User login
Most GI Service Chiefs Support POCUS Training, But Uptake Is Slow
, according to a national survey.
Low POCUS uptake may be explained by substantial barriers to implementation, including lack of trained instructors, necessary equipment, and support staff, lead author Keerthi Thallapureddy, MD, of the University of Texas Health San Antonio, and colleagues, reported.
“POCUS is being increasingly used by gastroenterologists due to its portability and real-time diagnostic ability,” the investigators wrote in Gastro Hep Advances, but “there is limited understanding of how gastroenterologists use POCUS.”
To learn more, the investigators conducted a nationwide survey of the VA healthcare system. Separate questionnaires were sent to chiefs of staff (n = 130) and GI service chiefs (n = 117), yielding response rates of 100% and 79%, respectively.
Respondents represented a wide distribution of geographic regions and institutional complexity levels, with 80% of GI groups based at high-complexity centers and 92% in urban locations. A minority (8%) reported the presence of a liver transplant program.
Data collection focused on the prevalence of POCUS use, types of clinical applications, institutional policies and training processes, and perceived or actual barriers to wider adoption. Barriers were sorted into three categories: training, equipment, and infrastructure.
Of the 93 GI service chiefs who participated in the survey, 44% reported that at least 1 gastroenterologist at their facility currently uses POCUS. Most common procedural uses were paracentesis (23%) and liver biopsy (13%), while ascites assessment (19%) and biliary visualization (7%) were the most common diagnostic uses.
Among the same respondents, 69% said they would support sending clinicians to a POCUS training course, and 37% said their teams had expressed an active interest in pursuing such training. Only 17% of facilities had a formal process in place to obtain POCUS training, and an equal proportion had implemented a facility-wide policy to guide its use.
Barriers to implementation were widespread and often multifactorial.
Most challenges related to training: 48% of sites reported a lack of trained providers, 28% cited insufficient funding for training, 24% noted a lack of training opportunities, and 14% reported difficulty securing travel funds.
Equipment limitations were also common, with 41% of sites lacking ultrasound machines and 27% lacking funding to purchase them.
Institutional infrastructure posed further hurdles. Nearly a quarter of GI chiefs (23%) reported lacking a clinician champion to lead implementation, while others cited a lack of support staff, simulation space, privileging criteria, image archiving capabilities, or standardized reporting forms.
“Our findings on current POCUS use, training, barriers, and infrastructure can guide expansion of POCUS use and training among GI groups,” Dr. Thallapureddy and colleagues wrote, noting that early efforts to expand access to GI-specific POCUS training are already underway.
They cited growing interest from national organizations such as the American Gastroenterological Association and the American Association for the Study of Liver Diseases, the latter of which piloted training workshops at the 2024 Liver Meeting. Similarly, the International Bowel Ultrasound Group now offers a 3-part certification program in intestinal ultrasound and is developing additional online and interactive modules to improve training accessibility.
The study was supported by the US Department of Veterans Affairs, Quality Enhancement Research Initiative Partnered Evaluation Initiative Grant, and the VA National Center for Patient Safety. The investigators reported no conflicts of interest.
, according to a national survey.
Low POCUS uptake may be explained by substantial barriers to implementation, including lack of trained instructors, necessary equipment, and support staff, lead author Keerthi Thallapureddy, MD, of the University of Texas Health San Antonio, and colleagues, reported.
“POCUS is being increasingly used by gastroenterologists due to its portability and real-time diagnostic ability,” the investigators wrote in Gastro Hep Advances, but “there is limited understanding of how gastroenterologists use POCUS.”
To learn more, the investigators conducted a nationwide survey of the VA healthcare system. Separate questionnaires were sent to chiefs of staff (n = 130) and GI service chiefs (n = 117), yielding response rates of 100% and 79%, respectively.
Respondents represented a wide distribution of geographic regions and institutional complexity levels, with 80% of GI groups based at high-complexity centers and 92% in urban locations. A minority (8%) reported the presence of a liver transplant program.
Data collection focused on the prevalence of POCUS use, types of clinical applications, institutional policies and training processes, and perceived or actual barriers to wider adoption. Barriers were sorted into three categories: training, equipment, and infrastructure.
Of the 93 GI service chiefs who participated in the survey, 44% reported that at least 1 gastroenterologist at their facility currently uses POCUS. Most common procedural uses were paracentesis (23%) and liver biopsy (13%), while ascites assessment (19%) and biliary visualization (7%) were the most common diagnostic uses.
Among the same respondents, 69% said they would support sending clinicians to a POCUS training course, and 37% said their teams had expressed an active interest in pursuing such training. Only 17% of facilities had a formal process in place to obtain POCUS training, and an equal proportion had implemented a facility-wide policy to guide its use.
Barriers to implementation were widespread and often multifactorial.
Most challenges related to training: 48% of sites reported a lack of trained providers, 28% cited insufficient funding for training, 24% noted a lack of training opportunities, and 14% reported difficulty securing travel funds.
Equipment limitations were also common, with 41% of sites lacking ultrasound machines and 27% lacking funding to purchase them.
Institutional infrastructure posed further hurdles. Nearly a quarter of GI chiefs (23%) reported lacking a clinician champion to lead implementation, while others cited a lack of support staff, simulation space, privileging criteria, image archiving capabilities, or standardized reporting forms.
“Our findings on current POCUS use, training, barriers, and infrastructure can guide expansion of POCUS use and training among GI groups,” Dr. Thallapureddy and colleagues wrote, noting that early efforts to expand access to GI-specific POCUS training are already underway.
They cited growing interest from national organizations such as the American Gastroenterological Association and the American Association for the Study of Liver Diseases, the latter of which piloted training workshops at the 2024 Liver Meeting. Similarly, the International Bowel Ultrasound Group now offers a 3-part certification program in intestinal ultrasound and is developing additional online and interactive modules to improve training accessibility.
The study was supported by the US Department of Veterans Affairs, Quality Enhancement Research Initiative Partnered Evaluation Initiative Grant, and the VA National Center for Patient Safety. The investigators reported no conflicts of interest.
, according to a national survey.
Low POCUS uptake may be explained by substantial barriers to implementation, including lack of trained instructors, necessary equipment, and support staff, lead author Keerthi Thallapureddy, MD, of the University of Texas Health San Antonio, and colleagues, reported.
“POCUS is being increasingly used by gastroenterologists due to its portability and real-time diagnostic ability,” the investigators wrote in Gastro Hep Advances, but “there is limited understanding of how gastroenterologists use POCUS.”
To learn more, the investigators conducted a nationwide survey of the VA healthcare system. Separate questionnaires were sent to chiefs of staff (n = 130) and GI service chiefs (n = 117), yielding response rates of 100% and 79%, respectively.
Respondents represented a wide distribution of geographic regions and institutional complexity levels, with 80% of GI groups based at high-complexity centers and 92% in urban locations. A minority (8%) reported the presence of a liver transplant program.
Data collection focused on the prevalence of POCUS use, types of clinical applications, institutional policies and training processes, and perceived or actual barriers to wider adoption. Barriers were sorted into three categories: training, equipment, and infrastructure.
Of the 93 GI service chiefs who participated in the survey, 44% reported that at least 1 gastroenterologist at their facility currently uses POCUS. Most common procedural uses were paracentesis (23%) and liver biopsy (13%), while ascites assessment (19%) and biliary visualization (7%) were the most common diagnostic uses.
Among the same respondents, 69% said they would support sending clinicians to a POCUS training course, and 37% said their teams had expressed an active interest in pursuing such training. Only 17% of facilities had a formal process in place to obtain POCUS training, and an equal proportion had implemented a facility-wide policy to guide its use.
Barriers to implementation were widespread and often multifactorial.
Most challenges related to training: 48% of sites reported a lack of trained providers, 28% cited insufficient funding for training, 24% noted a lack of training opportunities, and 14% reported difficulty securing travel funds.
Equipment limitations were also common, with 41% of sites lacking ultrasound machines and 27% lacking funding to purchase them.
Institutional infrastructure posed further hurdles. Nearly a quarter of GI chiefs (23%) reported lacking a clinician champion to lead implementation, while others cited a lack of support staff, simulation space, privileging criteria, image archiving capabilities, or standardized reporting forms.
“Our findings on current POCUS use, training, barriers, and infrastructure can guide expansion of POCUS use and training among GI groups,” Dr. Thallapureddy and colleagues wrote, noting that early efforts to expand access to GI-specific POCUS training are already underway.
They cited growing interest from national organizations such as the American Gastroenterological Association and the American Association for the Study of Liver Diseases, the latter of which piloted training workshops at the 2024 Liver Meeting. Similarly, the International Bowel Ultrasound Group now offers a 3-part certification program in intestinal ultrasound and is developing additional online and interactive modules to improve training accessibility.
The study was supported by the US Department of Veterans Affairs, Quality Enhancement Research Initiative Partnered Evaluation Initiative Grant, and the VA National Center for Patient Safety. The investigators reported no conflicts of interest.
FROM GASTRO HEP ADVANCES
AGA Women’s Committee Outlines Roadmap Towards Gender Equity
Despite the increasing number of women joining the field, GI remains one of the most male-dominated medical subspecialties. and has highlighted future directions to achieve gender equality in GI.
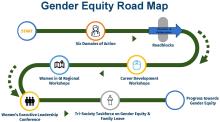
The AGA Gender Equity Framework outlines six domains of action, the current state and desired future state: bias & gender disparities, leadership & career advancement, wellness & balance, retention & recruitment, mentorship & sponsorship, and recognition.
Based on the desired future state, the group created a roadmap towards gender equity with measurable tactics. Career development workshops, including the Women in GI regional workshops and Women’s Executive Leadership Conference, are both crucial tactics.
AGA outlined a few key areas for future gender equity efforts to focus on:
- Clearer GI-specific transparency guidelines regarding recruitment, salary, promotions, funding, and leadership.
- Pathway and research programs that help students from underrepresented backgrounds get involved and stay engaged in GI.
- Support networks (through GI societies, institutions, or other organizations) that help women connect, collaborate, and grow their careers.
The AGA Women’s Committee, along with other AGA committees, will continue to work to achieve the vision laid out in the AGA Gender Equity Framework and Gender Equity Road Map.
Despite the increasing number of women joining the field, GI remains one of the most male-dominated medical subspecialties. and has highlighted future directions to achieve gender equality in GI.
The AGA Gender Equity Framework outlines six domains of action, the current state and desired future state: bias & gender disparities, leadership & career advancement, wellness & balance, retention & recruitment, mentorship & sponsorship, and recognition.
Based on the desired future state, the group created a roadmap towards gender equity with measurable tactics. Career development workshops, including the Women in GI regional workshops and Women’s Executive Leadership Conference, are both crucial tactics.
AGA outlined a few key areas for future gender equity efforts to focus on:
- Clearer GI-specific transparency guidelines regarding recruitment, salary, promotions, funding, and leadership.
- Pathway and research programs that help students from underrepresented backgrounds get involved and stay engaged in GI.
- Support networks (through GI societies, institutions, or other organizations) that help women connect, collaborate, and grow their careers.
The AGA Women’s Committee, along with other AGA committees, will continue to work to achieve the vision laid out in the AGA Gender Equity Framework and Gender Equity Road Map.
Despite the increasing number of women joining the field, GI remains one of the most male-dominated medical subspecialties. and has highlighted future directions to achieve gender equality in GI.
The AGA Gender Equity Framework outlines six domains of action, the current state and desired future state: bias & gender disparities, leadership & career advancement, wellness & balance, retention & recruitment, mentorship & sponsorship, and recognition.
Based on the desired future state, the group created a roadmap towards gender equity with measurable tactics. Career development workshops, including the Women in GI regional workshops and Women’s Executive Leadership Conference, are both crucial tactics.
AGA outlined a few key areas for future gender equity efforts to focus on:
- Clearer GI-specific transparency guidelines regarding recruitment, salary, promotions, funding, and leadership.
- Pathway and research programs that help students from underrepresented backgrounds get involved and stay engaged in GI.
- Support networks (through GI societies, institutions, or other organizations) that help women connect, collaborate, and grow their careers.
The AGA Women’s Committee, along with other AGA committees, will continue to work to achieve the vision laid out in the AGA Gender Equity Framework and Gender Equity Road Map.
Evolving Standards of Practice: Esophageal Varices and Barrett’s Esophagus
Dear colleagues,
In the dynamic field of medicine, long-held practices are being reevaluated in light of new evidence and evolving standards of practice.
Dr. Anahita Rabiee discusses the importance of prioritizing non-selective beta blockers (NSBB) over endoscopic variceal ligation (EVL) in the primary prophylaxis of variceal bleeding in patients with compensated cirrhosis. Drawing on data from the PREDESCI trial and real-world experience, she argues that NSBB address the upstream driver—portal hypertension—more broadly and effectively than EVL. In a complementary piece, Dr. Tarek Sawas explores the nuanced landscape of screening and surveillance in Barrett’s esophagus. From how to manage irregular Z-lines, to rethinking the need for 1-year follow-up endoscopies and interpreting the implications of the BOSS trial, Dr. Sawas advocates for a more personalized, risk-based approach.
We hope these perspectives spark dialogue and reflection in your own practice. Join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Choose NSBBs, Not EVL, in Patients with Compensated Cirrhosis
BY ANAHITA RABIEE, MD, MHS
I strongly favor the use of non selective beta blockers (NSBBs) in patients with compensated cirrhosis, rather than endoscopy and esophageal variceal ligation (EVL) for primary prophylaxis.
Since the results of PREDESCI trial (β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (CSPH)) were published in 2019, there has been much debate on the role of screening endoscopy and EVL for primary prophylaxis. While many argue that a single randomized trial should not overturn long standing practice, several compelling reasons convince me to choose NSBBs, when possible.
Recent guidance from major liver societies now recommends NSBBs as first line therapy for CSPH. Yet, adoption in clinical practice remains inconsistent.
Here is why I believe NSBB represent a better solution:
Treating Upstream, Not Just a Local Treatment
NSBBs such as propranolol and nadolol decrease portal pressure by decreasing portal venous inflow through β1 and β2 adrenergic blockade. Carvedilol is often preferred given its additional α1 adrenergic blocking activity making it the most effective one in decreasing the portal pressure. Therefore, NSBBs address the upstream driver of decompensation by decreasing portal pressures.
EVL, in contrast, is a local fix that only prevents variceal bleeding. Ascites, not variceal bleeding, is the most common initial decompensating event and is associated with high mortality. Preventing all forms of decompensation is clearly preferable to preventing just one.
Broader Eligibility, More Patients Benefit
CSPH is defined as hepatic venous pressure gradient (HVPG)>10 mmHg, the threshold where increased portal venous inflow secondary to splanchnic vasodilation and hyperdynamic circulation drives the increase in portal hypertension. This threshold has been shown to strongly predict decompensation in patients with compensated disease.
While all patients with varices have CSPH, not all patients with CSPH have varices. They can be identified by other non invasive criteria such as cross sectional imaging showing collaterals, or liver stiffness and platelet thresholds that have been previously validated. By restricting intervention to those with large varices and offering only EVL, we miss the opportunity to intervene earlier and to a broader group that would benefit from this treatment.
Comprehensive Protection Without Repeated Endoscopies
Once on an appropriate NSBB dose, patients are protected against variceal bleeding (at least as effectively as EVL). This eliminates the need for repeated surveillance endoscopies to identify and treat large varices in otherwise compensated patients.
Better Tolerated and – In Many Cases – Overlaps With Existing Medication List!
While overtreatment is a concern, regular endoscopies every two years are also burdensome. Many patients already need beta blockers for cardiac conditions such as atrial fibrillation, ischemic heart disease or hypertension. Carvedilol, in particular, offers dual benefit for both hepatologists and cardiologists.
It is important to emphasize that these arguments apply to compensated cirrhosis. In decompensated disease, the approach changes. After a variceal bleed, both NSBBs and EVL are required for secondary prophylaxis. In patients with prior ascites but no variceal bleed, the benefit of NSBBs is less pronounced since decompensation has already occurred. In this setting, NSBBs can still be used selectively, but only if systolic blood pressure remains above 90 mmHg.
The evidence supporting NSBBs over EVL in compensated cirrhosis is not perfect, but few things in medicine are. Given current data, NSBBs should be the first line therapy in compensated cirrhosis with CSPH. Once a patient is on an appropriate and tolerated NSBB dose, routine endoscopic surveillance is unnecessary. Endoscopy should be reserved for those who cannot tolerate NSBBs, in whom EVL is then indicated if large varices are present.
Dr. Rabiee is based at the Yale School of Medicine, New Haven, Connecticut, and the Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut. She has no disclosures in regard to this article.
Rethinking Screening and Surveillance in Barrett’s Esophagus: Navigating Controversies and Nuances
BY TAREK SAWAS, MD, MPH
Barrett’s esophagus (BE) is a precursor to esophageal adenocarcinoma (EAC). Despite our comprehensive guidelines, many of the day-to-day decisions still rely on clinical judgment and honest conversations with patients. This article explores common scenarios in which management decisions are nuanced and the right answer remains debatable.
Irregular Z-Line/Ultrashort Segment BE: Leave Or Watch It?
Few findings provoke more confusion than irregular Z-line or intestinal metaplasia (IM) < 1 cm at the gastroesophageal junction (GEJ). For years, we have debated whether these subtle changes represent a precursor to EAC or simply a benign variant. We have wrestled with how to handle these cases from whether we should take biopsies to how to perform surveillance.
The American College of Gastroenterology (ACG) guideline suggests that irregular Z-lines should not be routinely biopsied or surveyed. Similarly, the upcoming American Gastroenterology Association (AGA) surveillance guideline suggests against surveillance of IM<1 cm citing the low individual annual risk of progression to high-grade dysplasia (HGD) and EAC of 0.23% per year which is lower than that of non-dysplastic Barrett’s esophagus (NDBE). However, this is not the entire picture.
Despite the low per-patient risk, IM<1cm is highly prevalent with columnar mucosa observed in approximately 15% of patients undergoing upper endoscopy. This paradox is unsettling. While any one patient with IM<1 cm is unlikely to progress to EAC, the group accounts for a meaningful share of the EAC burden. Some experts have argued that this justifies routine biopsy and surveillance in all patients with visible columnar mucosa regardless of length. However, this approach risks overwhelming our surveillance infrastructure.
A recent decision modeling analysis suggested that at the lowest progression rates, either no surveillance or one-time endoscopy can be considered. Based on these data, I do not regularly biopsy ultrashort segments unless the mucosa appears suspicious. In those with IM<1 cm detected during a high-quality endoscopic exam, no follow-up is needed. However, if the exam is suboptimal, I perform a 1-time high-quality repeat exam. If there is no evidence of dysplasia then I do not pursue any further surveillance.
The One-Year Follow-Up Endoscopy: Is It Necessary?
Another controversy is the one-year follow-up endoscopy after an initial diagnosis of NDBE. Proponents of this approach cite the high proportion of post endoscopy esophageal neoplasia and cancer (PEEN/PEEC) detected in the first year after diagnosis (missed HGD/EAC). In fact, PEEN account for about a quarter of all HGD/EAC cases diagnosed during surveillance.
While this approach might mitigate PEEN/PEEC risk, it may not be necessary if the index endoscopy is high quality. To ensure high quality exams, several best practices have been proposed including:
- Use of high-definition white light endoscopy (HD-WLE) with chromoendoscopy (virtual or dye based)
- Appropriate inspection time (1 minute per cm of circumferential BE)
- Accurate documentation using the Prague criteria
- Adherence to the Seattle protocol with additional targeted biopsies
If the index endoscopy meets these quality metrics, I typically do not bring the patient back at one year. However, if the exam quality is in question, then I repeat it at one year to establish a reliable baseline and rule out prevalent neoplasia.
Surveillance In NDBE: After BOSS, Do We Rethink Everything?
The recently published BOSS trial (Barrett’s Oesophagus Surveillance Study) has reignited the debate over the value of endoscopic surveillance in NDBE. In this study, 3,453 patients with NDBE across the UK were randomized to either surveillance endoscopy every two years or endoscopy only as clinically indicated. After a median follow-up of 12.8 years, the trial found no significant difference in all-cause mortality between the two groups.
While these findings are important, they should be interpreted with caution. First, the primary endpoint, all-cause mortality, is not optimal for evaluating surveillance for EAC. Surveillance is not intended to reduce all-cause mortality but rather to reduce EAC–related mortality. Second, a substantial number of patients in the no surveillance group still underwent endoscopy at intervals that were not meaningfully different from those in the surveillance group. If both groups receive similar exposure to endoscopy, the comparison loses power. Lastly, the trial was underpowered due to overestimation of progression risk during its initial design. As we have since learned, the risk of progression of NDBE is lower than originally assumed.
So where do we stand now? For me, the BOSS trial does not negate the value of surveillance. it reminds us that a one-size-fits-all approach is inefficient, and our strategy must be risk based. For low-risk individuals, particularly older adults with short-segment NDBE, surveillance may offer little benefit. But in healthier, younger patients with longer segments or additional risk factors, surveillance remains an essential tool for early neoplasia detection.
When to Stop Surveillance
Perhaps the most under-discussed point is when to stop surveillance. Existing guidelines do not account for competing mortality risks unrelated to EAC or provide specific recommendations regarding cessation of surveillance. The desired benefits of surveillance likely diminish with advanced age and greater comorbidity because of lower life expectancy and ineligibility for definitive therapy for EAC.
A recent modeling study found that the optimal ages for last surveillance were 81, 80, 77, and 73 years for men with no, mild, moderate, and severe comorbidity respectively and 75, 73, 73, and 69 years for women. In my practice, I discuss surveillance cessation in patients older than 75 based on their comorbidities. If the risk of progression is outweighed by the risk of the procedure or by the reality of limited life expectancy, we should not hesitate to consider surveillance cessation.
In summary, high-quality endoscopic exam in appropriately selected patients remains the cornerstone of BE surveillance. A more personalized, risk-based approach is needed taking into account competing comorbidities. Emerging technology through risk stratification tools such as biomarkers and artificial intelligence may refine our approach and help address the current limitations.
Dr. Sawas is based at the University of Texas Southwestern, Dallas, Texas. He has no disclosures in regard to this article.
Dear colleagues,
In the dynamic field of medicine, long-held practices are being reevaluated in light of new evidence and evolving standards of practice.
Dr. Anahita Rabiee discusses the importance of prioritizing non-selective beta blockers (NSBB) over endoscopic variceal ligation (EVL) in the primary prophylaxis of variceal bleeding in patients with compensated cirrhosis. Drawing on data from the PREDESCI trial and real-world experience, she argues that NSBB address the upstream driver—portal hypertension—more broadly and effectively than EVL. In a complementary piece, Dr. Tarek Sawas explores the nuanced landscape of screening and surveillance in Barrett’s esophagus. From how to manage irregular Z-lines, to rethinking the need for 1-year follow-up endoscopies and interpreting the implications of the BOSS trial, Dr. Sawas advocates for a more personalized, risk-based approach.
We hope these perspectives spark dialogue and reflection in your own practice. Join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Choose NSBBs, Not EVL, in Patients with Compensated Cirrhosis
BY ANAHITA RABIEE, MD, MHS
I strongly favor the use of non selective beta blockers (NSBBs) in patients with compensated cirrhosis, rather than endoscopy and esophageal variceal ligation (EVL) for primary prophylaxis.
Since the results of PREDESCI trial (β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (CSPH)) were published in 2019, there has been much debate on the role of screening endoscopy and EVL for primary prophylaxis. While many argue that a single randomized trial should not overturn long standing practice, several compelling reasons convince me to choose NSBBs, when possible.
Recent guidance from major liver societies now recommends NSBBs as first line therapy for CSPH. Yet, adoption in clinical practice remains inconsistent.
Here is why I believe NSBB represent a better solution:
Treating Upstream, Not Just a Local Treatment
NSBBs such as propranolol and nadolol decrease portal pressure by decreasing portal venous inflow through β1 and β2 adrenergic blockade. Carvedilol is often preferred given its additional α1 adrenergic blocking activity making it the most effective one in decreasing the portal pressure. Therefore, NSBBs address the upstream driver of decompensation by decreasing portal pressures.
EVL, in contrast, is a local fix that only prevents variceal bleeding. Ascites, not variceal bleeding, is the most common initial decompensating event and is associated with high mortality. Preventing all forms of decompensation is clearly preferable to preventing just one.
Broader Eligibility, More Patients Benefit
CSPH is defined as hepatic venous pressure gradient (HVPG)>10 mmHg, the threshold where increased portal venous inflow secondary to splanchnic vasodilation and hyperdynamic circulation drives the increase in portal hypertension. This threshold has been shown to strongly predict decompensation in patients with compensated disease.
While all patients with varices have CSPH, not all patients with CSPH have varices. They can be identified by other non invasive criteria such as cross sectional imaging showing collaterals, or liver stiffness and platelet thresholds that have been previously validated. By restricting intervention to those with large varices and offering only EVL, we miss the opportunity to intervene earlier and to a broader group that would benefit from this treatment.
Comprehensive Protection Without Repeated Endoscopies
Once on an appropriate NSBB dose, patients are protected against variceal bleeding (at least as effectively as EVL). This eliminates the need for repeated surveillance endoscopies to identify and treat large varices in otherwise compensated patients.
Better Tolerated and – In Many Cases – Overlaps With Existing Medication List!
While overtreatment is a concern, regular endoscopies every two years are also burdensome. Many patients already need beta blockers for cardiac conditions such as atrial fibrillation, ischemic heart disease or hypertension. Carvedilol, in particular, offers dual benefit for both hepatologists and cardiologists.
It is important to emphasize that these arguments apply to compensated cirrhosis. In decompensated disease, the approach changes. After a variceal bleed, both NSBBs and EVL are required for secondary prophylaxis. In patients with prior ascites but no variceal bleed, the benefit of NSBBs is less pronounced since decompensation has already occurred. In this setting, NSBBs can still be used selectively, but only if systolic blood pressure remains above 90 mmHg.
The evidence supporting NSBBs over EVL in compensated cirrhosis is not perfect, but few things in medicine are. Given current data, NSBBs should be the first line therapy in compensated cirrhosis with CSPH. Once a patient is on an appropriate and tolerated NSBB dose, routine endoscopic surveillance is unnecessary. Endoscopy should be reserved for those who cannot tolerate NSBBs, in whom EVL is then indicated if large varices are present.
Dr. Rabiee is based at the Yale School of Medicine, New Haven, Connecticut, and the Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut. She has no disclosures in regard to this article.
Rethinking Screening and Surveillance in Barrett’s Esophagus: Navigating Controversies and Nuances
BY TAREK SAWAS, MD, MPH
Barrett’s esophagus (BE) is a precursor to esophageal adenocarcinoma (EAC). Despite our comprehensive guidelines, many of the day-to-day decisions still rely on clinical judgment and honest conversations with patients. This article explores common scenarios in which management decisions are nuanced and the right answer remains debatable.
Irregular Z-Line/Ultrashort Segment BE: Leave Or Watch It?
Few findings provoke more confusion than irregular Z-line or intestinal metaplasia (IM) < 1 cm at the gastroesophageal junction (GEJ). For years, we have debated whether these subtle changes represent a precursor to EAC or simply a benign variant. We have wrestled with how to handle these cases from whether we should take biopsies to how to perform surveillance.
The American College of Gastroenterology (ACG) guideline suggests that irregular Z-lines should not be routinely biopsied or surveyed. Similarly, the upcoming American Gastroenterology Association (AGA) surveillance guideline suggests against surveillance of IM<1 cm citing the low individual annual risk of progression to high-grade dysplasia (HGD) and EAC of 0.23% per year which is lower than that of non-dysplastic Barrett’s esophagus (NDBE). However, this is not the entire picture.
Despite the low per-patient risk, IM<1cm is highly prevalent with columnar mucosa observed in approximately 15% of patients undergoing upper endoscopy. This paradox is unsettling. While any one patient with IM<1 cm is unlikely to progress to EAC, the group accounts for a meaningful share of the EAC burden. Some experts have argued that this justifies routine biopsy and surveillance in all patients with visible columnar mucosa regardless of length. However, this approach risks overwhelming our surveillance infrastructure.
A recent decision modeling analysis suggested that at the lowest progression rates, either no surveillance or one-time endoscopy can be considered. Based on these data, I do not regularly biopsy ultrashort segments unless the mucosa appears suspicious. In those with IM<1 cm detected during a high-quality endoscopic exam, no follow-up is needed. However, if the exam is suboptimal, I perform a 1-time high-quality repeat exam. If there is no evidence of dysplasia then I do not pursue any further surveillance.
The One-Year Follow-Up Endoscopy: Is It Necessary?
Another controversy is the one-year follow-up endoscopy after an initial diagnosis of NDBE. Proponents of this approach cite the high proportion of post endoscopy esophageal neoplasia and cancer (PEEN/PEEC) detected in the first year after diagnosis (missed HGD/EAC). In fact, PEEN account for about a quarter of all HGD/EAC cases diagnosed during surveillance.
While this approach might mitigate PEEN/PEEC risk, it may not be necessary if the index endoscopy is high quality. To ensure high quality exams, several best practices have been proposed including:
- Use of high-definition white light endoscopy (HD-WLE) with chromoendoscopy (virtual or dye based)
- Appropriate inspection time (1 minute per cm of circumferential BE)
- Accurate documentation using the Prague criteria
- Adherence to the Seattle protocol with additional targeted biopsies
If the index endoscopy meets these quality metrics, I typically do not bring the patient back at one year. However, if the exam quality is in question, then I repeat it at one year to establish a reliable baseline and rule out prevalent neoplasia.
Surveillance In NDBE: After BOSS, Do We Rethink Everything?
The recently published BOSS trial (Barrett’s Oesophagus Surveillance Study) has reignited the debate over the value of endoscopic surveillance in NDBE. In this study, 3,453 patients with NDBE across the UK were randomized to either surveillance endoscopy every two years or endoscopy only as clinically indicated. After a median follow-up of 12.8 years, the trial found no significant difference in all-cause mortality between the two groups.
While these findings are important, they should be interpreted with caution. First, the primary endpoint, all-cause mortality, is not optimal for evaluating surveillance for EAC. Surveillance is not intended to reduce all-cause mortality but rather to reduce EAC–related mortality. Second, a substantial number of patients in the no surveillance group still underwent endoscopy at intervals that were not meaningfully different from those in the surveillance group. If both groups receive similar exposure to endoscopy, the comparison loses power. Lastly, the trial was underpowered due to overestimation of progression risk during its initial design. As we have since learned, the risk of progression of NDBE is lower than originally assumed.
So where do we stand now? For me, the BOSS trial does not negate the value of surveillance. it reminds us that a one-size-fits-all approach is inefficient, and our strategy must be risk based. For low-risk individuals, particularly older adults with short-segment NDBE, surveillance may offer little benefit. But in healthier, younger patients with longer segments or additional risk factors, surveillance remains an essential tool for early neoplasia detection.
When to Stop Surveillance
Perhaps the most under-discussed point is when to stop surveillance. Existing guidelines do not account for competing mortality risks unrelated to EAC or provide specific recommendations regarding cessation of surveillance. The desired benefits of surveillance likely diminish with advanced age and greater comorbidity because of lower life expectancy and ineligibility for definitive therapy for EAC.
A recent modeling study found that the optimal ages for last surveillance were 81, 80, 77, and 73 years for men with no, mild, moderate, and severe comorbidity respectively and 75, 73, 73, and 69 years for women. In my practice, I discuss surveillance cessation in patients older than 75 based on their comorbidities. If the risk of progression is outweighed by the risk of the procedure or by the reality of limited life expectancy, we should not hesitate to consider surveillance cessation.
In summary, high-quality endoscopic exam in appropriately selected patients remains the cornerstone of BE surveillance. A more personalized, risk-based approach is needed taking into account competing comorbidities. Emerging technology through risk stratification tools such as biomarkers and artificial intelligence may refine our approach and help address the current limitations.
Dr. Sawas is based at the University of Texas Southwestern, Dallas, Texas. He has no disclosures in regard to this article.
Dear colleagues,
In the dynamic field of medicine, long-held practices are being reevaluated in light of new evidence and evolving standards of practice.
Dr. Anahita Rabiee discusses the importance of prioritizing non-selective beta blockers (NSBB) over endoscopic variceal ligation (EVL) in the primary prophylaxis of variceal bleeding in patients with compensated cirrhosis. Drawing on data from the PREDESCI trial and real-world experience, she argues that NSBB address the upstream driver—portal hypertension—more broadly and effectively than EVL. In a complementary piece, Dr. Tarek Sawas explores the nuanced landscape of screening and surveillance in Barrett’s esophagus. From how to manage irregular Z-lines, to rethinking the need for 1-year follow-up endoscopies and interpreting the implications of the BOSS trial, Dr. Sawas advocates for a more personalized, risk-based approach.
We hope these perspectives spark dialogue and reflection in your own practice. Join the conversation on X at @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, and chief of endoscopy at West Haven VA Medical Center, both in Connecticut. He is an associate editor for GI & Hepatology News.
Choose NSBBs, Not EVL, in Patients with Compensated Cirrhosis
BY ANAHITA RABIEE, MD, MHS
I strongly favor the use of non selective beta blockers (NSBBs) in patients with compensated cirrhosis, rather than endoscopy and esophageal variceal ligation (EVL) for primary prophylaxis.
Since the results of PREDESCI trial (β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (CSPH)) were published in 2019, there has been much debate on the role of screening endoscopy and EVL for primary prophylaxis. While many argue that a single randomized trial should not overturn long standing practice, several compelling reasons convince me to choose NSBBs, when possible.
Recent guidance from major liver societies now recommends NSBBs as first line therapy for CSPH. Yet, adoption in clinical practice remains inconsistent.
Here is why I believe NSBB represent a better solution:
Treating Upstream, Not Just a Local Treatment
NSBBs such as propranolol and nadolol decrease portal pressure by decreasing portal venous inflow through β1 and β2 adrenergic blockade. Carvedilol is often preferred given its additional α1 adrenergic blocking activity making it the most effective one in decreasing the portal pressure. Therefore, NSBBs address the upstream driver of decompensation by decreasing portal pressures.
EVL, in contrast, is a local fix that only prevents variceal bleeding. Ascites, not variceal bleeding, is the most common initial decompensating event and is associated with high mortality. Preventing all forms of decompensation is clearly preferable to preventing just one.
Broader Eligibility, More Patients Benefit
CSPH is defined as hepatic venous pressure gradient (HVPG)>10 mmHg, the threshold where increased portal venous inflow secondary to splanchnic vasodilation and hyperdynamic circulation drives the increase in portal hypertension. This threshold has been shown to strongly predict decompensation in patients with compensated disease.
While all patients with varices have CSPH, not all patients with CSPH have varices. They can be identified by other non invasive criteria such as cross sectional imaging showing collaterals, or liver stiffness and platelet thresholds that have been previously validated. By restricting intervention to those with large varices and offering only EVL, we miss the opportunity to intervene earlier and to a broader group that would benefit from this treatment.
Comprehensive Protection Without Repeated Endoscopies
Once on an appropriate NSBB dose, patients are protected against variceal bleeding (at least as effectively as EVL). This eliminates the need for repeated surveillance endoscopies to identify and treat large varices in otherwise compensated patients.
Better Tolerated and – In Many Cases – Overlaps With Existing Medication List!
While overtreatment is a concern, regular endoscopies every two years are also burdensome. Many patients already need beta blockers for cardiac conditions such as atrial fibrillation, ischemic heart disease or hypertension. Carvedilol, in particular, offers dual benefit for both hepatologists and cardiologists.
It is important to emphasize that these arguments apply to compensated cirrhosis. In decompensated disease, the approach changes. After a variceal bleed, both NSBBs and EVL are required for secondary prophylaxis. In patients with prior ascites but no variceal bleed, the benefit of NSBBs is less pronounced since decompensation has already occurred. In this setting, NSBBs can still be used selectively, but only if systolic blood pressure remains above 90 mmHg.
The evidence supporting NSBBs over EVL in compensated cirrhosis is not perfect, but few things in medicine are. Given current data, NSBBs should be the first line therapy in compensated cirrhosis with CSPH. Once a patient is on an appropriate and tolerated NSBB dose, routine endoscopic surveillance is unnecessary. Endoscopy should be reserved for those who cannot tolerate NSBBs, in whom EVL is then indicated if large varices are present.
Dr. Rabiee is based at the Yale School of Medicine, New Haven, Connecticut, and the Veterans Affairs Connecticut Healthcare System, West Haven, Connecticut. She has no disclosures in regard to this article.
Rethinking Screening and Surveillance in Barrett’s Esophagus: Navigating Controversies and Nuances
BY TAREK SAWAS, MD, MPH
Barrett’s esophagus (BE) is a precursor to esophageal adenocarcinoma (EAC). Despite our comprehensive guidelines, many of the day-to-day decisions still rely on clinical judgment and honest conversations with patients. This article explores common scenarios in which management decisions are nuanced and the right answer remains debatable.
Irregular Z-Line/Ultrashort Segment BE: Leave Or Watch It?
Few findings provoke more confusion than irregular Z-line or intestinal metaplasia (IM) < 1 cm at the gastroesophageal junction (GEJ). For years, we have debated whether these subtle changes represent a precursor to EAC or simply a benign variant. We have wrestled with how to handle these cases from whether we should take biopsies to how to perform surveillance.
The American College of Gastroenterology (ACG) guideline suggests that irregular Z-lines should not be routinely biopsied or surveyed. Similarly, the upcoming American Gastroenterology Association (AGA) surveillance guideline suggests against surveillance of IM<1 cm citing the low individual annual risk of progression to high-grade dysplasia (HGD) and EAC of 0.23% per year which is lower than that of non-dysplastic Barrett’s esophagus (NDBE). However, this is not the entire picture.
Despite the low per-patient risk, IM<1cm is highly prevalent with columnar mucosa observed in approximately 15% of patients undergoing upper endoscopy. This paradox is unsettling. While any one patient with IM<1 cm is unlikely to progress to EAC, the group accounts for a meaningful share of the EAC burden. Some experts have argued that this justifies routine biopsy and surveillance in all patients with visible columnar mucosa regardless of length. However, this approach risks overwhelming our surveillance infrastructure.
A recent decision modeling analysis suggested that at the lowest progression rates, either no surveillance or one-time endoscopy can be considered. Based on these data, I do not regularly biopsy ultrashort segments unless the mucosa appears suspicious. In those with IM<1 cm detected during a high-quality endoscopic exam, no follow-up is needed. However, if the exam is suboptimal, I perform a 1-time high-quality repeat exam. If there is no evidence of dysplasia then I do not pursue any further surveillance.
The One-Year Follow-Up Endoscopy: Is It Necessary?
Another controversy is the one-year follow-up endoscopy after an initial diagnosis of NDBE. Proponents of this approach cite the high proportion of post endoscopy esophageal neoplasia and cancer (PEEN/PEEC) detected in the first year after diagnosis (missed HGD/EAC). In fact, PEEN account for about a quarter of all HGD/EAC cases diagnosed during surveillance.
While this approach might mitigate PEEN/PEEC risk, it may not be necessary if the index endoscopy is high quality. To ensure high quality exams, several best practices have been proposed including:
- Use of high-definition white light endoscopy (HD-WLE) with chromoendoscopy (virtual or dye based)
- Appropriate inspection time (1 minute per cm of circumferential BE)
- Accurate documentation using the Prague criteria
- Adherence to the Seattle protocol with additional targeted biopsies
If the index endoscopy meets these quality metrics, I typically do not bring the patient back at one year. However, if the exam quality is in question, then I repeat it at one year to establish a reliable baseline and rule out prevalent neoplasia.
Surveillance In NDBE: After BOSS, Do We Rethink Everything?
The recently published BOSS trial (Barrett’s Oesophagus Surveillance Study) has reignited the debate over the value of endoscopic surveillance in NDBE. In this study, 3,453 patients with NDBE across the UK were randomized to either surveillance endoscopy every two years or endoscopy only as clinically indicated. After a median follow-up of 12.8 years, the trial found no significant difference in all-cause mortality between the two groups.
While these findings are important, they should be interpreted with caution. First, the primary endpoint, all-cause mortality, is not optimal for evaluating surveillance for EAC. Surveillance is not intended to reduce all-cause mortality but rather to reduce EAC–related mortality. Second, a substantial number of patients in the no surveillance group still underwent endoscopy at intervals that were not meaningfully different from those in the surveillance group. If both groups receive similar exposure to endoscopy, the comparison loses power. Lastly, the trial was underpowered due to overestimation of progression risk during its initial design. As we have since learned, the risk of progression of NDBE is lower than originally assumed.
So where do we stand now? For me, the BOSS trial does not negate the value of surveillance. it reminds us that a one-size-fits-all approach is inefficient, and our strategy must be risk based. For low-risk individuals, particularly older adults with short-segment NDBE, surveillance may offer little benefit. But in healthier, younger patients with longer segments or additional risk factors, surveillance remains an essential tool for early neoplasia detection.
When to Stop Surveillance
Perhaps the most under-discussed point is when to stop surveillance. Existing guidelines do not account for competing mortality risks unrelated to EAC or provide specific recommendations regarding cessation of surveillance. The desired benefits of surveillance likely diminish with advanced age and greater comorbidity because of lower life expectancy and ineligibility for definitive therapy for EAC.
A recent modeling study found that the optimal ages for last surveillance were 81, 80, 77, and 73 years for men with no, mild, moderate, and severe comorbidity respectively and 75, 73, 73, and 69 years for women. In my practice, I discuss surveillance cessation in patients older than 75 based on their comorbidities. If the risk of progression is outweighed by the risk of the procedure or by the reality of limited life expectancy, we should not hesitate to consider surveillance cessation.
In summary, high-quality endoscopic exam in appropriately selected patients remains the cornerstone of BE surveillance. A more personalized, risk-based approach is needed taking into account competing comorbidities. Emerging technology through risk stratification tools such as biomarkers and artificial intelligence may refine our approach and help address the current limitations.
Dr. Sawas is based at the University of Texas Southwestern, Dallas, Texas. He has no disclosures in regard to this article.
IBD Medications Show No Link with Breast Cancer Recurrence
, according to investigators.
These findings diminish concerns that IBD therapy could theoretically reactivate dormant micrometastases, lead author Guillaume Le Cosquer, MD, of Toulouse University Hospital, Toulouse, France, and colleagues, reported.
“In patients with IBD, medical management of subjects with a history of breast cancer is a frequent and unresolved problem for clinicians,” the investigators wrote in Clinical Gastroenterology and Hepatology (2024 Nov. doi: 10.1016/j.cgh.2024.09.034).
Previous studies have reported that conventional immunosuppressants and biologics do not increase risk of incident cancer among IBD patients with a prior nondigestive malignancy; however, recent guidelines from the European Crohn’s and Colitis Organisation (ECCO) suggest that data are insufficient to make associated recommendations, prompting the present study.
“[T]he major strength of our work is that it is the first to focus on the most frequent cancer (breast cancer) in patients with IBD only, with the longest follow-up after breast cancer in patients with IBD ever published,” Dr. Le Cosquer and colleagues noted.
The dataset included 207 patients with IBD and a history of breast cancer, drawn from 7 tertiary centers across France.
The index date was the time of breast cancer diagnosis, and patients were followed for a median of 71 months. The median time from cancer diagnosis to initiation of IBD treatment was 28 months.
First-line post-cancer treatments included conventional immunosuppressants (19.3%), anti–tumor necrosis factor (anti-TNF) agents (19.8%), vedolizumab (7.2%), and ustekinumab (1.9%). Approximately half (51.6%) received no immunosuppressive therapy during follow-up.
Over the study period, 42 incident cancers were recorded (20.3%), among which 34 were breast cancer recurrences. Adjusted incidence rates per 1000 person-years were 10.2 (95% CI, 6.0–16.4) in the untreated group and 28.9 (95% CI, 11.6–59.6) in patients exposed to immunosuppressive or biologic therapies (P = .0519). Incident cancer–free survival did not differ significantly between treated and untreated groups (P = .4796).
On multivariable analysis, independent predictors of incident cancer included T4d stage (P = .036), triple-negative status (P = .016), and follow-up duration shorter than 71 months (P = .005).
“[I]mmunosuppressant and biologic use in selected patients with IBD with prior breast cancer does not seem to increase the risk of incident cancer,” the investigators wrote, noting that the main predictors of cancer recurrence were known poor prognostic features of breast cancer.
Dr. Le Cosquer and colleagues acknowledged a lack of prospective safety data for biologic therapies among patients with prior malignancy, as these individuals are often excluded from clinical trials. Still, they underscored alignment between their findings and earlier retrospective studies, including analyses from the SAPPHIRE registry and Medicare data, which also found no significant increase in breast cancer recurrence with anti-TNF agents or newer biologics such as vedolizumab and ustekinumab.
“Our findings will help clinicians to make decisions in multidisciplinary meetings to start immunosuppressants or biologics in case of IBD flare-up in these patients,” they concluded.
The investigators disclosed relationships with AbbVie, Janssen, Takeda, and others.
Patients with inflammatory bowel disease (IBD) are at risk for a host of other illnesses, including cancer, at rates similar to or greater than the general population. When faced with uncertainty about drug safety with a cancer diagnosis, the reflex is to avoid the therapy altogether. This may lead to significant flares which may in turn lead to difficulty in tolerating cancer therapy and a shortened and lower quality life.
Le Cosquer et al. address the question of the risk of incident cancer among patients with a history of breast cancer. The authors found that the risk was related to poor prognostic factors for breast cancer and not IBD therapy. This should be interpreted with caution as the numbers, though the largest reported, are 207 patients. After propensity score matching, crude incidence rates per 1000 person years appeared greater in the treatment arm (28.9) versus the untreated arm (10.2), P = .0519. With a greater number of patients, it is conceivable the difference is significant.
On the flip side, prior to diagnosis, the majority of IBD patients received immunosuppressant or biologic therapy; however, after the index cancer, 51.6% of patients received no treatment. The survival curves show a near 25% difference in favor of treated patients after 300 months, albeit with very small numbers, raising the question of whether withholding IBD therapy is more harmful.
It is reassuring that the multiple papers cited in the article have not shown an increase in solid organ tumors to date. However, the practitioner needs to balance maintenance of IBD remission and overall health with the risk of complications in the patient with underlying malignancy. This complex decision making will shift over time and should involve the patient, the oncologist, and the gastroenterologist. In my practice, thiopurines are avoided and anti-integrins and IL-23s are preferred. However, anti-TNF agents and JAK-inhibitors are used when the patients’ overall benefit from disease control outweighs their (theoretical) risk for recurrence, infection, and thromboembolism.
Uma Mahadevan, MD, AGAF, is the Lynne and Marc Benioff Professor of Gastroenterology, and director of the Colitis and Crohn’s Disease Center at the University of California, San Francisco. She declared research support from the Leona M. and Harry B. Helmsley Trust, and has served as a consultant for multiple pharmaceutical firms.
Patients with inflammatory bowel disease (IBD) are at risk for a host of other illnesses, including cancer, at rates similar to or greater than the general population. When faced with uncertainty about drug safety with a cancer diagnosis, the reflex is to avoid the therapy altogether. This may lead to significant flares which may in turn lead to difficulty in tolerating cancer therapy and a shortened and lower quality life.
Le Cosquer et al. address the question of the risk of incident cancer among patients with a history of breast cancer. The authors found that the risk was related to poor prognostic factors for breast cancer and not IBD therapy. This should be interpreted with caution as the numbers, though the largest reported, are 207 patients. After propensity score matching, crude incidence rates per 1000 person years appeared greater in the treatment arm (28.9) versus the untreated arm (10.2), P = .0519. With a greater number of patients, it is conceivable the difference is significant.
On the flip side, prior to diagnosis, the majority of IBD patients received immunosuppressant or biologic therapy; however, after the index cancer, 51.6% of patients received no treatment. The survival curves show a near 25% difference in favor of treated patients after 300 months, albeit with very small numbers, raising the question of whether withholding IBD therapy is more harmful.
It is reassuring that the multiple papers cited in the article have not shown an increase in solid organ tumors to date. However, the practitioner needs to balance maintenance of IBD remission and overall health with the risk of complications in the patient with underlying malignancy. This complex decision making will shift over time and should involve the patient, the oncologist, and the gastroenterologist. In my practice, thiopurines are avoided and anti-integrins and IL-23s are preferred. However, anti-TNF agents and JAK-inhibitors are used when the patients’ overall benefit from disease control outweighs their (theoretical) risk for recurrence, infection, and thromboembolism.
Uma Mahadevan, MD, AGAF, is the Lynne and Marc Benioff Professor of Gastroenterology, and director of the Colitis and Crohn’s Disease Center at the University of California, San Francisco. She declared research support from the Leona M. and Harry B. Helmsley Trust, and has served as a consultant for multiple pharmaceutical firms.
Patients with inflammatory bowel disease (IBD) are at risk for a host of other illnesses, including cancer, at rates similar to or greater than the general population. When faced with uncertainty about drug safety with a cancer diagnosis, the reflex is to avoid the therapy altogether. This may lead to significant flares which may in turn lead to difficulty in tolerating cancer therapy and a shortened and lower quality life.
Le Cosquer et al. address the question of the risk of incident cancer among patients with a history of breast cancer. The authors found that the risk was related to poor prognostic factors for breast cancer and not IBD therapy. This should be interpreted with caution as the numbers, though the largest reported, are 207 patients. After propensity score matching, crude incidence rates per 1000 person years appeared greater in the treatment arm (28.9) versus the untreated arm (10.2), P = .0519. With a greater number of patients, it is conceivable the difference is significant.
On the flip side, prior to diagnosis, the majority of IBD patients received immunosuppressant or biologic therapy; however, after the index cancer, 51.6% of patients received no treatment. The survival curves show a near 25% difference in favor of treated patients after 300 months, albeit with very small numbers, raising the question of whether withholding IBD therapy is more harmful.
It is reassuring that the multiple papers cited in the article have not shown an increase in solid organ tumors to date. However, the practitioner needs to balance maintenance of IBD remission and overall health with the risk of complications in the patient with underlying malignancy. This complex decision making will shift over time and should involve the patient, the oncologist, and the gastroenterologist. In my practice, thiopurines are avoided and anti-integrins and IL-23s are preferred. However, anti-TNF agents and JAK-inhibitors are used when the patients’ overall benefit from disease control outweighs their (theoretical) risk for recurrence, infection, and thromboembolism.
Uma Mahadevan, MD, AGAF, is the Lynne and Marc Benioff Professor of Gastroenterology, and director of the Colitis and Crohn’s Disease Center at the University of California, San Francisco. She declared research support from the Leona M. and Harry B. Helmsley Trust, and has served as a consultant for multiple pharmaceutical firms.
, according to investigators.
These findings diminish concerns that IBD therapy could theoretically reactivate dormant micrometastases, lead author Guillaume Le Cosquer, MD, of Toulouse University Hospital, Toulouse, France, and colleagues, reported.
“In patients with IBD, medical management of subjects with a history of breast cancer is a frequent and unresolved problem for clinicians,” the investigators wrote in Clinical Gastroenterology and Hepatology (2024 Nov. doi: 10.1016/j.cgh.2024.09.034).
Previous studies have reported that conventional immunosuppressants and biologics do not increase risk of incident cancer among IBD patients with a prior nondigestive malignancy; however, recent guidelines from the European Crohn’s and Colitis Organisation (ECCO) suggest that data are insufficient to make associated recommendations, prompting the present study.
“[T]he major strength of our work is that it is the first to focus on the most frequent cancer (breast cancer) in patients with IBD only, with the longest follow-up after breast cancer in patients with IBD ever published,” Dr. Le Cosquer and colleagues noted.
The dataset included 207 patients with IBD and a history of breast cancer, drawn from 7 tertiary centers across France.
The index date was the time of breast cancer diagnosis, and patients were followed for a median of 71 months. The median time from cancer diagnosis to initiation of IBD treatment was 28 months.
First-line post-cancer treatments included conventional immunosuppressants (19.3%), anti–tumor necrosis factor (anti-TNF) agents (19.8%), vedolizumab (7.2%), and ustekinumab (1.9%). Approximately half (51.6%) received no immunosuppressive therapy during follow-up.
Over the study period, 42 incident cancers were recorded (20.3%), among which 34 were breast cancer recurrences. Adjusted incidence rates per 1000 person-years were 10.2 (95% CI, 6.0–16.4) in the untreated group and 28.9 (95% CI, 11.6–59.6) in patients exposed to immunosuppressive or biologic therapies (P = .0519). Incident cancer–free survival did not differ significantly between treated and untreated groups (P = .4796).
On multivariable analysis, independent predictors of incident cancer included T4d stage (P = .036), triple-negative status (P = .016), and follow-up duration shorter than 71 months (P = .005).
“[I]mmunosuppressant and biologic use in selected patients with IBD with prior breast cancer does not seem to increase the risk of incident cancer,” the investigators wrote, noting that the main predictors of cancer recurrence were known poor prognostic features of breast cancer.
Dr. Le Cosquer and colleagues acknowledged a lack of prospective safety data for biologic therapies among patients with prior malignancy, as these individuals are often excluded from clinical trials. Still, they underscored alignment between their findings and earlier retrospective studies, including analyses from the SAPPHIRE registry and Medicare data, which also found no significant increase in breast cancer recurrence with anti-TNF agents or newer biologics such as vedolizumab and ustekinumab.
“Our findings will help clinicians to make decisions in multidisciplinary meetings to start immunosuppressants or biologics in case of IBD flare-up in these patients,” they concluded.
The investigators disclosed relationships with AbbVie, Janssen, Takeda, and others.
, according to investigators.
These findings diminish concerns that IBD therapy could theoretically reactivate dormant micrometastases, lead author Guillaume Le Cosquer, MD, of Toulouse University Hospital, Toulouse, France, and colleagues, reported.
“In patients with IBD, medical management of subjects with a history of breast cancer is a frequent and unresolved problem for clinicians,” the investigators wrote in Clinical Gastroenterology and Hepatology (2024 Nov. doi: 10.1016/j.cgh.2024.09.034).
Previous studies have reported that conventional immunosuppressants and biologics do not increase risk of incident cancer among IBD patients with a prior nondigestive malignancy; however, recent guidelines from the European Crohn’s and Colitis Organisation (ECCO) suggest that data are insufficient to make associated recommendations, prompting the present study.
“[T]he major strength of our work is that it is the first to focus on the most frequent cancer (breast cancer) in patients with IBD only, with the longest follow-up after breast cancer in patients with IBD ever published,” Dr. Le Cosquer and colleagues noted.
The dataset included 207 patients with IBD and a history of breast cancer, drawn from 7 tertiary centers across France.
The index date was the time of breast cancer diagnosis, and patients were followed for a median of 71 months. The median time from cancer diagnosis to initiation of IBD treatment was 28 months.
First-line post-cancer treatments included conventional immunosuppressants (19.3%), anti–tumor necrosis factor (anti-TNF) agents (19.8%), vedolizumab (7.2%), and ustekinumab (1.9%). Approximately half (51.6%) received no immunosuppressive therapy during follow-up.
Over the study period, 42 incident cancers were recorded (20.3%), among which 34 were breast cancer recurrences. Adjusted incidence rates per 1000 person-years were 10.2 (95% CI, 6.0–16.4) in the untreated group and 28.9 (95% CI, 11.6–59.6) in patients exposed to immunosuppressive or biologic therapies (P = .0519). Incident cancer–free survival did not differ significantly between treated and untreated groups (P = .4796).
On multivariable analysis, independent predictors of incident cancer included T4d stage (P = .036), triple-negative status (P = .016), and follow-up duration shorter than 71 months (P = .005).
“[I]mmunosuppressant and biologic use in selected patients with IBD with prior breast cancer does not seem to increase the risk of incident cancer,” the investigators wrote, noting that the main predictors of cancer recurrence were known poor prognostic features of breast cancer.
Dr. Le Cosquer and colleagues acknowledged a lack of prospective safety data for biologic therapies among patients with prior malignancy, as these individuals are often excluded from clinical trials. Still, they underscored alignment between their findings and earlier retrospective studies, including analyses from the SAPPHIRE registry and Medicare data, which also found no significant increase in breast cancer recurrence with anti-TNF agents or newer biologics such as vedolizumab and ustekinumab.
“Our findings will help clinicians to make decisions in multidisciplinary meetings to start immunosuppressants or biologics in case of IBD flare-up in these patients,” they concluded.
The investigators disclosed relationships with AbbVie, Janssen, Takeda, and others.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Chemotherapy Linked to Brain Atrophy in Patients With Breast Cancer
Patients with breast cancer who undergo chemotherapy may face an increased risk for brain atrophy and cognitive decline, new findings from a pilot study suggest.
Memory problems in patients with cancer may not stem solely from stress or anxiety related to their diagnosis but could reflect underlying changes in brain structure, study investigator Paul Edison, PhD, MPhil, professor of neuroscience and clinical professor of neurology at Imperial College London, England, told this news organization.
While the findings suggest that chemotherapy may contribute to neuronal damage, the researchers noted that many aspects of the relationship between treatment and brain changes remain unclear.
Edison highlighted three key areas that require further investigation — uncovering the mechanisms driving brain atrophy, determining the proportion of patients affected, and identifying effective prevention strategies.
Another investigator on the study, Laura Kenny, MD, PhD, associate professor and consultant medical oncologist at Imperial College London, noted that the issue has received limited attention to date but expressed hope that the findings will raise awareness and encourage further research, given its clinical importance.
The findings were presented on July 29 at the Alzheimer’s Association International Conference (AAIC) 2025.
Investigating Cognitive Impact
Advances in chemotherapeutic agents have improved survival rates in patients with cancer. However, challenges persist regarding the long-term impact of these drugs.
Chemotherapy-associated cognitive impairment, often referred to as “brain fog” or “chemobrain,” affects approximately one third of patients with breast cancer following treatment.
While cognitive decline resolves within 12 months for some patients, others experience persistent effects that may elevate the risk for neurodegenerative conditions, Edison explained.
To evaluate the impact of chemotherapy on the brain, investigators studied 328 women with nonmetastatic breast cancer who had undergone chemotherapy within the past 12 months.
Patients received either anthracycline — a drug derived from the Streptomyces peucetius bacterium — or taxanes such as docetaxel and paclitaxel, both commonly used in breast cancer treatment, or a combination of these agents. In addition, some patients may also have had hormone therapy at some point during treatment, said Kenny.
Participants completed neurocognitive prescreening tests every 3 months using a specialized artificial intelligence–driven platform, allowing them to take detailed memory assessments online from home.
Among those prescreened, 18 individuals with lower neurocognitive scores (mean age, 55 years) and 19 cognitively normal control individuals without breast cancer (mean age, 67 years) underwent comprehensive, in-person, neurocognitive evaluations and MRI scans.
Researchers analyzed the scans using region of interest (ROI) and voxel-based morphometry (VBM), which uses sophisticated computer software, to assess gray matter volumes and surface areas.
The ROI analysis revealed significant reductions in gray matter volume (measured in mm3) and surface area (measured in mm2) among patients experiencing chemobrain, particularly affecting the isthmus cingulate and pars opercularis, with changes extending into the orbitofrontal and temporal regions.
Significant Atrophy
The VBM analysis confirmed significant atrophy in the frontal, parietal, and cingulate regions of patients with chemobrain compared with control individuals (P < .05). Edison noted that this pattern overlaps with brain changes typically observed in Alzheimer’s disease and vascular cognitive impairment.
For both analyses, “we demonstrated there is some amount of shrinkage in the brain among patients with chemobrain,” he said. “The fact that controls are older means the results are even more significant as there’s more brain atrophy as people age.”
Some of the affected brain regions may be linked to impaired memory, a hallmark of Alzheimer’s disease, but Edison cautioned that given the small sample size this finding should be interpreted with caution.
While the analysis demonstrated overall lower brain volumes in patients with “chemobrain” compared with controls, Edison emphasized that this finding reflects a single time point and does not indicate brain shrinkage over time.
Other events, including stroke — can also cause brain changes.
Edison highlighted the importance of determining the significance of these brain changes, how they affect patients and whether they can be prevented.
In-person neurocognitive testing revealed significantly reduced semantic and verbal fluency, as well as lower Mini-Mental State Examination scores in patients with chemobrain. Edison noted that these results support the MRI findings.
The team plans to follow patients to track brain changes and memory recovery, Kenny said. While patients with breast cancer are a common focus, the researchers intend to expand the study to other cancers in both men and women, said Kenny.
Based on discussions with her oncology colleagues, Kenny noted that many patients anecdotally report experiencing memory problems during chemotherapy.
More Research Needed
Commenting for this news organization, Rebecca M. Edelmayer, PhD, vice president, scientific engagement, at the Alzheimer’s Association, said the research may help shed light on why women are more likely to develop dementia than men.
For years now, experts have been trying to figure out what puts women at higher risk for AD and other dementias, said Edelmayer.
“We still don’t understand whether this involves biologically driven risk factors or socially driven risk factors.”
Research linking treatments for other health conditions to increased memory problems may offer some clues, she noted, suggesting a potential avenue for further investigation into the intersection of chemotherapy and neurodegenerative diseases such as Alzheimer’s.
However, Edelmayer emphasized that this line of research is still in its infancy. Much more work is needed to determine whether there is a direct cause-and-effect relationship with specific chemotherapy drugs, and whether some patients may already be predisposed or at higher risk for cognitive decline, she said.
Also commenting for this news organization, Eric Brown, MD, associate scientist and associate chief of geriatric psychiatry at the Centre for Addiction and Mental Health in Toronto, raised concerns about the study’s design.
One issue, he noted, is that the researchers did not image all patients who received chemotherapy but instead selected those with the most significant cognitive impairment. As a result, the findings may not have reflected outcomes in the average post-chemotherapy patients but rather represent the most severely affected subgroup.
Brown pointed out that the study did not clarify whether this subgroup had comorbid conditions. It’s possible, he said, that some individuals may have had Alzheimer’s disease or other forms of dementia unrelated to chemotherapy.
He agreed that tracking longitudinal changes in both cognitive scores and neuroimaging — comparing patients who receive chemotherapy with those who do not — would be a valuable next step.
The investigators, Edelmayer, and Brown reported no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
Patients with breast cancer who undergo chemotherapy may face an increased risk for brain atrophy and cognitive decline, new findings from a pilot study suggest.
Memory problems in patients with cancer may not stem solely from stress or anxiety related to their diagnosis but could reflect underlying changes in brain structure, study investigator Paul Edison, PhD, MPhil, professor of neuroscience and clinical professor of neurology at Imperial College London, England, told this news organization.
While the findings suggest that chemotherapy may contribute to neuronal damage, the researchers noted that many aspects of the relationship between treatment and brain changes remain unclear.
Edison highlighted three key areas that require further investigation — uncovering the mechanisms driving brain atrophy, determining the proportion of patients affected, and identifying effective prevention strategies.
Another investigator on the study, Laura Kenny, MD, PhD, associate professor and consultant medical oncologist at Imperial College London, noted that the issue has received limited attention to date but expressed hope that the findings will raise awareness and encourage further research, given its clinical importance.
The findings were presented on July 29 at the Alzheimer’s Association International Conference (AAIC) 2025.
Investigating Cognitive Impact
Advances in chemotherapeutic agents have improved survival rates in patients with cancer. However, challenges persist regarding the long-term impact of these drugs.
Chemotherapy-associated cognitive impairment, often referred to as “brain fog” or “chemobrain,” affects approximately one third of patients with breast cancer following treatment.
While cognitive decline resolves within 12 months for some patients, others experience persistent effects that may elevate the risk for neurodegenerative conditions, Edison explained.
To evaluate the impact of chemotherapy on the brain, investigators studied 328 women with nonmetastatic breast cancer who had undergone chemotherapy within the past 12 months.
Patients received either anthracycline — a drug derived from the Streptomyces peucetius bacterium — or taxanes such as docetaxel and paclitaxel, both commonly used in breast cancer treatment, or a combination of these agents. In addition, some patients may also have had hormone therapy at some point during treatment, said Kenny.
Participants completed neurocognitive prescreening tests every 3 months using a specialized artificial intelligence–driven platform, allowing them to take detailed memory assessments online from home.
Among those prescreened, 18 individuals with lower neurocognitive scores (mean age, 55 years) and 19 cognitively normal control individuals without breast cancer (mean age, 67 years) underwent comprehensive, in-person, neurocognitive evaluations and MRI scans.
Researchers analyzed the scans using region of interest (ROI) and voxel-based morphometry (VBM), which uses sophisticated computer software, to assess gray matter volumes and surface areas.
The ROI analysis revealed significant reductions in gray matter volume (measured in mm3) and surface area (measured in mm2) among patients experiencing chemobrain, particularly affecting the isthmus cingulate and pars opercularis, with changes extending into the orbitofrontal and temporal regions.
Significant Atrophy
The VBM analysis confirmed significant atrophy in the frontal, parietal, and cingulate regions of patients with chemobrain compared with control individuals (P < .05). Edison noted that this pattern overlaps with brain changes typically observed in Alzheimer’s disease and vascular cognitive impairment.
For both analyses, “we demonstrated there is some amount of shrinkage in the brain among patients with chemobrain,” he said. “The fact that controls are older means the results are even more significant as there’s more brain atrophy as people age.”
Some of the affected brain regions may be linked to impaired memory, a hallmark of Alzheimer’s disease, but Edison cautioned that given the small sample size this finding should be interpreted with caution.
While the analysis demonstrated overall lower brain volumes in patients with “chemobrain” compared with controls, Edison emphasized that this finding reflects a single time point and does not indicate brain shrinkage over time.
Other events, including stroke — can also cause brain changes.
Edison highlighted the importance of determining the significance of these brain changes, how they affect patients and whether they can be prevented.
In-person neurocognitive testing revealed significantly reduced semantic and verbal fluency, as well as lower Mini-Mental State Examination scores in patients with chemobrain. Edison noted that these results support the MRI findings.
The team plans to follow patients to track brain changes and memory recovery, Kenny said. While patients with breast cancer are a common focus, the researchers intend to expand the study to other cancers in both men and women, said Kenny.
Based on discussions with her oncology colleagues, Kenny noted that many patients anecdotally report experiencing memory problems during chemotherapy.
More Research Needed
Commenting for this news organization, Rebecca M. Edelmayer, PhD, vice president, scientific engagement, at the Alzheimer’s Association, said the research may help shed light on why women are more likely to develop dementia than men.
For years now, experts have been trying to figure out what puts women at higher risk for AD and other dementias, said Edelmayer.
“We still don’t understand whether this involves biologically driven risk factors or socially driven risk factors.”
Research linking treatments for other health conditions to increased memory problems may offer some clues, she noted, suggesting a potential avenue for further investigation into the intersection of chemotherapy and neurodegenerative diseases such as Alzheimer’s.
However, Edelmayer emphasized that this line of research is still in its infancy. Much more work is needed to determine whether there is a direct cause-and-effect relationship with specific chemotherapy drugs, and whether some patients may already be predisposed or at higher risk for cognitive decline, she said.
Also commenting for this news organization, Eric Brown, MD, associate scientist and associate chief of geriatric psychiatry at the Centre for Addiction and Mental Health in Toronto, raised concerns about the study’s design.
One issue, he noted, is that the researchers did not image all patients who received chemotherapy but instead selected those with the most significant cognitive impairment. As a result, the findings may not have reflected outcomes in the average post-chemotherapy patients but rather represent the most severely affected subgroup.
Brown pointed out that the study did not clarify whether this subgroup had comorbid conditions. It’s possible, he said, that some individuals may have had Alzheimer’s disease or other forms of dementia unrelated to chemotherapy.
He agreed that tracking longitudinal changes in both cognitive scores and neuroimaging — comparing patients who receive chemotherapy with those who do not — would be a valuable next step.
The investigators, Edelmayer, and Brown reported no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
Patients with breast cancer who undergo chemotherapy may face an increased risk for brain atrophy and cognitive decline, new findings from a pilot study suggest.
Memory problems in patients with cancer may not stem solely from stress or anxiety related to their diagnosis but could reflect underlying changes in brain structure, study investigator Paul Edison, PhD, MPhil, professor of neuroscience and clinical professor of neurology at Imperial College London, England, told this news organization.
While the findings suggest that chemotherapy may contribute to neuronal damage, the researchers noted that many aspects of the relationship between treatment and brain changes remain unclear.
Edison highlighted three key areas that require further investigation — uncovering the mechanisms driving brain atrophy, determining the proportion of patients affected, and identifying effective prevention strategies.
Another investigator on the study, Laura Kenny, MD, PhD, associate professor and consultant medical oncologist at Imperial College London, noted that the issue has received limited attention to date but expressed hope that the findings will raise awareness and encourage further research, given its clinical importance.
The findings were presented on July 29 at the Alzheimer’s Association International Conference (AAIC) 2025.
Investigating Cognitive Impact
Advances in chemotherapeutic agents have improved survival rates in patients with cancer. However, challenges persist regarding the long-term impact of these drugs.
Chemotherapy-associated cognitive impairment, often referred to as “brain fog” or “chemobrain,” affects approximately one third of patients with breast cancer following treatment.
While cognitive decline resolves within 12 months for some patients, others experience persistent effects that may elevate the risk for neurodegenerative conditions, Edison explained.
To evaluate the impact of chemotherapy on the brain, investigators studied 328 women with nonmetastatic breast cancer who had undergone chemotherapy within the past 12 months.
Patients received either anthracycline — a drug derived from the Streptomyces peucetius bacterium — or taxanes such as docetaxel and paclitaxel, both commonly used in breast cancer treatment, or a combination of these agents. In addition, some patients may also have had hormone therapy at some point during treatment, said Kenny.
Participants completed neurocognitive prescreening tests every 3 months using a specialized artificial intelligence–driven platform, allowing them to take detailed memory assessments online from home.
Among those prescreened, 18 individuals with lower neurocognitive scores (mean age, 55 years) and 19 cognitively normal control individuals without breast cancer (mean age, 67 years) underwent comprehensive, in-person, neurocognitive evaluations and MRI scans.
Researchers analyzed the scans using region of interest (ROI) and voxel-based morphometry (VBM), which uses sophisticated computer software, to assess gray matter volumes and surface areas.
The ROI analysis revealed significant reductions in gray matter volume (measured in mm3) and surface area (measured in mm2) among patients experiencing chemobrain, particularly affecting the isthmus cingulate and pars opercularis, with changes extending into the orbitofrontal and temporal regions.
Significant Atrophy
The VBM analysis confirmed significant atrophy in the frontal, parietal, and cingulate regions of patients with chemobrain compared with control individuals (P < .05). Edison noted that this pattern overlaps with brain changes typically observed in Alzheimer’s disease and vascular cognitive impairment.
For both analyses, “we demonstrated there is some amount of shrinkage in the brain among patients with chemobrain,” he said. “The fact that controls are older means the results are even more significant as there’s more brain atrophy as people age.”
Some of the affected brain regions may be linked to impaired memory, a hallmark of Alzheimer’s disease, but Edison cautioned that given the small sample size this finding should be interpreted with caution.
While the analysis demonstrated overall lower brain volumes in patients with “chemobrain” compared with controls, Edison emphasized that this finding reflects a single time point and does not indicate brain shrinkage over time.
Other events, including stroke — can also cause brain changes.
Edison highlighted the importance of determining the significance of these brain changes, how they affect patients and whether they can be prevented.
In-person neurocognitive testing revealed significantly reduced semantic and verbal fluency, as well as lower Mini-Mental State Examination scores in patients with chemobrain. Edison noted that these results support the MRI findings.
The team plans to follow patients to track brain changes and memory recovery, Kenny said. While patients with breast cancer are a common focus, the researchers intend to expand the study to other cancers in both men and women, said Kenny.
Based on discussions with her oncology colleagues, Kenny noted that many patients anecdotally report experiencing memory problems during chemotherapy.
More Research Needed
Commenting for this news organization, Rebecca M. Edelmayer, PhD, vice president, scientific engagement, at the Alzheimer’s Association, said the research may help shed light on why women are more likely to develop dementia than men.
For years now, experts have been trying to figure out what puts women at higher risk for AD and other dementias, said Edelmayer.
“We still don’t understand whether this involves biologically driven risk factors or socially driven risk factors.”
Research linking treatments for other health conditions to increased memory problems may offer some clues, she noted, suggesting a potential avenue for further investigation into the intersection of chemotherapy and neurodegenerative diseases such as Alzheimer’s.
However, Edelmayer emphasized that this line of research is still in its infancy. Much more work is needed to determine whether there is a direct cause-and-effect relationship with specific chemotherapy drugs, and whether some patients may already be predisposed or at higher risk for cognitive decline, she said.
Also commenting for this news organization, Eric Brown, MD, associate scientist and associate chief of geriatric psychiatry at the Centre for Addiction and Mental Health in Toronto, raised concerns about the study’s design.
One issue, he noted, is that the researchers did not image all patients who received chemotherapy but instead selected those with the most significant cognitive impairment. As a result, the findings may not have reflected outcomes in the average post-chemotherapy patients but rather represent the most severely affected subgroup.
Brown pointed out that the study did not clarify whether this subgroup had comorbid conditions. It’s possible, he said, that some individuals may have had Alzheimer’s disease or other forms of dementia unrelated to chemotherapy.
He agreed that tracking longitudinal changes in both cognitive scores and neuroimaging — comparing patients who receive chemotherapy with those who do not — would be a valuable next step.
The investigators, Edelmayer, and Brown reported no relevant conflicts of interest. A version of this article first appeared on Medscape.com.
FROM AAIC 2025
These Two Simple Interventions May Cut Colorectal Cancer Recurrence Risk
This transcript has been edited for clarity.
New guidelines have lowered the age to begin screening for colon cancer to 45 years old. Although this change is positive, we’re still seeing advanced cancer in younger patients who haven’t been screened in time.
Once diagnosed, these patients undergo surgery and chemotherapy and often return to us asking, “What can I do now to help myself?”
Two recent studies highlight interventions that are simple, affordable, and actionable today: exercise and aspirin. Let’s take a closer look at the results.
Exercise’s Risk Reduction Potential
The idea that exercise reduces cancer recurrence and mortality is supported by observational data. The mechanistic effects behind this have been ascribed to metabolic growth factors, inflammatory changes, immune function changes, and perhaps even positive impact on sleep.
A study just published in The New England Journal of Medicine examined structured exercise after adjuvant chemotherapy for colon cancer. The phase 3 randomized CHALLENGE trial, mostly conducted at Canadian and Australian centers, recruited patients with resected stage II or III colon cancer (9.8% and 90.2%, respectively) who had completed adjuvant chemotherapy. Patients with recurrences within a year of diagnosis were excluded, as they were more likely to have highly aggressive, biologically active disease.
Patients were randomized to receive healthcare education materials alone or in conjunction with a structured exercise program over a 3-year follow-up period.
The exercise intervention, delivered in person or virtually, focused on increasing recreational aerobic activity over baseline by at least 10 metabolic equivalent task (MET). An increment of 10 MET hours per week is not too vigorous. It is essentially the equivalent of adding about 45-60 minutes of brisk walking or 25-30 minutes of jogging 3-4 times a week.
Patients were asked to increase MET over the first 6 months and then maintain or further increase the amount over the next 2.5 years. They were permitted to structure their own exercise program by choosing the type, frequency, intensity, and duration of aerobic exercise.
The primary endpoint was disease-free survival, with secondary endpoints assessing overall survival, patient-reported outcomes, and other outcomes. Although designed to detect differences at 3 years, follow-up was also performed out to 5 and 8 years.
At a median follow-up of 7.9 years, exercise reduced the relative risk of disease recurrence, new primary cancer, or death by 28% (P = .02). This benefit persisted — and even strengthened — over time, with disease-free survival increasing by 6.4 and 7.1 percentage points at 5 and 8 years, respectively.
Musculoskeletal adverse events were slightly higher in the exercise group compared with the health education group (18.5% vs 11.5%, respectively), but only 10% were directly attributed to the exercise.
There are considerations when interpreting these results. First, there was an attrition over time for compliance and training. It would be interesting to see whether that impacted the results. Second, it’s unclear whether patient pedigree or a genomic pathway may predispose to a benefit here for the exercise group.
But overall, this phase 3 trial provides class 1 evidence supporting exercise as a low-cost, high-impact intervention to reduce cancer recurrence.
Adjuvant Aspirin in Colon Cancer Subset
That’s a perfect segue into another recent study looking at the effects of adjuvant aspirin on the prevention of recurrence.
The ALASCCA trial— conducted across centers in Sweden, Denmark, Finland, and Norway — assessed patients with stage I-III rectal cancer or stage II-III colon cancer. It focused on a subset of patients with an oncogenic abnormality called PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha).
PIK3CA occurs in approximately a third of colon cancers and is associated with significant chemotherapy resistance and a higher rate of disease progression.
Of the included patients, 1103 (37%) had alterations in the PIK3CA pathway. Researchers randomized patients to receive either 160 mg of aspirin or placebo daily for 3 years, starting within 3 months of surgery.
Among patients with PIK3CA mutations, aspirin dramatically reduced the risk for time to recurrence by nearly 50% at 3 years (P = .044). Adverse events associated with aspirin were minimal, including one case each of gastrointestinal bleeding, hematoma, and allergic reaction.
There is no evidence that higher aspirin doses provide greater prevention of colorectal cancer recurrence. The 160-mg use in the current study is fairly normal, roughly equivalent to two low-dose (81-mg) aspirin tablets.
Now, it’s worth noting that the use of aspirin for the primary prevention of cardiovascular disease was initially recommended by the US Preventive Services Task Force in 2016. This recommendation was then recanted in 2022, when the same group reported limited net benefit to this approach.
Two Proactive Actions
These studies highlight 2 interventions — exercise and aspirin — that are low cost, accessible, and appeal to patients eager to help prevent their cancer from recurring.
Exercise is broadly beneficial and can be recommended immediately.
For aspirin, patients should work with their oncologist to determine their PIK3CA mutation status, as this subgroup appears to benefit the most.
These findings offer patients meaningful, proactive interventions they can apply to support their recovery and reduce the risk of recurrence. Hopefully these new findings will help guide your clinical conversations.
Johnson is a regular contributor to Medscape. He is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He disclosed that he is an adviser for ISOThrive.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
New guidelines have lowered the age to begin screening for colon cancer to 45 years old. Although this change is positive, we’re still seeing advanced cancer in younger patients who haven’t been screened in time.
Once diagnosed, these patients undergo surgery and chemotherapy and often return to us asking, “What can I do now to help myself?”
Two recent studies highlight interventions that are simple, affordable, and actionable today: exercise and aspirin. Let’s take a closer look at the results.
Exercise’s Risk Reduction Potential
The idea that exercise reduces cancer recurrence and mortality is supported by observational data. The mechanistic effects behind this have been ascribed to metabolic growth factors, inflammatory changes, immune function changes, and perhaps even positive impact on sleep.
A study just published in The New England Journal of Medicine examined structured exercise after adjuvant chemotherapy for colon cancer. The phase 3 randomized CHALLENGE trial, mostly conducted at Canadian and Australian centers, recruited patients with resected stage II or III colon cancer (9.8% and 90.2%, respectively) who had completed adjuvant chemotherapy. Patients with recurrences within a year of diagnosis were excluded, as they were more likely to have highly aggressive, biologically active disease.
Patients were randomized to receive healthcare education materials alone or in conjunction with a structured exercise program over a 3-year follow-up period.
The exercise intervention, delivered in person or virtually, focused on increasing recreational aerobic activity over baseline by at least 10 metabolic equivalent task (MET). An increment of 10 MET hours per week is not too vigorous. It is essentially the equivalent of adding about 45-60 minutes of brisk walking or 25-30 minutes of jogging 3-4 times a week.
Patients were asked to increase MET over the first 6 months and then maintain or further increase the amount over the next 2.5 years. They were permitted to structure their own exercise program by choosing the type, frequency, intensity, and duration of aerobic exercise.
The primary endpoint was disease-free survival, with secondary endpoints assessing overall survival, patient-reported outcomes, and other outcomes. Although designed to detect differences at 3 years, follow-up was also performed out to 5 and 8 years.
At a median follow-up of 7.9 years, exercise reduced the relative risk of disease recurrence, new primary cancer, or death by 28% (P = .02). This benefit persisted — and even strengthened — over time, with disease-free survival increasing by 6.4 and 7.1 percentage points at 5 and 8 years, respectively.
Musculoskeletal adverse events were slightly higher in the exercise group compared with the health education group (18.5% vs 11.5%, respectively), but only 10% were directly attributed to the exercise.
There are considerations when interpreting these results. First, there was an attrition over time for compliance and training. It would be interesting to see whether that impacted the results. Second, it’s unclear whether patient pedigree or a genomic pathway may predispose to a benefit here for the exercise group.
But overall, this phase 3 trial provides class 1 evidence supporting exercise as a low-cost, high-impact intervention to reduce cancer recurrence.
Adjuvant Aspirin in Colon Cancer Subset
That’s a perfect segue into another recent study looking at the effects of adjuvant aspirin on the prevention of recurrence.
The ALASCCA trial— conducted across centers in Sweden, Denmark, Finland, and Norway — assessed patients with stage I-III rectal cancer or stage II-III colon cancer. It focused on a subset of patients with an oncogenic abnormality called PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha).
PIK3CA occurs in approximately a third of colon cancers and is associated with significant chemotherapy resistance and a higher rate of disease progression.
Of the included patients, 1103 (37%) had alterations in the PIK3CA pathway. Researchers randomized patients to receive either 160 mg of aspirin or placebo daily for 3 years, starting within 3 months of surgery.
Among patients with PIK3CA mutations, aspirin dramatically reduced the risk for time to recurrence by nearly 50% at 3 years (P = .044). Adverse events associated with aspirin were minimal, including one case each of gastrointestinal bleeding, hematoma, and allergic reaction.
There is no evidence that higher aspirin doses provide greater prevention of colorectal cancer recurrence. The 160-mg use in the current study is fairly normal, roughly equivalent to two low-dose (81-mg) aspirin tablets.
Now, it’s worth noting that the use of aspirin for the primary prevention of cardiovascular disease was initially recommended by the US Preventive Services Task Force in 2016. This recommendation was then recanted in 2022, when the same group reported limited net benefit to this approach.
Two Proactive Actions
These studies highlight 2 interventions — exercise and aspirin — that are low cost, accessible, and appeal to patients eager to help prevent their cancer from recurring.
Exercise is broadly beneficial and can be recommended immediately.
For aspirin, patients should work with their oncologist to determine their PIK3CA mutation status, as this subgroup appears to benefit the most.
These findings offer patients meaningful, proactive interventions they can apply to support their recovery and reduce the risk of recurrence. Hopefully these new findings will help guide your clinical conversations.
Johnson is a regular contributor to Medscape. He is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He disclosed that he is an adviser for ISOThrive.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
New guidelines have lowered the age to begin screening for colon cancer to 45 years old. Although this change is positive, we’re still seeing advanced cancer in younger patients who haven’t been screened in time.
Once diagnosed, these patients undergo surgery and chemotherapy and often return to us asking, “What can I do now to help myself?”
Two recent studies highlight interventions that are simple, affordable, and actionable today: exercise and aspirin. Let’s take a closer look at the results.
Exercise’s Risk Reduction Potential
The idea that exercise reduces cancer recurrence and mortality is supported by observational data. The mechanistic effects behind this have been ascribed to metabolic growth factors, inflammatory changes, immune function changes, and perhaps even positive impact on sleep.
A study just published in The New England Journal of Medicine examined structured exercise after adjuvant chemotherapy for colon cancer. The phase 3 randomized CHALLENGE trial, mostly conducted at Canadian and Australian centers, recruited patients with resected stage II or III colon cancer (9.8% and 90.2%, respectively) who had completed adjuvant chemotherapy. Patients with recurrences within a year of diagnosis were excluded, as they were more likely to have highly aggressive, biologically active disease.
Patients were randomized to receive healthcare education materials alone or in conjunction with a structured exercise program over a 3-year follow-up period.
The exercise intervention, delivered in person or virtually, focused on increasing recreational aerobic activity over baseline by at least 10 metabolic equivalent task (MET). An increment of 10 MET hours per week is not too vigorous. It is essentially the equivalent of adding about 45-60 minutes of brisk walking or 25-30 minutes of jogging 3-4 times a week.
Patients were asked to increase MET over the first 6 months and then maintain or further increase the amount over the next 2.5 years. They were permitted to structure their own exercise program by choosing the type, frequency, intensity, and duration of aerobic exercise.
The primary endpoint was disease-free survival, with secondary endpoints assessing overall survival, patient-reported outcomes, and other outcomes. Although designed to detect differences at 3 years, follow-up was also performed out to 5 and 8 years.
At a median follow-up of 7.9 years, exercise reduced the relative risk of disease recurrence, new primary cancer, or death by 28% (P = .02). This benefit persisted — and even strengthened — over time, with disease-free survival increasing by 6.4 and 7.1 percentage points at 5 and 8 years, respectively.
Musculoskeletal adverse events were slightly higher in the exercise group compared with the health education group (18.5% vs 11.5%, respectively), but only 10% were directly attributed to the exercise.
There are considerations when interpreting these results. First, there was an attrition over time for compliance and training. It would be interesting to see whether that impacted the results. Second, it’s unclear whether patient pedigree or a genomic pathway may predispose to a benefit here for the exercise group.
But overall, this phase 3 trial provides class 1 evidence supporting exercise as a low-cost, high-impact intervention to reduce cancer recurrence.
Adjuvant Aspirin in Colon Cancer Subset
That’s a perfect segue into another recent study looking at the effects of adjuvant aspirin on the prevention of recurrence.
The ALASCCA trial— conducted across centers in Sweden, Denmark, Finland, and Norway — assessed patients with stage I-III rectal cancer or stage II-III colon cancer. It focused on a subset of patients with an oncogenic abnormality called PIK3CA (phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha).
PIK3CA occurs in approximately a third of colon cancers and is associated with significant chemotherapy resistance and a higher rate of disease progression.
Of the included patients, 1103 (37%) had alterations in the PIK3CA pathway. Researchers randomized patients to receive either 160 mg of aspirin or placebo daily for 3 years, starting within 3 months of surgery.
Among patients with PIK3CA mutations, aspirin dramatically reduced the risk for time to recurrence by nearly 50% at 3 years (P = .044). Adverse events associated with aspirin were minimal, including one case each of gastrointestinal bleeding, hematoma, and allergic reaction.
There is no evidence that higher aspirin doses provide greater prevention of colorectal cancer recurrence. The 160-mg use in the current study is fairly normal, roughly equivalent to two low-dose (81-mg) aspirin tablets.
Now, it’s worth noting that the use of aspirin for the primary prevention of cardiovascular disease was initially recommended by the US Preventive Services Task Force in 2016. This recommendation was then recanted in 2022, when the same group reported limited net benefit to this approach.
Two Proactive Actions
These studies highlight 2 interventions — exercise and aspirin — that are low cost, accessible, and appeal to patients eager to help prevent their cancer from recurring.
Exercise is broadly beneficial and can be recommended immediately.
For aspirin, patients should work with their oncologist to determine their PIK3CA mutation status, as this subgroup appears to benefit the most.
These findings offer patients meaningful, proactive interventions they can apply to support their recovery and reduce the risk of recurrence. Hopefully these new findings will help guide your clinical conversations.
Johnson is a regular contributor to Medscape. He is professor of medicine and chief of gastroenterology at Eastern Virginia Medical School in Norfolk, and a past president of the American College of Gastroenterology. His primary focus is the clinical practice of gastroenterology. He has published extensively in the internal medicine/gastroenterology literature, with principal research interests in esophageal and colon disease, and more recently in sleep and microbiome effects on gastrointestinal health and disease. He disclosed that he is an adviser for ISOThrive.
A version of this article appeared on Medscape.com.
Endometrial Cancer: 5 Things to Know
Endometrial cancer is a common type of gynecologic cancer, and its incidence is rising steadily in the United States and globally. Most cases are endometrioid adenocarcinomas, arising from the inner lining of the uterus — the endometrium. While many patients are diagnosed early because of noticeable symptoms like abnormal bleeding, trends in both incidence and mortality are concerning, especially given the persistent racial and socioeconomic disparities in outcomes.
In addition to being the most common uterine malignancy, endometrial cancer is at the forefront of precision oncology in gynecology. The traditional classification systems based on histology and hormone dependence are now being augmented by molecular subtyping that better informs prognosis and treatment. As diagnostic tools, genetic testing, and therapeutic strategies advance, the management of endometrial cancer is becoming increasingly personalized.
Here are five things to know about endometrial cancer:
1. Endometrial cancer is one of the few cancers with increasing mortality.
Endometrial cancer accounts for the majority of uterine cancers in the United States with an overall lifetime risk for women of about 1 in 40. Since the mid-2000s, incidence rates have risen steadily, by > 1% per year, reflecting both lifestyle and environmental factors. Importantly, the disease tends to be diagnosed at an early stage due to the presence of warning signs like postmenopausal bleeding, which contributes to relatively favorable survival outcomes when caught early.
However, mortality trends continue to evolve. From 1999 to 2013, death rates from endometrial cancer in the US declined slightly, but since 2013, they have increased sharply — by > 8% annually — according to recent data. This upward trend in mortality disproportionately affects non-Hispanic Black women, who experience the highest mortality rate (4.7 per 100,000) among all racial and ethnic groups. This disparity is likely caused by a complex interplay of factors, including delays in diagnosis, more aggressive tumor biology, and inequities in access to care. Addressing these disparities remains a key priority in improving outcomes.
2. Risk factors go beyond hormones and age.
Risk factors for endometrial cancer include prolonged exposure to unopposed estrogen, which can result from estrogen-only hormone replacement therapy, higher BMI, and early menarche or late menopause. Nulliparity (having never been pregnant) and older age also increase risk, as does tamoxifen use — a medication commonly prescribed for breast cancer prevention. These factors cumulatively increase endometrial proliferation and the potential for atypical cellular changes. Endometrial hyperplasia, a known precursor to cancer, is often linked to these hormonal imbalances and may require surveillance or treatment.
Beyond estrogen’s influence, a growing body of research is uncovering additional risk contributors. Women with polycystic ovary syndrome (PCOS), metabolic syndrome, or diabetes face elevated risk of developing endometrial cancer. Genetic syndromes, particularly Lynch and Cowden syndromes, are associated with significantly increased lifetime risks of endometrial cancer. Environmental exposures, such as the use of hair relaxers, are being investigated as emerging risk factors. Additionally, race remains a risk marker, with Black women not only experiencing higher mortality but also more aggressive subtypes of the disease. These complex, overlapping risks highlight the importance of individualized risk assessment and early intervention strategies.
3. Postmenopausal bleeding is the hallmark symptom — but not the only one.
In endometrial cancer, the majority of cases are diagnosed at an early stage, largely because of the hallmark symptom of postmenopausal bleeding. In addition to bleeding, patients may present with vaginal discharge, pyometra, and even pain and abdominal distension in advanced disease. Any bleeding in a postmenopausal woman should prompt evaluation, as it may signal endometrial hyperplasia or carcinoma. In premenopausal women, irregular or heavy menstrual bleeding may raise suspicion, particularly when accompanied by risk factors such as PCOS.
The diagnostic workup for suspected endometrial cancer in women, particularly those presenting with postmenopausal bleeding, begins with a focused clinical assessment and frequently includes transvaginal ultrasound (TVUS) to evaluate the endometrium. While TVUS can aid in identifying structural abnormalities or suggest malignancy, endometrial sampling is warranted in all postmenopausal women with abnormal bleeding, regardless of endometrial thickness. Office-based biopsy is the preferred initial approach due to its convenience and diagnostic yield; however, if the sample is nondiagnostic or technically difficult to obtain, hysteroscopy with directed biopsy or dilation and curettage should be pursued.
4. Classification systems are evolving to include molecular subtypes.
Historically, endometrial cancers were classified using the World Health Organization system based on histology and by hormone dependence: Type 1 (estrogen-dependent, typically endometrioid and low grade) and Type 2 (non-estrogen dependent, often serous and high grade). Type 1 cancers tend to have a better prognosis and slower progression, while Type 2 cancers are more aggressive and require intensive treatment. While helpful, this binary classification does not fully capture the biological diversity or treatment responsiveness of the disease.
The field is now moving toward molecular classification, which offers a more nuanced understanding. The four main molecular subtypes include: polymerase epsilon (POLE)-mutant, mismatch repair (MMR)-deficient, p53-abnormal, and no specific molecular profile (NSMP). These groups differ in prognosis and therapeutic implications. POLE-mutant tumors with extremely high mutational burdens generally have excellent outcomes and may not require aggressive adjuvant therapy. In contrast, p53-abnormal tumors are associated with chromosomal instability, TP53 mutations, and poor outcomes, necessitating more aggressive multimodal treatment. MMR-deficient tumors are particularly responsive to immunotherapy. These molecular distinctions are changing how clinicians approach risk stratification and management in patients with endometrial cancer.
5. Treatment is increasingly personalized — and immunotherapy is expanding.
The cornerstone of treatment for early-stage endometrial cancer is surgical: total hysterectomy with bilateral salpingo-oophorectomy, often with sentinel node mapping or lymphadenectomy. Adjuvant therapy depends on factors such as stage, grade, histology, and molecular subtype. Fertility-sparing management with progestin therapy is an option for highly selected patients with early-stage, low-grade tumors. Clinical guidelines recommend that fertility desires be addressed prior to initiating treatment, as standard surgical management typically results in loss of reproductive capacity.
For advanced or recurrent disease, treatment becomes more complex and increasingly individualized. Chemotherapy, often with carboplatin and paclitaxel, is standard for stage III/IV and recurrent disease. Molecular findings now guide additional therapy: For instance, MMR-deficient tumors may respond to checkpoint inhibitors. As targeted agents and combination regimens continue to emerge, treatment of endometrial is increasingly focused on precision medicine.
Markman is professor of medical oncology and therapeutics research and President of Medicine & Science at City of Hope in Atlanta and Chicago. He has disclosed relevant financial relationships with AstraZeneca, GSK and Myriad.
A version of this article first appeared on Medscape.com.
Endometrial cancer is a common type of gynecologic cancer, and its incidence is rising steadily in the United States and globally. Most cases are endometrioid adenocarcinomas, arising from the inner lining of the uterus — the endometrium. While many patients are diagnosed early because of noticeable symptoms like abnormal bleeding, trends in both incidence and mortality are concerning, especially given the persistent racial and socioeconomic disparities in outcomes.
In addition to being the most common uterine malignancy, endometrial cancer is at the forefront of precision oncology in gynecology. The traditional classification systems based on histology and hormone dependence are now being augmented by molecular subtyping that better informs prognosis and treatment. As diagnostic tools, genetic testing, and therapeutic strategies advance, the management of endometrial cancer is becoming increasingly personalized.
Here are five things to know about endometrial cancer:
1. Endometrial cancer is one of the few cancers with increasing mortality.
Endometrial cancer accounts for the majority of uterine cancers in the United States with an overall lifetime risk for women of about 1 in 40. Since the mid-2000s, incidence rates have risen steadily, by > 1% per year, reflecting both lifestyle and environmental factors. Importantly, the disease tends to be diagnosed at an early stage due to the presence of warning signs like postmenopausal bleeding, which contributes to relatively favorable survival outcomes when caught early.
However, mortality trends continue to evolve. From 1999 to 2013, death rates from endometrial cancer in the US declined slightly, but since 2013, they have increased sharply — by > 8% annually — according to recent data. This upward trend in mortality disproportionately affects non-Hispanic Black women, who experience the highest mortality rate (4.7 per 100,000) among all racial and ethnic groups. This disparity is likely caused by a complex interplay of factors, including delays in diagnosis, more aggressive tumor biology, and inequities in access to care. Addressing these disparities remains a key priority in improving outcomes.
2. Risk factors go beyond hormones and age.
Risk factors for endometrial cancer include prolonged exposure to unopposed estrogen, which can result from estrogen-only hormone replacement therapy, higher BMI, and early menarche or late menopause. Nulliparity (having never been pregnant) and older age also increase risk, as does tamoxifen use — a medication commonly prescribed for breast cancer prevention. These factors cumulatively increase endometrial proliferation and the potential for atypical cellular changes. Endometrial hyperplasia, a known precursor to cancer, is often linked to these hormonal imbalances and may require surveillance or treatment.
Beyond estrogen’s influence, a growing body of research is uncovering additional risk contributors. Women with polycystic ovary syndrome (PCOS), metabolic syndrome, or diabetes face elevated risk of developing endometrial cancer. Genetic syndromes, particularly Lynch and Cowden syndromes, are associated with significantly increased lifetime risks of endometrial cancer. Environmental exposures, such as the use of hair relaxers, are being investigated as emerging risk factors. Additionally, race remains a risk marker, with Black women not only experiencing higher mortality but also more aggressive subtypes of the disease. These complex, overlapping risks highlight the importance of individualized risk assessment and early intervention strategies.
3. Postmenopausal bleeding is the hallmark symptom — but not the only one.
In endometrial cancer, the majority of cases are diagnosed at an early stage, largely because of the hallmark symptom of postmenopausal bleeding. In addition to bleeding, patients may present with vaginal discharge, pyometra, and even pain and abdominal distension in advanced disease. Any bleeding in a postmenopausal woman should prompt evaluation, as it may signal endometrial hyperplasia or carcinoma. In premenopausal women, irregular or heavy menstrual bleeding may raise suspicion, particularly when accompanied by risk factors such as PCOS.
The diagnostic workup for suspected endometrial cancer in women, particularly those presenting with postmenopausal bleeding, begins with a focused clinical assessment and frequently includes transvaginal ultrasound (TVUS) to evaluate the endometrium. While TVUS can aid in identifying structural abnormalities or suggest malignancy, endometrial sampling is warranted in all postmenopausal women with abnormal bleeding, regardless of endometrial thickness. Office-based biopsy is the preferred initial approach due to its convenience and diagnostic yield; however, if the sample is nondiagnostic or technically difficult to obtain, hysteroscopy with directed biopsy or dilation and curettage should be pursued.
4. Classification systems are evolving to include molecular subtypes.
Historically, endometrial cancers were classified using the World Health Organization system based on histology and by hormone dependence: Type 1 (estrogen-dependent, typically endometrioid and low grade) and Type 2 (non-estrogen dependent, often serous and high grade). Type 1 cancers tend to have a better prognosis and slower progression, while Type 2 cancers are more aggressive and require intensive treatment. While helpful, this binary classification does not fully capture the biological diversity or treatment responsiveness of the disease.
The field is now moving toward molecular classification, which offers a more nuanced understanding. The four main molecular subtypes include: polymerase epsilon (POLE)-mutant, mismatch repair (MMR)-deficient, p53-abnormal, and no specific molecular profile (NSMP). These groups differ in prognosis and therapeutic implications. POLE-mutant tumors with extremely high mutational burdens generally have excellent outcomes and may not require aggressive adjuvant therapy. In contrast, p53-abnormal tumors are associated with chromosomal instability, TP53 mutations, and poor outcomes, necessitating more aggressive multimodal treatment. MMR-deficient tumors are particularly responsive to immunotherapy. These molecular distinctions are changing how clinicians approach risk stratification and management in patients with endometrial cancer.
5. Treatment is increasingly personalized — and immunotherapy is expanding.
The cornerstone of treatment for early-stage endometrial cancer is surgical: total hysterectomy with bilateral salpingo-oophorectomy, often with sentinel node mapping or lymphadenectomy. Adjuvant therapy depends on factors such as stage, grade, histology, and molecular subtype. Fertility-sparing management with progestin therapy is an option for highly selected patients with early-stage, low-grade tumors. Clinical guidelines recommend that fertility desires be addressed prior to initiating treatment, as standard surgical management typically results in loss of reproductive capacity.
For advanced or recurrent disease, treatment becomes more complex and increasingly individualized. Chemotherapy, often with carboplatin and paclitaxel, is standard for stage III/IV and recurrent disease. Molecular findings now guide additional therapy: For instance, MMR-deficient tumors may respond to checkpoint inhibitors. As targeted agents and combination regimens continue to emerge, treatment of endometrial is increasingly focused on precision medicine.
Markman is professor of medical oncology and therapeutics research and President of Medicine & Science at City of Hope in Atlanta and Chicago. He has disclosed relevant financial relationships with AstraZeneca, GSK and Myriad.
A version of this article first appeared on Medscape.com.
Endometrial cancer is a common type of gynecologic cancer, and its incidence is rising steadily in the United States and globally. Most cases are endometrioid adenocarcinomas, arising from the inner lining of the uterus — the endometrium. While many patients are diagnosed early because of noticeable symptoms like abnormal bleeding, trends in both incidence and mortality are concerning, especially given the persistent racial and socioeconomic disparities in outcomes.
In addition to being the most common uterine malignancy, endometrial cancer is at the forefront of precision oncology in gynecology. The traditional classification systems based on histology and hormone dependence are now being augmented by molecular subtyping that better informs prognosis and treatment. As diagnostic tools, genetic testing, and therapeutic strategies advance, the management of endometrial cancer is becoming increasingly personalized.
Here are five things to know about endometrial cancer:
1. Endometrial cancer is one of the few cancers with increasing mortality.
Endometrial cancer accounts for the majority of uterine cancers in the United States with an overall lifetime risk for women of about 1 in 40. Since the mid-2000s, incidence rates have risen steadily, by > 1% per year, reflecting both lifestyle and environmental factors. Importantly, the disease tends to be diagnosed at an early stage due to the presence of warning signs like postmenopausal bleeding, which contributes to relatively favorable survival outcomes when caught early.
However, mortality trends continue to evolve. From 1999 to 2013, death rates from endometrial cancer in the US declined slightly, but since 2013, they have increased sharply — by > 8% annually — according to recent data. This upward trend in mortality disproportionately affects non-Hispanic Black women, who experience the highest mortality rate (4.7 per 100,000) among all racial and ethnic groups. This disparity is likely caused by a complex interplay of factors, including delays in diagnosis, more aggressive tumor biology, and inequities in access to care. Addressing these disparities remains a key priority in improving outcomes.
2. Risk factors go beyond hormones and age.
Risk factors for endometrial cancer include prolonged exposure to unopposed estrogen, which can result from estrogen-only hormone replacement therapy, higher BMI, and early menarche or late menopause. Nulliparity (having never been pregnant) and older age also increase risk, as does tamoxifen use — a medication commonly prescribed for breast cancer prevention. These factors cumulatively increase endometrial proliferation and the potential for atypical cellular changes. Endometrial hyperplasia, a known precursor to cancer, is often linked to these hormonal imbalances and may require surveillance or treatment.
Beyond estrogen’s influence, a growing body of research is uncovering additional risk contributors. Women with polycystic ovary syndrome (PCOS), metabolic syndrome, or diabetes face elevated risk of developing endometrial cancer. Genetic syndromes, particularly Lynch and Cowden syndromes, are associated with significantly increased lifetime risks of endometrial cancer. Environmental exposures, such as the use of hair relaxers, are being investigated as emerging risk factors. Additionally, race remains a risk marker, with Black women not only experiencing higher mortality but also more aggressive subtypes of the disease. These complex, overlapping risks highlight the importance of individualized risk assessment and early intervention strategies.
3. Postmenopausal bleeding is the hallmark symptom — but not the only one.
In endometrial cancer, the majority of cases are diagnosed at an early stage, largely because of the hallmark symptom of postmenopausal bleeding. In addition to bleeding, patients may present with vaginal discharge, pyometra, and even pain and abdominal distension in advanced disease. Any bleeding in a postmenopausal woman should prompt evaluation, as it may signal endometrial hyperplasia or carcinoma. In premenopausal women, irregular or heavy menstrual bleeding may raise suspicion, particularly when accompanied by risk factors such as PCOS.
The diagnostic workup for suspected endometrial cancer in women, particularly those presenting with postmenopausal bleeding, begins with a focused clinical assessment and frequently includes transvaginal ultrasound (TVUS) to evaluate the endometrium. While TVUS can aid in identifying structural abnormalities or suggest malignancy, endometrial sampling is warranted in all postmenopausal women with abnormal bleeding, regardless of endometrial thickness. Office-based biopsy is the preferred initial approach due to its convenience and diagnostic yield; however, if the sample is nondiagnostic or technically difficult to obtain, hysteroscopy with directed biopsy or dilation and curettage should be pursued.
4. Classification systems are evolving to include molecular subtypes.
Historically, endometrial cancers were classified using the World Health Organization system based on histology and by hormone dependence: Type 1 (estrogen-dependent, typically endometrioid and low grade) and Type 2 (non-estrogen dependent, often serous and high grade). Type 1 cancers tend to have a better prognosis and slower progression, while Type 2 cancers are more aggressive and require intensive treatment. While helpful, this binary classification does not fully capture the biological diversity or treatment responsiveness of the disease.
The field is now moving toward molecular classification, which offers a more nuanced understanding. The four main molecular subtypes include: polymerase epsilon (POLE)-mutant, mismatch repair (MMR)-deficient, p53-abnormal, and no specific molecular profile (NSMP). These groups differ in prognosis and therapeutic implications. POLE-mutant tumors with extremely high mutational burdens generally have excellent outcomes and may not require aggressive adjuvant therapy. In contrast, p53-abnormal tumors are associated with chromosomal instability, TP53 mutations, and poor outcomes, necessitating more aggressive multimodal treatment. MMR-deficient tumors are particularly responsive to immunotherapy. These molecular distinctions are changing how clinicians approach risk stratification and management in patients with endometrial cancer.
5. Treatment is increasingly personalized — and immunotherapy is expanding.
The cornerstone of treatment for early-stage endometrial cancer is surgical: total hysterectomy with bilateral salpingo-oophorectomy, often with sentinel node mapping or lymphadenectomy. Adjuvant therapy depends on factors such as stage, grade, histology, and molecular subtype. Fertility-sparing management with progestin therapy is an option for highly selected patients with early-stage, low-grade tumors. Clinical guidelines recommend that fertility desires be addressed prior to initiating treatment, as standard surgical management typically results in loss of reproductive capacity.
For advanced or recurrent disease, treatment becomes more complex and increasingly individualized. Chemotherapy, often with carboplatin and paclitaxel, is standard for stage III/IV and recurrent disease. Molecular findings now guide additional therapy: For instance, MMR-deficient tumors may respond to checkpoint inhibitors. As targeted agents and combination regimens continue to emerge, treatment of endometrial is increasingly focused on precision medicine.
Markman is professor of medical oncology and therapeutics research and President of Medicine & Science at City of Hope in Atlanta and Chicago. He has disclosed relevant financial relationships with AstraZeneca, GSK and Myriad.
A version of this article first appeared on Medscape.com.
Earlier Vaccinations Helped Limit Marine Adenovirus Outbreak
Earlier Vaccinations Helped Limit Marine Adenovirus Outbreak
During an adenovirus (AdV) outbreak among recruits and staff at the Marine Corps Recruit Depot (MCRD) in San Diego, an investigation revealed that the earlier individuals working at the site received vaccination, the better. The clinical team found that accelerating the vaccination schedule could help prevent further outbreaks, medical separations, and training disruption.
From July 1, 2024, through September 23, 2024, a total of 212 trainees and staff developed AdV and 28 were hospitalized. Nine patients were hospitalized with AdV pneumonia within a 2-week period; 3 were admitted to the intensive care unit. Outpatient acute respiratory disease (ARD) cases also increased, with recruits accounting for nearly 97% of the AdV outbreak cases.
AdV is a frequent cause of illness among military recruits. Research has found that up to 80% of cases of febrile ARD in recruits are due to AdV, and 20% result in hospitalization.
The military developed and implemented a live, oral vaccine against AdV serotypes 4 and 7 (most common in recruits) starting in the 1970s, reducing febrile respiratory illness in recruit training sites by 50% and AdV infection by > 90%. However, the manufacturer halted production of the vaccine in 1995. By 1999, vaccine supply was depleted, and ARD cases rose. A replacement vaccine introduced in 2011 proved 99% effective, leading to a dramatic 100-fold decline in AdV disease among military trainees.
While the vaccine is effective, outbreaks are still possible among closely congregating groups like military trainees. AdV pneumonia cases spiked as the virus spread through the training companies and into new companies when they arrived at the MCRD in early July 2024. Most new infections were in recruits who had missed the AdV vaccination day.
Early symptoms of AdV may be very mild, and some recruits were likely already symptomatic when vaccinated. Aggressive environmental cleaning, separation of sick and well recruits, masking, and other nonpharmaceutical interventions did not slow the spread.
The preventive medicine and public health teams noted that AdV vaccination was being administered 11 days postarrival, to allow for pregnancy testing, and for assessing vaccine titers. US Department of Defense regulations do not dictate precise vaccination schedules. Implementation of the regulation varies among military training sites.
After reviewing other training sites’ vaccine timing schedules (most required vaccination by day 6 postarrival) and determining the time required for immunity, the medical teams at MCRD recommended shifting AdV vaccine administration, along with other standard vaccines, from day 11 to day 1 postarrival. Two weeks after the schedule change, overall incidence began declining rapidly.
Nearly 75% of patients had coinfections with other respiratory pathogens, most notably seasonal coronaviruses, COVID-19, and rhinovirus/enterovirus, suggesting that infection with AdV may increase susceptibility to other viruses, a finding that has not been identified in previous AdV outbreaks. Newly increased testing sensitivity associated with multiplex respiratory pathogen PCR availability may have been a factor in coinfection identification during this outbreak.
AdV is a significant medical threat to military recruits. Early vaccination, the investigators advise, should remain “a central tenet for prevention and control of communicable diseases in these high-risk, congregate settings.”
During an adenovirus (AdV) outbreak among recruits and staff at the Marine Corps Recruit Depot (MCRD) in San Diego, an investigation revealed that the earlier individuals working at the site received vaccination, the better. The clinical team found that accelerating the vaccination schedule could help prevent further outbreaks, medical separations, and training disruption.
From July 1, 2024, through September 23, 2024, a total of 212 trainees and staff developed AdV and 28 were hospitalized. Nine patients were hospitalized with AdV pneumonia within a 2-week period; 3 were admitted to the intensive care unit. Outpatient acute respiratory disease (ARD) cases also increased, with recruits accounting for nearly 97% of the AdV outbreak cases.
AdV is a frequent cause of illness among military recruits. Research has found that up to 80% of cases of febrile ARD in recruits are due to AdV, and 20% result in hospitalization.
The military developed and implemented a live, oral vaccine against AdV serotypes 4 and 7 (most common in recruits) starting in the 1970s, reducing febrile respiratory illness in recruit training sites by 50% and AdV infection by > 90%. However, the manufacturer halted production of the vaccine in 1995. By 1999, vaccine supply was depleted, and ARD cases rose. A replacement vaccine introduced in 2011 proved 99% effective, leading to a dramatic 100-fold decline in AdV disease among military trainees.
While the vaccine is effective, outbreaks are still possible among closely congregating groups like military trainees. AdV pneumonia cases spiked as the virus spread through the training companies and into new companies when they arrived at the MCRD in early July 2024. Most new infections were in recruits who had missed the AdV vaccination day.
Early symptoms of AdV may be very mild, and some recruits were likely already symptomatic when vaccinated. Aggressive environmental cleaning, separation of sick and well recruits, masking, and other nonpharmaceutical interventions did not slow the spread.
The preventive medicine and public health teams noted that AdV vaccination was being administered 11 days postarrival, to allow for pregnancy testing, and for assessing vaccine titers. US Department of Defense regulations do not dictate precise vaccination schedules. Implementation of the regulation varies among military training sites.
After reviewing other training sites’ vaccine timing schedules (most required vaccination by day 6 postarrival) and determining the time required for immunity, the medical teams at MCRD recommended shifting AdV vaccine administration, along with other standard vaccines, from day 11 to day 1 postarrival. Two weeks after the schedule change, overall incidence began declining rapidly.
Nearly 75% of patients had coinfections with other respiratory pathogens, most notably seasonal coronaviruses, COVID-19, and rhinovirus/enterovirus, suggesting that infection with AdV may increase susceptibility to other viruses, a finding that has not been identified in previous AdV outbreaks. Newly increased testing sensitivity associated with multiplex respiratory pathogen PCR availability may have been a factor in coinfection identification during this outbreak.
AdV is a significant medical threat to military recruits. Early vaccination, the investigators advise, should remain “a central tenet for prevention and control of communicable diseases in these high-risk, congregate settings.”
During an adenovirus (AdV) outbreak among recruits and staff at the Marine Corps Recruit Depot (MCRD) in San Diego, an investigation revealed that the earlier individuals working at the site received vaccination, the better. The clinical team found that accelerating the vaccination schedule could help prevent further outbreaks, medical separations, and training disruption.
From July 1, 2024, through September 23, 2024, a total of 212 trainees and staff developed AdV and 28 were hospitalized. Nine patients were hospitalized with AdV pneumonia within a 2-week period; 3 were admitted to the intensive care unit. Outpatient acute respiratory disease (ARD) cases also increased, with recruits accounting for nearly 97% of the AdV outbreak cases.
AdV is a frequent cause of illness among military recruits. Research has found that up to 80% of cases of febrile ARD in recruits are due to AdV, and 20% result in hospitalization.
The military developed and implemented a live, oral vaccine against AdV serotypes 4 and 7 (most common in recruits) starting in the 1970s, reducing febrile respiratory illness in recruit training sites by 50% and AdV infection by > 90%. However, the manufacturer halted production of the vaccine in 1995. By 1999, vaccine supply was depleted, and ARD cases rose. A replacement vaccine introduced in 2011 proved 99% effective, leading to a dramatic 100-fold decline in AdV disease among military trainees.
While the vaccine is effective, outbreaks are still possible among closely congregating groups like military trainees. AdV pneumonia cases spiked as the virus spread through the training companies and into new companies when they arrived at the MCRD in early July 2024. Most new infections were in recruits who had missed the AdV vaccination day.
Early symptoms of AdV may be very mild, and some recruits were likely already symptomatic when vaccinated. Aggressive environmental cleaning, separation of sick and well recruits, masking, and other nonpharmaceutical interventions did not slow the spread.
The preventive medicine and public health teams noted that AdV vaccination was being administered 11 days postarrival, to allow for pregnancy testing, and for assessing vaccine titers. US Department of Defense regulations do not dictate precise vaccination schedules. Implementation of the regulation varies among military training sites.
After reviewing other training sites’ vaccine timing schedules (most required vaccination by day 6 postarrival) and determining the time required for immunity, the medical teams at MCRD recommended shifting AdV vaccine administration, along with other standard vaccines, from day 11 to day 1 postarrival. Two weeks after the schedule change, overall incidence began declining rapidly.
Nearly 75% of patients had coinfections with other respiratory pathogens, most notably seasonal coronaviruses, COVID-19, and rhinovirus/enterovirus, suggesting that infection with AdV may increase susceptibility to other viruses, a finding that has not been identified in previous AdV outbreaks. Newly increased testing sensitivity associated with multiplex respiratory pathogen PCR availability may have been a factor in coinfection identification during this outbreak.
AdV is a significant medical threat to military recruits. Early vaccination, the investigators advise, should remain “a central tenet for prevention and control of communicable diseases in these high-risk, congregate settings.”
Earlier Vaccinations Helped Limit Marine Adenovirus Outbreak
Earlier Vaccinations Helped Limit Marine Adenovirus Outbreak
Novel Peptides Expressed in HIV Could Drive Treatment
Genetic sequencing of peptides in rebound virus in individuals with HIV who had analytic treatment interruptions (ATIs) confirmed the peptides’ expression in HIV-1 infection, according to data presented at the International AIDS Society Conference on HIV Science.
Previous research has shown that HIV-specific CD8 T-cell responses directed against five genetically conserved HIV-1 protein regions (Gag, Pol, Vif, Vpr, and Env) are associated with viral control, Josefina Marín-Rojas, PhD, Faculty of Medicine and Health, University of Sydney, and colleagues wrote in their abstract.
However, data on whether these peptides are expressed in rebound virus among individuals with HIV who experienced ATI are limited, they wrote.
The researchers applied an immunoinformatics analysis pipeline (IMAP) to select 182 peptides (IMAP-peptides) from structurally important and mutation-intolerant regions of HIV-1 proteins, senior author Sarah Palmer, PhD, co-director of the Centre for Virus Research at the Westmead Institute for Medical Research and professor in the Faculty of Medicine and Health at the University of Sydney, said in an interview.
“Our studies indicate if the immune system targets these structurally important and mutation-intolerant regions of HIV-1 proteins, this can contribute to virological control in the absence of HIV-1 therapy,” she explained.
The researchers reviewed data from the PULSE clinical trial, which included 68 men who have sex with men living with HIV in Australia. The men underwent three consecutive ATIs. A total of seven participants’ transiently controlled HIV rebound during the third ATI. The researchers examined whether the IMAP peptides were present in the HIV-1 RNA sequences of the rebound virus in four noncontrollers (patients who had viral rebound in all three ATIs) and five of the seven transient controllers who showed viral control during the third ATI.
The technique of near full-length HIV-1 RNA sequencing of rebound virus from three noncontrollers and two transient controllers identified the Gag, Pol, Vif, Vpr, and Env IMAP-peptides in 52%-100% of the viral sequences obtained from these participants across three ATI timepoints.
“We assumed that cells from people living with HIV that experience virological control after treatment interruption would have the immune response to our IMAP-peptides that we observed; however, we are amazed and encouraged by the level and extent of this immune response,” Palmer told this news organization.
The researchers also compared CD8 T-cell response between the IMAP peptides and a control peptide pool without the IMAP peptides.
The CD8 T-cells from three transient controllers had a 15- to 53-fold higher effector response to the IMAP-peptides than the CD8 T-cells from two noncontrollers, the researchers wrote in their abstract. The relative response to the IMAP-peptides in noncontrollers was 20 times lower than that to the control peptides, but the IMAP-peptide response in the transient controllers group was similar to that in the control group, the authors noted.
The results highlight the potential of IMAP in developing treatment strategies. Although the results are too preliminary to impact clinical practice at this time, the findings from the current study could lead to the development of an mRNA vaccine to clear HIV-infected cells from people living with HIV, Palmer told this news organization.
“Our next steps include developing and testing mRNA vaccine constructs that contain our IMAP-peptides to assess the immune response of cells from people living with HIV to these vaccines,” Palmer said. “From there we will conduct studies of the most promising mRNA vaccine constructs in a humanized mouse model,” she said.
Data Enhance Understanding of Immunity
The current study may provide information that can significantly impact understanding of the immune responses to HIV, David J. Cennimo, MD, associate professor of medicine and pediatrics in the Division of Infectious Disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“The investigators looked at highly conserved regions of multiple HIV proteins,” said Cennimo, who was not involved in the study. “Conserved regions and antibody responses to them may play a role in controlling HIV viral replication and rebound,” Cennimo told this news organization. “The investigators showed these regions were present in rebounding viremia, and individuals that exhibited greater immune recognition of these regions suppressed rebound viremia longer, and perhaps targeting these regions could impact HIV prevention or cure strategies,” he said.
Secondarily, the study showed the success of the novel technique (IMAP) to identify conserved peptides, said Cennimo. The technique could potentially be applied to other viruses that mutate to escape host response, he said.The study was funded by the U.S. National Institutes of Health, the Foundation for AIDS Research, the Australian National Health and Medical Research Council, and Sandra and David Ansley. The researchers and Cennimo disclosed no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Genetic sequencing of peptides in rebound virus in individuals with HIV who had analytic treatment interruptions (ATIs) confirmed the peptides’ expression in HIV-1 infection, according to data presented at the International AIDS Society Conference on HIV Science.
Previous research has shown that HIV-specific CD8 T-cell responses directed against five genetically conserved HIV-1 protein regions (Gag, Pol, Vif, Vpr, and Env) are associated with viral control, Josefina Marín-Rojas, PhD, Faculty of Medicine and Health, University of Sydney, and colleagues wrote in their abstract.
However, data on whether these peptides are expressed in rebound virus among individuals with HIV who experienced ATI are limited, they wrote.
The researchers applied an immunoinformatics analysis pipeline (IMAP) to select 182 peptides (IMAP-peptides) from structurally important and mutation-intolerant regions of HIV-1 proteins, senior author Sarah Palmer, PhD, co-director of the Centre for Virus Research at the Westmead Institute for Medical Research and professor in the Faculty of Medicine and Health at the University of Sydney, said in an interview.
“Our studies indicate if the immune system targets these structurally important and mutation-intolerant regions of HIV-1 proteins, this can contribute to virological control in the absence of HIV-1 therapy,” she explained.
The researchers reviewed data from the PULSE clinical trial, which included 68 men who have sex with men living with HIV in Australia. The men underwent three consecutive ATIs. A total of seven participants’ transiently controlled HIV rebound during the third ATI. The researchers examined whether the IMAP peptides were present in the HIV-1 RNA sequences of the rebound virus in four noncontrollers (patients who had viral rebound in all three ATIs) and five of the seven transient controllers who showed viral control during the third ATI.
The technique of near full-length HIV-1 RNA sequencing of rebound virus from three noncontrollers and two transient controllers identified the Gag, Pol, Vif, Vpr, and Env IMAP-peptides in 52%-100% of the viral sequences obtained from these participants across three ATI timepoints.
“We assumed that cells from people living with HIV that experience virological control after treatment interruption would have the immune response to our IMAP-peptides that we observed; however, we are amazed and encouraged by the level and extent of this immune response,” Palmer told this news organization.
The researchers also compared CD8 T-cell response between the IMAP peptides and a control peptide pool without the IMAP peptides.
The CD8 T-cells from three transient controllers had a 15- to 53-fold higher effector response to the IMAP-peptides than the CD8 T-cells from two noncontrollers, the researchers wrote in their abstract. The relative response to the IMAP-peptides in noncontrollers was 20 times lower than that to the control peptides, but the IMAP-peptide response in the transient controllers group was similar to that in the control group, the authors noted.
The results highlight the potential of IMAP in developing treatment strategies. Although the results are too preliminary to impact clinical practice at this time, the findings from the current study could lead to the development of an mRNA vaccine to clear HIV-infected cells from people living with HIV, Palmer told this news organization.
“Our next steps include developing and testing mRNA vaccine constructs that contain our IMAP-peptides to assess the immune response of cells from people living with HIV to these vaccines,” Palmer said. “From there we will conduct studies of the most promising mRNA vaccine constructs in a humanized mouse model,” she said.
Data Enhance Understanding of Immunity
The current study may provide information that can significantly impact understanding of the immune responses to HIV, David J. Cennimo, MD, associate professor of medicine and pediatrics in the Division of Infectious Disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“The investigators looked at highly conserved regions of multiple HIV proteins,” said Cennimo, who was not involved in the study. “Conserved regions and antibody responses to them may play a role in controlling HIV viral replication and rebound,” Cennimo told this news organization. “The investigators showed these regions were present in rebounding viremia, and individuals that exhibited greater immune recognition of these regions suppressed rebound viremia longer, and perhaps targeting these regions could impact HIV prevention or cure strategies,” he said.
Secondarily, the study showed the success of the novel technique (IMAP) to identify conserved peptides, said Cennimo. The technique could potentially be applied to other viruses that mutate to escape host response, he said.The study was funded by the U.S. National Institutes of Health, the Foundation for AIDS Research, the Australian National Health and Medical Research Council, and Sandra and David Ansley. The researchers and Cennimo disclosed no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Genetic sequencing of peptides in rebound virus in individuals with HIV who had analytic treatment interruptions (ATIs) confirmed the peptides’ expression in HIV-1 infection, according to data presented at the International AIDS Society Conference on HIV Science.
Previous research has shown that HIV-specific CD8 T-cell responses directed against five genetically conserved HIV-1 protein regions (Gag, Pol, Vif, Vpr, and Env) are associated with viral control, Josefina Marín-Rojas, PhD, Faculty of Medicine and Health, University of Sydney, and colleagues wrote in their abstract.
However, data on whether these peptides are expressed in rebound virus among individuals with HIV who experienced ATI are limited, they wrote.
The researchers applied an immunoinformatics analysis pipeline (IMAP) to select 182 peptides (IMAP-peptides) from structurally important and mutation-intolerant regions of HIV-1 proteins, senior author Sarah Palmer, PhD, co-director of the Centre for Virus Research at the Westmead Institute for Medical Research and professor in the Faculty of Medicine and Health at the University of Sydney, said in an interview.
“Our studies indicate if the immune system targets these structurally important and mutation-intolerant regions of HIV-1 proteins, this can contribute to virological control in the absence of HIV-1 therapy,” she explained.
The researchers reviewed data from the PULSE clinical trial, which included 68 men who have sex with men living with HIV in Australia. The men underwent three consecutive ATIs. A total of seven participants’ transiently controlled HIV rebound during the third ATI. The researchers examined whether the IMAP peptides were present in the HIV-1 RNA sequences of the rebound virus in four noncontrollers (patients who had viral rebound in all three ATIs) and five of the seven transient controllers who showed viral control during the third ATI.
The technique of near full-length HIV-1 RNA sequencing of rebound virus from three noncontrollers and two transient controllers identified the Gag, Pol, Vif, Vpr, and Env IMAP-peptides in 52%-100% of the viral sequences obtained from these participants across three ATI timepoints.
“We assumed that cells from people living with HIV that experience virological control after treatment interruption would have the immune response to our IMAP-peptides that we observed; however, we are amazed and encouraged by the level and extent of this immune response,” Palmer told this news organization.
The researchers also compared CD8 T-cell response between the IMAP peptides and a control peptide pool without the IMAP peptides.
The CD8 T-cells from three transient controllers had a 15- to 53-fold higher effector response to the IMAP-peptides than the CD8 T-cells from two noncontrollers, the researchers wrote in their abstract. The relative response to the IMAP-peptides in noncontrollers was 20 times lower than that to the control peptides, but the IMAP-peptide response in the transient controllers group was similar to that in the control group, the authors noted.
The results highlight the potential of IMAP in developing treatment strategies. Although the results are too preliminary to impact clinical practice at this time, the findings from the current study could lead to the development of an mRNA vaccine to clear HIV-infected cells from people living with HIV, Palmer told this news organization.
“Our next steps include developing and testing mRNA vaccine constructs that contain our IMAP-peptides to assess the immune response of cells from people living with HIV to these vaccines,” Palmer said. “From there we will conduct studies of the most promising mRNA vaccine constructs in a humanized mouse model,” she said.
Data Enhance Understanding of Immunity
The current study may provide information that can significantly impact understanding of the immune responses to HIV, David J. Cennimo, MD, associate professor of medicine and pediatrics in the Division of Infectious Disease at Rutgers New Jersey Medical School, Newark, New Jersey, said in an interview.
“The investigators looked at highly conserved regions of multiple HIV proteins,” said Cennimo, who was not involved in the study. “Conserved regions and antibody responses to them may play a role in controlling HIV viral replication and rebound,” Cennimo told this news organization. “The investigators showed these regions were present in rebounding viremia, and individuals that exhibited greater immune recognition of these regions suppressed rebound viremia longer, and perhaps targeting these regions could impact HIV prevention or cure strategies,” he said.
Secondarily, the study showed the success of the novel technique (IMAP) to identify conserved peptides, said Cennimo. The technique could potentially be applied to other viruses that mutate to escape host response, he said.The study was funded by the U.S. National Institutes of Health, the Foundation for AIDS Research, the Australian National Health and Medical Research Council, and Sandra and David Ansley. The researchers and Cennimo disclosed no financial conflicts of interest.
A version of this article first appeared on Medscape.com.
Alarming Rise in Early-Onset GI Cancers Calls for Early Screening, Lifestyle Change
, said the authors of a JAMA review.
In the US, early-onset GI cancers are increasing faster than any other type of early-onset cancer, including breast cancer. The trend is not limited to colorectal cancer (CRC). Gastric, pancreatic, esophageal, as well as many biliary tract and appendix cancers, are also on the rise in young adults, Kimmie Ng, MD, MPH, and Thejus Jayakrishnan, MD, both with Dana-Farber Cancer Institute, Boston, noted in their article.
The increase in early-onset GI cancers follows a “birth cohort effect,” with generational variation in risk, suggesting a potential association with changes in environmental exposures, Ng explained in an accompanying JAMA podcast.
All these GI cancers link strongly to multiple modifiable risk factors, and it is a “top area of investigation to determine exactly what environmental exposures are at play,” Ng added.
For many of these GI cancers, obesity has been the “leading hypothesis” given that rising rates seem to parallel the increase in incidence of these early-onset GI cancers, Ng explained.
“But we also have evidence, particularly strong for colorectal cancer, that dietary patterns, such as consuming a Western diet, as well as sedentary behavior and lifestyles seem to be associated with a significantly higher risk of developing these cancers at an age under 50,” Ng said.
Rising Incidence
Globally, among early-onset GI cancers reported in 2022, CRC was the most common (54%), followed by gastric cancer (24%), esophageal cancer (13%), and pancreatic cancer (9%).
In the US in 2022, 20,805 individuals were diagnosed with early-onset CRC, 2689 with early-onset gastric cancer, 2657 with early-onset pancreatic cancer, and 875 with early-onset esophageal cancer.
Since the mid-1990s, CRC among adults of all ages in the US declined by 1.3%-4.2% annually but early-onset CRC increased by roughly 2% per year in both men and women, and currently makes up about 14% of all CRC cases.
Early-onset pancreatic cancer and esophageal cancer each currently make up about 5% of all cases of these cancers in the US.
Between 2010 and 2019, the number of newly diagnosed cases of early-onset GI cancers rose by nearly about 15%, with Black, Hispanic, Indigenous ancestry, and women disproportionately affected, Ng and coauthors noted in a related review published in the British Journal of Surgery.
Modifiable and Nonmodifiable Risk Factors
Along with obesity and poor diet, other modifiable risk factors for early-onset GI cancers include sedentary lifestyle, cigarette smoking, and alcohol consumption.
Nonmodifiable risk factors include family history, hereditary cancer syndromes such as Lynch syndrome and inflammatory bowel disease.
Roughly 15%-30% of early-onset GI cancers have pathogenic germline variants in genes such as DNA mismatch repair genes and BRCA1/2.
All individuals with early-onset GI cancers should undergo germline and somatic genetic testing to guide treatment, screen for other cancers (eg, endometrial cancer in Lynch syndrome), and assess familial risk, Ng and Jayakrishnan advised.
Treatment Challenges
Treatment for early-onset GI cancers is generally similar to later-onset GI cancers and prognosis for patients with early-onset GI cancers is “similar to or worse” than that for patients with later-onset GI cancers, highlighting the need for improved methods of prevention and early detection, the authors said.
Ng noted that younger cancer patients often face more challenges after diagnosis than older patients and benefit from multidisciplinary care, including referral for fertility counseling and preservation if appropriate, and psychosocial support.
“It is very difficult and challenging to receive a cancer diagnosis no matter what age you are, but when a person is diagnosed in their 20s, 30s, or 40s, there are unique challenges,” Ng said.
Studies have documented “much higher levels of psychosocial distress, depression and anxiety” in early-onset cancer patients, “and they also often experience more financial toxicity, disruptions in their education as well as their career and there may be fertility concerns,” Ng added.
Diagnostic Delays and Screening
Currently, screening is not recommended for most early-onset GI cancers — with the exception of CRC, with screening recommended for average-risk adults in the US starting at age 45.
Yet, despite this recommendation, fewer than 1 in 5 (19.7%) US adults aged 45-49 years were screened in 2021, indicating a significant gap in early detection efforts.
High-risk individuals, such as those with Lynch syndrome, a first-degree relative with CRC, or advanced colorectal adenoma, should begin CRC screening earlier, at an age determined by the specific risk factor.
“Studies have shown significant delays in diagnosis among younger patients. It’s important that prompt diagnosis happens so that these patients do not end up being diagnosed with advanced or metastatic stages of cancer, as they often are,” Ng said.
“Screening adherence is absolutely critical,” co-author Jayakrishnan added in a news release.
“We have strong evidence that colorectal cancer screening saves lives by reducing both the number of people who develop colorectal cancer and the number of people who die from it. Each missed screening is a lost opportunity to detect cancer early when it is more treatable, or to prevent cancer altogether by identifying and removing precancerous polyps,” Jayakrishnan said.This research had no funding. Ng reported receipt of nonfinancial support from Pharmavite, institutional grants from Janssen, and personal fees from Bayer, Seagen, GlaxoSmithKline, Pfizer, CytomX, Jazz Pharmaceuticals, Revolution Medicines, Redesign Health, AbbVie, Etiome, and CRICO. Ng is an associate editor of JAMA but was not involved in any of the decisions regarding review of the manuscript or its acceptance. Jayakrishnan had no disclosures.
A version of this article appeared on Medscape.com.
, said the authors of a JAMA review.
In the US, early-onset GI cancers are increasing faster than any other type of early-onset cancer, including breast cancer. The trend is not limited to colorectal cancer (CRC). Gastric, pancreatic, esophageal, as well as many biliary tract and appendix cancers, are also on the rise in young adults, Kimmie Ng, MD, MPH, and Thejus Jayakrishnan, MD, both with Dana-Farber Cancer Institute, Boston, noted in their article.
The increase in early-onset GI cancers follows a “birth cohort effect,” with generational variation in risk, suggesting a potential association with changes in environmental exposures, Ng explained in an accompanying JAMA podcast.
All these GI cancers link strongly to multiple modifiable risk factors, and it is a “top area of investigation to determine exactly what environmental exposures are at play,” Ng added.
For many of these GI cancers, obesity has been the “leading hypothesis” given that rising rates seem to parallel the increase in incidence of these early-onset GI cancers, Ng explained.
“But we also have evidence, particularly strong for colorectal cancer, that dietary patterns, such as consuming a Western diet, as well as sedentary behavior and lifestyles seem to be associated with a significantly higher risk of developing these cancers at an age under 50,” Ng said.
Rising Incidence
Globally, among early-onset GI cancers reported in 2022, CRC was the most common (54%), followed by gastric cancer (24%), esophageal cancer (13%), and pancreatic cancer (9%).
In the US in 2022, 20,805 individuals were diagnosed with early-onset CRC, 2689 with early-onset gastric cancer, 2657 with early-onset pancreatic cancer, and 875 with early-onset esophageal cancer.
Since the mid-1990s, CRC among adults of all ages in the US declined by 1.3%-4.2% annually but early-onset CRC increased by roughly 2% per year in both men and women, and currently makes up about 14% of all CRC cases.
Early-onset pancreatic cancer and esophageal cancer each currently make up about 5% of all cases of these cancers in the US.
Between 2010 and 2019, the number of newly diagnosed cases of early-onset GI cancers rose by nearly about 15%, with Black, Hispanic, Indigenous ancestry, and women disproportionately affected, Ng and coauthors noted in a related review published in the British Journal of Surgery.
Modifiable and Nonmodifiable Risk Factors
Along with obesity and poor diet, other modifiable risk factors for early-onset GI cancers include sedentary lifestyle, cigarette smoking, and alcohol consumption.
Nonmodifiable risk factors include family history, hereditary cancer syndromes such as Lynch syndrome and inflammatory bowel disease.
Roughly 15%-30% of early-onset GI cancers have pathogenic germline variants in genes such as DNA mismatch repair genes and BRCA1/2.
All individuals with early-onset GI cancers should undergo germline and somatic genetic testing to guide treatment, screen for other cancers (eg, endometrial cancer in Lynch syndrome), and assess familial risk, Ng and Jayakrishnan advised.
Treatment Challenges
Treatment for early-onset GI cancers is generally similar to later-onset GI cancers and prognosis for patients with early-onset GI cancers is “similar to or worse” than that for patients with later-onset GI cancers, highlighting the need for improved methods of prevention and early detection, the authors said.
Ng noted that younger cancer patients often face more challenges after diagnosis than older patients and benefit from multidisciplinary care, including referral for fertility counseling and preservation if appropriate, and psychosocial support.
“It is very difficult and challenging to receive a cancer diagnosis no matter what age you are, but when a person is diagnosed in their 20s, 30s, or 40s, there are unique challenges,” Ng said.
Studies have documented “much higher levels of psychosocial distress, depression and anxiety” in early-onset cancer patients, “and they also often experience more financial toxicity, disruptions in their education as well as their career and there may be fertility concerns,” Ng added.
Diagnostic Delays and Screening
Currently, screening is not recommended for most early-onset GI cancers — with the exception of CRC, with screening recommended for average-risk adults in the US starting at age 45.
Yet, despite this recommendation, fewer than 1 in 5 (19.7%) US adults aged 45-49 years were screened in 2021, indicating a significant gap in early detection efforts.
High-risk individuals, such as those with Lynch syndrome, a first-degree relative with CRC, or advanced colorectal adenoma, should begin CRC screening earlier, at an age determined by the specific risk factor.
“Studies have shown significant delays in diagnosis among younger patients. It’s important that prompt diagnosis happens so that these patients do not end up being diagnosed with advanced or metastatic stages of cancer, as they often are,” Ng said.
“Screening adherence is absolutely critical,” co-author Jayakrishnan added in a news release.
“We have strong evidence that colorectal cancer screening saves lives by reducing both the number of people who develop colorectal cancer and the number of people who die from it. Each missed screening is a lost opportunity to detect cancer early when it is more treatable, or to prevent cancer altogether by identifying and removing precancerous polyps,” Jayakrishnan said.This research had no funding. Ng reported receipt of nonfinancial support from Pharmavite, institutional grants from Janssen, and personal fees from Bayer, Seagen, GlaxoSmithKline, Pfizer, CytomX, Jazz Pharmaceuticals, Revolution Medicines, Redesign Health, AbbVie, Etiome, and CRICO. Ng is an associate editor of JAMA but was not involved in any of the decisions regarding review of the manuscript or its acceptance. Jayakrishnan had no disclosures.
A version of this article appeared on Medscape.com.
, said the authors of a JAMA review.
In the US, early-onset GI cancers are increasing faster than any other type of early-onset cancer, including breast cancer. The trend is not limited to colorectal cancer (CRC). Gastric, pancreatic, esophageal, as well as many biliary tract and appendix cancers, are also on the rise in young adults, Kimmie Ng, MD, MPH, and Thejus Jayakrishnan, MD, both with Dana-Farber Cancer Institute, Boston, noted in their article.
The increase in early-onset GI cancers follows a “birth cohort effect,” with generational variation in risk, suggesting a potential association with changes in environmental exposures, Ng explained in an accompanying JAMA podcast.
All these GI cancers link strongly to multiple modifiable risk factors, and it is a “top area of investigation to determine exactly what environmental exposures are at play,” Ng added.
For many of these GI cancers, obesity has been the “leading hypothesis” given that rising rates seem to parallel the increase in incidence of these early-onset GI cancers, Ng explained.
“But we also have evidence, particularly strong for colorectal cancer, that dietary patterns, such as consuming a Western diet, as well as sedentary behavior and lifestyles seem to be associated with a significantly higher risk of developing these cancers at an age under 50,” Ng said.
Rising Incidence
Globally, among early-onset GI cancers reported in 2022, CRC was the most common (54%), followed by gastric cancer (24%), esophageal cancer (13%), and pancreatic cancer (9%).
In the US in 2022, 20,805 individuals were diagnosed with early-onset CRC, 2689 with early-onset gastric cancer, 2657 with early-onset pancreatic cancer, and 875 with early-onset esophageal cancer.
Since the mid-1990s, CRC among adults of all ages in the US declined by 1.3%-4.2% annually but early-onset CRC increased by roughly 2% per year in both men and women, and currently makes up about 14% of all CRC cases.
Early-onset pancreatic cancer and esophageal cancer each currently make up about 5% of all cases of these cancers in the US.
Between 2010 and 2019, the number of newly diagnosed cases of early-onset GI cancers rose by nearly about 15%, with Black, Hispanic, Indigenous ancestry, and women disproportionately affected, Ng and coauthors noted in a related review published in the British Journal of Surgery.
Modifiable and Nonmodifiable Risk Factors
Along with obesity and poor diet, other modifiable risk factors for early-onset GI cancers include sedentary lifestyle, cigarette smoking, and alcohol consumption.
Nonmodifiable risk factors include family history, hereditary cancer syndromes such as Lynch syndrome and inflammatory bowel disease.
Roughly 15%-30% of early-onset GI cancers have pathogenic germline variants in genes such as DNA mismatch repair genes and BRCA1/2.
All individuals with early-onset GI cancers should undergo germline and somatic genetic testing to guide treatment, screen for other cancers (eg, endometrial cancer in Lynch syndrome), and assess familial risk, Ng and Jayakrishnan advised.
Treatment Challenges
Treatment for early-onset GI cancers is generally similar to later-onset GI cancers and prognosis for patients with early-onset GI cancers is “similar to or worse” than that for patients with later-onset GI cancers, highlighting the need for improved methods of prevention and early detection, the authors said.
Ng noted that younger cancer patients often face more challenges after diagnosis than older patients and benefit from multidisciplinary care, including referral for fertility counseling and preservation if appropriate, and psychosocial support.
“It is very difficult and challenging to receive a cancer diagnosis no matter what age you are, but when a person is diagnosed in their 20s, 30s, or 40s, there are unique challenges,” Ng said.
Studies have documented “much higher levels of psychosocial distress, depression and anxiety” in early-onset cancer patients, “and they also often experience more financial toxicity, disruptions in their education as well as their career and there may be fertility concerns,” Ng added.
Diagnostic Delays and Screening
Currently, screening is not recommended for most early-onset GI cancers — with the exception of CRC, with screening recommended for average-risk adults in the US starting at age 45.
Yet, despite this recommendation, fewer than 1 in 5 (19.7%) US adults aged 45-49 years were screened in 2021, indicating a significant gap in early detection efforts.
High-risk individuals, such as those with Lynch syndrome, a first-degree relative with CRC, or advanced colorectal adenoma, should begin CRC screening earlier, at an age determined by the specific risk factor.
“Studies have shown significant delays in diagnosis among younger patients. It’s important that prompt diagnosis happens so that these patients do not end up being diagnosed with advanced or metastatic stages of cancer, as they often are,” Ng said.
“Screening adherence is absolutely critical,” co-author Jayakrishnan added in a news release.
“We have strong evidence that colorectal cancer screening saves lives by reducing both the number of people who develop colorectal cancer and the number of people who die from it. Each missed screening is a lost opportunity to detect cancer early when it is more treatable, or to prevent cancer altogether by identifying and removing precancerous polyps,” Jayakrishnan said.This research had no funding. Ng reported receipt of nonfinancial support from Pharmavite, institutional grants from Janssen, and personal fees from Bayer, Seagen, GlaxoSmithKline, Pfizer, CytomX, Jazz Pharmaceuticals, Revolution Medicines, Redesign Health, AbbVie, Etiome, and CRICO. Ng is an associate editor of JAMA but was not involved in any of the decisions regarding review of the manuscript or its acceptance. Jayakrishnan had no disclosures.
A version of this article appeared on Medscape.com.