User login
Bullous Dermatoses and Quality of Life: A Summary of Tools to Assess Psychosocial Health
Autoimmune bullous dermatoses (ABDs) develop due to antibodies directed against antigens within the epidermis or at the dermoepidermal junction. They are categorized histologically by the location of acantholysis (separation of keratinocytes), clinical presentation, and presence of autoantibodies. The most common ABDs include pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid (BP). These conditions present on a spectrum of symptoms and severity.1
Although multiple studies have evaluated the impact of bullous dermatoses on mental health, most were designed with a small sample size, thus limiting the generalizability of each study. Sebaratnam et al2 summarized several studies in 2012. In this review, we will analyze additional relevant literature and systematically combine the data to determine the psychological burden of disease of ABDs. We also will discuss the existing questionnaires frequently used in the dermatology setting to assess adverse psychosocial symptoms.
Methods
We searched PubMed, MEDLINE, and Google Scholar for articles published within the last 15 years using the terms bullous pemphigoid, pemphigus, quality of life, anxiety, and depression. We reviewed the citations in each article to further our search.
Criteria for Inclusion and Exclusion—Studies that utilized validated questionnaires to evaluate the effects of pemphigus vulgaris, pemphigus foliaceus, and/or BP on mental health were included. All research participants were 18 years and older. For the questionnaires administered, each study must have included numerical scores in the results. The studies all reported statistically significant results (P<.05), but no studies were excluded on the basis of statistical significance.
Studies were excluded if they did not use a validated questionnaire to examine quality of life (QOL) or psychological status. We also excluded database, retrospective, qualitative, and observational studies. We did not include studies with a sample size less than 20. Studies that administered questionnaires that were uncommon in this realm of research such as the Attitude to Appearance Scale or The Anxiety Questionnaire also were excluded. We did not exclude articles based on their primary language.
Results
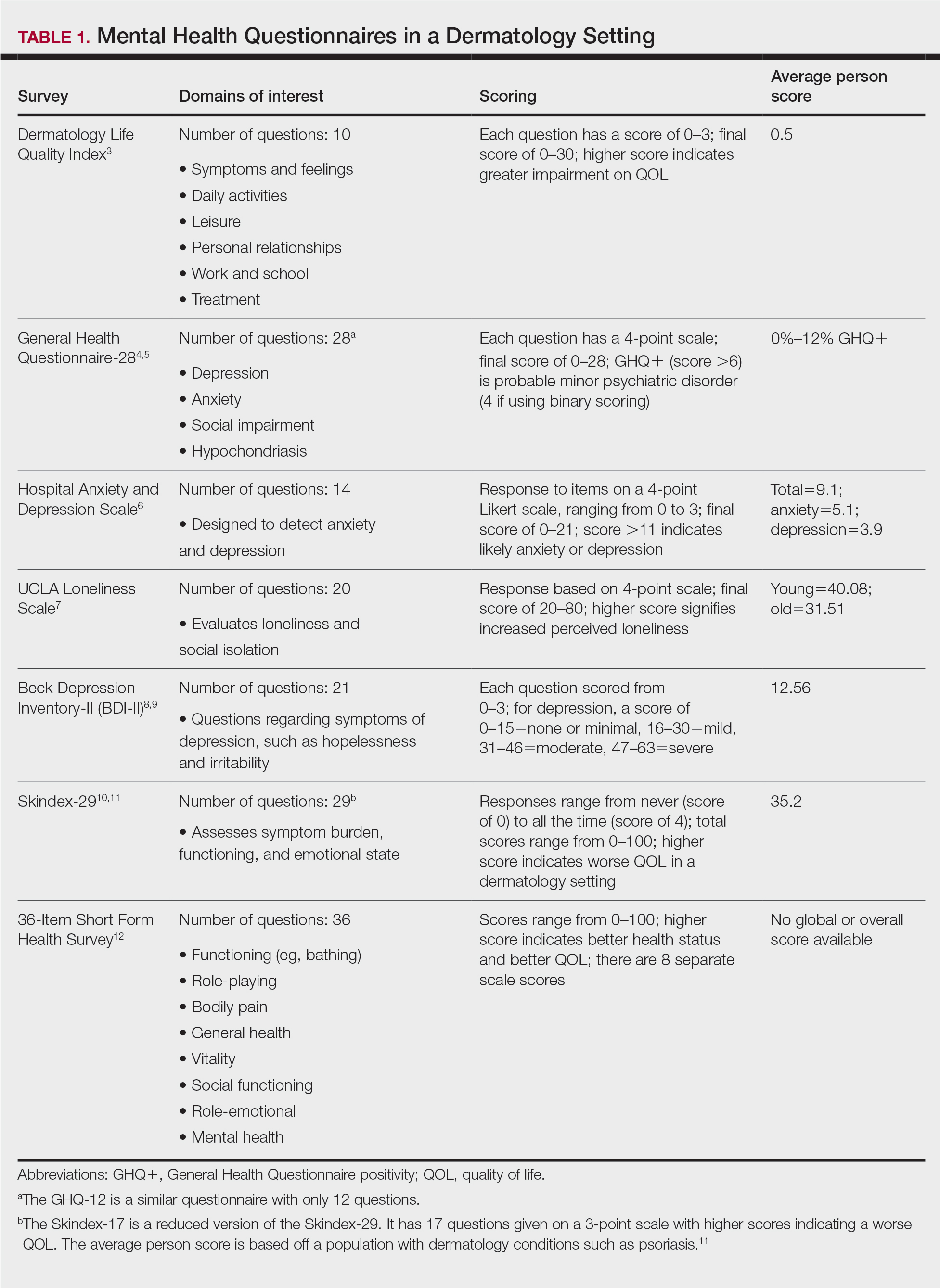
A total of 13 studies met the inclusion criteria with a total of 1716 participants enrolled in the trials. The questionnaires most commonly used are summarized in Table 1. Tables 2 and 3 demonstrate the studies that evaluate QOL and psychological state in patients with bullous dermatoses, respectively.
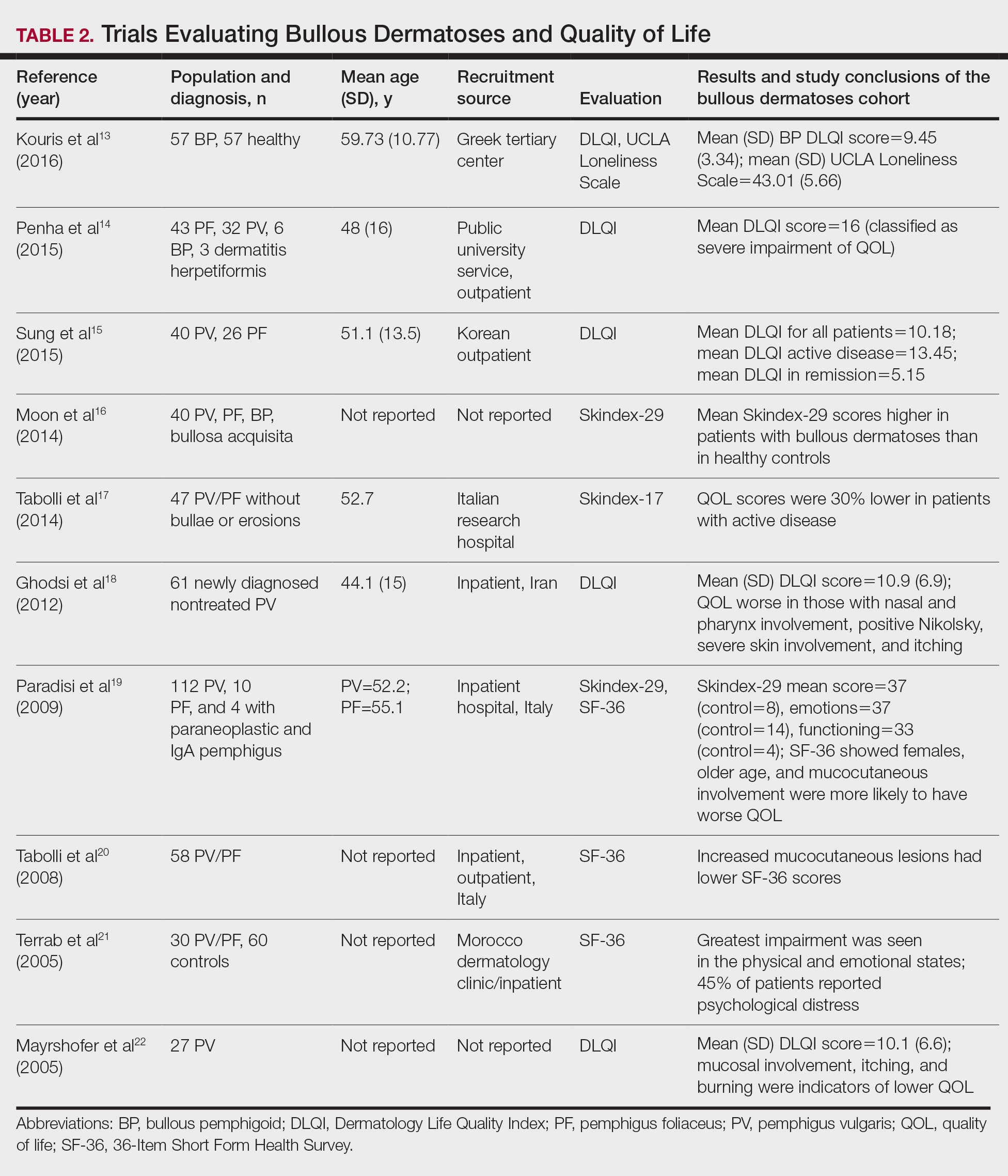
The Dermatology Life Quality Index (DLQI) was the most utilized method for analyzing QOL followed by the Skindex-17, Skindex-29, and 36-Item Short Form Health Survey. The DLQI is a skin-specific measurement tool with higher scores translating to greater impairment in QOL. Healthy patients have an average score of 0.5.3 The mean DLQI scores for ABD patients as seen in Table 2 were 9.45, 10.18, 16, 10.9, and 10.1.13-15,18,22 The most commonly reported concerns among patients included feelings about appearance and disturbances in daily activities.18 Symptoms of mucosal involvement, itching, and burning also were indicators of lower QOL.15,18,20,22 Furthermore, women consistently had lower scores than men.15,17,19,25 Multiple studies concluded that severity of the disease correlated with a lower QOL, though the subtype of pemphigus did not have an effect on QOL scores.15,19,20,21 Lastly, recent onset of symptoms was associated with a worse QOL score.15,18-20 Age, education level, and marital status did not have an effect on QOL.
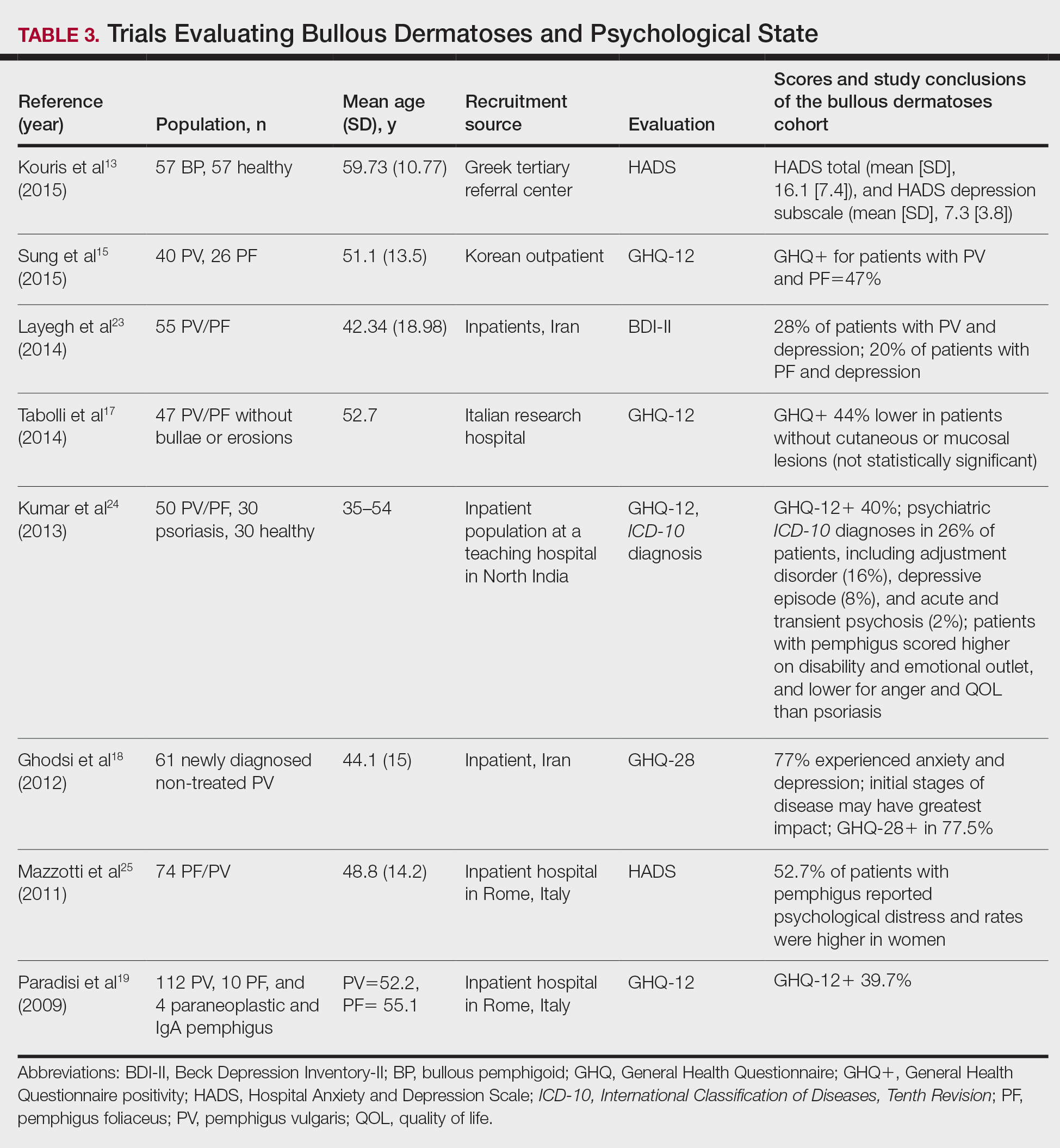
To evaluate psychological state, the General Health Questionnaire (GHQ)-28 and -12 primarily were used, in addition to the Hospital Anxiety and Depression Scale; the International Classification of Diseases, Tenth Revision; the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and the Beck Depression Inventory-II. As seen in Table 3, GHQ-12 positivity, reflecting probable minor nonpsychotic psychiatric disorders such as depression and anxiety, was identified in 47%, 39.7%, and 40% of patients with pemphigus15,19,24; GHQ-28 positivity was seen in 77.5% of pemphigus patients.18 In the average population, GHQ positivity was found in up to 12% of patients.26,27 Similar to the QOL scores, no significant differences were seen based on subtype of pemphigus for symptoms of depression or anxiety.20,23
Comment
Mental Health of Patients With ABDs—Immunobullous diseases are painful, potentially lifelong conditions that have no definitive cure. These conditions are characterized by bullae and erosions of the skin and mucosae that physically are disabling and often create a stigma for patients. Across multiple different validated psychosocial assessments, the 13 studies included in this review consistently reported that ABDs have a negative effect on mental well-being of patients that is more pronounced in women and worse at the onset of symptoms.13-25
QOL Scores in Patients With ABDs—Quality of life is a broad term that encompasses a general sense of psychological and overall well-being. A score of approximately 10 on the DLQI most often was reported in patients with ABDs, which translates to a moderate impact on QOL. Incomparison, a large cohort study reported the mean (SD) DLQI scores for patients with atopic dermatitis and psoriasis as 7.31 (5.98) and 5.93 (5.66), respectively.28 In another study, Penha et al14 found that patients with psoriasis have a mean DLQI score of 10. Reasons for the similarly low QOL scores in patients with ABDs include long hospitalization periods, disease chronicity, social anxiety, inability to control symptoms, difficulty with activities of daily living, and the belief that the disease is incurable.17,19,23 Although there is a need for increased family and social support with performing necessary daily tasks, personal relationships often are negatively affected, resulting in social isolation, loneliness, and worsening of cutaneous symptoms.
Severity of cutaneous disease and recent onset of symptoms correlated with worse QOL scores. Tabolli et al20 proposed the reason for this relates to not having had enough time to find the best treatment regimen. We believe there also may be an element of habituation involved, whereby patients become accustomed to the appearance of the lesions over time and therefore they become less distressing. Interestingly, Tabolli et al17 determined that patients in the quiescent phase of the disease—without any mucosal or cutaneous lesions—still maintained lower QOL scores than the average population, particularly on the psychosocial section of the 36-Item Short Form Health Survey, which may be due to a concern of disease relapse or from adverse effects of treatment. Providers should monitor patients for mental health complications not only in the disease infancy but throughout the disease course.
Future Directions—Cause and effect of the relationship between the psychosocial variables and ABD disease state has yet to be determined. Most studies included in this review were cross-sectional in design. Although many studies concluded that bullous dermatoses were the cause of impaired QOL, Ren and colleagues29 proposed that medications used to treat neuropsychiatric disorders may trigger the autoimmune antigens of BP. Possible triggers for BP have been reported including hydrochlorothiazide, ciprofloxacin, and dipeptidyl peptidase-4 inhibitors.27,30-32 A longitudinal study design would better evaluate the causal relationship.
The effects of the medications were included in 2 cases, one in which the steroid dose was not found to have a significant impact on rates of depression23 and another in which patients treated with a higher dose of corticosteroids (>10 mg) had worse QOL scores.17 Sung et al15 suggested this may be because patients who took higher doses of steroids had worse symptoms and therefore also had a worse QOL. It also is possible that those patients taking higher doses had increased side effects.17 Further studies that evaluate treatment modalities and timing in relation to the disease onset would be helpful.
Study Limitations—There are potential barriers to combining these data. Multiple different questionnaires were used, and it was difficult to ascertain if all the participants were experiencing active disease. Additionally, questionnaires are not always the best proxy for what is happening in everyday life. Lastly, the sample size of each individual study was small, and the studies only included adults.
Conclusion
As demonstrated by the 13 studies in this review, patients with ABDs have lower QOL scores and higher numbers of psychological symptoms. Clinicians should be mindful of this at-risk population and create opportunities in clinic to discuss personal hardship associated with the disease process and recommend psychiatric intervention if indicated. Additionally, family members often are overburdened with the chronicity of ABDs, and they should not be forgotten. Using one of the aforementioned questionnaires is a practical way to screen patients for lower QOL scores. We agree with Paradisi and colleagues19 that although these questionnaires may be helpful, clinicians still need to determine if the use of a dermatologic QOL evaluation tool in clinical practice improves patient satisfaction.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489. https://doi.org/10.1016/j.autrev.2014.01.047
- Sebaratnam DF, McMillan JR, Werth VP, et al. Quality of life in patients with bullous dermatoses. Clin Dermatol. 2012;30:103-107. doi:10.1016/j.clindermatol.2011.03.016
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. Oxford University Press; 1972.
- Cano A, Sprafkin RP, Scaturo DJ, et al. Mental health screening in primary care: a comparison of 3 brief measures of psychological distress. Prim Care Companion J Clin Psychiatry. 2001;3:206-210.
- Zigmond A, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-370.
- Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20-40. doi:10.1207/s15327752jpa6601_2
- Beck A, Alford B. Depression: Causes and Treatment. 2nd ed. Philadelphia University of Pennsylvania Press; 2009.
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, et al. Psychometric properties of a Persian-language version of the Beck Depression Inventory—Second Edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185-192. doi:10.1002/da.20070
- Chren MM, Lasek RJ, Sahay AP, et al. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105-110.
- Nijsten TEC, Sampogna F, Chren M, et al. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol. 2006;126:1244-1250. https://doi.org/10.1038/sj.jid.5700212
- Ware JE Jr, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid: a case control study. An Bras Dermatol. 2016;91:601-603. doi:10.1590/abd1806-4841.2016493
- Penha MA, Farat JG, Miot HA, et al. Quality of life index in autoimmune bullous dermatosis patients. An Bras Dermatol. 2015;90:190-194. https://dx.doi.org/10.1590/abd1806-4841.20153372
- Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol. 2015;27:492-498.
- Moon SH, Kwon HI, Park HC, et al. Assessment of the quality of life in autoimmune blistering skin disease patients. Korean J Dermatol. 2014;52:402-409.
- Tabolli S, Pagliarello C, Paradisi A, et al. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170:1087-1091. doi:10.1111/bjd.12836
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and general health questionnaires. J Dermatol. 2012;39:141-144. doi:10.1111/j.1346-8138.2011.01382
- Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269. doi:10.1016/j.jaad.2008.09.014
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034. doi:10.1111/j.1365-2133.2008.08481.x
- Terrab Z, Benchikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris: results from the German Bullous Skin Disease (BSD) Study Group [in German]. J Dtsch Dermatol Ges. 2005;3:431-435. doi:10.1111/j.1610-0387.2005.05722.x
- Layegh P, Mokhber N, Javidi Z, et al. Depression in patients with pemphigus: is it a major concern? J Dermatol. 2014;40:434-437. doi:10.1111/1346-8138.12067
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151-156. doi:10.1016/j.ajp.2012.10.005
- Mazzotti E, Mozzetta A, Antinone V, et al. Psychological distress and investment in one’s appearance in patients with pemphigus. J Eur Acad Dermatol Venereol. 2011;25:285-289. doi:10.1111/j.1468-3083.2010.03780.x
- Regier DA, Boyd JH, Burke JD, et al. One-month prevalence of mental disorders in the United States: based on five epidemiologic catchment area sites. Arch Gen Psychiatr. 1988;45:977-986. doi:10.1001/archpsyc.1988.01800350011002
- Cozzani E, Chinazzo C, Burlando M, et al. Ciprofloxacin as a trigger for bullous pemphigoid: the second case in the literature. Am J Ther. 2016;23:E1202-E1204. doi:10.1097/MJT.0000000000000283
- Lundberg L, Johannesson M, Silverdahl M, et al. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000;80:430-434.
- Ren Z, Hsu DY, Brieva J, et al. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176:87-99. doi:10.1111/bjd.14821
- Warner C, Kwak Y, Glover MH, et al. Bullous pemphigoid induced by hydrochlorothiazide therapy. J Drugs Dermatol. 2014;13:360-362.
- Mendonca FM, Martin-Gutierrez FJ, Rios-Martin JJ, et al. Three cases of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors—one due to linagliptin. Dermatology. 2016;232:249-253. doi:10.1159/000443330
- Attaway A, Mersfelder TL, Vaishnav S, et al. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors: a case report and review of literature. J Dermatol Case Rep. 2014;8:24-28.
Autoimmune bullous dermatoses (ABDs) develop due to antibodies directed against antigens within the epidermis or at the dermoepidermal junction. They are categorized histologically by the location of acantholysis (separation of keratinocytes), clinical presentation, and presence of autoantibodies. The most common ABDs include pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid (BP). These conditions present on a spectrum of symptoms and severity.1
Although multiple studies have evaluated the impact of bullous dermatoses on mental health, most were designed with a small sample size, thus limiting the generalizability of each study. Sebaratnam et al2 summarized several studies in 2012. In this review, we will analyze additional relevant literature and systematically combine the data to determine the psychological burden of disease of ABDs. We also will discuss the existing questionnaires frequently used in the dermatology setting to assess adverse psychosocial symptoms.
Methods
We searched PubMed, MEDLINE, and Google Scholar for articles published within the last 15 years using the terms bullous pemphigoid, pemphigus, quality of life, anxiety, and depression. We reviewed the citations in each article to further our search.
Criteria for Inclusion and Exclusion—Studies that utilized validated questionnaires to evaluate the effects of pemphigus vulgaris, pemphigus foliaceus, and/or BP on mental health were included. All research participants were 18 years and older. For the questionnaires administered, each study must have included numerical scores in the results. The studies all reported statistically significant results (P<.05), but no studies were excluded on the basis of statistical significance.
Studies were excluded if they did not use a validated questionnaire to examine quality of life (QOL) or psychological status. We also excluded database, retrospective, qualitative, and observational studies. We did not include studies with a sample size less than 20. Studies that administered questionnaires that were uncommon in this realm of research such as the Attitude to Appearance Scale or The Anxiety Questionnaire also were excluded. We did not exclude articles based on their primary language.

Results
A total of 13 studies met the inclusion criteria with a total of 1716 participants enrolled in the trials. The questionnaires most commonly used are summarized in Table 1. Tables 2 and 3 demonstrate the studies that evaluate QOL and psychological state in patients with bullous dermatoses, respectively.

The Dermatology Life Quality Index (DLQI) was the most utilized method for analyzing QOL followed by the Skindex-17, Skindex-29, and 36-Item Short Form Health Survey. The DLQI is a skin-specific measurement tool with higher scores translating to greater impairment in QOL. Healthy patients have an average score of 0.5.3 The mean DLQI scores for ABD patients as seen in Table 2 were 9.45, 10.18, 16, 10.9, and 10.1.13-15,18,22 The most commonly reported concerns among patients included feelings about appearance and disturbances in daily activities.18 Symptoms of mucosal involvement, itching, and burning also were indicators of lower QOL.15,18,20,22 Furthermore, women consistently had lower scores than men.15,17,19,25 Multiple studies concluded that severity of the disease correlated with a lower QOL, though the subtype of pemphigus did not have an effect on QOL scores.15,19,20,21 Lastly, recent onset of symptoms was associated with a worse QOL score.15,18-20 Age, education level, and marital status did not have an effect on QOL.

To evaluate psychological state, the General Health Questionnaire (GHQ)-28 and -12 primarily were used, in addition to the Hospital Anxiety and Depression Scale; the International Classification of Diseases, Tenth Revision; the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and the Beck Depression Inventory-II. As seen in Table 3, GHQ-12 positivity, reflecting probable minor nonpsychotic psychiatric disorders such as depression and anxiety, was identified in 47%, 39.7%, and 40% of patients with pemphigus15,19,24; GHQ-28 positivity was seen in 77.5% of pemphigus patients.18 In the average population, GHQ positivity was found in up to 12% of patients.26,27 Similar to the QOL scores, no significant differences were seen based on subtype of pemphigus for symptoms of depression or anxiety.20,23
Comment
Mental Health of Patients With ABDs—Immunobullous diseases are painful, potentially lifelong conditions that have no definitive cure. These conditions are characterized by bullae and erosions of the skin and mucosae that physically are disabling and often create a stigma for patients. Across multiple different validated psychosocial assessments, the 13 studies included in this review consistently reported that ABDs have a negative effect on mental well-being of patients that is more pronounced in women and worse at the onset of symptoms.13-25
QOL Scores in Patients With ABDs—Quality of life is a broad term that encompasses a general sense of psychological and overall well-being. A score of approximately 10 on the DLQI most often was reported in patients with ABDs, which translates to a moderate impact on QOL. Incomparison, a large cohort study reported the mean (SD) DLQI scores for patients with atopic dermatitis and psoriasis as 7.31 (5.98) and 5.93 (5.66), respectively.28 In another study, Penha et al14 found that patients with psoriasis have a mean DLQI score of 10. Reasons for the similarly low QOL scores in patients with ABDs include long hospitalization periods, disease chronicity, social anxiety, inability to control symptoms, difficulty with activities of daily living, and the belief that the disease is incurable.17,19,23 Although there is a need for increased family and social support with performing necessary daily tasks, personal relationships often are negatively affected, resulting in social isolation, loneliness, and worsening of cutaneous symptoms.
Severity of cutaneous disease and recent onset of symptoms correlated with worse QOL scores. Tabolli et al20 proposed the reason for this relates to not having had enough time to find the best treatment regimen. We believe there also may be an element of habituation involved, whereby patients become accustomed to the appearance of the lesions over time and therefore they become less distressing. Interestingly, Tabolli et al17 determined that patients in the quiescent phase of the disease—without any mucosal or cutaneous lesions—still maintained lower QOL scores than the average population, particularly on the psychosocial section of the 36-Item Short Form Health Survey, which may be due to a concern of disease relapse or from adverse effects of treatment. Providers should monitor patients for mental health complications not only in the disease infancy but throughout the disease course.
Future Directions—Cause and effect of the relationship between the psychosocial variables and ABD disease state has yet to be determined. Most studies included in this review were cross-sectional in design. Although many studies concluded that bullous dermatoses were the cause of impaired QOL, Ren and colleagues29 proposed that medications used to treat neuropsychiatric disorders may trigger the autoimmune antigens of BP. Possible triggers for BP have been reported including hydrochlorothiazide, ciprofloxacin, and dipeptidyl peptidase-4 inhibitors.27,30-32 A longitudinal study design would better evaluate the causal relationship.
The effects of the medications were included in 2 cases, one in which the steroid dose was not found to have a significant impact on rates of depression23 and another in which patients treated with a higher dose of corticosteroids (>10 mg) had worse QOL scores.17 Sung et al15 suggested this may be because patients who took higher doses of steroids had worse symptoms and therefore also had a worse QOL. It also is possible that those patients taking higher doses had increased side effects.17 Further studies that evaluate treatment modalities and timing in relation to the disease onset would be helpful.
Study Limitations—There are potential barriers to combining these data. Multiple different questionnaires were used, and it was difficult to ascertain if all the participants were experiencing active disease. Additionally, questionnaires are not always the best proxy for what is happening in everyday life. Lastly, the sample size of each individual study was small, and the studies only included adults.
Conclusion
As demonstrated by the 13 studies in this review, patients with ABDs have lower QOL scores and higher numbers of psychological symptoms. Clinicians should be mindful of this at-risk population and create opportunities in clinic to discuss personal hardship associated with the disease process and recommend psychiatric intervention if indicated. Additionally, family members often are overburdened with the chronicity of ABDs, and they should not be forgotten. Using one of the aforementioned questionnaires is a practical way to screen patients for lower QOL scores. We agree with Paradisi and colleagues19 that although these questionnaires may be helpful, clinicians still need to determine if the use of a dermatologic QOL evaluation tool in clinical practice improves patient satisfaction.
Autoimmune bullous dermatoses (ABDs) develop due to antibodies directed against antigens within the epidermis or at the dermoepidermal junction. They are categorized histologically by the location of acantholysis (separation of keratinocytes), clinical presentation, and presence of autoantibodies. The most common ABDs include pemphigus vulgaris, pemphigus foliaceus, and bullous pemphigoid (BP). These conditions present on a spectrum of symptoms and severity.1
Although multiple studies have evaluated the impact of bullous dermatoses on mental health, most were designed with a small sample size, thus limiting the generalizability of each study. Sebaratnam et al2 summarized several studies in 2012. In this review, we will analyze additional relevant literature and systematically combine the data to determine the psychological burden of disease of ABDs. We also will discuss the existing questionnaires frequently used in the dermatology setting to assess adverse psychosocial symptoms.
Methods
We searched PubMed, MEDLINE, and Google Scholar for articles published within the last 15 years using the terms bullous pemphigoid, pemphigus, quality of life, anxiety, and depression. We reviewed the citations in each article to further our search.
Criteria for Inclusion and Exclusion—Studies that utilized validated questionnaires to evaluate the effects of pemphigus vulgaris, pemphigus foliaceus, and/or BP on mental health were included. All research participants were 18 years and older. For the questionnaires administered, each study must have included numerical scores in the results. The studies all reported statistically significant results (P<.05), but no studies were excluded on the basis of statistical significance.
Studies were excluded if they did not use a validated questionnaire to examine quality of life (QOL) or psychological status. We also excluded database, retrospective, qualitative, and observational studies. We did not include studies with a sample size less than 20. Studies that administered questionnaires that were uncommon in this realm of research such as the Attitude to Appearance Scale or The Anxiety Questionnaire also were excluded. We did not exclude articles based on their primary language.

Results
A total of 13 studies met the inclusion criteria with a total of 1716 participants enrolled in the trials. The questionnaires most commonly used are summarized in Table 1. Tables 2 and 3 demonstrate the studies that evaluate QOL and psychological state in patients with bullous dermatoses, respectively.

The Dermatology Life Quality Index (DLQI) was the most utilized method for analyzing QOL followed by the Skindex-17, Skindex-29, and 36-Item Short Form Health Survey. The DLQI is a skin-specific measurement tool with higher scores translating to greater impairment in QOL. Healthy patients have an average score of 0.5.3 The mean DLQI scores for ABD patients as seen in Table 2 were 9.45, 10.18, 16, 10.9, and 10.1.13-15,18,22 The most commonly reported concerns among patients included feelings about appearance and disturbances in daily activities.18 Symptoms of mucosal involvement, itching, and burning also were indicators of lower QOL.15,18,20,22 Furthermore, women consistently had lower scores than men.15,17,19,25 Multiple studies concluded that severity of the disease correlated with a lower QOL, though the subtype of pemphigus did not have an effect on QOL scores.15,19,20,21 Lastly, recent onset of symptoms was associated with a worse QOL score.15,18-20 Age, education level, and marital status did not have an effect on QOL.

To evaluate psychological state, the General Health Questionnaire (GHQ)-28 and -12 primarily were used, in addition to the Hospital Anxiety and Depression Scale; the International Classification of Diseases, Tenth Revision; the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; and the Beck Depression Inventory-II. As seen in Table 3, GHQ-12 positivity, reflecting probable minor nonpsychotic psychiatric disorders such as depression and anxiety, was identified in 47%, 39.7%, and 40% of patients with pemphigus15,19,24; GHQ-28 positivity was seen in 77.5% of pemphigus patients.18 In the average population, GHQ positivity was found in up to 12% of patients.26,27 Similar to the QOL scores, no significant differences were seen based on subtype of pemphigus for symptoms of depression or anxiety.20,23
Comment
Mental Health of Patients With ABDs—Immunobullous diseases are painful, potentially lifelong conditions that have no definitive cure. These conditions are characterized by bullae and erosions of the skin and mucosae that physically are disabling and often create a stigma for patients. Across multiple different validated psychosocial assessments, the 13 studies included in this review consistently reported that ABDs have a negative effect on mental well-being of patients that is more pronounced in women and worse at the onset of symptoms.13-25
QOL Scores in Patients With ABDs—Quality of life is a broad term that encompasses a general sense of psychological and overall well-being. A score of approximately 10 on the DLQI most often was reported in patients with ABDs, which translates to a moderate impact on QOL. Incomparison, a large cohort study reported the mean (SD) DLQI scores for patients with atopic dermatitis and psoriasis as 7.31 (5.98) and 5.93 (5.66), respectively.28 In another study, Penha et al14 found that patients with psoriasis have a mean DLQI score of 10. Reasons for the similarly low QOL scores in patients with ABDs include long hospitalization periods, disease chronicity, social anxiety, inability to control symptoms, difficulty with activities of daily living, and the belief that the disease is incurable.17,19,23 Although there is a need for increased family and social support with performing necessary daily tasks, personal relationships often are negatively affected, resulting in social isolation, loneliness, and worsening of cutaneous symptoms.
Severity of cutaneous disease and recent onset of symptoms correlated with worse QOL scores. Tabolli et al20 proposed the reason for this relates to not having had enough time to find the best treatment regimen. We believe there also may be an element of habituation involved, whereby patients become accustomed to the appearance of the lesions over time and therefore they become less distressing. Interestingly, Tabolli et al17 determined that patients in the quiescent phase of the disease—without any mucosal or cutaneous lesions—still maintained lower QOL scores than the average population, particularly on the psychosocial section of the 36-Item Short Form Health Survey, which may be due to a concern of disease relapse or from adverse effects of treatment. Providers should monitor patients for mental health complications not only in the disease infancy but throughout the disease course.
Future Directions—Cause and effect of the relationship between the psychosocial variables and ABD disease state has yet to be determined. Most studies included in this review were cross-sectional in design. Although many studies concluded that bullous dermatoses were the cause of impaired QOL, Ren and colleagues29 proposed that medications used to treat neuropsychiatric disorders may trigger the autoimmune antigens of BP. Possible triggers for BP have been reported including hydrochlorothiazide, ciprofloxacin, and dipeptidyl peptidase-4 inhibitors.27,30-32 A longitudinal study design would better evaluate the causal relationship.
The effects of the medications were included in 2 cases, one in which the steroid dose was not found to have a significant impact on rates of depression23 and another in which patients treated with a higher dose of corticosteroids (>10 mg) had worse QOL scores.17 Sung et al15 suggested this may be because patients who took higher doses of steroids had worse symptoms and therefore also had a worse QOL. It also is possible that those patients taking higher doses had increased side effects.17 Further studies that evaluate treatment modalities and timing in relation to the disease onset would be helpful.
Study Limitations—There are potential barriers to combining these data. Multiple different questionnaires were used, and it was difficult to ascertain if all the participants were experiencing active disease. Additionally, questionnaires are not always the best proxy for what is happening in everyday life. Lastly, the sample size of each individual study was small, and the studies only included adults.
Conclusion
As demonstrated by the 13 studies in this review, patients with ABDs have lower QOL scores and higher numbers of psychological symptoms. Clinicians should be mindful of this at-risk population and create opportunities in clinic to discuss personal hardship associated with the disease process and recommend psychiatric intervention if indicated. Additionally, family members often are overburdened with the chronicity of ABDs, and they should not be forgotten. Using one of the aforementioned questionnaires is a practical way to screen patients for lower QOL scores. We agree with Paradisi and colleagues19 that although these questionnaires may be helpful, clinicians still need to determine if the use of a dermatologic QOL evaluation tool in clinical practice improves patient satisfaction.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489. https://doi.org/10.1016/j.autrev.2014.01.047
- Sebaratnam DF, McMillan JR, Werth VP, et al. Quality of life in patients with bullous dermatoses. Clin Dermatol. 2012;30:103-107. doi:10.1016/j.clindermatol.2011.03.016
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. Oxford University Press; 1972.
- Cano A, Sprafkin RP, Scaturo DJ, et al. Mental health screening in primary care: a comparison of 3 brief measures of psychological distress. Prim Care Companion J Clin Psychiatry. 2001;3:206-210.
- Zigmond A, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-370.
- Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20-40. doi:10.1207/s15327752jpa6601_2
- Beck A, Alford B. Depression: Causes and Treatment. 2nd ed. Philadelphia University of Pennsylvania Press; 2009.
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, et al. Psychometric properties of a Persian-language version of the Beck Depression Inventory—Second Edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185-192. doi:10.1002/da.20070
- Chren MM, Lasek RJ, Sahay AP, et al. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105-110.
- Nijsten TEC, Sampogna F, Chren M, et al. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol. 2006;126:1244-1250. https://doi.org/10.1038/sj.jid.5700212
- Ware JE Jr, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid: a case control study. An Bras Dermatol. 2016;91:601-603. doi:10.1590/abd1806-4841.2016493
- Penha MA, Farat JG, Miot HA, et al. Quality of life index in autoimmune bullous dermatosis patients. An Bras Dermatol. 2015;90:190-194. https://dx.doi.org/10.1590/abd1806-4841.20153372
- Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol. 2015;27:492-498.
- Moon SH, Kwon HI, Park HC, et al. Assessment of the quality of life in autoimmune blistering skin disease patients. Korean J Dermatol. 2014;52:402-409.
- Tabolli S, Pagliarello C, Paradisi A, et al. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170:1087-1091. doi:10.1111/bjd.12836
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and general health questionnaires. J Dermatol. 2012;39:141-144. doi:10.1111/j.1346-8138.2011.01382
- Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269. doi:10.1016/j.jaad.2008.09.014
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034. doi:10.1111/j.1365-2133.2008.08481.x
- Terrab Z, Benchikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris: results from the German Bullous Skin Disease (BSD) Study Group [in German]. J Dtsch Dermatol Ges. 2005;3:431-435. doi:10.1111/j.1610-0387.2005.05722.x
- Layegh P, Mokhber N, Javidi Z, et al. Depression in patients with pemphigus: is it a major concern? J Dermatol. 2014;40:434-437. doi:10.1111/1346-8138.12067
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151-156. doi:10.1016/j.ajp.2012.10.005
- Mazzotti E, Mozzetta A, Antinone V, et al. Psychological distress and investment in one’s appearance in patients with pemphigus. J Eur Acad Dermatol Venereol. 2011;25:285-289. doi:10.1111/j.1468-3083.2010.03780.x
- Regier DA, Boyd JH, Burke JD, et al. One-month prevalence of mental disorders in the United States: based on five epidemiologic catchment area sites. Arch Gen Psychiatr. 1988;45:977-986. doi:10.1001/archpsyc.1988.01800350011002
- Cozzani E, Chinazzo C, Burlando M, et al. Ciprofloxacin as a trigger for bullous pemphigoid: the second case in the literature. Am J Ther. 2016;23:E1202-E1204. doi:10.1097/MJT.0000000000000283
- Lundberg L, Johannesson M, Silverdahl M, et al. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000;80:430-434.
- Ren Z, Hsu DY, Brieva J, et al. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176:87-99. doi:10.1111/bjd.14821
- Warner C, Kwak Y, Glover MH, et al. Bullous pemphigoid induced by hydrochlorothiazide therapy. J Drugs Dermatol. 2014;13:360-362.
- Mendonca FM, Martin-Gutierrez FJ, Rios-Martin JJ, et al. Three cases of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors—one due to linagliptin. Dermatology. 2016;232:249-253. doi:10.1159/000443330
- Attaway A, Mersfelder TL, Vaishnav S, et al. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors: a case report and review of literature. J Dermatol Case Rep. 2014;8:24-28.
- Baum S, Sakka N, Artsi O, et al. Diagnosis and classification of autoimmune blistering diseases. Autoimmun Rev. 2014;13:482-489. https://doi.org/10.1016/j.autrev.2014.01.047
- Sebaratnam DF, McMillan JR, Werth VP, et al. Quality of life in patients with bullous dermatoses. Clin Dermatol. 2012;30:103-107. doi:10.1016/j.clindermatol.2011.03.016
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)—a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Goldberg DP. The Detection of Psychiatric Illness by Questionnaire. Oxford University Press; 1972.
- Cano A, Sprafkin RP, Scaturo DJ, et al. Mental health screening in primary care: a comparison of 3 brief measures of psychological distress. Prim Care Companion J Clin Psychiatry. 2001;3:206-210.
- Zigmond A, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361-370.
- Russell DW. UCLA Loneliness Scale (Version 3): reliability, validity, and factor structure. J Pers Assess. 1996;66:20-40. doi:10.1207/s15327752jpa6601_2
- Beck A, Alford B. Depression: Causes and Treatment. 2nd ed. Philadelphia University of Pennsylvania Press; 2009.
- Ghassemzadeh H, Mojtabai R, Karamghadiri N, et al. Psychometric properties of a Persian-language version of the Beck Depression Inventory—Second Edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185-192. doi:10.1002/da.20070
- Chren MM, Lasek RJ, Sahay AP, et al. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105-110.
- Nijsten TEC, Sampogna F, Chren M, et al. Testing and reducing Skindex-29 using Rasch analysis: Skindex-17. J Invest Dermatol. 2006;126:1244-1250. https://doi.org/10.1038/sj.jid.5700212
- Ware JE Jr, Sherbourne C. The MOS 36-item short-form health survey (SF-36): I. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Kouris A, Platsidaki E, Christodoulou C, et al. Quality of life, depression, anxiety and loneliness in patients with bullous pemphigoid: a case control study. An Bras Dermatol. 2016;91:601-603. doi:10.1590/abd1806-4841.2016493
- Penha MA, Farat JG, Miot HA, et al. Quality of life index in autoimmune bullous dermatosis patients. An Bras Dermatol. 2015;90:190-194. https://dx.doi.org/10.1590/abd1806-4841.20153372
- Sung JY, Roh MR, Kim SC. Quality of life assessment in Korean patients with pemphigus. Ann Dermatol. 2015;27:492-498.
- Moon SH, Kwon HI, Park HC, et al. Assessment of the quality of life in autoimmune blistering skin disease patients. Korean J Dermatol. 2014;52:402-409.
- Tabolli S, Pagliarello C, Paradisi A, et al. Burden of disease during quiescent periods in patients with pemphigus. Br J Dermatol. 2014;170:1087-1091. doi:10.1111/bjd.12836
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and general health questionnaires. J Dermatol. 2012;39:141-144. doi:10.1111/j.1346-8138.2011.01382
- Paradisi A, Sampogna F, Di Pietro C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269. doi:10.1016/j.jaad.2008.09.014
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034. doi:10.1111/j.1365-2133.2008.08481.x
- Terrab Z, Benchikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris: results from the German Bullous Skin Disease (BSD) Study Group [in German]. J Dtsch Dermatol Ges. 2005;3:431-435. doi:10.1111/j.1610-0387.2005.05722.x
- Layegh P, Mokhber N, Javidi Z, et al. Depression in patients with pemphigus: is it a major concern? J Dermatol. 2014;40:434-437. doi:10.1111/1346-8138.12067
- Kumar V, Mattoo SK, Handa S. Psychiatric morbidity in pemphigus and psoriasis: a comparative study from India. Asian J Psychiatr. 2013;6:151-156. doi:10.1016/j.ajp.2012.10.005
- Mazzotti E, Mozzetta A, Antinone V, et al. Psychological distress and investment in one’s appearance in patients with pemphigus. J Eur Acad Dermatol Venereol. 2011;25:285-289. doi:10.1111/j.1468-3083.2010.03780.x
- Regier DA, Boyd JH, Burke JD, et al. One-month prevalence of mental disorders in the United States: based on five epidemiologic catchment area sites. Arch Gen Psychiatr. 1988;45:977-986. doi:10.1001/archpsyc.1988.01800350011002
- Cozzani E, Chinazzo C, Burlando M, et al. Ciprofloxacin as a trigger for bullous pemphigoid: the second case in the literature. Am J Ther. 2016;23:E1202-E1204. doi:10.1097/MJT.0000000000000283
- Lundberg L, Johannesson M, Silverdahl M, et al. Health-related quality of life in patients with psoriasis and atopic dermatitis measured with SF-36, DLQI and a subjective measure of disease activity. Acta Derm Venereol. 2000;80:430-434.
- Ren Z, Hsu DY, Brieva J, et al. Hospitalization, inpatient burden and comorbidities associated with bullous pemphigoid in the U.S.A. Br J Dermatol. 2017;176:87-99. doi:10.1111/bjd.14821
- Warner C, Kwak Y, Glover MH, et al. Bullous pemphigoid induced by hydrochlorothiazide therapy. J Drugs Dermatol. 2014;13:360-362.
- Mendonca FM, Martin-Gutierrez FJ, Rios-Martin JJ, et al. Three cases of bullous pemphigoid associated with dipeptidyl peptidase-4 inhibitors—one due to linagliptin. Dermatology. 2016;232:249-253. doi:10.1159/000443330
- Attaway A, Mersfelder TL, Vaishnav S, et al. Bullous pemphigoid associated with dipeptidyl peptidase IV inhibitors: a case report and review of literature. J Dermatol Case Rep. 2014;8:24-28.
Practice Points
- Autoimmune bullous dermatoses cause cutaneous lesions that are painful and disfiguring. These conditions affect a patient’s ability to perform everyday tasks, and individual lesions can take years to heal.
- Providers should take necessary steps to address patient well-being, especially at disease onset in patients with bullous dermatoses.
A Fatal Case of Hemophagocytic Lymphohistiocytosis Secondary to Anti-MDA5–Positive Dermatomyositis
To the Editor:
Dermatomyositis (DM) is an idiopathic inflammatory myopathy characterized by bilateral, symmetrical, proximal muscle weakness and classic cutaneous manifestations.1 Patients with antibodies directed against melanoma differentiation–associated gene 5, MDA5, have a distinct presentation due to vasculopathy with more severe cutaneous ulcerations, palmar papules, alopecia, and an elevated risk of rapidly progressive interstitial lung disease.2 A ferritin level greater than 1600 ng/mL portends an increased risk for pulmonary disease and therefore can be of prognostic value.3 Further, patients with anti-MDA5 DM are at a lower risk of malignancy and are more likely to test negative for antinuclear antibodies in comparison to other patients with DM.2,4
Hemophagocytic lymphohistiocytosis (HLH), also known as hemophagocytic syndrome, is a potentially lethal condition whereby uncontrolled activation of histiocytes in the reticuloendothelial system causes hemophagocytosis and a hyperinflammatory state. Patients present with fever, splenomegaly, cytopenia, and hyperferritinemia.5 Autoimmune‐associated hemophagocytic syndrome (AAHS) describes HLH that develops in association with autoimmune conditions, most commonly systemic lupus erythematosus and adult-onset Still disease. Cases reported in association with DM exist but are few in number, and there is no standard-of-care treatment.6 We report a case of a woman with anti-MDA5 DM complicated by HLH and DM-associated liver injury.
A 50-year-old woman presented as a direct admit from the rheumatology clinic for diffuse muscle weakness of 8 months’ duration, 40-pound unintentional weight loss, pruritic rash, bilateral joint pains, dry eyes, dry mouth, and altered mental status. Four months prior, she presented to an outside hospital and was given a diagnosis of probable Sjögren syndrome and autoimmune hepatitis vs drug-induced liver injury. At that time, a workup was notable for antibodies against Sjögren syndrome–related antigen A, anti–smooth muscle antibodies, and transaminitis. Ultrasonography of the right upper quadrant revealed hepatic steatosis. The patient was started on oral prednisone and pilocarpine but had been off all medications for 1 month when she presented to our hospital.
On hospital admission, physical examination revealed a violaceous heliotrope rash; a v-sign on the chest; shawl sign; palmar papules with pits at the fingertips; and periungual erythema and ulcerations along the metacarpophalangeal joints, elbows, lateral feet, and upper eyelids (Figure 1). Laboratory workup showed the following results: white blood cell count, 4100/μL (reference range, 4000–11,000/μL); hemoglobin, 11.6 g/dL (reference range, 12–16 g/dL); platelet count, 100,000/μL (reference range, 150,000–450,000/μL); lactate dehydrogenase, 510 U/L (reference range, 80–225 U/L); alkaline phosphatase (ALP), 766 U/L (reference range, 30–120 U/L); alanine aminotransferase (ALT), 88 U/L (reference range, 10–40 U/L); aspartate aminotransferase (AST), 544 U/L (reference range, 10–40 U/L); total bilirubin, 4.2 mg/dL (reference range, 0.3–1.0 mg/dL); direct bilirubin, 3.7 mg/dL (reference range, 0.1–0.3 mg/dL); aldolase, 20.2 U/L (reference range, 1–7.5 U/L), creatine kinase, 180 U/L (reference range, 30–135 U/L); γ-glutamyltransferase (GGT), 2743 U/L (reference range, 8–40 U/L); high sensitivity C-reactive protein, 122.9 mg/L (low-risk reference range, <1.0 mg/L); triglycerides, 534 mg/dL (reference range, <150 mg/dL); ferritin, 3784 ng/mL (reference range, 24–307 ng/mL); antinuclear antibody, negative titer; antimitochondrial antibody, negative titer; soluble IL-2 receptor (CD25), 7000 U/mL (reference range, 189–846 U/mL); anti-Sjögren syndrome–related antigen A antibody, positive.
Magnetic resonance imaging of the shoulders showed diffuse soft-tissue edema. Computed tomography (CT) of the chest demonstrated parabronchial thickening and parenchymal bands suggestive of DM. An age-appropriate malignancy workup was negative, and results from a liver biopsy showed diffuse steatosis with no histologic evidence of autoimmune hepatitis. Punch biopsy results from a plaque on the left knee revealed vacuolar interface dermatitis with increased dermal mucin on colloidal iron staining, indicative of connective tissue disease (Figure 2). The patient was treated with intravenous (IV) methylprednisolone 250 mg twice daily for 2 days followed by oral prednisone 50 mg daily with IV immunoglobulin (IVIG) 0.4 mg/kg daily for 5 days. The patient’s symptoms improved, and she was discharged on oral prednisone 50 mg and mycophenolate mofetil 1000 mg twice daily with a plan for outpatient IVIG.
Two days after discharge, the patient was re-admitted for worsening muscle weakness; recalcitrant rash; new-onset hypophonia, dysphagia, and odynophagia; and intermittent fevers. Myositis panel results were positive for MDA5. Additionally, workup for HLH, which was initiated during the first hospital admission, revealed that she met 6 of 8 diagnostic criteria: intermittent fevers (maximum temperature, 38.2 °C), splenomegaly (12.6 cm on CT scan of abdomen), cytopenia in 2 cell lines (anemia, thrombocytopenia), hypertriglyceridemia, hyperferritinemia, and elevated IL-2 receptor (CD25). Based on these findings, the patient was diagnosed with anti-MDA5 DM associated with HLH.
The patient was started on IV methylprednisolone 1000 mg daily and received 1 rituximab infusion. Two days later, she experienced worsening fever with tachycardia, and a chest radiograph showed bibasilar infiltrates concerning for aspiration pneumonia, with sputum cultures growing Staphylococcus aureus. Due to the infection, the dosage of methylprednisolone was decreased to 16 mg 3 times daily and rituximab was stopped. The hematology department was consulted for the patient’s HLH, and due to her profound weakness and sepsis, the decision was made to hold initiation of etoposide, which, in addition to glucocorticoids, is considered first-line therapy for HLH. She subsequently experienced worsening hypoxia requiring intubation and received a second course of IVIG. Two days later, CT of the chest revealed progressive ground-glass opacities in the lower lobes of the lungs. The patient was then started on plasmapheresis every other day, hydroxychloroquine 200 mg daily, and IV methylprednisolone 1000 mg daily. Over the subsequent 6 days, she developed worsening renal failure, liver dysfunction, profound thrombocytopenia (13/μL), and acidemia. After extensive discussion with her family, the patient was transitioned to comfort care, and she died 33 days after the initial admission to our hospital.
Our case is a collection of several rare presentations: anti-MDA5 DM, with HLH and AAHS as complications of anti-MDA5 DM, and DM-associated liver injury. Anti-MDA5 DM is frequently refractory to conventional therapy, including high-dose glucocorticoids, cyclophosphamide, oral tacrolimus, and cyclosporine, and there currently is no single treatment algorithm.2 Lake and colleagues7 highlighted the importance of personalizing treatment of anti-MDA5 DM, as it can be one of the most aggressive rheumatologic diseases. We initially chose to treat our patient with high-dose methylprednisolone, IVIG, and rituximab. Kampylafka et al8 performed a retrospective analysis of the use of IVIG for DM as compared to standard therapy and demonstrated improved muscle and cutaneous involvement from a collection of 50 patients. Case reports have specifically revealed efficacy for the use of IVIG in patients with anti-MDA5 DM.9,10 Additionally, rituximab—an anti–B lymphocyte therapy—has been shown to be an effective supplemental therapy for cases of aggressive anti-MDA5 DM with associated interstitial lung disease, especially when conventional therapy has failed.11,12 Our patient’s sepsis secondary to S aureus pneumonia limited her to only receiving 1 dose of rituximab.
One promising treatment approach for anti-MDA5 DM recently published by Tsuji et al13 involves the use of combination therapy. In this prospective multicenter trial, patients were initially treated with a combination of high-dose glucocorticoids, oral tacrolimus, and IV cyclophosphamide. Plasmapheresis was then started for patients without symptomatic improvement. This method was compared to the more traditional step-up approach of high-dose steroids followed by another immunosuppressant. At 1-year follow-up, the combination therapy group demonstrated an 85% survival rate compared to 33% of historical controls.13
We suspect that our patient developed HLH and AAHS secondary to her underlying anti-MDA5 DM. Kumakura and Murakawa6 reported that among 116 cases of AAHS, 6.9% of cases were associated with DM, most commonly anti-Jo-1 DM. Hemophagocytic lymphohistiocytosis associated with anti-MDA5 DM has been described in only a few cases.14-16 The diagnosis of HLH is critical, as the treatments for HLH and DM differ. Both diseases manifest with hyperferritinemia—greater than 500 ng/mL in the case of HLH and 3784 ng/mL in our patient. Therefore, HLH can be easily overlooked. It is possible the rates of HLH associated with anti-MDA5 DM are higher than reported given their similar presentations.
Analogous to our case, Fujita et al15 reported a case of HLH associated with anti-MDA5 DM successfully treated with IV cyclophosphamide pulse therapy and plasmapheresis. The rationale for using plasmapheresis in anti-MDA5 DM is based on its success in patients with other antibody-mediated conditions such as Goodpasture syndrome and granulomatosis with polyangiitis.7 It is thought to expedite response to traditional treatment, and in the case described by Fujita et al,15 the patient received plasmapheresis 6 times total over the course of 9 days. The patient’s clinical symptoms, as well as platelet levels, liver enzymes, and ferritin value, improved.15 Our patient received 3 days of plasmapheresis with no improvement when the decision was made to discontinue plasmapheresis given her worsening clinical state.
Additionally, our patient had elevated hepatic enzymes (ALT, AST, ALP, GGT), and results of a liver biopsy demonstrated diffuse steatosis. We speculate her transaminitis was a complication of anti-MDA5 DM. Hepatocellular damage accompanying DM has been investigated in multiple studies and is most often defined as an elevated ALT.17-20 Improvement in ALT levels has been seen with DM treatment. However, investigators note that creatine kinase (CK) values often do not correlate with the resolution of the transaminitis, suggesting that CK denotes muscle damage whereas ALT represents separate liver damage.18-21
Nagashima et al22 highlighted that among 50 patients with DM without malignancy, only 20% presented with a transaminitis or elevated bilirubin. However, among those with liver injury, all were positive for antibodies against MDA5.22 The patients with anti-MDA5 DM liver dysfunction had higher ALT, ALP, and GGT levels compared to those without liver dysfunction. Similarly, in a retrospective review of 14 patients with anti-MDA5 DM, Gono and colleagues3 found elevated GGT levels and lower CK levels in comparison to patients with anti-aminoacyl-transfer RNA synthetase DM. Although liver enzymes can be elevated in patients with DM secondary to muscle damage, the authors argue that the specificity of GGT to the liver suggests intrinsic liver damage.3
The mechanism behind liver disease in anti-MDA5 DM is unclear, but it is hypothesized to be similar to nonalcoholic steatohepatitis.22 Other studies have revealed drug-induced hepatitis, hepatic congestion, nonspecific reactive hepatitis, metastatic liver tumor, primary biliary cholangitis, and autoimmune hepatitis as the etiology behind liver disease in their patients with DM.17-19 Liver biopsy results from patients with anti-MDA5 DM most commonly reveal hepatic steatosis, as seen in our patient, as well as hepatocyte ballooning and increased pigmented macrophages.22
We presented a case of anti-MDA5 DM complicated by HLH. Our patient had a fatal outcome despite aggressive treatment with high-dose methylprednisolone, IVIG, rituximab, and plasmapheresis. It is accepted that anti-MDA5 DM affects the lungs and skin, and our patient’s presentation also suggests liver involvement. In our case, onset of symptoms to fatality was approximately 1 year. It is essential to consider the diagnosis of HLH in all cases of anti-MDA5 DM given clinical disease overlap. Our patient could have benefited from earlier disease recognition and thus earlier aggressive therapy.
1. Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292:344-347.
2. Kurtzman DJB, Vleugels RA. Anti-melanoma differentiation-associated gene 5 (MDA5) dermatomyositis: a concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol. 2018;78:776-785.
3. Gono T, Kawaguchi Y, Satoh T, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford). 2010;49:1713-1719.
4. Fiorentino D, Chung L, Zwerner J, et al. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65:25-34.
5. Sepulveda FE, de Saint Basile G. Hemophagocytic syndrome: primary forms and predisposing conditions. Curr Opin Immunol. 2017;49:20-26.
6. Kumakura S, Murakawa Y. Clinical characteristics and treatment outcomes of autoimmune-associated hemophagocytic syndrome in adults. Arthritis Rheum. 2014;66:2297-2307.
7. Lake M, George G, Summer R. Time to personalize the treatment of anti-MDA-5 associated lung disease. Ann Rheum Dis. 2019;78:E52.
8. Kampylafka EI, Kosmidis ML, Panagiotakos DB, et al. The effect of intravenous immunoglobulin (IVIG) treatment on patients with dermatomyositis: a 4-year follow-up study. Clin Exp Rheumatol. 2012;30:397-401.
9. Koguchi-Yoshioka H, Okiyama N, Iwamoto K, et al. Intravenous immunoglobulin contributes to the control of antimelanoma differentiation-associated protein 5 antibody-associated dermatomyositis with palmar violaceous macules/papules. Br J Dermatol. 2017;177:1442-1446.
10. Hamada-Ode K, Taniguchi Y, Kimata T, et al. High-dose intravenous immunoglobulin therapy for rapidly progressive interstitial pneumonitis accompanied by anti-melanoma differentiation-associated gene 5 antibody-positive amyopathic dermatomyositis. Eur J Rheumatol. 2015;2:83-85.
11. So H, Wong VTL, Lao VWN, et al. Rituximab for refractory rapidly progressive interstitial lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis. Clin Rheumatol. 2018;37:1983-1989.
12. Koichi Y, Aya Y, Megumi U, et al. A case of anti-MDA5-positive rapidly progressive interstitial lung disease in a patient with clinically amyopathic dermatomyositis ameliorated by rituximab, in addition to standard immunosuppressive treatment. Mod Rheumatol. 2017;27:536-540.
13. Tsuji H, Nakashima R, Hosono Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. 2020;72:488-498.
14. Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune-associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol. 2020;49:244-246.
15. Fujita Y, Fukui S, Suzuki T, et al. Anti-MDA5 antibody-positive dermatomyositis complicated by autoimmune-associated hemophagocytic syndrome that was successfully treated with immunosuppressive therapy and plasmapheresis. Intern Med. 2018;57:3473-3478.
16. Gono T, Miyake K, Kawaguchi Y, et al. Hyperferritinaemia and macrophage activation in a patient with interstitial lung disease with clinically amyopathic DM. Rheumatology (Oxford). 2012;51:1336-1338.
17. Wada T, Abe G, Kudou, T, et al. Liver damage in patients with polymyositis and dermatomyositis. Kitasato Med Journal. 2016;46:40-46.
18. Takahashi A, Abe K, Yokokawa J, et al. Clinical features of liver dysfunction in collagen diseases. Hepatol Res. 2010;40:1092-1097.
19. Matsumoto T, Kobayashi S, Shimizu H, et al. The liver in collagen diseases: pathologic study of 160 cases with particular reference to hepatic arteritis, primary biliary cirrhosis, autoimmune hepatitis and nodular regenerative hyperplasia of the liver. Liver. 2000;20:366-373.
20. Shi Q, Niu J, Huang X, et al. Do muscle enzyme changes forecast liver injury in polymyositis/dermatomyositis patients treated with methylprednisolone and methotrexate? Ann Clin Lab Sci. 2016;46:266-269.
21. Noda S, Asano Y, Tamaki Z, et al. A case of dermatomyositis with “liver disease associated with rheumatoid diseases” positive for anti-liver-kidney microsome-1 antibody. Clin Rheumatol. 2010;29:941-943.
22. Nagashima T, Kamata Y, Iwamoto M, et al. Liver dysfunction in anti-melanoma differentiation-associated gene 5 antibody-positive patients with dermatomyositis. Rheumatol Int. 2019;39:901-909.
To the Editor:
Dermatomyositis (DM) is an idiopathic inflammatory myopathy characterized by bilateral, symmetrical, proximal muscle weakness and classic cutaneous manifestations.1 Patients with antibodies directed against melanoma differentiation–associated gene 5, MDA5, have a distinct presentation due to vasculopathy with more severe cutaneous ulcerations, palmar papules, alopecia, and an elevated risk of rapidly progressive interstitial lung disease.2 A ferritin level greater than 1600 ng/mL portends an increased risk for pulmonary disease and therefore can be of prognostic value.3 Further, patients with anti-MDA5 DM are at a lower risk of malignancy and are more likely to test negative for antinuclear antibodies in comparison to other patients with DM.2,4
Hemophagocytic lymphohistiocytosis (HLH), also known as hemophagocytic syndrome, is a potentially lethal condition whereby uncontrolled activation of histiocytes in the reticuloendothelial system causes hemophagocytosis and a hyperinflammatory state. Patients present with fever, splenomegaly, cytopenia, and hyperferritinemia.5 Autoimmune‐associated hemophagocytic syndrome (AAHS) describes HLH that develops in association with autoimmune conditions, most commonly systemic lupus erythematosus and adult-onset Still disease. Cases reported in association with DM exist but are few in number, and there is no standard-of-care treatment.6 We report a case of a woman with anti-MDA5 DM complicated by HLH and DM-associated liver injury.
A 50-year-old woman presented as a direct admit from the rheumatology clinic for diffuse muscle weakness of 8 months’ duration, 40-pound unintentional weight loss, pruritic rash, bilateral joint pains, dry eyes, dry mouth, and altered mental status. Four months prior, she presented to an outside hospital and was given a diagnosis of probable Sjögren syndrome and autoimmune hepatitis vs drug-induced liver injury. At that time, a workup was notable for antibodies against Sjögren syndrome–related antigen A, anti–smooth muscle antibodies, and transaminitis. Ultrasonography of the right upper quadrant revealed hepatic steatosis. The patient was started on oral prednisone and pilocarpine but had been off all medications for 1 month when she presented to our hospital.
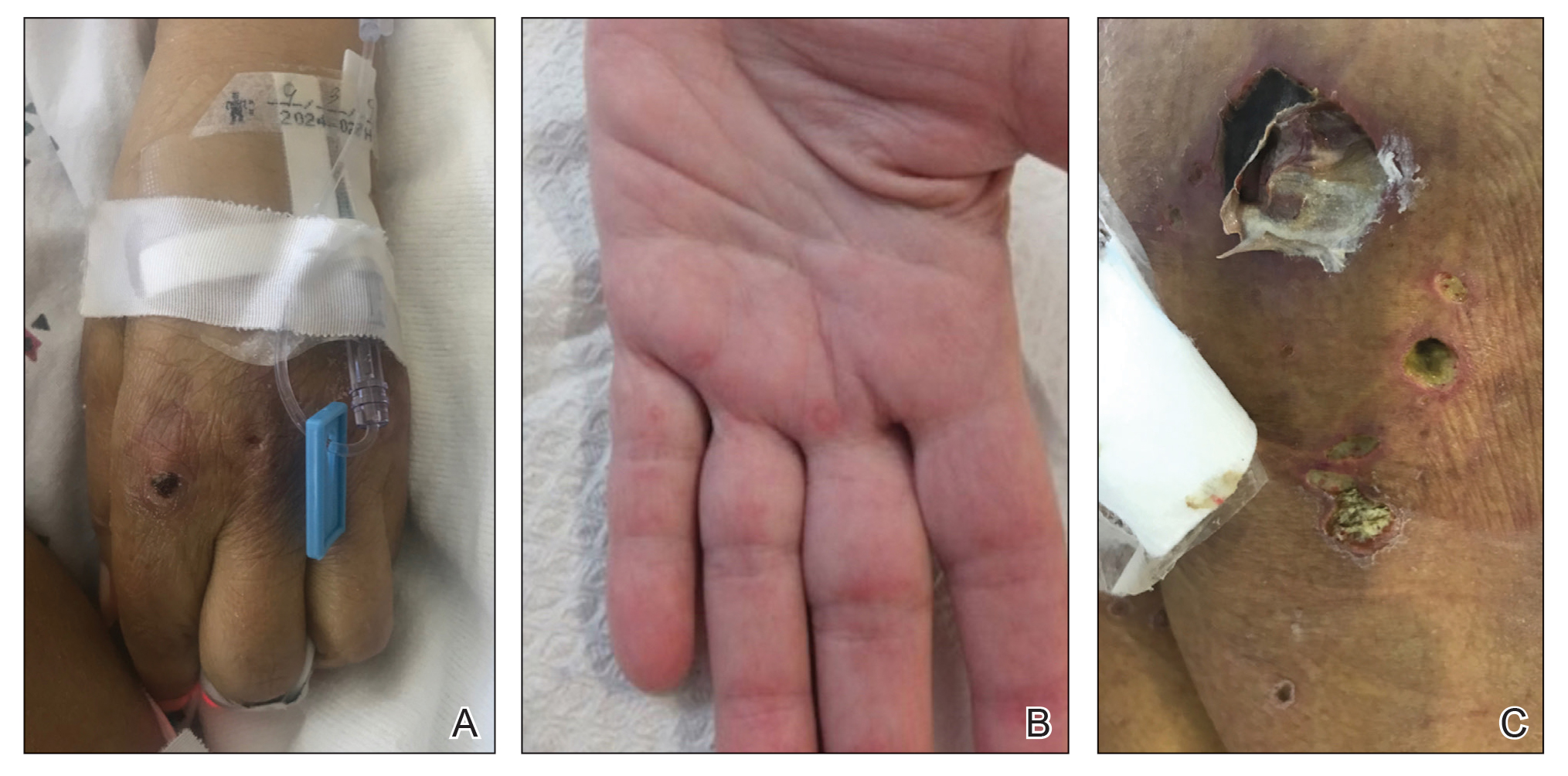
On hospital admission, physical examination revealed a violaceous heliotrope rash; a v-sign on the chest; shawl sign; palmar papules with pits at the fingertips; and periungual erythema and ulcerations along the metacarpophalangeal joints, elbows, lateral feet, and upper eyelids (Figure 1). Laboratory workup showed the following results: white blood cell count, 4100/μL (reference range, 4000–11,000/μL); hemoglobin, 11.6 g/dL (reference range, 12–16 g/dL); platelet count, 100,000/μL (reference range, 150,000–450,000/μL); lactate dehydrogenase, 510 U/L (reference range, 80–225 U/L); alkaline phosphatase (ALP), 766 U/L (reference range, 30–120 U/L); alanine aminotransferase (ALT), 88 U/L (reference range, 10–40 U/L); aspartate aminotransferase (AST), 544 U/L (reference range, 10–40 U/L); total bilirubin, 4.2 mg/dL (reference range, 0.3–1.0 mg/dL); direct bilirubin, 3.7 mg/dL (reference range, 0.1–0.3 mg/dL); aldolase, 20.2 U/L (reference range, 1–7.5 U/L), creatine kinase, 180 U/L (reference range, 30–135 U/L); γ-glutamyltransferase (GGT), 2743 U/L (reference range, 8–40 U/L); high sensitivity C-reactive protein, 122.9 mg/L (low-risk reference range, <1.0 mg/L); triglycerides, 534 mg/dL (reference range, <150 mg/dL); ferritin, 3784 ng/mL (reference range, 24–307 ng/mL); antinuclear antibody, negative titer; antimitochondrial antibody, negative titer; soluble IL-2 receptor (CD25), 7000 U/mL (reference range, 189–846 U/mL); anti-Sjögren syndrome–related antigen A antibody, positive.

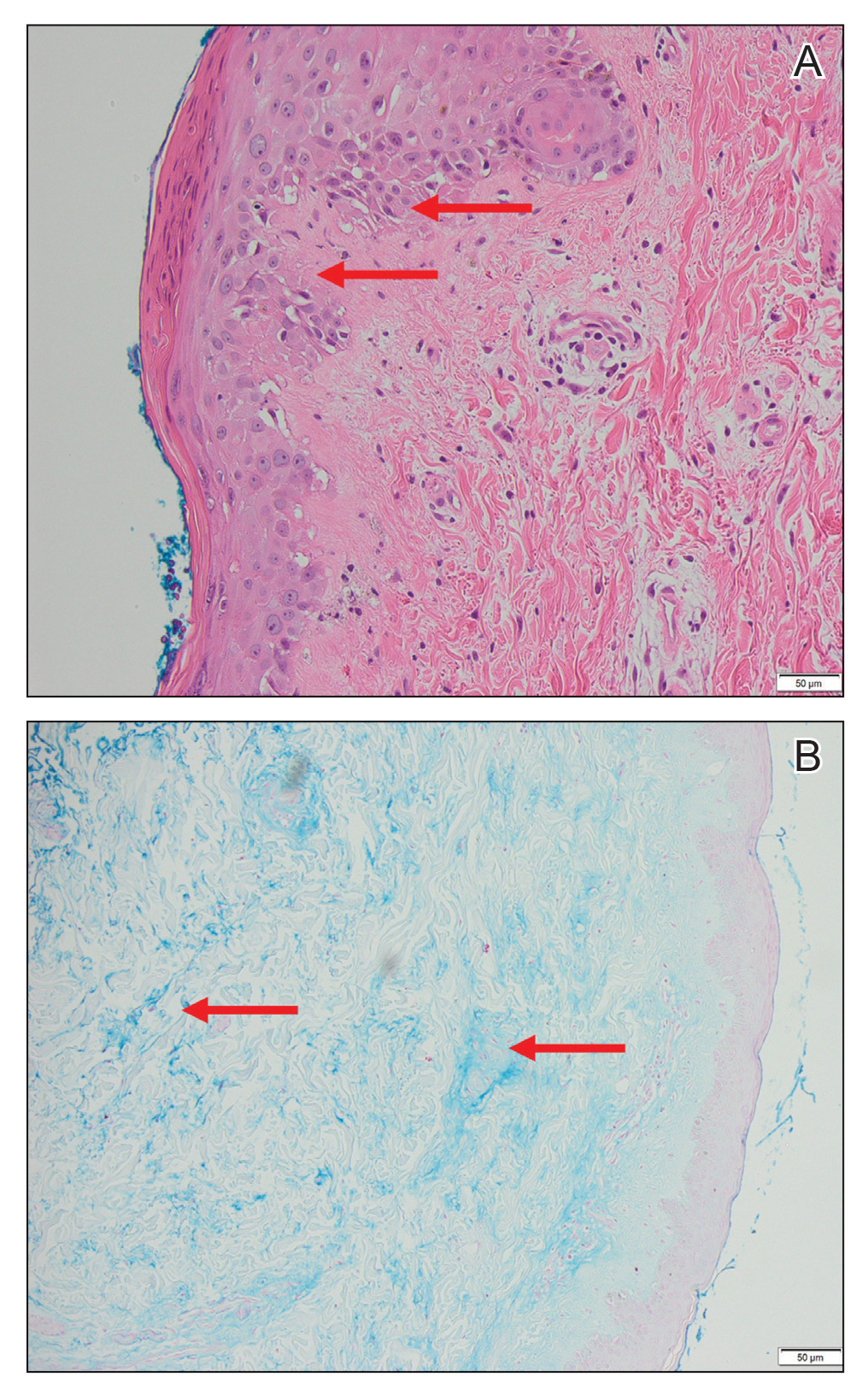
Magnetic resonance imaging of the shoulders showed diffuse soft-tissue edema. Computed tomography (CT) of the chest demonstrated parabronchial thickening and parenchymal bands suggestive of DM. An age-appropriate malignancy workup was negative, and results from a liver biopsy showed diffuse steatosis with no histologic evidence of autoimmune hepatitis. Punch biopsy results from a plaque on the left knee revealed vacuolar interface dermatitis with increased dermal mucin on colloidal iron staining, indicative of connective tissue disease (Figure 2). The patient was treated with intravenous (IV) methylprednisolone 250 mg twice daily for 2 days followed by oral prednisone 50 mg daily with IV immunoglobulin (IVIG) 0.4 mg/kg daily for 5 days. The patient’s symptoms improved, and she was discharged on oral prednisone 50 mg and mycophenolate mofetil 1000 mg twice daily with a plan for outpatient IVIG.

Two days after discharge, the patient was re-admitted for worsening muscle weakness; recalcitrant rash; new-onset hypophonia, dysphagia, and odynophagia; and intermittent fevers. Myositis panel results were positive for MDA5. Additionally, workup for HLH, which was initiated during the first hospital admission, revealed that she met 6 of 8 diagnostic criteria: intermittent fevers (maximum temperature, 38.2 °C), splenomegaly (12.6 cm on CT scan of abdomen), cytopenia in 2 cell lines (anemia, thrombocytopenia), hypertriglyceridemia, hyperferritinemia, and elevated IL-2 receptor (CD25). Based on these findings, the patient was diagnosed with anti-MDA5 DM associated with HLH.
The patient was started on IV methylprednisolone 1000 mg daily and received 1 rituximab infusion. Two days later, she experienced worsening fever with tachycardia, and a chest radiograph showed bibasilar infiltrates concerning for aspiration pneumonia, with sputum cultures growing Staphylococcus aureus. Due to the infection, the dosage of methylprednisolone was decreased to 16 mg 3 times daily and rituximab was stopped. The hematology department was consulted for the patient’s HLH, and due to her profound weakness and sepsis, the decision was made to hold initiation of etoposide, which, in addition to glucocorticoids, is considered first-line therapy for HLH. She subsequently experienced worsening hypoxia requiring intubation and received a second course of IVIG. Two days later, CT of the chest revealed progressive ground-glass opacities in the lower lobes of the lungs. The patient was then started on plasmapheresis every other day, hydroxychloroquine 200 mg daily, and IV methylprednisolone 1000 mg daily. Over the subsequent 6 days, she developed worsening renal failure, liver dysfunction, profound thrombocytopenia (13/μL), and acidemia. After extensive discussion with her family, the patient was transitioned to comfort care, and she died 33 days after the initial admission to our hospital.
Our case is a collection of several rare presentations: anti-MDA5 DM, with HLH and AAHS as complications of anti-MDA5 DM, and DM-associated liver injury. Anti-MDA5 DM is frequently refractory to conventional therapy, including high-dose glucocorticoids, cyclophosphamide, oral tacrolimus, and cyclosporine, and there currently is no single treatment algorithm.2 Lake and colleagues7 highlighted the importance of personalizing treatment of anti-MDA5 DM, as it can be one of the most aggressive rheumatologic diseases. We initially chose to treat our patient with high-dose methylprednisolone, IVIG, and rituximab. Kampylafka et al8 performed a retrospective analysis of the use of IVIG for DM as compared to standard therapy and demonstrated improved muscle and cutaneous involvement from a collection of 50 patients. Case reports have specifically revealed efficacy for the use of IVIG in patients with anti-MDA5 DM.9,10 Additionally, rituximab—an anti–B lymphocyte therapy—has been shown to be an effective supplemental therapy for cases of aggressive anti-MDA5 DM with associated interstitial lung disease, especially when conventional therapy has failed.11,12 Our patient’s sepsis secondary to S aureus pneumonia limited her to only receiving 1 dose of rituximab.
One promising treatment approach for anti-MDA5 DM recently published by Tsuji et al13 involves the use of combination therapy. In this prospective multicenter trial, patients were initially treated with a combination of high-dose glucocorticoids, oral tacrolimus, and IV cyclophosphamide. Plasmapheresis was then started for patients without symptomatic improvement. This method was compared to the more traditional step-up approach of high-dose steroids followed by another immunosuppressant. At 1-year follow-up, the combination therapy group demonstrated an 85% survival rate compared to 33% of historical controls.13
We suspect that our patient developed HLH and AAHS secondary to her underlying anti-MDA5 DM. Kumakura and Murakawa6 reported that among 116 cases of AAHS, 6.9% of cases were associated with DM, most commonly anti-Jo-1 DM. Hemophagocytic lymphohistiocytosis associated with anti-MDA5 DM has been described in only a few cases.14-16 The diagnosis of HLH is critical, as the treatments for HLH and DM differ. Both diseases manifest with hyperferritinemia—greater than 500 ng/mL in the case of HLH and 3784 ng/mL in our patient. Therefore, HLH can be easily overlooked. It is possible the rates of HLH associated with anti-MDA5 DM are higher than reported given their similar presentations.
Analogous to our case, Fujita et al15 reported a case of HLH associated with anti-MDA5 DM successfully treated with IV cyclophosphamide pulse therapy and plasmapheresis. The rationale for using plasmapheresis in anti-MDA5 DM is based on its success in patients with other antibody-mediated conditions such as Goodpasture syndrome and granulomatosis with polyangiitis.7 It is thought to expedite response to traditional treatment, and in the case described by Fujita et al,15 the patient received plasmapheresis 6 times total over the course of 9 days. The patient’s clinical symptoms, as well as platelet levels, liver enzymes, and ferritin value, improved.15 Our patient received 3 days of plasmapheresis with no improvement when the decision was made to discontinue plasmapheresis given her worsening clinical state.
Additionally, our patient had elevated hepatic enzymes (ALT, AST, ALP, GGT), and results of a liver biopsy demonstrated diffuse steatosis. We speculate her transaminitis was a complication of anti-MDA5 DM. Hepatocellular damage accompanying DM has been investigated in multiple studies and is most often defined as an elevated ALT.17-20 Improvement in ALT levels has been seen with DM treatment. However, investigators note that creatine kinase (CK) values often do not correlate with the resolution of the transaminitis, suggesting that CK denotes muscle damage whereas ALT represents separate liver damage.18-21
Nagashima et al22 highlighted that among 50 patients with DM without malignancy, only 20% presented with a transaminitis or elevated bilirubin. However, among those with liver injury, all were positive for antibodies against MDA5.22 The patients with anti-MDA5 DM liver dysfunction had higher ALT, ALP, and GGT levels compared to those without liver dysfunction. Similarly, in a retrospective review of 14 patients with anti-MDA5 DM, Gono and colleagues3 found elevated GGT levels and lower CK levels in comparison to patients with anti-aminoacyl-transfer RNA synthetase DM. Although liver enzymes can be elevated in patients with DM secondary to muscle damage, the authors argue that the specificity of GGT to the liver suggests intrinsic liver damage.3
The mechanism behind liver disease in anti-MDA5 DM is unclear, but it is hypothesized to be similar to nonalcoholic steatohepatitis.22 Other studies have revealed drug-induced hepatitis, hepatic congestion, nonspecific reactive hepatitis, metastatic liver tumor, primary biliary cholangitis, and autoimmune hepatitis as the etiology behind liver disease in their patients with DM.17-19 Liver biopsy results from patients with anti-MDA5 DM most commonly reveal hepatic steatosis, as seen in our patient, as well as hepatocyte ballooning and increased pigmented macrophages.22
We presented a case of anti-MDA5 DM complicated by HLH. Our patient had a fatal outcome despite aggressive treatment with high-dose methylprednisolone, IVIG, rituximab, and plasmapheresis. It is accepted that anti-MDA5 DM affects the lungs and skin, and our patient’s presentation also suggests liver involvement. In our case, onset of symptoms to fatality was approximately 1 year. It is essential to consider the diagnosis of HLH in all cases of anti-MDA5 DM given clinical disease overlap. Our patient could have benefited from earlier disease recognition and thus earlier aggressive therapy.
To the Editor:
Dermatomyositis (DM) is an idiopathic inflammatory myopathy characterized by bilateral, symmetrical, proximal muscle weakness and classic cutaneous manifestations.1 Patients with antibodies directed against melanoma differentiation–associated gene 5, MDA5, have a distinct presentation due to vasculopathy with more severe cutaneous ulcerations, palmar papules, alopecia, and an elevated risk of rapidly progressive interstitial lung disease.2 A ferritin level greater than 1600 ng/mL portends an increased risk for pulmonary disease and therefore can be of prognostic value.3 Further, patients with anti-MDA5 DM are at a lower risk of malignancy and are more likely to test negative for antinuclear antibodies in comparison to other patients with DM.2,4
Hemophagocytic lymphohistiocytosis (HLH), also known as hemophagocytic syndrome, is a potentially lethal condition whereby uncontrolled activation of histiocytes in the reticuloendothelial system causes hemophagocytosis and a hyperinflammatory state. Patients present with fever, splenomegaly, cytopenia, and hyperferritinemia.5 Autoimmune‐associated hemophagocytic syndrome (AAHS) describes HLH that develops in association with autoimmune conditions, most commonly systemic lupus erythematosus and adult-onset Still disease. Cases reported in association with DM exist but are few in number, and there is no standard-of-care treatment.6 We report a case of a woman with anti-MDA5 DM complicated by HLH and DM-associated liver injury.
A 50-year-old woman presented as a direct admit from the rheumatology clinic for diffuse muscle weakness of 8 months’ duration, 40-pound unintentional weight loss, pruritic rash, bilateral joint pains, dry eyes, dry mouth, and altered mental status. Four months prior, she presented to an outside hospital and was given a diagnosis of probable Sjögren syndrome and autoimmune hepatitis vs drug-induced liver injury. At that time, a workup was notable for antibodies against Sjögren syndrome–related antigen A, anti–smooth muscle antibodies, and transaminitis. Ultrasonography of the right upper quadrant revealed hepatic steatosis. The patient was started on oral prednisone and pilocarpine but had been off all medications for 1 month when she presented to our hospital.
On hospital admission, physical examination revealed a violaceous heliotrope rash; a v-sign on the chest; shawl sign; palmar papules with pits at the fingertips; and periungual erythema and ulcerations along the metacarpophalangeal joints, elbows, lateral feet, and upper eyelids (Figure 1). Laboratory workup showed the following results: white blood cell count, 4100/μL (reference range, 4000–11,000/μL); hemoglobin, 11.6 g/dL (reference range, 12–16 g/dL); platelet count, 100,000/μL (reference range, 150,000–450,000/μL); lactate dehydrogenase, 510 U/L (reference range, 80–225 U/L); alkaline phosphatase (ALP), 766 U/L (reference range, 30–120 U/L); alanine aminotransferase (ALT), 88 U/L (reference range, 10–40 U/L); aspartate aminotransferase (AST), 544 U/L (reference range, 10–40 U/L); total bilirubin, 4.2 mg/dL (reference range, 0.3–1.0 mg/dL); direct bilirubin, 3.7 mg/dL (reference range, 0.1–0.3 mg/dL); aldolase, 20.2 U/L (reference range, 1–7.5 U/L), creatine kinase, 180 U/L (reference range, 30–135 U/L); γ-glutamyltransferase (GGT), 2743 U/L (reference range, 8–40 U/L); high sensitivity C-reactive protein, 122.9 mg/L (low-risk reference range, <1.0 mg/L); triglycerides, 534 mg/dL (reference range, <150 mg/dL); ferritin, 3784 ng/mL (reference range, 24–307 ng/mL); antinuclear antibody, negative titer; antimitochondrial antibody, negative titer; soluble IL-2 receptor (CD25), 7000 U/mL (reference range, 189–846 U/mL); anti-Sjögren syndrome–related antigen A antibody, positive.

Magnetic resonance imaging of the shoulders showed diffuse soft-tissue edema. Computed tomography (CT) of the chest demonstrated parabronchial thickening and parenchymal bands suggestive of DM. An age-appropriate malignancy workup was negative, and results from a liver biopsy showed diffuse steatosis with no histologic evidence of autoimmune hepatitis. Punch biopsy results from a plaque on the left knee revealed vacuolar interface dermatitis with increased dermal mucin on colloidal iron staining, indicative of connective tissue disease (Figure 2). The patient was treated with intravenous (IV) methylprednisolone 250 mg twice daily for 2 days followed by oral prednisone 50 mg daily with IV immunoglobulin (IVIG) 0.4 mg/kg daily for 5 days. The patient’s symptoms improved, and she was discharged on oral prednisone 50 mg and mycophenolate mofetil 1000 mg twice daily with a plan for outpatient IVIG.

Two days after discharge, the patient was re-admitted for worsening muscle weakness; recalcitrant rash; new-onset hypophonia, dysphagia, and odynophagia; and intermittent fevers. Myositis panel results were positive for MDA5. Additionally, workup for HLH, which was initiated during the first hospital admission, revealed that she met 6 of 8 diagnostic criteria: intermittent fevers (maximum temperature, 38.2 °C), splenomegaly (12.6 cm on CT scan of abdomen), cytopenia in 2 cell lines (anemia, thrombocytopenia), hypertriglyceridemia, hyperferritinemia, and elevated IL-2 receptor (CD25). Based on these findings, the patient was diagnosed with anti-MDA5 DM associated with HLH.
The patient was started on IV methylprednisolone 1000 mg daily and received 1 rituximab infusion. Two days later, she experienced worsening fever with tachycardia, and a chest radiograph showed bibasilar infiltrates concerning for aspiration pneumonia, with sputum cultures growing Staphylococcus aureus. Due to the infection, the dosage of methylprednisolone was decreased to 16 mg 3 times daily and rituximab was stopped. The hematology department was consulted for the patient’s HLH, and due to her profound weakness and sepsis, the decision was made to hold initiation of etoposide, which, in addition to glucocorticoids, is considered first-line therapy for HLH. She subsequently experienced worsening hypoxia requiring intubation and received a second course of IVIG. Two days later, CT of the chest revealed progressive ground-glass opacities in the lower lobes of the lungs. The patient was then started on plasmapheresis every other day, hydroxychloroquine 200 mg daily, and IV methylprednisolone 1000 mg daily. Over the subsequent 6 days, she developed worsening renal failure, liver dysfunction, profound thrombocytopenia (13/μL), and acidemia. After extensive discussion with her family, the patient was transitioned to comfort care, and she died 33 days after the initial admission to our hospital.
Our case is a collection of several rare presentations: anti-MDA5 DM, with HLH and AAHS as complications of anti-MDA5 DM, and DM-associated liver injury. Anti-MDA5 DM is frequently refractory to conventional therapy, including high-dose glucocorticoids, cyclophosphamide, oral tacrolimus, and cyclosporine, and there currently is no single treatment algorithm.2 Lake and colleagues7 highlighted the importance of personalizing treatment of anti-MDA5 DM, as it can be one of the most aggressive rheumatologic diseases. We initially chose to treat our patient with high-dose methylprednisolone, IVIG, and rituximab. Kampylafka et al8 performed a retrospective analysis of the use of IVIG for DM as compared to standard therapy and demonstrated improved muscle and cutaneous involvement from a collection of 50 patients. Case reports have specifically revealed efficacy for the use of IVIG in patients with anti-MDA5 DM.9,10 Additionally, rituximab—an anti–B lymphocyte therapy—has been shown to be an effective supplemental therapy for cases of aggressive anti-MDA5 DM with associated interstitial lung disease, especially when conventional therapy has failed.11,12 Our patient’s sepsis secondary to S aureus pneumonia limited her to only receiving 1 dose of rituximab.
One promising treatment approach for anti-MDA5 DM recently published by Tsuji et al13 involves the use of combination therapy. In this prospective multicenter trial, patients were initially treated with a combination of high-dose glucocorticoids, oral tacrolimus, and IV cyclophosphamide. Plasmapheresis was then started for patients without symptomatic improvement. This method was compared to the more traditional step-up approach of high-dose steroids followed by another immunosuppressant. At 1-year follow-up, the combination therapy group demonstrated an 85% survival rate compared to 33% of historical controls.13
We suspect that our patient developed HLH and AAHS secondary to her underlying anti-MDA5 DM. Kumakura and Murakawa6 reported that among 116 cases of AAHS, 6.9% of cases were associated with DM, most commonly anti-Jo-1 DM. Hemophagocytic lymphohistiocytosis associated with anti-MDA5 DM has been described in only a few cases.14-16 The diagnosis of HLH is critical, as the treatments for HLH and DM differ. Both diseases manifest with hyperferritinemia—greater than 500 ng/mL in the case of HLH and 3784 ng/mL in our patient. Therefore, HLH can be easily overlooked. It is possible the rates of HLH associated with anti-MDA5 DM are higher than reported given their similar presentations.
Analogous to our case, Fujita et al15 reported a case of HLH associated with anti-MDA5 DM successfully treated with IV cyclophosphamide pulse therapy and plasmapheresis. The rationale for using plasmapheresis in anti-MDA5 DM is based on its success in patients with other antibody-mediated conditions such as Goodpasture syndrome and granulomatosis with polyangiitis.7 It is thought to expedite response to traditional treatment, and in the case described by Fujita et al,15 the patient received plasmapheresis 6 times total over the course of 9 days. The patient’s clinical symptoms, as well as platelet levels, liver enzymes, and ferritin value, improved.15 Our patient received 3 days of plasmapheresis with no improvement when the decision was made to discontinue plasmapheresis given her worsening clinical state.
Additionally, our patient had elevated hepatic enzymes (ALT, AST, ALP, GGT), and results of a liver biopsy demonstrated diffuse steatosis. We speculate her transaminitis was a complication of anti-MDA5 DM. Hepatocellular damage accompanying DM has been investigated in multiple studies and is most often defined as an elevated ALT.17-20 Improvement in ALT levels has been seen with DM treatment. However, investigators note that creatine kinase (CK) values often do not correlate with the resolution of the transaminitis, suggesting that CK denotes muscle damage whereas ALT represents separate liver damage.18-21
Nagashima et al22 highlighted that among 50 patients with DM without malignancy, only 20% presented with a transaminitis or elevated bilirubin. However, among those with liver injury, all were positive for antibodies against MDA5.22 The patients with anti-MDA5 DM liver dysfunction had higher ALT, ALP, and GGT levels compared to those without liver dysfunction. Similarly, in a retrospective review of 14 patients with anti-MDA5 DM, Gono and colleagues3 found elevated GGT levels and lower CK levels in comparison to patients with anti-aminoacyl-transfer RNA synthetase DM. Although liver enzymes can be elevated in patients with DM secondary to muscle damage, the authors argue that the specificity of GGT to the liver suggests intrinsic liver damage.3
The mechanism behind liver disease in anti-MDA5 DM is unclear, but it is hypothesized to be similar to nonalcoholic steatohepatitis.22 Other studies have revealed drug-induced hepatitis, hepatic congestion, nonspecific reactive hepatitis, metastatic liver tumor, primary biliary cholangitis, and autoimmune hepatitis as the etiology behind liver disease in their patients with DM.17-19 Liver biopsy results from patients with anti-MDA5 DM most commonly reveal hepatic steatosis, as seen in our patient, as well as hepatocyte ballooning and increased pigmented macrophages.22
We presented a case of anti-MDA5 DM complicated by HLH. Our patient had a fatal outcome despite aggressive treatment with high-dose methylprednisolone, IVIG, rituximab, and plasmapheresis. It is accepted that anti-MDA5 DM affects the lungs and skin, and our patient’s presentation also suggests liver involvement. In our case, onset of symptoms to fatality was approximately 1 year. It is essential to consider the diagnosis of HLH in all cases of anti-MDA5 DM given clinical disease overlap. Our patient could have benefited from earlier disease recognition and thus earlier aggressive therapy.
1. Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292:344-347.
2. Kurtzman DJB, Vleugels RA. Anti-melanoma differentiation-associated gene 5 (MDA5) dermatomyositis: a concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol. 2018;78:776-785.
3. Gono T, Kawaguchi Y, Satoh T, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford). 2010;49:1713-1719.
4. Fiorentino D, Chung L, Zwerner J, et al. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65:25-34.
5. Sepulveda FE, de Saint Basile G. Hemophagocytic syndrome: primary forms and predisposing conditions. Curr Opin Immunol. 2017;49:20-26.
6. Kumakura S, Murakawa Y. Clinical characteristics and treatment outcomes of autoimmune-associated hemophagocytic syndrome in adults. Arthritis Rheum. 2014;66:2297-2307.
7. Lake M, George G, Summer R. Time to personalize the treatment of anti-MDA-5 associated lung disease. Ann Rheum Dis. 2019;78:E52.
8. Kampylafka EI, Kosmidis ML, Panagiotakos DB, et al. The effect of intravenous immunoglobulin (IVIG) treatment on patients with dermatomyositis: a 4-year follow-up study. Clin Exp Rheumatol. 2012;30:397-401.
9. Koguchi-Yoshioka H, Okiyama N, Iwamoto K, et al. Intravenous immunoglobulin contributes to the control of antimelanoma differentiation-associated protein 5 antibody-associated dermatomyositis with palmar violaceous macules/papules. Br J Dermatol. 2017;177:1442-1446.
10. Hamada-Ode K, Taniguchi Y, Kimata T, et al. High-dose intravenous immunoglobulin therapy for rapidly progressive interstitial pneumonitis accompanied by anti-melanoma differentiation-associated gene 5 antibody-positive amyopathic dermatomyositis. Eur J Rheumatol. 2015;2:83-85.
11. So H, Wong VTL, Lao VWN, et al. Rituximab for refractory rapidly progressive interstitial lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis. Clin Rheumatol. 2018;37:1983-1989.
12. Koichi Y, Aya Y, Megumi U, et al. A case of anti-MDA5-positive rapidly progressive interstitial lung disease in a patient with clinically amyopathic dermatomyositis ameliorated by rituximab, in addition to standard immunosuppressive treatment. Mod Rheumatol. 2017;27:536-540.
13. Tsuji H, Nakashima R, Hosono Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. 2020;72:488-498.
14. Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune-associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol. 2020;49:244-246.
15. Fujita Y, Fukui S, Suzuki T, et al. Anti-MDA5 antibody-positive dermatomyositis complicated by autoimmune-associated hemophagocytic syndrome that was successfully treated with immunosuppressive therapy and plasmapheresis. Intern Med. 2018;57:3473-3478.
16. Gono T, Miyake K, Kawaguchi Y, et al. Hyperferritinaemia and macrophage activation in a patient with interstitial lung disease with clinically amyopathic DM. Rheumatology (Oxford). 2012;51:1336-1338.
17. Wada T, Abe G, Kudou, T, et al. Liver damage in patients with polymyositis and dermatomyositis. Kitasato Med Journal. 2016;46:40-46.
18. Takahashi A, Abe K, Yokokawa J, et al. Clinical features of liver dysfunction in collagen diseases. Hepatol Res. 2010;40:1092-1097.
19. Matsumoto T, Kobayashi S, Shimizu H, et al. The liver in collagen diseases: pathologic study of 160 cases with particular reference to hepatic arteritis, primary biliary cirrhosis, autoimmune hepatitis and nodular regenerative hyperplasia of the liver. Liver. 2000;20:366-373.
20. Shi Q, Niu J, Huang X, et al. Do muscle enzyme changes forecast liver injury in polymyositis/dermatomyositis patients treated with methylprednisolone and methotrexate? Ann Clin Lab Sci. 2016;46:266-269.
21. Noda S, Asano Y, Tamaki Z, et al. A case of dermatomyositis with “liver disease associated with rheumatoid diseases” positive for anti-liver-kidney microsome-1 antibody. Clin Rheumatol. 2010;29:941-943.
22. Nagashima T, Kamata Y, Iwamoto M, et al. Liver dysfunction in anti-melanoma differentiation-associated gene 5 antibody-positive patients with dermatomyositis. Rheumatol Int. 2019;39:901-909.
1. Bohan A, Peter JB. Polymyositis and dermatomyositis. N Engl J Med. 1975;292:344-347.
2. Kurtzman DJB, Vleugels RA. Anti-melanoma differentiation-associated gene 5 (MDA5) dermatomyositis: a concise review with an emphasis on distinctive clinical features. J Am Acad Dermatol. 2018;78:776-785.
3. Gono T, Kawaguchi Y, Satoh T, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford). 2010;49:1713-1719.
4. Fiorentino D, Chung L, Zwerner J, et al. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol. 2011;65:25-34.
5. Sepulveda FE, de Saint Basile G. Hemophagocytic syndrome: primary forms and predisposing conditions. Curr Opin Immunol. 2017;49:20-26.
6. Kumakura S, Murakawa Y. Clinical characteristics and treatment outcomes of autoimmune-associated hemophagocytic syndrome in adults. Arthritis Rheum. 2014;66:2297-2307.
7. Lake M, George G, Summer R. Time to personalize the treatment of anti-MDA-5 associated lung disease. Ann Rheum Dis. 2019;78:E52.
8. Kampylafka EI, Kosmidis ML, Panagiotakos DB, et al. The effect of intravenous immunoglobulin (IVIG) treatment on patients with dermatomyositis: a 4-year follow-up study. Clin Exp Rheumatol. 2012;30:397-401.
9. Koguchi-Yoshioka H, Okiyama N, Iwamoto K, et al. Intravenous immunoglobulin contributes to the control of antimelanoma differentiation-associated protein 5 antibody-associated dermatomyositis with palmar violaceous macules/papules. Br J Dermatol. 2017;177:1442-1446.
10. Hamada-Ode K, Taniguchi Y, Kimata T, et al. High-dose intravenous immunoglobulin therapy for rapidly progressive interstitial pneumonitis accompanied by anti-melanoma differentiation-associated gene 5 antibody-positive amyopathic dermatomyositis. Eur J Rheumatol. 2015;2:83-85.
11. So H, Wong VTL, Lao VWN, et al. Rituximab for refractory rapidly progressive interstitial lung disease related to anti-MDA5 antibody-positive amyopathic dermatomyositis. Clin Rheumatol. 2018;37:1983-1989.
12. Koichi Y, Aya Y, Megumi U, et al. A case of anti-MDA5-positive rapidly progressive interstitial lung disease in a patient with clinically amyopathic dermatomyositis ameliorated by rituximab, in addition to standard immunosuppressive treatment. Mod Rheumatol. 2017;27:536-540.
13. Tsuji H, Nakashima R, Hosono Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti-melanoma differentiation-associated gene 5-positive dermatomyositis. Arthritis Rheumatol. 2020;72:488-498.
14. Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune-associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol. 2020;49:244-246.
15. Fujita Y, Fukui S, Suzuki T, et al. Anti-MDA5 antibody-positive dermatomyositis complicated by autoimmune-associated hemophagocytic syndrome that was successfully treated with immunosuppressive therapy and plasmapheresis. Intern Med. 2018;57:3473-3478.
16. Gono T, Miyake K, Kawaguchi Y, et al. Hyperferritinaemia and macrophage activation in a patient with interstitial lung disease with clinically amyopathic DM. Rheumatology (Oxford). 2012;51:1336-1338.
17. Wada T, Abe G, Kudou, T, et al. Liver damage in patients with polymyositis and dermatomyositis. Kitasato Med Journal. 2016;46:40-46.
18. Takahashi A, Abe K, Yokokawa J, et al. Clinical features of liver dysfunction in collagen diseases. Hepatol Res. 2010;40:1092-1097.
19. Matsumoto T, Kobayashi S, Shimizu H, et al. The liver in collagen diseases: pathologic study of 160 cases with particular reference to hepatic arteritis, primary biliary cirrhosis, autoimmune hepatitis and nodular regenerative hyperplasia of the liver. Liver. 2000;20:366-373.
20. Shi Q, Niu J, Huang X, et al. Do muscle enzyme changes forecast liver injury in polymyositis/dermatomyositis patients treated with methylprednisolone and methotrexate? Ann Clin Lab Sci. 2016;46:266-269.
21. Noda S, Asano Y, Tamaki Z, et al. A case of dermatomyositis with “liver disease associated with rheumatoid diseases” positive for anti-liver-kidney microsome-1 antibody. Clin Rheumatol. 2010;29:941-943.
22. Nagashima T, Kamata Y, Iwamoto M, et al. Liver dysfunction in anti-melanoma differentiation-associated gene 5 antibody-positive patients with dermatomyositis. Rheumatol Int. 2019;39:901-909.
PRACTICE POINTS
- Anti-MDA5 (melanoma differentiation–associated gene 5 antibody)–positive dermatomyositis associated with hemophagocytic lymphohistiocytosis is a rare and aggressive condition associated with a poor prognosis, and there is no standard treatment.
- Dermatomyositis-associated liver injury is not well defined.