User login
What treatments are effective for varicose veins?
For larger trunk varicose veins, as in the saphenous vein, therapeutic options include conservative measures (such as leg elevation and compression stockings), injection sclerotherapy, and surgical vein ligation, with or without stripping.Long-term outcomes appear superior with surgical treatment.
For mid-sized reticular veins and spider telangiectasias, several options are available, including sclerotherapy, laser ablation, and thermal ablation. However, no randomized trials have compared the relative effectiveness of these treatments.
Venotonic medications (primarily plantderived and synthetic flavonoids, such as horse chestnut seed extract, that improve venous tone) provide symptom relief. Head-to-head comparisons are needed to identify the most efficacious therapies (strength of recommendation: C, based on case series and extrapolations from small trials.)
Evidence summary
Graduated elastic compression stockings improve lower-extremity hemodynamics (including reflux and residual volume measured by color flow duplex scanning) in patients with varicosities, and can improve symptoms such as swelling, discomfort, and leg tightness.1,2
A Cochrane review concluded that existing evidence supports the use of sclerotherapy for recurrent varicose veins after surgery and for relatively minor “thread” veins.3 Data did not show that any particular type of sclerosant or pressure dressing or duration of post-treatment compression have significant effect on outcomes, such as disappearance of varicosities and cosmetic improvement.3
A Cochrane protocol is in progress regarding comparison of the outcomes of surgery and sclerotherapy.4 Few randomized trials have compared surgery and sclerotherapy.
Belcaro reported results of a 10-year randomized trial including 121 subjects, 96 of whom completed the study.5 Surgery consisted of ligation of the saphenopopliteal junction without stripping. At 10 years, 16.1% of patients receiving surgery plus sclerotherapy had distal venous incompetence (assessed with color duplex scanning and ambulatory venous pressure measurement), compared with 36.4% of those who underwent surgery alone and 43.8% of those who received sclerotherapy alone. The authors concluded that long-term outcomes (defined as saphenofemoral junction competence) are superior with strategies that included surgery, but at greater cost.
Beresford and colleagues also concluded that surgery lessened the likelihood of additional treatment.6 Another randomized trial showed that saphenous vein stripping reduced by two thirds the need for reoperation due to recurrent saphenofemoral incompetence, compared with saphenofemoral junction ligation alone.7
A meta-analysis studied the effectiveness of venotonic medications (such as rutoside, flunarizine, and dihydroergotamine) in chronic venous insufficiency.8 These agents significantly reduced pain, leg heaviness, cramps, and paresthesias. However, a Cochrane Collaboration reviewer questioned the validity of pooling results from this heterogeneous group of studies into a single meta-analysis.9
A Cochrane Review did find that horse chestnut seed extract significantly improves leg pain, edema, pruritus, and lower leg volume and circumference, but suggests that larger randomized trials are needed to establish conclusively this agent’s efficacy.10
Recommendations from others
A recent clinical review indicated that patients whose main symptoms are swelling or aching can be treated with compression stockings alone; trunk varicosities should be treated with saphenofemoral or saphenopopliteal ligation, plus stripping of the long saphenous vein for long saphenous varicosities.11 They suggest that sclerotherapy should be reserved for varicosities that persist after surgery.
The Venous Insufficiency Epidemiologic and Economic Studies (VEINES) program recommends sclerotherapy for telangiectasias and reticular veins, and surgery for saphenous varicosities.12 However, they noted the need for randomized trials to compare therapies.
Alan Adelman, MD, MS
Penn State University, State College, Pa
Choosing the best treatment for varicose veins can be complicated. Symptoms and the type of varicose veins (truncal varices, reticular varices, or telangiectasia) can guide the clinician in selecting therapy. Asymptomatic varicosities can usually be observed without treatment. Patients with symptomatic varicosities may be treated conservatively before referring for invasive treatment.
Surgery is probably the best treatment for truncal varices, whereas sclerotherapy is better for reticular veins or telangiectasia. The long-term risks and benefits of newer modalities such as laser and thermal ablation need further evaluation. Regardless of the treatment chosen, patients with varicose veins should first undergo a thorough investigation.
1. Weiss RA, Duffy D. Clinical benefits of lightweight compression: reduction of venous-related symptoms by ready-to-wear lightweight gradient compression hosiery. Dermatol Surg 1999;25:701-704.
2. Labropoulos N, Leon M, Volteas N, Nicolaides AN. Acute and long-term effect of elastic stockings in patients with varicose veins. Int Angiol 1994;13:119-123.
3. Tisi PV, Beverley CA. Injection sclerotherapy for varicose veins (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
4. Michaels JA, Kendall RJ. Surgery for varicose veins (Protocol for a Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
5. Belcaro G, Nicolaides AN, Ricci A, et al. Endovascular sclerotherapy, surgery, and surgery plus sclerotherapy in superficial venous incompetence: a randomized, 10-year follow-up trial—final results. Angiology 2000;51:529-534.
6. Beresford SAA, Chant ADB, Jones HO, Piachaud D, Weddell JM. Varicose veins: a comparison of surgery and injection/compression sclerotherapy. Five-year follow-up. Lancet. 1978;1:921-924.
7. Dwerryhouse S, Davies B, Harradine K, Earnshaw JJ. Stripping the long saphenous vein reduces the rate of reoperation for recurrent varicose veins: five-year results of a randomized trial. J Vasc Surg 1999;29:589-592.
8. Boada JN, Nazco GJ. Therapeutic effect of venotonics in chronic venous insufficiency: a meta-analysis. Clin Drug Invest 1999;18:413-432.
9. Therapeutic effect of venotonics in chronic venous insufficiency: a meta-analysis In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
10. Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
11. London NJM, Nash R. ABC of arterial and venous disease. Varicose veins. BMJ 2000;320:1391-1394.
12. Kurz X, Kahn SR, Abenhaim L, et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidence-based report of the VEINES task force. Int Angiol 1999;18:83-102.
For larger trunk varicose veins, as in the saphenous vein, therapeutic options include conservative measures (such as leg elevation and compression stockings), injection sclerotherapy, and surgical vein ligation, with or without stripping.Long-term outcomes appear superior with surgical treatment.
For mid-sized reticular veins and spider telangiectasias, several options are available, including sclerotherapy, laser ablation, and thermal ablation. However, no randomized trials have compared the relative effectiveness of these treatments.
Venotonic medications (primarily plantderived and synthetic flavonoids, such as horse chestnut seed extract, that improve venous tone) provide symptom relief. Head-to-head comparisons are needed to identify the most efficacious therapies (strength of recommendation: C, based on case series and extrapolations from small trials.)
Evidence summary
Graduated elastic compression stockings improve lower-extremity hemodynamics (including reflux and residual volume measured by color flow duplex scanning) in patients with varicosities, and can improve symptoms such as swelling, discomfort, and leg tightness.1,2
A Cochrane review concluded that existing evidence supports the use of sclerotherapy for recurrent varicose veins after surgery and for relatively minor “thread” veins.3 Data did not show that any particular type of sclerosant or pressure dressing or duration of post-treatment compression have significant effect on outcomes, such as disappearance of varicosities and cosmetic improvement.3
A Cochrane protocol is in progress regarding comparison of the outcomes of surgery and sclerotherapy.4 Few randomized trials have compared surgery and sclerotherapy.
Belcaro reported results of a 10-year randomized trial including 121 subjects, 96 of whom completed the study.5 Surgery consisted of ligation of the saphenopopliteal junction without stripping. At 10 years, 16.1% of patients receiving surgery plus sclerotherapy had distal venous incompetence (assessed with color duplex scanning and ambulatory venous pressure measurement), compared with 36.4% of those who underwent surgery alone and 43.8% of those who received sclerotherapy alone. The authors concluded that long-term outcomes (defined as saphenofemoral junction competence) are superior with strategies that included surgery, but at greater cost.
Beresford and colleagues also concluded that surgery lessened the likelihood of additional treatment.6 Another randomized trial showed that saphenous vein stripping reduced by two thirds the need for reoperation due to recurrent saphenofemoral incompetence, compared with saphenofemoral junction ligation alone.7
A meta-analysis studied the effectiveness of venotonic medications (such as rutoside, flunarizine, and dihydroergotamine) in chronic venous insufficiency.8 These agents significantly reduced pain, leg heaviness, cramps, and paresthesias. However, a Cochrane Collaboration reviewer questioned the validity of pooling results from this heterogeneous group of studies into a single meta-analysis.9
A Cochrane Review did find that horse chestnut seed extract significantly improves leg pain, edema, pruritus, and lower leg volume and circumference, but suggests that larger randomized trials are needed to establish conclusively this agent’s efficacy.10
Recommendations from others
A recent clinical review indicated that patients whose main symptoms are swelling or aching can be treated with compression stockings alone; trunk varicosities should be treated with saphenofemoral or saphenopopliteal ligation, plus stripping of the long saphenous vein for long saphenous varicosities.11 They suggest that sclerotherapy should be reserved for varicosities that persist after surgery.
The Venous Insufficiency Epidemiologic and Economic Studies (VEINES) program recommends sclerotherapy for telangiectasias and reticular veins, and surgery for saphenous varicosities.12 However, they noted the need for randomized trials to compare therapies.
Alan Adelman, MD, MS
Penn State University, State College, Pa
Choosing the best treatment for varicose veins can be complicated. Symptoms and the type of varicose veins (truncal varices, reticular varices, or telangiectasia) can guide the clinician in selecting therapy. Asymptomatic varicosities can usually be observed without treatment. Patients with symptomatic varicosities may be treated conservatively before referring for invasive treatment.
Surgery is probably the best treatment for truncal varices, whereas sclerotherapy is better for reticular veins or telangiectasia. The long-term risks and benefits of newer modalities such as laser and thermal ablation need further evaluation. Regardless of the treatment chosen, patients with varicose veins should first undergo a thorough investigation.
For larger trunk varicose veins, as in the saphenous vein, therapeutic options include conservative measures (such as leg elevation and compression stockings), injection sclerotherapy, and surgical vein ligation, with or without stripping.Long-term outcomes appear superior with surgical treatment.
For mid-sized reticular veins and spider telangiectasias, several options are available, including sclerotherapy, laser ablation, and thermal ablation. However, no randomized trials have compared the relative effectiveness of these treatments.
Venotonic medications (primarily plantderived and synthetic flavonoids, such as horse chestnut seed extract, that improve venous tone) provide symptom relief. Head-to-head comparisons are needed to identify the most efficacious therapies (strength of recommendation: C, based on case series and extrapolations from small trials.)
Evidence summary
Graduated elastic compression stockings improve lower-extremity hemodynamics (including reflux and residual volume measured by color flow duplex scanning) in patients with varicosities, and can improve symptoms such as swelling, discomfort, and leg tightness.1,2
A Cochrane review concluded that existing evidence supports the use of sclerotherapy for recurrent varicose veins after surgery and for relatively minor “thread” veins.3 Data did not show that any particular type of sclerosant or pressure dressing or duration of post-treatment compression have significant effect on outcomes, such as disappearance of varicosities and cosmetic improvement.3
A Cochrane protocol is in progress regarding comparison of the outcomes of surgery and sclerotherapy.4 Few randomized trials have compared surgery and sclerotherapy.
Belcaro reported results of a 10-year randomized trial including 121 subjects, 96 of whom completed the study.5 Surgery consisted of ligation of the saphenopopliteal junction without stripping. At 10 years, 16.1% of patients receiving surgery plus sclerotherapy had distal venous incompetence (assessed with color duplex scanning and ambulatory venous pressure measurement), compared with 36.4% of those who underwent surgery alone and 43.8% of those who received sclerotherapy alone. The authors concluded that long-term outcomes (defined as saphenofemoral junction competence) are superior with strategies that included surgery, but at greater cost.
Beresford and colleagues also concluded that surgery lessened the likelihood of additional treatment.6 Another randomized trial showed that saphenous vein stripping reduced by two thirds the need for reoperation due to recurrent saphenofemoral incompetence, compared with saphenofemoral junction ligation alone.7
A meta-analysis studied the effectiveness of venotonic medications (such as rutoside, flunarizine, and dihydroergotamine) in chronic venous insufficiency.8 These agents significantly reduced pain, leg heaviness, cramps, and paresthesias. However, a Cochrane Collaboration reviewer questioned the validity of pooling results from this heterogeneous group of studies into a single meta-analysis.9
A Cochrane Review did find that horse chestnut seed extract significantly improves leg pain, edema, pruritus, and lower leg volume and circumference, but suggests that larger randomized trials are needed to establish conclusively this agent’s efficacy.10
Recommendations from others
A recent clinical review indicated that patients whose main symptoms are swelling or aching can be treated with compression stockings alone; trunk varicosities should be treated with saphenofemoral or saphenopopliteal ligation, plus stripping of the long saphenous vein for long saphenous varicosities.11 They suggest that sclerotherapy should be reserved for varicosities that persist after surgery.
The Venous Insufficiency Epidemiologic and Economic Studies (VEINES) program recommends sclerotherapy for telangiectasias and reticular veins, and surgery for saphenous varicosities.12 However, they noted the need for randomized trials to compare therapies.
Alan Adelman, MD, MS
Penn State University, State College, Pa
Choosing the best treatment for varicose veins can be complicated. Symptoms and the type of varicose veins (truncal varices, reticular varices, or telangiectasia) can guide the clinician in selecting therapy. Asymptomatic varicosities can usually be observed without treatment. Patients with symptomatic varicosities may be treated conservatively before referring for invasive treatment.
Surgery is probably the best treatment for truncal varices, whereas sclerotherapy is better for reticular veins or telangiectasia. The long-term risks and benefits of newer modalities such as laser and thermal ablation need further evaluation. Regardless of the treatment chosen, patients with varicose veins should first undergo a thorough investigation.
1. Weiss RA, Duffy D. Clinical benefits of lightweight compression: reduction of venous-related symptoms by ready-to-wear lightweight gradient compression hosiery. Dermatol Surg 1999;25:701-704.
2. Labropoulos N, Leon M, Volteas N, Nicolaides AN. Acute and long-term effect of elastic stockings in patients with varicose veins. Int Angiol 1994;13:119-123.
3. Tisi PV, Beverley CA. Injection sclerotherapy for varicose veins (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
4. Michaels JA, Kendall RJ. Surgery for varicose veins (Protocol for a Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
5. Belcaro G, Nicolaides AN, Ricci A, et al. Endovascular sclerotherapy, surgery, and surgery plus sclerotherapy in superficial venous incompetence: a randomized, 10-year follow-up trial—final results. Angiology 2000;51:529-534.
6. Beresford SAA, Chant ADB, Jones HO, Piachaud D, Weddell JM. Varicose veins: a comparison of surgery and injection/compression sclerotherapy. Five-year follow-up. Lancet. 1978;1:921-924.
7. Dwerryhouse S, Davies B, Harradine K, Earnshaw JJ. Stripping the long saphenous vein reduces the rate of reoperation for recurrent varicose veins: five-year results of a randomized trial. J Vasc Surg 1999;29:589-592.
8. Boada JN, Nazco GJ. Therapeutic effect of venotonics in chronic venous insufficiency: a meta-analysis. Clin Drug Invest 1999;18:413-432.
9. Therapeutic effect of venotonics in chronic venous insufficiency: a meta-analysis In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
10. Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
11. London NJM, Nash R. ABC of arterial and venous disease. Varicose veins. BMJ 2000;320:1391-1394.
12. Kurz X, Kahn SR, Abenhaim L, et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidence-based report of the VEINES task force. Int Angiol 1999;18:83-102.
1. Weiss RA, Duffy D. Clinical benefits of lightweight compression: reduction of venous-related symptoms by ready-to-wear lightweight gradient compression hosiery. Dermatol Surg 1999;25:701-704.
2. Labropoulos N, Leon M, Volteas N, Nicolaides AN. Acute and long-term effect of elastic stockings in patients with varicose veins. Int Angiol 1994;13:119-123.
3. Tisi PV, Beverley CA. Injection sclerotherapy for varicose veins (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
4. Michaels JA, Kendall RJ. Surgery for varicose veins (Protocol for a Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
5. Belcaro G, Nicolaides AN, Ricci A, et al. Endovascular sclerotherapy, surgery, and surgery plus sclerotherapy in superficial venous incompetence: a randomized, 10-year follow-up trial—final results. Angiology 2000;51:529-534.
6. Beresford SAA, Chant ADB, Jones HO, Piachaud D, Weddell JM. Varicose veins: a comparison of surgery and injection/compression sclerotherapy. Five-year follow-up. Lancet. 1978;1:921-924.
7. Dwerryhouse S, Davies B, Harradine K, Earnshaw JJ. Stripping the long saphenous vein reduces the rate of reoperation for recurrent varicose veins: five-year results of a randomized trial. J Vasc Surg 1999;29:589-592.
8. Boada JN, Nazco GJ. Therapeutic effect of venotonics in chronic venous insufficiency: a meta-analysis. Clin Drug Invest 1999;18:413-432.
9. Therapeutic effect of venotonics in chronic venous insufficiency: a meta-analysis In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
10. Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency (Cochrane Review). In: The Cochrane Library, Issue 2, 2002. Oxford: Update Software.
11. London NJM, Nash R. ABC of arterial and venous disease. Varicose veins. BMJ 2000;320:1391-1394.
12. Kurz X, Kahn SR, Abenhaim L, et al. Chronic venous disorders of the leg: epidemiology, outcomes, diagnosis and management. Summary of an evidence-based report of the VEINES task force. Int Angiol 1999;18:83-102.
Evidence-based answers from the Family Physicians Inquiries Network
Does surgery for carpal tunnel syndrome improve outcomes?
Good evidence supports the use of surgery for carpal tunnel syndrome over nonsurgical therapies such as wrist splints, nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, occupational therapy, local steroid injections, work modification, and oral vitamin B6 (Grade of Recommendation: A, based on extrapolation from a systematic review of 1 randomized controlled trial [RCT], 1 additional recent RCT, and 2 cohort studies). Surgery is likely worth the extra costs when conservative therapy (up to 3 months) fails to improve symptoms and return of function, because delayed surgery is as successful as surgery performed shortly after diagnosis. Closed endoscopic release and open release surgery are equally effective therapies for controlling symptoms (Grade of Recommendation: C, based on extrapolation from a systematic review of RCTs). However, whether endoscopic release results in more rapid regain of function and return to work is unclear.
See the Patient Information at the end of this article.
Evidence summary
A recent Cochrane review based on only 1 RCT of 22 patients published in 1964 concluded that surgical treatment of carpal tunnel syndrome appears to be more effective than wrist splinting.1 A well-designed RCT of 176 patients published since that Cochrane review stated that with regard to overall improvement of symptoms and function status, surgical treatment of carpal tunnel syndrome was more effective than wrist splinting 18 months posttreatment.2 The investigators found that surgery resulted in worse short-term outcomes at 1 month follow-up (29% vs 42% success), but by 3 months the improvement in all outcomes was greater in the surgery group (80% vs 54% success). The number needed to treat (NNT) over 18 months was only 2 patients in the treatment-received (per protocol) analysis (92% vs 37% success) and 7 in the intention-to-treat analysis (90% vs 75% success). Patients in the conservative treatment group who underwent surgery after splinting had failed had a higher success rate after 18 months follow-up than patients who did not have surgery (94% vs 62% success rate; NNT = 3).
One cohort study of 90 patients concluded that with respect to symptom control and return to function, open release surgery was as effective as local steroid injection at 1 month follow-up.3 However, at 4 to 6 months after the operation, surgery patients were found to have significantly improved symptom and function scores, with continued improvement compared with patients who received the steroid injection. One other cohort study of 429 patients found that surgery (open or closed endoscopic) was more effective with respect to symptom relief and functional status than various nonsurgical therapies (NSAIDs, splints, physical or occupational therapy, local steroid injections, work modification, or vitamin B6) at 30 months follow-up.4 In both cohort studies, the patients’ pretreatment symptom and functioning scores were worse in the surgery group than in the nonsurgical group. The investigators in the first study3 did not report controlling for these scores. In the second study,4 the authors controlled for functional status scores, but not for symptom severity.
One recent systematic review of 14 RCTs comparing types of surgical therapies for carpal tunnel syndrome concluded that none of the alternative surgical procedures, including closed endoscopic release, appeared to give better symptom relief than open release; and that the evidence is conflicting as to whether endoscopic release results in earlier return to work or improved level of function.5
Recommendations from others
The American Society of Plastic and Reconstructive Surgeons recommends surgical release in the following situations6: (1) failed or incomplete conservative therapy; (2) motor weakness or thenar atrophy; (3) lumbrical pattern symptoms (occur when the metacarpophalangeal joints are held at 90 degrees, eg, driving, letter writing, holding a magazine, pinching, using a small tool); (4) severe pattern on electrical studies (not defined); (5) space-occupying lesions requiring excision; (6) acute carpal tunnel syndrome with symptoms lasting longer than 6 to 8 hours; and (7) progressive or severe symptoms lasting longer than 12 months. The Society did not recommend one surgical procedure over another.
Maureen O’Reilly Brown, MD, MPH
Swedish Family Medicine Residency Program Seattle, Washington
In my practice, many patients have carpal tunnel syndrome and we regularly struggle with the question of whether and when to suggest surgical consultation. This review will make that struggle easier. With at least 33% of cases responding to splinting alone, an initial trial of conservative treatment seems appropriate for most patients. However, early surgical referral when a conservative approach has failed can now be easily justified, given the 90% or better success rate with surgery. The authors also include guidelines from the American Society of Plastic and Reconstructive Surgeons, which may be helpful in selecting which patients should go directly to surgical release.
Patient Information
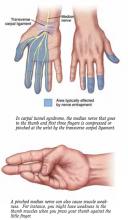
What is carpal tunnel syndrome?
Carpal tunnel syndrome is felt as pain, tingling, a burning sensation, or loss of sensation that occurs throughout all or part of the hand. These symptoms may be worse at night and can wake you from sleep. You may feel the pain in just the hand, or it may travel up the arm.
How it’s diagnosed
Carpal tunnel syndrome can be challenging to diagnose.
Your doctor will ask you to describe your symptoms and may ask you to perform specific motions with your hand or wrist to see how they affect your symptoms.
Your doctor may arrange for a nerve conduction study—a test to determine how well the nerves in your hand are working. The test can detect if the pressure on the nerve is enough to affect how well it works.
How it’s treated
Your doctor may ask you to wear wrist splints at night or during work, and may advise you to reduce those activities that make the problem worse. Steroid injections into the carpal tunnel may also help. If such conservative treatment does not help, your doctor may talk to you about a simple surgical procedure to relieve pressure on the nerve. The surgeon cuts the ligament over the carpal tunnel, which releases the pressure on the nerve. This surgery works well to relieve the symptoms of carpal tunnel syndrome.
The carpal “tunnel” is the space in which nerves, tendons, and blood vessels pass through the bones of the wrist. Anything that narrows the tunnel, such as swelling of tendons, can compress the nerve and cause carpal tunnel syndrome.
1. Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev 2002;(2):CD001552.-
2. Gerritsen AA, de Vet HC, Scholten RJ, Bertelsmann FW, de Krom MC, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA 2002;288:1245-51.
3. Demirci S, Kutluhan S, Koyuncuoglu HR, et al. Comparison of open carpal tunnel release and local steroid treatment outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int 2002;22:33-7.
4. Katz JN, Keller RB, Simmons BP, et al. Maine carpal tunnel study: outcomes of operative and nonoperative therapy for carpal tunnel syndrome in a community-based cohort. J Hand Surg [Am] 1998;23:697-710.
5. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg 2001;88:1285-95.
6. American Society of Plastic and Reconstructive Surgeons. Carpal Tunnel Syndrome (Guidelines). Arlington Heights, IL: American Society of Plastic and Reconstructive Surgeons; 1998.
Good evidence supports the use of surgery for carpal tunnel syndrome over nonsurgical therapies such as wrist splints, nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, occupational therapy, local steroid injections, work modification, and oral vitamin B6 (Grade of Recommendation: A, based on extrapolation from a systematic review of 1 randomized controlled trial [RCT], 1 additional recent RCT, and 2 cohort studies). Surgery is likely worth the extra costs when conservative therapy (up to 3 months) fails to improve symptoms and return of function, because delayed surgery is as successful as surgery performed shortly after diagnosis. Closed endoscopic release and open release surgery are equally effective therapies for controlling symptoms (Grade of Recommendation: C, based on extrapolation from a systematic review of RCTs). However, whether endoscopic release results in more rapid regain of function and return to work is unclear.
See the Patient Information at the end of this article.
Evidence summary
A recent Cochrane review based on only 1 RCT of 22 patients published in 1964 concluded that surgical treatment of carpal tunnel syndrome appears to be more effective than wrist splinting.1 A well-designed RCT of 176 patients published since that Cochrane review stated that with regard to overall improvement of symptoms and function status, surgical treatment of carpal tunnel syndrome was more effective than wrist splinting 18 months posttreatment.2 The investigators found that surgery resulted in worse short-term outcomes at 1 month follow-up (29% vs 42% success), but by 3 months the improvement in all outcomes was greater in the surgery group (80% vs 54% success). The number needed to treat (NNT) over 18 months was only 2 patients in the treatment-received (per protocol) analysis (92% vs 37% success) and 7 in the intention-to-treat analysis (90% vs 75% success). Patients in the conservative treatment group who underwent surgery after splinting had failed had a higher success rate after 18 months follow-up than patients who did not have surgery (94% vs 62% success rate; NNT = 3).
One cohort study of 90 patients concluded that with respect to symptom control and return to function, open release surgery was as effective as local steroid injection at 1 month follow-up.3 However, at 4 to 6 months after the operation, surgery patients were found to have significantly improved symptom and function scores, with continued improvement compared with patients who received the steroid injection. One other cohort study of 429 patients found that surgery (open or closed endoscopic) was more effective with respect to symptom relief and functional status than various nonsurgical therapies (NSAIDs, splints, physical or occupational therapy, local steroid injections, work modification, or vitamin B6) at 30 months follow-up.4 In both cohort studies, the patients’ pretreatment symptom and functioning scores were worse in the surgery group than in the nonsurgical group. The investigators in the first study3 did not report controlling for these scores. In the second study,4 the authors controlled for functional status scores, but not for symptom severity.
One recent systematic review of 14 RCTs comparing types of surgical therapies for carpal tunnel syndrome concluded that none of the alternative surgical procedures, including closed endoscopic release, appeared to give better symptom relief than open release; and that the evidence is conflicting as to whether endoscopic release results in earlier return to work or improved level of function.5
Recommendations from others
The American Society of Plastic and Reconstructive Surgeons recommends surgical release in the following situations6: (1) failed or incomplete conservative therapy; (2) motor weakness or thenar atrophy; (3) lumbrical pattern symptoms (occur when the metacarpophalangeal joints are held at 90 degrees, eg, driving, letter writing, holding a magazine, pinching, using a small tool); (4) severe pattern on electrical studies (not defined); (5) space-occupying lesions requiring excision; (6) acute carpal tunnel syndrome with symptoms lasting longer than 6 to 8 hours; and (7) progressive or severe symptoms lasting longer than 12 months. The Society did not recommend one surgical procedure over another.
Maureen O’Reilly Brown, MD, MPH
Swedish Family Medicine Residency Program Seattle, Washington
In my practice, many patients have carpal tunnel syndrome and we regularly struggle with the question of whether and when to suggest surgical consultation. This review will make that struggle easier. With at least 33% of cases responding to splinting alone, an initial trial of conservative treatment seems appropriate for most patients. However, early surgical referral when a conservative approach has failed can now be easily justified, given the 90% or better success rate with surgery. The authors also include guidelines from the American Society of Plastic and Reconstructive Surgeons, which may be helpful in selecting which patients should go directly to surgical release.
Patient Information
What is carpal tunnel syndrome?
Carpal tunnel syndrome is felt as pain, tingling, a burning sensation, or loss of sensation that occurs throughout all or part of the hand. These symptoms may be worse at night and can wake you from sleep. You may feel the pain in just the hand, or it may travel up the arm.
How it’s diagnosed
Carpal tunnel syndrome can be challenging to diagnose.
Your doctor will ask you to describe your symptoms and may ask you to perform specific motions with your hand or wrist to see how they affect your symptoms.
Your doctor may arrange for a nerve conduction study—a test to determine how well the nerves in your hand are working. The test can detect if the pressure on the nerve is enough to affect how well it works.
How it’s treated
Your doctor may ask you to wear wrist splints at night or during work, and may advise you to reduce those activities that make the problem worse. Steroid injections into the carpal tunnel may also help. If such conservative treatment does not help, your doctor may talk to you about a simple surgical procedure to relieve pressure on the nerve. The surgeon cuts the ligament over the carpal tunnel, which releases the pressure on the nerve. This surgery works well to relieve the symptoms of carpal tunnel syndrome.
The carpal “tunnel” is the space in which nerves, tendons, and blood vessels pass through the bones of the wrist. Anything that narrows the tunnel, such as swelling of tendons, can compress the nerve and cause carpal tunnel syndrome.
Good evidence supports the use of surgery for carpal tunnel syndrome over nonsurgical therapies such as wrist splints, nonsteroidal anti-inflammatory drugs (NSAIDs), physical therapy, occupational therapy, local steroid injections, work modification, and oral vitamin B6 (Grade of Recommendation: A, based on extrapolation from a systematic review of 1 randomized controlled trial [RCT], 1 additional recent RCT, and 2 cohort studies). Surgery is likely worth the extra costs when conservative therapy (up to 3 months) fails to improve symptoms and return of function, because delayed surgery is as successful as surgery performed shortly after diagnosis. Closed endoscopic release and open release surgery are equally effective therapies for controlling symptoms (Grade of Recommendation: C, based on extrapolation from a systematic review of RCTs). However, whether endoscopic release results in more rapid regain of function and return to work is unclear.
See the Patient Information at the end of this article.
Evidence summary
A recent Cochrane review based on only 1 RCT of 22 patients published in 1964 concluded that surgical treatment of carpal tunnel syndrome appears to be more effective than wrist splinting.1 A well-designed RCT of 176 patients published since that Cochrane review stated that with regard to overall improvement of symptoms and function status, surgical treatment of carpal tunnel syndrome was more effective than wrist splinting 18 months posttreatment.2 The investigators found that surgery resulted in worse short-term outcomes at 1 month follow-up (29% vs 42% success), but by 3 months the improvement in all outcomes was greater in the surgery group (80% vs 54% success). The number needed to treat (NNT) over 18 months was only 2 patients in the treatment-received (per protocol) analysis (92% vs 37% success) and 7 in the intention-to-treat analysis (90% vs 75% success). Patients in the conservative treatment group who underwent surgery after splinting had failed had a higher success rate after 18 months follow-up than patients who did not have surgery (94% vs 62% success rate; NNT = 3).
One cohort study of 90 patients concluded that with respect to symptom control and return to function, open release surgery was as effective as local steroid injection at 1 month follow-up.3 However, at 4 to 6 months after the operation, surgery patients were found to have significantly improved symptom and function scores, with continued improvement compared with patients who received the steroid injection. One other cohort study of 429 patients found that surgery (open or closed endoscopic) was more effective with respect to symptom relief and functional status than various nonsurgical therapies (NSAIDs, splints, physical or occupational therapy, local steroid injections, work modification, or vitamin B6) at 30 months follow-up.4 In both cohort studies, the patients’ pretreatment symptom and functioning scores were worse in the surgery group than in the nonsurgical group. The investigators in the first study3 did not report controlling for these scores. In the second study,4 the authors controlled for functional status scores, but not for symptom severity.
One recent systematic review of 14 RCTs comparing types of surgical therapies for carpal tunnel syndrome concluded that none of the alternative surgical procedures, including closed endoscopic release, appeared to give better symptom relief than open release; and that the evidence is conflicting as to whether endoscopic release results in earlier return to work or improved level of function.5
Recommendations from others
The American Society of Plastic and Reconstructive Surgeons recommends surgical release in the following situations6: (1) failed or incomplete conservative therapy; (2) motor weakness or thenar atrophy; (3) lumbrical pattern symptoms (occur when the metacarpophalangeal joints are held at 90 degrees, eg, driving, letter writing, holding a magazine, pinching, using a small tool); (4) severe pattern on electrical studies (not defined); (5) space-occupying lesions requiring excision; (6) acute carpal tunnel syndrome with symptoms lasting longer than 6 to 8 hours; and (7) progressive or severe symptoms lasting longer than 12 months. The Society did not recommend one surgical procedure over another.
Maureen O’Reilly Brown, MD, MPH
Swedish Family Medicine Residency Program Seattle, Washington
In my practice, many patients have carpal tunnel syndrome and we regularly struggle with the question of whether and when to suggest surgical consultation. This review will make that struggle easier. With at least 33% of cases responding to splinting alone, an initial trial of conservative treatment seems appropriate for most patients. However, early surgical referral when a conservative approach has failed can now be easily justified, given the 90% or better success rate with surgery. The authors also include guidelines from the American Society of Plastic and Reconstructive Surgeons, which may be helpful in selecting which patients should go directly to surgical release.
Patient Information
What is carpal tunnel syndrome?
Carpal tunnel syndrome is felt as pain, tingling, a burning sensation, or loss of sensation that occurs throughout all or part of the hand. These symptoms may be worse at night and can wake you from sleep. You may feel the pain in just the hand, or it may travel up the arm.
How it’s diagnosed
Carpal tunnel syndrome can be challenging to diagnose.
Your doctor will ask you to describe your symptoms and may ask you to perform specific motions with your hand or wrist to see how they affect your symptoms.
Your doctor may arrange for a nerve conduction study—a test to determine how well the nerves in your hand are working. The test can detect if the pressure on the nerve is enough to affect how well it works.
How it’s treated
Your doctor may ask you to wear wrist splints at night or during work, and may advise you to reduce those activities that make the problem worse. Steroid injections into the carpal tunnel may also help. If such conservative treatment does not help, your doctor may talk to you about a simple surgical procedure to relieve pressure on the nerve. The surgeon cuts the ligament over the carpal tunnel, which releases the pressure on the nerve. This surgery works well to relieve the symptoms of carpal tunnel syndrome.
The carpal “tunnel” is the space in which nerves, tendons, and blood vessels pass through the bones of the wrist. Anything that narrows the tunnel, such as swelling of tendons, can compress the nerve and cause carpal tunnel syndrome.
1. Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev 2002;(2):CD001552.-
2. Gerritsen AA, de Vet HC, Scholten RJ, Bertelsmann FW, de Krom MC, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA 2002;288:1245-51.
3. Demirci S, Kutluhan S, Koyuncuoglu HR, et al. Comparison of open carpal tunnel release and local steroid treatment outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int 2002;22:33-7.
4. Katz JN, Keller RB, Simmons BP, et al. Maine carpal tunnel study: outcomes of operative and nonoperative therapy for carpal tunnel syndrome in a community-based cohort. J Hand Surg [Am] 1998;23:697-710.
5. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg 2001;88:1285-95.
6. American Society of Plastic and Reconstructive Surgeons. Carpal Tunnel Syndrome (Guidelines). Arlington Heights, IL: American Society of Plastic and Reconstructive Surgeons; 1998.
1. Verdugo RJ, Salinas RS, Castillo J, Cea JG. Surgical versus non-surgical treatment for carpal tunnel syndrome. Cochrane Database Syst Rev 2002;(2):CD001552.-
2. Gerritsen AA, de Vet HC, Scholten RJ, Bertelsmann FW, de Krom MC, Bouter LM. Splinting vs surgery in the treatment of carpal tunnel syndrome: a randomized controlled trial. JAMA 2002;288:1245-51.
3. Demirci S, Kutluhan S, Koyuncuoglu HR, et al. Comparison of open carpal tunnel release and local steroid treatment outcomes in idiopathic carpal tunnel syndrome. Rheumatol Int 2002;22:33-7.
4. Katz JN, Keller RB, Simmons BP, et al. Maine carpal tunnel study: outcomes of operative and nonoperative therapy for carpal tunnel syndrome in a community-based cohort. J Hand Surg [Am] 1998;23:697-710.
5. Gerritsen AA, Uitdehaag BM, van Geldere D, Scholten RJ, de Vet HC, Bouter LM. Systematic review of randomized clinical trials of surgical treatment for carpal tunnel syndrome. Br J Surg 2001;88:1285-95.
6. American Society of Plastic and Reconstructive Surgeons. Carpal Tunnel Syndrome (Guidelines). Arlington Heights, IL: American Society of Plastic and Reconstructive Surgeons; 1998.
Evidence-based answers from the Family Physicians Inquiries Network
Does a low-salt diet reduce morbidity and mortality in congestive heart failure?
No randomized controlled trials (RCTs) have addressed the independent role of sodium restriction in the morbidity or mortality of congestive heart failure. However, current guidelines recommend sodium restriction for secondary prevention of congestive heart failure exacerbation. (Grade of recommendation: D.) Clinical trials of multifactorial, nondrug interventions have shown an association of sodium restriction with reduced morbidity and improved quality of life in some populations with congestive heart failure. (Grade of recommendation: C.)
Evidence summary
Sodium restriction is a mainstay of nonpharmacologic therapy for congestive heart failure, although no evidence proves that sodium restriction alone reduces morbidity and mortality.1 Sodium restriction reduces hypertension2,3 and left ventricular hypertrophy,4 both risk factors for congestive heart failure.
Studies of multifactorial interventions correlate reduced congestive heart failure morbidity with sodium restriction or dietary counseling. These results cannot be generalized to sodium restriction independent of the other nondrug interventions. A small RCT compared a program of exercise, cognitive therapy/stress management, salt restriction, and weight reduction to treating congestive heart failure with digoxin or placebo.5 The nondrug interventions improved functional capacity, body weight, and mood but not ejection fraction in patients with congestive heart failure.5 A systematic review of 6 RCTs showed that multidisciplinary heart failure disease management programs, which emphasized dietary counseling and/or sodium intake reduction, improved functional capacity, patient satisfaction, and quality of life.6
A large RCT that investigated how sodium reduction affects hypertension and frequency of cardiovascular events (including congestive heart failure) in the elderly did not show a significant difference in primary prevention of cardiovascular events between the sodium-restricted group and controls.3,7 Two prospective cohort studies linked high sodium intake to cardiovascular mortality and all-cause mortality in overweight persons independent of other cardiovascular risk factors.8,9
Recommendations from others
Physiological principles, observational studies, common practice, and expert opinion support sodium restriction for reducing edema and the need for diuretic agents in patients with congestive heart failure.1 No clinical trial evidence favors a 2-g over a 3- to 4-g sodium restriction. See Table for common recommendations.
TABLE
Recommended sodium restrictions
| Patient populations with congestive heart failure | Sodium restriction |
|---|---|
| Older adult1 | 1.6 g Na |
| With fluid retention or hypertension11 | Moderate sodium reduction |
| At risk for or with asymptomatic heart failure11 | Prudent dietary salt reduction |
| Older adult nursing home residents12 | Low salt |
| Taking diuretics10 | 2 g Na |
Clinical Commentary by John Tipton, MD, at http://www.fpin.org.
1. Aronow WS. J Am Geriatr Soc 1997;45:1252-7.
2. Johnson AG, Nguyen TV, Davis D. J Hypertens 2001;19:1053-60.
3. Appel LJ, Espeland MA, Easter L, et al. Arch Intern Med 2001;161:685-93.
4. Beil AH, Schmieder RE. Blood Press Suppl 1995;2:30-4.
5. Kostis JB, Rosen RC, Cosgrove NM, et al. Chest 1994;106:996-1001.
6. Rich MW. J Card Fail 1999;5:64-75.
7. Whelton PK, Appel LJ, Espeland MA, et al. JAMA 1998;279:839-46.
8. Tuomilehto J, Jousilahti P, Rastenyte D, et al. Lancet 2001;357:848-51.
9. He J, Ogden LG, Vupputuri S, et al. JAMA 1999;282:2027-34.
10. Heart failure—systolic dysfunction. August 1999. Available at: http://cme.med.umich.edu/pdf/guideline/heart.pdf.
11. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). November 1,1995 (revised December 2001). Available at: http://www.ngc.gov/VIEWS/summary.asp?guideline=2340.
12. American Medical Directors Association. Heart failure. 1996. Available at: http://www.guideline.gov/FRAMESETS/guideline_fs.asp?guideline=001035&.
No randomized controlled trials (RCTs) have addressed the independent role of sodium restriction in the morbidity or mortality of congestive heart failure. However, current guidelines recommend sodium restriction for secondary prevention of congestive heart failure exacerbation. (Grade of recommendation: D.) Clinical trials of multifactorial, nondrug interventions have shown an association of sodium restriction with reduced morbidity and improved quality of life in some populations with congestive heart failure. (Grade of recommendation: C.)
Evidence summary
Sodium restriction is a mainstay of nonpharmacologic therapy for congestive heart failure, although no evidence proves that sodium restriction alone reduces morbidity and mortality.1 Sodium restriction reduces hypertension2,3 and left ventricular hypertrophy,4 both risk factors for congestive heart failure.
Studies of multifactorial interventions correlate reduced congestive heart failure morbidity with sodium restriction or dietary counseling. These results cannot be generalized to sodium restriction independent of the other nondrug interventions. A small RCT compared a program of exercise, cognitive therapy/stress management, salt restriction, and weight reduction to treating congestive heart failure with digoxin or placebo.5 The nondrug interventions improved functional capacity, body weight, and mood but not ejection fraction in patients with congestive heart failure.5 A systematic review of 6 RCTs showed that multidisciplinary heart failure disease management programs, which emphasized dietary counseling and/or sodium intake reduction, improved functional capacity, patient satisfaction, and quality of life.6
A large RCT that investigated how sodium reduction affects hypertension and frequency of cardiovascular events (including congestive heart failure) in the elderly did not show a significant difference in primary prevention of cardiovascular events between the sodium-restricted group and controls.3,7 Two prospective cohort studies linked high sodium intake to cardiovascular mortality and all-cause mortality in overweight persons independent of other cardiovascular risk factors.8,9
Recommendations from others
Physiological principles, observational studies, common practice, and expert opinion support sodium restriction for reducing edema and the need for diuretic agents in patients with congestive heart failure.1 No clinical trial evidence favors a 2-g over a 3- to 4-g sodium restriction. See Table for common recommendations.
TABLE
Recommended sodium restrictions
| Patient populations with congestive heart failure | Sodium restriction |
|---|---|
| Older adult1 | 1.6 g Na |
| With fluid retention or hypertension11 | Moderate sodium reduction |
| At risk for or with asymptomatic heart failure11 | Prudent dietary salt reduction |
| Older adult nursing home residents12 | Low salt |
| Taking diuretics10 | 2 g Na |
Clinical Commentary by John Tipton, MD, at http://www.fpin.org.
No randomized controlled trials (RCTs) have addressed the independent role of sodium restriction in the morbidity or mortality of congestive heart failure. However, current guidelines recommend sodium restriction for secondary prevention of congestive heart failure exacerbation. (Grade of recommendation: D.) Clinical trials of multifactorial, nondrug interventions have shown an association of sodium restriction with reduced morbidity and improved quality of life in some populations with congestive heart failure. (Grade of recommendation: C.)
Evidence summary
Sodium restriction is a mainstay of nonpharmacologic therapy for congestive heart failure, although no evidence proves that sodium restriction alone reduces morbidity and mortality.1 Sodium restriction reduces hypertension2,3 and left ventricular hypertrophy,4 both risk factors for congestive heart failure.
Studies of multifactorial interventions correlate reduced congestive heart failure morbidity with sodium restriction or dietary counseling. These results cannot be generalized to sodium restriction independent of the other nondrug interventions. A small RCT compared a program of exercise, cognitive therapy/stress management, salt restriction, and weight reduction to treating congestive heart failure with digoxin or placebo.5 The nondrug interventions improved functional capacity, body weight, and mood but not ejection fraction in patients with congestive heart failure.5 A systematic review of 6 RCTs showed that multidisciplinary heart failure disease management programs, which emphasized dietary counseling and/or sodium intake reduction, improved functional capacity, patient satisfaction, and quality of life.6
A large RCT that investigated how sodium reduction affects hypertension and frequency of cardiovascular events (including congestive heart failure) in the elderly did not show a significant difference in primary prevention of cardiovascular events between the sodium-restricted group and controls.3,7 Two prospective cohort studies linked high sodium intake to cardiovascular mortality and all-cause mortality in overweight persons independent of other cardiovascular risk factors.8,9
Recommendations from others
Physiological principles, observational studies, common practice, and expert opinion support sodium restriction for reducing edema and the need for diuretic agents in patients with congestive heart failure.1 No clinical trial evidence favors a 2-g over a 3- to 4-g sodium restriction. See Table for common recommendations.
TABLE
Recommended sodium restrictions
| Patient populations with congestive heart failure | Sodium restriction |
|---|---|
| Older adult1 | 1.6 g Na |
| With fluid retention or hypertension11 | Moderate sodium reduction |
| At risk for or with asymptomatic heart failure11 | Prudent dietary salt reduction |
| Older adult nursing home residents12 | Low salt |
| Taking diuretics10 | 2 g Na |
Clinical Commentary by John Tipton, MD, at http://www.fpin.org.
1. Aronow WS. J Am Geriatr Soc 1997;45:1252-7.
2. Johnson AG, Nguyen TV, Davis D. J Hypertens 2001;19:1053-60.
3. Appel LJ, Espeland MA, Easter L, et al. Arch Intern Med 2001;161:685-93.
4. Beil AH, Schmieder RE. Blood Press Suppl 1995;2:30-4.
5. Kostis JB, Rosen RC, Cosgrove NM, et al. Chest 1994;106:996-1001.
6. Rich MW. J Card Fail 1999;5:64-75.
7. Whelton PK, Appel LJ, Espeland MA, et al. JAMA 1998;279:839-46.
8. Tuomilehto J, Jousilahti P, Rastenyte D, et al. Lancet 2001;357:848-51.
9. He J, Ogden LG, Vupputuri S, et al. JAMA 1999;282:2027-34.
10. Heart failure—systolic dysfunction. August 1999. Available at: http://cme.med.umich.edu/pdf/guideline/heart.pdf.
11. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). November 1,1995 (revised December 2001). Available at: http://www.ngc.gov/VIEWS/summary.asp?guideline=2340.
12. American Medical Directors Association. Heart failure. 1996. Available at: http://www.guideline.gov/FRAMESETS/guideline_fs.asp?guideline=001035&.
1. Aronow WS. J Am Geriatr Soc 1997;45:1252-7.
2. Johnson AG, Nguyen TV, Davis D. J Hypertens 2001;19:1053-60.
3. Appel LJ, Espeland MA, Easter L, et al. Arch Intern Med 2001;161:685-93.
4. Beil AH, Schmieder RE. Blood Press Suppl 1995;2:30-4.
5. Kostis JB, Rosen RC, Cosgrove NM, et al. Chest 1994;106:996-1001.
6. Rich MW. J Card Fail 1999;5:64-75.
7. Whelton PK, Appel LJ, Espeland MA, et al. JAMA 1998;279:839-46.
8. Tuomilehto J, Jousilahti P, Rastenyte D, et al. Lancet 2001;357:848-51.
9. He J, Ogden LG, Vupputuri S, et al. JAMA 1999;282:2027-34.
10. Heart failure—systolic dysfunction. August 1999. Available at: http://cme.med.umich.edu/pdf/guideline/heart.pdf.
11. ACC/AHA guidelines for the evaluation and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure). November 1,1995 (revised December 2001). Available at: http://www.ngc.gov/VIEWS/summary.asp?guideline=2340.
12. American Medical Directors Association. Heart failure. 1996. Available at: http://www.guideline.gov/FRAMESETS/guideline_fs.asp?guideline=001035&.
Evidence-based answers from the Family Physicians Inquiries Network
Are any oral iron formulations better tolerated than ferrous sulfate?
Ferrous salt preparations (ferrous sulfate, ferrous gluconate, and ferrous fumarate) are equally tolerable. (Grade of recommendation: A, based on randomized controlled trial.) Controlled-release iron preparations cause less nausea and epigastric pain than conventional ferrous sulfate (grade of recommendation: A, based on randomized controlled trials), although the discontinuation rates between the 2 iron formulations were similar. Ferrous sulfate remains the standard first-line treatment of iron-deficiency anemia given its general tolerability, effectiveness, and low cost.
Evidence summary
A randomized, double-blinded, placebo-controlled study in 1496 subjects examined side-effect rates of 3 iron salt formulations using equal dosages of elemental iron (Table).1 Gastrointestinal (GI) side-effect rates were not significantly different. The side-effect rate in the ferrous sulfate group (23%) was significantly different from that of the placebo group (14%); thus, for every 11 patients treated with ferrous sulfate, 1 patient would have GI side effects attributable to the iron salt (number needed to harm [NNH] = 11).
Two formulations—controlled-release iron preparations and polysaccharide–iron complexes—decrease the amount of iron presented to the proximal GI tract. Three large randomized trials assessed tolerability of controlled-release iron preparations compared with ferrous sulfate.2–4 The only double-blinded study found a lower rate of nausea and epigastric pain in the controlled-release iron formulation among 1376 blood donors receiving 200 mg/day elemental iron (3.3% vs 6.4%, P < .05, NNH = ~32).2 A nonblinded randomized trial of 543 non-anemic adult patients taking 50 mg/day elemental iron also found a lower rate of stomach-related side effects in the controlled-release group (12.2% vs 27.2%, P < .001, NNH = ~7).3 However, none of the 3 studies showed a difference in the discontinuation rates between the 2 iron formulations. Comparative constipation rates among the trials were conflicting.
Two small, nonblinded, randomized trials of polysaccharide–iron complexes reported conflicting results. A study of 159 subjects found fewer subjects discontinuing the polysaccharide–iron complex taken with meals than ferrous sulfate taken on an empty stomach.5 A study of 60 subjects taking both preparations on an empty stomach found no difference in side-effect rates.6 Two small, randomized, blinded studies found no difference in rates of GI side effects between carbonyl iron and ferrous sulfate.7,8
TABLE
Representative average wholesale prices* for various iron supplement formulations
| Iron supplement group | Generic or brand name | Dosage | Cost of 1-month course |
|---|---|---|---|
| Ferrous salts | Ferrous sulfate (generic) | Tablet: 325 mg po tid | $0.63 to $5.11 (90 tabs) |
| Ferrous fumarate (generic) | Tablet: 300 mg (99 mg iron) po bid | $1.80 (60 tabs) | |
| Ferrous gluconate (generic) | Tablet: 325 mg (36 mg iron) po tid | $2.70 to $5.00 (90 tabs) | |
| Controlled-release | Slow FE (Novartis) | Tablet: 160 mg (50 mg iron) po tid | $18.92 (90 tabs) |
| Ferro-Grad-500 (Abbott) | Tablet: 105 mg iron po bid | $31.84 (60 tabs) | |
| Polysaccharide–iron complex | Niferex-150 (Schwarz Pharma) | Capsule: 150 mg iron po qd | $10.50 (30 caps) |
| Carbonyl iron | Feosol (SmithKline Beecham) | Tablet: 50 mg iron po tid | $18.38 (90 tabs) |
| *2001 Drug Topics, Red Book. Daily dosages given here deliver 150 to 210 mg of elemental iron and are for comparison of average costs. Actual dosage should be adjusted according to the calculated need for iron replacement and the results of laboratory monitoring. | |||
Recommendations from others
Wintrobe’s Clinical Hematology9 and Williams Hematology10 recommend ferrous sulfate as the standard oral iron preparation, and assert that claims of improved tolerability of one oral iron preparation over another have not been substantiated.
Clinical Commentary by Andrea Gordon, MD, at http://www.fpin.org.
1. Hallberg L, Ryttinger L, Solvell L. Side-effects of oral iron therapy. A double-blind study of different iron compounds in tablet form. Acta Med Scand Suppl 1966;459:3-10.
2. Rybo G, Solvell L. Side-effect studies on a new sustained release iron preparation. Scand J Haematol 1971;8:257-64.
3. Brock C, Curry H, Hanna C, Knipfer M, Taylor L. Adverse effects of iron supplementation: a comparative trial of a wax-matrix iron preparation and conventional ferrous sulfate tablets. Clin Ther 1985;7:568-73.
4. Elwood PC, Williams G. A comparative trial of slow-release and conventional iron preparations. Practitioner 1970;204:812-5.
5. Jacobs P, Coghlan P. Comparative bioavailability of ferric polymaltose and ferrous sulphate in iron-deficient blood donors. J Clin Apheresis 1993;8:89-95.
6. Sas G, Nemesanszky E, Brauer H, Scheffer K. On the therapeutic effects of trivalent and divalent iron in iron deficiency anaemia. Arzneimittel-Forschung 1984;34:1575-9.
7. Gordeuk VR, Brittenham GM, Hughes M, Keating LJ, Opplt JJ. High-dose carbonyl iron for iron deficiency anemia: a randomized double-blind trial. Am J Clin Nutr 1987;46:1029-34.
8. Devasthali SD, Gordeuk VR, Brittenham GM, Bravo JR, Hughes MA, Keating LJ. Bioavailability of carbonyl iron: a randomized, double-blind study. Eur J Haematol 1991;46:272-8.
9. Richard LG. Wintrobe’s Clinical Hematology. 10th ed. Baltimore: Williams &; Wilkins; 1999;979-1010.
10. Fairbanks VF, Beutler E. Williams Hematology. 6th ed. New York: McGraw-Hill; 2001;447-70.
Ferrous salt preparations (ferrous sulfate, ferrous gluconate, and ferrous fumarate) are equally tolerable. (Grade of recommendation: A, based on randomized controlled trial.) Controlled-release iron preparations cause less nausea and epigastric pain than conventional ferrous sulfate (grade of recommendation: A, based on randomized controlled trials), although the discontinuation rates between the 2 iron formulations were similar. Ferrous sulfate remains the standard first-line treatment of iron-deficiency anemia given its general tolerability, effectiveness, and low cost.
Evidence summary
A randomized, double-blinded, placebo-controlled study in 1496 subjects examined side-effect rates of 3 iron salt formulations using equal dosages of elemental iron (Table).1 Gastrointestinal (GI) side-effect rates were not significantly different. The side-effect rate in the ferrous sulfate group (23%) was significantly different from that of the placebo group (14%); thus, for every 11 patients treated with ferrous sulfate, 1 patient would have GI side effects attributable to the iron salt (number needed to harm [NNH] = 11).
Two formulations—controlled-release iron preparations and polysaccharide–iron complexes—decrease the amount of iron presented to the proximal GI tract. Three large randomized trials assessed tolerability of controlled-release iron preparations compared with ferrous sulfate.2–4 The only double-blinded study found a lower rate of nausea and epigastric pain in the controlled-release iron formulation among 1376 blood donors receiving 200 mg/day elemental iron (3.3% vs 6.4%, P < .05, NNH = ~32).2 A nonblinded randomized trial of 543 non-anemic adult patients taking 50 mg/day elemental iron also found a lower rate of stomach-related side effects in the controlled-release group (12.2% vs 27.2%, P < .001, NNH = ~7).3 However, none of the 3 studies showed a difference in the discontinuation rates between the 2 iron formulations. Comparative constipation rates among the trials were conflicting.
Two small, nonblinded, randomized trials of polysaccharide–iron complexes reported conflicting results. A study of 159 subjects found fewer subjects discontinuing the polysaccharide–iron complex taken with meals than ferrous sulfate taken on an empty stomach.5 A study of 60 subjects taking both preparations on an empty stomach found no difference in side-effect rates.6 Two small, randomized, blinded studies found no difference in rates of GI side effects between carbonyl iron and ferrous sulfate.7,8
TABLE
Representative average wholesale prices* for various iron supplement formulations
| Iron supplement group | Generic or brand name | Dosage | Cost of 1-month course |
|---|---|---|---|
| Ferrous salts | Ferrous sulfate (generic) | Tablet: 325 mg po tid | $0.63 to $5.11 (90 tabs) |
| Ferrous fumarate (generic) | Tablet: 300 mg (99 mg iron) po bid | $1.80 (60 tabs) | |
| Ferrous gluconate (generic) | Tablet: 325 mg (36 mg iron) po tid | $2.70 to $5.00 (90 tabs) | |
| Controlled-release | Slow FE (Novartis) | Tablet: 160 mg (50 mg iron) po tid | $18.92 (90 tabs) |
| Ferro-Grad-500 (Abbott) | Tablet: 105 mg iron po bid | $31.84 (60 tabs) | |
| Polysaccharide–iron complex | Niferex-150 (Schwarz Pharma) | Capsule: 150 mg iron po qd | $10.50 (30 caps) |
| Carbonyl iron | Feosol (SmithKline Beecham) | Tablet: 50 mg iron po tid | $18.38 (90 tabs) |
| *2001 Drug Topics, Red Book. Daily dosages given here deliver 150 to 210 mg of elemental iron and are for comparison of average costs. Actual dosage should be adjusted according to the calculated need for iron replacement and the results of laboratory monitoring. | |||
Recommendations from others
Wintrobe’s Clinical Hematology9 and Williams Hematology10 recommend ferrous sulfate as the standard oral iron preparation, and assert that claims of improved tolerability of one oral iron preparation over another have not been substantiated.
Clinical Commentary by Andrea Gordon, MD, at http://www.fpin.org.
Ferrous salt preparations (ferrous sulfate, ferrous gluconate, and ferrous fumarate) are equally tolerable. (Grade of recommendation: A, based on randomized controlled trial.) Controlled-release iron preparations cause less nausea and epigastric pain than conventional ferrous sulfate (grade of recommendation: A, based on randomized controlled trials), although the discontinuation rates between the 2 iron formulations were similar. Ferrous sulfate remains the standard first-line treatment of iron-deficiency anemia given its general tolerability, effectiveness, and low cost.
Evidence summary
A randomized, double-blinded, placebo-controlled study in 1496 subjects examined side-effect rates of 3 iron salt formulations using equal dosages of elemental iron (Table).1 Gastrointestinal (GI) side-effect rates were not significantly different. The side-effect rate in the ferrous sulfate group (23%) was significantly different from that of the placebo group (14%); thus, for every 11 patients treated with ferrous sulfate, 1 patient would have GI side effects attributable to the iron salt (number needed to harm [NNH] = 11).
Two formulations—controlled-release iron preparations and polysaccharide–iron complexes—decrease the amount of iron presented to the proximal GI tract. Three large randomized trials assessed tolerability of controlled-release iron preparations compared with ferrous sulfate.2–4 The only double-blinded study found a lower rate of nausea and epigastric pain in the controlled-release iron formulation among 1376 blood donors receiving 200 mg/day elemental iron (3.3% vs 6.4%, P < .05, NNH = ~32).2 A nonblinded randomized trial of 543 non-anemic adult patients taking 50 mg/day elemental iron also found a lower rate of stomach-related side effects in the controlled-release group (12.2% vs 27.2%, P < .001, NNH = ~7).3 However, none of the 3 studies showed a difference in the discontinuation rates between the 2 iron formulations. Comparative constipation rates among the trials were conflicting.
Two small, nonblinded, randomized trials of polysaccharide–iron complexes reported conflicting results. A study of 159 subjects found fewer subjects discontinuing the polysaccharide–iron complex taken with meals than ferrous sulfate taken on an empty stomach.5 A study of 60 subjects taking both preparations on an empty stomach found no difference in side-effect rates.6 Two small, randomized, blinded studies found no difference in rates of GI side effects between carbonyl iron and ferrous sulfate.7,8
TABLE
Representative average wholesale prices* for various iron supplement formulations
| Iron supplement group | Generic or brand name | Dosage | Cost of 1-month course |
|---|---|---|---|
| Ferrous salts | Ferrous sulfate (generic) | Tablet: 325 mg po tid | $0.63 to $5.11 (90 tabs) |
| Ferrous fumarate (generic) | Tablet: 300 mg (99 mg iron) po bid | $1.80 (60 tabs) | |
| Ferrous gluconate (generic) | Tablet: 325 mg (36 mg iron) po tid | $2.70 to $5.00 (90 tabs) | |
| Controlled-release | Slow FE (Novartis) | Tablet: 160 mg (50 mg iron) po tid | $18.92 (90 tabs) |
| Ferro-Grad-500 (Abbott) | Tablet: 105 mg iron po bid | $31.84 (60 tabs) | |
| Polysaccharide–iron complex | Niferex-150 (Schwarz Pharma) | Capsule: 150 mg iron po qd | $10.50 (30 caps) |
| Carbonyl iron | Feosol (SmithKline Beecham) | Tablet: 50 mg iron po tid | $18.38 (90 tabs) |
| *2001 Drug Topics, Red Book. Daily dosages given here deliver 150 to 210 mg of elemental iron and are for comparison of average costs. Actual dosage should be adjusted according to the calculated need for iron replacement and the results of laboratory monitoring. | |||
Recommendations from others
Wintrobe’s Clinical Hematology9 and Williams Hematology10 recommend ferrous sulfate as the standard oral iron preparation, and assert that claims of improved tolerability of one oral iron preparation over another have not been substantiated.
Clinical Commentary by Andrea Gordon, MD, at http://www.fpin.org.
1. Hallberg L, Ryttinger L, Solvell L. Side-effects of oral iron therapy. A double-blind study of different iron compounds in tablet form. Acta Med Scand Suppl 1966;459:3-10.
2. Rybo G, Solvell L. Side-effect studies on a new sustained release iron preparation. Scand J Haematol 1971;8:257-64.
3. Brock C, Curry H, Hanna C, Knipfer M, Taylor L. Adverse effects of iron supplementation: a comparative trial of a wax-matrix iron preparation and conventional ferrous sulfate tablets. Clin Ther 1985;7:568-73.
4. Elwood PC, Williams G. A comparative trial of slow-release and conventional iron preparations. Practitioner 1970;204:812-5.
5. Jacobs P, Coghlan P. Comparative bioavailability of ferric polymaltose and ferrous sulphate in iron-deficient blood donors. J Clin Apheresis 1993;8:89-95.
6. Sas G, Nemesanszky E, Brauer H, Scheffer K. On the therapeutic effects of trivalent and divalent iron in iron deficiency anaemia. Arzneimittel-Forschung 1984;34:1575-9.
7. Gordeuk VR, Brittenham GM, Hughes M, Keating LJ, Opplt JJ. High-dose carbonyl iron for iron deficiency anemia: a randomized double-blind trial. Am J Clin Nutr 1987;46:1029-34.
8. Devasthali SD, Gordeuk VR, Brittenham GM, Bravo JR, Hughes MA, Keating LJ. Bioavailability of carbonyl iron: a randomized, double-blind study. Eur J Haematol 1991;46:272-8.
9. Richard LG. Wintrobe’s Clinical Hematology. 10th ed. Baltimore: Williams &; Wilkins; 1999;979-1010.
10. Fairbanks VF, Beutler E. Williams Hematology. 6th ed. New York: McGraw-Hill; 2001;447-70.
1. Hallberg L, Ryttinger L, Solvell L. Side-effects of oral iron therapy. A double-blind study of different iron compounds in tablet form. Acta Med Scand Suppl 1966;459:3-10.
2. Rybo G, Solvell L. Side-effect studies on a new sustained release iron preparation. Scand J Haematol 1971;8:257-64.
3. Brock C, Curry H, Hanna C, Knipfer M, Taylor L. Adverse effects of iron supplementation: a comparative trial of a wax-matrix iron preparation and conventional ferrous sulfate tablets. Clin Ther 1985;7:568-73.
4. Elwood PC, Williams G. A comparative trial of slow-release and conventional iron preparations. Practitioner 1970;204:812-5.
5. Jacobs P, Coghlan P. Comparative bioavailability of ferric polymaltose and ferrous sulphate in iron-deficient blood donors. J Clin Apheresis 1993;8:89-95.
6. Sas G, Nemesanszky E, Brauer H, Scheffer K. On the therapeutic effects of trivalent and divalent iron in iron deficiency anaemia. Arzneimittel-Forschung 1984;34:1575-9.
7. Gordeuk VR, Brittenham GM, Hughes M, Keating LJ, Opplt JJ. High-dose carbonyl iron for iron deficiency anemia: a randomized double-blind trial. Am J Clin Nutr 1987;46:1029-34.
8. Devasthali SD, Gordeuk VR, Brittenham GM, Bravo JR, Hughes MA, Keating LJ. Bioavailability of carbonyl iron: a randomized, double-blind study. Eur J Haematol 1991;46:272-8.
9. Richard LG. Wintrobe’s Clinical Hematology. 10th ed. Baltimore: Williams &; Wilkins; 1999;979-1010.
10. Fairbanks VF, Beutler E. Williams Hematology. 6th ed. New York: McGraw-Hill; 2001;447-70.
Evidence-based answers from the Family Physicians Inquiries Network