User login
Atopic Dermatitis Topical Therapies: Study of YouTube Videos as a Source of Patient Information
To the Editor:
Atopic dermatitis (eczema) affects approximately 20% of children worldwide.1 In atopic dermatitis management, patient education is crucial for optimal outcomes.2 The COVID-19 pandemic has impacted patient-physician interactions. To ensure safety of patients and physicians, visits may have been canceled, postponed, or conducted virtually, leaving less time for discussion and questions.3 As a consequence, patients may seek information about atopic dermatitis from alternative sources, including YouTube videos. We performed a cross-sectional study to analyze YouTube videos about topical treatments for atopic dermatitis.
During the week of July 16, 2020, we performed 4 private browser YouTube searches with default filters using the following terms: eczema topicals, eczema topical treatments, atopic dermatitis topicals, and atopic dermatitis topical treatments. For video selection, we defined topical treatments as topical corticosteroids, topical calcineurin inhibitors, crisaborole, emollients, wet wraps, and any prospective treatment topically administered. For each of the 4 searches, 2 researchers (A.M. and A.T.) independently examined the top 75 videos, yielding a total of 300 videos. Of them, 98 videos were duplicates, 19 videos were not about atopic dermatitis, and 91 videos were not about topical treatments, leaving a total of 92 videos for analysis (Figure 1).
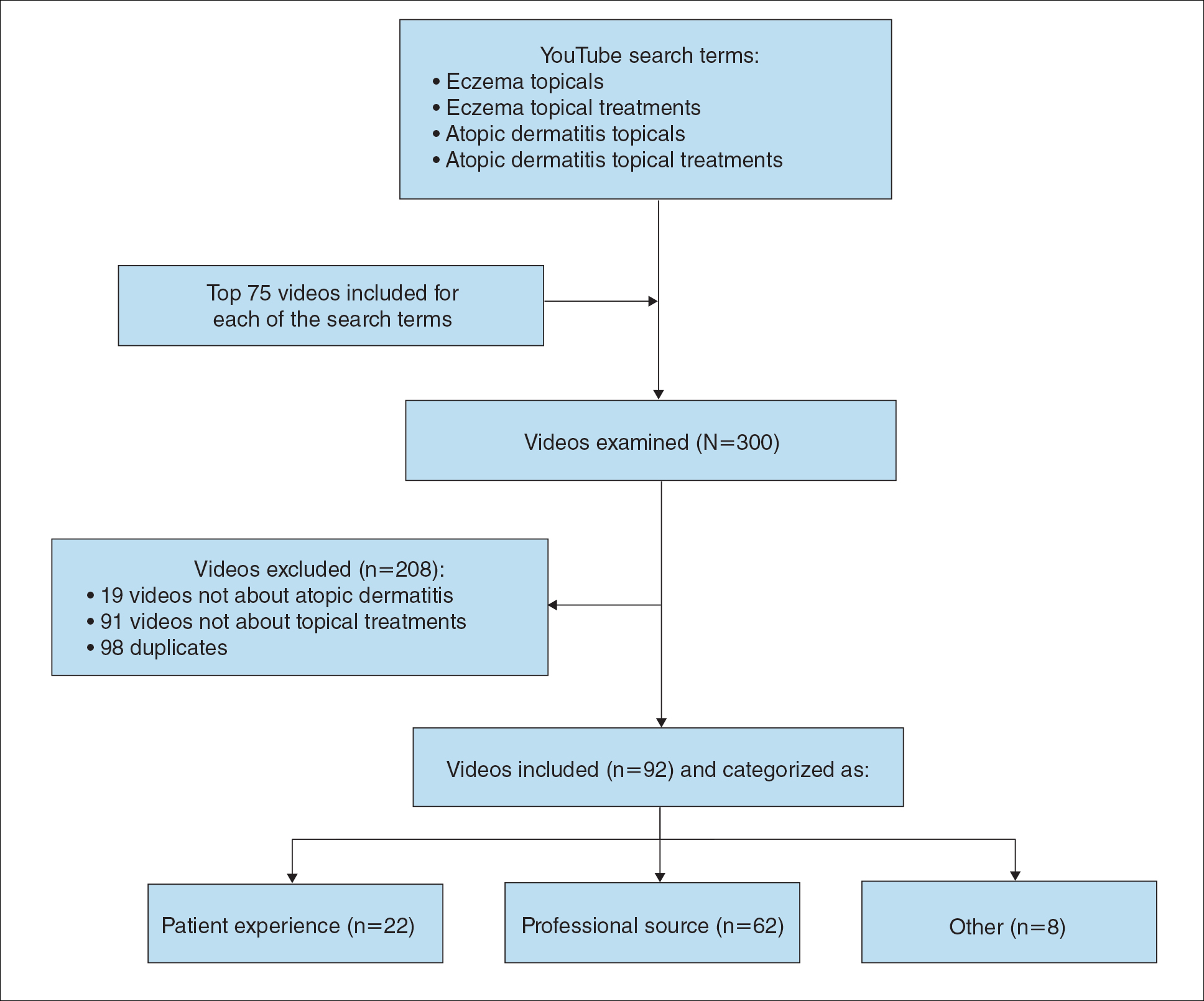
For the 92 included videos, the length; upload year; number of views, likes, dislikes, and comments; interaction ratio (IR)(the sum of likes, dislikes, and comments divided by the number of views); and video content were determined. The videos were placed into mutually exclusive categories as follows: (1) patient experience, defined as a video about patient perspective; (2) professional source, defined as a video featuring a physician, physician extender, pharmacist, or scientist, or produced by a formal organization; or (3) other. The DISCERN Instrument was used for grading the reliability and quality of the 92 included videos. This instrument consists of 16 questions with the responses rated on a scale of 1 to 5.4 For analysis of DISCERN scores, patient experience and other videos were grouped together as nonprofessional source videos. A 2-sample t-test was used to compare DISCERN scores between professional source and nonprofessional source videos.
Most videos were uploaded in 2017 (n=19), 2018 (n=23), and 2019 (n=25), but 20 were uploaded in 2012-2016 and 5 were uploaded in 2020. The 92 videos had a mean length of 8 minutes and 35 seconds (range, 30 seconds to 62 minutes and 23 seconds).
Patient experience videos accounted for 23.9% (n=22) of videos. These videos discussed topical steroid withdrawal (TSW)(n=16), instructions for making emollients (n=2), and treatment successes (n=4). Professional source videos represented 67.4% (n=62) of videos. Of them, 40.3% (n=25) were physician oriented, defined as having extensive medical terminology or qualifying for continuing medical education credit. Three (4.8%) of the professional source videos were sponsored by a drug company. Other constituted the remaining 8.7% (n=8) of videos. Patient experience videos had more views (median views [interquartile range], 6865 [10,307]) and higher engagement (median IR [interquartile range], 0.038 [0.022]) than professional source videos (views: median views [interquartile range], 1052.5 [10,610.5]; engagement: median IR [interquartile range], 0.006 [0.008]).
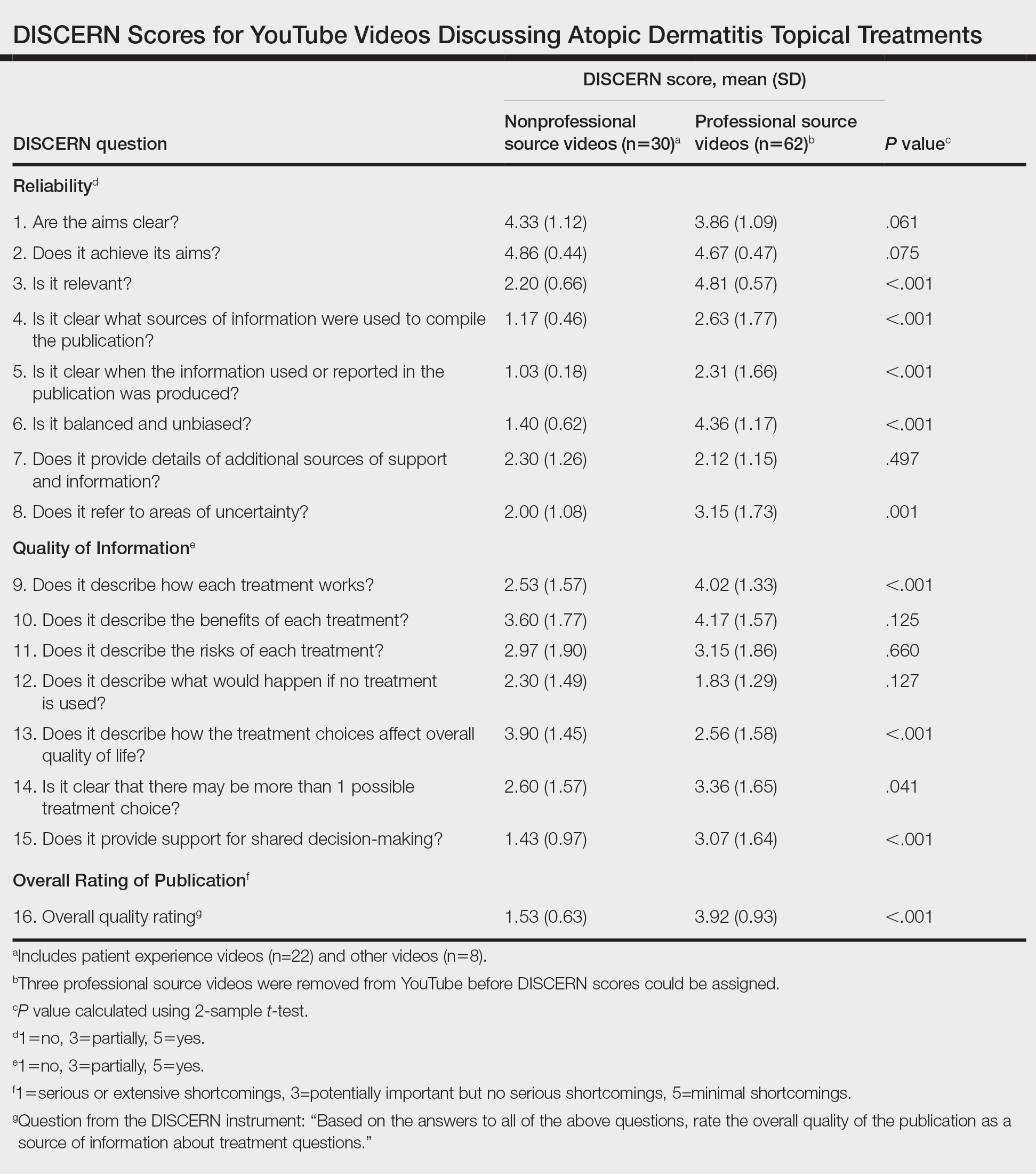
Although less popular, professional source videos had a significantly higher DISCERN overall quality rating score (question 16) compared to those categorized as nonprofessional source (3.92 vs 1.53; P<.001). In contrast, nonprofessional source videos scored significantly higher on the quality-of-life question (question 13) compared to professional source videos (3.90 vs 2.56; P<.001)(eTable). (Three professional source videos were removed from YouTube before DISCERN scores could be assigned.)
Notably, 20.7% (n=19) of the 92 videos discussed TSW, and most of them were patient experiences (n=16). Other categories included topical steroids excluding TSW (n=11), steroid phobia (n=2), topical calcineurin inhibitors (n=2), crisaborole (n=6), news broadcast (n=7), wet wraps (n=5), product advertisement (n=7), and research (n=11)(Figure 2). Interestingly, there were no videos focusing on the calcineurin inhibitor black box warning.

Similar to prior studies, our results indicate preference for patient-generated videos over videos produced by or including a professional source.5 Additionally, only 3 of 19 videos about TSW were from a professional source, increasing the potential for patient misconceptions about topical corticosteroids. Future studies should examine the educational impact of patient-generated videos as well as features that make the patient experience videos more desirable for viewing.
- Mueller SM, Hongler VNS, Jungo P, et al. Fiction, falsehoods, and few facts: cross-sectional study on the content-related quality of atopic eczema-related videos on YouTube. J Med Internet Res. 2020;22:e15599. doi:10.2196/15599
- Torres T, Ferreira EO, Gonçalo M, et al. Update on atopic dermatitis. Acta Med Port. 2019;32:606-613. doi:10.20344/amp.11963
- Vogler SA, Lightner AL. Rethinking how we care for our patients in a time of social distancing during the COVID-19 pandemic. Br J Surg. 2020;107:937-939. doi:10.1002/bjs.11636
- The DISCERN Instrument. discern online. Accessed January 22, 2021. http://www.discern.org.uk/discern_instrument.php
- Pithadia DJ, Reynolds KA, Lee EB, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube? [published online April 16, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1755418
To the Editor:
Atopic dermatitis (eczema) affects approximately 20% of children worldwide.1 In atopic dermatitis management, patient education is crucial for optimal outcomes.2 The COVID-19 pandemic has impacted patient-physician interactions. To ensure safety of patients and physicians, visits may have been canceled, postponed, or conducted virtually, leaving less time for discussion and questions.3 As a consequence, patients may seek information about atopic dermatitis from alternative sources, including YouTube videos. We performed a cross-sectional study to analyze YouTube videos about topical treatments for atopic dermatitis.
During the week of July 16, 2020, we performed 4 private browser YouTube searches with default filters using the following terms: eczema topicals, eczema topical treatments, atopic dermatitis topicals, and atopic dermatitis topical treatments. For video selection, we defined topical treatments as topical corticosteroids, topical calcineurin inhibitors, crisaborole, emollients, wet wraps, and any prospective treatment topically administered. For each of the 4 searches, 2 researchers (A.M. and A.T.) independently examined the top 75 videos, yielding a total of 300 videos. Of them, 98 videos were duplicates, 19 videos were not about atopic dermatitis, and 91 videos were not about topical treatments, leaving a total of 92 videos for analysis (Figure 1).

For the 92 included videos, the length; upload year; number of views, likes, dislikes, and comments; interaction ratio (IR)(the sum of likes, dislikes, and comments divided by the number of views); and video content were determined. The videos were placed into mutually exclusive categories as follows: (1) patient experience, defined as a video about patient perspective; (2) professional source, defined as a video featuring a physician, physician extender, pharmacist, or scientist, or produced by a formal organization; or (3) other. The DISCERN Instrument was used for grading the reliability and quality of the 92 included videos. This instrument consists of 16 questions with the responses rated on a scale of 1 to 5.4 For analysis of DISCERN scores, patient experience and other videos were grouped together as nonprofessional source videos. A 2-sample t-test was used to compare DISCERN scores between professional source and nonprofessional source videos.
Most videos were uploaded in 2017 (n=19), 2018 (n=23), and 2019 (n=25), but 20 were uploaded in 2012-2016 and 5 were uploaded in 2020. The 92 videos had a mean length of 8 minutes and 35 seconds (range, 30 seconds to 62 minutes and 23 seconds).
Patient experience videos accounted for 23.9% (n=22) of videos. These videos discussed topical steroid withdrawal (TSW)(n=16), instructions for making emollients (n=2), and treatment successes (n=4). Professional source videos represented 67.4% (n=62) of videos. Of them, 40.3% (n=25) were physician oriented, defined as having extensive medical terminology or qualifying for continuing medical education credit. Three (4.8%) of the professional source videos were sponsored by a drug company. Other constituted the remaining 8.7% (n=8) of videos. Patient experience videos had more views (median views [interquartile range], 6865 [10,307]) and higher engagement (median IR [interquartile range], 0.038 [0.022]) than professional source videos (views: median views [interquartile range], 1052.5 [10,610.5]; engagement: median IR [interquartile range], 0.006 [0.008]).
Although less popular, professional source videos had a significantly higher DISCERN overall quality rating score (question 16) compared to those categorized as nonprofessional source (3.92 vs 1.53; P<.001). In contrast, nonprofessional source videos scored significantly higher on the quality-of-life question (question 13) compared to professional source videos (3.90 vs 2.56; P<.001)(eTable). (Three professional source videos were removed from YouTube before DISCERN scores could be assigned.)

Notably, 20.7% (n=19) of the 92 videos discussed TSW, and most of them were patient experiences (n=16). Other categories included topical steroids excluding TSW (n=11), steroid phobia (n=2), topical calcineurin inhibitors (n=2), crisaborole (n=6), news broadcast (n=7), wet wraps (n=5), product advertisement (n=7), and research (n=11)(Figure 2). Interestingly, there were no videos focusing on the calcineurin inhibitor black box warning.

Similar to prior studies, our results indicate preference for patient-generated videos over videos produced by or including a professional source.5 Additionally, only 3 of 19 videos about TSW were from a professional source, increasing the potential for patient misconceptions about topical corticosteroids. Future studies should examine the educational impact of patient-generated videos as well as features that make the patient experience videos more desirable for viewing.
To the Editor:
Atopic dermatitis (eczema) affects approximately 20% of children worldwide.1 In atopic dermatitis management, patient education is crucial for optimal outcomes.2 The COVID-19 pandemic has impacted patient-physician interactions. To ensure safety of patients and physicians, visits may have been canceled, postponed, or conducted virtually, leaving less time for discussion and questions.3 As a consequence, patients may seek information about atopic dermatitis from alternative sources, including YouTube videos. We performed a cross-sectional study to analyze YouTube videos about topical treatments for atopic dermatitis.
During the week of July 16, 2020, we performed 4 private browser YouTube searches with default filters using the following terms: eczema topicals, eczema topical treatments, atopic dermatitis topicals, and atopic dermatitis topical treatments. For video selection, we defined topical treatments as topical corticosteroids, topical calcineurin inhibitors, crisaborole, emollients, wet wraps, and any prospective treatment topically administered. For each of the 4 searches, 2 researchers (A.M. and A.T.) independently examined the top 75 videos, yielding a total of 300 videos. Of them, 98 videos were duplicates, 19 videos were not about atopic dermatitis, and 91 videos were not about topical treatments, leaving a total of 92 videos for analysis (Figure 1).

For the 92 included videos, the length; upload year; number of views, likes, dislikes, and comments; interaction ratio (IR)(the sum of likes, dislikes, and comments divided by the number of views); and video content were determined. The videos were placed into mutually exclusive categories as follows: (1) patient experience, defined as a video about patient perspective; (2) professional source, defined as a video featuring a physician, physician extender, pharmacist, or scientist, or produced by a formal organization; or (3) other. The DISCERN Instrument was used for grading the reliability and quality of the 92 included videos. This instrument consists of 16 questions with the responses rated on a scale of 1 to 5.4 For analysis of DISCERN scores, patient experience and other videos were grouped together as nonprofessional source videos. A 2-sample t-test was used to compare DISCERN scores between professional source and nonprofessional source videos.
Most videos were uploaded in 2017 (n=19), 2018 (n=23), and 2019 (n=25), but 20 were uploaded in 2012-2016 and 5 were uploaded in 2020. The 92 videos had a mean length of 8 minutes and 35 seconds (range, 30 seconds to 62 minutes and 23 seconds).
Patient experience videos accounted for 23.9% (n=22) of videos. These videos discussed topical steroid withdrawal (TSW)(n=16), instructions for making emollients (n=2), and treatment successes (n=4). Professional source videos represented 67.4% (n=62) of videos. Of them, 40.3% (n=25) were physician oriented, defined as having extensive medical terminology or qualifying for continuing medical education credit. Three (4.8%) of the professional source videos were sponsored by a drug company. Other constituted the remaining 8.7% (n=8) of videos. Patient experience videos had more views (median views [interquartile range], 6865 [10,307]) and higher engagement (median IR [interquartile range], 0.038 [0.022]) than professional source videos (views: median views [interquartile range], 1052.5 [10,610.5]; engagement: median IR [interquartile range], 0.006 [0.008]).
Although less popular, professional source videos had a significantly higher DISCERN overall quality rating score (question 16) compared to those categorized as nonprofessional source (3.92 vs 1.53; P<.001). In contrast, nonprofessional source videos scored significantly higher on the quality-of-life question (question 13) compared to professional source videos (3.90 vs 2.56; P<.001)(eTable). (Three professional source videos were removed from YouTube before DISCERN scores could be assigned.)

Notably, 20.7% (n=19) of the 92 videos discussed TSW, and most of them were patient experiences (n=16). Other categories included topical steroids excluding TSW (n=11), steroid phobia (n=2), topical calcineurin inhibitors (n=2), crisaborole (n=6), news broadcast (n=7), wet wraps (n=5), product advertisement (n=7), and research (n=11)(Figure 2). Interestingly, there were no videos focusing on the calcineurin inhibitor black box warning.

Similar to prior studies, our results indicate preference for patient-generated videos over videos produced by or including a professional source.5 Additionally, only 3 of 19 videos about TSW were from a professional source, increasing the potential for patient misconceptions about topical corticosteroids. Future studies should examine the educational impact of patient-generated videos as well as features that make the patient experience videos more desirable for viewing.
- Mueller SM, Hongler VNS, Jungo P, et al. Fiction, falsehoods, and few facts: cross-sectional study on the content-related quality of atopic eczema-related videos on YouTube. J Med Internet Res. 2020;22:e15599. doi:10.2196/15599
- Torres T, Ferreira EO, Gonçalo M, et al. Update on atopic dermatitis. Acta Med Port. 2019;32:606-613. doi:10.20344/amp.11963
- Vogler SA, Lightner AL. Rethinking how we care for our patients in a time of social distancing during the COVID-19 pandemic. Br J Surg. 2020;107:937-939. doi:10.1002/bjs.11636
- The DISCERN Instrument. discern online. Accessed January 22, 2021. http://www.discern.org.uk/discern_instrument.php
- Pithadia DJ, Reynolds KA, Lee EB, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube? [published online April 16, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1755418
- Mueller SM, Hongler VNS, Jungo P, et al. Fiction, falsehoods, and few facts: cross-sectional study on the content-related quality of atopic eczema-related videos on YouTube. J Med Internet Res. 2020;22:e15599. doi:10.2196/15599
- Torres T, Ferreira EO, Gonçalo M, et al. Update on atopic dermatitis. Acta Med Port. 2019;32:606-613. doi:10.20344/amp.11963
- Vogler SA, Lightner AL. Rethinking how we care for our patients in a time of social distancing during the COVID-19 pandemic. Br J Surg. 2020;107:937-939. doi:10.1002/bjs.11636
- The DISCERN Instrument. discern online. Accessed January 22, 2021. http://www.discern.org.uk/discern_instrument.php
- Pithadia DJ, Reynolds KA, Lee EB, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube? [published online April 16, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1755418
Practice Points
- YouTube is a readily accessible resource for educating patients about topical treatments for atopic dermatitis.
- Although professional source videos comprised a larger percentage of the videos included within our study, patient experience videos had a higher number of views and engagement.
- Twenty-one percent (19/92) of the videos examined in our study discussed topical steroid withdrawal, and the majority of them were patient experience videos.
Home Phototherapy During the COVID-19 Pandemic
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
Office-based phototherapy practices have closed or are operating below capacity because of the coronavirus disease 2019 (COVID-19) pandemic.1 Social distancing measures to reduce virus transmission are a significant driving factor.1-3 In the age of biologics, other options requiring fewer patient visits are available, such as UVB phototherapy. UV phototherapy is considered first line when more than 10% of the body surface area is affected.4 Although phototherapy often is performed in the office, it also may be delivered at home.2 Home-based phototherapy is safe, effective, and similar in cost to office-based phototherapy.4 Currently, there are limited COVID-19–specific guidelines for home-based phototherapy.
The risks and sequelae of COVID-19 are still being investigated, with cases varying by location. As such, local and national public health recommendations are evolving. Dermatologists must make individualized decisions about practice services, as local restrictions differ. As office-based phototherapy services may struggle to implement mitigation strategies, home-based phototherapy is an increasingly viable treatment option.1,4,5 Patient benefits of home therapy include improved treatment compliance; greater patient satisfaction; reduced travel/waiting time; and reduced long-term cost, including co-pays, depending on insurance coverage.2,4
We aim to provide recommendations on home-based phototherapy during the pandemic. Throughout the decision-making process, careful consideration of safety, risks, benefits, and treatment options for physicians, staff, and patients will be vital to the successful implementation of home-based phototherapy. Our recommendations are based on maximizing benefits and minimizing risks.
Considerations for Physicians
Physicians should take the following steps when assessing if home phototherapy is an option for each patient.1,2,4
• Determine patient eligibility for phototherapy treatment if currently not on phototherapy
• Carefully review patient and provider requirements for home phototherapy supplier
• Review patient history of treatment compliance
• Determine insurance coverage and consider exclusion criteria
• Review prior treatments
• Provide education on side effects
• Provide education on signs of adequate treatment response
• Indicate the type of UV light and unit on the prescription
• Consider whether the patient is in the maintenance or initiation phase when providing recommendations
• Work with the supplier if the light therapy unit is denied by submitting an appeal or prescribing a different unit
• Follow up with telemedicine to assess treatment effectiveness and monitor for adverse effects
Considerations for Patients
Counsel patients to weigh the risks and benefits of home phototherapy prescription and usage.1,2,4
• Evaluate cost
• Carefully review patient and provider requirements for home phototherapy supplier
• Ensure a complete understanding of treatment schedule
• Properly utilize protective equipment (eg, genital shields for men, eye shields for all)
• Avoid sharing phototherapy units with household members
• Disinfect and maintain units
• Maintain proper ventilation of spaces
• Maintain treatment log
• Attend follow-up
Treatment Alternatives
For patients with severe psoriasis, there are alternative treatments to office and home phototherapy. Biologics, immunosuppressive therapies, and other treatment options may be considered on a case-by-case basis.3,4,6 Currently, recommendations for the risk of COVID-19 with biologics or systemic immunosuppressive therapies remains inconsistent and should be carefully considered when providing alternative treatments.7-11
Final Thoughts
As restrictions are lifted according to local public health measures, prepandemic office phototherapy practices may resume operations. Home phototherapy is a practical and effective alternative for treatment of psoriasis when access to the office setting is limited.
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
- Lim HW, Feldman SR, Van Voorhees AS, et al. Recommendations for phototherapy during the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:287-288.
Anderson KL, Feldman SR. A guide to prescribing home phototherapy for patients with psoriasis: the appropriate patient, the type of unit, the treatment regimen, and the potential obstacles. J Am Acad Dermatol. 2015;72:868.E1-878.E1. - Palmore TN, Smith BA. Coronavirus disease 2019 (COVID-19): infection control in health care and home settings. UpToDate. Updated January 7, 2021. Accessed January 25, 2021.https://www.uptodate.com/contents/coronavirus-disease-2019-covid-19-infection-control-in-health-care-and-home-settings
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomised controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Sadeghinia A, Daneshpazhooh M. Immunosuppressive drugs for patients with psoriasis during the COVID-19 pandemic era. a review [published online November 3, 2020]. Dermatol Ther. 2020:E14498. doi:10.1111/dth.14498
- Damiani G, Pacifico A, Bragazzi NL, et al. Biologics increase the risk of SARS-CoV-2 infection and hospitalization, but not ICU admission and death: real-life data from a large cohort during red-zone declaration. Dermatol Ther. 2020;33:E13475.
- Lebwohl M, Rivera-Oyola R, Murrell DF. Should biologics for psoriasis be interrupted in the era of COVID-19? J Am Acad Dermatol. 2020;82:1217-1218.
- Mehta P, Ciurtin C, Scully M, et al. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56:2001919.
- Walz L, Cohen AJ, Rebaza AP, et al. JAK-inhibitor and type I interferon ability to produce favorable clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. BMC Infect Dis. 2021;21:47.
- Carugno A, Gambini DM, Raponi F, et al. COVID-19 and biologics for psoriasis: a high-epidemic area experience-Bergamo, Lombardy, Italy. J Am Acad Dermatol. 2020;83:292-294.
- Gisondi P, Piaserico S, Naldi L, et al. Incidence rates of hospitalization and death from COVID-19 in patients with psoriasis receiving biological treatment: a Northern Italy experience [published online November 5, 2020]. J Allergy Clin Immunol. doi:10.1016/j.jaci.2020.10.032
Practice Points
- Home phototherapy is a safe and effective option for patients with psoriasis during the coronavirus disease 2019 (COVID-19) pandemic.
- Although a consensus has not been reached with systemic immunosuppressive therapies for patients with psoriasis and the risk of COVID-19, we continue to recommend caution and careful monitoring of clinical outcomes for patients.