not appreciate the seriousness of childhood infectious diseases or the full value of vaccines. As family physicians, we can help address this problem and recommend vaccines to our patients with confidence.
1. Mellerson JL, Maxwell CB, Knighton CL, et al. Vaccine coverage for selected vaccines and exemption rates among children in kindergarten—United States, 2017-18 school year. MMWR Morb Mortal Wkly Rep. 2018;67:1115-1122.
2. CDC. Ten great public health achievements—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241-243.
3. Whitney CG, Zhou F, Singleton J, et al. Benefits from immunization during the Vaccines for Children Program era—United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63:352-355.
4. CDC. Complications of measles. https://www.cdc.gov/measles/symptoms/complications.html. Accessed July 16, 2019.
5. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398-4405.
6. IOM (Institute of Medicine). Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press; 2012.
7. McCarthy NL, Gee J, Sukumaran L, et al. Vaccination and 30-day mortality risk in children, adolescents, and young adults. Pediatrics. 2016;137:1-8.
8. HRSA. Data and Statistics. https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/monthly-stats-may-2019.pdf. Accessed July 16, 2019.
9. Pickering LK, Orenstein WA, Sun W, et al. FDA licensure of and ACIP recommendations for vaccines. Vaccine. 2017;37:5027-5036.
10. Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann Intern Med. 2009;150:45-49.
11. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
12. Lee G, Carr W. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018:76:1271-1272.
1. Mellerson JL, Maxwell CB, Knighton CL, et al. Vaccine coverage for selected vaccines and exemption rates among children in kindergarten—United States, 2017-18 school year. MMWR Morb Mortal Wkly Rep. 2018;67:1115-1122.
2. CDC. Ten great public health achievements—United States, 1900-1999. MMWR Morb Mortal Wkly Rep. 1999;48:241-243.
3. Whitney CG, Zhou F, Singleton J, et al. Benefits from immunization during the Vaccines for Children Program era—United States, 1994-2013. MMWR Morb Mortal Wkly Rep. 2014;63:352-355.
4. CDC. Complications of measles. https://www.cdc.gov/measles/symptoms/complications.html. Accessed July 16, 2019.
5. Shimabukuro TT, Nguyen M, Martin D, et al. Safety monitoring in the Vaccine Adverse Event Reporting System (VAERS). Vaccine. 2015;33:4398-4405.
6. IOM (Institute of Medicine). Adverse Effects of Vaccines: Evidence and Causality. Washington, DC: The National Academies Press; 2012.
7. McCarthy NL, Gee J, Sukumaran L, et al. Vaccination and 30-day mortality risk in children, adolescents, and young adults. Pediatrics. 2016;137:1-8.
8. HRSA. Data and Statistics. https://www.hrsa.gov/sites/default/files/hrsa/vaccine-compensation/data/monthly-stats-may-2019.pdf. Accessed July 16, 2019.
9. Pickering LK, Orenstein WA, Sun W, et al. FDA licensure of and ACIP recommendations for vaccines. Vaccine. 2017;37:5027-5036.
10. Smith JC, Snider DE, Pickering LK. Immunization policy development in the United States: the role of the Advisory Committee on Immunization Practices. Ann Intern Med. 2009;150:45-49.
11. Ahmed F, Temte JL, Campos-Outcalt D, et al; for the ACIP Evidence Based Recommendations Work Group (EBRWG). Methods for developing evidence-based recommendations by the Advisory Committee on Immunization Practices (ACIP) of the U.S. Centers for Disease Control and Prevention (CDC). Vaccine. 2011;29:9171-9176.
12. Lee G, Carr W. Updated framework for development of evidence-based recommendations by the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2018:76:1271-1272.
MMR vaccine maintains effectiveness after 10 years
according to Stephane Carryn, PhD, of GlaxoSmithKline and associates.
In a phase 3, observer-blind, randomized study published in Vaccine, 1,887 children aged 12-22 months received two doses of the MMR plus varicella (MMRV) vaccine Priorix-Tetra, one dose of the MMR vaccine Priorix with one dose of the varicella vaccine Varilrix delivered separately, or two doses of the MMR vaccine. Blood samples were collected at baseline and at days 42 and 84, then at year 1, 2, 4, 6, 8, and 10.
Antimeasles and antirubella antibodies declined moderately over the 10-year study period, but seropositivity remained high at 10 years, with about 94.0% of children remaining seropositive for antimeasles antibodies and about 96.6% remaining seropositive for antirubella antibodies. Children who received Priorix-Tetra had antimeasles antibody titers twice as high as those who received Priorix throughout the study. In addition, children who received a second dose of MMR vaccine later in life saw only a transient benefit in antimeasles and antirubella titers.
Antimumps antibody titer levels remained stable over the course of the study, and the seropositivity rate was about 90.0% at 10 years. Children who received a second MMR vaccine later in life saw a boosting effect in seropositivity and antimumps antibody titer levels.
“The responses obtained after receipt of one or two doses of MMR-containing vaccines remain well above the seropositivity thresholds up to 10 years post vaccination, regardless of the vaccine given and the schedule used,” the investigators concluded.
The study was funded and supported by GlaxoSmithKline. The study authors reported being employed by GlaxoSmithKline; two also reported owning shares in the company.
SOURCE: Carryn S et al. Vaccine. 2019 Jul 22. doi: 10.1016/j.vaccine.2019.07.049.
according to Stephane Carryn, PhD, of GlaxoSmithKline and associates.
In a phase 3, observer-blind, randomized study published in Vaccine, 1,887 children aged 12-22 months received two doses of the MMR plus varicella (MMRV) vaccine Priorix-Tetra, one dose of the MMR vaccine Priorix with one dose of the varicella vaccine Varilrix delivered separately, or two doses of the MMR vaccine. Blood samples were collected at baseline and at days 42 and 84, then at year 1, 2, 4, 6, 8, and 10.
Antimeasles and antirubella antibodies declined moderately over the 10-year study period, but seropositivity remained high at 10 years, with about 94.0% of children remaining seropositive for antimeasles antibodies and about 96.6% remaining seropositive for antirubella antibodies. Children who received Priorix-Tetra had antimeasles antibody titers twice as high as those who received Priorix throughout the study. In addition, children who received a second dose of MMR vaccine later in life saw only a transient benefit in antimeasles and antirubella titers.
Antimumps antibody titer levels remained stable over the course of the study, and the seropositivity rate was about 90.0% at 10 years. Children who received a second MMR vaccine later in life saw a boosting effect in seropositivity and antimumps antibody titer levels.
“The responses obtained after receipt of one or two doses of MMR-containing vaccines remain well above the seropositivity thresholds up to 10 years post vaccination, regardless of the vaccine given and the schedule used,” the investigators concluded.
The study was funded and supported by GlaxoSmithKline. The study authors reported being employed by GlaxoSmithKline; two also reported owning shares in the company.
SOURCE: Carryn S et al. Vaccine. 2019 Jul 22. doi: 10.1016/j.vaccine.2019.07.049.
according to Stephane Carryn, PhD, of GlaxoSmithKline and associates.
In a phase 3, observer-blind, randomized study published in Vaccine, 1,887 children aged 12-22 months received two doses of the MMR plus varicella (MMRV) vaccine Priorix-Tetra, one dose of the MMR vaccine Priorix with one dose of the varicella vaccine Varilrix delivered separately, or two doses of the MMR vaccine. Blood samples were collected at baseline and at days 42 and 84, then at year 1, 2, 4, 6, 8, and 10.
Antimeasles and antirubella antibodies declined moderately over the 10-year study period, but seropositivity remained high at 10 years, with about 94.0% of children remaining seropositive for antimeasles antibodies and about 96.6% remaining seropositive for antirubella antibodies. Children who received Priorix-Tetra had antimeasles antibody titers twice as high as those who received Priorix throughout the study. In addition, children who received a second dose of MMR vaccine later in life saw only a transient benefit in antimeasles and antirubella titers.
Antimumps antibody titer levels remained stable over the course of the study, and the seropositivity rate was about 90.0% at 10 years. Children who received a second MMR vaccine later in life saw a boosting effect in seropositivity and antimumps antibody titer levels.
“The responses obtained after receipt of one or two doses of MMR-containing vaccines remain well above the seropositivity thresholds up to 10 years post vaccination, regardless of the vaccine given and the schedule used,” the investigators concluded.
The study was funded and supported by GlaxoSmithKline. The study authors reported being employed by GlaxoSmithKline; two also reported owning shares in the company.
SOURCE: Carryn S et al. Vaccine. 2019 Jul 22. doi: 10.1016/j.vaccine.2019.07.049.
FROM VACCINE
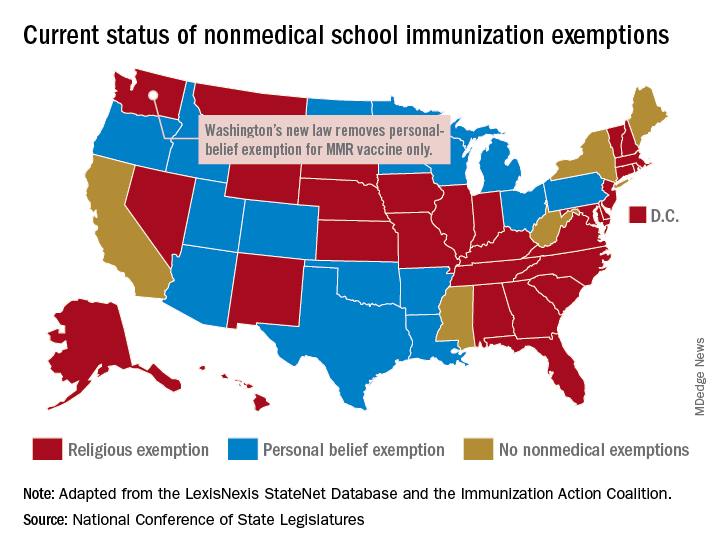
Washington State removes exemption for MMR vaccine
Washington state parents may no longer cite personal or philosophical objections to refuse the MMR vaccine for their children, effective July 28, according to the state’s department of health.
“In Washington state we believe in our doctors. We believe in our nurses. We believe in our educators. We believe in science and we love our children,” Gov. Jay Inslee (D) said when he signed the bill into law on May 10. “And that is why in Washington State, we are against measles.”
The new law applies only to the MMR vaccine and “does not change religious and medical exemption laws. Children who have one of these types of exemptions on file are not affected by the new law,” the health department said.
Washington is one of 45 states that allows religious exemptions from school immunization requirements, according to the National Conference of State Legislatures, which also reported that 15 of those states allow personal-belief exemptions.
The five states that do not allow any form of nonmedical exemption are California, Maine, Mississippi, New York, and West Virginia.
Washington state parents may no longer cite personal or philosophical objections to refuse the MMR vaccine for their children, effective July 28, according to the state’s department of health.

“In Washington state we believe in our doctors. We believe in our nurses. We believe in our educators. We believe in science and we love our children,” Gov. Jay Inslee (D) said when he signed the bill into law on May 10. “And that is why in Washington State, we are against measles.”
The new law applies only to the MMR vaccine and “does not change religious and medical exemption laws. Children who have one of these types of exemptions on file are not affected by the new law,” the health department said.
Washington is one of 45 states that allows religious exemptions from school immunization requirements, according to the National Conference of State Legislatures, which also reported that 15 of those states allow personal-belief exemptions.
The five states that do not allow any form of nonmedical exemption are California, Maine, Mississippi, New York, and West Virginia.
Washington state parents may no longer cite personal or philosophical objections to refuse the MMR vaccine for their children, effective July 28, according to the state’s department of health.

“In Washington state we believe in our doctors. We believe in our nurses. We believe in our educators. We believe in science and we love our children,” Gov. Jay Inslee (D) said when he signed the bill into law on May 10. “And that is why in Washington State, we are against measles.”
The new law applies only to the MMR vaccine and “does not change religious and medical exemption laws. Children who have one of these types of exemptions on file are not affected by the new law,” the health department said.
Washington is one of 45 states that allows religious exemptions from school immunization requirements, according to the National Conference of State Legislatures, which also reported that 15 of those states allow personal-belief exemptions.
The five states that do not allow any form of nonmedical exemption are California, Maine, Mississippi, New York, and West Virginia.
Pertussis: Comparison studies show DTwP more durable
Children primed with DTaP vaccines have a weaker response to the pertussis component of the Tdap booster vaccine, compared with children primed with the whole-cell vaccine (DTwP), according to a study in Vaccine.
Michael D. Decker, MD, and colleagues conducted a study in children aged 11-12 years who had been primed with DTaP (NCT01629589) that essentially mirrored one from 6 years earlier in children primed with DTwP when it was still the more commonly used vaccine (NCT00319553). This later study randomized 211 patients to Tdap5 and 212 to Tdap3, both licensed Tdap vaccines that had been used and compared in the earlier study. The only 35% as high for Tdap5 (31.0 vs. 86.7 endotoxin units/mL, respectively; 95% confidence interval, 30%-40%) and 32% as high (44.1 vs. 136 endotoxin units/mL; 95% CI, 28%-38%) for Tdap3.
The authors noted that, because studies including children primed with DTwP are usually much older, comparisons like the one made in this study can be unreliable because of various possible confounding factors – such as changes in manufacturing process, different assays used, changing characteristics in study populations or pertussis transmission, and so on – cannot be entirely excluded. However, one of the strengths of this study, they suggested, is that “all were randomized experimental studies conducted by Sanofi Pasteur using similar procedures (including time of sera collection), and sera from all were assayed by a single laboratory (GCI) employing consistent, [Food and Drug Administration]–accepted assays.”
They did note that estimates of mean pertussis antibodies was limited by sample sizes; however, they believed the results were sufficient for the comparisons in the study.
All authors of the study were employees of Sanofi Pasteur, which funded the study and also manufactures the Tdap5 vaccine.
SOURCE: Decker MD et al. Vaccine. 2019 Jul 10. doi: 10.1016/j.vaccine.2019.07.015.
Children primed with DTaP vaccines have a weaker response to the pertussis component of the Tdap booster vaccine, compared with children primed with the whole-cell vaccine (DTwP), according to a study in Vaccine.
Michael D. Decker, MD, and colleagues conducted a study in children aged 11-12 years who had been primed with DTaP (NCT01629589) that essentially mirrored one from 6 years earlier in children primed with DTwP when it was still the more commonly used vaccine (NCT00319553). This later study randomized 211 patients to Tdap5 and 212 to Tdap3, both licensed Tdap vaccines that had been used and compared in the earlier study. The only 35% as high for Tdap5 (31.0 vs. 86.7 endotoxin units/mL, respectively; 95% confidence interval, 30%-40%) and 32% as high (44.1 vs. 136 endotoxin units/mL; 95% CI, 28%-38%) for Tdap3.
The authors noted that, because studies including children primed with DTwP are usually much older, comparisons like the one made in this study can be unreliable because of various possible confounding factors – such as changes in manufacturing process, different assays used, changing characteristics in study populations or pertussis transmission, and so on – cannot be entirely excluded. However, one of the strengths of this study, they suggested, is that “all were randomized experimental studies conducted by Sanofi Pasteur using similar procedures (including time of sera collection), and sera from all were assayed by a single laboratory (GCI) employing consistent, [Food and Drug Administration]–accepted assays.”
They did note that estimates of mean pertussis antibodies was limited by sample sizes; however, they believed the results were sufficient for the comparisons in the study.
All authors of the study were employees of Sanofi Pasteur, which funded the study and also manufactures the Tdap5 vaccine.
SOURCE: Decker MD et al. Vaccine. 2019 Jul 10. doi: 10.1016/j.vaccine.2019.07.015.
Children primed with DTaP vaccines have a weaker response to the pertussis component of the Tdap booster vaccine, compared with children primed with the whole-cell vaccine (DTwP), according to a study in Vaccine.
Michael D. Decker, MD, and colleagues conducted a study in children aged 11-12 years who had been primed with DTaP (NCT01629589) that essentially mirrored one from 6 years earlier in children primed with DTwP when it was still the more commonly used vaccine (NCT00319553). This later study randomized 211 patients to Tdap5 and 212 to Tdap3, both licensed Tdap vaccines that had been used and compared in the earlier study. The only 35% as high for Tdap5 (31.0 vs. 86.7 endotoxin units/mL, respectively; 95% confidence interval, 30%-40%) and 32% as high (44.1 vs. 136 endotoxin units/mL; 95% CI, 28%-38%) for Tdap3.
The authors noted that, because studies including children primed with DTwP are usually much older, comparisons like the one made in this study can be unreliable because of various possible confounding factors – such as changes in manufacturing process, different assays used, changing characteristics in study populations or pertussis transmission, and so on – cannot be entirely excluded. However, one of the strengths of this study, they suggested, is that “all were randomized experimental studies conducted by Sanofi Pasteur using similar procedures (including time of sera collection), and sera from all were assayed by a single laboratory (GCI) employing consistent, [Food and Drug Administration]–accepted assays.”
They did note that estimates of mean pertussis antibodies was limited by sample sizes; however, they believed the results were sufficient for the comparisons in the study.
All authors of the study were employees of Sanofi Pasteur, which funded the study and also manufactures the Tdap5 vaccine.
SOURCE: Decker MD et al. Vaccine. 2019 Jul 10. doi: 10.1016/j.vaccine.2019.07.015.
FROM VACCINE
Pentavalent DTaP-Hb-Hib vaccine is found noninferior to comparator
The to a similar, commercially available vaccine in infants, according to a study in Vaccine.
In this phase 3, randomized, single-blind, multicenter, noninferiority study, Sai Krishna Susarla of Human Biologicals Institute, which developed the test vaccine, and colleagues randomized 405 infants aged 6-8 weeks 2:1 to three doses of either the test vaccine or the comparator, Pentavac SD (Serum Institute of India). The percentages of seroconversion for diphtheria, pertussis, hepatitis B, and Hib were 98.44%, 92.61%, 99.22%, and 95.72% for the test vaccine, respectively, and 90.0%, 89.23%, 100%, and 90.77% for the comparator. In keeping with some previous studies, the percentages for tetanus were low at 50.97% with the test vaccine and 30.23% with the comparator. Despite the low seroconversion for tetanus, the test vaccine was determined to be noninferior to the comparator for it and the other four diseases it targets. The safety profile was also found to be comparable.
Although the study’s major limitation is that it was conducted in only one country, “the strength of the study is considered to be good” because “compliance to protocol was good, deviations were minimal, and ... very few subjects were withdrawn,” the researchers wrote.
Some of the researchers were employees of the sponsor, Human Biologicals Institute, which developed the test vaccine. Other researchers had no financial interest in the test vaccine and were unrelated to the sponsor, but did receive research grants for conducting the study at their respective sites.
SOURCE: Susarla SK et al. Vaccine. 2019 Jul 19. doi: 10.1016/j.vaccine.2019.06.067.
The to a similar, commercially available vaccine in infants, according to a study in Vaccine.
In this phase 3, randomized, single-blind, multicenter, noninferiority study, Sai Krishna Susarla of Human Biologicals Institute, which developed the test vaccine, and colleagues randomized 405 infants aged 6-8 weeks 2:1 to three doses of either the test vaccine or the comparator, Pentavac SD (Serum Institute of India). The percentages of seroconversion for diphtheria, pertussis, hepatitis B, and Hib were 98.44%, 92.61%, 99.22%, and 95.72% for the test vaccine, respectively, and 90.0%, 89.23%, 100%, and 90.77% for the comparator. In keeping with some previous studies, the percentages for tetanus were low at 50.97% with the test vaccine and 30.23% with the comparator. Despite the low seroconversion for tetanus, the test vaccine was determined to be noninferior to the comparator for it and the other four diseases it targets. The safety profile was also found to be comparable.
Although the study’s major limitation is that it was conducted in only one country, “the strength of the study is considered to be good” because “compliance to protocol was good, deviations were minimal, and ... very few subjects were withdrawn,” the researchers wrote.
Some of the researchers were employees of the sponsor, Human Biologicals Institute, which developed the test vaccine. Other researchers had no financial interest in the test vaccine and were unrelated to the sponsor, but did receive research grants for conducting the study at their respective sites.
SOURCE: Susarla SK et al. Vaccine. 2019 Jul 19. doi: 10.1016/j.vaccine.2019.06.067.
The to a similar, commercially available vaccine in infants, according to a study in Vaccine.
In this phase 3, randomized, single-blind, multicenter, noninferiority study, Sai Krishna Susarla of Human Biologicals Institute, which developed the test vaccine, and colleagues randomized 405 infants aged 6-8 weeks 2:1 to three doses of either the test vaccine or the comparator, Pentavac SD (Serum Institute of India). The percentages of seroconversion for diphtheria, pertussis, hepatitis B, and Hib were 98.44%, 92.61%, 99.22%, and 95.72% for the test vaccine, respectively, and 90.0%, 89.23%, 100%, and 90.77% for the comparator. In keeping with some previous studies, the percentages for tetanus were low at 50.97% with the test vaccine and 30.23% with the comparator. Despite the low seroconversion for tetanus, the test vaccine was determined to be noninferior to the comparator for it and the other four diseases it targets. The safety profile was also found to be comparable.
Although the study’s major limitation is that it was conducted in only one country, “the strength of the study is considered to be good” because “compliance to protocol was good, deviations were minimal, and ... very few subjects were withdrawn,” the researchers wrote.
Some of the researchers were employees of the sponsor, Human Biologicals Institute, which developed the test vaccine. Other researchers had no financial interest in the test vaccine and were unrelated to the sponsor, but did receive research grants for conducting the study at their respective sites.
SOURCE: Susarla SK et al. Vaccine. 2019 Jul 19. doi: 10.1016/j.vaccine.2019.06.067.
FROM VACCINE
PHiD-CV with 4CMenB safe, effective for infants
Concomitant administration of pneumococcal and meningococcal vaccines is not only safe but also offers the potential to improve vaccine uptake and reduce the number of doctors’ visits required for routine vaccination, advised Marco Aurelio P. Safadi, MD, PhD, of Santa Casa de São Paulo School of Medical Sciences, Brazil, and associates.
In a post hoc analysis of a phase 3b open-label study, Dr. Safadi and associates sought to evaluate immune response in pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) administered concomitantly with either meningococcal serogroup B (4CMenB) vaccine and CRM-conjugated meningococcal serogroup C vaccine (MenC-CRM) or with MenC-CRM alone using reduced schedules in 213 healthy infants aged 83-104 days. Study participants were enrolled and randomized to one of two groups between April 2011 and December 2014 at four sites in Brazil (Vaccine. 2019 Jul 18. doi: 10.1016/j.vaccine.2019.07.021).
Similar immune response was seen with vaccine serotypes and vaccine-related pneumococcal serotypes 6A and 19A in children who had received concomitant administration of PHiD-CV, 4CMenB, and MenC-CRM without 4CMenB.
Dr. Safadi and associates pointed out that PHiD-CV was given in accordance with a 3+1 dosing schedule, while 4CMenB used a reduced 2+1 schedule, which was observed to produce an immune response and provide an acceptable safety profile.
The findings yielded valuable information for the 2+1 PHiD-CV vaccination schedule, which was recently introduced in Brazil, the researchers said. The post-booster results further reflect the “immunogenicity following 3-dose priming.”
The post hoc nature of this study design effectively demonstrated that or with MenC-CRM alone, they explained.
The study was supported by GlaxoSmithKline (GSK) Biologicals. Three authors are employees of the GSK group of companies, and three others received a grant from the GSK companies, two of whom received compensation from other pharmaceutical companies. The institution of one of the authors received clinical trial fees from the GSK companies, and received personal fees/nonfinancial support/grants/other from the GSK companies and many other pharmaceutical companies.
Concomitant administration of pneumococcal and meningococcal vaccines is not only safe but also offers the potential to improve vaccine uptake and reduce the number of doctors’ visits required for routine vaccination, advised Marco Aurelio P. Safadi, MD, PhD, of Santa Casa de São Paulo School of Medical Sciences, Brazil, and associates.
In a post hoc analysis of a phase 3b open-label study, Dr. Safadi and associates sought to evaluate immune response in pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) administered concomitantly with either meningococcal serogroup B (4CMenB) vaccine and CRM-conjugated meningococcal serogroup C vaccine (MenC-CRM) or with MenC-CRM alone using reduced schedules in 213 healthy infants aged 83-104 days. Study participants were enrolled and randomized to one of two groups between April 2011 and December 2014 at four sites in Brazil (Vaccine. 2019 Jul 18. doi: 10.1016/j.vaccine.2019.07.021).
Similar immune response was seen with vaccine serotypes and vaccine-related pneumococcal serotypes 6A and 19A in children who had received concomitant administration of PHiD-CV, 4CMenB, and MenC-CRM without 4CMenB.
Dr. Safadi and associates pointed out that PHiD-CV was given in accordance with a 3+1 dosing schedule, while 4CMenB used a reduced 2+1 schedule, which was observed to produce an immune response and provide an acceptable safety profile.
The findings yielded valuable information for the 2+1 PHiD-CV vaccination schedule, which was recently introduced in Brazil, the researchers said. The post-booster results further reflect the “immunogenicity following 3-dose priming.”
The post hoc nature of this study design effectively demonstrated that or with MenC-CRM alone, they explained.
The study was supported by GlaxoSmithKline (GSK) Biologicals. Three authors are employees of the GSK group of companies, and three others received a grant from the GSK companies, two of whom received compensation from other pharmaceutical companies. The institution of one of the authors received clinical trial fees from the GSK companies, and received personal fees/nonfinancial support/grants/other from the GSK companies and many other pharmaceutical companies.
Concomitant administration of pneumococcal and meningococcal vaccines is not only safe but also offers the potential to improve vaccine uptake and reduce the number of doctors’ visits required for routine vaccination, advised Marco Aurelio P. Safadi, MD, PhD, of Santa Casa de São Paulo School of Medical Sciences, Brazil, and associates.
In a post hoc analysis of a phase 3b open-label study, Dr. Safadi and associates sought to evaluate immune response in pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) administered concomitantly with either meningococcal serogroup B (4CMenB) vaccine and CRM-conjugated meningococcal serogroup C vaccine (MenC-CRM) or with MenC-CRM alone using reduced schedules in 213 healthy infants aged 83-104 days. Study participants were enrolled and randomized to one of two groups between April 2011 and December 2014 at four sites in Brazil (Vaccine. 2019 Jul 18. doi: 10.1016/j.vaccine.2019.07.021).
Similar immune response was seen with vaccine serotypes and vaccine-related pneumococcal serotypes 6A and 19A in children who had received concomitant administration of PHiD-CV, 4CMenB, and MenC-CRM without 4CMenB.
Dr. Safadi and associates pointed out that PHiD-CV was given in accordance with a 3+1 dosing schedule, while 4CMenB used a reduced 2+1 schedule, which was observed to produce an immune response and provide an acceptable safety profile.
The findings yielded valuable information for the 2+1 PHiD-CV vaccination schedule, which was recently introduced in Brazil, the researchers said. The post-booster results further reflect the “immunogenicity following 3-dose priming.”
The post hoc nature of this study design effectively demonstrated that or with MenC-CRM alone, they explained.
The study was supported by GlaxoSmithKline (GSK) Biologicals. Three authors are employees of the GSK group of companies, and three others received a grant from the GSK companies, two of whom received compensation from other pharmaceutical companies. The institution of one of the authors received clinical trial fees from the GSK companies, and received personal fees/nonfinancial support/grants/other from the GSK companies and many other pharmaceutical companies.
FROM VACCINE
New measles outbreaks reported in Los Angeles and El Paso
according to the Centers for Disease Control and Prevention.
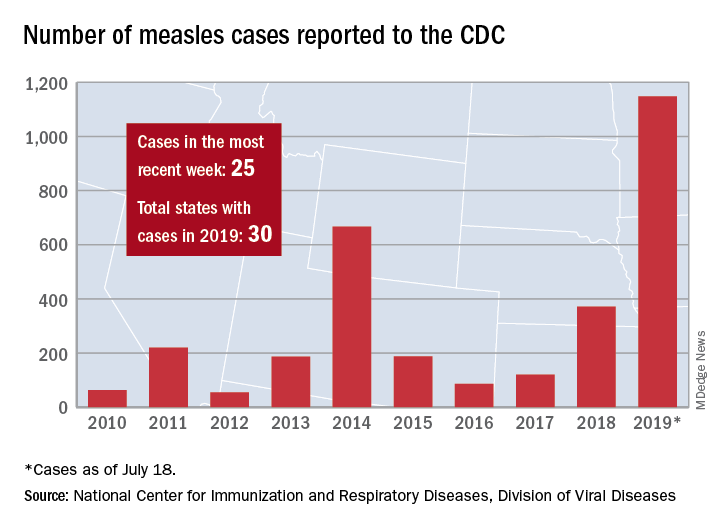
The total number of confirmed cases of measles in the United States is now up to 1,148 for the year, which is 25 more than the previous week, the CDC said on July 22. The highest 1-week total for the year was the 90 cases reported during the week of April 11.
The number of outbreaks is back up to five as California returned to the list after a 1-week absence and El Paso, Tex., made its first appearance of the year. The current outbreak in California – the state’s fifth – is occurring in Los Angeles, which is now up to 16 total cases in 2019. El Paso just reported its fourth case on July 17, and the city’s health department noted that “it had been more than 25 years since El Paso saw its last case of measles before these four recent cases.” Outbreaks also are ongoing in Rockland County, N.Y.; New York City; and three counties in Washington State.
States that joined the ranks of the measles-infected during this most recent reporting week were Alaska and Ohio, which brings the total number to 30 for the year, the CDC said.
The Alaska Department of Health and Social Services said that it “has confirmed a single case of measles in an unvaccinated teenager from the Kenai Peninsula who recently traveled out of state to Arizona via Seattle.” The Ohio case is a “young adult from Stark County [who] recently traveled to a state with confirmed measles cases,” according to the state’s health department.
according to the Centers for Disease Control and Prevention.

The total number of confirmed cases of measles in the United States is now up to 1,148 for the year, which is 25 more than the previous week, the CDC said on July 22. The highest 1-week total for the year was the 90 cases reported during the week of April 11.
The number of outbreaks is back up to five as California returned to the list after a 1-week absence and El Paso, Tex., made its first appearance of the year. The current outbreak in California – the state’s fifth – is occurring in Los Angeles, which is now up to 16 total cases in 2019. El Paso just reported its fourth case on July 17, and the city’s health department noted that “it had been more than 25 years since El Paso saw its last case of measles before these four recent cases.” Outbreaks also are ongoing in Rockland County, N.Y.; New York City; and three counties in Washington State.
States that joined the ranks of the measles-infected during this most recent reporting week were Alaska and Ohio, which brings the total number to 30 for the year, the CDC said.
The Alaska Department of Health and Social Services said that it “has confirmed a single case of measles in an unvaccinated teenager from the Kenai Peninsula who recently traveled out of state to Arizona via Seattle.” The Ohio case is a “young adult from Stark County [who] recently traveled to a state with confirmed measles cases,” according to the state’s health department.
according to the Centers for Disease Control and Prevention.

The total number of confirmed cases of measles in the United States is now up to 1,148 for the year, which is 25 more than the previous week, the CDC said on July 22. The highest 1-week total for the year was the 90 cases reported during the week of April 11.
The number of outbreaks is back up to five as California returned to the list after a 1-week absence and El Paso, Tex., made its first appearance of the year. The current outbreak in California – the state’s fifth – is occurring in Los Angeles, which is now up to 16 total cases in 2019. El Paso just reported its fourth case on July 17, and the city’s health department noted that “it had been more than 25 years since El Paso saw its last case of measles before these four recent cases.” Outbreaks also are ongoing in Rockland County, N.Y.; New York City; and three counties in Washington State.
States that joined the ranks of the measles-infected during this most recent reporting week were Alaska and Ohio, which brings the total number to 30 for the year, the CDC said.
The Alaska Department of Health and Social Services said that it “has confirmed a single case of measles in an unvaccinated teenager from the Kenai Peninsula who recently traveled out of state to Arizona via Seattle.” The Ohio case is a “young adult from Stark County [who] recently traveled to a state with confirmed measles cases,” according to the state’s health department.
Adjuvanted flu vaccine performs better than others in young children
according to an industry-funded synthesis of six studies.
The vaccine “offers significant advances over conventional inactivated influenza vaccines and presents an acceptable safety profile in children 6 months through 5 years of age,” Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and associates wrote in the analysis, published in the International Journal of Infectious Diseases. “The noteworthy increases in antibody responses and decreases in influenza cases following vaccination suggest an alternative for use in a population that is heavily impacted by influenza disease.”
Children are, of course, vulnerable to flu. The Centers for Disease Control and Prevention reported that 186 children died of flu during the landmark 2017-2018 flu season. That’s the highest number of pediatric flu deaths since they became a notifiable condition in 2004 (exclusive of the 2009 pandemic, when 358 pediatric deaths were reported from April 15, 2009, to October 2, 2010).The CDC said the vaccine during that flu season had an overall effectiveness level of 40%. According to research of others, however, flu vaccines are less effective in younger children than in adolescents and adults (Vaccine. 2014;32[31]:3886-94; Cochrane Database Syst Rev. 2008. doi: 10.1002/14651858.CD004879.pub3).
Fluad – a MF59-adjuvanted inactivated trivalent seasonal influenza vaccine – is used in adults over 65 in the United States and 29 other countries, and it is approved for children aged 6 months through 23 months in Canada.
Dr. Patel and associates examined the results of six studies – one phase 1b, three phase 2, and two phase 3 – that tested Fluad with or without other vaccines in 11,942 children aged 6 months to 5 years. The studies, mostly multicenter, were conducted in various countries, mainly in Europe and South and Central America, from 2006 to 2012.
In general, children in the intervention groups in the studies received two doses of the Fluad vaccine 4 weeks apart: two 0.25-mL doses for children aged 6-35 months and two 0.5-mL doses for those aged 3 years or older. In most of the studies, parallel control groups received nonadjuvanted trivalent or quadrivalent influenza vaccines.
Most participants (93%-94%) completed the studies. Solicited adverse effects were common in all groups (72% in the Fluad group vs. 67% who received IIV3 vaccines), and generally mild to moderate and resolved in 1-3 days. Unsolicited adverse effects were similar (55% and 62%, respectively) in the two flu vaccine groups. The authors wrote that “these data reflect a safety profile consistent with other licensed inactivated influenza vaccines administered to children.”
As for results, Dr. Patel and colleagues said, “HI [hemagglutination inhibition] antibody responses to both homologous and heterologous influenza strains are higher following vaccination with aIIV3, and this increase in immunogenicity is observed across all age subgroups in children aged 6 months through 5 years, and most profound in the children 6 to 36 months.”
For example, in one of the phase 3 studies when the influenza viruses were antigenically matched (homologous) for A/H1N1 among the children aged 6-35 months seroconversion was 100% for allV3 (Fluad) and 38% for IIV3-1/IIV3-4 (trivalent/quadrivalent flu vaccines); among children aged 3-5 years seroconversion was 100% for allV3 and 82% for IIV3-1/IIV3-4. For AH3N2 homologous among children aged 6-35 months, seroconversion was 98% for allV3 and 44% for IIV3-1/IIV3-4. For the B strain homologous among children aged 6-35 months, seroconversion was 88% for allV3 and 19% for IIV3-1/IIV3-4; among children aged 3-5 years seroconversion for B was 99% for allV3 and 59% for IIV3-1/IIV3-4.
In the same study when the influenza viruses were antigenically mismatched (heterologous) for A/H1N1 among children of all ages 6 months to greater than 72 months, seroconversion was 96% for allV3 (Fluad) and 44% for IIV3-1/IIV3-4; for A/H3N2 it was 98% for allV3 and 49% for IIV3-1/IIV3-4, and for the B strain it was 10% for allV3 and 3% for IIV3-1/IIV3-4.
They added that “in addition, aIIV3 had the fastest onset of immunogenicity and longest persistence of immune response, which has implications for the real-world clinical setting, where the influenza season might start earlier than expected or last longer, and second (follow-up) vaccinations may be missed.”
Dr. Patel and associates said the MF59 adjuvant in Fluad “recruits immune cells (primarily monocytes, macrophages, neutrophils, and dendritic cells) at the site of injection and differentiates them into antigen-presenting cells. With an MF59-adjuvanted vaccine, more antigen is transported from the injection site to the draining lymph node, wherein MF59 leads to T-cell activation and an increased B-cell expansion and a greater number and diversity of antibodies.”
According to goodrx.com, one syringe of Fluad 0.5 mL costs $45-$74 with coupon. The same dose of Fluzone Quadrivalent, a flu vaccine recently approved by the Food and Drug Administration for use in young children aged 6-35 months, costs $31 with coupon.
The study was funded by Novartis Vaccines and Diagnostics and Seqirus (formerly part of Novartis Vaccines and Diagnostics). The study authors disclosed employment by Novartis and Seqirus.
SOURCE: Patel SS et al. Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.05.009.
according to an industry-funded synthesis of six studies.
The vaccine “offers significant advances over conventional inactivated influenza vaccines and presents an acceptable safety profile in children 6 months through 5 years of age,” Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and associates wrote in the analysis, published in the International Journal of Infectious Diseases. “The noteworthy increases in antibody responses and decreases in influenza cases following vaccination suggest an alternative for use in a population that is heavily impacted by influenza disease.”
Children are, of course, vulnerable to flu. The Centers for Disease Control and Prevention reported that 186 children died of flu during the landmark 2017-2018 flu season. That’s the highest number of pediatric flu deaths since they became a notifiable condition in 2004 (exclusive of the 2009 pandemic, when 358 pediatric deaths were reported from April 15, 2009, to October 2, 2010).The CDC said the vaccine during that flu season had an overall effectiveness level of 40%. According to research of others, however, flu vaccines are less effective in younger children than in adolescents and adults (Vaccine. 2014;32[31]:3886-94; Cochrane Database Syst Rev. 2008. doi: 10.1002/14651858.CD004879.pub3).
Fluad – a MF59-adjuvanted inactivated trivalent seasonal influenza vaccine – is used in adults over 65 in the United States and 29 other countries, and it is approved for children aged 6 months through 23 months in Canada.
Dr. Patel and associates examined the results of six studies – one phase 1b, three phase 2, and two phase 3 – that tested Fluad with or without other vaccines in 11,942 children aged 6 months to 5 years. The studies, mostly multicenter, were conducted in various countries, mainly in Europe and South and Central America, from 2006 to 2012.
In general, children in the intervention groups in the studies received two doses of the Fluad vaccine 4 weeks apart: two 0.25-mL doses for children aged 6-35 months and two 0.5-mL doses for those aged 3 years or older. In most of the studies, parallel control groups received nonadjuvanted trivalent or quadrivalent influenza vaccines.
Most participants (93%-94%) completed the studies. Solicited adverse effects were common in all groups (72% in the Fluad group vs. 67% who received IIV3 vaccines), and generally mild to moderate and resolved in 1-3 days. Unsolicited adverse effects were similar (55% and 62%, respectively) in the two flu vaccine groups. The authors wrote that “these data reflect a safety profile consistent with other licensed inactivated influenza vaccines administered to children.”
As for results, Dr. Patel and colleagues said, “HI [hemagglutination inhibition] antibody responses to both homologous and heterologous influenza strains are higher following vaccination with aIIV3, and this increase in immunogenicity is observed across all age subgroups in children aged 6 months through 5 years, and most profound in the children 6 to 36 months.”
For example, in one of the phase 3 studies when the influenza viruses were antigenically matched (homologous) for A/H1N1 among the children aged 6-35 months seroconversion was 100% for allV3 (Fluad) and 38% for IIV3-1/IIV3-4 (trivalent/quadrivalent flu vaccines); among children aged 3-5 years seroconversion was 100% for allV3 and 82% for IIV3-1/IIV3-4. For AH3N2 homologous among children aged 6-35 months, seroconversion was 98% for allV3 and 44% for IIV3-1/IIV3-4. For the B strain homologous among children aged 6-35 months, seroconversion was 88% for allV3 and 19% for IIV3-1/IIV3-4; among children aged 3-5 years seroconversion for B was 99% for allV3 and 59% for IIV3-1/IIV3-4.
In the same study when the influenza viruses were antigenically mismatched (heterologous) for A/H1N1 among children of all ages 6 months to greater than 72 months, seroconversion was 96% for allV3 (Fluad) and 44% for IIV3-1/IIV3-4; for A/H3N2 it was 98% for allV3 and 49% for IIV3-1/IIV3-4, and for the B strain it was 10% for allV3 and 3% for IIV3-1/IIV3-4.
They added that “in addition, aIIV3 had the fastest onset of immunogenicity and longest persistence of immune response, which has implications for the real-world clinical setting, where the influenza season might start earlier than expected or last longer, and second (follow-up) vaccinations may be missed.”
Dr. Patel and associates said the MF59 adjuvant in Fluad “recruits immune cells (primarily monocytes, macrophages, neutrophils, and dendritic cells) at the site of injection and differentiates them into antigen-presenting cells. With an MF59-adjuvanted vaccine, more antigen is transported from the injection site to the draining lymph node, wherein MF59 leads to T-cell activation and an increased B-cell expansion and a greater number and diversity of antibodies.”
According to goodrx.com, one syringe of Fluad 0.5 mL costs $45-$74 with coupon. The same dose of Fluzone Quadrivalent, a flu vaccine recently approved by the Food and Drug Administration for use in young children aged 6-35 months, costs $31 with coupon.
The study was funded by Novartis Vaccines and Diagnostics and Seqirus (formerly part of Novartis Vaccines and Diagnostics). The study authors disclosed employment by Novartis and Seqirus.
SOURCE: Patel SS et al. Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.05.009.
according to an industry-funded synthesis of six studies.
The vaccine “offers significant advances over conventional inactivated influenza vaccines and presents an acceptable safety profile in children 6 months through 5 years of age,” Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and associates wrote in the analysis, published in the International Journal of Infectious Diseases. “The noteworthy increases in antibody responses and decreases in influenza cases following vaccination suggest an alternative for use in a population that is heavily impacted by influenza disease.”
Children are, of course, vulnerable to flu. The Centers for Disease Control and Prevention reported that 186 children died of flu during the landmark 2017-2018 flu season. That’s the highest number of pediatric flu deaths since they became a notifiable condition in 2004 (exclusive of the 2009 pandemic, when 358 pediatric deaths were reported from April 15, 2009, to October 2, 2010).The CDC said the vaccine during that flu season had an overall effectiveness level of 40%. According to research of others, however, flu vaccines are less effective in younger children than in adolescents and adults (Vaccine. 2014;32[31]:3886-94; Cochrane Database Syst Rev. 2008. doi: 10.1002/14651858.CD004879.pub3).
Fluad – a MF59-adjuvanted inactivated trivalent seasonal influenza vaccine – is used in adults over 65 in the United States and 29 other countries, and it is approved for children aged 6 months through 23 months in Canada.
Dr. Patel and associates examined the results of six studies – one phase 1b, three phase 2, and two phase 3 – that tested Fluad with or without other vaccines in 11,942 children aged 6 months to 5 years. The studies, mostly multicenter, were conducted in various countries, mainly in Europe and South and Central America, from 2006 to 2012.
In general, children in the intervention groups in the studies received two doses of the Fluad vaccine 4 weeks apart: two 0.25-mL doses for children aged 6-35 months and two 0.5-mL doses for those aged 3 years or older. In most of the studies, parallel control groups received nonadjuvanted trivalent or quadrivalent influenza vaccines.
Most participants (93%-94%) completed the studies. Solicited adverse effects were common in all groups (72% in the Fluad group vs. 67% who received IIV3 vaccines), and generally mild to moderate and resolved in 1-3 days. Unsolicited adverse effects were similar (55% and 62%, respectively) in the two flu vaccine groups. The authors wrote that “these data reflect a safety profile consistent with other licensed inactivated influenza vaccines administered to children.”
As for results, Dr. Patel and colleagues said, “HI [hemagglutination inhibition] antibody responses to both homologous and heterologous influenza strains are higher following vaccination with aIIV3, and this increase in immunogenicity is observed across all age subgroups in children aged 6 months through 5 years, and most profound in the children 6 to 36 months.”
For example, in one of the phase 3 studies when the influenza viruses were antigenically matched (homologous) for A/H1N1 among the children aged 6-35 months seroconversion was 100% for allV3 (Fluad) and 38% for IIV3-1/IIV3-4 (trivalent/quadrivalent flu vaccines); among children aged 3-5 years seroconversion was 100% for allV3 and 82% for IIV3-1/IIV3-4. For AH3N2 homologous among children aged 6-35 months, seroconversion was 98% for allV3 and 44% for IIV3-1/IIV3-4. For the B strain homologous among children aged 6-35 months, seroconversion was 88% for allV3 and 19% for IIV3-1/IIV3-4; among children aged 3-5 years seroconversion for B was 99% for allV3 and 59% for IIV3-1/IIV3-4.
In the same study when the influenza viruses were antigenically mismatched (heterologous) for A/H1N1 among children of all ages 6 months to greater than 72 months, seroconversion was 96% for allV3 (Fluad) and 44% for IIV3-1/IIV3-4; for A/H3N2 it was 98% for allV3 and 49% for IIV3-1/IIV3-4, and for the B strain it was 10% for allV3 and 3% for IIV3-1/IIV3-4.
They added that “in addition, aIIV3 had the fastest onset of immunogenicity and longest persistence of immune response, which has implications for the real-world clinical setting, where the influenza season might start earlier than expected or last longer, and second (follow-up) vaccinations may be missed.”
Dr. Patel and associates said the MF59 adjuvant in Fluad “recruits immune cells (primarily monocytes, macrophages, neutrophils, and dendritic cells) at the site of injection and differentiates them into antigen-presenting cells. With an MF59-adjuvanted vaccine, more antigen is transported from the injection site to the draining lymph node, wherein MF59 leads to T-cell activation and an increased B-cell expansion and a greater number and diversity of antibodies.”
According to goodrx.com, one syringe of Fluad 0.5 mL costs $45-$74 with coupon. The same dose of Fluzone Quadrivalent, a flu vaccine recently approved by the Food and Drug Administration for use in young children aged 6-35 months, costs $31 with coupon.
The study was funded by Novartis Vaccines and Diagnostics and Seqirus (formerly part of Novartis Vaccines and Diagnostics). The study authors disclosed employment by Novartis and Seqirus.
SOURCE: Patel SS et al. Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.05.009.
FROM INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
Adjuvanted influenza vaccine appears safe for at-risk children
according to a study in the International Journal of Infectious Diseases.
Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and colleagues performed a retrospective analysis on an integrated dataset that drew from six randomized clinical trials comparing aIIV3 with nonadjuvanted trivalent inactivated influenza vaccine (IIV3). The dataset comprised 10,794 patients aged 6 months through 5 years, of whom 373 (3%) were deemed at risk of influenza complications after review of their medical history for conditions such as heart disease, asthma, and endocrine disorders.
The rates of solicited adverse events (such as erythema, diarrhea, fever, and localized swelling) were 74% in the aIIV3 group and 73% in the IIV3 group. The rates for any unsolicited adverse events (such as upper respiratory tract infection) for aIIV3 and IIV3 were 54% and 59%, respectively (Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.04.023).
One of the six studies included in the dataset randomized 2,655 children for immunogenicity analyses, of whom 103 (4%) were deemed at risk. Hemagglutination inhibition assay geometric mean titers against homologous A/H1N1, A/H3N2, and B strains 21 days after the second of two doses of vaccines were two to three times higher in the aIIV3 than in the IIV3 group, which suggests that aIIV3 is more immunogenic than IIV3. As the investigators noted, this is likely because the adjuvanted vaccine induces a greater magnitude of immune response to the vaccine, something already lower in children than in adults, as well as more breadth of response, meaning the response goes beyond strains included in the vaccines.
The small number of at-risk children in the study poses a limitation on its findings. Dr. Patel and associates said that, regardless, the results of immunogenicity analyses were strong. “Overall, this analysis indicates that aIIV3 has a similar safety profile in young children with underlying medical conditions, consistent with other licensed inactivated influenza vaccines.”
Novartis Vaccines and Diagnostics originally funded the study, but was later acquired by CSL Group and now operates as Seqirus, which continued funding for the study. The authors were employees of one or the other of these companies.
according to a study in the International Journal of Infectious Diseases.
Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and colleagues performed a retrospective analysis on an integrated dataset that drew from six randomized clinical trials comparing aIIV3 with nonadjuvanted trivalent inactivated influenza vaccine (IIV3). The dataset comprised 10,794 patients aged 6 months through 5 years, of whom 373 (3%) were deemed at risk of influenza complications after review of their medical history for conditions such as heart disease, asthma, and endocrine disorders.
The rates of solicited adverse events (such as erythema, diarrhea, fever, and localized swelling) were 74% in the aIIV3 group and 73% in the IIV3 group. The rates for any unsolicited adverse events (such as upper respiratory tract infection) for aIIV3 and IIV3 were 54% and 59%, respectively (Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.04.023).
One of the six studies included in the dataset randomized 2,655 children for immunogenicity analyses, of whom 103 (4%) were deemed at risk. Hemagglutination inhibition assay geometric mean titers against homologous A/H1N1, A/H3N2, and B strains 21 days after the second of two doses of vaccines were two to three times higher in the aIIV3 than in the IIV3 group, which suggests that aIIV3 is more immunogenic than IIV3. As the investigators noted, this is likely because the adjuvanted vaccine induces a greater magnitude of immune response to the vaccine, something already lower in children than in adults, as well as more breadth of response, meaning the response goes beyond strains included in the vaccines.
The small number of at-risk children in the study poses a limitation on its findings. Dr. Patel and associates said that, regardless, the results of immunogenicity analyses were strong. “Overall, this analysis indicates that aIIV3 has a similar safety profile in young children with underlying medical conditions, consistent with other licensed inactivated influenza vaccines.”
Novartis Vaccines and Diagnostics originally funded the study, but was later acquired by CSL Group and now operates as Seqirus, which continued funding for the study. The authors were employees of one or the other of these companies.
according to a study in the International Journal of Infectious Diseases.
Sanjay S. Patel, PhD, of Novartis Vaccines and Diagnostics, Cambridge, Mass., and colleagues performed a retrospective analysis on an integrated dataset that drew from six randomized clinical trials comparing aIIV3 with nonadjuvanted trivalent inactivated influenza vaccine (IIV3). The dataset comprised 10,794 patients aged 6 months through 5 years, of whom 373 (3%) were deemed at risk of influenza complications after review of their medical history for conditions such as heart disease, asthma, and endocrine disorders.
The rates of solicited adverse events (such as erythema, diarrhea, fever, and localized swelling) were 74% in the aIIV3 group and 73% in the IIV3 group. The rates for any unsolicited adverse events (such as upper respiratory tract infection) for aIIV3 and IIV3 were 54% and 59%, respectively (Int J Infect Dis. 2019. doi: 10.1016/j.ijid.2019.04.023).
One of the six studies included in the dataset randomized 2,655 children for immunogenicity analyses, of whom 103 (4%) were deemed at risk. Hemagglutination inhibition assay geometric mean titers against homologous A/H1N1, A/H3N2, and B strains 21 days after the second of two doses of vaccines were two to three times higher in the aIIV3 than in the IIV3 group, which suggests that aIIV3 is more immunogenic than IIV3. As the investigators noted, this is likely because the adjuvanted vaccine induces a greater magnitude of immune response to the vaccine, something already lower in children than in adults, as well as more breadth of response, meaning the response goes beyond strains included in the vaccines.
The small number of at-risk children in the study poses a limitation on its findings. Dr. Patel and associates said that, regardless, the results of immunogenicity analyses were strong. “Overall, this analysis indicates that aIIV3 has a similar safety profile in young children with underlying medical conditions, consistent with other licensed inactivated influenza vaccines.”
Novartis Vaccines and Diagnostics originally funded the study, but was later acquired by CSL Group and now operates as Seqirus, which continued funding for the study. The authors were employees of one or the other of these companies.
FROM THE INTERNATIONAL JOURNAL OF INFECTIOUS DISEASES
New oral polio vaccine is noninferior to currently licensed vaccine
according to Khalequ Zaman, MBBS, PhD, of the International Center for Diarrheal Disease Research in Dhaka, Bangladesh, and associates.
In the first part of the observer-blind, randomized, controlled study, 40 patients aged 5-6 years received either the new vaccine (BBio bOPV) or the licensed vaccine (SII bOPV). In the second part, 1,080 patients aged 6-8 weeks received either BBio bOPV or SII bOPV at age 6, 10, and 14 weeks. Blood samples were taken to assess neutralizing antibody responses against poliovirus types 1 and 3, and safety also was assessed.
In the first part of the study, 12 adverse events were reported, none of which were serious and none of which were related to the vaccines. All participants demonstrated seroprotective titers against both poliovirus types 1 month after vaccination.
In the second part, more than 96% of infants demonstrated seroprotection and seroconversion against both poliovirus types. Geometric mean titers were equivalent in both groups. A total of 387 participants had at least one adverse event, and 18 serious adverse events were reported. None of these were related to the vaccines.
“The BBio bOPV has been proven safe and immunogenic in the target infant population in Bangladesh. As the use of bOPV is expected to be continued until at least 2022, availability of new bOPV bulk manufacturer will be helpful in securing adequate supplies of bOPV for global demand in the polio endgame strategy,” the investigators concluded.
The study was funded by Bilthoven Biologicals. Four coauthors are employed by Bilthoven, manufacturer of the study vaccine; two others are employed by the Serum Institute of India, which provided the control vaccine.
SOURCE: Zaman K et al. Vaccine. 2019 Jun 22. doi: 10.1016/j.vaccine.2019.06.048.
according to Khalequ Zaman, MBBS, PhD, of the International Center for Diarrheal Disease Research in Dhaka, Bangladesh, and associates.
In the first part of the observer-blind, randomized, controlled study, 40 patients aged 5-6 years received either the new vaccine (BBio bOPV) or the licensed vaccine (SII bOPV). In the second part, 1,080 patients aged 6-8 weeks received either BBio bOPV or SII bOPV at age 6, 10, and 14 weeks. Blood samples were taken to assess neutralizing antibody responses against poliovirus types 1 and 3, and safety also was assessed.
In the first part of the study, 12 adverse events were reported, none of which were serious and none of which were related to the vaccines. All participants demonstrated seroprotective titers against both poliovirus types 1 month after vaccination.
In the second part, more than 96% of infants demonstrated seroprotection and seroconversion against both poliovirus types. Geometric mean titers were equivalent in both groups. A total of 387 participants had at least one adverse event, and 18 serious adverse events were reported. None of these were related to the vaccines.
“The BBio bOPV has been proven safe and immunogenic in the target infant population in Bangladesh. As the use of bOPV is expected to be continued until at least 2022, availability of new bOPV bulk manufacturer will be helpful in securing adequate supplies of bOPV for global demand in the polio endgame strategy,” the investigators concluded.
The study was funded by Bilthoven Biologicals. Four coauthors are employed by Bilthoven, manufacturer of the study vaccine; two others are employed by the Serum Institute of India, which provided the control vaccine.
SOURCE: Zaman K et al. Vaccine. 2019 Jun 22. doi: 10.1016/j.vaccine.2019.06.048.
according to Khalequ Zaman, MBBS, PhD, of the International Center for Diarrheal Disease Research in Dhaka, Bangladesh, and associates.
In the first part of the observer-blind, randomized, controlled study, 40 patients aged 5-6 years received either the new vaccine (BBio bOPV) or the licensed vaccine (SII bOPV). In the second part, 1,080 patients aged 6-8 weeks received either BBio bOPV or SII bOPV at age 6, 10, and 14 weeks. Blood samples were taken to assess neutralizing antibody responses against poliovirus types 1 and 3, and safety also was assessed.
In the first part of the study, 12 adverse events were reported, none of which were serious and none of which were related to the vaccines. All participants demonstrated seroprotective titers against both poliovirus types 1 month after vaccination.
In the second part, more than 96% of infants demonstrated seroprotection and seroconversion against both poliovirus types. Geometric mean titers were equivalent in both groups. A total of 387 participants had at least one adverse event, and 18 serious adverse events were reported. None of these were related to the vaccines.
“The BBio bOPV has been proven safe and immunogenic in the target infant population in Bangladesh. As the use of bOPV is expected to be continued until at least 2022, availability of new bOPV bulk manufacturer will be helpful in securing adequate supplies of bOPV for global demand in the polio endgame strategy,” the investigators concluded.
The study was funded by Bilthoven Biologicals. Four coauthors are employed by Bilthoven, manufacturer of the study vaccine; two others are employed by the Serum Institute of India, which provided the control vaccine.
SOURCE: Zaman K et al. Vaccine. 2019 Jun 22. doi: 10.1016/j.vaccine.2019.06.048.
FROM VACCINE