User login
Official Newspaper of the American College of Surgeons
Ranked: State of the states’ health care
In the wild world of health care rankings, a year can make a big difference … or not.
Iowa fell from second to ninth over the course of the last year while Connecticut and South Dakota moved out of the top 10 to make way for Colorado and Maryland, according to WalletHub.
There was less movement at the other end of the rankings, however, with no change at all in the bottom five: Louisiana finished 51st again (the rankings include the District of Columbia), preceded by fellow repeaters Mississippi (50), Alaska (49), Arkansas (48), and North Carolina (47). Texas and Nevada did manage to move on up out of the bottom 11 – to 38th and 40th, respectively – at the expense of Oklahoma and Tennessee, WalletHub reported.
For 2018, the company compared the states and D.C. “across 40 measures of cost, accessibility and outcome,” which is five more measures than last year and a possible explanation for the changes at the top. The cost dimension’s five metrics included cost of medical visits and share of high out-of-pocket medical spending. The accessibility dimension consisted of 21 metrics, including average emergency department wait time and share of insured children. The outcomes dimension included 14 metrics, among them maternal mortality rate and share of adults with type 2 diabetes.
Vermont did well in both the outcomes (first) and cost (third) dimensions but only middle of the pack (23rd) in access. The District of Columbia was ranked first in cost and Maine was the leader in access. The lowest-ranked states in each category were Alaska (cost), Texas (access), and Mississippi (outcomes), according to the WalletHub analysis, which was based on data from such sources as the Centers for Disease Control and Prevention, the Health Resources & Services Administration, and the United Health Foundation.
In the wild world of health care rankings, a year can make a big difference … or not.
Iowa fell from second to ninth over the course of the last year while Connecticut and South Dakota moved out of the top 10 to make way for Colorado and Maryland, according to WalletHub.
There was less movement at the other end of the rankings, however, with no change at all in the bottom five: Louisiana finished 51st again (the rankings include the District of Columbia), preceded by fellow repeaters Mississippi (50), Alaska (49), Arkansas (48), and North Carolina (47). Texas and Nevada did manage to move on up out of the bottom 11 – to 38th and 40th, respectively – at the expense of Oklahoma and Tennessee, WalletHub reported.
For 2018, the company compared the states and D.C. “across 40 measures of cost, accessibility and outcome,” which is five more measures than last year and a possible explanation for the changes at the top. The cost dimension’s five metrics included cost of medical visits and share of high out-of-pocket medical spending. The accessibility dimension consisted of 21 metrics, including average emergency department wait time and share of insured children. The outcomes dimension included 14 metrics, among them maternal mortality rate and share of adults with type 2 diabetes.
Vermont did well in both the outcomes (first) and cost (third) dimensions but only middle of the pack (23rd) in access. The District of Columbia was ranked first in cost and Maine was the leader in access. The lowest-ranked states in each category were Alaska (cost), Texas (access), and Mississippi (outcomes), according to the WalletHub analysis, which was based on data from such sources as the Centers for Disease Control and Prevention, the Health Resources & Services Administration, and the United Health Foundation.
In the wild world of health care rankings, a year can make a big difference … or not.
Iowa fell from second to ninth over the course of the last year while Connecticut and South Dakota moved out of the top 10 to make way for Colorado and Maryland, according to WalletHub.
There was less movement at the other end of the rankings, however, with no change at all in the bottom five: Louisiana finished 51st again (the rankings include the District of Columbia), preceded by fellow repeaters Mississippi (50), Alaska (49), Arkansas (48), and North Carolina (47). Texas and Nevada did manage to move on up out of the bottom 11 – to 38th and 40th, respectively – at the expense of Oklahoma and Tennessee, WalletHub reported.
For 2018, the company compared the states and D.C. “across 40 measures of cost, accessibility and outcome,” which is five more measures than last year and a possible explanation for the changes at the top. The cost dimension’s five metrics included cost of medical visits and share of high out-of-pocket medical spending. The accessibility dimension consisted of 21 metrics, including average emergency department wait time and share of insured children. The outcomes dimension included 14 metrics, among them maternal mortality rate and share of adults with type 2 diabetes.
Vermont did well in both the outcomes (first) and cost (third) dimensions but only middle of the pack (23rd) in access. The District of Columbia was ranked first in cost and Maine was the leader in access. The lowest-ranked states in each category were Alaska (cost), Texas (access), and Mississippi (outcomes), according to the WalletHub analysis, which was based on data from such sources as the Centers for Disease Control and Prevention, the Health Resources & Services Administration, and the United Health Foundation.
Some doctors are warming to single-payer medicine
When the American Medical Association – one of the nation’s most powerful health care groups – met in Chicago this June, its medical student caucus seized an opportunity for change.
Though they had tried for years to advance a resolution calling on the organization to drop its decades-long opposition to single-payer health care, this was the first time it got a full hearing. The debate grew heated – older physicians warned their pay would decrease, calling younger advocates naive to single-payer’s consequences. But this time, by the meeting’s end, the AMA’s older members had agreed to at least study the possibility of changing its stance.
“We believe health care is a human right, maybe more so than past generations,” said Brad Zehr, MD, a pathology resident at Ohio State University, who was part of the debate. “There’s a generational shift happening, where we see universal health care as a requirement.”
The ins and outs of the AMA’s policymaking may sound like inside baseball. But this year’s youth uprising at the nexus of the medical establishment speaks to a cultural shift in the medical profession, and one with big political implications.
Amid Republican attacks on the Affordable Care Act, an increasing number of Democrats – both candidates and Congress members – are putting forth proposals that would vastly increase the government’s role in running the health system. These include single-payer, Medicare-for-all, or an option for anyone to buy in to the Medicare program. At least 70 House Democrats have signed on to the new “Medicare-for-all” caucus.
Organized medicine, and previous generations of doctors, had for the most part staunchly opposed any such plan. The AMA has thwarted public health insurance proposals since the 1930s and long been considered one of the policy’s most powerful opponents.
But the battle lines are shifting as younger doctors flip their views, a change that will likely assume greater significance as the next generation of physicians takes on leadership roles. The AMA did not make anyone available for comment.
Many younger physicians are “accepting of single-payer,” said Christian Pean, MD, a third-year orthopedic surgery resident at New York University.
In prior generations, “intelligent, motivated, quantitative” students pursued medicine, both for the income and because of the workplace independence – running practices with minimal government interference, said Steven Schroeder, MD, professor of medicine at the University of California, San Francisco.
In his 50 years of teaching, students’ attitudes have changed, he said. “The ‘Oh, keep government out of my work’ feeling is not as strong as it was with maybe older cohorts,” said Dr. Schroeder. “Students come in saying, ‘We want to make a difference through social justice. That’s why we’re here.’ ”
Though single-payer health care long has been dismissed as a left-wing pipe dream, polling suggests a slim majority of Americans now support the idea – though it is not clear people know what the term means.
A full single-payer system means everyone gets coverage from the same insurance plan, usually sponsored by the government. “Medicare for all,” a phrase that gained currency with the presidential campaign of Sen. Bernie Sanders (I-Vt.), means everyone gets Medicare, but, depending on the proposal, it may or may not allow private insurers to offer Medicare as well. (Sen. Sanders’ plan, which eliminates deductibles and expands benefits, would get rid of private insurers.)
Meanwhile, lots of countries achieve universal health care – everyone is covered somehow – but the method can vary. For example, France requires all citizens purchase coverage, which is sold through nonprofits. In Germany, most people get insurance from a government-run “public option,” while others purchase private plans. In England, health care is provided through the tax-funded National Health System.
American skeptics often use the phrase “socialized medicine” pejoratively to describe all of these models.
“Few really understand what you mean when you say single-payer,” said Frank Opelka, MD, medical director of quality and health policy for the American College of Surgeons, which opposes such a policy. “What they mean is, ‘I don’t think the current system is working.’ ”
But the willingness to explore previously unthinkable ideas is evident in young doctors’ ranks.
Recent surveys through LinkedIn, Merritt Hawkins, and NEJM Catalyst indicate growing support. In the March NEJM Catalyst survey, 61% of 607 respondents said single-payer would make it easier to deliver cost-effective, quality health care.
Delving further, that survey shows support is stronger among younger physicians, said Namita Mohta, MD, a hospitalist at Brigham and Women’s Hospital, Boston, and clinical editor at NEJM Catalyst.
But it’s unclear whether these findings reflect young doctors’ feelings about the policy or whether they are tapping in to broader frustrations with the American health system.
Much like the general public, doctors often use terms like single-payer, Medicare for all, and universal health care interchangeably.
“Our younger generation is less afraid to come out and say we want universal health care,” said Anna Yap, MD, an emergency medicine resident at UCLA, who served as a medical student delegate to the AMA until this past June. “But how? It’s different in what forms we see.”
Younger doctors also pointed to growing concern about how best to keep patients healthy. They cited research that broadly suggests having health insurance tracks with better health outcomes.
“Medical students, I would say, are very interested in public health and improving social determinants of health – one of them being access to health insurance,” said Jerome Jeevarajan, MD, a neurology resident at the University of Texas–Houston, referring to nonmedical factors that improve health, such as food or housing.
Some of the shift in opinion has to do with the changing realities of medical practice. Doctors now are more likely to end up working for large health systems or hospitals, rather than starting a practice. Combined with the increasing complexity of billing private insurance, many said, that means contracting with the government may feel like less of an intrusion.
The debate is, at this point, still theoretical. Republicans – who control the White House and both houses of Congress – sharply oppose single-payer. Meanwhile, single-state efforts in California, Colorado, and New York have fallen flat.
Also, doctors represent only one part of the sprawling health care industrial complex. Other health care interests – including private insurance, the drug industry, and hospital trade groups – have been slower to warm to catchphrases like single-payer or universal health care, all of which would likely mean a drop in income.
But increasingly physicians seem to be switching sides in the debate, and young physicians want to be part of the discussion.
“There’s tremendous potential ... to be at the table if single-payer becomes a significant part of the political discourse, and create a system that is more equitable,” Dr. Pean said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
When the American Medical Association – one of the nation’s most powerful health care groups – met in Chicago this June, its medical student caucus seized an opportunity for change.
Though they had tried for years to advance a resolution calling on the organization to drop its decades-long opposition to single-payer health care, this was the first time it got a full hearing. The debate grew heated – older physicians warned their pay would decrease, calling younger advocates naive to single-payer’s consequences. But this time, by the meeting’s end, the AMA’s older members had agreed to at least study the possibility of changing its stance.
“We believe health care is a human right, maybe more so than past generations,” said Brad Zehr, MD, a pathology resident at Ohio State University, who was part of the debate. “There’s a generational shift happening, where we see universal health care as a requirement.”
The ins and outs of the AMA’s policymaking may sound like inside baseball. But this year’s youth uprising at the nexus of the medical establishment speaks to a cultural shift in the medical profession, and one with big political implications.
Amid Republican attacks on the Affordable Care Act, an increasing number of Democrats – both candidates and Congress members – are putting forth proposals that would vastly increase the government’s role in running the health system. These include single-payer, Medicare-for-all, or an option for anyone to buy in to the Medicare program. At least 70 House Democrats have signed on to the new “Medicare-for-all” caucus.
Organized medicine, and previous generations of doctors, had for the most part staunchly opposed any such plan. The AMA has thwarted public health insurance proposals since the 1930s and long been considered one of the policy’s most powerful opponents.
But the battle lines are shifting as younger doctors flip their views, a change that will likely assume greater significance as the next generation of physicians takes on leadership roles. The AMA did not make anyone available for comment.
Many younger physicians are “accepting of single-payer,” said Christian Pean, MD, a third-year orthopedic surgery resident at New York University.
In prior generations, “intelligent, motivated, quantitative” students pursued medicine, both for the income and because of the workplace independence – running practices with minimal government interference, said Steven Schroeder, MD, professor of medicine at the University of California, San Francisco.
In his 50 years of teaching, students’ attitudes have changed, he said. “The ‘Oh, keep government out of my work’ feeling is not as strong as it was with maybe older cohorts,” said Dr. Schroeder. “Students come in saying, ‘We want to make a difference through social justice. That’s why we’re here.’ ”
Though single-payer health care long has been dismissed as a left-wing pipe dream, polling suggests a slim majority of Americans now support the idea – though it is not clear people know what the term means.
A full single-payer system means everyone gets coverage from the same insurance plan, usually sponsored by the government. “Medicare for all,” a phrase that gained currency with the presidential campaign of Sen. Bernie Sanders (I-Vt.), means everyone gets Medicare, but, depending on the proposal, it may or may not allow private insurers to offer Medicare as well. (Sen. Sanders’ plan, which eliminates deductibles and expands benefits, would get rid of private insurers.)
Meanwhile, lots of countries achieve universal health care – everyone is covered somehow – but the method can vary. For example, France requires all citizens purchase coverage, which is sold through nonprofits. In Germany, most people get insurance from a government-run “public option,” while others purchase private plans. In England, health care is provided through the tax-funded National Health System.
American skeptics often use the phrase “socialized medicine” pejoratively to describe all of these models.
“Few really understand what you mean when you say single-payer,” said Frank Opelka, MD, medical director of quality and health policy for the American College of Surgeons, which opposes such a policy. “What they mean is, ‘I don’t think the current system is working.’ ”
But the willingness to explore previously unthinkable ideas is evident in young doctors’ ranks.
Recent surveys through LinkedIn, Merritt Hawkins, and NEJM Catalyst indicate growing support. In the March NEJM Catalyst survey, 61% of 607 respondents said single-payer would make it easier to deliver cost-effective, quality health care.
Delving further, that survey shows support is stronger among younger physicians, said Namita Mohta, MD, a hospitalist at Brigham and Women’s Hospital, Boston, and clinical editor at NEJM Catalyst.
But it’s unclear whether these findings reflect young doctors’ feelings about the policy or whether they are tapping in to broader frustrations with the American health system.
Much like the general public, doctors often use terms like single-payer, Medicare for all, and universal health care interchangeably.
“Our younger generation is less afraid to come out and say we want universal health care,” said Anna Yap, MD, an emergency medicine resident at UCLA, who served as a medical student delegate to the AMA until this past June. “But how? It’s different in what forms we see.”
Younger doctors also pointed to growing concern about how best to keep patients healthy. They cited research that broadly suggests having health insurance tracks with better health outcomes.
“Medical students, I would say, are very interested in public health and improving social determinants of health – one of them being access to health insurance,” said Jerome Jeevarajan, MD, a neurology resident at the University of Texas–Houston, referring to nonmedical factors that improve health, such as food or housing.
Some of the shift in opinion has to do with the changing realities of medical practice. Doctors now are more likely to end up working for large health systems or hospitals, rather than starting a practice. Combined with the increasing complexity of billing private insurance, many said, that means contracting with the government may feel like less of an intrusion.
The debate is, at this point, still theoretical. Republicans – who control the White House and both houses of Congress – sharply oppose single-payer. Meanwhile, single-state efforts in California, Colorado, and New York have fallen flat.
Also, doctors represent only one part of the sprawling health care industrial complex. Other health care interests – including private insurance, the drug industry, and hospital trade groups – have been slower to warm to catchphrases like single-payer or universal health care, all of which would likely mean a drop in income.
But increasingly physicians seem to be switching sides in the debate, and young physicians want to be part of the discussion.
“There’s tremendous potential ... to be at the table if single-payer becomes a significant part of the political discourse, and create a system that is more equitable,” Dr. Pean said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
When the American Medical Association – one of the nation’s most powerful health care groups – met in Chicago this June, its medical student caucus seized an opportunity for change.
Though they had tried for years to advance a resolution calling on the organization to drop its decades-long opposition to single-payer health care, this was the first time it got a full hearing. The debate grew heated – older physicians warned their pay would decrease, calling younger advocates naive to single-payer’s consequences. But this time, by the meeting’s end, the AMA’s older members had agreed to at least study the possibility of changing its stance.
“We believe health care is a human right, maybe more so than past generations,” said Brad Zehr, MD, a pathology resident at Ohio State University, who was part of the debate. “There’s a generational shift happening, where we see universal health care as a requirement.”
The ins and outs of the AMA’s policymaking may sound like inside baseball. But this year’s youth uprising at the nexus of the medical establishment speaks to a cultural shift in the medical profession, and one with big political implications.
Amid Republican attacks on the Affordable Care Act, an increasing number of Democrats – both candidates and Congress members – are putting forth proposals that would vastly increase the government’s role in running the health system. These include single-payer, Medicare-for-all, or an option for anyone to buy in to the Medicare program. At least 70 House Democrats have signed on to the new “Medicare-for-all” caucus.
Organized medicine, and previous generations of doctors, had for the most part staunchly opposed any such plan. The AMA has thwarted public health insurance proposals since the 1930s and long been considered one of the policy’s most powerful opponents.
But the battle lines are shifting as younger doctors flip their views, a change that will likely assume greater significance as the next generation of physicians takes on leadership roles. The AMA did not make anyone available for comment.
Many younger physicians are “accepting of single-payer,” said Christian Pean, MD, a third-year orthopedic surgery resident at New York University.
In prior generations, “intelligent, motivated, quantitative” students pursued medicine, both for the income and because of the workplace independence – running practices with minimal government interference, said Steven Schroeder, MD, professor of medicine at the University of California, San Francisco.
In his 50 years of teaching, students’ attitudes have changed, he said. “The ‘Oh, keep government out of my work’ feeling is not as strong as it was with maybe older cohorts,” said Dr. Schroeder. “Students come in saying, ‘We want to make a difference through social justice. That’s why we’re here.’ ”
Though single-payer health care long has been dismissed as a left-wing pipe dream, polling suggests a slim majority of Americans now support the idea – though it is not clear people know what the term means.
A full single-payer system means everyone gets coverage from the same insurance plan, usually sponsored by the government. “Medicare for all,” a phrase that gained currency with the presidential campaign of Sen. Bernie Sanders (I-Vt.), means everyone gets Medicare, but, depending on the proposal, it may or may not allow private insurers to offer Medicare as well. (Sen. Sanders’ plan, which eliminates deductibles and expands benefits, would get rid of private insurers.)
Meanwhile, lots of countries achieve universal health care – everyone is covered somehow – but the method can vary. For example, France requires all citizens purchase coverage, which is sold through nonprofits. In Germany, most people get insurance from a government-run “public option,” while others purchase private plans. In England, health care is provided through the tax-funded National Health System.
American skeptics often use the phrase “socialized medicine” pejoratively to describe all of these models.
“Few really understand what you mean when you say single-payer,” said Frank Opelka, MD, medical director of quality and health policy for the American College of Surgeons, which opposes such a policy. “What they mean is, ‘I don’t think the current system is working.’ ”
But the willingness to explore previously unthinkable ideas is evident in young doctors’ ranks.
Recent surveys through LinkedIn, Merritt Hawkins, and NEJM Catalyst indicate growing support. In the March NEJM Catalyst survey, 61% of 607 respondents said single-payer would make it easier to deliver cost-effective, quality health care.
Delving further, that survey shows support is stronger among younger physicians, said Namita Mohta, MD, a hospitalist at Brigham and Women’s Hospital, Boston, and clinical editor at NEJM Catalyst.
But it’s unclear whether these findings reflect young doctors’ feelings about the policy or whether they are tapping in to broader frustrations with the American health system.
Much like the general public, doctors often use terms like single-payer, Medicare for all, and universal health care interchangeably.
“Our younger generation is less afraid to come out and say we want universal health care,” said Anna Yap, MD, an emergency medicine resident at UCLA, who served as a medical student delegate to the AMA until this past June. “But how? It’s different in what forms we see.”
Younger doctors also pointed to growing concern about how best to keep patients healthy. They cited research that broadly suggests having health insurance tracks with better health outcomes.
“Medical students, I would say, are very interested in public health and improving social determinants of health – one of them being access to health insurance,” said Jerome Jeevarajan, MD, a neurology resident at the University of Texas–Houston, referring to nonmedical factors that improve health, such as food or housing.
Some of the shift in opinion has to do with the changing realities of medical practice. Doctors now are more likely to end up working for large health systems or hospitals, rather than starting a practice. Combined with the increasing complexity of billing private insurance, many said, that means contracting with the government may feel like less of an intrusion.
The debate is, at this point, still theoretical. Republicans – who control the White House and both houses of Congress – sharply oppose single-payer. Meanwhile, single-state efforts in California, Colorado, and New York have fallen flat.
Also, doctors represent only one part of the sprawling health care industrial complex. Other health care interests – including private insurance, the drug industry, and hospital trade groups – have been slower to warm to catchphrases like single-payer or universal health care, all of which would likely mean a drop in income.
But increasingly physicians seem to be switching sides in the debate, and young physicians want to be part of the discussion.
“There’s tremendous potential ... to be at the table if single-payer becomes a significant part of the political discourse, and create a system that is more equitable,” Dr. Pean said.
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
PhRMA spending leads health-sector lobbying efforts
The Pharmaceutical Research and Manufacturers of America (PhRMA) led the way on health-sector lobbying in the first half of 2018 with spending that’s on pace to top its previous 1-year high, according to the Center for Responsive Politics.
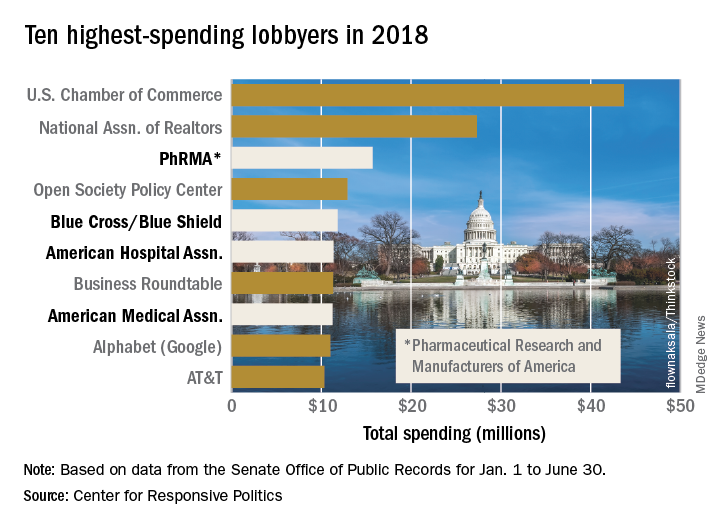
PhRMA spent over $15.7 million on lobbying through the end of June, and equaling that amount over the second half of the year would eclipse the $27.4 million the organization spent in 2009. PhRMA’s total for the year so far puts it third among all entities: The U.S. Chamber of Commerce was first with $43.7 million and the National Association of Realtors was second at $27.3 million, the center reported on OpenSecrets.org. The National Association of Realtors has been second in spending every year since 2012, and the chamber has been first every year since 2001.
The health sector’s three other representatives in the lobbying Top 10 for the first half of this year are Blue Cross/Blue Shield in fifth with $11.8 million in spending, the American Hospital Association in sixth ($11.4 million), and the American Medical Association in eighth ($11.2 million), based on the center’s analysis of data from the Senate Office of Public Records. The four current health sector representatives have all been in the top 10 every year since 2013.
Total spending for the health sector through June was $290.3 million, which was first among the 13 sectors into which the center separates the U.S. economy; spending for lobbying among all sectors was $1.69 billion. The health sector was ranked first in spending each of the 2 previous years and has never been lower than third since the center’s record keeping began in 2000, according to OpenSecrets.org.
The Pharmaceutical Research and Manufacturers of America (PhRMA) led the way on health-sector lobbying in the first half of 2018 with spending that’s on pace to top its previous 1-year high, according to the Center for Responsive Politics.

PhRMA spent over $15.7 million on lobbying through the end of June, and equaling that amount over the second half of the year would eclipse the $27.4 million the organization spent in 2009. PhRMA’s total for the year so far puts it third among all entities: The U.S. Chamber of Commerce was first with $43.7 million and the National Association of Realtors was second at $27.3 million, the center reported on OpenSecrets.org. The National Association of Realtors has been second in spending every year since 2012, and the chamber has been first every year since 2001.
The health sector’s three other representatives in the lobbying Top 10 for the first half of this year are Blue Cross/Blue Shield in fifth with $11.8 million in spending, the American Hospital Association in sixth ($11.4 million), and the American Medical Association in eighth ($11.2 million), based on the center’s analysis of data from the Senate Office of Public Records. The four current health sector representatives have all been in the top 10 every year since 2013.
Total spending for the health sector through June was $290.3 million, which was first among the 13 sectors into which the center separates the U.S. economy; spending for lobbying among all sectors was $1.69 billion. The health sector was ranked first in spending each of the 2 previous years and has never been lower than third since the center’s record keeping began in 2000, according to OpenSecrets.org.
The Pharmaceutical Research and Manufacturers of America (PhRMA) led the way on health-sector lobbying in the first half of 2018 with spending that’s on pace to top its previous 1-year high, according to the Center for Responsive Politics.

PhRMA spent over $15.7 million on lobbying through the end of June, and equaling that amount over the second half of the year would eclipse the $27.4 million the organization spent in 2009. PhRMA’s total for the year so far puts it third among all entities: The U.S. Chamber of Commerce was first with $43.7 million and the National Association of Realtors was second at $27.3 million, the center reported on OpenSecrets.org. The National Association of Realtors has been second in spending every year since 2012, and the chamber has been first every year since 2001.
The health sector’s three other representatives in the lobbying Top 10 for the first half of this year are Blue Cross/Blue Shield in fifth with $11.8 million in spending, the American Hospital Association in sixth ($11.4 million), and the American Medical Association in eighth ($11.2 million), based on the center’s analysis of data from the Senate Office of Public Records. The four current health sector representatives have all been in the top 10 every year since 2013.
Total spending for the health sector through June was $290.3 million, which was first among the 13 sectors into which the center separates the U.S. economy; spending for lobbying among all sectors was $1.69 billion. The health sector was ranked first in spending each of the 2 previous years and has never been lower than third since the center’s record keeping began in 2000, according to OpenSecrets.org.
Neoadjuvant-treated N2 rectal cancer linked to PCR failure
Orlando – Clinical an analysis of a large, multicenter database has suggested.
In multivariate regression, pretreatment N2 stage was the only variable significantly associated with failure of achieving pathologic complete response, according to Ebram Salama, MD, of Sir Mortimer B. Davis Jewish General Hospital at McGill University, Montreal.
“We should be reconsidering putting these patients in watch-and-wait protocols,” Dr. Salama said in an oral abstract presentation at the American College of Surgeons Quality and Safety Conference.
The analysis included 369 elective cases of cT2-4 N0-2 rectal cancer that were treated with neoadjuvant chemoradiotherapy during 2016 from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) proctectomy-specific database.
Of those cases, 53 (14.4%) achieved PCR, a proportion consistent with what has been reported previously in medical literature, Dr. Salama noted during his presentation.
The multivariate analysis revealed that pretreatment N2 stage was a negative predictor of PCR with an odds ratio of 0.18 (95% confidence interval, 0.04-0.82; P = .026), according to presented data.
By contrast, Dr. Salama said, there were no significant associations between response and other variables, including pretreatment N1 stage, pretreatment T stage, tumor location, gender, or body mass index.
Dr. Salama acknowledged limitations of this retrospective study, including a lack of data on other variables of interest, such as carcinoembryonic antigen, tumor size, imaging characteristics, molecular markers, and the time interval between chemoradiotherapy and surgery.
“We obviously need more data to evaluate other predictive factors in achieving a complete pathological response,” he said, adding that it’s also unclear whether the results of the present study could be generalized to institutions not participating in ACS NSQIP.
Dr. Salama presented the research on behalf of Nathalie Wong-Chong, MD, also of McGill University. He had no conflicts of interest to report for his presentation.
Orlando – Clinical an analysis of a large, multicenter database has suggested.
In multivariate regression, pretreatment N2 stage was the only variable significantly associated with failure of achieving pathologic complete response, according to Ebram Salama, MD, of Sir Mortimer B. Davis Jewish General Hospital at McGill University, Montreal.
“We should be reconsidering putting these patients in watch-and-wait protocols,” Dr. Salama said in an oral abstract presentation at the American College of Surgeons Quality and Safety Conference.
The analysis included 369 elective cases of cT2-4 N0-2 rectal cancer that were treated with neoadjuvant chemoradiotherapy during 2016 from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) proctectomy-specific database.
Of those cases, 53 (14.4%) achieved PCR, a proportion consistent with what has been reported previously in medical literature, Dr. Salama noted during his presentation.
The multivariate analysis revealed that pretreatment N2 stage was a negative predictor of PCR with an odds ratio of 0.18 (95% confidence interval, 0.04-0.82; P = .026), according to presented data.
By contrast, Dr. Salama said, there were no significant associations between response and other variables, including pretreatment N1 stage, pretreatment T stage, tumor location, gender, or body mass index.
Dr. Salama acknowledged limitations of this retrospective study, including a lack of data on other variables of interest, such as carcinoembryonic antigen, tumor size, imaging characteristics, molecular markers, and the time interval between chemoradiotherapy and surgery.
“We obviously need more data to evaluate other predictive factors in achieving a complete pathological response,” he said, adding that it’s also unclear whether the results of the present study could be generalized to institutions not participating in ACS NSQIP.
Dr. Salama presented the research on behalf of Nathalie Wong-Chong, MD, also of McGill University. He had no conflicts of interest to report for his presentation.
Orlando – Clinical an analysis of a large, multicenter database has suggested.
In multivariate regression, pretreatment N2 stage was the only variable significantly associated with failure of achieving pathologic complete response, according to Ebram Salama, MD, of Sir Mortimer B. Davis Jewish General Hospital at McGill University, Montreal.
“We should be reconsidering putting these patients in watch-and-wait protocols,” Dr. Salama said in an oral abstract presentation at the American College of Surgeons Quality and Safety Conference.
The analysis included 369 elective cases of cT2-4 N0-2 rectal cancer that were treated with neoadjuvant chemoradiotherapy during 2016 from the American College of Surgeons National Surgical Quality Improvement Program (NSQIP) proctectomy-specific database.
Of those cases, 53 (14.4%) achieved PCR, a proportion consistent with what has been reported previously in medical literature, Dr. Salama noted during his presentation.
The multivariate analysis revealed that pretreatment N2 stage was a negative predictor of PCR with an odds ratio of 0.18 (95% confidence interval, 0.04-0.82; P = .026), according to presented data.
By contrast, Dr. Salama said, there were no significant associations between response and other variables, including pretreatment N1 stage, pretreatment T stage, tumor location, gender, or body mass index.
Dr. Salama acknowledged limitations of this retrospective study, including a lack of data on other variables of interest, such as carcinoembryonic antigen, tumor size, imaging characteristics, molecular markers, and the time interval between chemoradiotherapy and surgery.
“We obviously need more data to evaluate other predictive factors in achieving a complete pathological response,” he said, adding that it’s also unclear whether the results of the present study could be generalized to institutions not participating in ACS NSQIP.
Dr. Salama presented the research on behalf of Nathalie Wong-Chong, MD, also of McGill University. He had no conflicts of interest to report for his presentation.
REPORTING FROM ACSQSC 2018
Key clinical point: N2 disease may be a negative predictor of pathological complete response after neoadjuvant chemoradiotherapy for rectal cancer.
Major finding: Pretreatment N2 stage was a negative predictor of complete pathological response, with an odds ratio of 0.18 (95% confidence interval, 0.04-0.82; P = .026).
Study details: A study of 369 elective cases of cT2-4 N0-2 rectal cancer treated with neoadjuvant chemoradiotherapy from 2016 in the ACS NSQIP proctectomy-specific database.
Disclosures: Dr. Salama had no conflicts of interest to report for his presentation.
Opioid use has not declined meaningfully
Opioid use has not significantly declined over the past 10 years despite efforts to educate prescribers about the risks of opioid abuse, with over half of disabled Medicare beneficiaries using opioids each year, according to a recent retrospective cohort study published in the BMJ.
“We found very high prevalence of opioid use and opioid doses in disabled Medicare beneficiaries, most likely reflecting the high burden of illness in this population,” Molly M. Jeffery, PhD, from the Mayo Clinic, Rochester, Minn., and her associates wrote in their study.
The investigators evaluated pharmaceutical and medical claims data from 48 million individuals who were commercially insured or were Medicare Advantage recipients (both those eligible because they were older than 65 years and those under 65 years old who still were eligible because of disability). The researchers found that 52% of disabled Medicare patients, 26% of aged Medicare patients, and 14% of commercially insured patients used opioids annually within the study period.
In the commercially insured group, there was little fluctuation in patient opioid prevalence by quarter, with an average daily dose of 17 mg morphine equivalents (MME) during 2011-2016; 6% of patients used opioids quarterly at the beginning and end of the study. There was an increase of quarterly opioid prevalence in the aged Medicare group from 11% to 14% at the beginning and end of the study period. Average daily dose also increased during this period for the aged Medicare group from 18 MME in 2011 to 20 MME in 2016.
Researchers said commercial beneficiaries between 45 years and 54 years old had the highest prevalence of opioid use. The disabled Medicare group saw the greatest increase among groups in opioid prevalence and average daily dose, with a 26% prevalence in 2007 and 53 MME average daily dose, which increased to a prevalence of 39% and an average daily dose of 56 MME in 2016.
“Doctors and patients should consider whether long-term opioid use is improving the patient’s ability to function and, if not, should consider other treatments either as an addition or replacement to opioid use,” Dr. Jeffery and her colleagues wrote. “Evidence-based approaches are needed to improve both the safety of opioid use and patient outcomes including pain management and ability to function.”
The researchers noted limitations in the study, such as not including people with Medicaid, fee-for-service Medicare, or the uninsured. In addition, the data reviewed did not indicate the prevalence of chronic pain or pain duration in the patient population studied, they said.
The authors reported no relevant financial disclosures.
SOURCE: Jeffery MM et al. BMJ. 2018 Aug 1. doi: 10.1136/bmj.k2833.
Opioid use has not significantly declined over the past 10 years despite efforts to educate prescribers about the risks of opioid abuse, with over half of disabled Medicare beneficiaries using opioids each year, according to a recent retrospective cohort study published in the BMJ.
“We found very high prevalence of opioid use and opioid doses in disabled Medicare beneficiaries, most likely reflecting the high burden of illness in this population,” Molly M. Jeffery, PhD, from the Mayo Clinic, Rochester, Minn., and her associates wrote in their study.
The investigators evaluated pharmaceutical and medical claims data from 48 million individuals who were commercially insured or were Medicare Advantage recipients (both those eligible because they were older than 65 years and those under 65 years old who still were eligible because of disability). The researchers found that 52% of disabled Medicare patients, 26% of aged Medicare patients, and 14% of commercially insured patients used opioids annually within the study period.
In the commercially insured group, there was little fluctuation in patient opioid prevalence by quarter, with an average daily dose of 17 mg morphine equivalents (MME) during 2011-2016; 6% of patients used opioids quarterly at the beginning and end of the study. There was an increase of quarterly opioid prevalence in the aged Medicare group from 11% to 14% at the beginning and end of the study period. Average daily dose also increased during this period for the aged Medicare group from 18 MME in 2011 to 20 MME in 2016.
Researchers said commercial beneficiaries between 45 years and 54 years old had the highest prevalence of opioid use. The disabled Medicare group saw the greatest increase among groups in opioid prevalence and average daily dose, with a 26% prevalence in 2007 and 53 MME average daily dose, which increased to a prevalence of 39% and an average daily dose of 56 MME in 2016.
“Doctors and patients should consider whether long-term opioid use is improving the patient’s ability to function and, if not, should consider other treatments either as an addition or replacement to opioid use,” Dr. Jeffery and her colleagues wrote. “Evidence-based approaches are needed to improve both the safety of opioid use and patient outcomes including pain management and ability to function.”
The researchers noted limitations in the study, such as not including people with Medicaid, fee-for-service Medicare, or the uninsured. In addition, the data reviewed did not indicate the prevalence of chronic pain or pain duration in the patient population studied, they said.
The authors reported no relevant financial disclosures.
SOURCE: Jeffery MM et al. BMJ. 2018 Aug 1. doi: 10.1136/bmj.k2833.
Opioid use has not significantly declined over the past 10 years despite efforts to educate prescribers about the risks of opioid abuse, with over half of disabled Medicare beneficiaries using opioids each year, according to a recent retrospective cohort study published in the BMJ.
“We found very high prevalence of opioid use and opioid doses in disabled Medicare beneficiaries, most likely reflecting the high burden of illness in this population,” Molly M. Jeffery, PhD, from the Mayo Clinic, Rochester, Minn., and her associates wrote in their study.
The investigators evaluated pharmaceutical and medical claims data from 48 million individuals who were commercially insured or were Medicare Advantage recipients (both those eligible because they were older than 65 years and those under 65 years old who still were eligible because of disability). The researchers found that 52% of disabled Medicare patients, 26% of aged Medicare patients, and 14% of commercially insured patients used opioids annually within the study period.
In the commercially insured group, there was little fluctuation in patient opioid prevalence by quarter, with an average daily dose of 17 mg morphine equivalents (MME) during 2011-2016; 6% of patients used opioids quarterly at the beginning and end of the study. There was an increase of quarterly opioid prevalence in the aged Medicare group from 11% to 14% at the beginning and end of the study period. Average daily dose also increased during this period for the aged Medicare group from 18 MME in 2011 to 20 MME in 2016.
Researchers said commercial beneficiaries between 45 years and 54 years old had the highest prevalence of opioid use. The disabled Medicare group saw the greatest increase among groups in opioid prevalence and average daily dose, with a 26% prevalence in 2007 and 53 MME average daily dose, which increased to a prevalence of 39% and an average daily dose of 56 MME in 2016.
“Doctors and patients should consider whether long-term opioid use is improving the patient’s ability to function and, if not, should consider other treatments either as an addition or replacement to opioid use,” Dr. Jeffery and her colleagues wrote. “Evidence-based approaches are needed to improve both the safety of opioid use and patient outcomes including pain management and ability to function.”
The researchers noted limitations in the study, such as not including people with Medicaid, fee-for-service Medicare, or the uninsured. In addition, the data reviewed did not indicate the prevalence of chronic pain or pain duration in the patient population studied, they said.
The authors reported no relevant financial disclosures.
SOURCE: Jeffery MM et al. BMJ. 2018 Aug 1. doi: 10.1136/bmj.k2833.
FROM THE BMJ
Key clinical point: The highest prevalence was seen among disabled patients with Medicare Advantage.
Major finding: Of those studied, annual prevalence of opioid use was 14% for commercial beneficiaries, 26% for aged Medicare beneficiaries, and 52% for disabled Medicare beneficiaries.
Study details: An observational cohort study of claims data from 48 million people who had commercial insurance or Medicare Advantage between January 2007 and December 2016.
Disclosures: The authors reported no relevant financial disclosures.
Source: Jeffery MM et al. BMJ. 2018 Aug 1. doi: 10.1136/bmj.k2833.
To tame prescription prices, HHS dips a toe into drug importation stream
It came as something of a surprise when Health & Human Services Secretary Alex Azar announced that the administration was exploring the importation of prescription drugs to fight high domestic prices. Sec. Azar and Scott Gottlieb, MD, commissioner of the Food and Drug Administration, who also endorsed the new proposal, had previously opposed the idea.
But drug prices in the United States have continued to rise and more than 80% of Americans say the government should take action. President Donald Trump has said drugmakers are “getting away with murder” and has angrily tweeted at companies about individual price hikes.
Although candidate Trump supported the idea of allowing patients to import medicines, since he was elected, he has not mentioned that option – which is strongly opposed by drug companies.
Now, determined to explore more avenues to curb price hikes, the administration is signaling that it is willing to consider what the industry regards as something of a nuclear option to address a recalcitrant problem. Carefully tailored to focus solely on specific situations in which a high-priced drug is made by only one company, it is finding support where broader proposals have failed.
“They’re approaching it incrementally and wisely, they’re focusing on prices where there’s a need,” said Dan Mendelson, the founder of health care consultant company Avalere and an official in the Clinton White House. “It is certainly more narrow than the way others have conceptualized it.”
Far from a blanket legalization of imported medicines, the working group Sec. Azar convened will study importation to combat sudden price increases in specific drugs. The focus is on temporarily bringing in cheaper similar or identical drugs to introduce competition into the U.S. market. The medicines must be off patent and have only one manufacturer here.
The secretary’s memo said the effort is designed to avoid the kind of overnight increases seen with Daraprim in 2015. That price hike was engineered by “pharma bro” Martin Shkreli, then CEO of Turing Pharmaceuticals. He purchased the rights to the single-sourced medication that treats parasitic infections and began charging $750 for a pill that formerly cost $13.50 and costs a little more than a dollar in much of the world. Turing was the only U.S. producer.
“This is a workable solution to a discrete problem,” said Ameet Sarpatwari, PhD, JD, an instructor in medicine at Harvard Medical School in Boston.
But those who support more sweeping importation policies decried the plan’s limited scope and suspected the announcement was part theatrics and part a threatening signal to drugmakers.
“This could just be a dog-and-pony show, where they’re calling in an expert group to explore avenues of importation – but when all is said and done, they find that they don’t want to do this,” said Gabriel Levitt, the cofounder of PharmacyChecker.com, a private company that verifies international online pharmacies and compares prescription drug prices for consumers.
“At that point, we’ll learn that the exercise was lip service,” he added. “Frankly, there’s a good chance that that is the case.”
This isn’t the first time officials have suggested importing drugs from other countries to find better prices. Bills have been offered in Congress to allow it, and George W. Bush administration officials investigated the issue and produced two reports questioning the safety of such efforts in 2004.
Overall, the measure is by no means a silver bullet to the larger problem of rising drug prices, said Rachel Sachs, JD, an associate professor of law at Washington University School of Law in St. Louis.
“It’s a really smart move to solve one of the many drug pricing problems we observe, but, of course, it won’t address every problem,” Sachs said.
Mr. Mendelson suggested this working group might be an effort to placate patients who have seen little movement to bring down drug costs, despite the president’s repeated promises to provide help.
“If the goal is to make policy changes that are visible and help with the 2018 and 2020 election, I think it’s right up there with a lot of the things they’re doing,” Mendelson said. “If the goal is truly to help consumers with drug prices, not so much.”
In addition, since the group’s work applies primarily to the generic drug market, a new policy would stop short of taming the price spirals and high launch prices of blockbuster brand-name drugs, which Harvard’s Sarpatwari said were the “elephants in the room” of the drug pricing debate.
Mr. Levitt pointed out that, while a big overnight increase on a drug might trigger action to allow importation, the move would do nothing to stop the yearly increases that drug companies tack on to medicines. Depending on how possible regulations are written, such increases might even be encouraged. The administration has been pressuring pharmaceutical makers to hold down those rising prices but finding tepid support among the companies.
Even if the policy targets just a slice of the overall problem, it could still make a difference for Americans struggling to pay for off-patent drugs and provide more competition.
“If Azar is serious about this proposal, even though it’s very limited in scope, it could help deter the most egregious forms of drug price gouging where there are single-source meds,” Mr. Levitt said.
KHN’s coverage of prescription drug development, costs and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
It came as something of a surprise when Health & Human Services Secretary Alex Azar announced that the administration was exploring the importation of prescription drugs to fight high domestic prices. Sec. Azar and Scott Gottlieb, MD, commissioner of the Food and Drug Administration, who also endorsed the new proposal, had previously opposed the idea.
But drug prices in the United States have continued to rise and more than 80% of Americans say the government should take action. President Donald Trump has said drugmakers are “getting away with murder” and has angrily tweeted at companies about individual price hikes.
Although candidate Trump supported the idea of allowing patients to import medicines, since he was elected, he has not mentioned that option – which is strongly opposed by drug companies.
Now, determined to explore more avenues to curb price hikes, the administration is signaling that it is willing to consider what the industry regards as something of a nuclear option to address a recalcitrant problem. Carefully tailored to focus solely on specific situations in which a high-priced drug is made by only one company, it is finding support where broader proposals have failed.
“They’re approaching it incrementally and wisely, they’re focusing on prices where there’s a need,” said Dan Mendelson, the founder of health care consultant company Avalere and an official in the Clinton White House. “It is certainly more narrow than the way others have conceptualized it.”
Far from a blanket legalization of imported medicines, the working group Sec. Azar convened will study importation to combat sudden price increases in specific drugs. The focus is on temporarily bringing in cheaper similar or identical drugs to introduce competition into the U.S. market. The medicines must be off patent and have only one manufacturer here.
The secretary’s memo said the effort is designed to avoid the kind of overnight increases seen with Daraprim in 2015. That price hike was engineered by “pharma bro” Martin Shkreli, then CEO of Turing Pharmaceuticals. He purchased the rights to the single-sourced medication that treats parasitic infections and began charging $750 for a pill that formerly cost $13.50 and costs a little more than a dollar in much of the world. Turing was the only U.S. producer.
“This is a workable solution to a discrete problem,” said Ameet Sarpatwari, PhD, JD, an instructor in medicine at Harvard Medical School in Boston.
But those who support more sweeping importation policies decried the plan’s limited scope and suspected the announcement was part theatrics and part a threatening signal to drugmakers.
“This could just be a dog-and-pony show, where they’re calling in an expert group to explore avenues of importation – but when all is said and done, they find that they don’t want to do this,” said Gabriel Levitt, the cofounder of PharmacyChecker.com, a private company that verifies international online pharmacies and compares prescription drug prices for consumers.
“At that point, we’ll learn that the exercise was lip service,” he added. “Frankly, there’s a good chance that that is the case.”
This isn’t the first time officials have suggested importing drugs from other countries to find better prices. Bills have been offered in Congress to allow it, and George W. Bush administration officials investigated the issue and produced two reports questioning the safety of such efforts in 2004.
Overall, the measure is by no means a silver bullet to the larger problem of rising drug prices, said Rachel Sachs, JD, an associate professor of law at Washington University School of Law in St. Louis.
“It’s a really smart move to solve one of the many drug pricing problems we observe, but, of course, it won’t address every problem,” Sachs said.
Mr. Mendelson suggested this working group might be an effort to placate patients who have seen little movement to bring down drug costs, despite the president’s repeated promises to provide help.
“If the goal is to make policy changes that are visible and help with the 2018 and 2020 election, I think it’s right up there with a lot of the things they’re doing,” Mendelson said. “If the goal is truly to help consumers with drug prices, not so much.”
In addition, since the group’s work applies primarily to the generic drug market, a new policy would stop short of taming the price spirals and high launch prices of blockbuster brand-name drugs, which Harvard’s Sarpatwari said were the “elephants in the room” of the drug pricing debate.
Mr. Levitt pointed out that, while a big overnight increase on a drug might trigger action to allow importation, the move would do nothing to stop the yearly increases that drug companies tack on to medicines. Depending on how possible regulations are written, such increases might even be encouraged. The administration has been pressuring pharmaceutical makers to hold down those rising prices but finding tepid support among the companies.
Even if the policy targets just a slice of the overall problem, it could still make a difference for Americans struggling to pay for off-patent drugs and provide more competition.
“If Azar is serious about this proposal, even though it’s very limited in scope, it could help deter the most egregious forms of drug price gouging where there are single-source meds,” Mr. Levitt said.
KHN’s coverage of prescription drug development, costs and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
It came as something of a surprise when Health & Human Services Secretary Alex Azar announced that the administration was exploring the importation of prescription drugs to fight high domestic prices. Sec. Azar and Scott Gottlieb, MD, commissioner of the Food and Drug Administration, who also endorsed the new proposal, had previously opposed the idea.
But drug prices in the United States have continued to rise and more than 80% of Americans say the government should take action. President Donald Trump has said drugmakers are “getting away with murder” and has angrily tweeted at companies about individual price hikes.
Although candidate Trump supported the idea of allowing patients to import medicines, since he was elected, he has not mentioned that option – which is strongly opposed by drug companies.
Now, determined to explore more avenues to curb price hikes, the administration is signaling that it is willing to consider what the industry regards as something of a nuclear option to address a recalcitrant problem. Carefully tailored to focus solely on specific situations in which a high-priced drug is made by only one company, it is finding support where broader proposals have failed.
“They’re approaching it incrementally and wisely, they’re focusing on prices where there’s a need,” said Dan Mendelson, the founder of health care consultant company Avalere and an official in the Clinton White House. “It is certainly more narrow than the way others have conceptualized it.”
Far from a blanket legalization of imported medicines, the working group Sec. Azar convened will study importation to combat sudden price increases in specific drugs. The focus is on temporarily bringing in cheaper similar or identical drugs to introduce competition into the U.S. market. The medicines must be off patent and have only one manufacturer here.
The secretary’s memo said the effort is designed to avoid the kind of overnight increases seen with Daraprim in 2015. That price hike was engineered by “pharma bro” Martin Shkreli, then CEO of Turing Pharmaceuticals. He purchased the rights to the single-sourced medication that treats parasitic infections and began charging $750 for a pill that formerly cost $13.50 and costs a little more than a dollar in much of the world. Turing was the only U.S. producer.
“This is a workable solution to a discrete problem,” said Ameet Sarpatwari, PhD, JD, an instructor in medicine at Harvard Medical School in Boston.
But those who support more sweeping importation policies decried the plan’s limited scope and suspected the announcement was part theatrics and part a threatening signal to drugmakers.
“This could just be a dog-and-pony show, where they’re calling in an expert group to explore avenues of importation – but when all is said and done, they find that they don’t want to do this,” said Gabriel Levitt, the cofounder of PharmacyChecker.com, a private company that verifies international online pharmacies and compares prescription drug prices for consumers.
“At that point, we’ll learn that the exercise was lip service,” he added. “Frankly, there’s a good chance that that is the case.”
This isn’t the first time officials have suggested importing drugs from other countries to find better prices. Bills have been offered in Congress to allow it, and George W. Bush administration officials investigated the issue and produced two reports questioning the safety of such efforts in 2004.
Overall, the measure is by no means a silver bullet to the larger problem of rising drug prices, said Rachel Sachs, JD, an associate professor of law at Washington University School of Law in St. Louis.
“It’s a really smart move to solve one of the many drug pricing problems we observe, but, of course, it won’t address every problem,” Sachs said.
Mr. Mendelson suggested this working group might be an effort to placate patients who have seen little movement to bring down drug costs, despite the president’s repeated promises to provide help.
“If the goal is to make policy changes that are visible and help with the 2018 and 2020 election, I think it’s right up there with a lot of the things they’re doing,” Mendelson said. “If the goal is truly to help consumers with drug prices, not so much.”
In addition, since the group’s work applies primarily to the generic drug market, a new policy would stop short of taming the price spirals and high launch prices of blockbuster brand-name drugs, which Harvard’s Sarpatwari said were the “elephants in the room” of the drug pricing debate.
Mr. Levitt pointed out that, while a big overnight increase on a drug might trigger action to allow importation, the move would do nothing to stop the yearly increases that drug companies tack on to medicines. Depending on how possible regulations are written, such increases might even be encouraged. The administration has been pressuring pharmaceutical makers to hold down those rising prices but finding tepid support among the companies.
Even if the policy targets just a slice of the overall problem, it could still make a difference for Americans struggling to pay for off-patent drugs and provide more competition.
“If Azar is serious about this proposal, even though it’s very limited in scope, it could help deter the most egregious forms of drug price gouging where there are single-source meds,” Mr. Levitt said.
KHN’s coverage of prescription drug development, costs and pricing is supported in part by the Laura and John Arnold Foundation. Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Study models surveillance interval after ablation of Barrett’s esophagus
Surveillance endoscopy should be spaced at 1 and 3 years after complete eradication of low-grade intestinal metaplasia and at 3 months, 6 months, 1 year, and then annually in cases of high-grade dysplasia, researchers wrote in Gastroenterology.
This “much-attenuated schedule of surveillance endoscopy would provide protection from invasive adenocarcinoma,” wrote Cary C. Cotton, MD, of the department of medicine at the University of North Carolina at Chapel Hill, with his associates. Following this schedule could prevent unnecessary endoscopies while still detecting unresectable cancers at rates under 1 in 1,000 endoscopies, they added.
Barrett’s esophagus recurs in at least one in four cases after successful radiofrequency ablation, the researchers noted. Therefore, surveillance endoscopy is recommended after complete eradication of intestinal metaplasia, but only expert opinion informs the frequency of surveillance. This study modeled and validated rates of neoplastic recurrence by using data from the United States Radiofrequency Ablation Registry during 2004-2013 and from the United Kingdom National Halo Registry during 2007-2015.
In line with prior research, predictors of neoplastic recurrence included baseline histologic grade, age, sex, endoscopic mucosal resection, and baseline length of Barrett’s esophagus, the researchers said. The strongest predictor of recurrence was the most severe histologic grade identified before complete eradication of intestinal metaplasia. After controlling for covariates, a model based only on most-severe baseline histology predicted neoplastic recurrence with a C statistic of 0.892 (95% confidence interval, 0.86-0.92) in the United States Radiofrequency Ablation Registry.
Dysplasia recurred at similar rates regardless of whether patients had nondysplastic Barrett’s esophagus or indeterminate dysplasia. Recurrence rates also were similar between patients with high-grade dysplasia and patients with intramucosal carcinoma. Thus, the researchers identified three risk groups based on most-severe baseline histology: dysplastic Barrett’s esophagus or indefinite for dysplasia, low-grade dysplasia, and high-grade lesions or intramucosal adenocarcinoma.
The annual rate of any-grade neoplastic recurrence was 0.19% in the lowest-risk group, 1.98% in the intermediate-risk group, and 5.93% in the highest-risk group. “In the higher-risk groups, neoplastic recurrence occurred at a higher rate in the first year, but at a constant estimated rate thereafter,” the investigators wrote. Among 114 initial cases of neoplastic recurrence, 1.8% were esophageal adenocarcinoma and another 1.8% of patients developed esophageal adenocarcinoma within 6 months.
The researchers then modeled surveillance intervals by assuming a 2.9% rate of neoplastic recurrence per visit, which yielded a 0.1% rate of invasive adenocarcinoma. “This level of risk tolerance was chosen so that the risk of complications from surveillance endoscopy – approximately one in 1,000 in this patient population – would roughly approximate the risk of invasive carcinoma discovered at the exam,” they wrote. For patients at higher risk of endoscopic complications, they set the rate of neoplastic recurrence at 5.7%, which yielded a 0.2% rate of invasive cancer. “As would be expected, the higher the risk tolerance, the longer the period between endoscopic surveillance intervals.”
Based on the model, the researchers proposed surveillance intervals of 1 year, followed by 3 years, followed by more than 5 years for patients with completely eradicated low-grade dysplasia. For cases of high-grade dysplasia or carcinoma in situ, the proposed surveillance intervals were 3 months, 6 months, 1 year, and then annually. The model also performed well when applied to data from the United Kingdom National Halo Registry, the investigators said, noting that their approach was the first to directly establish an evidence base for surveillance practices in Barrett’s esophagus.
The National Institutes of Health and Barrx/Covidien/Medtronic provided funding. Dr. Cotton reported having no relevant disclosures. Three coinvestigators disclosed ties to Pentax Europe, Medtronic, Beamline Diagnostics, C2 Therapeutics, Boston Scientific, and CDx Medical.
SOURCE: Cotton CC et al. Gastroenterology. 2018 Apr 12. doi: 10.1053/j.gastro.2018.04.011.
Surveillance endoscopy should be spaced at 1 and 3 years after complete eradication of low-grade intestinal metaplasia and at 3 months, 6 months, 1 year, and then annually in cases of high-grade dysplasia, researchers wrote in Gastroenterology.
This “much-attenuated schedule of surveillance endoscopy would provide protection from invasive adenocarcinoma,” wrote Cary C. Cotton, MD, of the department of medicine at the University of North Carolina at Chapel Hill, with his associates. Following this schedule could prevent unnecessary endoscopies while still detecting unresectable cancers at rates under 1 in 1,000 endoscopies, they added.
Barrett’s esophagus recurs in at least one in four cases after successful radiofrequency ablation, the researchers noted. Therefore, surveillance endoscopy is recommended after complete eradication of intestinal metaplasia, but only expert opinion informs the frequency of surveillance. This study modeled and validated rates of neoplastic recurrence by using data from the United States Radiofrequency Ablation Registry during 2004-2013 and from the United Kingdom National Halo Registry during 2007-2015.
In line with prior research, predictors of neoplastic recurrence included baseline histologic grade, age, sex, endoscopic mucosal resection, and baseline length of Barrett’s esophagus, the researchers said. The strongest predictor of recurrence was the most severe histologic grade identified before complete eradication of intestinal metaplasia. After controlling for covariates, a model based only on most-severe baseline histology predicted neoplastic recurrence with a C statistic of 0.892 (95% confidence interval, 0.86-0.92) in the United States Radiofrequency Ablation Registry.
Dysplasia recurred at similar rates regardless of whether patients had nondysplastic Barrett’s esophagus or indeterminate dysplasia. Recurrence rates also were similar between patients with high-grade dysplasia and patients with intramucosal carcinoma. Thus, the researchers identified three risk groups based on most-severe baseline histology: dysplastic Barrett’s esophagus or indefinite for dysplasia, low-grade dysplasia, and high-grade lesions or intramucosal adenocarcinoma.
The annual rate of any-grade neoplastic recurrence was 0.19% in the lowest-risk group, 1.98% in the intermediate-risk group, and 5.93% in the highest-risk group. “In the higher-risk groups, neoplastic recurrence occurred at a higher rate in the first year, but at a constant estimated rate thereafter,” the investigators wrote. Among 114 initial cases of neoplastic recurrence, 1.8% were esophageal adenocarcinoma and another 1.8% of patients developed esophageal adenocarcinoma within 6 months.
The researchers then modeled surveillance intervals by assuming a 2.9% rate of neoplastic recurrence per visit, which yielded a 0.1% rate of invasive adenocarcinoma. “This level of risk tolerance was chosen so that the risk of complications from surveillance endoscopy – approximately one in 1,000 in this patient population – would roughly approximate the risk of invasive carcinoma discovered at the exam,” they wrote. For patients at higher risk of endoscopic complications, they set the rate of neoplastic recurrence at 5.7%, which yielded a 0.2% rate of invasive cancer. “As would be expected, the higher the risk tolerance, the longer the period between endoscopic surveillance intervals.”
Based on the model, the researchers proposed surveillance intervals of 1 year, followed by 3 years, followed by more than 5 years for patients with completely eradicated low-grade dysplasia. For cases of high-grade dysplasia or carcinoma in situ, the proposed surveillance intervals were 3 months, 6 months, 1 year, and then annually. The model also performed well when applied to data from the United Kingdom National Halo Registry, the investigators said, noting that their approach was the first to directly establish an evidence base for surveillance practices in Barrett’s esophagus.
The National Institutes of Health and Barrx/Covidien/Medtronic provided funding. Dr. Cotton reported having no relevant disclosures. Three coinvestigators disclosed ties to Pentax Europe, Medtronic, Beamline Diagnostics, C2 Therapeutics, Boston Scientific, and CDx Medical.
SOURCE: Cotton CC et al. Gastroenterology. 2018 Apr 12. doi: 10.1053/j.gastro.2018.04.011.
Surveillance endoscopy should be spaced at 1 and 3 years after complete eradication of low-grade intestinal metaplasia and at 3 months, 6 months, 1 year, and then annually in cases of high-grade dysplasia, researchers wrote in Gastroenterology.
This “much-attenuated schedule of surveillance endoscopy would provide protection from invasive adenocarcinoma,” wrote Cary C. Cotton, MD, of the department of medicine at the University of North Carolina at Chapel Hill, with his associates. Following this schedule could prevent unnecessary endoscopies while still detecting unresectable cancers at rates under 1 in 1,000 endoscopies, they added.
Barrett’s esophagus recurs in at least one in four cases after successful radiofrequency ablation, the researchers noted. Therefore, surveillance endoscopy is recommended after complete eradication of intestinal metaplasia, but only expert opinion informs the frequency of surveillance. This study modeled and validated rates of neoplastic recurrence by using data from the United States Radiofrequency Ablation Registry during 2004-2013 and from the United Kingdom National Halo Registry during 2007-2015.
In line with prior research, predictors of neoplastic recurrence included baseline histologic grade, age, sex, endoscopic mucosal resection, and baseline length of Barrett’s esophagus, the researchers said. The strongest predictor of recurrence was the most severe histologic grade identified before complete eradication of intestinal metaplasia. After controlling for covariates, a model based only on most-severe baseline histology predicted neoplastic recurrence with a C statistic of 0.892 (95% confidence interval, 0.86-0.92) in the United States Radiofrequency Ablation Registry.
Dysplasia recurred at similar rates regardless of whether patients had nondysplastic Barrett’s esophagus or indeterminate dysplasia. Recurrence rates also were similar between patients with high-grade dysplasia and patients with intramucosal carcinoma. Thus, the researchers identified three risk groups based on most-severe baseline histology: dysplastic Barrett’s esophagus or indefinite for dysplasia, low-grade dysplasia, and high-grade lesions or intramucosal adenocarcinoma.
The annual rate of any-grade neoplastic recurrence was 0.19% in the lowest-risk group, 1.98% in the intermediate-risk group, and 5.93% in the highest-risk group. “In the higher-risk groups, neoplastic recurrence occurred at a higher rate in the first year, but at a constant estimated rate thereafter,” the investigators wrote. Among 114 initial cases of neoplastic recurrence, 1.8% were esophageal adenocarcinoma and another 1.8% of patients developed esophageal adenocarcinoma within 6 months.
The researchers then modeled surveillance intervals by assuming a 2.9% rate of neoplastic recurrence per visit, which yielded a 0.1% rate of invasive adenocarcinoma. “This level of risk tolerance was chosen so that the risk of complications from surveillance endoscopy – approximately one in 1,000 in this patient population – would roughly approximate the risk of invasive carcinoma discovered at the exam,” they wrote. For patients at higher risk of endoscopic complications, they set the rate of neoplastic recurrence at 5.7%, which yielded a 0.2% rate of invasive cancer. “As would be expected, the higher the risk tolerance, the longer the period between endoscopic surveillance intervals.”
Based on the model, the researchers proposed surveillance intervals of 1 year, followed by 3 years, followed by more than 5 years for patients with completely eradicated low-grade dysplasia. For cases of high-grade dysplasia or carcinoma in situ, the proposed surveillance intervals were 3 months, 6 months, 1 year, and then annually. The model also performed well when applied to data from the United Kingdom National Halo Registry, the investigators said, noting that their approach was the first to directly establish an evidence base for surveillance practices in Barrett’s esophagus.
The National Institutes of Health and Barrx/Covidien/Medtronic provided funding. Dr. Cotton reported having no relevant disclosures. Three coinvestigators disclosed ties to Pentax Europe, Medtronic, Beamline Diagnostics, C2 Therapeutics, Boston Scientific, and CDx Medical.
SOURCE: Cotton CC et al. Gastroenterology. 2018 Apr 12. doi: 10.1053/j.gastro.2018.04.011.
FROM GASTROENTEROLOGY
Key clinical point: Baseline histologic grade was the most important predictor of recurrence after radiofrequency ablation of Barrett’s esophagus.
Major finding: The proposed surveillance intervals were 1 year, followed by 3 years, followed by more than 5 years for patients with completely eradicated low-grade dysplasia. For cases of high-grade dysplasia or carcinoma in situ, the proposed surveillance intervals were 3 months, 6 months, 1 year, and then annually.
Study details: An analysis of data from the United States Radiofrequency Ablation Registry and the United Kingdom National Halo Registry.
Disclosures: The National Institutes of Health and Barrx/Covidien/Medtronic provided funding. Dr. Cotton reported having no relevant disclosures. Three coinvestigators disclosed ties to Pentax Europe, Medtronic, Beamline Diagnostics, C2 Therapeutics, Boston Scientific, and CDx Medical.
Source: Cotton CC et al. Gastroenterology. 2018 Apr 12. doi: 10.1053/j.gastro.2018.04.011.
CMS to resume risk adjustment payments for 2017
The Centers for Medicare & Medicaid Services has resumed the risk adjustment payment program from calendar year 2017 and is taking steps to ensure the program’s operation for 2018 and beyond.
CMS posted a final rule on July 24 that reissues, with additional explanation, the risk adjustment methodology that was used to calculate payments made to insurers for 2017.
The risk adjustment payment program uses statewide average premiums to draw money from health insurance plans within a state that have low levels of high-need patients and funnels that money to plans in that state with high amounts of high-need patients as a way to minimize adverse selection and to spread risk.
CMS halted the risk adjustment program earlier in the month following legal action. In January 2018, the U.S. District Court for the District of Massachusetts ruled that the CMS was acting within its authority in promulgating its methodology based on statewide average premiums. A month later, the U.S. District Court for the District of New Mexico ruled that the methodology was invalid and barred the CMS from collecting or making payments, which prompted the agency to temporarily halt the program.
“This final rule will restore operation of the risk adjustment program and mitigate some of the uncertainty caused by the New Mexico litigation,” CMS Administrator Seema Verma said in a statement. “Issuers that had expressed concern about having to withdraw from markets or becoming insolvent should be assured by our actions today.”
CMS said the final rule provides a fuller explanation to support the 2017 risk adjustment methodology. The agency dispensed with the normal notice and comment period before issuing the final rule to help ensure timely payments are made by the program while working to resolve the legal questions. A motion to reconsider was filed in New Mexico on June 21, but a ruling on that is not expected until early September, which prompted the unusual move to issue a final rule without any notice or comment period.
CMS added that there will be a notice of proposed rule making with the normal comment period related to the risk adjustment payments for the 2018 calendar year. The existing rule was also vacated by the New Mexico court. The risk adjustment methodology for 2019 and beyond was finalized in a rule issued on April 17.
The Centers for Medicare & Medicaid Services has resumed the risk adjustment payment program from calendar year 2017 and is taking steps to ensure the program’s operation for 2018 and beyond.
CMS posted a final rule on July 24 that reissues, with additional explanation, the risk adjustment methodology that was used to calculate payments made to insurers for 2017.
The risk adjustment payment program uses statewide average premiums to draw money from health insurance plans within a state that have low levels of high-need patients and funnels that money to plans in that state with high amounts of high-need patients as a way to minimize adverse selection and to spread risk.
CMS halted the risk adjustment program earlier in the month following legal action. In January 2018, the U.S. District Court for the District of Massachusetts ruled that the CMS was acting within its authority in promulgating its methodology based on statewide average premiums. A month later, the U.S. District Court for the District of New Mexico ruled that the methodology was invalid and barred the CMS from collecting or making payments, which prompted the agency to temporarily halt the program.
“This final rule will restore operation of the risk adjustment program and mitigate some of the uncertainty caused by the New Mexico litigation,” CMS Administrator Seema Verma said in a statement. “Issuers that had expressed concern about having to withdraw from markets or becoming insolvent should be assured by our actions today.”
CMS said the final rule provides a fuller explanation to support the 2017 risk adjustment methodology. The agency dispensed with the normal notice and comment period before issuing the final rule to help ensure timely payments are made by the program while working to resolve the legal questions. A motion to reconsider was filed in New Mexico on June 21, but a ruling on that is not expected until early September, which prompted the unusual move to issue a final rule without any notice or comment period.
CMS added that there will be a notice of proposed rule making with the normal comment period related to the risk adjustment payments for the 2018 calendar year. The existing rule was also vacated by the New Mexico court. The risk adjustment methodology for 2019 and beyond was finalized in a rule issued on April 17.
The Centers for Medicare & Medicaid Services has resumed the risk adjustment payment program from calendar year 2017 and is taking steps to ensure the program’s operation for 2018 and beyond.
CMS posted a final rule on July 24 that reissues, with additional explanation, the risk adjustment methodology that was used to calculate payments made to insurers for 2017.
The risk adjustment payment program uses statewide average premiums to draw money from health insurance plans within a state that have low levels of high-need patients and funnels that money to plans in that state with high amounts of high-need patients as a way to minimize adverse selection and to spread risk.
CMS halted the risk adjustment program earlier in the month following legal action. In January 2018, the U.S. District Court for the District of Massachusetts ruled that the CMS was acting within its authority in promulgating its methodology based on statewide average premiums. A month later, the U.S. District Court for the District of New Mexico ruled that the methodology was invalid and barred the CMS from collecting or making payments, which prompted the agency to temporarily halt the program.
“This final rule will restore operation of the risk adjustment program and mitigate some of the uncertainty caused by the New Mexico litigation,” CMS Administrator Seema Verma said in a statement. “Issuers that had expressed concern about having to withdraw from markets or becoming insolvent should be assured by our actions today.”
CMS said the final rule provides a fuller explanation to support the 2017 risk adjustment methodology. The agency dispensed with the normal notice and comment period before issuing the final rule to help ensure timely payments are made by the program while working to resolve the legal questions. A motion to reconsider was filed in New Mexico on June 21, but a ruling on that is not expected until early September, which prompted the unusual move to issue a final rule without any notice or comment period.
CMS added that there will be a notice of proposed rule making with the normal comment period related to the risk adjustment payments for the 2018 calendar year. The existing rule was also vacated by the New Mexico court. The risk adjustment methodology for 2019 and beyond was finalized in a rule issued on April 17.
ERAS adoption for colectomy yielded state-wide outcome improvements
ORLANDO – Widespread implementation of an analysis shows.
The benefits were particularly pronounced in the subset of patients undergoing laparoscopic colectomy in the retrospective analysis, which included data for treated at four institutions in the Virginia Surgical Quality Collaborative Workgroup.
While the benefits of enhanced recovery after surgery (ERAS) are well known, most of the published data has come from single-institution experiences, according to investigator Traci L. Hedrick, MD, FACS, of the University of Virginia, Charlottesville.
At the American College of Surgeons Quality and Safety Conference, Dr. Hedrick presented risk-adjusted National Surgical Quality Improvement Program (NSQIP) data for 2,971 consecutive procedures during 2012-2016 at the University of Virginia, Winchester Medical Center, Carilion Clinic, and Inova Fairfax.
“Institutions came and went from the collaborative during this time period, so we focused on those institutions that maintained in the collaborative throughout the entire study protocol,” Dr. Hedrick said in her presentation.
Of the 2,971 procedures, about half (1,460) were performed after implementation of enhanced recovery protocols. Laparoscopic and open procedures were analyzed separately due to a substantial shift toward laparoscopic procedures, mainly during the 2012-2014 period, Dr. Hedrick said.
Among laparoscopic cases, there was a significant 1-day reduction in median length of stay, dropping from 4 days for pre–enhanced recovery protocol cases to 3 days for post–enhanced recovery protocol cases, Dr. Hedrick reported.
Observed morbidity also dropped significantly from 14.8% to 8.9% for the pre– and post–enhanced recovery cases, and the readmission rate fell significantly from 13% to 8.8%.
For open cases, there was a significant 1-day drop in median length of stay, from 4 to 3 days, but no significant differences in observed morbidity or readmission rates, according to Dr. Hedrick.
“As more of the patients were done laparoscopically, that really selected out the more complicated patients that were undergoing open procedures,” she said.
The protocols implemented by institutions in the Virginia collaborative group were generally uniform in important tenants of enhanced recovery, such as opioid minimization and avoidance of fasting, but specific elements were left up to each institution to improve buy-in, according to Dr. Hedrick.
“A lot of our protocols are very similar, particularly with regards to the order set,” Dr. Hedrick explained, “[but] I really am a firm believer in not being very strict about exactly what to use, because it’s so dependent on preference at the local level.”
The Virginia Surgical Quality Collaborative Workgroup is one of 20 regional ACS NSQIP collaboratives with the objective of improving surgical outcomes through multi-institutional collaboration, Dr. Hedrick said.
Dr. Hedrick and her coinvestigators had no relevant disclosures to report.
ORLANDO – Widespread implementation of an analysis shows.
The benefits were particularly pronounced in the subset of patients undergoing laparoscopic colectomy in the retrospective analysis, which included data for treated at four institutions in the Virginia Surgical Quality Collaborative Workgroup.
While the benefits of enhanced recovery after surgery (ERAS) are well known, most of the published data has come from single-institution experiences, according to investigator Traci L. Hedrick, MD, FACS, of the University of Virginia, Charlottesville.
At the American College of Surgeons Quality and Safety Conference, Dr. Hedrick presented risk-adjusted National Surgical Quality Improvement Program (NSQIP) data for 2,971 consecutive procedures during 2012-2016 at the University of Virginia, Winchester Medical Center, Carilion Clinic, and Inova Fairfax.
“Institutions came and went from the collaborative during this time period, so we focused on those institutions that maintained in the collaborative throughout the entire study protocol,” Dr. Hedrick said in her presentation.
Of the 2,971 procedures, about half (1,460) were performed after implementation of enhanced recovery protocols. Laparoscopic and open procedures were analyzed separately due to a substantial shift toward laparoscopic procedures, mainly during the 2012-2014 period, Dr. Hedrick said.
Among laparoscopic cases, there was a significant 1-day reduction in median length of stay, dropping from 4 days for pre–enhanced recovery protocol cases to 3 days for post–enhanced recovery protocol cases, Dr. Hedrick reported.
Observed morbidity also dropped significantly from 14.8% to 8.9% for the pre– and post–enhanced recovery cases, and the readmission rate fell significantly from 13% to 8.8%.
For open cases, there was a significant 1-day drop in median length of stay, from 4 to 3 days, but no significant differences in observed morbidity or readmission rates, according to Dr. Hedrick.
“As more of the patients were done laparoscopically, that really selected out the more complicated patients that were undergoing open procedures,” she said.
The protocols implemented by institutions in the Virginia collaborative group were generally uniform in important tenants of enhanced recovery, such as opioid minimization and avoidance of fasting, but specific elements were left up to each institution to improve buy-in, according to Dr. Hedrick.
“A lot of our protocols are very similar, particularly with regards to the order set,” Dr. Hedrick explained, “[but] I really am a firm believer in not being very strict about exactly what to use, because it’s so dependent on preference at the local level.”
The Virginia Surgical Quality Collaborative Workgroup is one of 20 regional ACS NSQIP collaboratives with the objective of improving surgical outcomes through multi-institutional collaboration, Dr. Hedrick said.
Dr. Hedrick and her coinvestigators had no relevant disclosures to report.
ORLANDO – Widespread implementation of an analysis shows.
The benefits were particularly pronounced in the subset of patients undergoing laparoscopic colectomy in the retrospective analysis, which included data for treated at four institutions in the Virginia Surgical Quality Collaborative Workgroup.
While the benefits of enhanced recovery after surgery (ERAS) are well known, most of the published data has come from single-institution experiences, according to investigator Traci L. Hedrick, MD, FACS, of the University of Virginia, Charlottesville.
At the American College of Surgeons Quality and Safety Conference, Dr. Hedrick presented risk-adjusted National Surgical Quality Improvement Program (NSQIP) data for 2,971 consecutive procedures during 2012-2016 at the University of Virginia, Winchester Medical Center, Carilion Clinic, and Inova Fairfax.
“Institutions came and went from the collaborative during this time period, so we focused on those institutions that maintained in the collaborative throughout the entire study protocol,” Dr. Hedrick said in her presentation.
Of the 2,971 procedures, about half (1,460) were performed after implementation of enhanced recovery protocols. Laparoscopic and open procedures were analyzed separately due to a substantial shift toward laparoscopic procedures, mainly during the 2012-2014 period, Dr. Hedrick said.
Among laparoscopic cases, there was a significant 1-day reduction in median length of stay, dropping from 4 days for pre–enhanced recovery protocol cases to 3 days for post–enhanced recovery protocol cases, Dr. Hedrick reported.
Observed morbidity also dropped significantly from 14.8% to 8.9% for the pre– and post–enhanced recovery cases, and the readmission rate fell significantly from 13% to 8.8%.
For open cases, there was a significant 1-day drop in median length of stay, from 4 to 3 days, but no significant differences in observed morbidity or readmission rates, according to Dr. Hedrick.
“As more of the patients were done laparoscopically, that really selected out the more complicated patients that were undergoing open procedures,” she said.
The protocols implemented by institutions in the Virginia collaborative group were generally uniform in important tenants of enhanced recovery, such as opioid minimization and avoidance of fasting, but specific elements were left up to each institution to improve buy-in, according to Dr. Hedrick.
“A lot of our protocols are very similar, particularly with regards to the order set,” Dr. Hedrick explained, “[but] I really am a firm believer in not being very strict about exactly what to use, because it’s so dependent on preference at the local level.”
The Virginia Surgical Quality Collaborative Workgroup is one of 20 regional ACS NSQIP collaboratives with the objective of improving surgical outcomes through multi-institutional collaboration, Dr. Hedrick said.
Dr. Hedrick and her coinvestigators had no relevant disclosures to report.
REPORTING FROM ACSQSC 2018
Key clinical point: State-wide adoption of ERAS for colectomy yielded state-wide outcome improvements .
Major finding: Morbidity also dropped significantly from 14.8% to 8.9% and the readmission rate fell significantly from 13% to 8.8%.
Study details: Risk adjusted NSQIP data in 2,971 colectomy patients from four hospitals in the Virginia Surgical Quality Collaborative Workgroup.
Disclosures: Dr. Hedrick and coinvestigators had no relevant disclosures to report.
Anemia in hiatal hernia patients doubled postop complications risk
but it turns out to be associated with elevated risk of postoperative complications, according to findings published in Surgical Endoscopy.
In a retrospective study of 263 patients who underwent HHR, 27% were anemic. Anemia in these patients was associated with 2.6-fold greater odds of postoperative complications, reported Guillaume S. Chevrollier, MD, of the department of surgery at Jefferson Medical College, Philadelphia, and his coauthors.
Investigators identified 263 patients for study who underwent HHR between January 2011 and April 2017. Preoperative data included a full physical examination, chest x-ray, esophagogastroduodenoscopy, esophageal manometry, 24-hour pH study, and routine blood work.
Patient data were also assessed for identification of Cameron lesions, defined as either linear erosions or ulcers present at the diaphragmatic hiatus. Baseline data collected for analysis included age, sex, body mass index, Charlson Comorbidity Index, hernia type, hernia size, surgical approach, and urgency of repair.
Preoperative anemia was defined as serum hemoglobin levels less than 13 mg/dL in men and less than 12 mg/dL in women, in accordance with World Health Organization criteria. Outcomes of anemic and nonanemic patients were compared and included measures such as estimated blood loss, operative times, need for blood transfusion, intensive care unit admission, and postoperative complications. Postoperative complications were assessed for severity using the Clavien-Dindo Scale, Dr. Chevrollier and his colleagues wrote.
In total, 70 patients (27%) were anemic before their hernia repair surgery. A majority of patients (54%) were aged 65 years or older, of whom 29% were anemic. Large hernias were most common (60%), followed by moderate size (18%), giant (14%), and small (8%).
Sixty-four patients (24%) developed postoperative complications. Among anemic patients, 41% developed one or more complications, compared with just 18% of nonanemic patients (P less than .01). Anemia was associated with 2.6-fold greater odds of postoperative complications in adjusted multivariable analysis (odds ratio, 2.57; 95% confidence interval, 1.36-4.86; P less than .01), the authors reported.
“Heightened awareness for the presence and the implications of preoperative anemia in patients undergoing HHR is necessary,” the authors wrote. “Consideration for treatment of anemia prior to elective repair is likely warranted.”
No disclosures or conflicts of interest were reported.
SOURCE: Chevrollier G et al. Surg Endosc. 2018 Jul 11. doi: 10.1007/s00464-018-6328-4.
but it turns out to be associated with elevated risk of postoperative complications, according to findings published in Surgical Endoscopy.
In a retrospective study of 263 patients who underwent HHR, 27% were anemic. Anemia in these patients was associated with 2.6-fold greater odds of postoperative complications, reported Guillaume S. Chevrollier, MD, of the department of surgery at Jefferson Medical College, Philadelphia, and his coauthors.
Investigators identified 263 patients for study who underwent HHR between January 2011 and April 2017. Preoperative data included a full physical examination, chest x-ray, esophagogastroduodenoscopy, esophageal manometry, 24-hour pH study, and routine blood work.
Patient data were also assessed for identification of Cameron lesions, defined as either linear erosions or ulcers present at the diaphragmatic hiatus. Baseline data collected for analysis included age, sex, body mass index, Charlson Comorbidity Index, hernia type, hernia size, surgical approach, and urgency of repair.
Preoperative anemia was defined as serum hemoglobin levels less than 13 mg/dL in men and less than 12 mg/dL in women, in accordance with World Health Organization criteria. Outcomes of anemic and nonanemic patients were compared and included measures such as estimated blood loss, operative times, need for blood transfusion, intensive care unit admission, and postoperative complications. Postoperative complications were assessed for severity using the Clavien-Dindo Scale, Dr. Chevrollier and his colleagues wrote.
In total, 70 patients (27%) were anemic before their hernia repair surgery. A majority of patients (54%) were aged 65 years or older, of whom 29% were anemic. Large hernias were most common (60%), followed by moderate size (18%), giant (14%), and small (8%).
Sixty-four patients (24%) developed postoperative complications. Among anemic patients, 41% developed one or more complications, compared with just 18% of nonanemic patients (P less than .01). Anemia was associated with 2.6-fold greater odds of postoperative complications in adjusted multivariable analysis (odds ratio, 2.57; 95% confidence interval, 1.36-4.86; P less than .01), the authors reported.
“Heightened awareness for the presence and the implications of preoperative anemia in patients undergoing HHR is necessary,” the authors wrote. “Consideration for treatment of anemia prior to elective repair is likely warranted.”
No disclosures or conflicts of interest were reported.
SOURCE: Chevrollier G et al. Surg Endosc. 2018 Jul 11. doi: 10.1007/s00464-018-6328-4.
but it turns out to be associated with elevated risk of postoperative complications, according to findings published in Surgical Endoscopy.
In a retrospective study of 263 patients who underwent HHR, 27% were anemic. Anemia in these patients was associated with 2.6-fold greater odds of postoperative complications, reported Guillaume S. Chevrollier, MD, of the department of surgery at Jefferson Medical College, Philadelphia, and his coauthors.
Investigators identified 263 patients for study who underwent HHR between January 2011 and April 2017. Preoperative data included a full physical examination, chest x-ray, esophagogastroduodenoscopy, esophageal manometry, 24-hour pH study, and routine blood work.
Patient data were also assessed for identification of Cameron lesions, defined as either linear erosions or ulcers present at the diaphragmatic hiatus. Baseline data collected for analysis included age, sex, body mass index, Charlson Comorbidity Index, hernia type, hernia size, surgical approach, and urgency of repair.
Preoperative anemia was defined as serum hemoglobin levels less than 13 mg/dL in men and less than 12 mg/dL in women, in accordance with World Health Organization criteria. Outcomes of anemic and nonanemic patients were compared and included measures such as estimated blood loss, operative times, need for blood transfusion, intensive care unit admission, and postoperative complications. Postoperative complications were assessed for severity using the Clavien-Dindo Scale, Dr. Chevrollier and his colleagues wrote.
In total, 70 patients (27%) were anemic before their hernia repair surgery. A majority of patients (54%) were aged 65 years or older, of whom 29% were anemic. Large hernias were most common (60%), followed by moderate size (18%), giant (14%), and small (8%).
Sixty-four patients (24%) developed postoperative complications. Among anemic patients, 41% developed one or more complications, compared with just 18% of nonanemic patients (P less than .01). Anemia was associated with 2.6-fold greater odds of postoperative complications in adjusted multivariable analysis (odds ratio, 2.57; 95% confidence interval, 1.36-4.86; P less than .01), the authors reported.
“Heightened awareness for the presence and the implications of preoperative anemia in patients undergoing HHR is necessary,” the authors wrote. “Consideration for treatment of anemia prior to elective repair is likely warranted.”
No disclosures or conflicts of interest were reported.
SOURCE: Chevrollier G et al. Surg Endosc. 2018 Jul 11. doi: 10.1007/s00464-018-6328-4.
FROM SURGICAL ENDOSCOPY
Key clinical point: Anemia is common in patients undergoing hiatal hernia repair and is associated with greater risk of postoperative complications.
Major finding: Of patients in the study, 27% were anemic; anemia was associated with 2.6-fold greater odds of postoperative complications.
Study details: A retrospective analysis of 263 patients who underwent hiatal hernia repair between January 2011 and April 2017.
Disclosures: No disclosures or conflicts of interest were reported.
Source: Chevrollier G et al. Surg Endosc. 2018 Jul 11. doi: 10.1007/s00464-018-6328-4.