User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Outcomes and Aseptic Survivorship of Revision Total Knee Arthroplasty
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
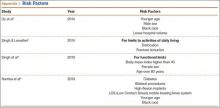
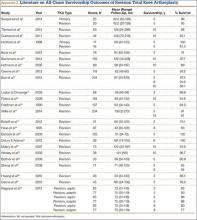
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
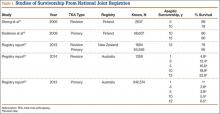
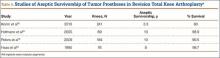
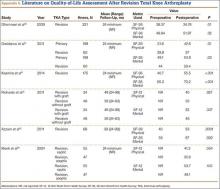
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
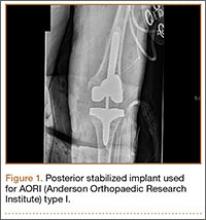
Posterior stabilized implants
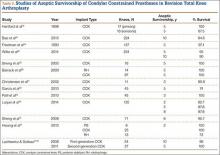
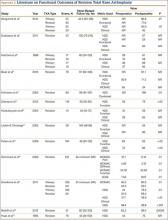
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
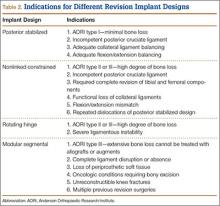
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
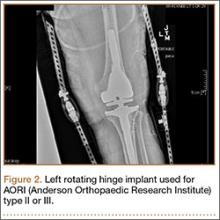
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
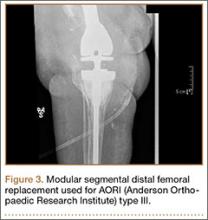
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
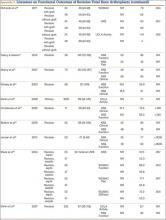
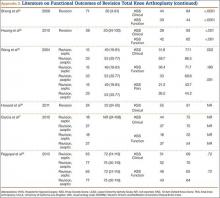
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
Posterior stabilized implants
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
Over the past 3 decades, total knee arthroplasty (TKA) has been considered a safe and effective treatment for end-stage knee arthritis.1 However, as the population, the incidence of obesity, and life expectancy continue to increase, the number of TKAs will rise as well.2,3 It is expected that over the next 16 years, the number of TKAs performed annually will exceed 3 million in the United States alone.4 This projection represents an over 600% increase from 2005 figures.5 Given the demographic shift expected over the next 2 decades, patients are anticipated to undergo these procedures at younger ages compared with previous generations, such that those age 65 years or younger will account for more than 55% of primary TKAs.6 More important, given this exponential growth in primary TKAs, there will be a concordant rise in revision procedures. It is expected that, the annual number has roughly doubled from that recorded for 2005.4
Compared with primary TKAs, however, revision TKAs have had less promising results, with survivorship as low as 60% over shorter periods.7,8 In addition, recent studies have found an even higher degree of dissatisfaction and functional limitations among revision TKA patients than among primary TKA patients, 15% to 30% of whom are unhappy with their procedures.9-11 These shortcomings of revision TKAs are thought to result from several factors, including poor bone quality, insufficient bone stock, ligamentous instability, soft-tissue incompetence, infection, malalignment, problems with extensor mechanisms, and substantial pain of uncertain etiology.
Despite there being several complex factors that can lead to worse outcomes with revision TKAs, surgeons are expected to produce results equivalent to those of primary TKAs. It is therefore imperative to delineate the objective and subjective outcomes of revision techniques to identify areas in need of improvement. In this article, we provide a concise overview of revision TKA outcomes in order to stimulate manufacturers, surgeons, and hospitals to improve on implant designs, surgical techniques, and care guidelines for revision TKA. We review the evidence on 5 points: aseptic survivorship, functional outcomes, patient satisfaction, quality of life (QOL), and economic impact. In addition, we compare available outcome data for revision and primary TKAs.
1. Aseptic survivorship
Fehring and colleagues12 in 2001 and Sharkey and colleagues13 in 2002 evaluated mechanisms of failure for revision TKA and reported many failures resulted from infection or were associated with the implant, and occurred within 2 years after the primary procedure. More recently, Dy and colleagues14 found the most common reason for revision was aseptic loosening, followed by infection. The present review focuses on aseptic femoral and tibial revision.
The failure rate for revision TKA is substantially higher than for primary TKA with the same type of prosthesis because of the complexity of the revision procedure, the increasing constraint of the implant design, and the higher degree of bone loss. (Appendix 1 lists risk factors for revision surgery. Appendix 2 is a complete list of survivorship outcomes of revision TKA.)
Sheng and colleagues15 in 2006 and Koskinen and colleagues16 in 2008 analyzed Finnish Arthroplasty Register data to determine failure rates for revision and primary TKA. Sheng and colleagues15 examined survivorship of 2637 revision TKAs (performed between 1990 and 2002) for all-cause endpoints after first revision procedure. Survivorship rates were 89% (5 years) and 79% (10 years), while Koskinen and colleagues16 noted all-cause survival rates of 80% at 15 years. More recently, in 2013, the New Zealand Orthopaedic Association17 analyzed New Zealand Joint Registry data for revision and re-revision rates (rates of revision per 100 component years) for 64,556 primary TKAs performed between 1999 and 2012. During the period studied, 1684 revisions were performed, reflecting a 2.6% revision rate, a 0.50% rate of revision per 100 component years, and a 13-year Kaplan-Meier survivorship of 94.5%. The most common reasons for revision were pain, deep infection, and tibial component loosening (Table 1).
Posterior stabilized implants
Laskin and Ohnsorge18 retrospectively reviewed the cases of 58 patients who underwent unilateral revision TKA (with a posterior stabilized implant), of which 42% were for coronal instability and 44% for a loose tibial component. At minimum 4-year follow-up, 52 of the 58 patients had anteroposterior instability of less than 5 mm. In addition, 5 years after surgery, aseptic survivorship was 96%. Meijer and colleagues19 conducted a retrospective comparative study of 69 revision TKAs (65 patients) in which 9 knees received a primary implant and 60 received a revision implant with stems and augmentation (60 = 37 posterior stabilized, 20 constrained, 3 rotating hinge). Survival rates for the primary implants were 100% (1 year), 73% (2 years), and 44% (5 years), and survival rates for the revision implants were significantly better: 95% (1 year), 92% (2 years), and 92% (5 years) (hazard ratio, 5.87; P = .008). The authors therefore indicated that it was unclear whether using a primary implant should still be an option in revision TKA and, if it is used, whether it should be limited to less complex situations in which bone loss and ligament damage are minimal (Table 2).
Constrained and semiconstrained implants
In a study of 234 knees (209 patients) with soft-tissue deficiency, Wilke and colleagues20 evaluated the long-term survivorship of revision TKA with use of a semiconstrained modular fixed-bearing implant system. Overall Kaplan-Meier survival rates were 91% (5 years) and 81% (10 years) at a mean follow-up of 9 years. When aseptic revision was evaluated, however, the survival rates increased to 95% (5 years) and 90% (10 years). The authors noted that male sex was the only variable that significantly increased the risk for re-revision (hazard ratio, 2.07; P = .02), which they attributed to potentially higher activity levels. In 2006 and 2011, Lachiewicz and Soileau21,22 evaluated the survival of first- and second-generation constrained condylar prostheses in primary TKA cases with severe valgus deformities, incompetent collateral ligaments, or severe flexion contractures. Of the 54 knees (44 patients) with first-generation prostheses, 42 (34 patients) had a mean follow-up of 9 years (range, 5-16 years). Ten-year survival with failure, defined as component revision for loosening, was 96%. The 27 TKAs using second-generation prostheses had a mean follow-up of about 5 years (range, 2-12 years). At final follow-up, there were no revisions for loosening or patellar problems, but 6 knees (22%) required lateral retinacular release of the patella (Table 3).
Rotating hinge implants
Neumann and colleagues23 evaluated the clinical and radiographic outcomes of 24 rotating hinge prostheses used for aseptic loosening with substantial bone loss and collateral ligament instability. At a mean follow-up of 56 months (range, 3-5 years), there was no evidence of loosening of any implants, and nonprogressive radiolucent lines were found in only 2 tibial components. Kowalczewski and colleagues24 evaluated the clinical and radiologic outcomes of 12 primary TKAs using a rotating hinge knee prosthesis at a minimum follow-up of 10 years. By most recent follow-up, no implants had been revised for loosening, and only 3 had nonprogressive radiolucent lines (Table 4).
Endoprostheses (modular segmental implants)
In a systematic review of 9 studies, Korim and colleagues25 evaluated 241 endoprostheses used for limb salvage under nononcologic conditions. Mean follow-up was about 3 years (range, 1-5 years). The devices were used to treat various conditions, including periprosthetic fracture, bone loss with aseptic loosening, and ligament insufficiency. The overall reoperation rate was 17% (41/241 cases). Mechanical failures were less frequent (6%-19%) (Table 5).
2. Functional outcomes
The goal in both primary and revision TKA is to restore the function and mobility of the knee and to alleviate pain. Whereas primary TKAs are realistically predictable and reproducible in their outcomes, revision TKAs are vastly more complicated, which can result in worse postoperative outcomes and function. In addition, revision TKAs may require extensive surgical exposure, which causes more tissue and muscle damage, prolonging rehabilitation. (Appendix 3 is a complete list of studies of functional outcomes of revision TKA.)
This discrepancy in functional outcomes between primary and revision TKA begins as early as the postoperative inpatient rehabilitation period. Using the functional independence measurement (FIM), which estimates performance of activities of daily living, mobility, and cognition, Vincent and colleagues26 evaluated the functional improvement produced by revision versus primary TKA during inpatient rehabilitation. They compared 424 consecutive primary TKAs with 138 revision TKAs. For both groups, FIM scores increased significantly (P = .015) between admission and discharge. On discharge, however, FIM scores were significantly (P = .01) higher for the primary group than the revision group (29 and 27 points, respectively). Furthermore, in the evaluation of mechanisms of failure, patients who had revision TKA for mechanical or pain-related problems did markedly better than those who had revision TKA for infection.
Compared with primary knee implants, revision implants require increasing constraint. We assume increasing constraint affects knee biomechanics, leading to worsening functional outcomes. In a study of 60 revision TKAs (57 patients) using posterior stabilized, condylar constrained, or rotating hinge prostheses, Vasso and colleagues27 examined functional outcomes at a median follow-up of 9 years (range, 4-12 years). At most recent follow-up, mean International Knee Society (IKS) Knee and Function scores were 81 (range, 48-97) and 79 (range, 56-92), mean Hospital for Special Surgery (HSS) score was 84 (range, 62-98), and mean range of motion (ROM) was 121° (range, 98°-132°) (P < .001). Although there were no significant differences in IKS and HSS scores between prosthesis types, ROM was significantly (P < .01) wider in the posterior stabilized group than in the condylar constrained and rotating hinge groups (127° vs 112° and 108°), suggesting increasing constraint resulted in decreased ROM. Several studies have found increasing constraint might lead to reduced function.28-30
However, Hwang and colleagues31 evaluated functional outcomes in 36 revision TKAs and noted that the cemented posterior stabilized (n = 8), condylar constrained (n = 25), and rotating hinge (n = 13) prostheses used did not differ in their mean Knee Society scores (78, 81, and 83, respectively).
There remains a marked disparity in patient limitations seen after revision versus primary TKA. Given the positive results being obtained with newer implants, studies might suggest recent generations of prostheses have allowed designs to be comparable. As design development continues, we may come closer to achieving outcomes comparable to those of primary TKA.
3. Patient satisfaction
Several recent reports have shown that 10% to 25% of patients who underwent primary TKA were dissatisfied with their surgery30,32; other studies have found patient satisfaction often correlating to function and pain.33-35 Given the worse outcomes for revision TKA (outlined in the preceding section), the substantial pain accompanying a second, more complex procedure, and the extensive rehabilitation expected, we suspect patients who undergo revision TKA are even less satisfied with their surgery than their primary counterparts are. (See Appendix 4 for a complete list of studies of patient satisfaction after revision TKA.)
Barrack and colleagues32 evaluated a consecutive series of 238 patients followed up for at least 1 year after revision TKA. Patients were asked to rate their degree of satisfaction with both their primary procedure and the revision and to indicate their expectations regarding their revision prosthesis. Mean satisfaction score was 7.4 (maximum = 10), with 13% of patients dissatisfied, 18% somewhat satisfied, and 69% satisfied. Seventy-four percent of patients expected their revision prosthesis to last longer than the primary prosthesis.
Greidanus and colleagues36 evaluated patient satisfaction in 60 revision TKA cases and 199 primary TKA cases at 2-year follow-up. The primary TKA group had significantly (P < .01) higher satisfaction scores in a comparison with the revision TKA group: Global (86 vs 73), Pain Relief (88 vs 70), Function (83 vs 67), and Recreation (77 vs 62). These findings support the satisfaction rates reported by Dahm and colleagues33,34: 91% for primary TKA patients and 77% for revision TKA patients.
4. Quality of life
Procedure complexity leads to reduced survivorship, function, and mobility, longer rehabilitation, and decreased QOL for revision TKA patients relative to primary TKA patients.37 (See Appendix 5 for a complete list of studies of QOL outcomes of revision TKA.)
Greidanus and colleagues36 evaluated joint-specific QOL (using the 12-item Oxford Knee Score; OKS) and generic QOL (using the 12-Item Short Form Health Survey; SF-12) in 60 revision TKA cases and 199 primary TKA cases at a mean follow-up of 2 years. (The OKS survey is used to evaluate patient perspectives on TKA outcomes,38 and the multipurpose SF-12 questionnaire is used to assess mental and physical function and general health-related QOL.39) Compared with the revision TKA group, the primary TKA group had significantly higher OKS after surgery (78 vs 68; P = .01) as well as significantly higher SF-12 scores: Global (84 vs 72; P = .01), Mental (54 vs 50; P = .03), and Physical (43 vs 37; P = .01). Similarly, Ghomrawi and colleagues40 evaluated patterns of improvement in 308 patients (318 knees) who had revision TKA. At 24-month follow-up, mean SF-36 Physical and Mental scores were 35 and 52, respectively.
Deehan and colleagues41 used the Nottingham Health Profile (NHP) to compare 94 patients’ health-related QOL scores before revision TKA with their scores 3 months, 1 year, and 5 years after revision. NHP Pain subscale scores were significantly lower 3 and 12 months after surgery than before surgery, but this difference was no longer seen at the 5-year follow-up. There was no significant improvement in scores on the other 5 NHP subscales (Sleep, Energy, Emotion, Mobility, Social Isolation) at any time points.
As shown in the literature, patients’ QOL outcomes improve after revision TKA, but these gains are not at the level of patients who undergo primary TKA.36,41 Given that revision surgery is more extensive, and that perhaps revision patients have poorer muscle function, they usually do not return to the level they attained after their index procedure.
5. Economic impact
Consistent with the outcomes already described, the economic impact of revision TKAs is excess expenditures and costs to patients and health care institutions.42 The sources of this impact are higher implant costs, extra operative trays and times, longer hospital stays, more rehabilitation, and increased medication use.43 Revision TKA costs range from $49,000 to more than $100,000—a tremendous increase over primary TKA costs ($25,000-$30,000).43-45 Furthermore, the annual economic burden associated with revision TKA, now $2.7 billion, is expected to exceed $13 billion by 2030.46 In the United States, about $23.2 billion will be spent on 926,527 primary TKAs in 2015; significantly, the costs associated with revising just 10% of these cases account for almost 50% of the total cost of the primary procedures.46
In a retrospective cost-identification multicenter cohort study, Bozic and colleagues47 found that both-component and single-component revisions, compared with primary procedures, were associated with significantly increased operative time (~265 and 221 minutes vs 200 minutes), use of allograft bone (23% and 14% vs 1%), length of stay (5.4 and 5.7 days vs 5.0 days), and percentage of patients discharged to extended-care facilities (26% and 26% vs 25%) (P < .0001). Hospital costs for both- and single-component revisions were 138% and 114% higher than costs for primary procedures (P < .0001). More recently, Kallala and colleagues44 analyzed UK National Health Service data and compared the costs of revision for infection with revision for other causes (pain, instability, aseptic loosening, fracture). Mean length of stay associated with revision for infection (21.5 days) was more than double that associated with revision for aseptic loosening (9.5 days; P < .0001), and mean cost of revision for septic causes (£30,011) was more than 3 times that of revision for other causes (£9655; P < .0001). The authors concluded that the higher costs of revision knee surgery have a considerable economic impact, especially in infection cases.
With more extensive procedures, long-stem or more constrained prostheses are often needed to obtain adequate fixation and stability. The resulting increased, substantial economic burden is felt by patients and the health care system. Given that health care reimbursements are declining, hospitals that perform revision TKAs can sustain marked financial losses. Some centers are asking whether it is cost-effective to continue to perform these types of procedures. We must find new ways to provide revision procedures using less costly implants and tools so that centers will continue to make these procedures available to patients.
Conclusion
Given the exponential growth in primary TKAs, there will be a concordant increase in revision TKAs in the decades to come. This review provides a concise overview of revision TKA outcomes. Given the low level of evidence regarding revision TKAs, we need further higher quality studies of their prostheses and outcomes. Specifically, we need systematic reviews and meta-analyses to provide higher quality evidence regarding outcomes of using individual prosthetic designs.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
1. Cram P, Lu X, Kates SL, Singh JA, Li Y, Wolf BR. Total knee arthroplasty volume, utilization, and outcomes among Medicare beneficiaries, 1991–2010. JAMA. 2012;308(12):1227-1236.
2. Crowninshield RD, Rosenberg AG, Sporer SM. Changing demographics of patients with total joint replacement. Clin Orthop Relat Res. 2006;443:266-272.
3. Ravi B, Croxford R, Reichmann WM, Losina E, Katz JN, Hawker GA. The changing demographics of total joint arthroplasty recipients in the United States and Ontario from 2001 to 2007. Best Pract Res Clin Rheumatol. 2012;26(5):637-647.
4. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
5. Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24(2):195-203.
6. Kurtz SM, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467(10):2606-2612.
7. Bryan RS, Rand JA. Revision total knee arthroplasty. Clin Orthop Relat Res. 1982;170:116-122.
8. Rand JA, Bryan RS. Revision after total knee arthroplasty. Orthop Clin North Am. 1982;13(1):201-212.
9. Bozic KJ, Kurtz SM, Lau E, et al. The epidemiology of revision total knee arthroplasty in the United States. Clin Orthop Relat Res. 2010;468(1):45-51.
10. Parvizi J, Nunley RM, Berend KR, et al. High level of residual symptoms in young patients after total knee arthroplasty. Clin Orthop Relat Res. 2014;472(1):133-137.
11. Ali A, Sundberg M, Robertsson O, et al. Dissatisfied patients after total knee arthroplasty: a registry study involving 114 patients with 8-13 years of followup. Acta Orthop. 2014;85(3):229-233.
12. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
13. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
14. Dy CJ, Marx RG, Bozic KJ, Pan TJ, Padgett DE, Lyman S. Risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1198-1207.
15. Sheng PY, Konttinen L, Lehto M, et al. Revision total knee arthroplasty: 1990 through 2002. A review of the Finnish Arthroplasty Registry. J Bone Joint Surg Am. 2006;88(7):1425-1430.
16. Koskinen E, Eskelinen A, Paavolainen P, Pulkkinen P, Remes V. Comparison of survival and cost-effectiveness between unicondylar arthroplasty and total knee arthroplasty in patients with primary osteoarthritis: a follow-up study of 50,493 knee replacements from the Finnish Arthroplasty Register. Acta Orthop. 2008;79(4):499-507.
17. New Zealand Orthopaedic Association. The New Zealand Joint Registry Fourteen Year Report (January 1999 to December 2012). http://www.nzoa.org.nz/system/files/NJR%2014%20Year%20Report.pdf. Published November 2013. Accessed December 16, 2015.
18. Laskin RS, Ohnsorge J. The use of standard posterior stabilized implants in revision total knee arthroplasty. Clin Orthop Relat Res. 2005;(440):122-125.
19. Meijer MF, Reininga IH, Boerboom AL, Stevens M, Bulstra SK. Poorer survival after a primary implant during revision total knee arthroplasty. Int Orthop. 2013;37(3):415-419.
20. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
21. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
22. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
23. Neumann DR, Hofstaedter T, Dorn U. Follow-up of a modular rotating hinge knee system in salvage revision total knee arthroplasty. J Arthroplasty. 2012;27(5):814-819.
24. Kowalczewski J, Marczak D, Synder M, Sibinski M. Primary rotating-hinge total knee arthroplasty: good outcomes at mid-term follow-up. J Arthroplasty. 2014;29(6):1202-1206.
25. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. Knee. 2013;20(6):367-375.
26. Vincent KR, Vincent HK, Lee LW, Alfano AP. Inpatient rehabilitation outcomes in primary and revision total knee arthroplasty patients. Clin Orthop Relat Res. 2006;(446):201-207.
27. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
28. Baier C, Luring C, Schaumburger J, et al. Assessing patient-oriented results after revision total knee arthroplasty. J Orthop Sci. 2013;18(6):955-961.
29. Hartford JM, Goodman SB, Schurman DJ, Knoblick G. Complex primary and revision total knee arthroplasty using the condylar constrained prosthesis: an average 5-year follow-up. J Arthroplasty. 1998;13(4):380-387.
30. Haidukewych GJ, Jacofsky DJ, Pagnano MW, Trousdale RT. Functional results after revision of well-fixed components for stiffness after primary total knee arthroplasty. J Arthroplasty. 2005;20(2):133-138.
31. Hwang SC, Kong JY, Nam DC, et al. Revision total knee arthroplasty with a cemented posterior stabilized, condylar constrained or fully constrained prosthesis: a minimum 2-year follow-up analysis. Clin Orthop Surg. 2010;2(2):112-120.
32. Barrack RL, McClure JT, Burak CF, Clohisy JC, Parvizi J, Sharkey P. Revision total knee arthroplasty: the patient’s perspective. Clin Orthop Relat Res. 2007;464:146-150.
33. Dahm DL, Barnes SA, Harrington JR, Berry DJ. Patient reported activity after revision total knee arthroplasty. J Arthroplasty. 2007;22(6 suppl 2):106-110.
34. Dahm DL, Barnes SA, Harrington JR, Sayeed SA, Berry DJ. Patient-reported activity level after total knee arthroplasty. J Arthroplasty. 2008;23(3):401-407.
35. Richards CJ, Garbuz DS, Pugh L, Masri BA. Revision total knee arthroplasty: clinical outcome comparison with and without the use of femoral head structural allograft. J Arthroplasty. 2011;26(8):1299-1304.
36. Greidanus NV, Peterson RC, Masri BA, Garbuz DS. Quality of life outcomes in revision versus primary total knee arthroplasty. J Arthroplasty. 2011;26(4):615-620.
37. Ethgen O, Bruyere O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
38. Murray DW, Fitzpatrick R, Rogers K, et al. The use of the Oxford hip and knee scores. J Bone Joint Surg Br. 2007;89(8):1010-1014.
39. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233.
40. Ghomrawi HM, Kane RL, Eberly LE, Bershadsky B, Saleh KJ; North American Knee Arthroplasty Revision Study Group. Patterns of functional improvement after revision knee arthroplasty. J Bone Joint Surg Am. 2009;91(12):2838-2845.
41. Deehan DJ, Murray JD, Birdsall PD, Pinder IM. Quality of life after knee revision arthroplasty. Acta Orthop. 2006;77(5):761-766.
42. Kapadia BH, McElroy MJ, Issa K, Johnson AJ, Bozic KJ, Mont MA. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplasty. 2014;29(5):929-932.
43. Bhandari M, Smith J, Miller LE, Block JE. Clinical and economic burden of revision knee arthroplasty. Clin Med Insights Arthritis Musculoskelet Disord. 2012;5:89-94.
44. Kallala RF, Vanhegan IS, Ibrahim MS, Sarmah S, Haddad FS. Financial analysis of revision knee surgery based on NHS tariffs and hospital costs: does it pay to provide a revision service? Bone Joint J Br. 2015;97(2):197-201.
45. Ong KL, Mowat FS, Chan N, Lau E, Halpern MT, Kurtz SM. Economic burden of revision hip and knee arthroplasty in Medicare enrollees. Clin Orthop Relat Res. 2006;446:22-28.
46. Kurtz SM, Ong KL, Lau E, Bozic KJ. Impact of the economic downturn on total joint replacement demand in the United States: updated projections to 2021. J Bone Joint Surg Am. 2014;96(8):624-630.
47. Bozic KJ, Durbhakula S, Berry DJ, et al. Differences in patient and procedure characteristics and hospital resource use in primary and revision total joint arthroplasty: a multicenter study. J Arthroplasty. 2005;20(7 suppl 3):17-25.
48. Lee KJ, Moon JY, Song EK, Lim HA, Seon JK. Minimum Two-year Results of Revision Total Knee Arthroplasty Following Infectious or Non-infectious Causes. Knee Surg Relat Res. 2012;24(4):227-234.
49. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
50. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
51. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
52. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
53. Mabry TM, Vessely MB, Schleck CD, Harmsen WS, Berry DJ. Revision total knee arthroplasty with modular cemented stems: long-term follow-up. J Arthroplasty. 2007;22(6 Suppl 2):100-105.
54. Gudnason A, Milbrink J, Hailer NP. Implant survival and outcome after rotating-hinge total knee revision arthroplasty: a minimum 6-year follow-up. Arch Orthop Trauma Surg. 2011;131(11):1601-1607.
55. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;430:125-131.
56. Greene JW, Reynolds SM, Stimac JD, Malkani AL, Massini MA. Midterm results of hybrid cement technique in revision total knee arthroplasty. J Arthroplasty. 2013;28(4):570-574.
57. Dalury DF, Adams MJ. Minimum 6-year follow-up of revision total knee arthroplasty without patella reimplantation. Journal Arthroplasty. 2012;27(8 Suppl):91-94.
58. Whaley AL, Trousdale RT, Rand JA, Hanssen AD. Cemented long-stem revision total knee arthroplasty. J Arthroplasty. 2003;18(5):592-599.
59. Friedman RJ, Hirst P, Poss R, Kelley K, Sledge CB. Results of revision total knee arthroplasty performed for aseptic loosening. Clinical Orthop Relat Res. 1990;255:235-241.
60. Barrack RL, Rorabeck C, Partington P, Sawhney J, Engh G. The results of retaining a well-fixed patellar component in revision total knee arthroplasty. J Arthroplasty. 2000;15(4):413-417.
61. Christensen CP, Crawford JJ, Olin MD, Vail TP. Revision of the stiff total knee arthroplasty. J Arthroplasty. 2002;17(4):409-415.
62. Garcia RM, Hardy BT, Kraay MJ, Goldberg VM. Revision total knee arthroplasty for aseptic and septic causes in patients with rheumatoid arthritis. Clin Orthop Relat Res. 2010;468(1):82-89.
63. Patil N, Lee K, Huddleston JI, Harris AH, Goodman SB. Aseptic versus septic revision total knee arthroplasty: patient satisfaction, outcome and quality of life improvement. Knee. 2010;17(3):200-203.
64. Luque R, Rizo B, Urda A, et al. Predictive factors for failure after total knee replacement revision. Int Orthop. 2014;38(2):429-435.
65. Bistolfi A, Massazza G, Rosso F, Crova M. Rotating-hinge total knee for revision total knee arthroplasty. Orthopedics. 2012;35(3):e325-e330.
66. Bottner F, Laskin R, Windsor RE, Haas SB. Hybrid component fixation in revision total knee arthroplasty. Clin Orthop Relat Res. 2006;446:127-131.
67. Jensen CL, Winther N, Schroder HM, Petersen MM. Outcome of revision total knee arthroplasty with the use of trabecular metal cone for reconstruction of severe bone loss at the proximal tibia. Knee. 2014;21(6):1233-1237.
68. Howard JL, Kudera J, Lewallen DG, Hanssen AD. Early results of the use of tantalum femoral cones for revision total knee arthroplasty. J Bone Joint Surg Am. 2011;93(5):478-484.
69. Yang JH, Yoon JR, Oh CH, Kim TS. Hybrid component fixation in total knee arthroplasty: minimum of 10-year follow-up study. J Arthroplasty. 2012;27(6):1111-1118.
70. Peters CL, Erickson JA, Gililland JM. Clinical and radiographic results of 184 consecutive revision total knee arthroplasties placed with modular cementless stems. J Arthroplasty. 2009;24(6 Suppl):48-53.
71. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2014. 2014.
72. Registry AOANJR. Hip and Knee Arthroplasty. Annual Report 2013. 2013.
Implant Designs in Revision Total Knee Arthroplasty
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
Before 1990, a considerable number of revisions were performed, largely for implant-associated failures, in the first few years after index primary knee arthroplasties.1,2 Since then, surgeons, manufacturers, and hospitals have collaborated to improve implant designs, techniques, and care guidelines.3,4 Despite the substantial improvements in designs, which led to implant longevity of more than 15 years in many cases, these devices still have limited life spans. Large studies have estimated that the risk for revision required after primary knee arthroplasty ranges from as low as 5% at 15 years to up to 9% at 10 years.4,5
The surgical goals of revision total knee arthroplasty (TKA) are to obtain stable fixation of the prosthesis to host bone, to obtain a stable range of motion compatible with the patient’s activities of daily living, and to achieve these goals while using the smallest amount of prosthetic augments and constraint so that the soft tissues may share in load transfer.6 As prosthetic constraint increases, the soft tissues participate less in load sharing, and increasing stresses are put on the implant–bone interface, which further increases the risk for early implant loosening.7 Hence, as characteristics of a revision implant become more constrained, there is often a higher rate of aseptic loosening expected.8
Controversy remains regarding the ideal implant type for revision TKA. To ensure the success of revision surgery and to reduce the risks for postoperative dissatisfaction, complications, and re-revision, orthopedists must understand the types of revision implant designs available, particularly as each has its own indications and potential complications.
In this article, we review the classification systems used for revision TKA as well as the types of prosthetic designs that can be used: posterior stabilized, nonlinked constrained, rotating hinge, and modular segmental.
1. Classification of bone loss and soft-tissue integrity
To further understand revision TKA, we must consider the complexity level of these cases, particularly by evaluating degree of bone loss and soft-tissue deficiency. The most accepted way to assess bone loss both before and during surgery is to use the AORI (Anderson Orthopaedic Research Institute) classification system.9 Bone loss can be classified into 3 types: I, in which metaphyseal bone is intact and small bone defects do not compromise component stability; II, in which metaphyseal bone is damaged and cancellous bone loss requires cement fill, augments, or bone graft; and III, in which metaphyseal bone is deficient, and lost bone comprises a major portion of condyle or plateau and occasionally requires bone grafts or custom implants (Table 1). These patterns of bone loss are occasionally associated with detachment of the collateral ligament or patellar tendon.
In addition to understanding bone loss in revision TKA, surgeons must be aware of soft-tissue deficiencies (eg, collateral ligaments, extensor mechanism), which also influence type and amount of prosthesis constraint. Specifically, constraint choice depends on amount of bone loss and on the condition of stabilizing tissues, such as the collateral ligaments. Under conditions of minimal bone loss and intact peripheral ligaments, a less constrained device, such as a primary posterior stabilized system, can be considered. When ligaments are present but insufficient, a semiconstrained device is recommended. In the presence of medial collateral ligament attenuation or complete medial or lateral collateral ligament dysfunction, a fully constrained prosthesis is required.8 Therefore, amount of bone loss or soft-tissue deficiency often dictates which prosthesis to use.
For radiographic classification, the Knee Society roentgenographic evaluation and scoring system10 has been implemented to allow for uniform reporting of radiographic results and to ensure adequate preoperative planning and postoperative assessment of component alignment. This system incorporates the evaluation of alignment in the coronal, sagittal, and patellofemoral planes and assesses radiolucency using zones dividing the implant–bone interface into segments to allow for easier classification of areas of lucency. More recently, a modified version of the Knee Society system was constructed.11 This modification simplifies zone classifications and accommodates more complex revision knee designs and stem extensions.
2. Posterior stabilized designs
Cruciate-retaining prostheses are seldom applicable in the revision TKA setting because of frequent damage to the posterior cruciate ligament, except in the case of simple polyethylene exchanges or, potentially, revisions of failed unicompartmental TKAs. Thus, posterior stabilized designs are the first-line choice for revision TKA (Figure 1). These prostheses are indicated only when the posterior cruciate ligament is incompetent and in the setting of adequate flexion and extension and medial and lateral collateral ligament balancing.
However, studies have shown that posterior stabilized TKAs have a limited role in revision TKAs, as the amount of ligamentous and bony damage is often underestimated in these patients, and use of a primary implant in a revision setting often requires additional augments, all of which may have contributed to the high failure rate. Thus, this design should be used only when the patient has adequate bone stock (AORI type I) and collateral ligament tension. This situation further emphasizes the importance of performing intraoperative testing for ligamentous balance and bone deficit evaluation in order to determine the most appropriate implant (Table 2).
3. Nonlinked constrained designs
Nonlinked constrained (condylar constrained) designs are the devices most commonly used for revision TKAs (>50% of revision knees). These prostheses provide increased articular constraint, which is required in patients with persistent instability, despite appropriate soft-tissue balancing. Increased articular constraint allows for more knee stability by providing progressive varus-valgus, coronal, and rotational stability with the aid of taller and wider tibial posts.12 Specifically, these implants incorporate a tibial post that fits closely between the femoral condyles, allowing for less motion compared with a standard posterior stabilized design.12
In addition, these designs may be used with augments, stems, and allografts when bone loss is more substantial. In particular, stem extensions allow for load distribution to the diaphyseal regions of the tibia and femur and thereby aid in reducing the increased stress at the bone–implant interface, which is a common concern with these implants. However, these extensions cost more, require intramedullary invasion, and are associated with higher rates of leg and thigh pain.12
These prostheses are often implicated in cases involving a high degree of bone loss (eg, AORI type II or III). They are ideally used in cases in which complete revision of both tibial and femoral components is needed and are indicated in cases of incompetent posterior cruciate ligament, partial functional loss of medial or lateral collateral ligaments, or flexion-extension mismatch.13 Furthermore, use of a constrained prosthesis is recommended in the setting of varus or valgus instability, or repeated dislocations of a posterior stabilized design (Table 2).
Ten-year survivorship ranges from 85% to 96%, but this is substantially lower than the 95% to 96% for condylar constrained prostheses used in primary TKAs.14-17 Moreover, the large discrepancy between survivorship of primary TKA and revision TKA with a constrained prosthesis further affirms that the complexity of revision surgery, rather than the prosthesis used, may have more deleterious effects on outcomes. However, surgeons must be aware that increased constraint leads to increased stress on the prosthetic interfaces with associated aseptic loosening and early failure, and this continues to be a legitimate concern.
4. Rotating hinge designs
Many patients who undergo revision TKA can be managed with a posterior stabilizing or nonlinked constrained design. However, in patients who present with severe ligamentous instability and bone loss (AORI type II or III), a rotating hinge prostheses, or highly constrained device, is often recommended (Figure 2).18 By using a rotating mobile-bearing platform, this prosthesis permits axial rotation through a metal-reinforced polyethylene-post articulation in the tibial tray. In addition, it involves use of modular diaphyseal-engaging stems and diaphyseal sleeves, which allow for the bypass of bony defects and areas of bone loss (Table 2).
However, the rigid biomechanics of hinged prostheses is associated with increased risk for aseptic loosening (aseptic 10-year survival, 60%-80%), imparted by the transfer of stresses across the bone. The higher risk for early loosening, osteolysis, and excessive wear—caused by the highly restricted biomechanics of early generations of fixed hinged designs—has led to the development of new devices with mobile mechanics. Prosthetic designs have been improved with an added rotational axis to reduce torsional stress, a patellar resurfacing option, and better stem fixation and patellofemoral kinematics. Overall, these are aimed to improve rates of instability and aseptic loosening, with promising results demonstrated in the literature.
5. Modular segmental arthroplasty designs
Segmental arthroplasty prostheses, which typically are end-of-the-line revision TKA options, are applicable only in cases of extensive bone loss (more than can be treated with allografts or augments; AORI type 3), complete ligamentous disruption/absence, loss of periprosthetic soft tissue, and multiple previous revision procedures (Figure 3). Despite the limited indications for these prostheses, they yield quick return to function without graft nonunion or resorption, and they augment ingrowth/ongrowth. Furthermore, the next surgical option could be fusion or amputation. When failures were specifically evaluated for aseptic loosening across 4 studies, the survival rate ranged from 83% to 99.5%, with the most frequent complication being infection (up to 33% in one series).6,19-21
The major roles for segmental arthroplasty prostheses in primary TKAs are in the setting of oncologic conditions that require bony excision, or unreconstuctable fractures about the knee. Used after ancillary metastatic disease, these prostheses demonstrate positive results, according to several reports.22,23 In the setting of revision TKA, however, these prostheses should be used only when other surgical options are unfeasible, given the high risk for infection and the re-revision rates. Currently, revision TKAs with tumor prostheses have a high failure rate (up to 50%) because of the extensive surgery and the lack of bony and soft-tissue support (Table 2).
Conclusion
Orthopedists performing revision TKAs must consider bone stock and remaining ligament stability. In particular, they should choose implants for least constraint and adequate knee stability, as these are essential in minimizing the stresses on the implant–bone interface. Ultimately, functional outcomes, survivorship, and postoperative satisfaction determine the success of these designs. However, predictors of outcomes of revision surgery are often multifactorial, and surgeons must also consider procedure complexity and patient-specific characteristics.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
1. Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315-318.
2. Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award paper. Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7-13.
3. Schroer WC, Berend KR, Lombardi AV, et al. Why are total knees failing today? Etiology of total knee revision in 2010 and 2011. J Arthroplasty. 2013;28(8 suppl):116-119.
4. Kim TK. CORR Insights(®): risk factors for revision within 10 years of total knee arthroplasty. Clin Orthop Relat Res. 2014;472(4):1208-1209.
5. Sheng PY, Jämsen E, Lehto MU, Konttinen YT, Pajamäki J, Halonen P. Revision total knee arthroplasty with the Total Condylar III system in inflammatory arthritis. J Bone Joint Surg Br. 2005;87(9):1222-1224.
6. Haas SB, Insall JN, Montgomery W 3rd, Windsor RE. Revision total knee arthroplasty with use of modular components with stems inserted without cement. J Bone Joint Surg Am. 1995;77(11):1700-1707.
7. Dennis DA. A stepwise approach to revision total knee arthroplasty. J Arthroplasty. 2007;22(4 suppl 1):32-38.
8. Vasso M, Beaufils P, Schiavone Panni A. Constraint choice in revision knee arthroplasty. Int Orthop. 2013;37(7):1279-1284.
9. Engh GA, Ammeen DJ. Bone loss with revision total knee arthroplasty: defect classification and alternatives for reconstruction. Instr Course Lect. 1999;48:167-175.
10. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
11. Meneghini RM, Mont MA, Backstein DB, Bourne RB, Dennis DA, Scuderi GR. Development of a modern Knee Society radiographic evaluation system and methodology for total knee arthroplasty. J Arthroplasty. 2015;30(12):2311-2314.
12. Nam D, Umunna BP, Cross MB, Reinhardt KR, Duggal S, Cornell CN. Clinical results and failure mechanisms of a nonmodular constrained knee without stem extensions. HSS J. 2012;8(2):96-102.
13. Lombardi AV Jr, Berend KR. The role of implant constraint in revision TKA: striking the balance. Orthopedics. 2006;29(9):847-849.
14. Lachiewicz PF, Soileau ES. Results of a second-generation constrained condylar prosthesis in primary total knee arthroplasty. J Arthroplasty. 2011;26(8):1228-1231.
15. Bae DK, Song SJ, Heo DB, Lee SH, Song WJ. Long-term survival rate of implants and modes of failure after revision total knee arthroplasty by a single surgeon. J Arthroplasty. 2013;28(7):1130-1134.
16. Wilke BK, Wagner ER, Trousdale RT. Long-term survival of semi-constrained total knee arthroplasty for revision surgery. J Arthroplasty. 2014;29(5):1005-1008.
17. Lachiewicz PF, Soileau ES. Ten-year survival and clinical results of constrained components in primary total knee arthroplasty. J Arthroplasty. 2006;21(6):803-808.
18. Jones RE. Total knee arthroplasty with modular rotating-platform hinge. Orthopedics. 2006;29(9 suppl):S80-S82.
19. Korim MT, Esler CN, Reddy VR, Ashford RU. A systematic review of endoprosthetic replacement for non-tumour indications around the knee joint. The Knee. 2013;20:367-375.
20. Hofmann AA, Goldberg T, Tanner AM, Kurtin SM. Treatment of infected total knee arthroplasty using an articulating spacer: 2- to 12-year experience. Clin Orthop Relat Res. 2005;(430):125-131.
21. Peters CL, Erickson J, Kloepper RG, Mohr RA. Revision total knee arthroplasty with modular components inserted with metaphyseal cement and stems without cement. J Arthroplasty. 2005;20:302-308.
22. Pala E, Trovarelli G, Calabro T, Angelini A, Abati CN, Ruggieri P. Survival of modern knee tumor megaprostheses: failures, functional results, and a comparative statistical analysis. Clinical Orthop Relat Res. 2015;473:891-899.
23. Angelini A, Henderson E, Trovarelli G, Ruggieri P. Is there a role for knee arthrodesis with modular endoprostheses for tumor and revision of failed endoprostheses? Clin Orthop Relat Res. 2013;471(10):3326-3335.
Nonoperative Treatment of Rotator Cuff Tears
Rotator cuff disease is extremely common, yet indications for surgery are not well established. Unfortunately, data on the natural history of patients with rotator cuff disease are lacking, as are high-level studies evaluating the effectiveness of rotator cuff repair. This deficit is highlighted by the recent American Academy of Orthopaedic Surgeons clinical practice guideline on optimizing the management of rotator cuff problems,1 in which none of the position statements were based on high-level evidence, and 22 of 25 statements were inconclusive or based on weak evidence or represented the panel’s consensus opinion. Although the traditional teaching is that rotator cuff tears (RCTs) should be surgically repaired, the present article reviews the evidence supporting physical therapy as a treatment for atraumatic full-thickness RCTs.
1. Less than 5% of people with RCTs undergo surgery
Studies on symptomatic and asymptomatic patients have found a high incidence of RCTs in the population at large.2,3 By conservative estimate, 10% of people older than 65 years have full-thickness RCTs. Therefore, the 2010 US Census4 finding of 57 million people over age 65 years translates to 5.7 million with full-thickness RCTs. In the United States, about 275,000 rotator cuff surgeries are performed annually.5 That is, less than 5% of people with RCTs undergo surgery each year.
2. Symptoms do not correlate well with RCT severity
Pain is statistically more likely in patients who experience RCT progression than in those who do not.6-8 However, RCTs may progress without pain, or there may be pain without progression, making pain a poor sign of RCT progression.9 The Multicenter Orthopaedic Outcome Network (MOON) Shoulder Group, studying a cohort of patients with atraumatic full-thickness RCTs, found no relationship between RCT severity and pain,10 symptom duration,11 or activity level,12 suggesting the relationship between RCTs and symptoms is not robust.
3. The high failure rates of surgical repairs do not affect patient-reported outcomes
Postoperative imaging has demonstrated high failure rates for rotator cuff repairs, yet patient-reported outcome scores do not differ between cases of intact and failed repairs.13,14 Strength is better, however, in intact repairs.14
4. Physical therapy is effective in treating atraumatic RCTs
The MOON Shoulder Group conducted a prospective cohort study to determine the predictors of failed physical therapy for atraumatic full-thickness RCTs and to help define the indications for rotator cuff surgery.15 All enrolled patients started with a well-defined physical therapy program, and they could opt out and have surgery at any time. The physical therapy program, derived from a systematic review of the literature, was found to be effective in more than 80% of patients with follow-up of 2 years or longer.15 The most important predictor of failed nonoperative treatment was patient expectations: For a patient who thought physical therapy would work, it worked; for a patient who thought it would not work, surgery was the more likely choice. No measure of pain or RCT severity predicted the need for surgery.16 For 2 randomized trials that compared surgery and physical therapy, the success of nonoperative treatment was similar: 76% (Moosmayer and colleagues17) and 92% (Kukkonen and colleagues18).
5. What are the indications for surgery?
These data suggest that physical therapy is reasonable for patients with atraumatic RCTs. Some data suggest that traumatic RCTs should be treated with surgery and that it should be performed early.19 Other data suggest strength is better after rotator cuff repair.13,14 What, then, are the indications for surgery? Patients with acute tears probably should have surgery; patients concerned about weakness should consider surgery but should keep in mind that its benefit depends on an intact rotator cuff repair; and patients with low expectations about the effectiveness of physical therapy probably should consider surgery.
When discussing options with a patient, you might approach informed consent as follows:
“Mr. Smith, you have a rotator cuff tear. So do at least 6 million other Americans over age 60 years. Only 5% of those undergo surgery. If your problem is weakness or functional loss, you should have surgery, though there is about a 30% chance the repair will fail. I don’t know how to predict the outcome of repair yet, but I worry your atraumatic tear is at risk for repair failure.
“If your problem is pain, you have an 80% chance of improving with physical therapy, and pain relief seems to last at least 2 years. If you go with physical therapy, however, there is a risk your tear could progress and start causing symptoms. I don’t yet know how likely it is your tear will progress or, if it does progress, how likely it is the tear will cause symptoms. I wish we had better information to help you make your decision.”
1. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
2. Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88(2):116-121.
3. Teunis, T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff pathology with increasing age. J Shoulder Elbow Surg. 2014;23(12):1913-1921.
4. Werner CA. The older population: 2010 (2010 Census briefs). US Census Bureau website. http://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf. Published November 2011. Accessed December 13, 2015.
5. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
6. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92(16):2623-2633.
7. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
8. Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39(4):710-714.
9. Kuhn JE. Are atraumatic rotator cuff tears painful? A model to describe the relationship between pain and rotator cuff tears. Minerva Orthop Traumatol. 2015;66:51-61.
10. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity: a cross-sectional study of 393 patients with a symptomatic atraumatic full-thickness rotator cuff tear. J Bone Joint Surg Am. 2014;96(10):793-800.
11. MOON Shoulder Group: Unruh KP, Kuhn JE, Sanders R, et al. The duration of symptoms does not correlate with rotator cuff tear severity or other patient-related features: a cross-sectional study of patients with atraumatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2014;23(7):1052-1058.
12. Brophy RH, Dunn WR, Kuhn JE; MOON Shoulder Group. Shoulder activity level is not associated with the severity of symptomatic, atraumatic rotator cuff tears in patients electing nonoperative treatment. Am J Sports Med. 2014;42(5):1150-1154.
13. Slabaugh MA, Nho SJ, Grumet RC, et al. Does the literature confirm superior clinical results in radiographically healed rotator cuffs after rotator cuff repair? Arthroscopy. 2010;26(3):393-403.
14. Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96(4):265-271.
15. Kuhn JE, Dunn WR, Sanders R, et al; MOON Shoulder Group. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. J Shoulder Elbow Surg. 2013;22(10):1371-1379.
16. Dunn WR, Kuhn JE, Sanders R, et al. Defining indications for rotator cuff repair: predictors of failure of nonoperative treatment of chronic, symptomatic full-thickness rotator cuff tears. Paper presented at: Open Meeting of the American Shoulder and Elbow Surgeons; March 23, 2013; Chicago, IL.
17. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears: a randomized controlled study in 103 cases with a five-year follow-up. J Bone Joint Surg Am. 2014;96(18):1504-1514.
18. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of non-traumatic rotator cuff tears: a randomised controlled trial with one-year clinical results. Bone Joint J Br. 2014;96(1):75-81.
19. Oh LS, Wolf BR, Hall MP, Levy BA, Marx RG. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;(455):52-63.
Rotator cuff disease is extremely common, yet indications for surgery are not well established. Unfortunately, data on the natural history of patients with rotator cuff disease are lacking, as are high-level studies evaluating the effectiveness of rotator cuff repair. This deficit is highlighted by the recent American Academy of Orthopaedic Surgeons clinical practice guideline on optimizing the management of rotator cuff problems,1 in which none of the position statements were based on high-level evidence, and 22 of 25 statements were inconclusive or based on weak evidence or represented the panel’s consensus opinion. Although the traditional teaching is that rotator cuff tears (RCTs) should be surgically repaired, the present article reviews the evidence supporting physical therapy as a treatment for atraumatic full-thickness RCTs.
1. Less than 5% of people with RCTs undergo surgery
Studies on symptomatic and asymptomatic patients have found a high incidence of RCTs in the population at large.2,3 By conservative estimate, 10% of people older than 65 years have full-thickness RCTs. Therefore, the 2010 US Census4 finding of 57 million people over age 65 years translates to 5.7 million with full-thickness RCTs. In the United States, about 275,000 rotator cuff surgeries are performed annually.5 That is, less than 5% of people with RCTs undergo surgery each year.
2. Symptoms do not correlate well with RCT severity
Pain is statistically more likely in patients who experience RCT progression than in those who do not.6-8 However, RCTs may progress without pain, or there may be pain without progression, making pain a poor sign of RCT progression.9 The Multicenter Orthopaedic Outcome Network (MOON) Shoulder Group, studying a cohort of patients with atraumatic full-thickness RCTs, found no relationship between RCT severity and pain,10 symptom duration,11 or activity level,12 suggesting the relationship between RCTs and symptoms is not robust.
3. The high failure rates of surgical repairs do not affect patient-reported outcomes
Postoperative imaging has demonstrated high failure rates for rotator cuff repairs, yet patient-reported outcome scores do not differ between cases of intact and failed repairs.13,14 Strength is better, however, in intact repairs.14
4. Physical therapy is effective in treating atraumatic RCTs
The MOON Shoulder Group conducted a prospective cohort study to determine the predictors of failed physical therapy for atraumatic full-thickness RCTs and to help define the indications for rotator cuff surgery.15 All enrolled patients started with a well-defined physical therapy program, and they could opt out and have surgery at any time. The physical therapy program, derived from a systematic review of the literature, was found to be effective in more than 80% of patients with follow-up of 2 years or longer.15 The most important predictor of failed nonoperative treatment was patient expectations: For a patient who thought physical therapy would work, it worked; for a patient who thought it would not work, surgery was the more likely choice. No measure of pain or RCT severity predicted the need for surgery.16 For 2 randomized trials that compared surgery and physical therapy, the success of nonoperative treatment was similar: 76% (Moosmayer and colleagues17) and 92% (Kukkonen and colleagues18).
5. What are the indications for surgery?
These data suggest that physical therapy is reasonable for patients with atraumatic RCTs. Some data suggest that traumatic RCTs should be treated with surgery and that it should be performed early.19 Other data suggest strength is better after rotator cuff repair.13,14 What, then, are the indications for surgery? Patients with acute tears probably should have surgery; patients concerned about weakness should consider surgery but should keep in mind that its benefit depends on an intact rotator cuff repair; and patients with low expectations about the effectiveness of physical therapy probably should consider surgery.
When discussing options with a patient, you might approach informed consent as follows:
“Mr. Smith, you have a rotator cuff tear. So do at least 6 million other Americans over age 60 years. Only 5% of those undergo surgery. If your problem is weakness or functional loss, you should have surgery, though there is about a 30% chance the repair will fail. I don’t know how to predict the outcome of repair yet, but I worry your atraumatic tear is at risk for repair failure.
“If your problem is pain, you have an 80% chance of improving with physical therapy, and pain relief seems to last at least 2 years. If you go with physical therapy, however, there is a risk your tear could progress and start causing symptoms. I don’t yet know how likely it is your tear will progress or, if it does progress, how likely it is the tear will cause symptoms. I wish we had better information to help you make your decision.”
Rotator cuff disease is extremely common, yet indications for surgery are not well established. Unfortunately, data on the natural history of patients with rotator cuff disease are lacking, as are high-level studies evaluating the effectiveness of rotator cuff repair. This deficit is highlighted by the recent American Academy of Orthopaedic Surgeons clinical practice guideline on optimizing the management of rotator cuff problems,1 in which none of the position statements were based on high-level evidence, and 22 of 25 statements were inconclusive or based on weak evidence or represented the panel’s consensus opinion. Although the traditional teaching is that rotator cuff tears (RCTs) should be surgically repaired, the present article reviews the evidence supporting physical therapy as a treatment for atraumatic full-thickness RCTs.
1. Less than 5% of people with RCTs undergo surgery
Studies on symptomatic and asymptomatic patients have found a high incidence of RCTs in the population at large.2,3 By conservative estimate, 10% of people older than 65 years have full-thickness RCTs. Therefore, the 2010 US Census4 finding of 57 million people over age 65 years translates to 5.7 million with full-thickness RCTs. In the United States, about 275,000 rotator cuff surgeries are performed annually.5 That is, less than 5% of people with RCTs undergo surgery each year.
2. Symptoms do not correlate well with RCT severity
Pain is statistically more likely in patients who experience RCT progression than in those who do not.6-8 However, RCTs may progress without pain, or there may be pain without progression, making pain a poor sign of RCT progression.9 The Multicenter Orthopaedic Outcome Network (MOON) Shoulder Group, studying a cohort of patients with atraumatic full-thickness RCTs, found no relationship between RCT severity and pain,10 symptom duration,11 or activity level,12 suggesting the relationship between RCTs and symptoms is not robust.
3. The high failure rates of surgical repairs do not affect patient-reported outcomes
Postoperative imaging has demonstrated high failure rates for rotator cuff repairs, yet patient-reported outcome scores do not differ between cases of intact and failed repairs.13,14 Strength is better, however, in intact repairs.14
4. Physical therapy is effective in treating atraumatic RCTs
The MOON Shoulder Group conducted a prospective cohort study to determine the predictors of failed physical therapy for atraumatic full-thickness RCTs and to help define the indications for rotator cuff surgery.15 All enrolled patients started with a well-defined physical therapy program, and they could opt out and have surgery at any time. The physical therapy program, derived from a systematic review of the literature, was found to be effective in more than 80% of patients with follow-up of 2 years or longer.15 The most important predictor of failed nonoperative treatment was patient expectations: For a patient who thought physical therapy would work, it worked; for a patient who thought it would not work, surgery was the more likely choice. No measure of pain or RCT severity predicted the need for surgery.16 For 2 randomized trials that compared surgery and physical therapy, the success of nonoperative treatment was similar: 76% (Moosmayer and colleagues17) and 92% (Kukkonen and colleagues18).
5. What are the indications for surgery?
These data suggest that physical therapy is reasonable for patients with atraumatic RCTs. Some data suggest that traumatic RCTs should be treated with surgery and that it should be performed early.19 Other data suggest strength is better after rotator cuff repair.13,14 What, then, are the indications for surgery? Patients with acute tears probably should have surgery; patients concerned about weakness should consider surgery but should keep in mind that its benefit depends on an intact rotator cuff repair; and patients with low expectations about the effectiveness of physical therapy probably should consider surgery.
When discussing options with a patient, you might approach informed consent as follows:
“Mr. Smith, you have a rotator cuff tear. So do at least 6 million other Americans over age 60 years. Only 5% of those undergo surgery. If your problem is weakness or functional loss, you should have surgery, though there is about a 30% chance the repair will fail. I don’t know how to predict the outcome of repair yet, but I worry your atraumatic tear is at risk for repair failure.
“If your problem is pain, you have an 80% chance of improving with physical therapy, and pain relief seems to last at least 2 years. If you go with physical therapy, however, there is a risk your tear could progress and start causing symptoms. I don’t yet know how likely it is your tear will progress or, if it does progress, how likely it is the tear will cause symptoms. I wish we had better information to help you make your decision.”
1. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
2. Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88(2):116-121.
3. Teunis, T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff pathology with increasing age. J Shoulder Elbow Surg. 2014;23(12):1913-1921.
4. Werner CA. The older population: 2010 (2010 Census briefs). US Census Bureau website. http://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf. Published November 2011. Accessed December 13, 2015.
5. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
6. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92(16):2623-2633.
7. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
8. Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39(4):710-714.
9. Kuhn JE. Are atraumatic rotator cuff tears painful? A model to describe the relationship between pain and rotator cuff tears. Minerva Orthop Traumatol. 2015;66:51-61.
10. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity: a cross-sectional study of 393 patients with a symptomatic atraumatic full-thickness rotator cuff tear. J Bone Joint Surg Am. 2014;96(10):793-800.
11. MOON Shoulder Group: Unruh KP, Kuhn JE, Sanders R, et al. The duration of symptoms does not correlate with rotator cuff tear severity or other patient-related features: a cross-sectional study of patients with atraumatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2014;23(7):1052-1058.
12. Brophy RH, Dunn WR, Kuhn JE; MOON Shoulder Group. Shoulder activity level is not associated with the severity of symptomatic, atraumatic rotator cuff tears in patients electing nonoperative treatment. Am J Sports Med. 2014;42(5):1150-1154.
13. Slabaugh MA, Nho SJ, Grumet RC, et al. Does the literature confirm superior clinical results in radiographically healed rotator cuffs after rotator cuff repair? Arthroscopy. 2010;26(3):393-403.
14. Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96(4):265-271.
15. Kuhn JE, Dunn WR, Sanders R, et al; MOON Shoulder Group. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. J Shoulder Elbow Surg. 2013;22(10):1371-1379.
16. Dunn WR, Kuhn JE, Sanders R, et al. Defining indications for rotator cuff repair: predictors of failure of nonoperative treatment of chronic, symptomatic full-thickness rotator cuff tears. Paper presented at: Open Meeting of the American Shoulder and Elbow Surgeons; March 23, 2013; Chicago, IL.
17. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears: a randomized controlled study in 103 cases with a five-year follow-up. J Bone Joint Surg Am. 2014;96(18):1504-1514.
18. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of non-traumatic rotator cuff tears: a randomised controlled trial with one-year clinical results. Bone Joint J Br. 2014;96(1):75-81.
19. Oh LS, Wolf BR, Hall MP, Levy BA, Marx RG. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;(455):52-63.
1. Pedowitz RA, Yamaguchi K, Ahmad CS, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: optimizing the management of rotator cuff problems. J Bone Joint Surg Am. 2012;94(2):163-167.
2. Reilly P, Macleod I, Macfarlane R, Windley J, Emery RJ. Dead men and radiologists don’t lie: a review of cadaveric and radiological studies of rotator cuff tear prevalence. Ann R Coll Surg Engl. 2006;88(2):116-121.
3. Teunis, T, Lubberts B, Reilly BT, Ring D. A systematic review and pooled analysis of the prevalence of rotator cuff pathology with increasing age. J Shoulder Elbow Surg. 2014;23(12):1913-1921.
4. Werner CA. The older population: 2010 (2010 Census briefs). US Census Bureau website. http://www.census.gov/prod/cen2010/briefs/c2010br-09.pdf. Published November 2011. Accessed December 13, 2015.
5. Colvin AC, Egorova N, Harrison AK, Moskowitz A, Flatow EL. National trends in rotator cuff repair. J Bone Joint Surg Am. 2012;94(3):227-233.
6. Mall NA, Kim HM, Keener JD, et al. Symptomatic progression of asymptomatic rotator cuff tears: a prospective study of clinical and sonographic variables. J Bone Joint Surg Am. 2010;92(16):2623-2633.
7. Moosmayer S, Tariq R, Stiris M, Smith HJ. The natural history of asymptomatic rotator cuff tears: a three-year follow-up of fifty cases. J Bone Joint Surg Am. 2013;95(14):1249-1255.
8. Safran O, Schroeder J, Bloom R, Weil Y, Milgrom C. Natural history of nonoperatively treated symptomatic rotator cuff tears in patients 60 years old or younger. Am J Sports Med. 2011;39(4):710-714.
9. Kuhn JE. Are atraumatic rotator cuff tears painful? A model to describe the relationship between pain and rotator cuff tears. Minerva Orthop Traumatol. 2015;66:51-61.
10. Dunn WR, Kuhn JE, Sanders R, et al. Symptoms of pain do not correlate with rotator cuff tear severity: a cross-sectional study of 393 patients with a symptomatic atraumatic full-thickness rotator cuff tear. J Bone Joint Surg Am. 2014;96(10):793-800.
11. MOON Shoulder Group: Unruh KP, Kuhn JE, Sanders R, et al. The duration of symptoms does not correlate with rotator cuff tear severity or other patient-related features: a cross-sectional study of patients with atraumatic, full-thickness rotator cuff tears. J Shoulder Elbow Surg. 2014;23(7):1052-1058.
12. Brophy RH, Dunn WR, Kuhn JE; MOON Shoulder Group. Shoulder activity level is not associated with the severity of symptomatic, atraumatic rotator cuff tears in patients electing nonoperative treatment. Am J Sports Med. 2014;42(5):1150-1154.
13. Slabaugh MA, Nho SJ, Grumet RC, et al. Does the literature confirm superior clinical results in radiographically healed rotator cuffs after rotator cuff repair? Arthroscopy. 2010;26(3):393-403.
14. Russell RD, Knight JR, Mulligan E, Khazzam MS. Structural integrity after rotator cuff repair does not correlate with patient function and pain: a meta-analysis. J Bone Joint Surg Am. 2014;96(4):265-271.
15. Kuhn JE, Dunn WR, Sanders R, et al; MOON Shoulder Group. Effectiveness of physical therapy in treating atraumatic full-thickness rotator cuff tears: a multicenter prospective cohort study. J Shoulder Elbow Surg. 2013;22(10):1371-1379.
16. Dunn WR, Kuhn JE, Sanders R, et al. Defining indications for rotator cuff repair: predictors of failure of nonoperative treatment of chronic, symptomatic full-thickness rotator cuff tears. Paper presented at: Open Meeting of the American Shoulder and Elbow Surgeons; March 23, 2013; Chicago, IL.
17. Moosmayer S, Lund G, Seljom US, et al. Tendon repair compared with physiotherapy in the treatment of rotator cuff tears: a randomized controlled study in 103 cases with a five-year follow-up. J Bone Joint Surg Am. 2014;96(18):1504-1514.
18. Kukkonen J, Joukainen A, Lehtinen J, et al. Treatment of non-traumatic rotator cuff tears: a randomised controlled trial with one-year clinical results. Bone Joint J Br. 2014;96(1):75-81.
19. Oh LS, Wolf BR, Hall MP, Levy BA, Marx RG. Indications for rotator cuff repair: a systematic review. Clin Orthop Relat Res. 2007;(455):52-63.
Adipose Flap Versus Fascial Sling for Anterior Subcutaneous Transposition of the Ulnar Nerve
Compression of the ulnar nerve at the elbow, also referred to as cubital tunnel syndrome (CuTS), is the second most common peripheral nerve compression syndrome in the upper extremity.1,2 Although the ulnar nerve can be compressed at 5 different sites, including arcade of Struthers, medial intermuscular septum, medial epicondyle, and deep flexor aponeurosis, the cubital tunnel is most commonly affected.3 Patients typically present with paresthesias in the fourth and fifth digits and weakness of hand muscle intrinsics. Activity-related pain or pain at the medial elbow can also occur in more advanced pathology.4 It is estimated that conservative therapy fails and surgical intervention is required in up to 30% of patients with CuTS.1 Surgical approaches range from in situ decompression to transposition techniques, but there is no consensus in the orthopedic community as to which technique offers the best results. In a 2008 meta-analysis, Macadam and colleagues5 found no statistical differences in outcomes among the various surgical approaches. Nevertheless, subcutaneous transposition of the ulnar nerve at the elbow is a popular option.6
Despite the widespread success of surgical intervention for CuTS, persistent or recurrent pain occurs in 9.9% to 21.0% of cases.7-10 In addition, several investigators have cited perineural scarring as a major cause of recurrent symptoms after primary surgery.11-14 Filippi and colleagues11 noted that patients who required reoperation after primary anterior transposition had “serious epineural fibrosis and fibrosis around the transposed ulnar nerve.” At our institution, we have similarly found that scarring of the fascial sling around the ulnar nerve led to recurrence of CuTS within 4 months after initial surgery (Figure 1).
We therefore prefer to use a vascularized adipose flap to secure the anteriorly transposed ulnar nerve. This flap provides a pliable, vascularized adipose environment for the nerve, which helps reduce nerve adherence and may enhance nerve recovery.15 In the study reported here, we retrospectively reviewed the long-term outcomes of ulnar nerve anterior subcutaneous transposition secured with either an adipose flap or a fascial sling. We hypothesized that patients in the 2 groups (adipose flap, fascial sling) would have equivalent outcomes.
Materials and Methods
After obtaining institutional review board approval, we reviewed the medical and surgical records of 104 patients (107 limbs) who underwent transposition of the ulnar nerve secured with either an adipose flap (27 limbs) or a fascial sling (80 limbs) over a 14-year period. The fascial sling cohort was used as a comparison group, matched to the adipose flap cohort by sex, age at time of surgery, hand dominance, symptom duration, and length of follow-up (Table 1). Patients were indicated for surgery and were included in the study if they had a history and physical examination consistent with primary CuTS, symptom duration longer than 1 year, and failed conservative management, including activity modification, night splinting, elbow pads, occupational therapy, and home exercise regimen. Electrodiagnostic testing was used at the discretion of the attending surgeon when the diagnosis was not clear from the history and physical examination. All fascial sling procedures were performed at our institution by 1 of 3 fellowship-trained hand surgeons, including Dr. Rosenwasser. The adipose flap modification was performed only by Dr. Rosenwasser. Of the 27 patients in the adipose flap group, 23 underwent surgery for primary CuTS and were included in the study; the other 4 (revision cases) were excluded; 1 patient subsequently died of a cause unrelated to the surgical procedure, and 6 were lost to follow-up. Of the 80 patients in the fascial sling group, 30 underwent surgery for primary CuTS; 5 died before follow-up, and 8 declined to participate.
Thirty-three patients (16 adipose flap, 17 fascial sling) met the inclusion criteria. Of the 16 adipose flap patients, 15 underwent the physical examination and completed the questionnaire, and 1 was interviewed by telephone. Similarly, of the 17 fascial sling patients, 15 underwent the physical examination and completed the questionnaire, and 2 were interviewed by telephone. There were no bilateral cases. Conservative management (activity modification, night splinting, elbow pads, occupational therapy, home exercise) failed in all cases.
A trained study team member who was not part of the surgical team performed follow-up evaluations using objective outcome measures and subjective questionnaires. Patients were assessed at a mean follow-up of 5.6 years (range, 1.6-15.9 years). Patients completed the DASH (Disabilities of the Arm, Shoulder, and Hand) questionnaire16 and visual analog scales (VASs) for pain, numbness, tingling, and weakness in the ulnar nerve distribution. They also rated the presence of night symptoms that were interfering with sleep. The Modified Bishop Rating Scale (MBRS) was used to quantify patient self-reported data17,18 (Figure 2). The MBRS measures overall satisfaction, symptom improvement, presence of residual symptoms, ability to engage in activities, work capability, and subjective changes in strength and sensibility.
In the physical examinations, we tested for Tinel, Wartenberg, and Froment signs; performed an elbow flexion test; and measured elbow range of motion for flexion and extension as well as forearm pronation and supination. We also evaluated lateral pinch strength and grip strength, using a Jamar hydraulic pinch gauge and a Jamar dynamometer (Therapeutic Equipment Corp) and taking the average of 3 assessments. Fifth-digit abduction strength was graded on a standard muscle strength scale. Two-point discrimination was measured at the middle, ring, and small digits of the operated and contralateral hands.19
Surgical Technique
Standard ulnar nerve decompression with anterior subcutaneous transposition and the following modifications were performed on all patients.20 A posteromedial incision parallel to the intermuscular septum was developed and the ulnar nerve identified. Minimizing stripping of the vascular mesentery, the dissection continued along the course of the nerve, and the medial intermuscular septum was excised to prevent secondary compression after transposition. The ulnar nerve was mobilized and transposed anterior to the medial epicondyle (Figure 3). For patients who received the fascial sling, a fascial sleeve was elevated from the flexor-pronator mass and sutured to the edge of the retinaculum securing the nerve. For patients who received the adipose flap, the flap with its vascular pedicle intact was elevated from the subcutaneous tissue of the anterior skin overlying the transposed nerve. The adipose tissue was sharply dissected in half while sufficient subcutaneous tissue was kept between the skin and the flap. A plane was developed based on an anterior adipose pedicle, which included a cutaneous artery and a vein that would supply the vascularized adipose flap. The flap was elevated and wrapped around the nerve without tension while the ulnar nerve was protected from being kinked by the construct. The flap was sutured to the anterior subcutaneous tissue to create a tunnel of adipose tissue surrounding the nerve along its length (Figure 4). The elbow was then flexed and extended to ensure free nerve gliding before wound closure.
The patient was allowed to move the elbow within the bulky dressings immediately after surgery. After 2 weeks, sutures were removed. Formal occupational therapy is not needed for these patients, except in the presence of significant weakness.
Results
As mentioned, the 2 groups were matched on demographics: age at time of surgery, sex, symptom duration, and length of follow-up (Table 1).
For the 16 adipose flap patients (Table 2), mean DASH score was 19.9 (range, 0-71.7). Seven of these patients reported upper extremity pain with a mean VAS score of 1.7 (range, 0-8); 4 patients reported pain in the wrist and fourth and fifth digits; only 1 patient reported pain that occasionally woke the patient from sleep. Constant numbness was present in 6 patients. Four patients reported constant mild tingling in the hand, and 11 reported intermittent tingling. Eleven patients (68.7%) reported operated-arm weakness with a mean VAS score of 3.4 (range, 0-8). In patients who had a physical examination, mean elbow flexion–extension arc of motion was 134° (range, 95°-150°), representing 99% of the motion of the contralateral arm. Mean pronation–supination arc was 174° (range, 150°-180°), accounting for 104% of the contralateral arm. Mean lateral pinch strength was 73% of the contralateral arm, and mean grip strength was 114% of the contralateral arm. The Tinel sign was present in 2 patients, the Froment sign was present in 3 patients, and the elbow flexion test was positive in 2 patients. No patient had a positive Wartenberg sign. On the MBRS, 10 patients had an excellent score, and 6 had a good score.
For the 17 fascial sling patients (Table 2), mean DASH score was 22.7 (range, 0-63.3). Three patients reported upper extremity pain with a mean VAS score of 1.4 (range, 0-7); 3 patients reported pain that occasionally woke them from sleep. Seven patients had constant numbness in the distribution of the ulnar nerve. Two patients had constant paresthesias, and 7 had intermittent paresthesias. Nine patients (52.9%) reported arm weakness with a mean VAS score of 2.5 (range, 0-8). Mean elbow flexion–extension arc of motion was 136° (range, 100°-150°), representing 100% of the contralateral arm. Mean pronation–supination arc was 187° (range, 155°-225°), accounting for 102% of the contralateral arm. Mean lateral pinch strength was 93% of the contralateral arm, and mean grip strength was 80% of the contralateral arm. The Tinel sign was present in 6 patients, the Froment sign in 3 patients, and the Wartenberg sign in 2 patients. The elbow flexion test was positive in 4 patients. On the MBRS, 10 patients had an excellent score, and 7 had a good score.
There was no recurrence of CuTS in either group. One adipose flap patient developed a wound infection that required reoperation.
Discussion
Ulnar neuropathy was described by Magee and Phalen21 in 1949 and termed cubital tunnel syndrome by Feindel and Stratford22 in 1958. Since then, numerous procedures, including in situ decompression, medial epicondylectomy, and endoscopic decompression,23,24 have been advocated for the treatment of this condition. In addition, anterior transposition, which involves securing the ulnar nerve in a submuscular, intramuscular, or subcutaneous sleeve,6 remains a popular option. Despite more than half a century of surgical treatment for this condition, there is no consensus about which procedure offers the best outcomes. Bartels and colleagues8 retrospectively reviewed surgical treatments for CuTS, examining 3148 arms over a 27-year period. They found simple decompression and anterior intramuscular transposition had the best results, followed by medial epicondylectomy and anterior subcutaneous transposition, with anterior submuscular transposition yielding the poorest outcomes. Despite these findings, the operative groups’ recurrence rates remained significant. These results were challenged in a 2008 meta-analysis5 that found no significant difference among simple decompression, subcutaneous transposition, and submuscular transposition and instead demonstrated trends toward better outcomes with anterior transposition. Osterman and Davis7 reported a 5% to 15% rate of unsatisfactory outcomes with anterior subcutaneous transposition, a popular technique used by surgeons at our institution.
The causes for failure or recurrence of ulnar neuropathy after surgical intervention are multifactorial and include preexisting medical conditions and improper operative technique. It is well established that failure to excise all 5 anatomical points of entrapment, or creation of new points of tension during surgery, leads to poor outcomes.12 Nevertheless, the contribution of perineural scarring to postoperative recurrent ulnar neuropathy is currently being recognized: Gabel and Amadio13 described postoperative fibrosis in one-third of their patients with surgically treated recurrent CuTS, Rogers and colleagues14 noted dense perineural fibrosis after intramuscular and subcutaneous transposition procedures, Filippi and colleagues11 cited serious epineural fibrosis and fibrosis around the ulnar nerve as the main findings in their study of 22 patients with recurrent ulnar neuropathy, and Vogel and colleagues12 found that 88% of their patients with persistent CuTS after surgery exhibited perineural scarring.
We think that use of a scar tissue barrier during ulnar nerve transposition reduces the incidence of cicatrix and produces better outcomes—a position largely echoed by the orthopedic community, as fascial, fasciocutaneous, free, and venous flaps have all been used for such purposes.25,26 Vein wrapping has demonstrated good recovery of a nerve after perineural scarring.27 Advocates of intramuscular transposition argue that their technique provides the nerve with a vascularized tunnel, as segmental vascular stripping is an inevitability in transposition. However, this technique increases the incidence of scarring and potential muscle damage.28,29 We think the pedicled adipofascial flap benefits the peripheral nerve by providing a scar tissue barrier and an optimal milieu for vascular regeneration. Kilic and colleagues15 demonstrated the regenerative effects of adipose tissue flaps on peripheral nerves after crush injuries in a rat model, and Strickland and colleagues30 retrospectively examined the effects of hypothenar fat flaps on recalcitrant carpal tunnel syndrome, showing excellent results for this procedure. It is hypothesized that adipose tissue provides not only adipose-derived stem cells but also a rich vascular bed on which nerves will regenerate.
For all patients in the present study, symptoms improved, though the adipose flap and fascial sling groups were not significantly different in their outcomes. We used the MBRS to quantify and compare the groups’ patient-rated outcomes. No statistically significant difference was found between the adipose flap and fascial sling groups. On the MBRS, excellent and good outcomes were reported by 62.5% and 37.5% of the adipose flap patients, respectively, and 59% and 41% of the fascial sling patients (Table 3). Likewise, objective measurements did not show a significant difference between the 2 interventions—indicating that, compared with the current standard of care, adipose flaps are more efficacious in securing the anteriorly transposed nerve.
Complications of the adipose flap technique are consistent with those reported for other techniques for anterior transposition of the ulnar nerve. The most common complication is hematoma, which can be avoided with meticulous hemostasis. Damage of the medial antebrachial cutaneous nerve or motor branches to the flexor carpi ulnaris has been reported for the fascial technique (we have not had such outcomes at our institution). Contraindications to the adipofascial technique include insufficient subcutaneous adipose tissue for covering the ulnar nerve.
This study was limited by its retrospective setup, which reduced access to preoperative objective and subjective data. The small sample size also limited our ability to demonstrate the advantageous effects of an adipofascial flap in preventing postoperative perineural scarring.
The adipose flap technique is a viable option for securing the anteriorly transposed ulnar nerve. Outcomes in this study demonstrated an efficacy comparable to that of the fascial sling technique. Symptoms resolve or improve, and the majority of patients are satisfied with long-term surgical outcomes. The adipofascial flap may have additional advantages, as it provides a pliable, vascular fat envelope mimicking the natural fatty environment of peripheral nerves.
1. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77(2):263-265.
2. Robertson C, Saratsiotis J. A review of compression ulnar neuropathy at the elbow. J Manipulative Physiol Ther. 2005;28(5):345.
3. Posner MA. Compressive ulnar neuropathies at the elbow: I. Etiology and diagnosis. J Am Acad Orthop Surg. 1998;6(5):282-288.
4. Piligian G, Herbert R, Hearns M, Dropkin J, Landsbergis P, Cherniack M. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med. 2000;37(1):75-93.
5. Macadam SA, Gandhi R, Bezuhly M, Lefaivre KA. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. J Hand Surg Am. 2008;33(8):1314.e1-e12.
6. Soltani AM, Best MJ, Francis CS, Allan BJ, Panthaki ZJ. Trends in the surgical treatment of cubital tunnel syndrome: an analysis of the National Survey of Ambulatory Surgery database. J Hand Surg Am. 2013;38(8):1551-1556.
7. Osterman AL, Davis CA. Subcutaneous transposition of the ulnar nerve for treatment of cubital tunnel syndrome. Hand Clin. 1996;12(2):421-433.
8. Bartels RH, Menovsky T, Van Overbeeke JJ, Verhagen WI. Surgical management of ulnar nerve compression at the elbow: an analysis of the literature. J Neurosurg. 1998;89(5):722-727.
9. Seradge H, Owen W. Cubital tunnel release with medial epicondylectomy factors influencing the outcome. J Hand Surg Am. 1998;23(3):483-491.
10. Schnabl SM, Kisslinger F, Schramm A, et al. Subjective outcome, neurophysiological investigations, postoperative complications and recurrence rate of partial medial epicondylectomy in cubital tunnel syndrome. Arch Orthop Trauma Surg. 2011;131(8):1027-1033.
11. Filippi R, Charalampaki P, Reisch R, Koch D, Grunert P. Recurrent cubital tunnel syndrome. Etiology and treatment. Minim Invasive Neurosurg. 2001;44(4):197-201.
12. Vogel RB, Nossaman BC, Rayan GM. Revision anterior submuscular transposition of the ulnar nerve for failed subcutaneous transposition. Br J Plast Surg. 2004;57(4):311-316.
13. Gabel GT, Amadio PC. Reoperation for failed decompression of the ulnar nerve in the region of the elbow. J Bone Joint Surg Am. 1990;72(2):213-219.
14. Rogers MR, Bergfield TG, Aulicino PL. The failed ulnar nerve transposition. Etiology and treatment. Clin Orthop. 1991;269:193-200.
15. Kilic A, Ojo B, Rajfer RA, et al. Effect of white adipose tissue flap and insulin-like growth factor-1 on nerve regeneration in rats. Microsurgery. 2013;33(5):367-375.
16. Ebersole GC, Davidge K, Damiano M, Mackinnon SE. Validity and responsiveness of the DASH questionnaire as an outcome measure following ulnar nerve transposition for cubital tunnel syndrome. Plast Reconstr Surg. 2013;132(1):81e-90e.
17. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
18. Dützmann S, Martin KD, Sobottka S, et al. Open vs retractor-endoscopic in situ decompression of the ulnar nerve in cubital tunnel syndrome: a retrospective cohort study. Neurosurgery. 2013;72(4):605-616.
19. Dellon AL, Mackinnon SE, Crosby PM. Reliability of two-point discrimination measurements. J Hand Surg Am. 1987;12(5 pt 1):693-696.
20. Danoff JR, Lombardi JM, Rosenwasser MP. Use of a pedicled adipose flap as a sling for anterior subcutaneous transposition of the ulnar nerve. J Hand Surg Am. 2014;39(3):552-555.
21. Magee RB, Phalen GS. Tardy ulnar palsy. Am J Surg. 1949;78(4):470-474.
22. Feindel W, Stratford J. Cubital tunnel compression in tardy ulnar palsy. Can Med Assoc J. 1958;78(5):351-353.
23. Tsai TM, Bonczar M, Tsuruta T, Syed SA. A new operative technique: cubital tunnel decompression with endoscopic assistance. Hand Clin. 1995;11(1):71-80.
24. Hoffmann R, Siemionow M. The endoscopic management of cubital tunnel syndrome. J Hand Surg Br. 2006;31(1):23-29.
25. Luchetti R, Riccio M, Papini Zorli I, Fairplay T. Protective coverage of the median nerve using fascial, fasciocutaneous or island flaps. Handchir Mikrochir Plast Chir. 2006;38(5):317-330.
26. Kokkalis ZT, Jain S, Sotereanos DG. Vein wrapping at cubital tunnel for ulnar nerve problems. J Shoulder Elbow Surg. 2010;19(2):91-97.
27. Masear VR, Colgin S. The treatment of epineural scarring with allograft vein wrapping. Hand Clin. 1996;12(4):773-779.
28. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
29. Lundborg G. Surgical treatment for ulnar nerve entrapment at the elbow. J Hand Surg Br. 1992;17(3):245-247.
30. Strickland JW, Idler RS, Lourie GM, Plancher KD. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J Hand Surg Am. 1996;21(5):840-848.
Compression of the ulnar nerve at the elbow, also referred to as cubital tunnel syndrome (CuTS), is the second most common peripheral nerve compression syndrome in the upper extremity.1,2 Although the ulnar nerve can be compressed at 5 different sites, including arcade of Struthers, medial intermuscular septum, medial epicondyle, and deep flexor aponeurosis, the cubital tunnel is most commonly affected.3 Patients typically present with paresthesias in the fourth and fifth digits and weakness of hand muscle intrinsics. Activity-related pain or pain at the medial elbow can also occur in more advanced pathology.4 It is estimated that conservative therapy fails and surgical intervention is required in up to 30% of patients with CuTS.1 Surgical approaches range from in situ decompression to transposition techniques, but there is no consensus in the orthopedic community as to which technique offers the best results. In a 2008 meta-analysis, Macadam and colleagues5 found no statistical differences in outcomes among the various surgical approaches. Nevertheless, subcutaneous transposition of the ulnar nerve at the elbow is a popular option.6
Despite the widespread success of surgical intervention for CuTS, persistent or recurrent pain occurs in 9.9% to 21.0% of cases.7-10 In addition, several investigators have cited perineural scarring as a major cause of recurrent symptoms after primary surgery.11-14 Filippi and colleagues11 noted that patients who required reoperation after primary anterior transposition had “serious epineural fibrosis and fibrosis around the transposed ulnar nerve.” At our institution, we have similarly found that scarring of the fascial sling around the ulnar nerve led to recurrence of CuTS within 4 months after initial surgery (Figure 1).
We therefore prefer to use a vascularized adipose flap to secure the anteriorly transposed ulnar nerve. This flap provides a pliable, vascularized adipose environment for the nerve, which helps reduce nerve adherence and may enhance nerve recovery.15 In the study reported here, we retrospectively reviewed the long-term outcomes of ulnar nerve anterior subcutaneous transposition secured with either an adipose flap or a fascial sling. We hypothesized that patients in the 2 groups (adipose flap, fascial sling) would have equivalent outcomes.
Materials and Methods
After obtaining institutional review board approval, we reviewed the medical and surgical records of 104 patients (107 limbs) who underwent transposition of the ulnar nerve secured with either an adipose flap (27 limbs) or a fascial sling (80 limbs) over a 14-year period. The fascial sling cohort was used as a comparison group, matched to the adipose flap cohort by sex, age at time of surgery, hand dominance, symptom duration, and length of follow-up (Table 1). Patients were indicated for surgery and were included in the study if they had a history and physical examination consistent with primary CuTS, symptom duration longer than 1 year, and failed conservative management, including activity modification, night splinting, elbow pads, occupational therapy, and home exercise regimen. Electrodiagnostic testing was used at the discretion of the attending surgeon when the diagnosis was not clear from the history and physical examination. All fascial sling procedures were performed at our institution by 1 of 3 fellowship-trained hand surgeons, including Dr. Rosenwasser. The adipose flap modification was performed only by Dr. Rosenwasser. Of the 27 patients in the adipose flap group, 23 underwent surgery for primary CuTS and were included in the study; the other 4 (revision cases) were excluded; 1 patient subsequently died of a cause unrelated to the surgical procedure, and 6 were lost to follow-up. Of the 80 patients in the fascial sling group, 30 underwent surgery for primary CuTS; 5 died before follow-up, and 8 declined to participate.
Thirty-three patients (16 adipose flap, 17 fascial sling) met the inclusion criteria. Of the 16 adipose flap patients, 15 underwent the physical examination and completed the questionnaire, and 1 was interviewed by telephone. Similarly, of the 17 fascial sling patients, 15 underwent the physical examination and completed the questionnaire, and 2 were interviewed by telephone. There were no bilateral cases. Conservative management (activity modification, night splinting, elbow pads, occupational therapy, home exercise) failed in all cases.
A trained study team member who was not part of the surgical team performed follow-up evaluations using objective outcome measures and subjective questionnaires. Patients were assessed at a mean follow-up of 5.6 years (range, 1.6-15.9 years). Patients completed the DASH (Disabilities of the Arm, Shoulder, and Hand) questionnaire16 and visual analog scales (VASs) for pain, numbness, tingling, and weakness in the ulnar nerve distribution. They also rated the presence of night symptoms that were interfering with sleep. The Modified Bishop Rating Scale (MBRS) was used to quantify patient self-reported data17,18 (Figure 2). The MBRS measures overall satisfaction, symptom improvement, presence of residual symptoms, ability to engage in activities, work capability, and subjective changes in strength and sensibility.
In the physical examinations, we tested for Tinel, Wartenberg, and Froment signs; performed an elbow flexion test; and measured elbow range of motion for flexion and extension as well as forearm pronation and supination. We also evaluated lateral pinch strength and grip strength, using a Jamar hydraulic pinch gauge and a Jamar dynamometer (Therapeutic Equipment Corp) and taking the average of 3 assessments. Fifth-digit abduction strength was graded on a standard muscle strength scale. Two-point discrimination was measured at the middle, ring, and small digits of the operated and contralateral hands.19
Surgical Technique
Standard ulnar nerve decompression with anterior subcutaneous transposition and the following modifications were performed on all patients.20 A posteromedial incision parallel to the intermuscular septum was developed and the ulnar nerve identified. Minimizing stripping of the vascular mesentery, the dissection continued along the course of the nerve, and the medial intermuscular septum was excised to prevent secondary compression after transposition. The ulnar nerve was mobilized and transposed anterior to the medial epicondyle (Figure 3). For patients who received the fascial sling, a fascial sleeve was elevated from the flexor-pronator mass and sutured to the edge of the retinaculum securing the nerve. For patients who received the adipose flap, the flap with its vascular pedicle intact was elevated from the subcutaneous tissue of the anterior skin overlying the transposed nerve. The adipose tissue was sharply dissected in half while sufficient subcutaneous tissue was kept between the skin and the flap. A plane was developed based on an anterior adipose pedicle, which included a cutaneous artery and a vein that would supply the vascularized adipose flap. The flap was elevated and wrapped around the nerve without tension while the ulnar nerve was protected from being kinked by the construct. The flap was sutured to the anterior subcutaneous tissue to create a tunnel of adipose tissue surrounding the nerve along its length (Figure 4). The elbow was then flexed and extended to ensure free nerve gliding before wound closure.
The patient was allowed to move the elbow within the bulky dressings immediately after surgery. After 2 weeks, sutures were removed. Formal occupational therapy is not needed for these patients, except in the presence of significant weakness.
Results
As mentioned, the 2 groups were matched on demographics: age at time of surgery, sex, symptom duration, and length of follow-up (Table 1).
For the 16 adipose flap patients (Table 2), mean DASH score was 19.9 (range, 0-71.7). Seven of these patients reported upper extremity pain with a mean VAS score of 1.7 (range, 0-8); 4 patients reported pain in the wrist and fourth and fifth digits; only 1 patient reported pain that occasionally woke the patient from sleep. Constant numbness was present in 6 patients. Four patients reported constant mild tingling in the hand, and 11 reported intermittent tingling. Eleven patients (68.7%) reported operated-arm weakness with a mean VAS score of 3.4 (range, 0-8). In patients who had a physical examination, mean elbow flexion–extension arc of motion was 134° (range, 95°-150°), representing 99% of the motion of the contralateral arm. Mean pronation–supination arc was 174° (range, 150°-180°), accounting for 104% of the contralateral arm. Mean lateral pinch strength was 73% of the contralateral arm, and mean grip strength was 114% of the contralateral arm. The Tinel sign was present in 2 patients, the Froment sign was present in 3 patients, and the elbow flexion test was positive in 2 patients. No patient had a positive Wartenberg sign. On the MBRS, 10 patients had an excellent score, and 6 had a good score.
For the 17 fascial sling patients (Table 2), mean DASH score was 22.7 (range, 0-63.3). Three patients reported upper extremity pain with a mean VAS score of 1.4 (range, 0-7); 3 patients reported pain that occasionally woke them from sleep. Seven patients had constant numbness in the distribution of the ulnar nerve. Two patients had constant paresthesias, and 7 had intermittent paresthesias. Nine patients (52.9%) reported arm weakness with a mean VAS score of 2.5 (range, 0-8). Mean elbow flexion–extension arc of motion was 136° (range, 100°-150°), representing 100% of the contralateral arm. Mean pronation–supination arc was 187° (range, 155°-225°), accounting for 102% of the contralateral arm. Mean lateral pinch strength was 93% of the contralateral arm, and mean grip strength was 80% of the contralateral arm. The Tinel sign was present in 6 patients, the Froment sign in 3 patients, and the Wartenberg sign in 2 patients. The elbow flexion test was positive in 4 patients. On the MBRS, 10 patients had an excellent score, and 7 had a good score.
There was no recurrence of CuTS in either group. One adipose flap patient developed a wound infection that required reoperation.
Discussion
Ulnar neuropathy was described by Magee and Phalen21 in 1949 and termed cubital tunnel syndrome by Feindel and Stratford22 in 1958. Since then, numerous procedures, including in situ decompression, medial epicondylectomy, and endoscopic decompression,23,24 have been advocated for the treatment of this condition. In addition, anterior transposition, which involves securing the ulnar nerve in a submuscular, intramuscular, or subcutaneous sleeve,6 remains a popular option. Despite more than half a century of surgical treatment for this condition, there is no consensus about which procedure offers the best outcomes. Bartels and colleagues8 retrospectively reviewed surgical treatments for CuTS, examining 3148 arms over a 27-year period. They found simple decompression and anterior intramuscular transposition had the best results, followed by medial epicondylectomy and anterior subcutaneous transposition, with anterior submuscular transposition yielding the poorest outcomes. Despite these findings, the operative groups’ recurrence rates remained significant. These results were challenged in a 2008 meta-analysis5 that found no significant difference among simple decompression, subcutaneous transposition, and submuscular transposition and instead demonstrated trends toward better outcomes with anterior transposition. Osterman and Davis7 reported a 5% to 15% rate of unsatisfactory outcomes with anterior subcutaneous transposition, a popular technique used by surgeons at our institution.
The causes for failure or recurrence of ulnar neuropathy after surgical intervention are multifactorial and include preexisting medical conditions and improper operative technique. It is well established that failure to excise all 5 anatomical points of entrapment, or creation of new points of tension during surgery, leads to poor outcomes.12 Nevertheless, the contribution of perineural scarring to postoperative recurrent ulnar neuropathy is currently being recognized: Gabel and Amadio13 described postoperative fibrosis in one-third of their patients with surgically treated recurrent CuTS, Rogers and colleagues14 noted dense perineural fibrosis after intramuscular and subcutaneous transposition procedures, Filippi and colleagues11 cited serious epineural fibrosis and fibrosis around the ulnar nerve as the main findings in their study of 22 patients with recurrent ulnar neuropathy, and Vogel and colleagues12 found that 88% of their patients with persistent CuTS after surgery exhibited perineural scarring.
We think that use of a scar tissue barrier during ulnar nerve transposition reduces the incidence of cicatrix and produces better outcomes—a position largely echoed by the orthopedic community, as fascial, fasciocutaneous, free, and venous flaps have all been used for such purposes.25,26 Vein wrapping has demonstrated good recovery of a nerve after perineural scarring.27 Advocates of intramuscular transposition argue that their technique provides the nerve with a vascularized tunnel, as segmental vascular stripping is an inevitability in transposition. However, this technique increases the incidence of scarring and potential muscle damage.28,29 We think the pedicled adipofascial flap benefits the peripheral nerve by providing a scar tissue barrier and an optimal milieu for vascular regeneration. Kilic and colleagues15 demonstrated the regenerative effects of adipose tissue flaps on peripheral nerves after crush injuries in a rat model, and Strickland and colleagues30 retrospectively examined the effects of hypothenar fat flaps on recalcitrant carpal tunnel syndrome, showing excellent results for this procedure. It is hypothesized that adipose tissue provides not only adipose-derived stem cells but also a rich vascular bed on which nerves will regenerate.
For all patients in the present study, symptoms improved, though the adipose flap and fascial sling groups were not significantly different in their outcomes. We used the MBRS to quantify and compare the groups’ patient-rated outcomes. No statistically significant difference was found between the adipose flap and fascial sling groups. On the MBRS, excellent and good outcomes were reported by 62.5% and 37.5% of the adipose flap patients, respectively, and 59% and 41% of the fascial sling patients (Table 3). Likewise, objective measurements did not show a significant difference between the 2 interventions—indicating that, compared with the current standard of care, adipose flaps are more efficacious in securing the anteriorly transposed nerve.
Complications of the adipose flap technique are consistent with those reported for other techniques for anterior transposition of the ulnar nerve. The most common complication is hematoma, which can be avoided with meticulous hemostasis. Damage of the medial antebrachial cutaneous nerve or motor branches to the flexor carpi ulnaris has been reported for the fascial technique (we have not had such outcomes at our institution). Contraindications to the adipofascial technique include insufficient subcutaneous adipose tissue for covering the ulnar nerve.
This study was limited by its retrospective setup, which reduced access to preoperative objective and subjective data. The small sample size also limited our ability to demonstrate the advantageous effects of an adipofascial flap in preventing postoperative perineural scarring.
The adipose flap technique is a viable option for securing the anteriorly transposed ulnar nerve. Outcomes in this study demonstrated an efficacy comparable to that of the fascial sling technique. Symptoms resolve or improve, and the majority of patients are satisfied with long-term surgical outcomes. The adipofascial flap may have additional advantages, as it provides a pliable, vascular fat envelope mimicking the natural fatty environment of peripheral nerves.
Compression of the ulnar nerve at the elbow, also referred to as cubital tunnel syndrome (CuTS), is the second most common peripheral nerve compression syndrome in the upper extremity.1,2 Although the ulnar nerve can be compressed at 5 different sites, including arcade of Struthers, medial intermuscular septum, medial epicondyle, and deep flexor aponeurosis, the cubital tunnel is most commonly affected.3 Patients typically present with paresthesias in the fourth and fifth digits and weakness of hand muscle intrinsics. Activity-related pain or pain at the medial elbow can also occur in more advanced pathology.4 It is estimated that conservative therapy fails and surgical intervention is required in up to 30% of patients with CuTS.1 Surgical approaches range from in situ decompression to transposition techniques, but there is no consensus in the orthopedic community as to which technique offers the best results. In a 2008 meta-analysis, Macadam and colleagues5 found no statistical differences in outcomes among the various surgical approaches. Nevertheless, subcutaneous transposition of the ulnar nerve at the elbow is a popular option.6
Despite the widespread success of surgical intervention for CuTS, persistent or recurrent pain occurs in 9.9% to 21.0% of cases.7-10 In addition, several investigators have cited perineural scarring as a major cause of recurrent symptoms after primary surgery.11-14 Filippi and colleagues11 noted that patients who required reoperation after primary anterior transposition had “serious epineural fibrosis and fibrosis around the transposed ulnar nerve.” At our institution, we have similarly found that scarring of the fascial sling around the ulnar nerve led to recurrence of CuTS within 4 months after initial surgery (Figure 1).
We therefore prefer to use a vascularized adipose flap to secure the anteriorly transposed ulnar nerve. This flap provides a pliable, vascularized adipose environment for the nerve, which helps reduce nerve adherence and may enhance nerve recovery.15 In the study reported here, we retrospectively reviewed the long-term outcomes of ulnar nerve anterior subcutaneous transposition secured with either an adipose flap or a fascial sling. We hypothesized that patients in the 2 groups (adipose flap, fascial sling) would have equivalent outcomes.
Materials and Methods
After obtaining institutional review board approval, we reviewed the medical and surgical records of 104 patients (107 limbs) who underwent transposition of the ulnar nerve secured with either an adipose flap (27 limbs) or a fascial sling (80 limbs) over a 14-year period. The fascial sling cohort was used as a comparison group, matched to the adipose flap cohort by sex, age at time of surgery, hand dominance, symptom duration, and length of follow-up (Table 1). Patients were indicated for surgery and were included in the study if they had a history and physical examination consistent with primary CuTS, symptom duration longer than 1 year, and failed conservative management, including activity modification, night splinting, elbow pads, occupational therapy, and home exercise regimen. Electrodiagnostic testing was used at the discretion of the attending surgeon when the diagnosis was not clear from the history and physical examination. All fascial sling procedures were performed at our institution by 1 of 3 fellowship-trained hand surgeons, including Dr. Rosenwasser. The adipose flap modification was performed only by Dr. Rosenwasser. Of the 27 patients in the adipose flap group, 23 underwent surgery for primary CuTS and were included in the study; the other 4 (revision cases) were excluded; 1 patient subsequently died of a cause unrelated to the surgical procedure, and 6 were lost to follow-up. Of the 80 patients in the fascial sling group, 30 underwent surgery for primary CuTS; 5 died before follow-up, and 8 declined to participate.
Thirty-three patients (16 adipose flap, 17 fascial sling) met the inclusion criteria. Of the 16 adipose flap patients, 15 underwent the physical examination and completed the questionnaire, and 1 was interviewed by telephone. Similarly, of the 17 fascial sling patients, 15 underwent the physical examination and completed the questionnaire, and 2 were interviewed by telephone. There were no bilateral cases. Conservative management (activity modification, night splinting, elbow pads, occupational therapy, home exercise) failed in all cases.
A trained study team member who was not part of the surgical team performed follow-up evaluations using objective outcome measures and subjective questionnaires. Patients were assessed at a mean follow-up of 5.6 years (range, 1.6-15.9 years). Patients completed the DASH (Disabilities of the Arm, Shoulder, and Hand) questionnaire16 and visual analog scales (VASs) for pain, numbness, tingling, and weakness in the ulnar nerve distribution. They also rated the presence of night symptoms that were interfering with sleep. The Modified Bishop Rating Scale (MBRS) was used to quantify patient self-reported data17,18 (Figure 2). The MBRS measures overall satisfaction, symptom improvement, presence of residual symptoms, ability to engage in activities, work capability, and subjective changes in strength and sensibility.
In the physical examinations, we tested for Tinel, Wartenberg, and Froment signs; performed an elbow flexion test; and measured elbow range of motion for flexion and extension as well as forearm pronation and supination. We also evaluated lateral pinch strength and grip strength, using a Jamar hydraulic pinch gauge and a Jamar dynamometer (Therapeutic Equipment Corp) and taking the average of 3 assessments. Fifth-digit abduction strength was graded on a standard muscle strength scale. Two-point discrimination was measured at the middle, ring, and small digits of the operated and contralateral hands.19
Surgical Technique
Standard ulnar nerve decompression with anterior subcutaneous transposition and the following modifications were performed on all patients.20 A posteromedial incision parallel to the intermuscular septum was developed and the ulnar nerve identified. Minimizing stripping of the vascular mesentery, the dissection continued along the course of the nerve, and the medial intermuscular septum was excised to prevent secondary compression after transposition. The ulnar nerve was mobilized and transposed anterior to the medial epicondyle (Figure 3). For patients who received the fascial sling, a fascial sleeve was elevated from the flexor-pronator mass and sutured to the edge of the retinaculum securing the nerve. For patients who received the adipose flap, the flap with its vascular pedicle intact was elevated from the subcutaneous tissue of the anterior skin overlying the transposed nerve. The adipose tissue was sharply dissected in half while sufficient subcutaneous tissue was kept between the skin and the flap. A plane was developed based on an anterior adipose pedicle, which included a cutaneous artery and a vein that would supply the vascularized adipose flap. The flap was elevated and wrapped around the nerve without tension while the ulnar nerve was protected from being kinked by the construct. The flap was sutured to the anterior subcutaneous tissue to create a tunnel of adipose tissue surrounding the nerve along its length (Figure 4). The elbow was then flexed and extended to ensure free nerve gliding before wound closure.
The patient was allowed to move the elbow within the bulky dressings immediately after surgery. After 2 weeks, sutures were removed. Formal occupational therapy is not needed for these patients, except in the presence of significant weakness.
Results
As mentioned, the 2 groups were matched on demographics: age at time of surgery, sex, symptom duration, and length of follow-up (Table 1).
For the 16 adipose flap patients (Table 2), mean DASH score was 19.9 (range, 0-71.7). Seven of these patients reported upper extremity pain with a mean VAS score of 1.7 (range, 0-8); 4 patients reported pain in the wrist and fourth and fifth digits; only 1 patient reported pain that occasionally woke the patient from sleep. Constant numbness was present in 6 patients. Four patients reported constant mild tingling in the hand, and 11 reported intermittent tingling. Eleven patients (68.7%) reported operated-arm weakness with a mean VAS score of 3.4 (range, 0-8). In patients who had a physical examination, mean elbow flexion–extension arc of motion was 134° (range, 95°-150°), representing 99% of the motion of the contralateral arm. Mean pronation–supination arc was 174° (range, 150°-180°), accounting for 104% of the contralateral arm. Mean lateral pinch strength was 73% of the contralateral arm, and mean grip strength was 114% of the contralateral arm. The Tinel sign was present in 2 patients, the Froment sign was present in 3 patients, and the elbow flexion test was positive in 2 patients. No patient had a positive Wartenberg sign. On the MBRS, 10 patients had an excellent score, and 6 had a good score.
For the 17 fascial sling patients (Table 2), mean DASH score was 22.7 (range, 0-63.3). Three patients reported upper extremity pain with a mean VAS score of 1.4 (range, 0-7); 3 patients reported pain that occasionally woke them from sleep. Seven patients had constant numbness in the distribution of the ulnar nerve. Two patients had constant paresthesias, and 7 had intermittent paresthesias. Nine patients (52.9%) reported arm weakness with a mean VAS score of 2.5 (range, 0-8). Mean elbow flexion–extension arc of motion was 136° (range, 100°-150°), representing 100% of the contralateral arm. Mean pronation–supination arc was 187° (range, 155°-225°), accounting for 102% of the contralateral arm. Mean lateral pinch strength was 93% of the contralateral arm, and mean grip strength was 80% of the contralateral arm. The Tinel sign was present in 6 patients, the Froment sign in 3 patients, and the Wartenberg sign in 2 patients. The elbow flexion test was positive in 4 patients. On the MBRS, 10 patients had an excellent score, and 7 had a good score.
There was no recurrence of CuTS in either group. One adipose flap patient developed a wound infection that required reoperation.
Discussion
Ulnar neuropathy was described by Magee and Phalen21 in 1949 and termed cubital tunnel syndrome by Feindel and Stratford22 in 1958. Since then, numerous procedures, including in situ decompression, medial epicondylectomy, and endoscopic decompression,23,24 have been advocated for the treatment of this condition. In addition, anterior transposition, which involves securing the ulnar nerve in a submuscular, intramuscular, or subcutaneous sleeve,6 remains a popular option. Despite more than half a century of surgical treatment for this condition, there is no consensus about which procedure offers the best outcomes. Bartels and colleagues8 retrospectively reviewed surgical treatments for CuTS, examining 3148 arms over a 27-year period. They found simple decompression and anterior intramuscular transposition had the best results, followed by medial epicondylectomy and anterior subcutaneous transposition, with anterior submuscular transposition yielding the poorest outcomes. Despite these findings, the operative groups’ recurrence rates remained significant. These results were challenged in a 2008 meta-analysis5 that found no significant difference among simple decompression, subcutaneous transposition, and submuscular transposition and instead demonstrated trends toward better outcomes with anterior transposition. Osterman and Davis7 reported a 5% to 15% rate of unsatisfactory outcomes with anterior subcutaneous transposition, a popular technique used by surgeons at our institution.
The causes for failure or recurrence of ulnar neuropathy after surgical intervention are multifactorial and include preexisting medical conditions and improper operative technique. It is well established that failure to excise all 5 anatomical points of entrapment, or creation of new points of tension during surgery, leads to poor outcomes.12 Nevertheless, the contribution of perineural scarring to postoperative recurrent ulnar neuropathy is currently being recognized: Gabel and Amadio13 described postoperative fibrosis in one-third of their patients with surgically treated recurrent CuTS, Rogers and colleagues14 noted dense perineural fibrosis after intramuscular and subcutaneous transposition procedures, Filippi and colleagues11 cited serious epineural fibrosis and fibrosis around the ulnar nerve as the main findings in their study of 22 patients with recurrent ulnar neuropathy, and Vogel and colleagues12 found that 88% of their patients with persistent CuTS after surgery exhibited perineural scarring.
We think that use of a scar tissue barrier during ulnar nerve transposition reduces the incidence of cicatrix and produces better outcomes—a position largely echoed by the orthopedic community, as fascial, fasciocutaneous, free, and venous flaps have all been used for such purposes.25,26 Vein wrapping has demonstrated good recovery of a nerve after perineural scarring.27 Advocates of intramuscular transposition argue that their technique provides the nerve with a vascularized tunnel, as segmental vascular stripping is an inevitability in transposition. However, this technique increases the incidence of scarring and potential muscle damage.28,29 We think the pedicled adipofascial flap benefits the peripheral nerve by providing a scar tissue barrier and an optimal milieu for vascular regeneration. Kilic and colleagues15 demonstrated the regenerative effects of adipose tissue flaps on peripheral nerves after crush injuries in a rat model, and Strickland and colleagues30 retrospectively examined the effects of hypothenar fat flaps on recalcitrant carpal tunnel syndrome, showing excellent results for this procedure. It is hypothesized that adipose tissue provides not only adipose-derived stem cells but also a rich vascular bed on which nerves will regenerate.
For all patients in the present study, symptoms improved, though the adipose flap and fascial sling groups were not significantly different in their outcomes. We used the MBRS to quantify and compare the groups’ patient-rated outcomes. No statistically significant difference was found between the adipose flap and fascial sling groups. On the MBRS, excellent and good outcomes were reported by 62.5% and 37.5% of the adipose flap patients, respectively, and 59% and 41% of the fascial sling patients (Table 3). Likewise, objective measurements did not show a significant difference between the 2 interventions—indicating that, compared with the current standard of care, adipose flaps are more efficacious in securing the anteriorly transposed nerve.
Complications of the adipose flap technique are consistent with those reported for other techniques for anterior transposition of the ulnar nerve. The most common complication is hematoma, which can be avoided with meticulous hemostasis. Damage of the medial antebrachial cutaneous nerve or motor branches to the flexor carpi ulnaris has been reported for the fascial technique (we have not had such outcomes at our institution). Contraindications to the adipofascial technique include insufficient subcutaneous adipose tissue for covering the ulnar nerve.
This study was limited by its retrospective setup, which reduced access to preoperative objective and subjective data. The small sample size also limited our ability to demonstrate the advantageous effects of an adipofascial flap in preventing postoperative perineural scarring.
The adipose flap technique is a viable option for securing the anteriorly transposed ulnar nerve. Outcomes in this study demonstrated an efficacy comparable to that of the fascial sling technique. Symptoms resolve or improve, and the majority of patients are satisfied with long-term surgical outcomes. The adipofascial flap may have additional advantages, as it provides a pliable, vascular fat envelope mimicking the natural fatty environment of peripheral nerves.
1. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77(2):263-265.
2. Robertson C, Saratsiotis J. A review of compression ulnar neuropathy at the elbow. J Manipulative Physiol Ther. 2005;28(5):345.
3. Posner MA. Compressive ulnar neuropathies at the elbow: I. Etiology and diagnosis. J Am Acad Orthop Surg. 1998;6(5):282-288.
4. Piligian G, Herbert R, Hearns M, Dropkin J, Landsbergis P, Cherniack M. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med. 2000;37(1):75-93.
5. Macadam SA, Gandhi R, Bezuhly M, Lefaivre KA. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. J Hand Surg Am. 2008;33(8):1314.e1-e12.
6. Soltani AM, Best MJ, Francis CS, Allan BJ, Panthaki ZJ. Trends in the surgical treatment of cubital tunnel syndrome: an analysis of the National Survey of Ambulatory Surgery database. J Hand Surg Am. 2013;38(8):1551-1556.
7. Osterman AL, Davis CA. Subcutaneous transposition of the ulnar nerve for treatment of cubital tunnel syndrome. Hand Clin. 1996;12(2):421-433.
8. Bartels RH, Menovsky T, Van Overbeeke JJ, Verhagen WI. Surgical management of ulnar nerve compression at the elbow: an analysis of the literature. J Neurosurg. 1998;89(5):722-727.
9. Seradge H, Owen W. Cubital tunnel release with medial epicondylectomy factors influencing the outcome. J Hand Surg Am. 1998;23(3):483-491.
10. Schnabl SM, Kisslinger F, Schramm A, et al. Subjective outcome, neurophysiological investigations, postoperative complications and recurrence rate of partial medial epicondylectomy in cubital tunnel syndrome. Arch Orthop Trauma Surg. 2011;131(8):1027-1033.
11. Filippi R, Charalampaki P, Reisch R, Koch D, Grunert P. Recurrent cubital tunnel syndrome. Etiology and treatment. Minim Invasive Neurosurg. 2001;44(4):197-201.
12. Vogel RB, Nossaman BC, Rayan GM. Revision anterior submuscular transposition of the ulnar nerve for failed subcutaneous transposition. Br J Plast Surg. 2004;57(4):311-316.
13. Gabel GT, Amadio PC. Reoperation for failed decompression of the ulnar nerve in the region of the elbow. J Bone Joint Surg Am. 1990;72(2):213-219.
14. Rogers MR, Bergfield TG, Aulicino PL. The failed ulnar nerve transposition. Etiology and treatment. Clin Orthop. 1991;269:193-200.
15. Kilic A, Ojo B, Rajfer RA, et al. Effect of white adipose tissue flap and insulin-like growth factor-1 on nerve regeneration in rats. Microsurgery. 2013;33(5):367-375.
16. Ebersole GC, Davidge K, Damiano M, Mackinnon SE. Validity and responsiveness of the DASH questionnaire as an outcome measure following ulnar nerve transposition for cubital tunnel syndrome. Plast Reconstr Surg. 2013;132(1):81e-90e.
17. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
18. Dützmann S, Martin KD, Sobottka S, et al. Open vs retractor-endoscopic in situ decompression of the ulnar nerve in cubital tunnel syndrome: a retrospective cohort study. Neurosurgery. 2013;72(4):605-616.
19. Dellon AL, Mackinnon SE, Crosby PM. Reliability of two-point discrimination measurements. J Hand Surg Am. 1987;12(5 pt 1):693-696.
20. Danoff JR, Lombardi JM, Rosenwasser MP. Use of a pedicled adipose flap as a sling for anterior subcutaneous transposition of the ulnar nerve. J Hand Surg Am. 2014;39(3):552-555.
21. Magee RB, Phalen GS. Tardy ulnar palsy. Am J Surg. 1949;78(4):470-474.
22. Feindel W, Stratford J. Cubital tunnel compression in tardy ulnar palsy. Can Med Assoc J. 1958;78(5):351-353.
23. Tsai TM, Bonczar M, Tsuruta T, Syed SA. A new operative technique: cubital tunnel decompression with endoscopic assistance. Hand Clin. 1995;11(1):71-80.
24. Hoffmann R, Siemionow M. The endoscopic management of cubital tunnel syndrome. J Hand Surg Br. 2006;31(1):23-29.
25. Luchetti R, Riccio M, Papini Zorli I, Fairplay T. Protective coverage of the median nerve using fascial, fasciocutaneous or island flaps. Handchir Mikrochir Plast Chir. 2006;38(5):317-330.
26. Kokkalis ZT, Jain S, Sotereanos DG. Vein wrapping at cubital tunnel for ulnar nerve problems. J Shoulder Elbow Surg. 2010;19(2):91-97.
27. Masear VR, Colgin S. The treatment of epineural scarring with allograft vein wrapping. Hand Clin. 1996;12(4):773-779.
28. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
29. Lundborg G. Surgical treatment for ulnar nerve entrapment at the elbow. J Hand Surg Br. 1992;17(3):245-247.
30. Strickland JW, Idler RS, Lourie GM, Plancher KD. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J Hand Surg Am. 1996;21(5):840-848.
1. Latinovic R, Gulliford MC, Hughes RA. Incidence of common compressive neuropathies in primary care. J Neurol Neurosurg Psychiatry. 2006;77(2):263-265.
2. Robertson C, Saratsiotis J. A review of compression ulnar neuropathy at the elbow. J Manipulative Physiol Ther. 2005;28(5):345.
3. Posner MA. Compressive ulnar neuropathies at the elbow: I. Etiology and diagnosis. J Am Acad Orthop Surg. 1998;6(5):282-288.
4. Piligian G, Herbert R, Hearns M, Dropkin J, Landsbergis P, Cherniack M. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am J Ind Med. 2000;37(1):75-93.
5. Macadam SA, Gandhi R, Bezuhly M, Lefaivre KA. Simple decompression versus anterior subcutaneous and submuscular transposition of the ulnar nerve for cubital tunnel syndrome: a meta-analysis. J Hand Surg Am. 2008;33(8):1314.e1-e12.
6. Soltani AM, Best MJ, Francis CS, Allan BJ, Panthaki ZJ. Trends in the surgical treatment of cubital tunnel syndrome: an analysis of the National Survey of Ambulatory Surgery database. J Hand Surg Am. 2013;38(8):1551-1556.
7. Osterman AL, Davis CA. Subcutaneous transposition of the ulnar nerve for treatment of cubital tunnel syndrome. Hand Clin. 1996;12(2):421-433.
8. Bartels RH, Menovsky T, Van Overbeeke JJ, Verhagen WI. Surgical management of ulnar nerve compression at the elbow: an analysis of the literature. J Neurosurg. 1998;89(5):722-727.
9. Seradge H, Owen W. Cubital tunnel release with medial epicondylectomy factors influencing the outcome. J Hand Surg Am. 1998;23(3):483-491.
10. Schnabl SM, Kisslinger F, Schramm A, et al. Subjective outcome, neurophysiological investigations, postoperative complications and recurrence rate of partial medial epicondylectomy in cubital tunnel syndrome. Arch Orthop Trauma Surg. 2011;131(8):1027-1033.
11. Filippi R, Charalampaki P, Reisch R, Koch D, Grunert P. Recurrent cubital tunnel syndrome. Etiology and treatment. Minim Invasive Neurosurg. 2001;44(4):197-201.
12. Vogel RB, Nossaman BC, Rayan GM. Revision anterior submuscular transposition of the ulnar nerve for failed subcutaneous transposition. Br J Plast Surg. 2004;57(4):311-316.
13. Gabel GT, Amadio PC. Reoperation for failed decompression of the ulnar nerve in the region of the elbow. J Bone Joint Surg Am. 1990;72(2):213-219.
14. Rogers MR, Bergfield TG, Aulicino PL. The failed ulnar nerve transposition. Etiology and treatment. Clin Orthop. 1991;269:193-200.
15. Kilic A, Ojo B, Rajfer RA, et al. Effect of white adipose tissue flap and insulin-like growth factor-1 on nerve regeneration in rats. Microsurgery. 2013;33(5):367-375.
16. Ebersole GC, Davidge K, Damiano M, Mackinnon SE. Validity and responsiveness of the DASH questionnaire as an outcome measure following ulnar nerve transposition for cubital tunnel syndrome. Plast Reconstr Surg. 2013;132(1):81e-90e.
17. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
18. Dützmann S, Martin KD, Sobottka S, et al. Open vs retractor-endoscopic in situ decompression of the ulnar nerve in cubital tunnel syndrome: a retrospective cohort study. Neurosurgery. 2013;72(4):605-616.
19. Dellon AL, Mackinnon SE, Crosby PM. Reliability of two-point discrimination measurements. J Hand Surg Am. 1987;12(5 pt 1):693-696.
20. Danoff JR, Lombardi JM, Rosenwasser MP. Use of a pedicled adipose flap as a sling for anterior subcutaneous transposition of the ulnar nerve. J Hand Surg Am. 2014;39(3):552-555.
21. Magee RB, Phalen GS. Tardy ulnar palsy. Am J Surg. 1949;78(4):470-474.
22. Feindel W, Stratford J. Cubital tunnel compression in tardy ulnar palsy. Can Med Assoc J. 1958;78(5):351-353.
23. Tsai TM, Bonczar M, Tsuruta T, Syed SA. A new operative technique: cubital tunnel decompression with endoscopic assistance. Hand Clin. 1995;11(1):71-80.
24. Hoffmann R, Siemionow M. The endoscopic management of cubital tunnel syndrome. J Hand Surg Br. 2006;31(1):23-29.
25. Luchetti R, Riccio M, Papini Zorli I, Fairplay T. Protective coverage of the median nerve using fascial, fasciocutaneous or island flaps. Handchir Mikrochir Plast Chir. 2006;38(5):317-330.
26. Kokkalis ZT, Jain S, Sotereanos DG. Vein wrapping at cubital tunnel for ulnar nerve problems. J Shoulder Elbow Surg. 2010;19(2):91-97.
27. Masear VR, Colgin S. The treatment of epineural scarring with allograft vein wrapping. Hand Clin. 1996;12(4):773-779.
28. Kleinman WB, Bishop AT. Anterior intramuscular transposition of the ulnar nerve. J Hand Surg Am. 1989;14(6):972-979.
29. Lundborg G. Surgical treatment for ulnar nerve entrapment at the elbow. J Hand Surg Br. 1992;17(3):245-247.
30. Strickland JW, Idler RS, Lourie GM, Plancher KD. The hypothenar fat pad flap for management of recalcitrant carpal tunnel syndrome. J Hand Surg Am. 1996;21(5):840-848.
Distal Ulna Fracture With Delayed Ulnar Nerve Palsy in a Baseball Player
Ulnar nerve injury leads to clawing of the ulnar digits and loss of digital abduction and adduction because of paralysis of the ulnar innervated extrinsic and intrinsic muscles. Isolated motor paralysis without sensory deficit can occur from compression within the Guyon canal.1 Cubital tunnel at the elbow is the most common site for ulnar nerve compression.2 Compression at both levels can be encountered in sports-related activities. Nerve compression in the Guyon canal can occur with bicycling and is known as cyclist’s palsy,3-6 but it can also develop from canoeing.7 Cubital tunnel syndrome is the most common neuropathy of the elbow among throwing athletes, especially in baseball pitchers and can result from nerve traction and compression within the fibro-osseous tunnel or subluxation out of the tunnel.2 Both compression syndromes can develop from repetitive stress and/or pressure to the nerve in the retrocondylar groove.
Ulnar nerve palsy may be associated with forearm fractures, which is usually caused by simultaneous ulna and radius fractures, especially in children.8-12 To our knowledge, there are no reports in the literature of an ulnar nerve palsy associated with an isolated ulnar shaft fracture in an adult. We report a case of delayed ulnar nerve palsy after an ulnar shaft fracture in a baseball player. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old, right hand–dominant college baseball player was batting right-handed in an intrasquad scrimmage when a high and inside pitched ball from a right-handed pitcher struck the volar-ulnar aspect of his right forearm. Examination in the training room and emergency department revealed moderate swelling and ecchymosis over the distal third of the ulna. He had a normal neurovascular examination, including normal sensation to light touch and normal finger abduction/adduction and wrist flexion/extension. He was otherwise healthy. Radiographs of the right forearm showed a minimally displaced transverse fracture of the distal third of the ulna (Figures 1A, 1B).
The patient was initially treated with a well-padded, removable, long-arm posterior splint for 2 weeks with serial examinations each day in the training room. At 2-week follow-up, he reported less pain and swelling but stated that his hand had “felt funny” the past several days. Examination revealed clawing of the ulnar digits with paresthesias in the ulnar nerve distribution (Figures 2A, 2B). His extrinsic muscle function was normal. Radiographs showed stable fracture alignment. Ulnar neuropathy was diagnosed, and treatment was observation with a plan for electromyography (EMG) at 6 weeks after injury if there were no signs of nerve recovery. Physical therapy was instituted and focused on improving intrinsic muscle and proprioceptive functions with the goal of an expeditious, but safe, return to playing baseball. Three weeks after his injury, the patient had decreased tenderness at his fracture site and was given a forearm pad and sleeve for light, noncontact baseball activity (Figure 3). A long velcro wrist splint was used during conditioning and when not playing baseball. Forearm supination and pronation were limited initially because of patient discomfort and to prevent torsional fracture displacement or delayed healing. Six weeks after his injury, the patient returned to hitting and was showing early signs of improved sensation and intrinsic hand strength. He had progressed to a light throwing program and reported difficult hand coordination, poor ball control, and overall difficulty in accurately throwing over the next 3 to 4 months. Because of his difficulty with ball control, the patient began a progressive return to full-game activity over 6 weeks, which initially included a return to batting only, then playing in the outfield, and, eventually, a return to his normal position in the infield. Serial radiographs continued to show good fracture alignment with appropriate new bone formation (Figures 4A, 4B). Normal motor strength was noted at 3 months after injury and normal sensation at 4 months after injury.
By the end of his summer league, 6 months after his injury, the patient was named Most Valuable Player and had a batting average over .400. He reported near-normal hand function. One year after injury, his examination revealed normal hand function (Figure 5), including normal sensation to light touch, 5/5 intrinsic hand function, and symmetric grip strength. Radiographs showed a healed fracture (Figures 6A, 6B). The patient has gone on to play more than 9 years of professional baseball.
Discussion
The ulnar nerve has a course that runs down the volar compartment of the distal forearm. The flexor carpi ulnaris provides coverage to the nerve in this area. Proximal to the wrist, the nerve emerges from under the flexor carpi ulnaris tendon and passes deep to the flexor retinaculum, which is the distal extension of the antebrachial fascia and blends distally into the palmar carpal ligament.13 In our patient, the most likely cause of this presentation of ulnar neuropathy was the direct blow to the nerve from the high-intensity impact of a thrown baseball to this superficial and exposed area of the forearm. Since the patient presented with delayed paresthesias and ulnar clawing 2 weeks after injury, possible contributing causes could be evolving pressure or nerve damage from a perineural hematoma and/or intraneural hematoma or increased local pressure from intramuscular hemorrhage.14 There are both acute and chronic cases of ulnar nerve entrapment by bone or scar tissue that resolved by surgical decompression.8-12 Surgical exploration was not deemed necessary in our case because the fracture was minimally displaced, and the patient regained sensation and motor function over the course of 3 to 4 months.
Nerve injuries can be classified as neurapraxia, axonotmesis, or neurotmesis. Neurapraxia is the mildest form of nerve injury and neurotmesis the most severe. Neurapraxia may be associated with a temporary block to conduction or nerve demyelination without axonal disruption. Spontaneous recovery takes 2 weeks to 2 months. Axonotmesis involves an actual loss of axonal continuity; however, connective tissue supporting structures remain intact and allow axonal regeneration. Finally, neurotmesis is transection of the peripheral nerve, and spontaneous regeneration is not possible. The mechanism of injury in our patient suggests that the pathology was neurapraxia.1,15
Management of these injuries should proceed according to basic extremity injury–care practices. Initial care should include thorough neurovascular and radiographic evaluations. If nerve deficits are present with a closed injury and minimal fracture displacement, treatment can include observation and serial examinations with a baseline EMG, or waiting until 4 to 6 weeks after injury to obtain an EMG if there are no signs of nerve recovery. Early EMG testing and surgical exploration may be warranted if there is a concern for nerve disruption or entrapment, such as marked fracture displacement or an open injury. Additional early-care measures should include swelling control modalities and immobilization based on the type of fracture. Ultrasound was not readily available at the time of our patient’s injury, but it may be a helpful adjunct in guiding decision-making regarding whether to perform early surgical exploration for hematoma evacuation or nerve injury.16-18 Our case report was intended to provide an awareness of the unusual association between an isolated ulnar shaft fracture and a delayed ulnar nerve palsy in an athlete. Nerve injuries may be unrecognized in some patients in a trauma situation, since the focus is usually on the fracture and the typical patient does not have to return to high-demand, coordinated athletic activity, such as throwing a ball. Because of the possible delayed presentation of these nerve injuries, close observation of nerve function after ulna fractures from blunt trauma is warranted.
1. Dhillon MS, Chu ML, Posner MA. Demyelinating focal motor neuropathy of the ulnar nerve masquerading as compression in Guyon’s canal: a case report. J Hand Surg Am. 2003;28(1):48-51.
2. Hariri S, McAdams TR. Nerve injuries about the elbow. Clin Sports Med. 2010;29(4):655-675.
3. Akuthota V, Plastaras C, Lindberg K, Tobey J, Press J, Garvan C. The effect of long-distance bicycling on ulnar and median nerves: an electrophysiologic evaluation of cyclist palsy. Am J Sports Med. 2005;33(8):1224-1230.
4. Capitani D, Beer S. Handlebar palsy--a compression syndrome of the deep terminal (motor) branch of the ulnar nerve in biking. J Neurol. 2002;249(10):1441-1445.
5. Patterson JM, Jaggars MM, Boyer MI. Ulnar and median nerve palsy in long-distance cyclists. A prospective study. Am J Sports Med. 2003;31(4):585-589.
6. Slane J, Timmerman M, Ploeg HL, Thelen DG. The influence of glove and hand position on pressure over the ulnar nerve during cycling. Clin Biomech (Bristol, Avon). 2011;26(6):642-648.
7. Paul F, Diesta FJ, Ratzlaff T, Vogel HP, Zipp F. Combined ulnar nerve palsy in Guyon’s canal and distal median nerve irritation following excessive canoeing. Clinical Neurophysiology. 2007;118(4):e81-e82.
8. Hirasawa H, Sakai A, Toba N, Kamiuttanai M, Nakamura T, Tanaka K. Bony entrapment of ulnar nerve after closed forearm fracture: a case report. J Orthop Surg (Hong Kong). 2004;12(1):122-125.
9. Dahlin LB, Düppe H. Injuries to the nerves associated with fractured forearms in children. Scand J Plast Reconstr Surg Hand Surg. 2007;41(4):207-210.
10. Neiman R, Maiocco B, Deeney VF. Ulnar nerve injury after closed forearm fractures in children. J Pediatr Orthop. 1998;18(5):683-685.
11. Pai VS. Injury of the ulnar nerve associated with fracture of the ulna: A case report. J Orthop Surgery. 1999;7(2):73.
12. Suganuma S, Tada K, Hayashi H, Segawa T, Tsuchiya H. Ulnar nerve palsy associated with closed midshaft forearm fractures. Orthopedics. 2012;35(11):e1680-e1683.
13. Ombaba J, Kuo M, Rayan G. Anatomy of the ulnar tunnel and the influence of wrist motion on its morphology. J Hand Surg Am. 2010;35A:760-768.
14. Vijayakumar R, Nesathurai S, Abbott KM, Eustace S. Ulnar neuropathy resulting from diffuse intramuscular hemorrhage: a case report. Arch Phys Med Rehabil. 2000;81(8):1127-1130.
15. Browner, Bruce. Skeletal Trauma: Basic Science, Management, and Reconstruction [eBook]. 4th ed. Philadelphia, PA: WB Saunders Company; 2009:1487.
16. Koenig RW, Pedro MT, Heinen CP, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. 2009;26(2):E13.
17. Zhu J, Liu F, Li D, Shao J, Hu B. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011;21(5):1097-1101.
18. Lee FC, Singh H, Nazarian LN, Ratliff JK. High-resolution ultrasonography in the diagnosis and intra-operative management of peripheral nerve lesions. J Neurosurg. 2011;114(1):206-221.
Ulnar nerve injury leads to clawing of the ulnar digits and loss of digital abduction and adduction because of paralysis of the ulnar innervated extrinsic and intrinsic muscles. Isolated motor paralysis without sensory deficit can occur from compression within the Guyon canal.1 Cubital tunnel at the elbow is the most common site for ulnar nerve compression.2 Compression at both levels can be encountered in sports-related activities. Nerve compression in the Guyon canal can occur with bicycling and is known as cyclist’s palsy,3-6 but it can also develop from canoeing.7 Cubital tunnel syndrome is the most common neuropathy of the elbow among throwing athletes, especially in baseball pitchers and can result from nerve traction and compression within the fibro-osseous tunnel or subluxation out of the tunnel.2 Both compression syndromes can develop from repetitive stress and/or pressure to the nerve in the retrocondylar groove.
Ulnar nerve palsy may be associated with forearm fractures, which is usually caused by simultaneous ulna and radius fractures, especially in children.8-12 To our knowledge, there are no reports in the literature of an ulnar nerve palsy associated with an isolated ulnar shaft fracture in an adult. We report a case of delayed ulnar nerve palsy after an ulnar shaft fracture in a baseball player. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old, right hand–dominant college baseball player was batting right-handed in an intrasquad scrimmage when a high and inside pitched ball from a right-handed pitcher struck the volar-ulnar aspect of his right forearm. Examination in the training room and emergency department revealed moderate swelling and ecchymosis over the distal third of the ulna. He had a normal neurovascular examination, including normal sensation to light touch and normal finger abduction/adduction and wrist flexion/extension. He was otherwise healthy. Radiographs of the right forearm showed a minimally displaced transverse fracture of the distal third of the ulna (Figures 1A, 1B).
The patient was initially treated with a well-padded, removable, long-arm posterior splint for 2 weeks with serial examinations each day in the training room. At 2-week follow-up, he reported less pain and swelling but stated that his hand had “felt funny” the past several days. Examination revealed clawing of the ulnar digits with paresthesias in the ulnar nerve distribution (Figures 2A, 2B). His extrinsic muscle function was normal. Radiographs showed stable fracture alignment. Ulnar neuropathy was diagnosed, and treatment was observation with a plan for electromyography (EMG) at 6 weeks after injury if there were no signs of nerve recovery. Physical therapy was instituted and focused on improving intrinsic muscle and proprioceptive functions with the goal of an expeditious, but safe, return to playing baseball. Three weeks after his injury, the patient had decreased tenderness at his fracture site and was given a forearm pad and sleeve for light, noncontact baseball activity (Figure 3). A long velcro wrist splint was used during conditioning and when not playing baseball. Forearm supination and pronation were limited initially because of patient discomfort and to prevent torsional fracture displacement or delayed healing. Six weeks after his injury, the patient returned to hitting and was showing early signs of improved sensation and intrinsic hand strength. He had progressed to a light throwing program and reported difficult hand coordination, poor ball control, and overall difficulty in accurately throwing over the next 3 to 4 months. Because of his difficulty with ball control, the patient began a progressive return to full-game activity over 6 weeks, which initially included a return to batting only, then playing in the outfield, and, eventually, a return to his normal position in the infield. Serial radiographs continued to show good fracture alignment with appropriate new bone formation (Figures 4A, 4B). Normal motor strength was noted at 3 months after injury and normal sensation at 4 months after injury.
By the end of his summer league, 6 months after his injury, the patient was named Most Valuable Player and had a batting average over .400. He reported near-normal hand function. One year after injury, his examination revealed normal hand function (Figure 5), including normal sensation to light touch, 5/5 intrinsic hand function, and symmetric grip strength. Radiographs showed a healed fracture (Figures 6A, 6B). The patient has gone on to play more than 9 years of professional baseball.
Discussion
The ulnar nerve has a course that runs down the volar compartment of the distal forearm. The flexor carpi ulnaris provides coverage to the nerve in this area. Proximal to the wrist, the nerve emerges from under the flexor carpi ulnaris tendon and passes deep to the flexor retinaculum, which is the distal extension of the antebrachial fascia and blends distally into the palmar carpal ligament.13 In our patient, the most likely cause of this presentation of ulnar neuropathy was the direct blow to the nerve from the high-intensity impact of a thrown baseball to this superficial and exposed area of the forearm. Since the patient presented with delayed paresthesias and ulnar clawing 2 weeks after injury, possible contributing causes could be evolving pressure or nerve damage from a perineural hematoma and/or intraneural hematoma or increased local pressure from intramuscular hemorrhage.14 There are both acute and chronic cases of ulnar nerve entrapment by bone or scar tissue that resolved by surgical decompression.8-12 Surgical exploration was not deemed necessary in our case because the fracture was minimally displaced, and the patient regained sensation and motor function over the course of 3 to 4 months.
Nerve injuries can be classified as neurapraxia, axonotmesis, or neurotmesis. Neurapraxia is the mildest form of nerve injury and neurotmesis the most severe. Neurapraxia may be associated with a temporary block to conduction or nerve demyelination without axonal disruption. Spontaneous recovery takes 2 weeks to 2 months. Axonotmesis involves an actual loss of axonal continuity; however, connective tissue supporting structures remain intact and allow axonal regeneration. Finally, neurotmesis is transection of the peripheral nerve, and spontaneous regeneration is not possible. The mechanism of injury in our patient suggests that the pathology was neurapraxia.1,15
Management of these injuries should proceed according to basic extremity injury–care practices. Initial care should include thorough neurovascular and radiographic evaluations. If nerve deficits are present with a closed injury and minimal fracture displacement, treatment can include observation and serial examinations with a baseline EMG, or waiting until 4 to 6 weeks after injury to obtain an EMG if there are no signs of nerve recovery. Early EMG testing and surgical exploration may be warranted if there is a concern for nerve disruption or entrapment, such as marked fracture displacement or an open injury. Additional early-care measures should include swelling control modalities and immobilization based on the type of fracture. Ultrasound was not readily available at the time of our patient’s injury, but it may be a helpful adjunct in guiding decision-making regarding whether to perform early surgical exploration for hematoma evacuation or nerve injury.16-18 Our case report was intended to provide an awareness of the unusual association between an isolated ulnar shaft fracture and a delayed ulnar nerve palsy in an athlete. Nerve injuries may be unrecognized in some patients in a trauma situation, since the focus is usually on the fracture and the typical patient does not have to return to high-demand, coordinated athletic activity, such as throwing a ball. Because of the possible delayed presentation of these nerve injuries, close observation of nerve function after ulna fractures from blunt trauma is warranted.
Ulnar nerve injury leads to clawing of the ulnar digits and loss of digital abduction and adduction because of paralysis of the ulnar innervated extrinsic and intrinsic muscles. Isolated motor paralysis without sensory deficit can occur from compression within the Guyon canal.1 Cubital tunnel at the elbow is the most common site for ulnar nerve compression.2 Compression at both levels can be encountered in sports-related activities. Nerve compression in the Guyon canal can occur with bicycling and is known as cyclist’s palsy,3-6 but it can also develop from canoeing.7 Cubital tunnel syndrome is the most common neuropathy of the elbow among throwing athletes, especially in baseball pitchers and can result from nerve traction and compression within the fibro-osseous tunnel or subluxation out of the tunnel.2 Both compression syndromes can develop from repetitive stress and/or pressure to the nerve in the retrocondylar groove.
Ulnar nerve palsy may be associated with forearm fractures, which is usually caused by simultaneous ulna and radius fractures, especially in children.8-12 To our knowledge, there are no reports in the literature of an ulnar nerve palsy associated with an isolated ulnar shaft fracture in an adult. We report a case of delayed ulnar nerve palsy after an ulnar shaft fracture in a baseball player. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old, right hand–dominant college baseball player was batting right-handed in an intrasquad scrimmage when a high and inside pitched ball from a right-handed pitcher struck the volar-ulnar aspect of his right forearm. Examination in the training room and emergency department revealed moderate swelling and ecchymosis over the distal third of the ulna. He had a normal neurovascular examination, including normal sensation to light touch and normal finger abduction/adduction and wrist flexion/extension. He was otherwise healthy. Radiographs of the right forearm showed a minimally displaced transverse fracture of the distal third of the ulna (Figures 1A, 1B).
The patient was initially treated with a well-padded, removable, long-arm posterior splint for 2 weeks with serial examinations each day in the training room. At 2-week follow-up, he reported less pain and swelling but stated that his hand had “felt funny” the past several days. Examination revealed clawing of the ulnar digits with paresthesias in the ulnar nerve distribution (Figures 2A, 2B). His extrinsic muscle function was normal. Radiographs showed stable fracture alignment. Ulnar neuropathy was diagnosed, and treatment was observation with a plan for electromyography (EMG) at 6 weeks after injury if there were no signs of nerve recovery. Physical therapy was instituted and focused on improving intrinsic muscle and proprioceptive functions with the goal of an expeditious, but safe, return to playing baseball. Three weeks after his injury, the patient had decreased tenderness at his fracture site and was given a forearm pad and sleeve for light, noncontact baseball activity (Figure 3). A long velcro wrist splint was used during conditioning and when not playing baseball. Forearm supination and pronation were limited initially because of patient discomfort and to prevent torsional fracture displacement or delayed healing. Six weeks after his injury, the patient returned to hitting and was showing early signs of improved sensation and intrinsic hand strength. He had progressed to a light throwing program and reported difficult hand coordination, poor ball control, and overall difficulty in accurately throwing over the next 3 to 4 months. Because of his difficulty with ball control, the patient began a progressive return to full-game activity over 6 weeks, which initially included a return to batting only, then playing in the outfield, and, eventually, a return to his normal position in the infield. Serial radiographs continued to show good fracture alignment with appropriate new bone formation (Figures 4A, 4B). Normal motor strength was noted at 3 months after injury and normal sensation at 4 months after injury.
By the end of his summer league, 6 months after his injury, the patient was named Most Valuable Player and had a batting average over .400. He reported near-normal hand function. One year after injury, his examination revealed normal hand function (Figure 5), including normal sensation to light touch, 5/5 intrinsic hand function, and symmetric grip strength. Radiographs showed a healed fracture (Figures 6A, 6B). The patient has gone on to play more than 9 years of professional baseball.
Discussion
The ulnar nerve has a course that runs down the volar compartment of the distal forearm. The flexor carpi ulnaris provides coverage to the nerve in this area. Proximal to the wrist, the nerve emerges from under the flexor carpi ulnaris tendon and passes deep to the flexor retinaculum, which is the distal extension of the antebrachial fascia and blends distally into the palmar carpal ligament.13 In our patient, the most likely cause of this presentation of ulnar neuropathy was the direct blow to the nerve from the high-intensity impact of a thrown baseball to this superficial and exposed area of the forearm. Since the patient presented with delayed paresthesias and ulnar clawing 2 weeks after injury, possible contributing causes could be evolving pressure or nerve damage from a perineural hematoma and/or intraneural hematoma or increased local pressure from intramuscular hemorrhage.14 There are both acute and chronic cases of ulnar nerve entrapment by bone or scar tissue that resolved by surgical decompression.8-12 Surgical exploration was not deemed necessary in our case because the fracture was minimally displaced, and the patient regained sensation and motor function over the course of 3 to 4 months.
Nerve injuries can be classified as neurapraxia, axonotmesis, or neurotmesis. Neurapraxia is the mildest form of nerve injury and neurotmesis the most severe. Neurapraxia may be associated with a temporary block to conduction or nerve demyelination without axonal disruption. Spontaneous recovery takes 2 weeks to 2 months. Axonotmesis involves an actual loss of axonal continuity; however, connective tissue supporting structures remain intact and allow axonal regeneration. Finally, neurotmesis is transection of the peripheral nerve, and spontaneous regeneration is not possible. The mechanism of injury in our patient suggests that the pathology was neurapraxia.1,15
Management of these injuries should proceed according to basic extremity injury–care practices. Initial care should include thorough neurovascular and radiographic evaluations. If nerve deficits are present with a closed injury and minimal fracture displacement, treatment can include observation and serial examinations with a baseline EMG, or waiting until 4 to 6 weeks after injury to obtain an EMG if there are no signs of nerve recovery. Early EMG testing and surgical exploration may be warranted if there is a concern for nerve disruption or entrapment, such as marked fracture displacement or an open injury. Additional early-care measures should include swelling control modalities and immobilization based on the type of fracture. Ultrasound was not readily available at the time of our patient’s injury, but it may be a helpful adjunct in guiding decision-making regarding whether to perform early surgical exploration for hematoma evacuation or nerve injury.16-18 Our case report was intended to provide an awareness of the unusual association between an isolated ulnar shaft fracture and a delayed ulnar nerve palsy in an athlete. Nerve injuries may be unrecognized in some patients in a trauma situation, since the focus is usually on the fracture and the typical patient does not have to return to high-demand, coordinated athletic activity, such as throwing a ball. Because of the possible delayed presentation of these nerve injuries, close observation of nerve function after ulna fractures from blunt trauma is warranted.
1. Dhillon MS, Chu ML, Posner MA. Demyelinating focal motor neuropathy of the ulnar nerve masquerading as compression in Guyon’s canal: a case report. J Hand Surg Am. 2003;28(1):48-51.
2. Hariri S, McAdams TR. Nerve injuries about the elbow. Clin Sports Med. 2010;29(4):655-675.
3. Akuthota V, Plastaras C, Lindberg K, Tobey J, Press J, Garvan C. The effect of long-distance bicycling on ulnar and median nerves: an electrophysiologic evaluation of cyclist palsy. Am J Sports Med. 2005;33(8):1224-1230.
4. Capitani D, Beer S. Handlebar palsy--a compression syndrome of the deep terminal (motor) branch of the ulnar nerve in biking. J Neurol. 2002;249(10):1441-1445.
5. Patterson JM, Jaggars MM, Boyer MI. Ulnar and median nerve palsy in long-distance cyclists. A prospective study. Am J Sports Med. 2003;31(4):585-589.
6. Slane J, Timmerman M, Ploeg HL, Thelen DG. The influence of glove and hand position on pressure over the ulnar nerve during cycling. Clin Biomech (Bristol, Avon). 2011;26(6):642-648.
7. Paul F, Diesta FJ, Ratzlaff T, Vogel HP, Zipp F. Combined ulnar nerve palsy in Guyon’s canal and distal median nerve irritation following excessive canoeing. Clinical Neurophysiology. 2007;118(4):e81-e82.
8. Hirasawa H, Sakai A, Toba N, Kamiuttanai M, Nakamura T, Tanaka K. Bony entrapment of ulnar nerve after closed forearm fracture: a case report. J Orthop Surg (Hong Kong). 2004;12(1):122-125.
9. Dahlin LB, Düppe H. Injuries to the nerves associated with fractured forearms in children. Scand J Plast Reconstr Surg Hand Surg. 2007;41(4):207-210.
10. Neiman R, Maiocco B, Deeney VF. Ulnar nerve injury after closed forearm fractures in children. J Pediatr Orthop. 1998;18(5):683-685.
11. Pai VS. Injury of the ulnar nerve associated with fracture of the ulna: A case report. J Orthop Surgery. 1999;7(2):73.
12. Suganuma S, Tada K, Hayashi H, Segawa T, Tsuchiya H. Ulnar nerve palsy associated with closed midshaft forearm fractures. Orthopedics. 2012;35(11):e1680-e1683.
13. Ombaba J, Kuo M, Rayan G. Anatomy of the ulnar tunnel and the influence of wrist motion on its morphology. J Hand Surg Am. 2010;35A:760-768.
14. Vijayakumar R, Nesathurai S, Abbott KM, Eustace S. Ulnar neuropathy resulting from diffuse intramuscular hemorrhage: a case report. Arch Phys Med Rehabil. 2000;81(8):1127-1130.
15. Browner, Bruce. Skeletal Trauma: Basic Science, Management, and Reconstruction [eBook]. 4th ed. Philadelphia, PA: WB Saunders Company; 2009:1487.
16. Koenig RW, Pedro MT, Heinen CP, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. 2009;26(2):E13.
17. Zhu J, Liu F, Li D, Shao J, Hu B. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011;21(5):1097-1101.
18. Lee FC, Singh H, Nazarian LN, Ratliff JK. High-resolution ultrasonography in the diagnosis and intra-operative management of peripheral nerve lesions. J Neurosurg. 2011;114(1):206-221.
1. Dhillon MS, Chu ML, Posner MA. Demyelinating focal motor neuropathy of the ulnar nerve masquerading as compression in Guyon’s canal: a case report. J Hand Surg Am. 2003;28(1):48-51.
2. Hariri S, McAdams TR. Nerve injuries about the elbow. Clin Sports Med. 2010;29(4):655-675.
3. Akuthota V, Plastaras C, Lindberg K, Tobey J, Press J, Garvan C. The effect of long-distance bicycling on ulnar and median nerves: an electrophysiologic evaluation of cyclist palsy. Am J Sports Med. 2005;33(8):1224-1230.
4. Capitani D, Beer S. Handlebar palsy--a compression syndrome of the deep terminal (motor) branch of the ulnar nerve in biking. J Neurol. 2002;249(10):1441-1445.
5. Patterson JM, Jaggars MM, Boyer MI. Ulnar and median nerve palsy in long-distance cyclists. A prospective study. Am J Sports Med. 2003;31(4):585-589.
6. Slane J, Timmerman M, Ploeg HL, Thelen DG. The influence of glove and hand position on pressure over the ulnar nerve during cycling. Clin Biomech (Bristol, Avon). 2011;26(6):642-648.
7. Paul F, Diesta FJ, Ratzlaff T, Vogel HP, Zipp F. Combined ulnar nerve palsy in Guyon’s canal and distal median nerve irritation following excessive canoeing. Clinical Neurophysiology. 2007;118(4):e81-e82.
8. Hirasawa H, Sakai A, Toba N, Kamiuttanai M, Nakamura T, Tanaka K. Bony entrapment of ulnar nerve after closed forearm fracture: a case report. J Orthop Surg (Hong Kong). 2004;12(1):122-125.
9. Dahlin LB, Düppe H. Injuries to the nerves associated with fractured forearms in children. Scand J Plast Reconstr Surg Hand Surg. 2007;41(4):207-210.
10. Neiman R, Maiocco B, Deeney VF. Ulnar nerve injury after closed forearm fractures in children. J Pediatr Orthop. 1998;18(5):683-685.
11. Pai VS. Injury of the ulnar nerve associated with fracture of the ulna: A case report. J Orthop Surgery. 1999;7(2):73.
12. Suganuma S, Tada K, Hayashi H, Segawa T, Tsuchiya H. Ulnar nerve palsy associated with closed midshaft forearm fractures. Orthopedics. 2012;35(11):e1680-e1683.
13. Ombaba J, Kuo M, Rayan G. Anatomy of the ulnar tunnel and the influence of wrist motion on its morphology. J Hand Surg Am. 2010;35A:760-768.
14. Vijayakumar R, Nesathurai S, Abbott KM, Eustace S. Ulnar neuropathy resulting from diffuse intramuscular hemorrhage: a case report. Arch Phys Med Rehabil. 2000;81(8):1127-1130.
15. Browner, Bruce. Skeletal Trauma: Basic Science, Management, and Reconstruction [eBook]. 4th ed. Philadelphia, PA: WB Saunders Company; 2009:1487.
16. Koenig RW, Pedro MT, Heinen CP, et al. High-resolution ultrasonography in evaluating peripheral nerve entrapment and trauma. Neurosurg Focus. 2009;26(2):E13.
17. Zhu J, Liu F, Li D, Shao J, Hu B. Preliminary study of the types of traumatic peripheral nerve injuries by ultrasound. Eur Radiol. 2011;21(5):1097-1101.
18. Lee FC, Singh H, Nazarian LN, Ratliff JK. High-resolution ultrasonography in the diagnosis and intra-operative management of peripheral nerve lesions. J Neurosurg. 2011;114(1):206-221.
Minimum 5-Year Results With Duracon Press-Fit Metal-Backed Patellae
The metal-backed patella was originally designed to address the shortcomings of cemented, all-polyethylene patellae: deformation, aseptic loosening, stress fractures of polyethylene, and possible thermal damage from bone cement.1-3 Several long-term studies have found very good outcomes with use of all-polyethylene patellae.4-6 However, complications of using an all-polyethylene patella reportedly accounted for up to half of all knee revisions, and during revision surgery patellar bone stock was often found to have been compromised.7
The intention behind the design of press-fit metal-backed patellae was to address the shortcomings of all-polyethylene patellae by eliminating the need for bone cement and providing stiffness that would help resist polyethylene deformation while decreasing implant–bone interface stresses.8 However, early design iterations of metal-backed patellae demonstrated short-term failures—most commonly, local polyethylene wear damaging the locking mechanism and subsequent dissociation or fracture from the metal baseplate; polyethylene delamination from the metal baseplate; and failure of interface fixation.9,10 On the other hand, good fixation with bony ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9,11-13 Overall, however, negative outcomes reported for metal-backed patellae led many surgeons to abandon these components and return to using cemented all-polyethylene patellae.
Negative outcomes of earlier metal-backed patellae designs have overshadowed reports of positive outcomes achieved with careful attention paid to component design, patellar tracking, and surgical technique.2,3,14 Subsequent design improvements (eg, a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15 The advantages of using a metal-backed patella (eg, uniform load sharing, decreased polyethylene deformation, potential for biological fixation) may be unjustly outweighed by the fear of patellar component failure.3
Our 30-plus years of experience with metal-backed patellar components reflect the evolving effect of component design on outcome. Much as reported elsewhere, we found earlier component failures were caused by poor locking mechanisms, thin polyethylene, poor tracking, and minimal femur contact. Over the past decade, however, our outcomes with Duracon metal-backed patellae (Stryker) have been encouraging. We think these positive outcomes, seen over minimum 5-year follow-up, are largely attributable to the thicker polyethylene and improved articular conformity of this component relative to earlier designs. We have also found it helpful to adhere to certain criteria when implanting metal-backed patellae, and we think adhering to these criteria, along with improved component design, indicates use of press-fit metal-backed patellae. In this article, we report our failure incidence with use of this device at minimum 5-year follow-up.
Materials and Methods
In this single-center study, we performed clinical and independent radiographic reviews of 88 primary press-fit metal-backed patellae with minimum 5-year follow-up. All components were the same design (Duracon metal-backed patella) from the same manufacturer (Stryker).
This study, which began in September 2003, was reviewed and approved by the Western Institutional Review Board (WIRB). Either the investigator (Dr. Hedley) or the clinical study coordinator gave study candidates a full explanation of the study and answered any questions. Patients who still wanted to participate in the study signed WIRB consent forms after their index surgery but before minimum 5-year follow-up.
Device Description
This Duracon patella has a porous-coated cobalt-chromium metal back intended for press-fit fixation, 3 cobalt-chromium porous-coated pegs, and a preassembled polyethylene anterior surface (Figure 1). Four sizes are available to fit the peripheral shape of the resected patella.
This patella has 3 styles: symmetric, asymmetric, and conversion. In this study, we used only the asymmetric and conversion styles. The design of each style incorporates medial/lateral facets intended to conform to the convex intercondylar radii of the femoral component, thereby allowing the patella to ride deeply in the recessed patellofemoral groove. The asymmetric patella is a resurfacing component with a generous polyethylene thickness (4.6 mm at its thinnest) and a larger lateral facet for more bone coverage. The asymmetric patella naturally medializes component placement. The articulating surface of the conversion patella is identical to that of the asymmetric patella. However, the conversion patella allows for exchange of the polyethylene portion of the implant without revising a stable, well-fixed metal baseplate.
Patient Selection
Candidates were recruited from a group of metal-backed patella patients within Dr. Hedley’s medical practice. All candidates had undergone primary total knee arthroplasty and received a Duracon press-fit metal-backed patella. All recruited patients had undergone primary knee arthroplasty at least 5 years before clinical and radiographic evaluation. Patients were included in the study if they had a diagnosis of noninflammatory degenerative joint disease (eg, osteoarthritis, traumatic arthritis, avascular necrosis). Patients with body mass index higher than 40 were excluded from the study.
Surgical Technique
The patella is everted completely or as much as feasible. Debridement is done circumferentially around the patella. Adherent fat and pseudomeniscus are stripped back until the surgeon sees the entry point of the quadriceps tendon fibers above and the patella tendon fibers below. The cut is then made at this level to remove as much bone as needed to restore the normal height of the patella with the implant in place. The cut is usually made by hand—without guides but with the patella stabilized with a towel clip above and below to prevent any movement during the action.
The desired cut must be absolutely planar, and this should be checked by placing the edge of the blade across the interface. Repeated passes with the saw blade are needed if the cut is not 100% planar. Once the cut is made, the patella is sized with the patella sizers and drill guide. After the appropriate size is selected, the patella is drilled with a bit that is slightly undersized from the size of the pegs (1/32 inch smaller than the bit supplied by the manufacturer).
Once the patella is prepared, the rest of the knee arthroplasty is performed. The patella is press-fit as the last component to be inserted.
Radiologic Review
Radiographic analysis was performed by an independent reviewer according to the current Knee Society total knee arthroplasty roentgenographic evaluation and scoring system (Figure 2).16 The reviewer was an orthopedist specializing in hip and knee surgery. Radiographs the reviewer deemed questionable were shown to another independent hip and knee surgeon for validation. In all cases, the second reviewer confirmed the first reviewer’s initial recorded observations.
KSS (Knee Society Scale), WOMAC (Western Ontario and McMaster Universities Arthritis Index), and SF-36 (36-Item Short Form Health Survey) were also used to evaluate effectiveness in this protocol.
Survivorship Calculations
Kaplan-Meier survivorship was determined for all metal-backed patellae. For survival analysis, only knees with radiographic data were included (74 knees). Mean follow-up was 75.8 months (range, 60-105 months).
Seventy-four patients (88 knees) met the study criteria (Table). At minimum 5-year follow-up, complete data were acquired for 59 patients (72 knees). Of the total group, 14 knees did not have radiographic data. Those knees were categorized as lost to follow-up and were excluded from the survivorship analysis. The status of patients enrolled in the study at minimum 5-year follow-up is shown in the Table.
Mann-Whitney U test (nonparametric t test) was used to compare WOMAC and SF-36 scores between the “complete” and the “WOMAC and SF-36 only” data groups.
Statistical Analysis
Kaplan-Meier survivorship probabilities (asymmetric method) were calculated using SAS Version 9.2 (SAS Institute); 95% pointwise confidence limits were used.
The Mann-Whitney U test is a nonparametric analogue to the independent-samples t test. It was used here to compare WOMAC and SF-36 scores of patients with “complete” data with scores of patients with “WOMAC and SF-36 only” data. In either group, for patients who had primary bilateral knee arthroplasty, mean WOMAC and SF-36 scores were used.
Comparisons were made between the unilateral and bilateral knee arthroplasty groups. There were no differences in age, height, or weight (Mann-Whitney U test) or in sex, primary diagnosis, or number of patients lost to follow-up (Fisher exact test). Fisher exact test (vs χ2 test) was used for the contingency table analysis because of small cell sizes (eg, ≤10 females in ‘‘both knees” group), suggesting the unilateral and bilateral patients did not differ in demographics.
For all patient-reported questionnaires, bilateral patients were given the opportunity to note any differences between their knee arthroplasties, but none of these patients made any special notations. We interpreted this to mean that all survey responses from bilateral patients were applicable to both knee arthroplasties.
Results
Seventy-four patients (88 knees) were enrolled in the study: 31 women (41.2%) and 43 men (58.1%). At time of surgery, mean age was 59.7 years (range, 40-86 years), and mean body mass index was 30.6 (range, 19.1-39.6). Eighty-three knees were diagnosed with osteoarthritis, and 5 knees were diagnosed with posttraumatic arthritis. Mean time to follow-up was 74.8 months (range, 60-105 months). Fourteen knees (14 patients) were considered lost to follow-up. However, 8 patients (8 knees) were contacted by telephone about the status of their knee(s), and all 8 completed and returned the minimum 5-year follow-up WOMAC and SF-36 forms; they did not return for their minimum 5-year clinical or radiographic evaluations.
Asymmetric patellae were used in 24 knees, conversion patellae in 64 knees (88 knees total). Forty-nine months after surgery, 1 patella was revised for loosening at its interface with the bone. The 51-year-old active female patient’s asymmetric patella was revised to a conversion patella. The decision to implant another metal-backed device was based on its high density; proper intrusion of acrylic cement would have been questionable. Some early wear was observed on the tibial insert, which was replaced. Sixty-eight months after the revision, the patient was asymptomatic, with a KSS Pain score of 96 and a KSS Function score of 100 (Figure 3). Another revision, for tibial insert exchange only, was performed 48 months after surgery. During this revision, the patella was evaluated and found to be well fixed and functioning normally.
Survivorship of the Duracon metal-backed patella at minimum 5-year follow-up was estimated to be 93.95%, with bounds of 73.61% and 98.74%.
Radiographic analysis revealed no radiolucencies larger than 1 mm (Figure 4). Seventeen 1-mm radiolucencies were recorded: 6 (35.3%) in zone 1, 2 (11.8%) in zone 2, and 9 (52.9%) in zone 4. Twelve (70.6%) of the 17 radiolucencies were in the left knee. Nine radiolucencies were in women and 8 in men. Most (55.6%) of the women’s radiolucencies were in zone 1, and most (75.0%) of the men’s were in zone 4. There were no loose beads other than in the case that was later revised.
KSS, WOMAC, and SF-36 scores and radiographic reviews were used to evaluate effectiveness in accordance with the protocol. At minimum 5-year follow-up, mean KSS Pain score was 94.10 (range, 55-100), and mean KSS Function score was 92.67 (range, 60-100). Mean WOMAC score was 2.21 (range, 0-19.70), mean SF-36 Physical score was 83.65 (range, 30.70-100), and mean SF-36 Mental score was 89.41 (range, 1.4-100).
The preceding calculations do not include WOMAC and SF-36 data for the 8 patients (8 knees) who were counted as lost to follow-up but who submitted minimum 5-year follow-up data. We compared these 8 patients with the 60 patients (74 knees) who had complete WOMAC and SF-36 data at the end of the study in order to determine whether there were any statistically significant differences between the 2 groups’ mean scores. No statistically significant differences were detected in any WOMAC or SF-36 category (α = 0.05).
Discussion
Metal-backed patellar components were originally designed to address the shortcomings (eg, fracture, deformation, aseptic loosening) of cemented all-polyethylene patellae.1-3 It was thought that the stiffness of the metal could help resist polyethylene deformation and that the press-fit interface with bone might eliminate issues related to bone cement.8 However, short-term failures were reported with early metal-backed designs.9,10 At the same time, good fixation with bone ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9-12,17 Further, reports of poor outcomes with some metal-backed patella designs overshadowed reports of positive outcomes.2,3 In all reports (of both poor and positive outcomes), component design, patellar tracking, and surgical technique were cited as contributing to implant success.2,3,14,17,18 Subsequent design improvements (eg, use of a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15
Our early results are similar to those reported in the literature, and we observed markedly better outcomes that we think resulted from component design improvements. Over the past decade, this has been particularly true with our use of the Duracon metal-backed patella, which has thicker polyethylene, better articular conformity, and a third stabilizing peg, all of which were previously noted as contributing to a successful metal-backed patellar component.2,12,14,15,19 In our study, all 72 knees radiographically evaluated and independently reviewed at minimum 5-year follow-up had well-fixed press-fit metal-backed patellae. Seventeen patellae had 1-mm radiolucencies; the other 59 had no radiolucencies in any zone around the patella–bone interface.
One of the most important aspects of removing a metal-backed patellar component from a patella is that the remaining bone stock is often far superior to the stock available after revision of a cemented patella. Careful removal should leave an excellent bony bed for reimplantation.
We think that surgeons should adhere to certain indications and contraindications when implanting metal-backed patellae and that doing so can contribute to successful outcomes. Type of bone stock available should be considered, as successful biological fixation relies on a good blood supply. A dense (or thin) patella in which intrusion of acrylic cement is improbable or impossible may favor use of a metal-backed patella. Cement is not an adhesive but a grout, so successful cementation requires intrusion of cement into the interstices of the cancellous bone. As adequate intrusion of cement into dense bone is not possible, cementation may not be the best option. Some patellae have failed because of peg “shear-off,”9 likely caused not by failure of peg strength but by failure of cement fixation at the nonpeg interface.20,21 Polyethylene pegs fail when used as the sole method of fixation (they were never designed for that). In addition, we think younger patients are often indicated for a metal-backed patella because, over the long term, loosening of a cemented patella (and the accompanying stress shielding and osteolysis) may cause severe patellar bone destruction. Last, we have found that abnormally high or small patellae are not good candidates for cement fixation because they tend to work themselves loose riding on and off the superior flange. These types of patellae appear to have a much sturdier and longer lasting interface than cement, once biological fixation has occurred.
In summary, we think the indications for a metal-backed implant are a patella that is dense or sclerotic; a patella that is thin, abnormally high, or small; and a younger patient. In addition, a metal-backed implant is not indicated for soft, osteoporotic bone.
This study had a few limitations. Fourteen knees (14 patients), or 15.9% of all knees in the study, were categorized as lost to follow-up. Comparing the WOMAC and SF-36 scores of 8 patients (8 knees) who completed minimum 5-year follow-up but were not clinically evaluated with the scores of patients who had complete data, we found no statistically significant differences in any category. However, 5-year follow-up clinical data were available for those 8 patients. Nevertheless, 74 knees were available for radiologic evaluation, and during telephone interviews all 8 patients indicated they had their original implant(s) and were asymptomatic.
Our experience with the Duracon metal-backed patella has been encouraging. In the study reported here, there were no failures caused by dissociation of plastic. We think that, because the porous coating is under almost constant compression, biological fixation is likely in most instances, as observed in our minimum 5-year radiologic results. Given our minimum 5-year follow-up results with uncemented metal-backed patellae, we think their use may be a viable alternative to use of all-polyethylene patellae.
1. Firestone TP, Teeny SM, Krackow KA, Hungerford DS. The clinical and roentgenographic results of cementless porous-coated patellar fixation. Clin Orthop Relat Res. 1991;273:184-189.
2. Laskin RS, Bucknell A. The use of metal-backed patellar prostheses in total knee arthroplasty. Clin Orthop Relat Res. 1990;260:52-55.
3. Evanich CJ, Tkach TK, von Glinski S, Camargo MP, Hofmann AA. 6- to 10-year experience using countersunk metal-backed patellas. J Arthroplasty. 1997;12(2):149-154.
4. Schwartz AJ, Della Vale CJ, Rosenberg AG, Jacobs JJ, Berger RA, Galante JO. Cruciate-retaining TKA using a third-generation system with a four-pegged tibial component: a minimum 10-year followup note. Clin Orthop Relat Res. 2010;468(8):2160-2167.
5. Bisschop R, Brouwer RW, Van Raay JJ. Total knee arthroplasty in younger patients: a 13-year follow-up study. Orthopedics. 2010;33(12):876-880.
6. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. A study of patients followed for a minimum of fifteen years. J Bone Joint Surg Am. 2005;87(3):598-603.
7. Brick GW, Scott RD. The patellofemoral component of total knee arthroplasty. Clin Orthop Relat Res. 1988;231)163-178.
8. Garcia RM, Kraay MJ, Goldberg VM. Isolated all-polyethylene patellar revisions for metal-backed patellar failure. Clin Orthop Relat Res. 2008;466(11):2784-2789.
9. Rosenberg AG, Andriacchi TP, Barden R, Galante JO. Patellar component failure in cementless total knee arthroplasty. Clin Orthop Relat Res. 1988;(236):106-114.
10. Stulberg SD, Stulberg BN, Hamati Y, Tsao A. Failure mechanisms of metal-backed patellar components. Clin Orthop Relat Res. 1988;236:88-105.
11. Sundfeldt M, Johansson CB, Regner L, Albrektsson T, Carlsson LV. Long-term results of a cementless knee prosthesis with a metal-backed patellar component: clinical and radiological follow-up with histology from retrieved components. J Long Term Eff Med Implants. 2003;13(4):341-354.
12. Kraay MJ, Darr OJ, Salata MJ, Goldberg VM. Outcome of metal-backed cementless patellar components: the effect of implant design. Clin Orthop Relat Res. 2001;392:239-244.
13. Jensen LN, Lund B, Gotfredsen K. Bone growth into a revised porous-coated patellar implant. Acta Orthop Scand. 1990;61(3):213-216.
14. Hsu HP, Walker PS. Wear and deformation of patellar components in total knee arthroplasty. Clin Orthop Relat Res. 1989;246:260-265.
15. Jordan LR, Sorrells RB, Jordan LC, Olivo JL. The long-term results of a metal-backed mobile bearing patella. Clin Orthop Relat Res. 2005;436:111-118.
16. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
17. Bayley JC, Scott RD, Ewald FC, Holmes GB Jr. Failure of the metal-backed patellar component after total knee replacement. J Bone Joint Surg Am. 1988;70(5):668-674.
18. Lombardi AV Jr, Engh GA, Volz RG, Albrigo JL, Brainard BJ. Fracture/dissociation of the polyethylene in metal-backed patellar components in total knee arthroplasty. J Bone Joint Surg Am. 1988;70(5):675-679.
19. Moreland JR. Mechanisms of failure in total knee arthroplasty. Clin Orthop Relat Res. 1988;226:49-64.
20. Francke EI, Lachiewicz PF. Failure of a cemented all-polyethylene patellar component of a press-fit condylar total knee arthroplasty. J Arthroplasty. 2000;15(2):234-237.
21. Stulberg BN, Wright TM, Stoller AP, Mimnaugh KL, Mason JJ. Bilateral patellar component shear failure of highly cross-linked polyethylene components: report of a case and laboratory analysis of failure mechanisms. J Arthroplasty. 2012;27(5):789-796.
The metal-backed patella was originally designed to address the shortcomings of cemented, all-polyethylene patellae: deformation, aseptic loosening, stress fractures of polyethylene, and possible thermal damage from bone cement.1-3 Several long-term studies have found very good outcomes with use of all-polyethylene patellae.4-6 However, complications of using an all-polyethylene patella reportedly accounted for up to half of all knee revisions, and during revision surgery patellar bone stock was often found to have been compromised.7
The intention behind the design of press-fit metal-backed patellae was to address the shortcomings of all-polyethylene patellae by eliminating the need for bone cement and providing stiffness that would help resist polyethylene deformation while decreasing implant–bone interface stresses.8 However, early design iterations of metal-backed patellae demonstrated short-term failures—most commonly, local polyethylene wear damaging the locking mechanism and subsequent dissociation or fracture from the metal baseplate; polyethylene delamination from the metal baseplate; and failure of interface fixation.9,10 On the other hand, good fixation with bony ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9,11-13 Overall, however, negative outcomes reported for metal-backed patellae led many surgeons to abandon these components and return to using cemented all-polyethylene patellae.
Negative outcomes of earlier metal-backed patellae designs have overshadowed reports of positive outcomes achieved with careful attention paid to component design, patellar tracking, and surgical technique.2,3,14 Subsequent design improvements (eg, a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15 The advantages of using a metal-backed patella (eg, uniform load sharing, decreased polyethylene deformation, potential for biological fixation) may be unjustly outweighed by the fear of patellar component failure.3
Our 30-plus years of experience with metal-backed patellar components reflect the evolving effect of component design on outcome. Much as reported elsewhere, we found earlier component failures were caused by poor locking mechanisms, thin polyethylene, poor tracking, and minimal femur contact. Over the past decade, however, our outcomes with Duracon metal-backed patellae (Stryker) have been encouraging. We think these positive outcomes, seen over minimum 5-year follow-up, are largely attributable to the thicker polyethylene and improved articular conformity of this component relative to earlier designs. We have also found it helpful to adhere to certain criteria when implanting metal-backed patellae, and we think adhering to these criteria, along with improved component design, indicates use of press-fit metal-backed patellae. In this article, we report our failure incidence with use of this device at minimum 5-year follow-up.
Materials and Methods
In this single-center study, we performed clinical and independent radiographic reviews of 88 primary press-fit metal-backed patellae with minimum 5-year follow-up. All components were the same design (Duracon metal-backed patella) from the same manufacturer (Stryker).
This study, which began in September 2003, was reviewed and approved by the Western Institutional Review Board (WIRB). Either the investigator (Dr. Hedley) or the clinical study coordinator gave study candidates a full explanation of the study and answered any questions. Patients who still wanted to participate in the study signed WIRB consent forms after their index surgery but before minimum 5-year follow-up.
Device Description
This Duracon patella has a porous-coated cobalt-chromium metal back intended for press-fit fixation, 3 cobalt-chromium porous-coated pegs, and a preassembled polyethylene anterior surface (Figure 1). Four sizes are available to fit the peripheral shape of the resected patella.
This patella has 3 styles: symmetric, asymmetric, and conversion. In this study, we used only the asymmetric and conversion styles. The design of each style incorporates medial/lateral facets intended to conform to the convex intercondylar radii of the femoral component, thereby allowing the patella to ride deeply in the recessed patellofemoral groove. The asymmetric patella is a resurfacing component with a generous polyethylene thickness (4.6 mm at its thinnest) and a larger lateral facet for more bone coverage. The asymmetric patella naturally medializes component placement. The articulating surface of the conversion patella is identical to that of the asymmetric patella. However, the conversion patella allows for exchange of the polyethylene portion of the implant without revising a stable, well-fixed metal baseplate.
Patient Selection
Candidates were recruited from a group of metal-backed patella patients within Dr. Hedley’s medical practice. All candidates had undergone primary total knee arthroplasty and received a Duracon press-fit metal-backed patella. All recruited patients had undergone primary knee arthroplasty at least 5 years before clinical and radiographic evaluation. Patients were included in the study if they had a diagnosis of noninflammatory degenerative joint disease (eg, osteoarthritis, traumatic arthritis, avascular necrosis). Patients with body mass index higher than 40 were excluded from the study.
Surgical Technique
The patella is everted completely or as much as feasible. Debridement is done circumferentially around the patella. Adherent fat and pseudomeniscus are stripped back until the surgeon sees the entry point of the quadriceps tendon fibers above and the patella tendon fibers below. The cut is then made at this level to remove as much bone as needed to restore the normal height of the patella with the implant in place. The cut is usually made by hand—without guides but with the patella stabilized with a towel clip above and below to prevent any movement during the action.
The desired cut must be absolutely planar, and this should be checked by placing the edge of the blade across the interface. Repeated passes with the saw blade are needed if the cut is not 100% planar. Once the cut is made, the patella is sized with the patella sizers and drill guide. After the appropriate size is selected, the patella is drilled with a bit that is slightly undersized from the size of the pegs (1/32 inch smaller than the bit supplied by the manufacturer).
Once the patella is prepared, the rest of the knee arthroplasty is performed. The patella is press-fit as the last component to be inserted.
Radiologic Review
Radiographic analysis was performed by an independent reviewer according to the current Knee Society total knee arthroplasty roentgenographic evaluation and scoring system (Figure 2).16 The reviewer was an orthopedist specializing in hip and knee surgery. Radiographs the reviewer deemed questionable were shown to another independent hip and knee surgeon for validation. In all cases, the second reviewer confirmed the first reviewer’s initial recorded observations.
KSS (Knee Society Scale), WOMAC (Western Ontario and McMaster Universities Arthritis Index), and SF-36 (36-Item Short Form Health Survey) were also used to evaluate effectiveness in this protocol.
Survivorship Calculations
Kaplan-Meier survivorship was determined for all metal-backed patellae. For survival analysis, only knees with radiographic data were included (74 knees). Mean follow-up was 75.8 months (range, 60-105 months).
Seventy-four patients (88 knees) met the study criteria (Table). At minimum 5-year follow-up, complete data were acquired for 59 patients (72 knees). Of the total group, 14 knees did not have radiographic data. Those knees were categorized as lost to follow-up and were excluded from the survivorship analysis. The status of patients enrolled in the study at minimum 5-year follow-up is shown in the Table.
Mann-Whitney U test (nonparametric t test) was used to compare WOMAC and SF-36 scores between the “complete” and the “WOMAC and SF-36 only” data groups.
Statistical Analysis
Kaplan-Meier survivorship probabilities (asymmetric method) were calculated using SAS Version 9.2 (SAS Institute); 95% pointwise confidence limits were used.
The Mann-Whitney U test is a nonparametric analogue to the independent-samples t test. It was used here to compare WOMAC and SF-36 scores of patients with “complete” data with scores of patients with “WOMAC and SF-36 only” data. In either group, for patients who had primary bilateral knee arthroplasty, mean WOMAC and SF-36 scores were used.
Comparisons were made between the unilateral and bilateral knee arthroplasty groups. There were no differences in age, height, or weight (Mann-Whitney U test) or in sex, primary diagnosis, or number of patients lost to follow-up (Fisher exact test). Fisher exact test (vs χ2 test) was used for the contingency table analysis because of small cell sizes (eg, ≤10 females in ‘‘both knees” group), suggesting the unilateral and bilateral patients did not differ in demographics.
For all patient-reported questionnaires, bilateral patients were given the opportunity to note any differences between their knee arthroplasties, but none of these patients made any special notations. We interpreted this to mean that all survey responses from bilateral patients were applicable to both knee arthroplasties.
Results
Seventy-four patients (88 knees) were enrolled in the study: 31 women (41.2%) and 43 men (58.1%). At time of surgery, mean age was 59.7 years (range, 40-86 years), and mean body mass index was 30.6 (range, 19.1-39.6). Eighty-three knees were diagnosed with osteoarthritis, and 5 knees were diagnosed with posttraumatic arthritis. Mean time to follow-up was 74.8 months (range, 60-105 months). Fourteen knees (14 patients) were considered lost to follow-up. However, 8 patients (8 knees) were contacted by telephone about the status of their knee(s), and all 8 completed and returned the minimum 5-year follow-up WOMAC and SF-36 forms; they did not return for their minimum 5-year clinical or radiographic evaluations.
Asymmetric patellae were used in 24 knees, conversion patellae in 64 knees (88 knees total). Forty-nine months after surgery, 1 patella was revised for loosening at its interface with the bone. The 51-year-old active female patient’s asymmetric patella was revised to a conversion patella. The decision to implant another metal-backed device was based on its high density; proper intrusion of acrylic cement would have been questionable. Some early wear was observed on the tibial insert, which was replaced. Sixty-eight months after the revision, the patient was asymptomatic, with a KSS Pain score of 96 and a KSS Function score of 100 (Figure 3). Another revision, for tibial insert exchange only, was performed 48 months after surgery. During this revision, the patella was evaluated and found to be well fixed and functioning normally.
Survivorship of the Duracon metal-backed patella at minimum 5-year follow-up was estimated to be 93.95%, with bounds of 73.61% and 98.74%.
Radiographic analysis revealed no radiolucencies larger than 1 mm (Figure 4). Seventeen 1-mm radiolucencies were recorded: 6 (35.3%) in zone 1, 2 (11.8%) in zone 2, and 9 (52.9%) in zone 4. Twelve (70.6%) of the 17 radiolucencies were in the left knee. Nine radiolucencies were in women and 8 in men. Most (55.6%) of the women’s radiolucencies were in zone 1, and most (75.0%) of the men’s were in zone 4. There were no loose beads other than in the case that was later revised.
KSS, WOMAC, and SF-36 scores and radiographic reviews were used to evaluate effectiveness in accordance with the protocol. At minimum 5-year follow-up, mean KSS Pain score was 94.10 (range, 55-100), and mean KSS Function score was 92.67 (range, 60-100). Mean WOMAC score was 2.21 (range, 0-19.70), mean SF-36 Physical score was 83.65 (range, 30.70-100), and mean SF-36 Mental score was 89.41 (range, 1.4-100).
The preceding calculations do not include WOMAC and SF-36 data for the 8 patients (8 knees) who were counted as lost to follow-up but who submitted minimum 5-year follow-up data. We compared these 8 patients with the 60 patients (74 knees) who had complete WOMAC and SF-36 data at the end of the study in order to determine whether there were any statistically significant differences between the 2 groups’ mean scores. No statistically significant differences were detected in any WOMAC or SF-36 category (α = 0.05).
Discussion
Metal-backed patellar components were originally designed to address the shortcomings (eg, fracture, deformation, aseptic loosening) of cemented all-polyethylene patellae.1-3 It was thought that the stiffness of the metal could help resist polyethylene deformation and that the press-fit interface with bone might eliminate issues related to bone cement.8 However, short-term failures were reported with early metal-backed designs.9,10 At the same time, good fixation with bone ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9-12,17 Further, reports of poor outcomes with some metal-backed patella designs overshadowed reports of positive outcomes.2,3 In all reports (of both poor and positive outcomes), component design, patellar tracking, and surgical technique were cited as contributing to implant success.2,3,14,17,18 Subsequent design improvements (eg, use of a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15
Our early results are similar to those reported in the literature, and we observed markedly better outcomes that we think resulted from component design improvements. Over the past decade, this has been particularly true with our use of the Duracon metal-backed patella, which has thicker polyethylene, better articular conformity, and a third stabilizing peg, all of which were previously noted as contributing to a successful metal-backed patellar component.2,12,14,15,19 In our study, all 72 knees radiographically evaluated and independently reviewed at minimum 5-year follow-up had well-fixed press-fit metal-backed patellae. Seventeen patellae had 1-mm radiolucencies; the other 59 had no radiolucencies in any zone around the patella–bone interface.
One of the most important aspects of removing a metal-backed patellar component from a patella is that the remaining bone stock is often far superior to the stock available after revision of a cemented patella. Careful removal should leave an excellent bony bed for reimplantation.
We think that surgeons should adhere to certain indications and contraindications when implanting metal-backed patellae and that doing so can contribute to successful outcomes. Type of bone stock available should be considered, as successful biological fixation relies on a good blood supply. A dense (or thin) patella in which intrusion of acrylic cement is improbable or impossible may favor use of a metal-backed patella. Cement is not an adhesive but a grout, so successful cementation requires intrusion of cement into the interstices of the cancellous bone. As adequate intrusion of cement into dense bone is not possible, cementation may not be the best option. Some patellae have failed because of peg “shear-off,”9 likely caused not by failure of peg strength but by failure of cement fixation at the nonpeg interface.20,21 Polyethylene pegs fail when used as the sole method of fixation (they were never designed for that). In addition, we think younger patients are often indicated for a metal-backed patella because, over the long term, loosening of a cemented patella (and the accompanying stress shielding and osteolysis) may cause severe patellar bone destruction. Last, we have found that abnormally high or small patellae are not good candidates for cement fixation because they tend to work themselves loose riding on and off the superior flange. These types of patellae appear to have a much sturdier and longer lasting interface than cement, once biological fixation has occurred.
In summary, we think the indications for a metal-backed implant are a patella that is dense or sclerotic; a patella that is thin, abnormally high, or small; and a younger patient. In addition, a metal-backed implant is not indicated for soft, osteoporotic bone.
This study had a few limitations. Fourteen knees (14 patients), or 15.9% of all knees in the study, were categorized as lost to follow-up. Comparing the WOMAC and SF-36 scores of 8 patients (8 knees) who completed minimum 5-year follow-up but were not clinically evaluated with the scores of patients who had complete data, we found no statistically significant differences in any category. However, 5-year follow-up clinical data were available for those 8 patients. Nevertheless, 74 knees were available for radiologic evaluation, and during telephone interviews all 8 patients indicated they had their original implant(s) and were asymptomatic.
Our experience with the Duracon metal-backed patella has been encouraging. In the study reported here, there were no failures caused by dissociation of plastic. We think that, because the porous coating is under almost constant compression, biological fixation is likely in most instances, as observed in our minimum 5-year radiologic results. Given our minimum 5-year follow-up results with uncemented metal-backed patellae, we think their use may be a viable alternative to use of all-polyethylene patellae.
The metal-backed patella was originally designed to address the shortcomings of cemented, all-polyethylene patellae: deformation, aseptic loosening, stress fractures of polyethylene, and possible thermal damage from bone cement.1-3 Several long-term studies have found very good outcomes with use of all-polyethylene patellae.4-6 However, complications of using an all-polyethylene patella reportedly accounted for up to half of all knee revisions, and during revision surgery patellar bone stock was often found to have been compromised.7
The intention behind the design of press-fit metal-backed patellae was to address the shortcomings of all-polyethylene patellae by eliminating the need for bone cement and providing stiffness that would help resist polyethylene deformation while decreasing implant–bone interface stresses.8 However, early design iterations of metal-backed patellae demonstrated short-term failures—most commonly, local polyethylene wear damaging the locking mechanism and subsequent dissociation or fracture from the metal baseplate; polyethylene delamination from the metal baseplate; and failure of interface fixation.9,10 On the other hand, good fixation with bony ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9,11-13 Overall, however, negative outcomes reported for metal-backed patellae led many surgeons to abandon these components and return to using cemented all-polyethylene patellae.
Negative outcomes of earlier metal-backed patellae designs have overshadowed reports of positive outcomes achieved with careful attention paid to component design, patellar tracking, and surgical technique.2,3,14 Subsequent design improvements (eg, a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15 The advantages of using a metal-backed patella (eg, uniform load sharing, decreased polyethylene deformation, potential for biological fixation) may be unjustly outweighed by the fear of patellar component failure.3
Our 30-plus years of experience with metal-backed patellar components reflect the evolving effect of component design on outcome. Much as reported elsewhere, we found earlier component failures were caused by poor locking mechanisms, thin polyethylene, poor tracking, and minimal femur contact. Over the past decade, however, our outcomes with Duracon metal-backed patellae (Stryker) have been encouraging. We think these positive outcomes, seen over minimum 5-year follow-up, are largely attributable to the thicker polyethylene and improved articular conformity of this component relative to earlier designs. We have also found it helpful to adhere to certain criteria when implanting metal-backed patellae, and we think adhering to these criteria, along with improved component design, indicates use of press-fit metal-backed patellae. In this article, we report our failure incidence with use of this device at minimum 5-year follow-up.
Materials and Methods
In this single-center study, we performed clinical and independent radiographic reviews of 88 primary press-fit metal-backed patellae with minimum 5-year follow-up. All components were the same design (Duracon metal-backed patella) from the same manufacturer (Stryker).
This study, which began in September 2003, was reviewed and approved by the Western Institutional Review Board (WIRB). Either the investigator (Dr. Hedley) or the clinical study coordinator gave study candidates a full explanation of the study and answered any questions. Patients who still wanted to participate in the study signed WIRB consent forms after their index surgery but before minimum 5-year follow-up.
Device Description
This Duracon patella has a porous-coated cobalt-chromium metal back intended for press-fit fixation, 3 cobalt-chromium porous-coated pegs, and a preassembled polyethylene anterior surface (Figure 1). Four sizes are available to fit the peripheral shape of the resected patella.
This patella has 3 styles: symmetric, asymmetric, and conversion. In this study, we used only the asymmetric and conversion styles. The design of each style incorporates medial/lateral facets intended to conform to the convex intercondylar radii of the femoral component, thereby allowing the patella to ride deeply in the recessed patellofemoral groove. The asymmetric patella is a resurfacing component with a generous polyethylene thickness (4.6 mm at its thinnest) and a larger lateral facet for more bone coverage. The asymmetric patella naturally medializes component placement. The articulating surface of the conversion patella is identical to that of the asymmetric patella. However, the conversion patella allows for exchange of the polyethylene portion of the implant without revising a stable, well-fixed metal baseplate.
Patient Selection
Candidates were recruited from a group of metal-backed patella patients within Dr. Hedley’s medical practice. All candidates had undergone primary total knee arthroplasty and received a Duracon press-fit metal-backed patella. All recruited patients had undergone primary knee arthroplasty at least 5 years before clinical and radiographic evaluation. Patients were included in the study if they had a diagnosis of noninflammatory degenerative joint disease (eg, osteoarthritis, traumatic arthritis, avascular necrosis). Patients with body mass index higher than 40 were excluded from the study.
Surgical Technique
The patella is everted completely or as much as feasible. Debridement is done circumferentially around the patella. Adherent fat and pseudomeniscus are stripped back until the surgeon sees the entry point of the quadriceps tendon fibers above and the patella tendon fibers below. The cut is then made at this level to remove as much bone as needed to restore the normal height of the patella with the implant in place. The cut is usually made by hand—without guides but with the patella stabilized with a towel clip above and below to prevent any movement during the action.
The desired cut must be absolutely planar, and this should be checked by placing the edge of the blade across the interface. Repeated passes with the saw blade are needed if the cut is not 100% planar. Once the cut is made, the patella is sized with the patella sizers and drill guide. After the appropriate size is selected, the patella is drilled with a bit that is slightly undersized from the size of the pegs (1/32 inch smaller than the bit supplied by the manufacturer).
Once the patella is prepared, the rest of the knee arthroplasty is performed. The patella is press-fit as the last component to be inserted.
Radiologic Review
Radiographic analysis was performed by an independent reviewer according to the current Knee Society total knee arthroplasty roentgenographic evaluation and scoring system (Figure 2).16 The reviewer was an orthopedist specializing in hip and knee surgery. Radiographs the reviewer deemed questionable were shown to another independent hip and knee surgeon for validation. In all cases, the second reviewer confirmed the first reviewer’s initial recorded observations.
KSS (Knee Society Scale), WOMAC (Western Ontario and McMaster Universities Arthritis Index), and SF-36 (36-Item Short Form Health Survey) were also used to evaluate effectiveness in this protocol.
Survivorship Calculations
Kaplan-Meier survivorship was determined for all metal-backed patellae. For survival analysis, only knees with radiographic data were included (74 knees). Mean follow-up was 75.8 months (range, 60-105 months).
Seventy-four patients (88 knees) met the study criteria (Table). At minimum 5-year follow-up, complete data were acquired for 59 patients (72 knees). Of the total group, 14 knees did not have radiographic data. Those knees were categorized as lost to follow-up and were excluded from the survivorship analysis. The status of patients enrolled in the study at minimum 5-year follow-up is shown in the Table.
Mann-Whitney U test (nonparametric t test) was used to compare WOMAC and SF-36 scores between the “complete” and the “WOMAC and SF-36 only” data groups.
Statistical Analysis
Kaplan-Meier survivorship probabilities (asymmetric method) were calculated using SAS Version 9.2 (SAS Institute); 95% pointwise confidence limits were used.
The Mann-Whitney U test is a nonparametric analogue to the independent-samples t test. It was used here to compare WOMAC and SF-36 scores of patients with “complete” data with scores of patients with “WOMAC and SF-36 only” data. In either group, for patients who had primary bilateral knee arthroplasty, mean WOMAC and SF-36 scores were used.
Comparisons were made between the unilateral and bilateral knee arthroplasty groups. There were no differences in age, height, or weight (Mann-Whitney U test) or in sex, primary diagnosis, or number of patients lost to follow-up (Fisher exact test). Fisher exact test (vs χ2 test) was used for the contingency table analysis because of small cell sizes (eg, ≤10 females in ‘‘both knees” group), suggesting the unilateral and bilateral patients did not differ in demographics.
For all patient-reported questionnaires, bilateral patients were given the opportunity to note any differences between their knee arthroplasties, but none of these patients made any special notations. We interpreted this to mean that all survey responses from bilateral patients were applicable to both knee arthroplasties.
Results
Seventy-four patients (88 knees) were enrolled in the study: 31 women (41.2%) and 43 men (58.1%). At time of surgery, mean age was 59.7 years (range, 40-86 years), and mean body mass index was 30.6 (range, 19.1-39.6). Eighty-three knees were diagnosed with osteoarthritis, and 5 knees were diagnosed with posttraumatic arthritis. Mean time to follow-up was 74.8 months (range, 60-105 months). Fourteen knees (14 patients) were considered lost to follow-up. However, 8 patients (8 knees) were contacted by telephone about the status of their knee(s), and all 8 completed and returned the minimum 5-year follow-up WOMAC and SF-36 forms; they did not return for their minimum 5-year clinical or radiographic evaluations.
Asymmetric patellae were used in 24 knees, conversion patellae in 64 knees (88 knees total). Forty-nine months after surgery, 1 patella was revised for loosening at its interface with the bone. The 51-year-old active female patient’s asymmetric patella was revised to a conversion patella. The decision to implant another metal-backed device was based on its high density; proper intrusion of acrylic cement would have been questionable. Some early wear was observed on the tibial insert, which was replaced. Sixty-eight months after the revision, the patient was asymptomatic, with a KSS Pain score of 96 and a KSS Function score of 100 (Figure 3). Another revision, for tibial insert exchange only, was performed 48 months after surgery. During this revision, the patella was evaluated and found to be well fixed and functioning normally.
Survivorship of the Duracon metal-backed patella at minimum 5-year follow-up was estimated to be 93.95%, with bounds of 73.61% and 98.74%.
Radiographic analysis revealed no radiolucencies larger than 1 mm (Figure 4). Seventeen 1-mm radiolucencies were recorded: 6 (35.3%) in zone 1, 2 (11.8%) in zone 2, and 9 (52.9%) in zone 4. Twelve (70.6%) of the 17 radiolucencies were in the left knee. Nine radiolucencies were in women and 8 in men. Most (55.6%) of the women’s radiolucencies were in zone 1, and most (75.0%) of the men’s were in zone 4. There were no loose beads other than in the case that was later revised.
KSS, WOMAC, and SF-36 scores and radiographic reviews were used to evaluate effectiveness in accordance with the protocol. At minimum 5-year follow-up, mean KSS Pain score was 94.10 (range, 55-100), and mean KSS Function score was 92.67 (range, 60-100). Mean WOMAC score was 2.21 (range, 0-19.70), mean SF-36 Physical score was 83.65 (range, 30.70-100), and mean SF-36 Mental score was 89.41 (range, 1.4-100).
The preceding calculations do not include WOMAC and SF-36 data for the 8 patients (8 knees) who were counted as lost to follow-up but who submitted minimum 5-year follow-up data. We compared these 8 patients with the 60 patients (74 knees) who had complete WOMAC and SF-36 data at the end of the study in order to determine whether there were any statistically significant differences between the 2 groups’ mean scores. No statistically significant differences were detected in any WOMAC or SF-36 category (α = 0.05).
Discussion
Metal-backed patellar components were originally designed to address the shortcomings (eg, fracture, deformation, aseptic loosening) of cemented all-polyethylene patellae.1-3 It was thought that the stiffness of the metal could help resist polyethylene deformation and that the press-fit interface with bone might eliminate issues related to bone cement.8 However, short-term failures were reported with early metal-backed designs.9,10 At the same time, good fixation with bone ingrowth was observed in both titanium and cobalt-chromium porous-coated patellae.1,3,9-12,17 Further, reports of poor outcomes with some metal-backed patella designs overshadowed reports of positive outcomes.2,3 In all reports (of both poor and positive outcomes), component design, patellar tracking, and surgical technique were cited as contributing to implant success.2,3,14,17,18 Subsequent design improvements (eg, use of a third stabilizing peg, thicker polyethylene, improved conformity) produced excellent outcomes.8,12,15
Our early results are similar to those reported in the literature, and we observed markedly better outcomes that we think resulted from component design improvements. Over the past decade, this has been particularly true with our use of the Duracon metal-backed patella, which has thicker polyethylene, better articular conformity, and a third stabilizing peg, all of which were previously noted as contributing to a successful metal-backed patellar component.2,12,14,15,19 In our study, all 72 knees radiographically evaluated and independently reviewed at minimum 5-year follow-up had well-fixed press-fit metal-backed patellae. Seventeen patellae had 1-mm radiolucencies; the other 59 had no radiolucencies in any zone around the patella–bone interface.
One of the most important aspects of removing a metal-backed patellar component from a patella is that the remaining bone stock is often far superior to the stock available after revision of a cemented patella. Careful removal should leave an excellent bony bed for reimplantation.
We think that surgeons should adhere to certain indications and contraindications when implanting metal-backed patellae and that doing so can contribute to successful outcomes. Type of bone stock available should be considered, as successful biological fixation relies on a good blood supply. A dense (or thin) patella in which intrusion of acrylic cement is improbable or impossible may favor use of a metal-backed patella. Cement is not an adhesive but a grout, so successful cementation requires intrusion of cement into the interstices of the cancellous bone. As adequate intrusion of cement into dense bone is not possible, cementation may not be the best option. Some patellae have failed because of peg “shear-off,”9 likely caused not by failure of peg strength but by failure of cement fixation at the nonpeg interface.20,21 Polyethylene pegs fail when used as the sole method of fixation (they were never designed for that). In addition, we think younger patients are often indicated for a metal-backed patella because, over the long term, loosening of a cemented patella (and the accompanying stress shielding and osteolysis) may cause severe patellar bone destruction. Last, we have found that abnormally high or small patellae are not good candidates for cement fixation because they tend to work themselves loose riding on and off the superior flange. These types of patellae appear to have a much sturdier and longer lasting interface than cement, once biological fixation has occurred.
In summary, we think the indications for a metal-backed implant are a patella that is dense or sclerotic; a patella that is thin, abnormally high, or small; and a younger patient. In addition, a metal-backed implant is not indicated for soft, osteoporotic bone.
This study had a few limitations. Fourteen knees (14 patients), or 15.9% of all knees in the study, were categorized as lost to follow-up. Comparing the WOMAC and SF-36 scores of 8 patients (8 knees) who completed minimum 5-year follow-up but were not clinically evaluated with the scores of patients who had complete data, we found no statistically significant differences in any category. However, 5-year follow-up clinical data were available for those 8 patients. Nevertheless, 74 knees were available for radiologic evaluation, and during telephone interviews all 8 patients indicated they had their original implant(s) and were asymptomatic.
Our experience with the Duracon metal-backed patella has been encouraging. In the study reported here, there were no failures caused by dissociation of plastic. We think that, because the porous coating is under almost constant compression, biological fixation is likely in most instances, as observed in our minimum 5-year radiologic results. Given our minimum 5-year follow-up results with uncemented metal-backed patellae, we think their use may be a viable alternative to use of all-polyethylene patellae.
1. Firestone TP, Teeny SM, Krackow KA, Hungerford DS. The clinical and roentgenographic results of cementless porous-coated patellar fixation. Clin Orthop Relat Res. 1991;273:184-189.
2. Laskin RS, Bucknell A. The use of metal-backed patellar prostheses in total knee arthroplasty. Clin Orthop Relat Res. 1990;260:52-55.
3. Evanich CJ, Tkach TK, von Glinski S, Camargo MP, Hofmann AA. 6- to 10-year experience using countersunk metal-backed patellas. J Arthroplasty. 1997;12(2):149-154.
4. Schwartz AJ, Della Vale CJ, Rosenberg AG, Jacobs JJ, Berger RA, Galante JO. Cruciate-retaining TKA using a third-generation system with a four-pegged tibial component: a minimum 10-year followup note. Clin Orthop Relat Res. 2010;468(8):2160-2167.
5. Bisschop R, Brouwer RW, Van Raay JJ. Total knee arthroplasty in younger patients: a 13-year follow-up study. Orthopedics. 2010;33(12):876-880.
6. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. A study of patients followed for a minimum of fifteen years. J Bone Joint Surg Am. 2005;87(3):598-603.
7. Brick GW, Scott RD. The patellofemoral component of total knee arthroplasty. Clin Orthop Relat Res. 1988;231)163-178.
8. Garcia RM, Kraay MJ, Goldberg VM. Isolated all-polyethylene patellar revisions for metal-backed patellar failure. Clin Orthop Relat Res. 2008;466(11):2784-2789.
9. Rosenberg AG, Andriacchi TP, Barden R, Galante JO. Patellar component failure in cementless total knee arthroplasty. Clin Orthop Relat Res. 1988;(236):106-114.
10. Stulberg SD, Stulberg BN, Hamati Y, Tsao A. Failure mechanisms of metal-backed patellar components. Clin Orthop Relat Res. 1988;236:88-105.
11. Sundfeldt M, Johansson CB, Regner L, Albrektsson T, Carlsson LV. Long-term results of a cementless knee prosthesis with a metal-backed patellar component: clinical and radiological follow-up with histology from retrieved components. J Long Term Eff Med Implants. 2003;13(4):341-354.
12. Kraay MJ, Darr OJ, Salata MJ, Goldberg VM. Outcome of metal-backed cementless patellar components: the effect of implant design. Clin Orthop Relat Res. 2001;392:239-244.
13. Jensen LN, Lund B, Gotfredsen K. Bone growth into a revised porous-coated patellar implant. Acta Orthop Scand. 1990;61(3):213-216.
14. Hsu HP, Walker PS. Wear and deformation of patellar components in total knee arthroplasty. Clin Orthop Relat Res. 1989;246:260-265.
15. Jordan LR, Sorrells RB, Jordan LC, Olivo JL. The long-term results of a metal-backed mobile bearing patella. Clin Orthop Relat Res. 2005;436:111-118.
16. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
17. Bayley JC, Scott RD, Ewald FC, Holmes GB Jr. Failure of the metal-backed patellar component after total knee replacement. J Bone Joint Surg Am. 1988;70(5):668-674.
18. Lombardi AV Jr, Engh GA, Volz RG, Albrigo JL, Brainard BJ. Fracture/dissociation of the polyethylene in metal-backed patellar components in total knee arthroplasty. J Bone Joint Surg Am. 1988;70(5):675-679.
19. Moreland JR. Mechanisms of failure in total knee arthroplasty. Clin Orthop Relat Res. 1988;226:49-64.
20. Francke EI, Lachiewicz PF. Failure of a cemented all-polyethylene patellar component of a press-fit condylar total knee arthroplasty. J Arthroplasty. 2000;15(2):234-237.
21. Stulberg BN, Wright TM, Stoller AP, Mimnaugh KL, Mason JJ. Bilateral patellar component shear failure of highly cross-linked polyethylene components: report of a case and laboratory analysis of failure mechanisms. J Arthroplasty. 2012;27(5):789-796.
1. Firestone TP, Teeny SM, Krackow KA, Hungerford DS. The clinical and roentgenographic results of cementless porous-coated patellar fixation. Clin Orthop Relat Res. 1991;273:184-189.
2. Laskin RS, Bucknell A. The use of metal-backed patellar prostheses in total knee arthroplasty. Clin Orthop Relat Res. 1990;260:52-55.
3. Evanich CJ, Tkach TK, von Glinski S, Camargo MP, Hofmann AA. 6- to 10-year experience using countersunk metal-backed patellas. J Arthroplasty. 1997;12(2):149-154.
4. Schwartz AJ, Della Vale CJ, Rosenberg AG, Jacobs JJ, Berger RA, Galante JO. Cruciate-retaining TKA using a third-generation system with a four-pegged tibial component: a minimum 10-year followup note. Clin Orthop Relat Res. 2010;468(8):2160-2167.
5. Bisschop R, Brouwer RW, Van Raay JJ. Total knee arthroplasty in younger patients: a 13-year follow-up study. Orthopedics. 2010;33(12):876-880.
6. Dixon MC, Brown RR, Parsch D, Scott RD. Modular fixed-bearing total knee arthroplasty with retention of the posterior cruciate ligament. A study of patients followed for a minimum of fifteen years. J Bone Joint Surg Am. 2005;87(3):598-603.
7. Brick GW, Scott RD. The patellofemoral component of total knee arthroplasty. Clin Orthop Relat Res. 1988;231)163-178.
8. Garcia RM, Kraay MJ, Goldberg VM. Isolated all-polyethylene patellar revisions for metal-backed patellar failure. Clin Orthop Relat Res. 2008;466(11):2784-2789.
9. Rosenberg AG, Andriacchi TP, Barden R, Galante JO. Patellar component failure in cementless total knee arthroplasty. Clin Orthop Relat Res. 1988;(236):106-114.
10. Stulberg SD, Stulberg BN, Hamati Y, Tsao A. Failure mechanisms of metal-backed patellar components. Clin Orthop Relat Res. 1988;236:88-105.
11. Sundfeldt M, Johansson CB, Regner L, Albrektsson T, Carlsson LV. Long-term results of a cementless knee prosthesis with a metal-backed patellar component: clinical and radiological follow-up with histology from retrieved components. J Long Term Eff Med Implants. 2003;13(4):341-354.
12. Kraay MJ, Darr OJ, Salata MJ, Goldberg VM. Outcome of metal-backed cementless patellar components: the effect of implant design. Clin Orthop Relat Res. 2001;392:239-244.
13. Jensen LN, Lund B, Gotfredsen K. Bone growth into a revised porous-coated patellar implant. Acta Orthop Scand. 1990;61(3):213-216.
14. Hsu HP, Walker PS. Wear and deformation of patellar components in total knee arthroplasty. Clin Orthop Relat Res. 1989;246:260-265.
15. Jordan LR, Sorrells RB, Jordan LC, Olivo JL. The long-term results of a metal-backed mobile bearing patella. Clin Orthop Relat Res. 2005;436:111-118.
16. Ewald FC. The Knee Society total knee arthroplasty roentgenographic evaluation and scoring system. Clin Orthop Relat Res. 1989;248:9-12.
17. Bayley JC, Scott RD, Ewald FC, Holmes GB Jr. Failure of the metal-backed patellar component after total knee replacement. J Bone Joint Surg Am. 1988;70(5):668-674.
18. Lombardi AV Jr, Engh GA, Volz RG, Albrigo JL, Brainard BJ. Fracture/dissociation of the polyethylene in metal-backed patellar components in total knee arthroplasty. J Bone Joint Surg Am. 1988;70(5):675-679.
19. Moreland JR. Mechanisms of failure in total knee arthroplasty. Clin Orthop Relat Res. 1988;226:49-64.
20. Francke EI, Lachiewicz PF. Failure of a cemented all-polyethylene patellar component of a press-fit condylar total knee arthroplasty. J Arthroplasty. 2000;15(2):234-237.
21. Stulberg BN, Wright TM, Stoller AP, Mimnaugh KL, Mason JJ. Bilateral patellar component shear failure of highly cross-linked polyethylene components: report of a case and laboratory analysis of failure mechanisms. J Arthroplasty. 2012;27(5):789-796.
Transitions
This month will mark the end of my 10-year tenure as Editor-in-Chief of The American Journal of Orthopedics (AJO). Every successful organization goes through periodic transitions, where past successes are reviewed and future challenges addressed. AJO is no different. I would like to reflect on these past 10 years, share some highlights, and acknowledge those who have contributed to the success of AJO.
The Editorial Staff of the Journal has consistently performed beyond the call of duty, producing outstanding issues month after month. Of this excellent team, several members deserve special mention: Group Editor Glenn Williams, Managing Editor Joseph Kinsley, and Assistant Editor Kellie DeSantis. I offer my gratitude to all for a job well done.
I am particularly proud that under my leadership, the Editorial Board nearly doubled and clinical submissions increased almost 4-fold. I want to thank all members of the Editorial Board. Your expertise and dedication has enabled AJO to accommodate the vast increase in submissions and greatly improved the quality of papers published in recent years. I have so appreciated your efforts and hard work.
The addition of the Residency Advisory Board several years ago provided an excellent forum for orthopedic surgeons in training to share their thoughts on subjects of particular interest and address issues not typically covered during the course of their clinical training; such as the importance of mentorship, how to organize a practice, and how to decipher an employment contract for one’s first “real” job. I have been immensely impressed with residents’ insights and their willingness to share them with their colleagues. I hope that this experience at AJO will encourage them to join other Editorial Boards during their professional careers.
Over these past 10 years, I have tried to satisfy the mission of The American Journal of Orthopedics: “… to provide timely, practical, and readable technical information of the highest caliber to the orthopedic surgeon involved in the everyday practice of orthopedics.” To this end, I expanded the “expert opinion” 5 Points series originally introduced by John Gould, MD, my predecessor as Editor-in-Chief. In addition, I added the Practice Management articles, prepared by Karen Zupko and her associates, which have been especially informative and popular.
I have thoroughly enjoyed sharing with you my nonclinical editorials touching on the “topics of the day.” Among my favorites were “Customer Satisfaction: Are Hospitals ‘Hospitable’?”1 which anticipated the growing influence of patient satisfaction scores in our professional lives; and “Are Surgeons Accepting Bribes?”,2 addressing a subject that predated the 2007 Deferred Prosecution Agreement3 which has transformed the relationships between orthopedic surgeons and the orthopedic device industry. I hope you have enjoyed reading my musings as much as I enjoyed writing them.
Our incoming Editor-in-Chief is Bryan T. Hanypsiak, MD, Director of Sports Medicine, Peconic Bay Medical Center in Riverhead, New York, and Chairman of the September 2015 Innovative Techniques: The Knee Course, sponsored by AJO in Las Vegas. Bryan will remain committed to the original mission of AJO and its high editorial and peer reviewed standards. However, the format will change with the March 2016 issue and have the feel of a clinical magazine. I have every confidence that, with Dr. Hanypsiak’s leadership and the support of the publisher, the AJO brand will continue to thrive in an ever-changing and challenging marketplace.
Finally, I wish to thank all the readers of AJO who have supported the Journal, one of only a handful of orthopedic publications that still caters to the professional interests of the general orthopedic surgeon with a comprehensive scope of clinical and non-clinical topics. Leading the Board as Editor-in-Chief has been one of the most fulfilling and rewarding activities of my professional career, and it has truly been my honor to serve you. I wish all of you the best.
1. McCann PD. Customer satisfaction: are hospitals “hospitable”? Am J Orthop. 2006;35(2):59.
2. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
3. Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed January 11, 2016.
This month will mark the end of my 10-year tenure as Editor-in-Chief of The American Journal of Orthopedics (AJO). Every successful organization goes through periodic transitions, where past successes are reviewed and future challenges addressed. AJO is no different. I would like to reflect on these past 10 years, share some highlights, and acknowledge those who have contributed to the success of AJO.
The Editorial Staff of the Journal has consistently performed beyond the call of duty, producing outstanding issues month after month. Of this excellent team, several members deserve special mention: Group Editor Glenn Williams, Managing Editor Joseph Kinsley, and Assistant Editor Kellie DeSantis. I offer my gratitude to all for a job well done.
I am particularly proud that under my leadership, the Editorial Board nearly doubled and clinical submissions increased almost 4-fold. I want to thank all members of the Editorial Board. Your expertise and dedication has enabled AJO to accommodate the vast increase in submissions and greatly improved the quality of papers published in recent years. I have so appreciated your efforts and hard work.
The addition of the Residency Advisory Board several years ago provided an excellent forum for orthopedic surgeons in training to share their thoughts on subjects of particular interest and address issues not typically covered during the course of their clinical training; such as the importance of mentorship, how to organize a practice, and how to decipher an employment contract for one’s first “real” job. I have been immensely impressed with residents’ insights and their willingness to share them with their colleagues. I hope that this experience at AJO will encourage them to join other Editorial Boards during their professional careers.
Over these past 10 years, I have tried to satisfy the mission of The American Journal of Orthopedics: “… to provide timely, practical, and readable technical information of the highest caliber to the orthopedic surgeon involved in the everyday practice of orthopedics.” To this end, I expanded the “expert opinion” 5 Points series originally introduced by John Gould, MD, my predecessor as Editor-in-Chief. In addition, I added the Practice Management articles, prepared by Karen Zupko and her associates, which have been especially informative and popular.
I have thoroughly enjoyed sharing with you my nonclinical editorials touching on the “topics of the day.” Among my favorites were “Customer Satisfaction: Are Hospitals ‘Hospitable’?”1 which anticipated the growing influence of patient satisfaction scores in our professional lives; and “Are Surgeons Accepting Bribes?”,2 addressing a subject that predated the 2007 Deferred Prosecution Agreement3 which has transformed the relationships between orthopedic surgeons and the orthopedic device industry. I hope you have enjoyed reading my musings as much as I enjoyed writing them.
Our incoming Editor-in-Chief is Bryan T. Hanypsiak, MD, Director of Sports Medicine, Peconic Bay Medical Center in Riverhead, New York, and Chairman of the September 2015 Innovative Techniques: The Knee Course, sponsored by AJO in Las Vegas. Bryan will remain committed to the original mission of AJO and its high editorial and peer reviewed standards. However, the format will change with the March 2016 issue and have the feel of a clinical magazine. I have every confidence that, with Dr. Hanypsiak’s leadership and the support of the publisher, the AJO brand will continue to thrive in an ever-changing and challenging marketplace.
Finally, I wish to thank all the readers of AJO who have supported the Journal, one of only a handful of orthopedic publications that still caters to the professional interests of the general orthopedic surgeon with a comprehensive scope of clinical and non-clinical topics. Leading the Board as Editor-in-Chief has been one of the most fulfilling and rewarding activities of my professional career, and it has truly been my honor to serve you. I wish all of you the best.
This month will mark the end of my 10-year tenure as Editor-in-Chief of The American Journal of Orthopedics (AJO). Every successful organization goes through periodic transitions, where past successes are reviewed and future challenges addressed. AJO is no different. I would like to reflect on these past 10 years, share some highlights, and acknowledge those who have contributed to the success of AJO.
The Editorial Staff of the Journal has consistently performed beyond the call of duty, producing outstanding issues month after month. Of this excellent team, several members deserve special mention: Group Editor Glenn Williams, Managing Editor Joseph Kinsley, and Assistant Editor Kellie DeSantis. I offer my gratitude to all for a job well done.
I am particularly proud that under my leadership, the Editorial Board nearly doubled and clinical submissions increased almost 4-fold. I want to thank all members of the Editorial Board. Your expertise and dedication has enabled AJO to accommodate the vast increase in submissions and greatly improved the quality of papers published in recent years. I have so appreciated your efforts and hard work.
The addition of the Residency Advisory Board several years ago provided an excellent forum for orthopedic surgeons in training to share their thoughts on subjects of particular interest and address issues not typically covered during the course of their clinical training; such as the importance of mentorship, how to organize a practice, and how to decipher an employment contract for one’s first “real” job. I have been immensely impressed with residents’ insights and their willingness to share them with their colleagues. I hope that this experience at AJO will encourage them to join other Editorial Boards during their professional careers.
Over these past 10 years, I have tried to satisfy the mission of The American Journal of Orthopedics: “… to provide timely, practical, and readable technical information of the highest caliber to the orthopedic surgeon involved in the everyday practice of orthopedics.” To this end, I expanded the “expert opinion” 5 Points series originally introduced by John Gould, MD, my predecessor as Editor-in-Chief. In addition, I added the Practice Management articles, prepared by Karen Zupko and her associates, which have been especially informative and popular.
I have thoroughly enjoyed sharing with you my nonclinical editorials touching on the “topics of the day.” Among my favorites were “Customer Satisfaction: Are Hospitals ‘Hospitable’?”1 which anticipated the growing influence of patient satisfaction scores in our professional lives; and “Are Surgeons Accepting Bribes?”,2 addressing a subject that predated the 2007 Deferred Prosecution Agreement3 which has transformed the relationships between orthopedic surgeons and the orthopedic device industry. I hope you have enjoyed reading my musings as much as I enjoyed writing them.
Our incoming Editor-in-Chief is Bryan T. Hanypsiak, MD, Director of Sports Medicine, Peconic Bay Medical Center in Riverhead, New York, and Chairman of the September 2015 Innovative Techniques: The Knee Course, sponsored by AJO in Las Vegas. Bryan will remain committed to the original mission of AJO and its high editorial and peer reviewed standards. However, the format will change with the March 2016 issue and have the feel of a clinical magazine. I have every confidence that, with Dr. Hanypsiak’s leadership and the support of the publisher, the AJO brand will continue to thrive in an ever-changing and challenging marketplace.
Finally, I wish to thank all the readers of AJO who have supported the Journal, one of only a handful of orthopedic publications that still caters to the professional interests of the general orthopedic surgeon with a comprehensive scope of clinical and non-clinical topics. Leading the Board as Editor-in-Chief has been one of the most fulfilling and rewarding activities of my professional career, and it has truly been my honor to serve you. I wish all of you the best.
1. McCann PD. Customer satisfaction: are hospitals “hospitable”? Am J Orthop. 2006;35(2):59.
2. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
3. Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed January 11, 2016.
1. McCann PD. Customer satisfaction: are hospitals “hospitable”? Am J Orthop. 2006;35(2):59.
2. McCann PD. Are surgeons accepting bribes? Am J Orthop. 2006;35(3):114.
3. Five companies in hip and knee replacement industry avoid prosecution by agreeing to compliance rules and monitoring [press release]. US Department of Justice website. http://www.justice.gov/usao/nj/Press/files/pdffiles/Older/hips0927.rel.pdf. Published September 27, 2007. Accessed January 11, 2016.
Subpectoral Biceps Tenodesis
Tendinopathy of the long head of the biceps brachii (LHB) is a common source of anterior shoulder pain. The LHB tendon is an intra-articular yet extrasynovial structure, ensheathed by the synovial lining of the articular capsule.1 Branches of the anterior circumflex humeral artery course along the bicipital groove, but the gliding undersurface of the LHB remains avascular.2 Tendon irritation is most common within the groove and usually produces “tendinosis,” characterized by collagen fiber atrophy, fibrinoid necrosis, and fibrocyte proliferation.1 Neviaser and colleagues3 correlated such changes in the LHB tendon with rotator cuff pathology, as the 2 often coexist. Primary LHB tendinitis is less common and associated with younger patients who engage in overhead activities, such as baseball and volleyball.4
Nonoperative management, which is trialed initially, consists of rest, use of nonsteroidal anti-inflammatory drugs, and physical therapy. Corticosteroid injections are administered through the subacromial space or glenohumeral joint, which is continuous with the LHB sheath. Some physicians give ultrasound-guided injections into the LHB sheath. For fear of tendon atrophy from corticosteroid injections, some physicians prefer iontophoresis with a topical steroid over the bicipital groove. If conservative measures fail, the physician can choose from 2 primary surgical options: biceps tenotomy and tenodesis. Tenodesis can be performed within the groove (suprapectoral) or subpectoral. In this review, we highlight 5 key features of subpectoral biceps tenodesis to guide treatment and improve outcomes.
Examination and Indications
Management of LHB tendinopathy begins with a complete physical examination. Tenderness over the bicipital groove is the most consistent finding, but this region may be difficult to localize in large individuals. The arm should be internally rotated 10° to orient the groove anterior and palpated 7 cm below the acromion.5 Anterior shoulder pain after resisted elevation with the elbow extended and supinated represents a positive Speed test. A positive Yergason test produces pain with resisted forearm supination while the elbow is flexed to 90°.
Evaluation of biceps instability is important in deciding which type of management (operative or nonoperative) is appropriate for a patient. Medial biceps subluxation may be detected by bringing the flexed arm from abduction, external rotation into cross-body adduction, internal rotation with decreased arm flexion.6 Another maneuver that elicits biceps irritation is combined abduction–extension, which places tension on the biceps tendon. Similarly, coracoid impingement may disrupt the subscapularis roof of the biceps sheath and cause LHB instability. Dines and colleagues7 reproduced the painful clicking of coracoid impingement by placing the shoulder in forward elevation, internal rotation, and varying degrees of adduction. Belly-press, lift-off, and internal rotation strength are other tests that assess subscapularis integrity. Rotator cuff impingement signs should be evaluated, and the contralateral shoulder should be examined for comparison.
Plain radiographs may show a pathology, such as anterior acromial spurring or posterior overgrowth of the coracoid, for which surgery is more suited. T2-weighted magnetic resonance imaging (MRI) may show an increased LHB signal, but this has shown poor concordance with arthroscopic findings of biceps pathology.8 Magnetic resonance arthrography can better detect medial dislocation of the LHB tendon from subscapularis tears. Ultrasound is cost-effective but highly operator-dependent.
Indications for biceps tenotomy or tenodesis include failed conservative management, partial-thickness LHB tears more than 25% to 50% in diameter, and medial subluxation of the LHB tendon with or without a subscapularis tear. Superior labrum anterior to posterior (SLAP) tears in older patients are a relative indication. Intraoperative findings may also indicate the need for LHB surgery. During the diagnostic arthroscopy, the LHB tendon should be evaluated for synovial inflammation or fraying (Figures 1A, 1B). This may need to be done under dry conditions, as pump pressure can compress and blunt the inflamed appearance. The O’Brien maneuver can be performed to demonstrate incarceration of the LHB tendon within the anterior glenohumeral joint. A probe should be placed through an anterior portal to pull the intertubercular LHB tendon into view, as this region is most commonly inflamed (Figure 2). Probing of the tendon also allows assessment of the stability of the biceps sling.
Surgical Technique
When biceps surgery is indicated, the surgeon must choose between tenotomy and tenodesis. Tenotomy is a low-demand procedure indicated for low-demand patients. A “Popeye” deformity may occur in up to 62% of patients, but Boileau and colleagues9 reported that none of their patients were bothered by it. Another concern after tenotomy is fatigue-cramping of the biceps muscle belly. Kelly and colleagues10 reported that up to 40% of patients had soreness and decreased strength with elbow flexion. Such cramping is more common in patients under age 60 years. For these reasons, biceps tenotomy should be reserved for older, low-demand patients who are not concerned about cosmesis and less likely to comply with postoperative motion restrictions.2 We tend to perform tenotomy in obese patients, who may have a Popeye deformity that is not detectable, and in patients with diabetes; the goal is to avoid a wound infection resulting from the close proximity of tenodesis incision and axilla.
Biceps tenodesis should preserve the length–tension relationship of the biceps muscle and maintain its normal contour. Tenodesis location may be proximal or distal. Proximal fixation can be performed arthroscopically, and its advocates argue that keeping the LHB tendon within the bicipital groove preserves muscle strength. Boileau and Neyton11 found biceps strength to be 90% that of the contralateral arm after arthroscopic tenodesis. The bicipital groove, however, is lined with synovium and is a primary site of LHB pathology. Up to 78% of intra-articular biceps tears extend through the groove outside the joint.12 Proximal tenodesis thus retains a major pain generator. In a retrospective study of 188 patients, Sanders and colleagues13,14 found a 36% revision rate after proximal arthroscopic tenodesis and a 13% rate after proximal open tenodesis with an intact biceps sheath—significantly lower than the 3% after distal tenodesis outside the bicipital groove.1 For this reason, we advocate distal biceps tenodesis beneath the pectoralis major tendon. After tenotomy with an arthroscopic basket (Figure 3), the LHB tendon is retracted out of the glenohumeral joint by extending the elbow. For the mini-open incision, the head of the bed is lowered from the beach-chair position to 30°. The arm is abducted on a Mayo stand, and the inferior border of the pectoralis major tendon is palpated. A 3-cm vertical incision is made along the medial arm starting 1 cm superior to the inferior pectoralis edge. The subcutaneous tissues are mobilized, and dissection is carried down to the pectoralis major and coracobrachialis tendons. Visualization of the cephalic vein indicates that the exposure is too far lateral. The horizontal fibers of the pectoralis major are identified, and a small incision through the inferior overlying fascia is directed laterally and then distally in line with the long axis of the humerus. Digital palpation helps identify the anterior humerus and fusiform LHB tendon running vertically within the intertubercular groove (Figure 4). Cephalad retraction of the pectoralis major allows direct visualization of the LHB tendon. A right-angle clamp is positioned deep to the LHB tendon and directed medial to lateral to retrieve the LHB tendon out of the incision.
No. 2 looped Fiberwire (Arthrex) is then whip-stitched from the top of the myotendinous junction up 20 mm (Figure 5). The remaining 2 to 3 cm of LHB tendon proximal to the whip-stitching may be excised to remove inflammatory tissue. The pectoralis major is retracted superiorly with an Army-Navy retractor while a pointed Hohmann retractor is placed laterally. Medial retraction of the conjoined tendon should be done carefully with a Chandler elevator and minimal levering. In a cadaveric study, Dickens and colleagues15 found that the musculocutaneous nerve, radial nerve, and deep brachial artery were all within 1 cm of the standard medial retractor. Compared with internal rotation of the arm, external rotation moves the musculocutaneous nerve 11 mm farther from the tenodesis site.15
Once exposure is adequate, the appropriate length–tension of the LHB tendon must be established. The inferior edge of the pectoralis major is used as a landmark. Anatomical studies have shown that the top of the LHB myotendinous junction lies 20 to 31 mm proximal to the inferior pectoralis edge.16,17 Therefore, the tenodesis site should be 2 to 3 cm superior to the inferior pectoralis edge and centered on the humerus. Overall, the subpectoral location offers unique landmarks for LHB length-tensioning and provides soft-tissue coverage of the tenodesis site.
After identification of the appropriate tenodesis site, the surgeon chooses from a variety of fixation techniques. The “bone-tunnel technique” involves drilling an 8-mm unicortical hole through the anterior humerus followed by 2 smaller suture tunnels inferior to it; the LHB tendon with Krackow stitches is passed retrograde through the large hole by pulling the sutures through the smaller tunnels and tying them down.18 Despite the ease of performing this type of fixation, Mazzocca and colleagues19 found more cyclic displacement with bone tunnels than with interference screws and suture anchors. Other, less common techniques include the keyhole method (passing a rolled knot of LHB tendon through a keyhole in the bone)20 and soft-tissue tenodesis to the rotator interval or conjoined tendon.21,22 Recently, however, attention has turned mostly to interference screw and suture anchor fixation.
Multiple laboratory studies have demonstrated the superiority of interference screw fixation. Kilicoglu and colleagues23 and Ozalay and colleagues24 evaluated various fixation types in a sheep model, and both groups found the highest loads to failure with interference screws. Patzer and colleagues25 compared interference screws and knotless suture anchors in a human cadaveric study and noted significantly higher failure loads with interference screws. Some authors26,27 have presented conflicting laboratory data, and Millett and colleagues28 reported no difference in clinical outcomes between interference screws and suture anchors. However, these studies have not demonstrated inferiority of interference screws, and, in light of other evidence suggesting its biomechanical superiority, we prefer interference screw fixation.19,23-25,29
Exposing the bony surface for fixation involves electrocautery and subsequent use of a periosteal elevator to reflect a 1-cm periosteal window. A guide wire is drilled unicortically through the anterior cortex at the tenodesis site and is overreamed with an 8-mm cannulated reamer (Figure 6). This tunnel is then tapped, and bone debris is irrigated and suctioned from the wound. Cadaveric studies have shown no difference in failure loads with varying screw lengths or diameters.29,30 We use an 8×12-mm BioTenodesis screw (Arthrex) to match the typical width of the LHB tendon (Figures 7A-7C). One suture limb from the tendon whip-stitch is passed through the BioTenodesis screw and screwdriver. An assistant then uses a right-angle clamp as a pulley on the tendon so that the tendon may be visualized and “dunked” into the tunnel under direct visualization. As the screw is inserted, axial pressure is applied and the insertion paddle firmly held. Care should be taken to avoid overtightening the screw lest it become intramedullary. After the screw is flush to bone, the 2 whip-stitch suture limbs are tied for additional fixation.
Postoperative Rehabilitation
The optimal postoperative protocol for subpectoral biceps tenodesis has not been rigorously studied and is guided by the procedures performed with the biceps tenodesis. For the immediate postoperative period, Provencher and colleagues5 and Mazzocca and colleagues31 recommended immobilization in a sling during sleep and during the day if the patient is out in public or having difficulty maintaining the elbow flexed passively.
For isolated biceps tenodesis cases, passive- and active-assisted range of motion (ROM) of the glenohumeral, elbow, and wrist joints are permitted during the initial 4 weeks. At 3 weeks, the sling is discontinued and active ROM permitted. At 6 weeks, strengthening of the biceps, rotator cuff, deltoid, and periscapular muscles may begin with isometric contractions and progress to elastic bands and handheld weights. The same protocol is used if acromioplasty is performed at time of tenodesis. These patients may progress to active-assisted and active ROM earlier than 4 weeks if advised of the risks. However, sustained isometric biceps contraction, biceps strengthening, and resisted supination should not be performed until 6 weeks after surgery. If rotator cuff repair is performed, the patient is immobilized in a sling and passive ROM of the glenohumeral, elbow, and wrist joints is permitted during the first 6 weeks. The patient may progress to active-assisted and active ROM over the next 6 weeks, after motion is restored but before formal strengthening is initiated.32 For overhead athletes, Werner and colleagues33 advocated a throwing program starting 3 to 4 months after surgery.
Outcomes and Complications
Mini-open subpectoral biceps tenodesis is a safe, reliable, and effective treatment for LHB tendon pathology. This procedure provides excellent pain relief and functional outcomes32,34,35 and has a low complication rate.5,35-40 At a mean of 29 months after biceps tenodesis with an interference screw, Mazzocca and colleagues32 found statistically significant improvements on all clinical outcome measures: Rowe, American Shoulder and Elbow Surgeons (ASES), Simple Shoulder Test (SST), Constant-Murley, and Single Assessment Numeric Evaluation (SANE). Biceps symmetry was restored in 35 of 41 patients. Millett and colleagues28 reported that subpectoral biceps tenodesis relieved pain and improved function as measured by visual analog scale pain, ASES scores, and abbreviated Constant scores. Werner and colleagues34 compared open subpectoral and arthroscopic suprapectoral techniques and found excellent clinical and functional outcomes with both techniques at a mean of 3.1 years. There were no significant differences in ROM, strength, or clinical outcome scores between the 2 techniques.
Potential complications include hematoma, seroma, hardware failure, reaction to biodegradable screw, persistent anterior shoulder pain, stiffness, humeral fracture, reflex sympathetic dystrophy, infection, nerve injury, and brachial artery injury. The musculocutaneous nerve can be lacerated during screw placement or even avulsed if the surgeon attempts to retrieve the LHB tendon blindly.41 In the most comprehensive study of tenodesis complications, Nho and colleagues35 recorded a 2% complication rate in 353 patients over 3 years. Persistent bicipital pain and fixation failure causing a Popeye deformity were the 2 most common complications (0.57% each). In a study of 103 patients, Abtahi and colleagues39 found a 7% complication rate, with 4 superficial wound infections and 2 temporary nerve palsies. Millett and colleagues28 reported low complication rates with both interference screw and suture anchor fixation. Neither technique had a fixation failure, and persistent bicipital groove tenderness occurred in just 3% of patients after interference screw fixation and in 7% after suture anchor fixation. Mazzocca and colleagues32 documented 1 fixation failure (2%) 1 year after interference screw fixation.
Werner and colleagues34 encountered stiffness more than any other complication and found it to be more common in their arthroscopic group (9.4%) than in their open group (6.0%). They used intra-articular corticosteroid injections and physical therapy to successfully treat all cases of postoperative stiffness. Humeral fracture is uncommon after tenodesis.37,42 In a recent biomechanical study, however, Euler and colleagues40 found a significant reduction (25%) in humeral strength after a laterally eccentric, malpositioned biceps tenodesis. This decreased osseous strength may increase susceptibility to humeral shaft fracture, especially when interference screw fixation is used. Sears and colleagues37 and Dein and colleagues42 presented case reports of humeral fracture after biceps tenodesis with an interference screw.
For patients with fixation failure or continued anterior shoulder pain, revision biceps tenodesis is safe and effective. Heckman and colleagues43 and Gregory and colleagues44 showed revision tenodesis can lead to excellent pain relief and functional outcomes, for it allows complete removal of the biceps from the groove and preserves biceps function. Gregory and colleagues44 revised subpectoral biceps tenodesis for either continued pain or fixation failure and found significant improvements in pain and function a mean of 33.4 months after surgery. Anthony and colleagues45 performed biceps tenodesis for failed surgical tenotomies and autorupture of the LHB tendon. In their study of 11 patients, this surgery resulted in symptom improvement, patient satisfaction, resolution of Popeye deformity, and predictable return to activity.
Conclusion
LHB tendon pathology is a significant source of anterior shoulder pain and functional limitation. Diagnosis and treatment of this pathology can be challenging, and it is important to identify any concomitant pathologies or other pain sources. After failed nonoperative management, surgeons have the option of mini-open subpectoral biceps tenodesis—a safe, reliable, and effective treatment with excellent outcomes. Although multiple fixation options are available, we think that, based on the current literature, fixation with a bioabsorbable interference screw remains the best option. This procedure has demonstrated efficacy for revision biceps tenodesis, failed biceps tenotomy, and autorupture of the biceps.
1. Friedman DJ, Dunn JC, Higgins LD, Warner JJP. Proximal biceps tendon: injuries and management. Sports Med Arthrosc. 2008;16(3):162-169.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Neviaser TJ, Neviaser RJ, Neviaser JS, Neviaser JS. The four-in-one arthroplasty for the painful arc syndrome. Clin Orthop Relat Res. 1982;163:107-112.
4. Patton WC, McCluskey GM 3rd. Biceps tendinitis and subluxation. Clin Sports Med. 2001;20(3):505-529.
5. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
6. Bennett WF. Arthroscopic repair of isolated subscapularis tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(2):131-143.
7. Dines DM, Warren RF, Inglis AE, Pavlov H. The coracoid impingement syndrome. Bone Joint J Br. 1990;72(2):314-316.
8. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
9. Boileau P, Baqué F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747-757.
10. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
11. Boileau P, Neyton L. Arthroscopic tenodesis for lesions of the long head of the biceps. Oper Orthop Traumatol. 2005;17(6):601-623.
12. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
13. Sanders B, Lavery K, Pennington S, Warner JJP. Biceps tendon tenodesis: success with proximal versus distal fixation (SS-16). Arthroscopy. 2008;24(6 suppl):e9.
14. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
15. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
16. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
17. Jarrett CD, McClelland WB, Xerogeanes JW. Minimally invasive proximal biceps tenodesis: an anatomical study for optimal placement and safe surgical technique. J Shoulder Elbow Surg. 2011;20(3):477-480.
18. Mazzocca AD, Noerdlinger MA, Romeo AA. Mini open and subpectoral biceps tenodesis. Oper Tech Sports Med. 2003;11(1):24-31.
19. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
20. Froimson AI, O I. Keyhole tenodesis of biceps origin at the shoulder. Clin Orthop Relat Res. 1975;(112):245-249.
21. Sekiya JK, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
22. Verma NN, Drakos M, O’Brien SJ. Arthroscopic transfer of the long head biceps to the conjoint tendon. Arthroscopy. 2005;21(6):764.
23. Kilicoglu O, Koyuncu O, Demirhan M, et al. Time-dependent changes in failure loads of 3 biceps tenodesis techniques: in vivo study in a sheep model. Am J Sports Med. 2005;33(10):1536-1544.
24. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
25. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
26. Buchholz A, Martetschläger F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
27. Tashjian RZ, Henninger HB. Biomechanical evaluation of subpectoral biceps tenodesis: dual suture anchor versus interference screw fixation. J Shoulder Elbow Surg. 2013;22(10):1408-1412.
28. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJP. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9(1):121.
29. Sethi PM, Rajaram A, Beitzel K, Hackett TR, Chowaniec DM, Mazzocca AD. Biomechanical performance of subpectoral biceps tenodesis: a comparison of interference screw fixation, cortical button fixation, and interference screw diameter. J Shoulder Elbow Surg. 2013;22(4):451-457.
30. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
31. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
32. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
33. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
34. Werner BC, Evans CL, Holzgrefe RE, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of minimum 2-year clinical outcomes. Am J Sports Med. 2014;42(11):2583-2590.
35. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
36. Rhee PC, Spinner RJ, Bishop AT, Shin AY. Iatrogenic brachial plexus injuries associated with open subpectoral biceps tenodesis: a report of 4 cases. Am J Sports Med. 2013;41(9):2048-2053.
37. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
38. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
39. Abtahi AM, Granger EK, Tashjian RZ. Complications after subpectoral biceps tenodesis using a dual suture anchor technique. Int J Shoulder Surg. 2014;8(2):47-50.
40. Euler SA, Smith SD, Williams BT, Dornan GJ, Millett PJ, Wijdicks CA. Biomechanical analysis of subpectoral biceps tenodesis: effect of screw malpositioning on proximal humeral strength. Am J Sports Med. 2015;43(1):69-74.
41. Carofino BC, Brogan DM, Kircher MF, et al. Iatrogenic nerve injuries during shoulder surgery. J Bone Joint Surg Am. 2013;95(18):1667-1674.
42. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
43. Heckman DS, Creighton RA, Romeo AA. Management of failed biceps tenodesis or tenotomy: causation and treatment. Sports Med Arthrosc. 2010;18(3):173-180.
44. Gregory JM, Harwood DP, Gochanour E, Sherman SL, Romeo AA. Clinical outcomes of revision biceps tenodesis. Int J Shoulder Surg. 2012;6(2):45-50.
45. Anthony SG, McCormick F, Gross DJ, Golijanin P, Provencher MT. Biceps tenodesis for long head of the biceps after auto-rupture or failed surgical tenotomy: results in an active population. J Shoulder Elbow Surg. 2015;24(2):e36-e40.
Tendinopathy of the long head of the biceps brachii (LHB) is a common source of anterior shoulder pain. The LHB tendon is an intra-articular yet extrasynovial structure, ensheathed by the synovial lining of the articular capsule.1 Branches of the anterior circumflex humeral artery course along the bicipital groove, but the gliding undersurface of the LHB remains avascular.2 Tendon irritation is most common within the groove and usually produces “tendinosis,” characterized by collagen fiber atrophy, fibrinoid necrosis, and fibrocyte proliferation.1 Neviaser and colleagues3 correlated such changes in the LHB tendon with rotator cuff pathology, as the 2 often coexist. Primary LHB tendinitis is less common and associated with younger patients who engage in overhead activities, such as baseball and volleyball.4
Nonoperative management, which is trialed initially, consists of rest, use of nonsteroidal anti-inflammatory drugs, and physical therapy. Corticosteroid injections are administered through the subacromial space or glenohumeral joint, which is continuous with the LHB sheath. Some physicians give ultrasound-guided injections into the LHB sheath. For fear of tendon atrophy from corticosteroid injections, some physicians prefer iontophoresis with a topical steroid over the bicipital groove. If conservative measures fail, the physician can choose from 2 primary surgical options: biceps tenotomy and tenodesis. Tenodesis can be performed within the groove (suprapectoral) or subpectoral. In this review, we highlight 5 key features of subpectoral biceps tenodesis to guide treatment and improve outcomes.
Examination and Indications
Management of LHB tendinopathy begins with a complete physical examination. Tenderness over the bicipital groove is the most consistent finding, but this region may be difficult to localize in large individuals. The arm should be internally rotated 10° to orient the groove anterior and palpated 7 cm below the acromion.5 Anterior shoulder pain after resisted elevation with the elbow extended and supinated represents a positive Speed test. A positive Yergason test produces pain with resisted forearm supination while the elbow is flexed to 90°.
Evaluation of biceps instability is important in deciding which type of management (operative or nonoperative) is appropriate for a patient. Medial biceps subluxation may be detected by bringing the flexed arm from abduction, external rotation into cross-body adduction, internal rotation with decreased arm flexion.6 Another maneuver that elicits biceps irritation is combined abduction–extension, which places tension on the biceps tendon. Similarly, coracoid impingement may disrupt the subscapularis roof of the biceps sheath and cause LHB instability. Dines and colleagues7 reproduced the painful clicking of coracoid impingement by placing the shoulder in forward elevation, internal rotation, and varying degrees of adduction. Belly-press, lift-off, and internal rotation strength are other tests that assess subscapularis integrity. Rotator cuff impingement signs should be evaluated, and the contralateral shoulder should be examined for comparison.
Plain radiographs may show a pathology, such as anterior acromial spurring or posterior overgrowth of the coracoid, for which surgery is more suited. T2-weighted magnetic resonance imaging (MRI) may show an increased LHB signal, but this has shown poor concordance with arthroscopic findings of biceps pathology.8 Magnetic resonance arthrography can better detect medial dislocation of the LHB tendon from subscapularis tears. Ultrasound is cost-effective but highly operator-dependent.
Indications for biceps tenotomy or tenodesis include failed conservative management, partial-thickness LHB tears more than 25% to 50% in diameter, and medial subluxation of the LHB tendon with or without a subscapularis tear. Superior labrum anterior to posterior (SLAP) tears in older patients are a relative indication. Intraoperative findings may also indicate the need for LHB surgery. During the diagnostic arthroscopy, the LHB tendon should be evaluated for synovial inflammation or fraying (Figures 1A, 1B). This may need to be done under dry conditions, as pump pressure can compress and blunt the inflamed appearance. The O’Brien maneuver can be performed to demonstrate incarceration of the LHB tendon within the anterior glenohumeral joint. A probe should be placed through an anterior portal to pull the intertubercular LHB tendon into view, as this region is most commonly inflamed (Figure 2). Probing of the tendon also allows assessment of the stability of the biceps sling.
Surgical Technique
When biceps surgery is indicated, the surgeon must choose between tenotomy and tenodesis. Tenotomy is a low-demand procedure indicated for low-demand patients. A “Popeye” deformity may occur in up to 62% of patients, but Boileau and colleagues9 reported that none of their patients were bothered by it. Another concern after tenotomy is fatigue-cramping of the biceps muscle belly. Kelly and colleagues10 reported that up to 40% of patients had soreness and decreased strength with elbow flexion. Such cramping is more common in patients under age 60 years. For these reasons, biceps tenotomy should be reserved for older, low-demand patients who are not concerned about cosmesis and less likely to comply with postoperative motion restrictions.2 We tend to perform tenotomy in obese patients, who may have a Popeye deformity that is not detectable, and in patients with diabetes; the goal is to avoid a wound infection resulting from the close proximity of tenodesis incision and axilla.
Biceps tenodesis should preserve the length–tension relationship of the biceps muscle and maintain its normal contour. Tenodesis location may be proximal or distal. Proximal fixation can be performed arthroscopically, and its advocates argue that keeping the LHB tendon within the bicipital groove preserves muscle strength. Boileau and Neyton11 found biceps strength to be 90% that of the contralateral arm after arthroscopic tenodesis. The bicipital groove, however, is lined with synovium and is a primary site of LHB pathology. Up to 78% of intra-articular biceps tears extend through the groove outside the joint.12 Proximal tenodesis thus retains a major pain generator. In a retrospective study of 188 patients, Sanders and colleagues13,14 found a 36% revision rate after proximal arthroscopic tenodesis and a 13% rate after proximal open tenodesis with an intact biceps sheath—significantly lower than the 3% after distal tenodesis outside the bicipital groove.1 For this reason, we advocate distal biceps tenodesis beneath the pectoralis major tendon. After tenotomy with an arthroscopic basket (Figure 3), the LHB tendon is retracted out of the glenohumeral joint by extending the elbow. For the mini-open incision, the head of the bed is lowered from the beach-chair position to 30°. The arm is abducted on a Mayo stand, and the inferior border of the pectoralis major tendon is palpated. A 3-cm vertical incision is made along the medial arm starting 1 cm superior to the inferior pectoralis edge. The subcutaneous tissues are mobilized, and dissection is carried down to the pectoralis major and coracobrachialis tendons. Visualization of the cephalic vein indicates that the exposure is too far lateral. The horizontal fibers of the pectoralis major are identified, and a small incision through the inferior overlying fascia is directed laterally and then distally in line with the long axis of the humerus. Digital palpation helps identify the anterior humerus and fusiform LHB tendon running vertically within the intertubercular groove (Figure 4). Cephalad retraction of the pectoralis major allows direct visualization of the LHB tendon. A right-angle clamp is positioned deep to the LHB tendon and directed medial to lateral to retrieve the LHB tendon out of the incision.
No. 2 looped Fiberwire (Arthrex) is then whip-stitched from the top of the myotendinous junction up 20 mm (Figure 5). The remaining 2 to 3 cm of LHB tendon proximal to the whip-stitching may be excised to remove inflammatory tissue. The pectoralis major is retracted superiorly with an Army-Navy retractor while a pointed Hohmann retractor is placed laterally. Medial retraction of the conjoined tendon should be done carefully with a Chandler elevator and minimal levering. In a cadaveric study, Dickens and colleagues15 found that the musculocutaneous nerve, radial nerve, and deep brachial artery were all within 1 cm of the standard medial retractor. Compared with internal rotation of the arm, external rotation moves the musculocutaneous nerve 11 mm farther from the tenodesis site.15
Once exposure is adequate, the appropriate length–tension of the LHB tendon must be established. The inferior edge of the pectoralis major is used as a landmark. Anatomical studies have shown that the top of the LHB myotendinous junction lies 20 to 31 mm proximal to the inferior pectoralis edge.16,17 Therefore, the tenodesis site should be 2 to 3 cm superior to the inferior pectoralis edge and centered on the humerus. Overall, the subpectoral location offers unique landmarks for LHB length-tensioning and provides soft-tissue coverage of the tenodesis site.
After identification of the appropriate tenodesis site, the surgeon chooses from a variety of fixation techniques. The “bone-tunnel technique” involves drilling an 8-mm unicortical hole through the anterior humerus followed by 2 smaller suture tunnels inferior to it; the LHB tendon with Krackow stitches is passed retrograde through the large hole by pulling the sutures through the smaller tunnels and tying them down.18 Despite the ease of performing this type of fixation, Mazzocca and colleagues19 found more cyclic displacement with bone tunnels than with interference screws and suture anchors. Other, less common techniques include the keyhole method (passing a rolled knot of LHB tendon through a keyhole in the bone)20 and soft-tissue tenodesis to the rotator interval or conjoined tendon.21,22 Recently, however, attention has turned mostly to interference screw and suture anchor fixation.
Multiple laboratory studies have demonstrated the superiority of interference screw fixation. Kilicoglu and colleagues23 and Ozalay and colleagues24 evaluated various fixation types in a sheep model, and both groups found the highest loads to failure with interference screws. Patzer and colleagues25 compared interference screws and knotless suture anchors in a human cadaveric study and noted significantly higher failure loads with interference screws. Some authors26,27 have presented conflicting laboratory data, and Millett and colleagues28 reported no difference in clinical outcomes between interference screws and suture anchors. However, these studies have not demonstrated inferiority of interference screws, and, in light of other evidence suggesting its biomechanical superiority, we prefer interference screw fixation.19,23-25,29
Exposing the bony surface for fixation involves electrocautery and subsequent use of a periosteal elevator to reflect a 1-cm periosteal window. A guide wire is drilled unicortically through the anterior cortex at the tenodesis site and is overreamed with an 8-mm cannulated reamer (Figure 6). This tunnel is then tapped, and bone debris is irrigated and suctioned from the wound. Cadaveric studies have shown no difference in failure loads with varying screw lengths or diameters.29,30 We use an 8×12-mm BioTenodesis screw (Arthrex) to match the typical width of the LHB tendon (Figures 7A-7C). One suture limb from the tendon whip-stitch is passed through the BioTenodesis screw and screwdriver. An assistant then uses a right-angle clamp as a pulley on the tendon so that the tendon may be visualized and “dunked” into the tunnel under direct visualization. As the screw is inserted, axial pressure is applied and the insertion paddle firmly held. Care should be taken to avoid overtightening the screw lest it become intramedullary. After the screw is flush to bone, the 2 whip-stitch suture limbs are tied for additional fixation.
Postoperative Rehabilitation
The optimal postoperative protocol for subpectoral biceps tenodesis has not been rigorously studied and is guided by the procedures performed with the biceps tenodesis. For the immediate postoperative period, Provencher and colleagues5 and Mazzocca and colleagues31 recommended immobilization in a sling during sleep and during the day if the patient is out in public or having difficulty maintaining the elbow flexed passively.
For isolated biceps tenodesis cases, passive- and active-assisted range of motion (ROM) of the glenohumeral, elbow, and wrist joints are permitted during the initial 4 weeks. At 3 weeks, the sling is discontinued and active ROM permitted. At 6 weeks, strengthening of the biceps, rotator cuff, deltoid, and periscapular muscles may begin with isometric contractions and progress to elastic bands and handheld weights. The same protocol is used if acromioplasty is performed at time of tenodesis. These patients may progress to active-assisted and active ROM earlier than 4 weeks if advised of the risks. However, sustained isometric biceps contraction, biceps strengthening, and resisted supination should not be performed until 6 weeks after surgery. If rotator cuff repair is performed, the patient is immobilized in a sling and passive ROM of the glenohumeral, elbow, and wrist joints is permitted during the first 6 weeks. The patient may progress to active-assisted and active ROM over the next 6 weeks, after motion is restored but before formal strengthening is initiated.32 For overhead athletes, Werner and colleagues33 advocated a throwing program starting 3 to 4 months after surgery.
Outcomes and Complications
Mini-open subpectoral biceps tenodesis is a safe, reliable, and effective treatment for LHB tendon pathology. This procedure provides excellent pain relief and functional outcomes32,34,35 and has a low complication rate.5,35-40 At a mean of 29 months after biceps tenodesis with an interference screw, Mazzocca and colleagues32 found statistically significant improvements on all clinical outcome measures: Rowe, American Shoulder and Elbow Surgeons (ASES), Simple Shoulder Test (SST), Constant-Murley, and Single Assessment Numeric Evaluation (SANE). Biceps symmetry was restored in 35 of 41 patients. Millett and colleagues28 reported that subpectoral biceps tenodesis relieved pain and improved function as measured by visual analog scale pain, ASES scores, and abbreviated Constant scores. Werner and colleagues34 compared open subpectoral and arthroscopic suprapectoral techniques and found excellent clinical and functional outcomes with both techniques at a mean of 3.1 years. There were no significant differences in ROM, strength, or clinical outcome scores between the 2 techniques.
Potential complications include hematoma, seroma, hardware failure, reaction to biodegradable screw, persistent anterior shoulder pain, stiffness, humeral fracture, reflex sympathetic dystrophy, infection, nerve injury, and brachial artery injury. The musculocutaneous nerve can be lacerated during screw placement or even avulsed if the surgeon attempts to retrieve the LHB tendon blindly.41 In the most comprehensive study of tenodesis complications, Nho and colleagues35 recorded a 2% complication rate in 353 patients over 3 years. Persistent bicipital pain and fixation failure causing a Popeye deformity were the 2 most common complications (0.57% each). In a study of 103 patients, Abtahi and colleagues39 found a 7% complication rate, with 4 superficial wound infections and 2 temporary nerve palsies. Millett and colleagues28 reported low complication rates with both interference screw and suture anchor fixation. Neither technique had a fixation failure, and persistent bicipital groove tenderness occurred in just 3% of patients after interference screw fixation and in 7% after suture anchor fixation. Mazzocca and colleagues32 documented 1 fixation failure (2%) 1 year after interference screw fixation.
Werner and colleagues34 encountered stiffness more than any other complication and found it to be more common in their arthroscopic group (9.4%) than in their open group (6.0%). They used intra-articular corticosteroid injections and physical therapy to successfully treat all cases of postoperative stiffness. Humeral fracture is uncommon after tenodesis.37,42 In a recent biomechanical study, however, Euler and colleagues40 found a significant reduction (25%) in humeral strength after a laterally eccentric, malpositioned biceps tenodesis. This decreased osseous strength may increase susceptibility to humeral shaft fracture, especially when interference screw fixation is used. Sears and colleagues37 and Dein and colleagues42 presented case reports of humeral fracture after biceps tenodesis with an interference screw.
For patients with fixation failure or continued anterior shoulder pain, revision biceps tenodesis is safe and effective. Heckman and colleagues43 and Gregory and colleagues44 showed revision tenodesis can lead to excellent pain relief and functional outcomes, for it allows complete removal of the biceps from the groove and preserves biceps function. Gregory and colleagues44 revised subpectoral biceps tenodesis for either continued pain or fixation failure and found significant improvements in pain and function a mean of 33.4 months after surgery. Anthony and colleagues45 performed biceps tenodesis for failed surgical tenotomies and autorupture of the LHB tendon. In their study of 11 patients, this surgery resulted in symptom improvement, patient satisfaction, resolution of Popeye deformity, and predictable return to activity.
Conclusion
LHB tendon pathology is a significant source of anterior shoulder pain and functional limitation. Diagnosis and treatment of this pathology can be challenging, and it is important to identify any concomitant pathologies or other pain sources. After failed nonoperative management, surgeons have the option of mini-open subpectoral biceps tenodesis—a safe, reliable, and effective treatment with excellent outcomes. Although multiple fixation options are available, we think that, based on the current literature, fixation with a bioabsorbable interference screw remains the best option. This procedure has demonstrated efficacy for revision biceps tenodesis, failed biceps tenotomy, and autorupture of the biceps.
Tendinopathy of the long head of the biceps brachii (LHB) is a common source of anterior shoulder pain. The LHB tendon is an intra-articular yet extrasynovial structure, ensheathed by the synovial lining of the articular capsule.1 Branches of the anterior circumflex humeral artery course along the bicipital groove, but the gliding undersurface of the LHB remains avascular.2 Tendon irritation is most common within the groove and usually produces “tendinosis,” characterized by collagen fiber atrophy, fibrinoid necrosis, and fibrocyte proliferation.1 Neviaser and colleagues3 correlated such changes in the LHB tendon with rotator cuff pathology, as the 2 often coexist. Primary LHB tendinitis is less common and associated with younger patients who engage in overhead activities, such as baseball and volleyball.4
Nonoperative management, which is trialed initially, consists of rest, use of nonsteroidal anti-inflammatory drugs, and physical therapy. Corticosteroid injections are administered through the subacromial space or glenohumeral joint, which is continuous with the LHB sheath. Some physicians give ultrasound-guided injections into the LHB sheath. For fear of tendon atrophy from corticosteroid injections, some physicians prefer iontophoresis with a topical steroid over the bicipital groove. If conservative measures fail, the physician can choose from 2 primary surgical options: biceps tenotomy and tenodesis. Tenodesis can be performed within the groove (suprapectoral) or subpectoral. In this review, we highlight 5 key features of subpectoral biceps tenodesis to guide treatment and improve outcomes.
Examination and Indications
Management of LHB tendinopathy begins with a complete physical examination. Tenderness over the bicipital groove is the most consistent finding, but this region may be difficult to localize in large individuals. The arm should be internally rotated 10° to orient the groove anterior and palpated 7 cm below the acromion.5 Anterior shoulder pain after resisted elevation with the elbow extended and supinated represents a positive Speed test. A positive Yergason test produces pain with resisted forearm supination while the elbow is flexed to 90°.
Evaluation of biceps instability is important in deciding which type of management (operative or nonoperative) is appropriate for a patient. Medial biceps subluxation may be detected by bringing the flexed arm from abduction, external rotation into cross-body adduction, internal rotation with decreased arm flexion.6 Another maneuver that elicits biceps irritation is combined abduction–extension, which places tension on the biceps tendon. Similarly, coracoid impingement may disrupt the subscapularis roof of the biceps sheath and cause LHB instability. Dines and colleagues7 reproduced the painful clicking of coracoid impingement by placing the shoulder in forward elevation, internal rotation, and varying degrees of adduction. Belly-press, lift-off, and internal rotation strength are other tests that assess subscapularis integrity. Rotator cuff impingement signs should be evaluated, and the contralateral shoulder should be examined for comparison.
Plain radiographs may show a pathology, such as anterior acromial spurring or posterior overgrowth of the coracoid, for which surgery is more suited. T2-weighted magnetic resonance imaging (MRI) may show an increased LHB signal, but this has shown poor concordance with arthroscopic findings of biceps pathology.8 Magnetic resonance arthrography can better detect medial dislocation of the LHB tendon from subscapularis tears. Ultrasound is cost-effective but highly operator-dependent.
Indications for biceps tenotomy or tenodesis include failed conservative management, partial-thickness LHB tears more than 25% to 50% in diameter, and medial subluxation of the LHB tendon with or without a subscapularis tear. Superior labrum anterior to posterior (SLAP) tears in older patients are a relative indication. Intraoperative findings may also indicate the need for LHB surgery. During the diagnostic arthroscopy, the LHB tendon should be evaluated for synovial inflammation or fraying (Figures 1A, 1B). This may need to be done under dry conditions, as pump pressure can compress and blunt the inflamed appearance. The O’Brien maneuver can be performed to demonstrate incarceration of the LHB tendon within the anterior glenohumeral joint. A probe should be placed through an anterior portal to pull the intertubercular LHB tendon into view, as this region is most commonly inflamed (Figure 2). Probing of the tendon also allows assessment of the stability of the biceps sling.
Surgical Technique
When biceps surgery is indicated, the surgeon must choose between tenotomy and tenodesis. Tenotomy is a low-demand procedure indicated for low-demand patients. A “Popeye” deformity may occur in up to 62% of patients, but Boileau and colleagues9 reported that none of their patients were bothered by it. Another concern after tenotomy is fatigue-cramping of the biceps muscle belly. Kelly and colleagues10 reported that up to 40% of patients had soreness and decreased strength with elbow flexion. Such cramping is more common in patients under age 60 years. For these reasons, biceps tenotomy should be reserved for older, low-demand patients who are not concerned about cosmesis and less likely to comply with postoperative motion restrictions.2 We tend to perform tenotomy in obese patients, who may have a Popeye deformity that is not detectable, and in patients with diabetes; the goal is to avoid a wound infection resulting from the close proximity of tenodesis incision and axilla.
Biceps tenodesis should preserve the length–tension relationship of the biceps muscle and maintain its normal contour. Tenodesis location may be proximal or distal. Proximal fixation can be performed arthroscopically, and its advocates argue that keeping the LHB tendon within the bicipital groove preserves muscle strength. Boileau and Neyton11 found biceps strength to be 90% that of the contralateral arm after arthroscopic tenodesis. The bicipital groove, however, is lined with synovium and is a primary site of LHB pathology. Up to 78% of intra-articular biceps tears extend through the groove outside the joint.12 Proximal tenodesis thus retains a major pain generator. In a retrospective study of 188 patients, Sanders and colleagues13,14 found a 36% revision rate after proximal arthroscopic tenodesis and a 13% rate after proximal open tenodesis with an intact biceps sheath—significantly lower than the 3% after distal tenodesis outside the bicipital groove.1 For this reason, we advocate distal biceps tenodesis beneath the pectoralis major tendon. After tenotomy with an arthroscopic basket (Figure 3), the LHB tendon is retracted out of the glenohumeral joint by extending the elbow. For the mini-open incision, the head of the bed is lowered from the beach-chair position to 30°. The arm is abducted on a Mayo stand, and the inferior border of the pectoralis major tendon is palpated. A 3-cm vertical incision is made along the medial arm starting 1 cm superior to the inferior pectoralis edge. The subcutaneous tissues are mobilized, and dissection is carried down to the pectoralis major and coracobrachialis tendons. Visualization of the cephalic vein indicates that the exposure is too far lateral. The horizontal fibers of the pectoralis major are identified, and a small incision through the inferior overlying fascia is directed laterally and then distally in line with the long axis of the humerus. Digital palpation helps identify the anterior humerus and fusiform LHB tendon running vertically within the intertubercular groove (Figure 4). Cephalad retraction of the pectoralis major allows direct visualization of the LHB tendon. A right-angle clamp is positioned deep to the LHB tendon and directed medial to lateral to retrieve the LHB tendon out of the incision.
No. 2 looped Fiberwire (Arthrex) is then whip-stitched from the top of the myotendinous junction up 20 mm (Figure 5). The remaining 2 to 3 cm of LHB tendon proximal to the whip-stitching may be excised to remove inflammatory tissue. The pectoralis major is retracted superiorly with an Army-Navy retractor while a pointed Hohmann retractor is placed laterally. Medial retraction of the conjoined tendon should be done carefully with a Chandler elevator and minimal levering. In a cadaveric study, Dickens and colleagues15 found that the musculocutaneous nerve, radial nerve, and deep brachial artery were all within 1 cm of the standard medial retractor. Compared with internal rotation of the arm, external rotation moves the musculocutaneous nerve 11 mm farther from the tenodesis site.15
Once exposure is adequate, the appropriate length–tension of the LHB tendon must be established. The inferior edge of the pectoralis major is used as a landmark. Anatomical studies have shown that the top of the LHB myotendinous junction lies 20 to 31 mm proximal to the inferior pectoralis edge.16,17 Therefore, the tenodesis site should be 2 to 3 cm superior to the inferior pectoralis edge and centered on the humerus. Overall, the subpectoral location offers unique landmarks for LHB length-tensioning and provides soft-tissue coverage of the tenodesis site.
After identification of the appropriate tenodesis site, the surgeon chooses from a variety of fixation techniques. The “bone-tunnel technique” involves drilling an 8-mm unicortical hole through the anterior humerus followed by 2 smaller suture tunnels inferior to it; the LHB tendon with Krackow stitches is passed retrograde through the large hole by pulling the sutures through the smaller tunnels and tying them down.18 Despite the ease of performing this type of fixation, Mazzocca and colleagues19 found more cyclic displacement with bone tunnels than with interference screws and suture anchors. Other, less common techniques include the keyhole method (passing a rolled knot of LHB tendon through a keyhole in the bone)20 and soft-tissue tenodesis to the rotator interval or conjoined tendon.21,22 Recently, however, attention has turned mostly to interference screw and suture anchor fixation.
Multiple laboratory studies have demonstrated the superiority of interference screw fixation. Kilicoglu and colleagues23 and Ozalay and colleagues24 evaluated various fixation types in a sheep model, and both groups found the highest loads to failure with interference screws. Patzer and colleagues25 compared interference screws and knotless suture anchors in a human cadaveric study and noted significantly higher failure loads with interference screws. Some authors26,27 have presented conflicting laboratory data, and Millett and colleagues28 reported no difference in clinical outcomes between interference screws and suture anchors. However, these studies have not demonstrated inferiority of interference screws, and, in light of other evidence suggesting its biomechanical superiority, we prefer interference screw fixation.19,23-25,29
Exposing the bony surface for fixation involves electrocautery and subsequent use of a periosteal elevator to reflect a 1-cm periosteal window. A guide wire is drilled unicortically through the anterior cortex at the tenodesis site and is overreamed with an 8-mm cannulated reamer (Figure 6). This tunnel is then tapped, and bone debris is irrigated and suctioned from the wound. Cadaveric studies have shown no difference in failure loads with varying screw lengths or diameters.29,30 We use an 8×12-mm BioTenodesis screw (Arthrex) to match the typical width of the LHB tendon (Figures 7A-7C). One suture limb from the tendon whip-stitch is passed through the BioTenodesis screw and screwdriver. An assistant then uses a right-angle clamp as a pulley on the tendon so that the tendon may be visualized and “dunked” into the tunnel under direct visualization. As the screw is inserted, axial pressure is applied and the insertion paddle firmly held. Care should be taken to avoid overtightening the screw lest it become intramedullary. After the screw is flush to bone, the 2 whip-stitch suture limbs are tied for additional fixation.
Postoperative Rehabilitation
The optimal postoperative protocol for subpectoral biceps tenodesis has not been rigorously studied and is guided by the procedures performed with the biceps tenodesis. For the immediate postoperative period, Provencher and colleagues5 and Mazzocca and colleagues31 recommended immobilization in a sling during sleep and during the day if the patient is out in public or having difficulty maintaining the elbow flexed passively.
For isolated biceps tenodesis cases, passive- and active-assisted range of motion (ROM) of the glenohumeral, elbow, and wrist joints are permitted during the initial 4 weeks. At 3 weeks, the sling is discontinued and active ROM permitted. At 6 weeks, strengthening of the biceps, rotator cuff, deltoid, and periscapular muscles may begin with isometric contractions and progress to elastic bands and handheld weights. The same protocol is used if acromioplasty is performed at time of tenodesis. These patients may progress to active-assisted and active ROM earlier than 4 weeks if advised of the risks. However, sustained isometric biceps contraction, biceps strengthening, and resisted supination should not be performed until 6 weeks after surgery. If rotator cuff repair is performed, the patient is immobilized in a sling and passive ROM of the glenohumeral, elbow, and wrist joints is permitted during the first 6 weeks. The patient may progress to active-assisted and active ROM over the next 6 weeks, after motion is restored but before formal strengthening is initiated.32 For overhead athletes, Werner and colleagues33 advocated a throwing program starting 3 to 4 months after surgery.
Outcomes and Complications
Mini-open subpectoral biceps tenodesis is a safe, reliable, and effective treatment for LHB tendon pathology. This procedure provides excellent pain relief and functional outcomes32,34,35 and has a low complication rate.5,35-40 At a mean of 29 months after biceps tenodesis with an interference screw, Mazzocca and colleagues32 found statistically significant improvements on all clinical outcome measures: Rowe, American Shoulder and Elbow Surgeons (ASES), Simple Shoulder Test (SST), Constant-Murley, and Single Assessment Numeric Evaluation (SANE). Biceps symmetry was restored in 35 of 41 patients. Millett and colleagues28 reported that subpectoral biceps tenodesis relieved pain and improved function as measured by visual analog scale pain, ASES scores, and abbreviated Constant scores. Werner and colleagues34 compared open subpectoral and arthroscopic suprapectoral techniques and found excellent clinical and functional outcomes with both techniques at a mean of 3.1 years. There were no significant differences in ROM, strength, or clinical outcome scores between the 2 techniques.
Potential complications include hematoma, seroma, hardware failure, reaction to biodegradable screw, persistent anterior shoulder pain, stiffness, humeral fracture, reflex sympathetic dystrophy, infection, nerve injury, and brachial artery injury. The musculocutaneous nerve can be lacerated during screw placement or even avulsed if the surgeon attempts to retrieve the LHB tendon blindly.41 In the most comprehensive study of tenodesis complications, Nho and colleagues35 recorded a 2% complication rate in 353 patients over 3 years. Persistent bicipital pain and fixation failure causing a Popeye deformity were the 2 most common complications (0.57% each). In a study of 103 patients, Abtahi and colleagues39 found a 7% complication rate, with 4 superficial wound infections and 2 temporary nerve palsies. Millett and colleagues28 reported low complication rates with both interference screw and suture anchor fixation. Neither technique had a fixation failure, and persistent bicipital groove tenderness occurred in just 3% of patients after interference screw fixation and in 7% after suture anchor fixation. Mazzocca and colleagues32 documented 1 fixation failure (2%) 1 year after interference screw fixation.
Werner and colleagues34 encountered stiffness more than any other complication and found it to be more common in their arthroscopic group (9.4%) than in their open group (6.0%). They used intra-articular corticosteroid injections and physical therapy to successfully treat all cases of postoperative stiffness. Humeral fracture is uncommon after tenodesis.37,42 In a recent biomechanical study, however, Euler and colleagues40 found a significant reduction (25%) in humeral strength after a laterally eccentric, malpositioned biceps tenodesis. This decreased osseous strength may increase susceptibility to humeral shaft fracture, especially when interference screw fixation is used. Sears and colleagues37 and Dein and colleagues42 presented case reports of humeral fracture after biceps tenodesis with an interference screw.
For patients with fixation failure or continued anterior shoulder pain, revision biceps tenodesis is safe and effective. Heckman and colleagues43 and Gregory and colleagues44 showed revision tenodesis can lead to excellent pain relief and functional outcomes, for it allows complete removal of the biceps from the groove and preserves biceps function. Gregory and colleagues44 revised subpectoral biceps tenodesis for either continued pain or fixation failure and found significant improvements in pain and function a mean of 33.4 months after surgery. Anthony and colleagues45 performed biceps tenodesis for failed surgical tenotomies and autorupture of the LHB tendon. In their study of 11 patients, this surgery resulted in symptom improvement, patient satisfaction, resolution of Popeye deformity, and predictable return to activity.
Conclusion
LHB tendon pathology is a significant source of anterior shoulder pain and functional limitation. Diagnosis and treatment of this pathology can be challenging, and it is important to identify any concomitant pathologies or other pain sources. After failed nonoperative management, surgeons have the option of mini-open subpectoral biceps tenodesis—a safe, reliable, and effective treatment with excellent outcomes. Although multiple fixation options are available, we think that, based on the current literature, fixation with a bioabsorbable interference screw remains the best option. This procedure has demonstrated efficacy for revision biceps tenodesis, failed biceps tenotomy, and autorupture of the biceps.
1. Friedman DJ, Dunn JC, Higgins LD, Warner JJP. Proximal biceps tendon: injuries and management. Sports Med Arthrosc. 2008;16(3):162-169.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Neviaser TJ, Neviaser RJ, Neviaser JS, Neviaser JS. The four-in-one arthroplasty for the painful arc syndrome. Clin Orthop Relat Res. 1982;163:107-112.
4. Patton WC, McCluskey GM 3rd. Biceps tendinitis and subluxation. Clin Sports Med. 2001;20(3):505-529.
5. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
6. Bennett WF. Arthroscopic repair of isolated subscapularis tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(2):131-143.
7. Dines DM, Warren RF, Inglis AE, Pavlov H. The coracoid impingement syndrome. Bone Joint J Br. 1990;72(2):314-316.
8. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
9. Boileau P, Baqué F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747-757.
10. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
11. Boileau P, Neyton L. Arthroscopic tenodesis for lesions of the long head of the biceps. Oper Orthop Traumatol. 2005;17(6):601-623.
12. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
13. Sanders B, Lavery K, Pennington S, Warner JJP. Biceps tendon tenodesis: success with proximal versus distal fixation (SS-16). Arthroscopy. 2008;24(6 suppl):e9.
14. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
15. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
16. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
17. Jarrett CD, McClelland WB, Xerogeanes JW. Minimally invasive proximal biceps tenodesis: an anatomical study for optimal placement and safe surgical technique. J Shoulder Elbow Surg. 2011;20(3):477-480.
18. Mazzocca AD, Noerdlinger MA, Romeo AA. Mini open and subpectoral biceps tenodesis. Oper Tech Sports Med. 2003;11(1):24-31.
19. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
20. Froimson AI, O I. Keyhole tenodesis of biceps origin at the shoulder. Clin Orthop Relat Res. 1975;(112):245-249.
21. Sekiya JK, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
22. Verma NN, Drakos M, O’Brien SJ. Arthroscopic transfer of the long head biceps to the conjoint tendon. Arthroscopy. 2005;21(6):764.
23. Kilicoglu O, Koyuncu O, Demirhan M, et al. Time-dependent changes in failure loads of 3 biceps tenodesis techniques: in vivo study in a sheep model. Am J Sports Med. 2005;33(10):1536-1544.
24. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
25. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
26. Buchholz A, Martetschläger F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
27. Tashjian RZ, Henninger HB. Biomechanical evaluation of subpectoral biceps tenodesis: dual suture anchor versus interference screw fixation. J Shoulder Elbow Surg. 2013;22(10):1408-1412.
28. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJP. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9(1):121.
29. Sethi PM, Rajaram A, Beitzel K, Hackett TR, Chowaniec DM, Mazzocca AD. Biomechanical performance of subpectoral biceps tenodesis: a comparison of interference screw fixation, cortical button fixation, and interference screw diameter. J Shoulder Elbow Surg. 2013;22(4):451-457.
30. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
31. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
32. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
33. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
34. Werner BC, Evans CL, Holzgrefe RE, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of minimum 2-year clinical outcomes. Am J Sports Med. 2014;42(11):2583-2590.
35. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
36. Rhee PC, Spinner RJ, Bishop AT, Shin AY. Iatrogenic brachial plexus injuries associated with open subpectoral biceps tenodesis: a report of 4 cases. Am J Sports Med. 2013;41(9):2048-2053.
37. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
38. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
39. Abtahi AM, Granger EK, Tashjian RZ. Complications after subpectoral biceps tenodesis using a dual suture anchor technique. Int J Shoulder Surg. 2014;8(2):47-50.
40. Euler SA, Smith SD, Williams BT, Dornan GJ, Millett PJ, Wijdicks CA. Biomechanical analysis of subpectoral biceps tenodesis: effect of screw malpositioning on proximal humeral strength. Am J Sports Med. 2015;43(1):69-74.
41. Carofino BC, Brogan DM, Kircher MF, et al. Iatrogenic nerve injuries during shoulder surgery. J Bone Joint Surg Am. 2013;95(18):1667-1674.
42. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
43. Heckman DS, Creighton RA, Romeo AA. Management of failed biceps tenodesis or tenotomy: causation and treatment. Sports Med Arthrosc. 2010;18(3):173-180.
44. Gregory JM, Harwood DP, Gochanour E, Sherman SL, Romeo AA. Clinical outcomes of revision biceps tenodesis. Int J Shoulder Surg. 2012;6(2):45-50.
45. Anthony SG, McCormick F, Gross DJ, Golijanin P, Provencher MT. Biceps tenodesis for long head of the biceps after auto-rupture or failed surgical tenotomy: results in an active population. J Shoulder Elbow Surg. 2015;24(2):e36-e40.
1. Friedman DJ, Dunn JC, Higgins LD, Warner JJP. Proximal biceps tendon: injuries and management. Sports Med Arthrosc. 2008;16(3):162-169.
2. Nho SJ, Strauss EJ, Lenart BA, et al. Long head of the biceps tendinopathy: diagnosis and management. J Am Acad Orthop Surg. 2010;18(11):645-656.
3. Neviaser TJ, Neviaser RJ, Neviaser JS, Neviaser JS. The four-in-one arthroplasty for the painful arc syndrome. Clin Orthop Relat Res. 1982;163:107-112.
4. Patton WC, McCluskey GM 3rd. Biceps tendinitis and subluxation. Clin Sports Med. 2001;20(3):505-529.
5. Provencher MT, LeClere LE, Romeo AA. Subpectoral biceps tenodesis. Sports Med Arthrosc. 2008;16(3):170-176.
6. Bennett WF. Arthroscopic repair of isolated subscapularis tears: a prospective cohort with 2- to 4-year follow-up. Arthroscopy. 2003;19(2):131-143.
7. Dines DM, Warren RF, Inglis AE, Pavlov H. The coracoid impingement syndrome. Bone Joint J Br. 1990;72(2):314-316.
8. Mohtadi NG, Vellet AD, Clark ML, et al. A prospective, double-blind comparison of magnetic resonance imaging and arthroscopy in the evaluation of patients presenting with shoulder pain. J Shoulder Elbow Surg. 2004;13(3):258-265.
9. Boileau P, Baqué F, Valerio L, Ahrens P, Chuinard C, Trojani C. Isolated arthroscopic biceps tenotomy or tenodesis improves symptoms in patients with massive irreparable rotator cuff tears. J Bone Joint Surg Am. 2007;89(4):747-757.
10. Kelly AM, Drakos MC, Fealy S, Taylor SA, O’Brien SJ. Arthroscopic release of the long head of the biceps tendon: functional outcome and clinical results. Am J Sports Med. 2005;33(2):208-213.
11. Boileau P, Neyton L. Arthroscopic tenodesis for lesions of the long head of the biceps. Oper Orthop Traumatol. 2005;17(6):601-623.
12. Moon SC, Cho NS, Rhee YG. Analysis of “hidden lesions” of the extra-articular biceps after subpectoral biceps tenodesis: the subpectoral portion as the optimal tenodesis site. Am J Sports Med. 2015;43(1):63-68.
13. Sanders B, Lavery K, Pennington S, Warner JJP. Biceps tendon tenodesis: success with proximal versus distal fixation (SS-16). Arthroscopy. 2008;24(6 suppl):e9.
14. Sanders B, Lavery KP, Pennington S, Warner JJ. Clinical success of biceps tenodesis with and without release of the transverse humeral ligament. J Shoulder Elbow Surg. 2012;21(1):66-71.
15. Dickens JF, Kilcoyne KG, Tintle SM, Giuliani J, Schaefer RA, Rue JP. Subpectoral biceps tenodesis: an anatomic study and evaluation of at-risk structures. Am J Sports Med. 2012;40(10):2337-2341.
16. Denard PJ, Dai X, Hanypsiak BT, Burkhart SS. Anatomy of the biceps tendon: implications for restoring physiological length–tension relation during biceps tenodesis with interference screw fixation. Arthroscopy. 2012;28(10):1352-1358.
17. Jarrett CD, McClelland WB, Xerogeanes JW. Minimally invasive proximal biceps tenodesis: an anatomical study for optimal placement and safe surgical technique. J Shoulder Elbow Surg. 2011;20(3):477-480.
18. Mazzocca AD, Noerdlinger MA, Romeo AA. Mini open and subpectoral biceps tenodesis. Oper Tech Sports Med. 2003;11(1):24-31.
19. Mazzocca AD, Bicos J, Santangelo S, Romeo AA, Arciero RA. The biomechanical evaluation of four fixation techniques for proximal biceps tenodesis. Arthroscopy. 2005;21(11):1296-1306.
20. Froimson AI, O I. Keyhole tenodesis of biceps origin at the shoulder. Clin Orthop Relat Res. 1975;(112):245-249.
21. Sekiya JK, Elkousy HA, Rodosky MW. Arthroscopic biceps tenodesis using the percutaneous intra-articular transtendon technique. Arthroscopy. 2003;19(10):1137-1141.
22. Verma NN, Drakos M, O’Brien SJ. Arthroscopic transfer of the long head biceps to the conjoint tendon. Arthroscopy. 2005;21(6):764.
23. Kilicoglu O, Koyuncu O, Demirhan M, et al. Time-dependent changes in failure loads of 3 biceps tenodesis techniques: in vivo study in a sheep model. Am J Sports Med. 2005;33(10):1536-1544.
24. Ozalay M, Akpinar S, Karaeminogullari O, et al. Mechanical strength of four different biceps tenodesis techniques. Arthroscopy. 2005;21(8):992-998.
25. Patzer T, Santo G, Olender GD, Wellmann M, Hurschler C, Schofer MD. Suprapectoral or subpectoral position for biceps tenodesis: biomechanical comparison of four different techniques in both positions. J Shoulder Elbow Surg. 2012;21(1):116-125.
26. Buchholz A, Martetschläger F, Siebenlist S, et al. Biomechanical comparison of intramedullary cortical button fixation and interference screw technique for subpectoral biceps tenodesis. Arthroscopy. 2013;29(5):845-853.
27. Tashjian RZ, Henninger HB. Biomechanical evaluation of subpectoral biceps tenodesis: dual suture anchor versus interference screw fixation. J Shoulder Elbow Surg. 2013;22(10):1408-1412.
28. Millett PJ, Sanders B, Gobezie R, Braun S, Warner JJP. Interference screw vs. suture anchor fixation for open subpectoral biceps tenodesis: does it matter? BMC Musculoskelet Disord. 2008;9(1):121.
29. Sethi PM, Rajaram A, Beitzel K, Hackett TR, Chowaniec DM, Mazzocca AD. Biomechanical performance of subpectoral biceps tenodesis: a comparison of interference screw fixation, cortical button fixation, and interference screw diameter. J Shoulder Elbow Surg. 2013;22(4):451-457.
30. Slabaugh MA, Frank RM, Van Thiel GS, et al. Biceps tenodesis with interference screw fixation: a biomechanical comparison of screw length and diameter. Arthroscopy. 2011;27(2):161-166.
31. Mazzocca AD, Rios CG, Romeo AA, Arciero RA. Subpectoral biceps tenodesis with interference screw fixation. Arthroscopy. 2005;21(7):896.
32. Mazzocca AD, Cote MP, Arciero CL, Romeo AA, Arciero RA. Clinical outcomes after subpectoral biceps tenodesis with an interference screw. Am J Sports Med. 2008;36(10):1922-1929.
33. Werner BC, Brockmeier SF, Miller MD. Etiology, diagnosis, and management of failed SLAP repair. J Am Acad Orthop Surg. 2014;22(9):554-565.
34. Werner BC, Evans CL, Holzgrefe RE, et al. Arthroscopic suprapectoral and open subpectoral biceps tenodesis: a comparison of minimum 2-year clinical outcomes. Am J Sports Med. 2014;42(11):2583-2590.
35. Nho SJ, Reiff SN, Verma NN, Slabaugh MA, Mazzocca AD, Romeo AA. Complications associated with subpectoral biceps tenodesis: low rates of incidence following surgery. J Shoulder Elbow Surg. 2010;19(5):764-768.
36. Rhee PC, Spinner RJ, Bishop AT, Shin AY. Iatrogenic brachial plexus injuries associated with open subpectoral biceps tenodesis: a report of 4 cases. Am J Sports Med. 2013;41(9):2048-2053.
37. Sears BW, Spencer EE, Getz CL. Humeral fracture following subpectoral biceps tenodesis in 2 active, healthy patients. J Shoulder Elbow Surg. 2011;20(6):e7-e11.
38. Ding DY, Gupta A, Snir N, Wolfson T, Meislin RJ. Nerve proximity during bicortical drilling for subpectoral biceps tenodesis: a cadaveric study. Arthroscopy. 2014;30(8):942-946.
39. Abtahi AM, Granger EK, Tashjian RZ. Complications after subpectoral biceps tenodesis using a dual suture anchor technique. Int J Shoulder Surg. 2014;8(2):47-50.
40. Euler SA, Smith SD, Williams BT, Dornan GJ, Millett PJ, Wijdicks CA. Biomechanical analysis of subpectoral biceps tenodesis: effect of screw malpositioning on proximal humeral strength. Am J Sports Med. 2015;43(1):69-74.
41. Carofino BC, Brogan DM, Kircher MF, et al. Iatrogenic nerve injuries during shoulder surgery. J Bone Joint Surg Am. 2013;95(18):1667-1674.
42. Dein EJ, Huri G, Gordon JC, McFarland EG. A humerus fracture in a baseball pitcher after biceps tenodesis. Am J Sports Med. 2014;42(4):877-879.
43. Heckman DS, Creighton RA, Romeo AA. Management of failed biceps tenodesis or tenotomy: causation and treatment. Sports Med Arthrosc. 2010;18(3):173-180.
44. Gregory JM, Harwood DP, Gochanour E, Sherman SL, Romeo AA. Clinical outcomes of revision biceps tenodesis. Int J Shoulder Surg. 2012;6(2):45-50.
45. Anthony SG, McCormick F, Gross DJ, Golijanin P, Provencher MT. Biceps tenodesis for long head of the biceps after auto-rupture or failed surgical tenotomy: results in an active population. J Shoulder Elbow Surg. 2015;24(2):e36-e40.
Navigating the Alphabet Soup of Labroligamentous Pathology of the Shoulder
The widespread use of eponyms and acronyms to describe labroligamentous findings in the shoulder has made interpretation of shoulder magnetic resonance imaging (MRI) reports challenging. We review and discuss the appearance of these lesions on shoulder MRI to help the orthopedic surgeon understand these entities as imaging findings.
Glenolabral articular disruption (GLAD) occurs secondary to impaction of the humeral head on the glenoid articular cartilage. There is a resultant defect in the glenoid articular cartilage, which extends to the glenoid labrum. A GLAD lesion is diagnosed only if the glenohumeral ligament and scapular periosteum remain intact1 (Figure 1).
Complete detachment of the anteroinferior labrum with tearing of the anterior glenoid periosteum represents a Bankart lesion. Cartilaginous Bankart lesions are caused by an anterior glenohumeral dislocation with resultant avulsion of the anteroinferior labrum and disruption of the scapular periosteum because of acute traction on the anterior band of the inferior glenohumeral ligament (Figure 2). Anterior instability, caused by disruption of the anterior labroligamentous complex, results. Osseous Bankart lesions occur when the anterior displaced humeral head impacts the anterior inferior glenoid rim, causing a fracture (Figure 3). This loss of the glenoid articular surface area can result in glenohumeral instability. Posterior shoulder dislocations can result in corresponding findings in the posterior inferior glenoid labrum (reverse Bankart lesion) and anterior medial humeral head (reverse Hill-Sachs lesion) (Figure 2).
A variant of the Bankart lesion is the anterior labroligamentous periosteal sleeve avulsion (ALPSA). This refers to a medially displaced tear of the anterior labrum with intact periosteal stripping along the medial glenoid2with medial rotation and inferior displacement of the anterior inferior labrum along the scapular neck. An ALPSA lesion can heal via the intact periosteal blood supply. If not repaired, anterior instability will result because of malposition of the labrum, causing a patulous anterior capsule.3 When a corresponding lesion occurs in the posterior labrum because of a posterior dislocation, it is called a posterior labrocapsular periosteal sleeve avulsion (POLPSA) (Figure 4).
Another variant of the Bankart lesion is the Perthes lesion, which is a nondisplaced tear of the anteroinferior labrum with periosteal stripping. This differs from the ALPSA because the detached labrum and periosteum are held in anatomic position, possibly making the lesion difficult to detect on magnetic resonance arthrography (MRA).3 Obtaining images in the abduction external rotation (ABER) position exerts traction on the anterior inferior joint capsule and may make the Perthes lesion more conspicuous.4 When this occurs in the posterior labrum, it is called a reverse Perthes lesion (Figure 5).
In a patient with anterior glenohumeral instability without a Bankart lesion, pathology of the anterior band of the inferior glenohumeral ligament (IGHL) at its humeral attachment must be suspected. Humeral avulsion of the IGHL (HAGL) or its variants can be overlooked on arthroscopy. HAGL is diagnosed on MRA when the normally U-shaped IGHL takes on a J-shape, and joint fluid extravasates across the torn humeral attachment (Figure 6). If there is an avulsed bony fragment from the medial humeral neck, the lesion is termed a bony HAGL (BHAGL). In addition to the findings of a HAGL, a BHAGL shows the osseous fragment and donor site on MRI. Since a BHAGL is a bony avulsion, it can even be suggested on radiography if a bony fragment is seen adjacent to the medial humeral neck.5 These lesions are highly associated with other shoulder injuries, particularly Hill-Sachs deformities and subscapularis tendon tears, and it is imperative, therefore, to search for additional injuries if a HAGL-type injury is seen.6
A more uncommon type of HAGL can occur in the setting of posterior capsulolabral injury. A posterior-band IGHL avulsion from the humerus (PHAGL) has similar imaging findings to a HAGL, except that it involves the posterior band of the IGHL. PHAGLs are usually not associated with an acute injury and are thought to be related to repetitive microtrauma, perhaps since the posterior band of the IGHL is the thinnest portion of the IGHL complex.7
A Kim lesion is an arthroscopic finding described in patients with posterior instability as a superficial defect at the undersurface of the posterior labrum and adjacent glenoid cartilage without detachment or extension to the chondrolabral junction.8 It is, by its nature, a concealed finding on routine MRI but can be more conspicuous in FADIR (flexed, adducted, internally rotated) positioning on MRA, which exerts traction on the posterior joint capsule, allowing intra-articular contrast to fill the tear (Figure 7).
This list describes several of the most commonly encountered acronyms in shoulder MRI. A review of SLAP (superior labrum anterior to posterior) lesions was described in a previous article in the journal’s Imaging Series.9 A thorough understanding of these lesions is helpful in interpreting reports and determining the appropriate treatment for patients with shoulder injuries.
1. Sanders TG, Tirman PF, Linares R, Feller JF, Richardson R. The glenolabral articular disruption lesion: MR arthrography with arthroscopic correlation. AJR Am J Roentgenol. 1999;172(1):171-175.
2. Beltran J, Jbara M, Maimon R. Shoulder: labrum and bicipital tendon. Top Magn Reson Imaging. 2003;14(1):35-50.
3. Waldt S, Burkart A, Imhoff AB, Bruegel M, Rummeny EJ, Woertler K. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-583.
4. Schreinemachers SA, van der Hulst VP, Willems J, Bipat S, van der Woude H. Is a single direct MR arthrography series in ABER position as accurate in detecting anteroinferior labroligamentous lesions as conventional MR arthrography? Skeletal Radiol. 2009;38(7):675-683.
5. Bui-Mansfield LT, Taylor DC, Uhorchak JM, Tenuta JT. Humeral avulsions of the glenohumeral ligament: imaging features and a review of the literature. AJR Am J Roentgenol. 2002;179(3):649-655.
6. Magee T. Prevalence of HAGL lesions and associated abnormalities on shoulder MR examination. Skeletal Radiol. 2014;43(3):307-313.
7. Chung CB, Sorenson S, Dwek JR, Resnick D. Humeral avulsion of the posterior band of the inferior glenohumeral ligament: MR arthrography and clinical correlation in 17 patients. AJR Am J Roentgenol. 2004;183(2):355-359.
8. Kim SH, Ha KI, Yoo JC, Noh KC. Kim’s lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy. 2004;20(7):712-720.
9. Grubin J, Maderazo A, Fitzpatrick D. Imaging evaluation of superior labral anteroposterior (SLAP) tears. Am J Orthop. 2015;44(10):476-477.
The widespread use of eponyms and acronyms to describe labroligamentous findings in the shoulder has made interpretation of shoulder magnetic resonance imaging (MRI) reports challenging. We review and discuss the appearance of these lesions on shoulder MRI to help the orthopedic surgeon understand these entities as imaging findings.
Glenolabral articular disruption (GLAD) occurs secondary to impaction of the humeral head on the glenoid articular cartilage. There is a resultant defect in the glenoid articular cartilage, which extends to the glenoid labrum. A GLAD lesion is diagnosed only if the glenohumeral ligament and scapular periosteum remain intact1 (Figure 1).
Complete detachment of the anteroinferior labrum with tearing of the anterior glenoid periosteum represents a Bankart lesion. Cartilaginous Bankart lesions are caused by an anterior glenohumeral dislocation with resultant avulsion of the anteroinferior labrum and disruption of the scapular periosteum because of acute traction on the anterior band of the inferior glenohumeral ligament (Figure 2). Anterior instability, caused by disruption of the anterior labroligamentous complex, results. Osseous Bankart lesions occur when the anterior displaced humeral head impacts the anterior inferior glenoid rim, causing a fracture (Figure 3). This loss of the glenoid articular surface area can result in glenohumeral instability. Posterior shoulder dislocations can result in corresponding findings in the posterior inferior glenoid labrum (reverse Bankart lesion) and anterior medial humeral head (reverse Hill-Sachs lesion) (Figure 2).
A variant of the Bankart lesion is the anterior labroligamentous periosteal sleeve avulsion (ALPSA). This refers to a medially displaced tear of the anterior labrum with intact periosteal stripping along the medial glenoid2with medial rotation and inferior displacement of the anterior inferior labrum along the scapular neck. An ALPSA lesion can heal via the intact periosteal blood supply. If not repaired, anterior instability will result because of malposition of the labrum, causing a patulous anterior capsule.3 When a corresponding lesion occurs in the posterior labrum because of a posterior dislocation, it is called a posterior labrocapsular periosteal sleeve avulsion (POLPSA) (Figure 4).
Another variant of the Bankart lesion is the Perthes lesion, which is a nondisplaced tear of the anteroinferior labrum with periosteal stripping. This differs from the ALPSA because the detached labrum and periosteum are held in anatomic position, possibly making the lesion difficult to detect on magnetic resonance arthrography (MRA).3 Obtaining images in the abduction external rotation (ABER) position exerts traction on the anterior inferior joint capsule and may make the Perthes lesion more conspicuous.4 When this occurs in the posterior labrum, it is called a reverse Perthes lesion (Figure 5).
In a patient with anterior glenohumeral instability without a Bankart lesion, pathology of the anterior band of the inferior glenohumeral ligament (IGHL) at its humeral attachment must be suspected. Humeral avulsion of the IGHL (HAGL) or its variants can be overlooked on arthroscopy. HAGL is diagnosed on MRA when the normally U-shaped IGHL takes on a J-shape, and joint fluid extravasates across the torn humeral attachment (Figure 6). If there is an avulsed bony fragment from the medial humeral neck, the lesion is termed a bony HAGL (BHAGL). In addition to the findings of a HAGL, a BHAGL shows the osseous fragment and donor site on MRI. Since a BHAGL is a bony avulsion, it can even be suggested on radiography if a bony fragment is seen adjacent to the medial humeral neck.5 These lesions are highly associated with other shoulder injuries, particularly Hill-Sachs deformities and subscapularis tendon tears, and it is imperative, therefore, to search for additional injuries if a HAGL-type injury is seen.6
A more uncommon type of HAGL can occur in the setting of posterior capsulolabral injury. A posterior-band IGHL avulsion from the humerus (PHAGL) has similar imaging findings to a HAGL, except that it involves the posterior band of the IGHL. PHAGLs are usually not associated with an acute injury and are thought to be related to repetitive microtrauma, perhaps since the posterior band of the IGHL is the thinnest portion of the IGHL complex.7
A Kim lesion is an arthroscopic finding described in patients with posterior instability as a superficial defect at the undersurface of the posterior labrum and adjacent glenoid cartilage without detachment or extension to the chondrolabral junction.8 It is, by its nature, a concealed finding on routine MRI but can be more conspicuous in FADIR (flexed, adducted, internally rotated) positioning on MRA, which exerts traction on the posterior joint capsule, allowing intra-articular contrast to fill the tear (Figure 7).
This list describes several of the most commonly encountered acronyms in shoulder MRI. A review of SLAP (superior labrum anterior to posterior) lesions was described in a previous article in the journal’s Imaging Series.9 A thorough understanding of these lesions is helpful in interpreting reports and determining the appropriate treatment for patients with shoulder injuries.
The widespread use of eponyms and acronyms to describe labroligamentous findings in the shoulder has made interpretation of shoulder magnetic resonance imaging (MRI) reports challenging. We review and discuss the appearance of these lesions on shoulder MRI to help the orthopedic surgeon understand these entities as imaging findings.
Glenolabral articular disruption (GLAD) occurs secondary to impaction of the humeral head on the glenoid articular cartilage. There is a resultant defect in the glenoid articular cartilage, which extends to the glenoid labrum. A GLAD lesion is diagnosed only if the glenohumeral ligament and scapular periosteum remain intact1 (Figure 1).
Complete detachment of the anteroinferior labrum with tearing of the anterior glenoid periosteum represents a Bankart lesion. Cartilaginous Bankart lesions are caused by an anterior glenohumeral dislocation with resultant avulsion of the anteroinferior labrum and disruption of the scapular periosteum because of acute traction on the anterior band of the inferior glenohumeral ligament (Figure 2). Anterior instability, caused by disruption of the anterior labroligamentous complex, results. Osseous Bankart lesions occur when the anterior displaced humeral head impacts the anterior inferior glenoid rim, causing a fracture (Figure 3). This loss of the glenoid articular surface area can result in glenohumeral instability. Posterior shoulder dislocations can result in corresponding findings in the posterior inferior glenoid labrum (reverse Bankart lesion) and anterior medial humeral head (reverse Hill-Sachs lesion) (Figure 2).
A variant of the Bankart lesion is the anterior labroligamentous periosteal sleeve avulsion (ALPSA). This refers to a medially displaced tear of the anterior labrum with intact periosteal stripping along the medial glenoid2with medial rotation and inferior displacement of the anterior inferior labrum along the scapular neck. An ALPSA lesion can heal via the intact periosteal blood supply. If not repaired, anterior instability will result because of malposition of the labrum, causing a patulous anterior capsule.3 When a corresponding lesion occurs in the posterior labrum because of a posterior dislocation, it is called a posterior labrocapsular periosteal sleeve avulsion (POLPSA) (Figure 4).
Another variant of the Bankart lesion is the Perthes lesion, which is a nondisplaced tear of the anteroinferior labrum with periosteal stripping. This differs from the ALPSA because the detached labrum and periosteum are held in anatomic position, possibly making the lesion difficult to detect on magnetic resonance arthrography (MRA).3 Obtaining images in the abduction external rotation (ABER) position exerts traction on the anterior inferior joint capsule and may make the Perthes lesion more conspicuous.4 When this occurs in the posterior labrum, it is called a reverse Perthes lesion (Figure 5).
In a patient with anterior glenohumeral instability without a Bankart lesion, pathology of the anterior band of the inferior glenohumeral ligament (IGHL) at its humeral attachment must be suspected. Humeral avulsion of the IGHL (HAGL) or its variants can be overlooked on arthroscopy. HAGL is diagnosed on MRA when the normally U-shaped IGHL takes on a J-shape, and joint fluid extravasates across the torn humeral attachment (Figure 6). If there is an avulsed bony fragment from the medial humeral neck, the lesion is termed a bony HAGL (BHAGL). In addition to the findings of a HAGL, a BHAGL shows the osseous fragment and donor site on MRI. Since a BHAGL is a bony avulsion, it can even be suggested on radiography if a bony fragment is seen adjacent to the medial humeral neck.5 These lesions are highly associated with other shoulder injuries, particularly Hill-Sachs deformities and subscapularis tendon tears, and it is imperative, therefore, to search for additional injuries if a HAGL-type injury is seen.6
A more uncommon type of HAGL can occur in the setting of posterior capsulolabral injury. A posterior-band IGHL avulsion from the humerus (PHAGL) has similar imaging findings to a HAGL, except that it involves the posterior band of the IGHL. PHAGLs are usually not associated with an acute injury and are thought to be related to repetitive microtrauma, perhaps since the posterior band of the IGHL is the thinnest portion of the IGHL complex.7
A Kim lesion is an arthroscopic finding described in patients with posterior instability as a superficial defect at the undersurface of the posterior labrum and adjacent glenoid cartilage without detachment or extension to the chondrolabral junction.8 It is, by its nature, a concealed finding on routine MRI but can be more conspicuous in FADIR (flexed, adducted, internally rotated) positioning on MRA, which exerts traction on the posterior joint capsule, allowing intra-articular contrast to fill the tear (Figure 7).
This list describes several of the most commonly encountered acronyms in shoulder MRI. A review of SLAP (superior labrum anterior to posterior) lesions was described in a previous article in the journal’s Imaging Series.9 A thorough understanding of these lesions is helpful in interpreting reports and determining the appropriate treatment for patients with shoulder injuries.
1. Sanders TG, Tirman PF, Linares R, Feller JF, Richardson R. The glenolabral articular disruption lesion: MR arthrography with arthroscopic correlation. AJR Am J Roentgenol. 1999;172(1):171-175.
2. Beltran J, Jbara M, Maimon R. Shoulder: labrum and bicipital tendon. Top Magn Reson Imaging. 2003;14(1):35-50.
3. Waldt S, Burkart A, Imhoff AB, Bruegel M, Rummeny EJ, Woertler K. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-583.
4. Schreinemachers SA, van der Hulst VP, Willems J, Bipat S, van der Woude H. Is a single direct MR arthrography series in ABER position as accurate in detecting anteroinferior labroligamentous lesions as conventional MR arthrography? Skeletal Radiol. 2009;38(7):675-683.
5. Bui-Mansfield LT, Taylor DC, Uhorchak JM, Tenuta JT. Humeral avulsions of the glenohumeral ligament: imaging features and a review of the literature. AJR Am J Roentgenol. 2002;179(3):649-655.
6. Magee T. Prevalence of HAGL lesions and associated abnormalities on shoulder MR examination. Skeletal Radiol. 2014;43(3):307-313.
7. Chung CB, Sorenson S, Dwek JR, Resnick D. Humeral avulsion of the posterior band of the inferior glenohumeral ligament: MR arthrography and clinical correlation in 17 patients. AJR Am J Roentgenol. 2004;183(2):355-359.
8. Kim SH, Ha KI, Yoo JC, Noh KC. Kim’s lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy. 2004;20(7):712-720.
9. Grubin J, Maderazo A, Fitzpatrick D. Imaging evaluation of superior labral anteroposterior (SLAP) tears. Am J Orthop. 2015;44(10):476-477.
1. Sanders TG, Tirman PF, Linares R, Feller JF, Richardson R. The glenolabral articular disruption lesion: MR arthrography with arthroscopic correlation. AJR Am J Roentgenol. 1999;172(1):171-175.
2. Beltran J, Jbara M, Maimon R. Shoulder: labrum and bicipital tendon. Top Magn Reson Imaging. 2003;14(1):35-50.
3. Waldt S, Burkart A, Imhoff AB, Bruegel M, Rummeny EJ, Woertler K. Anterior shoulder instability: accuracy of MR arthrography in the classification of anteroinferior labroligamentous injuries. Radiology. 2005;237(2):578-583.
4. Schreinemachers SA, van der Hulst VP, Willems J, Bipat S, van der Woude H. Is a single direct MR arthrography series in ABER position as accurate in detecting anteroinferior labroligamentous lesions as conventional MR arthrography? Skeletal Radiol. 2009;38(7):675-683.
5. Bui-Mansfield LT, Taylor DC, Uhorchak JM, Tenuta JT. Humeral avulsions of the glenohumeral ligament: imaging features and a review of the literature. AJR Am J Roentgenol. 2002;179(3):649-655.
6. Magee T. Prevalence of HAGL lesions and associated abnormalities on shoulder MR examination. Skeletal Radiol. 2014;43(3):307-313.
7. Chung CB, Sorenson S, Dwek JR, Resnick D. Humeral avulsion of the posterior band of the inferior glenohumeral ligament: MR arthrography and clinical correlation in 17 patients. AJR Am J Roentgenol. 2004;183(2):355-359.
8. Kim SH, Ha KI, Yoo JC, Noh KC. Kim’s lesion: an incomplete and concealed avulsion of the posteroinferior labrum in posterior or multidirectional posteroinferior instability of the shoulder. Arthroscopy. 2004;20(7):712-720.
9. Grubin J, Maderazo A, Fitzpatrick D. Imaging evaluation of superior labral anteroposterior (SLAP) tears. Am J Orthop. 2015;44(10):476-477.
Lateral Ulnar Collateral Ligament Reconstruction: An Analysis of Ulnar Tunnel Locations
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
Posterolateral rotatory instability (PLRI) of the elbow is well recognized1 and is the most common type of chronic elbow instability. PLRI is often an end result of traumatic elbow dislocation.2 The “essential lesion” in patients with PLRI of the elbow is injury to the lateral ulnar collateral ligament (LUCL).1 However, more recent research has emphasized the importance of other ligaments in the lateral ligament complex (radial collateral and annular ligaments) in preventing PLRI.3-5 Nevertheless, when conservative treatment fails, the most commonly used surgical treatment involves LUCL reconstruction.1,6-11
Numerous techniques for LUCL reconstruction have been described.1,7-9,11-13 The chosen technique ideally restores normal anatomy. Therefore, the isometric point of origin at the lateral epicondyle and insertion at the supinator tubercle are important landmarks for creating tunnels that reproduce isometry, function, and normal anatomy. Most often, 2 tunnels are created in the ulna to secure the graft. It has been our experience that ulnar tunnel creation can affect the length of the bony bridge and the orientation of the graft.
We conducted a study to identify the precise proximal ulna tunnel location—anterior to posterior, with the distal tunnel at the supinator tubercle on the crest—that allows for the largest bony bridge and most geometrically favorable construct. We hypothesized that a most posteriorly placed proximal tunnel would increase bony bridge size and allow for a more isosceles graft configuration. An isosceles configuration with the humerus tunnel at the isometric location would allow for anterior and posterior bands of the same length with theoretically equal force distribution.
Methods
After obtaining institutional review board approval, we retrospectively reviewed the cases of 17 adults with elbow computed tomography (CT) scans for inclusion in this study. The scans were previously performed for diagnostic workup of several pathologies, including valgus instability, olecranon stress fracture, and valgus extension overload. The scan protocol involved 0.5-mm axial cuts with inclusion of the distal humerus through the proximal radius and ulna in the DICOM (Digital Imaging and Communications in Medicine) format. Exclusion criteria included poor CT quality, inadequate visualization of the entire supinator crest, and age under 18 years. Fifteen patients with adequate CT scans met the inclusion criteria. MIMICS (Materialise’s Interactive Medical Image Control System) software was used to convert scans into patient-specific 3-dimensional (3-D) computer models. (Use of this software to produce anatomically accurate models has been verified in shoulder14 and elbow15 models.) These models were uploaded into Magics rapid prototyping software (Materialise) and manipulated for simulated tunnel drilling by precise bone subtraction methods. This software was used to define an ulnar Cartesian coordinate system with anatomical landmarks as reference points in order to standardize the position of each model (Figure 1).16 The y-axis was defined by the longitudinal axis of the ulna, and the x-axis was the transepicondylar axis, defined as the perpendicular line connecting the y-axis with the supinator crest. The z-axis was then established as the line perpendicular to the x- and y-axes—yielding a 3-D coordinate system that allowed us to manipulate the models in standardized fashion, maintaining the exact positions of the ulna while making measurements.
Surgical simulations were performed in the rapid prototyping software by creating a cylinder and placing it at the desired location of each tunnel. Cylinder diameter was 4 mm, matching the diameter of the drill we use to create each tunnel in our practice. The cylinder was inserted into the bone, perpendicular to the surface of the ulna at the point of insertion, so the cylinder’s deepest point entered the medullary canal of the ulna. Using a Boolean operation in the rapid prototyping software, we subtracted cylinder from bone to create a tunnel (Figure 2).15
In a previous study,17 we determined that the radial head junction is reproducibly about 15 mm proximal to the distinct supinator tubercle, which may be absent or not readily appreciated in up to 50% of cases. Therefore, proximal ulnar tunnels were placed 0, 5, and 10 mm posterior to the supinator crest at the radial head junction. Distal tunnels were placed 15 mm anterior to the radial head junction on the supinator crest (Figure 2). The bony bridges created by these tunnels were measured, as was the distance between the distal tunnel and the supinator tubercle.
Ideal graft configuration was described as an isosceles triangle with ulna tunnels perpendicular to the humeral tunnel (Figure 3).11 Location of the humeral origin in the sagittal plane was determined by finding the isometric point of the lateral humerus using only bony landmarks. Similar techniques have been used to find the isometric point on the medial epicondyle for medial ulnar collateral ligament reconstruction.15,18 With a circle fit into the trochlear notch of the ulna, the isometric point can be determined by the center of the circle. This point was then superimposed on the humerus to identify the starting point (Figure 4). In our simulation, we measured the isosceles configuration by drawing a line between the proximal and distal tunnels, and then another line connecting the bisecting point of the first line with the isometric point on the humerus from which the graft would originate. The angle between the 2 lines was measured; if isosceles, the angle was 90° (Figure 5). Length of the more proximal limb of the graft and the more distal limb of the graft was determined by measuring the distance from the isometric point to the proximal and distal tunnels, respectively (Figure 6).
One-way analysis of variance was used to compare all the tunnels’ bony bridge sizes, graft lengths, and angles to the isometric point. For all comparisons, statistical significance was set at P < .05. As no other studies have compared bony bridges by varying tunnel creation parameters, and as the present study is observational and not comparative, no power analysis was performed.
Results
Bony bridges were significantly longer, and angles more perpendicular, with increasing distance from the proximal tunnel to the supinator crest (Table 1, Figure 5, Figure 7). The bony bridge 0 mm posterior to the supinator crest yielded a mean (SE) bony bridge length of 11.0 (0.2) mm. This proximal tunnel also yielded the smallest mean (SE) perpendicular angle to the isometric point, 131.2° (1.9°). The tunnel most posterior to the supinator crest yielded the longest mean (SE) bony bridge, 13.7 (0.2) mm, and the largest mean (SE) degree of perpendicularity, 95.8° (1.4°). The differences between all tunnels’ bony bridges and isometric angles were statistically significant (P < .00001). The difference between the more distal limb and the more proximal limb of the graft was smallest in the more posteriorly placed proximal tunnel (Table 2, Figure 8). In fact, there was no statistical difference between the proximal and distal limbs of the graft when the proximal tunnel was placed 10 mm posterior to the supinator crest: Mean (SE) was 9.4 (0.5) mm at 0 mm (P < .00001) and 1.1 (0.6) mm at 10 mm (P = .24).
Discussion
PLRI of the elbow is best initially managed nonoperatively. However, when nonoperative management fails, the LUCL is often surgically reconstructed. Reconstruction methods vary by fixation method, graft choice, and bone tunnels.1,7-9,11-13 In 1991, O’Driscoll and colleagues1 described a “yoke” technique for LUCL reconstruction. Since then, the docking technique7 and other techniques have been developed. All these techniques emphasize maximizing anatomical precision and isometry with careful placement of tunnels or fixation devices. The humeral fixation site, at the anterior inferior aspect of the lateral epicondyle at the point of isometry, can be accessed relatively reproducibly. By contrast, the ulnar points of fixation are more variable, because of increased bone stock and overlying soft-tissue and bony anatomy.
Among the challenges in determining the points of ulnar fixation is the bony anatomy that is often used for landmarks. In the literature, the supinator crest or the supintor tubercle is the landmark for placing the distal tunnel.1,7-9,11-13 This is a problem for 2 reasons. First, the supintor crest, a longitudinal structure on the lateral aspect of the ulna, originates from the radial head junction and extends tens of millimeters distally; further specification is needed to guide these ulnar tunnels. The second reason is that use of the supinator tubercle, a prominence on the supinator crest, adds specificity to the location of the ulnar tunnels. During surgery, however, the supinator tubercle may not be a reliable, independently prominent structure; instead, it may be indistinguishable from the supinator crest, on which it rests. One study determined that only about 50% of computer models of patient ulnas had a distinct prominence that could be classified as the supinator tubercle.17 The percentage presumably is lower during surgery, with limited exposure and overlying soft tissues.
In a study of patients with a prominent tubercle, mean (SE) distance from radial head junction to tubercle was 15 (2) mm.17 This finding led us to use the radial head junction as the primary bony landmark in determining the location of the proximal tunnel and placing the distal tunnel 15 mm distally—achieving the same fixation described in the literature but using more distinct landmarks. Our study thus provided a reliable, verified approach to locating the ulnar tunnels in the proximal-distal axis.
We also explored the anterior-posterior orientation of the proximal ulnar tunnel. The 2 primary considerations surrounding the varied proximal tunnel placements were the bony bridge formed between the proximal and distal tunnels and the perpendicularity of the triangle formed by the fixation points. Maximizing the bony bridge is obviously ideal in securing and preventing fixation blowout. Achieving an isoceles reconstruction has been reported in the literature on the various fixation techniques for LUCL reconstruction.11 Although the biomechanical advantage of this fixation type is not fully clear, we assume the construct produces graft stands of equal length, tension, and stability. In addition, the larger footprint created by an isoceles reconstructed ligament increases the stability of the radial head.
Results of the present study showed that the more posterior the proximal ulnar tunnel, the longer the bony bridge and the more isoceles the reconstruction. The difference in bony bridge distance from the most anterior to the most posterior tunnel was about 2 mm, or 18%. For every 1 mm of posteriorization, the bony bridge was 0.2 mm longer. The line from the isometric point of humeral fixation bisecting the proximal and distal tunnels was also more perpendicular with the most posterior tunnel, by about 40°. The resulting proximal and distal limbs of the reconstruction were equal in length, as demonstrated by the smaller difference between the limbs. We assume this isoceles reconstruction more likely applies uniform restraint on the radial head. Thus, an effort should be made to posteriorize the proximal ulnar tunnel during reconstruction.
The study was limited by the number of patient-specific elbow models used. However, given the statistical consistency of measurements, sample size was sufficient. Another limitation, inherent to the model, was that only bony anatomy was incorporated. However, the overlying muscles, tendons, and ligaments can significantly alter tunnel placement, and this study provided other means and cues using more reliable landmarks to adequately place the tunnels. As this was a simulation study, we cannot confirm whether these results would make a difference clinically. The strengths of this study include development and verification of reliable landmarks that can be used to guide ulnar tunnel locations during LUCL reconstruction; these landmarks have been used for medial ulnar collateral ligament reconstruction.15 Other strengths include precise and accurate placement of tunnels and measurement of resulting bony bridges—accomplished independently and without compromising specimen quality.
Conclusion
We recommend drilling the proximal ulnar tunnel posterior to the supinator crest at the level of the radial head junction. A reasonable goal is 10 mm posterior to the crest, though the overlying soft tissue must be considered, and care should be taken to aim the drill anteriorly, toward the ulna’s intramedullary canal, to avoid posterior cortical breach. The distal ulnar tunnel should be drilled just posterior to the supinator crest, 15 mm distal to the radial head junction.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.
1. O’Driscoll SW, Bell DF, Morrey BF. Posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 1991;73(3):440-446.
2. O’Driscoll SW. Classification and evaluation of recurrent instability of the elbow. Clin Orthop Relat Res. 2000;370:34-43.
3. Takigawa N, Ryu J, Kish VL, Kinoshita M, Abe M. Functional anatomy of the lateral collateral ligament complex of the elbow: morphology and strain. J Hand Surg Br. 2005;30(2):143-147.
4. McAdams TR, Masters GW, Srivastava S. The effect of arthroscopic sectioning of the lateral ligament complex of the elbow on posterolateral rotatory stability. J Shoulder Elbow Surg. 2005;14(3):298-301.
5. Dunning CE, Zarzour ZD, Patterson SD, Johnson JA, King GJ. Ligamentous stabilizers against posterolateral rotatory instability of the elbow. J Bone Joint Surg Am. 2001;83(12):1823-1828.
6. Eygendaal D. Ligamentous reconstruction around the elbow using triceps tendon. Acta Orthop Scand. 2004;75(5):516-523.
7. Jones KJ, Dodson CC, Osbahr DC, et al. The docking technique for lateral ulnar collateral ligament reconstruction: surgical technique and clinical outcomes. J Shoulder Elbow Surg. 2012;21(3):389-395.
8. Lee BP, Teo LH. Surgical reconstruction for posterolateral rotatory instability of the elbow. J Shoulder Elbow Surg. 2003;12(5):476-479.
9. Lin KY, Shen PH, Lee CH, Pan RY, Lin LC, Shen HC. Functional outcomes of surgical reconstruction for posterolateral rotatory instability of the elbow. Injury. 2012;43(10):1657-1661.
10. Olsen BS, Søjbjerg JO. The treatment of recurrent posterolateral instability of the elbow. J Bone Joint Surg Br. 2003;85(3):342-346.
11. Sanchez-Sotelo J, Morrey BF, O’Driscoll SW. Ligamentous repair and reconstruction for posterolateral rotatory instability of the elbow. J Bone Joint Surg Br. 2005;87(1):54-61.
12. Savoie FH 3rd, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Hand Clin. 2009;25(3):323-329.
13. Savoie FH 3rd, O’Brien MJ, Field LD, Gurley DJ. Arthroscopic and open radial ulnohumeral ligament reconstruction for posterolateral rotatory instability of the elbow. Clin Sports Med. 2010;29(4):611-618.
14. Bryce CD, Pennypacker JL, Kulkarni N, et al. Validation of three-dimensional models of in situ scapulae. J Shoulder Elbow Surg. 2008;17(5):825-832.
15. Byram IR, Khanna K, Gardner TR, Ahmad CS. Characterizing bone tunnel placement in medial ulnar collateral ligament reconstruction using patient-specific 3-dimensional computed tomography modeling. Am J Sports Med. 2013;41(4):894-902.
16. Shiba R, Sorbie C, Siu DW, Bryant JT, Cooke TD, Wevers HW. Geometry of the humeroulnar joint. J Orthop Res. 1988;6(6):897-906.
17. Anakwenze OA, Khanna K, Levine WN, Ahmad CS. Characterization of the supinator tubercle for lateral ulnar collateral ligament reconstruction. Orthop J Sports Med. 2014;2(4):2325967114530969. doi:10.1177/2325967114530969.
18. Sasashige Y, Ochi M, Ikuta Y. Optimal attachment site for reconstruction of the ulnar collateral ligament. A cadaver study. Arch Orthop Trauma Surg. 1994;113(5):265-270.