User login
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
Prurigo nodularis (PN) is a chronic, severely pruritic neuroimmunologic skin disorder characterized by multiple firm hyperkeratotic nodules and intense pruritus, often leading to considerable impairment in quality of life and increased rates of depression and anxiety.1 It is considered difficult to treat due to its complex pathogenesis, the severity and chronicity of pruritus, and the limited efficacy of conventional therapies.2,3 The disease is driven by a self-perpetuating itch-scratch cycle, underpinned by dysregulation of both immune and neural pathways including type 2 (interleukin [IL] 4, IL-13, IL-31), Th17, and Th22 cytokines as well as neuropeptides and altered cutaneous nerve architecture.1,3 This results in persistent severe pruritus and nodular lesions that are highly refractory to standard treatments.1 Conventional therapies (eg, locally acting agents, phototherapy, and systemic immunomodulators and neuromodulators) have varied efficacy and notable adverse effect profiles.3 While the approval of targeted biologics has transformed the therapeutic landscape, several other treatment options also are being explored in clinical trials. Herein, we review all recently approved therapies as well as emerging treatments currently under investigation.
Dupilumab
Dupilumab, the first therapy for PN approved by the US Food and Drug Administration (FDA) in 2022—is a monoclonal antibody that inhibits signaling of IL-4 and IL-13, key drivers of type 2 inflammation implicated in PN pathogenesis.4,5 In 2 pivotal phase 3 randomized controlled trials (LIBERTY-PN PRIME and PRIME2),5 dupilumab demonstrated notable efficacy in adults with moderate to severe PN. A reduction of 4 points or more on the Worst Itch Numeric Rating Scale (WI-NRS) was achieved by 60.0% (45/75) of patients treated with dupilumab at week 24 compared with 18.4% (14/76) receiving placebo in the PRIME trial. In PRIME2, the same outcome was achieved by 37.2% (29/78) of patients receiving dupilumab at week 12 compared with 22.0% (18/82) of patients receiving placebo.5 Dupilumab also led to a greater proportion of patients achieving a substantial reduction in nodule count (≤5 nodules) and improved quality of life compared with placebo.5,6 The safety profile of dupilumab for treatment of PN was favorable and consistent with prior experience in atopic dermatitis; conjunctivitis was the most common adverse event.5,6
Nemolizumab
Nemolizumab, an IL-31 receptor A antagonist, is the most recent agent approved by the FDA for PN in 2024.7 In the OLYMPIA 1 and OLYMPIA 2 phase 3 trials,8 nemolizumab produced a clinically meaningful reduction in itch (defined as a ≥4-point improvement in the Peak Pruritus Numerical Rating Scale score) in 56.3% (103/183) of patients at week 16 compared with 20.9% (19/91) receiving placebo. Additionally, 37.7% (69/183) of patients receiving nemolizumab achieved clear or almost clear skin (Investigator’s Global Assessment score of 0 or 1 with a ≥2-point reduction) vs 11.0% with placebo (both P<.001). Benefits were observed as early as week 4, including rapid improvements in itch, sleep disturbance, and nodule count.8 Nemolizumab also improved quality of life and reduced symptoms of anxiety and depression. The safety profile was favorable, with headache and atopic dermatitis the most common adverse events; serious adverse events were infrequent and similar between groups.8
Abrocitinib
Abrocitinib, an oral selective Janus kinase 1 inhibitor, is an investigational therapy for PN and currently has not been approved by the FDA for this indication. In a phase 2 open-label trial, abrocitinib 200 mg daily for 12 weeks led to a 78.3% reduction in weekly Peak Pruritus Numerical Rating Scale scores in PN, with 80.0% (8/10) of patients achieving a clinically meaningful improvement of 4 points or higher. Nodule counts and quality of life also improved, with an onset of itch relief as early as week 2. The safety profile was favorable, with acneform eruptions the most common adverse event and no serious adverse events reported9; however, these results are based on small, nonrandomized studies and require confirmation in larger randomized controlled trials before abrocitinib can be considered a standard therapy for PN.
Cryosim-1
Transient receptor potential melastatin 8 (TRPM8) is a cold-sensing ion channel found in unmyelinated sensory neurons within the dorsal root and trigeminal ganglia.10 It is activated by cool temperatures (15-28 °C) and compounds such as menthol, leading to calcium influx and a cooling sensation. In a randomized, double-blind, vehicle-controlled trial, researchers investigated the efficacy of cryosim-1 (a synthetic TRPM8 agonist) in treating PN.10 Thirty patients were enrolled, with 18 (60.0%) receiving cryosim-1 and 12 (40.0%) receiving placebo over 8 weeks. By week 8, cryosim-1 significantly reduced itch severity (mean numerical rating scale score postapplication, 2.8 vs 4.3; P=.031) and improved sleep disturbances (2.2 vs 4.2; P=.031) compared to placebo. Patients reported higher satisfaction with itch relief, and no adverse effects were observed. The study concluded that cryosim-1 is a safe, effective topical therapy for PN, likely working by interrupting the itch-scratch cycle and potentially modulating inflammatory pathways involved in chronic itch.10
Nalbuphine
Nalbuphine is a κ opioid receptor agonist and μ opioid receptor antagonist that has been investigated for the treatment of PN.11 In a phase 2 randomized controlled trial, oral nalbuphine extended release (NAL-ER) 162 mg twice daily provided measurable antipruritic efficacy, with 44.4% (8/18) of patients achieving at least a 30% reduction in 7-day WI-NRS at week 10 compared with 36.4% (8/22) in the placebo group. Among those who completed the study, 66.7% (8/12) of patients receiving NAL-ER 162 mg achieved significant itch reduction vs 40% (8/20) receiving placebo (P=.03). At least a 50% reduction in WI-NRS was achieved by 33.3% (6/18) of patients receiving NAL-ER 162 mg twice daily. Extended open-label treatment was associated with further improvements in itch and lesion activity. Adverse events were mostly mild to moderate (eg, nausea, dizziness, headache, and fatigue) and occurred during dose titration. Physiologic opioid withdrawal symptoms were limited and resolved within a few days of discontinuing the medication.11
Final Thoughts
In conclusion, PN remains one of the most challenging chronic dermatologic conditions to manage and is driven by a complex interplay of neuroimmune mechanisms and resistance to many conventional therapies. The approval of dupilumab and nemolizumab has marked a pivotal shift in the therapeutic landscape, offering hope to patients who previously had limited options5,8; however, the burden of PN remains substantial, and many patients continue to experience relentless itch, poor sleep, and reduced quality of life.1 Emerging therapies such as TRPM8 agonists, Janus kinase inhibitors, and opioid modulators represent promising additions to the treatment options, targeting novel pathways beyond traditional immunosuppression.9-11
- Williams KA, Huang AH, Belzberg M, et al. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83:1567-1575. doi:10.1016/j.jaad.2020.04.182
- Gründel S, Pereira MP, Storck M, et al. Analysis of 325 patients with chronic nodular prurigo: clinics, burden of disease and course of treatment. Acta Derm Venereol. 2020;100:adv00269. doi:10.2340/00015555-3571
- Liao V, Cornman HL, Ma E, et al. Prurigo nodularis: new insights into pathogenesis and novel therapeutics. Br J Dermatol. 2024;190:798-810. doi:10.1093/bjd/ljae052
- Elmariah SB, Tao L, Valdes-Rodriguez R, et al. Individual article: management of prurigo nodularis. J Drugs Dermatol. 2023;22:SF365502s15-SF365502s22. doi:10.36849/JDD.SF365502
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Cao P, Xu W, Jiang S, et al. Dupilumab for the treatment of prurigo nodularis: a systematic review. Front Immunol. 2023;14:1092685. doi:10.3389/fimmu.2023.1092685
- Dagenet CB, Saadi C, Phillips MA, et al. Landscape of prurigo nodularis clinical trials. JAAD Rev. 2024;2:127-136. doi:10.1016/j.jdrv.2024.09.006
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Kwatra SG, Bordeaux ZA, Parthasarathy V, et al. Efficacy and safety of abrocitinib in prurigo nodularis and chronic pruritus of unknown origin: a nonrandomized controlled trial. JAMA Dermatol. 2024;160:717-724. doi:10.1001/jamadermatol.2024.1464
- Choi ME, Lee JH, Jung CJ, et al. A randomized, double-blinded, vehicle-controlled clinical trial of topical cryosim-1, a synthetic TRPM8 agonist, in prurigo nodularis. J Cosmet Dermatol. 2024;23:931-937. doi:10.1111/jocd.16079
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
Prurigo nodularis (PN) is a chronic, severely pruritic neuroimmunologic skin disorder characterized by multiple firm hyperkeratotic nodules and intense pruritus, often leading to considerable impairment in quality of life and increased rates of depression and anxiety.1 It is considered difficult to treat due to its complex pathogenesis, the severity and chronicity of pruritus, and the limited efficacy of conventional therapies.2,3 The disease is driven by a self-perpetuating itch-scratch cycle, underpinned by dysregulation of both immune and neural pathways including type 2 (interleukin [IL] 4, IL-13, IL-31), Th17, and Th22 cytokines as well as neuropeptides and altered cutaneous nerve architecture.1,3 This results in persistent severe pruritus and nodular lesions that are highly refractory to standard treatments.1 Conventional therapies (eg, locally acting agents, phototherapy, and systemic immunomodulators and neuromodulators) have varied efficacy and notable adverse effect profiles.3 While the approval of targeted biologics has transformed the therapeutic landscape, several other treatment options also are being explored in clinical trials. Herein, we review all recently approved therapies as well as emerging treatments currently under investigation.
Dupilumab
Dupilumab, the first therapy for PN approved by the US Food and Drug Administration (FDA) in 2022—is a monoclonal antibody that inhibits signaling of IL-4 and IL-13, key drivers of type 2 inflammation implicated in PN pathogenesis.4,5 In 2 pivotal phase 3 randomized controlled trials (LIBERTY-PN PRIME and PRIME2),5 dupilumab demonstrated notable efficacy in adults with moderate to severe PN. A reduction of 4 points or more on the Worst Itch Numeric Rating Scale (WI-NRS) was achieved by 60.0% (45/75) of patients treated with dupilumab at week 24 compared with 18.4% (14/76) receiving placebo in the PRIME trial. In PRIME2, the same outcome was achieved by 37.2% (29/78) of patients receiving dupilumab at week 12 compared with 22.0% (18/82) of patients receiving placebo.5 Dupilumab also led to a greater proportion of patients achieving a substantial reduction in nodule count (≤5 nodules) and improved quality of life compared with placebo.5,6 The safety profile of dupilumab for treatment of PN was favorable and consistent with prior experience in atopic dermatitis; conjunctivitis was the most common adverse event.5,6
Nemolizumab
Nemolizumab, an IL-31 receptor A antagonist, is the most recent agent approved by the FDA for PN in 2024.7 In the OLYMPIA 1 and OLYMPIA 2 phase 3 trials,8 nemolizumab produced a clinically meaningful reduction in itch (defined as a ≥4-point improvement in the Peak Pruritus Numerical Rating Scale score) in 56.3% (103/183) of patients at week 16 compared with 20.9% (19/91) receiving placebo. Additionally, 37.7% (69/183) of patients receiving nemolizumab achieved clear or almost clear skin (Investigator’s Global Assessment score of 0 or 1 with a ≥2-point reduction) vs 11.0% with placebo (both P<.001). Benefits were observed as early as week 4, including rapid improvements in itch, sleep disturbance, and nodule count.8 Nemolizumab also improved quality of life and reduced symptoms of anxiety and depression. The safety profile was favorable, with headache and atopic dermatitis the most common adverse events; serious adverse events were infrequent and similar between groups.8
Abrocitinib
Abrocitinib, an oral selective Janus kinase 1 inhibitor, is an investigational therapy for PN and currently has not been approved by the FDA for this indication. In a phase 2 open-label trial, abrocitinib 200 mg daily for 12 weeks led to a 78.3% reduction in weekly Peak Pruritus Numerical Rating Scale scores in PN, with 80.0% (8/10) of patients achieving a clinically meaningful improvement of 4 points or higher. Nodule counts and quality of life also improved, with an onset of itch relief as early as week 2. The safety profile was favorable, with acneform eruptions the most common adverse event and no serious adverse events reported9; however, these results are based on small, nonrandomized studies and require confirmation in larger randomized controlled trials before abrocitinib can be considered a standard therapy for PN.
Cryosim-1
Transient receptor potential melastatin 8 (TRPM8) is a cold-sensing ion channel found in unmyelinated sensory neurons within the dorsal root and trigeminal ganglia.10 It is activated by cool temperatures (15-28 °C) and compounds such as menthol, leading to calcium influx and a cooling sensation. In a randomized, double-blind, vehicle-controlled trial, researchers investigated the efficacy of cryosim-1 (a synthetic TRPM8 agonist) in treating PN.10 Thirty patients were enrolled, with 18 (60.0%) receiving cryosim-1 and 12 (40.0%) receiving placebo over 8 weeks. By week 8, cryosim-1 significantly reduced itch severity (mean numerical rating scale score postapplication, 2.8 vs 4.3; P=.031) and improved sleep disturbances (2.2 vs 4.2; P=.031) compared to placebo. Patients reported higher satisfaction with itch relief, and no adverse effects were observed. The study concluded that cryosim-1 is a safe, effective topical therapy for PN, likely working by interrupting the itch-scratch cycle and potentially modulating inflammatory pathways involved in chronic itch.10
Nalbuphine
Nalbuphine is a κ opioid receptor agonist and μ opioid receptor antagonist that has been investigated for the treatment of PN.11 In a phase 2 randomized controlled trial, oral nalbuphine extended release (NAL-ER) 162 mg twice daily provided measurable antipruritic efficacy, with 44.4% (8/18) of patients achieving at least a 30% reduction in 7-day WI-NRS at week 10 compared with 36.4% (8/22) in the placebo group. Among those who completed the study, 66.7% (8/12) of patients receiving NAL-ER 162 mg achieved significant itch reduction vs 40% (8/20) receiving placebo (P=.03). At least a 50% reduction in WI-NRS was achieved by 33.3% (6/18) of patients receiving NAL-ER 162 mg twice daily. Extended open-label treatment was associated with further improvements in itch and lesion activity. Adverse events were mostly mild to moderate (eg, nausea, dizziness, headache, and fatigue) and occurred during dose titration. Physiologic opioid withdrawal symptoms were limited and resolved within a few days of discontinuing the medication.11
Final Thoughts
In conclusion, PN remains one of the most challenging chronic dermatologic conditions to manage and is driven by a complex interplay of neuroimmune mechanisms and resistance to many conventional therapies. The approval of dupilumab and nemolizumab has marked a pivotal shift in the therapeutic landscape, offering hope to patients who previously had limited options5,8; however, the burden of PN remains substantial, and many patients continue to experience relentless itch, poor sleep, and reduced quality of life.1 Emerging therapies such as TRPM8 agonists, Janus kinase inhibitors, and opioid modulators represent promising additions to the treatment options, targeting novel pathways beyond traditional immunosuppression.9-11
Prurigo nodularis (PN) is a chronic, severely pruritic neuroimmunologic skin disorder characterized by multiple firm hyperkeratotic nodules and intense pruritus, often leading to considerable impairment in quality of life and increased rates of depression and anxiety.1 It is considered difficult to treat due to its complex pathogenesis, the severity and chronicity of pruritus, and the limited efficacy of conventional therapies.2,3 The disease is driven by a self-perpetuating itch-scratch cycle, underpinned by dysregulation of both immune and neural pathways including type 2 (interleukin [IL] 4, IL-13, IL-31), Th17, and Th22 cytokines as well as neuropeptides and altered cutaneous nerve architecture.1,3 This results in persistent severe pruritus and nodular lesions that are highly refractory to standard treatments.1 Conventional therapies (eg, locally acting agents, phototherapy, and systemic immunomodulators and neuromodulators) have varied efficacy and notable adverse effect profiles.3 While the approval of targeted biologics has transformed the therapeutic landscape, several other treatment options also are being explored in clinical trials. Herein, we review all recently approved therapies as well as emerging treatments currently under investigation.
Dupilumab
Dupilumab, the first therapy for PN approved by the US Food and Drug Administration (FDA) in 2022—is a monoclonal antibody that inhibits signaling of IL-4 and IL-13, key drivers of type 2 inflammation implicated in PN pathogenesis.4,5 In 2 pivotal phase 3 randomized controlled trials (LIBERTY-PN PRIME and PRIME2),5 dupilumab demonstrated notable efficacy in adults with moderate to severe PN. A reduction of 4 points or more on the Worst Itch Numeric Rating Scale (WI-NRS) was achieved by 60.0% (45/75) of patients treated with dupilumab at week 24 compared with 18.4% (14/76) receiving placebo in the PRIME trial. In PRIME2, the same outcome was achieved by 37.2% (29/78) of patients receiving dupilumab at week 12 compared with 22.0% (18/82) of patients receiving placebo.5 Dupilumab also led to a greater proportion of patients achieving a substantial reduction in nodule count (≤5 nodules) and improved quality of life compared with placebo.5,6 The safety profile of dupilumab for treatment of PN was favorable and consistent with prior experience in atopic dermatitis; conjunctivitis was the most common adverse event.5,6
Nemolizumab
Nemolizumab, an IL-31 receptor A antagonist, is the most recent agent approved by the FDA for PN in 2024.7 In the OLYMPIA 1 and OLYMPIA 2 phase 3 trials,8 nemolizumab produced a clinically meaningful reduction in itch (defined as a ≥4-point improvement in the Peak Pruritus Numerical Rating Scale score) in 56.3% (103/183) of patients at week 16 compared with 20.9% (19/91) receiving placebo. Additionally, 37.7% (69/183) of patients receiving nemolizumab achieved clear or almost clear skin (Investigator’s Global Assessment score of 0 or 1 with a ≥2-point reduction) vs 11.0% with placebo (both P<.001). Benefits were observed as early as week 4, including rapid improvements in itch, sleep disturbance, and nodule count.8 Nemolizumab also improved quality of life and reduced symptoms of anxiety and depression. The safety profile was favorable, with headache and atopic dermatitis the most common adverse events; serious adverse events were infrequent and similar between groups.8
Abrocitinib
Abrocitinib, an oral selective Janus kinase 1 inhibitor, is an investigational therapy for PN and currently has not been approved by the FDA for this indication. In a phase 2 open-label trial, abrocitinib 200 mg daily for 12 weeks led to a 78.3% reduction in weekly Peak Pruritus Numerical Rating Scale scores in PN, with 80.0% (8/10) of patients achieving a clinically meaningful improvement of 4 points or higher. Nodule counts and quality of life also improved, with an onset of itch relief as early as week 2. The safety profile was favorable, with acneform eruptions the most common adverse event and no serious adverse events reported9; however, these results are based on small, nonrandomized studies and require confirmation in larger randomized controlled trials before abrocitinib can be considered a standard therapy for PN.
Cryosim-1
Transient receptor potential melastatin 8 (TRPM8) is a cold-sensing ion channel found in unmyelinated sensory neurons within the dorsal root and trigeminal ganglia.10 It is activated by cool temperatures (15-28 °C) and compounds such as menthol, leading to calcium influx and a cooling sensation. In a randomized, double-blind, vehicle-controlled trial, researchers investigated the efficacy of cryosim-1 (a synthetic TRPM8 agonist) in treating PN.10 Thirty patients were enrolled, with 18 (60.0%) receiving cryosim-1 and 12 (40.0%) receiving placebo over 8 weeks. By week 8, cryosim-1 significantly reduced itch severity (mean numerical rating scale score postapplication, 2.8 vs 4.3; P=.031) and improved sleep disturbances (2.2 vs 4.2; P=.031) compared to placebo. Patients reported higher satisfaction with itch relief, and no adverse effects were observed. The study concluded that cryosim-1 is a safe, effective topical therapy for PN, likely working by interrupting the itch-scratch cycle and potentially modulating inflammatory pathways involved in chronic itch.10
Nalbuphine
Nalbuphine is a κ opioid receptor agonist and μ opioid receptor antagonist that has been investigated for the treatment of PN.11 In a phase 2 randomized controlled trial, oral nalbuphine extended release (NAL-ER) 162 mg twice daily provided measurable antipruritic efficacy, with 44.4% (8/18) of patients achieving at least a 30% reduction in 7-day WI-NRS at week 10 compared with 36.4% (8/22) in the placebo group. Among those who completed the study, 66.7% (8/12) of patients receiving NAL-ER 162 mg achieved significant itch reduction vs 40% (8/20) receiving placebo (P=.03). At least a 50% reduction in WI-NRS was achieved by 33.3% (6/18) of patients receiving NAL-ER 162 mg twice daily. Extended open-label treatment was associated with further improvements in itch and lesion activity. Adverse events were mostly mild to moderate (eg, nausea, dizziness, headache, and fatigue) and occurred during dose titration. Physiologic opioid withdrawal symptoms were limited and resolved within a few days of discontinuing the medication.11
Final Thoughts
In conclusion, PN remains one of the most challenging chronic dermatologic conditions to manage and is driven by a complex interplay of neuroimmune mechanisms and resistance to many conventional therapies. The approval of dupilumab and nemolizumab has marked a pivotal shift in the therapeutic landscape, offering hope to patients who previously had limited options5,8; however, the burden of PN remains substantial, and many patients continue to experience relentless itch, poor sleep, and reduced quality of life.1 Emerging therapies such as TRPM8 agonists, Janus kinase inhibitors, and opioid modulators represent promising additions to the treatment options, targeting novel pathways beyond traditional immunosuppression.9-11
- Williams KA, Huang AH, Belzberg M, et al. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83:1567-1575. doi:10.1016/j.jaad.2020.04.182
- Gründel S, Pereira MP, Storck M, et al. Analysis of 325 patients with chronic nodular prurigo: clinics, burden of disease and course of treatment. Acta Derm Venereol. 2020;100:adv00269. doi:10.2340/00015555-3571
- Liao V, Cornman HL, Ma E, et al. Prurigo nodularis: new insights into pathogenesis and novel therapeutics. Br J Dermatol. 2024;190:798-810. doi:10.1093/bjd/ljae052
- Elmariah SB, Tao L, Valdes-Rodriguez R, et al. Individual article: management of prurigo nodularis. J Drugs Dermatol. 2023;22:SF365502s15-SF365502s22. doi:10.36849/JDD.SF365502
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Cao P, Xu W, Jiang S, et al. Dupilumab for the treatment of prurigo nodularis: a systematic review. Front Immunol. 2023;14:1092685. doi:10.3389/fimmu.2023.1092685
- Dagenet CB, Saadi C, Phillips MA, et al. Landscape of prurigo nodularis clinical trials. JAAD Rev. 2024;2:127-136. doi:10.1016/j.jdrv.2024.09.006
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Kwatra SG, Bordeaux ZA, Parthasarathy V, et al. Efficacy and safety of abrocitinib in prurigo nodularis and chronic pruritus of unknown origin: a nonrandomized controlled trial. JAMA Dermatol. 2024;160:717-724. doi:10.1001/jamadermatol.2024.1464
- Choi ME, Lee JH, Jung CJ, et al. A randomized, double-blinded, vehicle-controlled clinical trial of topical cryosim-1, a synthetic TRPM8 agonist, in prurigo nodularis. J Cosmet Dermatol. 2024;23:931-937. doi:10.1111/jocd.16079
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
- Williams KA, Huang AH, Belzberg M, et al. Prurigo nodularis: pathogenesis and management. J Am Acad Dermatol. 2020;83:1567-1575. doi:10.1016/j.jaad.2020.04.182
- Gründel S, Pereira MP, Storck M, et al. Analysis of 325 patients with chronic nodular prurigo: clinics, burden of disease and course of treatment. Acta Derm Venereol. 2020;100:adv00269. doi:10.2340/00015555-3571
- Liao V, Cornman HL, Ma E, et al. Prurigo nodularis: new insights into pathogenesis and novel therapeutics. Br J Dermatol. 2024;190:798-810. doi:10.1093/bjd/ljae052
- Elmariah SB, Tao L, Valdes-Rodriguez R, et al. Individual article: management of prurigo nodularis. J Drugs Dermatol. 2023;22:SF365502s15-SF365502s22. doi:10.36849/JDD.SF365502
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Cao P, Xu W, Jiang S, et al. Dupilumab for the treatment of prurigo nodularis: a systematic review. Front Immunol. 2023;14:1092685. doi:10.3389/fimmu.2023.1092685
- Dagenet CB, Saadi C, Phillips MA, et al. Landscape of prurigo nodularis clinical trials. JAAD Rev. 2024;2:127-136. doi:10.1016/j.jdrv.2024.09.006
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589. doi:10.1056/NEJMoa2301333
- Kwatra SG, Bordeaux ZA, Parthasarathy V, et al. Efficacy and safety of abrocitinib in prurigo nodularis and chronic pruritus of unknown origin: a nonrandomized controlled trial. JAMA Dermatol. 2024;160:717-724. doi:10.1001/jamadermatol.2024.1464
- Choi ME, Lee JH, Jung CJ, et al. A randomized, double-blinded, vehicle-controlled clinical trial of topical cryosim-1, a synthetic TRPM8 agonist, in prurigo nodularis. J Cosmet Dermatol. 2024;23:931-937. doi:10.1111/jocd.16079
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open-label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
From Refractory to Responsive: The Expanding Therapeutic Landscape of Prurigo Nodularis
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).
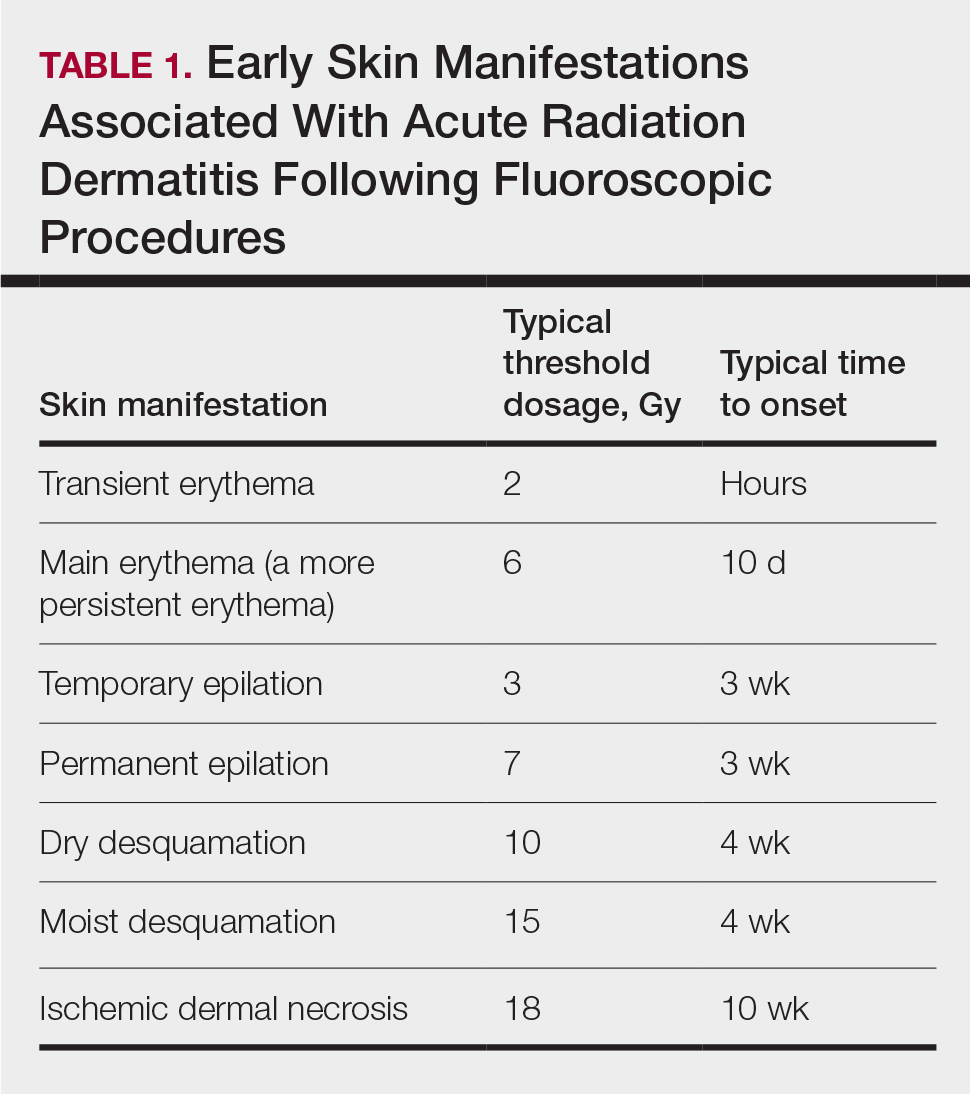
Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
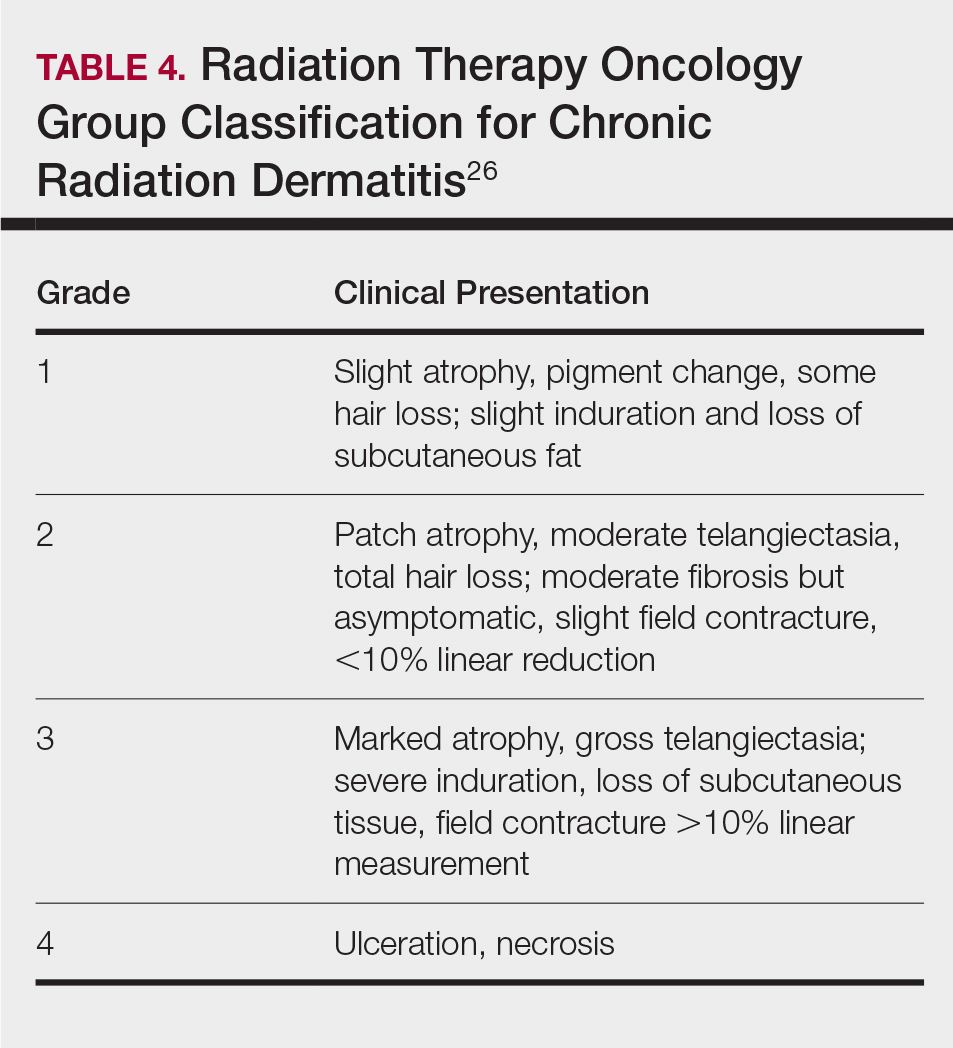
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27

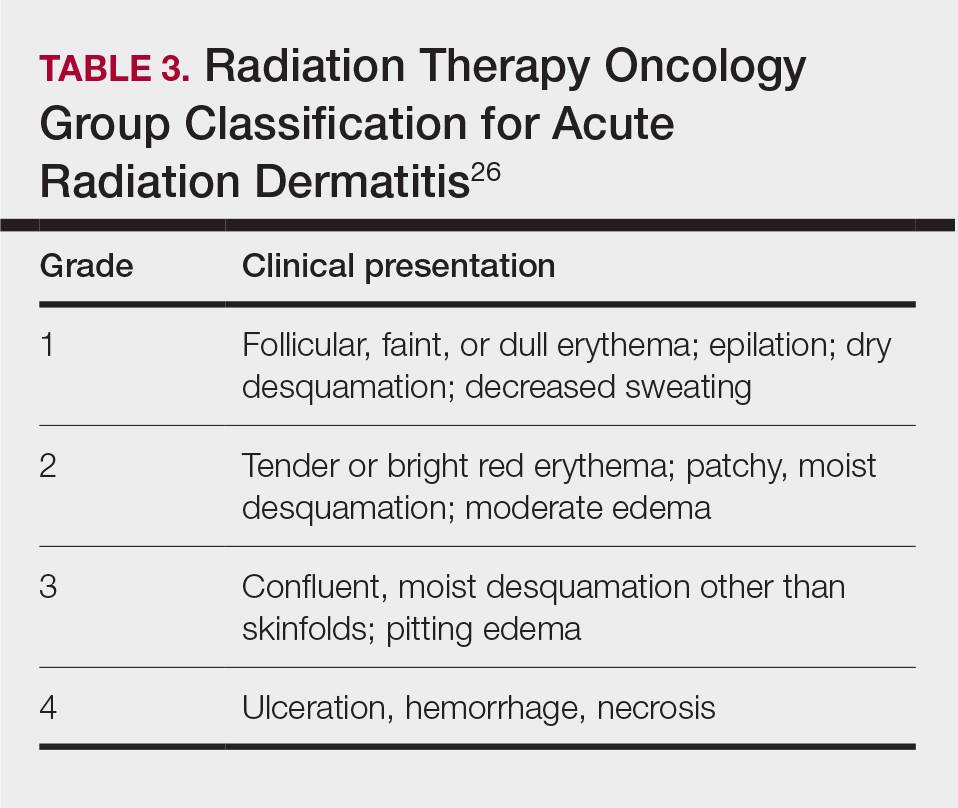
Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45
Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).

Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27



Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
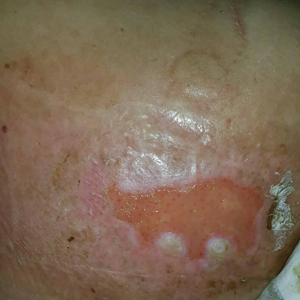
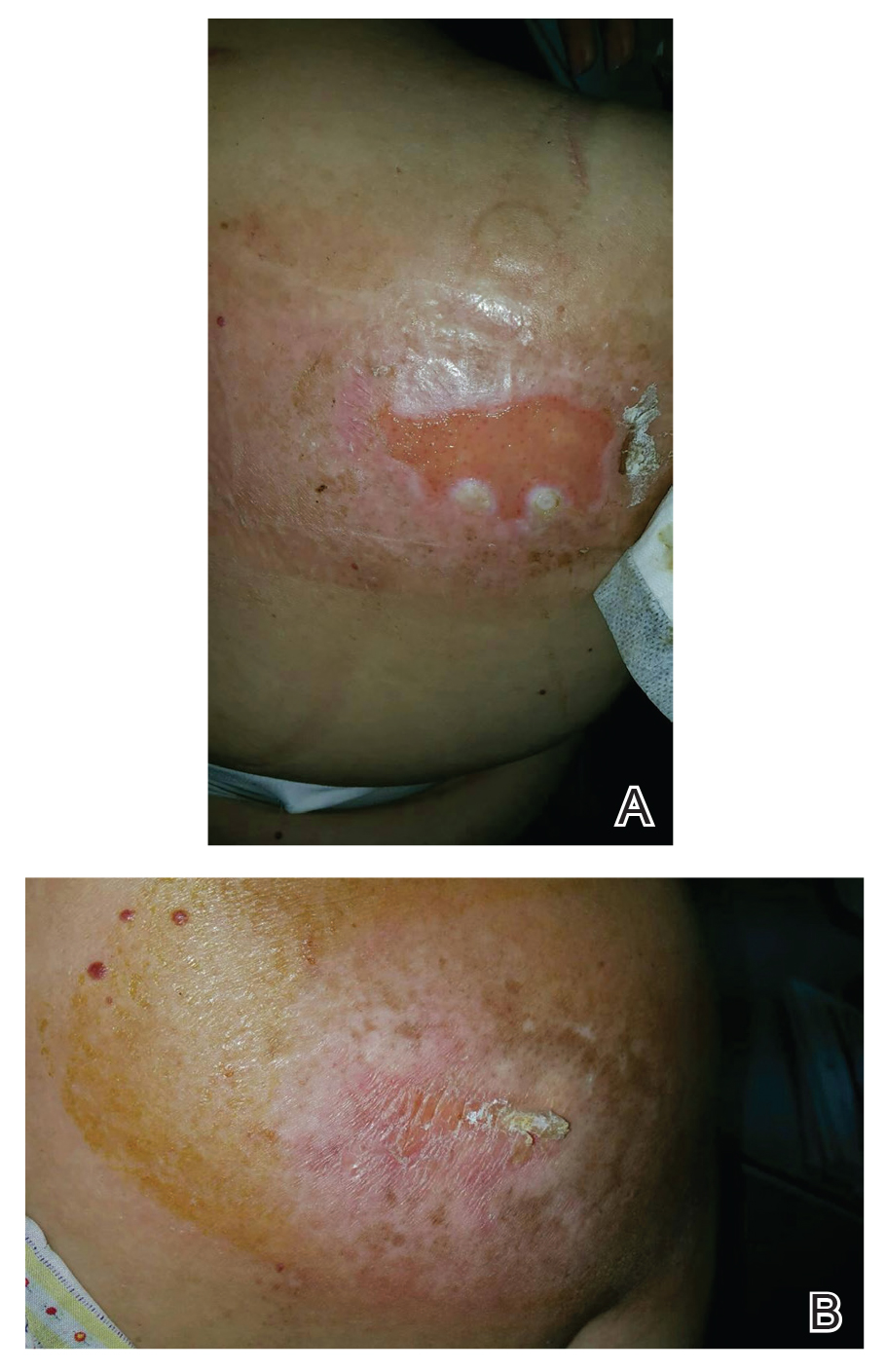
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45

Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
Fluoroscopy is an imaging technique that allows for real-time visualization of internal structures in the body using continuous radiography beams. More than 1 million fluoroscopy-guided procedures are performed annually in the United States.1 Utilization of these procedures continues to increase, and so does the probability of related complications, as prolonged exposure to ionizing radiation can cause skin injuries.2 Fortunately, the incidence of radiation-induced skin injuries compared with the total number of fluoroscopic procedures performed remains small,2 although one study suggested the incidence may be as high as 8.9% in at-risk populations.3
Radiation dermatitis is well recognized in dermatology as a complication of oncologic management; however, radiation dermatitis as a complication of fluoroscopic procedures is underrecognized.4 Fluoroscopy-induced radiation dermatitis can be categorized as acute, subacute, or chronic.5 Common fluoroscopic procedures that have been associated with fluoroscopy-induced radiation dermatitis include interventional cardiac procedures, neurovascular procedures, transjugular intrahepatic portosystemic shunt procedures, and endovascular abdominal aortic aneurysm repairs.6,7
Patients with fluoroscopy-induced radiation dermatitis, particularly fluoroscopy-induced chronic radiation dermatitis (FICRD), can present to dermatology up to several years after the initial fluoroscopy procedure with no awareness of the association between the procedure and their skin findings. This presents a diagnostic challenge, and FICRD often is overlooked.5,8-10
We conducted a literature search of PubMed articles indexed for MEDLINE using the search terms fluoroscopy and dermatitis. In this reappraisal, we will provide a comprehensive overview of fluoroscopy-induced radiation dermatitis with an emphasis on FICRD, covering its clinical manifestations, pathophysiology, risk factors, differential diagnosis, histology, and management. The aim of this review is to highlight the salient features and mimickers of FICRD and inform readers how to approach suspected cases, leading to accurate diagnosis and effective management.
Pathophysiology
Fluoroscopy-induced radiation dermatitis is the result of dose-dependent radiation-induced tissue damage. As the peak skin dosage (PSD) of radiation increases over the course of a procedure or multiple procedures, the severity of skin injury predictably increases. During fluoroscopic procedures, the standard irradiation dosage ranges from 0.02 Gy/min to 0.05 Gy/min.11 Transient skin changes may start to be seen around 2 Gy of cumulative exposure. Fluoroscopic procedures typically range in duration from 60 to 120 minutes; however, complex cases may exceed that. Additionally, multiple procedures performed within shorter intervals can result in greater PSD accumulation. Shorter intervals between procedures do not allow enough time for damage repair from the previous procedure and can result in further severe damage when the skin is re-exposed to radiation.2 The American College of Radiology recommends medical follow-up after 10 Gy of cumulative exposure, while cumulative exposure above 15 Gy within a 6- to 12-month period is defined as a sentinel event, according to The Joint Commission.12-14
Depending on the patient’s total radiation dosage during one or more procedures, the result of the tissue damage manifests differently at varying times: early skin changes are categorized as fluoroscopy-induced acute radiation dermatitis, and late skin changes are categorized as FICRD (Table 1).

Clinical Manifestations
Acute radiation dermatitis from fluoroscopic procedures manifests within hours to days up to 90 days following radiation exposure and can be characterized by erythema with blistering, desquamation, epilation, pigmentation changes, and even necrosis if the accumulated dosage exceeds 15 Gy.15 Chronic radiation dermatitis (which as related to fluoroscopic procedures is termed FICRD) has a longer onset of weeks to years and is clinically characterized by telangiectasias, permanent erythema, dermal atrophy, or ulcerations. Clinically, subacute radiation dermatitis shares features of both acute and chronic radiation dermatitis; therefore, it is differentiated based on its histologic features.5,16
Although fluoroscopy-induced acute radiation dermatitis (Table 1) may precede FICRD, acute manifestations of fluoroscopy-related dermatitis can be subtle and often manifest in areas not easily visualized. Because referrals to dermatologists for full-skin examinations after fluoroscopy procedures are not standard, patients may not be aware of the association between these procedures and the development of skin lesions. Nonetheless, some patients may report a history of skin changes such as redness days or weeks after a fluoroscopic procedure with accompanying pain and pruritus limited to the fluoroscopy-exposed region, which tend to self-resolve.17 The risk for FICRD is thought to increase if a history of fluoroscopy-induced acute radiation dermatitis is present.18
The location of the skin findings correlates to the area exposed to prolonged radiation during the procedure(s). The most common areas include the scapular and subscapular regions, the right lateral trunk inferior to the axilla, the mid back, and the right anterolateral chest.16,19,20 These regions are associated with more complex (eg, cardiac) procedures that have been reported to lead to prolonged radiation exposure. The skin findings in FICRD are described as geometric, corresponding to the squarish or rectangular radiography beam that is directed at the patient. Additionally, radiography beams spread outward as they travel in space; therefore, skin injuries are common at the region more distal to the path of origination of the beam.21-23 Subsequently, a geometric, dyspigmented, indurated or atrophic plaque with telangiectasias and erosions or ulcerations with progressive worsening is a common manifestation of FICRD.5,16,23 Patients also commonly present with pruritus or severe pain associated with the lesion.24,25
Dermatologic Manifestations of FICRD
Skin responses seen weeks to years after a fluoroscopic procedure and typically after cumulative radiation exposure of 10 Gy or greater are categorized as FICRD (Table 2). These changes also can be clinically graded based on the Radiation Therapy Oncology Group classification of radiation dermatitis (Tables 3 and 4).26 Chronic changes in the skin largely result from remodeling of the vasculature and the subcutaneous tissue over time. Unlike acute changes, chronic changes typically persist and continue to worsen.27



Telangiectasias—Anywhere from months to 1 year after exposure to 10 Gy of radiation, proliferation of atypical superficial vessels in the dermis can be seen, typically manifesting as telangiectasias on physical examination. Telangiectasias can increase with time and can even exhibit a dose-dependent relationship to the radiation exposure.28
Atrophy—Atrophic-appearing skin after radiation exposure is the result of direct injury to both the epidermis and fibroblasts in the dermis. The destruction of keratinocytes leads to a thin epidermis, and destruction of dermal fibroblasts causes insufficient collagen production.29 Clinically, this process manifests as an atrophic plaque that can be seen 12 weeks to 1 year after the procedure.
Fibrosis—Approximately 1 year after the exposure, the initial damage can lead to disruption of molecular pathways, causing fibrosis. Transforming growth factor (TGF) β1 is the main factor involved.29 Damage to the endothelial cells results in increased TGF-β1 levels, which causes increased stimulation of remaining atypical fibroblasts and thus increased irregular collagen deposition.30 Further adding to this knowledge, Wei et al31 recently proposed that damage to the epidermal keratinocytes leads to disruption of yes-associated protein 1, which is a protective factor released from keratinocytes that regulates the dermal fibroblasts. However, extensive damage to the keratinocytes can lead to lower yes-associated protein 1 levels and its downstream activity, leading to increased levels of TGF-β1 and fibroblast activity.31 Clinically, this fibrotic stage is seen as indurated plaques in patients.
Necrosis—There are 2 forms of necrosis that can be seen. Ischemic dermal necrosis typically occurs in the acute phase after 10 weeks and approximately 18 Gy of cumulative exposure. It results from substantial skin damage, including microvascular damage and reduction in dermal capillaries, leading to ischemia of the tissue.2 Late dermal necrosis is the process seen in the chronic stage of FICRD and radiation dermatitis not related to fluoroscopy. It results from the inability of the fibrotic dermis to vascularly support the epidermis above it.2 It can be seen anywhere from 1 to 4 years after the procedure. This stage clinically manifests as worsening ulcerations with major pain and increased risk for secondary infections.16
Dyspigmentation—Dyspigmentation at the site of the radiation exposure can be seen acutely and chronically. Dosage above 15 to 18 Gy can lead to destruction of melanocytes, which can cause hypopigmentation in exposed areas. However, melanocytes are relatively resistant to radiation; therefore, dosages below the threshold of destruction of 15 to 18 Gy can cause melanocytic hyperactivity leading to hyperpigmentation.32 Hence, pigmentary changes can vary greatly. Classically, a central area of hypopigmentation with surrounding hyperpigmentation is seen.
Histology
Histologic appearance of radiation dermatitis varies depending on its stage. Acute radiation dermatitis primarily demonstrates superficial dermal edema, damage to the basal cell layer, small vessel dilation with thrombi, and hemorrhage along with a sparse inflammatory cell infiltrate.33 Histology typically is the only way to characterize subacute radiation dermatitis.5 Lichenoid tissue reaction is its characteristic feature. Mononuclear cells are found adjected to necrotic keratinocytes along with prominent vacuolization of the basal cell layer.33
The key histologic features of chronic radiation dermatitis include epidermal atrophy, hyperkeratosis, telangiectasias, loss of adnexal structures, and dermal fibrosis along with sparse atypical stellate fibroblasts.34 However, clinical context of fluoroscopic exposure is required for the dermatopathologist to differentiate chronic radiation dermatitis from its histologic differential of morphea and lichen sclerosus. In a cross-sectional study, only 1 of 6 cases (16.7%) was correctly diagnosed as chronic radiation dermatitis in the absence of correlating clinical history.35
Risk Factors for FICRD
Since the diagnosis of FICRD can be a clinical challenge, understanding the risk factors can be helpful. The general likelihood of developing FICRD is related to the duration, frequency, interval, intensity, and area of radiation exposure. Procedures exceeding the normal duration of 60 to 120 minutes have been well documented as a substantial risk factor for radiation dermatitis and FICRD.36-38 The risk tends to be higher in longer procedures because they result in more radiation exposure and higher accumulated PSD. Obesity (ie, body mass index >26) is the major risk factor that has been associated with longer procedure times, as higher radiation dosages are necessary to penetrate the body of a larger patient and a larger skin surface area is exposed.37-39
Other risk factors associated with FICRD relate to how prone a patient is to radiation-induced DNA damage. Older patients are at higher risk due to lower intrinsic ability of the tissue to repair itself.11 Patients with a history of connective tissue diseases—particularly lupus, scleroderma, and mixed connective tissue disease—are at an increased risk.40 Furthermore, patients with genetic disorders that impair DNA repair are more susceptible to radiation-induced DNA damage; therefore, patients with ataxia-telangiectasia, xeroderma pigmentosum, Fanconi anemia, and hereditary nevoid basal cell carcinoma are at higher risk for FICRD.39 Similarly, medications that can affect DNA repair also have been shown to be risk factors. These medications include chemotherapeutic agents such as actinomycin D, cyclophosphamide, doxorubicin, methotrexate, and 5-fluorouracil.2,39 Diabetes, hyperthyroidism, and tobacco use also have been shown to increase a patient’s risk for FICRD.39 It also is reasonable to believe that patients with defects in fibroblasts or with elastin or collagen disorders (eg, Ehlers-Danlos syndrome) would be at higher risk, but there are no known studies highlighting the association in the literature.
Differential Diagnosis of FICRD
Acute allergic or irritant contact dermatitis manifests with a localized area of erythematous skin accompanied by pruritus.41 Patients with FICRD can present with a localized area of erythema and hyperpigmentation with minimal atrophy. The lesion may accompany substantial pruritus, which can favor the more common diagnosis of contact dermatitis.35,42,43
Fixed-drug eruption manifests as a well-defined, hyperpigmented plaque in a fixed location that occurs upon ingestion of a drug.44 Fluoroscopy-induced chronic radiation dermatitis lesions are well demarcated and geometrically shaped and therefore can mimic lesions seen in fixed-drug eruptions.45 Additionally, the patient population undergoing fluoroscopic procedures tends to have major comorbidities requiring multiple medications.4
Decubitus ulcers are a result of vascular compromise to an area of skin due to constant pressure and are most commonly seen in the sacral region of patients with obesity.46 Ulcerated FICRD lesions can manifest on the lower midback. These lesions can be seen after endovascular repair of abdominal aortic aneurysm or prostatic artery embolization.20,21 The location of these lesions can mimic decubitus ulcers if fluoroscopic history is unknown. As mentioned, obesity also increases the risk for FICRD.
Morphea can manifest as a localized area of induration and hyperpigmentation of the skin.47 When FICRD has progressed to dermal fibrosis, patients can present with indurated plaques without ulcerations, which can be hard to differentiate from morphea.16,48 However, the presence of ulcerations or hyperkeratosis can differentiate morphea from FICRD.16
Ultimately, it is the location of FICRD lesions that remains the biggest diagnostic clue. Any suspicious lesion present on the scapular or subscapular areas, anterolateral chest, and/or mid back should prompt an investigation into recent or remote history of fluoroscopic procedures.
Management of FICRD
Diagnosis of FICRD should be made clinically based on the history and physical examination whenever possible, since a biopsy is not recommended.35 Wound healing in FICRD is delayed, and biopsies can lead to ulcerations or secondary infections.17 Therefore, it is important to remain suspicious for FICRD. Management of FICRD should correspond to the clinical findings outlined by a recent Delphi consensus survey.49 Regardless, the core of FICRD management framework should always include good hygiene, maintenance of skin hydration to improve epithelialization, and sufficient photoprotection.49,50
Among the first signs of FICRD are telangiectasias. Although asymptomatic, their appearance can be distressing for patients. Pulsed dye laser therapy is a first-line option that has been studied and has shown clinical efficacy for treatment of telangiectasias and vascular changes in patients with FICRD.49,51
If patients develop fibrotic changes, treatment options are limited. Fibrosis is hard to reverse, and the management approach is limited to symptomatic relief. Mechanical and deep-friction massages have been shown to be effective at reducing skin induration in patients.52 Fractional ablative lasers also may be utilized for skin contractures, especially if range of motion is affected.53,54 Although it comes with its own challenges, autologous fat grafting has shown promise in reducing postradiation fibrosis and inducing angiogenesis in tissue.55 Oral pentoxifylline also has shown mild efficacy, as it may be able to suppress TGF-β1 levels.53 However, prevention of fibrotic changes may be the most important. Wei et al31 suggested that low-dose oral prednisolone at 5 mg twice daily for 3 weeks might be an option to prevent the progression of skin changes and even reverse fibrosis to an extent; however, further evidence regarding its efficacy still is necessary. Additionally, no evidence was identified to support the use of topical corticosteroids for fibrotic changes seen in FICRD.56
Patients with FICRD or even acute radiation dermatitis after fluoroscopy tend to develop superficial ulcerations from minor traumas. Good wound hygiene, antiseptic care, and absorbent dressings, such as hydrogel and hydrocolloid, may be sufficient for treating these wounds, as seen in the Figure.42,48 However, once patients develop refractory ulcerations or necrosis, treatment options are then limited to surgical removal with a flap or graft.5,33,42,45

Risk for basal cell carcinomas and squamous cell carcinomas is higher in patients with radiation exposure; however, the exact risk from fluoroscopic procedures is unknown. One study demonstrated an increased risk of 6.9% in development of skin cancer after a median radiation exposure of 15.5 Gy and a mean latency period of 38.3 years,57 and in another retrospective study, the risk was higher in Fitzpatrick skin types I and II.58 Unlike the development of radiodermatitis itself, which shows a dose-dependent response, development of skin cancers follows a stochastic pattern (not dose dependent).59 Therefore, it is important to identify these high-risk patients and establish follow-up.
Conclusion
Fluoroscopy-induced chronic radiation dermatitis can be a diagnostic challenge, as skin changes may not be readily associated with the procedure by patients. Therefore, any lesion with a geometric shape and accompanying chronic radiation dermatitis features located on the scapular or subscapular areas, anterolateral chest, and midback should prompt an investigation into history of fluoroscopic procedures. Treatment of chronic skin changes in FICRD depends on the clinical manifestations. Good hygiene, skin hydration, and sufficient photoprotection are crucial. Finally, long-term monitoring with skin examinations is important to assess for the development of skin cancers in the treated area.
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
- Benjamin EJ, Muntner P, Alonso A, et al. Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation. 2019;139:E56-E528. doi:10.1161/CIR.0000000000000659. Published correction appears in Circulation. 2020;141:E33.
- Koenig TR, Wolff D, Mettler FA, et al. Skin injuries from fluoroscopically guided procedures: part 1, characteristics of radiation injury. AJR Am J Roentgenol. 2001;177:3-11. doi:10.2214/ajr.177.1.1770003
- Guesnier-Dopagne M, Boyer L, Pereira B, et al. Incidence of chronic radiodermatitis after fluoroscopically guided interventions: a retrospective study. J Vasc Interv Radiol. 2019;30:692-698.e13. doi:10.1016/j.jvir.2019.01.010
- Cunha N, Cardoso P, Cabete J. Subacute radiation dermatitis following an interventional cardiology procedure. Cutan Ocul Toxicol. 2017;36:297-299. doi:10.1080/15569527.2016.1254649
- Frazier TH, Richardson JB, Fabré VC, et al. Fluoroscopy-induced chronic radiation skin injury: a disease perhaps often overlooked. Arch Dermatol. 2007;143:637-640. doi:10.1001/archderm.143.5.637
- Koenig TR, Mettler FA, Wagner LK. Skin injuries from fluoroscopically guided procedures: part 2, review of 73 cases and recommendations for minimizing dose delivered to patient. AJR Am J Roentgenol. 2001;177:13-20. doi:10.2214/ajr.177.1.1770013
- Shope TB. Radiation-induced skin injuries from fluoroscopy. Radiographics. 1996;16:1195-1199. doi:10.1148/radiographics.16.5.8888398
- Tchanque-Fossuo CN, Isseroff RR, Silverstein MA. Fluoroscopy induced chronic radiation dermatitis should be included in the differential diagnosis of notalgia paresthetica. Dermatol Online J. 2016;22:13030/qt0kh726m9.
- Berlin L. Radiation-induced skin injuries and fluoroscopy. AJR Am J Roentgenol. 2001;177:21-25. doi:10.2214/ajr.177.1.1770021
- Tchanque-Fossuo CN, Kamangar F, Ho B, et al. Fluoroscopy-induced radionecrosis. Dermatol Online J. 2016;22:13030/qt68w910t2.
- Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol. 1994;5:71-84. doi:10.1016/s1051-0443(94)71456-1
- Balter S, Hopewell JW, Miller DL, et al. Fluoroscopically guided interventional procedures: a review of radiation effects on patients’ skin and hair. Radiology. 2010;254:326-341. doi:10.1148/radiol.2542082312
- Vance AZ, Weinberg BD, Arbique GM, et al. Fluoroscopic sentinel events in neuroendovascular procedures: how to screen, prevent, and address occurrence. AJNR Am J Neuroradiol. 2013;34:1513-1515. doi:10.3174/ajnr.A3185
- Aerts A, Decraene T, van den Oord JJ, et al. Chronic radiodermatitis following percutaneous coronary interventions: a report of two cases. J Eur Acad Dermatol Venereol. 2003;17:340-343. doi:10.1046/j.1468-3083.2003.00687.x
- Rosenthal A, Israilevich R, Moy R. Management of acute radiation dermatitis: a review of the literature and proposal for treatment algorithm. J Am Acad Dermatol. 2019;81:558-567. doi:10.1016/j.jaad.2019.02.047
- Boncher J, Bergfeld WF. Fluoroscopy-induced chronic radiation dermatitis: a report of two additional cases and a brief review of the literature. J Cutan Pathol. 2012;39:63-67. doi:10.1111/j.1600-0560.2011.01754.x
- Spiker A, Zinn Z, Carter WH, et al. Fluoroscopy-induced chronic radiation dermatitis. Am J Cardiol. 2012;110:1861-1863. doi:10.1016/j.amjcard.2012.08.023
- Batrani M, Kubba A, Sundharam J. Fluoroscopy-induced chronic radiation dermatitis masquerading as morphea: a diagnostic pitfall. Indian J Pathol Microbiol. 2018;61:393-396. doi:10.4103/IJPM.IJPM_566_17
- Jeskowiak A, Hubmer M, Prenner G, et al. Radiation induced cutaneous ulcer on the back in a patient with congenital anomaly of the upper cava system. Interact Cardiovasc Thorac Surg. 2011;12:290-292.
- Laborda A, De Assis AM, Ioakeim I, et al. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38:755-759. doi:10.1007/s00270-015-1083-6
- Lyons AB, Harvey VM, Gusev J. Fluoroscopy-induced chronic radiation dermatitis (FICRD) after endovascular abdominal aortic aneurysm endoleak repair. JAAD Case Rep. 2015;1:403-405. doi:10.1016/j.jdcr.2015.09.022
- Mossman KL. Analysis of risk in computerized tomography and other diagnostic radiology procedures. Comput Radiol. 1982;6:251-256. doi:10.1016/0730-4862(82)90109-3
- Henry MF, Maender JL, Shen Y, et al. Fluoroscopy-induced chronic radiation dermatitis: a report of three cases. Dermatol Online J. 2009;15:3.
- Balter S, Miller DL. Patient skin reactions from interventional fluoroscopy procedures. AJR Am J Roentgenol. 2014;202:W335-W342. doi:10.2214/AJR.13.12029
- Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45:109-114. doi:10.4103/0970-0358.96599
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. doi:10.1016/0360-3016(95)00060-C
- Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer. 2013;21:2933-2948. doi:10.1007/s00520-013-1896-2
- Turesson I, Notter G. The predictive value of skin telangiectasia for late radiation effects in different normal tissues. Int J Radiat Oncol Biol Phys. 1986;12:603-609. doi:10.1016/0360-3016(86)90069-6
- Hegedus F, Mathew LM, Schwartz RA. Radiation dermatitis: an overview. Int J Dermatol. 2017;56:909-914. doi:10.1111/ijd.13371
- Denham JW, Hauer-Jensen M. The radiotherapeutic injury—a complex ‘wound.’ Radiother Oncol. 2002;63:129-145. doi:10.1016/s0167-8140(02)00060-9
- Wei KC, Lai SF, Huang WL, et al. An innovative targeted therapy for fluoroscopy-induced chronic radiation dermatitis. J Mol Med (Berl). 2022;100:135-146. doi:10.1007/s00109-021-02146-3
- Sitton E. Early and late radiation-induced skin alterations. part I: mechanisms of skin changes. Oncol Nurs Forum. 1992;19:801-807.
- Pruitt LG, Rogers W, Byarlay JA, et al. Subacute radiation dermatitis after fluoroscopy. J Cutan Pathol. 2016;43:1091-1095. doi:10.1111/cup.12815
- Anderson EB, Draft KS, Lee RA, et al. Update in dermatopathology. Am J Clin Pathol. 2006;125(Suppl):S50-S70. doi:10.1309/GMUFNP6LFMPNR86R
- Wei KC, Yang KC, Mar GY, et al. STROBE—radiation ulcer: an overlooked complication of fluoroscopic intervention: a cross-sectional study. Medicine (Baltimore). 2015;94:e2178. doi:10.1097/MD.0000000000002178
- Otterburn D, Losken A. Iatrogenic fluoroscopy injury to the skin. Ann Plast Surg. 2010;65:462-465. doi:10.1097/SAP.0b013e3181d6e2d3
- Cha MJ, Jo SJ, Cho Y, et al. Patient characteristics and the incidence of radiation-induced dermatitis following radiofrequency catheter ablation. Korean Circ J. 2016;46:646-653. doi:10.4070/kcj.2016.46.5.646
- Dehen L, Vilmer C, Humilière C, et al. Chronic radiodermatitis following cardiac catheterisation: a report of two cases and a brief review of the literature. Heart. 1999;81:308-312. doi:10.1136/hrt.81.3.308
- Brown KR, Rzucidlo E. Acute and chronic radiation injury. J Vasc Surg. 2011;53(Suppl 1):15S-21S. doi:10.1016/j.jvs.2010.06.175. Published correction appears in J Vasc Surg. 2012;55:627.
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Scheinman PL, Vocanson M, Thyssen JP, et al. Contact dermatitis. Nat Rev Dis Primers. 2021;7:38. doi:10.1038/s41572-021-00271-4
- Cheng TT, Yang HJ. Chronic radiation dermatitis induced by cardiac catheterization: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2022;31:147-149.
- Minni JP, Nowak M, Usmani A, et al. A unique case of subacute radiodermatitis. Cutis. 2013;91:230-232.
- Flowers H, Brodell R, Brents M, et al. Fixed drug eruptions: presentation, diagnosis, and management. South Med J. 2014;107:724-727. doi:10.14423/SMJ.0000000000000195
- Hashimoto I, Sedo H, Inatsugi K, et al. Severe radiation-induced injury after cardiac catheter ablation: a case requiring free anterolateral thigh flap and vastus lateralis muscle flap reconstruction on the upper arm. J Plast Reconstr Aesthet Surg. 2008;61:704-708. doi:10.1016/j.bjps.2007.01.003
- Mervis JS, Phillips TJ. Pressure ulcers: pathophysiology, epidemiology, risk factors, and presentation. J Am Acad Dermatol. 2019;81:881-890. doi:10.1016/j.jaad.2018.12.069
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Herz-Ruelas ME, Gómez-Flores M, Moxica-Del Angel J, et al. Ulcerated radiodermatitis induced after fluoroscopically guided stent implantation angioplasty. Case Rep Dermatol Med. 2014;2014:768624. doi:10.1155/2014/768624
- Wilson BN, Shah R, Menzer C, et al. Consensus on the clinical management of chronic radiation dermatitis and radiation fibrosis: a Delphi survey. Br J Dermatol. 2022;187:1054-1056. doi:10.1111/bjd.21852
- Khanna NR, Kumar DP, Laskar SG, et al. Radiation dermatitis: an overview. Indian J Burns. 2013;21:24-31. doi:10.4103/0971-653x.121877
- Spalek M. Chronic radiation-induced dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:473-482. doi:10.2147/CCID.S94320
- Bourgeois JF, Gourgou S, Kramar A, et al. A randomized, prospective study using the LPG technique in treating radiation-induced skin fibrosis: clinical and profilometric analysis. Skin Res Technol. 2008;14:71-76. doi:10.1111/j.1600-0846.2007.00263.x
- Borrelli MR, Shen AH, Lee GK, et al. Radiation-induced skinfibrosis: pathogenesis, current treatment options, and emerging therapeutics. Ann Plast Surg. 2019;83(4S Suppl 1):S59-S64. doi:10.1097/SAP.0000000000002098
- Wilson B, Shah R, Menzer C, et al. Laser therapy as a treatment for chronic radiation fibrosis. Lasers Surg Med. 2023;55:82-88. doi:10.1002/lsm.23617
- Rigotti G, Marchi A, Galiè M, et al. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: a healing process mediated by adipose-derived adult stem cells. Plast Reconstr Surg. 2007;119:1409-1422. doi:10.1097/01.prs.0000256047.47909.71
- Leventhal J, Young MR. Radiation dermatitis: recognition, prevention, and management. Oncology (Williston Park). 2017;31:885-899.
- van Vloten WA, Hermans J, van Daal WA. Radiation-induced skin cancer and radiodermatitis of the head and neck. Cancer. 1987;59:411-414. doi:10.1002/1097-0142(19870201)59:3<411::aid-cncr2820590310>3.0.co;2-z
- Davis MM, Hanke CW, Zollinger TW, et al. Skin cancer in patients with chronic radiation dermatitis. J Am Acad Dermatol. 1989;20:608-616. doi:10.1016/s0190-9622(89)70072-4
- Miller DL, Balter S, Schueler BA, et al. Clinical radiation management for fluoroscopically guided interventional procedures. Radiology. 2010;257:321-332. doi:10.1148/radiol.10091269
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
Fluoroscopy-Induced Chronic Radiation Dermatitis: A Comprehensive Review and Reappraisal
PRACTICE POINTS
- Fluoroscopy-induced chronic radiation dermatitis poses diagnostic challenges, as patients often are unable to associate a history of fluoroscopic procedures with the development of skin lesions.
- Scapular and subscapular lesions as well as those on the anterolateral chest and mid back should prompt clinicians to inquire about the patient’s history of fluoroscopic procedures.
- Because lesions can remain refractory to treatment, longterm monitoring is necessary if they are not excised.
Prurigo Nodularis Mechanisms and Current Management Options
Prurigo nodularis (PN)(also called chronic nodular prurigo, prurigo nodularis of Hyde, or picker’s nodules) was first characterized by James Hyde in 1909.1-3 Prurigo nodularis manifests with symmetrical, intensely pruritic, eroded, or hyperkeratotic nodules or papules on the extremities and trunk.1,2,4,5 Studies have shown that individuals with PN experience pruritus, sleep loss, decreased social functioning from the appearance of the nodules, and a higher incidence of anxiety and depression, causing a negative impact on their quality of life.2,6 In addition, the manifestation of PN has been linked to neurologic and psychiatric disorders; however, PN also can be idiopathic and manifest without underlying illnesses.2,6,7
Prurigo nodularis has been associated with other dermatologic conditions such as atopic dermatitis (up to 50%), lichen planus, keratoacanthomas (KAs), and bullous pemphigoid.7-9 It also has been linked to systemic diseases in 38% to 50% of cases, including chronic kidney disease, liver disease, type 2 diabetes mellitus, malignancies (hematopoietic, liver, and skin), and HIV infection.6,8,10
The pathophysiology of PN is highly complex and has yet to be fully elucidated. It is thought to be due to dysregulation and interaction of the increase in neural and immunologic responses of proinflammatory and pruritogenic cytokines.2,11 Treatments aim to break the itch-scratch cycle that perpetuates this disorder; however, this proves difficult, as PN is associated with a higher itch intensity than atopic dermatitis and psoriasis.10 Therefore, most patients attempt multiple forms of treatment for PN, ranging from topical therapies, oral immunosuppressants, and phototherapy to the newest and only medication approved by the US Food and Drug Administration for the treatment of PN—dupilumab.1,7,11 Herein, we provide an updated review of PN with a focus on its epidemiology, histopathology and pathophysiology, comorbidities, clinical presentation, differential diagnosis, and current treatment options.
Epidemiology
There are few studies on the epidemiology of PN; however, middle-aged populations with underlying dermatologic or psychiatric disorders tend to be impacted most frequently.2,12,13 In 2016, it was estimated that almost 88,000 individuals had PN in the United States, with the majority being female; however, this estimate only took into account those aged 18 to 64 years and utilized data from IBM MarketScan Commercial Claims and Encounters Database (IBM Watson Health) from October 2015 to December 2016.14 More recently, a retrospective database analysis estimated the prevalence of PN in the United States to be anywhere from 36.7 to 43.9 cases per 100,000 individuals. However, this retrospective review utilized the International Classification of Diseases, Tenth Revision code; PN has 2 codes associated with the diagnosis, and the coding accuracy is unknown.15 Sutaria et al16 looked at racial disparities in patients with PN utilizing data from TriNetX and found that patients who received a diagnosis of PN were more likely to be women, non-Hispanic, and Black compared with control patients. However, these estimates are restricted to the health care organizations within this database.
In 2018, Poland reported an annual prevalence of 6.52 cases per 100,000 individuals,17 while England reported a yearly prevalence of 3.27 cases per 100,000 individuals.18 Both countries reported most cases were female. However, these studies are not without limitations. Poland only uses the primary diagnosis code for medical billing to simplify clinical coding, thus underestimating the actual prevalence; furthermore, clinical codes more often than not are assigned by someone other than the diagnosing physician, leaving room for error.17 In addition, England’s PN estimate utilized diagnosis data from primary care and inpatient datasets, leaving out outpatient datasets in which patients with PN may have been referred and obtained the diagnosis, potentially underestimating the prevalence in this population.18
In contrast, Korea estimated the annual prevalence of PN to be 4.82 cases per 1000 dermatology outpatients, with the majority being men, based on results from a cross-sectional study among outpatients from the Catholic Medical Center. Although this is the largest health organization in Korea, the scope of this study is limited and lacks data from other medical centers in Korea.19
Histopathology and Pathophysiology
Almost all cells in the skin are involved in PN: keratinocytes, mast cells, dendritic cells, endothelial cells, lymphocytes, eosinophils, collagen fibers, and nerve fibers.11,20 Classically, PN manifests as a dome-shaped lesion with hyperkeratosis, hypergranulosis, and psoriasiform epidermal hyperplasia with increased thickness of the papillary dermis consisting of coarse collagen with compact interstitial and circumvascular infiltration as well as increased lymphocytes and histocytes in the superficial dermis (Figure 1).20 Hyperkeratosis is thought to be due to either the alteration of keratinocyte structures from scratching or keratinocyte abnormalities triggering PN.21 However, the increase in keratinocytes, which secrete nerve growth factor, allows for neuronal hyperplasia within the dermis.22 Nerve growth factor can stimulate keratinocyte proliferation23 in addition to the upregulation of substance P (SP), a tachykinin that triggers vascular dilation and pruritus in the skin.24 The density of SP nerve fibers in the dermis increases in PN, causing proinflammatory effects, upregulating the immune response to promote endothelial hyperplasia and increased vascularization.25 The increase in these fibers may lead to pruritus associated with PN.2,26
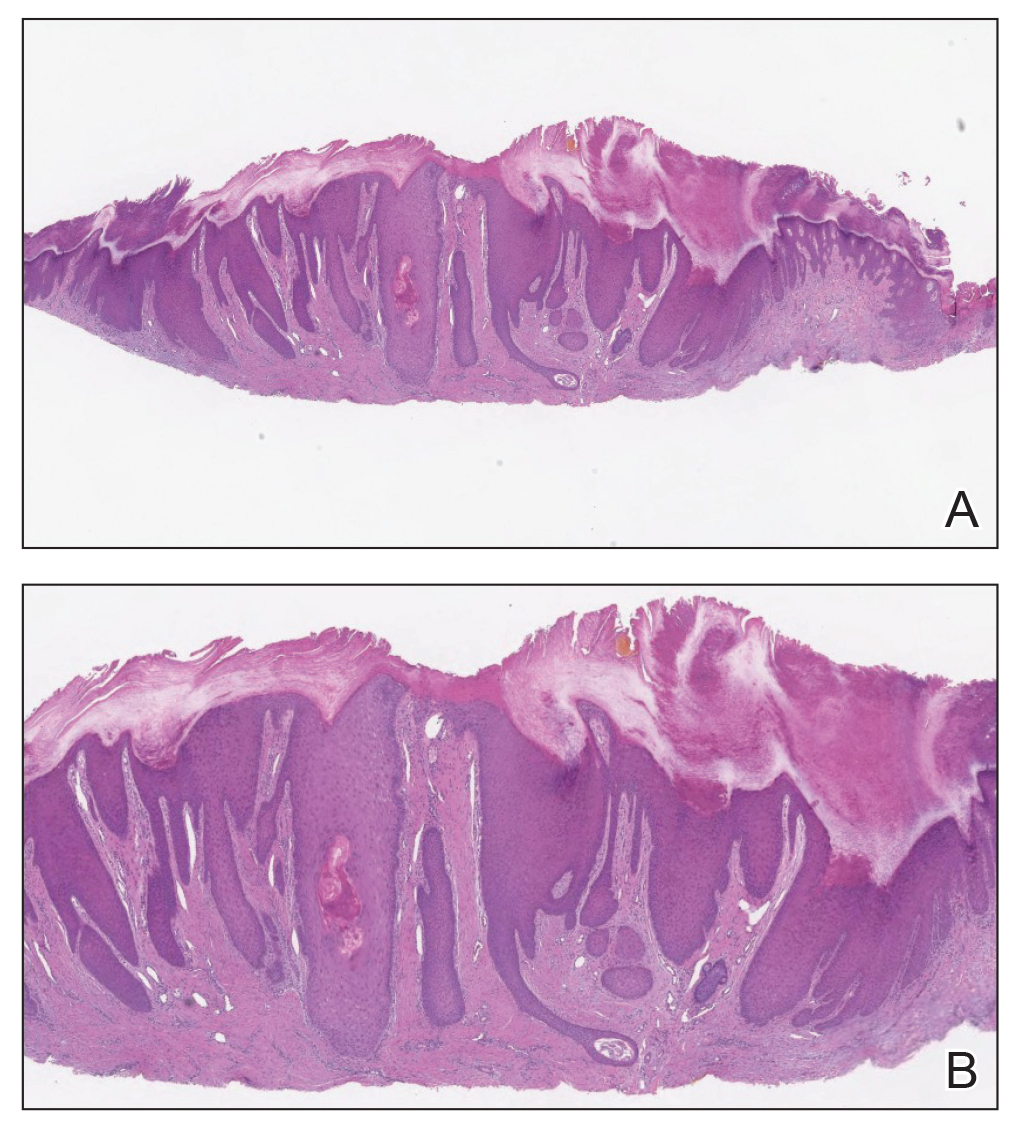
Many inflammatory cytokines and mediators also have been implicated in PN. Increased messenger RNA expression of IL-4, IL-17, IL-22, and IL-31 has been described in PN lesions.3,27 Furthermore, studies also have reported increased helper T cell (TH2) cytokines, including IL-4, IL-5, IL-10, and IL-13, in the dermis of PN lesions in patients without a history of atopy.3,28 These pruritogenic cytokines in conjunction with the SP fibers may create an intractable itch for those with PN. The interaction and culmination of the neural and immune responses make PN a complex condition to treat with the multifactorial interaction of systems.
Comorbidities
Prurigo nodularis has been associated with a wide array of comorbidities; however, the direction of the relationship between PN and these conditions makes it difficult to discern if PN is a primary or secondary condition.29 Prurigo nodularis commonly has been connected to other inflammatory dermatoses, with a link to atopic dermatitis being the strongest.5,29 However, PN also has been linked to other pruritic inflammatory cutaneous disorders, including psoriasis, cutaneous T-cell lymphoma, lichen planus, and dermatitis herpetiformis.14,29
Huang et al14 found an increased likelihood of psychiatric illnesses in patients with PN, including eating disorders, nonsuicidal self-injury disorder, attention-deficit/hyperactivity disorder, schizophrenia, mood disorders, anxiety, and substance abuse disorders. Treatments directed at the neural aspect of PN have included selective serotonin reuptake inhibitors (SSRIs), which also are utilized to treat these mental health disorders.
Furthermore, systemic diseases also have been found to be associated with PN, including hypertension, type 2 diabetes mellitus, chronic kidney disease, heart failure, cerebrovascular disease, coronary heart disease, and chronic obstructive pulmonary disease.14 The relationship between PN and systemic conditions may be due to increased systemic inflammation and dysregulation of neural and metabolic functions implicated in these conditions from increased pruritic manifestations.29,30 However, studies also have connected PN to infectious conditions such as HIV. One study found that patients with PN had 2.68 higher odds of infection with HIV compared to age- and sex-matched controls.14 It is unknown if these conditions contributed to the development of PN or PN contributed to the development of these disorders.
Clinical Presentations
Prurigo nodularis is a chronic inflammatory skin disease that typically manifests with multiple severely pruritic, dome-shaped, firm, hyperpigmented papulonodules with central scale or crust, often with erosion, due to chronic repetitive scratching and picking secondary to pruritic systemic or dermatologic diseases or psychological disorders (Figure 2).1,2,4,5,8,31 Most often, diagnosis of PN is based on history and physical examination of the lesion; however, biopsies may be performed. These nodules commonly manifest with ulceration distributed symmetrically on extensor extremities in easy-to-reach places, sparing the mid back (called the butterfly sign).8 Lesions—either a few or hundreds—can range from a few millimeters to 2 to 3 cm.8,32 The lesions differ in appearance depending on the pigment in the patient’s skin. In patients with darker skin tones, hyperpigmented or hypopigmented papulonodules are not uncommon, while those with fairer skin tones tend to present with erythema.31
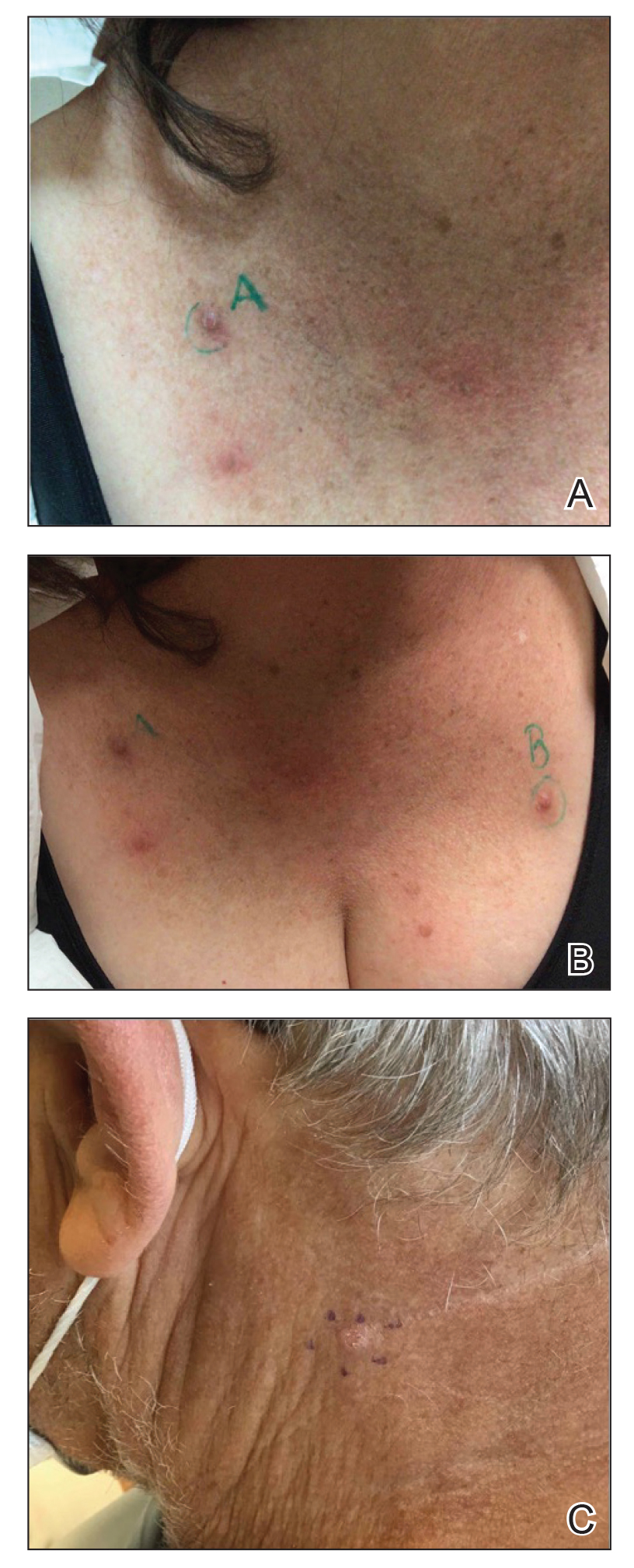
Differential Diagnosis
Because of the variation in manifestation of PN, these lesions may resemble other cutaneous conditions. If the lesions are hyperkeratotic, they can mimic hypertrophic lichen planus, which mainfests with hyperkeratotic plaques or nodules on the lower extremities.8,29 In addition, the histopathology of lichen planus resembles the appearance of PN, with epidermal hyperplasia, hypergranulosis, hyperkeratosis, and increased fibroblasts and capillaries.8,29
Pemphigoid nodularis is a rare subtype of bullous pemphigoid that exhibits characteristics of PN with pruritic plaques and erosions.8,29,33 The patient population for pemphigoid nodularis tends to be aged 50 to 60 years, and females are affected more frequently than males. However, pemphigoid nodularis may manifest with blistering and large plaques, which are not seen commonly with PN.29 On histopathology, pemphigoid nodularis deposits IgG and C3 on the basement membrane and has subepidermal clefting, unlike PN.7,29
Actinic prurigo manifests with pruritic papules or nodules post–UV exposure to unprotected skin.8,29,33 This rare condition usually manifests with cheilitis and conjunctivitis. Unlike PN, which commonly affects elderly populations, actinic prurigo typically is found in young females.8,29 Cytologic examination shows hyperkeratosis, spongiosis, and acanthosis of the epidermis with lymphocytic perivascular infiltration of the dermis.34
Neurotic excoriations also tend to mimic PN with raised excoriated lesions; however, this disorder is due to neurotic picking of the skin without associated pruritus or true hyperkeratosis.8,29,33 Histopathology shows epidermal crusting with inflammation of the upper dermis.35
Infiltrative cutaneous squamous cell carcinoma (SCC) may imitate PN in appearance. It manifests as tender, ulcerated, scaly plaques or nodules. Histopathology shows cytologic atypia with an infiltrative architectural pattern and presence of collections of compact keratin and parakeratin (called keratin pearls).
Keratoacanthomas can resemble PN lesions. They usually manifest as nodules measuring 1 to 2 cm in diameter and 0.5 cm thick, resembling crateriform tumors.36 On histopathology, KAs can resemble SCCs; however, KAs tend to manifest more frequently with a keratin-filled crater with a ground-glass appearance.36
Inverted follicular keratosis commonly manifests on the face in elderly men as a single, flesh-colored, verrucous papule that may resemble PN. However, cytology of inverted follicular keratosis is characterized by proliferation and squamous eddies.37 Consideration of the histologic findings and clinical appearance are important to differentiate between PN and cutaneous SCC.
Pseudoepitheliomatous hyperplasia is a benign condition that manifests as a plaque or nodule with crust, scale, or ulceration. Histologically, this condition presents with hyperplastic proliferation of the epidermis and adnexal epithelium.38 The clinical and histologic appearance can mimic PN and other cutaneous eruptions with epidermal hyperplasia.
In clinical cases that are resistant to treatment, biopsy is the best approach to diagnose the lesion. Due to similarities in physical appearance and superficial histologic presentation of PN, KAs from SCC, hypertrophic lichen planus, and other hyperkeratotic lesions, the biopsy should be taken at the base of the lesion to sample deeper layers of skin to differentiate these dermatologic disorders.
Management
Current treatments for PN yield varied results. Many patients with moderate to severe PN attempt multiple therapies before seeing improvement.31 Treatments include topical, oral, and injectable medications and are either directed at the neural or immune components of PN due to the interplay between increased nerve fibers in the lesions (neural axis) as well as increases in cytokines and other immunologic mediators (immune axis) of this condition. However, the FDA recently approved the first treatment for PN—dupilumab—which is an injectable IL-4 receptor antagonist directed at the immunologic interactions affiliated with PN.
Immune-Mediated Topical Therapies—Immunologic topical therapies include corticosteroids, calcipotriol, and calcineurin inhibitors. Studies that have analyzed these treatments are limited to case reports and small intraindividual and randomized controlled trials (Table 1). Topical therapies usually are first-line agents for most patients. Adverse effects include transient irritation of the skin.40,42,43
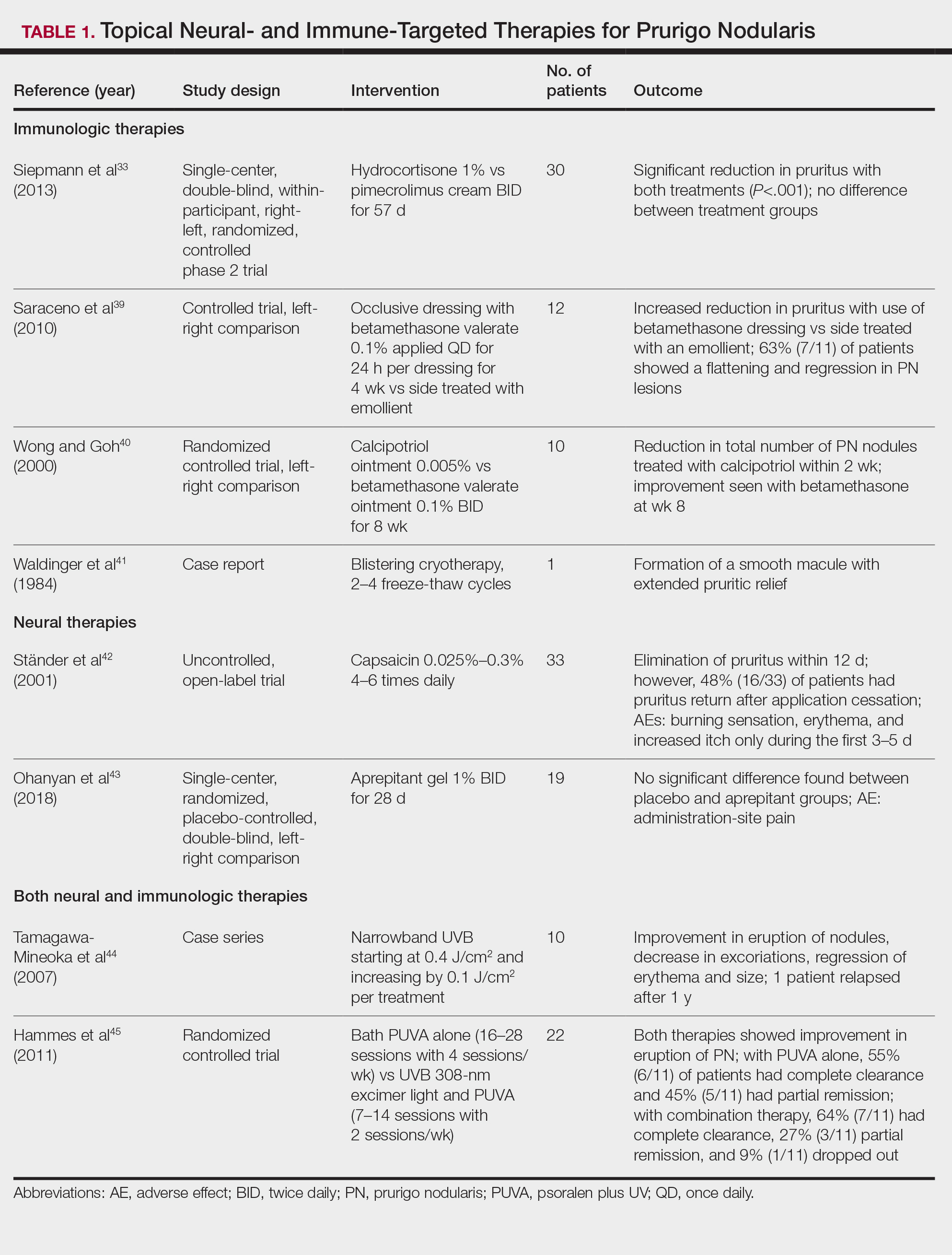
Cryotherapy is another topical and immunologic therapy for those with PN; however, this treatment is more appropriate for patients with fewer lesions due to the pain that accompanies lesions treated with liquid nitrogen. In addition, this therapy can cause dyspigmentation of the skin in the treated areas.41
Similar to cryotherapy, intralesional corticosteroid injections are appropriate for patients with few PN lesions. A recent report described intralesional corticosteroid injections of 2.5 mg/mL for a PN nodule with high efficacy.46,47 This treatment has not undergone trials, but success with this modality has been documented, with adverse effects including hyperpigmentation or hypopigmentation in the treated area and transient pain.46
Neural-Mediated Topical Therapies—Neural topical therapies include capsaicin and neurokinin-1 receptor antagonists, aprepitant43 and serlopitant. These treatment studies are limited to small open-label and randomized controlled trials. Adverse effects of these treatments include transient cutaneous pain at the site of topical administration. In addition, neural-mediated topical therapies have shown either limited improvements from baseline or return of symptoms after treatment cessation.42,43
Supplements—N-acetyl cysteine is an over-the-counter supplement that has been reported to improve symptoms in patients with skin-picking disorders.48 The mechanism of action includes antioxidant effects such as decreasing reactive oxygen species, decreasing inflammatory markers, regulating neurotransmitters, and inhibiting hyperkeratosis.49 N-acetyl cysteine has been poorly studied for its application in PN. A small study of 3 patients with subacute PN receiving 1200 mg of oral N-acetyl cysteine reported varying levels of improvement in skin appearance and reduction in skin picking.50
Phototherapy—Phototherapy, a typical first- or second-line treatment modality for PN, targets both the neural- and immune-mediated aspects associated with pruritus in PN (Table 1).51 UV light can penetrate through the epidermal layer of the skin and reach the keratinocytes, which play a role in the immune-related response of PN. In addition, the cutaneous sensory nerves are located in the upper dermal layer, from which nerve fibers grow and penetrate into the epidermis, thereby interacting with the keratinocytes where pruritic signals are transmitted from the periphery up to the brain.51
Studies analyzing the effects of phototherapy on PN are limited to case series and a small randomized controlled trial. However, this trial has shown improvements in pruritus in the participants. Adverse effects include transient burning and erythema at the treated sites.44,45
Immune-Mediated Oral Therapies—Immunologic-targeted oral therapies include bilastine, methotrexate, and cyclosporine (Table 2).52,53 Bilastine efficacy was analyzed in a small phase 3, open-label, multicenter study in Japan; however, patients were allowed to use topical steroids in conjunction with the oral antihistamine.54 Methotrexate and cyclosporine are immunosuppressive medications and were analyzed in small retrospective studies. Both treatments yielded notable relief for patients; however, 38.5% (15/39) of patients receiving methotrexate experienced adverse events, and 50.0% (4/8) experienced adverse events with cyclosporine.52,53
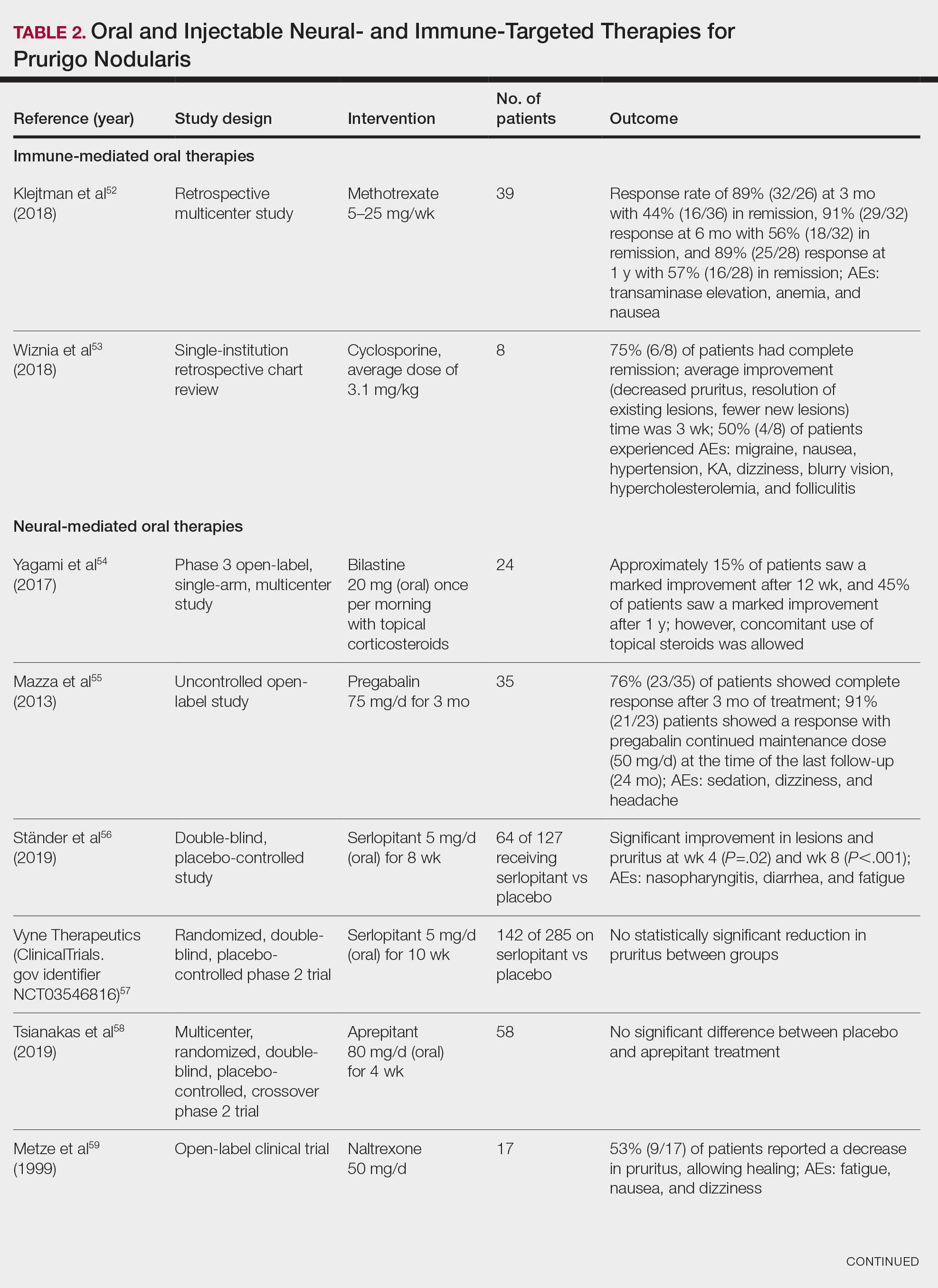
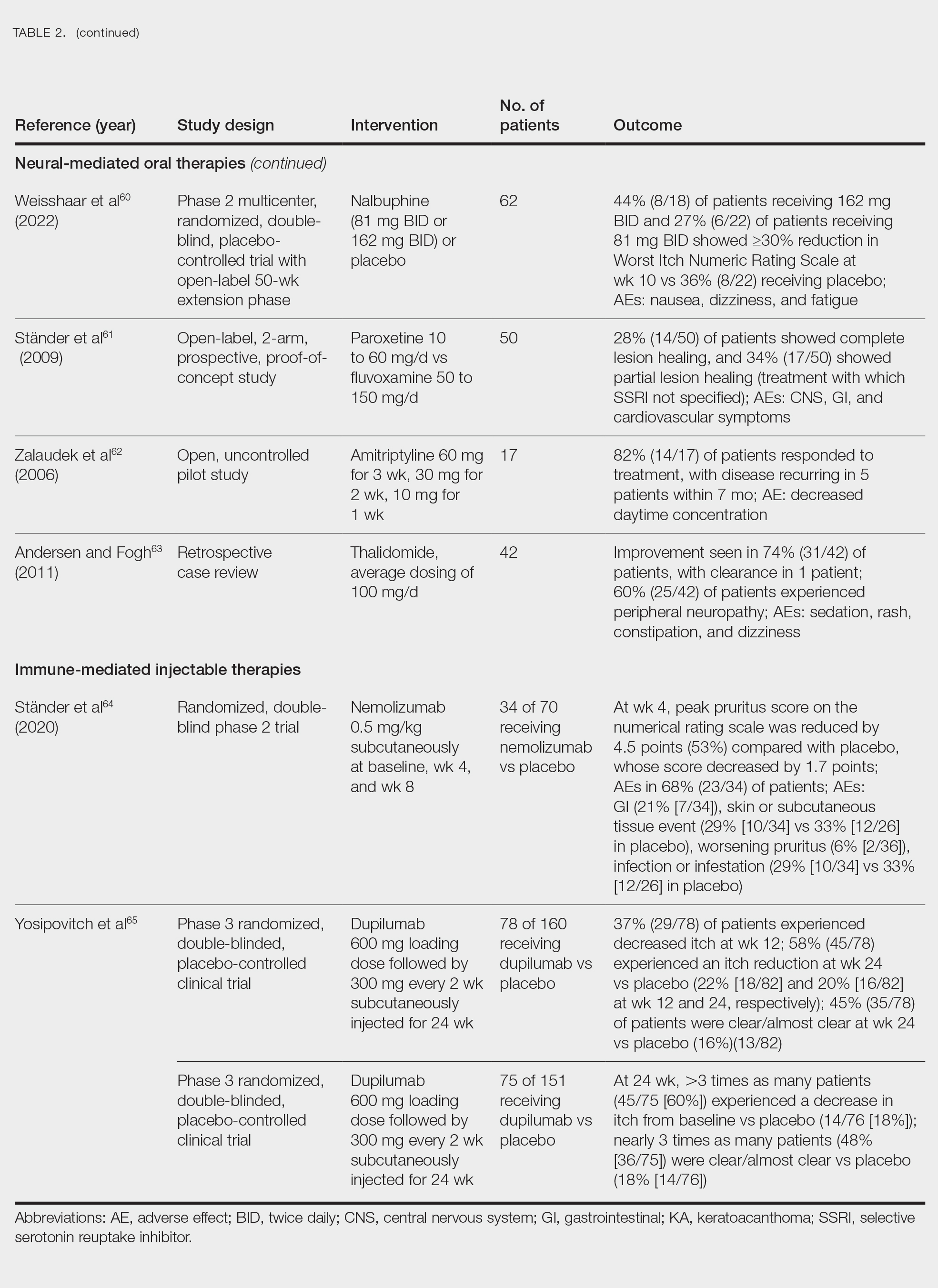
Neural-Mediated Oral Therapies—Neural-targeted oral therapies include pregabalin, serlopitant, aprepitant, naltrexone, nalbuphine, SSRIs (paroxetine and fluvoxamine), amitriptyline, and thalidomide. The research on these treatments ranges from case reviews to randomized controlled trials and open-label trials (Table 2).55-63
Thalidomide was studied in a small retrospective case review that showed notable improvement in PN. Dosages of thalidomide varied, but on average the dose was 100 mg/d. However, greater than 50% of patients experienced at least 1 adverse effect with this treatment.63
A study performed in Italy showed promising results for patients treated with pregabalin, with 70.0% (21/30) continuing to take pregabalin for almost 2 years following completion of the initial 3-month trial.55 Naltrexone decreased pruritus in more than half of patients (9/17).59 Amitriptyline yielded improvements in patients with PN; however, disease recurred in 5 patients (29%) after 7 months.62 A study performed in Germany reported promising results for paroxetine and fluvoxamine; however, some patients enrolled in the study had some form of psychiatric disorder.61
Serlopitant, aprepitant, and nalbuphine were studied in randomized controlled trials. The serlopitant trials were the largest of the neurally mediated oral medication studies; one showed substantial improvement in patients with PN,56 while the most recent trial did not show significant improvement (ClinicalTrials.gov identifier NCT03546816).57 On the other hand, aprepitant showed no major difference between the experimental and placebo groups.58 Nalbuphine 162 mg twice daily showed greater improvement in PN than nalbuphine 81 mg twice daily.60
Immune-Mediated Injectable Therapies—Immune-targeted injectables include nemolizumab and dupilumab (Table 2). Nemolizumab is an IL-31 antagonist that has been studied in a small randomized controlled trial that showed great success in decreasing pruritus associated with PN.64 IL-31 has been implicated in PN, and inhibition of the IL-31 receptor has been shown to disrupt the itch-scratch cycle of PN. Dupilumab is a monoclonal antibody against the IL-4 and IL-13 receptors, and it is the only FDA-approved treatment for PN.65 Blockage of these protein receptors decreases type 2 inflammation and chronic pruritus.66,67 Dupilumab is FDA approved for the treatment of atopic dermatitis and recently was approved for adults with PN. Dupilumab acts to block the shared α-subunit of the pruritogenic cytokines IL-4 and IL-13 pathways,29 thereby breaking the itch-scratch cycle associated with PN and allowing for the healing of these lesions. Results from 2 clinical trials showed substantially reduced itch in patients with PN.65 Dupilumab also was approved by the European Medicines Agency for moderate to severe PN.68
Conclusion
Prurigo nodularis is a chronic condition that affects patient quality of life and can mimic various dermatologic conditions. The epidemiology and pathophysiology of PN have not been fully expounded. More research should be conducted to determine the underpinnings of PN to help identify more consistently effective therapies for this complex condition.
- Durmaz K, Ataseven A, Ozer I, et al. Prurigo nodularis responding to intravenous immunoglobulins. Przegl Dermatol. 2022;109:159-162. doi:10.5114/dr.2022.117988
- Kowalski EH, Kneiber D, Valdebran M, et al. Treatment-resistant prurigo nodularis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:163-172. doi:10.2147/CCID.S188070
- Wong LS, Yen YT. Chronic nodular prurigo: an update on the pathogenesis and treatment. Int J Mol Sci. 2022;23:12390. doi:10.3390/ijms232012390
- Janmohamed SR, Gwillim EC, Yousaf M, et al. The impact of prurigo nodularis on quality of life: a systematic review and meta-analysis. Arch Dermatol Res. 2021;313:669-677. doi:10.1007/s00403-020-02148-0
- Zeidler C, Ständer S. The pathogenesis of prurigo nodularis - ‘super-itch’ in exploration. Eur J Pain. 2016;20:37-40. doi:10.1002/ejp.767
- Kwatra SG. Breaking the itch–scratch cycle in prurigo nodularis. N Engl J Med. 2020;382:757-758. doi:10.1056/NEJMe1916733
- Frølunde AS, Wiis MAK, Ben Abdallah H, et al. Non-atopic chronic nodular prurigo (prurigo nodularis hyde): a systematic review of best-evidenced treatment options. Dermatology. 2022;238:950-960. doi:10.1159/000523700
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
- Kowalski EH, Kneiber D, Valdebran M, et al. Distinguishing truly recalcitrant prurigo nodularis from poor treatment adherence: a response to treatment-resistant prurigo nodularis [Response to letter]. Clin Cosmet Investig Dermatol. 2019;12:371-372. doi:10.2147/CCID.S214195
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580. doi:10.1016/j.jaad.2021.05.036
- Labib A, Ju T, Vander Does A, et al. Immunotargets and therapy for prurigo nodularis. Immunotargets Ther. 2022;11:11-21. doi:10.2147/ITT.S316602
- Belzberg M, Alphonse MP, Brown I, et al. Prurigo nodularis is characterized by systemic and cutaneous T helper 22 immune polarization. J Invest Dermatol. 2021;141:2208-2218.e14. doi:10.1016/j.jid.2021.02.749
- Ständer S, Pereira MP, Berger T, et al. IFSI-guideline on chronic prurigo including prurigo nodularis. Itch. 2020;5:e42. doi:10.1097/itx.0000000000000042
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140:480-483.e4. doi:10.1016/j.jid.2019.07.697
- Ständer S, Augustin M, Berger T, et al. Prevalence of prurigo nodularis in the United States of America: a retrospective database analysis. JAAD Int. 2021;2:28-30. doi:10.1016/j.jdin.2020.10.009
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;86:487-490. doi:10.1016/j.jaad.2021.09.028
- Ryczek A, Reich A. Prevalence of prurigo nodularis in Poland. Acta Derm Venereol. 2020;100:adv00155. doi:10.2340/00015555-3518
- Morgan CL, Thomas M, Ständer S, et al. Epidemiology of prurigo nodularis in England: a retrospective database analysis. Br J Dermatol. 2022;187:188-195. doi:10.1111/bjd.21032
- Woo YR, Wang S, Sohn KA, et al. Epidemiology, comorbidities, and prescription patterns of Korean prurigo nodularis patients: a multi-institution study. J Clin Med Res. 2021;11:95. doi:10.3390/jcm11010095
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Yang LL, Jiang B, Chen SH, et al. Abnormal keratin expression pattern in prurigo nodularis epidermis. Skin Health Dis. 2022;2:e75. doi:10.1002/ski2.75
- Nockher WA, Renz H. Neurotrophins in allergic diseases: from neuronal growth factors to intercellular signaling molecules. J Allergy Clin Immunol. 2006;117:583-589. doi:10.1016/j.jaci.2005.11.049
- Di Marco E, Mathor M, Bondanza S, et al. Nerve growth factor binds to normal human keratinocytes through high and low affinity receptors and stimulates their growth by a novel autocrine loop. J Biol Chem. 1993;268:22838-22846.
- Hägermark O, Hökfelt T, Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978;71:233-235. doi:10.1111/1523-1747.ep12515092
- Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol. 2018;40:249-259. doi:10.1007/s00281-018-0675-z
- Haas S, Capellino S, Phan NQ, et al. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J Dermatol Sci. 2010;58:193-197. doi:10.1016/j.jdermsci.2010.03.020
- Park K, Mori T, Nakamura M, et al. Increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo. Eur J Dermatol. 2011;21:135-136.
- Tokura Y, Yagi H, Hanaoka K, et al. Subacute and chronic prurigo effectively treated with recombination interferon-gamma: implications for participation of Th2 cells in the pathogenesis of prurigo. Acta Derm Venereol. 1997;77:231-234. doi:10.2340/0001555577231234
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77. doi:10.1080/17512433.2021.1852080
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Bewley A, Homey B, Pink A. Prurigo nodularis: a review of IL-31RA blockade and other potential treatments. Dermatol Ther. 2022;12:2039-2048. doi:10.1007/s13555-022-00782-2
- Zeidler C, Yosipovitch G, Ständer S. Prurigo nodularis and its management. Dermatol Clin. 2018;36:189-197. doi:10.1016/j.det.2018.02.003
- Siepmann D, Lotts T, Blome C, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, double-blind phase II trial. Dermatology. 2013;227:353-360. doi:10.1159/000355671
- Valbuena MC, Muvdi S, Lim HW. Actinic prurigo. Dermatol Clin. 2014;32:335-344, viii. doi:10.1016/j.det.2014.03.010
- Aldhahwani R, Al Hawsawi KA. Neurotic excoriation presenting as solitary papule: case report. J Dermatol Dermatolog Surg. 2022;26:45. doi:10.4103/jdds.jdds_59_21
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233. doi:10.1016/j.jaad.2015.11.033
- Karadag AS, Ozlu E, Uzuncakmak TK, et al. Inverted follicular keratosis successfully treated with imiquimod. Indian Dermatol Online J. 2016;7:177-179. doi:10.4103/2229-5178.182354
- Nayak VN, Uma K, Girish HC, et al. Pseudoepitheliomatous hyperplasia in oral lesions: a review. J Int Oral Health. 2015;7:148-152.
- Saraceno R, Chiricozzi A, Nisticò SP, et al. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21:363-366. doi:10.3109/09546630903386606
- Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of prurigo nodularis. Arch Dermatol. 2000;136:807-808. doi:10.1001/archderm.136.6.807
- Waldinger TP, Wong RC, Taylor WB, et al. Cryotherapy improves prurigo nodularis. Arch Dermatol. 1984;120:1598-1600.
- Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471-478. doi:10.1067/mjd.2001.110059
- Ohanyan T, Schoepke N, Eirefelt S, et al. Role of substance P and its receptor neurokinin 1 in chronic prurigo: a randomized, proof-of-concept, controlled trial with topical aprepitant. Acta Derm Venereol. 2018;98:26-31. doi:10.2340/00015555-2780
- Tamagawa-Mineoka R, Katoh N, Ueda E, et al. Narrow-band ultraviolet B phototherapy in patients with recalcitrant nodular prurigo. J Dermatol. 2007;34:691-695. doi:10.1111/j.1346-8138.2007.00360.x
- Hammes S, Hermann J, Roos S, et al. UVB 308-nm excimer light and bath PUVA: combination therapy is very effective in the treatment of prurigo nodularis. J Eur Acad Dermatol Venereol. 2011;25:799-803. doi:10.1111/j.1468-3083.2010.03865.x
- Richards RN. Update on intralesional steroid: focus on dermatoses. J Cutan Med Surg. 2010;14:19-23. doi:10.2310/7750.2009.08082
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760. doi:10.1016/j.jaad.2020.07.025
- Grant JE Chamberlain SR Redden SA et al. N-Acetylcysteine in the treatment of excoriation disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73:490-496. doi:10.1001/jamapsychiatry.2016.0060
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659. doi: 10.4103/ijdvl.IJDVL_33_18.
- Taylor M, Bhagwandas K. Trichotillosis, skin picking and N-acetylcysteine. J Am Acad Dermatol. 2015;72(suppl 1):AB117. https://doi.org/10.1016/j.jaad.2015.02.482
- Legat FJ. The antipruritic effect of phototherapy. Front Med (Lausanne). 2018;5:333. doi:10.3389/fmed.2018.00333
- Klejtman T, Beylot-Barry M, Joly P, et al. Treatment of prurigo with methotrexate: a multicentre retrospective study of 39 cases. J Eur Acad Dermatol Venereol. 2018;32:437-440. doi:10.1111/jdv.14646
- Wiznia LE, Callahan SW, Cohen DE, et al. Rapid improvement of prurigo nodularis with cyclosporine treatment. J Am Acad Dermatol. 2018;78:1209-1211. doi:10.1016/j.jaad.2018.02.024
- Yagami A, Furue M, Togawa M, et al. One-year safety and efficacy study of bilastine treatment in Japanese patients with chronic spontaneous urticaria or pruritus associated with skin diseases. J Dermatol. 2017;44:375-385. doi:10.1111/1346-8138.13644
- Mazza M, Guerriero G, Marano G, et al. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38:16-18. doi:10.1111/jcpt.12005
- Ständer S, Kwon P, Hirman J, et al. Serlopitant reduced pruritus in patients with prurigo nodularis in a phase 2, randomized, placebo-controlled trial. J Am Acad Dermatol. 2019;80:1395-1402. doi:10.1016/j.jaad.2019.01.052
- Study of the efficacy, safety and tolerability of serlopitant for the treatment of pruritus (itch) with prurigo nodularis. ClinicalTrials.gov identifier: NCT03546816. Updated May 20, 2021. Accessed August 8, 2024. https://clinicaltrials.gov/study/NCT03546816
- Tsianakas A, Zeidler C, Riepe C, et al. Aprepitant in anti-histamine-refractory chronic nodular prurigo: a multicentre, randomized, double-blind, placebo-controlled, cross-over, phase-II trial (APREPRU). Acta Derm Venereol. 2019;99:379-385. doi:10.2340/00015555-3120
- Metze D, Reimann S, Beissert S, et al. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41:533-539.
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open‐label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
- Ständer S, Böckenholt B, Schürmeyer-Horst F, et al. Treatment of chronic pruritus with the selective serotonin re-uptake inhibitors paroxetine and fluvoxamine: results of an open-labelled, two-arm proof-of-concept study. Acta Derm Venereol. 2009;89:45-51. doi:10.2340/00015555-0553
- Zalaudek I, Petrillo G, Baldassarre MA, et al. Amitriptyline as therapeutic and not symptomatic approach in the treatment of prurigo nodularis. G Ital Dermatol Venereol. 2006;141:433-437.
- Andersen TP, Fogh K. Thalidomide in 42 patients with prurigo nodularis Hyde. Dermatology. 2011;223:107-112. doi:10.1159/000331577
- Ständer S, Yosipovitch G, Legat FJ, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020;382:706-716. doi:10.1056/NEJMoa1908316
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Mastorino L, Rosset F, Gelato F, et al. Chronic pruritus in atopic patients treated with dupilumab: real life response and related parameters in 354 patients. Pharmaceuticals (Basel). 2022;15:883. doi: 10.3390/ph15070883
- Kishi R, Toyama S, Tominaga M, et al. Effects of dupilumab on itch-related events in atopic dermatitis: implications for assessing treatment efficacy in clinical practice. Cells. 2023;12:239. doi: 10.3390/cells12020239
- Dupixent. European Medicines Agency website. Updated July 15, 2024. Accessed August 27, 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent
Prurigo nodularis (PN)(also called chronic nodular prurigo, prurigo nodularis of Hyde, or picker’s nodules) was first characterized by James Hyde in 1909.1-3 Prurigo nodularis manifests with symmetrical, intensely pruritic, eroded, or hyperkeratotic nodules or papules on the extremities and trunk.1,2,4,5 Studies have shown that individuals with PN experience pruritus, sleep loss, decreased social functioning from the appearance of the nodules, and a higher incidence of anxiety and depression, causing a negative impact on their quality of life.2,6 In addition, the manifestation of PN has been linked to neurologic and psychiatric disorders; however, PN also can be idiopathic and manifest without underlying illnesses.2,6,7
Prurigo nodularis has been associated with other dermatologic conditions such as atopic dermatitis (up to 50%), lichen planus, keratoacanthomas (KAs), and bullous pemphigoid.7-9 It also has been linked to systemic diseases in 38% to 50% of cases, including chronic kidney disease, liver disease, type 2 diabetes mellitus, malignancies (hematopoietic, liver, and skin), and HIV infection.6,8,10
The pathophysiology of PN is highly complex and has yet to be fully elucidated. It is thought to be due to dysregulation and interaction of the increase in neural and immunologic responses of proinflammatory and pruritogenic cytokines.2,11 Treatments aim to break the itch-scratch cycle that perpetuates this disorder; however, this proves difficult, as PN is associated with a higher itch intensity than atopic dermatitis and psoriasis.10 Therefore, most patients attempt multiple forms of treatment for PN, ranging from topical therapies, oral immunosuppressants, and phototherapy to the newest and only medication approved by the US Food and Drug Administration for the treatment of PN—dupilumab.1,7,11 Herein, we provide an updated review of PN with a focus on its epidemiology, histopathology and pathophysiology, comorbidities, clinical presentation, differential diagnosis, and current treatment options.
Epidemiology
There are few studies on the epidemiology of PN; however, middle-aged populations with underlying dermatologic or psychiatric disorders tend to be impacted most frequently.2,12,13 In 2016, it was estimated that almost 88,000 individuals had PN in the United States, with the majority being female; however, this estimate only took into account those aged 18 to 64 years and utilized data from IBM MarketScan Commercial Claims and Encounters Database (IBM Watson Health) from October 2015 to December 2016.14 More recently, a retrospective database analysis estimated the prevalence of PN in the United States to be anywhere from 36.7 to 43.9 cases per 100,000 individuals. However, this retrospective review utilized the International Classification of Diseases, Tenth Revision code; PN has 2 codes associated with the diagnosis, and the coding accuracy is unknown.15 Sutaria et al16 looked at racial disparities in patients with PN utilizing data from TriNetX and found that patients who received a diagnosis of PN were more likely to be women, non-Hispanic, and Black compared with control patients. However, these estimates are restricted to the health care organizations within this database.
In 2018, Poland reported an annual prevalence of 6.52 cases per 100,000 individuals,17 while England reported a yearly prevalence of 3.27 cases per 100,000 individuals.18 Both countries reported most cases were female. However, these studies are not without limitations. Poland only uses the primary diagnosis code for medical billing to simplify clinical coding, thus underestimating the actual prevalence; furthermore, clinical codes more often than not are assigned by someone other than the diagnosing physician, leaving room for error.17 In addition, England’s PN estimate utilized diagnosis data from primary care and inpatient datasets, leaving out outpatient datasets in which patients with PN may have been referred and obtained the diagnosis, potentially underestimating the prevalence in this population.18
In contrast, Korea estimated the annual prevalence of PN to be 4.82 cases per 1000 dermatology outpatients, with the majority being men, based on results from a cross-sectional study among outpatients from the Catholic Medical Center. Although this is the largest health organization in Korea, the scope of this study is limited and lacks data from other medical centers in Korea.19
Histopathology and Pathophysiology
Almost all cells in the skin are involved in PN: keratinocytes, mast cells, dendritic cells, endothelial cells, lymphocytes, eosinophils, collagen fibers, and nerve fibers.11,20 Classically, PN manifests as a dome-shaped lesion with hyperkeratosis, hypergranulosis, and psoriasiform epidermal hyperplasia with increased thickness of the papillary dermis consisting of coarse collagen with compact interstitial and circumvascular infiltration as well as increased lymphocytes and histocytes in the superficial dermis (Figure 1).20 Hyperkeratosis is thought to be due to either the alteration of keratinocyte structures from scratching or keratinocyte abnormalities triggering PN.21 However, the increase in keratinocytes, which secrete nerve growth factor, allows for neuronal hyperplasia within the dermis.22 Nerve growth factor can stimulate keratinocyte proliferation23 in addition to the upregulation of substance P (SP), a tachykinin that triggers vascular dilation and pruritus in the skin.24 The density of SP nerve fibers in the dermis increases in PN, causing proinflammatory effects, upregulating the immune response to promote endothelial hyperplasia and increased vascularization.25 The increase in these fibers may lead to pruritus associated with PN.2,26

Many inflammatory cytokines and mediators also have been implicated in PN. Increased messenger RNA expression of IL-4, IL-17, IL-22, and IL-31 has been described in PN lesions.3,27 Furthermore, studies also have reported increased helper T cell (TH2) cytokines, including IL-4, IL-5, IL-10, and IL-13, in the dermis of PN lesions in patients without a history of atopy.3,28 These pruritogenic cytokines in conjunction with the SP fibers may create an intractable itch for those with PN. The interaction and culmination of the neural and immune responses make PN a complex condition to treat with the multifactorial interaction of systems.
Comorbidities
Prurigo nodularis has been associated with a wide array of comorbidities; however, the direction of the relationship between PN and these conditions makes it difficult to discern if PN is a primary or secondary condition.29 Prurigo nodularis commonly has been connected to other inflammatory dermatoses, with a link to atopic dermatitis being the strongest.5,29 However, PN also has been linked to other pruritic inflammatory cutaneous disorders, including psoriasis, cutaneous T-cell lymphoma, lichen planus, and dermatitis herpetiformis.14,29
Huang et al14 found an increased likelihood of psychiatric illnesses in patients with PN, including eating disorders, nonsuicidal self-injury disorder, attention-deficit/hyperactivity disorder, schizophrenia, mood disorders, anxiety, and substance abuse disorders. Treatments directed at the neural aspect of PN have included selective serotonin reuptake inhibitors (SSRIs), which also are utilized to treat these mental health disorders.
Furthermore, systemic diseases also have been found to be associated with PN, including hypertension, type 2 diabetes mellitus, chronic kidney disease, heart failure, cerebrovascular disease, coronary heart disease, and chronic obstructive pulmonary disease.14 The relationship between PN and systemic conditions may be due to increased systemic inflammation and dysregulation of neural and metabolic functions implicated in these conditions from increased pruritic manifestations.29,30 However, studies also have connected PN to infectious conditions such as HIV. One study found that patients with PN had 2.68 higher odds of infection with HIV compared to age- and sex-matched controls.14 It is unknown if these conditions contributed to the development of PN or PN contributed to the development of these disorders.
Clinical Presentations
Prurigo nodularis is a chronic inflammatory skin disease that typically manifests with multiple severely pruritic, dome-shaped, firm, hyperpigmented papulonodules with central scale or crust, often with erosion, due to chronic repetitive scratching and picking secondary to pruritic systemic or dermatologic diseases or psychological disorders (Figure 2).1,2,4,5,8,31 Most often, diagnosis of PN is based on history and physical examination of the lesion; however, biopsies may be performed. These nodules commonly manifest with ulceration distributed symmetrically on extensor extremities in easy-to-reach places, sparing the mid back (called the butterfly sign).8 Lesions—either a few or hundreds—can range from a few millimeters to 2 to 3 cm.8,32 The lesions differ in appearance depending on the pigment in the patient’s skin. In patients with darker skin tones, hyperpigmented or hypopigmented papulonodules are not uncommon, while those with fairer skin tones tend to present with erythema.31

Differential Diagnosis
Because of the variation in manifestation of PN, these lesions may resemble other cutaneous conditions. If the lesions are hyperkeratotic, they can mimic hypertrophic lichen planus, which mainfests with hyperkeratotic plaques or nodules on the lower extremities.8,29 In addition, the histopathology of lichen planus resembles the appearance of PN, with epidermal hyperplasia, hypergranulosis, hyperkeratosis, and increased fibroblasts and capillaries.8,29
Pemphigoid nodularis is a rare subtype of bullous pemphigoid that exhibits characteristics of PN with pruritic plaques and erosions.8,29,33 The patient population for pemphigoid nodularis tends to be aged 50 to 60 years, and females are affected more frequently than males. However, pemphigoid nodularis may manifest with blistering and large plaques, which are not seen commonly with PN.29 On histopathology, pemphigoid nodularis deposits IgG and C3 on the basement membrane and has subepidermal clefting, unlike PN.7,29
Actinic prurigo manifests with pruritic papules or nodules post–UV exposure to unprotected skin.8,29,33 This rare condition usually manifests with cheilitis and conjunctivitis. Unlike PN, which commonly affects elderly populations, actinic prurigo typically is found in young females.8,29 Cytologic examination shows hyperkeratosis, spongiosis, and acanthosis of the epidermis with lymphocytic perivascular infiltration of the dermis.34
Neurotic excoriations also tend to mimic PN with raised excoriated lesions; however, this disorder is due to neurotic picking of the skin without associated pruritus or true hyperkeratosis.8,29,33 Histopathology shows epidermal crusting with inflammation of the upper dermis.35
Infiltrative cutaneous squamous cell carcinoma (SCC) may imitate PN in appearance. It manifests as tender, ulcerated, scaly plaques or nodules. Histopathology shows cytologic atypia with an infiltrative architectural pattern and presence of collections of compact keratin and parakeratin (called keratin pearls).
Keratoacanthomas can resemble PN lesions. They usually manifest as nodules measuring 1 to 2 cm in diameter and 0.5 cm thick, resembling crateriform tumors.36 On histopathology, KAs can resemble SCCs; however, KAs tend to manifest more frequently with a keratin-filled crater with a ground-glass appearance.36
Inverted follicular keratosis commonly manifests on the face in elderly men as a single, flesh-colored, verrucous papule that may resemble PN. However, cytology of inverted follicular keratosis is characterized by proliferation and squamous eddies.37 Consideration of the histologic findings and clinical appearance are important to differentiate between PN and cutaneous SCC.
Pseudoepitheliomatous hyperplasia is a benign condition that manifests as a plaque or nodule with crust, scale, or ulceration. Histologically, this condition presents with hyperplastic proliferation of the epidermis and adnexal epithelium.38 The clinical and histologic appearance can mimic PN and other cutaneous eruptions with epidermal hyperplasia.
In clinical cases that are resistant to treatment, biopsy is the best approach to diagnose the lesion. Due to similarities in physical appearance and superficial histologic presentation of PN, KAs from SCC, hypertrophic lichen planus, and other hyperkeratotic lesions, the biopsy should be taken at the base of the lesion to sample deeper layers of skin to differentiate these dermatologic disorders.
Management
Current treatments for PN yield varied results. Many patients with moderate to severe PN attempt multiple therapies before seeing improvement.31 Treatments include topical, oral, and injectable medications and are either directed at the neural or immune components of PN due to the interplay between increased nerve fibers in the lesions (neural axis) as well as increases in cytokines and other immunologic mediators (immune axis) of this condition. However, the FDA recently approved the first treatment for PN—dupilumab—which is an injectable IL-4 receptor antagonist directed at the immunologic interactions affiliated with PN.
Immune-Mediated Topical Therapies—Immunologic topical therapies include corticosteroids, calcipotriol, and calcineurin inhibitors. Studies that have analyzed these treatments are limited to case reports and small intraindividual and randomized controlled trials (Table 1). Topical therapies usually are first-line agents for most patients. Adverse effects include transient irritation of the skin.40,42,43

Cryotherapy is another topical and immunologic therapy for those with PN; however, this treatment is more appropriate for patients with fewer lesions due to the pain that accompanies lesions treated with liquid nitrogen. In addition, this therapy can cause dyspigmentation of the skin in the treated areas.41
Similar to cryotherapy, intralesional corticosteroid injections are appropriate for patients with few PN lesions. A recent report described intralesional corticosteroid injections of 2.5 mg/mL for a PN nodule with high efficacy.46,47 This treatment has not undergone trials, but success with this modality has been documented, with adverse effects including hyperpigmentation or hypopigmentation in the treated area and transient pain.46
Neural-Mediated Topical Therapies—Neural topical therapies include capsaicin and neurokinin-1 receptor antagonists, aprepitant43 and serlopitant. These treatment studies are limited to small open-label and randomized controlled trials. Adverse effects of these treatments include transient cutaneous pain at the site of topical administration. In addition, neural-mediated topical therapies have shown either limited improvements from baseline or return of symptoms after treatment cessation.42,43
Supplements—N-acetyl cysteine is an over-the-counter supplement that has been reported to improve symptoms in patients with skin-picking disorders.48 The mechanism of action includes antioxidant effects such as decreasing reactive oxygen species, decreasing inflammatory markers, regulating neurotransmitters, and inhibiting hyperkeratosis.49 N-acetyl cysteine has been poorly studied for its application in PN. A small study of 3 patients with subacute PN receiving 1200 mg of oral N-acetyl cysteine reported varying levels of improvement in skin appearance and reduction in skin picking.50
Phototherapy—Phototherapy, a typical first- or second-line treatment modality for PN, targets both the neural- and immune-mediated aspects associated with pruritus in PN (Table 1).51 UV light can penetrate through the epidermal layer of the skin and reach the keratinocytes, which play a role in the immune-related response of PN. In addition, the cutaneous sensory nerves are located in the upper dermal layer, from which nerve fibers grow and penetrate into the epidermis, thereby interacting with the keratinocytes where pruritic signals are transmitted from the periphery up to the brain.51
Studies analyzing the effects of phototherapy on PN are limited to case series and a small randomized controlled trial. However, this trial has shown improvements in pruritus in the participants. Adverse effects include transient burning and erythema at the treated sites.44,45
Immune-Mediated Oral Therapies—Immunologic-targeted oral therapies include bilastine, methotrexate, and cyclosporine (Table 2).52,53 Bilastine efficacy was analyzed in a small phase 3, open-label, multicenter study in Japan; however, patients were allowed to use topical steroids in conjunction with the oral antihistamine.54 Methotrexate and cyclosporine are immunosuppressive medications and were analyzed in small retrospective studies. Both treatments yielded notable relief for patients; however, 38.5% (15/39) of patients receiving methotrexate experienced adverse events, and 50.0% (4/8) experienced adverse events with cyclosporine.52,53


Neural-Mediated Oral Therapies—Neural-targeted oral therapies include pregabalin, serlopitant, aprepitant, naltrexone, nalbuphine, SSRIs (paroxetine and fluvoxamine), amitriptyline, and thalidomide. The research on these treatments ranges from case reviews to randomized controlled trials and open-label trials (Table 2).55-63
Thalidomide was studied in a small retrospective case review that showed notable improvement in PN. Dosages of thalidomide varied, but on average the dose was 100 mg/d. However, greater than 50% of patients experienced at least 1 adverse effect with this treatment.63
A study performed in Italy showed promising results for patients treated with pregabalin, with 70.0% (21/30) continuing to take pregabalin for almost 2 years following completion of the initial 3-month trial.55 Naltrexone decreased pruritus in more than half of patients (9/17).59 Amitriptyline yielded improvements in patients with PN; however, disease recurred in 5 patients (29%) after 7 months.62 A study performed in Germany reported promising results for paroxetine and fluvoxamine; however, some patients enrolled in the study had some form of psychiatric disorder.61
Serlopitant, aprepitant, and nalbuphine were studied in randomized controlled trials. The serlopitant trials were the largest of the neurally mediated oral medication studies; one showed substantial improvement in patients with PN,56 while the most recent trial did not show significant improvement (ClinicalTrials.gov identifier NCT03546816).57 On the other hand, aprepitant showed no major difference between the experimental and placebo groups.58 Nalbuphine 162 mg twice daily showed greater improvement in PN than nalbuphine 81 mg twice daily.60
Immune-Mediated Injectable Therapies—Immune-targeted injectables include nemolizumab and dupilumab (Table 2). Nemolizumab is an IL-31 antagonist that has been studied in a small randomized controlled trial that showed great success in decreasing pruritus associated with PN.64 IL-31 has been implicated in PN, and inhibition of the IL-31 receptor has been shown to disrupt the itch-scratch cycle of PN. Dupilumab is a monoclonal antibody against the IL-4 and IL-13 receptors, and it is the only FDA-approved treatment for PN.65 Blockage of these protein receptors decreases type 2 inflammation and chronic pruritus.66,67 Dupilumab is FDA approved for the treatment of atopic dermatitis and recently was approved for adults with PN. Dupilumab acts to block the shared α-subunit of the pruritogenic cytokines IL-4 and IL-13 pathways,29 thereby breaking the itch-scratch cycle associated with PN and allowing for the healing of these lesions. Results from 2 clinical trials showed substantially reduced itch in patients with PN.65 Dupilumab also was approved by the European Medicines Agency for moderate to severe PN.68
Conclusion
Prurigo nodularis is a chronic condition that affects patient quality of life and can mimic various dermatologic conditions. The epidemiology and pathophysiology of PN have not been fully expounded. More research should be conducted to determine the underpinnings of PN to help identify more consistently effective therapies for this complex condition.
Prurigo nodularis (PN)(also called chronic nodular prurigo, prurigo nodularis of Hyde, or picker’s nodules) was first characterized by James Hyde in 1909.1-3 Prurigo nodularis manifests with symmetrical, intensely pruritic, eroded, or hyperkeratotic nodules or papules on the extremities and trunk.1,2,4,5 Studies have shown that individuals with PN experience pruritus, sleep loss, decreased social functioning from the appearance of the nodules, and a higher incidence of anxiety and depression, causing a negative impact on their quality of life.2,6 In addition, the manifestation of PN has been linked to neurologic and psychiatric disorders; however, PN also can be idiopathic and manifest without underlying illnesses.2,6,7
Prurigo nodularis has been associated with other dermatologic conditions such as atopic dermatitis (up to 50%), lichen planus, keratoacanthomas (KAs), and bullous pemphigoid.7-9 It also has been linked to systemic diseases in 38% to 50% of cases, including chronic kidney disease, liver disease, type 2 diabetes mellitus, malignancies (hematopoietic, liver, and skin), and HIV infection.6,8,10
The pathophysiology of PN is highly complex and has yet to be fully elucidated. It is thought to be due to dysregulation and interaction of the increase in neural and immunologic responses of proinflammatory and pruritogenic cytokines.2,11 Treatments aim to break the itch-scratch cycle that perpetuates this disorder; however, this proves difficult, as PN is associated with a higher itch intensity than atopic dermatitis and psoriasis.10 Therefore, most patients attempt multiple forms of treatment for PN, ranging from topical therapies, oral immunosuppressants, and phototherapy to the newest and only medication approved by the US Food and Drug Administration for the treatment of PN—dupilumab.1,7,11 Herein, we provide an updated review of PN with a focus on its epidemiology, histopathology and pathophysiology, comorbidities, clinical presentation, differential diagnosis, and current treatment options.
Epidemiology
There are few studies on the epidemiology of PN; however, middle-aged populations with underlying dermatologic or psychiatric disorders tend to be impacted most frequently.2,12,13 In 2016, it was estimated that almost 88,000 individuals had PN in the United States, with the majority being female; however, this estimate only took into account those aged 18 to 64 years and utilized data from IBM MarketScan Commercial Claims and Encounters Database (IBM Watson Health) from October 2015 to December 2016.14 More recently, a retrospective database analysis estimated the prevalence of PN in the United States to be anywhere from 36.7 to 43.9 cases per 100,000 individuals. However, this retrospective review utilized the International Classification of Diseases, Tenth Revision code; PN has 2 codes associated with the diagnosis, and the coding accuracy is unknown.15 Sutaria et al16 looked at racial disparities in patients with PN utilizing data from TriNetX and found that patients who received a diagnosis of PN were more likely to be women, non-Hispanic, and Black compared with control patients. However, these estimates are restricted to the health care organizations within this database.
In 2018, Poland reported an annual prevalence of 6.52 cases per 100,000 individuals,17 while England reported a yearly prevalence of 3.27 cases per 100,000 individuals.18 Both countries reported most cases were female. However, these studies are not without limitations. Poland only uses the primary diagnosis code for medical billing to simplify clinical coding, thus underestimating the actual prevalence; furthermore, clinical codes more often than not are assigned by someone other than the diagnosing physician, leaving room for error.17 In addition, England’s PN estimate utilized diagnosis data from primary care and inpatient datasets, leaving out outpatient datasets in which patients with PN may have been referred and obtained the diagnosis, potentially underestimating the prevalence in this population.18
In contrast, Korea estimated the annual prevalence of PN to be 4.82 cases per 1000 dermatology outpatients, with the majority being men, based on results from a cross-sectional study among outpatients from the Catholic Medical Center. Although this is the largest health organization in Korea, the scope of this study is limited and lacks data from other medical centers in Korea.19
Histopathology and Pathophysiology
Almost all cells in the skin are involved in PN: keratinocytes, mast cells, dendritic cells, endothelial cells, lymphocytes, eosinophils, collagen fibers, and nerve fibers.11,20 Classically, PN manifests as a dome-shaped lesion with hyperkeratosis, hypergranulosis, and psoriasiform epidermal hyperplasia with increased thickness of the papillary dermis consisting of coarse collagen with compact interstitial and circumvascular infiltration as well as increased lymphocytes and histocytes in the superficial dermis (Figure 1).20 Hyperkeratosis is thought to be due to either the alteration of keratinocyte structures from scratching or keratinocyte abnormalities triggering PN.21 However, the increase in keratinocytes, which secrete nerve growth factor, allows for neuronal hyperplasia within the dermis.22 Nerve growth factor can stimulate keratinocyte proliferation23 in addition to the upregulation of substance P (SP), a tachykinin that triggers vascular dilation and pruritus in the skin.24 The density of SP nerve fibers in the dermis increases in PN, causing proinflammatory effects, upregulating the immune response to promote endothelial hyperplasia and increased vascularization.25 The increase in these fibers may lead to pruritus associated with PN.2,26

Many inflammatory cytokines and mediators also have been implicated in PN. Increased messenger RNA expression of IL-4, IL-17, IL-22, and IL-31 has been described in PN lesions.3,27 Furthermore, studies also have reported increased helper T cell (TH2) cytokines, including IL-4, IL-5, IL-10, and IL-13, in the dermis of PN lesions in patients without a history of atopy.3,28 These pruritogenic cytokines in conjunction with the SP fibers may create an intractable itch for those with PN. The interaction and culmination of the neural and immune responses make PN a complex condition to treat with the multifactorial interaction of systems.
Comorbidities
Prurigo nodularis has been associated with a wide array of comorbidities; however, the direction of the relationship between PN and these conditions makes it difficult to discern if PN is a primary or secondary condition.29 Prurigo nodularis commonly has been connected to other inflammatory dermatoses, with a link to atopic dermatitis being the strongest.5,29 However, PN also has been linked to other pruritic inflammatory cutaneous disorders, including psoriasis, cutaneous T-cell lymphoma, lichen planus, and dermatitis herpetiformis.14,29
Huang et al14 found an increased likelihood of psychiatric illnesses in patients with PN, including eating disorders, nonsuicidal self-injury disorder, attention-deficit/hyperactivity disorder, schizophrenia, mood disorders, anxiety, and substance abuse disorders. Treatments directed at the neural aspect of PN have included selective serotonin reuptake inhibitors (SSRIs), which also are utilized to treat these mental health disorders.
Furthermore, systemic diseases also have been found to be associated with PN, including hypertension, type 2 diabetes mellitus, chronic kidney disease, heart failure, cerebrovascular disease, coronary heart disease, and chronic obstructive pulmonary disease.14 The relationship between PN and systemic conditions may be due to increased systemic inflammation and dysregulation of neural and metabolic functions implicated in these conditions from increased pruritic manifestations.29,30 However, studies also have connected PN to infectious conditions such as HIV. One study found that patients with PN had 2.68 higher odds of infection with HIV compared to age- and sex-matched controls.14 It is unknown if these conditions contributed to the development of PN or PN contributed to the development of these disorders.
Clinical Presentations
Prurigo nodularis is a chronic inflammatory skin disease that typically manifests with multiple severely pruritic, dome-shaped, firm, hyperpigmented papulonodules with central scale or crust, often with erosion, due to chronic repetitive scratching and picking secondary to pruritic systemic or dermatologic diseases or psychological disorders (Figure 2).1,2,4,5,8,31 Most often, diagnosis of PN is based on history and physical examination of the lesion; however, biopsies may be performed. These nodules commonly manifest with ulceration distributed symmetrically on extensor extremities in easy-to-reach places, sparing the mid back (called the butterfly sign).8 Lesions—either a few or hundreds—can range from a few millimeters to 2 to 3 cm.8,32 The lesions differ in appearance depending on the pigment in the patient’s skin. In patients with darker skin tones, hyperpigmented or hypopigmented papulonodules are not uncommon, while those with fairer skin tones tend to present with erythema.31

Differential Diagnosis
Because of the variation in manifestation of PN, these lesions may resemble other cutaneous conditions. If the lesions are hyperkeratotic, they can mimic hypertrophic lichen planus, which mainfests with hyperkeratotic plaques or nodules on the lower extremities.8,29 In addition, the histopathology of lichen planus resembles the appearance of PN, with epidermal hyperplasia, hypergranulosis, hyperkeratosis, and increased fibroblasts and capillaries.8,29
Pemphigoid nodularis is a rare subtype of bullous pemphigoid that exhibits characteristics of PN with pruritic plaques and erosions.8,29,33 The patient population for pemphigoid nodularis tends to be aged 50 to 60 years, and females are affected more frequently than males. However, pemphigoid nodularis may manifest with blistering and large plaques, which are not seen commonly with PN.29 On histopathology, pemphigoid nodularis deposits IgG and C3 on the basement membrane and has subepidermal clefting, unlike PN.7,29
Actinic prurigo manifests with pruritic papules or nodules post–UV exposure to unprotected skin.8,29,33 This rare condition usually manifests with cheilitis and conjunctivitis. Unlike PN, which commonly affects elderly populations, actinic prurigo typically is found in young females.8,29 Cytologic examination shows hyperkeratosis, spongiosis, and acanthosis of the epidermis with lymphocytic perivascular infiltration of the dermis.34
Neurotic excoriations also tend to mimic PN with raised excoriated lesions; however, this disorder is due to neurotic picking of the skin without associated pruritus or true hyperkeratosis.8,29,33 Histopathology shows epidermal crusting with inflammation of the upper dermis.35
Infiltrative cutaneous squamous cell carcinoma (SCC) may imitate PN in appearance. It manifests as tender, ulcerated, scaly plaques or nodules. Histopathology shows cytologic atypia with an infiltrative architectural pattern and presence of collections of compact keratin and parakeratin (called keratin pearls).
Keratoacanthomas can resemble PN lesions. They usually manifest as nodules measuring 1 to 2 cm in diameter and 0.5 cm thick, resembling crateriform tumors.36 On histopathology, KAs can resemble SCCs; however, KAs tend to manifest more frequently with a keratin-filled crater with a ground-glass appearance.36
Inverted follicular keratosis commonly manifests on the face in elderly men as a single, flesh-colored, verrucous papule that may resemble PN. However, cytology of inverted follicular keratosis is characterized by proliferation and squamous eddies.37 Consideration of the histologic findings and clinical appearance are important to differentiate between PN and cutaneous SCC.
Pseudoepitheliomatous hyperplasia is a benign condition that manifests as a plaque or nodule with crust, scale, or ulceration. Histologically, this condition presents with hyperplastic proliferation of the epidermis and adnexal epithelium.38 The clinical and histologic appearance can mimic PN and other cutaneous eruptions with epidermal hyperplasia.
In clinical cases that are resistant to treatment, biopsy is the best approach to diagnose the lesion. Due to similarities in physical appearance and superficial histologic presentation of PN, KAs from SCC, hypertrophic lichen planus, and other hyperkeratotic lesions, the biopsy should be taken at the base of the lesion to sample deeper layers of skin to differentiate these dermatologic disorders.
Management
Current treatments for PN yield varied results. Many patients with moderate to severe PN attempt multiple therapies before seeing improvement.31 Treatments include topical, oral, and injectable medications and are either directed at the neural or immune components of PN due to the interplay between increased nerve fibers in the lesions (neural axis) as well as increases in cytokines and other immunologic mediators (immune axis) of this condition. However, the FDA recently approved the first treatment for PN—dupilumab—which is an injectable IL-4 receptor antagonist directed at the immunologic interactions affiliated with PN.
Immune-Mediated Topical Therapies—Immunologic topical therapies include corticosteroids, calcipotriol, and calcineurin inhibitors. Studies that have analyzed these treatments are limited to case reports and small intraindividual and randomized controlled trials (Table 1). Topical therapies usually are first-line agents for most patients. Adverse effects include transient irritation of the skin.40,42,43

Cryotherapy is another topical and immunologic therapy for those with PN; however, this treatment is more appropriate for patients with fewer lesions due to the pain that accompanies lesions treated with liquid nitrogen. In addition, this therapy can cause dyspigmentation of the skin in the treated areas.41
Similar to cryotherapy, intralesional corticosteroid injections are appropriate for patients with few PN lesions. A recent report described intralesional corticosteroid injections of 2.5 mg/mL for a PN nodule with high efficacy.46,47 This treatment has not undergone trials, but success with this modality has been documented, with adverse effects including hyperpigmentation or hypopigmentation in the treated area and transient pain.46
Neural-Mediated Topical Therapies—Neural topical therapies include capsaicin and neurokinin-1 receptor antagonists, aprepitant43 and serlopitant. These treatment studies are limited to small open-label and randomized controlled trials. Adverse effects of these treatments include transient cutaneous pain at the site of topical administration. In addition, neural-mediated topical therapies have shown either limited improvements from baseline or return of symptoms after treatment cessation.42,43
Supplements—N-acetyl cysteine is an over-the-counter supplement that has been reported to improve symptoms in patients with skin-picking disorders.48 The mechanism of action includes antioxidant effects such as decreasing reactive oxygen species, decreasing inflammatory markers, regulating neurotransmitters, and inhibiting hyperkeratosis.49 N-acetyl cysteine has been poorly studied for its application in PN. A small study of 3 patients with subacute PN receiving 1200 mg of oral N-acetyl cysteine reported varying levels of improvement in skin appearance and reduction in skin picking.50
Phototherapy—Phototherapy, a typical first- or second-line treatment modality for PN, targets both the neural- and immune-mediated aspects associated with pruritus in PN (Table 1).51 UV light can penetrate through the epidermal layer of the skin and reach the keratinocytes, which play a role in the immune-related response of PN. In addition, the cutaneous sensory nerves are located in the upper dermal layer, from which nerve fibers grow and penetrate into the epidermis, thereby interacting with the keratinocytes where pruritic signals are transmitted from the periphery up to the brain.51
Studies analyzing the effects of phototherapy on PN are limited to case series and a small randomized controlled trial. However, this trial has shown improvements in pruritus in the participants. Adverse effects include transient burning and erythema at the treated sites.44,45
Immune-Mediated Oral Therapies—Immunologic-targeted oral therapies include bilastine, methotrexate, and cyclosporine (Table 2).52,53 Bilastine efficacy was analyzed in a small phase 3, open-label, multicenter study in Japan; however, patients were allowed to use topical steroids in conjunction with the oral antihistamine.54 Methotrexate and cyclosporine are immunosuppressive medications and were analyzed in small retrospective studies. Both treatments yielded notable relief for patients; however, 38.5% (15/39) of patients receiving methotrexate experienced adverse events, and 50.0% (4/8) experienced adverse events with cyclosporine.52,53


Neural-Mediated Oral Therapies—Neural-targeted oral therapies include pregabalin, serlopitant, aprepitant, naltrexone, nalbuphine, SSRIs (paroxetine and fluvoxamine), amitriptyline, and thalidomide. The research on these treatments ranges from case reviews to randomized controlled trials and open-label trials (Table 2).55-63
Thalidomide was studied in a small retrospective case review that showed notable improvement in PN. Dosages of thalidomide varied, but on average the dose was 100 mg/d. However, greater than 50% of patients experienced at least 1 adverse effect with this treatment.63
A study performed in Italy showed promising results for patients treated with pregabalin, with 70.0% (21/30) continuing to take pregabalin for almost 2 years following completion of the initial 3-month trial.55 Naltrexone decreased pruritus in more than half of patients (9/17).59 Amitriptyline yielded improvements in patients with PN; however, disease recurred in 5 patients (29%) after 7 months.62 A study performed in Germany reported promising results for paroxetine and fluvoxamine; however, some patients enrolled in the study had some form of psychiatric disorder.61
Serlopitant, aprepitant, and nalbuphine were studied in randomized controlled trials. The serlopitant trials were the largest of the neurally mediated oral medication studies; one showed substantial improvement in patients with PN,56 while the most recent trial did not show significant improvement (ClinicalTrials.gov identifier NCT03546816).57 On the other hand, aprepitant showed no major difference between the experimental and placebo groups.58 Nalbuphine 162 mg twice daily showed greater improvement in PN than nalbuphine 81 mg twice daily.60
Immune-Mediated Injectable Therapies—Immune-targeted injectables include nemolizumab and dupilumab (Table 2). Nemolizumab is an IL-31 antagonist that has been studied in a small randomized controlled trial that showed great success in decreasing pruritus associated with PN.64 IL-31 has been implicated in PN, and inhibition of the IL-31 receptor has been shown to disrupt the itch-scratch cycle of PN. Dupilumab is a monoclonal antibody against the IL-4 and IL-13 receptors, and it is the only FDA-approved treatment for PN.65 Blockage of these protein receptors decreases type 2 inflammation and chronic pruritus.66,67 Dupilumab is FDA approved for the treatment of atopic dermatitis and recently was approved for adults with PN. Dupilumab acts to block the shared α-subunit of the pruritogenic cytokines IL-4 and IL-13 pathways,29 thereby breaking the itch-scratch cycle associated with PN and allowing for the healing of these lesions. Results from 2 clinical trials showed substantially reduced itch in patients with PN.65 Dupilumab also was approved by the European Medicines Agency for moderate to severe PN.68
Conclusion
Prurigo nodularis is a chronic condition that affects patient quality of life and can mimic various dermatologic conditions. The epidemiology and pathophysiology of PN have not been fully expounded. More research should be conducted to determine the underpinnings of PN to help identify more consistently effective therapies for this complex condition.
- Durmaz K, Ataseven A, Ozer I, et al. Prurigo nodularis responding to intravenous immunoglobulins. Przegl Dermatol. 2022;109:159-162. doi:10.5114/dr.2022.117988
- Kowalski EH, Kneiber D, Valdebran M, et al. Treatment-resistant prurigo nodularis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:163-172. doi:10.2147/CCID.S188070
- Wong LS, Yen YT. Chronic nodular prurigo: an update on the pathogenesis and treatment. Int J Mol Sci. 2022;23:12390. doi:10.3390/ijms232012390
- Janmohamed SR, Gwillim EC, Yousaf M, et al. The impact of prurigo nodularis on quality of life: a systematic review and meta-analysis. Arch Dermatol Res. 2021;313:669-677. doi:10.1007/s00403-020-02148-0
- Zeidler C, Ständer S. The pathogenesis of prurigo nodularis - ‘super-itch’ in exploration. Eur J Pain. 2016;20:37-40. doi:10.1002/ejp.767
- Kwatra SG. Breaking the itch–scratch cycle in prurigo nodularis. N Engl J Med. 2020;382:757-758. doi:10.1056/NEJMe1916733
- Frølunde AS, Wiis MAK, Ben Abdallah H, et al. Non-atopic chronic nodular prurigo (prurigo nodularis hyde): a systematic review of best-evidenced treatment options. Dermatology. 2022;238:950-960. doi:10.1159/000523700
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
- Kowalski EH, Kneiber D, Valdebran M, et al. Distinguishing truly recalcitrant prurigo nodularis from poor treatment adherence: a response to treatment-resistant prurigo nodularis [Response to letter]. Clin Cosmet Investig Dermatol. 2019;12:371-372. doi:10.2147/CCID.S214195
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580. doi:10.1016/j.jaad.2021.05.036
- Labib A, Ju T, Vander Does A, et al. Immunotargets and therapy for prurigo nodularis. Immunotargets Ther. 2022;11:11-21. doi:10.2147/ITT.S316602
- Belzberg M, Alphonse MP, Brown I, et al. Prurigo nodularis is characterized by systemic and cutaneous T helper 22 immune polarization. J Invest Dermatol. 2021;141:2208-2218.e14. doi:10.1016/j.jid.2021.02.749
- Ständer S, Pereira MP, Berger T, et al. IFSI-guideline on chronic prurigo including prurigo nodularis. Itch. 2020;5:e42. doi:10.1097/itx.0000000000000042
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140:480-483.e4. doi:10.1016/j.jid.2019.07.697
- Ständer S, Augustin M, Berger T, et al. Prevalence of prurigo nodularis in the United States of America: a retrospective database analysis. JAAD Int. 2021;2:28-30. doi:10.1016/j.jdin.2020.10.009
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;86:487-490. doi:10.1016/j.jaad.2021.09.028
- Ryczek A, Reich A. Prevalence of prurigo nodularis in Poland. Acta Derm Venereol. 2020;100:adv00155. doi:10.2340/00015555-3518
- Morgan CL, Thomas M, Ständer S, et al. Epidemiology of prurigo nodularis in England: a retrospective database analysis. Br J Dermatol. 2022;187:188-195. doi:10.1111/bjd.21032
- Woo YR, Wang S, Sohn KA, et al. Epidemiology, comorbidities, and prescription patterns of Korean prurigo nodularis patients: a multi-institution study. J Clin Med Res. 2021;11:95. doi:10.3390/jcm11010095
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Yang LL, Jiang B, Chen SH, et al. Abnormal keratin expression pattern in prurigo nodularis epidermis. Skin Health Dis. 2022;2:e75. doi:10.1002/ski2.75
- Nockher WA, Renz H. Neurotrophins in allergic diseases: from neuronal growth factors to intercellular signaling molecules. J Allergy Clin Immunol. 2006;117:583-589. doi:10.1016/j.jaci.2005.11.049
- Di Marco E, Mathor M, Bondanza S, et al. Nerve growth factor binds to normal human keratinocytes through high and low affinity receptors and stimulates their growth by a novel autocrine loop. J Biol Chem. 1993;268:22838-22846.
- Hägermark O, Hökfelt T, Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978;71:233-235. doi:10.1111/1523-1747.ep12515092
- Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol. 2018;40:249-259. doi:10.1007/s00281-018-0675-z
- Haas S, Capellino S, Phan NQ, et al. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J Dermatol Sci. 2010;58:193-197. doi:10.1016/j.jdermsci.2010.03.020
- Park K, Mori T, Nakamura M, et al. Increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo. Eur J Dermatol. 2011;21:135-136.
- Tokura Y, Yagi H, Hanaoka K, et al. Subacute and chronic prurigo effectively treated with recombination interferon-gamma: implications for participation of Th2 cells in the pathogenesis of prurigo. Acta Derm Venereol. 1997;77:231-234. doi:10.2340/0001555577231234
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77. doi:10.1080/17512433.2021.1852080
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Bewley A, Homey B, Pink A. Prurigo nodularis: a review of IL-31RA blockade and other potential treatments. Dermatol Ther. 2022;12:2039-2048. doi:10.1007/s13555-022-00782-2
- Zeidler C, Yosipovitch G, Ständer S. Prurigo nodularis and its management. Dermatol Clin. 2018;36:189-197. doi:10.1016/j.det.2018.02.003
- Siepmann D, Lotts T, Blome C, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, double-blind phase II trial. Dermatology. 2013;227:353-360. doi:10.1159/000355671
- Valbuena MC, Muvdi S, Lim HW. Actinic prurigo. Dermatol Clin. 2014;32:335-344, viii. doi:10.1016/j.det.2014.03.010
- Aldhahwani R, Al Hawsawi KA. Neurotic excoriation presenting as solitary papule: case report. J Dermatol Dermatolog Surg. 2022;26:45. doi:10.4103/jdds.jdds_59_21
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233. doi:10.1016/j.jaad.2015.11.033
- Karadag AS, Ozlu E, Uzuncakmak TK, et al. Inverted follicular keratosis successfully treated with imiquimod. Indian Dermatol Online J. 2016;7:177-179. doi:10.4103/2229-5178.182354
- Nayak VN, Uma K, Girish HC, et al. Pseudoepitheliomatous hyperplasia in oral lesions: a review. J Int Oral Health. 2015;7:148-152.
- Saraceno R, Chiricozzi A, Nisticò SP, et al. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21:363-366. doi:10.3109/09546630903386606
- Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of prurigo nodularis. Arch Dermatol. 2000;136:807-808. doi:10.1001/archderm.136.6.807
- Waldinger TP, Wong RC, Taylor WB, et al. Cryotherapy improves prurigo nodularis. Arch Dermatol. 1984;120:1598-1600.
- Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471-478. doi:10.1067/mjd.2001.110059
- Ohanyan T, Schoepke N, Eirefelt S, et al. Role of substance P and its receptor neurokinin 1 in chronic prurigo: a randomized, proof-of-concept, controlled trial with topical aprepitant. Acta Derm Venereol. 2018;98:26-31. doi:10.2340/00015555-2780
- Tamagawa-Mineoka R, Katoh N, Ueda E, et al. Narrow-band ultraviolet B phototherapy in patients with recalcitrant nodular prurigo. J Dermatol. 2007;34:691-695. doi:10.1111/j.1346-8138.2007.00360.x
- Hammes S, Hermann J, Roos S, et al. UVB 308-nm excimer light and bath PUVA: combination therapy is very effective in the treatment of prurigo nodularis. J Eur Acad Dermatol Venereol. 2011;25:799-803. doi:10.1111/j.1468-3083.2010.03865.x
- Richards RN. Update on intralesional steroid: focus on dermatoses. J Cutan Med Surg. 2010;14:19-23. doi:10.2310/7750.2009.08082
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760. doi:10.1016/j.jaad.2020.07.025
- Grant JE Chamberlain SR Redden SA et al. N-Acetylcysteine in the treatment of excoriation disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73:490-496. doi:10.1001/jamapsychiatry.2016.0060
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659. doi: 10.4103/ijdvl.IJDVL_33_18.
- Taylor M, Bhagwandas K. Trichotillosis, skin picking and N-acetylcysteine. J Am Acad Dermatol. 2015;72(suppl 1):AB117. https://doi.org/10.1016/j.jaad.2015.02.482
- Legat FJ. The antipruritic effect of phototherapy. Front Med (Lausanne). 2018;5:333. doi:10.3389/fmed.2018.00333
- Klejtman T, Beylot-Barry M, Joly P, et al. Treatment of prurigo with methotrexate: a multicentre retrospective study of 39 cases. J Eur Acad Dermatol Venereol. 2018;32:437-440. doi:10.1111/jdv.14646
- Wiznia LE, Callahan SW, Cohen DE, et al. Rapid improvement of prurigo nodularis with cyclosporine treatment. J Am Acad Dermatol. 2018;78:1209-1211. doi:10.1016/j.jaad.2018.02.024
- Yagami A, Furue M, Togawa M, et al. One-year safety and efficacy study of bilastine treatment in Japanese patients with chronic spontaneous urticaria or pruritus associated with skin diseases. J Dermatol. 2017;44:375-385. doi:10.1111/1346-8138.13644
- Mazza M, Guerriero G, Marano G, et al. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38:16-18. doi:10.1111/jcpt.12005
- Ständer S, Kwon P, Hirman J, et al. Serlopitant reduced pruritus in patients with prurigo nodularis in a phase 2, randomized, placebo-controlled trial. J Am Acad Dermatol. 2019;80:1395-1402. doi:10.1016/j.jaad.2019.01.052
- Study of the efficacy, safety and tolerability of serlopitant for the treatment of pruritus (itch) with prurigo nodularis. ClinicalTrials.gov identifier: NCT03546816. Updated May 20, 2021. Accessed August 8, 2024. https://clinicaltrials.gov/study/NCT03546816
- Tsianakas A, Zeidler C, Riepe C, et al. Aprepitant in anti-histamine-refractory chronic nodular prurigo: a multicentre, randomized, double-blind, placebo-controlled, cross-over, phase-II trial (APREPRU). Acta Derm Venereol. 2019;99:379-385. doi:10.2340/00015555-3120
- Metze D, Reimann S, Beissert S, et al. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41:533-539.
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open‐label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
- Ständer S, Böckenholt B, Schürmeyer-Horst F, et al. Treatment of chronic pruritus with the selective serotonin re-uptake inhibitors paroxetine and fluvoxamine: results of an open-labelled, two-arm proof-of-concept study. Acta Derm Venereol. 2009;89:45-51. doi:10.2340/00015555-0553
- Zalaudek I, Petrillo G, Baldassarre MA, et al. Amitriptyline as therapeutic and not symptomatic approach in the treatment of prurigo nodularis. G Ital Dermatol Venereol. 2006;141:433-437.
- Andersen TP, Fogh K. Thalidomide in 42 patients with prurigo nodularis Hyde. Dermatology. 2011;223:107-112. doi:10.1159/000331577
- Ständer S, Yosipovitch G, Legat FJ, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020;382:706-716. doi:10.1056/NEJMoa1908316
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Mastorino L, Rosset F, Gelato F, et al. Chronic pruritus in atopic patients treated with dupilumab: real life response and related parameters in 354 patients. Pharmaceuticals (Basel). 2022;15:883. doi: 10.3390/ph15070883
- Kishi R, Toyama S, Tominaga M, et al. Effects of dupilumab on itch-related events in atopic dermatitis: implications for assessing treatment efficacy in clinical practice. Cells. 2023;12:239. doi: 10.3390/cells12020239
- Dupixent. European Medicines Agency website. Updated July 15, 2024. Accessed August 27, 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent
- Durmaz K, Ataseven A, Ozer I, et al. Prurigo nodularis responding to intravenous immunoglobulins. Przegl Dermatol. 2022;109:159-162. doi:10.5114/dr.2022.117988
- Kowalski EH, Kneiber D, Valdebran M, et al. Treatment-resistant prurigo nodularis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:163-172. doi:10.2147/CCID.S188070
- Wong LS, Yen YT. Chronic nodular prurigo: an update on the pathogenesis and treatment. Int J Mol Sci. 2022;23:12390. doi:10.3390/ijms232012390
- Janmohamed SR, Gwillim EC, Yousaf M, et al. The impact of prurigo nodularis on quality of life: a systematic review and meta-analysis. Arch Dermatol Res. 2021;313:669-677. doi:10.1007/s00403-020-02148-0
- Zeidler C, Ständer S. The pathogenesis of prurigo nodularis - ‘super-itch’ in exploration. Eur J Pain. 2016;20:37-40. doi:10.1002/ejp.767
- Kwatra SG. Breaking the itch–scratch cycle in prurigo nodularis. N Engl J Med. 2020;382:757-758. doi:10.1056/NEJMe1916733
- Frølunde AS, Wiis MAK, Ben Abdallah H, et al. Non-atopic chronic nodular prurigo (prurigo nodularis hyde): a systematic review of best-evidenced treatment options. Dermatology. 2022;238:950-960. doi:10.1159/000523700
- Kwon CD, Khanna R, Williams KA, et al. Diagnostic workup and evaluation of patients with prurigo nodularis. Medicines (Basel). 2019;6:97. doi:10.3390/medicines6040097
- Kowalski EH, Kneiber D, Valdebran M, et al. Distinguishing truly recalcitrant prurigo nodularis from poor treatment adherence: a response to treatment-resistant prurigo nodularis [Response to letter]. Clin Cosmet Investig Dermatol. 2019;12:371-372. doi:10.2147/CCID.S214195
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580. doi:10.1016/j.jaad.2021.05.036
- Labib A, Ju T, Vander Does A, et al. Immunotargets and therapy for prurigo nodularis. Immunotargets Ther. 2022;11:11-21. doi:10.2147/ITT.S316602
- Belzberg M, Alphonse MP, Brown I, et al. Prurigo nodularis is characterized by systemic and cutaneous T helper 22 immune polarization. J Invest Dermatol. 2021;141:2208-2218.e14. doi:10.1016/j.jid.2021.02.749
- Ständer S, Pereira MP, Berger T, et al. IFSI-guideline on chronic prurigo including prurigo nodularis. Itch. 2020;5:e42. doi:10.1097/itx.0000000000000042
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Invest Dermatol. 2020;140:480-483.e4. doi:10.1016/j.jid.2019.07.697
- Ständer S, Augustin M, Berger T, et al. Prevalence of prurigo nodularis in the United States of America: a retrospective database analysis. JAAD Int. 2021;2:28-30. doi:10.1016/j.jdin.2020.10.009
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;86:487-490. doi:10.1016/j.jaad.2021.09.028
- Ryczek A, Reich A. Prevalence of prurigo nodularis in Poland. Acta Derm Venereol. 2020;100:adv00155. doi:10.2340/00015555-3518
- Morgan CL, Thomas M, Ständer S, et al. Epidemiology of prurigo nodularis in England: a retrospective database analysis. Br J Dermatol. 2022;187:188-195. doi:10.1111/bjd.21032
- Woo YR, Wang S, Sohn KA, et al. Epidemiology, comorbidities, and prescription patterns of Korean prurigo nodularis patients: a multi-institution study. J Clin Med Res. 2021;11:95. doi:10.3390/jcm11010095
- Weigelt N, Metze D, Ständer S. Prurigo nodularis: systematic analysis of 58 histological criteria in 136 patients. J Cutan Pathol. 2010;37:578-586. doi:10.1111/j.1600-0560.2009.01484.x
- Yang LL, Jiang B, Chen SH, et al. Abnormal keratin expression pattern in prurigo nodularis epidermis. Skin Health Dis. 2022;2:e75. doi:10.1002/ski2.75
- Nockher WA, Renz H. Neurotrophins in allergic diseases: from neuronal growth factors to intercellular signaling molecules. J Allergy Clin Immunol. 2006;117:583-589. doi:10.1016/j.jaci.2005.11.049
- Di Marco E, Mathor M, Bondanza S, et al. Nerve growth factor binds to normal human keratinocytes through high and low affinity receptors and stimulates their growth by a novel autocrine loop. J Biol Chem. 1993;268:22838-22846.
- Hägermark O, Hökfelt T, Pernow B. Flare and itch induced by substance P in human skin. J Invest Dermatol. 1978;71:233-235. doi:10.1111/1523-1747.ep12515092
- Choi JE, Di Nardo A. Skin neurogenic inflammation. Semin Immunopathol. 2018;40:249-259. doi:10.1007/s00281-018-0675-z
- Haas S, Capellino S, Phan NQ, et al. Low density of sympathetic nerve fibers relative to substance P-positive nerve fibers in lesional skin of chronic pruritus and prurigo nodularis. J Dermatol Sci. 2010;58:193-197. doi:10.1016/j.jdermsci.2010.03.020
- Park K, Mori T, Nakamura M, et al. Increased expression of mRNAs for IL-4, IL-17, IL-22 and IL-31 in skin lesions of subacute and chronic forms of prurigo. Eur J Dermatol. 2011;21:135-136.
- Tokura Y, Yagi H, Hanaoka K, et al. Subacute and chronic prurigo effectively treated with recombination interferon-gamma: implications for participation of Th2 cells in the pathogenesis of prurigo. Acta Derm Venereol. 1997;77:231-234. doi:10.2340/0001555577231234
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77. doi:10.1080/17512433.2021.1852080
- Huang AH, Williams KA, Kwatra SG. Prurigo nodularis: epidemiology and clinical features. J Am Acad Dermatol. 2020;83:1559-1565. doi:10.1016/j.jaad.2020.04.183
- Bewley A, Homey B, Pink A. Prurigo nodularis: a review of IL-31RA blockade and other potential treatments. Dermatol Ther. 2022;12:2039-2048. doi:10.1007/s13555-022-00782-2
- Zeidler C, Yosipovitch G, Ständer S. Prurigo nodularis and its management. Dermatol Clin. 2018;36:189-197. doi:10.1016/j.det.2018.02.003
- Siepmann D, Lotts T, Blome C, et al. Evaluation of the antipruritic effects of topical pimecrolimus in non-atopic prurigo nodularis: results of a randomized, hydrocortisone-controlled, double-blind phase II trial. Dermatology. 2013;227:353-360. doi:10.1159/000355671
- Valbuena MC, Muvdi S, Lim HW. Actinic prurigo. Dermatol Clin. 2014;32:335-344, viii. doi:10.1016/j.det.2014.03.010
- Aldhahwani R, Al Hawsawi KA. Neurotic excoriation presenting as solitary papule: case report. J Dermatol Dermatolog Surg. 2022;26:45. doi:10.4103/jdds.jdds_59_21
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233. doi:10.1016/j.jaad.2015.11.033
- Karadag AS, Ozlu E, Uzuncakmak TK, et al. Inverted follicular keratosis successfully treated with imiquimod. Indian Dermatol Online J. 2016;7:177-179. doi:10.4103/2229-5178.182354
- Nayak VN, Uma K, Girish HC, et al. Pseudoepitheliomatous hyperplasia in oral lesions: a review. J Int Oral Health. 2015;7:148-152.
- Saraceno R, Chiricozzi A, Nisticò SP, et al. An occlusive dressing containing betamethasone valerate 0.1% for the treatment of prurigo nodularis. J Dermatolog Treat. 2010;21:363-366. doi:10.3109/09546630903386606
- Wong SS, Goh CL. Double-blind, right/left comparison of calcipotriol ointment and betamethasone ointment in the treatment of prurigo nodularis. Arch Dermatol. 2000;136:807-808. doi:10.1001/archderm.136.6.807
- Waldinger TP, Wong RC, Taylor WB, et al. Cryotherapy improves prurigo nodularis. Arch Dermatol. 1984;120:1598-1600.
- Ständer S, Luger T, Metze D. Treatment of prurigo nodularis with topical capsaicin. J Am Acad Dermatol. 2001;44:471-478. doi:10.1067/mjd.2001.110059
- Ohanyan T, Schoepke N, Eirefelt S, et al. Role of substance P and its receptor neurokinin 1 in chronic prurigo: a randomized, proof-of-concept, controlled trial with topical aprepitant. Acta Derm Venereol. 2018;98:26-31. doi:10.2340/00015555-2780
- Tamagawa-Mineoka R, Katoh N, Ueda E, et al. Narrow-band ultraviolet B phototherapy in patients with recalcitrant nodular prurigo. J Dermatol. 2007;34:691-695. doi:10.1111/j.1346-8138.2007.00360.x
- Hammes S, Hermann J, Roos S, et al. UVB 308-nm excimer light and bath PUVA: combination therapy is very effective in the treatment of prurigo nodularis. J Eur Acad Dermatol Venereol. 2011;25:799-803. doi:10.1111/j.1468-3083.2010.03865.x
- Richards RN. Update on intralesional steroid: focus on dermatoses. J Cutan Med Surg. 2010;14:19-23. doi:10.2310/7750.2009.08082
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760. doi:10.1016/j.jaad.2020.07.025
- Grant JE Chamberlain SR Redden SA et al. N-Acetylcysteine in the treatment of excoriation disorder: a randomized clinical trial. JAMA Psychiatry. 2016;73:490-496. doi:10.1001/jamapsychiatry.2016.0060
- Adil M, Amin SS, Mohtashim M. N-acetylcysteine in dermatology. Indian J Dermatol Venereol Leprol. 2018;84:652-659. doi: 10.4103/ijdvl.IJDVL_33_18.
- Taylor M, Bhagwandas K. Trichotillosis, skin picking and N-acetylcysteine. J Am Acad Dermatol. 2015;72(suppl 1):AB117. https://doi.org/10.1016/j.jaad.2015.02.482
- Legat FJ. The antipruritic effect of phototherapy. Front Med (Lausanne). 2018;5:333. doi:10.3389/fmed.2018.00333
- Klejtman T, Beylot-Barry M, Joly P, et al. Treatment of prurigo with methotrexate: a multicentre retrospective study of 39 cases. J Eur Acad Dermatol Venereol. 2018;32:437-440. doi:10.1111/jdv.14646
- Wiznia LE, Callahan SW, Cohen DE, et al. Rapid improvement of prurigo nodularis with cyclosporine treatment. J Am Acad Dermatol. 2018;78:1209-1211. doi:10.1016/j.jaad.2018.02.024
- Yagami A, Furue M, Togawa M, et al. One-year safety and efficacy study of bilastine treatment in Japanese patients with chronic spontaneous urticaria or pruritus associated with skin diseases. J Dermatol. 2017;44:375-385. doi:10.1111/1346-8138.13644
- Mazza M, Guerriero G, Marano G, et al. Treatment of prurigo nodularis with pregabalin. J Clin Pharm Ther. 2013;38:16-18. doi:10.1111/jcpt.12005
- Ständer S, Kwon P, Hirman J, et al. Serlopitant reduced pruritus in patients with prurigo nodularis in a phase 2, randomized, placebo-controlled trial. J Am Acad Dermatol. 2019;80:1395-1402. doi:10.1016/j.jaad.2019.01.052
- Study of the efficacy, safety and tolerability of serlopitant for the treatment of pruritus (itch) with prurigo nodularis. ClinicalTrials.gov identifier: NCT03546816. Updated May 20, 2021. Accessed August 8, 2024. https://clinicaltrials.gov/study/NCT03546816
- Tsianakas A, Zeidler C, Riepe C, et al. Aprepitant in anti-histamine-refractory chronic nodular prurigo: a multicentre, randomized, double-blind, placebo-controlled, cross-over, phase-II trial (APREPRU). Acta Derm Venereol. 2019;99:379-385. doi:10.2340/00015555-3120
- Metze D, Reimann S, Beissert S, et al. Efficacy and safety of naltrexone, an oral opiate receptor antagonist, in the treatment of pruritus in internal and dermatological diseases. J Am Acad Dermatol. 1999;41:533-539.
- Weisshaar E, Szepietowski JC, Bernhard JD, et al. Efficacy and safety of oral nalbuphine extended release in prurigo nodularis: results of a phase 2 randomized controlled trial with an open‐label extension phase. J Eur Acad Dermatol Venereol. 2022;36:453-461. doi:10.1111/jdv.17816
- Ständer S, Böckenholt B, Schürmeyer-Horst F, et al. Treatment of chronic pruritus with the selective serotonin re-uptake inhibitors paroxetine and fluvoxamine: results of an open-labelled, two-arm proof-of-concept study. Acta Derm Venereol. 2009;89:45-51. doi:10.2340/00015555-0553
- Zalaudek I, Petrillo G, Baldassarre MA, et al. Amitriptyline as therapeutic and not symptomatic approach in the treatment of prurigo nodularis. G Ital Dermatol Venereol. 2006;141:433-437.
- Andersen TP, Fogh K. Thalidomide in 42 patients with prurigo nodularis Hyde. Dermatology. 2011;223:107-112. doi:10.1159/000331577
- Ständer S, Yosipovitch G, Legat FJ, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. 2020;382:706-716. doi:10.1056/NEJMoa1908316
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med. 2023;29:1180-1190. doi:10.1038/s41591-023-02320-9
- Mastorino L, Rosset F, Gelato F, et al. Chronic pruritus in atopic patients treated with dupilumab: real life response and related parameters in 354 patients. Pharmaceuticals (Basel). 2022;15:883. doi: 10.3390/ph15070883
- Kishi R, Toyama S, Tominaga M, et al. Effects of dupilumab on itch-related events in atopic dermatitis: implications for assessing treatment efficacy in clinical practice. Cells. 2023;12:239. doi: 10.3390/cells12020239
- Dupixent. European Medicines Agency website. Updated July 15, 2024. Accessed August 27, 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/dupixent
Practice Points
- Clinically, prurigo nodularis can mimic an array of dermatologic skin conditions and may be diagnosed more frequently in patients with comorbidities.
- Dupilumab is the first and only treatment for prurigo nodularis approved by the US Food and Drug Administration; however, many topical treatments are currently used as first-line therapies.
Olive Oil Shows Promise for Wound Healing of Ulcers
Olive oil is obtained by mechanical extraction from the fruit of the Olea europaea tree, which is believed to have originated from ancient Iran and Turkestan, later spreading to Anatolia, Syria, Palestine, and Israel. Mechanical extraction of the oil from the olive fruit involves pressure processing, centrifugation, and adhesion filtering.1 Refining of olive oil is done via alkali refining or physical refining, with physical refining being useful in removing oxidation by-products and pro-oxidant metals. Olive oil is composed mainly of triacylglycerols, which are glycerol esters attached to various fatty acids, with the most common fatty acid being the monounsaturated oleic acid. Additional fatty acids include palmitic acid, linoleic acid, stearic acid, and palmitoleic acid.2 Olive oil contains phenolic compounds, the main ones being oleuropein, hydroxytyrosol, and tyrosol. These phenolic compounds are proposed to be strong antioxidants and radical scavengers.3
Mediterranean countries are responsible for approximately 97% of the world’s olive cultivation.4 Olive oil historically was used as lamp fuel, lubricant, body ointment, and later as a source of edible oil.1 Recently, its potential uses in medicine have called for further exploration into other uses for olive oil.
The skin is the largest organ of the body and serves as a protective barrier against pathogens and harmful substances. Skin damage results in 3 main phases to aid in wound healing: inflammation, proliferation, and maturation. In proper skin healing, inflammation will stop once the harmful microbes are removed. However, an excess and prolongation of inflammation can result in delayed healing. Thus, interventions that can limit the amount of inflammation can help promote wound healing. Olive oil contains several anti-inflammatory molecules (compounds or chemicals), including phenolic compounds and omega-3 fatty acids.5 Studies also have shown that olive oil can promote re-epithelialization in tissues.6 Thus, use of olive oil in wound therapy has been of great interest.
This article will review studies that have investigated the use of olive oil for wound healing of diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers. To conduct a comprehensive scoping review of the literature on the effects of olive oil in wound healing, we utilized the resources of the Galter Health Sciences Library & Learning Center (Chicago, Illinois). Our search strategy was structured to encompass a range of relevant databases accessible through the library, including PubMed, Embase, and Web of Science. We formulated our search terms to be broad yet specific to our topic, combining keywords such as olive oil, wound healing, skin repair, and dermal therapy. The inclusion criteria were set to filter studies conducted from January 2000 to December 2019, focusing on clinical trials, observational studies, and review articles. We limited our search to articles published in English, which yielded a preliminary set of articles that were then screened based on their titles and abstracts. Full-text versions of potentially relevant studies were retrieved and assessed for eligibility. We included studies that specifically evaluated the effects of olive oil in wound healing, excluding those that did not directly relate to our research question or had insufficient data. The data extraction from these studies was conducted using a standardized form, capturing study design, population, intervention details, outcomes, and key findings. The synthesis of these data provided a comprehensive overview of the current evidence on the topic, aiding in the identification of gaps in knowledge and directions for future research.
Diabetic Foot Ulcers
Foot ulcers are common in patients with diabetes mellitus and are associated with notable morbidity and mortality. Foot ulcers can clinically manifest in various forms but are classically described as lesions with a deep sinus in the feet. Patients with diabetic foot ulcers are at risk for infection, and severe forms of the ulcers require amputation.7,8 Routine care of foot ulcers involves irrigation of the ulcer and surrounding area with normal saline solution daily, followed by a dressing with sterile gauze. Studies investigating the effect of olive oil on foot ulcers suggest that olive oil use for care and healing of foot ulcers is an area of interest.
A double-blind, randomized clinical trial investigated the effects of topical olive oil on diabetic foot ulcers.9 A total of 34 patients with foot ulcers of Wagner grades 1 (superficial ulcers that involved the skin but not underlying tissue) or 2 (deeper ulcers penetrating to the ligaments and muscles but not the bone) that had remained open and did not improve for more than 3 months were recruited. The patients were randomly assigned to receive topical olive oil and routine care (intervention group) or to receive routine care (control group). Patients who received olive oil had oil poured on their ulcers with gauze wrapped around the ulcer that was soaked with olive oil. The clinical characteristics of the diabetic ulcer (eg, site, grade, size, status of healing) were assessed. The study revealed that after 4 weeks, olive oil significantly decreased ulcer area (P=.01) and ulcer depth (P=.02) compared with the control. Furthermore, there was a significant difference (P=.003) in complete ulcer healing between the olive oil and control groups: 73.3% (11/15) of patients in the olive oil group had complete ulcer healing, whereas 13.3% (2/15) of patients in the control group had complete ulcer healing.9 The positive effect of olive oil on the healing of diabetic foot ulcers encourages further investigation as a possible therapy for foot ulcers.
Another randomized controlled trial of 45 patients with diabetic foot ulcers of Wagner grades 1 or 2 investigated the effect of olive oil.10 Patients were randomly assigned to 1 of 3 groups for 1 month: the olive oil group, the honey group, or the control group. Patients in the olive oil group had their wounds dressed using gauze with olive oil daily, the patients in the honey group had their wounds dressed using gauze with honey daily, and the control group had routine care consisting of irrigation with saline solution and dressing with a sterile gauze. This study calculated a wound healing score based on a predefined checklist for diabetic foot ulcers through 4 variables: wound grading, color, surrounding tissue status, and drainage. Each variable had a maximum score of 100, contributing to a total possible score of 400, which indicated complete healing. A score of 50 signified deterioration. Wound healing was categorized as follows: (1) complete healing is indicated by a total score of 400; (2) partial healing was indicated by an increase of at least 30 points from the initial score; (3) lack of healing occurred when there was no change or less than a 30-point increase from the initial score; and (4) aggravation was noted when the score decreased by at least 10 points from the initial assessment. The study revealed that olive oil and honey treatments resulted in an increase in mean score, which indicated better wound healing. Patients in the olive oil group had a mean score of 253.0 before the intervention and 330.5 after the intervention (P<.0001); patients in the honey group had a mean score of 267.5 before the intervention and 371.5 after the intervention (P<.0001).10
There also have been case reports on combined olive oil and honey in diabetic foot ulcer management. Haghighian et al11 presented a case of a diabetic foot wound that healed completely within 2 weeks after the combined use of olive oil and honey wax. Zahmatkesh and Rashidi12 observed the healing of a diabetic foot wound over a month with daily dressings of a mixture of heated honey and olive oil, resulting in granulation tissue formation within 5 days. Microvascular changes, such as capillary basement membrane thickening, pericyte degeneration, and impairment of vasodilation and constriction, may contribute to inflammation in blood vessels, which can delay the healing of diabetic foot ulcers.7 Because olive oil and honey contain compounds that have antioxidative, antimicrobial, and anti-inflammatory properties, both may play a role in notably reducing inflammation and promoting the healing of foot ulcers.13
Pressure Ulcers
A pressure ulcer is a superficial skin injury that is caused by a prolonged period of pressure on the skin, in which the skin becomes red but there is no rupture. Prolonged periods of immobility resulting in a reduction or pause of blood supply are common causes of pressure ulcers.14 Studies have suggested that topical olive oil may be effective in prevention of pressure ulcers and should be incorporated as part of standard-of-care measures.
In a randomized, single-blind trial, 72 patients with the first stage of bedsore—which is a pressure ulcer—in the sacral, shoulder, heel, or other areas were randomly assigned to either the intervention or control group.14 Patients in the intervention group had 15 mL of olive oil rubbed on the wound for 20 minutes daily and then washed with tepid water. The Pressure Ulcer Scale for Healing tool was utilized to assess the healing status of the pressure ulcer. This tool considers wound surface size, exudate rate, and tissue type to provide a score of 0 to 17 (0=healed ulcer; 17=progression of ulcer). The mean score (SD) was lower in the olive oil group at days 4 and 7 compared with the control group (day 4: 7.50 [2.823] vs 9.50 [1.732]; day 7: 5.44 [3.806] vs 8.83 [2.864])(P<.001). Furthermore, between days 1 and 7, there was significant improvement in the olive oil group (mean difference, 3.56; P<.001) but no significant change in the control group (mean difference, 0.75; P=.052).14 The results indicate that patients in the olive oil group had a better ulcer healing status compared with patients in the control group.
In a noninferiority, randomized, double-blind clinical trial, olive oil was compared to a recommended skin care measure of hyperoxygenated fatty acids (HOFAs) for the prevention of pressure ulcers.15 The study consisted of 571 residents from several nursing homes who were at risk for pressure ulcers. Either olive oil or HOFA was applied to areas at risk for pressure ulcers, with 2 sprays of 0.2 mL per spray to each area every 12 hours. The participants were followed up for 30 days or until a pressure ulcer developed. Researchers performed skin assessments; the Braden Scale was used to assess the risk for pressure ulcers. The incidence difference of pressure ulcers in the olive oil group and HOFA group did not exceed in the noninferiority margin of 7%. Furthermore, Kaplan-Meier survival curves for the time until pressure ulcer onset showed a nonsignificant difference between the 2 groups.15 These findings suggest that olive oil is as effective as HOFA for the prevention of pressure ulcers. Although the mechanism of olive oil on prevention of pressure ulcers has not yet been determined, it has been suggested that anti-inflammatory compounds in olive oil, such as polyphenol and oleocanthal compounds, play an anti-inflammatory role.
Perineal Ulcers
Episiotomy is a surgical incision that is made to open the vagina during birth to aid in delivery of the baby. In contrast to spontaneous vaginal tears, an episiotomy allows for easier repair and healing of the laceration.16 Studies were conducted to investigate the effect of olive oil on women with lacerations after an episiotomy.
A total of 90 primigravid women who had undergone episiotomy were recruited and randomly assigned to 1 of 2 interventions: cold compression with gel packs for 20 minutes within 12 hours after delivery for up to 10 days, if necessary, or topical olive oil twice daily within 12 hours after delivery for up to 10 days.17 Although there was no significant difference in the structural features of the wound, there was a significant difference in the redness severity. After 10 days, the mean REEDA (redness, edema, ecchymosis, discharge, and apposition) score (SD), which assesses tissue healing, was 0.47 (0.96) in patients who received cold compression with gel packs and 0.20 (0.50) in patients who received topical olive oil (P=.04).17 This study suggests that there is the potential for olive oil to be used for wound healing after episiotomy.
A double-blind trial consisted of 60 women who had mediolateral episiotomy or perineal tear grades 1 and 2 who were randomly assigned to 1 of 2 groups for 10 days: olive oil sitz bath or distilled water sitz bath (control group). The results showed a significant difference in pain severity after 5 and 10 days (P<.05), wound redness after 5 days (P<.0001), and redness (P<.000) and edema (P<.05) 10 days after delivery.18 This study encourages further investigation of the benefits of olive oil for care after an episiotomy.
Chronic Ulcers
Chronic ulcers are other persistent wounds that do not respond to standard treatments and pose a notable health burden. Their development is influenced by factors such as oxidative stress, microbial infections, and the body’s immune response. A case series was conducted to investigate the wound healing effects of olive oil on chronic ulcers.19 Fourteen patients who were diagnosed with 1 or more chronic skin ulcers that had not healed with conventional treatment, such as cleansing, debridement, or infection control, were recruited. The mean (SD) of the patients’
Final Thoughts
This review illuminated several key aspects of research on the role of olive oil in wound healing. Although the studies included in this review offer valuable insights, it is essential to acknowledge the variability in the quality of data presented. Several studies demonstrated robust methodology with clear definitions of outcomes and controlled conditions, providing high-quality evidence. However, other studies exhibited limitations, including small sample sizes and potential biases, which may affect the generalizability of the findings. Despite these limitations, the collective evidence suggests potential for olive oil in wound healing, warranting further investigation. Future research should aim for more standardized methodologies and larger, more diverse patient cohorts to validate these findings and explore the mechanisms underlying the therapeutic effects of olive oil.
- Emmons EW, Fedeli E, Firestone D. Olive oil introduction and history. In: Hui YH, ed. Bailey’s Industrial Oil & Fat Products, Vol. 2. Edible Oil and Fat Products: Edible Oils. 5th ed. John Wiley & Sons, Ltd; 241-269.
- Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. doi:10.3390/IJMS19030686
- Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636-644. doi:10.1016/S0955-2863(02)00229-2
- Rabiei Z, Enferadi ST. Traceability of origin and authenticity of olive oil. In: Boskou D, ed. Olive Oil: Constituents, Quality, Health Properties and Bioconversions. InTech; 2012.
- Wardhana, Surachmanto ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones. 2011;43:138-143.
- Aboui MM, Eidi A, Mortazavi P. Study of effect of olive oil on re-epithelialization of epithelial tissue in excision wound healing model in rats. J Comp Pathobiol. 2016;13:1875-1884.
- Aldana PC, Cartron AM, Khachemoune A. Reappraising diabetic foot ulcers: a focus on mechanisms of ulceration and clinical evaluation.Int J Low Extrem Wounds. 2022;21:294-302. doi:10.1177/1534734620944514
- Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21:255-264. doi:10.1007/s40257-019-00495-x
- Nasiri M, Fayazi S, Jahani S, et al. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14:38. doi:10.1186/S40200-015-0167-9
- Karimi Z, Behnammoghadam M, Rafiei H, et al. Impact of olive oil and honey on healing of diabetic foot: a randomized controlled trial. Clin Cosmet Investig Dermatol. 2019;12:347-354. doi:10.2147/CCID.S198577
- Haghighian HK, Koushan Y, Asgharzadeh A. Treatment of diabetic foot ulcer with propolis and olive oil: a case report. Knowl Health. 2012;6:35-38.
- Zahmatkesh M, Rashidi M. Case report of diabetic foot ulcer with topical honey and olive oil. J Med Plants. 2008;8:36-41.
- Cicerale S, Lucas LJ, Keast RS. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23:129-135. doi:10.1016/J.COPBIO.2011.09.006
- Miraj S, Pourafzali S, Ahmadabadi ZV, et al. Effect of olive oil in preventing the development of pressure ulcer grade one in intensive care unit patients. Int J Prev Med. 2020;11:23. doi:10.4103/IJPVM.IJPVM_545_18
- Díaz‐Valenzuela A, García‐Fernández FP, Carmona Fernández P, et al. Effectiveness and safety of olive oil preparation for topical use in pressure ulcer prevention: multicentre, controlled, randomised, and double‐blinded clinical trial. Int Wound J. 2019;16:1314-1322. doi:10.1111/IWJ.13191
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;CD000081. doi:10.1002/14651858.CD000081.PUB2
- Amani R, Kariman N, Mojab F, et al. Comparison of the effects of cold compress with gel packs and topical olive oil on episiotomy wound healing. J Babol Univ Med Sci. 2015;17:7-12. doi:10.22088/JBUMS.17.11.7
- Behmanesh F, Aghamohammadi A, Zeinalzadeh M, et al. Effects of olive oil sitz bath on improvement of perineal injury after delivery. Koomesh. 2013;14:309-315.
- Vitsos A, Tsagarousianos C, Vergos O, et al. Efficacy of a Ceratothoa oestroides olive oil extract in patients with chronic ulcers: a pilot study. Int J Low Extrem Wounds. 2019;18:309-316. doi:10.1177/1534734619856143
Olive oil is obtained by mechanical extraction from the fruit of the Olea europaea tree, which is believed to have originated from ancient Iran and Turkestan, later spreading to Anatolia, Syria, Palestine, and Israel. Mechanical extraction of the oil from the olive fruit involves pressure processing, centrifugation, and adhesion filtering.1 Refining of olive oil is done via alkali refining or physical refining, with physical refining being useful in removing oxidation by-products and pro-oxidant metals. Olive oil is composed mainly of triacylglycerols, which are glycerol esters attached to various fatty acids, with the most common fatty acid being the monounsaturated oleic acid. Additional fatty acids include palmitic acid, linoleic acid, stearic acid, and palmitoleic acid.2 Olive oil contains phenolic compounds, the main ones being oleuropein, hydroxytyrosol, and tyrosol. These phenolic compounds are proposed to be strong antioxidants and radical scavengers.3
Mediterranean countries are responsible for approximately 97% of the world’s olive cultivation.4 Olive oil historically was used as lamp fuel, lubricant, body ointment, and later as a source of edible oil.1 Recently, its potential uses in medicine have called for further exploration into other uses for olive oil.
The skin is the largest organ of the body and serves as a protective barrier against pathogens and harmful substances. Skin damage results in 3 main phases to aid in wound healing: inflammation, proliferation, and maturation. In proper skin healing, inflammation will stop once the harmful microbes are removed. However, an excess and prolongation of inflammation can result in delayed healing. Thus, interventions that can limit the amount of inflammation can help promote wound healing. Olive oil contains several anti-inflammatory molecules (compounds or chemicals), including phenolic compounds and omega-3 fatty acids.5 Studies also have shown that olive oil can promote re-epithelialization in tissues.6 Thus, use of olive oil in wound therapy has been of great interest.
This article will review studies that have investigated the use of olive oil for wound healing of diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers. To conduct a comprehensive scoping review of the literature on the effects of olive oil in wound healing, we utilized the resources of the Galter Health Sciences Library & Learning Center (Chicago, Illinois). Our search strategy was structured to encompass a range of relevant databases accessible through the library, including PubMed, Embase, and Web of Science. We formulated our search terms to be broad yet specific to our topic, combining keywords such as olive oil, wound healing, skin repair, and dermal therapy. The inclusion criteria were set to filter studies conducted from January 2000 to December 2019, focusing on clinical trials, observational studies, and review articles. We limited our search to articles published in English, which yielded a preliminary set of articles that were then screened based on their titles and abstracts. Full-text versions of potentially relevant studies were retrieved and assessed for eligibility. We included studies that specifically evaluated the effects of olive oil in wound healing, excluding those that did not directly relate to our research question or had insufficient data. The data extraction from these studies was conducted using a standardized form, capturing study design, population, intervention details, outcomes, and key findings. The synthesis of these data provided a comprehensive overview of the current evidence on the topic, aiding in the identification of gaps in knowledge and directions for future research.
Diabetic Foot Ulcers
Foot ulcers are common in patients with diabetes mellitus and are associated with notable morbidity and mortality. Foot ulcers can clinically manifest in various forms but are classically described as lesions with a deep sinus in the feet. Patients with diabetic foot ulcers are at risk for infection, and severe forms of the ulcers require amputation.7,8 Routine care of foot ulcers involves irrigation of the ulcer and surrounding area with normal saline solution daily, followed by a dressing with sterile gauze. Studies investigating the effect of olive oil on foot ulcers suggest that olive oil use for care and healing of foot ulcers is an area of interest.
A double-blind, randomized clinical trial investigated the effects of topical olive oil on diabetic foot ulcers.9 A total of 34 patients with foot ulcers of Wagner grades 1 (superficial ulcers that involved the skin but not underlying tissue) or 2 (deeper ulcers penetrating to the ligaments and muscles but not the bone) that had remained open and did not improve for more than 3 months were recruited. The patients were randomly assigned to receive topical olive oil and routine care (intervention group) or to receive routine care (control group). Patients who received olive oil had oil poured on their ulcers with gauze wrapped around the ulcer that was soaked with olive oil. The clinical characteristics of the diabetic ulcer (eg, site, grade, size, status of healing) were assessed. The study revealed that after 4 weeks, olive oil significantly decreased ulcer area (P=.01) and ulcer depth (P=.02) compared with the control. Furthermore, there was a significant difference (P=.003) in complete ulcer healing between the olive oil and control groups: 73.3% (11/15) of patients in the olive oil group had complete ulcer healing, whereas 13.3% (2/15) of patients in the control group had complete ulcer healing.9 The positive effect of olive oil on the healing of diabetic foot ulcers encourages further investigation as a possible therapy for foot ulcers.
Another randomized controlled trial of 45 patients with diabetic foot ulcers of Wagner grades 1 or 2 investigated the effect of olive oil.10 Patients were randomly assigned to 1 of 3 groups for 1 month: the olive oil group, the honey group, or the control group. Patients in the olive oil group had their wounds dressed using gauze with olive oil daily, the patients in the honey group had their wounds dressed using gauze with honey daily, and the control group had routine care consisting of irrigation with saline solution and dressing with a sterile gauze. This study calculated a wound healing score based on a predefined checklist for diabetic foot ulcers through 4 variables: wound grading, color, surrounding tissue status, and drainage. Each variable had a maximum score of 100, contributing to a total possible score of 400, which indicated complete healing. A score of 50 signified deterioration. Wound healing was categorized as follows: (1) complete healing is indicated by a total score of 400; (2) partial healing was indicated by an increase of at least 30 points from the initial score; (3) lack of healing occurred when there was no change or less than a 30-point increase from the initial score; and (4) aggravation was noted when the score decreased by at least 10 points from the initial assessment. The study revealed that olive oil and honey treatments resulted in an increase in mean score, which indicated better wound healing. Patients in the olive oil group had a mean score of 253.0 before the intervention and 330.5 after the intervention (P<.0001); patients in the honey group had a mean score of 267.5 before the intervention and 371.5 after the intervention (P<.0001).10
There also have been case reports on combined olive oil and honey in diabetic foot ulcer management. Haghighian et al11 presented a case of a diabetic foot wound that healed completely within 2 weeks after the combined use of olive oil and honey wax. Zahmatkesh and Rashidi12 observed the healing of a diabetic foot wound over a month with daily dressings of a mixture of heated honey and olive oil, resulting in granulation tissue formation within 5 days. Microvascular changes, such as capillary basement membrane thickening, pericyte degeneration, and impairment of vasodilation and constriction, may contribute to inflammation in blood vessels, which can delay the healing of diabetic foot ulcers.7 Because olive oil and honey contain compounds that have antioxidative, antimicrobial, and anti-inflammatory properties, both may play a role in notably reducing inflammation and promoting the healing of foot ulcers.13
Pressure Ulcers
A pressure ulcer is a superficial skin injury that is caused by a prolonged period of pressure on the skin, in which the skin becomes red but there is no rupture. Prolonged periods of immobility resulting in a reduction or pause of blood supply are common causes of pressure ulcers.14 Studies have suggested that topical olive oil may be effective in prevention of pressure ulcers and should be incorporated as part of standard-of-care measures.
In a randomized, single-blind trial, 72 patients with the first stage of bedsore—which is a pressure ulcer—in the sacral, shoulder, heel, or other areas were randomly assigned to either the intervention or control group.14 Patients in the intervention group had 15 mL of olive oil rubbed on the wound for 20 minutes daily and then washed with tepid water. The Pressure Ulcer Scale for Healing tool was utilized to assess the healing status of the pressure ulcer. This tool considers wound surface size, exudate rate, and tissue type to provide a score of 0 to 17 (0=healed ulcer; 17=progression of ulcer). The mean score (SD) was lower in the olive oil group at days 4 and 7 compared with the control group (day 4: 7.50 [2.823] vs 9.50 [1.732]; day 7: 5.44 [3.806] vs 8.83 [2.864])(P<.001). Furthermore, between days 1 and 7, there was significant improvement in the olive oil group (mean difference, 3.56; P<.001) but no significant change in the control group (mean difference, 0.75; P=.052).14 The results indicate that patients in the olive oil group had a better ulcer healing status compared with patients in the control group.
In a noninferiority, randomized, double-blind clinical trial, olive oil was compared to a recommended skin care measure of hyperoxygenated fatty acids (HOFAs) for the prevention of pressure ulcers.15 The study consisted of 571 residents from several nursing homes who were at risk for pressure ulcers. Either olive oil or HOFA was applied to areas at risk for pressure ulcers, with 2 sprays of 0.2 mL per spray to each area every 12 hours. The participants were followed up for 30 days or until a pressure ulcer developed. Researchers performed skin assessments; the Braden Scale was used to assess the risk for pressure ulcers. The incidence difference of pressure ulcers in the olive oil group and HOFA group did not exceed in the noninferiority margin of 7%. Furthermore, Kaplan-Meier survival curves for the time until pressure ulcer onset showed a nonsignificant difference between the 2 groups.15 These findings suggest that olive oil is as effective as HOFA for the prevention of pressure ulcers. Although the mechanism of olive oil on prevention of pressure ulcers has not yet been determined, it has been suggested that anti-inflammatory compounds in olive oil, such as polyphenol and oleocanthal compounds, play an anti-inflammatory role.
Perineal Ulcers
Episiotomy is a surgical incision that is made to open the vagina during birth to aid in delivery of the baby. In contrast to spontaneous vaginal tears, an episiotomy allows for easier repair and healing of the laceration.16 Studies were conducted to investigate the effect of olive oil on women with lacerations after an episiotomy.
A total of 90 primigravid women who had undergone episiotomy were recruited and randomly assigned to 1 of 2 interventions: cold compression with gel packs for 20 minutes within 12 hours after delivery for up to 10 days, if necessary, or topical olive oil twice daily within 12 hours after delivery for up to 10 days.17 Although there was no significant difference in the structural features of the wound, there was a significant difference in the redness severity. After 10 days, the mean REEDA (redness, edema, ecchymosis, discharge, and apposition) score (SD), which assesses tissue healing, was 0.47 (0.96) in patients who received cold compression with gel packs and 0.20 (0.50) in patients who received topical olive oil (P=.04).17 This study suggests that there is the potential for olive oil to be used for wound healing after episiotomy.
A double-blind trial consisted of 60 women who had mediolateral episiotomy or perineal tear grades 1 and 2 who were randomly assigned to 1 of 2 groups for 10 days: olive oil sitz bath or distilled water sitz bath (control group). The results showed a significant difference in pain severity after 5 and 10 days (P<.05), wound redness after 5 days (P<.0001), and redness (P<.000) and edema (P<.05) 10 days after delivery.18 This study encourages further investigation of the benefits of olive oil for care after an episiotomy.
Chronic Ulcers
Chronic ulcers are other persistent wounds that do not respond to standard treatments and pose a notable health burden. Their development is influenced by factors such as oxidative stress, microbial infections, and the body’s immune response. A case series was conducted to investigate the wound healing effects of olive oil on chronic ulcers.19 Fourteen patients who were diagnosed with 1 or more chronic skin ulcers that had not healed with conventional treatment, such as cleansing, debridement, or infection control, were recruited. The mean (SD) of the patients’
Final Thoughts
This review illuminated several key aspects of research on the role of olive oil in wound healing. Although the studies included in this review offer valuable insights, it is essential to acknowledge the variability in the quality of data presented. Several studies demonstrated robust methodology with clear definitions of outcomes and controlled conditions, providing high-quality evidence. However, other studies exhibited limitations, including small sample sizes and potential biases, which may affect the generalizability of the findings. Despite these limitations, the collective evidence suggests potential for olive oil in wound healing, warranting further investigation. Future research should aim for more standardized methodologies and larger, more diverse patient cohorts to validate these findings and explore the mechanisms underlying the therapeutic effects of olive oil.
Olive oil is obtained by mechanical extraction from the fruit of the Olea europaea tree, which is believed to have originated from ancient Iran and Turkestan, later spreading to Anatolia, Syria, Palestine, and Israel. Mechanical extraction of the oil from the olive fruit involves pressure processing, centrifugation, and adhesion filtering.1 Refining of olive oil is done via alkali refining or physical refining, with physical refining being useful in removing oxidation by-products and pro-oxidant metals. Olive oil is composed mainly of triacylglycerols, which are glycerol esters attached to various fatty acids, with the most common fatty acid being the monounsaturated oleic acid. Additional fatty acids include palmitic acid, linoleic acid, stearic acid, and palmitoleic acid.2 Olive oil contains phenolic compounds, the main ones being oleuropein, hydroxytyrosol, and tyrosol. These phenolic compounds are proposed to be strong antioxidants and radical scavengers.3
Mediterranean countries are responsible for approximately 97% of the world’s olive cultivation.4 Olive oil historically was used as lamp fuel, lubricant, body ointment, and later as a source of edible oil.1 Recently, its potential uses in medicine have called for further exploration into other uses for olive oil.
The skin is the largest organ of the body and serves as a protective barrier against pathogens and harmful substances. Skin damage results in 3 main phases to aid in wound healing: inflammation, proliferation, and maturation. In proper skin healing, inflammation will stop once the harmful microbes are removed. However, an excess and prolongation of inflammation can result in delayed healing. Thus, interventions that can limit the amount of inflammation can help promote wound healing. Olive oil contains several anti-inflammatory molecules (compounds or chemicals), including phenolic compounds and omega-3 fatty acids.5 Studies also have shown that olive oil can promote re-epithelialization in tissues.6 Thus, use of olive oil in wound therapy has been of great interest.
This article will review studies that have investigated the use of olive oil for wound healing of diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers. To conduct a comprehensive scoping review of the literature on the effects of olive oil in wound healing, we utilized the resources of the Galter Health Sciences Library & Learning Center (Chicago, Illinois). Our search strategy was structured to encompass a range of relevant databases accessible through the library, including PubMed, Embase, and Web of Science. We formulated our search terms to be broad yet specific to our topic, combining keywords such as olive oil, wound healing, skin repair, and dermal therapy. The inclusion criteria were set to filter studies conducted from January 2000 to December 2019, focusing on clinical trials, observational studies, and review articles. We limited our search to articles published in English, which yielded a preliminary set of articles that were then screened based on their titles and abstracts. Full-text versions of potentially relevant studies were retrieved and assessed for eligibility. We included studies that specifically evaluated the effects of olive oil in wound healing, excluding those that did not directly relate to our research question or had insufficient data. The data extraction from these studies was conducted using a standardized form, capturing study design, population, intervention details, outcomes, and key findings. The synthesis of these data provided a comprehensive overview of the current evidence on the topic, aiding in the identification of gaps in knowledge and directions for future research.
Diabetic Foot Ulcers
Foot ulcers are common in patients with diabetes mellitus and are associated with notable morbidity and mortality. Foot ulcers can clinically manifest in various forms but are classically described as lesions with a deep sinus in the feet. Patients with diabetic foot ulcers are at risk for infection, and severe forms of the ulcers require amputation.7,8 Routine care of foot ulcers involves irrigation of the ulcer and surrounding area with normal saline solution daily, followed by a dressing with sterile gauze. Studies investigating the effect of olive oil on foot ulcers suggest that olive oil use for care and healing of foot ulcers is an area of interest.
A double-blind, randomized clinical trial investigated the effects of topical olive oil on diabetic foot ulcers.9 A total of 34 patients with foot ulcers of Wagner grades 1 (superficial ulcers that involved the skin but not underlying tissue) or 2 (deeper ulcers penetrating to the ligaments and muscles but not the bone) that had remained open and did not improve for more than 3 months were recruited. The patients were randomly assigned to receive topical olive oil and routine care (intervention group) or to receive routine care (control group). Patients who received olive oil had oil poured on their ulcers with gauze wrapped around the ulcer that was soaked with olive oil. The clinical characteristics of the diabetic ulcer (eg, site, grade, size, status of healing) were assessed. The study revealed that after 4 weeks, olive oil significantly decreased ulcer area (P=.01) and ulcer depth (P=.02) compared with the control. Furthermore, there was a significant difference (P=.003) in complete ulcer healing between the olive oil and control groups: 73.3% (11/15) of patients in the olive oil group had complete ulcer healing, whereas 13.3% (2/15) of patients in the control group had complete ulcer healing.9 The positive effect of olive oil on the healing of diabetic foot ulcers encourages further investigation as a possible therapy for foot ulcers.
Another randomized controlled trial of 45 patients with diabetic foot ulcers of Wagner grades 1 or 2 investigated the effect of olive oil.10 Patients were randomly assigned to 1 of 3 groups for 1 month: the olive oil group, the honey group, or the control group. Patients in the olive oil group had their wounds dressed using gauze with olive oil daily, the patients in the honey group had their wounds dressed using gauze with honey daily, and the control group had routine care consisting of irrigation with saline solution and dressing with a sterile gauze. This study calculated a wound healing score based on a predefined checklist for diabetic foot ulcers through 4 variables: wound grading, color, surrounding tissue status, and drainage. Each variable had a maximum score of 100, contributing to a total possible score of 400, which indicated complete healing. A score of 50 signified deterioration. Wound healing was categorized as follows: (1) complete healing is indicated by a total score of 400; (2) partial healing was indicated by an increase of at least 30 points from the initial score; (3) lack of healing occurred when there was no change or less than a 30-point increase from the initial score; and (4) aggravation was noted when the score decreased by at least 10 points from the initial assessment. The study revealed that olive oil and honey treatments resulted in an increase in mean score, which indicated better wound healing. Patients in the olive oil group had a mean score of 253.0 before the intervention and 330.5 after the intervention (P<.0001); patients in the honey group had a mean score of 267.5 before the intervention and 371.5 after the intervention (P<.0001).10
There also have been case reports on combined olive oil and honey in diabetic foot ulcer management. Haghighian et al11 presented a case of a diabetic foot wound that healed completely within 2 weeks after the combined use of olive oil and honey wax. Zahmatkesh and Rashidi12 observed the healing of a diabetic foot wound over a month with daily dressings of a mixture of heated honey and olive oil, resulting in granulation tissue formation within 5 days. Microvascular changes, such as capillary basement membrane thickening, pericyte degeneration, and impairment of vasodilation and constriction, may contribute to inflammation in blood vessels, which can delay the healing of diabetic foot ulcers.7 Because olive oil and honey contain compounds that have antioxidative, antimicrobial, and anti-inflammatory properties, both may play a role in notably reducing inflammation and promoting the healing of foot ulcers.13
Pressure Ulcers
A pressure ulcer is a superficial skin injury that is caused by a prolonged period of pressure on the skin, in which the skin becomes red but there is no rupture. Prolonged periods of immobility resulting in a reduction or pause of blood supply are common causes of pressure ulcers.14 Studies have suggested that topical olive oil may be effective in prevention of pressure ulcers and should be incorporated as part of standard-of-care measures.
In a randomized, single-blind trial, 72 patients with the first stage of bedsore—which is a pressure ulcer—in the sacral, shoulder, heel, or other areas were randomly assigned to either the intervention or control group.14 Patients in the intervention group had 15 mL of olive oil rubbed on the wound for 20 minutes daily and then washed with tepid water. The Pressure Ulcer Scale for Healing tool was utilized to assess the healing status of the pressure ulcer. This tool considers wound surface size, exudate rate, and tissue type to provide a score of 0 to 17 (0=healed ulcer; 17=progression of ulcer). The mean score (SD) was lower in the olive oil group at days 4 and 7 compared with the control group (day 4: 7.50 [2.823] vs 9.50 [1.732]; day 7: 5.44 [3.806] vs 8.83 [2.864])(P<.001). Furthermore, between days 1 and 7, there was significant improvement in the olive oil group (mean difference, 3.56; P<.001) but no significant change in the control group (mean difference, 0.75; P=.052).14 The results indicate that patients in the olive oil group had a better ulcer healing status compared with patients in the control group.
In a noninferiority, randomized, double-blind clinical trial, olive oil was compared to a recommended skin care measure of hyperoxygenated fatty acids (HOFAs) for the prevention of pressure ulcers.15 The study consisted of 571 residents from several nursing homes who were at risk for pressure ulcers. Either olive oil or HOFA was applied to areas at risk for pressure ulcers, with 2 sprays of 0.2 mL per spray to each area every 12 hours. The participants were followed up for 30 days or until a pressure ulcer developed. Researchers performed skin assessments; the Braden Scale was used to assess the risk for pressure ulcers. The incidence difference of pressure ulcers in the olive oil group and HOFA group did not exceed in the noninferiority margin of 7%. Furthermore, Kaplan-Meier survival curves for the time until pressure ulcer onset showed a nonsignificant difference between the 2 groups.15 These findings suggest that olive oil is as effective as HOFA for the prevention of pressure ulcers. Although the mechanism of olive oil on prevention of pressure ulcers has not yet been determined, it has been suggested that anti-inflammatory compounds in olive oil, such as polyphenol and oleocanthal compounds, play an anti-inflammatory role.
Perineal Ulcers
Episiotomy is a surgical incision that is made to open the vagina during birth to aid in delivery of the baby. In contrast to spontaneous vaginal tears, an episiotomy allows for easier repair and healing of the laceration.16 Studies were conducted to investigate the effect of olive oil on women with lacerations after an episiotomy.
A total of 90 primigravid women who had undergone episiotomy were recruited and randomly assigned to 1 of 2 interventions: cold compression with gel packs for 20 minutes within 12 hours after delivery for up to 10 days, if necessary, or topical olive oil twice daily within 12 hours after delivery for up to 10 days.17 Although there was no significant difference in the structural features of the wound, there was a significant difference in the redness severity. After 10 days, the mean REEDA (redness, edema, ecchymosis, discharge, and apposition) score (SD), which assesses tissue healing, was 0.47 (0.96) in patients who received cold compression with gel packs and 0.20 (0.50) in patients who received topical olive oil (P=.04).17 This study suggests that there is the potential for olive oil to be used for wound healing after episiotomy.
A double-blind trial consisted of 60 women who had mediolateral episiotomy or perineal tear grades 1 and 2 who were randomly assigned to 1 of 2 groups for 10 days: olive oil sitz bath or distilled water sitz bath (control group). The results showed a significant difference in pain severity after 5 and 10 days (P<.05), wound redness after 5 days (P<.0001), and redness (P<.000) and edema (P<.05) 10 days after delivery.18 This study encourages further investigation of the benefits of olive oil for care after an episiotomy.
Chronic Ulcers
Chronic ulcers are other persistent wounds that do not respond to standard treatments and pose a notable health burden. Their development is influenced by factors such as oxidative stress, microbial infections, and the body’s immune response. A case series was conducted to investigate the wound healing effects of olive oil on chronic ulcers.19 Fourteen patients who were diagnosed with 1 or more chronic skin ulcers that had not healed with conventional treatment, such as cleansing, debridement, or infection control, were recruited. The mean (SD) of the patients’
Final Thoughts
This review illuminated several key aspects of research on the role of olive oil in wound healing. Although the studies included in this review offer valuable insights, it is essential to acknowledge the variability in the quality of data presented. Several studies demonstrated robust methodology with clear definitions of outcomes and controlled conditions, providing high-quality evidence. However, other studies exhibited limitations, including small sample sizes and potential biases, which may affect the generalizability of the findings. Despite these limitations, the collective evidence suggests potential for olive oil in wound healing, warranting further investigation. Future research should aim for more standardized methodologies and larger, more diverse patient cohorts to validate these findings and explore the mechanisms underlying the therapeutic effects of olive oil.
- Emmons EW, Fedeli E, Firestone D. Olive oil introduction and history. In: Hui YH, ed. Bailey’s Industrial Oil & Fat Products, Vol. 2. Edible Oil and Fat Products: Edible Oils. 5th ed. John Wiley & Sons, Ltd; 241-269.
- Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. doi:10.3390/IJMS19030686
- Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636-644. doi:10.1016/S0955-2863(02)00229-2
- Rabiei Z, Enferadi ST. Traceability of origin and authenticity of olive oil. In: Boskou D, ed. Olive Oil: Constituents, Quality, Health Properties and Bioconversions. InTech; 2012.
- Wardhana, Surachmanto ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones. 2011;43:138-143.
- Aboui MM, Eidi A, Mortazavi P. Study of effect of olive oil on re-epithelialization of epithelial tissue in excision wound healing model in rats. J Comp Pathobiol. 2016;13:1875-1884.
- Aldana PC, Cartron AM, Khachemoune A. Reappraising diabetic foot ulcers: a focus on mechanisms of ulceration and clinical evaluation.Int J Low Extrem Wounds. 2022;21:294-302. doi:10.1177/1534734620944514
- Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21:255-264. doi:10.1007/s40257-019-00495-x
- Nasiri M, Fayazi S, Jahani S, et al. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14:38. doi:10.1186/S40200-015-0167-9
- Karimi Z, Behnammoghadam M, Rafiei H, et al. Impact of olive oil and honey on healing of diabetic foot: a randomized controlled trial. Clin Cosmet Investig Dermatol. 2019;12:347-354. doi:10.2147/CCID.S198577
- Haghighian HK, Koushan Y, Asgharzadeh A. Treatment of diabetic foot ulcer with propolis and olive oil: a case report. Knowl Health. 2012;6:35-38.
- Zahmatkesh M, Rashidi M. Case report of diabetic foot ulcer with topical honey and olive oil. J Med Plants. 2008;8:36-41.
- Cicerale S, Lucas LJ, Keast RS. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23:129-135. doi:10.1016/J.COPBIO.2011.09.006
- Miraj S, Pourafzali S, Ahmadabadi ZV, et al. Effect of olive oil in preventing the development of pressure ulcer grade one in intensive care unit patients. Int J Prev Med. 2020;11:23. doi:10.4103/IJPVM.IJPVM_545_18
- Díaz‐Valenzuela A, García‐Fernández FP, Carmona Fernández P, et al. Effectiveness and safety of olive oil preparation for topical use in pressure ulcer prevention: multicentre, controlled, randomised, and double‐blinded clinical trial. Int Wound J. 2019;16:1314-1322. doi:10.1111/IWJ.13191
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;CD000081. doi:10.1002/14651858.CD000081.PUB2
- Amani R, Kariman N, Mojab F, et al. Comparison of the effects of cold compress with gel packs and topical olive oil on episiotomy wound healing. J Babol Univ Med Sci. 2015;17:7-12. doi:10.22088/JBUMS.17.11.7
- Behmanesh F, Aghamohammadi A, Zeinalzadeh M, et al. Effects of olive oil sitz bath on improvement of perineal injury after delivery. Koomesh. 2013;14:309-315.
- Vitsos A, Tsagarousianos C, Vergos O, et al. Efficacy of a Ceratothoa oestroides olive oil extract in patients with chronic ulcers: a pilot study. Int J Low Extrem Wounds. 2019;18:309-316. doi:10.1177/1534734619856143
- Emmons EW, Fedeli E, Firestone D. Olive oil introduction and history. In: Hui YH, ed. Bailey’s Industrial Oil & Fat Products, Vol. 2. Edible Oil and Fat Products: Edible Oils. 5th ed. John Wiley & Sons, Ltd; 241-269.
- Gorzynik-Debicka M, Przychodzen P, Cappello F, et al. Potential health benefits of olive oil and plant polyphenols. Int J Mol Sci. 2018;19:686. doi:10.3390/IJMS19030686
- Tuck KL, Hayball PJ. Major phenolic compounds in olive oil: metabolism and health effects. J Nutr Biochem. 2002;13:636-644. doi:10.1016/S0955-2863(02)00229-2
- Rabiei Z, Enferadi ST. Traceability of origin and authenticity of olive oil. In: Boskou D, ed. Olive Oil: Constituents, Quality, Health Properties and Bioconversions. InTech; 2012.
- Wardhana, Surachmanto ES, Datau EA. The role of omega-3 fatty acids contained in olive oil on chronic inflammation. Acta Med Indones. 2011;43:138-143.
- Aboui MM, Eidi A, Mortazavi P. Study of effect of olive oil on re-epithelialization of epithelial tissue in excision wound healing model in rats. J Comp Pathobiol. 2016;13:1875-1884.
- Aldana PC, Cartron AM, Khachemoune A. Reappraising diabetic foot ulcers: a focus on mechanisms of ulceration and clinical evaluation.Int J Low Extrem Wounds. 2022;21:294-302. doi:10.1177/1534734620944514
- Aldana PC, Khachemoune A. Diabetic foot ulcers: appraising standard of care and reviewing new trends in management. Am J Clin Dermatol. 2020;21:255-264. doi:10.1007/s40257-019-00495-x
- Nasiri M, Fayazi S, Jahani S, et al. The effect of topical olive oil on the healing of foot ulcer in patients with type 2 diabetes: a double-blind randomized clinical trial study in Iran. J Diabetes Metab Disord. 2015;14:38. doi:10.1186/S40200-015-0167-9
- Karimi Z, Behnammoghadam M, Rafiei H, et al. Impact of olive oil and honey on healing of diabetic foot: a randomized controlled trial. Clin Cosmet Investig Dermatol. 2019;12:347-354. doi:10.2147/CCID.S198577
- Haghighian HK, Koushan Y, Asgharzadeh A. Treatment of diabetic foot ulcer with propolis and olive oil: a case report. Knowl Health. 2012;6:35-38.
- Zahmatkesh M, Rashidi M. Case report of diabetic foot ulcer with topical honey and olive oil. J Med Plants. 2008;8:36-41.
- Cicerale S, Lucas LJ, Keast RS. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr Opin Biotechnol. 2012;23:129-135. doi:10.1016/J.COPBIO.2011.09.006
- Miraj S, Pourafzali S, Ahmadabadi ZV, et al. Effect of olive oil in preventing the development of pressure ulcer grade one in intensive care unit patients. Int J Prev Med. 2020;11:23. doi:10.4103/IJPVM.IJPVM_545_18
- Díaz‐Valenzuela A, García‐Fernández FP, Carmona Fernández P, et al. Effectiveness and safety of olive oil preparation for topical use in pressure ulcer prevention: multicentre, controlled, randomised, and double‐blinded clinical trial. Int Wound J. 2019;16:1314-1322. doi:10.1111/IWJ.13191
- Carroli G, Mignini L. Episiotomy for vaginal birth. Cochrane Database Syst Rev. 2009;CD000081. doi:10.1002/14651858.CD000081.PUB2
- Amani R, Kariman N, Mojab F, et al. Comparison of the effects of cold compress with gel packs and topical olive oil on episiotomy wound healing. J Babol Univ Med Sci. 2015;17:7-12. doi:10.22088/JBUMS.17.11.7
- Behmanesh F, Aghamohammadi A, Zeinalzadeh M, et al. Effects of olive oil sitz bath on improvement of perineal injury after delivery. Koomesh. 2013;14:309-315.
- Vitsos A, Tsagarousianos C, Vergos O, et al. Efficacy of a Ceratothoa oestroides olive oil extract in patients with chronic ulcers: a pilot study. Int J Low Extrem Wounds. 2019;18:309-316. doi:10.1177/1534734619856143
Practice Points
- Interventions that effectively reduce excessive and prolonged inflammation can help promote timely wound healing. Consider integrating anti-inflammatory treatments into wound care protocols to enhance healing outcomes.
- Utilization of olive oil in wound therapy, particularly for conditions such as diabetic foot ulcers, pressure ulcers, perineal ulcers, and chronic ulcers, has shown promise for promoting healing.
- Regularly review and incorporate findings from recent studies on the use of olive oil and other novel interventions in wound therapy to ensure the application of the most current and effective treatment strategies.
Periorbital Changes Induced by Prostaglandin Eye Drops
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.
The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.

The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
To the Editor:
A 42-year man presented with hollowing of the upper eyelid and skin discoloration of the left periorbital area of 10 years’ duration. He was a professional mixed martial arts fighter with a history of 2 surgeries for retinal detachment of the left eye 13 years prior to the current presentation. The patient also has macular scarring in the left eye. He denied a history of facial fracture, reconstructive surgery, or other medical conditions. His visual acuity was unknown; however, he did not require corrective glasses. He used 3 prescription ophthalmic eye drops—dorzolamide hydrochloride plus timolol maleate, 10 mL; brimonidine tartrate ophthalmic solution 0.15%, 5 mL; and latanoprost ophthalmic solution 0.005%, 125 μg/2.5 mL—in the left eye to lower intraocular pressure, as therapy for glaucoma. If left untreated, glaucoma can lead to vision loss.
Physical examination revealed periorbital hyperpigmentation on the left side; hypertrichosis and eyelash trichomegaly compared to the right side; and a deep left upper orbital sulcus compared to the right side (Figure). The patient was alert and oriented to person, place, and time. Extraocular movement was intact bilaterally, and his pupillary reflex was symmetric. No tenderness was noted over the affected area on palpation; no subcutaneous masses or lesions were observed or palpated. There was no ocular discharge, the conjunctiva was pink, and the sclera was white bilaterally.

The differential diagnosis included professional trauma-induced orbital changes, nevus of Ota (oculomucodermal melanocytosis), prostaglandin-associated periorbitopathy (PAP), and melasma. Although the patient sustained an injury that caused retinal detachment, he never experienced an orbital bone fracture; additionally, a fracture would not explain the skin discoloration or longer eyelashes. Periorbital nevus of Ota most commonly manifests as a unilateral scleral and brown-bluish skin discoloration but does not cause hollowing of the orbital sulcus or affect the length and thickness of eyelashes. Melasma—bilateral skin hyperpigmentation that most commonly affects women—can be induced by oral contraceptives, antibiotics, heat, sun exposure, and pregnancy. It does not affect the color of the iris or the depth of the scleral sulcus, and it does not increase the length and thickness of eyelashes. Based on the clinical presentation and a review of the eye drops used, he was diagnosed with PAP due to prolonged use of latanoprost ophthalmic solution. The patient was referred to an ophthalmologist for consideration of a switch to a different class of medication.
Of the 3 eye drops used by this patient, latanoprost, a prostaglandin analog, decreases intraocular pressure and is known to cause PAP. This condition comprises a constellation of changes, including upper eyelid ptosis, deepening of the upper eyelid sulcus, involution of dermatochalasis, periorbital fat atrophy, mild enophthalmos (sunken eye), inferior scleral show, increased prominence of eyelid vessels, and tight eyelids.1 Latanoprost most often produces these findings, but all prostaglandin ophthalmic medications can, including the dual-indication bimatoprost, which was approved by the US Food and Drug Administration to reduce elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension but also is used to grow darker, thicker, and longer eyelashes. Clinicians who prescribe bimatoprost for this cosmetic indication should be mindful of the potential for PAP and discuss it with patients.
The prescribing information (PI) for bimatoprost (Latisse; Allergan) does not list PAP as an adverse reaction observed in the 4-month multicenter, double-blind, randomized, vehicle-controlled study of bimatoprost (as Latisse) in 278 adults.2 The PI does list “periorbital and lid changes associated with periorbital fat atrophy and skin tightness resulting in deepening of eyelid sulcus and eyelid ptosis” as an adverse reaction in postmarketing experience. However, according to the PI, the frequency of these adverse reactions cannot be established, as the reporting of such incidents was voluntary and the size of the treated population was uncertain.2
Prostaglandins can cause periorbitopathy by several mechanisms; one speculated cause is that this group of medications might provoke smooth muscle contraction. Prostaglandin medications also have an affinity for fat cells1; atrophy of fat cells can lead to enophthalmos and deepening upper eyelid sulcus. In an observational study of 105 participants who were using a prostaglandin in 1 eye for longer than 1 month (the other eye was used as a control), the overall frequency of prostaglandin-associated periorbitopathy was 93.3% in the bimatoprost group, 41.4% in the latanoprost group, and 70% in the travoprost group, while the frequency of deepening of the upper eyelid sulcus was 80% in the bimatoprost group, 15.7% in the latanoprost group, and 45% in the travoprost group.3 These changes may not be as striking when a patient is using a prostaglandin ophthalmic medication in both eyes and may not be noticed even by the patient. It is prudent for the clinician to take a baseline photograph of the patient when these medications are prescribed to observe for early signs of periorbitopathy. These adverse effects may not be reversible when the medication is discontinued4 and have been observed as early as 4 to 6 weeks after the start of treatment.5
Our patient was counseled that his constellation of PAP findings potentially could be partially reversed over months or even a year or longer if the offending agent was discontinued. However, he was cautioned that cessation of latanoprost first needed to be discussed with his ophthalmologist, who would determine if there was a suitable alternative to a prostaglandin analog for him. The patient’s only concern was the aesthetic appearance of the left periorbital area. A hyaluronic acid filler or fat grafting can be considered for correction of orbital sulcus hollowing; however, we could not locate any long-term studies in which such corrective treatments were applied for PAP. Our patient continues to use latanoprost with no change in the frequency of use. There have been no further changes or progression in the physical appearance of the left eye or periorbital area. The patient has not undergone any corrective treatments.
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
- Berke SJ. PAP: new concerns for prostaglandin use. Rev Ophthalmol. 2012;19:70.
- Latisse (bimatoprost ophthalmic solution 0.03%). Package insert. Allergan; 2021. Accessed April 11, 2024. https://www.rxabbvie.com/pdf/latisse_pi.pdf
- Kucukevcilioglu M, Bayer A, Uysal Y, et al. Prostaglandin associated periorbitopathy in patients using bimatoprost, latanoprost and travoprost. Clin Exp Ophthalmol. 2014;42:126-131. doi:10.1111/ceo.12163
- Filippopoulos T, Paula JS, Torun N, et al. Periorbital changes associated with topical bimatoprost. Ophthalmic Plast Reconstr Surg. 2008;24:302-307. doi:10.1097/IOP.0b013e31817d81df
- Peplinski LS, Smith KA. Deepening of lid sulcus from topical bimatoprost therapy. Optom Vis Sci. 2004;81:574-577. doi:10.1097/01.opx.0000141791.16683.4a
PRACTICE POINTS
- Ask patients to provide photographs taken prior to noticed changes to assess progression if they are new to your practice.
- Take photographs of patients in good light against a solid-colored background to have a baseline. It may be helpful to update patient images annually.
- Discuss with patients the aesthetic changes that may occur with the use of prescription medications. Provide pamphlets with images to educate them on what to expect.
Vibrio vulnificus: Review of Mild to Life-threatening Skin Infections
Vibrio vulnificus is a member of the Vibrio genus. Most Vibrio species are nonpathogenic in humans; however, V vulnificus is one of the pathogenic strains.1 In Latin, the term vulnificus means “wounding,” and V vulnificus can cause life-threatening infections in patients. The mortality rate of V vulnificus infections is approximately 33% in the United States.2Vibrio vulnificus is a gram-negative bacterium that was first isolated by the Centers for Disease Control and Prevention in 1964 and was given its current name in 1979.3-6 It has been found in numerous organisms, including oysters, crabs, clams, shrimp, mussels, mullets, and sea bass.4 The vast majority of infections in the United States are due to oyster exposure and consumption.2,7Vibrio vulnificus is responsible for more than 95% of seafood-related deaths in the United States and has the highest mortality rate of all food-borne illness in the United States.2,5 It also has the highest per-case economic impact of all food-related diseases in the United States.1
What distinguishes a pathogenic vs nonpathogenic Vibrio isolate remains unknown; Vibrio species rapidly undergo horizontal gene transfer, making DNA isolation difficult.1 Some characteristics of V vulnificus that may confer virulence are the capsular polysaccharide, lipopolysaccharide, binding proteins, and tissue-degrading enzymes.1,5 First, encapsulated strains are more virulent and invasive than unencapsulated strains.1 The mucopolysaccharide capsule protects the bacterium from the immune system, allowing it to evade immune surveillance, cause more severe infection, and invade into the subcutaneous tissue.3,5 Second, production of sialic acid–like molecules alter the lipopolysaccharide, allowing for motility and biofilm formation.1 This allows the bacterium to survive in marine waters and within the bloodstream, the latter leading to sepsis in humans. Third, production of N-acetylglucosamine–binding protein A allows for adhesion to chitin. Shellfish consume chitin, and chitin accumulates in shellfish. N-acetylglucosamine–binding protein A also binds mucin; this may be how V vulnificus binds to mucin in the gastrointestinal tract in humans, causing gastroenteritis.1 Binding to the human mucosae also may allow the bacteria to gain access to the blood supply, leading to septicemia.4 Finally, tissue-degrading enzymes such as proteases are responsible for necrotizing wound infections associated with V vulnificus, as the enzymes allow for invasion into the skin and subcutaneous tissues. Proteases also increase vascular permeability and lead to edema.3 Hence, these virulence factors may provide V vulnificus the pathogenicity to cause infection in humans.
Three biotypes of V vulnificus have been discovered. Biotype 1 is the most common and is found worldwide in brackish water.8 It can cause the entire spectrum of illnesses, and it has a case fatality rate of 50% in humans. Biotype 1 is presumably responsible for all infections in the United States. Biotype 2 is found in the Far East and Western Europe; it inhabits a unique niche—saltwater used for eel farming. It typically causes infection in eels, but rarely it can cause wound infections in humans. Biotype 3 is found in freshwater fish farming in Israel, and it is a hybrid of biotypes 1 and 2.It can cause severe soft tissue infections in humans, sometimes requiring amputation.8
Epidemiology
Vibrio vulnificus is a motile, gram-negative, halophilic, aquatic bacterium.1,4,5,8,9 It is part of the normal estuarine microbiome and typically is found in warm coastal waters.1,5,10 The ideal conditions for growth and survival of V vulnificus are water temperatures at 18 °C (64.4 °F) and water salinities between 15 to 25 parts per thousand.2,8,9 These conditions are found in tropical and subtropical regions.2Vibrio vulnificus is found all over the world, including Denmark, Italy, Japan, Australia, Brazil, and the United States,2 where most infections come from oyster exposure and consumption in the Gulf of Mexico.2,8,11 The incidence of infection in the United States is highest between April and October.8,11
Some populations are at a higher risk of infection. Risk factors include male sex, liver cirrhosis, hemochromatosis, end-stage renal disease, immunosuppression, and diabetes mellitus.1,8,11 Healthy patients with no risk factors account for less than 5% of US V vulnificus infections.8
Male Predilection
Men are 6 times more likely to be affected by V vulnificus than women.Some hypotheses for this discrepancy are that estrogen is protective againstV vulnificus and that women may be less likely to engage in risky water activities and seafood handling.5 Additionally, older males (aged >60 years) are most often affected,1,8 likely due to the association between increasing age with number of comorbidities, such as diabetes mellitus, heart disease, and chronic disease.8
Iron Levels
Iron appears to play an important role in V vulnificus infection. Iron is essential for bacterial growth, and the ability to obtain iron from a host increases the organism’s pathogenicity.3Vibrio vulnificus rapidly grows when transferrin saturation exceeds 70%.8 Additionally, iron overload decreases the inoculum needed to cause sepsis in animal studies, which could play a role in human pathogenesis.4 Iron levels are elevated in patients with hemochromatosis due to increased iron absorption, cirrhosis and chronic liver disease due to impaired iron metabolism, and end-stage renal disease, especially in patients receiving parenteral iron.8
Immunosuppression
Patients who are immunocompromised and those with chronic liver disease are at an increased risk of infection because of neutrophils having decreased phagocytic activity.4
Diabetes Mellitus
Patients with diabetes mellitus may have peripheral neuropathy and may be unaware of pre-existing wounds that serve as entry points for V vulnificus.12
Etiology
Vibrio vulnificus infects humans via seafood consumption and handling as well as exposure to contaminated water.2,5 With respect to seafood consumption, raw shellfish are the primary type of seafood that harbor high levels of V vulnificus.5 Oysters are the most common etiology, but consumption of crabs, clams, and shrimp also can lead to infection.5,7Vibrio vulnificus contamination does not change the appearance, taste, or odor of shellfish, making it hard to detect.8 An inoculate of 1 million bacteria typically is necessary for infection after consumption.5 Contaminated seawater is another primary cause of V vulnificus infection. When open wounds are exposed to seawater harboring the bacteria, wound infections can arise.7 Infections can be acquired when swimming, fishing, or participating in water sports. Wound infections also occur while handling contaminated seafood, such as oyster shucking.5 There is a short incubation period for V vulnificus infections; the onset of symptoms and clinical outcome typically occur within 24 hours.5
Clinical Presentation
Vibrio vulnificus infections can have numerous clinical presentations, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis.1,8 There also is a spectrum of clinical outcomes; for instance, gastroenteritis typically is self-limited, whereas necrotizing fasciitis or sepsis can be fatal.2
Gastroenteritis
Vibrio vulnificus gastroenteritis is due to ingestion of contaminated shellfish.2,9 Symptoms typically are mild to moderate and include nausea, vomiting, diarrhea, fever, chills, abdominal pain, and cramping.2,4,8 Cases likely are underreported in the United States because gastroenteritis is self-limited, and many patients do not seek treatment.2,11
Wound Infections
Wound infections with V vulnificus have a cutaneous port of entry. Exposure to contaminated seawater or seafood can inoculate an open wound, leading to infection.7,8 Wound infections usually stem from 1 of 2 routes: (1) a pre-existing open wound gets infected while the patient is swimming in contaminated water, or (2) a traumatic injury occurs while the patient is handling contaminated shellfish, knives, or fishhooks. Many shellfish, such as oysters, have sharp points on their shells that can lacerate the skin.8 A wound on the hand can be contaminated by V vulnificus while handling contaminated seafood (eg, oyster shucking).13 Minor abrasions should not be dismissed; in fact, a small puncture or skin break often acts as the port of entry.9,11 Wound infections tend to arise within 7 days of exposure, though they can manifest up to 12 days after exposure.8 Wound infections can present as cellulitis, bullae, or ecchymoses.7 Lesions are exquisitely tender, and the skin is erythematous with marked surrounding soft tissue edema.3,4,8 Cellulitis typically arises first, with hemorrhagic bullae rapidly following.14 Lesions are limited to the affected extremity or area of inoculation.8 Systemic symptoms are rare, but fever and chills may accompany the infection.8,14 Unfortunately, lesions can become necrotic and progress rapidly to necrotizing fasciitis if left untreated.4,7,11 In these cases, secondary sepsis can occur.8
Necrotizing Fasciitis
Wound infections caused by V vulnificus can progress to necrotizing skin and soft tissue infections, such as necrotizing fasciitis and gangrene.5 Necrotizing fasciitis accounts for approximately one-third of V vulnificus infections.9 It usually stems from an open wound that is inoculated by contact with contaminated seafood or seawater.2,9 The wound infection begins as cellulitis with extreme tenderness, erythematous skin, and marked soft tissue edema, then rapidly progresses, becoming necrotic. These necrotic lesions present as black and purple eschars as the skin, blood supply, and subcutaneous tissues are infiltrated by the bacteria and destroyed. Lesions may have blistering or exudation. Many patients have accompanying systemic symptoms, including fever, chills, abdominal pain, diarrhea, hypotension, and sepsis.11,14 However, some patients may not present with systemic symptoms, so it is important to maintain a high index of suspicion even in the absence of these symptoms. The infection typically is limited to the affected extremity; necrotizing infections can lead to amputation and even death, depending on the extent of destruction and spread of the bacteria.11,13 The infection may spread beyond the inoculated extremity if the bacteria gains access to the bloodstream.8,9 In these cases, fulminant purpura or secondary septicemia can occur.8,15 Fatalityrates in the United States for necrotizing V vulnificus infections approach 30%.2 Necrotizing fasciitis accounts for approximately 8% of deaths associated with the pathogen in the United States.9
Interestingly, one reported case of necrotizing fasciitis associated with V vulnificus infection was triggered by acupuncture.16 The patient worked in a fish hatchery, where he was exposed to V vulnificus, and subsequent acupuncture led to the inoculation of bacteria into his bloodstream. This case raises the important point that we typically sequence the pathogenesis of V vulnificus infection as a patient having an open wound that is subsequently exposed to contaminated water; however, it also can follow the reverse sequence. Thus, proper cleansing of the skin after swimming in brackish water or handling shellfish is important to prevent V vulnificus infection.16 Additionally, dermatologists should be sure to cleanse patients’ skin thoroughly before performing procedures that could cause breaks in the skin.
Septicemia
Primary septicemia is the most common presentation of V vulnificus infection.2,8 Septicemia accounts for approximately 58% of V vulnificus infections in the United States.9 Infection typically occurs after ingestion of contaminated oysters, with subsequent absorption into the bloodstream through the ileum or cecum.2,8,9 Patients with chronic liver disease are 80 times more likely to develop primary sepsis than healthy individuals.8 Patients typically present with sudden-onset fever and chills, vomiting, diarrhea, and pain in the abdomen and/or extremities within hours to days of ingestion.4,8,9 The median time from ingestion to symptom onset is 18 hours.4,16 However, symptoms can be delayed up to 14 days.2 Progression is rapid; secondary lesions such as bullae, ecchymoses, cellulitis, purpura, macular or maculopapular eruptions, pustules, vasculitis, urticaria, and erythema multiforme–like lesions appear on the extremities within 24 hours of symptom onset. 2,3,4,8,17 Hemorrhagic bullae are the most common cutaneous manifestation of sepsis.4 Lesions are extremely tender to palpation.3 Cutaneous lesions can progress to necrotic ulcers, necrotizing fasciitis, gangrene, necrotizing vasculitis, or myonecrosis.4,8 Evidence of petechiae may indicate progression to disseminated intravascular coagulation (DIC). Elevated D-dimer and fibrin split products also may indicate DIC, and elevated creatine kinase may signify rhabdomyolysis.3 Unfortunately, septicemia has the worst outcomes of all V vulnificus presentations, with morality rates greater than 50% in the United States.1,2,4Vibrio vulnificus septicemia has a similar case-fatality rate to pathogens such as anthrax, Ebola virus disease, and the bubonic plague.5 Septicemia accounts for approximately 80% of the deaths associated with V vulnificus in the United States.8,9
Septicemia due to V vulnificus progresses to septic shock in two-thirds of cases.8 Septic shock presents with hypotension, mental status changes, and thrombocytopenia.2,8,17 Patients can become tachycardic, tachypneic, and hypoxic. Intubation may be required for resuscitation. In cases of septic shock secondary to V vulnificus infection, mortality rates reach 92%.3 Hypotension with a systolic blood pressure less than 90 mm Hg is a poor prognostic factor; patients presenting with hypotension secondary to V vulnificus infection have a fatality rate approaching 75% within 12 hours.2
Atypical Presentations
Rare atypical presentations of V vulnificus infection that have been reported in the literature include meningitis, corneal ulcers, epiglottitis, tonsillitis, spontaneous bacterial peritonitis, pneumonia, endometritis, septic arthritis, osteomyelitis, rhabdomyolysis endophthalmitis, and keratitis.2,4,6,13,18,19
Diagnosis
When diagnosing V vulnificus, providers need to obtain a thorough patient history, including any history of consumption or handling of raw seafood and recent water activities. Providers practicing in tropical climates or in warm summer months should keep V vulnificus in mind, as it is the ideal climate for the pathogen.9 Vital signs can range from unremarkable to fever, hypotension, tachycardia, and/or hypoxia. Skin examination may show exquisitely tender, erythematous skin with marked soft tissue edema, hemorrhagic bullae, ecchymoses, and/or necrosis. As physical examination findings can be nonspecific, wound cultures, blood cultures, and skin biopsies should be taken.
A wound culture and blood culture should be taken immediately if V vulnificus is suspected.8,11 A wound culture using discharge or fluid from necrotic or bullous lesions should be analyzed via gram stain.8,9 Gram stains of V vulnificus show short, slim, curved gram-negative rods under light microscopy.9,20 Special stains also can be done on cultures; V vulnificus is an oxidase-positive, lactose-positive, lysine-positive, salicin-positive, and arginine-negative organism. This knowledge can help differentiate V vulnificus from other gram-negative rods.13 Blood cultures will be positive in approximately 97% of patients with primary septicemia and 30% of patients with septicemia secondary to V vulnificus wound infections.3,9
Histologically, perilesional skin biopsies show epidermal necrosis with dermal and subcutaneous inflammation.12,17 There typically is an inflammatory infiltrate with neutrophilic abscesses and extensive tissue destruction in the subcutaneous tissue extending into the deep dermis.12,17 The superficial dermis is edematous but can lack the inflammatory infiltrate found in the subcutaneous tissue.17 Subepidermal bullae can form with numerous organisms within the fluid of the bullae. There also may be evidence of leukocytoclastic vasculitis with accompanying vessel wall necrosis. Fibrin clot formation and extravasated red blood cells may be visualized with few inflammatory cells but numerous organisms around the involved vessels.17
Management
Early diagnosis and treatment are vital.5,17 Cultures should be taken before aggressive treatment is started.3 Treatment is multifaceted; it requires antibiotics and wound care, except in cases of self-limited gastroenteritis.2,11 Aggressive debridement, fasciotomy, amputation, and supportive measures also may be necessary depending on the patient’s presentation.2,3,8,9 Establishing 2 peripheral intravenous lines is important in case rapid resuscitation becomes necessary.
Antibiotics
Primary cellulitis wound infections should be treated with doxycycline or a quinolone. If untreated, the wound can rapidly progress to necrotizing fasciitis.11 For necrotizing fasciitis and septicemia, broader-spectrum antibiotics are needed. For adults, ceftazidime plus doxycycline is the mainstay of antibiotic treatment for V vulnificus.2,9,11 For children, trimethoprim-sulfamethoxazole plus an aminoglycoside is preferred (Table).2,11
Antibiotic treatment has become more difficult as resistance arises. Antibiotic resistance likely is due to extensive antibiotic use in health care along with the agriculture and aquaculture industries using prophylactic and therapeutic antibiotics that wash into or are directly added to marine waters, where V vulnificus resides. Thus, antibiotic treatment should be tailored to the resistance profile of V vulnificus in various regions; for example, ceftazidime has an intermediate resistance profile in the United States, so cefotaxime and ceftriaxone may be better options.2
Wound Care
Wound infections must be extensively irrigated.9,21 For mild wound infections, proper wound care and oral antibiotics are appropriate (Table).21 Mild wounds should be irrigated thoroughly and followed by wound coverage to prevent progression, secondary infection, and necrosis. The dressing of choice will depend on the presenting lesion and provider preference; nonadherent, occlusive, or wet-to-dry dressings often are the best choices.22 Nonadherent dressings, such as petrolatum-covered gauze, do not pull off the newly formed epithelium when removed, making them beneficial to the skin’s healing process. Another option is occlusive dressings, which maintain a moist environment to hasten healing. They also enhance the autodigestion of necrotic tissue, which can be beneficial for necrotizing V vulnificus infections. Wet-to-dry dressings also may be used; these typically are comprised of gauze soaked with water, an astringent, and an antimicrobial or antiseptic solution. These dressings help to treat acute inflammation and also remove any exudate from the wound.22
Soft tissue and necrotizing infections require debridement.2,8 Early debridement decreases mortality rates.2,8,9 Necrotizing fasciitis often requires serial debridement to clear all the dead tissue and reduce the bacterial burden.8,9 Debridement prevents contiguous spread and metastatic seeding of the bacteria; it is important to prevent spread to the blood vessels, as vasculitis can necrose vessels, preventing antibiotics from reaching the dead tissue.17 Providers also should monitor for compartment syndrome, which should be treated with fasciotomy to decrease mortality.9,23 Many physicians leave V vulnificus–infected wounds open in order to heal by secondary intention.9 Hyperbaric oxygen therapy may be helpful as an adjunct to aggressive antimicrobial treatment for wound healing.8
Supportive Measures
Supportive care for dehydration, sepsis, DIC, and septic shock may be necessary, depending on the patient’s course. Treatment for severe V vulnificus infection includes intravenous fluids, crystalloids, oxygen, and/or intubation. Furthermore, if DIC develops, fresh frozen plasma, cryoprecipitate, a packed red blood cell transfusion, and/or anticoagulation may be required for resuscitation.3
Timing
Time to treatment and fatality rate are directly proportional in V vulnificus infection; the greater the delay in treatment, the higher the fatality rate.2 A 24-hour delay in antibiotic treatment is associated with a 33% case-fatality rate, and a 72-hour delay is associated with a 100% case-fatality rate.9 Even with early, appropriate treatment, mortality rates remain high.4
Prevention
Prevention of V vulnificus infections is an important consideration, especially for patients with chronic liver disease, immunosuppression, and hemochromatosis. Public education about the risks of eating raw shellfish is important.4 Oysters need to be treated properly to prevent growth and survival of V vulnificus.2 The most reliable method for destroying the bacteria is cooking shellfish.8,13 Only 15% of high-risk patients in the United States are aware of the risks associated with raw oyster consumption.3 High-risk patients should avoid eating raw oysters and shellfish and should cook seafood thoroughly before consumption.2,8 They also should wear protective clothing (ie, gloves) and eye protection when handling seafood and protective footwear (ie, wading shoes) while in seawater.2,8,13 It also is important to avoid contact with brackish water if one has any open wounds and to cleanse properly after exposure to brackish water or shellfish.2,8,16 Because severe V vulnificus infections can lead to death, prevention should be strongly encouraged by providers.2
Conclusion
Vibrio vulnificus infection typically occurs due to consumption of contaminated seafood or exposure to contaminated seawater. It most frequently affects older male patients with chronic liver disease, immunosuppression, hemochromatosis, or diabetes mellitus. Vibrio vulnificus can cause a vast spectrum of diseases, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis. Septicemia is the most common presentation of V vulnificus infection and accounts for the most fatalities from the bacteria. Septicemia often presents with fever, chills, vomiting, diarrhea, and hemorrhagic bullae. Vibrio vulnificus also commonly causes necrotizing fasciitis, which initially presents as cellulitis and rapidly progresses to hemorrhagic bullae or necrosis with accompanying systemic symptoms. Prompt diagnosis and treatment are vital to prevent mortality.
Interestingly, regions impacted by V vulnificus are expanding because of global warming.5,7Vibrio vulnificus thrives in warm waters, and increasing water temperatures are enhancing V vulnificus growth and survival.1,9 As global warming continues, the incidence of V vulnificus infections may rise. In fact, the number of infections increased by 78% between 1996 and 2006 in the United States.5 This rise likely was due to a combination of factors, including an aging population with more comorbidities, improvements in diagnosis, and climate change. Thus, as the number of V vulnificus infections rises, so too must providers’ suspicion for the pathogen.
- Phillips KE, Satchell KJF. Vibrio vulnificus: from oyster colonist to human pathogen [published online January 5, 2017]. PLOS Pathog. doi:10.1371/journal.ppat.1006053
- Heng SP, Letchumanan V, Deng CY, et al. Vibrio vulnificus: an environmental and clinical burden. Front Microbiol. 2017;8:997.
- Kumamoto KS, Vukich DJ. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1998;16:61-66.
- Borenstein M, Kerdel F. Infections with Vibrio vulnificus. Dermatol Clin. 2003;21:245-248.
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430.
- Kim SJ, Kim BC, Kim DC, et al. A fatal case of Vibrio vulnificus meningoencephalitis. Clin Microbiol Infect. 2003;9:568-571.
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733.
- Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus infection: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:E157-E166.
- Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014;21:207-213.
- Kikawa K, Yamasaki K, Sukiura T, et al. A successfully treated case of Vibrio vulnificus septicemia with shock. Jpn J Med. 1990;29:313-319.
- Perkins AP, Trimmier M. Recreational waterborne illnesses: recognition, treatment, and prevention. Am Fam Physician. 2017;95:554-560.
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145.
- Ulusarac O, Carter E. Varied clinical presentations of Vibrio vulnificus infections: a report of four unusual cases and review of the literature. South Med J. 2004;97:613-618.
- Bross MH, Soch K, Morales R, et al. Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician. 2007;76:539-544.
- Hori M, Nakayama A, Kitagawa D, et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen [published online May 19, 2017]. JMM Case Rep. doi:10.1099/jmmcr.0.005096
- Kotton Y, Soboh S, Bisharat N. Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infect Dis Rep. 2015;7:5901.
- Hoffman TJ, Nelson B, Darouiche R, et al. Vibrio vulnificus septicemia. Arch Intern Med. 1988;148:1825-1827.
- Alsaad AA, Sotello D, Kruse BT, et al. Vibrio vulnificus tonsillitis after swimming in the Gulf of Mexico [published online June 28, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-221161
- Tison DL, Kelly MT. Vibrio vulnificus endometritis. J Clin Microbiol. 1984;20:185-186.
- Beatty NL, Marquez J, Mohajer MA. Skin manifestations of primary Vibrio vulnificus septicemia. Am J Trop Med Hyg. 2017;97:1-2.
- Foote A, Henderson R, Lindberg A, et al. The Australian mid-west coastal marine wound infections study. Aust Fam Physician. 2017;46:923-927.
- Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Elsevier; 2019.
- Kim CS, Bae EH, Ma SK, et al. Severe septicemia, necrotizing fasciitis, and peritonitis due to Vibrio vulnificus in a patient undergoing continuous ambulatory peritoneal dialysis: a case report. BMC Infect Dis. 2015;15:422.
Vibrio vulnificus is a member of the Vibrio genus. Most Vibrio species are nonpathogenic in humans; however, V vulnificus is one of the pathogenic strains.1 In Latin, the term vulnificus means “wounding,” and V vulnificus can cause life-threatening infections in patients. The mortality rate of V vulnificus infections is approximately 33% in the United States.2Vibrio vulnificus is a gram-negative bacterium that was first isolated by the Centers for Disease Control and Prevention in 1964 and was given its current name in 1979.3-6 It has been found in numerous organisms, including oysters, crabs, clams, shrimp, mussels, mullets, and sea bass.4 The vast majority of infections in the United States are due to oyster exposure and consumption.2,7Vibrio vulnificus is responsible for more than 95% of seafood-related deaths in the United States and has the highest mortality rate of all food-borne illness in the United States.2,5 It also has the highest per-case economic impact of all food-related diseases in the United States.1
What distinguishes a pathogenic vs nonpathogenic Vibrio isolate remains unknown; Vibrio species rapidly undergo horizontal gene transfer, making DNA isolation difficult.1 Some characteristics of V vulnificus that may confer virulence are the capsular polysaccharide, lipopolysaccharide, binding proteins, and tissue-degrading enzymes.1,5 First, encapsulated strains are more virulent and invasive than unencapsulated strains.1 The mucopolysaccharide capsule protects the bacterium from the immune system, allowing it to evade immune surveillance, cause more severe infection, and invade into the subcutaneous tissue.3,5 Second, production of sialic acid–like molecules alter the lipopolysaccharide, allowing for motility and biofilm formation.1 This allows the bacterium to survive in marine waters and within the bloodstream, the latter leading to sepsis in humans. Third, production of N-acetylglucosamine–binding protein A allows for adhesion to chitin. Shellfish consume chitin, and chitin accumulates in shellfish. N-acetylglucosamine–binding protein A also binds mucin; this may be how V vulnificus binds to mucin in the gastrointestinal tract in humans, causing gastroenteritis.1 Binding to the human mucosae also may allow the bacteria to gain access to the blood supply, leading to septicemia.4 Finally, tissue-degrading enzymes such as proteases are responsible for necrotizing wound infections associated with V vulnificus, as the enzymes allow for invasion into the skin and subcutaneous tissues. Proteases also increase vascular permeability and lead to edema.3 Hence, these virulence factors may provide V vulnificus the pathogenicity to cause infection in humans.
Three biotypes of V vulnificus have been discovered. Biotype 1 is the most common and is found worldwide in brackish water.8 It can cause the entire spectrum of illnesses, and it has a case fatality rate of 50% in humans. Biotype 1 is presumably responsible for all infections in the United States. Biotype 2 is found in the Far East and Western Europe; it inhabits a unique niche—saltwater used for eel farming. It typically causes infection in eels, but rarely it can cause wound infections in humans. Biotype 3 is found in freshwater fish farming in Israel, and it is a hybrid of biotypes 1 and 2.It can cause severe soft tissue infections in humans, sometimes requiring amputation.8
Epidemiology
Vibrio vulnificus is a motile, gram-negative, halophilic, aquatic bacterium.1,4,5,8,9 It is part of the normal estuarine microbiome and typically is found in warm coastal waters.1,5,10 The ideal conditions for growth and survival of V vulnificus are water temperatures at 18 °C (64.4 °F) and water salinities between 15 to 25 parts per thousand.2,8,9 These conditions are found in tropical and subtropical regions.2Vibrio vulnificus is found all over the world, including Denmark, Italy, Japan, Australia, Brazil, and the United States,2 where most infections come from oyster exposure and consumption in the Gulf of Mexico.2,8,11 The incidence of infection in the United States is highest between April and October.8,11
Some populations are at a higher risk of infection. Risk factors include male sex, liver cirrhosis, hemochromatosis, end-stage renal disease, immunosuppression, and diabetes mellitus.1,8,11 Healthy patients with no risk factors account for less than 5% of US V vulnificus infections.8
Male Predilection
Men are 6 times more likely to be affected by V vulnificus than women.Some hypotheses for this discrepancy are that estrogen is protective againstV vulnificus and that women may be less likely to engage in risky water activities and seafood handling.5 Additionally, older males (aged >60 years) are most often affected,1,8 likely due to the association between increasing age with number of comorbidities, such as diabetes mellitus, heart disease, and chronic disease.8
Iron Levels
Iron appears to play an important role in V vulnificus infection. Iron is essential for bacterial growth, and the ability to obtain iron from a host increases the organism’s pathogenicity.3Vibrio vulnificus rapidly grows when transferrin saturation exceeds 70%.8 Additionally, iron overload decreases the inoculum needed to cause sepsis in animal studies, which could play a role in human pathogenesis.4 Iron levels are elevated in patients with hemochromatosis due to increased iron absorption, cirrhosis and chronic liver disease due to impaired iron metabolism, and end-stage renal disease, especially in patients receiving parenteral iron.8
Immunosuppression
Patients who are immunocompromised and those with chronic liver disease are at an increased risk of infection because of neutrophils having decreased phagocytic activity.4
Diabetes Mellitus
Patients with diabetes mellitus may have peripheral neuropathy and may be unaware of pre-existing wounds that serve as entry points for V vulnificus.12
Etiology
Vibrio vulnificus infects humans via seafood consumption and handling as well as exposure to contaminated water.2,5 With respect to seafood consumption, raw shellfish are the primary type of seafood that harbor high levels of V vulnificus.5 Oysters are the most common etiology, but consumption of crabs, clams, and shrimp also can lead to infection.5,7Vibrio vulnificus contamination does not change the appearance, taste, or odor of shellfish, making it hard to detect.8 An inoculate of 1 million bacteria typically is necessary for infection after consumption.5 Contaminated seawater is another primary cause of V vulnificus infection. When open wounds are exposed to seawater harboring the bacteria, wound infections can arise.7 Infections can be acquired when swimming, fishing, or participating in water sports. Wound infections also occur while handling contaminated seafood, such as oyster shucking.5 There is a short incubation period for V vulnificus infections; the onset of symptoms and clinical outcome typically occur within 24 hours.5
Clinical Presentation
Vibrio vulnificus infections can have numerous clinical presentations, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis.1,8 There also is a spectrum of clinical outcomes; for instance, gastroenteritis typically is self-limited, whereas necrotizing fasciitis or sepsis can be fatal.2
Gastroenteritis
Vibrio vulnificus gastroenteritis is due to ingestion of contaminated shellfish.2,9 Symptoms typically are mild to moderate and include nausea, vomiting, diarrhea, fever, chills, abdominal pain, and cramping.2,4,8 Cases likely are underreported in the United States because gastroenteritis is self-limited, and many patients do not seek treatment.2,11
Wound Infections
Wound infections with V vulnificus have a cutaneous port of entry. Exposure to contaminated seawater or seafood can inoculate an open wound, leading to infection.7,8 Wound infections usually stem from 1 of 2 routes: (1) a pre-existing open wound gets infected while the patient is swimming in contaminated water, or (2) a traumatic injury occurs while the patient is handling contaminated shellfish, knives, or fishhooks. Many shellfish, such as oysters, have sharp points on their shells that can lacerate the skin.8 A wound on the hand can be contaminated by V vulnificus while handling contaminated seafood (eg, oyster shucking).13 Minor abrasions should not be dismissed; in fact, a small puncture or skin break often acts as the port of entry.9,11 Wound infections tend to arise within 7 days of exposure, though they can manifest up to 12 days after exposure.8 Wound infections can present as cellulitis, bullae, or ecchymoses.7 Lesions are exquisitely tender, and the skin is erythematous with marked surrounding soft tissue edema.3,4,8 Cellulitis typically arises first, with hemorrhagic bullae rapidly following.14 Lesions are limited to the affected extremity or area of inoculation.8 Systemic symptoms are rare, but fever and chills may accompany the infection.8,14 Unfortunately, lesions can become necrotic and progress rapidly to necrotizing fasciitis if left untreated.4,7,11 In these cases, secondary sepsis can occur.8
Necrotizing Fasciitis
Wound infections caused by V vulnificus can progress to necrotizing skin and soft tissue infections, such as necrotizing fasciitis and gangrene.5 Necrotizing fasciitis accounts for approximately one-third of V vulnificus infections.9 It usually stems from an open wound that is inoculated by contact with contaminated seafood or seawater.2,9 The wound infection begins as cellulitis with extreme tenderness, erythematous skin, and marked soft tissue edema, then rapidly progresses, becoming necrotic. These necrotic lesions present as black and purple eschars as the skin, blood supply, and subcutaneous tissues are infiltrated by the bacteria and destroyed. Lesions may have blistering or exudation. Many patients have accompanying systemic symptoms, including fever, chills, abdominal pain, diarrhea, hypotension, and sepsis.11,14 However, some patients may not present with systemic symptoms, so it is important to maintain a high index of suspicion even in the absence of these symptoms. The infection typically is limited to the affected extremity; necrotizing infections can lead to amputation and even death, depending on the extent of destruction and spread of the bacteria.11,13 The infection may spread beyond the inoculated extremity if the bacteria gains access to the bloodstream.8,9 In these cases, fulminant purpura or secondary septicemia can occur.8,15 Fatalityrates in the United States for necrotizing V vulnificus infections approach 30%.2 Necrotizing fasciitis accounts for approximately 8% of deaths associated with the pathogen in the United States.9
Interestingly, one reported case of necrotizing fasciitis associated with V vulnificus infection was triggered by acupuncture.16 The patient worked in a fish hatchery, where he was exposed to V vulnificus, and subsequent acupuncture led to the inoculation of bacteria into his bloodstream. This case raises the important point that we typically sequence the pathogenesis of V vulnificus infection as a patient having an open wound that is subsequently exposed to contaminated water; however, it also can follow the reverse sequence. Thus, proper cleansing of the skin after swimming in brackish water or handling shellfish is important to prevent V vulnificus infection.16 Additionally, dermatologists should be sure to cleanse patients’ skin thoroughly before performing procedures that could cause breaks in the skin.
Septicemia
Primary septicemia is the most common presentation of V vulnificus infection.2,8 Septicemia accounts for approximately 58% of V vulnificus infections in the United States.9 Infection typically occurs after ingestion of contaminated oysters, with subsequent absorption into the bloodstream through the ileum or cecum.2,8,9 Patients with chronic liver disease are 80 times more likely to develop primary sepsis than healthy individuals.8 Patients typically present with sudden-onset fever and chills, vomiting, diarrhea, and pain in the abdomen and/or extremities within hours to days of ingestion.4,8,9 The median time from ingestion to symptom onset is 18 hours.4,16 However, symptoms can be delayed up to 14 days.2 Progression is rapid; secondary lesions such as bullae, ecchymoses, cellulitis, purpura, macular or maculopapular eruptions, pustules, vasculitis, urticaria, and erythema multiforme–like lesions appear on the extremities within 24 hours of symptom onset. 2,3,4,8,17 Hemorrhagic bullae are the most common cutaneous manifestation of sepsis.4 Lesions are extremely tender to palpation.3 Cutaneous lesions can progress to necrotic ulcers, necrotizing fasciitis, gangrene, necrotizing vasculitis, or myonecrosis.4,8 Evidence of petechiae may indicate progression to disseminated intravascular coagulation (DIC). Elevated D-dimer and fibrin split products also may indicate DIC, and elevated creatine kinase may signify rhabdomyolysis.3 Unfortunately, septicemia has the worst outcomes of all V vulnificus presentations, with morality rates greater than 50% in the United States.1,2,4Vibrio vulnificus septicemia has a similar case-fatality rate to pathogens such as anthrax, Ebola virus disease, and the bubonic plague.5 Septicemia accounts for approximately 80% of the deaths associated with V vulnificus in the United States.8,9
Septicemia due to V vulnificus progresses to septic shock in two-thirds of cases.8 Septic shock presents with hypotension, mental status changes, and thrombocytopenia.2,8,17 Patients can become tachycardic, tachypneic, and hypoxic. Intubation may be required for resuscitation. In cases of septic shock secondary to V vulnificus infection, mortality rates reach 92%.3 Hypotension with a systolic blood pressure less than 90 mm Hg is a poor prognostic factor; patients presenting with hypotension secondary to V vulnificus infection have a fatality rate approaching 75% within 12 hours.2
Atypical Presentations
Rare atypical presentations of V vulnificus infection that have been reported in the literature include meningitis, corneal ulcers, epiglottitis, tonsillitis, spontaneous bacterial peritonitis, pneumonia, endometritis, septic arthritis, osteomyelitis, rhabdomyolysis endophthalmitis, and keratitis.2,4,6,13,18,19
Diagnosis
When diagnosing V vulnificus, providers need to obtain a thorough patient history, including any history of consumption or handling of raw seafood and recent water activities. Providers practicing in tropical climates or in warm summer months should keep V vulnificus in mind, as it is the ideal climate for the pathogen.9 Vital signs can range from unremarkable to fever, hypotension, tachycardia, and/or hypoxia. Skin examination may show exquisitely tender, erythematous skin with marked soft tissue edema, hemorrhagic bullae, ecchymoses, and/or necrosis. As physical examination findings can be nonspecific, wound cultures, blood cultures, and skin biopsies should be taken.
A wound culture and blood culture should be taken immediately if V vulnificus is suspected.8,11 A wound culture using discharge or fluid from necrotic or bullous lesions should be analyzed via gram stain.8,9 Gram stains of V vulnificus show short, slim, curved gram-negative rods under light microscopy.9,20 Special stains also can be done on cultures; V vulnificus is an oxidase-positive, lactose-positive, lysine-positive, salicin-positive, and arginine-negative organism. This knowledge can help differentiate V vulnificus from other gram-negative rods.13 Blood cultures will be positive in approximately 97% of patients with primary septicemia and 30% of patients with septicemia secondary to V vulnificus wound infections.3,9
Histologically, perilesional skin biopsies show epidermal necrosis with dermal and subcutaneous inflammation.12,17 There typically is an inflammatory infiltrate with neutrophilic abscesses and extensive tissue destruction in the subcutaneous tissue extending into the deep dermis.12,17 The superficial dermis is edematous but can lack the inflammatory infiltrate found in the subcutaneous tissue.17 Subepidermal bullae can form with numerous organisms within the fluid of the bullae. There also may be evidence of leukocytoclastic vasculitis with accompanying vessel wall necrosis. Fibrin clot formation and extravasated red blood cells may be visualized with few inflammatory cells but numerous organisms around the involved vessels.17
Management
Early diagnosis and treatment are vital.5,17 Cultures should be taken before aggressive treatment is started.3 Treatment is multifaceted; it requires antibiotics and wound care, except in cases of self-limited gastroenteritis.2,11 Aggressive debridement, fasciotomy, amputation, and supportive measures also may be necessary depending on the patient’s presentation.2,3,8,9 Establishing 2 peripheral intravenous lines is important in case rapid resuscitation becomes necessary.
Antibiotics
Primary cellulitis wound infections should be treated with doxycycline or a quinolone. If untreated, the wound can rapidly progress to necrotizing fasciitis.11 For necrotizing fasciitis and septicemia, broader-spectrum antibiotics are needed. For adults, ceftazidime plus doxycycline is the mainstay of antibiotic treatment for V vulnificus.2,9,11 For children, trimethoprim-sulfamethoxazole plus an aminoglycoside is preferred (Table).2,11
Antibiotic treatment has become more difficult as resistance arises. Antibiotic resistance likely is due to extensive antibiotic use in health care along with the agriculture and aquaculture industries using prophylactic and therapeutic antibiotics that wash into or are directly added to marine waters, where V vulnificus resides. Thus, antibiotic treatment should be tailored to the resistance profile of V vulnificus in various regions; for example, ceftazidime has an intermediate resistance profile in the United States, so cefotaxime and ceftriaxone may be better options.2
Wound Care
Wound infections must be extensively irrigated.9,21 For mild wound infections, proper wound care and oral antibiotics are appropriate (Table).21 Mild wounds should be irrigated thoroughly and followed by wound coverage to prevent progression, secondary infection, and necrosis. The dressing of choice will depend on the presenting lesion and provider preference; nonadherent, occlusive, or wet-to-dry dressings often are the best choices.22 Nonadherent dressings, such as petrolatum-covered gauze, do not pull off the newly formed epithelium when removed, making them beneficial to the skin’s healing process. Another option is occlusive dressings, which maintain a moist environment to hasten healing. They also enhance the autodigestion of necrotic tissue, which can be beneficial for necrotizing V vulnificus infections. Wet-to-dry dressings also may be used; these typically are comprised of gauze soaked with water, an astringent, and an antimicrobial or antiseptic solution. These dressings help to treat acute inflammation and also remove any exudate from the wound.22
Soft tissue and necrotizing infections require debridement.2,8 Early debridement decreases mortality rates.2,8,9 Necrotizing fasciitis often requires serial debridement to clear all the dead tissue and reduce the bacterial burden.8,9 Debridement prevents contiguous spread and metastatic seeding of the bacteria; it is important to prevent spread to the blood vessels, as vasculitis can necrose vessels, preventing antibiotics from reaching the dead tissue.17 Providers also should monitor for compartment syndrome, which should be treated with fasciotomy to decrease mortality.9,23 Many physicians leave V vulnificus–infected wounds open in order to heal by secondary intention.9 Hyperbaric oxygen therapy may be helpful as an adjunct to aggressive antimicrobial treatment for wound healing.8
Supportive Measures
Supportive care for dehydration, sepsis, DIC, and septic shock may be necessary, depending on the patient’s course. Treatment for severe V vulnificus infection includes intravenous fluids, crystalloids, oxygen, and/or intubation. Furthermore, if DIC develops, fresh frozen plasma, cryoprecipitate, a packed red blood cell transfusion, and/or anticoagulation may be required for resuscitation.3
Timing
Time to treatment and fatality rate are directly proportional in V vulnificus infection; the greater the delay in treatment, the higher the fatality rate.2 A 24-hour delay in antibiotic treatment is associated with a 33% case-fatality rate, and a 72-hour delay is associated with a 100% case-fatality rate.9 Even with early, appropriate treatment, mortality rates remain high.4
Prevention
Prevention of V vulnificus infections is an important consideration, especially for patients with chronic liver disease, immunosuppression, and hemochromatosis. Public education about the risks of eating raw shellfish is important.4 Oysters need to be treated properly to prevent growth and survival of V vulnificus.2 The most reliable method for destroying the bacteria is cooking shellfish.8,13 Only 15% of high-risk patients in the United States are aware of the risks associated with raw oyster consumption.3 High-risk patients should avoid eating raw oysters and shellfish and should cook seafood thoroughly before consumption.2,8 They also should wear protective clothing (ie, gloves) and eye protection when handling seafood and protective footwear (ie, wading shoes) while in seawater.2,8,13 It also is important to avoid contact with brackish water if one has any open wounds and to cleanse properly after exposure to brackish water or shellfish.2,8,16 Because severe V vulnificus infections can lead to death, prevention should be strongly encouraged by providers.2
Conclusion
Vibrio vulnificus infection typically occurs due to consumption of contaminated seafood or exposure to contaminated seawater. It most frequently affects older male patients with chronic liver disease, immunosuppression, hemochromatosis, or diabetes mellitus. Vibrio vulnificus can cause a vast spectrum of diseases, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis. Septicemia is the most common presentation of V vulnificus infection and accounts for the most fatalities from the bacteria. Septicemia often presents with fever, chills, vomiting, diarrhea, and hemorrhagic bullae. Vibrio vulnificus also commonly causes necrotizing fasciitis, which initially presents as cellulitis and rapidly progresses to hemorrhagic bullae or necrosis with accompanying systemic symptoms. Prompt diagnosis and treatment are vital to prevent mortality.
Interestingly, regions impacted by V vulnificus are expanding because of global warming.5,7Vibrio vulnificus thrives in warm waters, and increasing water temperatures are enhancing V vulnificus growth and survival.1,9 As global warming continues, the incidence of V vulnificus infections may rise. In fact, the number of infections increased by 78% between 1996 and 2006 in the United States.5 This rise likely was due to a combination of factors, including an aging population with more comorbidities, improvements in diagnosis, and climate change. Thus, as the number of V vulnificus infections rises, so too must providers’ suspicion for the pathogen.
Vibrio vulnificus is a member of the Vibrio genus. Most Vibrio species are nonpathogenic in humans; however, V vulnificus is one of the pathogenic strains.1 In Latin, the term vulnificus means “wounding,” and V vulnificus can cause life-threatening infections in patients. The mortality rate of V vulnificus infections is approximately 33% in the United States.2Vibrio vulnificus is a gram-negative bacterium that was first isolated by the Centers for Disease Control and Prevention in 1964 and was given its current name in 1979.3-6 It has been found in numerous organisms, including oysters, crabs, clams, shrimp, mussels, mullets, and sea bass.4 The vast majority of infections in the United States are due to oyster exposure and consumption.2,7Vibrio vulnificus is responsible for more than 95% of seafood-related deaths in the United States and has the highest mortality rate of all food-borne illness in the United States.2,5 It also has the highest per-case economic impact of all food-related diseases in the United States.1
What distinguishes a pathogenic vs nonpathogenic Vibrio isolate remains unknown; Vibrio species rapidly undergo horizontal gene transfer, making DNA isolation difficult.1 Some characteristics of V vulnificus that may confer virulence are the capsular polysaccharide, lipopolysaccharide, binding proteins, and tissue-degrading enzymes.1,5 First, encapsulated strains are more virulent and invasive than unencapsulated strains.1 The mucopolysaccharide capsule protects the bacterium from the immune system, allowing it to evade immune surveillance, cause more severe infection, and invade into the subcutaneous tissue.3,5 Second, production of sialic acid–like molecules alter the lipopolysaccharide, allowing for motility and biofilm formation.1 This allows the bacterium to survive in marine waters and within the bloodstream, the latter leading to sepsis in humans. Third, production of N-acetylglucosamine–binding protein A allows for adhesion to chitin. Shellfish consume chitin, and chitin accumulates in shellfish. N-acetylglucosamine–binding protein A also binds mucin; this may be how V vulnificus binds to mucin in the gastrointestinal tract in humans, causing gastroenteritis.1 Binding to the human mucosae also may allow the bacteria to gain access to the blood supply, leading to septicemia.4 Finally, tissue-degrading enzymes such as proteases are responsible for necrotizing wound infections associated with V vulnificus, as the enzymes allow for invasion into the skin and subcutaneous tissues. Proteases also increase vascular permeability and lead to edema.3 Hence, these virulence factors may provide V vulnificus the pathogenicity to cause infection in humans.
Three biotypes of V vulnificus have been discovered. Biotype 1 is the most common and is found worldwide in brackish water.8 It can cause the entire spectrum of illnesses, and it has a case fatality rate of 50% in humans. Biotype 1 is presumably responsible for all infections in the United States. Biotype 2 is found in the Far East and Western Europe; it inhabits a unique niche—saltwater used for eel farming. It typically causes infection in eels, but rarely it can cause wound infections in humans. Biotype 3 is found in freshwater fish farming in Israel, and it is a hybrid of biotypes 1 and 2.It can cause severe soft tissue infections in humans, sometimes requiring amputation.8
Epidemiology
Vibrio vulnificus is a motile, gram-negative, halophilic, aquatic bacterium.1,4,5,8,9 It is part of the normal estuarine microbiome and typically is found in warm coastal waters.1,5,10 The ideal conditions for growth and survival of V vulnificus are water temperatures at 18 °C (64.4 °F) and water salinities between 15 to 25 parts per thousand.2,8,9 These conditions are found in tropical and subtropical regions.2Vibrio vulnificus is found all over the world, including Denmark, Italy, Japan, Australia, Brazil, and the United States,2 where most infections come from oyster exposure and consumption in the Gulf of Mexico.2,8,11 The incidence of infection in the United States is highest between April and October.8,11
Some populations are at a higher risk of infection. Risk factors include male sex, liver cirrhosis, hemochromatosis, end-stage renal disease, immunosuppression, and diabetes mellitus.1,8,11 Healthy patients with no risk factors account for less than 5% of US V vulnificus infections.8
Male Predilection
Men are 6 times more likely to be affected by V vulnificus than women.Some hypotheses for this discrepancy are that estrogen is protective againstV vulnificus and that women may be less likely to engage in risky water activities and seafood handling.5 Additionally, older males (aged >60 years) are most often affected,1,8 likely due to the association between increasing age with number of comorbidities, such as diabetes mellitus, heart disease, and chronic disease.8
Iron Levels
Iron appears to play an important role in V vulnificus infection. Iron is essential for bacterial growth, and the ability to obtain iron from a host increases the organism’s pathogenicity.3Vibrio vulnificus rapidly grows when transferrin saturation exceeds 70%.8 Additionally, iron overload decreases the inoculum needed to cause sepsis in animal studies, which could play a role in human pathogenesis.4 Iron levels are elevated in patients with hemochromatosis due to increased iron absorption, cirrhosis and chronic liver disease due to impaired iron metabolism, and end-stage renal disease, especially in patients receiving parenteral iron.8
Immunosuppression
Patients who are immunocompromised and those with chronic liver disease are at an increased risk of infection because of neutrophils having decreased phagocytic activity.4
Diabetes Mellitus
Patients with diabetes mellitus may have peripheral neuropathy and may be unaware of pre-existing wounds that serve as entry points for V vulnificus.12
Etiology
Vibrio vulnificus infects humans via seafood consumption and handling as well as exposure to contaminated water.2,5 With respect to seafood consumption, raw shellfish are the primary type of seafood that harbor high levels of V vulnificus.5 Oysters are the most common etiology, but consumption of crabs, clams, and shrimp also can lead to infection.5,7Vibrio vulnificus contamination does not change the appearance, taste, or odor of shellfish, making it hard to detect.8 An inoculate of 1 million bacteria typically is necessary for infection after consumption.5 Contaminated seawater is another primary cause of V vulnificus infection. When open wounds are exposed to seawater harboring the bacteria, wound infections can arise.7 Infections can be acquired when swimming, fishing, or participating in water sports. Wound infections also occur while handling contaminated seafood, such as oyster shucking.5 There is a short incubation period for V vulnificus infections; the onset of symptoms and clinical outcome typically occur within 24 hours.5
Clinical Presentation
Vibrio vulnificus infections can have numerous clinical presentations, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis.1,8 There also is a spectrum of clinical outcomes; for instance, gastroenteritis typically is self-limited, whereas necrotizing fasciitis or sepsis can be fatal.2
Gastroenteritis
Vibrio vulnificus gastroenteritis is due to ingestion of contaminated shellfish.2,9 Symptoms typically are mild to moderate and include nausea, vomiting, diarrhea, fever, chills, abdominal pain, and cramping.2,4,8 Cases likely are underreported in the United States because gastroenteritis is self-limited, and many patients do not seek treatment.2,11
Wound Infections
Wound infections with V vulnificus have a cutaneous port of entry. Exposure to contaminated seawater or seafood can inoculate an open wound, leading to infection.7,8 Wound infections usually stem from 1 of 2 routes: (1) a pre-existing open wound gets infected while the patient is swimming in contaminated water, or (2) a traumatic injury occurs while the patient is handling contaminated shellfish, knives, or fishhooks. Many shellfish, such as oysters, have sharp points on their shells that can lacerate the skin.8 A wound on the hand can be contaminated by V vulnificus while handling contaminated seafood (eg, oyster shucking).13 Minor abrasions should not be dismissed; in fact, a small puncture or skin break often acts as the port of entry.9,11 Wound infections tend to arise within 7 days of exposure, though they can manifest up to 12 days after exposure.8 Wound infections can present as cellulitis, bullae, or ecchymoses.7 Lesions are exquisitely tender, and the skin is erythematous with marked surrounding soft tissue edema.3,4,8 Cellulitis typically arises first, with hemorrhagic bullae rapidly following.14 Lesions are limited to the affected extremity or area of inoculation.8 Systemic symptoms are rare, but fever and chills may accompany the infection.8,14 Unfortunately, lesions can become necrotic and progress rapidly to necrotizing fasciitis if left untreated.4,7,11 In these cases, secondary sepsis can occur.8
Necrotizing Fasciitis
Wound infections caused by V vulnificus can progress to necrotizing skin and soft tissue infections, such as necrotizing fasciitis and gangrene.5 Necrotizing fasciitis accounts for approximately one-third of V vulnificus infections.9 It usually stems from an open wound that is inoculated by contact with contaminated seafood or seawater.2,9 The wound infection begins as cellulitis with extreme tenderness, erythematous skin, and marked soft tissue edema, then rapidly progresses, becoming necrotic. These necrotic lesions present as black and purple eschars as the skin, blood supply, and subcutaneous tissues are infiltrated by the bacteria and destroyed. Lesions may have blistering or exudation. Many patients have accompanying systemic symptoms, including fever, chills, abdominal pain, diarrhea, hypotension, and sepsis.11,14 However, some patients may not present with systemic symptoms, so it is important to maintain a high index of suspicion even in the absence of these symptoms. The infection typically is limited to the affected extremity; necrotizing infections can lead to amputation and even death, depending on the extent of destruction and spread of the bacteria.11,13 The infection may spread beyond the inoculated extremity if the bacteria gains access to the bloodstream.8,9 In these cases, fulminant purpura or secondary septicemia can occur.8,15 Fatalityrates in the United States for necrotizing V vulnificus infections approach 30%.2 Necrotizing fasciitis accounts for approximately 8% of deaths associated with the pathogen in the United States.9
Interestingly, one reported case of necrotizing fasciitis associated with V vulnificus infection was triggered by acupuncture.16 The patient worked in a fish hatchery, where he was exposed to V vulnificus, and subsequent acupuncture led to the inoculation of bacteria into his bloodstream. This case raises the important point that we typically sequence the pathogenesis of V vulnificus infection as a patient having an open wound that is subsequently exposed to contaminated water; however, it also can follow the reverse sequence. Thus, proper cleansing of the skin after swimming in brackish water or handling shellfish is important to prevent V vulnificus infection.16 Additionally, dermatologists should be sure to cleanse patients’ skin thoroughly before performing procedures that could cause breaks in the skin.
Septicemia
Primary septicemia is the most common presentation of V vulnificus infection.2,8 Septicemia accounts for approximately 58% of V vulnificus infections in the United States.9 Infection typically occurs after ingestion of contaminated oysters, with subsequent absorption into the bloodstream through the ileum or cecum.2,8,9 Patients with chronic liver disease are 80 times more likely to develop primary sepsis than healthy individuals.8 Patients typically present with sudden-onset fever and chills, vomiting, diarrhea, and pain in the abdomen and/or extremities within hours to days of ingestion.4,8,9 The median time from ingestion to symptom onset is 18 hours.4,16 However, symptoms can be delayed up to 14 days.2 Progression is rapid; secondary lesions such as bullae, ecchymoses, cellulitis, purpura, macular or maculopapular eruptions, pustules, vasculitis, urticaria, and erythema multiforme–like lesions appear on the extremities within 24 hours of symptom onset. 2,3,4,8,17 Hemorrhagic bullae are the most common cutaneous manifestation of sepsis.4 Lesions are extremely tender to palpation.3 Cutaneous lesions can progress to necrotic ulcers, necrotizing fasciitis, gangrene, necrotizing vasculitis, or myonecrosis.4,8 Evidence of petechiae may indicate progression to disseminated intravascular coagulation (DIC). Elevated D-dimer and fibrin split products also may indicate DIC, and elevated creatine kinase may signify rhabdomyolysis.3 Unfortunately, septicemia has the worst outcomes of all V vulnificus presentations, with morality rates greater than 50% in the United States.1,2,4Vibrio vulnificus septicemia has a similar case-fatality rate to pathogens such as anthrax, Ebola virus disease, and the bubonic plague.5 Septicemia accounts for approximately 80% of the deaths associated with V vulnificus in the United States.8,9
Septicemia due to V vulnificus progresses to septic shock in two-thirds of cases.8 Septic shock presents with hypotension, mental status changes, and thrombocytopenia.2,8,17 Patients can become tachycardic, tachypneic, and hypoxic. Intubation may be required for resuscitation. In cases of septic shock secondary to V vulnificus infection, mortality rates reach 92%.3 Hypotension with a systolic blood pressure less than 90 mm Hg is a poor prognostic factor; patients presenting with hypotension secondary to V vulnificus infection have a fatality rate approaching 75% within 12 hours.2
Atypical Presentations
Rare atypical presentations of V vulnificus infection that have been reported in the literature include meningitis, corneal ulcers, epiglottitis, tonsillitis, spontaneous bacterial peritonitis, pneumonia, endometritis, septic arthritis, osteomyelitis, rhabdomyolysis endophthalmitis, and keratitis.2,4,6,13,18,19
Diagnosis
When diagnosing V vulnificus, providers need to obtain a thorough patient history, including any history of consumption or handling of raw seafood and recent water activities. Providers practicing in tropical climates or in warm summer months should keep V vulnificus in mind, as it is the ideal climate for the pathogen.9 Vital signs can range from unremarkable to fever, hypotension, tachycardia, and/or hypoxia. Skin examination may show exquisitely tender, erythematous skin with marked soft tissue edema, hemorrhagic bullae, ecchymoses, and/or necrosis. As physical examination findings can be nonspecific, wound cultures, blood cultures, and skin biopsies should be taken.
A wound culture and blood culture should be taken immediately if V vulnificus is suspected.8,11 A wound culture using discharge or fluid from necrotic or bullous lesions should be analyzed via gram stain.8,9 Gram stains of V vulnificus show short, slim, curved gram-negative rods under light microscopy.9,20 Special stains also can be done on cultures; V vulnificus is an oxidase-positive, lactose-positive, lysine-positive, salicin-positive, and arginine-negative organism. This knowledge can help differentiate V vulnificus from other gram-negative rods.13 Blood cultures will be positive in approximately 97% of patients with primary septicemia and 30% of patients with septicemia secondary to V vulnificus wound infections.3,9
Histologically, perilesional skin biopsies show epidermal necrosis with dermal and subcutaneous inflammation.12,17 There typically is an inflammatory infiltrate with neutrophilic abscesses and extensive tissue destruction in the subcutaneous tissue extending into the deep dermis.12,17 The superficial dermis is edematous but can lack the inflammatory infiltrate found in the subcutaneous tissue.17 Subepidermal bullae can form with numerous organisms within the fluid of the bullae. There also may be evidence of leukocytoclastic vasculitis with accompanying vessel wall necrosis. Fibrin clot formation and extravasated red blood cells may be visualized with few inflammatory cells but numerous organisms around the involved vessels.17
Management
Early diagnosis and treatment are vital.5,17 Cultures should be taken before aggressive treatment is started.3 Treatment is multifaceted; it requires antibiotics and wound care, except in cases of self-limited gastroenteritis.2,11 Aggressive debridement, fasciotomy, amputation, and supportive measures also may be necessary depending on the patient’s presentation.2,3,8,9 Establishing 2 peripheral intravenous lines is important in case rapid resuscitation becomes necessary.
Antibiotics
Primary cellulitis wound infections should be treated with doxycycline or a quinolone. If untreated, the wound can rapidly progress to necrotizing fasciitis.11 For necrotizing fasciitis and septicemia, broader-spectrum antibiotics are needed. For adults, ceftazidime plus doxycycline is the mainstay of antibiotic treatment for V vulnificus.2,9,11 For children, trimethoprim-sulfamethoxazole plus an aminoglycoside is preferred (Table).2,11
Antibiotic treatment has become more difficult as resistance arises. Antibiotic resistance likely is due to extensive antibiotic use in health care along with the agriculture and aquaculture industries using prophylactic and therapeutic antibiotics that wash into or are directly added to marine waters, where V vulnificus resides. Thus, antibiotic treatment should be tailored to the resistance profile of V vulnificus in various regions; for example, ceftazidime has an intermediate resistance profile in the United States, so cefotaxime and ceftriaxone may be better options.2
Wound Care
Wound infections must be extensively irrigated.9,21 For mild wound infections, proper wound care and oral antibiotics are appropriate (Table).21 Mild wounds should be irrigated thoroughly and followed by wound coverage to prevent progression, secondary infection, and necrosis. The dressing of choice will depend on the presenting lesion and provider preference; nonadherent, occlusive, or wet-to-dry dressings often are the best choices.22 Nonadherent dressings, such as petrolatum-covered gauze, do not pull off the newly formed epithelium when removed, making them beneficial to the skin’s healing process. Another option is occlusive dressings, which maintain a moist environment to hasten healing. They also enhance the autodigestion of necrotic tissue, which can be beneficial for necrotizing V vulnificus infections. Wet-to-dry dressings also may be used; these typically are comprised of gauze soaked with water, an astringent, and an antimicrobial or antiseptic solution. These dressings help to treat acute inflammation and also remove any exudate from the wound.22
Soft tissue and necrotizing infections require debridement.2,8 Early debridement decreases mortality rates.2,8,9 Necrotizing fasciitis often requires serial debridement to clear all the dead tissue and reduce the bacterial burden.8,9 Debridement prevents contiguous spread and metastatic seeding of the bacteria; it is important to prevent spread to the blood vessels, as vasculitis can necrose vessels, preventing antibiotics from reaching the dead tissue.17 Providers also should monitor for compartment syndrome, which should be treated with fasciotomy to decrease mortality.9,23 Many physicians leave V vulnificus–infected wounds open in order to heal by secondary intention.9 Hyperbaric oxygen therapy may be helpful as an adjunct to aggressive antimicrobial treatment for wound healing.8
Supportive Measures
Supportive care for dehydration, sepsis, DIC, and septic shock may be necessary, depending on the patient’s course. Treatment for severe V vulnificus infection includes intravenous fluids, crystalloids, oxygen, and/or intubation. Furthermore, if DIC develops, fresh frozen plasma, cryoprecipitate, a packed red blood cell transfusion, and/or anticoagulation may be required for resuscitation.3
Timing
Time to treatment and fatality rate are directly proportional in V vulnificus infection; the greater the delay in treatment, the higher the fatality rate.2 A 24-hour delay in antibiotic treatment is associated with a 33% case-fatality rate, and a 72-hour delay is associated with a 100% case-fatality rate.9 Even with early, appropriate treatment, mortality rates remain high.4
Prevention
Prevention of V vulnificus infections is an important consideration, especially for patients with chronic liver disease, immunosuppression, and hemochromatosis. Public education about the risks of eating raw shellfish is important.4 Oysters need to be treated properly to prevent growth and survival of V vulnificus.2 The most reliable method for destroying the bacteria is cooking shellfish.8,13 Only 15% of high-risk patients in the United States are aware of the risks associated with raw oyster consumption.3 High-risk patients should avoid eating raw oysters and shellfish and should cook seafood thoroughly before consumption.2,8 They also should wear protective clothing (ie, gloves) and eye protection when handling seafood and protective footwear (ie, wading shoes) while in seawater.2,8,13 It also is important to avoid contact with brackish water if one has any open wounds and to cleanse properly after exposure to brackish water or shellfish.2,8,16 Because severe V vulnificus infections can lead to death, prevention should be strongly encouraged by providers.2
Conclusion
Vibrio vulnificus infection typically occurs due to consumption of contaminated seafood or exposure to contaminated seawater. It most frequently affects older male patients with chronic liver disease, immunosuppression, hemochromatosis, or diabetes mellitus. Vibrio vulnificus can cause a vast spectrum of diseases, including gastroenteritis, wound infections, necrotizing fasciitis, and sepsis. Septicemia is the most common presentation of V vulnificus infection and accounts for the most fatalities from the bacteria. Septicemia often presents with fever, chills, vomiting, diarrhea, and hemorrhagic bullae. Vibrio vulnificus also commonly causes necrotizing fasciitis, which initially presents as cellulitis and rapidly progresses to hemorrhagic bullae or necrosis with accompanying systemic symptoms. Prompt diagnosis and treatment are vital to prevent mortality.
Interestingly, regions impacted by V vulnificus are expanding because of global warming.5,7Vibrio vulnificus thrives in warm waters, and increasing water temperatures are enhancing V vulnificus growth and survival.1,9 As global warming continues, the incidence of V vulnificus infections may rise. In fact, the number of infections increased by 78% between 1996 and 2006 in the United States.5 This rise likely was due to a combination of factors, including an aging population with more comorbidities, improvements in diagnosis, and climate change. Thus, as the number of V vulnificus infections rises, so too must providers’ suspicion for the pathogen.
- Phillips KE, Satchell KJF. Vibrio vulnificus: from oyster colonist to human pathogen [published online January 5, 2017]. PLOS Pathog. doi:10.1371/journal.ppat.1006053
- Heng SP, Letchumanan V, Deng CY, et al. Vibrio vulnificus: an environmental and clinical burden. Front Microbiol. 2017;8:997.
- Kumamoto KS, Vukich DJ. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1998;16:61-66.
- Borenstein M, Kerdel F. Infections with Vibrio vulnificus. Dermatol Clin. 2003;21:245-248.
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430.
- Kim SJ, Kim BC, Kim DC, et al. A fatal case of Vibrio vulnificus meningoencephalitis. Clin Microbiol Infect. 2003;9:568-571.
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733.
- Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus infection: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:E157-E166.
- Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014;21:207-213.
- Kikawa K, Yamasaki K, Sukiura T, et al. A successfully treated case of Vibrio vulnificus septicemia with shock. Jpn J Med. 1990;29:313-319.
- Perkins AP, Trimmier M. Recreational waterborne illnesses: recognition, treatment, and prevention. Am Fam Physician. 2017;95:554-560.
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145.
- Ulusarac O, Carter E. Varied clinical presentations of Vibrio vulnificus infections: a report of four unusual cases and review of the literature. South Med J. 2004;97:613-618.
- Bross MH, Soch K, Morales R, et al. Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician. 2007;76:539-544.
- Hori M, Nakayama A, Kitagawa D, et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen [published online May 19, 2017]. JMM Case Rep. doi:10.1099/jmmcr.0.005096
- Kotton Y, Soboh S, Bisharat N. Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infect Dis Rep. 2015;7:5901.
- Hoffman TJ, Nelson B, Darouiche R, et al. Vibrio vulnificus septicemia. Arch Intern Med. 1988;148:1825-1827.
- Alsaad AA, Sotello D, Kruse BT, et al. Vibrio vulnificus tonsillitis after swimming in the Gulf of Mexico [published online June 28, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-221161
- Tison DL, Kelly MT. Vibrio vulnificus endometritis. J Clin Microbiol. 1984;20:185-186.
- Beatty NL, Marquez J, Mohajer MA. Skin manifestations of primary Vibrio vulnificus septicemia. Am J Trop Med Hyg. 2017;97:1-2.
- Foote A, Henderson R, Lindberg A, et al. The Australian mid-west coastal marine wound infections study. Aust Fam Physician. 2017;46:923-927.
- Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Elsevier; 2019.
- Kim CS, Bae EH, Ma SK, et al. Severe septicemia, necrotizing fasciitis, and peritonitis due to Vibrio vulnificus in a patient undergoing continuous ambulatory peritoneal dialysis: a case report. BMC Infect Dis. 2015;15:422.
- Phillips KE, Satchell KJF. Vibrio vulnificus: from oyster colonist to human pathogen [published online January 5, 2017]. PLOS Pathog. doi:10.1371/journal.ppat.1006053
- Heng SP, Letchumanan V, Deng CY, et al. Vibrio vulnificus: an environmental and clinical burden. Front Microbiol. 2017;8:997.
- Kumamoto KS, Vukich DJ. Clinical infections of Vibrio vulnificus: a case report and review of the literature. J Emerg Med. 1998;16:61-66.
- Borenstein M, Kerdel F. Infections with Vibrio vulnificus. Dermatol Clin. 2003;21:245-248.
- Baker-Austin C, Oliver JD. Vibrio vulnificus: new insights into a deadly opportunistic pathogen. Environ Microbiol. 2018;20:423-430.
- Kim SJ, Kim BC, Kim DC, et al. A fatal case of Vibrio vulnificus meningoencephalitis. Clin Microbiol Infect. 2003;9:568-571.
- Jones MK, Oliver JD. Vibrio vulnificus: disease and pathogenesis. Infect Immun. 2009;77:1723-1733.
- Horseman MA, Surani S. A comprehensive review of Vibrio vulnificus infection: an important cause of severe sepsis and skin and soft-tissue infection. Int J Infect Dis. 2011;15:E157-E166.
- Diaz JH. Skin and soft tissue infections following marine injuries and exposures in travelers. J Travel Med. 2014;21:207-213.
- Kikawa K, Yamasaki K, Sukiura T, et al. A successfully treated case of Vibrio vulnificus septicemia with shock. Jpn J Med. 1990;29:313-319.
- Perkins AP, Trimmier M. Recreational waterborne illnesses: recognition, treatment, and prevention. Am Fam Physician. 2017;95:554-560.
- Patel VJ, Gardner E, Burton CS. Vibrio vulnificus septicemia and leg ulcer. J Am Acad Dermatol. 2002;46(5 suppl):S144-S145.
- Ulusarac O, Carter E. Varied clinical presentations of Vibrio vulnificus infections: a report of four unusual cases and review of the literature. South Med J. 2004;97:613-618.
- Bross MH, Soch K, Morales R, et al. Vibrio vulnificus infection: diagnosis and treatment. Am Fam Physician. 2007;76:539-544.
- Hori M, Nakayama A, Kitagawa D, et al. A case of Vibrio vulnificus infection complicated with fulminant purpura: gene and biotype analysis of the pathogen [published online May 19, 2017]. JMM Case Rep. doi:10.1099/jmmcr.0.005096
- Kotton Y, Soboh S, Bisharat N. Vibrio vulnificus necrotizing fasciitis associated with acupuncture. Infect Dis Rep. 2015;7:5901.
- Hoffman TJ, Nelson B, Darouiche R, et al. Vibrio vulnificus septicemia. Arch Intern Med. 1988;148:1825-1827.
- Alsaad AA, Sotello D, Kruse BT, et al. Vibrio vulnificus tonsillitis after swimming in the Gulf of Mexico [published online June 28, 2017]. BMJ Case Rep. doi:10.1136/bcr-2017-221161
- Tison DL, Kelly MT. Vibrio vulnificus endometritis. J Clin Microbiol. 1984;20:185-186.
- Beatty NL, Marquez J, Mohajer MA. Skin manifestations of primary Vibrio vulnificus septicemia. Am J Trop Med Hyg. 2017;97:1-2.
- Foote A, Henderson R, Lindberg A, et al. The Australian mid-west coastal marine wound infections study. Aust Fam Physician. 2017;46:923-927.
- Marks JG Jr, Miller JJ. Lookingbill and Marks’ Principles of Dermatology. 6th ed. Elsevier; 2019.
- Kim CS, Bae EH, Ma SK, et al. Severe septicemia, necrotizing fasciitis, and peritonitis due to Vibrio vulnificus in a patient undergoing continuous ambulatory peritoneal dialysis: a case report. BMC Infect Dis. 2015;15:422.
Practice Points
- Vibrio vulnificus infection should be high on the differential for patients who present with chronic liver disease and immunosuppression; a history of raw seafood consumption or exposure to brackish water; and bullae, cellulitis, necrotic lesions, or sepsis.
- Time to treatment is directly proportional to mortality rates in V vulnificus infections, and prompt treatment with antibiotics, wound care, debridement, and supportive measures is necessary to decrease mortality rates.
- The incidence of V vulnificus infection is rising in the United States, likely due to a combination of factors, including an aging population with multiple comorbidities, improvements in diagnosis, and climate change.
Making the World's Skin Crawl: Dermatologic Implications of COVID-19
Coronaviruses (CoVs) are among the most common causes of the common cold but also can lead to severe respiratory disease.1 In recent years, CoVs have been responsible for outbreaks of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), caused by SARS-CoV and MERS-CoV, respectively. Severe acute respiratory syndrome emerged from China in 2002, and MERS started in Saudi Arabia in 2012. In December 2019, several cases of unexplained pneumonia were reported in Wuhan, China.1 A novel CoV--SARS-CoV-2--was isolated in these patients and is now known to cause coronavirus disease 19 (COVID-19).1 Coronavirus disease 19 can cause acute respiratory distress and multiorgan failure.1,2 It spread quickly throughout the world and was declared a pandemic by the World Health Organization on March 11, 2020. According to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html), there were approximately 14,500 COVID-19 cases diagnosed worldwide on February 1, 2020; by May 22, 2020, there were more than 5,159,600 cases. Thus, heightened measures for infection prevention and control were put in place around the globe in an attempt to slow the spread of disease.1
In this article, we describe the dermatologic implications of COVID-19, including the clinical manifestations of the disease, risk reduction techniques for patients and providers, personal protective equipment-associated adverse reactions, and the financial impact on dermatologists.
Clinical Manifestations
At the start of the COVID-19 outbreak, little was known about the skin manifestations of the disease. Providers speculated that COVID-19 could have nonspecific skin findings similar to many other viral illnesses.3,4 Research throughout the pandemic has found many cutaneous manifestations of the disease.3-6 A case report from Thailand described a patient who presented with petechiae in addition to fever and thrombocytopenia, which led to an initial misdiagnosis of Dengue fever; however, when the patient began having respiratory symptoms, the diagnosis of COVID-19 was discovered.5 Furthermore, a study from Italy (N=88) showed dermatologic findings in 20.4% (18/88) of patients, including erythematous rash (77.8% [14/18]), widespread urticaria (16.7% [3/18]), and chickenpoxlike vesicles (5.6% [1/18]). A recent study from Spain (N=375) found 5 cutaneous patterns associated with COVID-19: pseudochilblain--acral areas of erythema with vesicles and/or pustules--lesions (19%), vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%), and livedoid/necrotic lesions (6%).6 Pseudochilblain lesions appeared in younger patients, occurred later in the disease course, and were associated with less severe disease. Vesicular lesions often were found in middle-aged patients prior to the onset of other COVID-19 symptoms, and they were associated with intermediate disease severity. Urticarial and maculopapular lesions typically paralleled other COVID-19 symptoms in timing and were associated with more severe disease. Likewise, livedoid and necrotic lesions were associated with more severe disease; they occurred more frequently in older patients.6 Clinicians at Cleveland Clinic found similar cutaneous lesions in COVID-19 patients, including morbilliform rashes, acral purpura resembling perniosis, and livedoid lesions.3 Initial biopsies of these lesions pointed to viral exanthema and thrombotic vasculopathy as potential etiologies of morbilliform and livedoid lesions, respectively. Interestingly, patients may present with multiple cutaneous morphologies of the disease at the same time.3 The acral lesions ("COVID toes") have been popularized throughout the media and thus may be the best-known cutaneous manifestation of the disease at this time. New findings continuously arise, and further research is warranted as lesions that develop in hospitalized COVID-19 patients could be virus related or secondary to hospital-induced skin irritation, stressors, or medications.3 Importantly, clinicians should be aware of these cutaneous signs of COVID-19, especially when triaging patients.
Risk Reduction
The current health crisis could have a drastic impact on dermatology patients and providers. One factor that may increase COVID-19 risk in dermatology patients is immunosuppression. Many patients are on immunomodulators and biologics for skin conditions, which can cause immunosuppression directly and indirectly. Immunosuppression is a risk factor for severe disease in patients with COVID-19, so this population is at higher risk for serious infection.7 Telemedicine for nonemergent cases and follow-ups should be considered to decrease traffic in high-risk hospitals; to limit the number of people in waiting rooms; and to protect staff, providers, and patients alike.1 Recommendations for teledermatology consultation during this time include the following: First, have patients take photographs of their skin lesions and send them remotely to the consulting physician. If the lesion is easily recognizable, treatment recommendations can be made remotely; if the diagnosis is ambiguous, the dermatologist can set up an in-person appointment.1
Personal Protective Equipment
Moreover, the current need to wear personal protective equipment (PPE) and wash hands frequently may lead to skin disease among health care providers. Facial rashes may arise from wearing masks and goggles, and repeated handwashing and wearing gloves may lead to hand dermatitis.8 One study examined adverse skin reactions among health care workers (N=322) during the SARS outbreak in 2003. More than one-third (35.5%) of staff members who wore masks regularly during the outbreak reported adverse skin reactions, including acne (59.6%), facial itching (51.4%), and rash (35.8%).8 The acne etiology likely is multifactorial. Masks increase heat and humidity in the covered facial region, which can cause acne flare-ups due to increased sebum production and Cutibacterium acnes growth.8 Additionally, tight N95 masks may occlude the pilosebaceous glands, causing acne to flare. In the SARS study, facial itchiness and rashes likely were due to irritant contact dermatitis to the N95 masks. All of the respondents with adverse skin reactions from masks developed them after using N95 masks; those who wore surgical masks did not report reactions.8 Because N95 masks are recommended for health care workers caring for patients with highly transmissible respiratory infections such as SARS and COVID-19, it will be difficult to avoid wearing them during the current crisis. For this reason, topical retinoids and topical benzoyl peroxide should be the first-line treatment of mask-induced acne, and moisturization and topical corticosteroids should be used for facial erythema. Additionally, 21.4% of respondents reported adverse skin reactions from latex gloves during the SARS outbreak, including dry skin, itchiness, rash, and wheals.8 These skin reactions may have been type I IgE-mediated hypersensitivity reactions or irritant contact dermatitis due to latex sensitization and frequent handwashing. No respondents reported skin reactions to plastic gloves.8 For this reason, health care providers should consider wearing plastic gloves in lieu of or under latex gloves to prevent hand dermatitis during this time. Moisturization, barrier creams, and topical corticosteroids also can help treat hand dermatitis. Frequently changing PPE may help prevent skin disease among the frontline health care workers,8 which posed a problem at the beginning of the COVID-19 outbreak as there was a PPE shortage. With industry and individuals coming together to make and donate PPE, it is now more widely available for our frontline providers.
Financial Impact
Finally, the pandemic is having an immense financial impact on dermatology.9 At the onset of the outbreak, our role as health care providers was to help slow the spread of COVID-19; for this reason, most elective procedures were cancelled, and many outpatient clinics closed. Both elective procedures and outpatient visits are central to dermatology, so many dermatologists worked less or not at all during this time, leading to a loss of revenue. The goals of these measures were to reduce transmissibility of the disease, to prevent the health care system from being overwhelmed with critical COVID-19 cases, and to allocate resources to the frontline providers.9 Although these measures were beneficial for slowing the spread of disease, they were detrimental to some providers' and practices' financial stability. Many dermatology practices have begun to reopen with COVID-19 precautions in place. For example, practices are limiting the number of patients that can be in the office at one time, mandating temperature readings upon check-in, and requiring masks be worn throughout the entire visit. With continued recommendations for individuals to stay at home as much as possible, the number of patients being seen in dermatology clinics on a daily basis remains less than normal. One potential solution is telemedicine, which would allow patients' concerns to be addressed while keeping providers practicing with a normal patient volume during this time.9 Keeping providers financially afloat is vital for private practices to continue operating after the pandemic. Dermatology appointments are in high demand with long waiting lists during nonpandemic times; without dermatologists practicing at full capacity, there will be an accumulation of patients with dermatologic conditions with even longer waiting times after the pandemic. Telemedicine may help reduce this potential accumulation of patients and allow patients to be treated in a more timely manner while alleviating financial pressures for providers.
Final Thoughts
The COVID-19 pandemic has spread across the world, infecting millions of people. Although the trends have slowed, more than 106,100 cases are still being diagnosed daily according to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html). Patients with COVID-19 may present with a variety of cutaneous lesions. Wearing PPE to take care of COVID-19 patients may lead to skin irritation, so care should be taken to address these adverse skin reactions to maintain the safety of providers. Finally, dermatologists should consider telemedicine during this time to protect high-risk patients, prevent a postpandemic surge of patients, and alleviate financial stressors caused by COVID-19.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- Lippi G, Plebani M, Michael HB. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis [published online March 13, 2020]. Clin Chim Acta. doi:10.1016/j.cca.2020.03.022.
- Young S, Fernandez AP. Skin manifestations of COVID-19 [published online May 14, 2020]. Cleve Clin J Med. doi:10.3949/ccjm.87a.ccc031.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for Dengue [published online March 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.036.
- Casas CG, Catalá A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases [published online April 29, 2020]. Br J Dermatol. doi:10.1111/bjd.19163.
- Conforti C, Giuffrida R, Dianzani C, et al. COVID-19 and psoriasis: is it time to limited treatment with immunosuppressants? a call for action [published online March 11, 2020]. Dermatol Ther. doi:10.1111/dth.13298.
- Foo CC, Goon AT, Leow YH, et al. Adverse skin reactions to personal protective equipment against severe respiratory syndrome--a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294.
- Heymann WR. The profound dermatological manifestations of COVID-19 [published online March 18, 2020]. Dermatology World Insights and Inquiries. https://www.aad.org/dw/dw-insights-and-inquiries/2020-archive/march/dermatological-manifestations-covid-19. Accessed May 21, 2020.
Coronaviruses (CoVs) are among the most common causes of the common cold but also can lead to severe respiratory disease.1 In recent years, CoVs have been responsible for outbreaks of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), caused by SARS-CoV and MERS-CoV, respectively. Severe acute respiratory syndrome emerged from China in 2002, and MERS started in Saudi Arabia in 2012. In December 2019, several cases of unexplained pneumonia were reported in Wuhan, China.1 A novel CoV--SARS-CoV-2--was isolated in these patients and is now known to cause coronavirus disease 19 (COVID-19).1 Coronavirus disease 19 can cause acute respiratory distress and multiorgan failure.1,2 It spread quickly throughout the world and was declared a pandemic by the World Health Organization on March 11, 2020. According to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html), there were approximately 14,500 COVID-19 cases diagnosed worldwide on February 1, 2020; by May 22, 2020, there were more than 5,159,600 cases. Thus, heightened measures for infection prevention and control were put in place around the globe in an attempt to slow the spread of disease.1
In this article, we describe the dermatologic implications of COVID-19, including the clinical manifestations of the disease, risk reduction techniques for patients and providers, personal protective equipment-associated adverse reactions, and the financial impact on dermatologists.
Clinical Manifestations
At the start of the COVID-19 outbreak, little was known about the skin manifestations of the disease. Providers speculated that COVID-19 could have nonspecific skin findings similar to many other viral illnesses.3,4 Research throughout the pandemic has found many cutaneous manifestations of the disease.3-6 A case report from Thailand described a patient who presented with petechiae in addition to fever and thrombocytopenia, which led to an initial misdiagnosis of Dengue fever; however, when the patient began having respiratory symptoms, the diagnosis of COVID-19 was discovered.5 Furthermore, a study from Italy (N=88) showed dermatologic findings in 20.4% (18/88) of patients, including erythematous rash (77.8% [14/18]), widespread urticaria (16.7% [3/18]), and chickenpoxlike vesicles (5.6% [1/18]). A recent study from Spain (N=375) found 5 cutaneous patterns associated with COVID-19: pseudochilblain--acral areas of erythema with vesicles and/or pustules--lesions (19%), vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%), and livedoid/necrotic lesions (6%).6 Pseudochilblain lesions appeared in younger patients, occurred later in the disease course, and were associated with less severe disease. Vesicular lesions often were found in middle-aged patients prior to the onset of other COVID-19 symptoms, and they were associated with intermediate disease severity. Urticarial and maculopapular lesions typically paralleled other COVID-19 symptoms in timing and were associated with more severe disease. Likewise, livedoid and necrotic lesions were associated with more severe disease; they occurred more frequently in older patients.6 Clinicians at Cleveland Clinic found similar cutaneous lesions in COVID-19 patients, including morbilliform rashes, acral purpura resembling perniosis, and livedoid lesions.3 Initial biopsies of these lesions pointed to viral exanthema and thrombotic vasculopathy as potential etiologies of morbilliform and livedoid lesions, respectively. Interestingly, patients may present with multiple cutaneous morphologies of the disease at the same time.3 The acral lesions ("COVID toes") have been popularized throughout the media and thus may be the best-known cutaneous manifestation of the disease at this time. New findings continuously arise, and further research is warranted as lesions that develop in hospitalized COVID-19 patients could be virus related or secondary to hospital-induced skin irritation, stressors, or medications.3 Importantly, clinicians should be aware of these cutaneous signs of COVID-19, especially when triaging patients.
Risk Reduction
The current health crisis could have a drastic impact on dermatology patients and providers. One factor that may increase COVID-19 risk in dermatology patients is immunosuppression. Many patients are on immunomodulators and biologics for skin conditions, which can cause immunosuppression directly and indirectly. Immunosuppression is a risk factor for severe disease in patients with COVID-19, so this population is at higher risk for serious infection.7 Telemedicine for nonemergent cases and follow-ups should be considered to decrease traffic in high-risk hospitals; to limit the number of people in waiting rooms; and to protect staff, providers, and patients alike.1 Recommendations for teledermatology consultation during this time include the following: First, have patients take photographs of their skin lesions and send them remotely to the consulting physician. If the lesion is easily recognizable, treatment recommendations can be made remotely; if the diagnosis is ambiguous, the dermatologist can set up an in-person appointment.1
Personal Protective Equipment
Moreover, the current need to wear personal protective equipment (PPE) and wash hands frequently may lead to skin disease among health care providers. Facial rashes may arise from wearing masks and goggles, and repeated handwashing and wearing gloves may lead to hand dermatitis.8 One study examined adverse skin reactions among health care workers (N=322) during the SARS outbreak in 2003. More than one-third (35.5%) of staff members who wore masks regularly during the outbreak reported adverse skin reactions, including acne (59.6%), facial itching (51.4%), and rash (35.8%).8 The acne etiology likely is multifactorial. Masks increase heat and humidity in the covered facial region, which can cause acne flare-ups due to increased sebum production and Cutibacterium acnes growth.8 Additionally, tight N95 masks may occlude the pilosebaceous glands, causing acne to flare. In the SARS study, facial itchiness and rashes likely were due to irritant contact dermatitis to the N95 masks. All of the respondents with adverse skin reactions from masks developed them after using N95 masks; those who wore surgical masks did not report reactions.8 Because N95 masks are recommended for health care workers caring for patients with highly transmissible respiratory infections such as SARS and COVID-19, it will be difficult to avoid wearing them during the current crisis. For this reason, topical retinoids and topical benzoyl peroxide should be the first-line treatment of mask-induced acne, and moisturization and topical corticosteroids should be used for facial erythema. Additionally, 21.4% of respondents reported adverse skin reactions from latex gloves during the SARS outbreak, including dry skin, itchiness, rash, and wheals.8 These skin reactions may have been type I IgE-mediated hypersensitivity reactions or irritant contact dermatitis due to latex sensitization and frequent handwashing. No respondents reported skin reactions to plastic gloves.8 For this reason, health care providers should consider wearing plastic gloves in lieu of or under latex gloves to prevent hand dermatitis during this time. Moisturization, barrier creams, and topical corticosteroids also can help treat hand dermatitis. Frequently changing PPE may help prevent skin disease among the frontline health care workers,8 which posed a problem at the beginning of the COVID-19 outbreak as there was a PPE shortage. With industry and individuals coming together to make and donate PPE, it is now more widely available for our frontline providers.
Financial Impact
Finally, the pandemic is having an immense financial impact on dermatology.9 At the onset of the outbreak, our role as health care providers was to help slow the spread of COVID-19; for this reason, most elective procedures were cancelled, and many outpatient clinics closed. Both elective procedures and outpatient visits are central to dermatology, so many dermatologists worked less or not at all during this time, leading to a loss of revenue. The goals of these measures were to reduce transmissibility of the disease, to prevent the health care system from being overwhelmed with critical COVID-19 cases, and to allocate resources to the frontline providers.9 Although these measures were beneficial for slowing the spread of disease, they were detrimental to some providers' and practices' financial stability. Many dermatology practices have begun to reopen with COVID-19 precautions in place. For example, practices are limiting the number of patients that can be in the office at one time, mandating temperature readings upon check-in, and requiring masks be worn throughout the entire visit. With continued recommendations for individuals to stay at home as much as possible, the number of patients being seen in dermatology clinics on a daily basis remains less than normal. One potential solution is telemedicine, which would allow patients' concerns to be addressed while keeping providers practicing with a normal patient volume during this time.9 Keeping providers financially afloat is vital for private practices to continue operating after the pandemic. Dermatology appointments are in high demand with long waiting lists during nonpandemic times; without dermatologists practicing at full capacity, there will be an accumulation of patients with dermatologic conditions with even longer waiting times after the pandemic. Telemedicine may help reduce this potential accumulation of patients and allow patients to be treated in a more timely manner while alleviating financial pressures for providers.
Final Thoughts
The COVID-19 pandemic has spread across the world, infecting millions of people. Although the trends have slowed, more than 106,100 cases are still being diagnosed daily according to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html). Patients with COVID-19 may present with a variety of cutaneous lesions. Wearing PPE to take care of COVID-19 patients may lead to skin irritation, so care should be taken to address these adverse skin reactions to maintain the safety of providers. Finally, dermatologists should consider telemedicine during this time to protect high-risk patients, prevent a postpandemic surge of patients, and alleviate financial stressors caused by COVID-19.
Coronaviruses (CoVs) are among the most common causes of the common cold but also can lead to severe respiratory disease.1 In recent years, CoVs have been responsible for outbreaks of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), caused by SARS-CoV and MERS-CoV, respectively. Severe acute respiratory syndrome emerged from China in 2002, and MERS started in Saudi Arabia in 2012. In December 2019, several cases of unexplained pneumonia were reported in Wuhan, China.1 A novel CoV--SARS-CoV-2--was isolated in these patients and is now known to cause coronavirus disease 19 (COVID-19).1 Coronavirus disease 19 can cause acute respiratory distress and multiorgan failure.1,2 It spread quickly throughout the world and was declared a pandemic by the World Health Organization on March 11, 2020. According to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html), there were approximately 14,500 COVID-19 cases diagnosed worldwide on February 1, 2020; by May 22, 2020, there were more than 5,159,600 cases. Thus, heightened measures for infection prevention and control were put in place around the globe in an attempt to slow the spread of disease.1
In this article, we describe the dermatologic implications of COVID-19, including the clinical manifestations of the disease, risk reduction techniques for patients and providers, personal protective equipment-associated adverse reactions, and the financial impact on dermatologists.
Clinical Manifestations
At the start of the COVID-19 outbreak, little was known about the skin manifestations of the disease. Providers speculated that COVID-19 could have nonspecific skin findings similar to many other viral illnesses.3,4 Research throughout the pandemic has found many cutaneous manifestations of the disease.3-6 A case report from Thailand described a patient who presented with petechiae in addition to fever and thrombocytopenia, which led to an initial misdiagnosis of Dengue fever; however, when the patient began having respiratory symptoms, the diagnosis of COVID-19 was discovered.5 Furthermore, a study from Italy (N=88) showed dermatologic findings in 20.4% (18/88) of patients, including erythematous rash (77.8% [14/18]), widespread urticaria (16.7% [3/18]), and chickenpoxlike vesicles (5.6% [1/18]). A recent study from Spain (N=375) found 5 cutaneous patterns associated with COVID-19: pseudochilblain--acral areas of erythema with vesicles and/or pustules--lesions (19%), vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%), and livedoid/necrotic lesions (6%).6 Pseudochilblain lesions appeared in younger patients, occurred later in the disease course, and were associated with less severe disease. Vesicular lesions often were found in middle-aged patients prior to the onset of other COVID-19 symptoms, and they were associated with intermediate disease severity. Urticarial and maculopapular lesions typically paralleled other COVID-19 symptoms in timing and were associated with more severe disease. Likewise, livedoid and necrotic lesions were associated with more severe disease; they occurred more frequently in older patients.6 Clinicians at Cleveland Clinic found similar cutaneous lesions in COVID-19 patients, including morbilliform rashes, acral purpura resembling perniosis, and livedoid lesions.3 Initial biopsies of these lesions pointed to viral exanthema and thrombotic vasculopathy as potential etiologies of morbilliform and livedoid lesions, respectively. Interestingly, patients may present with multiple cutaneous morphologies of the disease at the same time.3 The acral lesions ("COVID toes") have been popularized throughout the media and thus may be the best-known cutaneous manifestation of the disease at this time. New findings continuously arise, and further research is warranted as lesions that develop in hospitalized COVID-19 patients could be virus related or secondary to hospital-induced skin irritation, stressors, or medications.3 Importantly, clinicians should be aware of these cutaneous signs of COVID-19, especially when triaging patients.
Risk Reduction
The current health crisis could have a drastic impact on dermatology patients and providers. One factor that may increase COVID-19 risk in dermatology patients is immunosuppression. Many patients are on immunomodulators and biologics for skin conditions, which can cause immunosuppression directly and indirectly. Immunosuppression is a risk factor for severe disease in patients with COVID-19, so this population is at higher risk for serious infection.7 Telemedicine for nonemergent cases and follow-ups should be considered to decrease traffic in high-risk hospitals; to limit the number of people in waiting rooms; and to protect staff, providers, and patients alike.1 Recommendations for teledermatology consultation during this time include the following: First, have patients take photographs of their skin lesions and send them remotely to the consulting physician. If the lesion is easily recognizable, treatment recommendations can be made remotely; if the diagnosis is ambiguous, the dermatologist can set up an in-person appointment.1
Personal Protective Equipment
Moreover, the current need to wear personal protective equipment (PPE) and wash hands frequently may lead to skin disease among health care providers. Facial rashes may arise from wearing masks and goggles, and repeated handwashing and wearing gloves may lead to hand dermatitis.8 One study examined adverse skin reactions among health care workers (N=322) during the SARS outbreak in 2003. More than one-third (35.5%) of staff members who wore masks regularly during the outbreak reported adverse skin reactions, including acne (59.6%), facial itching (51.4%), and rash (35.8%).8 The acne etiology likely is multifactorial. Masks increase heat and humidity in the covered facial region, which can cause acne flare-ups due to increased sebum production and Cutibacterium acnes growth.8 Additionally, tight N95 masks may occlude the pilosebaceous glands, causing acne to flare. In the SARS study, facial itchiness and rashes likely were due to irritant contact dermatitis to the N95 masks. All of the respondents with adverse skin reactions from masks developed them after using N95 masks; those who wore surgical masks did not report reactions.8 Because N95 masks are recommended for health care workers caring for patients with highly transmissible respiratory infections such as SARS and COVID-19, it will be difficult to avoid wearing them during the current crisis. For this reason, topical retinoids and topical benzoyl peroxide should be the first-line treatment of mask-induced acne, and moisturization and topical corticosteroids should be used for facial erythema. Additionally, 21.4% of respondents reported adverse skin reactions from latex gloves during the SARS outbreak, including dry skin, itchiness, rash, and wheals.8 These skin reactions may have been type I IgE-mediated hypersensitivity reactions or irritant contact dermatitis due to latex sensitization and frequent handwashing. No respondents reported skin reactions to plastic gloves.8 For this reason, health care providers should consider wearing plastic gloves in lieu of or under latex gloves to prevent hand dermatitis during this time. Moisturization, barrier creams, and topical corticosteroids also can help treat hand dermatitis. Frequently changing PPE may help prevent skin disease among the frontline health care workers,8 which posed a problem at the beginning of the COVID-19 outbreak as there was a PPE shortage. With industry and individuals coming together to make and donate PPE, it is now more widely available for our frontline providers.
Financial Impact
Finally, the pandemic is having an immense financial impact on dermatology.9 At the onset of the outbreak, our role as health care providers was to help slow the spread of COVID-19; for this reason, most elective procedures were cancelled, and many outpatient clinics closed. Both elective procedures and outpatient visits are central to dermatology, so many dermatologists worked less or not at all during this time, leading to a loss of revenue. The goals of these measures were to reduce transmissibility of the disease, to prevent the health care system from being overwhelmed with critical COVID-19 cases, and to allocate resources to the frontline providers.9 Although these measures were beneficial for slowing the spread of disease, they were detrimental to some providers' and practices' financial stability. Many dermatology practices have begun to reopen with COVID-19 precautions in place. For example, practices are limiting the number of patients that can be in the office at one time, mandating temperature readings upon check-in, and requiring masks be worn throughout the entire visit. With continued recommendations for individuals to stay at home as much as possible, the number of patients being seen in dermatology clinics on a daily basis remains less than normal. One potential solution is telemedicine, which would allow patients' concerns to be addressed while keeping providers practicing with a normal patient volume during this time.9 Keeping providers financially afloat is vital for private practices to continue operating after the pandemic. Dermatology appointments are in high demand with long waiting lists during nonpandemic times; without dermatologists practicing at full capacity, there will be an accumulation of patients with dermatologic conditions with even longer waiting times after the pandemic. Telemedicine may help reduce this potential accumulation of patients and allow patients to be treated in a more timely manner while alleviating financial pressures for providers.
Final Thoughts
The COVID-19 pandemic has spread across the world, infecting millions of people. Although the trends have slowed, more than 106,100 cases are still being diagnosed daily according to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html). Patients with COVID-19 may present with a variety of cutaneous lesions. Wearing PPE to take care of COVID-19 patients may lead to skin irritation, so care should be taken to address these adverse skin reactions to maintain the safety of providers. Finally, dermatologists should consider telemedicine during this time to protect high-risk patients, prevent a postpandemic surge of patients, and alleviate financial stressors caused by COVID-19.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- Lippi G, Plebani M, Michael HB. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis [published online March 13, 2020]. Clin Chim Acta. doi:10.1016/j.cca.2020.03.022.
- Young S, Fernandez AP. Skin manifestations of COVID-19 [published online May 14, 2020]. Cleve Clin J Med. doi:10.3949/ccjm.87a.ccc031.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for Dengue [published online March 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.036.
- Casas CG, Catalá A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases [published online April 29, 2020]. Br J Dermatol. doi:10.1111/bjd.19163.
- Conforti C, Giuffrida R, Dianzani C, et al. COVID-19 and psoriasis: is it time to limited treatment with immunosuppressants? a call for action [published online March 11, 2020]. Dermatol Ther. doi:10.1111/dth.13298.
- Foo CC, Goon AT, Leow YH, et al. Adverse skin reactions to personal protective equipment against severe respiratory syndrome--a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294.
- Heymann WR. The profound dermatological manifestations of COVID-19 [published online March 18, 2020]. Dermatology World Insights and Inquiries. https://www.aad.org/dw/dw-insights-and-inquiries/2020-archive/march/dermatological-manifestations-covid-19. Accessed May 21, 2020.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- Lippi G, Plebani M, Michael HB. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis [published online March 13, 2020]. Clin Chim Acta. doi:10.1016/j.cca.2020.03.022.
- Young S, Fernandez AP. Skin manifestations of COVID-19 [published online May 14, 2020]. Cleve Clin J Med. doi:10.3949/ccjm.87a.ccc031.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for Dengue [published online March 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.036.
- Casas CG, Catalá A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases [published online April 29, 2020]. Br J Dermatol. doi:10.1111/bjd.19163.
- Conforti C, Giuffrida R, Dianzani C, et al. COVID-19 and psoriasis: is it time to limited treatment with immunosuppressants? a call for action [published online March 11, 2020]. Dermatol Ther. doi:10.1111/dth.13298.
- Foo CC, Goon AT, Leow YH, et al. Adverse skin reactions to personal protective equipment against severe respiratory syndrome--a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294.
- Heymann WR. The profound dermatological manifestations of COVID-19 [published online March 18, 2020]. Dermatology World Insights and Inquiries. https://www.aad.org/dw/dw-insights-and-inquiries/2020-archive/march/dermatological-manifestations-covid-19. Accessed May 21, 2020.
Practice Points
- Clinicians should be aware of the skin manifesta-tions of coronavirus disease 19, especially when triaging patients.
- Health care providers may develop skin diseases from wearing the extensive personal protective equipment required during the current health crisis.
- Coronavirus disease 19 has had a substantial finan-cial impact on dermatologists, and telemedicine may be a potential solution.
Treatment Options for Pilonidal Sinus
Pilonidal disease was first described by Mayo1 in 1833 who hypothesized that the underlying etiology is incomplete separation of the mesoderm and ectoderm layers during embryogenesis. In 1880, Hodges2 coined the term pilonidal sinus; he postulated that sinus formation was incited by hair.2 Today, Hodges theory is known as the acquired theory: hair induces a foreign body response in surrounding tissue, leading to sinus formation. Although pilonidal cysts can occur anywhere on the body, they most commonly extend cephalad in the sacrococcygeal and upper gluteal cleft (Figure 1).3,4 An acute pilonidal cyst typically presents with pain, tenderness, and swelling, similar to the presentation of a superficial abscess in other locations; however, a clue to the diagnosis is the presence of cutaneous pits along the midline of the gluteal cleft.5 Chronic pilonidal disease varies based on the extent of inflammation and scarring; the underlying cavity communicates with the overlying skin through sinuses and often drains with pressure.6
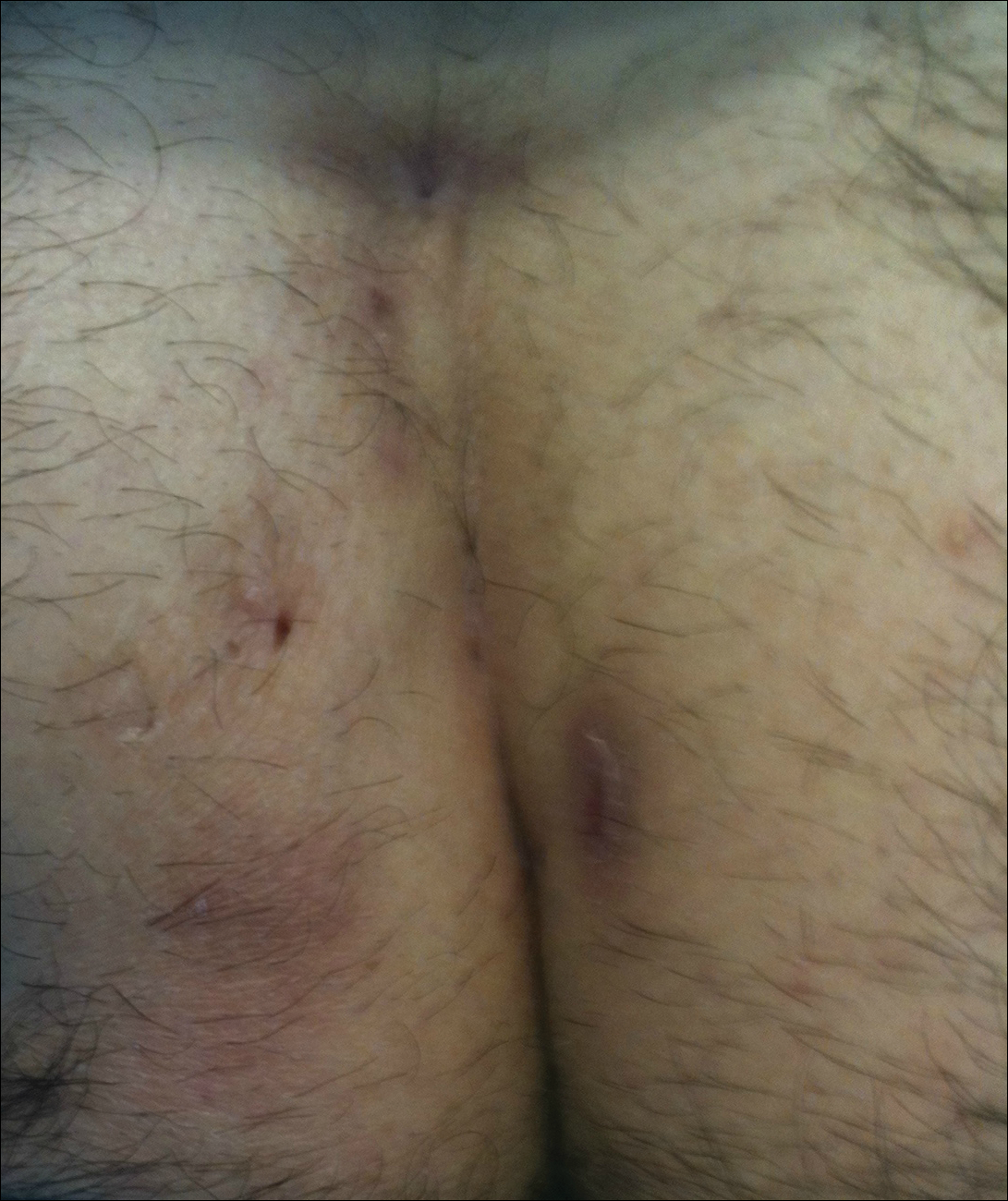
Pilonidal sinuses are rare before puberty or after 40 years of age7 and occur primarily in hirsute men. The ratio of men to women affected is between 3:1 and 4:1.8 Although pilonidal sinuses account for only 15% of anal suppurations, complications arising from pilonidal sinuses are a considerable cause of morbidity, resulting in loss of productivity in otherwise healthy individuals.9 Complications include chronic nonhealing wounds,10 as recurrent pilonidal sinuses tend to become colonized with gram-positive and facultative anaerobic bacteria, whereas primary pilonidal cysts more commonly become infected with anaerobic and gram-negative bacteria.11 Long-standing disease increases the risk of squamous cell carcinoma arising within sinus tracts.10,12
Histopathologically, pilonidal cysts are not true cysts because they lack an epithelial lining. Examination of the cavity commonly reveals hair, debris, and granulation tissue with surrounding foreign-body giant cells (Figure 2).5
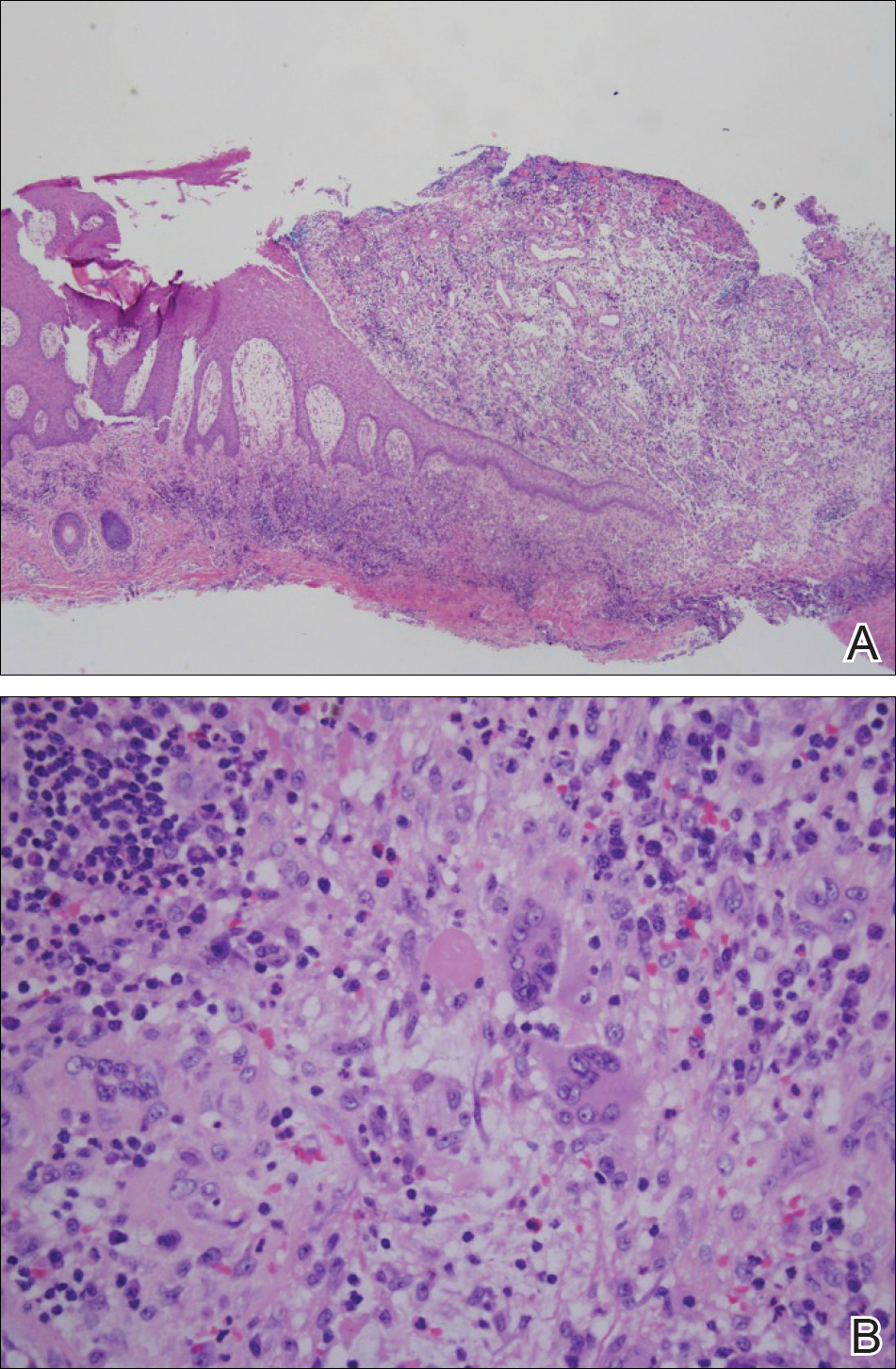
The preferred treatment of pilonidal cysts continues to be debated. In this article, we review evidence supporting current modalities including conservative and surgical techniques as well as novel laser therapy for the treatment of pilonidal disease.
Conservative Management Techniques
Phenol Injections
Liquid or crystallized phenol injections have been used for treatment of mild to moderate pilonidal cysts.13 Excess debris is removed by curettage, and phenol is administered through the existing orifices or pits without pressure. The phenol remains in the cavity for 1 to 3 minutes before aspiration. Remaining cyst contents are removed through tissue manipulation, and the sinus is washed with saline. Mean healing time is 20 days (range, +/−14 days).13
Classically, phenol injections have a failure rate of 30% to 40%, especially with multiple sinuses and suppurative disease6; however, the success rate improves with limited disease (ie, no more than 1–3 sinus pits).3 With multiple treatment sessions, a recurrence rate as low as 2% over 25 months has been reported.14 Phenol injection also has been proposed as an adjuvant therapy to pit excision to minimize the need for extensive surgery.15
Simple Incision and Drainage
Simple incision and drainage has a crucial role in the treatment of acute pilonidal disease to decrease pain and relieve tension. Off-midline incisions have been recommended for because the resulting closures fared better against sheer forces applied by the gluteal muscles on the cleft.6 Therefore, the incision often is made off-midline from the gluteal cleft even when the cyst lies directly on the gluteal cleft.
Rates of healing vary widely after incision and drainage, ranging from 45% to 82%.6 Primary pilonidal cysts may respond well, particularly if the cavity is abraded; in one series, 79% (58/73) of patients did not have a recurrence at the average follow-up of 60 months.16
Excision and Unroofing
Techniques for excision and unroofing without primary closure include 2 variants: wide and limited. The wide technique consists of an inwardly slanted excision that is deepest in the center of the cavity. The inward sloping angle of the incision aids in healing because it allows granulation to progress evenly from the base of the wound upward. The depth of the incision should spare the fascia and leave as much fatty tissue as possible while still resecting the entire cavity and associated pits.6 Limited incision techniques aim to shorten the healing period by making smaller incisions into the sinuses, pits, and secondary tracts, and they are frequently supplemented with curettage.6 Noteworthy disadvantages include prolonged healing time, need for professional wound management, and extended medical observation.5 The average duration of wound healing in a study of 300 patients was 5.4 weeks (range, +/−1.1 weeks),17 and the recurrence rate has ranged from 5% to 13%.18,19 Care must be taken to respond to numerous possible complications, including excessive exudation and granulation, superinfection, and walling off.6
Although the cost of treatment varies by hospital, location, and a patient’s insurance coverage, patient reports to the Pilonidal Support Alliance indicate that the cost of conservative management ranges from $500 to $2000.20
Excision and Primary Closure
An elliptical excision that includes some of the lateral margin is excised down to the level of the fascia. Adjacent lateral tracts may be excised by expanding the incision. To close the wound, edges are approximated with placement of deep and superficial sutures. Wound healing typically occurs faster than secondary granulation, as seen in one randomized controlled trial with a mean of 10 days for primary closure compared to 13 weeks for secondary intention.21 However, as with any surgical procedure, postoperative complications can delay wound healing.19 The recurrence rate after primary closure varies considerably, ranging from 10% to 38%.18,21-23 The average cost of an excision ranges from $3000 to $6000.20
A
Surgical Techniques
For severe or recurrent pilonidal disease, skin flaps often are required. Several flaps have been developed, including advancement, Bascom cleft lift, Karydakis, and modified Limberg flap. Flaps require a vascular pedicle but allow for closure without tension.26 The cost of a flap procedure, ranging from $10,000 to $30,000, is greater than the cost of excision or other conservative therapy20; however, with a lower recurrence rate of pilonidal disease following flap procedures compared to other treatments, patients may save more on treatment over the long-term.
Advancement Flaps
The most commonly used advancement flaps are the V-Y advancement flap and Z-plasty. The V-Y advancement flap creates a full-thickness V-shaped incision down to gluteal fascia that is closed to form a postrepair suture line in the shape of a Y.5 Depending on the size of the defect, the flaps may be utilized unilaterally or bilaterally. A defect as large as 8 to 10 cm can be covered unilaterally; however, defects larger than 10 cm commonly require a bilateral flap.26 The V-Y advancement flap failed to show superiority to primary closure techniques based on complications, recurrence, and patient satisfaction in a large randomized controlled trial.27
Performing a Z-plasty requires excision of diseased tissue with recruitment of lateral flaps incised down to the level of the fascia. The lateral edges are transposed to increase transverse length.26 No statistically significant difference in infection or recurrence rates was noted between excision alone and excision plus Z-plasty; however, wounds were reported to heal faster in patients receiving excision plus Z-plasty (41 vs 15 days).28
Cleft Lift Closure
In 1987, Bascom29 introduced the cleft lift closure for recurrent pilonidal disease. This technique aims to reduce or eliminate lateral gluteal forces on the wounds by filling the gluteal cleft.5 The sinus tracts are excised and a full-thickness skin flap is extended across the cleft and closed off-midline. The adipose tissue fills in the previous space of the gluteal cleft. In the initial study, no recurrences were reported in 30 patients who underwent this procedure at 2-year follow-up; similarly, in another case series of 26 patients who underwent the procedure, no recurrences were noted at a median follow-up of 3 years.30 Compared to excision with secondary wound healing and primary closure on the midline, the Bascom cleft lift demonstrated a decrease in wound healing time (62, 52, and 29 days, respectively).31
The classic Karydakis flap consists of an oblique elliptical excision of diseased tissue with fixation of the flap base to the sacral fascia (Figures 4 and 5). The flap is closed by suturing the edge off-midline.32 This technique prevents a midline wound and aims to remodel and flatten the natal cleft. Karydakis33 performed the most important study for treatment of pilonidal disease with the Karydakis flap, which included more than 5000 patients. The results showed a 0.9% recurrence rate and an 8.5% wound complication rate over a 2- to 20-year follow-up.33 These results have been substantiated by more recent studies, which produced similar results: a 1.8% to 5.3% infection rate and a recurrence rate of 0.9% to 4.4%.34,35
In the modified Karydakis flap, the same excision and closure is performed without tacking the flap to the sacral fascia, aiming to prevent formation of a new vulnerable raphe by flattening the natal cleft. The infection rate was similar to the classic Karydakis flap, and no recurrences were noted during a 20-month follow-up.36
Limberg Flap
The Limberg flap is derived from a rhomboid flap. In the classic Limberg flap, a midline rhomboid incision to the presacral fascia including the sinus is performed. The flap gains mobility by extending the excision laterally to the fascia of the gluteus maximus muscle. A variant of the original flap includes the modified Limberg flap, which lateralizes the midline sutures and flattens the intergluteal sulcus. Compared to the traditional Limberg approach, the modified Limberg flap was associated with a lower failure rate at both early and late time points and a lower rate of infection37,38; however, based on the data it is unclear when primary closure should be favored over a Limberg flap. Several studies show the recurrence rate to be identical; however, hospital stay and pain were reduced in the Limberg flap group compared to primary closure.39,40
Results from randomized controlled trials comparing the modified Limberg flap to the Karydakis flap vary. One of the largest prospective, randomized, controlled trials comparing the 2 flaps included 269 patients.Results showed a lower postoperative complication rate, lower pain scores, shorter operation time, and shorter hospital stay with the Karydakis flap compared to the Limberg flap, though no difference in recurrence was noted between the 2 groups.41
Tw
Overall, larger prospective trials are needed to clarify the differences in outcomes between flap techniques. In
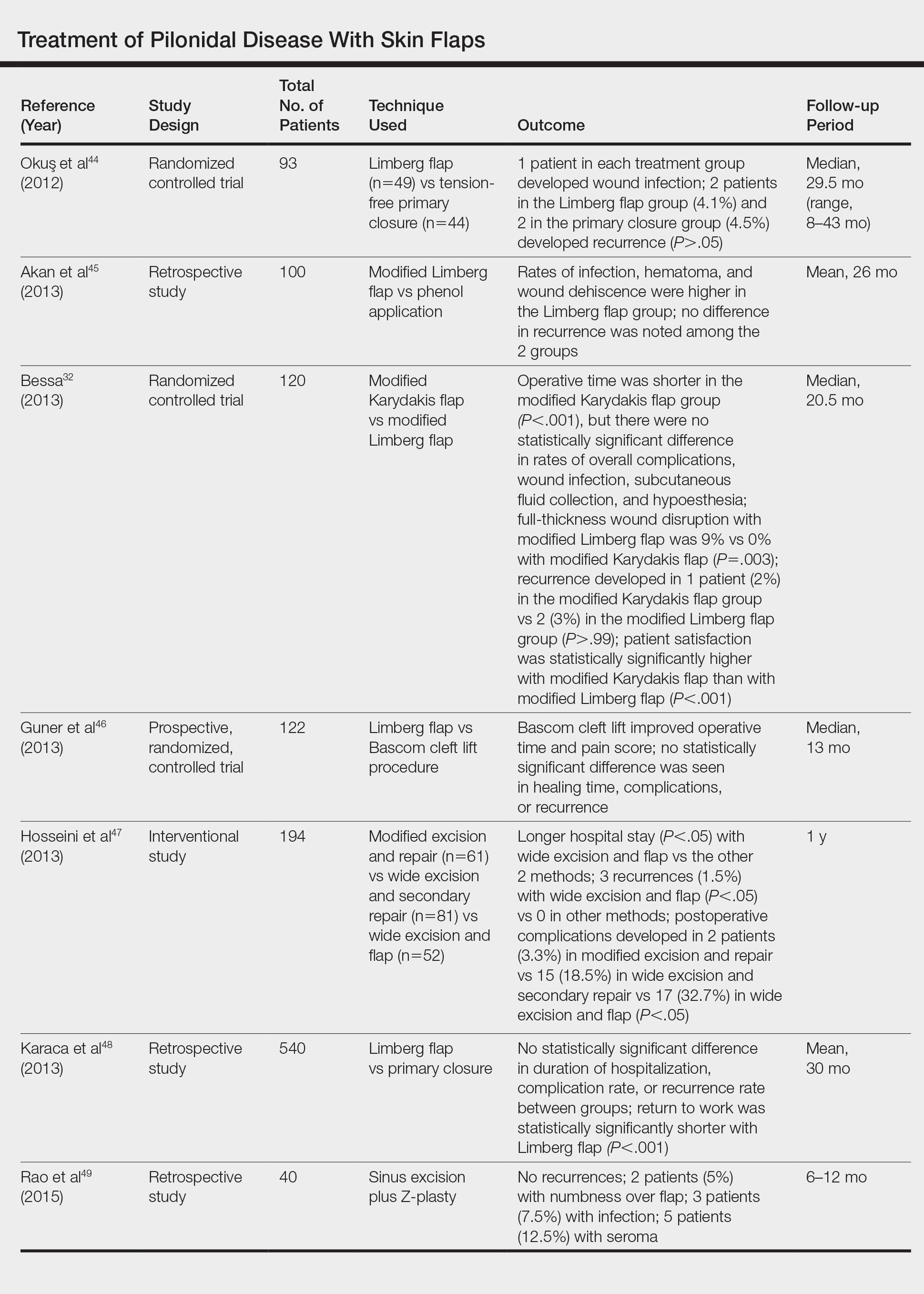
Laser Therapy
Lasers are emerging as primary and adjuvant treatment options for pilonidal sinuses. Depilation with alexandrite, diode, and Nd:YAG lasers has demonstrated the most consistent evidence.50-54 Th
Large randomized controlled trials are needed to fully determine the utility of laser therapy as a primary or adjuvant treatment in pilonidal disease; however, given that laser therapies address the core pathogenesis of pilonidal disease and generally are well tolerated, their use may be strongly considered.
Conclusion
With mild pilonidal disease, more conservative measures can be employed; however, in cases of recurrent or suppurative disease or extensive scarring, excision with flap closure typically is required. Although no single surgical procedure has been identified as superior, one review demonstrated that off-midline procedures are statistically superior to midline closure in healing time, surgical site infection, and recurrence rate.24 Novel techniques continue to emerge in the management of pilonidal disease, including laser therapy. This modality shows promise as either a primary or adjuvant treatment; however, large randomized controlled trials are needed to confirm early findings.
Given that pilonidal disease most commonly occurs in the actively employed population, we recommend that dermatologic surgeons discuss treatment options with patients who have pilonidal disease, taking into consideration cost, length of hospital stay, and recovery time when deciding on a treatment course.
- Mayo OH. Observations on Injuries and Diseases of the Rectum. London, England: Burgess and Hill; 1833.
- Hodges RM. Pilonidal sinus. Boston Med Surg J. 1880;103:485-486.
- Eryilmaz R, Okan I, Ozkan OV, et al. Interdigital pilonidal sinus: a case report and literature review. Dermatol Surg. 2012;38:1400-1403.
- Stone MS. Cysts with a lining of stratified epithelium. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Amsterdam, Netherlands: Elsevier Limited; 2012:1917-1929.
- Khanna A, Rombeau JL. Pilonidal disease. Clin Colon Rectal Surg. 2011;24:46-53.
- de Parades V, Bouchard D, Janier M, et al. Pilonidal sinus disease. J Visc Surg. 2013;150:237-247.
- Harris CL, Laforet K, Sibbald RG, et al. Twelve common mistakes in pilonidal sinus care. Adv Skin Wound Care. 2012;25:325-332.
- Lindholt-Jensen C, Lindholt J, Beyer M, et al. Nd-YAG laser treatment of primary and recurrent pilonidal sinus. Lasers Med Sci. 2012;27:505-508.
- Oueidat D, Rizkallah A, Dirani M, et al. 25 years’ experience in the management of pilonidal sinus disease. Open J Gastro. 2014;4:1-5.
- Gordon P, Grant L, Irwin T. Recurrent pilonidal sepsis. Ulster Med J. 2014;83:10-12.
- Ardelt M, Dittmar Y, Kocijan R, et al. Microbiology of the infected recurrent sacrococcygeal pilonidal sinus. Int Wound J. 2016;13:231-237.
- Eryilmaz R, Bilecik T, Okan I, et al. Recurrent squamous cell carcinoma arising in a neglected pilonidal sinus: report of a case and literature review. Int J Clin Exp Med. 2014;7:446-450.
- Kayaalp C, Aydin C. Review of phenol treatment in sacrococcygeal pilonidal disease. Tech Coloproctol. 2009;13:189-193.
- Dag A, Colak T, Turkmenoglu O, et al. Phenol procedure for pilonidal sinus disease and risk factors for treatment failure. Surgery. 2012;151:113-117.
- Olmez A, Kayaalp C, Aydin C. Treatment of pilonidal disease by combination of pit excision and phenol application. Tech Coloproctol. 2013;17:201-206.
- Jensen SL, Harling H. Prognosis after simple incision and drainage for a first-episode acute pilonidal abscess. Br J Surg. 1988;75:60-61.
- Kepenekci I, Demirkan A, Celasin H, et al. Unroofing and curettage for the treatment of acute and chronic pilonidal disease. World J Surg. 2010;34:153-157.
- Søndenaa K, Nesvik I, Anderson E, et al. Recurrent pilonidal sinus after excision with closed or open treatment: final results of a randomized trial. Eur J Surg. 1996;162:237-240.
- Spivak H, Brooks VL, Nussbaum M, et al. Treatment of chronic pilonidal disease. Dis Colon Rectum. 1996;39:1136-1139.
- Pilonidal surgery costs. Pilonidal Support Alliance website. https://www.pilonidal.org/treatments/surgical-costs/. Updated January 30, 2016. Accessed October 14, 2018.21. al-Hassan HK, Francis IM, Neglén P. Primary closure or secondary granulation after excision of pilonidal sinus? Acta Chir Scand. 1990;156:695-699.
- Khaira HS, Brown JH. Excision and primary suture of pilonidal sinus. Ann R Coll Surg Engl. 1995;77:242-244.
- Clothier PR, Haywood IR. The natural history of the post anal (pilonidal) sinus. Ann R Coll Surg Engl. 1984;66:201-203.
- Al-Khamis A, McCallum I, King PM, et al. Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database Syst Rev. 2010;1:CD006213.
- McCallum I, King PM, Bruce J. Healing by primary closure versus open healing after surgery for pilonidal sinus: systematic review and meta-analysis. BMJ. 2008;336:868-871.
- Lee PJ, Raniga S, Biyani DK, et al. Sacrococcygeal pilonidal disease. Colorect Dis. 2008;10:639-650.
- Nursal TZ, Ezer A, Calişkan K, et al. Prospective randomized controlled trial comparing V-Y advancement flaps with primary suture methods in pilonidal disease. Am J Surg. 2010;199:170-177.
- Fazeli MS, Adel MG, Lebaschi AH. Comparison of outcomes in Z-plasty and delayed healing by secondary intention of the wound after excision in the sacral pilonidal sinus: results of a randomized, clinical trial. Dis Col Rectum. 2006;49:1831-1836.
- Bascom JU. Repeat pilonidal operations. Am J Surg. 1987;154:118-122.
- Nordon IM, Senapati A, Cripps NP. A prospective randomized controlled trial of simple Bascom’s technique versus Bascom’s cleft closure in the treatment of chronic pilonidal disease. Am J Surg. 2009;197:189-192.
- Dudnik R, Veldkamp J, Nienhujis S, et al. Secondary healing versus midline closure and modified Bascom natal cleft lift for pilonidal sinus disease. Scand J Surg. 2011;100:110-113.
- Bessa SS. Comparison of short-term results between the modified Karydakis flap and the modified Limberg flap in the management of pilonidal sinus disease: a randomized controlled study. Dis Colon Rectum. 2013;56:491-498.
- Karydakis GE. Easy and successful treatment of pilonidal sinus after explanation of its causative process. Aust N Z J Surg. 1992;62:385-389.
- Kitchen PR. Pilonidal sinus: excision and primary closure with a lateralised wound - the Karydakis operation. Aust N Z J Surg. 1982;52:302-305.
- Akinci OF, Coskun A, Uzunköy A. Simple and effective surgical treatment of pilonidal sinus: asymmetric excision and primary closure using suction drain and subcuticular skin closure. Dis Colon Rectum. 2000;43:701-706.
- Bessa SS. Results of the lateral advancing flap operation (modified Karydakis procedure) for the management of pilonidal sinus disease. Dis Colon Rectum. 2007;50:1935-1940.
- Mentes BB, Leventoglu S, Chin A, et al. Modified Limberg transposition flap for sacrococcygeal pilonidal sinus. Surg Today. 2004;34:419-423.
- Cihan A, Ucan BH, Comert M, et al. Superiority of asymmetric modified Limberg flap for surgical treatment of pilonidal cyst disease. Dis Colon Rectum. 2006;49:244-249.
- Muzi MG, Milito G, Cadeddu F, et al. Randomized comparison of Limberg flap versus modified primary closure for treatment of pilonidal disease. Am J Surg. 2010;200:9-14.
- Tavassoli A, Noorshafiee S, Nazarzadeh R. Comparison of excision with primary repair versus Limberg flap. Int J Surg. 2011;9:343-346.
- Ates M, Dirican A, Sarac M, et al. Short and long-term results of the Karydakis flap versus the Limberg flap for treating pilonidal sinus disease: a prospective randomized study. Am J Surg. 2011;202:568-573.
- Can MF, Sevinc MM, Hancerliogullari O, et al. Multicenter prospective randomized trial comparing modified Limberg flap transposition and Karydakis flap reconstruction in patients with saccrococcygeal pilonidal disease. Am J Surg. 2010;200:318-327.
- Ersoy E, Devay AO, Aktimur R, et al. Comparison of short-term results after Limberg and Karydakis procedures for pilonidal disease: randomized prospective analysis of 100 patients. Colorectal Dis. 2009;11:705-710.
- Okuş A, Sevinç B, Karahan O, et al. Comparison of Limberg flap and tension-free primary closure during pilonidal sinus surgery. World J Surg. 2012;36:431-435.
- Akan K, Tihan D, Duman U, et al. Comparison of surgical Limberg flap technique and crystallized phenol application in the treatment of pilonidal sinus disease: a retrospective study. Ulus Cerrahi Derg. 2013;29:162-166.
- Guner A, Boz A, Ozkan OF, et al. Limberg flap versus Bascom cleft lift techniques for sacrococcygeal pilonidal sinus: prospective, randomized trial. World J Surg. 2013;37:2074-2080.
- Hosseini H, Heidari A, Jafarnejad B. Comparison of three surgical methods in treatment of patients with pilonidal sinus: modified excision and repair/wide excision/wide excision and flap in RASOUL, OMID and SADR hospitals (2004-2007). Indian J Surg. 2013;75:395-400.
- Karaca AS, Ali R, Capar M, et al. Comparison of Limberg flap and excision and primary closure of pilonidal sinus disease, in terms of quality of life and complications. J Korean Surg Soc. 2013;85:236-239.
- Rao J, Deora H, Mandia R. A retrospective study of 50 cases of pilonidal sinus with excision of tract and Z-plasty as treatment of choice for both primary and recurrent cases. Indian J Surg. 2015;77(suppl 2):691-693.
- Landa N, Aller O, Landa-Gundin N, et al. Successful treatment of recurrent pilonidal sinus with laser epilation. Dermatol Surg. 2005;31:726-728.
- Oram Y, Kahraman D, Karincaoğlu Y, et al. Evaluation of 60 patients with pilonidal sinus treated with laser epilation after surgery. Dermatol Surg. 2010;36:88-91.
- Benedetto AV, Lewis AT. Pilonidal sinus disease treated by depilation using an 800 nm diode laser and review of the literature. Dermatol Surg. 2005;31:587-591.
- Lindholt-Jensen CS, Lindholt JS, Beyer M, et al. Nd-YAG treatment of primary and recurrent pilonidal sinus. Lasers Med Sci. 2012;27:505-508.
- Jain V, Jain A. Use of lasers for the management of refractory cases of hidradenitis suppurativa and pilonidal sinus. J Cutan Aesthet. 2012;5:190-192.
Pilonidal disease was first described by Mayo1 in 1833 who hypothesized that the underlying etiology is incomplete separation of the mesoderm and ectoderm layers during embryogenesis. In 1880, Hodges2 coined the term pilonidal sinus; he postulated that sinus formation was incited by hair.2 Today, Hodges theory is known as the acquired theory: hair induces a foreign body response in surrounding tissue, leading to sinus formation. Although pilonidal cysts can occur anywhere on the body, they most commonly extend cephalad in the sacrococcygeal and upper gluteal cleft (Figure 1).3,4 An acute pilonidal cyst typically presents with pain, tenderness, and swelling, similar to the presentation of a superficial abscess in other locations; however, a clue to the diagnosis is the presence of cutaneous pits along the midline of the gluteal cleft.5 Chronic pilonidal disease varies based on the extent of inflammation and scarring; the underlying cavity communicates with the overlying skin through sinuses and often drains with pressure.6

Pilonidal sinuses are rare before puberty or after 40 years of age7 and occur primarily in hirsute men. The ratio of men to women affected is between 3:1 and 4:1.8 Although pilonidal sinuses account for only 15% of anal suppurations, complications arising from pilonidal sinuses are a considerable cause of morbidity, resulting in loss of productivity in otherwise healthy individuals.9 Complications include chronic nonhealing wounds,10 as recurrent pilonidal sinuses tend to become colonized with gram-positive and facultative anaerobic bacteria, whereas primary pilonidal cysts more commonly become infected with anaerobic and gram-negative bacteria.11 Long-standing disease increases the risk of squamous cell carcinoma arising within sinus tracts.10,12
Histopathologically, pilonidal cysts are not true cysts because they lack an epithelial lining. Examination of the cavity commonly reveals hair, debris, and granulation tissue with surrounding foreign-body giant cells (Figure 2).5

The preferred treatment of pilonidal cysts continues to be debated. In this article, we review evidence supporting current modalities including conservative and surgical techniques as well as novel laser therapy for the treatment of pilonidal disease.
Conservative Management Techniques
Phenol Injections
Liquid or crystallized phenol injections have been used for treatment of mild to moderate pilonidal cysts.13 Excess debris is removed by curettage, and phenol is administered through the existing orifices or pits without pressure. The phenol remains in the cavity for 1 to 3 minutes before aspiration. Remaining cyst contents are removed through tissue manipulation, and the sinus is washed with saline. Mean healing time is 20 days (range, +/−14 days).13
Classically, phenol injections have a failure rate of 30% to 40%, especially with multiple sinuses and suppurative disease6; however, the success rate improves with limited disease (ie, no more than 1–3 sinus pits).3 With multiple treatment sessions, a recurrence rate as low as 2% over 25 months has been reported.14 Phenol injection also has been proposed as an adjuvant therapy to pit excision to minimize the need for extensive surgery.15
Simple Incision and Drainage
Simple incision and drainage has a crucial role in the treatment of acute pilonidal disease to decrease pain and relieve tension. Off-midline incisions have been recommended for because the resulting closures fared better against sheer forces applied by the gluteal muscles on the cleft.6 Therefore, the incision often is made off-midline from the gluteal cleft even when the cyst lies directly on the gluteal cleft.
Rates of healing vary widely after incision and drainage, ranging from 45% to 82%.6 Primary pilonidal cysts may respond well, particularly if the cavity is abraded; in one series, 79% (58/73) of patients did not have a recurrence at the average follow-up of 60 months.16
Excision and Unroofing
Techniques for excision and unroofing without primary closure include 2 variants: wide and limited. The wide technique consists of an inwardly slanted excision that is deepest in the center of the cavity. The inward sloping angle of the incision aids in healing because it allows granulation to progress evenly from the base of the wound upward. The depth of the incision should spare the fascia and leave as much fatty tissue as possible while still resecting the entire cavity and associated pits.6 Limited incision techniques aim to shorten the healing period by making smaller incisions into the sinuses, pits, and secondary tracts, and they are frequently supplemented with curettage.6 Noteworthy disadvantages include prolonged healing time, need for professional wound management, and extended medical observation.5 The average duration of wound healing in a study of 300 patients was 5.4 weeks (range, +/−1.1 weeks),17 and the recurrence rate has ranged from 5% to 13%.18,19 Care must be taken to respond to numerous possible complications, including excessive exudation and granulation, superinfection, and walling off.6
Although the cost of treatment varies by hospital, location, and a patient’s insurance coverage, patient reports to the Pilonidal Support Alliance indicate that the cost of conservative management ranges from $500 to $2000.20
Excision and Primary Closure
An elliptical excision that includes some of the lateral margin is excised down to the level of the fascia. Adjacent lateral tracts may be excised by expanding the incision. To close the wound, edges are approximated with placement of deep and superficial sutures. Wound healing typically occurs faster than secondary granulation, as seen in one randomized controlled trial with a mean of 10 days for primary closure compared to 13 weeks for secondary intention.21 However, as with any surgical procedure, postoperative complications can delay wound healing.19 The recurrence rate after primary closure varies considerably, ranging from 10% to 38%.18,21-23 The average cost of an excision ranges from $3000 to $6000.20
A

Surgical Techniques
For severe or recurrent pilonidal disease, skin flaps often are required. Several flaps have been developed, including advancement, Bascom cleft lift, Karydakis, and modified Limberg flap. Flaps require a vascular pedicle but allow for closure without tension.26 The cost of a flap procedure, ranging from $10,000 to $30,000, is greater than the cost of excision or other conservative therapy20; however, with a lower recurrence rate of pilonidal disease following flap procedures compared to other treatments, patients may save more on treatment over the long-term.
Advancement Flaps
The most commonly used advancement flaps are the V-Y advancement flap and Z-plasty. The V-Y advancement flap creates a full-thickness V-shaped incision down to gluteal fascia that is closed to form a postrepair suture line in the shape of a Y.5 Depending on the size of the defect, the flaps may be utilized unilaterally or bilaterally. A defect as large as 8 to 10 cm can be covered unilaterally; however, defects larger than 10 cm commonly require a bilateral flap.26 The V-Y advancement flap failed to show superiority to primary closure techniques based on complications, recurrence, and patient satisfaction in a large randomized controlled trial.27
Performing a Z-plasty requires excision of diseased tissue with recruitment of lateral flaps incised down to the level of the fascia. The lateral edges are transposed to increase transverse length.26 No statistically significant difference in infection or recurrence rates was noted between excision alone and excision plus Z-plasty; however, wounds were reported to heal faster in patients receiving excision plus Z-plasty (41 vs 15 days).28
Cleft Lift Closure
In 1987, Bascom29 introduced the cleft lift closure for recurrent pilonidal disease. This technique aims to reduce or eliminate lateral gluteal forces on the wounds by filling the gluteal cleft.5 The sinus tracts are excised and a full-thickness skin flap is extended across the cleft and closed off-midline. The adipose tissue fills in the previous space of the gluteal cleft. In the initial study, no recurrences were reported in 30 patients who underwent this procedure at 2-year follow-up; similarly, in another case series of 26 patients who underwent the procedure, no recurrences were noted at a median follow-up of 3 years.30 Compared to excision with secondary wound healing and primary closure on the midline, the Bascom cleft lift demonstrated a decrease in wound healing time (62, 52, and 29 days, respectively).31
The classic Karydakis flap consists of an oblique elliptical excision of diseased tissue with fixation of the flap base to the sacral fascia (Figures 4 and 5). The flap is closed by suturing the edge off-midline.32 This technique prevents a midline wound and aims to remodel and flatten the natal cleft. Karydakis33 performed the most important study for treatment of pilonidal disease with the Karydakis flap, which included more than 5000 patients. The results showed a 0.9% recurrence rate and an 8.5% wound complication rate over a 2- to 20-year follow-up.33 These results have been substantiated by more recent studies, which produced similar results: a 1.8% to 5.3% infection rate and a recurrence rate of 0.9% to 4.4%.34,35


In the modified Karydakis flap, the same excision and closure is performed without tacking the flap to the sacral fascia, aiming to prevent formation of a new vulnerable raphe by flattening the natal cleft. The infection rate was similar to the classic Karydakis flap, and no recurrences were noted during a 20-month follow-up.36
Limberg Flap
The Limberg flap is derived from a rhomboid flap. In the classic Limberg flap, a midline rhomboid incision to the presacral fascia including the sinus is performed. The flap gains mobility by extending the excision laterally to the fascia of the gluteus maximus muscle. A variant of the original flap includes the modified Limberg flap, which lateralizes the midline sutures and flattens the intergluteal sulcus. Compared to the traditional Limberg approach, the modified Limberg flap was associated with a lower failure rate at both early and late time points and a lower rate of infection37,38; however, based on the data it is unclear when primary closure should be favored over a Limberg flap. Several studies show the recurrence rate to be identical; however, hospital stay and pain were reduced in the Limberg flap group compared to primary closure.39,40
Results from randomized controlled trials comparing the modified Limberg flap to the Karydakis flap vary. One of the largest prospective, randomized, controlled trials comparing the 2 flaps included 269 patients.Results showed a lower postoperative complication rate, lower pain scores, shorter operation time, and shorter hospital stay with the Karydakis flap compared to the Limberg flap, though no difference in recurrence was noted between the 2 groups.41
Tw
Overall, larger prospective trials are needed to clarify the differences in outcomes between flap techniques. In

Laser Therapy
Lasers are emerging as primary and adjuvant treatment options for pilonidal sinuses. Depilation with alexandrite, diode, and Nd:YAG lasers has demonstrated the most consistent evidence.50-54 Th
Large randomized controlled trials are needed to fully determine the utility of laser therapy as a primary or adjuvant treatment in pilonidal disease; however, given that laser therapies address the core pathogenesis of pilonidal disease and generally are well tolerated, their use may be strongly considered.
Conclusion
With mild pilonidal disease, more conservative measures can be employed; however, in cases of recurrent or suppurative disease or extensive scarring, excision with flap closure typically is required. Although no single surgical procedure has been identified as superior, one review demonstrated that off-midline procedures are statistically superior to midline closure in healing time, surgical site infection, and recurrence rate.24 Novel techniques continue to emerge in the management of pilonidal disease, including laser therapy. This modality shows promise as either a primary or adjuvant treatment; however, large randomized controlled trials are needed to confirm early findings.
Given that pilonidal disease most commonly occurs in the actively employed population, we recommend that dermatologic surgeons discuss treatment options with patients who have pilonidal disease, taking into consideration cost, length of hospital stay, and recovery time when deciding on a treatment course.
Pilonidal disease was first described by Mayo1 in 1833 who hypothesized that the underlying etiology is incomplete separation of the mesoderm and ectoderm layers during embryogenesis. In 1880, Hodges2 coined the term pilonidal sinus; he postulated that sinus formation was incited by hair.2 Today, Hodges theory is known as the acquired theory: hair induces a foreign body response in surrounding tissue, leading to sinus formation. Although pilonidal cysts can occur anywhere on the body, they most commonly extend cephalad in the sacrococcygeal and upper gluteal cleft (Figure 1).3,4 An acute pilonidal cyst typically presents with pain, tenderness, and swelling, similar to the presentation of a superficial abscess in other locations; however, a clue to the diagnosis is the presence of cutaneous pits along the midline of the gluteal cleft.5 Chronic pilonidal disease varies based on the extent of inflammation and scarring; the underlying cavity communicates with the overlying skin through sinuses and often drains with pressure.6

Pilonidal sinuses are rare before puberty or after 40 years of age7 and occur primarily in hirsute men. The ratio of men to women affected is between 3:1 and 4:1.8 Although pilonidal sinuses account for only 15% of anal suppurations, complications arising from pilonidal sinuses are a considerable cause of morbidity, resulting in loss of productivity in otherwise healthy individuals.9 Complications include chronic nonhealing wounds,10 as recurrent pilonidal sinuses tend to become colonized with gram-positive and facultative anaerobic bacteria, whereas primary pilonidal cysts more commonly become infected with anaerobic and gram-negative bacteria.11 Long-standing disease increases the risk of squamous cell carcinoma arising within sinus tracts.10,12
Histopathologically, pilonidal cysts are not true cysts because they lack an epithelial lining. Examination of the cavity commonly reveals hair, debris, and granulation tissue with surrounding foreign-body giant cells (Figure 2).5

The preferred treatment of pilonidal cysts continues to be debated. In this article, we review evidence supporting current modalities including conservative and surgical techniques as well as novel laser therapy for the treatment of pilonidal disease.
Conservative Management Techniques
Phenol Injections
Liquid or crystallized phenol injections have been used for treatment of mild to moderate pilonidal cysts.13 Excess debris is removed by curettage, and phenol is administered through the existing orifices or pits without pressure. The phenol remains in the cavity for 1 to 3 minutes before aspiration. Remaining cyst contents are removed through tissue manipulation, and the sinus is washed with saline. Mean healing time is 20 days (range, +/−14 days).13
Classically, phenol injections have a failure rate of 30% to 40%, especially with multiple sinuses and suppurative disease6; however, the success rate improves with limited disease (ie, no more than 1–3 sinus pits).3 With multiple treatment sessions, a recurrence rate as low as 2% over 25 months has been reported.14 Phenol injection also has been proposed as an adjuvant therapy to pit excision to minimize the need for extensive surgery.15
Simple Incision and Drainage
Simple incision and drainage has a crucial role in the treatment of acute pilonidal disease to decrease pain and relieve tension. Off-midline incisions have been recommended for because the resulting closures fared better against sheer forces applied by the gluteal muscles on the cleft.6 Therefore, the incision often is made off-midline from the gluteal cleft even when the cyst lies directly on the gluteal cleft.
Rates of healing vary widely after incision and drainage, ranging from 45% to 82%.6 Primary pilonidal cysts may respond well, particularly if the cavity is abraded; in one series, 79% (58/73) of patients did not have a recurrence at the average follow-up of 60 months.16
Excision and Unroofing
Techniques for excision and unroofing without primary closure include 2 variants: wide and limited. The wide technique consists of an inwardly slanted excision that is deepest in the center of the cavity. The inward sloping angle of the incision aids in healing because it allows granulation to progress evenly from the base of the wound upward. The depth of the incision should spare the fascia and leave as much fatty tissue as possible while still resecting the entire cavity and associated pits.6 Limited incision techniques aim to shorten the healing period by making smaller incisions into the sinuses, pits, and secondary tracts, and they are frequently supplemented with curettage.6 Noteworthy disadvantages include prolonged healing time, need for professional wound management, and extended medical observation.5 The average duration of wound healing in a study of 300 patients was 5.4 weeks (range, +/−1.1 weeks),17 and the recurrence rate has ranged from 5% to 13%.18,19 Care must be taken to respond to numerous possible complications, including excessive exudation and granulation, superinfection, and walling off.6
Although the cost of treatment varies by hospital, location, and a patient’s insurance coverage, patient reports to the Pilonidal Support Alliance indicate that the cost of conservative management ranges from $500 to $2000.20
Excision and Primary Closure
An elliptical excision that includes some of the lateral margin is excised down to the level of the fascia. Adjacent lateral tracts may be excised by expanding the incision. To close the wound, edges are approximated with placement of deep and superficial sutures. Wound healing typically occurs faster than secondary granulation, as seen in one randomized controlled trial with a mean of 10 days for primary closure compared to 13 weeks for secondary intention.21 However, as with any surgical procedure, postoperative complications can delay wound healing.19 The recurrence rate after primary closure varies considerably, ranging from 10% to 38%.18,21-23 The average cost of an excision ranges from $3000 to $6000.20
A

Surgical Techniques
For severe or recurrent pilonidal disease, skin flaps often are required. Several flaps have been developed, including advancement, Bascom cleft lift, Karydakis, and modified Limberg flap. Flaps require a vascular pedicle but allow for closure without tension.26 The cost of a flap procedure, ranging from $10,000 to $30,000, is greater than the cost of excision or other conservative therapy20; however, with a lower recurrence rate of pilonidal disease following flap procedures compared to other treatments, patients may save more on treatment over the long-term.
Advancement Flaps
The most commonly used advancement flaps are the V-Y advancement flap and Z-plasty. The V-Y advancement flap creates a full-thickness V-shaped incision down to gluteal fascia that is closed to form a postrepair suture line in the shape of a Y.5 Depending on the size of the defect, the flaps may be utilized unilaterally or bilaterally. A defect as large as 8 to 10 cm can be covered unilaterally; however, defects larger than 10 cm commonly require a bilateral flap.26 The V-Y advancement flap failed to show superiority to primary closure techniques based on complications, recurrence, and patient satisfaction in a large randomized controlled trial.27
Performing a Z-plasty requires excision of diseased tissue with recruitment of lateral flaps incised down to the level of the fascia. The lateral edges are transposed to increase transverse length.26 No statistically significant difference in infection or recurrence rates was noted between excision alone and excision plus Z-plasty; however, wounds were reported to heal faster in patients receiving excision plus Z-plasty (41 vs 15 days).28
Cleft Lift Closure
In 1987, Bascom29 introduced the cleft lift closure for recurrent pilonidal disease. This technique aims to reduce or eliminate lateral gluteal forces on the wounds by filling the gluteal cleft.5 The sinus tracts are excised and a full-thickness skin flap is extended across the cleft and closed off-midline. The adipose tissue fills in the previous space of the gluteal cleft. In the initial study, no recurrences were reported in 30 patients who underwent this procedure at 2-year follow-up; similarly, in another case series of 26 patients who underwent the procedure, no recurrences were noted at a median follow-up of 3 years.30 Compared to excision with secondary wound healing and primary closure on the midline, the Bascom cleft lift demonstrated a decrease in wound healing time (62, 52, and 29 days, respectively).31
The classic Karydakis flap consists of an oblique elliptical excision of diseased tissue with fixation of the flap base to the sacral fascia (Figures 4 and 5). The flap is closed by suturing the edge off-midline.32 This technique prevents a midline wound and aims to remodel and flatten the natal cleft. Karydakis33 performed the most important study for treatment of pilonidal disease with the Karydakis flap, which included more than 5000 patients. The results showed a 0.9% recurrence rate and an 8.5% wound complication rate over a 2- to 20-year follow-up.33 These results have been substantiated by more recent studies, which produced similar results: a 1.8% to 5.3% infection rate and a recurrence rate of 0.9% to 4.4%.34,35


In the modified Karydakis flap, the same excision and closure is performed without tacking the flap to the sacral fascia, aiming to prevent formation of a new vulnerable raphe by flattening the natal cleft. The infection rate was similar to the classic Karydakis flap, and no recurrences were noted during a 20-month follow-up.36
Limberg Flap
The Limberg flap is derived from a rhomboid flap. In the classic Limberg flap, a midline rhomboid incision to the presacral fascia including the sinus is performed. The flap gains mobility by extending the excision laterally to the fascia of the gluteus maximus muscle. A variant of the original flap includes the modified Limberg flap, which lateralizes the midline sutures and flattens the intergluteal sulcus. Compared to the traditional Limberg approach, the modified Limberg flap was associated with a lower failure rate at both early and late time points and a lower rate of infection37,38; however, based on the data it is unclear when primary closure should be favored over a Limberg flap. Several studies show the recurrence rate to be identical; however, hospital stay and pain were reduced in the Limberg flap group compared to primary closure.39,40
Results from randomized controlled trials comparing the modified Limberg flap to the Karydakis flap vary. One of the largest prospective, randomized, controlled trials comparing the 2 flaps included 269 patients.Results showed a lower postoperative complication rate, lower pain scores, shorter operation time, and shorter hospital stay with the Karydakis flap compared to the Limberg flap, though no difference in recurrence was noted between the 2 groups.41
Tw
Overall, larger prospective trials are needed to clarify the differences in outcomes between flap techniques. In

Laser Therapy
Lasers are emerging as primary and adjuvant treatment options for pilonidal sinuses. Depilation with alexandrite, diode, and Nd:YAG lasers has demonstrated the most consistent evidence.50-54 Th
Large randomized controlled trials are needed to fully determine the utility of laser therapy as a primary or adjuvant treatment in pilonidal disease; however, given that laser therapies address the core pathogenesis of pilonidal disease and generally are well tolerated, their use may be strongly considered.
Conclusion
With mild pilonidal disease, more conservative measures can be employed; however, in cases of recurrent or suppurative disease or extensive scarring, excision with flap closure typically is required. Although no single surgical procedure has been identified as superior, one review demonstrated that off-midline procedures are statistically superior to midline closure in healing time, surgical site infection, and recurrence rate.24 Novel techniques continue to emerge in the management of pilonidal disease, including laser therapy. This modality shows promise as either a primary or adjuvant treatment; however, large randomized controlled trials are needed to confirm early findings.
Given that pilonidal disease most commonly occurs in the actively employed population, we recommend that dermatologic surgeons discuss treatment options with patients who have pilonidal disease, taking into consideration cost, length of hospital stay, and recovery time when deciding on a treatment course.
- Mayo OH. Observations on Injuries and Diseases of the Rectum. London, England: Burgess and Hill; 1833.
- Hodges RM. Pilonidal sinus. Boston Med Surg J. 1880;103:485-486.
- Eryilmaz R, Okan I, Ozkan OV, et al. Interdigital pilonidal sinus: a case report and literature review. Dermatol Surg. 2012;38:1400-1403.
- Stone MS. Cysts with a lining of stratified epithelium. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Amsterdam, Netherlands: Elsevier Limited; 2012:1917-1929.
- Khanna A, Rombeau JL. Pilonidal disease. Clin Colon Rectal Surg. 2011;24:46-53.
- de Parades V, Bouchard D, Janier M, et al. Pilonidal sinus disease. J Visc Surg. 2013;150:237-247.
- Harris CL, Laforet K, Sibbald RG, et al. Twelve common mistakes in pilonidal sinus care. Adv Skin Wound Care. 2012;25:325-332.
- Lindholt-Jensen C, Lindholt J, Beyer M, et al. Nd-YAG laser treatment of primary and recurrent pilonidal sinus. Lasers Med Sci. 2012;27:505-508.
- Oueidat D, Rizkallah A, Dirani M, et al. 25 years’ experience in the management of pilonidal sinus disease. Open J Gastro. 2014;4:1-5.
- Gordon P, Grant L, Irwin T. Recurrent pilonidal sepsis. Ulster Med J. 2014;83:10-12.
- Ardelt M, Dittmar Y, Kocijan R, et al. Microbiology of the infected recurrent sacrococcygeal pilonidal sinus. Int Wound J. 2016;13:231-237.
- Eryilmaz R, Bilecik T, Okan I, et al. Recurrent squamous cell carcinoma arising in a neglected pilonidal sinus: report of a case and literature review. Int J Clin Exp Med. 2014;7:446-450.
- Kayaalp C, Aydin C. Review of phenol treatment in sacrococcygeal pilonidal disease. Tech Coloproctol. 2009;13:189-193.
- Dag A, Colak T, Turkmenoglu O, et al. Phenol procedure for pilonidal sinus disease and risk factors for treatment failure. Surgery. 2012;151:113-117.
- Olmez A, Kayaalp C, Aydin C. Treatment of pilonidal disease by combination of pit excision and phenol application. Tech Coloproctol. 2013;17:201-206.
- Jensen SL, Harling H. Prognosis after simple incision and drainage for a first-episode acute pilonidal abscess. Br J Surg. 1988;75:60-61.
- Kepenekci I, Demirkan A, Celasin H, et al. Unroofing and curettage for the treatment of acute and chronic pilonidal disease. World J Surg. 2010;34:153-157.
- Søndenaa K, Nesvik I, Anderson E, et al. Recurrent pilonidal sinus after excision with closed or open treatment: final results of a randomized trial. Eur J Surg. 1996;162:237-240.
- Spivak H, Brooks VL, Nussbaum M, et al. Treatment of chronic pilonidal disease. Dis Colon Rectum. 1996;39:1136-1139.
- Pilonidal surgery costs. Pilonidal Support Alliance website. https://www.pilonidal.org/treatments/surgical-costs/. Updated January 30, 2016. Accessed October 14, 2018.21. al-Hassan HK, Francis IM, Neglén P. Primary closure or secondary granulation after excision of pilonidal sinus? Acta Chir Scand. 1990;156:695-699.
- Khaira HS, Brown JH. Excision and primary suture of pilonidal sinus. Ann R Coll Surg Engl. 1995;77:242-244.
- Clothier PR, Haywood IR. The natural history of the post anal (pilonidal) sinus. Ann R Coll Surg Engl. 1984;66:201-203.
- Al-Khamis A, McCallum I, King PM, et al. Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database Syst Rev. 2010;1:CD006213.
- McCallum I, King PM, Bruce J. Healing by primary closure versus open healing after surgery for pilonidal sinus: systematic review and meta-analysis. BMJ. 2008;336:868-871.
- Lee PJ, Raniga S, Biyani DK, et al. Sacrococcygeal pilonidal disease. Colorect Dis. 2008;10:639-650.
- Nursal TZ, Ezer A, Calişkan K, et al. Prospective randomized controlled trial comparing V-Y advancement flaps with primary suture methods in pilonidal disease. Am J Surg. 2010;199:170-177.
- Fazeli MS, Adel MG, Lebaschi AH. Comparison of outcomes in Z-plasty and delayed healing by secondary intention of the wound after excision in the sacral pilonidal sinus: results of a randomized, clinical trial. Dis Col Rectum. 2006;49:1831-1836.
- Bascom JU. Repeat pilonidal operations. Am J Surg. 1987;154:118-122.
- Nordon IM, Senapati A, Cripps NP. A prospective randomized controlled trial of simple Bascom’s technique versus Bascom’s cleft closure in the treatment of chronic pilonidal disease. Am J Surg. 2009;197:189-192.
- Dudnik R, Veldkamp J, Nienhujis S, et al. Secondary healing versus midline closure and modified Bascom natal cleft lift for pilonidal sinus disease. Scand J Surg. 2011;100:110-113.
- Bessa SS. Comparison of short-term results between the modified Karydakis flap and the modified Limberg flap in the management of pilonidal sinus disease: a randomized controlled study. Dis Colon Rectum. 2013;56:491-498.
- Karydakis GE. Easy and successful treatment of pilonidal sinus after explanation of its causative process. Aust N Z J Surg. 1992;62:385-389.
- Kitchen PR. Pilonidal sinus: excision and primary closure with a lateralised wound - the Karydakis operation. Aust N Z J Surg. 1982;52:302-305.
- Akinci OF, Coskun A, Uzunköy A. Simple and effective surgical treatment of pilonidal sinus: asymmetric excision and primary closure using suction drain and subcuticular skin closure. Dis Colon Rectum. 2000;43:701-706.
- Bessa SS. Results of the lateral advancing flap operation (modified Karydakis procedure) for the management of pilonidal sinus disease. Dis Colon Rectum. 2007;50:1935-1940.
- Mentes BB, Leventoglu S, Chin A, et al. Modified Limberg transposition flap for sacrococcygeal pilonidal sinus. Surg Today. 2004;34:419-423.
- Cihan A, Ucan BH, Comert M, et al. Superiority of asymmetric modified Limberg flap for surgical treatment of pilonidal cyst disease. Dis Colon Rectum. 2006;49:244-249.
- Muzi MG, Milito G, Cadeddu F, et al. Randomized comparison of Limberg flap versus modified primary closure for treatment of pilonidal disease. Am J Surg. 2010;200:9-14.
- Tavassoli A, Noorshafiee S, Nazarzadeh R. Comparison of excision with primary repair versus Limberg flap. Int J Surg. 2011;9:343-346.
- Ates M, Dirican A, Sarac M, et al. Short and long-term results of the Karydakis flap versus the Limberg flap for treating pilonidal sinus disease: a prospective randomized study. Am J Surg. 2011;202:568-573.
- Can MF, Sevinc MM, Hancerliogullari O, et al. Multicenter prospective randomized trial comparing modified Limberg flap transposition and Karydakis flap reconstruction in patients with saccrococcygeal pilonidal disease. Am J Surg. 2010;200:318-327.
- Ersoy E, Devay AO, Aktimur R, et al. Comparison of short-term results after Limberg and Karydakis procedures for pilonidal disease: randomized prospective analysis of 100 patients. Colorectal Dis. 2009;11:705-710.
- Okuş A, Sevinç B, Karahan O, et al. Comparison of Limberg flap and tension-free primary closure during pilonidal sinus surgery. World J Surg. 2012;36:431-435.
- Akan K, Tihan D, Duman U, et al. Comparison of surgical Limberg flap technique and crystallized phenol application in the treatment of pilonidal sinus disease: a retrospective study. Ulus Cerrahi Derg. 2013;29:162-166.
- Guner A, Boz A, Ozkan OF, et al. Limberg flap versus Bascom cleft lift techniques for sacrococcygeal pilonidal sinus: prospective, randomized trial. World J Surg. 2013;37:2074-2080.
- Hosseini H, Heidari A, Jafarnejad B. Comparison of three surgical methods in treatment of patients with pilonidal sinus: modified excision and repair/wide excision/wide excision and flap in RASOUL, OMID and SADR hospitals (2004-2007). Indian J Surg. 2013;75:395-400.
- Karaca AS, Ali R, Capar M, et al. Comparison of Limberg flap and excision and primary closure of pilonidal sinus disease, in terms of quality of life and complications. J Korean Surg Soc. 2013;85:236-239.
- Rao J, Deora H, Mandia R. A retrospective study of 50 cases of pilonidal sinus with excision of tract and Z-plasty as treatment of choice for both primary and recurrent cases. Indian J Surg. 2015;77(suppl 2):691-693.
- Landa N, Aller O, Landa-Gundin N, et al. Successful treatment of recurrent pilonidal sinus with laser epilation. Dermatol Surg. 2005;31:726-728.
- Oram Y, Kahraman D, Karincaoğlu Y, et al. Evaluation of 60 patients with pilonidal sinus treated with laser epilation after surgery. Dermatol Surg. 2010;36:88-91.
- Benedetto AV, Lewis AT. Pilonidal sinus disease treated by depilation using an 800 nm diode laser and review of the literature. Dermatol Surg. 2005;31:587-591.
- Lindholt-Jensen CS, Lindholt JS, Beyer M, et al. Nd-YAG treatment of primary and recurrent pilonidal sinus. Lasers Med Sci. 2012;27:505-508.
- Jain V, Jain A. Use of lasers for the management of refractory cases of hidradenitis suppurativa and pilonidal sinus. J Cutan Aesthet. 2012;5:190-192.
- Mayo OH. Observations on Injuries and Diseases of the Rectum. London, England: Burgess and Hill; 1833.
- Hodges RM. Pilonidal sinus. Boston Med Surg J. 1880;103:485-486.
- Eryilmaz R, Okan I, Ozkan OV, et al. Interdigital pilonidal sinus: a case report and literature review. Dermatol Surg. 2012;38:1400-1403.
- Stone MS. Cysts with a lining of stratified epithelium. In: Bolognia JL, Jorizzo JL, Schaffer JV, eds. Dermatology. 3rd ed. Amsterdam, Netherlands: Elsevier Limited; 2012:1917-1929.
- Khanna A, Rombeau JL. Pilonidal disease. Clin Colon Rectal Surg. 2011;24:46-53.
- de Parades V, Bouchard D, Janier M, et al. Pilonidal sinus disease. J Visc Surg. 2013;150:237-247.
- Harris CL, Laforet K, Sibbald RG, et al. Twelve common mistakes in pilonidal sinus care. Adv Skin Wound Care. 2012;25:325-332.
- Lindholt-Jensen C, Lindholt J, Beyer M, et al. Nd-YAG laser treatment of primary and recurrent pilonidal sinus. Lasers Med Sci. 2012;27:505-508.
- Oueidat D, Rizkallah A, Dirani M, et al. 25 years’ experience in the management of pilonidal sinus disease. Open J Gastro. 2014;4:1-5.
- Gordon P, Grant L, Irwin T. Recurrent pilonidal sepsis. Ulster Med J. 2014;83:10-12.
- Ardelt M, Dittmar Y, Kocijan R, et al. Microbiology of the infected recurrent sacrococcygeal pilonidal sinus. Int Wound J. 2016;13:231-237.
- Eryilmaz R, Bilecik T, Okan I, et al. Recurrent squamous cell carcinoma arising in a neglected pilonidal sinus: report of a case and literature review. Int J Clin Exp Med. 2014;7:446-450.
- Kayaalp C, Aydin C. Review of phenol treatment in sacrococcygeal pilonidal disease. Tech Coloproctol. 2009;13:189-193.
- Dag A, Colak T, Turkmenoglu O, et al. Phenol procedure for pilonidal sinus disease and risk factors for treatment failure. Surgery. 2012;151:113-117.
- Olmez A, Kayaalp C, Aydin C. Treatment of pilonidal disease by combination of pit excision and phenol application. Tech Coloproctol. 2013;17:201-206.
- Jensen SL, Harling H. Prognosis after simple incision and drainage for a first-episode acute pilonidal abscess. Br J Surg. 1988;75:60-61.
- Kepenekci I, Demirkan A, Celasin H, et al. Unroofing and curettage for the treatment of acute and chronic pilonidal disease. World J Surg. 2010;34:153-157.
- Søndenaa K, Nesvik I, Anderson E, et al. Recurrent pilonidal sinus after excision with closed or open treatment: final results of a randomized trial. Eur J Surg. 1996;162:237-240.
- Spivak H, Brooks VL, Nussbaum M, et al. Treatment of chronic pilonidal disease. Dis Colon Rectum. 1996;39:1136-1139.
- Pilonidal surgery costs. Pilonidal Support Alliance website. https://www.pilonidal.org/treatments/surgical-costs/. Updated January 30, 2016. Accessed October 14, 2018.21. al-Hassan HK, Francis IM, Neglén P. Primary closure or secondary granulation after excision of pilonidal sinus? Acta Chir Scand. 1990;156:695-699.
- Khaira HS, Brown JH. Excision and primary suture of pilonidal sinus. Ann R Coll Surg Engl. 1995;77:242-244.
- Clothier PR, Haywood IR. The natural history of the post anal (pilonidal) sinus. Ann R Coll Surg Engl. 1984;66:201-203.
- Al-Khamis A, McCallum I, King PM, et al. Healing by primary versus secondary intention after surgical treatment for pilonidal sinus. Cochrane Database Syst Rev. 2010;1:CD006213.
- McCallum I, King PM, Bruce J. Healing by primary closure versus open healing after surgery for pilonidal sinus: systematic review and meta-analysis. BMJ. 2008;336:868-871.
- Lee PJ, Raniga S, Biyani DK, et al. Sacrococcygeal pilonidal disease. Colorect Dis. 2008;10:639-650.
- Nursal TZ, Ezer A, Calişkan K, et al. Prospective randomized controlled trial comparing V-Y advancement flaps with primary suture methods in pilonidal disease. Am J Surg. 2010;199:170-177.
- Fazeli MS, Adel MG, Lebaschi AH. Comparison of outcomes in Z-plasty and delayed healing by secondary intention of the wound after excision in the sacral pilonidal sinus: results of a randomized, clinical trial. Dis Col Rectum. 2006;49:1831-1836.
- Bascom JU. Repeat pilonidal operations. Am J Surg. 1987;154:118-122.
- Nordon IM, Senapati A, Cripps NP. A prospective randomized controlled trial of simple Bascom’s technique versus Bascom’s cleft closure in the treatment of chronic pilonidal disease. Am J Surg. 2009;197:189-192.
- Dudnik R, Veldkamp J, Nienhujis S, et al. Secondary healing versus midline closure and modified Bascom natal cleft lift for pilonidal sinus disease. Scand J Surg. 2011;100:110-113.
- Bessa SS. Comparison of short-term results between the modified Karydakis flap and the modified Limberg flap in the management of pilonidal sinus disease: a randomized controlled study. Dis Colon Rectum. 2013;56:491-498.
- Karydakis GE. Easy and successful treatment of pilonidal sinus after explanation of its causative process. Aust N Z J Surg. 1992;62:385-389.
- Kitchen PR. Pilonidal sinus: excision and primary closure with a lateralised wound - the Karydakis operation. Aust N Z J Surg. 1982;52:302-305.
- Akinci OF, Coskun A, Uzunköy A. Simple and effective surgical treatment of pilonidal sinus: asymmetric excision and primary closure using suction drain and subcuticular skin closure. Dis Colon Rectum. 2000;43:701-706.
- Bessa SS. Results of the lateral advancing flap operation (modified Karydakis procedure) for the management of pilonidal sinus disease. Dis Colon Rectum. 2007;50:1935-1940.
- Mentes BB, Leventoglu S, Chin A, et al. Modified Limberg transposition flap for sacrococcygeal pilonidal sinus. Surg Today. 2004;34:419-423.
- Cihan A, Ucan BH, Comert M, et al. Superiority of asymmetric modified Limberg flap for surgical treatment of pilonidal cyst disease. Dis Colon Rectum. 2006;49:244-249.
- Muzi MG, Milito G, Cadeddu F, et al. Randomized comparison of Limberg flap versus modified primary closure for treatment of pilonidal disease. Am J Surg. 2010;200:9-14.
- Tavassoli A, Noorshafiee S, Nazarzadeh R. Comparison of excision with primary repair versus Limberg flap. Int J Surg. 2011;9:343-346.
- Ates M, Dirican A, Sarac M, et al. Short and long-term results of the Karydakis flap versus the Limberg flap for treating pilonidal sinus disease: a prospective randomized study. Am J Surg. 2011;202:568-573.
- Can MF, Sevinc MM, Hancerliogullari O, et al. Multicenter prospective randomized trial comparing modified Limberg flap transposition and Karydakis flap reconstruction in patients with saccrococcygeal pilonidal disease. Am J Surg. 2010;200:318-327.
- Ersoy E, Devay AO, Aktimur R, et al. Comparison of short-term results after Limberg and Karydakis procedures for pilonidal disease: randomized prospective analysis of 100 patients. Colorectal Dis. 2009;11:705-710.
- Okuş A, Sevinç B, Karahan O, et al. Comparison of Limberg flap and tension-free primary closure during pilonidal sinus surgery. World J Surg. 2012;36:431-435.
- Akan K, Tihan D, Duman U, et al. Comparison of surgical Limberg flap technique and crystallized phenol application in the treatment of pilonidal sinus disease: a retrospective study. Ulus Cerrahi Derg. 2013;29:162-166.
- Guner A, Boz A, Ozkan OF, et al. Limberg flap versus Bascom cleft lift techniques for sacrococcygeal pilonidal sinus: prospective, randomized trial. World J Surg. 2013;37:2074-2080.
- Hosseini H, Heidari A, Jafarnejad B. Comparison of three surgical methods in treatment of patients with pilonidal sinus: modified excision and repair/wide excision/wide excision and flap in RASOUL, OMID and SADR hospitals (2004-2007). Indian J Surg. 2013;75:395-400.
- Karaca AS, Ali R, Capar M, et al. Comparison of Limberg flap and excision and primary closure of pilonidal sinus disease, in terms of quality of life and complications. J Korean Surg Soc. 2013;85:236-239.
- Rao J, Deora H, Mandia R. A retrospective study of 50 cases of pilonidal sinus with excision of tract and Z-plasty as treatment of choice for both primary and recurrent cases. Indian J Surg. 2015;77(suppl 2):691-693.
- Landa N, Aller O, Landa-Gundin N, et al. Successful treatment of recurrent pilonidal sinus with laser epilation. Dermatol Surg. 2005;31:726-728.
- Oram Y, Kahraman D, Karincaoğlu Y, et al. Evaluation of 60 patients with pilonidal sinus treated with laser epilation after surgery. Dermatol Surg. 2010;36:88-91.
- Benedetto AV, Lewis AT. Pilonidal sinus disease treated by depilation using an 800 nm diode laser and review of the literature. Dermatol Surg. 2005;31:587-591.
- Lindholt-Jensen CS, Lindholt JS, Beyer M, et al. Nd-YAG treatment of primary and recurrent pilonidal sinus. Lasers Med Sci. 2012;27:505-508.
- Jain V, Jain A. Use of lasers for the management of refractory cases of hidradenitis suppurativa and pilonidal sinus. J Cutan Aesthet. 2012;5:190-192.
Practice Points
- Mild pilonidal disease can be treated with conservative measures, including phenol injection and simple excision and drainage. Recurrent disease or the presence of extensive scarring or suppurative disease typically necessitates excision with flap closure.
- Off-midline procedures have been shown to be statistically superior to midline closure with regard to healing time, infection at the surgical site, and rate of recurrence.
- Laser excision holds promise as a primary or adjuvant treatment of pilonidal disease; however, large randomized controlled trials are needed to confirm early findings.
Paraneoplastic Palmoplantar Keratoderma Secondary to Metastatic Uterine Adenocarcinoma
Paraneoplastic palmoplantar keratoderma (PPK) is an acquired dermatosis that presents with hyperkeratosis of the palms and soles in association with visceral malignancies, such as esophageal, gastric, pulmonary, and bladder carcinomas. This condition may either be acquired or inherited.1
Case Report
A 72-year-old woman was referred to our dermatology clinic for evaluation of a nonpruritic hyperkeratotic eruption predominantly on the palms and soles of 2 to 3 months’ duration (Figure 1A). Review of systems was remarkable for chronic anxiety, unintentional weight loss of 10 lb over the last 6 months, and a mild cough of 10 days’ duration. The differential diagnosis included eczematous dermatitis, tinea manuum, new-onset palmoplantar psoriasis, and PPK.
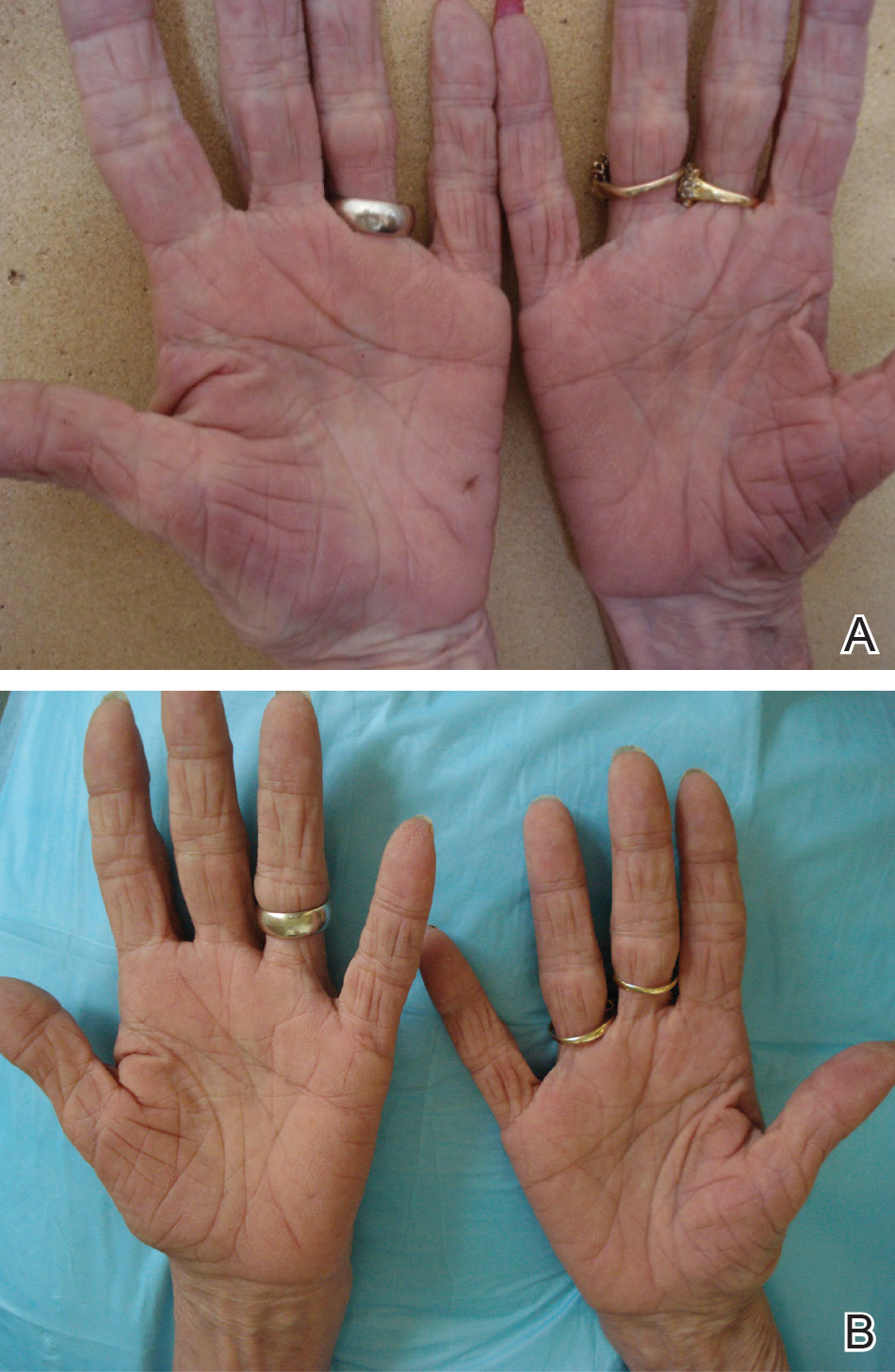
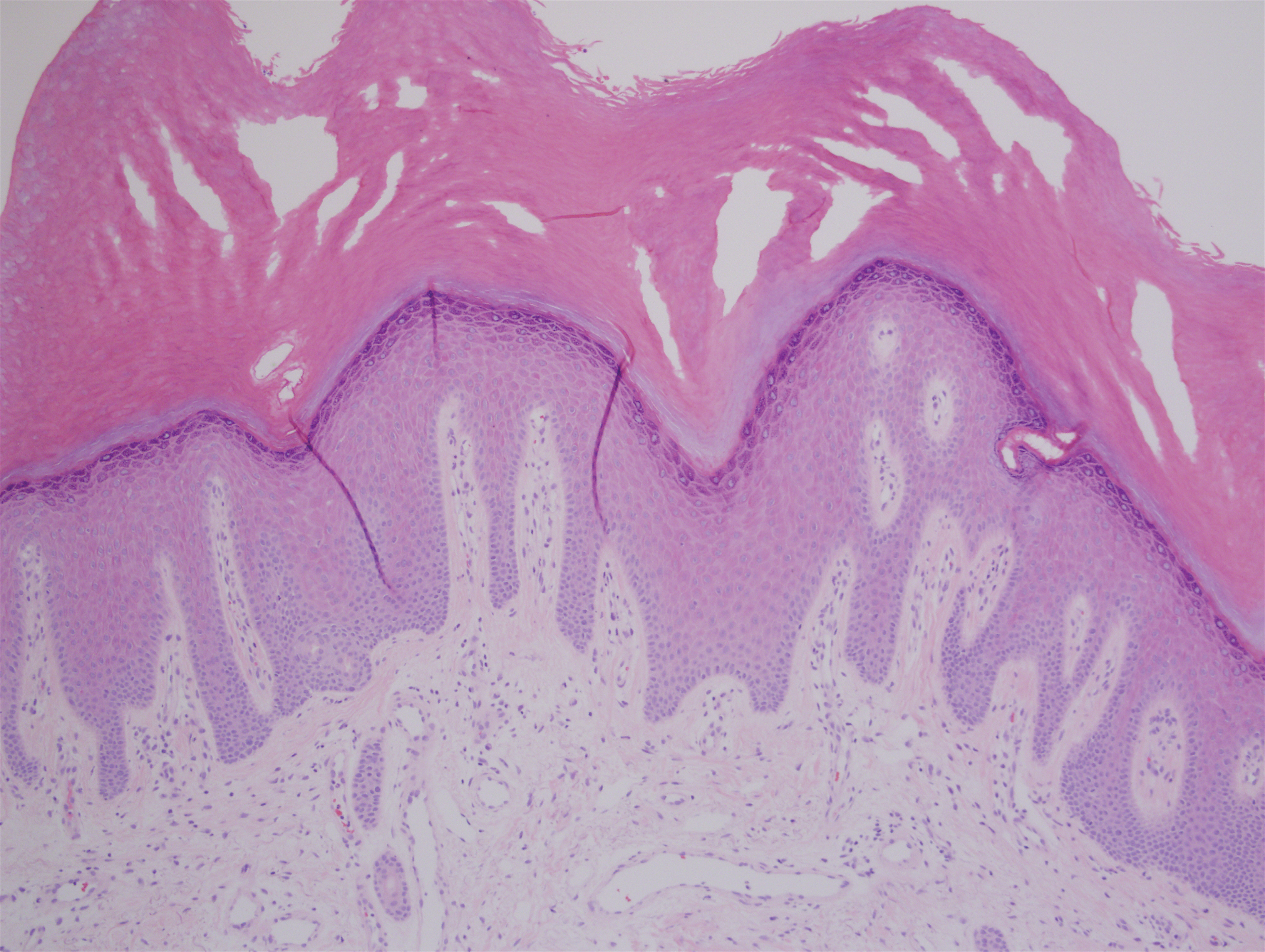
A punch biopsy of the medial hypothenar eminence of the left hand was performed, revealing notable lichenified hyperkeratosis with vascular ectasia (Figure 2). Periodic acid–Schiff staining was negative for fungal elements. Given the suspicion of PPK, multiple carcinoma markers were ordered. Cancer antigen 125 measured at 68 U/mL (reference range upper limit, 21 U/mL). Cancer antigen 27-29 was 50 U/mL (reference range, <38 U/mL) and cancer antigen 19-9 was 24 U/mL (reference range, <37 U/mL). Computed tomography of the chest revealed a large mass in the left lower lung associated with hilar lymphadenopathy. The patient was referred to oncology for further evaluation. Computed tomography–guided biopsy revealed metastatic uterine adenocarcinoma, which prompted subsequent chemotherapy. The combination of visceral malignancy with PPK led to the diagnosis of acquired PPK secondary to uterine cancer. After the completion of chemotherapy, the palmar dermatosis notably decreased (Figure 1B).
Comment
Paraneoplastic PPK is not uncommon. Ninety percent of acquired diffuse PPK is secondary to cancer,2 which occurs more frequently in male patients. Associated visceral malignancies include localized esophageal,3 myeloma,4 pulmonary, urinary/bladder,5 and gastric carcinoma.6 Paraneoplastic PPK in women is rare but has been linked to ovarian and breast carcinoma.7
The findings under light microscopy include thickening of any or all of the cell layers of the epidermis, which can include hyperkeratosis, acanthosis, and papillomatosis (Figure 2). A moderate amount of mononuclear cell infiltrates also can be visualized.
Palmoplantar keratoderma associated with uterine malignancy is rare. However, many other paraneoplastic dermatoses resulting from uterine cancer have been described as well as nonuterine gynecological malignancies (Table).8-17
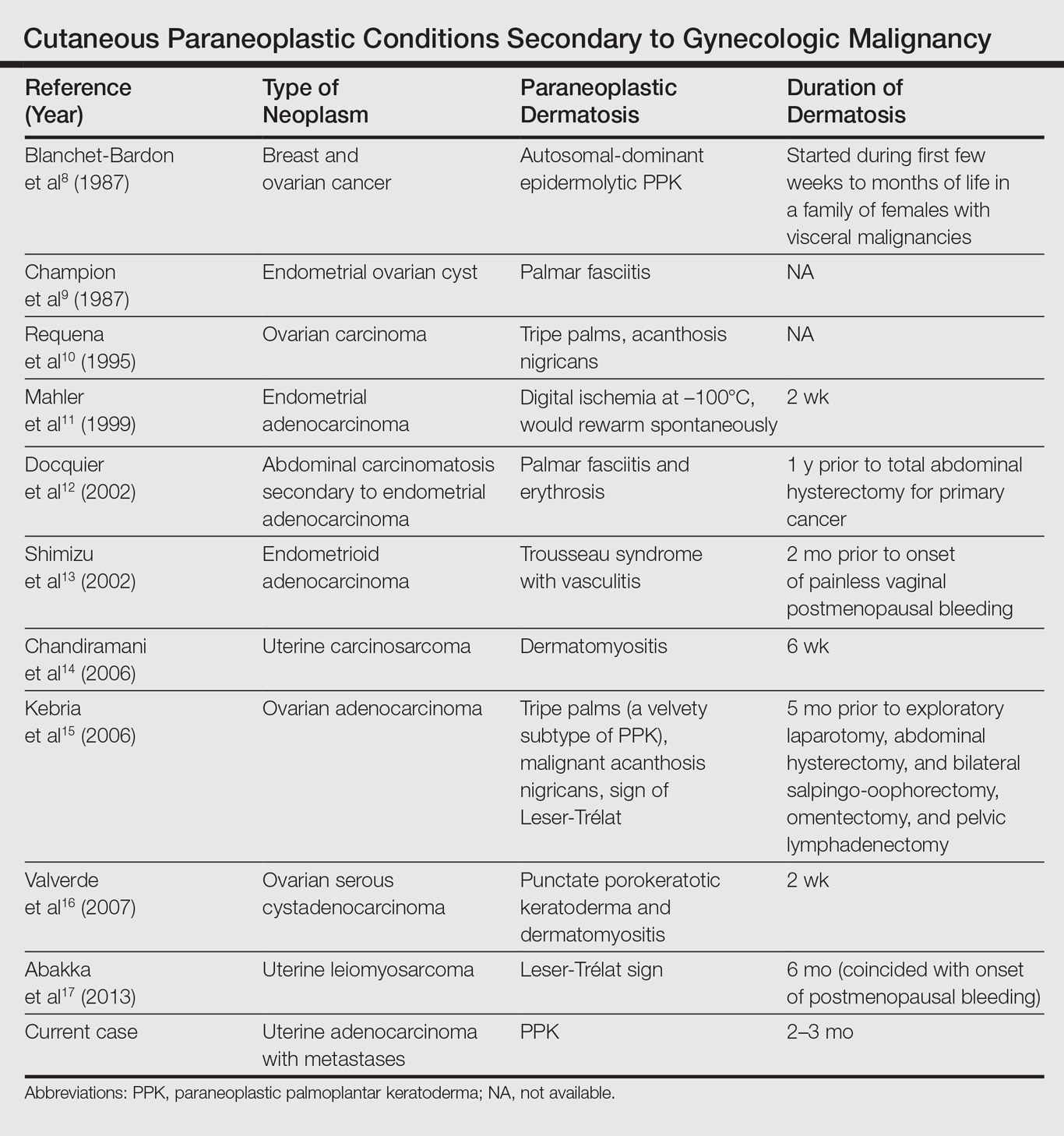
The first step in managing acquired PPK is to determine its etiology via a complete history and a total-body skin examination. If findings are consistent with a hereditary PPK, then genetic workup is advised. Other suspected etiologies should be investigated via imaging and laboratory analysis.18
The first approach in managing acquired PPK is to treat the underlying cause. In prior cases, complete resolution of skin findings resulted once the malignancy or associated dermatosis had been treated.8-17 Adjunctive medication includes topical keratolytics (eg, urea, salicylic acid, lactic acid), topical retinoids, topical psoralen plus UVA, and topical corticosteroids.18 Vitamin A analogues have been found to be an effective treatment of many hyperkeratotic dermatoses.19 Isotretinoin and etretinate have been used to treat the cutaneous findings and prevent the onset and progression of esophageal malignancy of the inherited forms of PPK. The oral retinoid acitretin has been shown to rapidly resolve lesions, have persistent effects after 5 months of cessation, and have minimal side effects. Thus, it has been suggested as the first-line treatment of chronic PPK.19 One study found no response to topical keratolytics (urea cream and salicylic acid ointment) and a 2-week course of oral prednisone; however, low-dose oral acitretin 10 mg once daily resulted in notable improvement over several weeks.7 Physical debridement also may be necessary.18
Conclusion
Palmoplantar keratoderma is a condition that presents with hyperkeratosis of the palms and soles. Acquired PPK often occurs as a paraneoplastic response as well as a stigma of other dermatoses. It occurs more frequently in male patients. Reports of PPK secondary to uterine cancer are not common in the literature. Management of PPK includes a complete history and total-body skin examination. After appropriate imaging and laboratory analysis, treatment of the underlying cause is the best approach. Adjunctive medications include topical keratolytics, topical retinoids, topical psoralen plus UVA, and topical corticosteroids. Oral isotretinoin and etretinate have demonstrated promising results.
- Zamiri M, van Steensel MA, Munro CS. Inherited palmoplantar keratodermas. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:538-548.
- Cohen PR, Grossman ME, Silvers DN. Tripe palms and cancer. Clin Dermatol. 1993;11:165-173.
- Belmar P, Marquet A, Martín-Sáez E. Symmetric palmar hyperkeratosis and esophageal carcinoma [in Spanish]. Actas Dermosifiliogr. 2008;99:149-150.
- Smith CH, Barker JN, Hay RJ. Diffuse plane xanthomatosis and acquired palmoplantar keratoderma in association with myeloma. Br J Dermatol. 1995;132:286-289.
- Küchmeister B, Rasokat H. Acquired disseminated papulous palmar keratoses—a paraneoplastic syndrome in cancers of the urinary bladder and lung? [in German]. Z Hautkr. 1984;59:1123-1124.
- Stieler K, Blume-Peytavi U, Vogel A, et al. Hyperkeratoses as paraneoplastic syndrome [published online June 1, 2012]. J Dtsch Dermatol Ges. 2012;10:593-595.
- Vignale RA, Espasandín J, Paciel J, et al. Diagnostic value of keratosis palmaris as indicative sign of visceral cancer [in Spanish]. Med Cutan Ibero Lat Am. 1983;11:287-292.
- Blanchet-Bardon C, Nazzaro V, Chevrant-Breton J, et al. Hereditary epidermolytic palmoplantar keratoderma associated with breast and ovarian cancer in a large kindred. Br J Dermatol. 1987;117:363-370.
- Champion GD, Saxon JA, Kossard S. The syndrome of palmar fibromatosis (fasciitis) and polyarthritis. J Rheumatol. 1987;14:1196-1198.
- Requena L, Aguilar A, Renedo G, et al. Tripe palms: a cutaneous marker of internal malignancy. J Dermatol. 1995;22:492-495.
- Mahler V, Neureiter D, Kirchner T, et al. Digital ischemia as paraneoplastic marker of metastatic endometrial carcinoma [in German]. Hautarzt. 1999;50:748-752.
- Docquier Ch, Majois F, Mitine C. Palmar fasciitis and arthritis: association with endometrial adenocarcinoma. Clin Rheumatol. 2002;21:63-65.
- Shimizu Y, Uchiyama S, Mori G, et al. A young patient with endometrioid adenocarcinoma who suffered Trousseau’s syndrome associated with vasculitis [in Japanese]. Rinsho Shinkeigaku. 2002;42:227-232.
- Chandiramani M, Joynson C, Panchal R, et al. Dermatomyositis as a paraneoplastic syndrome in carcinosarcoma of uterine origin. Clin Oncol (R Coll Radiol). 2006;18:641-648.
- Kebria MM, Belinson J, Kim R, et al. Malignant acanthosis nigricans, tripe palms and the sign of Leser-Trélat, a hint to the diagnosis of early stage ovarian cancer: a case report and review of the literature [published online January 27, 2006]. Gynecol Oncol. 2006;101:353-355.
- Valverde R, Sánchez-Caminero MP, Calzado L, et al. Dermatomyositis and punctate porokeratotic keratoderma as paraneoplastic syndrome of ovarian carcinoma [in Spanish]. Actas Dermosifiliogr. 2007;98:358-360.
- Abakka S, Elhalouat H, Khoummane N, et al. Uterine leiomyosarcoma and Leser-Trélat sign. Lancet. 2013;381:88.
- Patel S, Zirwas M, English JC 3rd. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Capella GL, Fracchiolla C, Frigerio E, et al. A controlled study of comparative efficacy of oral retinoids and topical betamethasone/salicylic acid for chronic hyperkeratotic palmoplantar dermatitis. J Dermatolog Treat. 2004;15:88-93.
Paraneoplastic palmoplantar keratoderma (PPK) is an acquired dermatosis that presents with hyperkeratosis of the palms and soles in association with visceral malignancies, such as esophageal, gastric, pulmonary, and bladder carcinomas. This condition may either be acquired or inherited.1
Case Report
A 72-year-old woman was referred to our dermatology clinic for evaluation of a nonpruritic hyperkeratotic eruption predominantly on the palms and soles of 2 to 3 months’ duration (Figure 1A). Review of systems was remarkable for chronic anxiety, unintentional weight loss of 10 lb over the last 6 months, and a mild cough of 10 days’ duration. The differential diagnosis included eczematous dermatitis, tinea manuum, new-onset palmoplantar psoriasis, and PPK.


A punch biopsy of the medial hypothenar eminence of the left hand was performed, revealing notable lichenified hyperkeratosis with vascular ectasia (Figure 2). Periodic acid–Schiff staining was negative for fungal elements. Given the suspicion of PPK, multiple carcinoma markers were ordered. Cancer antigen 125 measured at 68 U/mL (reference range upper limit, 21 U/mL). Cancer antigen 27-29 was 50 U/mL (reference range, <38 U/mL) and cancer antigen 19-9 was 24 U/mL (reference range, <37 U/mL). Computed tomography of the chest revealed a large mass in the left lower lung associated with hilar lymphadenopathy. The patient was referred to oncology for further evaluation. Computed tomography–guided biopsy revealed metastatic uterine adenocarcinoma, which prompted subsequent chemotherapy. The combination of visceral malignancy with PPK led to the diagnosis of acquired PPK secondary to uterine cancer. After the completion of chemotherapy, the palmar dermatosis notably decreased (Figure 1B).
Comment
Paraneoplastic PPK is not uncommon. Ninety percent of acquired diffuse PPK is secondary to cancer,2 which occurs more frequently in male patients. Associated visceral malignancies include localized esophageal,3 myeloma,4 pulmonary, urinary/bladder,5 and gastric carcinoma.6 Paraneoplastic PPK in women is rare but has been linked to ovarian and breast carcinoma.7
The findings under light microscopy include thickening of any or all of the cell layers of the epidermis, which can include hyperkeratosis, acanthosis, and papillomatosis (Figure 2). A moderate amount of mononuclear cell infiltrates also can be visualized.
Palmoplantar keratoderma associated with uterine malignancy is rare. However, many other paraneoplastic dermatoses resulting from uterine cancer have been described as well as nonuterine gynecological malignancies (Table).8-17

The first step in managing acquired PPK is to determine its etiology via a complete history and a total-body skin examination. If findings are consistent with a hereditary PPK, then genetic workup is advised. Other suspected etiologies should be investigated via imaging and laboratory analysis.18
The first approach in managing acquired PPK is to treat the underlying cause. In prior cases, complete resolution of skin findings resulted once the malignancy or associated dermatosis had been treated.8-17 Adjunctive medication includes topical keratolytics (eg, urea, salicylic acid, lactic acid), topical retinoids, topical psoralen plus UVA, and topical corticosteroids.18 Vitamin A analogues have been found to be an effective treatment of many hyperkeratotic dermatoses.19 Isotretinoin and etretinate have been used to treat the cutaneous findings and prevent the onset and progression of esophageal malignancy of the inherited forms of PPK. The oral retinoid acitretin has been shown to rapidly resolve lesions, have persistent effects after 5 months of cessation, and have minimal side effects. Thus, it has been suggested as the first-line treatment of chronic PPK.19 One study found no response to topical keratolytics (urea cream and salicylic acid ointment) and a 2-week course of oral prednisone; however, low-dose oral acitretin 10 mg once daily resulted in notable improvement over several weeks.7 Physical debridement also may be necessary.18
Conclusion
Palmoplantar keratoderma is a condition that presents with hyperkeratosis of the palms and soles. Acquired PPK often occurs as a paraneoplastic response as well as a stigma of other dermatoses. It occurs more frequently in male patients. Reports of PPK secondary to uterine cancer are not common in the literature. Management of PPK includes a complete history and total-body skin examination. After appropriate imaging and laboratory analysis, treatment of the underlying cause is the best approach. Adjunctive medications include topical keratolytics, topical retinoids, topical psoralen plus UVA, and topical corticosteroids. Oral isotretinoin and etretinate have demonstrated promising results.
Paraneoplastic palmoplantar keratoderma (PPK) is an acquired dermatosis that presents with hyperkeratosis of the palms and soles in association with visceral malignancies, such as esophageal, gastric, pulmonary, and bladder carcinomas. This condition may either be acquired or inherited.1
Case Report
A 72-year-old woman was referred to our dermatology clinic for evaluation of a nonpruritic hyperkeratotic eruption predominantly on the palms and soles of 2 to 3 months’ duration (Figure 1A). Review of systems was remarkable for chronic anxiety, unintentional weight loss of 10 lb over the last 6 months, and a mild cough of 10 days’ duration. The differential diagnosis included eczematous dermatitis, tinea manuum, new-onset palmoplantar psoriasis, and PPK.


A punch biopsy of the medial hypothenar eminence of the left hand was performed, revealing notable lichenified hyperkeratosis with vascular ectasia (Figure 2). Periodic acid–Schiff staining was negative for fungal elements. Given the suspicion of PPK, multiple carcinoma markers were ordered. Cancer antigen 125 measured at 68 U/mL (reference range upper limit, 21 U/mL). Cancer antigen 27-29 was 50 U/mL (reference range, <38 U/mL) and cancer antigen 19-9 was 24 U/mL (reference range, <37 U/mL). Computed tomography of the chest revealed a large mass in the left lower lung associated with hilar lymphadenopathy. The patient was referred to oncology for further evaluation. Computed tomography–guided biopsy revealed metastatic uterine adenocarcinoma, which prompted subsequent chemotherapy. The combination of visceral malignancy with PPK led to the diagnosis of acquired PPK secondary to uterine cancer. After the completion of chemotherapy, the palmar dermatosis notably decreased (Figure 1B).
Comment
Paraneoplastic PPK is not uncommon. Ninety percent of acquired diffuse PPK is secondary to cancer,2 which occurs more frequently in male patients. Associated visceral malignancies include localized esophageal,3 myeloma,4 pulmonary, urinary/bladder,5 and gastric carcinoma.6 Paraneoplastic PPK in women is rare but has been linked to ovarian and breast carcinoma.7
The findings under light microscopy include thickening of any or all of the cell layers of the epidermis, which can include hyperkeratosis, acanthosis, and papillomatosis (Figure 2). A moderate amount of mononuclear cell infiltrates also can be visualized.
Palmoplantar keratoderma associated with uterine malignancy is rare. However, many other paraneoplastic dermatoses resulting from uterine cancer have been described as well as nonuterine gynecological malignancies (Table).8-17

The first step in managing acquired PPK is to determine its etiology via a complete history and a total-body skin examination. If findings are consistent with a hereditary PPK, then genetic workup is advised. Other suspected etiologies should be investigated via imaging and laboratory analysis.18
The first approach in managing acquired PPK is to treat the underlying cause. In prior cases, complete resolution of skin findings resulted once the malignancy or associated dermatosis had been treated.8-17 Adjunctive medication includes topical keratolytics (eg, urea, salicylic acid, lactic acid), topical retinoids, topical psoralen plus UVA, and topical corticosteroids.18 Vitamin A analogues have been found to be an effective treatment of many hyperkeratotic dermatoses.19 Isotretinoin and etretinate have been used to treat the cutaneous findings and prevent the onset and progression of esophageal malignancy of the inherited forms of PPK. The oral retinoid acitretin has been shown to rapidly resolve lesions, have persistent effects after 5 months of cessation, and have minimal side effects. Thus, it has been suggested as the first-line treatment of chronic PPK.19 One study found no response to topical keratolytics (urea cream and salicylic acid ointment) and a 2-week course of oral prednisone; however, low-dose oral acitretin 10 mg once daily resulted in notable improvement over several weeks.7 Physical debridement also may be necessary.18
Conclusion
Palmoplantar keratoderma is a condition that presents with hyperkeratosis of the palms and soles. Acquired PPK often occurs as a paraneoplastic response as well as a stigma of other dermatoses. It occurs more frequently in male patients. Reports of PPK secondary to uterine cancer are not common in the literature. Management of PPK includes a complete history and total-body skin examination. After appropriate imaging and laboratory analysis, treatment of the underlying cause is the best approach. Adjunctive medications include topical keratolytics, topical retinoids, topical psoralen plus UVA, and topical corticosteroids. Oral isotretinoin and etretinate have demonstrated promising results.
- Zamiri M, van Steensel MA, Munro CS. Inherited palmoplantar keratodermas. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:538-548.
- Cohen PR, Grossman ME, Silvers DN. Tripe palms and cancer. Clin Dermatol. 1993;11:165-173.
- Belmar P, Marquet A, Martín-Sáez E. Symmetric palmar hyperkeratosis and esophageal carcinoma [in Spanish]. Actas Dermosifiliogr. 2008;99:149-150.
- Smith CH, Barker JN, Hay RJ. Diffuse plane xanthomatosis and acquired palmoplantar keratoderma in association with myeloma. Br J Dermatol. 1995;132:286-289.
- Küchmeister B, Rasokat H. Acquired disseminated papulous palmar keratoses—a paraneoplastic syndrome in cancers of the urinary bladder and lung? [in German]. Z Hautkr. 1984;59:1123-1124.
- Stieler K, Blume-Peytavi U, Vogel A, et al. Hyperkeratoses as paraneoplastic syndrome [published online June 1, 2012]. J Dtsch Dermatol Ges. 2012;10:593-595.
- Vignale RA, Espasandín J, Paciel J, et al. Diagnostic value of keratosis palmaris as indicative sign of visceral cancer [in Spanish]. Med Cutan Ibero Lat Am. 1983;11:287-292.
- Blanchet-Bardon C, Nazzaro V, Chevrant-Breton J, et al. Hereditary epidermolytic palmoplantar keratoderma associated with breast and ovarian cancer in a large kindred. Br J Dermatol. 1987;117:363-370.
- Champion GD, Saxon JA, Kossard S. The syndrome of palmar fibromatosis (fasciitis) and polyarthritis. J Rheumatol. 1987;14:1196-1198.
- Requena L, Aguilar A, Renedo G, et al. Tripe palms: a cutaneous marker of internal malignancy. J Dermatol. 1995;22:492-495.
- Mahler V, Neureiter D, Kirchner T, et al. Digital ischemia as paraneoplastic marker of metastatic endometrial carcinoma [in German]. Hautarzt. 1999;50:748-752.
- Docquier Ch, Majois F, Mitine C. Palmar fasciitis and arthritis: association with endometrial adenocarcinoma. Clin Rheumatol. 2002;21:63-65.
- Shimizu Y, Uchiyama S, Mori G, et al. A young patient with endometrioid adenocarcinoma who suffered Trousseau’s syndrome associated with vasculitis [in Japanese]. Rinsho Shinkeigaku. 2002;42:227-232.
- Chandiramani M, Joynson C, Panchal R, et al. Dermatomyositis as a paraneoplastic syndrome in carcinosarcoma of uterine origin. Clin Oncol (R Coll Radiol). 2006;18:641-648.
- Kebria MM, Belinson J, Kim R, et al. Malignant acanthosis nigricans, tripe palms and the sign of Leser-Trélat, a hint to the diagnosis of early stage ovarian cancer: a case report and review of the literature [published online January 27, 2006]. Gynecol Oncol. 2006;101:353-355.
- Valverde R, Sánchez-Caminero MP, Calzado L, et al. Dermatomyositis and punctate porokeratotic keratoderma as paraneoplastic syndrome of ovarian carcinoma [in Spanish]. Actas Dermosifiliogr. 2007;98:358-360.
- Abakka S, Elhalouat H, Khoummane N, et al. Uterine leiomyosarcoma and Leser-Trélat sign. Lancet. 2013;381:88.
- Patel S, Zirwas M, English JC 3rd. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Capella GL, Fracchiolla C, Frigerio E, et al. A controlled study of comparative efficacy of oral retinoids and topical betamethasone/salicylic acid for chronic hyperkeratotic palmoplantar dermatitis. J Dermatolog Treat. 2004;15:88-93.
- Zamiri M, van Steensel MA, Munro CS. Inherited palmoplantar keratodermas. In: Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:538-548.
- Cohen PR, Grossman ME, Silvers DN. Tripe palms and cancer. Clin Dermatol. 1993;11:165-173.
- Belmar P, Marquet A, Martín-Sáez E. Symmetric palmar hyperkeratosis and esophageal carcinoma [in Spanish]. Actas Dermosifiliogr. 2008;99:149-150.
- Smith CH, Barker JN, Hay RJ. Diffuse plane xanthomatosis and acquired palmoplantar keratoderma in association with myeloma. Br J Dermatol. 1995;132:286-289.
- Küchmeister B, Rasokat H. Acquired disseminated papulous palmar keratoses—a paraneoplastic syndrome in cancers of the urinary bladder and lung? [in German]. Z Hautkr. 1984;59:1123-1124.
- Stieler K, Blume-Peytavi U, Vogel A, et al. Hyperkeratoses as paraneoplastic syndrome [published online June 1, 2012]. J Dtsch Dermatol Ges. 2012;10:593-595.
- Vignale RA, Espasandín J, Paciel J, et al. Diagnostic value of keratosis palmaris as indicative sign of visceral cancer [in Spanish]. Med Cutan Ibero Lat Am. 1983;11:287-292.
- Blanchet-Bardon C, Nazzaro V, Chevrant-Breton J, et al. Hereditary epidermolytic palmoplantar keratoderma associated with breast and ovarian cancer in a large kindred. Br J Dermatol. 1987;117:363-370.
- Champion GD, Saxon JA, Kossard S. The syndrome of palmar fibromatosis (fasciitis) and polyarthritis. J Rheumatol. 1987;14:1196-1198.
- Requena L, Aguilar A, Renedo G, et al. Tripe palms: a cutaneous marker of internal malignancy. J Dermatol. 1995;22:492-495.
- Mahler V, Neureiter D, Kirchner T, et al. Digital ischemia as paraneoplastic marker of metastatic endometrial carcinoma [in German]. Hautarzt. 1999;50:748-752.
- Docquier Ch, Majois F, Mitine C. Palmar fasciitis and arthritis: association with endometrial adenocarcinoma. Clin Rheumatol. 2002;21:63-65.
- Shimizu Y, Uchiyama S, Mori G, et al. A young patient with endometrioid adenocarcinoma who suffered Trousseau’s syndrome associated with vasculitis [in Japanese]. Rinsho Shinkeigaku. 2002;42:227-232.
- Chandiramani M, Joynson C, Panchal R, et al. Dermatomyositis as a paraneoplastic syndrome in carcinosarcoma of uterine origin. Clin Oncol (R Coll Radiol). 2006;18:641-648.
- Kebria MM, Belinson J, Kim R, et al. Malignant acanthosis nigricans, tripe palms and the sign of Leser-Trélat, a hint to the diagnosis of early stage ovarian cancer: a case report and review of the literature [published online January 27, 2006]. Gynecol Oncol. 2006;101:353-355.
- Valverde R, Sánchez-Caminero MP, Calzado L, et al. Dermatomyositis and punctate porokeratotic keratoderma as paraneoplastic syndrome of ovarian carcinoma [in Spanish]. Actas Dermosifiliogr. 2007;98:358-360.
- Abakka S, Elhalouat H, Khoummane N, et al. Uterine leiomyosarcoma and Leser-Trélat sign. Lancet. 2013;381:88.
- Patel S, Zirwas M, English JC 3rd. Acquired palmoplantar keratoderma. Am J Clin Dermatol. 2007;8:1-11.
- Capella GL, Fracchiolla C, Frigerio E, et al. A controlled study of comparative efficacy of oral retinoids and topical betamethasone/salicylic acid for chronic hyperkeratotic palmoplantar dermatitis. J Dermatolog Treat. 2004;15:88-93.
Practice Points
- Paraneoplastic palmoplantar keratoderma (PPK) is an acquired dermatosis that presents with hyperkeratosis of the palms and soles in association with visceral malignancies (eg, esophageal, gastric, pulmonary, and urinary/bladder carcinomas).
- Palmoplantar keratoderma secondary to uterine cancer is rare.
- Light microscopy shows thickening of any or all of the cell layers of the epidermis (hyperkeratosis, acanthosis, and papillomatosis) and mononuclear cells.
- Management of acquired PPK includes treatment of the underlying malignancy. Adjunctive vitamin A analogues may be of additional utility.
Pits on the soles of the feet
A 22-year-old man came to the office with feet that were malodorous, had a rash, and were sweaty. The odor is made worse by any exercise that leads to a lot of foot sweating. His friends and family complain when he removes his shoes. He reported that everyone once left a public locker room after he removed his shoes. He is so embarrassed by this problem that he has waited months before seeking help.
The young man admits to wearing shoes that don’t let his feet breathe well, but he finds these to be the most comfortable shoes he has. He doesn’t like that his socks get wet easily from his excessive sweating. Aside from the malodor and hyperhidrosis of the feet, he denies any pain or severe pruritus.
On examination, the foul odor was immediately apparent. Multiple cribriform pits were noted on the pressure-bearing areas of the soles (FIGURE). There was scaling of the skin on the soles and around the toes. There was no lymphadenopathy. No other skin or mucosal areas involvements. His family history and review of systems were unremarkable.
FIGURE
Multiple pits on the sole
What is your diagnosis?
How would you manage this condition?
Diagnosis: Pitted keratolysis
Pitted keratolysis (PK), also known as keratolysis plantare sulcatum, is a skin disorder characterized by pits and collarettes from bacterial infection. PK is a superficial infection, confined to the stratum corneum.
Micrococcus sedentarius, a Gram-positive Staphylococcus-related bacterium, Dermatophilus congolensis, a Gram-positive facultative anaerobic Actinomyces species, and several Corynebacterium species have all been identified as causative agents of PK. These bacteria make proteinases that destroy the stratum corneum and open small tunnels and pits in the skin.1-4
Clinical picture of PK
The plantar aspects of the feet are most commonly affected by PK, pressure-bearing areas such as the ventral aspect of the toes and the ball of the foot in particular. Some patients develop lesions on the interdigital surfaces. The localized absence of the stratum corneum leads to a punched-out appearance of the skin.
Prolonged time of occlusion and hyperhidrosis often lead to increased skin surface pH. This triggers bacterial infections, resulting in PK. Malodor is common, presumed to be due the production of sulfur-compound byproducts such as thiols, sulfides, and thioesters.2-4 Often asymptomatic, a patient with PK may develop varying degrees of discomfort, ranging from mild burning sensation to severe tenderness and limitation of function.
The diagnosis is often clinical and seldom poses a challenge; skin biopsy is rarely performed. In recent reports, the use of transmission electron microscopy and scanning electron microscopy showed bacteria in the stratum corneum with typical transversal septations. Tunnel-like spaces were built inside the stratum corneum, where the bacteria exhibited a hairy surface.
The differential diagnosis for PK may include the following, especially when the soles are involved: candidal infections, basal cell nevus syndrome, and keratolysis exfoliativa.
Management: Good foot hygiene, topical medications
After clinical diagnosis of PK, most of the dermatologists and other practitioners with expertise in skin diseases management initiate empiric treatment. Management should include instructing patients to wear well-fitted shoes, avoid prolonged periods of occlusion, and use absorbent 100% cotton socks with frequent sock changes.
Topical erythromycin, clindamycin, and fucidic acid applied to the entire plantar surfaces of the feet are very effective. Topical mupirocin, benzoyl peroxide wash or gel, clotrimazole, miconazole, and Whitfield’s ointment are also effective. Successful treatment with topical antiseptics, such as glutaraldehyde and formaldehyde, has also been reported. Oral erythromycin is another option, especially for resistant cases. This usually clears both the lesions and odor in 3 to 4 weeks.
In addition, applying antiperspirants such as aluminum chloride 20% solution helps reduce hyperhidrosis. Inert antiseptic foot powders may also be used. Recently, plantar hyperhidrosis and pitted keratolysis have been successfully treated with botulinum toxin injection (Botox).5
Along with good foot hygiene, our patient was advised to use topical fucidic acid cream and 20% aluminum chloride solution for 2 weeks. On his 2-week follow-up visit, the lesions were almost completely resolved, the malodor was gone, and the hyperhidrosis had decreased.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, Wellman Center for Photomedicine, Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: [email protected]
1. Zaias N, Taplin D, Rebell G. Pitted keratolysis. Arch Dermatol 1965;92:151-154.
2. Longshaw CM, Wright JD, Farrell AM, Holland KT. Kytococcus sedentarius, the organism associated with pitted keratolysis, produces two keratin-degrading enzymes. J Appl Microbiol 2002;93:810-816.
3. Wohlrab J, Rohrbach D, Marsch WC. Keratolysis sulcata (pitted keratolysis): clinical symptoms with different histological correlates. Br J Dermatol 2000;143:1348-1349.
4. de Almeida HL, Jr, de Castro LA, Rocha NE, Abrantes VL. Ultrastructure of pitted keratolysis. Int J Dermatol 2000;39:698-701.
5. Tamura BM, Cuce LC, Souza RL, Levites J. Plantar hyperhidrosis and pitted keratolysis treated with botulinum toxin injection. Dermatol Surg 2004;30:1510-1514.
A 22-year-old man came to the office with feet that were malodorous, had a rash, and were sweaty. The odor is made worse by any exercise that leads to a lot of foot sweating. His friends and family complain when he removes his shoes. He reported that everyone once left a public locker room after he removed his shoes. He is so embarrassed by this problem that he has waited months before seeking help.
The young man admits to wearing shoes that don’t let his feet breathe well, but he finds these to be the most comfortable shoes he has. He doesn’t like that his socks get wet easily from his excessive sweating. Aside from the malodor and hyperhidrosis of the feet, he denies any pain or severe pruritus.
On examination, the foul odor was immediately apparent. Multiple cribriform pits were noted on the pressure-bearing areas of the soles (FIGURE). There was scaling of the skin on the soles and around the toes. There was no lymphadenopathy. No other skin or mucosal areas involvements. His family history and review of systems were unremarkable.
FIGURE
Multiple pits on the sole
What is your diagnosis?
How would you manage this condition?
Diagnosis: Pitted keratolysis
Pitted keratolysis (PK), also known as keratolysis plantare sulcatum, is a skin disorder characterized by pits and collarettes from bacterial infection. PK is a superficial infection, confined to the stratum corneum.
Micrococcus sedentarius, a Gram-positive Staphylococcus-related bacterium, Dermatophilus congolensis, a Gram-positive facultative anaerobic Actinomyces species, and several Corynebacterium species have all been identified as causative agents of PK. These bacteria make proteinases that destroy the stratum corneum and open small tunnels and pits in the skin.1-4
Clinical picture of PK
The plantar aspects of the feet are most commonly affected by PK, pressure-bearing areas such as the ventral aspect of the toes and the ball of the foot in particular. Some patients develop lesions on the interdigital surfaces. The localized absence of the stratum corneum leads to a punched-out appearance of the skin.
Prolonged time of occlusion and hyperhidrosis often lead to increased skin surface pH. This triggers bacterial infections, resulting in PK. Malodor is common, presumed to be due the production of sulfur-compound byproducts such as thiols, sulfides, and thioesters.2-4 Often asymptomatic, a patient with PK may develop varying degrees of discomfort, ranging from mild burning sensation to severe tenderness and limitation of function.
The diagnosis is often clinical and seldom poses a challenge; skin biopsy is rarely performed. In recent reports, the use of transmission electron microscopy and scanning electron microscopy showed bacteria in the stratum corneum with typical transversal septations. Tunnel-like spaces were built inside the stratum corneum, where the bacteria exhibited a hairy surface.
The differential diagnosis for PK may include the following, especially when the soles are involved: candidal infections, basal cell nevus syndrome, and keratolysis exfoliativa.
Management: Good foot hygiene, topical medications
After clinical diagnosis of PK, most of the dermatologists and other practitioners with expertise in skin diseases management initiate empiric treatment. Management should include instructing patients to wear well-fitted shoes, avoid prolonged periods of occlusion, and use absorbent 100% cotton socks with frequent sock changes.
Topical erythromycin, clindamycin, and fucidic acid applied to the entire plantar surfaces of the feet are very effective. Topical mupirocin, benzoyl peroxide wash or gel, clotrimazole, miconazole, and Whitfield’s ointment are also effective. Successful treatment with topical antiseptics, such as glutaraldehyde and formaldehyde, has also been reported. Oral erythromycin is another option, especially for resistant cases. This usually clears both the lesions and odor in 3 to 4 weeks.
In addition, applying antiperspirants such as aluminum chloride 20% solution helps reduce hyperhidrosis. Inert antiseptic foot powders may also be used. Recently, plantar hyperhidrosis and pitted keratolysis have been successfully treated with botulinum toxin injection (Botox).5
Along with good foot hygiene, our patient was advised to use topical fucidic acid cream and 20% aluminum chloride solution for 2 weeks. On his 2-week follow-up visit, the lesions were almost completely resolved, the malodor was gone, and the hyperhidrosis had decreased.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, Wellman Center for Photomedicine, Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: [email protected]
A 22-year-old man came to the office with feet that were malodorous, had a rash, and were sweaty. The odor is made worse by any exercise that leads to a lot of foot sweating. His friends and family complain when he removes his shoes. He reported that everyone once left a public locker room after he removed his shoes. He is so embarrassed by this problem that he has waited months before seeking help.
The young man admits to wearing shoes that don’t let his feet breathe well, but he finds these to be the most comfortable shoes he has. He doesn’t like that his socks get wet easily from his excessive sweating. Aside from the malodor and hyperhidrosis of the feet, he denies any pain or severe pruritus.
On examination, the foul odor was immediately apparent. Multiple cribriform pits were noted on the pressure-bearing areas of the soles (FIGURE). There was scaling of the skin on the soles and around the toes. There was no lymphadenopathy. No other skin or mucosal areas involvements. His family history and review of systems were unremarkable.
FIGURE
Multiple pits on the sole
What is your diagnosis?
How would you manage this condition?
Diagnosis: Pitted keratolysis
Pitted keratolysis (PK), also known as keratolysis plantare sulcatum, is a skin disorder characterized by pits and collarettes from bacterial infection. PK is a superficial infection, confined to the stratum corneum.
Micrococcus sedentarius, a Gram-positive Staphylococcus-related bacterium, Dermatophilus congolensis, a Gram-positive facultative anaerobic Actinomyces species, and several Corynebacterium species have all been identified as causative agents of PK. These bacteria make proteinases that destroy the stratum corneum and open small tunnels and pits in the skin.1-4
Clinical picture of PK
The plantar aspects of the feet are most commonly affected by PK, pressure-bearing areas such as the ventral aspect of the toes and the ball of the foot in particular. Some patients develop lesions on the interdigital surfaces. The localized absence of the stratum corneum leads to a punched-out appearance of the skin.
Prolonged time of occlusion and hyperhidrosis often lead to increased skin surface pH. This triggers bacterial infections, resulting in PK. Malodor is common, presumed to be due the production of sulfur-compound byproducts such as thiols, sulfides, and thioesters.2-4 Often asymptomatic, a patient with PK may develop varying degrees of discomfort, ranging from mild burning sensation to severe tenderness and limitation of function.
The diagnosis is often clinical and seldom poses a challenge; skin biopsy is rarely performed. In recent reports, the use of transmission electron microscopy and scanning electron microscopy showed bacteria in the stratum corneum with typical transversal septations. Tunnel-like spaces were built inside the stratum corneum, where the bacteria exhibited a hairy surface.
The differential diagnosis for PK may include the following, especially when the soles are involved: candidal infections, basal cell nevus syndrome, and keratolysis exfoliativa.
Management: Good foot hygiene, topical medications
After clinical diagnosis of PK, most of the dermatologists and other practitioners with expertise in skin diseases management initiate empiric treatment. Management should include instructing patients to wear well-fitted shoes, avoid prolonged periods of occlusion, and use absorbent 100% cotton socks with frequent sock changes.
Topical erythromycin, clindamycin, and fucidic acid applied to the entire plantar surfaces of the feet are very effective. Topical mupirocin, benzoyl peroxide wash or gel, clotrimazole, miconazole, and Whitfield’s ointment are also effective. Successful treatment with topical antiseptics, such as glutaraldehyde and formaldehyde, has also been reported. Oral erythromycin is another option, especially for resistant cases. This usually clears both the lesions and odor in 3 to 4 weeks.
In addition, applying antiperspirants such as aluminum chloride 20% solution helps reduce hyperhidrosis. Inert antiseptic foot powders may also be used. Recently, plantar hyperhidrosis and pitted keratolysis have been successfully treated with botulinum toxin injection (Botox).5
Along with good foot hygiene, our patient was advised to use topical fucidic acid cream and 20% aluminum chloride solution for 2 weeks. On his 2-week follow-up visit, the lesions were almost completely resolved, the malodor was gone, and the hyperhidrosis had decreased.
CORRESPONDING AUTHOR
Amor Khachemoune, MD, Wellman Center for Photomedicine, Department of Dermatology, Massachusetts General Hospital, Harvard Medical School, 40 Blossom Street (BAR 314), Boston, MA 02114. E-mail: [email protected]
1. Zaias N, Taplin D, Rebell G. Pitted keratolysis. Arch Dermatol 1965;92:151-154.
2. Longshaw CM, Wright JD, Farrell AM, Holland KT. Kytococcus sedentarius, the organism associated with pitted keratolysis, produces two keratin-degrading enzymes. J Appl Microbiol 2002;93:810-816.
3. Wohlrab J, Rohrbach D, Marsch WC. Keratolysis sulcata (pitted keratolysis): clinical symptoms with different histological correlates. Br J Dermatol 2000;143:1348-1349.
4. de Almeida HL, Jr, de Castro LA, Rocha NE, Abrantes VL. Ultrastructure of pitted keratolysis. Int J Dermatol 2000;39:698-701.
5. Tamura BM, Cuce LC, Souza RL, Levites J. Plantar hyperhidrosis and pitted keratolysis treated with botulinum toxin injection. Dermatol Surg 2004;30:1510-1514.
1. Zaias N, Taplin D, Rebell G. Pitted keratolysis. Arch Dermatol 1965;92:151-154.
2. Longshaw CM, Wright JD, Farrell AM, Holland KT. Kytococcus sedentarius, the organism associated with pitted keratolysis, produces two keratin-degrading enzymes. J Appl Microbiol 2002;93:810-816.
3. Wohlrab J, Rohrbach D, Marsch WC. Keratolysis sulcata (pitted keratolysis): clinical symptoms with different histological correlates. Br J Dermatol 2000;143:1348-1349.
4. de Almeida HL, Jr, de Castro LA, Rocha NE, Abrantes VL. Ultrastructure of pitted keratolysis. Int J Dermatol 2000;39:698-701.
5. Tamura BM, Cuce LC, Souza RL, Levites J. Plantar hyperhidrosis and pitted keratolysis treated with botulinum toxin injection. Dermatol Surg 2004;30:1510-1514.