User login
The American Journal of Orthopedics is an Index Medicus publication that is valued by orthopedic surgeons for its peer-reviewed, practice-oriented clinical information. Most articles are written by specialists at leading teaching institutions and help incorporate the latest technology into everyday practice.
Reconstructive Shelf Arthroplasty as a Salvage Procedure for Complex Fifth Tarsometatarsal Joint Complex Injuries: A Case Review and Discussion
Fractures of the cuboid bone are uncommon, with an annual incidence of approximately 1.8 per 100,000.1 This is largely attributed to the inherent stability provided by its anatomy and position in the foot’s lateral column, where it functions as a link between the lateral column and transverse plantar arch.2 Regarding its anatomy, the cuboid is a pyramidal-shaped bone with 6 bony surfaces that provide tremendous stability—3 of these are articular, 3 nonarticular.
Although the cuboid bone is susceptible to low-energy avulsion injuries, injuries that occur in the setting of high-energy trauma are most concerning, as they often occur concurrently with other midfoot fractures and dislocations. These less common crush injuries are associated with comminution, articular disruption, and shortening of the lateral column.3-5 Avulsion injuries occur via a twisting mechanism, while the more complex nutcracker fracture evolves via longitudinal compression of the lateral column, with the foot in a position of forced plantarflexion.6 Other comminuted fractures occur from direct impact on the lateral aspect of the foot.
Management of cuboid fractures varies according to etiology, fracture displacement, and articular involvement. Conservative management is reserved solely for stable, nondisplaced fractures.7 Unstable fracture-dislocations and those with associated lateral column shortening necessitate operative treatment, which attempts to restore anatomy, stability, and length of the foot’s lateral column.7-9 However, with the exception of open injuries, fractures tenting the skin, and injuries with concomitant compartment syndrome, the high-energy nature of cuboid fractures often precludes early surgical intervention, as the foot’s soft-tissue envelope is too compromised. For this reason, operative intervention is often performed on a delayed basis only after recovery of the soft tissue.
In this case report and literature review, we describe a reconstructive shelf arthroplasty of the fifth tarsometatarsal (TMT) joint as a primary intervention for crush-type cuboid fractures with associated joint subsidence and lateral column shortening. The shelf arthroplasty, which was first credited to Konig in 1891, has historically been described as a remodeling operation using bone graft wedges for the treatment of nonconcentric acetabular dysplasia.10 Although bone grafting is recognized as an effective means of addressing osseous voids in the setting of comminuted cuboid fractures, its specific application in the form of a shelf arthroplasty has not been described.11 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
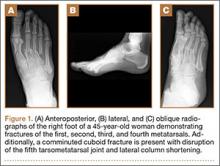
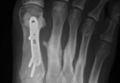
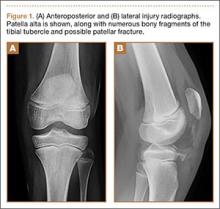
An otherwise healthy 45-year-old woman presented to our institution’s emergency department (ED) complaining of right foot pain after a motor vehicle accident. She was the restrained driver in a head-on collision. Primary survey revealed a swollen, ecchymotic, and tender right foot. Radiographs demonstrated fractures of her first, second, third, and fourth metatarsals, and a comminuted cuboid fracture with lateral column shortening and disruption of the fifth TMT joint (Figure 1).
Due to swelling, initial management consisted of soft-tissue management through the use of a well-padded splint. As this was her only injury, she was instructed to remain non-weight-bearing, ambulate with crutches, and return to our outpatient office for close follow-up. The need for delayed surgical intervention of her multiple foot injuries, due to her compromised soft-tissue envelope, was discussed prior to discharge.
Surgical intervention was performed 15 days after the injury, when the soft-tissue swelling had dissipated. The surgical plan included fixation of the multiple metatarsal fractures and lateral column reconstruction and stabilization. With regard to the lateral column, we obtained patient consent for several possible procedures, including fifth TMT joint closed reduction and percutaneous pinning, open reduction and internal fixation (ORIF), and TMT joint reconstruction with iliac crest bone graft (ICBG).
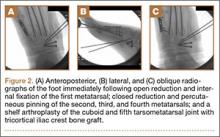
The metatarsals were addressed first via a dorsomedial incision, using a 5-hole 2.7-mm Limited Contact Dynamic Compression Plate (Synthes) to stabilize the first metatarsal and 2.0-mm Kirschner wires (K-wires) to maintain the length and alignment of the second, third, and fourth metatarsals (Figure 2). Closed reduction and percutaneous pinning of the fifth metatarsal was then attempted but abandoned because of persistent instability and subsidence of the cuboid in the proximal and plantar direction. ORIF was then attempted through a dorsolateral incision extending from just distal to the sinus tarsi to the base of the fourth metatarsal. However, the lateral cuboid was too comminuted to accommodate any fixation and prevent fifth TMT joint subluxation and lateral column shortening.
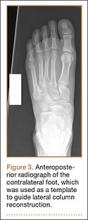
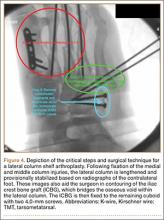
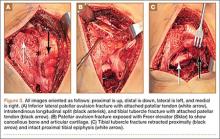
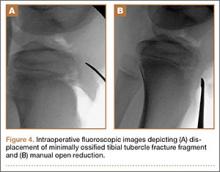
Autograft reconstruction of the lateral column was therefore performed, using radiographs of the patient’s uninjured, contralateral foot as a template for our lateral column shelf arthroplasty (Figure 3). Based on this template, the length and alignment of the lateral column were provisionally maintained with two 2.0-mm K-wires placed between the fifth metatarsal and intact cuboid (Figure 4). Tricortical ICBG was then harvested through an anterior approach to the iliac crest and contoured accordingly to fill the osseous void. To facilitate graft incorporation, comminuted fragments of cuboid bone were removed, with the remaining bone decorticated. The graft was then fixed to the remaining cuboid with two 4.0-mm partially threaded cannulated screws (Synthes; Figures 2, 4). This construct restored the length of the lateral column and effectively buttressed the fifth TMT joint, preventing subsidence and dislocation of the TMT joint.
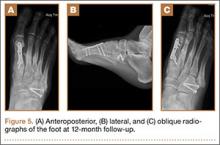
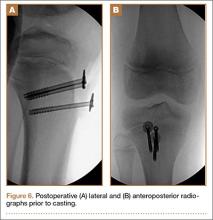
After a 2-day postoperative course in the hospital, the patient was discharged. She remained non-weight-bearing in a splint with Robert Jones cotton bandage. At her 2-week postoperative visit, all hardware was intact and there was no evidence of infection. Her sutures were removed and she was placed in a new splint. At the patient’s 5-week postoperative visit, all K-wires were removed. At this time she remained non-weight-bearing but was transitioned into a controlled ankle movement (CAM) boot and was allowed to begin active and passive ankle exercises. At her 10-week follow-up, radiographs revealed appropriate interval healing and callus formation. The patient began weight-bearing as tolerated in the CAM boot at that time. At 12 weeks, she was transitioned into a hard-soled shoe for comfort and was allowed to ambulate in the footwear of her choice as tolerated. Her activity levels were slowly advanced, and, at her 12-month follow-up, the patient had returned to playing tennis in her recreational league with no residual sequelae (Figure 5).
Discussion
Although rare, cuboid fractures are critical to identify and can result in significant disability, as they are frequently associated with additional foot trauma, as demonstrated in this case.1-4When isolated cuboid fractures are present, further imaging must be performed, including additional radiographic views and computed tomography, to search for other injuries, such as TMT joint complex disruption.
Only those cuboid fractures that are low-energy, stable, or nondisplaced can be effectively managed conservatively.12In the presence of instability, articular incongruity, or lateral column shortening, operative intervention is warranted. Arthritic degeneration, pain, and deformity result from residual incongruity at the calcaneocuboid or TMT joints, or when lateral column length is not restored.4-6,13 The latter leads to forefoot abduction and lateral subluxation of the lesser metatarsals, with ensuing posttraumatic pes planus or planovalgus deformity, which often necessitates secondary reconstructive procedures or arthrodesis.14,15 Stable reduction and restoration of lateral column length can be challenging, particularly in the setting of comminution and bone loss. Common methods of treatment involve lifting the dorsolateral cortex of the cuboid and buttressing the impacted articular surface with bone graft or bone graft substitutes. Fixation can be achieved with K-wires, small fragment plates and screws, and distraction external fixation.11 The latter is a particularly beneficial technique, as it can be used independent of or in conjunction with ORIF.
In a study by Weber and Locher,11 the short-term to midterm results of cuboid ORIF were assessed in 12 patients. Results were found to be good with respect to restoration of length, joint reconstruction, and overall return to function.11 Admittedly, these authors at times employed a similar but conceptually different approach to our patient. In their 7 patients with severe comminution and lateral column shortening, corticocancellous ICBG was used. However, Weber and Locher11did not describe this as a shelf arthroplasty, but instead as an adjunct to primary ORIF.
In our case, the tricortical ICBG shelf arthroplasty was used as it is in the hip, as a salvage procedure. Although little is known about outcomes following shelf arthroplasty for lateral column reconstruction in the foot, a 50% failure rate has been observed in the hip.16 As such, our preference was to perform an anatomic ORIF of the cuboid and lateral column, with the shelf arthroplasty only indicated if we were unable to achieve this. We believe that the need for tricortical ICBG in the treatment of cuboid fractures is indicative of a more severe injury and that it is a less optimal and more technically demanding intervention compared with primary ORIF. Furthermore, in other studies devoted to the treatment of cuboid fractures, patients requiring reconstruction with structural graft are not included in primary ORIF cohorts.17
As in the hip, suboptimal outcomes may occur when shelf arthroplasty is performed in the foot. There are additional considerations unique to the foot that surgeons must also contemplate when considering shelf arthroplasty. As demonstrated in the literature for adult-acquired flatfoot deformity, lateral column reconstruction is challenging and controversial and is associated with overload, pain, and the need to remove prominent hardware.18 These complications may also occur after shelf arthroplasty for cuboid fractures.
The work by Weber and Locher11 did not elucidate such considerations, and outcomes of ORIF and ICBG reconstruction were not compared. This is a limitation of their study, as differences in functional outcomes between the 2 procedures remain unknown. Given the degree of comminution that precludes ORIF and necessitates a graft reconstruction, we believe that the description of the shelf arthroplasty as a salvage procedure more accurately reflects the severity of injury. This may have implications regarding outcomes and patient expectations that the orthopedic surgeon must address. Future studies must further evaluate the outcomes of this technique, independent of and in comparison with ORIF.
Conclusion
In this case, we describe shelf arthroplasty for cuboid fractures. It is a reconstructive salvage procedure that is indicated when ORIF cannot be achieved. This useful approach to a complex injury must remain in the armamentarium of orthopedic surgeons. As we have demonstrated, it can effectively restore a damaged lateral column, providing length and, in our case, enabling the patient to return to her pre-injury level of activity.
1. Court-Brown C, Zinna S, Ekrol I. Classification and epidemiology of midfoot fractures. Foot. 2006;16(3):138-141.
2. Sarrafian SK. Osteology. In: Kelikian AS, ed. Sarrafian’s Anatomy of the Foot and Ankle. Philadelphia, PA: Lippincott; 1993:65-70.
3. Davis CA, Lubowitz J, Thordarson DB. Midtarsal fracture subluxation. Case report and review of the literature. Clin Orthop Relat Res. 1993;(292):264-268.
4. Dewar FP, Evans DC. Occult fracture-subluxation of the midtarsal joint. J Bone Joint Surg Br. 1968;50(2):386-388.
5. Sangeorzan BJ, Swiontkowski MF. Displaced fractures of the cuboid. J Bone Joint Surg Br. 1990;72(3):376-378.
6. Hermel MB, Gershon-Cohen J. The nutcracker fracture of the cuboid by indirect violence. Radiology. 1953;60(6):850-854.
7. Early J, Reid J. Fractures and dislocations of the midfoot and forefoot. In: Heckman JD, Bucholz RW, Court-Brown CM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:2120-2126.
8. Richter M, Wippermann B, Krettek C, Schratt HE, Hufner T, Therman H. Fractures and fracture dislocations of the midfoot: occurrence, causes and long-term results. Foot Ankle Int. 2001;22(5):392-398.
9. Borrelli J Jr, De S, VanPelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20(7):472-477.
10. Love BRT, Stevens PM, Williams PF. A long-term review of shelf arthroplasty. J Bone Joint Surg Br. 1980;62(3):321-325.
11. Weber M, Locher S. Reconstruction of the cuboid in compression fractures: short to midterm results in 12 patients. Foot Ankle Int. 2002;23(11):1008-1013.
12. Ebizie AO. Crush fractures of the cuboid from indirect violence. Injury. 1991;22(5):414-416.
13. Berlet GC, Hodges Davis W, Anderson RB. Tendon arthroplasty for basal fourth and fifth metatarsal arthritis. Foot Ankle Int. 2002;23(5):440-444.
14. Brunet JA, Wiley JJ. The late results of tarsometatarsal joint injuries. J Bone Joint Surg Br. 1987;69(3):437-440.
15. DeAsla R, Deland J. Anatomy and biomechanics of the foot and ankle. In: Thordarson DB, Tornetta P, Einhorn TA, eds. Orthopaedic Surgery Essentials: Foot & Ankle. Philadelphia, PA: Lippincott William & Wilkins; 2004:18-23.
16. Berton C, Bocquet D, Krantz N, Cotton A, Migaud H, Girard J. Shelf arthroplasties long-term outcome: influence of labral tears. A prospective study at a minimal 16 years’ follows up. Orthop Traumatol Surg Res. 2010;96(7):753-759.
17. van Raaij TM, Duffy PJ, Buckley RE. Displaced isolated cuboid fractures: results of four cases with operative treatment. Foot Ankle Int. 2010;31(3):242-246.
18. Grier KM, Walling AK. The use of tricortical autograft versus allograft in lateral column lengthening for adult acquired flatfoot deformity: an analysis of union rates and complications. Foot Ankle Int. 2010;31(9):760-769.
Fractures of the cuboid bone are uncommon, with an annual incidence of approximately 1.8 per 100,000.1 This is largely attributed to the inherent stability provided by its anatomy and position in the foot’s lateral column, where it functions as a link between the lateral column and transverse plantar arch.2 Regarding its anatomy, the cuboid is a pyramidal-shaped bone with 6 bony surfaces that provide tremendous stability—3 of these are articular, 3 nonarticular.
Although the cuboid bone is susceptible to low-energy avulsion injuries, injuries that occur in the setting of high-energy trauma are most concerning, as they often occur concurrently with other midfoot fractures and dislocations. These less common crush injuries are associated with comminution, articular disruption, and shortening of the lateral column.3-5 Avulsion injuries occur via a twisting mechanism, while the more complex nutcracker fracture evolves via longitudinal compression of the lateral column, with the foot in a position of forced plantarflexion.6 Other comminuted fractures occur from direct impact on the lateral aspect of the foot.
Management of cuboid fractures varies according to etiology, fracture displacement, and articular involvement. Conservative management is reserved solely for stable, nondisplaced fractures.7 Unstable fracture-dislocations and those with associated lateral column shortening necessitate operative treatment, which attempts to restore anatomy, stability, and length of the foot’s lateral column.7-9 However, with the exception of open injuries, fractures tenting the skin, and injuries with concomitant compartment syndrome, the high-energy nature of cuboid fractures often precludes early surgical intervention, as the foot’s soft-tissue envelope is too compromised. For this reason, operative intervention is often performed on a delayed basis only after recovery of the soft tissue.
In this case report and literature review, we describe a reconstructive shelf arthroplasty of the fifth tarsometatarsal (TMT) joint as a primary intervention for crush-type cuboid fractures with associated joint subsidence and lateral column shortening. The shelf arthroplasty, which was first credited to Konig in 1891, has historically been described as a remodeling operation using bone graft wedges for the treatment of nonconcentric acetabular dysplasia.10 Although bone grafting is recognized as an effective means of addressing osseous voids in the setting of comminuted cuboid fractures, its specific application in the form of a shelf arthroplasty has not been described.11 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 45-year-old woman presented to our institution’s emergency department (ED) complaining of right foot pain after a motor vehicle accident. She was the restrained driver in a head-on collision. Primary survey revealed a swollen, ecchymotic, and tender right foot. Radiographs demonstrated fractures of her first, second, third, and fourth metatarsals, and a comminuted cuboid fracture with lateral column shortening and disruption of the fifth TMT joint (Figure 1).
Due to swelling, initial management consisted of soft-tissue management through the use of a well-padded splint. As this was her only injury, she was instructed to remain non-weight-bearing, ambulate with crutches, and return to our outpatient office for close follow-up. The need for delayed surgical intervention of her multiple foot injuries, due to her compromised soft-tissue envelope, was discussed prior to discharge.
Surgical intervention was performed 15 days after the injury, when the soft-tissue swelling had dissipated. The surgical plan included fixation of the multiple metatarsal fractures and lateral column reconstruction and stabilization. With regard to the lateral column, we obtained patient consent for several possible procedures, including fifth TMT joint closed reduction and percutaneous pinning, open reduction and internal fixation (ORIF), and TMT joint reconstruction with iliac crest bone graft (ICBG).
The metatarsals were addressed first via a dorsomedial incision, using a 5-hole 2.7-mm Limited Contact Dynamic Compression Plate (Synthes) to stabilize the first metatarsal and 2.0-mm Kirschner wires (K-wires) to maintain the length and alignment of the second, third, and fourth metatarsals (Figure 2). Closed reduction and percutaneous pinning of the fifth metatarsal was then attempted but abandoned because of persistent instability and subsidence of the cuboid in the proximal and plantar direction. ORIF was then attempted through a dorsolateral incision extending from just distal to the sinus tarsi to the base of the fourth metatarsal. However, the lateral cuboid was too comminuted to accommodate any fixation and prevent fifth TMT joint subluxation and lateral column shortening.
Autograft reconstruction of the lateral column was therefore performed, using radiographs of the patient’s uninjured, contralateral foot as a template for our lateral column shelf arthroplasty (Figure 3). Based on this template, the length and alignment of the lateral column were provisionally maintained with two 2.0-mm K-wires placed between the fifth metatarsal and intact cuboid (Figure 4). Tricortical ICBG was then harvested through an anterior approach to the iliac crest and contoured accordingly to fill the osseous void. To facilitate graft incorporation, comminuted fragments of cuboid bone were removed, with the remaining bone decorticated. The graft was then fixed to the remaining cuboid with two 4.0-mm partially threaded cannulated screws (Synthes; Figures 2, 4). This construct restored the length of the lateral column and effectively buttressed the fifth TMT joint, preventing subsidence and dislocation of the TMT joint.
After a 2-day postoperative course in the hospital, the patient was discharged. She remained non-weight-bearing in a splint with Robert Jones cotton bandage. At her 2-week postoperative visit, all hardware was intact and there was no evidence of infection. Her sutures were removed and she was placed in a new splint. At the patient’s 5-week postoperative visit, all K-wires were removed. At this time she remained non-weight-bearing but was transitioned into a controlled ankle movement (CAM) boot and was allowed to begin active and passive ankle exercises. At her 10-week follow-up, radiographs revealed appropriate interval healing and callus formation. The patient began weight-bearing as tolerated in the CAM boot at that time. At 12 weeks, she was transitioned into a hard-soled shoe for comfort and was allowed to ambulate in the footwear of her choice as tolerated. Her activity levels were slowly advanced, and, at her 12-month follow-up, the patient had returned to playing tennis in her recreational league with no residual sequelae (Figure 5).
Discussion
Although rare, cuboid fractures are critical to identify and can result in significant disability, as they are frequently associated with additional foot trauma, as demonstrated in this case.1-4When isolated cuboid fractures are present, further imaging must be performed, including additional radiographic views and computed tomography, to search for other injuries, such as TMT joint complex disruption.
Only those cuboid fractures that are low-energy, stable, or nondisplaced can be effectively managed conservatively.12In the presence of instability, articular incongruity, or lateral column shortening, operative intervention is warranted. Arthritic degeneration, pain, and deformity result from residual incongruity at the calcaneocuboid or TMT joints, or when lateral column length is not restored.4-6,13 The latter leads to forefoot abduction and lateral subluxation of the lesser metatarsals, with ensuing posttraumatic pes planus or planovalgus deformity, which often necessitates secondary reconstructive procedures or arthrodesis.14,15 Stable reduction and restoration of lateral column length can be challenging, particularly in the setting of comminution and bone loss. Common methods of treatment involve lifting the dorsolateral cortex of the cuboid and buttressing the impacted articular surface with bone graft or bone graft substitutes. Fixation can be achieved with K-wires, small fragment plates and screws, and distraction external fixation.11 The latter is a particularly beneficial technique, as it can be used independent of or in conjunction with ORIF.
In a study by Weber and Locher,11 the short-term to midterm results of cuboid ORIF were assessed in 12 patients. Results were found to be good with respect to restoration of length, joint reconstruction, and overall return to function.11 Admittedly, these authors at times employed a similar but conceptually different approach to our patient. In their 7 patients with severe comminution and lateral column shortening, corticocancellous ICBG was used. However, Weber and Locher11did not describe this as a shelf arthroplasty, but instead as an adjunct to primary ORIF.
In our case, the tricortical ICBG shelf arthroplasty was used as it is in the hip, as a salvage procedure. Although little is known about outcomes following shelf arthroplasty for lateral column reconstruction in the foot, a 50% failure rate has been observed in the hip.16 As such, our preference was to perform an anatomic ORIF of the cuboid and lateral column, with the shelf arthroplasty only indicated if we were unable to achieve this. We believe that the need for tricortical ICBG in the treatment of cuboid fractures is indicative of a more severe injury and that it is a less optimal and more technically demanding intervention compared with primary ORIF. Furthermore, in other studies devoted to the treatment of cuboid fractures, patients requiring reconstruction with structural graft are not included in primary ORIF cohorts.17
As in the hip, suboptimal outcomes may occur when shelf arthroplasty is performed in the foot. There are additional considerations unique to the foot that surgeons must also contemplate when considering shelf arthroplasty. As demonstrated in the literature for adult-acquired flatfoot deformity, lateral column reconstruction is challenging and controversial and is associated with overload, pain, and the need to remove prominent hardware.18 These complications may also occur after shelf arthroplasty for cuboid fractures.
The work by Weber and Locher11 did not elucidate such considerations, and outcomes of ORIF and ICBG reconstruction were not compared. This is a limitation of their study, as differences in functional outcomes between the 2 procedures remain unknown. Given the degree of comminution that precludes ORIF and necessitates a graft reconstruction, we believe that the description of the shelf arthroplasty as a salvage procedure more accurately reflects the severity of injury. This may have implications regarding outcomes and patient expectations that the orthopedic surgeon must address. Future studies must further evaluate the outcomes of this technique, independent of and in comparison with ORIF.
Conclusion
In this case, we describe shelf arthroplasty for cuboid fractures. It is a reconstructive salvage procedure that is indicated when ORIF cannot be achieved. This useful approach to a complex injury must remain in the armamentarium of orthopedic surgeons. As we have demonstrated, it can effectively restore a damaged lateral column, providing length and, in our case, enabling the patient to return to her pre-injury level of activity.
Fractures of the cuboid bone are uncommon, with an annual incidence of approximately 1.8 per 100,000.1 This is largely attributed to the inherent stability provided by its anatomy and position in the foot’s lateral column, where it functions as a link between the lateral column and transverse plantar arch.2 Regarding its anatomy, the cuboid is a pyramidal-shaped bone with 6 bony surfaces that provide tremendous stability—3 of these are articular, 3 nonarticular.
Although the cuboid bone is susceptible to low-energy avulsion injuries, injuries that occur in the setting of high-energy trauma are most concerning, as they often occur concurrently with other midfoot fractures and dislocations. These less common crush injuries are associated with comminution, articular disruption, and shortening of the lateral column.3-5 Avulsion injuries occur via a twisting mechanism, while the more complex nutcracker fracture evolves via longitudinal compression of the lateral column, with the foot in a position of forced plantarflexion.6 Other comminuted fractures occur from direct impact on the lateral aspect of the foot.
Management of cuboid fractures varies according to etiology, fracture displacement, and articular involvement. Conservative management is reserved solely for stable, nondisplaced fractures.7 Unstable fracture-dislocations and those with associated lateral column shortening necessitate operative treatment, which attempts to restore anatomy, stability, and length of the foot’s lateral column.7-9 However, with the exception of open injuries, fractures tenting the skin, and injuries with concomitant compartment syndrome, the high-energy nature of cuboid fractures often precludes early surgical intervention, as the foot’s soft-tissue envelope is too compromised. For this reason, operative intervention is often performed on a delayed basis only after recovery of the soft tissue.
In this case report and literature review, we describe a reconstructive shelf arthroplasty of the fifth tarsometatarsal (TMT) joint as a primary intervention for crush-type cuboid fractures with associated joint subsidence and lateral column shortening. The shelf arthroplasty, which was first credited to Konig in 1891, has historically been described as a remodeling operation using bone graft wedges for the treatment of nonconcentric acetabular dysplasia.10 Although bone grafting is recognized as an effective means of addressing osseous voids in the setting of comminuted cuboid fractures, its specific application in the form of a shelf arthroplasty has not been described.11 The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 45-year-old woman presented to our institution’s emergency department (ED) complaining of right foot pain after a motor vehicle accident. She was the restrained driver in a head-on collision. Primary survey revealed a swollen, ecchymotic, and tender right foot. Radiographs demonstrated fractures of her first, second, third, and fourth metatarsals, and a comminuted cuboid fracture with lateral column shortening and disruption of the fifth TMT joint (Figure 1).
Due to swelling, initial management consisted of soft-tissue management through the use of a well-padded splint. As this was her only injury, she was instructed to remain non-weight-bearing, ambulate with crutches, and return to our outpatient office for close follow-up. The need for delayed surgical intervention of her multiple foot injuries, due to her compromised soft-tissue envelope, was discussed prior to discharge.
Surgical intervention was performed 15 days after the injury, when the soft-tissue swelling had dissipated. The surgical plan included fixation of the multiple metatarsal fractures and lateral column reconstruction and stabilization. With regard to the lateral column, we obtained patient consent for several possible procedures, including fifth TMT joint closed reduction and percutaneous pinning, open reduction and internal fixation (ORIF), and TMT joint reconstruction with iliac crest bone graft (ICBG).
The metatarsals were addressed first via a dorsomedial incision, using a 5-hole 2.7-mm Limited Contact Dynamic Compression Plate (Synthes) to stabilize the first metatarsal and 2.0-mm Kirschner wires (K-wires) to maintain the length and alignment of the second, third, and fourth metatarsals (Figure 2). Closed reduction and percutaneous pinning of the fifth metatarsal was then attempted but abandoned because of persistent instability and subsidence of the cuboid in the proximal and plantar direction. ORIF was then attempted through a dorsolateral incision extending from just distal to the sinus tarsi to the base of the fourth metatarsal. However, the lateral cuboid was too comminuted to accommodate any fixation and prevent fifth TMT joint subluxation and lateral column shortening.
Autograft reconstruction of the lateral column was therefore performed, using radiographs of the patient’s uninjured, contralateral foot as a template for our lateral column shelf arthroplasty (Figure 3). Based on this template, the length and alignment of the lateral column were provisionally maintained with two 2.0-mm K-wires placed between the fifth metatarsal and intact cuboid (Figure 4). Tricortical ICBG was then harvested through an anterior approach to the iliac crest and contoured accordingly to fill the osseous void. To facilitate graft incorporation, comminuted fragments of cuboid bone were removed, with the remaining bone decorticated. The graft was then fixed to the remaining cuboid with two 4.0-mm partially threaded cannulated screws (Synthes; Figures 2, 4). This construct restored the length of the lateral column and effectively buttressed the fifth TMT joint, preventing subsidence and dislocation of the TMT joint.
After a 2-day postoperative course in the hospital, the patient was discharged. She remained non-weight-bearing in a splint with Robert Jones cotton bandage. At her 2-week postoperative visit, all hardware was intact and there was no evidence of infection. Her sutures were removed and she was placed in a new splint. At the patient’s 5-week postoperative visit, all K-wires were removed. At this time she remained non-weight-bearing but was transitioned into a controlled ankle movement (CAM) boot and was allowed to begin active and passive ankle exercises. At her 10-week follow-up, radiographs revealed appropriate interval healing and callus formation. The patient began weight-bearing as tolerated in the CAM boot at that time. At 12 weeks, she was transitioned into a hard-soled shoe for comfort and was allowed to ambulate in the footwear of her choice as tolerated. Her activity levels were slowly advanced, and, at her 12-month follow-up, the patient had returned to playing tennis in her recreational league with no residual sequelae (Figure 5).
Discussion
Although rare, cuboid fractures are critical to identify and can result in significant disability, as they are frequently associated with additional foot trauma, as demonstrated in this case.1-4When isolated cuboid fractures are present, further imaging must be performed, including additional radiographic views and computed tomography, to search for other injuries, such as TMT joint complex disruption.
Only those cuboid fractures that are low-energy, stable, or nondisplaced can be effectively managed conservatively.12In the presence of instability, articular incongruity, or lateral column shortening, operative intervention is warranted. Arthritic degeneration, pain, and deformity result from residual incongruity at the calcaneocuboid or TMT joints, or when lateral column length is not restored.4-6,13 The latter leads to forefoot abduction and lateral subluxation of the lesser metatarsals, with ensuing posttraumatic pes planus or planovalgus deformity, which often necessitates secondary reconstructive procedures or arthrodesis.14,15 Stable reduction and restoration of lateral column length can be challenging, particularly in the setting of comminution and bone loss. Common methods of treatment involve lifting the dorsolateral cortex of the cuboid and buttressing the impacted articular surface with bone graft or bone graft substitutes. Fixation can be achieved with K-wires, small fragment plates and screws, and distraction external fixation.11 The latter is a particularly beneficial technique, as it can be used independent of or in conjunction with ORIF.
In a study by Weber and Locher,11 the short-term to midterm results of cuboid ORIF were assessed in 12 patients. Results were found to be good with respect to restoration of length, joint reconstruction, and overall return to function.11 Admittedly, these authors at times employed a similar but conceptually different approach to our patient. In their 7 patients with severe comminution and lateral column shortening, corticocancellous ICBG was used. However, Weber and Locher11did not describe this as a shelf arthroplasty, but instead as an adjunct to primary ORIF.
In our case, the tricortical ICBG shelf arthroplasty was used as it is in the hip, as a salvage procedure. Although little is known about outcomes following shelf arthroplasty for lateral column reconstruction in the foot, a 50% failure rate has been observed in the hip.16 As such, our preference was to perform an anatomic ORIF of the cuboid and lateral column, with the shelf arthroplasty only indicated if we were unable to achieve this. We believe that the need for tricortical ICBG in the treatment of cuboid fractures is indicative of a more severe injury and that it is a less optimal and more technically demanding intervention compared with primary ORIF. Furthermore, in other studies devoted to the treatment of cuboid fractures, patients requiring reconstruction with structural graft are not included in primary ORIF cohorts.17
As in the hip, suboptimal outcomes may occur when shelf arthroplasty is performed in the foot. There are additional considerations unique to the foot that surgeons must also contemplate when considering shelf arthroplasty. As demonstrated in the literature for adult-acquired flatfoot deformity, lateral column reconstruction is challenging and controversial and is associated with overload, pain, and the need to remove prominent hardware.18 These complications may also occur after shelf arthroplasty for cuboid fractures.
The work by Weber and Locher11 did not elucidate such considerations, and outcomes of ORIF and ICBG reconstruction were not compared. This is a limitation of their study, as differences in functional outcomes between the 2 procedures remain unknown. Given the degree of comminution that precludes ORIF and necessitates a graft reconstruction, we believe that the description of the shelf arthroplasty as a salvage procedure more accurately reflects the severity of injury. This may have implications regarding outcomes and patient expectations that the orthopedic surgeon must address. Future studies must further evaluate the outcomes of this technique, independent of and in comparison with ORIF.
Conclusion
In this case, we describe shelf arthroplasty for cuboid fractures. It is a reconstructive salvage procedure that is indicated when ORIF cannot be achieved. This useful approach to a complex injury must remain in the armamentarium of orthopedic surgeons. As we have demonstrated, it can effectively restore a damaged lateral column, providing length and, in our case, enabling the patient to return to her pre-injury level of activity.
1. Court-Brown C, Zinna S, Ekrol I. Classification and epidemiology of midfoot fractures. Foot. 2006;16(3):138-141.
2. Sarrafian SK. Osteology. In: Kelikian AS, ed. Sarrafian’s Anatomy of the Foot and Ankle. Philadelphia, PA: Lippincott; 1993:65-70.
3. Davis CA, Lubowitz J, Thordarson DB. Midtarsal fracture subluxation. Case report and review of the literature. Clin Orthop Relat Res. 1993;(292):264-268.
4. Dewar FP, Evans DC. Occult fracture-subluxation of the midtarsal joint. J Bone Joint Surg Br. 1968;50(2):386-388.
5. Sangeorzan BJ, Swiontkowski MF. Displaced fractures of the cuboid. J Bone Joint Surg Br. 1990;72(3):376-378.
6. Hermel MB, Gershon-Cohen J. The nutcracker fracture of the cuboid by indirect violence. Radiology. 1953;60(6):850-854.
7. Early J, Reid J. Fractures and dislocations of the midfoot and forefoot. In: Heckman JD, Bucholz RW, Court-Brown CM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:2120-2126.
8. Richter M, Wippermann B, Krettek C, Schratt HE, Hufner T, Therman H. Fractures and fracture dislocations of the midfoot: occurrence, causes and long-term results. Foot Ankle Int. 2001;22(5):392-398.
9. Borrelli J Jr, De S, VanPelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20(7):472-477.
10. Love BRT, Stevens PM, Williams PF. A long-term review of shelf arthroplasty. J Bone Joint Surg Br. 1980;62(3):321-325.
11. Weber M, Locher S. Reconstruction of the cuboid in compression fractures: short to midterm results in 12 patients. Foot Ankle Int. 2002;23(11):1008-1013.
12. Ebizie AO. Crush fractures of the cuboid from indirect violence. Injury. 1991;22(5):414-416.
13. Berlet GC, Hodges Davis W, Anderson RB. Tendon arthroplasty for basal fourth and fifth metatarsal arthritis. Foot Ankle Int. 2002;23(5):440-444.
14. Brunet JA, Wiley JJ. The late results of tarsometatarsal joint injuries. J Bone Joint Surg Br. 1987;69(3):437-440.
15. DeAsla R, Deland J. Anatomy and biomechanics of the foot and ankle. In: Thordarson DB, Tornetta P, Einhorn TA, eds. Orthopaedic Surgery Essentials: Foot & Ankle. Philadelphia, PA: Lippincott William & Wilkins; 2004:18-23.
16. Berton C, Bocquet D, Krantz N, Cotton A, Migaud H, Girard J. Shelf arthroplasties long-term outcome: influence of labral tears. A prospective study at a minimal 16 years’ follows up. Orthop Traumatol Surg Res. 2010;96(7):753-759.
17. van Raaij TM, Duffy PJ, Buckley RE. Displaced isolated cuboid fractures: results of four cases with operative treatment. Foot Ankle Int. 2010;31(3):242-246.
18. Grier KM, Walling AK. The use of tricortical autograft versus allograft in lateral column lengthening for adult acquired flatfoot deformity: an analysis of union rates and complications. Foot Ankle Int. 2010;31(9):760-769.
1. Court-Brown C, Zinna S, Ekrol I. Classification and epidemiology of midfoot fractures. Foot. 2006;16(3):138-141.
2. Sarrafian SK. Osteology. In: Kelikian AS, ed. Sarrafian’s Anatomy of the Foot and Ankle. Philadelphia, PA: Lippincott; 1993:65-70.
3. Davis CA, Lubowitz J, Thordarson DB. Midtarsal fracture subluxation. Case report and review of the literature. Clin Orthop Relat Res. 1993;(292):264-268.
4. Dewar FP, Evans DC. Occult fracture-subluxation of the midtarsal joint. J Bone Joint Surg Br. 1968;50(2):386-388.
5. Sangeorzan BJ, Swiontkowski MF. Displaced fractures of the cuboid. J Bone Joint Surg Br. 1990;72(3):376-378.
6. Hermel MB, Gershon-Cohen J. The nutcracker fracture of the cuboid by indirect violence. Radiology. 1953;60(6):850-854.
7. Early J, Reid J. Fractures and dislocations of the midfoot and forefoot. In: Heckman JD, Bucholz RW, Court-Brown CM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2009:2120-2126.
8. Richter M, Wippermann B, Krettek C, Schratt HE, Hufner T, Therman H. Fractures and fracture dislocations of the midfoot: occurrence, causes and long-term results. Foot Ankle Int. 2001;22(5):392-398.
9. Borrelli J Jr, De S, VanPelt M. Fracture of the cuboid. J Am Acad Orthop Surg. 2012;20(7):472-477.
10. Love BRT, Stevens PM, Williams PF. A long-term review of shelf arthroplasty. J Bone Joint Surg Br. 1980;62(3):321-325.
11. Weber M, Locher S. Reconstruction of the cuboid in compression fractures: short to midterm results in 12 patients. Foot Ankle Int. 2002;23(11):1008-1013.
12. Ebizie AO. Crush fractures of the cuboid from indirect violence. Injury. 1991;22(5):414-416.
13. Berlet GC, Hodges Davis W, Anderson RB. Tendon arthroplasty for basal fourth and fifth metatarsal arthritis. Foot Ankle Int. 2002;23(5):440-444.
14. Brunet JA, Wiley JJ. The late results of tarsometatarsal joint injuries. J Bone Joint Surg Br. 1987;69(3):437-440.
15. DeAsla R, Deland J. Anatomy and biomechanics of the foot and ankle. In: Thordarson DB, Tornetta P, Einhorn TA, eds. Orthopaedic Surgery Essentials: Foot & Ankle. Philadelphia, PA: Lippincott William & Wilkins; 2004:18-23.
16. Berton C, Bocquet D, Krantz N, Cotton A, Migaud H, Girard J. Shelf arthroplasties long-term outcome: influence of labral tears. A prospective study at a minimal 16 years’ follows up. Orthop Traumatol Surg Res. 2010;96(7):753-759.
17. van Raaij TM, Duffy PJ, Buckley RE. Displaced isolated cuboid fractures: results of four cases with operative treatment. Foot Ankle Int. 2010;31(3):242-246.
18. Grier KM, Walling AK. The use of tricortical autograft versus allograft in lateral column lengthening for adult acquired flatfoot deformity: an analysis of union rates and complications. Foot Ankle Int. 2010;31(9):760-769.
Definitive Fixation of Hand and Wrist Fractures in the Emergency Department
A mentor—now in his 60s—related his experiences as a resident. On call as a second-year resident, he would often be alone at a busy trauma center with no backup. When a case came in, he would quickly read about it in the library, then manage it in the emergency department (ED) if possible, or, if necessary, take the patient to the operating room (OR).
In the era of improved patient care, increased supervision, and decreased autonomy, this is not the reality anymore.1 In theory, more reliable patient care is the result; however, the pendulum may have swung too far.
There are a number of injuries that are amenable to definitive fixation in the ED, but not as limited an array of injuries as we have perhaps grown accustomed to. Hand injuries are among the most common orthopedic injuries seen in the ED, with fractures of the metacarpals and phalanges constituting nearly one-half of all hand injuries.2 The authors recently attended an excellent instructional course lecture on “The Lost and Found Art of Percutaneous Pinning in the Hand and Wrist” at the annual conference of the American Academy of Orthopaedic Surgeons.3 The presenters itemized a comprehensive list of fractures and simple dislocations of the hand, which could be simply, safely, effectively, and definitively managed through percutaneous pinning techniques. A significant number of unstable fractures of the phalanges and metacarpals can be treated in the ED under mini–C-arm fluoroscopy without an admission and trip to the OR.3,4 Most phalangeal and metacarpal fractures are nondisplaced or minimally displaced and stable, and can often be handled with a combination of closed reduction, buddy-taping, and splinting.5 The indications for percutaneous versus internal fixation depend on a number of factors, including bone quality, degree of comminution, quality of the soft-tissue envelope, articular involvement, acuity of presentation, and goals for motion.6,7
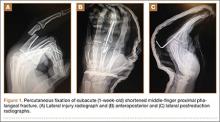
Many simple injury patterns involving unstable fractures or dislocations may be definitively managed in the ED with percutaneous pinning (eg, injuries that are unstable with closed reduction alone but that do not necessitate soft-tissue dissection). These include but are not limited to bony mallet injuries, unstable transverse or oblique fractures or fracture-dislocations of the phalanges and metacarpals, carpometacarpal fracture- dislocations, and underlying fractures that need protection of nail-bed repairs, soft-tissue flaps, or extensor tendon injuries (Figures 1, 2).7,8 The techniques for specific fracture types are beyond the scope of this article but are readily available.5,6
There are certain situations that undoubtedly warrant surgery in the OR, such as neurovascular injury necessitating microvascular repair, flexor tendon laceration, severely comminuted or segmental fractures, irreducible dislocations, and fractures with severe soft-tissue injury or contamination not amenable to primary irrigation, débridement, and closure at bedside.4,7,8
You might ask, “Why would one treat an operative injury in the ED and not formally in the OR?,” and we submit that there are a number of reasons.
First, and most important, with increasing health care costs and decreasing reimbursements, physicians are faced with providing safe but economical care. Percutaneous Kirschner wire (K-wire) fixation is dramatically more cost-effective when performed in the ED than in the OR. The cost of a procedure performed in either setting is similarly dependent on a variety of factors, generally including complexity of the patient or procedure, costs of supplies and pharmacologic agents, fixed versus variable overhead costs, and the professional fees of providers and ancillary personnel.9,10
While the patient is not charged per hour in the ED, it is estimated that ORs in the United States cost, on average, $62 per minute, ranging from as low as $22 to as high as $133 per minute.9 Additionally, the number of personnel involved in running an OR exceeds those for a similar procedure performed in the ED, considering (at a minimum) the orthopedic surgeon, anesthesiologist, scrub and radiology technicians, and nursing personnel required before, during, and after an operation.
While analgesia and procedural sedation can be performed similarly in either setting, it is our experience that patients are managed much more often in the ED with local anesthesia under direct care of only the orthopedic provider, whereas intravenous sedation and general anesthesia are far more commonly implemented in the OR. There are exceptions for pediatric patients or those who are unable to tolerate the procedure under only local anesthesia. Local anesthesia or even intravenous conscious sedation entails less risk as well as lower associated drug costs.11
The difference in risk is especially true for sicker patients undergoing minimally invasive procedures.11 Although administration of adequate procedural analgesia grows increasingly difficult the more proximal the injury, the hand and the fingers are easily and reliably anesthetized with well-placed wrist or digital blocks, with infrequent complications.12 Application of a lidocaine/bupivacaine mixture provides up to 6 to 8 hours of analgesia. A small tourniquet alternative, such as the finger of a sterile glove or phlebotomy tourniquet, applied to the base of the finger or the wrist additionally provides a relatively bloodless field and effectively acts as a Bier block.
Percutaneous pins are much more forgiving than rigid internal fixation. If the initial placement of a pin is unsatisfactory, the pin can be reinserted at little cost.12 Conversely, it may not be possible to reposition a misplaced screw or screw with inadequate purchase and still maintain adequate fixation. While percutaneous pin fixation is not as rigid as screw fixation, the degree of stability provided is adequate for the small forces affecting the hand in most cases. Accordingly, there is a very low incidence of fibrous union or nonunion.13,14 With an increasing appreciation of soft-tissue handling over the past few decades, another significant advantage of K-wire fixation is the obviation of soft-tissue dissection, preserving the biology to maximize healing and minimize adverse sequelae.12 Percutaneous fixation has been shown to achieve functional outcomes comparable to open reduction with internal fixation of operative phalangeal and metacarpal fractures, without soft-tissue disruption, scarring, or implant irritation, and with minimal risk of infection.3,13,15,16 Ultimate range of motion after percutaneous fixation is comparable, if not superior, to that of internal fixation, despite the initial advantage of rigid internal fixation secondary to decreased scarring and lack of indwelling hardware.16,17
While the risk of infection, perhaps the primary concern with percutaneous fixation, has been cited as high as 7%, osteomyelitis is exceedingly rare (<0.5%).3,13,14 Furthermore, pins are often left in place for 3 to 6 weeks, and infection has been found to occur most often at a mean of 10 weeks.7,13 Infection can also be mitigated by intelligent pin placement, relief of residual tension, and splint immobilization.4,15 Pin loosening has similarly been reported in up to 4% of cases in large retrospective studies, occurring at an average of 8 weeks, by which time most pins would have been extricated.13 Other complications related to impaling adjacent neurovascular or tendinous structures have also been cited but are rare.13 A 12-month prospective study of 75 patients specifically evaluating the outcomes after closed reduction with percutaneous fixation of unstable hand fractures in the ED reported only 6 complications at final follow-up.4 Complications were all minor, with no cases of nonunion, delayed union, malunion, pin-tract infection, pyarthrosis, or cellulitis, even in the setting of open fractures. Three patients required revision in the OR for pin migration, initial malreduction, and bone loss in the setting of comminution, respectively. The authors credited their low complication rate to supplementary immobilization.
In conclusion, many unstable simple fractures and dislocations of the hand and wrist can be safely and effectively treated in the ED. While it may seem daunting for a junior resident who is unfamiliar with percutaneous techniques, the authors advocate learning from a more senior mentor. The only additional training required is an understanding of how to apply this skill set in a different setting.
1. Levine WN, Spang RC 3rd. ACGME duty hour requirements: perceptions and impact on resident training and patient care. J Am Acad Orthop Surg. 2014;22(9):535-544.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Catalano LW 3rd, Glickel SZ, Strauch RJ, Barron AO. The lost and found art of percutaneous pinning in the hand and wrist. Instructional Course Lectures. Annual Meeting of the American Academy of Orthopaedic Surgeons; March 24, 2015; Las Vegas, NV.
4. Starker I, Eaton RG. Kirschner wire placement in the emergency room. Is there a risk? J Hand Surg Br. 1995;20(4):535-538.
5. Meals C, Meals R. Hand fractures: a review of current treatment strategies. J Hand Surg Am. 2013;38(5):1021-1031.
6. Henry MH. Fractures of the proximal phalanx and metacarpals in the hand: preferred methods of stabilization. J Am Acad Orthop Surg. 2008;16(10):586-595.
7. Klein DM, Belsole RJ. Percutaneous treatment of carpal, metacarpal, and phalangeal injuries. Clin Orthop Relat Res. 2000;(375):116-125.
8. Bernstein ML, Chung KC. Hand fractures and their management: an international view. Injury. 2006;37(11):1043-1048.
9. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
10. Williams RM. The costs of visits to emergency departments. N Engl J Med. 1996;334(10):642-646.
11. Bodenham AR, Howell SJ. General anesthesia vs local anaesthesia: an ongoing story. Br J Anaesth. 2009;103(6):785-789.
12. Stern PJ. Management of fractures of the hand over the last 25 years. J Hand Surg Am. 2000;25(5):817-823.
13. Botte MJ, Davis JL, Rose BA, et al. Complications of smooth pin fixation of fractures and dislocations in the hand and wrist. Clin Orthop Relat Res. 1992;(276):194-201.
14. Wray RC Jr, Glunk R. Treatment of delayed union, nonunion, and malunion of the phalanges of the hand. Ann Plast Surg. 1989;22(1):14-18.
15. Hsu LP, Schwartz EG, Kalainov DM, Chen F, Makowiec RL. Complications of K-wire fixation in procedures involving the hand and wrist. J Hand Surg Am. 2011;36(4):610-616.
16. Stem PJ, Wieser MJ, Reilly DG. Complications of plate fixation in the hand skeleton. Clin Orthop Relat Res. 1987;(214):59-65.
17. Page SM, Stern PJ. Complications and range of motion following plate fixation of metacarpal and phalangeal fractures. J Hand Surg Am. 1998;23(5):827-832.
A mentor—now in his 60s—related his experiences as a resident. On call as a second-year resident, he would often be alone at a busy trauma center with no backup. When a case came in, he would quickly read about it in the library, then manage it in the emergency department (ED) if possible, or, if necessary, take the patient to the operating room (OR).
In the era of improved patient care, increased supervision, and decreased autonomy, this is not the reality anymore.1 In theory, more reliable patient care is the result; however, the pendulum may have swung too far.
There are a number of injuries that are amenable to definitive fixation in the ED, but not as limited an array of injuries as we have perhaps grown accustomed to. Hand injuries are among the most common orthopedic injuries seen in the ED, with fractures of the metacarpals and phalanges constituting nearly one-half of all hand injuries.2 The authors recently attended an excellent instructional course lecture on “The Lost and Found Art of Percutaneous Pinning in the Hand and Wrist” at the annual conference of the American Academy of Orthopaedic Surgeons.3 The presenters itemized a comprehensive list of fractures and simple dislocations of the hand, which could be simply, safely, effectively, and definitively managed through percutaneous pinning techniques. A significant number of unstable fractures of the phalanges and metacarpals can be treated in the ED under mini–C-arm fluoroscopy without an admission and trip to the OR.3,4 Most phalangeal and metacarpal fractures are nondisplaced or minimally displaced and stable, and can often be handled with a combination of closed reduction, buddy-taping, and splinting.5 The indications for percutaneous versus internal fixation depend on a number of factors, including bone quality, degree of comminution, quality of the soft-tissue envelope, articular involvement, acuity of presentation, and goals for motion.6,7
Many simple injury patterns involving unstable fractures or dislocations may be definitively managed in the ED with percutaneous pinning (eg, injuries that are unstable with closed reduction alone but that do not necessitate soft-tissue dissection). These include but are not limited to bony mallet injuries, unstable transverse or oblique fractures or fracture-dislocations of the phalanges and metacarpals, carpometacarpal fracture- dislocations, and underlying fractures that need protection of nail-bed repairs, soft-tissue flaps, or extensor tendon injuries (Figures 1, 2).7,8 The techniques for specific fracture types are beyond the scope of this article but are readily available.5,6
There are certain situations that undoubtedly warrant surgery in the OR, such as neurovascular injury necessitating microvascular repair, flexor tendon laceration, severely comminuted or segmental fractures, irreducible dislocations, and fractures with severe soft-tissue injury or contamination not amenable to primary irrigation, débridement, and closure at bedside.4,7,8
You might ask, “Why would one treat an operative injury in the ED and not formally in the OR?,” and we submit that there are a number of reasons.
First, and most important, with increasing health care costs and decreasing reimbursements, physicians are faced with providing safe but economical care. Percutaneous Kirschner wire (K-wire) fixation is dramatically more cost-effective when performed in the ED than in the OR. The cost of a procedure performed in either setting is similarly dependent on a variety of factors, generally including complexity of the patient or procedure, costs of supplies and pharmacologic agents, fixed versus variable overhead costs, and the professional fees of providers and ancillary personnel.9,10
While the patient is not charged per hour in the ED, it is estimated that ORs in the United States cost, on average, $62 per minute, ranging from as low as $22 to as high as $133 per minute.9 Additionally, the number of personnel involved in running an OR exceeds those for a similar procedure performed in the ED, considering (at a minimum) the orthopedic surgeon, anesthesiologist, scrub and radiology technicians, and nursing personnel required before, during, and after an operation.
While analgesia and procedural sedation can be performed similarly in either setting, it is our experience that patients are managed much more often in the ED with local anesthesia under direct care of only the orthopedic provider, whereas intravenous sedation and general anesthesia are far more commonly implemented in the OR. There are exceptions for pediatric patients or those who are unable to tolerate the procedure under only local anesthesia. Local anesthesia or even intravenous conscious sedation entails less risk as well as lower associated drug costs.11
The difference in risk is especially true for sicker patients undergoing minimally invasive procedures.11 Although administration of adequate procedural analgesia grows increasingly difficult the more proximal the injury, the hand and the fingers are easily and reliably anesthetized with well-placed wrist or digital blocks, with infrequent complications.12 Application of a lidocaine/bupivacaine mixture provides up to 6 to 8 hours of analgesia. A small tourniquet alternative, such as the finger of a sterile glove or phlebotomy tourniquet, applied to the base of the finger or the wrist additionally provides a relatively bloodless field and effectively acts as a Bier block.
Percutaneous pins are much more forgiving than rigid internal fixation. If the initial placement of a pin is unsatisfactory, the pin can be reinserted at little cost.12 Conversely, it may not be possible to reposition a misplaced screw or screw with inadequate purchase and still maintain adequate fixation. While percutaneous pin fixation is not as rigid as screw fixation, the degree of stability provided is adequate for the small forces affecting the hand in most cases. Accordingly, there is a very low incidence of fibrous union or nonunion.13,14 With an increasing appreciation of soft-tissue handling over the past few decades, another significant advantage of K-wire fixation is the obviation of soft-tissue dissection, preserving the biology to maximize healing and minimize adverse sequelae.12 Percutaneous fixation has been shown to achieve functional outcomes comparable to open reduction with internal fixation of operative phalangeal and metacarpal fractures, without soft-tissue disruption, scarring, or implant irritation, and with minimal risk of infection.3,13,15,16 Ultimate range of motion after percutaneous fixation is comparable, if not superior, to that of internal fixation, despite the initial advantage of rigid internal fixation secondary to decreased scarring and lack of indwelling hardware.16,17
While the risk of infection, perhaps the primary concern with percutaneous fixation, has been cited as high as 7%, osteomyelitis is exceedingly rare (<0.5%).3,13,14 Furthermore, pins are often left in place for 3 to 6 weeks, and infection has been found to occur most often at a mean of 10 weeks.7,13 Infection can also be mitigated by intelligent pin placement, relief of residual tension, and splint immobilization.4,15 Pin loosening has similarly been reported in up to 4% of cases in large retrospective studies, occurring at an average of 8 weeks, by which time most pins would have been extricated.13 Other complications related to impaling adjacent neurovascular or tendinous structures have also been cited but are rare.13 A 12-month prospective study of 75 patients specifically evaluating the outcomes after closed reduction with percutaneous fixation of unstable hand fractures in the ED reported only 6 complications at final follow-up.4 Complications were all minor, with no cases of nonunion, delayed union, malunion, pin-tract infection, pyarthrosis, or cellulitis, even in the setting of open fractures. Three patients required revision in the OR for pin migration, initial malreduction, and bone loss in the setting of comminution, respectively. The authors credited their low complication rate to supplementary immobilization.
In conclusion, many unstable simple fractures and dislocations of the hand and wrist can be safely and effectively treated in the ED. While it may seem daunting for a junior resident who is unfamiliar with percutaneous techniques, the authors advocate learning from a more senior mentor. The only additional training required is an understanding of how to apply this skill set in a different setting.
A mentor—now in his 60s—related his experiences as a resident. On call as a second-year resident, he would often be alone at a busy trauma center with no backup. When a case came in, he would quickly read about it in the library, then manage it in the emergency department (ED) if possible, or, if necessary, take the patient to the operating room (OR).
In the era of improved patient care, increased supervision, and decreased autonomy, this is not the reality anymore.1 In theory, more reliable patient care is the result; however, the pendulum may have swung too far.
There are a number of injuries that are amenable to definitive fixation in the ED, but not as limited an array of injuries as we have perhaps grown accustomed to. Hand injuries are among the most common orthopedic injuries seen in the ED, with fractures of the metacarpals and phalanges constituting nearly one-half of all hand injuries.2 The authors recently attended an excellent instructional course lecture on “The Lost and Found Art of Percutaneous Pinning in the Hand and Wrist” at the annual conference of the American Academy of Orthopaedic Surgeons.3 The presenters itemized a comprehensive list of fractures and simple dislocations of the hand, which could be simply, safely, effectively, and definitively managed through percutaneous pinning techniques. A significant number of unstable fractures of the phalanges and metacarpals can be treated in the ED under mini–C-arm fluoroscopy without an admission and trip to the OR.3,4 Most phalangeal and metacarpal fractures are nondisplaced or minimally displaced and stable, and can often be handled with a combination of closed reduction, buddy-taping, and splinting.5 The indications for percutaneous versus internal fixation depend on a number of factors, including bone quality, degree of comminution, quality of the soft-tissue envelope, articular involvement, acuity of presentation, and goals for motion.6,7
Many simple injury patterns involving unstable fractures or dislocations may be definitively managed in the ED with percutaneous pinning (eg, injuries that are unstable with closed reduction alone but that do not necessitate soft-tissue dissection). These include but are not limited to bony mallet injuries, unstable transverse or oblique fractures or fracture-dislocations of the phalanges and metacarpals, carpometacarpal fracture- dislocations, and underlying fractures that need protection of nail-bed repairs, soft-tissue flaps, or extensor tendon injuries (Figures 1, 2).7,8 The techniques for specific fracture types are beyond the scope of this article but are readily available.5,6
There are certain situations that undoubtedly warrant surgery in the OR, such as neurovascular injury necessitating microvascular repair, flexor tendon laceration, severely comminuted or segmental fractures, irreducible dislocations, and fractures with severe soft-tissue injury or contamination not amenable to primary irrigation, débridement, and closure at bedside.4,7,8
You might ask, “Why would one treat an operative injury in the ED and not formally in the OR?,” and we submit that there are a number of reasons.
First, and most important, with increasing health care costs and decreasing reimbursements, physicians are faced with providing safe but economical care. Percutaneous Kirschner wire (K-wire) fixation is dramatically more cost-effective when performed in the ED than in the OR. The cost of a procedure performed in either setting is similarly dependent on a variety of factors, generally including complexity of the patient or procedure, costs of supplies and pharmacologic agents, fixed versus variable overhead costs, and the professional fees of providers and ancillary personnel.9,10
While the patient is not charged per hour in the ED, it is estimated that ORs in the United States cost, on average, $62 per minute, ranging from as low as $22 to as high as $133 per minute.9 Additionally, the number of personnel involved in running an OR exceeds those for a similar procedure performed in the ED, considering (at a minimum) the orthopedic surgeon, anesthesiologist, scrub and radiology technicians, and nursing personnel required before, during, and after an operation.
While analgesia and procedural sedation can be performed similarly in either setting, it is our experience that patients are managed much more often in the ED with local anesthesia under direct care of only the orthopedic provider, whereas intravenous sedation and general anesthesia are far more commonly implemented in the OR. There are exceptions for pediatric patients or those who are unable to tolerate the procedure under only local anesthesia. Local anesthesia or even intravenous conscious sedation entails less risk as well as lower associated drug costs.11
The difference in risk is especially true for sicker patients undergoing minimally invasive procedures.11 Although administration of adequate procedural analgesia grows increasingly difficult the more proximal the injury, the hand and the fingers are easily and reliably anesthetized with well-placed wrist or digital blocks, with infrequent complications.12 Application of a lidocaine/bupivacaine mixture provides up to 6 to 8 hours of analgesia. A small tourniquet alternative, such as the finger of a sterile glove or phlebotomy tourniquet, applied to the base of the finger or the wrist additionally provides a relatively bloodless field and effectively acts as a Bier block.
Percutaneous pins are much more forgiving than rigid internal fixation. If the initial placement of a pin is unsatisfactory, the pin can be reinserted at little cost.12 Conversely, it may not be possible to reposition a misplaced screw or screw with inadequate purchase and still maintain adequate fixation. While percutaneous pin fixation is not as rigid as screw fixation, the degree of stability provided is adequate for the small forces affecting the hand in most cases. Accordingly, there is a very low incidence of fibrous union or nonunion.13,14 With an increasing appreciation of soft-tissue handling over the past few decades, another significant advantage of K-wire fixation is the obviation of soft-tissue dissection, preserving the biology to maximize healing and minimize adverse sequelae.12 Percutaneous fixation has been shown to achieve functional outcomes comparable to open reduction with internal fixation of operative phalangeal and metacarpal fractures, without soft-tissue disruption, scarring, or implant irritation, and with minimal risk of infection.3,13,15,16 Ultimate range of motion after percutaneous fixation is comparable, if not superior, to that of internal fixation, despite the initial advantage of rigid internal fixation secondary to decreased scarring and lack of indwelling hardware.16,17
While the risk of infection, perhaps the primary concern with percutaneous fixation, has been cited as high as 7%, osteomyelitis is exceedingly rare (<0.5%).3,13,14 Furthermore, pins are often left in place for 3 to 6 weeks, and infection has been found to occur most often at a mean of 10 weeks.7,13 Infection can also be mitigated by intelligent pin placement, relief of residual tension, and splint immobilization.4,15 Pin loosening has similarly been reported in up to 4% of cases in large retrospective studies, occurring at an average of 8 weeks, by which time most pins would have been extricated.13 Other complications related to impaling adjacent neurovascular or tendinous structures have also been cited but are rare.13 A 12-month prospective study of 75 patients specifically evaluating the outcomes after closed reduction with percutaneous fixation of unstable hand fractures in the ED reported only 6 complications at final follow-up.4 Complications were all minor, with no cases of nonunion, delayed union, malunion, pin-tract infection, pyarthrosis, or cellulitis, even in the setting of open fractures. Three patients required revision in the OR for pin migration, initial malreduction, and bone loss in the setting of comminution, respectively. The authors credited their low complication rate to supplementary immobilization.
In conclusion, many unstable simple fractures and dislocations of the hand and wrist can be safely and effectively treated in the ED. While it may seem daunting for a junior resident who is unfamiliar with percutaneous techniques, the authors advocate learning from a more senior mentor. The only additional training required is an understanding of how to apply this skill set in a different setting.
1. Levine WN, Spang RC 3rd. ACGME duty hour requirements: perceptions and impact on resident training and patient care. J Am Acad Orthop Surg. 2014;22(9):535-544.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Catalano LW 3rd, Glickel SZ, Strauch RJ, Barron AO. The lost and found art of percutaneous pinning in the hand and wrist. Instructional Course Lectures. Annual Meeting of the American Academy of Orthopaedic Surgeons; March 24, 2015; Las Vegas, NV.
4. Starker I, Eaton RG. Kirschner wire placement in the emergency room. Is there a risk? J Hand Surg Br. 1995;20(4):535-538.
5. Meals C, Meals R. Hand fractures: a review of current treatment strategies. J Hand Surg Am. 2013;38(5):1021-1031.
6. Henry MH. Fractures of the proximal phalanx and metacarpals in the hand: preferred methods of stabilization. J Am Acad Orthop Surg. 2008;16(10):586-595.
7. Klein DM, Belsole RJ. Percutaneous treatment of carpal, metacarpal, and phalangeal injuries. Clin Orthop Relat Res. 2000;(375):116-125.
8. Bernstein ML, Chung KC. Hand fractures and their management: an international view. Injury. 2006;37(11):1043-1048.
9. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
10. Williams RM. The costs of visits to emergency departments. N Engl J Med. 1996;334(10):642-646.
11. Bodenham AR, Howell SJ. General anesthesia vs local anaesthesia: an ongoing story. Br J Anaesth. 2009;103(6):785-789.
12. Stern PJ. Management of fractures of the hand over the last 25 years. J Hand Surg Am. 2000;25(5):817-823.
13. Botte MJ, Davis JL, Rose BA, et al. Complications of smooth pin fixation of fractures and dislocations in the hand and wrist. Clin Orthop Relat Res. 1992;(276):194-201.
14. Wray RC Jr, Glunk R. Treatment of delayed union, nonunion, and malunion of the phalanges of the hand. Ann Plast Surg. 1989;22(1):14-18.
15. Hsu LP, Schwartz EG, Kalainov DM, Chen F, Makowiec RL. Complications of K-wire fixation in procedures involving the hand and wrist. J Hand Surg Am. 2011;36(4):610-616.
16. Stem PJ, Wieser MJ, Reilly DG. Complications of plate fixation in the hand skeleton. Clin Orthop Relat Res. 1987;(214):59-65.
17. Page SM, Stern PJ. Complications and range of motion following plate fixation of metacarpal and phalangeal fractures. J Hand Surg Am. 1998;23(5):827-832.
1. Levine WN, Spang RC 3rd. ACGME duty hour requirements: perceptions and impact on resident training and patient care. J Am Acad Orthop Surg. 2014;22(9):535-544.
2. Chung KC, Spilson SV. The frequency and epidemiology of hand and forearm fractures in the United States. J Hand Surg Am. 2001;26(5):908-915.
3. Catalano LW 3rd, Glickel SZ, Strauch RJ, Barron AO. The lost and found art of percutaneous pinning in the hand and wrist. Instructional Course Lectures. Annual Meeting of the American Academy of Orthopaedic Surgeons; March 24, 2015; Las Vegas, NV.
4. Starker I, Eaton RG. Kirschner wire placement in the emergency room. Is there a risk? J Hand Surg Br. 1995;20(4):535-538.
5. Meals C, Meals R. Hand fractures: a review of current treatment strategies. J Hand Surg Am. 2013;38(5):1021-1031.
6. Henry MH. Fractures of the proximal phalanx and metacarpals in the hand: preferred methods of stabilization. J Am Acad Orthop Surg. 2008;16(10):586-595.
7. Klein DM, Belsole RJ. Percutaneous treatment of carpal, metacarpal, and phalangeal injuries. Clin Orthop Relat Res. 2000;(375):116-125.
8. Bernstein ML, Chung KC. Hand fractures and their management: an international view. Injury. 2006;37(11):1043-1048.
9. Macario A. What does one minute of operating room time cost? J Clin Anesth. 2010;22(4):233-236.
10. Williams RM. The costs of visits to emergency departments. N Engl J Med. 1996;334(10):642-646.
11. Bodenham AR, Howell SJ. General anesthesia vs local anaesthesia: an ongoing story. Br J Anaesth. 2009;103(6):785-789.
12. Stern PJ. Management of fractures of the hand over the last 25 years. J Hand Surg Am. 2000;25(5):817-823.
13. Botte MJ, Davis JL, Rose BA, et al. Complications of smooth pin fixation of fractures and dislocations in the hand and wrist. Clin Orthop Relat Res. 1992;(276):194-201.
14. Wray RC Jr, Glunk R. Treatment of delayed union, nonunion, and malunion of the phalanges of the hand. Ann Plast Surg. 1989;22(1):14-18.
15. Hsu LP, Schwartz EG, Kalainov DM, Chen F, Makowiec RL. Complications of K-wire fixation in procedures involving the hand and wrist. J Hand Surg Am. 2011;36(4):610-616.
16. Stem PJ, Wieser MJ, Reilly DG. Complications of plate fixation in the hand skeleton. Clin Orthop Relat Res. 1987;(214):59-65.
17. Page SM, Stern PJ. Complications and range of motion following plate fixation of metacarpal and phalangeal fractures. J Hand Surg Am. 1998;23(5):827-832.
Phenotype HNPP (Hereditary Neuropathy With Liability to Pressure Palsies) Induced by Medical Procedures
PMP22 is a tetra-span membrane protein primarily expressed in myelinating Schwann cells. Heterozygous deletion of the PMP22 gene (1 copy) causes HNPP (hereditary neuropathy with liability to pressure palsies).1 Interestingly, a reciprocal genetic disorder with 3 copies of human PMP22 causes the most common inherited neuropathy, Charcot-Marie-Tooth disease type 1A (CMT1A).2,3 As the reciprocal mutations occur at initiation of gestation, it is expected that HNPP and CMT1A have a similar prevalence. However, studies have shown HNPP prevalence of 2 to 5 cases per 100,000, far below the CMT1A prevalence of 1:5000.4 This finding prompted speculation that many patients with HNPP may be undiagnosed because of the subtlety of the phenotypes.5
Patients with HNPP typically present with focal sensory loss and muscle weakness related to mechanical stress–induced failure of action potential propagation.6,7 In this article, we report the case of an asymptomatic woman with the HNPP mutation. Her focal neurologic deficits occurred only after total knee arthroplasty (TKA), which in healthy patients is not expected to induce focal sensory and motor symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
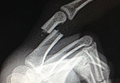
The patient, a healthy 57-year-old woman, had a normal developmental history. For decades, she had practiced ballet without any physical difficulties. She underwent left TKA and woke up with a footdrop on the left side. The left foot was less sensitive to temperature. Ankle strength returned 2 months later. There was no family history of HNPP.
The patient was examined by a local neurologist, who found steppage gait, weak ankle dorsiflexion (4 on Medical Research Council scale), and diminished touch on the lateral aspect of the left leg. Deep tendon reflexes were present in the arms but not the legs.
A nerve conduction study (NCS) performed after the footdrop revealed prolonged distal latency and decreased amplitude in the left peroneal and tibial nerves. The left sural nerve was normal. Needle electromyogram revealed denervation changes in the muscles innervated by the left peroneal nerve (Table). In addition, we also performed an NCS on the arm (Table), which was unaffected by the surgical procedure. This NCS revealed severely prolonged distal latency across the left wrist in the median nerve and focal slowing of conduction velocity of the ulnar nerve across the left elbow. These changes provide evidence of asymptomatic carpal tunnel syndrome and ulnar nerve entrapment, typical electrophysiologic abnormalities of HNPP.8As there was no explanation for the footdrop from the surgery, we had a DNA test performed (Athena Diagnostics). This test identified a heterozygous deletion of chromosome 17p12 containing the PMP22 gene, the HNPP mutation.
Discussion
This case had several important features. First, though the patient developed an electrophysiologic phenotype of HNPP, she was completely asymptomatic clinically and very athletic before her medical procedure. She would not have been diagnosed with HNPP if her clinical deficits had not been induced by TKA. Therefore, the prevalence of HNPP is likely underestimated. Second, for patients with the HNPP mutation, there may be serious neurologic consequences of certain medical procedures. The diagnosis of HNPP should be pursued if there is no explanation from the medical procedure per se. In addition, patients with a family history of HNPP should be carefully evaluated before any procedure that may put them at risk for severe peripheral nerve damage, and they should be counseled regarding the risks. It is important to determine the prevalence of HNPP among patients who develop footdrop after knee arthroplasty, as this information could potentially be used to revise ideas about the etiology of peripheral nerve complications of knee arthroplasty. We now describe possible revisions of these ideas.
Footdrop is a rare complication of TKA. Retrospective studies have found its incidence ranging from 0.3% to 1.3%.9-11 The investigators in those studies postulated 3 main causes for peroneal nerve palsy. First, traction may put pressure on the peroneal nerve during normalization of the mechanical axis of a valgus knee. Our patient did not have a valgus knee. Second, epidural hematoma by anesthetic procedure may compress the spinal roots. Our patient received general anesthesia during the procedure; epidural or spinal anesthesia was not used. Third, postoperative dressing may compress the nerve. Our patient did not develop any signs of constrictive dressing, such as inordinate pain, which can be relieved by removing the dressing, and swelling of the leg distal to the dressing. Therefore, her footdrop likely was not a complication of surgery.
This case demonstrates how a patient with undiagnosed HNPP can manifest the HNPP phenotype only after undergoing a particular surgical procedure. HNPP is unfamiliar to most orthopedic surgeons.
1. Chance PF, Alderson MK, Leppig KA, et al. DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell. 1993;72(1):143-151.
2. Lupski JR, de Oca-Luna RM, Slaugenhaupt S, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66(2):219-232.
3. Raeymaekers P, Timmerman V, Nelis E, et al. Estimation of the size of the chromosome 17p11.2 duplication in Charcot-Marie-Tooth neuropathy type 1a (CMT1a). HMSN Collaborative Research Group. J Med Genet. 1992;29(1):5-11.
4. Meretoja P, Silander K, Kalimo H, Aula P, Meretoja A, Savontaus ML. Epidemiology of hereditary neuropathy with liability to pressure palsies (HNPP) in south western Finland. Neuromuscul Disord. 1997;7(8):529-532.
5. Li J, Parker B, Martyn C, Natarajan C, Guo J. The PMP22 gene and its related diseases. Mol Neurobiol. 2013;47(2):673-698.
6. Bai Y, Zhang X, Katona I, et al. Conduction block in PMP22 deficiency. J Neurosci. 2010;30(2):600-608.
7. Guo J, Wang L, Zhang Y, et al. Abnormal junctions and permeability of myelin in PMP22-deficient nerves. Ann Neurol. 2014;75(2):255-265.
8. Li J, Krajewski K, Shy ME, Lewis RA. Hereditary neuropathy with liability to pressure palsy: the electrophysiology fits the name. Neurology. 2002;58(12):1769-1773.
9. Rose HA, Hood RW, Otis JC, Ranawat CS, Insall JN. Peroneal-nerve palsy following total knee arthroplasty. A review of the Hospital for Special Surgery experience. J Bone Joint Surg Am. 1982;64(3):347-351.
10. Schinsky MF, Macaulay W, Parks ML, Kiernan H, Nercessian OA. Nerve injury after primary total knee arthroplasty. J Arthroplasty. 2001;16(8):1048-1054.
11. Nercessian OA, Ugwonali OF, Park S. Peroneal nerve palsy after total knee arthroplasty. J Arthroplasty. 2005;20(8):1068-1073.
PMP22 is a tetra-span membrane protein primarily expressed in myelinating Schwann cells. Heterozygous deletion of the PMP22 gene (1 copy) causes HNPP (hereditary neuropathy with liability to pressure palsies).1 Interestingly, a reciprocal genetic disorder with 3 copies of human PMP22 causes the most common inherited neuropathy, Charcot-Marie-Tooth disease type 1A (CMT1A).2,3 As the reciprocal mutations occur at initiation of gestation, it is expected that HNPP and CMT1A have a similar prevalence. However, studies have shown HNPP prevalence of 2 to 5 cases per 100,000, far below the CMT1A prevalence of 1:5000.4 This finding prompted speculation that many patients with HNPP may be undiagnosed because of the subtlety of the phenotypes.5
Patients with HNPP typically present with focal sensory loss and muscle weakness related to mechanical stress–induced failure of action potential propagation.6,7 In this article, we report the case of an asymptomatic woman with the HNPP mutation. Her focal neurologic deficits occurred only after total knee arthroplasty (TKA), which in healthy patients is not expected to induce focal sensory and motor symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient, a healthy 57-year-old woman, had a normal developmental history. For decades, she had practiced ballet without any physical difficulties. She underwent left TKA and woke up with a footdrop on the left side. The left foot was less sensitive to temperature. Ankle strength returned 2 months later. There was no family history of HNPP.
The patient was examined by a local neurologist, who found steppage gait, weak ankle dorsiflexion (4 on Medical Research Council scale), and diminished touch on the lateral aspect of the left leg. Deep tendon reflexes were present in the arms but not the legs.
A nerve conduction study (NCS) performed after the footdrop revealed prolonged distal latency and decreased amplitude in the left peroneal and tibial nerves. The left sural nerve was normal. Needle electromyogram revealed denervation changes in the muscles innervated by the left peroneal nerve (Table). In addition, we also performed an NCS on the arm (Table), which was unaffected by the surgical procedure. This NCS revealed severely prolonged distal latency across the left wrist in the median nerve and focal slowing of conduction velocity of the ulnar nerve across the left elbow. These changes provide evidence of asymptomatic carpal tunnel syndrome and ulnar nerve entrapment, typical electrophysiologic abnormalities of HNPP.8As there was no explanation for the footdrop from the surgery, we had a DNA test performed (Athena Diagnostics). This test identified a heterozygous deletion of chromosome 17p12 containing the PMP22 gene, the HNPP mutation.
Discussion
This case had several important features. First, though the patient developed an electrophysiologic phenotype of HNPP, she was completely asymptomatic clinically and very athletic before her medical procedure. She would not have been diagnosed with HNPP if her clinical deficits had not been induced by TKA. Therefore, the prevalence of HNPP is likely underestimated. Second, for patients with the HNPP mutation, there may be serious neurologic consequences of certain medical procedures. The diagnosis of HNPP should be pursued if there is no explanation from the medical procedure per se. In addition, patients with a family history of HNPP should be carefully evaluated before any procedure that may put them at risk for severe peripheral nerve damage, and they should be counseled regarding the risks. It is important to determine the prevalence of HNPP among patients who develop footdrop after knee arthroplasty, as this information could potentially be used to revise ideas about the etiology of peripheral nerve complications of knee arthroplasty. We now describe possible revisions of these ideas.
Footdrop is a rare complication of TKA. Retrospective studies have found its incidence ranging from 0.3% to 1.3%.9-11 The investigators in those studies postulated 3 main causes for peroneal nerve palsy. First, traction may put pressure on the peroneal nerve during normalization of the mechanical axis of a valgus knee. Our patient did not have a valgus knee. Second, epidural hematoma by anesthetic procedure may compress the spinal roots. Our patient received general anesthesia during the procedure; epidural or spinal anesthesia was not used. Third, postoperative dressing may compress the nerve. Our patient did not develop any signs of constrictive dressing, such as inordinate pain, which can be relieved by removing the dressing, and swelling of the leg distal to the dressing. Therefore, her footdrop likely was not a complication of surgery.
This case demonstrates how a patient with undiagnosed HNPP can manifest the HNPP phenotype only after undergoing a particular surgical procedure. HNPP is unfamiliar to most orthopedic surgeons.
PMP22 is a tetra-span membrane protein primarily expressed in myelinating Schwann cells. Heterozygous deletion of the PMP22 gene (1 copy) causes HNPP (hereditary neuropathy with liability to pressure palsies).1 Interestingly, a reciprocal genetic disorder with 3 copies of human PMP22 causes the most common inherited neuropathy, Charcot-Marie-Tooth disease type 1A (CMT1A).2,3 As the reciprocal mutations occur at initiation of gestation, it is expected that HNPP and CMT1A have a similar prevalence. However, studies have shown HNPP prevalence of 2 to 5 cases per 100,000, far below the CMT1A prevalence of 1:5000.4 This finding prompted speculation that many patients with HNPP may be undiagnosed because of the subtlety of the phenotypes.5
Patients with HNPP typically present with focal sensory loss and muscle weakness related to mechanical stress–induced failure of action potential propagation.6,7 In this article, we report the case of an asymptomatic woman with the HNPP mutation. Her focal neurologic deficits occurred only after total knee arthroplasty (TKA), which in healthy patients is not expected to induce focal sensory and motor symptoms. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
The patient, a healthy 57-year-old woman, had a normal developmental history. For decades, she had practiced ballet without any physical difficulties. She underwent left TKA and woke up with a footdrop on the left side. The left foot was less sensitive to temperature. Ankle strength returned 2 months later. There was no family history of HNPP.
The patient was examined by a local neurologist, who found steppage gait, weak ankle dorsiflexion (4 on Medical Research Council scale), and diminished touch on the lateral aspect of the left leg. Deep tendon reflexes were present in the arms but not the legs.
A nerve conduction study (NCS) performed after the footdrop revealed prolonged distal latency and decreased amplitude in the left peroneal and tibial nerves. The left sural nerve was normal. Needle electromyogram revealed denervation changes in the muscles innervated by the left peroneal nerve (Table). In addition, we also performed an NCS on the arm (Table), which was unaffected by the surgical procedure. This NCS revealed severely prolonged distal latency across the left wrist in the median nerve and focal slowing of conduction velocity of the ulnar nerve across the left elbow. These changes provide evidence of asymptomatic carpal tunnel syndrome and ulnar nerve entrapment, typical electrophysiologic abnormalities of HNPP.8As there was no explanation for the footdrop from the surgery, we had a DNA test performed (Athena Diagnostics). This test identified a heterozygous deletion of chromosome 17p12 containing the PMP22 gene, the HNPP mutation.
Discussion
This case had several important features. First, though the patient developed an electrophysiologic phenotype of HNPP, she was completely asymptomatic clinically and very athletic before her medical procedure. She would not have been diagnosed with HNPP if her clinical deficits had not been induced by TKA. Therefore, the prevalence of HNPP is likely underestimated. Second, for patients with the HNPP mutation, there may be serious neurologic consequences of certain medical procedures. The diagnosis of HNPP should be pursued if there is no explanation from the medical procedure per se. In addition, patients with a family history of HNPP should be carefully evaluated before any procedure that may put them at risk for severe peripheral nerve damage, and they should be counseled regarding the risks. It is important to determine the prevalence of HNPP among patients who develop footdrop after knee arthroplasty, as this information could potentially be used to revise ideas about the etiology of peripheral nerve complications of knee arthroplasty. We now describe possible revisions of these ideas.
Footdrop is a rare complication of TKA. Retrospective studies have found its incidence ranging from 0.3% to 1.3%.9-11 The investigators in those studies postulated 3 main causes for peroneal nerve palsy. First, traction may put pressure on the peroneal nerve during normalization of the mechanical axis of a valgus knee. Our patient did not have a valgus knee. Second, epidural hematoma by anesthetic procedure may compress the spinal roots. Our patient received general anesthesia during the procedure; epidural or spinal anesthesia was not used. Third, postoperative dressing may compress the nerve. Our patient did not develop any signs of constrictive dressing, such as inordinate pain, which can be relieved by removing the dressing, and swelling of the leg distal to the dressing. Therefore, her footdrop likely was not a complication of surgery.
This case demonstrates how a patient with undiagnosed HNPP can manifest the HNPP phenotype only after undergoing a particular surgical procedure. HNPP is unfamiliar to most orthopedic surgeons.
1. Chance PF, Alderson MK, Leppig KA, et al. DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell. 1993;72(1):143-151.
2. Lupski JR, de Oca-Luna RM, Slaugenhaupt S, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66(2):219-232.
3. Raeymaekers P, Timmerman V, Nelis E, et al. Estimation of the size of the chromosome 17p11.2 duplication in Charcot-Marie-Tooth neuropathy type 1a (CMT1a). HMSN Collaborative Research Group. J Med Genet. 1992;29(1):5-11.
4. Meretoja P, Silander K, Kalimo H, Aula P, Meretoja A, Savontaus ML. Epidemiology of hereditary neuropathy with liability to pressure palsies (HNPP) in south western Finland. Neuromuscul Disord. 1997;7(8):529-532.
5. Li J, Parker B, Martyn C, Natarajan C, Guo J. The PMP22 gene and its related diseases. Mol Neurobiol. 2013;47(2):673-698.
6. Bai Y, Zhang X, Katona I, et al. Conduction block in PMP22 deficiency. J Neurosci. 2010;30(2):600-608.
7. Guo J, Wang L, Zhang Y, et al. Abnormal junctions and permeability of myelin in PMP22-deficient nerves. Ann Neurol. 2014;75(2):255-265.
8. Li J, Krajewski K, Shy ME, Lewis RA. Hereditary neuropathy with liability to pressure palsy: the electrophysiology fits the name. Neurology. 2002;58(12):1769-1773.
9. Rose HA, Hood RW, Otis JC, Ranawat CS, Insall JN. Peroneal-nerve palsy following total knee arthroplasty. A review of the Hospital for Special Surgery experience. J Bone Joint Surg Am. 1982;64(3):347-351.
10. Schinsky MF, Macaulay W, Parks ML, Kiernan H, Nercessian OA. Nerve injury after primary total knee arthroplasty. J Arthroplasty. 2001;16(8):1048-1054.
11. Nercessian OA, Ugwonali OF, Park S. Peroneal nerve palsy after total knee arthroplasty. J Arthroplasty. 2005;20(8):1068-1073.
1. Chance PF, Alderson MK, Leppig KA, et al. DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell. 1993;72(1):143-151.
2. Lupski JR, de Oca-Luna RM, Slaugenhaupt S, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66(2):219-232.
3. Raeymaekers P, Timmerman V, Nelis E, et al. Estimation of the size of the chromosome 17p11.2 duplication in Charcot-Marie-Tooth neuropathy type 1a (CMT1a). HMSN Collaborative Research Group. J Med Genet. 1992;29(1):5-11.
4. Meretoja P, Silander K, Kalimo H, Aula P, Meretoja A, Savontaus ML. Epidemiology of hereditary neuropathy with liability to pressure palsies (HNPP) in south western Finland. Neuromuscul Disord. 1997;7(8):529-532.
5. Li J, Parker B, Martyn C, Natarajan C, Guo J. The PMP22 gene and its related diseases. Mol Neurobiol. 2013;47(2):673-698.
6. Bai Y, Zhang X, Katona I, et al. Conduction block in PMP22 deficiency. J Neurosci. 2010;30(2):600-608.
7. Guo J, Wang L, Zhang Y, et al. Abnormal junctions and permeability of myelin in PMP22-deficient nerves. Ann Neurol. 2014;75(2):255-265.
8. Li J, Krajewski K, Shy ME, Lewis RA. Hereditary neuropathy with liability to pressure palsy: the electrophysiology fits the name. Neurology. 2002;58(12):1769-1773.
9. Rose HA, Hood RW, Otis JC, Ranawat CS, Insall JN. Peroneal-nerve palsy following total knee arthroplasty. A review of the Hospital for Special Surgery experience. J Bone Joint Surg Am. 1982;64(3):347-351.
10. Schinsky MF, Macaulay W, Parks ML, Kiernan H, Nercessian OA. Nerve injury after primary total knee arthroplasty. J Arthroplasty. 2001;16(8):1048-1054.
11. Nercessian OA, Ugwonali OF, Park S. Peroneal nerve palsy after total knee arthroplasty. J Arthroplasty. 2005;20(8):1068-1073.
Can Activity Aid Knees In Staying Lubricated?
SAN JOSE—A research team from the University of Delaware has proposed a mechanism that may explain how motion can cause cartilage to reabsorb fluid that leaks out over time.
About 80% of cartilage tissue is composed of synovial fluid that is essential for weight bearing and lubrication of joints. Synovial fluid is easily squeezed out of porous cartilage, decreasing its thickness and leading to joint degradation and osteoarthritis. Yet despite the constant fluid leakage, or deflation, the symptoms of osteoarthritis typically take decades to manifest. The investigator’s study is the latest to determine why this occurs.
Lead researcher David Burris, PhD, Associate Professor of Mechanical Engineering, and his research team tested their theory that the reabsorption process was driven by hydrodynamic pressurization, which occurs when the relative motion of 2 surfaces causes fluid between them to accelerate in the shape of a triangular wedge.
By modeling joint movement using cartilage samples, the team demonstrated that with increased motion (e.g. typical walking speed) the fluid lost as a result of deflation was counteracted by fluid regained through pressurization.
The conclusion, said Dr. Burris, is that, “It is activity itself that combats the natural deflation process associated with interstitial lubrication.” The team presented their findings at the AVS 62nd International Symposium and Exhibition.
The investigators also used in-situ measurements to demonstrate the same fluid recovery process previously observed in-vivo. The researchers presented evidence that fluid drawn into the contact by sliding is pressurized elastohydrodynamically and subsequently forced into the porous articular surfaces to restore hydration.
The new mechanism, which study authors call tribological rehydration, suggests that motion is the engine by which the joint maintains long-term function and health.
“We observed a dynamic competition between input and output [of synovial fluid],” Dr. Burris said. “We know that cartilage thickness is maintained over decades in the joint and this is the first direct insight into why. It is activity itself that combats the natural deflation process associated with interstitial lubrication.”
SAN JOSE—A research team from the University of Delaware has proposed a mechanism that may explain how motion can cause cartilage to reabsorb fluid that leaks out over time.
About 80% of cartilage tissue is composed of synovial fluid that is essential for weight bearing and lubrication of joints. Synovial fluid is easily squeezed out of porous cartilage, decreasing its thickness and leading to joint degradation and osteoarthritis. Yet despite the constant fluid leakage, or deflation, the symptoms of osteoarthritis typically take decades to manifest. The investigator’s study is the latest to determine why this occurs.
Lead researcher David Burris, PhD, Associate Professor of Mechanical Engineering, and his research team tested their theory that the reabsorption process was driven by hydrodynamic pressurization, which occurs when the relative motion of 2 surfaces causes fluid between them to accelerate in the shape of a triangular wedge.
By modeling joint movement using cartilage samples, the team demonstrated that with increased motion (e.g. typical walking speed) the fluid lost as a result of deflation was counteracted by fluid regained through pressurization.
The conclusion, said Dr. Burris, is that, “It is activity itself that combats the natural deflation process associated with interstitial lubrication.” The team presented their findings at the AVS 62nd International Symposium and Exhibition.
The investigators also used in-situ measurements to demonstrate the same fluid recovery process previously observed in-vivo. The researchers presented evidence that fluid drawn into the contact by sliding is pressurized elastohydrodynamically and subsequently forced into the porous articular surfaces to restore hydration.
The new mechanism, which study authors call tribological rehydration, suggests that motion is the engine by which the joint maintains long-term function and health.
“We observed a dynamic competition between input and output [of synovial fluid],” Dr. Burris said. “We know that cartilage thickness is maintained over decades in the joint and this is the first direct insight into why. It is activity itself that combats the natural deflation process associated with interstitial lubrication.”
SAN JOSE—A research team from the University of Delaware has proposed a mechanism that may explain how motion can cause cartilage to reabsorb fluid that leaks out over time.
About 80% of cartilage tissue is composed of synovial fluid that is essential for weight bearing and lubrication of joints. Synovial fluid is easily squeezed out of porous cartilage, decreasing its thickness and leading to joint degradation and osteoarthritis. Yet despite the constant fluid leakage, or deflation, the symptoms of osteoarthritis typically take decades to manifest. The investigator’s study is the latest to determine why this occurs.
Lead researcher David Burris, PhD, Associate Professor of Mechanical Engineering, and his research team tested their theory that the reabsorption process was driven by hydrodynamic pressurization, which occurs when the relative motion of 2 surfaces causes fluid between them to accelerate in the shape of a triangular wedge.
By modeling joint movement using cartilage samples, the team demonstrated that with increased motion (e.g. typical walking speed) the fluid lost as a result of deflation was counteracted by fluid regained through pressurization.
The conclusion, said Dr. Burris, is that, “It is activity itself that combats the natural deflation process associated with interstitial lubrication.” The team presented their findings at the AVS 62nd International Symposium and Exhibition.
The investigators also used in-situ measurements to demonstrate the same fluid recovery process previously observed in-vivo. The researchers presented evidence that fluid drawn into the contact by sliding is pressurized elastohydrodynamically and subsequently forced into the porous articular surfaces to restore hydration.
The new mechanism, which study authors call tribological rehydration, suggests that motion is the engine by which the joint maintains long-term function and health.
“We observed a dynamic competition between input and output [of synovial fluid],” Dr. Burris said. “We know that cartilage thickness is maintained over decades in the joint and this is the first direct insight into why. It is activity itself that combats the natural deflation process associated with interstitial lubrication.”
Does Medication Use Decrease After Hip-Replacement Surgery?
Results of a new study provide information on the trajectories of prescription drug use before and after total hip arthroplasty (THA). The study was published online ahead of print November 14 in Pain.
Researchers merged Norwegian national joint replacement and prescription databases to analyze the medication use of nearly 40,000 patients undergoing THA from 2005 to 2011. The patients’ average age was 68.5 and about three-fourths of patients underwent THA because of primary osteoarthritis.
The investigators analyzed trends in prescription drug use over 2 years: 4 quarters before and 4 quarters after hip-replacement surgery. The study focused on analgesics and hypnotics as well as drugs to treat anxiety and depression.
Overall, about half of patients filled a prescription for an analgesic in the year before surgery. Analgesic use included nonsteroidal anti-inflammatory drugs (NSAIDs) in 38% of patients, opioids in 16%, and other non-opioid analgesics in 12%.
Use of pain medications continued to increase during the last quarter before THA and then increased dramatically in the first quarter after surgery. The sharpest increases were for opioids, which increased to 28% in the last quarter before THA, then to 65% in the first quarter afterward; non-opioid analgesics increased to 21% and then to 60.5%.
The percentage of patients who filled prescriptions for hypnotics also increased from the quarter before to the quarter after surgery—from 14% to 25%. Analysis of the dosage showed a similar pattern.
With continued follow-up after THA, medication use decreased. By 1 year after THA, opioid use had decreased to 14%, NSAID use had decreased to 18%, and non-opioid analgesic use had decreased to 13%. Use of hypnotic drugs also decreased, along with medications to treat anxiety. There was little or no change in the use of antidepressants.
“Patients with chronic pain are frequent users of analgesic and psychotropic drugs and thereby risk adverse drug events,” said Tone Blågestad, a PhD candidate from the Department of Clinical Psychology at the University of Bergen in Norway, and coauthors. They cited special concern about the potential for serious adverse effects of opioids, including drug overdose.
The results suggest that use of pain medications increases in the year before THA, with a further increase immediately afterward, followed by a long-term decrease. That pattern is consistent with previous studies on pain scores in the period before and after THA.
Hypnotic drug use shows a similar trend, suggesting that sleep problems get worse, then improve with long-term pain relief after THA. The lack of change in antidepressant use suggests that depression in patients undergoing hip replacement isn’t necessarily related to hip pain.
“Overall, the present results extend the positive effects of THA to include reduced reliance on medication to alleviate symptoms,” said Ms. Blågestad and colleagues. The finding that hypnotics follow the same prescription trajectory as analgesics highlights the link between pain and sleep. The researchers add, “Our results warrant attention to the increased risk of adverse medication effects occurring with the increased use of both opioids and hypnotics in the recovery phase.”
Suggested Reading
Blågestad T, Nordhus IH, Grønli J, et al. Prescription trajectories and effect of total hip arthroplasty on the use of analgesics, hypnotics, antidepressants and anxiolytics: results from a population of total hip arthroplasty patients. Pain. 2015 Nov 14. [Epub ahead of print].
Results of a new study provide information on the trajectories of prescription drug use before and after total hip arthroplasty (THA). The study was published online ahead of print November 14 in Pain.
Researchers merged Norwegian national joint replacement and prescription databases to analyze the medication use of nearly 40,000 patients undergoing THA from 2005 to 2011. The patients’ average age was 68.5 and about three-fourths of patients underwent THA because of primary osteoarthritis.
The investigators analyzed trends in prescription drug use over 2 years: 4 quarters before and 4 quarters after hip-replacement surgery. The study focused on analgesics and hypnotics as well as drugs to treat anxiety and depression.
Overall, about half of patients filled a prescription for an analgesic in the year before surgery. Analgesic use included nonsteroidal anti-inflammatory drugs (NSAIDs) in 38% of patients, opioids in 16%, and other non-opioid analgesics in 12%.
Use of pain medications continued to increase during the last quarter before THA and then increased dramatically in the first quarter after surgery. The sharpest increases were for opioids, which increased to 28% in the last quarter before THA, then to 65% in the first quarter afterward; non-opioid analgesics increased to 21% and then to 60.5%.
The percentage of patients who filled prescriptions for hypnotics also increased from the quarter before to the quarter after surgery—from 14% to 25%. Analysis of the dosage showed a similar pattern.
With continued follow-up after THA, medication use decreased. By 1 year after THA, opioid use had decreased to 14%, NSAID use had decreased to 18%, and non-opioid analgesic use had decreased to 13%. Use of hypnotic drugs also decreased, along with medications to treat anxiety. There was little or no change in the use of antidepressants.
“Patients with chronic pain are frequent users of analgesic and psychotropic drugs and thereby risk adverse drug events,” said Tone Blågestad, a PhD candidate from the Department of Clinical Psychology at the University of Bergen in Norway, and coauthors. They cited special concern about the potential for serious adverse effects of opioids, including drug overdose.
The results suggest that use of pain medications increases in the year before THA, with a further increase immediately afterward, followed by a long-term decrease. That pattern is consistent with previous studies on pain scores in the period before and after THA.
Hypnotic drug use shows a similar trend, suggesting that sleep problems get worse, then improve with long-term pain relief after THA. The lack of change in antidepressant use suggests that depression in patients undergoing hip replacement isn’t necessarily related to hip pain.
“Overall, the present results extend the positive effects of THA to include reduced reliance on medication to alleviate symptoms,” said Ms. Blågestad and colleagues. The finding that hypnotics follow the same prescription trajectory as analgesics highlights the link between pain and sleep. The researchers add, “Our results warrant attention to the increased risk of adverse medication effects occurring with the increased use of both opioids and hypnotics in the recovery phase.”
Results of a new study provide information on the trajectories of prescription drug use before and after total hip arthroplasty (THA). The study was published online ahead of print November 14 in Pain.
Researchers merged Norwegian national joint replacement and prescription databases to analyze the medication use of nearly 40,000 patients undergoing THA from 2005 to 2011. The patients’ average age was 68.5 and about three-fourths of patients underwent THA because of primary osteoarthritis.
The investigators analyzed trends in prescription drug use over 2 years: 4 quarters before and 4 quarters after hip-replacement surgery. The study focused on analgesics and hypnotics as well as drugs to treat anxiety and depression.
Overall, about half of patients filled a prescription for an analgesic in the year before surgery. Analgesic use included nonsteroidal anti-inflammatory drugs (NSAIDs) in 38% of patients, opioids in 16%, and other non-opioid analgesics in 12%.
Use of pain medications continued to increase during the last quarter before THA and then increased dramatically in the first quarter after surgery. The sharpest increases were for opioids, which increased to 28% in the last quarter before THA, then to 65% in the first quarter afterward; non-opioid analgesics increased to 21% and then to 60.5%.
The percentage of patients who filled prescriptions for hypnotics also increased from the quarter before to the quarter after surgery—from 14% to 25%. Analysis of the dosage showed a similar pattern.
With continued follow-up after THA, medication use decreased. By 1 year after THA, opioid use had decreased to 14%, NSAID use had decreased to 18%, and non-opioid analgesic use had decreased to 13%. Use of hypnotic drugs also decreased, along with medications to treat anxiety. There was little or no change in the use of antidepressants.
“Patients with chronic pain are frequent users of analgesic and psychotropic drugs and thereby risk adverse drug events,” said Tone Blågestad, a PhD candidate from the Department of Clinical Psychology at the University of Bergen in Norway, and coauthors. They cited special concern about the potential for serious adverse effects of opioids, including drug overdose.
The results suggest that use of pain medications increases in the year before THA, with a further increase immediately afterward, followed by a long-term decrease. That pattern is consistent with previous studies on pain scores in the period before and after THA.
Hypnotic drug use shows a similar trend, suggesting that sleep problems get worse, then improve with long-term pain relief after THA. The lack of change in antidepressant use suggests that depression in patients undergoing hip replacement isn’t necessarily related to hip pain.
“Overall, the present results extend the positive effects of THA to include reduced reliance on medication to alleviate symptoms,” said Ms. Blågestad and colleagues. The finding that hypnotics follow the same prescription trajectory as analgesics highlights the link between pain and sleep. The researchers add, “Our results warrant attention to the increased risk of adverse medication effects occurring with the increased use of both opioids and hypnotics in the recovery phase.”
Suggested Reading
Blågestad T, Nordhus IH, Grønli J, et al. Prescription trajectories and effect of total hip arthroplasty on the use of analgesics, hypnotics, antidepressants and anxiolytics: results from a population of total hip arthroplasty patients. Pain. 2015 Nov 14. [Epub ahead of print].
Suggested Reading
Blågestad T, Nordhus IH, Grønli J, et al. Prescription trajectories and effect of total hip arthroplasty on the use of analgesics, hypnotics, antidepressants and anxiolytics: results from a population of total hip arthroplasty patients. Pain. 2015 Nov 14. [Epub ahead of print].
Combined Tibial Tubercle Avulsion Fracture and Patellar Avulsion Fracture: An Unusual Variant in an Adolescent Patient
Tibial tubercle fractures are rare injuries accounting for less than 1% of all pediatric physeal injuries.1 The original classification scheme for such fractures was proposed by Watson-Jones.2 Initially modified by Ogden and colleagues,3 the classification system has had numerous additions and modifications as new patterns of injury have been identified.4-6 Patellar fractures are also rare in children, making up 1% of all pediatric fractures, with less than 2% of these occurring in skeletally immature children.7
We present a case of an unreported combined tibial tubercle avulsion fracture and patellar avulsion fracture in an adolescent boy. The patient and his guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy presented to the emergency department with acute onset of right-knee pain and inability to ambulate after falling off a skateboard on the day of the injury. The patient was otherwise healthy and had no noteworthy medical or surgical history, including no prior fractures. On physical examination, he was noted to have a large right-knee effusion presumed to be hemarthrosis, and inability to perform a straight-leg raise against gravity. There were no neurologic deficits and his leg compartments were soft. Plain radiographs showed patella alta and numerous bony fragments believed to represent a complex tibial tubercle fracture. One bony fragment was identified closer to the patella, suggesting a possible concurrent patellar fracture (Figures 1A, 1B). A computed tomography (CT) scan further characterized both the tibial tubercle avulsion fracture and the lateral inferior pole patellar avulsion fracture (Figures 2A, 2B). The patient’s knee was immobilized, and he was admitted for soft-tissue rest and overnight observation to ensure that compartment syndrome did not develop.
Five days after injury, open reduction and internal fixation were performed. After limb exsanguination and tourniquet insufflation, the fracture was visualized through a direct midline approach. The patient was found to have a Z-type injury pattern to the extensor mechanism: an inferior lateral patellar avulsion fracture, longitudinal splits of the patellar tendon, and 2 large, mainly cartilaginous tibial tubercle fracture fragments, 1 of which extended into the proximal tibial epiphysis (Ogden type III) (Figures 3A-3C). Under direct visualization, the tibial tubercle fragments were reduced and stabilized with 3 cannulated 3.5-mm titanium, partially threaded screws with washers. Smaller screws were used to prevent fragmentation of these mostly cartilaginous fragments. Anatomic reduction was ensured along the articular surface, visualized through an arthrotomy, as well as on the distal cortex (Figures 4A, 4B). The patellar avulsion fracture included a very small section of articular surface and the decision was made to preserve the fragment. Because the patellar fragment was too small for screw fixation, the fracture was secured with suture fixation through bone tunnels over a patellar bony bridge using size 2 Phantom Fiber suture (Tornier) (Figure 5). Vicryl was used to repair the longitudinal patellar tendon split as well as the capsular and paratenon traumatic tears. Layered closure was completed and intraoperative radiographs were obtained (Figures 6A, 6B) prior to placement of a cylinder cast in full extension. Postoperatively, the patient remained overnight for observation and physical therapy evaluation. He was encouraged to bear weight in his cylinder cast as tolerated with crutches to assist with ambulation.
Postoperatively, the patient was maintained in full extension in the cylinder cast for 4 weeks. After cast removal, the patient was placed in a range-of-motion brace locked in extension for ambulation. He started physical therapy and was allowed to perform prone active-knee flexion limited to 90º, with passive extension, for an additional 4 weeks. At 8 weeks, the patient was allowed full-knee motion both active and passive, and the brace was discontinued. At his 18-week follow-up appointment, the patient reported successful return to all his normal activities, including skateboarding, with no apparent limitation in motion or weight-bearing. Examination at that time demonstrated knee range of motion from 5º in hyperextension to 135º in flexion, with his left knee having 5º in hyperextension and 145º in flexion. The patient appeared to have no gait abnormalities, and radiographs showed healed fractures. Because of a concern that continued compression across his tibial physis could lead to greater risk of growth arrest, the decision was made to remove the implants when radiographs showed healing. The patient returned to surgery at 20 weeks for implant removal. At 6 weeks after implant removal, the patient had returned to full activity with no residual pain and full-knee flexion equal to the uninvolved left knee. He was able to perform a stable single-leg squat on his affected leg, and his single-leg hop for distance was the same as his uninvolved leg. He was allowed to return to full sports activity. The patient will be followed with serial radiographs at 4 months, 8 months, and 12 months to look for premature physeal arrest. If an arrest occurs, treatment will be dictated by the extent of the arrest and the potential to cause either limb-length difference or angular deformity.
Discussion
Tibial tubercle fractures typically result from quadriceps contraction during sporting activities, predominantly in adolescent boys with open physes. Numerous modifications and additions have been made to the original classification of such fractures by Watson-Jones,2 most notably by Ogden and colleagues3 in 1980. These additions have included combined tendon avulsions and tubercle fractures as described by Frankl and coauthors,4 complete proximal tibial physeal separation now classified as type 4 by Ryu and Debenham,5 and a “Y” fracture configuration now termed type 5 by McKoy and Stanitski.6 Pandya and colleagues8 reported on 41 tibial tubercle fractures and described a new classification scheme based on the known anatomical closure pattern of the proximal tibial physis and tibial tubercle apophysis. The authors stressed the role of advanced imaging, such as CT or magnetic resonance imaging, in preoperative management of these complex high-energy fractures in adolescents, and the need for intraoperative arthroscopy or arthrotomy to ensure anatomical reduction of the articular involvement.
Tibial tubercle fractures and extensor mechanism injuries that do not fit these classification patterns have also been described. In 1979, Houghton and Ackroyd9 reported 3 cases of acute loss of extensor mechanism secondary to a traumatic patellar sleeve avulsion. In 1995, Berg10 described an ipsilateral inferior pole osteochondral patellar avulsion fracture with patellar tendon avulsion without fracture at the tubercle in a 12-year-old boy. Another variant was described in a 2002 case series of 3 adolescent boys who underwent operative fixation for tibial metaphyseal partial-sleeve avulsion injuries.11
Conclusion
We report a case of combined ipsilateral inferior lateral patellar avulsion fracture and an intra-articular tibial tubercle avulsion fracture with intervening longitudinal patellar tendon split. Preoperative standard radiographs were confusing, given the bony fragment high up by the patella, but use of advanced imaging, in this case CT, allowed us to fully characterize the origin of fracture fragments and realize we were dealing with a unique fracture pattern previously unreported in a pediatric patient. The CT findings allowed us to be better prepared preoperatively by having options for fixation of the patellar fracture, and the extent of articular involvement led us to decide that intra-articular evaluation would be required. Through the use of an open arthrotomy, anatomical articular reduction could be visualized and stabilized with screw fixation of the large, mostly cartilaginous tubercle fracture. Following the principles described by Pandya and colleagues,8 anatomical reduction was achieved, and, 6 months after the original surgery, the patient had return of full motion, clinical and radiographic union, and no clinical pain or limp, with no retained metallic implants across the tibial apophysis. Longer-term follow-up as planned will demonstrate any growth abnormality that would require further surgical intervention.
1. Mosier SM, Stanitski CL. Acute tibial tubercle avulsion fractures. J Pediatr Orthop. 2004;24(2):181-184.
2. Watson-Jones R. Fractures and Joint Injuries. Baltimore, MD: Lippincott Williams & Wilkins; 1955.
3. Ogden JA, Tross RB, Murphy MJ. Fractures of the tibial tuberosity in adolescents. J Bone Joint Surg Am. 1980;62(2):205-215.
4. Frankl U, Wasilewski SA, Healy WL. Avulsion fracture of the tibial tubercle with avulsion of the patellar ligament. Report of two cases. J Bone Joint Surg Am. 1990;72(9):1411-1413.
5. Ryu RK, Debenham JO. An unusual avulsion fracture of the proximal tibial epiphysis. Case report and proposed addition to the Watson-Jones classification. Clin Orthop Relat Res. 1985;(194):181-184.
6. McKoy BE, Stanitski CL. Acute tibial tubercle avulsion fractures. Orthop Clin North Am. 2003;34(3):397-403.
7. Hunt DM, Somashekar N. A review of sleeve fractures of the patella in children. Knee. 2005;12:3-7.
8. Pandya NK, Edmonds EW, Roocroft JH, Mubarak SJ. Tibial tubercle fractures: complications, classification, and the need for intra-articular assessment. J Pediatr Orthop. 2012;32(8):749-759.
9. Houghton GR, Ackroyd CE. Sleeve fractures of the patella in children: a report of three cases. J Bone Joint Surg Br. 1979;61(2):165-168.
10. Berg EE. Bipolar infrapatellar tendon rupture. J Pediatr Orthop. 1995;15(3):302-303.
11. Davidson D, Letts M. Partial sleeve fractures of the tibia in children: an unusual fracture pattern. J Pediatr Orthop. 2002;22(1):36-40.
Tibial tubercle fractures are rare injuries accounting for less than 1% of all pediatric physeal injuries.1 The original classification scheme for such fractures was proposed by Watson-Jones.2 Initially modified by Ogden and colleagues,3 the classification system has had numerous additions and modifications as new patterns of injury have been identified.4-6 Patellar fractures are also rare in children, making up 1% of all pediatric fractures, with less than 2% of these occurring in skeletally immature children.7
We present a case of an unreported combined tibial tubercle avulsion fracture and patellar avulsion fracture in an adolescent boy. The patient and his guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy presented to the emergency department with acute onset of right-knee pain and inability to ambulate after falling off a skateboard on the day of the injury. The patient was otherwise healthy and had no noteworthy medical or surgical history, including no prior fractures. On physical examination, he was noted to have a large right-knee effusion presumed to be hemarthrosis, and inability to perform a straight-leg raise against gravity. There were no neurologic deficits and his leg compartments were soft. Plain radiographs showed patella alta and numerous bony fragments believed to represent a complex tibial tubercle fracture. One bony fragment was identified closer to the patella, suggesting a possible concurrent patellar fracture (Figures 1A, 1B). A computed tomography (CT) scan further characterized both the tibial tubercle avulsion fracture and the lateral inferior pole patellar avulsion fracture (Figures 2A, 2B). The patient’s knee was immobilized, and he was admitted for soft-tissue rest and overnight observation to ensure that compartment syndrome did not develop.
Five days after injury, open reduction and internal fixation were performed. After limb exsanguination and tourniquet insufflation, the fracture was visualized through a direct midline approach. The patient was found to have a Z-type injury pattern to the extensor mechanism: an inferior lateral patellar avulsion fracture, longitudinal splits of the patellar tendon, and 2 large, mainly cartilaginous tibial tubercle fracture fragments, 1 of which extended into the proximal tibial epiphysis (Ogden type III) (Figures 3A-3C). Under direct visualization, the tibial tubercle fragments were reduced and stabilized with 3 cannulated 3.5-mm titanium, partially threaded screws with washers. Smaller screws were used to prevent fragmentation of these mostly cartilaginous fragments. Anatomic reduction was ensured along the articular surface, visualized through an arthrotomy, as well as on the distal cortex (Figures 4A, 4B). The patellar avulsion fracture included a very small section of articular surface and the decision was made to preserve the fragment. Because the patellar fragment was too small for screw fixation, the fracture was secured with suture fixation through bone tunnels over a patellar bony bridge using size 2 Phantom Fiber suture (Tornier) (Figure 5). Vicryl was used to repair the longitudinal patellar tendon split as well as the capsular and paratenon traumatic tears. Layered closure was completed and intraoperative radiographs were obtained (Figures 6A, 6B) prior to placement of a cylinder cast in full extension. Postoperatively, the patient remained overnight for observation and physical therapy evaluation. He was encouraged to bear weight in his cylinder cast as tolerated with crutches to assist with ambulation.
Postoperatively, the patient was maintained in full extension in the cylinder cast for 4 weeks. After cast removal, the patient was placed in a range-of-motion brace locked in extension for ambulation. He started physical therapy and was allowed to perform prone active-knee flexion limited to 90º, with passive extension, for an additional 4 weeks. At 8 weeks, the patient was allowed full-knee motion both active and passive, and the brace was discontinued. At his 18-week follow-up appointment, the patient reported successful return to all his normal activities, including skateboarding, with no apparent limitation in motion or weight-bearing. Examination at that time demonstrated knee range of motion from 5º in hyperextension to 135º in flexion, with his left knee having 5º in hyperextension and 145º in flexion. The patient appeared to have no gait abnormalities, and radiographs showed healed fractures. Because of a concern that continued compression across his tibial physis could lead to greater risk of growth arrest, the decision was made to remove the implants when radiographs showed healing. The patient returned to surgery at 20 weeks for implant removal. At 6 weeks after implant removal, the patient had returned to full activity with no residual pain and full-knee flexion equal to the uninvolved left knee. He was able to perform a stable single-leg squat on his affected leg, and his single-leg hop for distance was the same as his uninvolved leg. He was allowed to return to full sports activity. The patient will be followed with serial radiographs at 4 months, 8 months, and 12 months to look for premature physeal arrest. If an arrest occurs, treatment will be dictated by the extent of the arrest and the potential to cause either limb-length difference or angular deformity.
Discussion
Tibial tubercle fractures typically result from quadriceps contraction during sporting activities, predominantly in adolescent boys with open physes. Numerous modifications and additions have been made to the original classification of such fractures by Watson-Jones,2 most notably by Ogden and colleagues3 in 1980. These additions have included combined tendon avulsions and tubercle fractures as described by Frankl and coauthors,4 complete proximal tibial physeal separation now classified as type 4 by Ryu and Debenham,5 and a “Y” fracture configuration now termed type 5 by McKoy and Stanitski.6 Pandya and colleagues8 reported on 41 tibial tubercle fractures and described a new classification scheme based on the known anatomical closure pattern of the proximal tibial physis and tibial tubercle apophysis. The authors stressed the role of advanced imaging, such as CT or magnetic resonance imaging, in preoperative management of these complex high-energy fractures in adolescents, and the need for intraoperative arthroscopy or arthrotomy to ensure anatomical reduction of the articular involvement.
Tibial tubercle fractures and extensor mechanism injuries that do not fit these classification patterns have also been described. In 1979, Houghton and Ackroyd9 reported 3 cases of acute loss of extensor mechanism secondary to a traumatic patellar sleeve avulsion. In 1995, Berg10 described an ipsilateral inferior pole osteochondral patellar avulsion fracture with patellar tendon avulsion without fracture at the tubercle in a 12-year-old boy. Another variant was described in a 2002 case series of 3 adolescent boys who underwent operative fixation for tibial metaphyseal partial-sleeve avulsion injuries.11
Conclusion
We report a case of combined ipsilateral inferior lateral patellar avulsion fracture and an intra-articular tibial tubercle avulsion fracture with intervening longitudinal patellar tendon split. Preoperative standard radiographs were confusing, given the bony fragment high up by the patella, but use of advanced imaging, in this case CT, allowed us to fully characterize the origin of fracture fragments and realize we were dealing with a unique fracture pattern previously unreported in a pediatric patient. The CT findings allowed us to be better prepared preoperatively by having options for fixation of the patellar fracture, and the extent of articular involvement led us to decide that intra-articular evaluation would be required. Through the use of an open arthrotomy, anatomical articular reduction could be visualized and stabilized with screw fixation of the large, mostly cartilaginous tubercle fracture. Following the principles described by Pandya and colleagues,8 anatomical reduction was achieved, and, 6 months after the original surgery, the patient had return of full motion, clinical and radiographic union, and no clinical pain or limp, with no retained metallic implants across the tibial apophysis. Longer-term follow-up as planned will demonstrate any growth abnormality that would require further surgical intervention.
Tibial tubercle fractures are rare injuries accounting for less than 1% of all pediatric physeal injuries.1 The original classification scheme for such fractures was proposed by Watson-Jones.2 Initially modified by Ogden and colleagues,3 the classification system has had numerous additions and modifications as new patterns of injury have been identified.4-6 Patellar fractures are also rare in children, making up 1% of all pediatric fractures, with less than 2% of these occurring in skeletally immature children.7
We present a case of an unreported combined tibial tubercle avulsion fracture and patellar avulsion fracture in an adolescent boy. The patient and his guardian provided written informed consent for print and electronic publication of this case report.
Case Report
A 12-year-old boy presented to the emergency department with acute onset of right-knee pain and inability to ambulate after falling off a skateboard on the day of the injury. The patient was otherwise healthy and had no noteworthy medical or surgical history, including no prior fractures. On physical examination, he was noted to have a large right-knee effusion presumed to be hemarthrosis, and inability to perform a straight-leg raise against gravity. There were no neurologic deficits and his leg compartments were soft. Plain radiographs showed patella alta and numerous bony fragments believed to represent a complex tibial tubercle fracture. One bony fragment was identified closer to the patella, suggesting a possible concurrent patellar fracture (Figures 1A, 1B). A computed tomography (CT) scan further characterized both the tibial tubercle avulsion fracture and the lateral inferior pole patellar avulsion fracture (Figures 2A, 2B). The patient’s knee was immobilized, and he was admitted for soft-tissue rest and overnight observation to ensure that compartment syndrome did not develop.
Five days after injury, open reduction and internal fixation were performed. After limb exsanguination and tourniquet insufflation, the fracture was visualized through a direct midline approach. The patient was found to have a Z-type injury pattern to the extensor mechanism: an inferior lateral patellar avulsion fracture, longitudinal splits of the patellar tendon, and 2 large, mainly cartilaginous tibial tubercle fracture fragments, 1 of which extended into the proximal tibial epiphysis (Ogden type III) (Figures 3A-3C). Under direct visualization, the tibial tubercle fragments were reduced and stabilized with 3 cannulated 3.5-mm titanium, partially threaded screws with washers. Smaller screws were used to prevent fragmentation of these mostly cartilaginous fragments. Anatomic reduction was ensured along the articular surface, visualized through an arthrotomy, as well as on the distal cortex (Figures 4A, 4B). The patellar avulsion fracture included a very small section of articular surface and the decision was made to preserve the fragment. Because the patellar fragment was too small for screw fixation, the fracture was secured with suture fixation through bone tunnels over a patellar bony bridge using size 2 Phantom Fiber suture (Tornier) (Figure 5). Vicryl was used to repair the longitudinal patellar tendon split as well as the capsular and paratenon traumatic tears. Layered closure was completed and intraoperative radiographs were obtained (Figures 6A, 6B) prior to placement of a cylinder cast in full extension. Postoperatively, the patient remained overnight for observation and physical therapy evaluation. He was encouraged to bear weight in his cylinder cast as tolerated with crutches to assist with ambulation.
Postoperatively, the patient was maintained in full extension in the cylinder cast for 4 weeks. After cast removal, the patient was placed in a range-of-motion brace locked in extension for ambulation. He started physical therapy and was allowed to perform prone active-knee flexion limited to 90º, with passive extension, for an additional 4 weeks. At 8 weeks, the patient was allowed full-knee motion both active and passive, and the brace was discontinued. At his 18-week follow-up appointment, the patient reported successful return to all his normal activities, including skateboarding, with no apparent limitation in motion or weight-bearing. Examination at that time demonstrated knee range of motion from 5º in hyperextension to 135º in flexion, with his left knee having 5º in hyperextension and 145º in flexion. The patient appeared to have no gait abnormalities, and radiographs showed healed fractures. Because of a concern that continued compression across his tibial physis could lead to greater risk of growth arrest, the decision was made to remove the implants when radiographs showed healing. The patient returned to surgery at 20 weeks for implant removal. At 6 weeks after implant removal, the patient had returned to full activity with no residual pain and full-knee flexion equal to the uninvolved left knee. He was able to perform a stable single-leg squat on his affected leg, and his single-leg hop for distance was the same as his uninvolved leg. He was allowed to return to full sports activity. The patient will be followed with serial radiographs at 4 months, 8 months, and 12 months to look for premature physeal arrest. If an arrest occurs, treatment will be dictated by the extent of the arrest and the potential to cause either limb-length difference or angular deformity.
Discussion
Tibial tubercle fractures typically result from quadriceps contraction during sporting activities, predominantly in adolescent boys with open physes. Numerous modifications and additions have been made to the original classification of such fractures by Watson-Jones,2 most notably by Ogden and colleagues3 in 1980. These additions have included combined tendon avulsions and tubercle fractures as described by Frankl and coauthors,4 complete proximal tibial physeal separation now classified as type 4 by Ryu and Debenham,5 and a “Y” fracture configuration now termed type 5 by McKoy and Stanitski.6 Pandya and colleagues8 reported on 41 tibial tubercle fractures and described a new classification scheme based on the known anatomical closure pattern of the proximal tibial physis and tibial tubercle apophysis. The authors stressed the role of advanced imaging, such as CT or magnetic resonance imaging, in preoperative management of these complex high-energy fractures in adolescents, and the need for intraoperative arthroscopy or arthrotomy to ensure anatomical reduction of the articular involvement.
Tibial tubercle fractures and extensor mechanism injuries that do not fit these classification patterns have also been described. In 1979, Houghton and Ackroyd9 reported 3 cases of acute loss of extensor mechanism secondary to a traumatic patellar sleeve avulsion. In 1995, Berg10 described an ipsilateral inferior pole osteochondral patellar avulsion fracture with patellar tendon avulsion without fracture at the tubercle in a 12-year-old boy. Another variant was described in a 2002 case series of 3 adolescent boys who underwent operative fixation for tibial metaphyseal partial-sleeve avulsion injuries.11
Conclusion
We report a case of combined ipsilateral inferior lateral patellar avulsion fracture and an intra-articular tibial tubercle avulsion fracture with intervening longitudinal patellar tendon split. Preoperative standard radiographs were confusing, given the bony fragment high up by the patella, but use of advanced imaging, in this case CT, allowed us to fully characterize the origin of fracture fragments and realize we were dealing with a unique fracture pattern previously unreported in a pediatric patient. The CT findings allowed us to be better prepared preoperatively by having options for fixation of the patellar fracture, and the extent of articular involvement led us to decide that intra-articular evaluation would be required. Through the use of an open arthrotomy, anatomical articular reduction could be visualized and stabilized with screw fixation of the large, mostly cartilaginous tubercle fracture. Following the principles described by Pandya and colleagues,8 anatomical reduction was achieved, and, 6 months after the original surgery, the patient had return of full motion, clinical and radiographic union, and no clinical pain or limp, with no retained metallic implants across the tibial apophysis. Longer-term follow-up as planned will demonstrate any growth abnormality that would require further surgical intervention.
1. Mosier SM, Stanitski CL. Acute tibial tubercle avulsion fractures. J Pediatr Orthop. 2004;24(2):181-184.
2. Watson-Jones R. Fractures and Joint Injuries. Baltimore, MD: Lippincott Williams & Wilkins; 1955.
3. Ogden JA, Tross RB, Murphy MJ. Fractures of the tibial tuberosity in adolescents. J Bone Joint Surg Am. 1980;62(2):205-215.
4. Frankl U, Wasilewski SA, Healy WL. Avulsion fracture of the tibial tubercle with avulsion of the patellar ligament. Report of two cases. J Bone Joint Surg Am. 1990;72(9):1411-1413.
5. Ryu RK, Debenham JO. An unusual avulsion fracture of the proximal tibial epiphysis. Case report and proposed addition to the Watson-Jones classification. Clin Orthop Relat Res. 1985;(194):181-184.
6. McKoy BE, Stanitski CL. Acute tibial tubercle avulsion fractures. Orthop Clin North Am. 2003;34(3):397-403.
7. Hunt DM, Somashekar N. A review of sleeve fractures of the patella in children. Knee. 2005;12:3-7.
8. Pandya NK, Edmonds EW, Roocroft JH, Mubarak SJ. Tibial tubercle fractures: complications, classification, and the need for intra-articular assessment. J Pediatr Orthop. 2012;32(8):749-759.
9. Houghton GR, Ackroyd CE. Sleeve fractures of the patella in children: a report of three cases. J Bone Joint Surg Br. 1979;61(2):165-168.
10. Berg EE. Bipolar infrapatellar tendon rupture. J Pediatr Orthop. 1995;15(3):302-303.
11. Davidson D, Letts M. Partial sleeve fractures of the tibia in children: an unusual fracture pattern. J Pediatr Orthop. 2002;22(1):36-40.
1. Mosier SM, Stanitski CL. Acute tibial tubercle avulsion fractures. J Pediatr Orthop. 2004;24(2):181-184.
2. Watson-Jones R. Fractures and Joint Injuries. Baltimore, MD: Lippincott Williams & Wilkins; 1955.
3. Ogden JA, Tross RB, Murphy MJ. Fractures of the tibial tuberosity in adolescents. J Bone Joint Surg Am. 1980;62(2):205-215.
4. Frankl U, Wasilewski SA, Healy WL. Avulsion fracture of the tibial tubercle with avulsion of the patellar ligament. Report of two cases. J Bone Joint Surg Am. 1990;72(9):1411-1413.
5. Ryu RK, Debenham JO. An unusual avulsion fracture of the proximal tibial epiphysis. Case report and proposed addition to the Watson-Jones classification. Clin Orthop Relat Res. 1985;(194):181-184.
6. McKoy BE, Stanitski CL. Acute tibial tubercle avulsion fractures. Orthop Clin North Am. 2003;34(3):397-403.
7. Hunt DM, Somashekar N. A review of sleeve fractures of the patella in children. Knee. 2005;12:3-7.
8. Pandya NK, Edmonds EW, Roocroft JH, Mubarak SJ. Tibial tubercle fractures: complications, classification, and the need for intra-articular assessment. J Pediatr Orthop. 2012;32(8):749-759.
9. Houghton GR, Ackroyd CE. Sleeve fractures of the patella in children: a report of three cases. J Bone Joint Surg Br. 1979;61(2):165-168.
10. Berg EE. Bipolar infrapatellar tendon rupture. J Pediatr Orthop. 1995;15(3):302-303.
11. Davidson D, Letts M. Partial sleeve fractures of the tibia in children: an unusual fracture pattern. J Pediatr Orthop. 2002;22(1):36-40.
Risk-Stratified VTE Prophylaxis Following Total Joint Replacement Leads to Significant Hospital Cost Reductions and Prevents Deep Vein Thrombosis
DALLAS—Medical Compression Systems, Inc. (Concord, Massachusetts), announced new data that further validates the use of their ActiveCare deep vein thrombosis prophylaxis compression system following total joint replacement procedures. The study results demonstrate that a risk-stratification protocol using a synchronized mobile compression and an aspirin regimen is associated with low rates of venous thromboembolism, lower rates of adverse events, and reduced overall costs compared with a group treated with aggressive anticoagulant agents. Data were presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
“We’ve established through previous studies that prophylactic treatment with mobile compression and aspirin following total joint replacement can reduce the occurrence of venous thromboembolism and decrease adverse events, infections, and bleeding complications in patients undergoing total joint replacement,” said Richard Iorio, MD, primary study author and Professor of Orthopedic Surgery at NYU School of Medicine in New York.
The study was designed to determine if utilizing a risk-based venous thromboembolism chemoprophylaxis protocol would improve prevention of deep vein thrombosis and pulmonary embolism, quality metrics, and bleeding-related complications in patients undergoing total joint arthroplasty.
The retrospective review evaluated 2,611 patients that were divided into 2 cohorts. Cohort 1 included 1,203 patients who were previously treated with standard aggressive chemoprophylaxis agents (Enoxaparin, Rivaroxaban, Warfarin). Cohort 2 consisted of a risk-stratified group of patients either undergoing treatment with prophylactic synchronized mobile compression and aspirin (n=843) or aggressive prophylaxis (n=565).
Results demonstrated that patients in the risk-stratified protocol had a lower incidence of venous thromboembolism than the group treated with anticoagulation. Patients in this group also experienced fewer adverse events, readmissions, infections, and bleeding-related complications. Hospital costs were significantly lower in the synchronized mobile compression and aspirin subgroup of cohort 2 and overall costs were lower in the risk-stratified cohort, though they did not reach statistical significance.
“These results are significant in that they represent a large study population of more than 2,600 patients and are the first to demonstrate significant reductions in hospital costs, which support the hypothesis that a risk stratification protocol can advance patient-specific therapy and enhance the delivery of value-based care,” Dr. Iorio said.
DALLAS—Medical Compression Systems, Inc. (Concord, Massachusetts), announced new data that further validates the use of their ActiveCare deep vein thrombosis prophylaxis compression system following total joint replacement procedures. The study results demonstrate that a risk-stratification protocol using a synchronized mobile compression and an aspirin regimen is associated with low rates of venous thromboembolism, lower rates of adverse events, and reduced overall costs compared with a group treated with aggressive anticoagulant agents. Data were presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
“We’ve established through previous studies that prophylactic treatment with mobile compression and aspirin following total joint replacement can reduce the occurrence of venous thromboembolism and decrease adverse events, infections, and bleeding complications in patients undergoing total joint replacement,” said Richard Iorio, MD, primary study author and Professor of Orthopedic Surgery at NYU School of Medicine in New York.
The study was designed to determine if utilizing a risk-based venous thromboembolism chemoprophylaxis protocol would improve prevention of deep vein thrombosis and pulmonary embolism, quality metrics, and bleeding-related complications in patients undergoing total joint arthroplasty.
The retrospective review evaluated 2,611 patients that were divided into 2 cohorts. Cohort 1 included 1,203 patients who were previously treated with standard aggressive chemoprophylaxis agents (Enoxaparin, Rivaroxaban, Warfarin). Cohort 2 consisted of a risk-stratified group of patients either undergoing treatment with prophylactic synchronized mobile compression and aspirin (n=843) or aggressive prophylaxis (n=565).
Results demonstrated that patients in the risk-stratified protocol had a lower incidence of venous thromboembolism than the group treated with anticoagulation. Patients in this group also experienced fewer adverse events, readmissions, infections, and bleeding-related complications. Hospital costs were significantly lower in the synchronized mobile compression and aspirin subgroup of cohort 2 and overall costs were lower in the risk-stratified cohort, though they did not reach statistical significance.
“These results are significant in that they represent a large study population of more than 2,600 patients and are the first to demonstrate significant reductions in hospital costs, which support the hypothesis that a risk stratification protocol can advance patient-specific therapy and enhance the delivery of value-based care,” Dr. Iorio said.
DALLAS—Medical Compression Systems, Inc. (Concord, Massachusetts), announced new data that further validates the use of their ActiveCare deep vein thrombosis prophylaxis compression system following total joint replacement procedures. The study results demonstrate that a risk-stratification protocol using a synchronized mobile compression and an aspirin regimen is associated with low rates of venous thromboembolism, lower rates of adverse events, and reduced overall costs compared with a group treated with aggressive anticoagulant agents. Data were presented at the 25th Annual Meeting of the American Association of Hip and Knee Surgeons.
“We’ve established through previous studies that prophylactic treatment with mobile compression and aspirin following total joint replacement can reduce the occurrence of venous thromboembolism and decrease adverse events, infections, and bleeding complications in patients undergoing total joint replacement,” said Richard Iorio, MD, primary study author and Professor of Orthopedic Surgery at NYU School of Medicine in New York.
The study was designed to determine if utilizing a risk-based venous thromboembolism chemoprophylaxis protocol would improve prevention of deep vein thrombosis and pulmonary embolism, quality metrics, and bleeding-related complications in patients undergoing total joint arthroplasty.
The retrospective review evaluated 2,611 patients that were divided into 2 cohorts. Cohort 1 included 1,203 patients who were previously treated with standard aggressive chemoprophylaxis agents (Enoxaparin, Rivaroxaban, Warfarin). Cohort 2 consisted of a risk-stratified group of patients either undergoing treatment with prophylactic synchronized mobile compression and aspirin (n=843) or aggressive prophylaxis (n=565).
Results demonstrated that patients in the risk-stratified protocol had a lower incidence of venous thromboembolism than the group treated with anticoagulation. Patients in this group also experienced fewer adverse events, readmissions, infections, and bleeding-related complications. Hospital costs were significantly lower in the synchronized mobile compression and aspirin subgroup of cohort 2 and overall costs were lower in the risk-stratified cohort, though they did not reach statistical significance.
“These results are significant in that they represent a large study population of more than 2,600 patients and are the first to demonstrate significant reductions in hospital costs, which support the hypothesis that a risk stratification protocol can advance patient-specific therapy and enhance the delivery of value-based care,” Dr. Iorio said.
Pure Intrathoracic Scapular Dislocation
Scapular dislocation, which is also termed locked scapula or scapulothoracic dislocation, is an unusual condition that can be described as extrathoracic or intrathoracic dislocation, depending on the penetration of scapula into the thoracic cavity.
There have been 3 reported cases of intrathoracic scapular dislocations in the literature,1-3all associated with a preexisting condition (eg, sternoclavicular separation, prior rib fracture, thoracotomy for a lung transplant procedure, or surgical resection of superior ribs during breast or pulmonary tumor excisions). Three published cases of intrathoracic scapular impaction involve comminuted scapular fractures with intrathoracic impaction of the inferior fragment through intercostal space.4-6
Here we report an intrathoracic scapular dislocation that was not associated with fracture of the scapula or predisposing factors. To our knowledge, this is the first case of pure intrathoracic dislocation. The possibility of intrathoracic scapular dislocation should be considered as part of the differential diagnosis even in patients with a negative anamnesis for predisposing factors, such as lung or chest surgery. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 29-year-old woman presented to the emergency department after a motor vehicle accident. She had tenderness over the left shoulder and left elbow with decreased range of motion; however, motor and sensory examination of the wrist and fingers were normal. No distal neurovascular deficit was noted.
Physical examination revealed pain on pelvic compression. We observed an asymmetrical appearance between shoulders; the left shoulder was superior when compared with the right side (Figure 1). Palpation of the scapula aggravated the pain. The inferior angle of the left scapula was not palpable, and the medial border was palpated through the intercostal space between the third and fourth ribs.
Initial radiographs showed additional left olecranon and bilateral ramus pubis fractures. A chest radiograph showed nondisplaced fractures of the second and third ribs without any obvious hemothorax or pneumothorax. No other pathology involving the chest, such as resection of the ribs or congenital anomaly, was observed. The patient reported no history of thoracic trauma or lung surgery. There were no fractures of the scapula, humerus, or clavicles. Thoracic computed tomography was performed, and 3-dimensional (3D) reconstruction showed that the inferior angle of scapula penetrated into the thoracic cavity through the third intercostal space (Figure 2).
Given the intrathoracic scapular dislocation diagnosis, closed reduction under sedation was planned. The patient was placed in the supine position, and reduction was performed by applying pressure on the shoulder anteriorly. This maneuver increased deformity. At the same time, another physician pulled the spine of the scapula superiorly, releasing the scapula out of the thoracic cavity. When the arm was slightly lowered to neutral position, scapular deformity was no longer present (Figure 3). A shoulder sling was applied, and the patient was hospitalized for surgical fixation of pelvic and olecranon fractures. The arm was immobilized in a sling for 1 week, and shoulder exercises were started immediately afterward.
At 1-month follow-up, full shoulder range of motion was achieved, although rehabilitation for the elbow continued. Final follow-up examination at 4 months revealed no difference between shoulders, and no recurrence occurred.
Discussion
Intrathoracic scapular dislocation is a rare injury. There are only a few cases reported in the literature, and most of them are well associated with a predisposing factor. Nettrour and colleagues1 described the first intrathoracic scapular dislocation, which occurred 6 weeks after sternoclavicular separation and fracture of a rib. In the case reports of Ward and colleagues2 and Fowler and colleagues,3 the predisposing factor was resection of the ribs due to pancoast tumor and breast carcinoma, respectively. The mechanism of these dislocations depends on a weak area over the thoracic cage, creating a fulcrum point for levering the scapula into the thoracic cavity.
There are other cases of scapular dislocations that are accompanied by a comminuted fracture of scapula; a review of the literature revealed 3 cases.4-6 In our opinion, fracture of the inferior pole of the scapula leads to injury of the soft tissues and also results in intrathoracic impaction by creating a weak area over the thoracic cavity. This mechanism can be referred to as penetration.
Our case is singular because it is the first case that is not associated with fracture of the scapula or predisposing factors. Consequently, we report the first pure intrathoracic scapular dislocation in the literature. It is important to suspect intrathoracic scapular dislocation in the case of deformity (Figure 1), even in the absence of any predisposing factors or scapular fracture.
Although plain radiographs may not be elucidative, 3D reconstruction of computed tomography (Figure 2) reveals the pathology and plays an important role in guiding treatment.
In the treatment of our patient, relying on the unique dislocation mechanism without any fracture of the scapula or ribs, we started early active shoulder movement after 1 week of immobilization in a shoulder sling, which prevented recurrence of dislocation. In addition to presenting the first pure intrathoracic scapular dislocation, this case demonstrated satisfactory clinical results with short-term immobilization and early rehabilitation.
Conclusion
Contrary to the literature, the possibility of intrathoracic scapular dislocation should be considered in the differential diagnosis even in patients with a negative anamnesis for predisposing factors, such as lung or chest surgery, and when no fractures are detected. Shoulder or thorax computed tomography, especially 3D reconstructions, are helpful in diagnosing the condition and in guiding treatment. Closed reduction under sedation followed by early rehabilitation is an appropriate treatment method, which resulted in a full return of function in 1 month in our patient.
1. Nettrour LF, Krufky EL, Mueller RE, Raycroft JF. Locked scapula: intrathoracic dislocation of the inferior angle. A case report. J Bone Joint Surg Am. 1972;54(2):413-416.
2. Ward WG, Weaver JP, Garrett WE Jr. Locked scapula: A case report. J Bone Joint Surg Am. 1989;71(10):1558-1159.
3. Fowler TT, Taylor BC, Fankhauser RA. Recurrent low-energy intrathoracic dislocation of the scapula. Am J Orthop. 2013;42(1):E1-E4.
4. Blue JM, Anglen JO, Helikson MA. Fracture of the scapula with intrathoracic penetration. A case report. J Bone Joint Surg Am. 1997;79(7):1076-1078.
5. Schwartzbach CC, Seoudi H, Ross AE, Hendershot K, Robinson L, Malekzadeh A. Fracture of the scapula with intrathoracic penetration in a skeletally mature patient. A case report. J Bone Joint Surg Am. 2006;88(12):2735-2738.
6. Porte AN, Wirtzfeld DA, Mann C. Intrathoracic scapular impaction: an unusual complication of scapular fractures. Can J Surg. 2009;52(3):E62-E63.
Scapular dislocation, which is also termed locked scapula or scapulothoracic dislocation, is an unusual condition that can be described as extrathoracic or intrathoracic dislocation, depending on the penetration of scapula into the thoracic cavity.
There have been 3 reported cases of intrathoracic scapular dislocations in the literature,1-3all associated with a preexisting condition (eg, sternoclavicular separation, prior rib fracture, thoracotomy for a lung transplant procedure, or surgical resection of superior ribs during breast or pulmonary tumor excisions). Three published cases of intrathoracic scapular impaction involve comminuted scapular fractures with intrathoracic impaction of the inferior fragment through intercostal space.4-6
Here we report an intrathoracic scapular dislocation that was not associated with fracture of the scapula or predisposing factors. To our knowledge, this is the first case of pure intrathoracic dislocation. The possibility of intrathoracic scapular dislocation should be considered as part of the differential diagnosis even in patients with a negative anamnesis for predisposing factors, such as lung or chest surgery. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 29-year-old woman presented to the emergency department after a motor vehicle accident. She had tenderness over the left shoulder and left elbow with decreased range of motion; however, motor and sensory examination of the wrist and fingers were normal. No distal neurovascular deficit was noted.
Physical examination revealed pain on pelvic compression. We observed an asymmetrical appearance between shoulders; the left shoulder was superior when compared with the right side (Figure 1). Palpation of the scapula aggravated the pain. The inferior angle of the left scapula was not palpable, and the medial border was palpated through the intercostal space between the third and fourth ribs.
Initial radiographs showed additional left olecranon and bilateral ramus pubis fractures. A chest radiograph showed nondisplaced fractures of the second and third ribs without any obvious hemothorax or pneumothorax. No other pathology involving the chest, such as resection of the ribs or congenital anomaly, was observed. The patient reported no history of thoracic trauma or lung surgery. There were no fractures of the scapula, humerus, or clavicles. Thoracic computed tomography was performed, and 3-dimensional (3D) reconstruction showed that the inferior angle of scapula penetrated into the thoracic cavity through the third intercostal space (Figure 2).
Given the intrathoracic scapular dislocation diagnosis, closed reduction under sedation was planned. The patient was placed in the supine position, and reduction was performed by applying pressure on the shoulder anteriorly. This maneuver increased deformity. At the same time, another physician pulled the spine of the scapula superiorly, releasing the scapula out of the thoracic cavity. When the arm was slightly lowered to neutral position, scapular deformity was no longer present (Figure 3). A shoulder sling was applied, and the patient was hospitalized for surgical fixation of pelvic and olecranon fractures. The arm was immobilized in a sling for 1 week, and shoulder exercises were started immediately afterward.
At 1-month follow-up, full shoulder range of motion was achieved, although rehabilitation for the elbow continued. Final follow-up examination at 4 months revealed no difference between shoulders, and no recurrence occurred.
Discussion
Intrathoracic scapular dislocation is a rare injury. There are only a few cases reported in the literature, and most of them are well associated with a predisposing factor. Nettrour and colleagues1 described the first intrathoracic scapular dislocation, which occurred 6 weeks after sternoclavicular separation and fracture of a rib. In the case reports of Ward and colleagues2 and Fowler and colleagues,3 the predisposing factor was resection of the ribs due to pancoast tumor and breast carcinoma, respectively. The mechanism of these dislocations depends on a weak area over the thoracic cage, creating a fulcrum point for levering the scapula into the thoracic cavity.
There are other cases of scapular dislocations that are accompanied by a comminuted fracture of scapula; a review of the literature revealed 3 cases.4-6 In our opinion, fracture of the inferior pole of the scapula leads to injury of the soft tissues and also results in intrathoracic impaction by creating a weak area over the thoracic cavity. This mechanism can be referred to as penetration.
Our case is singular because it is the first case that is not associated with fracture of the scapula or predisposing factors. Consequently, we report the first pure intrathoracic scapular dislocation in the literature. It is important to suspect intrathoracic scapular dislocation in the case of deformity (Figure 1), even in the absence of any predisposing factors or scapular fracture.
Although plain radiographs may not be elucidative, 3D reconstruction of computed tomography (Figure 2) reveals the pathology and plays an important role in guiding treatment.
In the treatment of our patient, relying on the unique dislocation mechanism without any fracture of the scapula or ribs, we started early active shoulder movement after 1 week of immobilization in a shoulder sling, which prevented recurrence of dislocation. In addition to presenting the first pure intrathoracic scapular dislocation, this case demonstrated satisfactory clinical results with short-term immobilization and early rehabilitation.
Conclusion
Contrary to the literature, the possibility of intrathoracic scapular dislocation should be considered in the differential diagnosis even in patients with a negative anamnesis for predisposing factors, such as lung or chest surgery, and when no fractures are detected. Shoulder or thorax computed tomography, especially 3D reconstructions, are helpful in diagnosing the condition and in guiding treatment. Closed reduction under sedation followed by early rehabilitation is an appropriate treatment method, which resulted in a full return of function in 1 month in our patient.
Scapular dislocation, which is also termed locked scapula or scapulothoracic dislocation, is an unusual condition that can be described as extrathoracic or intrathoracic dislocation, depending on the penetration of scapula into the thoracic cavity.
There have been 3 reported cases of intrathoracic scapular dislocations in the literature,1-3all associated with a preexisting condition (eg, sternoclavicular separation, prior rib fracture, thoracotomy for a lung transplant procedure, or surgical resection of superior ribs during breast or pulmonary tumor excisions). Three published cases of intrathoracic scapular impaction involve comminuted scapular fractures with intrathoracic impaction of the inferior fragment through intercostal space.4-6
Here we report an intrathoracic scapular dislocation that was not associated with fracture of the scapula or predisposing factors. To our knowledge, this is the first case of pure intrathoracic dislocation. The possibility of intrathoracic scapular dislocation should be considered as part of the differential diagnosis even in patients with a negative anamnesis for predisposing factors, such as lung or chest surgery. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 29-year-old woman presented to the emergency department after a motor vehicle accident. She had tenderness over the left shoulder and left elbow with decreased range of motion; however, motor and sensory examination of the wrist and fingers were normal. No distal neurovascular deficit was noted.
Physical examination revealed pain on pelvic compression. We observed an asymmetrical appearance between shoulders; the left shoulder was superior when compared with the right side (Figure 1). Palpation of the scapula aggravated the pain. The inferior angle of the left scapula was not palpable, and the medial border was palpated through the intercostal space between the third and fourth ribs.
Initial radiographs showed additional left olecranon and bilateral ramus pubis fractures. A chest radiograph showed nondisplaced fractures of the second and third ribs without any obvious hemothorax or pneumothorax. No other pathology involving the chest, such as resection of the ribs or congenital anomaly, was observed. The patient reported no history of thoracic trauma or lung surgery. There were no fractures of the scapula, humerus, or clavicles. Thoracic computed tomography was performed, and 3-dimensional (3D) reconstruction showed that the inferior angle of scapula penetrated into the thoracic cavity through the third intercostal space (Figure 2).
Given the intrathoracic scapular dislocation diagnosis, closed reduction under sedation was planned. The patient was placed in the supine position, and reduction was performed by applying pressure on the shoulder anteriorly. This maneuver increased deformity. At the same time, another physician pulled the spine of the scapula superiorly, releasing the scapula out of the thoracic cavity. When the arm was slightly lowered to neutral position, scapular deformity was no longer present (Figure 3). A shoulder sling was applied, and the patient was hospitalized for surgical fixation of pelvic and olecranon fractures. The arm was immobilized in a sling for 1 week, and shoulder exercises were started immediately afterward.
At 1-month follow-up, full shoulder range of motion was achieved, although rehabilitation for the elbow continued. Final follow-up examination at 4 months revealed no difference between shoulders, and no recurrence occurred.
Discussion
Intrathoracic scapular dislocation is a rare injury. There are only a few cases reported in the literature, and most of them are well associated with a predisposing factor. Nettrour and colleagues1 described the first intrathoracic scapular dislocation, which occurred 6 weeks after sternoclavicular separation and fracture of a rib. In the case reports of Ward and colleagues2 and Fowler and colleagues,3 the predisposing factor was resection of the ribs due to pancoast tumor and breast carcinoma, respectively. The mechanism of these dislocations depends on a weak area over the thoracic cage, creating a fulcrum point for levering the scapula into the thoracic cavity.
There are other cases of scapular dislocations that are accompanied by a comminuted fracture of scapula; a review of the literature revealed 3 cases.4-6 In our opinion, fracture of the inferior pole of the scapula leads to injury of the soft tissues and also results in intrathoracic impaction by creating a weak area over the thoracic cavity. This mechanism can be referred to as penetration.
Our case is singular because it is the first case that is not associated with fracture of the scapula or predisposing factors. Consequently, we report the first pure intrathoracic scapular dislocation in the literature. It is important to suspect intrathoracic scapular dislocation in the case of deformity (Figure 1), even in the absence of any predisposing factors or scapular fracture.
Although plain radiographs may not be elucidative, 3D reconstruction of computed tomography (Figure 2) reveals the pathology and plays an important role in guiding treatment.
In the treatment of our patient, relying on the unique dislocation mechanism without any fracture of the scapula or ribs, we started early active shoulder movement after 1 week of immobilization in a shoulder sling, which prevented recurrence of dislocation. In addition to presenting the first pure intrathoracic scapular dislocation, this case demonstrated satisfactory clinical results with short-term immobilization and early rehabilitation.
Conclusion
Contrary to the literature, the possibility of intrathoracic scapular dislocation should be considered in the differential diagnosis even in patients with a negative anamnesis for predisposing factors, such as lung or chest surgery, and when no fractures are detected. Shoulder or thorax computed tomography, especially 3D reconstructions, are helpful in diagnosing the condition and in guiding treatment. Closed reduction under sedation followed by early rehabilitation is an appropriate treatment method, which resulted in a full return of function in 1 month in our patient.
1. Nettrour LF, Krufky EL, Mueller RE, Raycroft JF. Locked scapula: intrathoracic dislocation of the inferior angle. A case report. J Bone Joint Surg Am. 1972;54(2):413-416.
2. Ward WG, Weaver JP, Garrett WE Jr. Locked scapula: A case report. J Bone Joint Surg Am. 1989;71(10):1558-1159.
3. Fowler TT, Taylor BC, Fankhauser RA. Recurrent low-energy intrathoracic dislocation of the scapula. Am J Orthop. 2013;42(1):E1-E4.
4. Blue JM, Anglen JO, Helikson MA. Fracture of the scapula with intrathoracic penetration. A case report. J Bone Joint Surg Am. 1997;79(7):1076-1078.
5. Schwartzbach CC, Seoudi H, Ross AE, Hendershot K, Robinson L, Malekzadeh A. Fracture of the scapula with intrathoracic penetration in a skeletally mature patient. A case report. J Bone Joint Surg Am. 2006;88(12):2735-2738.
6. Porte AN, Wirtzfeld DA, Mann C. Intrathoracic scapular impaction: an unusual complication of scapular fractures. Can J Surg. 2009;52(3):E62-E63.
1. Nettrour LF, Krufky EL, Mueller RE, Raycroft JF. Locked scapula: intrathoracic dislocation of the inferior angle. A case report. J Bone Joint Surg Am. 1972;54(2):413-416.
2. Ward WG, Weaver JP, Garrett WE Jr. Locked scapula: A case report. J Bone Joint Surg Am. 1989;71(10):1558-1159.
3. Fowler TT, Taylor BC, Fankhauser RA. Recurrent low-energy intrathoracic dislocation of the scapula. Am J Orthop. 2013;42(1):E1-E4.
4. Blue JM, Anglen JO, Helikson MA. Fracture of the scapula with intrathoracic penetration. A case report. J Bone Joint Surg Am. 1997;79(7):1076-1078.
5. Schwartzbach CC, Seoudi H, Ross AE, Hendershot K, Robinson L, Malekzadeh A. Fracture of the scapula with intrathoracic penetration in a skeletally mature patient. A case report. J Bone Joint Surg Am. 2006;88(12):2735-2738.
6. Porte AN, Wirtzfeld DA, Mann C. Intrathoracic scapular impaction: an unusual complication of scapular fractures. Can J Surg. 2009;52(3):E62-E63.
Web Page Content and Quality Assessed for Shoulder Replacement
The Internet is becoming a primary source for obtaining medical information. This growing trend may have serious implications for the medical field. As patients increasingly regard the Internet as an essential tool for obtaining health-related information, questions have been raised regarding the quality of medical information available on the Internet.1 Studies have shown that health-related sites often present inaccurate, inconsistent, and outdated information that may have a negative impact on health care decisions made by patients.2
According to the US Census Bureau, 71.7% of American households report having access to the Internet.3 Of those who have access to Internet, approximately 72% have sought health information online over the last year.4 Among people older than age 65 years living in the United States, there has been a growing trend toward using the Internet, from 14% in 2000 to almost 60% in 2013, according to the Pew Research Internet Project.5 Most medical websites are viewed for information on diseases and treatment options.6 Since most patients want to be informed about treatment options, as well as risks and benefits for each treatment, access to credible information is essential for proper decision-making.7
To assess the quality of information on the Internet, we used DISCERN, a standardized questionnaire to aid consumers in judging Internet content.8 The DISCERN instrument, available at www.discern.org.uk, was designed by an expert group in the United Kingdom. First, an expert panel developed and tested the instrument, and then health care providers and self-help group members tested it further.8,9 The questionnaire had been found to have good interrater reliability, regardless of use by health professionals or consumers.8-10
More than 53,000 shoulder arthroplasties are performed in the United States annually, and the number is growing, with the main goal of pain relief from glenohumeral degenerative joint disease.11,12 The Internet has become a quasi–second opinion for patients trying to participate in their care. Given the prevalence of shoulder-related surgeries, it is critical to analyze and become familiar with the quality of information that patients read online in order to direct them to nonbiased, all-inclusive websites. In this study, we provide a summary assessment and comparison of the quality of online information pertaining to shoulder replacement, using medical (total shoulder replacement) and nontechnical (shoulder replacement) search terms.
Methods
Websites were identified using 3 search engines (Google, Yahoo, and Bing) and 2 search terms, shoulder replacement (SR) and total shoulder arthroplasty (TSA), on January 17, 2014. These 3 search engines were used because 77% of health care–related information online searches begin through a search engine (Google, Bing, Yahoo); only 13% begin at a health care–specialized website.4 These search terms were used after consulting with orthopedic residents and attending physicians in a focus group regarding the terminology used with patients. The first 30 websites in each search engine were identified consecutively and evaluated for category and quality of information using the DISCERN instrument.
A total of 180 websites (90 per search term) were reviewed. Each website was evaluated independently by 3 medical students. In the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram, we recorded how websites were identified, screened, and included (Figure 1).13 Websites that were duplicated within each search term and those that were inaccessible were used to determine the total number of noncommercial versus commercial websites, but were excluded from the final analysis. The first part of the analysis involved determining the type of website (eg, commercial vs noncommercial) based upon the html endings. All .com endings were classified as commercial websites; noncommercial included .gov, .org, .edu, and .net endings. Next, each website was categorized based on the target audience. Websites were grouped into health professional–oriented information, patient-oriented, advertisement, or “other.” These classifications were based on those described in previous works.14,15 The “other” category included images, YouTube videos, another search engine, and open forums, which were also excluded from the final analysis because they were not easily evaluable with the DISCERN instrument. Websites were considered health professional–oriented if they included journal articles, scholarly articles, and/or rehabilitation protocols. Patient-directed websites clearly stated the information was directed to patients or provided a general overview. Advertisement included sites that displayed ads or products for sale. Websites were evaluated for quality using the DISCERN instrument (Figure 2).
DISCERN has 3 subdivision scores: the reliable score (composed of the first 8 questions), the treatment options (the next 7 questions), and 1 final question that addresses the overall quality of the website and is rated independently of the first 15 questions. DISCERN uses 2 scales, a binary scale anchored on both extremes with the number 1 equaling complete absence of the criteria being measured, and the number 5 at the upper extreme, representing completeness of the quality being assessed. In between 1 and 5 is a partial ordinal scale measuring from 2 to 4, which indicates the information is present to some extent but not complete. The ordinal scale allows ranking of the criteria being assessed. Summarizing values from each of the 2 scales poses some concern: the scale is not a true binary scale because of the ordinal scale of the middle numbers (2-4), and as such, is not amenable to being an interval scale to calculate arithmetic means. To summarize the values from the 2 scales, we calculated the harmonic mean, the arithmetic mean, the geometric mean, and the median. The means were empirically compared with the median, and we used the harmonic mean to summarize scale values because it was the best approximation of the medians.
Results
A total of 90 websites were assessed with the search term total shoulder arthroplasty and another 90 with shoulder replacement. When 37 duplicate websites for TSA and 52 for SR were eliminated, 53 (59%) and 38 (42%) unique websites were evaluated for each search term, respectively (Figure 1). (These unique websites are included in the Appendix.) Between the 2 search terms, 20 websites were duplicated. Figure 3 shows the distribution of websites by category. Total shoulder arthroplasty provided the highest percentage of health professional–oriented information; SR had the greatest percentage of patient-oriented information. Both TSA and SR had nearly the same number of advertisements and websites labeled “other.” The percentage of noncommercial websites from each search engine is represented in Figure 4. For SR, Google had 40% (12/30) noncommercial websites compared with Yahoo at 53% (16/30) and Bing at 46% (14/30). Total shoulder arthroplasty had 43% (13/30) noncommercial websites on Google, 27% (8/30) on Yahoo, and 40% (12/30) on Bing. In total, SR had more noncommercial websites, 47% (42/90), compared with 37% (33/90) for TSA.
The mean of all 3 raters for reliablity (DISCERN questions 1-8) and treatment options (DISCERN questions 9-15) is represented in the Table. For both search terms, we found that websites identified as health professional–oriented had the highest reliable mean scores, followed by patient-oriented, and advertisement at the lowest (SR: P = .054; TSA: P = .134). For SR, treatment mean scores demonstrated similar results with health professional–oriented websites receiving the highest, followed by patient-oriented and advertisement (P = .005). However, the treatment mean scores for TSA differed with patient-oriented websites receiving higher scores than health professional–oriented websites, but this was not statistically significant (P= .407). Regarding search terms, there were no significant differences between mean reliable and treatment scores across all categories.
The average overall DISCERN score for TSA websites was 2.5 (range, 1-5), compared with 2.3 (range, 1-5) for SR websites. The overall reliable score (DISCERN questions 1-8) for TSA websites was 2.6 and 2.5 for SR websites (P < .001). For TSA websites, 38% (20/53) were classified as good, having an overall DISCERN score ≥3, versus 26% (10/38) of SR websites. The overall DISCERN score for health professional–oriented websites was 2.7, patient-oriented websites received a score of 2.6, and advertisements had the lowest score at 2.4.
Discussion
Both patients and health professionals obtain information on health care subjects through the Internet, which has become the primary resource for patients.15,16 However, there are no strict regulations of the content being written. This creates a challenge for the typical user to find credible and evidence-based information, which is important because misleading information could cause undue anxiety, among other effects.17,18 The aims of this study were to determine the quality of Internet information for shoulder replacement surgeries using the medical terminology total shoulder arthroplasty (TSA) and the nontechnical term shoulder replacement (SR), and to compare the results.
After analyzing the types of websites returned for both total shoulder arthroplasty and shoulder replacement (Figure 4), it was interesting to find that using nonmedical terminology as the search term provided more noncommercial websites compared with total shoulder arthroplasty. Furthermore, Yahoo provided the highest yield of noncommercial websites at 16, with Bing at 14, when using SR as the search term. We believe the increase in noncommercial websites returned for SR was greater than for TSA because SR yielded more patient-oriented websites, which usually had html endings of .edu and .org, as shown in Figure 3 (48% of SR websites offered patient-oriented information).
Although there were more noncommercial websites for SR, the majority of the DISCERN values between the 2 search terms did not differ significantly. This is a direct result of the number of sites (20) that were duplicated across both search terms. However as seen in the Table, TSA had similar reliable mean scores for advertisements and patient-oriented websites but a slightly higher reliable score for health professional–oriented websites. We correlated this with the increased number of health professional–oriented websites returned when using TSA as the search term (Figure 3). The health professional–oriented websites explained their aims and cited their sources more consistently than did patient-oriented sites and advertisements, resulting in higher reliable scores. Although patient-oriented websites frequently lacked citations, they provided information about multiple treatment options, which were more relevant to consumers. This resulted in nearly equivalent reliable scores. Treatment means for advertisements in both SR and TSA were similar. However, treatment means for professional-oriented websites in TSA were lower than those for SR because health professional–oriented websites often were only moderately relevant to consumers, with their focus usually on 1 treatment option or on rehabilitation protocols. Although the DISCERN scores were similar between the search terms, total shoulder arthroplasty provided more websites (20) classified as good—overall DISCERN score, ≥3—than SR did (10). Advertisement websites had similar overall DISCERN scores, which we anticipated because most of the advertisements were duplicated across the search terms.
Using the 2 search terms, academic websites and commercial websites, such as WebMD, consistently received higher reliable and overall DISCERN scores. Advertisement websites, which need to deliver a clear message, frequently scored high on explicitly stating their aims and relevance to consumers, but focused on their products without discussing the benefits of other treatment options. This is significant because Internet search engines, such as Google, offer sponsor links for which organizations pay to appear at the top of the search results. This creates the potential for consumers to receive biased information because most individuals only visit the top 10 websites generated by a search engine.19
We concluded that the quality of online information relating to SR and TSA was highly variable and frequently of moderate-to-poor quality, with most overall DISCERN scores <3. The quality of information found online for this study using the DISCERN instrument is consistent with those studies using DISCERN to evaluate other medical conditions (eg, bunions, chronic pain, general anesthesia, and anterior cruciate ligament reconstruction).2,9,15,19 These studies also concluded that online information varies tremendously in quality and completeness.
This study has several limitations. Websites were searched at a single time point and, because Internet resources are frequently updated, the results of this study could vary. Furthermore, although Google, Yahoo, and Bing are 3 of the most popular search engines, these are not the only resources patients use when searching the Internet for health-related information. Other search engines, such as Pubmed.gov and MSN.com, could provide additional websites for Internet users. Lastly, although DISCERN is validated to address the quality of information available online, it does not evaluate the accuracy of the information.8 Our use of DISCERN involves 2 scales, a binary yes/no (ratings, 1 and 5) and an ordinal scale (ratings, 2-4). As such, a single mean summary statistic cannot be calculated.
Conclusion
The information available on the Internet pertaining to TSA and SR is highly variable and provides mostly moderate-to-poor quality information based on the DISCERN instrument. Many websites failed to describe the benefits and the risks of different treatment options, including nonoperative management. Health care professionals should be aware that patients often refer to the Internet as a primary resource for obtaining medical information. It is important to direct patients to websites that provide accurate information, because patients who educate themselves about their conditions and actively participate in decision-making may have improved health outcomes.20-22 Overall, academic websites and commercial websites, such as WebMD and OrthoInfo, generally had higher DISCERN scores when using either search term. Of major concern is the potential for misleading advertisements or incorrect information that can negatively affect health outcomes. This study found that using nonmedical terminology (SR) provided more noncommercial and patient-oriented websites, especially through Yahoo. This study highlights the need for more comprehensive online information pertaining to shoulder replacement that can better serve as a resource for Internet users.
1. Eysenbach G, Powell J, Kuss O, Sa ER. Empirical studies assessing the quality of health information for consumers on the world wide web: a systematic review. JAMA. 2002;287(20):2691-2700.
2. Bruce-Brand RA, Baker JF, Byrne DP, Hogan NA, McCarthy T. Assessment of the quality and content of information on anterior cruciate ligament reconstruction on the internet. Arthroscopy. 2013;29(6):1095-1100.
3. Computer and internet use in the United States: population characteristics. US Census Bureau website. http://www.census.gov/hhes/computer/. Accessed December 11, 2015.
4. Fox S, Duggan M. Health online 2013. Pew Research Center website. http://pewinternet.org/Reports/2013/Health-online.aspx. Published January 15, 2013. Accessed November 24, 2015.
5. Smith A. Older adults and technology use. Pew Research Center website. http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use. Published April 3, 2014. Accessed November 24, 2015.
6. Shuyler KS, Knight KM. What are patients seeking when they turn to the internet? Qualitative content analysis of questions asked by visitors to an orthopaedics web site. J Med Internet Res. 2003;5(4):e24.
7. Meredith P, Emberton M, Wood C, Smith J. Comparison of patients’ needs for information on prostate surgery with printed materials provided by surgeons. Qual Health Care. 1995;4(1):18-23.
8. Charnock D, Shepperd S, Needham G, Gann R. DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. J Epidemiol Community Health. 1999;53(2):105-111.
9. Kaicker J, Debono VB, Dang W, Buckley N, Thabane L. Assessment of the quality and variability of health information on chronic pain websites using the DISCERN instrument. BMC Med. 2010;8(1):59.
10. Griffiths KM, Christensen H. Website quality indicators for consumers. J Med Internet Res. 2005;7(5):e55.
11. Wiater JM. Shoulder joint replacement. American Academy of Orthopedic Surgeons website. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Updated December 2011. Accessed November 24, 2015.
12. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the united states. J Bone Joint Surg Am. 2011;93(24):2249-2254.
13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-W94.
14. Nason GJ, Baker JF, Byrne DP, Noel J, Moore D, Kiely PJ. Scoliosis-specific information on the internet: has the “information highway” led to better information provision? Spine. 2012;37(21):E1364-E1369.
15. Starman JS, Gettys FK, Capo JA, Fleischli JE, Norton HJ, Karunakar MA. Quality and content of internet-based information for ten common orthopaedic sports medicine diagnoses. J Bone Joint Surg Am. 2010;92(7):1612-1618.
16. Bernstein J, Ahn J, Veillette C. The future of orthopaedic information management. J Bone Joint Surg Am. 2012;94(13):e95.
17. Berland GK, Elliott MN, Morales LS, et al. Health information on the Internet: accessibility, quality, and readability in English and Spanish. JAMA. 2001;285(20):2612-2621.
18. Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ. 1990;301(6752):575-580.
19. Chong YM, Fraval A, Chandrananth J, Plunkett V, Tran P. Assessment of the quality of web-based information on bunions. Foot Ankle Int. 2013;34(8):1134-1139.
20. Brody DS, Miller SM, Lerman CE, Smith DG, Caputo GC. Patient perception of involvement in medical care. J Gen Intern Med. 1989;4(6):506-511.
21. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528.
22. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27(3 suppl):S110-S127.
The Internet is becoming a primary source for obtaining medical information. This growing trend may have serious implications for the medical field. As patients increasingly regard the Internet as an essential tool for obtaining health-related information, questions have been raised regarding the quality of medical information available on the Internet.1 Studies have shown that health-related sites often present inaccurate, inconsistent, and outdated information that may have a negative impact on health care decisions made by patients.2
According to the US Census Bureau, 71.7% of American households report having access to the Internet.3 Of those who have access to Internet, approximately 72% have sought health information online over the last year.4 Among people older than age 65 years living in the United States, there has been a growing trend toward using the Internet, from 14% in 2000 to almost 60% in 2013, according to the Pew Research Internet Project.5 Most medical websites are viewed for information on diseases and treatment options.6 Since most patients want to be informed about treatment options, as well as risks and benefits for each treatment, access to credible information is essential for proper decision-making.7
To assess the quality of information on the Internet, we used DISCERN, a standardized questionnaire to aid consumers in judging Internet content.8 The DISCERN instrument, available at www.discern.org.uk, was designed by an expert group in the United Kingdom. First, an expert panel developed and tested the instrument, and then health care providers and self-help group members tested it further.8,9 The questionnaire had been found to have good interrater reliability, regardless of use by health professionals or consumers.8-10
More than 53,000 shoulder arthroplasties are performed in the United States annually, and the number is growing, with the main goal of pain relief from glenohumeral degenerative joint disease.11,12 The Internet has become a quasi–second opinion for patients trying to participate in their care. Given the prevalence of shoulder-related surgeries, it is critical to analyze and become familiar with the quality of information that patients read online in order to direct them to nonbiased, all-inclusive websites. In this study, we provide a summary assessment and comparison of the quality of online information pertaining to shoulder replacement, using medical (total shoulder replacement) and nontechnical (shoulder replacement) search terms.
Methods
Websites were identified using 3 search engines (Google, Yahoo, and Bing) and 2 search terms, shoulder replacement (SR) and total shoulder arthroplasty (TSA), on January 17, 2014. These 3 search engines were used because 77% of health care–related information online searches begin through a search engine (Google, Bing, Yahoo); only 13% begin at a health care–specialized website.4 These search terms were used after consulting with orthopedic residents and attending physicians in a focus group regarding the terminology used with patients. The first 30 websites in each search engine were identified consecutively and evaluated for category and quality of information using the DISCERN instrument.
A total of 180 websites (90 per search term) were reviewed. Each website was evaluated independently by 3 medical students. In the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram, we recorded how websites were identified, screened, and included (Figure 1).13 Websites that were duplicated within each search term and those that were inaccessible were used to determine the total number of noncommercial versus commercial websites, but were excluded from the final analysis. The first part of the analysis involved determining the type of website (eg, commercial vs noncommercial) based upon the html endings. All .com endings were classified as commercial websites; noncommercial included .gov, .org, .edu, and .net endings. Next, each website was categorized based on the target audience. Websites were grouped into health professional–oriented information, patient-oriented, advertisement, or “other.” These classifications were based on those described in previous works.14,15 The “other” category included images, YouTube videos, another search engine, and open forums, which were also excluded from the final analysis because they were not easily evaluable with the DISCERN instrument. Websites were considered health professional–oriented if they included journal articles, scholarly articles, and/or rehabilitation protocols. Patient-directed websites clearly stated the information was directed to patients or provided a general overview. Advertisement included sites that displayed ads or products for sale. Websites were evaluated for quality using the DISCERN instrument (Figure 2).
DISCERN has 3 subdivision scores: the reliable score (composed of the first 8 questions), the treatment options (the next 7 questions), and 1 final question that addresses the overall quality of the website and is rated independently of the first 15 questions. DISCERN uses 2 scales, a binary scale anchored on both extremes with the number 1 equaling complete absence of the criteria being measured, and the number 5 at the upper extreme, representing completeness of the quality being assessed. In between 1 and 5 is a partial ordinal scale measuring from 2 to 4, which indicates the information is present to some extent but not complete. The ordinal scale allows ranking of the criteria being assessed. Summarizing values from each of the 2 scales poses some concern: the scale is not a true binary scale because of the ordinal scale of the middle numbers (2-4), and as such, is not amenable to being an interval scale to calculate arithmetic means. To summarize the values from the 2 scales, we calculated the harmonic mean, the arithmetic mean, the geometric mean, and the median. The means were empirically compared with the median, and we used the harmonic mean to summarize scale values because it was the best approximation of the medians.
Results
A total of 90 websites were assessed with the search term total shoulder arthroplasty and another 90 with shoulder replacement. When 37 duplicate websites for TSA and 52 for SR were eliminated, 53 (59%) and 38 (42%) unique websites were evaluated for each search term, respectively (Figure 1). (These unique websites are included in the Appendix.) Between the 2 search terms, 20 websites were duplicated. Figure 3 shows the distribution of websites by category. Total shoulder arthroplasty provided the highest percentage of health professional–oriented information; SR had the greatest percentage of patient-oriented information. Both TSA and SR had nearly the same number of advertisements and websites labeled “other.” The percentage of noncommercial websites from each search engine is represented in Figure 4. For SR, Google had 40% (12/30) noncommercial websites compared with Yahoo at 53% (16/30) and Bing at 46% (14/30). Total shoulder arthroplasty had 43% (13/30) noncommercial websites on Google, 27% (8/30) on Yahoo, and 40% (12/30) on Bing. In total, SR had more noncommercial websites, 47% (42/90), compared with 37% (33/90) for TSA.
The mean of all 3 raters for reliablity (DISCERN questions 1-8) and treatment options (DISCERN questions 9-15) is represented in the Table. For both search terms, we found that websites identified as health professional–oriented had the highest reliable mean scores, followed by patient-oriented, and advertisement at the lowest (SR: P = .054; TSA: P = .134). For SR, treatment mean scores demonstrated similar results with health professional–oriented websites receiving the highest, followed by patient-oriented and advertisement (P = .005). However, the treatment mean scores for TSA differed with patient-oriented websites receiving higher scores than health professional–oriented websites, but this was not statistically significant (P= .407). Regarding search terms, there were no significant differences between mean reliable and treatment scores across all categories.
The average overall DISCERN score for TSA websites was 2.5 (range, 1-5), compared with 2.3 (range, 1-5) for SR websites. The overall reliable score (DISCERN questions 1-8) for TSA websites was 2.6 and 2.5 for SR websites (P < .001). For TSA websites, 38% (20/53) were classified as good, having an overall DISCERN score ≥3, versus 26% (10/38) of SR websites. The overall DISCERN score for health professional–oriented websites was 2.7, patient-oriented websites received a score of 2.6, and advertisements had the lowest score at 2.4.
Discussion
Both patients and health professionals obtain information on health care subjects through the Internet, which has become the primary resource for patients.15,16 However, there are no strict regulations of the content being written. This creates a challenge for the typical user to find credible and evidence-based information, which is important because misleading information could cause undue anxiety, among other effects.17,18 The aims of this study were to determine the quality of Internet information for shoulder replacement surgeries using the medical terminology total shoulder arthroplasty (TSA) and the nontechnical term shoulder replacement (SR), and to compare the results.
After analyzing the types of websites returned for both total shoulder arthroplasty and shoulder replacement (Figure 4), it was interesting to find that using nonmedical terminology as the search term provided more noncommercial websites compared with total shoulder arthroplasty. Furthermore, Yahoo provided the highest yield of noncommercial websites at 16, with Bing at 14, when using SR as the search term. We believe the increase in noncommercial websites returned for SR was greater than for TSA because SR yielded more patient-oriented websites, which usually had html endings of .edu and .org, as shown in Figure 3 (48% of SR websites offered patient-oriented information).
Although there were more noncommercial websites for SR, the majority of the DISCERN values between the 2 search terms did not differ significantly. This is a direct result of the number of sites (20) that were duplicated across both search terms. However as seen in the Table, TSA had similar reliable mean scores for advertisements and patient-oriented websites but a slightly higher reliable score for health professional–oriented websites. We correlated this with the increased number of health professional–oriented websites returned when using TSA as the search term (Figure 3). The health professional–oriented websites explained their aims and cited their sources more consistently than did patient-oriented sites and advertisements, resulting in higher reliable scores. Although patient-oriented websites frequently lacked citations, they provided information about multiple treatment options, which were more relevant to consumers. This resulted in nearly equivalent reliable scores. Treatment means for advertisements in both SR and TSA were similar. However, treatment means for professional-oriented websites in TSA were lower than those for SR because health professional–oriented websites often were only moderately relevant to consumers, with their focus usually on 1 treatment option or on rehabilitation protocols. Although the DISCERN scores were similar between the search terms, total shoulder arthroplasty provided more websites (20) classified as good—overall DISCERN score, ≥3—than SR did (10). Advertisement websites had similar overall DISCERN scores, which we anticipated because most of the advertisements were duplicated across the search terms.
Using the 2 search terms, academic websites and commercial websites, such as WebMD, consistently received higher reliable and overall DISCERN scores. Advertisement websites, which need to deliver a clear message, frequently scored high on explicitly stating their aims and relevance to consumers, but focused on their products without discussing the benefits of other treatment options. This is significant because Internet search engines, such as Google, offer sponsor links for which organizations pay to appear at the top of the search results. This creates the potential for consumers to receive biased information because most individuals only visit the top 10 websites generated by a search engine.19
We concluded that the quality of online information relating to SR and TSA was highly variable and frequently of moderate-to-poor quality, with most overall DISCERN scores <3. The quality of information found online for this study using the DISCERN instrument is consistent with those studies using DISCERN to evaluate other medical conditions (eg, bunions, chronic pain, general anesthesia, and anterior cruciate ligament reconstruction).2,9,15,19 These studies also concluded that online information varies tremendously in quality and completeness.
This study has several limitations. Websites were searched at a single time point and, because Internet resources are frequently updated, the results of this study could vary. Furthermore, although Google, Yahoo, and Bing are 3 of the most popular search engines, these are not the only resources patients use when searching the Internet for health-related information. Other search engines, such as Pubmed.gov and MSN.com, could provide additional websites for Internet users. Lastly, although DISCERN is validated to address the quality of information available online, it does not evaluate the accuracy of the information.8 Our use of DISCERN involves 2 scales, a binary yes/no (ratings, 1 and 5) and an ordinal scale (ratings, 2-4). As such, a single mean summary statistic cannot be calculated.
Conclusion
The information available on the Internet pertaining to TSA and SR is highly variable and provides mostly moderate-to-poor quality information based on the DISCERN instrument. Many websites failed to describe the benefits and the risks of different treatment options, including nonoperative management. Health care professionals should be aware that patients often refer to the Internet as a primary resource for obtaining medical information. It is important to direct patients to websites that provide accurate information, because patients who educate themselves about their conditions and actively participate in decision-making may have improved health outcomes.20-22 Overall, academic websites and commercial websites, such as WebMD and OrthoInfo, generally had higher DISCERN scores when using either search term. Of major concern is the potential for misleading advertisements or incorrect information that can negatively affect health outcomes. This study found that using nonmedical terminology (SR) provided more noncommercial and patient-oriented websites, especially through Yahoo. This study highlights the need for more comprehensive online information pertaining to shoulder replacement that can better serve as a resource for Internet users.
The Internet is becoming a primary source for obtaining medical information. This growing trend may have serious implications for the medical field. As patients increasingly regard the Internet as an essential tool for obtaining health-related information, questions have been raised regarding the quality of medical information available on the Internet.1 Studies have shown that health-related sites often present inaccurate, inconsistent, and outdated information that may have a negative impact on health care decisions made by patients.2
According to the US Census Bureau, 71.7% of American households report having access to the Internet.3 Of those who have access to Internet, approximately 72% have sought health information online over the last year.4 Among people older than age 65 years living in the United States, there has been a growing trend toward using the Internet, from 14% in 2000 to almost 60% in 2013, according to the Pew Research Internet Project.5 Most medical websites are viewed for information on diseases and treatment options.6 Since most patients want to be informed about treatment options, as well as risks and benefits for each treatment, access to credible information is essential for proper decision-making.7
To assess the quality of information on the Internet, we used DISCERN, a standardized questionnaire to aid consumers in judging Internet content.8 The DISCERN instrument, available at www.discern.org.uk, was designed by an expert group in the United Kingdom. First, an expert panel developed and tested the instrument, and then health care providers and self-help group members tested it further.8,9 The questionnaire had been found to have good interrater reliability, regardless of use by health professionals or consumers.8-10
More than 53,000 shoulder arthroplasties are performed in the United States annually, and the number is growing, with the main goal of pain relief from glenohumeral degenerative joint disease.11,12 The Internet has become a quasi–second opinion for patients trying to participate in their care. Given the prevalence of shoulder-related surgeries, it is critical to analyze and become familiar with the quality of information that patients read online in order to direct them to nonbiased, all-inclusive websites. In this study, we provide a summary assessment and comparison of the quality of online information pertaining to shoulder replacement, using medical (total shoulder replacement) and nontechnical (shoulder replacement) search terms.
Methods
Websites were identified using 3 search engines (Google, Yahoo, and Bing) and 2 search terms, shoulder replacement (SR) and total shoulder arthroplasty (TSA), on January 17, 2014. These 3 search engines were used because 77% of health care–related information online searches begin through a search engine (Google, Bing, Yahoo); only 13% begin at a health care–specialized website.4 These search terms were used after consulting with orthopedic residents and attending physicians in a focus group regarding the terminology used with patients. The first 30 websites in each search engine were identified consecutively and evaluated for category and quality of information using the DISCERN instrument.
A total of 180 websites (90 per search term) were reviewed. Each website was evaluated independently by 3 medical students. In the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram, we recorded how websites were identified, screened, and included (Figure 1).13 Websites that were duplicated within each search term and those that were inaccessible were used to determine the total number of noncommercial versus commercial websites, but were excluded from the final analysis. The first part of the analysis involved determining the type of website (eg, commercial vs noncommercial) based upon the html endings. All .com endings were classified as commercial websites; noncommercial included .gov, .org, .edu, and .net endings. Next, each website was categorized based on the target audience. Websites were grouped into health professional–oriented information, patient-oriented, advertisement, or “other.” These classifications were based on those described in previous works.14,15 The “other” category included images, YouTube videos, another search engine, and open forums, which were also excluded from the final analysis because they were not easily evaluable with the DISCERN instrument. Websites were considered health professional–oriented if they included journal articles, scholarly articles, and/or rehabilitation protocols. Patient-directed websites clearly stated the information was directed to patients or provided a general overview. Advertisement included sites that displayed ads or products for sale. Websites were evaluated for quality using the DISCERN instrument (Figure 2).
DISCERN has 3 subdivision scores: the reliable score (composed of the first 8 questions), the treatment options (the next 7 questions), and 1 final question that addresses the overall quality of the website and is rated independently of the first 15 questions. DISCERN uses 2 scales, a binary scale anchored on both extremes with the number 1 equaling complete absence of the criteria being measured, and the number 5 at the upper extreme, representing completeness of the quality being assessed. In between 1 and 5 is a partial ordinal scale measuring from 2 to 4, which indicates the information is present to some extent but not complete. The ordinal scale allows ranking of the criteria being assessed. Summarizing values from each of the 2 scales poses some concern: the scale is not a true binary scale because of the ordinal scale of the middle numbers (2-4), and as such, is not amenable to being an interval scale to calculate arithmetic means. To summarize the values from the 2 scales, we calculated the harmonic mean, the arithmetic mean, the geometric mean, and the median. The means were empirically compared with the median, and we used the harmonic mean to summarize scale values because it was the best approximation of the medians.
Results
A total of 90 websites were assessed with the search term total shoulder arthroplasty and another 90 with shoulder replacement. When 37 duplicate websites for TSA and 52 for SR were eliminated, 53 (59%) and 38 (42%) unique websites were evaluated for each search term, respectively (Figure 1). (These unique websites are included in the Appendix.) Between the 2 search terms, 20 websites were duplicated. Figure 3 shows the distribution of websites by category. Total shoulder arthroplasty provided the highest percentage of health professional–oriented information; SR had the greatest percentage of patient-oriented information. Both TSA and SR had nearly the same number of advertisements and websites labeled “other.” The percentage of noncommercial websites from each search engine is represented in Figure 4. For SR, Google had 40% (12/30) noncommercial websites compared with Yahoo at 53% (16/30) and Bing at 46% (14/30). Total shoulder arthroplasty had 43% (13/30) noncommercial websites on Google, 27% (8/30) on Yahoo, and 40% (12/30) on Bing. In total, SR had more noncommercial websites, 47% (42/90), compared with 37% (33/90) for TSA.
The mean of all 3 raters for reliablity (DISCERN questions 1-8) and treatment options (DISCERN questions 9-15) is represented in the Table. For both search terms, we found that websites identified as health professional–oriented had the highest reliable mean scores, followed by patient-oriented, and advertisement at the lowest (SR: P = .054; TSA: P = .134). For SR, treatment mean scores demonstrated similar results with health professional–oriented websites receiving the highest, followed by patient-oriented and advertisement (P = .005). However, the treatment mean scores for TSA differed with patient-oriented websites receiving higher scores than health professional–oriented websites, but this was not statistically significant (P= .407). Regarding search terms, there were no significant differences between mean reliable and treatment scores across all categories.
The average overall DISCERN score for TSA websites was 2.5 (range, 1-5), compared with 2.3 (range, 1-5) for SR websites. The overall reliable score (DISCERN questions 1-8) for TSA websites was 2.6 and 2.5 for SR websites (P < .001). For TSA websites, 38% (20/53) were classified as good, having an overall DISCERN score ≥3, versus 26% (10/38) of SR websites. The overall DISCERN score for health professional–oriented websites was 2.7, patient-oriented websites received a score of 2.6, and advertisements had the lowest score at 2.4.
Discussion
Both patients and health professionals obtain information on health care subjects through the Internet, which has become the primary resource for patients.15,16 However, there are no strict regulations of the content being written. This creates a challenge for the typical user to find credible and evidence-based information, which is important because misleading information could cause undue anxiety, among other effects.17,18 The aims of this study were to determine the quality of Internet information for shoulder replacement surgeries using the medical terminology total shoulder arthroplasty (TSA) and the nontechnical term shoulder replacement (SR), and to compare the results.
After analyzing the types of websites returned for both total shoulder arthroplasty and shoulder replacement (Figure 4), it was interesting to find that using nonmedical terminology as the search term provided more noncommercial websites compared with total shoulder arthroplasty. Furthermore, Yahoo provided the highest yield of noncommercial websites at 16, with Bing at 14, when using SR as the search term. We believe the increase in noncommercial websites returned for SR was greater than for TSA because SR yielded more patient-oriented websites, which usually had html endings of .edu and .org, as shown in Figure 3 (48% of SR websites offered patient-oriented information).
Although there were more noncommercial websites for SR, the majority of the DISCERN values between the 2 search terms did not differ significantly. This is a direct result of the number of sites (20) that were duplicated across both search terms. However as seen in the Table, TSA had similar reliable mean scores for advertisements and patient-oriented websites but a slightly higher reliable score for health professional–oriented websites. We correlated this with the increased number of health professional–oriented websites returned when using TSA as the search term (Figure 3). The health professional–oriented websites explained their aims and cited their sources more consistently than did patient-oriented sites and advertisements, resulting in higher reliable scores. Although patient-oriented websites frequently lacked citations, they provided information about multiple treatment options, which were more relevant to consumers. This resulted in nearly equivalent reliable scores. Treatment means for advertisements in both SR and TSA were similar. However, treatment means for professional-oriented websites in TSA were lower than those for SR because health professional–oriented websites often were only moderately relevant to consumers, with their focus usually on 1 treatment option or on rehabilitation protocols. Although the DISCERN scores were similar between the search terms, total shoulder arthroplasty provided more websites (20) classified as good—overall DISCERN score, ≥3—than SR did (10). Advertisement websites had similar overall DISCERN scores, which we anticipated because most of the advertisements were duplicated across the search terms.
Using the 2 search terms, academic websites and commercial websites, such as WebMD, consistently received higher reliable and overall DISCERN scores. Advertisement websites, which need to deliver a clear message, frequently scored high on explicitly stating their aims and relevance to consumers, but focused on their products without discussing the benefits of other treatment options. This is significant because Internet search engines, such as Google, offer sponsor links for which organizations pay to appear at the top of the search results. This creates the potential for consumers to receive biased information because most individuals only visit the top 10 websites generated by a search engine.19
We concluded that the quality of online information relating to SR and TSA was highly variable and frequently of moderate-to-poor quality, with most overall DISCERN scores <3. The quality of information found online for this study using the DISCERN instrument is consistent with those studies using DISCERN to evaluate other medical conditions (eg, bunions, chronic pain, general anesthesia, and anterior cruciate ligament reconstruction).2,9,15,19 These studies also concluded that online information varies tremendously in quality and completeness.
This study has several limitations. Websites were searched at a single time point and, because Internet resources are frequently updated, the results of this study could vary. Furthermore, although Google, Yahoo, and Bing are 3 of the most popular search engines, these are not the only resources patients use when searching the Internet for health-related information. Other search engines, such as Pubmed.gov and MSN.com, could provide additional websites for Internet users. Lastly, although DISCERN is validated to address the quality of information available online, it does not evaluate the accuracy of the information.8 Our use of DISCERN involves 2 scales, a binary yes/no (ratings, 1 and 5) and an ordinal scale (ratings, 2-4). As such, a single mean summary statistic cannot be calculated.
Conclusion
The information available on the Internet pertaining to TSA and SR is highly variable and provides mostly moderate-to-poor quality information based on the DISCERN instrument. Many websites failed to describe the benefits and the risks of different treatment options, including nonoperative management. Health care professionals should be aware that patients often refer to the Internet as a primary resource for obtaining medical information. It is important to direct patients to websites that provide accurate information, because patients who educate themselves about their conditions and actively participate in decision-making may have improved health outcomes.20-22 Overall, academic websites and commercial websites, such as WebMD and OrthoInfo, generally had higher DISCERN scores when using either search term. Of major concern is the potential for misleading advertisements or incorrect information that can negatively affect health outcomes. This study found that using nonmedical terminology (SR) provided more noncommercial and patient-oriented websites, especially through Yahoo. This study highlights the need for more comprehensive online information pertaining to shoulder replacement that can better serve as a resource for Internet users.
1. Eysenbach G, Powell J, Kuss O, Sa ER. Empirical studies assessing the quality of health information for consumers on the world wide web: a systematic review. JAMA. 2002;287(20):2691-2700.
2. Bruce-Brand RA, Baker JF, Byrne DP, Hogan NA, McCarthy T. Assessment of the quality and content of information on anterior cruciate ligament reconstruction on the internet. Arthroscopy. 2013;29(6):1095-1100.
3. Computer and internet use in the United States: population characteristics. US Census Bureau website. http://www.census.gov/hhes/computer/. Accessed December 11, 2015.
4. Fox S, Duggan M. Health online 2013. Pew Research Center website. http://pewinternet.org/Reports/2013/Health-online.aspx. Published January 15, 2013. Accessed November 24, 2015.
5. Smith A. Older adults and technology use. Pew Research Center website. http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use. Published April 3, 2014. Accessed November 24, 2015.
6. Shuyler KS, Knight KM. What are patients seeking when they turn to the internet? Qualitative content analysis of questions asked by visitors to an orthopaedics web site. J Med Internet Res. 2003;5(4):e24.
7. Meredith P, Emberton M, Wood C, Smith J. Comparison of patients’ needs for information on prostate surgery with printed materials provided by surgeons. Qual Health Care. 1995;4(1):18-23.
8. Charnock D, Shepperd S, Needham G, Gann R. DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. J Epidemiol Community Health. 1999;53(2):105-111.
9. Kaicker J, Debono VB, Dang W, Buckley N, Thabane L. Assessment of the quality and variability of health information on chronic pain websites using the DISCERN instrument. BMC Med. 2010;8(1):59.
10. Griffiths KM, Christensen H. Website quality indicators for consumers. J Med Internet Res. 2005;7(5):e55.
11. Wiater JM. Shoulder joint replacement. American Academy of Orthopedic Surgeons website. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Updated December 2011. Accessed November 24, 2015.
12. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the united states. J Bone Joint Surg Am. 2011;93(24):2249-2254.
13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-W94.
14. Nason GJ, Baker JF, Byrne DP, Noel J, Moore D, Kiely PJ. Scoliosis-specific information on the internet: has the “information highway” led to better information provision? Spine. 2012;37(21):E1364-E1369.
15. Starman JS, Gettys FK, Capo JA, Fleischli JE, Norton HJ, Karunakar MA. Quality and content of internet-based information for ten common orthopaedic sports medicine diagnoses. J Bone Joint Surg Am. 2010;92(7):1612-1618.
16. Bernstein J, Ahn J, Veillette C. The future of orthopaedic information management. J Bone Joint Surg Am. 2012;94(13):e95.
17. Berland GK, Elliott MN, Morales LS, et al. Health information on the Internet: accessibility, quality, and readability in English and Spanish. JAMA. 2001;285(20):2612-2621.
18. Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ. 1990;301(6752):575-580.
19. Chong YM, Fraval A, Chandrananth J, Plunkett V, Tran P. Assessment of the quality of web-based information on bunions. Foot Ankle Int. 2013;34(8):1134-1139.
20. Brody DS, Miller SM, Lerman CE, Smith DG, Caputo GC. Patient perception of involvement in medical care. J Gen Intern Med. 1989;4(6):506-511.
21. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528.
22. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27(3 suppl):S110-S127.
1. Eysenbach G, Powell J, Kuss O, Sa ER. Empirical studies assessing the quality of health information for consumers on the world wide web: a systematic review. JAMA. 2002;287(20):2691-2700.
2. Bruce-Brand RA, Baker JF, Byrne DP, Hogan NA, McCarthy T. Assessment of the quality and content of information on anterior cruciate ligament reconstruction on the internet. Arthroscopy. 2013;29(6):1095-1100.
3. Computer and internet use in the United States: population characteristics. US Census Bureau website. http://www.census.gov/hhes/computer/. Accessed December 11, 2015.
4. Fox S, Duggan M. Health online 2013. Pew Research Center website. http://pewinternet.org/Reports/2013/Health-online.aspx. Published January 15, 2013. Accessed November 24, 2015.
5. Smith A. Older adults and technology use. Pew Research Center website. http://www.pewinternet.org/2014/04/03/older-adults-and-technology-use. Published April 3, 2014. Accessed November 24, 2015.
6. Shuyler KS, Knight KM. What are patients seeking when they turn to the internet? Qualitative content analysis of questions asked by visitors to an orthopaedics web site. J Med Internet Res. 2003;5(4):e24.
7. Meredith P, Emberton M, Wood C, Smith J. Comparison of patients’ needs for information on prostate surgery with printed materials provided by surgeons. Qual Health Care. 1995;4(1):18-23.
8. Charnock D, Shepperd S, Needham G, Gann R. DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. J Epidemiol Community Health. 1999;53(2):105-111.
9. Kaicker J, Debono VB, Dang W, Buckley N, Thabane L. Assessment of the quality and variability of health information on chronic pain websites using the DISCERN instrument. BMC Med. 2010;8(1):59.
10. Griffiths KM, Christensen H. Website quality indicators for consumers. J Med Internet Res. 2005;7(5):e55.
11. Wiater JM. Shoulder joint replacement. American Academy of Orthopedic Surgeons website. http://orthoinfo.aaos.org/topic.cfm?topic=A00094. Updated December 2011. Accessed November 24, 2015.
12. Kim SH, Wise BL, Zhang Y, Szabo RM. Increasing incidence of shoulder arthroplasty in the united states. J Bone Joint Surg Am. 2011;93(24):2249-2254.
13. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65-W94.
14. Nason GJ, Baker JF, Byrne DP, Noel J, Moore D, Kiely PJ. Scoliosis-specific information on the internet: has the “information highway” led to better information provision? Spine. 2012;37(21):E1364-E1369.
15. Starman JS, Gettys FK, Capo JA, Fleischli JE, Norton HJ, Karunakar MA. Quality and content of internet-based information for ten common orthopaedic sports medicine diagnoses. J Bone Joint Surg Am. 2010;92(7):1612-1618.
16. Bernstein J, Ahn J, Veillette C. The future of orthopaedic information management. J Bone Joint Surg Am. 2012;94(13):e95.
17. Berland GK, Elliott MN, Morales LS, et al. Health information on the Internet: accessibility, quality, and readability in English and Spanish. JAMA. 2001;285(20):2612-2621.
18. Fallowfield LJ, Hall A, Maguire GP, Baum M. Psychological outcomes of different treatment policies in women with early breast cancer outside a clinical trial. BMJ. 1990;301(6752):575-580.
19. Chong YM, Fraval A, Chandrananth J, Plunkett V, Tran P. Assessment of the quality of web-based information on bunions. Foot Ankle Int. 2013;34(8):1134-1139.
20. Brody DS, Miller SM, Lerman CE, Smith DG, Caputo GC. Patient perception of involvement in medical care. J Gen Intern Med. 1989;4(6):506-511.
21. Greenfield S, Kaplan S, Ware JE Jr. Expanding patient involvement in care. Effects on patient outcomes. Ann Intern Med. 1985;102(4):520-528.
22. Kaplan SH, Greenfield S, Ware JE Jr. Assessing the effects of physician-patient interactions on the outcomes of chronic disease. Med Care. 1989;27(3 suppl):S110-S127.
Incidence, Risk Factors, and Outcome Trends of Acute Kidney Injury in Elective Total Hip and Knee Arthroplasty
Degenerative arthritis is a widespread chronic condition with an incidence of almost 43 million and annual health care costs of $60 billion in the United States alone.1 Although many cases can be managed symptomatically with medical therapy and intra-articular injections,2 many patients experience disease progression resulting in decreased ambulatory ability and work productivity. For these patients, elective hip and knee arthroplasties can drastically improve quality of life and functionality.3,4 Over the past decade, there has been a marked increase in the number of primary and revision total hip and knee arthroplasties performed in the United States. By 2030, the demand for primary total hip arthroplasties will grow an estimated 174%, to 572,000 procedures. Likewise, the demand for primary total knee arthroplasties is projected to grow by 673%, to 3.48 million procedures.5 However, though better surgical techniques and technology have led to improved functional outcomes, there is still substantial risk for complications in the perioperative period, especially in the geriatric population, in which substantial comorbidities are common.6-9
Acute kidney injury (AKI) is a common public health problem in hospitalized patients and in patients undergoing procedures. More than one-third of all AKI cases occur in surgical settings.10,11 Over the past decade, both community-acquired and in-hospital AKIs rapidly increased in incidence in all major clinical settings.12-14 Patients with AKI have high rates of adverse outcomes during hospitalization and discharge.11,15 Sequelae of AKIs include worsening chronic kidney disease (CKD) and progression to end-stage renal disease, necessitating either long-term dialysis or transplantation.12 This in turn leads to exacerbated disability, diminished quality of life, and disproportionate burden on health care resources.
Much of our knowledge about postoperative AKI has been derived from cardiovascular, thoracic, and abdominal surgery settings. However, there is a paucity of data on epidemiology and trends for either AKI or associated outcomes in patients undergoing major orthopedic surgery. The few studies to date either were single-center or had inadequate sample sizes for appropriately powered analysis of the risk factors and outcomes related to AKI.16
In the study reported here, we analyzed a large cohort of patients from a nationwide multicenter database to determine the incidence of and risk factors for AKI. We also examined the mortality and adverse discharges associated with AKI after major joint surgery. Lastly, we assessed temporal trends in both incidence and outcomes of AKI, including the death risk attributable to AKI.
Methods
Database
We extracted our study cohort from the Nationwide Inpatient Sample (NIS) and the National Inpatient Sample of Healthcare Cost and Utilization Project (HCUP) compiled by the Agency for Healthcare Research and Quality.17 NIS, the largest inpatient care database in the United States, stores data from almost 8 million stays in about 1000 hospitals across the country each year. Its participating hospital pool consists of about 20% of US community hospitals, resulting in a sampling frame comprising about 90% of all hospital discharges in the United States. This allows for calculation of precise, weighted nationwide estimates. Data elements within NIS are drawn from hospital discharge abstracts that indicate all procedures performed. NIS also stores information on patient characteristics, length of stay (LOS), discharge disposition, postoperative morbidity, and observed in-hospital mortality. However, it stores no information on long-term follow-up or complications after discharge.
Data Analysis
For the period 2002–2012, we queried the NIS database for hip and knee arthroplasties with primary diagnosis codes for osteoarthritis and secondary codes for AKI. We excluded patients under age 18 years and patients with diagnosis codes for hip and knee fracture/necrosis, inflammatory/infectious arthritis, or bone neoplasms (Table 1). We then extracted baseline characteristics of the study population. Patient-level characteristics included age, sex, race, quartile classification of median household income according to postal (ZIP) code, and primary payer (Medicare/Medicaid, private insurance, self-pay, no charge). Hospital-level characteristics included hospital location (urban, rural), hospital bed size (small, medium, large), region (Northeast, Midwest/North Central, South, West), and teaching status. We defined illness severity and likelihood of death using Deyo’s modification of the Charlson Comorbidity Index (CCI), which draws on principal and secondary ICD-9-CM (International Classification of Diseases, Ninth Revision-Clinical Modification) diagnosis codes, procedure codes, and patient demographics to estimate a patient’s mortality risk. This method reliably predicts mortality and readmission in the orthopedic population.18,19 We assessed the effect of AKI on 4 outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of stay. Discharge disposition was grouped by either (a) home or short-term facility or (b) adverse discharge. Home or short-term facility covered routine, short-term hospital, against medical advice, home intravenous provider, another rehabilitation facility, another institution for outpatient services, institution for outpatient services, discharged alive, and destination unknown; adverse discharge covered skilled nursing facility, intermediate care, hospice home, hospice medical facility, long-term care hospital, and certified nursing facility. This dichotomization of discharge disposition is often used in studies of NIS data.20
Statistical Analyses
We compared the baseline characteristics of hospitalized patients with and without AKI. To test for significance, we used the χ2 test for categorical variables, the Student t test for normally distributed continuous variables, the Wilcoxon rank sum test for non-normally distributed continuous variables, and the Cochran-Armitage test for trends in AKI incidence. We used survey logistic regression models to calculate adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) in order to estimate the predictors of AKI and the impact of AKI on hospital outcomes. We constructed final models after adjusting for confounders, testing for potential interactions, and ensuring no multicolinearity between covariates. Last, we computed the risk proportion of death attributable to AKI, indicating the proportion of deaths that could potentially be avoided if AKI and its complications were abrogated.21
We performed all statistical analyses with SAS Version 9.3 (SAS Institute) using designated weight values to produce weighted national estimates. The threshold for statistical significance was set at P < .01 (with ORs and 95% CIs that excluded 1).
Results
AKI Incidence, Risk Factors, and Trends
We identified 7,235,251 patients who underwent elective hip or knee arthroplasty for osteoarthritis between 2002 and 2012—an estimate consistent with data from the Centers for Disease Control and Prevention.22 Of that total, 94,367 (1.3%) had AKI. The proportion of discharges diagnosed with AKI increased rapidly over the decade, from 0.5% in 2002 to 1.8% to 1.9% in the period 2010–2012. This upward trend was highly significant (Ptrend < .001) (Figure 1). Patients with AKI (vs patients without AKI) were more likely to be older (mean age, 70 vs 66 years; P < .001), male (50.8% vs 38.4%; P < .001), and black (10.07% vs 5.15%; P<. 001). They were also found to have a significantly higher comorbidity score (mean CCI, 2.8 vs 1.5; P < .001) and higher proportions of comorbidities, including hypertension, CKD, atrial fibrillation, diabetes mellitus (DM), congestive heart failure, chronic liver disease, and hepatitis C virus infection. In addition, AKI was associated with perioperative myocardial infarction (MI), sepsis, cardiac catheterization, and blood transfusion. Regarding socioeconomic characteristics, patients with AKI were more likely to have Medicare/Medicaid insurance (72.26% vs 58.06%; P < .001) and to belong to the extremes of income categories (Table 2).
Using multivariable logistic regression, we found that increased age (1.11 increase in adjusted OR for every year older; 95% CI, 1.09-1.14; P < .001), male sex (adjusted OR, 1.65; 95% CI, 1.60-1.71; P < .001), and black race (adjusted OR, 1.57; 95% CI, 1.45-1.69; P < .001) were significantly associated with postoperative AKI. Regarding comorbidities, baseline CKD (adjusted OR, 8.64; 95% CI, 8.14-9.18; P < .001) and congestive heart failure (adjusted OR, 2.74; 95% CI, 2.57-2.92; P< .0001) were most significantly associated with AKI. Perioperative events, including sepsis (adjusted OR, 35.64; 95% CI, 30.28-41.96; P < .0001), MI (adjusted OR, 6.14; 95% CI, 5.17-7.28; P < .0001), and blood transfusion (adjusted OR, 2.28; 95% CI, 2.15-2.42; P < .0001), were also strongly associated with postoperative AKI. Last, compared with urban hospitals and small hospital bed size, rural hospitals (adjusted OR, 0.70; 95% CI, 0.60-0.81; P< .001) and large bed size (adjusted OR, 0.82; 95% CI, 0.70-0.93; P = .003) were associated with lower probability of developing AKI (Table 3).
Figure 2 elucidates the frequency of AKI based on a combination of key preoperative comorbid conditions and postoperative complications—demonstrating that the proportion of AKI cases associated with other postoperative complications is significantly higher in the CKD and concomitant DM/CKD patient populations. Patients hospitalized with CKD exhibited higher rates of AKI in cases involving blood transfusion (20.9% vs 1.8%; P < .001), acute MI (48.9% vs 13.8%; P < .001), and sepsis (74.7% vs 36.3%;P< .001) relative to patients without CKD. Similarly, patients with concomitant DM/CKD exhibited higher rates of AKI in cases involving blood transfusion (23% vs 1.9%; P< .001), acute MI (51.1% vs 12.1%; P< .001), and sepsis (75% vs 38.2%; P < .001) relative to patients without either condition. However, patients hospitalized with DM alone exhibited only marginally higher rates of AKI in cases involving blood transfusion (4.7% vs 2%; P < .01) and acute MI (19.2% vs 16.7%; P< .01) and a lower rate in cases involving sepsis (38.2% vs 41.7%; P < .01) relative to patients without DM. These data suggest that CKD is the most significant clinically relevant risk factor for AKI and that CKD may synergize with DM to raise the risk for AKI.
Outcomes
We then analyzed the impact of AKI on hospital outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of care. Mortality was significantly higher in patients with AKI than in patients without it (2.08% vs 0.06%; P < .001). Even after adjusting for confounders (eg, demographics, comorbidity burden, perioperative sepsis, hospital-level characteristics), AKI was still associated with strikingly higher odds of in-hospital death (adjusted OR, 11.32; 95% CI, 9.34-13.74; P < .001). However, analysis of temporal trends indicated that the odds for adjusted mortality associated with AKI decreased from 18.09 to 9.45 (Ptrend = .01) over the period 2002–2012 (Figure 3). This decrease in odds of death was countered by an increase in incidence of AKI, resulting in a stable attributable risk proportion (97.9% in 2002 to 97.3% in 2012; Ptrend = .90) (Table 4). Regarding discharge disposition, patients with AKI were much less likely to be discharged home (41.35% vs 62.59%; P < .001) and more likely to be discharged to long-term care (56.37% vs 37.03%; P< .001). After adjustment for confounders, AKI was associated with significantly increased odds of adverse discharge (adjusted OR, 2.24; 95% CI, 2.12-2.36; P< .001). Analysis of temporal trends revealed no appreciable decrease in the adjusted odds of adverse discharge between 2002 (adjusted OR, 1.87; 95% CI, 1.37-2.55; P < .001) and 2012 (adjusted OR, 1.93; 95% CI, 1.76-2.11; P < .001) (Figure 4, Table 5). Last, both mean LOS (5 days vs 3 days; P < .001) and mean cost of hospitalization (US $22,269 vs $15,757; P < .001) were significantly higher in patients with AKI.
Discussion
In this study, we found that the incidence of AKI among hospitalized patients increased 4-fold between 2002 and 2012. Moreover, we identified numerous patient-specific, hospital-specific, perioperative risk factors for AKI. Most important, we found that AKI was associated with a strikingly higher risk of in-hospital death, and surviving patients were more likely to experience adverse discharge. Although the adjusted mortality rate associated with AKI decreased over that decade, the attributable risk proportion remained stable.
Few studies have addressed this significant public health concern. In one recent study in Australia, Kimmel and colleagues16 identified risk factors for AKI but lacked data on AKI outcomes. In a study of complications and mortality occurring after orthopedic surgery, Belmont and colleagues22 categorized complications as either local or systemic but did not examine renal complications. Only 2 other major studies have been conducted on renal outcomes associated with major joint surgery, and both were limited to patients with acute hip fractures. The first included acute fracture surgery patients and omitted elective joint surgery patients, and it evaluated admission renal function but not postoperative AKI.22 The second study had a sample size of only 170 patients.23 Thus, the literature leaves us with a crucial knowledge gap in renal outcomes and their postoperative impact in elective arthroplasties.
The present study filled this information gap by examining the incidence, risk factors, outcomes, and temporal trends of AKI after elective hip and knee arthroplasties. The increasing incidence of AKI in this surgical setting is similar to that of AKI in other surgical settings (cardiac and noncardiac).21 Although our analysis was limited by lack of perioperative management data, patients undergoing elective joint arthroplasty can experience kidney dysfunction for several reasons, including volume depletion, postoperative sepsis, and influence of medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), especially in older patients with more comorbidities and a higher burden of CKD. Each of these factors can cause renal dysfunction in patients having orthopedic procedures.24 Moreover, NSAID use among elective joint arthroplasty patients is likely higher because of an emphasis on multimodal analgesia, as recent randomized controlled trials have demonstrated the efficacy of NSAID use in controlling pain without increasing bleeding.25-27 Our results also demonstrated that the absolute incidence of AKI after orthopedic surgery is relatively low. One possible explanation for this phenomenon is that the definitions used were based on ICD-9-CM codes that underestimate the true incidence of AKI.
Consistent with other studies, we found that certain key preoperative comorbid conditions and postoperative events were associated with higher AKI risk. We stratified the rate of AKI associated with each postoperative event (sepsis, acute MI, cardiac catheterization, need for transfusion) by DM/CKD comorbidity. CKD was associated with significantly higher AKI risk across all postoperative complications. This information may provide clinicians with bedside information that can be used to determine which patients may be at higher or lower risk for AKI.
Our analysis of patient outcomes revealed that, though AKI was relatively uncommon, it increased the risk for death during hospitalization more than 10-fold between 2002 and 2012. Although the adjusted OR of in-hospital mortality decreased over the decade studied, the concurrent increase in AKI incidence caused the attributable risk of death associated with AKI to essentially remain the same. This observation is consistent with recent reports from cardiac surgery settings.21 These data together suggest that ameliorating occurrences of AKI would decrease mortality and increase quality of care for patients undergoing elective joint surgeries.
We also examined the effect of AKI on resource use by studying LOS, costs, and risk for adverse discharge. Much as in other surgical settings, AKI increased both LOS and overall hospitalization costs. More important, AKI was associated with increased adverse discharge (discharge to long-term care or nursing homes). Although exact reasons are unclear, we can speculate that postoperative renal dysfunction precludes early rehabilitation, impeding desired functional outcome and disposition.28,29 Given the projected increases in primary and revision hip and knee arthroplasties,5 these data predict that the impact of AKI on health outcomes will increase alarmingly in coming years.
There are limitations to our study. First, it was based on administrative data and lacked patient-level and laboratory data. As reported, the sensitivity of AKI codes remains moderate,30 so the true burden may be higher than indicated here. As the definition of AKI was based on administrative coding, we also could not estimate severity, though previous studies have found that administrative codes typically capture a more severe form of disease.31 Another limitation is that, because the data were deidentified, we could not delineate the risk for recurrent AKI in repeated surgical procedures, though this cohort unlikely was large enough to qualitatively affect our results. The third limitation is that, though we used CCI to adjust for the comorbidity burden, we were unable to account for other unmeasured confounders associated with increased AKI incidence, such as specific medication use. In addition, given the lack of patient-level data, we could not analyze the specific factors responsible for AKI in the perioperative period. Nevertheless, the strengths of a nationally representative sample, such as large sample size and generalizability, outweigh these limitations.
Conclusion
AKI is potentially an important quality indicator of elective joint surgery, and reducing its incidence is therefore essential for quality improvement. Given that hip and knee arthroplasties are projected to increase exponentially, as is the burden of comorbid conditions in this population, postoperative AKI will continue to have an incremental impact on health and health care resources. Thus, a carefully planned approach of interdisciplinary perioperative care is warranted to reduce both the risk and the consequences of this devastating condition.
1. Reginster JY. The prevalence and burden of arthritis. Rheumatology. 2002;41(supp 1):3-6.
2. Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265-2268.
3. Kawasaki M, Hasegawa Y, Sakano S, Torii Y, Warashina H. Quality of life after several treatments for osteoarthritis of the hip. J Orthop Sci. 2003;8(1):32-35.
4. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
5. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
6. Matlock D, Earnest M, Epstein A. Utilization of elective hip and knee arthroplasty by age and payer. Clin Orthop Relat Res. 2008;466(4):914-919.
7. Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;(369):39-48.
8. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
9. Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am. 2001;83(8):1157-1161.
10. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
11. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67-75.
12. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891-898.
13. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
14. Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112-1119.
15. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20.
16. Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J. 2014;7(6):546-551.
17. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) databases, 2002–2012. Rockville, MD: Agency for Healthcare Research and Quality.
18. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11(4):203-209.
19. Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(5):1638-1644.
20. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370.
21. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28.
22. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
23. Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41(4):335-338.
24. Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101.
25. Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskelet Disord. 2008;9:77.
26. Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28(8):1274-1277.
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
28. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
29. Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014;93(11):962-970.
30. Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688-1694.
31. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682-689.
Degenerative arthritis is a widespread chronic condition with an incidence of almost 43 million and annual health care costs of $60 billion in the United States alone.1 Although many cases can be managed symptomatically with medical therapy and intra-articular injections,2 many patients experience disease progression resulting in decreased ambulatory ability and work productivity. For these patients, elective hip and knee arthroplasties can drastically improve quality of life and functionality.3,4 Over the past decade, there has been a marked increase in the number of primary and revision total hip and knee arthroplasties performed in the United States. By 2030, the demand for primary total hip arthroplasties will grow an estimated 174%, to 572,000 procedures. Likewise, the demand for primary total knee arthroplasties is projected to grow by 673%, to 3.48 million procedures.5 However, though better surgical techniques and technology have led to improved functional outcomes, there is still substantial risk for complications in the perioperative period, especially in the geriatric population, in which substantial comorbidities are common.6-9
Acute kidney injury (AKI) is a common public health problem in hospitalized patients and in patients undergoing procedures. More than one-third of all AKI cases occur in surgical settings.10,11 Over the past decade, both community-acquired and in-hospital AKIs rapidly increased in incidence in all major clinical settings.12-14 Patients with AKI have high rates of adverse outcomes during hospitalization and discharge.11,15 Sequelae of AKIs include worsening chronic kidney disease (CKD) and progression to end-stage renal disease, necessitating either long-term dialysis or transplantation.12 This in turn leads to exacerbated disability, diminished quality of life, and disproportionate burden on health care resources.
Much of our knowledge about postoperative AKI has been derived from cardiovascular, thoracic, and abdominal surgery settings. However, there is a paucity of data on epidemiology and trends for either AKI or associated outcomes in patients undergoing major orthopedic surgery. The few studies to date either were single-center or had inadequate sample sizes for appropriately powered analysis of the risk factors and outcomes related to AKI.16
In the study reported here, we analyzed a large cohort of patients from a nationwide multicenter database to determine the incidence of and risk factors for AKI. We also examined the mortality and adverse discharges associated with AKI after major joint surgery. Lastly, we assessed temporal trends in both incidence and outcomes of AKI, including the death risk attributable to AKI.
Methods
Database
We extracted our study cohort from the Nationwide Inpatient Sample (NIS) and the National Inpatient Sample of Healthcare Cost and Utilization Project (HCUP) compiled by the Agency for Healthcare Research and Quality.17 NIS, the largest inpatient care database in the United States, stores data from almost 8 million stays in about 1000 hospitals across the country each year. Its participating hospital pool consists of about 20% of US community hospitals, resulting in a sampling frame comprising about 90% of all hospital discharges in the United States. This allows for calculation of precise, weighted nationwide estimates. Data elements within NIS are drawn from hospital discharge abstracts that indicate all procedures performed. NIS also stores information on patient characteristics, length of stay (LOS), discharge disposition, postoperative morbidity, and observed in-hospital mortality. However, it stores no information on long-term follow-up or complications after discharge.
Data Analysis
For the period 2002–2012, we queried the NIS database for hip and knee arthroplasties with primary diagnosis codes for osteoarthritis and secondary codes for AKI. We excluded patients under age 18 years and patients with diagnosis codes for hip and knee fracture/necrosis, inflammatory/infectious arthritis, or bone neoplasms (Table 1). We then extracted baseline characteristics of the study population. Patient-level characteristics included age, sex, race, quartile classification of median household income according to postal (ZIP) code, and primary payer (Medicare/Medicaid, private insurance, self-pay, no charge). Hospital-level characteristics included hospital location (urban, rural), hospital bed size (small, medium, large), region (Northeast, Midwest/North Central, South, West), and teaching status. We defined illness severity and likelihood of death using Deyo’s modification of the Charlson Comorbidity Index (CCI), which draws on principal and secondary ICD-9-CM (International Classification of Diseases, Ninth Revision-Clinical Modification) diagnosis codes, procedure codes, and patient demographics to estimate a patient’s mortality risk. This method reliably predicts mortality and readmission in the orthopedic population.18,19 We assessed the effect of AKI on 4 outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of stay. Discharge disposition was grouped by either (a) home or short-term facility or (b) adverse discharge. Home or short-term facility covered routine, short-term hospital, against medical advice, home intravenous provider, another rehabilitation facility, another institution for outpatient services, institution for outpatient services, discharged alive, and destination unknown; adverse discharge covered skilled nursing facility, intermediate care, hospice home, hospice medical facility, long-term care hospital, and certified nursing facility. This dichotomization of discharge disposition is often used in studies of NIS data.20
Statistical Analyses
We compared the baseline characteristics of hospitalized patients with and without AKI. To test for significance, we used the χ2 test for categorical variables, the Student t test for normally distributed continuous variables, the Wilcoxon rank sum test for non-normally distributed continuous variables, and the Cochran-Armitage test for trends in AKI incidence. We used survey logistic regression models to calculate adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) in order to estimate the predictors of AKI and the impact of AKI on hospital outcomes. We constructed final models after adjusting for confounders, testing for potential interactions, and ensuring no multicolinearity between covariates. Last, we computed the risk proportion of death attributable to AKI, indicating the proportion of deaths that could potentially be avoided if AKI and its complications were abrogated.21
We performed all statistical analyses with SAS Version 9.3 (SAS Institute) using designated weight values to produce weighted national estimates. The threshold for statistical significance was set at P < .01 (with ORs and 95% CIs that excluded 1).
Results
AKI Incidence, Risk Factors, and Trends
We identified 7,235,251 patients who underwent elective hip or knee arthroplasty for osteoarthritis between 2002 and 2012—an estimate consistent with data from the Centers for Disease Control and Prevention.22 Of that total, 94,367 (1.3%) had AKI. The proportion of discharges diagnosed with AKI increased rapidly over the decade, from 0.5% in 2002 to 1.8% to 1.9% in the period 2010–2012. This upward trend was highly significant (Ptrend < .001) (Figure 1). Patients with AKI (vs patients without AKI) were more likely to be older (mean age, 70 vs 66 years; P < .001), male (50.8% vs 38.4%; P < .001), and black (10.07% vs 5.15%; P<. 001). They were also found to have a significantly higher comorbidity score (mean CCI, 2.8 vs 1.5; P < .001) and higher proportions of comorbidities, including hypertension, CKD, atrial fibrillation, diabetes mellitus (DM), congestive heart failure, chronic liver disease, and hepatitis C virus infection. In addition, AKI was associated with perioperative myocardial infarction (MI), sepsis, cardiac catheterization, and blood transfusion. Regarding socioeconomic characteristics, patients with AKI were more likely to have Medicare/Medicaid insurance (72.26% vs 58.06%; P < .001) and to belong to the extremes of income categories (Table 2).
Using multivariable logistic regression, we found that increased age (1.11 increase in adjusted OR for every year older; 95% CI, 1.09-1.14; P < .001), male sex (adjusted OR, 1.65; 95% CI, 1.60-1.71; P < .001), and black race (adjusted OR, 1.57; 95% CI, 1.45-1.69; P < .001) were significantly associated with postoperative AKI. Regarding comorbidities, baseline CKD (adjusted OR, 8.64; 95% CI, 8.14-9.18; P < .001) and congestive heart failure (adjusted OR, 2.74; 95% CI, 2.57-2.92; P< .0001) were most significantly associated with AKI. Perioperative events, including sepsis (adjusted OR, 35.64; 95% CI, 30.28-41.96; P < .0001), MI (adjusted OR, 6.14; 95% CI, 5.17-7.28; P < .0001), and blood transfusion (adjusted OR, 2.28; 95% CI, 2.15-2.42; P < .0001), were also strongly associated with postoperative AKI. Last, compared with urban hospitals and small hospital bed size, rural hospitals (adjusted OR, 0.70; 95% CI, 0.60-0.81; P< .001) and large bed size (adjusted OR, 0.82; 95% CI, 0.70-0.93; P = .003) were associated with lower probability of developing AKI (Table 3).
Figure 2 elucidates the frequency of AKI based on a combination of key preoperative comorbid conditions and postoperative complications—demonstrating that the proportion of AKI cases associated with other postoperative complications is significantly higher in the CKD and concomitant DM/CKD patient populations. Patients hospitalized with CKD exhibited higher rates of AKI in cases involving blood transfusion (20.9% vs 1.8%; P < .001), acute MI (48.9% vs 13.8%; P < .001), and sepsis (74.7% vs 36.3%;P< .001) relative to patients without CKD. Similarly, patients with concomitant DM/CKD exhibited higher rates of AKI in cases involving blood transfusion (23% vs 1.9%; P< .001), acute MI (51.1% vs 12.1%; P< .001), and sepsis (75% vs 38.2%; P < .001) relative to patients without either condition. However, patients hospitalized with DM alone exhibited only marginally higher rates of AKI in cases involving blood transfusion (4.7% vs 2%; P < .01) and acute MI (19.2% vs 16.7%; P< .01) and a lower rate in cases involving sepsis (38.2% vs 41.7%; P < .01) relative to patients without DM. These data suggest that CKD is the most significant clinically relevant risk factor for AKI and that CKD may synergize with DM to raise the risk for AKI.
Outcomes
We then analyzed the impact of AKI on hospital outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of care. Mortality was significantly higher in patients with AKI than in patients without it (2.08% vs 0.06%; P < .001). Even after adjusting for confounders (eg, demographics, comorbidity burden, perioperative sepsis, hospital-level characteristics), AKI was still associated with strikingly higher odds of in-hospital death (adjusted OR, 11.32; 95% CI, 9.34-13.74; P < .001). However, analysis of temporal trends indicated that the odds for adjusted mortality associated with AKI decreased from 18.09 to 9.45 (Ptrend = .01) over the period 2002–2012 (Figure 3). This decrease in odds of death was countered by an increase in incidence of AKI, resulting in a stable attributable risk proportion (97.9% in 2002 to 97.3% in 2012; Ptrend = .90) (Table 4). Regarding discharge disposition, patients with AKI were much less likely to be discharged home (41.35% vs 62.59%; P < .001) and more likely to be discharged to long-term care (56.37% vs 37.03%; P< .001). After adjustment for confounders, AKI was associated with significantly increased odds of adverse discharge (adjusted OR, 2.24; 95% CI, 2.12-2.36; P< .001). Analysis of temporal trends revealed no appreciable decrease in the adjusted odds of adverse discharge between 2002 (adjusted OR, 1.87; 95% CI, 1.37-2.55; P < .001) and 2012 (adjusted OR, 1.93; 95% CI, 1.76-2.11; P < .001) (Figure 4, Table 5). Last, both mean LOS (5 days vs 3 days; P < .001) and mean cost of hospitalization (US $22,269 vs $15,757; P < .001) were significantly higher in patients with AKI.
Discussion
In this study, we found that the incidence of AKI among hospitalized patients increased 4-fold between 2002 and 2012. Moreover, we identified numerous patient-specific, hospital-specific, perioperative risk factors for AKI. Most important, we found that AKI was associated with a strikingly higher risk of in-hospital death, and surviving patients were more likely to experience adverse discharge. Although the adjusted mortality rate associated with AKI decreased over that decade, the attributable risk proportion remained stable.
Few studies have addressed this significant public health concern. In one recent study in Australia, Kimmel and colleagues16 identified risk factors for AKI but lacked data on AKI outcomes. In a study of complications and mortality occurring after orthopedic surgery, Belmont and colleagues22 categorized complications as either local or systemic but did not examine renal complications. Only 2 other major studies have been conducted on renal outcomes associated with major joint surgery, and both were limited to patients with acute hip fractures. The first included acute fracture surgery patients and omitted elective joint surgery patients, and it evaluated admission renal function but not postoperative AKI.22 The second study had a sample size of only 170 patients.23 Thus, the literature leaves us with a crucial knowledge gap in renal outcomes and their postoperative impact in elective arthroplasties.
The present study filled this information gap by examining the incidence, risk factors, outcomes, and temporal trends of AKI after elective hip and knee arthroplasties. The increasing incidence of AKI in this surgical setting is similar to that of AKI in other surgical settings (cardiac and noncardiac).21 Although our analysis was limited by lack of perioperative management data, patients undergoing elective joint arthroplasty can experience kidney dysfunction for several reasons, including volume depletion, postoperative sepsis, and influence of medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), especially in older patients with more comorbidities and a higher burden of CKD. Each of these factors can cause renal dysfunction in patients having orthopedic procedures.24 Moreover, NSAID use among elective joint arthroplasty patients is likely higher because of an emphasis on multimodal analgesia, as recent randomized controlled trials have demonstrated the efficacy of NSAID use in controlling pain without increasing bleeding.25-27 Our results also demonstrated that the absolute incidence of AKI after orthopedic surgery is relatively low. One possible explanation for this phenomenon is that the definitions used were based on ICD-9-CM codes that underestimate the true incidence of AKI.
Consistent with other studies, we found that certain key preoperative comorbid conditions and postoperative events were associated with higher AKI risk. We stratified the rate of AKI associated with each postoperative event (sepsis, acute MI, cardiac catheterization, need for transfusion) by DM/CKD comorbidity. CKD was associated with significantly higher AKI risk across all postoperative complications. This information may provide clinicians with bedside information that can be used to determine which patients may be at higher or lower risk for AKI.
Our analysis of patient outcomes revealed that, though AKI was relatively uncommon, it increased the risk for death during hospitalization more than 10-fold between 2002 and 2012. Although the adjusted OR of in-hospital mortality decreased over the decade studied, the concurrent increase in AKI incidence caused the attributable risk of death associated with AKI to essentially remain the same. This observation is consistent with recent reports from cardiac surgery settings.21 These data together suggest that ameliorating occurrences of AKI would decrease mortality and increase quality of care for patients undergoing elective joint surgeries.
We also examined the effect of AKI on resource use by studying LOS, costs, and risk for adverse discharge. Much as in other surgical settings, AKI increased both LOS and overall hospitalization costs. More important, AKI was associated with increased adverse discharge (discharge to long-term care or nursing homes). Although exact reasons are unclear, we can speculate that postoperative renal dysfunction precludes early rehabilitation, impeding desired functional outcome and disposition.28,29 Given the projected increases in primary and revision hip and knee arthroplasties,5 these data predict that the impact of AKI on health outcomes will increase alarmingly in coming years.
There are limitations to our study. First, it was based on administrative data and lacked patient-level and laboratory data. As reported, the sensitivity of AKI codes remains moderate,30 so the true burden may be higher than indicated here. As the definition of AKI was based on administrative coding, we also could not estimate severity, though previous studies have found that administrative codes typically capture a more severe form of disease.31 Another limitation is that, because the data were deidentified, we could not delineate the risk for recurrent AKI in repeated surgical procedures, though this cohort unlikely was large enough to qualitatively affect our results. The third limitation is that, though we used CCI to adjust for the comorbidity burden, we were unable to account for other unmeasured confounders associated with increased AKI incidence, such as specific medication use. In addition, given the lack of patient-level data, we could not analyze the specific factors responsible for AKI in the perioperative period. Nevertheless, the strengths of a nationally representative sample, such as large sample size and generalizability, outweigh these limitations.
Conclusion
AKI is potentially an important quality indicator of elective joint surgery, and reducing its incidence is therefore essential for quality improvement. Given that hip and knee arthroplasties are projected to increase exponentially, as is the burden of comorbid conditions in this population, postoperative AKI will continue to have an incremental impact on health and health care resources. Thus, a carefully planned approach of interdisciplinary perioperative care is warranted to reduce both the risk and the consequences of this devastating condition.
Degenerative arthritis is a widespread chronic condition with an incidence of almost 43 million and annual health care costs of $60 billion in the United States alone.1 Although many cases can be managed symptomatically with medical therapy and intra-articular injections,2 many patients experience disease progression resulting in decreased ambulatory ability and work productivity. For these patients, elective hip and knee arthroplasties can drastically improve quality of life and functionality.3,4 Over the past decade, there has been a marked increase in the number of primary and revision total hip and knee arthroplasties performed in the United States. By 2030, the demand for primary total hip arthroplasties will grow an estimated 174%, to 572,000 procedures. Likewise, the demand for primary total knee arthroplasties is projected to grow by 673%, to 3.48 million procedures.5 However, though better surgical techniques and technology have led to improved functional outcomes, there is still substantial risk for complications in the perioperative period, especially in the geriatric population, in which substantial comorbidities are common.6-9
Acute kidney injury (AKI) is a common public health problem in hospitalized patients and in patients undergoing procedures. More than one-third of all AKI cases occur in surgical settings.10,11 Over the past decade, both community-acquired and in-hospital AKIs rapidly increased in incidence in all major clinical settings.12-14 Patients with AKI have high rates of adverse outcomes during hospitalization and discharge.11,15 Sequelae of AKIs include worsening chronic kidney disease (CKD) and progression to end-stage renal disease, necessitating either long-term dialysis or transplantation.12 This in turn leads to exacerbated disability, diminished quality of life, and disproportionate burden on health care resources.
Much of our knowledge about postoperative AKI has been derived from cardiovascular, thoracic, and abdominal surgery settings. However, there is a paucity of data on epidemiology and trends for either AKI or associated outcomes in patients undergoing major orthopedic surgery. The few studies to date either were single-center or had inadequate sample sizes for appropriately powered analysis of the risk factors and outcomes related to AKI.16
In the study reported here, we analyzed a large cohort of patients from a nationwide multicenter database to determine the incidence of and risk factors for AKI. We also examined the mortality and adverse discharges associated with AKI after major joint surgery. Lastly, we assessed temporal trends in both incidence and outcomes of AKI, including the death risk attributable to AKI.
Methods
Database
We extracted our study cohort from the Nationwide Inpatient Sample (NIS) and the National Inpatient Sample of Healthcare Cost and Utilization Project (HCUP) compiled by the Agency for Healthcare Research and Quality.17 NIS, the largest inpatient care database in the United States, stores data from almost 8 million stays in about 1000 hospitals across the country each year. Its participating hospital pool consists of about 20% of US community hospitals, resulting in a sampling frame comprising about 90% of all hospital discharges in the United States. This allows for calculation of precise, weighted nationwide estimates. Data elements within NIS are drawn from hospital discharge abstracts that indicate all procedures performed. NIS also stores information on patient characteristics, length of stay (LOS), discharge disposition, postoperative morbidity, and observed in-hospital mortality. However, it stores no information on long-term follow-up or complications after discharge.
Data Analysis
For the period 2002–2012, we queried the NIS database for hip and knee arthroplasties with primary diagnosis codes for osteoarthritis and secondary codes for AKI. We excluded patients under age 18 years and patients with diagnosis codes for hip and knee fracture/necrosis, inflammatory/infectious arthritis, or bone neoplasms (Table 1). We then extracted baseline characteristics of the study population. Patient-level characteristics included age, sex, race, quartile classification of median household income according to postal (ZIP) code, and primary payer (Medicare/Medicaid, private insurance, self-pay, no charge). Hospital-level characteristics included hospital location (urban, rural), hospital bed size (small, medium, large), region (Northeast, Midwest/North Central, South, West), and teaching status. We defined illness severity and likelihood of death using Deyo’s modification of the Charlson Comorbidity Index (CCI), which draws on principal and secondary ICD-9-CM (International Classification of Diseases, Ninth Revision-Clinical Modification) diagnosis codes, procedure codes, and patient demographics to estimate a patient’s mortality risk. This method reliably predicts mortality and readmission in the orthopedic population.18,19 We assessed the effect of AKI on 4 outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of stay. Discharge disposition was grouped by either (a) home or short-term facility or (b) adverse discharge. Home or short-term facility covered routine, short-term hospital, against medical advice, home intravenous provider, another rehabilitation facility, another institution for outpatient services, institution for outpatient services, discharged alive, and destination unknown; adverse discharge covered skilled nursing facility, intermediate care, hospice home, hospice medical facility, long-term care hospital, and certified nursing facility. This dichotomization of discharge disposition is often used in studies of NIS data.20
Statistical Analyses
We compared the baseline characteristics of hospitalized patients with and without AKI. To test for significance, we used the χ2 test for categorical variables, the Student t test for normally distributed continuous variables, the Wilcoxon rank sum test for non-normally distributed continuous variables, and the Cochran-Armitage test for trends in AKI incidence. We used survey logistic regression models to calculate adjusted odds ratios (ORs) with 95% confidence intervals (95% CIs) in order to estimate the predictors of AKI and the impact of AKI on hospital outcomes. We constructed final models after adjusting for confounders, testing for potential interactions, and ensuring no multicolinearity between covariates. Last, we computed the risk proportion of death attributable to AKI, indicating the proportion of deaths that could potentially be avoided if AKI and its complications were abrogated.21
We performed all statistical analyses with SAS Version 9.3 (SAS Institute) using designated weight values to produce weighted national estimates. The threshold for statistical significance was set at P < .01 (with ORs and 95% CIs that excluded 1).
Results
AKI Incidence, Risk Factors, and Trends
We identified 7,235,251 patients who underwent elective hip or knee arthroplasty for osteoarthritis between 2002 and 2012—an estimate consistent with data from the Centers for Disease Control and Prevention.22 Of that total, 94,367 (1.3%) had AKI. The proportion of discharges diagnosed with AKI increased rapidly over the decade, from 0.5% in 2002 to 1.8% to 1.9% in the period 2010–2012. This upward trend was highly significant (Ptrend < .001) (Figure 1). Patients with AKI (vs patients without AKI) were more likely to be older (mean age, 70 vs 66 years; P < .001), male (50.8% vs 38.4%; P < .001), and black (10.07% vs 5.15%; P<. 001). They were also found to have a significantly higher comorbidity score (mean CCI, 2.8 vs 1.5; P < .001) and higher proportions of comorbidities, including hypertension, CKD, atrial fibrillation, diabetes mellitus (DM), congestive heart failure, chronic liver disease, and hepatitis C virus infection. In addition, AKI was associated with perioperative myocardial infarction (MI), sepsis, cardiac catheterization, and blood transfusion. Regarding socioeconomic characteristics, patients with AKI were more likely to have Medicare/Medicaid insurance (72.26% vs 58.06%; P < .001) and to belong to the extremes of income categories (Table 2).
Using multivariable logistic regression, we found that increased age (1.11 increase in adjusted OR for every year older; 95% CI, 1.09-1.14; P < .001), male sex (adjusted OR, 1.65; 95% CI, 1.60-1.71; P < .001), and black race (adjusted OR, 1.57; 95% CI, 1.45-1.69; P < .001) were significantly associated with postoperative AKI. Regarding comorbidities, baseline CKD (adjusted OR, 8.64; 95% CI, 8.14-9.18; P < .001) and congestive heart failure (adjusted OR, 2.74; 95% CI, 2.57-2.92; P< .0001) were most significantly associated with AKI. Perioperative events, including sepsis (adjusted OR, 35.64; 95% CI, 30.28-41.96; P < .0001), MI (adjusted OR, 6.14; 95% CI, 5.17-7.28; P < .0001), and blood transfusion (adjusted OR, 2.28; 95% CI, 2.15-2.42; P < .0001), were also strongly associated with postoperative AKI. Last, compared with urban hospitals and small hospital bed size, rural hospitals (adjusted OR, 0.70; 95% CI, 0.60-0.81; P< .001) and large bed size (adjusted OR, 0.82; 95% CI, 0.70-0.93; P = .003) were associated with lower probability of developing AKI (Table 3).
Figure 2 elucidates the frequency of AKI based on a combination of key preoperative comorbid conditions and postoperative complications—demonstrating that the proportion of AKI cases associated with other postoperative complications is significantly higher in the CKD and concomitant DM/CKD patient populations. Patients hospitalized with CKD exhibited higher rates of AKI in cases involving blood transfusion (20.9% vs 1.8%; P < .001), acute MI (48.9% vs 13.8%; P < .001), and sepsis (74.7% vs 36.3%;P< .001) relative to patients without CKD. Similarly, patients with concomitant DM/CKD exhibited higher rates of AKI in cases involving blood transfusion (23% vs 1.9%; P< .001), acute MI (51.1% vs 12.1%; P< .001), and sepsis (75% vs 38.2%; P < .001) relative to patients without either condition. However, patients hospitalized with DM alone exhibited only marginally higher rates of AKI in cases involving blood transfusion (4.7% vs 2%; P < .01) and acute MI (19.2% vs 16.7%; P< .01) and a lower rate in cases involving sepsis (38.2% vs 41.7%; P < .01) relative to patients without DM. These data suggest that CKD is the most significant clinically relevant risk factor for AKI and that CKD may synergize with DM to raise the risk for AKI.
Outcomes
We then analyzed the impact of AKI on hospital outcomes, including in-hospital mortality, discharge disposition, LOS, and cost of care. Mortality was significantly higher in patients with AKI than in patients without it (2.08% vs 0.06%; P < .001). Even after adjusting for confounders (eg, demographics, comorbidity burden, perioperative sepsis, hospital-level characteristics), AKI was still associated with strikingly higher odds of in-hospital death (adjusted OR, 11.32; 95% CI, 9.34-13.74; P < .001). However, analysis of temporal trends indicated that the odds for adjusted mortality associated with AKI decreased from 18.09 to 9.45 (Ptrend = .01) over the period 2002–2012 (Figure 3). This decrease in odds of death was countered by an increase in incidence of AKI, resulting in a stable attributable risk proportion (97.9% in 2002 to 97.3% in 2012; Ptrend = .90) (Table 4). Regarding discharge disposition, patients with AKI were much less likely to be discharged home (41.35% vs 62.59%; P < .001) and more likely to be discharged to long-term care (56.37% vs 37.03%; P< .001). After adjustment for confounders, AKI was associated with significantly increased odds of adverse discharge (adjusted OR, 2.24; 95% CI, 2.12-2.36; P< .001). Analysis of temporal trends revealed no appreciable decrease in the adjusted odds of adverse discharge between 2002 (adjusted OR, 1.87; 95% CI, 1.37-2.55; P < .001) and 2012 (adjusted OR, 1.93; 95% CI, 1.76-2.11; P < .001) (Figure 4, Table 5). Last, both mean LOS (5 days vs 3 days; P < .001) and mean cost of hospitalization (US $22,269 vs $15,757; P < .001) were significantly higher in patients with AKI.
Discussion
In this study, we found that the incidence of AKI among hospitalized patients increased 4-fold between 2002 and 2012. Moreover, we identified numerous patient-specific, hospital-specific, perioperative risk factors for AKI. Most important, we found that AKI was associated with a strikingly higher risk of in-hospital death, and surviving patients were more likely to experience adverse discharge. Although the adjusted mortality rate associated with AKI decreased over that decade, the attributable risk proportion remained stable.
Few studies have addressed this significant public health concern. In one recent study in Australia, Kimmel and colleagues16 identified risk factors for AKI but lacked data on AKI outcomes. In a study of complications and mortality occurring after orthopedic surgery, Belmont and colleagues22 categorized complications as either local or systemic but did not examine renal complications. Only 2 other major studies have been conducted on renal outcomes associated with major joint surgery, and both were limited to patients with acute hip fractures. The first included acute fracture surgery patients and omitted elective joint surgery patients, and it evaluated admission renal function but not postoperative AKI.22 The second study had a sample size of only 170 patients.23 Thus, the literature leaves us with a crucial knowledge gap in renal outcomes and their postoperative impact in elective arthroplasties.
The present study filled this information gap by examining the incidence, risk factors, outcomes, and temporal trends of AKI after elective hip and knee arthroplasties. The increasing incidence of AKI in this surgical setting is similar to that of AKI in other surgical settings (cardiac and noncardiac).21 Although our analysis was limited by lack of perioperative management data, patients undergoing elective joint arthroplasty can experience kidney dysfunction for several reasons, including volume depletion, postoperative sepsis, and influence of medications, such as nonsteroidal anti-inflammatory drugs (NSAIDs), especially in older patients with more comorbidities and a higher burden of CKD. Each of these factors can cause renal dysfunction in patients having orthopedic procedures.24 Moreover, NSAID use among elective joint arthroplasty patients is likely higher because of an emphasis on multimodal analgesia, as recent randomized controlled trials have demonstrated the efficacy of NSAID use in controlling pain without increasing bleeding.25-27 Our results also demonstrated that the absolute incidence of AKI after orthopedic surgery is relatively low. One possible explanation for this phenomenon is that the definitions used were based on ICD-9-CM codes that underestimate the true incidence of AKI.
Consistent with other studies, we found that certain key preoperative comorbid conditions and postoperative events were associated with higher AKI risk. We stratified the rate of AKI associated with each postoperative event (sepsis, acute MI, cardiac catheterization, need for transfusion) by DM/CKD comorbidity. CKD was associated with significantly higher AKI risk across all postoperative complications. This information may provide clinicians with bedside information that can be used to determine which patients may be at higher or lower risk for AKI.
Our analysis of patient outcomes revealed that, though AKI was relatively uncommon, it increased the risk for death during hospitalization more than 10-fold between 2002 and 2012. Although the adjusted OR of in-hospital mortality decreased over the decade studied, the concurrent increase in AKI incidence caused the attributable risk of death associated with AKI to essentially remain the same. This observation is consistent with recent reports from cardiac surgery settings.21 These data together suggest that ameliorating occurrences of AKI would decrease mortality and increase quality of care for patients undergoing elective joint surgeries.
We also examined the effect of AKI on resource use by studying LOS, costs, and risk for adverse discharge. Much as in other surgical settings, AKI increased both LOS and overall hospitalization costs. More important, AKI was associated with increased adverse discharge (discharge to long-term care or nursing homes). Although exact reasons are unclear, we can speculate that postoperative renal dysfunction precludes early rehabilitation, impeding desired functional outcome and disposition.28,29 Given the projected increases in primary and revision hip and knee arthroplasties,5 these data predict that the impact of AKI on health outcomes will increase alarmingly in coming years.
There are limitations to our study. First, it was based on administrative data and lacked patient-level and laboratory data. As reported, the sensitivity of AKI codes remains moderate,30 so the true burden may be higher than indicated here. As the definition of AKI was based on administrative coding, we also could not estimate severity, though previous studies have found that administrative codes typically capture a more severe form of disease.31 Another limitation is that, because the data were deidentified, we could not delineate the risk for recurrent AKI in repeated surgical procedures, though this cohort unlikely was large enough to qualitatively affect our results. The third limitation is that, though we used CCI to adjust for the comorbidity burden, we were unable to account for other unmeasured confounders associated with increased AKI incidence, such as specific medication use. In addition, given the lack of patient-level data, we could not analyze the specific factors responsible for AKI in the perioperative period. Nevertheless, the strengths of a nationally representative sample, such as large sample size and generalizability, outweigh these limitations.
Conclusion
AKI is potentially an important quality indicator of elective joint surgery, and reducing its incidence is therefore essential for quality improvement. Given that hip and knee arthroplasties are projected to increase exponentially, as is the burden of comorbid conditions in this population, postoperative AKI will continue to have an incremental impact on health and health care resources. Thus, a carefully planned approach of interdisciplinary perioperative care is warranted to reduce both the risk and the consequences of this devastating condition.
1. Reginster JY. The prevalence and burden of arthritis. Rheumatology. 2002;41(supp 1):3-6.
2. Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265-2268.
3. Kawasaki M, Hasegawa Y, Sakano S, Torii Y, Warashina H. Quality of life after several treatments for osteoarthritis of the hip. J Orthop Sci. 2003;8(1):32-35.
4. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
5. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
6. Matlock D, Earnest M, Epstein A. Utilization of elective hip and knee arthroplasty by age and payer. Clin Orthop Relat Res. 2008;466(4):914-919.
7. Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;(369):39-48.
8. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
9. Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am. 2001;83(8):1157-1161.
10. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
11. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67-75.
12. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891-898.
13. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
14. Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112-1119.
15. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20.
16. Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J. 2014;7(6):546-551.
17. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) databases, 2002–2012. Rockville, MD: Agency for Healthcare Research and Quality.
18. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11(4):203-209.
19. Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(5):1638-1644.
20. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370.
21. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28.
22. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
23. Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41(4):335-338.
24. Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101.
25. Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskelet Disord. 2008;9:77.
26. Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28(8):1274-1277.
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
28. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
29. Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014;93(11):962-970.
30. Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688-1694.
31. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682-689.
1. Reginster JY. The prevalence and burden of arthritis. Rheumatology. 2002;41(supp 1):3-6.
2. Kullenberg B, Runesson R, Tuvhag R, Olsson C, Resch S. Intraarticular corticosteroid injection: pain relief in osteoarthritis of the hip? J Rheumatol. 2004;31(11):2265-2268.
3. Kawasaki M, Hasegawa Y, Sakano S, Torii Y, Warashina H. Quality of life after several treatments for osteoarthritis of the hip. J Orthop Sci. 2003;8(1):32-35.
4. Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974.
5. Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785.
6. Matlock D, Earnest M, Epstein A. Utilization of elective hip and knee arthroplasty by age and payer. Clin Orthop Relat Res. 2008;466(4):914-919.
7. Parvizi J, Holiday AD, Ereth MH, Lewallen DG. The Frank Stinchfield Award. Sudden death during primary hip arthroplasty. Clin Orthop Relat Res. 1999;(369):39-48.
8. Parvizi J, Mui A, Purtill JJ, Sharkey PF, Hozack WJ, Rothman RH. Total joint arthroplasty: when do fatal or near-fatal complications occur? J Bone Joint Surg Am. 2007;89(1):27-32.
9. Parvizi J, Sullivan TA, Trousdale RT, Lewallen DG. Thirty-day mortality after total knee arthroplasty. J Bone Joint Surg Am. 2001;83(8):1157-1161.
10. Uchino S, Kellum JA, Bellomo R, et al; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA. 2005;294(7):813-818.
11. Thakar CV. Perioperative acute kidney injury. Adv Chronic Kidney Dis. 2013;20(1):67-75.
12. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin J Am Soc Nephrol. 2009;4(5):891-898.
13. Rewa O, Bagshaw SM. Acute kidney injury—epidemiology, outcomes and economics. Nat Rev Nephrol. 2014;10(4):193-207.
14. Thakar CV, Worley S, Arrigain S, Yared JP, Paganini EP. Influence of renal dysfunction on mortality after cardiac surgery: modifying effect of preoperative renal function. Kidney Int. 2005;67(3):1112-1119.
15. Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clin J Am Soc Nephrol. 2014;9(1):12-20.
16. Kimmel LA, Wilson S, Janardan JD, Liew SM, Walker RG. Incidence of acute kidney injury following total joint arthroplasty: a retrospective review by RIFLE criteria. Clin Kidney J. 2014;7(6):546-551.
17. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project (HCUP) databases, 2002–2012. Rockville, MD: Agency for Healthcare Research and Quality.
18. Bjorgul K, Novicoff WM, Saleh KJ. Evaluating comorbidities in total hip and knee arthroplasty: available instruments. J Orthop Traumatol. 2010;11(4):203-209.
19. Voskuijl T, Hageman M, Ring D. Higher Charlson Comorbidity Index Scores are associated with readmission after orthopaedic surgery. Clin Orthop Relat Res. 2014;472(5):1638-1644.
20. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365-3370.
21. Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95(1):20-28.
22. Belmont PJ Jr, Goodman GP, Waterman BR, Bader JO, Schoenfeld AJ. Thirty-day postoperative complications and mortality following total knee arthroplasty: incidence and risk factors among a national sample of 15,321 patients. J Bone Joint Surg Am. 2014;96(1):20-26.
23. Bennet SJ, Berry OM, Goddard J, Keating JF. Acute renal dysfunction following hip fracture. Injury. 2010;41(4):335-338.
24. Kateros K, Doulgerakis C, Galanakos SP, Sakellariou VI, Papadakis SA, Macheras GA. Analysis of kidney dysfunction in orthopaedic patients. BMC Nephrol. 2012;13:101.
25. Huang YM, Wang CM, Wang CT, Lin WP, Horng LC, Jiang CC. Perioperative celecoxib administration for pain management after total knee arthroplasty—a randomized, controlled study. BMC Musculoskelet Disord. 2008;9:77.
26. Kelley TC, Adams MJ, Mulliken BD, Dalury DF. Efficacy of multimodal perioperative analgesia protocol with periarticular medication injection in total knee arthroplasty: a randomized, double-blinded study. J Arthroplasty. 2013;28(8):1274-1277.
27. Lamplot JD, Wagner ER, Manning DW. Multimodal pain management in total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty. 2014;29(2):329-334.
28. Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852.
29. Pua YH, Ong PH. Association of early ambulation with length of stay and costs in total knee arthroplasty: retrospective cohort study. Am J Phys Med Rehabil. 2014;93(11):962-970.
30. Waikar SS, Wald R, Chertow GM, et al. Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol. 2006;17(6):1688-1694.
31. Grams ME, Waikar SS, MacMahon B, Whelton S, Ballew SH, Coresh J. Performance and limitations of administrative data in the identification of AKI. Clin J Am Soc Nephrol. 2014;9(4):682-689.