User login
How best to treat “long-haulers” with reactive arthritis?
In the June Photo Rounds column, “Foot rash and joint pain” (J Fam Pract. 2021;70:249-251), Badon et al presented a case of chlamydia-associated reactive arthritis (ReA), formerly called Reiter syndrome, in a 21-year-old man following Chlamydia trachomatis urethritis. We would like to point out that, contrary to the conventional definition of ReA, in which the causative pathogen can’t be cultured from the affected joints,1 chlamydia-associated ReA is associated with evidence of chronic joint infection that, while not cultivable, can be confirmed by real-time polymerase chain reaction testing of metabolically active pathogens in synovial tissue and/or fluid.2
C trachomatis and C pneumoniae are the most frequent causative pathogens to elicit ReA.3 Short-course antibiotics and anti-inflammatory treatments can palliate ReA, but these treatments often do not provide a cure.3 Two controlled clinical trials demonstrated that chlamydia-associated ReA can be treated successfully with longer-term combination antibiotic therapy.4,5 ReA is usually diagnosed in the acute stage (first 6 months) and can become chronic in 30% of cases.6 It would be interesting to know the long-term treatment and outcome data for the case patient.
David L. Hahn, MD, MS
Alan P. Hudson, PhD
Charles Stratton, MD
Wilmore Webley, PhD
Judith Whittum-Hudson, PhD
1. Yu D, van Tubergenm A. Reactive arthritis. UpToDate. Updated 2021. Accessed August 10, 2021. www.uptodate.com/contents/reactive-arthritis
2. Gérard HC, Carter JD, Hudson AP. Chlamydia trachomatis is present and metabolically active during the remitting phase in synovial tissues from patients with chronic chlamydia-induced reactive arthritis. Am J Med Sci. 2013;346:22-25. doi: 10.1097/MAJ.0b013e3182648740
3. Zeidler H, Hudson AP. New insights into chlamydia and arthritis. Promise of a cure? Ann Rheum Dis. 2014;73:637-644. doi: 10.1136/annrheumdis-2013-204110
4. Carter JD, Valeriano J, Vasey FB. Doxycycline versus doxycycline and rifampin in undifferentiated spondyloarthropathy, with special reference to chlamydia-induced arthritis. A prospective, randomized 9-month comparison. J Rheumatol. 2004;31:1973-1980.
5. Carter JD, Espinoza LR, Inman RD, et al. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective trial. Arthritis Rheum. 2010;62:1298-1307. doi: 10.1002/art.27394
6. Carter JD, Inman RD, Whittum-Hudson J, et al. Chlamydia and chronic arthritis. Ann Med. 2012;44:784-792. doi: 10.3109/07853890.2011.606830
In the June Photo Rounds column, “Foot rash and joint pain” (J Fam Pract. 2021;70:249-251), Badon et al presented a case of chlamydia-associated reactive arthritis (ReA), formerly called Reiter syndrome, in a 21-year-old man following Chlamydia trachomatis urethritis. We would like to point out that, contrary to the conventional definition of ReA, in which the causative pathogen can’t be cultured from the affected joints,1 chlamydia-associated ReA is associated with evidence of chronic joint infection that, while not cultivable, can be confirmed by real-time polymerase chain reaction testing of metabolically active pathogens in synovial tissue and/or fluid.2
C trachomatis and C pneumoniae are the most frequent causative pathogens to elicit ReA.3 Short-course antibiotics and anti-inflammatory treatments can palliate ReA, but these treatments often do not provide a cure.3 Two controlled clinical trials demonstrated that chlamydia-associated ReA can be treated successfully with longer-term combination antibiotic therapy.4,5 ReA is usually diagnosed in the acute stage (first 6 months) and can become chronic in 30% of cases.6 It would be interesting to know the long-term treatment and outcome data for the case patient.
David L. Hahn, MD, MS
Alan P. Hudson, PhD
Charles Stratton, MD
Wilmore Webley, PhD
Judith Whittum-Hudson, PhD
In the June Photo Rounds column, “Foot rash and joint pain” (J Fam Pract. 2021;70:249-251), Badon et al presented a case of chlamydia-associated reactive arthritis (ReA), formerly called Reiter syndrome, in a 21-year-old man following Chlamydia trachomatis urethritis. We would like to point out that, contrary to the conventional definition of ReA, in which the causative pathogen can’t be cultured from the affected joints,1 chlamydia-associated ReA is associated with evidence of chronic joint infection that, while not cultivable, can be confirmed by real-time polymerase chain reaction testing of metabolically active pathogens in synovial tissue and/or fluid.2
C trachomatis and C pneumoniae are the most frequent causative pathogens to elicit ReA.3 Short-course antibiotics and anti-inflammatory treatments can palliate ReA, but these treatments often do not provide a cure.3 Two controlled clinical trials demonstrated that chlamydia-associated ReA can be treated successfully with longer-term combination antibiotic therapy.4,5 ReA is usually diagnosed in the acute stage (first 6 months) and can become chronic in 30% of cases.6 It would be interesting to know the long-term treatment and outcome data for the case patient.
David L. Hahn, MD, MS
Alan P. Hudson, PhD
Charles Stratton, MD
Wilmore Webley, PhD
Judith Whittum-Hudson, PhD
1. Yu D, van Tubergenm A. Reactive arthritis. UpToDate. Updated 2021. Accessed August 10, 2021. www.uptodate.com/contents/reactive-arthritis
2. Gérard HC, Carter JD, Hudson AP. Chlamydia trachomatis is present and metabolically active during the remitting phase in synovial tissues from patients with chronic chlamydia-induced reactive arthritis. Am J Med Sci. 2013;346:22-25. doi: 10.1097/MAJ.0b013e3182648740
3. Zeidler H, Hudson AP. New insights into chlamydia and arthritis. Promise of a cure? Ann Rheum Dis. 2014;73:637-644. doi: 10.1136/annrheumdis-2013-204110
4. Carter JD, Valeriano J, Vasey FB. Doxycycline versus doxycycline and rifampin in undifferentiated spondyloarthropathy, with special reference to chlamydia-induced arthritis. A prospective, randomized 9-month comparison. J Rheumatol. 2004;31:1973-1980.
5. Carter JD, Espinoza LR, Inman RD, et al. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective trial. Arthritis Rheum. 2010;62:1298-1307. doi: 10.1002/art.27394
6. Carter JD, Inman RD, Whittum-Hudson J, et al. Chlamydia and chronic arthritis. Ann Med. 2012;44:784-792. doi: 10.3109/07853890.2011.606830
1. Yu D, van Tubergenm A. Reactive arthritis. UpToDate. Updated 2021. Accessed August 10, 2021. www.uptodate.com/contents/reactive-arthritis
2. Gérard HC, Carter JD, Hudson AP. Chlamydia trachomatis is present and metabolically active during the remitting phase in synovial tissues from patients with chronic chlamydia-induced reactive arthritis. Am J Med Sci. 2013;346:22-25. doi: 10.1097/MAJ.0b013e3182648740
3. Zeidler H, Hudson AP. New insights into chlamydia and arthritis. Promise of a cure? Ann Rheum Dis. 2014;73:637-644. doi: 10.1136/annrheumdis-2013-204110
4. Carter JD, Valeriano J, Vasey FB. Doxycycline versus doxycycline and rifampin in undifferentiated spondyloarthropathy, with special reference to chlamydia-induced arthritis. A prospective, randomized 9-month comparison. J Rheumatol. 2004;31:1973-1980.
5. Carter JD, Espinoza LR, Inman RD, et al. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective trial. Arthritis Rheum. 2010;62:1298-1307. doi: 10.1002/art.27394
6. Carter JD, Inman RD, Whittum-Hudson J, et al. Chlamydia and chronic arthritis. Ann Med. 2012;44:784-792. doi: 10.3109/07853890.2011.606830
Prolonged azithromycin Tx for asthma?
In “Asthma: Newer Tx options mean more targeted therapy” (J Fam Pract. 2020;65:135-144), Rali et al recommend azithromycin as an add-on therapy to ICS-LABA for a select group of patients with uncontrolled persistent asthma (neutrophilic phenotype)—a Grade C recommendation. However, the best available evidence demonstrates that azithromycin is equally efficacious for uncontrolled persistent eosinophilic asthma.1,2 Thus, family physicians need not refer patients for bronchoscopy to identify the inflammatory “phenotype.”
An important unanswered question is whether azithromycin needs to be administered continuously. Emerging evidence indicates that some patients may experience prolonged benefit after time-limited azithromycin treatment. This suggests that the mechanism of action, which has been described as anti-inflammatory, is (at least in part) antimicrobial.3
For azithromycin-treated asthma patients who experience a significant clinical response after 3 to 6 months of treatment, I recommend that the prescribing clinician try taking the patient off azithromycin to assess whether clinical improvement persists or wanes. Nothing is lost, and much is gained, by this approach; patients who relapse can resume azithromycin, and patients who remain improved are spared exposure to an unnecessary and prolonged treatment.
David L. Hahn, MD, MS
Madison, WI
1. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390: 659-668.
2. Gibson PG, Yang IA, Upham JW, et al. Efficacy of azithromycin in severe asthma from the AMAZES randomised trial. ERJ Open Res. 2019;5.
3. Hahn D. When guideline treatment of asthma fails, consider a macrolide antibiotic. J Fam Pract. 2019;68:536-545.
In “Asthma: Newer Tx options mean more targeted therapy” (J Fam Pract. 2020;65:135-144), Rali et al recommend azithromycin as an add-on therapy to ICS-LABA for a select group of patients with uncontrolled persistent asthma (neutrophilic phenotype)—a Grade C recommendation. However, the best available evidence demonstrates that azithromycin is equally efficacious for uncontrolled persistent eosinophilic asthma.1,2 Thus, family physicians need not refer patients for bronchoscopy to identify the inflammatory “phenotype.”
An important unanswered question is whether azithromycin needs to be administered continuously. Emerging evidence indicates that some patients may experience prolonged benefit after time-limited azithromycin treatment. This suggests that the mechanism of action, which has been described as anti-inflammatory, is (at least in part) antimicrobial.3
For azithromycin-treated asthma patients who experience a significant clinical response after 3 to 6 months of treatment, I recommend that the prescribing clinician try taking the patient off azithromycin to assess whether clinical improvement persists or wanes. Nothing is lost, and much is gained, by this approach; patients who relapse can resume azithromycin, and patients who remain improved are spared exposure to an unnecessary and prolonged treatment.
David L. Hahn, MD, MS
Madison, WI
In “Asthma: Newer Tx options mean more targeted therapy” (J Fam Pract. 2020;65:135-144), Rali et al recommend azithromycin as an add-on therapy to ICS-LABA for a select group of patients with uncontrolled persistent asthma (neutrophilic phenotype)—a Grade C recommendation. However, the best available evidence demonstrates that azithromycin is equally efficacious for uncontrolled persistent eosinophilic asthma.1,2 Thus, family physicians need not refer patients for bronchoscopy to identify the inflammatory “phenotype.”
An important unanswered question is whether azithromycin needs to be administered continuously. Emerging evidence indicates that some patients may experience prolonged benefit after time-limited azithromycin treatment. This suggests that the mechanism of action, which has been described as anti-inflammatory, is (at least in part) antimicrobial.3
For azithromycin-treated asthma patients who experience a significant clinical response after 3 to 6 months of treatment, I recommend that the prescribing clinician try taking the patient off azithromycin to assess whether clinical improvement persists or wanes. Nothing is lost, and much is gained, by this approach; patients who relapse can resume azithromycin, and patients who remain improved are spared exposure to an unnecessary and prolonged treatment.
David L. Hahn, MD, MS
Madison, WI
1. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390: 659-668.
2. Gibson PG, Yang IA, Upham JW, et al. Efficacy of azithromycin in severe asthma from the AMAZES randomised trial. ERJ Open Res. 2019;5.
3. Hahn D. When guideline treatment of asthma fails, consider a macrolide antibiotic. J Fam Pract. 2019;68:536-545.
1. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390: 659-668.
2. Gibson PG, Yang IA, Upham JW, et al. Efficacy of azithromycin in severe asthma from the AMAZES randomised trial. ERJ Open Res. 2019;5.
3. Hahn D. When guideline treatment of asthma fails, consider a macrolide antibiotic. J Fam Pract. 2019;68:536-545.
When guideline treatment of asthma fails, consider a macrolide antibiotic
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3
This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29
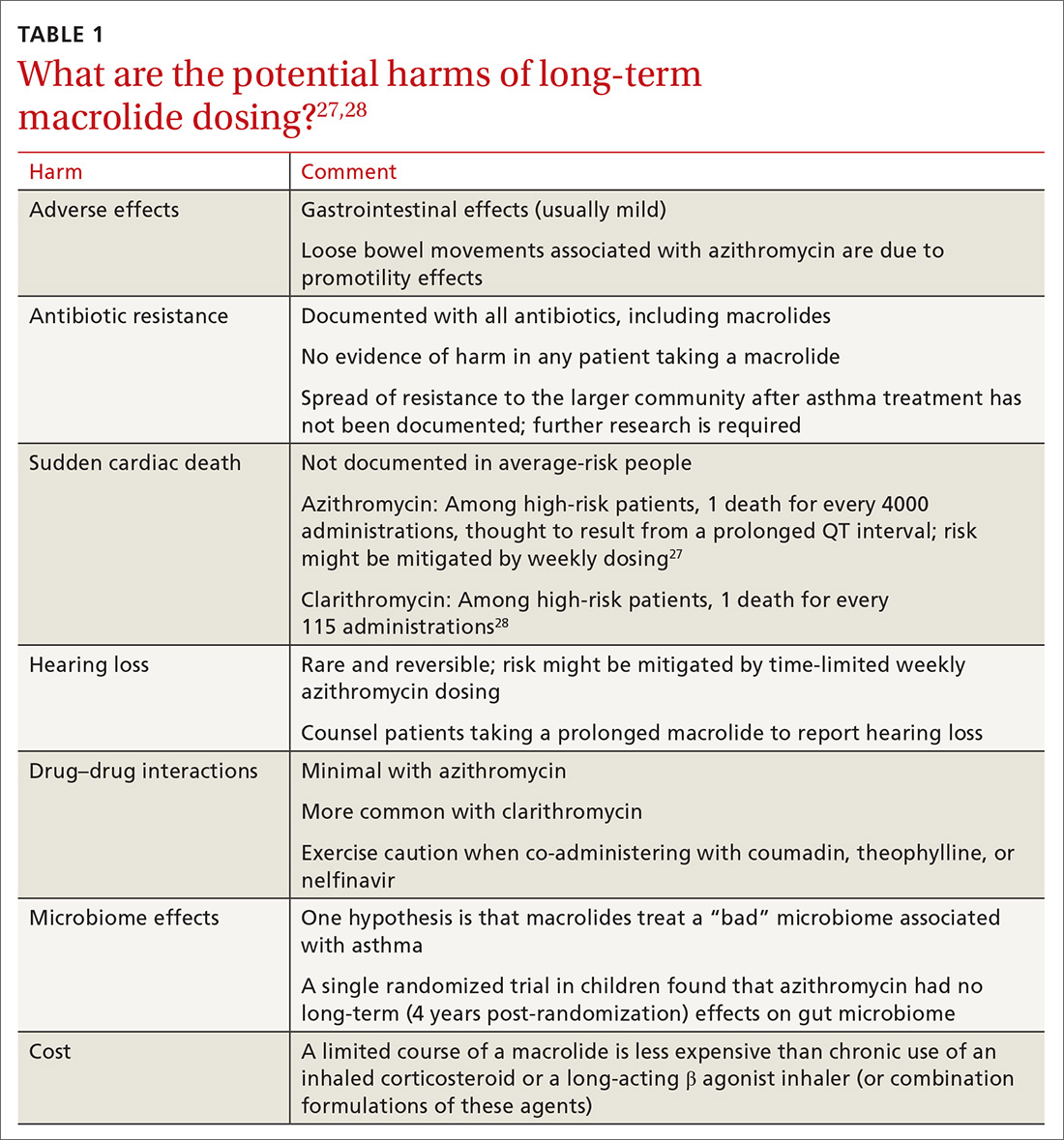
Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
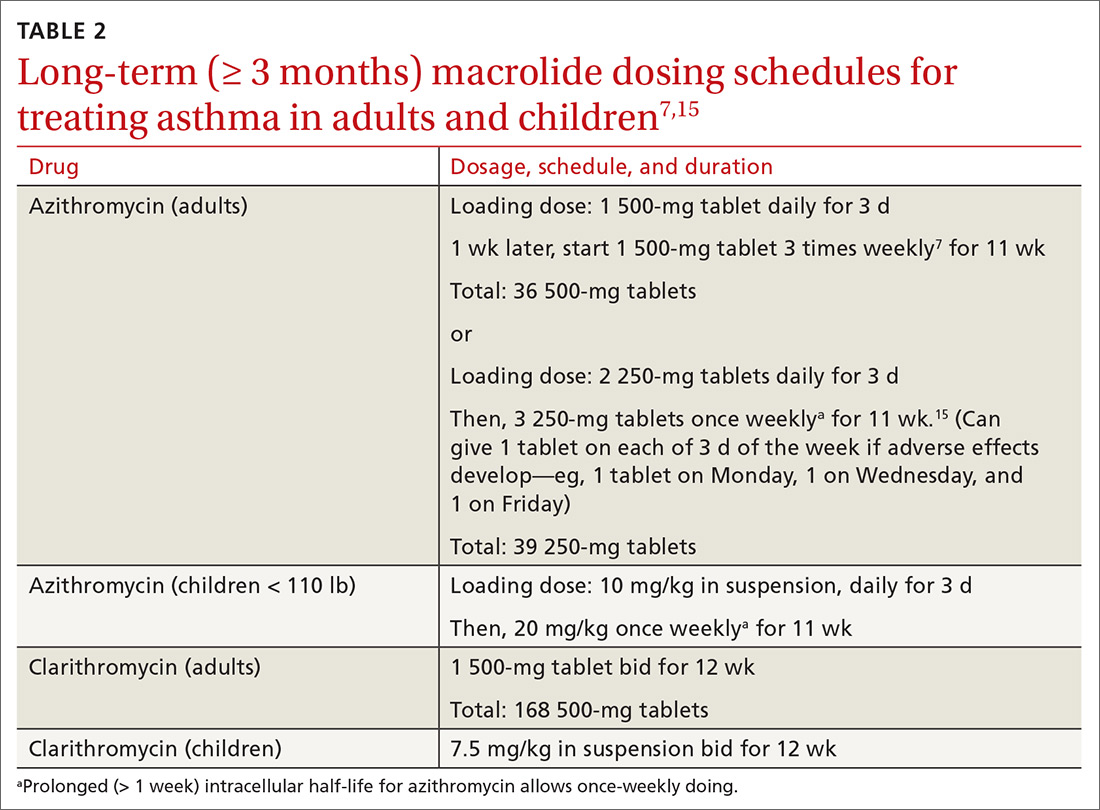
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.
Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3

This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29

Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.

Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
In vitro laboratory and in vivo animal models support the biologic plausibility that chronic infection is a potential cause of asthma.1,2 Arising from that hypothesis, macrolide antibiotics have been the subject of clinical trials and other studies to determine whether these drugs are efficacious in the long-term management of asthma in adults and children. Macrolides might also have immunomodulatory and antiviral properties that can benefit patients with asthma.3

This article looks at the evidence and clinical scenarios for the use of macrolides in asthma, provides proposed dosing schedules, and reviews associated concerns, including adverse effects, risk of bacterial resistance, and cost.
3 cases to consider
CASE 1 Paul D developed severe, refractory asthma at 30 years of age after an acute respiratory illness. At age 40, he was treated with 14 weekly doses of azithromycin. His asthma resolved slowly over 12 months.
Outcome. Mr. D has remained free of symptoms of asthma for more than 20 years.
CASE 2 Casey K developed severe wheezing at 18 months of age after an acute respiratory illness. Refractory asthma symptoms persisted until 6 years of age, at which time he was given 12 weekly doses of azithromycin. Asthma symptoms gradually resolved.
Outcome. Casey was able to resume normal physical activities, including competitive swimming.
CASE 3 Amy S, who had no history of respiratory problems, presented at 30 years of age with a 3-month history of wheezing and dyspnea after an acute respiratory illness. She was treated symptomatically with bronchodilators; wheezing failed to resolve. After 6 months of persistent wheezing that significantly affected her exercise capacity, Ms. S was given a diagnosis of persistent asthma and received 12 weekly doses of azithromycin.
[polldaddy:10475438]
Continue to: Outcome...
Outcome. Ms. S’s symptoms resolved completely within months.
Evidence of benefit of macrolides in asthma
These 3 cases, taken from my practice (but with names changed), demonstrate the therapeutic potential of macrolide antibiotics for patients with asthma under specific clinical circumstances. The cases are referenced again in the following examination of the literature on macrolides for asthma
SIDEBAR
Macrolides for Asthma: Registry of Clinical Experience
More information is needed about the “real world” effectiveness of antibiotic treatment for severe refractory and new-onset asthma. If you are a prescribing clinician who cares for patients with asthma and you are considering prescribing antibiotics for asthma, you are invited to document your outcomes by entering prospective, de-identified patient data into a human subjects committee-approved online registry. To gain access to the registry, and for more information, contact the author at [email protected] or visit https://www.fammed.wisc.edu/wren/resources/macrolides-for-asthma/ .
Meta-analysis. Reiter et al4 performed a meta-analysis of 12 randomized clinical trials of macrolides for long-term management of asthma in children and adults. Prolonged treatment was defined as > 3 weeks of continuous administration of a macrolide. The pooled effect of macrolides on forced expiratory volume in 1 second (FEV1) was not significant; however, a significant effect on peak expiratory flow, symptom scores, quality of life, and airway hyperreactivity was observed.
Comment: The study’s authors concluded: “Macrolides may therefore be beneficial as adjunct asthma therapy. Future trials, focusing on long-term safety and effectiveness, should use standardized outcomes and procedures.”
Cochrane meta-analysis. Kew et al5 performed a meta-analysis of 23 studies of macrolides for managing chronic asthma for the Cochrane Database of Systematic Reviews. In their review, they reported
- no significant effects of macrolides on asthma exacerbations, asthma control, quality of life, and rescue medication use; and
- significant effects of macrolides for asthma symptoms and FEV1.
Continue to: Two within-study subgroup...
Two within-study subgroup analyses showed a possible benefit of macrolides for non-eosinophilic asthma, defined by a predominance of neutrophils in a bronchoalveolar lavage specimen. Kew et al5 noted that (1) most of the evidence examined in the review was of low quality and (2) inclusion criteria, interventions, and outcomes were highly variable.
Comment: The validity of a meta-analysis depends on the validity and similarity of underlying trials. Both meta-analyses just described were characterized by (1) grouping trials of older and newer macrolides and (2) significant selection bias in the underlying trials.
Selection bias is prevalent in asthma research and is a major contributor to uncertainty: Randomized controlled trials upon which guideline treatments are based have systematically excluded > 90% of people with asthma.6 Exclusions include past or current smoking, the asthma–chronic obstructive pulmonary disease (COPD) overlap syndrome, severe asthma, and acute respiratory illness; these exclusion criteria have also been applied to studies of macrolides. Importantly, patients in the excluded groups are probably those most likely to respond to a macrolide.2 Pragmatic effectiveness studies (broad eligibility criteria, adequate duration of azithromycin treatment, a posttreatment observation period, and pre-specified biomarker subgroup analyses) have been recommended to address the hypothesis of what has been termed infectious asthma.2
Inconsistent evidence, the generally poor quality of underlying studies, and uncertainty about which subgroup(s) of asthma patients might benefit all contribute to a strength of recommendation of “B” for treating asthma with macrolides. Two recent randomized trials7,8 that were not included in the cited meta-analyses, along with other evidence,2 point to 2 groups of patients who are candidates for a trial of azithromycin: those with severe refractory asthma and those with new-onset asthma.
Clinical trial in adults. Gibson et al7 conducted a randomized, double-blind, placebo-controlled trial of azithromycin 500 mg 3 times a week or placebo for 1 year in 420 adults who had uncontrolled persistent asthma despite taking medium-to-high doses of an inhaled corticosteroid (ICS) plus a long-acting β agonist (LABA) (the AMAZES [Asthma and Macrolides: The Azithromycin Efficacy and Safety] trial; Level 1 study). The mean baseline asthma control questionnaire score was 1.5, equivalent to an Asthma Control Test (ACT) score* of 15.9
Continue to: Azithromycin reduced the frequency...
Azithromycin reduced the frequency of asthma exacerbations (to 1.07 per patient–year for azithromycin, compared with 1.86 per patient–year for placebo [incidence rate ratio = 0.59; 95% confidence interval (CI), 0.47-0.74]). The percentage of patients experiencing at least 1 exacerbation was reduced with azithromycin treatment (61% of patients in the placebo group experienced ≥ 1 exacerbation, compared with 44% in the azithromycin group [P < .0001; number needed to treat = 6]). Asthma quality of life was also improved by azithromycin (P = .001).
There was no significant difference between azithromycin and placebo in the overall rate of serious adverse events. Diarrhea that did not require treatment discontinuation was more common in patients treated with azithromycin (34%) than in the placebo group (19%). There was no posttreatment observation period to assess whether these azithromycin benefits waned or persisted after treatment was stopped.
Other evidence10 indicates that at least some patients who respond to azithromycin will experience persistent improvement after antibiotic treatment is completed (see CASE 1).
Pediatric clinical trial. Stokholm et al8 performed a randomized, double-blind, placebo-controlled trial of azithromycin in children 1 to 3 years of age who had been given a diagnosis of recurrent asthma-like symptoms (Level 1 study). Treatment was a 3-day course of azithromycin oral solution, 10 mg/kg/d, or placebo. Random allocation was performed for 158 asthma-like episodes in 72 children.
Azithromycin reduced the wheezing episode to a mean duration of 3.4 days, compared with 7.7 days for placebo (risk reduction = 63.3%; 95% CI, 56%-69.3% [P < .0001]). Effect size increased with early initiation of treatment: ie, an 83% reduction in episode duration was seen when treatment was initiated before Day 6 of the episode, compared with a 36% reduction if treatment was initiated on or after Day 6 (P < .0001).
Continue to: No differences between...
No differences between the randomized groups were observed in clinical adverse effects.
Comment: The brief course of azithromycin provided to patients in this trial did not have a significant impact on time to next episode of troublesome lung symptoms in individual children. Previous clinical observations have suggested that a longer duration of treatment (3-6 months) might be required to achieve lasting improvement or remission in selected patients with asthma (see CASE 2).10,11 The short-term benefit of azithromycin for acute wheezing is limited to children: Two comparable acute dosing trials in adults have shown little12 or no13 short-term benefit; however, these negative findings have been hypothesized to be the result of selection bias.14
Other evidence is worth examining
Other studies not included in the meta-analyses of randomized controlled trials provide additional evidence to support a recommendation of a trial of azithromycin in patients with severe, refractory, or new-onset asthma.
Nonrandomized controlled evidence. AZMATICS (AZithroMycin/Asthma Trial In Community Settings)15 is the sole randomized, double-blind, placebo-controlled trial of long-term azithromycin that included a 9-month posttreatment observation period. Seventy-five participants were randomized to receive a loading dose of 600 mg of azithromycin or placebo once daily for 3 days in Week 1. They then received either azithromycin 600 mg or placebo once weekly for 11 weeks. Posttreatment observation was performed until 48 weeks after randomization.
However, many eligible subjects, whom the principal investigator believed were ideal candidates for randomization, declined randomization because they did not want to risk receiving placebo. To accommodate those patients, the protocol was amended to include an open-label (OL) azithromycin arm, in which each participant’s personal physician prescribed azithromycin 750 mg for 11 weeks after a loading dose16 (OL cohort only, Level 2 study: controlled, nonrandomized, nonblinded). The OL group had (1) a higher baseline prevalence of severe, persistent asthma (32%) than the randomized group (8%) (P = .012); and (2) worse asthma quality of life than the randomized patients (P = .023). The OL group represented selection bias attributable to patient preference.
Continue to: The less severely...
The less severely affected randomized group of the trial did not exhibit significant effects attributable to azithromycin. The more severely affected OL cohort demonstrated significant, and large, azithromycin treatment effects for asthma symptoms, asthma quality of life, and asthma control (P < .05 for both groups; number needed to treat [NNT] = 3) that persisted during the posttreatment observation period.
Comment: The authors concluded: “Pending further randomized trials and given the relative safety of azithromycin and the significant disease burden from severe, refractory asthma, prescribing prolonged azithromycin therapy to patients with uncontrolled asthma may be considered by managing clinicians, particularly for patients who have failed to respond to conventional treatment and as an alternative to instituting immunomodulatory agents.”15
Before-and-after trial. Forty-six patients with moderate or severe chronic, persistent, stable asthma were selected as a cohort unlikely to experience spontaneous remission (ie, patients in exacerbation were excluded) (Level 2 study: prospective cohort).17 Subjects were treated for a median of 4 weeks (range, 3 to 9 weeks) with oral doxycycline, 100 mg bid; azithromycin, 1000 mg, once weekly; or erythromycin, 1000 mg/d in divided doses. Average duration of posttreatment follow-up was 6 months. All subjects were positive for antibodies to Chlamydia pneumoniae.
Four patients with diagnosed acuteC pneumoniae respiratory infection developed chronic asthma, which disappeared in each case after treatment. Of the other 42 seroreactive patients who were treated a mean of 6 years after they developed chronic asthma, 21 had either complete remission of asthma symptoms (n = 3) or major persistent clinical improvement (n = 18). Clinical improvement was more likely to occur in patients with early disease (P = .01) and before development of fixed airway obstruction (P < .01).
These results are consistent with the hypothesis that chronic infection of the lower respiratory tract contributes to the development and progression of asthma.17 Although clinical improvement was more likely in early asthma compared with asthma with fixed airway obstruction, improvement was nevertheless noted in the latter group.
Continue to: Physicians should also note...
Physicians should also note the landmark trial of azithromycin in severe, smoking-associated COPD, which found a clinically significant benefit in reducing exacerbations and improving quality of life (NNT = 3, to prevent 1 exacerbation).18
Case series. In a prospective case series (Level 2 study: prospective cohort), 163 primary care outpatients (adolescents and adults) who had acute wheezing illnesses or chronic asthma were evaluated for C pneumoniae infection by serologic testing.19 A subgroup of this cohort also had nasopharyngeal cultures tested for C pneumoniae.
Twenty patients (12%) were given a diagnosis of C pneumoniae infection defined by serology (n = 15), culture isolation (n = 3), or both (n = 2). Of the 20, 10 wheezed for the first time—6 of whom subsequently developed chronic asthma (n = 5) or chronic bronchitis (n = 1), with a serologic profile suggesting chronic infection. The other 10 patients who had a diagnosis of C pneumoniae infection already had a diagnosis of chronic asthma. In patients with established chronic asthma, initial serologic findings suggested chronic, rather than acute, C pneumoniae infection.
Tx recommendations: When to consider azithromycin
Randomized7 and nonrandomized15 evidence supports treating severely uncontrolled or refractory asthma (strength of recommendation [SOR], B); no comparable randomized trials of azithromycin have been conducted for new-onset asthma (SOR, C). Consider prescribing empiric azithromycin for patients with new-onset asthma in the context of shared decision making about potential benefits, harms, and consequences of chronic asthma (SOR, C).
It is important to note that wheezing is frequently associated with uncomplicated acute bronchitis that resolves spontaneously without antibiotic treatment.11 Azithromycin treatment for new-onset asthma should therefore be reserved for patients in whom apparent uncomplicated acute bronchitis fails to resolve after 3 to 6 months, and whose illness is diagnosable as asthma (see CASE 3).10
Continue to: Do biomarkers predict response?
Do biomarkers predict response?
Confirming C pneumoniae infection by bronchoscopy before beginning treatment has been recommended20 but might be impractical; also, diagnostic testing for C pneumoniae is limited in availability and has potentially low sensitivity for diagnosing chronic deep lung infection.
So should you test for C pneumoniae biomarkers (or for biomarkers of Mycoplasma pneumoniae, another atypical infection implicated in the pathogenesis of asthma21) before initiating treatment? Azithromycin has antimicrobial, immunomodulatory, and potential antiviral properties.3 The body of evidence reviewed here indicates that the effects of macrolides on asthma might be, at least in part, antimicrobial. However, there is no direct evidence that the benefit of azithromycin in asthma is limited to patients who have positive infection biomarkers.22 Therefore, infection biomarker testing as a decision aid cannot be recommended at this time (although future research might alter this recommendation).
Acute bronchitis and asthma-onset associated with an acute lower respiratory tract infection have been statistically associated with biomarkers of C pneumoniae infection.23 However, C pneumoniae biomarkers are also prevalent in patients who have asthma that is not associated with an infectious onset.23 Several other matters are worth noting:
- C pneumoniae-specific IgA23 and IgE24 are promising biomarkers that deserve further investigation.
- M pneumoniae infection has also been associated with asthma and a response to antibiotic therapy.21,25
- Noneosinophilic severe asthma is another potential predictive characteristic.26 The applicability of this biomarker to primary care practice is limited, however, by the invasive nature of bronchoscopy and by the uncertain validity of the diagnostic concept: There is no guarantee that dynamic inflammatory infiltrates remain stable over a lifetime. Furthermore, the AMAZES Trial7 reported that azithromycin benefit was comparable in eosinophilic and noneosinophilic asthma.
Potential for harm withlong-term macrolide use?
Controversies about the role of macrolides in asthma involve uncertainty about who might benefit from treatment and the potential harms of macrolides use (TABLE 127,28 and discussed below).29

Adverse effects. The newer macrolides azithromycin and clarithromycin offer favorable safety and tolerability profiles, compared with those of older agents.30 In clinical trials of azithromycin, gastrointestinal symptoms (nausea, vomiting, abdominal pain, and diarrhea) were usually mild or moderate and rarely (< 2% of subjects) required discontinuation of study medication.31,32Clostridium difficile diarrhea has not been reported in any of the large clinical trials, in which thousands of patients received azithromycin for 3 to 12 months.31,32 The major clinical “side effects” attributable to azithromycin are a significant reduction, compared to placebo, in acute respiratory illness, bronchitis, pneumonia, and sinusitis.31,32
Continue to: Antibiotic resistance
Antibiotic resistance. Exposure of populations to macrolides can increase the percentage of macrolide-resistant bacterial respiratory pathogens33; policies aimed at decreasing inappropriate macrolide prescribing can significantly lower that percentage.34 There is no evidence, however, of any detrimental effects of macrolide resistance in individual patients receiving azithromycin.33
In trials of azithromycin for the treatment of trachoma in Africa, significantly fewer deaths occurred in villages where subjects were treated with azithromycin than in villages where azithromycin therapy was not provided.35 In the United States, weekly azithromycin treatment for 3 to 12 months in adults with heart disease resulted in fewer cases of acute bronchitis and pneumonia, compared with the placebo-treated groups31,32; similar benefit for azithromycin was seen in children who had recurrent lung infection.8,36
Nevertheless, concern over the spread of macrolide-resistant bacteria to the surrounding community is a concern and a possibility—and should be the subject of future research.
Sudden cardiac death. In a Medicaid population, the risk of sudden cardiac death from taking a macrolide among patients at high risk of cardiovascular disease was 1 in every 4000 administrations.27 Compare that level of risk with the 1 in 167 risk of an acute cardiovascular event in patients with COPD who start taking a LABA.37 There is no detectable increase in the risk of sudden cardiac death when taking azithromycin in the general (ie, average cardiovascular risk) population38,39 or when azithromycin is coadministered with a LABA.3
Hearing loss. An excess of 18 (< 1%) patients affected by hearing loss, 7 of whom sought medical attention, was reported among 2004 patients who had stable coronary artery disease and had been treated once weekly with azithromycin for 12 months (P = .02, compared with placebo).32 In another study, hearing test changes leading to discontinuation of azithromycin were detected in an excess of 32
Continue to: Physicians who prescribe...
Physicians who prescribe long-term azithromycin should instruct patients to report any hearing loss.
Drug–drug interactions. Azithromycin is free of the drug–drug interactions characteristic of conventional macrolides, such as clarithromycin.40 Nevertheless:
- Caution is advised when giving azithromycin in conjunction with coumadin or theophylline.
- Giving azithromycin with antacids that contain aluminum or magnesium salts can reduce the rate, although not the extent, of the absorption of azithromycin.
- The serum concentration of azithromycin is markedly increased when it is given with nelfinavir.40
Microbiome effects. The host microbiome can have a significant effect on the risk of asthma.2 A cross-sectional study indicated that lower respiratory bacterial burden is greater in patients with asthma, compared with that of healthy control subjects, and correlates with bronchial hyperresponsiveness.41 Early colonization of the infant nasopharynx, particularly with Streptococcus spp, is a predictor of asthma risk.42,43 Bacterial pathogens in the nasopharyngeal biome at the time of upper respiratory viral infection are significant determinants of risk for the spread of infection to the lower airways, suggesting that these microorganisms contribute to the risk of persistent asthma.41
Investigators have speculated that, rather than increasing the risk of asthma by disrupting the “healthy” microbiome, azithromycin might be helpful in treating an “unhealthy” microbiome.42,43 Recently, it was shown in a randomized trial that azithromycin induced a perturbation in the gut microbiota of children 14 days after randomization, although the drug did not have a long-lasting effect on the composition of gut microbiota.44
What about cost?
Inhaled corticosteroids and combination formulations of an ICS and a LABA are expensive and must be taken for the long term. A 3-month course of generic azithromycin—comparable to what was used in the OL subgroup of AZMATICS15—costs about as much as 1 ICS and LABA combination inhaler. Using published results,15,45 a pilot cost-effectiveness analysis in patients with persistent asthma compared doubling the ICS dosage, adding salmeterol, adding tiotropium, or prescribing 3 months of azithromycin. In the long run, azithromycin was 10 to 20 times as cost-effective as the other 3 therapeutic options for improving asthma quality-of-life outcomes.* However, reliable cost-effectiveness analyses require more, and better, evidence.
Continue to: Recommendations to reflect on for your practice
Recommendations to reflect on for your practice
Table 27,15 outlines selected long-term (≥ 3 months) macrolide dosing schedules in the management of asthma. Consider a trial of azithromycin for your patients
- whose asthma is refractory (poorly controlled persistent asthma), despite treatment with either an ICS and LABA combination or an ICS and long-acting muscarinic antagonist combination; and
- who have new-onset asthma.

Last, there is no evidence for or against prescribing azithromycin for patients who have chronic asthma that is not refractory but is uncontrolled because they are not being treated according to guidelines.
*Data available from the author upon request. See “Correspondence,” at end of article.
CORRESPONDENCE
David L. Hahn, MD, MS, Department of Family Medicine & Community Health, University of Wisconsin School of Medicine & Public Health, 1100 Delaplaine Court, Madison, WI 53715; [email protected].
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
1. Hahn DL. Role of Chlamydia pneumoniae as an inducer of asthma. In: Friedman H, Yamamoto Y, Bendinelli M, eds. Chlamydia Pneumoniae: Infection and Disease. New York: Kluwer Academic/Plenum Publishers; 2004:239-262.
2. Webley WC, Hahn DL. Infection-mediated asthma: etiology, mechanisms and treatment options, with focus on Chlamydia pneumoniae and macrolides. Respir Res. 2017;18:98.
3. Wong EH, Porter JD, Edwards MR, et al. The role of macrolides in asthma: current evidence and future directions. Lancet Respir Med. 2014;2:657-670.
4. Reiter J, Demirel N, Mendy A, et al. Macrolides for the long-term management of asthma—a meta-analysis of randomized clinical trials. Allergy. 2013;68:1040-1049.
5. Kew KM, Undela K, Kotortsi I, et al. Macrolides for chronic asthma. Cochrane Database Syst Rev. 2015(9):CD002997.
6. Travers J, Marsh S, Williams M, et al. External validity of randomised controlled trials in asthma: to whom do the results of the trials apply? Thorax. 2007;62:219-223.
7. Gibson PG, Yang IA, Upham JW, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659-668.
8. Stokholm J, Chawes BL, Vissing NH, et al. Azithromycin for episodes with asthma-like symptoms in young children aged 1-3 years: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2016;4:19-26.
9. Korn S, Both J, Jung M, et al. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol. 2011;107:474-479.
10. Hahn DL. A Cure for Asthma? What Your Doctor Isn’t Telling You—and Why. Durham, North Carolina: Peoples Pharmacy Press; 2013.
11. Hahn DL. Acute asthmatic bronchitis: a new twist to an old problem. J Fam Pract. 1994;39:431-435.
12. Johnston SL, Blasi F, Black PN, et al; TELICAST Investigators. The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354:1589-1600.
13. Johnston SL, Szigeti M, Cross M, et al. Azithromycin for acute exacerbations of asthma: the AZALEA Randomized Clinical Trial. JAMA Intern Med. 2016;176:1630-1637.
14. Brusselle GG, Van Braeckel E. AZALEA trial highlights antibiotic overuse in acute asthma attacks. JAMA Intern Med. 2016;176:1637-1638.
15. Hahn DL, Grasmick M, Hetzel S, et al; AZMATICS (AZithroMycinAsthma Trial In Community Settings) Study Group. Azithromycin for bronchial asthma in adults: an effectiveness trial. J Am Board Fam Med. 2012;25:442-459.
16. Hahn DL. An unanticipated effect of clinical trial registration. BMJ.com. November 2, 2007. https://www.bmj.com/rapid-response/2011/11/01/unanticipated-effect-clinical-trial-registration. Accessed November 2, 2019.
17. Hahn DL. Treatment of Chlamydia pneumoniae infection in adult asthma: a before-after trial. J Fam Pract. 1995;41:345-351.
18. Albert RK, Connett J, Bailey WC, et al; COPD Clinical Research Network. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689-698.
19. Hahn DL, McDonald R. Can acute Chlamydia pneumoniae infection initiate chronic asthma? Ann Allergy Asthma Immunol. 1998;81:339-344.
20. Rollins DR, Beuther DA, Martin RJ. Update on infection and antibiotics in asthma. Curr Allergy Asthma Rep. 2010;10:67-73.
21. Martin RJ, Kraft M, Chu HW, et al. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595-601.
22. Hahn DL, Plane MB, Mahdi OS, et al. Secondary outcomes of a pilot randomized trial of azithromycin treatment for asthma. PLoS Clin Trials. 2006;1:e11.
23. Hahn DL, Peeling RW, Dillon E, et al. Serologic markers for Chlamydia pneumoniae in asthma. Ann Allergy Asthma Immunol. 2000;84: 227-233.
24. Hahn DL, Schure A, Patel K, et al. Chlamydia pneumoniae-specific IgE is prevalent in asthma and is associated with disease severity. PLoS One. 2012;7:e35945.
25. Kraft M, Cassell GH, Pak J, et al. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782-1788.
26. Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax. 2013;68:322-329.
27. Ray WA, Murray KT, Hall K, et al. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890.
28. Jespersen CM, Als-Nielsen B, Damgaard M, et al. Randomised placebo controlled multicentre trial to assess short term clarithromycin for patients with stable coronary heart disease: CLARICOR trial. BMJ. 2006;332:22-27.
29. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43:343-373.
30. Jackson LA, Stewart DK, Wang SP, et al. Safety and effect on antiChlamydia pneumoniae antibody titres of a 1 month course of daily azithromycin in adults with coronary artery disease. J Antimicrob Chemother. 1999;44:411-414.
31. O’Connor CM, Dunne MW, Pfeffer MA, et al; Investigators in the WIZARD Study. Azithromycin for the secondary prevention of coronary heart disease events: the WIZARD study: a randomized controlled trial. JAMA. 2003;290:1459-1466.
32. Grayston JT, Kronmal RA, Jackson LA, et al; ACES Investigators. Azithromycin for the secondary prevention of coronary events. N Engl J Med. 2005;352:1637-1645.
33. Skalet AH, Cevallos V, Ayele B, et al. Antibiotic selection pressure and macrolide resistance in nasopharyngeal Streptococcus pneumoniae: a cluster-randomized clinical trial. PLoS Med. 2010;7:e1000377.
34. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. Finnish Study Group for Antimicrobial Resistance. N Engl J Med. 1997;337:441-446.
35. Keenan JD, Emerson PM, Gaynor BD, et al. Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Intern Med. 2013;173:821-833.
36. Bacharier LB, Guilbert TW, Mauger DT, et al. Early administration of azithromycin and prevention of severe lower respiratory tract illnesses in preschool children with a history of such illnesses: a randomized clinical trial. JAMA. 2015;314:2034-2044.
37. Wang MT, Liou JT, Lin CW, et al. Association of cardiovascular risk with inhaled long-acting bronchodilators in patients with chronic obstructive pulmonary disease: a nested case-control study. JAMA Intern Med. 2018;178:229-238.
38. Svanström H, Pasternak B, Hviid A. Use of azithromycin and death from cardiovascular causes. N Engl J Med. 2013;368:1704-1712.
39. Khosropour CM, Capizzi JD, Schafer SD, et al. Lack of association between azithromycin and death from cardiovascular causes. N Engl J Med. 2014;370:1961-1962.
40. Bakheit AH, Al-Hadiya BM, Abd-Elgalil AA. Azithromycin. Profiles Drug Subst Excip Relat Methodol. 2014;39:1-40.
41. Huang YJ, Nelson CE, Brodie EL, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol. 2011;127:372-381.e1-3.
42. Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978.
43. Teo SM, Mok D, Pham K, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe. 2015;17:704-715.
44. Wei S, Mortensen MS, Stokholm J, et al. Short- and long-term impacts of azithromycin treatment on the gut microbiota in children: a double-blind, randomized, placebo-controlled trial. EBioMedicine. 2018;38:265-272.
45. Peters SP, Kunselman SJ, Icitovic N, et al; National Heart, Lung, and Blood Institute Asthma Clinical Research Network. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. New Engl J Med. 2010;363:1715-1726.
PRACTICE RECOMMENDATIONS
› Consider a trial of azithromycin for patients who have poorly controlled persistent asthma and are not responding to guideline treatment with the combination of an inhaled corticosteroid and either a long-acting bronchodilator or long-acting muscarinic antagonist. B
› Consider a trial of azithromycin in addition to first-line guideline therapy for patients who have new-onset asthma. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Public reporting needs reform!
Like many of my colleagues, I support President Obama’s call to demonstrate value as part of health care reform. One way to do that is through public reporting. The rationale is that public scrutiny of outcomes will motivate the health care “industry” to improve the “product” (outcomes), rather than accelerating valueless economic activity (process) that often benefits providers more than patients.
Fair enough. But does the existing system of quality indicators support the goals of reform identified by the Institute of Medicine (IOM)?1 That is, does it make the system safer, more effective and efficient, timely, equitable, and patient-centered?
Not necessarily.
The reason is 2-fold. First, the best quality indicators are patient-oriented outcomes (eg, quality of life, morbidity, mortality), but that’s not what’s being reported. Second, many publicly reported surrogate measures are more harmful than helpful, and in need of serious reform themselves.
My experience
The Wisconsin Collaborative for Healthcare Quality (WCHQ), which I’ve been involved with for nearly 10 years, is composed of health care organizations, mine included, committed to voluntary reporting of quality metrics. The TABLE features a list of the metrics, chosen by the WCHQ, that are reported.
I’ve rated each metric on 2 criteria:
- How good is the evidence for the screening tool or intervention? (There is good evidence for colorectal cancer screening, for example, but evidence for low-density lipoprotein [LDL] testing is poor.)
- How good is the quality indicator itself, including the frequency? (There’s good evidence for Pap testing within 3 years, whereas twice-yearly HbA1c testing is opinion-based.)
TABLE 1
How do the “quality indicators” rate?
| SCREENING TOOL/INTERVENTION (RECOMMENDED METRIC) | SCREENING TOOL SOR | METRIC SOR |
|---|---|---|
| Colorectal cancer screening (various modalities and frequencies) | A | A |
| Pap smear (within 3 years) | A | A |
| Tobacco use (documented in the past year) | A | B |
| DM2: BP control (last BP <130/80) | A | B |
| BP control in nondiabetics (last BP <140/90) | A | B |
| DM2: HbA1c testing (at least twice yearly) | B | C |
| DM2: blood sugar control (HbA1c <7) | B | C |
| Pneumococcal vaccine (once after age 65) | B | C |
| Mammography (within 2 years, women ages 40-69) | B | C |
| Postpartum care (21-56 days after delivery) | C | C |
| DM2: kidney function monitored (creatinine yearly) | C | C |
| CVD: LDL testing (yearly) | C | C |
| CVD: LDL control (LDL<100 mg/dL) | C | C |
| DM2: LDL testing (yearly) | C | C |
| DM2: LDL control (LDL<100 mg/dL) | C | C |
| BP, blood pressure; CVD, cardiovascular disease; DM2, type 2 diabetes; HbA1c, glycosylated hemoglobin; LDL, low-density lipoproteins, SOR, strength of recommendation. | ||
| Strength of recommendation (SOR): A Good-quality patient-oriented evidence B Inconsistent or limited-quality patient-oriented evidence C Consensus, usual practice, opinion, disease-oriented evidence, case series Source: Wisconsin Collaborative for Healthcare Quality (http://www.wchq.org/). | ||
Some worrisome examples
While the ratings are partly subjective, they’re meant to illustrate that not all publicly reported metrics are supported by good evidence.
This is particularly troubling, given the fact that acting on fair or poor evidence may cause more harm than good. Consider these worrisome examples:
LDL control <100 mg/dL. I’ve known patients who had their first myocardial infarction when their LDL cholesterol was <100 mg/dL. After the event, these patients weren’t given a statin because they were already “at goal”; they subsequently had a reinfarction.
LDL should not be used as a quality indicator in secondary prevention for (at least) 2 reasons: First, some LDL-lowering drugs are harmful or have no net benefit (eg, estrogen in women, fibrates).2 Second, statin benefit may or may not be related to lipid lowering, and the magnitude of benefit is not related to any arbitrary LDL goal.3
There is clear, compelling evidence supporting near-universal statin therapy for patients at high cardiovascular risk regardless of their LDL cholesterol values—but a lack of evidence that titrating lipid therapy to achieve proposed low LDL levels is beneficial or safe.4 Receiving the maximum tolerated dose of statin is therefore the appropriate evidence-based surrogate quality indicator, not LDL.
Mammography. The Cochrane collaboration has concluded that “for every 2000 women invited for [mammography] screening throughout 10 years, 1 will have her life prolonged,” and 10 healthy women who would not have been diagnosed without the screening will be treated unnecessarily.5 The Cochrane review thus concluded that it’s not clear whether mammography screening does more good than harm.5
The US Preventive Services Task Force (USPSTF) recently downgraded mammography screening for women over age 50 from an A- to a B-rated recommendation. The fine balance between benefit and harm in this and other USPSTF B-rated preventive measures requires that clinicians educate patients and elicit their preferences. But this doesn’t occur when health plans strive to outdo one another in achieving higher publicly reported screening goals.6 Documentation of valid shared decision-making, not screening rates, is the appropriate quality indicator.
The evidence vs the “business” of medicine
I have no illusions that my recommendations will be adopted easily—or soon. After all, we practice in an environment in which evidence-based practice recommendations can conflict with financial and operational goals perceived as necessary to survive. However, I believe that evidence trumps business in achieving the IOM goals.
It remains to be seen whether we can simultaneously move toward valid evidence-based public reporting and health-care financial reform. But one thing is clear: To insist that evidence-based patient-oriented quality indicators are too difficult to measure, or to ignore or deny the evidence, puts the lie to claims of patient-centered care and, ultimately, to long-needed health care reform looming on the horizon.
1. Institute of Medicine Crossing the Quality Chasm. Washington, DC: National Academy of Science; 2001.
2. Studer M, Briel M, Leimenstoll B, et al. Effect of different antilipidemic agents and diets on mortality: a systematic review. Arch Intern Med. 2005;165:725-730.
3. Baigent C, Keech A, Kearney PM, et al. for the Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267-1278.
4. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
5. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2006;(4):CD001877.-
6. Jørgensen KJ, Gøtzsche PC. Content of invitations for publicly funded screening mammography. BMJ. 2006;332:538-541.
Like many of my colleagues, I support President Obama’s call to demonstrate value as part of health care reform. One way to do that is through public reporting. The rationale is that public scrutiny of outcomes will motivate the health care “industry” to improve the “product” (outcomes), rather than accelerating valueless economic activity (process) that often benefits providers more than patients.
Fair enough. But does the existing system of quality indicators support the goals of reform identified by the Institute of Medicine (IOM)?1 That is, does it make the system safer, more effective and efficient, timely, equitable, and patient-centered?
Not necessarily.
The reason is 2-fold. First, the best quality indicators are patient-oriented outcomes (eg, quality of life, morbidity, mortality), but that’s not what’s being reported. Second, many publicly reported surrogate measures are more harmful than helpful, and in need of serious reform themselves.
My experience
The Wisconsin Collaborative for Healthcare Quality (WCHQ), which I’ve been involved with for nearly 10 years, is composed of health care organizations, mine included, committed to voluntary reporting of quality metrics. The TABLE features a list of the metrics, chosen by the WCHQ, that are reported.
I’ve rated each metric on 2 criteria:
- How good is the evidence for the screening tool or intervention? (There is good evidence for colorectal cancer screening, for example, but evidence for low-density lipoprotein [LDL] testing is poor.)
- How good is the quality indicator itself, including the frequency? (There’s good evidence for Pap testing within 3 years, whereas twice-yearly HbA1c testing is opinion-based.)
TABLE 1
How do the “quality indicators” rate?
| SCREENING TOOL/INTERVENTION (RECOMMENDED METRIC) | SCREENING TOOL SOR | METRIC SOR |
|---|---|---|
| Colorectal cancer screening (various modalities and frequencies) | A | A |
| Pap smear (within 3 years) | A | A |
| Tobacco use (documented in the past year) | A | B |
| DM2: BP control (last BP <130/80) | A | B |
| BP control in nondiabetics (last BP <140/90) | A | B |
| DM2: HbA1c testing (at least twice yearly) | B | C |
| DM2: blood sugar control (HbA1c <7) | B | C |
| Pneumococcal vaccine (once after age 65) | B | C |
| Mammography (within 2 years, women ages 40-69) | B | C |
| Postpartum care (21-56 days after delivery) | C | C |
| DM2: kidney function monitored (creatinine yearly) | C | C |
| CVD: LDL testing (yearly) | C | C |
| CVD: LDL control (LDL<100 mg/dL) | C | C |
| DM2: LDL testing (yearly) | C | C |
| DM2: LDL control (LDL<100 mg/dL) | C | C |
| BP, blood pressure; CVD, cardiovascular disease; DM2, type 2 diabetes; HbA1c, glycosylated hemoglobin; LDL, low-density lipoproteins, SOR, strength of recommendation. | ||
| Strength of recommendation (SOR): A Good-quality patient-oriented evidence B Inconsistent or limited-quality patient-oriented evidence C Consensus, usual practice, opinion, disease-oriented evidence, case series Source: Wisconsin Collaborative for Healthcare Quality (http://www.wchq.org/). | ||
Some worrisome examples
While the ratings are partly subjective, they’re meant to illustrate that not all publicly reported metrics are supported by good evidence.
This is particularly troubling, given the fact that acting on fair or poor evidence may cause more harm than good. Consider these worrisome examples:
LDL control <100 mg/dL. I’ve known patients who had their first myocardial infarction when their LDL cholesterol was <100 mg/dL. After the event, these patients weren’t given a statin because they were already “at goal”; they subsequently had a reinfarction.
LDL should not be used as a quality indicator in secondary prevention for (at least) 2 reasons: First, some LDL-lowering drugs are harmful or have no net benefit (eg, estrogen in women, fibrates).2 Second, statin benefit may or may not be related to lipid lowering, and the magnitude of benefit is not related to any arbitrary LDL goal.3
There is clear, compelling evidence supporting near-universal statin therapy for patients at high cardiovascular risk regardless of their LDL cholesterol values—but a lack of evidence that titrating lipid therapy to achieve proposed low LDL levels is beneficial or safe.4 Receiving the maximum tolerated dose of statin is therefore the appropriate evidence-based surrogate quality indicator, not LDL.
Mammography. The Cochrane collaboration has concluded that “for every 2000 women invited for [mammography] screening throughout 10 years, 1 will have her life prolonged,” and 10 healthy women who would not have been diagnosed without the screening will be treated unnecessarily.5 The Cochrane review thus concluded that it’s not clear whether mammography screening does more good than harm.5
The US Preventive Services Task Force (USPSTF) recently downgraded mammography screening for women over age 50 from an A- to a B-rated recommendation. The fine balance between benefit and harm in this and other USPSTF B-rated preventive measures requires that clinicians educate patients and elicit their preferences. But this doesn’t occur when health plans strive to outdo one another in achieving higher publicly reported screening goals.6 Documentation of valid shared decision-making, not screening rates, is the appropriate quality indicator.
The evidence vs the “business” of medicine
I have no illusions that my recommendations will be adopted easily—or soon. After all, we practice in an environment in which evidence-based practice recommendations can conflict with financial and operational goals perceived as necessary to survive. However, I believe that evidence trumps business in achieving the IOM goals.
It remains to be seen whether we can simultaneously move toward valid evidence-based public reporting and health-care financial reform. But one thing is clear: To insist that evidence-based patient-oriented quality indicators are too difficult to measure, or to ignore or deny the evidence, puts the lie to claims of patient-centered care and, ultimately, to long-needed health care reform looming on the horizon.
Like many of my colleagues, I support President Obama’s call to demonstrate value as part of health care reform. One way to do that is through public reporting. The rationale is that public scrutiny of outcomes will motivate the health care “industry” to improve the “product” (outcomes), rather than accelerating valueless economic activity (process) that often benefits providers more than patients.
Fair enough. But does the existing system of quality indicators support the goals of reform identified by the Institute of Medicine (IOM)?1 That is, does it make the system safer, more effective and efficient, timely, equitable, and patient-centered?
Not necessarily.
The reason is 2-fold. First, the best quality indicators are patient-oriented outcomes (eg, quality of life, morbidity, mortality), but that’s not what’s being reported. Second, many publicly reported surrogate measures are more harmful than helpful, and in need of serious reform themselves.
My experience
The Wisconsin Collaborative for Healthcare Quality (WCHQ), which I’ve been involved with for nearly 10 years, is composed of health care organizations, mine included, committed to voluntary reporting of quality metrics. The TABLE features a list of the metrics, chosen by the WCHQ, that are reported.
I’ve rated each metric on 2 criteria:
- How good is the evidence for the screening tool or intervention? (There is good evidence for colorectal cancer screening, for example, but evidence for low-density lipoprotein [LDL] testing is poor.)
- How good is the quality indicator itself, including the frequency? (There’s good evidence for Pap testing within 3 years, whereas twice-yearly HbA1c testing is opinion-based.)
TABLE 1
How do the “quality indicators” rate?
| SCREENING TOOL/INTERVENTION (RECOMMENDED METRIC) | SCREENING TOOL SOR | METRIC SOR |
|---|---|---|
| Colorectal cancer screening (various modalities and frequencies) | A | A |
| Pap smear (within 3 years) | A | A |
| Tobacco use (documented in the past year) | A | B |
| DM2: BP control (last BP <130/80) | A | B |
| BP control in nondiabetics (last BP <140/90) | A | B |
| DM2: HbA1c testing (at least twice yearly) | B | C |
| DM2: blood sugar control (HbA1c <7) | B | C |
| Pneumococcal vaccine (once after age 65) | B | C |
| Mammography (within 2 years, women ages 40-69) | B | C |
| Postpartum care (21-56 days after delivery) | C | C |
| DM2: kidney function monitored (creatinine yearly) | C | C |
| CVD: LDL testing (yearly) | C | C |
| CVD: LDL control (LDL<100 mg/dL) | C | C |
| DM2: LDL testing (yearly) | C | C |
| DM2: LDL control (LDL<100 mg/dL) | C | C |
| BP, blood pressure; CVD, cardiovascular disease; DM2, type 2 diabetes; HbA1c, glycosylated hemoglobin; LDL, low-density lipoproteins, SOR, strength of recommendation. | ||
| Strength of recommendation (SOR): A Good-quality patient-oriented evidence B Inconsistent or limited-quality patient-oriented evidence C Consensus, usual practice, opinion, disease-oriented evidence, case series Source: Wisconsin Collaborative for Healthcare Quality (http://www.wchq.org/). | ||
Some worrisome examples
While the ratings are partly subjective, they’re meant to illustrate that not all publicly reported metrics are supported by good evidence.
This is particularly troubling, given the fact that acting on fair or poor evidence may cause more harm than good. Consider these worrisome examples:
LDL control <100 mg/dL. I’ve known patients who had their first myocardial infarction when their LDL cholesterol was <100 mg/dL. After the event, these patients weren’t given a statin because they were already “at goal”; they subsequently had a reinfarction.
LDL should not be used as a quality indicator in secondary prevention for (at least) 2 reasons: First, some LDL-lowering drugs are harmful or have no net benefit (eg, estrogen in women, fibrates).2 Second, statin benefit may or may not be related to lipid lowering, and the magnitude of benefit is not related to any arbitrary LDL goal.3
There is clear, compelling evidence supporting near-universal statin therapy for patients at high cardiovascular risk regardless of their LDL cholesterol values—but a lack of evidence that titrating lipid therapy to achieve proposed low LDL levels is beneficial or safe.4 Receiving the maximum tolerated dose of statin is therefore the appropriate evidence-based surrogate quality indicator, not LDL.
Mammography. The Cochrane collaboration has concluded that “for every 2000 women invited for [mammography] screening throughout 10 years, 1 will have her life prolonged,” and 10 healthy women who would not have been diagnosed without the screening will be treated unnecessarily.5 The Cochrane review thus concluded that it’s not clear whether mammography screening does more good than harm.5
The US Preventive Services Task Force (USPSTF) recently downgraded mammography screening for women over age 50 from an A- to a B-rated recommendation. The fine balance between benefit and harm in this and other USPSTF B-rated preventive measures requires that clinicians educate patients and elicit their preferences. But this doesn’t occur when health plans strive to outdo one another in achieving higher publicly reported screening goals.6 Documentation of valid shared decision-making, not screening rates, is the appropriate quality indicator.
The evidence vs the “business” of medicine
I have no illusions that my recommendations will be adopted easily—or soon. After all, we practice in an environment in which evidence-based practice recommendations can conflict with financial and operational goals perceived as necessary to survive. However, I believe that evidence trumps business in achieving the IOM goals.
It remains to be seen whether we can simultaneously move toward valid evidence-based public reporting and health-care financial reform. But one thing is clear: To insist that evidence-based patient-oriented quality indicators are too difficult to measure, or to ignore or deny the evidence, puts the lie to claims of patient-centered care and, ultimately, to long-needed health care reform looming on the horizon.
1. Institute of Medicine Crossing the Quality Chasm. Washington, DC: National Academy of Science; 2001.
2. Studer M, Briel M, Leimenstoll B, et al. Effect of different antilipidemic agents and diets on mortality: a systematic review. Arch Intern Med. 2005;165:725-730.
3. Baigent C, Keech A, Kearney PM, et al. for the Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267-1278.
4. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
5. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2006;(4):CD001877.-
6. Jørgensen KJ, Gøtzsche PC. Content of invitations for publicly funded screening mammography. BMJ. 2006;332:538-541.
1. Institute of Medicine Crossing the Quality Chasm. Washington, DC: National Academy of Science; 2001.
2. Studer M, Briel M, Leimenstoll B, et al. Effect of different antilipidemic agents and diets on mortality: a systematic review. Arch Intern Med. 2005;165:725-730.
3. Baigent C, Keech A, Kearney PM, et al. for the Cholesterol Treatment Trialists’ (CTT) Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267-1278.
4. Hayward RA, Hofer TP, Vijan S. Narrative review: lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520-530.
5. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2006;(4):CD001877.-
6. Jørgensen KJ, Gøtzsche PC. Content of invitations for publicly funded screening mammography. BMJ. 2006;332:538-541.
“Should I have a mammogram?” Handout helps women decide
I was disappointed, recently, when I read a handout from the American College of Physicians (ACP) that was designed to help physicians talk to patients about the benefits and harms of screening mammography in women ages 40 to 49. The problem: The summary simply didn’t provide enough relevant information to help patients make an informed choice.
The patient handout was part of a collection of ACP articles that appeared in the Annals of Internal Medicine. (See related POEM.) The first article was a systematic review of screening mammography in women 40 to 49 years of age documenting current data on benefit and harms.1
The ACP also published a clinical practice guideline that included the recommendation that “clinicians should inform women 40 to 49 years of age about the potential benefits and harms of screening mammography.”2 Accompanying that article was a summary for patients.3 Unfortunately, though, this summary failed to provide answers to the following questions: (1) What is my risk of dying of breast cancer if I am not screened? (2) What is the quantitative benefit if I am screened? (3) What is the quantitative harm if I am screened?
Filling the gap between recommendations and practice
To provide patients with the information they need to make an informed choice, I developed a one-page patient information sheet (PATIENT HANDOUT). My handout outlines the benefit and harms as numbers per 1000 women over 10 years, as was done in a recently validated patient education pamphlet.4
I used a readily available epidemiological source5 for the base-case (without screening) breast cancer death risks, and calculated the putative decreases in breast cancer deaths due to screening using the 15% relative risk reduction adopted by the ACP,2 the USPSTF,6 and the Cochrane Collaboration editors.7
PATIENT HANDOUT
Weighing benefit and harms: Mammography for women, ages 40–49
For every 1000 women in their 40s, what are the benefits and harms of mammogram screening over a period of 10 years?
| WITH MAMMOGRAMS OVER 10 YEARS | WITHOUT MAMMOGRAMS OVER 10 YEARS | LIVES SAVED PER 1000 WOMEN SCREENED WITH MAMMOGRAMS OVER 10 YEARS | |
|---|---|---|---|
| BENEFIT OF MAMOGRAMS | Breast cancer death | Breast cancer death | |
| • Less likely to die from breast cancer1 | Black women 3.6 White women 2.1 Other women 1.3 | Black women 4.2 White women 2.5 Other women 1.5 | Less than 1 Less than 1 Less than 1 |
| HARMS OF MAMOGRAMS | Harms of screening | ||
| • More likely to have false alarms2 | 450 | None | |
| • More likely to have unnecessary diagnosis and treatment for breast cancer3 | 5 | None | |
| • More likely to have pain or discomfort from mammography4 | 320-550 | None | |
| • More likely to have radiation exposure5 | 1000 | None | |
| • More likely to have false reassurance6 | Uncertain | None | |
| 1The benefit of mammograms may be larger if you are at higher risk, or the benefit of mammograms may be smaller or even zero if the best quality research studies are correct. | |||
| 2False alarms are abnormal results that are not cancer, leading to unnecessary repeat mammograms, biopsies, and worry. | |||
| 3Not all cancer detected by mammography will cause symptoms or death. This is because not all cancers will continue to grow or spread. Doctors cannot always tell which cancers detected by mammography need treatment and which do not need treatment. | |||
| 4Many women complain of temporary discomfort or pain during mammography because the breasts are squeezed. | |||
| 5All mammography uses radiation. Doctors do not know whether this radiation causes cancer, but most doctors believe the harm is very small or nonexistent. | |||
| 6Some women who develop breast cancer before their next screening mammogram might delay treatment because their previous mammogram was normal. | |||
The science behind the bullet points
I kept the grid simple, but recognize that my colleagues would appreciate knowing where the numbers came from, and what was the basis for certain explanatory statements. So here is some background on the first three bullets.
- “Benefit: Less likely to die from breast cancer.” The explanation (#1) that is tagged to this statement notes that the benefit of mammograms may be larger if a woman is at higher risk. This statement was taken from the ACP meta-analysis and guideline.1,2 The second part of this explanatory sentence, which indicates that “the benefit of mammograms may be smaller or even zero if the best quality research studies are correct” is based on the results of the Cochrane Review7 and is acknowledged in the ACP guideline.2
- “Harm: More likely to have false alarms (false positive mammograms).” I presented the percentage of false positive mammograms as 45% based on the 5-year (30%) and 10-year (56%) false-positive percentages reported for the Harvard Pilgrim Health Care study by the ACP.2
- “Harm: More likely to have unnecessary diagnosis and treatment for breast cancer.” I listed the number of women likely to suffer this harm as 5, based on the Cochrane Collaboration Review on screening for breast cancer with mammography.7
Is it easier to just order the test?
In talking with my colleagues, I gather that many are aware that controversies surround the “B”-rated USPSTF recommendation for mammography for women ages 40 to 49. However, my colleagues often lack the time to discuss the details with each patient, so they tell me that it’s “just easier to order the test.”
I disagree. Physicians can use the patient information sheet I’ve developed to provide basic relevant information to women who are trying to decide whether or not to get a mammogram. If my sheet doesn’t meet your needs, consider others. (See Fast Track, below right.)
The ACP may have fallen short with its mammography screening summary for patients, but your discussion with patients need not.
Acknowledgments
The author would like to acknowledge Phil Colmenares, MD, MPH, for his valuable comments and input into the design of the patient information handout.
1. Armstrong K, Moye E, Williams S, Berlin JA. Screening mammography for women 40 to 49 years of age: A systematic review for the American College of Physicians. Ann Int Med 2007;146:516-526.
2. Qaseem A, Snow V, Sherif K, Aronson M, Weiss KB, Owens DK. Screening mammography for women 40 to 49 years of age: A clinical practice guideline from the American College of Physicians. Ann Int Med 2007;146:511-515.
3. Summaries for Patients: Screening mammography in women age 40 to 49 years. Ann Int Med 2007;146:1-20.
4. Woloshin S, Schwartz LM, Welch HG. The effectiveness of a primer to help people understand risk. Two randomized trials in distinct populations. Ann Int Med 2007;146:256-265
5. Marbella AM, Layde PM. Racial trends in age-specific breast cancer mortality rates in US women. Am J Public Health 2001;91:118-121.
6. USPSTF. Screening for breast cancer: recommendations and rationale. Ann Int Med 2002;137:344-346.
7. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev 2006;(4):CD001877.
I was disappointed, recently, when I read a handout from the American College of Physicians (ACP) that was designed to help physicians talk to patients about the benefits and harms of screening mammography in women ages 40 to 49. The problem: The summary simply didn’t provide enough relevant information to help patients make an informed choice.
The patient handout was part of a collection of ACP articles that appeared in the Annals of Internal Medicine. (See related POEM.) The first article was a systematic review of screening mammography in women 40 to 49 years of age documenting current data on benefit and harms.1
The ACP also published a clinical practice guideline that included the recommendation that “clinicians should inform women 40 to 49 years of age about the potential benefits and harms of screening mammography.”2 Accompanying that article was a summary for patients.3 Unfortunately, though, this summary failed to provide answers to the following questions: (1) What is my risk of dying of breast cancer if I am not screened? (2) What is the quantitative benefit if I am screened? (3) What is the quantitative harm if I am screened?
Filling the gap between recommendations and practice
To provide patients with the information they need to make an informed choice, I developed a one-page patient information sheet (PATIENT HANDOUT). My handout outlines the benefit and harms as numbers per 1000 women over 10 years, as was done in a recently validated patient education pamphlet.4
I used a readily available epidemiological source5 for the base-case (without screening) breast cancer death risks, and calculated the putative decreases in breast cancer deaths due to screening using the 15% relative risk reduction adopted by the ACP,2 the USPSTF,6 and the Cochrane Collaboration editors.7
PATIENT HANDOUT
Weighing benefit and harms: Mammography for women, ages 40–49
For every 1000 women in their 40s, what are the benefits and harms of mammogram screening over a period of 10 years?
| WITH MAMMOGRAMS OVER 10 YEARS | WITHOUT MAMMOGRAMS OVER 10 YEARS | LIVES SAVED PER 1000 WOMEN SCREENED WITH MAMMOGRAMS OVER 10 YEARS | |
|---|---|---|---|
| BENEFIT OF MAMOGRAMS | Breast cancer death | Breast cancer death | |
| • Less likely to die from breast cancer1 | Black women 3.6 White women 2.1 Other women 1.3 | Black women 4.2 White women 2.5 Other women 1.5 | Less than 1 Less than 1 Less than 1 |
| HARMS OF MAMOGRAMS | Harms of screening | ||
| • More likely to have false alarms2 | 450 | None | |
| • More likely to have unnecessary diagnosis and treatment for breast cancer3 | 5 | None | |
| • More likely to have pain or discomfort from mammography4 | 320-550 | None | |
| • More likely to have radiation exposure5 | 1000 | None | |
| • More likely to have false reassurance6 | Uncertain | None | |
| 1The benefit of mammograms may be larger if you are at higher risk, or the benefit of mammograms may be smaller or even zero if the best quality research studies are correct. | |||
| 2False alarms are abnormal results that are not cancer, leading to unnecessary repeat mammograms, biopsies, and worry. | |||
| 3Not all cancer detected by mammography will cause symptoms or death. This is because not all cancers will continue to grow or spread. Doctors cannot always tell which cancers detected by mammography need treatment and which do not need treatment. | |||
| 4Many women complain of temporary discomfort or pain during mammography because the breasts are squeezed. | |||
| 5All mammography uses radiation. Doctors do not know whether this radiation causes cancer, but most doctors believe the harm is very small or nonexistent. | |||
| 6Some women who develop breast cancer before their next screening mammogram might delay treatment because their previous mammogram was normal. | |||
The science behind the bullet points
I kept the grid simple, but recognize that my colleagues would appreciate knowing where the numbers came from, and what was the basis for certain explanatory statements. So here is some background on the first three bullets.
- “Benefit: Less likely to die from breast cancer.” The explanation (#1) that is tagged to this statement notes that the benefit of mammograms may be larger if a woman is at higher risk. This statement was taken from the ACP meta-analysis and guideline.1,2 The second part of this explanatory sentence, which indicates that “the benefit of mammograms may be smaller or even zero if the best quality research studies are correct” is based on the results of the Cochrane Review7 and is acknowledged in the ACP guideline.2
- “Harm: More likely to have false alarms (false positive mammograms).” I presented the percentage of false positive mammograms as 45% based on the 5-year (30%) and 10-year (56%) false-positive percentages reported for the Harvard Pilgrim Health Care study by the ACP.2
- “Harm: More likely to have unnecessary diagnosis and treatment for breast cancer.” I listed the number of women likely to suffer this harm as 5, based on the Cochrane Collaboration Review on screening for breast cancer with mammography.7
Is it easier to just order the test?
In talking with my colleagues, I gather that many are aware that controversies surround the “B”-rated USPSTF recommendation for mammography for women ages 40 to 49. However, my colleagues often lack the time to discuss the details with each patient, so they tell me that it’s “just easier to order the test.”
I disagree. Physicians can use the patient information sheet I’ve developed to provide basic relevant information to women who are trying to decide whether or not to get a mammogram. If my sheet doesn’t meet your needs, consider others. (See Fast Track, below right.)
The ACP may have fallen short with its mammography screening summary for patients, but your discussion with patients need not.
Acknowledgments
The author would like to acknowledge Phil Colmenares, MD, MPH, for his valuable comments and input into the design of the patient information handout.
I was disappointed, recently, when I read a handout from the American College of Physicians (ACP) that was designed to help physicians talk to patients about the benefits and harms of screening mammography in women ages 40 to 49. The problem: The summary simply didn’t provide enough relevant information to help patients make an informed choice.
The patient handout was part of a collection of ACP articles that appeared in the Annals of Internal Medicine. (See related POEM.) The first article was a systematic review of screening mammography in women 40 to 49 years of age documenting current data on benefit and harms.1
The ACP also published a clinical practice guideline that included the recommendation that “clinicians should inform women 40 to 49 years of age about the potential benefits and harms of screening mammography.”2 Accompanying that article was a summary for patients.3 Unfortunately, though, this summary failed to provide answers to the following questions: (1) What is my risk of dying of breast cancer if I am not screened? (2) What is the quantitative benefit if I am screened? (3) What is the quantitative harm if I am screened?
Filling the gap between recommendations and practice
To provide patients with the information they need to make an informed choice, I developed a one-page patient information sheet (PATIENT HANDOUT). My handout outlines the benefit and harms as numbers per 1000 women over 10 years, as was done in a recently validated patient education pamphlet.4
I used a readily available epidemiological source5 for the base-case (without screening) breast cancer death risks, and calculated the putative decreases in breast cancer deaths due to screening using the 15% relative risk reduction adopted by the ACP,2 the USPSTF,6 and the Cochrane Collaboration editors.7
PATIENT HANDOUT
Weighing benefit and harms: Mammography for women, ages 40–49
For every 1000 women in their 40s, what are the benefits and harms of mammogram screening over a period of 10 years?
| WITH MAMMOGRAMS OVER 10 YEARS | WITHOUT MAMMOGRAMS OVER 10 YEARS | LIVES SAVED PER 1000 WOMEN SCREENED WITH MAMMOGRAMS OVER 10 YEARS | |
|---|---|---|---|
| BENEFIT OF MAMOGRAMS | Breast cancer death | Breast cancer death | |
| • Less likely to die from breast cancer1 | Black women 3.6 White women 2.1 Other women 1.3 | Black women 4.2 White women 2.5 Other women 1.5 | Less than 1 Less than 1 Less than 1 |
| HARMS OF MAMOGRAMS | Harms of screening | ||
| • More likely to have false alarms2 | 450 | None | |
| • More likely to have unnecessary diagnosis and treatment for breast cancer3 | 5 | None | |
| • More likely to have pain or discomfort from mammography4 | 320-550 | None | |
| • More likely to have radiation exposure5 | 1000 | None | |
| • More likely to have false reassurance6 | Uncertain | None | |
| 1The benefit of mammograms may be larger if you are at higher risk, or the benefit of mammograms may be smaller or even zero if the best quality research studies are correct. | |||
| 2False alarms are abnormal results that are not cancer, leading to unnecessary repeat mammograms, biopsies, and worry. | |||
| 3Not all cancer detected by mammography will cause symptoms or death. This is because not all cancers will continue to grow or spread. Doctors cannot always tell which cancers detected by mammography need treatment and which do not need treatment. | |||
| 4Many women complain of temporary discomfort or pain during mammography because the breasts are squeezed. | |||
| 5All mammography uses radiation. Doctors do not know whether this radiation causes cancer, but most doctors believe the harm is very small or nonexistent. | |||
| 6Some women who develop breast cancer before their next screening mammogram might delay treatment because their previous mammogram was normal. | |||
The science behind the bullet points
I kept the grid simple, but recognize that my colleagues would appreciate knowing where the numbers came from, and what was the basis for certain explanatory statements. So here is some background on the first three bullets.
- “Benefit: Less likely to die from breast cancer.” The explanation (#1) that is tagged to this statement notes that the benefit of mammograms may be larger if a woman is at higher risk. This statement was taken from the ACP meta-analysis and guideline.1,2 The second part of this explanatory sentence, which indicates that “the benefit of mammograms may be smaller or even zero if the best quality research studies are correct” is based on the results of the Cochrane Review7 and is acknowledged in the ACP guideline.2
- “Harm: More likely to have false alarms (false positive mammograms).” I presented the percentage of false positive mammograms as 45% based on the 5-year (30%) and 10-year (56%) false-positive percentages reported for the Harvard Pilgrim Health Care study by the ACP.2
- “Harm: More likely to have unnecessary diagnosis and treatment for breast cancer.” I listed the number of women likely to suffer this harm as 5, based on the Cochrane Collaboration Review on screening for breast cancer with mammography.7
Is it easier to just order the test?
In talking with my colleagues, I gather that many are aware that controversies surround the “B”-rated USPSTF recommendation for mammography for women ages 40 to 49. However, my colleagues often lack the time to discuss the details with each patient, so they tell me that it’s “just easier to order the test.”
I disagree. Physicians can use the patient information sheet I’ve developed to provide basic relevant information to women who are trying to decide whether or not to get a mammogram. If my sheet doesn’t meet your needs, consider others. (See Fast Track, below right.)
The ACP may have fallen short with its mammography screening summary for patients, but your discussion with patients need not.
Acknowledgments
The author would like to acknowledge Phil Colmenares, MD, MPH, for his valuable comments and input into the design of the patient information handout.
1. Armstrong K, Moye E, Williams S, Berlin JA. Screening mammography for women 40 to 49 years of age: A systematic review for the American College of Physicians. Ann Int Med 2007;146:516-526.
2. Qaseem A, Snow V, Sherif K, Aronson M, Weiss KB, Owens DK. Screening mammography for women 40 to 49 years of age: A clinical practice guideline from the American College of Physicians. Ann Int Med 2007;146:511-515.
3. Summaries for Patients: Screening mammography in women age 40 to 49 years. Ann Int Med 2007;146:1-20.
4. Woloshin S, Schwartz LM, Welch HG. The effectiveness of a primer to help people understand risk. Two randomized trials in distinct populations. Ann Int Med 2007;146:256-265
5. Marbella AM, Layde PM. Racial trends in age-specific breast cancer mortality rates in US women. Am J Public Health 2001;91:118-121.
6. USPSTF. Screening for breast cancer: recommendations and rationale. Ann Int Med 2002;137:344-346.
7. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev 2006;(4):CD001877.
1. Armstrong K, Moye E, Williams S, Berlin JA. Screening mammography for women 40 to 49 years of age: A systematic review for the American College of Physicians. Ann Int Med 2007;146:516-526.
2. Qaseem A, Snow V, Sherif K, Aronson M, Weiss KB, Owens DK. Screening mammography for women 40 to 49 years of age: A clinical practice guideline from the American College of Physicians. Ann Int Med 2007;146:511-515.
3. Summaries for Patients: Screening mammography in women age 40 to 49 years. Ann Int Med 2007;146:1-20.
4. Woloshin S, Schwartz LM, Welch HG. The effectiveness of a primer to help people understand risk. Two randomized trials in distinct populations. Ann Int Med 2007;146:256-265
5. Marbella AM, Layde PM. Racial trends in age-specific breast cancer mortality rates in US women. Am J Public Health 2001;91:118-121.
6. USPSTF. Screening for breast cancer: recommendations and rationale. Ann Int Med 2002;137:344-346.
7. Gotzsche PC, Nielsen M. Screening for breast cancer with mammography. Cochrane Database Syst Rev 2006;(4):CD001877.
How practice-based research changed the way I manage depression
A psychiatrist colleague once remarked that I did a better-than-average job diagnosing depression. Maybe so, but I was never quite sure if my patients really met established diagnostic criteria, since I usually “gestalted” the diagnosis. I would talk to my patients about depression so they would understand and accept it as a treatable medical condition, not a moral failing.
Recommending appropriate therapy was fairly easy, since information on pharmacotherapy is so prevalent. But I rarely learned how well my depressed patients were doing, since I practiced the “no news is good news” model of chronic disease management.
Then I was invited to participate in a practice-based study of depression.1 I learned a better way to manage depression, which was more effective, satisfying, and—believe it or not—more efficient than the old way. I learned that successful management also saved time and money by reducing fruitless diagnostic testing and return visits for unmet needs.
The 5 Linked Tasks Of Successful Management
Successful management of depression requires completion of 5 linked tasks: recognition, diagnosis, treatment, systematic follow-up, and outcome assessment. Most important for the busy practitioner, these tasks can be completed without disrupting “usual” patient care.
What specific tools did I acquire? I’ll describe them according to tasks; the first task—recognition—deserves special emphasis and is discussed last.
Diagnosis: 9 simple questions
Whenever I suspect the possibility of depression in a patient, I ask the 9 simple questions in the PRIME-MD Patient Health Questionnaire (PHQ-9).2,3 The PHQ-9 instrument is the best available depression screening tool for primary care.4 An affirmative answer to either question A or B plus a total of 5 positive “nearly every day” replies indicates a diagnosis of major depression. Fewer symptoms indicate minor depression, for which therapy is individualized (Figure 1).
It takes about 1 or 2 minutes to make a PHQ-9 diagnosis of depression; listening to the patient’s story fleshes out the diagnosis and may reveal underlying comorbidities (such as alcohol use or physical abuse) that guide the next task.
I minimize diagnostic testing. In 16 months, 1 of 95 patients in my practice with major depression turned out to have a significant underlying medical illness responsible for his symptoms. I diagnosed a lymphoma during systematic follow-up when treatment failed.
FIGURE 1
PRIME-MD Patient Health Questionnaire (PHQ-9)
Treatment: What to discuss
I use most of the office visit to discuss the diagnosis, patient preferences for treatment (or no treatment), and pharmacotherapy indications, expectations, and side effects. In my experience, this discussion is usually completed within the allotted time of the appointment.
Follow-up: Make 3 appointments
I ask patients to make 3 follow-up appointments: at 1 week (to assess medication side effects, if medication is prescribed), at 4 weeks (to assess early response), and at 8 weeks (to assess maintenance of response). After the week-8 visit, I ask patients to return every 3 months, or sooner if not at goal.
If the patient declines pharmacologic treatment, or if referral for counseling is the sole treatment decision, the schedule of follow-up visits may vary. Visits would then be spent assessing whether the patient is improving or in need of additional treatment.
Outcome assessment: Take “emotional temperature”
I obtain a quantitative “depression score” (Figure 2) at the initial diagnostic visit and at each follow-up visit. This ad hoc scoring system, developed by my psychiatrist partners, complements a patient’s report of progress. Scores collected from individual patients can also be used to assess overall improvement in the practice population of depressed patients.
I have found that patients understand and support having their “emotional temperature” taken at each visit. The PHQ-9 can be used to quantitate improvement during follow-up visits.3
FIGURE 2
Depression Scoring System
Recognition: Pandora’s box?
No matter how efficient, practical, and “userfriendly,” a protocol is of no value if unused. I suspect that, consciously or unconsciously, many physicians avoid opening the “Pandora’s box” of depression, because they fear disrupting the office schedule.
In my practice, recognizing and managing depression—and other unexpected patient issues—necessitated, I now realize, a complete re-engineering of the way I practiced medicine. Suppose you went to a shoe store on Friday to buy a pair of hiking boots for a weekend outing and were told, “Sorry, you’ll have to come back next week: we sell hiking boots only on Tuesdays and Thursdays.” Think how absurd that would be, and then realize it is exactly how most doctors schedule patient visits—on the “quota system,” despite good evidence that patients’ primary care needs cannot be conveniently categorized and scheduled.
All I know about my schedule at the start of a day is that I will encounter 25 to 30 patients, and that I will leave the office when it closes. I will not know in advance the complaints, treatments, and time I should spend with each patient. This flexible scheduling works well and liberates me to address patients’ spontaneous needs as they arise during the office encounter.
Case Finding: Don’t Trust Appearances
For depression, the case-finding approach is preferable to screening the entire practice.5 I no longer trust appearances to recognize depression. While participating in the practice-based study of depression,1 I encountered a hardworking, cheerful female executive who had undergone many diagnostic procedures and who did not at all appear depressed—but she was. She got better after treatment and expressed profound gratitude. Not all severely affected patients present saying, “I’m depressed, please treat me.”
Unexplained somatic complaints
In my practice, one third of depressed patients complain only of a variety of somatic symptoms. I tell medical students, “If you can’t figure out what’s going on in an office encounter within minutes (1 to 3 minutes, depending on the level of training), think depression or alcohol abuse.” This clinical pearl is memorable and mostly accurate; the differential diagnosis can be expanded throughout training.
Stress? Suspect depression
Two thirds of my patients with diagnosed depression allude to “stress,” but often only in a very roundabout manner. So, whenever a patient utters the word “stress” during an office encounter, out comes the PHQ-9. I cannot think of a simpler technique for recognizing depression. In general, it is also well to remember that patients suffering from chronic diseases will have a higher-than-average likelihood of associated depression.
Managing depression should be simple
The management of depression should resemble the management of diabetes: astute case finding (mass screening for diabetes is not recommended), clearly defined diagnosis (blood sugar level), effective treatment (both behavioral and pharmacologic), systematic follow-up, and a quantitative outcome (glycosylated hemoglobin level). Diabetes was formerly managed as an acute illness, since once upon a time it was acceptable just to keep patients out of ketoacidosis. Today we treat diabetes better, and patients do better. Now it is time to do better with depression.
Correspondence
David L. Hahn, MD, MS, Arcand Park Clinic, 3434 East Washington Ave, Madison WI 53704. E-mail: [email protected]
1. Katzelnick DJ, Kobak KA, Greist JH, Jefferson JW, Henk HJ. Effect of primary care treatment of depression on service use by patients with high medical expenditures. Psychiatr Serv 1997;48:59-64.
2. Spitzer RL, Williams JBW, Kroenke K. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994;272:1749-1756.
3. Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study. JAMA 1999;282:1737-1744.
4. Nease Jr DE, Malouin JM. Depression screening: a practical strategy. J Fam Pract 2003;52:118-126.
5. Valenstein M, Vijan S, Zeber JE, Boehm K, Buttar A. The cost-utility of screening for depression in primary care. Ann Intern Med 2001;134:345-360.
A psychiatrist colleague once remarked that I did a better-than-average job diagnosing depression. Maybe so, but I was never quite sure if my patients really met established diagnostic criteria, since I usually “gestalted” the diagnosis. I would talk to my patients about depression so they would understand and accept it as a treatable medical condition, not a moral failing.
Recommending appropriate therapy was fairly easy, since information on pharmacotherapy is so prevalent. But I rarely learned how well my depressed patients were doing, since I practiced the “no news is good news” model of chronic disease management.
Then I was invited to participate in a practice-based study of depression.1 I learned a better way to manage depression, which was more effective, satisfying, and—believe it or not—more efficient than the old way. I learned that successful management also saved time and money by reducing fruitless diagnostic testing and return visits for unmet needs.
The 5 Linked Tasks Of Successful Management
Successful management of depression requires completion of 5 linked tasks: recognition, diagnosis, treatment, systematic follow-up, and outcome assessment. Most important for the busy practitioner, these tasks can be completed without disrupting “usual” patient care.
What specific tools did I acquire? I’ll describe them according to tasks; the first task—recognition—deserves special emphasis and is discussed last.
Diagnosis: 9 simple questions
Whenever I suspect the possibility of depression in a patient, I ask the 9 simple questions in the PRIME-MD Patient Health Questionnaire (PHQ-9).2,3 The PHQ-9 instrument is the best available depression screening tool for primary care.4 An affirmative answer to either question A or B plus a total of 5 positive “nearly every day” replies indicates a diagnosis of major depression. Fewer symptoms indicate minor depression, for which therapy is individualized (Figure 1).
It takes about 1 or 2 minutes to make a PHQ-9 diagnosis of depression; listening to the patient’s story fleshes out the diagnosis and may reveal underlying comorbidities (such as alcohol use or physical abuse) that guide the next task.
I minimize diagnostic testing. In 16 months, 1 of 95 patients in my practice with major depression turned out to have a significant underlying medical illness responsible for his symptoms. I diagnosed a lymphoma during systematic follow-up when treatment failed.
FIGURE 1
PRIME-MD Patient Health Questionnaire (PHQ-9)
Treatment: What to discuss
I use most of the office visit to discuss the diagnosis, patient preferences for treatment (or no treatment), and pharmacotherapy indications, expectations, and side effects. In my experience, this discussion is usually completed within the allotted time of the appointment.
Follow-up: Make 3 appointments
I ask patients to make 3 follow-up appointments: at 1 week (to assess medication side effects, if medication is prescribed), at 4 weeks (to assess early response), and at 8 weeks (to assess maintenance of response). After the week-8 visit, I ask patients to return every 3 months, or sooner if not at goal.
If the patient declines pharmacologic treatment, or if referral for counseling is the sole treatment decision, the schedule of follow-up visits may vary. Visits would then be spent assessing whether the patient is improving or in need of additional treatment.
Outcome assessment: Take “emotional temperature”
I obtain a quantitative “depression score” (Figure 2) at the initial diagnostic visit and at each follow-up visit. This ad hoc scoring system, developed by my psychiatrist partners, complements a patient’s report of progress. Scores collected from individual patients can also be used to assess overall improvement in the practice population of depressed patients.
I have found that patients understand and support having their “emotional temperature” taken at each visit. The PHQ-9 can be used to quantitate improvement during follow-up visits.3
FIGURE 2
Depression Scoring System
Recognition: Pandora’s box?
No matter how efficient, practical, and “userfriendly,” a protocol is of no value if unused. I suspect that, consciously or unconsciously, many physicians avoid opening the “Pandora’s box” of depression, because they fear disrupting the office schedule.
In my practice, recognizing and managing depression—and other unexpected patient issues—necessitated, I now realize, a complete re-engineering of the way I practiced medicine. Suppose you went to a shoe store on Friday to buy a pair of hiking boots for a weekend outing and were told, “Sorry, you’ll have to come back next week: we sell hiking boots only on Tuesdays and Thursdays.” Think how absurd that would be, and then realize it is exactly how most doctors schedule patient visits—on the “quota system,” despite good evidence that patients’ primary care needs cannot be conveniently categorized and scheduled.
All I know about my schedule at the start of a day is that I will encounter 25 to 30 patients, and that I will leave the office when it closes. I will not know in advance the complaints, treatments, and time I should spend with each patient. This flexible scheduling works well and liberates me to address patients’ spontaneous needs as they arise during the office encounter.
Case Finding: Don’t Trust Appearances
For depression, the case-finding approach is preferable to screening the entire practice.5 I no longer trust appearances to recognize depression. While participating in the practice-based study of depression,1 I encountered a hardworking, cheerful female executive who had undergone many diagnostic procedures and who did not at all appear depressed—but she was. She got better after treatment and expressed profound gratitude. Not all severely affected patients present saying, “I’m depressed, please treat me.”
Unexplained somatic complaints
In my practice, one third of depressed patients complain only of a variety of somatic symptoms. I tell medical students, “If you can’t figure out what’s going on in an office encounter within minutes (1 to 3 minutes, depending on the level of training), think depression or alcohol abuse.” This clinical pearl is memorable and mostly accurate; the differential diagnosis can be expanded throughout training.
Stress? Suspect depression
Two thirds of my patients with diagnosed depression allude to “stress,” but often only in a very roundabout manner. So, whenever a patient utters the word “stress” during an office encounter, out comes the PHQ-9. I cannot think of a simpler technique for recognizing depression. In general, it is also well to remember that patients suffering from chronic diseases will have a higher-than-average likelihood of associated depression.
Managing depression should be simple
The management of depression should resemble the management of diabetes: astute case finding (mass screening for diabetes is not recommended), clearly defined diagnosis (blood sugar level), effective treatment (both behavioral and pharmacologic), systematic follow-up, and a quantitative outcome (glycosylated hemoglobin level). Diabetes was formerly managed as an acute illness, since once upon a time it was acceptable just to keep patients out of ketoacidosis. Today we treat diabetes better, and patients do better. Now it is time to do better with depression.
Correspondence
David L. Hahn, MD, MS, Arcand Park Clinic, 3434 East Washington Ave, Madison WI 53704. E-mail: [email protected]
A psychiatrist colleague once remarked that I did a better-than-average job diagnosing depression. Maybe so, but I was never quite sure if my patients really met established diagnostic criteria, since I usually “gestalted” the diagnosis. I would talk to my patients about depression so they would understand and accept it as a treatable medical condition, not a moral failing.
Recommending appropriate therapy was fairly easy, since information on pharmacotherapy is so prevalent. But I rarely learned how well my depressed patients were doing, since I practiced the “no news is good news” model of chronic disease management.
Then I was invited to participate in a practice-based study of depression.1 I learned a better way to manage depression, which was more effective, satisfying, and—believe it or not—more efficient than the old way. I learned that successful management also saved time and money by reducing fruitless diagnostic testing and return visits for unmet needs.
The 5 Linked Tasks Of Successful Management
Successful management of depression requires completion of 5 linked tasks: recognition, diagnosis, treatment, systematic follow-up, and outcome assessment. Most important for the busy practitioner, these tasks can be completed without disrupting “usual” patient care.
What specific tools did I acquire? I’ll describe them according to tasks; the first task—recognition—deserves special emphasis and is discussed last.
Diagnosis: 9 simple questions
Whenever I suspect the possibility of depression in a patient, I ask the 9 simple questions in the PRIME-MD Patient Health Questionnaire (PHQ-9).2,3 The PHQ-9 instrument is the best available depression screening tool for primary care.4 An affirmative answer to either question A or B plus a total of 5 positive “nearly every day” replies indicates a diagnosis of major depression. Fewer symptoms indicate minor depression, for which therapy is individualized (Figure 1).
It takes about 1 or 2 minutes to make a PHQ-9 diagnosis of depression; listening to the patient’s story fleshes out the diagnosis and may reveal underlying comorbidities (such as alcohol use or physical abuse) that guide the next task.
I minimize diagnostic testing. In 16 months, 1 of 95 patients in my practice with major depression turned out to have a significant underlying medical illness responsible for his symptoms. I diagnosed a lymphoma during systematic follow-up when treatment failed.
FIGURE 1
PRIME-MD Patient Health Questionnaire (PHQ-9)
Treatment: What to discuss
I use most of the office visit to discuss the diagnosis, patient preferences for treatment (or no treatment), and pharmacotherapy indications, expectations, and side effects. In my experience, this discussion is usually completed within the allotted time of the appointment.
Follow-up: Make 3 appointments
I ask patients to make 3 follow-up appointments: at 1 week (to assess medication side effects, if medication is prescribed), at 4 weeks (to assess early response), and at 8 weeks (to assess maintenance of response). After the week-8 visit, I ask patients to return every 3 months, or sooner if not at goal.
If the patient declines pharmacologic treatment, or if referral for counseling is the sole treatment decision, the schedule of follow-up visits may vary. Visits would then be spent assessing whether the patient is improving or in need of additional treatment.
Outcome assessment: Take “emotional temperature”
I obtain a quantitative “depression score” (Figure 2) at the initial diagnostic visit and at each follow-up visit. This ad hoc scoring system, developed by my psychiatrist partners, complements a patient’s report of progress. Scores collected from individual patients can also be used to assess overall improvement in the practice population of depressed patients.
I have found that patients understand and support having their “emotional temperature” taken at each visit. The PHQ-9 can be used to quantitate improvement during follow-up visits.3
FIGURE 2
Depression Scoring System
Recognition: Pandora’s box?
No matter how efficient, practical, and “userfriendly,” a protocol is of no value if unused. I suspect that, consciously or unconsciously, many physicians avoid opening the “Pandora’s box” of depression, because they fear disrupting the office schedule.
In my practice, recognizing and managing depression—and other unexpected patient issues—necessitated, I now realize, a complete re-engineering of the way I practiced medicine. Suppose you went to a shoe store on Friday to buy a pair of hiking boots for a weekend outing and were told, “Sorry, you’ll have to come back next week: we sell hiking boots only on Tuesdays and Thursdays.” Think how absurd that would be, and then realize it is exactly how most doctors schedule patient visits—on the “quota system,” despite good evidence that patients’ primary care needs cannot be conveniently categorized and scheduled.
All I know about my schedule at the start of a day is that I will encounter 25 to 30 patients, and that I will leave the office when it closes. I will not know in advance the complaints, treatments, and time I should spend with each patient. This flexible scheduling works well and liberates me to address patients’ spontaneous needs as they arise during the office encounter.
Case Finding: Don’t Trust Appearances
For depression, the case-finding approach is preferable to screening the entire practice.5 I no longer trust appearances to recognize depression. While participating in the practice-based study of depression,1 I encountered a hardworking, cheerful female executive who had undergone many diagnostic procedures and who did not at all appear depressed—but she was. She got better after treatment and expressed profound gratitude. Not all severely affected patients present saying, “I’m depressed, please treat me.”
Unexplained somatic complaints
In my practice, one third of depressed patients complain only of a variety of somatic symptoms. I tell medical students, “If you can’t figure out what’s going on in an office encounter within minutes (1 to 3 minutes, depending on the level of training), think depression or alcohol abuse.” This clinical pearl is memorable and mostly accurate; the differential diagnosis can be expanded throughout training.
Stress? Suspect depression
Two thirds of my patients with diagnosed depression allude to “stress,” but often only in a very roundabout manner. So, whenever a patient utters the word “stress” during an office encounter, out comes the PHQ-9. I cannot think of a simpler technique for recognizing depression. In general, it is also well to remember that patients suffering from chronic diseases will have a higher-than-average likelihood of associated depression.
Managing depression should be simple
The management of depression should resemble the management of diabetes: astute case finding (mass screening for diabetes is not recommended), clearly defined diagnosis (blood sugar level), effective treatment (both behavioral and pharmacologic), systematic follow-up, and a quantitative outcome (glycosylated hemoglobin level). Diabetes was formerly managed as an acute illness, since once upon a time it was acceptable just to keep patients out of ketoacidosis. Today we treat diabetes better, and patients do better. Now it is time to do better with depression.
Correspondence
David L. Hahn, MD, MS, Arcand Park Clinic, 3434 East Washington Ave, Madison WI 53704. E-mail: [email protected]
1. Katzelnick DJ, Kobak KA, Greist JH, Jefferson JW, Henk HJ. Effect of primary care treatment of depression on service use by patients with high medical expenditures. Psychiatr Serv 1997;48:59-64.
2. Spitzer RL, Williams JBW, Kroenke K. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994;272:1749-1756.
3. Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study. JAMA 1999;282:1737-1744.
4. Nease Jr DE, Malouin JM. Depression screening: a practical strategy. J Fam Pract 2003;52:118-126.
5. Valenstein M, Vijan S, Zeber JE, Boehm K, Buttar A. The cost-utility of screening for depression in primary care. Ann Intern Med 2001;134:345-360.
1. Katzelnick DJ, Kobak KA, Greist JH, Jefferson JW, Henk HJ. Effect of primary care treatment of depression on service use by patients with high medical expenditures. Psychiatr Serv 1997;48:59-64.
2. Spitzer RL, Williams JBW, Kroenke K. Utility of a new procedure for diagnosing mental disorders in primary care. The PRIME-MD 1000 study. JAMA 1994;272:1749-1756.
3. Spitzer RL, Kroenke K, Williams JBW. Validation and utility of a self-report version of PRIME-MD: the PHQ Primary Care Study. JAMA 1999;282:1737-1744.
4. Nease Jr DE, Malouin JM. Depression screening: a practical strategy. J Fam Pract 2003;52:118-126.
5. Valenstein M, Vijan S, Zeber JE, Boehm K, Buttar A. The cost-utility of screening for depression in primary care. Ann Intern Med 2001;134:345-360.
The Delivery of Clinical Preventive Services Acute Care Intervention
METHODS: We performed a cross-sectional audit of the computerized billing data of all adult outpatients seen at least once by any primary care provider in 1995 (N = 75,621). Delivery of preventive services was stratified by age, sex, visit frequency, insurance status (FFS or HMO), and visit type (acute care only or scheduled preventive visit).
RESULTS: Insurance status and visit type were the strongest predictors of clinical preventive service delivery. Patients with FFS coverage received 6% to 13% (absolute difference) fewer of these services than HMO patients. Acute-care-only patients received 9% to 45% fewer services than patients who scheduled preventive visits. The combination of these factors was associated with profound differences.
CONCLUSIONS: Having insurance to pay for preventive services is an important factor in the delivery of such care. Encouraging all patients to schedule preventive visits has been suggested as a strategy for increasing delivery, but that is not practical in this setting. Assessing the need for preventive services and offering them during acute care visits has equal potential for increasing delivery.
Preventive care to the well patient has become an accepted activity in primary care.1 Clinical preventive services require a high standard of proof of effectiveness.2 Although evidence-based guidelines1 and goals3 have been published, optimal delivery has been achieved only infrequently.4 The formidable list of its potential barriers4 includes physician factors (lack of consensus, motivation, compensation, time), patient factors (age, race, sex, patient acceptance, insurance status, visit frequency, not scheduling preventive visits), and system factors (absence of paramedical assistance, disorganized medical records, fragmentation of care, lack of a systematic delivery program). Strategies to improve the delivery of preventive services must recognize the complexity and uniqueness of each office practice5-7 and advocate the use of quality improvement methodologies.8
Previous studies have shown that insurance coverage significantly affects preventive service delivery,9 but the fear that health maintenance organization (HMO) patients would receive fewer preventive services than fee-for-service (FFS) patients10 is unfounded.11 The favorable effect of HMO enrollment on preventive service delivery is probably because of insurance coverage rather than self-selection.11 Many studies of FFS and HMO care delivery have compared one physician group seeing FFS patients exclusively with another group seeing HMO patients.12-18 In that study design, differences may be related to confounding physician and system factors rather than the type of insurance coverage.19
Physicians have been trained to deliver preventive care during the annual complete physical examination. The effectiveness of the traditional complete physical as a vehicle for preventive service delivery has been questioned recently,20 however, and delivery of preventive services during acute care visits has been advocated as a more effective alternative.21,22 Indeed, systematic delivery strategies that include the offer of these services during acute care visits have achieved the highest levels of preventive service delivery in primary care settings.4,23 But these studies were performed in a small number of private practice settings, and it is unclear whether the same results apply in the larger organized systems of care.24
As part of a program to increase delivery of adult clinical preventive services, we determined the major factors associated with the performance of specified preventive services in a large physician-owned health delivery system that included both HMO and FFS patients. We particularly sought to measure the quantity of preventive services associated with insurance status (HMO vs FFS) and visit type (preventive visits vs acute care only).
Methods
Practice Setting
We studied a multispecialty group practice with 15 offices located in Dane County, Wisconsin. The service area is representative of the county and contains one midsize city (190,000 population), several smaller cities and towns, and a surrounding rural area. During the audit period there was a county population of 351,362, of whom 149,225 (42%) were enrolled in HMOs, 7027 (2%) were covered by Medicaid, and 42,193 (12%) were uninsured. The group practice owned and operated its own prepaid health plan (DeanCare HMO), which was clinically managed by the physician-owners who were compensated for their services on the basis of a discounted FFS formula that did not provide financial incentives to manage HMO and FFS patients differently. The HMO paid for all preventive services deemed appropriate by the physician, while many FFS insurance carriers did not.
Audit of Computerized Billing Records
The group practice computerized billing system contained a record for every encounter. The records include provider and patient codes, the site of service, diagnosis codes, and any billable tests obtained within the group’s clinical laboratory and radiology facilities. Only group practice sites that used these facilities exclusively were audited. Patient demographic information was obtained by cross-referencing the patient code with a master enrollment file. Computerized billing data for 1985 to 1995 were audited. For each patient, yearly data were abstracted that included a flag for the presence/absence (1/0) of a billable preventive procedure. From this data file, a set of positive criteria was constructed to determine the prevalence of preventive service delivery on the basis of age, sex, and frequency criteria Table 1. Positive criteria were modeled after those developed by the National Committee on Quality Assurance.25
The audited population included all adult outpatients (aged 18 years and older) who visited a defined panel of primary care providers at 10 sites at least once in 1995. In addition to age and sex, the following data were abstracted: billable preventive procedures (Papanicolaou test, screening mammogram, cholesterol test, tetanus immunization, fecal occult blood test, and sigmoidoscopy), total visits to primary care providers, insurance type (HMO, FFS, Medicare, Medicaid, or workers’ compensation), and visit type (acute care–only or scheduled preventive care visit). Cholesterol testing as part of a chemistry panel was counted separately from single cholesterol determinations or lipid profiles, which were classified as screening.
Computer Audit Validation Using Medical Record Reviews
At one study site, the computer audit results were validated by manual review of 200 randomly selected medical records. The validation audit found good agreement (±2.5%) for documentation of preventive procedures; the proportion of positive criteria for the computerized audit was always within 2.5% of that for the manual chart review of the same patient population. Computerized visit frequency counts were highly correlated with medical record review counts (R = 0.93; P <.0001). Medical record review also showed that, except for sigmoidoscopy, approximately 98% of audited preventive procedures had been performed for screening, not for symptoms. (Sixty-seven percent of sigmoidoscopies had been done for screening.)
A separate review of a stratified sample of 245 medical records4 found evidence that screening was performed outside the system of care (and not recorded in the transaction files) in 0.5% or fewer of preventive procedures; the only exception was Papanicolaou tests, which had been obtained outside the system in 13.8% of women aged 20 to 29 years and 4.5% of those aged 30 to 39 years.
Statistical Testing
We explored univariate associations between age, sex, visit frequency, insurance type, and visit type using analysis of variance for continuous dependent variables and the Fisher exact test for binary categorical variables. We used logistic regression to test whether preventive services were independently associated with those same variables. For the statistical testing, visit frequency was defined as all visits to primary care providers in 1995, and presence of a visit scheduled specifically for preventive care was coded as positive if one occurred during 1994 or 1995 (P <.05 was reported as significant).
Results
Thirty-five family practice physicians, 33 general internists, 15 obstetrician/gynecologists, and 12 physician assistants supervised by the primary care physicians made up the primary care provider panel. This panel encountered 75,621 outpatients at least once in 1995. Characteristics of this patient group are presented in Table 2.
Age was positively associated with men (mean = 47 years vs 45 years for women), visit frequency (R = .073), HMO insurance (mean = 45 years vs 41 years for FFS) and acute care–visits (mean = 48 years vs 43 years for preventive care visit) (P <.001 for all comparisons). Women were positively associated with visit frequency (mean = 3.4 visits per year vs 2.6 visits for men) and preventive care visits (52% of all women scheduled such visits vs 22% of men ).
HMO membership was equal for both sexes. Visit frequency was positively associated with HMO membership (mean = 3.2 visits vs 2.6 for FFS) and preventive care visits (mean = 3.1 visits vs 3.0 for acute care only) (P <.001 for both). HMO members scheduled more preventive visits than FFS patients in 1995 (36% and 31%, respectively; P <.001).
Table 3 presents the results for the Papanicolaou test, mammography, cholesterol, tetanus immunization, fecal occult blood testing, and sigmoidoscopy. In general, older patients received more preventive services than younger patients; differences by sex were inconsistent. Patients with 3 or more visits had consistently more positive criteria (7% to 12% more) than patients encountered only once or twice in 1995. HMO patients had 6% to 13% more screening than FFS patients. Associations with visit type were greatest: Patients who scheduled at least one preventive visit had 9% to 45% more positive criteria than patients seen only for acute care. Because of the large sample size, all these differences were statistically significant. Even a trivial positive association of age and mammography use achieved statistical significance (P = .01) in the multivariate model. Logistic regression analyses showed that, for every preventive service audited, the associations of visit frequency and type were statistically independent (P <.001 in all cases).
Table 4 illustrates the profound interaction between insurance type and visit type. HMO patients who scheduled a preventive visit had the highest rates of screening, while FFS patients seen only for acute care had rates that were 14% to 50% lower. FFS patients who scheduled a preventive visit had rates approaching the high rates of screening of the HMO/preventive visit group. Interestingly, HMO patients seen only for acute care had low rates of screening, closer to those of FFS/acute care–only than to the HMO patients who scheduled a preventive visit.
Discussion
The audit methodology we used in this study measured the delivery of adult clinical preventive services in a multispecialty, multisite group practice health system that treats both FFS and HMO patients. The audited group included all patients having at least one face-to-face encounter with a primary care provider in 1995. This methodology excluded patients who were not seen during the audited year. Peripheral sites where billable services were not recorded were also excluded. Positive audit criteria were determined according to nationally recognized norms.25
Limitations
Two random medical record reviews validated the computerized billing file audit showing that most recorded preventive procedures were performed for screening, and few patients had received any preventive services elsewhere. (The exceptions were the approximately 33% of sigmoidoscopies that were done for symptoms and a small percentage of Papanicolaou tests that were performed outside the system.) The magnitude of these discrepancies was insufficient to alter the conclusions of our study. It is possible that physicians did not code for some preventive visits scheduled by FFS patients whose insurance did not cover prevention. If so, our audit could have underestimated the association of scheduling preventive visits with the delivery of such services for FFS patients. There was no incentive for physicians to undercode preventive visits for HMO patients. The system of care did not record assignment of patients to individual physicians. Therefore, it was not possible to audit individual providers. The system’s database did not record demographic information, such as education, income, ethnic origin, or race. Thus, we could not analyze health system performance in regard to socioeconomic status, which is an important additional predictor of preventive service use.26 Nonbillable services, such as smoking cessation counseling and blood pressure testing, were not measurable using our methodology.
FFS Versus HMO Insurance
With one exception,27 studies of HMO and FFS care have compared one group treating HMO patients with another group providing care for FFS patients. That type of study design raises the concern that system factors (different provider group attitudes, training, system access, protocols, and so forth) were responsible for the differences reported between HMO and FFS care. It is unlikely that this happened in our study, because both HMO and FFS patients were seen within the same system of care by the same physicians whose compensation formula did not discriminate between insurance types. In this system of care, HMO members had insurance to pay for any preventive service offered by the physician, while coverage for prevention was not uniformly available to FFS policy holders.
Our results that show HMO patients received more preventive services than FFS patients agree with the results of the National Health Interview Survey (NHIS),11 which included a representative sample of the US population. It is unlikely that HMO self-selection accounted for the higher preventive services delivery rates for HMO patients in these studies. After controlling for factors correlated with selection into HMOs, health status, and use of medical services, results of the NHIS were not altered. In our study, only half of the HMO patients scheduled a preventive visit; the other half had lower screening rates Table 4.
The most important determinant of access to health care is having health insurance.28 We believe the most simple explanation for the superiority of preventive service delivery to HMO patients in our system of care is that FFS patients without preventive coverage are reluctant to pay out-of-pocket expenses for these services. This belief is supported by the results of a recent study in managed care settings showing that physician compensation method was not significantly related to use, while plan benefit level was positively related to increased service delivery.9 Thus, providing insurance coverage to pay for preventive care is one potential strategy for increasing delivery.
Preventive Visits Versus Acute Care
Visit frequency has previously been shown to have a positive association with the delivery of preventive services.4,29 Our study confirms the association with visit frequency, but found a statistically independent and greater association with visit type. Similar to results in other primary care studies,30 we found that a scheduled preventive visit was strongly predictive of the delivery of preventive services.
One strategy, then, for rectifying this discrepancy is to insist that all patients schedule a yearly preventive visit. Currently only one third of patients in primary care settings31 schedule such a visit. A simple calculation demonstrates, however, that if primary physicians in our audit spent 30 additional minutes each year performing a complete physical examination for the approximately 54,000 patients currently seen for acute care only, there would be little or no time remaining to care for sick patients. Since the audited group represents only a part of the whole, the systemwide impact would be even greater. Additionally, when one author (D.L.H.) systematically invited all adults aged older than 50 years to schedule a complete physical examination, most did not do so (unpublished observations).
We agree with Frame20 that it is neither feasible nor necessary to insist that all patients schedule preventive visits to receive preventive care. The delivery of preventive services during acute care visits has been advocated as a necessary strategy to deliver adequate services to entire patient populations.21,22 People want physicians to provide more preventive services,32 and many patients who have not scheduled preventive visits will accept them if the physician offers.4,33 Preventive services delivery during illness visits is common in community practice,33 though it is more prevalent in high-risk than average-risk patients.34 Other primary care–based studies show that the best results are accomplished when these services are systematically offered to all patients during illness visits, regardless of risk status.4,23,35,36 Offering preventive care during acute care visits can be integrated into office practice4,33,34 and can be effective.4,36
Conclusions
Coordinating efforts to offer evidence-based preventive services to all patients seen for acute care visits is a potential strategy to increase the delivery of this care. Implementation of this strategy could result in increased screening for patients enrolled in HMOs and for FFS patients with coverage. In 1996, Dean Health System began implementing a systemwide adult health maintenance guideline that emphasizes assessment and delivery during acute care visits and includes provider reports of guideline adherence. After 3 years of implementation, providers reported that more than 60,000 patients (mean = 1775 per month) were treated in accordance with the guideline. Follow-up audits are planned to monitor the outcome of this strategy to assess and offer preventive services during acute care visits.
Acknowledgments
We would like to thank Kurt C. Stange, MD, PhD, for constructive suggestions during the preparation of this manuscript, and Marcie Berger, MD; Jonathon Berkhoff, MD; Gene Bettinger; and Suzanne Hallett for technical assistance.
1. United States Preventive Services Task Force. Guide to clinical preventive services. Baltimore, Md: Williams & Wilkins; 1989.
2. Frame PS, Carlson SJ. A critical review of periodic health screening using specific criteria. Part 1: selected diseases of respiratory, cardiovascular, and central nervous systems. J Fam Pract 1975;2:29-36.
3. Greenwald P, Weisburger EK, eds. Cancer control objectives for the nation: 1985-2000. Objectives for screening and recommended actions. Washington, DC: US Department of Health and Human Services, Public Health Service, National Institutes of Health; 1986. Publication no. 86-2880; 2:30.
4. Hahn DL, Berger MG. Implementation of a systematic health maintenance protocol in a private practice. J Fam Pract 1990;31:492-504.
5. Pommerenke FA, Dietrich A. Improving and maintaining preventive services. Part 1: applying the patient model. J Fam Pract 1992;34:86-91.
6. Pommerenke FA, Dietrich A. Improving and maintaining preventive services. Part 2: practical principles for primary care. J Fam Pract 1992;34:92-7.
7. Carney PA, Dietrich AJ, Keller A, et al. Tools, teamwork, and tenacity: an office system for cancer prevention. J Fam Pract 1992;35:388-94.
8. Davis JE, McBride PE, Bobula JA. Improving prevention in primary care: physicians, patients, and process. J Fam Pract 1992;35:385-7.
9. Conrad DA, Maynard C, Cheadle A, et al. Primary care physician compensation method in medical groups: does it influence the use and cost of health services for enrollees in managed care organizations? JAMA 1998;279:853-8.
10. Jackson B, Jensen J. Most administrators fear quality will be hurt by prospective payment. Mod Health 1984;14:108-10.
11. Bernstein AB, Thompson GB, Harlan LC. Differences in rates of cancer screening by usual source of medical care: data from the 1987 National Health Interview Survey. Med Care 1991;29:196-209.
12. LoGerfo J, Efird RA, Diehr PK, et al. Rates of surgical care in prepaid group practices and the independent setting: what are the reasons for the differences? Med Care 1979;17:1-10.
13. Wilner S, Schoenbaum SC, Monson RR, et al. A comparison of the quality of maternity care between a health-maintenance organization and fee-for-service practices. N Engl J Med 1981;304:784-7.
14. Wright CH, Gardin TH, Wright CL. Obstetric care in a health maintenance organization and a private fee-for-service practice: a comparative analysis. Am J Obstet Gynecol 1984;149:848-56.
15. Francis AM, Polissar L, Lorenz AB. Care of patients with colorectal cancer: a comparison of a health maintenance organization and fee-for-service practices. Med Care 1984;22:418-29.
16. Yelin EH, Henke CJ, Kramer JS, et al. A comparison of the treatment of rheumatoid arthritis in health maintenance organizations and fee-for-service practices. N Engl J Med 1985;312:962-7.
17. Epstein A, Begg CB, McNeil BJ. The use of ambulatory testing in prepaid and fee-for-service group practices. N Engl J Med 1986;314:1089-94.
18. Seller RH, Lobley M. Efficient diagnosis of common complaints: a comparative study in the United States and England. J Fam Pract 1991;33:41-6.
19. Kravitz RL, Greenfield S, Rogers W, et al. Differences in the mix of patients among medical specialties and systems of care. JAMA 1992;267:1617-23.
20. Frame PS. The complete annual physical examination refuses to die. J Fam Pract 1995;40:543-5.
21. Canadian. Task Force on the Periodic Health Examination. The periodic health examination. Can Med Assoc J 1979;121:1193-254.
22. Battista RN. Adult cancer prevention in primary care: patterns of practice in Quebec. Am J Public Health 1983;73:1036-9.
23. Frame PS, Kowulich BA, Llewellyn AM. Improving physician compliance with a health maintenance protocol. J Fam Pract 1984;19:341-4.
24. Scheckler WE, Schultz R. Rapid change to HMO systems: profile of the Dane County, Wisconsin, experience. J Fam Pract 1987;24:417-24.
25. National Committee for Quality Assurance. HEDIS 3.0. Volume 2: technical specifications. Washington, DC: National Committee for Quality Assurance; 1997.
26. Katz SJ, Hofer TP. Socioeconomic disparities in preventive care persist despite universal coverage. JAMA 1994;272:530-4.
27. Udvarhelyi IS, Jennison K, Phillips RS, et al. Comparison of the quality of ambulatory care for fee-for-service and prepaid patients. Ann Intern Med 1991;115:394-400.
28. Stewart AL, Grumbach K, Osmond DH, et al. Primary care and patient perceptions of access to care. J Fam Pract 1997;44:177-85.
29. Frank E, Winkleby MA, Altman DG, et al. Predictors of physicians’ smoking cessation advice. JAMA 1991;266:3139-44.
30. Williams RB, Boles M, Johnson RE. A patient-initiated system for preventive health care: a randomized trial in community-based primary care practices. Arch Fam Med 1998;7:338-45.
31. Preisser JS, Cohen SJ, Wofford JL, et al. Physician and patient predictors of health maintenance visits. Arch Fam Med 1998;7:346-51.
32. Cogswell B, Eggert M. People want doctors to give more preventive care. Arch Fam Med 1993;2:611-9.
33. Stange KC, Flocke SA, Goodwin MA. Opportunistic preventive services delivery: are time limitations and patient satisfaction barriers? J Fam Pract 1998;46:412-24.
34. Flocke SA, Stange KC, Goodwin MA. Patient and visit characteristics associated with opportunistic preventive services delivery. J Fam Pract 1998;47:202-8.
35. Hahn DL. Feasibility of sigmoidoscopic screening for bowel cancer in a primary care setting. J Am Board Fam Pract 1989;2:25-9.
36. Hahn DL. Systematic cholesterol screening during acute care visits. J Am Board Fam Pract 1993;6:529-36.
METHODS: We performed a cross-sectional audit of the computerized billing data of all adult outpatients seen at least once by any primary care provider in 1995 (N = 75,621). Delivery of preventive services was stratified by age, sex, visit frequency, insurance status (FFS or HMO), and visit type (acute care only or scheduled preventive visit).
RESULTS: Insurance status and visit type were the strongest predictors of clinical preventive service delivery. Patients with FFS coverage received 6% to 13% (absolute difference) fewer of these services than HMO patients. Acute-care-only patients received 9% to 45% fewer services than patients who scheduled preventive visits. The combination of these factors was associated with profound differences.
CONCLUSIONS: Having insurance to pay for preventive services is an important factor in the delivery of such care. Encouraging all patients to schedule preventive visits has been suggested as a strategy for increasing delivery, but that is not practical in this setting. Assessing the need for preventive services and offering them during acute care visits has equal potential for increasing delivery.
Preventive care to the well patient has become an accepted activity in primary care.1 Clinical preventive services require a high standard of proof of effectiveness.2 Although evidence-based guidelines1 and goals3 have been published, optimal delivery has been achieved only infrequently.4 The formidable list of its potential barriers4 includes physician factors (lack of consensus, motivation, compensation, time), patient factors (age, race, sex, patient acceptance, insurance status, visit frequency, not scheduling preventive visits), and system factors (absence of paramedical assistance, disorganized medical records, fragmentation of care, lack of a systematic delivery program). Strategies to improve the delivery of preventive services must recognize the complexity and uniqueness of each office practice5-7 and advocate the use of quality improvement methodologies.8
Previous studies have shown that insurance coverage significantly affects preventive service delivery,9 but the fear that health maintenance organization (HMO) patients would receive fewer preventive services than fee-for-service (FFS) patients10 is unfounded.11 The favorable effect of HMO enrollment on preventive service delivery is probably because of insurance coverage rather than self-selection.11 Many studies of FFS and HMO care delivery have compared one physician group seeing FFS patients exclusively with another group seeing HMO patients.12-18 In that study design, differences may be related to confounding physician and system factors rather than the type of insurance coverage.19
Physicians have been trained to deliver preventive care during the annual complete physical examination. The effectiveness of the traditional complete physical as a vehicle for preventive service delivery has been questioned recently,20 however, and delivery of preventive services during acute care visits has been advocated as a more effective alternative.21,22 Indeed, systematic delivery strategies that include the offer of these services during acute care visits have achieved the highest levels of preventive service delivery in primary care settings.4,23 But these studies were performed in a small number of private practice settings, and it is unclear whether the same results apply in the larger organized systems of care.24
As part of a program to increase delivery of adult clinical preventive services, we determined the major factors associated with the performance of specified preventive services in a large physician-owned health delivery system that included both HMO and FFS patients. We particularly sought to measure the quantity of preventive services associated with insurance status (HMO vs FFS) and visit type (preventive visits vs acute care only).
Methods
Practice Setting
We studied a multispecialty group practice with 15 offices located in Dane County, Wisconsin. The service area is representative of the county and contains one midsize city (190,000 population), several smaller cities and towns, and a surrounding rural area. During the audit period there was a county population of 351,362, of whom 149,225 (42%) were enrolled in HMOs, 7027 (2%) were covered by Medicaid, and 42,193 (12%) were uninsured. The group practice owned and operated its own prepaid health plan (DeanCare HMO), which was clinically managed by the physician-owners who were compensated for their services on the basis of a discounted FFS formula that did not provide financial incentives to manage HMO and FFS patients differently. The HMO paid for all preventive services deemed appropriate by the physician, while many FFS insurance carriers did not.
Audit of Computerized Billing Records
The group practice computerized billing system contained a record for every encounter. The records include provider and patient codes, the site of service, diagnosis codes, and any billable tests obtained within the group’s clinical laboratory and radiology facilities. Only group practice sites that used these facilities exclusively were audited. Patient demographic information was obtained by cross-referencing the patient code with a master enrollment file. Computerized billing data for 1985 to 1995 were audited. For each patient, yearly data were abstracted that included a flag for the presence/absence (1/0) of a billable preventive procedure. From this data file, a set of positive criteria was constructed to determine the prevalence of preventive service delivery on the basis of age, sex, and frequency criteria Table 1. Positive criteria were modeled after those developed by the National Committee on Quality Assurance.25
The audited population included all adult outpatients (aged 18 years and older) who visited a defined panel of primary care providers at 10 sites at least once in 1995. In addition to age and sex, the following data were abstracted: billable preventive procedures (Papanicolaou test, screening mammogram, cholesterol test, tetanus immunization, fecal occult blood test, and sigmoidoscopy), total visits to primary care providers, insurance type (HMO, FFS, Medicare, Medicaid, or workers’ compensation), and visit type (acute care–only or scheduled preventive care visit). Cholesterol testing as part of a chemistry panel was counted separately from single cholesterol determinations or lipid profiles, which were classified as screening.
Computer Audit Validation Using Medical Record Reviews
At one study site, the computer audit results were validated by manual review of 200 randomly selected medical records. The validation audit found good agreement (±2.5%) for documentation of preventive procedures; the proportion of positive criteria for the computerized audit was always within 2.5% of that for the manual chart review of the same patient population. Computerized visit frequency counts were highly correlated with medical record review counts (R = 0.93; P <.0001). Medical record review also showed that, except for sigmoidoscopy, approximately 98% of audited preventive procedures had been performed for screening, not for symptoms. (Sixty-seven percent of sigmoidoscopies had been done for screening.)
A separate review of a stratified sample of 245 medical records4 found evidence that screening was performed outside the system of care (and not recorded in the transaction files) in 0.5% or fewer of preventive procedures; the only exception was Papanicolaou tests, which had been obtained outside the system in 13.8% of women aged 20 to 29 years and 4.5% of those aged 30 to 39 years.
Statistical Testing
We explored univariate associations between age, sex, visit frequency, insurance type, and visit type using analysis of variance for continuous dependent variables and the Fisher exact test for binary categorical variables. We used logistic regression to test whether preventive services were independently associated with those same variables. For the statistical testing, visit frequency was defined as all visits to primary care providers in 1995, and presence of a visit scheduled specifically for preventive care was coded as positive if one occurred during 1994 or 1995 (P <.05 was reported as significant).
Results
Thirty-five family practice physicians, 33 general internists, 15 obstetrician/gynecologists, and 12 physician assistants supervised by the primary care physicians made up the primary care provider panel. This panel encountered 75,621 outpatients at least once in 1995. Characteristics of this patient group are presented in Table 2.
Age was positively associated with men (mean = 47 years vs 45 years for women), visit frequency (R = .073), HMO insurance (mean = 45 years vs 41 years for FFS) and acute care–visits (mean = 48 years vs 43 years for preventive care visit) (P <.001 for all comparisons). Women were positively associated with visit frequency (mean = 3.4 visits per year vs 2.6 visits for men) and preventive care visits (52% of all women scheduled such visits vs 22% of men ).
HMO membership was equal for both sexes. Visit frequency was positively associated with HMO membership (mean = 3.2 visits vs 2.6 for FFS) and preventive care visits (mean = 3.1 visits vs 3.0 for acute care only) (P <.001 for both). HMO members scheduled more preventive visits than FFS patients in 1995 (36% and 31%, respectively; P <.001).
Table 3 presents the results for the Papanicolaou test, mammography, cholesterol, tetanus immunization, fecal occult blood testing, and sigmoidoscopy. In general, older patients received more preventive services than younger patients; differences by sex were inconsistent. Patients with 3 or more visits had consistently more positive criteria (7% to 12% more) than patients encountered only once or twice in 1995. HMO patients had 6% to 13% more screening than FFS patients. Associations with visit type were greatest: Patients who scheduled at least one preventive visit had 9% to 45% more positive criteria than patients seen only for acute care. Because of the large sample size, all these differences were statistically significant. Even a trivial positive association of age and mammography use achieved statistical significance (P = .01) in the multivariate model. Logistic regression analyses showed that, for every preventive service audited, the associations of visit frequency and type were statistically independent (P <.001 in all cases).
Table 4 illustrates the profound interaction between insurance type and visit type. HMO patients who scheduled a preventive visit had the highest rates of screening, while FFS patients seen only for acute care had rates that were 14% to 50% lower. FFS patients who scheduled a preventive visit had rates approaching the high rates of screening of the HMO/preventive visit group. Interestingly, HMO patients seen only for acute care had low rates of screening, closer to those of FFS/acute care–only than to the HMO patients who scheduled a preventive visit.
Discussion
The audit methodology we used in this study measured the delivery of adult clinical preventive services in a multispecialty, multisite group practice health system that treats both FFS and HMO patients. The audited group included all patients having at least one face-to-face encounter with a primary care provider in 1995. This methodology excluded patients who were not seen during the audited year. Peripheral sites where billable services were not recorded were also excluded. Positive audit criteria were determined according to nationally recognized norms.25
Limitations
Two random medical record reviews validated the computerized billing file audit showing that most recorded preventive procedures were performed for screening, and few patients had received any preventive services elsewhere. (The exceptions were the approximately 33% of sigmoidoscopies that were done for symptoms and a small percentage of Papanicolaou tests that were performed outside the system.) The magnitude of these discrepancies was insufficient to alter the conclusions of our study. It is possible that physicians did not code for some preventive visits scheduled by FFS patients whose insurance did not cover prevention. If so, our audit could have underestimated the association of scheduling preventive visits with the delivery of such services for FFS patients. There was no incentive for physicians to undercode preventive visits for HMO patients. The system of care did not record assignment of patients to individual physicians. Therefore, it was not possible to audit individual providers. The system’s database did not record demographic information, such as education, income, ethnic origin, or race. Thus, we could not analyze health system performance in regard to socioeconomic status, which is an important additional predictor of preventive service use.26 Nonbillable services, such as smoking cessation counseling and blood pressure testing, were not measurable using our methodology.
FFS Versus HMO Insurance
With one exception,27 studies of HMO and FFS care have compared one group treating HMO patients with another group providing care for FFS patients. That type of study design raises the concern that system factors (different provider group attitudes, training, system access, protocols, and so forth) were responsible for the differences reported between HMO and FFS care. It is unlikely that this happened in our study, because both HMO and FFS patients were seen within the same system of care by the same physicians whose compensation formula did not discriminate between insurance types. In this system of care, HMO members had insurance to pay for any preventive service offered by the physician, while coverage for prevention was not uniformly available to FFS policy holders.
Our results that show HMO patients received more preventive services than FFS patients agree with the results of the National Health Interview Survey (NHIS),11 which included a representative sample of the US population. It is unlikely that HMO self-selection accounted for the higher preventive services delivery rates for HMO patients in these studies. After controlling for factors correlated with selection into HMOs, health status, and use of medical services, results of the NHIS were not altered. In our study, only half of the HMO patients scheduled a preventive visit; the other half had lower screening rates Table 4.
The most important determinant of access to health care is having health insurance.28 We believe the most simple explanation for the superiority of preventive service delivery to HMO patients in our system of care is that FFS patients without preventive coverage are reluctant to pay out-of-pocket expenses for these services. This belief is supported by the results of a recent study in managed care settings showing that physician compensation method was not significantly related to use, while plan benefit level was positively related to increased service delivery.9 Thus, providing insurance coverage to pay for preventive care is one potential strategy for increasing delivery.
Preventive Visits Versus Acute Care
Visit frequency has previously been shown to have a positive association with the delivery of preventive services.4,29 Our study confirms the association with visit frequency, but found a statistically independent and greater association with visit type. Similar to results in other primary care studies,30 we found that a scheduled preventive visit was strongly predictive of the delivery of preventive services.
One strategy, then, for rectifying this discrepancy is to insist that all patients schedule a yearly preventive visit. Currently only one third of patients in primary care settings31 schedule such a visit. A simple calculation demonstrates, however, that if primary physicians in our audit spent 30 additional minutes each year performing a complete physical examination for the approximately 54,000 patients currently seen for acute care only, there would be little or no time remaining to care for sick patients. Since the audited group represents only a part of the whole, the systemwide impact would be even greater. Additionally, when one author (D.L.H.) systematically invited all adults aged older than 50 years to schedule a complete physical examination, most did not do so (unpublished observations).
We agree with Frame20 that it is neither feasible nor necessary to insist that all patients schedule preventive visits to receive preventive care. The delivery of preventive services during acute care visits has been advocated as a necessary strategy to deliver adequate services to entire patient populations.21,22 People want physicians to provide more preventive services,32 and many patients who have not scheduled preventive visits will accept them if the physician offers.4,33 Preventive services delivery during illness visits is common in community practice,33 though it is more prevalent in high-risk than average-risk patients.34 Other primary care–based studies show that the best results are accomplished when these services are systematically offered to all patients during illness visits, regardless of risk status.4,23,35,36 Offering preventive care during acute care visits can be integrated into office practice4,33,34 and can be effective.4,36
Conclusions
Coordinating efforts to offer evidence-based preventive services to all patients seen for acute care visits is a potential strategy to increase the delivery of this care. Implementation of this strategy could result in increased screening for patients enrolled in HMOs and for FFS patients with coverage. In 1996, Dean Health System began implementing a systemwide adult health maintenance guideline that emphasizes assessment and delivery during acute care visits and includes provider reports of guideline adherence. After 3 years of implementation, providers reported that more than 60,000 patients (mean = 1775 per month) were treated in accordance with the guideline. Follow-up audits are planned to monitor the outcome of this strategy to assess and offer preventive services during acute care visits.
Acknowledgments
We would like to thank Kurt C. Stange, MD, PhD, for constructive suggestions during the preparation of this manuscript, and Marcie Berger, MD; Jonathon Berkhoff, MD; Gene Bettinger; and Suzanne Hallett for technical assistance.
METHODS: We performed a cross-sectional audit of the computerized billing data of all adult outpatients seen at least once by any primary care provider in 1995 (N = 75,621). Delivery of preventive services was stratified by age, sex, visit frequency, insurance status (FFS or HMO), and visit type (acute care only or scheduled preventive visit).
RESULTS: Insurance status and visit type were the strongest predictors of clinical preventive service delivery. Patients with FFS coverage received 6% to 13% (absolute difference) fewer of these services than HMO patients. Acute-care-only patients received 9% to 45% fewer services than patients who scheduled preventive visits. The combination of these factors was associated with profound differences.
CONCLUSIONS: Having insurance to pay for preventive services is an important factor in the delivery of such care. Encouraging all patients to schedule preventive visits has been suggested as a strategy for increasing delivery, but that is not practical in this setting. Assessing the need for preventive services and offering them during acute care visits has equal potential for increasing delivery.
Preventive care to the well patient has become an accepted activity in primary care.1 Clinical preventive services require a high standard of proof of effectiveness.2 Although evidence-based guidelines1 and goals3 have been published, optimal delivery has been achieved only infrequently.4 The formidable list of its potential barriers4 includes physician factors (lack of consensus, motivation, compensation, time), patient factors (age, race, sex, patient acceptance, insurance status, visit frequency, not scheduling preventive visits), and system factors (absence of paramedical assistance, disorganized medical records, fragmentation of care, lack of a systematic delivery program). Strategies to improve the delivery of preventive services must recognize the complexity and uniqueness of each office practice5-7 and advocate the use of quality improvement methodologies.8
Previous studies have shown that insurance coverage significantly affects preventive service delivery,9 but the fear that health maintenance organization (HMO) patients would receive fewer preventive services than fee-for-service (FFS) patients10 is unfounded.11 The favorable effect of HMO enrollment on preventive service delivery is probably because of insurance coverage rather than self-selection.11 Many studies of FFS and HMO care delivery have compared one physician group seeing FFS patients exclusively with another group seeing HMO patients.12-18 In that study design, differences may be related to confounding physician and system factors rather than the type of insurance coverage.19
Physicians have been trained to deliver preventive care during the annual complete physical examination. The effectiveness of the traditional complete physical as a vehicle for preventive service delivery has been questioned recently,20 however, and delivery of preventive services during acute care visits has been advocated as a more effective alternative.21,22 Indeed, systematic delivery strategies that include the offer of these services during acute care visits have achieved the highest levels of preventive service delivery in primary care settings.4,23 But these studies were performed in a small number of private practice settings, and it is unclear whether the same results apply in the larger organized systems of care.24
As part of a program to increase delivery of adult clinical preventive services, we determined the major factors associated with the performance of specified preventive services in a large physician-owned health delivery system that included both HMO and FFS patients. We particularly sought to measure the quantity of preventive services associated with insurance status (HMO vs FFS) and visit type (preventive visits vs acute care only).
Methods
Practice Setting
We studied a multispecialty group practice with 15 offices located in Dane County, Wisconsin. The service area is representative of the county and contains one midsize city (190,000 population), several smaller cities and towns, and a surrounding rural area. During the audit period there was a county population of 351,362, of whom 149,225 (42%) were enrolled in HMOs, 7027 (2%) were covered by Medicaid, and 42,193 (12%) were uninsured. The group practice owned and operated its own prepaid health plan (DeanCare HMO), which was clinically managed by the physician-owners who were compensated for their services on the basis of a discounted FFS formula that did not provide financial incentives to manage HMO and FFS patients differently. The HMO paid for all preventive services deemed appropriate by the physician, while many FFS insurance carriers did not.
Audit of Computerized Billing Records
The group practice computerized billing system contained a record for every encounter. The records include provider and patient codes, the site of service, diagnosis codes, and any billable tests obtained within the group’s clinical laboratory and radiology facilities. Only group practice sites that used these facilities exclusively were audited. Patient demographic information was obtained by cross-referencing the patient code with a master enrollment file. Computerized billing data for 1985 to 1995 were audited. For each patient, yearly data were abstracted that included a flag for the presence/absence (1/0) of a billable preventive procedure. From this data file, a set of positive criteria was constructed to determine the prevalence of preventive service delivery on the basis of age, sex, and frequency criteria Table 1. Positive criteria were modeled after those developed by the National Committee on Quality Assurance.25
The audited population included all adult outpatients (aged 18 years and older) who visited a defined panel of primary care providers at 10 sites at least once in 1995. In addition to age and sex, the following data were abstracted: billable preventive procedures (Papanicolaou test, screening mammogram, cholesterol test, tetanus immunization, fecal occult blood test, and sigmoidoscopy), total visits to primary care providers, insurance type (HMO, FFS, Medicare, Medicaid, or workers’ compensation), and visit type (acute care–only or scheduled preventive care visit). Cholesterol testing as part of a chemistry panel was counted separately from single cholesterol determinations or lipid profiles, which were classified as screening.
Computer Audit Validation Using Medical Record Reviews
At one study site, the computer audit results were validated by manual review of 200 randomly selected medical records. The validation audit found good agreement (±2.5%) for documentation of preventive procedures; the proportion of positive criteria for the computerized audit was always within 2.5% of that for the manual chart review of the same patient population. Computerized visit frequency counts were highly correlated with medical record review counts (R = 0.93; P <.0001). Medical record review also showed that, except for sigmoidoscopy, approximately 98% of audited preventive procedures had been performed for screening, not for symptoms. (Sixty-seven percent of sigmoidoscopies had been done for screening.)
A separate review of a stratified sample of 245 medical records4 found evidence that screening was performed outside the system of care (and not recorded in the transaction files) in 0.5% or fewer of preventive procedures; the only exception was Papanicolaou tests, which had been obtained outside the system in 13.8% of women aged 20 to 29 years and 4.5% of those aged 30 to 39 years.
Statistical Testing
We explored univariate associations between age, sex, visit frequency, insurance type, and visit type using analysis of variance for continuous dependent variables and the Fisher exact test for binary categorical variables. We used logistic regression to test whether preventive services were independently associated with those same variables. For the statistical testing, visit frequency was defined as all visits to primary care providers in 1995, and presence of a visit scheduled specifically for preventive care was coded as positive if one occurred during 1994 or 1995 (P <.05 was reported as significant).
Results
Thirty-five family practice physicians, 33 general internists, 15 obstetrician/gynecologists, and 12 physician assistants supervised by the primary care physicians made up the primary care provider panel. This panel encountered 75,621 outpatients at least once in 1995. Characteristics of this patient group are presented in Table 2.
Age was positively associated with men (mean = 47 years vs 45 years for women), visit frequency (R = .073), HMO insurance (mean = 45 years vs 41 years for FFS) and acute care–visits (mean = 48 years vs 43 years for preventive care visit) (P <.001 for all comparisons). Women were positively associated with visit frequency (mean = 3.4 visits per year vs 2.6 visits for men) and preventive care visits (52% of all women scheduled such visits vs 22% of men ).
HMO membership was equal for both sexes. Visit frequency was positively associated with HMO membership (mean = 3.2 visits vs 2.6 for FFS) and preventive care visits (mean = 3.1 visits vs 3.0 for acute care only) (P <.001 for both). HMO members scheduled more preventive visits than FFS patients in 1995 (36% and 31%, respectively; P <.001).
Table 3 presents the results for the Papanicolaou test, mammography, cholesterol, tetanus immunization, fecal occult blood testing, and sigmoidoscopy. In general, older patients received more preventive services than younger patients; differences by sex were inconsistent. Patients with 3 or more visits had consistently more positive criteria (7% to 12% more) than patients encountered only once or twice in 1995. HMO patients had 6% to 13% more screening than FFS patients. Associations with visit type were greatest: Patients who scheduled at least one preventive visit had 9% to 45% more positive criteria than patients seen only for acute care. Because of the large sample size, all these differences were statistically significant. Even a trivial positive association of age and mammography use achieved statistical significance (P = .01) in the multivariate model. Logistic regression analyses showed that, for every preventive service audited, the associations of visit frequency and type were statistically independent (P <.001 in all cases).
Table 4 illustrates the profound interaction between insurance type and visit type. HMO patients who scheduled a preventive visit had the highest rates of screening, while FFS patients seen only for acute care had rates that were 14% to 50% lower. FFS patients who scheduled a preventive visit had rates approaching the high rates of screening of the HMO/preventive visit group. Interestingly, HMO patients seen only for acute care had low rates of screening, closer to those of FFS/acute care–only than to the HMO patients who scheduled a preventive visit.
Discussion
The audit methodology we used in this study measured the delivery of adult clinical preventive services in a multispecialty, multisite group practice health system that treats both FFS and HMO patients. The audited group included all patients having at least one face-to-face encounter with a primary care provider in 1995. This methodology excluded patients who were not seen during the audited year. Peripheral sites where billable services were not recorded were also excluded. Positive audit criteria were determined according to nationally recognized norms.25
Limitations
Two random medical record reviews validated the computerized billing file audit showing that most recorded preventive procedures were performed for screening, and few patients had received any preventive services elsewhere. (The exceptions were the approximately 33% of sigmoidoscopies that were done for symptoms and a small percentage of Papanicolaou tests that were performed outside the system.) The magnitude of these discrepancies was insufficient to alter the conclusions of our study. It is possible that physicians did not code for some preventive visits scheduled by FFS patients whose insurance did not cover prevention. If so, our audit could have underestimated the association of scheduling preventive visits with the delivery of such services for FFS patients. There was no incentive for physicians to undercode preventive visits for HMO patients. The system of care did not record assignment of patients to individual physicians. Therefore, it was not possible to audit individual providers. The system’s database did not record demographic information, such as education, income, ethnic origin, or race. Thus, we could not analyze health system performance in regard to socioeconomic status, which is an important additional predictor of preventive service use.26 Nonbillable services, such as smoking cessation counseling and blood pressure testing, were not measurable using our methodology.
FFS Versus HMO Insurance
With one exception,27 studies of HMO and FFS care have compared one group treating HMO patients with another group providing care for FFS patients. That type of study design raises the concern that system factors (different provider group attitudes, training, system access, protocols, and so forth) were responsible for the differences reported between HMO and FFS care. It is unlikely that this happened in our study, because both HMO and FFS patients were seen within the same system of care by the same physicians whose compensation formula did not discriminate between insurance types. In this system of care, HMO members had insurance to pay for any preventive service offered by the physician, while coverage for prevention was not uniformly available to FFS policy holders.
Our results that show HMO patients received more preventive services than FFS patients agree with the results of the National Health Interview Survey (NHIS),11 which included a representative sample of the US population. It is unlikely that HMO self-selection accounted for the higher preventive services delivery rates for HMO patients in these studies. After controlling for factors correlated with selection into HMOs, health status, and use of medical services, results of the NHIS were not altered. In our study, only half of the HMO patients scheduled a preventive visit; the other half had lower screening rates Table 4.
The most important determinant of access to health care is having health insurance.28 We believe the most simple explanation for the superiority of preventive service delivery to HMO patients in our system of care is that FFS patients without preventive coverage are reluctant to pay out-of-pocket expenses for these services. This belief is supported by the results of a recent study in managed care settings showing that physician compensation method was not significantly related to use, while plan benefit level was positively related to increased service delivery.9 Thus, providing insurance coverage to pay for preventive care is one potential strategy for increasing delivery.
Preventive Visits Versus Acute Care
Visit frequency has previously been shown to have a positive association with the delivery of preventive services.4,29 Our study confirms the association with visit frequency, but found a statistically independent and greater association with visit type. Similar to results in other primary care studies,30 we found that a scheduled preventive visit was strongly predictive of the delivery of preventive services.
One strategy, then, for rectifying this discrepancy is to insist that all patients schedule a yearly preventive visit. Currently only one third of patients in primary care settings31 schedule such a visit. A simple calculation demonstrates, however, that if primary physicians in our audit spent 30 additional minutes each year performing a complete physical examination for the approximately 54,000 patients currently seen for acute care only, there would be little or no time remaining to care for sick patients. Since the audited group represents only a part of the whole, the systemwide impact would be even greater. Additionally, when one author (D.L.H.) systematically invited all adults aged older than 50 years to schedule a complete physical examination, most did not do so (unpublished observations).
We agree with Frame20 that it is neither feasible nor necessary to insist that all patients schedule preventive visits to receive preventive care. The delivery of preventive services during acute care visits has been advocated as a necessary strategy to deliver adequate services to entire patient populations.21,22 People want physicians to provide more preventive services,32 and many patients who have not scheduled preventive visits will accept them if the physician offers.4,33 Preventive services delivery during illness visits is common in community practice,33 though it is more prevalent in high-risk than average-risk patients.34 Other primary care–based studies show that the best results are accomplished when these services are systematically offered to all patients during illness visits, regardless of risk status.4,23,35,36 Offering preventive care during acute care visits can be integrated into office practice4,33,34 and can be effective.4,36
Conclusions
Coordinating efforts to offer evidence-based preventive services to all patients seen for acute care visits is a potential strategy to increase the delivery of this care. Implementation of this strategy could result in increased screening for patients enrolled in HMOs and for FFS patients with coverage. In 1996, Dean Health System began implementing a systemwide adult health maintenance guideline that emphasizes assessment and delivery during acute care visits and includes provider reports of guideline adherence. After 3 years of implementation, providers reported that more than 60,000 patients (mean = 1775 per month) were treated in accordance with the guideline. Follow-up audits are planned to monitor the outcome of this strategy to assess and offer preventive services during acute care visits.
Acknowledgments
We would like to thank Kurt C. Stange, MD, PhD, for constructive suggestions during the preparation of this manuscript, and Marcie Berger, MD; Jonathon Berkhoff, MD; Gene Bettinger; and Suzanne Hallett for technical assistance.
1. United States Preventive Services Task Force. Guide to clinical preventive services. Baltimore, Md: Williams & Wilkins; 1989.
2. Frame PS, Carlson SJ. A critical review of periodic health screening using specific criteria. Part 1: selected diseases of respiratory, cardiovascular, and central nervous systems. J Fam Pract 1975;2:29-36.
3. Greenwald P, Weisburger EK, eds. Cancer control objectives for the nation: 1985-2000. Objectives for screening and recommended actions. Washington, DC: US Department of Health and Human Services, Public Health Service, National Institutes of Health; 1986. Publication no. 86-2880; 2:30.
4. Hahn DL, Berger MG. Implementation of a systematic health maintenance protocol in a private practice. J Fam Pract 1990;31:492-504.
5. Pommerenke FA, Dietrich A. Improving and maintaining preventive services. Part 1: applying the patient model. J Fam Pract 1992;34:86-91.
6. Pommerenke FA, Dietrich A. Improving and maintaining preventive services. Part 2: practical principles for primary care. J Fam Pract 1992;34:92-7.
7. Carney PA, Dietrich AJ, Keller A, et al. Tools, teamwork, and tenacity: an office system for cancer prevention. J Fam Pract 1992;35:388-94.
8. Davis JE, McBride PE, Bobula JA. Improving prevention in primary care: physicians, patients, and process. J Fam Pract 1992;35:385-7.
9. Conrad DA, Maynard C, Cheadle A, et al. Primary care physician compensation method in medical groups: does it influence the use and cost of health services for enrollees in managed care organizations? JAMA 1998;279:853-8.
10. Jackson B, Jensen J. Most administrators fear quality will be hurt by prospective payment. Mod Health 1984;14:108-10.
11. Bernstein AB, Thompson GB, Harlan LC. Differences in rates of cancer screening by usual source of medical care: data from the 1987 National Health Interview Survey. Med Care 1991;29:196-209.
12. LoGerfo J, Efird RA, Diehr PK, et al. Rates of surgical care in prepaid group practices and the independent setting: what are the reasons for the differences? Med Care 1979;17:1-10.
13. Wilner S, Schoenbaum SC, Monson RR, et al. A comparison of the quality of maternity care between a health-maintenance organization and fee-for-service practices. N Engl J Med 1981;304:784-7.
14. Wright CH, Gardin TH, Wright CL. Obstetric care in a health maintenance organization and a private fee-for-service practice: a comparative analysis. Am J Obstet Gynecol 1984;149:848-56.
15. Francis AM, Polissar L, Lorenz AB. Care of patients with colorectal cancer: a comparison of a health maintenance organization and fee-for-service practices. Med Care 1984;22:418-29.
16. Yelin EH, Henke CJ, Kramer JS, et al. A comparison of the treatment of rheumatoid arthritis in health maintenance organizations and fee-for-service practices. N Engl J Med 1985;312:962-7.
17. Epstein A, Begg CB, McNeil BJ. The use of ambulatory testing in prepaid and fee-for-service group practices. N Engl J Med 1986;314:1089-94.
18. Seller RH, Lobley M. Efficient diagnosis of common complaints: a comparative study in the United States and England. J Fam Pract 1991;33:41-6.
19. Kravitz RL, Greenfield S, Rogers W, et al. Differences in the mix of patients among medical specialties and systems of care. JAMA 1992;267:1617-23.
20. Frame PS. The complete annual physical examination refuses to die. J Fam Pract 1995;40:543-5.
21. Canadian. Task Force on the Periodic Health Examination. The periodic health examination. Can Med Assoc J 1979;121:1193-254.
22. Battista RN. Adult cancer prevention in primary care: patterns of practice in Quebec. Am J Public Health 1983;73:1036-9.
23. Frame PS, Kowulich BA, Llewellyn AM. Improving physician compliance with a health maintenance protocol. J Fam Pract 1984;19:341-4.
24. Scheckler WE, Schultz R. Rapid change to HMO systems: profile of the Dane County, Wisconsin, experience. J Fam Pract 1987;24:417-24.
25. National Committee for Quality Assurance. HEDIS 3.0. Volume 2: technical specifications. Washington, DC: National Committee for Quality Assurance; 1997.
26. Katz SJ, Hofer TP. Socioeconomic disparities in preventive care persist despite universal coverage. JAMA 1994;272:530-4.
27. Udvarhelyi IS, Jennison K, Phillips RS, et al. Comparison of the quality of ambulatory care for fee-for-service and prepaid patients. Ann Intern Med 1991;115:394-400.
28. Stewart AL, Grumbach K, Osmond DH, et al. Primary care and patient perceptions of access to care. J Fam Pract 1997;44:177-85.
29. Frank E, Winkleby MA, Altman DG, et al. Predictors of physicians’ smoking cessation advice. JAMA 1991;266:3139-44.
30. Williams RB, Boles M, Johnson RE. A patient-initiated system for preventive health care: a randomized trial in community-based primary care practices. Arch Fam Med 1998;7:338-45.
31. Preisser JS, Cohen SJ, Wofford JL, et al. Physician and patient predictors of health maintenance visits. Arch Fam Med 1998;7:346-51.
32. Cogswell B, Eggert M. People want doctors to give more preventive care. Arch Fam Med 1993;2:611-9.
33. Stange KC, Flocke SA, Goodwin MA. Opportunistic preventive services delivery: are time limitations and patient satisfaction barriers? J Fam Pract 1998;46:412-24.
34. Flocke SA, Stange KC, Goodwin MA. Patient and visit characteristics associated with opportunistic preventive services delivery. J Fam Pract 1998;47:202-8.
35. Hahn DL. Feasibility of sigmoidoscopic screening for bowel cancer in a primary care setting. J Am Board Fam Pract 1989;2:25-9.
36. Hahn DL. Systematic cholesterol screening during acute care visits. J Am Board Fam Pract 1993;6:529-36.
1. United States Preventive Services Task Force. Guide to clinical preventive services. Baltimore, Md: Williams & Wilkins; 1989.
2. Frame PS, Carlson SJ. A critical review of periodic health screening using specific criteria. Part 1: selected diseases of respiratory, cardiovascular, and central nervous systems. J Fam Pract 1975;2:29-36.
3. Greenwald P, Weisburger EK, eds. Cancer control objectives for the nation: 1985-2000. Objectives for screening and recommended actions. Washington, DC: US Department of Health and Human Services, Public Health Service, National Institutes of Health; 1986. Publication no. 86-2880; 2:30.
4. Hahn DL, Berger MG. Implementation of a systematic health maintenance protocol in a private practice. J Fam Pract 1990;31:492-504.
5. Pommerenke FA, Dietrich A. Improving and maintaining preventive services. Part 1: applying the patient model. J Fam Pract 1992;34:86-91.
6. Pommerenke FA, Dietrich A. Improving and maintaining preventive services. Part 2: practical principles for primary care. J Fam Pract 1992;34:92-7.
7. Carney PA, Dietrich AJ, Keller A, et al. Tools, teamwork, and tenacity: an office system for cancer prevention. J Fam Pract 1992;35:388-94.
8. Davis JE, McBride PE, Bobula JA. Improving prevention in primary care: physicians, patients, and process. J Fam Pract 1992;35:385-7.
9. Conrad DA, Maynard C, Cheadle A, et al. Primary care physician compensation method in medical groups: does it influence the use and cost of health services for enrollees in managed care organizations? JAMA 1998;279:853-8.
10. Jackson B, Jensen J. Most administrators fear quality will be hurt by prospective payment. Mod Health 1984;14:108-10.
11. Bernstein AB, Thompson GB, Harlan LC. Differences in rates of cancer screening by usual source of medical care: data from the 1987 National Health Interview Survey. Med Care 1991;29:196-209.
12. LoGerfo J, Efird RA, Diehr PK, et al. Rates of surgical care in prepaid group practices and the independent setting: what are the reasons for the differences? Med Care 1979;17:1-10.
13. Wilner S, Schoenbaum SC, Monson RR, et al. A comparison of the quality of maternity care between a health-maintenance organization and fee-for-service practices. N Engl J Med 1981;304:784-7.
14. Wright CH, Gardin TH, Wright CL. Obstetric care in a health maintenance organization and a private fee-for-service practice: a comparative analysis. Am J Obstet Gynecol 1984;149:848-56.
15. Francis AM, Polissar L, Lorenz AB. Care of patients with colorectal cancer: a comparison of a health maintenance organization and fee-for-service practices. Med Care 1984;22:418-29.
16. Yelin EH, Henke CJ, Kramer JS, et al. A comparison of the treatment of rheumatoid arthritis in health maintenance organizations and fee-for-service practices. N Engl J Med 1985;312:962-7.
17. Epstein A, Begg CB, McNeil BJ. The use of ambulatory testing in prepaid and fee-for-service group practices. N Engl J Med 1986;314:1089-94.
18. Seller RH, Lobley M. Efficient diagnosis of common complaints: a comparative study in the United States and England. J Fam Pract 1991;33:41-6.
19. Kravitz RL, Greenfield S, Rogers W, et al. Differences in the mix of patients among medical specialties and systems of care. JAMA 1992;267:1617-23.
20. Frame PS. The complete annual physical examination refuses to die. J Fam Pract 1995;40:543-5.
21. Canadian. Task Force on the Periodic Health Examination. The periodic health examination. Can Med Assoc J 1979;121:1193-254.
22. Battista RN. Adult cancer prevention in primary care: patterns of practice in Quebec. Am J Public Health 1983;73:1036-9.
23. Frame PS, Kowulich BA, Llewellyn AM. Improving physician compliance with a health maintenance protocol. J Fam Pract 1984;19:341-4.
24. Scheckler WE, Schultz R. Rapid change to HMO systems: profile of the Dane County, Wisconsin, experience. J Fam Pract 1987;24:417-24.
25. National Committee for Quality Assurance. HEDIS 3.0. Volume 2: technical specifications. Washington, DC: National Committee for Quality Assurance; 1997.
26. Katz SJ, Hofer TP. Socioeconomic disparities in preventive care persist despite universal coverage. JAMA 1994;272:530-4.
27. Udvarhelyi IS, Jennison K, Phillips RS, et al. Comparison of the quality of ambulatory care for fee-for-service and prepaid patients. Ann Intern Med 1991;115:394-400.
28. Stewart AL, Grumbach K, Osmond DH, et al. Primary care and patient perceptions of access to care. J Fam Pract 1997;44:177-85.
29. Frank E, Winkleby MA, Altman DG, et al. Predictors of physicians’ smoking cessation advice. JAMA 1991;266:3139-44.
30. Williams RB, Boles M, Johnson RE. A patient-initiated system for preventive health care: a randomized trial in community-based primary care practices. Arch Fam Med 1998;7:338-45.
31. Preisser JS, Cohen SJ, Wofford JL, et al. Physician and patient predictors of health maintenance visits. Arch Fam Med 1998;7:346-51.
32. Cogswell B, Eggert M. People want doctors to give more preventive care. Arch Fam Med 1993;2:611-9.
33. Stange KC, Flocke SA, Goodwin MA. Opportunistic preventive services delivery: are time limitations and patient satisfaction barriers? J Fam Pract 1998;46:412-24.
34. Flocke SA, Stange KC, Goodwin MA. Patient and visit characteristics associated with opportunistic preventive services delivery. J Fam Pract 1998;47:202-8.
35. Hahn DL. Feasibility of sigmoidoscopic screening for bowel cancer in a primary care setting. J Am Board Fam Pract 1989;2:25-9.
36. Hahn DL. Systematic cholesterol screening during acute care visits. J Am Board Fam Pract 1993;6:529-36.