User login
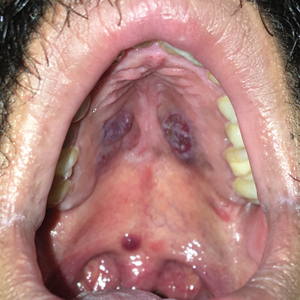
Violaceous Nodules on the Hard Palate
The Diagnosis: Kaposi Sarcoma
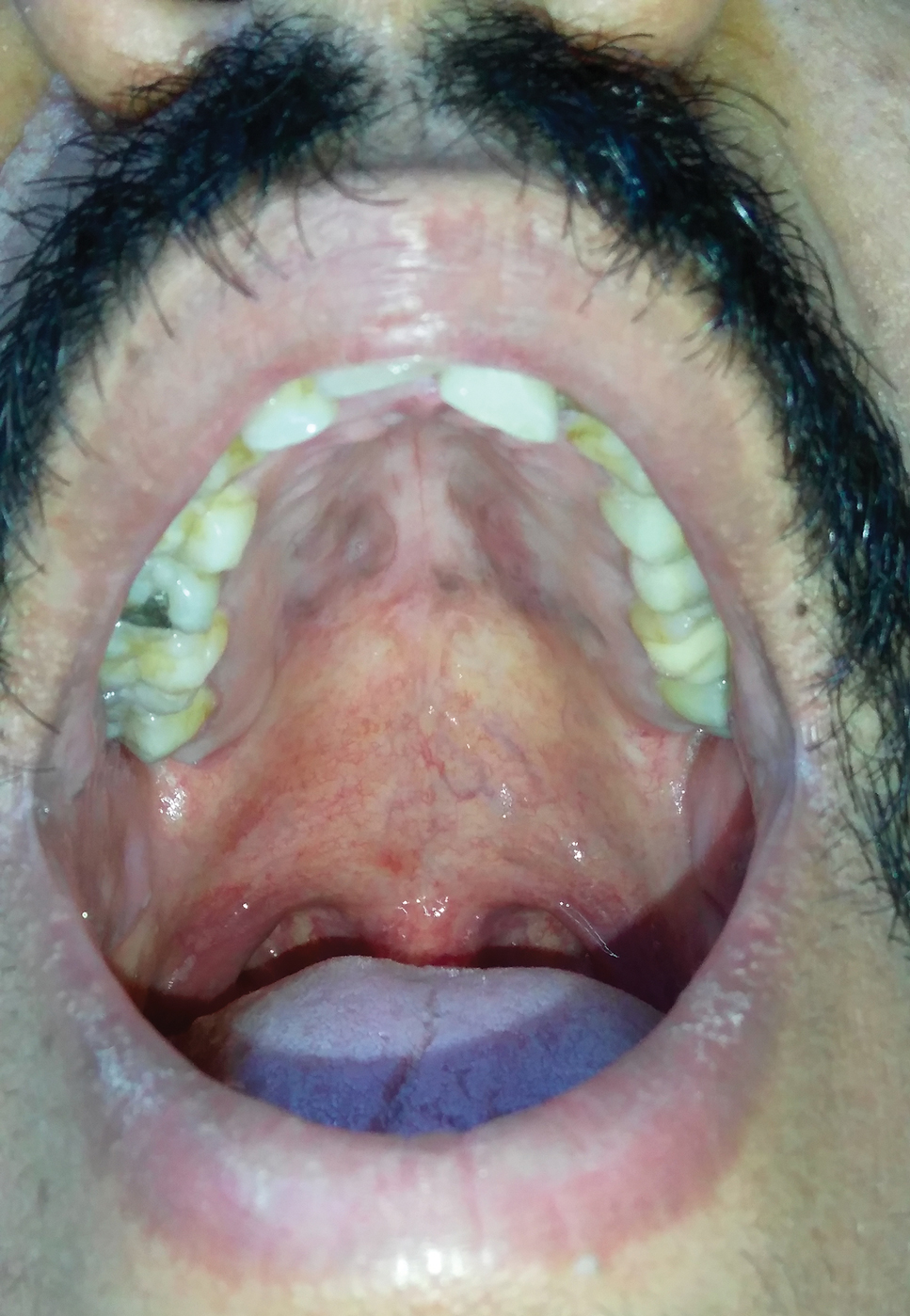
A 4-mm punch biopsy from the border of an ulcerated nodular lesion on the hard palate demonstrated diffusely distributed spindle cells, cleftlike microvascularity with extravasated erythrocytes, and widespread human herpesvirus 8 immunoreactivity on histopathology (Figure 1). Serologic tests were positive for human immunodeficiency virus (HIV) infection; HIV RNA was 14,584 IU/mL and the CD4 count was 254/mm3. The patient was diagnosed with Kaposi sarcoma (KS) and referred to the infectious disease department for initiation of antiviral therapy. Marked regression was detected after 6 months of highly active antiretroviral therapy (HAART) without any additional treatment (Figure 2).
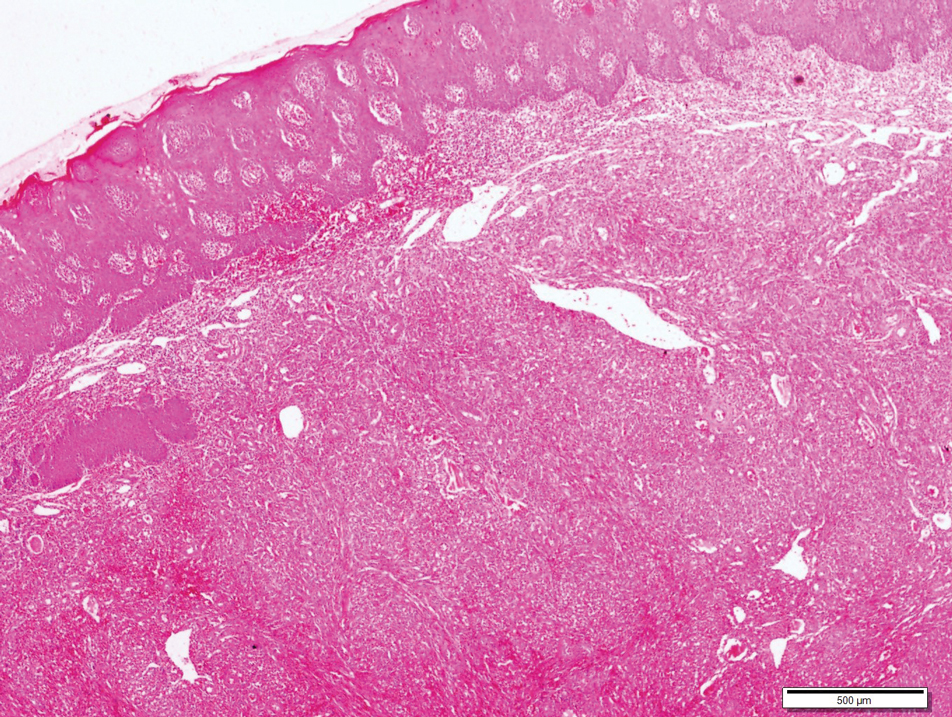
widespread human herpesvirus 8 immunoreactivity (H&E, original magnification ×4).
Kaposi sarcoma is a human herpesvirus 8-associated angioproliferative disorder with low-grade malignant potential. There are 4 well-known clinical types: classic, endemic, iatrogenic, and AIDS associated.1 Involvement of the oral cavity may be seen in all types but mostly is associated with the AIDS-associated type, which also could be a signal for undiagnosed asymptomatic HIV infection.2 Oral KS most often affects the hard and soft palate, gingiva, and dorsal tongue, with plaques or tumors ranging from nonpigmented to brownish red or violaceous. AIDS-associated KS is known to be related to cytokine expression, which is induced by HIV infection causing immune dysregulation by altering the expression of cytokines, including IL-1, tumor necrosis factor α, and IL-6.1 An in vitro study showed that cytokines secrete a number of angiogenic growth factors that, along with HIV proteins, induce and proliferate cells to become sarcoma cells. Integrins and the apoptosis process also are important in proliferation and neovascularization of KS tumor cells.3
Bacillary angiomatosis (BA) is a rare manifestation of infection caused by Bartonella species, which leads to vasoproliferative lesions of the skin and other organs. Bacillary angiomatosis affects individuals with advanced HIV or other immunocompromised individuals and may clinically mimic KS, which is similarly characterized by red-purple papules, nodules, or plaques. Differentiating BA from KS largely depends on histopathologic examination, with BA demonstrating protuberant endothelial cells surrounded by clumps of bacilli that are visible on Warthin-Starry silver stain.
Lymphangioma is a benign hamartomatous hyperplasia of the lymphatic vessels. The majority of lymphangiomas are superficial, but a few may extend deeply into the connective tissue. Intraoral lymphangiomas occur more frequently on the dorsum of the tongue, followed by the palate, buccal mucosa, gingiva, and lips. They may be differentiated with their soft quality, pebblelike surface, and translucent vesicles.
Malignant tumors of the oral cavity are rare, representing only 5% of tumors occurring in the body.4 Among malignant tumors of the oral cavity, squamous cell carcinomas are the most frequent type (90%-98%), and lymphomas and melanoma are the most outstanding among the remaining 2% to 10%. Both for lymphoma and mucosal melanoma, the most common sites of involvement are the soft tissues of the oral cavity, palatal mucosa, gingiva, tongue, cheeks, floor of the mouth, and lips.4 Although mucosal melanoma lesions usually are characterized by pigmented and ulcerated lesions, amelanotic variants also should be kept in mind. Histopathologic examination is mandatory for diagnosis.
Intralesional chemotherapy with vinblastine or bleomycin, radiotherapy, electrochemotherapy, systemic antiretroviral therapy (ie, HAART), and chemotherapy with daunorubicin and pegylated liposomal doxorubicin are the main treatment options.5,6 The immune system activator role of HAART leads to an increased CD4 count and reduces HIV proteins, which helps induction of the proliferation and neovascularization of KS tumor cells.3 This effect may help resolution of KS with localized involvement and allows physicians to utilize HAART without any other additional local and systemic chemotherapy treatment.
- Fatahzadeh M, Schwartz RA. Oral Kaposi's sarcoma: a review and update. Int J Dermatol. 2013;52:666-672.
- Martorano LM, Cannella JD, Lloyd JR. Mucocutaneous presentation of Kaposi sarcoma in an asymptomatic human immunodeficiency virus-positive man. Cutis. 2015;95:E19-E22.
- Stebbing J, Portsmouth S, Gazzard B. How does HAART lead to the resolution of Kaposi's sarcoma? J Antimicrobial Chemother. 2003;51:1095-1098.
- Guevara-Canales JO, Morales-Vadillo R, Sacsaquispe-Contreras SJ, et al. Malignant lymphoma of the oral cavity and the maxillofacial region: overall survivalprognostic factors. Med Oral Patol Oral Cir Bucal. 2013;18:E619-E626.
- Donato V, Guarnaccia R, Dognini J, et al. Radiation therapy in the treatment of HIV-related Kaposi's sarcoma. Anticancer Res. 2013;33:2153-2157.
- Gbabe OF, Okwundu CI, Dedicoat M, et al. Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults. Cochrane Database Syst Rev. 2014:CD003256.
The Diagnosis: Kaposi Sarcoma
A 4-mm punch biopsy from the border of an ulcerated nodular lesion on the hard palate demonstrated diffusely distributed spindle cells, cleftlike microvascularity with extravasated erythrocytes, and widespread human herpesvirus 8 immunoreactivity on histopathology (Figure 1). Serologic tests were positive for human immunodeficiency virus (HIV) infection; HIV RNA was 14,584 IU/mL and the CD4 count was 254/mm3. The patient was diagnosed with Kaposi sarcoma (KS) and referred to the infectious disease department for initiation of antiviral therapy. Marked regression was detected after 6 months of highly active antiretroviral therapy (HAART) without any additional treatment (Figure 2).

widespread human herpesvirus 8 immunoreactivity (H&E, original magnification ×4).

Kaposi sarcoma is a human herpesvirus 8-associated angioproliferative disorder with low-grade malignant potential. There are 4 well-known clinical types: classic, endemic, iatrogenic, and AIDS associated.1 Involvement of the oral cavity may be seen in all types but mostly is associated with the AIDS-associated type, which also could be a signal for undiagnosed asymptomatic HIV infection.2 Oral KS most often affects the hard and soft palate, gingiva, and dorsal tongue, with plaques or tumors ranging from nonpigmented to brownish red or violaceous. AIDS-associated KS is known to be related to cytokine expression, which is induced by HIV infection causing immune dysregulation by altering the expression of cytokines, including IL-1, tumor necrosis factor α, and IL-6.1 An in vitro study showed that cytokines secrete a number of angiogenic growth factors that, along with HIV proteins, induce and proliferate cells to become sarcoma cells. Integrins and the apoptosis process also are important in proliferation and neovascularization of KS tumor cells.3
Bacillary angiomatosis (BA) is a rare manifestation of infection caused by Bartonella species, which leads to vasoproliferative lesions of the skin and other organs. Bacillary angiomatosis affects individuals with advanced HIV or other immunocompromised individuals and may clinically mimic KS, which is similarly characterized by red-purple papules, nodules, or plaques. Differentiating BA from KS largely depends on histopathologic examination, with BA demonstrating protuberant endothelial cells surrounded by clumps of bacilli that are visible on Warthin-Starry silver stain.
Lymphangioma is a benign hamartomatous hyperplasia of the lymphatic vessels. The majority of lymphangiomas are superficial, but a few may extend deeply into the connective tissue. Intraoral lymphangiomas occur more frequently on the dorsum of the tongue, followed by the palate, buccal mucosa, gingiva, and lips. They may be differentiated with their soft quality, pebblelike surface, and translucent vesicles.
Malignant tumors of the oral cavity are rare, representing only 5% of tumors occurring in the body.4 Among malignant tumors of the oral cavity, squamous cell carcinomas are the most frequent type (90%-98%), and lymphomas and melanoma are the most outstanding among the remaining 2% to 10%. Both for lymphoma and mucosal melanoma, the most common sites of involvement are the soft tissues of the oral cavity, palatal mucosa, gingiva, tongue, cheeks, floor of the mouth, and lips.4 Although mucosal melanoma lesions usually are characterized by pigmented and ulcerated lesions, amelanotic variants also should be kept in mind. Histopathologic examination is mandatory for diagnosis.
Intralesional chemotherapy with vinblastine or bleomycin, radiotherapy, electrochemotherapy, systemic antiretroviral therapy (ie, HAART), and chemotherapy with daunorubicin and pegylated liposomal doxorubicin are the main treatment options.5,6 The immune system activator role of HAART leads to an increased CD4 count and reduces HIV proteins, which helps induction of the proliferation and neovascularization of KS tumor cells.3 This effect may help resolution of KS with localized involvement and allows physicians to utilize HAART without any other additional local and systemic chemotherapy treatment.
The Diagnosis: Kaposi Sarcoma
A 4-mm punch biopsy from the border of an ulcerated nodular lesion on the hard palate demonstrated diffusely distributed spindle cells, cleftlike microvascularity with extravasated erythrocytes, and widespread human herpesvirus 8 immunoreactivity on histopathology (Figure 1). Serologic tests were positive for human immunodeficiency virus (HIV) infection; HIV RNA was 14,584 IU/mL and the CD4 count was 254/mm3. The patient was diagnosed with Kaposi sarcoma (KS) and referred to the infectious disease department for initiation of antiviral therapy. Marked regression was detected after 6 months of highly active antiretroviral therapy (HAART) without any additional treatment (Figure 2).

widespread human herpesvirus 8 immunoreactivity (H&E, original magnification ×4).

Kaposi sarcoma is a human herpesvirus 8-associated angioproliferative disorder with low-grade malignant potential. There are 4 well-known clinical types: classic, endemic, iatrogenic, and AIDS associated.1 Involvement of the oral cavity may be seen in all types but mostly is associated with the AIDS-associated type, which also could be a signal for undiagnosed asymptomatic HIV infection.2 Oral KS most often affects the hard and soft palate, gingiva, and dorsal tongue, with plaques or tumors ranging from nonpigmented to brownish red or violaceous. AIDS-associated KS is known to be related to cytokine expression, which is induced by HIV infection causing immune dysregulation by altering the expression of cytokines, including IL-1, tumor necrosis factor α, and IL-6.1 An in vitro study showed that cytokines secrete a number of angiogenic growth factors that, along with HIV proteins, induce and proliferate cells to become sarcoma cells. Integrins and the apoptosis process also are important in proliferation and neovascularization of KS tumor cells.3
Bacillary angiomatosis (BA) is a rare manifestation of infection caused by Bartonella species, which leads to vasoproliferative lesions of the skin and other organs. Bacillary angiomatosis affects individuals with advanced HIV or other immunocompromised individuals and may clinically mimic KS, which is similarly characterized by red-purple papules, nodules, or plaques. Differentiating BA from KS largely depends on histopathologic examination, with BA demonstrating protuberant endothelial cells surrounded by clumps of bacilli that are visible on Warthin-Starry silver stain.
Lymphangioma is a benign hamartomatous hyperplasia of the lymphatic vessels. The majority of lymphangiomas are superficial, but a few may extend deeply into the connective tissue. Intraoral lymphangiomas occur more frequently on the dorsum of the tongue, followed by the palate, buccal mucosa, gingiva, and lips. They may be differentiated with their soft quality, pebblelike surface, and translucent vesicles.
Malignant tumors of the oral cavity are rare, representing only 5% of tumors occurring in the body.4 Among malignant tumors of the oral cavity, squamous cell carcinomas are the most frequent type (90%-98%), and lymphomas and melanoma are the most outstanding among the remaining 2% to 10%. Both for lymphoma and mucosal melanoma, the most common sites of involvement are the soft tissues of the oral cavity, palatal mucosa, gingiva, tongue, cheeks, floor of the mouth, and lips.4 Although mucosal melanoma lesions usually are characterized by pigmented and ulcerated lesions, amelanotic variants also should be kept in mind. Histopathologic examination is mandatory for diagnosis.
Intralesional chemotherapy with vinblastine or bleomycin, radiotherapy, electrochemotherapy, systemic antiretroviral therapy (ie, HAART), and chemotherapy with daunorubicin and pegylated liposomal doxorubicin are the main treatment options.5,6 The immune system activator role of HAART leads to an increased CD4 count and reduces HIV proteins, which helps induction of the proliferation and neovascularization of KS tumor cells.3 This effect may help resolution of KS with localized involvement and allows physicians to utilize HAART without any other additional local and systemic chemotherapy treatment.
- Fatahzadeh M, Schwartz RA. Oral Kaposi's sarcoma: a review and update. Int J Dermatol. 2013;52:666-672.
- Martorano LM, Cannella JD, Lloyd JR. Mucocutaneous presentation of Kaposi sarcoma in an asymptomatic human immunodeficiency virus-positive man. Cutis. 2015;95:E19-E22.
- Stebbing J, Portsmouth S, Gazzard B. How does HAART lead to the resolution of Kaposi's sarcoma? J Antimicrobial Chemother. 2003;51:1095-1098.
- Guevara-Canales JO, Morales-Vadillo R, Sacsaquispe-Contreras SJ, et al. Malignant lymphoma of the oral cavity and the maxillofacial region: overall survivalprognostic factors. Med Oral Patol Oral Cir Bucal. 2013;18:E619-E626.
- Donato V, Guarnaccia R, Dognini J, et al. Radiation therapy in the treatment of HIV-related Kaposi's sarcoma. Anticancer Res. 2013;33:2153-2157.
- Gbabe OF, Okwundu CI, Dedicoat M, et al. Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults. Cochrane Database Syst Rev. 2014:CD003256.
- Fatahzadeh M, Schwartz RA. Oral Kaposi's sarcoma: a review and update. Int J Dermatol. 2013;52:666-672.
- Martorano LM, Cannella JD, Lloyd JR. Mucocutaneous presentation of Kaposi sarcoma in an asymptomatic human immunodeficiency virus-positive man. Cutis. 2015;95:E19-E22.
- Stebbing J, Portsmouth S, Gazzard B. How does HAART lead to the resolution of Kaposi's sarcoma? J Antimicrobial Chemother. 2003;51:1095-1098.
- Guevara-Canales JO, Morales-Vadillo R, Sacsaquispe-Contreras SJ, et al. Malignant lymphoma of the oral cavity and the maxillofacial region: overall survivalprognostic factors. Med Oral Patol Oral Cir Bucal. 2013;18:E619-E626.
- Donato V, Guarnaccia R, Dognini J, et al. Radiation therapy in the treatment of HIV-related Kaposi's sarcoma. Anticancer Res. 2013;33:2153-2157.
- Gbabe OF, Okwundu CI, Dedicoat M, et al. Treatment of severe or progressive Kaposi's sarcoma in HIV-infected adults. Cochrane Database Syst Rev. 2014:CD003256.
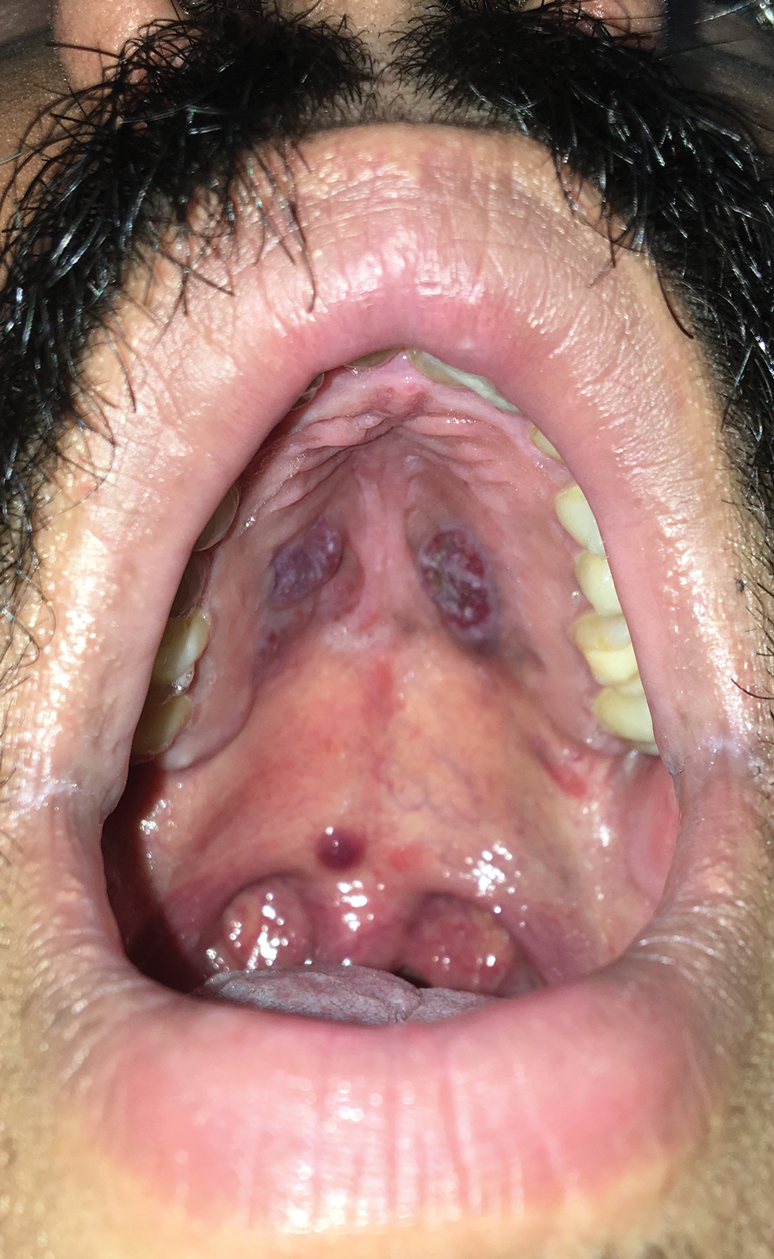
A 30-year-old man presented to our outpatient clinic with rapidly growing, ulcerated, violaceous lesions on the hard palate of 4 months' duration. Physical examination revealed approximately 2.0×1.5-cm, centrally ulcerated, violaceous, nodular lesions on the hard palate, as well as a 4-mm pinkish papular lesion on the soft palate.
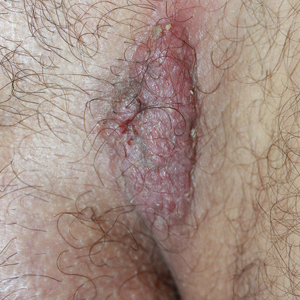
Solitary Exophytic Plaque on the Left Groin
The Diagnosis: Pemphigus Vegetans
A punch biopsy was taken from the verrucous plaque, and microscopic examination demonstrated prominent epidermal hyperplasia with intraepidermal eosinophilic microabscesses and a superficial dermatitis with abundant eosinophils (Figure 1A). Suprabasal acantholytic cleft formation was noted in a focal area (Figure 1B). Another punch biopsy was performed from the perilesional skin for direct immunofluorescence examination, which revealed intercellular deposits of IgG and C3 throughout the lower half of the epidermis (Figure 1C). Indirect immunofluorescence performed on monkey esophagus substrate showed circulating intercellular IgG antibodies in all the titers of up to 1/160 and an elevated level of IgG antidesmoglein 3 (anti-Dsg3) antibody (enzyme-linked immunosorbent assay index value, >200 RU/mL [reference range, <20 RU/mL]).

Because there was a solitary lesion, the decision was made to perform local treatment. One intralesional triamcinolone acetonide injection (20 mg/mL) resulted in remarkable flattening of the lesion (Figure 2). Subsequently, treatment was continued with clobetasol propionate ointment 3 times weekly for 1 month. During a follow-up period of 2 years, no signs of local relapse or new lesions elsewhere were noted, and the patient continued to be on long-term longitudinal evaluation.

Pemphigus vegetans (PV) is an uncommon variant of pemphigus, typically manifesting with vegetating erosions and plaques localized to the intertriginous areas of the body. Local factors such as semiocclusion, maceration, and/or bacterial or fungal colonization have been hypothesized to account for the distinctive localization and vegetation of the lesions.1,2 Traditionally, 2 clinical subtypes of PV have been described: (1) Hallopeau type presenting with pustules that later evolve into vegetating plaques, and (2) Neumann type that initially manifests as vesicles and bullae with a more disseminated distribution, transforming into hypertrophic masses with erosions.1-5 However, this distinction may not always be clear, and patients with features of both forms have been reported.2,5
At present, our case would best be regarded as a localized form of PV presenting with a solitary lesion. It may progress to more disseminated disease or remain localized during its course; the literature contains reports exemplifying both possibilities. In a large retrospective study from Tunisia encompassing almost 3 decades, the majority of the patients initially presented with unifocal involvement; however, the disease eventually became multifocal in almost all patients during the study period, emphasizing the need for long-term follow-up.2 There also are reports of PV confined to a single anatomic site, such as the scalp, sole, or vulva, that remained localized for years.2,4,6,7 Involvement of the oral mucosa is an important finding of PV and the presenting concern in approximately three-quarters of patients.2 Interestingly, the oral mucosa was not involved in our patient despite the high titer of anti-Dsg3 antibody, which suggests the need for the presence of other factors for clinical expression of the disease.
Although PV is considered a vegetating clinicomorphologic variant of pemphigus vulgaris, PV is histopathologically distinguished from pemphigus vulgaris by the presence of epidermal hyperplasia and intraepidermal eosinophilic microabscesses. Importantly, the epidermis displays signs of exuberant proliferation such as pseudoepitheliomatous hyperplasia and/or papillomatosis of a varying degree.1,2,5 Of note, suprabasal acantholysis is usually overshadowed by the changes in PV and presents only focally, as in our patient. The most common autoantibody profile is IgG targeting Dsg3; however, a spectrum of other autoantibodies has been identified, such as IgG antidesmocollin 3, IgA anti-Dsg3, and IgG anti-Dsg1.8,9
The most important differential diagnosis of PV is pyodermatitis-pyostomatitis vegetans. These 2 entities share many clinical and histopathological features; however, direct immunofluorescence is helpfulfor differentiation because it generally is negative in pyodermatitis-pyostomatitis vegetans.2,10 Furthermore, there is a well-established association between pyodermatitis-pyostomatitis vegetans and inflammatory bowel disorders, whereas PV has anecdotally been linked to malignancy, human immunodeficiency virus infection, and heroin abuse.1,2,10 Our patient was seronegative for human immunodeficiency virus and denied weight loss or loss of appetite. For those cases of PV involving a single anatomic site, the differential diagnosis is broader and encompasses dermatoses such as verrucae, syphilitic chancre, condylomata lata, granuloma inguinale, herpes simplex virus infection, and Kaposi sarcoma.
Treatment of PV is similar to pemphigus vulgaris and consists of a combination of systemic corticosteroids and steroid-sparing agents.1,5 On the other hand, more limited presentations of PV may be suitable for intralesional treatment with triamcinolone acetonide, thus avoiding potential adverse effects of systemic therapy.1,2 In our case with localized involvement, a favorable response was obtained with intralesional triamcinolone acetonide, and we plan to utilize systemic corticosteroids if the disease becomes generalized during follow-up.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Zaraa I, Sellami A, Bouguerra C, et al. Pemphigus vegetans: a clinical, histological, immunopathological and prognostic study. J Eur Acad Dermatol Venereol. 2011;25:1160-1167.
- Madan V, August PJ. Exophytic plaques, blisters, and mouth ulcers. pemphigus vegetans (PV), Neumann type. Arch Dermatol. 2009;145:715-720.
- Mori M, Mariotti G, Grandi V, et al. Pemphigus vegetans of the scalp. J Eur Acad Dermatol Venereol. 2016;30:368-370.
- Monshi B, Marker M, Feichtinger H, et al. Pemphigus vegetans--immunopathological findings in a rare variant of pemphigus vulgaris. J Dtsch Dermatol Ges. 2010;8:179-183.
- Jain VK, Dixit VB, Mohan H. Pemphigus vegetans in an unusual site. Int J Dermatol. 1989;28:352-353.
- Wong KT, Wong KK. A case of acantholytic dermatosis of the vulva with features of pemphigus vegetans. J Cutan Pathol. 1994;21:453-456.
- Morizane S, Yamamoto T, Hisamatsu Y, et al. Pemphigus vegetans with IgG and IgA antidesmoglein 3 antibodies. Br J Dermatol. 2005;153:1236-1237.
- Saruta H, Ishii N, Teye K, et al. Two cases of pemphigus vegetans with IgG anti-desmocollin 3 antibodies. JAMA Dermatol. 2013;149:1209-1213.
- Mehravaran M, Kemény L, Husz S, et al. Pyodermatitis-pyostomatitis vegetans. Br J Dermatol. 1997;137:266-269.
The Diagnosis: Pemphigus Vegetans
A punch biopsy was taken from the verrucous plaque, and microscopic examination demonstrated prominent epidermal hyperplasia with intraepidermal eosinophilic microabscesses and a superficial dermatitis with abundant eosinophils (Figure 1A). Suprabasal acantholytic cleft formation was noted in a focal area (Figure 1B). Another punch biopsy was performed from the perilesional skin for direct immunofluorescence examination, which revealed intercellular deposits of IgG and C3 throughout the lower half of the epidermis (Figure 1C). Indirect immunofluorescence performed on monkey esophagus substrate showed circulating intercellular IgG antibodies in all the titers of up to 1/160 and an elevated level of IgG antidesmoglein 3 (anti-Dsg3) antibody (enzyme-linked immunosorbent assay index value, >200 RU/mL [reference range, <20 RU/mL]).

Because there was a solitary lesion, the decision was made to perform local treatment. One intralesional triamcinolone acetonide injection (20 mg/mL) resulted in remarkable flattening of the lesion (Figure 2). Subsequently, treatment was continued with clobetasol propionate ointment 3 times weekly for 1 month. During a follow-up period of 2 years, no signs of local relapse or new lesions elsewhere were noted, and the patient continued to be on long-term longitudinal evaluation.

Pemphigus vegetans (PV) is an uncommon variant of pemphigus, typically manifesting with vegetating erosions and plaques localized to the intertriginous areas of the body. Local factors such as semiocclusion, maceration, and/or bacterial or fungal colonization have been hypothesized to account for the distinctive localization and vegetation of the lesions.1,2 Traditionally, 2 clinical subtypes of PV have been described: (1) Hallopeau type presenting with pustules that later evolve into vegetating plaques, and (2) Neumann type that initially manifests as vesicles and bullae with a more disseminated distribution, transforming into hypertrophic masses with erosions.1-5 However, this distinction may not always be clear, and patients with features of both forms have been reported.2,5
At present, our case would best be regarded as a localized form of PV presenting with a solitary lesion. It may progress to more disseminated disease or remain localized during its course; the literature contains reports exemplifying both possibilities. In a large retrospective study from Tunisia encompassing almost 3 decades, the majority of the patients initially presented with unifocal involvement; however, the disease eventually became multifocal in almost all patients during the study period, emphasizing the need for long-term follow-up.2 There also are reports of PV confined to a single anatomic site, such as the scalp, sole, or vulva, that remained localized for years.2,4,6,7 Involvement of the oral mucosa is an important finding of PV and the presenting concern in approximately three-quarters of patients.2 Interestingly, the oral mucosa was not involved in our patient despite the high titer of anti-Dsg3 antibody, which suggests the need for the presence of other factors for clinical expression of the disease.
Although PV is considered a vegetating clinicomorphologic variant of pemphigus vulgaris, PV is histopathologically distinguished from pemphigus vulgaris by the presence of epidermal hyperplasia and intraepidermal eosinophilic microabscesses. Importantly, the epidermis displays signs of exuberant proliferation such as pseudoepitheliomatous hyperplasia and/or papillomatosis of a varying degree.1,2,5 Of note, suprabasal acantholysis is usually overshadowed by the changes in PV and presents only focally, as in our patient. The most common autoantibody profile is IgG targeting Dsg3; however, a spectrum of other autoantibodies has been identified, such as IgG antidesmocollin 3, IgA anti-Dsg3, and IgG anti-Dsg1.8,9
The most important differential diagnosis of PV is pyodermatitis-pyostomatitis vegetans. These 2 entities share many clinical and histopathological features; however, direct immunofluorescence is helpfulfor differentiation because it generally is negative in pyodermatitis-pyostomatitis vegetans.2,10 Furthermore, there is a well-established association between pyodermatitis-pyostomatitis vegetans and inflammatory bowel disorders, whereas PV has anecdotally been linked to malignancy, human immunodeficiency virus infection, and heroin abuse.1,2,10 Our patient was seronegative for human immunodeficiency virus and denied weight loss or loss of appetite. For those cases of PV involving a single anatomic site, the differential diagnosis is broader and encompasses dermatoses such as verrucae, syphilitic chancre, condylomata lata, granuloma inguinale, herpes simplex virus infection, and Kaposi sarcoma.
Treatment of PV is similar to pemphigus vulgaris and consists of a combination of systemic corticosteroids and steroid-sparing agents.1,5 On the other hand, more limited presentations of PV may be suitable for intralesional treatment with triamcinolone acetonide, thus avoiding potential adverse effects of systemic therapy.1,2 In our case with localized involvement, a favorable response was obtained with intralesional triamcinolone acetonide, and we plan to utilize systemic corticosteroids if the disease becomes generalized during follow-up.
The Diagnosis: Pemphigus Vegetans
A punch biopsy was taken from the verrucous plaque, and microscopic examination demonstrated prominent epidermal hyperplasia with intraepidermal eosinophilic microabscesses and a superficial dermatitis with abundant eosinophils (Figure 1A). Suprabasal acantholytic cleft formation was noted in a focal area (Figure 1B). Another punch biopsy was performed from the perilesional skin for direct immunofluorescence examination, which revealed intercellular deposits of IgG and C3 throughout the lower half of the epidermis (Figure 1C). Indirect immunofluorescence performed on monkey esophagus substrate showed circulating intercellular IgG antibodies in all the titers of up to 1/160 and an elevated level of IgG antidesmoglein 3 (anti-Dsg3) antibody (enzyme-linked immunosorbent assay index value, >200 RU/mL [reference range, <20 RU/mL]).

Because there was a solitary lesion, the decision was made to perform local treatment. One intralesional triamcinolone acetonide injection (20 mg/mL) resulted in remarkable flattening of the lesion (Figure 2). Subsequently, treatment was continued with clobetasol propionate ointment 3 times weekly for 1 month. During a follow-up period of 2 years, no signs of local relapse or new lesions elsewhere were noted, and the patient continued to be on long-term longitudinal evaluation.

Pemphigus vegetans (PV) is an uncommon variant of pemphigus, typically manifesting with vegetating erosions and plaques localized to the intertriginous areas of the body. Local factors such as semiocclusion, maceration, and/or bacterial or fungal colonization have been hypothesized to account for the distinctive localization and vegetation of the lesions.1,2 Traditionally, 2 clinical subtypes of PV have been described: (1) Hallopeau type presenting with pustules that later evolve into vegetating plaques, and (2) Neumann type that initially manifests as vesicles and bullae with a more disseminated distribution, transforming into hypertrophic masses with erosions.1-5 However, this distinction may not always be clear, and patients with features of both forms have been reported.2,5
At present, our case would best be regarded as a localized form of PV presenting with a solitary lesion. It may progress to more disseminated disease or remain localized during its course; the literature contains reports exemplifying both possibilities. In a large retrospective study from Tunisia encompassing almost 3 decades, the majority of the patients initially presented with unifocal involvement; however, the disease eventually became multifocal in almost all patients during the study period, emphasizing the need for long-term follow-up.2 There also are reports of PV confined to a single anatomic site, such as the scalp, sole, or vulva, that remained localized for years.2,4,6,7 Involvement of the oral mucosa is an important finding of PV and the presenting concern in approximately three-quarters of patients.2 Interestingly, the oral mucosa was not involved in our patient despite the high titer of anti-Dsg3 antibody, which suggests the need for the presence of other factors for clinical expression of the disease.
Although PV is considered a vegetating clinicomorphologic variant of pemphigus vulgaris, PV is histopathologically distinguished from pemphigus vulgaris by the presence of epidermal hyperplasia and intraepidermal eosinophilic microabscesses. Importantly, the epidermis displays signs of exuberant proliferation such as pseudoepitheliomatous hyperplasia and/or papillomatosis of a varying degree.1,2,5 Of note, suprabasal acantholysis is usually overshadowed by the changes in PV and presents only focally, as in our patient. The most common autoantibody profile is IgG targeting Dsg3; however, a spectrum of other autoantibodies has been identified, such as IgG antidesmocollin 3, IgA anti-Dsg3, and IgG anti-Dsg1.8,9
The most important differential diagnosis of PV is pyodermatitis-pyostomatitis vegetans. These 2 entities share many clinical and histopathological features; however, direct immunofluorescence is helpfulfor differentiation because it generally is negative in pyodermatitis-pyostomatitis vegetans.2,10 Furthermore, there is a well-established association between pyodermatitis-pyostomatitis vegetans and inflammatory bowel disorders, whereas PV has anecdotally been linked to malignancy, human immunodeficiency virus infection, and heroin abuse.1,2,10 Our patient was seronegative for human immunodeficiency virus and denied weight loss or loss of appetite. For those cases of PV involving a single anatomic site, the differential diagnosis is broader and encompasses dermatoses such as verrucae, syphilitic chancre, condylomata lata, granuloma inguinale, herpes simplex virus infection, and Kaposi sarcoma.
Treatment of PV is similar to pemphigus vulgaris and consists of a combination of systemic corticosteroids and steroid-sparing agents.1,5 On the other hand, more limited presentations of PV may be suitable for intralesional treatment with triamcinolone acetonide, thus avoiding potential adverse effects of systemic therapy.1,2 In our case with localized involvement, a favorable response was obtained with intralesional triamcinolone acetonide, and we plan to utilize systemic corticosteroids if the disease becomes generalized during follow-up.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Zaraa I, Sellami A, Bouguerra C, et al. Pemphigus vegetans: a clinical, histological, immunopathological and prognostic study. J Eur Acad Dermatol Venereol. 2011;25:1160-1167.
- Madan V, August PJ. Exophytic plaques, blisters, and mouth ulcers. pemphigus vegetans (PV), Neumann type. Arch Dermatol. 2009;145:715-720.
- Mori M, Mariotti G, Grandi V, et al. Pemphigus vegetans of the scalp. J Eur Acad Dermatol Venereol. 2016;30:368-370.
- Monshi B, Marker M, Feichtinger H, et al. Pemphigus vegetans--immunopathological findings in a rare variant of pemphigus vulgaris. J Dtsch Dermatol Ges. 2010;8:179-183.
- Jain VK, Dixit VB, Mohan H. Pemphigus vegetans in an unusual site. Int J Dermatol. 1989;28:352-353.
- Wong KT, Wong KK. A case of acantholytic dermatosis of the vulva with features of pemphigus vegetans. J Cutan Pathol. 1994;21:453-456.
- Morizane S, Yamamoto T, Hisamatsu Y, et al. Pemphigus vegetans with IgG and IgA antidesmoglein 3 antibodies. Br J Dermatol. 2005;153:1236-1237.
- Saruta H, Ishii N, Teye K, et al. Two cases of pemphigus vegetans with IgG anti-desmocollin 3 antibodies. JAMA Dermatol. 2013;149:1209-1213.
- Mehravaran M, Kemény L, Husz S, et al. Pyodermatitis-pyostomatitis vegetans. Br J Dermatol. 1997;137:266-269.
- Ruocco V, Ruocco E, Caccavale S, et al. Pemphigus vegetans of the folds (intertriginous areas). Clin Dermatol. 2015;33:471-476.
- Zaraa I, Sellami A, Bouguerra C, et al. Pemphigus vegetans: a clinical, histological, immunopathological and prognostic study. J Eur Acad Dermatol Venereol. 2011;25:1160-1167.
- Madan V, August PJ. Exophytic plaques, blisters, and mouth ulcers. pemphigus vegetans (PV), Neumann type. Arch Dermatol. 2009;145:715-720.
- Mori M, Mariotti G, Grandi V, et al. Pemphigus vegetans of the scalp. J Eur Acad Dermatol Venereol. 2016;30:368-370.
- Monshi B, Marker M, Feichtinger H, et al. Pemphigus vegetans--immunopathological findings in a rare variant of pemphigus vulgaris. J Dtsch Dermatol Ges. 2010;8:179-183.
- Jain VK, Dixit VB, Mohan H. Pemphigus vegetans in an unusual site. Int J Dermatol. 1989;28:352-353.
- Wong KT, Wong KK. A case of acantholytic dermatosis of the vulva with features of pemphigus vegetans. J Cutan Pathol. 1994;21:453-456.
- Morizane S, Yamamoto T, Hisamatsu Y, et al. Pemphigus vegetans with IgG and IgA antidesmoglein 3 antibodies. Br J Dermatol. 2005;153:1236-1237.
- Saruta H, Ishii N, Teye K, et al. Two cases of pemphigus vegetans with IgG anti-desmocollin 3 antibodies. JAMA Dermatol. 2013;149:1209-1213.
- Mehravaran M, Kemény L, Husz S, et al. Pyodermatitis-pyostomatitis vegetans. Br J Dermatol. 1997;137:266-269.
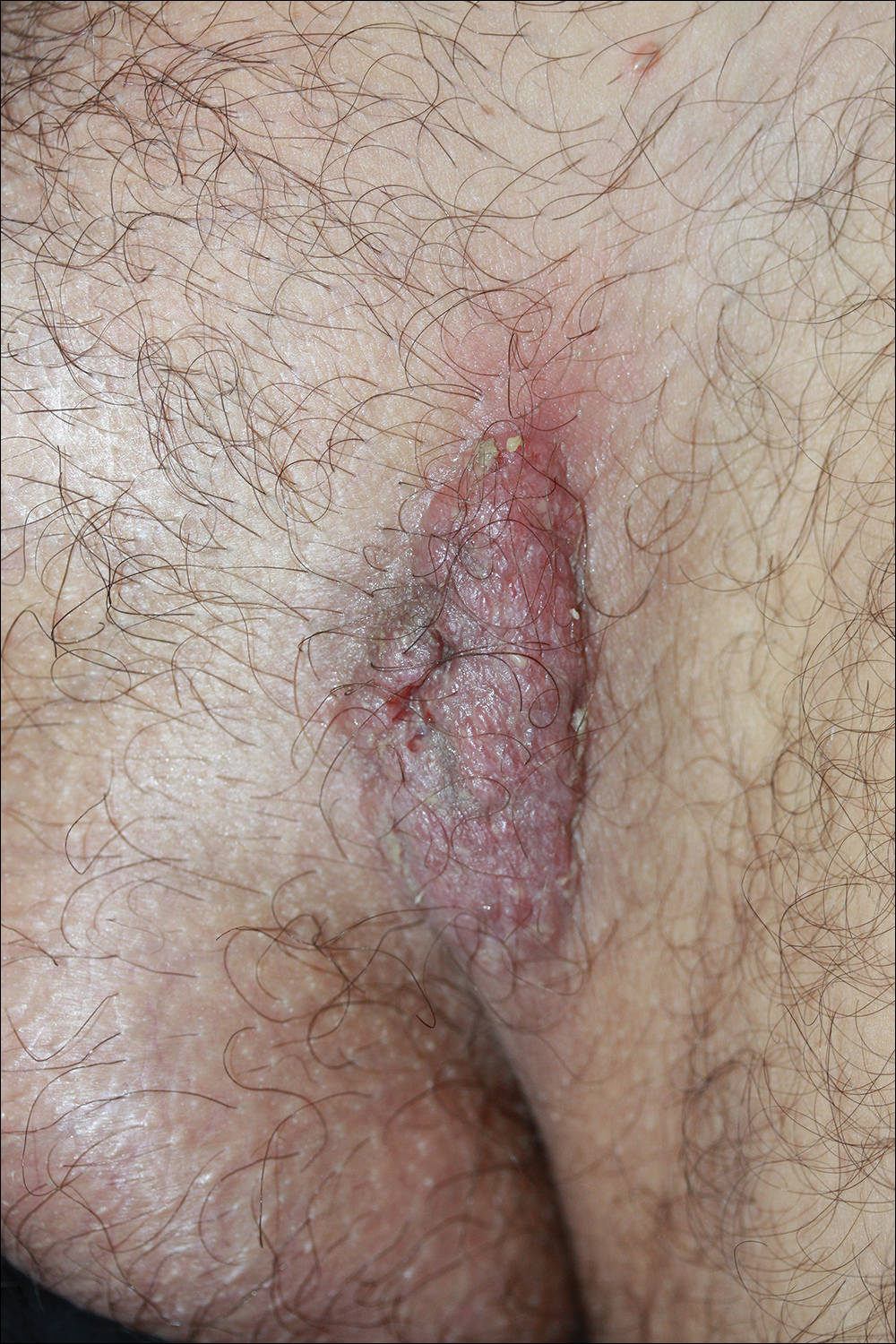
A 40-year-old otherwise healthy man presented with an exophytic plaque on the left groin of 1 month's duration. The lesion reportedly emerged as pustules that slowly expanded and coalesced. At an outside institution, cryotherapy was planned for a presumed diagnosis of condyloma acuminatum; however, the patient decided to get a second opinion. He denied recent intake of new drugs. Six months prior he had traveled to China and engaged in unprotected sexual intercourse. Physical examination revealed an approximately 4×2-cm exophytic plaque with a partially eroded and exudative surface on the left inguinal fold. Dermatologic examination, including the oral mucosa, was otherwise normal. Complete blood cell count and sexually transmitted disease panel were unremarkable.