User login
DAPT duration: How low can you go?
DENVER – Six months of dual-antiplatelet therapy proved equivalent in terms of safety, efficacy, and bleeding risk to the guideline-recommended standard 12 months in ST-elevation MI patients after primary PCI with a second-generation drug-eluting stent in the randomized DAPT-STEMI trial.
“This trial, for the first time, showed that in the modern DES [drug-eluting stent] era, event-free STEMI patients do not benefit from a prolonged DAPT beyond 6 months, as currently recommended, and sets the stage for further dedicated research in this important topic,” Elvin Kedhi, MD, PhD, declared in presenting the DAPT-STEMI results at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The final analysis took place at 24 months post STEMI; that is, 18 months post randomization. Among the 861 completers, the composite primary outcome of death, MI, revascularization, stroke, and major bleeding during months 6-24 occurred in 4.8% of the SAPT group, a 27% relative risk reduction compared with the 6.6% rate in the DAPT group. Thus, 6 months of DAPT met the prespecified endpoint of noninferiority compared to the standard 12 months of DAPT, reported Dr. Kedhi, head of interventional cardiology and clinical research and innovation at the Isala Heart Center in Zwolle, The Netherlands.
The secondary composite endpoint of death, MI, stroke, stent thrombosis, or TIMI major bleeding occurred in 3.2% of the SAPT group and 4.3% of the DAPT group, for a 25% relative risk reduction.
All individual components of the composite endpoints occurred at the same or lower rate in the SAPT group compared with the DAPT arm, he noted at the meeting, which was sponsored by the Cardiovascular Research Foundation.
At a press conference where Dr. Kedhi presented the DAPT-STEMI results, discussant Dean J. Kereiakes, MD, explained why he didn’t find the study results surprising.
Press conference moderator Gary S. Mintz, MD, put the DAPT-STEMI findings in perspective: “The need for DAPT has decreased along with all the stent-related complications. There’s always been a greater focus on DAPT for preventing events and a relatively lesser focus on the adverse consequences of DAPT. And anybody who’s a clinician who takes care of patients knows that drug-related bleeding after stent implantation is not a trivial occurrence,” observed Dr. Mintz, chief medical officer at the Cardiovascular Research Foundation in Washington.
DAPT-STEMI isn’t the final word on DAPT duration
At a late-breaking clinical trials session, comoderator Eric D. Peterson, MD, noted that in earlier megatrials such as PEGASUS, DAPT, and PLATO, there were signals that extending DAPT beyond 12 months might be even more beneficial than the guideline-recommended 12 months.
“It seems somewhat counterintuitive that now you have better results with less. Any speculation as to why?” asked Dr. Peterson, executive director of the Duke Clinical Research Institute and professor of medicine at Duke University in Durham, N.C.
“It’s true that DAPT reduces the general risk of thromboembolic events, but it does so at a relative risk reduction rate of about 20%, while it augments the bleeding risk by over 200%. And ask yourself, what is the benefit of this 6 months of extra DAPT on the lifelong process of atherosclerosis? It’s almost invisible,” Dr. Kedhi explained.
Dmitriy N. Feldman, MD, of Cornell University in New York, was one of several discussants to note that DAPT-STEMI was statistically underpowered to reach definitive conclusions. But he nonetheless found the results encouraging.
“It’s very reassuring that the stent thrombosis rates are quite low: 0.7% and 0.9%. And with this DES system and 42% of patients receiving clopidogrel rather than ticagrelor or prasugrel we still see low event rates. This is a very select group – patients had to tolerate the first 6 months of DAPT without MACE events or bleeding. But it is reassuring that in patients who are able to do well at 6 months, this is an option,” the interventional cardiologist said.
Session moderator Gregg W. Stone, MD, called DAPT-STEMI “hypothesis-generating” in light of its limited size and statistical power.
“At least it raises the concept of shorter-duration DAPT, whereas I’d say before today it was not a concept. We were always talking about prolonging DAPT in the highest-thrombotic risk STEMI patients, and now we can at least think about shortening it, whether for all patients or for higher-bleeding-risk patients,” observed Dr. Stone, professor of medicine at Columbia University in New York.
As a matter of fact, DAPT durations even briefer than 6 months are under active investigation. Dr. Kedhi is co-principal investigator in the Onyx ONE clinical trial, a new prospective, 85-center, randomized, single-blind trial of a mere 1 month of DAPT in 2,000 high-bleeding-risk CAD patients undergoing PCI with the Resolute Onyx DES or the BioFreedom drug-coated stent.
The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. Dr. Kedhi reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
SOURCE: Kedhi, E. no abst.
DENVER – Six months of dual-antiplatelet therapy proved equivalent in terms of safety, efficacy, and bleeding risk to the guideline-recommended standard 12 months in ST-elevation MI patients after primary PCI with a second-generation drug-eluting stent in the randomized DAPT-STEMI trial.
“This trial, for the first time, showed that in the modern DES [drug-eluting stent] era, event-free STEMI patients do not benefit from a prolonged DAPT beyond 6 months, as currently recommended, and sets the stage for further dedicated research in this important topic,” Elvin Kedhi, MD, PhD, declared in presenting the DAPT-STEMI results at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The final analysis took place at 24 months post STEMI; that is, 18 months post randomization. Among the 861 completers, the composite primary outcome of death, MI, revascularization, stroke, and major bleeding during months 6-24 occurred in 4.8% of the SAPT group, a 27% relative risk reduction compared with the 6.6% rate in the DAPT group. Thus, 6 months of DAPT met the prespecified endpoint of noninferiority compared to the standard 12 months of DAPT, reported Dr. Kedhi, head of interventional cardiology and clinical research and innovation at the Isala Heart Center in Zwolle, The Netherlands.
The secondary composite endpoint of death, MI, stroke, stent thrombosis, or TIMI major bleeding occurred in 3.2% of the SAPT group and 4.3% of the DAPT group, for a 25% relative risk reduction.
All individual components of the composite endpoints occurred at the same or lower rate in the SAPT group compared with the DAPT arm, he noted at the meeting, which was sponsored by the Cardiovascular Research Foundation.
At a press conference where Dr. Kedhi presented the DAPT-STEMI results, discussant Dean J. Kereiakes, MD, explained why he didn’t find the study results surprising.
Press conference moderator Gary S. Mintz, MD, put the DAPT-STEMI findings in perspective: “The need for DAPT has decreased along with all the stent-related complications. There’s always been a greater focus on DAPT for preventing events and a relatively lesser focus on the adverse consequences of DAPT. And anybody who’s a clinician who takes care of patients knows that drug-related bleeding after stent implantation is not a trivial occurrence,” observed Dr. Mintz, chief medical officer at the Cardiovascular Research Foundation in Washington.
DAPT-STEMI isn’t the final word on DAPT duration
At a late-breaking clinical trials session, comoderator Eric D. Peterson, MD, noted that in earlier megatrials such as PEGASUS, DAPT, and PLATO, there were signals that extending DAPT beyond 12 months might be even more beneficial than the guideline-recommended 12 months.
“It seems somewhat counterintuitive that now you have better results with less. Any speculation as to why?” asked Dr. Peterson, executive director of the Duke Clinical Research Institute and professor of medicine at Duke University in Durham, N.C.
“It’s true that DAPT reduces the general risk of thromboembolic events, but it does so at a relative risk reduction rate of about 20%, while it augments the bleeding risk by over 200%. And ask yourself, what is the benefit of this 6 months of extra DAPT on the lifelong process of atherosclerosis? It’s almost invisible,” Dr. Kedhi explained.
Dmitriy N. Feldman, MD, of Cornell University in New York, was one of several discussants to note that DAPT-STEMI was statistically underpowered to reach definitive conclusions. But he nonetheless found the results encouraging.
“It’s very reassuring that the stent thrombosis rates are quite low: 0.7% and 0.9%. And with this DES system and 42% of patients receiving clopidogrel rather than ticagrelor or prasugrel we still see low event rates. This is a very select group – patients had to tolerate the first 6 months of DAPT without MACE events or bleeding. But it is reassuring that in patients who are able to do well at 6 months, this is an option,” the interventional cardiologist said.
Session moderator Gregg W. Stone, MD, called DAPT-STEMI “hypothesis-generating” in light of its limited size and statistical power.
“At least it raises the concept of shorter-duration DAPT, whereas I’d say before today it was not a concept. We were always talking about prolonging DAPT in the highest-thrombotic risk STEMI patients, and now we can at least think about shortening it, whether for all patients or for higher-bleeding-risk patients,” observed Dr. Stone, professor of medicine at Columbia University in New York.
As a matter of fact, DAPT durations even briefer than 6 months are under active investigation. Dr. Kedhi is co-principal investigator in the Onyx ONE clinical trial, a new prospective, 85-center, randomized, single-blind trial of a mere 1 month of DAPT in 2,000 high-bleeding-risk CAD patients undergoing PCI with the Resolute Onyx DES or the BioFreedom drug-coated stent.
The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. Dr. Kedhi reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
SOURCE: Kedhi, E. no abst.
DENVER – Six months of dual-antiplatelet therapy proved equivalent in terms of safety, efficacy, and bleeding risk to the guideline-recommended standard 12 months in ST-elevation MI patients after primary PCI with a second-generation drug-eluting stent in the randomized DAPT-STEMI trial.
“This trial, for the first time, showed that in the modern DES [drug-eluting stent] era, event-free STEMI patients do not benefit from a prolonged DAPT beyond 6 months, as currently recommended, and sets the stage for further dedicated research in this important topic,” Elvin Kedhi, MD, PhD, declared in presenting the DAPT-STEMI results at the Transcatheter Cardiovascular Therapeutics annual educational meeting.
The final analysis took place at 24 months post STEMI; that is, 18 months post randomization. Among the 861 completers, the composite primary outcome of death, MI, revascularization, stroke, and major bleeding during months 6-24 occurred in 4.8% of the SAPT group, a 27% relative risk reduction compared with the 6.6% rate in the DAPT group. Thus, 6 months of DAPT met the prespecified endpoint of noninferiority compared to the standard 12 months of DAPT, reported Dr. Kedhi, head of interventional cardiology and clinical research and innovation at the Isala Heart Center in Zwolle, The Netherlands.
The secondary composite endpoint of death, MI, stroke, stent thrombosis, or TIMI major bleeding occurred in 3.2% of the SAPT group and 4.3% of the DAPT group, for a 25% relative risk reduction.
All individual components of the composite endpoints occurred at the same or lower rate in the SAPT group compared with the DAPT arm, he noted at the meeting, which was sponsored by the Cardiovascular Research Foundation.
At a press conference where Dr. Kedhi presented the DAPT-STEMI results, discussant Dean J. Kereiakes, MD, explained why he didn’t find the study results surprising.
Press conference moderator Gary S. Mintz, MD, put the DAPT-STEMI findings in perspective: “The need for DAPT has decreased along with all the stent-related complications. There’s always been a greater focus on DAPT for preventing events and a relatively lesser focus on the adverse consequences of DAPT. And anybody who’s a clinician who takes care of patients knows that drug-related bleeding after stent implantation is not a trivial occurrence,” observed Dr. Mintz, chief medical officer at the Cardiovascular Research Foundation in Washington.
DAPT-STEMI isn’t the final word on DAPT duration
At a late-breaking clinical trials session, comoderator Eric D. Peterson, MD, noted that in earlier megatrials such as PEGASUS, DAPT, and PLATO, there were signals that extending DAPT beyond 12 months might be even more beneficial than the guideline-recommended 12 months.
“It seems somewhat counterintuitive that now you have better results with less. Any speculation as to why?” asked Dr. Peterson, executive director of the Duke Clinical Research Institute and professor of medicine at Duke University in Durham, N.C.
“It’s true that DAPT reduces the general risk of thromboembolic events, but it does so at a relative risk reduction rate of about 20%, while it augments the bleeding risk by over 200%. And ask yourself, what is the benefit of this 6 months of extra DAPT on the lifelong process of atherosclerosis? It’s almost invisible,” Dr. Kedhi explained.
Dmitriy N. Feldman, MD, of Cornell University in New York, was one of several discussants to note that DAPT-STEMI was statistically underpowered to reach definitive conclusions. But he nonetheless found the results encouraging.
“It’s very reassuring that the stent thrombosis rates are quite low: 0.7% and 0.9%. And with this DES system and 42% of patients receiving clopidogrel rather than ticagrelor or prasugrel we still see low event rates. This is a very select group – patients had to tolerate the first 6 months of DAPT without MACE events or bleeding. But it is reassuring that in patients who are able to do well at 6 months, this is an option,” the interventional cardiologist said.
Session moderator Gregg W. Stone, MD, called DAPT-STEMI “hypothesis-generating” in light of its limited size and statistical power.
“At least it raises the concept of shorter-duration DAPT, whereas I’d say before today it was not a concept. We were always talking about prolonging DAPT in the highest-thrombotic risk STEMI patients, and now we can at least think about shortening it, whether for all patients or for higher-bleeding-risk patients,” observed Dr. Stone, professor of medicine at Columbia University in New York.
As a matter of fact, DAPT durations even briefer than 6 months are under active investigation. Dr. Kedhi is co-principal investigator in the Onyx ONE clinical trial, a new prospective, 85-center, randomized, single-blind trial of a mere 1 month of DAPT in 2,000 high-bleeding-risk CAD patients undergoing PCI with the Resolute Onyx DES or the BioFreedom drug-coated stent.
The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. Dr. Kedhi reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
SOURCE: Kedhi, E. no abst.
REPORTING FROM TCT 2017
Key clinical point:
Major finding: The composite endpoint of death, MI, stroke, revascularization, and major bleeding 24 months after primary PCI with a second-generation DES was 6.6% in patients who got the standard 12 months of DAPT and 4.8% in those who got 6.
Study details: A prospective randomized international study that enrolled 1,100 STEMI patients who underwent primary PCI.
Disclosures: The DAPT-STEMI trial was funded by Maasstad Cardiovascular Research. The presenter reported receiving consultant fees and/or institutional grants from Medtronic, Abbott Vascular, Meril. OrbusNeich, Boston Scientific, AstraZeneca, and Pfizer.
Source: Kedhi, E. No abstract.
EVAR, venous CPT coding revamped for 2018
CHICAGO – Current Procedural Terminology coding for endovascular aneurysm repair has been totally overhauled for 2018 with the introduction of a family of 20 new codes and codes for other vascular procedures have also been updated.
The new EVAR CPT codes attempt to capture the work involved in performing the procedures based upon the anatomy of the aneurysm and the treated vessels rather than being device-based, as previously, Matthew J. Sideman, MD, explained in presenting the coding and reimbursement for 2018 at a symposium on vascular surgery sponsored by Northwestern University.
“The new EVAR codes for 2018 have got a lot of gains. There are some losses as well, but overall, I think it’s going to be very positive moving forward,” according to Dr. Sideman, a vascular surgeon at the University of Texas, San Antonio, who serves as chair of the Society for Vascular Surgery Coding and Reimbursement Committee and an adviser to the American Medical Association Relative Value Scale Update Committee (RUC).
“What we gained was a new code for ruptured aneurysm repair, a new code for enhanced fixation, a new code for percutaneous access, new codes for alternative access options, and now all the access codes are add-on codes. But what we traded off was loss due to bundling. So catheterization is now bundled into the main procedure, radiographic supervision and interpretation is now bundled. The big thing that really hurt was we lost all proximal extensions to the renal arteries and all distal extensions to the iliac bifurcations – they’re also bundled into the main procedure,” he said.
Restructuring the EVAR codes was a multiyear collaborative project of the SVS, the American College of Surgeons, the Society of Interventional Radiology, the Society of Thoracic Surgery, the American College of Cardiology, and the Society for Cardiovascular Angiography and Interventions. The impetus was twofold: recognition that the existing codes seriously undervalued the work involved in EVAR because, for example, they didn’t distinguish between ruptured and elective aneurysm repair, nor did they recognize the unique challenges and advantages of percutaneous access.
Also, representatives of the professional societies involved with vascular medicine recognized that they had to develop a detailed proposal for coding restructuring or matters might be taken out of their hands. Bundling of codes has become the prevailing dogma at the RUC and the Centers for Medicare and Medicaid. Their current policy is that when analysis of coding patterns indicates two codes are billed together at least 51% of the time, that’s considered a ‘typical’ situation and a new code must be created combining them. The harsh reality for clinicians is that under what Dr. Sideman called “RUC math,” the new bundled codes invariably pay less than the two old ones.
“There was a little bit of smoke and mirrors – ‘Look at the pretty flashing lights and not what’s going on behind over here’ – as we tried to maintain value as we bundled these EVAR codes,” Dr. Sideman recalled. “I can stand here and tell you I did my very best to push for the best values possible. It can be a painful process, but I thought we came out ok.”
How the new EVAR codes work
Dr. Sideman explained that the impact of the new EVAR codes will depend upon a surgeon’s practice pattern.
He offered as a concrete example a patient undergoing elective EVAR of the aorta and both iliac arteries with percutaneous access and placement of a bifurcated device with one docking limb. In 2017, this might have been handled using CPT codes 34802, 36200-50, and 75952-26, for a total of 31.05 Relative Value Units (RVUs) of work.
In 2018, however, this same surgical strategy would be coded as 34705 (elective endovascular repair of infrarenal aorta and/or iliac artery or arteries) plus 34713 x 2 (percutaneous access and closure), for a total of 34.58 RVUs. Thus, the surgeon would come out 3.53 RVUs ahead in 2018, which at a conversion factor of $35.78/RVU translates to an extra $126.30.
On the other hand, if the surgeon chose to use a bifurcated device with one docking limb, a left iliac bell-bottom extension, a right iliac bell-bottom extension, and percutaneous access, in 2017, this would have been coded as 34802, 34825, 34826, 36200-50, 75952-26, and 75953-26 x 2, for a total of 44.29 RVUs of work. In 2018, this same treatment strategy would be coded as 34705 plus 34713 x 2, for a total of 34.58 RVUs, or a knockdown of 9.71 fewer RVUs compared with the year before, which translates to $347.42 less.
“The more extensions you use, the more you’re going to come out behind going forward,” according to Dr. Sideman.
Other coding changes in 2018
Sclerotherapy of single and multiple veins (codes 36470 and 36471) got down-valued from 1.10 and 2.49 to 0.75 and 1.5 RVUs, respectively.
Angiography of the extremities (75710 and 75716) will be better reimbursed in 2018. In what Dr. Sideman called “a good win,” unilateral angiography will be rated as 1.75 RVUs, up from 1.14 in 2017, while bilateral angiography increased from 1.31 to 1.97 RVUs.
“The other nice thing I can tell you is that through campaigning and lobbying and comments to CMS [Centers for Medicare & Medicaid Services], we got them to reverse their recommendations from 2017 to 2018 on the dialysis family of codes,” the surgeon continued.
Reimbursement for the dialysis codes took a big hit from 2016 to 2017, amounting to several hundred million dollars less in reimbursement, but CMS has reversed its policy on that score. The RVUs for the various dialysis codes have increased from 2017 to 2018 by 5%-21%, with central venous angioplasty (CPT 36907) garnering the biggest increase.
Existing RVUs were retained for 2018 in three of the four selective catheter placement codes. However, reimbursement for 36215 (first order catheterization of the thoracic or brachiocephalic branch) dropped from 4.67 to 4.17 RVUs because physician surveys showed the time involved was less than previously rated. Once the RUC and CMS saw that the time involved in a procedure has decreased, it became impossible to maintain the RVU, Dr. Sideman explained.
And speaking of time involved in procedures, Dr. Sideman offered a final plea to his vascular medicine colleagues:
“When you get surveys from the RUC asking for your input, please, please, please, fill them out because that’s how we get our direct physician input into the valuation of codes.”
He reported having no financial conflicts of interest regarding his presentation.
A detailed listing of many of the codes and changes can be found at the American College of Radiology website, and the Society for Vascular Surgery has coding resources available on their website, as well.
CHICAGO – Current Procedural Terminology coding for endovascular aneurysm repair has been totally overhauled for 2018 with the introduction of a family of 20 new codes and codes for other vascular procedures have also been updated.
The new EVAR CPT codes attempt to capture the work involved in performing the procedures based upon the anatomy of the aneurysm and the treated vessels rather than being device-based, as previously, Matthew J. Sideman, MD, explained in presenting the coding and reimbursement for 2018 at a symposium on vascular surgery sponsored by Northwestern University.
“The new EVAR codes for 2018 have got a lot of gains. There are some losses as well, but overall, I think it’s going to be very positive moving forward,” according to Dr. Sideman, a vascular surgeon at the University of Texas, San Antonio, who serves as chair of the Society for Vascular Surgery Coding and Reimbursement Committee and an adviser to the American Medical Association Relative Value Scale Update Committee (RUC).
“What we gained was a new code for ruptured aneurysm repair, a new code for enhanced fixation, a new code for percutaneous access, new codes for alternative access options, and now all the access codes are add-on codes. But what we traded off was loss due to bundling. So catheterization is now bundled into the main procedure, radiographic supervision and interpretation is now bundled. The big thing that really hurt was we lost all proximal extensions to the renal arteries and all distal extensions to the iliac bifurcations – they’re also bundled into the main procedure,” he said.
Restructuring the EVAR codes was a multiyear collaborative project of the SVS, the American College of Surgeons, the Society of Interventional Radiology, the Society of Thoracic Surgery, the American College of Cardiology, and the Society for Cardiovascular Angiography and Interventions. The impetus was twofold: recognition that the existing codes seriously undervalued the work involved in EVAR because, for example, they didn’t distinguish between ruptured and elective aneurysm repair, nor did they recognize the unique challenges and advantages of percutaneous access.
Also, representatives of the professional societies involved with vascular medicine recognized that they had to develop a detailed proposal for coding restructuring or matters might be taken out of their hands. Bundling of codes has become the prevailing dogma at the RUC and the Centers for Medicare and Medicaid. Their current policy is that when analysis of coding patterns indicates two codes are billed together at least 51% of the time, that’s considered a ‘typical’ situation and a new code must be created combining them. The harsh reality for clinicians is that under what Dr. Sideman called “RUC math,” the new bundled codes invariably pay less than the two old ones.
“There was a little bit of smoke and mirrors – ‘Look at the pretty flashing lights and not what’s going on behind over here’ – as we tried to maintain value as we bundled these EVAR codes,” Dr. Sideman recalled. “I can stand here and tell you I did my very best to push for the best values possible. It can be a painful process, but I thought we came out ok.”
How the new EVAR codes work
Dr. Sideman explained that the impact of the new EVAR codes will depend upon a surgeon’s practice pattern.
He offered as a concrete example a patient undergoing elective EVAR of the aorta and both iliac arteries with percutaneous access and placement of a bifurcated device with one docking limb. In 2017, this might have been handled using CPT codes 34802, 36200-50, and 75952-26, for a total of 31.05 Relative Value Units (RVUs) of work.
In 2018, however, this same surgical strategy would be coded as 34705 (elective endovascular repair of infrarenal aorta and/or iliac artery or arteries) plus 34713 x 2 (percutaneous access and closure), for a total of 34.58 RVUs. Thus, the surgeon would come out 3.53 RVUs ahead in 2018, which at a conversion factor of $35.78/RVU translates to an extra $126.30.
On the other hand, if the surgeon chose to use a bifurcated device with one docking limb, a left iliac bell-bottom extension, a right iliac bell-bottom extension, and percutaneous access, in 2017, this would have been coded as 34802, 34825, 34826, 36200-50, 75952-26, and 75953-26 x 2, for a total of 44.29 RVUs of work. In 2018, this same treatment strategy would be coded as 34705 plus 34713 x 2, for a total of 34.58 RVUs, or a knockdown of 9.71 fewer RVUs compared with the year before, which translates to $347.42 less.
“The more extensions you use, the more you’re going to come out behind going forward,” according to Dr. Sideman.
Other coding changes in 2018
Sclerotherapy of single and multiple veins (codes 36470 and 36471) got down-valued from 1.10 and 2.49 to 0.75 and 1.5 RVUs, respectively.
Angiography of the extremities (75710 and 75716) will be better reimbursed in 2018. In what Dr. Sideman called “a good win,” unilateral angiography will be rated as 1.75 RVUs, up from 1.14 in 2017, while bilateral angiography increased from 1.31 to 1.97 RVUs.
“The other nice thing I can tell you is that through campaigning and lobbying and comments to CMS [Centers for Medicare & Medicaid Services], we got them to reverse their recommendations from 2017 to 2018 on the dialysis family of codes,” the surgeon continued.
Reimbursement for the dialysis codes took a big hit from 2016 to 2017, amounting to several hundred million dollars less in reimbursement, but CMS has reversed its policy on that score. The RVUs for the various dialysis codes have increased from 2017 to 2018 by 5%-21%, with central venous angioplasty (CPT 36907) garnering the biggest increase.
Existing RVUs were retained for 2018 in three of the four selective catheter placement codes. However, reimbursement for 36215 (first order catheterization of the thoracic or brachiocephalic branch) dropped from 4.67 to 4.17 RVUs because physician surveys showed the time involved was less than previously rated. Once the RUC and CMS saw that the time involved in a procedure has decreased, it became impossible to maintain the RVU, Dr. Sideman explained.
And speaking of time involved in procedures, Dr. Sideman offered a final plea to his vascular medicine colleagues:
“When you get surveys from the RUC asking for your input, please, please, please, fill them out because that’s how we get our direct physician input into the valuation of codes.”
He reported having no financial conflicts of interest regarding his presentation.
A detailed listing of many of the codes and changes can be found at the American College of Radiology website, and the Society for Vascular Surgery has coding resources available on their website, as well.
CHICAGO – Current Procedural Terminology coding for endovascular aneurysm repair has been totally overhauled for 2018 with the introduction of a family of 20 new codes and codes for other vascular procedures have also been updated.
The new EVAR CPT codes attempt to capture the work involved in performing the procedures based upon the anatomy of the aneurysm and the treated vessels rather than being device-based, as previously, Matthew J. Sideman, MD, explained in presenting the coding and reimbursement for 2018 at a symposium on vascular surgery sponsored by Northwestern University.
“The new EVAR codes for 2018 have got a lot of gains. There are some losses as well, but overall, I think it’s going to be very positive moving forward,” according to Dr. Sideman, a vascular surgeon at the University of Texas, San Antonio, who serves as chair of the Society for Vascular Surgery Coding and Reimbursement Committee and an adviser to the American Medical Association Relative Value Scale Update Committee (RUC).
“What we gained was a new code for ruptured aneurysm repair, a new code for enhanced fixation, a new code for percutaneous access, new codes for alternative access options, and now all the access codes are add-on codes. But what we traded off was loss due to bundling. So catheterization is now bundled into the main procedure, radiographic supervision and interpretation is now bundled. The big thing that really hurt was we lost all proximal extensions to the renal arteries and all distal extensions to the iliac bifurcations – they’re also bundled into the main procedure,” he said.
Restructuring the EVAR codes was a multiyear collaborative project of the SVS, the American College of Surgeons, the Society of Interventional Radiology, the Society of Thoracic Surgery, the American College of Cardiology, and the Society for Cardiovascular Angiography and Interventions. The impetus was twofold: recognition that the existing codes seriously undervalued the work involved in EVAR because, for example, they didn’t distinguish between ruptured and elective aneurysm repair, nor did they recognize the unique challenges and advantages of percutaneous access.
Also, representatives of the professional societies involved with vascular medicine recognized that they had to develop a detailed proposal for coding restructuring or matters might be taken out of their hands. Bundling of codes has become the prevailing dogma at the RUC and the Centers for Medicare and Medicaid. Their current policy is that when analysis of coding patterns indicates two codes are billed together at least 51% of the time, that’s considered a ‘typical’ situation and a new code must be created combining them. The harsh reality for clinicians is that under what Dr. Sideman called “RUC math,” the new bundled codes invariably pay less than the two old ones.
“There was a little bit of smoke and mirrors – ‘Look at the pretty flashing lights and not what’s going on behind over here’ – as we tried to maintain value as we bundled these EVAR codes,” Dr. Sideman recalled. “I can stand here and tell you I did my very best to push for the best values possible. It can be a painful process, but I thought we came out ok.”
How the new EVAR codes work
Dr. Sideman explained that the impact of the new EVAR codes will depend upon a surgeon’s practice pattern.
He offered as a concrete example a patient undergoing elective EVAR of the aorta and both iliac arteries with percutaneous access and placement of a bifurcated device with one docking limb. In 2017, this might have been handled using CPT codes 34802, 36200-50, and 75952-26, for a total of 31.05 Relative Value Units (RVUs) of work.
In 2018, however, this same surgical strategy would be coded as 34705 (elective endovascular repair of infrarenal aorta and/or iliac artery or arteries) plus 34713 x 2 (percutaneous access and closure), for a total of 34.58 RVUs. Thus, the surgeon would come out 3.53 RVUs ahead in 2018, which at a conversion factor of $35.78/RVU translates to an extra $126.30.
On the other hand, if the surgeon chose to use a bifurcated device with one docking limb, a left iliac bell-bottom extension, a right iliac bell-bottom extension, and percutaneous access, in 2017, this would have been coded as 34802, 34825, 34826, 36200-50, 75952-26, and 75953-26 x 2, for a total of 44.29 RVUs of work. In 2018, this same treatment strategy would be coded as 34705 plus 34713 x 2, for a total of 34.58 RVUs, or a knockdown of 9.71 fewer RVUs compared with the year before, which translates to $347.42 less.
“The more extensions you use, the more you’re going to come out behind going forward,” according to Dr. Sideman.
Other coding changes in 2018
Sclerotherapy of single and multiple veins (codes 36470 and 36471) got down-valued from 1.10 and 2.49 to 0.75 and 1.5 RVUs, respectively.
Angiography of the extremities (75710 and 75716) will be better reimbursed in 2018. In what Dr. Sideman called “a good win,” unilateral angiography will be rated as 1.75 RVUs, up from 1.14 in 2017, while bilateral angiography increased from 1.31 to 1.97 RVUs.
“The other nice thing I can tell you is that through campaigning and lobbying and comments to CMS [Centers for Medicare & Medicaid Services], we got them to reverse their recommendations from 2017 to 2018 on the dialysis family of codes,” the surgeon continued.
Reimbursement for the dialysis codes took a big hit from 2016 to 2017, amounting to several hundred million dollars less in reimbursement, but CMS has reversed its policy on that score. The RVUs for the various dialysis codes have increased from 2017 to 2018 by 5%-21%, with central venous angioplasty (CPT 36907) garnering the biggest increase.
Existing RVUs were retained for 2018 in three of the four selective catheter placement codes. However, reimbursement for 36215 (first order catheterization of the thoracic or brachiocephalic branch) dropped from 4.67 to 4.17 RVUs because physician surveys showed the time involved was less than previously rated. Once the RUC and CMS saw that the time involved in a procedure has decreased, it became impossible to maintain the RVU, Dr. Sideman explained.
And speaking of time involved in procedures, Dr. Sideman offered a final plea to his vascular medicine colleagues:
“When you get surveys from the RUC asking for your input, please, please, please, fill them out because that’s how we get our direct physician input into the valuation of codes.”
He reported having no financial conflicts of interest regarding his presentation.
A detailed listing of many of the codes and changes can be found at the American College of Radiology website, and the Society for Vascular Surgery has coding resources available on their website, as well.
EXPERT ANALYSIS FROM THE NORTHWESTERN VASCULAR SYMPOSIUM
Skin signs spotlight highest-risk SLE patients
GENEVA – Careful warranting prompt initiation of long-term antiplatelet therapy, Dan Lipsker, MD, PhD, said in a plenary lecture at the annual congress of the European Academy of Dermatology and Venereology.
“Those patients are at very high risk. Those are the lupus patients with the poorest prognosis. Those are the lupus patients who still die today,” said Dr. Lipsker, professor of dermatology at the University of Strasbourg (France).
Cutaneous clues suggestive of thrombosis in SLE patients include atrophie blanche, pseudo-Degos lesions, livedo racemosa, acral nonpalpable purpura or reticulate erythema, cutaneous necrosis, splinter hemorrhage, thrombophlebitis, and nailfold telangiectasias. These skin findings can occur simultaneously with or after potentially life-threatening thrombotic events, or in the best of all scenarios, beforehand.
Dr. Lipsker told his audience of dermatologists that, by demonstrating facility in identifying these cutaneous disorders, they can make themselves “indispensable” to the rheumatologists, nephrologists, internists, and/or pediatricians who often provide the bulk of specialized care for SLE patients.
“We know today that, 5 years after initial diagnosis of SLE, the chief causes of morbidity and mortality are thrombotic events. And it can be extremely difficult to distinguish between an acute autoimmune lupus flare and a thrombotic event when, for example, the CNS or eyes are involved. But you will find direct evidence of thrombosis by carefully examining the skin,” the dermatologist maintained.
Some of these cutaneous signs constitute unequivocal evidence of thrombosis in SLE patients. Others are more ambiguous but should raise suspicion of an ongoing systemic thrombotic process unless another explanation is found.
One example of a skin finding that always indicates thrombosis is pseudo-Degos lesions: ivory-colored or white depressed atrophic papules with a raised border composed of telangiectasias. Biopsy shows evidence of a cone-shaped dermal arteriolar infarct.
“We have biopsied dozens of patients with this presentation, and you always find occluded vessels without a single inflammatory cell. These lesions are usually painful, and when you put those patients on low-dose aspirin they do better,” Dr. Lipsker said.
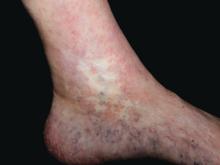
Atrophie blanche in a patient with SLE is also strong evidence of thrombotic vasculopathy. Atrophie blanche is a porcelain-white atrophic white scar with surrounding hyperpigmentation on the lower leg that occurs after skin injury in an area with venous insufficiency.
Livedo racemosa in a patient with SLE is also highly suggestive of a systemic thrombotic process. Characteristic of this dermatologic disorder is an irregular, netlike mottling surrounding pale skin.
“All of these skin signs allow identification of patients who have very high risk of thrombotic events,” Dr. Lipsker stressed.
He reported having no financial conflicts of interest regarding his presentation.
GENEVA – Careful warranting prompt initiation of long-term antiplatelet therapy, Dan Lipsker, MD, PhD, said in a plenary lecture at the annual congress of the European Academy of Dermatology and Venereology.
“Those patients are at very high risk. Those are the lupus patients with the poorest prognosis. Those are the lupus patients who still die today,” said Dr. Lipsker, professor of dermatology at the University of Strasbourg (France).
Cutaneous clues suggestive of thrombosis in SLE patients include atrophie blanche, pseudo-Degos lesions, livedo racemosa, acral nonpalpable purpura or reticulate erythema, cutaneous necrosis, splinter hemorrhage, thrombophlebitis, and nailfold telangiectasias. These skin findings can occur simultaneously with or after potentially life-threatening thrombotic events, or in the best of all scenarios, beforehand.
Dr. Lipsker told his audience of dermatologists that, by demonstrating facility in identifying these cutaneous disorders, they can make themselves “indispensable” to the rheumatologists, nephrologists, internists, and/or pediatricians who often provide the bulk of specialized care for SLE patients.
“We know today that, 5 years after initial diagnosis of SLE, the chief causes of morbidity and mortality are thrombotic events. And it can be extremely difficult to distinguish between an acute autoimmune lupus flare and a thrombotic event when, for example, the CNS or eyes are involved. But you will find direct evidence of thrombosis by carefully examining the skin,” the dermatologist maintained.
Some of these cutaneous signs constitute unequivocal evidence of thrombosis in SLE patients. Others are more ambiguous but should raise suspicion of an ongoing systemic thrombotic process unless another explanation is found.
One example of a skin finding that always indicates thrombosis is pseudo-Degos lesions: ivory-colored or white depressed atrophic papules with a raised border composed of telangiectasias. Biopsy shows evidence of a cone-shaped dermal arteriolar infarct.
“We have biopsied dozens of patients with this presentation, and you always find occluded vessels without a single inflammatory cell. These lesions are usually painful, and when you put those patients on low-dose aspirin they do better,” Dr. Lipsker said.
Atrophie blanche in a patient with SLE is also strong evidence of thrombotic vasculopathy. Atrophie blanche is a porcelain-white atrophic white scar with surrounding hyperpigmentation on the lower leg that occurs after skin injury in an area with venous insufficiency.
Livedo racemosa in a patient with SLE is also highly suggestive of a systemic thrombotic process. Characteristic of this dermatologic disorder is an irregular, netlike mottling surrounding pale skin.
“All of these skin signs allow identification of patients who have very high risk of thrombotic events,” Dr. Lipsker stressed.
He reported having no financial conflicts of interest regarding his presentation.
GENEVA – Careful warranting prompt initiation of long-term antiplatelet therapy, Dan Lipsker, MD, PhD, said in a plenary lecture at the annual congress of the European Academy of Dermatology and Venereology.
“Those patients are at very high risk. Those are the lupus patients with the poorest prognosis. Those are the lupus patients who still die today,” said Dr. Lipsker, professor of dermatology at the University of Strasbourg (France).
Cutaneous clues suggestive of thrombosis in SLE patients include atrophie blanche, pseudo-Degos lesions, livedo racemosa, acral nonpalpable purpura or reticulate erythema, cutaneous necrosis, splinter hemorrhage, thrombophlebitis, and nailfold telangiectasias. These skin findings can occur simultaneously with or after potentially life-threatening thrombotic events, or in the best of all scenarios, beforehand.
Dr. Lipsker told his audience of dermatologists that, by demonstrating facility in identifying these cutaneous disorders, they can make themselves “indispensable” to the rheumatologists, nephrologists, internists, and/or pediatricians who often provide the bulk of specialized care for SLE patients.
“We know today that, 5 years after initial diagnosis of SLE, the chief causes of morbidity and mortality are thrombotic events. And it can be extremely difficult to distinguish between an acute autoimmune lupus flare and a thrombotic event when, for example, the CNS or eyes are involved. But you will find direct evidence of thrombosis by carefully examining the skin,” the dermatologist maintained.
Some of these cutaneous signs constitute unequivocal evidence of thrombosis in SLE patients. Others are more ambiguous but should raise suspicion of an ongoing systemic thrombotic process unless another explanation is found.
One example of a skin finding that always indicates thrombosis is pseudo-Degos lesions: ivory-colored or white depressed atrophic papules with a raised border composed of telangiectasias. Biopsy shows evidence of a cone-shaped dermal arteriolar infarct.
“We have biopsied dozens of patients with this presentation, and you always find occluded vessels without a single inflammatory cell. These lesions are usually painful, and when you put those patients on low-dose aspirin they do better,” Dr. Lipsker said.
Atrophie blanche in a patient with SLE is also strong evidence of thrombotic vasculopathy. Atrophie blanche is a porcelain-white atrophic white scar with surrounding hyperpigmentation on the lower leg that occurs after skin injury in an area with venous insufficiency.
Livedo racemosa in a patient with SLE is also highly suggestive of a systemic thrombotic process. Characteristic of this dermatologic disorder is an irregular, netlike mottling surrounding pale skin.
“All of these skin signs allow identification of patients who have very high risk of thrombotic events,” Dr. Lipsker stressed.
He reported having no financial conflicts of interest regarding his presentation.
EXPERT ANALYSIS FROM THE EADV CONGRESS
FDA approves first therapy treatment for EGPA
The Food and Drug Administration announced Dec. 12 the approval of mepolizumab (Nucala) to treat adult patients with eosinophilic granulomatosis with polyangiitis (EGPA), making it the first FDA-approved therapy intended to treat this rare disease.
Approval was based on data from a 52-week clinical trial that compared mepolizumab with placebo, according to the FDA. Patients received 300 mg of mepolizumab once every 4 weeks while continuing stable daily oral corticosteroid therapy. Those patients receiving mepolizumab “achieved a significantly greater accrued time in remission compared with placebo,” and a significantly higher proportion of patients receiving 300 mg of mepolizumab had achieved remission at week 36 and week 48, the statement said. Additionally, significantly more patients treated with mepolizumab achieved remission within the first 24 weeks and remained in remission for the remainder of the 52-week study treatment period.
“The expanded indication of Nucala meets a critical, unmet need for EGPA patients. It’s notable that patients taking Nucala in clinical trials reported a significant improvement in their symptoms,” said Badrul Chowdhury, MD, PhD, director of the division of pulmonary, allergy, and rheumatology products in the FDA’s Center for Drug Evaluation and Research in the press release announcing the approval. EGPA was formerly known as Churg-Strauss syndrome, the statement pointed out.
Read the full press release on the FDA’s website.
SOURCE: FDA.gov
The Food and Drug Administration announced Dec. 12 the approval of mepolizumab (Nucala) to treat adult patients with eosinophilic granulomatosis with polyangiitis (EGPA), making it the first FDA-approved therapy intended to treat this rare disease.
Approval was based on data from a 52-week clinical trial that compared mepolizumab with placebo, according to the FDA. Patients received 300 mg of mepolizumab once every 4 weeks while continuing stable daily oral corticosteroid therapy. Those patients receiving mepolizumab “achieved a significantly greater accrued time in remission compared with placebo,” and a significantly higher proportion of patients receiving 300 mg of mepolizumab had achieved remission at week 36 and week 48, the statement said. Additionally, significantly more patients treated with mepolizumab achieved remission within the first 24 weeks and remained in remission for the remainder of the 52-week study treatment period.
“The expanded indication of Nucala meets a critical, unmet need for EGPA patients. It’s notable that patients taking Nucala in clinical trials reported a significant improvement in their symptoms,” said Badrul Chowdhury, MD, PhD, director of the division of pulmonary, allergy, and rheumatology products in the FDA’s Center for Drug Evaluation and Research in the press release announcing the approval. EGPA was formerly known as Churg-Strauss syndrome, the statement pointed out.
Read the full press release on the FDA’s website.
SOURCE: FDA.gov
The Food and Drug Administration announced Dec. 12 the approval of mepolizumab (Nucala) to treat adult patients with eosinophilic granulomatosis with polyangiitis (EGPA), making it the first FDA-approved therapy intended to treat this rare disease.
Approval was based on data from a 52-week clinical trial that compared mepolizumab with placebo, according to the FDA. Patients received 300 mg of mepolizumab once every 4 weeks while continuing stable daily oral corticosteroid therapy. Those patients receiving mepolizumab “achieved a significantly greater accrued time in remission compared with placebo,” and a significantly higher proportion of patients receiving 300 mg of mepolizumab had achieved remission at week 36 and week 48, the statement said. Additionally, significantly more patients treated with mepolizumab achieved remission within the first 24 weeks and remained in remission for the remainder of the 52-week study treatment period.
“The expanded indication of Nucala meets a critical, unmet need for EGPA patients. It’s notable that patients taking Nucala in clinical trials reported a significant improvement in their symptoms,” said Badrul Chowdhury, MD, PhD, director of the division of pulmonary, allergy, and rheumatology products in the FDA’s Center for Drug Evaluation and Research in the press release announcing the approval. EGPA was formerly known as Churg-Strauss syndrome, the statement pointed out.
Read the full press release on the FDA’s website.
SOURCE: FDA.gov
VTE rates in lenalidomide-treated NHL may warrant prophylaxis
ATLANTA – The rate of venous thromboembolism (VTE) in patients with non-Hodgkin lymphoma (NHL) treated with lenalidomide is similar to that seen in multiple myeloma, according to results of recent systematic review and meta-analysis of trials representing more than 10,000 treatment cycles.
Although rates of VTE for NHL and myeloma could not be directly compared statistically, the finding may have clinical implications for NHL patients, said lead study author Samuel Yamshon, MD, an internal medicine resident at Cornell University, New York.
“Although outpatient VTE prophylaxis is not currently recommended, it should be carefully considered in patients with lymphoma being treated with lenalidomide, especially those receiving lenalidomide as a single agent,” Dr. Yamshon said in a presentation of the results at the annual meeting of the American Society of Hematology.
The rate of thrombosis in patients with B cell NHL who received lenalidomide treatment was 0.75 events per 100 patient-cycles, according to results of the meta-analysis, which was based on 28 articles including 10,332 cycles of lenalidomide received by patients with B-cell NHL.
Reported rates of thrombosis in previously untreated myeloma patients treated with lenalidomide are between 0.7 and 0.8 per 100 patient-cycles, Dr. Yamshon said in his presentation.
Notably, single-agent lenalidomide was linked with a significantly increased risk of thrombosis compared with lenalidomide treatment in combinations. The relative risk of VTE for lenalidomide as a single agent versus lenalidomide in combination was 2.01 (95% confidence interval, 1.28-3.16; P = .002), according to the presented data.
The investigators were unsure why single-agent lenalidomide appeared to have caused increased rates of thrombosis compared to lenalidomide in combinations. “Perhaps patients treated with additional agents have a lower tumor burden, leading to less venous obstruction causing clots, or perhaps there’s a direct interaction between lenalidomide and tumor leading to effects on the vasculature and mediators of coagulation,” Dr. Yamshon said.
Chemotherapy and biologic combinations had somewhat different VTE rates when compared to single-agent lenalidomide. The rate in patients receiving lenalidomide alone was 1.06 events per 100 patient-cycles, compared with 0.73 and 0.41 events per 100 patient-cycles, respectively, for lenalidomide plus chemotherapy and lenalidomide plus biologics.
However, the lower event rate with lenalidomide and biologics compared with lenalidomide and chemotherapy was a “nonsignificant trend” that was likely caused by differences in patient characteristics between the two cohorts, according to Dr. Yamshon.
None of the studies included in the meta-analysis were prospectively designed to measure VTE as a primary or secondary outcome, Dr. Yamshon noted in a discussion of the study’s limitations.
Further studies are warranted to determine lenalidomide’s effect on the vasculature and how it effects mediators of coagulation, he added.
Based on the current results, Dr. Yamshon said it may be reasonable to consider VTE prophylaxis in NHL patients receiving lenalidomide.
“If we’re going to be recommending outpatient VTE prophylaxis in everyone on lenalidomide in multiple myeloma, and the rates (of VTE) are the same, I think it certainly makes sense based on the data to recommend it,” he said in a question-and-answer session.
Dr. Yamshon reported no conflicts related to the study. Coauthors reported disclosures related to Roche, Celgene, Seattle Genetics, Pharmacyclics, Cell Medica, Janssen, and AstraZeneca.
SOURCE: Yamshon S et al. Abstract 677.
ATLANTA – The rate of venous thromboembolism (VTE) in patients with non-Hodgkin lymphoma (NHL) treated with lenalidomide is similar to that seen in multiple myeloma, according to results of recent systematic review and meta-analysis of trials representing more than 10,000 treatment cycles.
Although rates of VTE for NHL and myeloma could not be directly compared statistically, the finding may have clinical implications for NHL patients, said lead study author Samuel Yamshon, MD, an internal medicine resident at Cornell University, New York.
“Although outpatient VTE prophylaxis is not currently recommended, it should be carefully considered in patients with lymphoma being treated with lenalidomide, especially those receiving lenalidomide as a single agent,” Dr. Yamshon said in a presentation of the results at the annual meeting of the American Society of Hematology.
The rate of thrombosis in patients with B cell NHL who received lenalidomide treatment was 0.75 events per 100 patient-cycles, according to results of the meta-analysis, which was based on 28 articles including 10,332 cycles of lenalidomide received by patients with B-cell NHL.
Reported rates of thrombosis in previously untreated myeloma patients treated with lenalidomide are between 0.7 and 0.8 per 100 patient-cycles, Dr. Yamshon said in his presentation.
Notably, single-agent lenalidomide was linked with a significantly increased risk of thrombosis compared with lenalidomide treatment in combinations. The relative risk of VTE for lenalidomide as a single agent versus lenalidomide in combination was 2.01 (95% confidence interval, 1.28-3.16; P = .002), according to the presented data.
The investigators were unsure why single-agent lenalidomide appeared to have caused increased rates of thrombosis compared to lenalidomide in combinations. “Perhaps patients treated with additional agents have a lower tumor burden, leading to less venous obstruction causing clots, or perhaps there’s a direct interaction between lenalidomide and tumor leading to effects on the vasculature and mediators of coagulation,” Dr. Yamshon said.
Chemotherapy and biologic combinations had somewhat different VTE rates when compared to single-agent lenalidomide. The rate in patients receiving lenalidomide alone was 1.06 events per 100 patient-cycles, compared with 0.73 and 0.41 events per 100 patient-cycles, respectively, for lenalidomide plus chemotherapy and lenalidomide plus biologics.
However, the lower event rate with lenalidomide and biologics compared with lenalidomide and chemotherapy was a “nonsignificant trend” that was likely caused by differences in patient characteristics between the two cohorts, according to Dr. Yamshon.
None of the studies included in the meta-analysis were prospectively designed to measure VTE as a primary or secondary outcome, Dr. Yamshon noted in a discussion of the study’s limitations.
Further studies are warranted to determine lenalidomide’s effect on the vasculature and how it effects mediators of coagulation, he added.
Based on the current results, Dr. Yamshon said it may be reasonable to consider VTE prophylaxis in NHL patients receiving lenalidomide.
“If we’re going to be recommending outpatient VTE prophylaxis in everyone on lenalidomide in multiple myeloma, and the rates (of VTE) are the same, I think it certainly makes sense based on the data to recommend it,” he said in a question-and-answer session.
Dr. Yamshon reported no conflicts related to the study. Coauthors reported disclosures related to Roche, Celgene, Seattle Genetics, Pharmacyclics, Cell Medica, Janssen, and AstraZeneca.
SOURCE: Yamshon S et al. Abstract 677.
ATLANTA – The rate of venous thromboembolism (VTE) in patients with non-Hodgkin lymphoma (NHL) treated with lenalidomide is similar to that seen in multiple myeloma, according to results of recent systematic review and meta-analysis of trials representing more than 10,000 treatment cycles.
Although rates of VTE for NHL and myeloma could not be directly compared statistically, the finding may have clinical implications for NHL patients, said lead study author Samuel Yamshon, MD, an internal medicine resident at Cornell University, New York.
“Although outpatient VTE prophylaxis is not currently recommended, it should be carefully considered in patients with lymphoma being treated with lenalidomide, especially those receiving lenalidomide as a single agent,” Dr. Yamshon said in a presentation of the results at the annual meeting of the American Society of Hematology.
The rate of thrombosis in patients with B cell NHL who received lenalidomide treatment was 0.75 events per 100 patient-cycles, according to results of the meta-analysis, which was based on 28 articles including 10,332 cycles of lenalidomide received by patients with B-cell NHL.
Reported rates of thrombosis in previously untreated myeloma patients treated with lenalidomide are between 0.7 and 0.8 per 100 patient-cycles, Dr. Yamshon said in his presentation.
Notably, single-agent lenalidomide was linked with a significantly increased risk of thrombosis compared with lenalidomide treatment in combinations. The relative risk of VTE for lenalidomide as a single agent versus lenalidomide in combination was 2.01 (95% confidence interval, 1.28-3.16; P = .002), according to the presented data.
The investigators were unsure why single-agent lenalidomide appeared to have caused increased rates of thrombosis compared to lenalidomide in combinations. “Perhaps patients treated with additional agents have a lower tumor burden, leading to less venous obstruction causing clots, or perhaps there’s a direct interaction between lenalidomide and tumor leading to effects on the vasculature and mediators of coagulation,” Dr. Yamshon said.
Chemotherapy and biologic combinations had somewhat different VTE rates when compared to single-agent lenalidomide. The rate in patients receiving lenalidomide alone was 1.06 events per 100 patient-cycles, compared with 0.73 and 0.41 events per 100 patient-cycles, respectively, for lenalidomide plus chemotherapy and lenalidomide plus biologics.
However, the lower event rate with lenalidomide and biologics compared with lenalidomide and chemotherapy was a “nonsignificant trend” that was likely caused by differences in patient characteristics between the two cohorts, according to Dr. Yamshon.
None of the studies included in the meta-analysis were prospectively designed to measure VTE as a primary or secondary outcome, Dr. Yamshon noted in a discussion of the study’s limitations.
Further studies are warranted to determine lenalidomide’s effect on the vasculature and how it effects mediators of coagulation, he added.
Based on the current results, Dr. Yamshon said it may be reasonable to consider VTE prophylaxis in NHL patients receiving lenalidomide.
“If we’re going to be recommending outpatient VTE prophylaxis in everyone on lenalidomide in multiple myeloma, and the rates (of VTE) are the same, I think it certainly makes sense based on the data to recommend it,” he said in a question-and-answer session.
Dr. Yamshon reported no conflicts related to the study. Coauthors reported disclosures related to Roche, Celgene, Seattle Genetics, Pharmacyclics, Cell Medica, Janssen, and AstraZeneca.
SOURCE: Yamshon S et al. Abstract 677.
AT ASH 2017
Key clinical point: The rates of VTE in patients on lenalidomide are similar whether they’re being treated for B cell non-Hodgkin lymphoma (NHL) or multiple myeloma, which suggests that VTE prophylaxis should be more carefully considered in B cell NHL patients.
Major finding: The rate of thrombosis in patients with B cell NHL who received lenalidomide treatment was 0.75 events per 100 patient-cycles.
Data source: A systematic review and meta-analysis of 28 articles including 10,332 cycles of lenalidomide received by patients with B cell NHL.
Disclosures: Authors of the study reported disclosures related to Roche, Celgene, Seattle Genetics, Pharmacyclics, Cell Medica, Janssen, and AstraZeneca.
Source: Yamshon S et al. Abstract 677.
Edoxaban noninferior to dalteparin for cancer-associated VTE
ATLANTA – Twelve months of daily treatment with the novel oral factor Xa inhibitor edoxaban was noninferior to standard subcutaneous therapy with dalteparin for treatment of venous thromboembolism in patients with cancer, according to late-breaking results from a randomized, open-label, blinded-outcomes trial.
Throughout follow-up, trial arms had nearly identical rates of survival free from recurrent VTE or major bleeding, Gary E. Raskob, PhD, reported during a late-breaking oral presentation at the annual meeting of the American Society of Hematology. “Edoxaban was associated with a lower rate of recurrent VTE, which was offset by a similar increase in risk of major bleeding,” he said. “Therefore, oral edoxaban was noninferior to subcutaneous dalteparin for the [combined] primary outcome.”
Venous thromboembolism affects about one in five patients with active cancer and is difficult to treat because patients face increased risks of recurrence and bleeding. The struggle to balance these risks fuels morbidity and mortality and can hamper cancer treatment, said Dr. Raskob of the University of Oklahoma, Oklahoma City.
Pharmacy and medical oncology societies recommend long-term low-molecular-weight heparin for cancer patients with VTE, but the daily burden of subcutaneous injections leads many to stop after about 2-4 months of treatment, Dr. Raskob said. “Direct oral anticoagulants may be an attractive alternative.”
For the trial, 1,446 adults with cancer and lower limb VTE from 114 clinics in North America, Europe, Australia, and New Zealand received either edoxaban (60 mg daily) or dalteparin (200 IU/kg for 30 days, followed by 150 IU/kg) for up to 12 months. Nearly all patients had active cancer. Tumor types reflected what’s most common in practice, such as malignancies of the lung, colon, and breast. About 50 patients had primary or metastatic brain cancers. Approximately two-thirds had pulmonary embolism with or without deep-vein thrombosis, while the rest had isolated deep-vein thrombosis.
After 12 months of follow-up, 12.8% of edoxaban patients had at least one recurrence of VTE or a major bleed, compared with 13.5% of dalteparin patients (hazard ratio, 0.97; 95% confidence interval, 0.70-1.36; P = .006 for noninferiority). Edoxaban also was noninferior to dalteparin after the first 6 months of treatment and in the per-protocol analysis (HRs, 1.0; P = .02 for noninferiority in each analysis). Thus, differences in efficacy did not only reflect better compliance to oral therapy, Dr. Raskob said.
He also reported on individual outcomes. In all, 10.3% of dalteparin recipients had a VTE recurrence, as did 6.5% of edoxaban recipients, for a risk difference of 3.8% (95% CI, 7.1%-0.4%). More than half of recurrences in each group were symptomatic, and none were fatal. Bleeding caused no deaths in either study arm, and each therapy conferred an identical chance of a grade 3-4 major bleed (2.3%).
Edoxaban was associated, however, with a greater frequency of major bleeds (33 events; 6.3%) than was dalteparin (17 events; 3.2%; risk difference, 3.1%; 95% CI, 0.5%-5.7%). In particular, patients who received edoxaban had a slightly higher rate of upper gastrointestinal bleeds. Most had gastric cancer.
Future studies should evaluate whether these patients should receive a lower dose of edoxaban, said Dr. Raskob. “We don’t yet fully know the minimum effective dose [of edoxaban] in cancer patients.”
He also addressed the idea that heparin has antineoplastic activity, calling it “one we should probably abandon. The concept originates from older trials in which researchers probably did not recognize that heparin was preventing fatal pulmonary embolism, he said.
The investigators soon will begin deeper analyses that should inform patient selection, he said. For now, he recommends discussing these findings with patients to help them make an informed choice between oral anticoagulation, with its ease of use but slightly higher rate of major bleeds, and subcutaneous heparin, with its lower bleeding rate and treatment burden.
Daiichi Sankyo provided funding. Dr. Raskob disclosed consulting relationships and honoraria from Daiichi Sankyo, Eli Lilly, Janssen, and several other pharmaceutical companies.
SOURCE: Raskob G et al. ASH Abstract LBA-6.
ATLANTA – Twelve months of daily treatment with the novel oral factor Xa inhibitor edoxaban was noninferior to standard subcutaneous therapy with dalteparin for treatment of venous thromboembolism in patients with cancer, according to late-breaking results from a randomized, open-label, blinded-outcomes trial.
Throughout follow-up, trial arms had nearly identical rates of survival free from recurrent VTE or major bleeding, Gary E. Raskob, PhD, reported during a late-breaking oral presentation at the annual meeting of the American Society of Hematology. “Edoxaban was associated with a lower rate of recurrent VTE, which was offset by a similar increase in risk of major bleeding,” he said. “Therefore, oral edoxaban was noninferior to subcutaneous dalteparin for the [combined] primary outcome.”
Venous thromboembolism affects about one in five patients with active cancer and is difficult to treat because patients face increased risks of recurrence and bleeding. The struggle to balance these risks fuels morbidity and mortality and can hamper cancer treatment, said Dr. Raskob of the University of Oklahoma, Oklahoma City.
Pharmacy and medical oncology societies recommend long-term low-molecular-weight heparin for cancer patients with VTE, but the daily burden of subcutaneous injections leads many to stop after about 2-4 months of treatment, Dr. Raskob said. “Direct oral anticoagulants may be an attractive alternative.”
For the trial, 1,446 adults with cancer and lower limb VTE from 114 clinics in North America, Europe, Australia, and New Zealand received either edoxaban (60 mg daily) or dalteparin (200 IU/kg for 30 days, followed by 150 IU/kg) for up to 12 months. Nearly all patients had active cancer. Tumor types reflected what’s most common in practice, such as malignancies of the lung, colon, and breast. About 50 patients had primary or metastatic brain cancers. Approximately two-thirds had pulmonary embolism with or without deep-vein thrombosis, while the rest had isolated deep-vein thrombosis.
After 12 months of follow-up, 12.8% of edoxaban patients had at least one recurrence of VTE or a major bleed, compared with 13.5% of dalteparin patients (hazard ratio, 0.97; 95% confidence interval, 0.70-1.36; P = .006 for noninferiority). Edoxaban also was noninferior to dalteparin after the first 6 months of treatment and in the per-protocol analysis (HRs, 1.0; P = .02 for noninferiority in each analysis). Thus, differences in efficacy did not only reflect better compliance to oral therapy, Dr. Raskob said.
He also reported on individual outcomes. In all, 10.3% of dalteparin recipients had a VTE recurrence, as did 6.5% of edoxaban recipients, for a risk difference of 3.8% (95% CI, 7.1%-0.4%). More than half of recurrences in each group were symptomatic, and none were fatal. Bleeding caused no deaths in either study arm, and each therapy conferred an identical chance of a grade 3-4 major bleed (2.3%).
Edoxaban was associated, however, with a greater frequency of major bleeds (33 events; 6.3%) than was dalteparin (17 events; 3.2%; risk difference, 3.1%; 95% CI, 0.5%-5.7%). In particular, patients who received edoxaban had a slightly higher rate of upper gastrointestinal bleeds. Most had gastric cancer.
Future studies should evaluate whether these patients should receive a lower dose of edoxaban, said Dr. Raskob. “We don’t yet fully know the minimum effective dose [of edoxaban] in cancer patients.”
He also addressed the idea that heparin has antineoplastic activity, calling it “one we should probably abandon. The concept originates from older trials in which researchers probably did not recognize that heparin was preventing fatal pulmonary embolism, he said.
The investigators soon will begin deeper analyses that should inform patient selection, he said. For now, he recommends discussing these findings with patients to help them make an informed choice between oral anticoagulation, with its ease of use but slightly higher rate of major bleeds, and subcutaneous heparin, with its lower bleeding rate and treatment burden.
Daiichi Sankyo provided funding. Dr. Raskob disclosed consulting relationships and honoraria from Daiichi Sankyo, Eli Lilly, Janssen, and several other pharmaceutical companies.
SOURCE: Raskob G et al. ASH Abstract LBA-6.
ATLANTA – Twelve months of daily treatment with the novel oral factor Xa inhibitor edoxaban was noninferior to standard subcutaneous therapy with dalteparin for treatment of venous thromboembolism in patients with cancer, according to late-breaking results from a randomized, open-label, blinded-outcomes trial.
Throughout follow-up, trial arms had nearly identical rates of survival free from recurrent VTE or major bleeding, Gary E. Raskob, PhD, reported during a late-breaking oral presentation at the annual meeting of the American Society of Hematology. “Edoxaban was associated with a lower rate of recurrent VTE, which was offset by a similar increase in risk of major bleeding,” he said. “Therefore, oral edoxaban was noninferior to subcutaneous dalteparin for the [combined] primary outcome.”
Venous thromboembolism affects about one in five patients with active cancer and is difficult to treat because patients face increased risks of recurrence and bleeding. The struggle to balance these risks fuels morbidity and mortality and can hamper cancer treatment, said Dr. Raskob of the University of Oklahoma, Oklahoma City.
Pharmacy and medical oncology societies recommend long-term low-molecular-weight heparin for cancer patients with VTE, but the daily burden of subcutaneous injections leads many to stop after about 2-4 months of treatment, Dr. Raskob said. “Direct oral anticoagulants may be an attractive alternative.”
For the trial, 1,446 adults with cancer and lower limb VTE from 114 clinics in North America, Europe, Australia, and New Zealand received either edoxaban (60 mg daily) or dalteparin (200 IU/kg for 30 days, followed by 150 IU/kg) for up to 12 months. Nearly all patients had active cancer. Tumor types reflected what’s most common in practice, such as malignancies of the lung, colon, and breast. About 50 patients had primary or metastatic brain cancers. Approximately two-thirds had pulmonary embolism with or without deep-vein thrombosis, while the rest had isolated deep-vein thrombosis.
After 12 months of follow-up, 12.8% of edoxaban patients had at least one recurrence of VTE or a major bleed, compared with 13.5% of dalteparin patients (hazard ratio, 0.97; 95% confidence interval, 0.70-1.36; P = .006 for noninferiority). Edoxaban also was noninferior to dalteparin after the first 6 months of treatment and in the per-protocol analysis (HRs, 1.0; P = .02 for noninferiority in each analysis). Thus, differences in efficacy did not only reflect better compliance to oral therapy, Dr. Raskob said.
He also reported on individual outcomes. In all, 10.3% of dalteparin recipients had a VTE recurrence, as did 6.5% of edoxaban recipients, for a risk difference of 3.8% (95% CI, 7.1%-0.4%). More than half of recurrences in each group were symptomatic, and none were fatal. Bleeding caused no deaths in either study arm, and each therapy conferred an identical chance of a grade 3-4 major bleed (2.3%).
Edoxaban was associated, however, with a greater frequency of major bleeds (33 events; 6.3%) than was dalteparin (17 events; 3.2%; risk difference, 3.1%; 95% CI, 0.5%-5.7%). In particular, patients who received edoxaban had a slightly higher rate of upper gastrointestinal bleeds. Most had gastric cancer.
Future studies should evaluate whether these patients should receive a lower dose of edoxaban, said Dr. Raskob. “We don’t yet fully know the minimum effective dose [of edoxaban] in cancer patients.”
He also addressed the idea that heparin has antineoplastic activity, calling it “one we should probably abandon. The concept originates from older trials in which researchers probably did not recognize that heparin was preventing fatal pulmonary embolism, he said.
The investigators soon will begin deeper analyses that should inform patient selection, he said. For now, he recommends discussing these findings with patients to help them make an informed choice between oral anticoagulation, with its ease of use but slightly higher rate of major bleeds, and subcutaneous heparin, with its lower bleeding rate and treatment burden.
Daiichi Sankyo provided funding. Dr. Raskob disclosed consulting relationships and honoraria from Daiichi Sankyo, Eli Lilly, Janssen, and several other pharmaceutical companies.
SOURCE: Raskob G et al. ASH Abstract LBA-6.
REPORTING FROM ASH 2017
Key clinical point: Oral anticoagulation with edoxaban is easier, but has a slightly higher rate of major bleeds than does subcutaneous heparin.
Major finding: .
Data source: A randomized, multicenter, open-label trial of 1,046 adults with cancer and VTE.
Disclosures: Daiichi Sankyo provided funding. Dr. Raskob disclosed consulting relationships and honoraria from Daiichi Sankyo, Eli Lilly, Janssen, and several other pharmaceutical companies.
Source: Raskob G et al. ASH Abstract LBA-6.
Pharmacomechanical thrombolysis does not reduce post-thrombotic syndrome risk
In patients with acute proximal deep vein thrombosis who were undergoing anticoagulation, adding pharmacomechanical catheter-directed thrombolysis did not reduce risk of the post-thrombotic syndrome, according to results of a phase 3, randomized, controlled trial.
Moreover, addition of pharmacomechanical thrombolysis increased risk of major bleeding risk, investigators wrote in a report published online Dec. 6 in the New England Journal of Medicine.
“Our trial, for uncertain reasons, did not confirm these findings,” wrote Suresh Vedantham, MD of Washington University, St. Louis, and his coauthors.
Post-thrombotic syndrome is associated with chronic limb swelling and pain, and can lead to leg ulcers, impaired quality of life, and major disability. About half of patients with proximal deep vein thrombosis (DVT) will develop the post-thrombotic syndrome within 2 years, despite use of anticoagulation therapy, Dr. Vedantham and his colleagues noted.
Pharmacomechanical thrombosis is the catheter-directed delivery of a fibrinolytic agent into the thrombus, along with aspiration or maceration of the thrombus. The goal of the treatment is to reduce the burden of thrombus, which in turn might reduce risk of the post-thrombotic syndrome.
However, in their randomized trial known as ATTRACT, which included 692 patients with an acute proximal DVT, rates of post-thrombotic syndrome between 6 to 24 months after intervention were 47% in the pharmacomechanical thrombolysis group and 48% in the control group (risk ratio, 0.96; 95% CI, 0.82-1.11; P = .56), according to the report (N Engl J Med. 2017;377:2240-52). Control group patients received no procedural intervention.
Major bleeds within 10 days of the intervention were 1.7% and 0.3% for the pharmacomechanical thrombolysis and control groups, respectively (P = .049).
By contrast, in the CAVENT trial, catheter-directed thrombolysis reduced the risk of the post-thrombotic syndrome over 5 years of follow-up (Lancet Haematol. 2016;3[2]:e64-71). Dr. Vedantham and his coauthors suggested that factors potentially explaining the difference in outcomes include the number of patients enrolled (692 in ATTRACT, versus 209 in CAVENT), or the greater use of mechanical therapies in ATTRACT versus longer recombinant tissue plasminogen activator infusions in CAVENT.
The study was supported by multiple sources, including the National Heart, Lung and Blood Institute (NHLBI), Boston Scientific, Covidien (now Medtronic), Genentech, and others. Dr. Vedantham reported receiving grant support from Cook Medical and Volcano. Some of the other authors reported financial ties to Abbott Vascular, Boston Scientific, Medtronic, and other pharmaceutical and device companies.
In patients with acute proximal deep vein thrombosis who were undergoing anticoagulation, adding pharmacomechanical catheter-directed thrombolysis did not reduce risk of the post-thrombotic syndrome, according to results of a phase 3, randomized, controlled trial.
Moreover, addition of pharmacomechanical thrombolysis increased risk of major bleeding risk, investigators wrote in a report published online Dec. 6 in the New England Journal of Medicine.
“Our trial, for uncertain reasons, did not confirm these findings,” wrote Suresh Vedantham, MD of Washington University, St. Louis, and his coauthors.
Post-thrombotic syndrome is associated with chronic limb swelling and pain, and can lead to leg ulcers, impaired quality of life, and major disability. About half of patients with proximal deep vein thrombosis (DVT) will develop the post-thrombotic syndrome within 2 years, despite use of anticoagulation therapy, Dr. Vedantham and his colleagues noted.
Pharmacomechanical thrombosis is the catheter-directed delivery of a fibrinolytic agent into the thrombus, along with aspiration or maceration of the thrombus. The goal of the treatment is to reduce the burden of thrombus, which in turn might reduce risk of the post-thrombotic syndrome.
However, in their randomized trial known as ATTRACT, which included 692 patients with an acute proximal DVT, rates of post-thrombotic syndrome between 6 to 24 months after intervention were 47% in the pharmacomechanical thrombolysis group and 48% in the control group (risk ratio, 0.96; 95% CI, 0.82-1.11; P = .56), according to the report (N Engl J Med. 2017;377:2240-52). Control group patients received no procedural intervention.
Major bleeds within 10 days of the intervention were 1.7% and 0.3% for the pharmacomechanical thrombolysis and control groups, respectively (P = .049).
By contrast, in the CAVENT trial, catheter-directed thrombolysis reduced the risk of the post-thrombotic syndrome over 5 years of follow-up (Lancet Haematol. 2016;3[2]:e64-71). Dr. Vedantham and his coauthors suggested that factors potentially explaining the difference in outcomes include the number of patients enrolled (692 in ATTRACT, versus 209 in CAVENT), or the greater use of mechanical therapies in ATTRACT versus longer recombinant tissue plasminogen activator infusions in CAVENT.
The study was supported by multiple sources, including the National Heart, Lung and Blood Institute (NHLBI), Boston Scientific, Covidien (now Medtronic), Genentech, and others. Dr. Vedantham reported receiving grant support from Cook Medical and Volcano. Some of the other authors reported financial ties to Abbott Vascular, Boston Scientific, Medtronic, and other pharmaceutical and device companies.
In patients with acute proximal deep vein thrombosis who were undergoing anticoagulation, adding pharmacomechanical catheter-directed thrombolysis did not reduce risk of the post-thrombotic syndrome, according to results of a phase 3, randomized, controlled trial.
Moreover, addition of pharmacomechanical thrombolysis increased risk of major bleeding risk, investigators wrote in a report published online Dec. 6 in the New England Journal of Medicine.
“Our trial, for uncertain reasons, did not confirm these findings,” wrote Suresh Vedantham, MD of Washington University, St. Louis, and his coauthors.
Post-thrombotic syndrome is associated with chronic limb swelling and pain, and can lead to leg ulcers, impaired quality of life, and major disability. About half of patients with proximal deep vein thrombosis (DVT) will develop the post-thrombotic syndrome within 2 years, despite use of anticoagulation therapy, Dr. Vedantham and his colleagues noted.
Pharmacomechanical thrombosis is the catheter-directed delivery of a fibrinolytic agent into the thrombus, along with aspiration or maceration of the thrombus. The goal of the treatment is to reduce the burden of thrombus, which in turn might reduce risk of the post-thrombotic syndrome.
However, in their randomized trial known as ATTRACT, which included 692 patients with an acute proximal DVT, rates of post-thrombotic syndrome between 6 to 24 months after intervention were 47% in the pharmacomechanical thrombolysis group and 48% in the control group (risk ratio, 0.96; 95% CI, 0.82-1.11; P = .56), according to the report (N Engl J Med. 2017;377:2240-52). Control group patients received no procedural intervention.
Major bleeds within 10 days of the intervention were 1.7% and 0.3% for the pharmacomechanical thrombolysis and control groups, respectively (P = .049).
By contrast, in the CAVENT trial, catheter-directed thrombolysis reduced the risk of the post-thrombotic syndrome over 5 years of follow-up (Lancet Haematol. 2016;3[2]:e64-71). Dr. Vedantham and his coauthors suggested that factors potentially explaining the difference in outcomes include the number of patients enrolled (692 in ATTRACT, versus 209 in CAVENT), or the greater use of mechanical therapies in ATTRACT versus longer recombinant tissue plasminogen activator infusions in CAVENT.
The study was supported by multiple sources, including the National Heart, Lung and Blood Institute (NHLBI), Boston Scientific, Covidien (now Medtronic), Genentech, and others. Dr. Vedantham reported receiving grant support from Cook Medical and Volcano. Some of the other authors reported financial ties to Abbott Vascular, Boston Scientific, Medtronic, and other pharmaceutical and device companies.
FROM NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point:
Major finding: Rates of post-thrombotic syndrome were 47% in the pharmacomechanical thrombolysis group, and 48% in the control group (risk ratio, 0.96; 95% CI, 0.82-1.11; P = .56).
Data source: A phase 3, multicenter, randomized, open-label, assessor-blinded, controlled clinical trial, including 692 patients with acute proximal deep vein thrombosis.
Disclosures: The study was supported by multiple sources, including the National Heart, Lung and Blood Institute (NHLBI), Boston Scientific, Covidien (now Medtronic), Genentech, and others. First author Suresh Vedantham, MD, reported receiving grant support from Cook Medical and Volcano. Some of the other authors reported financial ties to Abbott Vascular, Boston Scientific, Medtronic, and other pharmaceutical and device companies.
Major venous injury tied to adverse events in aortic reconstruction
Although uncommon, major venous injury during surgery for aortic reconstruction can result in massive blood loss resulting in increased morbidity and mortality, according to the results of a retrospective review conducted by Sachinder S. Hans, MD, and colleagues, and reported online in the Annals of Vascular Surgery.
Of 945 patients undergoing major aortic reconstruction, 723 (76.5%) underwent open abdominal aortic aneurysm (AAA) repair/iliac aneurysm repair; 222 patients (23.5%) underwent aortofemoral grafting (AFG). The number of units of packed red blood cells transfused, location of injured vessel, type of repair, postoperative morbidity, and mortality were collected in a vascular registry on a continuous basis. All patients identified with iliac vein/inferior vena cava/femoral vein injury had follow-up noninvasive venous examination of the lower extremities.
A total of 17 of 945 patients (1.9%) suffered 18 major venous injuries during aortic reconstruction according to Dr. Hans and his colleagues at St. John Macomb Hospital, Warren, Mich. These injuries comprised four inferior vena cava injuries, 10 iliac vein injuries, and four left renal vein injuries (Ann Vasc Surg. 2017. doi: 10.1016/j.avsg.2017.08.004).
Overall, 16 of the 18 injuries occurred during open AAA repair (7 for ruptured AAA, and 9 for intact). Two of the patients with venous injury died (11.8%), one from uncontrolled bleeding from a tear in the right iliac during repair of a ruptured AAA, and the second from disseminated intravascular complication following repair of ruptured AAA. The remaining two major venous injuries occurred during redo AFG (1 out of 6 total) and primary AFG (1 out of 216 total).
The following risk factors were also observed: The majority of the patients experiencing major venous injury were men (83%; P = .002), and the presence of periarterial inflammation (P = .006) and associated iliac aneurysm (P = .05) were significantly associated with major venous injury among the AAA patients.
The researchers suggested the following tips to lessen the likelihood of major venous injury: “Prevention of major venous injury is not always possible; however, keeping dissection plane close to arterial wall, avoiding passage of vessel loops or tapes around the neck of the aorta and iliac bifurcation, particularly in patients with surrounding inflammation and ligating venous tributaries crossing the aorta as they are joining the inferior vena cava may help reduce incidence of such injuries.”
They also suggested that surgeons should be cognizant of the serious complication that major venous injury was for patients undergoing aortic reconstruction, and to be aware that “the incidence of such injury is higher during the repair of ruptured AAA and redo aortofemoral grafting.”
The authors received no study funding and reported that they had no conflicts.
Although uncommon, major venous injury during surgery for aortic reconstruction can result in massive blood loss resulting in increased morbidity and mortality, according to the results of a retrospective review conducted by Sachinder S. Hans, MD, and colleagues, and reported online in the Annals of Vascular Surgery.
Of 945 patients undergoing major aortic reconstruction, 723 (76.5%) underwent open abdominal aortic aneurysm (AAA) repair/iliac aneurysm repair; 222 patients (23.5%) underwent aortofemoral grafting (AFG). The number of units of packed red blood cells transfused, location of injured vessel, type of repair, postoperative morbidity, and mortality were collected in a vascular registry on a continuous basis. All patients identified with iliac vein/inferior vena cava/femoral vein injury had follow-up noninvasive venous examination of the lower extremities.
A total of 17 of 945 patients (1.9%) suffered 18 major venous injuries during aortic reconstruction according to Dr. Hans and his colleagues at St. John Macomb Hospital, Warren, Mich. These injuries comprised four inferior vena cava injuries, 10 iliac vein injuries, and four left renal vein injuries (Ann Vasc Surg. 2017. doi: 10.1016/j.avsg.2017.08.004).
Overall, 16 of the 18 injuries occurred during open AAA repair (7 for ruptured AAA, and 9 for intact). Two of the patients with venous injury died (11.8%), one from uncontrolled bleeding from a tear in the right iliac during repair of a ruptured AAA, and the second from disseminated intravascular complication following repair of ruptured AAA. The remaining two major venous injuries occurred during redo AFG (1 out of 6 total) and primary AFG (1 out of 216 total).
The following risk factors were also observed: The majority of the patients experiencing major venous injury were men (83%; P = .002), and the presence of periarterial inflammation (P = .006) and associated iliac aneurysm (P = .05) were significantly associated with major venous injury among the AAA patients.
The researchers suggested the following tips to lessen the likelihood of major venous injury: “Prevention of major venous injury is not always possible; however, keeping dissection plane close to arterial wall, avoiding passage of vessel loops or tapes around the neck of the aorta and iliac bifurcation, particularly in patients with surrounding inflammation and ligating venous tributaries crossing the aorta as they are joining the inferior vena cava may help reduce incidence of such injuries.”
They also suggested that surgeons should be cognizant of the serious complication that major venous injury was for patients undergoing aortic reconstruction, and to be aware that “the incidence of such injury is higher during the repair of ruptured AAA and redo aortofemoral grafting.”
The authors received no study funding and reported that they had no conflicts.
Although uncommon, major venous injury during surgery for aortic reconstruction can result in massive blood loss resulting in increased morbidity and mortality, according to the results of a retrospective review conducted by Sachinder S. Hans, MD, and colleagues, and reported online in the Annals of Vascular Surgery.
Of 945 patients undergoing major aortic reconstruction, 723 (76.5%) underwent open abdominal aortic aneurysm (AAA) repair/iliac aneurysm repair; 222 patients (23.5%) underwent aortofemoral grafting (AFG). The number of units of packed red blood cells transfused, location of injured vessel, type of repair, postoperative morbidity, and mortality were collected in a vascular registry on a continuous basis. All patients identified with iliac vein/inferior vena cava/femoral vein injury had follow-up noninvasive venous examination of the lower extremities.
A total of 17 of 945 patients (1.9%) suffered 18 major venous injuries during aortic reconstruction according to Dr. Hans and his colleagues at St. John Macomb Hospital, Warren, Mich. These injuries comprised four inferior vena cava injuries, 10 iliac vein injuries, and four left renal vein injuries (Ann Vasc Surg. 2017. doi: 10.1016/j.avsg.2017.08.004).
Overall, 16 of the 18 injuries occurred during open AAA repair (7 for ruptured AAA, and 9 for intact). Two of the patients with venous injury died (11.8%), one from uncontrolled bleeding from a tear in the right iliac during repair of a ruptured AAA, and the second from disseminated intravascular complication following repair of ruptured AAA. The remaining two major venous injuries occurred during redo AFG (1 out of 6 total) and primary AFG (1 out of 216 total).
The following risk factors were also observed: The majority of the patients experiencing major venous injury were men (83%; P = .002), and the presence of periarterial inflammation (P = .006) and associated iliac aneurysm (P = .05) were significantly associated with major venous injury among the AAA patients.
The researchers suggested the following tips to lessen the likelihood of major venous injury: “Prevention of major venous injury is not always possible; however, keeping dissection plane close to arterial wall, avoiding passage of vessel loops or tapes around the neck of the aorta and iliac bifurcation, particularly in patients with surrounding inflammation and ligating venous tributaries crossing the aorta as they are joining the inferior vena cava may help reduce incidence of such injuries.”
They also suggested that surgeons should be cognizant of the serious complication that major venous injury was for patients undergoing aortic reconstruction, and to be aware that “the incidence of such injury is higher during the repair of ruptured AAA and redo aortofemoral grafting.”
The authors received no study funding and reported that they had no conflicts.
FROM THE ANNALS OF VASCULAR SURGERY
Key clinical point:
Major finding: A total of 17/945 patients suffered major venous injuries during aortic reconstruction.
Data source: A retrospective review of 945 patients undergoing aortic reconstruction at two sites.
Disclosures: The authors received no study funding and reported that they had no conflicts.
Pancreatic surgery: Similar outcomes with primary anastomosis, allografts
Pancreatic tumor involvement with the superior mesenteric vein/portal vein (SMV/PV) is common and requires exploration and resection, which has now become an integral part of routine surgical treatment. The short-term outcome of SMV/PV reconstruction with interposed cold-stored cadaveric venous allografts was found to be comparable to that of reconstruction with primary end-to-end anastomosis, according to the results of a study performed by Dyre Kleive, MD, and his colleagues.
In order to assess the optimal method of reconstructing the portal vein during pancreatic surgery, Dr. Kleive and his colleagues performed a retrospective review of all patients undergoing pancreatic surgery with venous resection and reconstruction at a single center between January 2006 and December 2015.
A total of 857 patients underwent open pancreatic surgery during the study period, of whom 171 (20%) had vascular resection and reconstruction. The study population comprised 42 patients treated with cold-stored interposition cadaveric allografts for reconstruction and 71 patients who had primary end-to-end anastomosis instead. Patients with other forms of reconstruction were excluded, according to an online report in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (2017. doi: 10.1016/j.jvsv.2017.09.003).
Early failure at the reconstruction site was defined as the presence of thrombosis or no flow or low flow within the first 30 days after surgery.
Patients in the allograft group had statistically significantly longer mean operative times, more intraoperative bleeding, more frequent use of neoadjuvant therapy, and a longer length of tumor-vein involvement than the anastomosis group.
However, there was no statistically significant difference in the number of patients with major complications (42.9% for allografts vs. 36.6% for anastomosis) or early failure at the reconstruction site (9.5% for allografts vs. 8.5% for anastomosis) between the two groups, Dr Kleive and his colleagues reported.
The proportion of patients with grade C stenosis at last available imaging scan was significantly higher in the allograft group (26/42 [61.9%] vs. 13 of 66 [19.7%] for the anastomosis group; P less than .01). A subgroup analysis of 10 patients in the allograft group showed the presence of donor-specific antibodies in all patients. This could indicate that graft rejection was a contributing factor to the statistically higher development of severe stenosis in allograft vs. anastomosis patients, the authors suggested.
“This study shows that the short-term outcome of SMV/PV reconstruction with interposed cold-stored cadaveric venous allografts is comparable to that of reconstruction with primary end-to-end anastomosis,” the researchers concluded.
Dr. Kleive and his colleagues reported that they had no conflicts of interest.
Pancreatic tumor involvement with the superior mesenteric vein/portal vein (SMV/PV) is common and requires exploration and resection, which has now become an integral part of routine surgical treatment. The short-term outcome of SMV/PV reconstruction with interposed cold-stored cadaveric venous allografts was found to be comparable to that of reconstruction with primary end-to-end anastomosis, according to the results of a study performed by Dyre Kleive, MD, and his colleagues.
In order to assess the optimal method of reconstructing the portal vein during pancreatic surgery, Dr. Kleive and his colleagues performed a retrospective review of all patients undergoing pancreatic surgery with venous resection and reconstruction at a single center between January 2006 and December 2015.
A total of 857 patients underwent open pancreatic surgery during the study period, of whom 171 (20%) had vascular resection and reconstruction. The study population comprised 42 patients treated with cold-stored interposition cadaveric allografts for reconstruction and 71 patients who had primary end-to-end anastomosis instead. Patients with other forms of reconstruction were excluded, according to an online report in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (2017. doi: 10.1016/j.jvsv.2017.09.003).
Early failure at the reconstruction site was defined as the presence of thrombosis or no flow or low flow within the first 30 days after surgery.
Patients in the allograft group had statistically significantly longer mean operative times, more intraoperative bleeding, more frequent use of neoadjuvant therapy, and a longer length of tumor-vein involvement than the anastomosis group.
However, there was no statistically significant difference in the number of patients with major complications (42.9% for allografts vs. 36.6% for anastomosis) or early failure at the reconstruction site (9.5% for allografts vs. 8.5% for anastomosis) between the two groups, Dr Kleive and his colleagues reported.
The proportion of patients with grade C stenosis at last available imaging scan was significantly higher in the allograft group (26/42 [61.9%] vs. 13 of 66 [19.7%] for the anastomosis group; P less than .01). A subgroup analysis of 10 patients in the allograft group showed the presence of donor-specific antibodies in all patients. This could indicate that graft rejection was a contributing factor to the statistically higher development of severe stenosis in allograft vs. anastomosis patients, the authors suggested.
“This study shows that the short-term outcome of SMV/PV reconstruction with interposed cold-stored cadaveric venous allografts is comparable to that of reconstruction with primary end-to-end anastomosis,” the researchers concluded.
Dr. Kleive and his colleagues reported that they had no conflicts of interest.
Pancreatic tumor involvement with the superior mesenteric vein/portal vein (SMV/PV) is common and requires exploration and resection, which has now become an integral part of routine surgical treatment. The short-term outcome of SMV/PV reconstruction with interposed cold-stored cadaveric venous allografts was found to be comparable to that of reconstruction with primary end-to-end anastomosis, according to the results of a study performed by Dyre Kleive, MD, and his colleagues.
In order to assess the optimal method of reconstructing the portal vein during pancreatic surgery, Dr. Kleive and his colleagues performed a retrospective review of all patients undergoing pancreatic surgery with venous resection and reconstruction at a single center between January 2006 and December 2015.
A total of 857 patients underwent open pancreatic surgery during the study period, of whom 171 (20%) had vascular resection and reconstruction. The study population comprised 42 patients treated with cold-stored interposition cadaveric allografts for reconstruction and 71 patients who had primary end-to-end anastomosis instead. Patients with other forms of reconstruction were excluded, according to an online report in the Journal of Vascular Surgery: Venous and Lymphatic Disorders (2017. doi: 10.1016/j.jvsv.2017.09.003).
Early failure at the reconstruction site was defined as the presence of thrombosis or no flow or low flow within the first 30 days after surgery.
Patients in the allograft group had statistically significantly longer mean operative times, more intraoperative bleeding, more frequent use of neoadjuvant therapy, and a longer length of tumor-vein involvement than the anastomosis group.
However, there was no statistically significant difference in the number of patients with major complications (42.9% for allografts vs. 36.6% for anastomosis) or early failure at the reconstruction site (9.5% for allografts vs. 8.5% for anastomosis) between the two groups, Dr Kleive and his colleagues reported.
The proportion of patients with grade C stenosis at last available imaging scan was significantly higher in the allograft group (26/42 [61.9%] vs. 13 of 66 [19.7%] for the anastomosis group; P less than .01). A subgroup analysis of 10 patients in the allograft group showed the presence of donor-specific antibodies in all patients. This could indicate that graft rejection was a contributing factor to the statistically higher development of severe stenosis in allograft vs. anastomosis patients, the authors suggested.
“This study shows that the short-term outcome of SMV/PV reconstruction with interposed cold-stored cadaveric venous allografts is comparable to that of reconstruction with primary end-to-end anastomosis,” the researchers concluded.
Dr. Kleive and his colleagues reported that they had no conflicts of interest.
FROM THE JOURNAL OF VASCULAR SURGERY: VENOUS AND LYMPHATIC DISEASES
Key clinical point:
Major finding: There was no statistically significant difference in the number of patients with major complications or early failure at the reconstruction site between the allograft and the anastomosis groups.
Data source: A retrospective review of all 171 patients undergoing pancreatic surgery with venous resection and reconstruction at a single center between January 2006 and December 2015.
Disclosures: The authors reported that they had no conflicts of interest.
Stenting improved outcomes for treating chronic IVC obstruction
Stenting improved outcomes in patients with chronic obstruction of the inferior vena cava (IVC), according to a retrospective analysis of medical records from March 2010 to September 2015.
In the study, 19 of 20 patients with chronic IVC obstruction had successful stent placement, with most resulting in improved and sustained clinical outcomes. The study, conducted by Ole Jørgen Grøtta, MD, and colleagues, was reported in the European Journal of Vascular and Endovascular Surgery (2017;54:620-8).
Dr. Grøtta and colleagues investigated 11 men and 9 women patients, with a median age 43years, and with venography-verified chronic IVC obstruction. Most patients also were screened and found to be positive for thrombophilia. Median follow-up was 25 months (range, 3-70 months).
The researchers reported on 13 patients with IVC occlusion and 7 with stenosis. Patient clinical status and symptom severity was assessed according to standardized CEAP (clinical, etiology, anatomy and pathophysiology) classification and the venous clinical severity score (VCSS), respectively. Sixteen patients presented chronic venous disease (CVD) symptoms in their lower limbs; of these, nine exhibited symptoms of an acute thrombosis and, based on their symptoms, seven were assigned to a specific CEAP category, ranging from C3-C6. The additional four patients included in the study had been referred to receive endovascular treatment intervention based on symptoms consistent with physical activity–related reduced cardiac preload.
A total of 13 of 19 (68%) patients showed sustained and significant clinical improvement, seen as a shift from the baseline VCSS score of 8.5 down to 7.0 (P = .007) at final follow-up. This included all four of the patients identified as having reduced cardiac preload problems. The authors also reported that here were no periprocedural or long-term complications.
The need for an alternative treatment for patients with chronic obstruction of the IVC is exemplified by typically poor sustained clinical improvement outcomes and/or gradual clinical deterioration when using the traditional conservative approach of anticoagulants combined with compression stockings, according to the authors.
“The endovascular approach with stent placement for chronic IVC obstructions is a safe treatment alternative that should be offered to patients who otherwise have little opportunity for clinical improvement,” they concluded.
The authors reported that they had no conflicts of interest.
Stenting improved outcomes in patients with chronic obstruction of the inferior vena cava (IVC), according to a retrospective analysis of medical records from March 2010 to September 2015.
In the study, 19 of 20 patients with chronic IVC obstruction had successful stent placement, with most resulting in improved and sustained clinical outcomes. The study, conducted by Ole Jørgen Grøtta, MD, and colleagues, was reported in the European Journal of Vascular and Endovascular Surgery (2017;54:620-8).
Dr. Grøtta and colleagues investigated 11 men and 9 women patients, with a median age 43years, and with venography-verified chronic IVC obstruction. Most patients also were screened and found to be positive for thrombophilia. Median follow-up was 25 months (range, 3-70 months).
The researchers reported on 13 patients with IVC occlusion and 7 with stenosis. Patient clinical status and symptom severity was assessed according to standardized CEAP (clinical, etiology, anatomy and pathophysiology) classification and the venous clinical severity score (VCSS), respectively. Sixteen patients presented chronic venous disease (CVD) symptoms in their lower limbs; of these, nine exhibited symptoms of an acute thrombosis and, based on their symptoms, seven were assigned to a specific CEAP category, ranging from C3-C6. The additional four patients included in the study had been referred to receive endovascular treatment intervention based on symptoms consistent with physical activity–related reduced cardiac preload.
A total of 13 of 19 (68%) patients showed sustained and significant clinical improvement, seen as a shift from the baseline VCSS score of 8.5 down to 7.0 (P = .007) at final follow-up. This included all four of the patients identified as having reduced cardiac preload problems. The authors also reported that here were no periprocedural or long-term complications.
The need for an alternative treatment for patients with chronic obstruction of the IVC is exemplified by typically poor sustained clinical improvement outcomes and/or gradual clinical deterioration when using the traditional conservative approach of anticoagulants combined with compression stockings, according to the authors.
“The endovascular approach with stent placement for chronic IVC obstructions is a safe treatment alternative that should be offered to patients who otherwise have little opportunity for clinical improvement,” they concluded.
The authors reported that they had no conflicts of interest.
Stenting improved outcomes in patients with chronic obstruction of the inferior vena cava (IVC), according to a retrospective analysis of medical records from March 2010 to September 2015.
In the study, 19 of 20 patients with chronic IVC obstruction had successful stent placement, with most resulting in improved and sustained clinical outcomes. The study, conducted by Ole Jørgen Grøtta, MD, and colleagues, was reported in the European Journal of Vascular and Endovascular Surgery (2017;54:620-8).
Dr. Grøtta and colleagues investigated 11 men and 9 women patients, with a median age 43years, and with venography-verified chronic IVC obstruction. Most patients also were screened and found to be positive for thrombophilia. Median follow-up was 25 months (range, 3-70 months).
The researchers reported on 13 patients with IVC occlusion and 7 with stenosis. Patient clinical status and symptom severity was assessed according to standardized CEAP (clinical, etiology, anatomy and pathophysiology) classification and the venous clinical severity score (VCSS), respectively. Sixteen patients presented chronic venous disease (CVD) symptoms in their lower limbs; of these, nine exhibited symptoms of an acute thrombosis and, based on their symptoms, seven were assigned to a specific CEAP category, ranging from C3-C6. The additional four patients included in the study had been referred to receive endovascular treatment intervention based on symptoms consistent with physical activity–related reduced cardiac preload.
A total of 13 of 19 (68%) patients showed sustained and significant clinical improvement, seen as a shift from the baseline VCSS score of 8.5 down to 7.0 (P = .007) at final follow-up. This included all four of the patients identified as having reduced cardiac preload problems. The authors also reported that here were no periprocedural or long-term complications.
The need for an alternative treatment for patients with chronic obstruction of the IVC is exemplified by typically poor sustained clinical improvement outcomes and/or gradual clinical deterioration when using the traditional conservative approach of anticoagulants combined with compression stockings, according to the authors.
“The endovascular approach with stent placement for chronic IVC obstructions is a safe treatment alternative that should be offered to patients who otherwise have little opportunity for clinical improvement,” they concluded.
The authors reported that they had no conflicts of interest.
FROM THE EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY
Key clinical point:
Major finding: Most patients showed a significant improvement from the baseline score of 8.5 score down to 7.0 (P = .007).
Data source: A retrospective analysis of 20 patients referred to the Norwegian National Unit for Reconstructive Deep Venous Surgery for stenting.
Disclosures: The authors reported they had no conflict of interest.