User login
A new standard for treatment of torus fractures of the wrist?
ILLUSTRATIVE CASE
A 9-year-old girl presents to your urgent care clinic after a fall while snowboarding for the first time. She reports falling forward onto her outstretched right hand and describes pain in her distal right forearm. She denies paresthesias, weakness, or lacerations. Physical examination reveals mild edema of the dorsal aspect of her distal right forearm and tenderness to palpation of the dorsal aspect of her distal radius. She denies tenderness to palpation of her ulna, anatomic snuffbox, hand, and elbow. Range of motion of the wrist is full on passive testing, but she declines active testing due to pain. Wrist radiographs reveal an uncomplicated torus fracture of the distal radius. Can immobilization with a soft bandage alone sufficiently treat this fracture?
Fractures of the distal radius are among the most common fractures of the upper extremity and commonly occur from a fall onto an outstretched hand.2 In the pediatric population, torus fractures, also known as buckle fractures, are the most common type of distal radius fracture, comprising an estimated 50% of pediatric wrist fractures.3,4 This is due to the presence of a
Pediatric torus fractures of the distal radius generally are treated with immobilization,2 traditionally through a
Despite common use of immobilization, torus fractures of the distal radius are anatomically stable, and displacement is unlikely to occur.7,8 As such, many studies have suggested that treatment of torus fractures with rigid immobilization in a cast or splint may not be necessary.9,10 However, a 2018 Cochrane review concluded that the quality of evidence illustrating similar recovery between treatments was low, leaving uncertainty as to the most appropriate management strategy.6 Less casting and follow-up imaging could have positive implications for patient satisfaction, health care–associated costs, and radiation exposure.10
This study, the Forearm Fracture Recovery in Children Evaluation (FORCE) trial, compared the traditional treatment of distal radius torus fractures with rigid immobilization to soft immobilization and immediate discharge.
STUDY SUMMARY
Providing quality evidence for a standard of care
FORCE was a randomized controlled equivalence trial (N = 965) across 23 emergency departments (EDs) in the United Kingdom that compared pain and function in pediatric patients with distal radius torus fractures treated with a soft bandage and immediate discharge vs rigid immobilization and routine follow-up.1 Patients included children ages 4 to 15 years presenting to the ED with a distal radius torus fracture, which was confirmed radiologically.
Patients with concomitant
Continue to: Patients were randomly assigned...
Patients were randomly assigned in a 1:1 ratio to receive treatment with either a soft bandage such as a gauze roller bandage (n = 489) or rigid immobilization (n = 476). For patients in the bandage group, a soft bandage was applied in the ED or provided for home application without planned clinical follow-up. Patients in the rigid immobilization group were treated in the ED with either a removable manufactured splint or a molded splint or cast, followed by the standard follow-up practice of the treating center. Patients in the soft bandage group were advised not to wear the bandage for more than 3 weeks. Blinding was not possible, but the treatment team did not take part in patient follow-up.
The primary outcome was change in pain 3 days after treatment, measured on the Wong-Baker FACES Pain Rating Scale (an ordinal assessment using 6 illustrated facial expressions translated to a numeric rating on a scale of 0-10, with higher scores indicating worse pain). This scale has an established minimum clinically important difference (MCID) value of 1 face (2 points).11 Per standard practice in equivalence trials, the equivalence margin was defined as half the MCID, with a value of 1.0 used in this study.
Secondary outcomes measured over the 6-week follow-up period included additional pain measurements using the Wong-Baker scale, measures of function and health-related quality of life, analgesia use, days of absence from school or childcare, complication rates, and patient satisfaction. This study used modified intention-to-treat and per-protocol analyses.
The mean age of participants was 9.6 years; 39% were girls and 61% were boys. In the bandage group, 94% opted to have the soft bandage applied in the ED, and 95% of the rigid immobilization group were treated with a removable wrist splint in the ED. At 3 days, pain scores improved by 3.2 points (standard deviation [SD] = 2.1) in the soft bandage group and 3.1 points (SD = 2.1) in the rigid immobilization group. The adjusted difference was –0.1 (95% CI, –0.37 to 0.17) in the intention-to-treat analysis and –0.06 (95% CI, –0.34 to 0.21) in the per-protocol analysis, which were both less than the predetermined equivalence margin. This equivalence margin also was met at all secondary time points (1 day, 7 days, 3 weeks, and 6 weeks after treatment) and in subgroup analysis of those 4 to 7 years and 8 to 15 years.
Use of any analgesia in the prior 24 hours was slightly higher in the soft bandage group on Day 1 (83% vs 78%; P = .04) and Day 3 (57% vs 51%; P = .05), but this difference was not seen on Day 7. Satisfaction, measured via a 7-point Likert scale (range from “extremely satisfied” to “extremely unsatisfied”), was slightly lower in the soft bandage group on Day 1 (median 2 [interquartile range = 1, 2] vs median 1 [interquartile range = 1, 2]; P < .0001) but was not different after 6 weeks. There were no measured differences in any other secondary outcomes, including function, quality of life, and complication rates.
Continue to: By the primary end point...
By the primary end point of 3 days, 36 patients (7%) in the soft bandage group returned to medical care requesting a change to rigid immobilization, compared with 1 patient (0.2%) in the rigid immobilization group declining intervention.
WHAT’S NEW
Equivalence in pain and function scores
This trial showed equivalence in pain at 3 days’ follow-up in children with distal radius torus fractures who were offered bandaging and then immediately discharged from the ED, compared with rigid immobilization and clinical follow-up. There were no significant differences in pain or function between groups during the 6 weeks following the initial injury. De-escalation of treatment offers an equivalent, resource-sparing alternative to traditional treatment of these fractures.
CAVEATS
Lack of masking likely introduced bias
There are no major caveats associated with managing distal radius torus fractures with a soft bandage and discharge from the ED, compared with the traditional treatment of rigid immobilization. However, bias was likely introduced in patient-reported outcomes due to the inability to mask patients and families to the treatment allocation. This may have led to overstating the severity of outcomes in the bandage group, given the strong preference for rigid immobilization, although equivalence was illustrated despite this potential bias.
CHALLENGES TO IMPLEMENTATION
Preferences may be difficult to change
Parents and clinicians demonstrated a preference for rigid immobilization, as shown in the imbalance in treatment crossovers, with 7% of children changing to the rigid immobilization group by the primary study end point of 3 days. The study authors hypothesized that crossovers may have been due to the perception by some parents that rigid immobilization is the gold standard of treatment, as well as clinicians’ seeking to escalate care for patients returning for follow-up. Policy and guideline changes, as well as physician efforts to educate patients on outcomes with soft bandage treatment, are likely to improve these misconceptions.
1. Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
2. Patel DS, Statuta SM, Ahmed N. Common fractures of the radius and ulna. Am Fam Physician. 2021;103:345-354.
3. Asokan A, Kheir N. Pediatric Torus Buckle Fracture. StatPearls Publishing; 2023.
4. Naranje SM, Erali RA, Warner WC Jr, et al. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop. 2016;36:e45-e48. doi: 10.1097/BPO.0000000000000595
5. Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19:77-81. doi: 10.1097/BPB.0b013e32832f067a
6. Handoll HHG, Elliott J, Iheozor-Ejiofor Z, et al. Interventions for treating wrist fractures in children. Cochrane Database Syst Rev. 2018;12:CD012470. doi: 10.1002/14651858.CD012470.pub2
7. Perry DC, Gibson P, Roland D, et al. What level of immobilisation is necessary for treatment of torus (buckle) fractures of the distal radius in children? BMJ. 2021;372:m4862. doi: 10.1136/bmj.m4862
8. Williams KG, Smith G, Luhmann SJ, et al. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr Emerg Care. 2013;29:555-559. doi: 10.1097/PEC.0b013e31828e56fb
9. Jiang N, Cao ZH, Ma YF, et al. Management of pediatric forearm torus fractures: a systematic review and meta-analysis. Pediatr Emerg Care. 2016;32:773-778. doi: 10.1097/PEC.0000000000000579
10. Williams BA, Alvarado CA, Montoya-Williams DC, et al. Buckling down on torus fractures: has evolving evidence affected practice? J Child Orthop. 2018;12:123-128. doi: 10.1302/1863-2548.12.170122
11. Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010;17:50-54. doi: 10.1111/j.1553-2712.2009.00620.x
ILLUSTRATIVE CASE
A 9-year-old girl presents to your urgent care clinic after a fall while snowboarding for the first time. She reports falling forward onto her outstretched right hand and describes pain in her distal right forearm. She denies paresthesias, weakness, or lacerations. Physical examination reveals mild edema of the dorsal aspect of her distal right forearm and tenderness to palpation of the dorsal aspect of her distal radius. She denies tenderness to palpation of her ulna, anatomic snuffbox, hand, and elbow. Range of motion of the wrist is full on passive testing, but she declines active testing due to pain. Wrist radiographs reveal an uncomplicated torus fracture of the distal radius. Can immobilization with a soft bandage alone sufficiently treat this fracture?
Fractures of the distal radius are among the most common fractures of the upper extremity and commonly occur from a fall onto an outstretched hand.2 In the pediatric population, torus fractures, also known as buckle fractures, are the most common type of distal radius fracture, comprising an estimated 50% of pediatric wrist fractures.3,4 This is due to the presence of a
Pediatric torus fractures of the distal radius generally are treated with immobilization,2 traditionally through a
Despite common use of immobilization, torus fractures of the distal radius are anatomically stable, and displacement is unlikely to occur.7,8 As such, many studies have suggested that treatment of torus fractures with rigid immobilization in a cast or splint may not be necessary.9,10 However, a 2018 Cochrane review concluded that the quality of evidence illustrating similar recovery between treatments was low, leaving uncertainty as to the most appropriate management strategy.6 Less casting and follow-up imaging could have positive implications for patient satisfaction, health care–associated costs, and radiation exposure.10
This study, the Forearm Fracture Recovery in Children Evaluation (FORCE) trial, compared the traditional treatment of distal radius torus fractures with rigid immobilization to soft immobilization and immediate discharge.
STUDY SUMMARY
Providing quality evidence for a standard of care
FORCE was a randomized controlled equivalence trial (N = 965) across 23 emergency departments (EDs) in the United Kingdom that compared pain and function in pediatric patients with distal radius torus fractures treated with a soft bandage and immediate discharge vs rigid immobilization and routine follow-up.1 Patients included children ages 4 to 15 years presenting to the ED with a distal radius torus fracture, which was confirmed radiologically.
Patients with concomitant
Continue to: Patients were randomly assigned...
Patients were randomly assigned in a 1:1 ratio to receive treatment with either a soft bandage such as a gauze roller bandage (n = 489) or rigid immobilization (n = 476). For patients in the bandage group, a soft bandage was applied in the ED or provided for home application without planned clinical follow-up. Patients in the rigid immobilization group were treated in the ED with either a removable manufactured splint or a molded splint or cast, followed by the standard follow-up practice of the treating center. Patients in the soft bandage group were advised not to wear the bandage for more than 3 weeks. Blinding was not possible, but the treatment team did not take part in patient follow-up.
The primary outcome was change in pain 3 days after treatment, measured on the Wong-Baker FACES Pain Rating Scale (an ordinal assessment using 6 illustrated facial expressions translated to a numeric rating on a scale of 0-10, with higher scores indicating worse pain). This scale has an established minimum clinically important difference (MCID) value of 1 face (2 points).11 Per standard practice in equivalence trials, the equivalence margin was defined as half the MCID, with a value of 1.0 used in this study.
Secondary outcomes measured over the 6-week follow-up period included additional pain measurements using the Wong-Baker scale, measures of function and health-related quality of life, analgesia use, days of absence from school or childcare, complication rates, and patient satisfaction. This study used modified intention-to-treat and per-protocol analyses.
The mean age of participants was 9.6 years; 39% were girls and 61% were boys. In the bandage group, 94% opted to have the soft bandage applied in the ED, and 95% of the rigid immobilization group were treated with a removable wrist splint in the ED. At 3 days, pain scores improved by 3.2 points (standard deviation [SD] = 2.1) in the soft bandage group and 3.1 points (SD = 2.1) in the rigid immobilization group. The adjusted difference was –0.1 (95% CI, –0.37 to 0.17) in the intention-to-treat analysis and –0.06 (95% CI, –0.34 to 0.21) in the per-protocol analysis, which were both less than the predetermined equivalence margin. This equivalence margin also was met at all secondary time points (1 day, 7 days, 3 weeks, and 6 weeks after treatment) and in subgroup analysis of those 4 to 7 years and 8 to 15 years.
Use of any analgesia in the prior 24 hours was slightly higher in the soft bandage group on Day 1 (83% vs 78%; P = .04) and Day 3 (57% vs 51%; P = .05), but this difference was not seen on Day 7. Satisfaction, measured via a 7-point Likert scale (range from “extremely satisfied” to “extremely unsatisfied”), was slightly lower in the soft bandage group on Day 1 (median 2 [interquartile range = 1, 2] vs median 1 [interquartile range = 1, 2]; P < .0001) but was not different after 6 weeks. There were no measured differences in any other secondary outcomes, including function, quality of life, and complication rates.
Continue to: By the primary end point...
By the primary end point of 3 days, 36 patients (7%) in the soft bandage group returned to medical care requesting a change to rigid immobilization, compared with 1 patient (0.2%) in the rigid immobilization group declining intervention.
WHAT’S NEW
Equivalence in pain and function scores
This trial showed equivalence in pain at 3 days’ follow-up in children with distal radius torus fractures who were offered bandaging and then immediately discharged from the ED, compared with rigid immobilization and clinical follow-up. There were no significant differences in pain or function between groups during the 6 weeks following the initial injury. De-escalation of treatment offers an equivalent, resource-sparing alternative to traditional treatment of these fractures.
CAVEATS
Lack of masking likely introduced bias
There are no major caveats associated with managing distal radius torus fractures with a soft bandage and discharge from the ED, compared with the traditional treatment of rigid immobilization. However, bias was likely introduced in patient-reported outcomes due to the inability to mask patients and families to the treatment allocation. This may have led to overstating the severity of outcomes in the bandage group, given the strong preference for rigid immobilization, although equivalence was illustrated despite this potential bias.
CHALLENGES TO IMPLEMENTATION
Preferences may be difficult to change
Parents and clinicians demonstrated a preference for rigid immobilization, as shown in the imbalance in treatment crossovers, with 7% of children changing to the rigid immobilization group by the primary study end point of 3 days. The study authors hypothesized that crossovers may have been due to the perception by some parents that rigid immobilization is the gold standard of treatment, as well as clinicians’ seeking to escalate care for patients returning for follow-up. Policy and guideline changes, as well as physician efforts to educate patients on outcomes with soft bandage treatment, are likely to improve these misconceptions.
ILLUSTRATIVE CASE
A 9-year-old girl presents to your urgent care clinic after a fall while snowboarding for the first time. She reports falling forward onto her outstretched right hand and describes pain in her distal right forearm. She denies paresthesias, weakness, or lacerations. Physical examination reveals mild edema of the dorsal aspect of her distal right forearm and tenderness to palpation of the dorsal aspect of her distal radius. She denies tenderness to palpation of her ulna, anatomic snuffbox, hand, and elbow. Range of motion of the wrist is full on passive testing, but she declines active testing due to pain. Wrist radiographs reveal an uncomplicated torus fracture of the distal radius. Can immobilization with a soft bandage alone sufficiently treat this fracture?
Fractures of the distal radius are among the most common fractures of the upper extremity and commonly occur from a fall onto an outstretched hand.2 In the pediatric population, torus fractures, also known as buckle fractures, are the most common type of distal radius fracture, comprising an estimated 50% of pediatric wrist fractures.3,4 This is due to the presence of a
Pediatric torus fractures of the distal radius generally are treated with immobilization,2 traditionally through a
Despite common use of immobilization, torus fractures of the distal radius are anatomically stable, and displacement is unlikely to occur.7,8 As such, many studies have suggested that treatment of torus fractures with rigid immobilization in a cast or splint may not be necessary.9,10 However, a 2018 Cochrane review concluded that the quality of evidence illustrating similar recovery between treatments was low, leaving uncertainty as to the most appropriate management strategy.6 Less casting and follow-up imaging could have positive implications for patient satisfaction, health care–associated costs, and radiation exposure.10
This study, the Forearm Fracture Recovery in Children Evaluation (FORCE) trial, compared the traditional treatment of distal radius torus fractures with rigid immobilization to soft immobilization and immediate discharge.
STUDY SUMMARY
Providing quality evidence for a standard of care
FORCE was a randomized controlled equivalence trial (N = 965) across 23 emergency departments (EDs) in the United Kingdom that compared pain and function in pediatric patients with distal radius torus fractures treated with a soft bandage and immediate discharge vs rigid immobilization and routine follow-up.1 Patients included children ages 4 to 15 years presenting to the ED with a distal radius torus fracture, which was confirmed radiologically.
Patients with concomitant
Continue to: Patients were randomly assigned...
Patients were randomly assigned in a 1:1 ratio to receive treatment with either a soft bandage such as a gauze roller bandage (n = 489) or rigid immobilization (n = 476). For patients in the bandage group, a soft bandage was applied in the ED or provided for home application without planned clinical follow-up. Patients in the rigid immobilization group were treated in the ED with either a removable manufactured splint or a molded splint or cast, followed by the standard follow-up practice of the treating center. Patients in the soft bandage group were advised not to wear the bandage for more than 3 weeks. Blinding was not possible, but the treatment team did not take part in patient follow-up.
The primary outcome was change in pain 3 days after treatment, measured on the Wong-Baker FACES Pain Rating Scale (an ordinal assessment using 6 illustrated facial expressions translated to a numeric rating on a scale of 0-10, with higher scores indicating worse pain). This scale has an established minimum clinically important difference (MCID) value of 1 face (2 points).11 Per standard practice in equivalence trials, the equivalence margin was defined as half the MCID, with a value of 1.0 used in this study.
Secondary outcomes measured over the 6-week follow-up period included additional pain measurements using the Wong-Baker scale, measures of function and health-related quality of life, analgesia use, days of absence from school or childcare, complication rates, and patient satisfaction. This study used modified intention-to-treat and per-protocol analyses.
The mean age of participants was 9.6 years; 39% were girls and 61% were boys. In the bandage group, 94% opted to have the soft bandage applied in the ED, and 95% of the rigid immobilization group were treated with a removable wrist splint in the ED. At 3 days, pain scores improved by 3.2 points (standard deviation [SD] = 2.1) in the soft bandage group and 3.1 points (SD = 2.1) in the rigid immobilization group. The adjusted difference was –0.1 (95% CI, –0.37 to 0.17) in the intention-to-treat analysis and –0.06 (95% CI, –0.34 to 0.21) in the per-protocol analysis, which were both less than the predetermined equivalence margin. This equivalence margin also was met at all secondary time points (1 day, 7 days, 3 weeks, and 6 weeks after treatment) and in subgroup analysis of those 4 to 7 years and 8 to 15 years.
Use of any analgesia in the prior 24 hours was slightly higher in the soft bandage group on Day 1 (83% vs 78%; P = .04) and Day 3 (57% vs 51%; P = .05), but this difference was not seen on Day 7. Satisfaction, measured via a 7-point Likert scale (range from “extremely satisfied” to “extremely unsatisfied”), was slightly lower in the soft bandage group on Day 1 (median 2 [interquartile range = 1, 2] vs median 1 [interquartile range = 1, 2]; P < .0001) but was not different after 6 weeks. There were no measured differences in any other secondary outcomes, including function, quality of life, and complication rates.
Continue to: By the primary end point...
By the primary end point of 3 days, 36 patients (7%) in the soft bandage group returned to medical care requesting a change to rigid immobilization, compared with 1 patient (0.2%) in the rigid immobilization group declining intervention.
WHAT’S NEW
Equivalence in pain and function scores
This trial showed equivalence in pain at 3 days’ follow-up in children with distal radius torus fractures who were offered bandaging and then immediately discharged from the ED, compared with rigid immobilization and clinical follow-up. There were no significant differences in pain or function between groups during the 6 weeks following the initial injury. De-escalation of treatment offers an equivalent, resource-sparing alternative to traditional treatment of these fractures.
CAVEATS
Lack of masking likely introduced bias
There are no major caveats associated with managing distal radius torus fractures with a soft bandage and discharge from the ED, compared with the traditional treatment of rigid immobilization. However, bias was likely introduced in patient-reported outcomes due to the inability to mask patients and families to the treatment allocation. This may have led to overstating the severity of outcomes in the bandage group, given the strong preference for rigid immobilization, although equivalence was illustrated despite this potential bias.
CHALLENGES TO IMPLEMENTATION
Preferences may be difficult to change
Parents and clinicians demonstrated a preference for rigid immobilization, as shown in the imbalance in treatment crossovers, with 7% of children changing to the rigid immobilization group by the primary study end point of 3 days. The study authors hypothesized that crossovers may have been due to the perception by some parents that rigid immobilization is the gold standard of treatment, as well as clinicians’ seeking to escalate care for patients returning for follow-up. Policy and guideline changes, as well as physician efforts to educate patients on outcomes with soft bandage treatment, are likely to improve these misconceptions.
1. Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
2. Patel DS, Statuta SM, Ahmed N. Common fractures of the radius and ulna. Am Fam Physician. 2021;103:345-354.
3. Asokan A, Kheir N. Pediatric Torus Buckle Fracture. StatPearls Publishing; 2023.
4. Naranje SM, Erali RA, Warner WC Jr, et al. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop. 2016;36:e45-e48. doi: 10.1097/BPO.0000000000000595
5. Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19:77-81. doi: 10.1097/BPB.0b013e32832f067a
6. Handoll HHG, Elliott J, Iheozor-Ejiofor Z, et al. Interventions for treating wrist fractures in children. Cochrane Database Syst Rev. 2018;12:CD012470. doi: 10.1002/14651858.CD012470.pub2
7. Perry DC, Gibson P, Roland D, et al. What level of immobilisation is necessary for treatment of torus (buckle) fractures of the distal radius in children? BMJ. 2021;372:m4862. doi: 10.1136/bmj.m4862
8. Williams KG, Smith G, Luhmann SJ, et al. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr Emerg Care. 2013;29:555-559. doi: 10.1097/PEC.0b013e31828e56fb
9. Jiang N, Cao ZH, Ma YF, et al. Management of pediatric forearm torus fractures: a systematic review and meta-analysis. Pediatr Emerg Care. 2016;32:773-778. doi: 10.1097/PEC.0000000000000579
10. Williams BA, Alvarado CA, Montoya-Williams DC, et al. Buckling down on torus fractures: has evolving evidence affected practice? J Child Orthop. 2018;12:123-128. doi: 10.1302/1863-2548.12.170122
11. Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010;17:50-54. doi: 10.1111/j.1553-2712.2009.00620.x
1. Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
2. Patel DS, Statuta SM, Ahmed N. Common fractures of the radius and ulna. Am Fam Physician. 2021;103:345-354.
3. Asokan A, Kheir N. Pediatric Torus Buckle Fracture. StatPearls Publishing; 2023.
4. Naranje SM, Erali RA, Warner WC Jr, et al. Epidemiology of pediatric fractures presenting to emergency departments in the United States. J Pediatr Orthop. 2016;36:e45-e48. doi: 10.1097/BPO.0000000000000595
5. Kennedy SA, Slobogean GP, Mulpuri K. Does degree of immobilization influence refracture rate in the forearm buckle fracture? J Pediatr Orthop B. 2010;19:77-81. doi: 10.1097/BPB.0b013e32832f067a
6. Handoll HHG, Elliott J, Iheozor-Ejiofor Z, et al. Interventions for treating wrist fractures in children. Cochrane Database Syst Rev. 2018;12:CD012470. doi: 10.1002/14651858.CD012470.pub2
7. Perry DC, Gibson P, Roland D, et al. What level of immobilisation is necessary for treatment of torus (buckle) fractures of the distal radius in children? BMJ. 2021;372:m4862. doi: 10.1136/bmj.m4862
8. Williams KG, Smith G, Luhmann SJ, et al. A randomized controlled trial of cast versus splint for distal radial buckle fracture: an evaluation of satisfaction, convenience, and preference. Pediatr Emerg Care. 2013;29:555-559. doi: 10.1097/PEC.0b013e31828e56fb
9. Jiang N, Cao ZH, Ma YF, et al. Management of pediatric forearm torus fractures: a systematic review and meta-analysis. Pediatr Emerg Care. 2016;32:773-778. doi: 10.1097/PEC.0000000000000579
10. Williams BA, Alvarado CA, Montoya-Williams DC, et al. Buckling down on torus fractures: has evolving evidence affected practice? J Child Orthop. 2018;12:123-128. doi: 10.1302/1863-2548.12.170122
11. Garra G, Singer AJ, Taira BR, et al. Validation of the Wong-Baker FACES Pain Rating Scale in pediatric emergency department patients. Acad Emerg Med. 2010;17:50-54. doi: 10.1111/j.1553-2712.2009.00620.x
PRACTICE CHANGER
For uncomplicated pediatric torus fractures of the distal radius, consider definitive management with soft bandage immobilization until pain resolution, rather than rigid immobilization and clinical follow-up.
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial with patient-oriented outcomes.1
Perry DC, Achten J, Knight R, et al; FORCE Collaborators in collaboration with PERUKI. Immobilisation of torus fractures of the wrist in children (FORCE): a randomised controlled equivalence trial in the UK. Lancet. 2022;400:39-47. doi: 10.1016/S0140-6736(22)01015-7
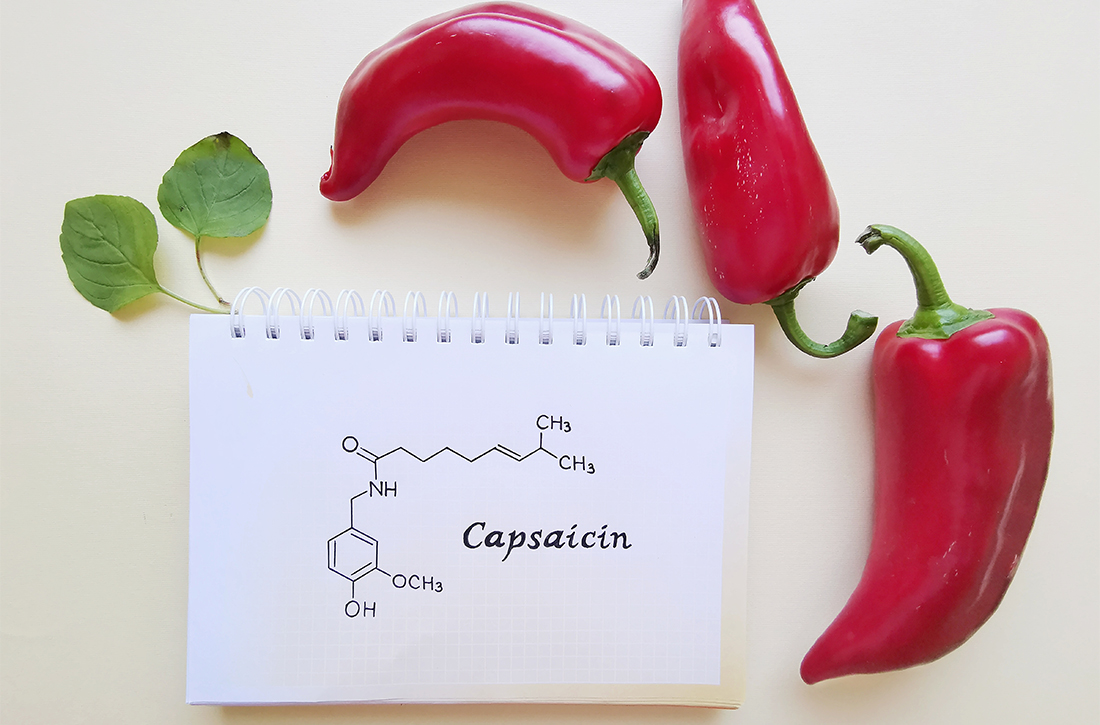
Time to consider topical capsaicin for acute trauma pain?
ILLUSTRATIVE CASE
A 23-year-old man with no significant past medical history presents to an urgent care center after a fall on his right arm while playing football. He reports a pain level of 6 using the visual analog scale (VAS). Physical exam reveals minor erythema and edema of his forearm with pain to palpation. Range of motion, strength, and sensation are intact. No lacerations are present. His vital signs are normal. No fracture is found on imaging. The physician decides that treatment with a topical analgesic is reasonable for this uncomplicated contusion of the right forearm. Is there a role for topical capsaicin in the treatment of this patient’s pain?
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) are effective for the treatment of acute non–low back pain musculoskeletal injuries.2 They are generally well tolerated and just as effective as oral NSAIDS or acetaminophen for localized injuries. Their ubiquitous availability, affordability, and low adverse effect profile make them an attractive first-line treatment option for acute musculoskeletal pain.
Capsaicin, a topical agent derived from a genus of red peppers, has been used for the treatment of neuropathic and chronic pain via its interactions with substance P, transient receptor potential vanilloid subtype 1 (TRPV1), and nociceptive nerve fibers.3,4 It has demonstrated effectiveness in the management of diabetic neuropathy, knee osteoarthritis, and postherpetic neuralgia, as well as various causes of pruritus.5,6
Although many studies have compared oral and topical NSAIDs, opiates, and acetaminophen, few studies have directly compared topical NSAIDs and capsaicin. This study compared the topical NSAID piroxicam with topical capsaicin.
STUDY SUMMARY
Topical capsaicin demonstrated superior pain reduction
This prospective, double-blind RCT compared the efficacy of topical capsaicin vs topical piroxicam for the treatment of acute pain following upper extremity blunt trauma. Patients (ages ≥ 18 years) who presented to a Turkish emergency department within 2 hours of upper extremity injury were randomized to receive either 0.05% capsaicin gel (n = 69) or 0.5% piroxicam gel (n = 67). Patients reported level 5 or higher pain on the VAS. Those with fractures, dislocations, skin disruption, or other trauma were excluded. Age, gender, pain duration, and mechanism of injury did not differ significantly between study groups.1
Blinding was ensured by placing the gels in opaque containers containing 30 mg of either capsaicin or piroxicam and dyeing the medicine with red and yellow food coloring. A thin layer of medication was applied to an area no larger than 5 × 5 cm on the upper extremity and rubbed for 1 minute. Patients were observed in the emergency department for 2 hours and discharged with instructions to apply the medication 3 times daily for 72 hours.
The investigators measured pain using VAS scores at 1 hour, 2 hours, 24 hours, and 72 hours after treatment. Topical capsaicin was superior to topical piroxicam at achieving both primary outcomes: a VAS score of ≤ 4 (85.5% vs 50.7%; number needed to treat [NNT] = 2.9; P < .001) and a > 50% reduction in VAS score (87% vs 62.7%; NNT = 4.1; P < .01) at the end of treatment.1 (These outcomes were based on earlier determinations of the minimal clinically important difference.7,8)
Additionally, capsaicin was more effective than piroxicam at each time interval. This difference was most pronounced at 72 hours, with a mean difference of delta VAS scores of 1.53 (95% CI, 0.85-2.221) and a mean percentage of the reduction in VAS scores of 19.7% (95% CI, 12.4%-27.2%) (P < .001).1
Reported adverse effects, such as burning, itching, and rash, were mild and infrequent and showed no significant difference between the treatment groups.
WHAT’S NEW
First study comparing topical capsaicin and a topical NSAID in acute trauma
Although both capsaicin and topical piroxicam have proven efficacy for the treatment of pain, this RCT is the first study to directly compare these agents in the setting of acute upper extremity blunt trauma. Capsaicin is currently more commonly prescribed as a treatment for chronic neuropathic pain.4,9 In this study, capsaicin demonstrated superior results in pain reduction at each assessed time interval and at the primary end point of 72 hours.
CAVEATS
Limited generalizability to lower extremity and truncal trauma
This RCT included a relatively small sample size (136 patients). Researchers evaluated only blunt upper extremity injuries; as such, the generalizability of the effectiveness of topical capsaicin in blunt lower extremity and truncal trauma is limited, especially over larger surface areas.
CHALLENGES TO IMPLEMENTATION
No major challenges found
There are no major challenges to implementing this inexpensive treatment.
1. Kocak AO, Dogruyol S, Akbas I, et al. Comparison of topical capsaicin and topical piroxicam in the treatment of acute trauma-induced pain: a randomized double-blind trial. Am J Emerg Med. 2020;38:1767-1771. doi: 10.1016/j.ajem.2020.05.104
2. Busse JW, Sadeghirad B, Oparin Y, et al. Management of acute pain from non–low back, musculoskeletal injuries: a systematic review and network meta-analysis of randomized trials. Ann Intern Med. 2020;173:730-738. doi: 10.7326/M19-3601
3. Chrubasik S, Weiser T, Beime B. Effectiveness and safety of topical capsaicin cream in the treatment of chronic soft tissue pain. Phytother Res. 2010;24:1877-1885. doi: 10.1002/ptr.3335
4. Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2012(9):CD010111. doi: 10.1002/14651858.CD010111
5. Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18:42-53. doi: 10.1016/j.jpain.2016.09.008
6. Papoiu ADP, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi: 10.1517/14656566.2010.481670
7. Kulkantrakorn K, Lorsuwansiri C, Meesawatsom P. 0.025% capsaicin gel for the treatment of painful diabetic neuropathy: a randomized, double-blind, crossover, placebo-controlled trial. Pain Pract. 2013;13:497-503. doi: 10.1111/papr.12013
8. Kocak AO, Ahiskalioglu A, Sengun E, et al. Comparison of intravenous NSAIDs and trigger point injection for low back pain in ED: a prospective randomized study. Am J Emerg Med. 2019;37:1927-1931. doi: 10.1016/j.ajem.2019.01.015
9. Derry S, Rice ASC, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;1(1):CD007393. doi: 10.1002/14651858.CD007393.pub4
ILLUSTRATIVE CASE
A 23-year-old man with no significant past medical history presents to an urgent care center after a fall on his right arm while playing football. He reports a pain level of 6 using the visual analog scale (VAS). Physical exam reveals minor erythema and edema of his forearm with pain to palpation. Range of motion, strength, and sensation are intact. No lacerations are present. His vital signs are normal. No fracture is found on imaging. The physician decides that treatment with a topical analgesic is reasonable for this uncomplicated contusion of the right forearm. Is there a role for topical capsaicin in the treatment of this patient’s pain?
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) are effective for the treatment of acute non–low back pain musculoskeletal injuries.2 They are generally well tolerated and just as effective as oral NSAIDS or acetaminophen for localized injuries. Their ubiquitous availability, affordability, and low adverse effect profile make them an attractive first-line treatment option for acute musculoskeletal pain.
Capsaicin, a topical agent derived from a genus of red peppers, has been used for the treatment of neuropathic and chronic pain via its interactions with substance P, transient receptor potential vanilloid subtype 1 (TRPV1), and nociceptive nerve fibers.3,4 It has demonstrated effectiveness in the management of diabetic neuropathy, knee osteoarthritis, and postherpetic neuralgia, as well as various causes of pruritus.5,6
Although many studies have compared oral and topical NSAIDs, opiates, and acetaminophen, few studies have directly compared topical NSAIDs and capsaicin. This study compared the topical NSAID piroxicam with topical capsaicin.
STUDY SUMMARY
Topical capsaicin demonstrated superior pain reduction
This prospective, double-blind RCT compared the efficacy of topical capsaicin vs topical piroxicam for the treatment of acute pain following upper extremity blunt trauma. Patients (ages ≥ 18 years) who presented to a Turkish emergency department within 2 hours of upper extremity injury were randomized to receive either 0.05% capsaicin gel (n = 69) or 0.5% piroxicam gel (n = 67). Patients reported level 5 or higher pain on the VAS. Those with fractures, dislocations, skin disruption, or other trauma were excluded. Age, gender, pain duration, and mechanism of injury did not differ significantly between study groups.1
Blinding was ensured by placing the gels in opaque containers containing 30 mg of either capsaicin or piroxicam and dyeing the medicine with red and yellow food coloring. A thin layer of medication was applied to an area no larger than 5 × 5 cm on the upper extremity and rubbed for 1 minute. Patients were observed in the emergency department for 2 hours and discharged with instructions to apply the medication 3 times daily for 72 hours.
The investigators measured pain using VAS scores at 1 hour, 2 hours, 24 hours, and 72 hours after treatment. Topical capsaicin was superior to topical piroxicam at achieving both primary outcomes: a VAS score of ≤ 4 (85.5% vs 50.7%; number needed to treat [NNT] = 2.9; P < .001) and a > 50% reduction in VAS score (87% vs 62.7%; NNT = 4.1; P < .01) at the end of treatment.1 (These outcomes were based on earlier determinations of the minimal clinically important difference.7,8)
Additionally, capsaicin was more effective than piroxicam at each time interval. This difference was most pronounced at 72 hours, with a mean difference of delta VAS scores of 1.53 (95% CI, 0.85-2.221) and a mean percentage of the reduction in VAS scores of 19.7% (95% CI, 12.4%-27.2%) (P < .001).1
Reported adverse effects, such as burning, itching, and rash, were mild and infrequent and showed no significant difference between the treatment groups.
WHAT’S NEW
First study comparing topical capsaicin and a topical NSAID in acute trauma
Although both capsaicin and topical piroxicam have proven efficacy for the treatment of pain, this RCT is the first study to directly compare these agents in the setting of acute upper extremity blunt trauma. Capsaicin is currently more commonly prescribed as a treatment for chronic neuropathic pain.4,9 In this study, capsaicin demonstrated superior results in pain reduction at each assessed time interval and at the primary end point of 72 hours.
CAVEATS
Limited generalizability to lower extremity and truncal trauma
This RCT included a relatively small sample size (136 patients). Researchers evaluated only blunt upper extremity injuries; as such, the generalizability of the effectiveness of topical capsaicin in blunt lower extremity and truncal trauma is limited, especially over larger surface areas.
CHALLENGES TO IMPLEMENTATION
No major challenges found
There are no major challenges to implementing this inexpensive treatment.
ILLUSTRATIVE CASE
A 23-year-old man with no significant past medical history presents to an urgent care center after a fall on his right arm while playing football. He reports a pain level of 6 using the visual analog scale (VAS). Physical exam reveals minor erythema and edema of his forearm with pain to palpation. Range of motion, strength, and sensation are intact. No lacerations are present. His vital signs are normal. No fracture is found on imaging. The physician decides that treatment with a topical analgesic is reasonable for this uncomplicated contusion of the right forearm. Is there a role for topical capsaicin in the treatment of this patient’s pain?
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) are effective for the treatment of acute non–low back pain musculoskeletal injuries.2 They are generally well tolerated and just as effective as oral NSAIDS or acetaminophen for localized injuries. Their ubiquitous availability, affordability, and low adverse effect profile make them an attractive first-line treatment option for acute musculoskeletal pain.
Capsaicin, a topical agent derived from a genus of red peppers, has been used for the treatment of neuropathic and chronic pain via its interactions with substance P, transient receptor potential vanilloid subtype 1 (TRPV1), and nociceptive nerve fibers.3,4 It has demonstrated effectiveness in the management of diabetic neuropathy, knee osteoarthritis, and postherpetic neuralgia, as well as various causes of pruritus.5,6
Although many studies have compared oral and topical NSAIDs, opiates, and acetaminophen, few studies have directly compared topical NSAIDs and capsaicin. This study compared the topical NSAID piroxicam with topical capsaicin.
STUDY SUMMARY
Topical capsaicin demonstrated superior pain reduction
This prospective, double-blind RCT compared the efficacy of topical capsaicin vs topical piroxicam for the treatment of acute pain following upper extremity blunt trauma. Patients (ages ≥ 18 years) who presented to a Turkish emergency department within 2 hours of upper extremity injury were randomized to receive either 0.05% capsaicin gel (n = 69) or 0.5% piroxicam gel (n = 67). Patients reported level 5 or higher pain on the VAS. Those with fractures, dislocations, skin disruption, or other trauma were excluded. Age, gender, pain duration, and mechanism of injury did not differ significantly between study groups.1
Blinding was ensured by placing the gels in opaque containers containing 30 mg of either capsaicin or piroxicam and dyeing the medicine with red and yellow food coloring. A thin layer of medication was applied to an area no larger than 5 × 5 cm on the upper extremity and rubbed for 1 minute. Patients were observed in the emergency department for 2 hours and discharged with instructions to apply the medication 3 times daily for 72 hours.
The investigators measured pain using VAS scores at 1 hour, 2 hours, 24 hours, and 72 hours after treatment. Topical capsaicin was superior to topical piroxicam at achieving both primary outcomes: a VAS score of ≤ 4 (85.5% vs 50.7%; number needed to treat [NNT] = 2.9; P < .001) and a > 50% reduction in VAS score (87% vs 62.7%; NNT = 4.1; P < .01) at the end of treatment.1 (These outcomes were based on earlier determinations of the minimal clinically important difference.7,8)
Additionally, capsaicin was more effective than piroxicam at each time interval. This difference was most pronounced at 72 hours, with a mean difference of delta VAS scores of 1.53 (95% CI, 0.85-2.221) and a mean percentage of the reduction in VAS scores of 19.7% (95% CI, 12.4%-27.2%) (P < .001).1
Reported adverse effects, such as burning, itching, and rash, were mild and infrequent and showed no significant difference between the treatment groups.
WHAT’S NEW
First study comparing topical capsaicin and a topical NSAID in acute trauma
Although both capsaicin and topical piroxicam have proven efficacy for the treatment of pain, this RCT is the first study to directly compare these agents in the setting of acute upper extremity blunt trauma. Capsaicin is currently more commonly prescribed as a treatment for chronic neuropathic pain.4,9 In this study, capsaicin demonstrated superior results in pain reduction at each assessed time interval and at the primary end point of 72 hours.
CAVEATS
Limited generalizability to lower extremity and truncal trauma
This RCT included a relatively small sample size (136 patients). Researchers evaluated only blunt upper extremity injuries; as such, the generalizability of the effectiveness of topical capsaicin in blunt lower extremity and truncal trauma is limited, especially over larger surface areas.
CHALLENGES TO IMPLEMENTATION
No major challenges found
There are no major challenges to implementing this inexpensive treatment.
1. Kocak AO, Dogruyol S, Akbas I, et al. Comparison of topical capsaicin and topical piroxicam in the treatment of acute trauma-induced pain: a randomized double-blind trial. Am J Emerg Med. 2020;38:1767-1771. doi: 10.1016/j.ajem.2020.05.104
2. Busse JW, Sadeghirad B, Oparin Y, et al. Management of acute pain from non–low back, musculoskeletal injuries: a systematic review and network meta-analysis of randomized trials. Ann Intern Med. 2020;173:730-738. doi: 10.7326/M19-3601
3. Chrubasik S, Weiser T, Beime B. Effectiveness and safety of topical capsaicin cream in the treatment of chronic soft tissue pain. Phytother Res. 2010;24:1877-1885. doi: 10.1002/ptr.3335
4. Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2012(9):CD010111. doi: 10.1002/14651858.CD010111
5. Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18:42-53. doi: 10.1016/j.jpain.2016.09.008
6. Papoiu ADP, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi: 10.1517/14656566.2010.481670
7. Kulkantrakorn K, Lorsuwansiri C, Meesawatsom P. 0.025% capsaicin gel for the treatment of painful diabetic neuropathy: a randomized, double-blind, crossover, placebo-controlled trial. Pain Pract. 2013;13:497-503. doi: 10.1111/papr.12013
8. Kocak AO, Ahiskalioglu A, Sengun E, et al. Comparison of intravenous NSAIDs and trigger point injection for low back pain in ED: a prospective randomized study. Am J Emerg Med. 2019;37:1927-1931. doi: 10.1016/j.ajem.2019.01.015
9. Derry S, Rice ASC, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;1(1):CD007393. doi: 10.1002/14651858.CD007393.pub4
1. Kocak AO, Dogruyol S, Akbas I, et al. Comparison of topical capsaicin and topical piroxicam in the treatment of acute trauma-induced pain: a randomized double-blind trial. Am J Emerg Med. 2020;38:1767-1771. doi: 10.1016/j.ajem.2020.05.104
2. Busse JW, Sadeghirad B, Oparin Y, et al. Management of acute pain from non–low back, musculoskeletal injuries: a systematic review and network meta-analysis of randomized trials. Ann Intern Med. 2020;173:730-738. doi: 10.7326/M19-3601
3. Chrubasik S, Weiser T, Beime B. Effectiveness and safety of topical capsaicin cream in the treatment of chronic soft tissue pain. Phytother Res. 2010;24:1877-1885. doi: 10.1002/ptr.3335
4. Derry S, Moore RA. Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2012(9):CD010111. doi: 10.1002/14651858.CD010111
5. Simpson DM, Robinson-Papp J, Van J, et al. Capsaicin 8% patch in painful diabetic peripheral neuropathy: a randomized, double-blind, placebo-controlled study. J Pain. 2017;18:42-53. doi: 10.1016/j.jpain.2016.09.008
6. Papoiu ADP, Yosipovitch G. Topical capsaicin. The fire of a ‘hot’ medicine is reignited. Expert Opin Pharmacother. 2010;11:1359-1371. doi: 10.1517/14656566.2010.481670
7. Kulkantrakorn K, Lorsuwansiri C, Meesawatsom P. 0.025% capsaicin gel for the treatment of painful diabetic neuropathy: a randomized, double-blind, crossover, placebo-controlled trial. Pain Pract. 2013;13:497-503. doi: 10.1111/papr.12013
8. Kocak AO, Ahiskalioglu A, Sengun E, et al. Comparison of intravenous NSAIDs and trigger point injection for low back pain in ED: a prospective randomized study. Am J Emerg Med. 2019;37:1927-1931. doi: 10.1016/j.ajem.2019.01.015
9. Derry S, Rice ASC, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2017;1(1):CD007393. doi: 10.1002/14651858.CD007393.pub4
PRACTICE CHANGER
Use topical capsaicin gel 0.05% for pain reduction in patients with isolated blunt injuries of the upper extremity without fracture.
STRENGTH OF RECOMMENDATION
B: Based on a single randomized controlled trial (RCT)1
Kocak AO, Dogruyol S, Akbas I, et al. Comparison of topical capsaicin and topical piroxicam in the treatment of acute trauma-induced pain: a randomized double-blind trial. Am J Emerg Med. 2020;38:1767-1771.