User login
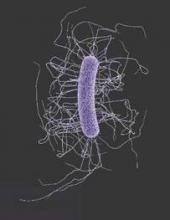
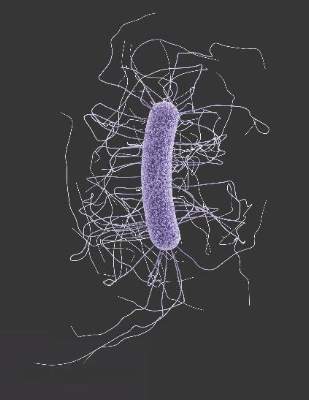
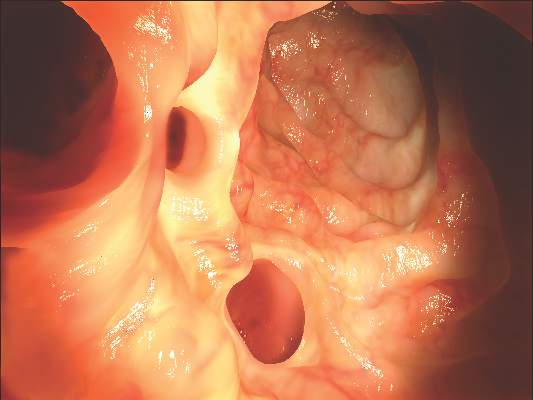
Fecal microbiota transplants achieved C. difficile resolution
An open-label study of fecal microbiota transfer in patients with recurrent Clostridium difficile infection has achieved clinical resolution in 96.7% of patients, according to a paper published online Feb. 8 in the Journal of Infectious Diseases.
Researchers treated 30 patients with two different dosing regimens of fractionated and encapsulated stool specimens from healthy donors, given after antibiotic therapy for a current episode of C. difficile infection.
At 8 weeks after dosing, 26 patients (86.7%) had had no C. difficile–positive diarrhea, and of the 4 who did have symptoms, 3 had early self-limiting diarrhea but tested negative for C. difficile carriage at 8 weeks, meaning that overall, 29 of the 30 patients achieved clinical resolution.
Patients also showed significant increases in microbial diversity from day 4 after treatment, and by week 8, the engrafted spore-forming bacteria made up around one-third of the total gut microbial carriage, with no serious adverse events related to the treatment (J Infect Diseases. 2016 Feb. 8. doi: 10.1093/infdis/jiv766).
“Although our study is limited by the lack of a placebo arm, the single clinical recurrence of C. difficile diarrhea in this study contrasts starkly with the recurrence rates documented in the placebo arms of three randomized, controlled trials involving patients with similar demographic characteristics and histories of recurrent episodes of [C. difficile infection],” wrote Dr. Sahil Khanna of the Mayo Clinic, Rochester, Minn., and coauthors.
The study was sponsored by Seres Therapeutics. One author reported a consultancy with Seres Therapeutics, and eight authors were employees of and held equity positions in Seres Therapeutics.
The development of SER-109 represents another major step toward fully engineered microbiota transfer, but we are still only halfway there.
Long-term safety databases are likely to play an important role in documenting currently unknown safety aspects of fecal microbiota transfer, and input from basic and clinical research is crucial to the identification of new indications.
Dr. Maria J.G.T. Vehreschild and Dr. Oliver A. Cornely are from the department of internal medicine at the University Hospital Cologne (Germany). These comments are adapted from an accompanying editorial (J Infect Diseases. 2016 Feb 8. doi: 10.1093/infdis/jiv768). The authors declared speakers bureau positions, lecture honoraria, research funding, and consultancies from a range of pharmaceutical companies. Dr. Cornely disclosed a consultancy with Seres.
The development of SER-109 represents another major step toward fully engineered microbiota transfer, but we are still only halfway there.
Long-term safety databases are likely to play an important role in documenting currently unknown safety aspects of fecal microbiota transfer, and input from basic and clinical research is crucial to the identification of new indications.
Dr. Maria J.G.T. Vehreschild and Dr. Oliver A. Cornely are from the department of internal medicine at the University Hospital Cologne (Germany). These comments are adapted from an accompanying editorial (J Infect Diseases. 2016 Feb 8. doi: 10.1093/infdis/jiv768). The authors declared speakers bureau positions, lecture honoraria, research funding, and consultancies from a range of pharmaceutical companies. Dr. Cornely disclosed a consultancy with Seres.
The development of SER-109 represents another major step toward fully engineered microbiota transfer, but we are still only halfway there.
Long-term safety databases are likely to play an important role in documenting currently unknown safety aspects of fecal microbiota transfer, and input from basic and clinical research is crucial to the identification of new indications.
Dr. Maria J.G.T. Vehreschild and Dr. Oliver A. Cornely are from the department of internal medicine at the University Hospital Cologne (Germany). These comments are adapted from an accompanying editorial (J Infect Diseases. 2016 Feb 8. doi: 10.1093/infdis/jiv768). The authors declared speakers bureau positions, lecture honoraria, research funding, and consultancies from a range of pharmaceutical companies. Dr. Cornely disclosed a consultancy with Seres.
An open-label study of fecal microbiota transfer in patients with recurrent Clostridium difficile infection has achieved clinical resolution in 96.7% of patients, according to a paper published online Feb. 8 in the Journal of Infectious Diseases.
Researchers treated 30 patients with two different dosing regimens of fractionated and encapsulated stool specimens from healthy donors, given after antibiotic therapy for a current episode of C. difficile infection.
At 8 weeks after dosing, 26 patients (86.7%) had had no C. difficile–positive diarrhea, and of the 4 who did have symptoms, 3 had early self-limiting diarrhea but tested negative for C. difficile carriage at 8 weeks, meaning that overall, 29 of the 30 patients achieved clinical resolution.
Patients also showed significant increases in microbial diversity from day 4 after treatment, and by week 8, the engrafted spore-forming bacteria made up around one-third of the total gut microbial carriage, with no serious adverse events related to the treatment (J Infect Diseases. 2016 Feb. 8. doi: 10.1093/infdis/jiv766).
“Although our study is limited by the lack of a placebo arm, the single clinical recurrence of C. difficile diarrhea in this study contrasts starkly with the recurrence rates documented in the placebo arms of three randomized, controlled trials involving patients with similar demographic characteristics and histories of recurrent episodes of [C. difficile infection],” wrote Dr. Sahil Khanna of the Mayo Clinic, Rochester, Minn., and coauthors.
The study was sponsored by Seres Therapeutics. One author reported a consultancy with Seres Therapeutics, and eight authors were employees of and held equity positions in Seres Therapeutics.
An open-label study of fecal microbiota transfer in patients with recurrent Clostridium difficile infection has achieved clinical resolution in 96.7% of patients, according to a paper published online Feb. 8 in the Journal of Infectious Diseases.
Researchers treated 30 patients with two different dosing regimens of fractionated and encapsulated stool specimens from healthy donors, given after antibiotic therapy for a current episode of C. difficile infection.
At 8 weeks after dosing, 26 patients (86.7%) had had no C. difficile–positive diarrhea, and of the 4 who did have symptoms, 3 had early self-limiting diarrhea but tested negative for C. difficile carriage at 8 weeks, meaning that overall, 29 of the 30 patients achieved clinical resolution.
Patients also showed significant increases in microbial diversity from day 4 after treatment, and by week 8, the engrafted spore-forming bacteria made up around one-third of the total gut microbial carriage, with no serious adverse events related to the treatment (J Infect Diseases. 2016 Feb. 8. doi: 10.1093/infdis/jiv766).
“Although our study is limited by the lack of a placebo arm, the single clinical recurrence of C. difficile diarrhea in this study contrasts starkly with the recurrence rates documented in the placebo arms of three randomized, controlled trials involving patients with similar demographic characteristics and histories of recurrent episodes of [C. difficile infection],” wrote Dr. Sahil Khanna of the Mayo Clinic, Rochester, Minn., and coauthors.
The study was sponsored by Seres Therapeutics. One author reported a consultancy with Seres Therapeutics, and eight authors were employees of and held equity positions in Seres Therapeutics.
FROM THE JOURNAL OF INFECTIOUS DISEASES
Key clinical point: Fecal microbiota transplants were highly efficacious in the treatment of recurrent Clostridium difficile infection in a study of 30 patients.
Major finding: Fecal microbiota transplants achieved clinical resolution of C. difficile infection in 96.7% of patients by 8 weeks.
Data source: An open-label study in 30 patients with recurrent C. difficile infection.
Disclosures: The study was sponsored by Seres Therapeutics. One author reported a consultancy with Seres Therapeutics, and eight authors were employees of and held equity positions in Seres Therapeutics.
Prison HIV screening links newly diagnosed to care
HIV opt-out screening in California state prisons achieves high rates of in-custody linkage to care and viral suppression for those diagnosed as HIV positive, and sustained viral suppression following release from prison, according to a study published in the Feb. 26 edition of Morbidity and Mortality Weekly Report.
Under the opt-out program, instituted in 2010, a medical care provider informs the inmate that an HIV test is routinely done, unless the inmate specifically declines the test. The 6-month assessment of the screening program in 2012 showed that 77% of the 17,436 inmates entering a reception center during that period were screened for HIV infection, with 135 (1%) testing positive, including 10 (0.1%) with newly diagnosed infection.
Kimberley D. Lucas of the California Correctional Health Care Services, and coauthors found that 134 (99%) of those who tested positive were linked to care within 90 days of their diagnosis and 122 (91%) began antiretroviral therapy; of those who initiated antiretroviral therapy (ART) and were still incarcerated the following year, 81 (98%) were still on the therapy and 71 (88%) had achieved viral suppression (Morb Mortal Wkly Rep. 2016 Feb 26;65[7]:178-81).
Thirty-nine inmates were released on antiretroviral therapy and 14 (36%) had uninterrupted linkage to care. The authors noted that continuity of care remained a challenge for individuals with a history of incarceration.
“The CCHCS HIV opt-out screening program demonstrated that identifying HIV infection at entry resulted in high rates of linkage to care, retention on ART, and significant reduction in viral loads during incarceration,” the researchers wrote.
No conflicts of interest were declared.
HIV opt-out screening in California state prisons achieves high rates of in-custody linkage to care and viral suppression for those diagnosed as HIV positive, and sustained viral suppression following release from prison, according to a study published in the Feb. 26 edition of Morbidity and Mortality Weekly Report.
Under the opt-out program, instituted in 2010, a medical care provider informs the inmate that an HIV test is routinely done, unless the inmate specifically declines the test. The 6-month assessment of the screening program in 2012 showed that 77% of the 17,436 inmates entering a reception center during that period were screened for HIV infection, with 135 (1%) testing positive, including 10 (0.1%) with newly diagnosed infection.
Kimberley D. Lucas of the California Correctional Health Care Services, and coauthors found that 134 (99%) of those who tested positive were linked to care within 90 days of their diagnosis and 122 (91%) began antiretroviral therapy; of those who initiated antiretroviral therapy (ART) and were still incarcerated the following year, 81 (98%) were still on the therapy and 71 (88%) had achieved viral suppression (Morb Mortal Wkly Rep. 2016 Feb 26;65[7]:178-81).
Thirty-nine inmates were released on antiretroviral therapy and 14 (36%) had uninterrupted linkage to care. The authors noted that continuity of care remained a challenge for individuals with a history of incarceration.
“The CCHCS HIV opt-out screening program demonstrated that identifying HIV infection at entry resulted in high rates of linkage to care, retention on ART, and significant reduction in viral loads during incarceration,” the researchers wrote.
No conflicts of interest were declared.
HIV opt-out screening in California state prisons achieves high rates of in-custody linkage to care and viral suppression for those diagnosed as HIV positive, and sustained viral suppression following release from prison, according to a study published in the Feb. 26 edition of Morbidity and Mortality Weekly Report.
Under the opt-out program, instituted in 2010, a medical care provider informs the inmate that an HIV test is routinely done, unless the inmate specifically declines the test. The 6-month assessment of the screening program in 2012 showed that 77% of the 17,436 inmates entering a reception center during that period were screened for HIV infection, with 135 (1%) testing positive, including 10 (0.1%) with newly diagnosed infection.
Kimberley D. Lucas of the California Correctional Health Care Services, and coauthors found that 134 (99%) of those who tested positive were linked to care within 90 days of their diagnosis and 122 (91%) began antiretroviral therapy; of those who initiated antiretroviral therapy (ART) and were still incarcerated the following year, 81 (98%) were still on the therapy and 71 (88%) had achieved viral suppression (Morb Mortal Wkly Rep. 2016 Feb 26;65[7]:178-81).
Thirty-nine inmates were released on antiretroviral therapy and 14 (36%) had uninterrupted linkage to care. The authors noted that continuity of care remained a challenge for individuals with a history of incarceration.
“The CCHCS HIV opt-out screening program demonstrated that identifying HIV infection at entry resulted in high rates of linkage to care, retention on ART, and significant reduction in viral loads during incarceration,” the researchers wrote.
No conflicts of interest were declared.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Key clinical point: HIV opt-out screening in California state prisons achieves high rates of in-custody linkage to care and viral suppression for those diagnosed as HIV positive.
Major finding: More than three-quarters of inmates were screened and 99% of those testing positive were linked to care within 90 days.
Data source: Retrospective analysis of 6 months of the HIV opt-out screening program among 17,436 inmates.
Disclosures: No conflicts of interest were declared.
Portal inflammation in pediatric NAFLD linked to fibrosis
Portal inflammation in children with nonalcoholic fatty liver disease is associated with more than a threefold greater risk of more advanced fibrosis, according to a paper published in Hepatology.
A cross-sectional study in 430 children with nonalcoholic fatty liver disease – 12% with type 1 disease, 22% with type 2, and 66% with an overlap of both – found that the presence of portal inflammation was associated with a significant independent association with stage 2-4 fibrosis (odds ratio, 3.70; 95% confidence interval, 1.40-5.21; P = .003), after adjustment for age and sex.
Stage 2-4 fibrosis was associated with a greater incidence of steatosis (OR, 1.81; P less than .0001), lobular inflammation (OR, 1.40; P less than .0001) and a twofold greater incidence of ballooning (P less than .0001).
While children with type 2 or overlap nonalcoholic fatty liver disease typically had lower alanine aminotransferase, aspartate aminotransferase, and bilirubin scores than those with type 1, they had a higher adjusted body mass index and waist circumference, lower HDL cholesterol and higher triglycerides (Hepatology. March 2016;63:745-53).
“Our data highlight that patients with type 2 and overlap NAFLD, in particular portal inflammation and high BMI or waist circumference, may be at increased risk of hepatic or metabolic complications,” wrote Dr. Jake P. Mann from the University of Cambridge, England, and his coauthors, suggesting that an elevated waist circumference could serve as a noninvasive indicator of risk of portal inflammation and fibrosis.
No conflicts of interest were declared.
Portal inflammation in children with nonalcoholic fatty liver disease is associated with more than a threefold greater risk of more advanced fibrosis, according to a paper published in Hepatology.
A cross-sectional study in 430 children with nonalcoholic fatty liver disease – 12% with type 1 disease, 22% with type 2, and 66% with an overlap of both – found that the presence of portal inflammation was associated with a significant independent association with stage 2-4 fibrosis (odds ratio, 3.70; 95% confidence interval, 1.40-5.21; P = .003), after adjustment for age and sex.
Stage 2-4 fibrosis was associated with a greater incidence of steatosis (OR, 1.81; P less than .0001), lobular inflammation (OR, 1.40; P less than .0001) and a twofold greater incidence of ballooning (P less than .0001).
While children with type 2 or overlap nonalcoholic fatty liver disease typically had lower alanine aminotransferase, aspartate aminotransferase, and bilirubin scores than those with type 1, they had a higher adjusted body mass index and waist circumference, lower HDL cholesterol and higher triglycerides (Hepatology. March 2016;63:745-53).
“Our data highlight that patients with type 2 and overlap NAFLD, in particular portal inflammation and high BMI or waist circumference, may be at increased risk of hepatic or metabolic complications,” wrote Dr. Jake P. Mann from the University of Cambridge, England, and his coauthors, suggesting that an elevated waist circumference could serve as a noninvasive indicator of risk of portal inflammation and fibrosis.
No conflicts of interest were declared.
Portal inflammation in children with nonalcoholic fatty liver disease is associated with more than a threefold greater risk of more advanced fibrosis, according to a paper published in Hepatology.
A cross-sectional study in 430 children with nonalcoholic fatty liver disease – 12% with type 1 disease, 22% with type 2, and 66% with an overlap of both – found that the presence of portal inflammation was associated with a significant independent association with stage 2-4 fibrosis (odds ratio, 3.70; 95% confidence interval, 1.40-5.21; P = .003), after adjustment for age and sex.
Stage 2-4 fibrosis was associated with a greater incidence of steatosis (OR, 1.81; P less than .0001), lobular inflammation (OR, 1.40; P less than .0001) and a twofold greater incidence of ballooning (P less than .0001).
While children with type 2 or overlap nonalcoholic fatty liver disease typically had lower alanine aminotransferase, aspartate aminotransferase, and bilirubin scores than those with type 1, they had a higher adjusted body mass index and waist circumference, lower HDL cholesterol and higher triglycerides (Hepatology. March 2016;63:745-53).
“Our data highlight that patients with type 2 and overlap NAFLD, in particular portal inflammation and high BMI or waist circumference, may be at increased risk of hepatic or metabolic complications,” wrote Dr. Jake P. Mann from the University of Cambridge, England, and his coauthors, suggesting that an elevated waist circumference could serve as a noninvasive indicator of risk of portal inflammation and fibrosis.
No conflicts of interest were declared.
FROM HEPATOLOGY
Key clinical point: Portal inflammation in children with nonalcoholic fatty liver disease is associated with more advanced fibrosis and a worse metabolic phenotype.
Major finding: Portal inflammation in children with NAFLD is associated with a more than threefold greater risk of more advanced fibrosis.
Data source: A cross-sectional study in 430 children with NAFLD.
Disclosures: No conflicts of interest were declared.
Electronic program reduces cirrhosis readmission rates
An electronic quality improvement initiative for patients with cirrhosis has achieved significant reductions in 30-day readmission rates.
The program’s goals included universal use of rifaximin and goal-directed therapy in patients with hepatic encephalopathy, and timely administration of antibiotics and albumin for patients with spontaneous bacterial peritonitis.
Researchers enrolled 824 patients with decompensated cirrhosis or receiving liver transplants, who were admitted to an inpatient hepatology unit, according to a paper published online in Clinical Gastroenterology and Hepatology.
The initiative consisted of three phases; a year-long control period in which 626 admissions received usual care, then a nearly 1-year phase of the intervention involving a hand-held checklist, and a second 1.3-year long electronic phase in which the checklist was incorporated into the electronic provider order entry system.
The final electronic phase of the initiative was associated with a 40% lower odds of readmission within 30 days, compared with the control period, and 38% lower odds of readmission, compared with the hand-held checklist phase, with no significant impact on 90-day mortality rates.
Both the hand-held and electronic stages of the intervention were associated with significant increases in rifaximin use, compared with controls (78.1% for controls, 89.4% for hand-held, and 96.3% for electronic), and the use of rifaximin in patients with overt hepatic encephalopathy lowered the risk of 30-day readmission by 61%.
“Reduced readmissions were likely mediated in large part by the ability to obtain the medication as an outpatient; however, the role of rifaximin co-therapy during an admission for overt [hepatic encephalopathy] should be explored further,” wrote Dr. Elliot B. Tapper and coauthors from the Harvard Medical School.
Increased antibiotic prophylaxis for spontaneous bacterial peritonitis also showed an impact, with a 60% lower odds of 30-day readmission in those with a history of or index admission for peritonitis, even though the increase in secondary antibiotic prophylaxis was not significant for either phase.
The analysis also showed evidence that six or more cups lactulose on the day of admission for overt hepatic encephalopathy was associated with a significantly reduced risk of readmission within 30 days (Clin Gastroenterol Hepatol. 2015 Sep 22. doi: 10.1016/j.cgh.2015.08.041).
Researchers also noted that during the electronic phase of the intervention, the impact on readmission rates was greater among patients attending the liver service than it was among patients cared for by Beth Israel Deaconess Medical Center hospitalists or at a neighboring institution.
Readmissions were a major driver of the estimated $2 billion cost of hospital admissions for complications of chronic liver disease, the authors said.
“Our study advances the current literature on [quality improvement] for patients with cirrhosis by presenting an inexpensive, easy to implement, and generalizable approach.”
The study was supported by the Carl J. Shapiro Institute for Education and Research, Harvard Catalyst/The Harvard Clinical and Translational Science Center and Harvard University. No conflicts of interest were declared.
An electronic quality improvement initiative for patients with cirrhosis has achieved significant reductions in 30-day readmission rates.
The program’s goals included universal use of rifaximin and goal-directed therapy in patients with hepatic encephalopathy, and timely administration of antibiotics and albumin for patients with spontaneous bacterial peritonitis.
Researchers enrolled 824 patients with decompensated cirrhosis or receiving liver transplants, who were admitted to an inpatient hepatology unit, according to a paper published online in Clinical Gastroenterology and Hepatology.
The initiative consisted of three phases; a year-long control period in which 626 admissions received usual care, then a nearly 1-year phase of the intervention involving a hand-held checklist, and a second 1.3-year long electronic phase in which the checklist was incorporated into the electronic provider order entry system.
The final electronic phase of the initiative was associated with a 40% lower odds of readmission within 30 days, compared with the control period, and 38% lower odds of readmission, compared with the hand-held checklist phase, with no significant impact on 90-day mortality rates.
Both the hand-held and electronic stages of the intervention were associated with significant increases in rifaximin use, compared with controls (78.1% for controls, 89.4% for hand-held, and 96.3% for electronic), and the use of rifaximin in patients with overt hepatic encephalopathy lowered the risk of 30-day readmission by 61%.
“Reduced readmissions were likely mediated in large part by the ability to obtain the medication as an outpatient; however, the role of rifaximin co-therapy during an admission for overt [hepatic encephalopathy] should be explored further,” wrote Dr. Elliot B. Tapper and coauthors from the Harvard Medical School.
Increased antibiotic prophylaxis for spontaneous bacterial peritonitis also showed an impact, with a 60% lower odds of 30-day readmission in those with a history of or index admission for peritonitis, even though the increase in secondary antibiotic prophylaxis was not significant for either phase.
The analysis also showed evidence that six or more cups lactulose on the day of admission for overt hepatic encephalopathy was associated with a significantly reduced risk of readmission within 30 days (Clin Gastroenterol Hepatol. 2015 Sep 22. doi: 10.1016/j.cgh.2015.08.041).
Researchers also noted that during the electronic phase of the intervention, the impact on readmission rates was greater among patients attending the liver service than it was among patients cared for by Beth Israel Deaconess Medical Center hospitalists or at a neighboring institution.
Readmissions were a major driver of the estimated $2 billion cost of hospital admissions for complications of chronic liver disease, the authors said.
“Our study advances the current literature on [quality improvement] for patients with cirrhosis by presenting an inexpensive, easy to implement, and generalizable approach.”
The study was supported by the Carl J. Shapiro Institute for Education and Research, Harvard Catalyst/The Harvard Clinical and Translational Science Center and Harvard University. No conflicts of interest were declared.
An electronic quality improvement initiative for patients with cirrhosis has achieved significant reductions in 30-day readmission rates.
The program’s goals included universal use of rifaximin and goal-directed therapy in patients with hepatic encephalopathy, and timely administration of antibiotics and albumin for patients with spontaneous bacterial peritonitis.
Researchers enrolled 824 patients with decompensated cirrhosis or receiving liver transplants, who were admitted to an inpatient hepatology unit, according to a paper published online in Clinical Gastroenterology and Hepatology.
The initiative consisted of three phases; a year-long control period in which 626 admissions received usual care, then a nearly 1-year phase of the intervention involving a hand-held checklist, and a second 1.3-year long electronic phase in which the checklist was incorporated into the electronic provider order entry system.
The final electronic phase of the initiative was associated with a 40% lower odds of readmission within 30 days, compared with the control period, and 38% lower odds of readmission, compared with the hand-held checklist phase, with no significant impact on 90-day mortality rates.
Both the hand-held and electronic stages of the intervention were associated with significant increases in rifaximin use, compared with controls (78.1% for controls, 89.4% for hand-held, and 96.3% for electronic), and the use of rifaximin in patients with overt hepatic encephalopathy lowered the risk of 30-day readmission by 61%.
“Reduced readmissions were likely mediated in large part by the ability to obtain the medication as an outpatient; however, the role of rifaximin co-therapy during an admission for overt [hepatic encephalopathy] should be explored further,” wrote Dr. Elliot B. Tapper and coauthors from the Harvard Medical School.
Increased antibiotic prophylaxis for spontaneous bacterial peritonitis also showed an impact, with a 60% lower odds of 30-day readmission in those with a history of or index admission for peritonitis, even though the increase in secondary antibiotic prophylaxis was not significant for either phase.
The analysis also showed evidence that six or more cups lactulose on the day of admission for overt hepatic encephalopathy was associated with a significantly reduced risk of readmission within 30 days (Clin Gastroenterol Hepatol. 2015 Sep 22. doi: 10.1016/j.cgh.2015.08.041).
Researchers also noted that during the electronic phase of the intervention, the impact on readmission rates was greater among patients attending the liver service than it was among patients cared for by Beth Israel Deaconess Medical Center hospitalists or at a neighboring institution.
Readmissions were a major driver of the estimated $2 billion cost of hospital admissions for complications of chronic liver disease, the authors said.
“Our study advances the current literature on [quality improvement] for patients with cirrhosis by presenting an inexpensive, easy to implement, and generalizable approach.”
The study was supported by the Carl J. Shapiro Institute for Education and Research, Harvard Catalyst/The Harvard Clinical and Translational Science Center and Harvard University. No conflicts of interest were declared.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: An electronic quality improvement initiative for patients with cirrhosis has achieved significant reductions in 30-day readmission rates.
Major finding: An electronic quality improvement program is associated with a 40% reduction in 30-day readmission rates in patients with cirrhosis.
Data source: Prospective cohort study in 824 patients with decompensated cirrhosis or who received liver transplants.
Disclosures: The study was supported by the Carl J. Shapiro Institute for Education and Research, Harvard Catalyst: the Harvard Clinical and Translational Science Center, and Harvard University. No conflicts of interest were declared.
Zika virus in pregnancy linked to hydrops fetalis
Zika virus infection in pregnant women may be linked to hydrops fetalis and fetal demise, according to a case report published online Feb. 25 in PLOS Neglected Tropical Diseases.
A 20-year-old pregnant woman without history or signs of Zika virus infection was referred to the Hospital Geral Roberto Santos in Salvador, Brazil, in the 18th week of gestation because of low fetal weight, but by week 26 and 30, ultrasound examinations showed microcephaly, hydranencephaly, intracranial calcifications, destructive lesions of posterior fossa, and evidence of hydrothorax, ascites, and subcutaneous edema.
Ultrasound examination at week 32 showed fetal demise, and after delivery, researchers found evidence of Zika virus in the brain and in the cerebrospinal and amniotic fluid, but not in the heart, lung, liver, eye, or placenta (PLoS Negl Trop Dis. 2016 Feb 25. doi: 10.1371/journal.pntd.0004517).
“This case report of a fetus provides additional evidence for the link between ZIKV [Zika virus] infection and microcephaly,” wrote Dr. Manoel Sarno of Hospital Geral Roberto Santos, and his coauthors. “Furthermore, it serves as an alert to clinicians that in addition to central nervous system and ophthalmological manifestations, congenital ZIKV infection may cause hydrops fetalis and fetal demise.”
The researchers reported having no financial disclosures.
Zika virus infection in pregnant women may be linked to hydrops fetalis and fetal demise, according to a case report published online Feb. 25 in PLOS Neglected Tropical Diseases.
A 20-year-old pregnant woman without history or signs of Zika virus infection was referred to the Hospital Geral Roberto Santos in Salvador, Brazil, in the 18th week of gestation because of low fetal weight, but by week 26 and 30, ultrasound examinations showed microcephaly, hydranencephaly, intracranial calcifications, destructive lesions of posterior fossa, and evidence of hydrothorax, ascites, and subcutaneous edema.
Ultrasound examination at week 32 showed fetal demise, and after delivery, researchers found evidence of Zika virus in the brain and in the cerebrospinal and amniotic fluid, but not in the heart, lung, liver, eye, or placenta (PLoS Negl Trop Dis. 2016 Feb 25. doi: 10.1371/journal.pntd.0004517).
“This case report of a fetus provides additional evidence for the link between ZIKV [Zika virus] infection and microcephaly,” wrote Dr. Manoel Sarno of Hospital Geral Roberto Santos, and his coauthors. “Furthermore, it serves as an alert to clinicians that in addition to central nervous system and ophthalmological manifestations, congenital ZIKV infection may cause hydrops fetalis and fetal demise.”
The researchers reported having no financial disclosures.
Zika virus infection in pregnant women may be linked to hydrops fetalis and fetal demise, according to a case report published online Feb. 25 in PLOS Neglected Tropical Diseases.
A 20-year-old pregnant woman without history or signs of Zika virus infection was referred to the Hospital Geral Roberto Santos in Salvador, Brazil, in the 18th week of gestation because of low fetal weight, but by week 26 and 30, ultrasound examinations showed microcephaly, hydranencephaly, intracranial calcifications, destructive lesions of posterior fossa, and evidence of hydrothorax, ascites, and subcutaneous edema.
Ultrasound examination at week 32 showed fetal demise, and after delivery, researchers found evidence of Zika virus in the brain and in the cerebrospinal and amniotic fluid, but not in the heart, lung, liver, eye, or placenta (PLoS Negl Trop Dis. 2016 Feb 25. doi: 10.1371/journal.pntd.0004517).
“This case report of a fetus provides additional evidence for the link between ZIKV [Zika virus] infection and microcephaly,” wrote Dr. Manoel Sarno of Hospital Geral Roberto Santos, and his coauthors. “Furthermore, it serves as an alert to clinicians that in addition to central nervous system and ophthalmological manifestations, congenital ZIKV infection may cause hydrops fetalis and fetal demise.”
The researchers reported having no financial disclosures.
FROM PLOS NEGLECTED TROPICAL DISEASES
Key clinical point: Zika virus infection in pregnant women may be associated with hydrops fetalis and fetal demise.
Major finding: Congenital Zika virus infection was associated with microcephaly, hydranencephaly, and fetal demise in a single case.
Data source: Case report of a fetus with congenital Zika virus infection.
Disclosures: The researchers reported having no financial disclosures.
Downturn in reoperation after breast conservation surgery
Rates of reoperation after breast conservation surgery have declined significantly since 2003, but nearly 25% of women are still undergoing repeat operations, new data suggest.
Researchers analyzed data from a population-based sample of 89,448 women undergoing primary breast conservation surgery for breast cancer in New York State from 2003 to 2013.
According to a study published online Feb.17 in JAMA Surgery, the mean 90-day reoperation rate declined from 39.5% in 2003-2004 to 23.1% in 2011-2013 (P less than .001), with an overall rate of 30.9% for the entire study period.
“We believe that the findings of reduced occurrence of reoperations are encouraging and imply improvements in training and patient selection for BCS,” wrote Abby J. Isaacs of Cornell University, New York, and her coauthors.
Reoperation rates were highest in younger women (37.7%), compared with women aged over 65 years (26.3%).
However, the study also showed that women aged 50-64 years were less likely than were older or younger women to undergo breast conservation surgery, compared with mastectomy as a repeat procedure (JAMA Surg 2016 Feb 17. doi:10.1001/jamasurg.2015.5535).
Breast conservation surgery was more common than was mastectomy as a repeat procedure in women who were white, had commercial insurance, had low comorbidity scores, or had in situ disease.
Researchers observed significant surgeon-level variation in rates of reoperation after breast conservation surgery, which they described as “novel and unprecedented.” The rates of reoperation among surgeons ranged from 0% to nearly 100%.
Overall, the mean rate of reoperation for surgeons was 30.8%, but nearly 20% of surgeons had reoperation rates above 50% and 6.1% had reoperation rates above the 99.8% limits.
Similarly, 6% of surgeons had reoperation rates below the 95% confidence limits and 2.9% were below the 99.8% limits.
Reoperation rates were independently associated with surgical volume; surgeons performing fewer than 14 breast conservation surgery procedures had a mean reoperation rate of 35.2% while those who performed 34 or more procedures had a reoperation rate of 27.5%.
Over the entire study period, the overall rate of breast conservation surgery peaked in 2004 with more than 8,500 cases, then decreased to a mean number of 8,078 for 2011-2013.
The decrease in primary breast conservation surgery was significantly greater in women aged 20-49 years (P less than .001) with 1,416 women in this age group undergoing breast conservation surgery in 2013, compared with 3,644 women aged 65 years or older.
“The reduction in the use of BCS in women younger than 50 years and a corresponding reduction in overall reoperation rates over time implies that surgeons may be selecting more appropriate patients for the procedures,” the authors wrote.
They acknowledged that they did not have access to information on tumor size, grade, and staging, which are variables known to have an important influence on margin rates and reoperation rates. “We believe, however, in the context of physician-level outcomes, that these factors will not bias the results; the recommendation for BCS will be made by surgeons based on their knowledge of the patients’ disease, and we have no reason to believe that unknowable patient characteristics will be unbalanced between surgeons.”
Two authors reported involvement with the Food and Drug Administration–funded MDEpiNet Science and Infrastructure Center; no other conflicts of interest were declared.
The recent Society for Surgical Oncology–American Society for Radiation Oncology (SSO-ASTRO) consensus guidelines encourage the use of “no ink on tumor” as the current standard in an era of multimodal treatment and evolving understanding of tumor biology along with tumor burden.

|
Dr. E. Shelley Hwang |
Establishing a rational, evidence-based approach to reexcision as originally proposed by Fisher et al. and supported by the new SSO-ASTRO guidelines can provide substantial national cost savings by eliminating reexcisions for close but negative margins.
Dr. Uttara Nag and Dr. E. Shelley Hwang are with the department of health policy and management in the department of surgery, Duke University, Durham, N.C. These comments are taken from an editorial (JAMA Surg. 2016 Feb 17. doi:10.1001/jamasurg.2015.5555). No conflicts of interest were declared.
The recent Society for Surgical Oncology–American Society for Radiation Oncology (SSO-ASTRO) consensus guidelines encourage the use of “no ink on tumor” as the current standard in an era of multimodal treatment and evolving understanding of tumor biology along with tumor burden.

|
Dr. E. Shelley Hwang |
Establishing a rational, evidence-based approach to reexcision as originally proposed by Fisher et al. and supported by the new SSO-ASTRO guidelines can provide substantial national cost savings by eliminating reexcisions for close but negative margins.
Dr. Uttara Nag and Dr. E. Shelley Hwang are with the department of health policy and management in the department of surgery, Duke University, Durham, N.C. These comments are taken from an editorial (JAMA Surg. 2016 Feb 17. doi:10.1001/jamasurg.2015.5555). No conflicts of interest were declared.
The recent Society for Surgical Oncology–American Society for Radiation Oncology (SSO-ASTRO) consensus guidelines encourage the use of “no ink on tumor” as the current standard in an era of multimodal treatment and evolving understanding of tumor biology along with tumor burden.

|
Dr. E. Shelley Hwang |
Establishing a rational, evidence-based approach to reexcision as originally proposed by Fisher et al. and supported by the new SSO-ASTRO guidelines can provide substantial national cost savings by eliminating reexcisions for close but negative margins.
Dr. Uttara Nag and Dr. E. Shelley Hwang are with the department of health policy and management in the department of surgery, Duke University, Durham, N.C. These comments are taken from an editorial (JAMA Surg. 2016 Feb 17. doi:10.1001/jamasurg.2015.5555). No conflicts of interest were declared.
Rates of reoperation after breast conservation surgery have declined significantly since 2003, but nearly 25% of women are still undergoing repeat operations, new data suggest.
Researchers analyzed data from a population-based sample of 89,448 women undergoing primary breast conservation surgery for breast cancer in New York State from 2003 to 2013.
According to a study published online Feb.17 in JAMA Surgery, the mean 90-day reoperation rate declined from 39.5% in 2003-2004 to 23.1% in 2011-2013 (P less than .001), with an overall rate of 30.9% for the entire study period.
“We believe that the findings of reduced occurrence of reoperations are encouraging and imply improvements in training and patient selection for BCS,” wrote Abby J. Isaacs of Cornell University, New York, and her coauthors.
Reoperation rates were highest in younger women (37.7%), compared with women aged over 65 years (26.3%).
However, the study also showed that women aged 50-64 years were less likely than were older or younger women to undergo breast conservation surgery, compared with mastectomy as a repeat procedure (JAMA Surg 2016 Feb 17. doi:10.1001/jamasurg.2015.5535).
Breast conservation surgery was more common than was mastectomy as a repeat procedure in women who were white, had commercial insurance, had low comorbidity scores, or had in situ disease.
Researchers observed significant surgeon-level variation in rates of reoperation after breast conservation surgery, which they described as “novel and unprecedented.” The rates of reoperation among surgeons ranged from 0% to nearly 100%.
Overall, the mean rate of reoperation for surgeons was 30.8%, but nearly 20% of surgeons had reoperation rates above 50% and 6.1% had reoperation rates above the 99.8% limits.
Similarly, 6% of surgeons had reoperation rates below the 95% confidence limits and 2.9% were below the 99.8% limits.
Reoperation rates were independently associated with surgical volume; surgeons performing fewer than 14 breast conservation surgery procedures had a mean reoperation rate of 35.2% while those who performed 34 or more procedures had a reoperation rate of 27.5%.
Over the entire study period, the overall rate of breast conservation surgery peaked in 2004 with more than 8,500 cases, then decreased to a mean number of 8,078 for 2011-2013.
The decrease in primary breast conservation surgery was significantly greater in women aged 20-49 years (P less than .001) with 1,416 women in this age group undergoing breast conservation surgery in 2013, compared with 3,644 women aged 65 years or older.
“The reduction in the use of BCS in women younger than 50 years and a corresponding reduction in overall reoperation rates over time implies that surgeons may be selecting more appropriate patients for the procedures,” the authors wrote.
They acknowledged that they did not have access to information on tumor size, grade, and staging, which are variables known to have an important influence on margin rates and reoperation rates. “We believe, however, in the context of physician-level outcomes, that these factors will not bias the results; the recommendation for BCS will be made by surgeons based on their knowledge of the patients’ disease, and we have no reason to believe that unknowable patient characteristics will be unbalanced between surgeons.”
Two authors reported involvement with the Food and Drug Administration–funded MDEpiNet Science and Infrastructure Center; no other conflicts of interest were declared.
Rates of reoperation after breast conservation surgery have declined significantly since 2003, but nearly 25% of women are still undergoing repeat operations, new data suggest.
Researchers analyzed data from a population-based sample of 89,448 women undergoing primary breast conservation surgery for breast cancer in New York State from 2003 to 2013.
According to a study published online Feb.17 in JAMA Surgery, the mean 90-day reoperation rate declined from 39.5% in 2003-2004 to 23.1% in 2011-2013 (P less than .001), with an overall rate of 30.9% for the entire study period.
“We believe that the findings of reduced occurrence of reoperations are encouraging and imply improvements in training and patient selection for BCS,” wrote Abby J. Isaacs of Cornell University, New York, and her coauthors.
Reoperation rates were highest in younger women (37.7%), compared with women aged over 65 years (26.3%).
However, the study also showed that women aged 50-64 years were less likely than were older or younger women to undergo breast conservation surgery, compared with mastectomy as a repeat procedure (JAMA Surg 2016 Feb 17. doi:10.1001/jamasurg.2015.5535).
Breast conservation surgery was more common than was mastectomy as a repeat procedure in women who were white, had commercial insurance, had low comorbidity scores, or had in situ disease.
Researchers observed significant surgeon-level variation in rates of reoperation after breast conservation surgery, which they described as “novel and unprecedented.” The rates of reoperation among surgeons ranged from 0% to nearly 100%.
Overall, the mean rate of reoperation for surgeons was 30.8%, but nearly 20% of surgeons had reoperation rates above 50% and 6.1% had reoperation rates above the 99.8% limits.
Similarly, 6% of surgeons had reoperation rates below the 95% confidence limits and 2.9% were below the 99.8% limits.
Reoperation rates were independently associated with surgical volume; surgeons performing fewer than 14 breast conservation surgery procedures had a mean reoperation rate of 35.2% while those who performed 34 or more procedures had a reoperation rate of 27.5%.
Over the entire study period, the overall rate of breast conservation surgery peaked in 2004 with more than 8,500 cases, then decreased to a mean number of 8,078 for 2011-2013.
The decrease in primary breast conservation surgery was significantly greater in women aged 20-49 years (P less than .001) with 1,416 women in this age group undergoing breast conservation surgery in 2013, compared with 3,644 women aged 65 years or older.
“The reduction in the use of BCS in women younger than 50 years and a corresponding reduction in overall reoperation rates over time implies that surgeons may be selecting more appropriate patients for the procedures,” the authors wrote.
They acknowledged that they did not have access to information on tumor size, grade, and staging, which are variables known to have an important influence on margin rates and reoperation rates. “We believe, however, in the context of physician-level outcomes, that these factors will not bias the results; the recommendation for BCS will be made by surgeons based on their knowledge of the patients’ disease, and we have no reason to believe that unknowable patient characteristics will be unbalanced between surgeons.”
Two authors reported involvement with the Food and Drug Administration–funded MDEpiNet Science and Infrastructure Center; no other conflicts of interest were declared.
FROM JAMA SURGERY
Key clinical point: Rates of reoperation after breast conservation surgery are declining but vary significantly between surgeons.
Major finding: The mean 90-day reoperation rate after breast conservation surgery declined from 39.5% in 2003-2004 to 23.1% in 2011-2013.
Data source: Population-based sample of 89,448 women undergoing primary breast conservation surgery for breast cancer.
Disclosures: Two authors reported involvement with the Food and Drug Administration–funded MDEpiNet Science and Infrastructure Center; no other conflicts of interest were declared.
Consensus Statement Stresses "Meaningful" Glucose Monitoring
Outpatient blood glucose monitoring (BGM) needs to be meaningful, and the best way to achieve that is to individualize the process, so that changes in control, which may flag need for changes in hypoglycemia agents, may emerge, according to the American Association of Clinical Endocrinologists consensus statement, published in the February 2016 issue of Endocrine Practice.
“Meaningful monitoring” as discussed in the consensus statement comes down to an individualized approach to clinical management that is customized to patient preference and lifestyle.
For adults with type 1 diabetes mellitus, the statement endorsed existing clinical practice guidelines that recommend blood glucose testing upon awakening, after each meal, at bedtime, and before driving, with a frequency ranging from at least 4-6 each day to 10 or more times per day.
Blood glucose monitoring is also essential for achieving optimal control in children and adolescents with type 1 diabetes, but this can be particularly challenging in pediatric patients, according to the consensus statement’s authors (Endocr Pract. 2016, Feb;22[2]:231-61).
“Food intake and activity are unpredictable in very young patients, complicating parents’ efforts to regulate glucose levels,” wrote Dr. Timothy S. Bailey from the University of California, San Diego, and his coauthors.
“In adolescents, the emotional fatigue of managing their diabetes often leads to a reduced frequency of BGM, missed insulin doses, and markedly elevated hemoglobin A1c levels.”
Physicians, parents, and patients need to be cognizant of trends in glycemic control that might suggest the patient has outgrown the insulin dose and should be taught to make longer-term regimen adjustments. “Such pattern recognition requires maintaining and periodically reviewing an electronic or written log of blood glucose levels,” according to the consensus statement.
BGM was deemed essential to all patients with type 2 diabetes mellitus, even those not receiving insulin treatment, because it provides immediate feedback on glycemic control that is not provided with HbA1c measurement.
“Two of the goals for any BGM strategy are to empower patients to play a more active role in their diabetes management and to maximize the efficacy and safety of glucose-lowering therapies, including lifestyle management,” the authors wrote.
In patients on intensive insulin therapy with prandial and basal insulin, the authors recommended blood glucose monitoring be conducted when fasting, premeal, at bedtime and occasionally in the middle of the night.
In patients on basal insulin only or in combination with other diabetes medications, blood glucose testing was recommended at least when fasting and at bedtime.
When it comes to patients on noninsulin therapies for type 2 diabetes, guidelines advise that blood glucose monitoring be used only when patients or their caregivers are equipped to incorporate it and the resulting therapeutic adjustments into the diabetes care plan.
The only exception to this was in patients receiving noninsulin agents linked with a higher risk of hypoglycemia, such as sulfonylureas and glinides, in which case, they should test their fasting blood glucose at least once a day and periodically at other times, the authors recommended.
In pregnant women with gestational diabetes, the consensus was to test fasting and 1 hour postprandial if patients were not receiving insulin, and to test fasting, preprandial, and 1 hour postprandial in those receiving insulin.
The authors also noted that there is some variation in accuracy among the blood glucose monitors approved by the Food and Drug Administration, but the degree of accuracy required is dependent on the patient.
“As the technology advances, there is a vital need to integrate the multiple data inputs from insulin pumps, glucose sensors, glucose meters, and carbohydrate intake in a comprehensive and standardized way so clinicians and patients can make sense of it all.”
The consensus statement was produced by the American Association of Clinical Endocrinologists. All but one author declared fees, honoraria, and research grants from the pharmaceutical industry.
Outpatient blood glucose monitoring (BGM) needs to be meaningful, and the best way to achieve that is to individualize the process, so that changes in control, which may flag need for changes in hypoglycemia agents, may emerge, according to the American Association of Clinical Endocrinologists consensus statement, published in the February 2016 issue of Endocrine Practice.
“Meaningful monitoring” as discussed in the consensus statement comes down to an individualized approach to clinical management that is customized to patient preference and lifestyle.
For adults with type 1 diabetes mellitus, the statement endorsed existing clinical practice guidelines that recommend blood glucose testing upon awakening, after each meal, at bedtime, and before driving, with a frequency ranging from at least 4-6 each day to 10 or more times per day.
Blood glucose monitoring is also essential for achieving optimal control in children and adolescents with type 1 diabetes, but this can be particularly challenging in pediatric patients, according to the consensus statement’s authors (Endocr Pract. 2016, Feb;22[2]:231-61).
“Food intake and activity are unpredictable in very young patients, complicating parents’ efforts to regulate glucose levels,” wrote Dr. Timothy S. Bailey from the University of California, San Diego, and his coauthors.
“In adolescents, the emotional fatigue of managing their diabetes often leads to a reduced frequency of BGM, missed insulin doses, and markedly elevated hemoglobin A1c levels.”
Physicians, parents, and patients need to be cognizant of trends in glycemic control that might suggest the patient has outgrown the insulin dose and should be taught to make longer-term regimen adjustments. “Such pattern recognition requires maintaining and periodically reviewing an electronic or written log of blood glucose levels,” according to the consensus statement.
BGM was deemed essential to all patients with type 2 diabetes mellitus, even those not receiving insulin treatment, because it provides immediate feedback on glycemic control that is not provided with HbA1c measurement.
“Two of the goals for any BGM strategy are to empower patients to play a more active role in their diabetes management and to maximize the efficacy and safety of glucose-lowering therapies, including lifestyle management,” the authors wrote.
In patients on intensive insulin therapy with prandial and basal insulin, the authors recommended blood glucose monitoring be conducted when fasting, premeal, at bedtime and occasionally in the middle of the night.
In patients on basal insulin only or in combination with other diabetes medications, blood glucose testing was recommended at least when fasting and at bedtime.
When it comes to patients on noninsulin therapies for type 2 diabetes, guidelines advise that blood glucose monitoring be used only when patients or their caregivers are equipped to incorporate it and the resulting therapeutic adjustments into the diabetes care plan.
The only exception to this was in patients receiving noninsulin agents linked with a higher risk of hypoglycemia, such as sulfonylureas and glinides, in which case, they should test their fasting blood glucose at least once a day and periodically at other times, the authors recommended.
In pregnant women with gestational diabetes, the consensus was to test fasting and 1 hour postprandial if patients were not receiving insulin, and to test fasting, preprandial, and 1 hour postprandial in those receiving insulin.
The authors also noted that there is some variation in accuracy among the blood glucose monitors approved by the Food and Drug Administration, but the degree of accuracy required is dependent on the patient.
“As the technology advances, there is a vital need to integrate the multiple data inputs from insulin pumps, glucose sensors, glucose meters, and carbohydrate intake in a comprehensive and standardized way so clinicians and patients can make sense of it all.”
The consensus statement was produced by the American Association of Clinical Endocrinologists. All but one author declared fees, honoraria, and research grants from the pharmaceutical industry.
Outpatient blood glucose monitoring (BGM) needs to be meaningful, and the best way to achieve that is to individualize the process, so that changes in control, which may flag need for changes in hypoglycemia agents, may emerge, according to the American Association of Clinical Endocrinologists consensus statement, published in the February 2016 issue of Endocrine Practice.
“Meaningful monitoring” as discussed in the consensus statement comes down to an individualized approach to clinical management that is customized to patient preference and lifestyle.
For adults with type 1 diabetes mellitus, the statement endorsed existing clinical practice guidelines that recommend blood glucose testing upon awakening, after each meal, at bedtime, and before driving, with a frequency ranging from at least 4-6 each day to 10 or more times per day.
Blood glucose monitoring is also essential for achieving optimal control in children and adolescents with type 1 diabetes, but this can be particularly challenging in pediatric patients, according to the consensus statement’s authors (Endocr Pract. 2016, Feb;22[2]:231-61).
“Food intake and activity are unpredictable in very young patients, complicating parents’ efforts to regulate glucose levels,” wrote Dr. Timothy S. Bailey from the University of California, San Diego, and his coauthors.
“In adolescents, the emotional fatigue of managing their diabetes often leads to a reduced frequency of BGM, missed insulin doses, and markedly elevated hemoglobin A1c levels.”
Physicians, parents, and patients need to be cognizant of trends in glycemic control that might suggest the patient has outgrown the insulin dose and should be taught to make longer-term regimen adjustments. “Such pattern recognition requires maintaining and periodically reviewing an electronic or written log of blood glucose levels,” according to the consensus statement.
BGM was deemed essential to all patients with type 2 diabetes mellitus, even those not receiving insulin treatment, because it provides immediate feedback on glycemic control that is not provided with HbA1c measurement.
“Two of the goals for any BGM strategy are to empower patients to play a more active role in their diabetes management and to maximize the efficacy and safety of glucose-lowering therapies, including lifestyle management,” the authors wrote.
In patients on intensive insulin therapy with prandial and basal insulin, the authors recommended blood glucose monitoring be conducted when fasting, premeal, at bedtime and occasionally in the middle of the night.
In patients on basal insulin only or in combination with other diabetes medications, blood glucose testing was recommended at least when fasting and at bedtime.
When it comes to patients on noninsulin therapies for type 2 diabetes, guidelines advise that blood glucose monitoring be used only when patients or their caregivers are equipped to incorporate it and the resulting therapeutic adjustments into the diabetes care plan.
The only exception to this was in patients receiving noninsulin agents linked with a higher risk of hypoglycemia, such as sulfonylureas and glinides, in which case, they should test their fasting blood glucose at least once a day and periodically at other times, the authors recommended.
In pregnant women with gestational diabetes, the consensus was to test fasting and 1 hour postprandial if patients were not receiving insulin, and to test fasting, preprandial, and 1 hour postprandial in those receiving insulin.
The authors also noted that there is some variation in accuracy among the blood glucose monitors approved by the Food and Drug Administration, but the degree of accuracy required is dependent on the patient.
“As the technology advances, there is a vital need to integrate the multiple data inputs from insulin pumps, glucose sensors, glucose meters, and carbohydrate intake in a comprehensive and standardized way so clinicians and patients can make sense of it all.”
The consensus statement was produced by the American Association of Clinical Endocrinologists. All but one author declared fees, honoraria, and research grants from the pharmaceutical industry.
FROM ENDOCRINE PRACTICE
Consensus statement stresses ‘meaningful’ glucose monitoring
Outpatient blood glucose monitoring (BGM) needs to be meaningful, and the best way to achieve that is to individualize the process, so that changes in control, which may flag need for changes in hypoglycemia agents, may emerge, according to the American Association of Clinical Endocrinologists consensus statement, published in the February 2016 issue of Endocrine Practice.
“Meaningful monitoring” as discussed in the consensus statement comes down to an individualized approach to clinical management that is customized to patient preference and lifestyle.
For adults with type 1 diabetes mellitus, the statement endorsed existing clinical practice guidelines that recommend blood glucose testing upon awakening, after each meal, at bedtime, and before driving, with a frequency ranging from at least 4-6 each day to 10 or more times per day.
Blood glucose monitoring is also essential for achieving optimal control in children and adolescents with type 1 diabetes, but this can be particularly challenging in pediatric patients, according to the consensus statement’s authors (Endocr Pract. 2016, Feb;22[2]:231-61).
“Food intake and activity are unpredictable in very young patients, complicating parents’ efforts to regulate glucose levels,” wrote Dr. Timothy S. Bailey from the University of California, San Diego, and his coauthors.
“In adolescents, the emotional fatigue of managing their diabetes often leads to a reduced frequency of BGM, missed insulin doses, and markedly elevated hemoglobin A1c levels.”
Physicians, parents, and patients need to be cognizant of trends in glycemic control that might suggest the patient has outgrown the insulin dose and should be taught to make longer-term regimen adjustments. “Such pattern recognition requires maintaining and periodically reviewing an electronic or written log of blood glucose levels,” according to the consensus statement.
BGM was deemed essential to all patients with type 2 diabetes mellitus, even those not receiving insulin treatment, because it provides immediate feedback on glycemic control that is not provided with HbA1c measurement.
“Two of the goals for any BGM strategy are to empower patients to play a more active role in their diabetes management and to maximize the efficacy and safety of glucose-lowering therapies, including lifestyle management,” the authors wrote.
In patients on intensive insulin therapy with prandial and basal insulin, the authors recommended blood glucose monitoring be conducted when fasting, premeal, at bedtime and occasionally in the middle of the night.
In patients on basal insulin only or in combination with other diabetes medications, blood glucose testing was recommended at least when fasting and at bedtime.
When it comes to patients on noninsulin therapies for type 2 diabetes, guidelines advise that blood glucose monitoring be used only when patients or their caregivers are equipped to incorporate it and the resulting therapeutic adjustments into the diabetes care plan.
The only exception to this was in patients receiving noninsulin agents linked with a higher risk of hypoglycemia, such as sulfonylureas and glinides, in which case, they should test their fasting blood glucose at least once a day and periodically at other times, the authors recommended.
In pregnant women with gestational diabetes, the consensus was to test fasting and 1 hour postprandial if patients were not receiving insulin, and to test fasting, preprandial, and 1 hour postprandial in those receiving insulin.
The authors also noted that there is some variation in accuracy among the blood glucose monitors approved by the Food and Drug Administration, but the degree of accuracy required is dependent on the patient.
“As the technology advances, there is a vital need to integrate the multiple data inputs from insulin pumps, glucose sensors, glucose meters, and carbohydrate intake in a comprehensive and standardized way so clinicians and patients can make sense of it all.”
The consensus statement was produced by the American Association of Clinical Endocrinologists. All but one author declared fees, honoraria, and research grants from the pharmaceutical industry.
Outpatient blood glucose monitoring (BGM) needs to be meaningful, and the best way to achieve that is to individualize the process, so that changes in control, which may flag need for changes in hypoglycemia agents, may emerge, according to the American Association of Clinical Endocrinologists consensus statement, published in the February 2016 issue of Endocrine Practice.
“Meaningful monitoring” as discussed in the consensus statement comes down to an individualized approach to clinical management that is customized to patient preference and lifestyle.
For adults with type 1 diabetes mellitus, the statement endorsed existing clinical practice guidelines that recommend blood glucose testing upon awakening, after each meal, at bedtime, and before driving, with a frequency ranging from at least 4-6 each day to 10 or more times per day.
Blood glucose monitoring is also essential for achieving optimal control in children and adolescents with type 1 diabetes, but this can be particularly challenging in pediatric patients, according to the consensus statement’s authors (Endocr Pract. 2016, Feb;22[2]:231-61).
“Food intake and activity are unpredictable in very young patients, complicating parents’ efforts to regulate glucose levels,” wrote Dr. Timothy S. Bailey from the University of California, San Diego, and his coauthors.
“In adolescents, the emotional fatigue of managing their diabetes often leads to a reduced frequency of BGM, missed insulin doses, and markedly elevated hemoglobin A1c levels.”
Physicians, parents, and patients need to be cognizant of trends in glycemic control that might suggest the patient has outgrown the insulin dose and should be taught to make longer-term regimen adjustments. “Such pattern recognition requires maintaining and periodically reviewing an electronic or written log of blood glucose levels,” according to the consensus statement.
BGM was deemed essential to all patients with type 2 diabetes mellitus, even those not receiving insulin treatment, because it provides immediate feedback on glycemic control that is not provided with HbA1c measurement.
“Two of the goals for any BGM strategy are to empower patients to play a more active role in their diabetes management and to maximize the efficacy and safety of glucose-lowering therapies, including lifestyle management,” the authors wrote.
In patients on intensive insulin therapy with prandial and basal insulin, the authors recommended blood glucose monitoring be conducted when fasting, premeal, at bedtime and occasionally in the middle of the night.
In patients on basal insulin only or in combination with other diabetes medications, blood glucose testing was recommended at least when fasting and at bedtime.
When it comes to patients on noninsulin therapies for type 2 diabetes, guidelines advise that blood glucose monitoring be used only when patients or their caregivers are equipped to incorporate it and the resulting therapeutic adjustments into the diabetes care plan.
The only exception to this was in patients receiving noninsulin agents linked with a higher risk of hypoglycemia, such as sulfonylureas and glinides, in which case, they should test their fasting blood glucose at least once a day and periodically at other times, the authors recommended.
In pregnant women with gestational diabetes, the consensus was to test fasting and 1 hour postprandial if patients were not receiving insulin, and to test fasting, preprandial, and 1 hour postprandial in those receiving insulin.
The authors also noted that there is some variation in accuracy among the blood glucose monitors approved by the Food and Drug Administration, but the degree of accuracy required is dependent on the patient.
“As the technology advances, there is a vital need to integrate the multiple data inputs from insulin pumps, glucose sensors, glucose meters, and carbohydrate intake in a comprehensive and standardized way so clinicians and patients can make sense of it all.”
The consensus statement was produced by the American Association of Clinical Endocrinologists. All but one author declared fees, honoraria, and research grants from the pharmaceutical industry.
Outpatient blood glucose monitoring (BGM) needs to be meaningful, and the best way to achieve that is to individualize the process, so that changes in control, which may flag need for changes in hypoglycemia agents, may emerge, according to the American Association of Clinical Endocrinologists consensus statement, published in the February 2016 issue of Endocrine Practice.
“Meaningful monitoring” as discussed in the consensus statement comes down to an individualized approach to clinical management that is customized to patient preference and lifestyle.
For adults with type 1 diabetes mellitus, the statement endorsed existing clinical practice guidelines that recommend blood glucose testing upon awakening, after each meal, at bedtime, and before driving, with a frequency ranging from at least 4-6 each day to 10 or more times per day.
Blood glucose monitoring is also essential for achieving optimal control in children and adolescents with type 1 diabetes, but this can be particularly challenging in pediatric patients, according to the consensus statement’s authors (Endocr Pract. 2016, Feb;22[2]:231-61).
“Food intake and activity are unpredictable in very young patients, complicating parents’ efforts to regulate glucose levels,” wrote Dr. Timothy S. Bailey from the University of California, San Diego, and his coauthors.
“In adolescents, the emotional fatigue of managing their diabetes often leads to a reduced frequency of BGM, missed insulin doses, and markedly elevated hemoglobin A1c levels.”
Physicians, parents, and patients need to be cognizant of trends in glycemic control that might suggest the patient has outgrown the insulin dose and should be taught to make longer-term regimen adjustments. “Such pattern recognition requires maintaining and periodically reviewing an electronic or written log of blood glucose levels,” according to the consensus statement.
BGM was deemed essential to all patients with type 2 diabetes mellitus, even those not receiving insulin treatment, because it provides immediate feedback on glycemic control that is not provided with HbA1c measurement.
“Two of the goals for any BGM strategy are to empower patients to play a more active role in their diabetes management and to maximize the efficacy and safety of glucose-lowering therapies, including lifestyle management,” the authors wrote.
In patients on intensive insulin therapy with prandial and basal insulin, the authors recommended blood glucose monitoring be conducted when fasting, premeal, at bedtime and occasionally in the middle of the night.
In patients on basal insulin only or in combination with other diabetes medications, blood glucose testing was recommended at least when fasting and at bedtime.
When it comes to patients on noninsulin therapies for type 2 diabetes, guidelines advise that blood glucose monitoring be used only when patients or their caregivers are equipped to incorporate it and the resulting therapeutic adjustments into the diabetes care plan.
The only exception to this was in patients receiving noninsulin agents linked with a higher risk of hypoglycemia, such as sulfonylureas and glinides, in which case, they should test their fasting blood glucose at least once a day and periodically at other times, the authors recommended.
In pregnant women with gestational diabetes, the consensus was to test fasting and 1 hour postprandial if patients were not receiving insulin, and to test fasting, preprandial, and 1 hour postprandial in those receiving insulin.
The authors also noted that there is some variation in accuracy among the blood glucose monitors approved by the Food and Drug Administration, but the degree of accuracy required is dependent on the patient.
“As the technology advances, there is a vital need to integrate the multiple data inputs from insulin pumps, glucose sensors, glucose meters, and carbohydrate intake in a comprehensive and standardized way so clinicians and patients can make sense of it all.”
The consensus statement was produced by the American Association of Clinical Endocrinologists. All but one author declared fees, honoraria, and research grants from the pharmaceutical industry.
FROM ENDOCRINE PRACTICE
Key clinical point: Consensus statement on blood glucose monitoring calls for individualized approach to clinical management involving “meaningful monitoring.”
Major finding: Blood glucose monitoring is a cornerstone of diabetes management.
Data source: Consensus statement from the American Association of Clinical Endocrinologists.
Disclosures: The consensus statement was produced by the American Association of Clinical Endocrinologists. All but one author declared fees, honoraria, and research grants from the pharmaceutical industry.
Elevated cardiovascular risks linked to hidradenitis suppurativa
The inflammatory skin disease hidradenitis suppurativa is associated with significantly increased risks of adverse cardiovascular outcomes such as ischemic stroke, myocardial infarction, and cardiovascular mortality, according to results of a population-based study.
A population-based cohort study of 5,964 patients with hidradenitis suppurativa showed that, after adjusting for confounders such as age, sex, smoking, and other comorbidities, hidradenitis suppurativa was associated with a 57% greater risk of myocardial infarction, 33% greater risk of ischemic stroke, 53% increase in major adverse cardiovascular events, and 35% increase in all-cause mortality over a mean 7.1 years of follow-up.
The study, published online Feb. 17 in JAMA Dermatology, also showed a significant increase in cardiovascular-associated death, which was the only adverse outcome that remained significantly elevated (incidence rate ratio, 1.58) in patients with hidradenitis suppurativa when compared with a control group of individuals with severe psoriasis (JAMA Dermatol. 2016 Feb 17. doi: 10.1001/jamadermatol.2015.6264).
“Studies have suggested that, in hidradenitis suppurativa, atrophy of the sebaceous glands, follicular hyperkeratinization, and subsequent hair follicle destruction are associated with deep-seated inflammation, increased susceptibility to secondary infections, and chronic perpetuation of the inflammatory response,” wrote Dr. Alexander Egeberg of the University of Copenhagen and coauthors.
The researchers suggested that there was a “conspicuous absence” of research reports on the risk of cardiovascular disease in hidradenitis suppurativa, especially in light of accumulating evidence of the association between cardiovascular disease and other chronic inflammatory diseases such as psoriasis, rheumatoid arthritis, and inflammatory bowel disease.
No conflicts of interest were declared.
The inflammatory skin disease hidradenitis suppurativa is associated with significantly increased risks of adverse cardiovascular outcomes such as ischemic stroke, myocardial infarction, and cardiovascular mortality, according to results of a population-based study.
A population-based cohort study of 5,964 patients with hidradenitis suppurativa showed that, after adjusting for confounders such as age, sex, smoking, and other comorbidities, hidradenitis suppurativa was associated with a 57% greater risk of myocardial infarction, 33% greater risk of ischemic stroke, 53% increase in major adverse cardiovascular events, and 35% increase in all-cause mortality over a mean 7.1 years of follow-up.
The study, published online Feb. 17 in JAMA Dermatology, also showed a significant increase in cardiovascular-associated death, which was the only adverse outcome that remained significantly elevated (incidence rate ratio, 1.58) in patients with hidradenitis suppurativa when compared with a control group of individuals with severe psoriasis (JAMA Dermatol. 2016 Feb 17. doi: 10.1001/jamadermatol.2015.6264).
“Studies have suggested that, in hidradenitis suppurativa, atrophy of the sebaceous glands, follicular hyperkeratinization, and subsequent hair follicle destruction are associated with deep-seated inflammation, increased susceptibility to secondary infections, and chronic perpetuation of the inflammatory response,” wrote Dr. Alexander Egeberg of the University of Copenhagen and coauthors.
The researchers suggested that there was a “conspicuous absence” of research reports on the risk of cardiovascular disease in hidradenitis suppurativa, especially in light of accumulating evidence of the association between cardiovascular disease and other chronic inflammatory diseases such as psoriasis, rheumatoid arthritis, and inflammatory bowel disease.
No conflicts of interest were declared.
The inflammatory skin disease hidradenitis suppurativa is associated with significantly increased risks of adverse cardiovascular outcomes such as ischemic stroke, myocardial infarction, and cardiovascular mortality, according to results of a population-based study.
A population-based cohort study of 5,964 patients with hidradenitis suppurativa showed that, after adjusting for confounders such as age, sex, smoking, and other comorbidities, hidradenitis suppurativa was associated with a 57% greater risk of myocardial infarction, 33% greater risk of ischemic stroke, 53% increase in major adverse cardiovascular events, and 35% increase in all-cause mortality over a mean 7.1 years of follow-up.
The study, published online Feb. 17 in JAMA Dermatology, also showed a significant increase in cardiovascular-associated death, which was the only adverse outcome that remained significantly elevated (incidence rate ratio, 1.58) in patients with hidradenitis suppurativa when compared with a control group of individuals with severe psoriasis (JAMA Dermatol. 2016 Feb 17. doi: 10.1001/jamadermatol.2015.6264).
“Studies have suggested that, in hidradenitis suppurativa, atrophy of the sebaceous glands, follicular hyperkeratinization, and subsequent hair follicle destruction are associated with deep-seated inflammation, increased susceptibility to secondary infections, and chronic perpetuation of the inflammatory response,” wrote Dr. Alexander Egeberg of the University of Copenhagen and coauthors.
The researchers suggested that there was a “conspicuous absence” of research reports on the risk of cardiovascular disease in hidradenitis suppurativa, especially in light of accumulating evidence of the association between cardiovascular disease and other chronic inflammatory diseases such as psoriasis, rheumatoid arthritis, and inflammatory bowel disease.
No conflicts of interest were declared.
FROM JAMA DERMATOLOGY
Key clinical point: Hidradenitis suppurativa is associated with a significantly increased risk of adverse cardiovascular events and all-cause mortality.
Major finding: Individuals with hidradenitis suppurativa had a 57% greater risk of myocardial infarction and 33% greater risk of ischemic stroke, compared with the general population.
Data source: A population-based cohort study in 5,964 patients with hidradenitis suppurativa.
Disclosures: No conflicts of interest were declared.
Early elective colon resection common in diverticulitis
A significant number of elective colon resections for uncomplicated diverticulitis are done in individuals who have experienced fewer than three episodes.
Researchers analyzed nationwide data from 87,461 immunocompetent patients with at least one claim for diverticulitis, of whom 5,604 (6.4%) underwent a resection.
According to a paper published online Feb. 10 in JAMA Surgery, 94.9% of resections, in a final cohort of 3,054 patients, occurred in individuals with fewer than three episodes of diverticulitis, if only inpatient claims were counted (doi:10.1001/jamasurg.2015.5478).
If both inpatient and outpatient claims for diverticulitis were counted, 80.5% of patients who underwent resection had experienced fewer than three episodes, and if all types of claims (including antibiotic prescription claims for diverticulitis) were counted, that figure dropped to 56.3%.
Individuals who underwent early resection were slightly more likely to be male (risk ratio [RR], 1.07; 95% confidence interval [CI], 1.02-1.13; P = .004) but were of a similar age to those who underwent resection after three or more episodes of diverticulitis.
The mean time between the last two episodes of diverticulitis was longer in individuals who underwent early surgery compared to those who delayed surgery (157 days vs. 96 days; P less than .001).
Patients residing in the South were also significantly more likely to undergo early surgery than were those residing in an other regions, with 60.5% of policy holders there undergoing early surgery compared to 50.7% in the West.
Insurance status also influenced the likelihood of early surgery, as patients with HMO or capitated insurance plans were less likely to undergo early surgery than were patients with other plan types.
In the last decade, professional guidelines have moved toward recommending elective surgery for diverticulitis after three or more episodes, but at the same time, the incidence of elective resection has more than doubled, reported Dr. Vlad V. Simianu of the University of Washington, Seattle, and coauthors.
This study covered a period of data in which guidelines on elective resection have remained in a relatively steady state, offering an opportunity to assess guideline adherence.
“Within this context, the suspected drivers of early elective surgery (younger age, laparoscopy, more frequent episodes, and personal financial risk) were not found to be associated with earlier operations for diverticulitis,” the authors wrote.
The analysis found laparoscopy was not associated with early surgery, which the authors said challenged the hypothesis that the threshold for early surgical resection might be lowered by the availability of laparoscopy.
The lack of an age difference between those undergoing early resection also challenged the notion that younger patients may experience more severe diverticulitis and suffer a greater impact on their quality of life and that this may drive physicians to operate earlier.
The authors noted that patient factors such as quality of life and anxiety about future episodes of diverticulitis, and surgeon-related factors such as training, local practice, and referral patterns were not tested, and that these may account for some of decisions about early surgery.
The National Institute of Diabetes and Digestive and Kidney Diseases and the University of Washington supported the study. No conflicts of interest were declared.
A significant number of elective colon resections for uncomplicated diverticulitis are done in individuals who have experienced fewer than three episodes.
Researchers analyzed nationwide data from 87,461 immunocompetent patients with at least one claim for diverticulitis, of whom 5,604 (6.4%) underwent a resection.
According to a paper published online Feb. 10 in JAMA Surgery, 94.9% of resections, in a final cohort of 3,054 patients, occurred in individuals with fewer than three episodes of diverticulitis, if only inpatient claims were counted (doi:10.1001/jamasurg.2015.5478).
If both inpatient and outpatient claims for diverticulitis were counted, 80.5% of patients who underwent resection had experienced fewer than three episodes, and if all types of claims (including antibiotic prescription claims for diverticulitis) were counted, that figure dropped to 56.3%.
Individuals who underwent early resection were slightly more likely to be male (risk ratio [RR], 1.07; 95% confidence interval [CI], 1.02-1.13; P = .004) but were of a similar age to those who underwent resection after three or more episodes of diverticulitis.
The mean time between the last two episodes of diverticulitis was longer in individuals who underwent early surgery compared to those who delayed surgery (157 days vs. 96 days; P less than .001).
Patients residing in the South were also significantly more likely to undergo early surgery than were those residing in an other regions, with 60.5% of policy holders there undergoing early surgery compared to 50.7% in the West.
Insurance status also influenced the likelihood of early surgery, as patients with HMO or capitated insurance plans were less likely to undergo early surgery than were patients with other plan types.
In the last decade, professional guidelines have moved toward recommending elective surgery for diverticulitis after three or more episodes, but at the same time, the incidence of elective resection has more than doubled, reported Dr. Vlad V. Simianu of the University of Washington, Seattle, and coauthors.
This study covered a period of data in which guidelines on elective resection have remained in a relatively steady state, offering an opportunity to assess guideline adherence.
“Within this context, the suspected drivers of early elective surgery (younger age, laparoscopy, more frequent episodes, and personal financial risk) were not found to be associated with earlier operations for diverticulitis,” the authors wrote.
The analysis found laparoscopy was not associated with early surgery, which the authors said challenged the hypothesis that the threshold for early surgical resection might be lowered by the availability of laparoscopy.
The lack of an age difference between those undergoing early resection also challenged the notion that younger patients may experience more severe diverticulitis and suffer a greater impact on their quality of life and that this may drive physicians to operate earlier.
The authors noted that patient factors such as quality of life and anxiety about future episodes of diverticulitis, and surgeon-related factors such as training, local practice, and referral patterns were not tested, and that these may account for some of decisions about early surgery.
The National Institute of Diabetes and Digestive and Kidney Diseases and the University of Washington supported the study. No conflicts of interest were declared.
A significant number of elective colon resections for uncomplicated diverticulitis are done in individuals who have experienced fewer than three episodes.
Researchers analyzed nationwide data from 87,461 immunocompetent patients with at least one claim for diverticulitis, of whom 5,604 (6.4%) underwent a resection.
According to a paper published online Feb. 10 in JAMA Surgery, 94.9% of resections, in a final cohort of 3,054 patients, occurred in individuals with fewer than three episodes of diverticulitis, if only inpatient claims were counted (doi:10.1001/jamasurg.2015.5478).
If both inpatient and outpatient claims for diverticulitis were counted, 80.5% of patients who underwent resection had experienced fewer than three episodes, and if all types of claims (including antibiotic prescription claims for diverticulitis) were counted, that figure dropped to 56.3%.
Individuals who underwent early resection were slightly more likely to be male (risk ratio [RR], 1.07; 95% confidence interval [CI], 1.02-1.13; P = .004) but were of a similar age to those who underwent resection after three or more episodes of diverticulitis.
The mean time between the last two episodes of diverticulitis was longer in individuals who underwent early surgery compared to those who delayed surgery (157 days vs. 96 days; P less than .001).
Patients residing in the South were also significantly more likely to undergo early surgery than were those residing in an other regions, with 60.5% of policy holders there undergoing early surgery compared to 50.7% in the West.
Insurance status also influenced the likelihood of early surgery, as patients with HMO or capitated insurance plans were less likely to undergo early surgery than were patients with other plan types.
In the last decade, professional guidelines have moved toward recommending elective surgery for diverticulitis after three or more episodes, but at the same time, the incidence of elective resection has more than doubled, reported Dr. Vlad V. Simianu of the University of Washington, Seattle, and coauthors.
This study covered a period of data in which guidelines on elective resection have remained in a relatively steady state, offering an opportunity to assess guideline adherence.
“Within this context, the suspected drivers of early elective surgery (younger age, laparoscopy, more frequent episodes, and personal financial risk) were not found to be associated with earlier operations for diverticulitis,” the authors wrote.
The analysis found laparoscopy was not associated with early surgery, which the authors said challenged the hypothesis that the threshold for early surgical resection might be lowered by the availability of laparoscopy.
The lack of an age difference between those undergoing early resection also challenged the notion that younger patients may experience more severe diverticulitis and suffer a greater impact on their quality of life and that this may drive physicians to operate earlier.
The authors noted that patient factors such as quality of life and anxiety about future episodes of diverticulitis, and surgeon-related factors such as training, local practice, and referral patterns were not tested, and that these may account for some of decisions about early surgery.
The National Institute of Diabetes and Digestive and Kidney Diseases and the University of Washington supported the study. No conflicts of interest were declared.
FROM JAMA SURGERY
Key clinical point: A majority of elective colon resections for uncomplicated diverticulitis are done in individuals who have experienced fewer than three episodes.
Major finding: More than 90% of elective resections occur in patients who have experienced fewer than three inpatient-managed episodes of diverticulitis.
Data source: Retrospective cohort study of 87,461 patients who underwent surgical resection.
Disclosures: The National Institute of Diabetes and Digestive and Kidney Diseases and the University of Washington supported the study. No conflicts of interest were declared.