User login
Pneumococcal conjugate vaccine decreased risk of sinusitis, pneumonia in youngest kids
Introduction of two specific variations of pneumococcal conjugate vaccine in children no older than 5 years of age can decrease their chances of developing sinusitis and pneumonia, according to a new study published in Pediatrics (Pediatrics 2014 doi: 10.1542/peds.2013-4177).
“Streptococcus pneumoniae is a major cause of pneumonia and sinusitis,” according to Dr. Ann Lindstrand, of the Public Health Agency of Sweden in Solna, and her associates “Although pneumococcal conjugate vaccine (PCV) is effective against invasive pneumococcal disease, its effectiveness against pneumonia is less consistent, and its effect on sinusitis is not known.”
The retrospective, population-based study involved data from three pediatric hospitals in Stockholm County, Sweden. The investigators compared the incidence of sinusitis, pneumonia, and empyema in the years July 2003 to June 2007 with the years July 2008 to June 2012, excluding the year of PCV7 introduction.
The results showed that PCV7 and PCV13 vaccinations led to significant decreases in sinusitis and pneumonia cases for children at or under the age of 2, while a decrease, though less profound, was also noted in cases of sinusitis and pneumonia for children between 2 and 5 years of age.
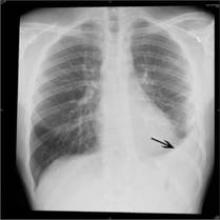
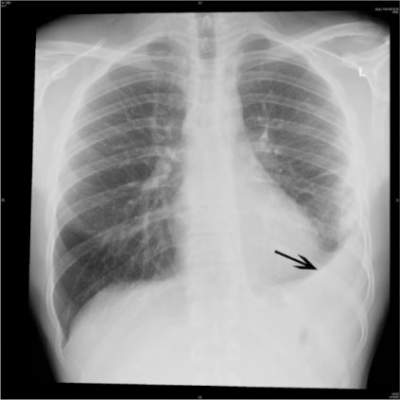
Following the introduction of PCV7 and PCV13 vaccinations, incidences of sinusitis dropped from 70 cases per 100,000 person-years to 24 cases per 100,000 for children ages 0-2 years (RR = 0.34, P < .001), while incidences for children ages 2-5 years dropped from 25 cases per 100,000 person-years to 18 cases per 100,000 person-years (RR = 0.76, P = 0.06). In cases of pneumonia, children ages 0-2 years saw incidence drop from 450 cases to 366 cases per 100,000 person-years (RR = 0.81, P , .001), while incidences in children ages 2-5 years decreased from 250 cases to 212 cases per 100,000 person-years (RR = 0.85, P = .002).
However, for children ages 5-18 years who received the vaccinations, hospitalizations for both sinusitis and pneumonia increased, albeit marginally. Similar increases were also noted in children who received the vaccines and developed empyema across all three age brackets. The increase in empyema hospitalizations as “not statistically significant,” according to Dr. Lindstrand and her associates.
“Pneumococcal disease is the most important vaccine-preventable disease in children, because it causes most child deaths. Many low- and middle-income countries are implementing PCV vaccination programs,” the study says. “This study adds evidence that PCV vaccine (PCV7 and PCV13) prevents severe sinusitis and pneumonia, with implications for global child survival. Specifically, we are the first to show great effectiveness against sinusitis in children [under] 5 years [of age].”
The authors reported that this study was supported by Stockholm County Council research funds, Foundation Samariten, Sachs’ Children’s Hospital, Swedish Research Council, Swedish Foundation for Strategic Research, Knut and Alice Wallenberg Foundation, and Sven Jerrings Foundation, Furthermore, the Stockholm County Council required that the company chosen to supply the vaccine was to give the county a 5% discount off the vaccine price for enabling an epidemiologic follow-up. Finally, Dr. Lindstrand disclosed that she received financial contributions for participation in two scientific conferences from GSK and Pfizer, with her employer financing the equivalent amount in accordance with Swedish rules for pharmaceutical sponsorship for medical education. She has also participated in one clinical vaccine trial in collaboration with GSK.
Introduction of two specific variations of pneumococcal conjugate vaccine in children no older than 5 years of age can decrease their chances of developing sinusitis and pneumonia, according to a new study published in Pediatrics (Pediatrics 2014 doi: 10.1542/peds.2013-4177).
“Streptococcus pneumoniae is a major cause of pneumonia and sinusitis,” according to Dr. Ann Lindstrand, of the Public Health Agency of Sweden in Solna, and her associates “Although pneumococcal conjugate vaccine (PCV) is effective against invasive pneumococcal disease, its effectiveness against pneumonia is less consistent, and its effect on sinusitis is not known.”
The retrospective, population-based study involved data from three pediatric hospitals in Stockholm County, Sweden. The investigators compared the incidence of sinusitis, pneumonia, and empyema in the years July 2003 to June 2007 with the years July 2008 to June 2012, excluding the year of PCV7 introduction.
The results showed that PCV7 and PCV13 vaccinations led to significant decreases in sinusitis and pneumonia cases for children at or under the age of 2, while a decrease, though less profound, was also noted in cases of sinusitis and pneumonia for children between 2 and 5 years of age.
Following the introduction of PCV7 and PCV13 vaccinations, incidences of sinusitis dropped from 70 cases per 100,000 person-years to 24 cases per 100,000 for children ages 0-2 years (RR = 0.34, P < .001), while incidences for children ages 2-5 years dropped from 25 cases per 100,000 person-years to 18 cases per 100,000 person-years (RR = 0.76, P = 0.06). In cases of pneumonia, children ages 0-2 years saw incidence drop from 450 cases to 366 cases per 100,000 person-years (RR = 0.81, P , .001), while incidences in children ages 2-5 years decreased from 250 cases to 212 cases per 100,000 person-years (RR = 0.85, P = .002).
However, for children ages 5-18 years who received the vaccinations, hospitalizations for both sinusitis and pneumonia increased, albeit marginally. Similar increases were also noted in children who received the vaccines and developed empyema across all three age brackets. The increase in empyema hospitalizations as “not statistically significant,” according to Dr. Lindstrand and her associates.
“Pneumococcal disease is the most important vaccine-preventable disease in children, because it causes most child deaths. Many low- and middle-income countries are implementing PCV vaccination programs,” the study says. “This study adds evidence that PCV vaccine (PCV7 and PCV13) prevents severe sinusitis and pneumonia, with implications for global child survival. Specifically, we are the first to show great effectiveness against sinusitis in children [under] 5 years [of age].”
The authors reported that this study was supported by Stockholm County Council research funds, Foundation Samariten, Sachs’ Children’s Hospital, Swedish Research Council, Swedish Foundation for Strategic Research, Knut and Alice Wallenberg Foundation, and Sven Jerrings Foundation, Furthermore, the Stockholm County Council required that the company chosen to supply the vaccine was to give the county a 5% discount off the vaccine price for enabling an epidemiologic follow-up. Finally, Dr. Lindstrand disclosed that she received financial contributions for participation in two scientific conferences from GSK and Pfizer, with her employer financing the equivalent amount in accordance with Swedish rules for pharmaceutical sponsorship for medical education. She has also participated in one clinical vaccine trial in collaboration with GSK.
Introduction of two specific variations of pneumococcal conjugate vaccine in children no older than 5 years of age can decrease their chances of developing sinusitis and pneumonia, according to a new study published in Pediatrics (Pediatrics 2014 doi: 10.1542/peds.2013-4177).
“Streptococcus pneumoniae is a major cause of pneumonia and sinusitis,” according to Dr. Ann Lindstrand, of the Public Health Agency of Sweden in Solna, and her associates “Although pneumococcal conjugate vaccine (PCV) is effective against invasive pneumococcal disease, its effectiveness against pneumonia is less consistent, and its effect on sinusitis is not known.”
The retrospective, population-based study involved data from three pediatric hospitals in Stockholm County, Sweden. The investigators compared the incidence of sinusitis, pneumonia, and empyema in the years July 2003 to June 2007 with the years July 2008 to June 2012, excluding the year of PCV7 introduction.
The results showed that PCV7 and PCV13 vaccinations led to significant decreases in sinusitis and pneumonia cases for children at or under the age of 2, while a decrease, though less profound, was also noted in cases of sinusitis and pneumonia for children between 2 and 5 years of age.
Following the introduction of PCV7 and PCV13 vaccinations, incidences of sinusitis dropped from 70 cases per 100,000 person-years to 24 cases per 100,000 for children ages 0-2 years (RR = 0.34, P < .001), while incidences for children ages 2-5 years dropped from 25 cases per 100,000 person-years to 18 cases per 100,000 person-years (RR = 0.76, P = 0.06). In cases of pneumonia, children ages 0-2 years saw incidence drop from 450 cases to 366 cases per 100,000 person-years (RR = 0.81, P , .001), while incidences in children ages 2-5 years decreased from 250 cases to 212 cases per 100,000 person-years (RR = 0.85, P = .002).
However, for children ages 5-18 years who received the vaccinations, hospitalizations for both sinusitis and pneumonia increased, albeit marginally. Similar increases were also noted in children who received the vaccines and developed empyema across all three age brackets. The increase in empyema hospitalizations as “not statistically significant,” according to Dr. Lindstrand and her associates.
“Pneumococcal disease is the most important vaccine-preventable disease in children, because it causes most child deaths. Many low- and middle-income countries are implementing PCV vaccination programs,” the study says. “This study adds evidence that PCV vaccine (PCV7 and PCV13) prevents severe sinusitis and pneumonia, with implications for global child survival. Specifically, we are the first to show great effectiveness against sinusitis in children [under] 5 years [of age].”
The authors reported that this study was supported by Stockholm County Council research funds, Foundation Samariten, Sachs’ Children’s Hospital, Swedish Research Council, Swedish Foundation for Strategic Research, Knut and Alice Wallenberg Foundation, and Sven Jerrings Foundation, Furthermore, the Stockholm County Council required that the company chosen to supply the vaccine was to give the county a 5% discount off the vaccine price for enabling an epidemiologic follow-up. Finally, Dr. Lindstrand disclosed that she received financial contributions for participation in two scientific conferences from GSK and Pfizer, with her employer financing the equivalent amount in accordance with Swedish rules for pharmaceutical sponsorship for medical education. She has also participated in one clinical vaccine trial in collaboration with GSK.
FROM PEDIATRICS
Key clinical point: Introduction of PCV7 and PCV13 into childhood vaccination program significantly reduced hospitalizations for sinusitis in children younger than 5 years of age.
Major finding: Incidences of sinusitis decreased from 70 cases per 100,000 population to 24 cases per 100,000 population in children ages 0-2 years old; incidences in children ages 2-5 years old decreased from 250 per 100,000 population to 212 per 100,000 population.
Data source: Retrospective population-based study (Pediatrics 2014;134:1–9).
Disclosures: The authors of the study reported several potential financial conflicts of interest.
Guidelines: Urinate 2 liters daily to stop kidney stones’ return
Patients who have had kidney stones at least once should increase their fluid consumption to produce at least 2 L of urine per day to prevent the condition from recurring, according to new guidelines published by the American College of Physicians.
If increased fluid intake doesn’t prevent recurrent nephrolithiasis, the updated ACP guidelines recommend adding treatment with a thiazide diuretic, citrate, or allopurinol. “Increased fluid intake spread throughout the day can decrease stone recurrence by at least half with virtually no side effects,” said Dr. David A. Fleming, ACP president, in a statement. “However, people who already drink the recommended amount of liquids, or when increased fluid intake is contraindicated, should not increase their fluid intake.”
The guidelines are based on a review of nephrolithiasis studies, in which the ACP’s director of clinical policy, Dr. Amir Qaseem, and his associates examined baseline stone composition, blood and urine chemistries, and final health outcomes, among other factors. Only English-language trials were used, and research was collected through searches of MEDLINE, the Cochrane Database of Systematic Reviews, Google Scholar, ClinicalTrials.gov, and Web of Science (Ann. Intern. Med. 2014;161:659-67).
Thiazide diuretics, citrates, or allopurinol significantly reduced recurrence of calcium-based stones (the most common type of kidney stone) in patients who had nephrolithiasis at least twice before, according to the analysis.
The ACP guidelines also caution against consuming non-fruit–flavored sodas and carbonated beverages, because they are acidified by phosphoric acid, and dietary animal protein and purines.
However, the guidelines encourage consumption of dietary oxalate – commonly found in chocolate, beets, nuts, rhubarb, spinach, strawberries, tea, and wheat bran – and support maintaining normal dietary calcium. There was no evidence to suggest a risk reduction advantage in drinking tap water rather than mineral water.
The recommendations do not apply to “patients with suspected hyperparathyroidism or other rare cases,” the guidelines authors noted.
Financial support for the guidelines came from the ACP operating budget. The authors had no other relevant financial disclosures.
Patients who have had kidney stones at least once should increase their fluid consumption to produce at least 2 L of urine per day to prevent the condition from recurring, according to new guidelines published by the American College of Physicians.
If increased fluid intake doesn’t prevent recurrent nephrolithiasis, the updated ACP guidelines recommend adding treatment with a thiazide diuretic, citrate, or allopurinol. “Increased fluid intake spread throughout the day can decrease stone recurrence by at least half with virtually no side effects,” said Dr. David A. Fleming, ACP president, in a statement. “However, people who already drink the recommended amount of liquids, or when increased fluid intake is contraindicated, should not increase their fluid intake.”
The guidelines are based on a review of nephrolithiasis studies, in which the ACP’s director of clinical policy, Dr. Amir Qaseem, and his associates examined baseline stone composition, blood and urine chemistries, and final health outcomes, among other factors. Only English-language trials were used, and research was collected through searches of MEDLINE, the Cochrane Database of Systematic Reviews, Google Scholar, ClinicalTrials.gov, and Web of Science (Ann. Intern. Med. 2014;161:659-67).
Thiazide diuretics, citrates, or allopurinol significantly reduced recurrence of calcium-based stones (the most common type of kidney stone) in patients who had nephrolithiasis at least twice before, according to the analysis.
The ACP guidelines also caution against consuming non-fruit–flavored sodas and carbonated beverages, because they are acidified by phosphoric acid, and dietary animal protein and purines.
However, the guidelines encourage consumption of dietary oxalate – commonly found in chocolate, beets, nuts, rhubarb, spinach, strawberries, tea, and wheat bran – and support maintaining normal dietary calcium. There was no evidence to suggest a risk reduction advantage in drinking tap water rather than mineral water.
The recommendations do not apply to “patients with suspected hyperparathyroidism or other rare cases,” the guidelines authors noted.
Financial support for the guidelines came from the ACP operating budget. The authors had no other relevant financial disclosures.
Patients who have had kidney stones at least once should increase their fluid consumption to produce at least 2 L of urine per day to prevent the condition from recurring, according to new guidelines published by the American College of Physicians.
If increased fluid intake doesn’t prevent recurrent nephrolithiasis, the updated ACP guidelines recommend adding treatment with a thiazide diuretic, citrate, or allopurinol. “Increased fluid intake spread throughout the day can decrease stone recurrence by at least half with virtually no side effects,” said Dr. David A. Fleming, ACP president, in a statement. “However, people who already drink the recommended amount of liquids, or when increased fluid intake is contraindicated, should not increase their fluid intake.”
The guidelines are based on a review of nephrolithiasis studies, in which the ACP’s director of clinical policy, Dr. Amir Qaseem, and his associates examined baseline stone composition, blood and urine chemistries, and final health outcomes, among other factors. Only English-language trials were used, and research was collected through searches of MEDLINE, the Cochrane Database of Systematic Reviews, Google Scholar, ClinicalTrials.gov, and Web of Science (Ann. Intern. Med. 2014;161:659-67).
Thiazide diuretics, citrates, or allopurinol significantly reduced recurrence of calcium-based stones (the most common type of kidney stone) in patients who had nephrolithiasis at least twice before, according to the analysis.
The ACP guidelines also caution against consuming non-fruit–flavored sodas and carbonated beverages, because they are acidified by phosphoric acid, and dietary animal protein and purines.
However, the guidelines encourage consumption of dietary oxalate – commonly found in chocolate, beets, nuts, rhubarb, spinach, strawberries, tea, and wheat bran – and support maintaining normal dietary calcium. There was no evidence to suggest a risk reduction advantage in drinking tap water rather than mineral water.
The recommendations do not apply to “patients with suspected hyperparathyroidism or other rare cases,” the guidelines authors noted.
Financial support for the guidelines came from the ACP operating budget. The authors had no other relevant financial disclosures.
FROM THE ANNALS OF INTERNAL MEDICINE
CDC: More Can Be Done to Prevent Cervical Cancer Fatalities
Close to 8 million women aged 21-65 years were not screened for cervical cancer in the past 5 years even though the disease claims nearly 4,000 lives per year, according to 2012 data, the Centers for Disease Control and Prevention reported.
In its November Vital Signs report, the CDC stated that it is committed to providing Americans with easier and more affordable access to screening and treatment options for human papillomavirus (HPV) and cervical cancer, reiterating that 93% of cervical cancers can be prevented with early screening and HPV vaccination.
“We know that cervical cancer screening works,” Dr. Ileana Arias, CDC deputy principal director, said during a telebriefing on Nov. 5. With new resources and increased awareness, “we can reach and actually go beyond the 2020 Health People Objective,” she said.
The Healthy People objective, a federal program designed to promote healthy living and disease prevention with specific goals, aims to reduce the number of invasive uterine cervical cancer cases from 7.9 per 100,000 females to 7.1 per 100,000 females, while increasing the average female screening percentage from 84.5% to 93.0%.
Dr. Arias explained that the Affordable Care Act will help eliminate some of the “financial barriers” that prevent girls and women from getting screened, which should be done every 3 years, and said that the ACA should cover preventative screening for no cost under most plans. The CDC points to a lack of health insurance as being a leading cause for nonscreening, as one out of every four women without insurance did not get screened in the last 5 years.
Other factors inhibiting more widespread HPV and cervical cancer screening include lack of awareness, knowledge, and transportation in rural and “lower resource” areas of the United States, as well as cultural beliefs. The American South continues to have the highest rates of cervical cancer incidence and death, while overall death rates across the country have remained stagnant from 2007 to 2011, making it difficult for the CDC to reach its 2020 Healthy People Objective.
The CDC recommends that all adolescents receive the HPV vaccine at around 11-12 years of age, although Dr. Arias pointed out that the vaccine could be administered to girls as young as 9 years and women as old as 26. According to CDC statistics, one out of every three girls and one out of every seven boys receive the vaccine.
“We continue to miss opportunities to screen for cervical cancers,” admitted Dr. Arias, calling on primary care providers and community outreach centers to advocate for screenings and make resources available to women and adolescents across the country.
The November Vital Signs report can be found in its entirety on the CDC website.
Close to 8 million women aged 21-65 years were not screened for cervical cancer in the past 5 years even though the disease claims nearly 4,000 lives per year, according to 2012 data, the Centers for Disease Control and Prevention reported.
In its November Vital Signs report, the CDC stated that it is committed to providing Americans with easier and more affordable access to screening and treatment options for human papillomavirus (HPV) and cervical cancer, reiterating that 93% of cervical cancers can be prevented with early screening and HPV vaccination.
“We know that cervical cancer screening works,” Dr. Ileana Arias, CDC deputy principal director, said during a telebriefing on Nov. 5. With new resources and increased awareness, “we can reach and actually go beyond the 2020 Health People Objective,” she said.
The Healthy People objective, a federal program designed to promote healthy living and disease prevention with specific goals, aims to reduce the number of invasive uterine cervical cancer cases from 7.9 per 100,000 females to 7.1 per 100,000 females, while increasing the average female screening percentage from 84.5% to 93.0%.
Dr. Arias explained that the Affordable Care Act will help eliminate some of the “financial barriers” that prevent girls and women from getting screened, which should be done every 3 years, and said that the ACA should cover preventative screening for no cost under most plans. The CDC points to a lack of health insurance as being a leading cause for nonscreening, as one out of every four women without insurance did not get screened in the last 5 years.
Other factors inhibiting more widespread HPV and cervical cancer screening include lack of awareness, knowledge, and transportation in rural and “lower resource” areas of the United States, as well as cultural beliefs. The American South continues to have the highest rates of cervical cancer incidence and death, while overall death rates across the country have remained stagnant from 2007 to 2011, making it difficult for the CDC to reach its 2020 Healthy People Objective.
The CDC recommends that all adolescents receive the HPV vaccine at around 11-12 years of age, although Dr. Arias pointed out that the vaccine could be administered to girls as young as 9 years and women as old as 26. According to CDC statistics, one out of every three girls and one out of every seven boys receive the vaccine.
“We continue to miss opportunities to screen for cervical cancers,” admitted Dr. Arias, calling on primary care providers and community outreach centers to advocate for screenings and make resources available to women and adolescents across the country.
The November Vital Signs report can be found in its entirety on the CDC website.
Close to 8 million women aged 21-65 years were not screened for cervical cancer in the past 5 years even though the disease claims nearly 4,000 lives per year, according to 2012 data, the Centers for Disease Control and Prevention reported.
In its November Vital Signs report, the CDC stated that it is committed to providing Americans with easier and more affordable access to screening and treatment options for human papillomavirus (HPV) and cervical cancer, reiterating that 93% of cervical cancers can be prevented with early screening and HPV vaccination.
“We know that cervical cancer screening works,” Dr. Ileana Arias, CDC deputy principal director, said during a telebriefing on Nov. 5. With new resources and increased awareness, “we can reach and actually go beyond the 2020 Health People Objective,” she said.
The Healthy People objective, a federal program designed to promote healthy living and disease prevention with specific goals, aims to reduce the number of invasive uterine cervical cancer cases from 7.9 per 100,000 females to 7.1 per 100,000 females, while increasing the average female screening percentage from 84.5% to 93.0%.
Dr. Arias explained that the Affordable Care Act will help eliminate some of the “financial barriers” that prevent girls and women from getting screened, which should be done every 3 years, and said that the ACA should cover preventative screening for no cost under most plans. The CDC points to a lack of health insurance as being a leading cause for nonscreening, as one out of every four women without insurance did not get screened in the last 5 years.
Other factors inhibiting more widespread HPV and cervical cancer screening include lack of awareness, knowledge, and transportation in rural and “lower resource” areas of the United States, as well as cultural beliefs. The American South continues to have the highest rates of cervical cancer incidence and death, while overall death rates across the country have remained stagnant from 2007 to 2011, making it difficult for the CDC to reach its 2020 Healthy People Objective.
The CDC recommends that all adolescents receive the HPV vaccine at around 11-12 years of age, although Dr. Arias pointed out that the vaccine could be administered to girls as young as 9 years and women as old as 26. According to CDC statistics, one out of every three girls and one out of every seven boys receive the vaccine.
“We continue to miss opportunities to screen for cervical cancers,” admitted Dr. Arias, calling on primary care providers and community outreach centers to advocate for screenings and make resources available to women and adolescents across the country.
The November Vital Signs report can be found in its entirety on the CDC website.
FROM A CDC TELEBRIEFING
CDC: More can be done to prevent cervical cancer fatalities
Close to 8 million women aged 21-65 years were not screened for cervical cancer in the past 5 years even though the disease claims nearly 4,000 lives per year, according to 2012 data, the Centers for Disease Control and Prevention reported.
In its November Vital Signs report, the CDC stated that it is committed to providing Americans with easier and more affordable access to screening and treatment options for human papillomavirus (HPV) and cervical cancer, reiterating that 93% of cervical cancers can be prevented with early screening and HPV vaccination.
“We know that cervical cancer screening works,” Dr. Ileana Arias, CDC deputy principal director, said during a telebriefing on Nov. 5. With new resources and increased awareness, “we can reach and actually go beyond the 2020 Health People Objective,” she said.
The Healthy People objective, a federal program designed to promote healthy living and disease prevention with specific goals, aims to reduce the number of invasive uterine cervical cancer cases from 7.9 per 100,000 females to 7.1 per 100,000 females, while increasing the average female screening percentage from 84.5% to 93.0%.
Dr. Arias explained that the Affordable Care Act will help eliminate some of the “financial barriers” that prevent girls and women from getting screened, which should be done every 3 years, and said that the ACA should cover preventative screening for no cost under most plans. The CDC points to a lack of health insurance as being a leading cause for nonscreening, as one out of every four women without insurance did not get screened in the last 5 years.
Other factors inhibiting more widespread HPV and cervical cancer screening include lack of awareness, knowledge, and transportation in rural and “lower resource” areas of the United States, as well as cultural beliefs. The American South continues to have the highest rates of cervical cancer incidence and death, while overall death rates across the country have remained stagnant from 2007 to 2011, making it difficult for the CDC to reach its 2020 Healthy People Objective.
The CDC recommends that all adolescents receive the HPV vaccine at around 11-12 years of age, although Dr. Arias pointed out that the vaccine could be administered to girls as young as 9 years and women as old as 26. According to CDC statistics, one out of every three girls and one out of every seven boys receive the vaccine.
“We continue to miss opportunities to screen for cervical cancers,” admitted Dr. Arias, calling on primary care providers and community outreach centers to advocate for screenings and make resources available to women and adolescents across the country.
The November Vital Signs report can be found in its entirety on the CDC website.
Close to 8 million women aged 21-65 years were not screened for cervical cancer in the past 5 years even though the disease claims nearly 4,000 lives per year, according to 2012 data, the Centers for Disease Control and Prevention reported.
In its November Vital Signs report, the CDC stated that it is committed to providing Americans with easier and more affordable access to screening and treatment options for human papillomavirus (HPV) and cervical cancer, reiterating that 93% of cervical cancers can be prevented with early screening and HPV vaccination.
“We know that cervical cancer screening works,” Dr. Ileana Arias, CDC deputy principal director, said during a telebriefing on Nov. 5. With new resources and increased awareness, “we can reach and actually go beyond the 2020 Health People Objective,” she said.
The Healthy People objective, a federal program designed to promote healthy living and disease prevention with specific goals, aims to reduce the number of invasive uterine cervical cancer cases from 7.9 per 100,000 females to 7.1 per 100,000 females, while increasing the average female screening percentage from 84.5% to 93.0%.
Dr. Arias explained that the Affordable Care Act will help eliminate some of the “financial barriers” that prevent girls and women from getting screened, which should be done every 3 years, and said that the ACA should cover preventative screening for no cost under most plans. The CDC points to a lack of health insurance as being a leading cause for nonscreening, as one out of every four women without insurance did not get screened in the last 5 years.
Other factors inhibiting more widespread HPV and cervical cancer screening include lack of awareness, knowledge, and transportation in rural and “lower resource” areas of the United States, as well as cultural beliefs. The American South continues to have the highest rates of cervical cancer incidence and death, while overall death rates across the country have remained stagnant from 2007 to 2011, making it difficult for the CDC to reach its 2020 Healthy People Objective.
The CDC recommends that all adolescents receive the HPV vaccine at around 11-12 years of age, although Dr. Arias pointed out that the vaccine could be administered to girls as young as 9 years and women as old as 26. According to CDC statistics, one out of every three girls and one out of every seven boys receive the vaccine.
“We continue to miss opportunities to screen for cervical cancers,” admitted Dr. Arias, calling on primary care providers and community outreach centers to advocate for screenings and make resources available to women and adolescents across the country.
The November Vital Signs report can be found in its entirety on the CDC website.
Close to 8 million women aged 21-65 years were not screened for cervical cancer in the past 5 years even though the disease claims nearly 4,000 lives per year, according to 2012 data, the Centers for Disease Control and Prevention reported.
In its November Vital Signs report, the CDC stated that it is committed to providing Americans with easier and more affordable access to screening and treatment options for human papillomavirus (HPV) and cervical cancer, reiterating that 93% of cervical cancers can be prevented with early screening and HPV vaccination.
“We know that cervical cancer screening works,” Dr. Ileana Arias, CDC deputy principal director, said during a telebriefing on Nov. 5. With new resources and increased awareness, “we can reach and actually go beyond the 2020 Health People Objective,” she said.
The Healthy People objective, a federal program designed to promote healthy living and disease prevention with specific goals, aims to reduce the number of invasive uterine cervical cancer cases from 7.9 per 100,000 females to 7.1 per 100,000 females, while increasing the average female screening percentage from 84.5% to 93.0%.
Dr. Arias explained that the Affordable Care Act will help eliminate some of the “financial barriers” that prevent girls and women from getting screened, which should be done every 3 years, and said that the ACA should cover preventative screening for no cost under most plans. The CDC points to a lack of health insurance as being a leading cause for nonscreening, as one out of every four women without insurance did not get screened in the last 5 years.
Other factors inhibiting more widespread HPV and cervical cancer screening include lack of awareness, knowledge, and transportation in rural and “lower resource” areas of the United States, as well as cultural beliefs. The American South continues to have the highest rates of cervical cancer incidence and death, while overall death rates across the country have remained stagnant from 2007 to 2011, making it difficult for the CDC to reach its 2020 Healthy People Objective.
The CDC recommends that all adolescents receive the HPV vaccine at around 11-12 years of age, although Dr. Arias pointed out that the vaccine could be administered to girls as young as 9 years and women as old as 26. According to CDC statistics, one out of every three girls and one out of every seven boys receive the vaccine.
“We continue to miss opportunities to screen for cervical cancers,” admitted Dr. Arias, calling on primary care providers and community outreach centers to advocate for screenings and make resources available to women and adolescents across the country.
The November Vital Signs report can be found in its entirety on the CDC website.
FROM A CDC TELEBRIEFING
Higher dose of mesalamine can decrease risk of relapse for patients with quiescent ulcerative colitis
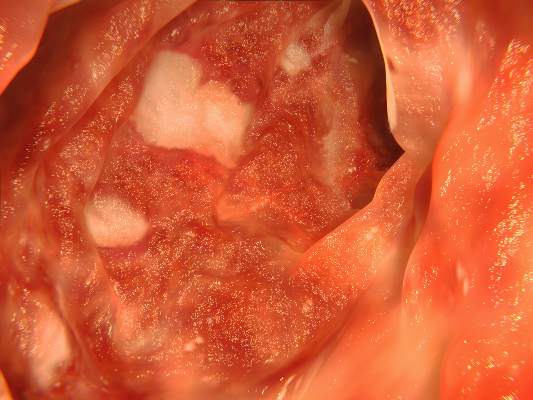
Increasing dosages of mesalamine by 2.4 g/d for patients with quiescent ulcerative colitis can dramatically reduce concentrations of fecal calprotectin and ultimately decrease the likelihood of relapse, according to the results of a new Dose Escalation and Remission (DEAR) study published in the November issue of Clinical Gastroenterology and Hepatology (2014;12:1887-93.e3).
In an open-label, randomized controlled trial, 119 patients with quiescent ulcerative colitis (UC) in remission were screened for Simple Clinical Colitis Activity Index scores, fecal calprotectin (FC) of 50 mcg/gram, and intake of no more than 3 g of mesalamine per day between October 2008 and March 2012. These subjects were divided into four groups based on FC levels, with Group 1 comprising 58 representing a baseline of FC < 50 mcg/gram, and groups 2-4 having 61 individuals with elevated baseline FC levels. Group 1 patients were retained for an observational follow-up study but were not included in the randomized controlled trial.
Participants were either told to maintain their current medication regimen or were prescribed a multimatrix mesalamine of either 2.4 or 4.8 g/d for 6 weeks, depending on their initial FC levels, after which point FC levels were taken. Participants in Group 2 with FC < 50 mcg/g were kept in an observational follow-up study group, while the rest of the participants were randomly assigned to continue their 2.4 g/d regimen, or increase their intake to 4.8 g/d.
Individuals in Group 3 with FC levels greater than 50 mcg/g were randomly assigned to either the 2.4- or 4.8-g/d regimen for 6 weeks; after that point, those whose FC levels had decreased below 50 continued with their regimen while those whose FC levels remained high were upped to 4.8 g/d. Subjects in Group 4 who were not already on a multimatrix mesalamine regimen and whose FC levels were above 50 mcg/g were randomized to continue their medication or add 2.4 g/d of mesalamine; after 6 weeks, if FC levels remained above 50, the dosage was increased to 4.8 g/d.
The primary outcome of continued remission with FC no greater than 50 mcg/g was observed in 3.8% of controls and 26.9% of participants in the dose escalation group (P = .0496). Others within the same group experienced secondary outcomes of FC levels below 100 mcg/gram (P = .04) and below 200 mcg/g (P = .005). Furthermore, among patients who were in remission during the randomization phase of the study, relapse occurred far sooner in those whose FC was above 200 mcg/g than those whose FC levels were below that mark.
“Our study adds to the evidence supporting FC concentration as a valid biomarker for UC,” said lead authors Dr. Mark T. Osterman and Dr. Faten N. Aberra of the University of Pennsylvania’s department of medicine, Philadelphia. “Perhaps more importantly, our results offer a novel way to use FC testing to positively impact outcomes in UC. We demonstrated efficacy of intervention to reduce FC (a surrogate for mucosal inflammation) before symptoms develop in patients at potentially increased risk of relapse.”
Dr. Osterman is a consultant for AbbVie, Elan, Janssen, and UCB and receives research funding from UCB. Dr. Aberra is a consultant for Janssen and research investigator for Amgen, Janssen, and UCB. Other coauthors disclosed ties to numerous pharmaceutical companies.
Increasing dosages of mesalamine by 2.4 g/d for patients with quiescent ulcerative colitis can dramatically reduce concentrations of fecal calprotectin and ultimately decrease the likelihood of relapse, according to the results of a new Dose Escalation and Remission (DEAR) study published in the November issue of Clinical Gastroenterology and Hepatology (2014;12:1887-93.e3).
In an open-label, randomized controlled trial, 119 patients with quiescent ulcerative colitis (UC) in remission were screened for Simple Clinical Colitis Activity Index scores, fecal calprotectin (FC) of 50 mcg/gram, and intake of no more than 3 g of mesalamine per day between October 2008 and March 2012. These subjects were divided into four groups based on FC levels, with Group 1 comprising 58 representing a baseline of FC < 50 mcg/gram, and groups 2-4 having 61 individuals with elevated baseline FC levels. Group 1 patients were retained for an observational follow-up study but were not included in the randomized controlled trial.
Participants were either told to maintain their current medication regimen or were prescribed a multimatrix mesalamine of either 2.4 or 4.8 g/d for 6 weeks, depending on their initial FC levels, after which point FC levels were taken. Participants in Group 2 with FC < 50 mcg/g were kept in an observational follow-up study group, while the rest of the participants were randomly assigned to continue their 2.4 g/d regimen, or increase their intake to 4.8 g/d.
Individuals in Group 3 with FC levels greater than 50 mcg/g were randomly assigned to either the 2.4- or 4.8-g/d regimen for 6 weeks; after that point, those whose FC levels had decreased below 50 continued with their regimen while those whose FC levels remained high were upped to 4.8 g/d. Subjects in Group 4 who were not already on a multimatrix mesalamine regimen and whose FC levels were above 50 mcg/g were randomized to continue their medication or add 2.4 g/d of mesalamine; after 6 weeks, if FC levels remained above 50, the dosage was increased to 4.8 g/d.
The primary outcome of continued remission with FC no greater than 50 mcg/g was observed in 3.8% of controls and 26.9% of participants in the dose escalation group (P = .0496). Others within the same group experienced secondary outcomes of FC levels below 100 mcg/gram (P = .04) and below 200 mcg/g (P = .005). Furthermore, among patients who were in remission during the randomization phase of the study, relapse occurred far sooner in those whose FC was above 200 mcg/g than those whose FC levels were below that mark.
“Our study adds to the evidence supporting FC concentration as a valid biomarker for UC,” said lead authors Dr. Mark T. Osterman and Dr. Faten N. Aberra of the University of Pennsylvania’s department of medicine, Philadelphia. “Perhaps more importantly, our results offer a novel way to use FC testing to positively impact outcomes in UC. We demonstrated efficacy of intervention to reduce FC (a surrogate for mucosal inflammation) before symptoms develop in patients at potentially increased risk of relapse.”
Dr. Osterman is a consultant for AbbVie, Elan, Janssen, and UCB and receives research funding from UCB. Dr. Aberra is a consultant for Janssen and research investigator for Amgen, Janssen, and UCB. Other coauthors disclosed ties to numerous pharmaceutical companies.
Increasing dosages of mesalamine by 2.4 g/d for patients with quiescent ulcerative colitis can dramatically reduce concentrations of fecal calprotectin and ultimately decrease the likelihood of relapse, according to the results of a new Dose Escalation and Remission (DEAR) study published in the November issue of Clinical Gastroenterology and Hepatology (2014;12:1887-93.e3).
In an open-label, randomized controlled trial, 119 patients with quiescent ulcerative colitis (UC) in remission were screened for Simple Clinical Colitis Activity Index scores, fecal calprotectin (FC) of 50 mcg/gram, and intake of no more than 3 g of mesalamine per day between October 2008 and March 2012. These subjects were divided into four groups based on FC levels, with Group 1 comprising 58 representing a baseline of FC < 50 mcg/gram, and groups 2-4 having 61 individuals with elevated baseline FC levels. Group 1 patients were retained for an observational follow-up study but were not included in the randomized controlled trial.
Participants were either told to maintain their current medication regimen or were prescribed a multimatrix mesalamine of either 2.4 or 4.8 g/d for 6 weeks, depending on their initial FC levels, after which point FC levels were taken. Participants in Group 2 with FC < 50 mcg/g were kept in an observational follow-up study group, while the rest of the participants were randomly assigned to continue their 2.4 g/d regimen, or increase their intake to 4.8 g/d.
Individuals in Group 3 with FC levels greater than 50 mcg/g were randomly assigned to either the 2.4- or 4.8-g/d regimen for 6 weeks; after that point, those whose FC levels had decreased below 50 continued with their regimen while those whose FC levels remained high were upped to 4.8 g/d. Subjects in Group 4 who were not already on a multimatrix mesalamine regimen and whose FC levels were above 50 mcg/g were randomized to continue their medication or add 2.4 g/d of mesalamine; after 6 weeks, if FC levels remained above 50, the dosage was increased to 4.8 g/d.
The primary outcome of continued remission with FC no greater than 50 mcg/g was observed in 3.8% of controls and 26.9% of participants in the dose escalation group (P = .0496). Others within the same group experienced secondary outcomes of FC levels below 100 mcg/gram (P = .04) and below 200 mcg/g (P = .005). Furthermore, among patients who were in remission during the randomization phase of the study, relapse occurred far sooner in those whose FC was above 200 mcg/g than those whose FC levels were below that mark.
“Our study adds to the evidence supporting FC concentration as a valid biomarker for UC,” said lead authors Dr. Mark T. Osterman and Dr. Faten N. Aberra of the University of Pennsylvania’s department of medicine, Philadelphia. “Perhaps more importantly, our results offer a novel way to use FC testing to positively impact outcomes in UC. We demonstrated efficacy of intervention to reduce FC (a surrogate for mucosal inflammation) before symptoms develop in patients at potentially increased risk of relapse.”
Dr. Osterman is a consultant for AbbVie, Elan, Janssen, and UCB and receives research funding from UCB. Dr. Aberra is a consultant for Janssen and research investigator for Amgen, Janssen, and UCB. Other coauthors disclosed ties to numerous pharmaceutical companies.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Increasing mesalamine dosage by 2.4 g/d significantly decreases risk of relapse for patients with UC.
Major finding: 26.9% of subjects with dose increase experienced drop in fecal calprotectin level below 50 mcg/g.
Data source: Dose Escalation and Remission (DEAR) study; open-label, randomized controlled trial.
Disclosures: Dr. Osterman is a consultant for AbbVie, Elan, Janssen, and UCB and receives research funding from UCB. Dr. Aberra is a consultant for Janssen and research investigator for Amgen, Janssen, and UCB. Other coauthors disclosed ties to numerous pharmaceutical companies.
AHA guidelines recommend Mediterranean diet to prevent stroke
Lifestyle modifications, including eating a Mediterranean or DASH-style diet, should be encouraged to lower an individual’s risk of first-time stroke, according to new guidelines for the primary prevention of stroke from the American Heart Association and American Stroke Association.
Mediterranean and DASH (Dietary Approaches to Stop Hypertension) dietary plans are characterized by their emphasis on fruits, vegetables, whole grains, legumes, nuts, seeds, poultry, and fish, while limiting red meats, sweets, and any foods with saturated fats. This new guidelines – which have been endorsed by the American Academy of Neurology, American Association of Neurological Surgeons, Congress of Neurological Surgeons, and Preventive Cardiovascular Nurses Association – suggest that adopting either of these diets in addition to a few other healthy living habits can dramatically reduce an individual’s odds of suffering a stroke (Stroke 2014;45 [doi: 10.1161/STR.0000000000000046]).
“We have a huge opportunity to improve how we prevent new strokes, because risk factors that can be changed or controlled – especially high blood pressure – account for 90% of strokes,” said Dr. James Meschia, chair of the writing committee and chairman of neurology at the Mayo Clinic in Jacksonville, Fla., in a statement. The last such guidelines were released 3 years ago (Stroke 2011;42:517-84)
The writing committee gathered data pertaining to the age, birth weight, race/ethnicity, and genetic factors, among several others. Studies examined included a U.S. Nationwide Inpatient Sample, which showed that stroke hospitalizations increased between 1998 and 2007 for individuals aged 25-34 years and 35-44 years; the Framingham Heart Study, which estimated that the odds of a middle-aged adult suffering a stroke are 1 in 6; an analysis of South Carolina Medicaid beneficiaries under age 50, which revealed that individuals born weighing less than 2,500 g were twice as likely to have a stroke as those born heavier; and an Atherosclerosis Risk in Communities (ARIC) study that showed Latino and African American populations being at higher risk for stroke due to hypertension, obesity, and diabetes.
Because blood pressure, hypertension, diabetes, and obesity are so commonly linked to stroke risk, the new American Heart Association/American Stroke Association (AHA/ASA) guidelines highly recommend a Mediterranean or DASH-style diet supplemented with nuts. Additionally, the guidelines advise health care professionals to advise patients to cut down on sodium intake, regularly monitor their blood pressure, talk to their physicians immediately if any medication does not do what it is intended to or creates negative side effects, and quit smoking, and, for women, consider an alternative to oral birth control pills.
Hypertension, “the most important, well-documented, modifiable stroke risk factor,” should be treated with antihypertensive medication to a target blood pressure of less than 140/90 mm Hg, the guidelines state.
Furthermore, the ASA/AHA guidelines continue to recommend regular physical exercise and acute monitoring of individuals’ cholesterol levels, such as LDL, HDL, and triglycerides, as failure to keep these numbers in check can easily lead to a serious stroke. The guidelines also state that although heavy alcohol consumption can increase the chance of stroke, “light to moderate” alcohol consumption can actually decrease the odds of suffering a stroke.
The AHA/ASA guidelines also examine factors such as migraines, which are associated with stroke in women under age 55, and hyperhomocysteinemia, which is also associated with an increased risk of stroke. Other factors like hypercoagulability and sleep apnea were not shown to have any identifiable relationship with an increased risk of stroke.
“As health professionals, we must ensure that progress in preventing stroke does not lead to complacency,” say the guidelines. “We must acknowledge that several recommendations remain vague because of suboptimal clinical trial evidence or, even more concerning, may be out of date and therefore irrelevant.”
Dr. Meschia and his associates warn that although medications are helpful, the best way to safeguard against a stroke is to change a person’s lifestyle into one of healthy eating and exercise habits. Unfortunately, say the authors, “it is easier to convince a patient to take a pill than to radically change his or her lifestyle, [but] we must expect the same standards of evidence for lifestyle interventions.”
Dr. Meschia disclosed that his research grant comes from the National Institute of Neurological Disorders and Stroke. He had no other relevant financial disclosures of interest. Several of the guidelines’ coauthors had disclosures of their own, which are listed in the statement.
Lifestyle modifications, including eating a Mediterranean or DASH-style diet, should be encouraged to lower an individual’s risk of first-time stroke, according to new guidelines for the primary prevention of stroke from the American Heart Association and American Stroke Association.
Mediterranean and DASH (Dietary Approaches to Stop Hypertension) dietary plans are characterized by their emphasis on fruits, vegetables, whole grains, legumes, nuts, seeds, poultry, and fish, while limiting red meats, sweets, and any foods with saturated fats. This new guidelines – which have been endorsed by the American Academy of Neurology, American Association of Neurological Surgeons, Congress of Neurological Surgeons, and Preventive Cardiovascular Nurses Association – suggest that adopting either of these diets in addition to a few other healthy living habits can dramatically reduce an individual’s odds of suffering a stroke (Stroke 2014;45 [doi: 10.1161/STR.0000000000000046]).
“We have a huge opportunity to improve how we prevent new strokes, because risk factors that can be changed or controlled – especially high blood pressure – account for 90% of strokes,” said Dr. James Meschia, chair of the writing committee and chairman of neurology at the Mayo Clinic in Jacksonville, Fla., in a statement. The last such guidelines were released 3 years ago (Stroke 2011;42:517-84)
The writing committee gathered data pertaining to the age, birth weight, race/ethnicity, and genetic factors, among several others. Studies examined included a U.S. Nationwide Inpatient Sample, which showed that stroke hospitalizations increased between 1998 and 2007 for individuals aged 25-34 years and 35-44 years; the Framingham Heart Study, which estimated that the odds of a middle-aged adult suffering a stroke are 1 in 6; an analysis of South Carolina Medicaid beneficiaries under age 50, which revealed that individuals born weighing less than 2,500 g were twice as likely to have a stroke as those born heavier; and an Atherosclerosis Risk in Communities (ARIC) study that showed Latino and African American populations being at higher risk for stroke due to hypertension, obesity, and diabetes.
Because blood pressure, hypertension, diabetes, and obesity are so commonly linked to stroke risk, the new American Heart Association/American Stroke Association (AHA/ASA) guidelines highly recommend a Mediterranean or DASH-style diet supplemented with nuts. Additionally, the guidelines advise health care professionals to advise patients to cut down on sodium intake, regularly monitor their blood pressure, talk to their physicians immediately if any medication does not do what it is intended to or creates negative side effects, and quit smoking, and, for women, consider an alternative to oral birth control pills.
Hypertension, “the most important, well-documented, modifiable stroke risk factor,” should be treated with antihypertensive medication to a target blood pressure of less than 140/90 mm Hg, the guidelines state.
Furthermore, the ASA/AHA guidelines continue to recommend regular physical exercise and acute monitoring of individuals’ cholesterol levels, such as LDL, HDL, and triglycerides, as failure to keep these numbers in check can easily lead to a serious stroke. The guidelines also state that although heavy alcohol consumption can increase the chance of stroke, “light to moderate” alcohol consumption can actually decrease the odds of suffering a stroke.
The AHA/ASA guidelines also examine factors such as migraines, which are associated with stroke in women under age 55, and hyperhomocysteinemia, which is also associated with an increased risk of stroke. Other factors like hypercoagulability and sleep apnea were not shown to have any identifiable relationship with an increased risk of stroke.
“As health professionals, we must ensure that progress in preventing stroke does not lead to complacency,” say the guidelines. “We must acknowledge that several recommendations remain vague because of suboptimal clinical trial evidence or, even more concerning, may be out of date and therefore irrelevant.”
Dr. Meschia and his associates warn that although medications are helpful, the best way to safeguard against a stroke is to change a person’s lifestyle into one of healthy eating and exercise habits. Unfortunately, say the authors, “it is easier to convince a patient to take a pill than to radically change his or her lifestyle, [but] we must expect the same standards of evidence for lifestyle interventions.”
Dr. Meschia disclosed that his research grant comes from the National Institute of Neurological Disorders and Stroke. He had no other relevant financial disclosures of interest. Several of the guidelines’ coauthors had disclosures of their own, which are listed in the statement.
Lifestyle modifications, including eating a Mediterranean or DASH-style diet, should be encouraged to lower an individual’s risk of first-time stroke, according to new guidelines for the primary prevention of stroke from the American Heart Association and American Stroke Association.
Mediterranean and DASH (Dietary Approaches to Stop Hypertension) dietary plans are characterized by their emphasis on fruits, vegetables, whole grains, legumes, nuts, seeds, poultry, and fish, while limiting red meats, sweets, and any foods with saturated fats. This new guidelines – which have been endorsed by the American Academy of Neurology, American Association of Neurological Surgeons, Congress of Neurological Surgeons, and Preventive Cardiovascular Nurses Association – suggest that adopting either of these diets in addition to a few other healthy living habits can dramatically reduce an individual’s odds of suffering a stroke (Stroke 2014;45 [doi: 10.1161/STR.0000000000000046]).
“We have a huge opportunity to improve how we prevent new strokes, because risk factors that can be changed or controlled – especially high blood pressure – account for 90% of strokes,” said Dr. James Meschia, chair of the writing committee and chairman of neurology at the Mayo Clinic in Jacksonville, Fla., in a statement. The last such guidelines were released 3 years ago (Stroke 2011;42:517-84)
The writing committee gathered data pertaining to the age, birth weight, race/ethnicity, and genetic factors, among several others. Studies examined included a U.S. Nationwide Inpatient Sample, which showed that stroke hospitalizations increased between 1998 and 2007 for individuals aged 25-34 years and 35-44 years; the Framingham Heart Study, which estimated that the odds of a middle-aged adult suffering a stroke are 1 in 6; an analysis of South Carolina Medicaid beneficiaries under age 50, which revealed that individuals born weighing less than 2,500 g were twice as likely to have a stroke as those born heavier; and an Atherosclerosis Risk in Communities (ARIC) study that showed Latino and African American populations being at higher risk for stroke due to hypertension, obesity, and diabetes.
Because blood pressure, hypertension, diabetes, and obesity are so commonly linked to stroke risk, the new American Heart Association/American Stroke Association (AHA/ASA) guidelines highly recommend a Mediterranean or DASH-style diet supplemented with nuts. Additionally, the guidelines advise health care professionals to advise patients to cut down on sodium intake, regularly monitor their blood pressure, talk to their physicians immediately if any medication does not do what it is intended to or creates negative side effects, and quit smoking, and, for women, consider an alternative to oral birth control pills.
Hypertension, “the most important, well-documented, modifiable stroke risk factor,” should be treated with antihypertensive medication to a target blood pressure of less than 140/90 mm Hg, the guidelines state.
Furthermore, the ASA/AHA guidelines continue to recommend regular physical exercise and acute monitoring of individuals’ cholesterol levels, such as LDL, HDL, and triglycerides, as failure to keep these numbers in check can easily lead to a serious stroke. The guidelines also state that although heavy alcohol consumption can increase the chance of stroke, “light to moderate” alcohol consumption can actually decrease the odds of suffering a stroke.
The AHA/ASA guidelines also examine factors such as migraines, which are associated with stroke in women under age 55, and hyperhomocysteinemia, which is also associated with an increased risk of stroke. Other factors like hypercoagulability and sleep apnea were not shown to have any identifiable relationship with an increased risk of stroke.
“As health professionals, we must ensure that progress in preventing stroke does not lead to complacency,” say the guidelines. “We must acknowledge that several recommendations remain vague because of suboptimal clinical trial evidence or, even more concerning, may be out of date and therefore irrelevant.”
Dr. Meschia and his associates warn that although medications are helpful, the best way to safeguard against a stroke is to change a person’s lifestyle into one of healthy eating and exercise habits. Unfortunately, say the authors, “it is easier to convince a patient to take a pill than to radically change his or her lifestyle, [but] we must expect the same standards of evidence for lifestyle interventions.”
Dr. Meschia disclosed that his research grant comes from the National Institute of Neurological Disorders and Stroke. He had no other relevant financial disclosures of interest. Several of the guidelines’ coauthors had disclosures of their own, which are listed in the statement.
FROM THE AMERICAN HEART ASSOCIATION AND THE AMERICAN STROKE ASSOCIATION
CDC confirms Ebola case in New York City
The Centers for Disease Control and Prevention confirmed Thursday that a medical aid worker who was hospitalized in New York City less than a week after returning from the West African nation of Guinea has tested positive for Ebola, marking the first case of the disease in the United States’ most populous city.
According to the CDC, the patient returned from Guinea on Oct. 17 and went through rigorous entry screening at JFK International Airport, at which time the patient did not exhibit any symptoms or signs of infection. On Thursday, the patient reported having a fever and was transported via a specially trained Hazardous Material Tactical (HAZ TAC) Unit to New York’s Bellevue Hospital, one of eight hospitals in the city designated to treat potential Ebola cases, where the patient currently remains in isolation.
The “CDC is in close communications with the New York City Health Department and Bellevue Hospital, and is providing technical assistance and resources,” the agency said in a statement, adding that a three-member CDC Ebola Response Team arrived in New York on Thursday as doctors conduct further testing and await lab results. This team will supplement an existing team of Ebola experts that CDC already had in place in New York and will work with Bellevue and other local hospitals to ensure that their Ebola protocols meet CDC standards.
The news of New York City’s first Ebola case comes shortly after the state of New York was designated as one of six that will launch CDC’s active monitoring program, which will require everyone coming to the United States from the Ebola-stricken nations of Guinea, Liberia, and Sierra Leone to remain in close daily contact with local health officials to ensure that they do not have or spread the disease. The other five states are Pennsylvania, Maryland, Virginia, New Jersey, and Georgia; together, these states account for 70% of incoming traffic from the afflicted West African nations.
The CDC and the New York City Health Department have interviewed the patient to gather information on any “close contacts and activities” since returning to the United States last week, according to the statement. To contract the disease, a person must come into contact with the bodily fluids of an infected individual who is symptomatic. People who come into contact with an Ebola patient but show no symptoms for 21 days are no longer at risk.
The Centers for Disease Control and Prevention confirmed Thursday that a medical aid worker who was hospitalized in New York City less than a week after returning from the West African nation of Guinea has tested positive for Ebola, marking the first case of the disease in the United States’ most populous city.
According to the CDC, the patient returned from Guinea on Oct. 17 and went through rigorous entry screening at JFK International Airport, at which time the patient did not exhibit any symptoms or signs of infection. On Thursday, the patient reported having a fever and was transported via a specially trained Hazardous Material Tactical (HAZ TAC) Unit to New York’s Bellevue Hospital, one of eight hospitals in the city designated to treat potential Ebola cases, where the patient currently remains in isolation.
The “CDC is in close communications with the New York City Health Department and Bellevue Hospital, and is providing technical assistance and resources,” the agency said in a statement, adding that a three-member CDC Ebola Response Team arrived in New York on Thursday as doctors conduct further testing and await lab results. This team will supplement an existing team of Ebola experts that CDC already had in place in New York and will work with Bellevue and other local hospitals to ensure that their Ebola protocols meet CDC standards.
The news of New York City’s first Ebola case comes shortly after the state of New York was designated as one of six that will launch CDC’s active monitoring program, which will require everyone coming to the United States from the Ebola-stricken nations of Guinea, Liberia, and Sierra Leone to remain in close daily contact with local health officials to ensure that they do not have or spread the disease. The other five states are Pennsylvania, Maryland, Virginia, New Jersey, and Georgia; together, these states account for 70% of incoming traffic from the afflicted West African nations.
The CDC and the New York City Health Department have interviewed the patient to gather information on any “close contacts and activities” since returning to the United States last week, according to the statement. To contract the disease, a person must come into contact with the bodily fluids of an infected individual who is symptomatic. People who come into contact with an Ebola patient but show no symptoms for 21 days are no longer at risk.
The Centers for Disease Control and Prevention confirmed Thursday that a medical aid worker who was hospitalized in New York City less than a week after returning from the West African nation of Guinea has tested positive for Ebola, marking the first case of the disease in the United States’ most populous city.
According to the CDC, the patient returned from Guinea on Oct. 17 and went through rigorous entry screening at JFK International Airport, at which time the patient did not exhibit any symptoms or signs of infection. On Thursday, the patient reported having a fever and was transported via a specially trained Hazardous Material Tactical (HAZ TAC) Unit to New York’s Bellevue Hospital, one of eight hospitals in the city designated to treat potential Ebola cases, where the patient currently remains in isolation.
The “CDC is in close communications with the New York City Health Department and Bellevue Hospital, and is providing technical assistance and resources,” the agency said in a statement, adding that a three-member CDC Ebola Response Team arrived in New York on Thursday as doctors conduct further testing and await lab results. This team will supplement an existing team of Ebola experts that CDC already had in place in New York and will work with Bellevue and other local hospitals to ensure that their Ebola protocols meet CDC standards.
The news of New York City’s first Ebola case comes shortly after the state of New York was designated as one of six that will launch CDC’s active monitoring program, which will require everyone coming to the United States from the Ebola-stricken nations of Guinea, Liberia, and Sierra Leone to remain in close daily contact with local health officials to ensure that they do not have or spread the disease. The other five states are Pennsylvania, Maryland, Virginia, New Jersey, and Georgia; together, these states account for 70% of incoming traffic from the afflicted West African nations.
The CDC and the New York City Health Department have interviewed the patient to gather information on any “close contacts and activities” since returning to the United States last week, according to the statement. To contract the disease, a person must come into contact with the bodily fluids of an infected individual who is symptomatic. People who come into contact with an Ebola patient but show no symptoms for 21 days are no longer at risk.
CDC announces monitoring of travelers from Ebola-stricken countries
Travelers arriving in the United States from any of three Ebola-stricken African countries will be monitored for symptoms for 21 days, the Centers for Disease Control and Prevention has announced.
The program, which will commence on Oct. 27, will require travelers from Liberia, Guinea, and Sierra Leone to check in with local public health officials on a daily basis to ensure that they have not contracted the Ebola virus. If Ebola symptoms are absent at the end of the 21-day monitoring period, the traveler is free of the virus and poses no threat to the general public.
“The bottom line here is that we have to keep up our guard against Ebola,” said Dr. Tom Frieden, director of the Centers for Disease Control and Prevention (CDC), during a teleconference with members of the media. “These additional steps will protect families, communities, and health care workers.”
The CDC and local health agencies will collect e-mail addresses, telephone numbers, and addresses from travelers to monitor them when they enter the United States. Similar information for a friend or relative in the United States also will be noted. Once within the country, passengers will be required to check in with local health agencies every day to report their temperature and any flulike symptoms, and will have to coordinate with the relevant public health officials if they plan to do any additional traveling within the United States.
Hospitals and other medical facilities are continuing to be instructed on the best ways to transport patients with suspected Ebola and care for those who are infected. A 24/7 CDC hotline also will be available for anyone who needs more information, and all travelers from the affected West African nations will be given Ebola “care kits” as well. Each kit includes a thermometer, temperature tracking log, and instructions on whom to contact if symptoms or fever develops.
The active monitoring programs will initially launch in just six states: New York, Pennsylvania, Maryland, Virginia, New Jersey, and Georgia. These states, according to the CDC, are where roughly 70% of travelers from West Africa end up, although the CDC will continue to work with all other states to set up active monitoring programs.
The announcement comes just 1 day after the CDC released from a 21-day monitoring period those individuals who came into contact with Thomas Eric Duncan, the first Ebola patient in the United States. Mr. Duncan died Oct. 8.
Travelers arriving in the United States from any of three Ebola-stricken African countries will be monitored for symptoms for 21 days, the Centers for Disease Control and Prevention has announced.
The program, which will commence on Oct. 27, will require travelers from Liberia, Guinea, and Sierra Leone to check in with local public health officials on a daily basis to ensure that they have not contracted the Ebola virus. If Ebola symptoms are absent at the end of the 21-day monitoring period, the traveler is free of the virus and poses no threat to the general public.
“The bottom line here is that we have to keep up our guard against Ebola,” said Dr. Tom Frieden, director of the Centers for Disease Control and Prevention (CDC), during a teleconference with members of the media. “These additional steps will protect families, communities, and health care workers.”
The CDC and local health agencies will collect e-mail addresses, telephone numbers, and addresses from travelers to monitor them when they enter the United States. Similar information for a friend or relative in the United States also will be noted. Once within the country, passengers will be required to check in with local health agencies every day to report their temperature and any flulike symptoms, and will have to coordinate with the relevant public health officials if they plan to do any additional traveling within the United States.
Hospitals and other medical facilities are continuing to be instructed on the best ways to transport patients with suspected Ebola and care for those who are infected. A 24/7 CDC hotline also will be available for anyone who needs more information, and all travelers from the affected West African nations will be given Ebola “care kits” as well. Each kit includes a thermometer, temperature tracking log, and instructions on whom to contact if symptoms or fever develops.
The active monitoring programs will initially launch in just six states: New York, Pennsylvania, Maryland, Virginia, New Jersey, and Georgia. These states, according to the CDC, are where roughly 70% of travelers from West Africa end up, although the CDC will continue to work with all other states to set up active monitoring programs.
The announcement comes just 1 day after the CDC released from a 21-day monitoring period those individuals who came into contact with Thomas Eric Duncan, the first Ebola patient in the United States. Mr. Duncan died Oct. 8.
Travelers arriving in the United States from any of three Ebola-stricken African countries will be monitored for symptoms for 21 days, the Centers for Disease Control and Prevention has announced.
The program, which will commence on Oct. 27, will require travelers from Liberia, Guinea, and Sierra Leone to check in with local public health officials on a daily basis to ensure that they have not contracted the Ebola virus. If Ebola symptoms are absent at the end of the 21-day monitoring period, the traveler is free of the virus and poses no threat to the general public.
“The bottom line here is that we have to keep up our guard against Ebola,” said Dr. Tom Frieden, director of the Centers for Disease Control and Prevention (CDC), during a teleconference with members of the media. “These additional steps will protect families, communities, and health care workers.”
The CDC and local health agencies will collect e-mail addresses, telephone numbers, and addresses from travelers to monitor them when they enter the United States. Similar information for a friend or relative in the United States also will be noted. Once within the country, passengers will be required to check in with local health agencies every day to report their temperature and any flulike symptoms, and will have to coordinate with the relevant public health officials if they plan to do any additional traveling within the United States.
Hospitals and other medical facilities are continuing to be instructed on the best ways to transport patients with suspected Ebola and care for those who are infected. A 24/7 CDC hotline also will be available for anyone who needs more information, and all travelers from the affected West African nations will be given Ebola “care kits” as well. Each kit includes a thermometer, temperature tracking log, and instructions on whom to contact if symptoms or fever develops.
The active monitoring programs will initially launch in just six states: New York, Pennsylvania, Maryland, Virginia, New Jersey, and Georgia. These states, according to the CDC, are where roughly 70% of travelers from West Africa end up, although the CDC will continue to work with all other states to set up active monitoring programs.
The announcement comes just 1 day after the CDC released from a 21-day monitoring period those individuals who came into contact with Thomas Eric Duncan, the first Ebola patient in the United States. Mr. Duncan died Oct. 8.
FROM A CDC TELECONFERENCE
Second child tests positive for HIV infection after apparent viral remission
An HIV-positive child, who was thought to have been cured of the virus after receiving intensive antiretroviral treatment for 3 years starting at birth, exhibited detectable signs of HIV infection just 2 weeks after termination of treatment.
The child, born to an HIV-positive mother from whom the infection was contracted, is the second reported instance of apparent HIV remission in a child after receiving early antiretroviral treatment (ART) at birth, following the case of the Mississippi infant. In both cases, intense ART was administered to the babies for prolonged periods of time beginning at birth, and traces of HIV were no longer evident in blood samples, only for the virus to return later.
“The so-called Mississippi child was believed to be cured thanks to early initiation of ART [which] raised the possibility of achieving a cure without procedures such as bone marrow transplant with CCR5-delta 32 cells,” according to a case report penned by Dr. Vania Giacomet and associates at the Luigi Sacco Hospital in Milan, Italy, where the most recent child was treated. “However, recent evidence of viral rebound in the Mississippi child shattered this hope.”
According to the case report published by The Lancet (Lancet 2014;384:1320), the latest child was born to an HIV-positive mother, who went into labor in December 2009 after 41 weeks’ gestation. HIV 1-2 antibody western blot and HIV-1 antigen p24 were positive in the newborn, who also had an HIV-RNA viral load of 152,560 copies per mL.
The infant was placed on a regimen of prophylaxis with zidovudine and nevirapine within 12 hours of birth, resulting in a viral load of 13,530 copies per mL after 4 days. Doctors then began a triple ART with ritonavir-boosted lopinavir, zidovudine, and lamivudine, which drastically decreased the infant’s viral load to 3,971 copies per mL at day 15, 49 copies per mL after 3 months, and “below assay detection” just 6 months after birth.
Doctors continued administering ART to the child for 3 years, reporting that the viral load remained undetectable and that CD4 T-cell counts were higher than usual. Furthermore, HIV antibodies also had vanished from the infant’s blood work, indicating that the infant was no longer seropositive. With the permission of the child’s mother, doctors terminated the treatment.
Two weeks after treatment ended, however, HIV tests on the child’s blood work came back positive, indicating that viral remission was no longer occurring. Because of this, doctors concluded that the ART was not sufficient to eliminate viral reservoirs within the child, thus allowing the disease to reemerge.
“The availability of many classes of potent antiretroviral drugs has substantially decreased HIV morbidity and mortality, but these drugs cannot eradicate the virus because they do not eliminate viral reservoirs,” the investigators said.
The case report also says that the infant’s in utero infection, low birth weight, and unusually high viral load likely “precluded long-lasting viral remission.” The authors note that the child’s case was not identical to that of the infant in Mississippi, namely because the Italian child’s immune system continued to show signs of responding to HIV infection even when viral load was undetectable; the Mississippi infant’s immune system did not.
No financial disclosures were reported.
An HIV-positive child, who was thought to have been cured of the virus after receiving intensive antiretroviral treatment for 3 years starting at birth, exhibited detectable signs of HIV infection just 2 weeks after termination of treatment.
The child, born to an HIV-positive mother from whom the infection was contracted, is the second reported instance of apparent HIV remission in a child after receiving early antiretroviral treatment (ART) at birth, following the case of the Mississippi infant. In both cases, intense ART was administered to the babies for prolonged periods of time beginning at birth, and traces of HIV were no longer evident in blood samples, only for the virus to return later.
“The so-called Mississippi child was believed to be cured thanks to early initiation of ART [which] raised the possibility of achieving a cure without procedures such as bone marrow transplant with CCR5-delta 32 cells,” according to a case report penned by Dr. Vania Giacomet and associates at the Luigi Sacco Hospital in Milan, Italy, where the most recent child was treated. “However, recent evidence of viral rebound in the Mississippi child shattered this hope.”
According to the case report published by The Lancet (Lancet 2014;384:1320), the latest child was born to an HIV-positive mother, who went into labor in December 2009 after 41 weeks’ gestation. HIV 1-2 antibody western blot and HIV-1 antigen p24 were positive in the newborn, who also had an HIV-RNA viral load of 152,560 copies per mL.
The infant was placed on a regimen of prophylaxis with zidovudine and nevirapine within 12 hours of birth, resulting in a viral load of 13,530 copies per mL after 4 days. Doctors then began a triple ART with ritonavir-boosted lopinavir, zidovudine, and lamivudine, which drastically decreased the infant’s viral load to 3,971 copies per mL at day 15, 49 copies per mL after 3 months, and “below assay detection” just 6 months after birth.
Doctors continued administering ART to the child for 3 years, reporting that the viral load remained undetectable and that CD4 T-cell counts were higher than usual. Furthermore, HIV antibodies also had vanished from the infant’s blood work, indicating that the infant was no longer seropositive. With the permission of the child’s mother, doctors terminated the treatment.
Two weeks after treatment ended, however, HIV tests on the child’s blood work came back positive, indicating that viral remission was no longer occurring. Because of this, doctors concluded that the ART was not sufficient to eliminate viral reservoirs within the child, thus allowing the disease to reemerge.
“The availability of many classes of potent antiretroviral drugs has substantially decreased HIV morbidity and mortality, but these drugs cannot eradicate the virus because they do not eliminate viral reservoirs,” the investigators said.
The case report also says that the infant’s in utero infection, low birth weight, and unusually high viral load likely “precluded long-lasting viral remission.” The authors note that the child’s case was not identical to that of the infant in Mississippi, namely because the Italian child’s immune system continued to show signs of responding to HIV infection even when viral load was undetectable; the Mississippi infant’s immune system did not.
No financial disclosures were reported.
An HIV-positive child, who was thought to have been cured of the virus after receiving intensive antiretroviral treatment for 3 years starting at birth, exhibited detectable signs of HIV infection just 2 weeks after termination of treatment.
The child, born to an HIV-positive mother from whom the infection was contracted, is the second reported instance of apparent HIV remission in a child after receiving early antiretroviral treatment (ART) at birth, following the case of the Mississippi infant. In both cases, intense ART was administered to the babies for prolonged periods of time beginning at birth, and traces of HIV were no longer evident in blood samples, only for the virus to return later.
“The so-called Mississippi child was believed to be cured thanks to early initiation of ART [which] raised the possibility of achieving a cure without procedures such as bone marrow transplant with CCR5-delta 32 cells,” according to a case report penned by Dr. Vania Giacomet and associates at the Luigi Sacco Hospital in Milan, Italy, where the most recent child was treated. “However, recent evidence of viral rebound in the Mississippi child shattered this hope.”
According to the case report published by The Lancet (Lancet 2014;384:1320), the latest child was born to an HIV-positive mother, who went into labor in December 2009 after 41 weeks’ gestation. HIV 1-2 antibody western blot and HIV-1 antigen p24 were positive in the newborn, who also had an HIV-RNA viral load of 152,560 copies per mL.
The infant was placed on a regimen of prophylaxis with zidovudine and nevirapine within 12 hours of birth, resulting in a viral load of 13,530 copies per mL after 4 days. Doctors then began a triple ART with ritonavir-boosted lopinavir, zidovudine, and lamivudine, which drastically decreased the infant’s viral load to 3,971 copies per mL at day 15, 49 copies per mL after 3 months, and “below assay detection” just 6 months after birth.
Doctors continued administering ART to the child for 3 years, reporting that the viral load remained undetectable and that CD4 T-cell counts were higher than usual. Furthermore, HIV antibodies also had vanished from the infant’s blood work, indicating that the infant was no longer seropositive. With the permission of the child’s mother, doctors terminated the treatment.
Two weeks after treatment ended, however, HIV tests on the child’s blood work came back positive, indicating that viral remission was no longer occurring. Because of this, doctors concluded that the ART was not sufficient to eliminate viral reservoirs within the child, thus allowing the disease to reemerge.
“The availability of many classes of potent antiretroviral drugs has substantially decreased HIV morbidity and mortality, but these drugs cannot eradicate the virus because they do not eliminate viral reservoirs,” the investigators said.
The case report also says that the infant’s in utero infection, low birth weight, and unusually high viral load likely “precluded long-lasting viral remission.” The authors note that the child’s case was not identical to that of the infant in Mississippi, namely because the Italian child’s immune system continued to show signs of responding to HIV infection even when viral load was undetectable; the Mississippi infant’s immune system did not.
No financial disclosures were reported.
FROM THE LANCET
Key clinical point: Early antiretroviral treatment did not eliminate viral reservoirs, leading to remeergence of HIV.
Major finding: Child began ART at birth and continued for 3 years, but showed detectable signs of HIV infection 2 weeks after termination of treatment.
Data source: Case report published in The Lancet.
Disclosures: No financial disclosures were reported.
Traumatic brain injury in adolescence increases likelihood of harmful behavior
A new study found that adolescents who suffer traumatic brain injury are more likely to engage in harmful behavior and that the risk of engaging in such behavior is higher among girls than boys.
The data come from the 2011 Ontario Student Drug Use and Health Survey, which anonymously polled 9,288 Ontario boys and girls between grades 7 and 12 using self-administered questionnaires. Subjects were asked whether they had a history of traumatic brain injury (TBI) – defined by the study as “a hit or blow to the head that resulted in a 5-minute loss of consciousness or at least one overnight hospitalization due to symptoms associated with it.” The questionnaires also asked the subjects whether they had experienced any of 13 distinct harmful outcomes over the past year, such as binge drinking, smoking of tobacco and cannabis products, drug abuse, psychological distress, declining grades, physical and cyberbullying, and thoughts of suicide.
The findings revealed that of the 13 harmful outcomes, boys were at a higher risk of engaging in 9. Girls, however, were likely to engage in all 13, indicating that sex plays a major role in the long-term effects of TBI when such incidents occur at a young age (PLoS One 2014 Sept. 30).
“Both boys and girls were more likely to engage in a variety of harmful behaviours if they reported a history of TBI, but girls engaged in all 13 harmful behaviours we looked for, whereas boys were at higher risk of engaging in only nine,” Gabriela Ilie, Ph.D., lead author of the study and a postdoctoral fellow at Toronto’s St. Michael’s Hospital, said in a statement. “Sex matters when it comes to traumatic brain injuries.”
The study found that girls and boys with a history of TBI were all at an elevated risk for alcohol consumption, binge drinking, cannabis smoking, declining grades, bullying others at school, being bullied online, drug abuse, and being treated for physical injuries. But female adolescents in particular were found to be additionally susceptible to elevated psychological distress, past-year suicidal ideation, being bullied at school in the past year, and past-year cigarette smoking.
Dr. Ilie and her colleagues also found that the risk of engaging in alcohol consumption, daily cigarette smoking, and being treated for physical injuries changed depending on the age of the subjects. The average age at which subjects began smoking was 14, while the age at which subjects had their first alcoholic drink skewed similarly young. For physical injury treatment, the risk ran higher for girls aged 11-13 years, and for boys aged 17-20 years.
Across the board, children with TBI presented higher incidences of all harmful behaviors than those without a history of TBI. The study also found that the majority of TBI came from playing team sports – 63% for males and 46.9% for females. Falls were responsible for 5.1% of males and 24.7% of females with TBI histories, with bicycle accidents being responsible for 8.1% of males with TBI and 1.8% of females with TBI.
The researchers cited several limitations of the study. The most salient, they said, was that the data were based on self-reports. In addition, their sample did not include “adolescents who might be at high risk of TBI.”
Still, Dr. Ilie and her colleagues hope that the findings of this study will help shed light on the need for greater action in recognizing and treating adolescent TBI, warning that failure to properly care for these injuries at the right time will significantly increase the risk of detrimental long-term consequences down the road.
“The results point to important opportunities for prevention, such as early-stage medical interventions, as well as diagnosis and rehabilitation approaches that consider the relationship between TBI and its risk factors,” the investigators said. “We know both adolescent boys and girls with TBI are vulnerable to the co-occurrence of harmful psychological and behavioural conditions. Given that most brain injuries among adolescents occur during sports, a more ecological approach to injury prevention requiring involvement by government, schools, and parents may be needed.”
The research was funded by a Canadian Institute of Health Research Team Grant in Traumatic Brain Injury and Violence and by the Ontario Neurotrauma Foundation, with additional funding from the Ontario Ministry of Health and Long-Term Care, the Social Sciences and Humanities Research Council, Industry Canada, and AUTO21, a member of the Networks of Centres of Excellence program that is administered and funded by the Natural Sciences and Engineering Research Council.
A new study found that adolescents who suffer traumatic brain injury are more likely to engage in harmful behavior and that the risk of engaging in such behavior is higher among girls than boys.
The data come from the 2011 Ontario Student Drug Use and Health Survey, which anonymously polled 9,288 Ontario boys and girls between grades 7 and 12 using self-administered questionnaires. Subjects were asked whether they had a history of traumatic brain injury (TBI) – defined by the study as “a hit or blow to the head that resulted in a 5-minute loss of consciousness or at least one overnight hospitalization due to symptoms associated with it.” The questionnaires also asked the subjects whether they had experienced any of 13 distinct harmful outcomes over the past year, such as binge drinking, smoking of tobacco and cannabis products, drug abuse, psychological distress, declining grades, physical and cyberbullying, and thoughts of suicide.
The findings revealed that of the 13 harmful outcomes, boys were at a higher risk of engaging in 9. Girls, however, were likely to engage in all 13, indicating that sex plays a major role in the long-term effects of TBI when such incidents occur at a young age (PLoS One 2014 Sept. 30).
“Both boys and girls were more likely to engage in a variety of harmful behaviours if they reported a history of TBI, but girls engaged in all 13 harmful behaviours we looked for, whereas boys were at higher risk of engaging in only nine,” Gabriela Ilie, Ph.D., lead author of the study and a postdoctoral fellow at Toronto’s St. Michael’s Hospital, said in a statement. “Sex matters when it comes to traumatic brain injuries.”
The study found that girls and boys with a history of TBI were all at an elevated risk for alcohol consumption, binge drinking, cannabis smoking, declining grades, bullying others at school, being bullied online, drug abuse, and being treated for physical injuries. But female adolescents in particular were found to be additionally susceptible to elevated psychological distress, past-year suicidal ideation, being bullied at school in the past year, and past-year cigarette smoking.
Dr. Ilie and her colleagues also found that the risk of engaging in alcohol consumption, daily cigarette smoking, and being treated for physical injuries changed depending on the age of the subjects. The average age at which subjects began smoking was 14, while the age at which subjects had their first alcoholic drink skewed similarly young. For physical injury treatment, the risk ran higher for girls aged 11-13 years, and for boys aged 17-20 years.
Across the board, children with TBI presented higher incidences of all harmful behaviors than those without a history of TBI. The study also found that the majority of TBI came from playing team sports – 63% for males and 46.9% for females. Falls were responsible for 5.1% of males and 24.7% of females with TBI histories, with bicycle accidents being responsible for 8.1% of males with TBI and 1.8% of females with TBI.
The researchers cited several limitations of the study. The most salient, they said, was that the data were based on self-reports. In addition, their sample did not include “adolescents who might be at high risk of TBI.”
Still, Dr. Ilie and her colleagues hope that the findings of this study will help shed light on the need for greater action in recognizing and treating adolescent TBI, warning that failure to properly care for these injuries at the right time will significantly increase the risk of detrimental long-term consequences down the road.
“The results point to important opportunities for prevention, such as early-stage medical interventions, as well as diagnosis and rehabilitation approaches that consider the relationship between TBI and its risk factors,” the investigators said. “We know both adolescent boys and girls with TBI are vulnerable to the co-occurrence of harmful psychological and behavioural conditions. Given that most brain injuries among adolescents occur during sports, a more ecological approach to injury prevention requiring involvement by government, schools, and parents may be needed.”
The research was funded by a Canadian Institute of Health Research Team Grant in Traumatic Brain Injury and Violence and by the Ontario Neurotrauma Foundation, with additional funding from the Ontario Ministry of Health and Long-Term Care, the Social Sciences and Humanities Research Council, Industry Canada, and AUTO21, a member of the Networks of Centres of Excellence program that is administered and funded by the Natural Sciences and Engineering Research Council.
A new study found that adolescents who suffer traumatic brain injury are more likely to engage in harmful behavior and that the risk of engaging in such behavior is higher among girls than boys.
The data come from the 2011 Ontario Student Drug Use and Health Survey, which anonymously polled 9,288 Ontario boys and girls between grades 7 and 12 using self-administered questionnaires. Subjects were asked whether they had a history of traumatic brain injury (TBI) – defined by the study as “a hit or blow to the head that resulted in a 5-minute loss of consciousness or at least one overnight hospitalization due to symptoms associated with it.” The questionnaires also asked the subjects whether they had experienced any of 13 distinct harmful outcomes over the past year, such as binge drinking, smoking of tobacco and cannabis products, drug abuse, psychological distress, declining grades, physical and cyberbullying, and thoughts of suicide.
The findings revealed that of the 13 harmful outcomes, boys were at a higher risk of engaging in 9. Girls, however, were likely to engage in all 13, indicating that sex plays a major role in the long-term effects of TBI when such incidents occur at a young age (PLoS One 2014 Sept. 30).
“Both boys and girls were more likely to engage in a variety of harmful behaviours if they reported a history of TBI, but girls engaged in all 13 harmful behaviours we looked for, whereas boys were at higher risk of engaging in only nine,” Gabriela Ilie, Ph.D., lead author of the study and a postdoctoral fellow at Toronto’s St. Michael’s Hospital, said in a statement. “Sex matters when it comes to traumatic brain injuries.”
The study found that girls and boys with a history of TBI were all at an elevated risk for alcohol consumption, binge drinking, cannabis smoking, declining grades, bullying others at school, being bullied online, drug abuse, and being treated for physical injuries. But female adolescents in particular were found to be additionally susceptible to elevated psychological distress, past-year suicidal ideation, being bullied at school in the past year, and past-year cigarette smoking.
Dr. Ilie and her colleagues also found that the risk of engaging in alcohol consumption, daily cigarette smoking, and being treated for physical injuries changed depending on the age of the subjects. The average age at which subjects began smoking was 14, while the age at which subjects had their first alcoholic drink skewed similarly young. For physical injury treatment, the risk ran higher for girls aged 11-13 years, and for boys aged 17-20 years.
Across the board, children with TBI presented higher incidences of all harmful behaviors than those without a history of TBI. The study also found that the majority of TBI came from playing team sports – 63% for males and 46.9% for females. Falls were responsible for 5.1% of males and 24.7% of females with TBI histories, with bicycle accidents being responsible for 8.1% of males with TBI and 1.8% of females with TBI.
The researchers cited several limitations of the study. The most salient, they said, was that the data were based on self-reports. In addition, their sample did not include “adolescents who might be at high risk of TBI.”
Still, Dr. Ilie and her colleagues hope that the findings of this study will help shed light on the need for greater action in recognizing and treating adolescent TBI, warning that failure to properly care for these injuries at the right time will significantly increase the risk of detrimental long-term consequences down the road.
“The results point to important opportunities for prevention, such as early-stage medical interventions, as well as diagnosis and rehabilitation approaches that consider the relationship between TBI and its risk factors,” the investigators said. “We know both adolescent boys and girls with TBI are vulnerable to the co-occurrence of harmful psychological and behavioural conditions. Given that most brain injuries among adolescents occur during sports, a more ecological approach to injury prevention requiring involvement by government, schools, and parents may be needed.”
The research was funded by a Canadian Institute of Health Research Team Grant in Traumatic Brain Injury and Violence and by the Ontario Neurotrauma Foundation, with additional funding from the Ontario Ministry of Health and Long-Term Care, the Social Sciences and Humanities Research Council, Industry Canada, and AUTO21, a member of the Networks of Centres of Excellence program that is administered and funded by the Natural Sciences and Engineering Research Council.
FROM PLOS ONE
Key clinical point: Traumatic brain injury in adolescence increases the risk of harmful behavior, particularly in females.
Major finding: Females with a history of TBI are at elevated risk for all 13 key harmful behavior parameters.
Data source: The findings are based on the 2011 Ontario Student Drug Use and Health Survey, developed by the Centre for Addiction and Mental Health.
Disclosures: The research was funded by a Canadian Institute of Health Research Team Grant in Traumatic Brain Injury and Violence and by the Ontario Neurotrauma Foundation, with additional funding from the Ontario Ministry of Health and Long-Term Care, the Social Sciences and Humanities Research Council, Industry Canada, and AUTO21, a member of the Networks of Centres of Excellence program that is administered and funded by the Natural Sciences and Engineering Research Council.