User login
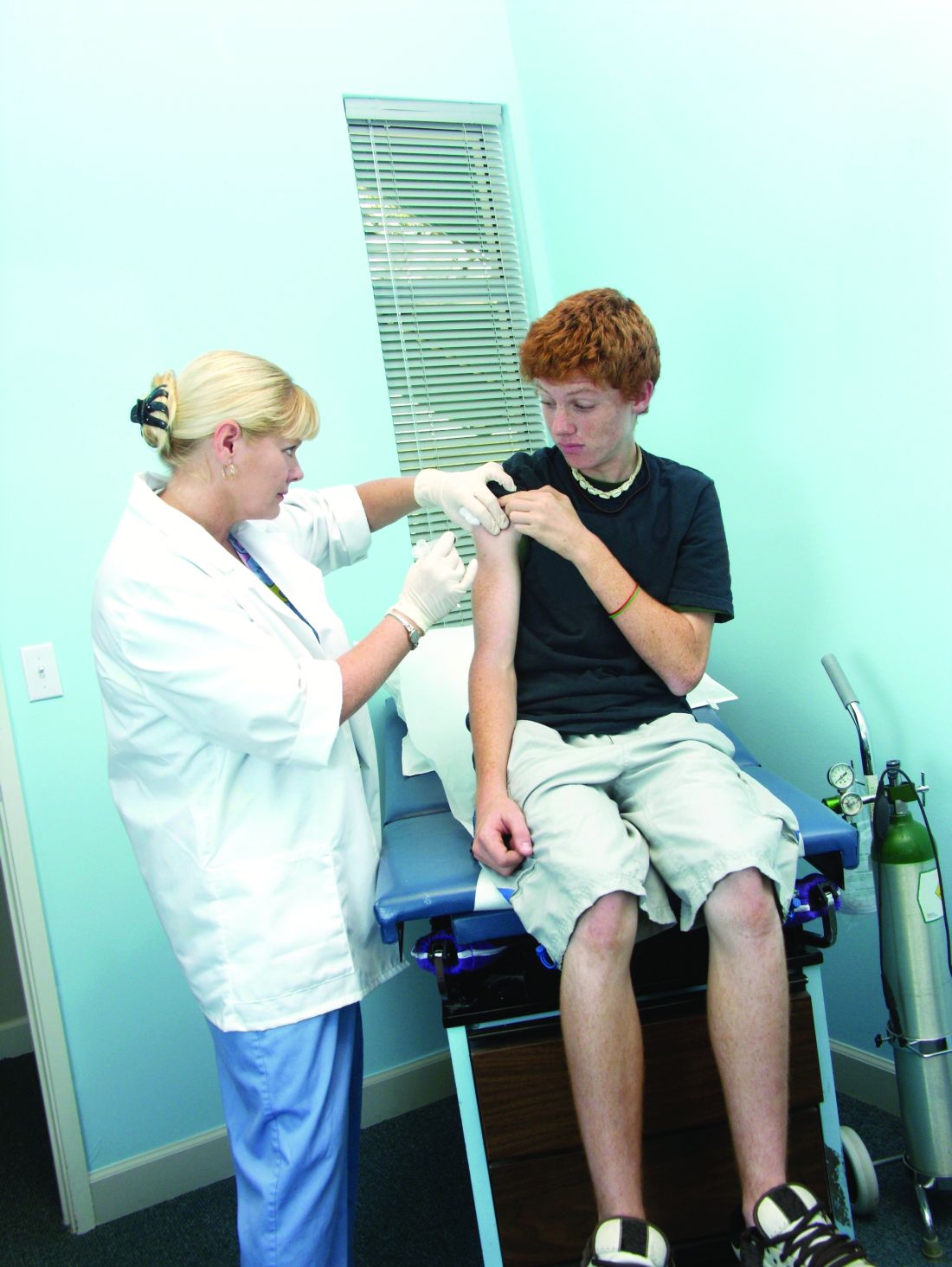
Fifty-one percent of U.S. adolescents fully vaccinated against HPV
according to a report published in Morbidity and Mortality Weekly Report.
Researchers analyzed data from 18,700 adolescents aged 13-17 years – 48% of whom were female – in the 2018 National Immunization Survey–Teen to discover that 51% of adolescents were up to date with the human papillomavirus (HPV) vaccine, and 68% had received at least one dose of the vaccine.
There was an increase in HPV vaccination coverage from 2017 to 2018, but this was attributable to a 4.4 percentage point increase in males who were up to date, compared with a 0.6 percentage point increase in females.
“Although HPV vaccination coverage improved, increases among all adolescents were modest compared with increases in previous years and were observed only among males,” wrote Tanja Y. Walker of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, and coauthors.
The number of adolescents who had at least one dose of the quadrivalent meningococcal conjugate (4MenB) vaccine increased by 1.5 percentage points to 86.6%, while among individuals aged 17 years, coverage with two or more doses of 4MenB vaccine increased by 6.5 percentage points to 50.8%. Tdap coverage remained the same at 89% (MMWR 2019;68(33):718-23).
However, the study saw no significant increases in coverage with three or more hepatitis B vaccine doses, two or more MMR vaccine doses, or with one or more varicella vaccine doses in adolescents without a history of varicella disease.
Adolescents with Medicaid had higher HPV vaccination coverage than did adolescents with private health insurance. Uninsured adolescents had lower coverage overall, ranging from 4 percentage points lower for one or more varicella vaccine doses to 19 percentage points lower for two or more 4MenB vaccines, compared with adolescents with private health insurance.
Vaccination rates were lower among adolescents outside metropolitan areas, particularly when it came to being up to date with HPV vaccination, where there was a 15 percentage point difference, and with two or more doses of the quadrivalent meningococcal conjugate vaccine, where there was a 20 percentage point difference.
Provider recommendations to parents were associated with a higher rate of coverage with one or more doses of the HPV vaccine, but the prevalence of provider recommendations varied significantly from state to state. Overall, 78% of parents said they received a provider recommendation for the adolescent HPV vaccine, but that figure was as low as 60% in Mississippi and as high as 91% in Massachusetts.
Parents living in nonmetropolitan areas were less likely to report receiving a provider recommendation than were those in metropolitan principal cities.
“Equipping providers with the tools they need to give strong recommendations that emphasize the importance of HPV vaccination in preventing cancer and effectively address parental concerns is a priority, especially in states where provider recommendations were less commonly reported,” Ms. Walker and associates said.
No conflicts of interest were declared.
according to a report published in Morbidity and Mortality Weekly Report.
Researchers analyzed data from 18,700 adolescents aged 13-17 years – 48% of whom were female – in the 2018 National Immunization Survey–Teen to discover that 51% of adolescents were up to date with the human papillomavirus (HPV) vaccine, and 68% had received at least one dose of the vaccine.
There was an increase in HPV vaccination coverage from 2017 to 2018, but this was attributable to a 4.4 percentage point increase in males who were up to date, compared with a 0.6 percentage point increase in females.
“Although HPV vaccination coverage improved, increases among all adolescents were modest compared with increases in previous years and were observed only among males,” wrote Tanja Y. Walker of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, and coauthors.
The number of adolescents who had at least one dose of the quadrivalent meningococcal conjugate (4MenB) vaccine increased by 1.5 percentage points to 86.6%, while among individuals aged 17 years, coverage with two or more doses of 4MenB vaccine increased by 6.5 percentage points to 50.8%. Tdap coverage remained the same at 89% (MMWR 2019;68(33):718-23).
However, the study saw no significant increases in coverage with three or more hepatitis B vaccine doses, two or more MMR vaccine doses, or with one or more varicella vaccine doses in adolescents without a history of varicella disease.
Adolescents with Medicaid had higher HPV vaccination coverage than did adolescents with private health insurance. Uninsured adolescents had lower coverage overall, ranging from 4 percentage points lower for one or more varicella vaccine doses to 19 percentage points lower for two or more 4MenB vaccines, compared with adolescents with private health insurance.
Vaccination rates were lower among adolescents outside metropolitan areas, particularly when it came to being up to date with HPV vaccination, where there was a 15 percentage point difference, and with two or more doses of the quadrivalent meningococcal conjugate vaccine, where there was a 20 percentage point difference.
Provider recommendations to parents were associated with a higher rate of coverage with one or more doses of the HPV vaccine, but the prevalence of provider recommendations varied significantly from state to state. Overall, 78% of parents said they received a provider recommendation for the adolescent HPV vaccine, but that figure was as low as 60% in Mississippi and as high as 91% in Massachusetts.
Parents living in nonmetropolitan areas were less likely to report receiving a provider recommendation than were those in metropolitan principal cities.
“Equipping providers with the tools they need to give strong recommendations that emphasize the importance of HPV vaccination in preventing cancer and effectively address parental concerns is a priority, especially in states where provider recommendations were less commonly reported,” Ms. Walker and associates said.
No conflicts of interest were declared.
according to a report published in Morbidity and Mortality Weekly Report.
Researchers analyzed data from 18,700 adolescents aged 13-17 years – 48% of whom were female – in the 2018 National Immunization Survey–Teen to discover that 51% of adolescents were up to date with the human papillomavirus (HPV) vaccine, and 68% had received at least one dose of the vaccine.
There was an increase in HPV vaccination coverage from 2017 to 2018, but this was attributable to a 4.4 percentage point increase in males who were up to date, compared with a 0.6 percentage point increase in females.
“Although HPV vaccination coverage improved, increases among all adolescents were modest compared with increases in previous years and were observed only among males,” wrote Tanja Y. Walker of the National Center for Immunization and Respiratory Diseases at the Centers for Disease Control and Prevention, and coauthors.
The number of adolescents who had at least one dose of the quadrivalent meningococcal conjugate (4MenB) vaccine increased by 1.5 percentage points to 86.6%, while among individuals aged 17 years, coverage with two or more doses of 4MenB vaccine increased by 6.5 percentage points to 50.8%. Tdap coverage remained the same at 89% (MMWR 2019;68(33):718-23).
However, the study saw no significant increases in coverage with three or more hepatitis B vaccine doses, two or more MMR vaccine doses, or with one or more varicella vaccine doses in adolescents without a history of varicella disease.
Adolescents with Medicaid had higher HPV vaccination coverage than did adolescents with private health insurance. Uninsured adolescents had lower coverage overall, ranging from 4 percentage points lower for one or more varicella vaccine doses to 19 percentage points lower for two or more 4MenB vaccines, compared with adolescents with private health insurance.
Vaccination rates were lower among adolescents outside metropolitan areas, particularly when it came to being up to date with HPV vaccination, where there was a 15 percentage point difference, and with two or more doses of the quadrivalent meningococcal conjugate vaccine, where there was a 20 percentage point difference.
Provider recommendations to parents were associated with a higher rate of coverage with one or more doses of the HPV vaccine, but the prevalence of provider recommendations varied significantly from state to state. Overall, 78% of parents said they received a provider recommendation for the adolescent HPV vaccine, but that figure was as low as 60% in Mississippi and as high as 91% in Massachusetts.
Parents living in nonmetropolitan areas were less likely to report receiving a provider recommendation than were those in metropolitan principal cities.
“Equipping providers with the tools they need to give strong recommendations that emphasize the importance of HPV vaccination in preventing cancer and effectively address parental concerns is a priority, especially in states where provider recommendations were less commonly reported,” Ms. Walker and associates said.
No conflicts of interest were declared.
FROM MMWR
Key clinical point: Slightly more than half of adolescents in the United States are fully vaccinated with the HPV vaccine.
Major finding: Rates of full HPV vaccination are 51% among adolescents aged 13-17 years.
Study details: Analysis of data from 18,700 adolescents aged 13-17 years in the 2018 National Immunization Survey–Teen.
Disclosures: No conflicts of interest were declared.
Source: Walker T et al. MMWR 2019 Aug 23;68(33):718-23.
EULAR updates vaccination recommendations for autoimmune inflammatory rheumatic disease patients
Vaccination status should be reviewed annually for patients with autoimmune inflammatory rheumatic diseases, according to updated recommendations from the European League Against Rheumatism.
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk for infections, and vaccination has been shown to reduce risk by “potentially translating into a lower rate of hospital admissions due to infections, emergency room visits, and the rate of invasive infectious diseases,” wrote Victoria Furer, MD, of Tel Aviv Sourasky Medical Center, and members of the task force that updated the recommendations, which were published in Annals of the Rheumatic Diseases.
However, AIIRD patients often go unvaccinated because of a lack of awareness or concerns about vaccine safety and efficacy, they said (Ann Rheum Dis. 2019 Aug 14. doi: 10.1136/annrheumdis-2019-215882).
The task force consisted of 21 experts, including patients, rheumatologists, immunologists, an infectious disease specialist, and health professionals in rheumatology representing eight countries. They evaluated data from four systematic literature reviews and developed nine recommendations based on six key principles.
“For each recommendation, the level of evidence for the incidence/prevalence of vaccine preventable infection in AIIRD, and efficacy/immunogenicity/safety of vaccination were stated, when available, followed by the strength of recommendation and the level of agreement,” the task force wrote.
These overarching principles start with an annual assessment of vaccination status by the AIIRD patient’s rheumatology team. Other principles include explanation of an individualized vaccination program to the patient as a foundation for joint decision-making, vaccinating patients during quiescent disease periods, vaccinating in advance of planned immunosuppression when possible, considering non-live vaccines for AIIRD patients also treated with systemic glucocorticoids and DMARDs, and considering live-attenuated vaccines with caution.
Several of the nine recommendations developed by the task force are modified from the previous recommendations issued in 2011. The task force made its recommendations with an eye toward optimizing individual risk stratification and avoiding “unnecessary” vaccination in AIIRD patients with low risk of infection as part of the update process. A notable change from the 2011 guidelines is the recommendation of both influenza and pneumococcal vaccinations for the majority of patients with AIIRD as opposed to all patients to emphasize the importance of individualized risk assessment, the task force noted.
The recommendations state that influenza vaccination and pneumococcal vaccination should be “strongly considered” for patients with AIIRD, and patients also should receive tetanus toxoid vaccination according to recommendations for the general population. However, clinicians should consider passive immunization for patients treated with B-cell depleting therapy, the task force wrote.
AIIRD patients at risk for hepatitis A and B should receive vaccinations for those diseases, with boosters or passive immunization if indicated, and high-risk patients may consider herpes zoster vaccination, according to the recommendations.
In addition, AIIRD patients – especially patients with systemic lupus erythematosus – should receive human papilloma virus vaccination according to recommendations for the general population, but AIIRD patients should avoid yellow fever vaccination, the task force stated. However, for AIIRD patients traveling to areas of yellow fever risk, “withholding immunosuppressive therapy to allow a safe vaccination or measuring serology in previously exposed patients may be considered.”
Finally, mothers treated with biologics during the second half of pregnancy should avoid live-attenuated vaccines for their newborns, and immunocompetent household members of AIIRD patients should be encouraged to follow national guidelines for routine vaccination with the exception of the oral polio vaccine, the task force concluded.
Vaccination status should be reviewed annually for patients with autoimmune inflammatory rheumatic diseases, according to updated recommendations from the European League Against Rheumatism.
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk for infections, and vaccination has been shown to reduce risk by “potentially translating into a lower rate of hospital admissions due to infections, emergency room visits, and the rate of invasive infectious diseases,” wrote Victoria Furer, MD, of Tel Aviv Sourasky Medical Center, and members of the task force that updated the recommendations, which were published in Annals of the Rheumatic Diseases.
However, AIIRD patients often go unvaccinated because of a lack of awareness or concerns about vaccine safety and efficacy, they said (Ann Rheum Dis. 2019 Aug 14. doi: 10.1136/annrheumdis-2019-215882).
The task force consisted of 21 experts, including patients, rheumatologists, immunologists, an infectious disease specialist, and health professionals in rheumatology representing eight countries. They evaluated data from four systematic literature reviews and developed nine recommendations based on six key principles.
“For each recommendation, the level of evidence for the incidence/prevalence of vaccine preventable infection in AIIRD, and efficacy/immunogenicity/safety of vaccination were stated, when available, followed by the strength of recommendation and the level of agreement,” the task force wrote.
These overarching principles start with an annual assessment of vaccination status by the AIIRD patient’s rheumatology team. Other principles include explanation of an individualized vaccination program to the patient as a foundation for joint decision-making, vaccinating patients during quiescent disease periods, vaccinating in advance of planned immunosuppression when possible, considering non-live vaccines for AIIRD patients also treated with systemic glucocorticoids and DMARDs, and considering live-attenuated vaccines with caution.
Several of the nine recommendations developed by the task force are modified from the previous recommendations issued in 2011. The task force made its recommendations with an eye toward optimizing individual risk stratification and avoiding “unnecessary” vaccination in AIIRD patients with low risk of infection as part of the update process. A notable change from the 2011 guidelines is the recommendation of both influenza and pneumococcal vaccinations for the majority of patients with AIIRD as opposed to all patients to emphasize the importance of individualized risk assessment, the task force noted.
The recommendations state that influenza vaccination and pneumococcal vaccination should be “strongly considered” for patients with AIIRD, and patients also should receive tetanus toxoid vaccination according to recommendations for the general population. However, clinicians should consider passive immunization for patients treated with B-cell depleting therapy, the task force wrote.
AIIRD patients at risk for hepatitis A and B should receive vaccinations for those diseases, with boosters or passive immunization if indicated, and high-risk patients may consider herpes zoster vaccination, according to the recommendations.
In addition, AIIRD patients – especially patients with systemic lupus erythematosus – should receive human papilloma virus vaccination according to recommendations for the general population, but AIIRD patients should avoid yellow fever vaccination, the task force stated. However, for AIIRD patients traveling to areas of yellow fever risk, “withholding immunosuppressive therapy to allow a safe vaccination or measuring serology in previously exposed patients may be considered.”
Finally, mothers treated with biologics during the second half of pregnancy should avoid live-attenuated vaccines for their newborns, and immunocompetent household members of AIIRD patients should be encouraged to follow national guidelines for routine vaccination with the exception of the oral polio vaccine, the task force concluded.
Vaccination status should be reviewed annually for patients with autoimmune inflammatory rheumatic diseases, according to updated recommendations from the European League Against Rheumatism.
Patients with autoimmune inflammatory rheumatic diseases (AIIRD) are at increased risk for infections, and vaccination has been shown to reduce risk by “potentially translating into a lower rate of hospital admissions due to infections, emergency room visits, and the rate of invasive infectious diseases,” wrote Victoria Furer, MD, of Tel Aviv Sourasky Medical Center, and members of the task force that updated the recommendations, which were published in Annals of the Rheumatic Diseases.
However, AIIRD patients often go unvaccinated because of a lack of awareness or concerns about vaccine safety and efficacy, they said (Ann Rheum Dis. 2019 Aug 14. doi: 10.1136/annrheumdis-2019-215882).
The task force consisted of 21 experts, including patients, rheumatologists, immunologists, an infectious disease specialist, and health professionals in rheumatology representing eight countries. They evaluated data from four systematic literature reviews and developed nine recommendations based on six key principles.
“For each recommendation, the level of evidence for the incidence/prevalence of vaccine preventable infection in AIIRD, and efficacy/immunogenicity/safety of vaccination were stated, when available, followed by the strength of recommendation and the level of agreement,” the task force wrote.
These overarching principles start with an annual assessment of vaccination status by the AIIRD patient’s rheumatology team. Other principles include explanation of an individualized vaccination program to the patient as a foundation for joint decision-making, vaccinating patients during quiescent disease periods, vaccinating in advance of planned immunosuppression when possible, considering non-live vaccines for AIIRD patients also treated with systemic glucocorticoids and DMARDs, and considering live-attenuated vaccines with caution.
Several of the nine recommendations developed by the task force are modified from the previous recommendations issued in 2011. The task force made its recommendations with an eye toward optimizing individual risk stratification and avoiding “unnecessary” vaccination in AIIRD patients with low risk of infection as part of the update process. A notable change from the 2011 guidelines is the recommendation of both influenza and pneumococcal vaccinations for the majority of patients with AIIRD as opposed to all patients to emphasize the importance of individualized risk assessment, the task force noted.
The recommendations state that influenza vaccination and pneumococcal vaccination should be “strongly considered” for patients with AIIRD, and patients also should receive tetanus toxoid vaccination according to recommendations for the general population. However, clinicians should consider passive immunization for patients treated with B-cell depleting therapy, the task force wrote.
AIIRD patients at risk for hepatitis A and B should receive vaccinations for those diseases, with boosters or passive immunization if indicated, and high-risk patients may consider herpes zoster vaccination, according to the recommendations.
In addition, AIIRD patients – especially patients with systemic lupus erythematosus – should receive human papilloma virus vaccination according to recommendations for the general population, but AIIRD patients should avoid yellow fever vaccination, the task force stated. However, for AIIRD patients traveling to areas of yellow fever risk, “withholding immunosuppressive therapy to allow a safe vaccination or measuring serology in previously exposed patients may be considered.”
Finally, mothers treated with biologics during the second half of pregnancy should avoid live-attenuated vaccines for their newborns, and immunocompetent household members of AIIRD patients should be encouraged to follow national guidelines for routine vaccination with the exception of the oral polio vaccine, the task force concluded.
FROM ANNALS OF THE RHEUMATIC DISEASES
No teen herd immunity for 4CMenB in landmark trial
LJUBLJANA, SLOVENIA – The 4CMenB vaccine didn’t affect carriage of disease-causing genogroups of Neisseria meningitidis in adolescents in the landmark Australian cluster-randomized trial of herd immunity known as the “B Part of It” study, Helen S. Marshall, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This was the largest-ever randomized trial of adolescents vaccinated against meningococcal disease, and the message, albeit somewhat disappointing, is clear: “MenB [Meningococcal serogroup B] vaccine programs should be designed to provide direct protection for those at highest risk of disease,” declared Dr. Marshall, professor of vaccinology and deputy director of the Robinson Research Institute at the University of Adelaide.
In other words, Youths in the age groups at highest risk of disease – infants and adolescents – need to routinely receive the vaccine.
The B Part of It study, whose sheer scope and rigor drew the attention of infectious disease clinical trialists the world over, randomized nearly 35,000 students at all high schools in the state of South Australia – whether urban, rural, or remote – to two doses of the 4CMenB vaccine known as Bexsero or to a nonvaccinated control group. This massive trial entailed training more than 250 nurses in the study procedures and involved 3,100 miles of travel to transport oropharyngeal swab samples obtained from students in outlying areas for centralized laboratory analysis using real-time polymerase chain reaction with meningococcal genotyping, culture for N. meningitidis, and whole-genome sequencing. Samples were obtained on day 1 of the study and 12 months later.
The investigators created widespread regional enthusiasm for this project through adept use of social media and other methods. As a result, 99.5% of students randomized to the intervention arm received one dose, while 97% got two doses. A gratifying unintended consequence of the study was that parents who’d never previously vaccinated their children enrolled them in B Part of It, Dr. Marshall noted.
The impetus for B Part of It was that, while the Australian national health insurance program covers a single dose of meningococcal conjugate MenACWY vaccine given at age 12 months and 14-19 years, MenB vaccine isn’t covered because of uncertainties about cost effectiveness and the vaccine’s impact on meningococcal carriage and herd immunity. B Part of It was designed to resolve those uncertainties.
South Australia has the highest rate of invasive meningococcal disease in the country, and more than 80% of cases there are caused by meningococcal serogroup B. Moreover, 75% of group B cases in South Australia involve the nasty hypervirulent New Zealand strain known as CC 41/44.
The primary outcome in B Part of It was the difference in carriage of the major disease-causing serotypes – groups A, B, C, W, X, and Y – between vaccinated and unvaccinated students at the 1-year follow-up mark. The carriage prevalence of all N. meningitidis in the vaccinated students went from 2.8% at baseline to 4.0% at 12 months, and similarly from 2.6% to 4.7% in unvaccinated controls. More importantly, the prevalence of disease-causing genotypes rose from 1.3% at baseline to 2.4% at follow-up in the vaccinated subjects, with a near-identical pattern seen in controls, where the prevalence rose from 1.4% to 2.4%. In an as-treated analysis, the rate of acquisition of carriage of disease-causing genotypes was identical at 2.0% in both study arms.
The 4CMenB vaccine proved reassuringly safe and effective in preventing meningococcal disease in vaccinated teens. With more than 58,000 doses of the vaccine given in the study, no new safety concerns or signals emerged. And the observed number of cases of invasive meningococcal disease in South Australian adolescent vaccine recipients to date has been significantly lower than expected.
Secondary and exploratory outcomes
Independent risk factors associated with N. meningitidis carriage in the study participants at the 1-year mark included smoking cigarettes or hookah, intimate kissing within the last week, and being in grades 11-12, as opposed to grade 10.
The vaccine had no significant impact on the carriage rate of the hypervirulent New Zealand serogroup B strain. Nor was there a vaccine impact on carriage density, as Mark McMillan, MD, reported elsewhere at ESPID 2019. But while the 4CMenB vaccine had minimal impact upon N. meningitidis carriage density, it was associated with a significant 41% increase in the likelihood of cleared carriage of disease-causing strains at 12 months, added Dr. McMillan, Dr. Marshall’s coinvestigator at University of Adelaide.
What’s next
The ongoing B Part of It School Leaver study is assessing carriage prevalence in vaccinated versus unvaccinated high schoolers in their first year after graduating.
In addition, the B Part of It investigators plan to prospectively study the impact of the 4CMen B vaccine on N. gonorrhoeae disease in an effort to confirm the intriguing findings of an earlier large, retrospective New Zealand case-control study. The Kiwis found that recipients of an outer membrane vesicle MenB vaccine had an adjusted 31% reduction in the risk of gonorrhea. This was the first-ever report of any vaccine effectiveness against this major global public health problem, in which antibiotic resistance is a growing concern (Lancet. 2017 Sep 30;390[10102]:1603-10). Dr. Marshall reported receiving research funding from GlaxoSmithKline, which markets Bexsero and was the major financial supporter of the B Part of It study.
But wait a minute...
Following Dr. Marshall’s report on the B Part of It study, outgoing ESPID president Adam Finn, MD, PhD, presented longitudinal data that he believes raise the possibility that protein-antigen vaccines such as Bexsero, which promote naturally acquired mucosal immunity, may impact on transmission population wide without reliably preventing acquisition. This would stand in stark contrast to conjugate meningococcus vaccines, which have a well-established massive impact on carriage and acquisition of N. meningitidis.
It may be that in studying throat carriage rates once in individuals immunized 12 months earlier, as in the B Part of It study, investigators are not asking the right question, proposed Dr. Finn, professor of pediatrics at the University of Bristol (England).
His research team has been obtaining throat swabs at monthly intervals in a population of 917 high schoolers aged 16-17 years. In 416 of the students, they also have collected saliva samples weekly both before and after immunization with 4CMenB vaccine, analyzing the samples for N. meningitidis by polymerase chain reaction. This is a novel method of studying meningococcal carriage they have found to be both reliable and far more acceptable to patients than oropharyngeal swabbing, which adolescents balk at if asked to do with any frequency (PLoS One. 2019 Feb 11;14[2]:e0209905).
Dr. Finn said that their findings, which need confirmation, suggest that N. meningitidis carriage is usually brief and dynamic. They also have found that carriage density varies markedly from month to month.
“We see much higher-density carriage in the adolescent population in the early months of the year in conjunction, we think, with viral infection with influenza and so forth,” he said, adding that this could have clinical implications. “It feels sort of intuitive that someone walking around with 1,000 or 10,000 times as many meningococci in their throat is more likely to be more infectious to people around them with a very small number, although this hasn’t been formally proven.”
He hopes that the Be on the TEAM (Teenagers Against Meningitis) study will help provide answers. The study is randomizing 24,000 U.K. high school students to vaccination with the meningococcal B protein–antigen vaccines Bexsero or Trumenba or to no vaccine in order to learn if there are significant herd immunity effects.
Dr. Finn’s meningococcal carriage research is funded by the Meningitis Research Foundation and the National Institute for Health Research. Dr. Marshall reported receiving research funding from GlaxoSmithKline, the major sponsor of the B Part of It study.
LJUBLJANA, SLOVENIA – The 4CMenB vaccine didn’t affect carriage of disease-causing genogroups of Neisseria meningitidis in adolescents in the landmark Australian cluster-randomized trial of herd immunity known as the “B Part of It” study, Helen S. Marshall, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This was the largest-ever randomized trial of adolescents vaccinated against meningococcal disease, and the message, albeit somewhat disappointing, is clear: “MenB [Meningococcal serogroup B] vaccine programs should be designed to provide direct protection for those at highest risk of disease,” declared Dr. Marshall, professor of vaccinology and deputy director of the Robinson Research Institute at the University of Adelaide.
In other words, Youths in the age groups at highest risk of disease – infants and adolescents – need to routinely receive the vaccine.
The B Part of It study, whose sheer scope and rigor drew the attention of infectious disease clinical trialists the world over, randomized nearly 35,000 students at all high schools in the state of South Australia – whether urban, rural, or remote – to two doses of the 4CMenB vaccine known as Bexsero or to a nonvaccinated control group. This massive trial entailed training more than 250 nurses in the study procedures and involved 3,100 miles of travel to transport oropharyngeal swab samples obtained from students in outlying areas for centralized laboratory analysis using real-time polymerase chain reaction with meningococcal genotyping, culture for N. meningitidis, and whole-genome sequencing. Samples were obtained on day 1 of the study and 12 months later.
The investigators created widespread regional enthusiasm for this project through adept use of social media and other methods. As a result, 99.5% of students randomized to the intervention arm received one dose, while 97% got two doses. A gratifying unintended consequence of the study was that parents who’d never previously vaccinated their children enrolled them in B Part of It, Dr. Marshall noted.
The impetus for B Part of It was that, while the Australian national health insurance program covers a single dose of meningococcal conjugate MenACWY vaccine given at age 12 months and 14-19 years, MenB vaccine isn’t covered because of uncertainties about cost effectiveness and the vaccine’s impact on meningococcal carriage and herd immunity. B Part of It was designed to resolve those uncertainties.
South Australia has the highest rate of invasive meningococcal disease in the country, and more than 80% of cases there are caused by meningococcal serogroup B. Moreover, 75% of group B cases in South Australia involve the nasty hypervirulent New Zealand strain known as CC 41/44.
The primary outcome in B Part of It was the difference in carriage of the major disease-causing serotypes – groups A, B, C, W, X, and Y – between vaccinated and unvaccinated students at the 1-year follow-up mark. The carriage prevalence of all N. meningitidis in the vaccinated students went from 2.8% at baseline to 4.0% at 12 months, and similarly from 2.6% to 4.7% in unvaccinated controls. More importantly, the prevalence of disease-causing genotypes rose from 1.3% at baseline to 2.4% at follow-up in the vaccinated subjects, with a near-identical pattern seen in controls, where the prevalence rose from 1.4% to 2.4%. In an as-treated analysis, the rate of acquisition of carriage of disease-causing genotypes was identical at 2.0% in both study arms.
The 4CMenB vaccine proved reassuringly safe and effective in preventing meningococcal disease in vaccinated teens. With more than 58,000 doses of the vaccine given in the study, no new safety concerns or signals emerged. And the observed number of cases of invasive meningococcal disease in South Australian adolescent vaccine recipients to date has been significantly lower than expected.
Secondary and exploratory outcomes
Independent risk factors associated with N. meningitidis carriage in the study participants at the 1-year mark included smoking cigarettes or hookah, intimate kissing within the last week, and being in grades 11-12, as opposed to grade 10.
The vaccine had no significant impact on the carriage rate of the hypervirulent New Zealand serogroup B strain. Nor was there a vaccine impact on carriage density, as Mark McMillan, MD, reported elsewhere at ESPID 2019. But while the 4CMenB vaccine had minimal impact upon N. meningitidis carriage density, it was associated with a significant 41% increase in the likelihood of cleared carriage of disease-causing strains at 12 months, added Dr. McMillan, Dr. Marshall’s coinvestigator at University of Adelaide.
What’s next
The ongoing B Part of It School Leaver study is assessing carriage prevalence in vaccinated versus unvaccinated high schoolers in their first year after graduating.
In addition, the B Part of It investigators plan to prospectively study the impact of the 4CMen B vaccine on N. gonorrhoeae disease in an effort to confirm the intriguing findings of an earlier large, retrospective New Zealand case-control study. The Kiwis found that recipients of an outer membrane vesicle MenB vaccine had an adjusted 31% reduction in the risk of gonorrhea. This was the first-ever report of any vaccine effectiveness against this major global public health problem, in which antibiotic resistance is a growing concern (Lancet. 2017 Sep 30;390[10102]:1603-10). Dr. Marshall reported receiving research funding from GlaxoSmithKline, which markets Bexsero and was the major financial supporter of the B Part of It study.
But wait a minute...
Following Dr. Marshall’s report on the B Part of It study, outgoing ESPID president Adam Finn, MD, PhD, presented longitudinal data that he believes raise the possibility that protein-antigen vaccines such as Bexsero, which promote naturally acquired mucosal immunity, may impact on transmission population wide without reliably preventing acquisition. This would stand in stark contrast to conjugate meningococcus vaccines, which have a well-established massive impact on carriage and acquisition of N. meningitidis.
It may be that in studying throat carriage rates once in individuals immunized 12 months earlier, as in the B Part of It study, investigators are not asking the right question, proposed Dr. Finn, professor of pediatrics at the University of Bristol (England).
His research team has been obtaining throat swabs at monthly intervals in a population of 917 high schoolers aged 16-17 years. In 416 of the students, they also have collected saliva samples weekly both before and after immunization with 4CMenB vaccine, analyzing the samples for N. meningitidis by polymerase chain reaction. This is a novel method of studying meningococcal carriage they have found to be both reliable and far more acceptable to patients than oropharyngeal swabbing, which adolescents balk at if asked to do with any frequency (PLoS One. 2019 Feb 11;14[2]:e0209905).
Dr. Finn said that their findings, which need confirmation, suggest that N. meningitidis carriage is usually brief and dynamic. They also have found that carriage density varies markedly from month to month.
“We see much higher-density carriage in the adolescent population in the early months of the year in conjunction, we think, with viral infection with influenza and so forth,” he said, adding that this could have clinical implications. “It feels sort of intuitive that someone walking around with 1,000 or 10,000 times as many meningococci in their throat is more likely to be more infectious to people around them with a very small number, although this hasn’t been formally proven.”
He hopes that the Be on the TEAM (Teenagers Against Meningitis) study will help provide answers. The study is randomizing 24,000 U.K. high school students to vaccination with the meningococcal B protein–antigen vaccines Bexsero or Trumenba or to no vaccine in order to learn if there are significant herd immunity effects.
Dr. Finn’s meningococcal carriage research is funded by the Meningitis Research Foundation and the National Institute for Health Research. Dr. Marshall reported receiving research funding from GlaxoSmithKline, the major sponsor of the B Part of It study.
LJUBLJANA, SLOVENIA – The 4CMenB vaccine didn’t affect carriage of disease-causing genogroups of Neisseria meningitidis in adolescents in the landmark Australian cluster-randomized trial of herd immunity known as the “B Part of It” study, Helen S. Marshall, MD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This was the largest-ever randomized trial of adolescents vaccinated against meningococcal disease, and the message, albeit somewhat disappointing, is clear: “MenB [Meningococcal serogroup B] vaccine programs should be designed to provide direct protection for those at highest risk of disease,” declared Dr. Marshall, professor of vaccinology and deputy director of the Robinson Research Institute at the University of Adelaide.
In other words, Youths in the age groups at highest risk of disease – infants and adolescents – need to routinely receive the vaccine.
The B Part of It study, whose sheer scope and rigor drew the attention of infectious disease clinical trialists the world over, randomized nearly 35,000 students at all high schools in the state of South Australia – whether urban, rural, or remote – to two doses of the 4CMenB vaccine known as Bexsero or to a nonvaccinated control group. This massive trial entailed training more than 250 nurses in the study procedures and involved 3,100 miles of travel to transport oropharyngeal swab samples obtained from students in outlying areas for centralized laboratory analysis using real-time polymerase chain reaction with meningococcal genotyping, culture for N. meningitidis, and whole-genome sequencing. Samples were obtained on day 1 of the study and 12 months later.
The investigators created widespread regional enthusiasm for this project through adept use of social media and other methods. As a result, 99.5% of students randomized to the intervention arm received one dose, while 97% got two doses. A gratifying unintended consequence of the study was that parents who’d never previously vaccinated their children enrolled them in B Part of It, Dr. Marshall noted.
The impetus for B Part of It was that, while the Australian national health insurance program covers a single dose of meningococcal conjugate MenACWY vaccine given at age 12 months and 14-19 years, MenB vaccine isn’t covered because of uncertainties about cost effectiveness and the vaccine’s impact on meningococcal carriage and herd immunity. B Part of It was designed to resolve those uncertainties.
South Australia has the highest rate of invasive meningococcal disease in the country, and more than 80% of cases there are caused by meningococcal serogroup B. Moreover, 75% of group B cases in South Australia involve the nasty hypervirulent New Zealand strain known as CC 41/44.
The primary outcome in B Part of It was the difference in carriage of the major disease-causing serotypes – groups A, B, C, W, X, and Y – between vaccinated and unvaccinated students at the 1-year follow-up mark. The carriage prevalence of all N. meningitidis in the vaccinated students went from 2.8% at baseline to 4.0% at 12 months, and similarly from 2.6% to 4.7% in unvaccinated controls. More importantly, the prevalence of disease-causing genotypes rose from 1.3% at baseline to 2.4% at follow-up in the vaccinated subjects, with a near-identical pattern seen in controls, where the prevalence rose from 1.4% to 2.4%. In an as-treated analysis, the rate of acquisition of carriage of disease-causing genotypes was identical at 2.0% in both study arms.
The 4CMenB vaccine proved reassuringly safe and effective in preventing meningococcal disease in vaccinated teens. With more than 58,000 doses of the vaccine given in the study, no new safety concerns or signals emerged. And the observed number of cases of invasive meningococcal disease in South Australian adolescent vaccine recipients to date has been significantly lower than expected.
Secondary and exploratory outcomes
Independent risk factors associated with N. meningitidis carriage in the study participants at the 1-year mark included smoking cigarettes or hookah, intimate kissing within the last week, and being in grades 11-12, as opposed to grade 10.
The vaccine had no significant impact on the carriage rate of the hypervirulent New Zealand serogroup B strain. Nor was there a vaccine impact on carriage density, as Mark McMillan, MD, reported elsewhere at ESPID 2019. But while the 4CMenB vaccine had minimal impact upon N. meningitidis carriage density, it was associated with a significant 41% increase in the likelihood of cleared carriage of disease-causing strains at 12 months, added Dr. McMillan, Dr. Marshall’s coinvestigator at University of Adelaide.
What’s next
The ongoing B Part of It School Leaver study is assessing carriage prevalence in vaccinated versus unvaccinated high schoolers in their first year after graduating.
In addition, the B Part of It investigators plan to prospectively study the impact of the 4CMen B vaccine on N. gonorrhoeae disease in an effort to confirm the intriguing findings of an earlier large, retrospective New Zealand case-control study. The Kiwis found that recipients of an outer membrane vesicle MenB vaccine had an adjusted 31% reduction in the risk of gonorrhea. This was the first-ever report of any vaccine effectiveness against this major global public health problem, in which antibiotic resistance is a growing concern (Lancet. 2017 Sep 30;390[10102]:1603-10). Dr. Marshall reported receiving research funding from GlaxoSmithKline, which markets Bexsero and was the major financial supporter of the B Part of It study.
But wait a minute...
Following Dr. Marshall’s report on the B Part of It study, outgoing ESPID president Adam Finn, MD, PhD, presented longitudinal data that he believes raise the possibility that protein-antigen vaccines such as Bexsero, which promote naturally acquired mucosal immunity, may impact on transmission population wide without reliably preventing acquisition. This would stand in stark contrast to conjugate meningococcus vaccines, which have a well-established massive impact on carriage and acquisition of N. meningitidis.
It may be that in studying throat carriage rates once in individuals immunized 12 months earlier, as in the B Part of It study, investigators are not asking the right question, proposed Dr. Finn, professor of pediatrics at the University of Bristol (England).
His research team has been obtaining throat swabs at monthly intervals in a population of 917 high schoolers aged 16-17 years. In 416 of the students, they also have collected saliva samples weekly both before and after immunization with 4CMenB vaccine, analyzing the samples for N. meningitidis by polymerase chain reaction. This is a novel method of studying meningococcal carriage they have found to be both reliable and far more acceptable to patients than oropharyngeal swabbing, which adolescents balk at if asked to do with any frequency (PLoS One. 2019 Feb 11;14[2]:e0209905).
Dr. Finn said that their findings, which need confirmation, suggest that N. meningitidis carriage is usually brief and dynamic. They also have found that carriage density varies markedly from month to month.
“We see much higher-density carriage in the adolescent population in the early months of the year in conjunction, we think, with viral infection with influenza and so forth,” he said, adding that this could have clinical implications. “It feels sort of intuitive that someone walking around with 1,000 or 10,000 times as many meningococci in their throat is more likely to be more infectious to people around them with a very small number, although this hasn’t been formally proven.”
He hopes that the Be on the TEAM (Teenagers Against Meningitis) study will help provide answers. The study is randomizing 24,000 U.K. high school students to vaccination with the meningococcal B protein–antigen vaccines Bexsero or Trumenba or to no vaccine in order to learn if there are significant herd immunity effects.
Dr. Finn’s meningococcal carriage research is funded by the Meningitis Research Foundation and the National Institute for Health Research. Dr. Marshall reported receiving research funding from GlaxoSmithKline, the major sponsor of the B Part of It study.
REPORTING FROM ESPID 2019
New measles outbreak reported in western N.Y.
A new measles outbreak in western New York has affected five people within a Mennonite community, according to the New York State Department of Health.
The five cases in Wyoming County, located east of Buffalo, were reported Aug. 8 and no further cases have been confirmed as of Aug. 16, the county health department said on its website.
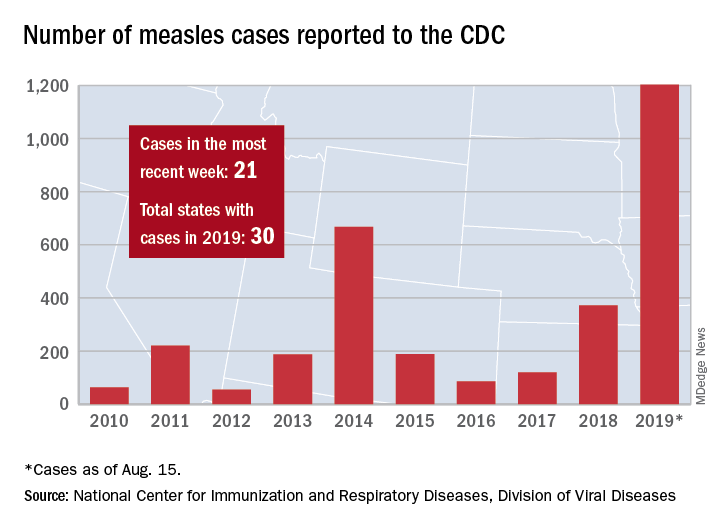
Those five cases, along with six new cases in Rockland County, N.Y., and 10 more around the country, brought the total for the Centers for Disease Control and Prevention’s latest reporting week to 21 and the total for the year to 1,203, the CDC said Aug. 19.
Along with Wyoming County and Rockland County (296 cases since Sept. 2018), the CDC currently is tracking outbreaks in New York City (653 cases since Sept. 2018), Washington state (85 cases in 2019; 13 in the current outbreak), California (65 cases in 2019; 5 in the current outbreak), and Texas (21 cases in 2019; 6 in the current outbreak).
“More than 75% of the cases this year are linked to outbreaks in New York and New York City,” the CDC said on its website, while also noting that “124 of the people who got measles this year were hospitalized, and 64 reported having complications, including pneumonia and encephalitis.”
A new measles outbreak in western New York has affected five people within a Mennonite community, according to the New York State Department of Health.

The five cases in Wyoming County, located east of Buffalo, were reported Aug. 8 and no further cases have been confirmed as of Aug. 16, the county health department said on its website.
Those five cases, along with six new cases in Rockland County, N.Y., and 10 more around the country, brought the total for the Centers for Disease Control and Prevention’s latest reporting week to 21 and the total for the year to 1,203, the CDC said Aug. 19.
Along with Wyoming County and Rockland County (296 cases since Sept. 2018), the CDC currently is tracking outbreaks in New York City (653 cases since Sept. 2018), Washington state (85 cases in 2019; 13 in the current outbreak), California (65 cases in 2019; 5 in the current outbreak), and Texas (21 cases in 2019; 6 in the current outbreak).
“More than 75% of the cases this year are linked to outbreaks in New York and New York City,” the CDC said on its website, while also noting that “124 of the people who got measles this year were hospitalized, and 64 reported having complications, including pneumonia and encephalitis.”
A new measles outbreak in western New York has affected five people within a Mennonite community, according to the New York State Department of Health.

The five cases in Wyoming County, located east of Buffalo, were reported Aug. 8 and no further cases have been confirmed as of Aug. 16, the county health department said on its website.
Those five cases, along with six new cases in Rockland County, N.Y., and 10 more around the country, brought the total for the Centers for Disease Control and Prevention’s latest reporting week to 21 and the total for the year to 1,203, the CDC said Aug. 19.
Along with Wyoming County and Rockland County (296 cases since Sept. 2018), the CDC currently is tracking outbreaks in New York City (653 cases since Sept. 2018), Washington state (85 cases in 2019; 13 in the current outbreak), California (65 cases in 2019; 5 in the current outbreak), and Texas (21 cases in 2019; 6 in the current outbreak).
“More than 75% of the cases this year are linked to outbreaks in New York and New York City,” the CDC said on its website, while also noting that “124 of the people who got measles this year were hospitalized, and 64 reported having complications, including pneumonia and encephalitis.”
Is your office ready for a case of measles?
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
Are rigid HPV vaccination schedules really necessary?
LJUBLJANA, SLOVENIA – Vladimir Gilca, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This novel observation from a post hoc analysis of two clinical trials conducted by the same research team has important potential implications for both clinical practice and public health, according to Dr. Gilca of the Quebec National Institute of Public Health and Laval University, Quebec City.
“A less rigid immunization schedule might facilitate the coadministration of HPV vaccine with other vaccines, such as meningococcal or Tdap, and reduce the number of vaccination visits. Also, our data support the decision to offer only one dose in cases of vaccine shortage, like we have presently in many countries around the world, with the possibility of giving the second dose several years later when the shortage is resolved,” he said.
He presented a comparison of anti-HPV geometric mean IgG antibody titers and their distribution in two clinical trials with serologic assays performed in the same lab using the same enzyme-linked immunosorbent assay procedures. In the first study, 173 boys and girls aged 9-10 years received two doses of a 9-valent HPV vaccine 6 months apart. In the second trial, 31 girls were vaccinated with one dose of a quadrivalent HPV vaccine at age 9-14 years and then received a dose of the 9-valent vaccine at a mean of 5.4 years and maximum of 8 years later. Blood samples were obtained before and 1 month after the second dose in both trials.
Despite the enormous differences in the time between the first and second doses in the two studies, 100% of subjects in both trials were seropositive to HPV 6, 11, 16, and 18, with similar geometric mean titers and titer distributions before dose number two. Moreover, 1 month after the second dose, the geometric mean titers jumped 40-91 times in study participants with a 6-month dosing interval, and similarly by 60-82 times in those with the far lengthier interval. Titer distributions after the second dose were equivalent in the two studies.
Dr. Gilca and coinvestigators looked at subgroups who received their second dose 3-4 years, 6, or 7-8 years after the first. The time difference didn’t affect the distribution of antibodies.
“We conclude that delayed administration of the second dose has no negative impact on the magnitude of the immune response,” he declared.
There are abundant precedents for this phenomenon of high immunogenicity of delayed doses of vaccine. Rabies, anthrax, hepatitis A and B, and tick-borne encephalitis vaccines have all been shown to elicit at least a similar magnitude of immune response after delayed administration of a second or third dose, compared with dosing at the guideline-recommended intervals, he noted.
Asked about the possible approach of giving just one dose of HPV vaccine, as was supported based upon retrospective data in a high-profile presentation earlier at ESPID 2019, Dr. Gilca replied, “The data we’ve seen so far show clinical noninferiority between one, two, and three doses. An approach that might be used by at least some countries is to give, for example, one dose of HPV vaccine in grade 4 and to then wait for confirmatory data about the efficacy of one dose, which we expect in the next 4-5 years. At least five or six clinical trials are ongoing on one dose versus two or three doses.”
He reported having no financial conflicts of interest regarding his presentation.
SOURCE: Gilca V et al. ESPID 2019, Abstract.
LJUBLJANA, SLOVENIA – Vladimir Gilca, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This novel observation from a post hoc analysis of two clinical trials conducted by the same research team has important potential implications for both clinical practice and public health, according to Dr. Gilca of the Quebec National Institute of Public Health and Laval University, Quebec City.
“A less rigid immunization schedule might facilitate the coadministration of HPV vaccine with other vaccines, such as meningococcal or Tdap, and reduce the number of vaccination visits. Also, our data support the decision to offer only one dose in cases of vaccine shortage, like we have presently in many countries around the world, with the possibility of giving the second dose several years later when the shortage is resolved,” he said.
He presented a comparison of anti-HPV geometric mean IgG antibody titers and their distribution in two clinical trials with serologic assays performed in the same lab using the same enzyme-linked immunosorbent assay procedures. In the first study, 173 boys and girls aged 9-10 years received two doses of a 9-valent HPV vaccine 6 months apart. In the second trial, 31 girls were vaccinated with one dose of a quadrivalent HPV vaccine at age 9-14 years and then received a dose of the 9-valent vaccine at a mean of 5.4 years and maximum of 8 years later. Blood samples were obtained before and 1 month after the second dose in both trials.
Despite the enormous differences in the time between the first and second doses in the two studies, 100% of subjects in both trials were seropositive to HPV 6, 11, 16, and 18, with similar geometric mean titers and titer distributions before dose number two. Moreover, 1 month after the second dose, the geometric mean titers jumped 40-91 times in study participants with a 6-month dosing interval, and similarly by 60-82 times in those with the far lengthier interval. Titer distributions after the second dose were equivalent in the two studies.
Dr. Gilca and coinvestigators looked at subgroups who received their second dose 3-4 years, 6, or 7-8 years after the first. The time difference didn’t affect the distribution of antibodies.
“We conclude that delayed administration of the second dose has no negative impact on the magnitude of the immune response,” he declared.
There are abundant precedents for this phenomenon of high immunogenicity of delayed doses of vaccine. Rabies, anthrax, hepatitis A and B, and tick-borne encephalitis vaccines have all been shown to elicit at least a similar magnitude of immune response after delayed administration of a second or third dose, compared with dosing at the guideline-recommended intervals, he noted.
Asked about the possible approach of giving just one dose of HPV vaccine, as was supported based upon retrospective data in a high-profile presentation earlier at ESPID 2019, Dr. Gilca replied, “The data we’ve seen so far show clinical noninferiority between one, two, and three doses. An approach that might be used by at least some countries is to give, for example, one dose of HPV vaccine in grade 4 and to then wait for confirmatory data about the efficacy of one dose, which we expect in the next 4-5 years. At least five or six clinical trials are ongoing on one dose versus two or three doses.”
He reported having no financial conflicts of interest regarding his presentation.
SOURCE: Gilca V et al. ESPID 2019, Abstract.
LJUBLJANA, SLOVENIA – Vladimir Gilca, MD, PhD, reported at the annual meeting of the European Society for Paediatric Infectious Diseases.
This novel observation from a post hoc analysis of two clinical trials conducted by the same research team has important potential implications for both clinical practice and public health, according to Dr. Gilca of the Quebec National Institute of Public Health and Laval University, Quebec City.
“A less rigid immunization schedule might facilitate the coadministration of HPV vaccine with other vaccines, such as meningococcal or Tdap, and reduce the number of vaccination visits. Also, our data support the decision to offer only one dose in cases of vaccine shortage, like we have presently in many countries around the world, with the possibility of giving the second dose several years later when the shortage is resolved,” he said.
He presented a comparison of anti-HPV geometric mean IgG antibody titers and their distribution in two clinical trials with serologic assays performed in the same lab using the same enzyme-linked immunosorbent assay procedures. In the first study, 173 boys and girls aged 9-10 years received two doses of a 9-valent HPV vaccine 6 months apart. In the second trial, 31 girls were vaccinated with one dose of a quadrivalent HPV vaccine at age 9-14 years and then received a dose of the 9-valent vaccine at a mean of 5.4 years and maximum of 8 years later. Blood samples were obtained before and 1 month after the second dose in both trials.
Despite the enormous differences in the time between the first and second doses in the two studies, 100% of subjects in both trials were seropositive to HPV 6, 11, 16, and 18, with similar geometric mean titers and titer distributions before dose number two. Moreover, 1 month after the second dose, the geometric mean titers jumped 40-91 times in study participants with a 6-month dosing interval, and similarly by 60-82 times in those with the far lengthier interval. Titer distributions after the second dose were equivalent in the two studies.
Dr. Gilca and coinvestigators looked at subgroups who received their second dose 3-4 years, 6, or 7-8 years after the first. The time difference didn’t affect the distribution of antibodies.
“We conclude that delayed administration of the second dose has no negative impact on the magnitude of the immune response,” he declared.
There are abundant precedents for this phenomenon of high immunogenicity of delayed doses of vaccine. Rabies, anthrax, hepatitis A and B, and tick-borne encephalitis vaccines have all been shown to elicit at least a similar magnitude of immune response after delayed administration of a second or third dose, compared with dosing at the guideline-recommended intervals, he noted.
Asked about the possible approach of giving just one dose of HPV vaccine, as was supported based upon retrospective data in a high-profile presentation earlier at ESPID 2019, Dr. Gilca replied, “The data we’ve seen so far show clinical noninferiority between one, two, and three doses. An approach that might be used by at least some countries is to give, for example, one dose of HPV vaccine in grade 4 and to then wait for confirmatory data about the efficacy of one dose, which we expect in the next 4-5 years. At least five or six clinical trials are ongoing on one dose versus two or three doses.”
He reported having no financial conflicts of interest regarding his presentation.
SOURCE: Gilca V et al. ESPID 2019, Abstract.
REPORTING FROM ESPID 2019
New RSV vaccine immunogenicity improved with protein engineering
Development of an effective respiratory syncytial virus (RSV) vaccine is feasible using a new technology that can contribute to development of other vaccines as well, according to results of a proof-of-concept study in Science.
The new method of protein engineering preserves the RSV antigen protein’s prefusion structure, including the epitope, thereby inducing antibodies that better “match,” and neutralize, the actual pathogen.
“Protein-based RSV vaccines have had a particularly complicated history, especially those in which the primary immunogen has been the fusion (F) glycoprotein, which exists in two major conformational states: prefusion (pre-F) and postfusion (post-F),” lead author Michelle Crank, MD, of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases in Bethesda, Md., and her colleagues explained in the paper.
Since the failure of the whole-inactivated RSV vaccine in the 1960s, researchers have focused on F subunit vaccine candidates, but these contain only post-F or “structurally undefined” F protein.
“Although the products are immunogenic, a substantial proportion of antibodies elicited are non- or poorly neutralizing, and field trials have shown no or minimal efficacy,” the authors wrote.
But now researchers have an “atomic-level understanding of F conformational states, antigenic sites, and the specificity of the human B cell repertoire and serum antibody response to infection.” Having developed a way to engineer proteins to retain the F protein’s prefusion conformation, the researchers developed the DS-Cav1 vaccine with an F protein from RSV subtype A.
In their phase 1, randomized, open-label clinical trial, the researchers tested the safety, tolerability and immunogenicity of DS-Cav1. The trial involved 90 healthy adults, aged 18-50, who had no abnormal findings in clinical lab tests, their medical history, or a physical exam.
The participants received two intramuscular doses, 12 weeks apart, of either 50 mcg, 150 mcg or 500 mcg of the vaccine. In each of these dosage groups, half the participants received a vaccine with 0.5 mcg of alum as an adjuvant, and half received a vaccine without any adjuvants. Each of the six randomized dosage-adjuvant groups had 15 participants.
The investigators report on safety and immunogenicity through 28 days after the first vaccine dose among the first 40 participants enrolled, each randomly assigned into four groups of 10 for the 50 mcg and 150 mcg doses with and without the adjuvant. Their primary immunogenicity endpoint was neutralizing activity from the vaccine.
Neutralizing activity with RSV A was seven times higher with 50 mcg and 12-15 times higher with 150 mcg at week 4 than at baseline (P less than .001).
“These increases in neutralizing activity were higher than those previously reported for F protein subunit vaccines and exceeded the threefold increase in neutralization reported after experimental human challenge with RSV,” the authors noted. Neutralization levels remained 5-10 times higher than baseline at week 12 (P less than .001).
Even with RSV B, neutralizing activity from DS-Cav1 was 4-6 times greater with 50 mcg and 9 times greater with 150 mcg, both with and without alum (P less than .001).
“The boost in neutralizing activity to subtype B after a single immunization with a subtype A–based F vaccine reflected the high conservation of F between subtypes and suggested that multiple prior infections by both RSV A and B subtypes establishes a broad preexisting B-cell repertoire,” the authors wrote.
The adjuvant had no clinically significant effect on immunogenicity, and no serious adverse events occurred in the groups.
The findings reveal that DS-Cav1 induces antibodies far more functionally effective than seen in previous RSV vaccines while opening the door to using similar techniques with other vaccines, the authors wrote. “We are now entering an era of vaccinology in which new technologies provide avenues to define the structural basis of antigenicity and to rapidly isolate and characterize human monoclonal antibodies,” the researchers wrote, marking “a step toward a future of precision vaccines.”
The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several of the study authors are inventors on patents for stabilizing the RSV F protein.
Development of an effective respiratory syncytial virus (RSV) vaccine is feasible using a new technology that can contribute to development of other vaccines as well, according to results of a proof-of-concept study in Science.
The new method of protein engineering preserves the RSV antigen protein’s prefusion structure, including the epitope, thereby inducing antibodies that better “match,” and neutralize, the actual pathogen.
“Protein-based RSV vaccines have had a particularly complicated history, especially those in which the primary immunogen has been the fusion (F) glycoprotein, which exists in two major conformational states: prefusion (pre-F) and postfusion (post-F),” lead author Michelle Crank, MD, of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases in Bethesda, Md., and her colleagues explained in the paper.
Since the failure of the whole-inactivated RSV vaccine in the 1960s, researchers have focused on F subunit vaccine candidates, but these contain only post-F or “structurally undefined” F protein.
“Although the products are immunogenic, a substantial proportion of antibodies elicited are non- or poorly neutralizing, and field trials have shown no or minimal efficacy,” the authors wrote.
But now researchers have an “atomic-level understanding of F conformational states, antigenic sites, and the specificity of the human B cell repertoire and serum antibody response to infection.” Having developed a way to engineer proteins to retain the F protein’s prefusion conformation, the researchers developed the DS-Cav1 vaccine with an F protein from RSV subtype A.
In their phase 1, randomized, open-label clinical trial, the researchers tested the safety, tolerability and immunogenicity of DS-Cav1. The trial involved 90 healthy adults, aged 18-50, who had no abnormal findings in clinical lab tests, their medical history, or a physical exam.
The participants received two intramuscular doses, 12 weeks apart, of either 50 mcg, 150 mcg or 500 mcg of the vaccine. In each of these dosage groups, half the participants received a vaccine with 0.5 mcg of alum as an adjuvant, and half received a vaccine without any adjuvants. Each of the six randomized dosage-adjuvant groups had 15 participants.
The investigators report on safety and immunogenicity through 28 days after the first vaccine dose among the first 40 participants enrolled, each randomly assigned into four groups of 10 for the 50 mcg and 150 mcg doses with and without the adjuvant. Their primary immunogenicity endpoint was neutralizing activity from the vaccine.
Neutralizing activity with RSV A was seven times higher with 50 mcg and 12-15 times higher with 150 mcg at week 4 than at baseline (P less than .001).
“These increases in neutralizing activity were higher than those previously reported for F protein subunit vaccines and exceeded the threefold increase in neutralization reported after experimental human challenge with RSV,” the authors noted. Neutralization levels remained 5-10 times higher than baseline at week 12 (P less than .001).
Even with RSV B, neutralizing activity from DS-Cav1 was 4-6 times greater with 50 mcg and 9 times greater with 150 mcg, both with and without alum (P less than .001).
“The boost in neutralizing activity to subtype B after a single immunization with a subtype A–based F vaccine reflected the high conservation of F between subtypes and suggested that multiple prior infections by both RSV A and B subtypes establishes a broad preexisting B-cell repertoire,” the authors wrote.
The adjuvant had no clinically significant effect on immunogenicity, and no serious adverse events occurred in the groups.
The findings reveal that DS-Cav1 induces antibodies far more functionally effective than seen in previous RSV vaccines while opening the door to using similar techniques with other vaccines, the authors wrote. “We are now entering an era of vaccinology in which new technologies provide avenues to define the structural basis of antigenicity and to rapidly isolate and characterize human monoclonal antibodies,” the researchers wrote, marking “a step toward a future of precision vaccines.”
The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several of the study authors are inventors on patents for stabilizing the RSV F protein.
Development of an effective respiratory syncytial virus (RSV) vaccine is feasible using a new technology that can contribute to development of other vaccines as well, according to results of a proof-of-concept study in Science.
The new method of protein engineering preserves the RSV antigen protein’s prefusion structure, including the epitope, thereby inducing antibodies that better “match,” and neutralize, the actual pathogen.
“Protein-based RSV vaccines have had a particularly complicated history, especially those in which the primary immunogen has been the fusion (F) glycoprotein, which exists in two major conformational states: prefusion (pre-F) and postfusion (post-F),” lead author Michelle Crank, MD, of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases in Bethesda, Md., and her colleagues explained in the paper.
Since the failure of the whole-inactivated RSV vaccine in the 1960s, researchers have focused on F subunit vaccine candidates, but these contain only post-F or “structurally undefined” F protein.
“Although the products are immunogenic, a substantial proportion of antibodies elicited are non- or poorly neutralizing, and field trials have shown no or minimal efficacy,” the authors wrote.
But now researchers have an “atomic-level understanding of F conformational states, antigenic sites, and the specificity of the human B cell repertoire and serum antibody response to infection.” Having developed a way to engineer proteins to retain the F protein’s prefusion conformation, the researchers developed the DS-Cav1 vaccine with an F protein from RSV subtype A.
In their phase 1, randomized, open-label clinical trial, the researchers tested the safety, tolerability and immunogenicity of DS-Cav1. The trial involved 90 healthy adults, aged 18-50, who had no abnormal findings in clinical lab tests, their medical history, or a physical exam.
The participants received two intramuscular doses, 12 weeks apart, of either 50 mcg, 150 mcg or 500 mcg of the vaccine. In each of these dosage groups, half the participants received a vaccine with 0.5 mcg of alum as an adjuvant, and half received a vaccine without any adjuvants. Each of the six randomized dosage-adjuvant groups had 15 participants.
The investigators report on safety and immunogenicity through 28 days after the first vaccine dose among the first 40 participants enrolled, each randomly assigned into four groups of 10 for the 50 mcg and 150 mcg doses with and without the adjuvant. Their primary immunogenicity endpoint was neutralizing activity from the vaccine.
Neutralizing activity with RSV A was seven times higher with 50 mcg and 12-15 times higher with 150 mcg at week 4 than at baseline (P less than .001).
“These increases in neutralizing activity were higher than those previously reported for F protein subunit vaccines and exceeded the threefold increase in neutralization reported after experimental human challenge with RSV,” the authors noted. Neutralization levels remained 5-10 times higher than baseline at week 12 (P less than .001).
Even with RSV B, neutralizing activity from DS-Cav1 was 4-6 times greater with 50 mcg and 9 times greater with 150 mcg, both with and without alum (P less than .001).
“The boost in neutralizing activity to subtype B after a single immunization with a subtype A–based F vaccine reflected the high conservation of F between subtypes and suggested that multiple prior infections by both RSV A and B subtypes establishes a broad preexisting B-cell repertoire,” the authors wrote.
The adjuvant had no clinically significant effect on immunogenicity, and no serious adverse events occurred in the groups.
The findings reveal that DS-Cav1 induces antibodies far more functionally effective than seen in previous RSV vaccines while opening the door to using similar techniques with other vaccines, the authors wrote. “We are now entering an era of vaccinology in which new technologies provide avenues to define the structural basis of antigenicity and to rapidly isolate and characterize human monoclonal antibodies,” the researchers wrote, marking “a step toward a future of precision vaccines.”
The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several of the study authors are inventors on patents for stabilizing the RSV F protein.
FROM SCIENCE
Key clinical point:
Major finding: Epitope-neutralizing activity is 5-10 times greater 12 weeks after baseline with a 50 mcg or 150 mcg with and without alum adjuvant.
Study details: The findings are based on a prespecified interim analysis of 90 healthy adult participants in a phase 1, randomized, trial of DS-Cav1.
Disclosures: The research was funded by the National Institutes of Health and the Bill & Melinda Gates Foundation. Several authors are inventors on patents for stabilizing the RSV F protein.
Source: Crank MC et al. Science. 2019;365(6452):505-9.
mRNA technology for respiratory vaccines impresses
LJUBLJANA, SLOVENIA – Encouraging safety and immunogenicity results reported from phase 1 studies of the first mRNA vaccines against the potentially pandemic H10N8 avian influenza and H7N9 influenza viruses suggest a bright future for what appears to be a breakthrough technology in vaccine development.
“We have developed an mRNA platform that has the potential to be quite applicable to the vaccine space. It’s an agile platform with the potential for relatively rapid development of vaccine antigen without the use of dedicated facilities, or growth in eggs, or insects, or mammalian cells,” Lori Panther, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We now have a platform that is relatively plug and play. If one has the mRNA sequence that you’re after to produce the protein that you’re after, it is a relatively repetitive process somewhat irrespective of the goal of the protein that you’re going to manufacture. We’re introducing an mRNA into our cellular machinery – the destination is the cellular ribosome – where it hopefully is able to be translated with fidelity into the target protein. Essentially it’s like the biological equivalent of a software hack for our own cells,” explained Dr. Panther, who is director of clinical development for infectious diseases at Moderna, in Cambridge, Mass.
Indeed, Moderna has numerous ongoing or recently completed phase 1 clinical trials of mRNA vaccines developed to protect against a raft of viral infections: respiratory syncytial virus, cytomegalovirus (NCT03382405), zika, chikungunya (NCT03829384), human metapneumovirus, and parainfluenza virus 3, as well as the aforementioned H10N8 and H7N9 influenza viruses. And an mRNA varicella zoster virus vaccine is in preclinical studies.
The mRNA vaccines closely mimic native viral infections, eliciting both B- and T-cell responses.
Moreover, the company also has ongoing phase 1 studies of mRNA-based cancer vaccines – therapies targeting solid tumors and lymphomas – as well as mRNA-directed increased production of relaxin as a treatment for heart failure and of vascular endothelial growth factor to treat myocardial ischemia.
“For the purposes of my company, the desired protein at this juncture could be an antibody, it could be a tumor antigen, it could be an enzyme that will replace an enzyme that’s lacking in somebody with an inborn error of metabolism. Or it could be a vaccine antigen target,” Dr. Panther said.
In addition to highlighting the results of the two phase 1 proof-of-concept studies of mRNA vaccines targeting the feared H10N8 and H7N9 influenza viruses, she presented interim results of an ongoing 1-year study of an mRNA vaccine that contains two antigens simultaneously targeting human metapneumovirus (hMPV) and parainfluenza virus 3 (PIV3).
“The rationale behind this study is that, taken together, these are two viruses that are responsible for a fair bit of disease burden in terms of lower respiratory tract infections and hospitalizations in children [younger] than 12 months of age, which will be the target population,” the infectious disease specialist noted.
The early positive results of the mRNA influenza vaccine studies were of particular interest to her audience of pediatric infectious disease specialists. Since the first human H7N9 infections were reported in China in 2013, five outbreaks have occurred involving more than 1,500 documented infections, resulting in more than 600 deaths. And ever since the virulent H10N8 avian influenza virus popped up on the radar in 2013, infectious disease physicians the world over have been waiting for the other shoe to drop.
There is obvious appeal to a novel, precise, and rapidly scalable technology such as that promised by intracellular delivery of mRNA in order to ramp up high-volume production of effective vaccines in the face of a looming pandemic threat. Elsewhere at the meeting, it was noted that, during the H1N1 pandemic of 2009, it took 6 months for the first vaccine doses to become available using current antiquated egg-based production methods. Another 2 months elapsed before the necessary millions of doses were produced.
The details of the two phase 1 studies of the mRNA vaccines against H7N9 and H10N8 influenza have recently been published (Vaccine. 2019 May 31;37[25]:3326-34). The vaccines, delivered in the conventional manner via injection into the deltoid muscle, were well tolerated, with the most common adverse events being the familiar ones: injection site pain, erythema, headache, fatigue, and myalgia. The immune response was robust and durable.
In response to an audience question, Dr. Panther said the mRNA vaccines are amenable to development as intranasal formulations.
The ongoing 12-month, phase 1, dose-ranging study of the mRNA hMPV/PIV3 virus vaccine includes 124 healthy adults at three U.S. sites who received two vaccinations on days 1 and 28. One month after a single vaccination, hMPV neutralizing antibody titers were 6.2-6.4 times those in the placebo arm; PIV3 neutralization titers were increased 3.3-fold. The second injection didn’t further boost antibody titers, suggesting that, at least in this study population of preexposed adults, a single vaccination is sufficient.
The use of mRNA technology has been a long time in coming. Dr. Panther explained why: “It’s a big trick to take an mRNA that by its own nature is a pretty fragile molecule and to get it past the degrading enzymes, like RNAses, that are out to chew it up immediately, and then to sneak it across the cellular membrane and into the cytoplasm, all the while avoiding the innate immune responses that exist solely to recognize RNA that looks foreign and chew it up.”
Moderna has accomplished this using a proprietary lipid nanoparticle delivery system.
“Essentially it’s a lipid shield that surrounds the mRNAs and ushers them past those enzymes and past the innate immune response that would otherwise destroy them,” according to Dr. Panther.
She and her colleagues believe they may eventually be able to change the nucleotide sequence of their manufactured mRNAs in order to expand the immunogenicity epitope and achieve a stronger immune response than would result from natural infection.
LJUBLJANA, SLOVENIA – Encouraging safety and immunogenicity results reported from phase 1 studies of the first mRNA vaccines against the potentially pandemic H10N8 avian influenza and H7N9 influenza viruses suggest a bright future for what appears to be a breakthrough technology in vaccine development.
“We have developed an mRNA platform that has the potential to be quite applicable to the vaccine space. It’s an agile platform with the potential for relatively rapid development of vaccine antigen without the use of dedicated facilities, or growth in eggs, or insects, or mammalian cells,” Lori Panther, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We now have a platform that is relatively plug and play. If one has the mRNA sequence that you’re after to produce the protein that you’re after, it is a relatively repetitive process somewhat irrespective of the goal of the protein that you’re going to manufacture. We’re introducing an mRNA into our cellular machinery – the destination is the cellular ribosome – where it hopefully is able to be translated with fidelity into the target protein. Essentially it’s like the biological equivalent of a software hack for our own cells,” explained Dr. Panther, who is director of clinical development for infectious diseases at Moderna, in Cambridge, Mass.
Indeed, Moderna has numerous ongoing or recently completed phase 1 clinical trials of mRNA vaccines developed to protect against a raft of viral infections: respiratory syncytial virus, cytomegalovirus (NCT03382405), zika, chikungunya (NCT03829384), human metapneumovirus, and parainfluenza virus 3, as well as the aforementioned H10N8 and H7N9 influenza viruses. And an mRNA varicella zoster virus vaccine is in preclinical studies.
The mRNA vaccines closely mimic native viral infections, eliciting both B- and T-cell responses.
Moreover, the company also has ongoing phase 1 studies of mRNA-based cancer vaccines – therapies targeting solid tumors and lymphomas – as well as mRNA-directed increased production of relaxin as a treatment for heart failure and of vascular endothelial growth factor to treat myocardial ischemia.
“For the purposes of my company, the desired protein at this juncture could be an antibody, it could be a tumor antigen, it could be an enzyme that will replace an enzyme that’s lacking in somebody with an inborn error of metabolism. Or it could be a vaccine antigen target,” Dr. Panther said.
In addition to highlighting the results of the two phase 1 proof-of-concept studies of mRNA vaccines targeting the feared H10N8 and H7N9 influenza viruses, she presented interim results of an ongoing 1-year study of an mRNA vaccine that contains two antigens simultaneously targeting human metapneumovirus (hMPV) and parainfluenza virus 3 (PIV3).
“The rationale behind this study is that, taken together, these are two viruses that are responsible for a fair bit of disease burden in terms of lower respiratory tract infections and hospitalizations in children [younger] than 12 months of age, which will be the target population,” the infectious disease specialist noted.
The early positive results of the mRNA influenza vaccine studies were of particular interest to her audience of pediatric infectious disease specialists. Since the first human H7N9 infections were reported in China in 2013, five outbreaks have occurred involving more than 1,500 documented infections, resulting in more than 600 deaths. And ever since the virulent H10N8 avian influenza virus popped up on the radar in 2013, infectious disease physicians the world over have been waiting for the other shoe to drop.
There is obvious appeal to a novel, precise, and rapidly scalable technology such as that promised by intracellular delivery of mRNA in order to ramp up high-volume production of effective vaccines in the face of a looming pandemic threat. Elsewhere at the meeting, it was noted that, during the H1N1 pandemic of 2009, it took 6 months for the first vaccine doses to become available using current antiquated egg-based production methods. Another 2 months elapsed before the necessary millions of doses were produced.
The details of the two phase 1 studies of the mRNA vaccines against H7N9 and H10N8 influenza have recently been published (Vaccine. 2019 May 31;37[25]:3326-34). The vaccines, delivered in the conventional manner via injection into the deltoid muscle, were well tolerated, with the most common adverse events being the familiar ones: injection site pain, erythema, headache, fatigue, and myalgia. The immune response was robust and durable.
In response to an audience question, Dr. Panther said the mRNA vaccines are amenable to development as intranasal formulations.
The ongoing 12-month, phase 1, dose-ranging study of the mRNA hMPV/PIV3 virus vaccine includes 124 healthy adults at three U.S. sites who received two vaccinations on days 1 and 28. One month after a single vaccination, hMPV neutralizing antibody titers were 6.2-6.4 times those in the placebo arm; PIV3 neutralization titers were increased 3.3-fold. The second injection didn’t further boost antibody titers, suggesting that, at least in this study population of preexposed adults, a single vaccination is sufficient.
The use of mRNA technology has been a long time in coming. Dr. Panther explained why: “It’s a big trick to take an mRNA that by its own nature is a pretty fragile molecule and to get it past the degrading enzymes, like RNAses, that are out to chew it up immediately, and then to sneak it across the cellular membrane and into the cytoplasm, all the while avoiding the innate immune responses that exist solely to recognize RNA that looks foreign and chew it up.”
Moderna has accomplished this using a proprietary lipid nanoparticle delivery system.
“Essentially it’s a lipid shield that surrounds the mRNAs and ushers them past those enzymes and past the innate immune response that would otherwise destroy them,” according to Dr. Panther.
She and her colleagues believe they may eventually be able to change the nucleotide sequence of their manufactured mRNAs in order to expand the immunogenicity epitope and achieve a stronger immune response than would result from natural infection.
LJUBLJANA, SLOVENIA – Encouraging safety and immunogenicity results reported from phase 1 studies of the first mRNA vaccines against the potentially pandemic H10N8 avian influenza and H7N9 influenza viruses suggest a bright future for what appears to be a breakthrough technology in vaccine development.
“We have developed an mRNA platform that has the potential to be quite applicable to the vaccine space. It’s an agile platform with the potential for relatively rapid development of vaccine antigen without the use of dedicated facilities, or growth in eggs, or insects, or mammalian cells,” Lori Panther, MD, said at the annual meeting of the European Society for Paediatric Infectious Diseases.
“We now have a platform that is relatively plug and play. If one has the mRNA sequence that you’re after to produce the protein that you’re after, it is a relatively repetitive process somewhat irrespective of the goal of the protein that you’re going to manufacture. We’re introducing an mRNA into our cellular machinery – the destination is the cellular ribosome – where it hopefully is able to be translated with fidelity into the target protein. Essentially it’s like the biological equivalent of a software hack for our own cells,” explained Dr. Panther, who is director of clinical development for infectious diseases at Moderna, in Cambridge, Mass.
Indeed, Moderna has numerous ongoing or recently completed phase 1 clinical trials of mRNA vaccines developed to protect against a raft of viral infections: respiratory syncytial virus, cytomegalovirus (NCT03382405), zika, chikungunya (NCT03829384), human metapneumovirus, and parainfluenza virus 3, as well as the aforementioned H10N8 and H7N9 influenza viruses. And an mRNA varicella zoster virus vaccine is in preclinical studies.
The mRNA vaccines closely mimic native viral infections, eliciting both B- and T-cell responses.
Moreover, the company also has ongoing phase 1 studies of mRNA-based cancer vaccines – therapies targeting solid tumors and lymphomas – as well as mRNA-directed increased production of relaxin as a treatment for heart failure and of vascular endothelial growth factor to treat myocardial ischemia.
“For the purposes of my company, the desired protein at this juncture could be an antibody, it could be a tumor antigen, it could be an enzyme that will replace an enzyme that’s lacking in somebody with an inborn error of metabolism. Or it could be a vaccine antigen target,” Dr. Panther said.
In addition to highlighting the results of the two phase 1 proof-of-concept studies of mRNA vaccines targeting the feared H10N8 and H7N9 influenza viruses, she presented interim results of an ongoing 1-year study of an mRNA vaccine that contains two antigens simultaneously targeting human metapneumovirus (hMPV) and parainfluenza virus 3 (PIV3).
“The rationale behind this study is that, taken together, these are two viruses that are responsible for a fair bit of disease burden in terms of lower respiratory tract infections and hospitalizations in children [younger] than 12 months of age, which will be the target population,” the infectious disease specialist noted.
The early positive results of the mRNA influenza vaccine studies were of particular interest to her audience of pediatric infectious disease specialists. Since the first human H7N9 infections were reported in China in 2013, five outbreaks have occurred involving more than 1,500 documented infections, resulting in more than 600 deaths. And ever since the virulent H10N8 avian influenza virus popped up on the radar in 2013, infectious disease physicians the world over have been waiting for the other shoe to drop.
There is obvious appeal to a novel, precise, and rapidly scalable technology such as that promised by intracellular delivery of mRNA in order to ramp up high-volume production of effective vaccines in the face of a looming pandemic threat. Elsewhere at the meeting, it was noted that, during the H1N1 pandemic of 2009, it took 6 months for the first vaccine doses to become available using current antiquated egg-based production methods. Another 2 months elapsed before the necessary millions of doses were produced.
The details of the two phase 1 studies of the mRNA vaccines against H7N9 and H10N8 influenza have recently been published (Vaccine. 2019 May 31;37[25]:3326-34). The vaccines, delivered in the conventional manner via injection into the deltoid muscle, were well tolerated, with the most common adverse events being the familiar ones: injection site pain, erythema, headache, fatigue, and myalgia. The immune response was robust and durable.
In response to an audience question, Dr. Panther said the mRNA vaccines are amenable to development as intranasal formulations.
The ongoing 12-month, phase 1, dose-ranging study of the mRNA hMPV/PIV3 virus vaccine includes 124 healthy adults at three U.S. sites who received two vaccinations on days 1 and 28. One month after a single vaccination, hMPV neutralizing antibody titers were 6.2-6.4 times those in the placebo arm; PIV3 neutralization titers were increased 3.3-fold. The second injection didn’t further boost antibody titers, suggesting that, at least in this study population of preexposed adults, a single vaccination is sufficient.
The use of mRNA technology has been a long time in coming. Dr. Panther explained why: “It’s a big trick to take an mRNA that by its own nature is a pretty fragile molecule and to get it past the degrading enzymes, like RNAses, that are out to chew it up immediately, and then to sneak it across the cellular membrane and into the cytoplasm, all the while avoiding the innate immune responses that exist solely to recognize RNA that looks foreign and chew it up.”
Moderna has accomplished this using a proprietary lipid nanoparticle delivery system.
“Essentially it’s a lipid shield that surrounds the mRNAs and ushers them past those enzymes and past the innate immune response that would otherwise destroy them,” according to Dr. Panther.
She and her colleagues believe they may eventually be able to change the nucleotide sequence of their manufactured mRNAs in order to expand the immunogenicity epitope and achieve a stronger immune response than would result from natural infection.
REPORTING FROM ESPID 2019
Vaccination is not associated with increased risk of MS
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
The analysis by Hapfelmeier et al. provides important evidence that vaccinations are not associated with multiple sclerosis (MS), said E. Ann Yeh, MD, a neurologist at the Hospital for Sick Children in Toronto, and Jennifer Graves, MD, PhD, a neurologist at the University of California, San Diego, in an accompanying editorial. On the contrary, the evidence supports a potential protective effect of vaccines on the risk of developing MS, they said.
“The reasons for this [finding] cannot be gleaned from this study and may range from biological to sociocultural/demographic reasons,” the authors added. “Infection, rather than vaccination, may be an MS trigger, or individuals obtaining vaccinations may be practicing other healthy behaviors protective for MS. These possibilities should be the subject of future studies.”
Until future studies are completed and their results published, the findings of Hapfelmeier et al. offer “strong evidence to share with worried patients and families when faced with the question of whether a vaccine in the recent or relatively distant past triggered the individual’s MS,” said Dr. Yeh and Dr. Graves.
The authors had various relationships with industry, including serving on advisory boards for and receiving funding from pharmaceutical companies.
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
(MS), according to an analysis published July 30 in Neurology. Although the results suggest that vaccination is associated with a lower likelihood of incident MS within the following 5 years, “these data alone do not allow for any conclusion regarding a possible protective effect of vaccinations regarding the development of MS,” wrote Alexander Hapfelmeier, PhD, of the Technical University of Munich and colleagues.
In recent years, researchers have proposed and investigated various potential environmental risk factors for the development of MS. Vaccination is one proposed environmental risk factor, but case reports and small studies have yielded conflicting results about its association with incident MS.
To examine this question more closely, Dr. Hapfelmeier and colleagues performed a systematic retrospective analysis of ambulatory claims data held by the Bavarian Association of Statutory Health Insurance Physicians. They reviewed the data to identify patients with new-onset MS and at least two ICD-10 diagnoses of the disorder. They next identified two control cohorts of participants diagnosed with other autoimmune diseases: Crohn’s disease and psoriasis. Finally, they randomly selected a third control cohort of patients without any of these diagnoses and matched them by age, sex, and district to patients with MS in a 5:1 ratio. Eligible participants were younger than 70 years.
Dr. Hapfelmeier and colleagues reviewed the incidence and frequency of vaccinations (such as those targeting tick-borne encephalitis, human papillomavirus, and influenza virus) in all cohorts. They created unconditional logistic regression models to assess the association between vaccination and MS. They also created separate models to contrast the MS cohort with each of the control cohorts.
The researchers included 12,262 patients with MS, 19,296 patients with Crohn’s disease, 112,292 patients with psoriasis, and 79,185 participants without these autoimmune diseases in their analysis. They found 456 participants with Crohn’s disease and psoriasis, 216 participants with MS and psoriasis, 48 participants with Crohn’s disease and MS, and 2 participants with Crohn’s disease, psoriasis, and MS. Dr. Hapfelmeier and colleagues allocated these participants to each of the respective cohorts and did not analyze them differently because of the comparatively small sample sizes.
The investigators analyzed the occurrence of vaccination in all participants during the 5 years before first diagnosis. Among patients who received vaccination, the odds ratio of MS was 0.870 in participants without autoimmune disease, 0.919 in participants with Crohn’s disease, and 0.973 in participants with psoriasis. Decreased risk of MS was most notable for vaccinations against influenza and tick-borne encephalitis. The results were consistent regardless of time frame, control cohort, and definition of MS.
The subjective definition of the MS cohort was a limitation of the study, but the authors addressed it by also using several strict definitions of that cohort. Another limitation is that the source data may reflect entry errors and incorrect coding.
A grant from the German Federal Ministry of Education and Research Competence Network MS supported the study. The authors had no conflicts that were relevant to the topic of the study.
SOURCE: Hapfelmeier A et al. Neurology. 2019 Jul 30. doi: 10.1212/WNL.0000000000008012.
FROM NEUROLOGY
Shoulder Injury Related to Vaccine Administration: A Rare Reaction
Localized reactions and transient pain at the site of vaccine administration are frequent and well-described occurrences that are typically short-lived and mild in nature. The most common findings at the injection site are soreness, erythema, and edema.1 Although less common, generalized shoulder dysfunction after vaccine administration also has been reported. Bodor and colleagues described a peri-articular inflammatory response that led to shoulder pain and weakness.2 A single case report by Kuether and colleagues described atraumatic osteonecrosis of the humeral head after H1N1 vaccine administration in the deltoid.3 In 2010, shoulder injury related to vaccine administration (SIRVA) was described by Atanasoff and colleagues as the rapid onset of shoulder pain and dysfunction persisting as a complication of deltoid muscle vaccination in a case series of 13 patients.4 In our report, we present a case of an active-duty male eventually diagnosed with SIRVA after influenza vaccination and discuss factors that may prevent vaccine-related shoulder injuries.
Case Presentation
A 31-year-old active-duty male presented to the Allergy clinic for evaluation of persistent left shoulder pain and decreased range of motion (ROM) following influenza vaccination 4 months prior. He reported a history of chronic low back and right shoulder pain. Although the patient had a traumatic injury to his right shoulder, which was corrected with surgery, he had no surgeries on the left shoulder. He reported no prior pain or known trauma to his left shoulder. He had no personal or family history of atopy or vaccine reactions.
The patient weighed 91 kg and received an intramuscular (IM) quadrivalent influenza vaccine with a 25-gauge, 1-inch needle during a mass influenza immunization. He recalled that the site of vaccination was slightly more than 3 cm below the top of the shoulder in a region correlating to the left deltoid. The vaccine was administered while he was standing with his arm extended, adducted, and internally rotated. The patient experienced intense pain immediately after the vaccination and noted decreased ROM. Initially, he dismissed the pain and decreased ROM as routine but sought medical attention when there was no improvement after 3 weeks.
Six weeks after the onset of symptoms, a magnetic resonance image (MRI) revealed tendinopathy of the left distal subscapularis, infraspinatus, supraspinatus, and teres minor tendon. These findings were suggestive of a small partial thickness tear of the supraspinatus (Figure 1), possible calcific tendinopathy of the distal teres minor (Figure 2), and underlying humeral head edema (Figure 3). The patient was evaluated by Orthopedics and experienced no relief from ibuprofen, celecoxib, and a steroid/lidocaine intra-articular injection. Laboratory studies included an unremarkable complete blood count and erythrocyte sedimentation rate. He was diagnosed with SIRVA and continued in physical therapy with incomplete resolution of symptoms 6 months postvaccination.
Discussion
According to a 2018 report issued by the Centers for Disease Control and Prevention, local reactions following immunizations are seen in up to 80% of administered vaccine doses.1 While most of these reactions are mild, transient, cutaneous reactions, rarely these also may persist and impact quality of life significantly. SIRVA is one such process that can lead to persistent musculoskeletal dysfunction. SIRVA presents as shoulder pain and limited ROM that occurs after the administration of an injectable vaccine. In 2011, the Institute of Medicine determined that evidence supported a causal relationship between vaccine administration and deltoid bursitis.5
In 2017, SIRVA was included in the Vaccine Injury Compensation Program (VICP), a federal program that can provide compensation to individuals injured by certain vaccines.6 A diagnosis of SIRVA can be considered in patients who experience pain within 48 hours of vaccination, have no prior history of pain or dysfunction of the affected shoulder prior to vaccine administration, and have symptoms limited to the shoulder in which the vaccine was administered where no other abnormality is present to explain these symptoms (eg, brachial neuritis, other neuropathy). Currently, patients with back pain or musculoskeletal complaints that do not include the shoulder following deltoid vaccination do not meet the reporting criteria for SIRVA in the VICP.6
The exact prevalence or incidence of SIRVA is unknown. In a 2017 systematic review of the literature and the Spanish Pharmacovigilance System database, Martín Arias and colleagues found 45 cases of new onset, unilateral shoulder dysfunction without associated neuropathy or autoimmune conditions following vaccine administration. They noted a female to male predominance (71.1% vs 28.9%) with a mean age of 53.6 years (range 22-89 y). Most of the cases occurred following influenza vaccine (62%); pneumococcal vaccine was the next most common (13%).7 Shoulder injury also has been reported after tetanus-diphtheria toxoids, human papilloma virus, and hepatitis A virus vaccines.4,7 The review noted that all patients had onset of pain within the first week following vaccination with the majority (81%) having pain in the first 24 hours. Two cases found in the Spanish database had pain onset 2 months postvaccination.7 Atanasoff and colleagues found that 93% of patients had pain onset within 24 hours of vaccination with 54% reporting immediate pain.4
The Vaccine Adverse Event Reporting System (VAERS) tracks reports of shoulder dysfunction following certain vaccinations, but the system is unable to establish causality. According to VAERS reporting, between 2010 and 2016, there were 1006 possible reports of shoulder dysfunction following inactivated influenza vaccination (IIV) compared with an estimated 130 million doses of IIV given each influenza season in the US.8
Bodor and Montalvo postulated that vaccine antigen was being over penetrated into the synovial space of the shoulder, as the subdeltoid/subacromial bursa is located a mere 0.8 to 1.6 cm below the skin surface in patients with healthy body mass index.2 Atanasoff and colleagues expounded that antibodies from previous vaccination or natural infection may then form antigen-antibody complexes, creating prolonged local immune and inflammatory responses leading to bursitis or tendonitis.4 Martín Arias and colleagues hypothesized that improper injection technique, including wrong insertion angle, incorrect needle type/size, and failure to account for the patient’s physical characteristics were the most likely causes of SIRVA.7
Proper vaccine administration ensures that vaccinations are delivered in a safe and efficacious manner. Safe vaccination practices include the use of trained personnel who receive comprehensive, competency-based training regarding vaccine administration.1 Aspiration prior to an injection is a practice that has not been evaluated fully. Given that the 2 routinely recommended locations for IM vaccines (deltoid muscle in adults or vastus lateralis muscle in infants) lack large blood vessels, the practice of aspiration prior to an IM vaccine is not currently deemed necessary.1 Additional safe vaccine practices include the selection of appropriate needle length for muscle penetration and that anatomic landmarks determine the location of vaccination.1 Despite this, in a survey of 100 medical professionals, half could not name any structure at risk from improper deltoid vaccination technique.9
Cook and colleagues used anthropomorphic data to evaluate the potential for injury to the subdeltoid/subacromial bursa and/or the axillary nerve.10 Based on these data, they recommended safe IM vaccine administration can be assured by using the midpoint of the deltoid muscle located midway between the acromion and deltoid tuberosity with the arm abducted to 60°.10,11 In 46% of SIRVA cases described by Atanasoff and colleagues, patients reported that the vaccine was administered “too high.”4 The study also recommended that the clinician and the patient be in the seated position to ensure proper needle angle and location of administration.4 For most adults, a 1-inch needle is appropriate for vaccine administration in the deltoid; however, in females weighing < 70 kg and males < 75 kg, a 5/8-inch needle is recommended to avoid injury.7
Our 91-kg patient was appropriately administered his vaccine with a 1-inch needle. As he experienced immediate pain, it is unlikely that his symptoms were due to an immune-mediated process, as this would not be expected to occur immediately. Improper location of vaccine administration is a proposed mechanism of injury for our patient, though this cannot be confirmed by history alone. His prior history of traumatic injury to the opposite shoulder could represent a confounding factor as no prior imaging was available for the vaccine-affected shoulder. A preexisting shoulder abnormality or injury cannot be completely excluded, and it is possible that an underlying prior shoulder injury was aggravated postvaccination.
Evaluation and Treatment
There is no standardized approach for the evaluation of SIRVA to date. Awareness of SIRVA and a high index of suspicion are necessary to evaluate patients with shoulder concerns postvaccination. Laboratory evaluation should be considered to evaluate for other potential diagnoses (eg, infection, rheumatologic concerns). Routine X-rays are not helpful in cases of SIRVA. Ultrasound may be considered as it can show bursa abnormalities consistent with bursitis.2 MRI of the affected shoulder may provide improved diagnostic capability if SIRVA is suspected. MRI findings vary but include intraosseous edema, bursitis, tendonitis, and rotator cuff tears.4,12 Complete rotator cuff tears were found in 15% of cases reviewed by Atanasoff and colleagues.4 While there is no recommended timing for MRI, 63% of MRIs were performed within 3 months of symptom onset.4 As SIRVA is not a neurologic injury, nerve conduction, electromyographic studies, and neurologic evaluation or testing are expected to be normal.
Treatment of SIRVA and other vaccine-related shoulder injuries typically have involved pain management (eg, nonsteroidal anti-inflammatory agents), intra-articular steroid injections, and physical therapy, though some patients never experience complete resolution of symptoms.2,4,7 Both patients with vaccination-related shoulder dysfunction described by Bodor and colleagues improved after intra-articular triamcinolone injections, with up to 3 injections before complete resolution of pain in one patient.2 Orthopedics evaluation may need to be considered for persistent symptoms. According to Atanasoff and colleagues, most patients were symptomatic for at least 6 months, and complete recovery was seen in less than one-third of patients.4 Although the development of SIRVA is not a contraindication to future doses of the presumed causative vaccine, subsequent vaccination should include careful consideration of other administration sites if possible (eg, vastus lateralis may be used for IM injections in adults) (Figure 4).
Reporting
A diagnosis or concern for SIRVA also should be reported to the VAERS, the national database established in order to detect possible safety problems with US-licensed vaccines. VAERS reports can be submitted by anyone with concerns for vaccine adverse reactions, including patients, caregivers, and health care professionals at vaers.hhs.gov/reportevent.html. Additional information regarding VICP can be obtained at www.hrsa.gov/vaccine-compensation/index.html.
Military-Specific Issues
The military values readiness, which includes ensuring that active-duty members remain up-to-date on life-saving vaccinations. Immunization is of critical importance to mobility and success of the overall mission. Mobility processing lines where immunizations can be provided to multiple active-duty members can be a successful strategy for mass immunizations. Although the quick administration of immunizations maintains readiness and provides a medically necessary service, it also may increase the chances of incorrect vaccine placement in the deltoid, causing long-term shoulder immobility that may impact a service member’s retainability. The benefits of mobility processing lines can continue to outweigh the risks of immunization administration by ensuring proper staff training, seating both the administrator and recipient of vaccination, and selecting a proper needle length and site of administration specific to each recipient.
Conclusion
Correct administration of vaccines is of utmost importance in preventing SIRVA and other vaccine-related shoulder dysfunctions. Proper staff training and refresher training can help prevent vaccine-related shoulder injuries. Additionally, clinicians should be aware of this potential complication and maintain a high index of suspicion when evaluating patients with postvaccination shoulder complaints.
1. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. https://www.cdc.gov/vaccines/pubs/pinkbook/vac-admin.html. Published 2015. Accessed June 3, 2019.
2. Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007;25(4):585-587.
3. Kuether G, Dietrich B, Smith T, Peter C, Gruessner S. Atraumatic osteonecrosis of the humeral head after influenza A-(H1N1) v-2009 vaccination. Vaccine. 2011;29(40):6830-6833.
4. Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28(51):8049-8052.
5. Institute of Medicine. Adverse effects of vaccines: evidence and causality. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2011/Adverse-Effects-of-Vaccines-Evidence-and-Causality/Vaccine-report-brief-FINAL.pdf. Published August 2011. Accessed June 3, 2019.
6. Health Resources and Services Administration, Health and Human Services Administration. National vaccine injury compensation program: revisions to the vaccine injury table. https://www.federalregister.gov/documents/2017/01/19/2017-00701/national-vaccine-injury-compensation-program-revisions-to-the-vaccine-injury-table. Published January 19, 2017. Accessed June 3, 2019.
7. Martín Arias LH, Sanz Fadrique R, Sáinz Gil M, Salgueiro-Vazquez ME. Risk of bursitis and other injuries and dysfunctions of the shoulder following vaccinations. Vaccine. 2017;35(37):4870-4876.
8. Centers for Disease Control and Prevention. Reports of shoulder dysfunction following inactivated influenza vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2010-2016. https://stacks.cdc.gov/view/cdc/57624. Published January 4, 2018. Accessed June 3, 2019.
9. McGarvey MA, Hooper AC. The deltoid intramuscular injection site in the adult. Current practice among general practitioners and practice nurses. Ir Med J. 2005;98(4):105-107.
10. Cook IF. An evidence based protocol for the prevention of upper arm injury related to vaccine administration (UAIRVA). Hum Vaccin. 2011;7(8):845-848.
11. Cook IF. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccin Immunother. 2015;11(5):1184-1191.
12. Okur G, Chaney KA, Lomasney LM. Magnetic resonance imaging of abnormal shoulder pain following influenza vaccination. Skeletal Radiol. 2014;43(9):1325-1331.
Localized reactions and transient pain at the site of vaccine administration are frequent and well-described occurrences that are typically short-lived and mild in nature. The most common findings at the injection site are soreness, erythema, and edema.1 Although less common, generalized shoulder dysfunction after vaccine administration also has been reported. Bodor and colleagues described a peri-articular inflammatory response that led to shoulder pain and weakness.2 A single case report by Kuether and colleagues described atraumatic osteonecrosis of the humeral head after H1N1 vaccine administration in the deltoid.3 In 2010, shoulder injury related to vaccine administration (SIRVA) was described by Atanasoff and colleagues as the rapid onset of shoulder pain and dysfunction persisting as a complication of deltoid muscle vaccination in a case series of 13 patients.4 In our report, we present a case of an active-duty male eventually diagnosed with SIRVA after influenza vaccination and discuss factors that may prevent vaccine-related shoulder injuries.
Case Presentation
A 31-year-old active-duty male presented to the Allergy clinic for evaluation of persistent left shoulder pain and decreased range of motion (ROM) following influenza vaccination 4 months prior. He reported a history of chronic low back and right shoulder pain. Although the patient had a traumatic injury to his right shoulder, which was corrected with surgery, he had no surgeries on the left shoulder. He reported no prior pain or known trauma to his left shoulder. He had no personal or family history of atopy or vaccine reactions.
The patient weighed 91 kg and received an intramuscular (IM) quadrivalent influenza vaccine with a 25-gauge, 1-inch needle during a mass influenza immunization. He recalled that the site of vaccination was slightly more than 3 cm below the top of the shoulder in a region correlating to the left deltoid. The vaccine was administered while he was standing with his arm extended, adducted, and internally rotated. The patient experienced intense pain immediately after the vaccination and noted decreased ROM. Initially, he dismissed the pain and decreased ROM as routine but sought medical attention when there was no improvement after 3 weeks.
Six weeks after the onset of symptoms, a magnetic resonance image (MRI) revealed tendinopathy of the left distal subscapularis, infraspinatus, supraspinatus, and teres minor tendon. These findings were suggestive of a small partial thickness tear of the supraspinatus (Figure 1), possible calcific tendinopathy of the distal teres minor (Figure 2), and underlying humeral head edema (Figure 3). The patient was evaluated by Orthopedics and experienced no relief from ibuprofen, celecoxib, and a steroid/lidocaine intra-articular injection. Laboratory studies included an unremarkable complete blood count and erythrocyte sedimentation rate. He was diagnosed with SIRVA and continued in physical therapy with incomplete resolution of symptoms 6 months postvaccination.
Discussion
According to a 2018 report issued by the Centers for Disease Control and Prevention, local reactions following immunizations are seen in up to 80% of administered vaccine doses.1 While most of these reactions are mild, transient, cutaneous reactions, rarely these also may persist and impact quality of life significantly. SIRVA is one such process that can lead to persistent musculoskeletal dysfunction. SIRVA presents as shoulder pain and limited ROM that occurs after the administration of an injectable vaccine. In 2011, the Institute of Medicine determined that evidence supported a causal relationship between vaccine administration and deltoid bursitis.5
In 2017, SIRVA was included in the Vaccine Injury Compensation Program (VICP), a federal program that can provide compensation to individuals injured by certain vaccines.6 A diagnosis of SIRVA can be considered in patients who experience pain within 48 hours of vaccination, have no prior history of pain or dysfunction of the affected shoulder prior to vaccine administration, and have symptoms limited to the shoulder in which the vaccine was administered where no other abnormality is present to explain these symptoms (eg, brachial neuritis, other neuropathy). Currently, patients with back pain or musculoskeletal complaints that do not include the shoulder following deltoid vaccination do not meet the reporting criteria for SIRVA in the VICP.6
The exact prevalence or incidence of SIRVA is unknown. In a 2017 systematic review of the literature and the Spanish Pharmacovigilance System database, Martín Arias and colleagues found 45 cases of new onset, unilateral shoulder dysfunction without associated neuropathy or autoimmune conditions following vaccine administration. They noted a female to male predominance (71.1% vs 28.9%) with a mean age of 53.6 years (range 22-89 y). Most of the cases occurred following influenza vaccine (62%); pneumococcal vaccine was the next most common (13%).7 Shoulder injury also has been reported after tetanus-diphtheria toxoids, human papilloma virus, and hepatitis A virus vaccines.4,7 The review noted that all patients had onset of pain within the first week following vaccination with the majority (81%) having pain in the first 24 hours. Two cases found in the Spanish database had pain onset 2 months postvaccination.7 Atanasoff and colleagues found that 93% of patients had pain onset within 24 hours of vaccination with 54% reporting immediate pain.4
The Vaccine Adverse Event Reporting System (VAERS) tracks reports of shoulder dysfunction following certain vaccinations, but the system is unable to establish causality. According to VAERS reporting, between 2010 and 2016, there were 1006 possible reports of shoulder dysfunction following inactivated influenza vaccination (IIV) compared with an estimated 130 million doses of IIV given each influenza season in the US.8
Bodor and Montalvo postulated that vaccine antigen was being over penetrated into the synovial space of the shoulder, as the subdeltoid/subacromial bursa is located a mere 0.8 to 1.6 cm below the skin surface in patients with healthy body mass index.2 Atanasoff and colleagues expounded that antibodies from previous vaccination or natural infection may then form antigen-antibody complexes, creating prolonged local immune and inflammatory responses leading to bursitis or tendonitis.4 Martín Arias and colleagues hypothesized that improper injection technique, including wrong insertion angle, incorrect needle type/size, and failure to account for the patient’s physical characteristics were the most likely causes of SIRVA.7
Proper vaccine administration ensures that vaccinations are delivered in a safe and efficacious manner. Safe vaccination practices include the use of trained personnel who receive comprehensive, competency-based training regarding vaccine administration.1 Aspiration prior to an injection is a practice that has not been evaluated fully. Given that the 2 routinely recommended locations for IM vaccines (deltoid muscle in adults or vastus lateralis muscle in infants) lack large blood vessels, the practice of aspiration prior to an IM vaccine is not currently deemed necessary.1 Additional safe vaccine practices include the selection of appropriate needle length for muscle penetration and that anatomic landmarks determine the location of vaccination.1 Despite this, in a survey of 100 medical professionals, half could not name any structure at risk from improper deltoid vaccination technique.9
Cook and colleagues used anthropomorphic data to evaluate the potential for injury to the subdeltoid/subacromial bursa and/or the axillary nerve.10 Based on these data, they recommended safe IM vaccine administration can be assured by using the midpoint of the deltoid muscle located midway between the acromion and deltoid tuberosity with the arm abducted to 60°.10,11 In 46% of SIRVA cases described by Atanasoff and colleagues, patients reported that the vaccine was administered “too high.”4 The study also recommended that the clinician and the patient be in the seated position to ensure proper needle angle and location of administration.4 For most adults, a 1-inch needle is appropriate for vaccine administration in the deltoid; however, in females weighing < 70 kg and males < 75 kg, a 5/8-inch needle is recommended to avoid injury.7
Our 91-kg patient was appropriately administered his vaccine with a 1-inch needle. As he experienced immediate pain, it is unlikely that his symptoms were due to an immune-mediated process, as this would not be expected to occur immediately. Improper location of vaccine administration is a proposed mechanism of injury for our patient, though this cannot be confirmed by history alone. His prior history of traumatic injury to the opposite shoulder could represent a confounding factor as no prior imaging was available for the vaccine-affected shoulder. A preexisting shoulder abnormality or injury cannot be completely excluded, and it is possible that an underlying prior shoulder injury was aggravated postvaccination.
Evaluation and Treatment
There is no standardized approach for the evaluation of SIRVA to date. Awareness of SIRVA and a high index of suspicion are necessary to evaluate patients with shoulder concerns postvaccination. Laboratory evaluation should be considered to evaluate for other potential diagnoses (eg, infection, rheumatologic concerns). Routine X-rays are not helpful in cases of SIRVA. Ultrasound may be considered as it can show bursa abnormalities consistent with bursitis.2 MRI of the affected shoulder may provide improved diagnostic capability if SIRVA is suspected. MRI findings vary but include intraosseous edema, bursitis, tendonitis, and rotator cuff tears.4,12 Complete rotator cuff tears were found in 15% of cases reviewed by Atanasoff and colleagues.4 While there is no recommended timing for MRI, 63% of MRIs were performed within 3 months of symptom onset.4 As SIRVA is not a neurologic injury, nerve conduction, electromyographic studies, and neurologic evaluation or testing are expected to be normal.
Treatment of SIRVA and other vaccine-related shoulder injuries typically have involved pain management (eg, nonsteroidal anti-inflammatory agents), intra-articular steroid injections, and physical therapy, though some patients never experience complete resolution of symptoms.2,4,7 Both patients with vaccination-related shoulder dysfunction described by Bodor and colleagues improved after intra-articular triamcinolone injections, with up to 3 injections before complete resolution of pain in one patient.2 Orthopedics evaluation may need to be considered for persistent symptoms. According to Atanasoff and colleagues, most patients were symptomatic for at least 6 months, and complete recovery was seen in less than one-third of patients.4 Although the development of SIRVA is not a contraindication to future doses of the presumed causative vaccine, subsequent vaccination should include careful consideration of other administration sites if possible (eg, vastus lateralis may be used for IM injections in adults) (Figure 4).
Reporting
A diagnosis or concern for SIRVA also should be reported to the VAERS, the national database established in order to detect possible safety problems with US-licensed vaccines. VAERS reports can be submitted by anyone with concerns for vaccine adverse reactions, including patients, caregivers, and health care professionals at vaers.hhs.gov/reportevent.html. Additional information regarding VICP can be obtained at www.hrsa.gov/vaccine-compensation/index.html.
Military-Specific Issues
The military values readiness, which includes ensuring that active-duty members remain up-to-date on life-saving vaccinations. Immunization is of critical importance to mobility and success of the overall mission. Mobility processing lines where immunizations can be provided to multiple active-duty members can be a successful strategy for mass immunizations. Although the quick administration of immunizations maintains readiness and provides a medically necessary service, it also may increase the chances of incorrect vaccine placement in the deltoid, causing long-term shoulder immobility that may impact a service member’s retainability. The benefits of mobility processing lines can continue to outweigh the risks of immunization administration by ensuring proper staff training, seating both the administrator and recipient of vaccination, and selecting a proper needle length and site of administration specific to each recipient.
Conclusion
Correct administration of vaccines is of utmost importance in preventing SIRVA and other vaccine-related shoulder dysfunctions. Proper staff training and refresher training can help prevent vaccine-related shoulder injuries. Additionally, clinicians should be aware of this potential complication and maintain a high index of suspicion when evaluating patients with postvaccination shoulder complaints.
Localized reactions and transient pain at the site of vaccine administration are frequent and well-described occurrences that are typically short-lived and mild in nature. The most common findings at the injection site are soreness, erythema, and edema.1 Although less common, generalized shoulder dysfunction after vaccine administration also has been reported. Bodor and colleagues described a peri-articular inflammatory response that led to shoulder pain and weakness.2 A single case report by Kuether and colleagues described atraumatic osteonecrosis of the humeral head after H1N1 vaccine administration in the deltoid.3 In 2010, shoulder injury related to vaccine administration (SIRVA) was described by Atanasoff and colleagues as the rapid onset of shoulder pain and dysfunction persisting as a complication of deltoid muscle vaccination in a case series of 13 patients.4 In our report, we present a case of an active-duty male eventually diagnosed with SIRVA after influenza vaccination and discuss factors that may prevent vaccine-related shoulder injuries.
Case Presentation
A 31-year-old active-duty male presented to the Allergy clinic for evaluation of persistent left shoulder pain and decreased range of motion (ROM) following influenza vaccination 4 months prior. He reported a history of chronic low back and right shoulder pain. Although the patient had a traumatic injury to his right shoulder, which was corrected with surgery, he had no surgeries on the left shoulder. He reported no prior pain or known trauma to his left shoulder. He had no personal or family history of atopy or vaccine reactions.
The patient weighed 91 kg and received an intramuscular (IM) quadrivalent influenza vaccine with a 25-gauge, 1-inch needle during a mass influenza immunization. He recalled that the site of vaccination was slightly more than 3 cm below the top of the shoulder in a region correlating to the left deltoid. The vaccine was administered while he was standing with his arm extended, adducted, and internally rotated. The patient experienced intense pain immediately after the vaccination and noted decreased ROM. Initially, he dismissed the pain and decreased ROM as routine but sought medical attention when there was no improvement after 3 weeks.
Six weeks after the onset of symptoms, a magnetic resonance image (MRI) revealed tendinopathy of the left distal subscapularis, infraspinatus, supraspinatus, and teres minor tendon. These findings were suggestive of a small partial thickness tear of the supraspinatus (Figure 1), possible calcific tendinopathy of the distal teres minor (Figure 2), and underlying humeral head edema (Figure 3). The patient was evaluated by Orthopedics and experienced no relief from ibuprofen, celecoxib, and a steroid/lidocaine intra-articular injection. Laboratory studies included an unremarkable complete blood count and erythrocyte sedimentation rate. He was diagnosed with SIRVA and continued in physical therapy with incomplete resolution of symptoms 6 months postvaccination.
Discussion
According to a 2018 report issued by the Centers for Disease Control and Prevention, local reactions following immunizations are seen in up to 80% of administered vaccine doses.1 While most of these reactions are mild, transient, cutaneous reactions, rarely these also may persist and impact quality of life significantly. SIRVA is one such process that can lead to persistent musculoskeletal dysfunction. SIRVA presents as shoulder pain and limited ROM that occurs after the administration of an injectable vaccine. In 2011, the Institute of Medicine determined that evidence supported a causal relationship between vaccine administration and deltoid bursitis.5
In 2017, SIRVA was included in the Vaccine Injury Compensation Program (VICP), a federal program that can provide compensation to individuals injured by certain vaccines.6 A diagnosis of SIRVA can be considered in patients who experience pain within 48 hours of vaccination, have no prior history of pain or dysfunction of the affected shoulder prior to vaccine administration, and have symptoms limited to the shoulder in which the vaccine was administered where no other abnormality is present to explain these symptoms (eg, brachial neuritis, other neuropathy). Currently, patients with back pain or musculoskeletal complaints that do not include the shoulder following deltoid vaccination do not meet the reporting criteria for SIRVA in the VICP.6
The exact prevalence or incidence of SIRVA is unknown. In a 2017 systematic review of the literature and the Spanish Pharmacovigilance System database, Martín Arias and colleagues found 45 cases of new onset, unilateral shoulder dysfunction without associated neuropathy or autoimmune conditions following vaccine administration. They noted a female to male predominance (71.1% vs 28.9%) with a mean age of 53.6 years (range 22-89 y). Most of the cases occurred following influenza vaccine (62%); pneumococcal vaccine was the next most common (13%).7 Shoulder injury also has been reported after tetanus-diphtheria toxoids, human papilloma virus, and hepatitis A virus vaccines.4,7 The review noted that all patients had onset of pain within the first week following vaccination with the majority (81%) having pain in the first 24 hours. Two cases found in the Spanish database had pain onset 2 months postvaccination.7 Atanasoff and colleagues found that 93% of patients had pain onset within 24 hours of vaccination with 54% reporting immediate pain.4
The Vaccine Adverse Event Reporting System (VAERS) tracks reports of shoulder dysfunction following certain vaccinations, but the system is unable to establish causality. According to VAERS reporting, between 2010 and 2016, there were 1006 possible reports of shoulder dysfunction following inactivated influenza vaccination (IIV) compared with an estimated 130 million doses of IIV given each influenza season in the US.8
Bodor and Montalvo postulated that vaccine antigen was being over penetrated into the synovial space of the shoulder, as the subdeltoid/subacromial bursa is located a mere 0.8 to 1.6 cm below the skin surface in patients with healthy body mass index.2 Atanasoff and colleagues expounded that antibodies from previous vaccination or natural infection may then form antigen-antibody complexes, creating prolonged local immune and inflammatory responses leading to bursitis or tendonitis.4 Martín Arias and colleagues hypothesized that improper injection technique, including wrong insertion angle, incorrect needle type/size, and failure to account for the patient’s physical characteristics were the most likely causes of SIRVA.7
Proper vaccine administration ensures that vaccinations are delivered in a safe and efficacious manner. Safe vaccination practices include the use of trained personnel who receive comprehensive, competency-based training regarding vaccine administration.1 Aspiration prior to an injection is a practice that has not been evaluated fully. Given that the 2 routinely recommended locations for IM vaccines (deltoid muscle in adults or vastus lateralis muscle in infants) lack large blood vessels, the practice of aspiration prior to an IM vaccine is not currently deemed necessary.1 Additional safe vaccine practices include the selection of appropriate needle length for muscle penetration and that anatomic landmarks determine the location of vaccination.1 Despite this, in a survey of 100 medical professionals, half could not name any structure at risk from improper deltoid vaccination technique.9
Cook and colleagues used anthropomorphic data to evaluate the potential for injury to the subdeltoid/subacromial bursa and/or the axillary nerve.10 Based on these data, they recommended safe IM vaccine administration can be assured by using the midpoint of the deltoid muscle located midway between the acromion and deltoid tuberosity with the arm abducted to 60°.10,11 In 46% of SIRVA cases described by Atanasoff and colleagues, patients reported that the vaccine was administered “too high.”4 The study also recommended that the clinician and the patient be in the seated position to ensure proper needle angle and location of administration.4 For most adults, a 1-inch needle is appropriate for vaccine administration in the deltoid; however, in females weighing < 70 kg and males < 75 kg, a 5/8-inch needle is recommended to avoid injury.7
Our 91-kg patient was appropriately administered his vaccine with a 1-inch needle. As he experienced immediate pain, it is unlikely that his symptoms were due to an immune-mediated process, as this would not be expected to occur immediately. Improper location of vaccine administration is a proposed mechanism of injury for our patient, though this cannot be confirmed by history alone. His prior history of traumatic injury to the opposite shoulder could represent a confounding factor as no prior imaging was available for the vaccine-affected shoulder. A preexisting shoulder abnormality or injury cannot be completely excluded, and it is possible that an underlying prior shoulder injury was aggravated postvaccination.
Evaluation and Treatment
There is no standardized approach for the evaluation of SIRVA to date. Awareness of SIRVA and a high index of suspicion are necessary to evaluate patients with shoulder concerns postvaccination. Laboratory evaluation should be considered to evaluate for other potential diagnoses (eg, infection, rheumatologic concerns). Routine X-rays are not helpful in cases of SIRVA. Ultrasound may be considered as it can show bursa abnormalities consistent with bursitis.2 MRI of the affected shoulder may provide improved diagnostic capability if SIRVA is suspected. MRI findings vary but include intraosseous edema, bursitis, tendonitis, and rotator cuff tears.4,12 Complete rotator cuff tears were found in 15% of cases reviewed by Atanasoff and colleagues.4 While there is no recommended timing for MRI, 63% of MRIs were performed within 3 months of symptom onset.4 As SIRVA is not a neurologic injury, nerve conduction, electromyographic studies, and neurologic evaluation or testing are expected to be normal.
Treatment of SIRVA and other vaccine-related shoulder injuries typically have involved pain management (eg, nonsteroidal anti-inflammatory agents), intra-articular steroid injections, and physical therapy, though some patients never experience complete resolution of symptoms.2,4,7 Both patients with vaccination-related shoulder dysfunction described by Bodor and colleagues improved after intra-articular triamcinolone injections, with up to 3 injections before complete resolution of pain in one patient.2 Orthopedics evaluation may need to be considered for persistent symptoms. According to Atanasoff and colleagues, most patients were symptomatic for at least 6 months, and complete recovery was seen in less than one-third of patients.4 Although the development of SIRVA is not a contraindication to future doses of the presumed causative vaccine, subsequent vaccination should include careful consideration of other administration sites if possible (eg, vastus lateralis may be used for IM injections in adults) (Figure 4).
Reporting
A diagnosis or concern for SIRVA also should be reported to the VAERS, the national database established in order to detect possible safety problems with US-licensed vaccines. VAERS reports can be submitted by anyone with concerns for vaccine adverse reactions, including patients, caregivers, and health care professionals at vaers.hhs.gov/reportevent.html. Additional information regarding VICP can be obtained at www.hrsa.gov/vaccine-compensation/index.html.
Military-Specific Issues
The military values readiness, which includes ensuring that active-duty members remain up-to-date on life-saving vaccinations. Immunization is of critical importance to mobility and success of the overall mission. Mobility processing lines where immunizations can be provided to multiple active-duty members can be a successful strategy for mass immunizations. Although the quick administration of immunizations maintains readiness and provides a medically necessary service, it also may increase the chances of incorrect vaccine placement in the deltoid, causing long-term shoulder immobility that may impact a service member’s retainability. The benefits of mobility processing lines can continue to outweigh the risks of immunization administration by ensuring proper staff training, seating both the administrator and recipient of vaccination, and selecting a proper needle length and site of administration specific to each recipient.
Conclusion
Correct administration of vaccines is of utmost importance in preventing SIRVA and other vaccine-related shoulder dysfunctions. Proper staff training and refresher training can help prevent vaccine-related shoulder injuries. Additionally, clinicians should be aware of this potential complication and maintain a high index of suspicion when evaluating patients with postvaccination shoulder complaints.
1. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. https://www.cdc.gov/vaccines/pubs/pinkbook/vac-admin.html. Published 2015. Accessed June 3, 2019.
2. Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007;25(4):585-587.
3. Kuether G, Dietrich B, Smith T, Peter C, Gruessner S. Atraumatic osteonecrosis of the humeral head after influenza A-(H1N1) v-2009 vaccination. Vaccine. 2011;29(40):6830-6833.
4. Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28(51):8049-8052.
5. Institute of Medicine. Adverse effects of vaccines: evidence and causality. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2011/Adverse-Effects-of-Vaccines-Evidence-and-Causality/Vaccine-report-brief-FINAL.pdf. Published August 2011. Accessed June 3, 2019.
6. Health Resources and Services Administration, Health and Human Services Administration. National vaccine injury compensation program: revisions to the vaccine injury table. https://www.federalregister.gov/documents/2017/01/19/2017-00701/national-vaccine-injury-compensation-program-revisions-to-the-vaccine-injury-table. Published January 19, 2017. Accessed June 3, 2019.
7. Martín Arias LH, Sanz Fadrique R, Sáinz Gil M, Salgueiro-Vazquez ME. Risk of bursitis and other injuries and dysfunctions of the shoulder following vaccinations. Vaccine. 2017;35(37):4870-4876.
8. Centers for Disease Control and Prevention. Reports of shoulder dysfunction following inactivated influenza vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2010-2016. https://stacks.cdc.gov/view/cdc/57624. Published January 4, 2018. Accessed June 3, 2019.
9. McGarvey MA, Hooper AC. The deltoid intramuscular injection site in the adult. Current practice among general practitioners and practice nurses. Ir Med J. 2005;98(4):105-107.
10. Cook IF. An evidence based protocol for the prevention of upper arm injury related to vaccine administration (UAIRVA). Hum Vaccin. 2011;7(8):845-848.
11. Cook IF. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccin Immunother. 2015;11(5):1184-1191.
12. Okur G, Chaney KA, Lomasney LM. Magnetic resonance imaging of abnormal shoulder pain following influenza vaccination. Skeletal Radiol. 2014;43(9):1325-1331.
1. Centers for Disease Control and Prevention. Epidemiology and prevention of vaccine-preventable diseases. https://www.cdc.gov/vaccines/pubs/pinkbook/vac-admin.html. Published 2015. Accessed June 3, 2019.
2. Bodor M, Montalvo E. Vaccination-related shoulder dysfunction. Vaccine. 2007;25(4):585-587.
3. Kuether G, Dietrich B, Smith T, Peter C, Gruessner S. Atraumatic osteonecrosis of the humeral head after influenza A-(H1N1) v-2009 vaccination. Vaccine. 2011;29(40):6830-6833.
4. Atanasoff S, Ryan T, Lightfoot R, Johann-Liang R. Shoulder injury related to vaccine administration (SIRVA). Vaccine. 2010;28(51):8049-8052.
5. Institute of Medicine. Adverse effects of vaccines: evidence and causality. http://www.nationalacademies.org/hmd/~/media/Files/Report%20Files/2011/Adverse-Effects-of-Vaccines-Evidence-and-Causality/Vaccine-report-brief-FINAL.pdf. Published August 2011. Accessed June 3, 2019.
6. Health Resources and Services Administration, Health and Human Services Administration. National vaccine injury compensation program: revisions to the vaccine injury table. https://www.federalregister.gov/documents/2017/01/19/2017-00701/national-vaccine-injury-compensation-program-revisions-to-the-vaccine-injury-table. Published January 19, 2017. Accessed June 3, 2019.
7. Martín Arias LH, Sanz Fadrique R, Sáinz Gil M, Salgueiro-Vazquez ME. Risk of bursitis and other injuries and dysfunctions of the shoulder following vaccinations. Vaccine. 2017;35(37):4870-4876.
8. Centers for Disease Control and Prevention. Reports of shoulder dysfunction following inactivated influenza vaccine in the Vaccine Adverse Event Reporting System (VAERS), 2010-2016. https://stacks.cdc.gov/view/cdc/57624. Published January 4, 2018. Accessed June 3, 2019.
9. McGarvey MA, Hooper AC. The deltoid intramuscular injection site in the adult. Current practice among general practitioners and practice nurses. Ir Med J. 2005;98(4):105-107.
10. Cook IF. An evidence based protocol for the prevention of upper arm injury related to vaccine administration (UAIRVA). Hum Vaccin. 2011;7(8):845-848.
11. Cook IF. Best vaccination practice and medically attended injection site events following deltoid intramuscular injection. Hum Vaccin Immunother. 2015;11(5):1184-1191.
12. Okur G, Chaney KA, Lomasney LM. Magnetic resonance imaging of abnormal shoulder pain following influenza vaccination. Skeletal Radiol. 2014;43(9):1325-1331.