User login
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disorder characterized by painful nodules, abscesses, and tunnels predominantly affecting intertriginous areas of the body.1,2 The condition poses significant challenges in terms of diagnosis, treatment, and quality of life for affected individuals. Various systemic therapies have been explored to manage this debilitating condition, with the emergence of biologic agents offering hope for improved outcomes. In 2015, adalimumab (ADA) was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of HS, followed by secukinumab in 2023 and bimekizumab in 2024. However, the off-label use of other biologics and/or tumor necrosis factor inhibitors such as infliximab (IFX) has become common practice.3
Although these therapies have demonstrated promising results in the treatment of HS, their widespread use may be hindered by accessibility and cost barriers. Orenstein et al analyzed data from the IBM Explorys platform from 2015 to 2020 and found that only 1.8% of patients diagnosed with HS had been prescribed ADA or IFX.4 More recently, Garg et al examined IBM MarketScan and IBM US Medicaid data from 2015 to 2018 to evaluate trends in clinical care and treatment. The prevalence of ADA and IFX prescriptions among patients with HS ranged from 2.3% to 8.0% (ADA) and 0.7% to 0.9% (IFX) for patients with commercial insurance, and 1.4% to 4.8% (ADA) and 0.5% to 0.7% (IFX) for patients with Medicaid.5 Biologics are often expensive, and the high cost associated with these therapies has been identified as a significant barrier to access for patients with HS, particularly those who lack adequate insurance coverage or face financial constraints.6
Furthermore, these barriers, particularly the financial barriers, are potentially compounded by the demographics of patients most notably affected by HS. In the US, a disproportionate incidence of HS has been noted in specific groups and age ranges, including women, individuals aged 18 to 29 years, and Black individuals.4 Orenstein et al found a statistically significant difference in use of ADA and IFX biologics based on age, sex, and race.4
The aim of this study was to examine the use of 2 biologics (ADA and IFX) in the Veterans Health Administration (VHA), a unique population in which financial barriers are reduced due to the single-payer government health care system structure. This design allowed for improved isolation and evaluation of variation in ADA and/or IFX prescription rates by demographics and health-related factors among patients with HS. To our knowledge, no studies have analyzed these metrics within the VHA.
Methods
This retrospective, cross-sectional analysis of VHA patients used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a data repository that provides access to longitudinal national electronic health record data for all veterans receiving care through VHA facilities. This study received ethical approval from institutional review boards at the Minneapolis Veterans Affairs Health Care System and VA Salt Lake City Healthcare System. Patient information was deidentified, and patient consent was not required.
Patients with HS were identified using ≥ 1 International Classification of Diseases (ICD) diagnostic code: (ICD-9 [705.83] or ICD-10 [L73.2]) between January 1, 2011, and December 31, 2021. The study included patients aged ≥ 18 years as of January 1, 2011, with ≥ 2 patient encounters during the postdiagnosis follow-up period, and with ≥ 1 encounter 6 months postindex. Patients with a biologic prescription prior to HS diagnosis were excluded. For this study, the term biologics refers to ADA and/or IFX prescriptions, unless otherwise specified. Only ADA and IFX were included in this analysis because ADA, a tumor necrosis factor (TNF)-á inhibitor, was the only FDA-approved medication at the time of the search, and IFX is another common TNF-α inhibitor used for the treatment of HS.
Statistical Analysis
We calculated logistic regression using SAS 9.4 (SAS Institute, Cary, NC). For each variable, the univariate relationship with biologic prescriptions was examined first, followed by the multivariate relationship controlling for all other variables. The following variables were controlled for in the multivariate models and were chosen a priori: sex, age, race, ethnicity, US region, hospital setting, current or previous tobacco use, obesity (defined as body mass index [BMI] ≥ 30), and Charlson Comorbidity Index (CCI).7
Results
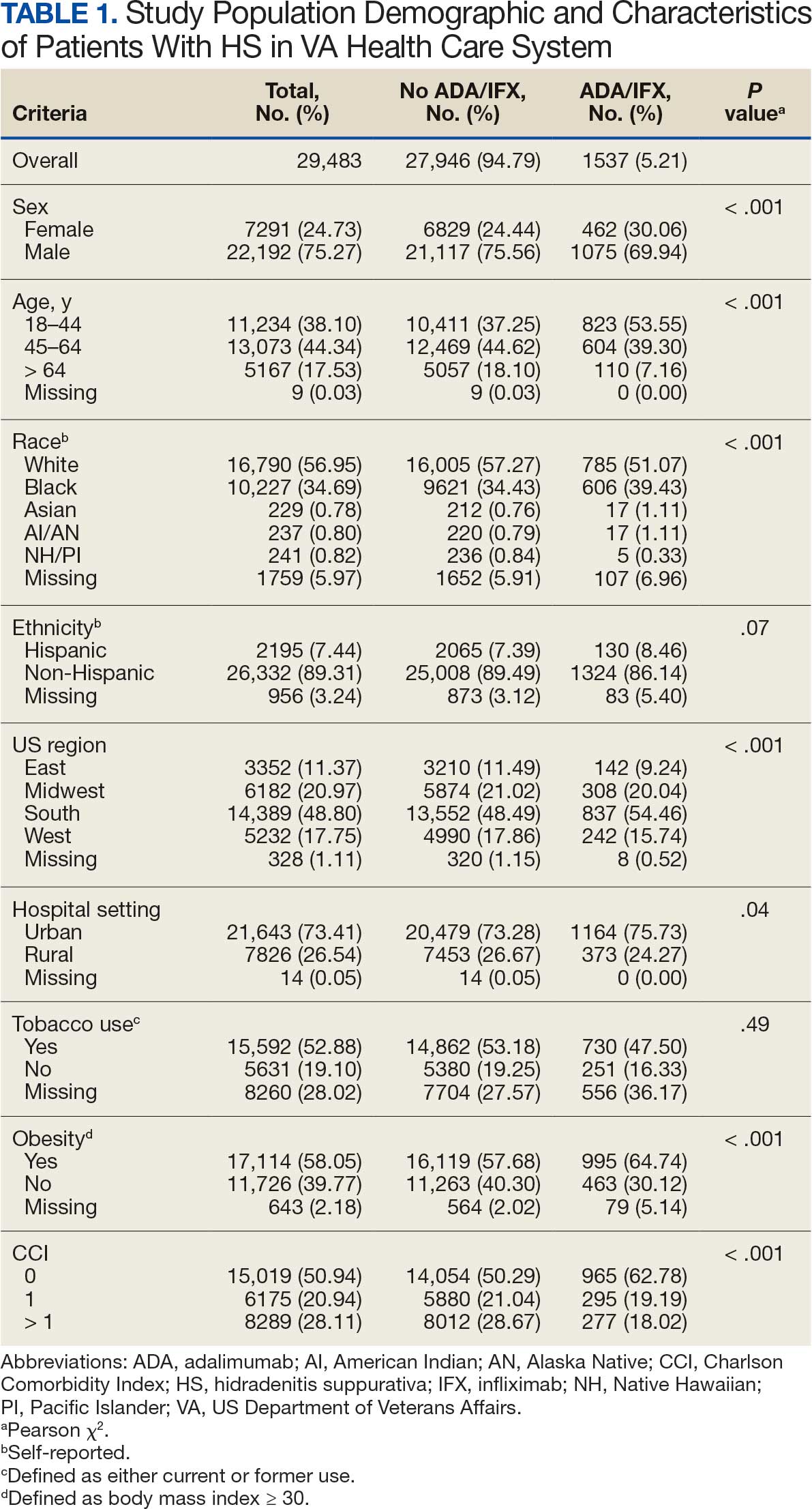
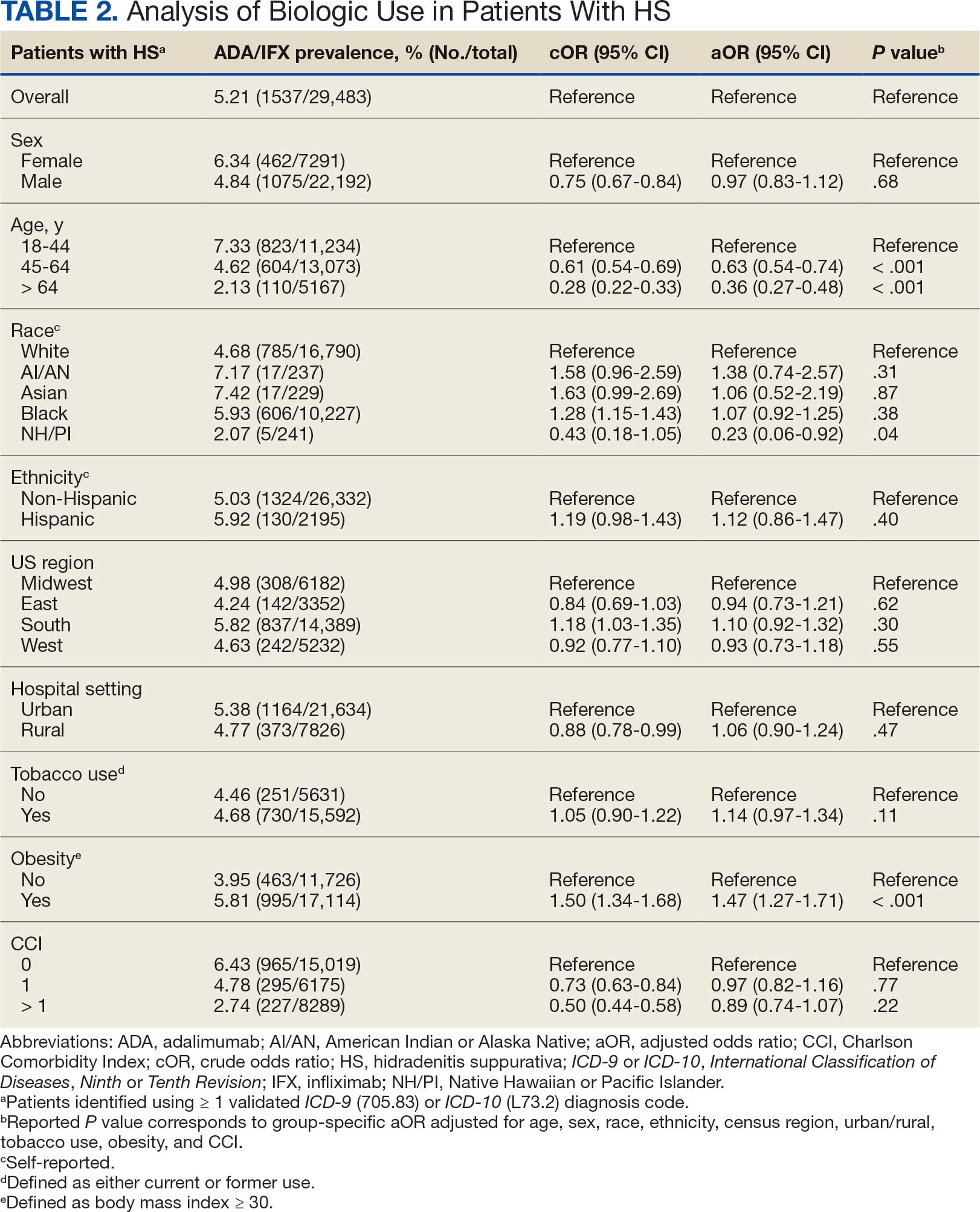
Using ICD codes, we identified 29,483 individuals with ≥ 1 HS diagnosis (Figure 1). Of those identified, 1537 patients (5.21%) had been prescribed ≥ 1 biologic. The cohort was predominantly White (60.56%), male (75.27%), obese (59.34%), and had a history of current or previous tobacco use (73.47%) (Table 1). There were significant adjusted differences in prescription rates among veterans with HS based on age, race, and BMI. Notably, there was an age-dependent reduction in the odds of being prescribed a biologic in patients with HS. Compared with patients aged 18 to 44 years, patients aged 45 to 64 years (adjusted odds ratio [aOR], 0.63; 95% CI, 0.54–0.74; P < .001) and patients aged ≥ 65 years (aOR, 0.36; 95% CI, 0.27–0.48; P < .001) had significantly lower odds of receiving a biologic prescription (Table 2). Compared with White patients with HS, Native Hawaiian (NH) or Pacific Islander (PI) patients were less likely to be prescribed a biologic (aOR, 0.23; 95% CI, 0.06–0.92; P = .04). Patients with obesity had significantly higher odds of receiving a biologic prescription compared with patients without obesity (aOR, 1.47; 95% CI, 1.27– 1.71; P < .001).
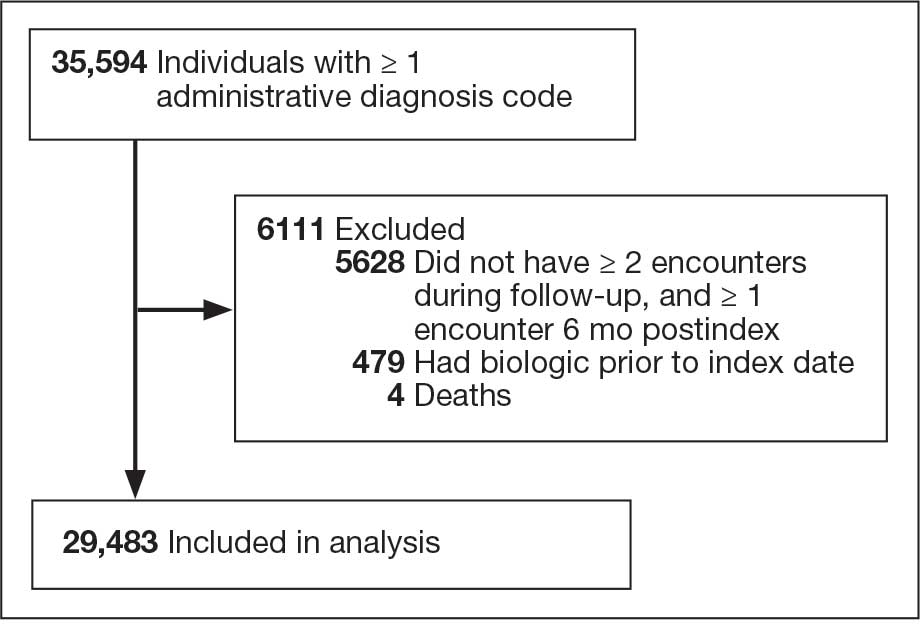
Included in Analysis.
After adjusting for the variables listed in Table 1, there were no significant differences in biologic prescription rates for men compared with women (aOR, 0.97; 95% CI, 0.83-1.12; P = .68). We observed slight variations in biologic prescriptions between US regions (Midwest 5.0%, East 4.2%, South 5.8%, West 4.6%), none of which were significantly different in the fully adjusted model. No statistically significant differences were found in biologic prescriptions between urban and rural VA settings (5.4% vs 4.8%; aOR, 1.06; 95% CI, 0.90–1.24; P = .47). Tobacco use was not associated with the rate of biologic prescription receipt (aOR, 1.14; 95% CI, 0.97–1.34; P = .11). After adjusting for other variables (as outlined in Table 2), no significant differences were found between CCI of 0 and 1 (aOR, 0.97; 95% CI, 0.82–1.16; P = .77) or between CCI of 0 and 2 (aOR, 0.89; 95% CI, 0.74–1.07; P = .22).7
Discussion
The aim of the study was to ascertain potential discrepancies in biologic prescription patterns among patients with HS in the VHA by demographic and lifestyle behavior modifiers. Veteran cohorts are unique in composition, consisting predominantly of older White men within a single-payer health care system. The prevalence of biologic prescriptions in this population was low (5.2%), consistent with prior studies (1.8%–8.9%).4,5
We found a significant difference in ADA/IFX prescription patterns between White patients and NH/PI patients (aOR, 0.23; 95% CI, 0.06-0.92; P = .04). Further replication of this result is needed due to the small number of NH/PI patients included in the study (n = 241). Notably, we did not find a significant difference in the odds of Black patients being prescribed a biologic compared with White patients (aOR, 1.07; 95% CI, 0.92–1.25; P = .38), consistent with prior studies.4
In line with prior studies, age was associated with the likelihood of receiving a biologic prescription.4 Using the multivariate model adjusting for variables listed in Table 1, including CCI, patients aged 45 to 64 years and > 64 years were less likely to be prescribed a biologic than patients aged 18 to 44 years. HS disease activity could be a potential confounding variable, as HS severity may subside in some people with increasing age or menopause.8
Because different regions in the US have different sociopolitical ideologies and governing legislation, we hypothesized that there may be dissimilarities in the prevalence rates of biologic prescribing across various US regions. However, no significant differences were found in prescription patterns among US regions or between rural and urban settings. Previous research has demonstrated discernible disparities in both dermatologic care and clinical outcomes based on hospital setting (ie, urban vs rural).9-11
Tobacco use has been demonstrated to be associated with the development of HS.12 In a large retrospective analysis, Garg et al reported increased odds of receiving a new HS diagnosis in known tobacco users (aOR, 1.9; 95% CI, 1.8–2.0).13 The extent to which tobacco use affects HS severity is less understood. While some studies have found an association between smoking and HS severity, other analyses have failed to find this association.14,15 The effects of smoking cessation on the disease course of HS are unknown.16 This analysis, found no significant difference in prescriptions for biologics among patients with HS comparing current or previous tobacco users with nonusers.
There is a known positive correlation between increasing BMI and HS prevalence and severity that may be explained by the downstream effects of adipose tissue secretion of proinflammatory mediators and insulin resistance in the setting of chronic inflammation.12 This analysis found that patients with HS and obesity were 1.47 times more likely to be prescribed a biologic than patients with HS without obesity, which may be confounded by increased HS severity among patients with obesity. The initial concern when analyzing tobacco use and obesity was that clinician bias may result in a decrease in the prevalence of biologic use in these demographics, which was not supported in this study.
Although we identified few disparities, the results demonstrated a substantial underutilization of biologic therapies (5.2%), similar to the other US civilian studies (1.8-8.9%).4,5 While there is no current universal, standardized severity scoring system to evaluate HS (it is difficult to objectively define moderate to severe HS), estimates have shown that 40.3% to 65.8% of patients with HS have Hurley stage II or III.17-19 Therefore, only a small percentage of patients with moderate to severe disease were prescribed the only FDA-approved medication during this time period. The persistence of this underutilization within a medical system that reduces financial barriers suggests that nonfinancial barriers have a notable role in the underutilization of biologics.
For instance, risk of adverse events, particularly lymphoma and infection, has been cited by patients as a reason to avoid biologics. Additionally, treatment fatigue reduced some patients’ willingness to try new treatments, as did lack of knowledge about treatment options.6,20 Other reported barriers included the frequency of injections and fear of needles.6 Additionally, within the VA, ADA may require prior authorization at the local facility level.21 An established relationship with a dermatologist has been shown to significantly increase the odds of being prescribed a biologic medication in the face of these barriers.4 Future system-wide quality improvement initiatives could be implemented to identify patients with HS not followed by dermatology, with the goal of establishing care with a dermatologist.
Limitations
Limitations to this study include an inability to categorize HS disease severity and assess the degree to which disease severity confounded study findings, particularly in relation to tobacco use and obesity. The generalizability of this study is also limited because of the demographic characteristics of the veteran patient population, which is predominantly older, White, and male, whereas HS disproportionately affects younger, Black, and female individuals in the US.22 Despite these limitations, this study contributes valuable insights into the use of biologic therapies for veteran populations with HS using a national dataset.
Conclusions
This study was performed within a single-payer government medical system, likely reducing or removing the financial barriers that some patient populations may face when pursuing biologics for HS treatment. However, the prevalence of biologic use in this population was low overall (5.2%), suggesting that other factors play a role in the underutilization of biologics in HS. Consistent with previous studies, younger individuals were more likely to be prescribed a biologic, and no difference in prescription rates between Black and White patients was observed. Unlike previous studies, no significant difference in prescription rates between men and women was observed.
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi:10.1016/j.jaad.2019.08.090
- Tchero H, Herlin C, Bekara F, et al. Hidradenitis suppurativa: a systematic review and meta-analysis of therapeutic interventions. Indian J Dermatol Venereol Leprol. 2019;85:248-257. doi:10.4103/ijdvl.IJDVL_69_18
- Shih T, Lee K, Grogan T, et al. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol Ther. 2022;35:e15691. doi:10.1111/dth.15691
- Orenstein LAV, Wright S, Strunk A, et al. Low prescription of tumor necrosis alpha inhibitors in hidradenitis suppurativa: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1399-1401. doi:10.1016/j.jaad.2020.07.108
- Garg A, Naik HB, Alavi A, et al. Real-world findings on the characteristics and treatment exposures of patients with hidradenitis suppurativa from US claims data. Dermatol Ther (Heidelb). 2023;13:581-594. doi:10.1007/s13555-022-00872-1
- De DR, Shih T, Fixsen D, et al. Biologic use in hidradenitis suppurativa: patient perspectives and barriers. J Dermatolog Treat. 2022;33:3060-3062. doi:10.1080/09546634.2022.2089336
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373- 383. doi:10.1016/0021-9681(87)90171-8
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389-392. doi:10.1046/j.1468-3083.2000.00087.x
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417-428.e2. doi:10.1016/j.anai.2020.12.020
- Wu YP, Parsons B, Jo Y, et al. Outdoor activities and sunburn among urban and rural families in a Western region of the US: implications for skin cancer prevention. Prev Med Rep. 2022;29:101914. doi:10.1016/j.pmedr.2022.101914
- Mannschreck DB, Li X, Okoye G. Rural melanoma patients in Maryland do not present with more advanced disease than urban patients. Dermatol Online J. 2021;27. doi:10.5070/D327553607
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population- based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Sartorius K, Emtestam L, Jemec GBE, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831- 839. doi:10.1111/j.1365-2133.2009.09198.x
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51-57. doi:10.1016/j.jaad.2009.02.013
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216- 221. doi:10.1136/postgradmedj-2013-131994
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population- based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Vanlaerhoven AMJD, Ardon CB, van Straalen KR, et al. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology. 2018;234:232-233. doi:10.1159/000491547
- Shahi V, Alikhan A, Vazquez BG, et al. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229:154-158. doi:10.1159/000363381
- Salame N, Sow YN, Siira MR, et al. Factors affecting treatment selection among patients with hidradenitis suppurativa. JAMA Dermatol. 2024;160:179. doi:10.1001/jamadermatol.2023.5425
- VA Formulary Advisor: ADALIMUMAB-BWWD INJ,SOLN. US Department of Veterans Affairs. Updated December 17, 2025. Accessed January 15, 2026. https://www.va.gov/formularyadvisor/drugs/4042383-ADALIMUMAB-BWWD-INJ-SOLN
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118- 122. doi:10.1016/j.jaad.2017.02.005
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disorder characterized by painful nodules, abscesses, and tunnels predominantly affecting intertriginous areas of the body.1,2 The condition poses significant challenges in terms of diagnosis, treatment, and quality of life for affected individuals. Various systemic therapies have been explored to manage this debilitating condition, with the emergence of biologic agents offering hope for improved outcomes. In 2015, adalimumab (ADA) was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of HS, followed by secukinumab in 2023 and bimekizumab in 2024. However, the off-label use of other biologics and/or tumor necrosis factor inhibitors such as infliximab (IFX) has become common practice.3
Although these therapies have demonstrated promising results in the treatment of HS, their widespread use may be hindered by accessibility and cost barriers. Orenstein et al analyzed data from the IBM Explorys platform from 2015 to 2020 and found that only 1.8% of patients diagnosed with HS had been prescribed ADA or IFX.4 More recently, Garg et al examined IBM MarketScan and IBM US Medicaid data from 2015 to 2018 to evaluate trends in clinical care and treatment. The prevalence of ADA and IFX prescriptions among patients with HS ranged from 2.3% to 8.0% (ADA) and 0.7% to 0.9% (IFX) for patients with commercial insurance, and 1.4% to 4.8% (ADA) and 0.5% to 0.7% (IFX) for patients with Medicaid.5 Biologics are often expensive, and the high cost associated with these therapies has been identified as a significant barrier to access for patients with HS, particularly those who lack adequate insurance coverage or face financial constraints.6
Furthermore, these barriers, particularly the financial barriers, are potentially compounded by the demographics of patients most notably affected by HS. In the US, a disproportionate incidence of HS has been noted in specific groups and age ranges, including women, individuals aged 18 to 29 years, and Black individuals.4 Orenstein et al found a statistically significant difference in use of ADA and IFX biologics based on age, sex, and race.4
The aim of this study was to examine the use of 2 biologics (ADA and IFX) in the Veterans Health Administration (VHA), a unique population in which financial barriers are reduced due to the single-payer government health care system structure. This design allowed for improved isolation and evaluation of variation in ADA and/or IFX prescription rates by demographics and health-related factors among patients with HS. To our knowledge, no studies have analyzed these metrics within the VHA.
Methods
This retrospective, cross-sectional analysis of VHA patients used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a data repository that provides access to longitudinal national electronic health record data for all veterans receiving care through VHA facilities. This study received ethical approval from institutional review boards at the Minneapolis Veterans Affairs Health Care System and VA Salt Lake City Healthcare System. Patient information was deidentified, and patient consent was not required.
Patients with HS were identified using ≥ 1 International Classification of Diseases (ICD) diagnostic code: (ICD-9 [705.83] or ICD-10 [L73.2]) between January 1, 2011, and December 31, 2021. The study included patients aged ≥ 18 years as of January 1, 2011, with ≥ 2 patient encounters during the postdiagnosis follow-up period, and with ≥ 1 encounter 6 months postindex. Patients with a biologic prescription prior to HS diagnosis were excluded. For this study, the term biologics refers to ADA and/or IFX prescriptions, unless otherwise specified. Only ADA and IFX were included in this analysis because ADA, a tumor necrosis factor (TNF)-á inhibitor, was the only FDA-approved medication at the time of the search, and IFX is another common TNF-α inhibitor used for the treatment of HS.
Statistical Analysis
We calculated logistic regression using SAS 9.4 (SAS Institute, Cary, NC). For each variable, the univariate relationship with biologic prescriptions was examined first, followed by the multivariate relationship controlling for all other variables. The following variables were controlled for in the multivariate models and were chosen a priori: sex, age, race, ethnicity, US region, hospital setting, current or previous tobacco use, obesity (defined as body mass index [BMI] ≥ 30), and Charlson Comorbidity Index (CCI).7
Results
Using ICD codes, we identified 29,483 individuals with ≥ 1 HS diagnosis (Figure 1). Of those identified, 1537 patients (5.21%) had been prescribed ≥ 1 biologic. The cohort was predominantly White (60.56%), male (75.27%), obese (59.34%), and had a history of current or previous tobacco use (73.47%) (Table 1). There were significant adjusted differences in prescription rates among veterans with HS based on age, race, and BMI. Notably, there was an age-dependent reduction in the odds of being prescribed a biologic in patients with HS. Compared with patients aged 18 to 44 years, patients aged 45 to 64 years (adjusted odds ratio [aOR], 0.63; 95% CI, 0.54–0.74; P < .001) and patients aged ≥ 65 years (aOR, 0.36; 95% CI, 0.27–0.48; P < .001) had significantly lower odds of receiving a biologic prescription (Table 2). Compared with White patients with HS, Native Hawaiian (NH) or Pacific Islander (PI) patients were less likely to be prescribed a biologic (aOR, 0.23; 95% CI, 0.06–0.92; P = .04). Patients with obesity had significantly higher odds of receiving a biologic prescription compared with patients without obesity (aOR, 1.47; 95% CI, 1.27– 1.71; P < .001).

Included in Analysis.
After adjusting for the variables listed in Table 1, there were no significant differences in biologic prescription rates for men compared with women (aOR, 0.97; 95% CI, 0.83-1.12; P = .68). We observed slight variations in biologic prescriptions between US regions (Midwest 5.0%, East 4.2%, South 5.8%, West 4.6%), none of which were significantly different in the fully adjusted model. No statistically significant differences were found in biologic prescriptions between urban and rural VA settings (5.4% vs 4.8%; aOR, 1.06; 95% CI, 0.90–1.24; P = .47). Tobacco use was not associated with the rate of biologic prescription receipt (aOR, 1.14; 95% CI, 0.97–1.34; P = .11). After adjusting for other variables (as outlined in Table 2), no significant differences were found between CCI of 0 and 1 (aOR, 0.97; 95% CI, 0.82–1.16; P = .77) or between CCI of 0 and 2 (aOR, 0.89; 95% CI, 0.74–1.07; P = .22).7


Discussion
The aim of the study was to ascertain potential discrepancies in biologic prescription patterns among patients with HS in the VHA by demographic and lifestyle behavior modifiers. Veteran cohorts are unique in composition, consisting predominantly of older White men within a single-payer health care system. The prevalence of biologic prescriptions in this population was low (5.2%), consistent with prior studies (1.8%–8.9%).4,5
We found a significant difference in ADA/IFX prescription patterns between White patients and NH/PI patients (aOR, 0.23; 95% CI, 0.06-0.92; P = .04). Further replication of this result is needed due to the small number of NH/PI patients included in the study (n = 241). Notably, we did not find a significant difference in the odds of Black patients being prescribed a biologic compared with White patients (aOR, 1.07; 95% CI, 0.92–1.25; P = .38), consistent with prior studies.4
In line with prior studies, age was associated with the likelihood of receiving a biologic prescription.4 Using the multivariate model adjusting for variables listed in Table 1, including CCI, patients aged 45 to 64 years and > 64 years were less likely to be prescribed a biologic than patients aged 18 to 44 years. HS disease activity could be a potential confounding variable, as HS severity may subside in some people with increasing age or menopause.8
Because different regions in the US have different sociopolitical ideologies and governing legislation, we hypothesized that there may be dissimilarities in the prevalence rates of biologic prescribing across various US regions. However, no significant differences were found in prescription patterns among US regions or between rural and urban settings. Previous research has demonstrated discernible disparities in both dermatologic care and clinical outcomes based on hospital setting (ie, urban vs rural).9-11
Tobacco use has been demonstrated to be associated with the development of HS.12 In a large retrospective analysis, Garg et al reported increased odds of receiving a new HS diagnosis in known tobacco users (aOR, 1.9; 95% CI, 1.8–2.0).13 The extent to which tobacco use affects HS severity is less understood. While some studies have found an association between smoking and HS severity, other analyses have failed to find this association.14,15 The effects of smoking cessation on the disease course of HS are unknown.16 This analysis, found no significant difference in prescriptions for biologics among patients with HS comparing current or previous tobacco users with nonusers.
There is a known positive correlation between increasing BMI and HS prevalence and severity that may be explained by the downstream effects of adipose tissue secretion of proinflammatory mediators and insulin resistance in the setting of chronic inflammation.12 This analysis found that patients with HS and obesity were 1.47 times more likely to be prescribed a biologic than patients with HS without obesity, which may be confounded by increased HS severity among patients with obesity. The initial concern when analyzing tobacco use and obesity was that clinician bias may result in a decrease in the prevalence of biologic use in these demographics, which was not supported in this study.
Although we identified few disparities, the results demonstrated a substantial underutilization of biologic therapies (5.2%), similar to the other US civilian studies (1.8-8.9%).4,5 While there is no current universal, standardized severity scoring system to evaluate HS (it is difficult to objectively define moderate to severe HS), estimates have shown that 40.3% to 65.8% of patients with HS have Hurley stage II or III.17-19 Therefore, only a small percentage of patients with moderate to severe disease were prescribed the only FDA-approved medication during this time period. The persistence of this underutilization within a medical system that reduces financial barriers suggests that nonfinancial barriers have a notable role in the underutilization of biologics.
For instance, risk of adverse events, particularly lymphoma and infection, has been cited by patients as a reason to avoid biologics. Additionally, treatment fatigue reduced some patients’ willingness to try new treatments, as did lack of knowledge about treatment options.6,20 Other reported barriers included the frequency of injections and fear of needles.6 Additionally, within the VA, ADA may require prior authorization at the local facility level.21 An established relationship with a dermatologist has been shown to significantly increase the odds of being prescribed a biologic medication in the face of these barriers.4 Future system-wide quality improvement initiatives could be implemented to identify patients with HS not followed by dermatology, with the goal of establishing care with a dermatologist.
Limitations
Limitations to this study include an inability to categorize HS disease severity and assess the degree to which disease severity confounded study findings, particularly in relation to tobacco use and obesity. The generalizability of this study is also limited because of the demographic characteristics of the veteran patient population, which is predominantly older, White, and male, whereas HS disproportionately affects younger, Black, and female individuals in the US.22 Despite these limitations, this study contributes valuable insights into the use of biologic therapies for veteran populations with HS using a national dataset.
Conclusions
This study was performed within a single-payer government medical system, likely reducing or removing the financial barriers that some patient populations may face when pursuing biologics for HS treatment. However, the prevalence of biologic use in this population was low overall (5.2%), suggesting that other factors play a role in the underutilization of biologics in HS. Consistent with previous studies, younger individuals were more likely to be prescribed a biologic, and no difference in prescription rates between Black and White patients was observed. Unlike previous studies, no significant difference in prescription rates between men and women was observed.
Hidradenitis suppurativa (HS) is a chronic, inflammatory skin disorder characterized by painful nodules, abscesses, and tunnels predominantly affecting intertriginous areas of the body.1,2 The condition poses significant challenges in terms of diagnosis, treatment, and quality of life for affected individuals. Various systemic therapies have been explored to manage this debilitating condition, with the emergence of biologic agents offering hope for improved outcomes. In 2015, adalimumab (ADA) was the first biologic approved by the US Food and Drug Administration (FDA) for the treatment of HS, followed by secukinumab in 2023 and bimekizumab in 2024. However, the off-label use of other biologics and/or tumor necrosis factor inhibitors such as infliximab (IFX) has become common practice.3
Although these therapies have demonstrated promising results in the treatment of HS, their widespread use may be hindered by accessibility and cost barriers. Orenstein et al analyzed data from the IBM Explorys platform from 2015 to 2020 and found that only 1.8% of patients diagnosed with HS had been prescribed ADA or IFX.4 More recently, Garg et al examined IBM MarketScan and IBM US Medicaid data from 2015 to 2018 to evaluate trends in clinical care and treatment. The prevalence of ADA and IFX prescriptions among patients with HS ranged from 2.3% to 8.0% (ADA) and 0.7% to 0.9% (IFX) for patients with commercial insurance, and 1.4% to 4.8% (ADA) and 0.5% to 0.7% (IFX) for patients with Medicaid.5 Biologics are often expensive, and the high cost associated with these therapies has been identified as a significant barrier to access for patients with HS, particularly those who lack adequate insurance coverage or face financial constraints.6
Furthermore, these barriers, particularly the financial barriers, are potentially compounded by the demographics of patients most notably affected by HS. In the US, a disproportionate incidence of HS has been noted in specific groups and age ranges, including women, individuals aged 18 to 29 years, and Black individuals.4 Orenstein et al found a statistically significant difference in use of ADA and IFX biologics based on age, sex, and race.4
The aim of this study was to examine the use of 2 biologics (ADA and IFX) in the Veterans Health Administration (VHA), a unique population in which financial barriers are reduced due to the single-payer government health care system structure. This design allowed for improved isolation and evaluation of variation in ADA and/or IFX prescription rates by demographics and health-related factors among patients with HS. To our knowledge, no studies have analyzed these metrics within the VHA.
Methods
This retrospective, cross-sectional analysis of VHA patients used data from the US Department of Veterans Affairs (VA) Corporate Data Warehouse, a data repository that provides access to longitudinal national electronic health record data for all veterans receiving care through VHA facilities. This study received ethical approval from institutional review boards at the Minneapolis Veterans Affairs Health Care System and VA Salt Lake City Healthcare System. Patient information was deidentified, and patient consent was not required.
Patients with HS were identified using ≥ 1 International Classification of Diseases (ICD) diagnostic code: (ICD-9 [705.83] or ICD-10 [L73.2]) between January 1, 2011, and December 31, 2021. The study included patients aged ≥ 18 years as of January 1, 2011, with ≥ 2 patient encounters during the postdiagnosis follow-up period, and with ≥ 1 encounter 6 months postindex. Patients with a biologic prescription prior to HS diagnosis were excluded. For this study, the term biologics refers to ADA and/or IFX prescriptions, unless otherwise specified. Only ADA and IFX were included in this analysis because ADA, a tumor necrosis factor (TNF)-á inhibitor, was the only FDA-approved medication at the time of the search, and IFX is another common TNF-α inhibitor used for the treatment of HS.
Statistical Analysis
We calculated logistic regression using SAS 9.4 (SAS Institute, Cary, NC). For each variable, the univariate relationship with biologic prescriptions was examined first, followed by the multivariate relationship controlling for all other variables. The following variables were controlled for in the multivariate models and were chosen a priori: sex, age, race, ethnicity, US region, hospital setting, current or previous tobacco use, obesity (defined as body mass index [BMI] ≥ 30), and Charlson Comorbidity Index (CCI).7
Results
Using ICD codes, we identified 29,483 individuals with ≥ 1 HS diagnosis (Figure 1). Of those identified, 1537 patients (5.21%) had been prescribed ≥ 1 biologic. The cohort was predominantly White (60.56%), male (75.27%), obese (59.34%), and had a history of current or previous tobacco use (73.47%) (Table 1). There were significant adjusted differences in prescription rates among veterans with HS based on age, race, and BMI. Notably, there was an age-dependent reduction in the odds of being prescribed a biologic in patients with HS. Compared with patients aged 18 to 44 years, patients aged 45 to 64 years (adjusted odds ratio [aOR], 0.63; 95% CI, 0.54–0.74; P < .001) and patients aged ≥ 65 years (aOR, 0.36; 95% CI, 0.27–0.48; P < .001) had significantly lower odds of receiving a biologic prescription (Table 2). Compared with White patients with HS, Native Hawaiian (NH) or Pacific Islander (PI) patients were less likely to be prescribed a biologic (aOR, 0.23; 95% CI, 0.06–0.92; P = .04). Patients with obesity had significantly higher odds of receiving a biologic prescription compared with patients without obesity (aOR, 1.47; 95% CI, 1.27– 1.71; P < .001).

Included in Analysis.
After adjusting for the variables listed in Table 1, there were no significant differences in biologic prescription rates for men compared with women (aOR, 0.97; 95% CI, 0.83-1.12; P = .68). We observed slight variations in biologic prescriptions between US regions (Midwest 5.0%, East 4.2%, South 5.8%, West 4.6%), none of which were significantly different in the fully adjusted model. No statistically significant differences were found in biologic prescriptions between urban and rural VA settings (5.4% vs 4.8%; aOR, 1.06; 95% CI, 0.90–1.24; P = .47). Tobacco use was not associated with the rate of biologic prescription receipt (aOR, 1.14; 95% CI, 0.97–1.34; P = .11). After adjusting for other variables (as outlined in Table 2), no significant differences were found between CCI of 0 and 1 (aOR, 0.97; 95% CI, 0.82–1.16; P = .77) or between CCI of 0 and 2 (aOR, 0.89; 95% CI, 0.74–1.07; P = .22).7


Discussion
The aim of the study was to ascertain potential discrepancies in biologic prescription patterns among patients with HS in the VHA by demographic and lifestyle behavior modifiers. Veteran cohorts are unique in composition, consisting predominantly of older White men within a single-payer health care system. The prevalence of biologic prescriptions in this population was low (5.2%), consistent with prior studies (1.8%–8.9%).4,5
We found a significant difference in ADA/IFX prescription patterns between White patients and NH/PI patients (aOR, 0.23; 95% CI, 0.06-0.92; P = .04). Further replication of this result is needed due to the small number of NH/PI patients included in the study (n = 241). Notably, we did not find a significant difference in the odds of Black patients being prescribed a biologic compared with White patients (aOR, 1.07; 95% CI, 0.92–1.25; P = .38), consistent with prior studies.4
In line with prior studies, age was associated with the likelihood of receiving a biologic prescription.4 Using the multivariate model adjusting for variables listed in Table 1, including CCI, patients aged 45 to 64 years and > 64 years were less likely to be prescribed a biologic than patients aged 18 to 44 years. HS disease activity could be a potential confounding variable, as HS severity may subside in some people with increasing age or menopause.8
Because different regions in the US have different sociopolitical ideologies and governing legislation, we hypothesized that there may be dissimilarities in the prevalence rates of biologic prescribing across various US regions. However, no significant differences were found in prescription patterns among US regions or between rural and urban settings. Previous research has demonstrated discernible disparities in both dermatologic care and clinical outcomes based on hospital setting (ie, urban vs rural).9-11
Tobacco use has been demonstrated to be associated with the development of HS.12 In a large retrospective analysis, Garg et al reported increased odds of receiving a new HS diagnosis in known tobacco users (aOR, 1.9; 95% CI, 1.8–2.0).13 The extent to which tobacco use affects HS severity is less understood. While some studies have found an association between smoking and HS severity, other analyses have failed to find this association.14,15 The effects of smoking cessation on the disease course of HS are unknown.16 This analysis, found no significant difference in prescriptions for biologics among patients with HS comparing current or previous tobacco users with nonusers.
There is a known positive correlation between increasing BMI and HS prevalence and severity that may be explained by the downstream effects of adipose tissue secretion of proinflammatory mediators and insulin resistance in the setting of chronic inflammation.12 This analysis found that patients with HS and obesity were 1.47 times more likely to be prescribed a biologic than patients with HS without obesity, which may be confounded by increased HS severity among patients with obesity. The initial concern when analyzing tobacco use and obesity was that clinician bias may result in a decrease in the prevalence of biologic use in these demographics, which was not supported in this study.
Although we identified few disparities, the results demonstrated a substantial underutilization of biologic therapies (5.2%), similar to the other US civilian studies (1.8-8.9%).4,5 While there is no current universal, standardized severity scoring system to evaluate HS (it is difficult to objectively define moderate to severe HS), estimates have shown that 40.3% to 65.8% of patients with HS have Hurley stage II or III.17-19 Therefore, only a small percentage of patients with moderate to severe disease were prescribed the only FDA-approved medication during this time period. The persistence of this underutilization within a medical system that reduces financial barriers suggests that nonfinancial barriers have a notable role in the underutilization of biologics.
For instance, risk of adverse events, particularly lymphoma and infection, has been cited by patients as a reason to avoid biologics. Additionally, treatment fatigue reduced some patients’ willingness to try new treatments, as did lack of knowledge about treatment options.6,20 Other reported barriers included the frequency of injections and fear of needles.6 Additionally, within the VA, ADA may require prior authorization at the local facility level.21 An established relationship with a dermatologist has been shown to significantly increase the odds of being prescribed a biologic medication in the face of these barriers.4 Future system-wide quality improvement initiatives could be implemented to identify patients with HS not followed by dermatology, with the goal of establishing care with a dermatologist.
Limitations
Limitations to this study include an inability to categorize HS disease severity and assess the degree to which disease severity confounded study findings, particularly in relation to tobacco use and obesity. The generalizability of this study is also limited because of the demographic characteristics of the veteran patient population, which is predominantly older, White, and male, whereas HS disproportionately affects younger, Black, and female individuals in the US.22 Despite these limitations, this study contributes valuable insights into the use of biologic therapies for veteran populations with HS using a national dataset.
Conclusions
This study was performed within a single-payer government medical system, likely reducing or removing the financial barriers that some patient populations may face when pursuing biologics for HS treatment. However, the prevalence of biologic use in this population was low overall (5.2%), suggesting that other factors play a role in the underutilization of biologics in HS. Consistent with previous studies, younger individuals were more likely to be prescribed a biologic, and no difference in prescription rates between Black and White patients was observed. Unlike previous studies, no significant difference in prescription rates between men and women was observed.
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi:10.1016/j.jaad.2019.08.090
- Tchero H, Herlin C, Bekara F, et al. Hidradenitis suppurativa: a systematic review and meta-analysis of therapeutic interventions. Indian J Dermatol Venereol Leprol. 2019;85:248-257. doi:10.4103/ijdvl.IJDVL_69_18
- Shih T, Lee K, Grogan T, et al. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol Ther. 2022;35:e15691. doi:10.1111/dth.15691
- Orenstein LAV, Wright S, Strunk A, et al. Low prescription of tumor necrosis alpha inhibitors in hidradenitis suppurativa: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1399-1401. doi:10.1016/j.jaad.2020.07.108
- Garg A, Naik HB, Alavi A, et al. Real-world findings on the characteristics and treatment exposures of patients with hidradenitis suppurativa from US claims data. Dermatol Ther (Heidelb). 2023;13:581-594. doi:10.1007/s13555-022-00872-1
- De DR, Shih T, Fixsen D, et al. Biologic use in hidradenitis suppurativa: patient perspectives and barriers. J Dermatolog Treat. 2022;33:3060-3062. doi:10.1080/09546634.2022.2089336
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373- 383. doi:10.1016/0021-9681(87)90171-8
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389-392. doi:10.1046/j.1468-3083.2000.00087.x
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417-428.e2. doi:10.1016/j.anai.2020.12.020
- Wu YP, Parsons B, Jo Y, et al. Outdoor activities and sunburn among urban and rural families in a Western region of the US: implications for skin cancer prevention. Prev Med Rep. 2022;29:101914. doi:10.1016/j.pmedr.2022.101914
- Mannschreck DB, Li X, Okoye G. Rural melanoma patients in Maryland do not present with more advanced disease than urban patients. Dermatol Online J. 2021;27. doi:10.5070/D327553607
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population- based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Sartorius K, Emtestam L, Jemec GBE, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831- 839. doi:10.1111/j.1365-2133.2009.09198.x
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51-57. doi:10.1016/j.jaad.2009.02.013
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216- 221. doi:10.1136/postgradmedj-2013-131994
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population- based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Vanlaerhoven AMJD, Ardon CB, van Straalen KR, et al. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology. 2018;234:232-233. doi:10.1159/000491547
- Shahi V, Alikhan A, Vazquez BG, et al. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229:154-158. doi:10.1159/000363381
- Salame N, Sow YN, Siira MR, et al. Factors affecting treatment selection among patients with hidradenitis suppurativa. JAMA Dermatol. 2024;160:179. doi:10.1001/jamadermatol.2023.5425
- VA Formulary Advisor: ADALIMUMAB-BWWD INJ,SOLN. US Department of Veterans Affairs. Updated December 17, 2025. Accessed January 15, 2026. https://www.va.gov/formularyadvisor/drugs/4042383-ADALIMUMAB-BWWD-INJ-SOLN
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118- 122. doi:10.1016/j.jaad.2017.02.005
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: epidemiology, clinical presentation, and pathogenesis. J Am Acad Dermatol. 2020;82:1045-1058. doi:10.1016/j.jaad.2019.08.090
- Tchero H, Herlin C, Bekara F, et al. Hidradenitis suppurativa: a systematic review and meta-analysis of therapeutic interventions. Indian J Dermatol Venereol Leprol. 2019;85:248-257. doi:10.4103/ijdvl.IJDVL_69_18
- Shih T, Lee K, Grogan T, et al. Infliximab in hidradenitis suppurativa: a systematic review and meta-analysis. Dermatol Ther. 2022;35:e15691. doi:10.1111/dth.15691
- Orenstein LAV, Wright S, Strunk A, et al. Low prescription of tumor necrosis alpha inhibitors in hidradenitis suppurativa: a cross-sectional analysis. J Am Acad Dermatol. 2021;84:1399-1401. doi:10.1016/j.jaad.2020.07.108
- Garg A, Naik HB, Alavi A, et al. Real-world findings on the characteristics and treatment exposures of patients with hidradenitis suppurativa from US claims data. Dermatol Ther (Heidelb). 2023;13:581-594. doi:10.1007/s13555-022-00872-1
- De DR, Shih T, Fixsen D, et al. Biologic use in hidradenitis suppurativa: patient perspectives and barriers. J Dermatolog Treat. 2022;33:3060-3062. doi:10.1080/09546634.2022.2089336
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373- 383. doi:10.1016/0021-9681(87)90171-8
- von der Werth JM, Williams HC. The natural history of hidradenitis suppurativa. J Eur Acad Dermatol Venereol. 2000;14:389-392. doi:10.1046/j.1468-3083.2000.00087.x
- Silverberg JI, Barbarot S, Gadkari A, et al. Atopic dermatitis in the pediatric population: a cross-sectional, international epidemiologic study. Ann Allergy Asthma Immunol. 2021;126:417-428.e2. doi:10.1016/j.anai.2020.12.020
- Wu YP, Parsons B, Jo Y, et al. Outdoor activities and sunburn among urban and rural families in a Western region of the US: implications for skin cancer prevention. Prev Med Rep. 2022;29:101914. doi:10.1016/j.pmedr.2022.101914
- Mannschreck DB, Li X, Okoye G. Rural melanoma patients in Maryland do not present with more advanced disease than urban patients. Dermatol Online J. 2021;27. doi:10.5070/D327553607
- Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/j.jaad.2021.01.059
- Garg A, Papagermanos V, Midura M, et al. Incidence of hidradenitis suppurativa among tobacco smokers: a population- based retrospective analysis in the U.S.A. Br J Dermatol. 2018;178:709-714. doi:10.1111/bjd.15939
- Sartorius K, Emtestam L, Jemec GBE, et al. Objective scoring of hidradenitis suppurativa reflecting the role of tobacco smoking and obesity. Br J Dermatol. 2009;161:831- 839. doi:10.1111/j.1365-2133.2009.09198.x
- Canoui-Poitrine F, Revuz JE, Wolkenstein P, et al. Clinical characteristics of a series of 302 French patients with hidradenitis suppurativa, with an analysis of factors associated with disease severity. J Am Acad Dermatol. 2009;61:51-57. doi:10.1016/j.jaad.2009.02.013
- Dufour DN, Emtestam L, Jemec GB. Hidradenitis suppurativa: a common and burdensome, yet under-recognised, inflammatory skin disease. Postgrad Med J. 2014;90:216- 221. doi:10.1136/postgradmedj-2013-131994
- Vazquez BG, Alikhan A, Weaver AL, et al. Incidence of hidradenitis suppurativa and associated factors: a population- based study of Olmsted County, Minnesota. J Invest Dermatol. 2013;133:97-103. doi:10.1038/jid.2012.255
- Vanlaerhoven AMJD, Ardon CB, van Straalen KR, et al. Hurley III hidradenitis suppurativa has an aggressive disease course. Dermatology. 2018;234:232-233. doi:10.1159/000491547
- Shahi V, Alikhan A, Vazquez BG, et al. Prevalence of hidradenitis suppurativa: a population-based study in Olmsted County, Minnesota. Dermatology. 2014;229:154-158. doi:10.1159/000363381
- Salame N, Sow YN, Siira MR, et al. Factors affecting treatment selection among patients with hidradenitis suppurativa. JAMA Dermatol. 2024;160:179. doi:10.1001/jamadermatol.2023.5425
- VA Formulary Advisor: ADALIMUMAB-BWWD INJ,SOLN. US Department of Veterans Affairs. Updated December 17, 2025. Accessed January 15, 2026. https://www.va.gov/formularyadvisor/drugs/4042383-ADALIMUMAB-BWWD-INJ-SOLN
- Garg A, Lavian J, Lin G, et al. Incidence of hidradenitis suppurativa in the United States: a sex- and age-adjusted population analysis. J Am Acad Dermatol. 2017;77:118- 122. doi:10.1016/j.jaad.2017.02.005
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Cross-Sectional Analysis of Biologic Use in the Treatment of Veterans With Hidradenitis Suppurativa
Cordlike Dermal Plaques and Nodules on the Neck and Hands
The Diagnosis: Fibroblastic Rheumatism
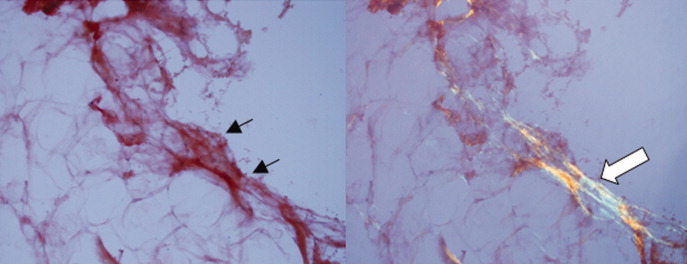
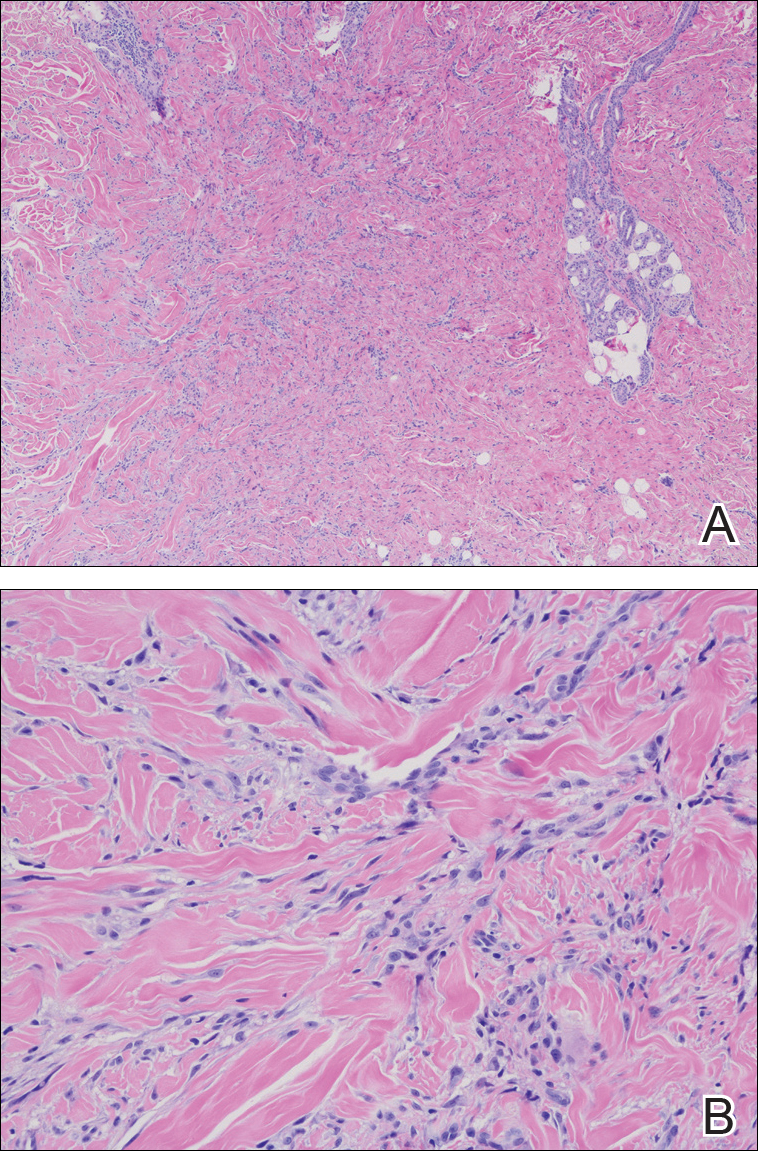
Routine histologic sections stained with hematoxylin and eosin demonstrated a noncircumscribed dermal proliferation of fibroblasts and myofibroblasts with thickened collagen bundles (Figure, A and B). Focally fragmented elastin fibers were noted with Verhoeff elastic tissue stain. Alcian blue stain did not show increased dermal mucin. With the clinical presentation and histologic findings described, we diagnosed the patient with fibroblastic rheumatism (FR). To date, the patient's condition has stabilized overall with skin lesions fading and minimal to no joint pain. Current therapies include adalimumab, mycophenolate mofetil 500 mg 3 times daily, and low-dose prednisone.
Fibroblastic rheumatism is a rare arthropathy with cutaneous findings initially described by Chaouat et al1 in 1980. Age of onset varies, and the condition also has been observed in pediatric patients.2 Fibroblastic rheumatism is characterized by sudden onset of firm, flesh-colored, subcutaneous nodules on periungual and periarticular surfaces.2 Neck lesions rarely are described,2-4 and cordlike plaques previously have not been reported in FR. Typically, patients develop diffusely swollen fingers, palmar thickening, sclerodactyly, and contractures. The eruption may be accompanied by Raynaud phenomenon as well as a progressive symmetric erosive arthropathy.2,5
The clinical course in FR is variable. The cutaneous findings spontaneously may regress in months to years.3,4 However, polyarthropathy often is destructive and progresses to disability.3 Response to therapy has been unpredictable, and the following treatments have been tried, generally with poor efficacy: aspirin, nonsteroidal anti-inflammatory drugs, hydroxychloroquine, colchicine, methotrexate, prednisone, infliximab, D-penicillamine, interferon alfa, and intensive physical therapy.2-4,6 Histologic characteristics may include thickened collagen bundles along with a fibroblastic and myofibroblastic proliferation. Elastic fibers may be decreased or absent.2,3,5
Clinical and histologic features in FR may mimic other entities; thus, clinical pathological correlation is essential in determining the correct diagnosis. Considerations in the differential diagnoses include multicentric reticulohistiocytosis (MRH), palisaded neutrophilic and granulomatous dermatitis, and scleroderma.
In MRH, a symmetric erosive arthritis of mainly distal interphalangeal joints typically precedes the cutaneous disease. Occurrence of arthritis mutilans is reported in approximately half of patients.4 Cutaneous manifestations typically include the presence of coral bead-like papules and nodules over the dorsal aspect of the hands, face, and neck. Unlike FR, MRH has a concomitant autoimmune disease in up to 20% of cases and an associated malignancy in up to 31% of cases, with breast and ovarian carcinomas most common. On histology, MRH is characterized by a nodular infiltrate of histiocytes and multinucleated giant cells with eosinophilic ground-glass cytoplasm.4 No notable collagen changes or fibroblastic proliferations typically are present.
Palisaded neutrophilic and granulomatous dermatitis, usually associated with rheumatoid arthritis or connective tissue disease, classically presents as annular plaques and indurated linear bands over the trunk and extremities. However, its clinical presentation is quite variable and may include pink to violaceous urticarialike; livedoid-appearing; or nonspecific papules, plaques, or nodules. Histology in palisaded neutrophilic and granulomatous dermatitis shows a dense dermal neutrophilic infiltrate associated with interstitial histiocytes having a palisading arrangement around degenerated collagen.7 No fibroblastic proliferation typically is present.
Scleroderma can be distinguished based on additional clinical and laboratory findings as well as histology showing thickened collagen bundles without fibroblastic proliferation.2 The histologic findings also may suggest inclusion of dermatofibroma or a scar in the differential diagnosis, though the clinical presentation of these entities would not support these diagnoses.
Acknowledgments
We thank the patient for granting permission to share this information. We also thank Sheng Chen, MD, PhD (Lake Success, New York), for his dermatopathological contributions to the case.
- Chaouat Y, Aron-Brunetiere R, Faures B, et al. Une nouvelle entité: le rhumatisme fibroblastique. a propos d'une observation [in French]. Rev Rhum Mal Osteoartic. 1980;47:34-35.
- Jurado SA, Alvin GG, Selim MA, et al. Fibroblastic rheumatism: a report of 4 cases with potential therapeutic implications. J Am Acad Dermatol. 2012;66:959-965.
- Colonna L, Barbieri C, Di Lella G, et al. Fibroblastic rheumatism: a case without rheumatological symptoms. Acta Derm Venereol. 2002;82:200-203.
- Trotta F, Colina M. Multicentric reticulohistiocytosis and fibroblastic rheumatism. Best Pract Res Clin Rheumatol. 2012;26:543-557.
- Lee JM, Sundel RP, Liang MG. Fibroblastic rheumatism: case report and review of the literature. Pediatr Dermatol. 2002;19:532-535.
- Kluger N, Dumas-Tesici A, Hamel D, et al. Fibroblastic rheumatism: fibromatosis rather than non-Langerhans cell histiocytosis. J Cutan Pathol. 2010;37:587-592.
- Stephenson SR, Campbell M, Dre GS, et al. Palisaded neutrophilic and granulomatous dermatitis presenting in a patient with rheumatoid arthritis on adalimumab. J Cutan Pathol. 2011;38:644-648.
The Diagnosis: Fibroblastic Rheumatism
Routine histologic sections stained with hematoxylin and eosin demonstrated a noncircumscribed dermal proliferation of fibroblasts and myofibroblasts with thickened collagen bundles (Figure, A and B). Focally fragmented elastin fibers were noted with Verhoeff elastic tissue stain. Alcian blue stain did not show increased dermal mucin. With the clinical presentation and histologic findings described, we diagnosed the patient with fibroblastic rheumatism (FR). To date, the patient's condition has stabilized overall with skin lesions fading and minimal to no joint pain. Current therapies include adalimumab, mycophenolate mofetil 500 mg 3 times daily, and low-dose prednisone.

Fibroblastic rheumatism is a rare arthropathy with cutaneous findings initially described by Chaouat et al1 in 1980. Age of onset varies, and the condition also has been observed in pediatric patients.2 Fibroblastic rheumatism is characterized by sudden onset of firm, flesh-colored, subcutaneous nodules on periungual and periarticular surfaces.2 Neck lesions rarely are described,2-4 and cordlike plaques previously have not been reported in FR. Typically, patients develop diffusely swollen fingers, palmar thickening, sclerodactyly, and contractures. The eruption may be accompanied by Raynaud phenomenon as well as a progressive symmetric erosive arthropathy.2,5
The clinical course in FR is variable. The cutaneous findings spontaneously may regress in months to years.3,4 However, polyarthropathy often is destructive and progresses to disability.3 Response to therapy has been unpredictable, and the following treatments have been tried, generally with poor efficacy: aspirin, nonsteroidal anti-inflammatory drugs, hydroxychloroquine, colchicine, methotrexate, prednisone, infliximab, D-penicillamine, interferon alfa, and intensive physical therapy.2-4,6 Histologic characteristics may include thickened collagen bundles along with a fibroblastic and myofibroblastic proliferation. Elastic fibers may be decreased or absent.2,3,5
Clinical and histologic features in FR may mimic other entities; thus, clinical pathological correlation is essential in determining the correct diagnosis. Considerations in the differential diagnoses include multicentric reticulohistiocytosis (MRH), palisaded neutrophilic and granulomatous dermatitis, and scleroderma.
In MRH, a symmetric erosive arthritis of mainly distal interphalangeal joints typically precedes the cutaneous disease. Occurrence of arthritis mutilans is reported in approximately half of patients.4 Cutaneous manifestations typically include the presence of coral bead-like papules and nodules over the dorsal aspect of the hands, face, and neck. Unlike FR, MRH has a concomitant autoimmune disease in up to 20% of cases and an associated malignancy in up to 31% of cases, with breast and ovarian carcinomas most common. On histology, MRH is characterized by a nodular infiltrate of histiocytes and multinucleated giant cells with eosinophilic ground-glass cytoplasm.4 No notable collagen changes or fibroblastic proliferations typically are present.
Palisaded neutrophilic and granulomatous dermatitis, usually associated with rheumatoid arthritis or connective tissue disease, classically presents as annular plaques and indurated linear bands over the trunk and extremities. However, its clinical presentation is quite variable and may include pink to violaceous urticarialike; livedoid-appearing; or nonspecific papules, plaques, or nodules. Histology in palisaded neutrophilic and granulomatous dermatitis shows a dense dermal neutrophilic infiltrate associated with interstitial histiocytes having a palisading arrangement around degenerated collagen.7 No fibroblastic proliferation typically is present.
Scleroderma can be distinguished based on additional clinical and laboratory findings as well as histology showing thickened collagen bundles without fibroblastic proliferation.2 The histologic findings also may suggest inclusion of dermatofibroma or a scar in the differential diagnosis, though the clinical presentation of these entities would not support these diagnoses.
Acknowledgments
We thank the patient for granting permission to share this information. We also thank Sheng Chen, MD, PhD (Lake Success, New York), for his dermatopathological contributions to the case.
The Diagnosis: Fibroblastic Rheumatism
Routine histologic sections stained with hematoxylin and eosin demonstrated a noncircumscribed dermal proliferation of fibroblasts and myofibroblasts with thickened collagen bundles (Figure, A and B). Focally fragmented elastin fibers were noted with Verhoeff elastic tissue stain. Alcian blue stain did not show increased dermal mucin. With the clinical presentation and histologic findings described, we diagnosed the patient with fibroblastic rheumatism (FR). To date, the patient's condition has stabilized overall with skin lesions fading and minimal to no joint pain. Current therapies include adalimumab, mycophenolate mofetil 500 mg 3 times daily, and low-dose prednisone.

Fibroblastic rheumatism is a rare arthropathy with cutaneous findings initially described by Chaouat et al1 in 1980. Age of onset varies, and the condition also has been observed in pediatric patients.2 Fibroblastic rheumatism is characterized by sudden onset of firm, flesh-colored, subcutaneous nodules on periungual and periarticular surfaces.2 Neck lesions rarely are described,2-4 and cordlike plaques previously have not been reported in FR. Typically, patients develop diffusely swollen fingers, palmar thickening, sclerodactyly, and contractures. The eruption may be accompanied by Raynaud phenomenon as well as a progressive symmetric erosive arthropathy.2,5
The clinical course in FR is variable. The cutaneous findings spontaneously may regress in months to years.3,4 However, polyarthropathy often is destructive and progresses to disability.3 Response to therapy has been unpredictable, and the following treatments have been tried, generally with poor efficacy: aspirin, nonsteroidal anti-inflammatory drugs, hydroxychloroquine, colchicine, methotrexate, prednisone, infliximab, D-penicillamine, interferon alfa, and intensive physical therapy.2-4,6 Histologic characteristics may include thickened collagen bundles along with a fibroblastic and myofibroblastic proliferation. Elastic fibers may be decreased or absent.2,3,5
Clinical and histologic features in FR may mimic other entities; thus, clinical pathological correlation is essential in determining the correct diagnosis. Considerations in the differential diagnoses include multicentric reticulohistiocytosis (MRH), palisaded neutrophilic and granulomatous dermatitis, and scleroderma.
In MRH, a symmetric erosive arthritis of mainly distal interphalangeal joints typically precedes the cutaneous disease. Occurrence of arthritis mutilans is reported in approximately half of patients.4 Cutaneous manifestations typically include the presence of coral bead-like papules and nodules over the dorsal aspect of the hands, face, and neck. Unlike FR, MRH has a concomitant autoimmune disease in up to 20% of cases and an associated malignancy in up to 31% of cases, with breast and ovarian carcinomas most common. On histology, MRH is characterized by a nodular infiltrate of histiocytes and multinucleated giant cells with eosinophilic ground-glass cytoplasm.4 No notable collagen changes or fibroblastic proliferations typically are present.
Palisaded neutrophilic and granulomatous dermatitis, usually associated with rheumatoid arthritis or connective tissue disease, classically presents as annular plaques and indurated linear bands over the trunk and extremities. However, its clinical presentation is quite variable and may include pink to violaceous urticarialike; livedoid-appearing; or nonspecific papules, plaques, or nodules. Histology in palisaded neutrophilic and granulomatous dermatitis shows a dense dermal neutrophilic infiltrate associated with interstitial histiocytes having a palisading arrangement around degenerated collagen.7 No fibroblastic proliferation typically is present.
Scleroderma can be distinguished based on additional clinical and laboratory findings as well as histology showing thickened collagen bundles without fibroblastic proliferation.2 The histologic findings also may suggest inclusion of dermatofibroma or a scar in the differential diagnosis, though the clinical presentation of these entities would not support these diagnoses.
Acknowledgments
We thank the patient for granting permission to share this information. We also thank Sheng Chen, MD, PhD (Lake Success, New York), for his dermatopathological contributions to the case.
- Chaouat Y, Aron-Brunetiere R, Faures B, et al. Une nouvelle entité: le rhumatisme fibroblastique. a propos d'une observation [in French]. Rev Rhum Mal Osteoartic. 1980;47:34-35.
- Jurado SA, Alvin GG, Selim MA, et al. Fibroblastic rheumatism: a report of 4 cases with potential therapeutic implications. J Am Acad Dermatol. 2012;66:959-965.
- Colonna L, Barbieri C, Di Lella G, et al. Fibroblastic rheumatism: a case without rheumatological symptoms. Acta Derm Venereol. 2002;82:200-203.
- Trotta F, Colina M. Multicentric reticulohistiocytosis and fibroblastic rheumatism. Best Pract Res Clin Rheumatol. 2012;26:543-557.
- Lee JM, Sundel RP, Liang MG. Fibroblastic rheumatism: case report and review of the literature. Pediatr Dermatol. 2002;19:532-535.
- Kluger N, Dumas-Tesici A, Hamel D, et al. Fibroblastic rheumatism: fibromatosis rather than non-Langerhans cell histiocytosis. J Cutan Pathol. 2010;37:587-592.
- Stephenson SR, Campbell M, Dre GS, et al. Palisaded neutrophilic and granulomatous dermatitis presenting in a patient with rheumatoid arthritis on adalimumab. J Cutan Pathol. 2011;38:644-648.
- Chaouat Y, Aron-Brunetiere R, Faures B, et al. Une nouvelle entité: le rhumatisme fibroblastique. a propos d'une observation [in French]. Rev Rhum Mal Osteoartic. 1980;47:34-35.
- Jurado SA, Alvin GG, Selim MA, et al. Fibroblastic rheumatism: a report of 4 cases with potential therapeutic implications. J Am Acad Dermatol. 2012;66:959-965.
- Colonna L, Barbieri C, Di Lella G, et al. Fibroblastic rheumatism: a case without rheumatological symptoms. Acta Derm Venereol. 2002;82:200-203.
- Trotta F, Colina M. Multicentric reticulohistiocytosis and fibroblastic rheumatism. Best Pract Res Clin Rheumatol. 2012;26:543-557.
- Lee JM, Sundel RP, Liang MG. Fibroblastic rheumatism: case report and review of the literature. Pediatr Dermatol. 2002;19:532-535.
- Kluger N, Dumas-Tesici A, Hamel D, et al. Fibroblastic rheumatism: fibromatosis rather than non-Langerhans cell histiocytosis. J Cutan Pathol. 2010;37:587-592.
- Stephenson SR, Campbell M, Dre GS, et al. Palisaded neutrophilic and granulomatous dermatitis presenting in a patient with rheumatoid arthritis on adalimumab. J Cutan Pathol. 2011;38:644-648.
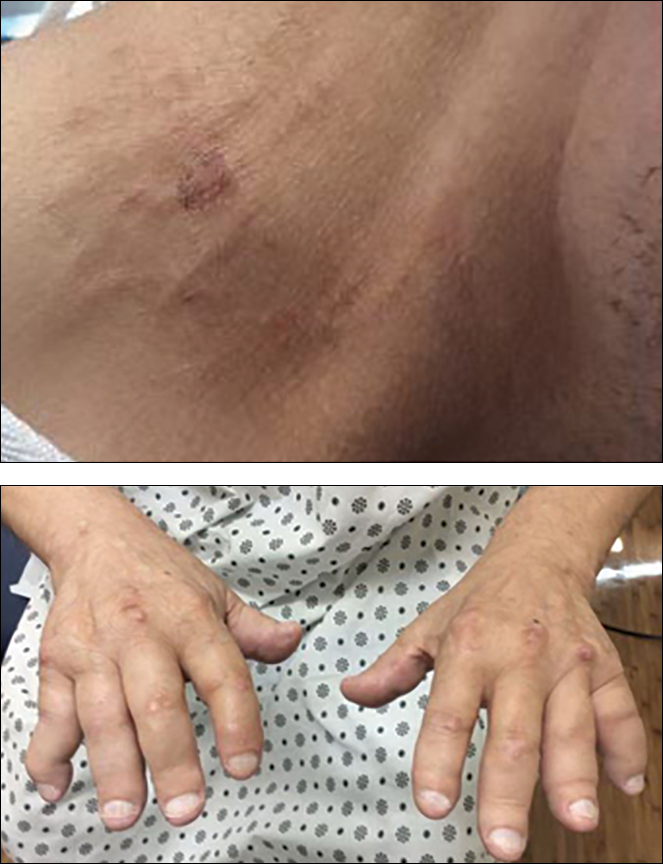
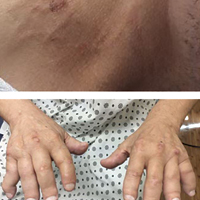
A 67-year-old man presented with asymptomatic plaques on the neck of 4 months' duration and nodules scattered over the hands, elbows, ears, and forehead of 3 years' duration. The eruption was associated with progressive thickening and contractures of the fingers, hand morning stiffness lasting less than 45 minutes, and Raynaud phenomenon. Physical examination revealed flesh-colored, firm, cordlike plaques on the neck bilaterally (top), with firm subcutaneous nodules on the helix and antihelix of the ears, forehead, elbows, and on the dorsal and ventral aspects of the hands (bottom). The largest nodules were approximately 5 cm. All fingers and first toes were thickened and firm with few contractile bands on the fingers. The patient had a persistently elevated erythrocyte sedimentation rate (80 mm/h)(reference range, 0-20 mm/h) and C-reactive protein level (3.27 mg/dL)(reference range, 0.00-0.40 mg/dL). Serologic workup was remarkable only for an antinuclear antibody titer of 1:80 (speckled). Plain radiographs confirmed an erosive arthropathy of the hands and feet. Erosions on the hands predominantly involved distal interphalangeal articulations, as well as, to a lesser extent, the proximal interphalangeal articulations, carpus, and the left distal radius. Erosive changes on the feet involved metatarsophalangeal, proximal interphalangeal, and distal interphalangeal articulations. Biopsies from the neck were performed for histopathologic correlation.
In the Face of It All
A 59 year‐old man was sent from urgent care clinic to the emergency room for further evaluation because of 1 month of diarrhea and an acute elevation in his serum creatinine.
Whereas acute diarrhea is commonly due to a self‐limited and often unspecified infection, diarrhea that extends beyond 23 weeks (chronic) warrants consideration of malabsorptive, inflammatory, infectious, and malignant processes. The acute renal failure likely is a consequence of dehydration, but the possibility of simultaneous gastrointestinal and renal involvement from a systemic process (eg, vasculitis) must be considered.
The patient's diarrhea began 1 month prior, shortly after having a milkshake at a fast food restaurant. The diarrhea was initially watery, occurred 8‐10 times per day, occasionally awakened him at night, and was associated with nausea. There was no mucus, blood, or steatorrhea until 1 day prior to presentation, when he developed epigastric pain and bloody stools. He denied any recent travel outside of Northern California and had no sick contacts. He had lost 10 pounds over the preceding month. He denied fevers, chills, vomiting, or jaundice, and had not taken antibiotics recently.
In the setting of chronic diarrhea, unintentional weight loss is an alarm feature but does not narrow the diagnostic possibilities significantly. The appearance of blood and pain on a single day after 1 month of symptoms renders their diagnostic value uncertain. For instance, rectal or hemorrhoidal bleeding would be a common occurrence after 1 month of frequent defecation. Sustained bloody stools might be seen in any form of erosive luminal disease, such as infection, inflammatory bowel disease, or neoplasm. Pain is compatible with inflammatory bowel disease, obstructing neoplasms, infections, or ischemia (eg, vasculitis). There are no fever or chills to support infection, and common gram‐negative enteric pathogens (such as Salmonella, Campylobacter, and Yersinia) usually do not produce symptoms for such an extended period. He has not taken antibiotics, which would predispose him to infection with Clostridum difficile, and he has no obvious exposure to parasites such as Entamoeba.
The patient had diabetes mellitus with microalbuminuria, chronic obstructive pulmonary disease, hypertension, hyperlipidemia, chronic low back pain, and gastritis, and had undergone a Billroth II procedure for a perforated gastric ulcer in the remote past. His medications included omeprazole, insulin glargine, simvastatin, lisinopril, amlodipine, and albuterol and beclomethasone metered‐dose inhalers. He had been married for 31 years, lived at home with his wife, was a former rigger in a shipyard and was on disability for chronic low back pain. He denied alcohol or intravenous drug use but had quit tobacco 5 years prior after more than 40 pack‐years of smoking. He had three healthy adult children and there was no family history of cancer, liver disease, or inflammatory bowel disease. There was no history of sexually transmitted diseases or unprotected sexual intercourse.
Bacterial overgrowth in the blind loop following a Billroth II operation can lead to malabsorption, but the diarrhea would not begin so abruptly this long after surgery. Medications are common causes of diarrhea. Proton‐pump inhibitors, by reducing gastric acidity, confer an increased risk of bacterial enteritis; they also are a risk factor for C difficile. Lisinopril may cause bowel angioedema months or years after initiation. Occult laxative use is a well‐recognized cause of chronic diarrhea and should also be considered. The most relevant element of his social history is the prolonged smoking and the attendant risk of cancer, although diarrhea is a rare paraneoplastic phenomenon.
On exam, temperature was 36.6C, blood pressure 125/78, pulse 88, respiratory rate 16 per minute, and oxygen saturation 97% while breathing room air. There was temporal wasting and mild scleral icterus, but no jaundice. Lungs were clear to auscultation and heart was regular in rate and rhythm without murmurs or gallops. There was no jugular venous distention. A large abdominal midline scar was present, bowel sounds were normoactive, and the abdomen was soft, nontender, and nondistended. The hard was regular in rate and rhythm the liver edge was 6 cm below costal margin; there was no splenomegaly. The patient was alert and oriented, with a normal neurologic exam.
The liver generally enlarges because of acute inflammation, congestion, or infiltration. Infiltration can be due to tumors, infections, hemochromatosis, amyloidosis, or sarcoidosis. A normal cardiac exam argues against hepatic congestion from right‐sided heart failure or pericardial disease.
The key elements of the case are diarrhea and hepatomegaly. Inflammatory bowel disease can be accompanied by sclerosing cholangitis, but this should not enlarge the liver. Mycobacterial infections and syphilis can infiltrate the liver and intestinal mucosa, causing diarrhea, but he lacks typical risk factors.
Malignancy is an increasing concern. Colon cancer commonly metastasizes to the liver and can occasionally be intensely secretory. Pancreatic cancer could account for these symptoms, especially if pancreatic exocrine insufficiency caused malabsorption. Various rare neuroendocrine tumors that arise in the pancreas can cause secretory diarrheas and liver metastases, such as carcinoid, VIPoma, and Zollinger‐Ellison syndrome.
Laboratory results revealed a serum sodium of 143 mmol/L, potassium 4.7 mmol/L, chloride 110 mmol/L, bicarbonate 25 mmol/L, urea nitrogen 24 mg/dL, and creatinine 2.5 mg/dL (baseline had been 1.2 mg/dL 2 months previously). Serum glucose was 108 mg/dL and calcium was 8.8 mg/dL. The total white blood cell count was 9300 per mm3 with a normal differential, hemoglobin was 14.4 g/dL, mean corpuscular volume was 87 fL, and the platelet count was normal. Total bilirubin was 3.7 mg/dL, and direct bilirubin was 3.1 mg/dL. Aspartate aminotransferase (AST) was 122 U/L (normal range, 831), alanine aminotransferase (ALT) 79 U/L (normal range, 731), alkaline phosphatase 1591 U/L (normal range, 39117), and gamma‐glutamyltransferase (GGT) 980 U/L (normal range, 57). Serum albumin was 2.5 mg/dL, prothrombin time was 16.4 seconds, and international normalized ratio (INR) was 1.6.
Urinalysis was normal except for trace hemoglobin, small bilirubin, and 70 mg/dL of protein; specific gravity was 1.007. Urine microscopy demonstrated no cells or casts. The ratio of protein to creatinine on a spot urine sample was less than 1. Chest x‐ray was normal. The electrocardiogram demonstrated sinus rhythm with an old right bundle branch block and normal QRS voltages.
The disproportionate elevation in alkaline phosphatase points to an infiltrative hepatopathy from a cancer originating in the gastrointestinal tract or infection. Other infiltrative processes such as sarcoidosis or amyloidosis usually have evidence of disease elsewhere before hepatic disease becomes apparent.
Mild proteinuria may be explained by diabetes. The specific gravity of 1.007 is atypical for dehydration and could suggest ischemic tubular injury. Although intrinsic renal diseases must continue to be entertained, hypovolemia (compounded by angiotensin‐converting enzyme [ACE] inhibitor use) is the leading explanation in light of the nondiagnostic renal studies. The preserved hemoglobin may simply indicate dehydration, but otherwise is somewhat reassuring in the context of bloody diarrhea.
The patient was admitted to the hospital. Three stool samples returned negative for C difficile toxin. No white cells were detected in the stool, and no ova or parasites were detected. Stool culture was negative for routine bacterial pathogens and for E coli O157. Tests for HIV and antinuclear antibodies (ANAs) and serologies for hepatitis A, B, and C were negative. Abdominal ultrasound demonstrated no intra‐ or extrahepatic bile duct dilatation; no hepatic masses were seen. Kidneys were normal in size and appearance without hydronephrosis. Computed tomography (CT) of the abdomen without intravenous contrast revealed normal‐appearing liver (with a 12‐cm span), spleen, biliary ducts, and pancreas, and there was no intra‐abdominal adenopathy.
The stool studies point away from infectious colitis. Infiltrative processes of the liver, including metastases, lymphoma, tuberculosis, syphilis, amyloidosis, and sarcoidosis, can be microscopic and therefore evade detection by ultrasound and CT scan. In conditions such as these, endoscopic retrograde cholangiopanccreatography/magnetic resonance cholangiopancreatography (ERCP/MRCP) or liver biopsy may be required. The CT is limited without contrast but does not suggest extrahepatic disease in the abdomen.
MRCP was performed, but was a technically suboptimal study due to the presence of ascites. The serum creatinine improved to 1.4 mg/dL over the next 4 days, and the patient's diarrhea decreased to two bowel movements daily with the use of loperamide. The patient was discharged home with outpatient gastroenterology follow‐up planned to discuss further evaluation of the abnormal liver enzymes.
Prior to being seen in the Gastroenterology Clinic, the patient's nonbloody diarrhea worsened. He felt weaker and continued to lose weight. He also noted new onset of bilateral lower face numbness and burning, which was followed by swelling of his lower lip 12 hours later. He returned to the hospital.
On examination, he was afebrile. His lower lip was markedly swollen and was drooping from his face. He could not move the lip to close his mouth. The upper lip and tongue were normal size and moved without restriction. Facial sensation was intact, but there was weakness when he attempted to wrinkle both of his brows and close his eyelids. The rest of his physical examination was unchanged.
The serum creatinine had risen to 3.6 mg/dL, and the complete blood count remained normal. Serum total bilirubin was 4.6 mg/dL, AST 87 U/L, ALT 76 U/L, and alkaline phosphatase 1910 U/L. The 24‐hour urine protein measurement was 86 mg.
Lip swelling suggests angioedema. ACE inhibitors are frequent offenders, and it would be important to know whether his lisinopril was restarted at discharge. ACE‐inhibitor angioedema can also affect the intestine, causing abdominal pain and diarrhea, but does not cause a systemic wasting illness or infiltrative hepatopathy. The difficulty moving the lip may reflect the physical effects of swelling, but generalized facial weakness supports a cranial neuropathy. Basilar meningitis may produce multiple cranial neuropathies, the etiologies of which are quite similar to the previously mentioned causes of infiltrative liver disease: sarcoidosis, syphilis, tuberculosis, or lymphoma.
The patient had not resumed lisinopril since his prior hospitalization. The lower lip swelling and paralysis persisted, and new sensory paresthesias developed over the right side of his chin. A consulting neurologist found normal language and speech and moderate dysarthria. Cranial nerve exam was normal except bilateral lower motor neuron facial nerve palsy was noted with bilateral facial droop, reduced strength of eyelid closure, and diminished forehead movement bilaterally; facial sensation was normal. Extremity motor exam revealed proximal iliopsoas muscle weakness bilaterally rated as 4/5 and was otherwise normal. Sensation to pinprick was diminished in a stocking/glove distribution. Deep‐tendon reflexes were normal and plantar response was down‐going bilaterally. Coordination was intact, Romberg was negative, and gait was slowed due to weakness.
Over the next several days, the patient continued to have diarrhea and facial symptoms. The serum total bilirubin increased to 14 mg/dL, alkaline phosphatase rose above 2,000 U/L, and serum creatinine increased to 5.5 mg/dL. Noncontrast CT scan of the head was normal.
Along with a mild peripheral sensory neuropathy, the exam indicates bilateral palsies of the facial nerve. Lyme disease is a frequent etiology, but this patient is not from an endemic area. I am most suspicious of bilateral infiltration of cranial nerve VII. I am thinking analogically to the numb chin syndrome, wherein lymphoma or breast cancer infiltration along the mental branch of V3 causes sensory loss, and perhaps these disorders can produce infiltrative facial neuropathy. At this point I am most concerned about lymphomatous meningitis with cranial nerve involvement. Cerebrospinal fluid (CSF) analysis (including cytology) would be informative.
Lumbar puncture demonstrated clear CSF with one white blood cell per mm3 and no red blood cells. Glucose was normal, and protein was 95.5 (normal range, 15‐45 mg/dL). Gram stain and culture for bacteria were negative, as were polymerase chain reaction (PCR) testing for herpes simplex virus, mycobacterial and fungal stains and cultures, and cytology. Transthoracic echocardiogram demonstrated severe concentric left ventricular (LV) hypertrophy, normal LV systolic function, and impaired LV relaxation. CT scan of the chest identified no adenopathy or other abnormality.
The CSF analysis does not support basilar meningitis, although the cytoalbuminologic dissociation makes me wonder whether there is some intrathecal antibody production or an autoimmune process we have yet to uncover. The absence of lymphadenopathy anywhere in the body and the negative CSF cytology now point away from lymphoma. As the case for lymphoma or an infection diminishes, systemic amyloidosis rises to the top of possibilities in this afebrile man who is losing weight, has infiltrative liver and nerve abnormalities, renal failure, cardiac enlargement, and suspected gastrointestinal luminal abnormality. Although the echocardiographic findings are most likely explained by hypertension, they are compatible with amyloid infiltration. A tissue specimen is needed, and either colonoscopy or liver biopsy should be suitable.
A pathologist performed a fat pad biopsy that demonstrated scant congophilic and birefringent material associated with blood vessels, suggestive of amyloid (Fig. 1). Colonoscopy demonstrated normal mucosa, and a rectal biopsy revealed congophilic material within the blood vessels consistent with amyloid (Fig. 2). No monoclonal band was present on serum protein electrophoresis. Urine protein electrophoresis identified a homogenous band in the gamma region, and urine kappa and lambda free light chains were increased: kappa was 10.7 mg/dL (normal range, 2), and lambda was 4.25 mg/dL (normal range, 2).
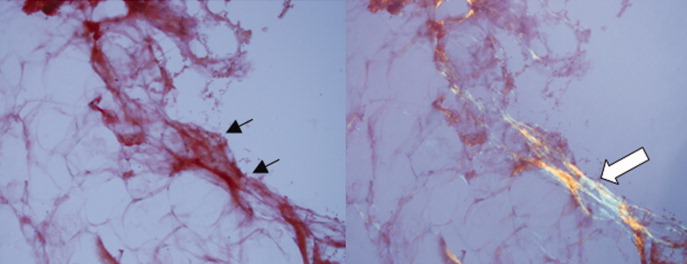
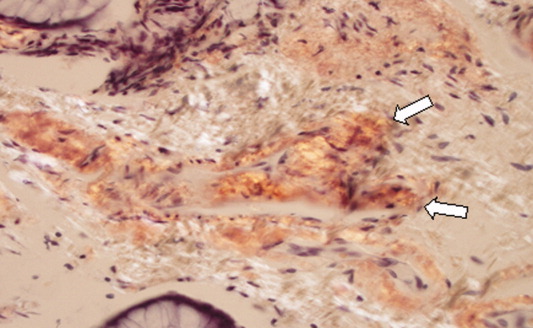
After extensive discussion among the patient, his wife, and a palliative care physician, the patient declined chemotherapy and elected to go home. Two days after discharge (7 weeks after his initial admission for diarrhea) he died in his sleep at home. Permission for a postmortem examination was not granted.
Discussion
Amyloidosis refers to abnormal extracellular deposition of fibril. There are many types of amyloidosis including primary amyloidosis (AL amyloidosis), secondary amyloidosis (AA amyloidosis), and hereditary causes. Systemic AL amyloidosis is a rare plasma cell disorder characterized by misfolding of insoluble extracellular fibrillar proteins derived from immunoglobulin light chains. These insoluble proteins typically deposit in the kidney, heart, and nervous system.1 Although the mechanism of organ dysfunction is debated, deposition of these proteins may disrupt the tissue architecture by interacting with local receptors and causing apoptosis.1
Table 1 indicates the most common findings in patients with AL amyloidosis.2 While our patient ultimately developed many common findings of AL amyloidosis, several features were atypical, including the marked hyperbilirubinemia, profound diarrhea, and bilateral facial diplegia.
| Organ Involvement | Incidence of Organ Involvement (%) | Symptoms | Signs | Laboratory/Test Finding |
|---|---|---|---|---|
| ||||
| General | Malaise, weight loss | |||
| Renal | 33 | Fatigue | Peripheral edema | Proteinuria with or without renal insufficiency, pleural effusion, hypercholesterolemia |
| Cardiac | 20 | Palpitations, dyspnea | Elevated jugular venous pressure, S3, peripheral edema, hepatomegaly | Low‐voltage or atrial fibrillation on electrocardiogram; echocardiogram: thickened ventricles, dilated atria |
| Neurological | 20 | Paresthesias, numbness, weakness, autonomic insufficiency | Carpal tunnel syndrome, postural hypotension | |
| Gastrointestinal and Hepatic | 16 | Diarrhea, nausea, weight loss | Macroglossia, hepatomegaly | Elevated alkaline phosphatase |
| Hematology | Rare | Bleeding | Periorbital purpura (raccoon eyes) | Prolonged prothrombin time, Factor X deficiency |
Up to 70% of patients with amyloidosis will have detectable liver deposits, typically involving portal vessels, portal stroma, central vein, and sinusoidal parenchyma.3 Clinically overt hepatic dysfunction from amyloid is less frequent,4 and the most characteristic findings are hepatomegaly with a markedly elevated serum alkaline phosphatase concentration; jaundice is rare. Palpable hepatic enlargement without abnormal liver enzymes should be interpreted with caution. The finding of a palpable liver edge correlates poorly with frank hepatomegaly, with a positive likelihood ratio of just 1.7.5 In the patient under discussion, suspected hepatomegaly was not confirmed on a subsequent CT scan. Nonetheless, the elevated alkaline phosphatase represented an important clue to potential infiltrative liver disease. In a series of amyloidosis patients from the Mayo Clinic, 81% had hepatomegaly on physical exam, and the mean alkaline phosphatase level was 1,029 U/L (normal, 250 U/L), while the mean serum bilirubin and AST levels were only modestly elevated, at 3.2 mg/dL and 72 U/L respectively. The prothrombin time was prolonged in 35% of patients.
Upper gastrointestinal tract involvement by AL amyloid may be found in up to a third of cases at autopsy, but clinically significant gastrointestinal features are seen in fewer than 5% of patients.6 Predominant intestinal manifestations are unintentional weight loss (average 7 kg) and diarrhea, nonspecific features that result in delayed diagnosis for a median of 7 months after symptom onset.7 Diarrhea in AL amyloid may stem from several mechanisms: small intestine mucosal infiltration, steatorrhea from pancreatic insufficiency, autonomic neuropathy leading to pseudo‐obstruction and bacterial overgrowth, bile acid malabsorption, or rapid transit time. Diarrhea in AL amyloid is often resistant to treatment and may be the primary cause of death.7
Systemic amyloidosis commonly produces peripheral neuropathies. Involvement of small unmyelinated fibers causes paresthesias and progressive sensory loss in a pattern that is usually distal, symmetric, and progressive.6, 9 Our patient presented with bilateral sensory paresthesias of the chin, suggesting the numb chin syndrome (NCS). NCS is characterized by facial numbness along the distribution of the mental branch of the trigeminal nerve. While dental disorders and infiltration from malignant tumors (mostly lung and breast cancer) account for most cases, amyloidosis and other infiltrative disorders are known to cause NCS as well.10, 11 Our patient's sensory paresthesias may have represented amyloid infiltration of peripheral nerves.
With the exception of carpal tunnel syndrome, motor or cranial neuropathy is uncommon in amyloid, and when present usually heralds advanced disease.12 Descriptions of bilateral facial weakness, also known as facial diplegia, from amyloidosis are limited to case reports.1315 Other causes of this rare finding include sarcoidosis, Guillain‐Barr syndrome, and Lyme disease.16
The diagnosis of primary amyloidosis requires histologic evidence of amyloid from a tissue biopsy specimen (demonstrating positive Congo red staining and pathognomonic green birefringence under cross‐polarized light microscopy), and the presence of a clonal plasma cell disorder. While biopsy of an affected organ is diagnostic, more easily obtained samples such as fat pad biopsy and rectal biopsy yield positive results in up to 80% of cases.2 Serum and urine protein electrophoresis with immunofixation identify an underlying plasma cell disorder in 90% of cases of primary amyloidosis. When these tests are inconclusive, serum or urine free light chain assays or bone marrow aspirate and biopsy are useful aids to detect underlying plasma cell dyscrasia.2 AL amyloidosis is a progressive disease with median survival of about 12 years.8 Poorer prognosis is associated with substantial echocardiographic findings, autonomic neuropathy, and liver involvement.2 Hyperbilirubinemia is associated with a poor prognosis, with a median survival of 8.5 months.4 Proteinuria or peripheral neuropathy portends a less ominous course.6
Treatment goals include reducing production and deposition of fibril proteins and contending with organ dysfunction (eg, congestive heart failure [CHF] management). Selected patients with AL amyloidosis may be candidates for high‐dose melphalan and autologous stem cell transplantation.
It would not be reasonable for clinicians to suspect amyloidosis in cases of diarrhea until two conditions are met: 1) the absence of evidence for the typical etiologies of diarrhea; and 2) the evolving picture of an infiltrative disorder. The latter was heralded by the elevated alkaline phosphatase, and was supported by the subsequent multiorgan involvement. Conceptualizing the disease as infiltrative still required a diligent exclusion of infection and invasive tumor cells, which invade disparate organs far more commonly than amyloidosis. Their absence and the organ pattern that is typical of AL amyloidosis (heart, kidney, and peripheral nerve involvement) allowed the discussant to reason by analogy that amyloidosis was also responsible for the most symptomatic phenomena, namely, the diarrhea and facial diplegia (and numb chin syndrome).
Key Teaching Points
-
Hospitalists should consider systemic amyloidosis in cases of unexplained diarrhea when other clinical features of AL amyloidosis are present, including nephrotic syndrome with or without renal insufficiency, cardiomyopathy, peripheral neuropathy, and hepatomegaly.
-
Hepatic amyloidosis should be suspected when weight loss, hepatomegaly, and elevated alkaline phosphatase are present. Although jaundice is rare in amyloidosis, liver involvement and hyperbilirubinemia portend a poorer prognosis.
-
Numb chin syndrome and bilateral facial diplegia are rare manifestations of AL amyloid deposition in peripheral nerves.
- ,.Molecular mechanisms of amyloidosis.N Engl J Med.2003;349(6):583–596.
- Guidelines Working Group of UK Myeloma Forum; British Committee for Standards in Haematology, British Society for Haematology.Guidelines on the diagnosis and management of AL amyloidosis.Br J Haematol.2004;125:681–700.
- ,.Hepatic amyloidosis: morphologic differences between systemic AL and AA types.Hum Pathol.1991;22(9):904–907.
- ,,, et al.Primary (AL) hepatic amyloidosis clinical features and natural history in 98 patients.Medicine.2003;82(5):291–298.
- .Evidence‐Based Physical Diagnosis.Philadelphia, PA:WB Saunders;2001:595–599.
- ,,, et al.Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis.Am J Hematol.2005;79:319–328.
- .Primary (AL) amyloidosis with gastrointestinal involvement.Scand J Gastroenterol.2009;44(6):708–711.
- ,.Gastrointestinal manifestations of amyloid.Am J Gastroenterol.2008;103:776–787.
- ,.Primary systemic amyloidosis: clinical and laboratory features in 474 cases.Semin Hematol.1995;32:45–59.
- ,,,,.Chin numbness: a symptom that should not be underestimated: a review of 12 cases.Am J Med Sci.2009;337:407–410.
- .Numb chin syndrome: a possible clue to serious illness.Hosp Physician.2000;54–56.
- .Autonomic peripheral neuropathy.Neurol Clin.2007;25:277–301.
- ,.Facial diplegia due to amyloidosis.South Med J.1986;79(11):1458–1459.
- ,,,,.Familial amyloidosis with cranial neuropathy and corneal lattice dystrophy.Neurology.1986;36:432–435.
- ,,.Crainal neuropathy associated with primary amyloidosis.Ann Neurol.1991;29:451–454.
- .Bilateral seventh nerve palsy: analysis of 43 cases and review of the literature.Neurology.1994;44:1198–202.
A 59 year‐old man was sent from urgent care clinic to the emergency room for further evaluation because of 1 month of diarrhea and an acute elevation in his serum creatinine.
Whereas acute diarrhea is commonly due to a self‐limited and often unspecified infection, diarrhea that extends beyond 23 weeks (chronic) warrants consideration of malabsorptive, inflammatory, infectious, and malignant processes. The acute renal failure likely is a consequence of dehydration, but the possibility of simultaneous gastrointestinal and renal involvement from a systemic process (eg, vasculitis) must be considered.
The patient's diarrhea began 1 month prior, shortly after having a milkshake at a fast food restaurant. The diarrhea was initially watery, occurred 8‐10 times per day, occasionally awakened him at night, and was associated with nausea. There was no mucus, blood, or steatorrhea until 1 day prior to presentation, when he developed epigastric pain and bloody stools. He denied any recent travel outside of Northern California and had no sick contacts. He had lost 10 pounds over the preceding month. He denied fevers, chills, vomiting, or jaundice, and had not taken antibiotics recently.
In the setting of chronic diarrhea, unintentional weight loss is an alarm feature but does not narrow the diagnostic possibilities significantly. The appearance of blood and pain on a single day after 1 month of symptoms renders their diagnostic value uncertain. For instance, rectal or hemorrhoidal bleeding would be a common occurrence after 1 month of frequent defecation. Sustained bloody stools might be seen in any form of erosive luminal disease, such as infection, inflammatory bowel disease, or neoplasm. Pain is compatible with inflammatory bowel disease, obstructing neoplasms, infections, or ischemia (eg, vasculitis). There are no fever or chills to support infection, and common gram‐negative enteric pathogens (such as Salmonella, Campylobacter, and Yersinia) usually do not produce symptoms for such an extended period. He has not taken antibiotics, which would predispose him to infection with Clostridum difficile, and he has no obvious exposure to parasites such as Entamoeba.
The patient had diabetes mellitus with microalbuminuria, chronic obstructive pulmonary disease, hypertension, hyperlipidemia, chronic low back pain, and gastritis, and had undergone a Billroth II procedure for a perforated gastric ulcer in the remote past. His medications included omeprazole, insulin glargine, simvastatin, lisinopril, amlodipine, and albuterol and beclomethasone metered‐dose inhalers. He had been married for 31 years, lived at home with his wife, was a former rigger in a shipyard and was on disability for chronic low back pain. He denied alcohol or intravenous drug use but had quit tobacco 5 years prior after more than 40 pack‐years of smoking. He had three healthy adult children and there was no family history of cancer, liver disease, or inflammatory bowel disease. There was no history of sexually transmitted diseases or unprotected sexual intercourse.
Bacterial overgrowth in the blind loop following a Billroth II operation can lead to malabsorption, but the diarrhea would not begin so abruptly this long after surgery. Medications are common causes of diarrhea. Proton‐pump inhibitors, by reducing gastric acidity, confer an increased risk of bacterial enteritis; they also are a risk factor for C difficile. Lisinopril may cause bowel angioedema months or years after initiation. Occult laxative use is a well‐recognized cause of chronic diarrhea and should also be considered. The most relevant element of his social history is the prolonged smoking and the attendant risk of cancer, although diarrhea is a rare paraneoplastic phenomenon.
On exam, temperature was 36.6C, blood pressure 125/78, pulse 88, respiratory rate 16 per minute, and oxygen saturation 97% while breathing room air. There was temporal wasting and mild scleral icterus, but no jaundice. Lungs were clear to auscultation and heart was regular in rate and rhythm without murmurs or gallops. There was no jugular venous distention. A large abdominal midline scar was present, bowel sounds were normoactive, and the abdomen was soft, nontender, and nondistended. The hard was regular in rate and rhythm the liver edge was 6 cm below costal margin; there was no splenomegaly. The patient was alert and oriented, with a normal neurologic exam.
The liver generally enlarges because of acute inflammation, congestion, or infiltration. Infiltration can be due to tumors, infections, hemochromatosis, amyloidosis, or sarcoidosis. A normal cardiac exam argues against hepatic congestion from right‐sided heart failure or pericardial disease.
The key elements of the case are diarrhea and hepatomegaly. Inflammatory bowel disease can be accompanied by sclerosing cholangitis, but this should not enlarge the liver. Mycobacterial infections and syphilis can infiltrate the liver and intestinal mucosa, causing diarrhea, but he lacks typical risk factors.
Malignancy is an increasing concern. Colon cancer commonly metastasizes to the liver and can occasionally be intensely secretory. Pancreatic cancer could account for these symptoms, especially if pancreatic exocrine insufficiency caused malabsorption. Various rare neuroendocrine tumors that arise in the pancreas can cause secretory diarrheas and liver metastases, such as carcinoid, VIPoma, and Zollinger‐Ellison syndrome.
Laboratory results revealed a serum sodium of 143 mmol/L, potassium 4.7 mmol/L, chloride 110 mmol/L, bicarbonate 25 mmol/L, urea nitrogen 24 mg/dL, and creatinine 2.5 mg/dL (baseline had been 1.2 mg/dL 2 months previously). Serum glucose was 108 mg/dL and calcium was 8.8 mg/dL. The total white blood cell count was 9300 per mm3 with a normal differential, hemoglobin was 14.4 g/dL, mean corpuscular volume was 87 fL, and the platelet count was normal. Total bilirubin was 3.7 mg/dL, and direct bilirubin was 3.1 mg/dL. Aspartate aminotransferase (AST) was 122 U/L (normal range, 831), alanine aminotransferase (ALT) 79 U/L (normal range, 731), alkaline phosphatase 1591 U/L (normal range, 39117), and gamma‐glutamyltransferase (GGT) 980 U/L (normal range, 57). Serum albumin was 2.5 mg/dL, prothrombin time was 16.4 seconds, and international normalized ratio (INR) was 1.6.
Urinalysis was normal except for trace hemoglobin, small bilirubin, and 70 mg/dL of protein; specific gravity was 1.007. Urine microscopy demonstrated no cells or casts. The ratio of protein to creatinine on a spot urine sample was less than 1. Chest x‐ray was normal. The electrocardiogram demonstrated sinus rhythm with an old right bundle branch block and normal QRS voltages.
The disproportionate elevation in alkaline phosphatase points to an infiltrative hepatopathy from a cancer originating in the gastrointestinal tract or infection. Other infiltrative processes such as sarcoidosis or amyloidosis usually have evidence of disease elsewhere before hepatic disease becomes apparent.
Mild proteinuria may be explained by diabetes. The specific gravity of 1.007 is atypical for dehydration and could suggest ischemic tubular injury. Although intrinsic renal diseases must continue to be entertained, hypovolemia (compounded by angiotensin‐converting enzyme [ACE] inhibitor use) is the leading explanation in light of the nondiagnostic renal studies. The preserved hemoglobin may simply indicate dehydration, but otherwise is somewhat reassuring in the context of bloody diarrhea.
The patient was admitted to the hospital. Three stool samples returned negative for C difficile toxin. No white cells were detected in the stool, and no ova or parasites were detected. Stool culture was negative for routine bacterial pathogens and for E coli O157. Tests for HIV and antinuclear antibodies (ANAs) and serologies for hepatitis A, B, and C were negative. Abdominal ultrasound demonstrated no intra‐ or extrahepatic bile duct dilatation; no hepatic masses were seen. Kidneys were normal in size and appearance without hydronephrosis. Computed tomography (CT) of the abdomen without intravenous contrast revealed normal‐appearing liver (with a 12‐cm span), spleen, biliary ducts, and pancreas, and there was no intra‐abdominal adenopathy.
The stool studies point away from infectious colitis. Infiltrative processes of the liver, including metastases, lymphoma, tuberculosis, syphilis, amyloidosis, and sarcoidosis, can be microscopic and therefore evade detection by ultrasound and CT scan. In conditions such as these, endoscopic retrograde cholangiopanccreatography/magnetic resonance cholangiopancreatography (ERCP/MRCP) or liver biopsy may be required. The CT is limited without contrast but does not suggest extrahepatic disease in the abdomen.
MRCP was performed, but was a technically suboptimal study due to the presence of ascites. The serum creatinine improved to 1.4 mg/dL over the next 4 days, and the patient's diarrhea decreased to two bowel movements daily with the use of loperamide. The patient was discharged home with outpatient gastroenterology follow‐up planned to discuss further evaluation of the abnormal liver enzymes.
Prior to being seen in the Gastroenterology Clinic, the patient's nonbloody diarrhea worsened. He felt weaker and continued to lose weight. He also noted new onset of bilateral lower face numbness and burning, which was followed by swelling of his lower lip 12 hours later. He returned to the hospital.
On examination, he was afebrile. His lower lip was markedly swollen and was drooping from his face. He could not move the lip to close his mouth. The upper lip and tongue were normal size and moved without restriction. Facial sensation was intact, but there was weakness when he attempted to wrinkle both of his brows and close his eyelids. The rest of his physical examination was unchanged.
The serum creatinine had risen to 3.6 mg/dL, and the complete blood count remained normal. Serum total bilirubin was 4.6 mg/dL, AST 87 U/L, ALT 76 U/L, and alkaline phosphatase 1910 U/L. The 24‐hour urine protein measurement was 86 mg.
Lip swelling suggests angioedema. ACE inhibitors are frequent offenders, and it would be important to know whether his lisinopril was restarted at discharge. ACE‐inhibitor angioedema can also affect the intestine, causing abdominal pain and diarrhea, but does not cause a systemic wasting illness or infiltrative hepatopathy. The difficulty moving the lip may reflect the physical effects of swelling, but generalized facial weakness supports a cranial neuropathy. Basilar meningitis may produce multiple cranial neuropathies, the etiologies of which are quite similar to the previously mentioned causes of infiltrative liver disease: sarcoidosis, syphilis, tuberculosis, or lymphoma.
The patient had not resumed lisinopril since his prior hospitalization. The lower lip swelling and paralysis persisted, and new sensory paresthesias developed over the right side of his chin. A consulting neurologist found normal language and speech and moderate dysarthria. Cranial nerve exam was normal except bilateral lower motor neuron facial nerve palsy was noted with bilateral facial droop, reduced strength of eyelid closure, and diminished forehead movement bilaterally; facial sensation was normal. Extremity motor exam revealed proximal iliopsoas muscle weakness bilaterally rated as 4/5 and was otherwise normal. Sensation to pinprick was diminished in a stocking/glove distribution. Deep‐tendon reflexes were normal and plantar response was down‐going bilaterally. Coordination was intact, Romberg was negative, and gait was slowed due to weakness.
Over the next several days, the patient continued to have diarrhea and facial symptoms. The serum total bilirubin increased to 14 mg/dL, alkaline phosphatase rose above 2,000 U/L, and serum creatinine increased to 5.5 mg/dL. Noncontrast CT scan of the head was normal.
Along with a mild peripheral sensory neuropathy, the exam indicates bilateral palsies of the facial nerve. Lyme disease is a frequent etiology, but this patient is not from an endemic area. I am most suspicious of bilateral infiltration of cranial nerve VII. I am thinking analogically to the numb chin syndrome, wherein lymphoma or breast cancer infiltration along the mental branch of V3 causes sensory loss, and perhaps these disorders can produce infiltrative facial neuropathy. At this point I am most concerned about lymphomatous meningitis with cranial nerve involvement. Cerebrospinal fluid (CSF) analysis (including cytology) would be informative.
Lumbar puncture demonstrated clear CSF with one white blood cell per mm3 and no red blood cells. Glucose was normal, and protein was 95.5 (normal range, 15‐45 mg/dL). Gram stain and culture for bacteria were negative, as were polymerase chain reaction (PCR) testing for herpes simplex virus, mycobacterial and fungal stains and cultures, and cytology. Transthoracic echocardiogram demonstrated severe concentric left ventricular (LV) hypertrophy, normal LV systolic function, and impaired LV relaxation. CT scan of the chest identified no adenopathy or other abnormality.
The CSF analysis does not support basilar meningitis, although the cytoalbuminologic dissociation makes me wonder whether there is some intrathecal antibody production or an autoimmune process we have yet to uncover. The absence of lymphadenopathy anywhere in the body and the negative CSF cytology now point away from lymphoma. As the case for lymphoma or an infection diminishes, systemic amyloidosis rises to the top of possibilities in this afebrile man who is losing weight, has infiltrative liver and nerve abnormalities, renal failure, cardiac enlargement, and suspected gastrointestinal luminal abnormality. Although the echocardiographic findings are most likely explained by hypertension, they are compatible with amyloid infiltration. A tissue specimen is needed, and either colonoscopy or liver biopsy should be suitable.
A pathologist performed a fat pad biopsy that demonstrated scant congophilic and birefringent material associated with blood vessels, suggestive of amyloid (Fig. 1). Colonoscopy demonstrated normal mucosa, and a rectal biopsy revealed congophilic material within the blood vessels consistent with amyloid (Fig. 2). No monoclonal band was present on serum protein electrophoresis. Urine protein electrophoresis identified a homogenous band in the gamma region, and urine kappa and lambda free light chains were increased: kappa was 10.7 mg/dL (normal range, 2), and lambda was 4.25 mg/dL (normal range, 2).


After extensive discussion among the patient, his wife, and a palliative care physician, the patient declined chemotherapy and elected to go home. Two days after discharge (7 weeks after his initial admission for diarrhea) he died in his sleep at home. Permission for a postmortem examination was not granted.
Discussion
Amyloidosis refers to abnormal extracellular deposition of fibril. There are many types of amyloidosis including primary amyloidosis (AL amyloidosis), secondary amyloidosis (AA amyloidosis), and hereditary causes. Systemic AL amyloidosis is a rare plasma cell disorder characterized by misfolding of insoluble extracellular fibrillar proteins derived from immunoglobulin light chains. These insoluble proteins typically deposit in the kidney, heart, and nervous system.1 Although the mechanism of organ dysfunction is debated, deposition of these proteins may disrupt the tissue architecture by interacting with local receptors and causing apoptosis.1
Table 1 indicates the most common findings in patients with AL amyloidosis.2 While our patient ultimately developed many common findings of AL amyloidosis, several features were atypical, including the marked hyperbilirubinemia, profound diarrhea, and bilateral facial diplegia.
| Organ Involvement | Incidence of Organ Involvement (%) | Symptoms | Signs | Laboratory/Test Finding |
|---|---|---|---|---|
| ||||
| General | Malaise, weight loss | |||
| Renal | 33 | Fatigue | Peripheral edema | Proteinuria with or without renal insufficiency, pleural effusion, hypercholesterolemia |
| Cardiac | 20 | Palpitations, dyspnea | Elevated jugular venous pressure, S3, peripheral edema, hepatomegaly | Low‐voltage or atrial fibrillation on electrocardiogram; echocardiogram: thickened ventricles, dilated atria |
| Neurological | 20 | Paresthesias, numbness, weakness, autonomic insufficiency | Carpal tunnel syndrome, postural hypotension | |
| Gastrointestinal and Hepatic | 16 | Diarrhea, nausea, weight loss | Macroglossia, hepatomegaly | Elevated alkaline phosphatase |
| Hematology | Rare | Bleeding | Periorbital purpura (raccoon eyes) | Prolonged prothrombin time, Factor X deficiency |
Up to 70% of patients with amyloidosis will have detectable liver deposits, typically involving portal vessels, portal stroma, central vein, and sinusoidal parenchyma.3 Clinically overt hepatic dysfunction from amyloid is less frequent,4 and the most characteristic findings are hepatomegaly with a markedly elevated serum alkaline phosphatase concentration; jaundice is rare. Palpable hepatic enlargement without abnormal liver enzymes should be interpreted with caution. The finding of a palpable liver edge correlates poorly with frank hepatomegaly, with a positive likelihood ratio of just 1.7.5 In the patient under discussion, suspected hepatomegaly was not confirmed on a subsequent CT scan. Nonetheless, the elevated alkaline phosphatase represented an important clue to potential infiltrative liver disease. In a series of amyloidosis patients from the Mayo Clinic, 81% had hepatomegaly on physical exam, and the mean alkaline phosphatase level was 1,029 U/L (normal, 250 U/L), while the mean serum bilirubin and AST levels were only modestly elevated, at 3.2 mg/dL and 72 U/L respectively. The prothrombin time was prolonged in 35% of patients.
Upper gastrointestinal tract involvement by AL amyloid may be found in up to a third of cases at autopsy, but clinically significant gastrointestinal features are seen in fewer than 5% of patients.6 Predominant intestinal manifestations are unintentional weight loss (average 7 kg) and diarrhea, nonspecific features that result in delayed diagnosis for a median of 7 months after symptom onset.7 Diarrhea in AL amyloid may stem from several mechanisms: small intestine mucosal infiltration, steatorrhea from pancreatic insufficiency, autonomic neuropathy leading to pseudo‐obstruction and bacterial overgrowth, bile acid malabsorption, or rapid transit time. Diarrhea in AL amyloid is often resistant to treatment and may be the primary cause of death.7
Systemic amyloidosis commonly produces peripheral neuropathies. Involvement of small unmyelinated fibers causes paresthesias and progressive sensory loss in a pattern that is usually distal, symmetric, and progressive.6, 9 Our patient presented with bilateral sensory paresthesias of the chin, suggesting the numb chin syndrome (NCS). NCS is characterized by facial numbness along the distribution of the mental branch of the trigeminal nerve. While dental disorders and infiltration from malignant tumors (mostly lung and breast cancer) account for most cases, amyloidosis and other infiltrative disorders are known to cause NCS as well.10, 11 Our patient's sensory paresthesias may have represented amyloid infiltration of peripheral nerves.
With the exception of carpal tunnel syndrome, motor or cranial neuropathy is uncommon in amyloid, and when present usually heralds advanced disease.12 Descriptions of bilateral facial weakness, also known as facial diplegia, from amyloidosis are limited to case reports.1315 Other causes of this rare finding include sarcoidosis, Guillain‐Barr syndrome, and Lyme disease.16
The diagnosis of primary amyloidosis requires histologic evidence of amyloid from a tissue biopsy specimen (demonstrating positive Congo red staining and pathognomonic green birefringence under cross‐polarized light microscopy), and the presence of a clonal plasma cell disorder. While biopsy of an affected organ is diagnostic, more easily obtained samples such as fat pad biopsy and rectal biopsy yield positive results in up to 80% of cases.2 Serum and urine protein electrophoresis with immunofixation identify an underlying plasma cell disorder in 90% of cases of primary amyloidosis. When these tests are inconclusive, serum or urine free light chain assays or bone marrow aspirate and biopsy are useful aids to detect underlying plasma cell dyscrasia.2 AL amyloidosis is a progressive disease with median survival of about 12 years.8 Poorer prognosis is associated with substantial echocardiographic findings, autonomic neuropathy, and liver involvement.2 Hyperbilirubinemia is associated with a poor prognosis, with a median survival of 8.5 months.4 Proteinuria or peripheral neuropathy portends a less ominous course.6
Treatment goals include reducing production and deposition of fibril proteins and contending with organ dysfunction (eg, congestive heart failure [CHF] management). Selected patients with AL amyloidosis may be candidates for high‐dose melphalan and autologous stem cell transplantation.
It would not be reasonable for clinicians to suspect amyloidosis in cases of diarrhea until two conditions are met: 1) the absence of evidence for the typical etiologies of diarrhea; and 2) the evolving picture of an infiltrative disorder. The latter was heralded by the elevated alkaline phosphatase, and was supported by the subsequent multiorgan involvement. Conceptualizing the disease as infiltrative still required a diligent exclusion of infection and invasive tumor cells, which invade disparate organs far more commonly than amyloidosis. Their absence and the organ pattern that is typical of AL amyloidosis (heart, kidney, and peripheral nerve involvement) allowed the discussant to reason by analogy that amyloidosis was also responsible for the most symptomatic phenomena, namely, the diarrhea and facial diplegia (and numb chin syndrome).
Key Teaching Points
-
Hospitalists should consider systemic amyloidosis in cases of unexplained diarrhea when other clinical features of AL amyloidosis are present, including nephrotic syndrome with or without renal insufficiency, cardiomyopathy, peripheral neuropathy, and hepatomegaly.
-
Hepatic amyloidosis should be suspected when weight loss, hepatomegaly, and elevated alkaline phosphatase are present. Although jaundice is rare in amyloidosis, liver involvement and hyperbilirubinemia portend a poorer prognosis.
-
Numb chin syndrome and bilateral facial diplegia are rare manifestations of AL amyloid deposition in peripheral nerves.
A 59 year‐old man was sent from urgent care clinic to the emergency room for further evaluation because of 1 month of diarrhea and an acute elevation in his serum creatinine.
Whereas acute diarrhea is commonly due to a self‐limited and often unspecified infection, diarrhea that extends beyond 23 weeks (chronic) warrants consideration of malabsorptive, inflammatory, infectious, and malignant processes. The acute renal failure likely is a consequence of dehydration, but the possibility of simultaneous gastrointestinal and renal involvement from a systemic process (eg, vasculitis) must be considered.
The patient's diarrhea began 1 month prior, shortly after having a milkshake at a fast food restaurant. The diarrhea was initially watery, occurred 8‐10 times per day, occasionally awakened him at night, and was associated with nausea. There was no mucus, blood, or steatorrhea until 1 day prior to presentation, when he developed epigastric pain and bloody stools. He denied any recent travel outside of Northern California and had no sick contacts. He had lost 10 pounds over the preceding month. He denied fevers, chills, vomiting, or jaundice, and had not taken antibiotics recently.
In the setting of chronic diarrhea, unintentional weight loss is an alarm feature but does not narrow the diagnostic possibilities significantly. The appearance of blood and pain on a single day after 1 month of symptoms renders their diagnostic value uncertain. For instance, rectal or hemorrhoidal bleeding would be a common occurrence after 1 month of frequent defecation. Sustained bloody stools might be seen in any form of erosive luminal disease, such as infection, inflammatory bowel disease, or neoplasm. Pain is compatible with inflammatory bowel disease, obstructing neoplasms, infections, or ischemia (eg, vasculitis). There are no fever or chills to support infection, and common gram‐negative enteric pathogens (such as Salmonella, Campylobacter, and Yersinia) usually do not produce symptoms for such an extended period. He has not taken antibiotics, which would predispose him to infection with Clostridum difficile, and he has no obvious exposure to parasites such as Entamoeba.
The patient had diabetes mellitus with microalbuminuria, chronic obstructive pulmonary disease, hypertension, hyperlipidemia, chronic low back pain, and gastritis, and had undergone a Billroth II procedure for a perforated gastric ulcer in the remote past. His medications included omeprazole, insulin glargine, simvastatin, lisinopril, amlodipine, and albuterol and beclomethasone metered‐dose inhalers. He had been married for 31 years, lived at home with his wife, was a former rigger in a shipyard and was on disability for chronic low back pain. He denied alcohol or intravenous drug use but had quit tobacco 5 years prior after more than 40 pack‐years of smoking. He had three healthy adult children and there was no family history of cancer, liver disease, or inflammatory bowel disease. There was no history of sexually transmitted diseases or unprotected sexual intercourse.
Bacterial overgrowth in the blind loop following a Billroth II operation can lead to malabsorption, but the diarrhea would not begin so abruptly this long after surgery. Medications are common causes of diarrhea. Proton‐pump inhibitors, by reducing gastric acidity, confer an increased risk of bacterial enteritis; they also are a risk factor for C difficile. Lisinopril may cause bowel angioedema months or years after initiation. Occult laxative use is a well‐recognized cause of chronic diarrhea and should also be considered. The most relevant element of his social history is the prolonged smoking and the attendant risk of cancer, although diarrhea is a rare paraneoplastic phenomenon.
On exam, temperature was 36.6C, blood pressure 125/78, pulse 88, respiratory rate 16 per minute, and oxygen saturation 97% while breathing room air. There was temporal wasting and mild scleral icterus, but no jaundice. Lungs were clear to auscultation and heart was regular in rate and rhythm without murmurs or gallops. There was no jugular venous distention. A large abdominal midline scar was present, bowel sounds were normoactive, and the abdomen was soft, nontender, and nondistended. The hard was regular in rate and rhythm the liver edge was 6 cm below costal margin; there was no splenomegaly. The patient was alert and oriented, with a normal neurologic exam.
The liver generally enlarges because of acute inflammation, congestion, or infiltration. Infiltration can be due to tumors, infections, hemochromatosis, amyloidosis, or sarcoidosis. A normal cardiac exam argues against hepatic congestion from right‐sided heart failure or pericardial disease.
The key elements of the case are diarrhea and hepatomegaly. Inflammatory bowel disease can be accompanied by sclerosing cholangitis, but this should not enlarge the liver. Mycobacterial infections and syphilis can infiltrate the liver and intestinal mucosa, causing diarrhea, but he lacks typical risk factors.
Malignancy is an increasing concern. Colon cancer commonly metastasizes to the liver and can occasionally be intensely secretory. Pancreatic cancer could account for these symptoms, especially if pancreatic exocrine insufficiency caused malabsorption. Various rare neuroendocrine tumors that arise in the pancreas can cause secretory diarrheas and liver metastases, such as carcinoid, VIPoma, and Zollinger‐Ellison syndrome.
Laboratory results revealed a serum sodium of 143 mmol/L, potassium 4.7 mmol/L, chloride 110 mmol/L, bicarbonate 25 mmol/L, urea nitrogen 24 mg/dL, and creatinine 2.5 mg/dL (baseline had been 1.2 mg/dL 2 months previously). Serum glucose was 108 mg/dL and calcium was 8.8 mg/dL. The total white blood cell count was 9300 per mm3 with a normal differential, hemoglobin was 14.4 g/dL, mean corpuscular volume was 87 fL, and the platelet count was normal. Total bilirubin was 3.7 mg/dL, and direct bilirubin was 3.1 mg/dL. Aspartate aminotransferase (AST) was 122 U/L (normal range, 831), alanine aminotransferase (ALT) 79 U/L (normal range, 731), alkaline phosphatase 1591 U/L (normal range, 39117), and gamma‐glutamyltransferase (GGT) 980 U/L (normal range, 57). Serum albumin was 2.5 mg/dL, prothrombin time was 16.4 seconds, and international normalized ratio (INR) was 1.6.
Urinalysis was normal except for trace hemoglobin, small bilirubin, and 70 mg/dL of protein; specific gravity was 1.007. Urine microscopy demonstrated no cells or casts. The ratio of protein to creatinine on a spot urine sample was less than 1. Chest x‐ray was normal. The electrocardiogram demonstrated sinus rhythm with an old right bundle branch block and normal QRS voltages.
The disproportionate elevation in alkaline phosphatase points to an infiltrative hepatopathy from a cancer originating in the gastrointestinal tract or infection. Other infiltrative processes such as sarcoidosis or amyloidosis usually have evidence of disease elsewhere before hepatic disease becomes apparent.
Mild proteinuria may be explained by diabetes. The specific gravity of 1.007 is atypical for dehydration and could suggest ischemic tubular injury. Although intrinsic renal diseases must continue to be entertained, hypovolemia (compounded by angiotensin‐converting enzyme [ACE] inhibitor use) is the leading explanation in light of the nondiagnostic renal studies. The preserved hemoglobin may simply indicate dehydration, but otherwise is somewhat reassuring in the context of bloody diarrhea.
The patient was admitted to the hospital. Three stool samples returned negative for C difficile toxin. No white cells were detected in the stool, and no ova or parasites were detected. Stool culture was negative for routine bacterial pathogens and for E coli O157. Tests for HIV and antinuclear antibodies (ANAs) and serologies for hepatitis A, B, and C were negative. Abdominal ultrasound demonstrated no intra‐ or extrahepatic bile duct dilatation; no hepatic masses were seen. Kidneys were normal in size and appearance without hydronephrosis. Computed tomography (CT) of the abdomen without intravenous contrast revealed normal‐appearing liver (with a 12‐cm span), spleen, biliary ducts, and pancreas, and there was no intra‐abdominal adenopathy.
The stool studies point away from infectious colitis. Infiltrative processes of the liver, including metastases, lymphoma, tuberculosis, syphilis, amyloidosis, and sarcoidosis, can be microscopic and therefore evade detection by ultrasound and CT scan. In conditions such as these, endoscopic retrograde cholangiopanccreatography/magnetic resonance cholangiopancreatography (ERCP/MRCP) or liver biopsy may be required. The CT is limited without contrast but does not suggest extrahepatic disease in the abdomen.
MRCP was performed, but was a technically suboptimal study due to the presence of ascites. The serum creatinine improved to 1.4 mg/dL over the next 4 days, and the patient's diarrhea decreased to two bowel movements daily with the use of loperamide. The patient was discharged home with outpatient gastroenterology follow‐up planned to discuss further evaluation of the abnormal liver enzymes.
Prior to being seen in the Gastroenterology Clinic, the patient's nonbloody diarrhea worsened. He felt weaker and continued to lose weight. He also noted new onset of bilateral lower face numbness and burning, which was followed by swelling of his lower lip 12 hours later. He returned to the hospital.
On examination, he was afebrile. His lower lip was markedly swollen and was drooping from his face. He could not move the lip to close his mouth. The upper lip and tongue were normal size and moved without restriction. Facial sensation was intact, but there was weakness when he attempted to wrinkle both of his brows and close his eyelids. The rest of his physical examination was unchanged.
The serum creatinine had risen to 3.6 mg/dL, and the complete blood count remained normal. Serum total bilirubin was 4.6 mg/dL, AST 87 U/L, ALT 76 U/L, and alkaline phosphatase 1910 U/L. The 24‐hour urine protein measurement was 86 mg.
Lip swelling suggests angioedema. ACE inhibitors are frequent offenders, and it would be important to know whether his lisinopril was restarted at discharge. ACE‐inhibitor angioedema can also affect the intestine, causing abdominal pain and diarrhea, but does not cause a systemic wasting illness or infiltrative hepatopathy. The difficulty moving the lip may reflect the physical effects of swelling, but generalized facial weakness supports a cranial neuropathy. Basilar meningitis may produce multiple cranial neuropathies, the etiologies of which are quite similar to the previously mentioned causes of infiltrative liver disease: sarcoidosis, syphilis, tuberculosis, or lymphoma.
The patient had not resumed lisinopril since his prior hospitalization. The lower lip swelling and paralysis persisted, and new sensory paresthesias developed over the right side of his chin. A consulting neurologist found normal language and speech and moderate dysarthria. Cranial nerve exam was normal except bilateral lower motor neuron facial nerve palsy was noted with bilateral facial droop, reduced strength of eyelid closure, and diminished forehead movement bilaterally; facial sensation was normal. Extremity motor exam revealed proximal iliopsoas muscle weakness bilaterally rated as 4/5 and was otherwise normal. Sensation to pinprick was diminished in a stocking/glove distribution. Deep‐tendon reflexes were normal and plantar response was down‐going bilaterally. Coordination was intact, Romberg was negative, and gait was slowed due to weakness.
Over the next several days, the patient continued to have diarrhea and facial symptoms. The serum total bilirubin increased to 14 mg/dL, alkaline phosphatase rose above 2,000 U/L, and serum creatinine increased to 5.5 mg/dL. Noncontrast CT scan of the head was normal.
Along with a mild peripheral sensory neuropathy, the exam indicates bilateral palsies of the facial nerve. Lyme disease is a frequent etiology, but this patient is not from an endemic area. I am most suspicious of bilateral infiltration of cranial nerve VII. I am thinking analogically to the numb chin syndrome, wherein lymphoma or breast cancer infiltration along the mental branch of V3 causes sensory loss, and perhaps these disorders can produce infiltrative facial neuropathy. At this point I am most concerned about lymphomatous meningitis with cranial nerve involvement. Cerebrospinal fluid (CSF) analysis (including cytology) would be informative.
Lumbar puncture demonstrated clear CSF with one white blood cell per mm3 and no red blood cells. Glucose was normal, and protein was 95.5 (normal range, 15‐45 mg/dL). Gram stain and culture for bacteria were negative, as were polymerase chain reaction (PCR) testing for herpes simplex virus, mycobacterial and fungal stains and cultures, and cytology. Transthoracic echocardiogram demonstrated severe concentric left ventricular (LV) hypertrophy, normal LV systolic function, and impaired LV relaxation. CT scan of the chest identified no adenopathy or other abnormality.
The CSF analysis does not support basilar meningitis, although the cytoalbuminologic dissociation makes me wonder whether there is some intrathecal antibody production or an autoimmune process we have yet to uncover. The absence of lymphadenopathy anywhere in the body and the negative CSF cytology now point away from lymphoma. As the case for lymphoma or an infection diminishes, systemic amyloidosis rises to the top of possibilities in this afebrile man who is losing weight, has infiltrative liver and nerve abnormalities, renal failure, cardiac enlargement, and suspected gastrointestinal luminal abnormality. Although the echocardiographic findings are most likely explained by hypertension, they are compatible with amyloid infiltration. A tissue specimen is needed, and either colonoscopy or liver biopsy should be suitable.
A pathologist performed a fat pad biopsy that demonstrated scant congophilic and birefringent material associated with blood vessels, suggestive of amyloid (Fig. 1). Colonoscopy demonstrated normal mucosa, and a rectal biopsy revealed congophilic material within the blood vessels consistent with amyloid (Fig. 2). No monoclonal band was present on serum protein electrophoresis. Urine protein electrophoresis identified a homogenous band in the gamma region, and urine kappa and lambda free light chains were increased: kappa was 10.7 mg/dL (normal range, 2), and lambda was 4.25 mg/dL (normal range, 2).


After extensive discussion among the patient, his wife, and a palliative care physician, the patient declined chemotherapy and elected to go home. Two days after discharge (7 weeks after his initial admission for diarrhea) he died in his sleep at home. Permission for a postmortem examination was not granted.
Discussion
Amyloidosis refers to abnormal extracellular deposition of fibril. There are many types of amyloidosis including primary amyloidosis (AL amyloidosis), secondary amyloidosis (AA amyloidosis), and hereditary causes. Systemic AL amyloidosis is a rare plasma cell disorder characterized by misfolding of insoluble extracellular fibrillar proteins derived from immunoglobulin light chains. These insoluble proteins typically deposit in the kidney, heart, and nervous system.1 Although the mechanism of organ dysfunction is debated, deposition of these proteins may disrupt the tissue architecture by interacting with local receptors and causing apoptosis.1
Table 1 indicates the most common findings in patients with AL amyloidosis.2 While our patient ultimately developed many common findings of AL amyloidosis, several features were atypical, including the marked hyperbilirubinemia, profound diarrhea, and bilateral facial diplegia.
| Organ Involvement | Incidence of Organ Involvement (%) | Symptoms | Signs | Laboratory/Test Finding |
|---|---|---|---|---|
| ||||
| General | Malaise, weight loss | |||
| Renal | 33 | Fatigue | Peripheral edema | Proteinuria with or without renal insufficiency, pleural effusion, hypercholesterolemia |
| Cardiac | 20 | Palpitations, dyspnea | Elevated jugular venous pressure, S3, peripheral edema, hepatomegaly | Low‐voltage or atrial fibrillation on electrocardiogram; echocardiogram: thickened ventricles, dilated atria |
| Neurological | 20 | Paresthesias, numbness, weakness, autonomic insufficiency | Carpal tunnel syndrome, postural hypotension | |
| Gastrointestinal and Hepatic | 16 | Diarrhea, nausea, weight loss | Macroglossia, hepatomegaly | Elevated alkaline phosphatase |
| Hematology | Rare | Bleeding | Periorbital purpura (raccoon eyes) | Prolonged prothrombin time, Factor X deficiency |
Up to 70% of patients with amyloidosis will have detectable liver deposits, typically involving portal vessels, portal stroma, central vein, and sinusoidal parenchyma.3 Clinically overt hepatic dysfunction from amyloid is less frequent,4 and the most characteristic findings are hepatomegaly with a markedly elevated serum alkaline phosphatase concentration; jaundice is rare. Palpable hepatic enlargement without abnormal liver enzymes should be interpreted with caution. The finding of a palpable liver edge correlates poorly with frank hepatomegaly, with a positive likelihood ratio of just 1.7.5 In the patient under discussion, suspected hepatomegaly was not confirmed on a subsequent CT scan. Nonetheless, the elevated alkaline phosphatase represented an important clue to potential infiltrative liver disease. In a series of amyloidosis patients from the Mayo Clinic, 81% had hepatomegaly on physical exam, and the mean alkaline phosphatase level was 1,029 U/L (normal, 250 U/L), while the mean serum bilirubin and AST levels were only modestly elevated, at 3.2 mg/dL and 72 U/L respectively. The prothrombin time was prolonged in 35% of patients.
Upper gastrointestinal tract involvement by AL amyloid may be found in up to a third of cases at autopsy, but clinically significant gastrointestinal features are seen in fewer than 5% of patients.6 Predominant intestinal manifestations are unintentional weight loss (average 7 kg) and diarrhea, nonspecific features that result in delayed diagnosis for a median of 7 months after symptom onset.7 Diarrhea in AL amyloid may stem from several mechanisms: small intestine mucosal infiltration, steatorrhea from pancreatic insufficiency, autonomic neuropathy leading to pseudo‐obstruction and bacterial overgrowth, bile acid malabsorption, or rapid transit time. Diarrhea in AL amyloid is often resistant to treatment and may be the primary cause of death.7
Systemic amyloidosis commonly produces peripheral neuropathies. Involvement of small unmyelinated fibers causes paresthesias and progressive sensory loss in a pattern that is usually distal, symmetric, and progressive.6, 9 Our patient presented with bilateral sensory paresthesias of the chin, suggesting the numb chin syndrome (NCS). NCS is characterized by facial numbness along the distribution of the mental branch of the trigeminal nerve. While dental disorders and infiltration from malignant tumors (mostly lung and breast cancer) account for most cases, amyloidosis and other infiltrative disorders are known to cause NCS as well.10, 11 Our patient's sensory paresthesias may have represented amyloid infiltration of peripheral nerves.
With the exception of carpal tunnel syndrome, motor or cranial neuropathy is uncommon in amyloid, and when present usually heralds advanced disease.12 Descriptions of bilateral facial weakness, also known as facial diplegia, from amyloidosis are limited to case reports.1315 Other causes of this rare finding include sarcoidosis, Guillain‐Barr syndrome, and Lyme disease.16
The diagnosis of primary amyloidosis requires histologic evidence of amyloid from a tissue biopsy specimen (demonstrating positive Congo red staining and pathognomonic green birefringence under cross‐polarized light microscopy), and the presence of a clonal plasma cell disorder. While biopsy of an affected organ is diagnostic, more easily obtained samples such as fat pad biopsy and rectal biopsy yield positive results in up to 80% of cases.2 Serum and urine protein electrophoresis with immunofixation identify an underlying plasma cell disorder in 90% of cases of primary amyloidosis. When these tests are inconclusive, serum or urine free light chain assays or bone marrow aspirate and biopsy are useful aids to detect underlying plasma cell dyscrasia.2 AL amyloidosis is a progressive disease with median survival of about 12 years.8 Poorer prognosis is associated with substantial echocardiographic findings, autonomic neuropathy, and liver involvement.2 Hyperbilirubinemia is associated with a poor prognosis, with a median survival of 8.5 months.4 Proteinuria or peripheral neuropathy portends a less ominous course.6
Treatment goals include reducing production and deposition of fibril proteins and contending with organ dysfunction (eg, congestive heart failure [CHF] management). Selected patients with AL amyloidosis may be candidates for high‐dose melphalan and autologous stem cell transplantation.
It would not be reasonable for clinicians to suspect amyloidosis in cases of diarrhea until two conditions are met: 1) the absence of evidence for the typical etiologies of diarrhea; and 2) the evolving picture of an infiltrative disorder. The latter was heralded by the elevated alkaline phosphatase, and was supported by the subsequent multiorgan involvement. Conceptualizing the disease as infiltrative still required a diligent exclusion of infection and invasive tumor cells, which invade disparate organs far more commonly than amyloidosis. Their absence and the organ pattern that is typical of AL amyloidosis (heart, kidney, and peripheral nerve involvement) allowed the discussant to reason by analogy that amyloidosis was also responsible for the most symptomatic phenomena, namely, the diarrhea and facial diplegia (and numb chin syndrome).
Key Teaching Points
-
Hospitalists should consider systemic amyloidosis in cases of unexplained diarrhea when other clinical features of AL amyloidosis are present, including nephrotic syndrome with or without renal insufficiency, cardiomyopathy, peripheral neuropathy, and hepatomegaly.
-
Hepatic amyloidosis should be suspected when weight loss, hepatomegaly, and elevated alkaline phosphatase are present. Although jaundice is rare in amyloidosis, liver involvement and hyperbilirubinemia portend a poorer prognosis.
-
Numb chin syndrome and bilateral facial diplegia are rare manifestations of AL amyloid deposition in peripheral nerves.
- ,.Molecular mechanisms of amyloidosis.N Engl J Med.2003;349(6):583–596.
- Guidelines Working Group of UK Myeloma Forum; British Committee for Standards in Haematology, British Society for Haematology.Guidelines on the diagnosis and management of AL amyloidosis.Br J Haematol.2004;125:681–700.
- ,.Hepatic amyloidosis: morphologic differences between systemic AL and AA types.Hum Pathol.1991;22(9):904–907.
- ,,, et al.Primary (AL) hepatic amyloidosis clinical features and natural history in 98 patients.Medicine.2003;82(5):291–298.
- .Evidence‐Based Physical Diagnosis.Philadelphia, PA:WB Saunders;2001:595–599.
- ,,, et al.Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis.Am J Hematol.2005;79:319–328.
- .Primary (AL) amyloidosis with gastrointestinal involvement.Scand J Gastroenterol.2009;44(6):708–711.
- ,.Gastrointestinal manifestations of amyloid.Am J Gastroenterol.2008;103:776–787.
- ,.Primary systemic amyloidosis: clinical and laboratory features in 474 cases.Semin Hematol.1995;32:45–59.
- ,,,,.Chin numbness: a symptom that should not be underestimated: a review of 12 cases.Am J Med Sci.2009;337:407–410.
- .Numb chin syndrome: a possible clue to serious illness.Hosp Physician.2000;54–56.
- .Autonomic peripheral neuropathy.Neurol Clin.2007;25:277–301.
- ,.Facial diplegia due to amyloidosis.South Med J.1986;79(11):1458–1459.
- ,,,,.Familial amyloidosis with cranial neuropathy and corneal lattice dystrophy.Neurology.1986;36:432–435.
- ,,.Crainal neuropathy associated with primary amyloidosis.Ann Neurol.1991;29:451–454.
- .Bilateral seventh nerve palsy: analysis of 43 cases and review of the literature.Neurology.1994;44:1198–202.
- ,.Molecular mechanisms of amyloidosis.N Engl J Med.2003;349(6):583–596.
- Guidelines Working Group of UK Myeloma Forum; British Committee for Standards in Haematology, British Society for Haematology.Guidelines on the diagnosis and management of AL amyloidosis.Br J Haematol.2004;125:681–700.
- ,.Hepatic amyloidosis: morphologic differences between systemic AL and AA types.Hum Pathol.1991;22(9):904–907.
- ,,, et al.Primary (AL) hepatic amyloidosis clinical features and natural history in 98 patients.Medicine.2003;82(5):291–298.
- .Evidence‐Based Physical Diagnosis.Philadelphia, PA:WB Saunders;2001:595–599.
- ,,, et al.Definition of organ involvement and treatment response in immunoglobulin light chain amyloidosis (AL): a consensus opinion from the 10th International Symposium on Amyloid and Amyloidosis.Am J Hematol.2005;79:319–328.
- .Primary (AL) amyloidosis with gastrointestinal involvement.Scand J Gastroenterol.2009;44(6):708–711.
- ,.Gastrointestinal manifestations of amyloid.Am J Gastroenterol.2008;103:776–787.
- ,.Primary systemic amyloidosis: clinical and laboratory features in 474 cases.Semin Hematol.1995;32:45–59.
- ,,,,.Chin numbness: a symptom that should not be underestimated: a review of 12 cases.Am J Med Sci.2009;337:407–410.
- .Numb chin syndrome: a possible clue to serious illness.Hosp Physician.2000;54–56.
- .Autonomic peripheral neuropathy.Neurol Clin.2007;25:277–301.
- ,.Facial diplegia due to amyloidosis.South Med J.1986;79(11):1458–1459.
- ,,,,.Familial amyloidosis with cranial neuropathy and corneal lattice dystrophy.Neurology.1986;36:432–435.
- ,,.Crainal neuropathy associated with primary amyloidosis.Ann Neurol.1991;29:451–454.
- .Bilateral seventh nerve palsy: analysis of 43 cases and review of the literature.Neurology.1994;44:1198–202.