User login
Update on the Standard of Care of Dermatologic Postprocedural Wounds
Blog: Ablative Fractional Resurfacing Improves Scar Mobility
When you think of ablative fractional resurfacing, you probably think of photo-damaged skin and acne scars, said Dr. Kenneth A. Arndt, a clinical professor of dermatology, emeritus, at Harvard.
So why would you use something that tightens skin to treat scars? When ablative fractional resurfacing is used on scars, it actually increases pliability, he said.
When you think of ablative fractional resurfacing, you probably think of photo-damaged skin and acne scars, said Dr. Kenneth A. Arndt, a clinical professor of dermatology, emeritus, at Harvard.
So why would you use something that tightens skin to treat scars? When ablative fractional resurfacing is used on scars, it actually increases pliability, he said.
When you think of ablative fractional resurfacing, you probably think of photo-damaged skin and acne scars, said Dr. Kenneth A. Arndt, a clinical professor of dermatology, emeritus, at Harvard.
So why would you use something that tightens skin to treat scars? When ablative fractional resurfacing is used on scars, it actually increases pliability, he said.
LET Gel Eases Pediatric Wound Suturing
STEAMBOAT SPRINGS, COLO. – Make lidocaine, epinephrine, and tetracaine gel your choice for pain control when repairing lacerations in children, Dr. Steven M. Selbst said.
Adoption of LET gel for routine use in wound repair may be the single most important change in practice that physicians can make in terms of analgesia for children, said Dr. Steven M. Selbst, professor and vice chair of pediatrics at Jefferson Medical College, Philadelphia.
"Even if you don’t suture wounds in your office, I think it’s key to try to make sure that the emergency department near your office uses LET in wound repair for children. It’s an incredible agent. I’ve been using it for 20 years, and I know it has been around for longer than that. I’ve seen so many anxious kids who are scared to death of having a wound repair with suturing that have had a completely painless repair with LET without any injection whatsoever. To me it’s amazing that some hospitals still don’t use LET," said Dr. Selbst, who is chair of the executive committee of the American Academy of Pediatrics Section on Pediatric Emergency Medicine.
The advantages of pharmacist-compounded LET gel over commercially available anesthetic creams, such as eutectic mixture of local anesthetics (EMLA) and lidocaine 4% (LMX-4), include much lower cost and a good anesthetic response within 20-30 minutes after LET is applied. In contrast, EMLA requires 60 minutes of contact, making it less practical for laceration repair. LET is as effective as tetracaine, adrenaline, and cocaine (TAC) solution, but it costs less and has less morbidity, he said at the meeting.
Once the treated site shows blanching due to LET’s vasoconstrictive activity, the physician can proceed with pain-free suturing, even on the face and scalp.
The gel formulation of LET contains 10 mL of injectable lidocaine 20%, 5 mL of racemic epinephrine, 12.5 mL of tetracaine hydrochloride 2%, 31.5 mg of sodium metabisulfite, and methylcellulose gel 5% added in sufficient quantity to bring the total volume to 50 mL. The ingredients are stirred or shaken until completely mixed, which takes about 2-3 minutes.
The LET gel remains stable for 4 weeks at room temperature or for 6 months if refrigerated.
"You can apply the gel directly to the wound or put it on cotton gauze and tape it to the wound. Use a generous amount," Dr. Selbst said.
Numerous studies have documented that inadequate pain control is far more common in children with painful conditions than in adults. Children with lower-extremity fractures, serious burns, or sickle cell crises were less than half as likely to get analgesics in the emergency department, compared with adults with the same conditions, according to an earlier study done by Dr. Selbst and a colleague. They also found that kids younger than 2 years got analgesics less frequently than older children (Ann. Emerg. Med. 1990;19:1010-3).
Recent studies indicate this gap has narrowed somewhat, although inadequate dosing of analgesics in children continues to be a problem. Possible explanations include the inability of infants and young children to verbalize, the disproved myth that babies don’t feel or remember pain, and fear of causing respiratory depression or addiction, although there is no evidence that giving a single dose of a narcotic for an acute painful condition is associated with an increased risk of addiction, Dr. Selbst emphasized.
He reported having no financial conflicts.
STEAMBOAT SPRINGS, COLO. – Make lidocaine, epinephrine, and tetracaine gel your choice for pain control when repairing lacerations in children, Dr. Steven M. Selbst said.
Adoption of LET gel for routine use in wound repair may be the single most important change in practice that physicians can make in terms of analgesia for children, said Dr. Steven M. Selbst, professor and vice chair of pediatrics at Jefferson Medical College, Philadelphia.
"Even if you don’t suture wounds in your office, I think it’s key to try to make sure that the emergency department near your office uses LET in wound repair for children. It’s an incredible agent. I’ve been using it for 20 years, and I know it has been around for longer than that. I’ve seen so many anxious kids who are scared to death of having a wound repair with suturing that have had a completely painless repair with LET without any injection whatsoever. To me it’s amazing that some hospitals still don’t use LET," said Dr. Selbst, who is chair of the executive committee of the American Academy of Pediatrics Section on Pediatric Emergency Medicine.
The advantages of pharmacist-compounded LET gel over commercially available anesthetic creams, such as eutectic mixture of local anesthetics (EMLA) and lidocaine 4% (LMX-4), include much lower cost and a good anesthetic response within 20-30 minutes after LET is applied. In contrast, EMLA requires 60 minutes of contact, making it less practical for laceration repair. LET is as effective as tetracaine, adrenaline, and cocaine (TAC) solution, but it costs less and has less morbidity, he said at the meeting.
Once the treated site shows blanching due to LET’s vasoconstrictive activity, the physician can proceed with pain-free suturing, even on the face and scalp.
The gel formulation of LET contains 10 mL of injectable lidocaine 20%, 5 mL of racemic epinephrine, 12.5 mL of tetracaine hydrochloride 2%, 31.5 mg of sodium metabisulfite, and methylcellulose gel 5% added in sufficient quantity to bring the total volume to 50 mL. The ingredients are stirred or shaken until completely mixed, which takes about 2-3 minutes.
The LET gel remains stable for 4 weeks at room temperature or for 6 months if refrigerated.
"You can apply the gel directly to the wound or put it on cotton gauze and tape it to the wound. Use a generous amount," Dr. Selbst said.
Numerous studies have documented that inadequate pain control is far more common in children with painful conditions than in adults. Children with lower-extremity fractures, serious burns, or sickle cell crises were less than half as likely to get analgesics in the emergency department, compared with adults with the same conditions, according to an earlier study done by Dr. Selbst and a colleague. They also found that kids younger than 2 years got analgesics less frequently than older children (Ann. Emerg. Med. 1990;19:1010-3).
Recent studies indicate this gap has narrowed somewhat, although inadequate dosing of analgesics in children continues to be a problem. Possible explanations include the inability of infants and young children to verbalize, the disproved myth that babies don’t feel or remember pain, and fear of causing respiratory depression or addiction, although there is no evidence that giving a single dose of a narcotic for an acute painful condition is associated with an increased risk of addiction, Dr. Selbst emphasized.
He reported having no financial conflicts.
STEAMBOAT SPRINGS, COLO. – Make lidocaine, epinephrine, and tetracaine gel your choice for pain control when repairing lacerations in children, Dr. Steven M. Selbst said.
Adoption of LET gel for routine use in wound repair may be the single most important change in practice that physicians can make in terms of analgesia for children, said Dr. Steven M. Selbst, professor and vice chair of pediatrics at Jefferson Medical College, Philadelphia.
"Even if you don’t suture wounds in your office, I think it’s key to try to make sure that the emergency department near your office uses LET in wound repair for children. It’s an incredible agent. I’ve been using it for 20 years, and I know it has been around for longer than that. I’ve seen so many anxious kids who are scared to death of having a wound repair with suturing that have had a completely painless repair with LET without any injection whatsoever. To me it’s amazing that some hospitals still don’t use LET," said Dr. Selbst, who is chair of the executive committee of the American Academy of Pediatrics Section on Pediatric Emergency Medicine.
The advantages of pharmacist-compounded LET gel over commercially available anesthetic creams, such as eutectic mixture of local anesthetics (EMLA) and lidocaine 4% (LMX-4), include much lower cost and a good anesthetic response within 20-30 minutes after LET is applied. In contrast, EMLA requires 60 minutes of contact, making it less practical for laceration repair. LET is as effective as tetracaine, adrenaline, and cocaine (TAC) solution, but it costs less and has less morbidity, he said at the meeting.
Once the treated site shows blanching due to LET’s vasoconstrictive activity, the physician can proceed with pain-free suturing, even on the face and scalp.
The gel formulation of LET contains 10 mL of injectable lidocaine 20%, 5 mL of racemic epinephrine, 12.5 mL of tetracaine hydrochloride 2%, 31.5 mg of sodium metabisulfite, and methylcellulose gel 5% added in sufficient quantity to bring the total volume to 50 mL. The ingredients are stirred or shaken until completely mixed, which takes about 2-3 minutes.
The LET gel remains stable for 4 weeks at room temperature or for 6 months if refrigerated.
"You can apply the gel directly to the wound or put it on cotton gauze and tape it to the wound. Use a generous amount," Dr. Selbst said.
Numerous studies have documented that inadequate pain control is far more common in children with painful conditions than in adults. Children with lower-extremity fractures, serious burns, or sickle cell crises were less than half as likely to get analgesics in the emergency department, compared with adults with the same conditions, according to an earlier study done by Dr. Selbst and a colleague. They also found that kids younger than 2 years got analgesics less frequently than older children (Ann. Emerg. Med. 1990;19:1010-3).
Recent studies indicate this gap has narrowed somewhat, although inadequate dosing of analgesics in children continues to be a problem. Possible explanations include the inability of infants and young children to verbalize, the disproved myth that babies don’t feel or remember pain, and fear of causing respiratory depression or addiction, although there is no evidence that giving a single dose of a narcotic for an acute painful condition is associated with an increased risk of addiction, Dr. Selbst emphasized.
He reported having no financial conflicts.
A MEETING ON PRACTICAL PEDIATRICS SPONSORED BY THE AMERICAN ACADEMY OF PEDIATRICS
'Transcutaneous Systemic' Clobetasol Reverses Bullous Pemphigoid
LISBON – An intensive regimen of whole-body topical clobetasol may be an effective and better tolerated alternative to standard, high-dose oral prednisone in patients with milder cases of bullous pemphigoid, according to Dr. Marcel F. Jonkman.
"In your daily practice you all use topical clobetasol, but not like this. I call it transcutaneous systemic clobetasol therapy. That means you put it on the entire geography, the whole body surface from your cheeks to your toes, not just on lesional skin," Dr. Jonkman explained at the annual congress of the European Academy of Dermatology and Venereology.
This is a laborious 4-month-long course of therapy. For mild disease – which Dr. Jonkman defined as fewer than 10 new bullae arising during the 3 days before first consultation – patients apply 20 g of clobetasol propionate cream once daily for the first month, then every other day for the second month, twice weekly in the third, and once per week for the fourth month.
Patients with severe bullous pemphigoid follow the same schedule, using 30 g per application rather than 20 g.
"That's really a lot of clobetasol. If you put on 25 g, I expect it's effectively equivalent to 35-40 mg of oral prednisone. Sometimes I get referred patients who are on 80 mg/day of prednisone and they respond to clobetasol therapy," said Dr. Jonkman, professor and chair of the department of dermatology at the University of Groningen (the Netherlands).
This is a decidedly off-label method of applying the superpotent fluorinated topical corticosteroid, he said. And make no mistake, the medication is absorbed systemically to a substantial degree. That's why it is so effective. Within about 5 days after starting daily therapy, the peripheral hypereosinophilia that marks bullous pemphigoid is reversed, with titers falling into the normal range.
Moreover, whole-body topical clobetasol is capable of inducing adrenal gland suppression and other familiar systemic side effects of high-dose corticosteroid therapy, said Dr. Jonkman. The main advantage of topical therapy is it avoids the severe gastritis that often results from long-term, high-dose oral prednisone.
Clinical Experience
He presented his personal experience using transcutaneous systemic clobetasol in 40 patients with mild, and 34 patients with severe, bullous pemphigoid. Patients in the mild disease group had a mean of 3.6 new blisters during the 3 days prior to first consultation; those in the severe bullous pemphigoid group averaged 36.7.
As a clinical pearl, Dr. Jonkman noted that all patients with severe disease and 39 of the 40 with mild disease had prominent itching. "That means if your patient does not have itch, he or she probably doesn’t have bullous pemphigoid. It's such a central part of the disease," he said.
Disease control (absence of new bullae for 3 consecutive days) was achieved in a mean of 26 days in 36 of 40 patients (90%) with mild disease and in 25 of 34 (73.5%) with severe disease. Thus, transcutaneous systemic clobetasol was less effective in patients with severe bullous pemphigoid, even though most of them were on additional medications, most often azathioprine.
"In severe bullous pemphigoid, trans-cutaneous systemic clobetasol is not sufficient, although it has added value as an adjunct," Dr. Jonkman said.
Thirty-six percent of patients in the mild disease group maintained a complete remission off therapy. Another 29% had a complete remission while on therapy only, 15% had a partial remission, and 20% had no remission.
In the severe bullous pemphigoid group, 6% maintained a complete remission off therapy, 35% had a complete remission while on therapy only, 29% had a partial remission, and 30% had no remission.
Relapse occurred in 56% of responders in both groups a mean of 2 months after they completed the 4-month treatment course. The relapses were mild and responded to another round of whole-body clobetasol or, if the patient preferred, oral therapy, according to Dr. Jonkman.
The most common side effect was skin atrophy, seen in 13 of the 74 patients. Striae and purpura were the other main adverse events.
Audience Questions
An audience member at the congress asked if the treatment could trigger steroid-induced diabetes. Dr. Jonkman replied that the complication hasn't arisen in his patients on transcutaneous systemic clobetasol therapy. He said he suspects it's because the 4-month treatment course is too brief to trigger the problem. He has found, however, that some patients with preexisting type 2 diabetes require an additional oral antidiabetic agent.
Another audience member asked if patients should apply the topical steroid on top of intact blisters and the wounds created by broken blisters. This should be done, Dr. Jonkman advised. "The medication won't work on top of an intact blister because it's too far away from the dermis. But because it's systemic therapy it will come from the blood into the skin," he explained.
A third audience member asked if Dr. Jonkman had ever used whole-body clobetasol in patients with pemphigus foliaceous. He replied that he hasn't because the opportunity hasn't arisen, although he said he suspects it would be effective in milder cases.
Background Studies
Dr. Jonkman credited French dermatologists with the idea of using topical corticosteroids to treat patients with bullous pemphigoid. In a landmark multicenter trial nearly a decade ago, researchers randomized 341 bullous pemphigoid patients to 40 g/day of topical clobetasol propionate cream or to oral prednisone for 1 year. Patients with moderate disease received 0.5 mg/kg per day of prednisone, while those with severe disease got 1 mg/kg.
The topical regimen proved superior to oral prednisone in the 188 patients with extensive disease. Disease was controlled at 3 weeks in 99% of patients on clobetasol, significantly higher than the 91% rate with prednisone. Moreover, the 1-year survival rate was 76% in the topical therapy arm, compared with 58% with oral therapy. Severe complications were half as common in the clobetasol arm.
In patients with moderate bullous pemphigoid, the two forms of therapy were similar in terms of disease control at 3 weeks, overall survival, and severe complications (N. Engl. J. Med. 2002;346:321-7).
Dr. Jonkman noted that more recently the French group tweaked their topical regimen, adopting a milder approach similar to his own. In a 312-patient multicenter trial, they randomized participants to 10-30 g/day of clobetasol tapered over a 4-month period or to their earlier regimen of 40 g/day for a full year. The investigators showed that the rate of death or life-threatening adverse events in the 134 patients with moderate disease was reduced by nearly half with the milder regimen (J. Invest. Dermatol. 2009;129:1681-7).
He reported having no relevant financial disclosures.
LISBON – An intensive regimen of whole-body topical clobetasol may be an effective and better tolerated alternative to standard, high-dose oral prednisone in patients with milder cases of bullous pemphigoid, according to Dr. Marcel F. Jonkman.
"In your daily practice you all use topical clobetasol, but not like this. I call it transcutaneous systemic clobetasol therapy. That means you put it on the entire geography, the whole body surface from your cheeks to your toes, not just on lesional skin," Dr. Jonkman explained at the annual congress of the European Academy of Dermatology and Venereology.
This is a laborious 4-month-long course of therapy. For mild disease – which Dr. Jonkman defined as fewer than 10 new bullae arising during the 3 days before first consultation – patients apply 20 g of clobetasol propionate cream once daily for the first month, then every other day for the second month, twice weekly in the third, and once per week for the fourth month.
Patients with severe bullous pemphigoid follow the same schedule, using 30 g per application rather than 20 g.
"That's really a lot of clobetasol. If you put on 25 g, I expect it's effectively equivalent to 35-40 mg of oral prednisone. Sometimes I get referred patients who are on 80 mg/day of prednisone and they respond to clobetasol therapy," said Dr. Jonkman, professor and chair of the department of dermatology at the University of Groningen (the Netherlands).
This is a decidedly off-label method of applying the superpotent fluorinated topical corticosteroid, he said. And make no mistake, the medication is absorbed systemically to a substantial degree. That's why it is so effective. Within about 5 days after starting daily therapy, the peripheral hypereosinophilia that marks bullous pemphigoid is reversed, with titers falling into the normal range.
Moreover, whole-body topical clobetasol is capable of inducing adrenal gland suppression and other familiar systemic side effects of high-dose corticosteroid therapy, said Dr. Jonkman. The main advantage of topical therapy is it avoids the severe gastritis that often results from long-term, high-dose oral prednisone.
Clinical Experience
He presented his personal experience using transcutaneous systemic clobetasol in 40 patients with mild, and 34 patients with severe, bullous pemphigoid. Patients in the mild disease group had a mean of 3.6 new blisters during the 3 days prior to first consultation; those in the severe bullous pemphigoid group averaged 36.7.
As a clinical pearl, Dr. Jonkman noted that all patients with severe disease and 39 of the 40 with mild disease had prominent itching. "That means if your patient does not have itch, he or she probably doesn’t have bullous pemphigoid. It's such a central part of the disease," he said.
Disease control (absence of new bullae for 3 consecutive days) was achieved in a mean of 26 days in 36 of 40 patients (90%) with mild disease and in 25 of 34 (73.5%) with severe disease. Thus, transcutaneous systemic clobetasol was less effective in patients with severe bullous pemphigoid, even though most of them were on additional medications, most often azathioprine.
"In severe bullous pemphigoid, trans-cutaneous systemic clobetasol is not sufficient, although it has added value as an adjunct," Dr. Jonkman said.
Thirty-six percent of patients in the mild disease group maintained a complete remission off therapy. Another 29% had a complete remission while on therapy only, 15% had a partial remission, and 20% had no remission.
In the severe bullous pemphigoid group, 6% maintained a complete remission off therapy, 35% had a complete remission while on therapy only, 29% had a partial remission, and 30% had no remission.
Relapse occurred in 56% of responders in both groups a mean of 2 months after they completed the 4-month treatment course. The relapses were mild and responded to another round of whole-body clobetasol or, if the patient preferred, oral therapy, according to Dr. Jonkman.
The most common side effect was skin atrophy, seen in 13 of the 74 patients. Striae and purpura were the other main adverse events.
Audience Questions
An audience member at the congress asked if the treatment could trigger steroid-induced diabetes. Dr. Jonkman replied that the complication hasn't arisen in his patients on transcutaneous systemic clobetasol therapy. He said he suspects it's because the 4-month treatment course is too brief to trigger the problem. He has found, however, that some patients with preexisting type 2 diabetes require an additional oral antidiabetic agent.
Another audience member asked if patients should apply the topical steroid on top of intact blisters and the wounds created by broken blisters. This should be done, Dr. Jonkman advised. "The medication won't work on top of an intact blister because it's too far away from the dermis. But because it's systemic therapy it will come from the blood into the skin," he explained.
A third audience member asked if Dr. Jonkman had ever used whole-body clobetasol in patients with pemphigus foliaceous. He replied that he hasn't because the opportunity hasn't arisen, although he said he suspects it would be effective in milder cases.
Background Studies
Dr. Jonkman credited French dermatologists with the idea of using topical corticosteroids to treat patients with bullous pemphigoid. In a landmark multicenter trial nearly a decade ago, researchers randomized 341 bullous pemphigoid patients to 40 g/day of topical clobetasol propionate cream or to oral prednisone for 1 year. Patients with moderate disease received 0.5 mg/kg per day of prednisone, while those with severe disease got 1 mg/kg.
The topical regimen proved superior to oral prednisone in the 188 patients with extensive disease. Disease was controlled at 3 weeks in 99% of patients on clobetasol, significantly higher than the 91% rate with prednisone. Moreover, the 1-year survival rate was 76% in the topical therapy arm, compared with 58% with oral therapy. Severe complications were half as common in the clobetasol arm.
In patients with moderate bullous pemphigoid, the two forms of therapy were similar in terms of disease control at 3 weeks, overall survival, and severe complications (N. Engl. J. Med. 2002;346:321-7).
Dr. Jonkman noted that more recently the French group tweaked their topical regimen, adopting a milder approach similar to his own. In a 312-patient multicenter trial, they randomized participants to 10-30 g/day of clobetasol tapered over a 4-month period or to their earlier regimen of 40 g/day for a full year. The investigators showed that the rate of death or life-threatening adverse events in the 134 patients with moderate disease was reduced by nearly half with the milder regimen (J. Invest. Dermatol. 2009;129:1681-7).
He reported having no relevant financial disclosures.
LISBON – An intensive regimen of whole-body topical clobetasol may be an effective and better tolerated alternative to standard, high-dose oral prednisone in patients with milder cases of bullous pemphigoid, according to Dr. Marcel F. Jonkman.
"In your daily practice you all use topical clobetasol, but not like this. I call it transcutaneous systemic clobetasol therapy. That means you put it on the entire geography, the whole body surface from your cheeks to your toes, not just on lesional skin," Dr. Jonkman explained at the annual congress of the European Academy of Dermatology and Venereology.
This is a laborious 4-month-long course of therapy. For mild disease – which Dr. Jonkman defined as fewer than 10 new bullae arising during the 3 days before first consultation – patients apply 20 g of clobetasol propionate cream once daily for the first month, then every other day for the second month, twice weekly in the third, and once per week for the fourth month.
Patients with severe bullous pemphigoid follow the same schedule, using 30 g per application rather than 20 g.
"That's really a lot of clobetasol. If you put on 25 g, I expect it's effectively equivalent to 35-40 mg of oral prednisone. Sometimes I get referred patients who are on 80 mg/day of prednisone and they respond to clobetasol therapy," said Dr. Jonkman, professor and chair of the department of dermatology at the University of Groningen (the Netherlands).
This is a decidedly off-label method of applying the superpotent fluorinated topical corticosteroid, he said. And make no mistake, the medication is absorbed systemically to a substantial degree. That's why it is so effective. Within about 5 days after starting daily therapy, the peripheral hypereosinophilia that marks bullous pemphigoid is reversed, with titers falling into the normal range.
Moreover, whole-body topical clobetasol is capable of inducing adrenal gland suppression and other familiar systemic side effects of high-dose corticosteroid therapy, said Dr. Jonkman. The main advantage of topical therapy is it avoids the severe gastritis that often results from long-term, high-dose oral prednisone.
Clinical Experience
He presented his personal experience using transcutaneous systemic clobetasol in 40 patients with mild, and 34 patients with severe, bullous pemphigoid. Patients in the mild disease group had a mean of 3.6 new blisters during the 3 days prior to first consultation; those in the severe bullous pemphigoid group averaged 36.7.
As a clinical pearl, Dr. Jonkman noted that all patients with severe disease and 39 of the 40 with mild disease had prominent itching. "That means if your patient does not have itch, he or she probably doesn’t have bullous pemphigoid. It's such a central part of the disease," he said.
Disease control (absence of new bullae for 3 consecutive days) was achieved in a mean of 26 days in 36 of 40 patients (90%) with mild disease and in 25 of 34 (73.5%) with severe disease. Thus, transcutaneous systemic clobetasol was less effective in patients with severe bullous pemphigoid, even though most of them were on additional medications, most often azathioprine.
"In severe bullous pemphigoid, trans-cutaneous systemic clobetasol is not sufficient, although it has added value as an adjunct," Dr. Jonkman said.
Thirty-six percent of patients in the mild disease group maintained a complete remission off therapy. Another 29% had a complete remission while on therapy only, 15% had a partial remission, and 20% had no remission.
In the severe bullous pemphigoid group, 6% maintained a complete remission off therapy, 35% had a complete remission while on therapy only, 29% had a partial remission, and 30% had no remission.
Relapse occurred in 56% of responders in both groups a mean of 2 months after they completed the 4-month treatment course. The relapses were mild and responded to another round of whole-body clobetasol or, if the patient preferred, oral therapy, according to Dr. Jonkman.
The most common side effect was skin atrophy, seen in 13 of the 74 patients. Striae and purpura were the other main adverse events.
Audience Questions
An audience member at the congress asked if the treatment could trigger steroid-induced diabetes. Dr. Jonkman replied that the complication hasn't arisen in his patients on transcutaneous systemic clobetasol therapy. He said he suspects it's because the 4-month treatment course is too brief to trigger the problem. He has found, however, that some patients with preexisting type 2 diabetes require an additional oral antidiabetic agent.
Another audience member asked if patients should apply the topical steroid on top of intact blisters and the wounds created by broken blisters. This should be done, Dr. Jonkman advised. "The medication won't work on top of an intact blister because it's too far away from the dermis. But because it's systemic therapy it will come from the blood into the skin," he explained.
A third audience member asked if Dr. Jonkman had ever used whole-body clobetasol in patients with pemphigus foliaceous. He replied that he hasn't because the opportunity hasn't arisen, although he said he suspects it would be effective in milder cases.
Background Studies
Dr. Jonkman credited French dermatologists with the idea of using topical corticosteroids to treat patients with bullous pemphigoid. In a landmark multicenter trial nearly a decade ago, researchers randomized 341 bullous pemphigoid patients to 40 g/day of topical clobetasol propionate cream or to oral prednisone for 1 year. Patients with moderate disease received 0.5 mg/kg per day of prednisone, while those with severe disease got 1 mg/kg.
The topical regimen proved superior to oral prednisone in the 188 patients with extensive disease. Disease was controlled at 3 weeks in 99% of patients on clobetasol, significantly higher than the 91% rate with prednisone. Moreover, the 1-year survival rate was 76% in the topical therapy arm, compared with 58% with oral therapy. Severe complications were half as common in the clobetasol arm.
In patients with moderate bullous pemphigoid, the two forms of therapy were similar in terms of disease control at 3 weeks, overall survival, and severe complications (N. Engl. J. Med. 2002;346:321-7).
Dr. Jonkman noted that more recently the French group tweaked their topical regimen, adopting a milder approach similar to his own. In a 312-patient multicenter trial, they randomized participants to 10-30 g/day of clobetasol tapered over a 4-month period or to their earlier regimen of 40 g/day for a full year. The investigators showed that the rate of death or life-threatening adverse events in the 134 patients with moderate disease was reduced by nearly half with the milder regimen (J. Invest. Dermatol. 2009;129:1681-7).
He reported having no relevant financial disclosures.
FROM THE ANNUAL CONGRESS OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Major Finding: Disease control was achieved in a mean of 26 days in 90% of patients with mild disease and in 73.5% with severe disease.
Data Source: A series of 74 bullous pemphigoid patients.
Disclosures: Dr. Jonkman reported no relevant financial disclosures.
Consider Granulation Options After Dermatologic Surgery
SAN DIEGO – To granulate or not to granulate? That’s the question clinicians may consider after performing dermatologic surgery.
The best sites for granulation are concavities of the temple, ear, eye, and nose, Dr. Malcolm S. Ke said at a meeting on superficial anatomy and cutaneous surgery. "Sometimes superficial convexities also do quite well, such as the nose, mucosal lip, ear, and scalp," he added. "Deeper convexities are less predictable."
Benefits of granulation include easy monitoring and a low infection rate. "There is no hematoma and no suture reactions," said Dr. Ke of the division of dermatology at the University of California, Los Angeles. "It’s one less procedure the patient has to tolerate, it is often less painful for the patient, and it can provide good cosmesis."
Drawbacks include the potential for a long healing time and the fact that wound care is patient dependent. "They have to take care of it every single day, and there is sometimes a less predictable outcome," Dr. Ke said.
Three main agents for preoperative wound care include alcohol 70%, lodophors, and chlorhexidine, "which is my favorite," he said. "It is bactericidal and antipseudomonal. It persists on the skin and is not absorbed, but it is not good for open wounds or around eyes."
Postoperatively, saline works well for keeping wounds clean. "A patient favorite is hydrogen peroxide, but it’s not that antimicrobial and it can be drying if you use it for a long time," Dr. Ke said.
Acetic acid and sodium hypochlorite are antipseudomonal options.
Topical antibiotics for consideration include bacitracin and polymyxin B (Polysporin) and bacitracin, neomycin, and polymyxin B (Neosporin), but both carry the risk of allergic reactions, he said. He also said he often recommends petroleum-based ointments such Vaseline or Aquaphor.
Mupirocin 2% "is a favorite for granulating wounds on the lower extremities when you worry about gram-positive and gram-negative coverage. I typically use silver sulfadiazine 1%."
Dr. Ke uses nonstick pads to cover wounds and occlusive dressings to match the wound type, including polyurethane foams, polyurethane films, hydrocolloids, hydrogels, alginates, and Unna’s boot. He sees patients every 1-2 weeks if necessary to check their wounds, "but if they need to be seen more often I’m there for them. You want to be supportive. Excess fibrin and debris can form on the area."
The meeting was sponsored by the University of California, San Diego School of Medicine and the Scripps Clinic. Dr. Ke said that he had no relevant financial conflicts to disclose.
SAN DIEGO – To granulate or not to granulate? That’s the question clinicians may consider after performing dermatologic surgery.
The best sites for granulation are concavities of the temple, ear, eye, and nose, Dr. Malcolm S. Ke said at a meeting on superficial anatomy and cutaneous surgery. "Sometimes superficial convexities also do quite well, such as the nose, mucosal lip, ear, and scalp," he added. "Deeper convexities are less predictable."
Benefits of granulation include easy monitoring and a low infection rate. "There is no hematoma and no suture reactions," said Dr. Ke of the division of dermatology at the University of California, Los Angeles. "It’s one less procedure the patient has to tolerate, it is often less painful for the patient, and it can provide good cosmesis."
Drawbacks include the potential for a long healing time and the fact that wound care is patient dependent. "They have to take care of it every single day, and there is sometimes a less predictable outcome," Dr. Ke said.
Three main agents for preoperative wound care include alcohol 70%, lodophors, and chlorhexidine, "which is my favorite," he said. "It is bactericidal and antipseudomonal. It persists on the skin and is not absorbed, but it is not good for open wounds or around eyes."
Postoperatively, saline works well for keeping wounds clean. "A patient favorite is hydrogen peroxide, but it’s not that antimicrobial and it can be drying if you use it for a long time," Dr. Ke said.
Acetic acid and sodium hypochlorite are antipseudomonal options.
Topical antibiotics for consideration include bacitracin and polymyxin B (Polysporin) and bacitracin, neomycin, and polymyxin B (Neosporin), but both carry the risk of allergic reactions, he said. He also said he often recommends petroleum-based ointments such Vaseline or Aquaphor.
Mupirocin 2% "is a favorite for granulating wounds on the lower extremities when you worry about gram-positive and gram-negative coverage. I typically use silver sulfadiazine 1%."
Dr. Ke uses nonstick pads to cover wounds and occlusive dressings to match the wound type, including polyurethane foams, polyurethane films, hydrocolloids, hydrogels, alginates, and Unna’s boot. He sees patients every 1-2 weeks if necessary to check their wounds, "but if they need to be seen more often I’m there for them. You want to be supportive. Excess fibrin and debris can form on the area."
The meeting was sponsored by the University of California, San Diego School of Medicine and the Scripps Clinic. Dr. Ke said that he had no relevant financial conflicts to disclose.
SAN DIEGO – To granulate or not to granulate? That’s the question clinicians may consider after performing dermatologic surgery.
The best sites for granulation are concavities of the temple, ear, eye, and nose, Dr. Malcolm S. Ke said at a meeting on superficial anatomy and cutaneous surgery. "Sometimes superficial convexities also do quite well, such as the nose, mucosal lip, ear, and scalp," he added. "Deeper convexities are less predictable."
Benefits of granulation include easy monitoring and a low infection rate. "There is no hematoma and no suture reactions," said Dr. Ke of the division of dermatology at the University of California, Los Angeles. "It’s one less procedure the patient has to tolerate, it is often less painful for the patient, and it can provide good cosmesis."
Drawbacks include the potential for a long healing time and the fact that wound care is patient dependent. "They have to take care of it every single day, and there is sometimes a less predictable outcome," Dr. Ke said.
Three main agents for preoperative wound care include alcohol 70%, lodophors, and chlorhexidine, "which is my favorite," he said. "It is bactericidal and antipseudomonal. It persists on the skin and is not absorbed, but it is not good for open wounds or around eyes."
Postoperatively, saline works well for keeping wounds clean. "A patient favorite is hydrogen peroxide, but it’s not that antimicrobial and it can be drying if you use it for a long time," Dr. Ke said.
Acetic acid and sodium hypochlorite are antipseudomonal options.
Topical antibiotics for consideration include bacitracin and polymyxin B (Polysporin) and bacitracin, neomycin, and polymyxin B (Neosporin), but both carry the risk of allergic reactions, he said. He also said he often recommends petroleum-based ointments such Vaseline or Aquaphor.
Mupirocin 2% "is a favorite for granulating wounds on the lower extremities when you worry about gram-positive and gram-negative coverage. I typically use silver sulfadiazine 1%."
Dr. Ke uses nonstick pads to cover wounds and occlusive dressings to match the wound type, including polyurethane foams, polyurethane films, hydrocolloids, hydrogels, alginates, and Unna’s boot. He sees patients every 1-2 weeks if necessary to check their wounds, "but if they need to be seen more often I’m there for them. You want to be supportive. Excess fibrin and debris can form on the area."
The meeting was sponsored by the University of California, San Diego School of Medicine and the Scripps Clinic. Dr. Ke said that he had no relevant financial conflicts to disclose.
EXPERT ANALYSIS FROM A MEETING ON SUPERFICIAL ANATOMY AND CUTANEOUS SURGERY
Antibiotic-Coated Monofilament Suture Effective, Cuts Costs
MIAMI BEACH – An antibiotic-coated monofilament suture provides strength, flexibility, and elasticity for dermatologic surgery, but requires an extra throw to prevent knot slippage and comes with an initial learning curve, Dr. Susan H. Weinkle said.
"I’ve been using the same sutures almost 30 years until about 9 months ago," Dr. Weinkle said at this year’s South Beach Symposium.
The Monocryl Plus (poliglecaprone 25, Ethicon) is an absorbable, monofilament suture coated with triclosan antibiotic. The antibiotic "is the plus" and it can lower wound closing costs, Dr. Weinkle said.
The suture can be buried or it can run along the surface of the skin, Dr. Weinkle said. This product is associated with low tissue reactivity, so you get wounds with very little inflammation. "If someone cannot get back to my office quickly enough for suture removal, there [still] is very little reaction."
Absorption of the suture begins in about 12 days and can take considerably longer. "That’s a good thing. It stays underneath for up to 120 days, which is longer than Vicryl would last," Dr. Weinkle said. "However, (the sutures) don’t absorb fast enough on the skin. You still need to see the patient back." She asks patients to return to check wound healing anyway, especially to rule out any hematoma "because everyone I do surgery on is on (a blood thinner)."
"When I sew with Vicryl (polyglactin 910 suture, Ethicon), I tend to only put three knots in the wound. Two throws to start, and then another one on top of that." With this monofilament suture, a fourth throw is generally required to secure the knot, Dr. Weinkle said.
Wound infection risk generally is lower with a monofilament suture. In contrast, although easier to use, braided or twisted multifilament sutures carry a higher infection risk. "In a braided suture you have many more areas for fluid and bacteria to get in – that is very important."
There is an initial learning curve with this monofilament suture and "you are not going to love it in the beginning," said Dr. Weinkle, a private practice dermatologist and Mohs surgeon in Bradenton, Fla. "I sent some of these to a colleague in town and he did not like them." She added: "That is only because he tried one pack. You need at least eight packages until you can actually bond with this suture."
This suture features good elasticity, an imperative for wounds where a lot of local anesthesia was injected and edema results when the wound is closed. The suture stretches, and when that edema dissipates, the suture needs to come back down, she explained.
Using one monofilament suture compared to a two-suture closure can save costs, Dr. Weinkle said. The cost of one monofilament suture to close both deeply and superficially is about $12, compared with almost $18 to use a combination of Vicryl and nylon sutures. "Say you work 48 weeks a year and you do 30 incisions a week." Save $6 on each of these 1,440 annual wound closures "and you're looking at a savings of $8,640."
How you buy this suture is also important, Dr. Weinkle said. "I have a low overhead because I check the prices." Multiple national distributors carry this suture. "Make sure whoever you are buying from knows you’re a surgeon. Otherwise, you may end up paying more money for the same packet of sutures because you’re a dermatologist – you're a different category," Dr. Weinkle said. Also consider joining a group purchasing organization.
"Talk to your rep, say you'd like some samples, and say you'll need at least eight packs," Dr. Weinkle said. "I'm telling you, this is going to save you money and do an even better job for your patients."
Dr. Weinkle said that she did not have any relevant financial disclosures.
Monocryl Plus, Vicryl, sutures
MIAMI BEACH – An antibiotic-coated monofilament suture provides strength, flexibility, and elasticity for dermatologic surgery, but requires an extra throw to prevent knot slippage and comes with an initial learning curve, Dr. Susan H. Weinkle said.
"I’ve been using the same sutures almost 30 years until about 9 months ago," Dr. Weinkle said at this year’s South Beach Symposium.
The Monocryl Plus (poliglecaprone 25, Ethicon) is an absorbable, monofilament suture coated with triclosan antibiotic. The antibiotic "is the plus" and it can lower wound closing costs, Dr. Weinkle said.
The suture can be buried or it can run along the surface of the skin, Dr. Weinkle said. This product is associated with low tissue reactivity, so you get wounds with very little inflammation. "If someone cannot get back to my office quickly enough for suture removal, there [still] is very little reaction."
Absorption of the suture begins in about 12 days and can take considerably longer. "That’s a good thing. It stays underneath for up to 120 days, which is longer than Vicryl would last," Dr. Weinkle said. "However, (the sutures) don’t absorb fast enough on the skin. You still need to see the patient back." She asks patients to return to check wound healing anyway, especially to rule out any hematoma "because everyone I do surgery on is on (a blood thinner)."
"When I sew with Vicryl (polyglactin 910 suture, Ethicon), I tend to only put three knots in the wound. Two throws to start, and then another one on top of that." With this monofilament suture, a fourth throw is generally required to secure the knot, Dr. Weinkle said.
Wound infection risk generally is lower with a monofilament suture. In contrast, although easier to use, braided or twisted multifilament sutures carry a higher infection risk. "In a braided suture you have many more areas for fluid and bacteria to get in – that is very important."
There is an initial learning curve with this monofilament suture and "you are not going to love it in the beginning," said Dr. Weinkle, a private practice dermatologist and Mohs surgeon in Bradenton, Fla. "I sent some of these to a colleague in town and he did not like them." She added: "That is only because he tried one pack. You need at least eight packages until you can actually bond with this suture."
This suture features good elasticity, an imperative for wounds where a lot of local anesthesia was injected and edema results when the wound is closed. The suture stretches, and when that edema dissipates, the suture needs to come back down, she explained.
Using one monofilament suture compared to a two-suture closure can save costs, Dr. Weinkle said. The cost of one monofilament suture to close both deeply and superficially is about $12, compared with almost $18 to use a combination of Vicryl and nylon sutures. "Say you work 48 weeks a year and you do 30 incisions a week." Save $6 on each of these 1,440 annual wound closures "and you're looking at a savings of $8,640."
How you buy this suture is also important, Dr. Weinkle said. "I have a low overhead because I check the prices." Multiple national distributors carry this suture. "Make sure whoever you are buying from knows you’re a surgeon. Otherwise, you may end up paying more money for the same packet of sutures because you’re a dermatologist – you're a different category," Dr. Weinkle said. Also consider joining a group purchasing organization.
"Talk to your rep, say you'd like some samples, and say you'll need at least eight packs," Dr. Weinkle said. "I'm telling you, this is going to save you money and do an even better job for your patients."
Dr. Weinkle said that she did not have any relevant financial disclosures.
MIAMI BEACH – An antibiotic-coated monofilament suture provides strength, flexibility, and elasticity for dermatologic surgery, but requires an extra throw to prevent knot slippage and comes with an initial learning curve, Dr. Susan H. Weinkle said.
"I’ve been using the same sutures almost 30 years until about 9 months ago," Dr. Weinkle said at this year’s South Beach Symposium.
The Monocryl Plus (poliglecaprone 25, Ethicon) is an absorbable, monofilament suture coated with triclosan antibiotic. The antibiotic "is the plus" and it can lower wound closing costs, Dr. Weinkle said.
The suture can be buried or it can run along the surface of the skin, Dr. Weinkle said. This product is associated with low tissue reactivity, so you get wounds with very little inflammation. "If someone cannot get back to my office quickly enough for suture removal, there [still] is very little reaction."
Absorption of the suture begins in about 12 days and can take considerably longer. "That’s a good thing. It stays underneath for up to 120 days, which is longer than Vicryl would last," Dr. Weinkle said. "However, (the sutures) don’t absorb fast enough on the skin. You still need to see the patient back." She asks patients to return to check wound healing anyway, especially to rule out any hematoma "because everyone I do surgery on is on (a blood thinner)."
"When I sew with Vicryl (polyglactin 910 suture, Ethicon), I tend to only put three knots in the wound. Two throws to start, and then another one on top of that." With this monofilament suture, a fourth throw is generally required to secure the knot, Dr. Weinkle said.
Wound infection risk generally is lower with a monofilament suture. In contrast, although easier to use, braided or twisted multifilament sutures carry a higher infection risk. "In a braided suture you have many more areas for fluid and bacteria to get in – that is very important."
There is an initial learning curve with this monofilament suture and "you are not going to love it in the beginning," said Dr. Weinkle, a private practice dermatologist and Mohs surgeon in Bradenton, Fla. "I sent some of these to a colleague in town and he did not like them." She added: "That is only because he tried one pack. You need at least eight packages until you can actually bond with this suture."
This suture features good elasticity, an imperative for wounds where a lot of local anesthesia was injected and edema results when the wound is closed. The suture stretches, and when that edema dissipates, the suture needs to come back down, she explained.
Using one monofilament suture compared to a two-suture closure can save costs, Dr. Weinkle said. The cost of one monofilament suture to close both deeply and superficially is about $12, compared with almost $18 to use a combination of Vicryl and nylon sutures. "Say you work 48 weeks a year and you do 30 incisions a week." Save $6 on each of these 1,440 annual wound closures "and you're looking at a savings of $8,640."
How you buy this suture is also important, Dr. Weinkle said. "I have a low overhead because I check the prices." Multiple national distributors carry this suture. "Make sure whoever you are buying from knows you’re a surgeon. Otherwise, you may end up paying more money for the same packet of sutures because you’re a dermatologist – you're a different category," Dr. Weinkle said. Also consider joining a group purchasing organization.
"Talk to your rep, say you'd like some samples, and say you'll need at least eight packs," Dr. Weinkle said. "I'm telling you, this is going to save you money and do an even better job for your patients."
Dr. Weinkle said that she did not have any relevant financial disclosures.
Monocryl Plus, Vicryl, sutures
Monocryl Plus, Vicryl, sutures
EXPERT ANALYSIS FROM THE SOUTH BEACH SYMPOSIUM
Ultrasound Shows Promise in Wound Healing
LOS ANGELES – New ultrasound devices have shown promise in healing wounds, according to Dr. Jonathan Rosenblum, a podiatrist at Shaare Zedek Medical Center, Jerusalem.
"In the right hands, with the right modality, it could be a sonic boom," he punned. He cautioned that "there is no good evidence yet for ultrasound for any aspect of wound care." But in his presentation at the Diabetic Foot Global Conference, he said that many encouraging cases have been reported, along with impressive laboratory research, and that he hopes to launch randomized, controlled trials soon.
Dr. Rosenblum first became interested in the technology when he tried it out on a painful venous ulcer and found that the treatment not only reduced pain but seemed to speed the healing. "With a simple saline dressing and no compression, within 10 days we went from a nasty, sloughy wound bed to a soft epithelial covering," he said at the conference, which was presented by Valley Presbyterian Hospital.
He has since tried it out on a wide variety of wounds with good success.
Researchers have experimented with ultrasound therapy using longitudinal, shear, and acoustic waves, he said. And they’ve tried high and low frequency, and high and low intensity.
Some high-frequency ultrasound devices are being used to treat pain in soft tissue, said Dr. Rosenblum. But they aren’t effective for wound care because the energy isn’t focused on the dermis, he said. "A lot of it is being wasted deeper than you need it, and you’re not getting the effect that you want."
To address that problem several years ago, inventors experimented with low-frequency devices, but these machines were too large to be commercially viable, said Dr. Rosenblum. "They took up whole rooms," he said. "These were 6-foot-tall devices."
More recently, smaller devices have been created using surface acoustic waves, a technology that is also used in some touch screens, he said. It is this technology that looks promising for wound care, said Dr. Rosenblum.
Experiments by John Loike, Ph.D., at Columbia University in New York have shown that the migration of neutrophils and epithelial cells can be significantly influenced by this type of ultrasound waves, said Dr. Rosenblum.
Other researchers have shown increased local uptake of systemic gentamicin in pseudomonas biofilms, increasing the kill rate of the antibiotic.
In addition to fighting pathogens, ultrasound may spur skin growth, said Dr. Rosenblum. "It has been shown effective in all types of collagen synthesis, including cartilage, tendon, [and] skin," he said. And it has shown capacity to reawaken senescent cells, he added.
So how can ultrasound cause these effects?
One possibility is the heat generated by the energy from the waves, said Dr. Rosenblum, which could affect various aspects of healing. For example, collagenase is sensitive to temperature.
But heat itself is probably not the whole story, he said. One other possibility is that ultrasound may stimulate cells to produce nitrous oxide. In addition to being a powerful analgesic, ultrasound is a potent vasodilator.
"We came to the conclusion that ultrasound may be beneficial to wound healing," Dr. Rosenblum concluded. "I’d like to see a couple of good studies that could change that to ‘is beneficial to wound healing.’ "
Dr. Rosenblum disclosed that he an independent consultant to NanoVibronix and a consultant to BRH Health.
LOS ANGELES – New ultrasound devices have shown promise in healing wounds, according to Dr. Jonathan Rosenblum, a podiatrist at Shaare Zedek Medical Center, Jerusalem.
"In the right hands, with the right modality, it could be a sonic boom," he punned. He cautioned that "there is no good evidence yet for ultrasound for any aspect of wound care." But in his presentation at the Diabetic Foot Global Conference, he said that many encouraging cases have been reported, along with impressive laboratory research, and that he hopes to launch randomized, controlled trials soon.
Dr. Rosenblum first became interested in the technology when he tried it out on a painful venous ulcer and found that the treatment not only reduced pain but seemed to speed the healing. "With a simple saline dressing and no compression, within 10 days we went from a nasty, sloughy wound bed to a soft epithelial covering," he said at the conference, which was presented by Valley Presbyterian Hospital.
He has since tried it out on a wide variety of wounds with good success.
Researchers have experimented with ultrasound therapy using longitudinal, shear, and acoustic waves, he said. And they’ve tried high and low frequency, and high and low intensity.
Some high-frequency ultrasound devices are being used to treat pain in soft tissue, said Dr. Rosenblum. But they aren’t effective for wound care because the energy isn’t focused on the dermis, he said. "A lot of it is being wasted deeper than you need it, and you’re not getting the effect that you want."
To address that problem several years ago, inventors experimented with low-frequency devices, but these machines were too large to be commercially viable, said Dr. Rosenblum. "They took up whole rooms," he said. "These were 6-foot-tall devices."
More recently, smaller devices have been created using surface acoustic waves, a technology that is also used in some touch screens, he said. It is this technology that looks promising for wound care, said Dr. Rosenblum.
Experiments by John Loike, Ph.D., at Columbia University in New York have shown that the migration of neutrophils and epithelial cells can be significantly influenced by this type of ultrasound waves, said Dr. Rosenblum.
Other researchers have shown increased local uptake of systemic gentamicin in pseudomonas biofilms, increasing the kill rate of the antibiotic.
In addition to fighting pathogens, ultrasound may spur skin growth, said Dr. Rosenblum. "It has been shown effective in all types of collagen synthesis, including cartilage, tendon, [and] skin," he said. And it has shown capacity to reawaken senescent cells, he added.
So how can ultrasound cause these effects?
One possibility is the heat generated by the energy from the waves, said Dr. Rosenblum, which could affect various aspects of healing. For example, collagenase is sensitive to temperature.
But heat itself is probably not the whole story, he said. One other possibility is that ultrasound may stimulate cells to produce nitrous oxide. In addition to being a powerful analgesic, ultrasound is a potent vasodilator.
"We came to the conclusion that ultrasound may be beneficial to wound healing," Dr. Rosenblum concluded. "I’d like to see a couple of good studies that could change that to ‘is beneficial to wound healing.’ "
Dr. Rosenblum disclosed that he an independent consultant to NanoVibronix and a consultant to BRH Health.
LOS ANGELES – New ultrasound devices have shown promise in healing wounds, according to Dr. Jonathan Rosenblum, a podiatrist at Shaare Zedek Medical Center, Jerusalem.
"In the right hands, with the right modality, it could be a sonic boom," he punned. He cautioned that "there is no good evidence yet for ultrasound for any aspect of wound care." But in his presentation at the Diabetic Foot Global Conference, he said that many encouraging cases have been reported, along with impressive laboratory research, and that he hopes to launch randomized, controlled trials soon.
Dr. Rosenblum first became interested in the technology when he tried it out on a painful venous ulcer and found that the treatment not only reduced pain but seemed to speed the healing. "With a simple saline dressing and no compression, within 10 days we went from a nasty, sloughy wound bed to a soft epithelial covering," he said at the conference, which was presented by Valley Presbyterian Hospital.
He has since tried it out on a wide variety of wounds with good success.
Researchers have experimented with ultrasound therapy using longitudinal, shear, and acoustic waves, he said. And they’ve tried high and low frequency, and high and low intensity.
Some high-frequency ultrasound devices are being used to treat pain in soft tissue, said Dr. Rosenblum. But they aren’t effective for wound care because the energy isn’t focused on the dermis, he said. "A lot of it is being wasted deeper than you need it, and you’re not getting the effect that you want."
To address that problem several years ago, inventors experimented with low-frequency devices, but these machines were too large to be commercially viable, said Dr. Rosenblum. "They took up whole rooms," he said. "These were 6-foot-tall devices."
More recently, smaller devices have been created using surface acoustic waves, a technology that is also used in some touch screens, he said. It is this technology that looks promising for wound care, said Dr. Rosenblum.
Experiments by John Loike, Ph.D., at Columbia University in New York have shown that the migration of neutrophils and epithelial cells can be significantly influenced by this type of ultrasound waves, said Dr. Rosenblum.
Other researchers have shown increased local uptake of systemic gentamicin in pseudomonas biofilms, increasing the kill rate of the antibiotic.
In addition to fighting pathogens, ultrasound may spur skin growth, said Dr. Rosenblum. "It has been shown effective in all types of collagen synthesis, including cartilage, tendon, [and] skin," he said. And it has shown capacity to reawaken senescent cells, he added.
So how can ultrasound cause these effects?
One possibility is the heat generated by the energy from the waves, said Dr. Rosenblum, which could affect various aspects of healing. For example, collagenase is sensitive to temperature.
But heat itself is probably not the whole story, he said. One other possibility is that ultrasound may stimulate cells to produce nitrous oxide. In addition to being a powerful analgesic, ultrasound is a potent vasodilator.
"We came to the conclusion that ultrasound may be beneficial to wound healing," Dr. Rosenblum concluded. "I’d like to see a couple of good studies that could change that to ‘is beneficial to wound healing.’ "
Dr. Rosenblum disclosed that he an independent consultant to NanoVibronix and a consultant to BRH Health.
EXPERT ANALYSIS FROM THE DIABETIC FOOT GLOBAL CONFERENCE
Combined Use of Skin Needling and Platelet-Rich Plasma in Acne Scarring Treatment
Methicillin-Resistant Staphylococcus aureus Superinfection Delaying the Diagnosis of an Atypical Mycobacteria Infection: Report of a Case
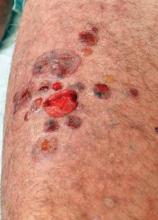
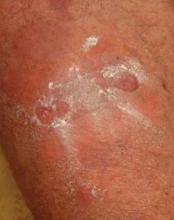
Dapsone Gel Effective for Chronic Atrophie Blanche Ulcerations
NEW ORLEANS – Topical dapsone is a novel and effective treatment for atrophie blanche ulcerations.
Atrophie blanche (also known as livedoid vasculopathy) is a therapeutic challenge. The etiology of this chronic and painful condition is unclear. The current standard care, which consists of low-dose aspirin, pentoxifylline, pain medications, and conservative wound dressings, has a suboptimal success rate in reducing pain and healing the ulcers, Dr. Erik Maus observed at the annual meeting of the American Academy of Dermatology.
He reported on 10 patients with biopsy-confirmed – and/or clinical findings consistent with – atrophie blanche, all of whom had failed to respond adequately to conventional measures. At the Memorial Hermann Center for Wound Healing at the University of Texas, Houston, they were placed on dapsone gel 5% (Aczone) twice daily, usually in conjunction with compression stockings or compression bandages when edema or venous stasis was present. The clinical results were impressive, according to Dr. Maus of the university.
One patient quickly discontinued topical dapsone after reporting feeling vaguely ill. Of the nine patients who continued on the drug, seven (78%) experienced rapid and steady cutaneous healing and reduced pain; their longstanding wounds closed in 6-20 weeks.
Patients described the topical therapy as easy to use. No one reported discomfort in applying dapsone gel to open wounds.
One patient relapsed when she ran out of the medication. Healing resumed when she restarted treatment.
Dapsone gel's likely mechanism of action in atrophie blanche involves inhibition of neutrophilic migration to the ulcerated sites, Dr. Maus speculated.
Atrophie blanche is characterized by painful, persistent ulcerations of the ankle and dorsal surface of the foot. The condition affects mainly young to middle-aged women. Areas of healed ulceration are marked by ivory-colored atrophic scars surrounded by sclerotic skin with hyperpigmentation and peripheral telangiectasias. Although altered local or systemic control of coagulation is believed to play a role in some cases, the etiology of atrophie blanche often remains obscure.
This study was conducted without corporate sponsorship. Dr. Maus declared having no relevant financial interests.
NEW ORLEANS – Topical dapsone is a novel and effective treatment for atrophie blanche ulcerations.
Atrophie blanche (also known as livedoid vasculopathy) is a therapeutic challenge. The etiology of this chronic and painful condition is unclear. The current standard care, which consists of low-dose aspirin, pentoxifylline, pain medications, and conservative wound dressings, has a suboptimal success rate in reducing pain and healing the ulcers, Dr. Erik Maus observed at the annual meeting of the American Academy of Dermatology.
He reported on 10 patients with biopsy-confirmed – and/or clinical findings consistent with – atrophie blanche, all of whom had failed to respond adequately to conventional measures. At the Memorial Hermann Center for Wound Healing at the University of Texas, Houston, they were placed on dapsone gel 5% (Aczone) twice daily, usually in conjunction with compression stockings or compression bandages when edema or venous stasis was present. The clinical results were impressive, according to Dr. Maus of the university.
One patient quickly discontinued topical dapsone after reporting feeling vaguely ill. Of the nine patients who continued on the drug, seven (78%) experienced rapid and steady cutaneous healing and reduced pain; their longstanding wounds closed in 6-20 weeks.
Patients described the topical therapy as easy to use. No one reported discomfort in applying dapsone gel to open wounds.
One patient relapsed when she ran out of the medication. Healing resumed when she restarted treatment.
Dapsone gel's likely mechanism of action in atrophie blanche involves inhibition of neutrophilic migration to the ulcerated sites, Dr. Maus speculated.
Atrophie blanche is characterized by painful, persistent ulcerations of the ankle and dorsal surface of the foot. The condition affects mainly young to middle-aged women. Areas of healed ulceration are marked by ivory-colored atrophic scars surrounded by sclerotic skin with hyperpigmentation and peripheral telangiectasias. Although altered local or systemic control of coagulation is believed to play a role in some cases, the etiology of atrophie blanche often remains obscure.
This study was conducted without corporate sponsorship. Dr. Maus declared having no relevant financial interests.
NEW ORLEANS – Topical dapsone is a novel and effective treatment for atrophie blanche ulcerations.
Atrophie blanche (also known as livedoid vasculopathy) is a therapeutic challenge. The etiology of this chronic and painful condition is unclear. The current standard care, which consists of low-dose aspirin, pentoxifylline, pain medications, and conservative wound dressings, has a suboptimal success rate in reducing pain and healing the ulcers, Dr. Erik Maus observed at the annual meeting of the American Academy of Dermatology.
He reported on 10 patients with biopsy-confirmed – and/or clinical findings consistent with – atrophie blanche, all of whom had failed to respond adequately to conventional measures. At the Memorial Hermann Center for Wound Healing at the University of Texas, Houston, they were placed on dapsone gel 5% (Aczone) twice daily, usually in conjunction with compression stockings or compression bandages when edema or venous stasis was present. The clinical results were impressive, according to Dr. Maus of the university.
One patient quickly discontinued topical dapsone after reporting feeling vaguely ill. Of the nine patients who continued on the drug, seven (78%) experienced rapid and steady cutaneous healing and reduced pain; their longstanding wounds closed in 6-20 weeks.
Patients described the topical therapy as easy to use. No one reported discomfort in applying dapsone gel to open wounds.
One patient relapsed when she ran out of the medication. Healing resumed when she restarted treatment.
Dapsone gel's likely mechanism of action in atrophie blanche involves inhibition of neutrophilic migration to the ulcerated sites, Dr. Maus speculated.
Atrophie blanche is characterized by painful, persistent ulcerations of the ankle and dorsal surface of the foot. The condition affects mainly young to middle-aged women. Areas of healed ulceration are marked by ivory-colored atrophic scars surrounded by sclerotic skin with hyperpigmentation and peripheral telangiectasias. Although altered local or systemic control of coagulation is believed to play a role in some cases, the etiology of atrophie blanche often remains obscure.
This study was conducted without corporate sponsorship. Dr. Maus declared having no relevant financial interests.
FROM THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF DERMATOLOGY
Major Finding: Of the nine patients who continued on topical dapsone, seven (78%) experienced rapid and steady cutaneous healing and reduced pain; their longstanding wounds closed in 6-20 weeks.
Data Source: A study of 10 patients with atrophie blanche ulcerations treated with dapsone gel 5% (Aczone) twice daily, usually in conjunction with compression stockings or compression bandages when edema or venous stasis was present.
Disclosures: This study was conducted without corporate sponsorship. Dr. Maus declared having no relevant financial interests.