User login
Facing systemic racism in health care: Inequities in medical education

Resources:
- The Pulse of Perseverance: Three Black Doctors on Their Journey to Success
Pierre Johnson, MD; Maxime Madhere, MD, Joseph Semien Jr, MD - Mindset: The New Psychology of Success
Carol S. Dweck

Resources:
- The Pulse of Perseverance: Three Black Doctors on Their Journey to Success
Pierre Johnson, MD; Maxime Madhere, MD, Joseph Semien Jr, MD - Mindset: The New Psychology of Success
Carol S. Dweck

Resources:
- The Pulse of Perseverance: Three Black Doctors on Their Journey to Success
Pierre Johnson, MD; Maxime Madhere, MD, Joseph Semien Jr, MD - Mindset: The New Psychology of Success
Carol S. Dweck
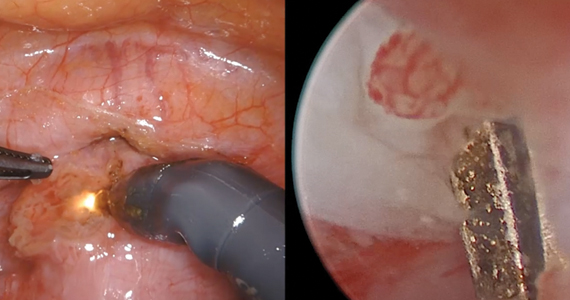
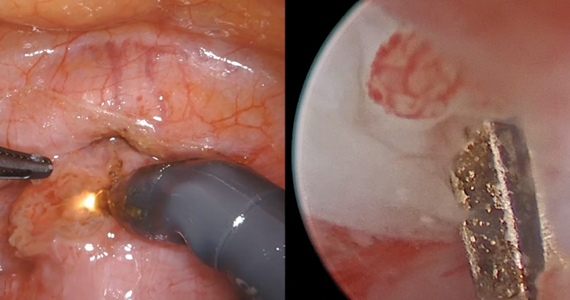
Isthmocele repair: Simultaneous hysteroscopy and robotic-assisted laparoscopy

An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues
- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.

An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues


An isthmocele is a pouch-like anterior uterine wall defect at the site of a previous cesarean scar. The incidence is not well known, but it is estimated in the literature to be between 19% and 88%.1 Issues arising from an isthmocele may include abnormal uterine bleeding; abdominal pain; diminished fertility; ectopic pregnancy; or obstetric complications, such as uterine rupture. Repair of an isthmocele may be indicated for symptomatic relief and preservation of fertility. Multiple surgical approaches have been described in the literature, including laparoscopic, hysteroscopic, and vaginal approaches.
The objective of this video is to illustrate the use of robotic-assisted laparoscopy with simultaneous hysteroscopy as a feasible and safe approach for the repair of an isthmocele. Here we illustrate the key surgical steps of this approach, including:
- presurgical planning with magnetic resonance imaging
- diagnostic hysteroscopy for confirmation of isthmocele
- simultaneous laparoscopy for identification of borders
- strategic hysterotomy
- excision of scar tissue
- imbricated, tension-free closure.
We hope that you find this video useful to your clinical practice.
>> Dr. Arnold P. Advincula, and colleagues

- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.
- Tower AM, Frishman GN. Cesarean scar defects: an underrecognized cause of abnormal uterine bleeding and other gynecologic complications. J Minim Invasive Gynecol. 2013;20:562-572. doi: 10.1016/j.jmig.2013.03.008.
COVID-19 and pregnancy: Is miscarriage a risk?

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020

- Are you treating pregnant patients with COVID-19? Take this brief survey: https://www.surveymonkey.com/r/CDZ7VFK
- Enroll your patients in PRIORITY: Pregnancy Coronavirus Outcomes Registry
- Second-Trimester Miscarriage in a Pregnant Woman With SARS-CoV-2 Infection JAMA. April 30, 2020
Double Masking and Decontamination: A Doctor's COVID-19 Routine

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about the PPE and decontamination processes she's using. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
PPE Routine at the Hospital
Dr Gary Ferenchick: You've developed your own PPE and decontamination routines. It's about protecting yourself at work but also about protecting your loved ones by not carrying the virus home. Could you walk us through it? I'll show it on the screen.
Dr Hannah Ferenchick: At work I wear scrubs, and I try to minimize any additional clothing. I don't wear a jacket over my scrubs, and I don't wear any T-shirts under my scrubs. If I'm going to be in a situation that might involve exposure to patient secretions or bodily fluids, then I also wear shoe covers.
Because so many of our patients are infected and we may be called upon at any time to do an aerosol-generating procedure, in the ED we have all taken to wearing N95 masks for our entire shift. I wear a fitted N95 mask. I cover that with a surgical mask.
We are anticipating N95 shortages because our use of the masks has increased exponentially. Every hospital has to think about how to protect their healthcare workers while conserving PPE. We cover the N95 mask with a surgical mask, so that if there is any soiling or droplets reaching the mask, we are able to change the surgical mask and continue to use the same N95.
In addition, eye protection is important. Generally throughout the shift I wear my own goggles. If I'm going to be involved in any procedure with the potential for aerosolization (intubation, performing CPR, bronchoscopy) then I wear a creation of my own, which is a welder's shield.
Many of our providers have chosen to use their own equipment, although we are still able to use hospital-provided equipment. There is probably no difference in effectiveness between these devices.
Cell Phones and Stethoscopes
I carry a personal cell phone at work (which I often use to look things up, use the calculator, and for other purposes), and I'm cognizant that when I touch it, I am potentially transmitting pathogens to my phone or its cover. So I've taken to keeping my phone in a plastic sandwich bag, which I disinfect a couple of times throughout the shift. The phone still works normally.
After my shift, in my "decontamination phase," I remove the phone from the plastic bag and disinfect the phone again.
I try to avoid bringing objects into the vicinity of the patient. That's different from my normal routine—I usually like to write down what the patient has told me—but unfortunately, carrying pen and paper or a clipboard into a patient's room is not feasible at this point. During this time, I've also avoided using my personal stethoscope.
There's also transmission risk associated with shared equipment. We share hospital-provided phones and they must be disinfected. We are each disinfecting our own workspaces: computer, keyboard, mouse, and countertop.
Obviously you are trying to minimize any contact with your mouth or face. You don't want to rub your eyes, touch your nose, or eat anything with your hands while you are at work. The assumption is that you are doing very frequent hand hygiene.
Decontamination Routine
One of our concerns as healthcare providers is the possibility that we could, either asymptomatically or through the objects that we use at work, be bringing the disease home. We want to protect the people who may be at higher risk just because they live with a healthcare provider. These are the decontamination practices I've developed for my own situation, taken from best practices and suggestions from others.
I remove my dirty scrubs and leave them at work, and I change into a clean pair of scrubs or clean clothes. I disinfect any inanimate objects that my hands may have touched during the shift using alcohol, sanitizer wipes, bleach wipes, or hospital-grade chemical wipes.
To keep those objects clean after disinfecting, I place them in clean plastic bags away from other objects (eg, a wallet or purse) that may not be easy to disinfect. Then I store those bags in the trunk of my car for my next shift, so I'm not taking them into my home.
I also change my shoes, leaving my work shoes in the trunk of my car, and wear another pair of shoes into the house.
When I get home, I basically do everything again. I disinfect my phone, I wash my hands, and I shower immediately. At that point, I consider myself sufficiently "disinfected."
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about the PPE and decontamination processes she's using. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
PPE Routine at the Hospital
Dr Gary Ferenchick: You've developed your own PPE and decontamination routines. It's about protecting yourself at work but also about protecting your loved ones by not carrying the virus home. Could you walk us through it? I'll show it on the screen.
Dr Hannah Ferenchick: At work I wear scrubs, and I try to minimize any additional clothing. I don't wear a jacket over my scrubs, and I don't wear any T-shirts under my scrubs. If I'm going to be in a situation that might involve exposure to patient secretions or bodily fluids, then I also wear shoe covers.
Because so many of our patients are infected and we may be called upon at any time to do an aerosol-generating procedure, in the ED we have all taken to wearing N95 masks for our entire shift. I wear a fitted N95 mask. I cover that with a surgical mask.
We are anticipating N95 shortages because our use of the masks has increased exponentially. Every hospital has to think about how to protect their healthcare workers while conserving PPE. We cover the N95 mask with a surgical mask, so that if there is any soiling or droplets reaching the mask, we are able to change the surgical mask and continue to use the same N95.
In addition, eye protection is important. Generally throughout the shift I wear my own goggles. If I'm going to be involved in any procedure with the potential for aerosolization (intubation, performing CPR, bronchoscopy) then I wear a creation of my own, which is a welder's shield.
Many of our providers have chosen to use their own equipment, although we are still able to use hospital-provided equipment. There is probably no difference in effectiveness between these devices.
Cell Phones and Stethoscopes
I carry a personal cell phone at work (which I often use to look things up, use the calculator, and for other purposes), and I'm cognizant that when I touch it, I am potentially transmitting pathogens to my phone or its cover. So I've taken to keeping my phone in a plastic sandwich bag, which I disinfect a couple of times throughout the shift. The phone still works normally.
After my shift, in my "decontamination phase," I remove the phone from the plastic bag and disinfect the phone again.
I try to avoid bringing objects into the vicinity of the patient. That's different from my normal routine—I usually like to write down what the patient has told me—but unfortunately, carrying pen and paper or a clipboard into a patient's room is not feasible at this point. During this time, I've also avoided using my personal stethoscope.
There's also transmission risk associated with shared equipment. We share hospital-provided phones and they must be disinfected. We are each disinfecting our own workspaces: computer, keyboard, mouse, and countertop.
Obviously you are trying to minimize any contact with your mouth or face. You don't want to rub your eyes, touch your nose, or eat anything with your hands while you are at work. The assumption is that you are doing very frequent hand hygiene.
Decontamination Routine
One of our concerns as healthcare providers is the possibility that we could, either asymptomatically or through the objects that we use at work, be bringing the disease home. We want to protect the people who may be at higher risk just because they live with a healthcare provider. These are the decontamination practices I've developed for my own situation, taken from best practices and suggestions from others.
I remove my dirty scrubs and leave them at work, and I change into a clean pair of scrubs or clean clothes. I disinfect any inanimate objects that my hands may have touched during the shift using alcohol, sanitizer wipes, bleach wipes, or hospital-grade chemical wipes.
To keep those objects clean after disinfecting, I place them in clean plastic bags away from other objects (eg, a wallet or purse) that may not be easy to disinfect. Then I store those bags in the trunk of my car for my next shift, so I'm not taking them into my home.
I also change my shoes, leaving my work shoes in the trunk of my car, and wear another pair of shoes into the house.
When I get home, I basically do everything again. I disinfect my phone, I wash my hands, and I shower immediately. At that point, I consider myself sufficiently "disinfected."
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about the PPE and decontamination processes she's using. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
PPE Routine at the Hospital
Dr Gary Ferenchick: You've developed your own PPE and decontamination routines. It's about protecting yourself at work but also about protecting your loved ones by not carrying the virus home. Could you walk us through it? I'll show it on the screen.
Dr Hannah Ferenchick: At work I wear scrubs, and I try to minimize any additional clothing. I don't wear a jacket over my scrubs, and I don't wear any T-shirts under my scrubs. If I'm going to be in a situation that might involve exposure to patient secretions or bodily fluids, then I also wear shoe covers.
Because so many of our patients are infected and we may be called upon at any time to do an aerosol-generating procedure, in the ED we have all taken to wearing N95 masks for our entire shift. I wear a fitted N95 mask. I cover that with a surgical mask.
We are anticipating N95 shortages because our use of the masks has increased exponentially. Every hospital has to think about how to protect their healthcare workers while conserving PPE. We cover the N95 mask with a surgical mask, so that if there is any soiling or droplets reaching the mask, we are able to change the surgical mask and continue to use the same N95.
In addition, eye protection is important. Generally throughout the shift I wear my own goggles. If I'm going to be involved in any procedure with the potential for aerosolization (intubation, performing CPR, bronchoscopy) then I wear a creation of my own, which is a welder's shield.
Many of our providers have chosen to use their own equipment, although we are still able to use hospital-provided equipment. There is probably no difference in effectiveness between these devices.
Cell Phones and Stethoscopes
I carry a personal cell phone at work (which I often use to look things up, use the calculator, and for other purposes), and I'm cognizant that when I touch it, I am potentially transmitting pathogens to my phone or its cover. So I've taken to keeping my phone in a plastic sandwich bag, which I disinfect a couple of times throughout the shift. The phone still works normally.
After my shift, in my "decontamination phase," I remove the phone from the plastic bag and disinfect the phone again.
I try to avoid bringing objects into the vicinity of the patient. That's different from my normal routine—I usually like to write down what the patient has told me—but unfortunately, carrying pen and paper or a clipboard into a patient's room is not feasible at this point. During this time, I've also avoided using my personal stethoscope.
There's also transmission risk associated with shared equipment. We share hospital-provided phones and they must be disinfected. We are each disinfecting our own workspaces: computer, keyboard, mouse, and countertop.
Obviously you are trying to minimize any contact with your mouth or face. You don't want to rub your eyes, touch your nose, or eat anything with your hands while you are at work. The assumption is that you are doing very frequent hand hygiene.
Decontamination Routine
One of our concerns as healthcare providers is the possibility that we could, either asymptomatically or through the objects that we use at work, be bringing the disease home. We want to protect the people who may be at higher risk just because they live with a healthcare provider. These are the decontamination practices I've developed for my own situation, taken from best practices and suggestions from others.
I remove my dirty scrubs and leave them at work, and I change into a clean pair of scrubs or clean clothes. I disinfect any inanimate objects that my hands may have touched during the shift using alcohol, sanitizer wipes, bleach wipes, or hospital-grade chemical wipes.
To keep those objects clean after disinfecting, I place them in clean plastic bags away from other objects (eg, a wallet or purse) that may not be easy to disinfect. Then I store those bags in the trunk of my car for my next shift, so I'm not taking them into my home.
I also change my shoes, leaving my work shoes in the trunk of my car, and wear another pair of shoes into the house.
When I get home, I basically do everything again. I disinfect my phone, I wash my hands, and I shower immediately. At that point, I consider myself sufficiently "disinfected."
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.
Expert outlines strategies for managing migraine in women
Understanding the hormonal changes women go through over their lives can help physicians refine migraine treatment approaches.
“It’s so critical that you know what’s going on hormonally, both endogenously and exogenously, to better evaluate treatment,” according to Susan Hutchinson, MD, director of the Orange County Migraine & Headache Center in Irvine, Calif.
In an interview, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, talks with Dr. Hutchinson about estrogen treatment for menstrual migraine, the safety of new medications during pregnancy and breastfeeding, and the importance of a collaborative approach between a patient’s primary care, neurology, and ob.gyn. providers.

Understanding the hormonal changes women go through over their lives can help physicians refine migraine treatment approaches.
“It’s so critical that you know what’s going on hormonally, both endogenously and exogenously, to better evaluate treatment,” according to Susan Hutchinson, MD, director of the Orange County Migraine & Headache Center in Irvine, Calif.
In an interview, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, talks with Dr. Hutchinson about estrogen treatment for menstrual migraine, the safety of new medications during pregnancy and breastfeeding, and the importance of a collaborative approach between a patient’s primary care, neurology, and ob.gyn. providers.

Understanding the hormonal changes women go through over their lives can help physicians refine migraine treatment approaches.
“It’s so critical that you know what’s going on hormonally, both endogenously and exogenously, to better evaluate treatment,” according to Susan Hutchinson, MD, director of the Orange County Migraine & Headache Center in Irvine, Calif.
In an interview, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, talks with Dr. Hutchinson about estrogen treatment for menstrual migraine, the safety of new medications during pregnancy and breastfeeding, and the importance of a collaborative approach between a patient’s primary care, neurology, and ob.gyn. providers.

Small-molecule CGRP receptor antagonists may pose a risk during stroke

“It turns out that these drugs make the blood vessels of the brain dysfunctional, such that the collateral channels that bring in blood to the region that is having the stroke are impaired,” explains the study’s lead author, Cenk Ayata, MD, associate professor of neurology and radiology at Harvard Medical School, Boston.
In an interview, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, talks with Dr. Ayata about his study findings, and about CGRP receptor antagonists’ potential effects if a patient has an ischemic event.
This article was updated 4/21/20.

“It turns out that these drugs make the blood vessels of the brain dysfunctional, such that the collateral channels that bring in blood to the region that is having the stroke are impaired,” explains the study’s lead author, Cenk Ayata, MD, associate professor of neurology and radiology at Harvard Medical School, Boston.
In an interview, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, talks with Dr. Ayata about his study findings, and about CGRP receptor antagonists’ potential effects if a patient has an ischemic event.
This article was updated 4/21/20.

“It turns out that these drugs make the blood vessels of the brain dysfunctional, such that the collateral channels that bring in blood to the region that is having the stroke are impaired,” explains the study’s lead author, Cenk Ayata, MD, associate professor of neurology and radiology at Harvard Medical School, Boston.
In an interview, Alan M. Rapoport, MD, clinical professor of neurology at the University of California, Los Angeles, talks with Dr. Ayata about his study findings, and about CGRP receptor antagonists’ potential effects if a patient has an ischemic event.
This article was updated 4/21/20.
'Silent Hypoxemia' and Other Curious Clinical Observations in COVID-19

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about what's going on in Detroit, and also about PPE and decontamination processes. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
Dr Gary Ferenchick: We were talking earlier about some of the not-well-described clinical scenarios that patients with definitive COVID might present with. One of these was the idea of "silent hypoxemia." Could you describe that?
Dr Hannah Ferenchick: Silent hypoxemia is being described in many of these COVID patients. That means the patient is very hypoxemic—they may have an oxygen saturation of about 85% on room air, but clinically they look very comfortable—they are not dyspneic or tachypneic and may not even verbalize a significant sense of shortness of breath. It's not every patient, but it has been interesting to see patients sitting there looking fairly normal, with a resting oxygen saturation much lower than you would expect for someone who doesn't have underlying pulmonary disease or other symptoms.
Dr Gary Ferenchick: What abnormalities are you seeing on standard or not-so-standard lab tests?
Dr Hannah Ferenchick: Some of the characteristic lab findings we are seeing are lymphopenia and elevated inflammatory markers (eg, CRP). A couple of other atypical findings seem to be specific for COVID—elevated LDH, ferritin, CPK, and procalcitonin levels. Some of the hematologic markers that we look at—the coagulation profile studies—are also abnormal, showing thrombocytopenia and elevated D-dimer levels.
That constellation of symptoms represents more of a clinical picture. A lot of times we have only a very high clinical suspicion, because in many parts of the country it still takes days to get back a confirmatory PCR test.
Much like we do for the flu, the confirmatory test is a nasopharyngeal swab that is run for COVID/coronavirus PCR. Unfortunately the sensitivity of that test is not great. Some studies have quoted 75%-80%, so even a negative PCR does not necessarily rule out the disease, especially if you have a high clinical suspicion. A clinical suspicion is based on the typical symptoms. Many patients, although not all, will have symptoms of lower respiratory tract infection.
Dr Gary Ferenchick: So the right clinical scenario with the right hematologic/biochemical findings dramatically raises the chance that the patient has COVID?
Dr Hannah Ferenchick: Yes, and one thing that we have all been astonished by is how terrible some of these x-rays can look. There are a lot of typical findings on x-ray. Some describe them as looking like pulmonary edema, but the patient has no history of heart failure. Peripheral consolidation and ground-glass opacities are classically described. If you saw one of these x-rays from a patient with bacterial pneumonia, you would expect that patient to be very ill-appearing. Sometimes we get x-rays on patients who are sitting there, maybe mildly symptomatic on room air, and we are astonished by how terrible their x-rays look.
Unfortunately, imaging studies are something we haven't been able to rely on too much for diagnosis. Part of that is to maintain hospital safety, because to take a patient to CT scan, you have to consider the turnaround time for cleaning the CT scanner and the exposure of additional staff to a possibly infected patient. Some of those logistical considerations have limited the availability of radiography.
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine, at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about what's going on in Detroit, and also about PPE and decontamination processes. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
Dr Gary Ferenchick: We were talking earlier about some of the not-well-described clinical scenarios that patients with definitive COVID might present with. One of these was the idea of "silent hypoxemia." Could you describe that?
Dr Hannah Ferenchick: Silent hypoxemia is being described in many of these COVID patients. That means the patient is very hypoxemic—they may have an oxygen saturation of about 85% on room air, but clinically they look very comfortable—they are not dyspneic or tachypneic and may not even verbalize a significant sense of shortness of breath. It's not every patient, but it has been interesting to see patients sitting there looking fairly normal, with a resting oxygen saturation much lower than you would expect for someone who doesn't have underlying pulmonary disease or other symptoms.
Dr Gary Ferenchick: What abnormalities are you seeing on standard or not-so-standard lab tests?
Dr Hannah Ferenchick: Some of the characteristic lab findings we are seeing are lymphopenia and elevated inflammatory markers (eg, CRP). A couple of other atypical findings seem to be specific for COVID—elevated LDH, ferritin, CPK, and procalcitonin levels. Some of the hematologic markers that we look at—the coagulation profile studies—are also abnormal, showing thrombocytopenia and elevated D-dimer levels.
That constellation of symptoms represents more of a clinical picture. A lot of times we have only a very high clinical suspicion, because in many parts of the country it still takes days to get back a confirmatory PCR test.
Much like we do for the flu, the confirmatory test is a nasopharyngeal swab that is run for COVID/coronavirus PCR. Unfortunately the sensitivity of that test is not great. Some studies have quoted 75%-80%, so even a negative PCR does not necessarily rule out the disease, especially if you have a high clinical suspicion. A clinical suspicion is based on the typical symptoms. Many patients, although not all, will have symptoms of lower respiratory tract infection.
Dr Gary Ferenchick: So the right clinical scenario with the right hematologic/biochemical findings dramatically raises the chance that the patient has COVID?
Dr Hannah Ferenchick: Yes, and one thing that we have all been astonished by is how terrible some of these x-rays can look. There are a lot of typical findings on x-ray. Some describe them as looking like pulmonary edema, but the patient has no history of heart failure. Peripheral consolidation and ground-glass opacities are classically described. If you saw one of these x-rays from a patient with bacterial pneumonia, you would expect that patient to be very ill-appearing. Sometimes we get x-rays on patients who are sitting there, maybe mildly symptomatic on room air, and we are astonished by how terrible their x-rays look.
Unfortunately, imaging studies are something we haven't been able to rely on too much for diagnosis. Part of that is to maintain hospital safety, because to take a patient to CT scan, you have to consider the turnaround time for cleaning the CT scanner and the exposure of additional staff to a possibly infected patient. Some of those logistical considerations have limited the availability of radiography.
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine, at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.

This transcript has been edited for clarity.
Gary S. Ferenchick, MD, MS: I'm Gary Ferenchick with Hannah Ferenchick, who has agreed to join us to talk about what's going on in Detroit, and also about PPE and decontamination processes. Why don't you introduce yourself?
Hannah R.B. Ferenchick, MD: I am Hannah Ferenchick. I'm an ER physician and medical intensivist. I split my time between the medical ICU and the emergency department at Detroit Medical Center.
Dr Gary Ferenchick: We were talking earlier about some of the not-well-described clinical scenarios that patients with definitive COVID might present with. One of these was the idea of "silent hypoxemia." Could you describe that?
Dr Hannah Ferenchick: Silent hypoxemia is being described in many of these COVID patients. That means the patient is very hypoxemic—they may have an oxygen saturation of about 85% on room air, but clinically they look very comfortable—they are not dyspneic or tachypneic and may not even verbalize a significant sense of shortness of breath. It's not every patient, but it has been interesting to see patients sitting there looking fairly normal, with a resting oxygen saturation much lower than you would expect for someone who doesn't have underlying pulmonary disease or other symptoms.
Dr Gary Ferenchick: What abnormalities are you seeing on standard or not-so-standard lab tests?
Dr Hannah Ferenchick: Some of the characteristic lab findings we are seeing are lymphopenia and elevated inflammatory markers (eg, CRP). A couple of other atypical findings seem to be specific for COVID—elevated LDH, ferritin, CPK, and procalcitonin levels. Some of the hematologic markers that we look at—the coagulation profile studies—are also abnormal, showing thrombocytopenia and elevated D-dimer levels.
That constellation of symptoms represents more of a clinical picture. A lot of times we have only a very high clinical suspicion, because in many parts of the country it still takes days to get back a confirmatory PCR test.
Much like we do for the flu, the confirmatory test is a nasopharyngeal swab that is run for COVID/coronavirus PCR. Unfortunately the sensitivity of that test is not great. Some studies have quoted 75%-80%, so even a negative PCR does not necessarily rule out the disease, especially if you have a high clinical suspicion. A clinical suspicion is based on the typical symptoms. Many patients, although not all, will have symptoms of lower respiratory tract infection.
Dr Gary Ferenchick: So the right clinical scenario with the right hematologic/biochemical findings dramatically raises the chance that the patient has COVID?
Dr Hannah Ferenchick: Yes, and one thing that we have all been astonished by is how terrible some of these x-rays can look. There are a lot of typical findings on x-ray. Some describe them as looking like pulmonary edema, but the patient has no history of heart failure. Peripheral consolidation and ground-glass opacities are classically described. If you saw one of these x-rays from a patient with bacterial pneumonia, you would expect that patient to be very ill-appearing. Sometimes we get x-rays on patients who are sitting there, maybe mildly symptomatic on room air, and we are astonished by how terrible their x-rays look.
Unfortunately, imaging studies are something we haven't been able to rely on too much for diagnosis. Part of that is to maintain hospital safety, because to take a patient to CT scan, you have to consider the turnaround time for cleaning the CT scanner and the exposure of additional staff to a possibly infected patient. Some of those logistical considerations have limited the availability of radiography.
Gary S. Ferenchick, MD, MS, is a family physician and professor in the Department of Medicine at Michigan State University in East Lansing, Michigan. His daughter, Hannah R.B. Ferenchick, MD, is an assistant professor in the Department of Emergency Medicine, Division of Pulmonary & Critical Care and Sleep Medicine, at Wayne State University, Detroit, Michigan, and a medical intensivist and emergency medicine physician at Detroit Medical Center.
Critical care and COVID-19: Dr. Matt Aldrich
Matt Aldrich, MD, is an anesthesiologist and medical director of critical care at UCSF Health in San Francisco. Robert Wachter, MD,MHM, spoke with him about critical care issues in COVID-19, including clinical presentation, PPE in the ICU, whether the health system has enough ventilators for a surge, and ethical dilemmas that ICUs may face during the pandemic.
Matt Aldrich, MD, is an anesthesiologist and medical director of critical care at UCSF Health in San Francisco. Robert Wachter, MD,MHM, spoke with him about critical care issues in COVID-19, including clinical presentation, PPE in the ICU, whether the health system has enough ventilators for a surge, and ethical dilemmas that ICUs may face during the pandemic.
Matt Aldrich, MD, is an anesthesiologist and medical director of critical care at UCSF Health in San Francisco. Robert Wachter, MD,MHM, spoke with him about critical care issues in COVID-19, including clinical presentation, PPE in the ICU, whether the health system has enough ventilators for a surge, and ethical dilemmas that ICUs may face during the pandemic.
Treatment options for COVID-19: Dr. Annie Luetkemeyer
Annie Luetkemeyer, MD, professor of infectious diseases at UCSF, is an expert on the treatment of viral infections. Robert Wachter, MD, MHM, chair of the UCSF Department of Medicine, interviewed her about the evidence behind potential treatments for COVID-19 (including chloroquine/hydroxychloroquine, remdesivir, and others), as well as how to assess new and existing drugs in a pandemic.
Annie Luetkemeyer, MD, professor of infectious diseases at UCSF, is an expert on the treatment of viral infections. Robert Wachter, MD, MHM, chair of the UCSF Department of Medicine, interviewed her about the evidence behind potential treatments for COVID-19 (including chloroquine/hydroxychloroquine, remdesivir, and others), as well as how to assess new and existing drugs in a pandemic.
Annie Luetkemeyer, MD, professor of infectious diseases at UCSF, is an expert on the treatment of viral infections. Robert Wachter, MD, MHM, chair of the UCSF Department of Medicine, interviewed her about the evidence behind potential treatments for COVID-19 (including chloroquine/hydroxychloroquine, remdesivir, and others), as well as how to assess new and existing drugs in a pandemic.
Managing the COVID-19 isolation floor at UCSF Medical Center
Robert Wachter, MD, MHM, chair of the department of medicine at UCSF, interviewed Armond Esmaili, MD, a hospitalist and assistant professor of medicine at UCSF, who is the leader of the Respiratory Isolation Unit at UCSF Medical Center, where the institution's COVID-19 and rule-out COVID-19 patients are being cohorted.
Robert Wachter, MD, MHM, chair of the department of medicine at UCSF, interviewed Armond Esmaili, MD, a hospitalist and assistant professor of medicine at UCSF, who is the leader of the Respiratory Isolation Unit at UCSF Medical Center, where the institution's COVID-19 and rule-out COVID-19 patients are being cohorted.
Robert Wachter, MD, MHM, chair of the department of medicine at UCSF, interviewed Armond Esmaili, MD, a hospitalist and assistant professor of medicine at UCSF, who is the leader of the Respiratory Isolation Unit at UCSF Medical Center, where the institution's COVID-19 and rule-out COVID-19 patients are being cohorted.