User login
Robotic Pet Therapy in the Intensive Care Unit
Critical illness is commonly associated with interrelated conditions including pain, agitation, delirium, immobility, and sleep disruption (PADIS). Managing PADIS is often complex and includes pharmacologic and nonpharmacologic interventions.1 Incorporating multifaceted practices to enhance PADIS management has been shown to improve several intensive care unit (ICU)-related outcomes.2
Many pharmacologic PADIS treatments are ineffective or associated with adverse effects. For example, antipsychotics used for treating ICU-related delirium have not shown improved outcomes.3,4 Commonly used medications for agitation, such as benzodiazepines, increase delirium risk.5,6 Because of these limitations, several nonpharmacologic interventions for PADIS have been evaluated.
Pet therapy has been implemented in some ICU settings, but is not widely adopted.7 Also referred to as animal-assisted activities, animal-assisted therapy, or animal-assisted interventions, pet therapy typically involves interaction between a patient and a live animal (most commonly a dog) under the direction of an animal handler, with the intention of providing therapeutic benefit. Interactions frequently include meet and greet activities such as petting, but also could include walking or other activities. Pet therapy has been reported to reduce pain, agitation, and stress among ICU patients.8 Introducing a pet therapy program with live animals in the ICU could be challenging because of factors such as identifying trained, accredited animals and handlers, and managing infection control and other risks.9 As an alternative to live pets, robotic pet therapy has been shown to be beneficial—mostly outside the ICU—in settings such as long-term care.10,11 Although uncommon, robotic pets have been used in the ICU and hospital settings for therapeutic purposes.12 Robotic pets reduce many concerns associated with live animals while mimicking the behaviors of live animals and potentially offering many of the same benefits.
OBSERVATIONS
The North Florida/South Georgia Veterans Health System (NF/SGVHS) implemented a novel robotic pet therapy program for patients requiring ICU care to improve the treatment of PADIS. Funding was provided through a Veterans Health Administration Innovation Grant procured by a clinical pharmacy specialist as the program’s champion. Goals of the robotic pet therapy program include reductions in: distressing symptoms associated with PADIS, use of psychoactive drugs and physical restraints, and ICU length of stay. The ICU team developed standard operating procedures and an order menu, which were integrated into the ICU prescriber ordering menu. Patients were selected for pet therapy based on PADIS scores and potential for positive response to pet therapy as assessed by the ICU team.Patients in medical and surgical ICU settings were eligible for the program. The robotic pets used in the program were Joy for All Companion Pets (Ageless Innovation LLC). Robotic cats and dogs were available and pets were “adopted’ by each patient (Figure). As an infection control measure, pets were not reissued or shared amongpatients and pets could be cleaned with a disinfectant solution. Nurses were primarily responsible for monitoring and documenting responses to robotic pet therapy.
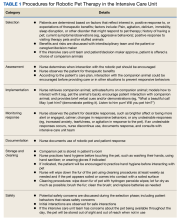
It was necessary to secure buy-in from several services to successfully implement the program. The critical care clinical pharmacy specialists were responsible for ordering, storing, and dispensing the robotic pets. The NF/SGVHS innovation specialist helped secure funding, procure the robotic pet, and promote the program. The standard operating procedures for the program were developed by a multidisciplinary team with input from critical care nurses, intensivists, pharmacists, patient safety, and infection control (Table 1). Success of the program also required buy-in from ICU team members.
Program Impact
A retrospective cohort study was conducted to assess for improvements in PADIS symptoms and medication use post-intervention. Patients were included if they received robotic pet therapy in the ICU from July 10, 2019, to February 1, 2021. Individuals aged < 18 years or > 89 years, were pregnant, or were not receiving ICU-level care were excluded. Outcomes assessed included improvement in pain scores, agitation scores, sleep quality, resolution of delirium, and use of pain or psychoactive medications during patients’ ICU stay.
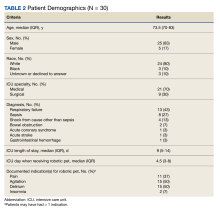
Thirty patients were included in the study (Table 2). After receiving a robotic pet, 9 (30%) patients recorded decreased pain scores, 15 (50%) recorded decreased agitation scores, 8 (27%) had resolution of delirium, and 2 (7%) described improvement in sleep. Pain medication use decreased in 12 (40%) patients and psychoactive medication use was reduced in 7 (23%) patients.
Limitations
The robotic pet therapy program has shown promising results; however, some aspects merit discussion. Evaluation of this program is limited by factors such as the observational study design, single-center patient sample, and lack of comparator group. Although no known adverse effects of robotic pet therapy were seen, it is possible that some patients may not have a favorable response. Challenges of implementing a robotic pet therapy program include cost and additional operational activities (storage, ordering, dispensing) necessary to maintain the program. Additional research is needed to evaluate the impact of robotic pet therapy on other outcomes including cost, ICU length of stay, and patient satisfaction.
CONCLUSIONS
Robotic pet therapy can be successfully implemented in the ICU and appears to provide a simple, safe, beneficial, nonpharmacologic intervention for PADIS. This study showed that many patients had favorable response to robotic pet therapy, indicating that it may be a viable alternative to traditional pet therapy. Other health systems could benefit from implementing programs similar to the robotic pet therapy program at NF/SGVHS.
Acknowledgments
The author would like to acknowledge Simran Panesar, PharmD, and Theresa Faison, PharmD, for their contributions to this project.
1. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825-e873. doi:10.1097/CCM.0000000000003299
2. Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. 2019;47:3-14. doi:10.1097/CCM.0000000000003482
3. Andersen-Ranberg NC, Poulsen LM, Perner A, et al; AID-ICU Trial Group. Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. 2022;387:2425-2435. doi:10.1056/NEJMoa2211868
4. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506-2516. doi:10.1056/NEJMoa1808217
5. Riker RR, Shehabi Y, Bokesch PM, et al; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-499. doi:10.1001/jama.2009.56
6. Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21-26. doi:10.1097/00000542-200601000-00005
7. Society of Critical Care Medicine. ICU liberation bundle. Accessed February 27, 2024. https://www.sccm.org/ICULiberation/Home/ABCDEF-Bundles
8. Lovell T, Ranse K. Animal-assisted activities in the intensive care unit: a scoping review. Intensive Crit Care Nurs. 2022;73:103304. doi:10.1016/j.iccn.2022.103304
9. Hosey MM, Jaskulski J, Wegener ST, Chlan LL, Needham DM. Animal-assisted intervention in the ICU: a tool for humanization. Crit Care. 2018;22:22. doi:10.1186/s13054-018-1946-8
10. Jøranson N, Pedersen I, Rokstad AM, Ihlebæk C. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J Am Med Dir Assoc. 2015;16:867-873. doi:10.1016/j.jamda.2015.05.002
11. Robinson H, Macdonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013;14:661-667. doi:10.1016/j.jamda.2013.02.007
12. Schulman-Marcus J, Mookherjee S, Rice L, Lyubarova R. New approaches for the treatment of delirium: a case for robotic pets. Am J Med. 2019;132:781-782. doi:10.1016/j.amjmed.2018.12.039
Critical illness is commonly associated with interrelated conditions including pain, agitation, delirium, immobility, and sleep disruption (PADIS). Managing PADIS is often complex and includes pharmacologic and nonpharmacologic interventions.1 Incorporating multifaceted practices to enhance PADIS management has been shown to improve several intensive care unit (ICU)-related outcomes.2
Many pharmacologic PADIS treatments are ineffective or associated with adverse effects. For example, antipsychotics used for treating ICU-related delirium have not shown improved outcomes.3,4 Commonly used medications for agitation, such as benzodiazepines, increase delirium risk.5,6 Because of these limitations, several nonpharmacologic interventions for PADIS have been evaluated.
Pet therapy has been implemented in some ICU settings, but is not widely adopted.7 Also referred to as animal-assisted activities, animal-assisted therapy, or animal-assisted interventions, pet therapy typically involves interaction between a patient and a live animal (most commonly a dog) under the direction of an animal handler, with the intention of providing therapeutic benefit. Interactions frequently include meet and greet activities such as petting, but also could include walking or other activities. Pet therapy has been reported to reduce pain, agitation, and stress among ICU patients.8 Introducing a pet therapy program with live animals in the ICU could be challenging because of factors such as identifying trained, accredited animals and handlers, and managing infection control and other risks.9 As an alternative to live pets, robotic pet therapy has been shown to be beneficial—mostly outside the ICU—in settings such as long-term care.10,11 Although uncommon, robotic pets have been used in the ICU and hospital settings for therapeutic purposes.12 Robotic pets reduce many concerns associated with live animals while mimicking the behaviors of live animals and potentially offering many of the same benefits.
OBSERVATIONS
The North Florida/South Georgia Veterans Health System (NF/SGVHS) implemented a novel robotic pet therapy program for patients requiring ICU care to improve the treatment of PADIS. Funding was provided through a Veterans Health Administration Innovation Grant procured by a clinical pharmacy specialist as the program’s champion. Goals of the robotic pet therapy program include reductions in: distressing symptoms associated with PADIS, use of psychoactive drugs and physical restraints, and ICU length of stay. The ICU team developed standard operating procedures and an order menu, which were integrated into the ICU prescriber ordering menu. Patients were selected for pet therapy based on PADIS scores and potential for positive response to pet therapy as assessed by the ICU team.Patients in medical and surgical ICU settings were eligible for the program. The robotic pets used in the program were Joy for All Companion Pets (Ageless Innovation LLC). Robotic cats and dogs were available and pets were “adopted’ by each patient (Figure). As an infection control measure, pets were not reissued or shared amongpatients and pets could be cleaned with a disinfectant solution. Nurses were primarily responsible for monitoring and documenting responses to robotic pet therapy.
It was necessary to secure buy-in from several services to successfully implement the program. The critical care clinical pharmacy specialists were responsible for ordering, storing, and dispensing the robotic pets. The NF/SGVHS innovation specialist helped secure funding, procure the robotic pet, and promote the program. The standard operating procedures for the program were developed by a multidisciplinary team with input from critical care nurses, intensivists, pharmacists, patient safety, and infection control (Table 1). Success of the program also required buy-in from ICU team members.
Program Impact
A retrospective cohort study was conducted to assess for improvements in PADIS symptoms and medication use post-intervention. Patients were included if they received robotic pet therapy in the ICU from July 10, 2019, to February 1, 2021. Individuals aged < 18 years or > 89 years, were pregnant, or were not receiving ICU-level care were excluded. Outcomes assessed included improvement in pain scores, agitation scores, sleep quality, resolution of delirium, and use of pain or psychoactive medications during patients’ ICU stay.
Thirty patients were included in the study (Table 2). After receiving a robotic pet, 9 (30%) patients recorded decreased pain scores, 15 (50%) recorded decreased agitation scores, 8 (27%) had resolution of delirium, and 2 (7%) described improvement in sleep. Pain medication use decreased in 12 (40%) patients and psychoactive medication use was reduced in 7 (23%) patients.
Limitations
The robotic pet therapy program has shown promising results; however, some aspects merit discussion. Evaluation of this program is limited by factors such as the observational study design, single-center patient sample, and lack of comparator group. Although no known adverse effects of robotic pet therapy were seen, it is possible that some patients may not have a favorable response. Challenges of implementing a robotic pet therapy program include cost and additional operational activities (storage, ordering, dispensing) necessary to maintain the program. Additional research is needed to evaluate the impact of robotic pet therapy on other outcomes including cost, ICU length of stay, and patient satisfaction.
CONCLUSIONS
Robotic pet therapy can be successfully implemented in the ICU and appears to provide a simple, safe, beneficial, nonpharmacologic intervention for PADIS. This study showed that many patients had favorable response to robotic pet therapy, indicating that it may be a viable alternative to traditional pet therapy. Other health systems could benefit from implementing programs similar to the robotic pet therapy program at NF/SGVHS.
Acknowledgments
The author would like to acknowledge Simran Panesar, PharmD, and Theresa Faison, PharmD, for their contributions to this project.
Critical illness is commonly associated with interrelated conditions including pain, agitation, delirium, immobility, and sleep disruption (PADIS). Managing PADIS is often complex and includes pharmacologic and nonpharmacologic interventions.1 Incorporating multifaceted practices to enhance PADIS management has been shown to improve several intensive care unit (ICU)-related outcomes.2
Many pharmacologic PADIS treatments are ineffective or associated with adverse effects. For example, antipsychotics used for treating ICU-related delirium have not shown improved outcomes.3,4 Commonly used medications for agitation, such as benzodiazepines, increase delirium risk.5,6 Because of these limitations, several nonpharmacologic interventions for PADIS have been evaluated.
Pet therapy has been implemented in some ICU settings, but is not widely adopted.7 Also referred to as animal-assisted activities, animal-assisted therapy, or animal-assisted interventions, pet therapy typically involves interaction between a patient and a live animal (most commonly a dog) under the direction of an animal handler, with the intention of providing therapeutic benefit. Interactions frequently include meet and greet activities such as petting, but also could include walking or other activities. Pet therapy has been reported to reduce pain, agitation, and stress among ICU patients.8 Introducing a pet therapy program with live animals in the ICU could be challenging because of factors such as identifying trained, accredited animals and handlers, and managing infection control and other risks.9 As an alternative to live pets, robotic pet therapy has been shown to be beneficial—mostly outside the ICU—in settings such as long-term care.10,11 Although uncommon, robotic pets have been used in the ICU and hospital settings for therapeutic purposes.12 Robotic pets reduce many concerns associated with live animals while mimicking the behaviors of live animals and potentially offering many of the same benefits.
OBSERVATIONS
The North Florida/South Georgia Veterans Health System (NF/SGVHS) implemented a novel robotic pet therapy program for patients requiring ICU care to improve the treatment of PADIS. Funding was provided through a Veterans Health Administration Innovation Grant procured by a clinical pharmacy specialist as the program’s champion. Goals of the robotic pet therapy program include reductions in: distressing symptoms associated with PADIS, use of psychoactive drugs and physical restraints, and ICU length of stay. The ICU team developed standard operating procedures and an order menu, which were integrated into the ICU prescriber ordering menu. Patients were selected for pet therapy based on PADIS scores and potential for positive response to pet therapy as assessed by the ICU team.Patients in medical and surgical ICU settings were eligible for the program. The robotic pets used in the program were Joy for All Companion Pets (Ageless Innovation LLC). Robotic cats and dogs were available and pets were “adopted’ by each patient (Figure). As an infection control measure, pets were not reissued or shared amongpatients and pets could be cleaned with a disinfectant solution. Nurses were primarily responsible for monitoring and documenting responses to robotic pet therapy.
It was necessary to secure buy-in from several services to successfully implement the program. The critical care clinical pharmacy specialists were responsible for ordering, storing, and dispensing the robotic pets. The NF/SGVHS innovation specialist helped secure funding, procure the robotic pet, and promote the program. The standard operating procedures for the program were developed by a multidisciplinary team with input from critical care nurses, intensivists, pharmacists, patient safety, and infection control (Table 1). Success of the program also required buy-in from ICU team members.
Program Impact
A retrospective cohort study was conducted to assess for improvements in PADIS symptoms and medication use post-intervention. Patients were included if they received robotic pet therapy in the ICU from July 10, 2019, to February 1, 2021. Individuals aged < 18 years or > 89 years, were pregnant, or were not receiving ICU-level care were excluded. Outcomes assessed included improvement in pain scores, agitation scores, sleep quality, resolution of delirium, and use of pain or psychoactive medications during patients’ ICU stay.
Thirty patients were included in the study (Table 2). After receiving a robotic pet, 9 (30%) patients recorded decreased pain scores, 15 (50%) recorded decreased agitation scores, 8 (27%) had resolution of delirium, and 2 (7%) described improvement in sleep. Pain medication use decreased in 12 (40%) patients and psychoactive medication use was reduced in 7 (23%) patients.
Limitations
The robotic pet therapy program has shown promising results; however, some aspects merit discussion. Evaluation of this program is limited by factors such as the observational study design, single-center patient sample, and lack of comparator group. Although no known adverse effects of robotic pet therapy were seen, it is possible that some patients may not have a favorable response. Challenges of implementing a robotic pet therapy program include cost and additional operational activities (storage, ordering, dispensing) necessary to maintain the program. Additional research is needed to evaluate the impact of robotic pet therapy on other outcomes including cost, ICU length of stay, and patient satisfaction.
CONCLUSIONS
Robotic pet therapy can be successfully implemented in the ICU and appears to provide a simple, safe, beneficial, nonpharmacologic intervention for PADIS. This study showed that many patients had favorable response to robotic pet therapy, indicating that it may be a viable alternative to traditional pet therapy. Other health systems could benefit from implementing programs similar to the robotic pet therapy program at NF/SGVHS.
Acknowledgments
The author would like to acknowledge Simran Panesar, PharmD, and Theresa Faison, PharmD, for their contributions to this project.
1. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825-e873. doi:10.1097/CCM.0000000000003299
2. Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. 2019;47:3-14. doi:10.1097/CCM.0000000000003482
3. Andersen-Ranberg NC, Poulsen LM, Perner A, et al; AID-ICU Trial Group. Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. 2022;387:2425-2435. doi:10.1056/NEJMoa2211868
4. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506-2516. doi:10.1056/NEJMoa1808217
5. Riker RR, Shehabi Y, Bokesch PM, et al; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-499. doi:10.1001/jama.2009.56
6. Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21-26. doi:10.1097/00000542-200601000-00005
7. Society of Critical Care Medicine. ICU liberation bundle. Accessed February 27, 2024. https://www.sccm.org/ICULiberation/Home/ABCDEF-Bundles
8. Lovell T, Ranse K. Animal-assisted activities in the intensive care unit: a scoping review. Intensive Crit Care Nurs. 2022;73:103304. doi:10.1016/j.iccn.2022.103304
9. Hosey MM, Jaskulski J, Wegener ST, Chlan LL, Needham DM. Animal-assisted intervention in the ICU: a tool for humanization. Crit Care. 2018;22:22. doi:10.1186/s13054-018-1946-8
10. Jøranson N, Pedersen I, Rokstad AM, Ihlebæk C. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J Am Med Dir Assoc. 2015;16:867-873. doi:10.1016/j.jamda.2015.05.002
11. Robinson H, Macdonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013;14:661-667. doi:10.1016/j.jamda.2013.02.007
12. Schulman-Marcus J, Mookherjee S, Rice L, Lyubarova R. New approaches for the treatment of delirium: a case for robotic pets. Am J Med. 2019;132:781-782. doi:10.1016/j.amjmed.2018.12.039
1. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825-e873. doi:10.1097/CCM.0000000000003299
2. Pun BT, Balas MC, Barnes-Daly MA, et al. Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative in over 15,000 adults. Crit Care Med. 2019;47:3-14. doi:10.1097/CCM.0000000000003482
3. Andersen-Ranberg NC, Poulsen LM, Perner A, et al; AID-ICU Trial Group. Haloperidol for the treatment of delirium in ICU patients. N Engl J Med. 2022;387:2425-2435. doi:10.1056/NEJMoa2211868
4. Girard TD, Exline MC, Carson SS, et al; MIND-USA Investigators. Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379:2506-2516. doi:10.1056/NEJMoa1808217
5. Riker RR, Shehabi Y, Bokesch PM, et al; SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489-499. doi:10.1001/jama.2009.56
6. Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transitioning to delirium in intensive care unit patients. Anesthesiology. 2006;104:21-26. doi:10.1097/00000542-200601000-00005
7. Society of Critical Care Medicine. ICU liberation bundle. Accessed February 27, 2024. https://www.sccm.org/ICULiberation/Home/ABCDEF-Bundles
8. Lovell T, Ranse K. Animal-assisted activities in the intensive care unit: a scoping review. Intensive Crit Care Nurs. 2022;73:103304. doi:10.1016/j.iccn.2022.103304
9. Hosey MM, Jaskulski J, Wegener ST, Chlan LL, Needham DM. Animal-assisted intervention in the ICU: a tool for humanization. Crit Care. 2018;22:22. doi:10.1186/s13054-018-1946-8
10. Jøranson N, Pedersen I, Rokstad AM, Ihlebæk C. Effects on symptoms of agitation and depression in persons with dementia participating in robot-assisted activity: a cluster-randomized controlled trial. J Am Med Dir Assoc. 2015;16:867-873. doi:10.1016/j.jamda.2015.05.002
11. Robinson H, Macdonald B, Kerse N, Broadbent E. The psychosocial effects of a companion robot: a randomized controlled trial. J Am Med Dir Assoc. 2013;14:661-667. doi:10.1016/j.jamda.2013.02.007
12. Schulman-Marcus J, Mookherjee S, Rice L, Lyubarova R. New approaches for the treatment of delirium: a case for robotic pets. Am J Med. 2019;132:781-782. doi:10.1016/j.amjmed.2018.12.039