User login
Cutaneous Squamous Cell Carcinoma With Perineural Invasion: A Case Report and Review of the Literature
Case Report
A 74-year-old man with a history of squamous cell carcinoma (SCC) on the right side of the temple that was treated with Mohs micrographic surgery (MMS) 3 years prior presented with a burning and tingling sensation of 3 months’ duration in the medial border of the repair scar. The patient denied prior anesthesia or muscle weakness of the face as well as any loss or change in vision.
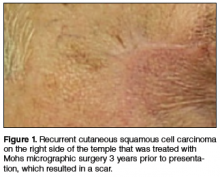
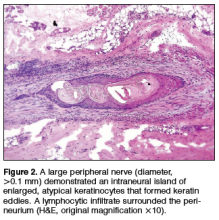
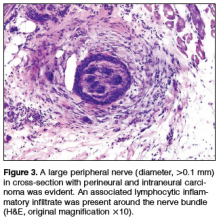
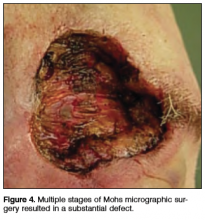
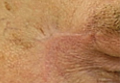
Physical examination revealed a well-healed advancement flap scar with induration at the medial border (Figure 1). Biopsy results were positive for recurrent SCC. Based on anatomic location, clinical symptoms, and tumor recurrence, treatment with MMS was initiated. Mohs sections demonstrated perineural invasion (PNI) (Figures 2 and 3). Multiple treatment stages were required for tumor clearance following the retrograde course of a nerve, which resulted in a substantial defect (Figure 4). The defect was allowed to heal by second intention followed by radiation therapy.
Comment
Incidence and Pathogenesis—Perineural invasion was first described by Cruveilhier1 in a report of invasion of the facial nerve in a patient with mammary carcinoma. Neumann2 reported the first case of a primary cutaneous lesion exhibiting PNI in a patient with a primary carcinoma of the lower lip with invasion and spread along the mental nerve. Perineural invasion is seen in approximately 5% of 200,000 total cases of cutaneous SCC reported annually in the United States.3,4 Other malignancies exhibit PNI more frequently, such as microcystic adnexal carcinoma of the skin, which has been reported to have an 80% rate of perineural growth.5
Perineural invasion can involve nerves of variable thickness, but invasion of larger nerves typically portends a poorer prognosis.6 Characteristics of cutaneous SCC that predispose the lesion to PNI include size greater than 2 cm, male gender, location on the face, and prior treatment of the lesion.6,7 In a study of cutaneous SCC, Leibovitch et al7 found PNI in 4.7% (36/772) of primary lesions and 6.9% (34/491) of recurrent lesions. In another study of 180 SCC tumors of the head and neck with PNI, Carter et al8 found that PNI was most commonly seen in tumors that were greater than 2.5 cm, suggesting that larger lesions have an increased predisposition for PNI.
The mechanism(s) by which PNI develops from these malignancies has not been fully elucidated, but some clues have been found. Vural et al9 showed a statistically significant difference (P<.01) in expression of neural cell adhesion molecules with 93% (38/41) of SCCs with PNI showing evidence of expression versus 36% (9/25) of SCCs without PNI. Chen-Tsai et al10 also suggested that levels of neural cell adhesion molecules may be a factor in determining the metastatic potential of cutaneous SCCs and that levels of neurotrophic tyrosine kinase receptor type 1 (TrkA) may predict PNI, but their study results lacked statistical power to form a firm conclusion.
Diagnosis and Prognosis—Perineural invasion can be diagnosed clinically, radiologically, or microscopically. On clinical examination, PNI is suggested by findings of neuropathy most frequently in cranial nerves V and/or VII, likely due to their extensive subcutaneous distribution.11 Common symptoms include pain, loss of motor skills, anesthesia, dysesthesia, and/or paresthesia (ie, tingling, burning, pricking, numbness).12,13 In a study of 72 cases, Goepfert et al14 found that only 40% (29/72) of patients with pathologically confirmed PNI presented with clinical symptoms and these patients had a poorer prognosis.
Radiologically, PNI can be identified via computed tomography or magnetic resonance imaging through findings of enlargement or abnormal enhancement of the nerve, obliteration of the normal fat plane surrounding the nerve, or erosion or enlargement of its related foramen.15 Magnetic resonance imaging is the preferred method for assessing enhancement of the nerve, while computed tomography is preferred to assess involvement of bone.16,17 Microscopically, there is some debate as to what defines PNI. Suggested findings include the presence of cells inside the epineurium, involvement of nerves outside the main bulk of the tumor, or presence of tumor cells surrounding a nerve.18
These definitions have prognostic significance. Mendenhall et al16 found that patients with radiologic evidence of PNI without clinical symptoms had a higher cure rate using surgery and postoperative irradiation compared to patients with clinical symptoms (80% vs 45%). Although prognosis generally is good in patients with cutaneous SCC without PNI, prognosis is notably poorer when PNI is present due to the association of this finding with increased tumor recurrence and both local and distant metastasis.13 Most frequently, cutaneous SCC with PNI spreads proximally, which can lead to invasion into the base of the brain, but also can extend distally, leading to increased local burden.12,19 In a study of 64 patients with mucosal SCC, Soo et al20 found that patients with lesions that exhibited PNI had a 5-year survival of 16% versus 44% in those without PNI. In their study of SCC of the head and neck, Goepfert et al14 reported that 46% (33/72) of patients with PNI had died or were alive with recurrence at 2 years’ follow-up versus 9.1% (41/448) of patients without PNI. In a systematic review of outcomes, Jambusaria-Pahlajani et al21 reported a disease-specific death rate of 16% for cutaneous SCC with PNI compared to 4% for SCC without PNI.
Perineural invasion can be further classified as clinical or microscopic (incidental) for prognostic purposes. A study by Garcia-Serra et al13 found that patients with clinical PNI had a notably poorer prognosis than those with microscopic (incidental) PNI. The clinical group achieved a local control rate of 55% at 5 years’ follow-up versus 87% in the microscopic group. McCord et al22 found a 5-year local control rate of 78% for microscopic (incidental) PNI versus 50% for clinical PNI; they also found that patients with radiologic evidence of PNI had a worse prognosis, noting that patients with radiologic evidence of PNI were nearly all clinically symptomatic.
Prognosis also is altered by the diameter of the nerve involved. In a study of 48 patients, Ross et al23 found that patients with cutaneous SCC involving small-caliber nerves (diameter, ≤0.1 mm) had a 0% disease-specific death rate versus 32% in those with large-caliber nerves (>0.1 mm). Perineural involvement of small-caliber nerves (<0.1 mm) was a positive prognostic indicator in that it was associated with smaller tumor diameter, more shallow invasion, and increased likelihood to be primary tumors.23 In a recent study, Jambusaria-Pahlajani et al24 investigated tumor staging for cutaneous SCC and reported that PNI is a statistically independent prognostic risk factor for nodal metastasis (subhazard ratio, 2.2 [95% confidence interval, 0.9-5.1]) and disease-specific death (subhazard ratio, 3.4 [95% confidence interval, 0.9-13.3]). Of interest, this increased risk applied only to PNI in nerves that were greater than 0.1 mm.24
Treatment Options—Management of confirmed cases of cutaneous SCC with PNI is difficult because of the nature of the lesions, including their increased propensity for metastasis, increased frequency of poorly differentiated cell types, highly aggressive nature, and the unique challenge of skip lesions.4,16 Skip lesions are found microscopically and show (or appear to show) neoplastic cells invading a nerve in a discontinuous fashion. This phenomenon has been suggested as one explanation for the relatively higher postsurgical recurrence rate of SCC with PNI compared to lesions without PNI.7 They are of particular interest when removing cutaneous SCC with PNI using MMS and attempting to define clear margins. Despite this limitation, MMS generally is accepted as the primary mode of excision of cutaneous SCCs with PNI, as it has the highest known cure rate.7 Cottel4 did not report any cases of local recurrence over 1 to 42 months in 17 patients who were treated with MMS, in contrast to Rowe et al25 who demonstrated that traditional surgical excision had a 47% (34/72) local recurrence rate; however, it bears noting that the varying follow-up periods in the Cottel4 study may underestimate recurrence rate. Leibovitch et al7 had similar findings in their prospective case series study of 70 patients, which revealed an 8% recurrence rate within 5 years in patients treated with MMS, a rate lower than other non-MMS modalities. In this same study, the authors noted that some researchers believe an additional level should be taken with MMS beyond the appearance of free margins in cases with PNI.7
Jambusaria-Pahlajani et al21 reported that PNI is one of the most common reasons cited for using adjuvant radiation therapy for cases of cutaneous SCC because of the known propensity of local recurrence; however, in 74 reviewed cases, there was no statistically significant difference in outcomes in cases of surgery alone versus surgery and adjuvant irradiation. Radiation therapy is a possible alternative primary treatment of cutaneous SCC with PNI, especially in cases of perineural involvement that is extensive or affects proximal portions of cranial nerves when surgery is a less viable option.17 Mendenhall et al16 suggested that patients with positive margins after excision who display extensive PNI should be treated with adjuvant irradiation locally and along the course of the involved nerve to the skull base.
Conclusion
Physicians should recognize the importance of early detection of PNI in cases of cutaneous SCC. A thorough history with good neurologic examination of the head and neck in patients with cutaneous SCC is imperative so patients can be treated earlier in the course of the lesion, increasing the likelihood of local control, minimizing the risk for future recurrence, and decreasing mortality.
1. Cruveilhier J. Maladies des nerfs. In: Cruveilhier J, ed. Anatomie Pathologique du Corps Humain. 2nd ed. Paris, France: JB Bailliere; 1835:1-3.
2. Neumann E. Secondare cancroid infiltration des nervus mentalis bei einem fall von lippincroid. Arch Pathol Anat. 1862;24:201-205.
3. Salasche S. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
4. Cottel WI. Perineural invasion by squamous-cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
5. Cooper PH, Mills SE, Leonard DD, et al. Sclerosing sweat duct (syringomatous) carcinoma. Am J Surg Pathol. 1985;9:422-433.
6. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
7. Leibovitch I, Huilgol SC, Selva D, et al. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia II. perineural invasion. J Am Acad Dermatol. 2005;53:261-266.
8. Carter RL, Foster CS, Dinsdale EA, et al. Perineural spread by squamous carcinomas of the head and neck: a morphological study using antiaxonal and antimyelin monoclonal antibodies. J Clin Pathol. 1983;36:269-275.
9. Vural E, Hutcheson J, Korourian S, et al. Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2000;122:717-720.
10. Chen-Tsai CP, Colome-Grimmer M, Wagner RF Jr. Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg. 2004;30:1009-1016.
11. McCord M, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with clinical perineural invasion. Int J Radiat Oncol Biol Phys. 2000;47:89-93.
12. Ampil FL, Hardin JC, Peskind SP, et al. Perineural invasion in skin cancer of the head and neck: a review of nine cases. J Oral Maxillofac Surg. 1995;53:34-38.
13. Garcia-Serra A, Hinerman RW, Mendenhall WM, et al. Carcinoma of the skin with perineural invasion. Head Neck. 2003;25:1027-1033.
14. Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
15. Galloway TJ, Morris CG, Mancuso AA, et al. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer. 2005;103:1254-1257.
16. Mendenhall WM, Amdur RJ, Williams LS, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 2002;24:78-83.
17. Williams LS, Mancuso AA, Mendenhall WM. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001;49:1061-1069.
18. Veness MJ, Biankin S. Perineural spread leading to orbital invasion from skin cancer. Australasian Radiol. 2000;44:296-302.
19. Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
20. Soo K, Carter RL, O’Brien CJ, et al. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope. 1986;96:1145-1148.
21. Jambusaria-Pahlajani A, Miller CJ, Quon H, et al. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35:574-584.
22. McCord MW, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with incidental microscopic perineural invasion. Int J Radiat Oncol Biol Phys. 1999;43:591-595.
23. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
24. Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
25. Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
Case Report
A 74-year-old man with a history of squamous cell carcinoma (SCC) on the right side of the temple that was treated with Mohs micrographic surgery (MMS) 3 years prior presented with a burning and tingling sensation of 3 months’ duration in the medial border of the repair scar. The patient denied prior anesthesia or muscle weakness of the face as well as any loss or change in vision.
Physical examination revealed a well-healed advancement flap scar with induration at the medial border (Figure 1). Biopsy results were positive for recurrent SCC. Based on anatomic location, clinical symptoms, and tumor recurrence, treatment with MMS was initiated. Mohs sections demonstrated perineural invasion (PNI) (Figures 2 and 3). Multiple treatment stages were required for tumor clearance following the retrograde course of a nerve, which resulted in a substantial defect (Figure 4). The defect was allowed to heal by second intention followed by radiation therapy.
Comment
Incidence and Pathogenesis—Perineural invasion was first described by Cruveilhier1 in a report of invasion of the facial nerve in a patient with mammary carcinoma. Neumann2 reported the first case of a primary cutaneous lesion exhibiting PNI in a patient with a primary carcinoma of the lower lip with invasion and spread along the mental nerve. Perineural invasion is seen in approximately 5% of 200,000 total cases of cutaneous SCC reported annually in the United States.3,4 Other malignancies exhibit PNI more frequently, such as microcystic adnexal carcinoma of the skin, which has been reported to have an 80% rate of perineural growth.5
Perineural invasion can involve nerves of variable thickness, but invasion of larger nerves typically portends a poorer prognosis.6 Characteristics of cutaneous SCC that predispose the lesion to PNI include size greater than 2 cm, male gender, location on the face, and prior treatment of the lesion.6,7 In a study of cutaneous SCC, Leibovitch et al7 found PNI in 4.7% (36/772) of primary lesions and 6.9% (34/491) of recurrent lesions. In another study of 180 SCC tumors of the head and neck with PNI, Carter et al8 found that PNI was most commonly seen in tumors that were greater than 2.5 cm, suggesting that larger lesions have an increased predisposition for PNI.
The mechanism(s) by which PNI develops from these malignancies has not been fully elucidated, but some clues have been found. Vural et al9 showed a statistically significant difference (P<.01) in expression of neural cell adhesion molecules with 93% (38/41) of SCCs with PNI showing evidence of expression versus 36% (9/25) of SCCs without PNI. Chen-Tsai et al10 also suggested that levels of neural cell adhesion molecules may be a factor in determining the metastatic potential of cutaneous SCCs and that levels of neurotrophic tyrosine kinase receptor type 1 (TrkA) may predict PNI, but their study results lacked statistical power to form a firm conclusion.
Diagnosis and Prognosis—Perineural invasion can be diagnosed clinically, radiologically, or microscopically. On clinical examination, PNI is suggested by findings of neuropathy most frequently in cranial nerves V and/or VII, likely due to their extensive subcutaneous distribution.11 Common symptoms include pain, loss of motor skills, anesthesia, dysesthesia, and/or paresthesia (ie, tingling, burning, pricking, numbness).12,13 In a study of 72 cases, Goepfert et al14 found that only 40% (29/72) of patients with pathologically confirmed PNI presented with clinical symptoms and these patients had a poorer prognosis.
Radiologically, PNI can be identified via computed tomography or magnetic resonance imaging through findings of enlargement or abnormal enhancement of the nerve, obliteration of the normal fat plane surrounding the nerve, or erosion or enlargement of its related foramen.15 Magnetic resonance imaging is the preferred method for assessing enhancement of the nerve, while computed tomography is preferred to assess involvement of bone.16,17 Microscopically, there is some debate as to what defines PNI. Suggested findings include the presence of cells inside the epineurium, involvement of nerves outside the main bulk of the tumor, or presence of tumor cells surrounding a nerve.18
These definitions have prognostic significance. Mendenhall et al16 found that patients with radiologic evidence of PNI without clinical symptoms had a higher cure rate using surgery and postoperative irradiation compared to patients with clinical symptoms (80% vs 45%). Although prognosis generally is good in patients with cutaneous SCC without PNI, prognosis is notably poorer when PNI is present due to the association of this finding with increased tumor recurrence and both local and distant metastasis.13 Most frequently, cutaneous SCC with PNI spreads proximally, which can lead to invasion into the base of the brain, but also can extend distally, leading to increased local burden.12,19 In a study of 64 patients with mucosal SCC, Soo et al20 found that patients with lesions that exhibited PNI had a 5-year survival of 16% versus 44% in those without PNI. In their study of SCC of the head and neck, Goepfert et al14 reported that 46% (33/72) of patients with PNI had died or were alive with recurrence at 2 years’ follow-up versus 9.1% (41/448) of patients without PNI. In a systematic review of outcomes, Jambusaria-Pahlajani et al21 reported a disease-specific death rate of 16% for cutaneous SCC with PNI compared to 4% for SCC without PNI.
Perineural invasion can be further classified as clinical or microscopic (incidental) for prognostic purposes. A study by Garcia-Serra et al13 found that patients with clinical PNI had a notably poorer prognosis than those with microscopic (incidental) PNI. The clinical group achieved a local control rate of 55% at 5 years’ follow-up versus 87% in the microscopic group. McCord et al22 found a 5-year local control rate of 78% for microscopic (incidental) PNI versus 50% for clinical PNI; they also found that patients with radiologic evidence of PNI had a worse prognosis, noting that patients with radiologic evidence of PNI were nearly all clinically symptomatic.
Prognosis also is altered by the diameter of the nerve involved. In a study of 48 patients, Ross et al23 found that patients with cutaneous SCC involving small-caliber nerves (diameter, ≤0.1 mm) had a 0% disease-specific death rate versus 32% in those with large-caliber nerves (>0.1 mm). Perineural involvement of small-caliber nerves (<0.1 mm) was a positive prognostic indicator in that it was associated with smaller tumor diameter, more shallow invasion, and increased likelihood to be primary tumors.23 In a recent study, Jambusaria-Pahlajani et al24 investigated tumor staging for cutaneous SCC and reported that PNI is a statistically independent prognostic risk factor for nodal metastasis (subhazard ratio, 2.2 [95% confidence interval, 0.9-5.1]) and disease-specific death (subhazard ratio, 3.4 [95% confidence interval, 0.9-13.3]). Of interest, this increased risk applied only to PNI in nerves that were greater than 0.1 mm.24
Treatment Options—Management of confirmed cases of cutaneous SCC with PNI is difficult because of the nature of the lesions, including their increased propensity for metastasis, increased frequency of poorly differentiated cell types, highly aggressive nature, and the unique challenge of skip lesions.4,16 Skip lesions are found microscopically and show (or appear to show) neoplastic cells invading a nerve in a discontinuous fashion. This phenomenon has been suggested as one explanation for the relatively higher postsurgical recurrence rate of SCC with PNI compared to lesions without PNI.7 They are of particular interest when removing cutaneous SCC with PNI using MMS and attempting to define clear margins. Despite this limitation, MMS generally is accepted as the primary mode of excision of cutaneous SCCs with PNI, as it has the highest known cure rate.7 Cottel4 did not report any cases of local recurrence over 1 to 42 months in 17 patients who were treated with MMS, in contrast to Rowe et al25 who demonstrated that traditional surgical excision had a 47% (34/72) local recurrence rate; however, it bears noting that the varying follow-up periods in the Cottel4 study may underestimate recurrence rate. Leibovitch et al7 had similar findings in their prospective case series study of 70 patients, which revealed an 8% recurrence rate within 5 years in patients treated with MMS, a rate lower than other non-MMS modalities. In this same study, the authors noted that some researchers believe an additional level should be taken with MMS beyond the appearance of free margins in cases with PNI.7
Jambusaria-Pahlajani et al21 reported that PNI is one of the most common reasons cited for using adjuvant radiation therapy for cases of cutaneous SCC because of the known propensity of local recurrence; however, in 74 reviewed cases, there was no statistically significant difference in outcomes in cases of surgery alone versus surgery and adjuvant irradiation. Radiation therapy is a possible alternative primary treatment of cutaneous SCC with PNI, especially in cases of perineural involvement that is extensive or affects proximal portions of cranial nerves when surgery is a less viable option.17 Mendenhall et al16 suggested that patients with positive margins after excision who display extensive PNI should be treated with adjuvant irradiation locally and along the course of the involved nerve to the skull base.
Conclusion
Physicians should recognize the importance of early detection of PNI in cases of cutaneous SCC. A thorough history with good neurologic examination of the head and neck in patients with cutaneous SCC is imperative so patients can be treated earlier in the course of the lesion, increasing the likelihood of local control, minimizing the risk for future recurrence, and decreasing mortality.
Case Report
A 74-year-old man with a history of squamous cell carcinoma (SCC) on the right side of the temple that was treated with Mohs micrographic surgery (MMS) 3 years prior presented with a burning and tingling sensation of 3 months’ duration in the medial border of the repair scar. The patient denied prior anesthesia or muscle weakness of the face as well as any loss or change in vision.
Physical examination revealed a well-healed advancement flap scar with induration at the medial border (Figure 1). Biopsy results were positive for recurrent SCC. Based on anatomic location, clinical symptoms, and tumor recurrence, treatment with MMS was initiated. Mohs sections demonstrated perineural invasion (PNI) (Figures 2 and 3). Multiple treatment stages were required for tumor clearance following the retrograde course of a nerve, which resulted in a substantial defect (Figure 4). The defect was allowed to heal by second intention followed by radiation therapy.
Comment
Incidence and Pathogenesis—Perineural invasion was first described by Cruveilhier1 in a report of invasion of the facial nerve in a patient with mammary carcinoma. Neumann2 reported the first case of a primary cutaneous lesion exhibiting PNI in a patient with a primary carcinoma of the lower lip with invasion and spread along the mental nerve. Perineural invasion is seen in approximately 5% of 200,000 total cases of cutaneous SCC reported annually in the United States.3,4 Other malignancies exhibit PNI more frequently, such as microcystic adnexal carcinoma of the skin, which has been reported to have an 80% rate of perineural growth.5
Perineural invasion can involve nerves of variable thickness, but invasion of larger nerves typically portends a poorer prognosis.6 Characteristics of cutaneous SCC that predispose the lesion to PNI include size greater than 2 cm, male gender, location on the face, and prior treatment of the lesion.6,7 In a study of cutaneous SCC, Leibovitch et al7 found PNI in 4.7% (36/772) of primary lesions and 6.9% (34/491) of recurrent lesions. In another study of 180 SCC tumors of the head and neck with PNI, Carter et al8 found that PNI was most commonly seen in tumors that were greater than 2.5 cm, suggesting that larger lesions have an increased predisposition for PNI.
The mechanism(s) by which PNI develops from these malignancies has not been fully elucidated, but some clues have been found. Vural et al9 showed a statistically significant difference (P<.01) in expression of neural cell adhesion molecules with 93% (38/41) of SCCs with PNI showing evidence of expression versus 36% (9/25) of SCCs without PNI. Chen-Tsai et al10 also suggested that levels of neural cell adhesion molecules may be a factor in determining the metastatic potential of cutaneous SCCs and that levels of neurotrophic tyrosine kinase receptor type 1 (TrkA) may predict PNI, but their study results lacked statistical power to form a firm conclusion.
Diagnosis and Prognosis—Perineural invasion can be diagnosed clinically, radiologically, or microscopically. On clinical examination, PNI is suggested by findings of neuropathy most frequently in cranial nerves V and/or VII, likely due to their extensive subcutaneous distribution.11 Common symptoms include pain, loss of motor skills, anesthesia, dysesthesia, and/or paresthesia (ie, tingling, burning, pricking, numbness).12,13 In a study of 72 cases, Goepfert et al14 found that only 40% (29/72) of patients with pathologically confirmed PNI presented with clinical symptoms and these patients had a poorer prognosis.
Radiologically, PNI can be identified via computed tomography or magnetic resonance imaging through findings of enlargement or abnormal enhancement of the nerve, obliteration of the normal fat plane surrounding the nerve, or erosion or enlargement of its related foramen.15 Magnetic resonance imaging is the preferred method for assessing enhancement of the nerve, while computed tomography is preferred to assess involvement of bone.16,17 Microscopically, there is some debate as to what defines PNI. Suggested findings include the presence of cells inside the epineurium, involvement of nerves outside the main bulk of the tumor, or presence of tumor cells surrounding a nerve.18
These definitions have prognostic significance. Mendenhall et al16 found that patients with radiologic evidence of PNI without clinical symptoms had a higher cure rate using surgery and postoperative irradiation compared to patients with clinical symptoms (80% vs 45%). Although prognosis generally is good in patients with cutaneous SCC without PNI, prognosis is notably poorer when PNI is present due to the association of this finding with increased tumor recurrence and both local and distant metastasis.13 Most frequently, cutaneous SCC with PNI spreads proximally, which can lead to invasion into the base of the brain, but also can extend distally, leading to increased local burden.12,19 In a study of 64 patients with mucosal SCC, Soo et al20 found that patients with lesions that exhibited PNI had a 5-year survival of 16% versus 44% in those without PNI. In their study of SCC of the head and neck, Goepfert et al14 reported that 46% (33/72) of patients with PNI had died or were alive with recurrence at 2 years’ follow-up versus 9.1% (41/448) of patients without PNI. In a systematic review of outcomes, Jambusaria-Pahlajani et al21 reported a disease-specific death rate of 16% for cutaneous SCC with PNI compared to 4% for SCC without PNI.
Perineural invasion can be further classified as clinical or microscopic (incidental) for prognostic purposes. A study by Garcia-Serra et al13 found that patients with clinical PNI had a notably poorer prognosis than those with microscopic (incidental) PNI. The clinical group achieved a local control rate of 55% at 5 years’ follow-up versus 87% in the microscopic group. McCord et al22 found a 5-year local control rate of 78% for microscopic (incidental) PNI versus 50% for clinical PNI; they also found that patients with radiologic evidence of PNI had a worse prognosis, noting that patients with radiologic evidence of PNI were nearly all clinically symptomatic.
Prognosis also is altered by the diameter of the nerve involved. In a study of 48 patients, Ross et al23 found that patients with cutaneous SCC involving small-caliber nerves (diameter, ≤0.1 mm) had a 0% disease-specific death rate versus 32% in those with large-caliber nerves (>0.1 mm). Perineural involvement of small-caliber nerves (<0.1 mm) was a positive prognostic indicator in that it was associated with smaller tumor diameter, more shallow invasion, and increased likelihood to be primary tumors.23 In a recent study, Jambusaria-Pahlajani et al24 investigated tumor staging for cutaneous SCC and reported that PNI is a statistically independent prognostic risk factor for nodal metastasis (subhazard ratio, 2.2 [95% confidence interval, 0.9-5.1]) and disease-specific death (subhazard ratio, 3.4 [95% confidence interval, 0.9-13.3]). Of interest, this increased risk applied only to PNI in nerves that were greater than 0.1 mm.24
Treatment Options—Management of confirmed cases of cutaneous SCC with PNI is difficult because of the nature of the lesions, including their increased propensity for metastasis, increased frequency of poorly differentiated cell types, highly aggressive nature, and the unique challenge of skip lesions.4,16 Skip lesions are found microscopically and show (or appear to show) neoplastic cells invading a nerve in a discontinuous fashion. This phenomenon has been suggested as one explanation for the relatively higher postsurgical recurrence rate of SCC with PNI compared to lesions without PNI.7 They are of particular interest when removing cutaneous SCC with PNI using MMS and attempting to define clear margins. Despite this limitation, MMS generally is accepted as the primary mode of excision of cutaneous SCCs with PNI, as it has the highest known cure rate.7 Cottel4 did not report any cases of local recurrence over 1 to 42 months in 17 patients who were treated with MMS, in contrast to Rowe et al25 who demonstrated that traditional surgical excision had a 47% (34/72) local recurrence rate; however, it bears noting that the varying follow-up periods in the Cottel4 study may underestimate recurrence rate. Leibovitch et al7 had similar findings in their prospective case series study of 70 patients, which revealed an 8% recurrence rate within 5 years in patients treated with MMS, a rate lower than other non-MMS modalities. In this same study, the authors noted that some researchers believe an additional level should be taken with MMS beyond the appearance of free margins in cases with PNI.7
Jambusaria-Pahlajani et al21 reported that PNI is one of the most common reasons cited for using adjuvant radiation therapy for cases of cutaneous SCC because of the known propensity of local recurrence; however, in 74 reviewed cases, there was no statistically significant difference in outcomes in cases of surgery alone versus surgery and adjuvant irradiation. Radiation therapy is a possible alternative primary treatment of cutaneous SCC with PNI, especially in cases of perineural involvement that is extensive or affects proximal portions of cranial nerves when surgery is a less viable option.17 Mendenhall et al16 suggested that patients with positive margins after excision who display extensive PNI should be treated with adjuvant irradiation locally and along the course of the involved nerve to the skull base.
Conclusion
Physicians should recognize the importance of early detection of PNI in cases of cutaneous SCC. A thorough history with good neurologic examination of the head and neck in patients with cutaneous SCC is imperative so patients can be treated earlier in the course of the lesion, increasing the likelihood of local control, minimizing the risk for future recurrence, and decreasing mortality.
1. Cruveilhier J. Maladies des nerfs. In: Cruveilhier J, ed. Anatomie Pathologique du Corps Humain. 2nd ed. Paris, France: JB Bailliere; 1835:1-3.
2. Neumann E. Secondare cancroid infiltration des nervus mentalis bei einem fall von lippincroid. Arch Pathol Anat. 1862;24:201-205.
3. Salasche S. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
4. Cottel WI. Perineural invasion by squamous-cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
5. Cooper PH, Mills SE, Leonard DD, et al. Sclerosing sweat duct (syringomatous) carcinoma. Am J Surg Pathol. 1985;9:422-433.
6. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
7. Leibovitch I, Huilgol SC, Selva D, et al. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia II. perineural invasion. J Am Acad Dermatol. 2005;53:261-266.
8. Carter RL, Foster CS, Dinsdale EA, et al. Perineural spread by squamous carcinomas of the head and neck: a morphological study using antiaxonal and antimyelin monoclonal antibodies. J Clin Pathol. 1983;36:269-275.
9. Vural E, Hutcheson J, Korourian S, et al. Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2000;122:717-720.
10. Chen-Tsai CP, Colome-Grimmer M, Wagner RF Jr. Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg. 2004;30:1009-1016.
11. McCord M, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with clinical perineural invasion. Int J Radiat Oncol Biol Phys. 2000;47:89-93.
12. Ampil FL, Hardin JC, Peskind SP, et al. Perineural invasion in skin cancer of the head and neck: a review of nine cases. J Oral Maxillofac Surg. 1995;53:34-38.
13. Garcia-Serra A, Hinerman RW, Mendenhall WM, et al. Carcinoma of the skin with perineural invasion. Head Neck. 2003;25:1027-1033.
14. Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
15. Galloway TJ, Morris CG, Mancuso AA, et al. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer. 2005;103:1254-1257.
16. Mendenhall WM, Amdur RJ, Williams LS, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 2002;24:78-83.
17. Williams LS, Mancuso AA, Mendenhall WM. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001;49:1061-1069.
18. Veness MJ, Biankin S. Perineural spread leading to orbital invasion from skin cancer. Australasian Radiol. 2000;44:296-302.
19. Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
20. Soo K, Carter RL, O’Brien CJ, et al. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope. 1986;96:1145-1148.
21. Jambusaria-Pahlajani A, Miller CJ, Quon H, et al. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35:574-584.
22. McCord MW, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with incidental microscopic perineural invasion. Int J Radiat Oncol Biol Phys. 1999;43:591-595.
23. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
24. Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
25. Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
1. Cruveilhier J. Maladies des nerfs. In: Cruveilhier J, ed. Anatomie Pathologique du Corps Humain. 2nd ed. Paris, France: JB Bailliere; 1835:1-3.
2. Neumann E. Secondare cancroid infiltration des nervus mentalis bei einem fall von lippincroid. Arch Pathol Anat. 1862;24:201-205.
3. Salasche S. Epidemiology of actinic keratoses and squamous cell carcinoma. J Am Acad Dermatol. 2000;42(1, pt 2):4-7.
4. Cottel WI. Perineural invasion by squamous-cell carcinoma. J Dermatol Surg Oncol. 1982;8:589-600.
5. Cooper PH, Mills SE, Leonard DD, et al. Sclerosing sweat duct (syringomatous) carcinoma. Am J Surg Pathol. 1985;9:422-433.
6. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
7. Leibovitch I, Huilgol SC, Selva D, et al. Cutaneous squamous cell carcinoma treated with Mohs micrographic surgery in Australia II. perineural invasion. J Am Acad Dermatol. 2005;53:261-266.
8. Carter RL, Foster CS, Dinsdale EA, et al. Perineural spread by squamous carcinomas of the head and neck: a morphological study using antiaxonal and antimyelin monoclonal antibodies. J Clin Pathol. 1983;36:269-275.
9. Vural E, Hutcheson J, Korourian S, et al. Correlation of neural cell adhesion molecules with perineural spread of squamous cell carcinoma of the head and neck. Otolaryngol Head Neck Surg. 2000;122:717-720.
10. Chen-Tsai CP, Colome-Grimmer M, Wagner RF Jr. Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg. 2004;30:1009-1016.
11. McCord M, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with clinical perineural invasion. Int J Radiat Oncol Biol Phys. 2000;47:89-93.
12. Ampil FL, Hardin JC, Peskind SP, et al. Perineural invasion in skin cancer of the head and neck: a review of nine cases. J Oral Maxillofac Surg. 1995;53:34-38.
13. Garcia-Serra A, Hinerman RW, Mendenhall WM, et al. Carcinoma of the skin with perineural invasion. Head Neck. 2003;25:1027-1033.
14. Goepfert H, Dichtel WJ, Medina JE, et al. Perineural invasion in squamous cell skin carcinoma of the head and neck. Am J Surg. 1984;148:542-547.
15. Galloway TJ, Morris CG, Mancuso AA, et al. Impact of radiographic findings on prognosis for skin carcinoma with clinical perineural invasion. Cancer. 2005;103:1254-1257.
16. Mendenhall WM, Amdur RJ, Williams LS, et al. Carcinoma of the skin of the head and neck with perineural invasion. Head Neck. 2002;24:78-83.
17. Williams LS, Mancuso AA, Mendenhall WM. Perineural spread of cutaneous squamous and basal cell carcinoma: CT and MR detection and its impact on patient management and prognosis. Int J Radiat Oncol Biol Phys. 2001;49:1061-1069.
18. Veness MJ, Biankin S. Perineural spread leading to orbital invasion from skin cancer. Australasian Radiol. 2000;44:296-302.
19. Feasel AM, Brown TJ, Bogle MA, et al. Perineural invasion of cutaneous malignancies. Dermatol Surg. 2001;27:531-542.
20. Soo K, Carter RL, O’Brien CJ, et al. Prognostic implications of perineural spread in squamous carcinomas of the head and neck. Laryngoscope. 1986;96:1145-1148.
21. Jambusaria-Pahlajani A, Miller CJ, Quon H, et al. Surgical monotherapy versus surgery plus adjuvant radiotherapy in high-risk cutaneous squamous cell carcinoma: a systematic review of outcomes. Dermatol Surg. 2009;35:574-584.
22. McCord MW, Mendenhall WM, Parsons JT, et al. Skin cancer of the head and neck with incidental microscopic perineural invasion. Int J Radiat Oncol Biol Phys. 1999;43:591-595.
23. Ross AS, Whalen FM, Elenitsas R, et al. Diameter of involved nerves predicts outcomes in cutaneous squamous cell carcinoma with perineural invasion: an investigator-blinded retrospective cohort study. Dermatol Surg. 2009;35:1859-1866.
24. Jambusaria-Pahlajani A, Kanetsky PA, Karia PS, et al. Evaluation of AJCC tumor staging for cutaneous squamous cell carcinoma and a proposed alternative tumor staging system. JAMA Dermatol. 2013;149:402-410.
25. Rowe DE, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. J Am Acad Dermatol. 1992;26:976-990.
Practice Points
• Patients with suspected cutaneous squamous cell carcinoma should be asked about neurological symptoms including pain, loss of motor skills, anesthesia, dysesthesia, and/or paresthesia, which may indicate perineural invasion.
• Patients with perineural invasion carry a much higher risk for local and distant recurrence and may require more aggressive treatment including Mohs micrographic surgery and adjuvant radiation.
Clear choices in managing epidermal tinea infections
- Potassium hydroxide preparation should be used as an aid to diagnosis for all erythrosquamous lesions (B).
- Fungal culture should be used in cases in which history, physical examination, and potassium hydroxide preparation fail to clearly exclude a diagnosis of tinea (B).
- Short-duration topical therapy with terbinafine, naftifine, and butenafine is efficacious for most epidermal tinea infections (A).
- Oral antifungal agents are important in the treatment of tinea infections thatare widespread, fail to respond to topical treatment, involve the thick stratum corneum of the soles and palms, or occur in immunosuppressed patients.
- Short courses of oral itraconazole and terbinafine are safe and effective in treating tinea infections (A).
Though findings on history and physical examination are sometimes sufficient to make a diagnosis of tinea infection, a potassium hydroxide (KOH) study usually is required for confirmation. Even the KOH study can be misleading, however, if a patient recently self-administered a topical antifungal agent. This article describes the varying appearance of tinea infections according to their anatomic location, and outlines a careful work-up.
Highly effective and affordable over-the-counter medications have proliferated, and short-course therapy is available. Based on systematic reviews of randomized, controlled studies, it is possible to recommend specific first-line therapies for tinea infections.
Manifestations of tinea infection
Clinical manifestations of tinea vary with anatomic location, duration of infection, and pathogen. In general, zoophilic dermatophytes evoke a more vigorous host response than the anthropophilic species.5 Shared features of many dermatophyte infections include erythema, scaling, pruritus, ring formation, and central clearing of lesions. Table 1 reviews published data on the diagnostic value of selected clinical signs in suspected tinea infection.6
TABLE 1
Diagnostic value of selected signs and symptoms in tinea infection
| Sign/symptom | Sensitivity | Specificity | PV+ | PV– | LR+ | LR– |
|---|---|---|---|---|---|---|
| Scaling | 77% | 20% | 17% | 80% | 0.96 | 1.15 |
| Erythema | 69% | 31% | 18% | 83% | 1.00 | 1.00 |
| Pruritus | 54% | 40% | 16% | 80% | 0.90 | 1.15 |
| Central clearing | 42% | 65% | 20% | 84% | 1.20 | 0.89 |
| Concentric rings | 27% | 80% | 23% | 84% | 1.35 | 0.91 |
| Maceration | 27% | 84% | 26% | 84% | 1.69 | 0.87 |
| Note: Signs and symptoms were compiled by 27 general practitioners prior to submission of skin for fungal culture. Specimens were taken from 148 consecutive patients with erythematosquamous lesions of glabrous skin. Culture results were considered the gold standard. | ||||||
| PV+, positive predictive value; PV–, negative predictive value; LR+, positive likelihood ratio; LR–, negative likelihood ratio. | ||||||
| Adapted from Lousbergh et al, Fam Pract 1999; 16:611–615.6 | ||||||
| Level of evidence=2b. For an explanation of levels of evidence, see page 865. | ||||||
Tinea pedis
Tinea of the foot may manifest as interdigital, plantar, or acute vesicular disease. Toe webs and soles of the feet are the sites most commonly affected. Tinea pedis occurs most commonly in postpubertal adolescents and adults, but may be seen in children.7
With interdigital infection, toe webs may become scaly, pruritic, and fissured. Interaction of bacteria and infecting dermatophytes may lead to a white, soggy maceration.8 Extension from the web space to the dorsal or plantar surface commonly occurs.
In chronic plantar or moccasin-type tinea pedis, the entire surface of the sole may be covered with fine, white scale and may assume a hyperkeratotic appearance. Chronic web infection may lead to acute inflammation characterized by vesicles, pustules, and bullae over the sole or the dorsum of the foot.5
Differential diagnosis. The differential diagnosis includes dry skin, pitted keratolysis, erythrasma, and contact dermatitis. Some conditions may appear very similar to tinea pedis, increasing the importance of KOH prep and fungal culture.
Tinea pedis may be distinguished from erythrasma by lack of bright coral appearance when examined with a Wood’s lamp. Tinea pedis infections also lack the well-demarcated erosions, or pits, of pitted keratolysis. Evidence of concurrent onychomycosis should increase the suspicion that tinea pedis is the correct diagnosis.
Tinea manuum
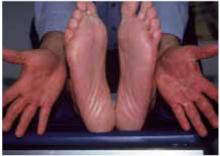
Tinea of the hand is usually analogous to moccasin-type tinea pedis. The palm appears hyperkeratotic and has very fine white scale that emphasizes the normal lines of the hand. Tinea of the dorsal surface of the hand usually occurs in the classic ringworm pattern. Tinea manuum is often seen in association with tinea pedis and onychomycosis. Many clinicians are familiar with the “one hand, two feet” syndrome, in which the palmar surfaces of both feet and one hand are infected (Figure 1).9 Onychomycosis often occurs in association with this presentation of tinea.
Differential diagnosis. The pattern of tinea manuum may be confused with those of eczema, contact dermatitis, palmar psoriasis, or even normal, rough hands. Unilateral involvement, presence of fingernail onychomycosis, lack of history indicating irritant or allergen exposure, and absence of psoriatic nail changes should increase the suspicion of palmar tinea manuum.
FIGURE 1
Tinea pedis
The common “1 hand, 2 feet” syndrome of tinea pedis. This syndrome usually requires systemic therapy.
Tinea cruris
Tinea of the groin is most common in adult males and is promoted by a warm, moist environment. Tinea cruris begins in the crural fold and spreads onto the thigh. The interior portion of the lesion is usually erythematous or slightly brown in light-skinned individuals. The leading edge often advances in a sharply demarcated semicircle with a raised, slightly scaling border. The lesion is most often bilateral, sparing the skin of the scrotum. Pruritus is common and increases as sweat macerates the irritated skin.
Differential diagnosis. Candidiasis, intertrigo, and erythrasma can cause similar lesions. It is helpful to recall that candidiasis may involve the scrotum and penis while tinea cruris does so rarely. An additional helpful feature is the characteristic bright coral appearance of erythrasma when examined with a Wood’s lamp, absent in cases of tinea cruris.
Tinea corporis and tinea faciale
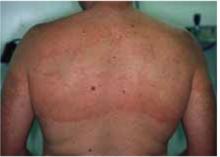
Tinea infections of the face and body begin as flat, scaly, and often pruritic macules that subsequently develop a raised border and begin to spread radially. As the ring expands, the central portion of the lesion often clears. This pattern leads to the formation of irregular circles that gives tinea corporis its common name, ringworm (Figure 2).
Tinea faciale is less common, but generally has a similar appearance with central clearing of lesions. Tinea faciale may not always exhibit the sharply demarcated border of tinea corporis.
Differential diagnosis. Eczema, impetigo, early pityriasis rosea, and localized psoriasis can mimic tinea corporis. History of exposure to persons or animals (typically house pets) with known ringworm should increase suspicion of tinea corporis. Current or past evidence of psoriasis or eczema should broaden the differential to include atypical presentations of these diseases.
FIGURE 2
Tinea corporis
Widespread tinea corporis. This patient would not be a candidate for topical treatment.
Diagnosing tinea: history and exam not enough
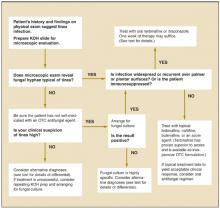
In some cases, findings on history and physical evaluation are so characteristic of tinea that they alone may allow the clinician to make a firm diagnosis. However, studies have shown that relying upon history and physical examination may result in a many missed diagnoses.6 Accordingly, consider tinea in all instances of papulosquamous skin disease and follow up appropriately. Figure 3 presents a simple algorithm for diagnosis and treatment of suspected tinea infection.
FIGURE 3
Evaluating possible tinea infection of the skin
Koh preparation
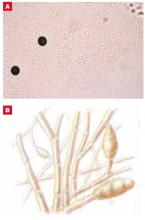
An important adjunct in diagnosing tinea is the office-based KOH wet mount preparation ( Figure 4 ), permitting direct visualization of fungal hyphae in keratinized material of the stratum corneum. Detailed protocols for preparation and interpretation of the KOH slide for diagnosis of dermatophytosis are well described in many other sources.10
FIGURE 4
KOH preparation
Potassium hydroxide dissolves epithelial cells to reveal hyphae (A), with their characteristic branching pattern (B). To increase the likelihood of detecting hyphae on microscopic exam of skin scrapings, begin with low light and low power. If fungal elements are detected, increase magnification to confirm the diagnosis. If fungal elements are not detected, yet clinical suspicion of tinea is high, consider arranging for a fungal culture.
Performing the test
The slide is prepared by combining skin scrapings from the leading edge of a lesion with a small amount of 20% potassium hydroxide. A cover slip is applied and the slide is gently heated prior to microscopy examination under high power. Table 2 summarizes the utility of clinical diagnosis and KOH preparation in diagnosis of tinea infection. History and physical examination should be combined with KOH preparation when making the diagnosis of epidermal dermatophyte infection (strength of recommendation [SOR]=B).6 (For an explanation of evidence ratings, see page 865.)
TABLE 2
Diagnostic value of clinical diagnosis and KOH prep in tinea infection
| Test | Quality | Sensitivity | Specificity | PV+ | PV– | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| a. Clinical diagnosis6 | 2b | 81% | 45% | 24 | 92% | 1.47 | 0.42 |
| b. KOH prep50 | 2b | 88% | 95% | 73% | 98% | 17.6 | 0.13 |
| c. KOH prep51 | 2b | 77% | 62% | 59% | 79% | 2.02 | 0.37 |
| a. The clinical diagnosis set was compiled by 27 general practitioners prior to submission of skin for fungal culture. Specimens were taken from consecutive patients with erythrosquamous lesions. Culture results were considered the gold standard. Study quality=2b. | |||||||
| b/c. Both studies of KOH preps were open analyses of patients with suspicious lesions. Paired fungal culture was initiated simultaneously with KOH prep and was considered the gold standard. Study quality=2b. For an explanation of levels of evidence, see page 865. | |||||||
Caveats
Though a rapid and inexpensive test, the KOH preparation has limitations. Some physicians have little experience interpreting KOH preparations, thus reducing the value of the test. Some patients with clinically significant tinea infections may be using over-the-counter topical antifungal medications, thus reducing the likelihood that fungal hyphae will be visualized at the office visit.
In cases where clinical suspicion of tinea infection is high, and the result of a KOH prep is negative, tissue should be submitted for fungal culture. Fungal culture is not typically available in the primary care office setting and usually requires submission of the sample to a reference or hospital laboratory.
Dermatophyte test medium
Dermatophyte test medium may be prepared and used in the office. It is mixed with a phenol indicator that turns red on exposure to alkaline metabolites of fungal growth. This medium is sensitive and usually exhibits color change within one week.11 Most primary care offices do not use dermatophyte test medium, however, because of its short shelf life and because the Clinical Laboratory Improvement Amendments classification requires that this test be performed by qualified laboratory personnel.
Treatment: topical and oral agents
Treatment of tinea infections may rely on antifungal medications singly or in combination. Antifungal agents are classified by their chemical structure—imidazoles, allylamines, benzylamines, and others—and act by different mechanisms to limit the availability of ergosterol, an essential component for normal function of fungal cell membranes.
Most tinea infections may be treated with topical agents alone. Oral therapy is required most often for:
- hyperkeratotic areas as on the palms or soles
- widespread or extensive infection
- immunocompromised patients
- persons intolerant of topical therapy
- failure of topical therapy
- chronic infection
Efforts to educate patients in proper hygiene and infection control may help. Specifically, remind patients that risk of tinea cruris and tinea pedis may be reduced if they wear nonocclusive footwear, use only clean and dry socks and undergarments, wear shower shoes, and apply absorbent powders. Eradication of fungal nail infections may help in controlling tinea pedis and tinea manuum. Caution in making contact with animals or people that have known tinea infections may reduce the incidence of tinea corporis.
Topical therapy
Topical antifungal agents are widely available in both prescription and over-the-counter forms. Preference of a specific agent may be difficult due to the limited number of head-to-head comparisons among these drugs. When recommending or prescribing an agent, consider efficacy, dosing regimen, cost, formulation, and availability. The most important topical antifungal agents are divided into 2 major classes—imidazoles and allylamines—although other agents are also used. Table 3 provides information on commonly used topical antifungal agents.
Imidazoles. Imidazoles are widely available as over-the-counter and prescription forms. Topical members of this class are clotrimazole, miconazole, econazole, ketoconazole, oxiconazole, and sulconazole. Miconazole and clotrimazole are available without a prescription. As a class, imidazoles are primarily fungistatic and are generally well-tolerated.12 Meta-analysis has failed to reveal significant differences in efficacy among members of this class.13 Imidazoles are efficacious with a pooled relative risk of failure to cure of 0.38 at 6 weeks after the initiation of therapy for tinea pedis (level of evidence [LOE]=1a).13
Current guidelines recommend twice-daily application for clotrimazole, miconazole, and econazole; ketoconazole, oxiconazole, and sulconazole may be applied once daily.14 When using imidazoles, usually prescribe a 2-week course for tinea cruris or tinea corporis, and a 4-week course for tinea pedis.15 In cases of inflammatory dermatophytosis, a combination agent containing clotrimazole and the corticosteroid betamethasone dipropionate may be used for a short initial period (LOE=4).16 Many experts recommend that combination steroid agents be used with caution, for no more than a few days, and with a plan for short-term follow-up.
Allylamines. A second, newer group of antifungal agents are the allylamines. Topical allylamines, including terbinafine and naftifine, are generally considered fungicidal. Terbinafine has recently been made available over-the-counter as 1% cream and 1% solution, while naftifine remains a prescription medication. Both agents are efficacious, with cure rates for dermatomycosis greater than 75%.17,18 Naftifine and terbinafine have exhibited long periods of activity in the skin and are therefore administered only once a day.19 Additionally, terbinafine has been shown effective in treating superficial mycoses in much shorter courses than typically required for imidazoles.20
Tinea infections are among the most common of all skin diseases. They are caused by dermatophytic fungi that digest keratin in the cells of the stratum corneum. Tinea infections are typically named according to the affected anatomic region: tinea corporis for the body, tinea pedis for the feet, tinea cruris for the groin, and so on.
The true prevalence of tinea is difficult to ascertain because many people self-treat or live with chronic infection. Approximately 8.6 million office visits occur each year for tinea infections. Family or general practitioners handle more than 35% of these visits. The estimated cost of office visits plus prescribed medications for cutaneous fungal infections for the 4-year period from 1990 to 1994 is just over $1 billion.1 Tinea infections occur in all age groups and in both genders; however, males have a higher incidence of tinea pedis and tinea cruris.
Dermatophytes affecting humans are from the genera Trichophyton, Microsporum, and Epidermophyton. Dermatophytes, which are ubiquitous in the environment, are categorized as geophilic, zoophilic, or anthropophilic according to their ecologic reservoir. Surveys of dermatophytes isolated from human patients in the United States from 1993 to 1995 indicate T tonsurans (44.9%), T rubrum (41.3%), T mentagrophytes (8.5%), and M canis (3.3%) are the most commonly encountered pathogens.2
The basic pathophysiology of tinea infection is inoculation of keratinized skin by dermatophytic fungi followed by release of keratinases and proteolytic enzymes. Symptoms follow as a result of host immune and epidermal response.3 Host factors, such as immunocompromised state and genetic susceptibility, play a role in infection. Warm temperature, moisture, and occlusion encourage dermatophyte growth.4
In a systematic review of available randomized controlled trials, allylamines were found to be more efficacious than imidazoles in treating tinea pedis (LOE=1a).13 Terbinafine has also been compared with imidazoles for treatment of tinea cruris and tinea corporis and found to be significantly more effective (LOE=1b).21
Previous pharmacoeconomic analyses of imidazole versus allylamine topical therapy for dermatophyte infection have presented conflicting conclusions, with some reporting that the greater cost of allylamine agents outweighs their slightly greater efficacy,22 while others concluded that the more efficacious medication initially is more cost effective.23 In the United States, terbinafine was approved for over-the-counter sale in 1999. Since then, the cost of terbinafine to consumers has declined significantly, to the point where it is now comparable to over-the-counter imidazoles (see Table 3 ). Allylamines have demonstrated some degree of intrinsic anti-inflammatory activity.24
Butenafine. Butenafine is a topical benzylamine antifungal structurally related to the allylamines. Butenafine is fungicidal and has efficacy similar to the allylamines. In a 1-week trial of therapy for tinea pedis with twice-daily butenafine, mycological cure rates in excess of 75% were observed (LOE=1b).25 In a trial of short-term therapy of tinea corporis, butenafine applied once-daily for 14 days led to high cure rates, symptom improvement, and increasing effectiveness 4 weeks after therapy was concluded (LOE=1b).26 In treatment of tinea cruris, butenafine was highly effective in achieving mycologic cure after 2 weeks of once-daily treatment (LOE=1b).27
Few trials directly compare butenafine and other topical agents, but in stand-alone or placebo-controlled trials, butenafine has demonstrated efficacy similar to the allylamines terbinafine and naftifine. Butenafine was approved for over-the-counter sale in the United States in 2000.
Ciclopirox. Ciclopirox is a substituted pyridone unrelated to the imidazole or allylamine agents. In double-blind, placebo-controlled trials, ciclopirox demonstrated significant efficacy in treating dermatophyte infections (LOE=1b).28 In head-to-head trials with the imidazole agent clotrimazole, ciclopirox demonstrated equivalent or slightly greater efficacy (LOE=1b).29,30
Ciclopirox applied twice daily is usually effective within 4 weeks of treatment. Ciclopirox is available only by prescription in the United States and is more expensive than most other topical antifungal agents. Ciclopirox has demonstrated intrinsic antiinflammatory activity equivalent to the allylamines (LOE=1b).31 Due to the availability of agents with comparable or superior efficacy at much lower costs, ciclopirox should not be considered a first- or second-line agent in treating epidermal tinea infections.
Tolnaftate. Tolnaftate is an over-the-counter antifungal agent that has been available in the US since 1965. Tolnaftate is inexpensive and has often been used in powder formulation as prophylaxis against tinea, especially tinea pedis.
In systematic reviews of available placebo-controlled trials, 1% tolnaftate was shown to be less effective against tinea pedis than the azoles and allylamines, with a number needed to treat of 3.6 for tinea pedis (LOE=1a).13 A comparative study has shown tolnaftate to be inferior to clotrimazole in the treatment of tinea pedis.32 With its demonstrated inferiority to the azoles and allylamines, tolnaftate should not be considered a first- or second-line treatment for tinea infections.
TABLE 3
Topical antifungal medications
| Agent | Formulation | Frequency* | Duration* | NNT † | Cost ‡ |
|---|---|---|---|---|---|
| IMIDAZOLES | |||||
| Clotrimazole | 1% cream | Twice daily | 2–4 weeks | 2.9 | $ 7.99 (15 g) |
| 1% solution | $ 6.99 (10 mL) | ||||
| 1% swabs | $ 6.99 (36 ea.) | ||||
| Econazole | 1% cream | Twice daily | 2–4 weeks | 2.6 | $16.85 (15 g) |
| Ketoconazole | 2% cream | Once daily | 2–4 weeks | No data available | $25.39 (15 g) |
| Miconazole | 2% cream | Twice daily | 2–4 weeks | 2.8 (at 8 weeks) | $ 6.99 (15 g) |
| 2% spray | $ 5.99 (3.5 oz) | ||||
| 2% powder | $ 5.99 (3.5 oz) | ||||
| Oxiconazole | 1% cream | Once to twice daily | 2–4 weeks | 2.9 | $20.27 (15 g) |
| 1% lotion | $34.19 (30 mL) | ||||
| Sulconazole | 1% cream | Once to twice daily | 2–4 weeks | 2.5 | $13.75 (15 g) |
| 1% solution | $26.57 (30 mL) | ||||
| ALLYLAMINES | |||||
| Naftifine | 1% cream | Once to twice daily | 1–4 weeks | 1.9 | $27.69 (15 g) |
| 1% gel | $51.39 (40 g) | ||||
| Terbinafine | 1% cream | Once to twice daily | 1–4 weeks | 1.6 (1.7 for tinea cruris/tinea corporis at 8 weeks)18 | $ 8.99 (12 g) |
| 1% solution | $ 9.99 (30 mL) | ||||
| BENZYLAMINE | |||||
| Butenafine | 1% cream | Once to twice daily | 1–4 weeks | 1.9 (1.4 for tinea corporis and 1.5 for tinea cruris)26,27 | $ 9.99 (12 g) |
| OTHER | |||||
| Ciclopirox | 0.77% cream | Twice daily | 2–4 weeks | 2.1 | $21.89 (15 g) |
| 0.77% lotion | $37.99 (30 mL) | ||||
| Tolnaftate | 1% powder | Twice daily | 4 weeks | 3.6 (at 8 weeks) | $ 3.99 (4 oz) |
| 1% spray | $ 5.49 (4 oz) | ||||
| 1% swabs | $ 6.99 (36 ea.) | ||||
| *Manufacturer guidelines. | |||||
| †NNT, number needed to treat. NNT is calculated from systematic review of all randomized controlled trials for tinea pedis at 6 weeks after the initiation of treatment33 except where otherwise noted. (See “Number needed to treat,” page 866.) | |||||
| ‡Lowest cost available (including generic agents) based upon internet listings of national on-line pharmacies: www.drugstore.com, www.eckerd.com, and www.walgreens.com as of May 2003. | |||||
Oral antifungal agents
Oral antifungal agents are used for dermatophyte infections that are widespread, chronic, or markedly inflammatory, or that affect hyperkeratotic areas as in palmar or plantar tinea. They are also use for those with immuno-suppression,16 and for those in whom treatment with topical drugs has been unsatisfactory. Agents include griseofulvin, the azoles (ketoconazole, itraconazole, and fluconazole), and the allylamine terbinafine. Table 4 summarizes pertinent comparative data regarding these agents.
Griseofulvin. Griseofulvin is the oldest of the systemic antifungal agents used for tinea infections, available for more than 40 years. Griseofulvin acts on susceptible fungal cells by inhibiting microtubule function. Griseofulvin is taken by adults once daily at 500 mg, for 4 to 6 weeks, and has demonstrated efficacy in the treatment of tinea infections.33
In a systematic review of comparative trials, oral griseofulvin was found to be significantly inferior to oral terbinafine in the treatment of tinea pedis (LOE=1a).33 Likewise, terbinafine was found to be superior to griseofulvin in the treatment of tinea corporis and tinea cruris (LOE=1b).34 In comparisons with ketoconazole, griseofulvin was equivalent in the treatment of dermatophytosis (LOE=2a).35
Griseofulvin has also been compared with itraconazole in various treatment schedules for tinea corporis, tinea cruris, tinea pedis, and tinea manus; it was found to be inferior in all treatment durations to a maximum of 3 months (LOE=1b).36 In head-to-head comparisons between griseofulvin and fluconazole in the treatment of tinea corporis and tinea cruris, outcomes were not statistically significantly different, although a trend toward better clinical cures in the fluconazole arm was identified (LOE=1b).37
Azoles. Studies have made limited comparisons among the azoles (ketoconazole, itraconazole, and fluconazole) in the treatment of dermatophytosis. Small comparisons with limited power between itraconazole and fluconazole38 and between ketoconazole and fluconazole39 have demonstrated similar cure rates of about 90% for all 3 agents (LOE=1b). In a placebo-controlled comparison for treatment of tinea cruris and tinea corporis, 100 mg/d of itraconazole for 14 days was highly curative (LOE=1b).40 In placebo-controlled trials of the treatment of tinea pedis, itraconazole was found to provide statistically significant cure rates in as little as 1 week (LOE=1b).41
Systematic review of trials between itraconazole (100 mg/d for 4 weeks) and terbinafine (250 mg/d for 2 weeks) for tinea pedis showed a non-statistically significant trend toward higher cure rate in those treated with terbinafine (LOE=1a).33
There are limited placebo-controlled trials evaluating fluconazole in the treatment of superficial fungal infections of the skin. In an open, noncomparative trial employing once-weekly dosing of fluconazole 150 mg for 1 to 4 weeks for tinea corporis and tinea cruris, clinical cure rate was 92% with long-term clinical cure rate of 88% (LOE=2b).42 Another study randomized 240 adults with skin dermatophytosis or cutaneous candidiasis to either flucona-zole 150 mg/wk or fluconazole 50 mg/d for a maximum of 4 weeks (non-tinea pedis) to 6 weeks (tinea pedis) with positive clinical response of greater than 90% in both arms of treatment (LOE=2b).43 Fluconazole has not been evaluated in direct comparison with terbinafine in the treatment of dermatophytic skin infections. Comparisons between azoles and griseofulvin have been discussed above.
Terbinafine. Oral terbinafine is effective in the treatment of skin dermatophytes. In a double-blind, placebo-controlled study of terbinafine 125 mg taken twice daily for 6 weeks, 65% of patients had mycologic cure at 2 weeks post-treatment (LOE=1b).44 In an open, noncon-trolled study of terbinafine 125 mg daily for one week, 100% mycologic cure was achieved in the treatment of tinea corporis and tinea cruris (LOE=2b).45
As noted above, terbinafine has shown efficacy superior to griseofulvin and itraconazole. No well-designed, comparative study was identified that showed any oral antifungal agent to be superior to terbinafine in the treatment of dermatophytic skin infections.
TABLE 4
Oral antifungal medications in the treatment of dermatomycosis
| Comparison (with treatment costs)* | SOR | Outcomes | NNT or reduction in risk of failure to cure | Comments |
|---|---|---|---|---|
| ITRACONAZOLE ($112–$223.99) vs placebo | A 40, 41 | Itraconazole with much greater efficacy than placebo | NNT = 1.7 at 8 weeks after 1 week of treatment for tinea pedis; 1.8 at 4 weeks after 2 weeks of treatment for tinea corporis and tinea cruris | In multiple, well-designed RCT for tinea pedis, tinea corporis and tinea cruris |
| TERBINAFINE ($112.99–$329.9) vs placebo | A 33, 44, 45 | Terbinafine with much greater efficacy than with placebo | NNT = 1.5 at 8 weeks after 2 weeks of treatment for tinea pedis | Systematic review of RCT for tinea pedis and multiple, non-comparative trials for tinea corporis and tinea cruris |
| ITRACONAZOLE ($112–$223.99) vs Griseofulvin ($50.99–$71.40) | A 36 | Itraconazole with greater efficacy than Griseofulvin | Risk difference = 19% in treatment of tinea cruris/corporis and 37% in treatment of tinea pedis/manus | Systematic review of RCT for tinea corporis, tinea cruris, tinea manuus, and tinea pedis |
| TERBINAFINE ($112.99–$223.99)vs Griseofulvin ($50.99–$71.40) | A 33 | Terbinafine with greater efficacy than Griseofulvin | Risk difference = 50% at 8 weeks post-treatment | Systematic review of RCT for tinea pedis |
| KETOCONAZOLE ($30.99–$61.99) vs Griseofulvin ($50.99–71.40) | A 33 | Approximately equal efficacy between Ketoconazole and Griseofulvin | Risk difference = No consistent difference favoring either agent | Systematic review of RCT for tinea pedis |
| FLUCONAZOLE ($13.99–$50.96) vs Griseofulvin ($50.99–$71.40) | A 37 | Non-significant trend toward greater efficacy with Fluconazole | Risk difference = 12% | RCT for tinea corporis and tinea cruris |
| FLUCONAZOLE ($13.99–$50.96) vs Ketoconazole ($30.99–$61.99) | B 39 | Approximately equal efficacy between Fluconazole and Ketoconazole | Risk difference = 4% favoring fluconazole at 7 weeks post-treatment | Small RCT for all dermatomycosis |
| ITRACONAZOLE ($112–$223.99) vs Fluconazole ($13.99–$50.96) | B 38 | Approximately equal efficacy between Fluconazole and Itraconazole | Risk difference = 5% favoring itraconazole at 10 weeks post-treatment | Small RCT for tinea manuum and tinea pedis |
| TERBINAFINE ($112.99–223.99) vs Itraconazole ($112–$223.99) | A 33 | Approximately equal efficacy between Terbinafine and Itraconazole | Risk difference = No consistent difference favoring either agent | Systematic review of RCT for tinea pedis |
| SOR, strength of recommendation; NNT, number needed to treat; RCT, randomized controlled trial | ||||
| SOR: A = Multiple RCT or a systematic review of RCT; B = Trials of moderate strength, as in open trials, noncomparative trials, or RCT with small size or poor follow-up. (See page 865 for full explanation of SOR ratings.) | ||||
| * Lowest cost available (including for generic agents) based upon internet listings of national on-line pharmacies, Drugstore.com, Eckerd.com, and Walgreens.com as of May 2003. | ||||
Summary of treatment recommendations
Based upon review of the available literature, it is recommended that clinicians consider the affordable and highly effective topical agents terbinafine and butenafine as first-line therapy for patients with uncomplicated epidermal tinea infections. While also highly effective, naftifine should be considered a second-line treatment due to its prescription-only availability and increased cost. The topical azoles clotrimazole and miconazole are marginally less effective than the allylamines, but are widely available and inexpensive. The prescription drug ciclopirox is slightly superior to the azoles, but its expense and availability make it a less desirable choice for most patients. Tolnaftate is cheap and widely available, but its relative inferiority to other topical agents limits its use in treatment of tinea infections. Tolnaftate may have some utility as a prophylactic against epidermal tinea infections in susceptible individuals.46
For patients with complicated epidermal tinea infections as described above, or for those with confirmed infection and treatment failure using topical agents, oral antifungal medications should be used. Terbinafine and itraconazole are both highly effective and safe in treating tinea infections. Despite their relatively high cost, either of these agents may be considered first-line therapies. Fluconazole has been evaluated in limited trials, but data at this time are insufficient to recommend that it be considered a first-line agent in treatment of epidermal tinea infection.
Griseofulvin may be considered a second-line agent as it has been shown to be clearly less efficacious than either terbinafine or itraconazole. Oral ketoconazole has a limited role in the treatment of tinea infections. It has demonstrated efficacy equivalent to or slightly superior to griseofulvin, but its association with potentially severe liver injury makes it a very problematic choice.
Adverse reactions
As with any systemic medication, adverse effects may occur with oral antifungal agents. Oral antifungal agents are generally considered safe in the treatment of skin dermatophytoses. In systemic review of trials of oral antifungal agents in the treatment of tinea pedis, the most commonly identified adverse events were gastrointestinal complaints such as diarrhea or nausea, headaches, and skin complaints.33
Of particular note, ketoconazole has been repeatedly related to potentially serious hepatotoxicity, especially when used in longer duration of treatment.47 One large cohort study found the incident rate of acute liver injury to be 134.1 per 100,000 person-months among ketoconazole users, compared with 10.4 and 2.5 for itraconazole and terbinafine, respectively.48 Accordingly, baseline and monthly laboratory studies are advised when using ketoconazole.49
Serious complications associated with the use of topical antifungal agents are rare. As with any topical medication, local irritation and dermatitis may occur.
Correspondence
Brian Thomas, MD, NOLF-IB, Bldg 184, Box 357140, San Diego, Ca 92135. E-mail: [email protected].
1. Smith ES, Fleischer AB, Friedman SR, Willford PM. Characteristics of office-based physician visits for cutaneous fungal infection: an analysis of 1990 to 1994 National Ambulatory Medical Care Survey Data. Cutis 2002;69:191-202.
2. Weitzman I, Chin NX, Kunjukunju N, Della-Latta P. A survey of dermatophytes isolated from human patients in the United States from 1993 to 1995. J Am Acad Dermatol 1998;39:255-261.
3. Roscoe J, Farmer ER. Diseases caused by fungi. In: Farmer ER, Hood AF, eds. Pathology of the Skin. 2nd ed. New York: McGraw-Hill; 2000;611-633.
4. Odom RB, James WD, Berger TG. Diseases resulting from fungi and yeasts. >In: Diseases of the Skin: Clinical Dermatology. 9th ed. Philadelphia: WB Saunders; 2000;358-415.
5. Habif TP. Superficial fungal infections. In: Clinical Dermatology. 3rd ed. St. Louis, Mo: Mosby; 1996;362-408.
6. Lousbergh D, Buntinx F, Pierard G. Diagnosing dermatomycosis in general practice. Fam Pract 1999;16:611-615.
7. Kearse HJ, Miller OF. Tinea pedis in prepubertal children: does it occur? J Am Acad Dermatol 1988;19:619-22.
8. Kates SG, Nordstrom KM, McGinley KJ, Leyden JJ. Microbial ecology of interdigital infections of toe web spaces. J Am Acad Dermatol 1990;22:578-582.
9. Lookingbill DP, Marks JG. Scaling papules, plaques, and patches. In: Principles of Dermatology. 2nd ed. Philadelphia: WB Saunders; 1993;136-159.
10. Rosen T. Dermatophytosis: diagnostic pointers and therapeutic pitfalls. Consultant 1997;37:1545-1557.
11. Sinski JT, Swanson JR, Kelley LM. Dermatophyte test medium: clinical and quantitative appraisal. J Inves Dermatol 1972;58:405-411.
12. Saag MS, Dismukes WE. Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother 1988;32:1-8.
13. Crawford F, Hart R, Bell-Syer S, Torgerson D, Young P, Russell I. Topical treatments for fungal infections of the skin and nails of the foot (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
14. Polak A. Antifungal activity of four antifungal drugs in the cutaneous retention time test. Sabouraudia 1984;22:501-503.
15. Gupta AK, Einarson TR, Summerbell RC, Shear NH. An overview of topical antifungal therapy in dermatomycosis.A North American perspective. Drugs 1998;55:645-674.
16. Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial mycotic infections of the skin: tinea corporis, tinea cruris, tinea faciei, tinea manuum, and tinea pedis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol 1996;34:282-286.
17. Jordon RE, Rapini RP, Rex IH, Jr, et al. Once-daily naftifine cream 1% in the treatment of tinea cruris and tinea corporis. Int J Dermatol 1990;29:441-442.
18. Budimulja U, Bramono K, Urip S, et al. Once daily treatment with terbinafine 1% cream (Lamisil) for one week is effective in the treatment of tinea corporis and tinea cruris. A placebo-controlled study. Mycoses 2001;44:300-306.
19. Schuster I, Schaude M, Schatz F, et al. Preclinical characteristics of allylamines. In: Berg D, Plempel M, eds. Sterol Biosynthesis Inhibitors. Chichester, England: Ellis Horwood; 1988;449-470.
20. Evans EG, Seaman RA, James IG. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br J Dermatol 1994;130:83-87
21. Bonifaz A, Saul A. Comparative study between terbinafine 1% emulsion-gel versus ketoconazole 2% cream in tinea cruris and tinea corporis. Eur J Dermatol 2000;10:107-109.
22. Chren MM, Landefeld CS. A cost analysis of topical drug regimens for dermatophyte infections. JAMA 1994;272:1922-1925.
23. Davis R, Balfour JA. Terbinafine. A pharmacoeconomic evaluation of its use in superficial fungal infections. Pharmacoeconomics. 1995;8:253-269.
24. Rosen T, Schell BJ, Orengo I. Anti-inflammatory activity of antifungal preparations. Int J Dermatol 1997;36:788-792.
25. Savin R, De Villez RL, Elewski B, et al. One-week therapy with twice-daily Butenafine 1% cream versus vehicle in the treatment of tinea pedis: a multicenter, double-blind trial. J Amer Acad Dermatol 1997;36:S15-S19.
26. Greer DL, Weiss J, Rodriguez DA, Hebert AA, Swinehart JM. A randomized trial to assess once-daily topical treatment of tinea corporis with butenafine, a new antifungal agent. J Amer Acad Dermatol 1997;37:231-235.
27. Lesher JL, Babel DE, Stewart DM, et al. Butenafine 1% cream in the treatment of tinea cruris: a multicenter, vehi-cle-controlled, double-blind trial. J Amer Acad Dermatol 1997;36:S20-S24.
28. Sehgal VN. Ciclopirox: a new topical pyrodonium antimy-cotic agent. A double-blind study in superficial dermato-mycoses. Br J Dermatol 1976;95:83-88.
29. Bogaert H, Cordero C, Ollague W, Savin RC, Shalita AR, Zaias N. Multicentre double-blind clinical trials of ciclopirox olamine cream 1% in the treatment of tinea corporis and tinea cruris. J Int Med Res 1986;14:210-216.
30. Beyer M. Selected double-blind comparative studies on the efficacy and tolerance of ciclopiroxolamine solution and cream [in German]. Arzneimittelforschung 1981;31:1378-1381.
31. Rosen T, Schell BJ, Orengo I. Anti-inflammatory activity of antifungal preparations. Int J Dermatol 1997;36:788-792.
32. Woscoff A, Carageli S. Treatment of tinea pedis with sulconazole nitrate 1% cream or miconazole nitrate 2% cream. Curr Ther Res 1986;39:753-757.
33. Bell-Syer SEM, Hart R, Crawford F, Torgerson DJ, Tyrell W, Russell I. Oral treatments for fungal infections of the skin of the foot (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
34. Voravutinon V. Oral treatment of tinea corporis and tinea cruris with terbinafine and griseofulvin: a randomized double-blind comparative study. J Med Assoc Thai. 1993;76:388-393.
35. Hay RJ, Clayton YM, Griffiths WA, Dowd PM. A comparative double-blind study of ketoconazole and griseofulvin in dermatophytosis. Br J Dermatol 1985;112:691-696.
36. Lachapelle JM, De Doncker P, Tennstedt D, Cauwenbergh G, Janssen PA. Itraconazole compared with griseo-fulvin in the treatment of tinea corporis/cruris and tinea pedis/mannus: An interpretation of the clinical results of all completed double-blind studies with respect to the pharmacokinetic profile. Dermatology 1992;184:45-50.
37. Faergemann J, Mork NJ, Haglund A, Odegard T. A multi-centre (double-blind) comparative study to assess the safety and efficacy of fluconazole and griseofulvin in the treatment of tinea corporis and tinea cruris. Br J Dermatol 1997;136:575-577.
38. Difonzo EM, Papini M, Cilli P, et al. A double blind study comparison of itraconazole and fluconazole in tinea pedis and tinea manuum. J Eur Acad Dermatol Venereol 1995;4:148-152.
39. Fischbein A, Haneke E, Lacner K. Comparative evaluation of oral fluconazole and oral ketoconazole in the treatment of fungal infections of the skin. Intl J Dermatol 1992;31:12-16.
40. Pariser DM, Pariser RJ, Ruoff G, Ray TL. Double-blind comparison of itraconazole and placebo in the treatment of tinea corporis and tinea cruris. J Amer Acad Dermatol 1994;31:232-234.
41. Svejgaard E, Avnstorp C, Wanscher B, Nilsson J, Heremans A. Efficacy and safety of short-term itracona-zole in tinea pedis: a double-blind, randomized, placebo-controlled trial. Dermatology 1998;197:368-372.
42. Suchil P, Gei FM, Robles M, Perera-Ramirez A, Welsh O, Male O. Once-weekly oral doses of fluconazole 150 mg in the treatment of tinea corporis/cruris and cutaneous candidiasis. Clin Exp Dermatol 1992;17:397-401.
43. Nozickova M, Koudelkova V, Kulikova Z, Malina L, Urbanowski S, Silny W. A comparison of the efficacy of oral fluconazole, 150 mg/week versus 50 mg/day, in the treatment of tinea corporis, tinea cruris, tinea pedis, and cutaneous candidiasis. Intl J Dermatol 1998;37:703-705.
44. Savin RC, Zaias N. Treatment of chronic moccasin-type tinea pedis with terbinafine: a double-blind, placebo-controlled trial. J Am Acad Dermatol 1990;23:804-807.
45. Farag A, Taha M, Halim S. One-week therapy with oral terbinafine in cases of tinea cruris/corporis. Br J Dermatol 1994;131:684-686.
46. Smith EB, Dickson JE, Knox JM. Tolnaftate powder in prophylaxis of tinea pedis. South Med J 1974;67:776-778.
47. Lewis JH, Zimmerman HJ, Benson GD, Ishak KG. Hepatic injury associated with ketoconazole therapy: analysis of 33 cases. Gastroenterology 1984;86:503-513.
48. Garcia Rodriguez LA, Duque A, Castellsague J, Perea-Gutthann S, Stricker BH. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol 1999;48:847-852.
49. Lake-Bakaar G, Scheuer PJ, Sherlock S. Hepatic reactions associated with ketoconazole in the United Kingdom. Br Med J (Clin Res Ed) 1987;294:419-422.
50. Haldane DJ, Robart E. A comparison of calcofluor white, potassium hydroxide, and culture for the laboratory diagnosis of superficial fungal infection. Diagn Microbiol Infect Dis 1990;13:337-339.
51. Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol 1993;129:510-511.
- Potassium hydroxide preparation should be used as an aid to diagnosis for all erythrosquamous lesions (B).
- Fungal culture should be used in cases in which history, physical examination, and potassium hydroxide preparation fail to clearly exclude a diagnosis of tinea (B).
- Short-duration topical therapy with terbinafine, naftifine, and butenafine is efficacious for most epidermal tinea infections (A).
- Oral antifungal agents are important in the treatment of tinea infections thatare widespread, fail to respond to topical treatment, involve the thick stratum corneum of the soles and palms, or occur in immunosuppressed patients.
- Short courses of oral itraconazole and terbinafine are safe and effective in treating tinea infections (A).
Though findings on history and physical examination are sometimes sufficient to make a diagnosis of tinea infection, a potassium hydroxide (KOH) study usually is required for confirmation. Even the KOH study can be misleading, however, if a patient recently self-administered a topical antifungal agent. This article describes the varying appearance of tinea infections according to their anatomic location, and outlines a careful work-up.
Highly effective and affordable over-the-counter medications have proliferated, and short-course therapy is available. Based on systematic reviews of randomized, controlled studies, it is possible to recommend specific first-line therapies for tinea infections.
Manifestations of tinea infection
Clinical manifestations of tinea vary with anatomic location, duration of infection, and pathogen. In general, zoophilic dermatophytes evoke a more vigorous host response than the anthropophilic species.5 Shared features of many dermatophyte infections include erythema, scaling, pruritus, ring formation, and central clearing of lesions. Table 1 reviews published data on the diagnostic value of selected clinical signs in suspected tinea infection.6
TABLE 1
Diagnostic value of selected signs and symptoms in tinea infection
| Sign/symptom | Sensitivity | Specificity | PV+ | PV– | LR+ | LR– |
|---|---|---|---|---|---|---|
| Scaling | 77% | 20% | 17% | 80% | 0.96 | 1.15 |
| Erythema | 69% | 31% | 18% | 83% | 1.00 | 1.00 |
| Pruritus | 54% | 40% | 16% | 80% | 0.90 | 1.15 |
| Central clearing | 42% | 65% | 20% | 84% | 1.20 | 0.89 |
| Concentric rings | 27% | 80% | 23% | 84% | 1.35 | 0.91 |
| Maceration | 27% | 84% | 26% | 84% | 1.69 | 0.87 |
| Note: Signs and symptoms were compiled by 27 general practitioners prior to submission of skin for fungal culture. Specimens were taken from 148 consecutive patients with erythematosquamous lesions of glabrous skin. Culture results were considered the gold standard. | ||||||
| PV+, positive predictive value; PV–, negative predictive value; LR+, positive likelihood ratio; LR–, negative likelihood ratio. | ||||||
| Adapted from Lousbergh et al, Fam Pract 1999; 16:611–615.6 | ||||||
| Level of evidence=2b. For an explanation of levels of evidence, see page 865. | ||||||
Tinea pedis
Tinea of the foot may manifest as interdigital, plantar, or acute vesicular disease. Toe webs and soles of the feet are the sites most commonly affected. Tinea pedis occurs most commonly in postpubertal adolescents and adults, but may be seen in children.7
With interdigital infection, toe webs may become scaly, pruritic, and fissured. Interaction of bacteria and infecting dermatophytes may lead to a white, soggy maceration.8 Extension from the web space to the dorsal or plantar surface commonly occurs.
In chronic plantar or moccasin-type tinea pedis, the entire surface of the sole may be covered with fine, white scale and may assume a hyperkeratotic appearance. Chronic web infection may lead to acute inflammation characterized by vesicles, pustules, and bullae over the sole or the dorsum of the foot.5
Differential diagnosis. The differential diagnosis includes dry skin, pitted keratolysis, erythrasma, and contact dermatitis. Some conditions may appear very similar to tinea pedis, increasing the importance of KOH prep and fungal culture.
Tinea pedis may be distinguished from erythrasma by lack of bright coral appearance when examined with a Wood’s lamp. Tinea pedis infections also lack the well-demarcated erosions, or pits, of pitted keratolysis. Evidence of concurrent onychomycosis should increase the suspicion that tinea pedis is the correct diagnosis.
Tinea manuum
Tinea of the hand is usually analogous to moccasin-type tinea pedis. The palm appears hyperkeratotic and has very fine white scale that emphasizes the normal lines of the hand. Tinea of the dorsal surface of the hand usually occurs in the classic ringworm pattern. Tinea manuum is often seen in association with tinea pedis and onychomycosis. Many clinicians are familiar with the “one hand, two feet” syndrome, in which the palmar surfaces of both feet and one hand are infected (Figure 1).9 Onychomycosis often occurs in association with this presentation of tinea.
Differential diagnosis. The pattern of tinea manuum may be confused with those of eczema, contact dermatitis, palmar psoriasis, or even normal, rough hands. Unilateral involvement, presence of fingernail onychomycosis, lack of history indicating irritant or allergen exposure, and absence of psoriatic nail changes should increase the suspicion of palmar tinea manuum.
FIGURE 1
Tinea pedis
The common “1 hand, 2 feet” syndrome of tinea pedis. This syndrome usually requires systemic therapy.
Tinea cruris
Tinea of the groin is most common in adult males and is promoted by a warm, moist environment. Tinea cruris begins in the crural fold and spreads onto the thigh. The interior portion of the lesion is usually erythematous or slightly brown in light-skinned individuals. The leading edge often advances in a sharply demarcated semicircle with a raised, slightly scaling border. The lesion is most often bilateral, sparing the skin of the scrotum. Pruritus is common and increases as sweat macerates the irritated skin.
Differential diagnosis. Candidiasis, intertrigo, and erythrasma can cause similar lesions. It is helpful to recall that candidiasis may involve the scrotum and penis while tinea cruris does so rarely. An additional helpful feature is the characteristic bright coral appearance of erythrasma when examined with a Wood’s lamp, absent in cases of tinea cruris.
Tinea corporis and tinea faciale
Tinea infections of the face and body begin as flat, scaly, and often pruritic macules that subsequently develop a raised border and begin to spread radially. As the ring expands, the central portion of the lesion often clears. This pattern leads to the formation of irregular circles that gives tinea corporis its common name, ringworm (Figure 2).
Tinea faciale is less common, but generally has a similar appearance with central clearing of lesions. Tinea faciale may not always exhibit the sharply demarcated border of tinea corporis.
Differential diagnosis. Eczema, impetigo, early pityriasis rosea, and localized psoriasis can mimic tinea corporis. History of exposure to persons or animals (typically house pets) with known ringworm should increase suspicion of tinea corporis. Current or past evidence of psoriasis or eczema should broaden the differential to include atypical presentations of these diseases.
FIGURE 2
Tinea corporis
Widespread tinea corporis. This patient would not be a candidate for topical treatment.
Diagnosing tinea: history and exam not enough
In some cases, findings on history and physical evaluation are so characteristic of tinea that they alone may allow the clinician to make a firm diagnosis. However, studies have shown that relying upon history and physical examination may result in a many missed diagnoses.6 Accordingly, consider tinea in all instances of papulosquamous skin disease and follow up appropriately. Figure 3 presents a simple algorithm for diagnosis and treatment of suspected tinea infection.
FIGURE 3
Evaluating possible tinea infection of the skin
Koh preparation
An important adjunct in diagnosing tinea is the office-based KOH wet mount preparation ( Figure 4 ), permitting direct visualization of fungal hyphae in keratinized material of the stratum corneum. Detailed protocols for preparation and interpretation of the KOH slide for diagnosis of dermatophytosis are well described in many other sources.10
FIGURE 4
KOH preparation
Potassium hydroxide dissolves epithelial cells to reveal hyphae (A), with their characteristic branching pattern (B). To increase the likelihood of detecting hyphae on microscopic exam of skin scrapings, begin with low light and low power. If fungal elements are detected, increase magnification to confirm the diagnosis. If fungal elements are not detected, yet clinical suspicion of tinea is high, consider arranging for a fungal culture.
Performing the test
The slide is prepared by combining skin scrapings from the leading edge of a lesion with a small amount of 20% potassium hydroxide. A cover slip is applied and the slide is gently heated prior to microscopy examination under high power. Table 2 summarizes the utility of clinical diagnosis and KOH preparation in diagnosis of tinea infection. History and physical examination should be combined with KOH preparation when making the diagnosis of epidermal dermatophyte infection (strength of recommendation [SOR]=B).6 (For an explanation of evidence ratings, see page 865.)
TABLE 2
Diagnostic value of clinical diagnosis and KOH prep in tinea infection
| Test | Quality | Sensitivity | Specificity | PV+ | PV– | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| a. Clinical diagnosis6 | 2b | 81% | 45% | 24 | 92% | 1.47 | 0.42 |
| b. KOH prep50 | 2b | 88% | 95% | 73% | 98% | 17.6 | 0.13 |
| c. KOH prep51 | 2b | 77% | 62% | 59% | 79% | 2.02 | 0.37 |
| a. The clinical diagnosis set was compiled by 27 general practitioners prior to submission of skin for fungal culture. Specimens were taken from consecutive patients with erythrosquamous lesions. Culture results were considered the gold standard. Study quality=2b. | |||||||
| b/c. Both studies of KOH preps were open analyses of patients with suspicious lesions. Paired fungal culture was initiated simultaneously with KOH prep and was considered the gold standard. Study quality=2b. For an explanation of levels of evidence, see page 865. | |||||||
Caveats
Though a rapid and inexpensive test, the KOH preparation has limitations. Some physicians have little experience interpreting KOH preparations, thus reducing the value of the test. Some patients with clinically significant tinea infections may be using over-the-counter topical antifungal medications, thus reducing the likelihood that fungal hyphae will be visualized at the office visit.
In cases where clinical suspicion of tinea infection is high, and the result of a KOH prep is negative, tissue should be submitted for fungal culture. Fungal culture is not typically available in the primary care office setting and usually requires submission of the sample to a reference or hospital laboratory.
Dermatophyte test medium
Dermatophyte test medium may be prepared and used in the office. It is mixed with a phenol indicator that turns red on exposure to alkaline metabolites of fungal growth. This medium is sensitive and usually exhibits color change within one week.11 Most primary care offices do not use dermatophyte test medium, however, because of its short shelf life and because the Clinical Laboratory Improvement Amendments classification requires that this test be performed by qualified laboratory personnel.
Treatment: topical and oral agents
Treatment of tinea infections may rely on antifungal medications singly or in combination. Antifungal agents are classified by their chemical structure—imidazoles, allylamines, benzylamines, and others—and act by different mechanisms to limit the availability of ergosterol, an essential component for normal function of fungal cell membranes.
Most tinea infections may be treated with topical agents alone. Oral therapy is required most often for:
- hyperkeratotic areas as on the palms or soles
- widespread or extensive infection
- immunocompromised patients
- persons intolerant of topical therapy
- failure of topical therapy
- chronic infection
Efforts to educate patients in proper hygiene and infection control may help. Specifically, remind patients that risk of tinea cruris and tinea pedis may be reduced if they wear nonocclusive footwear, use only clean and dry socks and undergarments, wear shower shoes, and apply absorbent powders. Eradication of fungal nail infections may help in controlling tinea pedis and tinea manuum. Caution in making contact with animals or people that have known tinea infections may reduce the incidence of tinea corporis.
Topical therapy
Topical antifungal agents are widely available in both prescription and over-the-counter forms. Preference of a specific agent may be difficult due to the limited number of head-to-head comparisons among these drugs. When recommending or prescribing an agent, consider efficacy, dosing regimen, cost, formulation, and availability. The most important topical antifungal agents are divided into 2 major classes—imidazoles and allylamines—although other agents are also used. Table 3 provides information on commonly used topical antifungal agents.
Imidazoles. Imidazoles are widely available as over-the-counter and prescription forms. Topical members of this class are clotrimazole, miconazole, econazole, ketoconazole, oxiconazole, and sulconazole. Miconazole and clotrimazole are available without a prescription. As a class, imidazoles are primarily fungistatic and are generally well-tolerated.12 Meta-analysis has failed to reveal significant differences in efficacy among members of this class.13 Imidazoles are efficacious with a pooled relative risk of failure to cure of 0.38 at 6 weeks after the initiation of therapy for tinea pedis (level of evidence [LOE]=1a).13
Current guidelines recommend twice-daily application for clotrimazole, miconazole, and econazole; ketoconazole, oxiconazole, and sulconazole may be applied once daily.14 When using imidazoles, usually prescribe a 2-week course for tinea cruris or tinea corporis, and a 4-week course for tinea pedis.15 In cases of inflammatory dermatophytosis, a combination agent containing clotrimazole and the corticosteroid betamethasone dipropionate may be used for a short initial period (LOE=4).16 Many experts recommend that combination steroid agents be used with caution, for no more than a few days, and with a plan for short-term follow-up.
Allylamines. A second, newer group of antifungal agents are the allylamines. Topical allylamines, including terbinafine and naftifine, are generally considered fungicidal. Terbinafine has recently been made available over-the-counter as 1% cream and 1% solution, while naftifine remains a prescription medication. Both agents are efficacious, with cure rates for dermatomycosis greater than 75%.17,18 Naftifine and terbinafine have exhibited long periods of activity in the skin and are therefore administered only once a day.19 Additionally, terbinafine has been shown effective in treating superficial mycoses in much shorter courses than typically required for imidazoles.20
Tinea infections are among the most common of all skin diseases. They are caused by dermatophytic fungi that digest keratin in the cells of the stratum corneum. Tinea infections are typically named according to the affected anatomic region: tinea corporis for the body, tinea pedis for the feet, tinea cruris for the groin, and so on.
The true prevalence of tinea is difficult to ascertain because many people self-treat or live with chronic infection. Approximately 8.6 million office visits occur each year for tinea infections. Family or general practitioners handle more than 35% of these visits. The estimated cost of office visits plus prescribed medications for cutaneous fungal infections for the 4-year period from 1990 to 1994 is just over $1 billion.1 Tinea infections occur in all age groups and in both genders; however, males have a higher incidence of tinea pedis and tinea cruris.
Dermatophytes affecting humans are from the genera Trichophyton, Microsporum, and Epidermophyton. Dermatophytes, which are ubiquitous in the environment, are categorized as geophilic, zoophilic, or anthropophilic according to their ecologic reservoir. Surveys of dermatophytes isolated from human patients in the United States from 1993 to 1995 indicate T tonsurans (44.9%), T rubrum (41.3%), T mentagrophytes (8.5%), and M canis (3.3%) are the most commonly encountered pathogens.2
The basic pathophysiology of tinea infection is inoculation of keratinized skin by dermatophytic fungi followed by release of keratinases and proteolytic enzymes. Symptoms follow as a result of host immune and epidermal response.3 Host factors, such as immunocompromised state and genetic susceptibility, play a role in infection. Warm temperature, moisture, and occlusion encourage dermatophyte growth.4
In a systematic review of available randomized controlled trials, allylamines were found to be more efficacious than imidazoles in treating tinea pedis (LOE=1a).13 Terbinafine has also been compared with imidazoles for treatment of tinea cruris and tinea corporis and found to be significantly more effective (LOE=1b).21
Previous pharmacoeconomic analyses of imidazole versus allylamine topical therapy for dermatophyte infection have presented conflicting conclusions, with some reporting that the greater cost of allylamine agents outweighs their slightly greater efficacy,22 while others concluded that the more efficacious medication initially is more cost effective.23 In the United States, terbinafine was approved for over-the-counter sale in 1999. Since then, the cost of terbinafine to consumers has declined significantly, to the point where it is now comparable to over-the-counter imidazoles (see Table 3 ). Allylamines have demonstrated some degree of intrinsic anti-inflammatory activity.24
Butenafine. Butenafine is a topical benzylamine antifungal structurally related to the allylamines. Butenafine is fungicidal and has efficacy similar to the allylamines. In a 1-week trial of therapy for tinea pedis with twice-daily butenafine, mycological cure rates in excess of 75% were observed (LOE=1b).25 In a trial of short-term therapy of tinea corporis, butenafine applied once-daily for 14 days led to high cure rates, symptom improvement, and increasing effectiveness 4 weeks after therapy was concluded (LOE=1b).26 In treatment of tinea cruris, butenafine was highly effective in achieving mycologic cure after 2 weeks of once-daily treatment (LOE=1b).27
Few trials directly compare butenafine and other topical agents, but in stand-alone or placebo-controlled trials, butenafine has demonstrated efficacy similar to the allylamines terbinafine and naftifine. Butenafine was approved for over-the-counter sale in the United States in 2000.
Ciclopirox. Ciclopirox is a substituted pyridone unrelated to the imidazole or allylamine agents. In double-blind, placebo-controlled trials, ciclopirox demonstrated significant efficacy in treating dermatophyte infections (LOE=1b).28 In head-to-head trials with the imidazole agent clotrimazole, ciclopirox demonstrated equivalent or slightly greater efficacy (LOE=1b).29,30
Ciclopirox applied twice daily is usually effective within 4 weeks of treatment. Ciclopirox is available only by prescription in the United States and is more expensive than most other topical antifungal agents. Ciclopirox has demonstrated intrinsic antiinflammatory activity equivalent to the allylamines (LOE=1b).31 Due to the availability of agents with comparable or superior efficacy at much lower costs, ciclopirox should not be considered a first- or second-line agent in treating epidermal tinea infections.
Tolnaftate. Tolnaftate is an over-the-counter antifungal agent that has been available in the US since 1965. Tolnaftate is inexpensive and has often been used in powder formulation as prophylaxis against tinea, especially tinea pedis.
In systematic reviews of available placebo-controlled trials, 1% tolnaftate was shown to be less effective against tinea pedis than the azoles and allylamines, with a number needed to treat of 3.6 for tinea pedis (LOE=1a).13 A comparative study has shown tolnaftate to be inferior to clotrimazole in the treatment of tinea pedis.32 With its demonstrated inferiority to the azoles and allylamines, tolnaftate should not be considered a first- or second-line treatment for tinea infections.
TABLE 3
Topical antifungal medications
| Agent | Formulation | Frequency* | Duration* | NNT † | Cost ‡ |
|---|---|---|---|---|---|
| IMIDAZOLES | |||||
| Clotrimazole | 1% cream | Twice daily | 2–4 weeks | 2.9 | $ 7.99 (15 g) |
| 1% solution | $ 6.99 (10 mL) | ||||
| 1% swabs | $ 6.99 (36 ea.) | ||||
| Econazole | 1% cream | Twice daily | 2–4 weeks | 2.6 | $16.85 (15 g) |
| Ketoconazole | 2% cream | Once daily | 2–4 weeks | No data available | $25.39 (15 g) |
| Miconazole | 2% cream | Twice daily | 2–4 weeks | 2.8 (at 8 weeks) | $ 6.99 (15 g) |
| 2% spray | $ 5.99 (3.5 oz) | ||||
| 2% powder | $ 5.99 (3.5 oz) | ||||
| Oxiconazole | 1% cream | Once to twice daily | 2–4 weeks | 2.9 | $20.27 (15 g) |
| 1% lotion | $34.19 (30 mL) | ||||
| Sulconazole | 1% cream | Once to twice daily | 2–4 weeks | 2.5 | $13.75 (15 g) |
| 1% solution | $26.57 (30 mL) | ||||
| ALLYLAMINES | |||||
| Naftifine | 1% cream | Once to twice daily | 1–4 weeks | 1.9 | $27.69 (15 g) |
| 1% gel | $51.39 (40 g) | ||||
| Terbinafine | 1% cream | Once to twice daily | 1–4 weeks | 1.6 (1.7 for tinea cruris/tinea corporis at 8 weeks)18 | $ 8.99 (12 g) |
| 1% solution | $ 9.99 (30 mL) | ||||
| BENZYLAMINE | |||||
| Butenafine | 1% cream | Once to twice daily | 1–4 weeks | 1.9 (1.4 for tinea corporis and 1.5 for tinea cruris)26,27 | $ 9.99 (12 g) |
| OTHER | |||||
| Ciclopirox | 0.77% cream | Twice daily | 2–4 weeks | 2.1 | $21.89 (15 g) |
| 0.77% lotion | $37.99 (30 mL) | ||||
| Tolnaftate | 1% powder | Twice daily | 4 weeks | 3.6 (at 8 weeks) | $ 3.99 (4 oz) |
| 1% spray | $ 5.49 (4 oz) | ||||
| 1% swabs | $ 6.99 (36 ea.) | ||||
| *Manufacturer guidelines. | |||||
| †NNT, number needed to treat. NNT is calculated from systematic review of all randomized controlled trials for tinea pedis at 6 weeks after the initiation of treatment33 except where otherwise noted. (See “Number needed to treat,” page 866.) | |||||
| ‡Lowest cost available (including generic agents) based upon internet listings of national on-line pharmacies: www.drugstore.com, www.eckerd.com, and www.walgreens.com as of May 2003. | |||||
Oral antifungal agents
Oral antifungal agents are used for dermatophyte infections that are widespread, chronic, or markedly inflammatory, or that affect hyperkeratotic areas as in palmar or plantar tinea. They are also use for those with immuno-suppression,16 and for those in whom treatment with topical drugs has been unsatisfactory. Agents include griseofulvin, the azoles (ketoconazole, itraconazole, and fluconazole), and the allylamine terbinafine. Table 4 summarizes pertinent comparative data regarding these agents.
Griseofulvin. Griseofulvin is the oldest of the systemic antifungal agents used for tinea infections, available for more than 40 years. Griseofulvin acts on susceptible fungal cells by inhibiting microtubule function. Griseofulvin is taken by adults once daily at 500 mg, for 4 to 6 weeks, and has demonstrated efficacy in the treatment of tinea infections.33
In a systematic review of comparative trials, oral griseofulvin was found to be significantly inferior to oral terbinafine in the treatment of tinea pedis (LOE=1a).33 Likewise, terbinafine was found to be superior to griseofulvin in the treatment of tinea corporis and tinea cruris (LOE=1b).34 In comparisons with ketoconazole, griseofulvin was equivalent in the treatment of dermatophytosis (LOE=2a).35
Griseofulvin has also been compared with itraconazole in various treatment schedules for tinea corporis, tinea cruris, tinea pedis, and tinea manus; it was found to be inferior in all treatment durations to a maximum of 3 months (LOE=1b).36 In head-to-head comparisons between griseofulvin and fluconazole in the treatment of tinea corporis and tinea cruris, outcomes were not statistically significantly different, although a trend toward better clinical cures in the fluconazole arm was identified (LOE=1b).37
Azoles. Studies have made limited comparisons among the azoles (ketoconazole, itraconazole, and fluconazole) in the treatment of dermatophytosis. Small comparisons with limited power between itraconazole and fluconazole38 and between ketoconazole and fluconazole39 have demonstrated similar cure rates of about 90% for all 3 agents (LOE=1b). In a placebo-controlled comparison for treatment of tinea cruris and tinea corporis, 100 mg/d of itraconazole for 14 days was highly curative (LOE=1b).40 In placebo-controlled trials of the treatment of tinea pedis, itraconazole was found to provide statistically significant cure rates in as little as 1 week (LOE=1b).41
Systematic review of trials between itraconazole (100 mg/d for 4 weeks) and terbinafine (250 mg/d for 2 weeks) for tinea pedis showed a non-statistically significant trend toward higher cure rate in those treated with terbinafine (LOE=1a).33
There are limited placebo-controlled trials evaluating fluconazole in the treatment of superficial fungal infections of the skin. In an open, noncomparative trial employing once-weekly dosing of fluconazole 150 mg for 1 to 4 weeks for tinea corporis and tinea cruris, clinical cure rate was 92% with long-term clinical cure rate of 88% (LOE=2b).42 Another study randomized 240 adults with skin dermatophytosis or cutaneous candidiasis to either flucona-zole 150 mg/wk or fluconazole 50 mg/d for a maximum of 4 weeks (non-tinea pedis) to 6 weeks (tinea pedis) with positive clinical response of greater than 90% in both arms of treatment (LOE=2b).43 Fluconazole has not been evaluated in direct comparison with terbinafine in the treatment of dermatophytic skin infections. Comparisons between azoles and griseofulvin have been discussed above.
Terbinafine. Oral terbinafine is effective in the treatment of skin dermatophytes. In a double-blind, placebo-controlled study of terbinafine 125 mg taken twice daily for 6 weeks, 65% of patients had mycologic cure at 2 weeks post-treatment (LOE=1b).44 In an open, noncon-trolled study of terbinafine 125 mg daily for one week, 100% mycologic cure was achieved in the treatment of tinea corporis and tinea cruris (LOE=2b).45
As noted above, terbinafine has shown efficacy superior to griseofulvin and itraconazole. No well-designed, comparative study was identified that showed any oral antifungal agent to be superior to terbinafine in the treatment of dermatophytic skin infections.
TABLE 4
Oral antifungal medications in the treatment of dermatomycosis
| Comparison (with treatment costs)* | SOR | Outcomes | NNT or reduction in risk of failure to cure | Comments |
|---|---|---|---|---|
| ITRACONAZOLE ($112–$223.99) vs placebo | A 40, 41 | Itraconazole with much greater efficacy than placebo | NNT = 1.7 at 8 weeks after 1 week of treatment for tinea pedis; 1.8 at 4 weeks after 2 weeks of treatment for tinea corporis and tinea cruris | In multiple, well-designed RCT for tinea pedis, tinea corporis and tinea cruris |
| TERBINAFINE ($112.99–$329.9) vs placebo | A 33, 44, 45 | Terbinafine with much greater efficacy than with placebo | NNT = 1.5 at 8 weeks after 2 weeks of treatment for tinea pedis | Systematic review of RCT for tinea pedis and multiple, non-comparative trials for tinea corporis and tinea cruris |
| ITRACONAZOLE ($112–$223.99) vs Griseofulvin ($50.99–$71.40) | A 36 | Itraconazole with greater efficacy than Griseofulvin | Risk difference = 19% in treatment of tinea cruris/corporis and 37% in treatment of tinea pedis/manus | Systematic review of RCT for tinea corporis, tinea cruris, tinea manuus, and tinea pedis |
| TERBINAFINE ($112.99–$223.99)vs Griseofulvin ($50.99–$71.40) | A 33 | Terbinafine with greater efficacy than Griseofulvin | Risk difference = 50% at 8 weeks post-treatment | Systematic review of RCT for tinea pedis |
| KETOCONAZOLE ($30.99–$61.99) vs Griseofulvin ($50.99–71.40) | A 33 | Approximately equal efficacy between Ketoconazole and Griseofulvin | Risk difference = No consistent difference favoring either agent | Systematic review of RCT for tinea pedis |
| FLUCONAZOLE ($13.99–$50.96) vs Griseofulvin ($50.99–$71.40) | A 37 | Non-significant trend toward greater efficacy with Fluconazole | Risk difference = 12% | RCT for tinea corporis and tinea cruris |
| FLUCONAZOLE ($13.99–$50.96) vs Ketoconazole ($30.99–$61.99) | B 39 | Approximately equal efficacy between Fluconazole and Ketoconazole | Risk difference = 4% favoring fluconazole at 7 weeks post-treatment | Small RCT for all dermatomycosis |
| ITRACONAZOLE ($112–$223.99) vs Fluconazole ($13.99–$50.96) | B 38 | Approximately equal efficacy between Fluconazole and Itraconazole | Risk difference = 5% favoring itraconazole at 10 weeks post-treatment | Small RCT for tinea manuum and tinea pedis |
| TERBINAFINE ($112.99–223.99) vs Itraconazole ($112–$223.99) | A 33 | Approximately equal efficacy between Terbinafine and Itraconazole | Risk difference = No consistent difference favoring either agent | Systematic review of RCT for tinea pedis |
| SOR, strength of recommendation; NNT, number needed to treat; RCT, randomized controlled trial | ||||
| SOR: A = Multiple RCT or a systematic review of RCT; B = Trials of moderate strength, as in open trials, noncomparative trials, or RCT with small size or poor follow-up. (See page 865 for full explanation of SOR ratings.) | ||||
| * Lowest cost available (including for generic agents) based upon internet listings of national on-line pharmacies, Drugstore.com, Eckerd.com, and Walgreens.com as of May 2003. | ||||
Summary of treatment recommendations
Based upon review of the available literature, it is recommended that clinicians consider the affordable and highly effective topical agents terbinafine and butenafine as first-line therapy for patients with uncomplicated epidermal tinea infections. While also highly effective, naftifine should be considered a second-line treatment due to its prescription-only availability and increased cost. The topical azoles clotrimazole and miconazole are marginally less effective than the allylamines, but are widely available and inexpensive. The prescription drug ciclopirox is slightly superior to the azoles, but its expense and availability make it a less desirable choice for most patients. Tolnaftate is cheap and widely available, but its relative inferiority to other topical agents limits its use in treatment of tinea infections. Tolnaftate may have some utility as a prophylactic against epidermal tinea infections in susceptible individuals.46
For patients with complicated epidermal tinea infections as described above, or for those with confirmed infection and treatment failure using topical agents, oral antifungal medications should be used. Terbinafine and itraconazole are both highly effective and safe in treating tinea infections. Despite their relatively high cost, either of these agents may be considered first-line therapies. Fluconazole has been evaluated in limited trials, but data at this time are insufficient to recommend that it be considered a first-line agent in treatment of epidermal tinea infection.
Griseofulvin may be considered a second-line agent as it has been shown to be clearly less efficacious than either terbinafine or itraconazole. Oral ketoconazole has a limited role in the treatment of tinea infections. It has demonstrated efficacy equivalent to or slightly superior to griseofulvin, but its association with potentially severe liver injury makes it a very problematic choice.
Adverse reactions
As with any systemic medication, adverse effects may occur with oral antifungal agents. Oral antifungal agents are generally considered safe in the treatment of skin dermatophytoses. In systemic review of trials of oral antifungal agents in the treatment of tinea pedis, the most commonly identified adverse events were gastrointestinal complaints such as diarrhea or nausea, headaches, and skin complaints.33
Of particular note, ketoconazole has been repeatedly related to potentially serious hepatotoxicity, especially when used in longer duration of treatment.47 One large cohort study found the incident rate of acute liver injury to be 134.1 per 100,000 person-months among ketoconazole users, compared with 10.4 and 2.5 for itraconazole and terbinafine, respectively.48 Accordingly, baseline and monthly laboratory studies are advised when using ketoconazole.49
Serious complications associated with the use of topical antifungal agents are rare. As with any topical medication, local irritation and dermatitis may occur.
Correspondence
Brian Thomas, MD, NOLF-IB, Bldg 184, Box 357140, San Diego, Ca 92135. E-mail: [email protected].
- Potassium hydroxide preparation should be used as an aid to diagnosis for all erythrosquamous lesions (B).
- Fungal culture should be used in cases in which history, physical examination, and potassium hydroxide preparation fail to clearly exclude a diagnosis of tinea (B).
- Short-duration topical therapy with terbinafine, naftifine, and butenafine is efficacious for most epidermal tinea infections (A).
- Oral antifungal agents are important in the treatment of tinea infections thatare widespread, fail to respond to topical treatment, involve the thick stratum corneum of the soles and palms, or occur in immunosuppressed patients.
- Short courses of oral itraconazole and terbinafine are safe and effective in treating tinea infections (A).
Though findings on history and physical examination are sometimes sufficient to make a diagnosis of tinea infection, a potassium hydroxide (KOH) study usually is required for confirmation. Even the KOH study can be misleading, however, if a patient recently self-administered a topical antifungal agent. This article describes the varying appearance of tinea infections according to their anatomic location, and outlines a careful work-up.
Highly effective and affordable over-the-counter medications have proliferated, and short-course therapy is available. Based on systematic reviews of randomized, controlled studies, it is possible to recommend specific first-line therapies for tinea infections.
Manifestations of tinea infection
Clinical manifestations of tinea vary with anatomic location, duration of infection, and pathogen. In general, zoophilic dermatophytes evoke a more vigorous host response than the anthropophilic species.5 Shared features of many dermatophyte infections include erythema, scaling, pruritus, ring formation, and central clearing of lesions. Table 1 reviews published data on the diagnostic value of selected clinical signs in suspected tinea infection.6
TABLE 1
Diagnostic value of selected signs and symptoms in tinea infection
| Sign/symptom | Sensitivity | Specificity | PV+ | PV– | LR+ | LR– |
|---|---|---|---|---|---|---|
| Scaling | 77% | 20% | 17% | 80% | 0.96 | 1.15 |
| Erythema | 69% | 31% | 18% | 83% | 1.00 | 1.00 |
| Pruritus | 54% | 40% | 16% | 80% | 0.90 | 1.15 |
| Central clearing | 42% | 65% | 20% | 84% | 1.20 | 0.89 |
| Concentric rings | 27% | 80% | 23% | 84% | 1.35 | 0.91 |
| Maceration | 27% | 84% | 26% | 84% | 1.69 | 0.87 |
| Note: Signs and symptoms were compiled by 27 general practitioners prior to submission of skin for fungal culture. Specimens were taken from 148 consecutive patients with erythematosquamous lesions of glabrous skin. Culture results were considered the gold standard. | ||||||
| PV+, positive predictive value; PV–, negative predictive value; LR+, positive likelihood ratio; LR–, negative likelihood ratio. | ||||||
| Adapted from Lousbergh et al, Fam Pract 1999; 16:611–615.6 | ||||||
| Level of evidence=2b. For an explanation of levels of evidence, see page 865. | ||||||
Tinea pedis
Tinea of the foot may manifest as interdigital, plantar, or acute vesicular disease. Toe webs and soles of the feet are the sites most commonly affected. Tinea pedis occurs most commonly in postpubertal adolescents and adults, but may be seen in children.7
With interdigital infection, toe webs may become scaly, pruritic, and fissured. Interaction of bacteria and infecting dermatophytes may lead to a white, soggy maceration.8 Extension from the web space to the dorsal or plantar surface commonly occurs.
In chronic plantar or moccasin-type tinea pedis, the entire surface of the sole may be covered with fine, white scale and may assume a hyperkeratotic appearance. Chronic web infection may lead to acute inflammation characterized by vesicles, pustules, and bullae over the sole or the dorsum of the foot.5
Differential diagnosis. The differential diagnosis includes dry skin, pitted keratolysis, erythrasma, and contact dermatitis. Some conditions may appear very similar to tinea pedis, increasing the importance of KOH prep and fungal culture.
Tinea pedis may be distinguished from erythrasma by lack of bright coral appearance when examined with a Wood’s lamp. Tinea pedis infections also lack the well-demarcated erosions, or pits, of pitted keratolysis. Evidence of concurrent onychomycosis should increase the suspicion that tinea pedis is the correct diagnosis.
Tinea manuum
Tinea of the hand is usually analogous to moccasin-type tinea pedis. The palm appears hyperkeratotic and has very fine white scale that emphasizes the normal lines of the hand. Tinea of the dorsal surface of the hand usually occurs in the classic ringworm pattern. Tinea manuum is often seen in association with tinea pedis and onychomycosis. Many clinicians are familiar with the “one hand, two feet” syndrome, in which the palmar surfaces of both feet and one hand are infected (Figure 1).9 Onychomycosis often occurs in association with this presentation of tinea.
Differential diagnosis. The pattern of tinea manuum may be confused with those of eczema, contact dermatitis, palmar psoriasis, or even normal, rough hands. Unilateral involvement, presence of fingernail onychomycosis, lack of history indicating irritant or allergen exposure, and absence of psoriatic nail changes should increase the suspicion of palmar tinea manuum.
FIGURE 1
Tinea pedis
The common “1 hand, 2 feet” syndrome of tinea pedis. This syndrome usually requires systemic therapy.
Tinea cruris
Tinea of the groin is most common in adult males and is promoted by a warm, moist environment. Tinea cruris begins in the crural fold and spreads onto the thigh. The interior portion of the lesion is usually erythematous or slightly brown in light-skinned individuals. The leading edge often advances in a sharply demarcated semicircle with a raised, slightly scaling border. The lesion is most often bilateral, sparing the skin of the scrotum. Pruritus is common and increases as sweat macerates the irritated skin.
Differential diagnosis. Candidiasis, intertrigo, and erythrasma can cause similar lesions. It is helpful to recall that candidiasis may involve the scrotum and penis while tinea cruris does so rarely. An additional helpful feature is the characteristic bright coral appearance of erythrasma when examined with a Wood’s lamp, absent in cases of tinea cruris.
Tinea corporis and tinea faciale
Tinea infections of the face and body begin as flat, scaly, and often pruritic macules that subsequently develop a raised border and begin to spread radially. As the ring expands, the central portion of the lesion often clears. This pattern leads to the formation of irregular circles that gives tinea corporis its common name, ringworm (Figure 2).
Tinea faciale is less common, but generally has a similar appearance with central clearing of lesions. Tinea faciale may not always exhibit the sharply demarcated border of tinea corporis.
Differential diagnosis. Eczema, impetigo, early pityriasis rosea, and localized psoriasis can mimic tinea corporis. History of exposure to persons or animals (typically house pets) with known ringworm should increase suspicion of tinea corporis. Current or past evidence of psoriasis or eczema should broaden the differential to include atypical presentations of these diseases.
FIGURE 2
Tinea corporis
Widespread tinea corporis. This patient would not be a candidate for topical treatment.
Diagnosing tinea: history and exam not enough
In some cases, findings on history and physical evaluation are so characteristic of tinea that they alone may allow the clinician to make a firm diagnosis. However, studies have shown that relying upon history and physical examination may result in a many missed diagnoses.6 Accordingly, consider tinea in all instances of papulosquamous skin disease and follow up appropriately. Figure 3 presents a simple algorithm for diagnosis and treatment of suspected tinea infection.
FIGURE 3
Evaluating possible tinea infection of the skin
Koh preparation
An important adjunct in diagnosing tinea is the office-based KOH wet mount preparation ( Figure 4 ), permitting direct visualization of fungal hyphae in keratinized material of the stratum corneum. Detailed protocols for preparation and interpretation of the KOH slide for diagnosis of dermatophytosis are well described in many other sources.10
FIGURE 4
KOH preparation
Potassium hydroxide dissolves epithelial cells to reveal hyphae (A), with their characteristic branching pattern (B). To increase the likelihood of detecting hyphae on microscopic exam of skin scrapings, begin with low light and low power. If fungal elements are detected, increase magnification to confirm the diagnosis. If fungal elements are not detected, yet clinical suspicion of tinea is high, consider arranging for a fungal culture.
Performing the test
The slide is prepared by combining skin scrapings from the leading edge of a lesion with a small amount of 20% potassium hydroxide. A cover slip is applied and the slide is gently heated prior to microscopy examination under high power. Table 2 summarizes the utility of clinical diagnosis and KOH preparation in diagnosis of tinea infection. History and physical examination should be combined with KOH preparation when making the diagnosis of epidermal dermatophyte infection (strength of recommendation [SOR]=B).6 (For an explanation of evidence ratings, see page 865.)
TABLE 2
Diagnostic value of clinical diagnosis and KOH prep in tinea infection
| Test | Quality | Sensitivity | Specificity | PV+ | PV– | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| a. Clinical diagnosis6 | 2b | 81% | 45% | 24 | 92% | 1.47 | 0.42 |
| b. KOH prep50 | 2b | 88% | 95% | 73% | 98% | 17.6 | 0.13 |
| c. KOH prep51 | 2b | 77% | 62% | 59% | 79% | 2.02 | 0.37 |
| a. The clinical diagnosis set was compiled by 27 general practitioners prior to submission of skin for fungal culture. Specimens were taken from consecutive patients with erythrosquamous lesions. Culture results were considered the gold standard. Study quality=2b. | |||||||
| b/c. Both studies of KOH preps were open analyses of patients with suspicious lesions. Paired fungal culture was initiated simultaneously with KOH prep and was considered the gold standard. Study quality=2b. For an explanation of levels of evidence, see page 865. | |||||||
Caveats
Though a rapid and inexpensive test, the KOH preparation has limitations. Some physicians have little experience interpreting KOH preparations, thus reducing the value of the test. Some patients with clinically significant tinea infections may be using over-the-counter topical antifungal medications, thus reducing the likelihood that fungal hyphae will be visualized at the office visit.
In cases where clinical suspicion of tinea infection is high, and the result of a KOH prep is negative, tissue should be submitted for fungal culture. Fungal culture is not typically available in the primary care office setting and usually requires submission of the sample to a reference or hospital laboratory.
Dermatophyte test medium
Dermatophyte test medium may be prepared and used in the office. It is mixed with a phenol indicator that turns red on exposure to alkaline metabolites of fungal growth. This medium is sensitive and usually exhibits color change within one week.11 Most primary care offices do not use dermatophyte test medium, however, because of its short shelf life and because the Clinical Laboratory Improvement Amendments classification requires that this test be performed by qualified laboratory personnel.
Treatment: topical and oral agents
Treatment of tinea infections may rely on antifungal medications singly or in combination. Antifungal agents are classified by their chemical structure—imidazoles, allylamines, benzylamines, and others—and act by different mechanisms to limit the availability of ergosterol, an essential component for normal function of fungal cell membranes.
Most tinea infections may be treated with topical agents alone. Oral therapy is required most often for:
- hyperkeratotic areas as on the palms or soles
- widespread or extensive infection
- immunocompromised patients
- persons intolerant of topical therapy
- failure of topical therapy
- chronic infection
Efforts to educate patients in proper hygiene and infection control may help. Specifically, remind patients that risk of tinea cruris and tinea pedis may be reduced if they wear nonocclusive footwear, use only clean and dry socks and undergarments, wear shower shoes, and apply absorbent powders. Eradication of fungal nail infections may help in controlling tinea pedis and tinea manuum. Caution in making contact with animals or people that have known tinea infections may reduce the incidence of tinea corporis.
Topical therapy
Topical antifungal agents are widely available in both prescription and over-the-counter forms. Preference of a specific agent may be difficult due to the limited number of head-to-head comparisons among these drugs. When recommending or prescribing an agent, consider efficacy, dosing regimen, cost, formulation, and availability. The most important topical antifungal agents are divided into 2 major classes—imidazoles and allylamines—although other agents are also used. Table 3 provides information on commonly used topical antifungal agents.
Imidazoles. Imidazoles are widely available as over-the-counter and prescription forms. Topical members of this class are clotrimazole, miconazole, econazole, ketoconazole, oxiconazole, and sulconazole. Miconazole and clotrimazole are available without a prescription. As a class, imidazoles are primarily fungistatic and are generally well-tolerated.12 Meta-analysis has failed to reveal significant differences in efficacy among members of this class.13 Imidazoles are efficacious with a pooled relative risk of failure to cure of 0.38 at 6 weeks after the initiation of therapy for tinea pedis (level of evidence [LOE]=1a).13
Current guidelines recommend twice-daily application for clotrimazole, miconazole, and econazole; ketoconazole, oxiconazole, and sulconazole may be applied once daily.14 When using imidazoles, usually prescribe a 2-week course for tinea cruris or tinea corporis, and a 4-week course for tinea pedis.15 In cases of inflammatory dermatophytosis, a combination agent containing clotrimazole and the corticosteroid betamethasone dipropionate may be used for a short initial period (LOE=4).16 Many experts recommend that combination steroid agents be used with caution, for no more than a few days, and with a plan for short-term follow-up.
Allylamines. A second, newer group of antifungal agents are the allylamines. Topical allylamines, including terbinafine and naftifine, are generally considered fungicidal. Terbinafine has recently been made available over-the-counter as 1% cream and 1% solution, while naftifine remains a prescription medication. Both agents are efficacious, with cure rates for dermatomycosis greater than 75%.17,18 Naftifine and terbinafine have exhibited long periods of activity in the skin and are therefore administered only once a day.19 Additionally, terbinafine has been shown effective in treating superficial mycoses in much shorter courses than typically required for imidazoles.20
Tinea infections are among the most common of all skin diseases. They are caused by dermatophytic fungi that digest keratin in the cells of the stratum corneum. Tinea infections are typically named according to the affected anatomic region: tinea corporis for the body, tinea pedis for the feet, tinea cruris for the groin, and so on.
The true prevalence of tinea is difficult to ascertain because many people self-treat or live with chronic infection. Approximately 8.6 million office visits occur each year for tinea infections. Family or general practitioners handle more than 35% of these visits. The estimated cost of office visits plus prescribed medications for cutaneous fungal infections for the 4-year period from 1990 to 1994 is just over $1 billion.1 Tinea infections occur in all age groups and in both genders; however, males have a higher incidence of tinea pedis and tinea cruris.
Dermatophytes affecting humans are from the genera Trichophyton, Microsporum, and Epidermophyton. Dermatophytes, which are ubiquitous in the environment, are categorized as geophilic, zoophilic, or anthropophilic according to their ecologic reservoir. Surveys of dermatophytes isolated from human patients in the United States from 1993 to 1995 indicate T tonsurans (44.9%), T rubrum (41.3%), T mentagrophytes (8.5%), and M canis (3.3%) are the most commonly encountered pathogens.2
The basic pathophysiology of tinea infection is inoculation of keratinized skin by dermatophytic fungi followed by release of keratinases and proteolytic enzymes. Symptoms follow as a result of host immune and epidermal response.3 Host factors, such as immunocompromised state and genetic susceptibility, play a role in infection. Warm temperature, moisture, and occlusion encourage dermatophyte growth.4
In a systematic review of available randomized controlled trials, allylamines were found to be more efficacious than imidazoles in treating tinea pedis (LOE=1a).13 Terbinafine has also been compared with imidazoles for treatment of tinea cruris and tinea corporis and found to be significantly more effective (LOE=1b).21
Previous pharmacoeconomic analyses of imidazole versus allylamine topical therapy for dermatophyte infection have presented conflicting conclusions, with some reporting that the greater cost of allylamine agents outweighs their slightly greater efficacy,22 while others concluded that the more efficacious medication initially is more cost effective.23 In the United States, terbinafine was approved for over-the-counter sale in 1999. Since then, the cost of terbinafine to consumers has declined significantly, to the point where it is now comparable to over-the-counter imidazoles (see Table 3 ). Allylamines have demonstrated some degree of intrinsic anti-inflammatory activity.24
Butenafine. Butenafine is a topical benzylamine antifungal structurally related to the allylamines. Butenafine is fungicidal and has efficacy similar to the allylamines. In a 1-week trial of therapy for tinea pedis with twice-daily butenafine, mycological cure rates in excess of 75% were observed (LOE=1b).25 In a trial of short-term therapy of tinea corporis, butenafine applied once-daily for 14 days led to high cure rates, symptom improvement, and increasing effectiveness 4 weeks after therapy was concluded (LOE=1b).26 In treatment of tinea cruris, butenafine was highly effective in achieving mycologic cure after 2 weeks of once-daily treatment (LOE=1b).27
Few trials directly compare butenafine and other topical agents, but in stand-alone or placebo-controlled trials, butenafine has demonstrated efficacy similar to the allylamines terbinafine and naftifine. Butenafine was approved for over-the-counter sale in the United States in 2000.
Ciclopirox. Ciclopirox is a substituted pyridone unrelated to the imidazole or allylamine agents. In double-blind, placebo-controlled trials, ciclopirox demonstrated significant efficacy in treating dermatophyte infections (LOE=1b).28 In head-to-head trials with the imidazole agent clotrimazole, ciclopirox demonstrated equivalent or slightly greater efficacy (LOE=1b).29,30
Ciclopirox applied twice daily is usually effective within 4 weeks of treatment. Ciclopirox is available only by prescription in the United States and is more expensive than most other topical antifungal agents. Ciclopirox has demonstrated intrinsic antiinflammatory activity equivalent to the allylamines (LOE=1b).31 Due to the availability of agents with comparable or superior efficacy at much lower costs, ciclopirox should not be considered a first- or second-line agent in treating epidermal tinea infections.
Tolnaftate. Tolnaftate is an over-the-counter antifungal agent that has been available in the US since 1965. Tolnaftate is inexpensive and has often been used in powder formulation as prophylaxis against tinea, especially tinea pedis.
In systematic reviews of available placebo-controlled trials, 1% tolnaftate was shown to be less effective against tinea pedis than the azoles and allylamines, with a number needed to treat of 3.6 for tinea pedis (LOE=1a).13 A comparative study has shown tolnaftate to be inferior to clotrimazole in the treatment of tinea pedis.32 With its demonstrated inferiority to the azoles and allylamines, tolnaftate should not be considered a first- or second-line treatment for tinea infections.
TABLE 3
Topical antifungal medications
| Agent | Formulation | Frequency* | Duration* | NNT † | Cost ‡ |
|---|---|---|---|---|---|
| IMIDAZOLES | |||||
| Clotrimazole | 1% cream | Twice daily | 2–4 weeks | 2.9 | $ 7.99 (15 g) |
| 1% solution | $ 6.99 (10 mL) | ||||
| 1% swabs | $ 6.99 (36 ea.) | ||||
| Econazole | 1% cream | Twice daily | 2–4 weeks | 2.6 | $16.85 (15 g) |
| Ketoconazole | 2% cream | Once daily | 2–4 weeks | No data available | $25.39 (15 g) |
| Miconazole | 2% cream | Twice daily | 2–4 weeks | 2.8 (at 8 weeks) | $ 6.99 (15 g) |
| 2% spray | $ 5.99 (3.5 oz) | ||||
| 2% powder | $ 5.99 (3.5 oz) | ||||
| Oxiconazole | 1% cream | Once to twice daily | 2–4 weeks | 2.9 | $20.27 (15 g) |
| 1% lotion | $34.19 (30 mL) | ||||
| Sulconazole | 1% cream | Once to twice daily | 2–4 weeks | 2.5 | $13.75 (15 g) |
| 1% solution | $26.57 (30 mL) | ||||
| ALLYLAMINES | |||||
| Naftifine | 1% cream | Once to twice daily | 1–4 weeks | 1.9 | $27.69 (15 g) |
| 1% gel | $51.39 (40 g) | ||||
| Terbinafine | 1% cream | Once to twice daily | 1–4 weeks | 1.6 (1.7 for tinea cruris/tinea corporis at 8 weeks)18 | $ 8.99 (12 g) |
| 1% solution | $ 9.99 (30 mL) | ||||
| BENZYLAMINE | |||||
| Butenafine | 1% cream | Once to twice daily | 1–4 weeks | 1.9 (1.4 for tinea corporis and 1.5 for tinea cruris)26,27 | $ 9.99 (12 g) |
| OTHER | |||||
| Ciclopirox | 0.77% cream | Twice daily | 2–4 weeks | 2.1 | $21.89 (15 g) |
| 0.77% lotion | $37.99 (30 mL) | ||||
| Tolnaftate | 1% powder | Twice daily | 4 weeks | 3.6 (at 8 weeks) | $ 3.99 (4 oz) |
| 1% spray | $ 5.49 (4 oz) | ||||
| 1% swabs | $ 6.99 (36 ea.) | ||||
| *Manufacturer guidelines. | |||||
| †NNT, number needed to treat. NNT is calculated from systematic review of all randomized controlled trials for tinea pedis at 6 weeks after the initiation of treatment33 except where otherwise noted. (See “Number needed to treat,” page 866.) | |||||
| ‡Lowest cost available (including generic agents) based upon internet listings of national on-line pharmacies: www.drugstore.com, www.eckerd.com, and www.walgreens.com as of May 2003. | |||||
Oral antifungal agents
Oral antifungal agents are used for dermatophyte infections that are widespread, chronic, or markedly inflammatory, or that affect hyperkeratotic areas as in palmar or plantar tinea. They are also use for those with immuno-suppression,16 and for those in whom treatment with topical drugs has been unsatisfactory. Agents include griseofulvin, the azoles (ketoconazole, itraconazole, and fluconazole), and the allylamine terbinafine. Table 4 summarizes pertinent comparative data regarding these agents.
Griseofulvin. Griseofulvin is the oldest of the systemic antifungal agents used for tinea infections, available for more than 40 years. Griseofulvin acts on susceptible fungal cells by inhibiting microtubule function. Griseofulvin is taken by adults once daily at 500 mg, for 4 to 6 weeks, and has demonstrated efficacy in the treatment of tinea infections.33
In a systematic review of comparative trials, oral griseofulvin was found to be significantly inferior to oral terbinafine in the treatment of tinea pedis (LOE=1a).33 Likewise, terbinafine was found to be superior to griseofulvin in the treatment of tinea corporis and tinea cruris (LOE=1b).34 In comparisons with ketoconazole, griseofulvin was equivalent in the treatment of dermatophytosis (LOE=2a).35
Griseofulvin has also been compared with itraconazole in various treatment schedules for tinea corporis, tinea cruris, tinea pedis, and tinea manus; it was found to be inferior in all treatment durations to a maximum of 3 months (LOE=1b).36 In head-to-head comparisons between griseofulvin and fluconazole in the treatment of tinea corporis and tinea cruris, outcomes were not statistically significantly different, although a trend toward better clinical cures in the fluconazole arm was identified (LOE=1b).37
Azoles. Studies have made limited comparisons among the azoles (ketoconazole, itraconazole, and fluconazole) in the treatment of dermatophytosis. Small comparisons with limited power between itraconazole and fluconazole38 and between ketoconazole and fluconazole39 have demonstrated similar cure rates of about 90% for all 3 agents (LOE=1b). In a placebo-controlled comparison for treatment of tinea cruris and tinea corporis, 100 mg/d of itraconazole for 14 days was highly curative (LOE=1b).40 In placebo-controlled trials of the treatment of tinea pedis, itraconazole was found to provide statistically significant cure rates in as little as 1 week (LOE=1b).41
Systematic review of trials between itraconazole (100 mg/d for 4 weeks) and terbinafine (250 mg/d for 2 weeks) for tinea pedis showed a non-statistically significant trend toward higher cure rate in those treated with terbinafine (LOE=1a).33
There are limited placebo-controlled trials evaluating fluconazole in the treatment of superficial fungal infections of the skin. In an open, noncomparative trial employing once-weekly dosing of fluconazole 150 mg for 1 to 4 weeks for tinea corporis and tinea cruris, clinical cure rate was 92% with long-term clinical cure rate of 88% (LOE=2b).42 Another study randomized 240 adults with skin dermatophytosis or cutaneous candidiasis to either flucona-zole 150 mg/wk or fluconazole 50 mg/d for a maximum of 4 weeks (non-tinea pedis) to 6 weeks (tinea pedis) with positive clinical response of greater than 90% in both arms of treatment (LOE=2b).43 Fluconazole has not been evaluated in direct comparison with terbinafine in the treatment of dermatophytic skin infections. Comparisons between azoles and griseofulvin have been discussed above.
Terbinafine. Oral terbinafine is effective in the treatment of skin dermatophytes. In a double-blind, placebo-controlled study of terbinafine 125 mg taken twice daily for 6 weeks, 65% of patients had mycologic cure at 2 weeks post-treatment (LOE=1b).44 In an open, noncon-trolled study of terbinafine 125 mg daily for one week, 100% mycologic cure was achieved in the treatment of tinea corporis and tinea cruris (LOE=2b).45
As noted above, terbinafine has shown efficacy superior to griseofulvin and itraconazole. No well-designed, comparative study was identified that showed any oral antifungal agent to be superior to terbinafine in the treatment of dermatophytic skin infections.
TABLE 4
Oral antifungal medications in the treatment of dermatomycosis
| Comparison (with treatment costs)* | SOR | Outcomes | NNT or reduction in risk of failure to cure | Comments |
|---|---|---|---|---|
| ITRACONAZOLE ($112–$223.99) vs placebo | A 40, 41 | Itraconazole with much greater efficacy than placebo | NNT = 1.7 at 8 weeks after 1 week of treatment for tinea pedis; 1.8 at 4 weeks after 2 weeks of treatment for tinea corporis and tinea cruris | In multiple, well-designed RCT for tinea pedis, tinea corporis and tinea cruris |
| TERBINAFINE ($112.99–$329.9) vs placebo | A 33, 44, 45 | Terbinafine with much greater efficacy than with placebo | NNT = 1.5 at 8 weeks after 2 weeks of treatment for tinea pedis | Systematic review of RCT for tinea pedis and multiple, non-comparative trials for tinea corporis and tinea cruris |
| ITRACONAZOLE ($112–$223.99) vs Griseofulvin ($50.99–$71.40) | A 36 | Itraconazole with greater efficacy than Griseofulvin | Risk difference = 19% in treatment of tinea cruris/corporis and 37% in treatment of tinea pedis/manus | Systematic review of RCT for tinea corporis, tinea cruris, tinea manuus, and tinea pedis |
| TERBINAFINE ($112.99–$223.99)vs Griseofulvin ($50.99–$71.40) | A 33 | Terbinafine with greater efficacy than Griseofulvin | Risk difference = 50% at 8 weeks post-treatment | Systematic review of RCT for tinea pedis |
| KETOCONAZOLE ($30.99–$61.99) vs Griseofulvin ($50.99–71.40) | A 33 | Approximately equal efficacy between Ketoconazole and Griseofulvin | Risk difference = No consistent difference favoring either agent | Systematic review of RCT for tinea pedis |
| FLUCONAZOLE ($13.99–$50.96) vs Griseofulvin ($50.99–$71.40) | A 37 | Non-significant trend toward greater efficacy with Fluconazole | Risk difference = 12% | RCT for tinea corporis and tinea cruris |
| FLUCONAZOLE ($13.99–$50.96) vs Ketoconazole ($30.99–$61.99) | B 39 | Approximately equal efficacy between Fluconazole and Ketoconazole | Risk difference = 4% favoring fluconazole at 7 weeks post-treatment | Small RCT for all dermatomycosis |
| ITRACONAZOLE ($112–$223.99) vs Fluconazole ($13.99–$50.96) | B 38 | Approximately equal efficacy between Fluconazole and Itraconazole | Risk difference = 5% favoring itraconazole at 10 weeks post-treatment | Small RCT for tinea manuum and tinea pedis |
| TERBINAFINE ($112.99–223.99) vs Itraconazole ($112–$223.99) | A 33 | Approximately equal efficacy between Terbinafine and Itraconazole | Risk difference = No consistent difference favoring either agent | Systematic review of RCT for tinea pedis |
| SOR, strength of recommendation; NNT, number needed to treat; RCT, randomized controlled trial | ||||
| SOR: A = Multiple RCT or a systematic review of RCT; B = Trials of moderate strength, as in open trials, noncomparative trials, or RCT with small size or poor follow-up. (See page 865 for full explanation of SOR ratings.) | ||||
| * Lowest cost available (including for generic agents) based upon internet listings of national on-line pharmacies, Drugstore.com, Eckerd.com, and Walgreens.com as of May 2003. | ||||
Summary of treatment recommendations
Based upon review of the available literature, it is recommended that clinicians consider the affordable and highly effective topical agents terbinafine and butenafine as first-line therapy for patients with uncomplicated epidermal tinea infections. While also highly effective, naftifine should be considered a second-line treatment due to its prescription-only availability and increased cost. The topical azoles clotrimazole and miconazole are marginally less effective than the allylamines, but are widely available and inexpensive. The prescription drug ciclopirox is slightly superior to the azoles, but its expense and availability make it a less desirable choice for most patients. Tolnaftate is cheap and widely available, but its relative inferiority to other topical agents limits its use in treatment of tinea infections. Tolnaftate may have some utility as a prophylactic against epidermal tinea infections in susceptible individuals.46
For patients with complicated epidermal tinea infections as described above, or for those with confirmed infection and treatment failure using topical agents, oral antifungal medications should be used. Terbinafine and itraconazole are both highly effective and safe in treating tinea infections. Despite their relatively high cost, either of these agents may be considered first-line therapies. Fluconazole has been evaluated in limited trials, but data at this time are insufficient to recommend that it be considered a first-line agent in treatment of epidermal tinea infection.
Griseofulvin may be considered a second-line agent as it has been shown to be clearly less efficacious than either terbinafine or itraconazole. Oral ketoconazole has a limited role in the treatment of tinea infections. It has demonstrated efficacy equivalent to or slightly superior to griseofulvin, but its association with potentially severe liver injury makes it a very problematic choice.
Adverse reactions
As with any systemic medication, adverse effects may occur with oral antifungal agents. Oral antifungal agents are generally considered safe in the treatment of skin dermatophytoses. In systemic review of trials of oral antifungal agents in the treatment of tinea pedis, the most commonly identified adverse events were gastrointestinal complaints such as diarrhea or nausea, headaches, and skin complaints.33
Of particular note, ketoconazole has been repeatedly related to potentially serious hepatotoxicity, especially when used in longer duration of treatment.47 One large cohort study found the incident rate of acute liver injury to be 134.1 per 100,000 person-months among ketoconazole users, compared with 10.4 and 2.5 for itraconazole and terbinafine, respectively.48 Accordingly, baseline and monthly laboratory studies are advised when using ketoconazole.49
Serious complications associated with the use of topical antifungal agents are rare. As with any topical medication, local irritation and dermatitis may occur.
Correspondence
Brian Thomas, MD, NOLF-IB, Bldg 184, Box 357140, San Diego, Ca 92135. E-mail: [email protected].
1. Smith ES, Fleischer AB, Friedman SR, Willford PM. Characteristics of office-based physician visits for cutaneous fungal infection: an analysis of 1990 to 1994 National Ambulatory Medical Care Survey Data. Cutis 2002;69:191-202.
2. Weitzman I, Chin NX, Kunjukunju N, Della-Latta P. A survey of dermatophytes isolated from human patients in the United States from 1993 to 1995. J Am Acad Dermatol 1998;39:255-261.
3. Roscoe J, Farmer ER. Diseases caused by fungi. In: Farmer ER, Hood AF, eds. Pathology of the Skin. 2nd ed. New York: McGraw-Hill; 2000;611-633.
4. Odom RB, James WD, Berger TG. Diseases resulting from fungi and yeasts. >In: Diseases of the Skin: Clinical Dermatology. 9th ed. Philadelphia: WB Saunders; 2000;358-415.
5. Habif TP. Superficial fungal infections. In: Clinical Dermatology. 3rd ed. St. Louis, Mo: Mosby; 1996;362-408.
6. Lousbergh D, Buntinx F, Pierard G. Diagnosing dermatomycosis in general practice. Fam Pract 1999;16:611-615.
7. Kearse HJ, Miller OF. Tinea pedis in prepubertal children: does it occur? J Am Acad Dermatol 1988;19:619-22.
8. Kates SG, Nordstrom KM, McGinley KJ, Leyden JJ. Microbial ecology of interdigital infections of toe web spaces. J Am Acad Dermatol 1990;22:578-582.
9. Lookingbill DP, Marks JG. Scaling papules, plaques, and patches. In: Principles of Dermatology. 2nd ed. Philadelphia: WB Saunders; 1993;136-159.
10. Rosen T. Dermatophytosis: diagnostic pointers and therapeutic pitfalls. Consultant 1997;37:1545-1557.
11. Sinski JT, Swanson JR, Kelley LM. Dermatophyte test medium: clinical and quantitative appraisal. J Inves Dermatol 1972;58:405-411.
12. Saag MS, Dismukes WE. Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother 1988;32:1-8.
13. Crawford F, Hart R, Bell-Syer S, Torgerson D, Young P, Russell I. Topical treatments for fungal infections of the skin and nails of the foot (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
14. Polak A. Antifungal activity of four antifungal drugs in the cutaneous retention time test. Sabouraudia 1984;22:501-503.
15. Gupta AK, Einarson TR, Summerbell RC, Shear NH. An overview of topical antifungal therapy in dermatomycosis.A North American perspective. Drugs 1998;55:645-674.
16. Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial mycotic infections of the skin: tinea corporis, tinea cruris, tinea faciei, tinea manuum, and tinea pedis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol 1996;34:282-286.
17. Jordon RE, Rapini RP, Rex IH, Jr, et al. Once-daily naftifine cream 1% in the treatment of tinea cruris and tinea corporis. Int J Dermatol 1990;29:441-442.
18. Budimulja U, Bramono K, Urip S, et al. Once daily treatment with terbinafine 1% cream (Lamisil) for one week is effective in the treatment of tinea corporis and tinea cruris. A placebo-controlled study. Mycoses 2001;44:300-306.
19. Schuster I, Schaude M, Schatz F, et al. Preclinical characteristics of allylamines. In: Berg D, Plempel M, eds. Sterol Biosynthesis Inhibitors. Chichester, England: Ellis Horwood; 1988;449-470.
20. Evans EG, Seaman RA, James IG. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br J Dermatol 1994;130:83-87
21. Bonifaz A, Saul A. Comparative study between terbinafine 1% emulsion-gel versus ketoconazole 2% cream in tinea cruris and tinea corporis. Eur J Dermatol 2000;10:107-109.
22. Chren MM, Landefeld CS. A cost analysis of topical drug regimens for dermatophyte infections. JAMA 1994;272:1922-1925.
23. Davis R, Balfour JA. Terbinafine. A pharmacoeconomic evaluation of its use in superficial fungal infections. Pharmacoeconomics. 1995;8:253-269.
24. Rosen T, Schell BJ, Orengo I. Anti-inflammatory activity of antifungal preparations. Int J Dermatol 1997;36:788-792.
25. Savin R, De Villez RL, Elewski B, et al. One-week therapy with twice-daily Butenafine 1% cream versus vehicle in the treatment of tinea pedis: a multicenter, double-blind trial. J Amer Acad Dermatol 1997;36:S15-S19.
26. Greer DL, Weiss J, Rodriguez DA, Hebert AA, Swinehart JM. A randomized trial to assess once-daily topical treatment of tinea corporis with butenafine, a new antifungal agent. J Amer Acad Dermatol 1997;37:231-235.
27. Lesher JL, Babel DE, Stewart DM, et al. Butenafine 1% cream in the treatment of tinea cruris: a multicenter, vehi-cle-controlled, double-blind trial. J Amer Acad Dermatol 1997;36:S20-S24.
28. Sehgal VN. Ciclopirox: a new topical pyrodonium antimy-cotic agent. A double-blind study in superficial dermato-mycoses. Br J Dermatol 1976;95:83-88.
29. Bogaert H, Cordero C, Ollague W, Savin RC, Shalita AR, Zaias N. Multicentre double-blind clinical trials of ciclopirox olamine cream 1% in the treatment of tinea corporis and tinea cruris. J Int Med Res 1986;14:210-216.
30. Beyer M. Selected double-blind comparative studies on the efficacy and tolerance of ciclopiroxolamine solution and cream [in German]. Arzneimittelforschung 1981;31:1378-1381.
31. Rosen T, Schell BJ, Orengo I. Anti-inflammatory activity of antifungal preparations. Int J Dermatol 1997;36:788-792.
32. Woscoff A, Carageli S. Treatment of tinea pedis with sulconazole nitrate 1% cream or miconazole nitrate 2% cream. Curr Ther Res 1986;39:753-757.
33. Bell-Syer SEM, Hart R, Crawford F, Torgerson DJ, Tyrell W, Russell I. Oral treatments for fungal infections of the skin of the foot (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
34. Voravutinon V. Oral treatment of tinea corporis and tinea cruris with terbinafine and griseofulvin: a randomized double-blind comparative study. J Med Assoc Thai. 1993;76:388-393.
35. Hay RJ, Clayton YM, Griffiths WA, Dowd PM. A comparative double-blind study of ketoconazole and griseofulvin in dermatophytosis. Br J Dermatol 1985;112:691-696.
36. Lachapelle JM, De Doncker P, Tennstedt D, Cauwenbergh G, Janssen PA. Itraconazole compared with griseo-fulvin in the treatment of tinea corporis/cruris and tinea pedis/mannus: An interpretation of the clinical results of all completed double-blind studies with respect to the pharmacokinetic profile. Dermatology 1992;184:45-50.
37. Faergemann J, Mork NJ, Haglund A, Odegard T. A multi-centre (double-blind) comparative study to assess the safety and efficacy of fluconazole and griseofulvin in the treatment of tinea corporis and tinea cruris. Br J Dermatol 1997;136:575-577.
38. Difonzo EM, Papini M, Cilli P, et al. A double blind study comparison of itraconazole and fluconazole in tinea pedis and tinea manuum. J Eur Acad Dermatol Venereol 1995;4:148-152.
39. Fischbein A, Haneke E, Lacner K. Comparative evaluation of oral fluconazole and oral ketoconazole in the treatment of fungal infections of the skin. Intl J Dermatol 1992;31:12-16.
40. Pariser DM, Pariser RJ, Ruoff G, Ray TL. Double-blind comparison of itraconazole and placebo in the treatment of tinea corporis and tinea cruris. J Amer Acad Dermatol 1994;31:232-234.
41. Svejgaard E, Avnstorp C, Wanscher B, Nilsson J, Heremans A. Efficacy and safety of short-term itracona-zole in tinea pedis: a double-blind, randomized, placebo-controlled trial. Dermatology 1998;197:368-372.
42. Suchil P, Gei FM, Robles M, Perera-Ramirez A, Welsh O, Male O. Once-weekly oral doses of fluconazole 150 mg in the treatment of tinea corporis/cruris and cutaneous candidiasis. Clin Exp Dermatol 1992;17:397-401.
43. Nozickova M, Koudelkova V, Kulikova Z, Malina L, Urbanowski S, Silny W. A comparison of the efficacy of oral fluconazole, 150 mg/week versus 50 mg/day, in the treatment of tinea corporis, tinea cruris, tinea pedis, and cutaneous candidiasis. Intl J Dermatol 1998;37:703-705.
44. Savin RC, Zaias N. Treatment of chronic moccasin-type tinea pedis with terbinafine: a double-blind, placebo-controlled trial. J Am Acad Dermatol 1990;23:804-807.
45. Farag A, Taha M, Halim S. One-week therapy with oral terbinafine in cases of tinea cruris/corporis. Br J Dermatol 1994;131:684-686.
46. Smith EB, Dickson JE, Knox JM. Tolnaftate powder in prophylaxis of tinea pedis. South Med J 1974;67:776-778.
47. Lewis JH, Zimmerman HJ, Benson GD, Ishak KG. Hepatic injury associated with ketoconazole therapy: analysis of 33 cases. Gastroenterology 1984;86:503-513.
48. Garcia Rodriguez LA, Duque A, Castellsague J, Perea-Gutthann S, Stricker BH. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol 1999;48:847-852.
49. Lake-Bakaar G, Scheuer PJ, Sherlock S. Hepatic reactions associated with ketoconazole in the United Kingdom. Br Med J (Clin Res Ed) 1987;294:419-422.
50. Haldane DJ, Robart E. A comparison of calcofluor white, potassium hydroxide, and culture for the laboratory diagnosis of superficial fungal infection. Diagn Microbiol Infect Dis 1990;13:337-339.
51. Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol 1993;129:510-511.
1. Smith ES, Fleischer AB, Friedman SR, Willford PM. Characteristics of office-based physician visits for cutaneous fungal infection: an analysis of 1990 to 1994 National Ambulatory Medical Care Survey Data. Cutis 2002;69:191-202.
2. Weitzman I, Chin NX, Kunjukunju N, Della-Latta P. A survey of dermatophytes isolated from human patients in the United States from 1993 to 1995. J Am Acad Dermatol 1998;39:255-261.
3. Roscoe J, Farmer ER. Diseases caused by fungi. In: Farmer ER, Hood AF, eds. Pathology of the Skin. 2nd ed. New York: McGraw-Hill; 2000;611-633.
4. Odom RB, James WD, Berger TG. Diseases resulting from fungi and yeasts. >In: Diseases of the Skin: Clinical Dermatology. 9th ed. Philadelphia: WB Saunders; 2000;358-415.
5. Habif TP. Superficial fungal infections. In: Clinical Dermatology. 3rd ed. St. Louis, Mo: Mosby; 1996;362-408.
6. Lousbergh D, Buntinx F, Pierard G. Diagnosing dermatomycosis in general practice. Fam Pract 1999;16:611-615.
7. Kearse HJ, Miller OF. Tinea pedis in prepubertal children: does it occur? J Am Acad Dermatol 1988;19:619-22.
8. Kates SG, Nordstrom KM, McGinley KJ, Leyden JJ. Microbial ecology of interdigital infections of toe web spaces. J Am Acad Dermatol 1990;22:578-582.
9. Lookingbill DP, Marks JG. Scaling papules, plaques, and patches. In: Principles of Dermatology. 2nd ed. Philadelphia: WB Saunders; 1993;136-159.
10. Rosen T. Dermatophytosis: diagnostic pointers and therapeutic pitfalls. Consultant 1997;37:1545-1557.
11. Sinski JT, Swanson JR, Kelley LM. Dermatophyte test medium: clinical and quantitative appraisal. J Inves Dermatol 1972;58:405-411.
12. Saag MS, Dismukes WE. Azole antifungal agents: emphasis on new triazoles. Antimicrob Agents Chemother 1988;32:1-8.
13. Crawford F, Hart R, Bell-Syer S, Torgerson D, Young P, Russell I. Topical treatments for fungal infections of the skin and nails of the foot (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
14. Polak A. Antifungal activity of four antifungal drugs in the cutaneous retention time test. Sabouraudia 1984;22:501-503.
15. Gupta AK, Einarson TR, Summerbell RC, Shear NH. An overview of topical antifungal therapy in dermatomycosis.A North American perspective. Drugs 1998;55:645-674.
16. Drake LA, Dinehart SM, Farmer ER, et al. Guidelines of care for superficial mycotic infections of the skin: tinea corporis, tinea cruris, tinea faciei, tinea manuum, and tinea pedis. Guidelines/Outcomes Committee. American Academy of Dermatology. J Am Acad Dermatol 1996;34:282-286.
17. Jordon RE, Rapini RP, Rex IH, Jr, et al. Once-daily naftifine cream 1% in the treatment of tinea cruris and tinea corporis. Int J Dermatol 1990;29:441-442.
18. Budimulja U, Bramono K, Urip S, et al. Once daily treatment with terbinafine 1% cream (Lamisil) for one week is effective in the treatment of tinea corporis and tinea cruris. A placebo-controlled study. Mycoses 2001;44:300-306.
19. Schuster I, Schaude M, Schatz F, et al. Preclinical characteristics of allylamines. In: Berg D, Plempel M, eds. Sterol Biosynthesis Inhibitors. Chichester, England: Ellis Horwood; 1988;449-470.
20. Evans EG, Seaman RA, James IG. Short-duration therapy with terbinafine 1% cream in dermatophyte skin infections. Br J Dermatol 1994;130:83-87
21. Bonifaz A, Saul A. Comparative study between terbinafine 1% emulsion-gel versus ketoconazole 2% cream in tinea cruris and tinea corporis. Eur J Dermatol 2000;10:107-109.
22. Chren MM, Landefeld CS. A cost analysis of topical drug regimens for dermatophyte infections. JAMA 1994;272:1922-1925.
23. Davis R, Balfour JA. Terbinafine. A pharmacoeconomic evaluation of its use in superficial fungal infections. Pharmacoeconomics. 1995;8:253-269.
24. Rosen T, Schell BJ, Orengo I. Anti-inflammatory activity of antifungal preparations. Int J Dermatol 1997;36:788-792.
25. Savin R, De Villez RL, Elewski B, et al. One-week therapy with twice-daily Butenafine 1% cream versus vehicle in the treatment of tinea pedis: a multicenter, double-blind trial. J Amer Acad Dermatol 1997;36:S15-S19.
26. Greer DL, Weiss J, Rodriguez DA, Hebert AA, Swinehart JM. A randomized trial to assess once-daily topical treatment of tinea corporis with butenafine, a new antifungal agent. J Amer Acad Dermatol 1997;37:231-235.
27. Lesher JL, Babel DE, Stewart DM, et al. Butenafine 1% cream in the treatment of tinea cruris: a multicenter, vehi-cle-controlled, double-blind trial. J Amer Acad Dermatol 1997;36:S20-S24.
28. Sehgal VN. Ciclopirox: a new topical pyrodonium antimy-cotic agent. A double-blind study in superficial dermato-mycoses. Br J Dermatol 1976;95:83-88.
29. Bogaert H, Cordero C, Ollague W, Savin RC, Shalita AR, Zaias N. Multicentre double-blind clinical trials of ciclopirox olamine cream 1% in the treatment of tinea corporis and tinea cruris. J Int Med Res 1986;14:210-216.
30. Beyer M. Selected double-blind comparative studies on the efficacy and tolerance of ciclopiroxolamine solution and cream [in German]. Arzneimittelforschung 1981;31:1378-1381.
31. Rosen T, Schell BJ, Orengo I. Anti-inflammatory activity of antifungal preparations. Int J Dermatol 1997;36:788-792.
32. Woscoff A, Carageli S. Treatment of tinea pedis with sulconazole nitrate 1% cream or miconazole nitrate 2% cream. Curr Ther Res 1986;39:753-757.
33. Bell-Syer SEM, Hart R, Crawford F, Torgerson DJ, Tyrell W, Russell I. Oral treatments for fungal infections of the skin of the foot (Cochrane Review). In: The Cochrane Library, Issue 1, 2003. Oxford: Update Software.
34. Voravutinon V. Oral treatment of tinea corporis and tinea cruris with terbinafine and griseofulvin: a randomized double-blind comparative study. J Med Assoc Thai. 1993;76:388-393.
35. Hay RJ, Clayton YM, Griffiths WA, Dowd PM. A comparative double-blind study of ketoconazole and griseofulvin in dermatophytosis. Br J Dermatol 1985;112:691-696.
36. Lachapelle JM, De Doncker P, Tennstedt D, Cauwenbergh G, Janssen PA. Itraconazole compared with griseo-fulvin in the treatment of tinea corporis/cruris and tinea pedis/mannus: An interpretation of the clinical results of all completed double-blind studies with respect to the pharmacokinetic profile. Dermatology 1992;184:45-50.
37. Faergemann J, Mork NJ, Haglund A, Odegard T. A multi-centre (double-blind) comparative study to assess the safety and efficacy of fluconazole and griseofulvin in the treatment of tinea corporis and tinea cruris. Br J Dermatol 1997;136:575-577.
38. Difonzo EM, Papini M, Cilli P, et al. A double blind study comparison of itraconazole and fluconazole in tinea pedis and tinea manuum. J Eur Acad Dermatol Venereol 1995;4:148-152.
39. Fischbein A, Haneke E, Lacner K. Comparative evaluation of oral fluconazole and oral ketoconazole in the treatment of fungal infections of the skin. Intl J Dermatol 1992;31:12-16.
40. Pariser DM, Pariser RJ, Ruoff G, Ray TL. Double-blind comparison of itraconazole and placebo in the treatment of tinea corporis and tinea cruris. J Amer Acad Dermatol 1994;31:232-234.
41. Svejgaard E, Avnstorp C, Wanscher B, Nilsson J, Heremans A. Efficacy and safety of short-term itracona-zole in tinea pedis: a double-blind, randomized, placebo-controlled trial. Dermatology 1998;197:368-372.
42. Suchil P, Gei FM, Robles M, Perera-Ramirez A, Welsh O, Male O. Once-weekly oral doses of fluconazole 150 mg in the treatment of tinea corporis/cruris and cutaneous candidiasis. Clin Exp Dermatol 1992;17:397-401.
43. Nozickova M, Koudelkova V, Kulikova Z, Malina L, Urbanowski S, Silny W. A comparison of the efficacy of oral fluconazole, 150 mg/week versus 50 mg/day, in the treatment of tinea corporis, tinea cruris, tinea pedis, and cutaneous candidiasis. Intl J Dermatol 1998;37:703-705.
44. Savin RC, Zaias N. Treatment of chronic moccasin-type tinea pedis with terbinafine: a double-blind, placebo-controlled trial. J Am Acad Dermatol 1990;23:804-807.
45. Farag A, Taha M, Halim S. One-week therapy with oral terbinafine in cases of tinea cruris/corporis. Br J Dermatol 1994;131:684-686.
46. Smith EB, Dickson JE, Knox JM. Tolnaftate powder in prophylaxis of tinea pedis. South Med J 1974;67:776-778.
47. Lewis JH, Zimmerman HJ, Benson GD, Ishak KG. Hepatic injury associated with ketoconazole therapy: analysis of 33 cases. Gastroenterology 1984;86:503-513.
48. Garcia Rodriguez LA, Duque A, Castellsague J, Perea-Gutthann S, Stricker BH. A cohort study on the risk of acute liver injury among users of ketoconazole and other antifungal drugs. Br J Clin Pharmacol 1999;48:847-852.
49. Lake-Bakaar G, Scheuer PJ, Sherlock S. Hepatic reactions associated with ketoconazole in the United Kingdom. Br Med J (Clin Res Ed) 1987;294:419-422.
50. Haldane DJ, Robart E. A comparison of calcofluor white, potassium hydroxide, and culture for the laboratory diagnosis of superficial fungal infection. Diagn Microbiol Infect Dis 1990;13:337-339.
51. Miller MA, Hodgson Y. Sensitivity and specificity of potassium hydroxide smears of skin scrapings for the diagnosis of tinea pedis. Arch Dermatol 1993;129:510-511.