User login
FDA update: Higher late mortality with paclitaxel-coated devices
Paclitaxel-coated devices, which are used to treat peripheral artery disease (PAD), appear to have a nearly 60% higher mortality risk than uncoated devices, according to a letter to health care providers from the Food and Drug Administration.
This letter updates details about long-term follow-up data and panel conclusions reviewed by the Food and Drug Administration, as well as recommendations from the agency regarding these devices. On Jan. 17, 2019, the FDA notified providers regarding an apparent increased late mortality risk seen with paclitaxel-eluting stents and paclitaxel-coated balloons placed in the femoropopliteal artery in patients with PAD. The agency issued an update March 15.
In a public meeting June 19-20, the Circulatory System Devices Panel of the Medical Devices Advisory Committee discussed long-term follow-up data that demonstrated a 57% relative increase in mortality among PAD patients treated with paclitaxel-coated devices when compared with those receiving uncoated devices. The panel concluded that the late mortality signal was real and warranted further study and action, a conclusion with which the FDA has concurred.
Among other recommendations issued by the FDA, health care professionals should continue to closely monitor patients who’ve already received the devices and fully discuss the risks and benefits of these devices with patients. The FDA has decided that, given the demonstrated short-term benefits of these devices, clinical studies may continue and should collect long-term safety and effectiveness data.
The magnitude of this late mortality signal should be interpreted with caution, the FDA noted in the update, because of the wide confidence intervals (although the relative risk was 1.57, the 95% confidence interval was 1.16-2.13, which translates to 16%-113% higher relative risk), pooling studies of different devices that weren’t meant to be combined, missing data, and other reasons.
The full letter, including more detailed data and the full list of recommendations, is available on the FDA’s website.
Paclitaxel-coated devices, which are used to treat peripheral artery disease (PAD), appear to have a nearly 60% higher mortality risk than uncoated devices, according to a letter to health care providers from the Food and Drug Administration.
This letter updates details about long-term follow-up data and panel conclusions reviewed by the Food and Drug Administration, as well as recommendations from the agency regarding these devices. On Jan. 17, 2019, the FDA notified providers regarding an apparent increased late mortality risk seen with paclitaxel-eluting stents and paclitaxel-coated balloons placed in the femoropopliteal artery in patients with PAD. The agency issued an update March 15.
In a public meeting June 19-20, the Circulatory System Devices Panel of the Medical Devices Advisory Committee discussed long-term follow-up data that demonstrated a 57% relative increase in mortality among PAD patients treated with paclitaxel-coated devices when compared with those receiving uncoated devices. The panel concluded that the late mortality signal was real and warranted further study and action, a conclusion with which the FDA has concurred.
Among other recommendations issued by the FDA, health care professionals should continue to closely monitor patients who’ve already received the devices and fully discuss the risks and benefits of these devices with patients. The FDA has decided that, given the demonstrated short-term benefits of these devices, clinical studies may continue and should collect long-term safety and effectiveness data.
The magnitude of this late mortality signal should be interpreted with caution, the FDA noted in the update, because of the wide confidence intervals (although the relative risk was 1.57, the 95% confidence interval was 1.16-2.13, which translates to 16%-113% higher relative risk), pooling studies of different devices that weren’t meant to be combined, missing data, and other reasons.
The full letter, including more detailed data and the full list of recommendations, is available on the FDA’s website.
Paclitaxel-coated devices, which are used to treat peripheral artery disease (PAD), appear to have a nearly 60% higher mortality risk than uncoated devices, according to a letter to health care providers from the Food and Drug Administration.
This letter updates details about long-term follow-up data and panel conclusions reviewed by the Food and Drug Administration, as well as recommendations from the agency regarding these devices. On Jan. 17, 2019, the FDA notified providers regarding an apparent increased late mortality risk seen with paclitaxel-eluting stents and paclitaxel-coated balloons placed in the femoropopliteal artery in patients with PAD. The agency issued an update March 15.
In a public meeting June 19-20, the Circulatory System Devices Panel of the Medical Devices Advisory Committee discussed long-term follow-up data that demonstrated a 57% relative increase in mortality among PAD patients treated with paclitaxel-coated devices when compared with those receiving uncoated devices. The panel concluded that the late mortality signal was real and warranted further study and action, a conclusion with which the FDA has concurred.
Among other recommendations issued by the FDA, health care professionals should continue to closely monitor patients who’ve already received the devices and fully discuss the risks and benefits of these devices with patients. The FDA has decided that, given the demonstrated short-term benefits of these devices, clinical studies may continue and should collect long-term safety and effectiveness data.
The magnitude of this late mortality signal should be interpreted with caution, the FDA noted in the update, because of the wide confidence intervals (although the relative risk was 1.57, the 95% confidence interval was 1.16-2.13, which translates to 16%-113% higher relative risk), pooling studies of different devices that weren’t meant to be combined, missing data, and other reasons.
The full letter, including more detailed data and the full list of recommendations, is available on the FDA’s website.
Lancet joins movement to reject ‘manels’
The Lancet Group’s 18 medical journals have committed to ensuring that their editorial advisory boards include at least 50% female members by the end of 2019 as just one component of the diversity and gender parity initiative unveiled Aug. 8.
“The case for gender equity and diversity is clear: Teams that are diverse in terms of gender, ethnicity, and social background produce better health science, are more highly cited, generate a broader range of ideas and innovations, and better represent society,” group editors wrote in their comment (Lancet. 2019 Aug 10;394:452-3). They emphasized the importance of increasing inclusion in science “across gender, ethnicity, geography, and other social categories.”
The Diversity Pledge states the group’s commitment “to increasing diversity and inclusion in research and publishing, and in particular to increasing the representation of women and colleagues from low-income and middle-income countries among our editorial advisers, peer reviewers, and authors.”
The No All-Male Panel Policy echoes a call from the National Institutes of Health for ending the “Manel Tradition,” as NIH Director Francis S. Collins, MD, PhD, wrote in early June. Recognizing the need “to combat cultural forces that tolerate gender harassment and limit the advancement of women,” Dr. Collins pledged to decline speaking invitations if “attention to inclusiveness” is not clear in the event’s agenda.
Discussion of “manels” – all-male panels – and the decision to boycott them has been picking up speed in scientific, medical and even business circles over the past several years. The BBC highlighted a popular blog that shamed events with all-male panels in 2015, and a 2018 study more formally concluded that male scientists had considerably more opportunities to speak and present at the world’s largest geophysical conference.
One business and development leader even included space on his website to allow other leaders to pledge not to “serve on a panel of two people or more unless there is at least one woman on the panel, not including the chair.” More than 2,000 leaders from across the globe already have signed.
Six months ago, the Lancet published a special theme issue on women in science, medicine, and global health. The editors noted in the issue that women comprise fewer than a third of authors and reviewers in high-impact medical journals – just one example of the underrepresentation of women and people in color in medical publishing. The group is now revamping their systems to address the disparities.
“An upcoming update of our online submission system will have a field for self-selected gender, so we can better track representation across genders among authors, reviewers, editors, and editorial advisers, along with country of origin,” the editors wrote.
But they acknowledged that their efforts are just one piece of the academic ecosystem and called on others’ participation. “We encourage other publishers, journals, and members of the science community to contribute to these pledges.”
SOURCE: Lancet. 2019 Aug 10;394:452-3.
The Lancet Group’s 18 medical journals have committed to ensuring that their editorial advisory boards include at least 50% female members by the end of 2019 as just one component of the diversity and gender parity initiative unveiled Aug. 8.
“The case for gender equity and diversity is clear: Teams that are diverse in terms of gender, ethnicity, and social background produce better health science, are more highly cited, generate a broader range of ideas and innovations, and better represent society,” group editors wrote in their comment (Lancet. 2019 Aug 10;394:452-3). They emphasized the importance of increasing inclusion in science “across gender, ethnicity, geography, and other social categories.”
The Diversity Pledge states the group’s commitment “to increasing diversity and inclusion in research and publishing, and in particular to increasing the representation of women and colleagues from low-income and middle-income countries among our editorial advisers, peer reviewers, and authors.”
The No All-Male Panel Policy echoes a call from the National Institutes of Health for ending the “Manel Tradition,” as NIH Director Francis S. Collins, MD, PhD, wrote in early June. Recognizing the need “to combat cultural forces that tolerate gender harassment and limit the advancement of women,” Dr. Collins pledged to decline speaking invitations if “attention to inclusiveness” is not clear in the event’s agenda.
Discussion of “manels” – all-male panels – and the decision to boycott them has been picking up speed in scientific, medical and even business circles over the past several years. The BBC highlighted a popular blog that shamed events with all-male panels in 2015, and a 2018 study more formally concluded that male scientists had considerably more opportunities to speak and present at the world’s largest geophysical conference.
One business and development leader even included space on his website to allow other leaders to pledge not to “serve on a panel of two people or more unless there is at least one woman on the panel, not including the chair.” More than 2,000 leaders from across the globe already have signed.
Six months ago, the Lancet published a special theme issue on women in science, medicine, and global health. The editors noted in the issue that women comprise fewer than a third of authors and reviewers in high-impact medical journals – just one example of the underrepresentation of women and people in color in medical publishing. The group is now revamping their systems to address the disparities.
“An upcoming update of our online submission system will have a field for self-selected gender, so we can better track representation across genders among authors, reviewers, editors, and editorial advisers, along with country of origin,” the editors wrote.
But they acknowledged that their efforts are just one piece of the academic ecosystem and called on others’ participation. “We encourage other publishers, journals, and members of the science community to contribute to these pledges.”
SOURCE: Lancet. 2019 Aug 10;394:452-3.
The Lancet Group’s 18 medical journals have committed to ensuring that their editorial advisory boards include at least 50% female members by the end of 2019 as just one component of the diversity and gender parity initiative unveiled Aug. 8.
“The case for gender equity and diversity is clear: Teams that are diverse in terms of gender, ethnicity, and social background produce better health science, are more highly cited, generate a broader range of ideas and innovations, and better represent society,” group editors wrote in their comment (Lancet. 2019 Aug 10;394:452-3). They emphasized the importance of increasing inclusion in science “across gender, ethnicity, geography, and other social categories.”
The Diversity Pledge states the group’s commitment “to increasing diversity and inclusion in research and publishing, and in particular to increasing the representation of women and colleagues from low-income and middle-income countries among our editorial advisers, peer reviewers, and authors.”
The No All-Male Panel Policy echoes a call from the National Institutes of Health for ending the “Manel Tradition,” as NIH Director Francis S. Collins, MD, PhD, wrote in early June. Recognizing the need “to combat cultural forces that tolerate gender harassment and limit the advancement of women,” Dr. Collins pledged to decline speaking invitations if “attention to inclusiveness” is not clear in the event’s agenda.
Discussion of “manels” – all-male panels – and the decision to boycott them has been picking up speed in scientific, medical and even business circles over the past several years. The BBC highlighted a popular blog that shamed events with all-male panels in 2015, and a 2018 study more formally concluded that male scientists had considerably more opportunities to speak and present at the world’s largest geophysical conference.
One business and development leader even included space on his website to allow other leaders to pledge not to “serve on a panel of two people or more unless there is at least one woman on the panel, not including the chair.” More than 2,000 leaders from across the globe already have signed.
Six months ago, the Lancet published a special theme issue on women in science, medicine, and global health. The editors noted in the issue that women comprise fewer than a third of authors and reviewers in high-impact medical journals – just one example of the underrepresentation of women and people in color in medical publishing. The group is now revamping their systems to address the disparities.
“An upcoming update of our online submission system will have a field for self-selected gender, so we can better track representation across genders among authors, reviewers, editors, and editorial advisers, along with country of origin,” the editors wrote.
But they acknowledged that their efforts are just one piece of the academic ecosystem and called on others’ participation. “We encourage other publishers, journals, and members of the science community to contribute to these pledges.”
SOURCE: Lancet. 2019 Aug 10;394:452-3.
FROM THE LANCET
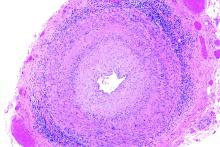
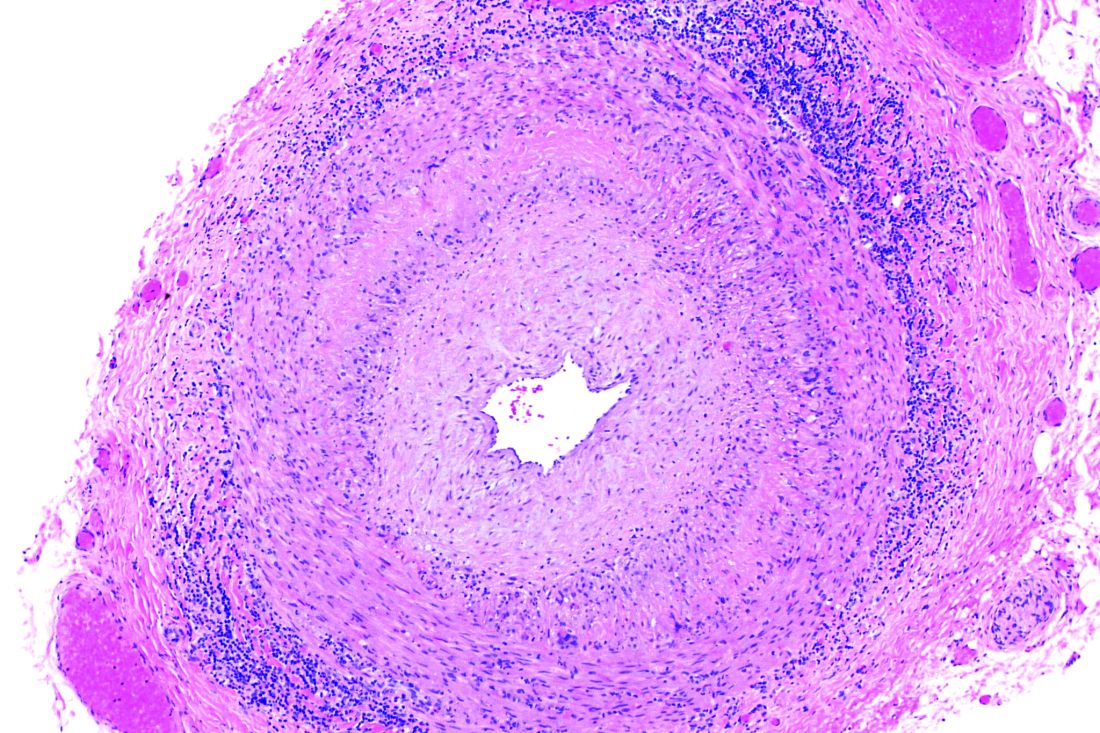
White and black patients have similar rates of giant cell arteritis
To determine the incidence of biopsy-proven GCA (BP-GCA) in a racially diverse cohort, Anna M. Gruener of Nottingham (England) University Hospitals NHS Trust and coauthors analyzed the medical records of more than 10 years of patients who underwent temporal artery biopsy at Johns Hopkins Wilmer Eye Institute in Baltimore. Of the 586 patients in the study, 167 (28.5%) were black, 382 (65.2%) were white, and 37 (6.3%) were other or unknown. The mean age was 70.5 years.
Of the 573 patients who were aged 50 years and older, 92 (16.1%) had a positive biopsy for BP-GCA; 14 were black (8.4% of all black patients), 75 were white (19.6% of all white patients), and 3 were other or unknown. The population-adjusted, age- and sex-standardized incidence rates per 100,000 were 3.1 (95% confidence interval, 1.0-5.2) for black patients and 3.6 (95% CI, 2.5-4.7) for white patients.
Overall, BP-GCA occurred more frequently in women than in men (incidence rate ratio, 1.9; 95% CI, 1.1-3.4; P = .03) but at similar levels in white and black patients (IRR, 1.2; 95% CI, 0.6-2.4; P = .66).
In an accompanying editorial, Michael K. Yoon, MD, and Joseph F. Rizzo III, MD, of Harvard Medical School, Boston, praised the researchers for conducting their study in a population with a large percentage of black patients, a noted weakness of earlier studies in this area (JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2933). That said, the two doctors also recognized the limitations of the work done by Gruener et al., including relying on U.S. Census data to calculate adjusted incidence rates instead of local racial distribution and also the potentially problematic choice to count patients with healed arteritis as having BP-GCA.
Still, Dr. Yoon and Dr. Rizzo commended Gruener et al. for questioning previous findings on GCA rates. “Although the authors’ methods are imperfect,” they wrote, “the studies that had previously established a low incidence of GCA in black patients were also flawed in design.”
The study had no outside funding source, and no conflicts of interest were reported.
SOURCE: Gruener AM et al. JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2919.
To determine the incidence of biopsy-proven GCA (BP-GCA) in a racially diverse cohort, Anna M. Gruener of Nottingham (England) University Hospitals NHS Trust and coauthors analyzed the medical records of more than 10 years of patients who underwent temporal artery biopsy at Johns Hopkins Wilmer Eye Institute in Baltimore. Of the 586 patients in the study, 167 (28.5%) were black, 382 (65.2%) were white, and 37 (6.3%) were other or unknown. The mean age was 70.5 years.
Of the 573 patients who were aged 50 years and older, 92 (16.1%) had a positive biopsy for BP-GCA; 14 were black (8.4% of all black patients), 75 were white (19.6% of all white patients), and 3 were other or unknown. The population-adjusted, age- and sex-standardized incidence rates per 100,000 were 3.1 (95% confidence interval, 1.0-5.2) for black patients and 3.6 (95% CI, 2.5-4.7) for white patients.
Overall, BP-GCA occurred more frequently in women than in men (incidence rate ratio, 1.9; 95% CI, 1.1-3.4; P = .03) but at similar levels in white and black patients (IRR, 1.2; 95% CI, 0.6-2.4; P = .66).
In an accompanying editorial, Michael K. Yoon, MD, and Joseph F. Rizzo III, MD, of Harvard Medical School, Boston, praised the researchers for conducting their study in a population with a large percentage of black patients, a noted weakness of earlier studies in this area (JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2933). That said, the two doctors also recognized the limitations of the work done by Gruener et al., including relying on U.S. Census data to calculate adjusted incidence rates instead of local racial distribution and also the potentially problematic choice to count patients with healed arteritis as having BP-GCA.
Still, Dr. Yoon and Dr. Rizzo commended Gruener et al. for questioning previous findings on GCA rates. “Although the authors’ methods are imperfect,” they wrote, “the studies that had previously established a low incidence of GCA in black patients were also flawed in design.”
The study had no outside funding source, and no conflicts of interest were reported.
SOURCE: Gruener AM et al. JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2919.
To determine the incidence of biopsy-proven GCA (BP-GCA) in a racially diverse cohort, Anna M. Gruener of Nottingham (England) University Hospitals NHS Trust and coauthors analyzed the medical records of more than 10 years of patients who underwent temporal artery biopsy at Johns Hopkins Wilmer Eye Institute in Baltimore. Of the 586 patients in the study, 167 (28.5%) were black, 382 (65.2%) were white, and 37 (6.3%) were other or unknown. The mean age was 70.5 years.
Of the 573 patients who were aged 50 years and older, 92 (16.1%) had a positive biopsy for BP-GCA; 14 were black (8.4% of all black patients), 75 were white (19.6% of all white patients), and 3 were other or unknown. The population-adjusted, age- and sex-standardized incidence rates per 100,000 were 3.1 (95% confidence interval, 1.0-5.2) for black patients and 3.6 (95% CI, 2.5-4.7) for white patients.
Overall, BP-GCA occurred more frequently in women than in men (incidence rate ratio, 1.9; 95% CI, 1.1-3.4; P = .03) but at similar levels in white and black patients (IRR, 1.2; 95% CI, 0.6-2.4; P = .66).
In an accompanying editorial, Michael K. Yoon, MD, and Joseph F. Rizzo III, MD, of Harvard Medical School, Boston, praised the researchers for conducting their study in a population with a large percentage of black patients, a noted weakness of earlier studies in this area (JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2933). That said, the two doctors also recognized the limitations of the work done by Gruener et al., including relying on U.S. Census data to calculate adjusted incidence rates instead of local racial distribution and also the potentially problematic choice to count patients with healed arteritis as having BP-GCA.
Still, Dr. Yoon and Dr. Rizzo commended Gruener et al. for questioning previous findings on GCA rates. “Although the authors’ methods are imperfect,” they wrote, “the studies that had previously established a low incidence of GCA in black patients were also flawed in design.”
The study had no outside funding source, and no conflicts of interest were reported.
SOURCE: Gruener AM et al. JAMA Ophthalmol. 2019 Aug 8. doi: 10.1001/jamaophthalmol.2019.2919.
FROM JAMA OPHTHALMOLOGY
Professional coaching keeps doctors in the game
Physicians who receive professional coaching are less emotionally exhausted and less vulnerable to burnout, according to the results of a pilot study.
“This intervention adds to the growing literature of evidence-based approaches to promote physician well-being and should be considered a complementary strategy to be deployed in combination with other organizational approaches to improve system-level drivers of work-related stressors,” wrote Liselotte N. Dyrbye, MD, of the Mayo Clinic in Rochester, Minn., and coauthors in JAMA Internal Medicine.
Dr. Dyrbye and colleagues conducted a randomized pilot study of 88 Mayo Clinic physicians in the departments of medicine, family medicine, and pediatrics. Half (n = 44) received 3.5 hours of sessions facilitated by a professional coach. The other half (n = 44) served as controls. Participants’ well-being – in regard to burnout, quality of life, resilience, job satisfaction, engagement, and meaning at work – was surveyed at baseline and the study’s completion.
Physicians in the coaching group participated in a 1-hour initial telephone session, designed to establish a relationship between the physician and coach, as well as to assess needs, set goals, identify values, and create an action plan. During follow-up sessions, coaches would check in, help plan and set goals, and suggest strategies/changes to incorporate into daily life. Physicians were permitted to ask for support on any issue, but also were expected to see as many patients as their colleagues outside of the study.
After 6 months, physicians in the coaching group saw a significant decrease in emotional exhaustion by a mean of 5.2 points, compared with an increase of 1.5 points in the control group. At 5 months, absolute rates of high emotional exhaustion decreased by 19.5% in the coaching group and increased by 9.8% in the control group and absolute rates of overall burnout decreased by 17.1% in the coaching group and increased by 4.9% in the control group. Quality of life and resilience scores also improved, though there were no notable differences between groups in measures of job satisfaction, engagement, and meaning at work.
The authors noted their study’s limitations, which included a modest sample size and a volunteer group of participants.
In addition, the lower percentage of men in the study – 48 of 88 participants were women – may be a result of factors that deserve further investigation. Finally, burnout rates among volunteers were higher than those among other physicians, suggesting that “the study appealed to those in greatest need of the intervention.”
The study was funded by the Mayo Clinic department of medicine’s Program on Physician Well-Being and the Physician Foundation. Two of the authors – Dr. Dyrbye and Tait D. Shanafelt, MD, of Stanford (Calif.) University – reported being the coinventors of, and receiving royalties for, the Physician Well-Being Index, Medical Student Well-Being Index, Nurse Well-Being Index, and the Well-Being Index.
SOURCE: Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2425.
Physicians who receive professional coaching are less emotionally exhausted and less vulnerable to burnout, according to the results of a pilot study.
“This intervention adds to the growing literature of evidence-based approaches to promote physician well-being and should be considered a complementary strategy to be deployed in combination with other organizational approaches to improve system-level drivers of work-related stressors,” wrote Liselotte N. Dyrbye, MD, of the Mayo Clinic in Rochester, Minn., and coauthors in JAMA Internal Medicine.
Dr. Dyrbye and colleagues conducted a randomized pilot study of 88 Mayo Clinic physicians in the departments of medicine, family medicine, and pediatrics. Half (n = 44) received 3.5 hours of sessions facilitated by a professional coach. The other half (n = 44) served as controls. Participants’ well-being – in regard to burnout, quality of life, resilience, job satisfaction, engagement, and meaning at work – was surveyed at baseline and the study’s completion.
Physicians in the coaching group participated in a 1-hour initial telephone session, designed to establish a relationship between the physician and coach, as well as to assess needs, set goals, identify values, and create an action plan. During follow-up sessions, coaches would check in, help plan and set goals, and suggest strategies/changes to incorporate into daily life. Physicians were permitted to ask for support on any issue, but also were expected to see as many patients as their colleagues outside of the study.
After 6 months, physicians in the coaching group saw a significant decrease in emotional exhaustion by a mean of 5.2 points, compared with an increase of 1.5 points in the control group. At 5 months, absolute rates of high emotional exhaustion decreased by 19.5% in the coaching group and increased by 9.8% in the control group and absolute rates of overall burnout decreased by 17.1% in the coaching group and increased by 4.9% in the control group. Quality of life and resilience scores also improved, though there were no notable differences between groups in measures of job satisfaction, engagement, and meaning at work.
The authors noted their study’s limitations, which included a modest sample size and a volunteer group of participants.
In addition, the lower percentage of men in the study – 48 of 88 participants were women – may be a result of factors that deserve further investigation. Finally, burnout rates among volunteers were higher than those among other physicians, suggesting that “the study appealed to those in greatest need of the intervention.”
The study was funded by the Mayo Clinic department of medicine’s Program on Physician Well-Being and the Physician Foundation. Two of the authors – Dr. Dyrbye and Tait D. Shanafelt, MD, of Stanford (Calif.) University – reported being the coinventors of, and receiving royalties for, the Physician Well-Being Index, Medical Student Well-Being Index, Nurse Well-Being Index, and the Well-Being Index.
SOURCE: Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2425.
Physicians who receive professional coaching are less emotionally exhausted and less vulnerable to burnout, according to the results of a pilot study.
“This intervention adds to the growing literature of evidence-based approaches to promote physician well-being and should be considered a complementary strategy to be deployed in combination with other organizational approaches to improve system-level drivers of work-related stressors,” wrote Liselotte N. Dyrbye, MD, of the Mayo Clinic in Rochester, Minn., and coauthors in JAMA Internal Medicine.
Dr. Dyrbye and colleagues conducted a randomized pilot study of 88 Mayo Clinic physicians in the departments of medicine, family medicine, and pediatrics. Half (n = 44) received 3.5 hours of sessions facilitated by a professional coach. The other half (n = 44) served as controls. Participants’ well-being – in regard to burnout, quality of life, resilience, job satisfaction, engagement, and meaning at work – was surveyed at baseline and the study’s completion.
Physicians in the coaching group participated in a 1-hour initial telephone session, designed to establish a relationship between the physician and coach, as well as to assess needs, set goals, identify values, and create an action plan. During follow-up sessions, coaches would check in, help plan and set goals, and suggest strategies/changes to incorporate into daily life. Physicians were permitted to ask for support on any issue, but also were expected to see as many patients as their colleagues outside of the study.
After 6 months, physicians in the coaching group saw a significant decrease in emotional exhaustion by a mean of 5.2 points, compared with an increase of 1.5 points in the control group. At 5 months, absolute rates of high emotional exhaustion decreased by 19.5% in the coaching group and increased by 9.8% in the control group and absolute rates of overall burnout decreased by 17.1% in the coaching group and increased by 4.9% in the control group. Quality of life and resilience scores also improved, though there were no notable differences between groups in measures of job satisfaction, engagement, and meaning at work.
The authors noted their study’s limitations, which included a modest sample size and a volunteer group of participants.
In addition, the lower percentage of men in the study – 48 of 88 participants were women – may be a result of factors that deserve further investigation. Finally, burnout rates among volunteers were higher than those among other physicians, suggesting that “the study appealed to those in greatest need of the intervention.”
The study was funded by the Mayo Clinic department of medicine’s Program on Physician Well-Being and the Physician Foundation. Two of the authors – Dr. Dyrbye and Tait D. Shanafelt, MD, of Stanford (Calif.) University – reported being the coinventors of, and receiving royalties for, the Physician Well-Being Index, Medical Student Well-Being Index, Nurse Well-Being Index, and the Well-Being Index.
SOURCE: Dyrbye LN et al. JAMA Intern Med. 2019 Aug 5. doi: 10.1001/jamainternmed.2019.2425.
FROM JAMA INTERNAL MEDICINE
Propose Ideas for Sessions for VAM 2020
The Society for Vascular Surgery seeks proposals for invited sessions for the 2020 Vascular Annual Meeting, to be held June 17 to 20 in Toronto, Ontario, Canada. Scientific sessions will be June 18 to 20 and exhibits will be open June 18 to 19. Proposals should include the session's educational benefit, a short outline of the program topic, session goals and target audience, among other information. Obtain the information/submission form here. Email completed forms to [email protected].
The Society for Vascular Surgery seeks proposals for invited sessions for the 2020 Vascular Annual Meeting, to be held June 17 to 20 in Toronto, Ontario, Canada. Scientific sessions will be June 18 to 20 and exhibits will be open June 18 to 19. Proposals should include the session's educational benefit, a short outline of the program topic, session goals and target audience, among other information. Obtain the information/submission form here. Email completed forms to [email protected].
The Society for Vascular Surgery seeks proposals for invited sessions for the 2020 Vascular Annual Meeting, to be held June 17 to 20 in Toronto, Ontario, Canada. Scientific sessions will be June 18 to 20 and exhibits will be open June 18 to 19. Proposals should include the session's educational benefit, a short outline of the program topic, session goals and target audience, among other information. Obtain the information/submission form here. Email completed forms to [email protected].
VAM on Demand Now Available
All who attended the 2019 Vascular Annual Meeting can now review sessions they attended, as well as “attend” those that they missed. Those who weren’t at VAM can now experience all they missed. Slides and audio presentations of nearly every session are included in the now available VAM on Demand. The cost for one year of access is $199 for VAM attendees and $499 for non-attendees. Those who purchased VAM on Demand before the meeting ended can visit this site to gain access by logging in with their SVS credentials.
All who attended the 2019 Vascular Annual Meeting can now review sessions they attended, as well as “attend” those that they missed. Those who weren’t at VAM can now experience all they missed. Slides and audio presentations of nearly every session are included in the now available VAM on Demand. The cost for one year of access is $199 for VAM attendees and $499 for non-attendees. Those who purchased VAM on Demand before the meeting ended can visit this site to gain access by logging in with their SVS credentials.
All who attended the 2019 Vascular Annual Meeting can now review sessions they attended, as well as “attend” those that they missed. Those who weren’t at VAM can now experience all they missed. Slides and audio presentations of nearly every session are included in the now available VAM on Demand. The cost for one year of access is $199 for VAM attendees and $499 for non-attendees. Those who purchased VAM on Demand before the meeting ended can visit this site to gain access by logging in with their SVS credentials.
Poll: Medicare-for-all sees slight drop in support
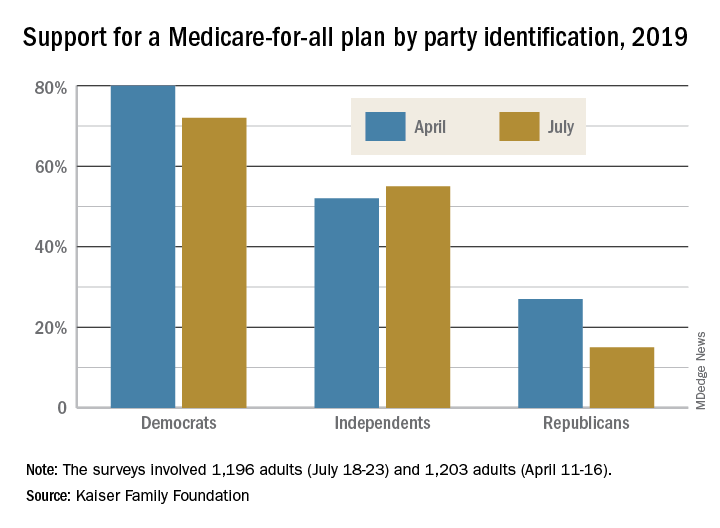
The two polls showed that Americans’ overall favorability for a national Medicare-for-all plan declined from 56% in April to 51% in July. That drop came from Democratic respondents, whose support went from 80% to 72%, and from Republicans, whose support declined from 27% to 15%. Overall favorability increased from 52% to 55% among independents, Kaiser reported in its latest Health Tracking Poll.
“The small dip in Medicare-for-all support may reflect recent debate over the role of private insurance, including employer-sponsored coverage, which would largely disappear under the leading Medicare-for-all plans but would continue under a public option,” Kaiser said in a statement accompanying the report.
When given a choice, 55% of Democrats and Democratic-leaning Independents (Republicans were not asked) would rather build on the existing Affordable Care Act, compared with 39% who want to replace it with Medicare-for-all, the Kaiser investigators said.
Support is currently greater for a public option that would give all residents the ability to choose a government-sponsored insurance plan. Almost two-thirds of those surveyed said that they favor such a proposal, with support at 85% for Democrats, 68% for independents, and 36% for Republicans, the report’s authors said.
The two polls were conducted among 1,196 adults during July 18-23, 2019, and 1,203 adults during April 11-16, 2019. The margin of sampling error for both polls was plus or minus 3 percentage points.

The two polls showed that Americans’ overall favorability for a national Medicare-for-all plan declined from 56% in April to 51% in July. That drop came from Democratic respondents, whose support went from 80% to 72%, and from Republicans, whose support declined from 27% to 15%. Overall favorability increased from 52% to 55% among independents, Kaiser reported in its latest Health Tracking Poll.
“The small dip in Medicare-for-all support may reflect recent debate over the role of private insurance, including employer-sponsored coverage, which would largely disappear under the leading Medicare-for-all plans but would continue under a public option,” Kaiser said in a statement accompanying the report.
When given a choice, 55% of Democrats and Democratic-leaning Independents (Republicans were not asked) would rather build on the existing Affordable Care Act, compared with 39% who want to replace it with Medicare-for-all, the Kaiser investigators said.
Support is currently greater for a public option that would give all residents the ability to choose a government-sponsored insurance plan. Almost two-thirds of those surveyed said that they favor such a proposal, with support at 85% for Democrats, 68% for independents, and 36% for Republicans, the report’s authors said.
The two polls were conducted among 1,196 adults during July 18-23, 2019, and 1,203 adults during April 11-16, 2019. The margin of sampling error for both polls was plus or minus 3 percentage points.

The two polls showed that Americans’ overall favorability for a national Medicare-for-all plan declined from 56% in April to 51% in July. That drop came from Democratic respondents, whose support went from 80% to 72%, and from Republicans, whose support declined from 27% to 15%. Overall favorability increased from 52% to 55% among independents, Kaiser reported in its latest Health Tracking Poll.
“The small dip in Medicare-for-all support may reflect recent debate over the role of private insurance, including employer-sponsored coverage, which would largely disappear under the leading Medicare-for-all plans but would continue under a public option,” Kaiser said in a statement accompanying the report.
When given a choice, 55% of Democrats and Democratic-leaning Independents (Republicans were not asked) would rather build on the existing Affordable Care Act, compared with 39% who want to replace it with Medicare-for-all, the Kaiser investigators said.
Support is currently greater for a public option that would give all residents the ability to choose a government-sponsored insurance plan. Almost two-thirds of those surveyed said that they favor such a proposal, with support at 85% for Democrats, 68% for independents, and 36% for Republicans, the report’s authors said.
The two polls were conducted among 1,196 adults during July 18-23, 2019, and 1,203 adults during April 11-16, 2019. The margin of sampling error for both polls was plus or minus 3 percentage points.
Dr. Alexa will see you now: Is Amazon primed to come to your rescue?
Now that it’s upending the way you play music, cook, shop, hear the news, and check the weather, the friendly voice emanating from your Amazon Alexa–enabled smart speaker is poised to wriggle its way into all things health care.
Amazon has big ambitions for its devices. It thinks Alexa, the virtual assistant inside them, could help doctors diagnose mental illness, autism, concussion, and Parkinson’s disease. It even hopes Alexa will detect when you’re having a heart attack.
At present, Alexa can perform a handful of health care–related tasks: “She” can track blood glucose levels, describe symptoms, access postsurgical care instructions, monitor home prescription deliveries, and make same-day appointments at the nearest urgent care center.
Amazon has partnered with numerous health care companies, including several in California, to let consumers and employees use Alexa for health care purposes. Workers at Cigna Corp. can manage their health improvement goals and earn wellness incentives with Alexa. And Alexa helps people who use Omron Healthcare’s blood pressure monitor, HeartGuide, track their readings.
But a flood of new opportunities is emerging since Alexa won permission to use protected patient health records controlled under the U.S. privacy law known as HIPAA.
Before, Alexa had been limited to providing generic responses about medical conditions. Now that it can transmit private patient information, Amazon has extended its Alexa Skills Kit, the software development tools used to add functions. Soon, the virtual assistant will be able to send and receive individualized patient records, allowing health care companies to create services for consumers to use at home.
Amazon’s efforts in this domain are important because, with its 100 million smart devices in use worldwide, it could radically change the way consumers get health information and even treatment – and not just tech-savvy consumers. Analysts expect that 55% of U.S. households will have smart speakers by 2022.
Some of Alexa’s new skills depend on a little-understood feature of the devices: They listen to every sound around them. They have to in order to be ready to respond to a request, like “Alexa, how many tablespoons in a half-pint?” or “Put carrots on the shopping list.”
University of Washington researchers recently published a study in which they taught Alexa and two other devices – an iPhone 5s and a Samsung Galaxy S4 – to listen for so-called agonal breathing, the distinct gasping sounds that are an early-warning sign in about half of all cardiac arrests. These devices correctly identified agonal breathing in 97% of instances, while registering a false positive only 0.2% of the time.
Earlier research had shown that a machine learning system could recognize cardiac arrest during 911 emergency calls more accurately and far faster than human dispatchers could.
Amazon, which declined to comment for this article, holds a patent on an acoustic technology that recognizes and could act on significant audio interruptions. Combined with patented technology from the University of Washington that differentiates coughs and sneezes from other background noises, for example, Alexa could discern when someone is ill and suggest solutions.
Because Amazon also holds patents on monitoring blood flow and heart rate through an Alexa-enabled camera, Alexa could send vitals to a doctor’s office before you head to your appointment and continue to monitor your condition after you get home.
“It opens possibilities to deliver care at a distance,” said Sandhya Pruthi, MD, lead investigator for several breast cancer prevention trials at the Mayo Clinic, which has been on the front lines of using voice assistants in health care. “Think about people living in small towns who aren’t always getting access to care and knowing when to get health care,” she said. “Could this be an opportunity, if someone had symptoms, to say, ‘It’s time for this to get checked out’?”
A growing number of clinics, hospitals, home health care providers and insurers have begun experimenting with products using Alexa:
- Livongo, a Mountain View, Calif.–based startup focused on managing chronic diseases, sells an Alexa-connected blood glucose monitor that can help diabetes patients track their condition.
- Home health care provider Libertana Home Health, based in Sherman Oaks, Calif., created an Alexa skill that lets elderly or frail residents connect with caregivers, sets up reminders about medications, reports their weight and blood pressure, and schedules appointments.
- Cedars-Sinai Medical Center in Los Angeles put Amazon devices loaded with a plug-in called Aiva into more than 100 rooms to connect patients with staff and to provide hands-free television controls. Unlike a static call button, the voice-controlled device can tell nurses why a patient needs help and can then tell the patient the status of their request.
- Boston Children’s Hospital, which offered the first Alexa health care software with an educational tool called Kids MD, now uses Alexa to share postsurgical recovery data between a patient’s home and the hospital.
Many medical technology companies are tantalized by the possibilities offered by Alexa and similar technologies for an aging population. A wearable device could transmit information about falls or an uneven gait. Alexa could potentially combat loneliness. It is learning how to make conversation.
“Alexa can couple a practical interaction around health care with an interaction that can engage the patient, even delight the patient,” said elder care advocate Laurie M. Orlov.
It and other voice assistants also might help bring some relief to doctors and other medical practitioners who commonly complain that entering medical information into EHRs is too time consuming and detracts from effective interactions with patients.
This technology could work in the background to take notes on doctor-patient meetings, even suggesting possible treatments. Several startup companies are working on such applications.
One such company is Suki, based in Redwood City, Calif., which bills itself as “Alexa for doctors.” Its artificial intelligence software listens in on interactions between doctors and patients to write up medical notes automatically.
Amazon devices will need to excel at conversational artificial intelligence, capable of relating an earlier phrase to a subsequent one, if it is to remain dominant in homes.
In a 2018 interview on Amazon’s corporate blog, Rohit Prasad, a company vice president who is head scientist for Amazon Alexa, described Alexa’s anticipated evolution using “federated learning” that lets algorithms make themselves smarter by incorporating input from a wide variety of sources.
“With these advances, we will see Alexa become more contextually aware in how she recognizes, understands and responds to requests from users,” Prasad said.
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation. KHN is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Now that it’s upending the way you play music, cook, shop, hear the news, and check the weather, the friendly voice emanating from your Amazon Alexa–enabled smart speaker is poised to wriggle its way into all things health care.
Amazon has big ambitions for its devices. It thinks Alexa, the virtual assistant inside them, could help doctors diagnose mental illness, autism, concussion, and Parkinson’s disease. It even hopes Alexa will detect when you’re having a heart attack.
At present, Alexa can perform a handful of health care–related tasks: “She” can track blood glucose levels, describe symptoms, access postsurgical care instructions, monitor home prescription deliveries, and make same-day appointments at the nearest urgent care center.
Amazon has partnered with numerous health care companies, including several in California, to let consumers and employees use Alexa for health care purposes. Workers at Cigna Corp. can manage their health improvement goals and earn wellness incentives with Alexa. And Alexa helps people who use Omron Healthcare’s blood pressure monitor, HeartGuide, track their readings.
But a flood of new opportunities is emerging since Alexa won permission to use protected patient health records controlled under the U.S. privacy law known as HIPAA.
Before, Alexa had been limited to providing generic responses about medical conditions. Now that it can transmit private patient information, Amazon has extended its Alexa Skills Kit, the software development tools used to add functions. Soon, the virtual assistant will be able to send and receive individualized patient records, allowing health care companies to create services for consumers to use at home.
Amazon’s efforts in this domain are important because, with its 100 million smart devices in use worldwide, it could radically change the way consumers get health information and even treatment – and not just tech-savvy consumers. Analysts expect that 55% of U.S. households will have smart speakers by 2022.
Some of Alexa’s new skills depend on a little-understood feature of the devices: They listen to every sound around them. They have to in order to be ready to respond to a request, like “Alexa, how many tablespoons in a half-pint?” or “Put carrots on the shopping list.”
University of Washington researchers recently published a study in which they taught Alexa and two other devices – an iPhone 5s and a Samsung Galaxy S4 – to listen for so-called agonal breathing, the distinct gasping sounds that are an early-warning sign in about half of all cardiac arrests. These devices correctly identified agonal breathing in 97% of instances, while registering a false positive only 0.2% of the time.
Earlier research had shown that a machine learning system could recognize cardiac arrest during 911 emergency calls more accurately and far faster than human dispatchers could.
Amazon, which declined to comment for this article, holds a patent on an acoustic technology that recognizes and could act on significant audio interruptions. Combined with patented technology from the University of Washington that differentiates coughs and sneezes from other background noises, for example, Alexa could discern when someone is ill and suggest solutions.
Because Amazon also holds patents on monitoring blood flow and heart rate through an Alexa-enabled camera, Alexa could send vitals to a doctor’s office before you head to your appointment and continue to monitor your condition after you get home.
“It opens possibilities to deliver care at a distance,” said Sandhya Pruthi, MD, lead investigator for several breast cancer prevention trials at the Mayo Clinic, which has been on the front lines of using voice assistants in health care. “Think about people living in small towns who aren’t always getting access to care and knowing when to get health care,” she said. “Could this be an opportunity, if someone had symptoms, to say, ‘It’s time for this to get checked out’?”
A growing number of clinics, hospitals, home health care providers and insurers have begun experimenting with products using Alexa:
- Livongo, a Mountain View, Calif.–based startup focused on managing chronic diseases, sells an Alexa-connected blood glucose monitor that can help diabetes patients track their condition.
- Home health care provider Libertana Home Health, based in Sherman Oaks, Calif., created an Alexa skill that lets elderly or frail residents connect with caregivers, sets up reminders about medications, reports their weight and blood pressure, and schedules appointments.
- Cedars-Sinai Medical Center in Los Angeles put Amazon devices loaded with a plug-in called Aiva into more than 100 rooms to connect patients with staff and to provide hands-free television controls. Unlike a static call button, the voice-controlled device can tell nurses why a patient needs help and can then tell the patient the status of their request.
- Boston Children’s Hospital, which offered the first Alexa health care software with an educational tool called Kids MD, now uses Alexa to share postsurgical recovery data between a patient’s home and the hospital.
Many medical technology companies are tantalized by the possibilities offered by Alexa and similar technologies for an aging population. A wearable device could transmit information about falls or an uneven gait. Alexa could potentially combat loneliness. It is learning how to make conversation.
“Alexa can couple a practical interaction around health care with an interaction that can engage the patient, even delight the patient,” said elder care advocate Laurie M. Orlov.
It and other voice assistants also might help bring some relief to doctors and other medical practitioners who commonly complain that entering medical information into EHRs is too time consuming and detracts from effective interactions with patients.
This technology could work in the background to take notes on doctor-patient meetings, even suggesting possible treatments. Several startup companies are working on such applications.
One such company is Suki, based in Redwood City, Calif., which bills itself as “Alexa for doctors.” Its artificial intelligence software listens in on interactions between doctors and patients to write up medical notes automatically.
Amazon devices will need to excel at conversational artificial intelligence, capable of relating an earlier phrase to a subsequent one, if it is to remain dominant in homes.
In a 2018 interview on Amazon’s corporate blog, Rohit Prasad, a company vice president who is head scientist for Amazon Alexa, described Alexa’s anticipated evolution using “federated learning” that lets algorithms make themselves smarter by incorporating input from a wide variety of sources.
“With these advances, we will see Alexa become more contextually aware in how she recognizes, understands and responds to requests from users,” Prasad said.
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation. KHN is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Now that it’s upending the way you play music, cook, shop, hear the news, and check the weather, the friendly voice emanating from your Amazon Alexa–enabled smart speaker is poised to wriggle its way into all things health care.
Amazon has big ambitions for its devices. It thinks Alexa, the virtual assistant inside them, could help doctors diagnose mental illness, autism, concussion, and Parkinson’s disease. It even hopes Alexa will detect when you’re having a heart attack.
At present, Alexa can perform a handful of health care–related tasks: “She” can track blood glucose levels, describe symptoms, access postsurgical care instructions, monitor home prescription deliveries, and make same-day appointments at the nearest urgent care center.
Amazon has partnered with numerous health care companies, including several in California, to let consumers and employees use Alexa for health care purposes. Workers at Cigna Corp. can manage their health improvement goals and earn wellness incentives with Alexa. And Alexa helps people who use Omron Healthcare’s blood pressure monitor, HeartGuide, track their readings.
But a flood of new opportunities is emerging since Alexa won permission to use protected patient health records controlled under the U.S. privacy law known as HIPAA.
Before, Alexa had been limited to providing generic responses about medical conditions. Now that it can transmit private patient information, Amazon has extended its Alexa Skills Kit, the software development tools used to add functions. Soon, the virtual assistant will be able to send and receive individualized patient records, allowing health care companies to create services for consumers to use at home.
Amazon’s efforts in this domain are important because, with its 100 million smart devices in use worldwide, it could radically change the way consumers get health information and even treatment – and not just tech-savvy consumers. Analysts expect that 55% of U.S. households will have smart speakers by 2022.
Some of Alexa’s new skills depend on a little-understood feature of the devices: They listen to every sound around them. They have to in order to be ready to respond to a request, like “Alexa, how many tablespoons in a half-pint?” or “Put carrots on the shopping list.”
University of Washington researchers recently published a study in which they taught Alexa and two other devices – an iPhone 5s and a Samsung Galaxy S4 – to listen for so-called agonal breathing, the distinct gasping sounds that are an early-warning sign in about half of all cardiac arrests. These devices correctly identified agonal breathing in 97% of instances, while registering a false positive only 0.2% of the time.
Earlier research had shown that a machine learning system could recognize cardiac arrest during 911 emergency calls more accurately and far faster than human dispatchers could.
Amazon, which declined to comment for this article, holds a patent on an acoustic technology that recognizes and could act on significant audio interruptions. Combined with patented technology from the University of Washington that differentiates coughs and sneezes from other background noises, for example, Alexa could discern when someone is ill and suggest solutions.
Because Amazon also holds patents on monitoring blood flow and heart rate through an Alexa-enabled camera, Alexa could send vitals to a doctor’s office before you head to your appointment and continue to monitor your condition after you get home.
“It opens possibilities to deliver care at a distance,” said Sandhya Pruthi, MD, lead investigator for several breast cancer prevention trials at the Mayo Clinic, which has been on the front lines of using voice assistants in health care. “Think about people living in small towns who aren’t always getting access to care and knowing when to get health care,” she said. “Could this be an opportunity, if someone had symptoms, to say, ‘It’s time for this to get checked out’?”
A growing number of clinics, hospitals, home health care providers and insurers have begun experimenting with products using Alexa:
- Livongo, a Mountain View, Calif.–based startup focused on managing chronic diseases, sells an Alexa-connected blood glucose monitor that can help diabetes patients track their condition.
- Home health care provider Libertana Home Health, based in Sherman Oaks, Calif., created an Alexa skill that lets elderly or frail residents connect with caregivers, sets up reminders about medications, reports their weight and blood pressure, and schedules appointments.
- Cedars-Sinai Medical Center in Los Angeles put Amazon devices loaded with a plug-in called Aiva into more than 100 rooms to connect patients with staff and to provide hands-free television controls. Unlike a static call button, the voice-controlled device can tell nurses why a patient needs help and can then tell the patient the status of their request.
- Boston Children’s Hospital, which offered the first Alexa health care software with an educational tool called Kids MD, now uses Alexa to share postsurgical recovery data between a patient’s home and the hospital.
Many medical technology companies are tantalized by the possibilities offered by Alexa and similar technologies for an aging population. A wearable device could transmit information about falls or an uneven gait. Alexa could potentially combat loneliness. It is learning how to make conversation.
“Alexa can couple a practical interaction around health care with an interaction that can engage the patient, even delight the patient,” said elder care advocate Laurie M. Orlov.
It and other voice assistants also might help bring some relief to doctors and other medical practitioners who commonly complain that entering medical information into EHRs is too time consuming and detracts from effective interactions with patients.
This technology could work in the background to take notes on doctor-patient meetings, even suggesting possible treatments. Several startup companies are working on such applications.
One such company is Suki, based in Redwood City, Calif., which bills itself as “Alexa for doctors.” Its artificial intelligence software listens in on interactions between doctors and patients to write up medical notes automatically.
Amazon devices will need to excel at conversational artificial intelligence, capable of relating an earlier phrase to a subsequent one, if it is to remain dominant in homes.
In a 2018 interview on Amazon’s corporate blog, Rohit Prasad, a company vice president who is head scientist for Amazon Alexa, described Alexa’s anticipated evolution using “federated learning” that lets algorithms make themselves smarter by incorporating input from a wide variety of sources.
“With these advances, we will see Alexa become more contextually aware in how she recognizes, understands and responds to requests from users,” Prasad said.
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation. KHN is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Become a Social Media Ambassador
If you are a medical student, a general surgery resident and/or a trainee who’d like to get more involved within the Society for Vascular Surgery, apply to be an SVS Social Media Ambassador. Ambassadors must be members of the SVS and understand the basic concept of social media. Once a part of the program, they are encouraged to be advocates for the SVS and provide honest feedback to the staff as to what they’d like to see on our channels. If you are interested, learn more about the program here and apply today.
If you are a medical student, a general surgery resident and/or a trainee who’d like to get more involved within the Society for Vascular Surgery, apply to be an SVS Social Media Ambassador. Ambassadors must be members of the SVS and understand the basic concept of social media. Once a part of the program, they are encouraged to be advocates for the SVS and provide honest feedback to the staff as to what they’d like to see on our channels. If you are interested, learn more about the program here and apply today.
If you are a medical student, a general surgery resident and/or a trainee who’d like to get more involved within the Society for Vascular Surgery, apply to be an SVS Social Media Ambassador. Ambassadors must be members of the SVS and understand the basic concept of social media. Once a part of the program, they are encouraged to be advocates for the SVS and provide honest feedback to the staff as to what they’d like to see on our channels. If you are interested, learn more about the program here and apply today.
Interested in joining an SVS Task Force?
The Society for Vascular Surgery is establishing a new Health Information Technology Task Force. The SVS Executive Board is seeking members with a desire to explore and advance technology-based health improvement for vascular patients. Volunteers should be interested in, as well as have some experience with, technology-related health improvements, medical informatics, etc. Selected task force members will collaborate with non-member industry technology experts, as well as SVS leadership. If you’d like to be considered, please email a brief statement of interest to [email protected]. Read all the details here.
The Society for Vascular Surgery is establishing a new Health Information Technology Task Force. The SVS Executive Board is seeking members with a desire to explore and advance technology-based health improvement for vascular patients. Volunteers should be interested in, as well as have some experience with, technology-related health improvements, medical informatics, etc. Selected task force members will collaborate with non-member industry technology experts, as well as SVS leadership. If you’d like to be considered, please email a brief statement of interest to [email protected]. Read all the details here.
The Society for Vascular Surgery is establishing a new Health Information Technology Task Force. The SVS Executive Board is seeking members with a desire to explore and advance technology-based health improvement for vascular patients. Volunteers should be interested in, as well as have some experience with, technology-related health improvements, medical informatics, etc. Selected task force members will collaborate with non-member industry technology experts, as well as SVS leadership. If you’d like to be considered, please email a brief statement of interest to [email protected]. Read all the details here.